(SAMPLE COPY, NOT FOR RESALE)
|
|
|
- Theodore Nelson
- 9 years ago
- Views:
Transcription
1 TriMark Publications April 2016 Volume: TMRPOC POINT OF CARE DIAGNOSTIC TESTING WORLD MARKETS (SAMPLE COPY, NOT FOR RESALE) Trends, Industry Participants, Product Overviews and Market Drivers
2 TABLE OF CONTENTS 1. Overview About This Report Scope of the Report Objectives Methodology Executive Summary Overview of Diagnostic Testing Point of Care Markets Key Issues in the POC Diagnostic Testing Sector Current Market Trends and Drivers Effectiveness of POCT New Growth Areas for POCT Advantages of POCT in a Clinical Setting Pricing and Reimbursement Issues Key Customer Segments Global Point of Care Marketplace Shifts from Central Lab Testing to POCT Geographical Distribution Challenges of POCT Key Issues for POCT Locations of POCT Milestones in Technology Development Drive for Decentralization from Lab to Patient Competitive Landscape for POCT The Future of POCT Summary Analysis of the Global POCT Market: Value, Growth Rates and Market Share Global POCT Market Global Market Revenues Analysis by Market Segment Global POC Blood Glucose Monitoring Systems Market Global POC Blood Gas and Electrolyte Analyzers Market Global POC Rapid Coagulation Analyzers Market Global POC Cardiac Markers Devices Market Global POC Substance Abuse Testing Device Market Global POC Infectious Disease Testing Device Market Global POC Urine Strip Testing Market Global POC Pregnancy Testing Device Market Global POC Fecal Occult Device Market Global POC Cholesterol Testing Market Global Market Share Analysis Global Market Size by Geographic Location Global Market Size by Analyte Market Segment Global POCT Testing by Location Analysis of the U.S. POCT Market: Value, Growth Rates and Market Share U.S. Market Revenues Analysis by Market Segment U.S. POC Blood Glucose Monitoring Systems Market U.S. POC Blood Gas and Electrolyte Analyzers Market U.S. POC Rapid Coagulation Analyzers Market U.S. POC Cardiac Markers Devices Market U.S. POC Substance Abuse Testing Device Market U.S. POC Infectious Disease Testing Device Market U.S. POC Urine Strip Testing Market U.S. POC Pregnancy Testing Device Market TriMark Publications, LLC. All rights reserved 1
3 4.1.9 U.S. POC Fecal Occult Device Market U.S. POC Cholesterol Testing Market U.S. Market Share Analysis U.S. Market Size by Analyte Market Segment Analysis of the European POCT Market: Value, Growth Rates and Market Shares European Market Revenues Analysis European Market Revenues Analysis by Geographic Region French Revenues for POCT Systems German Revenues for POCT Systems Italian Revenues for POCT Systems Spanish Revenues for POCT Systems U.K. Revenues for POCT Systems Benelux Revenues for POCT Systems Scandinavian Revenues for POCT Systems Rest of Europe Revenues for POCT Systems European Market Revenues Analysis by Market Segment European POC Blood Glucose Monitoring Systems Market European POC Blood Gas and Electrolyte Analyzers Market European POC Rapid Coagulation Analyzers Market European POC Cardiac Marker Market European POC Substance Abuse Testing Device Market European POC Infectious Disease Testing Market European POC Urine Strip Testing Market European POC Pregnancy Testing Device Market European POC Fecal Occult Blood Testing Market European POC Cholesterol Testing Market European Market Share Analysis Analysis of the POCT Markets for Japan, China and India: Value, Growth Rates and Market Share Summary Analysis of Japanese, Chinese and Indian POCT Market Revenues Summary of Asian (Japanese, Chinese and Indian) Market Revenues by Market Segment Asian (Japanese, Chinese and Indian) POC Blood Glucose Monitoring Systems Market Asian (Japanese, Chinese and Indian) POC Blood Gas and Electrolyte Analyzers Market Asian (Japanese, Chinese and Indian) POC Rapid Coagulation Analyzers Market Asian (Japanese, Chinese and Indian) POC Cardiac Markers Devices Market Asian (Japanese, Chinese and Indian) POC Substance Abuse Testing Device Market Asian (Japanese, Chinese and Indian) POC Infectious Disease Testing Device Market Asian (Japanese, Chinese and Indian) POC Urine Strip Testing Market Asian (Japanese, Chinese and Indian) POC Pregnancy Testing Device Market Asian (Japanese, Chinese and Indian) POC Fecal Occult Device Market Asian (Japanese, Chinese and Indian) POC Cholesterol Testing Market Asian (Japanese, Chinese and Indian) Market Share Analysis Summary Analysis of the Japanese POCT Market Summary Analysis of the Indian POCT Market Summary Analysis of the Chinese POCT Market Analysis of the Rest of World (ROW) POCT Market: Value, Growth Rates and Market Share Summary Analysis of the ROW POCT Market Revenues Summary of the ROW Market Revenues by Market Segment ROW POC Blood Glucose Monitoring Systems Market ROW POC Blood Gas and Electrolyte Analyzers Market ROW POC Rapid Coagulation Analyzers Market ROW POC Cardiac Markers Devices Market ROW POC Substance Abuse Testing Device Market TriMark Publications, LLC. All rights reserved 2
4 7.2.6 ROW POC Infectious Disease Testing Device Market ROW POC Urine Strip Testing Market ROW POC Pregnancy Testing Device Market ROW POC Fecal Occult Device Market ROW POC Cholesterol Testing Market ROW Market Share Analysis Summary Analysis of the ROW POCT Market Revenues Summary Analysis of the Argentinian POCT Market Summary Analysis of the Australian POCT Market Summary Analysis of the Brazilian POCT Market Summary Analysis of the Canadian POCT Market Summary Analysis of the Russian POCT Market Summary Analysis of the Middle East POCT Market Summary Analysis of the African POCT Market Summary Analysis of the Asia-Pacific POCT Market Review of the Market Segments, Reagents and Equipment Blood Glucose Monitoring Introduction to Blood Glucose Monitoring Types of Blood Glucose Testing Fasting Blood Sugar (FBS) or Fasting Plasma Glucose (FPG) Two-Hour Postprandial Blood Sugar or Two-Hour PC Random Blood Sugar (RBS) Glucose Urine Test Blood Glucose Test Strip Glycosylated Hemoglobin (HbA1c) Intravenous Glucose Tolerance Test (IVGTT) Oral Glucose Tolerance Test (OGTT) Segmentation of POC Blood Glucose Testing Continuous Blood Glucose Monitoring (CBGM) Self-Monitoring Blood Glucose (SMBG) Testing Minimally-Invasive and Non-Invasive Blood Glucose Testing Emerging Glucose Monitoring Technologies Optical Transducer Technologies Transdermal Sensor Technologies Invasive Glucose Sensor Technologies Review of Professional POC Blood Glucose Monitoring Systems New Guidance: Use of Blood Glucose Monitoring Systems for Professional and Home POC Review of POC Blood Glucose Monitoring Products Abaxis, Inc Abbott Alere Apex Biotechnology ARKRAY, Inc DiaSys EKF Diagnostics Fora Care HemoCue (part of Radiometer) HMD BioMedical Infopia Instrumentation Laboratory Jant Pharmacal LifeHealth (formerly International Technidyne Corp.) Medtronic Nova Biomedical Polymer Technology Systems TriMark Publications, LLC. All rights reserved 3
5 Radiometer Randox Laboratories Roche Siemens Stanbio Laboratory Techno Medica Co., Ltd LifeScan Professional POC Hemoglobin A1c (HbA1c) Testing A. Menarini Diagnostics Alere Apex Biotechnology Convergent Technologies GmbH & Co DiaSys Diagnostic Systems Diazyme Laboratories EKF Diagnostics Eurolyser Green Cross Medis (formerly Ceragem Medisys) HemoCue (a division of Radiometer) Infopia Roche SD Biosensor Siemens Healthcare Diagnostics Erba Diagnostics Market Analysis Worldwide Professional Glucose Testing Market U.S. Professional Glucose Testing Market Competitive Analysis for the Glucose POCT Sector Market Share for Glucose Testing Competitive Analysis for the Glucose POCT Sector Market Analysis Market Drivers Market Restraints POC Glucose Testing Assay Market and Technology Trends POC Glucose Testing Assay Market Trends POC Glucose Testing Assay Technology Trends POC Glucose Testing Assay Strategic Recommendations Blood Gas and Electrolytes Background to POC Blood Gas and Electrolyte Testing Acid-Base Balance and the Lungs Respiratory Acidosis Respiratory Alkalosis Metabolic (or Non-Respiratory) Acidosis Metabolic (or Non-Respiratory) Alkalosis Increasing Popularity of POC Blood Gas and Electrolyte Testing Market Segmentation (Segmentation by Type of Blood Gas Monitoring Equipment) Intermittent Blood Gas Monitoring Equipment Continuous Blood Gas Monitoring Equipment EABG Monitors IABG Monitors Portable Blood Gas Analyzers Patient-Attached On-Demand Blood Gas Analyzers Applications for POCT Blood Gas and Electrolyte Analyzers Emerging Technologies Whole-Blood Lactate Creatinine Electrolytes Point of Care Analysis: Comparison of POCT and Central Lab Results TriMark Publications, LLC. All rights reserved 4
6 8.2.8 Review of Selected Blood Gas and Electrolyte Analyzers Review of Company Products Roche Corporation Siemens Healthcare Diagnostics Instrumentation Laboratory Nova Biomedical Radiometer Abbott Point of Care Accriva Diagnostics Alere Opti Medical Market Share for Blood Gas and Electrolyte Testing Market Analysis Market Drivers Market Restraints POC Blood Gas and Electrolyte Testing Assay Market and Technology Trends POC Blood Gas and Electrolyte Testing Assay Market Trends POC Blood Gas and Electrolyte Testing Assay Technology Trends POC Blood Gas and Electrolyte Testing Assay Strategic Recommendations Rapid Coagulation Tests Background to Rapid Coagulation Tests Review of Selected POC Rapid Coagulation Analyzers Review of Company Products Roche Diagnostics Corp Abbott Laboratories Accriva Medtronic Alere Helena Laboratories Point of Care Sienco, Inc Instrumentation Laboratory s GEM PCL Plus Connectivity Issues Cost Benefits Quality Control Issues Certification for POC Coagulation Devices D-Dimer Testing Market Analysis Market Share for POCT Coagulation Testing Competitive Analysis of Sector Companies Market Drivers Market Restraints POC Coagulation Testing Assay Market Trends POC Coagulation Testing Assay Technology Trends POC Coagulation Testing Assay Strategic Recommendations SWOT Analysis: Summary of Strengths, Weaknesses, Opportunities and Threats in the Coagulation POC Market POC Rapid Cardiac Markers Background to POC Rapid Cardiac Marker Testing Cardiac Marker Tests Creatine Kinase (CK) Myoglobin Cardiac Troponins T (TnT), I (TnI) and C (TnC) C-Reactive Protein (CRP) Homocysteine Emerging Markers B-Type Natriuretic Peptide (BNP) TriMark Publications, LLC. All rights reserved 5
7 Myeloperoxidase (MPO) Ischemia-Modified Albumin (IMA) Glycogen Phosphorylase Isoenzyme BB (GPBB) Fatty Acid-Binding Proteins (FABPs) Review of Selected POC Cardiac Biomarker Analyzers Review of Company Products Abbott s i-stat System Alere s Triage System Alpha Scientific Boditech Med s i-chroma System BTNX Homemed LSI Medience (formerly Mitsubishi Chemical Medience Corp.) Nano-Ditech Princeton BioMeditech Radiometer (part of Danaher) Response Biomedical Corporation Roche Diagnostics cobas h 232 POC System Roche s CARDIAC TROP T Sensitive Test (Visual) Siemens Healthcare Diagnostics (f/k/a Bayer Healthcare Diagnostics, Diagnostic Products Corporation and Dade Behring) Trinity Biotech Trivitron Healthcare (formerly Ani Biotech) biomérieux Market Share and Sales for Cardiac Marker Testing Market Drivers Market Restraints POC Cardiac Marker Testing Assay Market and Technology Trends POC Cardiac Marker Testing Assay Market Trends POC Cardiac Marker Testing Assay Technology Trends POC Cardiac Marker Testing Assay Strategic Recommendations POCT Substance Abuse Testing Background to POC Substance Abuse Testing Substance Abuse Test Types Urine Substance/Drug Screening Hair Tests for Substance Abuse and Screening Blood Tests for Substance Abuse and Screening Saliva Tests for Substance Abuse and Screening Sweat Tests for Substance Abuse and Screening Alcohol Abuse and Screening Saliva Testing Qualitative Analysis Market Drivers Market Restraints Review of Selected POC Substance Abuse Analyzers Review of Company Products Alere Abbott Diagnostics BTNX, Inc BioScan Screening Systems, Inc American Bio Medica Corp Phamatech, Inc First Check Diagnostics Corp. (Alere, Inc.) OraSure Noble Medical, Inc Guangzhou Wondfo Biotech Co., Ltd TriMark Publications, LLC. All rights reserved 6
