Treatment in Relapsing MS: Choosing Among the Options. Donald Negroski, MD
|
|
|
- Rosanna Atkins
- 10 years ago
- Views:
Transcription
1 Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD
2 Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education and non-cme lectures Biogen, Teva, Bayer, Serono, Novartis, Genzyme, Questcor, Acorda, ONO, Chugia 3
3 Agenda I. Multiple Sclerosis Phenotypes II. Risk of Disease Progression III. Pathophysiology IV. Treatment Sequencing
4 What happens if you don t Treat MS? Difficulty with Thinking and Memory Loss of work Depression Sexual Dysfunction Stress on Relationships Relapsing Remitting MS years 50% SPMS and 50% need a cane 83% need cane 30 years from onset ~ 34% bed bound Weinshenker, 1989
5 MS Phenotypes RIS (radiological isolated syndrome) CIS (clinically isolated syndrome) CDMS (clinically definite MS) Relapsing forms and Progressive Forms: Active vs. Inactive Worsening vs No worsening of disability
6 1996 vs 2013 MS Phenotype Descriptions Progressive Disease 1995 MS Clinical Description Subtypes 2013 MS Disease Modifiers Phenotypes Progressive Disease Primary Progressive Progressive accumulation of disability from onset; with or without temporary plateaus, minor remissions and improvements Secondary Progressive Progressive accumulation of disability after initial relapsing course, with or without occasional relapses and minor remissions Progressive Relapsing Progressive accumulation of disability from onset but clear acute clinical attacks with or without full recovery Progressive accumulation of disability from onset PP Progressive Disease SP Progressive accumulation of disability after initial relapsing course Lublin FD, et al. Neurology. 2014;83(3): ; Miller AE. ACTRIMS/ECTRIMS 2014 [TC1]. Active* and with progression** Active but without progression Not active but with progression Not active and without progression (stable disease) *activity determined by clinical relapses assessed at least annually and/or MRI activity; ** progression measured by clinical evaluation, assessed at least annually
7 Factors Related to Risk of Disease Progression During First 5 Years after Diagnosis Non-Caucasian Multiple deficits at presentation Increased number of attacks Short interattack interval Disability High MRI lesion load Early motor/ cerebellar / bowel and bladder involvement Stroup, Neuro Report. 2014
8 1000-CIS: Factors that Determine Disease Course Early Changes to Predict Longterm Prognosis Study designed to determine factors that add value to clinical and brain MRI changes occurring during the first year, to predict conversion to CDMS and disability accumulation N = 1015 CIS patients (from 1995 till 2013) Demographic factors Topography CSF: OB Baseline Number of T2 12m New 12 Treatment factors Decreased Risk Increased Risk Key findings First year clinical and brain MRI changes further improve the estimation of individual prognosis Independent predictors of further attacks: Baseline lesions, new T2 during the first year, DMT before second attack Independent predictors of accumulation of disability: OB, new T2 and incomplete recovery Hour Tintore, et al. ACTRIMS-ECTRIMS; September 10-13, 2014; Boston, MA. PS9.4.
9 Relapse Recovery 18% (of 1562) patients with incomplete recovery reached EDSS 6 in ½ time of those with complete recovery Pyramidal, bowel/bladder, cognitive and cerebellar relapses had increased residual disability Relapses in patients on 1 st line therapy were often preceded by similar pre-treatment relapses. Confarvreau, Brain 2003, Kalincik, MSJ 2014, Deen, J Neuro Neurosur Psych 2008
