Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
|
|
|
- Valentine Thomas
- 8 years ago
- Views:
Transcription
1 Multiple Sclerosis Agents Page 1 of 13 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: See also: Multiple Sclerosis Agents Tysabri (natalizumab) medical policy Prior Authorization Form: Prime Therapeutics will review Prior Authorization requests. For information concerning Prior Authorization Prescription Drugs: Link to Drug List (Formulary): Professional Institutional Original Effective Date: January 1, 2012 Original Effective Date: January 1, 2012 Revision Date(s): November 1, 2012; July 8, 2013; January 1, 2014; April 1, 2014 Revision Date(s): November 1, 2012; July 8, 2013; January 1, 2014; April 1, 2014 Current Effective Date: April 1, 2014 Current Effective Date: April 1, 2014 State and Federal mandates and health plan member contract language, including specific provisions/exclusions, take precedence over Medical Policy and must be considered first in determining eligibility for coverage. To verify a member's benefits, contact Blue Cross and Blue Shield of Kansas Customer Service. The BCBSKS Medical Policies contained herein are for informational purposes and apply only to members who have health insurance through BCBSKS or who are covered by a self-insured group plan administered by BCBSKS. Medical Policy for FEP members is subject to FEP medical policy which may differ from BCBSKS Medical Policy. The medical policies do not constitute medical advice or medical care. Treating health care providers are independent contractors and are neither employees nor agents of Blue Cross and Blue Shield of Kansas and are solely responsible for diagnosis, treatment and medical advice. If your patient is covered under a different Blue Cross and Blue Shield plan, please refer to the Medical Policies of that plan.
2 Multiple Sclerosis Agents Page 2 of 13 DESCRIPTION The intent of the Multiple Sclerosis Agents Prior Authorization (PA) Program is to encourage appropriate selection of patients for therapy according to product labeling and/or clinical guidelines and/or clinical studies, and also to encourage use of 2 preferred multiple sclerosis (MS) agents. The program allows continuation of therapy with a nonpreferred MS agent when there is documentation that the patient is receiving the requested agent. Studies supporting concomitant therapy of any two disease modifying agents in MS have been limited, and because the risk for developing serious adverse effects may be higher with combination therapy, the criteria will allow coverage of only one disease modifying agent (DMA) at a time. The intent of the Multiple Sclerosis Agents Quantity Limit (QL) program is to encourage appropriate prescribing quantities as recommended by Food and Drug Administration (FDA) approved product labeling and/or clinical studies and/or guidelines. Requests for larger quantities will be reviewed when patient-specific documentation has been provided. Disease Modifying Agents (DMA) Preferred Agents Betaseron (interferon β-1b) Copaxone (glatiramer) Rebif (interferon β-1a) Tecfidera TM (dimethyl fumarate) Non-Preferred Agents Aubagio (teriflunomide) Avonex (interferon β-1a) Extavia (interferon β-1b) Gilenya TM (fingolimod) 1-7, 23, 25 FDA Approved Indications and Dosage Available Products Aubagio (teriflunomide) tablet Avonex (interferon β-1a) intramuscular injection Betaseron, Extavia (interferon β-1b) subcutaneous injection Copaxone (glatiramer acetate) subcutaneous injection Indication Relapsing forms of MS RRMS b RRMS b RRMS b 7 mg or 14 mg once daily Dosage and Administration 30 mcg intramuscularly once weekly Patients should be started at mg subcutaneously every other day, and increased over a six-week period to 0.25 mg every other day. See recommended titration table: Recommended Titration Dose Volume Weeks % mg 0.25 ml Weeks % mg 0.50 ml Weeks % mg 0.75 ml Week % 0.25 mg 1.0 ml 20 mg subcutaneously daily 40 mg three times weekly
3 Multiple Sclerosis Agents Page 3 of 13 Available Products Gilenya (fingolimod) tablet Rebif (interferon β-1a) subcutaneous injection Tecfidera (dimethyl fumarate) Tysabri (natalizumab) intravenous infusion Indication RRMS RRMS Relapsing forms of MS RRMS Moderate to severe CD 0.5 mg orally once daily Dosage and Administration 22 mcg or 44 mcg injected subcutaneously three times per week. Patients should be started at 20% of the prescribed dose three times a week and increased over a 4-week period to the targeted dose, either 22 mcg or 44 mcg three times a week. See recommended titration table: Recommended Titration Titration Dose for 22 mcg Weeks % 4.4 mcg 8.8 mcg Weeks % 11 mcg 22 mcg Weeks % 22 mcg 44 mcg Starting dose: 120 mg twice daily for 7 days Titration Dose for 44 mcg Maintenance dose: 240 mg twice daily 300 mg infused intravenously over approximately 1 hr, given at 4- week (28-day) intervals a, c RRMS- Relapsing-remitting multiple sclerosis; CD- Crohn s disease a-in Crohn s disease, discontinue in patients that have not experienced therapeutic benefit by 12 weeks of induction therapy, and in patients that cannot discontinue chronic concomitant steroids within six months of starting therapy. b-approved for patients with first clinical event suggestive of multiple sclerosis c-in adult patients with moderately to severely active disease who have had an inadequate response to, or are unable to tolerate, conventional CD therapies and inhibitors of tumor necrosis factor-alpha (TNF-a) POLICY Prior Authorization Criteria for Approval Through Preferred Agent Initial Evaluation The requested agent will be approved when ALL of the following are met: 1. ONE of the following: a. The patient is not currently being treated with a disease modifying agent (DMA) b. The patient is currently being treated with a DMA the DMA will be discontinued before starting the requested agent.
