PII S (99) CLINICAL INVESTIGATION
|
|
|
- Roger Lloyd
- 8 years ago
- Views:
Transcription
1 PII S (99) Int. J. Radiation Oncology Biol. Phys., Vol. 45, No. 5, pp , 1999 Copyright 1999 Elsevier Science Inc. Printed in the USA. All rights reserved /99/$ see front matter CLINICAL INVESTIGATION Lung HYPERFRACTIONATED ACCELERATED RADIOTHERAPY (HART) FOR INOPERABLE, NONMETASTATIC NON-SMALL CELL LUNG CARCINOMA OF THE LUNG (NSCLC): RESULTS OF A PHASE II STUDY FOR PATIENTS INELIGIBLE FOR COMBINATION RADIOCHEMOTHERAPY SOPHIE KOUTAïSSOFF, M.D.,* DANIELLE WELLMANN, M.D., PHILIPPE COUCKE, M.D.,* MAHMUT OZSAHIN, M.D., PH.D.,* SANDRO PAMPALLONA, SC.D., AND RENÉ-OLIVIER MIRIMANOFF, M.D.* *Department of Radiation Oncology, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland; Department of Radiation Oncology, Hôpital Cantonal Fribourg, Switzerland; and For Med, Statistics for Medicine, Evolène, Switzerland Purpose: To evaluate a hyperfractionated and accelerated radiotherapy (HART) protocol in patients with inoperable non-small cell lung carcinoma (NSCLC) who were ineligible for combination radiochemotherapy studies. Methods and Materials: From February 1989 through August 1994, 23 patients ineligible for available combined modality protocols in our institution were enrolled and treated with HART, consisting of 63 Gy given in 42 fractions of 1.5 Gy each, twice daily, with a minimum time interval of 6 h between fractions, 5 days a week, over an elapsed time of 4.2 weeks, or 29 days. There was no planned interruption. Results: The 1-, 2-, and 3-year survival rates were 61%, 39%, and 19%, respectively, with a median survival of 16.8 months. At the time of analysis, 4 patients are alive and 19 have died, 16 from NSCLC and 3 from cardiac disease. Overall response rate was 48%, with 22% of patients achieving a complete response and 26% a partial response. Correlation between acute response rate and survival was poor. First site of relapse was local-regional in 8 patients (35%), distant in 6 patients (26%), and local-regional and distant in 4 (17%) patients. One patient had Grade IV and 2 had Grade III esophagitis. One patient presented with chronic Grade III lung toxicity. There were no treatment-related deaths. Conclusion: In this group of 23 patients ineligible for radiochemotherapy, this HART regime was quite feasible and was followed by little toxicity. Results in this particularly poor prognosis NSCLC patient category should be compared to series with a similar patient profile; however, median survival is at least similar to that obtained in recent series of combination radiochemotherapy Elsevier Science Inc. Non-small cell lung cancer, Radiotherapy, Accelerated radiotherapy. INTRODUCTION In spite of the many advances in surgery, radiotherapy, chemotherapy, or their combinations, locally advanced Stage IIIA and IIIB non-small cell lung cancer (NSCLC) remains a disease category with a poor overall prognosis, and the optimal treatment remains unknown (1). With conventional radiotherapy (RT) alone, both local and distant failure rates are high, and the expected median survival is generally between 9 and 12 months (2, 3). To improve the local control, surgery following induction chemotherapy, radiotherapy, or both can be attempted (4, 5); however, this approach is applicable only to the subgroup of patients with potentially resectable tumors. Dose-escalation via three-dimensional (3D) planning and conformal radiotherapy is an important development, and is currently being investigated (6 8). Combination chemotherapy and radiotherapy have been extensively studied with a large Reprint requests to: R. O. Mirimanoff, Department of Radiation Oncology, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland. Tel: 41.21/ ; Fax: 41.21/ number of drug associations and radiochemotherapy schedules (9, 10). Meta-analyses have concluded that results favor combined cisplatin-based chemotherapy; however, the gain in terms of 3- and 5-year survival is quite modest (11, 12). Moreover, a substantial subset of patients cannot be treated with chemotherapy because of various medical or psychological reasons. Unconventionally fractionated radiotherapy, mainly hyperfractionated accelerated radiotherapy (HART) (13 15) and continuous hyperfractionated accelerated radiotherapy (CHART) (16, 17) offer interesting alternatives in locally advanced NSCLC. We report here our experience with a HART protocol in a Phase II study for patients with inoperable NSCLC who could not be enrolled in combination radiochemotherapy protocols ; miriman@chuv.hospvd.ch Accepted for publication 22 July
2 1152 I. J. Radiation Oncology Biology Physics Volume 45, Number 5, 1999 Table 1. Reasons for patient exclusion from combined radiochemotherapy GOTHA I and II studies Condition PATIENTS AND METHODS No. of patients Severe heart disease 12 Severe chronic lung disease 6 Age 70 years 1 Weight loss 10% in 6 months 1 Two pulmonary tumors 1 Patient refused chemotherapy 3 Eligibility and study parameters Between February 1989 and August 1994, 23 patients were entered in this Phase II trial. Patients with pathologically or cytologically documented squamous cell carcinoma, adenocarcinoma, large cell, or undifferentiated NSCLC with either inoperable Stage IIIA or IIIB (with the exception of patients with pleural or pericardial effusion), or medically inoperable Stage I or II were considered to be eligible. This series of 23 consecutive patients represents a subgroup of all inoperable NSCLC seen in our department, who could not be enrolled in on-going trials of combined radiochemotherapy available at this time, namely the GOTHA I and II trials (18, 19). These priority protocols in our department consisted of different schemes of combination cisplatin-based chemotherapy, rapidly alternated with hyperfractionated-accelerated RT. The reasons for ineligibility of these 23 patients from the combined modality trials are shown in Table 1, any of which representing exclusion criteria from GOTHA I and II. The majority of patients presented with chronic medical conditions, mainly heart and lung diseases, precluding the administration of aggressive radiochemotherapy schedules. A review was made retrospectively on the histological or cytological material, which was available for 21 patients. The diagnosis of NSCLC was confirmed in all cases. Two patients with Stage I and 1 patient with Stage II disease, who were considered as medically inoperable, were included in the study, and the remaining 20 patients had surgically inoperable Stage III disease. Thus, patients either with surgically unresectable or medically inoperable tumors were eligible for this Phase II trial, but those with previous radiotherapy, surgery, or previous chemotherapy were excluded. Other requirements for eligibility were a forced expiratory volume of 1.5 liters or more, and the absence of hematogenous metastases on work-up. The pretreatment evaluation included AP and lateral chest X-ray, thoracic computed tomography (CT), and bronchoscopy in all patients. Twenty-two (96%) patients had bone scintigram and 19 (83%) had upper abdominal CT. Ten (44%) patients underwent brain CT or magnetic resonance imaging (MRI). Treatment scheme The radiotherapy scheme was adapted from GOTHA I and II, the major difference being that it did not include any interruption. (In GOTHA I and II, a 2-week split was mandatory for the administration of the second cycle of chemotherapy) (18, 19). Thus, patients were treated with a HART without any planned interruption, consisting of 63 Gy delivered in 42 fractions of 1.5 Gy each, twice daily, with a minimum time interval of 6 h between fractions, 5 days a week. The scheduled overall duration of treatment was 29 days (4.2 weeks). A dose of 40.5 Gy was given to the first planning target volume, encompassing the primary tumor, draining ipsilateral hilar lymph nodes, mediastinum, thoracic inlet, and the supraclavicular fossae. This volume was treated with anterior-posterior parallel-opposed fields. An additional dose of 22.5 Gy was given to the second planning target volume, comprising only the primary tumor and grossly involved lymph nodes, to a total dose of 63 Gy. That volume was treated in 22 patients with oblique, parallelopposed, off-the-cord reduced fields, and in 1 patient with lateral parallel-opposed fields. Megavoltage (6 and 18 MV), isocentric technique, and cerrobend blocking were required. For planning purposes, simulator and dosimetry with treatment planning computers were mandatory. Evaluation of toxicity and response, patient follow-up Study endpoints were acute toxicity, late normal tissue damage, tumor response, and survival. Acute toxicity, using WHO grading, was defined as toxicity occurring during or at the end of RT. Chronic toxicity was defined as toxicity occurring after 90 days following the start of RT, or when acute reactions persisted over 90 days, using the RTOG scale. The assessment of tumor response was based on the comparison of the initial tumor measurements 4 8 weeks after completion of the radiotherapy. A complete response was defined as the total disappearance of the tumor on chest radiography and chest CT. A partial response was considered as a 50% or more reduction of the sum of the products of the greatest perpendicular diameters of the tumor, whereas progression corresponded to a 25% or more increase of the sum of these diameters. No change was defined as less than 50% reduction or less than 25% progression of the tumor. All patients were to be followed for the duration of their life, monthly during the first year, every three months in the second year, and yearly afterwards, and the date and site of first failure were registered. In case of failure, the choice of treatment was made on an individual basis. Statistical methods The overall survival (OS) was measured from the first day of treatment to death from any cause or to the date of last follow-up if the patient was alive. For the evaluation of progression-free survival (PFS), the date of first progression, or death from any cause or the date of last evaluation
