To link to this article: D OI: / URL:
|
|
|
- Matthew Tate
- 8 years ago
- Views:
Transcription
1 This article was downloaded by:[steele, Brooke] On: 6 F ebruary 2007 Access Details: [subscription number ] Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: Registered office: Mortimer House, Mortimer Street, London W1T 3JH, UK C omputer Methods in Biomechanics and Biomedical E ngine ering Publication details, including instructions for authors and subscription information: Fractal network model for simulating abdominal and lower extremity blood flow during resting and exercise conditions To link to this article: D OI: / URL: Full terms and conditions of use: This article maybe used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material. Taylor and Francis 2007
2 Computer Methods in Biomechanics and Biomedical Engineering, Vol. 10, No. 1, February 2007, Fractal network model for simulating abdominal and lower extremity blood flow during resting and exercise conditions BROOKE N. STEELE *, METTE S. OLUFSEN and CHARLES A. TAYLOR{k Joint Department of Biomedical Engineering, NC State University & UNC-Chapel Hill, Campus Box 7115, Raleigh, NC , USA Department of Mathematics, NC State University, Campus Box 8205, Raleigh, NC , USA {Departments of Mechanical Engineering, Bioengineering, and Surgery, James H. Clark Center, Room E350B, 318 Campus Drive, Stanford, CA , USA (Received 6 August 2006; in final form 26 September 2006) We present a one-dimensional (1D) fluid dynamic model that can predict blood flow and blood pressure during exercise using data collected at rest. To facilitate accurate prediction of blood flow, we developed an impedance boundary condition using morphologically derived structured trees. Our model was validated by computing blood flow through a model of large arteries extending from the thoracic aorta to the profunda arteries. The computed flow was compared against measured flow in the infrarenal (IR) aorta at rest and during exercise. Phase contrast-magnetic resonance imaging (PC-MRI) data was collected from 11 healthy volunteers at rest and during steady exercise. For each subject, an allometrically-scaled geometry of the large vessels was created. This geometry extends from the thoracic aorta to the femoral arteries and includes the celiac, superior mesenteric, renal, inferior mesenteric, internal iliac and profunda arteries. During rest, flow was simulated using measured supraceliac (SC) flow at the inlet and a uniform set of impedance boundary conditions at the 11 outlets. To simulate exercise, boundary conditions were modified. Inflow data collected during steady exercise was specified at the inlet and the outlet boundaries were adjusted as follows. The geometry of the structured trees used to compute impedance was scaled to simulate the effective change in the crosssectional area of resistance vessels and capillaries due to exercise. The resulting computed flow through the IR aorta was compared to measured flow. This method produces good results with a mean difference between paired data to be 1.1 ^ 7 cm 3 s 21 at rest and 4.0 ^ 15 cm 3 s 21 at exercise. While future work will improve on these results, this method provides groundwork with which to predict the flow distributions in a network due to physiologic regulation. Keywords: One-dimensional model; Arterial blood flow; Fractal; Structured tree; Impedance; Exercise 1. Introduction Numerous models have been used to describe the dynamics of blood flow and blood pressure in the cardiovascular system. These models include simple Windkessel models (Pater and van den Berg 1964; Westerhof et al. 1969; Noordergraaf 1978), non-linear one-dimensional (1D) models (Stergiopulos et al. 1992; Olufsen et al. 2000; Wan et al. 2002) and complex three-dimensional (3D) models (Taylor et al. 1996; Cebral et al. 2003). Each class of models is suited to answer a particular type of blood flow question. For example, the Windkessel can be used to describe the overall dynamics of blood flow in the systemic circulation (Olufsen et al. 2000; Olufsen and Nadim 2004) while spatial models (1D, 2D and 3D models) can describe blood flow and blood pressure through a given geometry. Spatial models span a limited region of interest. The remainder of the circulatory system is represented with a set of boundary conditions that are developed to approximate blood flow and blood pressure outside the modelled domain. *Corresponding author. Tel: þ Fax: þ bnsteel@ncsu.edu Tel: þ Fax: þ msolufse@ncsu.edu ktel: þ Fax: þ taylorca@stanford.edu Computer Methods in Biomechanics and Biomedical Engineering ISSN print/issn online q 2007 Taylor & Francis DOI: /
3 40 B. N. Steele et al. To describe boundary conditions for spatial models, researchers often prescribe blood flow or pressure profiles (Taylor et al. 1999a). Although this approach is simple, specifying flow or pressure will influence the fluid dynamics inside the model domain and is only appropriate when profiles and distribution between outlets is known. Often complete boundary profile information is not available, so constant relationships between pressure and flow are used (Wan et al. 2002). Resistance boundary conditions provide a convenient method to specify a boundary relationship without prescribing a pressure or flow waveform. However, pure resistance boundary conditions cannot account for non-proportional variations between pressures and flow as observed in compliant vessels. An alternative to the constant resistance boundary condition is the impedance boundary condition, which is the frequency analogue to resistance. Impedance has long been recognized as an important tool for evaluating the reflections and damping of flow and pressure waves (Taylor 1966; Brown 1996; Nichols and O Rourke 2005). Impedance boundary conditions are often implemented using simple three-element Windkessel model (Burattini et al. 1994; Manning et al. 2002). While useful, the Windkessel model has two limitations: (1) parameters cannot be specified as a function of model geometry; and (2) Windkessel models cannot account for flow and pressure wave changes including damping or amplification and dispersion that occur in a branched network of compliant blood vessels with spatially varying properties (Olufsen and Nadim 2004). An alternate method not subject to these limitations is to compute the impedance using a fractal network (Taylor 1966; Brown 1996; Olufsen 1999) representing the vascular bed. In this work, the objective is to extend the structured tree model developed by Olufsen (1999) to compute the impedances of vascular beds during rest and exercise. Short-term regulatory mechanisms in the body continuously alter the impedance of vascular beds to control the distribution of blood due to varying demands of organs and tissues. These regulatory mechanisms act on the vascular beds resulting in changes in vascular anatomy such as vasodilation or vasoconstriction and recruitment or closure of capillary beds by the opening and closing of pre-capillary sphincters. Following the onset of leg exercise, heart rate (HR) and cardiac output (CO) are increased and as a result, the aortic flow waveform is changed from tri- to bi-phasic as negative flows are eliminated. Impedance in the leg is decreased due to the dilation (3 5 times) of arterioles or recruitment of non-flowing capillaries to meet the metabolic demand of the active muscles. Meanwhile, the vascular beds that supply non-essential organs and inactive muscles reduce flow, using constriction of arterioles or pre-capillary sphincters to direct more of the CO to high-demand locations and maintain blood pressure. These impedance-regulating mechanisms can be incorporated by using geometric alterations in the structured tree impedance boundary. A number of in vitro and numerical studies have been performed to visualize the changes in flow features in the abdominal aorta during exercise (Pedersen et al. 1993; Moore and Ku 1994; Boutouyrie et al. 1998; Taylor et al. 1999b). In these studies, the goal was to understand current hemodynamic conditions with a prescribed, known outflow condition. This method would not be suitable in determining the change in flow features following a change in the geometry of the modelled region. The ability to simulate both rest and exercise is desired because diagnostic data required for modelling is primarily collected with the patient at rest and symptoms of lower extremity vascular disease are most evident during exercise. One of the most pronounced symptoms of lower extremity vascular disease is claudication, pain in the thigh and buttock during exercise due to diminished capacity to deliver blood to active muscle. Currently, the success rate of relieving claudication is not easily predicted as it is related to the location and extent of disease, the ability of proximal vessels to supply blood to the region, and the capacity of distal beds to accommodate runoff. As a consequence of this difficulty, potential negative outcomes include: (1) patient may be required to undergo a re-do operation to relieve symptoms following an under aggressive treatment; (2) patient may not benefit due to being a poor candidate; or (3) patient may suffer unnecessary complications from overaggressive treatment. Computational modelling for surgical planning in the scenario above, with the ability to model the exercise state based on data collected during rest, may improve the success rate and reduce the risk to patients suffering from claudication. In summary, this paper shows how to model blood flow in large vessels during rest and exercise. We demonstrate the effect of changing inlet HR, CO, and the geometry of the structured tree attached at the outlet. This model is validated against non-invasively recorded phase contrastmagnetic resonance imaging (PC-MRI) flow data for eleven healthy subjects during rest and exercise. 2. Methods 2.1 Governing equations Axisymmetric 1D equations for blood flow and pressure can be derived by appropriately integrating the 3D Navier Stokes equations over the vessel cross-section and neglecting in-plane components of velocity (Hughes and Lubliner 1973; Hughes 1974). This model is used to describe large vessels in which the blood flow is considered Newtonian, the fluid is considered incompressible and the vessel walls are assumed to be impermeable. We further assume that the velocity profile across the diameter of the vessels is parabolic (Wan et al. 2002). The resulting partial differential equations for conservation of mass (1) and balance of momentum (2)
4 Fractal network model for rest and exercise 41 are given by: q t þ z 4 q 2 3 s s t þ q z ¼ 0 ð1þ þ s p r z ¼ 28pn q s þ n 2 q z 2 : ð2þ pressure and flow of the form: Pðz; vþ ¼ Zðz; vþqðz; vþ, Qðz; vþ ¼ Pðz; vþyðz; vþ; where Yðz; vþ ¼ 1=Zðz; vþ: ð5þ The primary variables are cross-sectional area sðz; tþ (cm 2 ), volumetric flow rate, qðz; tþ (cm 3 s 21 ), and pressure, pðz; tþ (dynes s 21 cm 22 ); z (cm) is the axial location along the arteries and t (s) is time. The density of the fluid is given by r ¼ 1.06 g cm 23, the kinematic viscosity is given by n ¼ cm 2 s Constitutive equation The above system has three variables, but only two equations. Hence, to complete the system of equations, a constitutive relationship is needed. In this paper, we have used a model that describes pressure p as an elastic function of the cross-sectional area s. This equation, derived by Olufsen (1999), is given by: pðsðz; tþ; z; tþ ¼ p 0 þ 4 Eh 3 r 0 ðzþ s ffiffiffiffiffiffiffiffiffiffiffi! s 0 ðzþ 1 2 sðz; tþ where p 0 is the unstressed pressure, E (g s 22 cm 21 ) is Young s modulus, h (cm) is the thickness of the arterial wall, r 0 (z) (cm) is the radius of the unstressed vessel at location z, and s 0 (cm 2 ) is the cross-sectional area of the unstressed vessel. Young s modulus times the wall thickness over the radius is defined by: Eh r 0 ðzþ ¼ k 1e k 2r 0 ðzþ þ k 3 ; where k 1 ¼ g s 22 cm 21, k 2 ¼ cm 21, and k 3 ¼ g s 22 cm 21 are constants obtained from Olufsen (1999). This elastic model is only an approximation and hence, it does not reflect the viscoelastic nature of arteries. 2.3 Initial and boundary conditions Initially, the cross-sectional area is prescribed from model geometry, and the initial flow is set to zero. Since the above system of equations is hyperbolic, one boundary condition must be specified at each end for all vessels. There are three types of vessel endings: inlets, outlets and bifurcations. At the inlet, we specify a flow waveform qð0; tþ from data, and at the outlets, we use an expression for impedance obtained by solving the linearized version of the Navier Stokes equations in the structured tree using an approach first described by Womersley (1955) and Taylor (1966). The impedance is computed as a function of frequency, v (s 21 ). It provides a relation between ð3þ ð4þ Pðz; vþ; Qðz; vþ; Zðz; vþ and Yðz; vþ are frequency dependent pressure, flow, impedance and admittance, respectively. Since these expressions are applied as outlet conditions, for each outflow vessel they are calculated at z ¼ L: For each outlet, the relation between variables expressed in the time domain and their counterparts in the frequency domain is found using the Fourier transform. Hence, time dependent quantities can be obtained using the convolution theorem, i.e.: qðz; tþ ¼ 1 T ð T=2 2T=2 yðz; t 2 tþpðz; tþ dt; where z ¼ L and y is admittance in the time domain. In our implementation, we evaluate the flow waveform by computing the flow at discrete time points using the form: qðl; nþ ¼ XN21 yðl; jþpðl; n 2 jþ; j¼0 where N is the number of time steps per cardiac cycle and L is the length of the given vessel. Finally, bifurcation conditions are introduced to link properties of a parent vessel x p and daughter vessels x di, i ¼ 1; 2. For each bifurcation three relations must be obtained, an outlet condition for the parent vessel and an inlet condition for each daughter vessel. One equation is obtained by ensuring that flow is conserved, and two other relations are obtained by assuming continuity of pressure: q p ðl; tþ ¼ q d1 ð0; tþ þ q d2 ð0; tþ; p p ðl; tþ ¼ p d1 ð0; tþ ¼ p d2 ð0; tþ: Pressure losses associated with the formation of vortices downstream from the junctions are accommodated for by including a minor loss term applied in the proximal region of the junction vessels. For a detailed description, see Steele et al. (2003). To solve the system of equations (1) (3) combined with the inlet condition, outlet conditions (7), and bifurcation conditions (8), we employ a space-time finite element method that include Galerkin least squares stabilization in space and a discontinuous Galerkin method in time. We use a modified Newton Raphson technique to solve the resultant nonlinear equations for each time step (Wan et al. 2002). ð6þ ð7þ ð8þ
