EVIDENCE TABLE. Study Objective. (Purpose of Study)
|
|
|
- Maximillian Wilkins
- 8 years ago
- Views:
Transcription
1 . Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 997; (6): Greene FL, Page DL, Fleming ID, et al, eds, for the American Joint Committee on Cancer. AJCC Cancer Staging Manual. New York, NY: Springer-Verlag; Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 007; (8): Rosell R, Gomez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with nonsmall-cell lung cancer. N Engl J Med 994; 330(3): ,39 Present revisions in stage grouping of the TNM The TNM subsets in stage IIIB T4 any N subsets (T = primary tumor, N = regional M0, any T N3M0, and in stage IV any T lymph nodes, M = distant metastasis) in the any N M, remain the same. Analysis of International System for Staging Lung Cancer. database confirmed the validity of the TNM Study analyzed database representing all and stage grouping classification schema. clinical, surgical-pathologic, and follow-up information for patients treated for primary lung cancer. Revisions addressed two problems; the heterogeneity of end results existing for the TNM categories within stage groups, and a need for greater specificity in stage classification. 5 N/A Cancer staging manual. N/A N/A 5 67,75 cases Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumors. 60 Randomized trial to test the value of induction chemotherapy in resectable IIIA disease. Compared preoperative chemotherapy plus surgery with surgery alone. Suggestions include additional cutoffs for tumor size, with tumors >7 cm moving from T to T3; reassigning the category given to additional pulmonary nodules in some locations; and reclassifying pleural effusion as an M descriptor. In addition, it is suggested that Tb N0 M0 cases be moved from stage IB to stage IIA, Ta N M0 cases from stage IIB to stage IIA, and T4 N0- M0 cases from stage IIIB to stage IIIA. Median period of survival was 6 months for chemotherapy plus surgery compared with 8 months for surgery alone (P<0.00). The rate of recurrence was 56% in the group treated with chemotherapy plus surgery and 74% in the group treated with surgery alone. The prevalence of mutated K-ras oncogenes was 5% in the group treated with preoperative chemotherapy and 4% among those treated with surgery alone (P=0.05). Preoperative chemotherapy increases the median survival in patients with * See Last Page for Key 00 Review Gewanter Page
2 5. Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-smallcell lung cancer. J Natl Cancer Inst 994; 86(9): Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III nonsmall-cell lung cancer. J Clin Oncol 999; 7(9): Prospective, randomized study of patients with previously untreated, potentially resectable clinical stage IIIA NSCLC to compare the results of perioperative chemotherapy and surgery with those of surgery alone. 30 Prospective, randomized study to determine whether concurrent or sequential treatment with radiotherapy (RT) and chemotherapy improves survival in unresectable stage III Patient s treated with perioperative chemotherapy and surgery had an estimated median survival of 64 months compared with months for patients who had surgery alone (P<.008 by log-rank test; P<.08 by Wilcoxon test). The estimated -year and 3-year survival rates were 60% and 56% for the perioperative chemotherapy patients and 5% and 5% for those who had surgery alone, respectively. Perioperative chemotherapy and surgery was more effective than surgery alone. The median survival duration was better in patients receiving concurrent therapy (6.5 months), as compared with those receiving sequential therapy (3.3 months) (P=.03998). Two-, 3-, 4-, and 5-year survival rates in the concurrent group (34.6%,.3%, 6.9%, and 5.8%, respectively) were better than those in the sequential group (7.4%, 4.7%, 0.%, and 8.9%, respectively). Myelosuppression was significantly greater among patients on the concurrent arm than on the sequential arm (P=.000). In selected patients with unresectable stage III NSCLC, the concurrent approach yields a significantly increased response rate and enhanced median survival duration when compared with the sequential approach. * See Last Page for Key 00 Review Gewanter Page
3 7. Komaki R, Seiferheld W, Ettinger D, Lee JS, Movsas B, Sause W. Randomized phase II chemotherapy and radiotherapy trial for patients with locally advanced inoperable non-small-cell lung cancer: long-term follow-up of RTOG Int J Radiat Oncol Biol Phys 00; 53(3): Perez CA, Stanley K, Rubin P, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer 980; 45(): Cox JD, Azarnia N, Byhardt RW, Shin KH, Emami B, Pajak TF. A randomized phase I/II trial of hyperfractionated radiation therapy with total doses of 60.0 Gy to 79. Gy: possible survival benefit with greater than or equal to 69.6 Gy in favorable patients with Radiation Therapy Oncology Group stage III non-small-cell lung carcinoma: report of Radiation Therapy Oncology Group 83-. J Clin Oncol 990; 8(9): Prospective, randomized study to evaluate further the toxicity and efficacy of two different strategies of chemo-rt evaluated in two prior Radiation Therapy Oncology Group (RTOG) Phase II studies. 8 patients were treated in Arm and 8 patients in Arm. 365 Preliminary analysis of a prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. 848 (n=350 eligible for CALGB 84-33) Randomized trial to determine maximum tolerated dose of hyperfractionated RT and tumor control over a range of doses:. Gy/fx BID to doses of 60 Gy, 64.8 Gy, 69.6 Gy, 74.4 Gy, and 79. Gy. Incidence of acute esophagitis was higher in Arm than Arm (P<0.000). Incidence of acute hematologic toxicity was higher in Arm (P=0.0 for anemia and P=0.03 for other hematologic toxicities) than Arm. Analysis of late toxicity showed that chronic esophageal toxicity was significantly more frequent in Arm than in Arm (P=0.003). The time to in-field progression was significantly different (P 0.009), favoring Arm compared with Arm (6% vs 45% with failure in years and 30% vs 49% with failure in 4 years, respectively). The median -year and overall 5-year survival rates were similar between the two arms. Concurrent chemotherapy and hyperfractionated RT resulted in a significant prolongation of the time to infield progression, but with higher acute and chronic esophagitis. Data suggest patients treated with 5000 or 6000 rad have a better response, tumor control, and survival rate than those receiving lower doses. Patients with high performance status or with tumors in earlier stages have a -year survival rate of approximately 40%, in comparison with 0% for other patients. Additional follow-up of patients needed. Risks of severe or life-threatening pneumonitis were.6% for 60.0 to 64.8 Gy, 5.7% for 69.6 to 74.4 Gy, and 8.% for 79. Gy. Among Cancer and Leukemia Group B (CALGB) eligible patients, significant incurrence survival for 69.6 Gy group vs <69.6 Gy when analyzed by dose received. No further improvement with higher doses. * See Last Page for Key 00 Review Gewanter Page 3
4 0. Saunders M, Dische S, Barrett A, Harvey A, Griffiths G, Palmar M. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: mature data from the randomised multicentre trial. CHART Steering committee. Radiother Oncol 999; 5(): Murshed H, Liu HH, Liao Z, et al. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 004; 58(4): Randomized comparison of continuous hyperfractionated accelerated RT with conventional RT. CHART (54 Gy/36 fractions over days (.5 Gy TID) vs conventional (60 Gy/30 fractions over 6 weeks ( Gy daily). 3b 4 Comparative study to examine dosimetric improvements with respect to tumor-dose conformity and normal tissue sparing using IMRT vs 3D conformal RT (3D-CRT) for advanced-stage In the subgroup of patients with squamous cell cancer which accounted for 8% of the cases, there was a 30% reduction in the relative risk of death, which is equivalent to an absolute improvement in year survival of 3% from 0%-33% (P=0.0007) and a 7% reduction in the relative risk of local progression (P=0.0). In squamous carcinoma there was a 5% reduction in the relative risk of local and/or distant progression (P=0.05) and 4% reduction in the relative risk of metastasis (P=0.043). Analysis of mature data confirms that CHART is superior to conventional RT in achieving local tumor control and survival in locally advanced Using IMRT, the median absolute reduction in the percentage of lung volume irradiated to >0 and >0 Gy was 7% and 0%, respectively. This corresponded to a decrease of > Gy in the total lung mean dose and of 0% in the risk of radiation pneumonitis. The volumes of the heart and esophagus irradiated to >40-50 Gy and normal thoracic tissue volume irradiated to >0-40 Gy were reduced using the IMRT plans. A marginal increase occurred in the spinal cord maximal dose and lung volume >5 Gy in the IMRT plans, which could be have resulted from the significant increase in monitor units and thus leakage dose in IMRT. 3 * See Last Page for Key 00 Review Gewanter Page 4
5 . Yom SS, Liao Z, Liu HH, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-smallcell lung cancer patients treated with concurrent chemotherapy and intensitymodulated radiotherapy. Int J Radiat Oncol Biol Phys 007; 68(): Sura S, Gupta V, Yorke E, Jackson A, Amols H, Rosenzweig KE. Intensitymodulated radiation therapy (IMRT) for inoperable non-small cell lung cancer: the Memorial Sloan-Kettering Cancer Center (MSKCC) experience. Radiother Oncol 008; 87(): Bush DA, Slater JD, Shin BB, Cheek G, Miller DW, Slater JM. Hypofractionated proton beam radiotherapy for stage I lung cancer. Chest 004; 6(4): c 68 patients treated with IMRT patients had 3D-CRT Retrospective study to examine the rate of high-grade treatment-related pneumonitis in patients with advanced NSCLC treated with concurrent chemotherapy and IMRT. 3a 55 Retrospective review of patients with stage I- IIIB inoperable NSCLC treated with IMRT Prospective phase II trial to determine the efficacy and toxicity of high-dose hypofractionated proton beam RT for patients with clinical stage I lung cancer. Median follow-up durations for IMRT and 3D-CRT patients were 8 months and 9 months, respectively. The median IMRT and 3D-CRT doses were 63 Gy. The median gross tumor volume was 94 ml for IMRT, compared with 4 ml for 3D-CRT (P=0.00). Despite the IMRT group s larger gross tumor volume, the rate of grade 3 treatment-related pneumonitis at months was 8% compared with 3% for 3D-CRT (P=0.00). Study concludes that in advanced NSCLC patients treated with chemoradiation, IMRT resulted in significantly lower levels of grade 3 treatment-related pneumonitis compared with 3D-CRT. For median follow-up of 6 months, the -year local control and overall survival rates for stage I/II patients were 50% and 55%, respectively. For the stage III patients, -year local control and overall survival rates were 58% and 58%, respectively, with a median survival time of 5 months. IMRT treatment resulted in promising outcomes for inoperable NSCLC patients. The 3-year local control and disease-specific survival rates were 74%, and 7%, respectively. There was significant improvement in local tumor control in T vs T tumors (87% vs 49%), with a trend toward improved survival. Cox regression analysis revealed that patients with higher performance status, female gender, and smaller tumor sizes had significantly improved survival. 3 * See Last Page for Key 00 Review Gewanter Page 5
