Module 34. Heat Treatment of steel IV. Lecture 34. Heat Treatment of steel IV
|
|
|
- Marion Walker
- 8 years ago
- Views:
Transcription
1 Module 34 Heat reatment of steel IV Lecture 34 Heat reatment of steel IV 1
2 Keywords : Austenitization of hypo & hyper eutectoid steel, austenization temperature, effect of heat treatment on structure & properties of hypo & hyper eutectoid steel, tempering, effect of tempering on structure & properties of steel, tempering parameter, recalescence Introduction 2 he wide variety of phase transformations that takes place in steel makes it one of the most attractive engineering materials. We are now familiar with the basic principles of each of these. he Fe Fe 3 C phase diagram helps one guess the microstructure and properties of steel as a function of composition provided the cooling rate is extremely slow. It suggests that eutectoid austenite below a critical temperature (A 1 ) transforms into a mixture of ferrite and carbide. If the cooling rate is slow or if it is allowed to decompose at a temperature a little below A 1 it gives a coarse lamellar structure consisting of alternate layers of ferrite and cementite plates. his type of structure is known as pearlite. his is a diffusion controlled process where carbon atoms move over a long distance in austenite. As against this if it is cooled extremely fast the carbon in austenite (FCC) is not allowed to come out of the solid solution when it transforms into ferrite (which is BCC). he resultant structure gets distorted due the presence of excess carbon in its octahedral interstices. It consists of a homogeneous phase called martensite having BC crystal. It is extremely hard and brittle. It forms without any diffusion when the temperature drops below a critical temperature (M s ) and the process continues until the temperature reaches M f. Apart from the two extreme cases there is a range of temperature over which the austenite decomposes into a very fine mixture of ferrite and carbide. he carbide size and shape is distinctly different from that of pearlite. his structure is known as bainite. he structural features of steel as a function of time and temperature are represented with the help of a diagram. It depends on composition of the steel and the temperature at which austenitization has been done. We are familiar with the main features of such diagrams for hypo eutectoid, eutectoid and hyper eutectoid steels. hese are useful in planning heat treatment schedules based on isothermal holds at different temperatures. However most commonly used heat treatments processes adopt continuous cooling. A different version of transformation diagram is needed to guess the type of structure that is expected in steel on cooling at a given rate. hese are known as CC diagram. Like diagram it is a function of %C in steel and the temperature at which it is transformed into austenite. owards the end of the last module its usefulness in predicting the microstructure of steel during annealing, normalizing and hardening of eutectoid steel was explained. able 1 gives a comparison of the main characteristics of the 3 heat treatment processes with respect to eutectoid steel. Slide 1 shows the heating & cooling cycles used and the CC diagram with cooling curves for the 3 processes. In each of the three common heat treatment processes conversion of ferrite carbide structure into austenite is important. his occurs through a diffusion controlled process. Let us begin this module by looking at this process a little critically.
3 able 1 Characteristics Annealing Normalizing Hardening Cooling rate Furnace cooled Air cooled Water quenching Microstructure Coarse pearlite Fine pearlite Martensite Hardness R c (10 20) R c R c 64 Ductility Very good Good poor Residual stress Negligible Low Very high Structural stability Best Moderate Least Annealing, normalizing & hardening: eutectoid steel H: WQ t A: FC N: AC H M Log t Martensite N A Coarse Pearlite Fine Pearlite Slide 1 (R C ) H > (RC) N > (RC) A Austenitization of hypo & hyper eutectoid steel: 3 his is the first stage of transformation during annealing, normalizing or hardening of steel. he main purpose is to get a homogeneous austenite. At room temperature steel has two phases: ferrite and cementite. During heating when the temperature goes beyond A 1 cementite reacts with adjacent ferrite to form austenite. (a) cm C /cm C 910 (b) A 3 Austenitization temperature + cm A cm + cm A 1 Fig 1 0 C %C C /cm
4 Figure 1: shows what happens at the ferrite cementitite interface when the temperature goes beyond A 1, the lower critical temperature. iny nuclei of austenite form at the ferrite cementite boundary. Figure 1(a) gives an enlarged view of pearlite having alternate plates of cementite and ferrite. It shows the formation of a tiny austenite nucleus. It would setup a concentration gradient within the austenite grain. he origin of concentration gradient is easily understood from fig 1(b). he horizontal line at the austenitization temperature intersects A 3 at C. his represents the concentration of carbon in austenite at the ferrite / austenite boundary. Similarly the point of intersection of this line with A cm represents the concentration of carbon in austenite at the austenite / cementite interface. Note that C /cm > C. herefore carbon would diffuse from the austenite/ cementite interface to the austenite / ferrite interface. As a result the cm and ferrite regions would shrink and the austenite nucleus would grow. he diffusion of carbon in austenite would primarily determine the kinetics of transformation. he mass flow rate due to diffusion depends on diffusivity and concentration gradient. Exactly at A 1 there is hardly any concentration gradient. herefore the transformation rate is negligible. Some amount of superheating beyond A 1 is necessary for the transformation of pearlite to austenite. he extent of transformation is a function of both temperature and time. It can be represented in the form a time temperature transformation diagram. An example of such a diagram for eutectoid steel is given in fig 2. It helps in the estimation of the time needed to transform eutectoid steel into homogeneous austenite at a temperature above A1. Higher the temperature the shorter is the time required. 0% 100% Homogeneous + +cm het Austenitization temperature Fig 2 A 1 + cm Isothermal hold time to get homogeneous Log (time) 4
5 Hypo-eutectoid steel het hom A, N, H Coarse A 3 +cm A 3 A 1 +cm cm Slide 2 %C time Austenitization: aim to get fine homogeneous 5 Slide 2 shows the process of austenitization of hypo eutectoid steel of a particular composition. he aim of austenitization is to get homogeneous. Let us look at this process with respect to a particular grade of steel whose location on the relevant part of the phase diagram is shown by the dashed line in the sketch on the left in slide 2. he conversion of pearlite to austenite begins as soon as the temperature goes beyond A 1. he sketch on the right shows a time temperature diagram of this steel representing the various stages of transformation. he time it takes for this to begin at A 1 is extremely long but it decreases with increasing temperature. he structure now consists of ferrite, austenite and cementite. Although austenite has formed still there may be a significant amount of pearlite (recall that pearlite is a mixture of ferrite and cementite). he time at which pearlite disappears from the structure is also a function of temperature. It is longer at lower temperature. After the conversion of pearlite to austenite the structure consists of +. he amount of ferrite decreases with increasing temperature and time. he transformation diagram in slide 2 shows a line beyond which there is no ferrite. However still it may not be homogeneous austenite. he region is labeled as het. It takes a little more thermal exposure for the concentration of carbon in austenite to be uniform. he sketch includes a line that gives the time needed for this to happen as a function of temperature. If the samples are kept too long a time beyond that required for homogeneous austenite to form the grain may become unusually coarse. Coarse grain austenite on subsequent cooling gives relatively poor toughness. herefore too high a temperature or too long a time should be avoided. he phase diagram in slide 2 shows the range of recommended austenitization temperature for annealing (A), normalizing (N), and hardening (H) of hypo eutectoid steel. It is usually C higher than the upper critical temperature (A 3 ).
6 Hyper eutectoid steel A 3 +cm %C N A, H A cm A 1 M S M f + M Log (t) FC AC Slide 3 Normalizing: Air cooled from A Cm +30 C. Air cooling suppresses cm network formation. Cm network makes the steel brittle: unusable he phase diagram in slide 3 shows the recommended austenitization temperature for annealing (A), normalizing (N) and hardening (H) of hyper eutectoid steel. he purpose of annealing is to get a soft and ductile structure. If the steel is heated beyond A cm to form homogeneous austenite, on cooling it is likely to give a continuous network of pro eutectoid cementite around austenite before the remaining austenite transforms into coarse pearlite. his is because during annealing the component is furnace cooled (FC). he corresponding cooling curve has been superimposed on the diagram given in slide 3. Precipitation of proeutectoid cementite begins soon after the temperature drops below A cm. he grain boundaries are the most preferred site for precipitation. Cementite is brittle. his makes the structure prone to inter granular cracking. herefore the purpose of annealing is lost. As against this if austenitization is done at A C before slow furnace cooling begins the structure consists of globules of cementite surrounding the prior austenite grains which subsequently transforms into coarse pearlite. here is no chance of forming a brittle network around the prior austenite grain boundaries. herefore this is the recommended austenitization temperature for annealing of hyper eutectoid steel. 6 Pearlite Cementite Fig 3 (a) (b)
7 Figure 3(a) shows the microstructure of hyper eutectoid steel if it is furnace cooled (FC) from A cm + 30 C. his type of structure has poor resistance to withstand shock loading. It is responsible for poor ductility. herefore it should be avoided. Figure 3(b) shows the microstructure of hyper eutectoid steel when it is furnace cooled (FC) after soaking at A C. During soaking at a temperature higher than A 1 the pro eutectoid cementite has enough time to become spherical. he driving force for such a change is the decrease in total surface energy of carbide. his is because a sphere has the least surface area to volume ratio. he amount of pro eutectoid cementite may still be the same. However in the absence of a continuous grain boundary network of brittle cementite there is no easy path for a crack to grow. herefore such a microstructure has excellent toughness or resistance to crack growth. his is the most desired structure of annealed hyper eutectoid steel. he austenitization temperature for normalizing of hyper eutectoid steel is shown on the phase diagram in slide 3 by a bar labeled N. It is around A cm + 30 C. After homogenization the sample (or the job) is cooled in air. he cooling rate is relatively fast. he corresponding cooling curve is superimposed on the schematic diagram given in slide 3. he time allowed for the precipitation of pro eutectoid cementite is much less. herefore the amount of pro eutectoid cementite will be less than that in the case of annealing. he amount often is not high enough to form a continuous carbide net work. Figure 4 shows a schematic microstructure of normalized hyper eutectoid steel. Pearlite Cementite Fig 4: Shows the main features of the microstructure of normalized hyper eutectoid steel. It has broken network of pro eutectoid cementite around the prior austenite grain boundaries. he pearlite is expected to be much finer than that of the annealed steel. 7 he purpose of hardening is to get maximum possible hardness in steel. he temperature at which steel is austenitized has a marked influence on its hardness. It would have the maximum hardness if it has 100% martensitic structure and %C in martensite is greater than 0.6. he phase diagram in slide 3 gives the austenitizing temperature for hardening of hyper eutectoid steel. It is given by A C. It is same for all hyper eutectoid steel. On austenitization at this temperature it has at least 0.8%C in solid solution. On quenching therefore martensite is likely to have 0.8% C. his is high enough to give the maximum possible hardness. Recall that the M f temperature of steel deceases with increasing %C in austenite. If hyper eutectoid steel is austenitized above A cm it would have much higher amount of carbon in solid solution. Its M f temperature may even be lower than the room temperature. herefore even on quenching to room temperature the martensitic transformation may not be complete. here will be some amount of retained austenite. As a result the hardness will be lower than the expected value.