5 42 B. N. Steele et al. 2.4 Impedance boundary condition for vascular networks Vascular impedance is the resistance to blood flow through a vascular network. During steady state (i.e. at rest or during steady exercise), impedance can be computed from the structured trees that represent vascular beds and used as an outlet boundary condition. The vascular impedance at the root of the fractal tree is obtained in a recursive manner starting from the terminal branches where pressure is assumed to be 0 mmhg (figure 2). Along each vessel in the structured tree, impedance is computed from linear, axisymmetric, 1D equations for conservation of mass and momentum (Olufsen et al. 2000). Linearized equations are appropriate for use in arteries with diameter smaller than 2 mm where viscosity dominates (Olufsen and Nadim 2004) and the nonlinear advection effects can be neglected as a first approximation (Womersley 1957; Atabek and Lew 1966; Pedley 1980). The details of this computation are given in Olufsen et al. (2000). Briefly, the input impedance is computed at the beginning of each vessel z ¼ 0 as a function of the impedance at the end of a vessel z ¼ L : Zð0; vþ ¼ ig 21 sin ðvl=cþ þ ZðL; vþcosðvl=cþ cosðvl=cþ þ igzðl; vþsinðvl=cþ p L is vessel length, c ¼ ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi s 0 ð1 2 F J Þ=ðrCÞ is the wavepropagation velocity, where: ð9þ Zð0; 0Þ ¼ lim Zð0; vþ ¼ 8ml rr v!0 pr 3 þ ZðL; 0ÞF J ¼ 2 J 1ðw 0 Þ 0 w m J 0 ðw 0 Þ ð10þ J 0 (x) and J 1 (x) are the zero th and first order Bessel functions with w 2 0 ¼ i 3 w and w 2 ¼ r 2 0v=y: The compliance C is approximated as: C < 3s 0r 0 2Eh ; ð11þ 2.5 The geometry of the structured tree The small arteries that form the vascular bed are described using a bifurcating self-similar tree characterized by three parameters as described below (Olufsen 1999). The first parameter describes the branching relationship across bifurcations between the radius of the parent vessel r p and the radii of the daughter vessels r di ; i ¼ 1; 2: There are two methods for defining this relationship, the area ratio and the power law. The area ratio, h, is given by: h ¼ r2 d1 þ r2 d2 r 2 p The power law is defined by: r k p ¼ r k d1 þ r k d2 ð13þ ð14þ If k ¼ 2, then area will be conserved. Murray (1926) studied the physiologic organization of the vascular system and applied the principle of minimum work to examine the correlation between structure and function of the arteries. Murray derived that maximum efficiency for blood flow is attained when the relationship between flow and vessel radius is of the form q / r 3 ; or k ¼ 3: However, observations show that k is neither constant nor organ specific. It has also been suggested that k may vary depending on the radius of the branches involved. Several studies show that k varies from 2 to 3 with mean values given by 2.5, 2.7 and 2.9 (Iberall 1967; Zamir 1999; Karch et al. 2000). Zamir (1999) introduces the concept of sub-ranges of vessels based on diameter. In this work, we adopt this concept and use a three tiered structure with k ¼ 2:5 for small arteries r, 250 mm, k ¼ 2:7 for resistance arteries 250 mm, r, 50 mm; and k ¼ 2:9 for vessels r, 50 mm (table 1). The second parameter, g, is known as the bifurcation index or the asymmetry index. The asymmetry index describes the relative relationship between the daughter vessels: where s 0 is the reference cross-sectional area, Eh=r 0 is defined in equation (4), and g ¼ cc: The impedance at v ¼ 0 can be found as: Zð0; 0Þ ¼ lim v!0 ð12þ where l rr ¼ L=r is the length-to-radius ratio described below and viscosity, m ¼ g cm 21 s. g ¼ r d1 r d2 : ð15þ Assuming that r d1 # r d2 ; g is between 0 and 1. The asymmetry index varies widely throughout vascular beds and does not appear to be organ specific (Papageorgiou et al. 1990; Zamir 1999). We chose to vary the asymmetry ratio in the tiered system described above with g ¼ 0.4, 0.6 and 0.9 (table 1). Table 1. Parameters used to describe the structured tree. The tree is divided into three levels as a function of the vessel radius (second column). For each level, the parameters that describe the power exponent k (third column) and the asymmetry ratio g (last column) are varied. Level Radius Power exponent Asymmetry ratio Small arteries 250 mm, r k ¼ 2.50 g ¼ 0.4 Resistance vessels 50 mm, r, 250 mm k ¼ 2.76 g ¼ 0.6 Capillaries r, 50 mm k ¼ 2.90 g ¼ 0.9
6 Fractal network model for rest and exercise 43 Finally, the length of a given artery (between bifurcations) can be expressed as a function of the mean radius of the vessel. Iberall (1967) recommends the use of a length-to-radius ratio, l r r, of 50. This conclusion was drawn from analysis of data collected in several studies that produced a range of estimates. Zamir (1999) suggests that the mean l r r is 20 with a maximum of 70. Others (Suwa et al. 1963; Iberall 1967; Zamir 1999) have shown that that the l r r in the vascular bed is widely varied and that the value is organ specific. We elect to use l rr as a mechanism to vary the relative impedance between outlets. The properties described above are used in an asymmetric structured tree originally devised by Olufsen (1999). The limbs of the structured tree are systematically ordered to take advantage of pre-computed branches to minimize the computational cost in arriving at the root impedance. In the tree, the radii of successive daughter vessels (r d1 and r d2 ) were obtained by introduction of scaling parameters a and b for the radius of the root vessel (r root ) such that: r d1 ¼ ar root ; r d2 ¼ br root and r i; j ¼ a i b j2i r root ð16þ for the radius of the ith daughter vessel in the jth generation of the tree and i ¼ {0; 1;... ; j} (see figure 1). The power law, area and asymmetry ratios are combined to give: and h ¼ r2 d1 þ r2 d2 r 2 p 1 þ g ¼ ð1 þ g k=2 Þ 2=k ¼ ðar rootþ 2 þ ðbr root Þ 2 r 2 p a ¼ ð1 þ g k=2 Þ 2ð1=kÞ p and b ¼ a ffiffiffi g ð17þ ð18þ Olufsen used an asymmetry ratio g ¼ 0:41; a power k ¼ 2:76 and l r r ¼ 50: The terminal minimum radius ( mm) was used as the mechanism to vary the relative impedance between outlets. In our implementation of the structured tree, we developed a three-tiered model with variables r root and l rr to mimic the behaviour of a specific vascular bed. The tiers in table 1 were developed based on the literature described above and to provide asymmetry. These modifications to Olufsen s structured tree allow the extension of the structured tree to a minimum radius of 3 mm where pressure is set to be 0.0 mmhg. The extension of the structured tree to include resistance vessels facilitates the use of a scaling factor, described below to model physiologic regulation. Although the apparent viscosity of blood in microcirculation is a function of both vessel diameter and hematocrit (Fahraeus-Lindqvist effect) Pries et al. (1990), we do not include this variation in viscosity in this impedance model. 2.6 Modeling exercise During exercise, regional resistance vessels regulate the blood supply to active muscle and non-essential organs. To mimic this behaviour, the radii of the resistance vessels ðr, 300 mmþ were adjusted by a scaling factor, f, to simulate the increase or decrease in effective crosssectional area of the vascular bed during exercise. If f, 1; the effective vessel cross-section is decreased and if f. 1; the effective cross-sectional area is increased. In order to preserve the structure of the tree, the length and radii of the vessels and number of generations of the tree are determined before scaling vessel radii. 2.7 Application with non-invasively measured data The method described above is validated using flow data obtained non-invasively from 11 healthy subjects Taylor et al. (2002). Through-plane flow velocities were acquired using a Cine PC-MRI sequence on a 0.5 T open magnet (GE Signa SP, GE Medical Systems, Milwaukee, WI, USA). To minimize blood flow regulation associated with digestion, subjects were instructed to fast 2 h before scanning. Subjects were seated on a custom built magnetic Table 2. Standard dimensions for idealized model. Columns describe the large vessels in the idealized model with inlet and outlet radii and vessel lengths (cm) Vessel Inlet radius (cm) Outlet radius (cm) Length (cm) Figure 1. Structured tree and sub-unit. The root of the tree is the interface between the modeled domain and boundary condition. Radii of vessels determined using scaling parameter a i b j2i. In the convention shown, the leftmost daughter vessel assumes i ¼ j and the right most daughter vessel assumes i ¼ 0. Aorta Celiac Superior mesenteric Renal Inferior mesenteric Iliac Internal iliac Femoral Profunda
7 44 B. N. Steele et al. Table 3. Estimated flow distribution expressed as a percentage of CO. Structured tree boundary conditions were created for each outlet using the specified length-to-radius ratio. This uniform set of parameters was used for each subject. Target distribution in extremities estimated from measured data and physiologic blood pressure. Symmetry was assumed in right/left-paired vessels (renal, profunda, internal iliac, and femoral). Outlet Target CO (%) Length-to-radius ratio Celiac Superior mesenteric Renal (2) Inferior mesenteric 4 24 Internal iliac (2) 4 60 Profunda (2) 4 60 Femoral (2) 5 80 resonance (MR) compatible cycle ergometer with their torsos in the field of view of the magnet. To minimize movement, the subjects were securely strapped to the seat. PC-MRI images of velocity and cross-section were captured at the supraceliac (SC) and infrarenal (IR) levels of the abdominal aorta at rest and during moderate cycling exercise. The velocities were integrated over the crosssectional area of the vessel to compute the flow rate. The single cardiac cycle produced by PC-MRI is a composite of many gated cardiac cycles. Patient specific anatomy and pressure data were not acquired. In order to develop subject specific geometric models, an existing geometry of an idealized abdominal aorta with major branching vessels (Moore and Ku 1994) was scaled to match measured SC and IR cross-section areas. The idealized model includes one inlet vessel and 11 outlet vessels as shown in figure 2 and in table 2. The scaling was performed using an allometric scaling law (West et al. 1997): R ¼ R 0 M b ð19þ where R represents the desired radius (cm), R 0 is the known scaling constant (cm), M represents the body mass and b is the scaling exponent. The measured cross-sectional area of the aorta for one test subject matched the aorta of the idealized model. This subject was assumed ideal and the SC radius was used as the scaling constant Y 0. This subject s body mass was designated M 0. In order to balance the units, M ¼ M i =M 0 where M i is the test subjects body mass. Using the measured radius from the PC-MRI slice obtained at the SC aorta (R) during diastole, the size of the idealized aorta (R 0 ), and the mass for each subject (M), the scaling exponent, b, was found to be for the measured data. This scaling exponent was then used to scale the idealized model for all remaining vessels. The scaled idealized models represent the large vessels in the computational domain including the aorta (inlet), celiac, superior and inferior mesenteric, renal, iliac, internal iliac, femoral and profunda arteries (see figure 2 and table 2). Next, boundary conditions were determined. The inlet boundary condition for each subject was specified from the SC flow waveforms measured using PC-MRI. All outlet boundary conditions were specified using the modified structured tree with root radius set to the model domain boundary radius and neutral tone, f ¼ 1:0: Initially, visceral outlet boundaries were assigned l rr ¼ 20 and leg outlet boundaries were assigned l rr ¼ 70: The l rr values were adjusted so that the distribution of flow to the viscera matched distributions reported in the literature and to maintain a physiologic blood pressure of approximately 120/70 mmhg (see table 3). For normal, healthy individuals, it is assumed that approximately 20 27% of CO flows through the renal arteries (Ganong 1995) and approximately 27% flows through the celiac, superior mesenteric artery (SMA), and inferior mesenteric artery (IMA) combined. The resting, fasting mesenteric flow can be further estimated as 10% of the CO to the celiac artery, 13% to the SMA, and 4% to the IMA (Ganong 1995; Perko et al. 1998). Because CO was not measured, each subject s blood volume in litres was estimated as 7% of body mass (self reported) in kg. This value was used to estimate resting CO by assuming the entire blood volume is circulated in 1 min (table 4). Using this target CO, l rr values were determined for all outlets (table 3). These outlet boundary conditions were used to perform resting flow analysis for all subjects. Finally, steady exercise was simulated by modifying the boundary conditions. The inlet boundary condition for each subject was specified using the SC flow measured using PC- MRI during exercise. The outlet boundary conditions were modified by specifying a scaling factor, f, as described above. To determine appropriate factors, a series of analyses were performed on one data set. Initially, peripheral beds were dilated by a factor of 5 as described in Ganong (1995) to increase flow to the active muscles Table 4. Estimated resting CO based on weight and HR. Estimated SC aortic flow based on target percentage (66%) of estimated CO. Measured SC flow and error between measured and estimated SC flow. Subject Weight (kg) HR Estimated CO (l min 21 ) Estimated SC (l min 21 ) Measured SC (l min 21 ) Error (%)
8 Fractal network model for rest and exercise 45 Figure 2. Computational domain for the larger arteries. This model represents an idealized aorta and major branching vessels. Branching vessels include celiac, SMA, IMA, renal, internal iliac, profunda and femoral arteries. Note that the femoral arteries have been truncated in this image. Dimension for each of these vessels for a standard subject is shown in table 2. in the legs. After a cursory study, it was found that f ¼ 5:0 is the maximum effective dilation factor and alone did not create a large enough pressure drop to draw an appropriate amount of flow away from the viscera (as measured with PC-MRI) through the IR aorta. To decrease flow through the visceral vessels, a constriction factor was applied uniformly to all of the viscera beds in increments of 0.1 until the appropriate mean flow was computed in the IR aorta. This method provided a reasonable result, however, other combinations of parameters may provide similar results. Using this method, a constricting factor of f ¼ 0:7 and a dilating factor of f ¼ 5:0; applied to the viscera and lower extremity outlets respectively, were found to provide the appropriate flow redistribution, matching the measured IR aortic flow and approximating physiological pressures. This scheme was then applied to all data sets. 3. Results 3.1 Rest Pressure, flow and cross-sectional area were computed in the large arteries of the abdomen and legs for 11 healthy Figure 3. Flow comparisons for healthy young adults at rest for one cardiac cycle. Graphs show comparison between measured (thin line) and computed (bold line) IR aortic flow.
