Medtronic Product HCPCS and Outpatient Category C-Codes
|
|
|
- Quentin Craig
- 9 years ago
- Views:
Transcription
1 Product HCPCS and Outpatient Category C-Codes The following is a list of Neurological products grouped by type of therapy. This information will be helpful when providing patient care in the hospital outpatient setting. Neurostimulation Chronic Pain Product Brand Name and Synergy implantable neurostimulator and extension or 7489 E0756 C1767 C1883 Synergy Versitrel implantable neurostimulator and extension 7427V 7471 or 7489 E0756 C1767 C1883 Synergy EZ patient 7435 E0754 C1787 programmer (hand-held) Itrel 3 implantable neurostimulator and in-line connector extension E0756 C1767 C1883 Itrel 3 EZ patient programmer (hand-held) 7434A E0754 C1787 Mattrix receiver (2X4) 3272 E0757 (receiver) C1816 Mattrix transmitter kit 3210 E0758 (transmitter) Pisces Z-Quad lead kit 3890 E0752 C1778 Pisces Z-Quad compact lead kit 3891 E0752 C1778 Pisces Z-Quad Plus lead kit 3892 E0752 C1778 Pisces-Quad lead kit 3487A E0752 C1778 Pisces-Quad compact lead kit 3887 E0752 C1778 Pisces-Quad Plus lead kit 3888 E0752 C1778 Pisces-Octad lead kit 3898 E0752 C1778 Specify lead kit (CPT codes for a laminectomy are 3998 E0752 C1778 classifieds as inpatient only) Resume II lead kit 3587A E0752 C1778 Resume TL lead kit 3986A E0752 C1778 SymMix lead kit 3982A E0752 C1778 On-Point peripheral nerve stimulation lead kit 3987A E0752 C1778 Activa Therapy Parkinson s disease, Tremor Product Brand Name and Kinetra neurostimulator and extension Soletra neurostimulator and extension DBS lead kit (1.5 mm spaces) DBS lead kit (0.5 mm spaces) E0756 E0756 E0752 E0752 C1767 C1883 C1767 C1883 Not applicable (CPT code for DBS lead implantation is classified as inpatient only) Access Therapy Controller 7436 E0754 C1787 Access Review Therapy Controller 7438 E0754 C1787 Page 1 of 30
2 Drug Delivery Therapies Chronic pain, Cancer pain, and Intrathecal Baclofen Therapy Product Brand Name and SynchroMed EL infusion pump Programmable, 18mL. reservoir E0783 E0786(replacement) C1772 SynchroMed EL infusion pump. 8626L18 E0783 C1772 Programmable, 18mL. reservoir, and suture loops E0786(replacement) SynchroMed EL infusion pump E0783 C1772 Programmable, 10mL. reservoir E0786(replacement) SynchroMed EL infusion pump 8626L10 E0783 C1772 Programmable, 10mL. reservoir, and suture loops E0786(replacement) SynchroMed EL infusion pump, attached screened catheter access port E0783 C1772 Programmable, 18mL. reservoir E0786(replacement) SynchroMed EL infusion pump, attached screened catheter access port. 8627L18 E0783 C1772 Programmable, 18mL. reservoir, and suture loops E0786(replacement) SynchroMed EL infusion pump, attached screened catheter access port E0783 C1772 Programmable, 10mL. reservoir E0786(replacement) SynchroMed EL infusion pump w/ filter and attached screened catheter access port. 8627L10 E0783 C1772 Programmable, 10mL. reservoir, and suture loops E0786(replacement) SynchroMed II infusion pump with filter. Programmable, provided with mesh pouch, 20 ml E0783 C1772 Reservoir E0786(replacement) SynchroMed II infusion pump with filter. Programmable, provided with mesh pouch, 40 ml E0783 C1772 Reservoir E0786(replacement) IsoMed infusion pump, non-programmable E0782 C InDura IP intrathecal catheter 8709 E0785 (Replacement C1755 catheter only) 8731 intrathecal catheter 8731 E0785(Replacement C1755 catheter only) 8749 intrathecal catheter 8749 E0785(Replacement C1755 catheter only) Algoline intraspinal catheter 81102, E1399 C1755 Page 2 of 30
3 Drug Name Morphine Sulfate preservative-free, per 10mg N/A J2275 N/A Floxuridine, per 500mg N/A J9200 N/A Lioresal Intrathecal (baclofen injection) Screening ampule (50mcg) NCD Lioresal Intrathecal Refill Kit, one ampule of 10mg/20mL (500mcg/mL) NDC Lioresal Intrathecal Refill Kit, two ampules of 10mg/5mL (2,000 mcg/ml) NDC Lioresal Intrathecal Refill Kit, one ampule of 40mg/20mL (2,000mcg/mL) NDC Lioresal Intrathecal Refill Kit, two ampules of 20mg/40ml (500mcg/mL) NDC Lioresal Intrathecal Refill Kit, two ampules of 80mg/40mL (2,000mcg/mL) NDC s J0476 C J0475 C J0475 C J0475 C J0475 C J0475 C9009 Footnotes: 1 HCPCS 2004, Practice Management Information (PMIC), Los Angeles, CA Federal Register, Vol. 68, No. 216, November 7, 2003, # C-codes for device categories, as they existed on December 31, The use of the codes is not required and will not be enforced. Consult CMS Transmittal A-01-41, implementation date , for more information about Category C-Codes. Lioresal a registered trademark of Novartis Pharmaceuticals Corporation. Please read the attached Lioresal Intrathecal (baclofen injection) disclosure. Neurological Reimbursement Assistance for: Pain, ITB and Activa Therapies (800) :00 am 5:00 pm Central Time Monday through Friday or, [email protected] Note: This information is not intended to make recommendations regarding clinical practice. It is the responsibility of the provider/facility to determine appropriate coding and to understand and follow local carrier/payer coverage and coding policies. Page 3 of 30
4 SynchroMed and IsoMed Infusion Systems Product technical manual and the appropriate drug labeling must be reviewed for detailed disclosure prior to use. Indications: Chronic intrathecal infusion of preservative-free morphine sulfate sterile solution in the treatment of chronic intractable pain and chronic intravascular infusion of floxuridine (FUDR) for the treatment of primary or metastatic cancer. SynchroMed is also indicated for chronic intrathecal infusion of Lioresal Intrathecal (baclofen injection) for severe spasticity, chronic epidural infusion of preservative-free morphine sulfate sterile solution in the treatment of chronic intractable pain, chronic intravascular infusion of doxorubicin, cisplatin, or methotrexate for the treatment of primary or metastatic cancer, and chronic intravenous infusion of clindamycin for the treatment of osteomyelitis1. Contraindications: When infection is present2; when the pump cannot be implanted within 2.5 cm (1 inch ) from the surface of the skin; when body size is not sufficient to accept pump bulk and weight; when contraindications exist related to the drug. Blood sampling through the side catheter access port is contraindicated. Warnings: Use only with approved drugs. Improper use, calculation or programming errors, or component failure may result in loss of therapeutic effect, or clinically significant or fatal drug overdose or underdose symptoms. Clinically significant or fatal drug overdose may result from overpressurization of the pump reservoir, overheating of the pump during implant preparation or as a result of diathermy, improper pump preparation, improper injection of drug through the catheter access port or into the pump pocket, or failure to account for significant amounts of drug residing in the reservoir, pump tubing, catheter access port, or catheter. The effects of mixing drugs are unknown. Flow rate of the IsoMed pump may decrease or stop if drug precipitation occurs. An inflammatory mass that can result in serious neurological impairment, including paralysis, can occur at the tip of the implanted catheter. Patients on intraspinal opioid therapy should be monitored carefully at each visit for any new or changed neurological signs or symptoms. Timely treatment may minimize or avert permanent neurological injury. The effects of implanting the SynchroMed pump in patients with other implanted programmable devices are unknown. Do not use if sterility has been compromised or if the use by date has expired. Do not resterilize or reuse. Precautions: Only qualified personnel should implant, fill and refill the pumps; access the catheter access ports; or program the SynchroMed pump. Follow recommended procedures. Maintain strict aseptic techniques during all procedures to prevent infection. The catheter access port does not contain a bacterial filter. Consider use of peri- and postoperative antibiotics for pump implantation and any subsequent surgical procedures. Use caution in selecting an anatomical pump site appropriate to the size and mass of the patient. Initial fill and refill volumes must not exceed levels specified in the technical manuals. Do not allow pump to stop or run dry; if therapy is discontinued, maintain minimal flow of appropriate fluid. Do not expose pumps to temperatures above 43 degrees C (110 degrees F) or below 5 degrees C (40 degrees F). Avoid exposing pump to diathermy, therapeutic radiation, lithotripsy or pressure extremes. Do not implant a pump that has been dropped onto a hard surface or shows signs of damage. Follow manufacturer s instructions regarding drug preparation, dosage, and administration. FUDR should be used with added caution in patients with impaired hepatic or renal function. Systemic therapy should be considered for patients with known disease extending beyond an area capable of infusion. IsoMed pump flow rate will vary depending on factors such as body temperature, altitude, arterial pressure at the catheter tip, and solution viscosity. Advise patients of symptoms to report, activities to avoid, and the importance of keeping refill appointments. Magnetic Resonance Imaging (MRI): MRI will temporarily stop the SynchroMed pump motor and suspend drug infusion for the duration of MRI exposure. The SynchroMed pump should resume normal operation upon termination of MRI exposure. Exposure of IsoMed pumps to MRI fields of 1.5 T (Tesla) has demonstrated no impact to pump performance and a limited effect on the quality of the diagnostic information. During an MRI scan, the patient may experience heating or peripheral nerve stimulation at or near the pump implant site. In the unlikely event that this happens, the MRI scan parameters should be adjusted to reduce Specific Absorption Rate (SAR) for heating or db/dt for nerve stimulation or both. Upon completion of an MRI scan, the SynchroMed pump parameters should be confirmed using a clinician programmer. SynchroMed pump performance has not been established in >2.0 T (Tesla) MR scanners nor has IsoMed pump performance been established in >1.5 T (Tesla) MR scanners it is not recommended that patients have MRI scans using these scanners. Adverse Events: Include, but not limited to, clinically significant or fatal drug overdose or underdose, cessation or change in therapy, or a return of underlying symptoms due to an empty reservoir, component failure, misuse, misprogramming or miscommunication, or SynchroMed battery depletion; seroma/hematoma, infection, inflammation, tissue erosion, or pain at implant site; complete or partial catheter occlusion, kinking, breakage, leakage or disconnection; catheter dislodgment or migration; CSF leak/accumulation, internal/gi bleeding; arachnoiditis; radiculitis; meningitis; spinal headache; inflammatory mass; perforation of internal organs; drug toxicity and related side effects; and procedural complications. Rx Only Page 4 of 30
