How To Read Flow Cytometry Data
|
|
|
- Regina Dortha Stewart
- 3 years ago
- Views:
Transcription
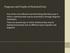
1 26 Nature Publishing Group Interpreting flow cytometry data: a guide for the perplexed Leonore A Herzenberg, James Tung, Wayne A Moore, Leonard A Herzenberg & David R Parks Recent advances in flow cytometry technologies are changing how researchers collect, look at and present their data. Recent advances in fluorescence-activated cell sorting (FACS) technology offer new and exciting approaches for understanding, monitoring and combating immune-related diseases. However, these technological advances also introduce considerable challenges for the producers, reviewers and readers of today s immunology literature. Although some laboratories have readily adopted today s improved FACS data acquisition and analysis methods, too many others have found it difficult to understand how or even believe that using the older methods can lead to serious misinterpretations of FACS data. In essence, results for the same sample can be very different (as described below) depending on whether the data for the sample are collected and displayed with the older or newer methods. In fact, disputes can arise over what might seem to be incompatible data, but the contradictions could simply reflect unrecognized differences in how the data are processed and displayed. These problems and limitations are becoming more apparent and more important as measurement technologies change and as the number of fluorescence labels being routinely measured increases. For example, when the older technology is used to display a FACS data set, a subset of cells without expression of either of two surface markers may remain mostly hidden. However, Leonore A. Herzenberg, James Tung, Wayne A. Moore, Leonard A. Herzenberg and David R. Parks are in the Department of Genetics, Stanford University School of Medicine, Stanford, California , USA. leeherz@stanford.edu with the newer technology, the complete subset is visible (Fig. 1, bottom left regions containing double-negative cells, logarithmic versus logicle ). Far from magically creating a new subset, the newer display method simply makes an appropriate place on the visual scale for cells whose fluorescence values are close to zero. The older methods typically assign such cells to the lowest value of the (logarithmic) visual scale and thus pile them up on the axis. In the absence of an understanding of these differences between old and new technologies (explained below), long-lasting disagreements could develop simply because different laboratories are using incommensurate methods. The discussion here focuses on closing this knowledge gap and making findings with the newer and better FACS technology more accessible both to FACS users and to investigators who need to understand and interpret the diverse FACS data in today s literature. The goal is to clear up misunderstandings and help a community schooled in the older FACS vernacular retrain in the modern idiom. Technical details are intentionally restricted to those essential to understanding how differences between the older and newer FACS technologies affect FACS-based findings. Though FACS aficionados may find this discussion useful, they will no doubt prefer the sources on which it is based 1 6. The logicle solution FACS data are commonly presented as onedimensional histograms or two-dimensional displays (dot displays or contour maps) with logarithmic axes that extend over a four- to five-decade range, representing cells with flourescence values that differ 1,- to 1,-fold between the lower and upper ends of the scale. Researchers use these logarithmic displays to visualize data during instrument setup and data collection as well as to analyze and publish FACS data. Over the years, these displays have become such a staple in FACS plots that published figures often (and inexcusably) omit axis values and even axis tick marks that identify the scale as logarithmic. Nonetheless, old habits are changing. Many investigators are now forsaking that well established technology in favor of newly introduced logicle (or bi-exponential ) displays, which were specifically designed for the display of FACS data so that they incorporate the useful features of logarithmic displays but also provide accurate visualization of populations with low or background fluorescence 1 (Fig. 1). Logicle displays provide good visualization of all subsets, regardless of the amount of cell-associated fluorescence. Logarithmic displays, in contrast, provide similar representation of subsets of cells with medium to large amounts of fluorescence associated with surface and internal markers. However, these latter widely used displays present deceptive, truncated views of subsets in data dimensions in which they have little or no cell-associated fluorescence. This occurs because logarithmic scales, by their nature, spread data at the lowest on-scale values and cannot accommodate values at or below zero. We demonstrate here the considerable difference between the logicle and logarithmic display methods by presenting the same data set plotted on logarithmic axes (Fig. 1, left) and logicle axes (Fig. 1, right) and visualized with color density plots (Fig. 1, top) and quantile contour maps (Fig. 1, middle). Although each of the four plots represents data for all of the cells in the sample, the minimally fluorescent NATURE IMMUNOLOGY VOLUME 7 NUMBER 7 JULY
2 26 Nature Publishing Group subset that is visible in the logicle displays (centered near zero on both axes) seems to be missing from the logarithmic displays. However, as indicated above, this subset is present but is represented mainly by data points and contours that are piled up on the plot axes. The location of the median fluorescence value in each dimension (Fig. 1, dark red crosses) further demonstrates the problem with logarithmic data visualization. By definition, half of the data values in each rectangular region are greater than the median and half are less than that value. However, whereas the locations of the median values in the logicle displays (Fig. 1, right) correspond to the visual centers of the subsets, the locations of the median values in the logarithmic displays (Fig. 1, left) are substantially offset from the apparent peak of the subset. In fact, each of the subsets in the logarithmic display is broken up into a false peak above the median value, a sparsely populated region between this peak and the baseline, and a pileup of a large fraction of the cells on the baseline. This artificial subdivision is also visible when data are plotted as a one-dimensional histogram on a logarithmic scale (Fig. 1, bottom left). The sharp rise at the lowest value on the scale reflects the pileup of the minimally fluorescent cells at the lowest value on the scale. This peak tends to be overlooked because it is nearly coincident with the axis. However, it represents roughly 4% of the minimally fluorescent population and 2% of the cells in the overall population. The valley and the false peak are also in the histogram, creating a total of three peaks rather than the two that actually exist. This problem does not occur in histograms plotted on logicle axes (Fig. 1, bottom right), in which the minimally fluorescent cells form a peak centered at or near zero and extending symmetrically above and below the peak center, thereby representing the cells whose measured fluorescence values are below zero. In two-dimensional logarithmic displays, cells whose fluorescence measurements are at or below zero in one or both dimensions are piled up on the axes and are represented by contours along both axes in contour displays (Fig. 1, middle left) and by colored dots, visible with keen eyes, on the axes in color density displays (Fig. 1, top left). The contours (and dots) range along a large proportion of both the x and y axes, indicating that cells with fluorescence values at or below zero in one dimension may have equivalently low fluorescence in the other dimension or may have very high fluorescence values in this second dimension. Surprisingly, given how modest the contours along the axes seem, they represent about 4% lgd CD5 3% 5% CD5 Logarithmic of the cells in the sample. Notably, these missing cells are readily visible in logicle displays, which enable visualization of fluorescent measurements for essentially all cells in the sample, including those whose fluorescence values are at or below zero in either dimension (Fig. 1, right) % 5% Logicle Figure 1 Logicle displays provide improved representation of cells with minimal fluorescence. Cells with minimal fluorescence can be visualized with logicle displays (right) but are piled up on the axis with logarithmic displays (left). The true center of each gated population (median fluorescence value in each dimension; dark red crosses) matches the visual peak for that population in logicle displays (right, top and middle) but does not match the visual peak in the logarithmic displays (left, top and middle). Because logarithmic scales cannot display cells with zero or negative values, these cells are piled up on the axis in the logarithmic displays. However, they are properly visualized in the logicle display (bottom right, red shaded region). Data provided by E. Ghosn (Stanford University, Stanford, California). The importance of seeing less than nothing As FACS instruments measure cell-associated fluorescence that can be essentially zero but not negative, how can a FACS data set contain values below zero and why are some cell populations found to be distributed symmetrically around zero? The answer lies in the way the FACS instrument collects data and routinely corrects (compensates for) the data for spectral overlap before visualization in histograms, dot plots and contour maps. In essence, background light and electronic noise make a small but important contribution to the overall signals the FACS instrument obtains for each cell. The FACS apparatus measures this background when no cells are present and subtracts it from the signals the detectors record for each cell. This instrument background correction introduces statistical variation, which may cause the measurements that the detectors record for cells that have no cell-associated fluorescence to be a little above or a little below the measurement set as the instrument zero. As a result, the corrected measurements distribute symmetrically around zero. This distribution is often broadened sub- 682 VOLUME 7 NUMBER 7 JULY 26 NATURE IMMUNOLOGY