8 he purpose of hardening will be defeated. his is why A cm +30 C is the optimum austenitization temperature for hardening of hyper eutectoid steel. Effect of heat treatment on the structure and properties of hypo eutectoid steel: Annealing, normalizing and hardening are the 3 most common heat treatments given to hypoeutectoid steel. During each of the 3 processes austenitization is done at a temperature C higher than the upper critical temperature of steel. Subsequently it is water quenched in the case of hardening, air cooled in the case of normalizing and furnace cooled in the case of annealing. he microstructures and properties after these heat treatments are going to be widely different. Slide 4 explains the origin of the marked difference in their structure and properties using an appropriate CC diagram. A 3 A 1 M s Martensite M f H Hardening Hypo-eutectoid steel P s + M f s N P f Log (time) A Normalizing Pro-eutectoid ferrite Coarse pearlite Annealing Fine pearlite GB ferrite Slide 4 8 he cooling rate in the case of annealing is extremely slow. he sample after austenitization remains within the furnace, only the power supply is cut off. he cooling rate corresponds to equilibrium cooling rate. he cooling curve during the process has been superimposed on the CC diagram of hypoeutectoid steel. It is labeled as A. he precipitation of pro eutectoid ferrite begins when the curve A intersects the curve labeled f s. It continues until the cooling curve intersects P s it denotes the onset of decomposition of austenite into pearlite. he entire transformation process is complete when the cooling curve intersects the line labeled P f. he final structure therefore consists of pro eutectoid ferrite and coarse pearlite. he amount of ferrite can be estimated using lever rule if the composition is known. Alternatively if the amount of pro eutectoid ferrite can be determined %C can be calculated using lever rule. his is possible because the cooling rate is extremely slow.
9 he cooling rate in the case of normalizing is moderate. he sample after austenitization is taken out of the furnace and allowed to cool in air. he cooling rate is higher than the equilibrium cooling rate. his is likely to affect diffusion controlled transformation. he cooling curve during the process has been superimposed on the CC diagram of hypoeutectoid steel. It is labeled as N. he precipitation of pro eutectoid ferrite begins when the curve N intersects the curve labeled f s. Note that the transformation begins at a temperature lower than that in the case of annealing. Precipitation of ferrite continues until the cooling curve intersects P s it denotes the onset of decomposition of austenite into pearlite. his occurs at a relatively lower temperature. he pearlite that forms is likely to be finer. he entire transformation process is complete when the cooling curve intersects the line labeled P f. he final structure therefore consists of pro eutectoid ferrite and fine pearlite. he amount of pro eutectoid ferrite is less than that you get during annealing. 9 he cooling rate in the case of hardening is extremely fast. he sample after austenitization is directly quenched in water. he temperature of the sample as it cools on quenching has been superimposed on the CC diagram in slide 3 in the form of a cooling curve labeled as H. It avoids the nose of the C curve. It only intersects the horizontal lines representing M s and M f temperatures. It suggests that austenite should transform completely into martensite. he process is athermal and there is no diffusion of carbon atoms within the lattice. he %C in martensite is exactly same as that in austenite before quenching. Recall that martensite can be considered as a super saturated solid solution of carbon in BCC ferrite. he presence of excess carbon within the interstitial site results in tetragonal distortion. his is responsible for the change over from body centered cubic (BCC) in ferrite to body centered tetragonal structure (BC) in martensite in the presence of excess carbon. he c/a ratio of BC martensite is a function of %C. It is around 1.05 in martensite having 0.8%C. Note that its crystal structure is marginally different from that of BCC. he morphology of martensite depends on its carbon content. If %C is less than 0.6 the austenite grains get subdivided into a large number of laths of martensite crystal. It %C is greater than 0.6 the austenite transforms into a large number of plates of martensite. he microstructure shown in slide 4 is that of plate or acicular martensite. he size of lath or plate is extremely small. herefore the 3 main factors responsible for the unusually high hardness of martensite are (i) solid solution strengthening due the presence of excess carbon in solid solution (ii) strengthening due to extremely fine laths / plates (grain size effect) (iii) asymmetric lattice distortion due to the presence of carbon atoms in octahedral interstices. Martensitic transformation is accompanied by volume expansion. he regions surrounding the laths or plates are highly stressed. his too may add to strengthening. Excessive stress may also suppress the transformation of austenite to martensite. Often after hardening steel may have some amount of untransformed austenite at room temperature even if it is lower than M f. In other words apart from being hard and brittle steel after hardening has
10 very high residual stress and unstable retained austenite. herefore it is given an additional heat treatment called tempering. Effect of heat treatment on the structure and properties of hyper eutectoid steel: Annealing, normalizing and hardening are also the 3 most common heat treatments given to hyper eutectoid steel. he main difference lies in the selection of appropriate austenitization temperature. Hyper eutectoid steel becomes 100% austenite on heating to a temperature a little beyond A cm, representing the temperature at which cementite dissolves in the matrix. During normalizing hyper eutectoid steel is soaked at A cm + 30 C so that it converts entirely into homogeneous austenite. Subsequently it is allowed to cool in air. As against this the soaking temperatures of hyper eutectoid steel for annealing and hardening are A C (look at slide 3). During this stage the room temperature structure gets converted into one consisting of globules of cementite dispersed within homogeneous austenite. Subsequently it is water quenched in the case of hardening, and furnace cooled in the case of annealing. he microstructures and properties after these heat treatments are going to be widely different. Slide 5 explains the origin of the marked difference in their structure and properties using an appropriate CC diagram. A cm A 1 M S M f Hyper-eutectoid steel: annealing & hardening + cm + M P s Normalizing Log(t) cm s P f P + cm FC AC Why austenitization above A Cm not recommended? A 1 M S M f M (b) +cm + cm WQ (a) P s + M P f P+ cm Annealing Hardening Log(t) (c) cm FC P Slide 5 10 Slide 5 gives two CC diagrams. One on the left is used to predict or describe the evolution of structural changes when hyper eutectoid steel is cooled after homogenization at A cm +30 C. It has two cooling curves superimposed on the CC diagram. One represents furnace cooling (FC:
11 extremely slow cooling) and other air cooling (AC: moderate cooling). Annealing means furnace cooling. If homogeneous austenite is allowed to cool in furnace the pro eutectoid cementite would precipitate along the austenite grain boundary forming a continuous network. When the temperature drops below A 1 the remaining austenite transforms into coarse pearlite. Such a structure is brittle. his is why hyper eutectoid steel is not heated beyond A cm if it is to be annealed. However in the case of normalizing it does not pose a problem because the cooling rate adopted is relatively fast to suppress complete precipitation of cementite. Look at the cooling curve labeled as AC. he time spent within the range of temperature where cm precipitates is very short. he amount of cm is not large enough to form a continuous network of brittle cm around the grains of austenite. One of the objectives of normalizing heat treatment is to break the continuous carbide network in cast hyper eutectoid steel. he transformation gets over by the time the cooling curve intersects the line denoted as P f. It falls within the fine pearlitic region of the CC diagram. he final structure of normalized hypereutectoid steel consists of discontinuous carbide network along prior austenite grain boundaries in a matrix of fine nodules of pearlite. It is expected to be moderately hard and ductile. Austenitization temperature for annealing of hyper eutectoid steel is A C. It is independent of %C. On completion of austenitization the microstructure consists of globules of cm in a matrix of austenite (see the sketch (a) in slide 5). he initial structure before the onset of cooling is different from that in the case of normalizing (here it is 100% austenite). his is why a different CC diagram is needed to interpret its microstructure. Slide 5 shows such a diagram. A cooling curve labeled as FC (furnace cooling) has been superimposed on this diagram. his corresponds to the one adopted during annealing. Note that the transformation of austenite to pearlite begins when the cooling curve intersects the line P s and it is completed at a temperature where the plot intersects the line P f. he transformation takes place in the temperature range where you expect coarse pearlite to form. he final structure of this steel after annealing consists of globules of cm in a matrix of coarse pearlite (see the sketch (c) in slide 5). It is expected to be soft and ductile. 11 Austenitization temperature for hardening of hyper eutectoid steel is A C. It is independent of %C. On completion of austenitization the microstructure consists of globules of cm in a matrix of austenite (see the sketch (a) in slide 5). he initial structure before the onset of cooling is similar to that in the case of annealing. his is why the CC diagram used to interpret its microstructure is same as that used in the case of annealing (see slide 5). A cooling curve labeled as WQ (water quenched) has been superimposed on this diagram. his corresponds to the one used during hardening. Note that the transformation of austenite to pearlite is totally suppressed. he curve does not intersect the Ps of the CC diagram. However diffusion less martensitic transformation takes place in this steel in the temperature range Ms