5 Page 5 of 30
6 Page 6 of 30
7 Bravo ph Monitoring System Gastroenterology Product Brand Name and Bravo ph Capsule with Delivery System 9012B1011 E1399 C9712 (box of 5) Bravo ph Monitoring System Note: Reference product directions for use including indications, contraindications, warnings and precautions. Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. InterStim Therapy Urology Product Brand Name and InterStim Test Kit (sacral nerve stimulation) 3065USC A4290 TL22 InterStim Neurostimulator 3023 E0756 C1767 InterStim Patient Programmer 3031A E0754 C1787 Lead Introducer E1399 C1894 All Extensions InterStim Leads E1399 C1897 InterStim Therapy for Urinary Control: Product technical manual must be reviewed prior to use for detailed disclosure. Indications: InterStim Therapy for Urinary Control is indicated for the treatment of urinary retention and the symptoms of overactive bladder, including urinary urge incontinence and significant symptoms of urgency-frequency alone or in combination, in patients who have failed or could not tolerate more conservative treatments. Contraindications: Patients are contraindicated for implant of the InterStim System if they have not demonstrated an appropriate response to test stimulation or are unable to operate the neurostimulator. Also, diathermy (e.g., shortwave diathermy, microwave diathermy or therapeutic ultrasound diathermy) is contraindicated because diathermy's energy can be transferred through the implanted system (or any of the separate implanted components), which can cause tissue damage and can result in severe injury or death. Diathermy can damage parts of the neurostimulation system. Precaution/Adverse Events: Warning: This therapy is not intended for patients with mechanical obstruction such as benign prostatic hypertrophy, cancer, or urethral stricture. Safety and effectiveness have not been established for: bilateral stimulation, patients with neurological disease origins such as multiple sclerosis, pregnancy and delivery, or for pediatric use under the age of 16. System may be affected by or adversely affect cardiac pacemakers or therapies, cardioverter defibrillators, electrocautery, external defibrillators, ultrasonic equipment, radiation therapy, magnetic resonance imaging (MRI), theft detectors and screening devices. Adverse events related to the therapy, device, or procedure can include pain at the implant sites, lead migration, infection or skin irritation, technical or device problems, transient electric shock, adverse change in bowel or voiding function, numbness, nerve injury, seroma at the neurostimulator site, change in menstrual cycle, and undesirable stimulation or sensations. CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician. E0752 C1883 C1778 Reimbursement Assistance for: Gastroenterology and Urology Therapies (877) x49705 or x :00 am 5:00 pm Central Time - Monday through Friday Note: This information is not intended to make recommendations regarding clinical practice. It is the responsibility of the provider/facility to determine appropriate coding and to understand and follow local carrier/payer coverage and coding policies. Page 7 of 30
8 Coronary and Non-coronary Stents Stent, non-coated/non-covered, with delivery system Vascular Temporary Occlusion and Aspiration Systems Product Brand Name and S660D2509W OTW coronary stent S660D2509W NA C1876 S660D2512W OTW coronary stent S660D2512W NA C1876 S660D2515W OTW coronary stent S660D2515W NA C1876 S660D2518W OTW coronary stent S660D2518W NA C1876 S660D2524W OTW coronary stent S660D2524W NA C1876 S mm x 9mm OTW coronary stent S660D27509W NA C1876 S mm x 12mm OTW coronary stent S660D27512W NA C1876 S mm x 15mm OTW coronary stent S660D27515W NA C1876 S mm x 18mm OTW coronary stent S660D27518W NA C1876 S mm x 24mm OTW coronary stent S660D27524W NA C x 12mm S660 Zipper Mx S627512ZP NA C x 15mm S660 Zipper Mx S627515ZP NA C x 18mm S660 Zipper Mx S627518ZP NA C x 24mm S660 Zipper Mx S627524ZP NA C mm x 09mm Driver OTW DRV30009W NA C mm x 12mm Driver OTW DRV30012W NA C mm x 15mm Driver OTW DRV30015W NA C mm x 18mm Driver OTW DRV30018W NA C mm x 24mm Driver OTW DRV30024W NA C mm x 30mm Driver OTW DRV30030W NA C mm x 09mm Driver OTW DRV35009W NA C mm x 12mm Driver OTW DRV35012W NA C mm x 15mm Driver OTW DRV35015W NA C mm x 18mm Driver OTW DRV35018W NA C mm x 24mm Driver OTW DRV35024W NA C mm x 30mm Driver OTW DRV35030W NA C mm x 09mm Driver OTW DRV40009W NA C mm x 12mm Driver OTW DRV40012W NA C mm x 15mm Driver OTW DRV40015W NA C mm x 18mm Driver OTW DRV40018W NA C mm x 24mm Driver OTW DRV40024W NA C mm x 30mm Driver OTW DRV40030W NA C mm x 09mm Driver Mx2 DRV30009MX NA C mm x 12mm Driver Mx2 DRV30012MX NA C mm x 15mm Driver Mx2 DRV30015MX NA C mm x 18mm Driver Mx2 DRV30018MX NA C mm x 24mm Driver Mx2 DRV30024MX NA C mm x 30mm Driver Mx2 DRV30030MX NA C1876 Page 8 of 30
9 3.50mm x 09mm Driver Mx2 DRV35009MX NA C mm x 12mm Driver Mx2 DRV35012MX NA C mm x 15mm Driver Mx2 DRV35015MX NA C mm x 18mm Driver Mx2 DRV35018MX NA C mm x 24mm Driver Mx2 DRV35024MX NA C mm x 30mm Driver Mx2 DRV35030MX NA C mm x 09mm Driver Mx2 DRV40009MX NA C mm x 12mm Driver Mx2 DRV40012MX NA C mm x 15mm Driver Mx2 DRV40015MX NA C mm x 18mm Driver Mx2 DRV40018MX NA C mm x 24mm Driver Mx2 DRV40024MX NA C mm x 30mm Driver Mx2 DRV40030MX NA C1876 ASSURANT - 6mm x 20mm FB620F NA C1876 ASSURANT PLUS - 6mm x 20mm FB620L NA C1876 ASSURANT - 7mm x 20mm FB720F NA C1876 ASSURANT PLUS - 7mm x 20mm FB720L NA C1876 ASSURANT - 8mm x 20mm FB820F NA C1876 ASSURANT PLUS - 8mm x 20mm FB820L NA C1876 ASSURANT - 6mm x 30mm FB630F NA C1876 ASSURANT PLUS - 6mm x 30mm FB630L NA C1876 ASSURANT - 7mm x 30mm FB730F NA C1876 ASSURANT PLUS - 7mm x 30mm FB730L NA C1876 ASSURANT - 8mm x 30mm FB830F NA C1876 ASSURANT PLUS - 8mm x 30mm FB830L NA C1876 ASSURANT - 9mm x 30mm FB930F NA C1876 ASSURANT PLUS - 9mm x 30mm FB930L NA C1876 ASSURANT - 10mm x 30mm FB1030F NA C1876 ASSURANT PLUS - 10mm x 30mm FB1030L NA C1876 ASSURANT - 6mm x 40mm FB640F NA C1876 ASSURANT - 7mm x 40mm FB740F NA C1876 ASSURANT - 8mm x 40mm FB840F NA C1876 ASSURANT - 9mm x 40mm FB940F NA C1876 ASSURANT - 10mm x 40mm FB1040F NA C1876 ASSURANT - 6mm x 60mm FB660F NA C1876 ASSURANT - 7mm x 60mm FB760F NA C1876 ASSURANT - 8mm x 60mm FB860F NA C1876 ASSURANT - 9mm x 60mm FB960F NA C1876 ASSURANT - 10mm x 60mm FB1060F NA C SM NA C1876 6mm x 20mm 7mm x 20mm 720SM NA C1876 Page 9 of 30
10 8mm x 20mm 9mm x 20mm 10mm x 20mm 6mm x 40mm 7mm x 40mm 8mm x 40mm 9mm x 40mm 10mm x 40mm 6mm x 60mm 7mm x 60mm 8mm x 60mm 9mm x 60mm 10mm x 60mm 6mm x 80mm 7mm x 80mm 8mm x 80mm 9mm x 80mm 10mm x 80mm 6mm x 20mm - Long 7mm x 20mm - Long 8mm x 20mm - Long 9mm x 20mm - Long 10mm x 20mm - Long 6mm x 40mm - Long 7mm x 40mm - Long 8mm x 40mm - Long 9mm x 40mm - Long 10mm x 40mm - Long 6mm x 60mm - Long 820SM NA C SM NA C SM NA C SM NA C SM NA C SM NA C SM NA C SM NA C SM NA C SM NA C SM NA C SM NA C SM NA C SM NA C SM NA C SM NA C SM NA C SM NA C SML NA C SML NA C SML NA C SML NA C SML NA C SML NA C SML NA C SML NA C SML NA C SML NA C SML NA C1876 Page 10 of 30
11 7mm x 60mm - Long 8mm x 60mm - Long 9mm x 60mm - Long 10mm x 60mm - Long 6mm x 80mm - Long 7mm x 80mm - Long 8mm x 80mm - Long 9mm x 80mm - Long 10mm x 80mm - Long 4mm x 12mm.018 OTW 80cm 4mm x 12mm.018 OTW 130cm 5mm x 12mm.018 OTW 80cm 5mm x 12mm.018 OTW 130cm 6mm x 12mm.018 OTW 80cm 6mm x 12mm.018 OTW 130cm 7mm x 12mm.018 OTW 80cm 7mm X 12mm.018 OTW 130cm 4mm X 18mm.018 OTW 80cm 4mm x 18mm.018 OTW 130cm 5mm x 18mm.018 OTW 80cm 5mm x 18mm.018 OTW 130cm 6mm x 18mm.018 OTW 80cm 6mm x 18mm.018 OTW 130cm 7mm x 18mm.018 OTW 80cm 7mm x 18mm.018 OTW 130cm 760SML NA C SML NA C SML NA C SML NA C SML NA C SML NA C SML NA C SML NA C SML NA C1876 XD412YF NA C1876 XD412YL NA C1876 XD512YF NA C1876 XD512YL NA C1876 XD612YF NA C1876 XD612YL NA C1876 XD712YF NA C1876 XD712YL NA C1876 XD418YF NA C1876 XD418YL NA C1876 XD518YF NA C1876 XD518YL NA C1876 XD618YF NA C1876 XD618YL NA C1876 XD718YF NA C1876 XD718YL NA C1876 Page 11 of 30
12 Pioneer Catheter Catheter, intravascular ultrasound Vascular Temporary Occlusion and Aspiration Systems Product Brand Name and Pioneer catheter, IVUS catheter TA-XP-001 NA C1753 The Pioneer catheter is a unique breakthrough for subintimal entrapment, allowing controlled and precise return to the true lumen within the peripheral vasculature. The tip of the catheter features a fully integrated 64-element phased array IVUS transducer enabling controlled targeting of a 24-gauge needle. The device allows you to choose an optimal needle depth, up to 7 mm, for controlled delivery of a inch guidewire into the true lumen, distal to the lesion. Embolic Protection Embolization protective system Vascular Temporary Occlusion and Aspiration Systems Product Brand Name and GUARDWIRE 2-5 BBE, 7F Rx GuardWire - embolic protection system GUARDWIRE 2-5 BBE, 7F OTW GuardWire - embolic protection system GUARDWIRE 3-6 BBE, 7F Rx GuardWire - embolic protection system GUARDWIRE 3-6 BBE 7F OTW GuardWire - embolic protection system C1884* - eligible for pass-through payment under the Medicare hospital OPPS. GUARDWIRE Temporary Occlusion and Aspiration System Distal Protection Devices Occlusion & Aspiration Systems GEZ5200US NA C1884* GEZ5300US NA C1884* GEZ6200US NA C1884* GEZ6300US NA C1884* Distal protection is provided by occluding the vessel to prevent particulate debris migrating distally into the myocardium, and aspirating the debris before restoring blood flow. This simple solution has been clinically proven to reduce cumulative MACE events by 42 percent at 30-day follow-up in SVG interventions (SAFER Trial, Circulation, May 2002). The GuardWire system consists of an ultra-low profile protection balloon (0.028" or ) mounted on a standard 0.014" guidewire, and the Export Aspiration Catheter ( F guide compatible). The GuardWire system can protect vessels from 2.5-6mm in 0.5mm increments, and the GuardWire lengths are 200 and 300 cm to support monorail and over-thewire therapy catheters. Page 12 of 30
