Physiology of cerebral venous blood flow: from experimental data in animals to normal function in humans
|
|
|
- Hillary Hudson
- 7 years ago
- Views:
Transcription
1 Brain Research Reviews 46 (2004) Review Physiology of cerebral venous blood flow: from experimental data in animals to normal function in humans B. Schaller* Max-Planck-Institute for Neurological Research, Gleueler Strasse 50, D Cologne, Germany Accepted 26 April 2004 Available online 13 November Abstract In contrast to the cerebroarterial system, the cerebrovenous system is not well examined and only partly understood. The cerebrovenous system represents a complex three-dimensional structure that is often asymmetric and considerably represent more variable pattern than the arterial anatomy. Particular emphasis is devoted to the venous return to extracranial drainage routes. As the state-of-theart-imaging methods are playing a greater role in visualizing the intracranial venous system at present, its clinically pertinent anatomy and physiology has gain increasing interest, even so only few data are available. For this reason, experimental research on specific biophysical (fluid dynamic, rheologic factors) and hemodynamic (venous pressure, cerebral venous blood flow) parameters of the cerebral venous system is more on the focus; especially as these parameters are different to the cerebral arterial system. Particular emphasis is devoted to the venous return to extracranial drainage routes. From the present point of view, it seems that the cerebrovenous system may be one of the most important factors that guarantee normal brain function. In the light of this increasing interest in the cerebral venous system, the authors have summarized the current knowledge of the physiology of the cerebrovenous system and discuss it is in the light of its clinical relevance. D 2004 Published by Elsevier B.V. Keywords: Cerebrovenous system; Physiology; Animal; Human; Anatomy; Brain function; Hemodynamic Contents 1. Introduction Anatomy of the venous system of the brain Superficial cortical veins and their intracranial draining routes Anastomotic veins Deep (medullary and subependymal vein) and their draining routes Extracranial draining pathways Venous drainage of the posterior fossa Brainstem Cerebellar hemisphere Bridging veins and posterior fossa venous anastomosis Major dural sinuses of the posterior fossa Abbreviations: BBB, blood brain barrier; BOLD, blood oxygen level-dependent; CBF, cerebral blood flow; CBFV, cerebral blood flow velocity; CBV, cerebral blood volume; CSF, cerebrospinal fluid; CT, computed tomography; CVS, cerebral venous system; ISS, inferior sagittal sinus; MR, magnetic resonance; PET, positron emission tomography; pco 2, partial pressure of carbon dioxide; rso 2, regional cerebral oxygen saturation; SjO 2, jugular bulb oxygen saturation; SSS, superior sagittal sinus * Tel.: ; fax: address: Bernhard.Schaller@pet.mpin-koeln.mpg.de /$ - see front matter D 2004 Published by Elsevier B.V. doi: /j.brainresrev
2 244 B. Schaller / Brain Research Reviews 46 (2004) Collateral venous pathways Anastomoses of superficial cerebral veins Classical description The superficial sylvian vein Regional anastomosis Anastomoses of deep cerebral veins transcerebral vein Anastomoses of intra- and extracranial veins Anastomoses of the posterior fossa Elementary fluid dynamics as related to cerebral venous system Rheologic factors Physiological characteristics of the cerebral venous system Physiological factors influencing the cerebral venous drainage Physiological parameters influencing the venous pressure Changes of venous pressure Cerebral venous blood flow Biological background Venous outflow as source of slow oscillation low ICP CBV and CBF relationship Cerebral venous oxygenation Correlation between superior sagittal sinus flow velocity and cerebral blood flow Effect of age on cerebral venous circulation Conclusion References Introduction The cerebral vasculature plays a crucial role in maintaining adequate brain perfusion to meet the metabolic needs for normal cerebral function. In the current literature, less space is devoted to physiological studies of the cerebral venous system (CVS) than to that of the cerebral arterial system until present times. Therefore, a substantial gap still exists between the results obtained at well-defined but singular time-points in animal experiments, on one hand, and the findings collected incidentally in patients at various time-points after a CVS event, on the other hand [2,3,44,102]. In addition, cerebral blood vessels have some structural characteristics that are very different from those of blood vessels in other parts of the human body [44,85]. The lack of parallelism between arterial and venous circulations or the multiplicity of individual and hemispheric variations in CVS organization explain why their systemization remains difficult [97]. Anatomical descriptions mainly based on embryological data make a clear, but distinct difference between superficial and deep veins [68]. State-of-the-art imaging modalities have failed to clearly visualize this topographical difference until recently. However, technical advances in state-ofthe-art imaging methods such as magnetic resonance (MR) imaging, computed tomography (CT) or positron emission tomography (PET) facilitated CVS examination, leading to an improved knowledge especially of physiology and biophysics of normal cerebral (venous) hemodynamics [97,96]. These physiological examinations are important, so that transient pathophysiological changes can be followed until the final state of damage or recovery after pathological CVS events and can be compared to normal function in different animal models [95]. These different circumstances let to an increased clinical interest to physiology and biophysics of CVS, even so only few experimental data are available. For this reason, we have summarized the current knowledge of physiology and biophysics of CVS and discuss it in the light of its clinical relevance. 2. Anatomy of the venous system of the brain The CVS is mainly composed of dural sinuses and cerebral veins [39,68,72]. The venous outflow from cerebral hemispheres consists mainly of two different vascular systems [1]: (i) the superficial (cortical) system reaches dural sinuses by cortical veins and drains blood mainly from cortex and subcortical white matter [7,68]; (ii) the deep (medullary and subependymal) system is composed by subependymal veins, internal cerebral veins, basal vein and great cerebral vein of Galen and their tributaries, and drains the deep white and gray matter surrounding the lateral and third ventricles or the basal cistern [68,77]. A few superficial medullary veins also contain blood from cortical regions [68]. However, superficial cortical veins drain centrifugally and deep veins course centripetally.
3 B. Schaller / Brain Research Reviews 46 (2004) Superficial cortical veins and their intracranial draining routes Superficial cortical veins are usually quite small in diameter and highly variable in topography [1,7,68], so that venous vascular territories demonstrate substantial differences in their extension (see Fig. 1). The superficial middle cerebral vein runs along the sylvian cistern and drains the anterior temporal lobe, parasylvian cortex and anterior inferior frontal lobes [88]. It subsequently empties into cavernous sinus, sphenoparietal sinus or pterygoid plexus [88]. It is formed by anastomosis of temporo-sylvian veins; these veins are connected with midline bridging veins on one side and juxta-basal temporal veins on the other [88] and enter predominantly the cavernous sinus, either directly or through the sphenoparietal sinus [88]. Many anatomical variations are possible: (i) in size: when very thin or even absent, neighboring veins become of greatest importance; Fig. 1. Schematic drawing. (A) Cerebral venous system. (B) Cerebral venous anastomosis. Legend: Cs, confluens sinuum; Ips, inferior petrosal sinus; Iss, inferior sagital sinus; Sc, cavernous sinus; Os, occipital sinus; Ss, sigmoid sinus; Sss, superior sagittal sinus; Ts, transverse sinus; Vai, V. anastomotica inferior (Labbe); Vas, V. anastomotica superior (Troland); Vb, V. basalis (Rosenthal); Vcm, V. cerebri magna (Galen); Vcms, V. cerebri media superficialis; Vji, V. jugularis interna. (ii) in termination: draining towards the pterygoid plexus or the superior petrosal sinus [51,88]. The superficial sylvian vein is dominant in % of the cases [4]. The superior sagittal sinus (SSS) starts at the foramen coecum, just anterior to the crista galli [69]. It courses along the gentle curvature of the inner table of the skull, within the leaves of the dura mater, to reach the confluence of sinuses: the torcular Herophili [6]. Along the way, the sinus receives venous tributaries of the superficial venous system (i.e. superficial cortical veins). Its vascular caliber increased form anterior to posterior, but irregularly because of venous lakes contained within the two layers of the dura mater adjacent to the sinus and because of the penetration of arachnoid villi inside its lumen [6]. The SSS is circulating in a more or less laminal way, accentuated by the existence of longitudinal septa, especially in midthird portion. The lateral sinuses have an average diameter of 8 10 mm, but with frequent difference in size between the two sides [88]. Lateral sinuses drain the SSS, equally on both sides in only 20% of cases and asymmetrically in more than 50%, depending upon configuration of torcular Herophili [104]. In extreme, one lateral sinus may drain SSS in totality (most often on right side) and the other one the straight sinus; this accounts for 20% of the cases [104]. The transverse sinus can be atretic or even totally absent on one side (17% of cases) [104] so that the remaining sigmoid sinus drains the inferior cerebral veins (i.e. the Labbé system). The sigmoid sinus drains the posterior fossa [88] receiving the superior and inferior petrosal sinuses and also (in constant) veins coming from the lateral aspect of pons and medulla oblongata [104]. Sigmoid sinus has frequent anastomosis with cutaneous venous network through mastoid emissary vein [104]. When the sigmoid segment of the lateral sinus is atretic, the transverse sinus with its affluent drains toward the opposite side [104]. The inferior sagittal sinus (ISS) follows a similar curve within the free margin of the falx cerebri [111]. Often, its anterior portion is deficient and the sinus appears more linear than curvilinear. In contrast to its superior counterpart, the ISS empties into straight sinus, at or near its communicating with the vein of Galen [111]. The straight sinus, therefore, drains the deep venous system through the vein of Galen as well as much of superficial venous system indirectly through the ISS and basal vein [104]. As are all other sinuses, the straight sinus is situated within dural leaves, in this case, of tentorium cerebelli and falx cerebri [111]. Posteriorly, straight sinus empties into torcular [111]. Another complex network of dural sinuses collects blood along the brain s ventral surface [6] including the cavernous, superior and inferior petrosal, and sphenoparietal sinuses. Cavernous sinus is a trabeculated dural venous packet along parasellar sphenoid bone (sphenoid sinus) [20]. The sinus extends from superior orbital fissure to petrous apex. Its venous inflow includes orbital venous drainage (superior and middle ophthalmid veins), anterior middle cranial fossa drainage (superior and inferior middle
4 246 B. Schaller / Brain Research Reviews 46 (2004) cerebral veins) and sphenoorbital sinus. From cavernous sinus, blood drains posterolaterally along superior petrosal sinus to transverse sinus and inferior-laterally along inferior petrosal sinus into jugular vein [104]. Deep sinuses also interconnect by way of the basilar venous plexus along the dorsal clivus and the intercavernous sinus among the pituitary infundibulum. Occasionally, a prominent venous plexus connects the cavernous sinuses along the floor of the sella turcica Anastomotic veins The vein of Trolard represents the largest anastomotic cortical vein [1] and courses cephalad from the sylvian fissure toward SSS [76]. Convexity veins often unite just lateral to the midline, forming a common trunk, before entering SSS [76]. Cortical veins typically enter SSS at an angle perpendicular to the sinus and in its postcentral region, but may, however, penetrate its central or precentral portion [76]. This is particularly true in the frontal region: The angle becomes increasingly more acute towards posteriorly, however, especially in the occipital region [76]. Often, cortical veins draining occipital lobes proceed anteriorly before entering the sagittal sinus at an acute angle, against the direction of blood flow in SSS [76]. The vein of Trolard is predominantly in the minor hemisphere (46 52% in the right and 18 24% in the left) [4]. Inferior (juxta-basal) veins are cortical bridging veins that channel into basal sinuses (or into the deep venous system) [106]. The vein of Labbé, along with temporal occipital veins, drains lateral temporo-occipital part of cortex and empties into the transverse sinus [106]. The vein of Labbé occasionally drains portions of inferiolateral frontal lobes and in this case can be seen coursing across sylvian fissure [61]. Typically, the vein of Labbé course from sylvian fissure posteriolaterally toward anterior portion of transverse sinus and creates an anastomosis between superficial sylvian vein and the transverse sinus before its junction to the sigmoid sinus. Numerous variations exist, besides this classical topography. The anterior inferiolateral vein usually demonstrates a course that runs posterior and inferior toward the so-called btransverse venous pointq [106]. It occasionally reaches the lateral and inferior temporal lobe margin and takes a more medial course to run along the tentorial surface towards the btransverse venous pointq [106]. In about 20 30% of the cases, this anterior inferiolateral vein can be found to be the prominent draining vein of the posterior temporal lobe region with an adjacent vein, which is located in the expected anatomic site of the vein of Labbé, and that plays a more minor role [106]. The veins of Trolard, Labbé and Sylvius are often reciprocal constructed: when one is large, the others are typically small, but is often not symmetric between the both hemispheres [1] Deep (medullary and subependymal vein) and their draining routes The subcortical and deep white matter are drained by deep medullary veins [1] that originate mm below the cortex and course centrally to the subependymal veins that surround the ventricles [33,49]. The subependymal veins drain from the deeper subcortical structures, such as internal and external capsule, the basal ganglia and the dorsal part of the diencephalon, to the vein of Galen, which curves around the posterior surface of the splenium of the corpus callosum before terminating at the confluence of sinuses [49]. Except from the anatomical variations of the basal veins, the system of the inner cerebral veins remains relatively constant compared to the superficial cortical venous system [51,88]. Medullar veins can be subdivided into two different subgroups: The superficial medullar veins drain 1 2 cm of white matter and course through the gray matter [76]. They are connected to the superficial cerebral veins. The deep medullar veins begin within the white matter deeper than superficial medullar veins and course toward lateral ventricles, emptying into the subependymal veins of the walls of the lateral ventricle [33]. A third group of medullar veins (btranscerebral veinsq) completely transverses the hemisphere to reach subependymal veins [39,50]. All these white matter veins run perpendicular to the long axis of the lateral ventricle, creating a fem-shaped appearance [59]. Three subependymal veins are commonly seen on the venous phase of a cerebral arteriogram [18,67]: (i) thalamostriate vein, (ii) septal vein and (iii) internal cerebral vein. The thalamostriate veins join the septal veins from the internal cerebral veins [99,106]. The point at which the anterior vein of the septum pellucidum joins the superior thalamostriate vein is called the venous angle and is usually located at the interventricular foramen [99]. The internal cerebral veins are paired and run within the velum interpositum posteriorly, above the roof of the third ventricle [99]. These veins are joined by basal veins of Rosenthal forming the vein of Galen [99]. The basal vein of Rosenthal represents the confluence of veins draining basal and medial parts of frontal lobe, temporal lobe, basal ganglia and insula [99]. Occasionally, basal vein of Rosenthal can drain aberrantly into a tentorial sinus or superior petrosal sinus [99] Extracranial draining pathways Venous outflow from the SSS and deep cerebral veins is usually directed via the confluens sinum toward the sigmoid sinuses and jugular veins. Interconnections with other basal venous structures permit additional drainage toward vertebral venous system [75]. This freely communicating, valveless system is present throughout the entire spinal column and may be divided into an internal intraspinal part, the epidural veins and an extraspinal paravertebral part. With increased intraabdominal and intrathoracic pressure,