3 HART for non-small cell lung cancer S. KOUTAïSSOFF et al Table 2. Patient characteristics in the HART study and in GOTHA I and II HART GOTHA I&II No. of patients Median age (years) 63.8 (46 75) 55.5 (28 70) Male/Female 22/1 7.3/1 Cell types Squamous cell 18 (78%) 66% Adenocarcinoma 2 (9%) 22% Large cell carcinoma 2 (9%) 14% Non-small cell undifferentiated 1 (4%) 4% Stage I 2 (9%) 0% II 1 (4%) 0% IIIa 5 (22%) 44% IIIb 15 (65%) 56% PS 0 2 (9%) 36% 1 13 (56%) 52% 2 8 (35%) 12% if the patient had no evidence of disease, was considered. The probability of survival was calculated according to the method of Kaplan-Meier (20) and the corresponding standard error by Greenwood formula (21). Cox regression analysis was used to test the simultaneous effect of age, performance status (0 vs. 1 vs. 2), and duration of treatment on the risk of death (22). RESULTS Patients Twenty-three patients were accrued between February 1989 and August 1994; all have been included in the analysis. Table 2 summarizes the characteristics of the patients: the median age was 63.8 years; the male to female ratio was 22:1; the WHO performance status was 0 in 2 patients, 1 in 13, and 2 in 8 patients, respectively. Histologic types consisted of squamous cell carcinoma in 18, adenocarcinoma in 2, large-cell carcinoma in 2, and undifferentiated non-small cell carcinoma in 1 patient. Three patients had Stage I and II, 5 had Stage IIIA, and 15 had Stage IIIB disease. Of these, 4 patients presented with a T2 tumor, 7 with a T3, and 12 with a T4 lesion. Ten patients had clinically and radiologically negative lymph nodes (NO). One patient underwent a blank thoracotomy before RT. Table 2 also compares patients of the present study to those included in GOTHA I and II studies (19). Feasibility and dose intensity All patients were irradiated to the first planning target volume with a median doses of 40.5 Gy (range 26 43). One patient refused any further RT because of fatigue, and another decided to have a break after he had received 40.5 Gy, also because of fatigue, but resumed his RT 2 weeks afterward. Twenty-two of 23 patients (96%) were irradiated to the second planning target volume, with a median dose of 22.5 Gy (range ). The median total dose was 63 Gy (range ), and the median duration of treatment was 30 days (range 23 46), one day longer than scheduled (29 days). Response, survival, and pattern of failure The overall response rate at first control after 4-8 weeks was 48%, with 5 (22%) patients achieving a complete response and 6 (26%) patients showing a partial response. Eight (35%) patients had no change, no patient was in progression, and 4 were inevaluable. The 1-, 2-, and 3-year survival rates were 61% ( 10), 39% ( 10), and 19% ( 8), respectively, with a median survival of 16.8 months. Apparently, there was no correlation between the degree of response and survival: among the seven patients surviving 2 years or more, only one had a complete response, two a partial response, and four had no change in their tumor size. Conversely, of the five patients who were scored as having a complete response, four died within 2 years. At the time of analysis, 16 (70%) patients were dead from their pulmonary carcinoma and three (13%) from other causes: two patients died of myocardial infarction and one of acute left cardiac insufficiency. Two (9%) patients were alive with disease and two (9%) were free of disease. The median progression-free survival was 10.2 months. The events contributing to the progression-free survival were 5 deaths as first event and 16 progressions. There were no differences in overall survival and progression-free survival attributable to age, performance status, and duration of treatment when such factors were considered in the Cox regression analyses; however, the number of patients in each category was quite small. Sites of first failure were as follows: 8 patients (35%) had local-regional failures, 4 (17%) distant failures, and 6 (26%) both local-regional and distant failures. Of the 14 patients with a local failure component, 10 had radiological tumor persistence at the time of first evaluation after treatment. Toxicity As seen in Table 3, acute toxicity was quite moderate. One patient had Grade IV acute esophagitis, requiring nasogastric tube feeding, and two patients had acute Grade III esophagitis; all of these eventually resolved. One patient had chronic Grade III dyspnea, the only significant chronic toxicity of the study. When considering either AP-PA field size or oblique boost field size, there was no significant influence on the occurrence of Grade III IV esophageal or lung toxicity. DISCUSSION For decades, especially the past 20 years, many efforts have been made to improve the results of radiotherapy for inoperable and locally advanced NSCLC. These have comprised various combinations of chemotherapy and radiotherapy (9, 10).
4 1154 I. J. Radiation Oncology Biology Physics Volume 45, Number 5, 1999 Table 3. Treatment scheme and results of the HART study compared to GOTHA I and II HART GOTHA I&II Study protocol HART alone Alternated chemotherapy and HART Radiotherapy 63 Gy, 1.5 Gy b.i.d., no split 63 Gy, 1.5 Gy b.i.d., 15-day split after 30 Gy Median RT treatment time (days) Chemotherapy None GOTHA I: cisplatin, mitomycin, vindesin GOTHA II: cisplatin, vinblastine Median survival (months) , 2-, 3-year survival 61%, 39%, 19% 56%, 27%, 17% Acute Grade III IV mucositis 3/23* (13%) 18/132 (14%) Late pulmonary toxicity 1/23 (4%) 8/132 (6%) * Two patients had Grade III and one patient had Grade IV esophagitis. One patient had Grade III late pulmonary toxicity. Several trials comparing radiotherapy alone to cisplatinbased chemotherapy associated with radiotherapy have shown that the combined modality was better; however, meta-analyses have indicated that the gain in 3- and 5-year survival was quite modest (11, 12). In addition, attempts were made to increase the intensity of radiotherapy either via the use of 3D conformal radiotherapy and dose escalation (6 8) or by the use of unconventionally fractionated radiotherapy (13 17, 23, 24). The latter approach is not only important in our efforts to overcome the still high local failure rates after conventional radiotherapy, as shown by recent studies, but also can present interesting options for patients who cannot receive (or who refuse) chemotherapy combined with radiotherapy. A number of unconventional schedules have been tested, including accelerated radiotherapy (AF), hyperfractionated radiotherapy (HF) mixed schedules of hyperfractionated accelerated radiotherapy (HART), and the continuous hyperfractionated accelerated radiotherapy (CHART). (13 17, 23, 34). In two of the studies, a three-time daily program gave accelerated hyperfractionation to gross tumor and conventional hyperfractionation to electively irradiated volumes (13, 15). The last two schemes (HART and CHART) represent an interesting compromise that takes advantage of decreased doses per fraction, which could reduce long-term normal tissue morbidity (25) and shorten overall treatment time, which could counteract cancer cell repopulation (26). Data on kinetics in NSCLLC seem to indicate a rapid cell proliferation, at least in an important subset of tumors (27, 28). In our scheme, a total dose of 63 Gy is given in 1.5 Gy twice-daily schedule, over an elapsed time of 29 days. Compared to a conventionally fractionated 2 Gy/fraction scheme, in which 63 Gy would be given in 44 days, the gain is 15 days. Assuming an average Tpot of 3 days, the overall gain would be approximately 10 Gy. Regarding late tissue damage, with an average / value of 3, decreasing the dose per fraction from 2 to 1.5 would represent a decrease in BED of approximately 20%. Taking this into account in this admittedly limited series of patients, we suggest that in terms of tolerance and survival, our results are comparable to those of our previously reported combined modality protocols, the GOTHA I and II (Table 3), and are also comparable to other radiochemotherapy regimes. Regarding tolerance, Grade III and IV acute mucositisesophagitis was seen in 13% and 14% of patients, respectively, in HART and GOTHA I and II, and Grade III IV lung toxicity was 4% and 6%, respectively. In other studies, using concomitant radiochemotherapy, Grade III IV mucositis-esophagitis ranged from 10% to 53% and Grade III IV lung toxicity was between 8 25% (29 31). In the CHART trial, 19% of patients had their diet reduced to fluids compared to 3% of patients treated conventionally; however, regarding symptomatic pneumonitis, it was 19% in the conventional and 10% in the CHART group (17). In a trial from Duke University, where a HART regime was given to 73.6 Gy, at 1.6 and 1.25 Gy/fraction, Grade III esophagal toxicity was 19% and the 2-year actuarial rate of severe lung toxicity was 20% (14). As shown in Table 3, median survival and 1-, 2-, and 3-year survival of this HART regime were fairly similar to those of GOTHA I and II, knowing that patients in HART were excluded from combination chemoradiotherapy studies (see Table 1), and they were on average older, with higher percentage of Stage IIIb disease and less favorable performance status (Table 2). In our series, the median survival of 16.8 months was better than the usual 9 12 months found in series of conventional radiotherapy alone, and is comparable to studies using combination radiochemotherapy (10, 29 31). In the CHART trial, the experimental arm demonstrated a significant improvement in survival of 8% at 1 year and 9% at 2 years over the conventional arm (17). In the Duke trial, median survival was 15.3 months and 2-year survival 46% (14). RTOG protocol compared doses of 60.0, 64.8, and 69.6 Gy using 1.2 Gy b.i.d. (23).