12 to Mf. he final structure of this steel after hardening consists of globules of cm in a matrix of martensite (see the sketch (b) in slide 5). %C in martensite is a little higher than that in eutectoid steel (0.8%C). he morphology of martensite depends on %C. If it is greater than 0.6 it is likely to have plate morphology. he speed at which the plates grow is extremely fast. It is accompanied by significant expansion in volume. he austenitic regions surrounding the plates get highly compressed. his may inhibit further transformation. his is why some amount of austenite may still exist in the tiny regions between the plates even if the room temperature is lower than Mf. Nevertheless the bulk of the microstructure would consist of plate martensite. It is very hard and brittle. It would also have high amount of residual stress. It may lead to cracking. herefore it is necessary to give an additional heat treatment. It is known as tempering. It improves the toughness (or the resistance to crack growth) of steel. empering: Steel must be cooled rapidly if it is to be hardened. he most common practice is to quench it in water after it has been austenitized at an appropriate temperature. When you quench a piece of steel of finite size its surface no doubt cools very fast but its core would cool slowly. At any instant the temperature () at the core would be higher than that at its surface. he difference between the two ( ) is a function of the size of the specimen (or the job / component). It increases with the increase in the size of the specimen. he thermal stress that develops is proportional to. herefore it is high in a thick component. Apart from this there would be additional stresses due to the change in volume associated with the transformation of austenite to martensite. he net stress is the sum of the two. Its magnitude might be high enough to cause distortion or cracking. his must be avoided. One obvious way is to adopt slow cooling. But it may not favor formation of martensitic structure. he other option is to heat the sample once again after quenching. he temperature should be high enough to relieve the stresses. he process is known as tempering. Apart from stress relief there are two more reasons that are worth noting. (i) High carbon marteniste is extremely brittle. empering would make it little more ductile and tough (higher impact / crack resistance). (ii) he presence of retained austenite poses an additional problem. It is unstable at room temperature. It may decompose into a ferrite carbide structure with time. Such a transformation is accompanied by an expansion in volume. his may lead to cracking or a change in the dimension of the component. 12
13 empering Hardening empering R C 2 1 Slide 6 time time 3 Objective: (i) Improve toughness & ductility (ii) Remove retained austenite to give structural / dimensional stability (iii) Remove residual stresses Slide 6 shows the heating and cooling cycle for tempering of steel. It always follows hardening heat treatment. It is an isothermal heat treatment process. During tempering local plastic deformation due to the presence of residual stresses and diffusion controlled phase transformation take place. he kinetics of transformation depends on both time and temperature. After a specific thermal exposure the sample is either oil quenched or air cooled. Slide 6 gives hardness (R c ) versus time plots at three different temperatures. he change in hardness is a good indicator of the kinetics of transformation that takes place during tempering. It drops with increasing time at a constant temperature. It is more prominent at higher temperatures. If the hardness is plotted against a combined time temperature parameter the hardness data fall on common master plot as shown in slide 7. One of these is called Holloman Jaffe tempering parameter. It is given by (C + log t) where is the temperature in K and t is time in hour and the constant C =
14 empering parameter R C 2 t 21 t 22 t 23 1 t 11 t 12 t 13 3 t 31 t 32 t 33 Slide 7 (C+log t) Holloman & Jaffe: C=14.3 Structural changes during tempering Stage I: HCM=LCM+ carbide R C I II III II: RA = + carbide III: carbide dissolves & M= +carbide Slide c/a 1.0 F(,t) M= +cm time Experimental techniques Microstructure X Ray diffraction Dilatometer 14 Slide 8 gives the structural changes that occur during tempering. Note that the hardness versus time temperature parameter plot can be divided into three stages. During stage I high carbon martensite transforms into a mixture of low carbon martensite and epsilon carbide (Fe 2.4 C). During stage II the retained austenite (RA) transforms into a mixture of ferrite and carbide. Its structure is similar to that of bainite. During stage III epsilon carbide is replaced by cm globules and low carbon martensite transforms into ferrite. When the globules of cementite coarsen there is a significant drop in the hardness of steel. he exact nature of the plot depends on the initial carbon content of martensite and the amount of retained austenite. Low carbon
15 martensite has lower hardness. In such a case precipitation of very fine epsilon carbides may be accompanied by a little increase in hardness. If %C is higher than 0.6 the hardness of martensite should be around R c 64. When it transforms into low carbon martensite it should be accompanied by a drop in hardness. However in the presence of very small precipitates the drop in hardness may not be significant. If hardened steel has a significant amount of retained austenite there may be an increase in hardness when it transforms into ferrite carbide aggregate. In short the trend shown by hardness plot may vary widely depending of the composition and microstructure of steel after hardening. Slide 9 gives a summary of the structural change expected in hardened steel as a function of tempering temperature. Apart from hardness there are several other ways of monitoring the transformations that occur during tempering. Slide 8 includes 3 such techniques. hese are examination of microstructure, X ray diffraction and dilatometer. he crystal structure of martensite is BC. X ray diffraction technique can be used to find its c/a ratio. here is a plot in slide 8 that shows how c/a ratio is expected to change as a function of time at a given tempering temperature. Structural changes during tempering emp range Microstructure Properties C LCM + carbide Slight increase in hardness C RA: decomposition Hardness starts decreasing C carbides pick up C to form Cm: not resolved in OM Marked decrease in hardness C Coarsening of Cm Fully softened Slide 9 empered martensite having Rc has excellent combination of strength & toughness. Problem? 15 he drop in hardness during tempering is accompanied by an increase in its ductility and toughness. empered martensitic structure is known to have the best combination of strength and toughness. It is possible to get same hardness in steel by giving different heat treatments. For example you can get a hardness of R c 35 in a given piece of steel either by normalizing or by hardening and tempering but hardened and tempered steel has much higher impact toughness than the one which is normalized. Sometimes tempering can make steel brittle. One of these is associated with the precipitation of carbide. It occurs at low temperature. he other takes
16 place on slow cooling through a temperature range of C after tempering. It is due to presence of a few unwanted alloy elements in steel. It can be avoided by fast cooling. Charpy V Notch (CVN) test is the best possible way to determine the susceptibility of a specific grade of steel to such embrittlement. It will be taken up later after we talk about the effect of alloy addition on structure and properties of steel. Recalescence: It is associated with reheating during cooling as a result of eutectoid transformation. It is important in steel having higher percentage of pearlite. he cooling curves used to describe the effect of cooling rate on the evolution of structures in steel were assumed to be smooth. However because of reheating effect that occurs due to phase transformation it is often difficult to maintain a constant cooling rate. It can easily be demonstrated in the laboratory by heating a piano wire made of high carbon steel, held straight between a pair of grips, by passing electrical current from a power source until it becomes red hot and then let it cool in air. During heating wire expands but when it converts to austenite it contracts. he two balance each other. herefore the wire remains straight. During cooling it becomes dull initially but again it gets heated when austenite begins to transform. his is because it is exothermic. he wire again becomes red & sags because of expansion due to both temperature increase & phase change. Recall that the transformation of austenite to pearlite or martensite is accompanied by an expansion in volume. his is illustrated with the help of sketches in fig 5. he effect is more prominent in high carbon steel. Piano wire becomes red when heated but remains straight. On cooling initially becomes dull grey but remains straight A 1 & t at which eutectoid transformation begins Fig 5 Becomes red hot again when it begins to transform & sags Log t Actual cooling curve 16 An idea about recalesence (the extent of reheating due to phase transformation) while steel is being cooled is of considerable importance in the design and selection of cooling scheme for continuous thermo mechanical treatments (M). For example during the production of high strength bars / rods after M have to be cooled rapidly. During this stage it is necessary to
17 take away the heat contained within the product as well as that being generated due to phase transformation. Dual phase steel: Ever since the oil crisis of 1970s there have considerable efforts world over for the development of high strength steel so that more fuel efficient automobiles could be produced by reducing their weight. Ever since, several new grades of high strength low alloy steel has been introduced. Although some of these will be covered in subsequent modules it may be worthwhile to mention about dual phase steel in this module because high strength in this grade was achieved by adopting a simple but novel heat treatment scheme. Bulk of the steel used in load bearing members of automobiles or critical structures are made of low carbon steel. Strength of such steel can be substantially increased by quenching it from a temperature a little above A 1. his would have a microstructure consisting of soft ferrite and hard martensite. he amount of ferrite in this steel is around 70 80% and the balance being martensite. he ratio of the two can be controlled by proper selection of the temperature within the ferrite austenite field of the phase diagram. he presence of hard martensite in a soft matrix made of ferrite gives it an optimum combination of strength and ductility. Besides these it has a smooth stress strain curve. Unlike most low carbon steel it does not exhibit yield point phenomenon. herefore it has excellent cold formability as well. he development took place almost concurrently during at Nippon steel, General Motors and Ford. It is commonly known as dual phase steel. Summary: 17 In this lecture we looked at annealing, normalizing and hardening; the three common methods of heat treatment of steel. he principles behind the selection of austenitization temperature have been explained. he process of austenization is a diffusion controlled phenomenon. Its kinetics depends on both temperature and time. herefore it can be represented by time temperature transformation diagram. It depends on the composition of steel. he importance of CC diagram in describing the evolution of microstructure during heat treatment has been emphasized. In order to harden steel it must be cooled rapidly. he final structure is unstable. It has retained austenite and high residual stresses. herefore it must be tempered as early as possible. he structural changes that take place during tempering have been explained. he problem of reheating during air cooling of high carbon steel has been discussed. Its occurrence is due to exothermic eutectoid transformation. A simple experimental technique to demonstrate this has been described. he phenomenon is known as recalesence.
