A practical approach to multicolor flow cytometry for immunophenotyping
|
|
|
- Walter Fisher
- 9 years ago
- Views:
Transcription
1 Journal of Immunological Methods 243 (2000) locate/ jim A practical approach to multicolor flow cytometry for immunophenotyping Nicole Baumgarth, Mario Roederer a, b, * a Department of Genetics, Stanford University Medical School, Beckman Center B-007, Stanford, CA , USA b Department of Stomatology, UCSF, San Francisco, CA, USA Abstract Through a series of novel developments in flow cytometry hardware, software, and dye-chemistry it is now possible to simultaneously measure up to distinct fluorescences and two scattered light parameters on each cell. Such advanced multicolor systems have a number of advantages over current two- and three-color flow cytometric measurements. They provide a large amount of novel information for each sample studied, an exquisitely accurate quantitation of even rare cell populations, and allow identification and characterization of novel cell subsets. In particular, this technology is proving crucial to identifying functionally homogeneous subsets of cells within the enormously complex immune system; such identification and enumeration is important for understanding disease pathogenesis. However, multicolor flow cytometry comes with a new and sometimes difficult set of technical problems that must be overcome by users to derive meaningful results. In this manuscript, we describe the basic aspects of multicolor flow cytometry, including the technical hurdles and artefacts that may occur, and provide some suggestions for how to best overcome these hurdles. While inspired by the -color technology that we currently use, these principles apply to all flow cytometric experiments in which more than one fluorescent dye is used Elsevier Science B.V. All rights reserved. Keywords: Flow cytometry; Immunofluorescence; Immunophenotyping; Multiparameter flow cytometry. Introduction Abbreviations: APC, allophycocyanin; A595, Alexa 595; CasB: Since the earliest application of flow cytometry to Cascade Blue; CasY, Cascade Yellow; Cy5.5APC, Cy7APC, the study of cells, there has been a drive to increase Cy5.5, Cy7 conjugates of allophycocyanin; Cy5PE, Cy5.5PE, the number of distinct measurements for each cell. Cy7PE, Cy5, Cy5.5, and Cy7 conjugates of phycoerythrin; This developmental effort blossomed in the late Cy5.5PerCP: Cy5.5 tandem conjugate of PerCP; FITC, fluorescein; PBP: phycobiliprotein (i.e., PE or APC); PE, phycoerythrin; 990s, when the number of independently measur- PerCP: peridinin chlorophyll protein; PFC, polychromatic flow able colors (each color corresponds to a distinct cytometry; PMT, photomultiplier tube; TR, Texas Red; TRPE, fluorescence-based measurement of a cellular protein Texas Red-conjugated phycoerythrin; SA, streptavidin or function) increased from five to (Roederer et *Corresponding author. Tel.: ; fax: -45- al., 997; Bigos et al., 999) address: [email protected] (M. Roederer). The success of this developmental effort was due Present address: Center for Comparative Medicine, University to the coordinated development of new hardware, of California, Davis, CA 9566, USA. new fluorochromes, and new software analysis tools / 00/ $ see front matter 2000 Elsevier Science B.V. All rights reserved. PII: S (00)
2 78 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) that significantly increase the quality and quantity of can be simultaneously measured. The -color PFC measurements. This increase comes with a price, that is currently in routine use at Stanford uses dyes however, as this new technology has its own set of excited by three different laser lines. The excitation technical problems and difficulties that users must be and emission spectra of these dyes and the filters that aware of and must overcome in order to derive were chosen to collect the emitted light from these meaningful results. Nonetheless, once these hurdles dyes are shown in Fig.. are overcome, this new technology is well worth the effort, as the information obtained from the measure- 2.. Characteristics of useful fluorochromes ments is not only novel but could not be obtained otherwise using standard flow cytometric techniques. When designing experiments for the flow cytome- As we outline below, setting up multicolor flow ter that include the use of new dyes, careful considcytometry is not simply achieved by adding new eration must be given to the choice of fluorochromes. reagents to existing reagent combinations, but re- Desirable fluorochromes for cytometric technologies quires a more involved process of quality control, have several properties: they (i) are biologically optimization, and debugging. Therefore, to dis- inert; (ii) have high cell-associated fluorescence tinguish multicolor flow cytometry with its unique intensities ( bright ); (iii) exhibit little spectral overbenefits and technical problems from current stan- lap amongst each other; and (iv) for immunodard flow cytometric technologies (i.e., using four or phenotyping, are easily conjugated to monoclonal fewer fluorescent dyes), we refer to it as poly- antibodies. chromatic flow cytometry (PFC) and will use the term throughout this manuscript Biological inertness Flow cytometers capable of collecting data for Most of the fluorochromes that are currently in use more than three or four colors are now becoming are biologically inert: i.e., they do not affect the more prevalent, as manufacturers have recognized cells, nor do they bind directly to cellular elements. the significant demand for the types of analysis There are, however, exceptions to this: the most afforded by this technology. Given the bewildering common example is that of the background binding array of fluorochromes, lasers, hardware, and soft- of Cy5PE (and other Cyanine PBP tandem dyes) to ware that might be used in PFC, we outline here the monocytes and B cells. This background binding is fundamental requirements, interactions, and prob- variable between species and can be extremely high lems associated with setting up this technology, so in some instances. For example, Cy5PE binds strongthat users can make educated decisions about instru- ly to B cells in mice with autoimmune disorders ment requirements and the design of their experi- (e.g., non-obese diabetic mice). While there are ments. Finally, we provide some examples in which methods available to reduce this background, it is of this technology has been of particular benefit. We concern when the particular cell types being studied hope to provide with this brief review some practical are those that interact nonspecifically with the tips and encouragement for those thinking of expand- fluorochrome. ing their current flow cytometric measurements. The benefits of true multicolor flow cytometry make this Cell-associated fluorescence intensity technique a particularly useful and probably soon an With regard to the high fluorescence intensities or irreplaceable tool for the study of cell biology and the brightness of a fluorochrome, it should be noted immunology. that the characterization of a fluorescence signal as bright, i.e., the difference between the unstained and the stained cells, is still empirical. Bright 2. Fluorochromes signals result from fluorochromes with the following characteristics: () a high extinction coefficient; (2) a The ability to measure multiple parameters of each high quantum yield; (3) an emission spectrum overcell is limited by the number of fluorochromes that lapping as little as possible with cellular auto-
3 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) Fig.. Shown are excitation and emission spectra of the dyes that we currently use for polychromatic flow cytometry (PFC). Indicated are the wavelengths of the different laser lines used for their excitation (407, 488 and 595 nm; other laser lines that can be used for some of the dyes are shown for reference), and the filters chosen for optimal light collection and minimal spillover. The fluorochromes, laser lines and filters are currently in routine use on a modified three-laser hybrid Becton-Dickinson/ Cytomation flow cytometer, described previously (Roederer et al., 997; Bigos et al., 999).
4 80 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) fluorescence; (4) measurable with high sensitivity detectors; and (5) the ability to conjugate multiple fluorochromes to each detecting unit (e.g., a high ratio of fluorochrome to antibody). The differences in brightness can be quantitated experimentally by conjugating the same monoclonal antibody to the various fluorochromes at optimized ratios. Fig. 2 shows histograms of human peripheral blood lymphocytes that were stained with different conjugates of an anti-cd8 monoclonal antibody, one conjugate for each of the fluorochromes that we currently use in PFC. As shown in this figure, the brightness is affected not only by the intensity with which the positive cells are stained, but also by the background of the negative cells. Although some of the conjugates appear to stain much brighter than others, all of these fluorochromes are useful for clearly distinguishing the positively stained cells from the unstained cells. It is important to point out that the brightness of a fluorochrome will differ depending on the instrument used. For example, conjugates of antibodies to PE generally result in much brighter staining when cells are analyzed with typical benchtop cytometers using flow cells as compared to cytometers using jet-in-air detection. This is due to a number of differences in the optical paths that affect the efficiency with which emitted light is collected by the detectors; however, other differences (notably the laser power) also influence sensitivity. Therefore, reagent brightness is a relative measurement and must be assessed by users on any given instrument. Fig. 2. An anti-human CD8 monoclonal antibody was conjugated at optimal fluorochrome antibody ratios to each of the indicated fluorochromes (Roederer, 997a,b). Human peripheral blood lymphocytes, purified by gradient centrifugation over Ficoll, were stained with each of the indicated anti-cd8 conjugates and with an antibody to the pan-t cell marker CD3, conjugated either to FITC (for CasB and CasY graphs) or to CasB (all others). Shown are histograms of cells that stained positively for CD3. The dashed line indicates levels of staining for cells stained with anti-cd3 only, and the solid line indicates the anti-cd8 fluorescence for cells stained with both antibodies. Empirically, the relative brightness of any of these fluorophores can be estimated based on the separation of CD8 positive and negative cells. The apparent difference in the number of cells between the positive and negative peaks for these dyes is a visual artefact of the scaling (note that the CD8-dim cells appear to be more prevalent in those plots where the CD8 peak is higher). There was no difference in the percent dim or bright-positive cells for any of distributions.
5 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) Spectral overlaps gle protein molecules (PE, APC) require a slightly The spectral overlaps that exist between dyes more complex procedure, but reactive fluorochromes currently represent the biggest hurdle in PFC analy- can be easily prepared, are stable for long periods of sis. Due to the similarities and overlaps in the time, can be used in a simple conjugation procedure. emission spectra of different dyes (see Fig. ), it is (3) Tandem dyes 2 (Cy5PE, Cy5.5PE, Cy7PE, not possible to choose emission filters that uniquely Cy5.5APC, Cy7APC, Cy5.5PerCP), require the use measure only one of the dyes in a multicolor of more complex procedures which involve first the experiment. The appropriate choice of filter, how- generation of the reactive tandems that can then, in a ever, can greatly reduce collection of light from second step, be used for conjugation to antibodies. other fluorochromes (i.e., reduce spillover ). Note Generating the reactive tandems requires careful from Fig. that a set of up to four dyes excited by testing of different conditions for each batch of three different lasers can be chosen that exhibit tandem dye to optimize the ratio of the two dyes essentially no spectral overlap; thus, four- or fewer- used. However, once the chemistry has been opticolor experiments can be carefully designed to avoid mized, a large batch of reactive dye can be prepared the problems caused by spillover. However, the for use in many conjugations. necessity of having three lasers for a four-color experiment renders this an impractical solution. Because of the spectral overlap, each fluorochrome 2.2. Other fluorochromes. will contribute a signal to several detectors, New fluorochromes for immunophenotyping appli- therefore the contribution in detectors not assigned to cations are constantly being developed. At the time that fluorochrome must be subtracted from the total of writing, several dyes exist that are useful for signal in those detectors. This process, termed com- immunophenotyping applications and which can be pensation, is discussed in detail below. used in addition to or instead of some dyes listed in Conjugation to antibodies Fig.. For many applications of flow cytometry, a further requirement for a fluorochrome is that it can be PerCP readily conjugated to antibodies. The dyes we This is a chlorophyll-like protein that can be commonly use (Figs. and 2) meet this criterion. directly conjugated to antibodies. It has an emission There are, however, varying degrees of difficulty spectrum similar to Cy5PE, but with no excitation in involved in carrying out the conjugation reactions. It the red, and hence very little spillover into the APC is likely that any laboratory carrying out flow detector compared to Cy5PE. On the other hand, it cytometric analyses with five or more dyes will need has several disadvantages: it is easily bleached by to conjugate antibodies, as commercial conjugates lasers, limiting its utility to low-power instruments are not yet available for a number of these dyes. such as benchtop instruments; it is less bright than Even if all of the dyes become available as antibody Cy5PE; and it is only available in conjugates from a conjugates, it is unlikely that every combination of single vendor (Becton-Dickinson, San Jose, CA). antibody and fluorochrome necessary for each user will be available Cy5.5PerCP Detailed procedures for conjugating the dyes can A relatively new tandem conjugate of PerCP that be found on the web (Roederer, 997a) or from has excitation and emission spectra very similar to commercial vendors who supply conjugation kits. Cy5.5PE. Unlike PerCP, it can be used in high power Briefly, the procedures for conjugating these dyes laser instruments as it does not bleach easily, but is fall into three classes. () Small organic molecules also only available in conjugated form through (FITC, TR, A595, A430, CasB, and CasY) are Becton-Dickinson. conjugated to antibodies in relatively simple, short reactions that require only one or two column 2 Cy5PE is the same as Tricolor, Cy7APC is the same as separations (or other desalting procedures). (2) Sin- Allo-7 and PharRed, and Cy5.5PerCP is the same as TruRed.