8 Pathtech Alfa Scientific Designs, Inc American Bio Medica Biophor Diagnostics Randox Laboratories Branan Medical Concateno LifeSign POC Pregnancy and Fertility Tests Background to POC Pregnancy and Fertility Tests Review of Selected POC Pregnancy Testing Devices Review of Company Products Quidel Abbott Diagnostics Alere Sutherland Health Group Ltd Sekisui Diagnostics, LLC Siemens Diagnostics Orgenics (A subsidiary of Alere) LifeSign Other Companies in this Sector Market Analysis Competitive Sector Analysis Fecal Occult Blood Background to POC Fecal Occult Blood Testing Review of Selected POC Fecal Occult Testing Devices Review of Company Products Helena Laboratories Biomerica Beckman Coulter Worldwide Medical Aerscher Diagnostics Enterix Medix Biochemica Orion Diagnostica ACON Laboratories Mission FOB Reagent Strips Alere s Products Immunostics Hema-Screen Princeton BioMeditech s BioSign ifobtest Quest Diagnostics InSure FIT Quidel s QuickVue ifob Exact Sciences Cologuard Epigenomics CTK Biotech Market Share for Fecal Occult Blood Testing Company Analysis for the Fecal Occult Blood POCT Sector Market Analysis Market Drivers Market Restraints POC Fecal Occult Blood Testing Assay Market and Technology Trends POC Fecal Occult Blood Testing Assay Market Trends Fecal Occult Blood Testing Assay Technology Trends POC Fecal Occult Blood Testing Assay Strategic Recommendations POC Infectious Disease Testing Background to POC Infectious Disease Testing Types of Diagnosis for Infectious Diseases TriMark Publications, LLC. All rights reserved 7
9 Microbial Culture Microscopy Biochemical Tests Immunologic Tests Molecular Diagnostics Testing Diagnostic Platforms for Infectious Diseases Centralized Laboratory Testing for Infectious Diseases POC Testing for Infectious Diseases Emerging Technologies Market Analysis Market Drivers Market Restraints Review of Selected POC Infectious Disease Testing Devices POC Infectious Disease Testing Devices and Assays in Development Cepheid s GeneXpert Omni Alere q Quidel Roche s cobas Liat System QuantuMDx s Q-POC SRI International Atlas Genetics io System Overview of POC Assays for Detection of Infectious Disease Anthrax and Other Bio-threats Campylobacter Clostridium difficile E. coli Gastrointestinal Infectious Agents Giardia and Cryptosporidium Entamoeba histolytica Rotavirus & Adenovirus Norovirus Hepatitis Herpes Simplex Virus Type Influenza Legionella Malaria Testing Meningitis Mononucleosis Procalcitonin (Detects Sepsis) Respiratory Tract Infections C-Reactive Proteins Pneumonia Respiratory Tract Panels RSV Streptococcus Rubella Sexually-Transmitted Diseases Chlamydia Gonorrhea Testing Syphilis Staphylococcus aureus and MRSA Streptococcus A Testing Tropical Diseases Chagas Disease Dengue Fever Ebola TriMark Publications, LLC. All rights reserved 8
10 Filariasis Onchocerciasis Zika Virus Tuberculosis Typhoid POC Urine Strip Testing Background to POC Urine Strip Testing Emerging Technologies Review of Selected POC Urine Strip Testing Devices POC Cholesterol Testing POC Cholesterol Testing Market Size and Development Background to POC Cholesterol Testing POC Cholesterol Testing Devices Review of Selected POC Cholesterol Testing Devices Review of Company Products Cholestech (Now Alere) Polymer Technology Systems Miraculins Abaxis, Inc Product Comparison of Leading Suppliers Launch Dates of Leading Products Worldwide Miscellaneous POC Tests Estriol POC H. pylori Testing Bacterial Contamination of Platelet Units POC Vaginal Fluid ph and Vaginitis Testing POC Cancer Testing Prostate-Specific Antigen (PSA) Bladder Cancer Other Rapid Cancer Tests Fetal Status (PROM) Hemodynamic Monitoring Heparin-Induced Thrombocytopenia Ruptured Fetal Membranes Alcohol Microalbumin Testing Prenatal, Labor and Delivery Testing Alere Triage PLGF Medix Biochemica Sperm Fertility Surgical Coagulation and Hemostasis Management Respiratory Tests C-Reactive Protein HIV FDA-Approved Rapid HIV Assays Alere biolytical Laboratories, Inc Bio-Rad Laboratories Chembio Diagnostics MedMira Laboratories, Inc OraSure Technologies Trinity Biotech Rapid HIV Assays Not Approved by the FDA (not commercially available in the U.S.) Alere biomérieux Bio-Rad TriMark Publications, LLC. All rights reserved 9
11 Core Diagnostics CTK Biotech EY Laboratories Hema Diagnostics Systems ImmunoScience InTec Products JAL Innovation J Mitra & Co Premier Medical Corporation Princeton BioMeditech Savyon Diagnostics Shanghai Kehua Span Diagnostics Tulip Group YD Diagnostics AIDScan POC Viral Load Technologies of the Future Cepheid Roche Wave 80 Biosciences POCT: Growth Regulators Moderators of Growth Personnel Acceptance Key People for POCT Information Management Issues Elements of Information Management for POCT: Information-Processing Capabilities Data Mining Middleware Web Portals POCT-1A Standard Key Elements for POCT POCT and Reimbursement Effectiveness of Clinical Outcomes Rapid Near-Patient Testing in Hospitals Satellite Facilities Regionalization of Laboratory Care Requirements for POCT Locations of POC for Patient Care Benefits of POCT Cost Elements of POCT Necessary Functions in POCT Turnaround Time (TAT) for POCT Clinical Laboratory Improvement Amendments (CLIA) Sexually-Transmitted Diseases in Underdeveloped Countries Sources of Error in POC Testing Business Trends in the POC Sector Sector Consolidation Diagnostic Testing Growth Trends Opportunities for Healthcare Stakeholders Acquisition, License Agreements, Internal Development and Partnerships Product Testing Depth in POCT Government Regulation U.S. Regulation Importing Medical Devices into the U.S Exporting Medical Devices from the U.S TriMark Publications, LLC. All rights reserved 10
12 E.U. Regulation Japanese Regulation Post-Filing Regulations for POC Devices Exporting Unapproved POCTs Analyte-Specific Reagents ( Home-Brew Tests) Medical Device Registration Product Labeling Punitive FDA Actions CLIA 88 and State Laboratory Laws Impact of Regulations on the Industry Minimizing Regulatory Barriers Waived Testing Technology Platform Innovations in POCT Latest POCT Technological Platforms Device Miniaturization and Microfluidic Technologies Minimally-Invasive and Non-Invasive POCT Technologies Libre Flash Glucose Monitor and OneTouch Verio Sync Meter Echo Therapeutics Advances in Wireless Technologies Automation of POCT Developments in New Genomic Technologies (Genotyping, Haplotyping and Sequencing Technologies) Advances in Informatics Technologies Pharmacogenetic Testing Multi-Assay Technologies in POCT Molecular Diagnostic Technology in POCT for Infectious Disease Smart Phone Technology Aquila Diagnostic Systems, Inc AgaMatrix Quick HIV and Syphilis Screening Accu-Check Connect System QuantuMDx Handheld, Portable Low Cost Rapid Devices Data Management and Connectivity Wireless LANs Connectivity Platforms Data Management Systems Alere RALS System International Technidyne Corporation HEMOCHRON Signature Elite Siemens Diagnostics Rapidpoint Radiometer HemoCue s DM Hemoglobin Roche Diagnostics Cobas IT Accriva BD.id Medical Implant Communications Service (MICS) Conworx Technology Telcor QML Medasys NoemaLife Telcare Advantages of POCT Connectivity Cost-Benefit Analysis of POCT and IT Connectivity Hospital Network Issues TriMark Publications, LLC. All rights reserved 11
13 13. Corporate Profiles Abaxis, Inc Abbott Laboratories AccuBioTech Co., Ltd Accriva Diagnostics ACON Laboratories, Inc Acrongenomics AgaMatrix Aerscher Diagnostics, LLC Akers Biosciences, Inc Alere Alfa Scientific Designs, Inc American Bio Medica Corporation Arkray, Inc Atlas Genetics Ltd Atonomics A/S Audit Diagnostics Augurix Diagnostics Ltd Axxin Beckman Coulter (Danaher Corporation) biolytical Laboratories Biomerica, Inc biomérieux BiOracle Bio-Rad Laboratories, Inc BioScan Screening Systems, Inc Calypte Biomedical Corporation Chembio Diagnostic, Inc Cholestech Claros Diagnostics (OPKO Healthcare) ERBA Diagnostics, Inc Daktari Diagnostics Dexcom Diagnostics for All Enigma Diagnostics Ltd EY Laboratoires, Inc Eurotrol Exalenz Bioscience GenBio Guided Therapeutics, Inc Helena Laboratories Hema Diagnostic Systems, LLC Instrumentation Laboratory (IL)/Werfen Group Jant Pharmacal Corporation Lein Applied Diagnostics LifeSign, LLC Medica Corporation Medix Biochemica MedMira Laboratories A. Menarini Diagnostics Micronics New Horizons Diagnostics Nova Biomedical OraSure Technologies, Inc Orion Diagnostica Polymedco, Inc TriMark Publications, LLC. All rights reserved 12
14 13.56 Polymer Technology Systems, Inc Prima Biomedical Company Qualigen Quest Diagnostics, Inc Quidel Corporation Radiometer Medical Response Biomedical Corp Roche Diagnostics Savyon Diagnostics Seegene Sekisui Diagnostics, LLC Shanghai Ruicare Medical Co., Ltd Shenzhen Fitconn Technology Co., Ltd Shionogi & Co., Ltd Siemens AG Siloam Biosciences, Inc Spectral Diagnostics, Inc StatSure Diagnostic Systems, Inc One Step Detect Associates, LLC Senseonics Telcare Trinity Biotech Plc Väsamed, Inc POCT Sector Trends and Forecasts Home Care Analysis as Part of Near-Patient Testing Non-Traditional Collection for POCT New Systems for Critical Care and Near-Patient Testing Utility of Near-Patient Testing in Critical-Care Settings Physician s Office Market Information Management Advances Test-Ordering Patterns and Demand for POCT Demand for Emergency Department Services Move Away from the Central Laboratory Healthcare Cost Controls Mergers & Acquisitions Competition for Services Drivers of POCT Confluence of New Technology Difficulties of Design for POC Products Point of Care Compliance The Biggest New Opportunities in POCT New Entrants to the Point of Care Market Alliance International Co., Ltd Analyticon Biotechnologies AG Ativa Medical Corporation Aquila Diagnostic Systems, Inc ArcDia International Oyo Ltd Artron Laboratories, Inc Atlas Link Biotech Co., Ltd Audit Diagnostics Autobio Diagnostics Co., Ltd Biocartis SA BioFire Diagnostics, Inc BioMedomics TriMark Publications, LLC. All rights reserved 13
15 15.13 Biosensia Ltd Curetis AG DxNA, LLC Echo Therapeutics, Inc Gentag, Inc Great Basin Corporation Immunexpress Group Integrated Diagnostics Integrity Applications MBio Diagnostics, Inc Menssana Research, Inc Metaara Medical Technologies, Inc QuantuMDx Group Venaxis, Inc Philips Business Group EBA Wave 80 Biosciences Veredus Laboratories Pte Ltd. 449 INDEX OF FIGURES Figure 2.1: Worldwide Distribution of IVD Testing, Figure 2.2: Top 13 Country IVD Testing Markets, Figure 2.3: Market Share for IVD Testing by Company, Figure 3.1: Total Global POCT Market, Figure 3.2: Worldwide Distribution of POCT, Figure 3.3: Summary of Global POCT Markets by Market Segment, Figure 4.1: U.S. Revenues for POCT Market, Figure 4.2: Summary of U.S. POCT Markets by Market Segment, Figure 5.1: European Revenues for POCT Market, Figure 6.1: Summary Analysis of Japanese, Chinese and Indian POCT Market, Figure 6.2: Japanese Revenues for POCT Market, Figure 6.3: Indian Revenues for POCT Market, Figure 6.4: Chinese Revenues for POCT Market, Figure 7.1: Summary Analysis of ROW POCT Market, Figure 7.2: Summary Analysis of Russian Revenues for POCT Products Market, Figure 7.3: Summary Analysis of Middle East Revenues for POCT Products Market, Figure 7.4: Summary Analysis of African Revenues for POCT Products Market, Figure 7.5: Summary Analysis of Latin America Revenues for POCT Products Market, Figure 8.1: Global Share of Glucose Testing Market by Company, Figure 8.2: Global POCT Share of Blood Gas and Electrolyte Testing Market, Figure 8.3: Global POCT Share of Coagulation Testing Market, Figure 8.4: Global POCT Share of Cardiac Marker Testing Market, Figure 8.5: Distribution of Infectious Diseases Tested at the Point of Care 259 Figure 8.6: Global POCT Share of HIV Testing Market, Figure 9.1: Annual Rate of Emergency Room Visits by Primary Expected Source of Payment 342 Figure 10.1: Conformity Assessment Route, Annexes and Quality System Standards by IVD Device Category 360 Figure 12.1: The Alere RALS Data Management System Connects to Most of the Major Instruments Used in Point of Care 385 INDEX OF TABLES Table 2.1: Summary of Global POCT Markets by Major Market Sub-Segment, 2015 and Table 2.2: Summary of U.S. POCT Markets by Major Market Sub-Segment, 2015 and TriMark Publications, LLC. All rights reserved 14