4 Multiple Sclerosis Agents Page 4 of ONE of the following: a. There is documentation that the patient is currently being treated with the requested agent (paid claim within the past 90 days, or patient claim within the past 120 days and physician states the patient is currently taking the requested medication in the past 90 days) b. The prescribing physician states the patient is using the preferred agent is at risk if therapy is changed c. ALL of the following: 1) The patient has an FDA labeled diagnosis for the requested agent 2) The patient does not have any FDA labeled contraindications to therapy with the requested agent, 3) If the agent is a nonpreferred agent and meets ONE of the following: a) The patient s medication history indicates use of 2 preferred agents for MS b) The patient has a documented intolerance, FDA labeled contraindication, or hypersensitivity to at least 2 preferred agents (i.e. Betaseron, Copaxone, Rebif, or Tecfidera) 4) If Gilenya, the request will be approved when the following additional criteria are met: The prescribing physician has performed an electrocardiogram within the past 6 months and has confirmed that the patient does not have ANY of the following prior to initiating treatment: a) Bradycardia (sitting heart rate <55 bpm) b) Congestive heart failure c) Sick sinus syndrome d) Ischemic cardiac disease e) Irregular heart beat f) Current neutropenia g) Current chronic or acute infection(s) 3. ONE of the following: a. The prescribed dosage is within the program limit (FDA approved labeled dosage)
5 Multiple Sclerosis Agents Page 5 of 13 b. The quantity (dose) requested is greater than the maximum dose recommended in FDA approved labeling, and the prescriber has submitted documentation in support of therapy with a higher dose for the intended diagnosis Length of Approval: 12 months Renewal Evaluation The requested agent will be renewed when ALL of the following are met: 1. The patient has been previously approved for the requested therapy through the PA process. 2. The patient is NOT currently being treated with an additional disease modifying agent (DMA) 3. The patient does not have any FDA labeled contraindications to therapy with the requested agent 4. ONE of the following: a. The prescribed dosage is within the program limit (FDA approved labeled dosage) b. The quantity (dose) requested is greater than the maximum dose recommended in FDA approved labeling, and the prescriber has submitted documentation in support of therapy with a higher dose for the intended diagnosis Length of approval: 12 months FDA Labeled Contraindications Brand (generic) Contraindications Aubagio (teriflunomide) Pregnancy, severe hepatic impairment Myocardial infarction, unstable angina, stroke, TIA, decompensated heart failure requiring hospitalization or Class III/IV heart failure in the last 6 months, history of Mobitz Type II Gilenya (fingolimod) second or third-degree AV block or sick sinus syndrome (unless functioning pacemaker), baseline QT interval 500 ms, or treatment with Class Ia or Class III anti-arrhythmic drugs (see chart below)
6 Multiple Sclerosis Agents Page 6 of 13 Class Ia antiarrhythmics Norpace (disopyramide) Pronestyl (procainamide) quinidine Class III antiarrhythmics Cordarone, Pacerone (amiodarone) Betapace (sotalol) Tikosyn (dofetilide) Multaq (dronedarone) Corvert (ibutilide) Quantity Limits for Disease Modifying Agents (DMA) Brand (generic) Quantity Limit Aubagio (teriflunomide) 7 mg tablet 1 tablet daily 14 mg tablet 1 tablet daily Avonex (interferon β-1a) 30 mcg vial 30 mcg/0.5 ml prefilled syringe 30 mcg/0.5 ml Autoinjector pen Betaseron (interferon β-1b) 0.3 mg vial + syringe with diluent Copaxone (glatiramer) 1 vial/week (1 package of 4 vials/28 days) 1 syringe/week (1 package of 4 syringes/28 days) 1 syringe/week (1 package of 4 syringes/28 days) 14 vial/syringe units (1 box)/28 days 20 mg/ml syringe 1 syringe/day (1 carton of 30 syringes/30 days) Copaxone (glatiramer) 40 mg/ml syringe 12 syringes/28 days Extavia (interferon β-1b) 0.3 mg vial + syringe with diluent 15 vial/syringe units (1 box)/30 days Gilenya TM (fingolimod) 0.5 mg tablet 1 tablet/day Brand (generic) Quantity Limit Rebif (interferon β-1a) 22 mcg/0.5 ml 3 syringes/week (1 carton 12 syringes/28 days) 44 mcg/0.5 ml 3 syringes/week (1 carton 12 syringes/28 days) Titration pack: (6 x 8.8 mcg/0.2 ml + 6 x 22 mcg/0.5 ml) 1 kit/28 days Tecfidera TM (dimethyl fumerate) Starter kit 1 kit / 180 days 120 mg capsules 14 capsules / 180 days 240 mg capsules 2 capsules daily
7 Multiple Sclerosis Agents Page 7 of 13 RATIONALE Injectable Disease Modifying Agents (DMAs) for Multiple Sclerosis (MS) DMAs for the treatment of MS reduce the number and severity of relapses, reduce the number of new lesions appearing on magnetic resonance imaging, and may reduce long-term progression of MS. 8,9 Guidelines from the United States and Europe recommend treatment for RRMS be initiated with either glatiramer or interferon beta (INFβ). The INFβ agents are considered appropriate for patients at high risk of developing clinically definite MS, or those who already have RRMS or secondary progressive MS and are experiencing relapses. There is a probable dose or frequency of dosing response curve associated with use of INFβ agents. Interferon beta-1a (Avonex, Rebif) has been associated with less neutralizing antibody formation than interferon beta-1b (Betaseron, Extavia). The clinical effects of these neutralizing antibodies are uncertain. Their presence has been associated with a possible decrease in interferon efficacy. The route of administration of the INFβ agents does not have apparent effects on efficacy but side effect profiles differ between routes of administration. Because glatiramer works by a different mechanism than interferons, the side effect profile is different from interferons and may make this agent an option for some patients unable to tolerate interferons. 9 Glatiramer is considered an appropriate option for patients with RRMS or those experiencing a first clinical episode with MRI imaging consistent with MS. Natalizumab is recommended for patients with relapsing forms of MS who have had an inadequate response to, or are unable to tolerate other MS therapies. 8,9 Concurrent use of more than one injectable DMA has been studied in clinical trials. The combinations of INFβ with natalizumab and glatiramer with natalizumab have been studied. Although a beneficial effect was seen (such as improved magnetic resonance imaging (MRI) parameters), there may be more adverse reactions associated with combination therapies. The study with a combination of INFβ and natalizumab was halted due to reported cases of progressive multifocal leukoencephalopathy (PML). 21 The adverse effects seen with combination therapies are similar to those reported with the individual agents, but it is unclear if the risk for developing these adverse effects is higher in combination therapy. Some of the clinical effects of glatiramer may occur by entry of regulatory glatiramer-reactive cells into the central nervous system (CNS) across a disrupted blood-brain-barrier (BBB) and effects on CNS resident cells. It is possible that combining glatiramer with therapies that close the BBB like INFβ and natalizumab may limit the effectiveness of glatiramer. 21 The benefits of combination therapies and the safety concerns associated with concurrent therapy still need further investigation. Oral DMAs for MS 10,23 Fingolimod, a sphingosine 1 phosphate (S1P) receptor modulator, is the first oral disease modifying therapy for RRMS. Fingolimod works by trapping lymphocytes (T-cells and B-cells) in the lymph nodes so that they cannot attack the central nervous system. Clinical studies have shown fingolimod to be effective in preventing MS relapses, and fingolimod was superior to Avonex in one comparative study. However, its place in therapy is undetermined. Fingolimod has not been studied in combination with (concurrently with) other DMAs. Higher doses (1.25 mg compared to 0.5 mg) of fingolimod were studied in clinical trials, but there was not a statistical difference in efficacy. More serious adverse events, including increased bradycardia, were reported with 1.25 mg. 1 Dose-related first dose bradycardia and atrioventricular heart block has been reported. Patients should receive their first dose of fingolimod under medical supervision and be monitored for six hours post dose. 5 Ophthalmic exams are recommended to detect