5 B. Schaller / Brain Research Reviews 46 (2004) blood is shunted through vertebral, prevertebral and epidural venous networks. At the craniocervical level, anterior sources of intraspinal system arise from basal plexus and inferior petrosal sinus [22]. A posterior communicating venous drainage exists with occipital sinus, which may be relevant in cases of lateral sinus hypoplasia [92]. The extraspinal system receives blood anteriorly from cavernous sinus via pterygoid plexus [92]. A dorsal part is connected to suboccipital venous plexus, which receives blood through mastoid and condylar emissaries [15]. Although the jugular veins were previously thought to be the predominant draining pathway [23], anatomical recent study-findings have demonstrated that this role is confined to the supine position; redirection of venous flow to the vertebral veins occurs in upright-position [92]. Rather than being a minor plexus that surrounds vertebral artery, vertebral veins in the cervical region represent large collecting vessels of the vertebral venous system; they are readily and receive their blood from condylar veins, from emissaries vein and through segmental connections [92]. 3. Venous drainage of the posterior fossa Venous drainage of the posterior fossa is as complex as that of the supratentorial compartment. In the posterior fossa, venous blood exits through galenic and petrosal systems and, to a lesser extent, tentorial veins and transverse sinuses [92]. Veins of the upper brainstem, dorsal cerebellum and vermis may drain through their various tributaries into the vein of Galen [22]. Included in this subgroup may be the precentral vein, a midline structured vessel that drains ventral and superior vermis [15] and initially parallels the anterior medullary velum only to turn posteriorly toward the quadrigeminal cistern at the colliculocentral point [92]. In the superior cerebellar cistern, this vein merges with the superior vermian veins as well as smaller midline and lateral tributaries, to reach vein of Galen [15]. The anterior pontomesencephalic vein, which lies ventral to brainstem, may drain rostrally to basal vein of Rosenthal (and, therefore, the vein of Galen) or petrosal venous system [15]. Posterior fossa veins are divided into four groups: (i) superficial, (ii) deep, (iii) brainstem and (iv) bridging veins. However, the veins of the posterior fossa terminate as bridging veins [1] Brainstem Generally, brainstem veins are named on the basis of whether they drain midbrain, pons or medulla and course transversely or horizontally [1,40]. The superior collector corresponds to the superior petrosal vein(s) so-called Dandy vein(s) which flow(s) to the superior petrosal sinus [1]. Its affluents consist of the vein of the lateral recess of the fourth ventricle, the lateral mesencephalic vein and the pontine vein. Superior petrosal veins also receive vein(s) of the superior aspect of cerebellar hemisphere. The inferior collector is much less important and corresponds to the inferior petrosal vein and its main affluents: the vein of the horizontal fissure, which drains the anterior part of cerebellar hemisphere [73]. The inferior petrosal veins often anastomoses with the vein of the lateral recess of the fourth ventricle. Venous drainage is toward the inferior petrosal sinus Cerebellar hemisphere The superficial veins drain cortical surface of cerebellum and are divided on the basis of whether they drain tentorial, petrosal or suboccipital surface and whether they drain hemisphere or vermis [90]: (i) veins of the posterior territory which drains toward torcular and median portion of transverse sinus directly or through medial intratentorial (small) sinuses; (ii) vein of superior territory, toward superior petrosal sinus; (iii) vein of anterior territory, toward inferior petrosal sinus [90]; (iv) veins of the superior aspect which drain to vein of Galen; (v) veins of inferior vermis, to torcular and/or medial portion of transverse sinuses. Constant precentral vermian vein runs anteriorly to culmen, up to the vein of Galen Bridging veins and posterior fossa venous anastomosis The terminal ends of veins draining brainstem and cerebellum form bridging veins that cross subarachnoid and subdural spaces to reach venous sinuses in the dura [105]. These bridging veins collect into three subgroups [105]: (i) a superior or galenic group that drains into the vein of Galen, (ii) an anterior or petrosal group that drains into petrosal sinuses and (iii) a posterior or tentorial group that drains into sinuses converging on the torcula. In the posterior fossa, venous anastomosis can be found longitudinally with upper cervical and foramen magnum veins (inferiorly) and the system of Galen (superiorly), as well as transversally between both sides across the ventral aspect of medulla, pons and mesencephalon [22]. Petrosal venous system drains the inferior, anterolateral portion of posterior fossa [22]. Specifically, anterior medullary vein (along the ventral medulla), anterior pontomesencephalic venous complex (including the transverse pontine veins), longitudinal lateral pontomesencephalic veins, anterolateral superior and inferior cerebellar hemispheric veins and the vein of the lateral recess of fourth ventricle, all reach the petrosal venous complex [105]. Finally, tentorium receives direct venous drainage from medial superior and inferior cerebellar hemispheres, and inferior vermian veins drains around vermis and ascends to straight sinus [105]. Posterior inferior cerebellar hemispheres may drain directly into the transverse sinus [105] Major dural sinuses of the posterior fossa Perioccipital sinus constitutes a venous intradural ring around foramen magnum [52]. Superior petrosal sinus
6 248 B. Schaller / Brain Research Reviews 46 (2004) courses along petrous ridges from the cavernous sinus to the transverse sinus [52]. The inferior petrosal sinus courses along the petro-clival suture; it drains retroclival sinus and cavernous sinus toward jugular foramen [52]. Retroclival sinus anastomoses both sinuses posteriorly to clivus, and dorsum sellae, it is connected with numerous and often voluminous extradural venous plexuses [52]. Small venous lakes can be more or less developed inside the tentorium. Part of these intratentorial sinuses have a median topography along the straight sinus; they drain cerebellar vemian afferent veins [52] draining cerebellar hemispheric veins and also occipital temporal veins (with sometimes the Labbe vein) toward transverse sinus. 4. Collateral venous pathways In addition to major anastomotic vascular venous channels that connect them both to meningeal veins and to intra- and extracranial veins, superficial cerebral veins widely anastomized with each other and build a fine network of venous vessels. This cortical drainage has a surprisingly regular organization [66]. Within grey matter, capillaries of 5 cm diameter (just wide enough to admit red cells) and mean length 200 Am drain into venules of Am diameter. These combine, at angles close to 908, to form larger venules. When a diameter of about cm is reached, the venule is termed a principal intracortical vein and typically leads directly to the cortical surface, at right angles to it. These veins often extend the full depth (about 3 mm) of the cortex, increasing in diameters as they approach the surface, swelled by incoming venules. Once the principal intracortical vein, spaced about cm apart, reach the surface they combine to form pial veins in a twodimensional drainage network on cortical surface [83]. Like intracortical venules, pial veins join nearly at right angles (Table 1). Table 1 Changes of cerebrovascular and cerebrometabolic parameters Primary reduction CBF CBV(ICP) AVDO 2 CMRO 2 = CPP (autoregulation intact) = + = CPP (autoregulation defective) + Blood viscosity = = (autoregulation intact) Blood viscosity + = (autoregulation defective) PaCO 2 + Conductive vessel diameter (vasospasm above ischemic threshold) + + CBF, cerebral blood flow; CBV, cerebral blood volume; ICP, intracranial pressure; AVDO 2, arteriovenous O 2 difference; CMRO 2, cerebral metabolic rate of oxygen; CPP, cerebral perfusion pressure; PaCO 2, arterial CO 2 tension Anastomoses of superficial cerebral veins Classical description On the lateral surface of each cerebral hemisphere two main anastomotic vascular-venous channels have been described [1]: (i) Labbe s vein and (ii) Trolard s vein. Both venous to venous anastomoses build a vascular connection between SSS and basal transverse sinus. Trolard s vein (vena anastomotica superior) links veins of the lateral sulcus to SSS. It runs along the surface of frontal and parietal lobe, at the level with the central region. According to Oka et al. [75], it usually corresponds to a postcentral vein. However, it is thought to be more frequent on non-dominant hemisphere [25]. Labbe s vein (vena anastomotica inferior) interconnects the veins of lateral sulcus and the transverse sinus. Oka et al. [75] demonstrated that this vein usually corresponds to a middle temporal vein and, less frequently, to a posterior temporal vein. It is thought to be more frequent on dominant hemisphere [25]. This is in accordance with an anatomical study of 175 brains, in which Delmas et al. [24] could show that Labbe s vein was more frequent located on the left side (42%) than on the right side (21%), whereas Trolard s vein was more frequently found on the right than on the left side The superficial sylvian vein In the embryo, the middle cerebral vein system is ramified [24]. Their tributary veins are arranged around middle cerebral veins at three branches [24]. As the opercula begins to develop, the veins of the hemispheric convexity get closer to each other [10] and eventually fuse to form superficial sylvian vein. This usually single vein runs along the whole length of sylvian fissure along lateral sulcus and receives fronto-sylvian, parieto-sylvian and temporo-sylvian veins. At the anterior end of the lateral sulcus, the superficial slyvian vein crosses subarachnoidal spaces to join cavernous sinus either directly or through spheno-parietal sinus [107]. Other modes of ending, described as para-cavernous sinus, are possible [2,107]; they run towards pterygoidal veins (spheno-basal sinus) or superior petrosal sinus (sphenopetrosal sinus). Among all the veins of the lateral brain surface, superficial slyvian vein is the most variable in both size and extend of venous anastomosis, which accounts for the great facilities of venous drainage of lateral sulcus and cavernous sinus Regional anastomosis Classical venous vascular arrangement does not account, by itself, for importance of fine and wide-spread anastomoses established by veins of the lateral surface of the hemisphere [1,35]. For this reason, Petit-Dutaillis et al. [82] described two types of cortical veins according to their mode of ending: (i) bipolar and (ii) unipolar. Bipolar veins have a central end in the superficial sylvian vein and a peripheral end in a venous sinus [82]. Unipolar veins only have a peripheral end. These types of ending veins are
7 B. Schaller / Brain Research Reviews 46 (2004) already found during fetal life [82]. In the 3-month-old fetus, there can be already found such a dual cortical venous system: the first one is central and joins the vein of lateral sulcus, whereas the second one is peripheral and joins dural sinuses [82]. In older fetus, both systems are anastomized [82]. In adults, the two-end arrangement is found only in certain topographical brain regions [82]. In other brain areas, veins revert to their bprimitiveq character. Depending on frequency of anastomoses with the sylvian system, Petit- Dutaillis et al. [82] have defined three venous brain areas, which lean against the above described unipolar bipolar venous system: (i) an bunipolar circulation areaq, where the regional veins are found to be little or not anastomized with the sylvian veins, this is the case in the occipital and parietal regions; (ii) a bbipolar circulation areaq, where anastomoses are constantly found as is the case in the rolandic and sylvian regions; and (iii) a blow circulation areaq, where the venous networks vary considerable in number, caliber and anastomoses, as in the frontal and anterior temporal regions Anastomoses of deep cerebral veins transcerebral vein Multiple venous anastomoses interconnecting deep cervical veins and other venous systems [107] are summarized in Table 2 borrowed from Mikhailov and Kagan [70]. Among these anastomoses, transcerebral veins have been described by Duret [27] and have given rise to important anatomical studies [35,70]. Their role has been considered in different studies [11,38,98] in that have been described two anastomotic systems that open after ligation of medial cerebral veins: (i) veins which cross hemispheric white Table 2 Anastomoses of deep cerebral veins (after Mikhailov and Kagan [70]) I. Deep cerebral veins superficial cerebral veins A. Extracerebral anastomoses 1. Medial Vs of hemisphere (Vs of corpus callosum SSS) 2. Inferior frontal Vs (RBV-SSS) 3. Inferior temporal Vs (RBV-LS) 4. V. of lateral sulcus (DSV-SSV) 5. Infero-medial occipital Vs (Vs of corpus callosum SSS+LS) B. Transcerebral anastomoses II. Deep cerebral anastomoses 1. Ventral Vs of pons 2. Superior cererbral Vs III. Deep cerebral veins dura mater sinuses 1. Spheno-parietal sinus 2. Cavernous sinus 3. Superior petrosal sinus IV. Deep cerebral veins with each other A. Extracerebral anastomoses 1. Plexus of lateral ventricle 2. Rosenthal s basal Vs V. Intracerebral anastomoses 1. Vs of corpus callosum 2. Lenticular Vs DSV: Deep sylvian vein, LS: lateral sinus, RBV: Rosenthal s basal vein, SSV: superficial sylvian vein, SSS: superior sagittal sinus, Vs: veins. matter and connect caudate longitudinal vein to cortical veins; (ii) veins which cross basal ganglia and connect subependymal veins of lateral ventricle to Rosenthal s basal vein; they are called external and internal lenticular veins. Similar to cisternal basal arteries, inner cerebral veins form a venous circuit. The basal vein of Rosenthal is the most important common final passage and represent the most prominent basal venous vessel, which can be separated into three segments: (i) anterior, (ii) middle and (iii) posterior [106]. Hypoplasia seldom occurs. Variations are restricted to anterior and posterior segments, whereas the middle segment of basal vein of Rosenthal is almost always standard in location and size, with a diameter of 2 3 mm. The deep middle cerebral vein is the second most important vein. It passes through the anterior perforated substance, where it joins anterior cerebral vein to form the anterior segment of the basal vein of Rosenthal [106]. On the basis of a microradiographic study performed after opafication, Hassler [39] considers that there is a brain area of habitual drainage of deep venous system complementing to some extent the superficial system. Moreover, this author has shown that injecting a radio-opaque substance into great cerebral vein of Galen promptly opacifies SSS and transverse sinuses [22] confirming the existence of transcerebral veins, the number of which is estimated at in each hemisphere [22]. Their usual caliber ranges from 0.25 to 0.35 mm, but wider veins have been rarely described [22]. They are sometimes located on the same sites as the white matter fibers [39,70] and seem to be influenced by locations of wide superficial veins. Goetzen [35] studied 100 injected hemispheres and concluded that deep veins of the brain tissue participate in the drainage of the cerebral cortex and, conversely, that the lateral superficial veins can drain lateral ventricle wall. For this reason, there are bvenous channelsq in hemispheric white matter, corresponding to centro-peripheral anastomoses [35]. These elements are characteristic of transcerebral anastomoses, as they demonstrate larger anastomotic network of cerebral veins compared to arteries [35]. Goetzen [35] observed centro-peripheral veins in 62% as against 3% for arterial centro-peripheral anastomoses Anastomoses of intra- and extracranial veins Batson [9] described vessel-connections between intraand extracranial veins at cranial base as a single large venous plexus, and this appears to be the most practical view: (i) The suboccipital venous plexus represents the cranial beginning of the posterior external vertebral plexus between the dorsal muscles; it is connected to the sigmoid sinuses via mastoid and condylar emissaries [80]. (ii) The posterior internal vertebral plexus receives blood from occipital sinus and thus from confluens sinuum (torcular) [80]. The torcular is often plexiform and asymmetrical, and when one lateral sinus is narrow or absent, occipital sinus is larger than usual; and in neonates the occipital sinus is very
8 250 B. Schaller / Brain Research Reviews 46 (2004) large [9,80]. (iii) The anterior internal vertebral plexus is a continuation of basal plexus, which lies on clivus and connects inferior petrosal sinuses and both cavernous sinuses [80]. (iv) The anterior external vertebral plexus is a continuation of large pterygoid plexus, which receive blood from cavernous sinuses and, via middle meningeal veins, from superior longitudinal sinus [9,80]. The pharyngeal venous plexuses may play a role in the anterior region that should not be ignored. Besides these affluent pathways, the outflow channels of vertebral plexuses can also be summarized [9]: (i) In the thoracolumbar area, outflow from vertebral plexuses takes place via the lumbo-azygos system, which also forms a collateral channel between inferior and superior vena cava. Valves in the azygos system are superior rudimentary and nonfunctioning. Obstruction of the superior vena cava cranially from the azygos vein, and is tolerated well, but obstruction involving connection of termination of azygos vein to heart leaves collateral flow only toward inferior vena cava, which results in insufficient cranial venous outflow [47]. (ii) In vertebral plexuses of cervical area, longitudinal collecting channels besides internal jugular vein on either side are deep cervical vein posteriorly between muscles, vertebral vein (through the transverse process of the cervical vertebrae) and, subcutaneously, external jugular vein [9]. These veins join subclavian and internal jugular veins in forming brachiocephalic vein [9] Anastomoses of the posterior fossa Deep venous system is not primarily vulnerable at the great vein of Galen, because channels towards basal vein can afford sufficient collateral flow [22]. The most vulnerable area is around the junction of internal cerebral and superior choroidal veins, because at this point principal flow and collateral flow can even be hindered at a distance, when obstruction of superior vena cava includes collateral outflow through azygos vein [80]. Anatomy of collateral venous pathways indicates location of vascular areas, where principal and collateral flow can be impaired simultaneously [80]. The most obvious example is the posterior cranial fossa, where flow both through compressible sigmoid sinus and towards vertebral plexuses can be impeded by a space-occupying lesion or a hypoplasia of chondrocranium [9] causing increased blood flow through cavernous sinus that has become connected to cerebral veins [52]. In most infants and some adults this is not case, so that vulnerability is increased. On the other hand, other routes from venous outflow from superficial veins may exist in infant, namely via occipital sinus, which is still large, and through open cranial sutures via scalp veins, although open cranial sutures via scalp veins, and these will always be insufficient [15]. Cerebrospinal fluid (CSF) pressure depends on a hydrostatic-osmotic pressure equilibrium with choroidal capillaries and veins [101]. Cranial enlargement will occur when cerebrospinal fluid pressure greatly exceeds atmospheric pressure, while cranial sutures are still open [101]. Cerebral surface retains its relationship to enlarging cranium and size of lateral ventricles increases [101]. But insufficient deep venous flow also causes periventricular atrophy, manifest as ventricular dilatation, when cranial vault is not enlarged [101]. 