What is Multiple Sclerosis? Disease Modifying Therapies. Best of all. Why is treatment so important? Outline and Expectations.
|
|
|
- Ralph Mathews
- 8 years ago
- Views:
Transcription
1 What is Multiple Sclerosis? Disease Modifying Therapies for Relapsing Remitting MS Jeanie Lynn Cote, MD Chronic, auto immune disorder. As a consequence of immune attacks, there is loss of myelin (fatty protective covering on nerve fibers) and over time destruction of neurons. This can produce an array of symptoms based on the areas of involvement. MRI will show accumulated lesions and global volume loss over time. Leading cause of disability in working aged. Why is treatment so important? Though study designs and specific results vary, in general disease modifying therapies (DMT s) Decrease the number of relapses Prolong time to the next relapse Delay disability progression Decrease new lesion formation on MRI Decrease brain volume loss* Best of all We have options! Many FDA approved DMTs Even more are coming Outline and Expectations The DMTs Discuss Clinical trial results Dosing Side effects Baseline and surveillance needs Touch upon upcoming treatments Recognize the available treatments for RRMS including generic and trade names and how supplied Be aware of common and more serious side effects Know what surveillance is required based on the treatment used Injections Interferons (5) Glatiramer Acetate Oral Teriflunomide Infusions Natalizumab Novantrone* 1
2 Injection Treatments Interferons Beta 1a (2) Beta 1b (2) Pegylated beta 1a Glatiramer Acetate Interferon 1993: Betaseron (250 mcg SQ, EOD) 1996: Avonex (30 mcg IM, weekly) 2002: Rebif (22 or 44 mcg SQ, TIW) 2009: Extavia (250 mcg SQ, EOD) 2014: Plegridy (125 mcg SQ, every 2 weeks) Seems to modify cytokines towards anti inflammatory production (IL 10, IL4), inhibits release of inflammatory cytokines, enhances regulatory T cell function. Interferon Will focus on trial data for IFN B 1a PRISMS 22 mcg, 44 mcg IFN B 1a (Rebif) and placebo Decreased relapse rate (27 and 32% respectively) Reduction in moderate to severe types of relapses Decrease in median MR lesion load and active T2 lesion (greater in 44 mcg dose) EVIDENCE IFN β 1a 44 μg s.c. t.i.w. vs IFN β 1a 30 μg i.m. q.w aka Rebif vs Avonex Overall, more apt to remain relapse free and with fewer new lesions with higher more frequent dosing. Interferon Side effects Injection site reactions including necrosis Flu like side effects Depression, suicidal ideation Hepatotoxicity Decreased counts Less common: Allergic reaction, thrombotic microangiopathy, thyroid abnormalities, seizure* Baseline CBCPD and LFTs Repeat at 1, 3 and 6 months after starting, and periodically thereafter. Copaxone (1996) Glatopa (2015) Glatiramer Acetate Subcutaneous Injection 20 mg daily 40 mg three times per week (2014) Polymer of four amino acids found in MBP Mechanism unknown, however does appear to shift T cells from pro inflammatory Th1 cells to regulatory Th2 cells Glatiramer Acetate Numerous trials Lowers relapse rate relative to placebo (~ 1/3). Lower rate of lesion formation (both T2 and Gd+). Delayed time to next relapse. PreCISe Early treatment can delay conversion to clinically definite MS (CDMS) by 45 % Study patients had CIS with 2 or more T2 lesions 2
3 Glatiramer Acetate Side Effects Injection Site Reaction Lipoatrophy Post injection flushing / chest pain / tachycardia / anxiety / itching / SOB. 16% Lasts minutes Serious allergic reaction rare Teriflunomide Oral Treatments Monitoring: No routine labs required. Gilenya (2010) 0.5 mg capsule daily Binds to the Sphingosine 1 P receptor. Prevents egress of lymphocytes from lymph nodes, reducing the number of lymphocytes in peripheral blood. FREEDOMS Comparison of 0.5 mg, 1.25 mg and placebo ARR.16,.18 and.4 respectively Relapses were reduced by about half on treatment Reduced risk of disability progression propor on of pa ents disease free over 2 years 30% reduction of brain volume loss. Decreased T2 and Gd+ lesions TRANSFORMS compared to weekly IFN beta 1a Needed to have 1 relapse in last year, or 2 in last 2 years More effective in reducing ARR, rate of lesion development, lower rate of brain atrophy Compared with interferon beta 1a, after switching to fingolimod there were no significant changes in EDSS Side effects Decrease in heart rate / AV conduction Macular edema Headache, nausea, GI upset, aches, LFT abnormalities PRES reported Decrease in PFTs reported Infection (including herpes) 3 Cases of PML 3
4 Prior to starting VZV IgG CBCPD and CMP EKG Baseline eye exam (OCT) Baseline skin exam* First Dose Monitoring 6 hours of monitoring after the first dose. EKG and vitals are tracked Additional observation needed if at 6 hours: PR <45 bpm Nadir has not yet occurred ECG shows new onset second degree or higher AV block May need repeated if doses skipped, based on how long someone has been on treatment. After starting CBCPD and CMP every six months Repeat eye exam at 3 4 months Periodic MR surveillance Aubagio (2012) Teriflunomide 7 or 14 mg daily tablets Pyrimidine synthesis inhibitor, however the exact mechanism is unknown. Appears to affect production of T and B cells. Teriflunomide TEMSO 7 mg and 14 mg of teriflunomide vs placebo Reduced ARR by ~30 % 14 mg risk of sustained disability progression ~ 30 % 40 to 67% in lesion volume compared to placebo Number of Gd+ lesions were reduced with both doses, and trend toward greater effect was observed with the higher dose Teriflunomide Side effects Hepatotoxicity Teratogenicity (X) Decreased WBC / infection Headache, diarrhea, nausea, alopecia Peripheral neuropathy One case of Klebsiella PNA ILD theoretical 4
