Nanotechnology & Society: Activity 1
|
|
|
- Theodora Clarke
- 10 years ago
- Views:
Transcription
1 Nanotechnology & Society: Activity 1 Water Clean-up Dirty Business I m Taylor. My friend Gabe and I love the water, but we know that keeping it clean is not always easy. Just upriver in Palmerton, Pennsylvania, sits an old zinc smelting plant that was one of the factories polluting the local rivers and streams. We headed to The Franklin in Philadelphia and learned that nanoiron is being used to clean up contaminants and keep our water safe. Because it is so small, nanoiron can seep through the soil and attach to pollutants both in the ground and in the water. Our question: Can nanoiron clean up the pollution in soil and prevent it from getting into drinking water? We met up with a researcher from Penn State University who helped us collect some soil samples for testing. We tested for metals like lead, nickel and copper both before and after treatment with nanoiron. Nano Matters The field of nanotechnology covers many disciplines of science and engineering. Numerous efforts are focused on providing solutions for some of the world s environmental problems: dirty drinking water, nonrenewable energy sources and pollution. Here, we focus on the use of nanoiron to neutralize heavy metal toxins and prevent them from entering the drinking water supply. Because of its small size, nanoiron has a large amount of exposed surface area available to neutralize many different types of pollutants such as herbicides, pesticides and other industrial waste. Nanoiron can be injected directly into the ground and maneuver through soil, sand, silt and even fractured rock to reach pollutants. This remediation technique is currently being used in sites across the country. The scientist featured in this segment is also doing work in Ghana to see if this technology can be used to help people around the world who struggle daily for clean water. 56
2 Icebreaker Make a model well. 20 minutes DragonflyTV Skill: Observing Guide your kids as they 1) Place the toilet paper tube (your well) upright in the center of the empty coffee can. 2) Hold the tube and pour gravel or small rocks around it, about 1 2 deep. Be careful not to get anything inside the tube. 3) Place a few drops of food coloring on top of the gravel. This represents pollutants present in the soil. 4) Pour sand on top of the gravel about 1 2 deep. Again, being careful not to get anything inside the tube. 5) Take a cup of water (representing rainwater) and slowly pour the water onto the sand until it just reaches the top. What happens inside the tube? You ll need: empty coffee can empty toilet paper tube sand gravel or aquarium rocks food coloring cup water Are you a nano-bit curious? In this segment, the kids investigate one way nanotechnologists are working to clean pollutants in water. When pollutants are present in the ground, they can make their way into the water we drink, just as you saw in the model of a well in this activity. When it rains, the water enters the ground and falls through the pores and cracks in the rocks and dirt until it reaches the groundwater reservoir below. The food coloring represents pollutants in the ground that can be carried into the groundwater and eventually enter water sources such as wells, rivers and streams. Nanoscientists are excited about possibilities such as nanoiron, because it is small enough to fit through the pores in the rocks and dirt to reach the contaminants both in the ground (preventing them from eventually entering the water supply) and in the groundwater itself. 57
3 Investigation Use this water filter to explore remediation techniques. 3 hours Preparation Drill a small hole in the cap of each soda bottle. This activity will work best if the holes are the same size. 1) Break into groups. Each group should make 3 groundwater models by taking old soda bottles and cutting off the bottoms. 2) Place the caps on the bottles. Flip the bottles (with cap sides down) and add rocks, gravel, sand and dirt to create your ground. (These items should be added in order. See the image on the page 60.) 3) The bottles need to sit above the clear cups. If you do not have ring stands to suspend them, take a piece of cardboard and cut out a hole so the bottle fits inside without falling through and place this over the cup. (See the illustration on page 60.) 4) In each of the 3 clear cups, add approximately 1/2 cup water plus 20 drops of iodine. Then, add about 6 drops of liquid starch to each cup until the color turns a dark purple (almost black). This represents the pollutants in the groundwater. Place these cups on a sheet of white paper so the reaction will be easier to see. 5) Take 1/2 cup of warm water and add 2 vitamin C tablets. Stir with a Popsicle stick to dissolve. (Note: the tablets do not have to completely dissolve for the reaction to work.) 6) Mix the clear Kool-Aid according to the recipe on the package. (You do not need to add sugar.) 7) To the top of each ground model (bottle) add one of the following: 1/2 cup of liquid vitamin C, 1/2 cup of lemon juice or 1/2 cup Kool-Aid. (It is best to do this sequentially, so you can watch each model separately.) You ll need: iodine (from a drugstore) water spray starch (liquid form not aerosol is best) rocks pebbles sand dirt clear cups old soda bottles (20 ounce) with caps ring stands or pieces of cardboard vitamin C tablets lemon juice Kool-Aid (clear) Popsicle sticks white paper Continued on next page... 58
4 Investigation Continued 8) Monitor as the drops begin to fall through the ground and reach the pollutants in the groundwater. You may want to swirl the cups containing pollutants. There are several ways to check the effectiveness of the reaction (changing from dark blue to clear): a. Count how many drops it takes for the color change to occur. b. Start a timer when each liquid is added and mark the time when the pollutants are completely neutralized (only accurate if drip rates are equivalent). c. Weigh each cup prior to the experiment. After the color has changed, remove the cup and weigh it again. Record the difference in weight. d. Measure the volume of liquid prior to the experiment. After the color has changed, measure the final volume of the liquid in a graduated cylinder. Record the difference in weight. 9) Which solution worked best? Why? This activity was created in part through the Penn State MRSEC and The Franklin Institute MPS: Internships in Public Science Education program. DFTV Science Helper The liquid starch in this experiment is not a requirement and only acts as an indicator. If you add vitamin C to iodine, the color will disappear, but it is hard to see the exact moment of the switch. When starch (a coiled molecule) is mixed with iodine, groups of three iodine atoms squeeze into the spaces within the molecule s coils, changing the color from brown to dark blue. The iodine can still react with the vitamin C, making it easier to determine the end point of the experiment. 59
5 vitamin c, lemon juice or Kool-Aid dirt sand gravel rocks startch-iodine mixture pollutants ring stand cardboard stand 60
6 Are you a nano-bit curious? You conducted a reduction-oxidation (redox) reaction in which vitamin C acted as a reducing agent. Vitamin C (ascorbic acid) gave up electrons to iodine (I3) and caused it to break up into 3 ions of charged I -. When this occurs, the iodine (or iodine/starch complex) loses its color (see Science Helper on page 59). Nanoiron is another example of a reducing agent. In the same way, nanoparticles of iron donate electrons to contaminants in the groundwater converting them to nontoxic forms. DFTV Kids Synthesize Data and Analysis From this graph, can you tell which substance is the most powerful reducing agent (i.e., caused the quickest color change)? Which is the weakest? Volume of liquid for complete color change (mililiters) Vitamin C Lemon Juice Kool-Aid Keep Exploring! This activity doubles as a great way to test how much vitamin C is in a substance. Is there any vitamin C in lemon-lime soda? White grape juice? Vitamin water? Sports drinks? Test them to see! 61
Mixtures. reflect. How is seawater different from pure water? How is it different from rocky soil?