13 PTCA Balloon Catheters Catheter, transluminal angioplasty, non-laser (may include guidance, infusion/perfusion capability) Vascular Temporary Occlusion and Aspiration Systems Product Brand Name and 1.5 x 10mm Stormer OTW Balloon D3S1510 NA C x 15mm Stormer OTW Balloon D3S1515 NA C x 20mm Stormer OTW Balloon D3S1520 NA C x 30mm Stormer OTW Balloon D3S1530 NA C x 10mm Stormer OTW Balloon D3S2010 NA C x 15mm Stormer OTW Balloon D3S2015 NA C x 20mm Stormer OTW Balloon D3S2020 NA C x 30mm Stormer OTW Balloon D3S2030 NA C x 10mm Stormer OTW Balloon D3S22510 NA C x 15mm Stormer OTW Balloon D3S22515 NA C x 20mm Stormer OTW Balloon D3S22520 NA C x 10mm Stormer OTW Balloon D3S2510 NA C x 15mm Stormer OTW Balloon D3S2515 NA C x 20mm Stormer OTW Balloon D3S2520 NA C x 30mm Stormer OTW Balloon D3S2530 NA C x 10mm Stormer OTW Balloon D3S27510 NA C x 15mm Stormer OTW Balloon D3S27515 NA C x 20mm Stormer OTW Balloon D3S27520 NA C x 10mm Stormer OTW Balloon D3S3010 NA C x 15mm Stormer OTW Balloon D3S3015 NA C x 20mm Stormer OTW Balloon D3S3020 NA C x 30mm Stormer OTW Balloon D3S3030 NA C x 10mm Stormer OTW Balloon D3S32510 NA C x 15mm Stormer OTW Balloon D3S32515 NA C x 20mm Stormer OTW Balloon D3S32520 NA C x 10mm Stormer OTW Balloon D3S3510 NA C x 15mm Stormer OTW Balloon D3S3515 NA C x 20mm Stormer OTW Balloon D3S3520 NA C x 30mm Stormer OTW Balloon D3S3530 NA C x 20mm Stormer OTW Balloon D3S37520 NA C x 10mm Stormer OTW Balloon D3S4010 NA C x 15mm Stormer OTW Balloon D3S4015 NA C x 20mm Stormer OTW Balloon D3S4020 NA C x 30mm Stormer OTW Balloon D3S4030 NA C x 10mm Stormer OTW Balloon D3S4010 NA C x 15mm Stormer OTW Balloon D3S4015 NA C x 20mm Stormer OTW Balloon D3S4020 NA C x 30mm Stormer OTW Balloon D3S4030 NA C x 6mm NC Stormer OTW Balloon NCS1506W NA C1725 NC Stormer 1.5 x 6mm Zipper Mx NCS1506ZP NA C x 11mm NC Stormer OTW Balloon NCS1511W NA C1725 NC Stormer 1.5 x 11mm Zipper Mx NCS1511ZP NA C1725 Page 13 of 30
14 1.5 x 16mm NC Stormer OTW Balloon NCS1516W NA C1725 NC Stormer 1.5 x 16mm Zipper Mx NCS1516ZP NA C x 21mm NC Stormer OTW Balloon NCS1521W NA C1725 NC Stormer 1.5 x 21mm Zipper Mx NCS1521ZP NA C x 6mm NC Stormer OTW Balloon NCS2006W NA C1725 NC Stormer 2.0 x 6mm Zipper Mx NCS2006ZP NA C x 11mm NC Stormer OTW Balloon NCS2011W NA C1725 NC Stormer 2.0 x 11mm Zipper Mx NCS2011ZP NA C x 16mm NC Stormer OTW Balloon NCS2016W NA C1725 NC Stormer 2.0 x 16mm Zipper Mx NCS2016ZP NA C x 21mm NC Stormer OTW Balloon NCS2021W NA C1725 NC Stormer 2.0 x 21mm Zipper Mx NCS2021ZP NA C x 6mm NC Stormer OTW Balloon NCS22506W NA C1725 NC Stormer 2.25 x 6mm Zipper Mx NCS22506ZP NA C x 11mm NC Stormer OTW Balloon NCS22511W NA C1725 NC Stormer 2.25 x 11mm Zipper Mx NCS22511ZP NA C x 16mm NC Stormer OTW Balloon NCS22516W NA C1725 NC Stormer 2.25 x 16mm Zipper Mx NCS22516ZP NA C x 21mm NC Stormer OTW Balloon NCS22521W NA C1725 NC Stormer 2.25 x 21mm Zipper Mx NCS22521ZP NA C x 6mm NC Stormer OTW Balloon NCS2506W NA C1725 NC Stormer 2.5 x 6mm Zipper Mx NCS2506ZP NA C x 11mm NC Stormer OTW Balloon NCS2511W NA C1725 NC Stormer 2.5 x 11mm Zipper Mx NCS2511ZP NA C x 16mm NC Stormer OTW Balloon NCS2516W NA C1725 NC Stormer 2.5 x 16mm Zipper Mx NCS2516ZP NA C x 21mm NC Stormer OTW Balloon NCS2521W NA C1725 NC Stormer 2.5 x 21mm Zipper Mx NCS2521ZP NA C x 26mm NC Stormer OTW Balloon NCS2526W NA C1725 NC Stormer 2.5 x 26mm Zipper Mx NCS2526ZP NA C x 31mm NC Stormer OTW Balloon NCS2531W NA C1725 NC Stormer 2.5 x 31mm Zipper Mx NCS2531ZP NA C x 6mm NC Stormer OTW Balloon NCS27506W NA C1725 NC Stormer 2.75 x 6mm Zipper Mx NCS27506ZP NA C x 11mm NC Stormer OTW Balloon NCS27511W NA C1725 NC Stormer 2.75 x 11mm Zipper Mx NCS27511ZP NA C x 16mm NC Stormer OTW Balloon NCS27516W NA C1725 NC Stormer 2.75 x 16mm Zipper Mx NCS27516ZP NA C x 21mm NC Stormer OTW Balloon NCS27521W NA C1725 NC Stormer 2.75 x 21mm Zipper Mx NCS27521ZP NA C x 6mm NC Stormer OTW Balloon NCS3006W NA C1725 NC Stormer 3.0 x 6mm Zipper Mx NCS3006ZP NA C x 11mm NC Stormer OTW Balloon NCS3011W NA C1725 NC Stormer 3.0 x 11mm Zipper Mx NCS3011ZP NA C x 16mm NC Stormer OTW Balloon NCS3016W NA C1725 NC Stormer 3.0 x 16mm Zipper Mx NCS3016ZP NA C x 21mm NC Stormer OTW Balloon NCS3021W NA C1725 NC Stormer 3.0 x 21mm Zipper Mx NCS3021ZP NA C x 26mm NC Stormer OTW Balloon NCS3026W NA C1725 NC Stormer 3.0 x 26mm Zipper Mx NCS3026ZP NA C x 31mm NC Stormer OTW Balloon NCS3031W NA C1725 Page 14 of 30
15 NC Stormer 3.0 x 31mm Zipper Mx NCS3031ZP NA C x 6mm NC Stormer OTW Balloon NCS32506W NA C1725 NC Stormer 3.25 x 6mm Zipper Mx NCS32506ZP NA C x 11mm NC Stormer OTW Balloon NCS32511W NA C1725 NC Stormer 3.25 x 11mm Zipper Mx NCS32511ZP NA C x 16mm NC Stormer OTW Balloon NCS32516W NA C1725 NC Stormer 3.25 x 16mm Zipper Mx NCS32516ZP NA C x 21mm NC Stormer OTW Balloon NCS32521W NA C1725 NC Stormer 3.25 x 21mm Zipper Mx NCS32521ZP NA C x 6mm NC Stormer OTW Balloon NCS3506W NA C1725 NC Stormer 3.5 x 6mm Zipper Mx NCS3506ZP NA C x 11mm NC Stormer OTW Balloon NCS3511W NA C1725 NC Stormer 3.5 x 11mm Zipper Mx NCS3511ZP NA C x 16mm NC Stormer OTW Balloon NCS3516W NA C1725 NC Stormer 3.5 x 16mm Zipper Mx NCS3516ZP NA C x 21mm NC Stormer OTW Balloon NCS3521W NA C1725 NC Stormer 3.5 x 21mm Zipper Mx NCS3521ZP NA C x 6mm NC Stormer OTW Balloon NCS37506W NA C1725 NC Stormer 3.75 x 6mm Zipper Mx NCS37506ZP NA C x 11mm NC Stormer OTW Balloon NCS37511W NA C1725 NC Stormer 3.75 x 11mm Zipper Mx NCS37511ZP NA C x 16mm NC Stormer OTW Balloon NCS37516W NA C1725 NC Stormer 3.75 x 16mm Zipper Mx NCS37516ZP NA C x 21mm NC Stormer OTW Balloon NCS37521W NA C1725 NC Stormer 3.75 x 21mm Zipper Mx NCS37521ZP NA C x 6mm NC Stormer OTW Balloon NCS4006W NA C1725 NC Stormer 4.0 x 6mm Zipper Mx NCS4006ZP NA C x 11mm NC Stormer OTW Balloon NCS4011W NA C1725 NC Stormer 4.0 x 11mm Zipper Mx NCS4011ZP NA C x 16mm NC Stormer OTW Balloon NCS4016W NA C1725 NC Stormer 4.0 x 16mm Zipper Mx NCS4016ZP NA C x 21mm NC Stormer OTW Balloon NCS4021W NA C1725 NC Stormer 4.0 x 21mm Zipper Mx NCS4021ZP NA C1725 Stormer 1.5 x 10mm Zipper Mx STR1510ZP NA C1725 Stormer 1.5 x 15mm Zipper Mx STR1515ZP NA C1725 Stormer 1.5 x 20mm Zipper Mx STR1520ZP NA C1725 Stormer 1.5 x 25mm Zipper Mx STR1525ZP NA C1725 Stormer 1.5 x 30mm Zipper Mx STR1530ZP NA C1725 Stormer 2.0 x 10mm Zipper Mx STR2010ZP NA C1725 Stormer 2.25 x 10mm Zipper Mx STR22510ZP NA C1725 Stormer 2.5 x 10mm Zipper Mx STR2510ZP NA C1725 Stormer 2.75 x 10mm Zipper Mx STR27510ZP NA C1725 Stormer 3.0 x 10mm Zipper Mx STR3010ZP NA C1725 Stormer 3.25 x 10mm Zipper Mx STR32510ZP NA C1725 Stormer 3.5 x 10mm Zipper Mx STR3510ZP NA C1725 Stormer 3.75 x 10mm Zipper Mx STR37510ZP NA C1725 Stormer 4.0 x 10mm Zipper Mx STR4010ZP NA C1725 Stormer 2.0 x 15mm Zipper Mx STR2015ZP NA C1725 Stormer 3.0 x 15mm Zipper Mx STR3015ZP NA C1725 Stormer 4.0 x 15mm Zipper Mx STR4015ZP NA C1725 Stormer 2.25 x 15mm Zipper Mx STR22515ZP NA C1725 Page 15 of 30
16 Stormer 2.5 x 15mm Zipper Mx STR2515ZP NA C1725 Stormer 3.5 x 15mm Zipper Mx STR3515ZP NA C1725 Stormer 2.75 x 15mm Zipper Mx STR27515ZP NA C1725 Stormer 3.25 x 15mm Zipper Mx STR32515ZP NA C1725 Stormer 3.75 x 15mm Zipper Mx STR37515ZP NA C1725 Stormer 2.0 x 20mm Zipper Mx STR2020ZP NA C1725 Stormer 2.25 x 20mm Zipper Mx STR22520ZP NA C1725 Stormer 2.5 x 20mm Zipper Mx STR2520ZP NA C1725 Stormer 2.75 x 20mm Zipper Mx STR27520ZP NA C1725 Stormer 3.0 x 20mm Zipper Mx STR3020ZP NA C1725 Stormer 3.25 x 20mm Zipper Mx STR32520ZP NA C1725 Stormer 3.5 x 20mm Zipper Mx STR3520ZP NA C1725 Stormer 3.75 x 20mm Zipper Mx STR37520ZO NA C1725 Stormer 4.0 x 20mm Zipper Mx STR4020ZP NA C1725 Stormer 2.0 x 25mm Zipper Mx STR2025ZP NA C1725 Stormer 2.25 x 25mm Zipper Mx STR22525ZP NA C1725 Stormer 2.5 x 25mm Zipper Mx STR2525ZP NA C1725 Stormer 2.75 x 25mm Zipper Mx STR27525ZP NA C1725 Stormer 3.0 x 25mm Zipper Mx STR3025ZP NA C1725 Stormer 3.25 x 25mm Zipper Mx STR32525ZP NA C1725 Stormer 3.5 x 25mm Zipper Mx STR3525ZP NA C1725 Stormer 3.75 x 25mm Zipper Mx STR37525ZP NA C1725 Stormer 4.0 x 25mm Zipper Mx STR4025ZP NA C1725 Stormer 2.0 x 30mm Zipper Mx STR2030ZP NA C1725 Stormer 2.25 x 30mm Zipper Mx STR22530ZP NA C1725 Stormer 2.5 x 30mm Zipper Mx STR2530ZP NA C1725 Stormer 2.75 x 30mm Zipper Mx STR27530ZP NA C1725 Stormer 3.0 x 30mm Zipper Mx STR3030ZP NA C1725 Stormer 3.25 x 30mm Zipper Mx STR32530ZP NA C1725 Stormer 3.5 x 30mm Zipper Mx STR3530ZP NA C1725 Stormer 3.75 x 30mm Zipper Mx STR37530ZP NA C1725 Stormer 4.0 x 30mm Zipper Mx STR4030ZP NA C1725 Sprinter 1.5 x 6mm OTW SPR1506W NA C1725 Sprinter 1.5 x 12mm OTW SPR1512W NA C1725 Sprinter 1.5 x 15mm OTW SPR1515W NA C1725 Sprinter 1.5 x 20mm OTW SPR1520W NA C1725 Sprinter 2.0 x 6mm OTW SPR2006W NA C1725 Sprinter 2.0 x 12mm OTW SPR2012W NA C1725 Sprinter 2.0 x 15mm OTW SPR2015W NA C1725 Sprinter 2.0 x 20mm OTW SPR2020W NA C1725 Sprinter 2.0 x 25mm OTW SPR2025W NA C1725 Sprinter 2.0 x 30mm OTW SPR2030W NA C1725 Sprinter 2.25 x 6mm OTW SPR22506W NA C1725 Sprinter 2.25 x 12mm OTW SPR22512W NA C1725 Sprinter 2.25 x 15mm OTW SPR22515W NA C1725 Sprinter 2.25 x 20mm OTW SPR22520W NA C1725 Sprinter 2.5 x 6mm OTW SPR2506W NA C1725 Sprinter 2.5 x 12mm OTW SPR2512W NA C1725 Sprinter 2.5 x 15mm OTW SPR2515W NA C1725 Sprinter 2.5 x 20mm OTW SPR2520W NA C1725 Sprinter 2.5 x 25mm OTW SPR2525W NA C1725 Page 16 of 30
17 Sprinter 2.5 x 30mm OTW SPR2530W NA C1725 Sprinter 2.75 x 6mm OTW SPR27506W NA C1725 Sprinter 2.75 x 12mm OTW SPR27512W NA C1725 Sprinter 2.75 x 15mm OTW SPR27515W NA C1725 Sprinter 2.75 x 20mm OTW SPR27520W NA C1725 Sprinter 3.0 x 6mm OTW SPR3006W NA C1725 Sprinter 3.0 x 12mm OTW SPR3012W NA C1725 Sprinter 3.0 x 15mm OTW SPR3015W NA C1725 Sprinter 3.0 x 20mm OTW SPR3020W NA C1725 Sprinter 3.0 x 25mm OTW SPR3025W NA C1725 Sprinter 3.0 x 30mm OTW SPR3030W NA C1725 Sprinter 3.25 x 12mm OTW SPR32512W NA C1725 Sprinter 3.25 x 15mm OTW SPR32515W NA C1725 Sprinter 3.25 x 20mm OTW SPR32520W NA C1725 Sprinter 3.5 x 6mm OTW SPR3506W NA C1725 Sprinter 3.5 x 12mm OTW SPR3512W NA C1725 Sprinter 3.5 x 15mm OTW SPR3515W NA C1725 Sprinter 3.5 x 20mm OTW SPR3520W NA C1725 Sprinter 3.5 x 25mm OTW SPR3525W NA C1725 Sprinter 3.5 x 30mm OTW SPR3530W NA C1725 Sprinter 3.75 x 12mm OTW SPR37512W NA C1725 Sprinter 3.75 x 15mm OTW SPR37515W NA C1725 Sprinter 3.75 x 20mm OTW SPR37520W NA C1725 Sprinter 4.0 x 6mm OTW SPR4006W NA C1725 Sprinter 4.0 x 12mm OTW SPR4012W NA C1725 Sprinter 4.0 x 15mm OTW SPR4015W NA C1725 Sprinter 4.0 x 20mm OTW SPR4020W NA C1725 Sprinter 4.0 x 25mm OTW SPR4025W NA C1725 Sprinter 4.0 x 30mm OTW SPR4030W NA C1725 Guide/Angiographic Catheters Catheter, guiding (may include infusion/perfusion capability) Vascular Temporary Occlusion and Aspiration Systems Product Brand Name and LAUNCHER 6F - 8F - guide catheter NA C1887 ZUMA 5F - 9F - guide catheter NA C1887 ZUMA 2 6F - 8F - guide catheter Multiple Model NA C1887 Numbers PRO-FLO 7F - angiographic catheter NA C1887 SITESEER - angiographic catheter NA C1887 Page 17 of 30