5 HART for non-small cell lung cancer S. KOUTAïSSOFF et al Thereafter, 2 arms of 74.4 and 79.2 supplanted the lowest arms. The 69.6 Gy was significantly better in favorable patients than all other arms, with 1-year survival rate of 58% and 3-year rate of 20% (23). In a study from Shanghai, in which a total dose of 74.3 Gy was applied in three daily fractions of 1.1 Gy, median survival was 22.6 months, and 1-, 2-, and 3-year actuarial survival was 72%, 47% and 28%, respectively (15). Other studies seem to disclose less convincing results with unconventionally fractionated radiotherapy. In a combined RTOG-ECOG Phase III randomized trial, standard radiation arm was compared to induction chemotherapy followed by radiation, and to twice-daily radiotherapy consisting of 1.2 Gy per fraction b.i.d. to a total dose of 69.6 Gy (24). This study demonstrated a significantly superior survival in the chemotherapy plus radiotherapy arm, but there was no statistically significant difference between the conventional and hyperfractionated-accelerated arm (24). Accelerated fractionation radiotherapy including concomitant boost was used in RTOG trials (32, 33). For 355 patients treated this way at doses of Gy in weeks, results disclosed a disappointing 9-month median survival, and a 2-year survival ranging from 6% to 21% (32). It is evident that a simple comparison between pilot studies, large Phase II, and randomized trials can be very misleading due to different methodologies, selection bias, different patient populations, small numbers, and other factors. Nevertheless, it is fair to say that the patients included in our HART study represented a subset with poor prognosis, inoperable NSCLC who could not be included in protocols of radiochemotherapy. In this regard, our radiotherapy scheme was well tolerated, presented the advantage of being given in 4 weeks only, and gave a rather encouraging 16.8-month median survival. Similar schemes deserve further investigation, and perhaps should be integrated also in combined modality protocols or in those including conformal radiotherapy with dose escalation. REFERENCES 1. Buccheri G, Ferrigno D. Therapeutic options for regionally advanced non-small cell lung cancer. Lung Cancer 1996; 14: Choi NC, Doucette JA. Improved survival of patients with unresectable non-small cell bronchogenic carcinoma by an innovative high dose en-bloc radiotherapeutic approach. Cancer 1981; 48: Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy: Report by the Radiation Therapy Oncology Group. Cancer 1987; 59: Green MR, Barkley JE. Intensity of neoadjuvant therapy in resectable non-small cell lung cancer. Lung Cancer 1991; 17: Ginsberg RJ. Neoadjuvant (induction) treatment for non-small cell lung cancer. Lung Cancer 1995; 12: Armstrong J, Raben A, Zelefski M, et al. Promising survival with three-dimensional conformal radiation therapy for nonsmall cell lung cancer. Radiother Oncol 1997; 44: Sibley GS, Mundt AJ, Shapiro C, et al. The treatment of stage III non-small cell lung cancer using high dose conformal radiotherapy. Int J Radiat Oncol Biol Phys 1995; 33: Rosenman J, Hazuka MB, Martel MK. Three dimensional treatment planning in lung cancer radiotherapy. In: Pass HI, Mitchell JB, Johnsons DH, et al., editors. Lung cancer: principles and practice. Philadelphia: Lippincott-Raven Publishers; p Mirimanoff RO. Concurrent chemotherapy and radiotherapy in locally advanced non-small cell lung cancer: A review. Lung Cancer 1994; 11: Komaki R. Combined chemotherapy and radiation therapy in surgically unresectable regionally advanced non-small cell lung cancer. Semin Radiat Oncol 1996; 6: Anonymous. Chemotherapy in non-small cell lung cancer: A meta-analysis using updated data or individual patients from 52 randomized clinical trials. Br Med J 1995; 311: Marino P, Praetoni A, Cantoni A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stage IIIa and IIIb non-small cell lung cancer: A meta-analysis. Cancer 1995; 76: Herskovic A, Orton C, Seyedsadr M, et al. Initial experience with practical hyperfractionated accelerated radiation therapy regimen. Int J Radiat Oncol Biol Phys 1991; 21: King SC, Acker JC, Kussin PS, et al. High-dose, hyperfractionated, accelerated radiotherapy using a concurrent boost for the treatment of non-small cell lung cancer: Unusual toxicity and promising early results. Int J Radiat Oncol Biol Phys 1996; 36: Fu XL, Jiang GL, Wang LJ, et al. Hyperfractionated accelerated radiotherapy for non-small cell lung cancer: Clinical phase I/II trial. Int J Radiat Oncol Biol Phys 1997; 39: Saunders MI, Dische S, Barrett A, et al. Randomised multicentre trials of CHART versus conventional radiotherapy in head and neck and non-small cell lung cancer: An interim report. Br J Cancer 1996; 73: Saunders MI, Dische S, Barrett A, et al. Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: A randomised multicentre trial. Lancet 1997; 350: Alberto P, Mirimanoff RO, Mermillod B, et al. Rapidly alternating combination of cisplatin-based chemotherapy and hyperfractionated accelerated radiotherapy in split-course for stage IIIA and stade IIIB non-small cell lung cancer: Results of a phase I II study by the GOTHA group. Eur J Cancer 1995; 31A: Mirimanoff RO, Moro D, Bolla M, et al. Alternating radiotherapy and chemotherapy for inoperable stage III non-small cell lung cancer: Long-term results of two phase II GOTHA trials. Int J Radiat Oncol Biol Phys 1998; 42: Kaplan EL, Meier P. Non-parametric estimation for incomplete observations. J Am Stat Assoc 1958; 53: Greenwood M. Reports on public health and medical subjects: The natural duration of cancer. HMSO 1926; 33: Cox DR. Regression models and lifetables. J Roy Stat Soc B 1972; 34: Cox J, Azarnia N, Byhardt RW, et al. N2 (clinical) non-small cell carcinoma of the lung: Prospective trials of radiation
6 1156 I. J. Radiation Oncology Biology Physics Volume 45, Number 5, 1999 therapy with total doses 60 Gy by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1991; 20: Sause WT, Scott C, Taylor S, et al. Radiation Therapy Oncology Group (RTOG) and Eastern Cooperative Oncology Group (ECOG) 4588: Preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. J Natl Cancer Inst 1995; 84: Withers HR. Biological basis of radiation therapy for cancer. Lancet 1992; 339: Wilson GD, McNally NJ, Dische S, et al. Measurement of cell kinetics in human tumors in vivo using bromodeoxyuridine incorporation and flow cytometry. Br J Cancer 1988; 58: Usada K, Saito Y, Sagawa M, et al. Tumor doubling time and prognostic assessment of patients with primary lung cancer. Cancer 1994; 74: Kerr KM, Lamb D. Actual growth rate and tumor cell proliferation in human pulmonary neoplasms. Br J Cancer 1984; 50: Lee JS, Scott C, Komaki R, et al. Concurrent chemoradiation therapy with oral etoposide and cisplatin for locally advanced inoperable non-small cell lung cancer: Radiation Therapy Oncology Group protocol J Clin Oncol 1996; 14: Le Péchoux C, Arriagada R, Le Chevalier T, et al. Concurrent cisplatin-vindesin and hyperfractionated thoracic radiotherapy in locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 1996; 35: Reboul F, Brewer Y, Vincent P, et al. Concurrent cisplatin, etoposide, and radiotherapy for unresectable stage III nonsmall cell lung cancer: A phase II study. Int J Radiat Oncol Biol Phys 1996; 35: Byhardt RW, Pajak TF, Emami B, et al. A phase I/II study to evaluate accelerated fractionation via concomitant boost for squamous, adeno, and large cell carcinoma of the lung: Report of Radiation Therapy Oncology Group Int J Radiat Oncol Biol Phys 1993; 26: Graham MV, Pajak TE, Herskovic AM, et al. Phase I/II study of treatment of locally advanced (T3/T4) non-oat cell lung cancer with concomitant boost radiotherapy by the Radiation Therapy Oncology Group (RTOG 83-12): Long-term results. Int J Radiat Oncol Biol Phys 1995; 31:
Stage IIIB disease includes patients with T4 tumors,
 Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
Table of Contents. Data Supplement 1: Summary of ASTRO Guideline Statements. Data Supplement 2: Definition of Terms
 Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
Radiation Therapy in the Treatment of
 Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Small Cell Lung Cancer
 Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
4.8 Lung cancer. 4.8.5 In the UK, three fractionation regimens are most commonly used:
 4.8 Lung cancer 4.8.1 In 2005, both NICE (National Institute for Health and Clinical Excellence) and SIGN (Scottish Intercollegiate Guidelines Network) published guidelines on the management of lung cancer.
4.8 Lung cancer 4.8.1 In 2005, both NICE (National Institute for Health and Clinical Excellence) and SIGN (Scottish Intercollegiate Guidelines Network) published guidelines on the management of lung cancer.
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
A new score predicting the survival of patients with spinal cord compression from myeloma
 A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
Altered Fractionation of Radical Radiation Therapy in the Management of Unresectable Non-Small Cell Lung Cancer
 Evidence-based Series #7-12 Version 2 A Quality Initiative of the Program in Evidence-based Care (PEBC), Cancer Care Ontario (CCO) Altered Fractionation of Radical Radiation Therapy in the Management of
Evidence-based Series #7-12 Version 2 A Quality Initiative of the Program in Evidence-based Care (PEBC), Cancer Care Ontario (CCO) Altered Fractionation of Radical Radiation Therapy in the Management of
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
T reatment O utcomes of T hree-dimensional Conformal Radiotherapy for Stage III Non-Small Cell Lung Cancer
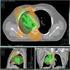 Cancer Res Treat. 2005;37(5):273-278 T reatment O utcomes of T hree-dimensional Conformal Radiotherapy for Stage III Non-Small Cell Lung Cancer Seung-Gu Yeo, M.D. 1, Moon-June Cho, M.D. 1,4, Sun-Young
Cancer Res Treat. 2005;37(5):273-278 T reatment O utcomes of T hree-dimensional Conformal Radiotherapy for Stage III Non-Small Cell Lung Cancer Seung-Gu Yeo, M.D. 1, Moon-June Cho, M.D. 1,4, Sun-Young
A new score predicting the survival of patients with spinal cord compression from myeloma
 A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
NCCN Non-Small Cell Lung Cancer V.1.2011 Update Meeting 07/09/10
 Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof.
 Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
5. Non small Cell Lung Cancer
 5. Non small Cell Lung Cancer Introduction The cancer registry in Sweden shows a continuous increase of lung cancer cases from 867 in 1958 when the registry started to 2 846 in 2000. The Swedish cancer
5. Non small Cell Lung Cancer Introduction The cancer registry in Sweden shows a continuous increase of lung cancer cases from 867 in 1958 when the registry started to 2 846 in 2000. The Swedish cancer
Lung Cancer Treatment Guidelines
 Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
Radiotherapy in locally advanced & metastatic NSC lung cancer
 Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines
 Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines April 2008 (presented at 6/12/08 cancer committee meeting) By Shelly Smits, RHIT, CCS, CTR Conclusions by Dr. Ian Thompson, MD Dr. James
Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines April 2008 (presented at 6/12/08 cancer committee meeting) By Shelly Smits, RHIT, CCS, CTR Conclusions by Dr. Ian Thompson, MD Dr. James
Helical TomoTherapy for Lung Cancer Radiotherapy: Good Science Pays Clinical Dividends Peter Hoban, Ph.D., TomoTherapy Inc.
 CLINICAL FEATURE Helical TomoTherapy for Lung Cancer Radiotherapy: Good Science Pays Clinical Dividends Peter Hoban, Ph.D., TomoTherapy Inc. The Challenge Lung cancer kills more people each year in the
CLINICAL FEATURE Helical TomoTherapy for Lung Cancer Radiotherapy: Good Science Pays Clinical Dividends Peter Hoban, Ph.D., TomoTherapy Inc. The Challenge Lung cancer kills more people each year in the
Concurrent Chemotherapy and Radiotherapy for Head and Neck Cancer
 Concurrent Chemotherapy and Radiotherapy for Head and Neck Cancer Ryan J. Burri; Nancy Y. Lee Published: 03/23/2009 Abstract and Introduction Abstract Head and neck cancer is best managed in a multidisciplinary
Concurrent Chemotherapy and Radiotherapy for Head and Neck Cancer Ryan J. Burri; Nancy Y. Lee Published: 03/23/2009 Abstract and Introduction Abstract Head and neck cancer is best managed in a multidisciplinary
L Lang-Lazdunski, A Bille, S Marshall, R Lal, D Landau, J Spicer
 Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine and systemic chemotherapy in malignant pleural mesothelioma. A 10-year experience. L Lang-Lazdunski, A Bille, S Marshall, R Lal,
Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine and systemic chemotherapy in malignant pleural mesothelioma. A 10-year experience. L Lang-Lazdunski, A Bille, S Marshall, R Lal,
Management of stage III A-B of NSCLC. Hamed ALHusaini Medical Oncologist
 Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Definitive Treatment of Poor-Risk Patients with Stage I Lung Cancer. A Single Institution Experience
 ORIGINAL ARTICLE Definitive Treatment of Poor-Risk Patients with Stage I Lung Cancer A Single Institution Experience Michael Hsie, MD,* Stefania Morbidini-Gaffney, MD,* Leslie J. Kohman, MD, Elisabeth
ORIGINAL ARTICLE Definitive Treatment of Poor-Risk Patients with Stage I Lung Cancer A Single Institution Experience Michael Hsie, MD,* Stefania Morbidini-Gaffney, MD,* Leslie J. Kohman, MD, Elisabeth
PET/CT in Lung Cancer
 PET/CT in Lung Cancer Rodolfo Núñez Miller, M.D. Nuclear Medicine and Diagnostic Imaging Section Division of Human Health International Atomic Energy Agency Vienna, Austria GLOBOCAN 2012 #1 #3 FDG-PET/CT
PET/CT in Lung Cancer Rodolfo Núñez Miller, M.D. Nuclear Medicine and Diagnostic Imaging Section Division of Human Health International Atomic Energy Agency Vienna, Austria GLOBOCAN 2012 #1 #3 FDG-PET/CT
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians
 Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
BASED ON THE RESULTS of the Radiation Therapy
 INT 0123 (Radiation Therapy Oncology Group 94-05) Phase III Trial of Combined-Modality Therapy for Esophageal Cancer: High-Dose Versus Standard-Dose Radiation Therapy By Bruce D. Minsky, Thomas F. Pajak,
INT 0123 (Radiation Therapy Oncology Group 94-05) Phase III Trial of Combined-Modality Therapy for Esophageal Cancer: High-Dose Versus Standard-Dose Radiation Therapy By Bruce D. Minsky, Thomas F. Pajak,
Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study
 Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Epidemiology, Staging and Treatment of Lung Cancer. Mark A. Socinski, MD
 Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
Adiuwantowe i neoadiuwantowe leczenie chorych na zaawansowanego raka żołądka
 Adiuwantowe i neoadiuwantowe leczenie chorych na zaawansowanego raka żołądka Neoadiuvant and adiuvant therapy for advanced gastric cancer Franco Roviello, IT Neoadjuvant and adjuvant therapy for advanced
Adiuwantowe i neoadiuwantowe leczenie chorych na zaawansowanego raka żołądka Neoadiuvant and adiuvant therapy for advanced gastric cancer Franco Roviello, IT Neoadjuvant and adjuvant therapy for advanced
Who may benefit from prophylactic cranial irradiation amongst stage III non-small cell lung cancer patients?