3 26 Nature Publishing Group Yellow PE Green FITC Logarithmic Logicle 1 Uncompensated 5 Compensated stantially by the effects of spectral overlap between dyes when two or more dyes are used to stain a cell suspension. During FACS data collection, separate detectors are dedicated to each fluorochrome and measure most light from the target fluorochrome. Some of the light from this fluorochrome, however, may be emitted and detected in the wavelength range assigned to a different fluorochrome. Fluorescence compensation is the process by which the amounts of spectral overlap are estimated and subtracted from the total detected signals to yield an estimate of the actual amount of each dye. Single-laser, two-color FACS experiments in the mid-197s showed that fluorescence compensation is important 6. It is now recognized as an obligate step in most FACS analyses. For measurement of the spectral overlaps, the fluorescence detected on all measurement channels is evaluated for compensation control samples, which have each been labeled with only one of the fluorochromes used in the composite stain set. These measurements are used to make the fluorescence compensation corrections. That is, they are used to estimate and subtract the contribution of the spectral overlap to the overall signal that each detector measures for cells stained with the composite stain set. When a population has little or no detectable cell-associated fluorescence for a particular dye, the subtraction of overlapping fluorescence by the fluorescence compensation process results in a population distributed symmetrically around zero (or cell autofluorescence) in the corresponding data channel. Statistical uncertainties inherent in primary and overlapping fluorescence measurements will determine the width of this distribution. As these uncertainties become more pronounced as the size of the correction increases, larger corrections will result in broader distributions that nonetheless will still be centered on the average autofluorescence of the cells (Fig. 2, bottom right, vertical dimension). Applying the compensation to the data for the compensation control samples and using logicle displays to visualize the results for these samples provide a straightforward way to determine whether the applied compensation is correct. This is demonstrated by the position of populations displaced from the origin along the x or y axis. A population centered above the unstained cell background indicates undercompensation; a population centered below the unstained cell background indicates overcompensation; and a population centered at the unstained background indicates that the compensation correction is, like Goldilocks porridge, just right. Collect, then compensate Given the difficulty or impossibility of setting correct compensation visually using logarithmic displays, the appropriate way to set correct compensation is to rely on values computed by software from appropriately gated compensation control samples. As noted above, logicle displays of compensation control data can be used to confirm that nothing has gone wrong in the process and the resulting compensation is correct. Setting compensation manually from logarithmic displays is in effect flying blind and routinely leads to substantial under- or overcompensation. This is a shocking realization, as the instrument-based method (compensate by eye first; collect the data after) was the only method Figure 2 Logicle displays are superior to logarithmic displays in determining whether compensation has been properly applied to the sample. Uncompensated data for a sample stained with fluorescein isothiocyanate conjugated antibody are presented in logarithmic (top left) and logicle (top right) displays. Fluorescent colors (yellow and green) are used to designate the axes because they represent uncompensated color measurements. Many events are piled up on the axis in the logarithmic display, whereas all events are visible in the logicle display. Bottom, compensated data for samples above. In a properly compensated sample, the location of the median phycoerythrin fluorescence in the FITC population by definition matches the location of the median phycoerythrin fluorescence in the FITC population. This match is apparent in the logicle display but not the logarithmic display (bottom right versus bottom left). In the logicle display (bottom right), the distribution of the phycoerythrin fluorescence in the FITC population is broader than the distribution of the low fluorescence measured for the FITC population. This difference is due to statistical variance of the fluorescence measurements, which increases with the amount of overlap fluorescence that must be subtracted. Red dashed lines above the populations (bottom right) indicate the thresholds (gates) needed to identify the PE events in the FITC and FITC populations in this simple analysis. With more complex stain sets, FMO controls are useful for setting these thresholds. available for many years and is still widely used today. Thus, much of the older FACS data, and a fair part of today s FACS data, have been compromised to some extent, particularly with respect to distinguishing dull (weakly positive) from negative populations or evaluating small differences in the expression of a marker on two subsets. The solution to this problem is simple and straightforward: data should be collected and stored uncompensated (that is, written to data files before compensation is applied); data for compensation samples should also be collected and written to data files; and the compensation should be computed and applied when the data are analyzed. This recommendation, which is made in the strongest terms, goes against common practice. It is time for that practice to change. The errors introduced by compensating the data before collection are no longer tolerable in an era where fluorescence compensation can readily be done after the (uncompensated) data have been collected and stored. Recognizing the necessity for this shift to post-collection compensation, FACS software designers have already introduced software packages to provide this (and related) functionality. Among those that are already familiar (Supplementary Note online), the FacsXpert protocol design software ( provides labels for plot axes and table columns and specifies the necessary compensation and other controls; the FlowJo FACS data analysis software ( computes and applies compensation corrections, enables visualization of all data on logicle axes and provides quantile ( probability ) contour displays; and the DIVA software on newer Becton-Dickinson NATURE IMMUNOLOGY VOLUME 7 NUMBER 7 JULY
4 26 Nature Publishing Group instruments ( provides utilities for viewing data on logicle scales during data collection while collecting and storing uncompensated data. For viewing data during cell sorting, it is of course necessary to use the compensation tools provided by the instrument (as the data must be compensated before its visualization). In addition, it is often desirable to monitor data during data collection, which again must be compensated with on board compensation utilities. As indicated above, some of the newer analyzers and cell sorters offer the ability to view data and sort cells with compensation in place while still allowing the collection and storage of uncompensated data (along with the compensation specification used on the instrument). However, with the older instruments, there is no choice but to use the instrumentbased compensation utility to approximate the compensation. Do not overstep the boundaries Although unstained cell populations are centered close to zero when displayed on the logicle scale, the width of these populations can vary considerably, depending on the size of the fluorescence correction. Therefore, determining whether cells are dully stained or belong to a broad unstained population is not as simple as drawing a threshold based on unstained cells as is done, for example, with the commonly used quadrant method for specifying gates. Similarly, basing a threshold on amounts obtained for cells stained with an isotype control can give an erroneous impression of the location of the true threshold with which to separate unstained and dully fluorescent cells. The best way to determine this threshold is to run fluorescence minus one (FMO) controls 2,7 for all dyes for which the threshold is in question; that is, where spectral overlap correction may lead to greater uncertainty as to what constitutes background staining. In an FMO control stain set, all reagents used in a given multicolor stain are included except the reagent for which the threshold is to be determined. Alternatively, an isotype control reagent is used to replace the antibody for which the threshold is sought (if the isotype control is assumed to have the same nonspecific stickiness as the staining antibody). To show the threshold that distinguishes dully fluorescent cells from cells that do not express the determinant detected by the reagent omitted from the FMO control, data are collected for cells stained with FMO control stain sets and are compensated, displayed and gated in the same way as cells stained with the full stain set. The background fluorescence for each subset in the display is demonstrated by the upper boundary Dot plots Quantile contours CD4 CD8 F4/ IgD 1 cells 5 cells 1.5 cells of the subset in the FMO control. We present here an FMO control in the simplest possible form (Fig. 2, bottom right). The threshold for identifying cells with phycoerythrin above background should be set differently for fluorescein isothiocyanate negative and fluorescein isothiocyanate positive populations (Fig. 2, red dashed lines). Let standards serve as a guide Running stable multicolor fluorescent microspheres ( beads ) and adjusting the FACS apparatus so that the bead fluorescence in each channel precisely match the fluorescence in previous experiments allows the instrument to be tuned to return data that are highly reproducible from one experiment to the next. Standardizing the instrument in this way is desirable but realistically is very hard to achieve without computer-based standardization tools. In the absence of such tools, the best that can be done (and should be done) is to run a multicolor bead sample, adjust the instrument so that the fluorescence in each channel is close to the desired value and collect a data set showing where the bead fluorescence was during that FACS assay. This procedure will bring the instrument into roughly standard conditions and provide a way of knowing how closely fluorescence on subsets can be expected to match Figure 3 Quantile contour plots provide more accurate data representation than dot plots. Samples containing 1, 5 and 1.5 events are plotted as quantile contour plots plus outliers (bottom) or as dot plots (top). The contours in the quantile contour plots (bottom) in each sample are preserved regardless of the numbers of events displayed. Color density plots (Fig. 1) are similar to quantile contour plots in this respect. In contrast, the commonly used dot plots (top) become saturated and populations become harder to separate as more events (cells) are displayed. In addition, lowfrequency populations may seem to be present at higher frequencies when large numbers of events are displayed. Some software packages offer the ability to display contour plots that include dots showing the location of cells outside the lowest contour. In the commonly used 5% quantile contour plots presented here (bottom), 95% of the cells are bounded by the contour lines. The remaining cells are displayed as dots that, as in all dot plots, increase in number as the number of cells analyzed increases. between experiments. The bead-based standardization method is now growing in popularity and is replacing the older method in which instruments are standardized by analyzing unstained cells and tuning the instrument so that these cells are in the center of the first decade of the logarithmic amplifier display. This cell-based method is flawed because of problems with displaying cells near the axes on logarithmic displays (as described above) and because it references data to the least accurate measurements being made; that is, to values for nonfluorescent or autofluorescent cells. Therefore, the bead-based method is the method of choice for instrument standardization. Follow the dots (with better displays) There are three readily available choices for displaying two-dimensional data. Perhaps the most commonly used, the monochrome dot plot, dates back to the use of oscilloscopes to visualize FACS populations and has been carried forward as the printed version of the screen view available on the primitive instrument. In its time, the dot plot was the best display that could be achieved. Today it is the worst. Watching dot plots develop as data is collected demonstrates the considerable limitations inherent in this type of display. 684 VOLUME 7 NUMBER 7 JULY 26 NATURE IMMUNOLOGY