18 Guide Wires Guide Wire Vascular Temporary Occlusion and Aspiration Systems Product Brand Name and Coronary and angiographic guide wires Multiple Model Numbers NA C1769 Introducers / Sheaths Introducer/sheath, other than guiding, other than electrophysilogical, non-laser Vascular Temporary Occlusion and Aspiration Systems Product Brand Name and INPUT TS - introducer and sheaths Multiple Model Numbers NA C1894 Reimbursement Assistance for: Vascular Therapies (707) Alex Au-Yeung 8:00 am 5:00 pm Pacific Time - Monday through Friday Note: This information is not intended to make recommendations regarding clinical practice. It is the responsibility of the provider/facility to determine appropriate coding and to understand and follow local carrier/payer coverage and coding policies. Page 18 of 30
19 Cardiac Rhythm Management Therapies Cardioverter Defibrillator, Dual Chamber (Implantable) Product Brand Name and Maximo DR 7278 C1721 Marquis DR 7274 C1721 Jewel AF 7250 C1721 GEM III DR 7275 C1721 GEM II DR 7273 C1721 GEM DR 7271 C1721 Intrinsic 7287, 7288 C1721 Cardiac Rhythm Management Therapies Cardioverter Defibrillator, Single Chamber (Implantable) Product Brand Name and Maximo VR 7232 C , 7230Cx, Marquis VR 7230B, 7230E C1722 GEM III VR 7231 C1722 GEM II VR 7229Cx C1722 GEM 7227, 7227B, 7227D, 7227E, C Cx ONYX VR 7290Cx C1722 Cardiac Rhythm Management Therapies Cardioverter Defibrillator, Other Than Single or Dual Chamber (Implantable) Product Brand Name and InSync II Marquis 7289 C1882 InSync Marquis 7277 C1882 InSync ICD 7272 C1882 GEM III AT 7276 C1882 Micro Jewel 7221B, 7221C, 7221Cx, 7221D, C E Micro Jewel II 7223Cx C1882 InSync II Protect 7295 C1882 InSync Maximo 7303 C1882 Page 19 of 30
20 Cardiac Rhythm Management Therapies Pacemaker, Dual Chamber, Rate-Responsive (Implantable) Product Brand Name and EnPulse EnPulse DR E1DR01, E1DR03, E1DR06, E1DR21 E2DR01, E2DR03, E2DR06, E2DR21 C1785 C1785 Vitatron C-Series C60DR C1785 AT500 DDDRP AT501 C1785 Kappa 900 DR KDR 901, KDR 903, KDR 906, KDR 921, KDR 92100, C1785 KDR 931, KDR 933 Kappa 800 DR KDR 801, KDR 803, KDR 806 C1785 Kappa 700 DR KDR 700, KDR 701, KDR 703, KDR 706, KDR 720, KDR C , KDR 730, KDR 731, KDR 733 Kappa 400 DR KDR 401, KDR 403 C1785 Selection AFm C1785 Diamond 3 DR 840 C1785 Diamond II DR 820E, 822E C1785 Kappa 650 DR KDR 651, KDR 653, KDR 656 C1785 Kappa 600 DR KDR 600, KDR 601, KDR 603, C1785 KDR 606 Vita 2 DR 830 C1785 Vita DR 810 C1785 SDR 303B, SDR Sigma 300 DR 303U, SDR 306U C1785 Sigma 200 DR SDR 203B C1785 Legacy II DR 828, 826 C1785 Clarity DR 860, 862, 865 C1785 Thera DR 7940, 7941, 7942, 7950, 7951, 7952, 7960i, C i, 7962i, 7964i, 7965i, 7966i, 7968i Preva DR 7088, 7089 C1785 Prodigy DR 7860, 7861, 7862 C1785 Legacy DR 850, 851, 852 C1785 Page 20 of 30
21 Cardiac Rhythm Management Therapies Pacemaker, Single Chamber, Rate-Responsive (Implantable) Product Brand Name and EnPulse E2SR01, E2SR03, E2SR06 C1786 Vitatron C-Series C20SR C1786 Kappa 900 SR KSR 901, KSR 903, KSR 906, C1786 KSR Kappa 700 SR KSR 700, KSR 701, KSR 703, C1786 KSR 706 Kappa 400 SR KSR 401, KSR 403 C1786 Topaz 3 SR 540 C1786 Topaz II SR 520, 521, 522 C1786 Sigma 300 SR SSR 303, SSR 303B, SSR C U, SSR 306U Vita 2 SR 530 C1786 Vita SR 310, 415 C1786 Sigma 200 SR SSR 203, SSR C B Legacy II SR 526, 528 C1786 Clarity SR 560, 562, 565 C1786 Prodigy SR 8158, 8160, 8161, 8162 C1786 Thera SR 8940, 8941, 8942, 8960i, C i, 8962i Preva SR 8088, 8089 C1786 Legacy SR 550, 551, 552 C1786 Page 21 of 30
22 Cardiac Rhythm Management Therapies Pacemaker, Dual Chamber, Non Rate-Responsive (Implantable) Product Brand Name and EnPulse E2VDD01 C2619 EnPulse D E2D01, E2D03, E2VDD01 C2619 Kappa 700 D KD 700, KD 701, KD 703, KD 706 C2619 Ruby 3 D Ruby 3 D C2619 Ruby II DDD 720 C2619 Sigma 300 D SD 303B C2619 Sigma 200 D SD 203B C2619 Legacy II D 728, 726 C2619 Thera D 7944, 7945, 7946, 7964i, C i, 7966i Preva D 7068 C2619 Legacy D 750, 751 C2619 Vita D 710 C2619 Prodigy D 7864, 7865, 7866 C2619 Kappa 900 VDD KVDD 901 C2619 KVDD 700, Kappa 700 VDD KVDD 701 C2619 Sigma 300 VDD SVDD 303 C2619 Thera VDD 8948, 8968i C2619 Prodigy VDD 8168 C2619 Vita VDD 410 C2619 Cardiac Rhythm Management Therapies Pacemaker, Single Chamber, Non Rate-Responsive (Implantable) Product Brand Name and Premier 8081 C2620 Sigma 300 S SS 303, SS 303B C2620 Jade 3 S 340 C2620 Jade II SSI 220 C2620 Sigma 200 S SS203 C2620 Legacy II S 126, 128 C2620 Sigma 100 S SS 103, SS 103B, SS 106U C2620 Thera S 8944, 8945, 8946, 8964i, C i, 8966i Prevail 8084, 8085, 8086 C2620 Legacy S 150, 151, 152 C2620 Prodigy S 8164, 8165, 8166 C2620 Page 22 of 30
23 Cardiac Rhythm Management Therapies Pacemaker, Other Than Single or Dual Chamber (Implantable) Product Brand Name and InSync III 8042 C2621 InSync 8040 C2621 Cardiac Rhythm Management Therapies Lead, Cardioverter Defibrillator, Endocardial Single Coil (Implantable) Product Brand Name and Sprint 6932, 6943 C1777 Fidelis 6930, 6931 C1777 Cardiac Rhythm Management Therapies Lead, Pacemaker, Transvenous VDD Single Pass Product Brand Name and CapSure VDD 5032, 5032L, 5032S, 5038, 5038L, 5038S C1779 Cardiac Rhythm Management Therapies Lead, Cardioverter Defibrillator, Endocardial Dual Coil (Implantable) Product Brand Name and Sprint Quattro Secure 6947 C1895 Sprint Quattro 6944 C1895 Sprint 6942, 6945 C1895 Fidelis 6948, 6949 C1895 Cardiac Rhythm Management Therapies Lead, Left Ventricular Coronary Venous System Product Brand Name and Attain OTW 4193 C1900 Attain LV 2187 C1900 Attain CS/CV 2188 C1900 Page 23 of 30
24 Cardiac Rhythm Management Therapies Lead, Cardioverter Defibrillator, Other Than Endocardial Single or Dual Coil (Implantable) Product Brand Name and 6996 SQ 6996 C1896 Transvene 6881, 6884, 6895, 6933, 6934S, C , 6937, 6939, 6963, 6966, 6999 Oval Patch Leads 6721L, 6721M, 6721S, 6939 C1896 CapSure Fix 6940 C1896 CapSure SP 5024M-110 C M, M, 5071 C1896 Cardiac Rhythm Management Therapies Lead, Pacemaker, Other Than Transvenous VDD Single Pass Product Brand Name and CapSure Sense 4074, 4574, 4073 C1898 Crystalline ICM 09B, ICM C JB, ICM 09 CapSure Z Novus 5054, 5554 C1898 CapSure Z Impulse II Impulse CapSure SP Novus CapSure SP CapSure Excellence + Excellence S+ Excellence PS+ 4033, 4034, 4533, 4534, 5033, C , 5534, 5554 IHP 09B, IHP 09JB C1898 IMG 49, IMG 49B, IMG 49JB C , 4592, 5092, 5592, 5594 C , 4024, 4523, 4524, 5023, 5023M, 5024, C M, 5523, 5523M, 5524, 5524M, , 4003M, 4004, 4004M, 4503, 4503M, C , 4504M, 5025, 5026, 5525 IMD 49, IMD 49B, IMD 49JB C1898 IME 49, IME 49B, IME 49JB C1898 IMK 49B, IMK 49JB C1898 Page 24 of 30
25 IML 49B, IML Excellence SS+ 49JB C1898 CapSureFix Novus 4076, 5076 C1898 CapSureFix 4067, 4068, 4568, 5058, 5067, C , 5568 (bipolar) SureFix 5072 C1898 Pirouet + IMU 49, IMU C B, IMU 49JB Pirouet S+ IMX 49, IMX 49B, IMX 49JB C1898 Crystalline ActFix ICF 09 C1898 CapSure Epi 4965, 4968 C M, 4058M, 4557M, 4558M, M, 4058M, 4557M, 4558M, 5058 C1898 Cardiac Rhythm Management Therapies Event Recorder, Cardiac (Implantable) Product Brand Name and Reveal, Reveal Plus 9525, 9526 C1764 Cardiac Rhythm Management Therapies Catheter, Electrophysiology, Diagnostic, Other Than 3D Mapping (19 or Fewer Electrodes) Product Brand Name and Torqr CS Torqr, Soloist Marinr CS Marinr EnCirclr AL 1045AL1, 1060AL1, 1045AL2, 1060AL2 C1730 C1730 C1730 C1730 C1730 Cardiac Rhythm Management Therapies Catheter, Electrophysiology, Diagnostic, Other Than 3D Mapping (20 or More Electrodes) Product Brand Name and Stable Mapr SM 04401SM47, 04402SM47 C1731 Page 25 of 30
26 Cardiac Rhythm Management Therapies Catheter, Electrophysiology, Diagnostic/Ablation, Other Than 3D or Vector Mapping, Other Than Cool Tip Product Brand Name and RF Enhancr RF Enhancr II RF Contactr RF Conductr RF Marinr MC 5F RF Marinr RF Performr , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , C1733 C1733 C1733 C1733 C1733 C1733 C1733 Page 26 of 30
27 Cardiac Rhythm Management Therapies Catheter, Guiding (may include infusion/perfusion capability) Product Brand Name and Attain Attain LDS Guide Catheter Attain Access Guide Catheter 6226DEF (includes guide wire), 6218A-AM, 6216A-MP, 6228CTH (includes guide wire) Item is a component of the Attain LDS Models 6216 and 6216A Item is a component of the Attain Access Models 6218 and 6218A C1887 C1887 C Guide Catheter 9210 C1887 Cardiac Rhythm Management Therapies Introducer/Sheath, Guiding, Intracardiac Electrophysiological, Fixed Curve, Peel-Away Product Brand Name and SafeSheath Worley SafeSheath Attain Sealing Adaptor Standard Wing SafeSheath Attain Sealing Adaptor Extended Wing C304-S59, C304- SelectSite L69 C1892 SafeSheath MultiSite (MSP) CSG/MSP C1892 CSF/Worley- 109M SS-SA-09MM SS-SA-EW- 09MM C1892 C1892 C1892 Cardiac Rhythm Management Therapies Introducer/Sheath, Guiding, Intracardiac Electrophysiological, Fixed Curve, Other Than Peel-Away Product Brand Name and Mullins , , , C1893 Page 27 of 30
28 Cardiac Rhythm Management Therapies Introducer/Sheath, Other Than Guiding, Intracardiac Electrophysiological, Non-Laser Product Brand Name and Attain LDS Introducer Introducer Item is a component of the Attain LDS Model S, 6208S, 6209S, 6210S, 6211S, 6212S, 6214S, 6207D, 6208D, 6209D, 6210D, 6211D, 6207BTK, 6208BTK, 6209BTK, 6210BTK, 6211BTK, 6212BTK, 6214BTK, 6207BTKD, 6208BTKD, 6209BTKD, 6210BTKD, 6211BTKD, HLS-1007M, HLS-1008M, HLS-1009M, HLS-10105M, HLS-1011M, HLS-2507, HLS-2508, HLS- 2509, HLS-25105, HLS-2511 C1894 C1894 Cardiac Rhythm Management Therapies Guide Wire Product Brand Name and Attain LDS Guide Wire Attain Access Item is a component of the Attain LDS Catheter Models 6216 and 6216A Item is a component of the Guide Catheter Attain Access Models 6218 and 6218A C1769 C1769 Page 28 of 30
29 Cardiac Rhythm Management Therapies Adaptor/Extension, Pacing Lead (Implantable) Product Brand Name and Adaptor 2872, M, , M, , M, M, M, 6707, 6726 C1883 See the appropriate technical manual for detailed information regarding indications, contraindictions, warnings, and precautions. Caution: Federal law (USA) restricts these devices to sale by or on the order of a physician. Reimbursement Assistance for: Cardiac Rhythm Management Therapies (800) x48892 Sara Mattson 8:00 am 5:00 pm Central Time - Monday through Friday Note: This information is not intended to make recommendations regarding clinical practice. It is the responsibility of the provider/facility to determine appropriate coding and to understand and follow local carrier/payer coverage and coding policies. Page 29 of 30
