Pharmacologic Therapies for Multiple Sclerosis: From injectable to oral agents
|
|
|
- Beverly Cunningham
- 9 years ago
- Views:
Transcription
1 Pharmacologic Therapies for Multiple Sclerosis: From injectable to oral agents KIRANPAL SINGH SANGHA, PHARM.D. CLINICAL PHARMACY SPECIALIST CNS THE UNIVERSITY OF CINCINNATI MEDICAL CENTER
2 Objectives List three oral therapies for Multiple Sclerosis (MS) Describe dosing of common injectable therapies for MS List a common adverse effect for the interferon products Describe storage of common agents for MS I have no conflicts of interest related to this presentation
3 What is Multiple Sclerosis? Most common disabling neurological disease of young adults Characterized by areas of inflammation, demyelination, axonal loss & gliosis in the CNS The cause is unknown, but immune mediated physiology is widely accepted
4 Potential Triggers - Multiple Sclerosis Genetic predisposition Infectious agent Abnormal immunologic response Environmental factors MS
5 Multiple Sclerosis Age of onset: 15 to 45 years Gender: 70% women Prevalence: 350,000 in the USA, about 2.5 million people worldwide Geography: incidence increases with distance from equator Quality of life disease Immunotherapies do not treat symptoms Many MS patients feel their symptoms are neglected by their physicians
6 Common Symptoms Spasticity Bladder symp. Incontinence Fatigue Visual symp. Optic neuritis, diplopia Bowel symp. Incontinence, constipation Cognitive symp. Depression & mood symp. Sexual dysfunction Pain
7 Why do we treat MS? Disease modifying therapies (DMT) have been shown to reduce rate of relapse & there is evidence of reduction of long term disability Studies with interferon-beta 1a IM, interferon-beta 1b, interferon beta 1a SQ and glatiramer acetate have shown that treating early (CIS) has been shown to delay progression to clinically definite MS MS is the most common cause of non traumatic neurologic disability in adults
8 Time line for Multiple Sclerosis 1986 Consortium of MS Centers founded (CMSC) Injectable DMT s: 1993 IFN-1b Betaseron 1995 IFN-1a Avonex 1996 Glatiramer 2002 IFN-1a Rebif Oral DMT s: 2010 Dalfampridine 2010 Fingolimod 2012 Teriflunomide 2013 Dimethyl fumarate 1950 s Interferons 1 st identified Future? MS Named 1916 James Dawson describes pathology MS 1970 s ACTH replaced by IV steriods Other DMT s: 2014 Alemtuzumab
9 Clinically Isolated Syndrome (CIS) CIS is a first episode of neurologic symptoms that lasts at least 24 hrs & is caused by inflammation or demyelination in the CNS Drug Interferon beta-1b Interferon beta 1a IM Glatiramer acetate Reduction in conversion to clinically definite MS at 3 or 5 yr follow-up 37% 35% 45%
10 MS Treatment -Acute Relapses IV Methylprednisolone 1 gm/day x 3-5 days Recent trials show equal efficacy of IV & PO dosage forms. Some Clinicians are using PO prednisone for relapses Clinical Pearl - PO Prednisone 1000 mg/day x 3-5 days if IV therapy is not an option If steroids fail plasmapheresis is an option
11 MS -Choosing initial DMT No consensus on initial therapy CMSC 2014 consensus statement says to initiate treatment with an FDA approved DMT Availability of oral agents presents alternatives to injectable agents Some suggest efficacy of the meds decreases if adherence is < 80% Risks of certain adverse effects increase with comorbidities The only drug that should not be used 1 st line is alemtuzumab.