5 Before starting CBCPD, LFTs, HCG TB testing Teriflunomide Monitoring ALT monthly for 6 months LFTs and CBCPD q6 month thereafter Rapid Elimination Activated Charcoal Cholestyramine Tecfidera (2013) 240 mg capsule, twice daily Dimethyl ester of fumaric acid. Shifts cytokine balance towards an anti inflammatory / Th2 state. Activates anti oxidant pathways to reduce oxidative stress. DEFINE Comparing BID and TID dosing to placebo BID ARR by ~ 50% at one year risk of sustained disability progression ~ 38 % CONFIRM Comparing 2 doses of Tecfidera, Copaxone and placebo Not designed to compare the effectiveness of Tecfidera to Copaxone. Significant reduction in disease activity on MR. relapse rates % for Tecfidera compared to placebo. If curious, Copaxone decreased rates ~ 29% compared to placebo. ENDORSE (Extension Study) Evaluating long terms safety and efficacy 2014: Case of PML in MS after 4 yrs of treatment Prolonged lymphopenia may have contributed Side effects Lymphopenia GI upset Flushing Anaphylaxis Pre testing CBCPD, HCG Surveillance CBCPD every 6 months, with particular attention to the lymphocyte count 5
6 Infusions Natalizumab (Tysabri) (Lemtrada) Mitoxantrone (Novantrone) Tysabri (2004) Natalizumab 300 mg IV infusion, monthly Must be TOUCH registered Monoclonal antibody, alpha 4 integrin inhibitor. Prevents migration of leukocytes across vascular endothelium to parenchyma. Natalizumab AFFIRM Reduced risk of disability progression over 2 years by 42% 54% Decreased ARR by 68% during a period of 2 years. 83 % reduction in the accumulation of new or enlarging T2 hyper intense lesions 92 % fewer Gd+ lesions Natalizumab (Tysabri) Side effects / risks Well tolerated Occasional headache / fatigue post infusion. More rarely HSV and VZV Meningitis / encephalitis Hepatotoxicity Allergic reaction / hypersensitivity PML Monitoring includes JCV Ab q 6 months. LFTs as indicated Progressive Multi Focal Leukoencephalopathy Opportunistic viral infection of the brain (JCV) Neuropsychiatric changes and evolving focal neurologic symptoms Progress over days to weeks May include progressive weakness on one side of the body, clumsiness, disturbance of vision, changes in thinking, memory or orientation leading to confusion and personality changes. PML MRI Single or multifocal lesions, large or confluent Indistinct borders Grey and white matter No mass effect No enhancement Most commonly subcortical WM, cerebellar hemispheres, cerebellar peduncles, brain stem Hyperintense on T2/FLAIR, hypointense on T1. JCV PCR in CSF 6
7 Natalizumab associated PML Risk influenced by: JCV status Duration of treatment Previous immunosuppression Natalizumab associated PML Management includes PLEX Improves survival Accelerates drug clearance and immune reconstitution Tysabri Exposure No prior Prior immunosuppresion immunosuppression 1 24 months < 1 / 1,000 1 / 1, months 3 / 1,000 (1:333) 12 / 1,000 (1:83) months 6 / 1,000 (1:140) 13 / 1,000 (1:75) Caution for IRIS PML and 3 post marketing reports of PML in patients on Not previously on Natalizumab One after ~ 2.5 years Another with probable PML after 4 years 3 rd with concomitant history of Crohn s and past chemo exposure Lemtrada (2014) Annual IV infusion Year 1: 5 daily infusions Year 2: 3 daily infusions *Herpetic prophylaxis for 2 mo or CD4 > 200 cells/ml CD52 directed monoclonal antibody (Ab), which binds to T and B lymphocytes, NK cells, monocytes and macrophages. Following binding there is Ab dependent cellular cytolysis and complement mediated lysis. CARE MS I 2 year trial Compared to IFN Beta 1 a (Rebif) in treatment naïve patients ARR of.18 vs 0.39 for Rebif (~ 50% reduction) 60% in severe relapses requiring steroids Significant decrease in frequency of Gd+ lesions 15% on Lemtrada vs 27% on Rebif Care MS II Compared to INF Beta 1 a (Rebif) In patients who had prior relapse on an injectable ~ 50 % improvement in ARR 65% relapse free at 2 years (47% with Rebif) Subset highly active disease, 24% relapse free at 2 years Decrease in brain volume loss Decrease in lesion formation in mean disability score (slight worsening on Rebif) Extension: 70% of EDSS stable to improved at year 3 7
8 Side Effects Other autoimmune disorders Thyroid (34%) Immune thrombocytopenia (ITP, 2%) Anti glomerular basement membrane disease (0.3%) Other cytopenias (0.2%) Infusion reactions Anaphylaxis, Headache, fatigue, rash etc. Increased risk of malignancy, including thyroid, melanoma, lymphoproliferative disease Infections Herpes, Nasopharyngitis, UTI, URI, flu, fungal (Candida, listeria) Pre testing Skin exam Thyroid function Blood count Renal function UA with cell counts VZV IgG (and provide vaccine if needed 6 weeks prior) Tb screening per local guidelines (per insert) Consider Hep B / C screening in high risk patients Surveillance is for 48 months following the last dose Annual skin exam Thyroid studies q3 month Monthly CBCPD Monthly S Cr Monthly UA with counts Annual HPV screening Mitoxantrone Novantrone (2000) RRMS and SPMS IV infusion provided q 3 months Lifetime dose limit of 140 mg/m2 Single dose is 12 mg/m2 Cardiac disease, liver failure and leukemia Rarely used today Other / Emerging Therapies Rituximab Ocrelizumab Ofatumumab Daclizumab 8
Progress in MS: Current and Emerging Therapies
 Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
A neurologist would assess your eligibility and suitability for the DMTs.
 Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Integrating New Treatments: A Case Based Approach
 Integrating New Treatments: A Case Based Approach JILL CONWAY, MD, MA, MSCE DIRECTOR, MS CENTER DIRECTOR, NEUROLOGY CLERKSHIP AT UNCSOM- CHARLOTTE CAMPUS CAROLINAS HEALTHCARE CENTER Objectives Provide
Integrating New Treatments: A Case Based Approach JILL CONWAY, MD, MA, MSCE DIRECTOR, MS CENTER DIRECTOR, NEUROLOGY CLERKSHIP AT UNCSOM- CHARLOTTE CAMPUS CAROLINAS HEALTHCARE CENTER Objectives Provide
New Treatment Options for MS Patients: Understanding risks versus benefits
 New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
Resources for the Primary Care Provider. Please print these out for reference
 Resources for the Primary Care Provider Please print these out for reference Resources for providers American Academy of Neurology www.aan.com Provides education and resources, such as guidelines for clinical
Resources for the Primary Care Provider Please print these out for reference Resources for providers American Academy of Neurology www.aan.com Provides education and resources, such as guidelines for clinical
Disease Modifying Therapies (DMTs) in Multiple Sclerosis
 Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
New treatments in MS What s here and what s nearly here
 5 th MS Research Day, June 14 th 2014 New treatments in MS What s here and what s nearly here David Miller Queen Square MS Centre at UCL and UCLH Course of MS and its treatment Relapsing remitting Disability
5 th MS Research Day, June 14 th 2014 New treatments in MS What s here and what s nearly here David Miller Queen Square MS Centre at UCL and UCLH Course of MS and its treatment Relapsing remitting Disability
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Disclosures. Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
 Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Personalised Medicine in MS
 Personalised Medicine in MS Supportive Evidence from Therapeutic Trials Ludwig Kappos Neurology and Department of Biomedicine University Hospital CH-4031 Basel LKappos@uhbs.ch Established partially effective
Personalised Medicine in MS Supportive Evidence from Therapeutic Trials Ludwig Kappos Neurology and Department of Biomedicine University Hospital CH-4031 Basel LKappos@uhbs.ch Established partially effective
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Multiple Sclerosis Update. Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center
 Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Lemtrada (alemtuzumab)
 Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Information About Medicines for Multiple Sclerosis
 Information About Medicines for Multiple Sclerosis Information About Medicines for Multiple Sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information About Medicines for Multiple Sclerosis Information About Medicines for Multiple Sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Understanding How Existing and Emerging MS Therapies Work
 Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
The MS Disease- Modifying Medications GENERAL INFORMATION
 The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
Information about medicines for multiple sclerosis
 Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Which injectable medication should I take for relapsing-remitting multiple sclerosis?
 Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
The MS Disease- Modifying Medications
 The MS Disease- Modifying Medications National MS Society 1 Current as of November 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
The MS Disease- Modifying Medications National MS Society 1 Current as of November 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
The MS Disease- Modifying Medications
 The MS Disease- Modifying Medications National MS Society 1 Current as of January 2015. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
The MS Disease- Modifying Medications National MS Society 1 Current as of January 2015. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
Treatment in Relapsing MS: Choosing Among the Options. Donald Negroski, MD
 Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio
 ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
Laquinimod Polman, C. et al. Neurology 2005;64:987-991
 Laquinimod Polman, C. et al. Neurology 2005;64:987-991 Multicenter, double-blind, randomized trial, patients with RR MS received 0.1 mg or 0.3 mg laquinimod or placebo as three daily tablets for 24 weeks
Laquinimod Polman, C. et al. Neurology 2005;64:987-991 Multicenter, double-blind, randomized trial, patients with RR MS received 0.1 mg or 0.3 mg laquinimod or placebo as three daily tablets for 24 weeks
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
Medication Policy Manual. Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014
 Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual. Topic: Gilenya, fingolimod Date of Origin: November 22, 2010
 Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Disease modifying drug therapy
 Disease modifying drug therapy New edition for 2014-15 We hope you find the information in this book helpful. If you would like to speak with someone about any aspect of MS, contact the MS Trust information
Disease modifying drug therapy New edition for 2014-15 We hope you find the information in this book helpful. If you would like to speak with someone about any aspect of MS, contact the MS Trust information
Multiple Sclerosis: An imaging review and update on new treatments.