18 Exercise: 1. Suggest a simple experimental method for determination of Ms temperature of steel if you have only facility for heat treatment and metallographic examination. 2. Which of the two would require more severe cooling rate to get a fully hardened structure? (a) 0.8 % carbon steel (b) 1.0 % C steel. 3. A piece of steel which was quenched after prolonged holding at 700⁰C was found to have ferrite martensite structure. Explain when would you expect this to happen? 4. List the factors that determine the strength of properly hardened steel % plain carbon steel in annealed condition has 25% coarse pearlite. If it is normalized (heat treatment) what changes would you expect in its microstructure? 6. A thin strip of 1.2% carbon steel is quenched in water from its fully austenitic state. What structure would you expect in this steel? Answer: 1. Austenitize a steel specimen, quench in baths maintained at a temperature close to its expected Ms, heat it again by placing it in another bath maintained at a little higher temperature, and then quench in water after holding it for some time. Repeat the same steps with different combination of the intermediate quenching temperatures. Polish etch and examine the microstructures under microscope. If martensite forms at the first step cooling it will get tempered when it is put in a higher temperature bath. empered martensite etches easily and it appears dark under microscope. Amount of tempered martensite can be estimated. Plot % tempered martensite against intermediate quenching temperature. Extrapolate it to 0% tempered martensite. his gives an estimate of Ms. he experimental scheme is shown in the following figure for eutectoid steel Ms Log t %M Ms emperature Austenitization temperatures for both are same for hardening heat treatment. (a) being an eutectoid steel will have homogeneous austenite before quenching. Where as in (b) there will be cementite globules present which would provide sites for nucleation of
19 pearlite. herefore the latter would need more severe quenching to get fully hardened structure. 3. Eutectoid temperature of steel is a function of its composition. For plain carbon steel it is around 720⁰C. o get martensite the steel should be heated beyond this temperature. However the presence of alloying elements which stabilize austenite (such as Mn, Ni) can bring down eutectoid temperature. In this case steel must be an alloy steel whose eutectoid temperature is less than 700⁰C. herefore it must have been quenched from ferrite austenite region and the austenite on quenching got transformed into martensite resulting in a ferrite martensite structure. 4. A steel has maximum strength if has 100% martensite. his is obtained by quenching steel from austenitic state. Strength of martensite depends primarily on its carbon content. However the cooling rate needed to get 100% martensite in plain carbon steel is often difficult to achieve. Presence of additional alloy elements decreases critical cooling rate. Alloy steels are easy to harden. herefore strength of hardened steel would depend on its carbon content, section size (thickness) and other alloy elements present & their amounts in the steel. 5. As against furnace cooling in the case of annealing the job is allowed to cool in air while normalizing. his being significantly faster normalized structure would have finer pearlite and a little less amount of ferrite. However the total amount of cementite would still be the same. Only the carbon content and therefore the amount of cementite in pearlite would decrease. 6. On quenching the microstructure of steel will consist of martensite and some retained austenite. Both M s & M f temperature of steel decreases with % carbon. Steels having beyond 0.7%C has subzero M f temperature. 19
Iron-Carbon Phase Diagram (a review) see Callister Chapter 9
 Iron-Carbon Phase Diagram (a review) see Callister Chapter 9 University of Tennessee, Dept. of Materials Science and Engineering 1 The Iron Iron Carbide (Fe Fe 3 C) Phase Diagram In their simplest form,
Iron-Carbon Phase Diagram (a review) see Callister Chapter 9 University of Tennessee, Dept. of Materials Science and Engineering 1 The Iron Iron Carbide (Fe Fe 3 C) Phase Diagram In their simplest form,
Chapter Outline: Phase Transformations in Metals
 Chapter Outline: Phase Transformations in Metals Heat Treatment (time and temperature) Microstructure Mechanical Properties Kinetics of phase transformations Multiphase Transformations Phase transformations
Chapter Outline: Phase Transformations in Metals Heat Treatment (time and temperature) Microstructure Mechanical Properties Kinetics of phase transformations Multiphase Transformations Phase transformations
Heat Treatment of Steel
 Heat Treatment of Steel Steels can be heat treated to produce a great variety of microstructures and properties. Generally, heat treatment uses phase transformation during heating and cooling to change
Heat Treatment of Steel Steels can be heat treated to produce a great variety of microstructures and properties. Generally, heat treatment uses phase transformation during heating and cooling to change
Heat Treatment of Steels : Spheroidize annealing. Heat Treatment of Steels : Normalizing
 Heat Treatment of Steels :Recrystallization annealing The carbon and alloy steels were treated at a temperature of about 700 C, which is about 20 C below the eutectoid temperature. The holding time should
Heat Treatment of Steels :Recrystallization annealing The carbon and alloy steels were treated at a temperature of about 700 C, which is about 20 C below the eutectoid temperature. The holding time should
LABORATORY EXPERIMENTS TESTING OF MATERIALS
 LABORATORY EXPERIMENTS TESTING OF MATERIALS 1. TENSION TEST: INTRODUCTION & THEORY The tension test is the most commonly used method to evaluate the mechanical properties of metals. Its main objective
LABORATORY EXPERIMENTS TESTING OF MATERIALS 1. TENSION TEST: INTRODUCTION & THEORY The tension test is the most commonly used method to evaluate the mechanical properties of metals. Its main objective
The mechanical properties of metal affected by heat treatment are:
 Training Objective After watching this video and reviewing the printed material, the student/trainee will learn the basic concepts of the heat treating processes as they pertain to carbon and alloy steels.
Training Objective After watching this video and reviewing the printed material, the student/trainee will learn the basic concepts of the heat treating processes as they pertain to carbon and alloy steels.
Alloys & Their Phase Diagrams
 Alloys & Their Phase Diagrams Objectives of the class Gibbs phase rule Introduction to phase diagram Practice phase diagram Lever rule Important Observation: One question in the midterm Consider the Earth
Alloys & Their Phase Diagrams Objectives of the class Gibbs phase rule Introduction to phase diagram Practice phase diagram Lever rule Important Observation: One question in the midterm Consider the Earth
HEAT TREATMENT OF STEEL
 HEAT TREATMENT OF STEEL Heat Treatment of Steel Most heat treating operations begin with heating the alloy into the austenitic phase field to dissolve the carbide in the iron. Steel heat treating practice
HEAT TREATMENT OF STEEL Heat Treatment of Steel Most heat treating operations begin with heating the alloy into the austenitic phase field to dissolve the carbide in the iron. Steel heat treating practice
Experiment: Heat Treatment - Quenching & Tempering
 Experiment: Heat Treatment - Quenching & Tempering Objectives 1) To investigate the conventional heat treatment procedures, such as quenching and annealing, used to alter the properties of steels. SAE
Experiment: Heat Treatment - Quenching & Tempering Objectives 1) To investigate the conventional heat treatment procedures, such as quenching and annealing, used to alter the properties of steels. SAE
Lecture 19: Eutectoid Transformation in Steels: a typical case of Cellular
 Lecture 19: Eutectoid Transformation in Steels: a typical case of Cellular Precipitation Today s topics Understanding of Cellular transformation (or precipitation): when applied to phase transformation
Lecture 19: Eutectoid Transformation in Steels: a typical case of Cellular Precipitation Today s topics Understanding of Cellular transformation (or precipitation): when applied to phase transformation
Introduction to Materials Science, Chapter 9, Phase Diagrams. Phase Diagrams. University of Tennessee, Dept. of Materials Science and Engineering 1
 Phase Diagrams University of Tennessee, Dept. of Materials Science and Engineering 1 Chapter Outline: Phase Diagrams Microstructure and Phase Transformations in Multicomponent Systems Definitions and basic
Phase Diagrams University of Tennessee, Dept. of Materials Science and Engineering 1 Chapter Outline: Phase Diagrams Microstructure and Phase Transformations in Multicomponent Systems Definitions and basic
The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = V S V C. = 2(sphere volume) = 2 = V C = 4R
 3.5 Show that the atomic packing factor for BCC is 0.68. The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = V S V C Since there are two spheres associated
3.5 Show that the atomic packing factor for BCC is 0.68. The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = V S V C Since there are two spheres associated
Continuous Cooling Transformation (CCT) Diagrams
 Continuous Cooling Transformation (CCT) Diagrams R. Manna Assistant Professor Centre of Advanced Study Department of Metallurgical Engineering Institute of Technology, Banaras Hindu University Varanasi-221
Continuous Cooling Transformation (CCT) Diagrams R. Manna Assistant Professor Centre of Advanced Study Department of Metallurgical Engineering Institute of Technology, Banaras Hindu University Varanasi-221
MSE 528 - PRECIPITATION HARDENING IN 7075 ALUMINUM ALLOY
 MSE 528 - PRECIPITATION HARDENING IN 7075 ALUMINUM ALLOY Objective To study the time and temperature variations in the hardness and electrical conductivity of Al-Zn-Mg-Cu high strength alloy on isothermal
MSE 528 - PRECIPITATION HARDENING IN 7075 ALUMINUM ALLOY Objective To study the time and temperature variations in the hardness and electrical conductivity of Al-Zn-Mg-Cu high strength alloy on isothermal
INFLUENCE OF THERMOMECHANICAL TREATMENT ON THE STEEL C45 FATIGUE PROPERTIES
 CO-MAT-TECH 2005 TRNAVA, 20-21 October 2005 INFLUENCE OF THERMOMECHANICAL TREATMENT ON THE STEEL C45 FATIGUE PROPERTIES Jiří MALINA 1+2, Hana STANKOVÁ 1+2, Jaroslav DRNEK 3, Zbyšek NOVÝ 3, Bohuslav MAŠEK
CO-MAT-TECH 2005 TRNAVA, 20-21 October 2005 INFLUENCE OF THERMOMECHANICAL TREATMENT ON THE STEEL C45 FATIGUE PROPERTIES Jiří MALINA 1+2, Hana STANKOVÁ 1+2, Jaroslav DRNEK 3, Zbyšek NOVÝ 3, Bohuslav MAŠEK
Materials Issues in Fatigue and Fracture
 Materials Issues in Fatigue and Fracture 5.1 Fundamental Concepts 5.2 Ensuring Infinite Life 5.3 Finite Life 5.4 Summary FCP 1 5.1 Fundamental Concepts Structural metals Process of fatigue A simple view
Materials Issues in Fatigue and Fracture 5.1 Fundamental Concepts 5.2 Ensuring Infinite Life 5.3 Finite Life 5.4 Summary FCP 1 5.1 Fundamental Concepts Structural metals Process of fatigue A simple view
9.11 Upon heating a lead-tin alloy of composition 30 wt% Sn-70 wt% Pb from 150 C and utilizing Figure
 9-13 9.8: 9.11 Upon heating a lead-tin alloy of composition 30 wt% Sn-70 wt% Pb from 150 C and utilizing Figure (a) The first liquid forms at the temperature at which a vertical line at this composition
9-13 9.8: 9.11 Upon heating a lead-tin alloy of composition 30 wt% Sn-70 wt% Pb from 150 C and utilizing Figure (a) The first liquid forms at the temperature at which a vertical line at this composition
X15TN TM. A high hardness, corrosion and fatigue resistance martensitic grade CONTINUOUS INNOVATION RESEARCH SERVICE.