5 26 Nature Publishing Group BOX 1 SUGGESTED GUIDELINES FOR FACS DATA PRESENTATION 4 Instrument: Identify the FACS instrument and the software used to collect, compensateand analyze the data. Include model and version number where more than one exists. Graphic displays: Choose smoothing, graph and display options according to the dictates of the study. Be consistent across all displays in an analysis. Indicate the number of cells for which data are displayed and, where applicable, the contour or color density intervals used in the figure. Scaling: Show all parts of the plot axis necessary to indicate the scaling that was used (such as log, linear or logicle ). Numerical values for axis ticks can be eliminated except when necessary to clarify the scaling. For univariate (onedimensional) histograms, the scale for the abscissa (y axis) should be linear and should begin at zero unless otherwise indicated. Numerical axis values should not be included with the zero-based linear axes but should be shown for other axes. Gating: Display the gates used at each step in the gating sequence when gates are set manually (subjective gating). Show data for control samples when these are used to set gates. If necessary, present this information in supplementary figures. When an algorithm is used to set gates, define it explicitly and state that it has been used. Gating is assumed to be subjective unless otherwise stated. Frequency measurements: Show the frequencies (or percentages) of cells in gates of importance in the study. Compute these values relative to the total number of cells presented in the display on which the values appear. If a different frequency computation is used, define the method that was used and where it was applied. The graph itself cannot convey this requisite information. Intensity measurements: Explicitly define the statistic applied (mean, median or a particular percentile). All statistics should be applied to the scaled intensity measurement rather than to channel numbers. Once a cell triggers the placement of a dot on the screen, hundreds or thousands of cells measured at the same fluorescence will effectively be invisible because they will all be represented on the screen by the initial dot. In addition, if enough cells are analyzed, sparse regions will become populated with enough dots at a density of one cell per dot to form a contiguous (black) region indistinguishable from and often merged with highly populated black regions saturated with thousands of dots and potentially thousands of signals per dot (Fig. 3). Curiously, although this problem with live dots plots is fairly well recognized, many investigators have adopted computer-generated dot plots as their preferred method for viewing and publishing data. Some of these investigators believe (erroneously) that dot plots provide the most accurate representation of the subsets that are present. However, quantile contour plots (sometimes called probability plots) and color density plots actually provide a much more accurate representation of the data. By representing the frequencies of cells present at each point in the plot, these plots avoid the dynamic range problems encountered with monochrome dot plots. Thus, they become more rather than less accurate as the number of cells for which data are collected increases (Fig. 3). Furthermore, because they facilitate the discrimination of densely and sparsely populated regions, these contour and color density plots enable precise localization of the peaks and valleys that distinguish subsets of cells. The FACS Tower of Babel From a reader s or a reviewer s point of view, the lesson here is that comparison of FACS data can be difficult even in the same laboratory when data are collected on the same FACS instrument. Dully stained cell populations can seem to be negative, unstained populations can seem to be positive, and irritating controversies can arise, all because one investigator used online data visualization and compensation methods based on logarithmically displayed data, whereas the other collected uncompensated data and used offline compensation and logicle visualizations for analysis. This gap can be even greater when the comparison is between uncompensated data collected on the older FACS machines with logarithmic amplifiers and peak detection electronics and data collected on the newer machines with linear digital signal processing. Even when the data are correctly compensated in both cases and when the data are visualized with the same analysis program, cell populations at both ends of the scale tend to be more accurately represented when data are collected with the newer machines, because the older electronic systems tend to render signals less accurately in the lowest and often also the highest regions. Thus, readers and reviewers accustomed to the older FACS instruments and the older displays may encounter surprises in data acquired with the newer technologies, whereas readers and reviewers steeped in data acquired with the newer technologies may miss data features they are accustomed to seeing. Overall, this means that as the field transitions from the older to the newer technologies (which may take some time), it will be necessary for investigators at all levels to recognize the differences between the technologies and for authors submitting papers to ensure that the information that accompanies FACS data is sufficient for reviewers to understand which technologies were used for data collection, compensation and display. Although this kind of information has sometimes been considered superfluous, it is now essential for making FACS data interpretable. Therefore, the development of a standard to specify the minimal acceptable information necessary to accompany FACS data is obviously in order, the sooner the better (Box 1). 1. Parks, D.R., Roederer, M. & Moore, W.A. Cytometry A (in the press). 2. Tung, J.W., Parks, D.R., Moore, W.A., Herzenberg, L.A. & Herzenberg, L.A. Clin. Immunol. 11, (24). 3. Tung, J.W., Parks, D.R., Moore, W.A., Herzenberg, L.A. & Herzenberg, L.A. Methods Mol. Biol. 271, (24). 4. Roederer, M., Darzynkiewicz, Z. & Parks, D.R. Methods Cell Biol. 75, (24). 5. Herzenberg, L.A. et al. Clin. Chem. 48, (22). 6. Loken, M.R., Parks, D.R. & Herzenberg, L.A. J. Histochem. Cytochem. 25, (1977). 7. Roederer, M. Cytometry 45, (21). NATURE IMMUNOLOGY VOLUME 7 NUMBER 7 JULY
APPLICATION INFORMATION
 DRAFT: Rev. D A-2045A APPLICATION INFORMATION Flow Cytometry 3-COLOR COMPENSATION Raquel Cabana,* Mark Cheetham, Jay Enten, Yong Song, Michael Thomas,* and Brendan S. Yee Beckman Coulter, Inc., Miami FL
DRAFT: Rev. D A-2045A APPLICATION INFORMATION Flow Cytometry 3-COLOR COMPENSATION Raquel Cabana,* Mark Cheetham, Jay Enten, Yong Song, Michael Thomas,* and Brendan S. Yee Beckman Coulter, Inc., Miami FL
Three Rules for Compensation Controls
 From FlowJo s Daily Dongle Three Rules for Compensation Controls First and foremost, there must be a single-stained control for every parameter in the experiment! In addition, there are three rules for
From FlowJo s Daily Dongle Three Rules for Compensation Controls First and foremost, there must be a single-stained control for every parameter in the experiment! In addition, there are three rules for
Fluorescence Compensation. Jennifer Wilshire, PhD jennifer@flowjo.com
 Fluorescence Compensation Jennifer Wilshire, PhD jennifer@flowjo.com Outline 1. Compensation Basics 2. Practical Applications -selecting comp controls (cells, stains?) 3. Multicolor Issues -spreading due
Fluorescence Compensation Jennifer Wilshire, PhD jennifer@flowjo.com Outline 1. Compensation Basics 2. Practical Applications -selecting comp controls (cells, stains?) 3. Multicolor Issues -spreading due
Compensation Basics - Bagwell. Compensation Basics. C. Bruce Bagwell MD, Ph.D. Verity Software House, Inc.
 Compensation Basics C. Bruce Bagwell MD, Ph.D. Verity Software House, Inc. 2003 1 Intrinsic or Autofluorescence p2 ac 1,2 c 1 ac 1,1 p1 In order to describe how the general process of signal cross-over
Compensation Basics C. Bruce Bagwell MD, Ph.D. Verity Software House, Inc. 2003 1 Intrinsic or Autofluorescence p2 ac 1,2 c 1 ac 1,1 p1 In order to describe how the general process of signal cross-over
Compensation in Flow Cytometry
 Compensation in UNIT.4 The term compensation, as it applies to flow cytometric analysis, refers to the process of correcting for fluorescence spillover, i.e., removing the signal of any given fluorochrome
Compensation in UNIT.4 The term compensation, as it applies to flow cytometric analysis, refers to the process of correcting for fluorescence spillover, i.e., removing the signal of any given fluorochrome
Selected Topics in Electrical Engineering: Flow Cytometry Data Analysis
 Selected Topics in Electrical Engineering: Flow Cytometry Data Analysis Bilge Karaçalı, PhD Department of Electrical and Electronics Engineering Izmir Institute of Technology Outline Compensation and gating
Selected Topics in Electrical Engineering: Flow Cytometry Data Analysis Bilge Karaçalı, PhD Department of Electrical and Electronics Engineering Izmir Institute of Technology Outline Compensation and gating
Standardization, Calibration and Quality Control
 Standardization, Calibration and Quality Control Ian Storie Flow cytometry has become an essential tool in the research and clinical diagnostic laboratory. The range of available flow-based diagnostic
Standardization, Calibration and Quality Control Ian Storie Flow cytometry has become an essential tool in the research and clinical diagnostic laboratory. The range of available flow-based diagnostic
Instructions for Use. CyAn ADP. High-speed Analyzer. Summit 4.3. 0000050G June 2008. Beckman Coulter, Inc. 4300 N. Harbor Blvd. Fullerton, CA 92835
 Instructions for Use CyAn ADP High-speed Analyzer Summit 4.3 0000050G June 2008 Beckman Coulter, Inc. 4300 N. Harbor Blvd. Fullerton, CA 92835 Overview Summit software is a Windows based application that
Instructions for Use CyAn ADP High-speed Analyzer Summit 4.3 0000050G June 2008 Beckman Coulter, Inc. 4300 N. Harbor Blvd. Fullerton, CA 92835 Overview Summit software is a Windows based application that
Introduction to flow cytometry
 Introduction to flow cytometry Flow cytometry is a popular laser-based technology. Discover more with our introduction to flow cytometry. Flow cytometry is now a widely used method for analyzing the expression
Introduction to flow cytometry Flow cytometry is a popular laser-based technology. Discover more with our introduction to flow cytometry. Flow cytometry is now a widely used method for analyzing the expression
COMPENSATION MIT Flow Cytometry Core Facility
 COMPENSATION MIT Flow Cytometry Core Facility Why do we need compensation? 1) Because the long emission spectrum tail of dyes causes overlap like with the fluorophores FITC and PE. 2) For sensitivity reasons,
COMPENSATION MIT Flow Cytometry Core Facility Why do we need compensation? 1) Because the long emission spectrum tail of dyes causes overlap like with the fluorophores FITC and PE. 2) For sensitivity reasons,
Immunophenotyping Flow Cytometry Tutorial. Contents. Experimental Requirements...1. Data Storage...2. Voltage Adjustments...3. Compensation...