8 Multiple Sclerosis Agents Page 8 of 13 macular edema as a greater incidence of macular edema was seen in the fingolimod-treated group compared to the placebo group in clinical trials. Dose-dependent decrease of pulmonary function (forced expiratory volume within one second [FEV1]) was observed in clinical trials with fingolimod. It is unclear whether pulmonary function changes will continue to worsen over time with uninterrupted fingolimod dosing. Long term safety data is not available for fingolimod as the longest phase 3 trial to date was 2 years in duration. Contraindications to therapy include patients in the last 6 months who have experienced myocardial infarction, unstable angina, stroke, TIA, decompensated heart failure requiring hospitalization or Class III/IV heart failure. Patients with Mobitz Type II second or third degree atrioventricular block (AV) or sick sinus syndrome, unless the patient has a functioning pacemaker, those with a baseline QTc interval 500 ms and those receiving treatment with Class Ia (e.g. disopyramide, procainamide, quinidine) or Class III (e.g. amiodarone, sotalol, dofetilide) anti-arrhythmic drugs. 5 A National MS Society consensus statement recommends changing from one disease modifying therapy to another only for medically appropriate reasons (e.g., lack of efficacy, adverse effects, or if better treatments options become available). 8 This consensus statement was written prior to the approval of the oral MS therapies. The European Medicines Agency review of fingolimod recommends it as second line therapy based on evaluation of quality, safety, and efficacy for patients with high disease activity despite treatment with a beta-interferon or for patients with rapidly evolving severe relapsing remitting MS. 22 Teriflunomide is a pyrimidine synthesis inhibitor. The exact mechanism for its therapeutic effect in MS is unknown but thought to reduce the number of activated lymphocytes in the CNS. Clinical trial results showed a significant reduction in annualized relapse rates at both doses of teriflunomide compared to placebo. There was not an active comparator in the study but reductions in annual relapse rates were similar (30% to 50%) to the injectable disease modifying agents. Teriflunomide is contraindicated in severe hepatic impairment and pregnancy. There is a boxed warning for hepatoxicity and teratogenicity. The most common adverse events include increased ALT, alopecia, diarrhea, influenza, nausea and paresthesia. 23 The therapeutic effects of dimethyl fumarate in MS is unknown but its metabolite has been shown to active the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway which is involved in the cellular response to oxidative stress. Clinical trial results for Study 1 which was a placebo controlled study, showed a significant reduction in the proportion of relapsing remitting patients with relapses at 2 years. The proportion of patients relapsing were 27% (n=410) versus 46% (n=408) [p=<0.0001] for the dimethyl fumarate and placebo groups respectively, with a relative risk reduction of 49%. The annualized relapse rate was for the treated group and for placebo [p<0.0001] with a relative risk reduction of 53%. Study 2 was also a placebo controlled study that included an open label active comparator with a primary endpoint of annualized relapse rate at 2 years. The results of this trial showed a statistically significant reduction in annualized relapse rates compared to placebo. The annualized relapse rate for dimethyl fumarate was (n=359) and (n=363) for placebo [p=<0.0001], with a relative risk reduction of 44%. The proportion of patients relapsing was similar to those in Study The most common adverse events ( 10% and 2% placebo) include flushing, abdominal pain, diarrhea, and nausea.
9 Multiple Sclerosis Agents Page 9 of 13 CODING The following codes for treatment and procedures applicable to this policy are included below for informational purposes. Inclusion or exclusion of a procedure, diagnosis or device code(s) does not constitute or imply member coverage or provider reimbursement. Please refer to the member's contract benefits in effect at the time of service to determine coverage or noncoverage of these services as it applies to an individual member. HCPCS J1595 Injection, glatiramer acetate, 20 mg J1826 interferon beta-1a, 30 mcg J1830 Injection interferon beta-1b, 0.25 mg (code may be used for Medicare when drug administered under the direct supervision of a physician, not for use when drug is self-administered) There are no specific J codes for the remaining drugs listed in this policy. REVISIONS Policy added to the bcbsks.com web site. Policy was effective January 1, Revised Title From: "Multiple Sclerosis Interferon Agents Step Therapy Program Summary" To: "Multiple Sclerosis Agents Prior Authorization (Through Preferred) Program Summary" Description section updated In Policy section: Expanded to the current policy language from: "Non-preferred Multiple Sclerosis Agents will be approved when BOTH of the following are met: 1. ONE of the following: a. The patient is not currently being treated with a disease modifying agent (DMA) (see description) for multiple sclerosis (MS) b. The patient is currently being treated with a DMA for MS the DMA will be discontinued before starting the requested agent 2. ONE of the following: a. The patient s medication history indicates use of a preferred multiple sclerosis agent b. There is documentation that the patient is currently using the requested nonpreferred multiple sclerosis agent c. The prescribing physician states the patient is using the requested nonpreferred multiple sclerosis agent is at risk if therapy is changed d. The patient has a documented intolerance, FDA labeled contraindication, or hypersensitivity to a preferred multiple sclerosis agent Length of approval: 12 months" Rationale section added References updated Revised title from "Multiple Sclerosis Agents Prior Authorization (Through referred) Program Summary" to "Multiple Sclerosis Agents (also addresses Tysabri's use in Crohn's disease)" In Description section: Description updated
10 Multiple Sclerosis Agents Page 10 of 13 Updated the Disease Modifying Agents (DMA) chart to remove the reference to "Target Drugs" in the title and added the drugs Aubagio (teriflunomide) and Tecfidera (dimethyl fumarate). Updated the FDA Approved Indication and Dosage chart including adding the drugs Aubagio (teriflunomide) and Tecfidera (dimethyl fumarate) to the chart. In Policy section: Added "Through Preferred Agents" to the header. Under the Initial Evaluation portion, added "ALL of" to read "The requested agent will be approved when ALL of the following are met:" Revised 1 a and 1 b to the current language from, "The requested agent will be approved when the following are met: 1. ONE of the following: a. The patient is not currently being treated with a n additional disease modifying agent (DMA) for (MS) b. The patient is currently being treated with a n additional DMA for MS the DMA will be discontinued before starting the requested agent." Added 2 c 2) "The patient does not have any contraindications to therapy with the requested agent " Revised 2 c 3) and 2 c 3) a) to the current language from, 3) The requested agent is a nonpreferred agent ONE of the following: a) The patient s medication history indicates use of a preferred agent for MS In 2 c 4) relocated the following contraindications for Gilenyto a chart titled FDA Labeled Contraindications, d) Prolonged QT interval 500 ms i) Use of antineoplastic, immunosuppressive, Class Ia (e.g. disopyramide, procainamide, quinidine) or Class III (e.g. amiodarone, dronedarone, sotalol, dofetilide, ibutilide) antiarrhythmics or immune modulating therapies j) Mobitz Type II second or third-degree AV block without a functioning pacemaker k) ANY of the following in the last 6 months: i. Myocardial infarction ii. Unstable angina iii. Stroke iv. TIA v. Decompensated heart failure requiring hospitalization Added indications 5 a and 5 b, " 5) If Tysabri, the request will be approved for moderate to severe Crohn s Disease (CD) when ONE of the following additional criteria is met: a The patient s medication history includes use of a conventional CD therapy (aminosalicylates, metronidazole, ciprofloxacin, corticosteroids, methotrexate, or immunomodulators such as azathioprine or 6- mercaptopurine) b the patient has a documented intolerance, FDA labeled contraindication, or hypersensitivity, to conventional CD therapy" Under the Renewal Evaluation added "ALL of" to read, "The requested agent will