12 Treatment DMT s 1 st generation -injectable: Beta interferon-1a (Avonex), beta interferon-1b (Betaseron), beta interferon-1a (Rebif), Glatiramer acetate (Copaxone) 2 nd generation injectable to oral agents: Mitoxantrone (Novantrone), Natalizumab (Tysabri), Alemtuzumab (Lemtrada) Oral agents fingolimod, teriflunomide, dimethyl fumarate. Dalfampridine Brand Names have been used in presentation to distinguish products
13 Pre-drug testing All drugs except glatiramer need baseline CBC with diff. & LFTs Fingolimod requires VZV IgG titers, baseline EKG & ophthalmologic exam for macular edema Teriflunomide PPD and negative pregnancy test Natalizumab JCV Ab status Fingolimod, dimethyl fumarate JCV Ab status
14 Follow-up testing Interferons, natalizumab, dimethy fumarate - CBC with diff, LFTs and clinic visits every 3 mo s for 1 year Fingolimod CBC with diff, LFTs & clinic visit 1 mo after starting then every 3 mo s for the first yr. If JCV Ab negative, yearly testing Teriflunomide LFTs monthly for 6 mo s, CBC with diff every 6 mo s. Natalizumab If JCV Ab negative, every 6 mo s. If JCV Ab positive every 3 mo s
15
16
17 Dosing DMTs -injectables Drug Dose Route Frequency β INFN-1a (Avonex) 30 mcg IM Weekly Peg INFN-beta1a 63 mcg x1 SQ (Plegridy) 94 mcg in 14 days then 125 mcg q 14 days β INFN-1b (Betaseron) 0.25 mg SQ Every other day Extavia β INFN-1a (Rebif) 44 mcg SQ Three times per week Glatiramer acetate 20 mg SQ Daily (Copaxone) or 40 mg SQ 3 x week
18 Dosing DMTs -Injectables Drug Dose Route Frequency Mitoxantrone 12 mg/m 2 IV Every 3 months Natalizumab (Tysabri) 300 mg IV Every 4 weeks Alemtuzumab 12 mg/day over 5 days IV Repeat in 1 year Dosing -Oral Agents Drug Dose Route Frequency Fingolimod 0.5 mg PO daily Teriflunomide 7 or 14 mg PO daily Dimethyl fumarate 120 x 7 days then 240 mg PO twice daily Dalfampridine 10 mg PO twice daily
19 Self assessment question Which of the following are oral disease modifying therapies for MS? a. Interferons, copaxone & alemtuzumab b. Copaxone, fingolimod & mitoxantrone c. Fingolimod, teriflunomide & dimethyl fumarate d. Alemtuzumab, natalizumab & copaxone
20
21 Injection Site reaction from Glatiramer Acetate
22 DMTs continued Natalizumab (Tysabri) Indicated: Monotherapy for relapsing forms of MS to slow disability & reduce frequency of relapses Should generally be used when other DMTs are ineffective or intolerable or in patients with aggressive disease Dose: 300 mg IV q 4 weeks. TOUCH prescribing program Adverse Effects: Anxiety, fatigue, peripheral edema, infusion related symmptoms, Hypersensitivity rxn, Immunosuppression / Infections & Progressive multifocal leukoencephalopathy (PML)
23 Alemtuzumab (Lemtrada) Alemtuzumab is a CD52-directed cytolytic antibody indicated for B-cell CLL & MS Restricted distribution program Dosing: 12 mg/day IV over 5 days and repeat in year for 3 days Premedicate with diphenhydramine & acetaminophen. Administer Bactrim & acyclovir for PCP & herpes prophylaxis.
24 Dalfampridine ER (Ampyra) US FDA approved 1/2010 Dalfampridine is a K channel blocker indicated to improve walking in pts with MS. Typical Dose: 10mg oral twice daily. Take tablets whole, do not crush or chew. PK: Relative Fo 96%, low ppb, Tmax 3-4hrs, Elimination T1/2 = 5-6 hrs, eliminated renally (>90 %) AEs: seizures, insomnia, dizziness, HA, nausea, contraindicated if Cr Cl < 50 ml/min Efficacy: A phase III study showed improved walking (25 ft) in 43% pts vs 9% placebo
25 Fingolimod (Gilenya) US FDA approved 9/2010 Fingolimod is a sphingosine 1-phosphate receptor modulator indicated to reduce the frequency of clinical exacerbations and to delay accumulation of physical disability with relapsing forms of MS Dose: 0.5 mg orally daily. Available: 0.5 mg caps - Pts need to be observed for 6 hrs after the first dose in an office or clinic for S/S bradycardia / heart block. If med stopped > 2 weeks then restart monitoring as new therapy
26 Fingolimod (Gilenya) Use caution in patients taking beta blockers or antiarrhythmics Live attenuated vaccines should be avoided during the first 2 mo s of therapy Antibody testing for Varicella Zoster virus perform at baseline. If VZV non-immune then vaccinate Ophthalmologic exam at baseline and every 3-4 mo s for macular edema Animal studies suggest teratogenicity: use effective contraception
27 Teriflunomide (Aubagio R ) US FDA approved 9/2012 Teriflunomide has selective reversible inhibition of dihydroorotate dehydrogenase that blocks pyrimidine synthesis in rapidly proliferating cells. For relapsing forms of MS It is the active metabolite of leflunomide Available: 7 and 14 mg tablets Adult dose: 7 or 14 mg orally once daily AE: HA., Incr LFT, alopecia, diarrhea, nausea, paresthesia