6 82 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) Alexa 430, 595 availability of laser lines on the machine in use (i.e., A series of new dyes termed Alexa dyes from is a 595-nm dye laser line available, or a 407-nm Molecular Probes (Eugene, OR) includes two that we violet-enhanced krypton laser line?). The useable set have tested for their usefulness in PFC. A430 is an of dyes also depends on the number of detectors that excellent replacement for CasY, in that it has less are available on the instrument to collect the excited non-specific interaction with CasB and is otherwise light (i.e., how many colors can be collected that are roughly similar in brightness (Baumgarth, unpub- excited by the first, second, and third laser line?). lished). A595 is spectrally similar to Texas Red (TR) Once those constraints have been determined, there but does not have its non-specific binding charac- can still be a wide range of possible combinations. teristics, leading to a somewhat better separation of Table lists a number of such combinations, given positive and negative signals than that seen with some constraints of laser and detector availability many TR conjugates. often seen in currently available machines TRPE This dye, also called Red 63 or ECD is 3. Hardware excited by a 488 nm laser line and can replace Cy5PE in a multicolor staining combination, as it has 3.. Lasers considerably less overlap into the APC channel than Cy5PE. It is however less bright than Cy5PE and it One of the single largest component costs of has somewhat more overlap into the PE channel; it cytometry instrumentation is the cost of the lasers. also displays considerably higher background bind- Newer diode lasers are becoming prevalent and these ing typical of TR conjugates. can be significantly cheaper than the older gas ion There are a number of other dyes that are com- lasers. When considering a solid-state laser, it is monly used to directly stain cellular components important to choose one that has a long life span and such as DNA (e.g., Hoechst, propidium iodide), provides a consistent power output, which therefore cellular membranes (e.g., CFSE, nile red), lysosomes excludes the very cheap solid-state lasers that are (e.g., CFDA), and dyes that can measure metabolic currently available. In addition, for current stream-inactivity of the cell (e.g., rhodamine 23). It is beyond air instrumentation it is desirable to have at least 50 the scope of this manuscript to cover fluorochromes mw of power for each laser line in use, since the that are not used for immunophenotyping. For a fluorescence signal (and thus sensitivity) increases detailed description of these therefore refer to (Sha- with laser power. For most purposes, it is not piro, 994). Importantly, however, the use of any necessary to have much more power than this, combination of dyes, irrespective of the measure- because most fluorochromes will saturate (are maximent for which it is used, will need to address the mally excited) at mw. Indeed, additional same issues such as reagent brightness, spillover, power may actually reduce relative signal to backand compensation as described here for antibody ground staining, because the fluorophore will satuconjugates. rate at much lower powers than background fluorescence. Table 2 lists some of the currently available 2.3. Fluorochrome combinations lasers and the lines that are often used in flow cytometry, their approximate costs and the dyes that Out of the dozen or more fluorochromes that can they can excite. be used for immunophenotyping (by virtue of their spectral and chemical properties discussed above), it 3.2. Optical setup is important to select the best combination for a particular experiment under consideration. This is Sensitive detection of fluorochromes requires true whether the experiment requires four or selection of appropriate filters that are placed before colors. To a large extent, the constraints on which each detector, or photomultiplier tube (PMT). Filters fluorochromes can be used will depend on the must be selected so as to collect as much emitted
7 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) Table Sample fluorochrome combinations a b c Dyes Lasers Combinations 4 2 (488647) FITC, PE, Cy5.5PE or Cy7PE, APC FITC, PE, Cy5PE, Cy5.5APC or Cy7APC FITC, PE, Cy5PE, APC FITC, PE, TRPE, APC (488) FITC, PE, Cy5PE, Cy5.5PE or Cy7PE 5 2 (488595) FITC, PE, Cy5.5PE or Cy7PE, TR, APC FITC, PE, Cy5PE, TR, Cy5.5APC or Cy7APC FITC, PE, Cy5PE, TR, APC 2 (488632) FITC, PE, Cy7PE, APC, Cy5.5APC FITC, PE, Cy5.5PE, APC, Cy7APC FITC, PE, Cy5PE, Cy5.5APC, Cy7APC FITC, PE, Cy5PE, APC, Cy5.5APC or Cy7APC FITC, PE, Cy5PE or Cy7PE, APC, Cy7APC (488) FITC, PE, Cy5PE, Cy5.5PE, Cy7PE 6 2 (488633) FITC, PE, Cy5.5PE, Cy7PE, APC, Cy5.5APC or Cy7APC FITC, PE, Cy5PE, Cy5.5PE or Cy7PE, Cy5.5APC, Cy7APC FITC, PE, Cy5PE, Cy5.5PE or Cy7PE, APC, Cy5.5APC or Cy7APC 2 (488595) FITC, PE, Cy5.5PE, Cy7PE, TR, APC FITC, PE, Cy5PE, Cy7PE, TR, Cy5.5APC FITC, PE, Cy5PE, Cy5.5PE, TR, Cy7APC FITC, PE, Cy5PE, Cy5.5PE or Cy7PE, TR, APC (488) FITC, PE, Cy5PE, TRPE, Cy5.5PE, Cy7PE 7 2 (488632) FITC, PE, Cy5.5PE, Cy7PE, APC, Cy5.5APC, Cy7APC FITC, PE, Cy5PE, Cy5.5PE or Cy7PE, APC, Cy5.5APC, Cy7APC 2 (488595) FITC, PE, Cy5.5PE, Cy7PE, TR, APC, Cy5.5APC or Cy7APC FITC, PE, Cy5.5PE or Cy7PE, TR, APC, Cy5.5APC, Cy7APC a Any of the listed combinations can be augmented with a 407 nm Krypton laser and the fluorochromes CasB and CasY (or A430) to increase the number of colors by two without significantly affecting compensation requirements. b Number of lasers, followed by excitation lines (see footnote a). c Combinations are listed in rough order of increasing quality of measurements. i.e., for any given set of combinations, the first ones will tend to have lower compensation requirements and higher brightness. There are many more possible combinations than can be listed in this table. The order does not take into account commercial availability or ease of conjugate preparation. Substitutions: PerCP can be substituted for Cy5PE on lower laser-power instruments; Cy5.5PerCp can be substituted for Cy5.5PE on any system; A595 can be substituted for TR on any system. TRPE could be added to most combinations not employing 595 lasers or used as a substitute for Cy5PE. Where two dyes are listed as A or B, either can be used in the combination listed, but A is preferred for reasons of brightness or compensation. light from the primary fluorochrome for high sen- filters, allowing for example to collect the FITC sitivity, but as little as possible from other fluoro- signals with a much wider bandpass filter than was chromes to reduce the compensation required. In previously possible (old, 530/ 30; new, 525/ 50) general, these two criteria work against each other; while still blocking scattered light from the 488-nm therefore, for any given set of fluorochromes used in laser line. an experiment, the optimal filter set may be different. For experiments using a small subset of these However, we have found that the filter set shown in fluorochromes, we found that using the widest Fig. and Table 3 is close to optimal for many possible bandpass that still excludes as many other experimental setups that are currently in use. fluorescences as possible yields the best results. A further important selection criterion is the Nonetheless, switching to wide-open filters will ability to block scattered light from the laser lines. generally only increase the measured signal by 20 Modern filters are considerably better than the old 00% compared to the bandpass filters shown in
8 84 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) Table 2 Lasers for flow cytometry a Laser type Cooling Line (nm) Approx. cost Fluorochromes Argon Ion Air 488 $0K FITC, PE, TRPE, PerCP, Cy5PE, Cy5.5PE, Cy5.5PerCP, Cy7PE, A488 Krypton Water 568 $30K PE, TRPE, PerCP, Cy5PE, Cy5.5PE, Cy5.5PerCP, Cy7PE, A568, TR, A APC, Cy5APC, Cy5.5APC, Cy7APC (Cy5PE, Cy5.5PE, e Cy7PE) Violet-enhanced Water $50K CasB, CasY, A430 b Krypton ion c d Dye Water 595 $5K TR, A595, APC, Cy5, Cy5.5APC, Cy7APC (TRPE, e Cy5PE, Cy5.5PE) Doubled Nd-YAG Air 532 $2K PE, TRPE, PerCP, Cy5PE, Cy5.5PE, Cy5.5PerCP, Cy7PE HeNe Air 632 $6K (50 mw) APC, Cy5, Cy5.5APC, Diode Air 635 $K (0 mw) e Cy7APC (Cy5PE, Cy5.5PE) a Except as noted, lasers are rated at 50 mw output or greater at the given line. b 405-nm diode lasers with sufficient power for cytometry will be available soon; currently, 4-mW lasers cost about $4K. c Currently, 5-mW HeNe lasers with a 594-nm line can be obtained for about $3K; this power level is probably too low except for bench-top (flow cell) cytometers. d A dye laser also requires a 5-W, 488-nm pump laser, adding another $20K to the cost of this laser. However, the 488-nm line of the pump laser (running in all lines mode) can be split off and used as the primary laser beam in the instrument, obviating the need to purchase the primary Argon laser. e Dyes in parentheses will also be excited by this laser, although their primary excitation is by other laser lines. Table 2. For flow cytometric experiments, 2-fold logarithmic conversion and/ or digitization, or postincreases in signal-to-background levels are marginal collection by software. compared to the optimizations that can be achieved While compensation is one of the most important in other parts of the experiment. processes required for proper data analysis in flow cytometry, it is also perhaps the least well understood. In order to properly design, implement, and 4. Compensation analyze multicolor experiments, users must be aware of the effects of compensation, understand how to apply it correctly, and recognize when data are not 4.. What is compensation? properly compensated. While we will discuss some of these topics here in brief, the reader is strongly Compensation is the process by which the spectral encouraged to read the texts (Roederer, 999) and overlap between different fluorochromes is mathe- web pages (Roederer, 997b) devoted to this topic matically eliminated. The algorithm of compensation for more in-depth information. is a straightforward application of linear algebra, and should not be thought of as a subtraction process Compensation complications Compensation between detectors can be performed either by hardware, after signal detection but before Currently, most instruments provide the capability
9 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) Table 3 reality only about 40 of these are non-zero). Furthera Optimal interference filters for fluorochromes more, because of the multiple interactions between Fluorochrome b Filter these controls, some settings may actually be less CasB 440/ 40 than zero; something that current hardware controls CasY, A / 90 do not allow. Finally, since setting interacting pair- FITC 525/ 50 wise compensation controls is an iterative process, it PE 575/ 25 would take virtually forever to manually set them Cy5PE 665/ 30 PerCP 680/ 30 properly. Cy5.5PE, Cy5.5PerCP 720/ 45 c An additional complication to compensation is Cy7PE d 785/ 45 brought about by the use of tandem dyes (e.g., TRPE, TR, A / 40 Cy5PE, Cy7APC, etc.). Tandem dyes are covalently APC 660/ 40 c linked combinations of donor (e.g., PE) and accep- Cy5.5APC 705/ 50 d Cy7APC 750LP tor (e.g., Cy5) dyes. The ratio of acceptor to donor is usually in the range of 3: to 0:, depending on Optimization is a function of maximizing the amount of signal the optimization strategy used by the manufacturer. collected while minimizing the amount of spillover from other fluorochromes. Different filters may be optimal for particular In fact, different tandem-conjugated antibodies from applications; see text. the same manufacturer often have slightly different b Filters are given as x/y, where x is the wavelength in ratios of acceptor to donor. The problem is that the nanometers of the center of the bandpass and y is the width in compensation required to correct tandem emissions nanometers of the band. LP, long pass filter. Filters have an extra in other fluorescence channels strongly depends on coating to block scattered light. c Use of a 720/45 filter for Cy5.5PE is optimal when Cy5PE is this ratio. Therefore, a different compensation setting also to be measured. For measuring Cy5.5PE (or Cy5.5PerCP) in may be needed for every different tandem conjugate the absence of Cy5PE, then a 705/ 50 is better. Because APC has used in a particular experiment. In essence, this a lower emission wavelength than Cy5PE, the 705/ 50 works well means that the compensation setting needs to be for Cy5.5APC in combination with APC. However, if Cy5.5APC adjusted for every different staining combination in is to be measured with Cy5, then the 720/ 45 filter should be selected. use. d The 750LP and the 785/ 50 are roughly equivalent for collecting Cy7 emission; either can be used. Given these complications, it is apparent that complete compensation on multicolor systems requires computer intervention. Currently no instrument is commercially available that provides users for compensating between several spectrally adjacent with the ability to automatically adjust compensation pairs of detectors: for example, between FITC and settings on a per-stain basis, nor even to provide PE, and between PE and Cy5PE. For a two-color complete compensation among all pairs of fluoresanalysis, such pairwise compensation is sufficient cence detectors. Therefore, the only solution as yet is and complete. However, the introduction of a third the use of software analysis programs that are fluorochrome introduces the potential for complex applied after data collection is completed for cominteractions that cannot by fully corrected by such pensation of the data. Examples of such software pair-wise compensations. For example, FITC has low include WinList (for PC) and FlowJo (for Macinbut measurable emission into the Cy5PE detector. tosh). This overlap may not be completely corrected by the The use of software to set compensation simplifies combination of the FITC PE and PE Cy5PE the process considerably. Computers can set the compensation settings, causing what appears to be compensation correctly (there is no subjective criterartefactual Cy5PE fluorescence when using a very ion applied) and can be instructed to appropriately bright FITC stain. Obviously, as more than three vary the compensation matrix according to the colors are used, these problems increase. specific fluorochromes used in any given sample. Indeed, for a typical -color stain, one would However, there are significant limitations to software potentially need to use up to 00 pair-wise com- compensation (see below). In the interim before pensation controls to fully compensate all colors (in complete automated hardware compensation is avail-
10 86 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) able, the optimal solution is to combine both hard- Examples for significant inter-laser compensations ware and software compensation to minimize the are shown in Table 4. Potential signal degradation limitations of each. due to inter-laser compensation can occur with dye combinations that are found at high levels on the 4.3. Limitations of compensation same cell. The principal limitation of software compensation Both available hardware and software compensa- is caused by the inaccurate conversion from linear to tion have significant limitations that place restrictions logarithmic signals by the electronics of the flow on the utility of PFC. Hopefully, some of these cytometer. Since this conversion is typically perlimitations will soon be obviated by new technology formed by analog circuitry, it is merely an approxidevelopments, which would enable the easier set-up mate conversion. The software, however, treats the of new reagent combinations for multicolor flow data values as if they were an exact conversion, cytometric measurements. Until then, it is important which can result in compensated values that are to understand these limitations and how they may be significantly inaccurate. The major inaccuracy in the best addressed. electronic linear to log conversion is due to the fact One limitation that is shared by both hardware and that the dynamic range of the output is not exactly software compensation is due to the low precision of four decades, as the software (and the user) is told to measurements in the far-red channels, Cy7APC, expect, but can range from 3.5 to 4.3 decades. The Cy5.5APC, and to a certain extent APC. In these effects of this error are insidious, in that data can be channels, for dimly stained cells, the actual number properly compensated at a particular intensity, underof photoelectrons detected by the PMT can be very low, due to the inefficiency of the currently available PMT to detect light at this wavelength. Even the Table 4 deep red-sensitive PMTs now available do not Significant intralaser compensations a overcome this problem. The unavoidable counting Fluorochrome Detector errors inherent in the measurement can lead to the introduction of significant artefacts. For example, if TR APC Cy5.5APC Cy7APC only five photoelectrons are collected, the counting First second laser compensation Œ] error is 5, or 45%. This error propagates to other PE channels that require compensation from this detec- Cy5PE Cy5.5PE tor. The result is that the broadening of populations Cy7PE. that occurs due to compensation (Roederer, 997b, 999) is significantly worse for the far-red emitting Second first laser compensation b fluorochromes. Cy5PE Cy5.5PE Cy7PE Hardware compensation operates on the analog TR 2.2. signals collected from the PMT, before further signal APC processing is done. For detectors with sufficient Cy5.5APC signal, this value is both precise and accurate, Cy7APC rendering compensation close to exact. Problems a Significant spillovers are those above 0.%. Values are given arise when compensation is required between signals as the percentage of the signal level in the primary detector for a given fluorochrome that is found in the detector listed in each propagated from different lasers, since the signals column. Only interlaser spillovers are shown; intralaser spillovers must be electronically delayed so that they can be are significantly higher but can be more easily compensated by presented to the analog compensation circuitry at the hardware. Values are given for a 488-nm first laser and a 595-nm same time. This electronic delay is difficult to second laser. Different laser lines can give qualitatively similar but perform precisely, without changing the shape of the quantitatively different results. Spillover values are system depen- dent and determined by optics, laser power, and gain of detector electronic pulse generated by the PMT. Therefore, (PMT type, voltage, amplifier) on each channel pair. hardware-based interlaser compensation is typically b There are no significant spillovers into the FITC or PE much less accurate than intra-laser compensation. channels from these dyes.