 Immunophenotyping Flow Cytometry Tutorial Contents Experimental Requirements...1 Data Storage...2 Voltage Adjustments...3 Compensation...5 Experimental Requirements For immunophenotyping with FITC and
Immunophenotyping Flow Cytometry Tutorial Contents Experimental Requirements...1 Data Storage...2 Voltage Adjustments...3 Compensation...5 Experimental Requirements For immunophenotyping with FITC and
Flow Data Analysis. Qianjun Zhang Application Scientist, Tree Star Inc. Oregon, USA FLOWJO CYTOMETRY DATA ANALYSIS SOFTWARE
 Flow Data Analysis Qianjun Zhang Application Scientist, Tree Star Inc. Oregon, USA Flow Data Analysis From Cells to Data - data measurement and standard Data display and gating - Data Display options -
Flow Data Analysis Qianjun Zhang Application Scientist, Tree Star Inc. Oregon, USA Flow Data Analysis From Cells to Data - data measurement and standard Data display and gating - Data Display options -
BD FACSDiva 4.1 - TUTORIAL TSRI FLOW CYTOMETRY CORE FACILITY
 BD FACSDiva 4.1 - TUTORIAL TSRI FLOW CYTOMETRY CORE FACILITY IMPORTANT NOTES BEFORE READING THIS TUTORIAL This is a very expensive piece of equipment so PLEASE treat it with respect! After you are done
BD FACSDiva 4.1 - TUTORIAL TSRI FLOW CYTOMETRY CORE FACILITY IMPORTANT NOTES BEFORE READING THIS TUTORIAL This is a very expensive piece of equipment so PLEASE treat it with respect! After you are done
Introduction to Flow Cytometry
 Introduction to Flow Cytometry presented by: Flow Cytometry y Core Facility Biomedical Instrumentation Center Uniformed Services University Topics Covered in this Lecture What is flow cytometry? Flow cytometer
Introduction to Flow Cytometry presented by: Flow Cytometry y Core Facility Biomedical Instrumentation Center Uniformed Services University Topics Covered in this Lecture What is flow cytometry? Flow cytometer
Compensation Controls Data Visualization Panel Development Gating Quantum Dots
 Compensation Controls Data Visualization Panel Development Gating Quantum Dots FITC Single Stain Control FITC PE Argon Laser FL1 FL2 450 500 550 600 FITC Compensation Control Unwanted signal detected in
Compensation Controls Data Visualization Panel Development Gating Quantum Dots FITC Single Stain Control FITC PE Argon Laser FL1 FL2 450 500 550 600 FITC Compensation Control Unwanted signal detected in
Application Note. Selecting Reagents for Multicolor Flow Cytometry. Holden Maecker and Joe Trotter BD Biosciences, San Jose
 Selecting Reagents for Multicolor Flow Cytometry Holden Maecker and Joe Trotter BD Biosciences, San Jose Contents 1 The basics: Know your instrument 2 Fluorochromes: Go for the bright 3 Colors and specificities:
Selecting Reagents for Multicolor Flow Cytometry Holden Maecker and Joe Trotter BD Biosciences, San Jose Contents 1 The basics: Know your instrument 2 Fluorochromes: Go for the bright 3 Colors and specificities:
Technical Bulletin. An Introduction to Compensation for Multicolor Assays on Digital Flow Cytometers
 An Introduction to Compensation for Multicolor Assays on Digital Flow Cytometers BD Biosciences, San Jose, CA Contents 2 Introduction to compensation 5 Tips for setting up compensation 10 Tools for easy,
An Introduction to Compensation for Multicolor Assays on Digital Flow Cytometers BD Biosciences, San Jose, CA Contents 2 Introduction to compensation 5 Tips for setting up compensation 10 Tools for easy,
FlowMergeCluster Documentation
 FlowMergeCluster Documentation Description: Author: Clustering of flow cytometry data using the FlowMerge algorithm. Josef Spidlen, jspidlen@bccrc.ca Please see the gp-flowcyt-help Google Group (https://groups.google.com/a/broadinstitute.org/forum/#!forum/gpflowcyt-help)
FlowMergeCluster Documentation Description: Author: Clustering of flow cytometry data using the FlowMerge algorithm. Josef Spidlen, jspidlen@bccrc.ca Please see the gp-flowcyt-help Google Group (https://groups.google.com/a/broadinstitute.org/forum/#!forum/gpflowcyt-help)
Using CyTOF Data with FlowJo Version 10.0.7. Revised 2/3/14
 Using CyTOF Data with FlowJo Version 10.0.7 Revised 2/3/14 Table of Contents 1. Background 2. Scaling and Display Preferences 2.1 Cytometer Based Preferences 2.2 Useful Display Preferences 3. Scale and
Using CyTOF Data with FlowJo Version 10.0.7 Revised 2/3/14 Table of Contents 1. Background 2. Scaling and Display Preferences 2.1 Cytometer Based Preferences 2.2 Useful Display Preferences 3. Scale and
Notes on titering antibodies
 Home Events This Week Bulletin Boards Lab Personnel Lab Policies Protocols MolBio Tetramers Proteins Flow Cytometry Immunoassays Instruments Computers Lab Publications Scientiific Literature Science on
Home Events This Week Bulletin Boards Lab Personnel Lab Policies Protocols MolBio Tetramers Proteins Flow Cytometry Immunoassays Instruments Computers Lab Publications Scientiific Literature Science on
BD FACSComp Software Tutorial
 BD FACSComp Software Tutorial This tutorial guides you through a BD FACSComp software lyse/no-wash assay setup run. If you are already familiar with previous versions of BD FACSComp software on Mac OS
BD FACSComp Software Tutorial This tutorial guides you through a BD FACSComp software lyse/no-wash assay setup run. If you are already familiar with previous versions of BD FACSComp software on Mac OS
Katharina Lückerath (AG Dr. Martin Zörnig) adapted from Dr. Jörg Hildmann BD Biosciences,Customer Service
 Introduction into Flow Cytometry Katharina Lückerath (AG Dr. Martin Zörnig) adapted from Dr. Jörg Hildmann BD Biosciences,Customer Service How does a FACS look like? FACSCalibur FACScan What is Flow Cytometry?
Introduction into Flow Cytometry Katharina Lückerath (AG Dr. Martin Zörnig) adapted from Dr. Jörg Hildmann BD Biosciences,Customer Service How does a FACS look like? FACSCalibur FACScan What is Flow Cytometry?
