How to S.E.A.R.C.H. SM for the Right MS Therapy For You!
|
|
|
- Brent Chandler
- 10 years ago
- Views:
Transcription
1 How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration (FDA) in This forever changed the landscape of how MS could be managed. The approval of Betaseron (interferon beta-1b) for RRMS ushered in a remarkable surge of MS treatments designed to reduce the number and severity of exacerbations and help lessen disease progression. Throughout the 1990s and into the 2000s, several effective medications for MS have become available, giving neurologists and patients a variety of treatment options for slowing disease activity. Along with these treatments, known as disease-modifying therapies (DMTs), came the expanded use and improved technology of magnetic resonance imaging (MRI). Through diagnostic and follow-up MRI scans of the brain and spinal cord, physicians could now better diagnose, treat, track and manage the ever-changing course of MS in a more definitive and proactive manner. As the recommendation and usage of MRIs grew, MSAA recognized the need to assist MS patients who required these valuable tests but lacked insurance coverage or the financial means to pay for the exams. As a result, MSAA developed and implemented the MRI Diagnostic Fund and MRI Institute programs. To date, these two programs have combined to serve nearly 5,000 MS clients who have benefited from an initial or follow-up MRI exam. For more details on these two programs, please visit the MSAA website, or call (800) , ext Advances in MRI techniques, along with years of consistent research data, have demonstrated that most patients who begin and maintain a DMT will experience fewer active lesions on the brain and spinal cord, fewer and less severe exacerbations, a reduction in symptoms, and a delay in disease progression and disability. In addition, more recent clinical trials have found that many of these DMTs also delay time to a second MS-like event, in cases of clinically isolated syndrome (CIS). CIS refers to the first presenting symptom of MS, prior to a confirmed diagnosis. These impressive results led the MS medical community to universally adopt and support the position of treating MS with approved DMTs as early as possible and for patients to maintain adherence. Since the mid-2000s, the issue of treatment adherence has been aggressively advocated by leaders in the MS medical and healthcare communities, including MSAA. 1
2 Framing the Discussion Supporting the critically important message for patients to begin and stay on an MS therapy, MSAA launched a national public awareness campaign in 2007 titled, These Treatments Work, Let Them Work for You. The campaign featured brochures, articles and other print materials distributed to MS patients and MS centers across the country. It also included a series of radio and television public service announcements and airport dioramas. Over the last few years, this national initiative has been widely successful, generating nearly 70,000 broadcasts and $6 million of free radio and television airtime to support the message of treatment adherence. While the issue of treatment adherence continues to gain awareness and momentum, MSAA also recognizes the complexity of the situation. Healthcare providers continue to encourage their patients to become more health literate and to take an active, decisionmaking role in selecting a treatment. In doing so, an extraordinary number of factors need to be considered when choosing an appropriate MS therapy or switching from one DMT to another. Among the numerous questions to consider include: What are the therapies? Am I a candidate? What should I know about each one? How will my body react to taking one of these medications? How are the different medications administered? What about the costs or insurance? Once I have begun taking a DMT, how do I know if the one I am prescribed is working? These and other important considerations require ongoing conversations with your doctor and other healthcare professionals. The treatment decision for each patient is unique and must be addressed individually between the person and his or her healthcare team. Additionally, patients must recognize the need to prioritize their issues, questions, and concerns in order to maximize the time with their doctor and healthcare team. With so much information to remember, organize, and prioritize, MSAA recognized the need to help frame these important discussions. By doing so, MSAA is able to support patients and their physicians in their S.E.A.R.C.H. SM for the most appropriate therapy for each individual. What is S.E.A.R.C.H. SM? Designed as a memory aid, the S.E.A.R.C.H. SM acronym represents the key areas that should be considered when searching for the most appropriate MS treatment. Each letter represents an important topic that must be considered by patients, physicians, and other healthcare and social service professionals. S.E.A.R.C.H. SM stands for: S. = Safety E. = Effectiveness A. = Affordability R. = Risks C. = Convenience H. = Health Outcomes (overall wellness and quality of life) 2