11 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) compensated at lower (or higher) intensities, and excessive concentrations of the conjugated antibody overcompensated at higher (or lower) intensities. The are used, or when conjugates are used that were less compensation that needs to be done by the prepared with a non-optimized ratio of fluorochrome software the smaller the extent of this problem is. to antibody. Titration of reagents and optimization of This again points to the importance of appropriate reagent conjugations are therefore particularly imreagent/ color choices, as described below. portant when attempting to scale up a one- or two- Given the respective limitations of hardware and color stain to a stain that contains three or more software compensation, our approach has been to use colors. Some reagents, such as Cy5PE, also exhibit a hardware compensation for as many pairs of signals certain degree of stickiness ; in the case of Cy5PE as possible (Bigos et al., 999). Currently we use 4 this is seen particularly with B cells. Finally, the hardware controls, dedicated to signals that require amount of cellular autofluorescence varies signifithe largest amount of intralaser compensation. Soft- cantly in different areas of the spectrum. In parware compensation is used following data acquisition ticular, autofluorescence overlaps greatly with FITC, to complete the compensation process. It is critical to CasB, and CasY. Therefore, highly autofluorescent set the pair-wise settings conservatively, i.e., to cells such as macrophages might appear as dull under-compensate considerably where necessary. positive in these detectors, despite the fact that they There are two reasons for this: inadvertent over- might not stain specifically with any of the reagents compensation cannot be corrected in software and used. thus causes irretrievable data error; and, given the The choice of fluorochrome/ conjugate used for multiple interactions, proper compensation between identifying a given marker will depend on the levels one pair of fluorochromes can lead to overcompensa- of expression and the cell types on which the marker tion of a different pair. For example, proper com- is expressed. If this information is not at hand, it is pensation of TR into APC often results in (unrecov- advisable to use one of the bright fluorochromes erable) overcompensation of Cy5PE into APC. such as PE, Cy5PE or APC, and use the duller stains for identification of surface markers that are known, such as the lineage markers for lymphocyte subset 5. Choosing the right fluorochrome staining. As an example, we show in Fig. 3 various dual-color staining combinations of splenic mouse As outlined above, fluorochromes differ considera- cells for CD9, a lineage marker for B cells, and bly in relative brightness. Conjugation of the CD5. CD5 is expressed at high levels on all T cells various fluorochromes to the same antibody and and at low levels on a small subset of B cells in the staining of the same population of cells can therefore spleen (Kantor and Herzenberg, 993). CD5 B result in large differences in resolution of positive cells express somewhat higher levels of CD9. As and negative events (Fig. 2). For single-color stain- apparent from the figure, resolution of the dully ing for a highly expressed marker such as CD8 on T stained CD5 B cells is achieved only when the cells (Fig. 2), the differences are merely cosmetic biotinylated CD5 antibody is revealed with strepand no alterations in the frequency of negative and tavidin (SA) conjugated to PE, Cy5PE or APC (see positive events are found after staining with different also Kantor and Roederer, 997). Staining with other conjugates. However, when staining for surface SA-fluorochrome conjugates does not reveal a dismarkers that exhibit high and low levels of expres- tinct CD5 B cell population. It is also useful to note sion, for example the expression of CD28 or FAS on that staining with SA-CasB resulted in an apparent T cells, a relatively dull fluorochrome can result in staining of a small population of CD9-dull cells. an underestimation of the size of the positive popula- However, this staining is in fact due to the autotion (false-negatives). fluorescence of macrophages which appears as a dull False-positive results are seen particularly with positive stain in many channels (data not shown). dyes and conjugates that have high background Hence, simply the choice of fluorochrome used staining. High background staining can occur when will determine whether this small B cell population
12 88 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) Fig. 3. Shown are 5% probability contour outlier plots of mouse spleen cells stained with a monoclonal antibody to the pan-b cell marker CD9, conjugated as indicated to either FITC or APC and with (upper panel) or without (lower panel) biotinylated anti-cd5 monoclonal antibody. CD5 recognizes all T cells and a small population of CD9 B cells. CD5 was revealed by adding streptavidin (SA) conjugated to the various fluorochromes indicated to the samples. Note that only staining with SA-PE, -Cy5PE or -APC resulted in a clear staining of the CD9 CD5 B cell population (indicated by the arrow). can be identified at all. Importantly, each of the reagents used was able to clearly separate the CD5 2 2 CD9 T cell populations from the CD5 cells. The general rule of thumb is therefore: the lower the expression level of a given marker, the more likely that the choice of a bright fluorochrome will help to reveal its expression. Fig. 2 might help to gauge the relative brightness of fluorochromes.
13 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) Choosing the right combinations of chosen for the other conjugates that do not stain the fluorochromes cells identified by the APC stain. The other crucial lesson from this experiment is What are the criteria for choosing the right that isotype controls are only appropriate when used combination of fluorochromes? As outlined above, in the context of every other antibody stain. That is, one major criterion is the expected levels of expres- a sample that was stained with isotype controls on all sion for the markers of interest. Markers such as channels would show a background of only 0.2 on CD5 on B cells can only be identified when a bright the APC channel, considerably less than the backconjugate is used; but identifying CD5 on T cells can ground observed on this channel given a full staining be accomplished with any fluorochrome. An un- set minus the APC stain (4.). This is why false known surface molecule is initially best studied with positives will be identified without the use of approa conjugate that uses one of the brightest fluoro- priate controls. chromes, PE, Cy5PE, or APC. Other considerations In the example shown in Fig. 3, i.e., measuring the include the spectral overlaps that exist between the low levels of CD5 on CD9 cells, one should various dyes in the staining combinations. Although choose to label CD9 (well-expressed on all B cells) hardware or software compensation can eliminate with a color that does not have a large compensation some of the associated problems, the careful choice component with the fluorochrome used for CD5. On of reagents can minimize the need for compensation. the other hand, CD8 (part of the dump channel) Fig. 4 shows an example of how the APC signal is could be assigned to such a color even though CD8 affected by using one to four very brightly staining is extremely highly expressed. This is because CD8 markers that all have significant spillover into the is not expressed on the cells of interest (CD9 this channel. This figure therefore shows an example cells) and thus compensating CD8 fluorescence of how best not to design an experiment. In this would have no impact on the quantitation of CD5 on example we chose to add antibodies to B cell B cells. markers conjugated to Cy5PE, TR, Cy5.5APC and The dyes excited by the violet-enhanced krypton Cy7APC to the staining mix and then looked at the laser are particularly useful for multicolor staining background APC levels. Remember that APC is one combinations. Although they appear rather dull of the dyes that are in general bright (see Figs. 2 mainly due to the large autofluorescence background and 3) and is therefore a good choice for identifying in these channels (Fig. 2), they have minimal overlap dimly expressed markers such as CD5 on B cells. As with any of the other fluorochromes and are easily we add staining reagents to the cells, the background compensated against each other. Therefore any levels of the APC signal increase significantly, from combination with a 407-nm line-excited dye should a mean fluorescence intensity of 0.2 to 4. (Fig. 4). work without problems, as long as the marker chosen This increase in mean fluorescence intensity at the for the CasB or CasY conjugation is expressed at APC detector is observed despite the fact that sufficiently high levels to separate the stained from software compensation was applied to the samples the unstained cell population. In many cases, signifiand each compensation pair seemed to have been cant compensation can also be avoided by using dyes compensated appropriately (not shown; see Fig. 5 for excited by different lasers (however, see Table 4 for explanation). Therefore, after addition of the Cy5PE, dyes that do require significant interlaser compensa- TR, Cy5.5APC and Cy7APC stains, a dim positive tion). staining with a reagent conjugated to APC could no In summary, the appropriate choice of reagents longer be distinguished from the increased back- and reagent combinations requires certain knowledge ground staining; the effective brightness of APC is of the characteristics of the individual fluorochromes, reduced 20-fold. Since a number of colors that are such as brightness and spillover into other chancurrently used for PFC show large overlaps into the nels, to avoid the use of inappropriate or useless APC detector, the usefulness of this detector is staining combinations. Typically, multiple different substantially compromised unless reagents are combinations of fluorochrome/ antibody pairings
14 90 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) Fig. 4. Shown in solid gray is the histogram of background APC signals (i.e., no APC reagent was added) of splenic mouse cells stained with FITC-anti-Ig-k and gated to identify all B cells (data not shown). Stepwise addition of other reagents (identified by in the table) that have considerable spectral spillover into the APC channel increased the background level of the APC signal. This is shown in the shift of the histogram plots to the right and the increase in mean fluorescence intensity level (but not median!) indicated in the table. This increase in the mean was observed despite appropriate software compensation and is a direct result of the broadening artefact of compensation (Roederer, 997a,b, 999); see also Fig. 5. Reagents used: Cy5PE-anti-B220, TR-anti-IgD, Cy5.5APC-anti-CD9, Cy7APC-anti-IgM.
15 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) Fig. 5. The effect of random noise added to an autofluorescence distribution. The process of fluorescence compensation introduces additional error into the distribution of measured fluorescence. Histograms in this figure represent a mathematical modeling of this process. (A) A typical distribution of cellular autofluorescence. The fluorescence distribution is log-normal, i.e., it is a Gaussian when viewed on a logarithmic axis. The distribution is shown only for the first 400 (of 024) channels of a typical measurement parameter; this represents the first.5 decades of fluorescence. (B) The distribution in (A), after instrumentation boundary conditions are imposed. Since there is no zero on a logarithmic axis, the low end of the fluorescence scale is arbitrary. Any events with fluorescence below the lowest value (0. here) are automatically assigned to channel zero and given a fluorescence value of 0.. This results in an artificial peak at channel zero, the sum of all events with fluorescence less than this (shown in white bars). This same artefact occurs in two-dimensional histograms used for contour plots or density plots, causing the appearance of an artificial peak (multiple contour lines) on the axis. This peak is an artefact of the measurement and visualization processes, and importantly, it does not represent a population of events distinct from those adjacent to it. (C) Effects of compensation. To model the error introduced by compensation, a random amount of fluorescence (averaging fluorescence units) was added to each event in the distribution shown in (B). The fluorescence of about 25% of all events is now shifted to below 0. units (open bins), consequently these events are forced into the single point at channel zero. Note the scale change of the vertical axis. The reason for the asymmetric effect on the distribution (i.e., the right edge of the histogram increased very little, but the left tail increased greatly, and the mode of the distribution has increased) is a consequence of the logarithmic scaling: a noise distribution that is linearly distributed was added to the log-normal distribution. Thus, as shown by the arrows, adding 0.4 units of fluorescence to an event at 0.45 (to 0.85) moves it much less distance (in the log domain) than subtracting the equivalent amount (to 0.05). Compensation introduces errors that are symmetrically distributed in the linear domain. Thus, one of the effects of compensation is to alter the autofluorescence distribution from log-normal to that shown in (C). It is important to note that the median fluorescence intensity in (C) is exactly the same as it is in (B), but the mean fluorescence has increased, and the mode (above channel 0) has increased even more. Because of our internal bias to use the mode (peak) to estimate a population s center rather than the median or mean, which is virtually impossible to estimate visually, the distribution appears to have moved substantially. Finally, note that placement of a positive gate at, for example, fluorescence unit would be appropriate for the uncompensated parameter (B), but not for the compensated parameter (C), even though both represent unstained distributions.