6 5. Nihei K, Ogino T, Ishikura S, Nishimura H. High-dose proton beam therapy for Stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 006; 65(): Chang J, Komaki R, Wen HY, et al. Toxicity and Patterns of Failure of Adaptive/Ablative Proton Therapy for Early-Stage, Medically Inoperable Non- Small Cell Lung Cancer. Int J Rad Oncol Biol Phys 00:In press. 7. Shioyama Y, Tokuuye K, Okumura T, et al. Clinical evaluation of proton radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 003; 56():7-3. 3a 37 To retrospectively study the safety and efficacy of high-dose proton beam therapy for stage I 8 To analyze the toxicity and patterns of failure of proton therapy given in ablative doses for medically inoperable early-stage 5 To evaluate the clinical results of proton RT for patients with Patient characteristics (number of patients) were as follows: Stage IA/IB 7/0; medically inoperable/refusal of surgery 3/4; total dose 70/80/88/94; Gy(E) 3/7/6/. With a median follow-up period of 4 months, the -year local progression-free and overall survival rates were 80% and 84%, respectively. The -year locoregional relapse-free survival rates in stage IA and stage IB were 79% and 60%, respectively. Proton beam therapy is a promising treatment modality. At a median follow-up time of 6.3 months (range, months), no patient had experienced grade 4 or 5 toxicity. The most common adverse effect was dermatitis (grade, 67%; grade 3, 7%), followed by grade fatigue (44%), grade pneumonitis (%), grade esophagitis (6%), and grade chest wall pain (6%). Rates of local control were 88.9%, regional lymph node failure.%, and distant metastasis 7.8%. Twelve patients (67%) were still alive at the last follow-up; five had died of metastatic disease and one of preexisting cardiac disease. 5-year overall survival rate was 9% for all patients, 70% for 9 stage IA patients, and 6% for 9 stage IB patients, respectively (IA vs IB: P< 0.05). The 5-year in-field local control rate was higher in patients with stage IA (89%) when compared with those with stage IB (39%). 47 patients (9%) experienced acute lung toxicity of grade or less; 3 had grade, had grade 3, and none experienced grade 4 or higher. Proton therapy is safe and effective treatment. 3 3 * See Last Page for Key 00 Review Gewanter Page 6
7 8. Chang JY, Komaki R, Bucci MK, et al. Failure Patterns and Toxicity of Concurrent Proton Therapy and Chemotherapy for Stage III Non small Cell Lung Cancer. International journal of radiation oncology, biology, physics 009; 75(3):S446-S Widesott L, Amichetti M, Schwarz M. Proton therapy in lung cancer: clinical outcomes and technical issues. A systematic review. Radiother Oncol 008; 86(): Dillman RO, Herndon J, Seagren SL, Eaton WL, Jr., Green MR. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst 996; 88(7): Analyze failure patterns, survival, and toxicity in a phase II study of patients with stage III NSCLC treated with dose escalated proton therapy and concurrent chemotherapy. 7 7 reports researchers Systematic review to determine whether proton therapy has a role in the treatment of NSCLC, to assess its safety and efficacy and to evaluate major technical issues related to this technique. 55 Present long-term survival results based on a median follow-up of more than 8 years for patients enrolled in CALGB 8433 trial. A randomized trial of induction chemotherapy plus high dose radiation vs radiation alone in stage III Rates of isolated local failure in the planned target volume (PTV) and in regional lymph nodes outside the PTV were both 3.3%; rate of distant metastasis was 0%; rate of distant metastasis + local/regional failure was 6.7%. Dose-escalated concurrent proton therapy and chemotherapy seems to improve local control with less toxicity than photon therapy. Longer follow-up is needed. Limited data available for use of proton therapy in clinical practice. Application of proton therapy to lung cancer presents technical challenges. Because of the small number of institutions involved in the treatment of this disease, number of patients, and methodological weaknesses of the trials it is therefore not possible to draw definitive conclusions about the superiority of proton therapy with respect to the photon techniques currently available for the treatment of Rate of tumor response was 56% for the chemotherapy-rt group and 43% for the RT group (P=.09). After more than 7 years of follow-up, the median survival remains greater for the chemotherapy-rt group (3.7 months) than for the RT group (9.6 months) (P=.0). The percentages of patients surviving after years through 7 were 54%, 6%, 4%, 9%, 7%, 3%, and 3% for the chemotherapy-rt group and 40%, 3%, 0%, 7%, 6%, 6%, and 6% for the RT group. Long-term follow-up confirms that patients with stage III NSCLC who receive 5 weeks of chemotherapy with cisplatin and vinblastine before RT have a 4.- month increase in median survival. 3 * See Last Page for Key 00 Review Gewanter Page 7
8 . Le Chevalier T, Arriagada R, Quoix E, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in unresectable non-small cell lung carcinoma. Lung Cancer 994; 0 Suppl :S Sause W, Kolesar P, Taylor SI, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 000; 7(): Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med 99; 36(8): (77 had RT alone and 76 had combined treatment) Randomized study comparing RT alone to combined RT and chemotherapy in unresectable squamous cell and large cell lung carcinoma. 458 Randomized study to determine if chemotherapy followed by RT resulted in superior survival to either hyperfractionated radiation or standard radiation in surgically unresectable 33 To examine effects of concomitant cisplatin and RT on inoperative Randomly assigned patients with nonmetastatic inoperable NSCLC to one of three treatments: RT for weeks (3 Gy given 0 times, in 5 fractions a week), followed by a three-week rest period and then RT for two more weeks (.5 Gy given 0 times, five fractions a week); RT on the same schedule, combined with 30 mg of cisplatin per square meter of body-surface area, given on the first day of each treatment week; or RT on the same schedule, combined with 6 mg of cisplatin per square meter, given daily before RT. -year survival rate was 4% for patients receiving RT vs % for patients receiving the combined treatment (P=0.0). The distant metastasis rate was significantly lower in the group receiving the combined treatment (P<0.00). Local control at year was poor in both groups (7% and 5%, respectively) and remains a major problem in locally advanced Overall survival was superior for chemotherapy and RT. The twice-daily RT arm was not superior in survival for those patients receiving standard radiation. Median survival for standard radiation was.4 months; for chemotherapy and irradiation, 3. months; and for hyperfractionated irradiation, months. The respective 5-year survivals were 5% for standard RT, 8% for chemotherapy followed by RT, and 6% for hyperfractionated irradiation. Survival was improved in the RT-dailycisplatin group vs RT group (P=0.009): survival in the RT-daily-cisplatin group was 54% at one year, 6% at two years, and 6% at three years, as compared with 46%, 3%, and % respectively, in the RT group. Survival in the RT-weekly-cisplatin group was intermediate (44%, 9%, and 3%) and not significantly different from survival in either of the other two groups. Survival without local recurrence was 59% at one year and 3% at two years in the RTdaily-cisplatin group; 4% and 30%, respectively, in the RT-weekly-cisplatin group; and 4% and 9%, respectively, in the RT group. * See Last Page for Key 00 Review Gewanter Page 8
9 4. Wolf M, Hans K, Becker H, et al. Radiotherapy alone versus chemotherapy with ifosfamide/vindesine followed by radiotherapy in unresectable locally advanced non-small cell lung cancer. Semin Oncol 994; (3 Suppl 4): Ansari R, Tokars R, Fisher W, et al. A phase III study of thoracic irradiation with or without concomitant cisplatin in locoregional unresectable non-small cell cancer. A Hoosier Oncology Group Protocol. Proc Am Soc Clin Oncol 99; 0:4. 6. Mattson K, Holsti LR, Holsti P, et al. Inoperable non-small cell lung cancer: radiation with or without chemotherapy. Eur J Cancer Clin Oncol 988; 4(3): Randomized, multicenter study to compare RT alone vs chemotherapy with ifosfamide/vindesine followed by RT in unresectable locally advanced 5 A Phase III study of thoracic irradiation with and without concomitant Cisplatin in locally advanced NSCLC; a Hoosier Oncology Group study. 38 Randomized, multicentre study of split-course RT, with or without combination chemotherapy in patients with inoperable Of the patients receiving chemotherapy, 5% had a partial remission after -cycles, 46% showed no change, and 9% had progressive disease. After RT, response rates were 49% in Arm A and 58% in Arm B, including a 0% complete remission rate in both groups. Median survival was 9 months vs 3.7 months and -year survival was % vs 4%, both in favor of the group receiving chemotherapy. Results indicate that chemotherapy is able to prolong survival. Overall response rate was 50% on the combination arm vs 38% on the RT-alone arm. The median progression-free survival time was 3 vs weeks, respectively (P=.0537). The median survival time was 43 weeks on the combination arm vs 46 weeks on the RT arm. The -, -, and 5-year survival rates were 43%, 8%, and 5% on the combination arm vs 45% 3%, and % on the RT arm, respectively. Cisplatin, administered every 3 weeks, does not significantly improve response rate, progression-free survival, or overall survival when added to thoracic RT for locally advanced unresectable No significant difference was apparent between the RT and the RT-chemotherapy arms with respect to objective response rates (CR + PR) (44% and 49%, respectively), median duration of response (78 and 30 days), local failure (3% and 0%), distant progression (3% and 0%) or median survival (3 and 3 days). The survival figures showed an almost significant (P=0.05) therapeutic advantage of the combined regimen with stage IIIM0 disease. Progressive disease was the cause of death in 9% and 88%. Chemotherapy did not contribute significantly to either local control or survival as compared to RT alone. * See Last Page for Key 00 Review Gewanter Page 9