16 Table 2.3: Summary of European POCT Markets by Major Market Sub-Segment, 2015 and Table 2.4: Summary of Asian POCT Markets by Major Market Sub-Segment, 2015 and Table 2.5: Summary of ROW POCT Markets by Major Market Sub-Segment, 2015 and Table 2.6: Worldwide Distribution of IVD Testing, Table 2.7: Top 13 Country IVD Testing Markets, Table 2.8: Company Market Share for IVD Testing Markets, Table 2.9: Competitive Landscape for POC Diagnostic Testing 42 Table 3.1: Total Global POCT Market, Table 3.2: Global Revenues for POC Blood Glucose Monitoring Systems, Table 3.3: Global Revenues for POC Blood Gas and Electrolyte Analyzers, Table 3.4: Global Revenues for POC Rapid Coagulation Analyzer Systems, Table 3.5: Global Revenues for POC Cardiac Marker Devices, Table 3.6: Global Revenues for POC Substance/Drug Abuse Testing Device Market, Table 3.7: Global Revenues for POC Infectious Disease Testing Devices Market, Table 3.8: Global Revenues for POC Urine Strip Testing Products Market, Table 3.9: Global Revenues for POC Pregnancy Testing Devices Market, Table 3.10: Global Revenues for POC Fecal Occult Testing Devices Market, Table 3.11: Global Revenues for POC Cholesterol Testing Products Market, Table 3.12: Global POCT Market Share Analysis, Table 3.13: Worldwide POCT Market Size by Geographic Location, Table 3.14: Worldwide Distribution of POCT, Table 3.15: Summary of Global POCT Markets by Market Segment, Table 3.16: Worldwide POCT by Performance Location, Table 4.1: U.S. Revenues for POCT Market, Table 4.2: U.S Revenues for POC Blood Glucose Monitoring Systems, Table 4.3: U.S. Revenues for POC Blood Gas and Electrolyte Analyzers, Table 4.4: U.S. Revenues for POC Rapid Coagulation Analyzer Systems, Table 4.5: U.S. Revenues for POC Cardiac Marker Devices, Table 4.6: Market Share of U.S. POCT Cardiac Marker Market, Table 4.7: U.S. Revenues for POC Substance/Drug Abuse Testing Device Market, Table 4.8: U.S. Revenues for POC Infectious Disease Testing Devices Market, Table 4.9: U.S. Revenues for POC Urine Strip Testing Products Market, Table 4.10: U.S. Revenues for POC Pregnancy Testing Devices Market, Table 4.11: U.S. Revenues for POC Fecal Occult Testing Devices Market, Table 4.12: U.S. Revenues for POC Cholesterol Testing Products Market, Table 4.13: U.S. POCT Market Share Analysis, Table 4.14: Summary of U.S. POCT Markets by Market Segment, Table 5.1: Models of Public-Private Partnership in Hospital Provision 67 Table 5.2: European Revenues for POCT Market, Table 5.3: French Revenues for POCT Systems, Table 5.4: German Revenues for POCT Systems, Table 5.5: Italian Revenues for POCT Systems, Table 5.6: Spanish Revenues for POCT Systems, Table 5.7: U.K. Revenues for POCT Systems, Table 5.8: Benelux Revenues for POCT Systems, Table 5.9: Scandinavian Revenues for POCT Systems, Table 5.10: Rest-of-Europe Revenues for POCT Systems, Table 5.11: European Revenues for POC Blood Glucose Monitoring Systems, Table 5.12: European Revenues for POC Blood Gas and Electrolyte Analyzers, Table 5.13: European Revenues for POC Rapid Coagulation Analyzer Systems, Table 5.14: European Revenues for POC Cardiac Markers, Table 5.15: European Revenues for POC Substance/Drug Abuse Testing Market, Table 5.16: European Revenues for POC Infectious Disease Testing Market, Table 5.17: European Revenues for POC Urine Strip Testing Products Market, Table 5.18: European Revenues for POC Pregnancy Testing Market, Table 5.19: European Revenues for POC Fecal Occult Blood Testing Market, TriMark Publications, LLC. All rights reserved 15
17 Table 5.20: European Revenues for POC Cholesterol Testing Products Market, Table 5.21: European Market Share Analysis, Table 6.1: Summary Analysis of Japanese, Chinese and Indian POCT Market, Table 6.2: Summary Analysis of Japanese, Chinese and Indian Revenues for POC Blood Glucose Monitoring Systems, Table 6.3: Summary Analysis of Japanese, Chinese and Indian Revenues for POC Blood Gas and Electrolyte Analyzers, Table 6.4: Summary Analysis of Japanese, Chinese and Indian Revenues for POC Rapid Coagulation Analyzer Systems, Table 6.5: Summary Analysis of Japanese, Chinese and Indian Revenues for POC Cardiac Marker Devices, Table 6.6: Summary Analysis of Japanese, Chinese and Indian Revenues for POC Substance/Drug Abuse Testing Device Market, Table 6.7: Summary Analysis of Japanese, Chinese and Indian Revenues for POC Infectious Disease Testing Devices Market, Table 6.8: Summary Analysis of Japanese, Chinese and Indian Revenues for POC Urine Strip Testing Products Market, Table 6.9: Summary Analysis of Japanese, Chinese and Indian Revenues for POC Pregnancy Testing Devices Market, Table 6.10: Summary Analysis of Japanese, Chinese and Indian Revenues for POC Fecal Occult Testing Devices Market, Table 6.11: Summary Analysis of Japanese, Chinese and Indian Revenues for POC Cholesterol Testing Products Market, Table 6.12: Summary Analysis of Japanese, Chinese and Indian Market Shares, Table 6.13: Japanese Population and Aging Demographics Forecast, Table 6.14: Japanese Revenues for POCT Market, Table 6.15: Indian Revenues for POCT Market, Table 6.16: Chinese Revenues for POCT Market, Table 7.1: Summary Analysis of ROW POCT Market, Table 7.2: Summary Analysis of ROW Revenues for POC Blood Glucose Monitoring Systems, Table 7.3: Summary Analysis of ROW Revenues for POC Blood Gas and Electrolyte Analyzers, Table 7.4: Summary Analysis of ROW Revenues for POC Rapid Coagulation Analyzer Systems, Table 7.5: Summary Analysis of ROW Revenues for POC Cardiac Marker Devices, Table 7.6: Summary Analysis of ROW Revenues for POC Substance/Drug Abuse Testing Device Market, Table 7.7: Summary Analysis of ROW Revenues for POC Infectious Disease Testing Devices Market, Table 7.8: Summary Analysis of ROW Revenues for POC Urine Strip Testing Products Market, Table 7.9: Summary Analysis of ROW Revenues for POC Pregnancy Testing Devices Market, Table 7.10: Summary Analysis of ROW Revenues for POC Fecal Occult Testing Devices Market, Table 7.11: Summary Analysis of ROW Revenues for POC Cholesterol Testing Products Market, Table 7.12: Summary Analysis of ROW Market Shares, Table 7.13: Summary Analysis of Argentinian Revenues for POCT Products Market, Table 7.14: Summary Analysis of Australian Revenues for POCT Products Market, Table 7.15: Summary Analysis of Brazilian Revenues for POCT Products Market, Table 7.16: Summary Analysis of Canadian Revenues for POCT Products Market, Table 7.17: Summary Analysis of Russian Revenues for POCT Products Market, Table 7.18: Summary Analysis of Middle East Revenues for POCT Products Market, Table 7.19: Summary Analysis of African Revenues for POCT Products Market, TriMark Publications, LLC. All rights reserved 16
18 Table 7.20: Summary Analysis of Asia (Other) Revenues for POCT Products Market, Table 7.21: Summary Analysis of Latin America (Other) Revenues for POCT Products Market, Table 8.1: Selected POC Blood Glucose and Blood Gas Meters Marketed for Professional Use, Table 8.2: POC HbA1c Testing Devices and Assays on the Market 143 Table 8.3: Global Revenues for Total Professional Blood Glucose Monitoring (Central Laboratory Testing and Hospital POC and POL), Table 8.4: SWOT Analysis: Summary of Strengths, Weaknesses, Opportunities and Threats of the Glucose Point of Care Market 150 Table 8.5: Global Share of Glucose Testing Market by Company, Table 8.6: Selected POC Blood Gas and Electrolyte Analyzers, Table 8.7: Market Share of Blood Gas and Electrolyte POCT Diagnostic Testing Companies Worldwide, Table 8.8: SWOT Analysis: Summary of Strengths, Weaknesses, Opportunities and Threats in the Blood Gas and Electrolyte POC Market 170 Table 8.9: Hospital Locations of Point of Care Coagulation Testing 172 Table 8.10: Selected POC Rapid Coagulation Analyzers, Table 8.11: Actalyke XL 185 Table 8.12: Actalyke Mini 185 Table 8.13: Connectivity Modes for Leading Coagulation POCT Analyzers 187 Table 8.14: Market Share for Coagulation POCT Diagnostic Testing Companies, Table 8.15: Drivers for Point of Care Coagulation Testing 192 Table 8.16: Barriers for Point of Care Coagulation Testing 192 Table 8.17: SWOT Analysis: Summary of Strengths, Weaknesses, Opportunities and Threats in the Coagulation POC Market 193 Table 8.18: Selected POC Cardiac Biomarkers, Table 8.19: Market Share of Cardiac Marker POCT Diagnostic Testing Companies Worldwide, Table 8.20: POC Cardiac Marker Testing Market: Market Drivers Ranked in Order of Impact 214 Table 8.21: POC Cardiac Marker Testing Market: Market Restraints Ranked in Order of Impact 215 Table 8.22: SWOT Analysis: Summary of Strengths, Weaknesses, Opportunities and Threats in the Cardiac Marker POC Market 216 Table 8.23: Drug Testing Needs Tier I for Rapid Serum POCT in the Emergency Department 223 Table 8.24: Stat Urine Testing Drug Recommendations 223 Table 8.25: Selected POC Substance/Drug Abuse Testing Devices, Table 8.26: Selected POC Pregnancy Testing Devices, Table 8.27: Selected POC Fecal Occult Testing Devices, Table 8.28: ColoCARE Fecal Occult Blood Test 249 Table 8.29: SWOT Analysis: Summary of Strengths, Weaknesses, Opportunities and Threats in the Fecal Occult Blood POC Market 254 Table 8.30: Selected POC Infectious Disease Testing Devices and Assays, Table 8.31: Influenza Diagnostic Rapid Tests 274 Table 8.32: Selected POC Urine Strip Testing, Table 8.33: Selected POC Cholesterol Testing Devices, Table 8.34: AmniSure 318 Table 8.35: Competitive Factors Related to HIV Tests 321 Table 8.36: Global POCT Market for Rapid HIV Testing, Table 8.37: Global POCT Share of HIV Testing Market, Table 8.38: Rapid HIV Immunoassay Tests 324 Table 9.1: POCT1-Compliant Connectivity POC Instruments 340 Table 9.2: POCT Clinical Outcomes in Diabetic Ketoacidosis 342 Table 9.3: POCT Clinical Outcomes in Arterial Blood-Gas Measurements 342 Table 9.4: POCT in Three Hospitals Cost Analysis with Labor Included 345 Table 9.5: POCT versus Central Lab Cost Analysis for Glucose Testing 345 Table 11.1: Common Genotype Techniques 375 Table 12.1: Applications for Wireless LAN Technology 381 Table 12.2: Customized Reports Must Support CAP and JCAHO Requirements TriMark Publications, LLC. All rights reserved 17
19 Table 14.1: Improvements in Achieving ED Operational Efficiency Using POCT 433 Table 14.2: Test Menu and TAT for ED 434 Table 14.3: Top Reasons Why POC Cardiac Marker Testing is Implemented in the ED 434 Table 14.4: Key Areas and Metrics that Hospitals Measure to Assess Efficiency of ED 435 Table 14.5: Estimates of Patient Costs by DRG TriMark Publications, LLC. All rights reserved 18
20 1. Overview Point of care testing (POCT) enables rapid diagnostic tests to be performed while the patient is at the point of care (POC) facility where results can be obtained immediately rather than waiting hours or even days for outside lab results to arrive. POCT covers: blood glucose testing, blood gas and electrolytes analysis, rapid coagulation testing, rapid cardiac markers diagnostics, drugs of abuse screening, urine strips testing, pregnancy testing, fecal occult blood analysis, hemoglobin diagnostics, infectious disease testing and cholesterol screening. This TriMark Publications report describes the POC testing segment of the diagnostic market. An analysis of analytes that are related to the common chemical constituents of blood, plasma/serum, urine and other body fluids at the point of care of the patient are addressed. The two most important areas where such tests are measured for immediate results in a POC setting are hospital emergency rooms and critical-care clinics. The third place where these tests are frequently measured in what is characterized as a near-patient setting is in physician s office labs (POLs). Other testing areas of interest for these analytes are satellite labs, critical-care units, neonatal intensive-care units (NICUs), intensive-care units (ICUs) and home testing locations. Home testing is not directly covered in this report except in cases when the products and companies in this market segment are also actively used in the hospital and physician s office POC settings. This report also examines the subsections of each POC market segment, including: glucose, blood gases, coagulation, cardiac markers, drugs of abuse, infectious disease and many others. Additionally, rapid detection of infectious pathogens (methicillin-resistant Staphylococcus aureus [MRSA], herpes simplex, avian flu, West Nile virus [WNV] and typhoid) is discussed. This examination of POCT focuses on the POC segments in important worldwide markets, such as the U.S., Japanese, European, Asian and Rest-of-the-World (ROW) markets. An extensive review of POCT in this report includes the market for clinical equipment and supplies as well as the market for screening reagents and instruments for analysis of individual components in blood, serum, urine and other body fluids. This report defines the dollar volume of sales, for both worldwide and U.S. markets, and it analyzes the factors that influence the size and the growth of the individual market segments. Most of the companies known to be developing instruments and reagents for the clinical POC market are examined in this study. Each company is discussed in depth with a section on the history of the company, the product line, a business and marketing analysis and a subjective commentary of the position of the company in its market. 1.1 About This Report A review of analytes that are related to the chemical and cellular constituents of blood, plasma or serum at the point of care of the patient is addressed in this study. The two most important areas where such tests are measured are in the hospital and the clinic environments (the emergency department and the critical-care section). Another important place where these tests are measured is in POLs. Newer testing areas of interest for these analytes are satellite labs, corporate facilities, law enforcement agencies and home testing locations. This report s emphasis is on companies that are actively developing and marketing clinical laboratory instrumentation, reagents and supplies for performing clinical diagnostic tests in the near-patient environment. The main objectives of this analysis are: For the general point of care testing market: The size of the market and distribution between different market segments. The expected growth over the next five years within each market segment. A comprehensive overview of the main POCT players. What kind of instruments do they have and what is range of analysis? Which analysis in POCT is expected to experience exceptional growth going forward? Identifying viable technology drivers through a comprehensive look at platform technologies for POCT. Understanding the different sectors of POCT, looking at the hospital market segment and, separately, at a description of the instruments, reagents and supplies marketed by major companies in each segment. Obtaining a complete understanding of the individual POC tests, from their basic principles to their clinical applications TriMark Publications, LLC. All rights reserved 19