16 92 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) must be evaluated in order to select the panel that of CD5 on a small B cell subpopulation in the spleen provides the most information. (Fig. 3), it would be difficult to accurately determine the frequency of these cells using this two-color stain. In Fig. 6 we outline how the simultaneous use 7. Enhanced accuracy of measurement with of four (Fig. 6B) and six (Fig. 6C) colors enhances multicolor flow cytometry the accuracy of the measurement and the amount of information that can be obtained from one stain (Fig. Despite the relatively good resolution that the use 6A). In this figure we compare the staining pattern of of SA-PE, Cy5PE and APC afforded for the staining spleen cells isolated from wild-type mice and gene Fig. 6. Shown are 5% probability contour outlier plots of (A) two-, (B) four- and (C) six-color stains of single cell suspensions prepared from mouse spleens derived from normal wild-type C57BL/6 mice and mice deficient in the signaling molecule vav (vav2 / 2). (A) CD9 2 and CD5 staining of splenic B cells shows a reduction in the number of CD5 CD9 T cells and CD9 CD5 B cells. (B) Addition of a dump channel (reagents to CD3, CD4, CD8 and F4/80) and a second marker (CD43) expressed on mouse B- cells identifies the lack of B- cells among the overall normal frequency of CD9 B cells in vav2 / 2 mice compared to their wild-type controls. (C) Addition of anti-igm, anti-igd and anti-cd2 in the staining cocktail reveals alterations in IgM and IgD expression of follicular B cells and the hi neg lo neg appearance of normal frequencies of marginal zone (CD2 CD43 ) and immature (CD2 CD43 ) B cells and the absence of B- cells lo hi lo (CD2 CD43 ) among IgM IgD cells.
17 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) targeted mice, deficient in vav, a signaling molecule stain therefore clearly reveals that vav2/ 2 mice important in appropriate signaling through the an- completely lack B- cells in the spleen, a conclusion tigen-receptor expressed by the B cell. The presence that could not have been drawn from any two-color of vav has been shown to be crucial for normal B analysis. cell development (Zhang et al., 995).Vav2/2 mice The use of six or seven colors (Fig. 6C) on the have splenic B cells that differ considerably in same cell samples shown in Fig. 6B reveals a much phenotype (Fig. 6) and function from their wild-type more detailed analysis of the effects of vav-deletion controls. Comparison of a two-color stain of CD9 on the other peripheral B cell subpopulations found and CD5 expression using Cy5PE for staining of in the spleen of mice. As shown in Fig. 6C, the CD5 (Fig. 6A) identifies a subpopulation of CD5 relative frequency of CD9 cells is identical in the (.4 units) CD9 (.0.9 units) cells in the spleens two mouse strains. However, staining of CD9 B of the wild-type mice. In the gene-targeted mice this cells for expression of surface IgM and IgD revealed population seems absent. However, it is not clear strong differences in the expression pattern of these from this stain whether the levels of CD5 expression molecules on cells from vav2/2 and control mice, are reduced, or whether this population of B cells is respectively. Normal splenic mouse B cells contain a missing in these mice. In fact, a somewhat more main population of cells expressing high levels of inexperienced investigator might not even see a IgD and low levels of IgM, which are the relatively difference in the CD5-staining patterns of the CD9 homogeneous, recirculating, follicular B cells. As cells for these two different mice. However, a clear seen in Fig. 6C, although the frequency of the cells 2 difference in the frequency of the CD5 CD9 T does not differ greatly in the vav2/ 2 mice, the cell population is easily demonstrated. levels of IgM expression are increased, indicating a Much better quantitation of the CD5 CD9 B maturation defect in the cells from these mice. cell population is achieved with a four-color stain, as Furthermore, the population of surface IgM- and shown in Fig. 6B. This four-color stain includes a IgD-negative cells, presumably cells that have underso-called dump channel, a combination of reagents gone isotype switching, is increased 2 3-fold (.5 conjugated to the same fluorochrome that are exhi lo 3.5%). Finally, the population of IgM IgD cells pressed on cells that are not of interest. The use of a contains at least three cell populations, as shown in dump channel is a particularly good way for enhanc- the lower panel of Fig. 6C. These can be differening the fidelity of a given stain, since not only tiated by their expression of CD2 and CD43 into stained cells, but also sticky and highly autofluoresneg neg immature B cells (CD2 CD43 ), marginal zone cent cells can be clearly separated from the cells of hi neg B cells (CD2 CD43 ) (Takahashi et al., 997) interest. Another reason for including a dump chanlo and B- cells (CD2 CD43 ) (Wells et al., 994). nel in a staining combination is the identification of As shown in the four-color stain, B- cells are small cell subsets, for example to identify the lineage completely absent in the vav2/ 2 mice, whereas the marker negative stem cells found in bone marrow or two other populations, marginal zone and immature to identify dendritic cells in peripheral lymphoid B, seem unaffected in these mice. With the help of tissues. In Fig. 6B, we show how B cells are this one six-color stain we can therefore identify all identified first by their expression of CD9 and the major known B cell populations present in the spleen lack of expression of various T cell markers (CD3, of mice, allowing us to clearly identify and quanti- CD4 and CD8) and a macrophage marker (F4/80). tate the effects of vav or any other gene of interest Simultaneous staining for CD5 and CD43 now on these cells. allows identification and accurate enumeration of a This example illustrates how adding more and population of CD5 and CD43 B cells that express more measurements on the same cell populations can CD9, since CD43 and CD5 co-expression is a significantly simplify the interpretation of the data. hallmark of (CD5 ) B- cells in the mouse (Wells Rather than trying to draw conclusions from a series et al., 994). Note that these markers can only be of two- (or even four)-color measurements that can used in combination with CD9, as both are also only show changes in bulk populations of B cells, or expressed on virtually all T cells. This four-color from changes in relative expression levels of certain
18 94 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) markers on any of several indistinguishable cell types, the use of PFC allows us to pinpoint precisely the defects in B cell development of these mice. tandem dyes that are prepared in the laboratory, stains that use tandem dyes from the same lot should suffice. Thus, careful inventory of conjugates in a laboratory that includes the lot number of a tandem dye for each antibody conjugate can greatly reduce 8. Staining controls the number of compensation control samples for each experiment. Control stains are important for all experiments using flow cytometry, but they become particularly 8.2. Isotype or unstained controls critical for complex multicolor stains. The higher the number of fluorochromes and antibodies used in each The use of isotype control antibodies is usually of stain the greater the risk for artifacts introduced by little value, since each antibody and antibody conjucompensation errors and/ or reagent interactions. In gate has very different characteristics in terms of general, two types of controls should be included and background staining, stickiness, etc. If nonspecific data collected with every experiment: compensation staining is suspected, the use of an Fc-block, such controls and staining controls. as anti-fc receptor (CD6/ CD35) antibodies (for It will become apparent from the discussion below staining mouse cells) might be useful. These antithat the number of control tubes might often exceed bodies are usually applied for 0 or 5 min before the number of sample tubes in a given experiment. It the stains are added. is necessary to invest this significant effort in the Since completely unstained cells have very differcontrols in order to ensure that the valuable sample ent levels of background than cells that have been that is stained with so many different markers does stained with multiple reagents (see Fig. 4 and its not suffer from experimental artefacts, or become discussion), the best control for any given marker of uninterpretable. interest in a multicolor staining combination is a stain that contains all reagents but the one of interest. 8.. Compensation controls For example, a five-color combination control would include one or more four-color combinations, each For each fluorochrome used in an experiment, one leaving out one of the reagents in the complete stain. should include a compensation control, i.e., a single In this way, positive versus negative gating can be color stain, for which data are collected. It is not more rigorously determined and applied. In some appropriate to skimp on the number of tubes col- cases, this gate may not be a one-dimensional lected by trying to design stains that can control for (histogram gate), but may require a sloping line in multiple compensation settings in a single tube. two dimensions because of the ever-increasing Ideally, the reagent used for the compensation sam- broadening imposed by compensation as signal ple should be the same as that used in the staining intensity increases. cocktail. For the non-tandem dyes (FITC, PE, TR, APC), however, a reagent that stains brightly on a good number of cells is sufficient and can be used 9. Advantages of PFC instead of a reagent that might only stain a very small subpopulation of cells. In general the best Most of the advantages of increasing the number result is obtained by using the brightest possible of colors in flow cytometry experiments are readily reagent as the compensation control. In contrast, and apparent. First, the information content increases as outlined above, the tandem-dyes (Cy5PE, Cy7PE, geometrically with the number of parameters simul- Cy7APC, etc.) may exhibit great lot-to-lot variations. taneously analyzed. Second, and just as important, As lot-numbers of the fluorochromes are not usually information can be obtained from multicolor experisupplied with the conjugate if bought from a com- ments that is not available in any other way. For mercial source, each different staining combination example, no combination of one-color stains can should have its own compensation control. For accurately enumerate or be used to isolate
19 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) CD3 CD4 CD82 T cells (excluding, for example, cytometry can result in the unambiguous identifica- CD3 CD4 CD8 T cells and small CD4 mono- tion of rare cell subsets. For example, using eightcytes). Third, detailed immunophenotyping can be color PFC we were able to identify a small populacombined with functional assays (DNA/Cell cycle, tion of follicular B cells in the mouse, with an as yet metabolic activity, cytokine assays, apoptosis assays, unknown function, that expresses high levels of the etc.) to provide a distribution of activities across nonclassical MHC molecule CD (Amano et al., highly defined cell subsets. Fourth, by staining for 998). multiple markers in one cocktail, far fewer samples Other examples include the use of 0-color PFC need to be prepared for each experiment. This is for the identification of antigen-specific lymphocytes. particularly important when analyzing precious sam- Multimers of soluble MHC-class I molecules loaded ples (e.g., pediatric samples, leukocytes isolated from with a peptide of known origin are now often used to biopsies, rare antigen-specific lymphocytes, mouse identify antigen-specific CD8 T cells in human tissues that yield small numbers of cells). And fifth, blood or various tissues in the mouse (Altman et al., less total reagents are used, since less duplication of 996). As the number of antigen-specific T cells is the same reagent among multiple tubes is required. often very small, even a small amount of contamina- A number of technical developments have also tion can lead to considerable errors in the quantitaallowed the increasing use of flow cytometry for tion of these cells which makes further analysis extensive functional studies on cells. Increasing the inaccurate. We recently showed that the use of PFC number of simultaneously measured parameters is allows the identification and functional characterizaparticularly useful for these applications, since one tion of gd T cells specific for a nonclassical MHC can combine detailed immunophenotyping with func- molecule T22. gd T cells constitute roughly % of tional studies. One example is the use of cytoplasmic the splenic cell population of which about 0.5%, or cytokine staining for the identification of cytokine 0.005% of all spleen cells, bind to the T22 tetramer profiles among bulk populations of lymphocytes. By (Crowley et al., 999). Using multicolor cytometry combining antibodies against a number of cell we could show not only that those cells bind to the surface markers together with antibodies specific for MHC multimer, but also that they alter their surface cytokines, we recently demonstrated that populations expression of activation markers such as CD69 and of human peripheral blood T cells classified by their CD62L after binding the MHC multimer (Crowley et differential expression of various activation al., 999). markers differ in their relative frequencies of IL-4 In a separate study, we also used the MHC and IFN-g producers. Importantly, the relative fre- multimer approach to identify potentially tumor-reacquencies of these phenotypically and functionally tive cytotoxic T cells in patients with metastatic distinct T cell subpopulations in the blood differed melanoma (Lee et al., 999). Because of the rarity of among people with various disease states (Mitra et these cells (0 to 0 of PBMC), standard al., 999). In the future, the use of additional techniques are unable to derive much information antibodies against chemokine- and homing-receptors about these cells. We were able to quantitate the will likely lead to the identification of further T cell expression of nearly 40 different cell surface ansubsets. When combined with antibodies against a tigens (to accurately pinpoint the T cell subset to number of cytokines, this is likely to yield further which the antigen-specific T cells belong), as well as important and novel information regarding the rela- determine their cytokine profile, showing that these tionship between T cell phenotype, function and the cells are functionally anergic (whereas viral antigenmigration pattern of these cells. Eventually, this specific T cells from the same patient samples were might lead to the identification of correlates of normally functional). We were even able to perform disease pathogenesis that will provide important serological T cell receptor repertoire measurement to information on which to base therapeutic interven- demonstrate that, in one patient, these cells (contions. stituting 2% of all CD8 T cells) expressed only a The increased quality of the information obtained single Vb gene. with PFC compared with two- or three-color flow Although in routine use for only 2 years, -color
20 96 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) PFC has proven to be a most valuable tool for the and Susan Wormsley, to PharMingen and to Becton study of different aspects of cellular immunology. Dickinson for significantly supporting our reagent These successes are almost certainly a harbinger of development program; and to both Becton Dickinson the new age of cytometry that we will enter as and Cytomation for instrumentation support. The instrumentation to support true multicolor flow cyto- vav2/ 2 mice were provided by the Crabtree laborametric measurements become commercially avail- tory at Stanford. Finally, we wish to thank the able. This new age will encompass considerably Herzenberg laboratory and the flow cytometry commore complex experiments, data analysis, and hur- munity at large for their support, enthusiasm, and dles. In the end, however, these complex analyses valuable ideas. will yield a more complete understanding of biology, especially immunobiology. Ultimately, PFC is a tool that will simplify complex biological processes so References that we may better comprehend their interactions, their functions, and their role in biology. Altman, J.D., Moss, P.A.H., Goulder, P.J.R., Barouch, D.H., We hope that with this brief outline on the McHeyzer-Williams, M.G., Bell, J.I., McMichael, A.J., Davis, advantages and pitfalls of multicolor flow cytometry M.M., 996. Phenotypic analysis of antigen-specific T lym- phocytes. Science 274, we can encourage and provide some guidance to an Amano, M., Baumgarth, N., Dck, M.D., Brossay, L., Kronenberg, increasing number of users for whom flow cytometry M., Herzenberg, L.A., Strober, S., 998. CD expression presents a new technology that allows them to defines subsets of follicular and marginal zone B cells in the address previously unanswerable questions. spleen: b2-microglobulin-dependent and independent forms. J. Immunol. 6, Bigos, M., Baumgarth, N., Jager, G.C., Herman, O.C., Nozaki, T., Acknowledgements Stovel, R.T., Parks, D.R., Herzenberg, L.A., 999. Nine color eleven parameter immunophenotyping using three laser flow cytometry. Cytometry 36, The development of multicolor flow cytometry Crowley, M.P., Fahrer, A.M., Baumgarth, N., Hampl, J., spanned more than half a decade and represents the Gutgemann, I., Teyton, L., Chien, L.-h., 999. A population of murine gd T cells that recognize a nonclassical MHC class I efforts of a large number of individuals. We thank in molecule. Science 287, 34. particular Drs. Leonard and Leonore Herzenberg in Kantor, A., Roederer, M., 997. FACS analysis of leukocytes. In: whose laboratory both fluorescence-activated flow Herzenberg, L.A., Weir, D.M., Herzenberg, L.A., Blackwell, cytometry and Polychromatic Flow Cytometry were C. (Eds.), Handbook of Experimental Immunology, 5th Edi- born. The work, continuous drive and implementa- tion. Blackwell Science, Cambridge, pp Kantor, A.B., Herzenberg, L.A., 993. Origin of murine B cell tion of technical improvements by Dr. David Parks, lineages. Annu. Rev. Immunol., Marty Bigos, Richard Stovel, Tom Nozaki, Wayne Lee, P.P., Yee, C., Savage, P.A., Fong, L., Brockstedt, D., Weber, Moore, and Adam Treister have led to the develop- J.S., Johnson, D., Swetter, S., Thompson, J., Greenberg, P.D., ment of both the hardware and software tools Roederer, M., Davis, M.M., 999. Characterization of circulat- necessary to make this technology work. We thank ing T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 5, Drs. Alan Waggoner, Aaron Kantor, Rachel Gerstein, Mitra, D.K., Rosa, S.C.D., Luk, A., Balamurugan, A., Khaitan, Michael Anderson, and Richard Haugland for their B.K., Tung, J., Mehra, N.K., Terr, A.I., O Garra, A., Herzecollaborative efforts in the development of many of nberg, L.A., Herzenberg, L.A., Roederer, M., 999. Differential the new fluorochromes we employ, as well as Drs. representations of memory T cell subsets are characteristic Randy Hardy and Alan Stall, who developed the of polarized immunity in Leprosy and atopic diseases. Int. Immunol., conjugation protocols that we routinely use. We also Roederer, M., 997a. Methods for fluorescent conjugation of like to acknowledge Drs. Stephen De Rosa, Dipendra monoclonal antibodies. abcon. Mitra, and Nobukazu Watanabe, who spearheaded Roederer, M., 997b. Compensation in flow cytometry: a perspective. many of the early research projects that helped focus compensation. the development efforts, and Gina Jaeger, Ometa Roederer, M., 999. Compensation. In: Robinson, J.P., Darzynk- iewicz, Z., Dean, P.N., Dressler, L.G., Rabinovitch, P.S., Herman, and Iwan Tjioe for expert technical assis- Stewart, C.C., Tanke, H.J., Wheeless, L.L. (Eds.), Current tance. We are especially grateful to Dr. Ernie Huang Protocols in Cytometry. Wiley, New York, in press.