 TM A high hardness, corrosion and fatigue resistance martensitic grade CONTINUOUS METALLURGICAL SPECIAL STEELS INNOVATION RESEARCH SERVICE DEVELOPMENT Enhancing your performance THE INDUSTRIAL ENVIRONMENT
TM A high hardness, corrosion and fatigue resistance martensitic grade CONTINUOUS METALLURGICAL SPECIAL STEELS INNOVATION RESEARCH SERVICE DEVELOPMENT Enhancing your performance THE INDUSTRIAL ENVIRONMENT
Massachusetts Institute of Technology Department of Mechanical Engineering Cambridge, MA 02139
 Massachusetts Institute of Technology Department of Mechanical Engineering Cambridge, MA 02139 2.002 Mechanics and Materials II Spring 2004 Laboratory Module No. 5 Heat Treatment of Plain Carbon and Low
Massachusetts Institute of Technology Department of Mechanical Engineering Cambridge, MA 02139 2.002 Mechanics and Materials II Spring 2004 Laboratory Module No. 5 Heat Treatment of Plain Carbon and Low
Phase Transformations in Metals and Alloys
 Phase Transformations in Metals and Alloys THIRD EDITION DAVID A. PORTER, KENNETH E. EASTERLING, and MOHAMED Y. SHERIF ( г йс) CRC Press ^ ^ ) Taylor & Francis Group Boca Raton London New York CRC Press
Phase Transformations in Metals and Alloys THIRD EDITION DAVID A. PORTER, KENNETH E. EASTERLING, and MOHAMED Y. SHERIF ( г йс) CRC Press ^ ^ ) Taylor & Francis Group Boca Raton London New York CRC Press
Chapter Outline Dislocations and Strengthening Mechanisms
 Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
8. Technical Heat Treatment
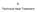 8. Technical Heat Treatment 8. Technical Heat Treatment 95 6 cm 4 2 0-2 -4 C 400 C C -6-14 -12-10 -8-6 -4-2 0 2 cm 6 723 C temperature C 1750 750 250 When welding a workpiece, not only the weld itself,
8. Technical Heat Treatment 8. Technical Heat Treatment 95 6 cm 4 2 0-2 -4 C 400 C C -6-14 -12-10 -8-6 -4-2 0 2 cm 6 723 C temperature C 1750 750 250 When welding a workpiece, not only the weld itself,
Full Density Properties of Low Alloy Steels
 Full Density Properties of Low Alloy Steels Michael L. Marucci & Arthur J. Rawlings Hoeganaes Corporation, Cinnaminson, NJ Presented at PM 2 TEC2005 International Conference on Powder Metallurgy and Particulate
Full Density Properties of Low Alloy Steels Michael L. Marucci & Arthur J. Rawlings Hoeganaes Corporation, Cinnaminson, NJ Presented at PM 2 TEC2005 International Conference on Powder Metallurgy and Particulate
Lecture: 33. Solidification of Weld Metal
 Lecture: 33 Solidification of Weld Metal This chapter presents common solidification mechanisms observed in weld metal and different modes of solidification. Influence of welding speed and heat input on
Lecture: 33 Solidification of Weld Metal This chapter presents common solidification mechanisms observed in weld metal and different modes of solidification. Influence of welding speed and heat input on
Fundamentals of the Heat Treating of Steel
 CHAPTER 2 Fundamentals of the Heat Treating of Steel BEFORE CONSIDERATION can be given to the heat treatment of steel or other iron-base alloys, it is helpful to explain what steel is. The common dictionary
CHAPTER 2 Fundamentals of the Heat Treating of Steel BEFORE CONSIDERATION can be given to the heat treatment of steel or other iron-base alloys, it is helpful to explain what steel is. The common dictionary
FATIGUE BEHAVIOUR ANALYSIS OF DIFFERENTLY HEAT TREATED MEDIUM CARBON STEEL. Master of Technology (Res.) Metallurgical and Materials Engineering
 FATIGUE BEHAVIOUR ANALYSIS OF DIFFERENTLY HEAT TREATED MEDIUM CARBON STEEL A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Technology (Res.) in Metallurgical and
FATIGUE BEHAVIOUR ANALYSIS OF DIFFERENTLY HEAT TREATED MEDIUM CARBON STEEL A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Technology (Res.) in Metallurgical and
ATI 2205 ATI 2205. Technical Data Sheet. Duplex Stainless Steel GENERAL PROPERTIES. (UNS S31803 and S32205)
 ATI 2205 Duplex Stainless Steel (UNS S31803 and S32205) GENERAL PROPERTIES ATI 2205 alloy (UNS S31803 and/or S32205) is a nitrogen-enhanced duplex stainless steel alloy. The nitrogen serves to significantly
ATI 2205 Duplex Stainless Steel (UNS S31803 and S32205) GENERAL PROPERTIES ATI 2205 alloy (UNS S31803 and/or S32205) is a nitrogen-enhanced duplex stainless steel alloy. The nitrogen serves to significantly
AUSTENITIC STAINLESS DAMASCENE STEEL
 AUSTENITIC STAINLESS DAMASCENE STEEL Damasteel s austenitic stainless Damascene Steel is a mix between types 304L and 316L stainless steels which are variations of the 18 percent chromium 8 percent nickel
AUSTENITIC STAINLESS DAMASCENE STEEL Damasteel s austenitic stainless Damascene Steel is a mix between types 304L and 316L stainless steels which are variations of the 18 percent chromium 8 percent nickel
Ch. 4: Imperfections in Solids Part 1. Dr. Feras Fraige
 Ch. 4: Imperfections in Solids Part 1 Dr. Feras Fraige Outline Defects in Solids 0D, Point defects vacancies Interstitials impurities, weight and atomic composition 1D, Dislocations edge screw 2D, Grain
Ch. 4: Imperfections in Solids Part 1 Dr. Feras Fraige Outline Defects in Solids 0D, Point defects vacancies Interstitials impurities, weight and atomic composition 1D, Dislocations edge screw 2D, Grain
PROPERTIES OF MATERIALS
 1 PROPERTIES OF MATERIALS 1.1 PROPERTIES OF MATERIALS Different materials possess different properties in varying degree and therefore behave in different ways under given conditions. These properties
1 PROPERTIES OF MATERIALS 1.1 PROPERTIES OF MATERIALS Different materials possess different properties in varying degree and therefore behave in different ways under given conditions. These properties
WJM Technologies excellence in material joining
 Girish P. Kelkar, Ph.D. (562) 743-7576 girish@welding-consultant.com www.welding-consultant.com Weld Cracks An Engineer s Worst Nightmare There are a variety of physical defects such as undercut, insufficient
Girish P. Kelkar, Ph.D. (562) 743-7576 girish@welding-consultant.com www.welding-consultant.com Weld Cracks An Engineer s Worst Nightmare There are a variety of physical defects such as undercut, insufficient
UDDEHOLM VANADIS 30 SUPERCLEAN
 UDDEHOLM VANADIS 30 SUPERCLEAN UDDEHOLMS AB No part of this publication may be reproduced or transmitted for commercial purposes without permission of the copyright holder. This information is based on
UDDEHOLM VANADIS 30 SUPERCLEAN UDDEHOLMS AB No part of this publication may be reproduced or transmitted for commercial purposes without permission of the copyright holder. This information is based on
Solution for Homework #1
 Solution for Homework #1 Chapter 2: Multiple Choice Questions (2.5, 2.6, 2.8, 2.11) 2.5 Which of the following bond types are classified as primary bonds (more than one)? (a) covalent bonding, (b) hydrogen
Solution for Homework #1 Chapter 2: Multiple Choice Questions (2.5, 2.6, 2.8, 2.11) 2.5 Which of the following bond types are classified as primary bonds (more than one)? (a) covalent bonding, (b) hydrogen
In order to solve this problem it is first necessary to use Equation 5.5: x 2 Dt. = 1 erf. = 1.30, and x = 2 mm = 2 10-3 m. Thus,
 5.3 (a) Compare interstitial and vacancy atomic mechanisms for diffusion. (b) Cite two reasons why interstitial diffusion is normally more rapid than vacancy diffusion. Solution (a) With vacancy diffusion,
5.3 (a) Compare interstitial and vacancy atomic mechanisms for diffusion. (b) Cite two reasons why interstitial diffusion is normally more rapid than vacancy diffusion. Solution (a) With vacancy diffusion,
AISI O1 Cold work tool steel
 T OOL STEEL FACTS AISI O1 Cold work tool steel Great Tooling Starts Here! This information is based on our present state of knowledge and is intended to provide general notes on our products and their
T OOL STEEL FACTS AISI O1 Cold work tool steel Great Tooling Starts Here! This information is based on our present state of knowledge and is intended to provide general notes on our products and their
INFLUENCE OF Cr, Mn AND Mo ON STRUCTURE AND PROPERTIES OF V MICROALLOYED MEDIUM CARBON FORGING STEELS
 Association of Metallurgical Engineers of Serbia AMES Scientific paper UDC: 669.15'26'74'28-194 INFLUENCE OF Cr, Mn AND Mo ON STRUCTURE AND PROPERTIES OF V MICROALLOYED MEDIUM CARBON FORGING STEELS Nenad
Association of Metallurgical Engineers of Serbia AMES Scientific paper UDC: 669.15'26'74'28-194 INFLUENCE OF Cr, Mn AND Mo ON STRUCTURE AND PROPERTIES OF V MICROALLOYED MEDIUM CARBON FORGING STEELS Nenad
HIGH STRENGTH DUCTILE IRON PRODUCED BY THE ENGINEERED COOLING: PROCESS CONCEPT
 IJMC14-244-2 HIGH STRENGTH DUCTILE IRON PRODUCED BY THE ENGINEERED COOLING: PROCESS CONCEPT Copyright 215 American Foundry Society Abstract Simon N. Lekakh Missouri University of Science and Technology,
IJMC14-244-2 HIGH STRENGTH DUCTILE IRON PRODUCED BY THE ENGINEERED COOLING: PROCESS CONCEPT Copyright 215 American Foundry Society Abstract Simon N. Lekakh Missouri University of Science and Technology,
North American Stainless
 North American Stainless Long Products Stainless Steel Grade Sheet 2205 UNS S2205 EN 1.4462 2304 UNS S2304 EN 1.4362 INTRODUCTION Types 2205 and 2304 are duplex stainless steel grades with a microstructure,
North American Stainless Long Products Stainless Steel Grade Sheet 2205 UNS S2205 EN 1.4462 2304 UNS S2304 EN 1.4362 INTRODUCTION Types 2205 and 2304 are duplex stainless steel grades with a microstructure,
Lecture slides on rolling By: Dr H N Dhakal Lecturer in Mechanical and Marine Engineering, School of Engineering, University of Plymouth
 Lecture slides on rolling By: Dr H N Dhakal Lecturer in Mechanical and Marine Engineering, School of Engineering, University of Plymouth Bulk deformation forming (rolling) Rolling is the process of reducing
Lecture slides on rolling By: Dr H N Dhakal Lecturer in Mechanical and Marine Engineering, School of Engineering, University of Plymouth Bulk deformation forming (rolling) Rolling is the process of reducing
Chapter Outline. Diffusion - how do atoms move through solids?