11 Multiple Sclerosis Agents Page 11 of 13 be renewed when ALL of the following are met:" In item 2 added "for the intended FDA labeled indication" to read, "The patient is NOT currently being treated with an additional disease modifying agent (DMA) for the intended FDA labeled indication." Added the following four charts: "FDA Labeled Contraindications", "Class Ia and Class II antiarrhythmics", Contraindicated as Concomitant Therapy", and "Quantity Limits" Rationale section updated Added Coding section Added HCPCS codes: J1595, J1826, J1830, J2323 Added the statement: "There are no specific J codes for the remaining drugs listed in this policy." References updated In Title section: Revised Title from: "Multiple Sclerosis Agents (also addresses Tysabri's use in Crohn's disease)", to: "Multiple Sclerosis Agents". Added the See also policy of "Tysabri (natalizumab)" In Description section: Description section updated Updated Disease Modifying Agents (DMA) chart changing Tecfidera (dimethyl fumarate) from a non-preferred to a preferred agent. Removed Tysabri (natalizumab) from the chart as it is now addressed in a stand-alone policy. In Policy section: In Item 2 a added look-back information In Initial Evaluation In 1 a removed "for the requested indication (MS or CD)" to read, "The patient is not currently being treated with a disease modifying agent (DMA)" In 1 b removed "for the requested indication" to read, "The patient is currently being treated with a DMA the DMA will be discontinued before starting the requested agent. In 2 c 2) added "FDA labeled" to read, "The patient does not have any FDA labeled contraindications to therapy with the requested agent" In 2 c 3) a) added "2" and removed "the requested FDA labeled indication (CD) to read, "The patient s medication history indicates use of 2 preferred agents for MS" In 2 c 3) b) added "at least 2" to read, "The patient has a documented intolerance, FDA labeled contraindication, or hypersensitivity to at least 2 preferred agents (i.e. Betaseron, Copaxone, Rebif, or Tecfidera)" Removed item 2 c 3) c) "If Gilenya, the patient s medication history indicates the use of Tysabri" In 2 c 4) added "performed an electrocardiogram within the past 6 months and has" to read, "The prescribing physician has performed an electrocardiogram within the past 6 months and has confirmed that the patient does not have ANY of the following prior to initiating treatment:" Removed indications for Tysabri in 2 c: "5) If Tysabri, the request will be approved for moderate to severe Crohn s Disease (CD) when ONE of the following additional criteria is met:
12 Multiple Sclerosis Agents Page 12 of 13 a) The patient s medication history includes use of a conventional CD therapy (aminosalicylates, metronidazole, ciprofloxacin, corticosteroids, methotrexate, or immunomodulators such as azathioprine or 6-mercaptopurine) b) the patient has a documented intolerance, FDA labeled contraindication, or hypersensitivity, to conventional CD therapy" In Renewal Evaluation In 2 removed "for the intended FDA labeled indication" to read, "The patient is NOT currently being treated with an additional disease modifying agent (DMA)" Added item 3 "The patient does not have any FDA labeled contraindications to therapy with the requested agent. " Updated the FDA Labeled Contraindications chart by removing Tysabri Removed the Contraindicated as Concomitant Therapy chart for Tysabri Updated the Quantity Limits chart Rationale section updated In Coding section: Removed HCPCS code: J2323 References updated Policy posted July 15, Administrative Update In Description section: Updated the FDA Approved Indications and Dosage to include an updated dosage for Copaxone (glatiramer acetate) of 40 mg three times weekly. In the Policy section: Removed the Contraindicated as Concomitant Therapy chart. In the Quantity Limits chart replaced "Target Drugs" with "Disease Modifying Agents (DMA)" Updated the Quantity Limits chart for Copaxone from 1 carton of mg/ml syringes/30 days to mg/ml syringes/28 days References updated REFERENCES 1. Avonex prescribing information. Biogen Idec, Inc. October Betaseron prescribing information. Bayer HealthCare Pharmaceuticals Inc. March Copaxone prescribing information. Teva Neurosciences, Inc. August Extavia prescribing information. Novartis. March Gilenya prescribing information. Novartis. May Rebif prescribing information. Serono, Inc./Pfizer Inc. February Tysabri prescribing information. Biogen Idec, Inc./Elan Pharmaceuticals, Inc. June National Multiple Sclerosis Society Disease Management Consensus Statement- Recommendations from the MS Information Sourcebook; 2007 Update. National Multiple Sclerosis Society. Available at: Accessed January 2, Goodin DS, Frohman EM, Garmany GP, et al. Disease modifying therapies in multiple sclerosis. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Reaffimed 9/24/2010. Neurology 2002;58; Available at Accessed September 24, 2010.
13 Multiple Sclerosis Agents Page 13 of Peripheral and Central Nervous System Drugs Advisory Committee Meeting. Fingolimod (NDA ) Background Package. June 10, Available at ripheralandcentralnervoussystemdrugsadvisorycommittee/ucm pdf. Accessed September 14, Marshall JK, Blackhouse G, Goeree R, Brazier N, Irvine EF, Faulkner L, Dipchand C, O Brian BJ. Infliximab for the Treatment of Crohn s Disease: A Systematic Review and Cost-Utility Analysis. Ottawa: Canadian Coordinating Office for Health Technology Assessment; Technology report no 24 Available at: Accessed February 1, Carter MJ, Lobo AJ, Travis SPL, on behalf of the IBD Section of the British Society of Gastroenterology. Gut. 2004;53(suppl V):v1-v Lichtenstein GR, Hanauer SB, Sandborn WJ and the Practice Parameters Committee of the American College of Gastroenterology. Management of Crohn s Disease in Adults. Online publication January 6, 2009; doi: /ajg Sandborn WJ, Feagan BG, Lichtenstein GR. Medical management of mild to moderate Crohn s disease: evidence-based treatment algorithms for induction and maintenance of remission. Aliment Pharmacol Ther. 2007;26: Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American Gastroenterological Association Institute Medical Position Statement on Corticosteroids, Immunomodulators, and Infliximab in Inflammatory Bowel Disease. Gastroenterology. 2006;130: American Gastroenterological Association Institute Medical Position Statement: Perianal Crohn s Disease. Gastroenterology. 2003;125: American Gastroenterological Association Institute. American Gastroenterological Association Consensus Development Conference on the Use of Biologics in the Treatment of Inflammatory Bowel Disease, June 21-23, Gastroenterology. 2007;133: Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369: Center for Drug Evaluation and Research. FDA Alert. August Available at: Accessed 12/19/ FDA MedWatch. Natalizumab MedWatch Alert. 2/5/2010. Available at: ucts/ucm htm. Accessed 12/19/ Graber J, McGraw C, Kimbrough D, Dhib-Jalbut S. Overlapping and distinct mechanisms of action of multiple sclerosis therapies. Clinical Neurology and Neurosurgery. 2010;112: European Medicines Agency (EMA). Assessment report: Gilenya. Doc Ref: EMA/108602/2011. February 17, Available at: Public_assessment_report/human/002202/WC pdf. Accessed on November 2, Aubagio prescribing information. Genzyme Corporation, Cambridge, MA. September Deleted. 25. Tecfidera prescribing information. Biogen Idec, Inc. March 2013.