21 Discovering feasible market opportunities by identifying high-growth applications in different analytical diagnostic areas, emphasizing the biggest and expanding markets. Focusing on global industry development through an in-depth analysis of the major world markets for POC technology, including growth forecasts. Presenting POCT market figures regarding the market s current value, market projections, market share, key players and sector growth rates. This information is the most currently available data derived from the global diagnostic industry. This study contains: A detailed analysis of recent trends in the POC marketplace. In-depth profiles of the leading companies with POC tools and technologies. A forecast for the POC market and diagnostic segments thereof. Views and predictions on the POC industry from leading industry experts. An analysis of potential new POC applications in the clinical sector. Market predictions and trends analysis concerning U.S. expenditures on POC solutions. Projections of POC market sizes for European and Asian markets. Projections for future applications of molecular diagnostic tests in POC-related screening. Analysis of commercial POC business strategies. The latest news and mergers and acquisitions (M&As) developments in the POC marketplace. A comprehensive overview of and insight into POC business strategies. An in-depth examination of the subsections of each market segment, including the POLs and clinic testing. Analysis includes charts and graphs measuring product growth and trends within the marketplace. Company-specific information, including sales figures, product pipeline status and research and development (R&D) trends, is provided. This review will also: Assess POC market drivers and bottlenecks from medical and scientific community perspectives. Discuss the potential benefits of POC for various sectors of the medical and scientific community. Establish the current total market size and future growth of the POC market and analyze the current size and growth of individual segments. Provide current and forecasted market shares by company. Discuss profit and business opportunities by segment. Provide strategic recommendations for near-term business opportunities. Assess current commercial uses of the POC market. The following questions will also be addressed in this analysis: What are the near-term business opportunities in the POCT market? What are the current and forecasted POCT market sizes in the U.S., the European Union (E.U.), Japan and other key country markets? What are the business models currently used by companies in the POCT market? How will stakeholders like manufacturers, regulators, physicians and patients influence this market? What are the drivers and restraints influencing the POCT market? What are the important technologies used in POCT? Who holds the proprietary rights to the POCT market technology platforms? In the U.S., Japan and the E.U., what regulatory processes apply to POCT technologies? How will new POC technologies change diagnostic screening testing paradigms? How will new POC technologies reduce healthcare expenditures and affect R&D spending? The report contains: A comprehensive overview of the several categories of POC technology platforms that are or will be revolutionizing the use of diagnostic tests in hospitals TriMark Publications, LLC. All rights reserved 20
22 A chapter on each of the important POC categories and applications. Full descriptions of the technologies involved and how they differ from existing and emerging technologies. Analysis of the technological approaches undertaken by various competitors, as well as industry and enduser responses to these products. Regulatory issues and legislation affecting the use and marketing of POC products. Market forecasts for each category of product, including profiles of selected competitors. 1.2 Scope of the Report The POCT diagnostic product markets in the U.S., Japan and Europe the world s three largest analytical markets are the focus of this study. Analysis of the diagnostic and POC activity of a number of other smaller country markets is also included. Primary attention is paid to the clinical market segment and, separately, to the instruments, reagents and supplies marketed by major companies in this segment. Market size, growth rates and market components for instruments, reagents, controls and consumables used in this area are also analyzed. In general, the non-professional (home care) market for self-testing is considered an entirely different market from professional (hospitals, clinics and doctor s offices) testing and is not considered in this report in any detailed way. Other related areas, e.g., infant jaundice evaluation, anthrax detection, homeland defense testing, bovine spongiform encephalopathy (BSE, i.e., Mad Cow Disease), tuberculosis and food pathogens, are also discussed. This report focuses on the point of care market. In this context, this phrase means professional testing, which extends beyond the hospital ER to doctor s offices and other medical facilities where trained personnel perform the tests. In the context of this report, and by general agreement within the diagnostics sector, this will exclude so called home testing. Over 90% of this type of testing is used for glucose measurements. More information on self-testing markets is available in a TriMark report entitled World Glucose Self-Testing Markets. The reader should consult other TriMark Publications reports at for detailed discussions of important individual market segments related to the POCT market such as POC Diagnostic Sector Testing Trends, Blood Glucose Testing and Diabetes Management, and Women s Health Diagnostic Testing. 1.3 Objectives The key objective of this study is to conduct a comprehensive review of the POCT market with particular emphasis on emerging trends in equipment and supplies using screening reagents and instruments for analysis of individual components in tissue samples, blood, serum or plasma. Also examined are the sub-segments of each market segment, including physician s office labs, specialty labs (e.g., NICUs) and critical-care laboratories. In addition, this report reviews a number of institutions using these forms of POCT and includes a discussion of the factors that influence their purchasing decisions. The report surveys almost all of the companies known to be marketing, manufacturing or developing instruments and reagents for the POC market. 1.4 Methodology The author of this report holds a Ph.D. in biochemistry from the University of Minnesota and has had post-doctoral experience at the University of Connecticut School of Medicine. He has taught at Quinnipiac University and the Tufts School of Medicine and has been a senior scientist at Pfizer Pharmaceutical Laboratories in drug development. He also has many decades of experience in science writing and as a medical industry analyst. He has over 30 years of experience in laboratory testing and instrument and reagent development technology as a licensed clinical laboratory director, as well as extensive experience in senior-level management positions in biotech and medical service companies. Company-specific information is obtained mainly from industry trade publications, academic journals, news and research articles, press releases and corporate websites as well as from annual reports for publicly-held firms. Additional sources of information include non-governmental organizations (NGOs) such as the World Health Organization (WHO) and governmental entities such as the U.S. Department of Health and Human Services (HHS), the National Institutes of Health (NIH), the U.S. Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC). Where possible and practicable, the most recent data available have been used TriMark Publications, LLC. All rights reserved 21
23 Some of the statistical information was taken from Biotechnology Associates databases and from TriMark s private data stores. The information in this study was obtained from sources that we believe to be reliable, but we do not guarantee the accuracy, adequacy or completeness of any information or omission or the results obtained from the use of such information. Key information from the business literature was used as a basis to conduct dialogue with and obtain expert opinion from market professionals regarding commercial potential and market sizes. Senior managers from major company players were interviewed for part of the information in this report. Primary Sources TriMark collects information from hundreds of Database Tables and many comprehensive multi-client research projects, as well as from Sector Snapshots that it publishes annually. TriMark extracts relevant data and analytics from its research as part of this data collection. Secondary Sources TriMark uses research publications, journals, magazines, newspapers, newsletters, industry reports, investment research reports, trade and industry association reports, government-affiliated trade releases and other published information as part of its secondary research materials. The information is then analyzed and translated by the Industry Research Group into a TriMark study. The Editorial Group reviews the complete package with product and market forecasts, critical industry trends, threats and opportunities, competitive strategies and market share determinations. TriMark Publications Report Research and Data Acquisition Structure The general sequence of research and analysis activity prior to the publication of every report includes the following items: Completing an extensive secondary research effort on an important market sector, including gathering all relevant information from corporate reporting, publicly-available databases, proprietary databases, direct meetings and personal interviews with key personnel. Formulating a study outline with the assigned writer, including the following: Market and product segment grouping and evaluating their relative significance. Key competitors evaluations, including their relative positions in the business and other relevant facts to prioritize diligence levels and assist in designing a primary research strategy. End-user research to evaluate analytical significance in market estimation. Supply chain research and analysis to identify any factors affecting the market. New technology platforms and cutting-edge applications. Identifying the key technology and market trends that drive or affect these markets. Assessing the regional significance for each product and market segment for proper emphasis of further regional/national primary and secondary research. Launching a combination of primary research activities, including two levels of questionnaires and executive-direct focused, company-specific and region-specific communications to qualified and experienced senior executives worldwide. Completing a confirmatory primary research assessment of the report s findings with the assistance of Expert Panel Partners from the industry being analyzed. The information is then analyzed and translated by the Industry Research Group into a TriMark study. The Editorial Group reviews the complete package with product and market forecasts, critical industry trends, threats and opportunities, competitive strategies and market share determinations TriMark Publications, LLC. All rights reserved 22