30 Xomed Therapies Ear, Nose and Throat/Ophthalmic Product Brand Name and EpiDisc Otologic Lamina C1763 Epifilm Otologic Lamina C1763 MeroGel Otologic Packing C1763 MeroGel Nasal & Sinus Packing C1763 MeroGel Nasal & Sinus Packing, Double Pack C1763 Netterville PhonoForm Silicone Block, Right C1878 Netterville PhonoForm Silicone Block, Left C1878 Netterville PhonoForm Silicone Block, Wedge C1878 GORE Thyroplasty Device.75cm x 40cm 1MDT201 C1878 GORE Thyroplasty Device.40cm x 20cm 1MDT202 C1878 GORE Thyroplasty Device.60cm x 20cm 1MDT203 C1878 Reimbursement Assistance for: Xomed Therapies (800) Kim Brew 8:00 am 5:00 pm Eastern Time - Monday through Friday Note: This information is not intended to make recommendations regarding clinical practice. It is the responsibility of the provider/facility to determine appropriate coding and to understand and follow local carrier/payer coverage and coding policies. Page 30 of 30
Cardiac Rhythm Disease Management Products
 Cardiac Rhythm Disease Management Products Medicare C-Code List for hospital outpatient device reporting Updated January 1, 2012 Documentation/Coding and Reimbursement Resources Cardiac Rhythm Disease
Cardiac Rhythm Disease Management Products Medicare C-Code List for hospital outpatient device reporting Updated January 1, 2012 Documentation/Coding and Reimbursement Resources Cardiac Rhythm Disease
 Epimed Would Like To Congratulate The 20th Annual Budapest Conference The New Shape of Pain Relief SmalleST. ThiNNeST. CoNTouRed design. Precision Novi is the world s smallest, thinnest 16 contact primary
Epimed Would Like To Congratulate The 20th Annual Budapest Conference The New Shape of Pain Relief SmalleST. ThiNNeST. CoNTouRed design. Precision Novi is the world s smallest, thinnest 16 contact primary
Physician Coding and Payment Guide 2015
 Targeted Drug Delivery Physician Coding and Payment Guide 2015 Flowonix Medical has compiled this coding information for your convenience. This information is gathered from third party sources and is subject
Targeted Drug Delivery Physician Coding and Payment Guide 2015 Flowonix Medical has compiled this coding information for your convenience. This information is gathered from third party sources and is subject
Ambulatory Surgery Center Coding and Payment Guide 2015
 Targeted Drug Delivery Ambulatory Surgery Center Coding and Payment Guide 2015 Flowonix Medical has compiled this coding information for your convenience. This information is gathered from third party
Targeted Drug Delivery Ambulatory Surgery Center Coding and Payment Guide 2015 Flowonix Medical has compiled this coding information for your convenience. This information is gathered from third party
Normal bladder function requires a coordinated effort between the brain, spinal cord, and the bladder.
 .. Urinary Incontinence Urinary incontinence is not an inevitable part of aging, and it is not a disease. The loss of bladder control - called urinary incontinence - affects between 13 and 17 million adult
.. Urinary Incontinence Urinary incontinence is not an inevitable part of aging, and it is not a disease. The loss of bladder control - called urinary incontinence - affects between 13 and 17 million adult
Christopher M. Wright, MD, MBA Pioneer Cardiovascular Consultants Tempe, Arizona
 Christopher M. Wright, MD, MBA Pioneer Cardiovascular Consultants Tempe, Arizona Areas to be covered Historical, current, and future treatments for various cardiovascular disease: Atherosclerosis (Coronary
Christopher M. Wright, MD, MBA Pioneer Cardiovascular Consultants Tempe, Arizona Areas to be covered Historical, current, and future treatments for various cardiovascular disease: Atherosclerosis (Coronary
Patient Information. PORT-A-CATH Implantable Venous Access Systems
 Patient Information PORT-A-CATH Implantable Venous Access Systems Your doctor has prescribed treatment that requires the frequent administration of medications or other fluids directly into your bloodstream
Patient Information PORT-A-CATH Implantable Venous Access Systems Your doctor has prescribed treatment that requires the frequent administration of medications or other fluids directly into your bloodstream
surg urin Surgery: Urinary System 1
 Surgery: Urinary System 1 This section contains information to assist providers in billing for surgical procedures related to the urinary system. Extracorporeal Shock Wave Lithotripsy Medi-Cal covers Extracorporeal
Surgery: Urinary System 1 This section contains information to assist providers in billing for surgical procedures related to the urinary system. Extracorporeal Shock Wave Lithotripsy Medi-Cal covers Extracorporeal
NeuroPace. RNS System Patient Manual
 NeuroPace RNS System Patient Manual CAUTION: Federal law restricts this device to sale by or on the order of a physician. 01/2013 DN 1013555 Rev 2 1 2013 NeuroPace, Inc. 2 This Manual supports: RNS Neurostimulator
NeuroPace RNS System Patient Manual CAUTION: Federal law restricts this device to sale by or on the order of a physician. 01/2013 DN 1013555 Rev 2 1 2013 NeuroPace, Inc. 2 This Manual supports: RNS Neurostimulator
Ch. 138 CARDIAC CATHETERIZATION SERVICES 28 138.1 CHAPTER 138. CARDIAC CATHETERIZATION SERVICES GENERAL PROVISIONS
 Ch. 138 CARDIAC CATHETERIZATION SERVICES 28 138.1 CHAPTER 138. CARDIAC CATHETERIZATION SERVICES Sec. 138.1 Principle. 138.2. Definitions. GENERAL PROVISIONS PROGRAM, SERVICE, PERSONNEL AND AGREEMENT REQUIREMENTS
Ch. 138 CARDIAC CATHETERIZATION SERVICES 28 138.1 CHAPTER 138. CARDIAC CATHETERIZATION SERVICES Sec. 138.1 Principle. 138.2. Definitions. GENERAL PROVISIONS PROGRAM, SERVICE, PERSONNEL AND AGREEMENT REQUIREMENTS
Instructions for Use. Device Description The AMPLATZER Vascular Plug II is a self-expandable nitinol mesh occlusion device (see Figure 1).
 Vascular Plug II Instructions for Use Device Description The AMPLATZER Vascular Plug II is a self-expandable nitinol mesh occlusion device (see Figure 1). A B Figure 1. AMPLATZER Vascular Plug II A. Nitinol
Vascular Plug II Instructions for Use Device Description The AMPLATZER Vascular Plug II is a self-expandable nitinol mesh occlusion device (see Figure 1). A B Figure 1. AMPLATZER Vascular Plug II A. Nitinol
Refer to Coaptite Injectable Implant Instructions for Use provided with product for complete instructions for use.
 Questions for my Doctor Refer to Coaptite Injectable Implant Instructions for Use provided with product for complete instructions for use. INDICATIONS: Coaptite Injectable Implant is indicated for soft
Questions for my Doctor Refer to Coaptite Injectable Implant Instructions for Use provided with product for complete instructions for use. INDICATIONS: Coaptite Injectable Implant is indicated for soft
Model 8709SC a n d model 8731sc Intrathecal Catheters
 Model 8709SC a n d model 8731sc Intrathecal Catheters Low Complication Catheter Implant Technique * Source: Follett KA, Burchiel K, Deer T, et al. Prevention of intrathecal drug delivery catheter-related
Model 8709SC a n d model 8731sc Intrathecal Catheters Low Complication Catheter Implant Technique * Source: Follett KA, Burchiel K, Deer T, et al. Prevention of intrathecal drug delivery catheter-related
URINARY INCONTINENCE
 URINARY INCONTINENCE What is urinary incontinence? Urinary incontinence is the uncontrollable loss of urine. The amount of urine leaked can vary from only a few drops when you cough or sneeze to entirely
URINARY INCONTINENCE What is urinary incontinence? Urinary incontinence is the uncontrollable loss of urine. The amount of urine leaked can vary from only a few drops when you cough or sneeze to entirely
Intrathecal Baclofen for CNS Spasticity
 Intrathecal Baclofen for CNS Spasticity Last Review Date: November 13, 2015 Number: MG.MM.ME.31bC5 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Intrathecal Baclofen for CNS Spasticity Last Review Date: November 13, 2015 Number: MG.MM.ME.31bC5 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Introduction. Planned surgical procedures
 Guidelines for the perioperative management of patients with implantable pacemakers or implantable cardioverter defibrillators, where the use of surgical diathermy/electrocautery is anticipated. Introduction
Guidelines for the perioperative management of patients with implantable pacemakers or implantable cardioverter defibrillators, where the use of surgical diathermy/electrocautery is anticipated. Introduction
CODING SHEETS CHRONIC INTRACTABLE SPASTICITY. Effective January 1, 2009 CODMAN 3000 NEUROMODULATION AND ONCOLOGY REIMBURSEMENT HOTLINE
 CODING SHEETS CHRONIC INTRACTABLE SPASTICITY Effective January 1, 2009 CODMAN 3000 NEUROMODULATION AND ONCOLOGY REIMBURSEMENT HOTLINE Phone: 800-609-1108 Email: [email protected] Fax: 303-703-1572 CODMAN
CODING SHEETS CHRONIC INTRACTABLE SPASTICITY Effective January 1, 2009 CODMAN 3000 NEUROMODULATION AND ONCOLOGY REIMBURSEMENT HOTLINE Phone: 800-609-1108 Email: [email protected] Fax: 303-703-1572 CODMAN
CODING SHEETS CHRONIC INTRACTABLE PAIN MANAGEMENT. Effective January 1, 2011 CODMAN 3000 NEUROMODULATION AND ONCOLOGY REIMBURSEMENT HOTLINE
 CODING SHEETS CHRONIC INTRACTABLE PAIN MANAGEMENT Effective January 1, 2011 CODMAN 3000 NEUROMODULATION AND ONCOLOGY REIMBURSEMENT HOTLINE Phone: 800-609-1108 Email: [email protected] Fax: 303-703-1572
CODING SHEETS CHRONIC INTRACTABLE PAIN MANAGEMENT Effective January 1, 2011 CODMAN 3000 NEUROMODULATION AND ONCOLOGY REIMBURSEMENT HOTLINE Phone: 800-609-1108 Email: [email protected] Fax: 303-703-1572
3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP
 PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
Aspira* Peritoneal Drainage Catheter
 Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Reimbursement Information For Electrophysiology and Arrhythmia Service Procedures 1
 GE Healthcare Information For Electrophysiology and Arrhythmia Procedures 1 2011 Update www.gehealthcare.com/reimbursement This overview addresses coding, coverage, and payment for electrophysiology procedures
GE Healthcare Information For Electrophysiology and Arrhythmia Procedures 1 2011 Update www.gehealthcare.com/reimbursement This overview addresses coding, coverage, and payment for electrophysiology procedures
MRI Case Study MRI Cervical Spine
 MRI Case Study MRI Cervical Spine Jewish Hospital Louisville, KY The Revo MRI SureScan pacing system is MR Conditional designed to allow patients to undergo MRI under the specified conditions for use.