5. Elementary fluid dynamics as related to cerebral venous system Cerebral veins were collapsible because of their specific cytoarchitectionial construction. Collapsible blood vessels or tubes are characterized by marked changes in their crosssectional configuration when transmural pressure is slightly positive or negative. Transmural pressure can drop either by lowering intraluminal pressure or by increasing external pressure or both. Internal pressure can change by variables in Bernoulli-Poiseuille equation (e.g. upstream or downstream resistance to flow, flow rate, acceleration or deceleration, negative gravitational pressure). It should be emphasized that changes in external forces, meaning increased compartment pressures, can affect blood flow simply by altering the bgeometryq of collapsible tubes, which affects variables in Bernoulli-Poisuille equation, such as viscous resistance, acceleration deceleration [108]. When transmural pressure is slightly positive, the vessel is circular and distends with increasing transmural pressure. However, slightly negativity of transmural pressure causes very marked collapse of the vessel and a reduction in its cross-sectional area or possibly complete closure [108] implying a great increase in viscous resistance to flow. The mathematical equations for resistance to flow of collapsible vessels that are flat, biconcave or dumbbell-shaped in crosssection have not been worked out to knowledge [108]. Such studies are pertinent to our discussion since blood flow in collapsible vessels, like veins, has flow characteristics somewhat similar to that of collapsible tubes [108]. The earliest study was carried out by Holt in 1941 using latex penrose drain tube placed horizontally in an enclosed jacket with flow derived from a Mariotte bottle which keeps energy head constant as liquid flows through tube system [48]. Energy gradient could be altered by elevating or lowering the temperature of a thick-walled outflow rubber tube. The pressure immediately upstream and downstream of the jacket and the jacket pressure were recorded with water manometers. Such studies were extended by Permutt et al. [81] in models in which external pressure compressing collapsible tube (called Starling resistor) was either below or above downstream pressure. Analysis of these experimental models led to the following conclusions: (i) when downstream pressure adjacent to collapsible tube is greater than external pressure on the tube, flow is proportional to the difference between upstream and downstream pressure and changes in external pressure on the collapsible tube have no
9 B. Schaller / Brain Research Reviews 46 (2004) influence on flow; (ii) when external pressure on collapsible tube is greater than downstream pressure, flow is proportional to the difference between upstream pressure and external pressure on the tube and changes in downstream pressure have no influence on flow [108]. The latter relationship was considered to be similar to a bwaterfallq. These concepts were applied to pulmonary circulation shown in constant, while pulmonary venous pressure is reduced to and below the alveolar pressure. If down-stream pressure is kept constant and up-stream pressure is increased, the pressure-flow curve is quite different and does not demonstrate flat portion, which is the basis of the bwaterfallq concept [46,81]. Subsequent experimental studies on collapsible tubes (penrose drain) were carried out by Katz et al. [57] using measurements of pressured gradients and flow together with photographs of tube contour at different perfusion pressures. Pressure gradients along the length of the tube were altered by varying constriction of orifices upstream or downstream to the tube [57]. This experimental approach is significantly different from the model of Permutt et al. [81] in which upstream pressure is held constant and downstream pressure is reduced, thereby increasing in pressure gradient. According to Katz et al. [57] these results on flow and on configuration of distensible tube vary depending on which pressure is primarily altered: upstream or downstream. When the penrose drain is fully open, flow increases with increase in pressure gradient between up-stream and downstream pressure. However, once the tube begins to collapse, flow diminishes progressively as perfusion pressure increases (called bnegative slopeq by these authors) until a maximum pressure gradient is reached, beyond which collapse is so severe that all flow stops. In some experiments, there was flutter in the partially collapsed tube in the region of negative slope [57]. Further studies on pressure-flow-through penrose tubing (Starling resistor) were conducted by Lyon et al. using different viscosities of liquids to vary Reynolds number [65,64] and demonstrated that the waterfall model proved adequate to describe flow in the Starling resistor only at very low Reynold s number (Reynold s numberb1) [65]. Since such low Reynold s number occur only in microvessels, they concluded that it is inappropriate to apply the waterfall model to flow through large collapsible veins [65,64]. In our opinion, data on penrose drain tubes cannot be applied without some reservation to vascular beds in vivo. 6. Rheologic factors Mean blood velocity amount accounts ~0.05 cm/s in microvessels of cerebral venous drainage [100] and does not exceed 10 cm/s of the vein s vessel of capacity [100]. At the present time, exact blood velocity in veins (variation between 30 and 50 cm/s in internal jugular vein) is unknown, so that it can be only said that blood velocity may be rapid in the sinus [100]. Physiology of venous pressure is better understood. It varies in capacity of venous vessels dependent on the patient s position, revealing the important role of position in CVS. In cerebral sinus, venous pressure may be about +1 mm Hg in the lying position and 3 mm Hg in the up-right position [42]. It is suggested that, in up-right position, there may be assumed a venous flow of ml/min corresponding to a displacement of approximately 80 ml [42]. In the main CVS (e.g. straight sinus, vein of Galen and basilar veins) the markedly cyclic changes are synchronous with arterial pulsations. The position of systolic arterial velocity pulse at the ascending part of venous waveform suggest that arterial systolic volume pulse might compress venous channels and thus increase velocity. This argument is, however, only valid, when venous volume flow is assumed to remain constant. Several factors might lead to a pulsatile venous pattern [19]: (i) increased systolic volume pulse due to increased cerebral blood flow (CBF); (ii) a large difference in systolic diastolic pressure, particularly with impaired autoregulation; (iii) increased stiffness of brain parenchyma due to edema or increased intracranial pressure (ICP); (iv) modulation of flow velocity by changes in intrathoracic pressure. With the exception of increased partial pressure of carbon dioxide ( pco 2 ), two factors were of major importance for venous flow velocity [53]: (i) diastolic arterial blood pressure and (ii) peak ventilation pressure. Stepwise discriminating analysis indicates that resistance index of pericallosal artery is closely related to diastolic blood pressure, whereas venous flow velocities and pulsatile factors were markedly below any level of significance [53]. A high resistance index of, for example, 1.00 or more, any thus merely indicated low diastolic blood pressure (below 25 mm Hg) and is of little or no additional value in such a case [53]. An increased peak ventilation pressure is significantly associated with a relative period of no recorded flow velocity in the basilar veins [34,86]. The other two factors as selected by discrimination analysis were time average velocity in pericallosal artery and straight sinus, but these F-values are not significant [34]. High ventilation pressure could thus impair cerebral venous drainage intermittently and not solely act through an increased ICP [19], possibly explaining a slight fall of time average arterial velocity with high ventilation pressure [19]. Transduceinduced pressure is another major factor influencing arterial and venous flow velocity in small premature, ventilated infants: diastolic arterial flow velocity decreases, as does systolic to a lesser degree, whereas flow velocity in the straight sinus rises [100], indicating that the straight sinus might be compressible [100]. One can assume that CVS has some kind of pulsation, which means that there can be demonstrated a hydraulic translation that can be observed at the exit of the sinus (named bsinus pulseq). In view of the frequency of the pulsation, the origin seems to be a residual arterial pressure.
10 252 B. Schaller / Brain Research Reviews 46 (2004) But the respiration, which means the thoraco-abdominal pump, lead to changes of the amplitude of these pulsations [16]. Among dural sinuses, the vein of Galen has lower amplitude pulsations than other dural sinuses suggesting that amplitude of intracranial venous pulsations might increase as flow runs from periphery toward proximal portion [100]. Physiologically, intracranial venous pulsation can be explained as passive pulsations transmitted from the heart or brain. The fact that brain pulsation exist is known and a pulsatile brain can be seen after craniotomy. Brain pulsations associates with cardiac cycles were proved by studying epidural pulse waves, CSF pulse waveforms and anterior frontal pulsations. Hirai et al. [45] investigated the origin of epidural-pressure pulse waveforms measuring epidural-pulse and pressure waveforms of cortical artery or vein simultaneously and found that pulsatile arterial blood flow into the brain can generate brain pulsations and that cortical venous pulse can be affected passively by brain pulsations [45]. CVS can also have a passive pulsation directly transmitted from the heart [45]. Whether cerebral venous flow is influenced by brain pulsation or heartbeat remains unclear, but it is reasonable to suggest that the amplitude of venous pulsations increases from distal to proximal portion of CVS. The veins, which have thin walls, are easily affected by the tissue-pressure outside of CVS. Intracranial dural sinuses (e.g. SSS) are outside the convexity between hemispheres; therefore, venous circulation can be subjected to a larger effect than arterial circulation because of the increased tissue-pressure around it [86]. However, SSS is within the dura mater that covers the buffer zone, which absorbs pressure transmitted to it by moderately increased ICP. When that pressure increases, it progressively obliterates this buffer zone; thus the pressure stretches the dura mater or sinus so that brain pulsations are not transmitted to them. That physical explanation might account for the flattered venous waveforms in cerebral sinus. ICP waveforms are influenced by intracranial constituents and compliance of the container [94], so disappearance of venous pulsations might indicate high ICP [37]. MR spectroscopy of venous signal (for detail description of the method, see Ref. [28]) demonstrates a considerably smaller proportion of heart rate-and respiration related oscillation, but a considerably higher proportion of low and very low frequencies, compared with all the other parameters [74]. The fixed diameter of the incompressible venous sinus may be one reason for this minor influence of heart action; also, the capillary system and venules cause a deceleration of the pulse wave before it reaches large cerebral veins. Respiratory effects on venous outflow via central venous pressure also seem to be of minor effect on oscillations in the venous sinus [103]. The higher proportion of low and very low frequencies may reflect a venous autoregulatory mechanism [103]. Apart from the description of low pulsatility, no comparable data for the venous CBF exists in the literature. 7. Physiological characteristics of the cerebral venous system CVS is maintained by non-contractile veins with some hinds of pulsative waves, which were consequently influenced among other things by pulsation of concomitant arteries. In effect, blood arrives at the exit of the capillaries with a certain pressure, as pressure and pulsability of cerebral arteries are key-source of cerebrovenous flow. Physiologically, CVS is mainly passive and influenced by different extravascular factors. Venous reflow of the brain is supported by an estimated pressure of 20F5 mm Hg in the subarachnoidal veins [31] and is maintained by the residual capillary pressure (vis a tergo), by the transmission-pressure of the ICP (vis a latere) or by the niveau of pressure in the venous sinus (vis a fronte) itself [31]. From this mathematical point of view, a venous reflow exist physiologically in the case that the arterial pressure is superior to the subarachnoidal pressure [31]. Based on principles of physics to intracranial contents, it can be hypothesized according to the Monro-Kellie hypothesis, that going out from an intact skull, the sum of brain volume plus CSF, plus cerebral blood volume (CBV) always remains constant [19]. Therefore, an increase in one of these parameters should cause a reduction in one or both of the remaining two. As the intracranial content is incompressible under physiological conditions, the blood circulating in the cranium is therefore of a constant volume at all times [19]. Arterial vasodilatation can only be produced by a volume reduction of the intracranial veins that, as far as it is concerned, can be achieved by lowering the venous pressure [109]. The subsequent volume compensation by blood can be achieved by variations of the cerebral fluid volume extra- and intracellularly [109]. For this reason, every increase of the volume of a part of the intracranial content would require volume compensation. CVS is primarily affected, as it maintains a pressure that is normally not largely different from that of CSF [98]. On the other hand, circulating arterial blood is not reduced as venous pressure is not as high as that of arteries [109]. Changes also take place at the spinal level. The vertebral canal is encased by bony and fibrous components that are though and only slightly elastic. While in the cranial cavity, the dura mater is closely applied to the inner surface of cranial bone; in the spinal canal, there is an epidural space between the dura mater and the fibroosteal canal, which is filled with fatty areolar tissue and epidural venous plexus. The capacity of the spinal venous system is not totally known because the numerous anastomoses, but varies between 200 and 1000 ml [112]. The biological significance of the tendency that small arteries pass superficial to veins more frequently than large arteries remains yet unclear. But it is easily understood that compression of veins by arteries is reduced, thus inducing less stasis of venous flow at locations where veins are crossed over by smaller arteries, as compared to locations
11 B. Schaller / Brain Research Reviews 46 (2004) where veins are crossed over by larger arteries. It has been proposed that a major route for drainage of interstitial fluid from the cortical gray matter in the periarterial space along the cerebral arteries and that this route plays an important role in protecting the cortex from edema in different cerebral diseases [110]. However, it seems more likely that the cortex, the most superficially situated tissue of the brain, is protected from excessive retention of interstitial fluid primarily by unhindered venous flow from the surface of the brain tissue to the dural venous sinuses Physiological factors influencing the cerebral venous drainage CVS is additionally influenced by the thoracic aspiration (subathmosphere pressure). At the time of expiration, the intrathoracal or pleural pressure is negative, approximately 5 cmh 2 O. The inspiration is the source of the respiratory muscular action which can generate an intrathoracal pressure still more negative ( 8 cmh 2 O). This intrapleural pressure decrease contains the pressure changes of intrathoracal veins being constantly dilatated and favors the venous return to the right heart [17]. The transmural venous pressure of the spinal venous system is determinated by the CSF pressure [56]. The intracranial and spinal liquor system communicate freely by the foramen magnum. For this reason, the pressure gradient between CSF and veins is the forcing power of spinal venous blood flow [56]. Under physiological conditions, there can be found in the horizontal position a pressure of 11 mm Hg at the level of craniocervical junction [1]. In the upright position, CSF pressure at occipital niveau is subathmospheric ( 5 cmh 2 O) and, at lower spinal level, it can be reached a pressure of cm H 2 O. From a physiological point of view, one should also consider that same postural influences affecting cerebral venous drainage might redirect the circulation of the internal and external vertebral venous system. Two different ways of cerebral venous reflow related to the posture is known in primates and in humans. In up-right positions, venous drainage is preferentially via the spinal venous system, while it is the anterior jugular system in the lying position [89]. The spinal venous system represents a main venous drainage system in humans, even in the sitting or lying position [89] Physiological parameters influencing the venous pressure Regarding the brain tissue, the cerebrospinal canal may also represent a column of fluid outside the true blood vessels, which could compensate automatically for the transmural pressure variations during shifts for recumbent to erect positions and vice versa. If so, transmural vascular venous pressure will be independent on the height of the organ above the heart and no venous collapse or transcapillary absorption will occur [56]. However, these physiological explanations are unlikely as the tissue pressure is shown to be roughly constant in, for example, the epidural space, independent of the position of the body. The interstitial and epidural pressure are close to the atmospheric pressure or even slightly negative. Furthermore, the cerebrospinal space is a closed and quite rigid cavity, and therefore the intracranial ventricular pressure will not decrease in relation to the elevation of the head. It is a well known fact that autoregulatory mechanisms protect organs such as the brain from variations in CBF and hydrostatic capillary pressure during alterations in arterial pressure. The results of Asgeirsson et al. demonstrate that organs positioned above the heart are protected from the venous side, during elevation, via variations in the outflow resistance [5]. Provided these results can be transferred to the damaged brain with impaired autoregulation and disrupted blood brain barrier (BBB), head elevation will not reduce intracranial hypertension through increase in venous drainage as previously suggested, but ICP may instead be reduced from the arterial side through transcapillary absorption [12]. However, a decrease in interstitial pressure causing transcapillary filtration may occur during head elevation due to the existence of the semi-rigid spinal canal [5]. The upright position evokes a venous resistance at the outlet of CVS and the size of this resistance is directly related to the height of the elevation [12]. The resistance starts to develop as soon as the elevation exceeds venous pressure level. This variable outflow resistance effectively isolates the organ from any hemodynamic influence of the decrease in venous hydrostatic pressure, except for the small volume changes inherent in variation in the venous outflow resistance per se [12]. 8. Changes of venous pressure With the assumption that the entire increase in right arterial pressure is transmitted through the jugular venous channel, the subsequent collapse of jugular veins, when the head is elevated, may be the main vascular resistance to pressure transmission upward [63]. One mechanisms by which the surrounding pressure of a vascular system can affect CBF is based on vascular waterfall model of the pulmonary circulation [63]. This model suggests that, if extravascular pressure exceeds intravascular pressure within a collapsible segment, there must be a certain point at which the surrounding pressure will exactly be equal to the intrasvascular pressure. Under these conditions, CBF through vascular segment proximal to that point will be proportional to arterial inflow pressure minus surrounding pressure. As long as outflow pressure remains less than surrounding pressure, outflow pressure will have no effect on altering CBF through vascular segment. However, when outflow pressure is equal to or higher than surrounding pressure, it becomes the bach pressure for CBF. Similarly, it