21 N. Baumgarth, M. Roederer / Journal of Immunological Methods 243 (2000) Roederer, M., DeRosa, S., Gerstein, R., Anderson, M., Bigos, M., levels during the development of autoimmunity in MRL/ lpr Stovel, R., Nozaki, T., Parks, D., Herzenberg, L., Herzenberg, mice. J. Immunol. 59, L., Color, 0-parameter flow cytometry to elucidate Wells, S.M., Kantor, A.B., Stall, A.M., 994. CD43 (S7) exprescomplex leukocyte heterogeneity. Cytometry 29, 2. sion identifies peripheral B cell subsets. J. Immunol. 53, Shapiro, H.M., 994. In: Practical Flow Cytometry. Wiley-Liss, New York. Zhang, R., Alt, F.W., Davidson, L., Orkin, S.H., Swat, W., 995. Takahashi, K., Kozono, Y., Waldschmidt, T.J., Berthiaume, D., Defective signaling through the T- and B-cell antigen re- Quigg, R.J., Baron, A., Holers, V.M., 997. Mouse comple- ceptors in lymphoid cells lacking the vav proto-oncogene. ment receptors type (CR;CD35) and type 2 (CR2;CD2). Nature 374, Expression on normal B cell subopulations and decreased
Introduction to flow cytometry
 Introduction to flow cytometry Flow cytometry is a popular laser-based technology. Discover more with our introduction to flow cytometry. Flow cytometry is now a widely used method for analyzing the expression
Introduction to flow cytometry Flow cytometry is a popular laser-based technology. Discover more with our introduction to flow cytometry. Flow cytometry is now a widely used method for analyzing the expression
APPLICATION INFORMATION
 DRAFT: Rev. D A-2045A APPLICATION INFORMATION Flow Cytometry 3-COLOR COMPENSATION Raquel Cabana,* Mark Cheetham, Jay Enten, Yong Song, Michael Thomas,* and Brendan S. Yee Beckman Coulter, Inc., Miami FL
DRAFT: Rev. D A-2045A APPLICATION INFORMATION Flow Cytometry 3-COLOR COMPENSATION Raquel Cabana,* Mark Cheetham, Jay Enten, Yong Song, Michael Thomas,* and Brendan S. Yee Beckman Coulter, Inc., Miami FL
Selected Topics in Electrical Engineering: Flow Cytometry Data Analysis
 Selected Topics in Electrical Engineering: Flow Cytometry Data Analysis Bilge Karaçalı, PhD Department of Electrical and Electronics Engineering Izmir Institute of Technology Outline Compensation and gating
Selected Topics in Electrical Engineering: Flow Cytometry Data Analysis Bilge Karaçalı, PhD Department of Electrical and Electronics Engineering Izmir Institute of Technology Outline Compensation and gating
Compensation in Flow Cytometry
 Compensation in UNIT.4 The term compensation, as it applies to flow cytometric analysis, refers to the process of correcting for fluorescence spillover, i.e., removing the signal of any given fluorochrome
Compensation in UNIT.4 The term compensation, as it applies to flow cytometric analysis, refers to the process of correcting for fluorescence spillover, i.e., removing the signal of any given fluorochrome
Three Rules for Compensation Controls
 From FlowJo s Daily Dongle Three Rules for Compensation Controls First and foremost, there must be a single-stained control for every parameter in the experiment! In addition, there are three rules for
From FlowJo s Daily Dongle Three Rules for Compensation Controls First and foremost, there must be a single-stained control for every parameter in the experiment! In addition, there are three rules for
Application Note. Selecting Reagents for Multicolor Flow Cytometry. Holden Maecker and Joe Trotter BD Biosciences, San Jose
 Selecting Reagents for Multicolor Flow Cytometry Holden Maecker and Joe Trotter BD Biosciences, San Jose Contents 1 The basics: Know your instrument 2 Fluorochromes: Go for the bright 3 Colors and specificities:
Selecting Reagents for Multicolor Flow Cytometry Holden Maecker and Joe Trotter BD Biosciences, San Jose Contents 1 The basics: Know your instrument 2 Fluorochromes: Go for the bright 3 Colors and specificities:
Multicolor Flow Cytometry: Setup and Optimization on the BD Accuri C6 Flow Cytometer
 Multicolor Flow Cytometry: Setup and Optimization on the BD Accuri C6 Flow Cytometer Presented by Clare Rogers, MS Senior Marketing Applications Specialist BD Biosciences 23-13660-00 Webinar Overview Multicolor
Multicolor Flow Cytometry: Setup and Optimization on the BD Accuri C6 Flow Cytometer Presented by Clare Rogers, MS Senior Marketing Applications Specialist BD Biosciences 23-13660-00 Webinar Overview Multicolor
How To Read Flow Cytometry Data
 26 Nature Publishing Group http://www.nature.com/natureimmunology Interpreting flow cytometry data: a guide for the perplexed Leonore A Herzenberg, James Tung, Wayne A Moore, Leonard A Herzenberg & David
26 Nature Publishing Group http://www.nature.com/natureimmunology Interpreting flow cytometry data: a guide for the perplexed Leonore A Herzenberg, James Tung, Wayne A Moore, Leonard A Herzenberg & David
Introduction to Flow Cytometry
 Introduction to Flow Cytometry presented by: Flow Cytometry y Core Facility Biomedical Instrumentation Center Uniformed Services University Topics Covered in this Lecture What is flow cytometry? Flow cytometer
Introduction to Flow Cytometry presented by: Flow Cytometry y Core Facility Biomedical Instrumentation Center Uniformed Services University Topics Covered in this Lecture What is flow cytometry? Flow cytometer
Compensation Controls Data Visualization Panel Development Gating Quantum Dots
 Compensation Controls Data Visualization Panel Development Gating Quantum Dots FITC Single Stain Control FITC PE Argon Laser FL1 FL2 450 500 550 600 FITC Compensation Control Unwanted signal detected in
Compensation Controls Data Visualization Panel Development Gating Quantum Dots FITC Single Stain Control FITC PE Argon Laser FL1 FL2 450 500 550 600 FITC Compensation Control Unwanted signal detected in
Technical Bulletin. An Introduction to Compensation for Multicolor Assays on Digital Flow Cytometers
 An Introduction to Compensation for Multicolor Assays on Digital Flow Cytometers BD Biosciences, San Jose, CA Contents 2 Introduction to compensation 5 Tips for setting up compensation 10 Tools for easy,
An Introduction to Compensation for Multicolor Assays on Digital Flow Cytometers BD Biosciences, San Jose, CA Contents 2 Introduction to compensation 5 Tips for setting up compensation 10 Tools for easy,
Katharina Lückerath (AG Dr. Martin Zörnig) adapted from Dr. Jörg Hildmann BD Biosciences,Customer Service
 Introduction into Flow Cytometry Katharina Lückerath (AG Dr. Martin Zörnig) adapted from Dr. Jörg Hildmann BD Biosciences,Customer Service How does a FACS look like? FACSCalibur FACScan What is Flow Cytometry?
Introduction into Flow Cytometry Katharina Lückerath (AG Dr. Martin Zörnig) adapted from Dr. Jörg Hildmann BD Biosciences,Customer Service How does a FACS look like? FACSCalibur FACScan What is Flow Cytometry?
Using the BD TM Cytometer Setup and Tracking (CS&T) System for Instrument Characterization and Performance Tracking
 Using the BD TM Cytometer Setup and Tracking (CS&T) System for Instrument Characterization and Performance Tracking Mark KuKuruga Senior Technical Applications Specialist BD Biosciences 23-14462-00 Outline
Using the BD TM Cytometer Setup and Tracking (CS&T) System for Instrument Characterization and Performance Tracking Mark KuKuruga Senior Technical Applications Specialist BD Biosciences 23-14462-00 Outline
Immunophenotyping peripheral blood cells
 IMMUNOPHENOTYPING Attune Accoustic Focusing Cytometer Immunophenotyping peripheral blood cells A no-lyse, no-wash, no cell loss method for immunophenotyping nucleated peripheral blood cells using the Attune
IMMUNOPHENOTYPING Attune Accoustic Focusing Cytometer Immunophenotyping peripheral blood cells A no-lyse, no-wash, no cell loss method for immunophenotyping nucleated peripheral blood cells using the Attune
Fluorescence Compensation. Jennifer Wilshire, PhD [email protected]
 Fluorescence Compensation Jennifer Wilshire, PhD [email protected] Outline 1. Compensation Basics 2. Practical Applications -selecting comp controls (cells, stains?) 3. Multicolor Issues -spreading due
Fluorescence Compensation Jennifer Wilshire, PhD [email protected] Outline 1. Compensation Basics 2. Practical Applications -selecting comp controls (cells, stains?) 3. Multicolor Issues -spreading due
COMPENSATION MIT Flow Cytometry Core Facility
 COMPENSATION MIT Flow Cytometry Core Facility Why do we need compensation? 1) Because the long emission spectrum tail of dyes causes overlap like with the fluorophores FITC and PE. 2) For sensitivity reasons,
COMPENSATION MIT Flow Cytometry Core Facility Why do we need compensation? 1) Because the long emission spectrum tail of dyes causes overlap like with the fluorophores FITC and PE. 2) For sensitivity reasons,
Using BD FACSDiva CST To. Evaluate Cytometer Performance, Create Custom Assay Settings. and
 Using BD FACSDiva CST To Evaluate Cytometer Performance, Create Custom Assay Settings and Implement Cross-Instrument and Cross-Site Standardization of Assays PART 2 Alan M. Stall Director, Advanced Cytometry
Using BD FACSDiva CST To Evaluate Cytometer Performance, Create Custom Assay Settings and Implement Cross-Instrument and Cross-Site Standardization of Assays PART 2 Alan M. Stall Director, Advanced Cytometry
BD FACSComp Software Tutorial
 BD FACSComp Software Tutorial This tutorial guides you through a BD FACSComp software lyse/no-wash assay setup run. If you are already familiar with previous versions of BD FACSComp software on Mac OS
BD FACSComp Software Tutorial This tutorial guides you through a BD FACSComp software lyse/no-wash assay setup run. If you are already familiar with previous versions of BD FACSComp software on Mac OS
Compensation Basics - Bagwell. Compensation Basics. C. Bruce Bagwell MD, Ph.D. Verity Software House, Inc.
