Introduction to Materials Science, Chapter 9, Phase Diagrams. Phase Diagrams. University of Tennessee, Dept. of Materials Science and Engineering 1
|
|
|
- Gertrude Lawson
- 7 years ago
- Views:
Transcription
1 Phase Diagrams University of Tennessee, Dept. of Materials Science and Engineering 1 Chapter Outline: Phase Diagrams Microstructure and Phase Transformations in Multicomponent Systems Definitions and basic concepts Phases and microstructure Binary isomorphous systems (complete solid solubility) Binary eutectic systems (limited solid solubility) Binary systems with intermediate phases/compounds The iron-carbon system (steel and cast iron) Not tested: 9.12 The Gibbs Phase Rule University of Tennessee, Dept. of Materials Science and Engineering 2
2 Definitions: Components and Phases Component - chemically recognizable species (Fe and C in carbon steel, H 2 O and NaCl in salted water). A binary alloy contains two components, a ternary alloy three, etc. Phase a portion of a system that has uniform physical and chemical characteristics. Two distinct phases in a system have distinct physical or chemical characteristics (e.g. water and ice) and are separated from each other by definite phase boundaries. A phase may contain one or more components. A single-phase system is called homogeneous, systems with two or more phases are mixtures or heterogeneous systems. University of Tennessee, Dept. of Materials Science and Engineering 3 Definitions: Solubility Limit Solvent - host or major component in solution, solute - minor component (Chapter 4). Solubility Limit of a component in a phase is the maximum amount of the component that can be dissolved in it (e.g. alcohol has unlimited solubility in water, sugar has a limited solubility, oil is insoluble). The same concepts apply to solid phases: Cu and Ni are mutually soluble in any amount (unlimited solid solubility), while C has a limited solubility in Fe. University of Tennessee, Dept. of Materials Science and Engineering 4
3 Microstructure The properties of an alloy depend not only on proportions of the phases but also on how they are arranged structurally at the microscopic level. Thus, the microstructure is specified by the number of phases, their proportions, and their arrangement in space. Microstructure of cast Iron This is an alloy of Fe with 4 wt.% C. There are several phases. The long gray regions are flakes of graphite. The matrix is a fine mixture of BCC Fe and Fe 3 C compound. Phase diagrams will help us to understand and predict the microstructures like the one shown in this page University of Tennessee, Dept. of Materials Science and Engineering 5 Equilibrium and Metastable States A system is at equilibrium if at constant temperature, pressure and composition the system is stable, not changing with time. Equilibrium is the state that is achieved given sufficient time. But the time to achieve equilibrium may be very long (the kinetics can be slow) that a state along the path to the equilibrium may appear to be stable. This is called a metastable state. In thermodynamics, equilibrium is described as the state of system that corresponds to the minimum of the thermodynamic function called the free energy of the system. Thermodynamics tells us that: Under conditions of a constant temperature and pressure and composition, the direction of any spontaneous change is toward a lower free energy. The state of stable thermodynamic equilibrium is the one with equilibrium minimum free energy. A system at a metastable state is trapped in a local minimum of free energy that is not the global one. metastable Free Energy University of Tennessee, Dept. of Materials Science and Engineering 6
4 Phase diagram A phase diagram - graphical representation of the combinations of temperature, pressure, composition, or other variables for which specific phases exist at equilibrium. For H 2 O, a typical diagram shows the temperature and pressure at which ice (solid),water (liquid) and steam (gas) exist. University of Tennessee, Dept. of Materials Science and Engineering 7 Phase diagram A phase diagrams show what phases exist at equilibrium and what phase transformations we can expect when we change one of the parameters of the system (T, P, composition). We will discuss phase diagrams for binary alloys only and will assume pressure to be constant at one atmosphere. Phase diagrams for materials with more than two components are complex and difficult to represent. University of Tennessee, Dept. of Materials Science and Engineering 8
5 Binary Isomorphous Systems (I) Isomorphous system - complete solid solubility of the two components (both in the liquid and solid phases). L α + L α Three phase region can be identified on the phase diagram: Liquid (L), solid + liquid (α +L), solid (α ) Liquidus line separates liquid from liquid + solid Solidus line separates solid from liquid + solid University of Tennessee, Dept. of Materials Science and Engineering 9 Binary Isomorphous Systems (II) Example of isomorphous system: Cu-Ni (the complete solubility occurs because both Cu and Ni have the same crystal structure, FCC, similar radii, electronegativity and valence). University of Tennessee, Dept. of Materials Science and Engineering 10
6 Binary Isomorphous Systems (III) In one-component system melting occurs at a well-defined melting temperature. In multi-component systems melting occurs over the range of temperatures, between the solidus and liquidus lines. Solid and liquid phases are in equilibrium in this temperature range. L α + L Liquid solution α Liquid solution + Crystallites of Solid solution Polycrystal Solid solution University of Tennessee, Dept. of Materials Science and Engineering 11 Interpretation of Phase Diagrams For a given temperature and composition we can use phase diagram to determine: 1) The phases that are present 2) Compositions of the phases 3) The relative fractions of the phases Finding the composition in a two phase region: 1. Locate composition and temperature in diagram 2. In two phase region draw the tie line or isotherm 3. Note intersection with phase boundaries. Read compositions at the intersections. The liquid and solid phases have these compositions. University of Tennessee, Dept. of Materials Science and Engineering 12
7 The Lever Rule Finding the amounts of phases in a two phase region: 1. Locate composition and temperature in diagram 2. In two phase region draw the tie line or isotherm 3. Fraction of a phase is determined by taking the length of the tie line to the phase boundary for the other phase, and dividing by the total length of tie line The lever rule is a mechanical analogy to the mass balance calculation. The tie line in the two-phase region is analogous to a lever balanced on a fulcrum. University of Tennessee, Dept. of Materials Science and Engineering 13 The Lever Rule Mass fractions: W L = S / (R+S) = (C α -C o ) / (C α -C L ) W α = R / (R+S) = (C o -C L ) / (C α -C L ) University of Tennessee, Dept. of Materials Science and Engineering 14
8 Derivation of the lever rule 1) All material must be in one phase or the other: W α + W L = 1 2) Mass of a component that is present in both phases equal to the mass of the component in one phase + mass of the component in the second phase: W α C α + W L C L = C o 3) Solution of these equations gives us the Lever rule. W L = (C α -C o ) / (C α -C L ) W α = (C o -C L ) / (C α -C L ) University of Tennessee, Dept. of Materials Science and Engineering 15 Phase compositions and amounts. An example. C o = 35 wt. %, C L = 31.5 wt. %, C α = 42.5 wt. % Mass fractions: W L = (C α -C o ) / (C α -C L ) = 0.68 W α = (C o -C L ) / (C α -C L ) = 0.32 University of Tennessee, Dept. of Materials Science and Engineering 16
9 Development of microstructure in isomorphous alloys Equilibrium (very slow) cooling Solidification in the solid + liquid phase occurs gradually upon cooling from the liquidus line. The composition of the solid and the liquid change gradually during cooling (as can be determined by the tie-line method.) Nuclei of the solid phase form and they grow to consume all the liquid at the solidus line. University of Tennessee, Dept. of Materials Science and Engineering 17 Development of microstructure in isomorphous alloys Equilibrium (very slow) cooling University of Tennessee, Dept. of Materials Science and Engineering 18
