PCORI Workshop on Treatment for Multiple Sclerosis. Breakout Group Topics and Questions Draft
|
|
|
- Howard Doyle
- 10 years ago
- Views:
Transcription
1 PCORI Workshop on Treatment for Multiple Sclerosis Breakout Group Topics and Questions Draft Group 1 - Comparison across DMTs, including differential effects in subgroups Consolidated straw man questions 1.a What are the comparative harms and benefits of the different disease-modifying therapies in newly diagnosed relapsing, remitting multiple sclerosis in terms of disease progression and disability? 1.b Among MS patients receiving a disease modifying therapy who experience a relapse, what are the benefits and harms of continuing the same therapy versus switching to a new medication or a similar medication (e.g., interferon) with a different dosage and administration frequency? Considerations Subgroups (examples) Relapsing remitting, primary progressive, secondary progressive Degree of underlying disease activity Low socioeconomic status or poor access to healthcare Sex Newly-diagnosed vs. established disease Original (refined) questions 1.1 How do the currently-approved DMTs compare in terms of slowing the progression of people living with MS from the relapsing-remitting (RR) form of the disease to the secondary-progressive (SP) form of the disease? 1.2 What is the comparative effectiveness of the various DMTs to treat symptoms and regenerate or enhance remyelination? 1.3 What is the effect of MS or its treatments on susceptibility to other autoimmune disorders? What is the effect of DMTs upon the risk of infections or malignancies? 1
2 1.4 Conduct a 3-5 year randomized, controlled trial to compare Glatiramer acetate (Copaxone), Fingolimod (Gilenya), Dimethyl fumarate (Tecfidera), Interferon Beta-1a (Avonex and Rebif), and Peginterferon beta-1a (Plegridy) in newly diagnosed relapsing, remitting multiple sclerosis for effects on disease progression and disability. 1.5 In patients with relapsing multiple sclerosis, what is the impact of step therapy for medications on relapse rates, patient daily function, and disability progression? 1.6 Among MS patients receiving a disease modifying therapy who experience a relapse, what are the benefits and risks of continuing same therapy versus switching to a new mechanism or similar mechanism with different dosage, i.e. low dose / low frequency vs. high dose / high frequency interferons? 1.7 In people with a diagnosis of relapsing MS, what are the comparative benefits of the current disease modifying therapies on disability progression, cognitive function, depression, and quality of life. 1.8 In patients with MS on Tysabri is there efficacy - by clinical relapses, disability progression, MRI activity - and safety of extended dosing of Natalizumab (NTZ)? OR What are the comparative benefits and risks of extended vs FDA approved dosing? 1.9 What is the risk of PML in patients on extended dosing of Natalizumab (NTZ) compared with FDA approved dosing? 1.10 In persons with relapsing-remitting MS, study the comparative effectiveness of currently approved therapies on MS symptoms such as 1) cognitive impairment, 2) fatigue, 3) pain, or 4) spasticity in RR-MS patients who experience such symptoms Once diagnosed with MS (any type), how should the patient be treated to help them return to normal or to be as functional as possible (QOL)? Questions about subgroups 1.12 Compare intensity of Primary Progressive symptoms and co-morbidities across ethnic and socio-economic groups before and after effective treatment of the comorbidity What is the risk/benefit of a given DMD (same as DMT) for patients by degree of underlying disease activity? 2
3 1.14 Compare outcomes for each of the DMDs in terms of QoL, job productivity, etc., in patients who are progressing due to an aggressive, malignant relapsing MS (e.g., having breakthrough relapses) versus patients who are primary progressive MS (i.e., experiencing disability but not having any relapses) 1.15 For people who are Medicaid beneficiaries who have MS; who have mental health and substance abuse diagnoses what is the rate of progression of their MS? 1.16 What is the comparative effectiveness of DMTs on MS-related symptoms in patients with MS? Does this effectiveness differ for different subgroups of MS patients (i.e. those with relapsing, remitting MS, those with secondary progressive MS, or those with primary progressive MS)? 1.17 Do DMTs or symptomatic treatments differ in effectiveness or safety by patient characteristics, MS type or other disease characteristics, genetic differences, or other patient subgroups? 3