 Compensation Basics C. Bruce Bagwell MD, Ph.D. Verity Software House, Inc. 2003 1 Intrinsic or Autofluorescence p2 ac 1,2 c 1 ac 1,1 p1 In order to describe how the general process of signal cross-over
Compensation Basics C. Bruce Bagwell MD, Ph.D. Verity Software House, Inc. 2003 1 Intrinsic or Autofluorescence p2 ac 1,2 c 1 ac 1,1 p1 In order to describe how the general process of signal cross-over
These particles have something in common
 These particles have something in common Blood cells Chromosomes Algae Protozoa Certain parameters of these particles can be measured with a flow cytometer Which parameters can be measured? the relative
These particles have something in common Blood cells Chromosomes Algae Protozoa Certain parameters of these particles can be measured with a flow cytometer Which parameters can be measured? the relative
MEASURABLE PARAMETERS: Flow cytometers are capable of measuring a variety of cellular characteristics such as:
 INTRODUCTION Flow Cytometry involves the use of a beam of laser light projected through a liquid stream that contains cells, or other particles, which when struck by the focused light give out signals
INTRODUCTION Flow Cytometry involves the use of a beam of laser light projected through a liquid stream that contains cells, or other particles, which when struck by the focused light give out signals
Standardization, Calibration and Quality Control
 Standardization, Calibration and Quality Control Ian Storie Flow cytometry has become an essential tool in the research and clinical diagnostic laboratory. The range of available flow-based diagnostic
Standardization, Calibration and Quality Control Ian Storie Flow cytometry has become an essential tool in the research and clinical diagnostic laboratory. The range of available flow-based diagnostic
No-wash, no-lyse detection of leukocytes in human whole blood on the Attune NxT Flow Cytometer
 APPLICATION NOTE Attune NxT Flow Cytometer No-wash, no-lyse detection of leukocytes in human whole blood on the Attune NxT Flow Cytometer Introduction Standard methods for isolating and detecting leukocytes
APPLICATION NOTE Attune NxT Flow Cytometer No-wash, no-lyse detection of leukocytes in human whole blood on the Attune NxT Flow Cytometer Introduction Standard methods for isolating and detecting leukocytes
123count ebeads Catalog Number: 01-1234 Also known as: Absolute cell count beads GPR: General Purpose Reagents. For Laboratory Use.
 Page 1 of 1 Catalog Number: 01-1234 Also known as: Absolute cell count beads GPR: General Purpose Reagents. For Laboratory Use. Normal human peripheral blood was stained with Anti- Human CD45 PE (cat.
Page 1 of 1 Catalog Number: 01-1234 Also known as: Absolute cell count beads GPR: General Purpose Reagents. For Laboratory Use. Normal human peripheral blood was stained with Anti- Human CD45 PE (cat.
Principles of Flowcytometry
 Objectives Introduction to Cell Markers: Principles of Flowcytometry Michelle Petrasich NZIMLS Scientific Meeting August 24, 2010, Paihia What are cell markers How do we detect them Production of Monoclonal
Objectives Introduction to Cell Markers: Principles of Flowcytometry Michelle Petrasich NZIMLS Scientific Meeting August 24, 2010, Paihia What are cell markers How do we detect them Production of Monoclonal
CELL CYCLE BASICS. G0/1 = 1X S Phase G2/M = 2X DYE FLUORESCENCE
 CELL CYCLE BASICS Analysis of a population of cells replication state can be achieved by fluorescence labeling of the nuclei of cells in suspension and then analyzing the fluorescence properties of each
CELL CYCLE BASICS Analysis of a population of cells replication state can be achieved by fluorescence labeling of the nuclei of cells in suspension and then analyzing the fluorescence properties of each
BD FACSDiva 4.1 - TUTORIAL TSRI FLOW CYTOMETRY CORE FACILITY
 BD FACSDiva 4.1 - TUTORIAL TSRI FLOW CYTOMETRY CORE FACILITY IMPORTANT NOTES BEFORE READING THIS TUTORIAL This is a very expensive piece of equipment so PLEASE treat it with respect! After you are done
BD FACSDiva 4.1 - TUTORIAL TSRI FLOW CYTOMETRY CORE FACILITY IMPORTANT NOTES BEFORE READING THIS TUTORIAL This is a very expensive piece of equipment so PLEASE treat it with respect! After you are done
Flow Data Analysis. Qianjun Zhang Application Scientist, Tree Star Inc. Oregon, USA FLOWJO CYTOMETRY DATA ANALYSIS SOFTWARE
 Flow Data Analysis Qianjun Zhang Application Scientist, Tree Star Inc. Oregon, USA Flow Data Analysis From Cells to Data - data measurement and standard Data display and gating - Data Display options -
Flow Data Analysis Qianjun Zhang Application Scientist, Tree Star Inc. Oregon, USA Flow Data Analysis From Cells to Data - data measurement and standard Data display and gating - Data Display options -
Zecotek S Light Projection Network Marketing
 White Paper Zecotek Visible Fiber Laser Platform Enabling the future of laser technology Zecotek Photonics Inc. (TSX- V: ZMS; Frankfurt: W1I) www.zecotek.com is a Canadian photonics technology company
White Paper Zecotek Visible Fiber Laser Platform Enabling the future of laser technology Zecotek Photonics Inc. (TSX- V: ZMS; Frankfurt: W1I) www.zecotek.com is a Canadian photonics technology company
Introduction to Flow Cytometry
 Outline Introduction to Flow Cytometry Basic Concept of Flow Cytometry Introduction to Instrument Subsystems Daisy Kuo Assistant Product Manager E-mail: [email protected] BDBiosciences Application Examples
Outline Introduction to Flow Cytometry Basic Concept of Flow Cytometry Introduction to Instrument Subsystems Daisy Kuo Assistant Product Manager E-mail: [email protected] BDBiosciences Application Examples
Instrument Characterization and Performance Tracking for Digital Flow Cytometers
 Instrument Characterization and Performance Tracking for Digital Flow Cytometers BD Biosciences Cytometer Set-up & Tracking (CS&T) System For Research Use Only. Not for use in diagnostic or therapeutic
Instrument Characterization and Performance Tracking for Digital Flow Cytometers BD Biosciences Cytometer Set-up & Tracking (CS&T) System For Research Use Only. Not for use in diagnostic or therapeutic
Notes on titering antibodies
 Home Events This Week Bulletin Boards Lab Personnel Lab Policies Protocols MolBio Tetramers Proteins Flow Cytometry Immunoassays Instruments Computers Lab Publications Scientiific Literature Science on
Home Events This Week Bulletin Boards Lab Personnel Lab Policies Protocols MolBio Tetramers Proteins Flow Cytometry Immunoassays Instruments Computers Lab Publications Scientiific Literature Science on
Technical Note. Roche Applied Science. No. LC 19/2004. Color Compensation
 Roche Applied Science Technical Note No. LC 19/2004 Purpose of this Note Color The LightCycler System is able to simultaneously detect and analyze more than one color in each capillary. Due to overlap
Roche Applied Science Technical Note No. LC 19/2004 Purpose of this Note Color The LightCycler System is able to simultaneously detect and analyze more than one color in each capillary. Due to overlap
Flow Cytometry A Basic Overview
 Flow Cytometry A Basic Overview Overview Flow cytometry is a powerful technology for investigating many aspects of cell biology and for isolating cells of interest. Flow cytometry utilizes highly focused,
Flow Cytometry A Basic Overview Overview Flow cytometry is a powerful technology for investigating many aspects of cell biology and for isolating cells of interest. Flow cytometry utilizes highly focused,
CyAn : 11 Parameter Desktop Flow Cytometer
 CyAn : 11 Parameter Desktop Flow Cytometer Cyan ADP 3 excitation lines 488nm, 635nm, and UV or violet 11 simultaneous parameters FSC, SSC, and 7-9 colors with simultaneous width, peak, area, and log on
CyAn : 11 Parameter Desktop Flow Cytometer Cyan ADP 3 excitation lines 488nm, 635nm, and UV or violet 11 simultaneous parameters FSC, SSC, and 7-9 colors with simultaneous width, peak, area, and log on
Uses of Flow Cytometry
 Uses of Flow Cytometry 1. Multicolour analysis... 2 2. Cell Cycle and Proliferation... 3 a. Analysis of Cellular DNA Content... 4 b. Cell Proliferation Assays... 5 3. Immunology... 6 4. Apoptosis... 7
Uses of Flow Cytometry 1. Multicolour analysis... 2 2. Cell Cycle and Proliferation... 3 a. Analysis of Cellular DNA Content... 4 b. Cell Proliferation Assays... 5 3. Immunology... 6 4. Apoptosis... 7
Using CyTOF Data with FlowJo Version 10.0.7. Revised 2/3/14
 Using CyTOF Data with FlowJo Version 10.0.7 Revised 2/3/14 Table of Contents 1. Background 2. Scaling and Display Preferences 2.1 Cytometer Based Preferences 2.2 Useful Display Preferences 3. Scale and
Using CyTOF Data with FlowJo Version 10.0.7 Revised 2/3/14 Table of Contents 1. Background 2. Scaling and Display Preferences 2.1 Cytometer Based Preferences 2.2 Useful Display Preferences 3. Scale and
Immunophenotyping Flow Cytometry Tutorial. Contents. Experimental Requirements...1. Data Storage...2. Voltage Adjustments...3. Compensation...
 Immunophenotyping Flow Cytometry Tutorial Contents Experimental Requirements...1 Data Storage...2 Voltage Adjustments...3 Compensation...5 Experimental Requirements For immunophenotyping with FITC and
Immunophenotyping Flow Cytometry Tutorial Contents Experimental Requirements...1 Data Storage...2 Voltage Adjustments...3 Compensation...5 Experimental Requirements For immunophenotyping with FITC and
The NIAID Flow Cytometry Advisory Committee; the Guidelines Subcommittee
 January, 1999 From: To: Subject: The NIAID Flow Cytometry Advisory Committee; the Guidelines Subcommittee NIAID DAIDS Flow Cytometry Laboratories Comparison study information for labs wishing to switch
January, 1999 From: To: Subject: The NIAID Flow Cytometry Advisory Committee; the Guidelines Subcommittee NIAID DAIDS Flow Cytometry Laboratories Comparison study information for labs wishing to switch
CELL CYCLE BASICS. G0/1 = 1X S Phase G2/M = 2X DYE FLUORESCENCE
 CELL CYCLE BASICS Analysis of a population of cells replication state can be achieved by fluorescence labeling of the nuclei of cells in suspension and then analyzing the fluorescence properties of each
CELL CYCLE BASICS Analysis of a population of cells replication state can be achieved by fluorescence labeling of the nuclei of cells in suspension and then analyzing the fluorescence properties of each
CyTOF2. Mass cytometry system. Unveil new cell types and function with high-parameter protein detection
 CyTOF2 Mass cytometry system Unveil new cell types and function with high-parameter protein detection DISCOVER MORE. IMAGINE MORE. MASS CYTOMETRY. THE FUTURE OF CYTOMETRY TODAY. Mass cytometry resolves
CyTOF2 Mass cytometry system Unveil new cell types and function with high-parameter protein detection DISCOVER MORE. IMAGINE MORE. MASS CYTOMETRY. THE FUTURE OF CYTOMETRY TODAY. Mass cytometry resolves
Technical Bulletin. Threshold and Analysis of Small Particles on the BD Accuri C6 Flow Cytometer
 Threshold and Analysis of Small Particles on the BD Accuri C6 Flow Cytometer Contents 2 Thresholds 2 Setting the Threshold When analyzing small particles, defined as particles smaller than 3.0 µm, on the
Threshold and Analysis of Small Particles on the BD Accuri C6 Flow Cytometer Contents 2 Thresholds 2 Setting the Threshold When analyzing small particles, defined as particles smaller than 3.0 µm, on the
Below is a list of things you should be aware of before you schedule your sort.
 Sorting at the Flow Cytometry Facility At the present time, the assistance of a trained cell sorter operator is needed for all sorting applications. As such, if you are planning a first time sort you will
Sorting at the Flow Cytometry Facility At the present time, the assistance of a trained cell sorter operator is needed for all sorting applications. As such, if you are planning a first time sort you will
Chapter 6. Antigen-Antibody Properties 10/3/2012. Antigen-Antibody Interactions: Principles and Applications. Precipitin reactions
 Chapter 6 Antigen-Antibody Interactions: Principles and Applications Antigen-Antibody Properties You must remember antibody affinity (single) VS avidity (multiple) High affinity: bound tightly and longer!
Chapter 6 Antigen-Antibody Interactions: Principles and Applications Antigen-Antibody Properties You must remember antibody affinity (single) VS avidity (multiple) High affinity: bound tightly and longer!
FlowSight. Flow cytometry with vision
 FlowSight Flow cytometry with vision Flow cytometry with vision Introducing FlowSight Capable: Sensitive and flexible for every need Intuitive: Easy-to-use, with imagery for every cell Affordable: Designed
FlowSight Flow cytometry with vision Flow cytometry with vision Introducing FlowSight Capable: Sensitive and flexible for every need Intuitive: Easy-to-use, with imagery for every cell Affordable: Designed
Boundary-breaking acoustic focusing cytometry
 Boundary-breaking acoustic focusing cytometry Introducing the Attune NxT Acoustic Focusing Cytometer a high-performance system that s flexible enough for any lab One of the main projects in my laboratory
Boundary-breaking acoustic focusing cytometry Introducing the Attune NxT Acoustic Focusing Cytometer a high-performance system that s flexible enough for any lab One of the main projects in my laboratory
Instructions for Use. CyAn ADP. High-speed Analyzer. Summit 4.3. 0000050G June 2008. Beckman Coulter, Inc. 4300 N. Harbor Blvd. Fullerton, CA 92835
 Instructions for Use CyAn ADP High-speed Analyzer Summit 4.3 0000050G June 2008 Beckman Coulter, Inc. 4300 N. Harbor Blvd. Fullerton, CA 92835 Overview Summit software is a Windows based application that
Instructions for Use CyAn ADP High-speed Analyzer Summit 4.3 0000050G June 2008 Beckman Coulter, Inc. 4300 N. Harbor Blvd. Fullerton, CA 92835 Overview Summit software is a Windows based application that
Compensation: An Instrumental Perspective
 Compensation: An Instrumental Perspective Why Digital? Visualization Issues Boston User s Group September 10, 2003 Joe Trotter [email protected] Review: Instrument Sensitivity Measuring Sensitivity -
Compensation: An Instrumental Perspective Why Digital? Visualization Issues Boston User s Group September 10, 2003 Joe Trotter [email protected] Review: Instrument Sensitivity Measuring Sensitivity -
Introduction to Flow Cytometry:
 Introduction to Flow Cytometry: A Learning Guide Manual Part Number: 11-11032-01 April, 2000 BD Biosciences 2350 Qume Drive San Jose, CA 95131-1807 1-800-448-2347 Introduction to Flow Cytometry: A Learning
Introduction to Flow Cytometry: A Learning Guide Manual Part Number: 11-11032-01 April, 2000 BD Biosciences 2350 Qume Drive San Jose, CA 95131-1807 1-800-448-2347 Introduction to Flow Cytometry: A Learning
Flow Cytometry for Everyone Else Susan McQuiston, J.D., MLS(ASCP), C.Cy.