 Chapter Outline iffusion - how do atoms move through solids? iffusion mechanisms Vacancy diffusion Interstitial diffusion Impurities The mathematics of diffusion Steady-state diffusion (Fick s first law)
Chapter Outline iffusion - how do atoms move through solids? iffusion mechanisms Vacancy diffusion Interstitial diffusion Impurities The mathematics of diffusion Steady-state diffusion (Fick s first law)
Martensite in Steels
 Materials Science & Metallurgy http://www.msm.cam.ac.uk/phase-trans/2002/martensite.html H. K. D. H. Bhadeshia Martensite in Steels The name martensite is after the German scientist Martens. It was used
Materials Science & Metallurgy http://www.msm.cam.ac.uk/phase-trans/2002/martensite.html H. K. D. H. Bhadeshia Martensite in Steels The name martensite is after the German scientist Martens. It was used
Simulations of the Effect of Section Size and Cooling on Sigma Phase Formation in Duplex Stainless Steels
 Simulations of the Effect of Section Size and Cooling on Sigma Phase Formation in Duplex Stainless Steels Richard A. Hardin and Christoph Beckermann Department of Mechanical and Industrial Engineering
Simulations of the Effect of Section Size and Cooling on Sigma Phase Formation in Duplex Stainless Steels Richard A. Hardin and Christoph Beckermann Department of Mechanical and Industrial Engineering
Chapter Outline Dislocations and Strengthening Mechanisms
 Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
CHROMIUM STEEL POWDERS FOR COMPONENTS. JEANETTE LEWENHAGEN Höganäs AB, Sweden
 CHROMIUM STEEL POWDERS FOR COMPONENTS JEANETTE LEWENHAGEN Höganäs AB, Sweden KEYWORDS Pre-alloyed steel powder, chromium, PM ABSTRACT Chromium as an alloying element is of great interest due to its low
CHROMIUM STEEL POWDERS FOR COMPONENTS JEANETTE LEWENHAGEN Höganäs AB, Sweden KEYWORDS Pre-alloyed steel powder, chromium, PM ABSTRACT Chromium as an alloying element is of great interest due to its low
Technical Data BLUE SHEET. Martensitic. stainless steels. Types 410, 420, 425 Mod, and 440A GENERAL PROPERTIES APPLICATIONS PRODUCT FORM
 Technical Data BLUE SHEET Allegheny Ludlum Corporation Pittsburgh, PA Martensitic Stainless Steels Types 410, 420, 425 Mod, and 440A GENERAL PROPERTIES Allegheny Ludlum Types 410, 420, 425 Modified, and
Technical Data BLUE SHEET Allegheny Ludlum Corporation Pittsburgh, PA Martensitic Stainless Steels Types 410, 420, 425 Mod, and 440A GENERAL PROPERTIES Allegheny Ludlum Types 410, 420, 425 Modified, and
Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras
 Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras Module - 2 Lecture - 2 Part 2 of 2 Review of Atomic Bonding II We will continue
Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras Module - 2 Lecture - 2 Part 2 of 2 Review of Atomic Bonding II We will continue
Defects Introduction. Bonding + Structure + Defects. Properties
 Defects Introduction Bonding + Structure + Defects Properties The processing determines the defects Composition Bonding type Structure of Crystalline Processing factors Defects Microstructure Types of
Defects Introduction Bonding + Structure + Defects Properties The processing determines the defects Composition Bonding type Structure of Crystalline Processing factors Defects Microstructure Types of
BINARY SYSTEMS. Definition of Composition: Atomic (molar) fraction. Atomic percent. Mass fraction. Mass percent (weight percent)
 BINARY SYSTEMS Definition of Composition: Atomic (molar) fraction Atomic percent Mass fraction Mass percent (weight percent) na =, x i n = A i i i Weight percent mainly in industry! x at % A = x 100 A
BINARY SYSTEMS Definition of Composition: Atomic (molar) fraction Atomic percent Mass fraction Mass percent (weight percent) na =, x i n = A i i i Weight percent mainly in industry! x at % A = x 100 A
Uddeholm Dievar is a specially developed steel grade by Uddeholm, which provides the best possible performance.
 Uddeholm Dievar is a specially developed steel grade by Uddeholm, which provides the best possible performance. The chemical composition and the very latest in production technique make the property profile
Uddeholm Dievar is a specially developed steel grade by Uddeholm, which provides the best possible performance. The chemical composition and the very latest in production technique make the property profile
Continuous Cooling Bainite Transformation Characteristics of a Low Carbon Microalloyed Steel under the Simulated Welding Thermal Cycle Process
 Available online at SciVerse ScienceDirect J. Mater. Sci. Technol., 2013, 29(5), 446e450 Continuous Cooling Bainite Transformation Characteristics of a Low Carbon Microalloyed Steel under the Simulated
Available online at SciVerse ScienceDirect J. Mater. Sci. Technol., 2013, 29(5), 446e450 Continuous Cooling Bainite Transformation Characteristics of a Low Carbon Microalloyed Steel under the Simulated
STUDY THE DAMPING EFFECT OF TWO TYPES OF METALS (CK 45, 40 X) USING OILS (ASTRALUBE AND SHIELD) IN TERMS OF HARDNESS AND TENSILE
 INTERNATIONAL JOURNAL OF MECHANICAL ENGINEERING AND TECHNOLOGY (IJMET) International Journal of Mechanical Engineering and Technology (IJMET), ISSN 976 634(Print), ISSN 976 634 (Print) ISSN 976 6359 (Online)
INTERNATIONAL JOURNAL OF MECHANICAL ENGINEERING AND TECHNOLOGY (IJMET) International Journal of Mechanical Engineering and Technology (IJMET), ISSN 976 634(Print), ISSN 976 634 (Print) ISSN 976 6359 (Online)
Effect of Temperature and Aging Time on 2024 Aluminum Behavior
 Proceedings of the XIth International Congress and Exposition June 2-5, 2008 Orlando, Florida USA 2008 Society for Experimental Mechanics Inc. Effect of Temperature and Aging Time on 2024 Aluminum Behavior
Proceedings of the XIth International Congress and Exposition June 2-5, 2008 Orlando, Florida USA 2008 Society for Experimental Mechanics Inc. Effect of Temperature and Aging Time on 2024 Aluminum Behavior
ROLLED STAINLESS STEEL PLATES, SECTIONS AND BARS
 STANDARD FOR CERTIFICATION No. 2.9 ROLLED STAINLESS STEEL PLATES, SECTIONS AND BARS OCTOBER 2008 Veritasveien 1, NO-1322 Høvik, Norway Tel.: +47 67 57 99 00 Fax: +47 67 57 99 11 FOREWORD (DNV) is an autonomous
STANDARD FOR CERTIFICATION No. 2.9 ROLLED STAINLESS STEEL PLATES, SECTIONS AND BARS OCTOBER 2008 Veritasveien 1, NO-1322 Høvik, Norway Tel.: +47 67 57 99 00 Fax: +47 67 57 99 11 FOREWORD (DNV) is an autonomous
ALLOY 2205 DATA SHEET
 ALLOY 2205 DATA SHEET UNS S32205, EN 1.4462 / UNS S31803 GENERAL PROPERTIES ////////////////////////////////////////////////////// //// 2205 (UNS designations S32205 / S31803) is a 22 % chromium, 3 % molybdenum,
ALLOY 2205 DATA SHEET UNS S32205, EN 1.4462 / UNS S31803 GENERAL PROPERTIES ////////////////////////////////////////////////////// //// 2205 (UNS designations S32205 / S31803) is a 22 % chromium, 3 % molybdenum,
Objective To conduct Charpy V-notch impact test and determine the ductile-brittle transition temperature of steels.