 JBUON 2013; 18(2): 453-458 ISSN: 1107-0625 www.jbuon.com E-mail: info@jbuon.com ORIGINAL ARTICLE Who may benefit from prophylactic cranial irradiation amongst stage III non-small cell lung cancer patients?
JBUON 2013; 18(2): 453-458 ISSN: 1107-0625 www.jbuon.com E-mail: info@jbuon.com ORIGINAL ARTICLE Who may benefit from prophylactic cranial irradiation amongst stage III non-small cell lung cancer patients?
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
The Need for Accurate Lung Cancer Staging
 The Need for Accurate Lung Cancer Staging Peter Baik, DO Thoracic Surgery Cancer Treatment Centers of America Oklahoma Osteopathic Association 115th Annual Convention Financial Disclosures: None 2 Objectives
The Need for Accurate Lung Cancer Staging Peter Baik, DO Thoracic Surgery Cancer Treatment Centers of America Oklahoma Osteopathic Association 115th Annual Convention Financial Disclosures: None 2 Objectives
Activity of pemetrexed in thoracic malignancies
 Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Special Article. Received 5 December 2014; revised 18 February 2015; accepted 25 February 2015. Practical Radiation Oncology (2015) 5, 141-148
 Practical Radiation Oncology (2015) 5, 141-148 www.practicalradonc.org Special Article Definitive radiation therapy in locally advanced non-small cell lung cancer: Executive summary of an American Society
Practical Radiation Oncology (2015) 5, 141-148 www.practicalradonc.org Special Article Definitive radiation therapy in locally advanced non-small cell lung cancer: Executive summary of an American Society
ORIGINAL ARTICLE THORACIC ONCOLOGY
 Ann Surg Oncol (2013) 20:1934 1940 DOI 10.1245/s10434-012-2800-x ORIGINAL ARTICLE THORACIC ONCOLOGY Predictors for Locoregional Recurrence for Clinical Stage III-N2 Non-small Cell Lung Cancer with Nodal
Ann Surg Oncol (2013) 20:1934 1940 DOI 10.1245/s10434-012-2800-x ORIGINAL ARTICLE THORACIC ONCOLOGY Predictors for Locoregional Recurrence for Clinical Stage III-N2 Non-small Cell Lung Cancer with Nodal
TITLE: Comparison of the dosimetric planning of partial breast irradiation with and without the aid of 3D virtual reality simulation (VRS) software.
 SAMPLE CLINICAL RESEARCH APPLICATION ABSTRACT: TITLE: Comparison of the dosimetric planning of partial breast irradiation with and without the aid of 3D virtual reality simulation (VRS) software. Hypothesis:
SAMPLE CLINICAL RESEARCH APPLICATION ABSTRACT: TITLE: Comparison of the dosimetric planning of partial breast irradiation with and without the aid of 3D virtual reality simulation (VRS) software. Hypothesis:
Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence
 Post- survival in completely resected stage I non-small cell lung cancer with local J-J Hung, 1,2,3 W-H Hsu, 3 C-C Hsieh, 3 B-S Huang, 3 M-H Huang, 3 J-S Liu, 2 Y-C Wu 3 See Editorial, p 185 c A supplementary
Post- survival in completely resected stage I non-small cell lung cancer with local J-J Hung, 1,2,3 W-H Hsu, 3 C-C Hsieh, 3 B-S Huang, 3 M-H Huang, 3 J-S Liu, 2 Y-C Wu 3 See Editorial, p 185 c A supplementary
Cesare Gridelli 1, Francesca Casaluce 2, Assunta Sgambato 2, Fabio Monaco 3, Cesare Guida 4
 Editorial Treatment of limited-stage small cell lung cancer in the elderly, chemotherapy vs. sequential chemoradiotherapy vs. concurrent chemoradiotherapy: that s the question Cesare Gridelli 1, Francesca
Editorial Treatment of limited-stage small cell lung cancer in the elderly, chemotherapy vs. sequential chemoradiotherapy vs. concurrent chemoradiotherapy: that s the question Cesare Gridelli 1, Francesca
Klaus Junker, MD; Kathrin Langner, MD; Folker Klinke, MD; Ulrich Bosse, MD; and Michael Thomas, MD
 Grading of Tumor Regression in Non-small Cell Lung Cancer* Morphology and Prognosis Klaus Junker, MD; Kathrin Langner, MD; Folker Klinke, MD; Ulrich Bosse, MD; and Michael Thomas, MD Objective: Different
Grading of Tumor Regression in Non-small Cell Lung Cancer* Morphology and Prognosis Klaus Junker, MD; Kathrin Langner, MD; Folker Klinke, MD; Ulrich Bosse, MD; and Michael Thomas, MD Objective: Different
Adjuvant Therapy Non Small Cell Lung Cancer. Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015
 Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
SAKK Lung Cancer Group. Current activities and future projects
 SAKK Lung Cancer Group Current activities and future projects SAKK Lung Cancer Group Open group of physicians interested in lung cancer Mostly Medical Oncologists, but also Thoracic Surgeons Radiation
SAKK Lung Cancer Group Current activities and future projects SAKK Lung Cancer Group Open group of physicians interested in lung cancer Mostly Medical Oncologists, but also Thoracic Surgeons Radiation
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer
 Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
POLICY A. INDICATIONS
 Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
Mesothelioma. 1. Introduction. 1.1 General Information and Aetiology
 Mesothelioma 1. Introduction 1.1 General Information and Aetiology Mesotheliomas are tumours that arise from the mesothelial cells of the pleura, peritoneum, pericardium or tunica vaginalis [1]. Most are
Mesothelioma 1. Introduction 1.1 General Information and Aetiology Mesotheliomas are tumours that arise from the mesothelial cells of the pleura, peritoneum, pericardium or tunica vaginalis [1]. Most are
Outcomes of Patients With Stage III Nonsmall Cell Lung Cancer Treated With Chemotherapy and Radiation With and Without Surgery
 Outcomes of Patients With Stage III Nonsmall Cell Lung Cancer Treated With Chemotherapy and Radiation With and Without Surgery Hale B. Caglar, MD 1 ; Elizabeth H. Baldini, MD, MPH 1 ; Megan Othus, MS 2
Outcomes of Patients With Stage III Nonsmall Cell Lung Cancer Treated With Chemotherapy and Radiation With and Without Surgery Hale B. Caglar, MD 1 ; Elizabeth H. Baldini, MD, MPH 1 ; Megan Othus, MS 2
Pulmonary function. Is patient potentially operable? Yes. tests 3. Yes. Pulmonary function. tests 3, if clinically indicated. Yes
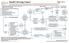 INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
Lung Cancer and Mesothelioma
 Lung Cancer and Mesothelioma Robert Kratzke, M.D. John C. Skoglund Professor of Lung Cancer Research Section of Heme/Onc/Transplant Department of Medicine University of Minnesota Medical School Malignant