9 46 B. N. Steele et al. Figure 4. Scatter plot comparison between predicted and measured flow (cm 3 s 21 ) waveforms. The identity line is shown. A correlation coefficient of 0.94 was computed. subjects. For each subject, the model geometry (see figure 2) was based on an idealized model scaled for each subject using body weight and an allometric scaling law. Inlet boundary conditions were obtained from non-invasively measured data and outlet boundary conditions were specified using structured trees with parameters based on approximations from literature (see table 3). PC-MRI was used to acquire non-invasive flow measurements for each subject at the IR aorta. Paired waveforms of computed and measured IR flow for each subject are shown in figure 3. A scatter plot of all paired data points is shown in figure 4. While overall means between paired measured and computed data are within 1.1 cm 3 s 21, the computed waveforms tend to overestimate the amplitude of the waveforms with a standard deviation of 7.00 cm 3 s 21. Mean flow distribution through each outlet is shown in figure 5. Gross distributions between the viscera and legs were determined by comparing SC and IR flow and were measured to be 30 ^ 6% and computed to be 28 ^ 2%. The measured SC inlet flow was less than estimate based on body weight for subjects 3, 5, 7 and 11 (table 4). The boundary condition scheme was not altered for these subjects. Although this reduced level of flow would undoubtedly result in a physiologic change in distribution, i.e. less flow to non-essential organs and resting muscle, there does not seem to be a large difference between measured and predicted infra renal flow. In figure 6, computed inlet pressures are shown with the corresponding measured inlet flow waveforms. As described previously, measured SC inlet flow is lower than estimated for several subjects and the boundary condition scheme was not altered for these subjects. Consequently, the computed pressures are lower than expected in subjects with lower than expected measured flow. 3.2 Exercise The computational models are modified to reflect the exercise state by adjusting the inlet boundary conditions to match measured data, with increased abdominal aortic flow rate and a shorter cardiac cycle. The outlet boundary conditions are modified to reflect constriction (f ¼ 0:7) of the visceral beds and dilation (f ¼ 5:0) of lower extremity vascular beds. Paired waveforms of computed and measured IR flow during lower extremity exercise are shown in figure 7. Figure 8 shows a scatter plot of all paired data points. The mean difference between paired data is 4.07 cm 3 s 21 with a standard deviation of 15 cm 3 s 21. While overall agreement is good, the computed waveform for subject 11 is underestimated. The computed mean outlet flow distribution during exercise is shown in figure 9. Gross distributions between the viscera and legs were determined by comparing SC and IR flow and were found to be 79 ^ 7% during exercise and computed to be 82 ^ 1%. In figure 10, the computed inlet pressures are shown with the corresponding measured inlet flow waveforms. 4. Discussion In this paper, we have described a method for implementing impedance boundary conditions in a 1D fluid dynamic model using scaleable structured trees to represent Figure 5. Computed mean flow values at outlet boundaries at rest as determined by uniform outlet boundary conditions. Sum of flow indicates mean measured superceliac flow for each subject. Paired arteries (renal, femoral, internal iliac and profunda) are summed.
10 Fractal network model for rest and exercise 47 Figure 6. Measured inlet flow boundary condition (cm 3 s 21 ) (thin line) and computed inlet pressures (mmhg) (bold line) at rest for one cardiac cycle. downstream vascular beds. The structured trees were scaled to simulate changes in the effective cross-sectional area of the resistance vessels during exercise. Using this method, we were able to predict changes in blood flow waveform and distribution. This model was validated by comparing computed flow waveforms to non-invasively measured data from 11 healthy subjects at rest and during moderate cycling exercise. Scatter plots and flow waveforms demonstrate good agreement between computed and measured data. This study lays the groundwork for modelling the redistribution of flow due to changes in peripheral impedance resulting from physiologic regulation. 4.1 Model limitations and future work As with any model study, this investigation was subject to errors associated with experimental methods and modelling assumptions. The experimental method used to measure blood flow, PC-MRI, may have contributed to observed difference between computed and measured values. Although PC-MRI is considered the gold standard for non-invasive measurement of blood flow, errors associated with this technique are estimated to be as much as 10 20% (Taylor et al. 2002). This uncertainty makes it difficult to quantify the error associated with the computational studies. The MR sequence used for this study involved constructing the recorded cardiac cycle from multiple cycles acquired over approximately 30 s. Beat-to-beat changes in HR and short-term regulation over the recording timeframe are not considered. The inherent error between the MRI flow measurements and assumptions are demonstrated in table 4. The estimation of flow does not consider the gender or ratio of body fat to lean muscle. Both of these factors will affect resting and exercise physiology. Modelling errors may result from assumptions made while describing the structured trees, creating the anatomic geometries, and specifying model parameters. First, when specifying the boundary conditions, subjects
11 48 B. N. Steele et al. Figure 7. Flow comparisons for healthy young adults during exercise for one cardiac cycle. Graphs show comparison between measured (thin line) and computed (bold line) IR aortic flow. Resting impedance boundary conditions were modified using a uniform exercise factor: f ¼ 0:7 for all viscera and f ¼ 5:0 for all lower extremity structured trees. were assigned a flow distribution (table 3). Distribution is difficult, if not impossible, to quantify as short-term regulatory effects may change flow through parallel networks on a beat-to-beat basis. Gross distributions between the viscera and legs were quantified by comparing SC and IR flow. During the exercise protocol, subjects were asked to exercise to a level of approximately 1.5 times their resting HR. However, change in HR does not correlate to change in CO. In the study, subjects with the same increase in HR (1.5 resting) experienced increases in CO ranging from times resting. Similarly, distribution between the viscera and legs during exercise was non-uniform with distributions ranging from 66% (subject 10) to 92% (subject 11). Clearly, cardiovascular regulation in response to exercise is not uniform, with potential differences based on a number of factors including athletic fitness, lean muscle mass, and level of effort. The variations in CO were minimized by using measured SC flow as the inlet boundary conditions for rest and exercise. The overall variations in distribution Figure 8. Comparison between predicted and measured flow (cm 3 s 21 ) waveform. The identity line is shown. A correlation coefficient of 0.90 was computed.
12 Fractal network model for rest and exercise 49 Figure 9. Predicted flow distribution for all outlets during exercise. Sum of flow indicates the mean measured SC flow. Uniform scaling of impedance boundary conditions used to simulate physiologic changes due to lower extremity exercise: f ¼ 0:7 for all viscera and f ¼ 5:0 for all lower extremity structured trees. between viscera and legs were minimal, with good agreement between measured and computed for 8 of 11 subjects. Second, because full geometric data sets were not available for the subjects, this study used idealized geometry (see figure 2 and table 2) that was scaled to the subject s body mass using an allometric scaling law. These scaled geometries may not have accurately reflected true subject geometry. A third source of modelling error is found in the geometry of the structured trees and scaling factors used to approximate physiological response to exercise. Variations Figure 10. Measured inlet flow q (cm 3 s 21 ) (thin line) and corresponding computed inlet pressures p (mmhg) (bold line) during exercise for one cardiac cycle. Uniform scaling of structured tree impedance boundary conditions used to simulate physiologic changes due to lower extremity exercise.