24 Finally, before publication, each market report is reviewed by a fact checker, and editor and a proof reader. The final copy is ultimately released by the Editor-in-Chief and management. 1.5 Executive Summary TriMark estimates that the professional global in vitro diagnostic (IVD) testing market was valued at approximately $62 billion in 2015 and that the professional POCT market sub-segment was valued at $12.91 billion in 2015, or the equivalent of 19.4% of the global IVD market. Note that the glucose self-testing market, estimated to be approximately $5 billion, is considered as a separate and independent market in this report. It is estimated that the global POCT market will exhibit a compound annual growth rate (CAGR) of 8.6% between 2012 and 2018 to $16.72 billion. The report provides a summary of the global POCT market divided into individual major market sub-segments, with market values for 2015 and market projections to With any growth rate estimate, glucose testing remains the leading point of care testing global segment, accounting for 40.3% of global near patient testing by Excluding glucose testing, only rapid cardiac markers rises above $2.8 billion in POC testing by 2018, accounting for an estimated 17.2% of total testing by Despite budget pressures on hospitals, point of care testing will continue to grow at a rate larger than central lab testing, powered by continuing trends for adoption and use of these platforms across disciplines, notably cardiac markers, coagulation and infectious disease. As such, several diagnostic companies have scheduled advanced multiplexing platforms in their development pipelines, including a broad range of immunoassays and molecular tests. The U.S. is recognized as the largest single country market for both IVD and POCT products. The POCT subsegment represented an estimated 20.0% of the U.S. IVD market in In 2015, the U.S. POCT market was valued at $4.65 billion. TriMark estimated that the U.S. POCT market would exhibit a CAGR of 7.7% between 2015 and TriMark has determined that Europe represents the largest regional market for both IVD and POCT products. The POCT sub-segment represented an estimated 26.0% of the European IVD market in In 2015, the European POCT market was valued at $5.31 billion. TriMark estimated that the European POCT market would exhibit a CAGR of 7.7% between 2015 and 2018 to $6.6 billion. Germany is widely recognized as the largest single market for both IVD and POCT products within Europe. The German POCT market in 2015 was valued at $1.25 billion. Asia (which includes Japan, China and India for the purposes of this report) represents a major market, but there are significant differences in growth rates for each of the individual countries. The POCT sub-segment represented an estimated 19.5% of the Asian IVD market in In 2015, the POCT market for Japan, China and India (referred to as the Asian POCT market in this report) was $2.19 billion. TriMark estimated that the Asian POCT market would exhibit a CAGR of 12.2% between 2015 and The Japanese POCT market has slowed recently and is expected to see a CAGR of 8.0%; it is estimated to have been valued at $1.33 billion in In contrast, the emerging markets of India and China are exhibiting higher CAGRs of 19.0% and 18.4%, respectively, between 2012 and 2018, and are valued at $277.6 million and $585.2 million in 2015, respectively. The report also provides a summary of the Asian POCT market segmented by individual major market sub-segment with market values for 2015 and market projections to Although the ROW segment represents the smallest geographic region, it is nevertheless a major growth opportunity and includes the emerging markets of Brazil and Russia. Included in this regional analysis are the Middle East, Africa, other Asian countries, Australia, Canada and other Latin American countries such as Mexico and Argentina. TriMark has determined that the IVD market for the ROW is valued at approximately $3.0 billion. The POCT market for the ROW was valued at $755.7 million in It is predicted that the market for POCT products in the ROW will be valued at $988.1 million by 2018 (a CAGR of 9.4%). Table 2.5 provides a summary of the ROW POCT market segmented by individual major market sub-segment with market values for 2015 and market projections to TriMark Publications, LLC. All rights reserved 23
25 Roche, Alere [now acquired by Abbott] and Abbott Laboratories are the three largest suppliers of POCT devices throughout the world and collectively comprise an estimated 49.6% of the global market. Although other companies, such as Nova, are becoming increasingly focused on POCT, increasing their product expansion and collaboration efforts to gain market share, the market remains extremely fragmented. Alere has sought to improve its market position through its acquisitions of companies like Biosite, Axis-Shield and its collaborations with SureStat and Chembio. Alere s HIV market share, on the other hand, could erode with the entry of Roche and Orasure and the advent of oral HIV tests. Nova Biomedical is becoming a reliable player with its new POCT platform. POCT dynamics are changing rapidly, and smaller players, including Cepheid, Tm Bioscience, Chembio, Response Biomedical and Orasure, are poised to deliver value with their innovative platforms and growing test menus. The market has been driven by a transition to fully-automated systems, real-time amplification, connectivity platforms and growing test development for POC platforms. TriMark estimates that future growth will stem from emerging applications like genotyping for identifying drugresistant strains; bioterrorism; testing applications within infectious disease like Influenza and HIV; and disease diagnostics and prognostic assays for disease applications like sepsis, cardiovascular disease (CHF) and coagulation testing. The industry consolidation is significant, as larger players like Alere want to move into faster-growing markets to expand their product offerings and/or geographical reach. Larger, established diagnostic players like Roche are eager to build out and extend their molecular diagnostic franchises for point of care technology and are willing to pay premium prices for good technology. Market growth will be paced by substantial gains in the cardiac markers market (primarily beta-natriuretic-peptide (BNP) testing), coagulation, infectious disease testing and continued moderate growth in the clinical chemistry subsegment the largest in the POCT market. The later constellation of routine tests, now dominated by central lab testing (ALT, AST, BUN, creatinine, etc.), will eventually migrate completely to near patient testing. The value of the global professional POC blood glucose analysis systems market in 2015 was $5.4 billion. By the end of the forecast period in 2018, it is predicted that this market will have increased in value to $6.75 billion (with an average CAGR of 7.3% from 2015 to 2018). Industry experts expect that the U.S. and Europe POC blood glucose testing market revenues will continue to be negatively impacted by pricing pressure and weak volume growth. In addition, the U.S. market may be affected by a proposed tightening of performance standards to address the limitations of some current blood glucose meters. However, the underlying market fundamentals remain strong given the rising number of people with diabetes globally, especially in Asia and the ROW. The value of the global POC blood gas and electrolyte analysis systems market in 2015 was $1.17 billion. By the end of the forecast period in 2018, it is predicted that this market will have increased in value to $1.51 billion with an average CAGR of 8.5% from 2012 to 2018). The value of the global POC rapid coagulation analyzers systems market increased to an estimated $779.1 million by By the end of the forecast period in 2018, it is predicted that the market will have increased in value to $1,043.7 million (with an average CAGR of 10.1% from 2012 to 2018). The value of the global POC cardiac marker analysis device market increased to an estimated $2.0 billion by By the end of the forecast period in 2018, it is predicted that the market will have increased in value to $2.87 billion (with an average CAGR of 12.8% from 2012 to 2018). The value of the global POC substance/drug abuse testing devices market increased to an estimated $622.3 million by By the end of the forecast period in 2018, it is predicted that the market will have increased in value to $832.2 million (with an average CAGR of 9.9% from 2012 to 2018). The global POC infectious disease testing devices market increased to an estimated $978.9 million by By the end of the forecast period in 2018, it is predicted that the market will have increased in value to $1,402.5 million (with an average CAGR of 12.1% from 2012 to 2018) TriMark Publications, LLC. All rights reserved 24
26 The value of the global POC urine strip testing products market increased to an estimated $382.3 million by By the end of the forecast period in 2018, it is predicted that the market will have increased in value to $448.8 million (with an average CAGR of 5.1% from 2012 to 2018). The value of the global POC pregnancy testing devices market increased to an estimated $445.7 million by By the end of the forecast period in 2018, it is predicted that the market will have increased in value to $516.8 million (with an average CAGR of 5.0% from 2012 to 2018). The value of the global POC fecal occult products market increased to an estimated $428.3 million by By the end of the forecast period in 2018, it is predicted that the market will have increased in value to $498.2 million (with an average CAGR of 5.2% from 2012 to 2018). The value of the global POC cholesterol testing products market increased to an estimated $606.3 million by By the end of the forecast period in 2018, it is predicted that the market will have increased in value to $750.9 million (with an average CAGR of 6.2% from 2012 to 2018) TriMark Publications, LLC. All rights reserved 25
Worldwide Markets and Emerging Technologies for Point-of-Care Testing, 2013-2019. February 2015
 Worldwide Markets and Emerging Technologies for Point-of-Care Testing, 2013-2019 February 2015 INTELAB CORPORATION MARKET AND TECHNOLOGY REPORTS Report 530 Copyright 2015 Worldwide Markets and Emerging
Worldwide Markets and Emerging Technologies for Point-of-Care Testing, 2013-2019 February 2015 INTELAB CORPORATION MARKET AND TECHNOLOGY REPORTS Report 530 Copyright 2015 Worldwide Markets and Emerging
(SAMPLE COPY, NOT FOR RESALE)
 TriMark Publications July 2011 Volume: TMRPOC11-0701 POINT OF CARE DIAGNOSTIC TESTING WORLD MARKETS (SAMPLE COPY, NOT FOR RESALE) Trends, Industry Participants, Product Overviews and Market Drivers TABLE
TriMark Publications July 2011 Volume: TMRPOC11-0701 POINT OF CARE DIAGNOSTIC TESTING WORLD MARKETS (SAMPLE COPY, NOT FOR RESALE) Trends, Industry Participants, Product Overviews and Market Drivers TABLE
POINT OF CARE DIAGNOSTIC TESTING AND SECTOR TRENDS (SAMPLE COPY, NOT FOR RESALE)
 TriMark Publications November 2011 Volume: TMRPOCST11-1101 POINT OF CARE DIAGNOSTIC TESTING AND SECTOR TRENDS (SAMPLE COPY, NOT FOR RESALE) Trends, Industry Participants, Product Overviews and Market Drivers
TriMark Publications November 2011 Volume: TMRPOCST11-1101 POINT OF CARE DIAGNOSTIC TESTING AND SECTOR TRENDS (SAMPLE COPY, NOT FOR RESALE) Trends, Industry Participants, Product Overviews and Market Drivers
Clinical Chemistry Analyzers
 Brochure More information from http://www.researchandmarkets.com/reports/562864/ Clinical Chemistry Analyzers Description: Clinical chemistry analysis is one of the most important areas within clinical
Brochure More information from http://www.researchandmarkets.com/reports/562864/ Clinical Chemistry Analyzers Description: Clinical chemistry analysis is one of the most important areas within clinical
(SAMPLE COPY, NOT FOR RESALE)
 TriMark Publications October 2010 Volume: TMRCIA10-1001 CLINICAL IMMUNOANALYZER MARKETS (SAMPLE COPY, NOT FOR RESALE) TABLE OF CONTENTS 1. Overview 7 1.1 Objectives 7 1.2 Methodology 8 1.3 Scope of the
TriMark Publications October 2010 Volume: TMRCIA10-1001 CLINICAL IMMUNOANALYZER MARKETS (SAMPLE COPY, NOT FOR RESALE) TABLE OF CONTENTS 1. Overview 7 1.1 Objectives 7 1.2 Methodology 8 1.3 Scope of the
(SAMPLE COPY, NOT FOR RESALE)
 TriMark Publications May 2016 Volume: TMRMDIDT16-0501 MOLECULAR DIAGNOSTICS IN INFECTIOUS DISEASE TESTING (SAMPLE COPY, NOT FOR RESALE) Trends, Industry Participants, Product Overviews and Market Drivers
TriMark Publications May 2016 Volume: TMRMDIDT16-0501 MOLECULAR DIAGNOSTICS IN INFECTIOUS DISEASE TESTING (SAMPLE COPY, NOT FOR RESALE) Trends, Industry Participants, Product Overviews and Market Drivers
IVD (In Vitro Diagnostic) Testing World Markets