 Multiple Sclerosis: An imaging review and update on new treatments. Dr Marcus Likeman Consultant Neuroradiologist North Bristol NHS Trust Bristol Royal Hospital for Children MRI appearances - White Matter
Multiple Sclerosis: An imaging review and update on new treatments. Dr Marcus Likeman Consultant Neuroradiologist North Bristol NHS Trust Bristol Royal Hospital for Children MRI appearances - White Matter
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru381 Topic: Lemtrada TM, alemtuzumab Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru381 Topic: Lemtrada TM, alemtuzumab Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
News on modifying diseases therapies. Michel CLANET CHU Toulouse France ECTRIMS
 News on modifying diseases therapies Michel CLANET CHU Toulouse France ECTRIMS Current treatment strategies Future oral treatments Future non oral treatments Drug safety and risks CIS at risk of MS Active
News on modifying diseases therapies Michel CLANET CHU Toulouse France ECTRIMS Current treatment strategies Future oral treatments Future non oral treatments Drug safety and risks CIS at risk of MS Active
National Multiple Sclerosis Society. Disease Modification in Multiple Sclerosis. Current as of January 2, 2013
 National Multiple Sclerosis Society Disease Modification in Multiple Sclerosis Current as of January 2, 2013 Since 1993, the U.S. Food and Drug Administration (FDA) has approved several medications for
National Multiple Sclerosis Society Disease Modification in Multiple Sclerosis Current as of January 2, 2013 Since 1993, the U.S. Food and Drug Administration (FDA) has approved several medications for
Relapsing-remitting multiple sclerosis Ambulatory with or without aid
 AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
Is the Grass Really Greener with New Oral Multiple Sclerosis Treatments? A Clinical Debate
 Is the Grass Really Greener with New Oral Multiple Sclerosis Treatments? A Clinical Debate Elizabeth Sebranek Evans, PharmD, BCPS, CGP Alana Whittaker, PharmD, BCPS Roseman University of Health Sciences
Is the Grass Really Greener with New Oral Multiple Sclerosis Treatments? A Clinical Debate Elizabeth Sebranek Evans, PharmD, BCPS, CGP Alana Whittaker, PharmD, BCPS Roseman University of Health Sciences
Altered Immune Response in MS
 How do the Current DMT s Affect the Altered Immune Response in MS Scott Newsome, D.O. Assistant Professor of Neurology Director, Neurology Outpatient Services Johns Hopkins University School of Medicine
How do the Current DMT s Affect the Altered Immune Response in MS Scott Newsome, D.O. Assistant Professor of Neurology Director, Neurology Outpatient Services Johns Hopkins University School of Medicine
Drug Class Review. Disease-modifying Drugs for Multiple Sclerosis. Single Drug Addendum: Fingolimod
 Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
Natalizumab and the Risk of PML
 Natalizumab and the Risk of PML Gloria von Geldern, MD Multiple Sclerosis Center Assistant Professor of Neurology University of Washington May 13, 2015 Conflict of Interest Dr. von Geldern has nothing
Natalizumab and the Risk of PML Gloria von Geldern, MD Multiple Sclerosis Center Assistant Professor of Neurology University of Washington May 13, 2015 Conflict of Interest Dr. von Geldern has nothing
New Developments in the Treatment and Management of Multiple Sclerosis
 New Developments in the Treatment and Management of Multiple Sclerosis Myla D. Goldman, MD, MS For a CME/CEU version of this article, please go to www.namcp.org/cmeonline.htm, and then click the activity
New Developments in the Treatment and Management of Multiple Sclerosis Myla D. Goldman, MD, MS For a CME/CEU version of this article, please go to www.namcp.org/cmeonline.htm, and then click the activity
CNS DEMYLINATING DISORDERS
 CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
The MS Disease- Modifying Drugs. Gener al information
 The MS Disease- Modifying Drugs Gener al information Current as of October 30, 2009. This online version is updated as breaking news requires. If you have downloaded and printed a copy from the web, please
The MS Disease- Modifying Drugs Gener al information Current as of October 30, 2009. This online version is updated as breaking news requires. If you have downloaded and printed a copy from the web, please
Pharmacologic Therapies for Multiple Sclerosis: From injectable to oral agents
 Pharmacologic Therapies for Multiple Sclerosis: From injectable to oral agents KIRANPAL SINGH SANGHA, PHARM.D. CLINICAL PHARMACY SPECIALIST CNS THE UNIVERSITY OF CINCINNATI MEDICAL CENTER Objectives List
Pharmacologic Therapies for Multiple Sclerosis: From injectable to oral agents KIRANPAL SINGH SANGHA, PHARM.D. CLINICAL PHARMACY SPECIALIST CNS THE UNIVERSITY OF CINCINNATI MEDICAL CENTER Objectives List
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS?
 Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
What is MS? 1. disease that affects the central nervous. Is a disease that affects both white and gray matter
 What is MS? 1 Neuron Damaged myelin due to inflammation MS is a chronic immunemediated disease that affects the central nervous system (CNS) Is a disease that affects both white and gray matter Interrupted
What is MS? 1 Neuron Damaged myelin due to inflammation MS is a chronic immunemediated disease that affects the central nervous system (CNS) Is a disease that affects both white and gray matter Interrupted
Treatments for MS: Immunotherapy. Gilenya (fingolimod) Glatiramer acetate (Copaxone )
 Treatments for MS: Immunotherapy There are currently several disease-modifying therapies approved for people with MS in Australia. These therapies, called immunotherapies, work to reduce disease activity
Treatments for MS: Immunotherapy There are currently several disease-modifying therapies approved for people with MS in Australia. These therapies, called immunotherapies, work to reduce disease activity
A blood sample will be collected annually for up to 2 years for JCV antibody testing.
 Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Disease modifying drug therapy
 Disease modifying drug therapy what you need to know Third Edition Karen Alldus Simon Webster SECTION 2 We hope you find the information in this book helpful. If you would like to speak with someone about
Disease modifying drug therapy what you need to know Third Edition Karen Alldus Simon Webster SECTION 2 We hope you find the information in this book helpful. If you would like to speak with someone about
Journal of Central Nervous System Disease
 Journal of Central Nervous System Disease Expert Review Open Access Full open access to this and thousands of other papers at http://www.la-press.com. New and Emerging Disease-Modifying Therapies for Relapsing-Remitting
Journal of Central Nervous System Disease Expert Review Open Access Full open access to this and thousands of other papers at http://www.la-press.com. New and Emerging Disease-Modifying Therapies for Relapsing-Remitting
Multiple sclerosis disease-modifying drugs second line treatments
 Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
Current and future options of MS treatment Prof. Dr. Karl Vass, AKH Wien
 Current and future options of MS treatment Prof. Dr. Karl Vass, AKH Wien European Health Forum, Gastein 6 th October 2010 Multiple Sclerosis is the most common neurological disorder in young Caucasian
Current and future options of MS treatment Prof. Dr. Karl Vass, AKH Wien European Health Forum, Gastein 6 th October 2010 Multiple Sclerosis is the most common neurological disorder in young Caucasian
Conflict of Interest Declaration. Overview of New Medications for Multiple Sclerosis. Assessment Question. Objectives 4/1/2011
 Conflict of Interest Declaration Overview of New Medications for Multiple Sclerosis I or my spouse have no actual or potential conflict of interest in relation to this activity. Crystal Obering, Pharm.D.,
Conflict of Interest Declaration Overview of New Medications for Multiple Sclerosis I or my spouse have no actual or potential conflict of interest in relation to this activity. Crystal Obering, Pharm.D.,
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents
 New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
5.07.09. Aubagio. Aubagio (teriflunomide) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Advanced medicine conference. Monday 20 Tuesday 21 June 2016
 Advanced medicine conference Monday 20 Tuesday 21 June 2016 Relapsing Multiple Sclerosis: new and emerging treatments Jeremy Chataway Queen Square MS Centre National Hospital for Neurology and Neurosurgery,
Advanced medicine conference Monday 20 Tuesday 21 June 2016 Relapsing Multiple Sclerosis: new and emerging treatments Jeremy Chataway Queen Square MS Centre National Hospital for Neurology and Neurosurgery,
Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis
 Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness
Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness
Growth in revenue from MS drugs has been driven largely by price increases over the last several years.
 March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
SECTION 2. Section 2 Multiple Sclerosis (MS) Drug Coverage
 SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
MEDICAL POLICY STATEMENT
 MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 10/01/2013 10/1/2015 08/25/2015 Policy Name Policy Number Multiple Sclerosis Therapy Class SRx-0022
MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 10/01/2013 10/1/2015 08/25/2015 Policy Name Policy Number Multiple Sclerosis Therapy Class SRx-0022
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
Oxford University Hospitals. NHS Trust. Department of Neurology Natalizumab (Tysabri) for Multiple Sclerosis. Information for patients
 Oxford University Hospitals NHS Trust Department of Neurology Natalizumab (Tysabri) for Multiple Sclerosis Information for patients page 2 What is Natalizumab and what is it used for? Natalizumab is an
Oxford University Hospitals NHS Trust Department of Neurology Natalizumab (Tysabri) for Multiple Sclerosis Information for patients page 2 What is Natalizumab and what is it used for? Natalizumab is an
Multiple Sclerosis (MS) Class Update
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
J.P. Morgan Cazenove Therapeutic Seminar
 Jannan, MS J.P. Morgan Cazenove Therapeutic Seminar David Meeker - CEO, Genzyme June 25, 2012 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private
Jannan, MS J.P. Morgan Cazenove Therapeutic Seminar David Meeker - CEO, Genzyme June 25, 2012 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private
PCORI Workshop on Treatment for Multiple Sclerosis. Breakout Group Topics and Questions Draft 3-27-15
 PCORI Workshop on Treatment for Multiple Sclerosis Breakout Group Topics and Questions Draft 3-27-15 Group 1 - Comparison across DMTs, including differential effects in subgroups Consolidated straw man
PCORI Workshop on Treatment for Multiple Sclerosis Breakout Group Topics and Questions Draft 3-27-15 Group 1 - Comparison across DMTs, including differential effects in subgroups Consolidated straw man
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
This information can also be found in the Summit 2011 Program on page 8.