13 50 B. N. Steele et al. in visceral tone due to chemical, neural, or metabolic factors were not characterized individually, but if quantified, could be incorporated into the model through scaling factors. While the factors used were adequate in determining the redistribution of flow from rest to exercise, the simulation overestimates the pressure during exercise. Studies run with alternate exercise factors and alternate resistance vessel radii values did not significantly alter exercise pressures. This is mainly because the dilation factor 5.0 produced the maximal decrease in impedance in the lower limbs. Additional impedance was required to further direct the desired flow away from the viscera outlets. The structured trees follow a simple bifurcating scheme and are generically defined from literature values. It is clear that structured trees must be tailored to more accurately reflect the organs they are perfusing and allow for a greater range of impedance regulation. One option is to adopt a methodology that would use a diameter-defined Strahler (Jiang et al. 1994) system with several higher-order branches emanating from a single lower-order vessel. In order to determine if any scheme accurately models a vascular bed, additional data regarding blood flow in resting and active muscle is required. Such data has been described in recent work using positron emission tomography and could be used in further studies to tune the vascular beds (Kalliokoski et al. 2000; Mizuno et al. 2003). Fourth, the purely elastic constitutive model used to describe vessel response to pressure is not ideal. The computation tend to overestimate flow during systole and underestimate flow during diastole, indicating that the compliance values for the resting case may be too low. It is possible to reduce the amplitude of the computed resting IR flow by modifying the compliance, but this change results in a drastic underestimation of exercise amplitude. A combination of structured tree modification as described above as well as a more sophisticated constitutive model is needed to more accurately predict blood pressure and flow wave propagation at rest as well as during an altered physiologic state. Finally, the physiologic changes accounted for in this study were limited to a single, steady level of exercise and were generated using a simple vasodilation/vasoconstriction scheme. Regulation mechanisms are more sophisticated, with pre-capillary sphincters that lead to the recruitment of collapsed capillary beds. It may be assumed that alternate levels of exercise, fitness (Wilmore et al. 1980; Wright et al. 2002), age, gender (Fu and Levine 2005), and disease may elicit different physiologic responses. By determining these relationships, it may be possible to predict the steady-state regulatory responses of vascular regions from diagnostic patient data collected at rest. Acknowledgements This work was done as part of the thesis work of Dr Steele under the guidance of Dr Taylor at Stanford University, and was supported in part by the National Science Foundation under Grant No , the Whitaker Foundation and the Ayers Foundation. References H. Atabek and H. Lew, Wave propagation through a viscous incompressible fluid contained in an initially stressed elastic tube, Biophys. J., 8, pp , P. Boutouyrie, S. Boumaza, P. Challande, P. Lacolley and S. Laurent, Smooth muscle tone and arterial wall viscosity: an in vivo/in vitro study, Hypertension, 32, pp , D.J. Brown, Input impedance and reflection coefficient in fractal-like models of asymmetrically branching compliant tubes, IEEE Trans. Biomed. Eng., 43, pp , R. Burattini, R. Fogliardi and K. Campbell, Lumped model of terminal aortic impedance in the dog, Ann. Biomed. Eng., 22, pp , J.R. Cebral, M.A. Castro, O. Soto, R. Lohner and N. Alperin, Bloodflow models of the circle of Willis from magnetic resonance data, J. Eng. Math., 47, p. 369, Q. Fu and B.D. Levine, Cardiovascular response to exercise in women, Med. Sci. Sports Exerc., 37, pp , W.F.M. Ganong, Review of Medical Physiology, Englewood Cliffs: Appleton & Lange, T.J.R. Hughes and J. Lubliner, On the one-dimensional theory of blood flow in the larger vessels, Math. Biosci., 18, pp , T.J.R. Hughes, A study of one-dimensional theory of arterial pulse propagation, PhD thesis, U.C., Berkeley A.S. Iberall, Anatomy and steady flow characteristics of the arterial system with an introduction to its pulsatile characteristics, Math. Biosci., 1, pp , Z.L. Jiang, G.S. Kassab and Y.C. Fung, Diameter-defined Strahler system and connectivity matrix of the pulmonary arterial tree, J. Appl. Physiol.: Resp. Environ. Exercise Physiol., 76, pp , K.K. Kalliokoski, J. Kemppainen, K. Larmola, et al. Muscle blood flow and flow heterogeneity during exercise studied with positron emission tomography in humans, Eur. J. Appl. Physiol., 83, pp , R. Karch, F. Neumann, M. Neumann and W. Schreiner, Staged growth of optimized arterial model trees, Ann. Biomed. Eng., 28, pp , T.S. Manning, B.E. Shykoff and J.L. Izzo, Jr, Validity and reliability of diastolic pulse contour analysis (Windkessel model) in humans, Hypertension, 39, pp , M. Mizuno, Y. Kimura, T. Iwakawa, et al. Regional differences in blood flow and oxygen consumption in resting muscle and their relationship during recovery from exhaustive exercise, J. Appl. Physiol. Resp. Environ. Exercise Physiol., 95, pp , J.E. Moore, Jr and D.N. Ku, Pulsatile velocity measurements in a model of the human abdominal aorta under resting conditions, J. Biomech. Eng., 116, pp , C.D. Murray, The physiological principle of minimum work. I. The vascular system and the cost of blood volume, Proc. Natl. Acad. Sci. USA, 12, pp , W.W. Nichols and M.F. O Rourke, McDonald s blood flow in arteries, Theoretical, Experimental and Clinical Principles, New York: Oxford University Press, A. Noordergraaf, Circulatory System Dynamics, New York: Academic Press, M.S. Olufsen, A structured tree outflow condition for blood flow in the larger systemic arteries, Am. J. Physiol., 276, pp. H257 H268, M.S. Olufsen, C.S. Peskin, W.Y. Kim, E.M. Pedersen, A. Nadim and J. Larsen, Numerical simulation and experimental validation of blood flow in arteries with structured-tree outflow conditions, Ann. Biomed. Eng., 28, pp , M.S. Olufsen and A. Nadim, On deriving lumped models for blood flow and pressure in the systemic arteries, Math. Biosci. Eng., 1, pp , G.L. Papageorgiou, B.N. Jones, V.J. Redding and N. Hudson, The area ratio of normal arterial junctions and its implications in pulse-wave reflections, Cardiovasc. Res., 24, pp , L. Pater and J.W. van den Berg, An electrical analogue of the entire human circulatory system, Med. Electron. Biol. Eng., 2, pp , 1964.
14 Fractal network model for rest and exercise 51 E.M. Pedersen, S. Hsing-Wen, A.C. Burlson and A.P. Yoganathan, Twodimensional velocity measurements in a pulsatile flow model of the normal abdominal aorta simulating different hemodynamic conditions, J. Biomech., 26, pp , T. Pedley, The Fluid Mechanics of Large Blood Vessels, Cambridge: Cambridge University Press, M.J. Perko, H.B. Nielsen, C. Skak, J.O. Clemmesen, T.V. Schroeder and N.H. Secher, Mesenteric, coeliac and splanchnic blood flow in humans during exercise, J. Physiol., 513, pp , A.R. Pries, T.W. Secomb, P. Gaehtgens and J.F. Gross, Blood flow in microvascular networks. Experiments and simulation, Circ. Res., 67, pp , B.N. Steele, J. Wan, J.P. Ku, T.J.R. Hughes and C.A. Taylor, In vivo validation of a one-dimensional finite element method for predicting blood flow in cardiovascular bypass grafts, IEEE Trans. Biomed. Eng., 50, pp , N. Stergiopulos, D.F. Young and T.R. Rogge, Computer simulation of arterial flow with applications to arterial and aortic stenoses, J. Biomech., 25, pp , N. Suwa, T. Nniwa, H. Fukasawa and Y. Sasaki, Estimation of intravascular blood pressure gradient by mathematical analysis of arterial casts, Tohoku J. Exp. Med., 79, pp , M.G. Taylor, Wave transmission through an assembly of randomly branching elastic tubes, Biophys. J., 6, pp , C.A. Taylor, T.J.R. Hughes and C.K. Zarins, Computational investigations in vascular disease, Comput. Phys., 10, pp , C.A. Taylor, M.T. Draney and J.P. Ku, et al. Predictive medicine: computational techniques in therapeutic decision-making, Comput. Aided. Surg., 4, pp , 1999a. C.A. Taylor, T.J.R. Hughes and C.K. Zarins, Effect of exercise on hemodynamic conditions in the abdominal aorta, J. Vasc. Surg., 29, pp , 1999b. C.A. Taylor, C.P. Cheng, L.A. Espinosa, B.T. Tang, D. Parker and R.J. Herfkens, In vivo quantification of blood flow and wall shear stress in the human abdominal aorta during lower limb exercise, Ann. Biomed. Eng., 30, pp , J. Wan, B.N. Steele, S.A. Spicer, et al. A one-dimensional finite element method for simulation-based medical planning for cardiovascular disease, Comput. Methods Biomech. Biomed. Engin., 5, pp , G.B. West, J.H. Brown and B.J. Enquist, A general model for the origin of allometric scaling laws in biology, Science, 276, pp , N. Westerhof, F. Bosman, C.J. De Vries and A. Noordergraaf, Analog studies of the human systemic arterial tree, J. Biomech., 2, pp , J.H. Wilmore, J.A. Davis, R.S. O Brien, P.A. Vodak, G.R. Walder and E.A. Amsterdam, Physiological alterations consequent to 20-week conditioning programs of bicycling, tennis, and jogging, Med. Sci. Sports and Exerc., 12, pp. 1 8, J.R. Womersley, Oscillatory motion of a viscous liquid in a thin-walled elastic tube -I: the linear approximation for long waves, Philos. Mag., 47, pp , J. R. Womersley, An elastic tube theory of pulse transmission and oscillatory flow in mammalian arteries, Wright Air Development Center, Wright Patterson Air Force Base, OH, A. Wright, F.E. Marino, D. Kay, et al. Influence of lean body mass on performance differences of male and female distance runners in warm, humid environments, Am. J. Phys. Anthropol., 118, pp , M. Zamir, On fractal properties of arterial trees, J. Theor. Biol., 197, pp , 1999.
Geometric multiscaling in the circulatory system
 Geometric multiscaling in the circulatory system Local: 3D FSI flow model Global: 1D network of arteries and veins (Euler hyperbolic system) Global: 0D capillary network (DAE system) Geometric multiscaling
Geometric multiscaling in the circulatory system Local: 3D FSI flow model Global: 1D network of arteries and veins (Euler hyperbolic system) Global: 0D capillary network (DAE system) Geometric multiscaling
AgoraLink Agora for Life Science Technologies Linköpings Universitet Kurs i Fysiologisk mätteknik Biofluidflöden
 AgoraLink Agora for Life Science Technologies Linköpings Universitet Kurs i Fysiologisk mätteknik Biofluidflöden Fysiologisk mätteknik Anatomy of the heart The complex myocardium structure right ventricle
AgoraLink Agora for Life Science Technologies Linköpings Universitet Kurs i Fysiologisk mätteknik Biofluidflöden Fysiologisk mätteknik Anatomy of the heart The complex myocardium structure right ventricle
NUMERICAL ANALYSIS OF THE EFFECTS OF WIND ON BUILDING STRUCTURES
 Vol. XX 2012 No. 4 28 34 J. ŠIMIČEK O. HUBOVÁ NUMERICAL ANALYSIS OF THE EFFECTS OF WIND ON BUILDING STRUCTURES Jozef ŠIMIČEK email: jozef.simicek@stuba.sk Research field: Statics and Dynamics Fluids mechanics
Vol. XX 2012 No. 4 28 34 J. ŠIMIČEK O. HUBOVÁ NUMERICAL ANALYSIS OF THE EFFECTS OF WIND ON BUILDING STRUCTURES Jozef ŠIMIČEK email: jozef.simicek@stuba.sk Research field: Statics and Dynamics Fluids mechanics
Modeling and Numerical Blood Flow Analysis of Tibial Artery using CFD
 Modeling and Numerical Blood Flow Analysis of Tibial Artery using CFD S.Manimaran Department of Biomedical Engineering C.Muralidharan M.E Assistant Professor Department of Biomedical Engineering Surendra
Modeling and Numerical Blood Flow Analysis of Tibial Artery using CFD S.Manimaran Department of Biomedical Engineering C.Muralidharan M.E Assistant Professor Department of Biomedical Engineering Surendra
PLEASE SCROLL DOWN FOR ARTICLE. Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
 This article was downloaded by: On: 6 January 2010 Access details: Access Details: Free Access Publisher Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered
This article was downloaded by: On: 6 January 2010 Access details: Access Details: Free Access Publisher Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered
Overview of the Cardiovascular System
 Overview of the Cardiovascular System 2 vascular (blood vessel) loops: Pulmonary circulation: from heart to lungs and back) Systemic circulation: from heart to other organs and back Flow through systemic
Overview of the Cardiovascular System 2 vascular (blood vessel) loops: Pulmonary circulation: from heart to lungs and back) Systemic circulation: from heart to other organs and back Flow through systemic
Fluids and Solids: Fundamentals
 Fluids and Solids: Fundamentals We normally recognize three states of matter: solid; liquid and gas. However, liquid and gas are both fluids: in contrast to solids they lack the ability to resist deformation.
Fluids and Solids: Fundamentals We normally recognize three states of matter: solid; liquid and gas. However, liquid and gas are both fluids: in contrast to solids they lack the ability to resist deformation.
Vascular System The heart can be thought of 2 separate pumps from the right ventricle, blood is pumped at a low pressure to the lungs and then back
 Vascular System The heart can be thought of 2 separate pumps from the right ventricle, blood is pumped at a low pressure to the lungs and then back to the left atria from the left ventricle, blood is pumped
Vascular System The heart can be thought of 2 separate pumps from the right ventricle, blood is pumped at a low pressure to the lungs and then back to the left atria from the left ventricle, blood is pumped
1. Fluids Mechanics and Fluid Properties. 1.1 Objectives of this section. 1.2 Fluids
 1. Fluids Mechanics and Fluid Properties What is fluid mechanics? As its name suggests it is the branch of applied mechanics concerned with the statics and dynamics of fluids - both liquids and gases.