3 The S.E.A.R.C.H. SM acronym is not only a useful tool to help frame and remember these important issues, but gives patients a way to start the conversation with their healthcare team, explains MSAA President and CEO Doug Franklin. Our goal is to foster a positive doctor-patient relationship and allow the dialog to take its own course. MSAA recognizes that MS is a uniquely individual disease that affects each person differently. We are not advocating any one treatment or approach, but rather looking to help guide the conversation between patients and their medical team toward issues that matter most. To assist with this conversation, MSAA has prepared a sampling of key questions within each aspect of S.E.A.R.C.H. SM These questions represent a broad overview of many different factors to consider and investigate. They also allow the flexibility for patients to adapt their specific medical history, current disease state, experiences and other physical, emotional, and financial aspects into the decision-making process. The S.E.A.R.C.H. SM Questions MSAA has developed the following S.E.A.R.C.H. SM questions to serve as a sample, or guide, for you to consider when evaluating your own healthcare needs. These S.E.A.R.C.H. SM questions merely reflect a starting point to help you think about your own medical situation, issues to prioritize, and ways to develop questions which address your specific healthcare needs. When using the S.E.A.R.C.H. SM model, it is also important to recognize that reviewing key topics and questions will likely require more than one office visit with members of your healthcare team. The S.E.A.R.C.H. SM framework can also be helpful when conducting your own research before or after visiting your healthcare provider. Please see a comprehensive resource guide at the end of this article. Safety What are the long-term safety profiles of these FDA-approved MS disease modifying therapies (DMTs)? What tests are required prior to taking DMTs? What tests are required while receiving DMTs? How will DMTs interact with my current medical treatments, other medical conditions, and any complementary and alternative medicines? Effectiveness How effective are these DMTs in reducing MS relapses, disability, and MRI activity? What are my realistic expectations regarding the effectiveness of these DMTs? How can I tell if my DMT is working? 3
4 Affordability (These questions could be directed to other healthcare team members including your social worker, insurance representative, MS organization, etc.) What are the costs and insurance coverage for these DMTs? Does the insurance coverage have caps, gaps or limitations? Are there assistance programs through the pharmaceutical companies, government, or charities? Risks What are the risks of side effects associated with these DMTs? How frequent and severe are the side effects? How soon do they subside? Can these side effects be managed, and if so, how? Convenience How are the DMTs administered? How often do I take these DMTs? Must I have regular tests or visits to other healthcare providers to monitor the effects of my treatment? Health Outcomes How will my general health and quality of life be affected by these DMTs? Will taking a DMT lower my immune system and cause other problems? Can these DMTs assist with my mobility, cognition, and other health factors? Maximizing S.E.A.R.C.H. SM As mentioned in the beginning of this article, the MS landscape has dramatically changed over the past two decades. With the recent introduction of an oral medication, and with new investigational drugs nearing completion of their trials, changes in this landscape continue to evolve at a rapid pace. Much like the design of a Global Positioning System (GPS), MS patients and their physicians can employ the S.E.A.R.C.H. SM model to navigate through this dynamic, ever-changing landscape to reach their desired destination. Also, similar to a GPS s feature to recalculate direction, patients can continue to utilize the S.E.A.R.C.H. SM tool to recalculate their decisions and adjust treatments if necessary in order to optimize health outcomes. Another way to derive maximum benefit from S.E.A.R.C.H. SM is to use it as a time saver. Unfortunately, doctors today face an increasing workload of patients, restrictive managed-care regulations, and other factors that prevent many physicians from spending as much time with their patients as they were able to do in the past. The reality of these brief and often rushed doctor visits can leave both the patient and physician feeling dissatisfied with the outcome and searching for a better way to manage their time. 4
5 As a neurologist, I find tremendous value in the S.E.A.R.C.H. SM model because it brings to light the key issues involved in treating MS in a way that focuses the conversation, explains MSAA Chief Medical Officer Dr. Jack Burks. Patients can present their questions and concerns in a clear cut, easy, and efficient way. I see S.E.A.R.C.H. SM as a template from which patients can choose the issues most important to them. Patients sometimes call or revisit me after an appointment with additional questions that they forgot to ask. S.E.A.R.C.H. SM will effectively reduce the time needed to cover the important topics. This will give patients more confidence in their medical decisions. The S.E.A.R.C.H. SM Toolkit In addition to this article, MSAA has produced a variety of informational tools to help people maximum their success with S.E.A.R.C.H. SM