 If you would like to receive CME credit for this activity, please visit: http://www.pesgce.com/pvasummit2011/ This information can also be found in the Summit 2011 Program on page 8. Newer Treatments:
If you would like to receive CME credit for this activity, please visit: http://www.pesgce.com/pvasummit2011/ This information can also be found in the Summit 2011 Program on page 8. Newer Treatments:
Economic Evaluation of Natalizumab (Tysabri) for the treatment of relapsing remitting multiple sclerosis that is rapidly evolving and severe or
 Economic Evaluation of Natalizumab (Tysabri) for the treatment of relapsing remitting multiple sclerosis that is rapidly evolving and severe or sub-optimally treated Summary In January 2007 Biogen Idec
Economic Evaluation of Natalizumab (Tysabri) for the treatment of relapsing remitting multiple sclerosis that is rapidly evolving and severe or sub-optimally treated Summary In January 2007 Biogen Idec
Published by MSAA in March 2013
 Published by MSAA in March 2013 Improving Lives Today! MSAA s MS Research Update is published annually as a service to the MS community. For additional information about MS as well as MSAA s programs and
Published by MSAA in March 2013 Improving Lives Today! MSAA s MS Research Update is published annually as a service to the MS community. For additional information about MS as well as MSAA s programs and
Medication Policy Manual. Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004
 Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Published by MSAA in February 2014
 Published by MSAA in February 2014 VITAMIN D MSAA s MS Research Update is published annually as a service to the MS community. This update provides an overview of the research behind the approved and experimental
Published by MSAA in February 2014 VITAMIN D MSAA s MS Research Update is published annually as a service to the MS community. This update provides an overview of the research behind the approved and experimental
UTAH MEDICAID DUR REPORT FEBRUARY 2015 MULTIPLE SCLEROSIS (MS): ORAL DISEASE-MODIFYING DRUGS
 UTAH MEDICAID DUR REPORT FEBRUARY 2015 MULTIPLE SCLEROSIS (MS): ORAL DISEASE-MODIFYING DRUGS Teriflunomide (Aubagio ) Fingolimod (Gilenya ) Dimethyl fumarate (Tecfidera ) Drug Regimen Review Center Joanita
UTAH MEDICAID DUR REPORT FEBRUARY 2015 MULTIPLE SCLEROSIS (MS): ORAL DISEASE-MODIFYING DRUGS Teriflunomide (Aubagio ) Fingolimod (Gilenya ) Dimethyl fumarate (Tecfidera ) Drug Regimen Review Center Joanita
MULTIPLE SCLEROSIS Management and Therapy
 MULTIPLE SCLEROSIS Management and Therapy 115 th Oklahoma Osteopathic Association Annual Convention April 30 th, 2015 Anthony J. Vaughn, M.D. ANTHONY J. VAUGHN, M.D. Assistant Professor Director, Clinical
MULTIPLE SCLEROSIS Management and Therapy 115 th Oklahoma Osteopathic Association Annual Convention April 30 th, 2015 Anthony J. Vaughn, M.D. ANTHONY J. VAUGHN, M.D. Assistant Professor Director, Clinical
Version History. Previous Versions. Drugs for MS.Drug facts box fingolimod Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Therapeutic Advances in the Management of Multiple Sclerosis
 Therapeutic Advances in the Management of Multiple Sclerosis A CME webcast jointly sponsored by the Postgraduate Institute for Medicine and AcademicCME Fred D. Lublin, MD Course Chair Moderator Saunders
Therapeutic Advances in the Management of Multiple Sclerosis A CME webcast jointly sponsored by the Postgraduate Institute for Medicine and AcademicCME Fred D. Lublin, MD Course Chair Moderator Saunders
Uncertainty in Benefit and Risk: Tysabri (natalizumab)
 Uncertainty in Benefit and Risk: Tysabri (natalizumab) Robert J. Temple, M.D. Deputy Center Director for Clinical Science Center for Drug Evaluation and Research U.S. Food and Drug Administration IOM Drug
Uncertainty in Benefit and Risk: Tysabri (natalizumab) Robert J. Temple, M.D. Deputy Center Director for Clinical Science Center for Drug Evaluation and Research U.S. Food and Drug Administration IOM Drug
Department of Health. Rheynn Slaynt. Clinical Recommendations Committee
 Recommendation 06/13 Department of Health Rheynn Slaynt Clinical Recommendations Committee The Isle of Man Department of Health recommend Gilenya (fingolimod) as a HIGH PRIORITY - as an option for the
Recommendation 06/13 Department of Health Rheynn Slaynt Clinical Recommendations Committee The Isle of Man Department of Health recommend Gilenya (fingolimod) as a HIGH PRIORITY - as an option for the
Managing Relapsing Remitting MS Risks & benefits of emerging therapies. Dr Mike Boggild The Walton Centre
 Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
Multiple Sclerosis Agents Step Therapy with Quantity Limit Program Summary
 Multiple Sclerosis Agents Step Therapy with Quantity Limit Program Summary OBJECTIVE The intent of the Multiple Sclerosis (MS) Agents Step Therapy (ST) program is to encourage the use of preferred multiple
Multiple Sclerosis Agents Step Therapy with Quantity Limit Program Summary OBJECTIVE The intent of the Multiple Sclerosis (MS) Agents Step Therapy (ST) program is to encourage the use of preferred multiple
Literature Scan: Oral Multiple Sclerosis Drugs
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Best practices for using MS disease modifying therapies
 Best practices for using MS disease modifying therapies CMSC Annual Meeting 2015 COREY C FORD, MD, PHD MS SPECIALTY CLINIC UNIVERSITY OF NEW MEXICO HSC MAY 30, 2015 Objectives Use best practices to select
Best practices for using MS disease modifying therapies CMSC Annual Meeting 2015 COREY C FORD, MD, PHD MS SPECIALTY CLINIC UNIVERSITY OF NEW MEXICO HSC MAY 30, 2015 Objectives Use best practices to select
Treating Relapsing Multiple Sclerosis: Risk Stratification and Mitigation. Existing and Emerging MS Therapies. The Challenges of MS Management
 2015 RECENT ADVANCES IN NEUROLOGY Treating Relapsing Multiple Sclerosis: Risk Stratification and Mitigation Bruce Cree, MD, PhD, MAS University of California San Francisco Disclosures Dr. Cree has received
2015 RECENT ADVANCES IN NEUROLOGY Treating Relapsing Multiple Sclerosis: Risk Stratification and Mitigation Bruce Cree, MD, PhD, MAS University of California San Francisco Disclosures Dr. Cree has received
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON
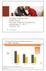 Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Eastern Health MS Service. Tysabri Therapy. Information for People with MS and their Families
 Eastern Health MS Service Tysabri Therapy Information for People with MS and their Families The Eastern Health MS Service has developed this information for you as a guide through what will happen to you
Eastern Health MS Service Tysabri Therapy Information for People with MS and their Families The Eastern Health MS Service has developed this information for you as a guide through what will happen to you
Multiple Sclerosis Treatment Experience Questionnaire (MSTEQ)
 Multiple Sclerosis Treatment Experience Questionnaire (MSTEQ) Name: Today s date (mm/dd/yy): It is important that you take your as prescribed by your physician. However, from to you may find it difficult
Multiple Sclerosis Treatment Experience Questionnaire (MSTEQ) Name: Today s date (mm/dd/yy): It is important that you take your as prescribed by your physician. However, from to you may find it difficult
Resources for the Patient. Please print these out and give them to your patients with MS
 Resources for the Patient Please print these out and give them to your patients with MS An internet program for persons with MS on the FCM website Multiple Sclerosis: A Toolbox of Information and Resources
Resources for the Patient Please print these out and give them to your patients with MS An internet program for persons with MS on the FCM website Multiple Sclerosis: A Toolbox of Information and Resources
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis
 Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Final Update 3 Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Final Update 3 Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness
Recent Advances in the Treatment & Management of Multiple Sclerosis
 Recent Advances in the Treatment & Management of Multiple Sclerosis Consultant: AbbVie, Accordant, Acorda, Bayer, Biogen, Genentech/Roche, Genzyme/Sanofi, Mylan, Novartis, Serono, Teva Research: Actelion,
Recent Advances in the Treatment & Management of Multiple Sclerosis Consultant: AbbVie, Accordant, Acorda, Bayer, Biogen, Genentech/Roche, Genzyme/Sanofi, Mylan, Novartis, Serono, Teva Research: Actelion,
RELAPSE MANAGEMENT. Pauline Shaw MS Nurse Specialist 25 th June 2010
 RELAPSE MANAGEMENT Pauline Shaw MS Nurse Specialist 25 th June 2010 AIMS OF SESSION Relapsing/Remitting MS Definition of relapse/relapse rate Relapse Management NICE Guidelines Regional Clinical Guidelines
RELAPSE MANAGEMENT Pauline Shaw MS Nurse Specialist 25 th June 2010 AIMS OF SESSION Relapsing/Remitting MS Definition of relapse/relapse rate Relapse Management NICE Guidelines Regional Clinical Guidelines
Has the medication received FDA approval? Yes the FDA approved dimethyl fumarate on March 27, 2013.