10 7. Morton RF, Jett JR, McGinnis WL, et al. Thoracic radiation therapy alone compared with combined chemoradiotherapy for locally unresectable non-small cell lung cancer. A randomized, phase III trial. Ann Intern Med 99; 5(9): Robinow JS, Shaw EG, Eagan RT, et al. Results of combination chemotherapy and thoracic radiation therapy for unresectable non-small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 989; 7(6): Trovo MG, Minatel E, Veronesi A, et al. Combined radiotherapy and chemotherapy versus radiotherapy alone in locally advanced epidermoid bronchogenic carcinoma. A randomized study. Cancer 990; 65(3): Groen HJ, van der Leest AH, Fokkema E, et al. Continuously infused carboplatin used as radiosensitizer in locally unresectable non-small-cell lung cancer: a multicenter phase III study. Ann Oncol 004; 5(3): Randomized, multicenter phase III trial. Thoracic RT alone compared with combined chemo-rt for locally advanced resectable 6 Results of combination chemotherapy and thoracic RT for unresectable NSCLC from a prospective, randomized trial. Randomized study comparing combined RT and chemotherapy vs RT alone in locally advanced epidermoid bronchogenic carcinoma. 60 Randomized, multicenter phase 3 trial to determine the radiosensitizing effect of prolonged exposure of carboplatin in patients with locally unresectable 3/ 56 patients treated with combination therapy and 37/58 treated with radiation only had major clinical responses. The median time to progression was 9 days with RT only compared with 99 days for combined modality therapy. The median survival time was 33 days compared with 37 days, respectively. The -, -, and 5-year survival rates after thoracic radiation only were 45% and 7%. With chemo-rt, the survival rates were 46%, %, and 5%, respectively. Median and 5-year survivals for the group were 4 months and 0%, respectively; for the evaluable subgroup (0 patients), they were 4.8 months and %, respectively. Median and 5-year survivals were 6. months and 8%, respectively, with VP-6 plus CAP (cytoxan, adriamycin, and cisplatin) plus thoracic RT. Data suggest combined treatment with V-CAP and thoracic RT yielded excellent results. Difference in response rate was not significant. Median time to progression was 5.9 and 7.0 months, respectively, for the radiation treatment and the combined treatment. Median survival was.74 and 0.03 months, respectively, without statistically significant differences between the two groups of patients. Study found no significant superiority of combined RT and chemotherapy treatment over RT alone. Median survival in the combination arm was.8 months and in the RT alone arm.7 months; progression-free survival was not different between arms [6.8 and 7.5 months, respectively (P=0.8)]. Addition of continuously administered carboplatin as radiosensitizer for locally unresectable NSCLC does not improve local tumor control or overall survival. * See Last Page for Key 00 Review Gewanter Page 0
11 3. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 5 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 995; 3(700): Marino P, Preatoni A, Cantoni A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer. A metaanalysis. Cancer 995; 76(4): Auperin A, Le Pechoux C, Pignon JP, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 764 patients. Ann Oncol 006; 7(3): ,387 Meta-analysis using available randomized trials to evaluate the effect of cytotoxic chemotherapy on survival in patients with 7,887 Meta-analysis study using clinical trials that evaluated combined RT plus chemotherapy vs RT alone in patients with stages IIIa and IIIb 7,764 Meta-analysis based on individual patient data from published and unpublished randomized trials which compared RT alone with the same RT combined with concomitant cisplatin- or carboplatin-based chemotherapy. Trials comparing surgery with surgery plus chemotherapy gave a hazard ratio of 0.87 (3% reduction in the risk of death, equivalent to an absolute benefit of 5% at 5- years). Trials comparing radical RT with radical RT plus chemotherapy gave a hazard ratio of 0.87 (3% reduction in the risk of death; absolute benefit of 4% at -years). Trials comparing supportive care with supportive care plus chemotherapy 0.73 (7% reduction in the risk of death; 0% improvement in survival at -year). For the cisplatin-based group, the estimated pooled odds ratio of death at year and years was 0.76 ( CI) and 0.70 ( CI), with a reduction in mortality of 4% and 30%, respectively. For the noncisplatin-based group, the estimated pooled odds ratio at and years was.05 ( CI) and 0.8 ( CI), with a reduction in mortality of 5% and 8%, respectively. The hazard ratio of death among patients treated with radio chemotherapy compared to RT alone was 0.89 (95% CI: ; P=0.0) corresponding to an absolute benefit of chemotherapy of 4% at years. There was some evidence of heterogeneity among trials and sensitivity analyses did not lead to consistent results. The combination of platin with etoposide seemed more effective than platin alone. * See Last Page for Key 00 Review Gewanter Page
12 34. Bonner JA, McGinnis WL, Stella PJ, et al. The possible advantage of hyperfractionated thoracic radiotherapy in the treatment of locally advanced nonsmall cell lung carcinoma: results of a North Central Cancer Treatment Group Phase III Study. Cancer 998; 8(6): Schild SE, Stella PJ, Geyer SM, et al. Phase III trial comparing chemotherapy plus once-daily or twice-daily radiotherapy in Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 00; 54(): Randomized trial to compare response rates, distant progression, and survival for patients with unresectable (stage IIIA or IIIB) NSCLC treated with standard fractionated thoracic radiotherapy (SFTRT) vs accelerated hyperfractionated thoracic radiotherapy (AHTRT) with or without combination etoposide and cisplatin chemotherapy. 34 Randomized, multicenter, Phase III trial to determine whether chemotherapy plus BID or q.d. RT resulted in superior survival for patients with stage III The improvement in survival associated with AHTRT (with or without chemotherapy) was statistically significant for the subgroup of patients with non-squamous cell carcinoma after adjustment for other potentially confounding factors (P =0.0). No differences in freedom from systemic progression or survival were found in a comparison of AHTRT with chemotherapy and AHTRT without chemotherapy. Results suggest that treatment of stage IIIA or IIIB NSCLC with AHTRT with or without chemotherapy may improve freedom from local progression and survival as compared with SFTRT. Statistical powers to detect the observed differences in median time to local progression and survival were approximately 55% and 35%, respectively. Incidence of severe ( grade 3) acute nonhematologic toxicity (q.d. RT, 53% vs BID RT, 65%) and severe ( grade 3) hematologic toxicities (thrombocytopenia, 4% q.d. RT vs 39% BID RT; neutropenia, 80% q.d. RT vs 8% BID RT) was not significantly different between the treatment arms. No significant differences were found between the q.d. and BID RT arms in terms of time to progression (P=0.9; median 9.4 and 9.6 months, respectively), overall survival (P=0.4; median 4 and 5 months and -year survival rate 37% and 40%, respectively), and cumulative incidence of local failure (P=0.6; -year rate 45% and 4%, respectively). Split-course BID RT plus etoposide and cisplatin was not superior to standard q.d. RT plus etoposide and cisplatin. The toxicity, tumor control, and survival rates were similar with either BID or q.d. RT. * See Last Page for Key 00 Review Gewanter Page
13 36. Movsas B, Scott C, Langer C, et al. Randomized trial of amifostine in locally advanced non-small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: radiation therapy oncology group trial J Clin Oncol 005; 3(0): Fournel P, Robinet G, Thomas P, et al. Randomized Phase III Trial of Sequential Chemoradiotherapy Compared With Concurrent Chemoradiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: Groupe Lyon-Saint-Etienne d'oncologie Thoracique-Groupe Francais de Pneumo- Cancerologie NPC 95-0 Study. J Clin Oncol 005; 3(5): Curran WJ, Jr., Scott C, Langer C, et al. Phase III Comparison of Sequential vs Concurrent Chemoradiation for Patients (Pts) with Unresected Stage III Non-Small Cell Lung Cancer (NSCLC): Initial Report of Radiation Therapy Oncology Group (RTOG) 940. Proc Am Soc Clin Oncol 000; 9:(abstr 89). 39. Rolland E, Le Pechoux C, Curran WJ, et al. Concomitant Radio-chemotherapy (CT-RT) versus Sequential CT-RT In Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): A Meta-Analysis Using Individual Patient Data (IPD) From Randomised Clinical Trials (RCTs). Int J Rad Oncol Biol Phys 007; 69(suppl 3):S5. 4 Randomized, multicenter trial to determine the ability of cytoprotectant, amifostine (AM), to reduce chemo-rt-induced esophagitis and evaluate its influence on quality of life and swallowing symptoms. 05 Randomized Phase III trial to compare the survival impact of concurrent vs sequential treatment with RT and chemotherapy in locally advanced 597 Phase III trial comparing two concurrent chemotherapy and thoracic RT regimens to a standard sequential chemotherapy and thoracic RT approach in patients with unresected stage III 7,307 patients from 7 trials Meta-analysis of randomized controlled trials that compared concomitant chemotherapy-rt with sequential chemotherapy-rt. AM resulted in increased acute nausea, vomiting, cardiovascular toxicity, and infection or febrile neutropenia. The rate of grade 3 esophagitis was 30% with AM vs 34% without AM. 6 toxic deaths in sequential arm and 0 in concurrent arm. Median survival was 4.5 months in the sequential arm and 6.3 months in the concurrent arm. Two-, 3-, and 4-year survival rates were better in the concurrent arm (39%, 5%, and %, respectively) than in the sequential arm (6%, 9%, and 4%, respectively). Esophageal toxicity was significantly more frequent in the concurrent arm than in the sequential arm (3% vs 3%). Study suggests concurrent chemoradiation is the optimal strategy in locally advanced Rates of grade 3-4 non-hematologic toxicity rates were higher with concurrent than sequential therapy, but late toxicity rates were similar. No difference in grade 5 toxicity rates is noted. Preliminary survival results for the concurrent platinum-based chemotherapy and daily thoracic RT arm are quite promising. Concomitant chemotherapy-rt, as compared to sequential chemotherapy-rt, improved survival of patients with locally advanced NSCLC, mainly due to the decrease of locoregional progression, at the cost of increased acute esophageal toxicity. * See Last Page for Key 00 Review Gewanter Page 3
14 40. Vokes EE, Herndon JE, II, Kelley MJ, et al. Induction Chemotherapy Followed by Chemoradiotherapy Compared With Chemoradiotherapy Alone for Regionally Advanced Unresectable Stage III Non- Small-Cell Lung Cancer: Cancer and Leukemia Group B. J Clin Oncol 007; 5(3): Gandara DR, Chansky K, Albain KS, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol 003; (0): Mina LA, Neubauer MA, Ansari RH, et al. Phase III trial of cisplatin (P) plus etoposide (E) plus concurrent chest radiation (XRT) with or without consolidation docetaxel (D) in patients (pts) with inoperable stage III non-small cell lung cancer (NSCLC): HOG LUN 0-4/USO-03--Updated results. J Clin Oncol 008; 6(0 suppl):(abstr 759). 366 Randomized study to determine whether induction chemotherapy before concurrent chemoradiotherapy would result in improved survival. Patients were randomly assigned to arm A (immediate concurrent chemoradiotherapy with carboplatin area under the concentration-time curve (AUC) of and paclitaxel 50 mg/m given weekly during 66 Gy of chest RT ) or arm B ( cycles of carboplatin AUC 6 and paclitaxel 00 mg/m administered every days followed by identical chemoradiotherapy) To test the concept of taxane sequencing in combined-modality therapy, this phase II trial (S9504) evaluated consolidation docetaxel after concurrent chemoradiotherapy in patients with pathologically documented stage IIIB 43 To present updated results from a previously reported randomized phase III trial comparing cisplatin (P) plus etoposide (E) plus concurrent chest radiation (XRT) with or without consolidation docetaxel in patients with inoperable stage III Grade 3 or 4 toxicities during induction chemotherapy on arm B consisted of neutropenia (8% and 0%, respectively). During concurrent chemoradiotherapy, there was no difference in severity of in-field toxicities of esophagitis (grade 3 and 4 were, respectively, 30% and % for arm A v 8% and 8% for arm B) and dyspnea (grade 3 and 4 were, respectively, % and 3% for arm A v 5% and 4% for arm B). Survival differences were not statistically significant (P=.3), with a median survival on arm A of months vs. 4 months on arm B and a -year survival of 9% and 3%. Addition of induction chemotherapy to concurrent chemoradiotherapy added toxicity and provided no survival benefit over concurrent chemoradiotherapy alone. Neutropenia during consolidation docetaxel was common (57% with grade 4) and most frequent during escalation to 00 mg/m. Median progression-free survival was 6 months, median survival was 6 months, and -, -, and 3-year survival rates were 76%, 54%, and 37%, respectively. Brain metastasis was the most common site of failure. In S909, median survival was 5 months and -, -, and 3-year survival rates were 58%, 34%, and 7%, respectively. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB NSCLC is feasible and generally tolerable, and results compare favorably with the predecessor trial S909. Updated results confirm prior conclusion that consolidation docetaxel does not improve survival following EP/XRT, is associated with significant toxicities and can no longer be considered as standard treatment for patients with inoperable stage III * See Last Page for Key 00 Review Gewanter Page 4