12 254 B. Schaller / Brain Research Reviews 46 (2004) has been demonstrated that cerebral vein collapses when it is elevated above the heart because surrounding pressure is greater than pressure inside the vein, thus creating a Starling resistor effect [65]. In a Starling resistor, defined as an easily collapsible vascular segment submitted to external pressure, CBF will start when upstream pressure is large enough to cause pressure inside the collapsed segment (intravascular pressure) to exceed external pressure (extravascular pressure) and thus open the vessels [65]. Starling resistor upstream pressure at which vessels collapse is often referred to as the closing pressure [65]. With relatively constant CBF, such as in a living subject, the upstream pressure remains almost constant at this level, despite small changes in downstream pressure [65]. Holt [48] demonstrated in dogs that, when a vein was held above heart level, arterial pressure had to be increased by several centimeters of water before there was any rise in peripheral venous pressure. Humans in the sitting position, the elevated cephalic vein partially collapsed at the point at which it entered the upper end of the thorax, and the venous pressure was not a function of right arterial pressure [48]. In the sitting or head-elevated position, cerebral venous blood exists through the internal jugular veins, emissary veins and vertebral venous plexus in humans. Epstein et al. [32] demonstrated in monkeys with erect position that cerebral venous blood drains primarily from the vertebral venous system with little from the jugular veins and that the proportion of outflow depended on the airway pressure. Eckenhoff [30] explained the cerebral venous outflow as a dynamic state: during expiration, high-intrathoracic pressure causes a major proportion of blood to drain via the vertebral plexus; during inspiration, low-intrathoracic pressure causes blood to drain freely from both the jugular veins and vertebral plexus. During forced expiration, high-intrathoracic pressure stops the return of blood via jugular veins [79], so that all cerebral venous blood flows into vertebral plexus or out into other venous beds [79]. The vertebral venous plexus has been described as an enormous, valveless, thinwalled vascular bed contained within the spinal canal that courses between and parallel to vertebral bodies [8]. Blood is free to flow in and out of these venous plexus and do not directly connect to the superior vena cava, so that it is not subjected to immediated intrathoracic pressure variations [65]. These two unique characteristics, its large capacitance and its indirect connection with the superior vena cava, may also partially explain why pressure transmission is less effective in the head-elevated position [65]. Confluens sinuum pressure is elevated by retroflexion and lowered by anteflexion of the neck [55]. Right rotatation of the neck causes slight elevation of confluens sinuum pressure while left rotation causes no marked change. The cause of this difference is not obvious [55]. Of extraordinary importance is the fact that confluens sinuum pressure is elevated without exception by jugular compression, especially when applied bilaterally [55]. In patients in supine position, confluens sinuum pressure is raised from 6.6F2 to 19.6F3.6 mm Hg by bilateral compression [55]. In the patients in the sitting position, bilateral jugular compression elevated confluens sinuum pressure from 5.5F1.3 to +6.7F4.2 mm Hg [55]. 9. Cerebral venous blood flow 9.1. Biological background Starling resistor formula has been used to represent cerebral venous outflow, wherein flow is independent of extracranial venous pressure and depends only on the upstream transmural pressure [65,93]. However, cerebral perfusion pressure, which corresponds to the upstream pressure for the whole brain, does not convincingly fit the waterfall model and completed cessation of CBF, when ICP increases to blood pressure levels, is difficult to imagine because of presence of a significant arteriovenous pressure difference along an open vascular system [71]. This discrepancy also was demonstrated experimentally by use of two collapsible tubes perfused in an enclosed chamber to simulate arterial and venous intracranial beds. Equalizing the chamber and inflow pressures produced on 80% CBF drop, but no complete cessation as would be predicted from the Starling model [71]. Unfortunately, this pathophysiological discrepancy is left unexplained. There is a close coupling relationship between ICP and cortical venous pressure, with cortical venous pressure ~2 5 mm Hg higher than ICP. This offset is not dependent on even large changes in ICP that might affect CBF and does not agree with the Starling resistor principle, in which flow is directly proportional to upstream transmural pressure, namely venous pressure minus ICP. The laboratory model indicated that this apparent contradiction is related to the place at which upstream pressure is measured. Provided an adequate level of CBF is maintained, the pressure just at the entrance to the collapsible part is changing in parallel with ICP after a sharp exponentional relationship between flow and pressures at this levels. Under the same conditions, the pressures separated from the collapsible segment by an additional resistance, followed the Starling formula. Pressure and flow in the bridging veins are related each other through the same exponential curve. Consequently, in humans, the change from severe hyperemia (total CBF, 1000 ml/min) to serve ischemia (CBF, 300 ml/min) produce only 1.5 mm Hg change in difference between ICP and pressure in the bridging veins. Unless specifically thought, such as small differences would go unnoticed in any clinical or experimental study, and pressures would be assumed to change bin parallelq. In contrast, the model predicts that at the level of small pial veins, effects of distal venous resistance would prevail and flow would obey the laws of a Starling resistor [65]. For flow changes from hyperemia to ischemia, pressure will change by up to 37 mm Hg, proportionally to CBF [65]. For a large CBF decrease,
13 B. Schaller / Brain Research Reviews 46 (2004) nonlinear logarithmic term becomes significant, and pressure coupling downstream and flow-pressure proportionally upstream cease [65]. Specifically, there will be non-zero flow for ICP as high as inflow pressure Venous outflow as source of slow oscillation Normal CVS exhibit natural slow variations in pressure and flow values. It is accepted that increased intracranial slow wave activity denotes an abnormality, although exact mechanism of this phenomenon is still disputed. An interesting features of the proposed flow-pressure model is that it is not continuous when ICP crosses outflow pressure (equivalent to SSS pressure). The amplitude of the fluctuations increased visibly with increased amplitude of pulsation transmitted downstream to inflow pressure (simulating cerebral intraparenchymal venous pressure) when proximal resistance is removed noncontinuous flow-pressure characteristics of the collapsible tube have been noted previously [43]. The measurement of these characteristics is difficult owing to possible hysteresis in collapsible tube behavior [13] and resulting self-generated flow [80]. In the formula suggested earlier, transition between linear and logarithmic models is immediate. Under physiological conditions, the influence of rapid change of behavior of venous outflow is likely to be small, because of provision of a pressure head for flow. The upstream system displays strong flowregulating properties. Therefore, transition between stable states will occur without much changes of flow and moderate change in transmural pressure. However, in systems in which the up-stream regulation of flow is disturbed, steep change in properties for venous outflow may add substantially to generation and transmission of pressure and flow waves low ICP Piechnik et al. [84] investigated low CBF behavior for negative downstream transmural pressures (ICPbSSS). Although such pressure distribution is not physiological during longer time periods, it may attain clinical importance by shunting for hydrocephalus with intracranial hypotension, as a result of overdrainage related to vertical body position, increased ICP wave activity or CSF leaks [21]. Under such experimental conditions, venous pressure in bridging veins and ICP become nonphysiologically dissociated [21]. An additional stress is placed on the walls of bridging veins that may be relevant to our understanding of postshunting complications in hydrocephalus: it is thought that artificially CSF overdrainage from cerebral ventricles produces their passive collapse [87]. These results in stretching of venous vessels in subarachnoid space, which increases the chance of their rupture and subsequently hemorrhage [91]. From our model, a complementary scenario may be proposed in which pial veins engorge because of unphysiologically large transmural pressures [84]. These changes in subarachnoid space and parenchyma may contribute to postshunting ventricular collapse. In addition, repeated stretching and relaxing of veins in the subarachnoid space from pressure fluctuations may produce an additional bwear-and-tearq effect, which could be significant in producing venous rupture in addition to increasing the stretch from collapsing brain [84] CBV and CBF relationship Venous regional cerebral blood volume (rcbv) changes are much less than arterial rcbv changes (see Fig. 2). The ratio of arterial and venous rcbv changes measured by nuclear magnetic resonance (NMR) is consistent with that determined by vessels diameter measurements [41]. It is well-known that hypercapnia similarly induces vascular vessel diameter changes in cerebrum and cerebellum. Furthermore, although differences in anesthesia under experimental or clinical conditions may affect vascular responses to hypercapnia, it is likely that the ratio between the diameter changes of arteries and veins will be preserved [41]. Translation of the vessel diameter data into accurate rcbv changes should take into account the fractional diameter changes represent by all size and distribution of vessels [41]. Thus, it is difficult to directly compare diameter changes obtained by videomicroscopy and with rcbv measured a diffusion-weighted by 19 F NMR method (diffusion-weighted NMR signals show two distinct pseudodiffusion (D*) components: the larger D* component predominantly reflects more oxygenated arterial blood, the smaller D* component represent (less) oxygenated venous blood [28]). However, the ratio of arterial and venous rcbv changes in the entire brain measured by 19 F NMR can be compared with the ratio of an arterial and venous diameter changes in small-size vessels measure by videomicroscopy, assuming larger arterial and venous blood vessels behave similarly and also assuming that the ratio of blood volume fractions in small and large vessels are similar in the arterial and venous trees [41]. Fig. 2. Schematic plot. Venous and arterial rcbv vs. rcbf in the brain. Legend: CBV, cerebral blood volume; CBF, cerebral blood flow.
14 256 B. Schaller / Brain Research Reviews 46 (2004) CBV through vessels, which can be determined by the product of the cross section of the blood vessel (A) and the linear velocity (V), should be conserved at various sections of blood vessels. Thus, CBF can be changed by the modulation of A and/or V. Since the changes of A is directly related to CBV, CBF and CBV changes are interrelated as CBF stim /CBF cont =(A stim /A cont )(V stim /V cont )=(CBV stim / CBV cont )(V stim /V cont ), where subscripts bstimq and bcontq indicated bstimulatedq and bcontrolq conditions, respectively. According to CBV and CBF observations during hypercapnia, it is expected that in case of venous blood velocity is much more than that of arterial blood velocity considering that the rcbv change in venous blood is much less than that of arterial blood. An increase in blood velocity is much less than that of arterial blood. An increase in blood velocity, especially in the venous blood pool, increases the dephasing of diffusion-weighted NMR signals, resulting in increased D* during hypercapnia. D*1 (fast-moving components) and D*2 (slow-moving components) are linearly correlated with pco 2 level, which is closely related to CBF [60]. In the study of Lee et al. [60], the D* values of arterial and venous blood did not show significant correlation with CBF due to a large variability between animals and trials. This needs to be further investigated. However, it is generally accepted that cerebral blood flow velocity (CBFV) functioned as an indicator of the cerebral blood volume, since the CBFV is proportional to the cerebral blood volume and corresponding changes. Until now, it has been assumed that relative total CBV change, which was answered by contrast agent-based methods or estimated from relative CBF changes using Grubb s equitation of CBF-total CBV relationship. Based on Grubb s equation and the findings of Lee et al., a 100% increase in CBF induces 31% total CBV increase [61]. However, venous rcbv change (e.g. 15%) is about half of the total venous rcbv change (e.g. 31%) [61]. An increase in venous blood volume decreases the blood oxygen leveldependent (BOLD) MR imaging signal (for detailed description of the method, see Ref. [36]), while an increase in venous oxygenation level increases the BOLD signal [61]. For a given BOLD signal changes, less blood oxygenation level change. Therefore, the venous oxygenation level change calculated from BOLD and total rcbv changes (instead of the venous CBV change) would be overestimated [61]. The overestimation of venous oxygenation level by using total rcbv during neural stimulation results in an underestimation of oxygen consumption changes determined from BOLD and CBF/CBV data [61]. These problems can be alleviated by replacing the total rcbv change with the venous rcbv change, i.e. approximately 50% of total rcbv change [61] Cerebral venous oxygenation Oxygen in the cerebral blood must cross the BBB to reach into the interstitial space and subsequently into the intracellular space to be utilized predominantly in the mitochondria. It has been proposed that the dissolved oxygen does not diffuse freely from the blood pool into the interstitial space because the BBB acts as a barrier to the passage of oxygen [12]. If this is the case, a large gradient of O 2 -concentration across the vascular-interstitial compartment would be theoretically expected. Venous hemoglobin saturation of oxygen is an important physiological parameter for assessment of tissue oxygenation [61]. The dynamic changes of blood oxygenation provide a passage of functional information, and this physiological variable could be used to assess the strength of brain activation directly. Nearinfrared spectroscopy is a tool that has the potential to provide similar but continuous, noninvasive and safe information about cerebral oxygenation status. Although estimates of regional cerebral oxygen saturation (rso 2 ) include arterial and capillary components, the largest contribution is considered to be venous, which has been estimated to represent 75% of the CBV [62]. Thus, changes in jugular bulb oxygen saturation (SjO 2 ) should be paralleled by changes in rso 2, although close agreement between the two parameters would not necessarily be expected as different entities are being measured [62]. The relationship between the two variables (as defined by linear regression analysis) varies considerably between patients [62] suggesting that it may not be possible to predict absolute SjO 2 for any given patient based solely on rso 2 measurements [62]. It should also be noted that magnitude and direction of the difference between rso 2 and SjO 2 varied with the absolute value of SjO 2. This means in practical terms, for high values of SjO 2, rso 2 runs low, whereas at low values it runs high. Thus, severe desaturation of jugular venous return might not always be recognized [62]. Conversely, it may be hard to be sure when cerebral metabolism has nearly ceased during profound hypothermia as rso 2 values rarely rose to more than 90%. In addition, it should be noted that intravascular oxygenation may not always accurately reflect intracellular oxygen availability. du Plessis et al. [26] recently described a dissociation of cerebral intravascular and mitochondrial oxygenation by comparing changes in hemoglobin O 2 saturation to changes in oxidized cytochrome aa3. Thus, measurement of cerebral intravascular oxygenation alone may be an inadequate method for assessing adequacy of cerebral protection during periods of decreased CBF [26]. The reasons for the variable relationship between rso 2 and SjO 2 between patients (despite the excellent correlation in individual patients) are not entirely clear. Some laboratories have reported the mean tissue po 2 (weighted average of all tissue compartments) to be close to, or below, venous po 2 [26], while other reported the mean tissue po 2 to fall in between arterial and venous po 2 [26]. In addition, little is known regarding how interstitial or tissue oxygen tension is modulated in response to changes in arterial oxygen tension and/or CBF [26]. Thus, the ability