 Flow Cytometry for Everyone Else Susan McQuiston, J.D., MLS(ASCP), C.Cy. At the end of this session, the participant will be able to: 1. Describe the components of a flow cytometer 2. Describe the gating
Flow Cytometry for Everyone Else Susan McQuiston, J.D., MLS(ASCP), C.Cy. At the end of this session, the participant will be able to: 1. Describe the components of a flow cytometer 2. Describe the gating
DNA Detection. Chapter 13
 DNA Detection Chapter 13 Detecting DNA molecules Once you have your DNA separated by size Now you need to be able to visualize the DNA on the gel somehow Original techniques: Radioactive label, silver
DNA Detection Chapter 13 Detecting DNA molecules Once you have your DNA separated by size Now you need to be able to visualize the DNA on the gel somehow Original techniques: Radioactive label, silver
Standard Operating Procedure
 1.0 Purpose: 1.1 The characterisation of of main leukocyte subsets in peripheral blood cells from mice by flow cytometry. Reliable values of frequencies of leukocyte clusters are very much dependent on
1.0 Purpose: 1.1 The characterisation of of main leukocyte subsets in peripheral blood cells from mice by flow cytometry. Reliable values of frequencies of leukocyte clusters are very much dependent on
Cell Cycle Tutorial. Contents
 Cell Cycle Tutorial Contents Experimental Requirements...2 DNA Dyes...2 Protocols...3 PI Parameter & Analysis Setup...4 PI Voltage Adjustments...6 7-AAD Parameter Setup...6 To-Pro3 Parameter Setup...6
Cell Cycle Tutorial Contents Experimental Requirements...2 DNA Dyes...2 Protocols...3 PI Parameter & Analysis Setup...4 PI Voltage Adjustments...6 7-AAD Parameter Setup...6 To-Pro3 Parameter Setup...6
Flow Cytometry. What is Flow Cytometry? What Can a Flow Cytometer Tell Us About a Cell? Particle Size. Flow = Fluid Cyto = Cell Metry = Measurement
 What is Flow Cytometry? Flow Cytometry Basic Principle and Applications BD Biosciences Daisy Kuo ([email protected]) Flow = Fluid Cyto = Cell Metry = Measurement A variety of measurements are made on cells,
What is Flow Cytometry? Flow Cytometry Basic Principle and Applications BD Biosciences Daisy Kuo ([email protected]) Flow = Fluid Cyto = Cell Metry = Measurement A variety of measurements are made on cells,
Four Color Compensation
 Cytometry (Communications in Clinical Cytometry) 38:161 175 (1999) Four Color Compensation Carleton C. Stewart* and Sigrid J. Stewart Laboratory of Flow Cytometry, Roswell Park Cancer Institute, Buffalo,
Cytometry (Communications in Clinical Cytometry) 38:161 175 (1999) Four Color Compensation Carleton C. Stewart* and Sigrid J. Stewart Laboratory of Flow Cytometry, Roswell Park Cancer Institute, Buffalo,
Stepcount. Product Description: Closed transparent tubes with a metal screen, including a white matrix at the bottom. Cat. Reference: STP-25T
 Product Description: Closed transparent tubes with a metal screen, including a white matrix at the bottom Cat. Reference: STP-25T Reagent provided:: 25 Stepcount tubes for 25 test INTENDED USE. Immunostep
Product Description: Closed transparent tubes with a metal screen, including a white matrix at the bottom Cat. Reference: STP-25T Reagent provided:: 25 Stepcount tubes for 25 test INTENDED USE. Immunostep
Multicolor Bead Flow Cytometry Standardization Heba Degheidy MD, PhD, QCYM DB/OSEL/CDRH/FDA Manager of MCM Flow Cytometry Facility
 Multicolor Bead Flow Cytometry Standardization Heba Degheidy MD, PhD, QCYM DB/OSEL/CDRH/FDA Manager of MCM Flow Cytometry Facility The mention of commercial products, their sources, or their use in connection
Multicolor Bead Flow Cytometry Standardization Heba Degheidy MD, PhD, QCYM DB/OSEL/CDRH/FDA Manager of MCM Flow Cytometry Facility The mention of commercial products, their sources, or their use in connection
FlowJo Basic Tutorial
 FlowJo Basic Tutorial FlowJo is software for the analysis of flow cytometry data. This tutorial will quickly introduce you to FlowJo using an example experiment, where a FITC anti-cd8 reagent is titrated
FlowJo Basic Tutorial FlowJo is software for the analysis of flow cytometry data. This tutorial will quickly introduce you to FlowJo using an example experiment, where a FITC anti-cd8 reagent is titrated
Introduction to Flow Cytometry -- BD LSR II. What is Flow Cytometry? What Can a Flow Cytometer Tell Us About a Cell? Particle Size
 Introduction to Flow Cytometry -- BD LSR II Daisy Kuo 郭 正 佼 Assistant Product Manager BD Biosciences [email protected] What is Flow Cytometry? Flow = Fluid Cyto = Cell Metry = Measurement A variety of measurements
Introduction to Flow Cytometry -- BD LSR II Daisy Kuo 郭 正 佼 Assistant Product Manager BD Biosciences [email protected] What is Flow Cytometry? Flow = Fluid Cyto = Cell Metry = Measurement A variety of measurements
Hoechst 33342 HSC Staining and Stem Cell Purification Protocol (see Goodell, M., et al. (1996) J Exp Med 183, 1797-806)
 Hoechst 33342 HSC Staining and Stem Cell Purification Protocol (see Goodell, M., et al. (1996) J Exp Med 183, 1797-86) The Hoechst purification was established for murine hematopoietic stem cells (HSC)
Hoechst 33342 HSC Staining and Stem Cell Purification Protocol (see Goodell, M., et al. (1996) J Exp Med 183, 1797-86) The Hoechst purification was established for murine hematopoietic stem cells (HSC)
Flow Cytometry. flow cytometer DNA apoptosis ph
 Flow Cytometry flow cytometer DNA apoptosis ph flow cytometry flow cytometer (a) (b) cell counting instrument (c) 1960 ink-jet technology 17 19 1940 1950 fluorescence microscopy fluorescent dye DNA polyclonal
Flow Cytometry flow cytometer DNA apoptosis ph flow cytometry flow cytometer (a) (b) cell counting instrument (c) 1960 ink-jet technology 17 19 1940 1950 fluorescence microscopy fluorescent dye DNA polyclonal
INSIDE THE BLACK BOX
 FLOW CYTOMETRY ESSENTIALS INSIDE THE BLACK BOX Alice L. Givan Englert Cell Analysis Laboratory of the Norris Cotton Cancer Center Dartmouth Medical School HOW NOT TO BE A FLOW CYTOMETRIST Drawing by Ben
FLOW CYTOMETRY ESSENTIALS INSIDE THE BLACK BOX Alice L. Givan Englert Cell Analysis Laboratory of the Norris Cotton Cancer Center Dartmouth Medical School HOW NOT TO BE A FLOW CYTOMETRIST Drawing by Ben
Zeiss 780 Training Notes
 Zeiss 780 Training Notes 780 Start Up Sequence Do you need the argon laser, 458,488,514nm lines? No Turn on the Systems PC Switch Turn on Main Power Switch Yes Turn on the laser main power switch and turn
Zeiss 780 Training Notes 780 Start Up Sequence Do you need the argon laser, 458,488,514nm lines? No Turn on the Systems PC Switch Turn on Main Power Switch Yes Turn on the laser main power switch and turn
use. 3,5 Apoptosis Viability Titration Assays
 Introduction Monitoring cell viability can provide critical insights into the effectiveness of biological protocols and the stability of biological systems including: the efficacy of cell harvesting procedures,
Introduction Monitoring cell viability can provide critical insights into the effectiveness of biological protocols and the stability of biological systems including: the efficacy of cell harvesting procedures,
Supplementary Material. Free-radical production after post-thaw incubation of ram spermatozoa is related to decreased in vivo fertility
 10.1071/RD14043_AC CSIRO 2015 Supplementary Material: Reproduction, Fertility and Development, 2015, 27(8), 1187 1196. Supplementary Material Free-radical production after post-thaw incubation of ram spermatozoa
10.1071/RD14043_AC CSIRO 2015 Supplementary Material: Reproduction, Fertility and Development, 2015, 27(8), 1187 1196. Supplementary Material Free-radical production after post-thaw incubation of ram spermatozoa
Experiment #5: Qualitative Absorption Spectroscopy
 Experiment #5: Qualitative Absorption Spectroscopy One of the most important areas in the field of analytical chemistry is that of spectroscopy. In general terms, spectroscopy deals with the interactions
Experiment #5: Qualitative Absorption Spectroscopy One of the most important areas in the field of analytical chemistry is that of spectroscopy. In general terms, spectroscopy deals with the interactions
Spherotech, Inc. 27845 Irma Lee Circle, Unit 101, Lake Forest, Illinois 60045 1
 78 Irma Lee Circle, Unit 0, Lake Forest, Illinois 00 SPHERO TM Technical Note STN- Rev B 008 DETERMINING PMT LINEARITY IN FLOW CYTOMETERS USING THE SPHERO TM PMT QUALITY CONTROL EXCEL TEMPLATE Introduction
78 Irma Lee Circle, Unit 0, Lake Forest, Illinois 00 SPHERO TM Technical Note STN- Rev B 008 DETERMINING PMT LINEARITY IN FLOW CYTOMETERS USING THE SPHERO TM PMT QUALITY CONTROL EXCEL TEMPLATE Introduction
Fluorescent dyes for use with the
 Detection of Multiple Reporter Dyes in Real-time, On-line PCR Analysis with the LightCycler System Gregor Sagner, Cornelia Goldstein, and Rob van Miltenburg Roche Molecular Biochemicals, Penzberg, Germany
Detection of Multiple Reporter Dyes in Real-time, On-line PCR Analysis with the LightCycler System Gregor Sagner, Cornelia Goldstein, and Rob van Miltenburg Roche Molecular Biochemicals, Penzberg, Germany
FLOW CYTOMETRY: PRINCIPLES AND APPLICATIONS. By: Douaa Moh. Sayed
 FLOW CYTOMETRY: PRINCIPLES AND APPLICATIONS By: Douaa Moh. Sayed Definition Flow cytometry is a technique for counting, examining, and sorting microscopic particles suspended in a stream of fluid. It allows
FLOW CYTOMETRY: PRINCIPLES AND APPLICATIONS By: Douaa Moh. Sayed Definition Flow cytometry is a technique for counting, examining, and sorting microscopic particles suspended in a stream of fluid. It allows
EdU Flow Cytometry Kit. User Manual
 User Manual Ordering information: (for detailed kit content see Table 2) EdU Flow Cytometry Kits for 50 assays: Product number EdU Used fluorescent dye BCK-FC488-50 10 mg 6-FAM Azide BCK-FC555-50 10 mg
User Manual Ordering information: (for detailed kit content see Table 2) EdU Flow Cytometry Kits for 50 assays: Product number EdU Used fluorescent dye BCK-FC488-50 10 mg 6-FAM Azide BCK-FC555-50 10 mg
PROTOCOL. Immunostaining for Flow Cytometry. Background. Materials and equipment required.
 PROTOCOL Immunostaining for Flow Cytometry 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 Background The combination of single cell analysis using flow cytometry and the specificity of antibody-based
PROTOCOL Immunostaining for Flow Cytometry 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 Background The combination of single cell analysis using flow cytometry and the specificity of antibody-based
Setting up and calibration of a flow cytometer for multicolor immunophenotyping
 Journal of Biological Regulators and Homeostatic Agents Setting up and calibration of a flow cytometer for multicolor immunophenotyping J. KRAAN 1, J.W. GRATAMA 1, M. KEENEY 2, J.L. D HAUTCOURT 3 1 Department
Journal of Biological Regulators and Homeostatic Agents Setting up and calibration of a flow cytometer for multicolor immunophenotyping J. KRAAN 1, J.W. GRATAMA 1, M. KEENEY 2, J.L. D HAUTCOURT 3 1 Department
Flow cytometry basics fluidics, optics, electronics...