Multiple Sclerosis Agents Step Therapy with Quantity Limit Program Summary
 Multiple Sclerosis Agents Step Therapy with Quantity Limit Program Summary OBJECTIVE The intent of the Multiple Sclerosis (MS) Agents Step Therapy (ST) program is to encourage the use of preferred multiple
Multiple Sclerosis Agents Step Therapy with Quantity Limit Program Summary OBJECTIVE The intent of the Multiple Sclerosis (MS) Agents Step Therapy (ST) program is to encourage the use of preferred multiple
Multiple Sclerosis Step Therapy and Quantity Limit Criteria
 Multiple Sclerosis Step Therapy and Quantity Limit Criteria Tysabri (natalizumab) will NOT be included in this step therapy program for Blue Cross and Blue Shield of Illinois because this plan does not
Multiple Sclerosis Step Therapy and Quantity Limit Criteria Tysabri (natalizumab) will NOT be included in this step therapy program for Blue Cross and Blue Shield of Illinois because this plan does not
MEDICAL POLICY STATEMENT
 MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 10/01/2013 10/1/2015 08/25/2015 Policy Name Policy Number Multiple Sclerosis Therapy Class SRx-0022
MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 10/01/2013 10/1/2015 08/25/2015 Policy Name Policy Number Multiple Sclerosis Therapy Class SRx-0022
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual. Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014
 Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual. Topic: Gilenya, fingolimod Date of Origin: November 22, 2010
 Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual. Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004
 Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Tysabri
 MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF I. Requirements for Prior Authorization of Tysabri A. Prescriptions That Require Prior Authorization All prescriptions for Tysabri must be prior authorized.
MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF I. Requirements for Prior Authorization of Tysabri A. Prescriptions That Require Prior Authorization All prescriptions for Tysabri must be prior authorized.
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
Lemtrada (alemtuzumab)
 Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Progress in MS: Current and Emerging Therapies
 Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
SECTION 2. Section 2 Multiple Sclerosis (MS) Drug Coverage
 SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
Relapsing-remitting multiple sclerosis Ambulatory with or without aid
 AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
Version History. Previous Versions. Drugs for MS.Drug facts box fingolimod Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
New Treatment Options for MS Patients: Understanding risks versus benefits
 New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
A neurologist would assess your eligibility and suitability for the DMTs.
 Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Growth in revenue from MS drugs has been driven largely by price increases over the last several years.
 March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
New treatments in MS What s here and what s nearly here
 5 th MS Research Day, June 14 th 2014 New treatments in MS What s here and what s nearly here David Miller Queen Square MS Centre at UCL and UCLH Course of MS and its treatment Relapsing remitting Disability
5 th MS Research Day, June 14 th 2014 New treatments in MS What s here and what s nearly here David Miller Queen Square MS Centre at UCL and UCLH Course of MS and its treatment Relapsing remitting Disability
The MS Disease- Modifying Medications GENERAL INFORMATION
 The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
National Multiple Sclerosis Society. Disease Modification in Multiple Sclerosis. Current as of January 2, 2013
 National Multiple Sclerosis Society Disease Modification in Multiple Sclerosis Current as of January 2, 2013 Since 1993, the U.S. Food and Drug Administration (FDA) has approved several medications for
National Multiple Sclerosis Society Disease Modification in Multiple Sclerosis Current as of January 2, 2013 Since 1993, the U.S. Food and Drug Administration (FDA) has approved several medications for
fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK
 fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK 08 August 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK 08 August 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
Multiple Sclerosis (MS) Class Update
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
Version History. Previous Versions. Policy Title. Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Novartis Gilenya FDO Program Clinical Protocol and Highlights from Prescribing Information (PI)
 Novartis Gilenya FDO Program Clinical Protocol and Highlights from Prescribing Information (PI) Highlights from Prescribing Information - the link to the full text PI is as follows: http://www.pharma.us.novartis.com/product/pi/pdf/gilenya.pdf
Novartis Gilenya FDO Program Clinical Protocol and Highlights from Prescribing Information (PI) Highlights from Prescribing Information - the link to the full text PI is as follows: http://www.pharma.us.novartis.com/product/pi/pdf/gilenya.pdf
The MS Disease- Modifying Drugs. Gener al information
 The MS Disease- Modifying Drugs Gener al information Current as of October 30, 2009. This online version is updated as breaking news requires. If you have downloaded and printed a copy from the web, please
The MS Disease- Modifying Drugs Gener al information Current as of October 30, 2009. This online version is updated as breaking news requires. If you have downloaded and printed a copy from the web, please
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
Conflict of Interest Declaration. Overview of New Medications for Multiple Sclerosis. Assessment Question. Objectives 4/1/2011
 Conflict of Interest Declaration Overview of New Medications for Multiple Sclerosis I or my spouse have no actual or potential conflict of interest in relation to this activity. Crystal Obering, Pharm.D.,
Conflict of Interest Declaration Overview of New Medications for Multiple Sclerosis I or my spouse have no actual or potential conflict of interest in relation to this activity. Crystal Obering, Pharm.D.,
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Drug Class Review. Disease-modifying Drugs for Multiple Sclerosis. Single Drug Addendum: Fingolimod
 Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
Pharmacotherapy of Multiple Sclerosis
 PHARMACY / MEDICAL POLICY 5.01.565 Pharmacotherapy of Multiple Sclerosis Effective Date: July 1, 2016 Last Revised: June 14, 2016 Replaces: Extracted from 5.01.550 RELATED MEDICAL POLICIES: 5.01.556 Rituximab:
PHARMACY / MEDICAL POLICY 5.01.565 Pharmacotherapy of Multiple Sclerosis Effective Date: July 1, 2016 Last Revised: June 14, 2016 Replaces: Extracted from 5.01.550 RELATED MEDICAL POLICIES: 5.01.556 Rituximab:
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Afrezza Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Afrezza (human insulin) Prime Therapeutics will review Prior Authorization requests Prior Authorization
Afrezza Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Afrezza (human insulin) Prime Therapeutics will review Prior Authorization requests Prior Authorization
Personalised Medicine in MS
 Personalised Medicine in MS Supportive Evidence from Therapeutic Trials Ludwig Kappos Neurology and Department of Biomedicine University Hospital CH-4031 Basel LKappos@uhbs.ch Established partially effective
Personalised Medicine in MS Supportive Evidence from Therapeutic Trials Ludwig Kappos Neurology and Department of Biomedicine University Hospital CH-4031 Basel LKappos@uhbs.ch Established partially effective
The MS Disease- Modifying Medications
 The MS Disease- Modifying Medications National MS Society 1 Current as of November 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
The MS Disease- Modifying Medications National MS Society 1 Current as of November 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
Literature Scan: Oral Multiple Sclerosis Drugs
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis
 Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness of teriflunomide
Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness of teriflunomide
Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis
 Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness
Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness
The submission positioned dimethyl fumarate as a first-line treatment option.