28 Dimethyl Fumarate (Tecfidera) US FDA approved 3/2013 Dimethyl fumarate (DMF) has been shown to activate the nuclear factor like 2 (Nrf2) pathway. The Nrf2 pathway is involved in the cellular response to oxidative stress Available: 120 & 240 mg delayed release caps. Swallow whole, do not crush/chew Adult Dose: Start 120 mg twice a day and may incr to 240 mg bid after 7 days
29 Dimethyl Fumarate Protect capsules from light. AE: flushing, abdominal pain, diarrhea & N Pregnancy: unknown effects Monitor CBC at baseline and at least annually. Withholding treatment is warranted with serious infections
30 Absolute contraindications Interferons: LFT s at baseline > 2 x ULN Fingolimod: LFT s at baseline > 2 x ULN, MI, stroke, TIA, unstable angina, decomp. heart failure within 6 mo s, hx of Mobitz type II, 3 rd degree AV block, sick sinus syndrome, baseline QTc > 500 ms, classs Ia or II antiarrhythmics, negative VZV titers, pregnancy Teriflunomide: positive PPD, LFT s > 2x ULN
31 MS Medications Storage Avonex* powder IFN-beta1a Avonex* prefilled Syringe Betaseron IFN-beta1b Refrigerate F or at room temp up to 30 days. After mixing use with in 6 hrs if kept in fridge Refrigerate F or at room temp up to 7 days Room temp. Use immediately after mixing, or within 3 hrs if kept in fridge Extavia IFN-beta1b Room temp up to 77 F. Use immediately after mixing or within 3 hrs if kept in fridge * Do not freeze, protect from heat and light
32 MS Medications Storage Glatiramer* Copaxone Rebif* IFN-beta1a Fingolimod Gilenya DMF Tecfidera Refrigerate F or at room temp up to 30 days Refrigerate F or at room temp up to 30 days Store in original blister pack at room temp Store at room temp. Protect from light, store in original container Alemtuzumab Store at room temp or refrigerate. Use Lemtrada within 8 hrs of dilution * Do not freeze, protect from heat and light
33 MS DMT Annual Drug Cost ** Approximate WAC or Manufacturer published wholesaler price ** Drug Annual Cost ($) Glatiramer (Copaxone) 65,104 Interferon beta-1a - Avonex - Rebif Interferon beta -1a pegylated -Plegridy Interferon beta-1b - Betaseron - Extavia 65,442 70,638 65,442 69,397 57,694
34 MS DMT Annual Drug Cost ** Approximate WAC or Manufacturer published wholesaler price ** Drug Annual Cost ($) Mitoxantrone 3167 Natalizumab Alemtuzumab Oral agents - Fingolimod - Teriflunomide - Dimethy fumarate 64,480 59,250 70,752 66,017 65,520
35 Self Assessment questions Which of the following are common adverse effects among the interferon products utilized for MS? a. Flushing b. Acute kidney injury c. Severe bradycardia d. Flu like symptoms Which of the following products can be stored at room temperature for over 30 days? a. Interferon beta-1a (Avonex) b. Glatiramaer (Copaxone) c. Interferon Beta 1b (Betaseron) d. Interferon-Beta 1a (Rebif)
36 Multiple Sclerosis Take home concepts MS symptoms are from lesions in the CNS Acute relapses: corticosteriods Traditional DMTs are injectable: β interferons, copaxone, mitoxantrone & natalizumab Newer DMTs are oral therapies: Fingolimod, Teriflunomide and dimethyl fumarate Ongoing research: More oral medications
37 Questions Kiranpal S. Sangha, Pharm.D. Clinical Pharmacy Specialist CNS The University of Cincinnati Medical Center Adjunct Asst. Professor Clinical Pharmacy, James L. Winkle UC College of Pharmacy Phone:
Progress in MS: Current and Emerging Therapies
 Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
MEDICAL POLICY STATEMENT
 MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 10/01/2013 10/1/2015 08/25/2015 Policy Name Policy Number Multiple Sclerosis Therapy Class SRx-0022
MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 10/01/2013 10/1/2015 08/25/2015 Policy Name Policy Number Multiple Sclerosis Therapy Class SRx-0022
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual. Topic: Gilenya, fingolimod Date of Origin: November 22, 2010
 Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Lemtrada (alemtuzumab)
 Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Information About Medicines for Multiple Sclerosis
 Information About Medicines for Multiple Sclerosis Information About Medicines for Multiple Sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information About Medicines for Multiple Sclerosis Information About Medicines for Multiple Sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Medication Policy Manual. Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014
 Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
New treatments in MS What s here and what s nearly here