Lung Cancer and Mesothelioma Robert Kratzke, M.D. John C. Skoglund Professor of Lung Cancer Research Section of Heme/Onc/Transplant Department of Medicine University of Minnesota Medical School Malignant
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline
 Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Stomach (Gastric) Cancer. Prof. M K Mahajan ACDT & RC Bathinda
 Stomach (Gastric) Cancer Prof. M K Mahajan ACDT & RC Bathinda Gastric Cancer Role of Radiation Layers of the Stomach Mucosa Submucosa Muscularis Serosa Stomach and Regional Lymph Nodes Stomach and Regional
Stomach (Gastric) Cancer Prof. M K Mahajan ACDT & RC Bathinda Gastric Cancer Role of Radiation Layers of the Stomach Mucosa Submucosa Muscularis Serosa Stomach and Regional Lymph Nodes Stomach and Regional
Survey on the treatment of non-small cell lung cancer (NSCLC) in England and Wales
 Eur Respir J 1997; 10: 1552 1558 DOI: 10.1183/09031936.97.10071552 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1997 European Respiratory Journal ISSN 0903-1936 Survey on the treatment
Eur Respir J 1997; 10: 1552 1558 DOI: 10.1183/09031936.97.10071552 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1997 European Respiratory Journal ISSN 0903-1936 Survey on the treatment
Efficacy of chemotherapy combined hyperfractionated accelerated radiotherapy on limited small cell lung cancer
 [Chinese Journal of Cancer 27:10, 353-357; October 2008]; 2008 Sun Yat-Sen University Cancer Center Clinical Research Paper Efficacy of chemotherapy combined hyperfractionated accelerated radiotherapy
[Chinese Journal of Cancer 27:10, 353-357; October 2008]; 2008 Sun Yat-Sen University Cancer Center Clinical Research Paper Efficacy of chemotherapy combined hyperfractionated accelerated radiotherapy
Radioterapia panencefalica. Umberto Ricardi
 Radioterapia panencefalica Umberto Ricardi Background Systemic disease to the brain is unfortunately a quite common event Radiotherapy, especially with the great technical development during the past decades,
Radioterapia panencefalica Umberto Ricardi Background Systemic disease to the brain is unfortunately a quite common event Radiotherapy, especially with the great technical development during the past decades,
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
B. Dingle MD, FRCPC, Brian Yaremko MD,FRCPC, R. Ash, MD, FRCPC, P. Truong, MD, FRCPC
 Lung Cancer B. Dingle MD, FRCPC, Brian Yaremko MD,FRCPC, R. Ash, MD, FRCPC, P. Truong, MD, FRCPC EPIDEMIOLOGY The estimated incidence of lung cancer in Canada for 2007 is 23,300 with 12,400 occurring in
Lung Cancer B. Dingle MD, FRCPC, Brian Yaremko MD,FRCPC, R. Ash, MD, FRCPC, P. Truong, MD, FRCPC EPIDEMIOLOGY The estimated incidence of lung cancer in Canada for 2007 is 23,300 with 12,400 occurring in
Extrapleural Pneumonectomy for Malignant Mesothelioma: Pro. Joon H. Lee 9/17/2012
 Extrapleural Pneumonectomy for Malignant Mesothelioma: Pro Joon H. Lee 9/17/2012 Malignant Pleural Mesothelioma (Epidemiology) Incidence: 7/mil (Japan) to 40/mil (Australia) Attributed secondary to asbestos
Extrapleural Pneumonectomy for Malignant Mesothelioma: Pro Joon H. Lee 9/17/2012 Malignant Pleural Mesothelioma (Epidemiology) Incidence: 7/mil (Japan) to 40/mil (Australia) Attributed secondary to asbestos
Radiotherapy in Plasmacytoma and Myeloma. David Cutter Multiple Myeloma NSSG Annual Meeting 14 th September 2015
 Radiotherapy in Plasmacytoma and Myeloma David Cutter Multiple Myeloma NSSG Annual Meeting 14 th September 2015 Contents Indications for radiotherapy: Palliation in Multiple Myeloma Solitary Bone Plasmacytoma
Radiotherapy in Plasmacytoma and Myeloma David Cutter Multiple Myeloma NSSG Annual Meeting 14 th September 2015 Contents Indications for radiotherapy: Palliation in Multiple Myeloma Solitary Bone Plasmacytoma
Particle Therapy for Lung Cancer. Bradford Hoppe MD, MPH Assistant Professor University of Florida bhoppe@floridaproton.org
 Particle Therapy for Lung Cancer Bradford Hoppe MD, MPH Assistant Professor University of Florida bhoppe@floridaproton.org Content Rationale for Particle Therapy in Lung Cancer Proof of Principle Treatment
Particle Therapy for Lung Cancer Bradford Hoppe MD, MPH Assistant Professor University of Florida bhoppe@floridaproton.org Content Rationale for Particle Therapy in Lung Cancer Proof of Principle Treatment
Lung Cancer. Public Outcomes Report. Submitted by Omar A. Majid, MD
 Public Outcomes Report Lung Cancer Submitted by Omar A. Majid, MD Lung cancer is the most common cancer-related cause of death among men and women. It has been estimated that there will be 226,1 new cases
Public Outcomes Report Lung Cancer Submitted by Omar A. Majid, MD Lung cancer is the most common cancer-related cause of death among men and women. It has been estimated that there will be 226,1 new cases
How TARGIT Intra-operative Radiotherapy can help Older Patients with Breast cancer
 How TARGIT Intra-operative Radiotherapy can help Older Patients with Breast cancer Jeffrey S Tobias, Jayant S Vaidya, Frederik Wenz and Michael Baum, University College Hospital, London, UK - on behalf
How TARGIT Intra-operative Radiotherapy can help Older Patients with Breast cancer Jeffrey S Tobias, Jayant S Vaidya, Frederik Wenz and Michael Baum, University College Hospital, London, UK - on behalf
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness
 Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness Investigators: Paul G. Shekelle, MD, PhD, Director Alicia R. Maher, MD Clinical
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness Investigators: Paul G. Shekelle, MD, PhD, Director Alicia R. Maher, MD Clinical
Management of Non-Small Cell Lung Cancer Guide for General Practitioners
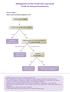 Management of n-small Cell Lung Cancer Guide for General Practitioners Clinical Stage I Cancer only in one lobe of lung and
Management of n-small Cell Lung Cancer Guide for General Practitioners Clinical Stage I Cancer only in one lobe of lung and
CANCER PULMON: ESTADIOS INICIALES POSTMUNDIAL PULMON DENVER 2015. 8-10-2015.Manuel Cobo Dols S. Oncología Médica HU Málaga Regional y VV
 CANCER PULMON: ESTADIOS INICIALES POSTMUNDIAL PULMON DENVER 2015 8-10-2015.Manuel Cobo Dols S. Oncología Médica HU Málaga Regional y VV Meta-analisis LACE: adyuvancia vs no adyuvancia Pignon JP, et al.
CANCER PULMON: ESTADIOS INICIALES POSTMUNDIAL PULMON DENVER 2015 8-10-2015.Manuel Cobo Dols S. Oncología Médica HU Málaga Regional y VV Meta-analisis LACE: adyuvancia vs no adyuvancia Pignon JP, et al.