 reflect Everything around us is made out of tiny bits of matter. These particles may combine in different ways to produce new materials. Sometimes we need to separate the parts of a material. If we know
reflect Everything around us is made out of tiny bits of matter. These particles may combine in different ways to produce new materials. Sometimes we need to separate the parts of a material. If we know
Chapter 3: Separating Mixtures (pg. 54 81)
 Chapter 3: Separating Mixtures (pg. 54 81) 3.2: Separating Mechanical Mixtures (PB Pg. 40 5 & TB Pg. 58 61): Name: Date: Check Your Understanding & Learning (PB pg. 40 & TB pg. 61): 1. What are four methods
Chapter 3: Separating Mixtures (pg. 54 81) 3.2: Separating Mechanical Mixtures (PB Pg. 40 5 & TB Pg. 58 61): Name: Date: Check Your Understanding & Learning (PB pg. 40 & TB pg. 61): 1. What are four methods
Lesson Plan: How Do We Clean Polluted Water?
 Lesson Plan: How Do We Clean Polluted Water? Oil Spill Cleanup / Phosphate Cleanup / Groundwater Contamination / Water Treatment Simulation Estimated Time: 2-4 days State Standards taught and addressed
Lesson Plan: How Do We Clean Polluted Water? Oil Spill Cleanup / Phosphate Cleanup / Groundwater Contamination / Water Treatment Simulation Estimated Time: 2-4 days State Standards taught and addressed
Acids and Bases. AND a widemouth container of the following solids:
 Acids and Bases GOAL To introduce students to acids and bases. MATERIALS: 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz. bottle of water - labeled Water 1 4 oz. bottle of
Acids and Bases GOAL To introduce students to acids and bases. MATERIALS: 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz. bottle of water - labeled Water 1 4 oz. bottle of
OBJECTIVES: Visitors learn what an antioxidant is and how it behaves. They also learn how to test for the presence of vitamin C..
 Vitamin C Visitors use iodine to compare the reactivity of two starch solutions one with vitamin C added, one without vitamin C. OBJECTIVES: Visitors learn what an antioxidant is and how it behaves. They
Vitamin C Visitors use iodine to compare the reactivity of two starch solutions one with vitamin C added, one without vitamin C. OBJECTIVES: Visitors learn what an antioxidant is and how it behaves. They
Determination of a Chemical Formula
 1 Determination of a Chemical Formula Introduction Molar Ratios Elements combine in fixed ratios to form compounds. For example, consider the compound TiCl 4 (titanium chloride). Each molecule of TiCl
1 Determination of a Chemical Formula Introduction Molar Ratios Elements combine in fixed ratios to form compounds. For example, consider the compound TiCl 4 (titanium chloride). Each molecule of TiCl
Integrated Physics & Chemistry Supply List (2010)
 Integrated Physics & Chemistry Supply List (2010) Integrated Physics and Chemistry is a physical science course covering basic concepts found in chemistry and physics. Topics included in the study are
Integrated Physics & Chemistry Supply List (2010) Integrated Physics and Chemistry is a physical science course covering basic concepts found in chemistry and physics. Topics included in the study are
Acids and Bases: Cabbage Juice ph Indicator
 Acids and Bases: Cabbage Juice ph Indicator Student Advanced Version Acids and bases are found in a variety of everyday items, including food and drink, medicine, and cleaning products. In this lab, we
Acids and Bases: Cabbage Juice ph Indicator Student Advanced Version Acids and bases are found in a variety of everyday items, including food and drink, medicine, and cleaning products. In this lab, we
Vitamin C Content of Fruit Juice
 1 Vitamin C Content of Fruit Juice Introduction Vitamin C Vitamins are organic compounds that have important biological functions. For instance, in humans they enable a variety of enzymes in the body to
1 Vitamin C Content of Fruit Juice Introduction Vitamin C Vitamins are organic compounds that have important biological functions. For instance, in humans they enable a variety of enzymes in the body to
ANALYSIS OF VITAMIN C
 Purpose To learn how to analyze food for vitamin C content and to examine various sources for vitamin C content. Caution Handle the glassware with caution to prevent breakage. When using a burner in the
Purpose To learn how to analyze food for vitamin C content and to examine various sources for vitamin C content. Caution Handle the glassware with caution to prevent breakage. When using a burner in the
Recovery of Elemental Copper from Copper (II) Nitrate
 Recovery of Elemental Copper from Copper (II) Nitrate Objectives: Challenge: Students should be able to - recognize evidence(s) of a chemical change - convert word equations into formula equations - perform
Recovery of Elemental Copper from Copper (II) Nitrate Objectives: Challenge: Students should be able to - recognize evidence(s) of a chemical change - convert word equations into formula equations - perform
Hydrogeology Experiment on Surface-Groundwater Interactions: How Do Our Actions Affect Water Quantity and Quality?