Visualization Quick Guide
 Visualization Quick Guide A best practice guide to help you find the right visualization for your data WHAT IS DOMO? Domo is a new form of business intelligence (BI) unlike anything before an executive
Visualization Quick Guide A best practice guide to help you find the right visualization for your data WHAT IS DOMO? Domo is a new form of business intelligence (BI) unlike anything before an executive
Technical Bulletin. Threshold and Analysis of Small Particles on the BD Accuri C6 Flow Cytometer
 Threshold and Analysis of Small Particles on the BD Accuri C6 Flow Cytometer Contents 2 Thresholds 2 Setting the Threshold When analyzing small particles, defined as particles smaller than 3.0 µm, on the
Threshold and Analysis of Small Particles on the BD Accuri C6 Flow Cytometer Contents 2 Thresholds 2 Setting the Threshold When analyzing small particles, defined as particles smaller than 3.0 µm, on the
BD LSR II and FACSDiVa Software. Dr. Jens Fleischer, Basel Dr. Norbert Leclere,, Berlin
 BD LSR II and FACSDiVa Software, Basel Dr. Norbert Leclere,, Berlin Overview Electronics Covers Fluidics Connectors Controlpanel Sample Port On/Off The Control Panel Ease of Use The basic procedures to
BD LSR II and FACSDiVa Software, Basel Dr. Norbert Leclere,, Berlin Overview Electronics Covers Fluidics Connectors Controlpanel Sample Port On/Off The Control Panel Ease of Use The basic procedures to
Cash Flow and Accounts Receivable Management for Dialysis
 Dialysis & Transplantation, Volume 13, Number 4, April 1984, p. 201 Cash Flow and Accounts Receivable Management for Dialysis John A. Sargent, PhD, President; Albert Grutze, Systems Manager, Quantitative
Dialysis & Transplantation, Volume 13, Number 4, April 1984, p. 201 Cash Flow and Accounts Receivable Management for Dialysis John A. Sargent, PhD, President; Albert Grutze, Systems Manager, Quantitative
Immunophenotyping peripheral blood cells
 IMMUNOPHENOTYPING Attune Accoustic Focusing Cytometer Immunophenotyping peripheral blood cells A no-lyse, no-wash, no cell loss method for immunophenotyping nucleated peripheral blood cells using the Attune
IMMUNOPHENOTYPING Attune Accoustic Focusing Cytometer Immunophenotyping peripheral blood cells A no-lyse, no-wash, no cell loss method for immunophenotyping nucleated peripheral blood cells using the Attune
These particles have something in common
 These particles have something in common Blood cells Chromosomes Algae Protozoa Certain parameters of these particles can be measured with a flow cytometer Which parameters can be measured? the relative
These particles have something in common Blood cells Chromosomes Algae Protozoa Certain parameters of these particles can be measured with a flow cytometer Which parameters can be measured? the relative
Demographics of Atlanta, Georgia:
 Demographics of Atlanta, Georgia: A Visual Analysis of the 2000 and 2010 Census Data 36-315 Final Project Rachel Cohen, Kathryn McKeough, Minnar Xie & David Zimmerman Ethnicities of Atlanta Figure 1: From
Demographics of Atlanta, Georgia: A Visual Analysis of the 2000 and 2010 Census Data 36-315 Final Project Rachel Cohen, Kathryn McKeough, Minnar Xie & David Zimmerman Ethnicities of Atlanta Figure 1: From
Data Visualization Techniques
 Data Visualization Techniques From Basics to Big Data with SAS Visual Analytics WHITE PAPER SAS White Paper Table of Contents Introduction.... 1 Generating the Best Visualizations for Your Data... 2 The
Data Visualization Techniques From Basics to Big Data with SAS Visual Analytics WHITE PAPER SAS White Paper Table of Contents Introduction.... 1 Generating the Best Visualizations for Your Data... 2 The
Using Excel (Microsoft Office 2007 Version) for Graphical Analysis of Data
 Using Excel (Microsoft Office 2007 Version) for Graphical Analysis of Data Introduction In several upcoming labs, a primary goal will be to determine the mathematical relationship between two variable
Using Excel (Microsoft Office 2007 Version) for Graphical Analysis of Data Introduction In several upcoming labs, a primary goal will be to determine the mathematical relationship between two variable
No-wash, no-lyse detection of leukocytes in human whole blood on the Attune NxT Flow Cytometer
 APPLICATION NOTE Attune NxT Flow Cytometer No-wash, no-lyse detection of leukocytes in human whole blood on the Attune NxT Flow Cytometer Introduction Standard methods for isolating and detecting leukocytes
APPLICATION NOTE Attune NxT Flow Cytometer No-wash, no-lyse detection of leukocytes in human whole blood on the Attune NxT Flow Cytometer Introduction Standard methods for isolating and detecting leukocytes
Stepcount. Product Description: Closed transparent tubes with a metal screen, including a white matrix at the bottom. Cat. Reference: STP-25T
 Product Description: Closed transparent tubes with a metal screen, including a white matrix at the bottom Cat. Reference: STP-25T Reagent provided:: 25 Stepcount tubes for 25 test INTENDED USE. Immunostep
Product Description: Closed transparent tubes with a metal screen, including a white matrix at the bottom Cat. Reference: STP-25T Reagent provided:: 25 Stepcount tubes for 25 test INTENDED USE. Immunostep
Using the BD TM Cytometer Setup and Tracking (CS&T) System for Instrument Characterization and Performance Tracking
 Using the BD TM Cytometer Setup and Tracking (CS&T) System for Instrument Characterization and Performance Tracking Mark KuKuruga Senior Technical Applications Specialist BD Biosciences 23-14462-00 Outline
Using the BD TM Cytometer Setup and Tracking (CS&T) System for Instrument Characterization and Performance Tracking Mark KuKuruga Senior Technical Applications Specialist BD Biosciences 23-14462-00 Outline
Data Exploration Data Visualization
 Data Exploration Data Visualization What is data exploration? A preliminary exploration of the data to better understand its characteristics. Key motivations of data exploration include Helping to select
Data Exploration Data Visualization What is data exploration? A preliminary exploration of the data to better understand its characteristics. Key motivations of data exploration include Helping to select
Spherotech, Inc. 27845 Irma Lee Circle, Unit 101, Lake Forest, Illinois 60045 1
 78 Irma Lee Circle, Unit 0, Lake Forest, Illinois 00 SPHERO TM Technical Note STN- Rev B 008 DETERMINING PMT LINEARITY IN FLOW CYTOMETERS USING THE SPHERO TM PMT QUALITY CONTROL EXCEL TEMPLATE Introduction
78 Irma Lee Circle, Unit 0, Lake Forest, Illinois 00 SPHERO TM Technical Note STN- Rev B 008 DETERMINING PMT LINEARITY IN FLOW CYTOMETERS USING THE SPHERO TM PMT QUALITY CONTROL EXCEL TEMPLATE Introduction
CELL CYCLE BASICS. G0/1 = 1X S Phase G2/M = 2X DYE FLUORESCENCE
 CELL CYCLE BASICS Analysis of a population of cells replication state can be achieved by fluorescence labeling of the nuclei of cells in suspension and then analyzing the fluorescence properties of each
CELL CYCLE BASICS Analysis of a population of cells replication state can be achieved by fluorescence labeling of the nuclei of cells in suspension and then analyzing the fluorescence properties of each
FlowJo Basic Tutorial
 FlowJo Basic Tutorial FlowJo is software for the analysis of flow cytometry data. This tutorial will quickly introduce you to FlowJo using an example experiment, where a FITC anti-cd8 reagent is titrated
FlowJo Basic Tutorial FlowJo is software for the analysis of flow cytometry data. This tutorial will quickly introduce you to FlowJo using an example experiment, where a FITC anti-cd8 reagent is titrated
Iris Sample Data Set. Basic Visualization Techniques: Charts, Graphs and Maps. Summary Statistics. Frequency and Mode
 Iris Sample Data Set Basic Visualization Techniques: Charts, Graphs and Maps CS598 Information Visualization Spring 2010 Many of the exploratory data techniques are illustrated with the Iris Plant data
Iris Sample Data Set Basic Visualization Techniques: Charts, Graphs and Maps CS598 Information Visualization Spring 2010 Many of the exploratory data techniques are illustrated with the Iris Plant data
PATHOGEN DETECTION SYSTEMS BY REAL TIME PCR. Results Interpretation Guide
 PATHOGEN DETECTION SYSTEMS BY REAL TIME PCR Results Interpretation Guide Pathogen Detection Systems by Real Time PCR Microbial offers real time PCR based systems for the detection of pathogenic bacteria
PATHOGEN DETECTION SYSTEMS BY REAL TIME PCR Results Interpretation Guide Pathogen Detection Systems by Real Time PCR Microbial offers real time PCR based systems for the detection of pathogenic bacteria
Using BD FACSDiva CST To. Evaluate Cytometer Performance, Create Custom Assay Settings. and
 Using BD FACSDiva CST To Evaluate Cytometer Performance, Create Custom Assay Settings and Implement Cross-Instrument and Cross-Site Standardization of Assays PART 2 Alan M. Stall Director, Advanced Cytometry
Using BD FACSDiva CST To Evaluate Cytometer Performance, Create Custom Assay Settings and Implement Cross-Instrument and Cross-Site Standardization of Assays PART 2 Alan M. Stall Director, Advanced Cytometry
STATS8: Introduction to Biostatistics. Data Exploration. Babak Shahbaba Department of Statistics, UCI
 STATS8: Introduction to Biostatistics Data Exploration Babak Shahbaba Department of Statistics, UCI Introduction After clearly defining the scientific problem, selecting a set of representative members
STATS8: Introduction to Biostatistics Data Exploration Babak Shahbaba Department of Statistics, UCI Introduction After clearly defining the scientific problem, selecting a set of representative members
Diagrams and Graphs of Statistical Data
 Diagrams and Graphs of Statistical Data One of the most effective and interesting alternative way in which a statistical data may be presented is through diagrams and graphs. There are several ways in
Diagrams and Graphs of Statistical Data One of the most effective and interesting alternative way in which a statistical data may be presented is through diagrams and graphs. There are several ways in
Summarizing and Displaying Categorical Data
 Summarizing and Displaying Categorical Data Categorical data can be summarized in a frequency distribution which counts the number of cases, or frequency, that fall into each category, or a relative frequency
Summarizing and Displaying Categorical Data Categorical data can be summarized in a frequency distribution which counts the number of cases, or frequency, that fall into each category, or a relative frequency
Real-time PCR: Understanding C t
 APPLICATION NOTE Real-Time PCR Real-time PCR: Understanding C t Real-time PCR, also called quantitative PCR or qpcr, can provide a simple and elegant method for determining the amount of a target sequence
APPLICATION NOTE Real-Time PCR Real-time PCR: Understanding C t Real-time PCR, also called quantitative PCR or qpcr, can provide a simple and elegant method for determining the amount of a target sequence
Data Visualization Techniques
 Data Visualization Techniques From Basics to Big Data with SAS Visual Analytics WHITE PAPER SAS White Paper Table of Contents Introduction.... 1 Generating the Best Visualizations for Your Data... 2 The
Data Visualization Techniques From Basics to Big Data with SAS Visual Analytics WHITE PAPER SAS White Paper Table of Contents Introduction.... 1 Generating the Best Visualizations for Your Data... 2 The
Below is a list of things you should be aware of before you schedule your sort.