 IMPACT TESTING Objective To conduct Charpy V-notch impact test and determine the ductile-brittle transition temperature of steels. Equipment Coolants Standard Charpy V-Notched Test specimens Impact tester
IMPACT TESTING Objective To conduct Charpy V-notch impact test and determine the ductile-brittle transition temperature of steels. Equipment Coolants Standard Charpy V-Notched Test specimens Impact tester
EFFECT OF COPPER ALLOY ADDITION METHOD ON THE DIMENSIONAL RESPONSE OF SINTERED FE-CU-C STEELS
 EFFECT OF COPPER ALLOY ADDITION METHOD ON THE DIMENSIONAL RESPONSE OF SINTERED FE-CU-C STEELS Michael L. Marucci and Francis G. Hanejko Hoeganaes Corporation Cinnaminson, NJ 08077 - USA Abstract Fe-Cu-C
EFFECT OF COPPER ALLOY ADDITION METHOD ON THE DIMENSIONAL RESPONSE OF SINTERED FE-CU-C STEELS Michael L. Marucci and Francis G. Hanejko Hoeganaes Corporation Cinnaminson, NJ 08077 - USA Abstract Fe-Cu-C
The Effect of Heat Treatment Atmosphere on Hardening of Surface Region of H13 Tool Steel. Dominique AU
 The Effect of Heat Treatment Atmosphere on Hardening of Surface Region of H13 Tool Steel Dominique AU Report submitted in partial fulfilment of the degree of Postgraduate diploma in Research Auckland University
The Effect of Heat Treatment Atmosphere on Hardening of Surface Region of H13 Tool Steel Dominique AU Report submitted in partial fulfilment of the degree of Postgraduate diploma in Research Auckland University
THREE MAIN SOLIDIFICATION REACTIONS OF VANADIUM MODIFIED T1 TUNGSTEN HIGH SPEED TOOL STEEL. Hossam Halfa
 THREE MAIN SOLIDIFICATION REACTIONS OF VANADIUM MODIFIED T1 TUNGSTEN HIGH SPEED TOOL STEEL Hossam Halfa Steel Technology Department, Central Metallurgical R&D Institute (CMRDI), Helwan, Egypt, hossamhalfa@cmrdi.sci.eg;
THREE MAIN SOLIDIFICATION REACTIONS OF VANADIUM MODIFIED T1 TUNGSTEN HIGH SPEED TOOL STEEL Hossam Halfa Steel Technology Department, Central Metallurgical R&D Institute (CMRDI), Helwan, Egypt, hossamhalfa@cmrdi.sci.eg;
North American Stainless
 North American Stainless Flat Products Stainless Steel Grade Sheet 430 (S43000)/ EN 1.4016 Introduction: SS430 is a low-carbon plain chromium, ferritic stainless steel without any stabilization of carbon
North American Stainless Flat Products Stainless Steel Grade Sheet 430 (S43000)/ EN 1.4016 Introduction: SS430 is a low-carbon plain chromium, ferritic stainless steel without any stabilization of carbon
Evaluation of the Susceptibility of Simulated Welds In HSLA-100 and HY-100 Steels to Hydrogen Induced Cracking
 Evaluation of the Susceptibility of Simulated Welds In HSLA-100 and HY-100 Steels to Hydrogen Induced Cracking R. E. Ricker, M. R. Stoudt, and D. J. Pitchure Materials Performance Group Metallurgy Division
Evaluation of the Susceptibility of Simulated Welds In HSLA-100 and HY-100 Steels to Hydrogen Induced Cracking R. E. Ricker, M. R. Stoudt, and D. J. Pitchure Materials Performance Group Metallurgy Division
High-Strength Low-Alloy Steels
 High-Strength Low-Alloy Steels Introduction and Overview High-strength low-alloy (HSLA) steels, or microalloyed steels, are designed to provide better mechanical properties and/or greater resistance to
High-Strength Low-Alloy Steels Introduction and Overview High-strength low-alloy (HSLA) steels, or microalloyed steels, are designed to provide better mechanical properties and/or greater resistance to
UDDEHOLM ELMAX SUPERCLEAN
 UDDEHOLM ELMAX SUPERCLEAN UDDEHOLMS AB No part of this publication may be reproduced or transmitted for commercial purposes without permission of the copyright holder. This information is based on our
UDDEHOLM ELMAX SUPERCLEAN UDDEHOLMS AB No part of this publication may be reproduced or transmitted for commercial purposes without permission of the copyright holder. This information is based on our
Mechanical Properties of Metals Mechanical Properties refers to the behavior of material when external forces are applied
 Mechanical Properties of Metals Mechanical Properties refers to the behavior of material when external forces are applied Stress and strain fracture or engineering point of view: allows to predict the
Mechanical Properties of Metals Mechanical Properties refers to the behavior of material when external forces are applied Stress and strain fracture or engineering point of view: allows to predict the
Lösungen Übung Verformung
 Lösungen Übung Verformung 1. (a) What is the meaning of T G? (b) To which materials does it apply? (c) What effect does it have on the toughness and on the stress- strain diagram? 2. Name the four main
Lösungen Übung Verformung 1. (a) What is the meaning of T G? (b) To which materials does it apply? (c) What effect does it have on the toughness and on the stress- strain diagram? 2. Name the four main
EFFECT OF SEVERE PLASTIC DEFORMATION ON STRUCTURE AND PROPERTIES OF AUSTENITIC AISI 316 GRADE STEEL
 EFFECT OF SEVERE PLASTIC DEFORMATION ON STRUCTURE AND PROPERTIES OF AUSTENITIC AISI 316 GRADE STEEL Ladislav KANDER a, Miroslav GREGER b a MATERIÁLOVÝ A METALURGICKÝ VÝZKUM, s.r.o., Ostrava, Czech Republic,
EFFECT OF SEVERE PLASTIC DEFORMATION ON STRUCTURE AND PROPERTIES OF AUSTENITIC AISI 316 GRADE STEEL Ladislav KANDER a, Miroslav GREGER b a MATERIÁLOVÝ A METALURGICKÝ VÝZKUM, s.r.o., Ostrava, Czech Republic,
Application of Direct Resistance Treatment to Localized Areas on Advanced High Strength Steel Sheet
 Application of Direct Resistance Treatment to Localized Areas on Advanced High Strength Steel Sheet Jerry E. Gould *, Mark Harris **, Dan Sakkinen**, and Warren Peterson * * EWI, Columbus OH ** Johnson
Application of Direct Resistance Treatment to Localized Areas on Advanced High Strength Steel Sheet Jerry E. Gould *, Mark Harris **, Dan Sakkinen**, and Warren Peterson * * EWI, Columbus OH ** Johnson
UDDEHOLM BALDER UDDEHOLM BALDER UDDEHOLM STEEL FOR INDEXABLE INSERT CUTTING TOOLS
 UDDEHOLM BALDER Reliable and efficient steel is essential for good results. The same goes for achieving high productivity and high availability. When choosing the right steel many parameters must be considered,
UDDEHOLM BALDER Reliable and efficient steel is essential for good results. The same goes for achieving high productivity and high availability. When choosing the right steel many parameters must be considered,
PRELIMINARY BROCHURE. Uddeholm Ramax HH
 PRELIMINARY BROCHURE Uddeholm Ramax HH Uddeholm Ramax HH Uddeholm Ramax HH provides several benefits: The product offers uniform hardness in all dimensions combined with excellent indentation resistance.
PRELIMINARY BROCHURE Uddeholm Ramax HH Uddeholm Ramax HH Uddeholm Ramax HH provides several benefits: The product offers uniform hardness in all dimensions combined with excellent indentation resistance.
Lecture 18 Strain Hardening And Recrystallization
 -138- Lecture 18 Strain Hardening And Recrystallization Strain Hardening We have previously seen that the flow stress (the stress necessary to produce a certain plastic strain rate) increases with increasing
-138- Lecture 18 Strain Hardening And Recrystallization Strain Hardening We have previously seen that the flow stress (the stress necessary to produce a certain plastic strain rate) increases with increasing
Time Temperature Transformation (TTT) Diagrams
 Tata Steel-TRAERF Faculty Fellowship Visiting Scholar Department of Materials Science and Metallurgy University of Cambridge, Pembroke Street, Cambridge, CB2 3QZ rm659@cam.ac.uk Time Temperature Transformation
Tata Steel-TRAERF Faculty Fellowship Visiting Scholar Department of Materials Science and Metallurgy University of Cambridge, Pembroke Street, Cambridge, CB2 3QZ rm659@cam.ac.uk Time Temperature Transformation
Structural Integrity Analysis
 Structural Integrity Analysis 1. STRESS CONCENTRATION Igor Kokcharov 1.1 STRESSES AND CONCENTRATORS 1.1.1 Stress An applied external force F causes inner forces in the carrying structure. Inner forces
Structural Integrity Analysis 1. STRESS CONCENTRATION Igor Kokcharov 1.1 STRESSES AND CONCENTRATORS 1.1.1 Stress An applied external force F causes inner forces in the carrying structure. Inner forces
Heat Treatment of Aluminum Foundry Alloys. Fred Major Rio Tinto Alcan
 Heat Treatment of Aluminum Foundry Alloys Fred Major Rio Tinto Alcan OUTLINE Basics of Heat Treatment (What is happening to the metal at each step). Atomic Structure of Aluminum Deformation Mechanisms
Heat Treatment of Aluminum Foundry Alloys Fred Major Rio Tinto Alcan OUTLINE Basics of Heat Treatment (What is happening to the metal at each step). Atomic Structure of Aluminum Deformation Mechanisms
Chapter 8. Phase Diagrams
 Phase Diagrams A phase in a material is a region that differ in its microstructure and or composition from another region Al Al 2 CuMg H 2 O(solid, ice) in H 2 O (liquid) 2 phases homogeneous in crystal
Phase Diagrams A phase in a material is a region that differ in its microstructure and or composition from another region Al Al 2 CuMg H 2 O(solid, ice) in H 2 O (liquid) 2 phases homogeneous in crystal
LOW CARBON MICROALLOYED COLD ROLLED, HOT DIP GALVANIZED DUAL PHASE STEEL FOR LARGER CROSS-SECTIONAL AREAS WITH IMPROVED PROPERTIES
 LOW CARBON MICROALLOYED COLD ROLLED, HOT DIP GALVANIZED DUAL PHASE STEEL FOR LARGER CROSS-SECTIONAL AREAS WITH IMPROVED PROPERTIES Hardy Mohrbacher NiobelCon bvba, Schilde, Belgium Thomas Schulz Salzgitter-Mannesmann
LOW CARBON MICROALLOYED COLD ROLLED, HOT DIP GALVANIZED DUAL PHASE STEEL FOR LARGER CROSS-SECTIONAL AREAS WITH IMPROVED PROPERTIES Hardy Mohrbacher NiobelCon bvba, Schilde, Belgium Thomas Schulz Salzgitter-Mannesmann
EXPERIMENTAL AND NUMERICAL ANALYSIS OF THE COLLAR PRODUCTION ON THE PIERCED FLAT SHEET METAL USING LASER FORMING PROCESS
 JOURNAL OF CURRENT RESEARCH IN SCIENCE (ISSN 2322-5009) CODEN (USA): JCRSDJ 2014, Vol. 2, No. 2, pp:277-284 Available at www.jcrs010.com ORIGINAL ARTICLE EXPERIMENTAL AND NUMERICAL ANALYSIS OF THE COLLAR
JOURNAL OF CURRENT RESEARCH IN SCIENCE (ISSN 2322-5009) CODEN (USA): JCRSDJ 2014, Vol. 2, No. 2, pp:277-284 Available at www.jcrs010.com ORIGINAL ARTICLE EXPERIMENTAL AND NUMERICAL ANALYSIS OF THE COLLAR
Solid shape molding is not desired in injection molding due to following reasons.