The S.E.A.R.C.H. SM tools are available as PDF documents at The first tool is a laminated wallet-size, reference card which includes the six key elements of S.E.A.R.C.H. SM. Designed as a simple guide that is convenient to carry and readily available, this foursided card provides a basic explanation of S.E.A.R.C.H. SM and offers suggested questions to begin the conversation with your healthcare team. As a secondary and more comprehensive tool to help organize and manage the many aspects of S.E.A.R.C.H. SM, MSAA has created a very useful patient workbook. The MSAA S.E.A.R.C.H. SM Patient Workbook serves as an effective tool to help you research, collect, organize, and store information about your decision to start an MS disease modifying therapy or re-evaluate your current treatment options. With so much information to manage, this Workbook offers you a convenient way to journal and maintain accurate notes on research information, key questions and answers from your healthcare providers, and additional resources. The Workbook concludes with a office visit questionnaire which serves as a guide to help prioritize your S.E.A.R.C.H. SM questions and maximize your office visit. As the S.E.A.R.C.H. SM campaign begins to gain awareness and momentum, MSAA plans to roll out additional activities and tools including an on-demand educational video, live webcasts, a series of in-person public education programs, a smart-phone application, and the development of additional support materials for healthcare professionals and patients. Treatment Chart Below please see an easy-to-follow reference chart on the currently approved and available MS disease modifying therapies. This chart does not address the issues of efficacy, safety, and risk. All of the disease-modifying therapies for MS have different benefits and risks. The effectiveness and side effects of each drug may vary from one patient to another. Additionally, patients who do not respond well to one DMT may benefit by switching to a different treatment. Individuals need to consult with their healthcare team to determine which treatment might be the best option for them. 5
6 Currently Approved MS Treatments DRUG FDA APPROVAL MECHANISM OF ACTION ADMINISTERED Avonex (interferon beta-1a) Biogen Idec MS in 1996 and for individuals with clinically isolated syndrome (CIS). Avonex is an interferon. Interferons appear to reduce inflammation by modulating a favorable balance between cells that increase inflammation and cells that decrease it. 30 micrograms taken via weekly intermuscular injections Betaseron (interferon beta-1b) Bayer Healthcare Pharmaceuticals MS in 1993 and for individuals with clinically isolated syndrome (CIS). Betaseron is an interferon. Interferons appear to reduce inflammation by modulating a favorable balance between cells that increase inflammation and cells that decrease it. 250 micrograms taken via subcutaneous injections every other day Copaxone (glatiramer acetate) Teva Neuroscience MS in 1996 and for individuals with clinically isolated syndrome (CIS). Copaxone is a synthetic polypeptide that mimics myelin basic protein, a key component of the myelin sheath that is damaged in MS. By a different mechanism of action than the interferons, Copaxone also appears to reduce inflammation by modulating a favorable balance between cells that increase inflammation and cells that decrease it. 20 milligrams taken via daily subcutaneous injections Extavia (interferon beta-1b) Novartis Pharmaceuticals Corp. MS in 2010 and for individuals with CIS. Extavia is an interferon beta-1b that is biologically identical to Betaseron and made in an identical process, but marketed by a different company. 250 micrograms taken via subcutaneous injections every other day Gilenya (fingolimod, FTY720) Novartis Pharmaceuticals Corp MS in 2010 Gilenya blocks potentially damaging T cells from leaving lymph nodes, thereby lowering their number in the blood, central nervous system and tissues. First oral DMT for MS. 0.5 mg capsule taken orally once per day Novantrone (mitoxantrone) EMD Serono, Inc. Approved for use in secondaryprogressive MS (SPMS), progressive-relapsing MS (PRMS) and worsening RRMS in 2000 Novantrone is an immunosuppressant that has been used for years to treat cancer. It targets rapidly dividing cells, including those believed to be involved in MS. IV infusion once every 3 months (for two to three years maximum). 12 mg/m2 approximately 5 to 15 minutes Rebif (interferon beta-1a) Parent companies: EMD Serono, Inc. and Pfizer Inc. MS in Rebif is an interferon. Interferons appear to reduce inflammation by modulating a favorable balance between cells that increase inflammation and cells that decrease it. 44 micrograms taken via subcutaneous injections three times weekly Tysabri (natalizumab) Parent companies: Biogen Idec and Elan Pharmaceuticals MS in 2006 This laboratory-produced monoclonal antibody acts against a molecule involved in the activation and function of lymphocytes and their migration into the central nervous system (CNS). It is thought to prevent damaging immune cells from crossing the blood-brain barrier. IV infusion every four weeks 300 milligrams (mg) over 1 hour 6