 Name: Period: Hydrogeology Experiment on Surface-Groundwater Interactions: How Do Our Actions Affect Water Quantity and Quality? Purpose/Objective: Students will learn how groundcover influences surface
Name: Period: Hydrogeology Experiment on Surface-Groundwater Interactions: How Do Our Actions Affect Water Quantity and Quality? Purpose/Objective: Students will learn how groundcover influences surface
Luminol Test PROCESS SKILLS SCIENCE TOPICS VOCABULARY
 EXPERIMENT: LUMINOL TEST Luminol Test Visitors mix a solution of luminol with fake blood (hydrogen peroxide) to produce a reaction that gives off blue light. OBJECTIVES: Visitors learn that some chemical
EXPERIMENT: LUMINOL TEST Luminol Test Visitors mix a solution of luminol with fake blood (hydrogen peroxide) to produce a reaction that gives off blue light. OBJECTIVES: Visitors learn that some chemical
Unit 1 - Pure Substances and Mixtures Chapter 2: Solutions
 2.1 Solutes & Solvents Vocabulary: Unit 1 - Pure Substances and Mixtures Chapter 2: Solutions solvent the larger part of a solution - the part of a solution into which the solutes dissolve solute the smaller
2.1 Solutes & Solvents Vocabulary: Unit 1 - Pure Substances and Mixtures Chapter 2: Solutions solvent the larger part of a solution - the part of a solution into which the solutes dissolve solute the smaller
Solutions and Suspensions
 Science Unit: Lesson 11: Matter Solutions and Suspensions School year: 2005/2006 Developed for: Developed by: Grade level: Duration of Lesson McBride Elementary School, Vancouver School District Catriona
Science Unit: Lesson 11: Matter Solutions and Suspensions School year: 2005/2006 Developed for: Developed by: Grade level: Duration of Lesson McBride Elementary School, Vancouver School District Catriona
Review and apply Investigation 5. Let s review Pages 311-312
 Review and apply Investigation 5 Let s review Pages 311-312 1. After you tested all the known powders with all the test liquids, describe what you did to identify the unknown powder. Students should have
Review and apply Investigation 5 Let s review Pages 311-312 1. After you tested all the known powders with all the test liquids, describe what you did to identify the unknown powder. Students should have
CHM 130LL: ph, Buffers, and Indicators
 CHM 130LL: ph, Buffers, and Indicators Many substances can be classified as acidic or basic. Acidic substances contain hydrogen ions, H +, while basic substances contain hydroxide ions, OH. The relative
CHM 130LL: ph, Buffers, and Indicators Many substances can be classified as acidic or basic. Acidic substances contain hydrogen ions, H +, while basic substances contain hydroxide ions, OH. The relative
The Amazing Elephant Toothpaste! Lesson Overview
 The Amazing Elephant Toothpaste! Lesson Overview Students will investigate chemical change. Suggested Grade Levels: 3-8 Standards for Lesson Content Standard A: Science as Inquiry Content Standard B: Physical
The Amazing Elephant Toothpaste! Lesson Overview Students will investigate chemical change. Suggested Grade Levels: 3-8 Standards for Lesson Content Standard A: Science as Inquiry Content Standard B: Physical
Human Impact on the Environment and Pollution 2 nd or 3 rd Grade Bret Underwood
 Human Impact on the Environment and Pollution 2 nd or 3 rd Grade Bret Underwood Benchmarks: SLC 14: Students identify and describe the relationship between human activities and the environment in terms
Human Impact on the Environment and Pollution 2 nd or 3 rd Grade Bret Underwood Benchmarks: SLC 14: Students identify and describe the relationship between human activities and the environment in terms
Water Treatment Filtration Lab. discharged into an aquatic ecosystem? We had to build a water filtration system with
 Water Treatment Filtration Lab Brandon Lyons P.5 APES Abstract: How could polluted water be remediated so that it could support life when it is discharged into an aquatic ecosystem? We had to build a water
Water Treatment Filtration Lab Brandon Lyons P.5 APES Abstract: How could polluted water be remediated so that it could support life when it is discharged into an aquatic ecosystem? We had to build a water
Lesson Plan for Lava Lamps
 Lesson Plan for Lava Lamps Written by Liz Roth-Johnson and Perry Roth-Johnson Introduction & Background Information Although we cannot see them with our eyes, all things are made up of molecules. Different
Lesson Plan for Lava Lamps Written by Liz Roth-Johnson and Perry Roth-Johnson Introduction & Background Information Although we cannot see them with our eyes, all things are made up of molecules. Different
Chemical versus Physical Changes
 Chemical versus Physical Changes Permission to Copy - This document may be reproduced for non-commercial educational purposes Copyright 2009 General Electric Company What are physical and chemical changes?
Chemical versus Physical Changes Permission to Copy - This document may be reproduced for non-commercial educational purposes Copyright 2009 General Electric Company What are physical and chemical changes?
Wetland or Marsh Water Filter
 Wetland or Marsh Water Filter Objectives: Students will plan and conduct a simple investigation Students will communicate their investigations and explanations Students will learn how a wetland works to
Wetland or Marsh Water Filter Objectives: Students will plan and conduct a simple investigation Students will communicate their investigations and explanations Students will learn how a wetland works to
Physical and Chemical Changes
 Physical and Chemical Changes Jana Barrow West Point Jr. High 2775 W 550 N 801-402-8100 West Point, UT 84015 [email protected] Eighth Grade Integrated Science Standard I: Students will understand the
Physical and Chemical Changes Jana Barrow West Point Jr. High 2775 W 550 N 801-402-8100 West Point, UT 84015 [email protected] Eighth Grade Integrated Science Standard I: Students will understand the
Properties of Acids and Bases
 Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? What
Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? What
Operation Oil Spill Cleanup
 Name Class Date Inquiry Lab Operation Oil Spill Cleanup DESIGN YOUR OWN Offshore oil drilling and the use of supertankers for transporting oil pose the risk of oil spills. Oil spills can damage commercial
Name Class Date Inquiry Lab Operation Oil Spill Cleanup DESIGN YOUR OWN Offshore oil drilling and the use of supertankers for transporting oil pose the risk of oil spills. Oil spills can damage commercial
SOLUBILITY OF A SALT IN WATER AT VARIOUS TEMPERATURES LAB
 SOLUBILITY OF A SALT IN WATER AT VARIOUS TEMPERATURES LAB Purpose: Most ionic compounds are considered by chemists to be salts and many of these are water soluble. In this lab, you will determine the solubility,
SOLUBILITY OF A SALT IN WATER AT VARIOUS TEMPERATURES LAB Purpose: Most ionic compounds are considered by chemists to be salts and many of these are water soluble. In this lab, you will determine the solubility,
The Acid Test Grade Nine
 Ohio Standards Connection: Physical Sciences Benchmark B Explain how atoms react with each other to form other substances and how molecules react with each other or other atoms to form even different substances.
Ohio Standards Connection: Physical Sciences Benchmark B Explain how atoms react with each other to form other substances and how molecules react with each other or other atoms to form even different substances.