 5 th MS Research Day, June 14 th 2014 New treatments in MS What s here and what s nearly here David Miller Queen Square MS Centre at UCL and UCLH Course of MS and its treatment Relapsing remitting Disability
5 th MS Research Day, June 14 th 2014 New treatments in MS What s here and what s nearly here David Miller Queen Square MS Centre at UCL and UCLH Course of MS and its treatment Relapsing remitting Disability
Information about medicines for multiple sclerosis
 Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
New Treatment Options for MS Patients: Understanding risks versus benefits
 New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
Multiple Sclerosis Update. Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center
 Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Relapsing-remitting multiple sclerosis Ambulatory with or without aid
 AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
The MS Disease- Modifying Medications GENERAL INFORMATION
 The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
Literature Scan: Oral Multiple Sclerosis Drugs
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru381 Topic: Lemtrada TM, alemtuzumab Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru381 Topic: Lemtrada TM, alemtuzumab Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio
 ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents
 New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
The MS Disease- Modifying Medications
 The MS Disease- Modifying Medications National MS Society 1 Current as of January 2015. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
The MS Disease- Modifying Medications National MS Society 1 Current as of January 2015. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
Medication Policy Manual. Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004
 Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Disease Modifying Therapies (DMTs) in Multiple Sclerosis
 Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
What is Multiple Sclerosis? Disease Modifying Therapies. Best of all. Why is treatment so important? Outline and Expectations.
 What is Multiple Sclerosis? Disease Modifying Therapies for Relapsing Remitting MS Jeanie Lynn Cote, MD Chronic, auto immune disorder. As a consequence of immune attacks, there is loss of myelin (fatty
What is Multiple Sclerosis? Disease Modifying Therapies for Relapsing Remitting MS Jeanie Lynn Cote, MD Chronic, auto immune disorder. As a consequence of immune attacks, there is loss of myelin (fatty
Multiple Sclerosis (MS) Class Update
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
A blood sample will be collected annually for up to 2 years for JCV antibody testing.
 Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
What is MS? 1. disease that affects the central nervous. Is a disease that affects both white and gray matter
 What is MS? 1 Neuron Damaged myelin due to inflammation MS is a chronic immunemediated disease that affects the central nervous system (CNS) Is a disease that affects both white and gray matter Interrupted
What is MS? 1 Neuron Damaged myelin due to inflammation MS is a chronic immunemediated disease that affects the central nervous system (CNS) Is a disease that affects both white and gray matter Interrupted
A neurologist would assess your eligibility and suitability for the DMTs.
 Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Pharmacotherapy of Multiple Sclerosis
 PHARMACY / MEDICAL POLICY 5.01.565 Pharmacotherapy of Multiple Sclerosis Effective Date: July 1, 2016 Last Revised: June 14, 2016 Replaces: Extracted from 5.01.550 RELATED MEDICAL POLICIES: 5.01.556 Rituximab:
PHARMACY / MEDICAL POLICY 5.01.565 Pharmacotherapy of Multiple Sclerosis Effective Date: July 1, 2016 Last Revised: June 14, 2016 Replaces: Extracted from 5.01.550 RELATED MEDICAL POLICIES: 5.01.556 Rituximab:
Study Support Materials Cover Sheet
 Study Support Materials Cover Sheet Document Title ESCALATE Patient Brochure Intended Audience This brochure is designed to be given to potentially eligible patients as a take-home summary of key information
Study Support Materials Cover Sheet Document Title ESCALATE Patient Brochure Intended Audience This brochure is designed to be given to potentially eligible patients as a take-home summary of key information
Treatment in Relapsing MS: Choosing Among the Options. Donald Negroski, MD
 Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
SECTION 2. Section 2 Multiple Sclerosis (MS) Drug Coverage
 SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
CNS DEMYLINATING DISORDERS
 CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS?
 Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
New Developments in the Treatment and Management of Multiple Sclerosis
 New Developments in the Treatment and Management of Multiple Sclerosis Myla D. Goldman, MD, MS For a CME/CEU version of this article, please go to www.namcp.org/cmeonline.htm, and then click the activity
New Developments in the Treatment and Management of Multiple Sclerosis Myla D. Goldman, MD, MS For a CME/CEU version of this article, please go to www.namcp.org/cmeonline.htm, and then click the activity
RISK EVALUATION AND MITIGATION STRATEGY (REMS)
 Initial REMS Approval: 9/2010 Most Recent Modification: 05/2015 0.5mg capsules Sphingosine 1-phosphate Receptor Modulator Novartis Pharmaceuticals Corporation One Health Plaza East Hanover, NJ 07936 RISK
Initial REMS Approval: 9/2010 Most Recent Modification: 05/2015 0.5mg capsules Sphingosine 1-phosphate Receptor Modulator Novartis Pharmaceuticals Corporation One Health Plaza East Hanover, NJ 07936 RISK
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
 Multiple Sclerosis Agents Page 1 of 13 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See also: Multiple Sclerosis Agents Tysabri (natalizumab) medical policy
Multiple Sclerosis Agents Page 1 of 13 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See also: Multiple Sclerosis Agents Tysabri (natalizumab) medical policy
Multiple Sclerosis: What You Need To Know. For Professionals
 Multiple Sclerosis: What You Need To Know For Professionals What will I learn today? The Basics: What is MS? Living with MS: A Family Affair We Can Help: The National MS Society What MS Is: MS is thought
Multiple Sclerosis: What You Need To Know For Professionals What will I learn today? The Basics: What is MS? Living with MS: A Family Affair We Can Help: The National MS Society What MS Is: MS is thought
National Multiple Sclerosis Society. Disease Modification in Multiple Sclerosis. Current as of January 2, 2013
 National Multiple Sclerosis Society Disease Modification in Multiple Sclerosis Current as of January 2, 2013 Since 1993, the U.S. Food and Drug Administration (FDA) has approved several medications for
National Multiple Sclerosis Society Disease Modification in Multiple Sclerosis Current as of January 2, 2013 Since 1993, the U.S. Food and Drug Administration (FDA) has approved several medications for
Novartis Gilenya FDO Program Clinical Protocol and Highlights from Prescribing Information (PI)
 Novartis Gilenya FDO Program Clinical Protocol and Highlights from Prescribing Information (PI) Highlights from Prescribing Information - the link to the full text PI is as follows: http://www.pharma.us.novartis.com/product/pi/pdf/gilenya.pdf
Novartis Gilenya FDO Program Clinical Protocol and Highlights from Prescribing Information (PI) Highlights from Prescribing Information - the link to the full text PI is as follows: http://www.pharma.us.novartis.com/product/pi/pdf/gilenya.pdf
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
Multiple Sclerosis. Multiple Sclerosis. In addition to help nursing professional to understand the signs and
 How to Receive Your CE Credits Read your selected course Completed the quiz at the end of the course with a 70% or greater. Complete the evaluation for your selected course. Print your Certificate CE s
How to Receive Your CE Credits Read your selected course Completed the quiz at the end of the course with a 70% or greater. Complete the evaluation for your selected course. Print your Certificate CE s
Drug Class Review. Disease-modifying Drugs for Multiple Sclerosis. Single Drug Addendum: Fingolimod
 Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES. I. Requirements for Prior Authorization of Multiple Sclerosis Agents
 MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Multiple Sclerosis Agents A. Prescriptions That Require Prior Authorization Prescriptions
MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Multiple Sclerosis Agents A. Prescriptions That Require Prior Authorization Prescriptions
Disclosures. Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
 Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Disease modifying drug therapy
 Disease modifying drug therapy New edition for 2014-15 We hope you find the information in this book helpful. If you would like to speak with someone about any aspect of MS, contact the MS Trust information
Disease modifying drug therapy New edition for 2014-15 We hope you find the information in this book helpful. If you would like to speak with someone about any aspect of MS, contact the MS Trust information
Is the Grass Really Greener with New Oral Multiple Sclerosis Treatments? A Clinical Debate
 Is the Grass Really Greener with New Oral Multiple Sclerosis Treatments? A Clinical Debate Elizabeth Sebranek Evans, PharmD, BCPS, CGP Alana Whittaker, PharmD, BCPS Roseman University of Health Sciences
Is the Grass Really Greener with New Oral Multiple Sclerosis Treatments? A Clinical Debate Elizabeth Sebranek Evans, PharmD, BCPS, CGP Alana Whittaker, PharmD, BCPS Roseman University of Health Sciences
Understanding How Existing and Emerging MS Therapies Work
 Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
Mellen Center Approaches: Choosing First-Line Treatment
 Cleveland Clinic Mellen Center for Multiple Sclerosis Treatment and Research 216.444.8600 Mellen Center Approaches: Choosing First-Line Treatment Q: Should my patient with newly diagnosed multiple sclerosis
Cleveland Clinic Mellen Center for Multiple Sclerosis Treatment and Research 216.444.8600 Mellen Center Approaches: Choosing First-Line Treatment Q: Should my patient with newly diagnosed multiple sclerosis
Which injectable medication should I take for relapsing-remitting multiple sclerosis?
 Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
PCORI Workshop on Treatment for Multiple Sclerosis. Breakout Group Topics and Questions Draft 3-27-15
 PCORI Workshop on Treatment for Multiple Sclerosis Breakout Group Topics and Questions Draft 3-27-15 Group 1 - Comparison across DMTs, including differential effects in subgroups Consolidated straw man
PCORI Workshop on Treatment for Multiple Sclerosis Breakout Group Topics and Questions Draft 3-27-15 Group 1 - Comparison across DMTs, including differential effects in subgroups Consolidated straw man
PharmaPoint: Multiple Sclerosis - United Kingdom Drug Forecast and Market Analysis to 2022. Multiple
 Brochure More information from http://www.researchandmarkets.com/reports/2541548/ PharmaPoint: Multiple Sclerosis - United Kingdom Drug Forecast and Market Analysis to 2022 Description: PharmaPoint: Multiple
Brochure More information from http://www.researchandmarkets.com/reports/2541548/ PharmaPoint: Multiple Sclerosis - United Kingdom Drug Forecast and Market Analysis to 2022 Description: PharmaPoint: Multiple
Treatments for MS: Immunotherapy. Gilenya (fingolimod) Glatiramer acetate (Copaxone )
 Treatments for MS: Immunotherapy There are currently several disease-modifying therapies approved for people with MS in Australia. These therapies, called immunotherapies, work to reduce disease activity
Treatments for MS: Immunotherapy There are currently several disease-modifying therapies approved for people with MS in Australia. These therapies, called immunotherapies, work to reduce disease activity
The Nuts and Bolts of Multiple Sclerosis. Rebecca Milholland, M.D., Ph.D. Center for Neurosciences
 The Nuts and Bolts of Multiple Sclerosis Rebecca Milholland, M.D., Ph.D. Center for Neurosciences Objectives Discuss which patients are at risk for Multiple Sclerosis Discuss the diagnostic criteria for
The Nuts and Bolts of Multiple Sclerosis Rebecca Milholland, M.D., Ph.D. Center for Neurosciences Objectives Discuss which patients are at risk for Multiple Sclerosis Discuss the diagnostic criteria for
SOUTH TAMPA MULTIPLE SCLEROSIS CENTER PATIENT/ CARE GIVER QUESTIONNAIRE
 SOUTH TAMPA MULTIPLE SCLEROSIS CENTER PATIENT/ CARE GIVER QUESTIONNAIRE DEMOGRAPHIC INFORMATION Patient Name: Date: Address: City: State: Zip Code Best Phone Number: Marital Status Phone (H): (W) (Cell):
SOUTH TAMPA MULTIPLE SCLEROSIS CENTER PATIENT/ CARE GIVER QUESTIONNAIRE DEMOGRAPHIC INFORMATION Patient Name: Date: Address: City: State: Zip Code Best Phone Number: Marital Status Phone (H): (W) (Cell):
Version History. Previous Versions. Drugs for MS.Drug facts box fingolimod Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
teriflunomide, 14mg, film-coated tablets (Aubagio ) SMC No. (940/14) Genzyme Ltd.
 teriflunomide, 14mg, film-coated tablets (Aubagio ) SMC No. (940/14) Genzyme Ltd. 10 January 2014 (Issued 07 February 2014) The Scottish Medicines Consortium (SMC) has completed its assessment of the above
teriflunomide, 14mg, film-coated tablets (Aubagio ) SMC No. (940/14) Genzyme Ltd. 10 January 2014 (Issued 07 February 2014) The Scottish Medicines Consortium (SMC) has completed its assessment of the above
How to S.E.A.R.C.H. SM for the Right MS Therapy For You!