1. Fluids Mechanics and Fluid Properties What is fluid mechanics? As its name suggests it is the branch of applied mechanics concerned with the statics and dynamics of fluids - both liquids and gases.
A Comparison of Analytical and Finite Element Solutions for Laminar Flow Conditions Near Gaussian Constrictions
 A Comparison of Analytical and Finite Element Solutions for Laminar Flow Conditions Near Gaussian Constrictions by Laura Noelle Race An Engineering Project Submitted to the Graduate Faculty of Rensselaer
A Comparison of Analytical and Finite Element Solutions for Laminar Flow Conditions Near Gaussian Constrictions by Laura Noelle Race An Engineering Project Submitted to the Graduate Faculty of Rensselaer
Predicting Aerobic Power (VO 2max ) Using The 1-Mile Walk Test
 USING A WALKING TEST 12/25/05 PAGE 1 Predicting Aerobic Power (VO 2max ) Using The 1-Mile Walk Test KEYWORDS 1. Predict VO 2max 2. Rockport 1-mile walk test 3. Self-paced test 4. L min -1 5. ml kg -1 1min
USING A WALKING TEST 12/25/05 PAGE 1 Predicting Aerobic Power (VO 2max ) Using The 1-Mile Walk Test KEYWORDS 1. Predict VO 2max 2. Rockport 1-mile walk test 3. Self-paced test 4. L min -1 5. ml kg -1 1min
TWO-DIMENSIONAL FINITE ELEMENT ANALYSIS OF FORCED CONVECTION FLOW AND HEAT TRANSFER IN A LAMINAR CHANNEL FLOW
 TWO-DIMENSIONAL FINITE ELEMENT ANALYSIS OF FORCED CONVECTION FLOW AND HEAT TRANSFER IN A LAMINAR CHANNEL FLOW Rajesh Khatri 1, 1 M.Tech Scholar, Department of Mechanical Engineering, S.A.T.I., vidisha
TWO-DIMENSIONAL FINITE ELEMENT ANALYSIS OF FORCED CONVECTION FLOW AND HEAT TRANSFER IN A LAMINAR CHANNEL FLOW Rajesh Khatri 1, 1 M.Tech Scholar, Department of Mechanical Engineering, S.A.T.I., vidisha
Physics 9e/Cutnell. correlated to the. College Board AP Physics 1 Course Objectives
 Physics 9e/Cutnell correlated to the College Board AP Physics 1 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring
Physics 9e/Cutnell correlated to the College Board AP Physics 1 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring
A LAMINAR FLOW ELEMENT WITH A LINEAR PRESSURE DROP VERSUS VOLUMETRIC FLOW. 1998 ASME Fluids Engineering Division Summer Meeting
 TELEDYNE HASTINGS TECHNICAL PAPERS INSTRUMENTS A LAMINAR FLOW ELEMENT WITH A LINEAR PRESSURE DROP VERSUS VOLUMETRIC FLOW Proceedings of FEDSM 98: June -5, 998, Washington, DC FEDSM98 49 ABSTRACT The pressure
TELEDYNE HASTINGS TECHNICAL PAPERS INSTRUMENTS A LAMINAR FLOW ELEMENT WITH A LINEAR PRESSURE DROP VERSUS VOLUMETRIC FLOW Proceedings of FEDSM 98: June -5, 998, Washington, DC FEDSM98 49 ABSTRACT The pressure
Differential Relations for Fluid Flow. Acceleration field of a fluid. The differential equation of mass conservation
 Differential Relations for Fluid Flow In this approach, we apply our four basic conservation laws to an infinitesimally small control volume. The differential approach provides point by point details of
Differential Relations for Fluid Flow In this approach, we apply our four basic conservation laws to an infinitesimally small control volume. The differential approach provides point by point details of
Signal Extraction Technology
 Signal Extraction Technology Technical bulletin Introduction Masimo SET pulse oximetry is a new and fundamentally distinct method of acquiring, processing and reporting arterial oxygen saturation and pulse
Signal Extraction Technology Technical bulletin Introduction Masimo SET pulse oximetry is a new and fundamentally distinct method of acquiring, processing and reporting arterial oxygen saturation and pulse
Mathematical Model of Blood Flow in Carotid Bifurcation. Phd student: Eng. Emanuel Muraca. 16/10/09 Milan
 Presented at the COMSOL Conference 2009 Milan Mathematical Model of Blood Flow in Carotid Bifurcation Phd student: Eng. Emanuel Muraca 16/10/09 Milan 1 Research s s goal The goal of this research is to
Presented at the COMSOL Conference 2009 Milan Mathematical Model of Blood Flow in Carotid Bifurcation Phd student: Eng. Emanuel Muraca 16/10/09 Milan 1 Research s s goal The goal of this research is to
The mhr model is described by 30 ordinary differential equations (ODEs): one. ion concentrations and 23 equations describing channel gating.
 On-line Supplement: Computer Modeling Chris Clausen, PhD and Ira S. Cohen, MD, PhD Computer models of canine ventricular action potentials The mhr model is described by 30 ordinary differential equations
On-line Supplement: Computer Modeling Chris Clausen, PhD and Ira S. Cohen, MD, PhD Computer models of canine ventricular action potentials The mhr model is described by 30 ordinary differential equations
Imaging of Thoracic Endovascular Stent-Grafts
 Imaging of Thoracic Endovascular Stent-Grafts Tariq Hameed, M.D. Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, Indiana Disclosures: No relevant financial
Imaging of Thoracic Endovascular Stent-Grafts Tariq Hameed, M.D. Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, Indiana Disclosures: No relevant financial
The Viscosity of Fluids
 Experiment #11 The Viscosity of Fluids References: 1. Your first year physics textbook. 2. D. Tabor, Gases, Liquids and Solids: and Other States of Matter (Cambridge Press, 1991). 3. J.R. Van Wazer et
Experiment #11 The Viscosity of Fluids References: 1. Your first year physics textbook. 2. D. Tabor, Gases, Liquids and Solids: and Other States of Matter (Cambridge Press, 1991). 3. J.R. Van Wazer et
Numerical Model for the Study of the Velocity Dependence Of the Ionisation Growth in Gas Discharge Plasma
 Journal of Basrah Researches ((Sciences)) Volume 37.Number 5.A ((2011)) Available online at: www.basra-science -journal.org ISSN 1817 2695 Numerical Model for the Study of the Velocity Dependence Of the
Journal of Basrah Researches ((Sciences)) Volume 37.Number 5.A ((2011)) Available online at: www.basra-science -journal.org ISSN 1817 2695 Numerical Model for the Study of the Velocity Dependence Of the
Published online: 17 Jun 2010.
 This article was downloaded by: [Sam Houston State University] On: 07 August 2014, At: 15:09 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered
This article was downloaded by: [Sam Houston State University] On: 07 August 2014, At: 15:09 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered
Viscoelastic changes in the blood and vascular wall in a pulsating circular flow
 Pol J Med Phys Eng 2010;16(1):43-53. PL ISSN 1425-4689 doi: 10.2478/v10013-010-0005-9 website: http://www.pjmpe.waw.pl Merab Beraia 1, Fridon Todua 2, Irina Khomeriki 3 Viscoelastic changes in the blood
Pol J Med Phys Eng 2010;16(1):43-53. PL ISSN 1425-4689 doi: 10.2478/v10013-010-0005-9 website: http://www.pjmpe.waw.pl Merab Beraia 1, Fridon Todua 2, Irina Khomeriki 3 Viscoelastic changes in the blood
Lecture 5 Hemodynamics. Description of fluid flow. The equation of continuity
 1 Lecture 5 Hemodynamics Description of fluid flow Hydrodynamics is the part of physics, which studies the motion of fluids. It is based on the laws of mechanics. Hemodynamics studies the motion of blood
1 Lecture 5 Hemodynamics Description of fluid flow Hydrodynamics is the part of physics, which studies the motion of fluids. It is based on the laws of mechanics. Hemodynamics studies the motion of blood
Abaqus/CFD Sample Problems. Abaqus 6.10
 Abaqus/CFD Sample Problems Abaqus 6.10 Contents 1. Oscillatory Laminar Plane Poiseuille Flow 2. Flow in Shear Driven Cavities 3. Buoyancy Driven Flow in Cavities 4. Turbulent Flow in a Rectangular Channel
Abaqus/CFD Sample Problems Abaqus 6.10 Contents 1. Oscillatory Laminar Plane Poiseuille Flow 2. Flow in Shear Driven Cavities 3. Buoyancy Driven Flow in Cavities 4. Turbulent Flow in a Rectangular Channel
Milwaukee School of Engineering Gerrits@msoe.edu. Case Study: Factors that Affect Blood Pressure Instructor Version
 Case Study: Factors that Affect Blood Pressure Instructor Version Goal This activity (case study and its associated questions) is designed to be a student-centered learning activity relating to the factors
Case Study: Factors that Affect Blood Pressure Instructor Version Goal This activity (case study and its associated questions) is designed to be a student-centered learning activity relating to the factors
CFD SIMULATION OF SDHW STORAGE TANK WITH AND WITHOUT HEATER
 International Journal of Advancements in Research & Technology, Volume 1, Issue2, July-2012 1 CFD SIMULATION OF SDHW STORAGE TANK WITH AND WITHOUT HEATER ABSTRACT (1) Mr. Mainak Bhaumik M.E. (Thermal Engg.)
International Journal of Advancements in Research & Technology, Volume 1, Issue2, July-2012 1 CFD SIMULATION OF SDHW STORAGE TANK WITH AND WITHOUT HEATER ABSTRACT (1) Mr. Mainak Bhaumik M.E. (Thermal Engg.)
Human Anatomy and Physiology II Laboratory
 Human Anatomy and Physiology II Laboratory The Circulation (Two Weeks) 1 This lab involves two weeks work studying the vasculature of the human body. Both weeks involve the exercise in the lab manual entitled
Human Anatomy and Physiology II Laboratory The Circulation (Two Weeks) 1 This lab involves two weeks work studying the vasculature of the human body. Both weeks involve the exercise in the lab manual entitled
2.2.1 Pressure and flow rate along a pipe: a few fundamental concepts
 1.1 INTRODUCTION Single-cell organisms live in direct contact with the environment from where they derive nutrients and into where they dispose of their waste. For living systems containing multiple cells,
1.1 INTRODUCTION Single-cell organisms live in direct contact with the environment from where they derive nutrients and into where they dispose of their waste. For living systems containing multiple cells,
BIOMEDICAL ULTRASOUND
 BIOMEDICAL ULTRASOUND Goals: To become familiar with: Ultrasound wave Wave propagation and Scattering Mechanisms of Tissue Damage Biomedical Ultrasound Transducers Biomedical Ultrasound Imaging Ultrasonic
BIOMEDICAL ULTRASOUND Goals: To become familiar with: Ultrasound wave Wave propagation and Scattering Mechanisms of Tissue Damage Biomedical Ultrasound Transducers Biomedical Ultrasound Imaging Ultrasonic
FLUID FLOW STREAMLINE LAMINAR FLOW TURBULENT FLOW REYNOLDS NUMBER
 VISUAL PHYSICS School of Physics University of Sydney Australia FLUID FLOW STREAMLINE LAMINAR FLOW TURBULENT FLOW REYNOLDS NUMBER? What type of fluid flow is observed? The above pictures show how the effect
VISUAL PHYSICS School of Physics University of Sydney Australia FLUID FLOW STREAMLINE LAMINAR FLOW TURBULENT FLOW REYNOLDS NUMBER? What type of fluid flow is observed? The above pictures show how the effect
INVESTIGATION OF FALLING BALL VISCOMETRY AND ITS ACCURACY GROUP R1 Evelyn Chou, Julia Glaser, Bella Goyal, Sherri Wykosky
 INVESTIGATION OF FALLING BALL VISCOMETRY AND ITS ACCURACY GROUP R1 Evelyn Chou, Julia Glaser, Bella Goyal, Sherri Wykosky ABSTRACT: A falling ball viscometer and its associated equations were studied in
INVESTIGATION OF FALLING BALL VISCOMETRY AND ITS ACCURACY GROUP R1 Evelyn Chou, Julia Glaser, Bella Goyal, Sherri Wykosky ABSTRACT: A falling ball viscometer and its associated equations were studied in
The Periodic Moving Average Filter for Removing Motion Artifacts from PPG Signals
 International Journal The of Periodic Control, Moving Automation, Average and Filter Systems, for Removing vol. 5, no. Motion 6, pp. Artifacts 71-76, from December PPG s 27 71 The Periodic Moving Average
International Journal The of Periodic Control, Moving Automation, Average and Filter Systems, for Removing vol. 5, no. Motion 6, pp. Artifacts 71-76, from December PPG s 27 71 The Periodic Moving Average
Page 1. Introduction The blood vessels of the body form a closed delivery system that begins and ends at the heart.