 Sorting at the Flow Cytometry Facility At the present time, the assistance of a trained cell sorter operator is needed for all sorting applications. As such, if you are planning a first time sort you will
Sorting at the Flow Cytometry Facility At the present time, the assistance of a trained cell sorter operator is needed for all sorting applications. As such, if you are planning a first time sort you will
International Year of Light 2015 Tech-Talks BREGENZ: Mehmet Arik Well-Being in Office Applications Light Measurement & Quality Parameters
 www.led-professional.com ISSN 1993-890X Trends & Technologies for Future Lighting Solutions ReviewJan/Feb 2015 Issue LpR 47 International Year of Light 2015 Tech-Talks BREGENZ: Mehmet Arik Well-Being in
www.led-professional.com ISSN 1993-890X Trends & Technologies for Future Lighting Solutions ReviewJan/Feb 2015 Issue LpR 47 International Year of Light 2015 Tech-Talks BREGENZ: Mehmet Arik Well-Being in
GETTING STARTED WITH LABVIEW POINT-BY-POINT VIS
 USER GUIDE GETTING STARTED WITH LABVIEW POINT-BY-POINT VIS Contents Using the LabVIEW Point-By-Point VI Libraries... 2 Initializing Point-By-Point VIs... 3 Frequently Asked Questions... 5 What Are the
USER GUIDE GETTING STARTED WITH LABVIEW POINT-BY-POINT VIS Contents Using the LabVIEW Point-By-Point VI Libraries... 2 Initializing Point-By-Point VIs... 3 Frequently Asked Questions... 5 What Are the
CELL CYCLE BASICS. G0/1 = 1X S Phase G2/M = 2X DYE FLUORESCENCE
 CELL CYCLE BASICS Analysis of a population of cells replication state can be achieved by fluorescence labeling of the nuclei of cells in suspension and then analyzing the fluorescence properties of each
CELL CYCLE BASICS Analysis of a population of cells replication state can be achieved by fluorescence labeling of the nuclei of cells in suspension and then analyzing the fluorescence properties of each
Supplementary Material. Free-radical production after post-thaw incubation of ram spermatozoa is related to decreased in vivo fertility
 10.1071/RD14043_AC CSIRO 2015 Supplementary Material: Reproduction, Fertility and Development, 2015, 27(8), 1187 1196. Supplementary Material Free-radical production after post-thaw incubation of ram spermatozoa
10.1071/RD14043_AC CSIRO 2015 Supplementary Material: Reproduction, Fertility and Development, 2015, 27(8), 1187 1196. Supplementary Material Free-radical production after post-thaw incubation of ram spermatozoa
Technical Bulletin. Standardizing Application Setup Across Multiple Flow Cytometers Using BD FACSDiva Version 6 Software. Abstract
 arch 212 Standardizing Application Setup Across ultiple Flow Cytometers Using BD FACSDiva Version 6 Ellen einelt, ervi Reunanen, ark Edinger, aria Jaimes, Alan Stall, Dennis Sasaki, Joe Trotter Contents
arch 212 Standardizing Application Setup Across ultiple Flow Cytometers Using BD FACSDiva Version 6 Ellen einelt, ervi Reunanen, ark Edinger, aria Jaimes, Alan Stall, Dennis Sasaki, Joe Trotter Contents
123count ebeads Catalog Number: 01-1234 Also known as: Absolute cell count beads GPR: General Purpose Reagents. For Laboratory Use.
 Page 1 of 1 Catalog Number: 01-1234 Also known as: Absolute cell count beads GPR: General Purpose Reagents. For Laboratory Use. Normal human peripheral blood was stained with Anti- Human CD45 PE (cat.
Page 1 of 1 Catalog Number: 01-1234 Also known as: Absolute cell count beads GPR: General Purpose Reagents. For Laboratory Use. Normal human peripheral blood was stained with Anti- Human CD45 PE (cat.
2.500 Threshold. 2.000 1000e - 001. Threshold. Exponential phase. Cycle Number
 application note Real-Time PCR: Understanding C T Real-Time PCR: Understanding C T 4.500 3.500 1000e + 001 4.000 3.000 1000e + 000 3.500 2.500 Threshold 3.000 2.000 1000e - 001 Rn 2500 Rn 1500 Rn 2000
application note Real-Time PCR: Understanding C T Real-Time PCR: Understanding C T 4.500 3.500 1000e + 001 4.000 3.000 1000e + 000 3.500 2.500 Threshold 3.000 2.000 1000e - 001 Rn 2500 Rn 1500 Rn 2000
Clustering & Visualization
 Chapter 5 Clustering & Visualization Clustering in high-dimensional databases is an important problem and there are a number of different clustering paradigms which are applicable to high-dimensional data.
Chapter 5 Clustering & Visualization Clustering in high-dimensional databases is an important problem and there are a number of different clustering paradigms which are applicable to high-dimensional data.
Compensation: An Instrumental Perspective
 Compensation: An Instrumental Perspective Why Digital? Visualization Issues Boston User s Group September 10, 2003 Joe Trotter Joe_Trotter@bd.com Review: Instrument Sensitivity Measuring Sensitivity -
Compensation: An Instrumental Perspective Why Digital? Visualization Issues Boston User s Group September 10, 2003 Joe Trotter Joe_Trotter@bd.com Review: Instrument Sensitivity Measuring Sensitivity -
PROTOCOL. Immunostaining for Flow Cytometry. Background. Materials and equipment required.