 Brochure More information from http://www.researchandmarkets.com/reports/3073218/ IVD (In Vitro Diagnostic) Testing World Markets Description: In vitro diagnostic (IVD) testing is experiencing a revival
Brochure More information from http://www.researchandmarkets.com/reports/3073218/ IVD (In Vitro Diagnostic) Testing World Markets Description: In vitro diagnostic (IVD) testing is experiencing a revival
(SAMPLE COPY, NOT FOR RESALE)
 TriMark Publications January 2015 Volume: TMRIVD15-0101 IN VITRO DIAGNOSTIC TESTING WORLD MARKETS (SAMPLE COPY, NOT FOR RESALE) Trends, Industry Participants, Product Overviews and Market Drivers TABLE
TriMark Publications January 2015 Volume: TMRIVD15-0101 IN VITRO DIAGNOSTIC TESTING WORLD MARKETS (SAMPLE COPY, NOT FOR RESALE) Trends, Industry Participants, Product Overviews and Market Drivers TABLE
IMMUNOCHEMICALS - A Global Strategic Business Report
 /wepdwukltuyot IMMUNOCHEMICALS - A Global Strategic Business Report 联 系 购 买 电 话 :010-82863480 公 司 名 称 : 佐 思 信 息 公 司 地 址 : 北 京 市 海 淀 区 苏 州 街 18 号 院 长 远 天 地 大 厦 A2 座 1008-1 室 (100080) 出 版 日 期 :April 2011
/wepdwukltuyot IMMUNOCHEMICALS - A Global Strategic Business Report 联 系 购 买 电 话 :010-82863480 公 司 名 称 : 佐 思 信 息 公 司 地 址 : 北 京 市 海 淀 区 苏 州 街 18 号 院 长 远 天 地 大 厦 A2 座 1008-1 室 (100080) 出 版 日 期 :April 2011
(SAMPLE COPY, NOT FOR RESALE)
 TriMark Publications July 2012 Volume: TMRCLTV112-0701 CLINICAL LABORATORY TESTING VOLUME 1: IVD REAGENTS AND INSTRUMENTS MARKETS (SAMPLE COPY, NOT FOR RESALE) TABLE OF CONTENTS 1. Overview 8 1.1 Objectives
TriMark Publications July 2012 Volume: TMRCLTV112-0701 CLINICAL LABORATORY TESTING VOLUME 1: IVD REAGENTS AND INSTRUMENTS MARKETS (SAMPLE COPY, NOT FOR RESALE) TABLE OF CONTENTS 1. Overview 8 1.1 Objectives
Table 40: Product Profile - MS9 Table 41: MS9 SWOT Analysis, 2013 Table 42: Septin-9 CRC Screening Marketed Laboratory-Developed Tests Table 43:
 LIST OF TABLES 1.1 List of Tables Table 1: Initial Presenting Symptoms of Colorectal Cancer Table 2: Guideline Organization Recommendations for Non-Invasive Tests Table 3: TNM Classification System to
LIST OF TABLES 1.1 List of Tables Table 1: Initial Presenting Symptoms of Colorectal Cancer Table 2: Guideline Organization Recommendations for Non-Invasive Tests Table 3: TNM Classification System to
Samsung POINT OF CARE Systems - Cardiac/Acute Care Biomarkers LABGEO IB Clinical Chemistry Analytes LABGEO PT
 Samsung POINT OF CARE Systems - Cardiac/Acute Care Biomarkers LABGEO IB Clinical Chemistry Analytes LABGEO PT Alexander Belenky, PhD Director -Product Development Samsung - Nexus-Dx Edward Brennan, PhD
Samsung POINT OF CARE Systems - Cardiac/Acute Care Biomarkers LABGEO IB Clinical Chemistry Analytes LABGEO PT Alexander Belenky, PhD Director -Product Development Samsung - Nexus-Dx Edward Brennan, PhD
Urinalysis Compliance Tools. POCC Webinar January 19, 2011 Dr. Susan Selgren
 Urinalysis Compliance Tools POCC Webinar January 19, 2011 Dr. Susan Selgren Learning Objectives Be able to review and improve upon a laboratory plan for compliance including: Competency Documentation Proficiency
Urinalysis Compliance Tools POCC Webinar January 19, 2011 Dr. Susan Selgren Learning Objectives Be able to review and improve upon a laboratory plan for compliance including: Competency Documentation Proficiency
SMF Awareness Seminar 2014
 SMF Awareness Seminar 2014 Clinical Evaluation for In Vitro Diagnostic Medical Devices Dr Jiang Naxin Health Sciences Authority Medical Device Branch 1 In vitro diagnostic product means Definition of IVD
SMF Awareness Seminar 2014 Clinical Evaluation for In Vitro Diagnostic Medical Devices Dr Jiang Naxin Health Sciences Authority Medical Device Branch 1 In vitro diagnostic product means Definition of IVD
China Medical Equipment Market Analysis and Forecasts to 2015
 Brochure More information from http://www.researchandmarkets.com/reports/1080808/ China Medical Equipment Market Analysis and Forecasts to 2015 Description: China Medical Equipment Market Analysis and
Brochure More information from http://www.researchandmarkets.com/reports/1080808/ China Medical Equipment Market Analysis and Forecasts to 2015 Description: China Medical Equipment Market Analysis and
The Future of Clinical Chemistry and Laboratory Medicine, The Diagnostics Industry View
 The Future of Clinical Chemistry and Laboratory Medicine, The Diagnostics Industry View Peng Yin, M.D., Ph.D., Director, Global Scientific Affairs Abbott Diagnostic Division (ADD) Agenda Lack of perceived
The Future of Clinical Chemistry and Laboratory Medicine, The Diagnostics Industry View Peng Yin, M.D., Ph.D., Director, Global Scientific Affairs Abbott Diagnostic Division (ADD) Agenda Lack of perceived
PROPOSED DOCUMENT. Global Harmonization Task Force. Title: Principles of In Vitro Diagnostic (IVD) Medical Devices Classification
 SG1(PD)/N045R12 PROPOSED DOCUMENT Global Harmonization Task Force Title: Principles of In Vitro Diagnostic (IVD) Medical Devices Classification Authoring Group: Study Group 1 of the Global Harmonization
SG1(PD)/N045R12 PROPOSED DOCUMENT Global Harmonization Task Force Title: Principles of In Vitro Diagnostic (IVD) Medical Devices Classification Authoring Group: Study Group 1 of the Global Harmonization
Diagnostics Division. Roland Diggelmann COO Roche Diagnostics. Picture
 Diagnostics Division Roland Diggelmann COO Roche Diagnostics Picture HY 2013: Roche Group highlights HY 2013 performance Strong Pharma performance, driven by US; solid growth for Diagnostics 12% core EPS
Diagnostics Division Roland Diggelmann COO Roche Diagnostics Picture HY 2013: Roche Group highlights HY 2013 performance Strong Pharma performance, driven by US; solid growth for Diagnostics 12% core EPS
Point of Care Testing and Informatics: How to Prepare for the POCT Explosion
 Point of Care Testing and Informatics: How to Prepare for the POCT Explosion Pathology Informatics Summit 2014 David McClintock, MD May 14, 2014 Disclosures No conflicts of interest with any content presented
Point of Care Testing and Informatics: How to Prepare for the POCT Explosion Pathology Informatics Summit 2014 David McClintock, MD May 14, 2014 Disclosures No conflicts of interest with any content presented
Global Clinical Laboratory Testing Market Report: 2012 Edition
 Brochure More information from http://www.researchandmarkets.com/reports/2165640/ Global Clinical Laboratory Testing Market Report: 2012 Edition Description: Clinical laboratory testing market is one of
Brochure More information from http://www.researchandmarkets.com/reports/2165640/ Global Clinical Laboratory Testing Market Report: 2012 Edition Description: Clinical laboratory testing market is one of
MEDICAL NUTRITION THERAPY (MNT) CLINICAL NUTRITION THERAPY Service Time CPT Code
 MEDICAL NUTRITION THERAPY (MNT) CLINICAL NUTRITION THERAPY Service Time CPT Code Initial Assessment And Intervention This Code Can Be Used Only Once A Year For First Appointment Medical Nutrition Therapy
MEDICAL NUTRITION THERAPY (MNT) CLINICAL NUTRITION THERAPY Service Time CPT Code Initial Assessment And Intervention This Code Can Be Used Only Once A Year For First Appointment Medical Nutrition Therapy
CANADIAN RULE BASED CLASSIFICATION SYSTEM (IVDD) Life Sciences British Columbia NRC-Industry Research Assistance Program
 CANADIAN RULE BASED CLASSIFICATION SYSTEM (IVDD) Life Sciences British Columbia NRC-Industry Research Assistance Program Health Canada Regulations on Medical Devices Vancouver, B.C. October 29, 2007 Nancy
CANADIAN RULE BASED CLASSIFICATION SYSTEM (IVDD) Life Sciences British Columbia NRC-Industry Research Assistance Program Health Canada Regulations on Medical Devices Vancouver, B.C. October 29, 2007 Nancy
MediPoint: In-Vitro Colorectal Cancer Screening Tests - Current and Future Players
 Brochure More information from http://www.researchandmarkets.com/reports/2669701/ MediPoint: In-Vitro Colorectal Cancer Screening Tests - Current and Future Players Description: MediPoint: In-Vitro Colorectal
Brochure More information from http://www.researchandmarkets.com/reports/2669701/ MediPoint: In-Vitro Colorectal Cancer Screening Tests - Current and Future Players Description: MediPoint: In-Vitro Colorectal
Intelligent Flow Meter Market by type, by Technology, by Application and Geography - Global Trends & Forecasts 2014-2020
 Brochure More information from http://www.researchandmarkets.com/reports/3098548/ Intelligent Flow Meter Market by type, by Technology, by Application and Geography - Global Trends & Forecasts 2014-2020
Brochure More information from http://www.researchandmarkets.com/reports/3098548/ Intelligent Flow Meter Market by type, by Technology, by Application and Geography - Global Trends & Forecasts 2014-2020
Quality Requirements of a Point of Care Testing Service
 Quality Requirements of a Point of Care Testing Service Adil I. Khan MSc, PhD Assistant Professor of Pathology Director of Point of Care Testing & Clinical Chemistry Laboratory, Temple University Episcopal
Quality Requirements of a Point of Care Testing Service Adil I. Khan MSc, PhD Assistant Professor of Pathology Director of Point of Care Testing & Clinical Chemistry Laboratory, Temple University Episcopal
TECHNOLOGIES, PRODUCTS & SERVICES for MOLECULAR DIAGNOSTICS, MDx ABA 298
 DIAGNOSTICS BUSINESS ANALYSIS SERIES: TECHNOLOGIES, PRODUCTS & SERVICES for MOLECULAR DIAGNOSTICS, MDx ABA 298 By ADAMS BUSINESS ASSOCIATES MAY 2014. May 2014 ABA 298 1 Technologies, Products & Services
DIAGNOSTICS BUSINESS ANALYSIS SERIES: TECHNOLOGIES, PRODUCTS & SERVICES for MOLECULAR DIAGNOSTICS, MDx ABA 298 By ADAMS BUSINESS ASSOCIATES MAY 2014. May 2014 ABA 298 1 Technologies, Products & Services
ADMINISTRATIVE MANUAL Policy and Procedure
 ADMINISTRATIVE MANUAL Policy and Procedure TITLE: Point of Care Testing NUMBER: CH 30-111 (Laboratory Diagnostic Bedside Testing) Effective Date: January 2014 Page 1 of 6 Applies To: Holders of Administrative
ADMINISTRATIVE MANUAL Policy and Procedure TITLE: Point of Care Testing NUMBER: CH 30-111 (Laboratory Diagnostic Bedside Testing) Effective Date: January 2014 Page 1 of 6 Applies To: Holders of Administrative
Managing POCT: Trying to Control Testing in an Out-of-Control Environment. William Clarke, PhD, MBA, DABCC Johns Hopkins School of Medicine 3/24/11
 Managing POCT: Trying to Control Testing in an Out-of-Control Environment William Clarke, PhD, MBA, DABCC Johns Hopkins School of Medicine 3/24/11 Learning Objectives Participants should be able to: Discuss
Managing POCT: Trying to Control Testing in an Out-of-Control Environment William Clarke, PhD, MBA, DABCC Johns Hopkins School of Medicine 3/24/11 Learning Objectives Participants should be able to: Discuss
Coding and Payment Guide for Laboratory Services. An essential coding, billing, and payment resource for laboratory and pathology services
 Coding and Payment Guide for Laboratory Services An essential coding, billing, and payment resource for laboratory and pathology services Contents Introduction............................... 1 Coding Systems...............................
Coding and Payment Guide for Laboratory Services An essential coding, billing, and payment resource for laboratory and pathology services Contents Introduction............................... 1 Coding Systems...............................
Molecular Diagnostics in Cancer Testing
 Product Sheet More information at http://www.biomarketgroup.com/market-research-report/molecular-diagnostics-in-cancertesting.html Molecular Diagnostics in Cancer Testing Published: 2015-AUG-01 Pages:
Product Sheet More information at http://www.biomarketgroup.com/market-research-report/molecular-diagnostics-in-cancertesting.html Molecular Diagnostics in Cancer Testing Published: 2015-AUG-01 Pages:
U.S. Clinical Laboratory and Pathology Testing 2013-2015:
 U.S. Clinical Laboratory and Pathology Testing 2013-2015: Market Analysis, Trends, and Forecasts Now more than ever, it s critical to understand the industry at a macro level. Prepare your organization
U.S. Clinical Laboratory and Pathology Testing 2013-2015: Market Analysis, Trends, and Forecasts Now more than ever, it s critical to understand the industry at a macro level. Prepare your organization
Rapid Screening Tests
 Rapid Screening Tests Infectious Diseases Cardiac Marker Tumor Markers Pregnancy Rheumatology Allergy Drugs of Abuse > For rapid and cost-effective diagnosis > Accurate, reliable results > Easy to use
Rapid Screening Tests Infectious Diseases Cardiac Marker Tumor Markers Pregnancy Rheumatology Allergy Drugs of Abuse > For rapid and cost-effective diagnosis > Accurate, reliable results > Easy to use
Product: Point-of-care immunoassay system yielding rapid labquality quantitative blood test results with unprecedented ease-ofuse.