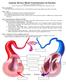 Anatomy Review: Blood Vessel Structure & Function Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction The blood vessels
Anatomy Review: Blood Vessel Structure & Function Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction The blood vessels
Incorporating Internal Gradient and Restricted Diffusion Effects in Nuclear Magnetic Resonance Log Interpretation
 The Open-Access Journal for the Basic Principles of Diffusion Theory, Experiment and Application Incorporating Internal Gradient and Restricted Diffusion Effects in Nuclear Magnetic Resonance Log Interpretation
The Open-Access Journal for the Basic Principles of Diffusion Theory, Experiment and Application Incorporating Internal Gradient and Restricted Diffusion Effects in Nuclear Magnetic Resonance Log Interpretation
Dynamic Process Modeling. Process Dynamics and Control
 Dynamic Process Modeling Process Dynamics and Control 1 Description of process dynamics Classes of models What do we need for control? Modeling for control Mechanical Systems Modeling Electrical circuits
Dynamic Process Modeling Process Dynamics and Control 1 Description of process dynamics Classes of models What do we need for control? Modeling for control Mechanical Systems Modeling Electrical circuits
Dimensional Analysis
 Dimensional Analysis An Important Example from Fluid Mechanics: Viscous Shear Forces V d t / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Ƭ = F/A = μ V/d More generally, the viscous
Dimensional Analysis An Important Example from Fluid Mechanics: Viscous Shear Forces V d t / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Ƭ = F/A = μ V/d More generally, the viscous
Modelling physiological features of Human body behavior in car crash simulations
 Modelling physiological features of Human body behavior in car crash simulations Michel BEHR, Yves GODIO, Maxime LLARI, Christian BRUNET Laboratoire de Biomécanique Appliquée (Marseille, France) Presented
Modelling physiological features of Human body behavior in car crash simulations Michel BEHR, Yves GODIO, Maxime LLARI, Christian BRUNET Laboratoire de Biomécanique Appliquée (Marseille, France) Presented
A Patient s Guide to Minimally Invasive Abdominal Aortic Aneurysm Repair
 A Patient s Guide to Minimally Invasive Abdominal Aortic Aneurysm Repair Table of Contents The AFX Endovascular AAA System............................................ 1 What is an Abdominal Aortic Aneurysm
A Patient s Guide to Minimally Invasive Abdominal Aortic Aneurysm Repair Table of Contents The AFX Endovascular AAA System............................................ 1 What is an Abdominal Aortic Aneurysm
Edwards FloTrac Sensor & Edwards Vigileo Monitor. Measuring Continuous Cardiac Output with the FloTrac Sensor and Vigileo Monitor
 Edwards FloTrac Sensor & Edwards Vigileo Monitor Measuring Continuous Cardiac Output with the FloTrac Sensor and Vigileo Monitor 1 Topics System Configuration Physiological Principles Pulse pressure relationship
Edwards FloTrac Sensor & Edwards Vigileo Monitor Measuring Continuous Cardiac Output with the FloTrac Sensor and Vigileo Monitor 1 Topics System Configuration Physiological Principles Pulse pressure relationship
Rock Bolt Condition Monitoring Using Ultrasonic Guided Waves
 Rock Bolt Condition Monitoring Using Ultrasonic Guided Waves Bennie Buys Department of Mechanical and Aeronautical Engineering University of Pretoria Introduction Rock Bolts and their associated problems
Rock Bolt Condition Monitoring Using Ultrasonic Guided Waves Bennie Buys Department of Mechanical and Aeronautical Engineering University of Pretoria Introduction Rock Bolts and their associated problems
Unsteady Pressure Measurements
 Quite often the measurements of pressures has to be conducted in unsteady conditions. Typical cases are those of -the measurement of time-varying pressure (with periodic oscillations or step changes) -the
Quite often the measurements of pressures has to be conducted in unsteady conditions. Typical cases are those of -the measurement of time-varying pressure (with periodic oscillations or step changes) -the
Turbulence Modeling in CFD Simulation of Intake Manifold for a 4 Cylinder Engine
 HEFAT2012 9 th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics 16 18 July 2012 Malta Turbulence Modeling in CFD Simulation of Intake Manifold for a 4 Cylinder Engine Dr MK
HEFAT2012 9 th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics 16 18 July 2012 Malta Turbulence Modeling in CFD Simulation of Intake Manifold for a 4 Cylinder Engine Dr MK
4.3 Results... 27 4.3.1 Drained Conditions... 27 4.3.2 Undrained Conditions... 28 4.4 References... 30 4.5 Data Files... 30 5 Undrained Analysis of
 Table of Contents 1 One Dimensional Compression of a Finite Layer... 3 1.1 Problem Description... 3 1.1.1 Uniform Mesh... 3 1.1.2 Graded Mesh... 5 1.2 Analytical Solution... 6 1.3 Results... 6 1.3.1 Uniform
Table of Contents 1 One Dimensional Compression of a Finite Layer... 3 1.1 Problem Description... 3 1.1.1 Uniform Mesh... 3 1.1.2 Graded Mesh... 5 1.2 Analytical Solution... 6 1.3 Results... 6 1.3.1 Uniform
Lecture 6 - Boundary Conditions. Applied Computational Fluid Dynamics
 Lecture 6 - Boundary Conditions Applied Computational Fluid Dynamics Instructor: André Bakker http://www.bakker.org André Bakker (2002-2006) Fluent Inc. (2002) 1 Outline Overview. Inlet and outlet boundaries.
Lecture 6 - Boundary Conditions Applied Computational Fluid Dynamics Instructor: André Bakker http://www.bakker.org André Bakker (2002-2006) Fluent Inc. (2002) 1 Outline Overview. Inlet and outlet boundaries.
EFSUMB EUROPEAN FEDERATION OF SOCIETIES FOR ULTRASOUND IN MEDICINE AND BIOLOGY Building a European Ultrasound Community
 MINIMUM TRAINING REQUIREMENTS FOR THE PRACTICE OF MEDICAL ULTRASOUND IN EUROPE Appendix 8: Vascular Ultrasound Level 1 Training and Practice Practical training should involve at least two half day ultrasound
MINIMUM TRAINING REQUIREMENTS FOR THE PRACTICE OF MEDICAL ULTRASOUND IN EUROPE Appendix 8: Vascular Ultrasound Level 1 Training and Practice Practical training should involve at least two half day ultrasound
Blood Vessels and Circulation
 13 Blood Vessels and Circulation FOCUS: Blood flows from the heart through the arterial blood vessels to capillaries, and from capillaries back to the heart through veins. The pulmonary circulation transports
13 Blood Vessels and Circulation FOCUS: Blood flows from the heart through the arterial blood vessels to capillaries, and from capillaries back to the heart through veins. The pulmonary circulation transports
Introduction to COMSOL. The Navier-Stokes Equations
 Flow Between Parallel Plates Modified from the COMSOL ChE Library module rev 10/13/08 Modified by Robert P. Hesketh, Chemical Engineering, Rowan University Fall 2008 Introduction to COMSOL The following
Flow Between Parallel Plates Modified from the COMSOL ChE Library module rev 10/13/08 Modified by Robert P. Hesketh, Chemical Engineering, Rowan University Fall 2008 Introduction to COMSOL The following
3 rd Russian-Bavarian Conference on Bio-Medical Engineering
 3 rd Russian-Bavarian Conference on Bio-Medical Engineering Blood Pressure Estimation based on Pulse Transit Time and Compensation of Vertical Position Dipl.-Inform. Med. Christian Douniama Dipl.-Ing.
3 rd Russian-Bavarian Conference on Bio-Medical Engineering Blood Pressure Estimation based on Pulse Transit Time and Compensation of Vertical Position Dipl.-Inform. Med. Christian Douniama Dipl.-Ing.