10 Proposed Pathophysiology of MS VCAM = vascular cell adhesion molecule.
11 APC=antigen-presenting cells Pathophysiology of MS
12 Evolving MS Treatment Landscape (Alemtuzumab) Lemtrada Laquinimod Betaseron (IFNβ-1b) Tysabri (natalizumab) Plegridy TM (PEG-IFNbeta-1a) Ocrelizumab Avonex (IFNβ-1a) Copaxone (glatiramer acetate) Novantrone (mitoxantrone) Extavia (IFNβ-1b) Gilenya (fingolimod) Aubagio (teriflunomide) Copaxone (GA 40 mg TIW) Tecfidera (dimethyl fumarate/) Dac-HYP Ofatumumab Rebif (IFNβ-1a) FDA-Approved Therapies Phase III Injectable Therapy Oral Therapy
13 Different MOA s Influence immune cell functioning Inhibit immune cell trafficking Inhibit cell replication Immune cell depletion/destruction
14 Treatment Sequencing/ Optimization for Relapsing MS Goal: NEIDA (No Evidence of Inflammatory Disease Activity): no sustained disability after 3 months, no relapses, no MRI activity (new/enlarging T2 or gd+ lesions) Proposed: NEDA-4: no annual brain volume loss > 0.4%, no disability after 6 months
15 Theoretical Reasons to Switch Attempt to limit tissue damage Prevent irreversible disability Examples of switches: Lateral move Induction Escalation 16
16 Use of MRI for Decisions Likely nonresponder to Interferon Tx if >1 relapse or 1 relapse and 4 new T2 lesions Patients with one or more new or enlarging T2 lesion during first year were twice as likely to experience a relapse during the second year (PBO pt in 3 trials) Rio, Mult Scler, 2009, Sormani, Nat Rev Neur 2013, Richert, Neurology, 2014
17 Limited Switch Studies Some benefit for treatment failure Less clear for tolerability Limited between classes Some evidence (2 studies) from injectable to Fingolimod Majority of Neurologists wait 6-12 months as a minimum tx duration before switching 57% of neurologist would switch with 3-5 new asymptomatic T2 lesions Stuve, JAMA Neuro, 2015, Tornatore, Neur Cl Pra 2012
18 Switching Therapy is Not Without Risks Breakthrough disease activity Timing of treatment Washout concerns Rebound disease Leaving patient untreated Montalban X. Presented at: ACTRIMS-ECTRIMS; September 10-13, 2014; Boston, MA; Parallel Session 9.2; Havla J, et al. Ther Clin Risk Manag. 2013;9:
19 Transitioning Between Therapies: Washout Considerations From Transitioning To Washout IFNβ or GA Any drug No washout period required Any drug IFNβ or GA No washout period required Natalizumab Teriflunomide Fingolimod DMF Fingolimod/natalizumab Fingolimod Teriflunomide DMF Other MS therapies Teriflunomide Natalizumab Alemtuzumab Natalizumab Fingolimod Alemtuzumab months after discontinuation of natalizumab At least 3.5 months is required to prevent possibility of concomitant immune effects. Caution is required if other therapies are started within this time period. Accelerated Elimination Procedure 1-2 month period needed for lymphocytes to return to normal range following discontinuation of fingolimod No washout period required 2-3 months for natalizumab Until > 800 lymphocytes for fingolimod Montalban X. Presented at: ACTRIMS-ECTRIMS; September 10-13, 2014; Boston, MA Parallel Session 9.2.
20 Treatment Decision Making in MS Define the MS Choose therapy Patient and disease profile MS prognosis Disease activity / type Shared goals for treatment Disease factors (stage, activity, severity) Patient factors (preference, comoribidities) Drug factors (efficacy, safety, cost, route) X Y Z yes no Monitor Monitor Clinical (relapse, disability progression, tolerability) MRI (T2 and T1-Gd, brain atrophy) Biomarkers (anti-jcv Ab, NAbs) Change therapy? Suboptimal response? Intolerable side effects? Giovannoni G, et al. Curr Opin Neurol. 2012;25(suppl1):AS20-S27; Dhib-Jalbut S. Presented at: ACTRIMS-ECTRIMS; September 10-13, 2014; Boston; Parallel Session 7.2
21 MS Treatment Algorithm (Europe) CIS at high risk of developing MS CIS fulfilling MS criteria (McDonald 2010) RRMS Interferon beta 1b Interferon beta 1a sc Interferon beta 1a im Glatiramer Acetate Teriflunomide DMF Rapidly evolving severe RRMS MILD Disease activity Natalizumab Fingolimod Alemtuzumab Montalban X. Presented at: ACTRIMS-ECTRIMS; September 10-13, 2014; Boston, MA. Parallel Session 9.2.
22 MS Treatment Algorithm (Europe) CIS at high risk of developing MS CIS fulfilling MS criteria (McDonald 2010) RRMS Interferon beta 1b Interferon beta 1a sc Interferon beta 1a im Glatiramer Acetate Teriflunomide DMF Rapidly evolving severe RRMS MODERATE/SEVERE Disease activity Natalizumab Fingolimod Alemtuzumab Montalban X. Presented at: ACTRIMS-ECTRIMS; September 10-13, 2014; Boston, MA. Parallel Session 9.2.