 Dimethyl Fumarate [formerly called BG-12] (Tecfidera ) CLINICIAN INFORMATION What is the medication? April 2013 Generic: Dimethyl Fumarate [formerly called BG-12] Brand name: Tecfidera The third oral medication
Dimethyl Fumarate [formerly called BG-12] (Tecfidera ) CLINICIAN INFORMATION What is the medication? April 2013 Generic: Dimethyl Fumarate [formerly called BG-12] Brand name: Tecfidera The third oral medication
Multiple Sclerosis (MS) is a disease of the central nervous system (including the brain and spinal cord) in which the nerves degenerate.
 What is Multiple Sclerosis? Multiple Sclerosis (MS) is a disease of the central nervous system (including the brain and spinal cord) in which the nerves degenerate. A disease of the central nervous system
What is Multiple Sclerosis? Multiple Sclerosis (MS) is a disease of the central nervous system (including the brain and spinal cord) in which the nerves degenerate. A disease of the central nervous system
How to S.E.A.R.C.H. for the Right MS Therapy for You!
 How to S.E.A.R.C.H. for the Right MS Therapy for You! How to S.E.A.R.C.H. for the Right MS Therapy for You! Copyright Multiple Sclerosis Association of America, 2012. All rights reserved. This booklet
How to S.E.A.R.C.H. for the Right MS Therapy for You! How to S.E.A.R.C.H. for the Right MS Therapy for You! Copyright Multiple Sclerosis Association of America, 2012. All rights reserved. This booklet
acquired chronic immune-mediated inflammatory condition of CNS. MS in children: 10% +secondary progressive MS: rare +primary progressive MS: rare
 Immunomodulatory Therapies in Pediatric MS Vuong Chinh Quyen Neurology Department Medscape Mar 8, 2013 Multiple Sclerosis in Children. Iran J Child Neurol. 2013 Spring Introduction acquired chronic immune-mediated
Immunomodulatory Therapies in Pediatric MS Vuong Chinh Quyen Neurology Department Medscape Mar 8, 2013 Multiple Sclerosis in Children. Iran J Child Neurol. 2013 Spring Introduction acquired chronic immune-mediated
How to S.E.A.R.C.H. SM for the Right MS Therapy For You!
 How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
- Patients treated with alemtuzumab in CARE-MS II were more than twice as likely to experience disability improvement compared to Rebif -
 PRESS RELEASE Significant Improvement in Disability Scores Observed in Multiple Sclerosis Patients Who Received Lemtrada TM* (Alemtuzumab) Compared With Rebif in Phase lll Trial - Patients treated with
PRESS RELEASE Significant Improvement in Disability Scores Observed in Multiple Sclerosis Patients Who Received Lemtrada TM* (Alemtuzumab) Compared With Rebif in Phase lll Trial - Patients treated with
Treatments-related side effects
 Treatments-related side effects MS: from the diagnosis to the disease management MSc, MD, José Flores Rivera Instituto Nacional de Neurología y Neurocirugía Safety issues: beta interferon Depression/suicidal
Treatments-related side effects MS: from the diagnosis to the disease management MSc, MD, José Flores Rivera Instituto Nacional de Neurología y Neurocirugía Safety issues: beta interferon Depression/suicidal
Natalizumab (Tysabri)
 Natalizumab (Tysabri) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Natalizumab (Tysabri) Date of issue: July 2010 Review date: July 2011 Contents Section
Natalizumab (Tysabri) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Natalizumab (Tysabri) Date of issue: July 2010 Review date: July 2011 Contents Section
Multiple Sclerosis: What You Need To Know. For Professionals
 Multiple Sclerosis: What You Need To Know For Professionals What will I learn today? The Basics: What is MS? Living with MS: A Family Affair We Can Help: The National MS Society What MS Is: MS is thought
Multiple Sclerosis: What You Need To Know For Professionals What will I learn today? The Basics: What is MS? Living with MS: A Family Affair We Can Help: The National MS Society What MS Is: MS is thought
teriflunomide, 14mg, film-coated tablets (Aubagio ) SMC No. (940/14) Genzyme Ltd.
 teriflunomide, 14mg, film-coated tablets (Aubagio ) SMC No. (940/14) Genzyme Ltd. 10 January 2014 (Issued 07 February 2014) The Scottish Medicines Consortium (SMC) has completed its assessment of the above
teriflunomide, 14mg, film-coated tablets (Aubagio ) SMC No. (940/14) Genzyme Ltd. 10 January 2014 (Issued 07 February 2014) The Scottish Medicines Consortium (SMC) has completed its assessment of the above
Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy
 Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy Klaus Schmierer, PhD FRCP Blizard Institute, Barts and The London School of Medicine & Dentistry Barts Health
Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy Klaus Schmierer, PhD FRCP Blizard Institute, Barts and The London School of Medicine & Dentistry Barts Health
Multiple Sclerosis - Relapsing and Remissioning
 DISEASE-MODIFYING THERAPIES IN RELAPSING-REMITTING MULTIPLE SCLEROSIS* Benjamin M. Greenberg, MD, MHS ABSTRACT Four major disease-modifying therapies are discussed within the context of relapsing and remitting
DISEASE-MODIFYING THERAPIES IN RELAPSING-REMITTING MULTIPLE SCLEROSIS* Benjamin M. Greenberg, MD, MHS ABSTRACT Four major disease-modifying therapies are discussed within the context of relapsing and remitting