A GROUNDWATER PROTECTION PLAN FOR HOME HEATING OIL TANKS
 A GROUNDWATER PROTECTION PLAN FOR HOME HEATING OIL TANKS What is a groundwater protection plan? A groundwater protection plan identifies the activities being conducted that can pollute groundwater and
A GROUNDWATER PROTECTION PLAN FOR HOME HEATING OIL TANKS What is a groundwater protection plan? A groundwater protection plan identifies the activities being conducted that can pollute groundwater and
Lesson Plan: How Do We Know What is Healthy Water?
 Lesson Plan: How Do We Know What is Healthy Water? Estimated Time: 1-3 days ph /Chlorine / Hardness State Standards taught and addressed Grade 8: Standards Taught (and evaluated at end of lesson) Science
Lesson Plan: How Do We Know What is Healthy Water? Estimated Time: 1-3 days ph /Chlorine / Hardness State Standards taught and addressed Grade 8: Standards Taught (and evaluated at end of lesson) Science
Lesson 4: What Makes Water Healthy?
 Lesson 4: What Makes Water Healthy? Activity: Students make observations and measurements of several water samples. This activity helps students think about different ways to determine water quality. Grade
Lesson 4: What Makes Water Healthy? Activity: Students make observations and measurements of several water samples. This activity helps students think about different ways to determine water quality. Grade
Wherever chemical solutions are involved, ph matters. Some
 47 Acids, Bases, and the ph Scale r e a d i n g Wherever chemical solutions are involved, ph matters. Some important chemical reactions, such as those involved in corrosion of iron or digestion of food,
47 Acids, Bases, and the ph Scale r e a d i n g Wherever chemical solutions are involved, ph matters. Some important chemical reactions, such as those involved in corrosion of iron or digestion of food,
Household Acids and Bases
 Household Acids and Bases GRADE LEVEL INDICATORS Experiment Demonstrate that the ph scale (0-14) is used to measure acidity and classify substances or solutions as acidic, basic, or neutral. 21 Develop
Household Acids and Bases GRADE LEVEL INDICATORS Experiment Demonstrate that the ph scale (0-14) is used to measure acidity and classify substances or solutions as acidic, basic, or neutral. 21 Develop
ACIDS AND BASES SAFETY PRECAUTIONS
 ACIDS AND BASES Mild acids and bases are used in cooking (their reaction makes biscuits and bread rise). Acids such as those in our stomachs eat away at food or digest it. Strong acids and bases are used
ACIDS AND BASES Mild acids and bases are used in cooking (their reaction makes biscuits and bread rise). Acids such as those in our stomachs eat away at food or digest it. Strong acids and bases are used
VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE. Acids and Bases. Fall 2012
 VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE Acids and Bases Fall 2012 GOAL: To introduce students to acids and bases. MATERIALS 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz.
VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE Acids and Bases Fall 2012 GOAL: To introduce students to acids and bases. MATERIALS 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz.
Vitamin C Content of Foods
 Vitamin C Content of Foods Experiment #11 Objective: To measure the heat and alkaline stability of vitamin C and its quantity in juices or tablets. Introduction Vitamin C is an essential component of the
Vitamin C Content of Foods Experiment #11 Objective: To measure the heat and alkaline stability of vitamin C and its quantity in juices or tablets. Introduction Vitamin C is an essential component of the
Chem 100 Lab Experiment #9 - ACID/BASE INDICATORS
 Lab #9 Chem 100 Lab Experiment #9 - ACID/BASE INDICATORS Name: Purpose: In this laboratory we will investigate how indicators can be used to test for the presence of acids or bases in a number of common
Lab #9 Chem 100 Lab Experiment #9 - ACID/BASE INDICATORS Name: Purpose: In this laboratory we will investigate how indicators can be used to test for the presence of acids or bases in a number of common
A PRIMER ON ph. A Presentation for ASTA Conference September 30-October 1, 2008
 A PRIMER ON ph A Presentation for ASTA Conference September 30-October 1, 2008 Rebecca Richardson Martha Gothard Director Science Specialist Regional In-Service Education Center AMSTI University of Montevallo
A PRIMER ON ph A Presentation for ASTA Conference September 30-October 1, 2008 Rebecca Richardson Martha Gothard Director Science Specialist Regional In-Service Education Center AMSTI University of Montevallo
We use in our daily life a large
 5 Acids, Bases and Salts We use in our daily life a large number of substances such as lemon, tamarind, common salt, sugar and vinegar. Do they have the same taste? Let us recall tastes of some edible
5 Acids, Bases and Salts We use in our daily life a large number of substances such as lemon, tamarind, common salt, sugar and vinegar. Do they have the same taste? Let us recall tastes of some edible
Dukes of Hazard Activity Time:
 Dukes of Hazard Activity Time: TEACHERS: Read Populution and Water Source Protection on page 80 of Peel Water Story book. Objectives: Students will: Identify household hazardous waste (HHW) materials around
Dukes of Hazard Activity Time: TEACHERS: Read Populution and Water Source Protection on page 80 of Peel Water Story book. Objectives: Students will: Identify household hazardous waste (HHW) materials around
How To Understand And Understand The Effects Of Pollution And Water Quality
 Lesson 2. Pollution and Water Quality Keywords: pollutants, water pollution, point source, non-point source, urban pollution, agricultural pollution, atmospheric pollution, smog, nutrient pollution, eutrophication,
Lesson 2. Pollution and Water Quality Keywords: pollutants, water pollution, point source, non-point source, urban pollution, agricultural pollution, atmospheric pollution, smog, nutrient pollution, eutrophication,
Return to Lab Menu. Acids and Bases in Your House
 Return to Lab Menu Acids and Bases in Your House OBJECTIVES Isolate a natural acid-base indicator. Determine the acid-base properties of common household solutions. INTRODUCTION Acids and bases are among
Return to Lab Menu Acids and Bases in Your House OBJECTIVES Isolate a natural acid-base indicator. Determine the acid-base properties of common household solutions. INTRODUCTION Acids and bases are among
Hands-On Labs SM-1 Lab Manual
 EXPERIMENT 4: Separation of a Mixture of Solids Read the entire experiment and organize time, materials, and work space before beginning. Remember to review the safety sections and wear goggles when appropriate.
EXPERIMENT 4: Separation of a Mixture of Solids Read the entire experiment and organize time, materials, and work space before beginning. Remember to review the safety sections and wear goggles when appropriate.