15 43. Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 009; 374(9687): Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 005; 353(): Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for Elderly, Advanced- Stage Non Small-Cell Lung Cancer Patients Treated With Bevacizumab in Combination With Carboplatin and Paclitaxel: Analysis of Eastern Cooperative Oncology Group Trial J Clin Oncol 008; 6(): Kelly K, Chansky K, Gaspar LE, et al. Phase III Trial of Maintenance Gefitinib or Placebo After Concurrent Chemoradiotherapy and Docetaxel Consolidation in Inoperable Stage III Non-Small-Cell Lung Cancer: SWOG S003. J Clin Oncol 008; 6(5): Blumenschein G, Moughan J, Curran WJ, et al. A phase II study of cetuximab (C5) in combination with chemoradiation (CRT) in patients (pts) with stage III A/B non-small cell lung cancer (NSCLC): An interim report of the RTOG 034 trial. J Clin Oncol 007; 5(8S): Phase III trial to compare concurrent chemotherapy and RT followed by resection with standard concurrent chemotherapy and definitive RT without resection. 73 To determine whether erlotinib prolongs survival in lung cancer after the failure of firstline or second-line chemotherapy. 3b 4 elderly patients To retrospectively analyze Eastern Cooperative Oncology Group (ECOG) 4599 to determine the outcome for elderly patients. According to ECOG 4599, bevacizumab (B) in combination with carboplatin (C) and paclitaxel (P) improves survival for advanced, nonsquamous 43 Randomized phase III trial to evaluate gefitinib (G) in a maintenance setting in stage III disease following definitive treatment Phase II trial testing the combination of C5 with CRT in unresectable stage III NSCLC with planned sample size of 84 patients. In group, 6 (8%) deaths were treatment related vs four (%) in group. In an exploratory analysis, OS was improved for patients who underwent lobectomy, but not pneumonectomy, vs chemotherapy plus RT. Chemotherapy plus RT with or without resection (preferably lobectomy) are options for patients with stage IIIA(N) Erlotinib can prolong survival. For elderly patients, there was a trend towards higher response rate (9% vs 7%; P=.067) and progression-free survival (5.9 vs 4.9 months; P=.063) with PCB compared with PC, although overall survival (PCB =.3 months; PC =. months; P=.4) was similar. Grade 3 to 5 toxicities occurred in 87% of elderly patients with PCB vs 6% with PC (P<.00), with seven treatment-related deaths in the PCB arm compared with two with PC. PCB was associated with a higher degree of toxicity, but no obvious improvement in survival compared with PC. For median follow-up time of 7 months, median survival time was 3 months for gefitinib (n=8) and 35 months for placebo (n=5; two-sided P=.03). The toxic death rate was % with gefitinib compared with 0% for placebo. Gefitinib did not improve survival. Decreased survival was due to cancer not gefitinib toxicity. Response rate is 6% (n=54) and month overall survival is 68%. Adverse events related to treatment include 0% of patients with grade 4 hematologic toxicities and 7 patients who had grade 3 esophagitis. Combination of C5 with CRT is feasible. * See Last Page for Key 00 Review Gewanter Page 5
16 48. Govindan R, Bogart J, Wang X, Hodgson L, Kratzke R, Vokes EE. Phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small cell lung cancer: CALGB J Clin Oncol 009; 7(5s):(suppl; abstr 7505). 49. Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 45 subjects in a Japanese multiinstitutional study. Cancer 004; 0(7): Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 006; 4(30): Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 53 patients. Radiology 007; 43(): Phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable 3a 45 Retrospective evaluation of stereotactic hypofractionated high-dose irradiation for stage I non-small-cell lung carcinoma Prospective phase trial using stereotactic body radiation therapy (SBRT) in medically inoperable early-stage lung cancer To retrospectively evaluate long-term safety and efficacy for all percutaneous CT-guided lung tumor radiofrequency ablations. The most common histological type was adenocarcinoma (46% in Arm A and 4% in Arm B). Updated toxicity data (grade 3 or greater, %) by arms (arm A/arm B) for 06 patients: neutropenia 40/47; febrile neutropenia 8/6, thrombocytopenia 36/34, nausea/vomiting 8/0, esophagitis 3/4, skin rash / and fatigue /7. The median follow up time is 7 months. Preliminary efficacy data by arms (arm A/arm B) for 99 patients: complete or partial response 73% (95% CI 59-83)/7% (95% CI 57-8%), median failure free survival (months).9 (95% CI )/0.3 (95% CI ); 8 month survival 57% (95% CI 4-79)/47% (95% CI 33-67) and median survival (months).3/8.7. Combination of pemetrexed, carboplatin and thoracic radiation meet the protocol-specified criteria for further study. Hypofractionated high-dose STI with BED <50 Gy was good for curative treatment of patients with stage I Local control and survival rates were better with BED 00 Gy compared with <00 Gy. Survival rates in medically operable, BED 00 Gy were excellent. Kaplan-Meier local control at years was 95%. Median survival was 3.6 months and -year overall survival was 54.7%. Grade 3 to 5 toxicity occurred in 4 patients. Among patients experiencing toxicity, the median time to observation was 0.5 months. Patients treated for tumors in the peripheral lung had - year freedom from severe toxicity of 83% compared with only 54% for patients with central tumors. Achieved high rates of local control. Radiofrequency ablation appears to be safe and linked with promising long-term survival and local tumor progression outcomes. * See Last Page for Key 00 Review Gewanter Page 6
17 5. Steinke K, Sewell PE, Dupuy D, et al. Pulmonary radiofrequency ablation--an international study survey. Anticancer Res 004; 4(): Yoshimatsu R, Yamagami T, Terayama K, Matsumoto T, Miura H, Nishimura T. Delayed and recurrent pneumothorax after radiofrequency ablation of lung tumors. Chest 009; 35(4): centers reported 493 percutaneous procedures Retrospectively survey experiences of 4 centers on use of percutaneous pulmonary radiofrequency ablation. 3c 68 Retrospective study to examine the rate of delayed or recurrent pneumothorax after radiofrequency ablation for lung tumors and the risk factors associated with its occurrence. Study was based on 94 consecutive sessions of percutaneous radiofrequency ablation of 0 lung tumors. Percutaneous pulmonary radiofrequency ablation is a safe, minimally invasive tool for local pulmonary tumor control with little mortality and morbidity, short hospital stay and gain in quality of life. Pneumothorax after radiofrequency ablation occurred in 8/94 ablation sessions (4.3%). 33/8 sessions had either delayed pneumothorax (n=0) or recurrent pneumothorax (n=3). Results showed that delayed or recurrent pneumothorax is relatively frequently encountered after radiofrequency ablation of lung tumors. * See Last Page for Key 00 Review Gewanter Page 7
18 ACR Appropriateness Criteria Table Key Key Numbers -7 are for studies of therapies while numbers 8-5 are used to describe studies of diagnostics.. Randomized Controlled Trial Treatment. Controlled Trial 3. Observation Study a. Cohort b. Cross-sectional c. Case-control 4. Clinical Series 5. Case reviews 6. Anecdotes 7. Reviews 8. Randomized Controlled Trial Diagnostic 9. Comparative Assessment 0. Clinical Assessment. Quantitative Review. Qualitative Review 3. Descriptive Study 4. Case Report 5. Other (Described in text) Key Category - The conclusions of the study are valid and strongly supported by study design, analysis and results. Category - The conclusions of the study are likely valid, but study design does not permit certainty. Category 3 - The conclusions of the study may be valid but the evidence supporting the conclusions is inconclusive or equivocal. Category 4 - The conclusions of the study may not be valid because the evidence may not be reliable given the study design or analysis. ACR Appropriateness Criteria Table Key
Stage IIIB disease includes patients with T4 tumors,
 Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
Management of stage III A-B of NSCLC. Hamed ALHusaini Medical Oncologist
 Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
NCCN Non-Small Cell Lung Cancer V.1.2011 Update Meeting 07/09/10
 Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
ACR Appropriateness Criteria Nonsurgical Treatment for Non-Small-Cell Lung Cancer: Good Performance Status/Definitive Intent
 ACR Appropriateness Criteria Nonsurgical Treatment for Non-Small-Cell Lung Cancer: Good Performance Status/Definitive Intent Richard M. Gewanter, MD, Kenneth E. Rosenzweig, MD, Joe Yujiao Chang, MD, PhD,
ACR Appropriateness Criteria Nonsurgical Treatment for Non-Small-Cell Lung Cancer: Good Performance Status/Definitive Intent Richard M. Gewanter, MD, Kenneth E. Rosenzweig, MD, Joe Yujiao Chang, MD, PhD,
Management of Stage III, N2 NSCLC: A Virtual Thoracic Oncology Tumor Board
 Management of Stage III, N2 NSCLC: A Virtual Thoracic Oncology Tumor Board Abstract Introduction Management of stage III non small-cell lung cancer (NSCLC) is complex and requires careful work-up, staging,
Management of Stage III, N2 NSCLC: A Virtual Thoracic Oncology Tumor Board Abstract Introduction Management of stage III non small-cell lung cancer (NSCLC) is complex and requires careful work-up, staging,
Table of Contents. Data Supplement 1: Summary of ASTRO Guideline Statements. Data Supplement 2: Definition of Terms
 Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Radiation Therapy in the Treatment of
 Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
4.8 Lung cancer. 4.8.5 In the UK, three fractionation regimens are most commonly used:
 4.8 Lung cancer 4.8.1 In 2005, both NICE (National Institute for Health and Clinical Excellence) and SIGN (Scottish Intercollegiate Guidelines Network) published guidelines on the management of lung cancer.