 Title Flow cytometry basics fluidics, optics, electronics... RNDr. Jan Svoboda, Ph.D. Cytometry and Microscopy Core Facility IMB, CAS, v.v.i Vídeňská 1083 Fluorescence Fluorescence occurs when a valence
Title Flow cytometry basics fluidics, optics, electronics... RNDr. Jan Svoboda, Ph.D. Cytometry and Microscopy Core Facility IMB, CAS, v.v.i Vídeňská 1083 Fluorescence Fluorescence occurs when a valence
Application Information Bulletin: Set-Up of the CytoFLEX Set-Up of the CytoFLEX* for Extracellular Vesicle Measurement
 Application Information Bulletin: Set-Up of the CytoFLEX Set-Up of the CytoFLEX* for Extracellular Vesicle Measurement Andreas Spittler, MD, Associate Professor for Pathophysiology, Medical University
Application Information Bulletin: Set-Up of the CytoFLEX Set-Up of the CytoFLEX* for Extracellular Vesicle Measurement Andreas Spittler, MD, Associate Professor for Pathophysiology, Medical University
Chapter 12 Filters for FISH Imaging
 Chapter 12 Filters for FISH Imaging Dan Osborn The application of in situ hybridization (ISH) has advanced from short lived, non-specific isotopic methods, to very specific, long lived, multiple color
Chapter 12 Filters for FISH Imaging Dan Osborn The application of in situ hybridization (ISH) has advanced from short lived, non-specific isotopic methods, to very specific, long lived, multiple color
CyFlow SL. Microbiology. Detection and Analysis of Microorganisms and Small Particles
 CyFlow SL Microbiology Detection and Analysis of Microorganisms and Small Particles 01 COMPANY Flow Cytometry made in Germany New sophisticated applications and increasing requirements for reliable results
CyFlow SL Microbiology Detection and Analysis of Microorganisms and Small Particles 01 COMPANY Flow Cytometry made in Germany New sophisticated applications and increasing requirements for reliable results
Automated Quadratic Characterization of Flow Cytometer Instrument Sensitivity (flowqb Package: Introductory Processing Using Data NIH))
 Automated Quadratic Characterization of Flow Cytometer Instrument Sensitivity (flowqb Package: Introductory Processing Using Data NIH)) October 14, 2013 1 Licensing Under the Artistic License, you are
Automated Quadratic Characterization of Flow Cytometer Instrument Sensitivity (flowqb Package: Introductory Processing Using Data NIH)) October 14, 2013 1 Licensing Under the Artistic License, you are
CyFlow SL. Healthcare Immunology. Portable FCM System for 3-colour Immunophenotyping
 CyFlow SL Healthcare Immunology Portable FCM System for 3-colour Immunophenotyping 01 COMPANY Flow Cytometry made in Germany New sophisticated applications and increasing requirements for reliable results
CyFlow SL Healthcare Immunology Portable FCM System for 3-colour Immunophenotyping 01 COMPANY Flow Cytometry made in Germany New sophisticated applications and increasing requirements for reliable results
Lab 2. Isolation of mononuclear cells from peripheral blood and separation into subpopulations
 Lab 2 Isolation of mononuclear cells from peripheral blood and separation into subpopulations Supervisors: Sissela Broos [email protected] tel: 222 96 78 Niclas Olsson [email protected]
Lab 2 Isolation of mononuclear cells from peripheral blood and separation into subpopulations Supervisors: Sissela Broos [email protected] tel: 222 96 78 Niclas Olsson [email protected]
Cytell Cell Imaging System
 Cytell Cell Imaging System Simplify your cell analysis and start changing tomorrow today www.gelifesciences.com/cytell The Cytell Cell Imaging System is an intuitive cell analysis instrument that streamlines
Cytell Cell Imaging System Simplify your cell analysis and start changing tomorrow today www.gelifesciences.com/cytell The Cytell Cell Imaging System is an intuitive cell analysis instrument that streamlines
Improved predictive modeling of white LEDs with accurate luminescence simulation and practical inputs
 Improved predictive modeling of white LEDs with accurate luminescence simulation and practical inputs TracePro Opto-Mechanical Design Software s Fluorescence Property Utility TracePro s Fluorescence Property
Improved predictive modeling of white LEDs with accurate luminescence simulation and practical inputs TracePro Opto-Mechanical Design Software s Fluorescence Property Utility TracePro s Fluorescence Property
Products - Microarray Scanners - SpotLight Two-Color Microarray Fluorescence Scanners New! SpotLight 2 now available!
 Products - Microarray Scanners - SpotLight Two-Color Microarray Fluorescence Scanners New! SpotLight 2 now available! Arrayit SpotLight Two-Color Microarray Scanners provide the market s most affordable
Products - Microarray Scanners - SpotLight Two-Color Microarray Fluorescence Scanners New! SpotLight 2 now available! Arrayit SpotLight Two-Color Microarray Scanners provide the market s most affordable
ArC Amine Reactive Compensation Bead Kit
 ArC Amine Reactive Compensation Bead Kit Catalog no. A1346 Table 1. Contents and storage information. Material Amount Composition Storage Stability ArC reactive beads (Component A) ArC negative beads (Component
ArC Amine Reactive Compensation Bead Kit Catalog no. A1346 Table 1. Contents and storage information. Material Amount Composition Storage Stability ArC reactive beads (Component A) ArC negative beads (Component
PATHOGEN DETECTION SYSTEMS BY REAL TIME PCR. Results Interpretation Guide
 PATHOGEN DETECTION SYSTEMS BY REAL TIME PCR Results Interpretation Guide Pathogen Detection Systems by Real Time PCR Microbial offers real time PCR based systems for the detection of pathogenic bacteria
PATHOGEN DETECTION SYSTEMS BY REAL TIME PCR Results Interpretation Guide Pathogen Detection Systems by Real Time PCR Microbial offers real time PCR based systems for the detection of pathogenic bacteria
Deep profiling of multitube flow cytometry data Supplemental information
 Deep profiling of multitube flow cytometry data Supplemental information Kieran O Neill et al December 19, 2014 1 Table S1: Markers in simulated multitube data. The data was split into three tubes, each
Deep profiling of multitube flow cytometry data Supplemental information Kieran O Neill et al December 19, 2014 1 Table S1: Markers in simulated multitube data. The data was split into three tubes, each
MACSQuantify Software guide. Basics of flow cytometric analysis and software guide. Version 1 EN Original instructions
 MACSQuantify Software guide Basics of flow cytometric analysis and software guide Version 1 EN Original instructions Miltenyi Biotec GmbH Friedrich-Ebert-Straße 68 51429 Bergisch Gladbach Germany Phone:
MACSQuantify Software guide Basics of flow cytometric analysis and software guide Version 1 EN Original instructions Miltenyi Biotec GmbH Friedrich-Ebert-Straße 68 51429 Bergisch Gladbach Germany Phone:
1. Instrument Layout pg 2. 2. Summit Overview pg 3. 3. Data Storage and Data Files pgs 6-7. 4. Saving Data pg 7. 5. Start Up pg 8
 Table of Contents 1. Instrument Layout pg 2 2. Summit Overview pg 3 3. Data Storage and Data Files pgs 6-7 4. Saving Data pg 7 5. Start Up pg 8 6. Choosing and Naming Parameters pg 9 7. Creating Histograms
Table of Contents 1. Instrument Layout pg 2 2. Summit Overview pg 3 3. Data Storage and Data Files pgs 6-7 4. Saving Data pg 7 5. Start Up pg 8 6. Choosing and Naming Parameters pg 9 7. Creating Histograms
Chapter 10 Immunofluorescence
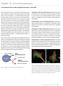 Chapter 10 Immunofluorescence J. Paul Robinson PhD, Jennifer Sturgis BS and George L. Kumar PhD Immunofluorescence (IF) is a common laboratory technique used in almost all aspects of biology. This technique
Chapter 10 Immunofluorescence J. Paul Robinson PhD, Jennifer Sturgis BS and George L. Kumar PhD Immunofluorescence (IF) is a common laboratory technique used in almost all aspects of biology. This technique
Technical Bulletin. Standardizing Application Setup Across Multiple Flow Cytometers Using BD FACSDiva Version 6 Software. Abstract
 arch 212 Standardizing Application Setup Across ultiple Flow Cytometers Using BD FACSDiva Version 6 Ellen einelt, ervi Reunanen, ark Edinger, aria Jaimes, Alan Stall, Dennis Sasaki, Joe Trotter Contents
arch 212 Standardizing Application Setup Across ultiple Flow Cytometers Using BD FACSDiva Version 6 Ellen einelt, ervi Reunanen, ark Edinger, aria Jaimes, Alan Stall, Dennis Sasaki, Joe Trotter Contents
Flow Cytometry Protocols
 METHODS IN MOLECULAR BIOLOGY TM TM Volume 263 Flow Cytometry Protocols SECOND EDITION Edited by Teresa S. Hawley Robert G. Hawley 1 Flow Cytometry An Introduction Alice L. Givan Summary A flow cytometer
METHODS IN MOLECULAR BIOLOGY TM TM Volume 263 Flow Cytometry Protocols SECOND EDITION Edited by Teresa S. Hawley Robert G. Hawley 1 Flow Cytometry An Introduction Alice L. Givan Summary A flow cytometer
A Basic Guide. Institute of Medical Biology, University of Southern Denmark. Ungated Gate 1 (= R1) Gate 4 (=R4) Gate3 (=R3) Gate 2 (= R2) R3 R4
 Flow Cytometry A Basic Guide R1 Ungated Gate 1 (= R1) R2 R3 R4 Gate 2 (= R2) Gate3 (=R3) Gate 4 (=R4) Graham Leslie, PhD Institute of Medical Biology, University of Southern Denmark Graham Leslie, PhD
Flow Cytometry A Basic Guide R1 Ungated Gate 1 (= R1) R2 R3 R4 Gate 2 (= R2) Gate3 (=R3) Gate 4 (=R4) Graham Leslie, PhD Institute of Medical Biology, University of Southern Denmark Graham Leslie, PhD
Technical Note. Roche Applied Science. No. LC 18/2004. Assay Formats for Use in Real-Time PCR
 Roche Applied Science Technical Note No. LC 18/2004 Purpose of this Note Assay Formats for Use in Real-Time PCR The LightCycler Instrument uses several detection channels to monitor the amplification of
Roche Applied Science Technical Note No. LC 18/2004 Purpose of this Note Assay Formats for Use in Real-Time PCR The LightCycler Instrument uses several detection channels to monitor the amplification of
DELPHI 27 V 2016 CYTOMETRY STRATEGIES IN THE DIAGNOSIS OF HEMATOLOGICAL DISEASES
 DELPHI 27 V 2016 CYTOMETRY STRATEGIES IN THE DIAGNOSIS OF HEMATOLOGICAL DISEASES CLAUDIO ORTOLANI UNIVERSITY OF URBINO - ITALY SUN TZU (544 b.c. 496 b.c) SUN TZU (544 b.c. 496 b.c.) THE ART OF CYTOMETRY
DELPHI 27 V 2016 CYTOMETRY STRATEGIES IN THE DIAGNOSIS OF HEMATOLOGICAL DISEASES CLAUDIO ORTOLANI UNIVERSITY OF URBINO - ITALY SUN TZU (544 b.c. 496 b.c) SUN TZU (544 b.c. 496 b.c.) THE ART OF CYTOMETRY
Spectrophotometry and the Beer-Lambert Law: An Important Analytical Technique in Chemistry
 Spectrophotometry and the Beer-Lambert Law: An Important Analytical Technique in Chemistry Jon H. Hardesty, PhD and Bassam Attili, PhD Collin College Department of Chemistry Introduction: In the last lab
Spectrophotometry and the Beer-Lambert Law: An Important Analytical Technique in Chemistry Jon H. Hardesty, PhD and Bassam Attili, PhD Collin College Department of Chemistry Introduction: In the last lab
International Beryllium Conference, Montreal, Canada March 10, 2005
 Alternative Lymphocyte Proliferation Tests: BrdU and Flow Cytometry Based Tests International Beryllium Conference, Montreal, Canada March 10, 2005 Tim K. Takaro Department of Environmental and Occupational
Alternative Lymphocyte Proliferation Tests: BrdU and Flow Cytometry Based Tests International Beryllium Conference, Montreal, Canada March 10, 2005 Tim K. Takaro Department of Environmental and Occupational
BD LSR II and FACSDiVa Software. Dr. Jens Fleischer, Basel Dr. Norbert Leclere,, Berlin
 BD LSR II and FACSDiVa Software, Basel Dr. Norbert Leclere,, Berlin Overview Electronics Covers Fluidics Connectors Controlpanel Sample Port On/Off The Control Panel Ease of Use The basic procedures to
BD LSR II and FACSDiVa Software, Basel Dr. Norbert Leclere,, Berlin Overview Electronics Covers Fluidics Connectors Controlpanel Sample Port On/Off The Control Panel Ease of Use The basic procedures to
Molecular Spectroscopy
 Molecular Spectroscopy UV-Vis Spectroscopy Absorption Characteristics of Some Common Chromophores UV-Vis Spectroscopy Absorption Characteristics of Aromatic Compounds UV-Vis Spectroscopy Effect of extended
Molecular Spectroscopy UV-Vis Spectroscopy Absorption Characteristics of Some Common Chromophores UV-Vis Spectroscopy Absorption Characteristics of Aromatic Compounds UV-Vis Spectroscopy Effect of extended
Fundamentals of modern UV-visible spectroscopy. Presentation Materials
 Fundamentals of modern UV-visible spectroscopy Presentation Materials The Electromagnetic Spectrum E = hν ν = c / λ 1 Electronic Transitions in Formaldehyde 2 Electronic Transitions and Spectra of Atoms
Fundamentals of modern UV-visible spectroscopy Presentation Materials The Electromagnetic Spectrum E = hν ν = c / λ 1 Electronic Transitions in Formaldehyde 2 Electronic Transitions and Spectra of Atoms
7.1 A Wide Variety of Protein Conjugates
 7.1 A Wide Variety of Protein Conjugates Overview of Molecular Probes Protein Conjugates Antibody and Avidin Conjugates The quality of a conjugate depends to a large degree on the quality of the protein
7.1 A Wide Variety of Protein Conjugates Overview of Molecular Probes Protein Conjugates Antibody and Avidin Conjugates The quality of a conjugate depends to a large degree on the quality of the protein