Current Status and Perspectives of Radiation Therapy for Breast Cancer
 Breast Cancer Current Status and Perspectives of Radiation Therapy for Breast Cancer JMAJ 45(10): 434 439, 2002 Masahiro HIRAOKA, Masaki KOKUBO, Chikako YAMAMOTO and Michihide MITSUMORI Department of Therapeutic
Breast Cancer Current Status and Perspectives of Radiation Therapy for Breast Cancer JMAJ 45(10): 434 439, 2002 Masahiro HIRAOKA, Masaki KOKUBO, Chikako YAMAMOTO and Michihide MITSUMORI Department of Therapeutic
Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma
 Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma Marc de Perrot, Ronald Feld, Natasha B Leighl, Andrew Hope, Thomas K Waddell, Shaf Keshavjee,
Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma Marc de Perrot, Ronald Feld, Natasha B Leighl, Andrew Hope, Thomas K Waddell, Shaf Keshavjee,
Controversies in Management of. Inoperable NSCLC. Inoperable NSCLC. Introduction:
 Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin
 COMPENDIA TRANSPARENCY TRACKING FORM DRUG: Docetaxel INDICATION: Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin COMPENDIA TRANSPARENCY REQUIREMENTS 1 Provide
COMPENDIA TRANSPARENCY TRACKING FORM DRUG: Docetaxel INDICATION: Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin COMPENDIA TRANSPARENCY REQUIREMENTS 1 Provide
If several different trials are mentioned in one publication, the data of each should be extracted in a separate data extraction form.
 General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
Mesothelioma. Malignant Pleural Mesothelioma
 Mesothelioma William G. Richards, PhD Brigham and Women s Hospital Malignant Pleural Mesothelioma 2,000-3,000 cases per year (USA) Increasing incidence Asbestos (50-80%, decreasing) 30-40 year latency
Mesothelioma William G. Richards, PhD Brigham and Women s Hospital Malignant Pleural Mesothelioma 2,000-3,000 cases per year (USA) Increasing incidence Asbestos (50-80%, decreasing) 30-40 year latency
Is an evidence-based approach realistic in non-small cell lung cancer (NSCLC)?
 Is an evidence-based approach realistic in non-small cell lung cancer (NSCLC)? Authors Key words P.A. Coucke, N. Barthelemy, L. Bosquee, J.P. van Meerbeeck NSCLC, sequential and concomitant chemo-radiotherapy,
Is an evidence-based approach realistic in non-small cell lung cancer (NSCLC)? Authors Key words P.A. Coucke, N. Barthelemy, L. Bosquee, J.P. van Meerbeeck NSCLC, sequential and concomitant chemo-radiotherapy,
Stage I, II Non Small Cell Lung Cancer
 Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
Cancer Treatments Subcommittee of PTAC Meeting held 18 September 2015. (minutes for web publishing)
 Cancer Treatments Subcommittee of PTAC Meeting held 18 September 2015 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the
Cancer Treatments Subcommittee of PTAC Meeting held 18 September 2015 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the
Management of Stage III, N2 NSCLC: A Virtual Thoracic Oncology Tumor Board
 Management of Stage III, N2 NSCLC: A Virtual Thoracic Oncology Tumor Board Abstract Introduction Management of stage III non small-cell lung cancer (NSCLC) is complex and requires careful work-up, staging,
Management of Stage III, N2 NSCLC: A Virtual Thoracic Oncology Tumor Board Abstract Introduction Management of stage III non small-cell lung cancer (NSCLC) is complex and requires careful work-up, staging,
7. Prostate cancer in PSA relapse
 7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
Objectives. Mylene T. Truong, MD. Malignant Pleural Mesothelioma Background
 Imaging of Pleural Tumors Mylene T. Truong, MD Imaging of Pleural Tumours Mylene T. Truong, M. D. University of Texas M.D. Anderson Cancer Center, Houston, TX Objectives To review tumors involving the
Imaging of Pleural Tumors Mylene T. Truong, MD Imaging of Pleural Tumours Mylene T. Truong, M. D. University of Texas M.D. Anderson Cancer Center, Houston, TX Objectives To review tumors involving the
Treatment of stage III non-small cell lung cancer and limited-disease small-cell lung cancer
 Treatment of stage III non-small cell lung cancer and limited-disease small-cell lung cancer ISBN/EAN 9789490122096 Cover photo: Monique Slagter-Verburg Layout: Karin van Rijnbach Print: Gildeprint Drukkerijen
Treatment of stage III non-small cell lung cancer and limited-disease small-cell lung cancer ISBN/EAN 9789490122096 Cover photo: Monique Slagter-Verburg Layout: Karin van Rijnbach Print: Gildeprint Drukkerijen
EVIDENCE TABLE. Study Objective. (Purpose of Study)
 . Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 997; (6):70-77.. Greene FL, Page DL, Fleming ID, et al, eds, for the American Joint Committee on Cancer. AJCC Cancer
. Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 997; (6):70-77.. Greene FL, Page DL, Fleming ID, et al, eds, for the American Joint Committee on Cancer. AJCC Cancer
INTRODUCTION. liver neoplasms, adrenal gland neoplasms, non small-cell lung cancer, radionuclide imaging, bisphosphonates,
 VOLUME 22 NUMBER 2 JANUARY 15 2004 JOURNAL OF CLINICAL ONCOLOGY A S C O S P E C I A L A R T I C L E American Society of Clinical Oncology Treatment of Unresectable Non Small-Cell Lung Cancer Guideline:
VOLUME 22 NUMBER 2 JANUARY 15 2004 JOURNAL OF CLINICAL ONCOLOGY A S C O S P E C I A L A R T I C L E American Society of Clinical Oncology Treatment of Unresectable Non Small-Cell Lung Cancer Guideline:
3.0 With final Comments for presentation at Sub Group Meeting 24. 24.11.10
 Guideline for the Treatment of Lung Cancer Version History 2.0 Endorsed by the Governance Committee as treatment of lung cancer 27.07.09 with radiotherapy and chemotherapy. 2.1 Re-written to include the
Guideline for the Treatment of Lung Cancer Version History 2.0 Endorsed by the Governance Committee as treatment of lung cancer 27.07.09 with radiotherapy and chemotherapy. 2.1 Re-written to include the
PROTON THERAPY FOR PROSTATE CANCER: THE INITIAL LOMA LINDA UNIVERSITY EXPERIENCE
 doi:10.1016/j.ijrobp.2003.10.011 Int. J. Radiation Oncology Biol. Phys., Vol. 59, No. 2, pp. 348 352, 2004 Copyright 2004 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/04/$ see front
doi:10.1016/j.ijrobp.2003.10.011 Int. J. Radiation Oncology Biol. Phys., Vol. 59, No. 2, pp. 348 352, 2004 Copyright 2004 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/04/$ see front
Intensity Modulated Radiation Therapy (IMRT) for Thyroid Cancer
 Thyroid Science 5(1):CLS1-8, 2010 www.thyroidscience.com Clinical and Laboratory Studies Intensity Modulated Radiation Therapy (IMRT) for Thyroid Cancer 1 2 5 Aruna Turaka, MD, Tianyu Li, MS, Jian Q. Yu,
Thyroid Science 5(1):CLS1-8, 2010 www.thyroidscience.com Clinical and Laboratory Studies Intensity Modulated Radiation Therapy (IMRT) for Thyroid Cancer 1 2 5 Aruna Turaka, MD, Tianyu Li, MS, Jian Q. Yu,
Targeted Therapy What the Surgeon Needs to Know
 Targeted Therapy What the Surgeon Needs to Know AATS Focus in Thoracic Surgery 2014 David R. Jones, M.D. Professor & Chief, Thoracic Surgery Memorial Sloan Kettering Cancer Center I have no disclosures
Targeted Therapy What the Surgeon Needs to Know AATS Focus in Thoracic Surgery 2014 David R. Jones, M.D. Professor & Chief, Thoracic Surgery Memorial Sloan Kettering Cancer Center I have no disclosures
Harmesh Naik, MD. Hope Cancer Clinic HOW DO I MANAGE STAGE 4 NSCLC IN 2012: STATE OF THE ART