4.8 Lung cancer 4.8.1 In 2005, both NICE (National Institute for Health and Clinical Excellence) and SIGN (Scottish Intercollegiate Guidelines Network) published guidelines on the management of lung cancer.
Adjuvant Therapy Non Small Cell Lung Cancer. Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015
 Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
Outcomes of Patients With Stage III Nonsmall Cell Lung Cancer Treated With Chemotherapy and Radiation With and Without Surgery
 Outcomes of Patients With Stage III Nonsmall Cell Lung Cancer Treated With Chemotherapy and Radiation With and Without Surgery Hale B. Caglar, MD 1 ; Elizabeth H. Baldini, MD, MPH 1 ; Megan Othus, MS 2
Outcomes of Patients With Stage III Nonsmall Cell Lung Cancer Treated With Chemotherapy and Radiation With and Without Surgery Hale B. Caglar, MD 1 ; Elizabeth H. Baldini, MD, MPH 1 ; Megan Othus, MS 2
American College of Radiology ACR Appropriateness Criteria
 American College of Radiology ACR Appropriateness Criteria Date of origin: 1996 Last review date: 2014 NONSURGICAL TREATMENT FOR LOCALLY ADVANCED NON SMALL-CELL LUNG CANCER: GOOD PERFORMANCE STATUS/DEFINITIVE
American College of Radiology ACR Appropriateness Criteria Date of origin: 1996 Last review date: 2014 NONSURGICAL TREATMENT FOR LOCALLY ADVANCED NON SMALL-CELL LUNG CANCER: GOOD PERFORMANCE STATUS/DEFINITIVE
Special Article. Received 5 December 2014; revised 18 February 2015; accepted 25 February 2015. Practical Radiation Oncology (2015) 5, 141-148
 Practical Radiation Oncology (2015) 5, 141-148 www.practicalradonc.org Special Article Definitive radiation therapy in locally advanced non-small cell lung cancer: Executive summary of an American Society
Practical Radiation Oncology (2015) 5, 141-148 www.practicalradonc.org Special Article Definitive radiation therapy in locally advanced non-small cell lung cancer: Executive summary of an American Society
Activity of pemetrexed in thoracic malignancies
 Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Is an evidence-based approach realistic in non-small cell lung cancer (NSCLC)?
 Is an evidence-based approach realistic in non-small cell lung cancer (NSCLC)? Authors Key words P.A. Coucke, N. Barthelemy, L. Bosquee, J.P. van Meerbeeck NSCLC, sequential and concomitant chemo-radiotherapy,
Is an evidence-based approach realistic in non-small cell lung cancer (NSCLC)? Authors Key words P.A. Coucke, N. Barthelemy, L. Bosquee, J.P. van Meerbeeck NSCLC, sequential and concomitant chemo-radiotherapy,
PII S0360-3016(99)00307-7 CLINICAL INVESTIGATION
 PII S0360-3016(99)00307-7 Int. J. Radiation Oncology Biol. Phys., Vol. 45, No. 5, pp. 1151 1156, 1999 Copyright 1999 Elsevier Science Inc. Printed in the USA. All rights reserved 0360-3016/99/$ see front
PII S0360-3016(99)00307-7 Int. J. Radiation Oncology Biol. Phys., Vol. 45, No. 5, pp. 1151 1156, 1999 Copyright 1999 Elsevier Science Inc. Printed in the USA. All rights reserved 0360-3016/99/$ see front
T reatment O utcomes of T hree-dimensional Conformal Radiotherapy for Stage III Non-Small Cell Lung Cancer
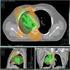 Cancer Res Treat. 2005;37(5):273-278 T reatment O utcomes of T hree-dimensional Conformal Radiotherapy for Stage III Non-Small Cell Lung Cancer Seung-Gu Yeo, M.D. 1, Moon-June Cho, M.D. 1,4, Sun-Young
Cancer Res Treat. 2005;37(5):273-278 T reatment O utcomes of T hree-dimensional Conformal Radiotherapy for Stage III Non-Small Cell Lung Cancer Seung-Gu Yeo, M.D. 1, Moon-June Cho, M.D. 1,4, Sun-Young
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
How To Improve Lung Cancer Survival With Radiation Therapy
 Clinical Controversies: Proton Therapy for Thoracic Tumors Dirk De Ruysscher, MD, PhD,* and Joe Y. Chang, MD, PhD Photon and proton therapy techniques have both improved dramatically over the past decade.
Clinical Controversies: Proton Therapy for Thoracic Tumors Dirk De Ruysscher, MD, PhD,* and Joe Y. Chang, MD, PhD Photon and proton therapy techniques have both improved dramatically over the past decade.
Radiotherapy in locally advanced & metastatic NSC lung cancer
 Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
Controversies in Management of. Inoperable NSCLC. Inoperable NSCLC. Introduction:
 Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Altered Fractionation of Radical Radiation Therapy in the Management of Unresectable Non-Small Cell Lung Cancer
 Evidence-based Series #7-12 Version 2 A Quality Initiative of the Program in Evidence-based Care (PEBC), Cancer Care Ontario (CCO) Altered Fractionation of Radical Radiation Therapy in the Management of
Evidence-based Series #7-12 Version 2 A Quality Initiative of the Program in Evidence-based Care (PEBC), Cancer Care Ontario (CCO) Altered Fractionation of Radical Radiation Therapy in the Management of
Multidisciplinary Therapy of Stage IIIA Non Small-Cell Lung Cancer: Long-term Outcome of Chemoradiation With or Without Surgery
 Special Report Multidisciplinary Therapy of Stage IIIA Non Small-Cell Lung Cancer: Long-term Outcome of Chemoradiation With or Without Surgery Charu Aggarwal, MD, Linna Li, MD, Hossein Borghaei, DO, Ranee
Special Report Multidisciplinary Therapy of Stage IIIA Non Small-Cell Lung Cancer: Long-term Outcome of Chemoradiation With or Without Surgery Charu Aggarwal, MD, Linna Li, MD, Hossein Borghaei, DO, Ranee
Helical TomoTherapy for Lung Cancer Radiotherapy: Good Science Pays Clinical Dividends Peter Hoban, Ph.D., TomoTherapy Inc.
 CLINICAL FEATURE Helical TomoTherapy for Lung Cancer Radiotherapy: Good Science Pays Clinical Dividends Peter Hoban, Ph.D., TomoTherapy Inc. The Challenge Lung cancer kills more people each year in the
CLINICAL FEATURE Helical TomoTherapy for Lung Cancer Radiotherapy: Good Science Pays Clinical Dividends Peter Hoban, Ph.D., TomoTherapy Inc. The Challenge Lung cancer kills more people each year in the
Concurrent Chemotherapy and Radiotherapy for Head and Neck Cancer
 Concurrent Chemotherapy and Radiotherapy for Head and Neck Cancer Ryan J. Burri; Nancy Y. Lee Published: 03/23/2009 Abstract and Introduction Abstract Head and neck cancer is best managed in a multidisciplinary
Concurrent Chemotherapy and Radiotherapy for Head and Neck Cancer Ryan J. Burri; Nancy Y. Lee Published: 03/23/2009 Abstract and Introduction Abstract Head and neck cancer is best managed in a multidisciplinary
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Pulmonary function. Is patient potentially operable? Yes. tests 3. Yes. Pulmonary function. tests 3, if clinically indicated. Yes
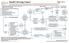 INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
Non-small Cell Lung Cancer: Locally Advanced
 Non-small Cell Lung Cancer: Locally Advanced Daniel W. Golden MD, PGY-5, Ryan Bair MD, PGY-3, and Matthew Koshy MD, Assistant Professor Pritzker School of Medicine, University of Chicago Chicago Clinical
Non-small Cell Lung Cancer: Locally Advanced Daniel W. Golden MD, PGY-5, Ryan Bair MD, PGY-3, and Matthew Koshy MD, Assistant Professor Pritzker School of Medicine, University of Chicago Chicago Clinical
Small Cell Lung Cancer
 Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study
 Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Lung Cancer and Mesothelioma
 Lung Cancer and Mesothelioma Robert Kratzke, M.D. John C. Skoglund Professor of Lung Cancer Research Section of Heme/Onc/Transplant Department of Medicine University of Minnesota Medical School Malignant
Lung Cancer and Mesothelioma Robert Kratzke, M.D. John C. Skoglund Professor of Lung Cancer Research Section of Heme/Onc/Transplant Department of Medicine University of Minnesota Medical School Malignant
Definitive Treatment of Poor-Risk Patients with Stage I Lung Cancer. A Single Institution Experience
 ORIGINAL ARTICLE Definitive Treatment of Poor-Risk Patients with Stage I Lung Cancer A Single Institution Experience Michael Hsie, MD,* Stefania Morbidini-Gaffney, MD,* Leslie J. Kohman, MD, Elisabeth
ORIGINAL ARTICLE Definitive Treatment of Poor-Risk Patients with Stage I Lung Cancer A Single Institution Experience Michael Hsie, MD,* Stefania Morbidini-Gaffney, MD,* Leslie J. Kohman, MD, Elisabeth
Who may benefit from prophylactic cranial irradiation amongst stage III non-small cell lung cancer patients?