7 MS Resource Guide MSAA: For more information on FDA-approved therapies, symptom management treatments, and MSAA programs and services, please access additional sections of this website or contact MSAA at (800) or MS Coalition: The MS Coalition is a collaborative network of independent MS organizations. The MS Coalition's mission is to increase opportunities for cooperation and provide greater opportunity to leverage the effective use of resources for the benefit of the MS community. Please visit: In addition to MSAA, the MS Coalition members include: (listed alphabetically) Accelerated Cure Project for Multiple Sclerosis Phone: (781) ; Website: Consortium of Multiple Sclerosis Centers (CMSC) Phone: (201) ; Website: or Can Do Multiple Sclerosis Phone: (800) ; Website: International Organization of Multiple Sclerosis Nurses Phone: (201) ; Website: Multiple Sclerosis Foundation Phone: (800) ; Website: National Multiple Sclerosis Society Phone: (800) ; Website: United Spinal Association Phone: (718) ; Website: Assistance Programs of Approved MS Therapies: The following pharmaceutical companies offer patient programs to provide information, instruction, and resources for advocacy and financial assistance. (listed alphabetically) Avonex - MS ActiveSource (800) Betaseron - Betaplus MS Support (800)
8 Copaxone - Shared Solutions (800) Extavia Patient Support Program (866) Gilenya Patient Support Program (877) Rebif - MS LifeLines (877) Novantrone - MS LifeLines (877) Tysabri (800) The MSAA S.E.A.R.C.H. SM initiative is made possible through unrestricted educational grants from Bayer Healthcare Pharmaceuticals, Biogen Idec, and Teva Neuroscience. MSAA is solely responsible for the development of S.E.A.R.C.H. SM and its content. 8
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
National Multiple Sclerosis Society. Disease Modification in Multiple Sclerosis. Current as of January 2, 2013
 National Multiple Sclerosis Society Disease Modification in Multiple Sclerosis Current as of January 2, 2013 Since 1993, the U.S. Food and Drug Administration (FDA) has approved several medications for
National Multiple Sclerosis Society Disease Modification in Multiple Sclerosis Current as of January 2, 2013 Since 1993, the U.S. Food and Drug Administration (FDA) has approved several medications for
Study Support Materials Cover Sheet
 Study Support Materials Cover Sheet Document Title ESCALATE Patient Brochure Intended Audience This brochure is designed to be given to potentially eligible patients as a take-home summary of key information
Study Support Materials Cover Sheet Document Title ESCALATE Patient Brochure Intended Audience This brochure is designed to be given to potentially eligible patients as a take-home summary of key information
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
What is Multiple Sclerosis? Gener al information
 What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
Multiple Sclerosis Drug Discoveries - What the Future Holds
 Brochure More information from http://www.researchandmarkets.com/reports/1408035/ Multiple Sclerosis Drug Discoveries - What the Future Holds Description: The recent approval in the US of Novartis' orally
Brochure More information from http://www.researchandmarkets.com/reports/1408035/ Multiple Sclerosis Drug Discoveries - What the Future Holds Description: The recent approval in the US of Novartis' orally
The MS Disease- Modifying Medications GENERAL INFORMATION
 The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
Medication Policy Manual. Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014
 Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual. Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004
 Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Multiple Sclerosis: What You Need To Know. For Professionals
 Multiple Sclerosis: What You Need To Know For Professionals What will I learn today? The Basics: What is MS? Living with MS: A Family Affair We Can Help: The National MS Society What MS Is: MS is thought
Multiple Sclerosis: What You Need To Know For Professionals What will I learn today? The Basics: What is MS? Living with MS: A Family Affair We Can Help: The National MS Society What MS Is: MS is thought
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
A neurologist would assess your eligibility and suitability for the DMTs.
 Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Relapsing-remitting multiple sclerosis Ambulatory with or without aid
 AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
Disease Modifying Therapies (DMTs) in Multiple Sclerosis
 Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Global Multiple Sclerosis Market: Trends and Opportunities (2013-18)
 Global Multiple Sclerosis Market: Trends and Opportunities (2013-18) Scope of the Report The report titled Global Multiple Sclerosis Market: Trends & Opportunities (2013-18) provides an insight into the
Global Multiple Sclerosis Market: Trends and Opportunities (2013-18) Scope of the Report The report titled Global Multiple Sclerosis Market: Trends & Opportunities (2013-18) provides an insight into the
Lemtrada (alemtuzumab)
 Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Progress in MS: Current and Emerging Therapies
 Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Growth in revenue from MS drugs has been driven largely by price increases over the last several years.