10 Development of microstructure in isomorphous alloys Non-equilibrium cooling Compositional changes require diffusion in solid and liquid phases Diffusion in the solid state is very slow. The new layers that solidify on top of the existing grains have the equilibrium composition at that temperature but once they are solid their composition does not change. Formation of layered (cored) grains and the invalidity of the tie-line method to determine the composition of the solid phase. The tie-line method still works for the liquid phase, where diffusion is fast. Average Ni content of solid grains is higher. Application of the lever rule gives us a greater proportion of liquid phase as compared to the one for equilibrium cooling at the same T. Solidus line is shifted to the right (higher Ni contents), solidification is complete at lower T, the outer part of the grains are richer in the low-melting component (Cu). Upon heating grain boundaries will melt first. This can lead to premature mechanical failure. University of Tennessee, Dept. of Materials Science and Engineering 19 Complete solidifcation occurs at lower temp. and higher Ni conc. than equilibrium Solid can t freeze fast enough: solidus line effectively shifted to higher Ni concentrations. Shift increases with faster cooling rates, slower diffusion University of Tennessee, Dept. of Materials Science and Engineering 20
11 Development of microstructure in isomorphous alloys Non-equilibrium cooling Note that the center of each grain is rich in higher mp constituent (freezes first), with compositional gradient to edge of grain: segregation The resulting microstructure is termed a cored structure On re-heating, GBs will melt first, as they are rich in lower mp constituent. This can lead to premature mechanical failure! University of Tennessee, Dept. of Materials Science and Engineering 21 Mechanical properties of isomorphous alloys Solid solution strengthening University of Tennessee, Dept. of Materials Science and Engineering 22
12 Binary Eutectic Systems (I) Copper Silver phase diagram Three single phase regions (α - solid solution of Ag in Cu matrix, β = solid solution of Cu in Ag marix, L - liquid) Three two-phase regions (α + L, β +L, α +β) Solvus line separates one solid solution from a mixture of solid solutions. The Solvus line shows limit of solubility University of Tennessee, Dept. of Materials Science and Engineering 23 Binary Eutectic Systems (II) Lead Tin phase diagram T E E Invariant or eutectic point Eutectic isotherm CE Eutectic or invariant point - Liquid and two solid phases co-exist in equilibrium at the eutectic composition CE and the eutectic temperature T E. Eutectic isotherm - the horizontal solidus line at T E. University of Tennessee, Dept. of Materials Science and Engineering 24
13 Binary Eutectic Systems (III) General Rules Eutectic reaction transition between liquid and mixture of two solid phases, α + β at eutectic concentration C E. The melting point of the eutectic alloy is lower than that of the components (eutectic = easy to melt in Greek). At most two phases can be in equilibrium within a phase field. Three phases (L, α, β) may be in equilibrium only only at a few points along the eutectic isotherm. Single-phase regions are separated by 2-phase regions. University of Tennessee, Dept. of Materials Science and Engineering 25 Binary Eutectic Systems (IV) Compositions and relative amounts of phases are determined from the same tie lines and lever rule, as for isomorphous alloys A B C For points A, B, and C calculate the compositions (wt. %) and relative amounts (mass fractions) of phases present. University of Tennessee, Dept. of Materials Science and Engineering 26
14 Development of microstructure in eutectic alloys (I) Several different types of microstructure can be formed in slow cooling an different compositions. Let s consider cooling of liquid lead tin system at different compositions. In this case of lead-rich alloy (0-2 wt. % of tin) solidification proceeds in the same manner as for isomorphous alloys (e.g. Cu-Ni) that we discussed earlier. L α+l α University of Tennessee, Dept. of Materials Science and Engineering 27 Development of microstructure in eutectic alloys (II) At compositions between the room temperature solubility limit and the maximum solid solubility at the eutectic temperature, β phase nucleates as the α solid solubility is exceeded upon crossing the solvus line. L α +L α α +β University of Tennessee, Dept. of Materials Science and Engineering 28
15 Development of microstructure in eutectic alloys (III) Solidification at the eutectic composition (I) No changes above the eutectic temperature T E. At T E the liquid transforms to α and β phases (eutectic reaction). L α +β University of Tennessee, Dept. of Materials Science and Engineering 29 Development of microstructure in eutectic alloys (IV) Solidification at the eutectic composition (II) Compositions of α and β phases are very different the eutectic reaction involves redistribution of Pb and Sn atoms by atomic diffusion. This simultaneous formation of α and β phases result in a layered (lamellar) microstructure that is called the eutectic structure. Formation of the eutectic structure in the lead-tin system. In the micrograph, the dark layers are lead-reach α phase, the light layers are the tin-reach β phase. University of Tennessee, Dept. of Materials Science and Engineering 30
16 Development of microstructure in eutectic alloys (V) Compositions other than eutectic but within the range of the eutectic isotherm Primary α phase is formed in the α + L region, and the eutectic structure that includes layers of α and β phases (called eutectic α and eutectic β phases) is formed upon crossing the eutectic isotherm. L α+ L α +β University of Tennessee, Dept. of Materials Science and Engineering 31 Development of microstructure in eutectic alloys (VI) Microconstituent element of the microstructure having a distinctive structure. In the case described in the previous page, microstructure consists of two microconstituents, primary α phase and the eutectic structure. Although the eutectic structure consists of two phases, it is a microconstituent with distinct lamellar structure and fixed ratio of the two phases. University of Tennessee, Dept. of Materials Science and Engineering 32
17 How to calculate relative amounts of microconstituents? Eutectic microconstituent forms from liquid having eutectic composition (61.9 wt% Sn) We can treat the eutectic as a separate phase and apply the lever rule to find the relative fractions of primary α phase (18.3 wt% Sn) and the eutectic structure (61.9 wt% Sn): W e = P / (P+Q) (eutectic) W α = Q / (P+Q) (primary) University of Tennessee, Dept. of Materials Science and Engineering 33 How to calculate the total amount of α phase (both eutectic and primary)? Fraction of α phase determined by application of the lever rule across the entire α + β phase field: W α = (Q+R) / (P+Q+R) (α phase) W β = P / (P+Q+R) (β phase) University of Tennessee, Dept. of Materials Science and Engineering 34
18 Phase Diagrams with Intermediate Phases Eutectic systems that we have studied so far have only two solid phases (α and β) that exist near the ends of phase diagrams. These phases are called terminal solid solutions. Some binary alloy systems have intermediate solid solution phases. In phase diagrams, these phases are separated from the composition extremes (0% and 100%). Example: in Cu-Zn, α and η are terminal solid solutions, β, β, γ, δ, ε are intermediate solid solutions. University of Tennessee, Dept. of Materials Science and Engineering 35 Phase Diagrams with Intermetallic Compounds Besides solid solutions, intermetallic compounds, that have precise chemical compositions can exist in some systems. When using the lever rules, intermetallic compounds are treated like any other phase, except they appear not as a wide region but as a vertical line. intermetallic compound This diagram can be thought of as two joined eutectic diagrams, for Mg-Mg 2 Pb and Mg 2 Pb-Pb. In this case compound Mg 2 Pb can be considered as a component. University of Tennessee, Dept. of Materials Science and Engineering 36
19 Peritectic Reactions A peritectic reaction - solid phase and liquid phase will together form a second solid phase at a particular temperature and composition upon cooling - e.g. L + α β These reactions are rather slow as the product phase will form at the boundary between the two reacting phases thus separating them, and slowing down any further reaction. Peritectics are not as common as eutectics and eutectiods, but do occur in some alloy systems. There is one in the Fe- C system that we will consider later. University of Tennessee, Dept. of Materials Science and Engineering 37 Peritectic Reactions An invariant point at which on heating a solid phase transforms into one solid and one liquid phase E.g at point P above: δ + L ε University of Tennessee, Dept. of Materials Science and Engineering 38