Determination of Vitamin C of Citrus Juices. by Dr. Walter Scharf and Dr. Charles Malerich Natural Sciences, Baruch College New York, NY 10010
 Determination of Vitamin C of Citrus Juices by Dr. Walter Scharf and Dr. Charles Malerich Natural Sciences, Baruch College New York, NY 10010 Introduction--Vitamin C is a water-soluble vitamin that is
Determination of Vitamin C of Citrus Juices by Dr. Walter Scharf and Dr. Charles Malerich Natural Sciences, Baruch College New York, NY 10010 Introduction--Vitamin C is a water-soluble vitamin that is
Chemical Changes. Measuring a Chemical Reaction. Name(s)
 Chemical Changes Name(s) In the particle model of matter, individual atoms can be bound tightly to other atoms to form molecules. For example, water molecules are made up of two hydrogen atoms bound to
Chemical Changes Name(s) In the particle model of matter, individual atoms can be bound tightly to other atoms to form molecules. For example, water molecules are made up of two hydrogen atoms bound to
Enzyme Pre-Lab. Using the Enzyme worksheet and Enzyme lab handout answer the Pre-Lab questions the pre-lab must be complete before beginning the lab.
 Enzyme Pre-Lab Using the Enzyme worksheet and Enzyme lab handout answer the Pre-Lab questions the pre-lab must be complete before beginning the lab. Background: In this investigation, you will study several
Enzyme Pre-Lab Using the Enzyme worksheet and Enzyme lab handout answer the Pre-Lab questions the pre-lab must be complete before beginning the lab. Background: In this investigation, you will study several
STANDARDIZATION OF A SODIUM HYDROXIDE SOLUTION EXPERIMENT 14
 STANDARDIZATION OF A SODIUM HYDROXIDE SOLUTION EXPERIMENT 14 OBJECTIVE The objective of this experiment will be the standardization of sodium hydroxide using potassium hydrogen phthalate by the titration
STANDARDIZATION OF A SODIUM HYDROXIDE SOLUTION EXPERIMENT 14 OBJECTIVE The objective of this experiment will be the standardization of sodium hydroxide using potassium hydrogen phthalate by the titration
Acids & Bases Around the House Use a ph indicator to find acids and bases
 Use a ph indicator to find acids and bases Description: Visitors predict whether various household solutions are acids or bases, and test their hypotheses using a universal ph indicator. Then, visitors
Use a ph indicator to find acids and bases Description: Visitors predict whether various household solutions are acids or bases, and test their hypotheses using a universal ph indicator. Then, visitors
TITRATION OF VITAMIN C
 TITRATION OF VITAMIN C Introduction: In this lab, we will be performing two different types of titrations on ascorbic acid, more commonly known as Vitamin C. The first will be an acid-base titration in
TITRATION OF VITAMIN C Introduction: In this lab, we will be performing two different types of titrations on ascorbic acid, more commonly known as Vitamin C. The first will be an acid-base titration in
ph Measurements of Common Substances
 Chem 100 Section Experiment 10 Name Partner s Name Introduction ph Measurements of Common Substances The concentration of an acid or base is frequently expressed as ph. Historically, ph stands for the
Chem 100 Section Experiment 10 Name Partner s Name Introduction ph Measurements of Common Substances The concentration of an acid or base is frequently expressed as ph. Historically, ph stands for the
ANSWER KEY. Acids, Bases, and Solutions. Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries,
 Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries, and grass 2. Answers will vary. Sample: Cut 5 g of cherries into small pieces and place in blender. Blend for two minutes,
Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries, and grass 2. Answers will vary. Sample: Cut 5 g of cherries into small pieces and place in blender. Blend for two minutes,
The Water Cycle Now You See It, Now You Don t
 The Water Cycle Now You See It, Now You Don t Unit: Salinity Patterns & the Water Cycle l Grade Level: Elementary l Time Required: Introduction - 30 min. - Activity as groups 45min Wrap Up 20 min l Content
The Water Cycle Now You See It, Now You Don t Unit: Salinity Patterns & the Water Cycle l Grade Level: Elementary l Time Required: Introduction - 30 min. - Activity as groups 45min Wrap Up 20 min l Content
Water Un-Mix-ology & Purification!
 Water Un-Mix-ology & Purification! Subject Area(s): Associated Unit: Associated Lesson: Activity Title : water, physical properties, temperature, mixing Properties of Water (Grade 4, NYC PS) Water Un-Mix-ology
Water Un-Mix-ology & Purification! Subject Area(s): Associated Unit: Associated Lesson: Activity Title : water, physical properties, temperature, mixing Properties of Water (Grade 4, NYC PS) Water Un-Mix-ology
Extraction: Separation of Acidic Substances
 Extraction: Separation of Acidic Substances Chemists frequently find it necessary to separate a mixture of compounds by moving a component from one solution or mixture to another. The process most often
Extraction: Separation of Acidic Substances Chemists frequently find it necessary to separate a mixture of compounds by moving a component from one solution or mixture to another. The process most often
Lesson Plan Title: Water filtration model
 Lesson Plan Title: Water filtration model Concept / Topic to Teach: How water is filtered naturally through the layers underneath the ground, and how to replicate/ represent this on a smaller scale. Target
Lesson Plan Title: Water filtration model Concept / Topic to Teach: How water is filtered naturally through the layers underneath the ground, and how to replicate/ represent this on a smaller scale. Target
Experiment 8 Synthesis of Aspirin
 Experiment 8 Synthesis of Aspirin Aspirin is an effective analgesic (pain reliever), antipyretic (fever reducer) and anti-inflammatory agent and is one of the most widely used non-prescription drugs. The
Experiment 8 Synthesis of Aspirin Aspirin is an effective analgesic (pain reliever), antipyretic (fever reducer) and anti-inflammatory agent and is one of the most widely used non-prescription drugs. The
Lead Testing and On Site Calibration for Water Testing Detection Range: 2 100ppb
 Document: AND Lead 100 7 2013 Lead Testing and On Site Calibration for Water Testing Detection Range: 2 100ppb July, 2013 Edition 1 ANDalyze, Inc., 2012. All rights reserved. Printed in USA. Table of Contents
Document: AND Lead 100 7 2013 Lead Testing and On Site Calibration for Water Testing Detection Range: 2 100ppb July, 2013 Edition 1 ANDalyze, Inc., 2012. All rights reserved. Printed in USA. Table of Contents
Determination of Ascorbic Acid in Vitamin C Tablets by Redox and Acid/Base Titrations
 hemistry 211 Spring 2011 Purpose: Determination of Ascorbic Acid in Vitamin Tablets by Redox and Acid/Base Titrations To determine the quantity of Vitamin (ascorbic acid) found in commercially available
hemistry 211 Spring 2011 Purpose: Determination of Ascorbic Acid in Vitamin Tablets by Redox and Acid/Base Titrations To determine the quantity of Vitamin (ascorbic acid) found in commercially available
CHECK LIST FOR COLONOSCOPY 2 DAY PREP