MRI Case Study MRI Cervical Spine Jewish Hospital Louisville, KY The Revo MRI SureScan pacing system is MR Conditional designed to allow patients to undergo MRI under the specified conditions for use.
INSERTABLE CARDIAC MONITORING SYSTEM. UNLOCK the ANSWER. Your heart and long-term monitoring
 INSERTABLE CARDIAC MONITORING SYSTEM UNLOCK the ANSWER Your heart and long-term monitoring UNLOCK the ANSWER Irregular heartbeats can be related to a variety of conditions, including unexplained fainting,
INSERTABLE CARDIAC MONITORING SYSTEM UNLOCK the ANSWER Your heart and long-term monitoring UNLOCK the ANSWER Irregular heartbeats can be related to a variety of conditions, including unexplained fainting,
X-Plain Trigeminal Neuralgia Reference Summary
 X-Plain Trigeminal Neuralgia Reference Summary Introduction Trigeminal neuralgia is a condition that affects about 40,000 patients in the US every year. Its treatment mostly involves the usage of oral
X-Plain Trigeminal Neuralgia Reference Summary Introduction Trigeminal neuralgia is a condition that affects about 40,000 patients in the US every year. Its treatment mostly involves the usage of oral
Management of Pacing Wires After Cardiac Surgery
 Management of Pacing Wires After Cardiac Surgery David E. Lizotte, Jr. PA C, MPAS, FAPACVS President, Association of Physician Assistants in Cardiovascular Surgery Conflicts: None Indications 2008 Journal
Management of Pacing Wires After Cardiac Surgery David E. Lizotte, Jr. PA C, MPAS, FAPACVS President, Association of Physician Assistants in Cardiovascular Surgery Conflicts: None Indications 2008 Journal
What is Multiple Sclerosis? Gener al information
 What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
PERIPHERALLY INSERTED CENTRAL CATHETERS (PICC) Fong So Kwan APN, Haematology unit Medical Department, QMH
 PERIPHERALLY INSERTED CENTRAL CATHETERS (PICC) Fong So Kwan APN, Haematology unit Medical Department, QMH 1 What is a PICC catheter? Primary vascular access device since their introduction in the mid-1970s,
PERIPHERALLY INSERTED CENTRAL CATHETERS (PICC) Fong So Kwan APN, Haematology unit Medical Department, QMH 1 What is a PICC catheter? Primary vascular access device since their introduction in the mid-1970s,
Neurostimulation: Orthopaedic Institute of Ohio 801 Medical Drive Lima, Ohio 45804 419-222-6622
 801 Medical Drive Lima, Ohio 45804 419-222-6622 Neurostimulation is the stimulation of the spinal cord by tiny electrical impulses. An implanted lead (a flexible insulated wire), which is powered by an
801 Medical Drive Lima, Ohio 45804 419-222-6622 Neurostimulation is the stimulation of the spinal cord by tiny electrical impulses. An implanted lead (a flexible insulated wire), which is powered by an
LifeStent XL Biliary Stent System
 B05688 Rev.2/07-13 LifeStent XL Biliary Stent System Recommended Guidewire Length Table Catheter Working Length Recommended Guidewire Length 130 cm 300 cm 80 cm 260 cm 130 cm & 80 cm 160 cm & 110 cm Figure
B05688 Rev.2/07-13 LifeStent XL Biliary Stent System Recommended Guidewire Length Table Catheter Working Length Recommended Guidewire Length 130 cm 300 cm 80 cm 260 cm 130 cm & 80 cm 160 cm & 110 cm Figure
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: spinal_cord_stimulation 3/1980 10/2015 10/2016 10/2015 Description of Procedure or Service Spinal cord stimulation
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: spinal_cord_stimulation 3/1980 10/2015 10/2016 10/2015 Description of Procedure or Service Spinal cord stimulation
Transforming Healthcare. Transforming Lives. Company Overview
 Transforming Healthcare. Transforming Lives. Company Overview Kobi Graham, Australia One of the more than 9 million people transformed by a Medtronic product or therapy. Innovating for life. The Medtronic
Transforming Healthcare. Transforming Lives. Company Overview Kobi Graham, Australia One of the more than 9 million people transformed by a Medtronic product or therapy. Innovating for life. The Medtronic
ON-Q * Catheters and Introducers
 Instructions For Use ON-Q * Catheters and Introducers MANUFACTURED BY: Kimberly-Clark 1400 Holcomb Bridge Road Roswell, GA 30076 USA Kimberly-Clark N.V. Da Vincilaan 1 1935 Zaventem, Belgium Figure 1 4
Instructions For Use ON-Q * Catheters and Introducers MANUFACTURED BY: Kimberly-Clark 1400 Holcomb Bridge Road Roswell, GA 30076 USA Kimberly-Clark N.V. Da Vincilaan 1 1935 Zaventem, Belgium Figure 1 4
Living with Your Pacemaker
 Brief Statement Additional Device Information An implantable pacemaker system relieves symptoms of heart rhythm disturbances. They do this by restoring normal heart rates. A normal heart rate provides
Brief Statement Additional Device Information An implantable pacemaker system relieves symptoms of heart rhythm disturbances. They do this by restoring normal heart rates. A normal heart rate provides
PATIENT INFORMATION BOOKLET
 PATIENT INFORMATION BOOKLET Wingspan Stent System with Gateway PTA Balloon Catheter TABLE OF CONTENTS Definitions... 2 What is the Purpose of This Booklet?... 3 What is an Intracranial Lesion?... 3 Who
PATIENT INFORMATION BOOKLET Wingspan Stent System with Gateway PTA Balloon Catheter TABLE OF CONTENTS Definitions... 2 What is the Purpose of This Booklet?... 3 What is an Intracranial Lesion?... 3 Who
Emergency Insertion of Temporary Trans-venous Pacing Catheter in ICU
 TARGET AUDIENCE Junior Medical Staff Critical Care Trained ICU Nurses PURPOSE This document is intended as a basic guide to managing patients who require insertion of Temporary Trans-venous Cardiac Pacing
TARGET AUDIENCE Junior Medical Staff Critical Care Trained ICU Nurses PURPOSE This document is intended as a basic guide to managing patients who require insertion of Temporary Trans-venous Cardiac Pacing
A4.7 Management of a totally occluded central catheter and persistent withdrawal occlusion (PWO)
 A4.7 Management of a totally occluded central catheter and persistent withdrawal occlusion (PWO) Types of Catheter Related Thrombotic A catheter-related thrombus may be intraluminal (inside the catheter)
A4.7 Management of a totally occluded central catheter and persistent withdrawal occlusion (PWO) Types of Catheter Related Thrombotic A catheter-related thrombus may be intraluminal (inside the catheter)
2014 Procedural Reimbursement Guide Select Percutaneous Coronary Interventions
 2014 Procedural Reimbursement Guide Select Percutaneous Coronary Interventions IC-221010-AA Jan 2014 Page 1 of 10 Interventional Cardiology This for interventional cardiology procedures provides coding
2014 Procedural Reimbursement Guide Select Percutaneous Coronary Interventions IC-221010-AA Jan 2014 Page 1 of 10 Interventional Cardiology This for interventional cardiology procedures provides coding
When Procedural Support really matters. Navien TM A+ Intracranial Support Catheter
 When Procedural Support really matters Navien TM A+ Intracranial Support Catheter Coils / Liquid Embolics DURABLE ovalization resistance, Maintains patent lumen in tortuosity for smooth micro catheter
When Procedural Support really matters Navien TM A+ Intracranial Support Catheter Coils / Liquid Embolics DURABLE ovalization resistance, Maintains patent lumen in tortuosity for smooth micro catheter
FAQ About Prostate Cancer Treatment and SpaceOAR System
 FAQ About Prostate Cancer Treatment and SpaceOAR System P. 4 Prostate Cancer Background SpaceOAR Frequently Asked Questions (FAQ) 1. What is prostate cancer? The vast majority of prostate cancers develop
FAQ About Prostate Cancer Treatment and SpaceOAR System P. 4 Prostate Cancer Background SpaceOAR Frequently Asked Questions (FAQ) 1. What is prostate cancer? The vast majority of prostate cancers develop
The science of medicine. The compassion to heal.
 A PATIENT S GUIDE TO ELECTROPHYSIOLOGY STUDIES OF THE HEART The science of medicine. The compassion to heal. This teaching booklet is designed to introduce you to electrophysiology studies of the heart.
A PATIENT S GUIDE TO ELECTROPHYSIOLOGY STUDIES OF THE HEART The science of medicine. The compassion to heal. This teaching booklet is designed to introduce you to electrophysiology studies of the heart.
Bard: Intermittent Catheters. A guide to. Bard: Pelvic Organ Prolapse. An REIMBURSEMENT. overview of OF INTERMITTENT. Prolapse CATHETERS
 Bard: Intermittent Catheters A guide to Bard: Pelvic Organ Prolapse An REIMBURSEMENT overview of Pelvic OF INTERMITTENT Organ Prolapse CATHETERS 1 Intermittent catheterization is a covered Medicare benefit
Bard: Intermittent Catheters A guide to Bard: Pelvic Organ Prolapse An REIMBURSEMENT overview of Pelvic OF INTERMITTENT Organ Prolapse CATHETERS 1 Intermittent catheterization is a covered Medicare benefit
InterStim CONSULTATION INFORMATION. Round Rock: 512-828-5522 North Austin: 512-231-1444 Westlake: 512-328-1313 www.urologyteam.com
 InterStim CONSULTATION INFORMATION Round Rock: 512-828-5522 North Austin: 512-231-1444 Westlake: 512-328-1313 www.urologyteam.com TABLE OF CONTENTS Section 1: Providers Dr. Melody Denson.p. 1 Dr. Bryan
InterStim CONSULTATION INFORMATION Round Rock: 512-828-5522 North Austin: 512-231-1444 Westlake: 512-328-1313 www.urologyteam.com TABLE OF CONTENTS Section 1: Providers Dr. Melody Denson.p. 1 Dr. Bryan
Within the Scope of Practice/Role of APRN RN _ X_LPN CNA
 Wyoming State Board of Nursing 130 Hobbs Avenue, Suite B Cheyenne, WY 82002 Phone (307) 777-7601 Fax (307) 777-3519 E-Mail: [email protected] Home Page: https://nursing-online.state.wy.us/ OPINION:
Wyoming State Board of Nursing 130 Hobbs Avenue, Suite B Cheyenne, WY 82002 Phone (307) 777-7601 Fax (307) 777-3519 E-Mail: [email protected] Home Page: https://nursing-online.state.wy.us/ OPINION:
Background. Rhythm Management. 2015 Billing and Coding Guide
 8 C-Codes Cardiac Rhythm Management Category Codes (C-Codes) for Medical Devices 8-2 Electrophysiology Category Codes (C-Codes) for Medical Devices 8-9 Background Ambulatory Payment Classifications (APC)
8 C-Codes Cardiac Rhythm Management Category Codes (C-Codes) for Medical Devices 8-2 Electrophysiology Category Codes (C-Codes) for Medical Devices 8-9 Background Ambulatory Payment Classifications (APC)
Scout Vessel Guard. A cover for vessels during anterior lumbar spine surgery.