20 Congruent Phase Transformations A congruent transformation involves no change in composition (e.g., allotropic transformation such as α-fe to γ-fe or melting transitions in pure solids). For an incongruent transformation, at least one phase changes composition (e.g. eutectic, eutectoid, peritectic reactions). Congruent melting of γ Ni-Ti University of Tennessee, Dept. of Materials Science and Engineering 39 Eutectoid Reactions (I) The eutectoid (eutectic-like in Greek) reaction is similar to the eutectic reaction but occurs from one solid phase to two new solid phases. Invariant point (the eutectoid) three solid phases are in equilibrium. Upon cooling, a solid phase transforms into two other solid phases (γ α + β) in the example below) University of Tennessee, Dept. of Materials Science and Engineering 40
21 Eutectoid Reactions (II) The above phase diagram contains both an eutectic reaction and its solid-state analog, an eutectoid reaction University of Tennessee, Dept. of Materials Science and Engineering 41 The Iron Iron Carbide (Fe Fe 3 C) Phase Diagram In their simplest form, steels are alloys of Iron (Fe) and Carbon (C). The Fe-C phase diagram is a fairly complex one, but we will only consider the steel part of the diagram, up to around 7% Carbon. University of Tennessee, Dept. of Materials Science and Engineering 42
22 Phases in Fe Fe 3 C Phase Diagram α-ferrite - solid solution of C in BCC Fe Stable form of iron at room temperature. The maximum solubility of C is wt% Transforms to FCC γ-austenite at 912 C γ-austenite - solid solution of C in FCC Fe The maximum solubility of C is 2.14 wt %. Transforms to BCC δ-ferrite at 1395 C Is not stable below the eutectic temperature (727 C) unless cooled rapidly (Chapter 10) δ-ferrite solid solution of C in BCC Fe The same structure as α-ferrite Stable only at high T, above 1394 C Melts at 1538 C Fe 3 C (iron carbide or cementite) This intermetallic compound is metastable, it remains as a compound indefinitely at room T, but decomposes (very slowly, within several years) into α-fe and C (graphite) at C Fe-C liquid solution University of Tennessee, Dept. of Materials Science and Engineering 43 A few comments on Fe Fe 3 C system C is an interstitial impurity in Fe. It forms a solid solution with α, γ, δ phases of iron Maximum solubility in BCC α-ferrite is limited (max wt% at 727 C) - BCC has relatively small interstitial positions Maximum solubility in FCC austenite is 2.14 wt% at 1147 C - FCC has larger interstitial positions Mechanical properties: Cementite is very hard and brittle - can strengthen steels. Mechanical properties also depend on the microstructure, that is, how ferrite and cementite are mixed. Magnetic properties: α -ferrite is magnetic below 768 C, austenite is non-magnetic Classification. Three types of ferrous alloys: Iron: less than wt % C in α ferrite at room T Steels: wt % C (usually < 1 wt % ) α-ferrite + Fe 3 C at room T (Chapter 12) Cast iron: wt % (usually < 4.5 wt %) University of Tennessee, Dept. of Materials Science and Engineering 44
23 Eutectic and eutectoid reactions in Fe Fe 3 C Eutectic: 4.30 wt% C, 1147 C L γ + Fe 3 C Eutectoid: 0.76 wt%c, 727 C γ(0.76 wt% C) α(0.022 wt% C) + Fe 3 C Eutectic and eutectoid reactions are very important in heat treatment of steels University of Tennessee, Dept. of Materials Science and Engineering 45 Development of Microstructure in Iron - Carbon alloys Microstructure depends on composition (carbon content) and heat treatment. In the discussion below we consider slow cooling in which equilibrium is maintained. Microstructure of eutectoid steel (I) University of Tennessee, Dept. of Materials Science and Engineering 46
24 Microstructure of eutectoid steel (II) When alloy of eutectoid composition (0.76 wt % C) is cooled slowly it forms perlite, a lamellar or layered structure of two phases: α-ferrite and cementite (Fe 3 C) The layers of alternating phases in pearlite are formed for the same reason as layered structure of eutectic structures: redistribution C atoms between ferrite (0.022 wt%) and cementite (6.7 wt%) by atomic diffusion. Mechanically, pearlite has properties intermediate to soft, ductile ferrite and hard, brittle cementite. In the micrograph, the dark areas are Fe 3 C layers, the light phase is α- ferrite University of Tennessee, Dept. of Materials Science and Engineering 47 Microstructure of hypoeutectoid steel (I) Compositions to the left of eutectoid ( wt % C) hypoeutectoid (less than eutectoid -Greek) alloys. γ α+ γ α+ Fe 3 C University of Tennessee, Dept. of Materials Science and Engineering 48
25 Microstructure of hypoeutectoid steel (II) Hypoeutectoid alloys contain proeutectoid ferrite (formed above the eutectoid temperature) plus the eutectoid perlite that contain eutectoid ferrite and cementite. University of Tennessee, Dept. of Materials Science and Engineering 49 Microstructure of hypereutectoid steel (I) Compositions to the right of eutectoid ( wt % C) hypereutectoid (more than eutectoid -Greek) alloys. γ γ+ Fe 3 C α+ Fe 3 C University of Tennessee, Dept. of Materials Science and Engineering 50
26 Microstructure of hypereutectoid steel (II) Hypereutectoid alloys contain proeutectoid cementite (formed above the eutectoid temperature) plus perlite that contain eutectoid ferrite and cementite. University of Tennessee, Dept. of Materials Science and Engineering 51 How to calculate the relative amounts of proeutectoid phase (α or Fe 3 C) and pearlite? Application of the lever rule with tie line that extends from the eutectoid composition (0.75 wt% C) to α (α + Fe 3 C) boundary (0.022 wt% C) for hypoeutectoid alloys and to (α + Fe 3 C) Fe 3 C boundary (6.7 wt% C) for hipereutectoid alloys. Fraction of α phase is determined by application of the lever rule University across of Tennessee, the entire Dept. (α of Materials + Fe Science and Engineering 3 C) phase field: 52
27 Example for hypereutectoid alloy with composition C 1 Fraction of pearlite: W P = X / (V+X) = (6.7 C 1 ) / ( ) Fraction of proeutectoid cementite: W Fe3C = V / (V+X) = (C ) / ( ) University of Tennessee, Dept. of Materials Science and Engineering 53 Summary Make sure you understand language and concepts: Austenite Cementite Component Congruent transformation Equilibrium Eutectic phase Eutectic reaction Eutectic structure Eutectoid reaction Ferrite Hypereutectoid alloy Hypoeutectoid alloy Intermediate solid solution Intermetallic compound Invariant point Isomorphous Lever rule Liquidus line Metastable Microconstituent Pearlite Peritectic reaction Phase Phase diagram Phase equilibrium Primary phase Proeutectoid cementite Proeutectoid ferrite Solidus line Solubility limit Solvus line System Terminal solid solution Tie line University of Tennessee, Dept. of Materials Science and Engineering 54
28 Reading for next class: Chapter 10: Phase Transformations in Metals Kinetics of phase transformations Multiphase Transformations Phase transformations in Fe-C alloys Isothermal Transformation Diagrams Mechanical Behavior Tempered Martensite Optional reading (Parts that are not covered / not tested): 10.6 Continuous Cooling Transformation Diagrams University of Tennessee, Dept. of Materials Science and Engineering 55
Iron-Carbon Phase Diagram (a review) see Callister Chapter 9
 Iron-Carbon Phase Diagram (a review) see Callister Chapter 9 University of Tennessee, Dept. of Materials Science and Engineering 1 The Iron Iron Carbide (Fe Fe 3 C) Phase Diagram In their simplest form,
Iron-Carbon Phase Diagram (a review) see Callister Chapter 9 University of Tennessee, Dept. of Materials Science and Engineering 1 The Iron Iron Carbide (Fe Fe 3 C) Phase Diagram In their simplest form,
Phase Equilibria & Phase Diagrams
 Phase Equilibria & Phase Diagrams Week7 Material Sciences and Engineering MatE271 1 Motivation Phase diagram (Ch 9) Temperature Time Kinematics (Ch 10) New structure, concentration (mixing level) (at what
Phase Equilibria & Phase Diagrams Week7 Material Sciences and Engineering MatE271 1 Motivation Phase diagram (Ch 9) Temperature Time Kinematics (Ch 10) New structure, concentration (mixing level) (at what
Binary phase diagrams
 inary phase diagrams inary phase diagrams and ibbs free energy curves inary solutions with unlimited solubility Relative proportion of phases (tie lines and the lever principle) Development of microstructure
inary phase diagrams inary phase diagrams and ibbs free energy curves inary solutions with unlimited solubility Relative proportion of phases (tie lines and the lever principle) Development of microstructure
Lecture 19: Eutectoid Transformation in Steels: a typical case of Cellular
 Lecture 19: Eutectoid Transformation in Steels: a typical case of Cellular Precipitation Today s topics Understanding of Cellular transformation (or precipitation): when applied to phase transformation
Lecture 19: Eutectoid Transformation in Steels: a typical case of Cellular Precipitation Today s topics Understanding of Cellular transformation (or precipitation): when applied to phase transformation
Chapter 8. Phase Diagrams