 CHECK LIST FOR COLONOSCOPY 2 DAY PREP Medication Sheet: Fill out & take to Day Surgery on day of procedure Pre-Register - by phone: 979-299-2888 Blood Thinning Medications: Hold for 5 days prior to procedure
CHECK LIST FOR COLONOSCOPY 2 DAY PREP Medication Sheet: Fill out & take to Day Surgery on day of procedure Pre-Register - by phone: 979-299-2888 Blood Thinning Medications: Hold for 5 days prior to procedure
Experiment 16-Acids, Bases and ph
 Definitions acid-an ionic compound that releases or reacts with water to form hydrogen ion (H + ) in aqueous solution. They taste sour and turn litmus red. Acids react with certain metals such as zinc,
Definitions acid-an ionic compound that releases or reacts with water to form hydrogen ion (H + ) in aqueous solution. They taste sour and turn litmus red. Acids react with certain metals such as zinc,
The Digestive System Grade 5
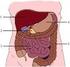 TEACHING LEARNING COLLABORATIVE (TLC) LIFE SCIENCE The Digestive System Grade 5 Created by: Shelly Bell (Kelseyville Elementary School), Bart Pontoni (Riviera Elementary School), Shane Lee (Pomo Elementary
TEACHING LEARNING COLLABORATIVE (TLC) LIFE SCIENCE The Digestive System Grade 5 Created by: Shelly Bell (Kelseyville Elementary School), Bart Pontoni (Riviera Elementary School), Shane Lee (Pomo Elementary
Oxygen Give and Take. Correlation to National Science Education Standards
 Chemistry and Environmental Sciences Oxygen Give and Take Summary This is a series of three activities followed by a worksheet. The concepts taught include gas production (O 2 and CO 2 ), chemical reactions,
Chemistry and Environmental Sciences Oxygen Give and Take Summary This is a series of three activities followed by a worksheet. The concepts taught include gas production (O 2 and CO 2 ), chemical reactions,
Acids, Bases, and ph
 CHAPTER 9 1 SECTION Acids, Bases, and Salts Acids, Bases, and ph KEY IDEAS As you read this section, keep these questions in mind: What properties do acids have? What properties do bases have? How can
CHAPTER 9 1 SECTION Acids, Bases, and Salts Acids, Bases, and ph KEY IDEAS As you read this section, keep these questions in mind: What properties do acids have? What properties do bases have? How can
Syllabus OC18 Use litmus or a universal indicator to test a variety of solutions, and classify these as acidic, basic or neutral
 Chemistry: 9. Acids and Bases Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC18 Use litmus or a universal indicator to test a variety
Chemistry: 9. Acids and Bases Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC18 Use litmus or a universal indicator to test a variety
Experiment 12- Classification of Matter Experiment
 Experiment 12- Classification of Matter Experiment Matter can be classified into two groups: mixtures and pure substances. Mixtures are the most common form of matter and consist of mixtures of pure substances.
Experiment 12- Classification of Matter Experiment Matter can be classified into two groups: mixtures and pure substances. Mixtures are the most common form of matter and consist of mixtures of pure substances.
1 of 8 5/10/2010 12:47 AM
 1 of 8 5/10/2010 12:47 AM All, There seems to be a lot of confusion on the various acid mixtures used in the different reactions. I've put together this quick list of reaction mixes. I know it's incomplete
1 of 8 5/10/2010 12:47 AM All, There seems to be a lot of confusion on the various acid mixtures used in the different reactions. I've put together this quick list of reaction mixes. I know it's incomplete
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
WASTEWATER TREATMENT OBJECTIVES
 WASTEWATER TREATMENT OBJECTIVES The student will do the following: 1. Define wastewater and list components of wastewater. 2. Describe the function of a wastewater treatment plant. 3. Create a wastewater
WASTEWATER TREATMENT OBJECTIVES The student will do the following: 1. Define wastewater and list components of wastewater. 2. Describe the function of a wastewater treatment plant. 3. Create a wastewater
Written By Kelly Lundstrom & Kennda Lynch January 31, 2012 Milk Dye ACTIVITY PLAN
 Milk Dye ACTIVITY PLAN Objective: Students will use the scientific method to test the difference between using whole milk and skim milk in this milk and food dye experiment. Students will explore ideas
Milk Dye ACTIVITY PLAN Objective: Students will use the scientific method to test the difference between using whole milk and skim milk in this milk and food dye experiment. Students will explore ideas
Introduction. ph = log [H + ]
![Introduction. ph = log [H + ] Introduction. ph = log [H + ]](/thumbs/40/21282159.jpg) Visualizing ph 2010, 1992 by David A. Katz. All rights reserved. Permission granted for classroom use. All reproductions must include original copyright. David A. Katz Chemist, Educator, Science Communicator,
Visualizing ph 2010, 1992 by David A. Katz. All rights reserved. Permission granted for classroom use. All reproductions must include original copyright. David A. Katz Chemist, Educator, Science Communicator,
Juice Titration. Background. Acid/Base Titration
 Juice Titration Background Acids in Juice Juice contains both citric and ascorbic acids. Citric acid is used as a natural preservative and provides a sour taste. Ascorbic acid is a water-soluble vitamin
Juice Titration Background Acids in Juice Juice contains both citric and ascorbic acids. Citric acid is used as a natural preservative and provides a sour taste. Ascorbic acid is a water-soluble vitamin
Kool Demo for Acid-Base Reactions
 Kool Demo for Acid-Base Reactions Kool Demo for Acid-Base Reactions Adapted from : http://www.stevespanglerscience.com/lab/experiments/color-changing-milk-of-magnesia Materials: Red cabbage juice indicator
Kool Demo for Acid-Base Reactions Kool Demo for Acid-Base Reactions Adapted from : http://www.stevespanglerscience.com/lab/experiments/color-changing-milk-of-magnesia Materials: Red cabbage juice indicator
Determination of Citric Acid in Powdered Drink Mixes
 Determination of Citric Acid in Powdered Drink Mixes Citric acid and its salts (sodium citrate and potassium citrate) are found in many foods, drinks, pharmaceuticals, shampoos, and cosmetics. The tartness
Determination of Citric Acid in Powdered Drink Mixes Citric acid and its salts (sodium citrate and potassium citrate) are found in many foods, drinks, pharmaceuticals, shampoos, and cosmetics. The tartness
Environmental Water Testing: Surface Water, Groundwater, Hard Water, Wastewater, & Seawater
 Document: AND Sol Env 08 2013 Environmental Water Testing: Surface Water, Groundwater, Hard Water, Wastewater, & Seawater Matrix specific sample preparation and testing methods for environmental waters
Document: AND Sol Env 08 2013 Environmental Water Testing: Surface Water, Groundwater, Hard Water, Wastewater, & Seawater Matrix specific sample preparation and testing methods for environmental waters
III. BACKGROUND KNOWLEDGE
 Chemical Bonds and Reactions (Water) Grade Level: 7 (Applied Technology) Presented by: James Rubright, Three Oaks Middle School, Fort Myers, Florida Length of Unit: Ten Daily Lessons and Activities I.