15 B. Schaller / Brain Research Reviews 46 (2004) to measure compartment-specific interstitial, arterial and venous oxygen tension in the brain could potentially shed light on the underlying mechanism by which oxygen passes from capillary across BBB and into the brain tissue. In the study of Duong et al. [29], the relationship between pio 2 and CBF was roughly linear for the CBF values of 1 2 ml/g/ min. At this range, a 100% increase in CBF resulted in 50% increase in oxygen delivery to the interstitial space, suggesting that the decreased transit time resulting from increased CBF had a significant effect on the oxygen passage across BBB [29]. The degree of coupling between neural activity and oxidative metabolism associated with increased neuronal activity, however, remains controversial [29]. When oxygen delivery increases via an augmentation in CBF with no change in oxygen demand, HbO 2 and a reciprocal decrease in deoxy-hb means that flow velocity increased without accompanying either vasodilatation or recruitment, which resulted in increases in venous oxygenation. This same pattern of changes in hemoglobulin oxygenation has also been observed in the activated area in near infrared spectroscopy studies [26], although the dilatative response of pial arterioles to neuronal activation has been determined videomicroscopically in several studies [26]. Kleinschmidt et al. [58] proposed two explanations for this absence of the thb response during activation: changes in local cerebral hematocrit associated with flow velocity changes, or a short interval between task performance, which does not allow for recovery of vasomotor tone. However, the mechanism of dilatation of pial arterioles are still controversial. There is no valid evidence to deny the possibility that, when increases in CBF are very small, the degree of dilatation of arterioles is too small to detect [58]. This possibility is supported by observation in microspectroscopy measurements through a cranial window and a thinned skull in rats pial arterioles did not show detectable dilatation, whereas optical and electrocortical signal changes were observed in capillary areas [58]. In general, activitydependent changes in rcbf for subtle cognitive tasks are small (b10%) [58]. Thus, an increase in flow velocity without detectable vasodilatation might account for the absence of an increase in thb during activation. 10. Correlation between superior sagittal sinus flow velocity and cerebral blood flow A significant linear correlation between peak velocity of SSS flow and both cortical and hemispheric blood flow is identified [54]. It has also been analyzed the correlation between the mean velocity of SSS flow by averaging the velocities obtained in each cardiac phase, and both cortical and hemispheric blood flow [54]. As indicated in the relation to the peak velocity, the mean velocity of SSS flow is a good indicative for CBF. It has been shown that the cross-sectional area of SSS does not change significantly during hyperventilation of hypercapnea, suggesting that SSS flow velocity (cm s 1 ) reflects SSS blood flow (ml s 1 ) [66]. Previous studies utilizing the arterial in-flow method involved prediction of CBF by carotid velocity measurements, and these studies have yielded a good correlation between actual CBF measured by the 133 Xe clearance method and carotid flow velocity assessed by the Doppler technique. The venous outflow method, performed in experimental animals, was also found to provide accurate estimates of CBF. However, in human measurements, the use of SSS flow velocity as an index of CBF may result in a relatively low estimate because of the influence of other venous drainage systems that were eliminated in the animal experiments. There have been a few studies using transcranial Doppler measurements of SSS using transcranial fants [19]. Bezinque et al. [14] showed that SSS flow velocity measured by Doppler technique at the anterior fontanel was about 15 cm s 1, which was near the lowest value for adult patients in our series, but they failed to obtain significant correlation between SSS flow velocity and clinical parameters, possibly because of the large fluctuations in both flow velocity and pressure in SSS of infants [14]. 11. Effect of age on cerebral venous circulation Documented morphological changes in the cerebral vasculature of the aging brain include, among other things, thinning of the endothelium, reduction in capillary lumen diameter and decreasing number of capillary endothelial cells. These structural and blood coagulative changes (such as reduced blood filterability associated with advancing age) might results in a shift of the thrombogenic/thrombolytic equilibrium. The different behavior of CVS in advanced age compared to young brain is not already understood [78]. There is a need of further physiological and biophysical studies to shed more light into this aspect, especially on its impact on the clinical routine [78]. In addition, these existing differences between different age stages in CVS may be also an important reason, whether experimental data on CVS can not be easily transferred to clinical condition [78]. This may be underlined by the fact, that for experimental research, there were used, in most of the cases, young animals, but the affected patients are almost in advanced aged [98]. 12. Conclusion The CVS represents an important to control not only in the physiological hemodynamic and metabolic course, but has also an impact on the pathophysiological changes, such as increased ICP. The cerebrovenous system seems to be more complex than the arterial one, even so only little of its
16 258 B. Schaller / Brain Research Reviews 46 (2004) physiological and biophysical behaviour is yet understood. The importance of the cerebrovenous system on the different physiological parameter of normal brain function can not be estimated, but it seems that it may be greater than previously assumed. Possibly, the CVS may be one of the most important factors to guarantee normal cerebral function. We could demonstrate the importance and the need of further experimental work on this subject, especially to better understand the relationship of different physiological factors on the CVS. In the future, different state-ofthe-art-imaging methods may play an important role in further understanding the complexity of the CVS. References [1] J. Anderweg, Intracranial venous pressure, hydrocephalus and effects of cerebrospinal fluid shunts, Child s. Nerv. Syst. 5 (1989) [2] R. Anxionnat, Anatomie et radio-anatomie du lobe temporal (MD thesis). Nancy, [3] M.L.J. Apuzzo, O.K. Chikovani, P.S. Gott, et al., Transcallocal, interfornical approaches for lesions affecting the third ventricle. Surgical considerations and consequences, Neurosurgery 10 (1982) [4] J. Auque, Th. Civit, Les veines superficielles, in: J. Auque (Ed.), Le Sacrifice Veineux, Neurochirurgie (Suppl.), vol. 1, 1996, pp [5] B. Asgeirsson, P.C. Grande, Local vascular response to elevation of an organ above the heart, Acta Physiol. Scand. 156 (1996) [6] R.H. Ayanzu, C.R. Bird, P.J. Keller, et al., Cerebral MR venography: normal anatomy and potential diagnostic pitfalls, AJNR 21 (2000) [7] I.H. Aydin, Y. Tuzun, E. Takci, et al., The anatomical variations of sylvian veinsand cisterns, Minim. Invasive Neurosurg. 40 (1997) [8] O.V. Batson, The vertebral vein system Caldwell lecture 1956, Am. J. Roentgenol. 78 (1957) [9] O.V. Batson, The vertebral system of veins as a menas for cancer dissemination, Prog. Clin. Cancer 3 (1967) [10] A. Beau, P. Rabischong, Les veines internes du cerveau, C. r. Assoc. Anat. 102 (1959) [11] T.H.B. Bedford, The venous system of the velum interposition of the Rhesus monkey and the effect of experimental occlusion of the great vein of Galen, Brain 57 (1934) [12] M. Bergsneider, A.A. Alwan, L. Falkson, et al., The relationship of pulsatile cerebrospinal fluid to cerebral blood flow and intracranial pressure: a new theoretical model, Acta Neurochir., Suppl. (Wien) 71 (1998) [13] C.D. Bertram, Unstable equilibrium behavior in collapsible tubes, J. Biochem. 19 (1986) [14] S.L. Bezinque, T.L. Slovis, A.S. Touchette, et al., Characterization of superior sagittal sinus blood flow velocity using color Doppler in neonates and infants, Pediatr. Radiol. 25 (1995) [15] S. Brew, Normal galenic drainage of the deep cerebral venous system, Child s Nerv. Syst. 20 (2004) [16] R.H. Britt, G.T. Rossi, Quantitative analysis of methods for reducing physiological brain pulsations, J. Neurosci. Methods 6 (1982) [17] P.E. Burrows, O. Konez, A. Birdorff, Venous variations of the brain and cranial vault, Neuroimaging Clin. N. Am. 13 (2003) [18] N.F. Capra, K.V. Anderson, Anatomy of the cerebral venous system, in: J.P. Kapp, H.H. Schmidek (Eds.), The Cerebral Venous System and its Disorders, Grune and Stratton, Philadelphia, 1984, pp [19] A. Carmelo, A. Ficola, M.L. Fravolini, et al., ICP and CBF regulation: a new hypothesis to explain the bwindkesselq phenomenon, Acta Neurochir., Suppl. 81 (2002) [20] A. Chanda, A. Nanda, Anatomical study of the orbitozygomatic transsellar-transcavernous-transclinoidal approach to the basilar artery bifurcation, J. Neurosurg. 97 (2002) [21] P.H. Chapman, E.R. Cosman, M.A. Arnold, The relationship between ventricular fluid pressure and body position in normal subjects and subjects with shunts. A telemetric study, Neurosurgery 26 (1990) [22] P. Chaynes, Microsurgical anatomy of the great cerebral vein of Galen and its tributaries, J. Neurosurg. 99 (2003) [23] F. Cowan, M. Thorensen, Ultrasound study of the cranial venous system in the human new-born infant and the adult, Acta Physiol. Scand. 117 (1983) [24] A. Delmas, B. Pertuiset, G. Bertrand, Les veines du lobe temporal, Rev. Oto-Neuro-Ophtalmol. 23 (1951) [25] G. DiChiro, Angiographic patterns of cerebral convexity veins and superficial dural sinuses, AJR 87 (1962) [26] A.J. du Plessis, Near-infrared spectroscopy for the in vivo study of cerebral hemodynamics and oxygenation, Curr. Opin. Pediatr. 7 (1995) [27] H. Duret, Recherches anatomiques sur la circulation veineuse de l encephale, Arch. Physiol. Norm. Pathol. 1 (1874) [28] T.Q. Duong, S.-G. Kim, In vivo MR measurements of regional arterial and venous blood volume fractions in intract rat brain, Magn. Reson. Med. 43 (2000) [29] T.Q. Duong, E. Yacoub, G. Ariany, et al., High-resolution spin-echo BOLD, and CBF fmri at 4 and 7 T, Magn. Reson. Med. 48 (2002) [30] J.E. Eckenhoff, The physiologic significance of the vertebral venous plexus, Surg. Gynecol. Obstet. 131 (1970) [31] J. Ekstedt, CSF hydrodynamic studies in man: 2. Normal hydrodynamic variables related to CSF pressure and flow, J. Neurol. Neurosurg. Psychiatry 41 (1978) [32] H.M. Epstein, H.W. Linde, A.R. Crampton, et al., The vertebral venous plexus as a major cerebral venous outflow tract, Anesthesiology 32 (1970) [33] D.P. Friedman, Abnormalities of the deep medullary white matter veins: MR imaging findings, Am. J. Roentgenol. 168 (1997) [34] D. Georgiadis, S. Schwarz, R. Kollmar, et al., Influence of inspiration: expiration ratio on intracraial and cerebral perfusion pressure in acute stroke patients, Intensive Care Med. 28 (2002) [35] B. Goetzen, Veines internes du cerveau humain. Morphologie et topographie des anastomoses veineuses centrol-peripheriques, Arch. Anat. Pathol. 12 (1964) [36] D.A. Hall, M.S. Goncalves, Smiths, et al., A method for determining venous contribution to BOLD contrast sensory activation, Magn. Reson. Imaging 20 (2002) [37] J. Hamer, E. Alberti, S. Hoyer, et al., Influence of systemic and cerebral vascular factors on the cerebrospinal fluid pulse waves, J. Neurosurg. 46 (1977) [38] M.K. Hammock, T.H. Milhorat, K. Earle, et al., Vein of Galen ligation in the primate, J. Neurosurg. 34 (1971) [39] O. Hassler, Deep cerebral venous system in man: a microangiographic study on its areas of drainage and its anastomoses with superficial cerebral veins, Neurology 16 (1966) [40] O. Hassler, Venous anatomy of human hindbrain. A stereomicroangiographic study of the venous angio-architecture and the venous areas of drainage, Arch. Neurol. 16 (1967) [41] G.M. Hathout, S.S. Gambhir, R.K. Gaopi, et al., A quantitative physiologic model of blood oxygenation for functional magnetic resonance imaging, Invest. Radiol. 30 (1995) [42] P. Haure, G.E. Cold, T.M. Hansen, et al., The ICP-lowering effect of 10 degrees reverse Trendelenburg position during craniotomy is
17 B. Schaller / Brain Research Reviews 46 (2004) stable during a 10-minute period, J. Neurosurg. Anesthesiol. 15 (2003) [43] M. Heil, T.J. Pedley, Stokes flow in collapsible tubes: computation and experiment, J. Fluid Mech. 353 (1997) [44] W.D. Heiss, R. Graf, K. Wienhard, Relevance of experimental ischemia in cats for stroke management: a comparative reevaluation, Cerebrovasc. Dis. 11 (2001) [45] O. Hirai, H. Honda, M. Ishikawa, et al., Epidural pulse waveform as an indicator of intracranial pressure dynamics, Surg. Neurol. 21 (1984) [46] J. Hlinka, S. Kamba, J. Petzelt, et al., Origin of the bwaterfallq effect in phonon dispersion of relaxar perovsites, Phys. Rev. Lett. 91 (2003) [47] O. Hoffmann, R. Klingelbiel, J.S. Braun, et al., Diagnostic pitfall: atypical cerebrovenous drainage via the vertebral venous system, AJNR 23 (2002) [48] J.P. Holt, The collapse factor in the measurement of venous pressure, Am. J. Physiol. 134 (1941) [49] I. Hooshmand, A.E. Rosenbaum, R.L. Stein, Radiographic anatomy of normal cerebral deep medullary veins. Critical for distinguishing them from their abnormal counterparts, Neuroradiology 7 (1974) [50] Y.P. Huang, B.S. Wolf, Veins of the white matter of the cerebral hemispheres (the medullary veins), AJR 92 (1964) [51] G. Huther, J. Dorth, H. Van der Loos, et al., Microanatomic and vascular aspects of the temporomesial region, Neurosurgery 43 (1998) [52] G. Iaconetta, M. Fusco, M. Samii, The sphenopetroclival venous gulf: a microanatomical study, J. Neurosurg. 99 (2003) [53] K. Ide, N.H. Secher, Cerebral blood flow and metabolism during exercise, Prog. Neurobiol. 61 (2000) [54] S. Inao, H. Kuchiwaki, J. Yoshida, et al., Magnetic resonace imaging quatification of superior sagittal sinus flow. Correlation to cerebral blood flow measured by xenon-enhanced computed tomography, Neurol. Res. 19 (1997) [55] T. Iwabuchi, E. Sobata, K. Ebina, et al., Dural sinus pressure: various aspects in human brain surgery in children and adults, Am. J. Physiol. 250 (1986) H389 H396. [56] Y. Kate, M. Mokay, R. Pucher, et al., Cerebrovascular response to change of cerebral venous pressure and cerebrospinal fluid pressure, Acta Neurochir. (Wien) 109 (1991) [57] M.G. Katz, V. Khazia, A. Steinmetz, et al., Distribution of cerebral flow using retrograde versus antegrade cerebral perfusion, Ann. Thorac. Surg. 67 (1999) [58] A. Kleinschmidt, A. Obrig, M. Requardt, et al., Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near-infrared spectroscopy, J. Cereb. Blood Flow Metab. 16 (1996) [59] C. Lee, M.A. Pennington, C.M. Kenney, MR evaluation of developmental venous anomalies: medullary venous anatomy of venous angiomas, AJNR 17 (1996) [60] S.P. Lee, T.Q. Duong, G. Yang, et al., Relative changes of cerebral arterial and venous blood volumes during increased cerebral blood flow: implications for BOLD FMRI, Magn. Reson. Med. 45 (2001) [61] M. Lemay, Left right dissymmetry, handedness, AJNR 13 (1992) [62] W. Lin, A. Celik, R.P. Paczynski, et al., Quantitative magnetic resonance imaging in experimental hypercapnia: improvement in the relation between changes in bran R2 and the oxygen saturation of venous blood after correction for changes in cerebral blood volume, J. Cereb. Blood Flow Metab. 19 (1999) [63] R. Lopez-Muniz, N.L. Stephens, B. Bromberger-Barnea, et al., Critical closure of pulmonary vessels analyzed in terms of Starling resistor models, J. Appl. Physiol. 24 (1968) [64] C.K. Lyon, J.B. Scott, C.Y. Wang, Flow through collapsible tubes at low Reynolds numbers. Applicability of the waterfall model, Circ. Res. 47 (1980) [65] C.K. Lyon, J.B. Scott, D.K. Anderson, et al., Flow through collapsible tubes at high Reynolds numbers, Circ. Res. 49 (1981) [66] H.P. Mattle, R.R. Edelman, M.A. Reis, et al., Flow quantification in the superior sagittal sinus using magnetic resonance, Neurology 40 (1990) [67] H.P. Mattle, K.U. Wentz, R.R. Edelman, et al., Cerebral venography with MR, Radiology 178 (1991) [68] J.F. Meder, J. Chiras, J. Roland, et al., Venous territories of the brain, J. Neuroradiol. 21 (1994) [69] N.R. Mehta, L. Jones, M.A. Kraut, et al., Physiologic variations in dural venous sinus flow on phase-contrast MR imaging, Am. J. Roentgenol. 175 (2000) [70] S.S. Mikhailov, I.I. Kagan, The anastomoses of the venous system of the brain and their role in the collateral circulation, Folia Morphol. 16 (1968) [71] J.D. Miller, A.E. Stanek, T.W. Langfitt, Cerebral blood flow regulation during experimental brain compression, J. Neurosurg. 39 (1973) [72] J.P. Muizelaar, Cerebral blood flow, cerebral blood volume, and cerebral metabolism after severe head injury, in: D.P. Becker, S.K. Gudeman (Eds.), Textbook of Head Injury, WB Saunders, Philadelphia, PA, 1989, pp [73] N. Muthukumar, P. Palaniappan, Tentorial venous sinuses: an anatomic study, Neurosurgery 42 (1998) [74] N. Nagdyman, T. Fleck, S. Barth, et al., Relation of cerebral tissue oxygenation index to central venous oxygen saturation in children, Intensive Care Med. 30 (2004) [75] K. Oka, A.L. Rhoton, M. Barry, et al., Microsurgical anatomy of the deep venous system of the brain, Neurosurgery 15 (1984) [76] T. Okudera, T. Ohta, Y.P. Huang, et al., Developmental and radiological anatomy of the superficial cerebral convexity vessels in the human fetus, J. Neuroradiol. 15 (1988) [77] M. Ono, A.L. Rhoton, D. Peace, et al., Microsurgical anatomy of the deep venous system of the brain, Neurosurgery 15 (1984) [78] H. Otsuka, H. Nakase, K. Nagata, et al., Effect of age on cerebral venous circulation disturbances in the rat, J. Neurosurg. 93 (2000) [79] J.L. Parker, C.J. Flucker, N. Harvey, et al., Comparison of external jugular and central venous pressures in mechanically ventilated patient, Anesthesia 57 (2002) [80] T.J. Pedley, X.Y. Luo, Modeling flow and oscillations in collapsible tubes, Theor. Comput. Fluid Dyn. 10 (1998) [81] S. Permutt, B. Bromberger-Barnea, H.N. Bane, Alveolar pressure, pulmonary venous pressure, and the vascular waterfall, Med. Thorac. 19 (1962) [82] D. Petit-Dutaillis, A. Delmas, B. Pertuiset, Le reseau veineux du cortex cerebral, Sem. Hop. Paris 26 (1950) [83] J.H. Piatt, J.X. Kellogg, A hazard of combining the infratentorial supracerebellar and the cerebellomedullary fissure approaches: cerebellar venous insufficiency, Pediatr. Neurosurg. 33 (2000) [84] S.K. Piechnik, M. Czosnyka, H.K. Richards, et al., Cerebral venous blood outflow: a theoretical model based on laboratory simulation, Neurosurgery 49 (2001) [85] K. Plaschke, C. Sommer, A. Fahrner, et al., Pronounced arterial collateralization was induced after permanent rat cerebral four-vessel occlusion. Relation to neuropathology and capillary ultrastructure, J. Neural Transm. 110 (2003) [86] T. Przybylowski, M.F. Bangash, K. Reichmuth, et al., Mechanisms of the cerebrovascular response to apnoea in humans, J. Physiol. 548 (2003) [87] R.H. Pudenz, E.L. Foltz, Hydrocephalus: overdrainage by ventricular shunts a review and recommendations, Surg. Neurol. 35 (1991) [88] R. Reisch, L. Vutskits, R. Filippi, et al., Topographic microsurgical anatomy of the parclinoid cartotid artery, Neurosurg. Rev. 25 (2002)