23 Practice Patterns Consensus of US Neurologists : Summary of 2014 Survey US MS specialists (n=239) 2 surveys developed by a steering committee of 6 MS experts independent of the CMSC and 2 representatives from large national health plan and pharmacy benefits managers Results show an earlier more aggressive approach to MS treatment RIS Treat if >2 new Gd+ lesions (80%) Follow-up MRI within 12 months (84%) Diagnosis CIS RRMS: Initiate treatment Treat if 2 nonenhancing T2 lesions (95%) or 1 Gd+ lesion in addition to nonenhancing T2 lesions (98%) Treat mild disease with injectable treatment (38%) or DMF (30%) (100%) JCV-, aggressive disease, treat with natalizumab Follow-up MRI within 12 months (75%) Follow-up MRI within 12 months (97%) Follow-up MRI within 12 months (97%) Consider switching to oral/iv DMT if 2 new T2 lesions (67%) or 1 new Gd+ lesion (52%) on therapy Tornatore C et al. Presented at: ACTRIMS-ECTRIMS; September, 2014; Boston; Poster 295.
24 Treatment in Relapsing MS: Choosing Among the Options
25
Disease Modifying Therapies (DMTs) in Multiple Sclerosis
 Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Multiple Sclerosis Update. Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center
 Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Using the MS Clinical Course Descriptions in Clinical Practice
 Using the MS Clinical Course Descriptions in Clinical Practice Mark J. Tullman, MD Director of Clinical Research The MS Center for Innovations in Care Missouri Baptist Medical Center Disclosures Consultant/speaking
Using the MS Clinical Course Descriptions in Clinical Practice Mark J. Tullman, MD Director of Clinical Research The MS Center for Innovations in Care Missouri Baptist Medical Center Disclosures Consultant/speaking
Progress in MS: Current and Emerging Therapies
 Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Treatment Optimization in MS: When to Start, When to Shift, when to Stop
 Treatment Optimization in MS: When to Start, When to Shift, when to Stop Mark S. Freedman MSc MD FAAN FANA FRCPC Director, Multiple Sclerosis Research Unit University of Ottawa Sr. Scientist, Ottawa Hospital
Treatment Optimization in MS: When to Start, When to Shift, when to Stop Mark S. Freedman MSc MD FAAN FANA FRCPC Director, Multiple Sclerosis Research Unit University of Ottawa Sr. Scientist, Ottawa Hospital
Medication Policy Manual. Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004
 Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Medication Policy Manual. Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014
 Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Relapsing-remitting multiple sclerosis Ambulatory with or without aid
 AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
Growth in revenue from MS drugs has been driven largely by price increases over the last several years.
 March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
Lemtrada (alemtuzumab)
 Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
MEDICAL POLICY STATEMENT
 MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 10/01/2013 10/1/2015 08/25/2015 Policy Name Policy Number Multiple Sclerosis Therapy Class SRx-0022
MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 10/01/2013 10/1/2015 08/25/2015 Policy Name Policy Number Multiple Sclerosis Therapy Class SRx-0022
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS?
 Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
Multiple Sclerosis Therapeutics to 2019 - Treatment Diversification, Increasing Efficacy, and Pipeline Innovation Combine to Drive Growth
 Brochure More information from http://www.researchandmarkets.com/reports/2640803/ Multiple Sclerosis Therapeutics to 2019 - Treatment Diversification, Increasing Efficacy, and Pipeline Innovation Combine
Brochure More information from http://www.researchandmarkets.com/reports/2640803/ Multiple Sclerosis Therapeutics to 2019 - Treatment Diversification, Increasing Efficacy, and Pipeline Innovation Combine
Medication Policy Manual. Topic: Gilenya, fingolimod Date of Origin: November 22, 2010
 Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
SECTION 2. Section 2 Multiple Sclerosis (MS) Drug Coverage
 SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON
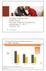 Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
PharmaPoint: Multiple Sclerosis - United Kingdom Drug Forecast and Market Analysis to 2022. Multiple
 Brochure More information from http://www.researchandmarkets.com/reports/2541548/ PharmaPoint: Multiple Sclerosis - United Kingdom Drug Forecast and Market Analysis to 2022 Description: PharmaPoint: Multiple
Brochure More information from http://www.researchandmarkets.com/reports/2541548/ PharmaPoint: Multiple Sclerosis - United Kingdom Drug Forecast and Market Analysis to 2022 Description: PharmaPoint: Multiple
What is MS? 1. disease that affects the central nervous. Is a disease that affects both white and gray matter
 What is MS? 1 Neuron Damaged myelin due to inflammation MS is a chronic immunemediated disease that affects the central nervous system (CNS) Is a disease that affects both white and gray matter Interrupted
What is MS? 1 Neuron Damaged myelin due to inflammation MS is a chronic immunemediated disease that affects the central nervous system (CNS) Is a disease that affects both white and gray matter Interrupted
How To Use A Drug In Multiple Sclerosis
 Revised (2009) guidelines for prescribing in multiple sclerosis INTRODUCTION In January 2001, the (ABN) first published guidelines for the use of licensed disease modifying treatments (ß-interferon and
Revised (2009) guidelines for prescribing in multiple sclerosis INTRODUCTION In January 2001, the (ABN) first published guidelines for the use of licensed disease modifying treatments (ß-interferon and
Best practices for using MS disease modifying therapies
 Best practices for using MS disease modifying therapies CMSC Annual Meeting 2015 COREY C FORD, MD, PHD MS SPECIALTY CLINIC UNIVERSITY OF NEW MEXICO HSC MAY 30, 2015 Objectives Use best practices to select
Best practices for using MS disease modifying therapies CMSC Annual Meeting 2015 COREY C FORD, MD, PHD MS SPECIALTY CLINIC UNIVERSITY OF NEW MEXICO HSC MAY 30, 2015 Objectives Use best practices to select
A neurologist would assess your eligibility and suitability for the DMTs.
 Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Multiple Sclerosis. Current and Future Players. GDHC1009FPR/ Published March 2013
 Multiple Sclerosis Current and Future Players GDHC1009FPR/ Published March 2013 Executive Summary Moderate Growth in the Multiple Sclerosis Market is Expected from 2012 2022 GlobalData estimates the 2012
Multiple Sclerosis Current and Future Players GDHC1009FPR/ Published March 2013 Executive Summary Moderate Growth in the Multiple Sclerosis Market is Expected from 2012 2022 GlobalData estimates the 2012
Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy
 Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy Klaus Schmierer, PhD FRCP Blizard Institute, Barts and The London School of Medicine & Dentistry Barts Health
Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy Klaus Schmierer, PhD FRCP Blizard Institute, Barts and The London School of Medicine & Dentistry Barts Health
Disclosures. Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
 Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
ß-interferon and. ABN Guidelines for 2007 Treatment of Multiple Sclerosis with. Glatiramer Acetate
 ABN Guidelines for 2007 Treatment of Multiple Sclerosis with ß-interferon and Glatiramer Acetate Published by the Association of British Neurologists Ormond House, 27 Boswell Street, London WC1N 3JZ Contents
ABN Guidelines for 2007 Treatment of Multiple Sclerosis with ß-interferon and Glatiramer Acetate Published by the Association of British Neurologists Ormond House, 27 Boswell Street, London WC1N 3JZ Contents
Multiple Sclerosis Drug Discoveries - What the Future Holds
 Brochure More information from http://www.researchandmarkets.com/reports/1408035/ Multiple Sclerosis Drug Discoveries - What the Future Holds Description: The recent approval in the US of Novartis' orally
Brochure More information from http://www.researchandmarkets.com/reports/1408035/ Multiple Sclerosis Drug Discoveries - What the Future Holds Description: The recent approval in the US of Novartis' orally
New treatments in MS What s here and what s nearly here
 5 th MS Research Day, June 14 th 2014 New treatments in MS What s here and what s nearly here David Miller Queen Square MS Centre at UCL and UCLH Course of MS and its treatment Relapsing remitting Disability
5 th MS Research Day, June 14 th 2014 New treatments in MS What s here and what s nearly here David Miller Queen Square MS Centre at UCL and UCLH Course of MS and its treatment Relapsing remitting Disability
How to S.E.A.R.C.H. SM for the Right MS Therapy For You!
 How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
A blood sample will be collected annually for up to 2 years for JCV antibody testing.
 Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Product Profiles: Multiple Sclerosis - Gilenya Raises Bar for New Market Entrants
 Brochure More information from http://www.researchandmarkets.com/reports/1841621/ Product Profiles: Multiple Sclerosis - Gilenya Raises Bar for New Market Entrants Description: Introduction Beginning with
Brochure More information from http://www.researchandmarkets.com/reports/1841621/ Product Profiles: Multiple Sclerosis - Gilenya Raises Bar for New Market Entrants Description: Introduction Beginning with
Study Support Materials Cover Sheet
 Study Support Materials Cover Sheet Document Title ESCALATE Patient Brochure Intended Audience This brochure is designed to be given to potentially eligible patients as a take-home summary of key information
Study Support Materials Cover Sheet Document Title ESCALATE Patient Brochure Intended Audience This brochure is designed to be given to potentially eligible patients as a take-home summary of key information
J.P. Morgan Cazenove Therapeutic Seminar
 Jannan, MS J.P. Morgan Cazenove Therapeutic Seminar David Meeker - CEO, Genzyme June 25, 2012 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private
Jannan, MS J.P. Morgan Cazenove Therapeutic Seminar David Meeker - CEO, Genzyme June 25, 2012 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private
Managing Relapsing Remitting MS Risks & benefits of emerging therapies. Dr Mike Boggild The Walton Centre
 Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
Which injectable medication should I take for relapsing-remitting multiple sclerosis?
 Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
A Letter From the MS Coalition
 0 A Letter From the MS Coalition The treatment of multiple sclerosis (MS) requires a comprehensive management strategy. One important component of that strategy is modifying the disease course. When deciding
0 A Letter From the MS Coalition The treatment of multiple sclerosis (MS) requires a comprehensive management strategy. One important component of that strategy is modifying the disease course. When deciding
Use of Observa,onal Data to Make Causal Inferences About Treatment Decisions in Mul,ple Sclerosis. Brian Healy, PhD
 Use of Observa,onal Data to Make Causal Inferences About Treatment Decisions in Mul,ple Sclerosis Brian Healy, PhD Disclosures n I receive research support from Merck Serono and Novar,s Outline n Background
Use of Observa,onal Data to Make Causal Inferences About Treatment Decisions in Mul,ple Sclerosis Brian Healy, PhD Disclosures n I receive research support from Merck Serono and Novar,s Outline n Background
What is Multiple Sclerosis? Gener al information
 What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
New Treatment Options for MS Patients: Understanding risks versus benefits
 New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
Multiple Sclerosis: An imaging review and update on new treatments.
 Multiple Sclerosis: An imaging review and update on new treatments. Dr Marcus Likeman Consultant Neuroradiologist North Bristol NHS Trust Bristol Royal Hospital for Children MRI appearances - White Matter
Multiple Sclerosis: An imaging review and update on new treatments. Dr Marcus Likeman Consultant Neuroradiologist North Bristol NHS Trust Bristol Royal Hospital for Children MRI appearances - White Matter
Multiple Sclerosis. Global Drug Forecast and Market Analysis to 2022 Event-Driven Update. GDHC34PIDR / Published April 2013
 Multiple Sclerosis Global Drug Forecast and Market Analysis to 2022 Event-Driven Update GDHC34PIDR / Published April 2013 Executive Summary The table below presents the key metrics for multiple sclerosis
Multiple Sclerosis Global Drug Forecast and Market Analysis to 2022 Event-Driven Update GDHC34PIDR / Published April 2013 Executive Summary The table below presents the key metrics for multiple sclerosis
Multiple Sclerosis (MS) Class Update
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
PCORI Workshop on Treatment for Multiple Sclerosis. Breakout Group Topics and Questions Draft 3-27-15
 PCORI Workshop on Treatment for Multiple Sclerosis Breakout Group Topics and Questions Draft 3-27-15 Group 1 - Comparison across DMTs, including differential effects in subgroups Consolidated straw man
PCORI Workshop on Treatment for Multiple Sclerosis Breakout Group Topics and Questions Draft 3-27-15 Group 1 - Comparison across DMTs, including differential effects in subgroups Consolidated straw man
A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market
 A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market Launch of Several Pipeline Oral Products Could Diminish the Market for Injectable Therapies NEC3-52 November 2014 Contents
A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market Launch of Several Pipeline Oral Products Could Diminish the Market for Injectable Therapies NEC3-52 November 2014 Contents
NHS BOURNEMOUTH AND POOLE AND NHS DORSET
 NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
New Developments in the Treatment and Management of Multiple Sclerosis
 New Developments in the Treatment and Management of Multiple Sclerosis Myla D. Goldman, MD, MS For a CME/CEU version of this article, please go to www.namcp.org/cmeonline.htm, and then click the activity
New Developments in the Treatment and Management of Multiple Sclerosis Myla D. Goldman, MD, MS For a CME/CEU version of this article, please go to www.namcp.org/cmeonline.htm, and then click the activity
acquired chronic immune-mediated inflammatory condition of CNS. MS in children: 10% +secondary progressive MS: rare +primary progressive MS: rare
 Immunomodulatory Therapies in Pediatric MS Vuong Chinh Quyen Neurology Department Medscape Mar 8, 2013 Multiple Sclerosis in Children. Iran J Child Neurol. 2013 Spring Introduction acquired chronic immune-mediated
Immunomodulatory Therapies in Pediatric MS Vuong Chinh Quyen Neurology Department Medscape Mar 8, 2013 Multiple Sclerosis in Children. Iran J Child Neurol. 2013 Spring Introduction acquired chronic immune-mediated