 Phase Diagrams A phase in a material is a region that differ in its microstructure and or composition from another region Al Al 2 CuMg H 2 O(solid, ice) in H 2 O (liquid) 2 phases homogeneous in crystal
Phase Diagrams A phase in a material is a region that differ in its microstructure and or composition from another region Al Al 2 CuMg H 2 O(solid, ice) in H 2 O (liquid) 2 phases homogeneous in crystal
9.11 Upon heating a lead-tin alloy of composition 30 wt% Sn-70 wt% Pb from 150 C and utilizing Figure
 9-13 9.8: 9.11 Upon heating a lead-tin alloy of composition 30 wt% Sn-70 wt% Pb from 150 C and utilizing Figure (a) The first liquid forms at the temperature at which a vertical line at this composition
9-13 9.8: 9.11 Upon heating a lead-tin alloy of composition 30 wt% Sn-70 wt% Pb from 150 C and utilizing Figure (a) The first liquid forms at the temperature at which a vertical line at this composition
BINARY SYSTEMS. Definition of Composition: Atomic (molar) fraction. Atomic percent. Mass fraction. Mass percent (weight percent)
 BINARY SYSTEMS Definition of Composition: Atomic (molar) fraction Atomic percent Mass fraction Mass percent (weight percent) na =, x i n = A i i i Weight percent mainly in industry! x at % A = x 100 A
BINARY SYSTEMS Definition of Composition: Atomic (molar) fraction Atomic percent Mass fraction Mass percent (weight percent) na =, x i n = A i i i Weight percent mainly in industry! x at % A = x 100 A
Chapter Outline: Phase Transformations in Metals
 Chapter Outline: Phase Transformations in Metals Heat Treatment (time and temperature) Microstructure Mechanical Properties Kinetics of phase transformations Multiphase Transformations Phase transformations
Chapter Outline: Phase Transformations in Metals Heat Treatment (time and temperature) Microstructure Mechanical Properties Kinetics of phase transformations Multiphase Transformations Phase transformations
Phase. Gibbs Phase rule
 Phase diagrams Phase A phase can be defined as a physically distinct and chemically homogeneous portion of a system that has a particular chemical composition and structure. Water in liquid or vapor state
Phase diagrams Phase A phase can be defined as a physically distinct and chemically homogeneous portion of a system that has a particular chemical composition and structure. Water in liquid or vapor state
Alloys & Their Phase Diagrams
 Alloys & Their Phase Diagrams Objectives of the class Gibbs phase rule Introduction to phase diagram Practice phase diagram Lever rule Important Observation: One question in the midterm Consider the Earth
Alloys & Their Phase Diagrams Objectives of the class Gibbs phase rule Introduction to phase diagram Practice phase diagram Lever rule Important Observation: One question in the midterm Consider the Earth
CHAPTER 9 Part 1. = 5 wt% Sn-95 wt% Pb C β. = 98 wt% Sn-2 wt% Pb. = 77 wt% Ag-23 wt% Cu. = 51 wt% Zn-49 wt% Cu C γ. = 58 wt% Zn-42 wt% Cu
 CHAPTER 9 Part 1 9.5 This problem asks that we cite the phase or phases present for several alloys at specified temperatures. (a) For an alloy composed of 15 wt% Sn-85 wt% Pb and at 100 C, from Figure
CHAPTER 9 Part 1 9.5 This problem asks that we cite the phase or phases present for several alloys at specified temperatures. (a) For an alloy composed of 15 wt% Sn-85 wt% Pb and at 100 C, from Figure
Phase Transformations in Metals and Alloys
 Phase Transformations in Metals and Alloys THIRD EDITION DAVID A. PORTER, KENNETH E. EASTERLING, and MOHAMED Y. SHERIF ( г йс) CRC Press ^ ^ ) Taylor & Francis Group Boca Raton London New York CRC Press
Phase Transformations in Metals and Alloys THIRD EDITION DAVID A. PORTER, KENNETH E. EASTERLING, and MOHAMED Y. SHERIF ( г йс) CRC Press ^ ^ ) Taylor & Francis Group Boca Raton London New York CRC Press
CHAPTER 8. Phase Diagrams 8-1
 CHAPTER 8 Phase Diagrams 8-1 Introducción Fase: Una region en un material que difiere en estructura y función de otra región. Diagramas de fase : Representan las fases presentes en el metal a diferentes
CHAPTER 8 Phase Diagrams 8-1 Introducción Fase: Una region en un material que difiere en estructura y función de otra región. Diagramas de fase : Representan las fases presentes en el metal a diferentes
Heat Treatment of Steel
 Heat Treatment of Steel Steels can be heat treated to produce a great variety of microstructures and properties. Generally, heat treatment uses phase transformation during heating and cooling to change
Heat Treatment of Steel Steels can be heat treated to produce a great variety of microstructures and properties. Generally, heat treatment uses phase transformation during heating and cooling to change
Heat Treatment of Steels : Spheroidize annealing. Heat Treatment of Steels : Normalizing
 Heat Treatment of Steels :Recrystallization annealing The carbon and alloy steels were treated at a temperature of about 700 C, which is about 20 C below the eutectoid temperature. The holding time should
Heat Treatment of Steels :Recrystallization annealing The carbon and alloy steels were treated at a temperature of about 700 C, which is about 20 C below the eutectoid temperature. The holding time should
Module 34. Heat Treatment of steel IV. Lecture 34. Heat Treatment of steel IV
 Module 34 Heat reatment of steel IV Lecture 34 Heat reatment of steel IV 1 Keywords : Austenitization of hypo & hyper eutectoid steel, austenization temperature, effect of heat treatment on structure &
Module 34 Heat reatment of steel IV Lecture 34 Heat reatment of steel IV 1 Keywords : Austenitization of hypo & hyper eutectoid steel, austenization temperature, effect of heat treatment on structure &
The mechanical properties of metal affected by heat treatment are:
 Training Objective After watching this video and reviewing the printed material, the student/trainee will learn the basic concepts of the heat treating processes as they pertain to carbon and alloy steels.
Training Objective After watching this video and reviewing the printed material, the student/trainee will learn the basic concepts of the heat treating processes as they pertain to carbon and alloy steels.
Ch. 4: Imperfections in Solids Part 1. Dr. Feras Fraige
 Ch. 4: Imperfections in Solids Part 1 Dr. Feras Fraige Outline Defects in Solids 0D, Point defects vacancies Interstitials impurities, weight and atomic composition 1D, Dislocations edge screw 2D, Grain
Ch. 4: Imperfections in Solids Part 1 Dr. Feras Fraige Outline Defects in Solids 0D, Point defects vacancies Interstitials impurities, weight and atomic composition 1D, Dislocations edge screw 2D, Grain
μ α =μ β = μ γ = =μ ω μ α =μ β =μ γ = =μ ω Thus for c components, the number of additional constraints is c(p 1) ( ) ( )
 Phase Diagrams 1 Gibbs Phase Rule The Gibbs phase rule describes the degrees of freedom available to describe a particular system with various phases and substances. To derive the phase rule, let us begin
Phase Diagrams 1 Gibbs Phase Rule The Gibbs phase rule describes the degrees of freedom available to describe a particular system with various phases and substances. To derive the phase rule, let us begin
The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = V S V C. = 2(sphere volume) = 2 = V C = 4R
 3.5 Show that the atomic packing factor for BCC is 0.68. The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = V S V C Since there are two spheres associated
3.5 Show that the atomic packing factor for BCC is 0.68. The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = V S V C Since there are two spheres associated
Defects Introduction. Bonding + Structure + Defects. Properties
 Defects Introduction Bonding + Structure + Defects Properties The processing determines the defects Composition Bonding type Structure of Crystalline Processing factors Defects Microstructure Types of
Defects Introduction Bonding + Structure + Defects Properties The processing determines the defects Composition Bonding type Structure of Crystalline Processing factors Defects Microstructure Types of
THREE MAIN SOLIDIFICATION REACTIONS OF VANADIUM MODIFIED T1 TUNGSTEN HIGH SPEED TOOL STEEL. Hossam Halfa
 THREE MAIN SOLIDIFICATION REACTIONS OF VANADIUM MODIFIED T1 TUNGSTEN HIGH SPEED TOOL STEEL Hossam Halfa Steel Technology Department, Central Metallurgical R&D Institute (CMRDI), Helwan, Egypt, hossamhalfa@cmrdi.sci.eg;
THREE MAIN SOLIDIFICATION REACTIONS OF VANADIUM MODIFIED T1 TUNGSTEN HIGH SPEED TOOL STEEL Hossam Halfa Steel Technology Department, Central Metallurgical R&D Institute (CMRDI), Helwan, Egypt, hossamhalfa@cmrdi.sci.eg;
LN 10. 3.091 Introduction to Solid State Chemistry. Lecture Notes No. 10 PHASE EQUILIBRIA AND PHASE DIAGRAMS
 3.091 Introduction to Solid State Chemistry Lecture Notes No. 10 PHASE EQUILIBRIA AND PHASE DIAGRAMS * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * Sources