 Scout Vessel Guard. A cover for vessels during anterior lumbar spine surgery. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Scout Vessel Guard 2
Scout Vessel Guard. A cover for vessels during anterior lumbar spine surgery. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Scout Vessel Guard 2
Spinal cord stimulation
 Spinal cord stimulation This leaflet aims to answer your questions about having spinal cord stimulation. It explains the benefits, risks and alternatives, as well as what you can expect when you come to
Spinal cord stimulation This leaflet aims to answer your questions about having spinal cord stimulation. It explains the benefits, risks and alternatives, as well as what you can expect when you come to
SUTTER MEDICAL CENTER, SACRAMENTO Department of Cardiovascular Disease Cardiology - Delineation of Privileges
 INITIAL: [ ] RENEWED: [ ] DATE: ADDITIONAL: [ ] Privileges are granted for Sutter General Hospital, Sutter Memorial Hospital, Sutter Center for Psychiatry, Sutter Oaks Midtown and the Capitol Pavilion
INITIAL: [ ] RENEWED: [ ] DATE: ADDITIONAL: [ ] Privileges are granted for Sutter General Hospital, Sutter Memorial Hospital, Sutter Center for Psychiatry, Sutter Oaks Midtown and the Capitol Pavilion
NOVOSTE BETA-CATH SYSTEM
 HOSPITAL INPATIENT AND OUTPATIENT BILLING GUIDE FOR THE NOVOSTE BETA-CATH SYSTEM INTRAVASCULAR BRACHYTHERAPY DEVICE This guide is intended solely for use as a tool to help hospital billing staff resolve
HOSPITAL INPATIENT AND OUTPATIENT BILLING GUIDE FOR THE NOVOSTE BETA-CATH SYSTEM INTRAVASCULAR BRACHYTHERAPY DEVICE This guide is intended solely for use as a tool to help hospital billing staff resolve
LOSS OF BLADDER CONTROL IS TREATABLE TAKE CONTROL AND RESTORE YOUR LIFESTYLE
 LOSS OF BLADDER CONTROL IS TREATABLE TAKE CONTROL AND RESTORE YOUR LIFESTYLE TALKING ABOUT STRESS INCONTINENCE (SUI) Millions of women suffer from stress incontinence (SUI). This condition results in accidental
LOSS OF BLADDER CONTROL IS TREATABLE TAKE CONTROL AND RESTORE YOUR LIFESTYLE TALKING ABOUT STRESS INCONTINENCE (SUI) Millions of women suffer from stress incontinence (SUI). This condition results in accidental
Bladder Health Promotion
 Bladder Health Promotion Community Awareness Presentation Content contributions provided by the Society of Urologic Nurses (SUNA) National Association for Continence (NAFC) Simon Foundation for Continence
Bladder Health Promotion Community Awareness Presentation Content contributions provided by the Society of Urologic Nurses (SUNA) National Association for Continence (NAFC) Simon Foundation for Continence
What Are Arrhythmias?
 What Are Arrhythmias? Many people have questions about what the word arrhythmia means, and arrhythmias can be a difficult subject to understand. The text below should give you a better understanding of
What Are Arrhythmias? Many people have questions about what the word arrhythmia means, and arrhythmias can be a difficult subject to understand. The text below should give you a better understanding of
Aspira* Pleural Drainage Catheter
 Aspira* Pleural Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Pleural Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid from the
Aspira* Pleural Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Pleural Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid from the
Urinary Diversion: Ileovesicostomy/Ileal Loop/Colon Loop
 Urinary Diversion: Ileovesicostomy/Ileal Loop/Colon Loop Why do I need this surgery? A urinary diversion is a surgical procedure that is performed to allow urine to safely pass from the kidneys into a
Urinary Diversion: Ileovesicostomy/Ileal Loop/Colon Loop Why do I need this surgery? A urinary diversion is a surgical procedure that is performed to allow urine to safely pass from the kidneys into a
1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System
 1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System NEVRO CORP. All questions or concerns about Nevro products should be forwarded to: Nevro Corp. 1800 Bridge Parkway
1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System NEVRO CORP. All questions or concerns about Nevro products should be forwarded to: Nevro Corp. 1800 Bridge Parkway
Complete Guide for Interventional Radiology
 2013 Complete Guide for Interventional Radiology Contents Introduction... 1 CPT Codes and Descriptions...1 Procedure Codes...2 Chapter 1: The Basics... 5 APC Basics Why Is This Important?...5 CCI Edits
2013 Complete Guide for Interventional Radiology Contents Introduction... 1 CPT Codes and Descriptions...1 Procedure Codes...2 Chapter 1: The Basics... 5 APC Basics Why Is This Important?...5 CCI Edits
Overactive Bladder (OAB)
 Overactive Bladder (OAB) Overactive bladder is a problem with bladder storage function that causes a sudden urge to urinate. The urge may be difficult to suppress, and overactive bladder can lead to the
Overactive Bladder (OAB) Overactive bladder is a problem with bladder storage function that causes a sudden urge to urinate. The urge may be difficult to suppress, and overactive bladder can lead to the
CARDIOLOGY Delineation of Privileges
 CARDIOLOGY Delineation of Privileges APPLICANT: INITIAL APPOINTMENT REQUIREMENTS: BASIC EDUCATION: M.D. or D.O. from an accredited school of medicine or osteopathy. Successful completion of an ACGME or
CARDIOLOGY Delineation of Privileges APPLICANT: INITIAL APPOINTMENT REQUIREMENTS: BASIC EDUCATION: M.D. or D.O. from an accredited school of medicine or osteopathy. Successful completion of an ACGME or
Patient Information Guide Morpheus CT Peripherally Inserted Central Catheter
 Patient Information Guide Morpheus CT Peripherally Inserted Central Catheter IC 192 Rev C A measure of flexibility and strength. Table of Contents 1. Introduction 2. What is the Morpheus CT PICC? 3. What
Patient Information Guide Morpheus CT Peripherally Inserted Central Catheter IC 192 Rev C A measure of flexibility and strength. Table of Contents 1. Introduction 2. What is the Morpheus CT PICC? 3. What
A Guide for Patients Living with a Biliary Metal Stent
 A Guide for Patients Living with a Biliary Metal Stent What is a biliary metal stent? A biliary metal stent (also known as a bile duct stent ) is a flexible metallic tube specially designed to hold your
A Guide for Patients Living with a Biliary Metal Stent What is a biliary metal stent? A biliary metal stent (also known as a bile duct stent ) is a flexible metallic tube specially designed to hold your
Talent Thoracic Stent Graft with THE Xcelerant Delivery System. Expanding the Indications for TEVAR
 Talent Thoracic with THE Xcelerant Delivery System Expanding the Indications for TEVAR Talent Thoracic Precise placement 1 Broad patient applicability 1 Excellent clinical outcomes 1, a + Xcelerant Delivery
Talent Thoracic with THE Xcelerant Delivery System Expanding the Indications for TEVAR Talent Thoracic Precise placement 1 Broad patient applicability 1 Excellent clinical outcomes 1, a + Xcelerant Delivery
Department of Neurosciences Dorsal Root Ganglion (DRG) Stimulation Information for patients
 Oxford University Hospitals NHS Trust Department of Neurosciences Dorsal Root Ganglion (DRG) Stimulation Information for patients We have recently seen you in clinic as you have had pain for a long period
Oxford University Hospitals NHS Trust Department of Neurosciences Dorsal Root Ganglion (DRG) Stimulation Information for patients We have recently seen you in clinic as you have had pain for a long period
Spinal Cord and Bladder Management Male: Intermittent Catheter
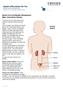 Spinal Cord and Bladder Management Male: Intermittent Catheter The 5 parts of the urinary system work together to get rid of waste and make urine. Urine is made in your kidneys and travels down 2 thin
Spinal Cord and Bladder Management Male: Intermittent Catheter The 5 parts of the urinary system work together to get rid of waste and make urine. Urine is made in your kidneys and travels down 2 thin
Bard LifeStar Biliary Stent System
 Bard LifeStar Biliary Stent System Instructions For Use (IFU) Bard LifeStar Biliary Stent System Delivery System Diagram B05686 Vers.1/08-11 1 Information for use Read the Bard LifeStar Biliary Stent System
Bard LifeStar Biliary Stent System Instructions For Use (IFU) Bard LifeStar Biliary Stent System Delivery System Diagram B05686 Vers.1/08-11 1 Information for use Read the Bard LifeStar Biliary Stent System
Baxter Elastomeric Pumps CLINICIAN GUIDE
 Baxter Elastomeric Pumps CLINICIAN GUIDE Portfolio Overview: Baxter Elastomeric Pumps are non-electronic medication pumps designed to provide ambulatory infusion therapy. Medication is delivered to the
Baxter Elastomeric Pumps CLINICIAN GUIDE Portfolio Overview: Baxter Elastomeric Pumps are non-electronic medication pumps designed to provide ambulatory infusion therapy. Medication is delivered to the
Empire BlueCross BlueShield Professional Reimbursement Policy
 Pressional Reimbursement Policy Subject: Bundled Services and Supplies NY Policy: 0008 Effective: 01/01/2014-02/23/2014 Coverage is subject to the terms, conditions, and limitations an individual member
Pressional Reimbursement Policy Subject: Bundled Services and Supplies NY Policy: 0008 Effective: 01/01/2014-02/23/2014 Coverage is subject to the terms, conditions, and limitations an individual member
PHENYLEPHRINE HYDROCHLORIDE INJECTION USP
 PRESCRIBING INFORMATION PHENYLEPHRINE HYDROCHLORIDE INJECTION USP 10 mg/ml Sandoz Canada Inc. Date of Preparation: September 1992 145 Jules-Léger Date of Revision : January 13, 2011 Boucherville, QC, Canada
PRESCRIBING INFORMATION PHENYLEPHRINE HYDROCHLORIDE INJECTION USP 10 mg/ml Sandoz Canada Inc. Date of Preparation: September 1992 145 Jules-Léger Date of Revision : January 13, 2011 Boucherville, QC, Canada
URINARY CATHETER CARE
 URINARY CATHETER CARE INTRODUCTION Urinary catheter care is a very important skill, and it is a skill that many certified nursing assistants (CNAs) must know. Competence at providing urinary catheter care
URINARY CATHETER CARE INTRODUCTION Urinary catheter care is a very important skill, and it is a skill that many certified nursing assistants (CNAs) must know. Competence at providing urinary catheter care
Understanding your Renal Stent Procedure. A patient Guide (COVER PAGE) TABLE OF CONTENTS (inside front page)
 Understanding your Renal Stent Procedure. A patient Guide (COVER PAGE) TABLE OF CONTENTS (inside front page) The Kidney and the Renal Arteries... 1 Renal Artery Disease... 2 Diagnosis of Renal.Artery Disease...
Understanding your Renal Stent Procedure. A patient Guide (COVER PAGE) TABLE OF CONTENTS (inside front page) The Kidney and the Renal Arteries... 1 Renal Artery Disease... 2 Diagnosis of Renal.Artery Disease...
NeuroStar TMS Therapy Patient Guide for Treating Depression
 NeuroStar TMS Therapy Patient Guide for Treating Depression This NeuroStar TMS Therapy Patient Guide for Treating Depression provides important safety and use information for you to consider about treating
NeuroStar TMS Therapy Patient Guide for Treating Depression This NeuroStar TMS Therapy Patient Guide for Treating Depression provides important safety and use information for you to consider about treating
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: infusion_therapy_in_the_home 3/1998 2/2016 2/2017 2/2016 Description of Procedure or Service Home infusion
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: infusion_therapy_in_the_home 3/1998 2/2016 2/2017 2/2016 Description of Procedure or Service Home infusion
VUMC Guidelines for Management of Indwelling Urinary Catheters. UC Access/ Maintenance
 VUMC Guidelines for Management of Indwelling Urinary Catheters UC Insertion Preparation & Procedure Indications for insertion and continued use of indwelling urinary catheters include: Urinary retention
VUMC Guidelines for Management of Indwelling Urinary Catheters UC Insertion Preparation & Procedure Indications for insertion and continued use of indwelling urinary catheters include: Urinary retention
Kaiser Oakland Urology
 Kaiser Oakland Urology The Main Purpose of Bladder Catheterization Complete Bladder Emptying! Help maintain a healthy bladder Help maintain healthy kidneys Reduce the chances of significant urinary tract
Kaiser Oakland Urology The Main Purpose of Bladder Catheterization Complete Bladder Emptying! Help maintain a healthy bladder Help maintain healthy kidneys Reduce the chances of significant urinary tract
Subject: Bundled Services and Supplies
 Blue Cross and Blue Shield of Georgia Inc, and Blue Cross Blue Shield Healthcare Plan of Georgia, Inc. (hereinafter referred to collectively as BCBSGA ) Professional Reimbursement Policy Subject: Bundled
Blue Cross and Blue Shield of Georgia Inc, and Blue Cross Blue Shield Healthcare Plan of Georgia, Inc. (hereinafter referred to collectively as BCBSGA ) Professional Reimbursement Policy Subject: Bundled
Patient Information Booklet. Endovascular Stent Grafts: A Treatment for Abdominal Aortic Aneurysms
 Patient Information Booklet Endovascular Stent Grafts: A Treatment for Abdominal Aortic Aneurysms TABLE OF CONTENTS Introduction 1 Glossary 2 Abdominal Aorta 4 Abdominal Aortic Aneurysm 5 Causes 6 Symptoms
Patient Information Booklet Endovascular Stent Grafts: A Treatment for Abdominal Aortic Aneurysms TABLE OF CONTENTS Introduction 1 Glossary 2 Abdominal Aorta 4 Abdominal Aortic Aneurysm 5 Causes 6 Symptoms
Ureteral Stenting and Nephrostomy
 Scan for mobile link. Ureteral Stenting and Nephrostomy Ureteral stenting and nephrostomy help restore urine flow through blocked ureters and return the kidney to normal function. Ureters are long, narrow
Scan for mobile link. Ureteral Stenting and Nephrostomy Ureteral stenting and nephrostomy help restore urine flow through blocked ureters and return the kidney to normal function. Ureters are long, narrow
150640_Brochure_B 4/12/07 2:58 PM Page 2. Patient Information. Freedom From an Enlarged Prostate
 150640_Brochure_B 4/12/07 2:58 PM Page 2 Patient Information Freedom From an Enlarged Prostate 150640_Brochure_B 4/12/07 2:58 PM Page 3 GreenLight Laser Therapy 1 150640_Brochure_B 4/12/07 2:58 PM Page
150640_Brochure_B 4/12/07 2:58 PM Page 2 Patient Information Freedom From an Enlarged Prostate 150640_Brochure_B 4/12/07 2:58 PM Page 3 GreenLight Laser Therapy 1 150640_Brochure_B 4/12/07 2:58 PM Page
0.9% Sodium Chloride injection may be used in most cases.