 JBUON 2013; 18(2): 453-458 ISSN: 1107-0625 www.jbuon.com E-mail: info@jbuon.com ORIGINAL ARTICLE Who may benefit from prophylactic cranial irradiation amongst stage III non-small cell lung cancer patients?
JBUON 2013; 18(2): 453-458 ISSN: 1107-0625 www.jbuon.com E-mail: info@jbuon.com ORIGINAL ARTICLE Who may benefit from prophylactic cranial irradiation amongst stage III non-small cell lung cancer patients?
The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy
 17 (Supplement 10): x108 x112, 2006 doi:10.1093/annonc/mdl247 The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy E. Felip & E. Vilar Oncology Department, Vall d
17 (Supplement 10): x108 x112, 2006 doi:10.1093/annonc/mdl247 The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy E. Felip & E. Vilar Oncology Department, Vall d
National Clinical Trials Network Groups Update Fall 2014
 National Clinical Trials Network Groups Update Fall 2014 Walter J Curran, Jr, MD An NRG Oncology Group Chair Executive Director Winship Cancer Institute of Emory University Atlanta, GA NCTN Groups Update
National Clinical Trials Network Groups Update Fall 2014 Walter J Curran, Jr, MD An NRG Oncology Group Chair Executive Director Winship Cancer Institute of Emory University Atlanta, GA NCTN Groups Update
Emerging Drug List GEFITINIB
 Generic (Trade Name): Manufacturer: Gefitinib (Iressa ) formerly referred to as ZD1839 AstraZeneca NO. 52 JANUARY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence:
Generic (Trade Name): Manufacturer: Gefitinib (Iressa ) formerly referred to as ZD1839 AstraZeneca NO. 52 JANUARY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence:
Efficacy of chemotherapy combined hyperfractionated accelerated radiotherapy on limited small cell lung cancer
 [Chinese Journal of Cancer 27:10, 353-357; October 2008]; 2008 Sun Yat-Sen University Cancer Center Clinical Research Paper Efficacy of chemotherapy combined hyperfractionated accelerated radiotherapy
[Chinese Journal of Cancer 27:10, 353-357; October 2008]; 2008 Sun Yat-Sen University Cancer Center Clinical Research Paper Efficacy of chemotherapy combined hyperfractionated accelerated radiotherapy
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof.
 Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
5. Non small Cell Lung Cancer
 5. Non small Cell Lung Cancer Introduction The cancer registry in Sweden shows a continuous increase of lung cancer cases from 867 in 1958 when the registry started to 2 846 in 2000. The Swedish cancer
5. Non small Cell Lung Cancer Introduction The cancer registry in Sweden shows a continuous increase of lung cancer cases from 867 in 1958 when the registry started to 2 846 in 2000. The Swedish cancer
SAKK Lung Cancer Group. Current activities and future projects
 SAKK Lung Cancer Group Current activities and future projects SAKK Lung Cancer Group Open group of physicians interested in lung cancer Mostly Medical Oncologists, but also Thoracic Surgeons Radiation
SAKK Lung Cancer Group Current activities and future projects SAKK Lung Cancer Group Open group of physicians interested in lung cancer Mostly Medical Oncologists, but also Thoracic Surgeons Radiation
Lung Cancer Treatment Guidelines
 Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
LUNG CANCER INTRODUCTION
 LUNG CANCER INTRODUCTION Lung cancer remains the leading cause of cancer death in the UK. Approximately 50% of those diagnosed present with stage IV disease. Significant advances in the management of lung
LUNG CANCER INTRODUCTION Lung cancer remains the leading cause of cancer death in the UK. Approximately 50% of those diagnosed present with stage IV disease. Significant advances in the management of lung
Cesare Gridelli 1, Francesca Casaluce 2, Assunta Sgambato 2, Fabio Monaco 3, Cesare Guida 4
 Editorial Treatment of limited-stage small cell lung cancer in the elderly, chemotherapy vs. sequential chemoradiotherapy vs. concurrent chemoradiotherapy: that s the question Cesare Gridelli 1, Francesca
Editorial Treatment of limited-stage small cell lung cancer in the elderly, chemotherapy vs. sequential chemoradiotherapy vs. concurrent chemoradiotherapy: that s the question Cesare Gridelli 1, Francesca
Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with relatively good performance status
 Available online at www.sciencedirect.com Journal of the Chinese Medical Association 74 (2011) 209e214 Original Article Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with
Available online at www.sciencedirect.com Journal of the Chinese Medical Association 74 (2011) 209e214 Original Article Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with
National Horizon Scanning Centre. Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer. December 2007
 Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Stomach (Gastric) Cancer. Prof. M K Mahajan ACDT & RC Bathinda
 Stomach (Gastric) Cancer Prof. M K Mahajan ACDT & RC Bathinda Gastric Cancer Role of Radiation Layers of the Stomach Mucosa Submucosa Muscularis Serosa Stomach and Regional Lymph Nodes Stomach and Regional
Stomach (Gastric) Cancer Prof. M K Mahajan ACDT & RC Bathinda Gastric Cancer Role of Radiation Layers of the Stomach Mucosa Submucosa Muscularis Serosa Stomach and Regional Lymph Nodes Stomach and Regional
Introduction. Acta Medica Mediterranea, 2015, 31: 883 BOZKURT DUMAN 5, SEMRA PAYDAŞ 4, VEHBI ERCOLAK 6 1
 Acta Medica Mediterranea, 2015, 31: 883 THE EFFECT OF CHEMORADIOTHERAPY ON SURVIVAL IN LOCALLY ADVANCED UNRESECTABLE NON-SMALL CELL LUNG CANCER PATIENTS: EXPERIENCE FROM THE SOUTHEAST REGION OF TURKEY
Acta Medica Mediterranea, 2015, 31: 883 THE EFFECT OF CHEMORADIOTHERAPY ON SURVIVAL IN LOCALLY ADVANCED UNRESECTABLE NON-SMALL CELL LUNG CANCER PATIENTS: EXPERIENCE FROM THE SOUTHEAST REGION OF TURKEY
Adiuwantowe i neoadiuwantowe leczenie chorych na zaawansowanego raka żołądka
 Adiuwantowe i neoadiuwantowe leczenie chorych na zaawansowanego raka żołądka Neoadiuvant and adiuvant therapy for advanced gastric cancer Franco Roviello, IT Neoadjuvant and adjuvant therapy for advanced
Adiuwantowe i neoadiuwantowe leczenie chorych na zaawansowanego raka żołądka Neoadiuvant and adiuvant therapy for advanced gastric cancer Franco Roviello, IT Neoadjuvant and adjuvant therapy for advanced
NON-SMALL CELL LUNG CANCER STAGE III
 NON-SMALL CELL LUNG CANCER STAGE III Effective Date: April, 2012 The recommendations contained in this guideline are a consensus of the Alberta Provincial Thoracic Tumour Team synthesis of currently accepted
NON-SMALL CELL LUNG CANCER STAGE III Effective Date: April, 2012 The recommendations contained in this guideline are a consensus of the Alberta Provincial Thoracic Tumour Team synthesis of currently accepted
Sur les nouveaux médicaments et les perspectives qu ils offrent (traitement à la carte et survie longue)
 Sur les nouveaux médicaments et les perspectives qu ils offrent (traitement à la carte et survie longue) Professeur Jean Trédaniel Unité de cancérologie thoracique Hôpital Saint-Louis Comparison of Four
Sur les nouveaux médicaments et les perspectives qu ils offrent (traitement à la carte et survie longue) Professeur Jean Trédaniel Unité de cancérologie thoracique Hôpital Saint-Louis Comparison of Four
Medication Policy Manual. Topic: Alimta, pemetrexed Date of Origin: May 12, 2010
 Medication Policy Manual Policy No: dru213 Topic: Alimta, pemetrexed Date of Origin: May 12, 2010 Committee Approval Date: February 17, 2015 Next Review Date: February 2016 Effective Date: March 1, 2015
Medication Policy Manual Policy No: dru213 Topic: Alimta, pemetrexed Date of Origin: May 12, 2010 Committee Approval Date: February 17, 2015 Next Review Date: February 2016 Effective Date: March 1, 2015
Particle Therapy for Lung Cancer. Bradford Hoppe MD, MPH Assistant Professor University of Florida bhoppe@floridaproton.org
 Particle Therapy for Lung Cancer Bradford Hoppe MD, MPH Assistant Professor University of Florida bhoppe@floridaproton.org Content Rationale for Particle Therapy in Lung Cancer Proof of Principle Treatment
Particle Therapy for Lung Cancer Bradford Hoppe MD, MPH Assistant Professor University of Florida bhoppe@floridaproton.org Content Rationale for Particle Therapy in Lung Cancer Proof of Principle Treatment
Lung Cancer and Postoperative Radiotherapy
 . Fernando HC, Goldstraw P. The accuracy of clinical evaluative intrathoracic staging in lung cancer as assessed by postsurgical pathologic staging. Cancer 990; 65():50-506.. Keller SM, Adak S, Wagner