3.091 Introduction to Solid State Chemistry Lecture Notes No. 10 PHASE EQUILIBRIA AND PHASE DIAGRAMS * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * Sources
MSE 528 - PRECIPITATION HARDENING IN 7075 ALUMINUM ALLOY
 MSE 528 - PRECIPITATION HARDENING IN 7075 ALUMINUM ALLOY Objective To study the time and temperature variations in the hardness and electrical conductivity of Al-Zn-Mg-Cu high strength alloy on isothermal
MSE 528 - PRECIPITATION HARDENING IN 7075 ALUMINUM ALLOY Objective To study the time and temperature variations in the hardness and electrical conductivity of Al-Zn-Mg-Cu high strength alloy on isothermal
Fundamentals of the Heat Treating of Steel
 CHAPTER 2 Fundamentals of the Heat Treating of Steel BEFORE CONSIDERATION can be given to the heat treatment of steel or other iron-base alloys, it is helpful to explain what steel is. The common dictionary
CHAPTER 2 Fundamentals of the Heat Treating of Steel BEFORE CONSIDERATION can be given to the heat treatment of steel or other iron-base alloys, it is helpful to explain what steel is. The common dictionary
In order to solve this problem it is first necessary to use Equation 5.5: x 2 Dt. = 1 erf. = 1.30, and x = 2 mm = 2 10-3 m. Thus,
 5.3 (a) Compare interstitial and vacancy atomic mechanisms for diffusion. (b) Cite two reasons why interstitial diffusion is normally more rapid than vacancy diffusion. Solution (a) With vacancy diffusion,
5.3 (a) Compare interstitial and vacancy atomic mechanisms for diffusion. (b) Cite two reasons why interstitial diffusion is normally more rapid than vacancy diffusion. Solution (a) With vacancy diffusion,
HEAT TREATMENT OF STEEL
 HEAT TREATMENT OF STEEL Heat Treatment of Steel Most heat treating operations begin with heating the alloy into the austenitic phase field to dissolve the carbide in the iron. Steel heat treating practice
HEAT TREATMENT OF STEEL Heat Treatment of Steel Most heat treating operations begin with heating the alloy into the austenitic phase field to dissolve the carbide in the iron. Steel heat treating practice
Thermodynamic database of the phase diagrams in copper base alloy systems
 Journal of Physics and Chemistry of Solids 66 (2005) 256 260 www.elsevier.com/locate/jpcs Thermodynamic database of the phase diagrams in copper base alloy systems C.P. Wang a, X.J. Liu b, M. Jiang b,
Journal of Physics and Chemistry of Solids 66 (2005) 256 260 www.elsevier.com/locate/jpcs Thermodynamic database of the phase diagrams in copper base alloy systems C.P. Wang a, X.J. Liu b, M. Jiang b,
REACTIONS IN THE SN CORNER OF THE CU-SN-ZN ALLOY SYSTEM
 REACTIONS IN THE SN CORNER OF THE CU-SN-ZN ALLOY SYSTEM D.D. Perovic, L Snugovsky and J.W. Rutter Department of Materials Science and Engineering University of Toronto Toronto, ON, Canada doug.perovic@utoronto.ca
REACTIONS IN THE SN CORNER OF THE CU-SN-ZN ALLOY SYSTEM D.D. Perovic, L Snugovsky and J.W. Rutter Department of Materials Science and Engineering University of Toronto Toronto, ON, Canada doug.perovic@utoronto.ca
The study about as cast microstructure and solidification
 The study about as cast microstructure and solidification process of Al-Mg-Si alloys Introduction High-strength and high formability aluminum alloys are the highly demanded alloy in automobile industry,
The study about as cast microstructure and solidification process of Al-Mg-Si alloys Introduction High-strength and high formability aluminum alloys are the highly demanded alloy in automobile industry,
m sugar + m water (wt%) = 77 wt% = m sugar + 1500 g 100 m' sugar m' sugar + 1500 g 100
 14:440:407 ch9 Question 9.1 Consider the sugar water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90 C (194 F)? (b) If the saturated liquid solution in part (a) is cooled
14:440:407 ch9 Question 9.1 Consider the sugar water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90 C (194 F)? (b) If the saturated liquid solution in part (a) is cooled
Experiment: Heat Treatment - Quenching & Tempering
 Experiment: Heat Treatment - Quenching & Tempering Objectives 1) To investigate the conventional heat treatment procedures, such as quenching and annealing, used to alter the properties of steels. SAE
Experiment: Heat Treatment - Quenching & Tempering Objectives 1) To investigate the conventional heat treatment procedures, such as quenching and annealing, used to alter the properties of steels. SAE
Kinetics of Phase Transformations: Nucleation & Growth
 Kinetics of Phase Transformations: Nucleation & Growth Radhika Barua Department of Chemical Engineering Northeastern University Boston, MA USA Thermodynamics of Phase Transformation Northeastern University
Kinetics of Phase Transformations: Nucleation & Growth Radhika Barua Department of Chemical Engineering Northeastern University Boston, MA USA Thermodynamics of Phase Transformation Northeastern University
Massachusetts Institute of Technology Department of Mechanical Engineering Cambridge, MA 02139
 Massachusetts Institute of Technology Department of Mechanical Engineering Cambridge, MA 02139 2.002 Mechanics and Materials II Spring 2004 Laboratory Module No. 5 Heat Treatment of Plain Carbon and Low
Massachusetts Institute of Technology Department of Mechanical Engineering Cambridge, MA 02139 2.002 Mechanics and Materials II Spring 2004 Laboratory Module No. 5 Heat Treatment of Plain Carbon and Low
Chapter 5: Diffusion. 5.1 Steady-State Diffusion
 : Diffusion Diffusion: the movement of particles in a solid from an area of high concentration to an area of low concentration, resulting in the uniform distribution of the substance Diffusion is process
: Diffusion Diffusion: the movement of particles in a solid from an area of high concentration to an area of low concentration, resulting in the uniform distribution of the substance Diffusion is process
Experiment 5: Phase diagram for a three-component system (Dated: April 12, 2010)
 Experiment 5: Phase diagram for a three-component system (Dated: April 12, 2010) I. INTRODUCTION It is sometimes necessary to know the mutual solubilities of liquids in a two-phase system. For example,
Experiment 5: Phase diagram for a three-component system (Dated: April 12, 2010) I. INTRODUCTION It is sometimes necessary to know the mutual solubilities of liquids in a two-phase system. For example,
Martensite in Steels
 Materials Science & Metallurgy http://www.msm.cam.ac.uk/phase-trans/2002/martensite.html H. K. D. H. Bhadeshia Martensite in Steels The name martensite is after the German scientist Martens. It was used
Materials Science & Metallurgy http://www.msm.cam.ac.uk/phase-trans/2002/martensite.html H. K. D. H. Bhadeshia Martensite in Steels The name martensite is after the German scientist Martens. It was used
SOLIDIFICATION. (a)formation of stable nuclei. Growth of a stable nucleus. (c) Grain structure
 SOLIDIFICATION Most metals are melted and then cast into semifinished or finished shape. Solidification of a metal can be divided into the following steps: Formation of a stable nucleus Growth of a stable
SOLIDIFICATION Most metals are melted and then cast into semifinished or finished shape. Solidification of a metal can be divided into the following steps: Formation of a stable nucleus Growth of a stable
Warm-Up 9/9. 1. Define the term matter. 2. Name something in this room that is not matter.
 Warm-Up 9/9 1. Define the term matter. 2. Name something in this room that is not matter. Warm-Up 9/16 1. List the three most important rules of lab safety. 2. Would you classify jello as a solid or a
Warm-Up 9/9 1. Define the term matter. 2. Name something in this room that is not matter. Warm-Up 9/16 1. List the three most important rules of lab safety. 2. Would you classify jello as a solid or a
Chapter Outline Dislocations and Strengthening Mechanisms
 Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Heterogeneous Homogenous. Mixtures; Solutions. Phases of matter: Solid. Phases of Matter: Liquid. Phases of Matter: Gas. Solid, Liquid, Gas
 Phases of matter: Solid Heterogeneous Homogenous Mixtures Solutions Phases of Matter: Liquid Atoms and molecules are more spaced out and now can move. The material can be slightly compressed into a smaller
Phases of matter: Solid Heterogeneous Homogenous Mixtures Solutions Phases of Matter: Liquid Atoms and molecules are more spaced out and now can move. The material can be slightly compressed into a smaller
Name Date Class CHAPTER 1 REVIEW. Answer the following questions in the space provided.
 CHAPTER 1 REVIEW Matter and Change SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a Technological development of a chemical product often (a) lags behind basic research
CHAPTER 1 REVIEW Matter and Change SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a Technological development of a chemical product often (a) lags behind basic research
Chapter Outline. Diffusion - how do atoms move through solids?