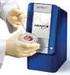 Mission Provide Clinicians, Patients and Payors with a Point-of-Care Diagnostic Solution Coupling Clinically Relevant Performance with Unparalleled Ease-of-Use and Cost Effectiveness Product: Point-of-care
Mission Provide Clinicians, Patients and Payors with a Point-of-Care Diagnostic Solution Coupling Clinically Relevant Performance with Unparalleled Ease-of-Use and Cost Effectiveness Product: Point-of-care
US Diabetic Devices Industry Research Report: KenResearch
 US Diabetic Devices Industry Research Report: KenResearch by KenResearch - Monday, February 24, 2014 https://www.kenresearch.com/blog/2014/02/us-diabetic-devices-industry-research-report-kenresearch/ The
US Diabetic Devices Industry Research Report: KenResearch by KenResearch - Monday, February 24, 2014 https://www.kenresearch.com/blog/2014/02/us-diabetic-devices-industry-research-report-kenresearch/ The
CAP Accreditation Checklists 2015 Edition
 CAP Accreditation Checklists 2015 Edition The College of American Pathologists (CAP) accreditation checklists contain the CAP accreditation program requirements, developed on more than 50 years of insight
CAP Accreditation Checklists 2015 Edition The College of American Pathologists (CAP) accreditation checklists contain the CAP accreditation program requirements, developed on more than 50 years of insight
Abbott Diagnostics. Durable Growth Business
 Abbott Diagnostics Durable Growth Business Forward-Looking Statement Some statements in this presentation may be forward-looking statements for purposes of the Private Securities Litigation Reform Act
Abbott Diagnostics Durable Growth Business Forward-Looking Statement Some statements in this presentation may be forward-looking statements for purposes of the Private Securities Litigation Reform Act
IT Risk Management. Agenda. Roche at a glance OSI Layers above 7 What we did What we plan to do how to not get shoot
 IT Risk Management 30. 2006 ISACA Switzerland Chapter After Hours Seminar Mario Walter Agenda Roche at a glance OSI Layers above 7 What we did What we plan to do how to not get shoot 2006 ISACA After Hours
IT Risk Management 30. 2006 ISACA Switzerland Chapter After Hours Seminar Mario Walter Agenda Roche at a glance OSI Layers above 7 What we did What we plan to do how to not get shoot 2006 ISACA After Hours
Alere Informatics, Inc. 2000 Holiday Drive Charlottesville, VA 22901 1.888.971.7953 www.rals.com. Release Summary for RALS System. Version 5.
 Release Summary for RALS System Version 5.6. AI-DSREL0174 v1.0 Page 1 of 12 February 17, 2015 Contents RALS New Devices Supported Page Quidel Sofia Analyzer.. 3 Roche Urisys 1100.. 4 RALS New System Features
Release Summary for RALS System Version 5.6. AI-DSREL0174 v1.0 Page 1 of 12 February 17, 2015 Contents RALS New Devices Supported Page Quidel Sofia Analyzer.. 3 Roche Urisys 1100.. 4 RALS New System Features
The Evolution of a Point of Care Company From R & D to Commercial Launch
 The Evolution of a Point of Care Company From R & D to Commercial Launch Karen Hedine, President Micronics, Inc. : Background Founded 1996 Technology platform : laminar flow diffusionbased microfluidics
The Evolution of a Point of Care Company From R & D to Commercial Launch Karen Hedine, President Micronics, Inc. : Background Founded 1996 Technology platform : laminar flow diffusionbased microfluidics
2.0 Rationale, Purpose and Scope... 5. 4.0 Definitions... 6. 5.0 General Principles... 7
 Table of Contents Principles of IVD Medical Devices Classification 1.0 Introduction... 4 2.0 Rationale, Purpose and Scope... 5 2.1 Rationale... 5 2.2 Purpose... 5 2.3 Scope... 5 3.0 References... 5 4.0
Table of Contents Principles of IVD Medical Devices Classification 1.0 Introduction... 4 2.0 Rationale, Purpose and Scope... 5 2.1 Rationale... 5 2.2 Purpose... 5 2.3 Scope... 5 3.0 References... 5 4.0
Identification of a problem, e.g., an outbreak Surveilance Intervention Effect
 EPIDEMIOLOGY EPIDEMIOLOGY Epidemiology is a cornerstone of the control of infectious diseases. Statens Serum Institut s epidemiological activities cover a wide field, from surveillance of diseases and
EPIDEMIOLOGY EPIDEMIOLOGY Epidemiology is a cornerstone of the control of infectious diseases. Statens Serum Institut s epidemiological activities cover a wide field, from surveillance of diseases and
BSI: An In Vitro Diagnostics Notified Body. A guide to the In Vitro Diagnostic Directive....making excellence a habit.
 BSI: An In Vitro Diagnostics Notified Body A guide to the In Vitro Diagnostic Directive...making excellence a habit. A BSI guide to the In Vitro Diagnostic Directive Introduction In Vitro Diagnostics (IVD)
BSI: An In Vitro Diagnostics Notified Body A guide to the In Vitro Diagnostic Directive...making excellence a habit. A BSI guide to the In Vitro Diagnostic Directive Introduction In Vitro Diagnostics (IVD)
Randox Laboratories Ltd. - Product Pipeline Analysis, 2014 Update
 Randox Laboratories Ltd. - Product Pipeline Analysis, 2014 Update Randox Laboratories Ltd. - Product Pipeline Analysis, 2014 Update Sector Publishing Intelligence Limited (SPi) has been marketing business
Randox Laboratories Ltd. - Product Pipeline Analysis, 2014 Update Randox Laboratories Ltd. - Product Pipeline Analysis, 2014 Update Sector Publishing Intelligence Limited (SPi) has been marketing business
Blood, Plasma, and Cellular Blood Components INTRODUCTION
 Blood, Plasma, and Cellular Blood Components INTRODUCTION This chapter of the Guideline provides recommendations to Sponsors of Requests for Revision for new monographs for blood, plasma, and cellular
Blood, Plasma, and Cellular Blood Components INTRODUCTION This chapter of the Guideline provides recommendations to Sponsors of Requests for Revision for new monographs for blood, plasma, and cellular
Commercially Available Rapid Hepatitis B Tests
 Type of Test: Lateral Flow Commercially Available Rapid Tests Manufacturer Name of Test Web Site Abbott Acon Laboratories, Inc. Akers Laboratories Alfa Scientific Designs Inc. AmeriTek, Inc. Beijing Blue
Type of Test: Lateral Flow Commercially Available Rapid Tests Manufacturer Name of Test Web Site Abbott Acon Laboratories, Inc. Akers Laboratories Alfa Scientific Designs Inc. AmeriTek, Inc. Beijing Blue
Point of Care HIV Viral Load Testing:
 Point of Care HIV Viral Load Testing: Lesley Scott, Natasha Gous, Shuqi Chen *, Wendy Stevens Department of Molecular Medicine and Haematology University of the Witwatersrand, National Health Laboratory
Point of Care HIV Viral Load Testing: Lesley Scott, Natasha Gous, Shuqi Chen *, Wendy Stevens Department of Molecular Medicine and Haematology University of the Witwatersrand, National Health Laboratory
Home Healthcare Software - Global Forecast to 2018
 Brochure More information from http://www.researchandmarkets.com/reports/2849039/ Home Healthcare Software - Global Forecast to 2018 Description: Home Healthcare Software - Product & Service Market - Application
Brochure More information from http://www.researchandmarkets.com/reports/2849039/ Home Healthcare Software - Global Forecast to 2018 Description: Home Healthcare Software - Product & Service Market - Application
MEDICAL DOCUMENT MANAGEMENT SYSTEMS MARKET
 Medical Devices 2014 MEDICAL DOCUMENT MANAGEMENT SYSTEMS MARKET BY SOLUTION (DOCUMENT SCANNING SOFTWARE, DOCUMENT MANAGEMENT SOFTWARE), BY APPLICATION MEDICAL RECORDS MANAGEMENT), BY MODE OF DELIVERY (WEB-BASED,
Medical Devices 2014 MEDICAL DOCUMENT MANAGEMENT SYSTEMS MARKET BY SOLUTION (DOCUMENT SCANNING SOFTWARE, DOCUMENT MANAGEMENT SOFTWARE), BY APPLICATION MEDICAL RECORDS MANAGEMENT), BY MODE OF DELIVERY (WEB-BASED,
PPS UNDERWRITING GUIDE FOR APPLICANTS
 PPS UNDERWRITING GUIDE FOR APPLICANTS UNDERWRITING guide 2013 WHAT HAPPENS WHEN YOU SUBMIT YOUR APPLICATION FOR INSURANCE? Once an application is submitted it is put through a number of processes to ensure
PPS UNDERWRITING GUIDE FOR APPLICANTS UNDERWRITING guide 2013 WHAT HAPPENS WHEN YOU SUBMIT YOUR APPLICATION FOR INSURANCE? Once an application is submitted it is put through a number of processes to ensure
EiCon G.m.b.H. Diagnostic Healthcare Consulting. Amtsgericht Lörrach : HRB-Nr.3002. E-Mail : [email protected]. Home page: http://www.eiconnet.
 EiCon G.m.b.H. Diagnostic Healthcare Consulting Amtsgericht Lörrach : HRB-Nr.3002 Unterbaselweg 55 D -79576 Weil am Rhein Germany Phone : (49) - 7621-798373 Fax : (49) - 7621-798374 Hauptstrasse 160 D-79576
EiCon G.m.b.H. Diagnostic Healthcare Consulting Amtsgericht Lörrach : HRB-Nr.3002 Unterbaselweg 55 D -79576 Weil am Rhein Germany Phone : (49) - 7621-798373 Fax : (49) - 7621-798374 Hauptstrasse 160 D-79576
Direct Testing Systems and Serology
 Direct Testing Systems and Serology Rapid Manual Tests 6-2 Serology Diagnostics 6-6 BD Diagnostics Diagnostic Systems Catalog 2005/2006 6-1 Rapid Manual Tests Meningitis Test Systems 252360 Directigen
Direct Testing Systems and Serology Rapid Manual Tests 6-2 Serology Diagnostics 6-6 BD Diagnostics Diagnostic Systems Catalog 2005/2006 6-1 Rapid Manual Tests Meningitis Test Systems 252360 Directigen
In Vitro Diagnostics Market to 2018 - Consolidation, Decentralization and Demand for Genetic Testing to Shape the Competitive Landscape
 Decentralization and Demand for Genetic Testing to Shape the Reference Code: GBIME0067MR Publication Date: March 2012 In Vitro Diagnostics Market Forecast to Increase at a CAGR of XX% during 2011 2018
Decentralization and Demand for Genetic Testing to Shape the Reference Code: GBIME0067MR Publication Date: March 2012 In Vitro Diagnostics Market Forecast to Increase at a CAGR of XX% during 2011 2018
Development and Validation of In Vitro Diagnostic Tests. YC Lee, Ph.D. CEO
 Development and Validation of In Vitro Diagnostic Tests YC Lee, Ph.D. CEO 1 Validation of In Vitro Diagnostic Tests Validated d Diagnostic Test should: Provides test results that identify if positive i
Development and Validation of In Vitro Diagnostic Tests YC Lee, Ph.D. CEO 1 Validation of In Vitro Diagnostic Tests Validated d Diagnostic Test should: Provides test results that identify if positive i
New Medicare Preventive
 New Medicare Preventive Services Screening Tests You Can Perform in the Office Charles B. Root, PhD Medicare is finally getting serious about preventive services. Until now, the limited preventive testing
New Medicare Preventive Services Screening Tests You Can Perform in the Office Charles B. Root, PhD Medicare is finally getting serious about preventive services. Until now, the limited preventive testing
phealthlab, a need for effective phealthcare!
 phealthlab, a need for effective phealthcare! Define an unmet need! Provide a simple solution for the user!! Lars-Olof Hansson Karolinska University Laboratory Karolinska Institution Stockholm, Sweden
phealthlab, a need for effective phealthcare! Define an unmet need! Provide a simple solution for the user!! Lars-Olof Hansson Karolinska University Laboratory Karolinska Institution Stockholm, Sweden
China In Vitro Diagnostics (IVD) Marketplace
 GENReports: Market & Tech Analysis China In Vitro Diagnostics (IVD) Marketplace > Enal Razvi, Ph.D. Managing Director SELECTBIO US [email protected] > Jeff Fan Biotechnology Analyst, SELECTBIO US Topic
GENReports: Market & Tech Analysis China In Vitro Diagnostics (IVD) Marketplace > Enal Razvi, Ph.D. Managing Director SELECTBIO US [email protected] > Jeff Fan Biotechnology Analyst, SELECTBIO US Topic
Phone: +44 20 8123 2220 Fax: +44 207 900 3970 [email protected] https://marketpublishers.com
 Event Management Software Market by Component, Software, Service, Deployment Mode (On-Premise, Cloud), Organization size, Verticals (Education, corporate, Third-Party Planners, Government, & others), and
Event Management Software Market by Component, Software, Service, Deployment Mode (On-Premise, Cloud), Organization size, Verticals (Education, corporate, Third-Party Planners, Government, & others), and
Manufacturing CUSTOM CHEMICALS AND SERVICES, SUPPORTING SCIENTIFIC ADVANCES FOR HUMAN HEALTH
 Manufacturing CUSTOM CHEMICALS AND SERVICES, SUPPORTING SCIENTIFIC ADVANCES FOR HUMAN HEALTH VWR enables the advancement of science by providing high-quality chemicals and services, customized to your
Manufacturing CUSTOM CHEMICALS AND SERVICES, SUPPORTING SCIENTIFIC ADVANCES FOR HUMAN HEALTH VWR enables the advancement of science by providing high-quality chemicals and services, customized to your
A Policy Primer on Diagnostics. June 2011
 A Policy Primer on Diagnostics June 2011 Table of Contents Overview of Diagnostics... 3 In-Vitro Diagnostic Tests... 5 Wide Range of Tests and Uses... 6 Federal Regulatory Oversight... 9 Laboratory Medicine...