. Fernando HC, Goldstraw P. The accuracy of clinical evaluative intrathoracic staging in lung cancer as assessed by postsurgical pathologic staging. Cancer 990; 65():50-506.. Keller SM, Adak S, Wagner
National Imaging Associates, Inc. Clinical guideline: NON SMALL CELL LUNG CANCER
 National Imaging Associates, Inc. Clinical guideline: NON SMALL CELL LUNG CANCER Original Date: March 2011 Page 1 of 10 Radiation Oncology Last Review Date: June 2012 Guideline Number: NIA_CG_122 Last
National Imaging Associates, Inc. Clinical guideline: NON SMALL CELL LUNG CANCER Original Date: March 2011 Page 1 of 10 Radiation Oncology Last Review Date: June 2012 Guideline Number: NIA_CG_122 Last
Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin
 COMPENDIA TRANSPARENCY TRACKING FORM DRUG: Docetaxel INDICATION: Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin COMPENDIA TRANSPARENCY REQUIREMENTS 1 Provide
COMPENDIA TRANSPARENCY TRACKING FORM DRUG: Docetaxel INDICATION: Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin COMPENDIA TRANSPARENCY REQUIREMENTS 1 Provide
Radiotherapy for Non-Small Cell Lung Cancer. Standard Treatment Options Radiotherapy Planning
 Radiotherapy for Non-Small Cell Lung Cancer I II Standard Treatment Options Radiotherapy Planning TNM Staging System Disease Staging - Management is based on disease stage - Stage I-II: early stage - Stage
Radiotherapy for Non-Small Cell Lung Cancer I II Standard Treatment Options Radiotherapy Planning TNM Staging System Disease Staging - Management is based on disease stage - Stage I-II: early stage - Stage
POLICY A. INDICATIONS
 Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
American College of Radiology ACR Appropriateness Criteria
 American College of Radiology ACR Appropriateness Criteria Date of origin: 1996 Last review date: 2012 NONSURGICAL TREATMENT FOR NON-SMALL-CELL LUNG CANCER: POOR PERFORMANCE STATUS OR PALLIATIVE INTENT
American College of Radiology ACR Appropriateness Criteria Date of origin: 1996 Last review date: 2012 NONSURGICAL TREATMENT FOR NON-SMALL-CELL LUNG CANCER: POOR PERFORMANCE STATUS OR PALLIATIVE INTENT
Cetuximab (Erbitux) MM.04.005 05/10/2005. HMO; PPO; QUEST Integration 01/01/2015 Section: Prescription Drugs Place(s) of Service: Office: Outpatient
 Cetuximab (Erbitux) Policy Number: Original Effective Date: MM.04.005 05/10/2005 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 01/01/2015 Section: Prescription Drugs Place(s)
Cetuximab (Erbitux) Policy Number: Original Effective Date: MM.04.005 05/10/2005 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 01/01/2015 Section: Prescription Drugs Place(s)
Mesothelioma. Malignant Pleural Mesothelioma
 Mesothelioma William G. Richards, PhD Brigham and Women s Hospital Malignant Pleural Mesothelioma 2,000-3,000 cases per year (USA) Increasing incidence Asbestos (50-80%, decreasing) 30-40 year latency
Mesothelioma William G. Richards, PhD Brigham and Women s Hospital Malignant Pleural Mesothelioma 2,000-3,000 cases per year (USA) Increasing incidence Asbestos (50-80%, decreasing) 30-40 year latency
Shifting the Paradigm for Maintenance Therapy for Non small-cell Lung Cancer
 J Hong Kong Col Radiol. 2010;13(Suppl):S16-21 ORIGINAL ARTICLE Shifting the Paradigm for Maintenance Therapy for Non small-cell Lung Cancer VHF Lee Department of Clinical Oncology, Queen Mary Hospital,
J Hong Kong Col Radiol. 2010;13(Suppl):S16-21 ORIGINAL ARTICLE Shifting the Paradigm for Maintenance Therapy for Non small-cell Lung Cancer VHF Lee Department of Clinical Oncology, Queen Mary Hospital,
ACR Appropriateness Criteria on Nonsurgical Treatment for Non Small-Cell Lung Cancer: Poor Performance Status or Palliative Intent
 ACR Appropriateness Criteria on Nonsurgical Treatment for Non Small-Cell Lung Cancer: Poor Performance Status or Palliative Intent Kenneth E. Rosenzweig, MD a, Benjamin Movsas, MD b, Jeff Bradley, MD c,
ACR Appropriateness Criteria on Nonsurgical Treatment for Non Small-Cell Lung Cancer: Poor Performance Status or Palliative Intent Kenneth E. Rosenzweig, MD a, Benjamin Movsas, MD b, Jeff Bradley, MD c,
INTRODUCTION. liver neoplasms, adrenal gland neoplasms, non small-cell lung cancer, radionuclide imaging, bisphosphonates,
 VOLUME 22 NUMBER 2 JANUARY 15 2004 JOURNAL OF CLINICAL ONCOLOGY A S C O S P E C I A L A R T I C L E American Society of Clinical Oncology Treatment of Unresectable Non Small-Cell Lung Cancer Guideline:
VOLUME 22 NUMBER 2 JANUARY 15 2004 JOURNAL OF CLINICAL ONCOLOGY A S C O S P E C I A L A R T I C L E American Society of Clinical Oncology Treatment of Unresectable Non Small-Cell Lung Cancer Guideline:
Targeted Therapy What the Surgeon Needs to Know
 Targeted Therapy What the Surgeon Needs to Know AATS Focus in Thoracic Surgery 2014 David R. Jones, M.D. Professor & Chief, Thoracic Surgery Memorial Sloan Kettering Cancer Center I have no disclosures
Targeted Therapy What the Surgeon Needs to Know AATS Focus in Thoracic Surgery 2014 David R. Jones, M.D. Professor & Chief, Thoracic Surgery Memorial Sloan Kettering Cancer Center I have no disclosures
Highlights in NSCLC From the 15th World Conference on Lung Cancer
 January 214 Volume 12, Issue 1, Supplement 1 A SPECIAL MEETING REVIEW EDITION Highlights in CLC From the 15th World Conference on Lung Cancer A Review of Selected Presentations From the 15th World Conference
January 214 Volume 12, Issue 1, Supplement 1 A SPECIAL MEETING REVIEW EDITION Highlights in CLC From the 15th World Conference on Lung Cancer A Review of Selected Presentations From the 15th World Conference
More than half of all lung cancer patients present with. New Combinations in the Treatment of Lung Cancer* A Time for Optimism
 New Combinations in the Treatment of Lung Cancer* A Time for Optimism Paul A. Bunn, Jr., MD; and Karen Kelly, MD Strides have been made in the treatment of lung cancer in the last decade that warrant a
New Combinations in the Treatment of Lung Cancer* A Time for Optimism Paul A. Bunn, Jr., MD; and Karen Kelly, MD Strides have been made in the treatment of lung cancer in the last decade that warrant a
Adjuvant Chemotherapy After Complete Resection of Non-Small Cell Lung Cancer
 REVIEW ARTICLE Adjuvant Chemotherapy After Complete Resection of Non-Small Cell Lung Cancer Eckart Laack, Carsten Bokemeyer, Dieter Kurt Hossfeld SUMMARY Introduction: In non-small cell lung cancer (NSCLC)
REVIEW ARTICLE Adjuvant Chemotherapy After Complete Resection of Non-Small Cell Lung Cancer Eckart Laack, Carsten Bokemeyer, Dieter Kurt Hossfeld SUMMARY Introduction: In non-small cell lung cancer (NSCLC)
SECOND-LINE CHEMOTHERAPY in advanced non
 Gemcitabine as Second-Line Treatment for Advanced Non Small-Cell Lung Cancer: A Phase II Trial By Lucio Crinò, Anna Maria Mosconi, Giorgio Scagliotti, Giovanni Selvaggi, Silvia Novello, Massimo Rinaldi,
Gemcitabine as Second-Line Treatment for Advanced Non Small-Cell Lung Cancer: A Phase II Trial By Lucio Crinò, Anna Maria Mosconi, Giorgio Scagliotti, Giovanni Selvaggi, Silvia Novello, Massimo Rinaldi,
A new score predicting the survival of patients with spinal cord compression from myeloma
 A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer
 Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
ORIGINAL ARTICLE THORACIC ONCOLOGY
 Ann Surg Oncol (2013) 20:1934 1940 DOI 10.1245/s10434-012-2800-x ORIGINAL ARTICLE THORACIC ONCOLOGY Predictors for Locoregional Recurrence for Clinical Stage III-N2 Non-small Cell Lung Cancer with Nodal
Ann Surg Oncol (2013) 20:1934 1940 DOI 10.1245/s10434-012-2800-x ORIGINAL ARTICLE THORACIC ONCOLOGY Predictors for Locoregional Recurrence for Clinical Stage III-N2 Non-small Cell Lung Cancer with Nodal
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline
 Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Scottish Medicines Consortium
 Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
CANCER PULMON: ESTADIOS INICIALES POSTMUNDIAL PULMON DENVER 2015. 8-10-2015.Manuel Cobo Dols S. Oncología Médica HU Málaga Regional y VV
 CANCER PULMON: ESTADIOS INICIALES POSTMUNDIAL PULMON DENVER 2015 8-10-2015.Manuel Cobo Dols S. Oncología Médica HU Málaga Regional y VV Meta-analisis LACE: adyuvancia vs no adyuvancia Pignon JP, et al.
CANCER PULMON: ESTADIOS INICIALES POSTMUNDIAL PULMON DENVER 2015 8-10-2015.Manuel Cobo Dols S. Oncología Médica HU Málaga Regional y VV Meta-analisis LACE: adyuvancia vs no adyuvancia Pignon JP, et al.