Exchange solutes and water with cells of the body
 Chapter 8 Heart and Blood Vessels Three Types of Blood Vessels Transport Blood Arteries Carry blood away from the heart Transport blood under high pressure Capillaries Exchange solutes and water with cells
Chapter 8 Heart and Blood Vessels Three Types of Blood Vessels Transport Blood Arteries Carry blood away from the heart Transport blood under high pressure Capillaries Exchange solutes and water with cells
Fellow TEE Review Workshop Hemodynamic Calculations 2013. Director, Intraoperative TEE Program. Johns Hopkins School of Medicine
 Fellow TEE Review Workshop Hemodynamic Calculations 2013 Mary Beth Brady, MD, FASE Director, Intraoperative TEE Program Johns Hopkins School of Medicine At the conclusion of the workshop, the participants
Fellow TEE Review Workshop Hemodynamic Calculations 2013 Mary Beth Brady, MD, FASE Director, Intraoperative TEE Program Johns Hopkins School of Medicine At the conclusion of the workshop, the participants
Multiphysics Software Applications in Reverse Engineering
 Multiphysics Software Applications in Reverse Engineering *W. Wang 1, K. Genc 2 1 University of Massachusetts Lowell, Lowell, MA, USA 2 Simpleware, Exeter, United Kingdom *Corresponding author: University
Multiphysics Software Applications in Reverse Engineering *W. Wang 1, K. Genc 2 1 University of Massachusetts Lowell, Lowell, MA, USA 2 Simpleware, Exeter, United Kingdom *Corresponding author: University
DEVELOPMENT OF HIGH SPEED RESPONSE LAMINAR FLOW METER FOR AIR CONDITIONING
 DEVELOPMENT OF HIGH SPEED RESPONSE LAMINAR FLOW METER FOR AIR CONDITIONING Toshiharu Kagawa 1, Yukako Saisu 2, Riki Nishimura 3 and Chongho Youn 4 ABSTRACT In this paper, we developed a new laminar flow
DEVELOPMENT OF HIGH SPEED RESPONSE LAMINAR FLOW METER FOR AIR CONDITIONING Toshiharu Kagawa 1, Yukako Saisu 2, Riki Nishimura 3 and Chongho Youn 4 ABSTRACT In this paper, we developed a new laminar flow
L r = L m /L p. L r = L p /L m
 NOTE: In the set of lectures 19/20 I defined the length ratio as L r = L m /L p The textbook by Finnermore & Franzini defines it as L r = L p /L m To avoid confusion let's keep the textbook definition,
NOTE: In the set of lectures 19/20 I defined the length ratio as L r = L m /L p The textbook by Finnermore & Franzini defines it as L r = L p /L m To avoid confusion let's keep the textbook definition,
Hemodynamic Monitoring: Principles to Practice M. L. Cheatham, MD, FACS, FCCM
 SUMMARY HEMODYNAMIC MONITORING: FROM PRINCIPLES TO PRACTICE Michael L. Cheatham, MD, FACS, FCCM Director, Surgical Intensive Care Units Orlando Regional Medical Center Orlando, Florida Fluid-filled catheters
SUMMARY HEMODYNAMIC MONITORING: FROM PRINCIPLES TO PRACTICE Michael L. Cheatham, MD, FACS, FCCM Director, Surgical Intensive Care Units Orlando Regional Medical Center Orlando, Florida Fluid-filled catheters
HEAT TRANSFER ANALYSIS IN A 3D SQUARE CHANNEL LAMINAR FLOW WITH USING BAFFLES 1 Vikram Bishnoi
 HEAT TRANSFER ANALYSIS IN A 3D SQUARE CHANNEL LAMINAR FLOW WITH USING BAFFLES 1 Vikram Bishnoi 2 Rajesh Dudi 1 Scholar and 2 Assistant Professor,Department of Mechanical Engineering, OITM, Hisar (Haryana)
HEAT TRANSFER ANALYSIS IN A 3D SQUARE CHANNEL LAMINAR FLOW WITH USING BAFFLES 1 Vikram Bishnoi 2 Rajesh Dudi 1 Scholar and 2 Assistant Professor,Department of Mechanical Engineering, OITM, Hisar (Haryana)
PLEASE SCROLL DOWN FOR ARTICLE
 This article was downloaded by: [University of Minnesota] On: 8 April 2009 Access details: Access Details: [subscription number 788736612] Publisher Taylor & Francis Informa Ltd Registered in England and
This article was downloaded by: [University of Minnesota] On: 8 April 2009 Access details: Access Details: [subscription number 788736612] Publisher Taylor & Francis Informa Ltd Registered in England and
Normal & Abnormal Intracardiac. Lancashire & South Cumbria Cardiac Network
 Normal & Abnormal Intracardiac Pressures Lancashire & South Cumbria Cardiac Network Principle Pressures recorded from catheter tip Electrical transducer - wheatstone bridge mechanical to electrical waveform
Normal & Abnormal Intracardiac Pressures Lancashire & South Cumbria Cardiac Network Principle Pressures recorded from catheter tip Electrical transducer - wheatstone bridge mechanical to electrical waveform
CE 6303 MECHANICS OF FLUIDS L T P C QUESTION BANK PART - A
 CE 6303 MECHANICS OF FLUIDS L T P C QUESTION BANK 3 0 0 3 UNIT I FLUID PROPERTIES AND FLUID STATICS PART - A 1. Define fluid and fluid mechanics. 2. Define real and ideal fluids. 3. Define mass density
CE 6303 MECHANICS OF FLUIDS L T P C QUESTION BANK 3 0 0 3 UNIT I FLUID PROPERTIES AND FLUID STATICS PART - A 1. Define fluid and fluid mechanics. 2. Define real and ideal fluids. 3. Define mass density
Patient Information Booklet. Endovascular Stent Grafts: A Treatment for Abdominal Aortic Aneurysms
 Patient Information Booklet Endovascular Stent Grafts: A Treatment for Abdominal Aortic Aneurysms TABLE OF CONTENTS Introduction 1 Glossary 2 Abdominal Aorta 4 Abdominal Aortic Aneurysm 5 Causes 6 Symptoms
Patient Information Booklet Endovascular Stent Grafts: A Treatment for Abdominal Aortic Aneurysms TABLE OF CONTENTS Introduction 1 Glossary 2 Abdominal Aorta 4 Abdominal Aortic Aneurysm 5 Causes 6 Symptoms
NUMERICAL INVESTIGATIONS ON HEAT TRANSFER IN FALLING FILMS AROUND TURBULENCE WIRES
 NUMERICAL INVESTIGATIONS ON HEAT TRANSFER IN FALLING FILMS AROUND TURBULENCE WIRES Abstract H. Raach and S. Somasundaram Thermal Process Engineering, University of Paderborn, Paderborn, Germany Turbulence
NUMERICAL INVESTIGATIONS ON HEAT TRANSFER IN FALLING FILMS AROUND TURBULENCE WIRES Abstract H. Raach and S. Somasundaram Thermal Process Engineering, University of Paderborn, Paderborn, Germany Turbulence
Ny teknologi: Fagdagene ved St. Olavs Hospital Lasse Løvstakken Dept. Circulation and Medical Imaging 11.06.2010
 1 Ny teknologi: Ultralyd måler m blodstrøm Fagdagene ved St. Olavs Hospital Lasse Løvstakken Dept. Circulation and Medical Imaging 11.06.2010 2 Conventional imaging methods of blood flow using ultrasound
1 Ny teknologi: Ultralyd måler m blodstrøm Fagdagene ved St. Olavs Hospital Lasse Løvstakken Dept. Circulation and Medical Imaging 11.06.2010 2 Conventional imaging methods of blood flow using ultrasound
Computer Aided Engineering (CAE) Techniques Applied To Hip Implant
 International Journal Of Computational Engineering Research (ijceronline.com) Vol. 3 Issue. 3 Computer Aided Engineering (CAE) Techniques Applied To Hip Implant 1, M. S. Abo_Elkhair, 2, M. E. Abo-Elnor,
International Journal Of Computational Engineering Research (ijceronline.com) Vol. 3 Issue. 3 Computer Aided Engineering (CAE) Techniques Applied To Hip Implant 1, M. S. Abo_Elkhair, 2, M. E. Abo-Elnor,
Published December 11, 2014 as 10.3174/ajnr.A4179
 Published December 11, 2014 as 10.3174/ajnr.A4179 ORIGINAL RESEARCH INTERVENTIONAL Artery Length Sensitivity in Patient-Specific Cerebral Aneurysm Simulations S. Hodis, S. Kargar, D.F. Kallmes, and D.
Published December 11, 2014 as 10.3174/ajnr.A4179 ORIGINAL RESEARCH INTERVENTIONAL Artery Length Sensitivity in Patient-Specific Cerebral Aneurysm Simulations S. Hodis, S. Kargar, D.F. Kallmes, and D.
Navier-Stokes Equation Solved in Comsol 4.1. Copyright Bruce A. Finlayson, 2010 See also Introduction to Chemical Engineering Computing, Wiley (2006).
 Introduction to Chemical Engineering Computing Copyright, Bruce A. Finlayson, 2004 1 Navier-Stokes Equation Solved in Comsol 4.1. Copyright Bruce A. Finlayson, 2010 See also Introduction to Chemical Engineering
Introduction to Chemical Engineering Computing Copyright, Bruce A. Finlayson, 2004 1 Navier-Stokes Equation Solved in Comsol 4.1. Copyright Bruce A. Finlayson, 2010 See also Introduction to Chemical Engineering
. Address the following issues in your solution:
 CM 3110 COMSOL INSTRUCTIONS Faith Morrison and Maria Tafur Department of Chemical Engineering Michigan Technological University, Houghton, MI USA 22 November 2012 Zhichao Wang edits 21 November 2013 revised
CM 3110 COMSOL INSTRUCTIONS Faith Morrison and Maria Tafur Department of Chemical Engineering Michigan Technological University, Houghton, MI USA 22 November 2012 Zhichao Wang edits 21 November 2013 revised
Effects of Cell Phone Radiation on the Head. BEE 4530 Computer-Aided Engineering: Applications to Biomedical Processes
 Effects of Cell Phone Radiation on the Head BEE 4530 Computer-Aided Engineering: Applications to Biomedical Processes Group 3 Angela Cai Youjin Cho Mytien Nguyen Praveen Polamraju Table of Contents I.
Effects of Cell Phone Radiation on the Head BEE 4530 Computer-Aided Engineering: Applications to Biomedical Processes Group 3 Angela Cai Youjin Cho Mytien Nguyen Praveen Polamraju Table of Contents I.
THEORY, SIMULATION, AND COMPENSATION OF PHYSIOLOGICAL MOTION ARTIFACTS IN FUNCTIONAL MRI. Douglas C. Noll* and Walter Schneider
 THEORY, SIMULATION, AND COMPENSATION OF PHYSIOLOGICAL MOTION ARTIFACTS IN FUNCTIONAL MRI Douglas C. Noll* and Walter Schneider Departments of *Radiology, *Electrical Engineering, and Psychology University
THEORY, SIMULATION, AND COMPENSATION OF PHYSIOLOGICAL MOTION ARTIFACTS IN FUNCTIONAL MRI Douglas C. Noll* and Walter Schneider Departments of *Radiology, *Electrical Engineering, and Psychology University
Normal Intracardiac Pressures. Lancashire & South Cumbria Cardiac Network
 Normal Intracardiac Pressures Lancashire & South Cumbria Cardiac Network Principle Pressures recorded from catheter tip Electrical transducer - wheatstone bridge mechanical to electrical waveform display
Normal Intracardiac Pressures Lancashire & South Cumbria Cardiac Network Principle Pressures recorded from catheter tip Electrical transducer - wheatstone bridge mechanical to electrical waveform display
AN EFFECT OF GRID QUALITY ON THE RESULTS OF NUMERICAL SIMULATIONS OF THE FLUID FLOW FIELD IN AN AGITATED VESSEL
 14 th European Conference on Mixing Warszawa, 10-13 September 2012 AN EFFECT OF GRID QUALITY ON THE RESULTS OF NUMERICAL SIMULATIONS OF THE FLUID FLOW FIELD IN AN AGITATED VESSEL Joanna Karcz, Lukasz Kacperski
14 th European Conference on Mixing Warszawa, 10-13 September 2012 AN EFFECT OF GRID QUALITY ON THE RESULTS OF NUMERICAL SIMULATIONS OF THE FLUID FLOW FIELD IN AN AGITATED VESSEL Joanna Karcz, Lukasz Kacperski
Viscous flow through pipes of various cross-sections
 IOP PUBLISHING Eur. J. Phys. 28 (2007 521 527 EUROPEAN JOURNAL OF PHYSICS doi:10.1088/0143-0807/28/3/014 Viscous flow through pipes of various cross-sections John Lekner School of Chemical and Physical
IOP PUBLISHING Eur. J. Phys. 28 (2007 521 527 EUROPEAN JOURNAL OF PHYSICS doi:10.1088/0143-0807/28/3/014 Viscous flow through pipes of various cross-sections John Lekner School of Chemical and Physical
Online publication date: 19 May 2010 PLEASE SCROLL DOWN FOR ARTICLE
 This article was downloaded by: [Patterson, David A.] On: 19 May 2010 Access details: Access Details: [subscription number 922426156] Publisher Routledge Informa Ltd Registered in England and Wales Registered
This article was downloaded by: [Patterson, David A.] On: 19 May 2010 Access details: Access Details: [subscription number 922426156] Publisher Routledge Informa Ltd Registered in England and Wales Registered
Direct Arterial Blood Pressure Monitoring Angel M. Rivera CVT, VTS (ECC) Animal Emergency Center Glendale, WI March 2003
 Direct Arterial Blood Pressure Monitoring Angel M. Rivera CVT, VTS (ECC) Animal Emergency Center Glendale, WI March 2003 Introduction Direct measurement of arterial blood pressure is obtained via a peripheral
Direct Arterial Blood Pressure Monitoring Angel M. Rivera CVT, VTS (ECC) Animal Emergency Center Glendale, WI March 2003 Introduction Direct measurement of arterial blood pressure is obtained via a peripheral
CHAPTER 9 CHANNELS APPENDIX A. Hydraulic Design Equations for Open Channel Flow
 CHAPTER 9 CHANNELS APPENDIX A Hydraulic Design Equations for Open Channel Flow SEPTEMBER 2009 CHAPTER 9 APPENDIX A Hydraulic Design Equations for Open Channel Flow Introduction The Equations presented
CHAPTER 9 CHANNELS APPENDIX A Hydraulic Design Equations for Open Channel Flow SEPTEMBER 2009 CHAPTER 9 APPENDIX A Hydraulic Design Equations for Open Channel Flow Introduction The Equations presented
Dimensional analysis is a method for reducing the number and complexity of experimental variables that affect a given physical phenomena.