 PLASTICS PART DESIGN and MOULDABILITY Injection molding is popular manufacturing method because of its high-speed production capability. Performance of plastics part is limited by its properties which
PLASTICS PART DESIGN and MOULDABILITY Injection molding is popular manufacturing method because of its high-speed production capability. Performance of plastics part is limited by its properties which
CORRELATION BETWEEN HARDNESS AND TENSILE PROPERTIES IN ULTRA-HIGH STRENGTH DUAL PHASE STEELS SHORT COMMUNICATION
 155 CORRELATION BETWEEN HARDNESS AND TENSILE PROPERTIES IN ULTRA-HIGH STRENGTH DUAL PHASE STEELS SHORT COMMUNICATION Martin Gaško 1,*, Gejza Rosenberg 1 1 Institute of materials research, Slovak Academy
155 CORRELATION BETWEEN HARDNESS AND TENSILE PROPERTIES IN ULTRA-HIGH STRENGTH DUAL PHASE STEELS SHORT COMMUNICATION Martin Gaško 1,*, Gejza Rosenberg 1 1 Institute of materials research, Slovak Academy
CONSOLIDATION AND HIGH STRAIN RATE MECHANICAL BEHAVIOR OF NANOCRYSTALLINE TANTALUM POWDER
 CONSOLIDATION AND HIGH STRAIN RATE MECHANICAL BEHAVIOR OF NANOCRYSTALLINE TANTALUM POWDER Sang H. Yoo, T.S. Sudarshan, Krupa Sethuram Materials Modification Inc, 2929-P1 Eskridge Rd, Fairfax, VA, 22031
CONSOLIDATION AND HIGH STRAIN RATE MECHANICAL BEHAVIOR OF NANOCRYSTALLINE TANTALUM POWDER Sang H. Yoo, T.S. Sudarshan, Krupa Sethuram Materials Modification Inc, 2929-P1 Eskridge Rd, Fairfax, VA, 22031
North American Stainless
 North American Stainless Long Products Stainless Steel Grade Sheet AISI 316 UNS S31600 EN 1.4401 AISI 316L UNS S31630 EN 1.4404 INTRODUCTION NAS provides 316 and 316L SS, which are molybdenum-bearing austenitic
North American Stainless Long Products Stainless Steel Grade Sheet AISI 316 UNS S31600 EN 1.4401 AISI 316L UNS S31630 EN 1.4404 INTRODUCTION NAS provides 316 and 316L SS, which are molybdenum-bearing austenitic
Effect of cooling rate on hardness and microstructure of AISI 1020, AISI 1040 and AISI 1060 Steels
 International Journal of Physical Sciences Vol. 4 (9), pp. 514-518, September, 2009 Available online at http://www.academicjournals.org/ijps ISSN 1992-1950 2009 Academic Journals Full Length Research Paper
International Journal of Physical Sciences Vol. 4 (9), pp. 514-518, September, 2009 Available online at http://www.academicjournals.org/ijps ISSN 1992-1950 2009 Academic Journals Full Length Research Paper
EDEXCEL NATIONAL CERTIFICATE/DIPLOMA MECHANICAL PRINCIPLES OUTCOME 2 ENGINEERING COMPONENTS TUTORIAL 1 STRUCTURAL MEMBERS
 ENGINEERING COMPONENTS EDEXCEL NATIONAL CERTIFICATE/DIPLOMA MECHANICAL PRINCIPLES OUTCOME ENGINEERING COMPONENTS TUTORIAL 1 STRUCTURAL MEMBERS Structural members: struts and ties; direct stress and strain,
ENGINEERING COMPONENTS EDEXCEL NATIONAL CERTIFICATE/DIPLOMA MECHANICAL PRINCIPLES OUTCOME ENGINEERING COMPONENTS TUTORIAL 1 STRUCTURAL MEMBERS Structural members: struts and ties; direct stress and strain,
RAMAX S Prehardened stainless holder steel
 T O O L S T E E L F A C T S RAMAX S Prehardened stainless holder steel Wherever tools are made Wherever tools are used This information is based on our present state of knowledge and is intended to provide
T O O L S T E E L F A C T S RAMAX S Prehardened stainless holder steel Wherever tools are made Wherever tools are used This information is based on our present state of knowledge and is intended to provide
Binary phase diagrams
 inary phase diagrams inary phase diagrams and ibbs free energy curves inary solutions with unlimited solubility Relative proportion of phases (tie lines and the lever principle) Development of microstructure
inary phase diagrams inary phase diagrams and ibbs free energy curves inary solutions with unlimited solubility Relative proportion of phases (tie lines and the lever principle) Development of microstructure
North American Stainless
 Introduction: North American Stainless Flat Products Stainless Steel Grade Sheet 309S (S30908)/ EN1.4833 SS309 is a highly alloyed austenitic stainless steel used for its excellent oxidation resistance,
Introduction: North American Stainless Flat Products Stainless Steel Grade Sheet 309S (S30908)/ EN1.4833 SS309 is a highly alloyed austenitic stainless steel used for its excellent oxidation resistance,
This is an author-deposited version published in: http://sam.ensam.eu Handle ID:.http://hdl.handle.net/10985/10324
 Science Arts & Métiers (SAM) is an open access repository that collects the work of Arts et Métiers ParisTech researchers and makes it freely available over the web where possible. This is an author-deposited
Science Arts & Métiers (SAM) is an open access repository that collects the work of Arts et Métiers ParisTech researchers and makes it freely available over the web where possible. This is an author-deposited
Heat Transfer Prof. Dr. Aloke Kumar Ghosal Department of Chemical Engineering Indian Institute of Technology, Guwahati
 Heat Transfer Prof. Dr. Aloke Kumar Ghosal Department of Chemical Engineering Indian Institute of Technology, Guwahati Module No. # 02 One Dimensional Steady State Heat Transfer Lecture No. # 05 Extended
Heat Transfer Prof. Dr. Aloke Kumar Ghosal Department of Chemical Engineering Indian Institute of Technology, Guwahati Module No. # 02 One Dimensional Steady State Heat Transfer Lecture No. # 05 Extended
STAVAX SUPREME. Stainless tool steel
 STAVAX SUPREME Stainless tool steel General Demands placed on plastic mould tooling are increasing. Such conditions require mould steels that possess a unique combination of toughness, corrosion resistance
STAVAX SUPREME Stainless tool steel General Demands placed on plastic mould tooling are increasing. Such conditions require mould steels that possess a unique combination of toughness, corrosion resistance
ME 612 Metal Forming and Theory of Plasticity. 1. Introduction
 Metal Forming and Theory of Plasticity Yrd.Doç. e mail: azsenalp@gyte.edu.tr Makine Mühendisliği Bölümü Gebze Yüksek Teknoloji Enstitüsü In general, it is possible to evaluate metal forming operations
Metal Forming and Theory of Plasticity Yrd.Doç. e mail: azsenalp@gyte.edu.tr Makine Mühendisliği Bölümü Gebze Yüksek Teknoloji Enstitüsü In general, it is possible to evaluate metal forming operations
FEM analysis of the forming process of automotive suspension springs
 FEM analysis of the forming process of automotive suspension springs Berti G. and Monti M. University of Padua, DTG, Stradella San Nicola 3, I-36100 Vicenza (Italy) guido.berti@unipd.it, manuel.monti@unipd.it.
FEM analysis of the forming process of automotive suspension springs Berti G. and Monti M. University of Padua, DTG, Stradella San Nicola 3, I-36100 Vicenza (Italy) guido.berti@unipd.it, manuel.monti@unipd.it.
SS-EN ISO 9001 SS-EN ISO 14001
 This information is based on our present state of knowledge and is intended to provide general notes on our products and their uses. It should not therefore be construed as a warranty of specific properties
This information is based on our present state of knowledge and is intended to provide general notes on our products and their uses. It should not therefore be construed as a warranty of specific properties
COMPAX SUPREME Mold Quality Tool Steel
 T OOL STEEL FACTS COMPAX Mold Quality Tool Steel Great Tooling Starts Here! 2 This information is based on our present state of knowledge and is intended to provide general notes on our products and their
T OOL STEEL FACTS COMPAX Mold Quality Tool Steel Great Tooling Starts Here! 2 This information is based on our present state of knowledge and is intended to provide general notes on our products and their
Characterization of Electronic Materials Using Thermal Analysis
 application Note Thermal Analysis Characterization of Electronic Materials Using Thermal Analysis Thermal analysis comprises a series of powerful techniques for the characterization of the thermal, physical,
application Note Thermal Analysis Characterization of Electronic Materials Using Thermal Analysis Thermal analysis comprises a series of powerful techniques for the characterization of the thermal, physical,
Simulation of Residual Stresses in an Induction Hardened Roll
 2.6.4 Simulation of Residual Stresses in an Induction Hardened Roll Ludwig Hellenthal, Clemens Groth Walzen Irle GmbH, Netphen-Deuz, Germany CADFEM GmbH, Burgdorf/Hannover, Germany Summary A heat treatment
2.6.4 Simulation of Residual Stresses in an Induction Hardened Roll Ludwig Hellenthal, Clemens Groth Walzen Irle GmbH, Netphen-Deuz, Germany CADFEM GmbH, Burgdorf/Hannover, Germany Summary A heat treatment
Section 4: NiResist Iron
 Section 4: NiResist Iron Section 4 Ni-Resist Description of Grades...4-2 201 (Type 1) Ni-Resist...4-3 202 (Type 2) Ni-Resist...4-6 Stock Listings...4-8 4-1 Ni-Resist Description of Grades Ni-Resist Dura-Bar
Section 4: NiResist Iron Section 4 Ni-Resist Description of Grades...4-2 201 (Type 1) Ni-Resist...4-3 202 (Type 2) Ni-Resist...4-6 Stock Listings...4-8 4-1 Ni-Resist Description of Grades Ni-Resist Dura-Bar
High-strength and ultrahigh-strength. Cut sheet from hot-rolled steel strip and heavy plate. voestalpine Steel Division www.voestalpine.
 High-strength and ultrahigh-strength TM steels Cut sheet from hot-rolled steel strip and heavy plate Josef Elmer, Key account manager voestalpine Steel Division www.voestalpine.com/steel Weight savings
High-strength and ultrahigh-strength TM steels Cut sheet from hot-rolled steel strip and heavy plate Josef Elmer, Key account manager voestalpine Steel Division www.voestalpine.com/steel Weight savings
Numerical Analysis of Independent Wire Strand Core (IWSC) Wire Rope
 Numerical Analysis of Independent Wire Strand Core (IWSC) Wire Rope Rakesh Sidharthan 1 Gnanavel B K 2 Assistant professor Mechanical, Department Professor, Mechanical Department, Gojan engineering college,
Numerical Analysis of Independent Wire Strand Core (IWSC) Wire Rope Rakesh Sidharthan 1 Gnanavel B K 2 Assistant professor Mechanical, Department Professor, Mechanical Department, Gojan engineering college,
MATERIALIZING VISIONS. Bohler-Uddeholm P20 Modified
 MATERIALIZING VISIONS Bohler-Uddeholm P20 Modified General Bohler-Uddeholm P20 Modified is a Cr-Mo-alloyed steel which is supplied in the hardened and tempered condition. P20 Modified offers the following
MATERIALIZING VISIONS Bohler-Uddeholm P20 Modified General Bohler-Uddeholm P20 Modified is a Cr-Mo-alloyed steel which is supplied in the hardened and tempered condition. P20 Modified offers the following
Stainless steel grade chart
 Stainless steel grade chart ATLAS STEELS METAL DISTRIBUTION Chemical analysis (%) specified C Si Mn P S Cr Mo Ni Other Austenitic stainless steels 253MA S30815 0.05 1.1-2.0 0.8 0.040 0.030 20.0-22.0 10.0-12.0
Stainless steel grade chart ATLAS STEELS METAL DISTRIBUTION Chemical analysis (%) specified C Si Mn P S Cr Mo Ni Other Austenitic stainless steels 253MA S30815 0.05 1.1-2.0 0.8 0.040 0.030 20.0-22.0 10.0-12.0