18 260 B. Schaller / Brain Research Reviews 46 (2004) [89] D.S.M. Ruit, P. Gailloud, D.A. Ruefenacht, et al., The craniocervical venous system in relation to cerebral venous drainage, AJNR 23 (2002) [90] K. Sakata, O. Al-Mefty, I. Yamamoto, Venous consideration in petrosal approach: microsurgical anatomy of the temporal bridging vein, Neurosurgery 47 (2000) [91] S. Samuelson, D.M. Long, S.N. Chou, Subdural hematoma as a complication of shunting procedures for normal pressure hydrocephalus, J. Neurosurg. 37 (1972) [92] D. San Millan Ruiz, P. Gailloud, D.A. Rufenacht, et al., The craniocervical venous system in relation to cerebral venous drainage, AJNR 23 (2002) [93] B. Schaller, R. Graf, Cerebral ischemia and reperfusion: the pathophysiological concept as basis of clinical therapy? J. Cereb. Blood Flow Metab. 24 (2004) [94] B. Schaller, R. Graf, Cerebral venous infarction: the pathophysiological concept, Cerebrovasc. Dis. 18 (2004) [95] B. Schaller, R. Graf, Y. Sanada, et al., Hemodynamic changes after occlusion of the posterior superior sagittal sinus. An experimental PET-study in cats, AJNR 24 (2003) [96] B. Schaller, R. Graf, A.H. Jacobs, Invited commentary: ischemic tolerance: a window to endogenous neuroprotection? Lancet 362 (2003) [97] B. Schaller, A.H. Jacobs, R. Graf, Hemispheric dominance for the cortical control of swallowing in humans: a contribution to better understand cortical organization?, Eur. J. Radiol. 51 (2004) [98] B. Schlesinger, The venous system of the brain with special reference of the Galenic system, Brain 62 (1939) [99] J.N. Scott, R.I. Farb, Imaging and anatomy of the normal intracranial venous system, Neuroimaging Clin. N. Am. 13 (2003) [100] A.R. Shakhnovich, V.A. Shakhnovich, A.A. Glashkina, Noninvasive assessment of the elastance and reserve capacity of the craniovertebral contents via flow velocity measurements in the straight sinus by TCD during body tilting test, J. Neuroimaging 9 (1999) [101] K. Shapira, A. Fried, F. Takei, et al., Effect of the skull and dura on neural axis pressure volume relationships and CSF hydrodynamics, J. Neurosurg. 63 (1985) [102] M. Sindou, F. Alaywan, P. Hallacq, Chirurgie des grands sinus veineux duraux intracraniens, in: J. Auque (Ed.), Le Sacrifice Veineux en Neurochirurgie, Neurochirurgia (Suppl.), vol. 1, 1996, pp [103] C. Strik, U. Koose, C. Kiefer, et al., Slow rhythmic oscillations in intracranial CSF and blood flow: registered by MRI, Acta Neurochir., Suppl. 81 (2002) [104] Y. Suzuki, H. Ikeda, M. Shimada, et al., Variations of the basal vein: identification using three-dimensional CT angiography, AJNR 22 (2001) [105] S. Takahashi, K. Kato, N. Tomura, et al., Dural arteriovenous fistula of the cavernous sinus with cortical venous reflux of the posterior fossa via a bridging vein, Radiat. Med. 19 (2001) [106] M. Teksum, S. Casey, A. McKinney, et al., Anatomy and frequency of large pontomesencephalic veins on 3D CT angiograms of the circle of Willis, AJNR 24 (2003) [107] J. Theron, Les affluents du sinus caverneux, Neurochirurgie 18 (1972) [108] M. Ursino, C.A. Lodi, A simple mathematical model of the interaction between intracranial pressure and cerebral hemodynamic, J. Appl. Physiol. 82 (1997) [109] E.P. Wei, H.A. Kontos, Responses of cerebral arterioles to increased venous pressure, Am. J. Physiol. 243 (1982) H442 H447. [110] R.O. Weller, Pathology of cerebrospinal fluid and interstitial fluid of the CNS: significance for Alzheimer disease, J. Neuropathol. Exp. Neurol. 57 (1998) [111] S.G. Wetzel, E. Kirsch, K.W. Stock, et al., Cerebral veins: comparative study of CT venography with intraarterial digital subtraction angiography, AJNR 20 (1999) [112] A. Zahkarov, C. Papaiconomon, L. Koh, et al., Intergrating the roles of extracranial lymphaties and intracranial veins in cerebrospinal fluid absorption in sheep, Micriovasc. Res. 67 (2004)
2401 : Anatomy/Physiology
 Dr. Chris Doumen Week 7 2401 : Anatomy/Physiology The Brain Central Nervous System TextBook Readings Pages 431 through 435 and 463-467 Make use of the figures in your textbook ; a picture is worth a thousand
Dr. Chris Doumen Week 7 2401 : Anatomy/Physiology The Brain Central Nervous System TextBook Readings Pages 431 through 435 and 463-467 Make use of the figures in your textbook ; a picture is worth a thousand
CSE511 Brain & Memory Modeling. Lect04: Brain & Spine Neuroanatomy
 CSE511 Brain & Memory Modeling CSE511 Brain & Memory Modeling Lect02: BOSS Discrete Event Simulator Lect04: Brain & Spine Neuroanatomy Appendix of Purves et al., 4e Larry Wittie Computer Science, StonyBrook
CSE511 Brain & Memory Modeling CSE511 Brain & Memory Modeling Lect02: BOSS Discrete Event Simulator Lect04: Brain & Spine Neuroanatomy Appendix of Purves et al., 4e Larry Wittie Computer Science, StonyBrook
Parts of the Brain. Chapter 1
 Chapter 1 Parts of the Brain Living creatures are made up of cells. Groups of cells, similar in appearance and with the same function, form tissue. The brain is a soft mass of supportive tissues and nerve
Chapter 1 Parts of the Brain Living creatures are made up of cells. Groups of cells, similar in appearance and with the same function, form tissue. The brain is a soft mass of supportive tissues and nerve
Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9 th ed.)
 BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9 th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9 th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic
BIOL 1108 Vertebrate Anatomy Lab
 BIOL 1108 Vertebrate Anatomy Lab This lab explores major organs associated with the circulatory, excretory, and nervous systems of mammals. Circulatory System Vertebrates are among the organisms that have
BIOL 1108 Vertebrate Anatomy Lab This lab explores major organs associated with the circulatory, excretory, and nervous systems of mammals. Circulatory System Vertebrates are among the organisms that have
Vascular System The heart can be thought of 2 separate pumps from the right ventricle, blood is pumped at a low pressure to the lungs and then back
 Vascular System The heart can be thought of 2 separate pumps from the right ventricle, blood is pumped at a low pressure to the lungs and then back to the left atria from the left ventricle, blood is pumped
Vascular System The heart can be thought of 2 separate pumps from the right ventricle, blood is pumped at a low pressure to the lungs and then back to the left atria from the left ventricle, blood is pumped
DISSECTION OF THE SHEEP'S BRAIN
 DISSECTION OF THE SHEEP'S BRAIN Introduction The purpose of the sheep brain dissection is to familiarize you with the threedimensional structure of the brain and teach you one of the great methods of studying
DISSECTION OF THE SHEEP'S BRAIN Introduction The purpose of the sheep brain dissection is to familiarize you with the threedimensional structure of the brain and teach you one of the great methods of studying
Objectives AXIAL SKELETON. 1. Frontal Bone. 2. Parietal Bones. 3. Temporal Bones. CRANIAL BONES (8 total flat bones w/ 2 paired)
 Objectives AXIAL SKELETON SKULL 1. On a skull or diagram, identify and name the bones of the skull 2. Identify the structure and function of the bones of the skull 3. Describe how a fetal skull differs
Objectives AXIAL SKELETON SKULL 1. On a skull or diagram, identify and name the bones of the skull 2. Identify the structure and function of the bones of the skull 3. Describe how a fetal skull differs
Practical class 3 THE HEART
 Practical class 3 THE HEART OBJECTIVES By the time you have completed this assignment and any necessary further reading or study you should be able to:- 1. Describe the fibrous pericardium and serous pericardium,
Practical class 3 THE HEART OBJECTIVES By the time you have completed this assignment and any necessary further reading or study you should be able to:- 1. Describe the fibrous pericardium and serous pericardium,
Hemorrhagic venous infarction Heather Borders, MD
 Hemorrhagic venous infarction Heather Borders, MD 12/13/2010 History 16 year old female with four day history of headache and acute change in mental status. History of two days of oral contraceptive use
Hemorrhagic venous infarction Heather Borders, MD 12/13/2010 History 16 year old female with four day history of headache and acute change in mental status. History of two days of oral contraceptive use
What role does the nucleolus have in cell functioning? Glial cells
 Nervous System Lab The nervous system of vertebrates can be divided into the central nervous system, which consists of the brain and spinal cord, and the peripheral nervous system, which contains nerves,
Nervous System Lab The nervous system of vertebrates can be divided into the central nervous system, which consists of the brain and spinal cord, and the peripheral nervous system, which contains nerves,
What Is an Arteriovenous Malformation (AVM)?
 What Is an Arteriovenous Malformation (AVM)? From the Cerebrovascular Imaging and Intervention Committee of the American Heart Association Cardiovascular Council Randall T. Higashida, M.D., Chair 1 What
What Is an Arteriovenous Malformation (AVM)? From the Cerebrovascular Imaging and Intervention Committee of the American Heart Association Cardiovascular Council Randall T. Higashida, M.D., Chair 1 What
BIO130 Chapter 14 The Brain and Cranial Nerves Lecture Outline
 BIO130 Chapter 14 The Brain and Cranial Nerves Lecture Outline Brain structure 1. Cerebrum Hemispheres: left & right Cerebral cortex Gyri Sulci Fissures Longitudinal fissure Corpus callosum Lobes Central
BIO130 Chapter 14 The Brain and Cranial Nerves Lecture Outline Brain structure 1. Cerebrum Hemispheres: left & right Cerebral cortex Gyri Sulci Fissures Longitudinal fissure Corpus callosum Lobes Central
Sheep Brain Dissection Picture Guide
 Sheep Brain Dissection Picture Guide Figure 1: Right Hemisphere of Sheep s Brain Figure 2: Underside of Sheep s Brain Figure 3: Saggital cut of Sheep s Brain to reveal subcortical structures Figure 4:
Sheep Brain Dissection Picture Guide Figure 1: Right Hemisphere of Sheep s Brain Figure 2: Underside of Sheep s Brain Figure 3: Saggital cut of Sheep s Brain to reveal subcortical structures Figure 4:
3) Cerebral Cortex & Functions of the 4 LOBES. 5) Cranial Nerves (Nerves In the Cranium, i.e., Head)
 Lecture 5 (Oct 8 th ): ANATOMY and FUNCTION OF THE NERVOUS SYSTEM Lecture Outline 1) Basic Divisions (CNS vs. PNS, Somatic vs. Autonomic) and Directional Terms 2) The Brain (Hindbrain/ Midbrain/ Forebrain)
Lecture 5 (Oct 8 th ): ANATOMY and FUNCTION OF THE NERVOUS SYSTEM Lecture Outline 1) Basic Divisions (CNS vs. PNS, Somatic vs. Autonomic) and Directional Terms 2) The Brain (Hindbrain/ Midbrain/ Forebrain)
LECTURE 16 NEUROPATHOPHYSIOLOGY (HEAD INJURY)
 LECTURE 16 Copyright 2000 by Bowman O. Davis, Jr. The approach and organization of this material was developed by Bowman O. Davis, Jr. for specific use in online instruction. All rights reserved. No part
LECTURE 16 Copyright 2000 by Bowman O. Davis, Jr. The approach and organization of this material was developed by Bowman O. Davis, Jr. for specific use in online instruction. All rights reserved. No part
Chapter 7: The Nervous System
 Chapter 7: The Nervous System I. Organization of the Nervous System Objectives: List the general functions of the nervous system Explain the structural and functional classifications of the nervous system
Chapter 7: The Nervous System I. Organization of the Nervous System Objectives: List the general functions of the nervous system Explain the structural and functional classifications of the nervous system
Anatomy of the Brain > 1. Figure 1. Eight bones form the skull and fourteen bones form the face.
 Anatomy of the Brain Overview The human brain is an amazing three-pound organ that controls all functions of the body, interprets information from the outside world, and embodies the essence of the mind
Anatomy of the Brain Overview The human brain is an amazing three-pound organ that controls all functions of the body, interprets information from the outside world, and embodies the essence of the mind
The Axial Skeleton Eighty bones segregated into three regions
 The Axial Skeleton Eighty bones segregated into three regions Skull Vertebral column Bony thorax Bones of the Axial Skeleton Figure 7.1 The Skull The skull, the body s most complex bony structure, is formed
The Axial Skeleton Eighty bones segregated into three regions Skull Vertebral column Bony thorax Bones of the Axial Skeleton Figure 7.1 The Skull The skull, the body s most complex bony structure, is formed
NEUROANATOMY 6 Limbic System
 NEUROANATOMY 6 Limbic System The Limbic System The part of the brain involved with learning, memory and emotion. It is affected in many neuropsychiatric diseases including schizophrenia, Alzheimer s disease
NEUROANATOMY 6 Limbic System The Limbic System The part of the brain involved with learning, memory and emotion. It is affected in many neuropsychiatric diseases including schizophrenia, Alzheimer s disease
CHAPTER 11: NERVOUS SYSTEM II: DIVISIONS OF THE NERVOUS SYSTEM OBJECTIVES: 1. Outline the major divisions of the nervous system.
 CHAPTER 11: NERVOUS II: DIVISIONS OF THE NERVOUS OBJECTIVES: 1. Outline the major divisions of the nervous system. NERVOUS CENTRAL NERVOUS (BRAIN & SPINAL CORD) (INTERNEURONS) PERIPHERAL NERVOUS (CRANIAL
CHAPTER 11: NERVOUS II: DIVISIONS OF THE NERVOUS OBJECTIVES: 1. Outline the major divisions of the nervous system. NERVOUS CENTRAL NERVOUS (BRAIN & SPINAL CORD) (INTERNEURONS) PERIPHERAL NERVOUS (CRANIAL
THE BRAIN, SPINAL CORD, AND CRANIAL NERVES
 THE BRAIN, SPINAL CORD, AND CRANIAL NERVES I. BRAIN ANATOMY A. Meninges (coverings) of the brain and spinal cord (Fig. [13.120 p. 452 [457]) Use text illustrations to study these. Note that the singular
THE BRAIN, SPINAL CORD, AND CRANIAL NERVES I. BRAIN ANATOMY A. Meninges (coverings) of the brain and spinal cord (Fig. [13.120 p. 452 [457]) Use text illustrations to study these. Note that the singular
Nervous System: PNS and CNS
 Nervous System: PNS and CNS Biology 105 Lecture 10 Chapter 8 Outline I. Central Nervous System vs Peripheral Nervous System II. Peripheral Nervous System A. Somatic Nervous System B. Autonomic Nervous
Nervous System: PNS and CNS Biology 105 Lecture 10 Chapter 8 Outline I. Central Nervous System vs Peripheral Nervous System II. Peripheral Nervous System A. Somatic Nervous System B. Autonomic Nervous
Biol 111 Comparative & Human Anatomy Lab 9: Circulatory System of the Cat Spring 2014
 Biol 111 Comparative & Human Anatomy Lab 9: Circulatory System of the Cat Spring 2014 Philip J. Bergmann Lab Objectives 1. To learn how blood flows through a dual circuit circulation with lungs. 2. To
Biol 111 Comparative & Human Anatomy Lab 9: Circulatory System of the Cat Spring 2014 Philip J. Bergmann Lab Objectives 1. To learn how blood flows through a dual circuit circulation with lungs. 2. To
Ventilation Perfusion Relationships
 Ventilation Perfusion Relationships VENTILATION PERFUSION RATIO Ideally, each alveolus in the lungs would receive the same amount of ventilation and pulmonary capillary blood flow (perfusion). In reality,
Ventilation Perfusion Relationships VENTILATION PERFUSION RATIO Ideally, each alveolus in the lungs would receive the same amount of ventilation and pulmonary capillary blood flow (perfusion). In reality,
THE BRAIN AND CRANIAL NERVES
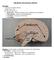 THE BRAIN AND CRANIAL NERVES The Brain - made up of a trillion neurons - weighs about 3 lbs - has four principle parts 1. Brain stem - medulla oblongata, pons, midbrain (mesencephalon) 2. Diencephalon
THE BRAIN AND CRANIAL NERVES The Brain - made up of a trillion neurons - weighs about 3 lbs - has four principle parts 1. Brain stem - medulla oblongata, pons, midbrain (mesencephalon) 2. Diencephalon
Chapter 3 The Anatomy of the Nervous System
 Chapter 3 The Anatomy of the Nervous System Systems, Structures, and Cells That Make Up Your Nervous System 1 General Layout of the Nervous System Central Nervous System (CNS) Brain (in the skull) Spinal
Chapter 3 The Anatomy of the Nervous System Systems, Structures, and Cells That Make Up Your Nervous System 1 General Layout of the Nervous System Central Nervous System (CNS) Brain (in the skull) Spinal
Distance Learning Program Anatomy of the Human Heart/Pig Heart Dissection Middle School/ High School
 Distance Learning Program Anatomy of the Human Heart/Pig Heart Dissection Middle School/ High School This guide is for middle and high school students participating in AIMS Anatomy of the Human Heart and
Distance Learning Program Anatomy of the Human Heart/Pig Heart Dissection Middle School/ High School This guide is for middle and high school students participating in AIMS Anatomy of the Human Heart and
1. Which of the following is NOT part of the diencephalon? a. Pineal gland b. Tectum c. Interthalamic adhesion d. Hypothalamus e.