 Harmesh Naik, MD. Hope Cancer Clinic HOW DO I MANAGE STAGE 4 NSCLC IN 2012: STATE OF THE ART Goals Discuss treatment options for stage 4 lung cancer: New and old Discuss new developments in personalized
Harmesh Naik, MD. Hope Cancer Clinic HOW DO I MANAGE STAGE 4 NSCLC IN 2012: STATE OF THE ART Goals Discuss treatment options for stage 4 lung cancer: New and old Discuss new developments in personalized
Malignant Mesothelioma State of the Art
 Malignant Mesothelioma State of the Art Paul Baas The Netherlands Cancer Institute August 12, 2011, Carlsbad, CA Summary Diagnosis; epithelial type subdivided Pleiomorphic vs other Staging: IASLC-IMIG
Malignant Mesothelioma State of the Art Paul Baas The Netherlands Cancer Institute August 12, 2011, Carlsbad, CA Summary Diagnosis; epithelial type subdivided Pleiomorphic vs other Staging: IASLC-IMIG
New Evaluation Criteria for Response and Toxicity in Lung Cancer Treatment
 Lung Cancer New Evaluation Criteria for Response and Toxicity in Lung Cancer Treatment JMAJ 46(12): 554 558, 2003 Masahiko SHIBUYA Chief, Division of Respiratory Medicine, Tokyo Metropolitan Komagome Hospital
Lung Cancer New Evaluation Criteria for Response and Toxicity in Lung Cancer Treatment JMAJ 46(12): 554 558, 2003 Masahiko SHIBUYA Chief, Division of Respiratory Medicine, Tokyo Metropolitan Komagome Hospital
LATE MORBIDITY PROFILES IN PROSTATE CANCER PATIENTS TREATED TO 79 84 GY BY A SIMPLE FOUR-FIELD COPLANAR BEAM ARRANGEMENT
 PII S0360-3016(02)03822-1 Int. J. Radiation Oncology Biol. Phys., Vol. 55, No. 1, pp. 71 77, 2003 Copyright 2003 Elsevier Science Inc. Printed in the USA. All rights reserved 0360-3016/03/$ see front matter
PII S0360-3016(02)03822-1 Int. J. Radiation Oncology Biol. Phys., Vol. 55, No. 1, pp. 71 77, 2003 Copyright 2003 Elsevier Science Inc. Printed in the USA. All rights reserved 0360-3016/03/$ see front matter
People Living with Cancer
 Patient Guide ASCOInformation for People Living with Cancer ADVANCED LUNG CANCER TREATMENT Recommendations of the American Society of Clinical Oncology Welcome The American Society of Clinical Oncology
Patient Guide ASCOInformation for People Living with Cancer ADVANCED LUNG CANCER TREATMENT Recommendations of the American Society of Clinical Oncology Welcome The American Society of Clinical Oncology
SBRT (Elekta), 45 Gy in fractions of 3 Gy 3x/week for 5 weeks (N=22) vs.
 Uitgangsvraag 6: Wat is de plaats van stereotactische radiotherapiebehandeling (SBRT) bij HCC patiënten? Primaire studies I Study ID II Method III Patient characteristics IV Intervention(s) V Results primary
Uitgangsvraag 6: Wat is de plaats van stereotactische radiotherapiebehandeling (SBRT) bij HCC patiënten? Primaire studies I Study ID II Method III Patient characteristics IV Intervention(s) V Results primary
Scottish Medicines Consortium
 Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Stage 3 Lung Cancer - Know YourSurviving Conditions
 Survival of Stage IIIb and IV Non-Small Cell Lung Cancer Patients on Best Supportive Care in Manitoba, Canada Erich Kliewer Alain Demers Sri Navaratnam Coreen Hildebrand Grace Musto Report for AstraZeneca
Survival of Stage IIIb and IV Non-Small Cell Lung Cancer Patients on Best Supportive Care in Manitoba, Canada Erich Kliewer Alain Demers Sri Navaratnam Coreen Hildebrand Grace Musto Report for AstraZeneca
New Trends & Current Research in the Treatment of Lung Cancer, Pt. II
 New Trends & Current esearch in the Treatment of Lung Cancer, Pt. II Howard (Jack) West, MD President & CEO, GACE Medical Director, Thoracic Oncology Program Swedish Cancer Institute Seattle, WA Cancer
New Trends & Current esearch in the Treatment of Lung Cancer, Pt. II Howard (Jack) West, MD President & CEO, GACE Medical Director, Thoracic Oncology Program Swedish Cancer Institute Seattle, WA Cancer
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Implementation Date: April 2015 Clinical Operations
 National Imaging Associates, Inc. Clinical guideline PROSTATE CANCER Original Date: March 2011 Page 1 of 5 Radiation Oncology Last Review Date: March 2015 Guideline Number: NIA_CG_124 Last Revised Date:
National Imaging Associates, Inc. Clinical guideline PROSTATE CANCER Original Date: March 2011 Page 1 of 5 Radiation Oncology Last Review Date: March 2015 Guideline Number: NIA_CG_124 Last Revised Date:
Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008
 Special Report Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008 Matthew B. Schabath, PhD, Zachary J. Thompson, PhD,
Special Report Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008 Matthew B. Schabath, PhD, Zachary J. Thompson, PhD,
CLINICAL POLICY Department: Medical Management Document Name: Opdivo Reference Number: CP.PHAR.121 Effective Date: 07/15
 Page: 1 of 6 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Page: 1 of 6 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
ACR Appropriateness Criteria on Nonsurgical Treatment for Non Small-Cell Lung Cancer: Poor Performance Status or Palliative Intent
 ACR Appropriateness Criteria on Nonsurgical Treatment for Non Small-Cell Lung Cancer: Poor Performance Status or Palliative Intent Kenneth E. Rosenzweig, MD a, Benjamin Movsas, MD b, Jeff Bradley, MD c,
ACR Appropriateness Criteria on Nonsurgical Treatment for Non Small-Cell Lung Cancer: Poor Performance Status or Palliative Intent Kenneth E. Rosenzweig, MD a, Benjamin Movsas, MD b, Jeff Bradley, MD c,
Radiation Therapy for Non-Small Cell Lung Cancer. Principles of Radiation Therapy for Non-small Cell Lung Cancer
 Chapter 4 Radiation Therapy for Non-Small Cell Lung Cancer Join Y. Luh, MD, FACP and Charles R. Thomas, Jr., MD Introduction Radiation is a form of energy that has both beneficial and harmful effects on
Chapter 4 Radiation Therapy for Non-Small Cell Lung Cancer Join Y. Luh, MD, FACP and Charles R. Thomas, Jr., MD Introduction Radiation is a form of energy that has both beneficial and harmful effects on
Neoplasms of the LUNG and PLEURA
 Neoplasms of the LUNG and PLEURA 2015-2016 FCDS Educational Webcast Series Steven Peace, BS, CTR September 19, 2015 2015 Focus o Anatomy o SSS 2000 o MPH Rules o AJCC TNM 1 Case 1 Case Vignette HISTORY:
Neoplasms of the LUNG and PLEURA 2015-2016 FCDS Educational Webcast Series Steven Peace, BS, CTR September 19, 2015 2015 Focus o Anatomy o SSS 2000 o MPH Rules o AJCC TNM 1 Case 1 Case Vignette HISTORY:
Moving forward, where are we with Clinical Trials?
 Moving forward, where are we with Clinical Trials? Dennis A. Wigle Division of Thoracic Surgery Mayo Clinic AATS/STS General Thoracic Surgery Symposium Sunday, April 27 th 2014 2012 MFMER slide-1 Where
Moving forward, where are we with Clinical Trials? Dennis A. Wigle Division of Thoracic Surgery Mayo Clinic AATS/STS General Thoracic Surgery Symposium Sunday, April 27 th 2014 2012 MFMER slide-1 Where
Small Cell Lung Cancer
 Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Male. Female. Death rates from lung cancer in USA
 Male Female Death rates from lung cancer in USA Smoking represents an interesting combination of an entrenched industry and a clearly drug-induced cancer Tobacco Use in the US, 1900-2000 5000 100 Per Capita
Male Female Death rates from lung cancer in USA Smoking represents an interesting combination of an entrenched industry and a clearly drug-induced cancer Tobacco Use in the US, 1900-2000 5000 100 Per Capita