A Policy Primer on Diagnostics June 2011 Table of Contents Overview of Diagnostics... 3 In-Vitro Diagnostic Tests... 5 Wide Range of Tests and Uses... 6 Federal Regulatory Oversight... 9 Laboratory Medicine...
Course outline. Code: MLS211 Title: Medical Biochemistry
 Course outline Code: MLS211 Title: Medical Biochemistry Faculty of: Science, Health, Education and Engineering Teaching Session: Semester 2 Year: 2015 Course Coordinator: Dr Mark Holmes Tel: 5430 2844
Course outline Code: MLS211 Title: Medical Biochemistry Faculty of: Science, Health, Education and Engineering Teaching Session: Semester 2 Year: 2015 Course Coordinator: Dr Mark Holmes Tel: 5430 2844
Personalized medicine in China s healthcare system
 Personalized medicine in China s healthcare system Jingmin Kan, Sam Linsen Netherlands office for Science and Technology, Guangzhou and Shanghai, China Content PERSONALIZED MEDICINE 2 FOCUS AT THE INDIVIDUAL
Personalized medicine in China s healthcare system Jingmin Kan, Sam Linsen Netherlands office for Science and Technology, Guangzhou and Shanghai, China Content PERSONALIZED MEDICINE 2 FOCUS AT THE INDIVIDUAL
Diagnosis of HIV-1 Infection. Estelle Piwowar-Manning HPTN Central Laboratory The Johns Hopkins University
 Diagnosis of HIV-1 Infection Estelle Piwowar-Manning HPTN Central Laboratory The Johns Hopkins University Tests Used to Diagnose HIV-1 Infection HIV antibody (today s topic) HIV p24 antigen HIV DNA HIV
Diagnosis of HIV-1 Infection Estelle Piwowar-Manning HPTN Central Laboratory The Johns Hopkins University Tests Used to Diagnose HIV-1 Infection HIV antibody (today s topic) HIV p24 antigen HIV DNA HIV
Unaccredited Point-of-Care Laboratory Testing Guideline for Physicians
 Unaccredited Point-of-Care Laboratory Testing Guideline for Physicians Prepared by the Advisory Committee on Laboratory Medicine College of Physicians & Surgeons of Alberta Serving the Public by guiding
Unaccredited Point-of-Care Laboratory Testing Guideline for Physicians Prepared by the Advisory Committee on Laboratory Medicine College of Physicians & Surgeons of Alberta Serving the Public by guiding
Expanding Access through Pharmacy-Based Point-of-Care Testing
 Expanding Access through Pharmacy-Based Point-of-Care Testing Donald G. Klepser, PhD Associate Professor, Department of Pharmacy Practice University of Nebraska Medical Center Doug Read, Pharm.D. Director,
Expanding Access through Pharmacy-Based Point-of-Care Testing Donald G. Klepser, PhD Associate Professor, Department of Pharmacy Practice University of Nebraska Medical Center Doug Read, Pharm.D. Director,
TRI-LEVEL CARDIAC CONTROL (CRD CONTROL 1, 2, 3)
 TRI-LEVEL CARDIAC CONTROL (CRD CONTROL 1, 2, 3) CAT NO. CQ3259 LOT NOS. 3190CK, 3191CK, 3192CK SIZE: 3 x 2ml EXPIRY: 2015-02 INTENDED USE This product is intended for in vitro diagnostic use in the quality
TRI-LEVEL CARDIAC CONTROL (CRD CONTROL 1, 2, 3) CAT NO. CQ3259 LOT NOS. 3190CK, 3191CK, 3192CK SIZE: 3 x 2ml EXPIRY: 2015-02 INTENDED USE This product is intended for in vitro diagnostic use in the quality
Determining Donor Eligibility Blood Donor vs. Stem Cell Donor. Wanda Koetz, RN, HPC Clinical Nurse Lead, Memorial Blood Centers ASFA - May 7, 2015
 Determining Donor Eligibility Blood Donor vs. Stem Cell Donor Wanda Koetz, RN, HPC Clinical Nurse Lead, Memorial Blood Centers ASFA - May 7, 2015 Objectives The learner will be able to: Define donor eligibility
Determining Donor Eligibility Blood Donor vs. Stem Cell Donor Wanda Koetz, RN, HPC Clinical Nurse Lead, Memorial Blood Centers ASFA - May 7, 2015 Objectives The learner will be able to: Define donor eligibility
Next Generation Sequencing in Public Health Laboratories. 2014 Survey Results
 Next Generation Sequencing in Public Health Laboratories 2014 Survey Results MAY 2015 This project was 100% funded with federal funds from a federal program of $215,972. This publication was supported
Next Generation Sequencing in Public Health Laboratories 2014 Survey Results MAY 2015 This project was 100% funded with federal funds from a federal program of $215,972. This publication was supported
Adding Management. www.starlims.com
 Adding Management to Your LIS Given the dynamic nature of their operations, today s clinical laboratories need to be nimble and agile. They need to cope with ever changing requirements brought about by
Adding Management to Your LIS Given the dynamic nature of their operations, today s clinical laboratories need to be nimble and agile. They need to cope with ever changing requirements brought about by
Adams Memorial Hospital Decatur, Indiana EXPLANATION OF LABORATORY TESTS
 Adams Memorial Hospital Decatur, Indiana EXPLANATION OF LABORATORY TESTS Your health is important to us! The test descriptions listed below are for educational purposes only. Laboratory test interpretation
Adams Memorial Hospital Decatur, Indiana EXPLANATION OF LABORATORY TESTS Your health is important to us! The test descriptions listed below are for educational purposes only. Laboratory test interpretation
TriMark Publications (SAMPLE COPY, NOT FOR RESALE)
 TriMark Publications November 2013 Volume: TMRMDC13-1101 MOLECULAR DIAGNOSTICS IN CANCER TESTING (SAMPLE COPY, NOT FOR RESALE) Trends, Industry Participants, Product Overviews and Market Drivers TABLE
TriMark Publications November 2013 Volume: TMRMDC13-1101 MOLECULAR DIAGNOSTICS IN CANCER TESTING (SAMPLE COPY, NOT FOR RESALE) Trends, Industry Participants, Product Overviews and Market Drivers TABLE
Internet of Things (IoT) in Energy Market by Systems & Solutions, by Application, by Services & by Regions - Global Forecast to 2020
 Brochure More information from http://www.researchandmarkets.com/reports/3443414/ Internet of Things (IoT) in Energy Market by Systems & Solutions, by Application, by Services & by Regions - Global Forecast
Brochure More information from http://www.researchandmarkets.com/reports/3443414/ Internet of Things (IoT) in Energy Market by Systems & Solutions, by Application, by Services & by Regions - Global Forecast
Medical technology makes healthcare smart. Frederik Horemans DSP Valley
 Medical technology makes healthcare smart Frederik Horemans DSP Valley DSP Valley, the cluster of excellence in smart electronic systems, acts as matchmaker for open innovation Strong technology focus
Medical technology makes healthcare smart Frederik Horemans DSP Valley DSP Valley, the cluster of excellence in smart electronic systems, acts as matchmaker for open innovation Strong technology focus
Florida Nursing Assistant Academy #2926 COURSE SYLLABUS
 HSW01 Healthcare Support Workers 90 Hours Knowledge of the health care delivery system and health occupations, oral and written communications skills, professional ethics and legal responsibilities, understanding
HSW01 Healthcare Support Workers 90 Hours Knowledge of the health care delivery system and health occupations, oral and written communications skills, professional ethics and legal responsibilities, understanding
Dental Implants and Prosthetics Market by Material, Stage, Connectors & Product Type - Global Forecast to 2020
 Brochure More information from http://www.researchandmarkets.com/reports/3288512/ Dental Implants and Prosthetics Market by Material, Stage, Connectors & Product Type - Global Forecast to 2020 Description:
Brochure More information from http://www.researchandmarkets.com/reports/3288512/ Dental Implants and Prosthetics Market by Material, Stage, Connectors & Product Type - Global Forecast to 2020 Description:
Global Learning Management System Market Analysis - Forecast (2014-2020)
 Brochure More information from http://www.researchandmarkets.com/reports/3506494/ Global Learning Management System Market Analysis - Forecast (2014-2020) Description: This analysis is one of the most
Brochure More information from http://www.researchandmarkets.com/reports/3506494/ Global Learning Management System Market Analysis - Forecast (2014-2020) Description: This analysis is one of the most
Customer Relationship Management (CRM) Analytics Global Market Analysis - Forecast (2014-2020)
 Brochure More information from http://www.researchandmarkets.com/reports/3164949/ Customer Relationship Management (CRM) Analytics Global Market Analysis - Forecast (2014-2020) Description: The report
Brochure More information from http://www.researchandmarkets.com/reports/3164949/ Customer Relationship Management (CRM) Analytics Global Market Analysis - Forecast (2014-2020) Description: The report
Guidance on the In Vitro Diagnostic Medical Devices Directive 98/79/EC
 Guidance on the In Vitro Diagnostic Medical Devices Directive 98/79/EC August 2013 Contents 1 Introduction...3 2 Scope of the directive...3 2.1 What is an in vitro diagnostic medical device?... 3 2.2 Specimen
Guidance on the In Vitro Diagnostic Medical Devices Directive 98/79/EC August 2013 Contents 1 Introduction...3 2 Scope of the directive...3 2.1 What is an in vitro diagnostic medical device?... 3 2.2 Specimen
FACULTY OF MEDICAL SCIENCE
 Doctor of Philosophy Program in Microbiology FACULTY OF MEDICAL SCIENCE Naresuan University 171 Doctor of Philosophy Program in Microbiology The time is critical now for graduate education and research
Doctor of Philosophy Program in Microbiology FACULTY OF MEDICAL SCIENCE Naresuan University 171 Doctor of Philosophy Program in Microbiology The time is critical now for graduate education and research
Russia Genetic Testing Market Outlook to 2021
 Russia Genetic Testing Market Outlook to 2021 GlobalData s new report, Russia Genetic Testing Market Outlook to 2021, provides key market data on the Russia Genetic Testing market. The report provides
Russia Genetic Testing Market Outlook to 2021 GlobalData s new report, Russia Genetic Testing Market Outlook to 2021, provides key market data on the Russia Genetic Testing market. The report provides
Guidance Document Infectious Substances
 Guidance Document Infectious Substances Note: 1. The following Guidance Document was developed by the ICAO DGP. The original ICAO document reflects references to the ICAO Technical Instructions these have
Guidance Document Infectious Substances Note: 1. The following Guidance Document was developed by the ICAO DGP. The original ICAO document reflects references to the ICAO Technical Instructions these have
The Medical Laboratory Licensing Regulations, 1995
 1 The Medical Laboratory Licensing Regulations, 1995 being Chapter M-9.2 Reg 1 (effective March 1, 1996) as amended by Saskatchewan Regulations 23/2004, 87/2007 and 88/2013. NOTE: This consolidation is
1 The Medical Laboratory Licensing Regulations, 1995 being Chapter M-9.2 Reg 1 (effective March 1, 1996) as amended by Saskatchewan Regulations 23/2004, 87/2007 and 88/2013. NOTE: This consolidation is
APPENDIX A GUIDANCE DOCUMENT
 APPENDIX A GUIDANCE DOCUMENT Infectious Substances International Civil Aviation Organization Technical Instructions for the Safe Transport of Dangerous Goods by Air, 2005-2006 Note: This guidance document
APPENDIX A GUIDANCE DOCUMENT Infectious Substances International Civil Aviation Organization Technical Instructions for the Safe Transport of Dangerous Goods by Air, 2005-2006 Note: This guidance document
Types of HIV test. Antigen - Any substance (such as an immunogen or a hapten) foreign to the body that stimulates an immune system response.
 Types of HIV test There are two basic categories of HIV test 4 th generation and 3 rd generation. Each individual test differs as to what it tests (whether antibodies and/or p24 antigens), how it tests
Types of HIV test There are two basic categories of HIV test 4 th generation and 3 rd generation. Each individual test differs as to what it tests (whether antibodies and/or p24 antigens), how it tests
Global Medical Practice Management Software Market Outlook: 2014-2020
 Brochure More information from http://www.researchandmarkets.com/reports/3159021/ Global Medical Practice Management Software Market Outlook: 2014-2020 Description: Medical practice management software
Brochure More information from http://www.researchandmarkets.com/reports/3159021/ Global Medical Practice Management Software Market Outlook: 2014-2020 Description: Medical practice management software