 Chapter Outline iffusion - how do atoms move through solids? iffusion mechanisms Vacancy diffusion Interstitial diffusion Impurities The mathematics of diffusion Steady-state diffusion (Fick s first law)
Chapter Outline iffusion - how do atoms move through solids? iffusion mechanisms Vacancy diffusion Interstitial diffusion Impurities The mathematics of diffusion Steady-state diffusion (Fick s first law)
8. Technical Heat Treatment
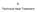 8. Technical Heat Treatment 8. Technical Heat Treatment 95 6 cm 4 2 0-2 -4 C 400 C C -6-14 -12-10 -8-6 -4-2 0 2 cm 6 723 C temperature C 1750 750 250 When welding a workpiece, not only the weld itself,
8. Technical Heat Treatment 8. Technical Heat Treatment 95 6 cm 4 2 0-2 -4 C 400 C C -6-14 -12-10 -8-6 -4-2 0 2 cm 6 723 C temperature C 1750 750 250 When welding a workpiece, not only the weld itself,
DIFFUSION IN SOLIDS. Materials often heat treated to improve properties. Atomic diffusion occurs during heat treatment
 DIFFUSION IN SOLIDS WHY STUDY DIFFUSION? Materials often heat treated to improve properties Atomic diffusion occurs during heat treatment Depending on situation higher or lower diffusion rates desired
DIFFUSION IN SOLIDS WHY STUDY DIFFUSION? Materials often heat treated to improve properties Atomic diffusion occurs during heat treatment Depending on situation higher or lower diffusion rates desired
LABORATORY EXPERIMENTS TESTING OF MATERIALS
 LABORATORY EXPERIMENTS TESTING OF MATERIALS 1. TENSION TEST: INTRODUCTION & THEORY The tension test is the most commonly used method to evaluate the mechanical properties of metals. Its main objective
LABORATORY EXPERIMENTS TESTING OF MATERIALS 1. TENSION TEST: INTRODUCTION & THEORY The tension test is the most commonly used method to evaluate the mechanical properties of metals. Its main objective
EXAMPLE EXERCISE 4.1 Change of Physical State
 EXAMPLE EXERCISE 4.1 Change of Physical State State the term that applies to each of the following changes of physical state: (a) Snow changes from a solid to a liquid. (b) Gasoline changes from a liquid
EXAMPLE EXERCISE 4.1 Change of Physical State State the term that applies to each of the following changes of physical state: (a) Snow changes from a solid to a liquid. (b) Gasoline changes from a liquid
Objectives/Introduction Extraction of zinc Physical properties of zinc Zinc casting alloys Wrought zinc alloys Engineering design with zinc alloys
 Lecture 7 Zinc and its alloys Subjects of interest Objectives/Introduction Extraction of zinc Physical properties of zinc Zinc casting alloys Wrought zinc alloys Engineering design with zinc alloys Objectives
Lecture 7 Zinc and its alloys Subjects of interest Objectives/Introduction Extraction of zinc Physical properties of zinc Zinc casting alloys Wrought zinc alloys Engineering design with zinc alloys Objectives
Metals and Non-metals. Comparison of physical properties of metals and non metals
 Metals and Non-metals Comparison of physical properties of metals and non metals PHYSICAL PROPERTY METALS NON-METALS Physical State Metallic lustre (having a shining surface) Mostly solids (Liquid -mercury)
Metals and Non-metals Comparison of physical properties of metals and non metals PHYSICAL PROPERTY METALS NON-METALS Physical State Metallic lustre (having a shining surface) Mostly solids (Liquid -mercury)
CHAPTER 3: MATTER. Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64
 CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
Lecture: 33. Solidification of Weld Metal
 Lecture: 33 Solidification of Weld Metal This chapter presents common solidification mechanisms observed in weld metal and different modes of solidification. Influence of welding speed and heat input on
Lecture: 33 Solidification of Weld Metal This chapter presents common solidification mechanisms observed in weld metal and different modes of solidification. Influence of welding speed and heat input on
Phase Diagrams & Thermodynamics
 Phase Diagrams & Thermodynamics A phase diagram is a graphical representation of the equilibrium state of a system using the intensive variables T and i while p is kept constant. The equilibrium may be
Phase Diagrams & Thermodynamics A phase diagram is a graphical representation of the equilibrium state of a system using the intensive variables T and i while p is kept constant. The equilibrium may be
Materials Issues in Fatigue and Fracture
 Materials Issues in Fatigue and Fracture 5.1 Fundamental Concepts 5.2 Ensuring Infinite Life 5.3 Finite Life 5.4 Summary FCP 1 5.1 Fundamental Concepts Structural metals Process of fatigue A simple view
Materials Issues in Fatigue and Fracture 5.1 Fundamental Concepts 5.2 Ensuring Infinite Life 5.3 Finite Life 5.4 Summary FCP 1 5.1 Fundamental Concepts Structural metals Process of fatigue A simple view
Interface Reaction and Mechanical Properties of Lead-free Sn Zn Alloy/Cu Joints
 Materials Transactions, Vol. 43, No. 8 (2002) pp. 1797 to 1801 Special Issue on Lead-Free Electronics Packaging c 2002 The Japan Institute of Metals Interface Reaction and Mechanical Properties of Lead-free
Materials Transactions, Vol. 43, No. 8 (2002) pp. 1797 to 1801 Special Issue on Lead-Free Electronics Packaging c 2002 The Japan Institute of Metals Interface Reaction and Mechanical Properties of Lead-free
Pure Substances, Mixtures, and Solutions
 Pure Substances, Mixtures, and Solutions Mixtures, elements, compounds Scientists like to classify things. One way that scientists classify matter is by its composition. Ultimately, all matter can be classified
Pure Substances, Mixtures, and Solutions Mixtures, elements, compounds Scientists like to classify things. One way that scientists classify matter is by its composition. Ultimately, all matter can be classified
Review - After School Matter Name: Review - After School Matter Tuesday, April 29, 2008
 Name: Review - After School Matter Tuesday, April 29, 2008 1. Figure 1 The graph represents the relationship between temperature and time as heat was added uniformly to a substance starting at a solid
Name: Review - After School Matter Tuesday, April 29, 2008 1. Figure 1 The graph represents the relationship between temperature and time as heat was added uniformly to a substance starting at a solid
CONCEPT CHECK QUESTIONS AND ANSWERS. Chapter 2 Atomic Structure and Interatomic Bonding
 CONCEPT CHECK QUESTIONS AND ANSWERS Chapter 2 Atomic Structure and Interatomic Bonding Concept Check 2.1 Question: Why are the atomic weights of the elements generally not integers? Cite two reasons. Answer:
CONCEPT CHECK QUESTIONS AND ANSWERS Chapter 2 Atomic Structure and Interatomic Bonding Concept Check 2.1 Question: Why are the atomic weights of the elements generally not integers? Cite two reasons. Answer:
Problems in Welding of High Strength Aluminium Alloys
 Singapore Welding Society Newsletter, September 1999 Problems in Welding of High Strength Aluminium Alloys Wei Zhou Nanyang Technological University, Singapore E-mail: WZhou@Cantab.Net Pure aluminium has
Singapore Welding Society Newsletter, September 1999 Problems in Welding of High Strength Aluminium Alloys Wei Zhou Nanyang Technological University, Singapore E-mail: WZhou@Cantab.Net Pure aluminium has
Section 4: NiResist Iron
 Section 4: NiResist Iron Section 4 Ni-Resist Description of Grades...4-2 201 (Type 1) Ni-Resist...4-3 202 (Type 2) Ni-Resist...4-6 Stock Listings...4-8 4-1 Ni-Resist Description of Grades Ni-Resist Dura-Bar
Section 4: NiResist Iron Section 4 Ni-Resist Description of Grades...4-2 201 (Type 1) Ni-Resist...4-3 202 (Type 2) Ni-Resist...4-6 Stock Listings...4-8 4-1 Ni-Resist Description of Grades Ni-Resist Dura-Bar
The Effect of Heat Treatment Atmosphere on Hardening of Surface Region of H13 Tool Steel. Dominique AU
 The Effect of Heat Treatment Atmosphere on Hardening of Surface Region of H13 Tool Steel Dominique AU Report submitted in partial fulfilment of the degree of Postgraduate diploma in Research Auckland University
The Effect of Heat Treatment Atmosphere on Hardening of Surface Region of H13 Tool Steel Dominique AU Report submitted in partial fulfilment of the degree of Postgraduate diploma in Research Auckland University
HIGH STRENGTH DUCTILE IRON PRODUCED BY THE ENGINEERED COOLING: PROCESS CONCEPT
 IJMC14-244-2 HIGH STRENGTH DUCTILE IRON PRODUCED BY THE ENGINEERED COOLING: PROCESS CONCEPT Copyright 215 American Foundry Society Abstract Simon N. Lekakh Missouri University of Science and Technology,
IJMC14-244-2 HIGH STRENGTH DUCTILE IRON PRODUCED BY THE ENGINEERED COOLING: PROCESS CONCEPT Copyright 215 American Foundry Society Abstract Simon N. Lekakh Missouri University of Science and Technology,
PRECIPITATION HARDENING OF ZINC ALLOYS CASTINGS PRECIPITAČNÍ VYTVRZOVÁNÍ ODLITKŮ SLITIN ZINKU
 PRECIPITATION HARDENING OF ZINC ALLOYS CASTINGS PRECIPITAČNÍ VYTVRZOVÁNÍ ODLITKŮ SLITIN ZINKU Iva NOVÁ a, Iva NOVÁKOVÁ b, Jiří MACHUTA c a Technická univerzita v Liberci, Studentská 2, 463 17 Liberec,
PRECIPITATION HARDENING OF ZINC ALLOYS CASTINGS PRECIPITAČNÍ VYTVRZOVÁNÍ ODLITKŮ SLITIN ZINKU Iva NOVÁ a, Iva NOVÁKOVÁ b, Jiří MACHUTA c a Technická univerzita v Liberci, Studentská 2, 463 17 Liberec,
Chapter Test A. Elements, Compounds, and Mixtures MULTIPLE CHOICE. chemically combined? MIXs2 a. element b. compound c. mixture d.