 Product: Dimethyl Fumarate, capsules, 120 mg and 240 mg, Tecfidera Sponsor: Biogen Idec Australia Pty Ltd Date of PBAC Consideration: July 2013 1. Purpose of Application The major submission sought an
Product: Dimethyl Fumarate, capsules, 120 mg and 240 mg, Tecfidera Sponsor: Biogen Idec Australia Pty Ltd Date of PBAC Consideration: July 2013 1. Purpose of Application The major submission sought an
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru381 Topic: Lemtrada TM, alemtuzumab Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru381 Topic: Lemtrada TM, alemtuzumab Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
5.07.09. Aubagio. Aubagio (teriflunomide) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Integrating New Treatments: A Case Based Approach
 Integrating New Treatments: A Case Based Approach JILL CONWAY, MD, MA, MSCE DIRECTOR, MS CENTER DIRECTOR, NEUROLOGY CLERKSHIP AT UNCSOM- CHARLOTTE CAMPUS CAROLINAS HEALTHCARE CENTER Objectives Provide
Integrating New Treatments: A Case Based Approach JILL CONWAY, MD, MA, MSCE DIRECTOR, MS CENTER DIRECTOR, NEUROLOGY CLERKSHIP AT UNCSOM- CHARLOTTE CAMPUS CAROLINAS HEALTHCARE CENTER Objectives Provide
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio
 ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
teriflunomide, 14mg, film-coated tablets (Aubagio ) SMC No. (940/14) Genzyme Ltd.
 teriflunomide, 14mg, film-coated tablets (Aubagio ) SMC No. (940/14) Genzyme Ltd. 10 January 2014 (Issued 07 February 2014) The Scottish Medicines Consortium (SMC) has completed its assessment of the above
teriflunomide, 14mg, film-coated tablets (Aubagio ) SMC No. (940/14) Genzyme Ltd. 10 January 2014 (Issued 07 February 2014) The Scottish Medicines Consortium (SMC) has completed its assessment of the above
Treatment in Relapsing MS: Choosing Among the Options. Donald Negroski, MD
 Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
Study Support Materials Cover Sheet
 Study Support Materials Cover Sheet Document Title ESCALATE Patient Brochure Intended Audience This brochure is designed to be given to potentially eligible patients as a take-home summary of key information
Study Support Materials Cover Sheet Document Title ESCALATE Patient Brochure Intended Audience This brochure is designed to be given to potentially eligible patients as a take-home summary of key information
The MS Disease- Modifying Medications
 The MS Disease- Modifying Medications National MS Society 1 Current as of January 2015. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
The MS Disease- Modifying Medications National MS Society 1 Current as of January 2015. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
Multiple Sclerosis in Practice. An Expert Commentary With Jeffrey Cohen, MD, PhD A Clinical Context Report
 Multiple Sclerosis in Practice An Expert Commentary With Jeffrey Cohen, MD, PhD A Clinical Context Report Clinical Context: Multiple Sclerosis in Practice Expert Commentary Jointly Sponsored by: and Clinical
Multiple Sclerosis in Practice An Expert Commentary With Jeffrey Cohen, MD, PhD A Clinical Context Report Clinical Context: Multiple Sclerosis in Practice Expert Commentary Jointly Sponsored by: and Clinical
NHS BOURNEMOUTH AND POOLE AND NHS DORSET
 NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
Pharmacologic Therapies for Multiple Sclerosis: From injectable to oral agents
 Pharmacologic Therapies for Multiple Sclerosis: From injectable to oral agents KIRANPAL SINGH SANGHA, PHARM.D. CLINICAL PHARMACY SPECIALIST CNS THE UNIVERSITY OF CINCINNATI MEDICAL CENTER Objectives List
Pharmacologic Therapies for Multiple Sclerosis: From injectable to oral agents KIRANPAL SINGH SANGHA, PHARM.D. CLINICAL PHARMACY SPECIALIST CNS THE UNIVERSITY OF CINCINNATI MEDICAL CENTER Objectives List
Which injectable medication should I take for relapsing-remitting multiple sclerosis?
 Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Therapeutic Class Overview Multiple Sclerosis Agents
 Therapeutic Class Overview Multiple Sclerosis Agents Therapeutic Class Overview/Summary: Several biologic response modifiers are Food and Drug Administration (FDA)- approved for the treatment of relapsing-remitting
Therapeutic Class Overview Multiple Sclerosis Agents Therapeutic Class Overview/Summary: Several biologic response modifiers are Food and Drug Administration (FDA)- approved for the treatment of relapsing-remitting
How to S.E.A.R.C.H. SM for the Right MS Therapy For You!
 How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
Information About Medicines for Multiple Sclerosis
 Information About Medicines for Multiple Sclerosis Information About Medicines for Multiple Sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information About Medicines for Multiple Sclerosis Information About Medicines for Multiple Sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
J.P. Morgan Cazenove Therapeutic Seminar
 Jannan, MS J.P. Morgan Cazenove Therapeutic Seminar David Meeker - CEO, Genzyme June 25, 2012 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private
Jannan, MS J.P. Morgan Cazenove Therapeutic Seminar David Meeker - CEO, Genzyme June 25, 2012 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private
Treatments for MS: Immunotherapy. Gilenya (fingolimod) Glatiramer acetate (Copaxone )
 Treatments for MS: Immunotherapy There are currently several disease-modifying therapies approved for people with MS in Australia. These therapies, called immunotherapies, work to reduce disease activity
Treatments for MS: Immunotherapy There are currently several disease-modifying therapies approved for people with MS in Australia. These therapies, called immunotherapies, work to reduce disease activity
A blood sample will be collected annually for up to 2 years for JCV antibody testing.
 Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
this 7^ day of September 2014 by Randall S. Gregg Special Deputy Director
 r STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v File No. 143525-001 Health Alliance Plan of Michigan,
r STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v File No. 143525-001 Health Alliance Plan of Michigan,
fingolimod (as hydrochloride), 0.5mg hard capsules (Gilenya ) SMC No. (763/12) Novartis Pharmaceuticals UK Ltd
 fingolimod (as hydrochloride), 0.5mg hard capsules (Gilenya ) SMC No. (763/12) Novartis Pharmaceuticals UK Ltd 10 February 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the
fingolimod (as hydrochloride), 0.5mg hard capsules (Gilenya ) SMC No. (763/12) Novartis Pharmaceuticals UK Ltd 10 February 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the
RISK EVALUATION AND MITIGATION STRATEGY (REMS)
 Initial REMS Approval: 9/2010 Most Recent Modification: 05/2015 0.5mg capsules Sphingosine 1-phosphate Receptor Modulator Novartis Pharmaceuticals Corporation One Health Plaza East Hanover, NJ 07936 RISK
Initial REMS Approval: 9/2010 Most Recent Modification: 05/2015 0.5mg capsules Sphingosine 1-phosphate Receptor Modulator Novartis Pharmaceuticals Corporation One Health Plaza East Hanover, NJ 07936 RISK
CLINICAL POLICY Department: Medical Management Document Name: MS Treatments
 Page: 1 of 21 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Page: 1 of 21 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Genzyme s Multiple Sclerosis Franchise Featured at AAN
 PRESS RELEASE Genzyme s Multiple Sclerosis Franchise Featured at AAN - Multiple Presentations Highlight Continuing Progress of AUBAGIO and LEMTRADA Programs - Paris, France March 13, 2013 Sanofi (EURONEXT:
PRESS RELEASE Genzyme s Multiple Sclerosis Franchise Featured at AAN - Multiple Presentations Highlight Continuing Progress of AUBAGIO and LEMTRADA Programs - Paris, France March 13, 2013 Sanofi (EURONEXT:
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents
 New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
New Developments in the Treatment and Management of Multiple Sclerosis
 New Developments in the Treatment and Management of Multiple Sclerosis Myla D. Goldman, MD, MS For a CME/CEU version of this article, please go to www.namcp.org/cmeonline.htm, and then click the activity
New Developments in the Treatment and Management of Multiple Sclerosis Myla D. Goldman, MD, MS For a CME/CEU version of this article, please go to www.namcp.org/cmeonline.htm, and then click the activity
CADTH Therapeutic Review
 Canadian Agency for Drugs and Technologies in Health Agence canadienne des médicaments et des technologies de la santé CADTH Therapeutic Review October 2013 Volume 1, Issue 2B Comparative Clinical and
Canadian Agency for Drugs and Technologies in Health Agence canadienne des médicaments et des technologies de la santé CADTH Therapeutic Review October 2013 Volume 1, Issue 2B Comparative Clinical and
Uncertainty in Benefit and Risk: Tysabri (natalizumab)
 Uncertainty in Benefit and Risk: Tysabri (natalizumab) Robert J. Temple, M.D. Deputy Center Director for Clinical Science Center for Drug Evaluation and Research U.S. Food and Drug Administration IOM Drug
Uncertainty in Benefit and Risk: Tysabri (natalizumab) Robert J. Temple, M.D. Deputy Center Director for Clinical Science Center for Drug Evaluation and Research U.S. Food and Drug Administration IOM Drug
Information about medicines for multiple sclerosis
 Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Resources for the Primary Care Provider. Please print these out for reference
 Resources for the Primary Care Provider Please print these out for reference Resources for providers American Academy of Neurology www.aan.com Provides education and resources, such as guidelines for clinical
Resources for the Primary Care Provider Please print these out for reference Resources for providers American Academy of Neurology www.aan.com Provides education and resources, such as guidelines for clinical
Patients with confirmed relapse 111 26 (23.4 %) 104 16 (15.4 %) 1.52 [0.87; 2.67] p = 0.143 Probability of a relapse by week 96
![Patients with confirmed relapse 111 26 (23.4 %) 104 16 (15.4 %) 1.52 [0.87; 2.67] p = 0.143 Probability of a relapse by week 96 Patients with confirmed relapse 111 26 (23.4 %) 104 16 (15.4 %) 1.52 [0.87; 2.67] p = 0.143 Probability of a relapse by week 96](/thumbs/33/15779352.jpg) Resolution by the Federal Joint Committee on an amendment to the Pharmaceutical Directive (AM-RL): Appendix XII Resolutions on the benefit assessment of pharmaceuticals with new active ingredients, in
Resolution by the Federal Joint Committee on an amendment to the Pharmaceutical Directive (AM-RL): Appendix XII Resolutions on the benefit assessment of pharmaceuticals with new active ingredients, in
Effective for dates of service on or after January 1, 2015 refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm
 Effective for dates of service on or after January 1, 2015 refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm Name of Policy: Tysabri (natalizumab) Policy #: 281 Latest Review Date: October
Effective for dates of service on or after January 1, 2015 refer to: https://www.bcbsal.org/providers/drugpolicies/index.cfm Name of Policy: Tysabri (natalizumab) Policy #: 281 Latest Review Date: October
Decisions relating to Multiple Sclerosis treatments
 10 October 2014 Decisions relating to Multiple Sclerosis treatments PHARMAC is pleased to announce that, from 1 November 2014, it will: list fingolimod (Gilenya); list natalizumab (Tysabri); and change
10 October 2014 Decisions relating to Multiple Sclerosis treatments PHARMAC is pleased to announce that, from 1 November 2014, it will: list fingolimod (Gilenya); list natalizumab (Tysabri); and change
CNS DEMYLINATING DISORDERS
 CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
How to S.E.A.R.C.H. for the Right MS Therapy for You!
 How to S.E.A.R.C.H. for the Right MS Therapy for You! How to S.E.A.R.C.H. for the Right MS Therapy for You! Copyright Multiple Sclerosis Association of America, 2012. All rights reserved. This booklet
How to S.E.A.R.C.H. for the Right MS Therapy for You! How to S.E.A.R.C.H. for the Right MS Therapy for You! Copyright Multiple Sclerosis Association of America, 2012. All rights reserved. This booklet
UTAH MEDICAID DUR REPORT FEBRUARY 2015 MULTIPLE SCLEROSIS (MS): ORAL DISEASE-MODIFYING DRUGS
 UTAH MEDICAID DUR REPORT FEBRUARY 2015 MULTIPLE SCLEROSIS (MS): ORAL DISEASE-MODIFYING DRUGS Teriflunomide (Aubagio ) Fingolimod (Gilenya ) Dimethyl fumarate (Tecfidera ) Drug Regimen Review Center Joanita
UTAH MEDICAID DUR REPORT FEBRUARY 2015 MULTIPLE SCLEROSIS (MS): ORAL DISEASE-MODIFYING DRUGS Teriflunomide (Aubagio ) Fingolimod (Gilenya ) Dimethyl fumarate (Tecfidera ) Drug Regimen Review Center Joanita
Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy
 Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy Klaus Schmierer, PhD FRCP Blizard Institute, Barts and The London School of Medicine & Dentistry Barts Health
Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy Klaus Schmierer, PhD FRCP Blizard Institute, Barts and The London School of Medicine & Dentistry Barts Health
peginterferon 63, 94 and 125 microgram solution for injection in pre-filled syringe (Plegridy ) SMC No. (1018/14) Biogen Idec Ltd.