 March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
Multiple Sclerosis. Current and Future Players. GDHC1009FPR/ Published March 2013
 Multiple Sclerosis Current and Future Players GDHC1009FPR/ Published March 2013 Executive Summary Moderate Growth in the Multiple Sclerosis Market is Expected from 2012 2022 GlobalData estimates the 2012
Multiple Sclerosis Current and Future Players GDHC1009FPR/ Published March 2013 Executive Summary Moderate Growth in the Multiple Sclerosis Market is Expected from 2012 2022 GlobalData estimates the 2012
Patient Group Input to CADTH
 Patient Group Input to CADTH Section 1 General Information Name of the drug CADTH is reviewing and indication(s) of interest Name of patient group/author of submission Patient group s contact information:
Patient Group Input to CADTH Section 1 General Information Name of the drug CADTH is reviewing and indication(s) of interest Name of patient group/author of submission Patient group s contact information:
Multiple Sclerosis Therapeutics to 2019 - Treatment Diversification, Increasing Efficacy, and Pipeline Innovation Combine to Drive Growth
 Brochure More information from http://www.researchandmarkets.com/reports/2640803/ Multiple Sclerosis Therapeutics to 2019 - Treatment Diversification, Increasing Efficacy, and Pipeline Innovation Combine
Brochure More information from http://www.researchandmarkets.com/reports/2640803/ Multiple Sclerosis Therapeutics to 2019 - Treatment Diversification, Increasing Efficacy, and Pipeline Innovation Combine
The MS Disease- Modifying Medications
 The MS Disease- Modifying Medications National MS Society 1 Current as of January 2015. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
The MS Disease- Modifying Medications National MS Society 1 Current as of January 2015. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
Information About Medicines for Multiple Sclerosis
 Information About Medicines for Multiple Sclerosis Information About Medicines for Multiple Sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information About Medicines for Multiple Sclerosis Information About Medicines for Multiple Sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Multiple Sclerosis Update. Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center
 Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Published by MSAA in February 2014
 Published by MSAA in February 2014 VITAMIN D MSAA s MS Research Update is published annually as a service to the MS community. This update provides an overview of the research behind the approved and experimental
Published by MSAA in February 2014 VITAMIN D MSAA s MS Research Update is published annually as a service to the MS community. This update provides an overview of the research behind the approved and experimental
SECTION 2. Section 2 Multiple Sclerosis (MS) Drug Coverage
 SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
A Letter From the MS Coalition
 0 A Letter From the MS Coalition The treatment of multiple sclerosis (MS) requires a comprehensive management strategy. One important component of that strategy is modifying the disease course. When deciding
0 A Letter From the MS Coalition The treatment of multiple sclerosis (MS) requires a comprehensive management strategy. One important component of that strategy is modifying the disease course. When deciding
New Treatment Options for MS Patients: Understanding risks versus benefits
 New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
Medication Policy Manual. Topic: Gilenya, fingolimod Date of Origin: November 22, 2010
 Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Ontario Reimburses CIS Indication for REBIF, a First-Line Treatment for Multiple Sclerosis
 May 25, 2015 Contact: Shikha Virdi 905-919-0200 ext. 5504 Ontario Reimburses CIS Indication for REBIF, a First-Line Treatment for Multiple Sclerosis Rebif now reimbursed under Ontario Drug Benefit Program
May 25, 2015 Contact: Shikha Virdi 905-919-0200 ext. 5504 Ontario Reimburses CIS Indication for REBIF, a First-Line Treatment for Multiple Sclerosis Rebif now reimbursed under Ontario Drug Benefit Program
The rising cost of prescription drugs to treat multiple sclerosis in upstate New York
 T H E F A C T S A B O U T The rising cost of prescription drugs to treat multiple sclerosis in upstate New York Per-person mean annual prescription drug cost to treat multiple sclerosis Annual drug cost
T H E F A C T S A B O U T The rising cost of prescription drugs to treat multiple sclerosis in upstate New York Per-person mean annual prescription drug cost to treat multiple sclerosis Annual drug cost
Product Profiles: Multiple Sclerosis - Gilenya Raises Bar for New Market Entrants
 Brochure More information from http://www.researchandmarkets.com/reports/1841621/ Product Profiles: Multiple Sclerosis - Gilenya Raises Bar for New Market Entrants Description: Introduction Beginning with
Brochure More information from http://www.researchandmarkets.com/reports/1841621/ Product Profiles: Multiple Sclerosis - Gilenya Raises Bar for New Market Entrants Description: Introduction Beginning with
Information about medicines for multiple sclerosis
 Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Treatment in Relapsing MS: Choosing Among the Options. Donald Negroski, MD
 Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
Betaferon (interferon beta 1b)
 Betaferon (interferon beta 1b) What is Betaferon? Betaferon (interferon beta-1b) is a type of medicine known as an interferon, which is used to treat MS. Interferons are proteins found naturally in the
Betaferon (interferon beta 1b) What is Betaferon? Betaferon (interferon beta-1b) is a type of medicine known as an interferon, which is used to treat MS. Interferons are proteins found naturally in the
Drug Class Review. Disease-modifying Drugs for Multiple Sclerosis. Single Drug Addendum: Fingolimod
 Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
Multiple Sclerosis (MS) Class Update
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
A blood sample will be collected annually for up to 2 years for JCV antibody testing.
 Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
PCORI Workshop on Treatment for Multiple Sclerosis. Breakout Group Topics and Questions Draft 3-27-15
 PCORI Workshop on Treatment for Multiple Sclerosis Breakout Group Topics and Questions Draft 3-27-15 Group 1 - Comparison across DMTs, including differential effects in subgroups Consolidated straw man
PCORI Workshop on Treatment for Multiple Sclerosis Breakout Group Topics and Questions Draft 3-27-15 Group 1 - Comparison across DMTs, including differential effects in subgroups Consolidated straw man
Multiple Sclerosis. Global Drug Forecast and Market Analysis to 2022 Event-Driven Update. GDHC34PIDR / Published April 2013
 Multiple Sclerosis Global Drug Forecast and Market Analysis to 2022 Event-Driven Update GDHC34PIDR / Published April 2013 Executive Summary The table below presents the key metrics for multiple sclerosis
Multiple Sclerosis Global Drug Forecast and Market Analysis to 2022 Event-Driven Update GDHC34PIDR / Published April 2013 Executive Summary The table below presents the key metrics for multiple sclerosis
What is MS? 1. disease that affects the central nervous. Is a disease that affects both white and gray matter
 What is MS? 1 Neuron Damaged myelin due to inflammation MS is a chronic immunemediated disease that affects the central nervous system (CNS) Is a disease that affects both white and gray matter Interrupted
What is MS? 1 Neuron Damaged myelin due to inflammation MS is a chronic immunemediated disease that affects the central nervous system (CNS) Is a disease that affects both white and gray matter Interrupted
CNS DEMYLINATING DISORDERS
 CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
MEDICAL POLICY STATEMENT
 MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 10/01/2013 10/1/2015 08/25/2015 Policy Name Policy Number Multiple Sclerosis Therapy Class SRx-0022
MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 10/01/2013 10/1/2015 08/25/2015 Policy Name Policy Number Multiple Sclerosis Therapy Class SRx-0022
Understanding How Existing and Emerging MS Therapies Work
 Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
Understanding. Multiple Sclerosis. Tim, diagnosed in 2004.
 Understanding Multiple Sclerosis Tim, diagnosed in 2004. What Is Multiple Sclerosis? Multiple sclerosis (MS) is a neurologic disorder that affects the central nervous system (CNS). The CNS includes the
Understanding Multiple Sclerosis Tim, diagnosed in 2004. What Is Multiple Sclerosis? Multiple sclerosis (MS) is a neurologic disorder that affects the central nervous system (CNS). The CNS includes the
Survey of 267 Patients Using Low Dose Naltrexone for Multiple Sclerosis
 Survey of 267 Patients Using Low Dose Naltrexone for Multiple Sclerosis Summary In order to stimulate interest among other academic researchers in LDN trials for MS, an online patient tracking system has
Survey of 267 Patients Using Low Dose Naltrexone for Multiple Sclerosis Summary In order to stimulate interest among other academic researchers in LDN trials for MS, an online patient tracking system has
Treatments for MS: Immunotherapy. Gilenya (fingolimod) Glatiramer acetate (Copaxone )
 Treatments for MS: Immunotherapy There are currently several disease-modifying therapies approved for people with MS in Australia. These therapies, called immunotherapies, work to reduce disease activity
Treatments for MS: Immunotherapy There are currently several disease-modifying therapies approved for people with MS in Australia. These therapies, called immunotherapies, work to reduce disease activity
Version History. Previous Versions. Drugs for MS.Drug facts box fingolimod Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON
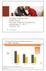 Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS?
 Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
MS Treatments Gilenya
 1 MSology Essentials Series Gilenya (fingolimod) Developed by MSology with the invaluable assistance of multiple sclerosis nurse advisors: Trudy Campbell Dalhousie MS Research Unit, Capital Health, Halifax,
1 MSology Essentials Series Gilenya (fingolimod) Developed by MSology with the invaluable assistance of multiple sclerosis nurse advisors: Trudy Campbell Dalhousie MS Research Unit, Capital Health, Halifax,
A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market
 A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market Launch of Several Pipeline Oral Products Could Diminish the Market for Injectable Therapies NEC3-52 November 2014 Contents
A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market Launch of Several Pipeline Oral Products Could Diminish the Market for Injectable Therapies NEC3-52 November 2014 Contents
fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK
 fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK 08 August 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK 08 August 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
Natalizumab (Tysabri)
 Natalizumab (Tysabri) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Natalizumab (Tysabri) Date of issue: July 2010 Review date: July 2011 Contents Section
Natalizumab (Tysabri) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Natalizumab (Tysabri) Date of issue: July 2010 Review date: July 2011 Contents Section
Multiple sclerosis disease-modifying drugs second line treatments
 Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
Disease modifying drug therapy. what you need to know
 Disease modifying drug therapy what you need to know Contact information Neurologist s name: Neurologist s secretary: Telephone number: MS specialist nurse: Telephone number: Manufacturer helpline number:
Disease modifying drug therapy what you need to know Contact information Neurologist s name: Neurologist s secretary: Telephone number: MS specialist nurse: Telephone number: Manufacturer helpline number:
PharmaPoint: Multiple Sclerosis - United Kingdom Drug Forecast and Market Analysis to 2022. Multiple
 Brochure More information from http://www.researchandmarkets.com/reports/2541548/ PharmaPoint: Multiple Sclerosis - United Kingdom Drug Forecast and Market Analysis to 2022 Description: PharmaPoint: Multiple
Brochure More information from http://www.researchandmarkets.com/reports/2541548/ PharmaPoint: Multiple Sclerosis - United Kingdom Drug Forecast and Market Analysis to 2022 Description: PharmaPoint: Multiple
All About Multiple Sclerosis
 Multiple Sclerosis Association of America All About Multiple Sclerosis Third Edition MSAA MSAA All About Multiple Sclerosis Written by: Susan Wells Courtney Edited by: Jack Burks, M.D. Andrea Borkowski
Multiple Sclerosis Association of America All About Multiple Sclerosis Third Edition MSAA MSAA All About Multiple Sclerosis Written by: Susan Wells Courtney Edited by: Jack Burks, M.D. Andrea Borkowski
New treatments in MS What s here and what s nearly here
 5 th MS Research Day, June 14 th 2014 New treatments in MS What s here and what s nearly here David Miller Queen Square MS Centre at UCL and UCLH Course of MS and its treatment Relapsing remitting Disability
5 th MS Research Day, June 14 th 2014 New treatments in MS What s here and what s nearly here David Miller Queen Square MS Centre at UCL and UCLH Course of MS and its treatment Relapsing remitting Disability
Clinical Trials of Disease Modifying Treatments
 MS CENTER CLINICAL RESEARCH The UCSF MS Center is an internationally recognized leader in multiple sclerosis clinical research. We conduct clinical trials involving the use of experimental treatments,
MS CENTER CLINICAL RESEARCH The UCSF MS Center is an internationally recognized leader in multiple sclerosis clinical research. We conduct clinical trials involving the use of experimental treatments,
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
Which injectable medication should I take for relapsing-remitting multiple sclerosis?
 Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy
 Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy Klaus Schmierer, PhD FRCP Blizard Institute, Barts and The London School of Medicine & Dentistry Barts Health
Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy Klaus Schmierer, PhD FRCP Blizard Institute, Barts and The London School of Medicine & Dentistry Barts Health
IF YOU ARE RECEIVING TREATMENT WITH TYSABRI FOR RELAPSING-REMITTING MS (NATALIZUMAB)
 IF YOU ARE RECEIVING (NATALIZUMAB) TREATMENT WITH TYSABRI FOR RELAPSING-REMITTING MS Read the patient information leaflet that accompanies the medicine carefully. 1 This brochure is a supplement to the
IF YOU ARE RECEIVING (NATALIZUMAB) TREATMENT WITH TYSABRI FOR RELAPSING-REMITTING MS Read the patient information leaflet that accompanies the medicine carefully. 1 This brochure is a supplement to the
Disease modifying drug therapy
 Disease modifying drug therapy what you need to know Third Edition Karen Alldus Simon Webster SECTION 2 We hope you find the information in this book helpful. If you would like to speak with someone about
Disease modifying drug therapy what you need to know Third Edition Karen Alldus Simon Webster SECTION 2 We hope you find the information in this book helpful. If you would like to speak with someone about