 How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
Uncertainty in Benefit and Risk: Tysabri (natalizumab)
 Uncertainty in Benefit and Risk: Tysabri (natalizumab) Robert J. Temple, M.D. Deputy Center Director for Clinical Science Center for Drug Evaluation and Research U.S. Food and Drug Administration IOM Drug
Uncertainty in Benefit and Risk: Tysabri (natalizumab) Robert J. Temple, M.D. Deputy Center Director for Clinical Science Center for Drug Evaluation and Research U.S. Food and Drug Administration IOM Drug
06/06/2012. The Impact of Multiple Sclerosis in the Pacific Northwest. James Bowen, MD. Swedish Neuroscience Institute
 The Impact of Multiple Sclerosis in the Pacific Northwest James Bowen, MD Multiple Sclerosis Center Multiple Sclerosis Center Swedish Neuroscience Institute 1 2 Motor Symptoms of MS Weakness Spasticity
The Impact of Multiple Sclerosis in the Pacific Northwest James Bowen, MD Multiple Sclerosis Center Multiple Sclerosis Center Swedish Neuroscience Institute 1 2 Motor Symptoms of MS Weakness Spasticity
Multiple sclerosis disease-modifying drugs second line treatments
 Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
Best practices for using MS disease modifying therapies
 Best practices for using MS disease modifying therapies CMSC Annual Meeting 2015 COREY C FORD, MD, PHD MS SPECIALTY CLINIC UNIVERSITY OF NEW MEXICO HSC MAY 30, 2015 Objectives Use best practices to select
Best practices for using MS disease modifying therapies CMSC Annual Meeting 2015 COREY C FORD, MD, PHD MS SPECIALTY CLINIC UNIVERSITY OF NEW MEXICO HSC MAY 30, 2015 Objectives Use best practices to select
this 7^ day of September 2014 by Randall S. Gregg Special Deputy Director
 r STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v File No. 143525-001 Health Alliance Plan of Michigan,
r STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v File No. 143525-001 Health Alliance Plan of Michigan,
Growth in revenue from MS drugs has been driven largely by price increases over the last several years.
 March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
Patients with confirmed relapse 111 26 (23.4 %) 104 16 (15.4 %) 1.52 [0.87; 2.67] p = 0.143 Probability of a relapse by week 96
![Patients with confirmed relapse 111 26 (23.4 %) 104 16 (15.4 %) 1.52 [0.87; 2.67] p = 0.143 Probability of a relapse by week 96 Patients with confirmed relapse 111 26 (23.4 %) 104 16 (15.4 %) 1.52 [0.87; 2.67] p = 0.143 Probability of a relapse by week 96](/thumbs/33/15779352.jpg) Resolution by the Federal Joint Committee on an amendment to the Pharmaceutical Directive (AM-RL): Appendix XII Resolutions on the benefit assessment of pharmaceuticals with new active ingredients, in
Resolution by the Federal Joint Committee on an amendment to the Pharmaceutical Directive (AM-RL): Appendix XII Resolutions on the benefit assessment of pharmaceuticals with new active ingredients, in
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON
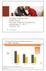 Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Current and future options of MS treatment Prof. Dr. Karl Vass, AKH Wien
 Current and future options of MS treatment Prof. Dr. Karl Vass, AKH Wien European Health Forum, Gastein 6 th October 2010 Multiple Sclerosis is the most common neurological disorder in young Caucasian
Current and future options of MS treatment Prof. Dr. Karl Vass, AKH Wien European Health Forum, Gastein 6 th October 2010 Multiple Sclerosis is the most common neurological disorder in young Caucasian
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Multiple Sclerosis Therapy Table of Contents Coverage Policy... 1 General Background... 9 Coding/Billing Information... 14 References... 14 Effective Date...
Cigna Drug and Biologic Coverage Policy Subject Multiple Sclerosis Therapy Table of Contents Coverage Policy... 1 General Background... 9 Coding/Billing Information... 14 References... 14 Effective Date...
The submission positioned dimethyl fumarate as a first-line treatment option.