 PROTOCOL Immunostaining for Flow Cytometry 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 Background The combination of single cell analysis using flow cytometry and the specificity of antibody-based
PROTOCOL Immunostaining for Flow Cytometry 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 Background The combination of single cell analysis using flow cytometry and the specificity of antibody-based
Data Mining: Exploring Data. Lecture Notes for Chapter 3. Introduction to Data Mining
 Data Mining: Exploring Data Lecture Notes for Chapter 3 Introduction to Data Mining by Tan, Steinbach, Kumar Tan,Steinbach, Kumar Introduction to Data Mining 8/05/2005 1 What is data exploration? A preliminary
Data Mining: Exploring Data Lecture Notes for Chapter 3 Introduction to Data Mining by Tan, Steinbach, Kumar Tan,Steinbach, Kumar Introduction to Data Mining 8/05/2005 1 What is data exploration? A preliminary
Instrument Characterization and Performance Tracking for Digital Flow Cytometers
 Instrument Characterization and Performance Tracking for Digital Flow Cytometers BD Biosciences Cytometer Set-up & Tracking (CS&T) System For Research Use Only. Not for use in diagnostic or therapeutic
Instrument Characterization and Performance Tracking for Digital Flow Cytometers BD Biosciences Cytometer Set-up & Tracking (CS&T) System For Research Use Only. Not for use in diagnostic or therapeutic
A practical approach to multicolor flow cytometry for immunophenotyping
 Journal of Immunological Methods 243 (2000) 77 97 www.elsevier.nl/ locate/ jim A practical approach to multicolor flow cytometry for immunophenotyping Nicole Baumgarth, Mario Roederer a, b, * a Department
Journal of Immunological Methods 243 (2000) 77 97 www.elsevier.nl/ locate/ jim A practical approach to multicolor flow cytometry for immunophenotyping Nicole Baumgarth, Mario Roederer a, b, * a Department
Drawing a histogram using Excel
 Drawing a histogram using Excel STEP 1: Examine the data to decide how many class intervals you need and what the class boundaries should be. (In an assignment you may be told what class boundaries to
Drawing a histogram using Excel STEP 1: Examine the data to decide how many class intervals you need and what the class boundaries should be. (In an assignment you may be told what class boundaries to
COM CO P 5318 Da t Da a t Explora Explor t a ion and Analysis y Chapte Chapt r e 3
 COMP 5318 Data Exploration and Analysis Chapter 3 What is data exploration? A preliminary exploration of the data to better understand its characteristics. Key motivations of data exploration include Helping
COMP 5318 Data Exploration and Analysis Chapter 3 What is data exploration? A preliminary exploration of the data to better understand its characteristics. Key motivations of data exploration include Helping
CSU, Fresno - Institutional Research, Assessment and Planning - Dmitri Rogulkin
 My presentation is about data visualization. How to use visual graphs and charts in order to explore data, discover meaning and report findings. The goal is to show that visual displays can be very effective
My presentation is about data visualization. How to use visual graphs and charts in order to explore data, discover meaning and report findings. The goal is to show that visual displays can be very effective
use. 3,5 Apoptosis Viability Titration Assays
 Introduction Monitoring cell viability can provide critical insights into the effectiveness of biological protocols and the stability of biological systems including: the efficacy of cell harvesting procedures,
Introduction Monitoring cell viability can provide critical insights into the effectiveness of biological protocols and the stability of biological systems including: the efficacy of cell harvesting procedures,
GEOENGINE MSc in Geomatics Engineering (Master Thesis) Anamelechi, Falasy Ebere
 Master s Thesis: ANAMELECHI, FALASY EBERE Analysis of a Raster DEM Creation for a Farm Management Information System based on GNSS and Total Station Coordinates Duration of the Thesis: 6 Months Completion
Master s Thesis: ANAMELECHI, FALASY EBERE Analysis of a Raster DEM Creation for a Farm Management Information System based on GNSS and Total Station Coordinates Duration of the Thesis: 6 Months Completion
Zecotek S Light Projection Network Marketing
 White Paper Zecotek Visible Fiber Laser Platform Enabling the future of laser technology Zecotek Photonics Inc. (TSX- V: ZMS; Frankfurt: W1I) www.zecotek.com is a Canadian photonics technology company
White Paper Zecotek Visible Fiber Laser Platform Enabling the future of laser technology Zecotek Photonics Inc. (TSX- V: ZMS; Frankfurt: W1I) www.zecotek.com is a Canadian photonics technology company
Using Excel for descriptive statistics
 FACT SHEET Using Excel for descriptive statistics Introduction Biologists no longer routinely plot graphs by hand or rely on calculators to carry out difficult and tedious statistical calculations. These
FACT SHEET Using Excel for descriptive statistics Introduction Biologists no longer routinely plot graphs by hand or rely on calculators to carry out difficult and tedious statistical calculations. These
Data Mining: Exploring Data. Lecture Notes for Chapter 3. Slides by Tan, Steinbach, Kumar adapted by Michael Hahsler
 Data Mining: Exploring Data Lecture Notes for Chapter 3 Slides by Tan, Steinbach, Kumar adapted by Michael Hahsler Topics Exploratory Data Analysis Summary Statistics Visualization What is data exploration?
Data Mining: Exploring Data Lecture Notes for Chapter 3 Slides by Tan, Steinbach, Kumar adapted by Michael Hahsler Topics Exploratory Data Analysis Summary Statistics Visualization What is data exploration?
BD CellQuest Pro Software Analysis Tutorial
 BD CellQuest Pro Analysis Tutorial This tutorial guides you through an analysis example using BD CellQuest Pro software. If you are already familiar with BD CellQuest Pro software on Mac OS 9, refer to
BD CellQuest Pro Analysis Tutorial This tutorial guides you through an analysis example using BD CellQuest Pro software. If you are already familiar with BD CellQuest Pro software on Mac OS 9, refer to
Chapter 6: Constructing and Interpreting Graphic Displays of Behavioral Data
 Chapter 6: Constructing and Interpreting Graphic Displays of Behavioral Data Chapter Focus Questions What are the benefits of graphic display and visual analysis of behavioral data? What are the fundamental
Chapter 6: Constructing and Interpreting Graphic Displays of Behavioral Data Chapter Focus Questions What are the benefits of graphic display and visual analysis of behavioral data? What are the fundamental
Deep profiling of multitube flow cytometry data Supplemental information
 Deep profiling of multitube flow cytometry data Supplemental information Kieran O Neill et al December 19, 2014 1 Table S1: Markers in simulated multitube data. The data was split into three tubes, each
Deep profiling of multitube flow cytometry data Supplemental information Kieran O Neill et al December 19, 2014 1 Table S1: Markers in simulated multitube data. The data was split into three tubes, each
MASCOT Search Results Interpretation
 The Mascot protein identification program (Matrix Science, Ltd.) uses statistical methods to assess the validity of a match. MS/MS data is not ideal. That is, there are unassignable peaks (noise) and usually
The Mascot protein identification program (Matrix Science, Ltd.) uses statistical methods to assess the validity of a match. MS/MS data is not ideal. That is, there are unassignable peaks (noise) and usually
GETTING STARTED. 6. Click the New Specimen icon.
 BD FACSDiva Software Basic Experiment Guide Please note, this guide is not intended to take the place of in-person training but is to be used as a reference as needed after completing training with Unified
BD FACSDiva Software Basic Experiment Guide Please note, this guide is not intended to take the place of in-person training but is to be used as a reference as needed after completing training with Unified
Exploratory Data Analysis
 Exploratory Data Analysis Paul Cohen ISTA 370 Spring, 2012 Paul Cohen ISTA 370 () Exploratory Data Analysis Spring, 2012 1 / 46 Outline Data, revisited The purpose of exploratory data analysis Learning
Exploratory Data Analysis Paul Cohen ISTA 370 Spring, 2012 Paul Cohen ISTA 370 () Exploratory Data Analysis Spring, 2012 1 / 46 Outline Data, revisited The purpose of exploratory data analysis Learning
GRADATION OF AGGREGATE FOR CONCRETE BLOCK
 GRADATION OF AGGREGATE FOR CONCRETE BLOCK Although numerous papers have been written concerning the proper gradation for concrete mixes, they have generally dealt with plastic mixes, and very little published
GRADATION OF AGGREGATE FOR CONCRETE BLOCK Although numerous papers have been written concerning the proper gradation for concrete mixes, they have generally dealt with plastic mixes, and very little published
How to operate the BD FACSCanto flow cytometer
 How to operate the BD FACSCanto flow cytometer Preface Dear colleague, the BD FACSCanto flow cytometer is use to operate, however, it is a delicate instrument for measuring fluorescence of single cells.
How to operate the BD FACSCanto flow cytometer Preface Dear colleague, the BD FACSCanto flow cytometer is use to operate, however, it is a delicate instrument for measuring fluorescence of single cells.