 Dimensional Analysis and Similarity Dimensional analysis is very useful for planning, presentation, and interpretation of experimental data. As discussed previously, most practical fluid mechanics problems
Dimensional Analysis and Similarity Dimensional analysis is very useful for planning, presentation, and interpretation of experimental data. As discussed previously, most practical fluid mechanics problems
AI CPT Codes. x x. 70336 MRI Magnetic resonance (eg, proton) imaging, temporomandibular joint(s)
 Code Category Description Auth Required Medicaid Medicare 0126T IMT Testing Common carotid intima-media thickness (IMT) study for evaluation of atherosclerotic burden or coronary heart disease risk factor
Code Category Description Auth Required Medicaid Medicare 0126T IMT Testing Common carotid intima-media thickness (IMT) study for evaluation of atherosclerotic burden or coronary heart disease risk factor
Development of Simulation Tools Software
 Development of Simulation Tools Software Vincent Luboz Department of Biosurgery and Surgical Technology Imperial College London BSc VR Surgical Simulation Software Slide 1 Contents Virtual Reality VR Surgical
Development of Simulation Tools Software Vincent Luboz Department of Biosurgery and Surgical Technology Imperial College London BSc VR Surgical Simulation Software Slide 1 Contents Virtual Reality VR Surgical
Adaptation of General Purpose CFD Code for Fusion MHD Applications*
 Adaptation of General Purpose CFD Code for Fusion MHD Applications* Andrei Khodak Princeton Plasma Physics Laboratory P.O. Box 451 Princeton, NJ, 08540 USA akhodak@pppl.gov Abstract Analysis of many fusion
Adaptation of General Purpose CFD Code for Fusion MHD Applications* Andrei Khodak Princeton Plasma Physics Laboratory P.O. Box 451 Princeton, NJ, 08540 USA akhodak@pppl.gov Abstract Analysis of many fusion
Computed Tomography, Head Or Brain; Without Contrast Material, Followed By Contrast Material(S) And Further Sections
 1199SEIU BENEFIT AND PENSION FUNDS High Tech Diagnostic Radiology and s # 1 70336 Magnetic Resonance (Eg, Proton) Imaging, Temporomandibular Joint(S) 2 70450 Computed Tomography, Head Or Brain; Without
1199SEIU BENEFIT AND PENSION FUNDS High Tech Diagnostic Radiology and s # 1 70336 Magnetic Resonance (Eg, Proton) Imaging, Temporomandibular Joint(S) 2 70450 Computed Tomography, Head Or Brain; Without
Appendix 4-C. Open Channel Theory
 4-C-1 Appendix 4-C Open Channel Theory 4-C-2 Appendix 4.C - Table of Contents 4.C.1 Open Channel Flow Theory 4-C-3 4.C.2 Concepts 4-C-3 4.C.2.1 Specific Energy 4-C-3 4.C.2.2 Velocity Distribution Coefficient
4-C-1 Appendix 4-C Open Channel Theory 4-C-2 Appendix 4.C - Table of Contents 4.C.1 Open Channel Flow Theory 4-C-3 4.C.2 Concepts 4-C-3 4.C.2.1 Specific Energy 4-C-3 4.C.2.2 Velocity Distribution Coefficient
Low-gradient severe aortic stenosis with normal LVEF: A disturbing clinical entity
 Low-gradient severe aortic stenosis with normal LVEF: A disturbing clinical entity Jean-Luc MONIN, MD, PhD Henri Mondor University Hospital Créteil, FRANCE Disclosures : None 77-year-old woman, mild dyspnea
Low-gradient severe aortic stenosis with normal LVEF: A disturbing clinical entity Jean-Luc MONIN, MD, PhD Henri Mondor University Hospital Créteil, FRANCE Disclosures : None 77-year-old woman, mild dyspnea
OEM MAXNIBP Frequently Asked Questions
 Frequently Asked Questions Why does the monitor sometimes inflate the BP cuff, then shortly thereafter reinflate the cuff? How will I know if the monitor is experiencing motion artifact during a measurement?
Frequently Asked Questions Why does the monitor sometimes inflate the BP cuff, then shortly thereafter reinflate the cuff? How will I know if the monitor is experiencing motion artifact during a measurement?
Basic Principles in Microfluidics
 Basic Principles in Microfluidics 1 Newton s Second Law for Fluidics Newton s 2 nd Law (F= ma) : Time rate of change of momentum of a system equal to net force acting on system!f = dp dt Sum of forces
Basic Principles in Microfluidics 1 Newton s Second Law for Fluidics Newton s 2 nd Law (F= ma) : Time rate of change of momentum of a system equal to net force acting on system!f = dp dt Sum of forces
Laminar Flow and Heat Transfer of Herschel-Bulkley Fluids in a Rectangular Duct; Finite-Element Analysis
 Tamkang Journal of Science and Engineering, Vol. 12, No. 1, pp. 99 107 (2009) 99 Laminar Flow and Heat Transfer of Herschel-Bulkley Fluids in a Rectangular Duct; Finite-Element Analysis M. E. Sayed-Ahmed
Tamkang Journal of Science and Engineering, Vol. 12, No. 1, pp. 99 107 (2009) 99 Laminar Flow and Heat Transfer of Herschel-Bulkley Fluids in a Rectangular Duct; Finite-Element Analysis M. E. Sayed-Ahmed
Principles of Medical Ultrasound. Pai-Chi Li Department of Electrical Engineering National Taiwan University
 Principles of Medical Ultrasound Pai-Chi Li Department of Electrical Engineering National Taiwan University What is Medical Ultrasound? Prevention: actions taken to avoid diseases. Diagnosis: the process
Principles of Medical Ultrasound Pai-Chi Li Department of Electrical Engineering National Taiwan University What is Medical Ultrasound? Prevention: actions taken to avoid diseases. Diagnosis: the process
(1) 2 TEST SETUP. Table 1 Summary of models used for calculating roughness parameters Model Published z 0 / H d/h
 Estimation of Surface Roughness using CFD Simulation Daniel Abdi a, Girma T. Bitsuamlak b a Research Assistant, Department of Civil and Environmental Engineering, FIU, Miami, FL, USA, dabdi001@fiu.edu
Estimation of Surface Roughness using CFD Simulation Daniel Abdi a, Girma T. Bitsuamlak b a Research Assistant, Department of Civil and Environmental Engineering, FIU, Miami, FL, USA, dabdi001@fiu.edu
Renal Blood Flow GFR. Glomerulus Fluid Flow and Forces. Renal Blood Flow (cont d)
 GFR Glomerular filtration rate: about 120 ml /minute (180 L a day) Decreases with age (about 10 ml/min for each decade over 40) GFR = Sum of the filtration of two million glomeruli Each glomerulus probably
GFR Glomerular filtration rate: about 120 ml /minute (180 L a day) Decreases with age (about 10 ml/min for each decade over 40) GFR = Sum of the filtration of two million glomeruli Each glomerulus probably
Fluid structure interaction of a vibrating circular plate in a bounded fluid volume: simulation and experiment
 Fluid Structure Interaction VI 3 Fluid structure interaction of a vibrating circular plate in a bounded fluid volume: simulation and experiment J. Hengstler & J. Dual Department of Mechanical and Process
Fluid Structure Interaction VI 3 Fluid structure interaction of a vibrating circular plate in a bounded fluid volume: simulation and experiment J. Hengstler & J. Dual Department of Mechanical and Process
du u U 0 U dy y b 0 b
 BASIC CONCEPTS/DEFINITIONS OF FLUID MECHANICS (by Marios M. Fyrillas) 1. Density (πυκνότητα) Symbol: 3 Units of measure: kg / m Equation: m ( m mass, V volume) V. Pressure (πίεση) Alternative definition:
BASIC CONCEPTS/DEFINITIONS OF FLUID MECHANICS (by Marios M. Fyrillas) 1. Density (πυκνότητα) Symbol: 3 Units of measure: kg / m Equation: m ( m mass, V volume) V. Pressure (πίεση) Alternative definition:
Algebra 1 2008. Academic Content Standards Grade Eight and Grade Nine Ohio. Grade Eight. Number, Number Sense and Operations Standard
 Academic Content Standards Grade Eight and Grade Nine Ohio Algebra 1 2008 Grade Eight STANDARDS Number, Number Sense and Operations Standard Number and Number Systems 1. Use scientific notation to express
Academic Content Standards Grade Eight and Grade Nine Ohio Algebra 1 2008 Grade Eight STANDARDS Number, Number Sense and Operations Standard Number and Number Systems 1. Use scientific notation to express
Ravi Kumar Singh*, K. B. Sahu**, Thakur Debasis Mishra***
 Ravi Kumar Singh, K. B. Sahu, Thakur Debasis Mishra / International Journal of Engineering Research and Applications (IJERA) ISSN: 48-96 www.ijera.com Vol. 3, Issue 3, May-Jun 3, pp.766-77 Analysis of
Ravi Kumar Singh, K. B. Sahu, Thakur Debasis Mishra / International Journal of Engineering Research and Applications (IJERA) ISSN: 48-96 www.ijera.com Vol. 3, Issue 3, May-Jun 3, pp.766-77 Analysis of
Numerical Analysis of Independent Wire Strand Core (IWSC) Wire Rope
 Numerical Analysis of Independent Wire Strand Core (IWSC) Wire Rope Rakesh Sidharthan 1 Gnanavel B K 2 Assistant professor Mechanical, Department Professor, Mechanical Department, Gojan engineering college,
Numerical Analysis of Independent Wire Strand Core (IWSC) Wire Rope Rakesh Sidharthan 1 Gnanavel B K 2 Assistant professor Mechanical, Department Professor, Mechanical Department, Gojan engineering college,
Thoracoabdominal aortic aneurysm
 Thoracoabdominal aortic aneurysm Patient (1) - 69 PMH: 2013 - MVP, aortic root replacement with biological valve (Perimount) and subtotal aortic arch replacement Analysis for oppressive chest complaints
Thoracoabdominal aortic aneurysm Patient (1) - 69 PMH: 2013 - MVP, aortic root replacement with biological valve (Perimount) and subtotal aortic arch replacement Analysis for oppressive chest complaints
Introduction to Engineering System Dynamics
 CHAPTER 0 Introduction to Engineering System Dynamics 0.1 INTRODUCTION The objective of an engineering analysis of a dynamic system is prediction of its behaviour or performance. Real dynamic systems are
CHAPTER 0 Introduction to Engineering System Dynamics 0.1 INTRODUCTION The objective of an engineering analysis of a dynamic system is prediction of its behaviour or performance. Real dynamic systems are
Quantitative Inventory Uncertainty
 Quantitative Inventory Uncertainty It is a requirement in the Product Standard and a recommendation in the Value Chain (Scope 3) Standard that companies perform and report qualitative uncertainty. This
Quantitative Inventory Uncertainty It is a requirement in the Product Standard and a recommendation in the Value Chain (Scope 3) Standard that companies perform and report qualitative uncertainty. This
CFD Analysis of Swept and Leaned Transonic Compressor Rotor
 CFD Analysis of Swept and Leaned Transonic Compressor Nivin Francis #1, J. Bruce Ralphin Rose *2 #1 Student, Department of Aeronautical Engineering& Regional Centre of Anna University Tirunelveli India
CFD Analysis of Swept and Leaned Transonic Compressor Nivin Francis #1, J. Bruce Ralphin Rose *2 #1 Student, Department of Aeronautical Engineering& Regional Centre of Anna University Tirunelveli India
Basic Equations, Boundary Conditions and Dimensionless Parameters
 Chapter 2 Basic Equations, Boundary Conditions and Dimensionless Parameters In the foregoing chapter, many basic concepts related to the present investigation and the associated literature survey were
Chapter 2 Basic Equations, Boundary Conditions and Dimensionless Parameters In the foregoing chapter, many basic concepts related to the present investigation and the associated literature survey were
Cardiorespiratory Fitness
 Cardiorespiratory Fitness Assessment Purpose Determine level of fitness & set goals Develop safe & effective exercise prescription Document improvements Motivation Provide info concerning health status
Cardiorespiratory Fitness Assessment Purpose Determine level of fitness & set goals Develop safe & effective exercise prescription Document improvements Motivation Provide info concerning health status
SCHWEITZER ENGINEERING LABORATORIES, COMERCIAL LTDA.
 Pocket book of Electrical Engineering Formulas Content 1. Elementary Algebra and Geometry 1. Fundamental Properties (real numbers) 1 2. Exponents 2 3. Fractional Exponents 2 4. Irrational Exponents 2 5.
Pocket book of Electrical Engineering Formulas Content 1. Elementary Algebra and Geometry 1. Fundamental Properties (real numbers) 1 2. Exponents 2 3. Fractional Exponents 2 4. Irrational Exponents 2 5.