 Product: Dimethyl Fumarate, capsules, 120 mg and 240 mg, Tecfidera Sponsor: Biogen Idec Australia Pty Ltd Date of PBAC Consideration: July 2013 1. Purpose of Application The major submission sought an
Product: Dimethyl Fumarate, capsules, 120 mg and 240 mg, Tecfidera Sponsor: Biogen Idec Australia Pty Ltd Date of PBAC Consideration: July 2013 1. Purpose of Application The major submission sought an
Multiple Sclerosis Drug Discoveries - What the Future Holds
 Brochure More information from http://www.researchandmarkets.com/reports/1408035/ Multiple Sclerosis Drug Discoveries - What the Future Holds Description: The recent approval in the US of Novartis' orally
Brochure More information from http://www.researchandmarkets.com/reports/1408035/ Multiple Sclerosis Drug Discoveries - What the Future Holds Description: The recent approval in the US of Novartis' orally
NHS BOURNEMOUTH AND POOLE AND NHS DORSET
 NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
Genzyme s Multiple Sclerosis Franchise Featured at AAN
 PRESS RELEASE Genzyme s Multiple Sclerosis Franchise Featured at AAN - Multiple Presentations Highlight Continuing Progress of AUBAGIO and LEMTRADA Programs - Paris, France March 13, 2013 Sanofi (EURONEXT:
PRESS RELEASE Genzyme s Multiple Sclerosis Franchise Featured at AAN - Multiple Presentations Highlight Continuing Progress of AUBAGIO and LEMTRADA Programs - Paris, France March 13, 2013 Sanofi (EURONEXT:
Patient Group Input to CADTH
 Patient Group Input to CADTH Section 1 General Information Name of the drug CADTH is reviewing and indication(s) of interest Name of patient group/author of submission Patient group s contact information:
Patient Group Input to CADTH Section 1 General Information Name of the drug CADTH is reviewing and indication(s) of interest Name of patient group/author of submission Patient group s contact information:
Laquinimod Polman, C. et al. Neurology 2005;64:987-991
 Laquinimod Polman, C. et al. Neurology 2005;64:987-991 Multicenter, double-blind, randomized trial, patients with RR MS received 0.1 mg or 0.3 mg laquinimod or placebo as three daily tablets for 24 weeks
Laquinimod Polman, C. et al. Neurology 2005;64:987-991 Multicenter, double-blind, randomized trial, patients with RR MS received 0.1 mg or 0.3 mg laquinimod or placebo as three daily tablets for 24 weeks
Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis
 Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness
Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness
5.07.09. Aubagio. Aubagio (teriflunomide) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Multiple Sclerosis (MS) is a disease of the central nervous system (including the brain and spinal cord) in which the nerves degenerate.
 What is Multiple Sclerosis? Multiple Sclerosis (MS) is a disease of the central nervous system (including the brain and spinal cord) in which the nerves degenerate. A disease of the central nervous system
What is Multiple Sclerosis? Multiple Sclerosis (MS) is a disease of the central nervous system (including the brain and spinal cord) in which the nerves degenerate. A disease of the central nervous system
acquired chronic immune-mediated inflammatory condition of CNS. MS in children: 10% +secondary progressive MS: rare +primary progressive MS: rare
 Immunomodulatory Therapies in Pediatric MS Vuong Chinh Quyen Neurology Department Medscape Mar 8, 2013 Multiple Sclerosis in Children. Iran J Child Neurol. 2013 Spring Introduction acquired chronic immune-mediated
Immunomodulatory Therapies in Pediatric MS Vuong Chinh Quyen Neurology Department Medscape Mar 8, 2013 Multiple Sclerosis in Children. Iran J Child Neurol. 2013 Spring Introduction acquired chronic immune-mediated
What is Multiple Sclerosis? Gener al information
 What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
MULTIPLE SCLEROSIS Management and Therapy
 MULTIPLE SCLEROSIS Management and Therapy 115 th Oklahoma Osteopathic Association Annual Convention April 30 th, 2015 Anthony J. Vaughn, M.D. ANTHONY J. VAUGHN, M.D. Assistant Professor Director, Clinical
MULTIPLE SCLEROSIS Management and Therapy 115 th Oklahoma Osteopathic Association Annual Convention April 30 th, 2015 Anthony J. Vaughn, M.D. ANTHONY J. VAUGHN, M.D. Assistant Professor Director, Clinical