Chemical Bonds and Reactions (Water) Grade Level: 7 (Applied Technology) Presented by: James Rubright, Three Oaks Middle School, Fort Myers, Florida Length of Unit: Ten Daily Lessons and Activities I.
Mixtures and Pure Substances
 Unit 2 Mixtures and Pure Substances Matter can be classified into two groups: mixtures and pure substances. Mixtures are the most common form of matter and consist of mixtures of pure substances. They
Unit 2 Mixtures and Pure Substances Matter can be classified into two groups: mixtures and pure substances. Mixtures are the most common form of matter and consist of mixtures of pure substances. They
Animal & Plant Cell Slides
 Animal & Plant Cell Slides Category: Biology Type: Class Experiment, 60 min class Materials: 2 Glass Slides 2 Cover Slips 1 Bottle of methylene blue (optional) 1 Plastic tray 1 Bottle of iodine 1 Plastic
Animal & Plant Cell Slides Category: Biology Type: Class Experiment, 60 min class Materials: 2 Glass Slides 2 Cover Slips 1 Bottle of methylene blue (optional) 1 Plastic tray 1 Bottle of iodine 1 Plastic
Experiment 18: ph Measurements of Common Substances. Experiment 17: Reactions of Acids with Common Substances
 Experiment 18: ph Measurements of Common Substances and Experiment 17: Reactions of Acids with Common Substances What is this lab about? You mean what ARE THESE labs about? Ok, so what are THESE labs about?
Experiment 18: ph Measurements of Common Substances and Experiment 17: Reactions of Acids with Common Substances What is this lab about? You mean what ARE THESE labs about? Ok, so what are THESE labs about?
SIXTH GRADE WATER 1 WEEK LESSON PLANS AND ACTIVITIES
 SIXTH GRADE WATER 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF SIXTH GRADE WATER WEEK 1. PRE: Evaluating components of the water cycle. LAB: Experimenting with porosity and permeability.
SIXTH GRADE WATER 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF SIXTH GRADE WATER WEEK 1. PRE: Evaluating components of the water cycle. LAB: Experimenting with porosity and permeability.
Pure Substances, Mixtures, and Solutions
 Pure Substances, Mixtures, and Solutions Mixtures, elements, compounds Scientists like to classify things. One way that scientists classify matter is by its composition. Ultimately, all matter can be classified
Pure Substances, Mixtures, and Solutions Mixtures, elements, compounds Scientists like to classify things. One way that scientists classify matter is by its composition. Ultimately, all matter can be classified
US Bank Tower Cincinnati Recycling
 US Bank Tower Cincinnati Recycling US Bank Tower Recycling US Bank Tower produces 335 tons of trash annually. Approximately 80% of what we throw away can be recycled, but we Need your participation to
US Bank Tower Cincinnati Recycling US Bank Tower Recycling US Bank Tower produces 335 tons of trash annually. Approximately 80% of what we throw away can be recycled, but we Need your participation to
Shampoo Properties Evaluation General Science
 / 10 Shampoo Properties Evaluation General Science Name It is difficult to obtain exact information on the formulation of commercial shampoos. These facts are held by the manufacturer to protect their
/ 10 Shampoo Properties Evaluation General Science Name It is difficult to obtain exact information on the formulation of commercial shampoos. These facts are held by the manufacturer to protect their
Lesson 10: Mixtures of Matter - Part 2
 Science Unit: Matter Lesson 10: Mixtures of Matter - Part 2 School year: 2004/2005 Developed for: Developed by: Grade level: Duration of lesson: Notes: Queen Alexandra Elementary School, Vancouver School
Science Unit: Matter Lesson 10: Mixtures of Matter - Part 2 School year: 2004/2005 Developed for: Developed by: Grade level: Duration of lesson: Notes: Queen Alexandra Elementary School, Vancouver School
CLEANING WATER. Student Section
 National Aeronautics and Space Administration CLEANING WATER Student Section Student Name This lesson challenges you to create and test a water filtration system. During this lesson, you will design and
National Aeronautics and Space Administration CLEANING WATER Student Section Student Name This lesson challenges you to create and test a water filtration system. During this lesson, you will design and
Thin Layer Chromatography.
 Thin Layer Chromatography. Thin layer chromatography, or TLC, is a method for analyzing mixtures by separating the compounds in the mixture. TLC can be used to help determine the number of components in
Thin Layer Chromatography. Thin layer chromatography, or TLC, is a method for analyzing mixtures by separating the compounds in the mixture. TLC can be used to help determine the number of components in
Minnesota Comprehensive Assessments-Series III
 Not for student use. Minnesota Comprehensive Assessments-Series III Science Item Sampler Script Grade 8 S ARE NOT SECURE TEST MATERIALS. THIS ITEM SAMPLER SCRIPT MAY BE COPIED OR DUPLICATED. MINNESOTA
Not for student use. Minnesota Comprehensive Assessments-Series III Science Item Sampler Script Grade 8 S ARE NOT SECURE TEST MATERIALS. THIS ITEM SAMPLER SCRIPT MAY BE COPIED OR DUPLICATED. MINNESOTA
experiment5 Understanding and applying the concept of limiting reagents. Learning how to perform a vacuum filtration.