 1. Which of the following is NOT part of the diencephalon? a. Pineal gland b. Tectum c. Interthalamic adhesion d. Hypothalamus e. Thalamus 2. The is the primary relay station for sensory information coming
1. Which of the following is NOT part of the diencephalon? a. Pineal gland b. Tectum c. Interthalamic adhesion d. Hypothalamus e. Thalamus 2. The is the primary relay station for sensory information coming
Sheep Brain Dissection
 Sheep Brain Dissection http://www.carolina.com/product/preserved+organisms/preserved+animals+%28mammal s%29/sheep+organs/preserved+sheep+dissection.do Michigan State University Neuroscience Program Brain
Sheep Brain Dissection http://www.carolina.com/product/preserved+organisms/preserved+animals+%28mammal s%29/sheep+organs/preserved+sheep+dissection.do Michigan State University Neuroscience Program Brain
Constituents within the skull include the brain (80%/1400ml), blood (10%/150ml) and cerebrospinal fluid (CSF 10%/150ml)
 Cerebral Blood Flow and Intracranial Pressure Dr Lisa Hill, SpR Anaesthesia, Royal Oldham Hospital, UK. Email lambpie10@hotmail.com Dr Carl Gwinnutt, Consultant Neuroanaesthetist, Hope Hospital, UK. Part
Cerebral Blood Flow and Intracranial Pressure Dr Lisa Hill, SpR Anaesthesia, Royal Oldham Hospital, UK. Email lambpie10@hotmail.com Dr Carl Gwinnutt, Consultant Neuroanaesthetist, Hope Hospital, UK. Part
Chapter 9 Nervous System
 Chapter 9 Nervous System Nervous System function: The nervous system is composed of neurons and neuroglia. at the ends of peripheral nerves gather information and convert it into nerve impulses. When sensory
Chapter 9 Nervous System Nervous System function: The nervous system is composed of neurons and neuroglia. at the ends of peripheral nerves gather information and convert it into nerve impulses. When sensory
Functions of Blood System. Blood Cells
 Functions of Blood System Transport: to and from tissue cells Nutrients to cells: amino acids, glucose, vitamins, minerals, lipids (as lipoproteins). Oxygen: by red blood corpuscles (oxyhaemoglobin - 4
Functions of Blood System Transport: to and from tissue cells Nutrients to cells: amino acids, glucose, vitamins, minerals, lipids (as lipoproteins). Oxygen: by red blood corpuscles (oxyhaemoglobin - 4
Cardiovascular Physiology
 Cardiovascular Physiology Heart Physiology for the heart to work properly contraction and relaxation of chambers must be coordinated cardiac muscle tissue differs from smooth and skeletal muscle tissues
Cardiovascular Physiology Heart Physiology for the heart to work properly contraction and relaxation of chambers must be coordinated cardiac muscle tissue differs from smooth and skeletal muscle tissues
Heart and Vascular System Practice Questions
 Heart and Vascular System Practice Questions Student: 1. The pulmonary veins are unusual as veins because they are transporting. A. oxygenated blood B. de-oxygenated blood C. high fat blood D. nutrient-rich
Heart and Vascular System Practice Questions Student: 1. The pulmonary veins are unusual as veins because they are transporting. A. oxygenated blood B. de-oxygenated blood C. high fat blood D. nutrient-rich
Page 1. Introduction The blood vessels of the body form a closed delivery system that begins and ends at the heart.
 Anatomy Review: Blood Vessel Structure & Function Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction The blood vessels
Anatomy Review: Blood Vessel Structure & Function Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction The blood vessels
CENTRAL NERVOUS SYSTEM. Sensory Pathway (PNS) OVERVIEW OF SPINAL CORD ANATOMY OF THE SPINAL CORD FUNCTIONS OF THE SPINAL CORD
 CENTRAL NERVOUS SYSTEM Central nervous system (CNS) brain and spinal cord enclosed in bony coverings Functions of the spinal cord spinal cord reflexes integration ti (summation of inhibitory and excitatory)
CENTRAL NERVOUS SYSTEM Central nervous system (CNS) brain and spinal cord enclosed in bony coverings Functions of the spinal cord spinal cord reflexes integration ti (summation of inhibitory and excitatory)
Overview of the Cardiovascular System
 Overview of the Cardiovascular System 2 vascular (blood vessel) loops: Pulmonary circulation: from heart to lungs and back) Systemic circulation: from heart to other organs and back Flow through systemic
Overview of the Cardiovascular System 2 vascular (blood vessel) loops: Pulmonary circulation: from heart to lungs and back) Systemic circulation: from heart to other organs and back Flow through systemic
1. Cerebrospainal Fluid (CSF) circulation in brain: Sites and mechanisms of CSF secretion, circulation and reabsorption. Physiological and modelling
 1. Cerebrospainal Fluid (CSF) circulation in brain: Sites and mechanisms of CSF secretion, circulation and reabsorption. Physiological and modelling description. Cerebrospinal space Brain lump in a box?
1. Cerebrospainal Fluid (CSF) circulation in brain: Sites and mechanisms of CSF secretion, circulation and reabsorption. Physiological and modelling description. Cerebrospinal space Brain lump in a box?
Doppler. Doppler. Doppler shift. Doppler Frequency. Doppler shift. Doppler shift. Chapter 19
 Doppler Doppler Chapter 19 A moving train with a trumpet player holding the same tone for a very long time travels from your left to your right. The tone changes relative the motion of you (receiver) and
Doppler Doppler Chapter 19 A moving train with a trumpet player holding the same tone for a very long time travels from your left to your right. The tone changes relative the motion of you (receiver) and
Introduction. I. Objectives. II. Introduction. A. To become familiar with the terms of direction and location.
 E X E R C I S E Introduction I. Objectives A. To become familiar with the terms of direction and location. B. To become familiar with different types of planes and sections. C. To learn the names and locations
E X E R C I S E Introduction I. Objectives A. To become familiar with the terms of direction and location. B. To become familiar with different types of planes and sections. C. To learn the names and locations
Cerebral blood flow (CBF) is dependent on a number of factors that can broadly be divided into:
 Cerebral Blood Flow and Intracranial Pressure Dr Lisa Hill, SpR Anaesthesia, Royal Oldham Hospital, UK. Email lambpie10@hotmail.com Dr Carl Gwinnutt, Consultant Neuroanaesthetist, Hope Hospital, UK. The
Cerebral Blood Flow and Intracranial Pressure Dr Lisa Hill, SpR Anaesthesia, Royal Oldham Hospital, UK. Email lambpie10@hotmail.com Dr Carl Gwinnutt, Consultant Neuroanaesthetist, Hope Hospital, UK. The
Note: The left and right sides of the heart must pump exactly the same volume of blood when averaged over a period of time
 page 1 HEART AS A PUMP A. Functional Anatomy of the Heart 1. Two pumps, arranged in series a. right heart: receives blood from the systemic circulation (via the great veins and vena cava) and pumps blood
page 1 HEART AS A PUMP A. Functional Anatomy of the Heart 1. Two pumps, arranged in series a. right heart: receives blood from the systemic circulation (via the great veins and vena cava) and pumps blood
Nervous System Organization. PNS and CNS. Nerves. Peripheral Nervous System. Peripheral Nervous System. Motor Component.
 Nervous System Organization PNS and CNS Chapters 8 and 9 Peripheral Nervous System (PNS) connects CNS to sensory receptors, muscles and glands Central Nervous System (CNS) control/integrating center brain
Nervous System Organization PNS and CNS Chapters 8 and 9 Peripheral Nervous System (PNS) connects CNS to sensory receptors, muscles and glands Central Nervous System (CNS) control/integrating center brain
Lab Exercise 9. Nervous Tissue. Brain. Cranial Nerves. Spinal Cord. Spinal Nerves
 Lab Exercise 9 Nervous Tissue Brain Cranial Nerves Spinal Cord Spinal Nerves Textbook Reference: See Chapter 11 for histology of nerve tissue and spinal cord See Chapter 12 for brain and spinal cord anatomy
Lab Exercise 9 Nervous Tissue Brain Cranial Nerves Spinal Cord Spinal Nerves Textbook Reference: See Chapter 11 for histology of nerve tissue and spinal cord See Chapter 12 for brain and spinal cord anatomy
13. Volume-pressure infusion tests: Typical patterns of infusion studies in different forms of CSF circulatory disorders.
 13. Volume-pressure infusion tests: Typical patterns of infusion studies in different forms of CSF circulatory disorders. Hydrocephalus is far more complex than disorder of CSF circulation CSF circulation
13. Volume-pressure infusion tests: Typical patterns of infusion studies in different forms of CSF circulatory disorders. Hydrocephalus is far more complex than disorder of CSF circulation CSF circulation
Subclavian Steal Syndrome By Marta Thorup
 Subclavian Steal Syndrome By Marta Thorup Definition Subclavian steal syndrome (SSS), is a constellation of signs and symptoms that arise from retrograde flow of blood in the vertebral artery, due to proximal
Subclavian Steal Syndrome By Marta Thorup Definition Subclavian steal syndrome (SSS), is a constellation of signs and symptoms that arise from retrograde flow of blood in the vertebral artery, due to proximal
Human Anatomy and Physiology The Respiratory System
 Human Anatomy and Physiology The Respiratory System Basic functions of the respiratory system: as a Gas exchange supply oxygen to aerobic tissues in the body and remove carbon dioxide waste product. in-
Human Anatomy and Physiology The Respiratory System Basic functions of the respiratory system: as a Gas exchange supply oxygen to aerobic tissues in the body and remove carbon dioxide waste product. in-
Administrative. Patient name Date compare with previous Position markers R-L, upright, supine Technical quality
 CHEST X-RAY Administrative Patient name Date compare with previous Position markers R-L, upright, supine Technical quality AP or PA ( with x-ray beam entering from back of patient, taken at 6 feet) Good
CHEST X-RAY Administrative Patient name Date compare with previous Position markers R-L, upright, supine Technical quality AP or PA ( with x-ray beam entering from back of patient, taken at 6 feet) Good
Section Four: Pulmonary Artery Waveform Interpretation
 Section Four: Pulmonary Artery Waveform Interpretation All hemodynamic pressures and waveforms are generated by pressure changes in the heart caused by myocardial contraction (systole) and relaxation/filling
Section Four: Pulmonary Artery Waveform Interpretation All hemodynamic pressures and waveforms are generated by pressure changes in the heart caused by myocardial contraction (systole) and relaxation/filling
Welcome to Anatomy & Physiology
 Welcome to Anatomy & Physiology Chapter 1 -Human Organization What do you need to do to pass this class? MEMORIZE! The Scope of Human Anatomy Human anatomy is the study of the structure of the human body.
Welcome to Anatomy & Physiology Chapter 1 -Human Organization What do you need to do to pass this class? MEMORIZE! The Scope of Human Anatomy Human anatomy is the study of the structure of the human body.
Lab 5 Overview of the Skeleton: Classification and Structure of Bones and Cartilages Exercise 9 The Axial Skeleton Exercise 10
 Lab 5 Overview of the Skeleton: Classification and Structure of Bones and Cartilages Exercise 9 The Axial Skeleton Exercise 10 Overview of the Skeleton Locate the important cartilages in the human skeleton
Lab 5 Overview of the Skeleton: Classification and Structure of Bones and Cartilages Exercise 9 The Axial Skeleton Exercise 10 Overview of the Skeleton Locate the important cartilages in the human skeleton
Transverse Sections of the Spinal Cord
 Transverse Sections of the Spinal Cord The spinal cord is perhaps the most simply arranged part of the CNS. Its basic structure, indicated in a schematic drawing of the eighth cervical segment (Figure
Transverse Sections of the Spinal Cord The spinal cord is perhaps the most simply arranged part of the CNS. Its basic structure, indicated in a schematic drawing of the eighth cervical segment (Figure
Autonomic Nervous System of the Neck. Adam Koleśnik, MD Department of Descriptive and Clinical Anatomy Center of Biostructure Research, MUW
 Autonomic Nervous System of the Neck Adam Koleśnik, MD Department of Descriptive and Clinical Anatomy Center of Biostructure Research, MUW Autonomic nervous system sympathetic parasympathetic enteric Autonomic
Autonomic Nervous System of the Neck Adam Koleśnik, MD Department of Descriptive and Clinical Anatomy Center of Biostructure Research, MUW Autonomic nervous system sympathetic parasympathetic enteric Autonomic
Airways Resistance and Airflow through the Tracheobronchial Tree
 Airways Resistance and Airflow through the Tracheobronchial Tree Lecturer: Sally Osborne, Ph.D. Department of Cellular & Physiological Sciences Email: sosborne@interchange.ubc.ca Useful links: www.sallyosborne.com
Airways Resistance and Airflow through the Tracheobronchial Tree Lecturer: Sally Osborne, Ph.D. Department of Cellular & Physiological Sciences Email: sosborne@interchange.ubc.ca Useful links: www.sallyosborne.com
FERNE / EMRA 2009 Mid-Atlantic Emergency Medicine Medical Student Symposium: ABCs of Head CT Interpretation; Heather M. Prendergast MD, MPH.
 ABCs of Head CT Interpretation in the Emergency Department: CT Interpretation Workshop Guide Heather M. Prendergast, MD, MPH, FACEP Associate Professor Department of Emergency Medicine University of Illinois
ABCs of Head CT Interpretation in the Emergency Department: CT Interpretation Workshop Guide Heather M. Prendergast, MD, MPH, FACEP Associate Professor Department of Emergency Medicine University of Illinois
Hole s Human Anatomy and Physiology Eleventh Edition. Mrs. Hummer Hanover Area Jr./Sr. High School. Chapter 1 Introduction to Anatomy and Physiology
 Hole s Human Anatomy and Physiology Eleventh Edition Mrs. Hummer Hanover Area Jr./Sr. High School Chapter 1 Introduction to Anatomy and Physiology 1 Chapter 1 Introduction to Human Anatomy and Physiology
Hole s Human Anatomy and Physiology Eleventh Edition Mrs. Hummer Hanover Area Jr./Sr. High School Chapter 1 Introduction to Anatomy and Physiology 1 Chapter 1 Introduction to Human Anatomy and Physiology
Human Anatomy & Physiology
 PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 7 The Skeleton: Part B Annie Leibovitz/Contact Press Images Vertebral
PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 7 The Skeleton: Part B Annie Leibovitz/Contact Press Images Vertebral
6.0 Management of Head Injuries for Maxillofacial SHOs
 6.0 Management of Head Injuries for Maxillofacial SHOs As a Maxillofacial SHO you are not required to manage established head injury, however an awareness of the process is essential when dealing with
6.0 Management of Head Injuries for Maxillofacial SHOs As a Maxillofacial SHO you are not required to manage established head injury, however an awareness of the process is essential when dealing with
Lectures of Human Anatomy
 Lectures of Human Anatomy Vertebral Column-I By DR. ABDEL-MONEM AWAD HEGAZY M.B. with honor 1983, Dipl."Gynecology and Obstetrics "1989, Master "Anatomy and Embryology" 1994, M.D. "Anatomy and Embryology"
Lectures of Human Anatomy Vertebral Column-I By DR. ABDEL-MONEM AWAD HEGAZY M.B. with honor 1983, Dipl."Gynecology and Obstetrics "1989, Master "Anatomy and Embryology" 1994, M.D. "Anatomy and Embryology"
20. Cerebral Compartmental Compliances
 20. Cerebral Compartmental Compliances CBF a (t) Cerebral arterial inflow Cerebral arterial blood volume (C a BV) mean CBF a 0 CBF v (t) Cerebral venous outflow CBV(t) mean CBV + - t t ΔCBV (CBF (t) CBF
20. Cerebral Compartmental Compliances CBF a (t) Cerebral arterial inflow Cerebral arterial blood volume (C a BV) mean CBF a 0 CBF v (t) Cerebral venous outflow CBV(t) mean CBV + - t t ΔCBV (CBF (t) CBF
Human Neuroanatomy. Grades 9-12. Driving Question: How did the evolution of the human brain impact the structure and function it has today?