06/06/2012. The Impact of Multiple Sclerosis in the Pacific Northwest. James Bowen, MD. Swedish Neuroscience Institute
 The Impact of Multiple Sclerosis in the Pacific Northwest James Bowen, MD Multiple Sclerosis Center Multiple Sclerosis Center Swedish Neuroscience Institute 1 2 Motor Symptoms of MS Weakness Spasticity
The Impact of Multiple Sclerosis in the Pacific Northwest James Bowen, MD Multiple Sclerosis Center Multiple Sclerosis Center Swedish Neuroscience Institute 1 2 Motor Symptoms of MS Weakness Spasticity
Review Date: March 2012. Issue Status: Approved Issue No: 2 Issue Date: March 2010
 Title: Multiple Sclerosis guidelines for the use of beta-interferon, glatiramer acetate, natalizumab, mitoxantrone and other disease Authors Name: Dr P Talbot Contact Name: Dr Paul Talbot Contact Phone
Title: Multiple Sclerosis guidelines for the use of beta-interferon, glatiramer acetate, natalizumab, mitoxantrone and other disease Authors Name: Dr P Talbot Contact Name: Dr Paul Talbot Contact Phone
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru381 Topic: Lemtrada TM, alemtuzumab Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru381 Topic: Lemtrada TM, alemtuzumab Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
MSTAC Initial Application
 MSTAC Initial Application Please send applications to: Facsimile 04 916 7571 Further Contact Details: Address The Co-ordinator MSTAC PHARMAC P O Box 10-254 WELLINGTON Phone 04 460 4990 Email [email protected]
MSTAC Initial Application Please send applications to: Facsimile 04 916 7571 Further Contact Details: Address The Co-ordinator MSTAC PHARMAC P O Box 10-254 WELLINGTON Phone 04 460 4990 Email [email protected]
Literature Scan: Oral Multiple Sclerosis Drugs
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Published by MSAA in February 2014
 Published by MSAA in February 2014 VITAMIN D MSAA s MS Research Update is published annually as a service to the MS community. This update provides an overview of the research behind the approved and experimental
Published by MSAA in February 2014 VITAMIN D MSAA s MS Research Update is published annually as a service to the MS community. This update provides an overview of the research behind the approved and experimental
Recent Advances in the Treatment & Management of Multiple Sclerosis
 Recent Advances in the Treatment & Management of Multiple Sclerosis Consultant: AbbVie, Accordant, Acorda, Bayer, Biogen, Genentech/Roche, Genzyme/Sanofi, Mylan, Novartis, Serono, Teva Research: Actelion,
Recent Advances in the Treatment & Management of Multiple Sclerosis Consultant: AbbVie, Accordant, Acorda, Bayer, Biogen, Genentech/Roche, Genzyme/Sanofi, Mylan, Novartis, Serono, Teva Research: Actelion,
MULTIPLE SCLEROSIS 2015. Mercedes P Jacobson, MD, Department of Neurology Temple University School of Medicine
 MULTIPLE SCLEROSIS 2015 Mercedes P Jacobson, MD, Department of Neurology Temple University School of Medicine Disclosure Research support, Sunovion and Marinus for epilepsy research Goals and Objectives
MULTIPLE SCLEROSIS 2015 Mercedes P Jacobson, MD, Department of Neurology Temple University School of Medicine Disclosure Research support, Sunovion and Marinus for epilepsy research Goals and Objectives
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
News on modifying diseases therapies. Michel CLANET CHU Toulouse France ECTRIMS
 News on modifying diseases therapies Michel CLANET CHU Toulouse France ECTRIMS Current treatment strategies Future oral treatments Future non oral treatments Drug safety and risks CIS at risk of MS Active
News on modifying diseases therapies Michel CLANET CHU Toulouse France ECTRIMS Current treatment strategies Future oral treatments Future non oral treatments Drug safety and risks CIS at risk of MS Active
Therapeutic Class Overview Multiple Sclerosis Agents
 Therapeutic Class Overview Multiple Sclerosis Agents Therapeutic Class Overview/Summary: Several biologic response modifiers are Food and Drug Administration (FDA)- approved for the treatment of relapsing-remitting
Therapeutic Class Overview Multiple Sclerosis Agents Therapeutic Class Overview/Summary: Several biologic response modifiers are Food and Drug Administration (FDA)- approved for the treatment of relapsing-remitting
What is Multiple Sclerosis? Disease Modifying Therapies. Best of all. Why is treatment so important? Outline and Expectations.