 peginterferon 63, 94 and 125 microgram solution for injection in pre-filled syringe (Plegridy ) SMC No. (1018/14) Biogen Idec Ltd. 05 December 2014 The Scottish Medicines Consortium (SMC) has completed
peginterferon 63, 94 and 125 microgram solution for injection in pre-filled syringe (Plegridy ) SMC No. (1018/14) Biogen Idec Ltd. 05 December 2014 The Scottish Medicines Consortium (SMC) has completed
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON
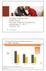 Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Understanding How Existing and Emerging MS Therapies Work
 Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
Multiple Sclerosis - Relapsing and Remissioning
 DISEASE-MODIFYING THERAPIES IN RELAPSING-REMITTING MULTIPLE SCLEROSIS* Benjamin M. Greenberg, MD, MHS ABSTRACT Four major disease-modifying therapies are discussed within the context of relapsing and remitting
DISEASE-MODIFYING THERAPIES IN RELAPSING-REMITTING MULTIPLE SCLEROSIS* Benjamin M. Greenberg, MD, MHS ABSTRACT Four major disease-modifying therapies are discussed within the context of relapsing and remitting
Disease modifying drug therapy
 Disease modifying drug therapy New edition for 2014-15 We hope you find the information in this book helpful. If you would like to speak with someone about any aspect of MS, contact the MS Trust information
Disease modifying drug therapy New edition for 2014-15 We hope you find the information in this book helpful. If you would like to speak with someone about any aspect of MS, contact the MS Trust information
Natalizumab (Tysabri)
 Natalizumab (Tysabri) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Natalizumab (Tysabri) Date of issue: July 2010 Review date: July 2011 Contents Section
Natalizumab (Tysabri) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Natalizumab (Tysabri) Date of issue: July 2010 Review date: July 2011 Contents Section
Disclosures. Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
 Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Multiple sclerosis disease-modifying drugs second line treatments
 Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
Disease Modifying Therapies (DMTs) in Multiple Sclerosis
 Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
Department of Health. Rheynn Slaynt. Clinical Recommendations Committee
 Recommendation 06/13 Department of Health Rheynn Slaynt Clinical Recommendations Committee The Isle of Man Department of Health recommend Gilenya (fingolimod) as a HIGH PRIORITY - as an option for the
Recommendation 06/13 Department of Health Rheynn Slaynt Clinical Recommendations Committee The Isle of Man Department of Health recommend Gilenya (fingolimod) as a HIGH PRIORITY - as an option for the
A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market
 A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market Launch of Several Pipeline Oral Products Could Diminish the Market for Injectable Therapies NEC3-52 November 2014 Contents
A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market Launch of Several Pipeline Oral Products Could Diminish the Market for Injectable Therapies NEC3-52 November 2014 Contents
Version History. Previous Versions. for secondary progressive MS (SPMS) Policy Title. Drugs for MS.Drug facts box Interferon beta 1b
 Version History Policy Title Drugs for MS.Drug facts box Interferon beta 1b for secondary progressive MS (SPMS) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review
Version History Policy Title Drugs for MS.Drug facts box Interferon beta 1b for secondary progressive MS (SPMS) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Multiple Sclerosis Therapy Table of Contents Coverage Policy... 1 General Background... 9 Coding/Billing Information... 14 References... 14 Effective Date...
Cigna Drug and Biologic Coverage Policy Subject Multiple Sclerosis Therapy Table of Contents Coverage Policy... 1 General Background... 9 Coding/Billing Information... 14 References... 14 Effective Date...
Multiple Sclerosis Agents Therapeutic Class Review (TCR) March 13, 2015
 Multiple Sclerosis Agents Therapeutic Class Review (TCR) March 13, 2015 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying,
Multiple Sclerosis Agents Therapeutic Class Review (TCR) March 13, 2015 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying,
Economic Evaluation of Natalizumab (Tysabri) for the treatment of relapsing remitting multiple sclerosis that is rapidly evolving and severe or
 Economic Evaluation of Natalizumab (Tysabri) for the treatment of relapsing remitting multiple sclerosis that is rapidly evolving and severe or sub-optimally treated Summary In January 2007 Biogen Idec
Economic Evaluation of Natalizumab (Tysabri) for the treatment of relapsing remitting multiple sclerosis that is rapidly evolving and severe or sub-optimally treated Summary In January 2007 Biogen Idec
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis
 Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Final Update 3 Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Final Update 3 Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness
Multiple Sclerosis Drug Discoveries - What the Future Holds
 Brochure More information from http://www.researchandmarkets.com/reports/1408035/ Multiple Sclerosis Drug Discoveries - What the Future Holds Description: The recent approval in the US of Novartis' orally
Brochure More information from http://www.researchandmarkets.com/reports/1408035/ Multiple Sclerosis Drug Discoveries - What the Future Holds Description: The recent approval in the US of Novartis' orally
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
Resources for the Patient. Please print these out and give them to your patients with MS
 Resources for the Patient Please print these out and give them to your patients with MS An internet program for persons with MS on the FCM website Multiple Sclerosis: A Toolbox of Information and Resources
Resources for the Patient Please print these out and give them to your patients with MS An internet program for persons with MS on the FCM website Multiple Sclerosis: A Toolbox of Information and Resources
Laquinimod Polman, C. et al. Neurology 2005;64:987-991
 Laquinimod Polman, C. et al. Neurology 2005;64:987-991 Multicenter, double-blind, randomized trial, patients with RR MS received 0.1 mg or 0.3 mg laquinimod or placebo as three daily tablets for 24 weeks
Laquinimod Polman, C. et al. Neurology 2005;64:987-991 Multicenter, double-blind, randomized trial, patients with RR MS received 0.1 mg or 0.3 mg laquinimod or placebo as three daily tablets for 24 weeks
1. Comparative effectiveness of alemtuzumab
 Cost-effectiveness of alemtuzumab (Lemtrada ) for the treatment of adult patients with relapsing remitting multiple sclerosis with active disease defined by clinical or imaging features The NCPE has issued
Cost-effectiveness of alemtuzumab (Lemtrada ) for the treatment of adult patients with relapsing remitting multiple sclerosis with active disease defined by clinical or imaging features The NCPE has issued
Multiple Sclerosis Therapeutics to 2019 - Treatment Diversification, Increasing Efficacy, and Pipeline Innovation Combine to Drive Growth
 Brochure More information from http://www.researchandmarkets.com/reports/2640803/ Multiple Sclerosis Therapeutics to 2019 - Treatment Diversification, Increasing Efficacy, and Pipeline Innovation Combine
Brochure More information from http://www.researchandmarkets.com/reports/2640803/ Multiple Sclerosis Therapeutics to 2019 - Treatment Diversification, Increasing Efficacy, and Pipeline Innovation Combine
What is Multiple Sclerosis? Gener al information
 What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
Clinical Commissioning Policy: Disease Modifying Therapies For patients With Multiple Sclerosis (MS) December 2012. Reference : NHSCB/D4/c/1
 Clinical Commissioning Policy: Disease Modifying Therapies For patients With Multiple Sclerosis (MS) December 2012 Reference : NHSCB/D4/c/1 NHS Commissioning Board Clinical Commissioning Policy: Disease
Clinical Commissioning Policy: Disease Modifying Therapies For patients With Multiple Sclerosis (MS) December 2012 Reference : NHSCB/D4/c/1 NHS Commissioning Board Clinical Commissioning Policy: Disease