Outline. Quantizing Intensities. Achromatic Light. Optical Illusion. Quantizing Intensities. CS 430/585 Computer Graphics I
 CS 430/585 Computer Graphics I Week 8, Lecture 15 Outline Light Physical Properties of Light and Color Eye Mechanism for Color Systems to Define Light and Color David Breen, William Regli and Maxim Peysakhov
CS 430/585 Computer Graphics I Week 8, Lecture 15 Outline Light Physical Properties of Light and Color Eye Mechanism for Color Systems to Define Light and Color David Breen, William Regli and Maxim Peysakhov
Big Data: Rethinking Text Visualization
 Big Data: Rethinking Text Visualization Dr. Anton Heijs anton.heijs@treparel.com Treparel April 8, 2013 Abstract In this white paper we discuss text visualization approaches and how these are important
Big Data: Rethinking Text Visualization Dr. Anton Heijs anton.heijs@treparel.com Treparel April 8, 2013 Abstract In this white paper we discuss text visualization approaches and how these are important
Statistics Chapter 2
 Statistics Chapter 2 Frequency Tables A frequency table organizes quantitative data. partitions data into classes (intervals). shows how many data values are in each class. Test Score Number of Students
Statistics Chapter 2 Frequency Tables A frequency table organizes quantitative data. partitions data into classes (intervals). shows how many data values are in each class. Test Score Number of Students
Grade 6 Mathematics Performance Level Descriptors
 Limited Grade 6 Mathematics Performance Level Descriptors A student performing at the Limited Level demonstrates a minimal command of Ohio s Learning Standards for Grade 6 Mathematics. A student at this
Limited Grade 6 Mathematics Performance Level Descriptors A student performing at the Limited Level demonstrates a minimal command of Ohio s Learning Standards for Grade 6 Mathematics. A student at this
Flow Cytometry A Basic Overview
 Flow Cytometry A Basic Overview Overview Flow cytometry is a powerful technology for investigating many aspects of cell biology and for isolating cells of interest. Flow cytometry utilizes highly focused,
Flow Cytometry A Basic Overview Overview Flow cytometry is a powerful technology for investigating many aspects of cell biology and for isolating cells of interest. Flow cytometry utilizes highly focused,
Analyzer Experiment setup guide for LSRII and Canto s
 Analyzer Experiment setup guide for LSRII and Canto s 1. Check the instrument configuration on our website to determine the most appropriate cytometer for your experiment, https://depts.washington.edu/flowlab/instrumentation.html,
Analyzer Experiment setup guide for LSRII and Canto s 1. Check the instrument configuration on our website to determine the most appropriate cytometer for your experiment, https://depts.washington.edu/flowlab/instrumentation.html,
Exploratory Spatial Data Analysis
 Exploratory Spatial Data Analysis Part II Dynamically Linked Views 1 Contents Introduction: why to use non-cartographic data displays Display linking by object highlighting Dynamic Query Object classification
Exploratory Spatial Data Analysis Part II Dynamically Linked Views 1 Contents Introduction: why to use non-cartographic data displays Display linking by object highlighting Dynamic Query Object classification
Validation of measurement procedures
 Validation of measurement procedures R. Haeckel and I.Püntmann Zentralkrankenhaus Bremen The new ISO standard 15189 which has already been accepted by most nations will soon become the basis for accreditation
Validation of measurement procedures R. Haeckel and I.Püntmann Zentralkrankenhaus Bremen The new ISO standard 15189 which has already been accepted by most nations will soon become the basis for accreditation
Exploratory Data Analysis
 Exploratory Data Analysis Johannes Schauer johannes.schauer@tugraz.at Institute of Statistics Graz University of Technology Steyrergasse 17/IV, 8010 Graz www.statistics.tugraz.at February 12, 2008 Introduction
Exploratory Data Analysis Johannes Schauer johannes.schauer@tugraz.at Institute of Statistics Graz University of Technology Steyrergasse 17/IV, 8010 Graz www.statistics.tugraz.at February 12, 2008 Introduction
Engineering Problem Solving and Excel. EGN 1006 Introduction to Engineering
 Engineering Problem Solving and Excel EGN 1006 Introduction to Engineering Mathematical Solution Procedures Commonly Used in Engineering Analysis Data Analysis Techniques (Statistics) Curve Fitting techniques
Engineering Problem Solving and Excel EGN 1006 Introduction to Engineering Mathematical Solution Procedures Commonly Used in Engineering Analysis Data Analysis Techniques (Statistics) Curve Fitting techniques
Chapter 6. Antigen-Antibody Properties 10/3/2012. Antigen-Antibody Interactions: Principles and Applications. Precipitin reactions
 Chapter 6 Antigen-Antibody Interactions: Principles and Applications Antigen-Antibody Properties You must remember antibody affinity (single) VS avidity (multiple) High affinity: bound tightly and longer!
Chapter 6 Antigen-Antibody Interactions: Principles and Applications Antigen-Antibody Properties You must remember antibody affinity (single) VS avidity (multiple) High affinity: bound tightly and longer!
Data representation and analysis in Excel
 Page 1 Data representation and analysis in Excel Let s Get Started! This course will teach you how to analyze data and make charts in Excel so that the data may be represented in a visual way that reflects
Page 1 Data representation and analysis in Excel Let s Get Started! This course will teach you how to analyze data and make charts in Excel so that the data may be represented in a visual way that reflects
NCSS Statistical Software Principal Components Regression. In ordinary least squares, the regression coefficients are estimated using the formula ( )
 Chapter 340 Principal Components Regression Introduction is a technique for analyzing multiple regression data that suffer from multicollinearity. When multicollinearity occurs, least squares estimates
Chapter 340 Principal Components Regression Introduction is a technique for analyzing multiple regression data that suffer from multicollinearity. When multicollinearity occurs, least squares estimates
Exploratory data analysis (Chapter 2) Fall 2011
 Exploratory data analysis (Chapter 2) Fall 2011 Data Examples Example 1: Survey Data 1 Data collected from a Stat 371 class in Fall 2005 2 They answered questions about their: gender, major, year in school,
Exploratory data analysis (Chapter 2) Fall 2011 Data Examples Example 1: Survey Data 1 Data collected from a Stat 371 class in Fall 2005 2 They answered questions about their: gender, major, year in school,
Atomic Force Microscope and Magnetic Force Microscope Background Information
 Atomic Force Microscope and Magnetic Force Microscope Background Information Lego Building Instructions There are several places to find the building instructions for building the Lego models of atomic
Atomic Force Microscope and Magnetic Force Microscope Background Information Lego Building Instructions There are several places to find the building instructions for building the Lego models of atomic
MiSeq: Imaging and Base Calling
 MiSeq: Imaging and Page Welcome Navigation Presenter Introduction MiSeq Sequencing Workflow Narration Welcome to MiSeq: Imaging and. This course takes 35 minutes to complete. Click Next to continue. Please
MiSeq: Imaging and Page Welcome Navigation Presenter Introduction MiSeq Sequencing Workflow Narration Welcome to MiSeq: Imaging and. This course takes 35 minutes to complete. Click Next to continue. Please
Application Note 10. Measurement of Cell Recovery. After Sorting with a Catcher-Tube-Based. Cell Sorter. Introduction
 Application Note 10 Measurement of Cell Recovery After Sorting with a Catcher-Tube-Based Cell Sorter Introduction In many experiments using sorted cells, it is important to be able to count the number
Application Note 10 Measurement of Cell Recovery After Sorting with a Catcher-Tube-Based Cell Sorter Introduction In many experiments using sorted cells, it is important to be able to count the number
SECTION 2-1: OVERVIEW SECTION 2-2: FREQUENCY DISTRIBUTIONS
 SECTION 2-1: OVERVIEW Chapter 2 Describing, Exploring and Comparing Data 19 In this chapter, we will use the capabilities of Excel to help us look more carefully at sets of data. We can do this by re-organizing
SECTION 2-1: OVERVIEW Chapter 2 Describing, Exploring and Comparing Data 19 In this chapter, we will use the capabilities of Excel to help us look more carefully at sets of data. We can do this by re-organizing
Exercise 1: How to Record and Present Your Data Graphically Using Excel Dr. Chris Paradise, edited by Steven J. Price
 Biology 1 Exercise 1: How to Record and Present Your Data Graphically Using Excel Dr. Chris Paradise, edited by Steven J. Price Introduction In this world of high technology and information overload scientists
Biology 1 Exercise 1: How to Record and Present Your Data Graphically Using Excel Dr. Chris Paradise, edited by Steven J. Price Introduction In this world of high technology and information overload scientists
Name Class Date. spectrum. White is not a color, but is a combination of all colors. Black is not a color; it is the absence of all light.
 Exercises 28.1 The Spectrum (pages 555 556) 1. Isaac Newton was the first person to do a systematic study of color. 2. Circle the letter of each statement that is true about Newton s study of color. a.
Exercises 28.1 The Spectrum (pages 555 556) 1. Isaac Newton was the first person to do a systematic study of color. 2. Circle the letter of each statement that is true about Newton s study of color. a.
Component Ordering in Independent Component Analysis Based on Data Power
 Component Ordering in Independent Component Analysis Based on Data Power Anne Hendrikse Raymond Veldhuis University of Twente University of Twente Fac. EEMCS, Signals and Systems Group Fac. EEMCS, Signals
Component Ordering in Independent Component Analysis Based on Data Power Anne Hendrikse Raymond Veldhuis University of Twente University of Twente Fac. EEMCS, Signals and Systems Group Fac. EEMCS, Signals
Excel -- Creating Charts
 Excel -- Creating Charts The saying goes, A picture is worth a thousand words, and so true. Professional looking charts give visual enhancement to your statistics, fiscal reports or presentation. Excel
Excel -- Creating Charts The saying goes, A picture is worth a thousand words, and so true. Professional looking charts give visual enhancement to your statistics, fiscal reports or presentation. Excel
Technical Note. Roche Applied Science. No. LC 18/2004. Assay Formats for Use in Real-Time PCR
 Roche Applied Science Technical Note No. LC 18/2004 Purpose of this Note Assay Formats for Use in Real-Time PCR The LightCycler Instrument uses several detection channels to monitor the amplification of
Roche Applied Science Technical Note No. LC 18/2004 Purpose of this Note Assay Formats for Use in Real-Time PCR The LightCycler Instrument uses several detection channels to monitor the amplification of
A Picture Really Is Worth a Thousand Words
 4 A Picture Really Is Worth a Thousand Words Difficulty Scale (pretty easy, but not a cinch) What you ll learn about in this chapter Why a picture is really worth a thousand words How to create a histogram
4 A Picture Really Is Worth a Thousand Words Difficulty Scale (pretty easy, but not a cinch) What you ll learn about in this chapter Why a picture is really worth a thousand words How to create a histogram