 What is Multiple Sclerosis? Disease Modifying Therapies for Relapsing Remitting MS Jeanie Lynn Cote, MD Chronic, auto immune disorder. As a consequence of immune attacks, there is loss of myelin (fatty
What is Multiple Sclerosis? Disease Modifying Therapies for Relapsing Remitting MS Jeanie Lynn Cote, MD Chronic, auto immune disorder. As a consequence of immune attacks, there is loss of myelin (fatty
Multiple Sclerosis in Practice. An Expert Commentary With Jeffrey Cohen, MD, PhD A Clinical Context Report
 Multiple Sclerosis in Practice An Expert Commentary With Jeffrey Cohen, MD, PhD A Clinical Context Report Clinical Context: Multiple Sclerosis in Practice Expert Commentary Jointly Sponsored by: and Clinical
Multiple Sclerosis in Practice An Expert Commentary With Jeffrey Cohen, MD, PhD A Clinical Context Report Clinical Context: Multiple Sclerosis in Practice Expert Commentary Jointly Sponsored by: and Clinical
Patient Group Input to CADTH
 Patient Group Input to CADTH Section 1 General Information Name of the drug CADTH is reviewing and indication(s) of interest Name of patient group/author of submission Patient group s contact information:
Patient Group Input to CADTH Section 1 General Information Name of the drug CADTH is reviewing and indication(s) of interest Name of patient group/author of submission Patient group s contact information:
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
Decisions relating to Multiple Sclerosis treatments
 10 October 2014 Decisions relating to Multiple Sclerosis treatments PHARMAC is pleased to announce that, from 1 November 2014, it will: list fingolimod (Gilenya); list natalizumab (Tysabri); and change
10 October 2014 Decisions relating to Multiple Sclerosis treatments PHARMAC is pleased to announce that, from 1 November 2014, it will: list fingolimod (Gilenya); list natalizumab (Tysabri); and change
Putting the Cart Back Behind the Horse: Converting a population based research database into an electronic clinical patient record
 Putting the Cart Back Behind the Horse: Converting a population based research database into an electronic clinical patient record The Story Crash course on Multiple Sclerosis A little bit of History of
Putting the Cart Back Behind the Horse: Converting a population based research database into an electronic clinical patient record The Story Crash course on Multiple Sclerosis A little bit of History of
CNS DEMYLINATING DISORDERS
 CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
Clinical Commissioning Policy: Disease Modifying Therapies For patients With Multiple Sclerosis (MS) December 2012. Reference : NHSCB/D4/c/1
 Clinical Commissioning Policy: Disease Modifying Therapies For patients With Multiple Sclerosis (MS) December 2012 Reference : NHSCB/D4/c/1 NHS Commissioning Board Clinical Commissioning Policy: Disease
Clinical Commissioning Policy: Disease Modifying Therapies For patients With Multiple Sclerosis (MS) December 2012 Reference : NHSCB/D4/c/1 NHS Commissioning Board Clinical Commissioning Policy: Disease
Multiple Sclerosis: What You Need To Know. For Professionals
 Multiple Sclerosis: What You Need To Know For Professionals What will I learn today? The Basics: What is MS? Living with MS: A Family Affair We Can Help: The National MS Society What MS Is: MS is thought
Multiple Sclerosis: What You Need To Know For Professionals What will I learn today? The Basics: What is MS? Living with MS: A Family Affair We Can Help: The National MS Society What MS Is: MS is thought
The MS Disease- Modifying Medications GENERAL INFORMATION
 The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
Rational basis for early treatment in MS. Bonaventura Casanova Estruch Unitat d Esclerosi Múltiple Hospital Universitari la Fe València
 Rational basis for early treatment in MS Bonaventura Casanova Estruch Unitat d Esclerosi Múltiple Hospital Universitari la Fe València Bonaventura Casanova Department of Neurology University Hospital La
Rational basis for early treatment in MS Bonaventura Casanova Estruch Unitat d Esclerosi Múltiple Hospital Universitari la Fe València Bonaventura Casanova Department of Neurology University Hospital La
Current and future options of MS treatment Prof. Dr. Karl Vass, AKH Wien
 Current and future options of MS treatment Prof. Dr. Karl Vass, AKH Wien European Health Forum, Gastein 6 th October 2010 Multiple Sclerosis is the most common neurological disorder in young Caucasian
Current and future options of MS treatment Prof. Dr. Karl Vass, AKH Wien European Health Forum, Gastein 6 th October 2010 Multiple Sclerosis is the most common neurological disorder in young Caucasian
Disclosure Statement. Multiple Sclerosis: Current Trends in Treatment. Epidemiology of MS. Multiple Sclerosis. Viral Link to MS.