 Assessment Chapter Test A Elements, Compounds, and Mixtures MULTIPLE CHOICE Write the letter of the correct answer in the space provided. 1. What is a pure substance made of two or more elements that are
Assessment Chapter Test A Elements, Compounds, and Mixtures MULTIPLE CHOICE Write the letter of the correct answer in the space provided. 1. What is a pure substance made of two or more elements that are
Crystal Structure of Aluminum, Zinc, and their Alloys By: Omar Fajardo Sebastian Henao Devin Baines ENGR45, F2014, SRJC
 Crystal Structure of Aluminum, Zinc, and their Alloys By: Omar Fajardo Sebastian Henao Devin Baines ENGR45, F2014, SRJC Purpose The purpose of this experiment was to examine and observe the microstructure
Crystal Structure of Aluminum, Zinc, and their Alloys By: Omar Fajardo Sebastian Henao Devin Baines ENGR45, F2014, SRJC Purpose The purpose of this experiment was to examine and observe the microstructure
Continuous Cooling Transformation (CCT) Diagrams
 Continuous Cooling Transformation (CCT) Diagrams R. Manna Assistant Professor Centre of Advanced Study Department of Metallurgical Engineering Institute of Technology, Banaras Hindu University Varanasi-221
Continuous Cooling Transformation (CCT) Diagrams R. Manna Assistant Professor Centre of Advanced Study Department of Metallurgical Engineering Institute of Technology, Banaras Hindu University Varanasi-221
Chapter Outline Dislocations and Strengthening Mechanisms
 Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Name: Unit 2- Elements, Compounds and Mixtures and Physical/Chemical Properties and Changes. Elements, Compounds and Mixtures
 Name: Unit 2- Elements, Compounds and Mixtures and Physical/Chemical Properties and Changes Day Page # Description IC/HW All 2 Warm-up IC 1 3 5 Matter Notes IC 1 6 Nuts & Bolts IC 1 7 Elements, Compounds
Name: Unit 2- Elements, Compounds and Mixtures and Physical/Chemical Properties and Changes Day Page # Description IC/HW All 2 Warm-up IC 1 3 5 Matter Notes IC 1 6 Nuts & Bolts IC 1 7 Elements, Compounds
FEATURES AND BENEFITS OF DIFFERENT PLATINUM ALLOYS. Kris Vaithinathan and Richard Lanam Engelhard Corporation
 FEATURES AND BENEFITS OF DIFFERENT PLATINUM ALLOYS Kris Vaithinathan and Richard Lanam Engelhard Corporation Introduction There has been a significant increase in the world wide use of platinum for jewelry
FEATURES AND BENEFITS OF DIFFERENT PLATINUM ALLOYS Kris Vaithinathan and Richard Lanam Engelhard Corporation Introduction There has been a significant increase in the world wide use of platinum for jewelry
BASIC CHEMICAL CONCEPTS
 CHEMISTRY EDUCATION: RESEARCH AND PRACTICE 2003, Vol. 4, No. 1, pp. 19-24 THE PRACTICE OF CHEMISTRY EDUCATION (PAPER) Concepts Peter G. NELSON University of Hull, Department of Chemistry BASIC CHEMICAL
CHEMISTRY EDUCATION: RESEARCH AND PRACTICE 2003, Vol. 4, No. 1, pp. 19-24 THE PRACTICE OF CHEMISTRY EDUCATION (PAPER) Concepts Peter G. NELSON University of Hull, Department of Chemistry BASIC CHEMICAL
Lecture 4: Thermodynamics of Diffusion: Spinodals
 Materials Science & Metallurgy Master of Philosophy, Materials Modelling, Course MP6, Kinetics and Microstructure Modelling, H. K. D. H. Bhadeshia Lecture 4: Thermodynamics of Diffusion: Spinodals Fick
Materials Science & Metallurgy Master of Philosophy, Materials Modelling, Course MP6, Kinetics and Microstructure Modelling, H. K. D. H. Bhadeshia Lecture 4: Thermodynamics of Diffusion: Spinodals Fick
Question Bank Electrolysis
 Question Bank Electrolysis 1. (a) What do you understand by the terms (i) electrolytes (ii) non-electrolytes? (b) Arrange electrolytes and non-electrolytes from the following substances (i) sugar solution
Question Bank Electrolysis 1. (a) What do you understand by the terms (i) electrolytes (ii) non-electrolytes? (b) Arrange electrolytes and non-electrolytes from the following substances (i) sugar solution
Interfacial Reaction between Sn Ag Co Solder and Metals
 Materials Transactions, Vol. 46, No. 11 (25) pp. 2394 to 2399 Special Issue on Lead-Free ing in Electronics III #25 The Japan Institute of Metals Interfacial Reaction between Sn Ag Co and Metals Hiroshi
Materials Transactions, Vol. 46, No. 11 (25) pp. 2394 to 2399 Special Issue on Lead-Free ing in Electronics III #25 The Japan Institute of Metals Interfacial Reaction between Sn Ag Co and Metals Hiroshi
A. Types of Mixtures:
 I. MIXTURES: SOLUTIONS 1) mixture = a blend of two or more kinds of matter, each of which retains its own identity and properties a) homogeneous mixture = a mixture that is uniform in composition throughout
I. MIXTURES: SOLUTIONS 1) mixture = a blend of two or more kinds of matter, each of which retains its own identity and properties a) homogeneous mixture = a mixture that is uniform in composition throughout
WJM Technologies excellence in material joining
 Girish P. Kelkar, Ph.D. (562) 743-7576 girish@welding-consultant.com www.welding-consultant.com Weld Cracks An Engineer s Worst Nightmare There are a variety of physical defects such as undercut, insufficient
Girish P. Kelkar, Ph.D. (562) 743-7576 girish@welding-consultant.com www.welding-consultant.com Weld Cracks An Engineer s Worst Nightmare There are a variety of physical defects such as undercut, insufficient
Ionic and Metallic Bonding
 Ionic and Metallic Bonding BNDING AND INTERACTINS 71 Ions For students using the Foundation edition, assign problems 1, 3 5, 7 12, 14, 15, 18 20 Essential Understanding Ions form when atoms gain or lose
Ionic and Metallic Bonding BNDING AND INTERACTINS 71 Ions For students using the Foundation edition, assign problems 1, 3 5, 7 12, 14, 15, 18 20 Essential Understanding Ions form when atoms gain or lose
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING
 CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
Soldering Definition and Differences
 Soldering Understanding the Basics Copyright 2014 ASM International M. Schwartz, editor All rights reserved www.asminternational.org Chapter 1 Soldering Definition and Differences SOLDER IS A FUSIBLE METAL
Soldering Understanding the Basics Copyright 2014 ASM International M. Schwartz, editor All rights reserved www.asminternational.org Chapter 1 Soldering Definition and Differences SOLDER IS A FUSIBLE METAL
LOGO. Modeling and Simulation of Microstructural Changes in Composite Sn-Ag-Cu Solder Alloys with Cu Nanoparticles
 Modeling and Simulation of Microstructural Changes in Composite Sn-Ag-Cu Solder Alloys with Cu Nanoparticles Yuanyuan Guan, A.Durga, Nele Moelans Dept. Metallurgy and Materials Engineering, K.U. Leuven,
Modeling and Simulation of Microstructural Changes in Composite Sn-Ag-Cu Solder Alloys with Cu Nanoparticles Yuanyuan Guan, A.Durga, Nele Moelans Dept. Metallurgy and Materials Engineering, K.U. Leuven,
FATIGUE BEHAVIOUR ANALYSIS OF DIFFERENTLY HEAT TREATED MEDIUM CARBON STEEL. Master of Technology (Res.) Metallurgical and Materials Engineering
 FATIGUE BEHAVIOUR ANALYSIS OF DIFFERENTLY HEAT TREATED MEDIUM CARBON STEEL A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Technology (Res.) in Metallurgical and
FATIGUE BEHAVIOUR ANALYSIS OF DIFFERENTLY HEAT TREATED MEDIUM CARBON STEEL A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Technology (Res.) in Metallurgical and
Name Class Date. What is ionic bonding? What happens to atoms that gain or lose electrons? What kinds of solids are formed from ionic bonds?
 CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
CHAPTER 1 2 Ionic Bonds SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is ionic bonding? What happens to atoms that gain or lose
Lecture 18 Strain Hardening And Recrystallization
 -138- Lecture 18 Strain Hardening And Recrystallization Strain Hardening We have previously seen that the flow stress (the stress necessary to produce a certain plastic strain rate) increases with increasing
-138- Lecture 18 Strain Hardening And Recrystallization Strain Hardening We have previously seen that the flow stress (the stress necessary to produce a certain plastic strain rate) increases with increasing
Corrosion-induced cracking of model train zincaluminium
 Nationaal Lucht- en Ruimtevaartlaboratorium National Aerospace Laboratory NLR Corrosion-induced cracking of model train zincaluminium die castings R.J.H. Wanhill and T. Hattenberg This report may be cited
Nationaal Lucht- en Ruimtevaartlaboratorium National Aerospace Laboratory NLR Corrosion-induced cracking of model train zincaluminium die castings R.J.H. Wanhill and T. Hattenberg This report may be cited
Full Density Properties of Low Alloy Steels
 Full Density Properties of Low Alloy Steels Michael L. Marucci & Arthur J. Rawlings Hoeganaes Corporation, Cinnaminson, NJ Presented at PM 2 TEC2005 International Conference on Powder Metallurgy and Particulate
Full Density Properties of Low Alloy Steels Michael L. Marucci & Arthur J. Rawlings Hoeganaes Corporation, Cinnaminson, NJ Presented at PM 2 TEC2005 International Conference on Powder Metallurgy and Particulate
EXPERIMENT 1 (Organic Chemistry I)
 EXPERIMENT 1 (Organic Chemistry I) Melting Point Determination Purpose a) Determine the purity of a substance using melting point as physical property b) Identify an unknown compound using its melting
EXPERIMENT 1 (Organic Chemistry I) Melting Point Determination Purpose a) Determine the purity of a substance using melting point as physical property b) Identify an unknown compound using its melting
Thermodynamics. Thermodynamics 1
 Thermodynamics 1 Thermodynamics Some Important Topics First Law of Thermodynamics Internal Energy U ( or E) Enthalpy H Second Law of Thermodynamics Entropy S Third law of Thermodynamics Absolute Entropy
Thermodynamics 1 Thermodynamics Some Important Topics First Law of Thermodynamics Internal Energy U ( or E) Enthalpy H Second Law of Thermodynamics Entropy S Third law of Thermodynamics Absolute Entropy
Work hard. Be nice. Name: Period: Date: UNIT 1: Introduction to Matter Lesson 4: A Fine Line Between Compounds and Mixtures
 Name: Period: Date: UNIT 1: Introduction to Matter Lesson 4: A Fine Line Between Compounds and Mixtures Do Now: PRE-READING OPEN-NOTES QUIZ! By the end of today, you will have an answer to: How do pure
Name: Period: Date: UNIT 1: Introduction to Matter Lesson 4: A Fine Line Between Compounds and Mixtures Do Now: PRE-READING OPEN-NOTES QUIZ! By the end of today, you will have an answer to: How do pure
The Periodic Table: Periodic trends
 Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Chapter 12 - Liquids and Solids
 Chapter 12 - Liquids and Solids 12-1 Liquids I. Properties of Liquids and the Kinetic Molecular Theory A. Fluids 1. Substances that can flow and therefore take the shape of their container B. Relative
Chapter 12 - Liquids and Solids 12-1 Liquids I. Properties of Liquids and the Kinetic Molecular Theory A. Fluids 1. Substances that can flow and therefore take the shape of their container B. Relative
Solution for Homework #1
 Solution for Homework #1 Chapter 2: Multiple Choice Questions (2.5, 2.6, 2.8, 2.11) 2.5 Which of the following bond types are classified as primary bonds (more than one)? (a) covalent bonding, (b) hydrogen
Solution for Homework #1 Chapter 2: Multiple Choice Questions (2.5, 2.6, 2.8, 2.11) 2.5 Which of the following bond types are classified as primary bonds (more than one)? (a) covalent bonding, (b) hydrogen
We will study the temperature-pressure diagram of nitrogen, in particular the triple point.
 K4. Triple Point of Nitrogen I. OBJECTIVE OF THE EXPERIMENT We will study the temperature-pressure diagram of nitrogen, in particular the triple point. II. BAKGROUND THOERY States of matter Matter is made
K4. Triple Point of Nitrogen I. OBJECTIVE OF THE EXPERIMENT We will study the temperature-pressure diagram of nitrogen, in particular the triple point. II. BAKGROUND THOERY States of matter Matter is made
2 MATTER. 2.1 Physical and Chemical Properties and Changes
 2 MATTER Matter is the material of which the universe is composed. It has two characteristics: It has mass; and It occupies space (i.e., it has a volume). Matter can be found in three generic states: Solid;
2 MATTER Matter is the material of which the universe is composed. It has two characteristics: It has mass; and It occupies space (i.e., it has a volume). Matter can be found in three generic states: Solid;
RAPIDLY SOLIDIFIED COPPER ALLOYS RIBBONS
 Association of Metallurgical Engineers of Serbia AMES Scientific paper UDC:669.35-153.881-412.2=20 RAPIDLY SOLIDIFIED COPPER ALLOYS RIBBONS M. ŠULER 1, L. KOSEC 1, A. C. KNEISSL 2, M. BIZJAK 1, K. RAIĆ
Association of Metallurgical Engineers of Serbia AMES Scientific paper UDC:669.35-153.881-412.2=20 RAPIDLY SOLIDIFIED COPPER ALLOYS RIBBONS M. ŠULER 1, L. KOSEC 1, A. C. KNEISSL 2, M. BIZJAK 1, K. RAIĆ
How do single crystals differ from polycrystalline samples? Why would one go to the effort of growing a single crystal?
 Crystal Growth How do single crystals differ from polycrystalline samples? Single crystal specimens maintain translational symmetry over macroscopic distances (crystal dimensions are typically 0.1 mm 10
Crystal Growth How do single crystals differ from polycrystalline samples? Single crystal specimens maintain translational symmetry over macroscopic distances (crystal dimensions are typically 0.1 mm 10
Periodic Table, Valency and Formula
 Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
Simulations of the Effect of Section Size and Cooling on Sigma Phase Formation in Duplex Stainless Steels
 Simulations of the Effect of Section Size and Cooling on Sigma Phase Formation in Duplex Stainless Steels Richard A. Hardin and Christoph Beckermann Department of Mechanical and Industrial Engineering
Simulations of the Effect of Section Size and Cooling on Sigma Phase Formation in Duplex Stainless Steels Richard A. Hardin and Christoph Beckermann Department of Mechanical and Industrial Engineering
CENTRIFUGAL CASTING. Email: amitjoshi@iitb.ac.in amitjoshi1000@yahoo.ca
 CENTRIFUGAL CASTING Amit M Joshi (B.Engg. Mechanical, A.M.I.Prod.E, A.I.E) Dept. of Metallurgical Engg. & Material Science, Indian Institute of Technology Bombay, India. Email: amitjoshi@iitb.ac.in amitjoshi1000@yahoo.ca
CENTRIFUGAL CASTING Amit M Joshi (B.Engg. Mechanical, A.M.I.Prod.E, A.I.E) Dept. of Metallurgical Engg. & Material Science, Indian Institute of Technology Bombay, India. Email: amitjoshi@iitb.ac.in amitjoshi1000@yahoo.ca
KINETIC MOLECULAR THEORY OF MATTER
 KINETIC MOLECULAR THEORY OF MATTER The kinetic-molecular theory is based on the idea that particles of matter are always in motion. The theory can be used to explain the properties of solids, liquids,
KINETIC MOLECULAR THEORY OF MATTER The kinetic-molecular theory is based on the idea that particles of matter are always in motion. The theory can be used to explain the properties of solids, liquids,
VYSOKÁ ŠKOLA BÁŇSKÁ TECHNICAL UNIVERSITY OF OSTRAVA COMPUTER SIMULATION AND MODELLING IN MATERIALS ENGINEERING. Study Support
 VYSOKÁ ŠKOLA BÁŇSKÁ TECHNICAL UNIVERSITY OF OSTRAVA FACULTY OF METALLURGY AND MATERIALS ENGINEERING COMPUTER SIMULATION AND MODELLING IN MATERIALS ENGINEERING Study Support Jaromír Drápala, Vlastimil Vodárek,
VYSOKÁ ŠKOLA BÁŇSKÁ TECHNICAL UNIVERSITY OF OSTRAVA FACULTY OF METALLURGY AND MATERIALS ENGINEERING COMPUTER SIMULATION AND MODELLING IN MATERIALS ENGINEERING Study Support Jaromír Drápala, Vlastimil Vodárek,
Copper and its alloys
 Copper and its alloys Lecture 4 Subjects of interest Introduction/Objectives Extraction of copper from ores and refining of copper Classification of copper alloys The wrought copper Copper zinc alloys
Copper and its alloys Lecture 4 Subjects of interest Introduction/Objectives Extraction of copper from ores and refining of copper Classification of copper alloys The wrought copper Copper zinc alloys
SINTERING OF POWDER PREMIXES A BRIEF OVERVIEW
 SINTERING OF POWDER PREMIXES A BRIEF OVERVIEW Kalathur S.Narasimhan and Frederick J.Semel Hoeganaes Corporation 1001 Taylors lane Cinnaminson,NJ 08077 Abstract Advances in the understanding of the sintering
SINTERING OF POWDER PREMIXES A BRIEF OVERVIEW Kalathur S.Narasimhan and Frederick J.Semel Hoeganaes Corporation 1001 Taylors lane Cinnaminson,NJ 08077 Abstract Advances in the understanding of the sintering