 Table 2. Alternatives to Heparin Sodium in Selected Situations 12-14 Situation Alternative Dose Maintain patency of peripheral venous catheters* 21-26 0.9% Sodium Chloride injection may be used in most
Table 2. Alternatives to Heparin Sodium in Selected Situations 12-14 Situation Alternative Dose Maintain patency of peripheral venous catheters* 21-26 0.9% Sodium Chloride injection may be used in most
A Patient s Guide to Minimally Invasive Abdominal Aortic Aneurysm Repair
 A Patient s Guide to Minimally Invasive Abdominal Aortic Aneurysm Repair Table of Contents The AFX Endovascular AAA System............................................ 1 What is an Abdominal Aortic Aneurysm
A Patient s Guide to Minimally Invasive Abdominal Aortic Aneurysm Repair Table of Contents The AFX Endovascular AAA System............................................ 1 What is an Abdominal Aortic Aneurysm
Coding for Ancillary Services and Providers
 Coding for Ancillary Services and Providers Joanne Mehmert, CPC Joanne Mehmert & Associates, LLC 816-436-4271 [email protected] 1 Today s Discussion Points Billing/payment for supplies New and revised HCPCS
Coding for Ancillary Services and Providers Joanne Mehmert, CPC Joanne Mehmert & Associates, LLC 816-436-4271 [email protected] 1 Today s Discussion Points Billing/payment for supplies New and revised HCPCS
Lumbar Laminectomy and Interspinous Process Fusion
 Lumbar Laminectomy and Interspinous Process Fusion Introduction Low back and leg pain caused by pinched nerves in the back is a common condition that limits your ability to move, walk, and work. This condition
Lumbar Laminectomy and Interspinous Process Fusion Introduction Low back and leg pain caused by pinched nerves in the back is a common condition that limits your ability to move, walk, and work. This condition
Urinary Incontinence FAQ Sheet
 Urinary Incontinence FAQ Sheet Are you reluctant to talk to your doctor about your bladder control problem? Don t be. There is help. Loss of bladder control is called urinary incontinence. It can happen
Urinary Incontinence FAQ Sheet Are you reluctant to talk to your doctor about your bladder control problem? Don t be. There is help. Loss of bladder control is called urinary incontinence. It can happen
Suffering from varicose veins? Patient Information. ELVeS Radial Minimally invasive laser therapy of venous insufficiency
 Suffering from varicose veins? Patient Information ELVeS Radial Minimally invasive laser therapy of venous insufficiency Do you suffer from heavy legs or visible veins? This makes diseases of the veins
Suffering from varicose veins? Patient Information ELVeS Radial Minimally invasive laser therapy of venous insufficiency Do you suffer from heavy legs or visible veins? This makes diseases of the veins
Automatic External Defibrillators
 Last Review Date: May 27, 2016 Number: MG.MM.DM.10dC2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: May 27, 2016 Number: MG.MM.DM.10dC2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Vaxcel PICCs Valved and Non-Valved. A Patient s Guide
 Vaxcel PICCs Valved and Non-Valved A Patient s Guide Information about your Vaxcel PICC is available by calling the Navilyst Medical Vascular Access Information Line 800.513.6876 Vaxcel Peripherally Inserted
Vaxcel PICCs Valved and Non-Valved A Patient s Guide Information about your Vaxcel PICC is available by calling the Navilyst Medical Vascular Access Information Line 800.513.6876 Vaxcel Peripherally Inserted
Pacemaker Counter and Histogram Operation and Interpretation. Percentage of total count. Figure 2. Detailed ALTRUA Paced and Sensed Event Counters
 A Closer Look SUMMARY This article provides information regarding the operation and interpretation of Event Counters and Histograms available in ALTRUA, INSIGNIA, PULSAR MAX II, and DISCOVERY II pacemakers.
A Closer Look SUMMARY This article provides information regarding the operation and interpretation of Event Counters and Histograms available in ALTRUA, INSIGNIA, PULSAR MAX II, and DISCOVERY II pacemakers.
OVER 45 YEARS TEXTILE GRAFT TECHNOLOGY EXPERIENCE MAQUET THE GOLD STANDARD
 OVER 45 YEARS TEXTILE GRAFT TECHNOLOGY EXPERIENCE MAQUET THE GOLD STANDARD A comprehensive, proven vascular graft portfolio and exceptional professional support make MAQUET Cardiovascular a valuable asset
OVER 45 YEARS TEXTILE GRAFT TECHNOLOGY EXPERIENCE MAQUET THE GOLD STANDARD A comprehensive, proven vascular graft portfolio and exceptional professional support make MAQUET Cardiovascular a valuable asset
Guy s, King s and St Thomas Cancer Centre The Cancer Outpatient Clinic Central venous catheter: Peripherally inserted central catheter
 Guy s, King s and St Thomas Cancer Centre The Cancer Outpatient Clinic Central venous catheter: Peripherally inserted central catheter This information leaflet aims to help answer some of the questions
Guy s, King s and St Thomas Cancer Centre The Cancer Outpatient Clinic Central venous catheter: Peripherally inserted central catheter This information leaflet aims to help answer some of the questions
Vagus Nerve Stimulation (VNS)
 Vagus Nerve Stimulation (VNS) Your doctor has suggested Vagus Nerve Stimulation (VNS) as an extra treatment to reduce the number of seizures you or your child has as well as how long they last. Either
Vagus Nerve Stimulation (VNS) Your doctor has suggested Vagus Nerve Stimulation (VNS) as an extra treatment to reduce the number of seizures you or your child has as well as how long they last. Either
Ultrafiltration Devices
 6 21 CFR 876.5820, Hemodialysis system and accessories. 7 21 CFR 876.5860, High permeability hemodialysis system. 10 21 CFR 876.5540, Blood access device and accessories. 8 21 CFR 876.5540, Blood access
6 21 CFR 876.5820, Hemodialysis system and accessories. 7 21 CFR 876.5860, High permeability hemodialysis system. 10 21 CFR 876.5540, Blood access device and accessories. 8 21 CFR 876.5540, Blood access
PHYSICIAN OFFICE BILLING INFORMATION SHEET FOR IMLYGIC (talimogene laherparepvec)
 PHYSICIAN OFFICE BILLING INFORMATION SHEET FOR IMLYGIC (talimogene laherparepvec) INDICATION IMLYGIC is a genetically modified oncolytic viral therapy indicated for the local treatment of unresectable
PHYSICIAN OFFICE BILLING INFORMATION SHEET FOR IMLYGIC (talimogene laherparepvec) INDICATION IMLYGIC is a genetically modified oncolytic viral therapy indicated for the local treatment of unresectable
Product Reference Guide
 Product Reference Guide EFFECTIVE AUGUST 2013 FEATUrInG MemoryGel Breast Implants and MemoryShape Breast Implants Important Safety Information: MENTOR MemoryGel Breast Implants, MENTOR MemoryShape Breast
Product Reference Guide EFFECTIVE AUGUST 2013 FEATUrInG MemoryGel Breast Implants and MemoryShape Breast Implants Important Safety Information: MENTOR MemoryGel Breast Implants, MENTOR MemoryShape Breast
Transcutaneous Electrical Nerve Stimulation Device LUMI-TENS
 Transcutaneous Electrical Nerve Stimulation Device LUMI-TENS Operation Manual Read Before Using LUMI-TENS-INS-LAB-RevA08 TABLE OF CONTENTS INTRODUCTION TO TENS INDICATIONS AND CONTRAINDICATIONS WARNINGS
Transcutaneous Electrical Nerve Stimulation Device LUMI-TENS Operation Manual Read Before Using LUMI-TENS-INS-LAB-RevA08 TABLE OF CONTENTS INTRODUCTION TO TENS INDICATIONS AND CONTRAINDICATIONS WARNINGS
Care of your peripherally inserted central catheter
 Care of your peripherally inserted central catheter A guide for patients and their carers We care, we discover, we teach Contents What is a PICC?.... 1 How is it put in?.... 1 What are the benefits of
Care of your peripherally inserted central catheter A guide for patients and their carers We care, we discover, we teach Contents What is a PICC?.... 1 How is it put in?.... 1 What are the benefits of
Solitaire FR Revascularization Device CODING AND REIMBURSEMENT GUIDE REIMBURSEMENT SUPPORT HOTLINE 877.278.7482
 Solitaire FR Revascularization Device TM CODING AND GUIDE INTRODUCTION: HOSPITAL INPATIENT CODING SOLITAIRE FR REVASCULARIZATION DEVICE DESCRIPTION The Solitaire FR revascularization device is a self-expanding
Solitaire FR Revascularization Device TM CODING AND GUIDE INTRODUCTION: HOSPITAL INPATIENT CODING SOLITAIRE FR REVASCULARIZATION DEVICE DESCRIPTION The Solitaire FR revascularization device is a self-expanding
Plasmax Plasma Concentration System BIOLOGICS
 Plasmax Plasma Concentration System BIOLOGICS Plasmax Plasma Concentration System Plasmax Plasma Concentrate System Contains Plasmax concentrator GPS III separator Provides 16ml of autologous output, 6ml
Plasmax Plasma Concentration System BIOLOGICS Plasmax Plasma Concentration System Plasmax Plasma Concentrate System Contains Plasmax concentrator GPS III separator Provides 16ml of autologous output, 6ml
Laser Treatment Policy
 Laser Treatment Policy Pursuant to federal law 21 CFR 812.2(c)7 and 812.3(b), physician(s) at this pain center may advise and use unapproved laser s on patients under one or more of the following conditions:
Laser Treatment Policy Pursuant to federal law 21 CFR 812.2(c)7 and 812.3(b), physician(s) at this pain center may advise and use unapproved laser s on patients under one or more of the following conditions:
PATIENT CARE MANUAL PROCEDURE
 PATIENT CARE MANUAL PROCEDURE NUMBER VII-E-5 PAGE 1 OF 7 APPROVED BY: CATEGORY: Tri-site Nursing Policy and Procedures Review Committee Body Systems; Genitourinary 1.0 GOALS To influence patient care providers
PATIENT CARE MANUAL PROCEDURE NUMBER VII-E-5 PAGE 1 OF 7 APPROVED BY: CATEGORY: Tri-site Nursing Policy and Procedures Review Committee Body Systems; Genitourinary 1.0 GOALS To influence patient care providers
Instructions and User Guide. Select Series: Vascular Access Ultrasound Training Models BPO100, BPBV110, BPP120, BPNB150. June 8, 2011 Rev 2.
 Instructions and User Guide Select Series: Vascular Access Ultrasound Training Models BPO100, BPBV110, BPP120, BPNB150 June 8, 2011 Rev 2.1 Contents 3 Chapter 1: Overview 3 Giving you the confidence only
Instructions and User Guide Select Series: Vascular Access Ultrasound Training Models BPO100, BPBV110, BPP120, BPNB150 June 8, 2011 Rev 2.1 Contents 3 Chapter 1: Overview 3 Giving you the confidence only
Central Venous Lines, PICCs, Ports and Pumps
 Central Venous Lines, PICCs, Ports and Pumps 2012 CODING AND REIMBURSEMENT GUIDE Placement of a non-tunneled or tunneled device requires that the site of entry, type of device, age of patient and tunneling
Central Venous Lines, PICCs, Ports and Pumps 2012 CODING AND REIMBURSEMENT GUIDE Placement of a non-tunneled or tunneled device requires that the site of entry, type of device, age of patient and tunneling