 Disclosure Statement Multiple Sclerosis: Current Trends in Treatment Member of Speaker s Bureau Biogen Idec Will discuss non FDA approved therapies Christine St Laurent MSN, RN, MSCN 19 th Annual Mud Season
Disclosure Statement Multiple Sclerosis: Current Trends in Treatment Member of Speaker s Bureau Biogen Idec Will discuss non FDA approved therapies Christine St Laurent MSN, RN, MSCN 19 th Annual Mud Season
RELAPSE MANAGEMENT. Pauline Shaw MS Nurse Specialist 25 th June 2010
 RELAPSE MANAGEMENT Pauline Shaw MS Nurse Specialist 25 th June 2010 AIMS OF SESSION Relapsing/Remitting MS Definition of relapse/relapse rate Relapse Management NICE Guidelines Regional Clinical Guidelines
RELAPSE MANAGEMENT Pauline Shaw MS Nurse Specialist 25 th June 2010 AIMS OF SESSION Relapsing/Remitting MS Definition of relapse/relapse rate Relapse Management NICE Guidelines Regional Clinical Guidelines
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio
 ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents
 New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
Pharmacotherapy of Multiple Sclerosis
 PHARMACY / MEDICAL POLICY 5.01.565 Pharmacotherapy of Multiple Sclerosis Effective Date: July 1, 2016 Last Revised: June 14, 2016 Replaces: Extracted from 5.01.550 RELATED MEDICAL POLICIES: 5.01.556 Rituximab:
PHARMACY / MEDICAL POLICY 5.01.565 Pharmacotherapy of Multiple Sclerosis Effective Date: July 1, 2016 Last Revised: June 14, 2016 Replaces: Extracted from 5.01.550 RELATED MEDICAL POLICIES: 5.01.556 Rituximab:
Is the Grass Really Greener with New Oral Multiple Sclerosis Treatments? A Clinical Debate
 Is the Grass Really Greener with New Oral Multiple Sclerosis Treatments? A Clinical Debate Elizabeth Sebranek Evans, PharmD, BCPS, CGP Alana Whittaker, PharmD, BCPS Roseman University of Health Sciences
Is the Grass Really Greener with New Oral Multiple Sclerosis Treatments? A Clinical Debate Elizabeth Sebranek Evans, PharmD, BCPS, CGP Alana Whittaker, PharmD, BCPS Roseman University of Health Sciences
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
Information about medicines for multiple sclerosis
 Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Uncertainty in Benefit and Risk: Tysabri (natalizumab)
 Uncertainty in Benefit and Risk: Tysabri (natalizumab) Robert J. Temple, M.D. Deputy Center Director for Clinical Science Center for Drug Evaluation and Research U.S. Food and Drug Administration IOM Drug
Uncertainty in Benefit and Risk: Tysabri (natalizumab) Robert J. Temple, M.D. Deputy Center Director for Clinical Science Center for Drug Evaluation and Research U.S. Food and Drug Administration IOM Drug
Disease modifying drug therapy
 Disease modifying drug therapy New edition for 2014-15 We hope you find the information in this book helpful. If you would like to speak with someone about any aspect of MS, contact the MS Trust information
Disease modifying drug therapy New edition for 2014-15 We hope you find the information in this book helpful. If you would like to speak with someone about any aspect of MS, contact the MS Trust information
National Multiple Sclerosis Society. Disease Modification in Multiple Sclerosis. Current as of January 2, 2013
 National Multiple Sclerosis Society Disease Modification in Multiple Sclerosis Current as of January 2, 2013 Since 1993, the U.S. Food and Drug Administration (FDA) has approved several medications for
National Multiple Sclerosis Society Disease Modification in Multiple Sclerosis Current as of January 2, 2013 Since 1993, the U.S. Food and Drug Administration (FDA) has approved several medications for
Drug Class Review. Disease-modifying Drugs for Multiple Sclerosis. Single Drug Addendum: Fingolimod
 Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