 81 experiment5 LECTURE AND LAB SKILLS EMPHASIZED Synthesizing an organic substance. Understanding and applying the concept of limiting reagents. Determining percent yield. Learning how to perform a vacuum
81 experiment5 LECTURE AND LAB SKILLS EMPHASIZED Synthesizing an organic substance. Understanding and applying the concept of limiting reagents. Determining percent yield. Learning how to perform a vacuum
KODAK Developer System Cleaner and Neutralizer
 KODAK Developer System Cleaner and Neutralizer TECHNICAL DATA / CHEMICAL March 2010 TI-2000 GENERAL INFORMATION KODAK Developer System Cleaner and Neutralizer is designed to remove the buildup of silver
KODAK Developer System Cleaner and Neutralizer TECHNICAL DATA / CHEMICAL March 2010 TI-2000 GENERAL INFORMATION KODAK Developer System Cleaner and Neutralizer is designed to remove the buildup of silver
SECOND GRADE 1 WEEK LESSON PLANS AND ACTIVITIES
 SECOND GRADE 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF SECOND GRADE WATER WEEK 1. PRE: Exploring the properties of water. LAB: Experimenting with different soap mixtures. POST: Analyzing
SECOND GRADE 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF SECOND GRADE WATER WEEK 1. PRE: Exploring the properties of water. LAB: Experimenting with different soap mixtures. POST: Analyzing
Sugar or Salt? Ionic and Covalent Bonds
 Lab 11 Sugar or Salt? Ionic and Covalent Bonds TN Standard 2.1: The student will investigate chemical bonding. Have you ever accidentally used salt instead of sugar? D rinking tea that has been sweetened
Lab 11 Sugar or Salt? Ionic and Covalent Bonds TN Standard 2.1: The student will investigate chemical bonding. Have you ever accidentally used salt instead of sugar? D rinking tea that has been sweetened
AP ENVIRONMENTAL SCIENCE 2007 SCORING GUIDELINES
 AP ENVIRONMENTAL SCIENCE 2007 SCORING GUIDELINES Question 1 Read the Fremont Examiner article below and answer the questions that follow. (a) Identify ONE component of the sewage that is targeted for removal
AP ENVIRONMENTAL SCIENCE 2007 SCORING GUIDELINES Question 1 Read the Fremont Examiner article below and answer the questions that follow. (a) Identify ONE component of the sewage that is targeted for removal
Solutions, Suspensions, and Colloids
 Movie Special Effects Activity 3 Solutions, Suspensions, and Colloids GOALS In this activity you will: Explore different ways that materials can be mixed together to make new materials. Test some materials
Movie Special Effects Activity 3 Solutions, Suspensions, and Colloids GOALS In this activity you will: Explore different ways that materials can be mixed together to make new materials. Test some materials
Chemical reaction (slow): Enzyme-catalyzed reaction (much faster):
 1 Enzymes Introduction Enzymes are Biological Catalysts Recall that a catalyst is an agent which speeds up a chemical reaction without actually being consumed or changed by the reaction. Enzymes are proteins
1 Enzymes Introduction Enzymes are Biological Catalysts Recall that a catalyst is an agent which speeds up a chemical reaction without actually being consumed or changed by the reaction. Enzymes are proteins
Chapter 5, Lesson 3 Why Does Water Dissolve Salt?
 Chapter 5, Lesson 3 Why Does Water Dissolve Salt? Key Concepts The polarity of water molecules enables water to dissolve many ionically bonded substances. Salt (sodium chloride) is made from positive sodium
Chapter 5, Lesson 3 Why Does Water Dissolve Salt? Key Concepts The polarity of water molecules enables water to dissolve many ionically bonded substances. Salt (sodium chloride) is made from positive sodium
SAMPLE ACTIVITY. from
 SAMPLE ACTIVITY from The Water SourceBook Distributed By Legacy, Inc., Partners in Environmental Education Funding for this project is made possible by proceeds from the sale of Alabama's Protect Our Environment
SAMPLE ACTIVITY from The Water SourceBook Distributed By Legacy, Inc., Partners in Environmental Education Funding for this project is made possible by proceeds from the sale of Alabama's Protect Our Environment
Fluoride Strengthens Teeth
 Fluoride Strengthens Teeth Two hard-boiled eggs Fluoride gel or solution, 4 to 6 oz. (from dental office) Three clean plastic containers Several cans of dark soda Water 1. Place a hard-boiled egg in one
Fluoride Strengthens Teeth Two hard-boiled eggs Fluoride gel or solution, 4 to 6 oz. (from dental office) Three clean plastic containers Several cans of dark soda Water 1. Place a hard-boiled egg in one
The Influence of Carbon Dioxide on Algae Growth
 The Influence of Carbon Dioxide on Algae Growth The first objective of this experiment is to show that increased atmospheric concentrations of carbon dioxide, CO 2, can stimulate algae growth. The second
The Influence of Carbon Dioxide on Algae Growth The first objective of this experiment is to show that increased atmospheric concentrations of carbon dioxide, CO 2, can stimulate algae growth. The second
Taking Apart the Pieces
 Lab 4 Taking Apart the Pieces How does starting your morning out right relate to relief from a headache? I t is a lazy Saturday morning and you ve just awakened to your favorite cereal Morning Trails and
Lab 4 Taking Apart the Pieces How does starting your morning out right relate to relief from a headache? I t is a lazy Saturday morning and you ve just awakened to your favorite cereal Morning Trails and
Neutralizing an Acid and a Base
 Balancing Act Teacher Information Objectives In this activity, students neutralize a base with an acid. Students determine the point of neutralization of an acid mixed with a base while they: Recognize
Balancing Act Teacher Information Objectives In this activity, students neutralize a base with an acid. Students determine the point of neutralization of an acid mixed with a base while they: Recognize
Determining the Quantity of Iron in a Vitamin Tablet. Evaluation copy
 Determining the Quantity of Iron in a Vitamin Tablet Computer 34 As biochemical research becomes more sophisticated, we are learning more about the role of metallic elements in the human body. For example,
Determining the Quantity of Iron in a Vitamin Tablet Computer 34 As biochemical research becomes more sophisticated, we are learning more about the role of metallic elements in the human body. For example,