 Human Neuroanatomy Grades 9-12 Driving Question: How did the evolution of the human brain impact the structure and function it has today? Objectives: Students will be able to Describe the basic parts and
Human Neuroanatomy Grades 9-12 Driving Question: How did the evolution of the human brain impact the structure and function it has today? Objectives: Students will be able to Describe the basic parts and
Anatomy and Terminology of the Spine. Bones of the Spine (Vertebrae)
 Anatomy and Terminology of the Spine The spine, also called the spinal column, vertebral column or backbone, consists of bones, intervertebral discs, ligaments, and joints. In addition, the spine serves
Anatomy and Terminology of the Spine The spine, also called the spinal column, vertebral column or backbone, consists of bones, intervertebral discs, ligaments, and joints. In addition, the spine serves
Provided by the American Venous Forum: veinforum.org
 CHAPTER 1 NORMAL VENOUS CIRCULATION Original author: Frank Padberg Abstracted by Teresa L.Carman Introduction The circulatory system is responsible for circulating (moving) blood throughout the body. The
CHAPTER 1 NORMAL VENOUS CIRCULATION Original author: Frank Padberg Abstracted by Teresa L.Carman Introduction The circulatory system is responsible for circulating (moving) blood throughout the body. The
Vocabulary & General Concepts of Brain Organization
 Vocabulary & General Concepts of Brain Organization Jeanette J. Norden, Ph.D. Professor Emerita Vanderbilt University School of Medicine Course Outline Lecture 1: Vocabulary & General Concepts of Brain
Vocabulary & General Concepts of Brain Organization Jeanette J. Norden, Ph.D. Professor Emerita Vanderbilt University School of Medicine Course Outline Lecture 1: Vocabulary & General Concepts of Brain
Pulmonary Ventilation
 Pulmonary Ventilation Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Pulmonary ventilation, or breathing, is the
Pulmonary Ventilation Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Pulmonary ventilation, or breathing, is the
Gas Exchange Graphics are used with permission of: adam.com (http://www.adam.com/) Benjamin Cummings Publishing Co (http://www.awl.
 Gas Exchange Graphics are used with permission of: adam.com (http://www.adam.com/) Benjamin Cummings Publishing Co (http://www.awl.com/bc) Page 1. Introduction Oxygen and carbon dioxide diffuse between
Gas Exchange Graphics are used with permission of: adam.com (http://www.adam.com/) Benjamin Cummings Publishing Co (http://www.awl.com/bc) Page 1. Introduction Oxygen and carbon dioxide diffuse between
Human Anatomy and Physiology II Laboratory
 Human Anatomy and Physiology II Laboratory The Circulation (Two Weeks) 1 This lab involves two weeks work studying the vasculature of the human body. Both weeks involve the exercise in the lab manual entitled
Human Anatomy and Physiology II Laboratory The Circulation (Two Weeks) 1 This lab involves two weeks work studying the vasculature of the human body. Both weeks involve the exercise in the lab manual entitled
6 Diagnosing Injuries of the Skull Base
 Flowchart Injuries of the Skull Base, Chapter 3, p. 17. Treatment of Injuries of the Skull Base, Chapter 17, p. 140. Surgical Anatomy n The bony architecture of the skull base can be divided into three
Flowchart Injuries of the Skull Base, Chapter 3, p. 17. Treatment of Injuries of the Skull Base, Chapter 17, p. 140. Surgical Anatomy n The bony architecture of the skull base can be divided into three
Chapter 15. Sympathetic Nervous System
 Chapter 15 Sympathetic Nervous System Somatic versus Autonomic Pathways Somatic efferent innervation ACh Myelinated fiber Somatic effectors (skeletal muscles) Autonomic efferent innervation ACh ACh or
Chapter 15 Sympathetic Nervous System Somatic versus Autonomic Pathways Somatic efferent innervation ACh Myelinated fiber Somatic effectors (skeletal muscles) Autonomic efferent innervation ACh ACh or
Fellow TEE Review Workshop Hemodynamic Calculations 2013. Director, Intraoperative TEE Program. Johns Hopkins School of Medicine
 Fellow TEE Review Workshop Hemodynamic Calculations 2013 Mary Beth Brady, MD, FASE Director, Intraoperative TEE Program Johns Hopkins School of Medicine At the conclusion of the workshop, the participants
Fellow TEE Review Workshop Hemodynamic Calculations 2013 Mary Beth Brady, MD, FASE Director, Intraoperative TEE Program Johns Hopkins School of Medicine At the conclusion of the workshop, the participants
RACE I Rapid Assessment by Cardiac Echo. Intensive Care Training Program Radboud University Medical Centre NIjmegen
 RACE I Rapid Assessment by Cardiac Echo Intensive Care Training Program Radboud University Medical Centre NIjmegen RACE Goal-directed study with specific questions Excludes Doppler ultrasound Perform 50
RACE I Rapid Assessment by Cardiac Echo Intensive Care Training Program Radboud University Medical Centre NIjmegen RACE Goal-directed study with specific questions Excludes Doppler ultrasound Perform 50
Gas Exchange. Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com)
 Gas Exchange Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Oxygen and carbon dioxide diffuse between the alveoli
Gas Exchange Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Oxygen and carbon dioxide diffuse between the alveoli
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 2 ORGANIZATION OF THE BODY
 ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 2 ORGANIZATION OF THE BODY Human beings are arguably the most complex organisms on this planet. Imagine billions of microscopic parts, each with its own identity,
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 2 ORGANIZATION OF THE BODY Human beings are arguably the most complex organisms on this planet. Imagine billions of microscopic parts, each with its own identity,
An introduction to fetal neurosonography using three-dimensional ultrasound
 An introduction to fetal neurosonography using three-dimensional ultrasound Taddei F, Fratelli N, Prefumo F, Franceshetti L, Signorelli M and Frusca T Maternal Fetal Medicine Unit, Department of Obstetrics
An introduction to fetal neurosonography using three-dimensional ultrasound Taddei F, Fratelli N, Prefumo F, Franceshetti L, Signorelli M and Frusca T Maternal Fetal Medicine Unit, Department of Obstetrics
CHAPTER 9 BODY ORGANIZATION
 CHAPTER 9 BODY ORGANIZATION Objectives Identify the meaning of 10 or more terms relating to the organization of the body Describe the properties of life Describe the function for the structures of the
CHAPTER 9 BODY ORGANIZATION Objectives Identify the meaning of 10 or more terms relating to the organization of the body Describe the properties of life Describe the function for the structures of the
3. The neuron has many branch-like extensions called that receive input from other neurons. a. glia b. dendrites c. axons d.
 Chapter Test 1. A cell that receives information and transmits it to other cells via an electrochemical process is called a(n) a. neuron b. hormone c. glia d. endorphin Answer: A difficulty: 1 factual
Chapter Test 1. A cell that receives information and transmits it to other cells via an electrochemical process is called a(n) a. neuron b. hormone c. glia d. endorphin Answer: A difficulty: 1 factual
Exchange solutes and water with cells of the body
 Chapter 8 Heart and Blood Vessels Three Types of Blood Vessels Transport Blood Arteries Carry blood away from the heart Transport blood under high pressure Capillaries Exchange solutes and water with cells
Chapter 8 Heart and Blood Vessels Three Types of Blood Vessels Transport Blood Arteries Carry blood away from the heart Transport blood under high pressure Capillaries Exchange solutes and water with cells
Laparoscopic Anatomy of the Pelvis
 2 Laparoscopic Anatomy of the Pelvis Intra-Abdominal Anatomy of the Male Pelvic Region Bladder Medial Umbilical Ligaments Lateral Umbilical Ligaments Spermatic Cords Iliac Vessels Ureters Seminal Vesicular
2 Laparoscopic Anatomy of the Pelvis Intra-Abdominal Anatomy of the Male Pelvic Region Bladder Medial Umbilical Ligaments Lateral Umbilical Ligaments Spermatic Cords Iliac Vessels Ureters Seminal Vesicular
3. Tunica adventitia is the outermost layer; it is composed of loosely woven connective tissue infiltrated by nerves, blood vessels and lymphatics
 Blood vessels and blood pressure I. Introduction - distribution of CO at rest II. General structure of blood vessel walls - walls are composed of three distinct layers: 1. Tunica intima is the innermost
Blood vessels and blood pressure I. Introduction - distribution of CO at rest II. General structure of blood vessel walls - walls are composed of three distinct layers: 1. Tunica intima is the innermost
Determinants of Blood Oxygen Content Instructor s Guide
 Determinants of Blood Oxygen Content Instructor s Guide Time to Complete This activity will take approximately 75 minutes, but can be shortened depending on how much time the instructor takes to review
Determinants of Blood Oxygen Content Instructor s Guide Time to Complete This activity will take approximately 75 minutes, but can be shortened depending on how much time the instructor takes to review
Chapter 2 - Anatomy & Physiology of the Respiratory System
 Chapter 2 - Anatomy & Physiology of the Respiratory System Written by - AH Kendrick & C Newall 2.1 Introduction 2.2 Gross Anatomy of the Lungs, 2.3 Anatomy of the Thorax, 2.4 Anatomy and Histology of the
Chapter 2 - Anatomy & Physiology of the Respiratory System Written by - AH Kendrick & C Newall 2.1 Introduction 2.2 Gross Anatomy of the Lungs, 2.3 Anatomy of the Thorax, 2.4 Anatomy and Histology of the
Chapter 9 - Nervous System
 Chapter 9 - Nervous System 9.1 Introduction (p. 215; Fig. 9.1) A. The nervous system is composed of neurons and neuroglia. 1. Neurons transmit nerve impulses along nerve fibers to other neurons. 2. Nerves
Chapter 9 - Nervous System 9.1 Introduction (p. 215; Fig. 9.1) A. The nervous system is composed of neurons and neuroglia. 1. Neurons transmit nerve impulses along nerve fibers to other neurons. 2. Nerves
Chapter 15. Autonomic Nervous System (ANS) and Visceral Reflexes. general properties Anatomy. Autonomic effects on target organs
 Chapter 15 Autonomic Nervous System (ANS) and Visceral Reflexes general properties Anatomy Autonomic effects on target organs Central control of autonomic function 15-1 Copyright (c) The McGraw-Hill Companies,
Chapter 15 Autonomic Nervous System (ANS) and Visceral Reflexes general properties Anatomy Autonomic effects on target organs Central control of autonomic function 15-1 Copyright (c) The McGraw-Hill Companies,
PE finding: Left side extremities mild weakness No traumatic wound No bloody otorrhea, nor rhinorrhea
 Case report A 82-year-old man was suffered from sudden onset spasm of extremities then he fell down to the ground with loss of consciousness. He recovered his consciousness 7-8 mins later but his conscious
Case report A 82-year-old man was suffered from sudden onset spasm of extremities then he fell down to the ground with loss of consciousness. He recovered his consciousness 7-8 mins later but his conscious
Urinary System Lab Guide
 Urinary System Lab Guide I. Prelab Questions Name 1. Describe the location of the kidneys. 2. Describe the following structures: a. renal cortex b. renal pyramid c. renal column d. minor calyx e. renal
Urinary System Lab Guide I. Prelab Questions Name 1. Describe the location of the kidneys. 2. Describe the following structures: a. renal cortex b. renal pyramid c. renal column d. minor calyx e. renal
Blood Vessels and Circulation
 13 Blood Vessels and Circulation FOCUS: Blood flows from the heart through the arterial blood vessels to capillaries, and from capillaries back to the heart through veins. The pulmonary circulation transports
13 Blood Vessels and Circulation FOCUS: Blood flows from the heart through the arterial blood vessels to capillaries, and from capillaries back to the heart through veins. The pulmonary circulation transports
Maxillary Sinus. (Antrum of Higmore)
 Maxillary Sinus (Antrum of Higmore) The maxillary sinus is a pneumatic space. It is the largest bilateral air sinus located in the body of the maxilla and opens in the middle nasal meatus of the nasal
Maxillary Sinus (Antrum of Higmore) The maxillary sinus is a pneumatic space. It is the largest bilateral air sinus located in the body of the maxilla and opens in the middle nasal meatus of the nasal
Nervous System sensor input integration motor output sensory organs central nervous system
 Nervous System Nervous system performs three overlapping functions of sensor input, integration, and motor output. This process is generally the same even at a very primitive level of nervous system, but
Nervous System Nervous system performs three overlapping functions of sensor input, integration, and motor output. This process is generally the same even at a very primitive level of nervous system, but
Common types of congenital heart defects
 Common types of congenital heart defects Congenital heart defects are abnormalities that develop before birth. They can occur in the heart's chambers, valves or blood vessels. A baby may be born with only
Common types of congenital heart defects Congenital heart defects are abnormalities that develop before birth. They can occur in the heart's chambers, valves or blood vessels. A baby may be born with only
Physics 9e/Cutnell. correlated to the. College Board AP Physics 1 Course Objectives
 Physics 9e/Cutnell correlated to the College Board AP Physics 1 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring
Physics 9e/Cutnell correlated to the College Board AP Physics 1 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring
Factors Affecting Blood Pressure. Vessel Elasticity Blood Volume Cardiac Output
 Factors that Affect Pressure Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction pressure is affected by several factors:
Factors that Affect Pressure Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction pressure is affected by several factors:
A. function: supplies body with oxygen and removes carbon dioxide. a. O2 diffuses from air into pulmonary capillary blood
 A. function: supplies body with oxygen and removes carbon dioxide 1. ventilation = movement of air into and out of lungs 2. diffusion: B. organization a. O2 diffuses from air into pulmonary capillary blood
A. function: supplies body with oxygen and removes carbon dioxide 1. ventilation = movement of air into and out of lungs 2. diffusion: B. organization a. O2 diffuses from air into pulmonary capillary blood
Basic Brain Information
 Basic Brain Information Brain facts Your brain weighs about 3lbs, or just under 1.5Kg It has the texture of blancmange Your brain is connected to your spinal cord by the brain stem Behind your brain stem
Basic Brain Information Brain facts Your brain weighs about 3lbs, or just under 1.5Kg It has the texture of blancmange Your brain is connected to your spinal cord by the brain stem Behind your brain stem
Clarification of Terms
 Shoulder Girdle Clarification of Terms Shoulder girdle = scapula and clavicle Shoulder joint (glenohumeral joint) = scapula and humerus What is the purpose (or function) of the shoulder and entire upper
Shoulder Girdle Clarification of Terms Shoulder girdle = scapula and clavicle Shoulder joint (glenohumeral joint) = scapula and humerus What is the purpose (or function) of the shoulder and entire upper
Mini-atlas of the Marmoset Brain
 Mini-atlas of the Marmoset Brain http://marmoset-brain.org Aya Senoo Tokyo University of Agriculture and Technology Hironobu Tokuno Tokyo Metropolitan Institute of Medical Science Charles Watson Curtin
Mini-atlas of the Marmoset Brain http://marmoset-brain.org Aya Senoo Tokyo University of Agriculture and Technology Hironobu Tokuno Tokyo Metropolitan Institute of Medical Science Charles Watson Curtin
The heart walls and coronary circulation
 CHAPTER 1 The heart walls and coronary circulation The heart is located in the central-left part of the thorax (lying on the diaphragm) and is oriented anteriorly, with the apex directed forward, downward,
CHAPTER 1 The heart walls and coronary circulation The heart is located in the central-left part of the thorax (lying on the diaphragm) and is oriented anteriorly, with the apex directed forward, downward,
DEVELOPMENT AND GROWTH OF THE MANDIBLE
 2012-2013 ORAL BIOLOGY DEVELOPMENT AND GROWTH OF THE MANDIBLE Ass. Prof. Dr. Heba M. Elsabaa Development and Growth of the Mandible DEVELOPMENT OF THE MANDIBLE The Mandible Is the largest and strongest
2012-2013 ORAL BIOLOGY DEVELOPMENT AND GROWTH OF THE MANDIBLE Ass. Prof. Dr. Heba M. Elsabaa Development and Growth of the Mandible DEVELOPMENT OF THE MANDIBLE The Mandible Is the largest and strongest
Student Academic Learning Services Page 1 of 8 Nervous System Quiz
 Student Academic Learning Services Page 1 of 8 Nervous System Quiz 1. The term central nervous system refers to the: A) autonomic and peripheral nervous systems B) brain, spinal cord, and cranial nerves
Student Academic Learning Services Page 1 of 8 Nervous System Quiz 1. The term central nervous system refers to the: A) autonomic and peripheral nervous systems B) brain, spinal cord, and cranial nerves
An Introduction to Anatomy and Physiology
 An Introduction to Anatomy and Physiology Objectives Define anatomy and physiology Identify the levels of organization in organisms from simplest to most complex Identify the organ systems of the human
An Introduction to Anatomy and Physiology Objectives Define anatomy and physiology Identify the levels of organization in organisms from simplest to most complex Identify the organ systems of the human