Chemotherapy in Advanced Non-Small Cell Lung Cancer: Optimal Treatment Approach for Elderly and Patients With Poor Performance Status
 Chemotherapy in Advanced Non-Small Cell Lung Cancer: Optimal Treatment Approach for Elderly and Patients With Poor Performance Status Tracey L. Evans, MD Abstract In spite of advances in molecular profiling
Chemotherapy in Advanced Non-Small Cell Lung Cancer: Optimal Treatment Approach for Elderly and Patients With Poor Performance Status Tracey L. Evans, MD Abstract In spite of advances in molecular profiling
Cancer Treatments Subcommittee of PTAC Meeting held 18 September 2015. (minutes for web publishing)
 Cancer Treatments Subcommittee of PTAC Meeting held 18 September 2015 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the
Cancer Treatments Subcommittee of PTAC Meeting held 18 September 2015 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the
Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence
 Post- survival in completely resected stage I non-small cell lung cancer with local J-J Hung, 1,2,3 W-H Hsu, 3 C-C Hsieh, 3 B-S Huang, 3 M-H Huang, 3 J-S Liu, 2 Y-C Wu 3 See Editorial, p 185 c A supplementary
Post- survival in completely resected stage I non-small cell lung cancer with local J-J Hung, 1,2,3 W-H Hsu, 3 C-C Hsieh, 3 B-S Huang, 3 M-H Huang, 3 J-S Liu, 2 Y-C Wu 3 See Editorial, p 185 c A supplementary
January 2013 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW. Summary. Contents
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
Implementation Date: April 2015 Clinical Operations
 National Imaging Associates, Inc. Clinical guideline PROSTATE CANCER Original Date: March 2011 Page 1 of 5 Radiation Oncology Last Review Date: March 2015 Guideline Number: NIA_CG_124 Last Revised Date:
National Imaging Associates, Inc. Clinical guideline PROSTATE CANCER Original Date: March 2011 Page 1 of 5 Radiation Oncology Last Review Date: March 2015 Guideline Number: NIA_CG_124 Last Revised Date:
Survival analysis of 220 patients with completely resected stage II non small cell lung cancer
 窑 Original Article 窑 Chinese Journal of Cancer Survival analysis of 22 patients with completely resected stage II non small cell lung cancer Yun Dai,2,3, Xiao Dong Su,2,3, Hao Long,2,3, Peng Lin,2,3, Jian
窑 Original Article 窑 Chinese Journal of Cancer Survival analysis of 22 patients with completely resected stage II non small cell lung cancer Yun Dai,2,3, Xiao Dong Su,2,3, Hao Long,2,3, Peng Lin,2,3, Jian
Non-Small Cell Lung Cancer
 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Non-Small Cell Lung Cancer Version 1.2015 NCCN.org NCCN Guidelines for Patients available at www.nccn.org/patients Continue CLINICAL ASSESSMENT
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Non-Small Cell Lung Cancer Version 1.2015 NCCN.org NCCN Guidelines for Patients available at www.nccn.org/patients Continue CLINICAL ASSESSMENT
Recent Trends in Management of Unresectable Non-Small Cell Lung Cancer (NSCLC)
 Bahrain Medical Bulletin, Vol.23, No.4, December 2001 Recent Trends in Management of Unresectable Non-Small Cell Lung Cancer (NSCLC) Jalal Al-Maskati, MBChB, ABIM * Lung cancer is a major health problem
Bahrain Medical Bulletin, Vol.23, No.4, December 2001 Recent Trends in Management of Unresectable Non-Small Cell Lung Cancer (NSCLC) Jalal Al-Maskati, MBChB, ABIM * Lung cancer is a major health problem
Elderly Patients with Advanced Non-Small Cell Lung Cancer: What Treatment?
 4 The Open Lung Cancer Journal, 2011, 4, 4-9 Open Access Elderly Patients with Advanced Non-Small Cell Lung Cancer: What Treatment? Antonio Rossi * Division of Medical Oncology, S.G. Moscati Hospital,
4 The Open Lung Cancer Journal, 2011, 4, 4-9 Open Access Elderly Patients with Advanced Non-Small Cell Lung Cancer: What Treatment? Antonio Rossi * Division of Medical Oncology, S.G. Moscati Hospital,
Moving towards novel treatments in mesothelioma. Paul Baas, The Netherlands Cancer Institute Rolf Stahel, University Clinic Zurich
 Moving towards novel treatments in mesothelioma Paul Baas, The Netherlands Cancer Institute Rolf Stahel, University Clinic Zurich Malignant pleural mesothelioma (MPM) has proven to be quite resistant to
Moving towards novel treatments in mesothelioma Paul Baas, The Netherlands Cancer Institute Rolf Stahel, University Clinic Zurich Malignant pleural mesothelioma (MPM) has proven to be quite resistant to
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
 Department of Veterans Affairs Health Services Research & Development Service Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
Department of Veterans Affairs Health Services Research & Development Service Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
Harmesh Naik, MD. Hope Cancer Clinic HOW DO I MANAGE STAGE 4 NSCLC IN 2012: STATE OF THE ART
 Harmesh Naik, MD. Hope Cancer Clinic HOW DO I MANAGE STAGE 4 NSCLC IN 2012: STATE OF THE ART Goals Discuss treatment options for stage 4 lung cancer: New and old Discuss new developments in personalized
Harmesh Naik, MD. Hope Cancer Clinic HOW DO I MANAGE STAGE 4 NSCLC IN 2012: STATE OF THE ART Goals Discuss treatment options for stage 4 lung cancer: New and old Discuss new developments in personalized
U.S. Food and Drug Administration
 U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
Small Cell Lung Cancer* State-of-the-Art Therapy 1994
 Small Cell Lung Cancer* State-of-the-Art Therapy 1994 Daniel C. Ihde, MD In the United States, small cell lung cancer (SCLC) accounts for about 20% of all cases of lung cancer. Without treatment, tumor
Small Cell Lung Cancer* State-of-the-Art Therapy 1994 Daniel C. Ihde, MD In the United States, small cell lung cancer (SCLC) accounts for about 20% of all cases of lung cancer. Without treatment, tumor
the standard of care 2009 5/1/2009 Mesothelioma: The standard of care take home messages PILC 2006 Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009
 Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
American College of Radiology ACR Appropriateness Criteria POSTOPERATIVE ADJUVANT THERAPY IN NON-SMALL-CELL LUNG CANCER
 American College of Radiology ACR Appropriateness Criteria Date of origin: 1996 Last review date: 2010 POSTOPERATIVE ADJUVANT THERAPY IN NON-SMALL-CELL LUNG CANCER Expert Panel on Radiation Oncology Lung:
American College of Radiology ACR Appropriateness Criteria Date of origin: 1996 Last review date: 2010 POSTOPERATIVE ADJUVANT THERAPY IN NON-SMALL-CELL LUNG CANCER Expert Panel on Radiation Oncology Lung:
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness
 Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness Investigators: Paul G. Shekelle, MD, PhD, Director Alicia R. Maher, MD Clinical
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness Investigators: Paul G. Shekelle, MD, PhD, Director Alicia R. Maher, MD Clinical
Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
 Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Treatment Paradigm in NSCLC Treatment
 Treatment Paradigm in NSCLC Treatment Era of Targeted Therapy Aumkhae Sookprasert, MD Medicine Department, KKU Which factors taken to be account in NSCLC treatment? 1. Staging 2. ECOG performance status
Treatment Paradigm in NSCLC Treatment Era of Targeted Therapy Aumkhae Sookprasert, MD Medicine Department, KKU Which factors taken to be account in NSCLC treatment? 1. Staging 2. ECOG performance status
SBRT (Elekta), 45 Gy in fractions of 3 Gy 3x/week for 5 weeks (N=22) vs.
 Uitgangsvraag 6: Wat is de plaats van stereotactische radiotherapiebehandeling (SBRT) bij HCC patiënten? Primaire studies I Study ID II Method III Patient characteristics IV Intervention(s) V Results primary
Uitgangsvraag 6: Wat is de plaats van stereotactische radiotherapiebehandeling (SBRT) bij HCC patiënten? Primaire studies I Study ID II Method III Patient characteristics IV Intervention(s) V Results primary
Chapter 7: Lung Cancer
 Chapter 7: Lung Cancer Contents Chapter 7: Lung Cancer... 1 Small Cell... 2 Good PS + Limited stage... 2 Cisplatin/etoposide... 2 Concurrent chemotherapy + XRT... 2 Good / Intermediate PS... 2 Carboplatin
Chapter 7: Lung Cancer Contents Chapter 7: Lung Cancer... 1 Small Cell... 2 Good PS + Limited stage... 2 Cisplatin/etoposide... 2 Concurrent chemotherapy + XRT... 2 Good / Intermediate PS... 2 Carboplatin
New Trends & Current Research in the Treatment of Lung Cancer, Pt. II
 New Trends & Current esearch in the Treatment of Lung Cancer, Pt. II Howard (Jack) West, MD President & CEO, GACE Medical Director, Thoracic Oncology Program Swedish Cancer Institute Seattle, WA Cancer
New Trends & Current esearch in the Treatment of Lung Cancer, Pt. II Howard (Jack) West, MD President & CEO, GACE Medical Director, Thoracic Oncology Program Swedish Cancer Institute Seattle, WA Cancer
Treatment Advances in Locally Advanced and Metastatic Non-Small Cell Lung Cancer. Veerle Surmont
 Treatment Advances in Locally Advanced and Metastatic Non-Small Cell Lung Cancer Veerle Surmont Cover design: Divine De Baets; Rotterdam-Gent Impressions Lay-out: Optima Grafische Communicatie Rotterdam
Treatment Advances in Locally Advanced and Metastatic Non-Small Cell Lung Cancer Veerle Surmont Cover design: Divine De Baets; Rotterdam-Gent Impressions Lay-out: Optima Grafische Communicatie Rotterdam
Treatment of Mesothelioma with Radiotherapy
 41 Treatment of Mesothelioma with Radiotherapy Ryan P. Smith and Stephen M. Hahn General Principles of Radiation Therapy Radiation therapy is a therapeutic modality that uses ionizing radiation to treat
41 Treatment of Mesothelioma with Radiotherapy Ryan P. Smith and Stephen M. Hahn General Principles of Radiation Therapy Radiation therapy is a therapeutic modality that uses ionizing radiation to treat