J.P. Morgan Cazenove Therapeutic Seminar
 Jannan, MS J.P. Morgan Cazenove Therapeutic Seminar David Meeker - CEO, Genzyme June 25, 2012 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private
Jannan, MS J.P. Morgan Cazenove Therapeutic Seminar David Meeker - CEO, Genzyme June 25, 2012 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private
Disclosures. Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
 Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Insight. Drug Class. Background, new developments, key strategies. Introduction. Faulty signals. 400,000 patients in the US 1
 Drug Class Insight Multiple Sclerosis Background, new developments, key strategies Introduction 400,000 patients in the US 1 Most people diagnosed between age 20 50 2 2-3x more women than men get MS 2
Drug Class Insight Multiple Sclerosis Background, new developments, key strategies Introduction 400,000 patients in the US 1 Most people diagnosed between age 20 50 2 2-3x more women than men get MS 2
Version History. Previous Versions. Policy Title. Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Disease Management Consensus Statement
 Expert Opinion Paper National Medical Advisory Board Disease Management Consensus Statement Treatment Recommendations for Clinicians This paper is currently undergoing updates from 2008 content. Visit
Expert Opinion Paper National Medical Advisory Board Disease Management Consensus Statement Treatment Recommendations for Clinicians This paper is currently undergoing updates from 2008 content. Visit
this 7^ day of September 2014 by Randall S. Gregg Special Deputy Director
 r STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v File No. 143525-001 Health Alliance Plan of Michigan,
r STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v File No. 143525-001 Health Alliance Plan of Michigan,
Multiple Sclerosis Treatments: World Market Outlook to 2011
 Brochure More information from http://www.researchandmarkets.com/reports/2860814/ Multiple Sclerosis Treatments: World Market Outlook to 2011 Description: Multiple sclerosis (MS) is a chronic demyelinating
Brochure More information from http://www.researchandmarkets.com/reports/2860814/ Multiple Sclerosis Treatments: World Market Outlook to 2011 Description: Multiple sclerosis (MS) is a chronic demyelinating
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Tysabri
 MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF I. Requirements for Prior Authorization of Tysabri A. Prescriptions That Require Prior Authorization All prescriptions for Tysabri must be prior authorized.
MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF I. Requirements for Prior Authorization of Tysabri A. Prescriptions That Require Prior Authorization All prescriptions for Tysabri must be prior authorized.
MS Treatments Aubagio TM
 1 MSology Essentials Series Aubagio TM (teriflunomide) Developed by MSology with the invaluable assistance of multiple sclerosis nurse advisors: Bonnie Blain Central Alberta MS Clinic, Red Deer, Alberta
1 MSology Essentials Series Aubagio TM (teriflunomide) Developed by MSology with the invaluable assistance of multiple sclerosis nurse advisors: Bonnie Blain Central Alberta MS Clinic, Red Deer, Alberta
Three years ago, the mantra for
 MANAGING MS: Trends, Issues, and Perspectives The entry of oral medications for multiple sclerosis into the marketplace and the high cost of biologic drugs are presenting new challenges for stakeholders.
MANAGING MS: Trends, Issues, and Perspectives The entry of oral medications for multiple sclerosis into the marketplace and the high cost of biologic drugs are presenting new challenges for stakeholders.
Understanding your Tecfidera treatment
 Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
Understanding your Tecfidera treatment
 Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
New Developments in the Treatment and Management of Multiple Sclerosis
 New Developments in the Treatment and Management of Multiple Sclerosis Myla D. Goldman, MD, MS For a CME/CEU version of this article, please go to www.namcp.org/cmeonline.htm, and then click the activity
New Developments in the Treatment and Management of Multiple Sclerosis Myla D. Goldman, MD, MS For a CME/CEU version of this article, please go to www.namcp.org/cmeonline.htm, and then click the activity
Life with MS: Mastering Early Treatment
 Life with MS: Mastering Early Treatment Essential Information About MS Multiple sclerosis (MS) is a disease that attacks the central nervous system (CNS). Approximately 2.5 million people worldwide and
Life with MS: Mastering Early Treatment Essential Information About MS Multiple sclerosis (MS) is a disease that attacks the central nervous system (CNS). Approximately 2.5 million people worldwide and
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook
 National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
06/06/2012. The Impact of Multiple Sclerosis in the Pacific Northwest. James Bowen, MD. Swedish Neuroscience Institute
 The Impact of Multiple Sclerosis in the Pacific Northwest James Bowen, MD Multiple Sclerosis Center Multiple Sclerosis Center Swedish Neuroscience Institute 1 2 Motor Symptoms of MS Weakness Spasticity
The Impact of Multiple Sclerosis in the Pacific Northwest James Bowen, MD Multiple Sclerosis Center Multiple Sclerosis Center Swedish Neuroscience Institute 1 2 Motor Symptoms of MS Weakness Spasticity