4 Group 2 - Care strategies Consolidated straw man questions 2.a In patients with relapsing-remitting, secondary progressive, and primary progressive, what are the comparative benefits and harms, in terms of both clinically-assessed outcomes and patient-reported outcomes, of treatment by either an MS specialist, general neurologist or other clinician? 2.b What are the comparative benefits and risks, in terms of both clinically-assessed outcomes and patient-reported outcomes, of treating to a standard of no-evidence of disease activity (NEDA) compared with other strategies in people with relapsing-remitting MS? 2.c What are the effects of different care strategies (e.g., solo practitioner, collaborative care, interdisciplinary care teams) in effectively treating MS-related symptoms in MS patients with and without comorbidities? Considerations What healthcare systems modifications would be useful, in terms of technology, personnel, or care models? Original (refined) questions 2.1 What are the comparative benefits and risks of MS specialist center vs. Community Neurologist based care for patients with progressive MS (SPMS/ PPMS)? This question should look at outcomes that include symptoms of depression, cognition, mobility, sphincter disturbance and employability. 2.2 What are the comparative benefits and risks of MS specialist center vs. Community Neurologist based care for patients with RRMS (EDSS 0-5.0)? This question should look at long term (3+ years) outcomes that include mobility, cognition, mood and employability. 2.3 In patients with MS and visual impairment, what is the effect on medication compliance of use of alternative prescribing instructions suitably for the visually impaired? 2.4 In patients with MS with mobility challenges, what is the effect on use of preventive screening and patient experience by providing accessible exam equipment, such as exam table scales, compared to usual care? 2.5 Compare intensity of Primary Progressive symptoms and co-morbidities across ethnic and socio-economic groups before and after effective treatment of the comorbidity. 4
5 2.6 What are the comparative benefits and risks, in terms of both clinically-assessed outcomes and patient-reported outcomes, of treatment by either an MS specialist, general neurologist or other clinician for people with RRMS, Secondary-Progressive MS (SPMS) or Primary-Progressive MS (PPMS)? 2.7 What are the comparative benefits and risks of MS specialist center vs. Community Neurologist based care for patients with progressive MS (SPMS/ PPMS)? This question should look at outcomes that include symptoms of depression, cognition, mobility, sphincter disturbance and employability. 2.8 What are the comparative benefits and risks, in terms of both clinically-assessed outcomes and patient-reported outcomes, of treating to a standard of NEDA compared with other strategies in people with relapsing-remitting MS? (Treating to a NEDA standard means changing/escalating treatment upon evidence of disease activity in the form of relapses, disability progression, and/or MRI lesion activity.) 2.9 Of the currently approved disease modifying drug therapies how can doctors help patients select the best, meaning maximum benefit and lowest risk, drug for their form or stage of MS? Should disease modifying therapies be just the start of treatment? Or should these drugs be used in conjunction with OT, PT, ST, and exercise therapy to help reduce de-conditioning and more progressive stages of MS? 2.10 What existing tools, or as-yet not developed tools, effectively measure MS-related symptoms? How can these tools be used to identify best practices to mitigate the impact of these symptoms on patients quality of life? 2.11 What are the comparative safety and effectiveness of strategies to help patients engage in key self-management behaviors for managing MS or MS-related symptoms? 2.12 How do people with MS find, assess, and integrate treatment information to manage their health? 2.13 What kind of information do people with MS seek to inform their self-care or shared decision making with their healthcare providers? 2.14 What is the comparative effectiveness of annual (once per annum) vs. semi-annual (twice per annum) cranial magnetic resonance imaging scanning for the management of MS disease modifying therapy in people with relapsing MS? 5
6 Group 3 - Non-pharmacologic and non-dmt therapy for specific symptoms and overall health Consolidated straw man questions 3.a For persons with MS who have moderate to severe fatigue, compare strategies for fatigue management education, including individual, group, online, medication vs aerobic exercise to counteract deconditioning for their effects on fatigue and quality of life. 3.b In people with relapse-remitting MS who are already receiving disease modifying therapies (DMT), compare the effectiveness of DMT alone or DMT plus physical rehabilitation interventions and other adjunctive therapies for decreasing the rate and accumulation of disability. Considerations Specific symptoms (examples): Fatigue Depression Disability Cognitive impairment In patients with multiple sclerosis and [specific characteristics], what is the comparative effectiveness of the following (examples): Rehabilitation for impairments, mobility, and function Different levels of exercise on prevention of disability and restoration of function Complementary and alternative medicine, occupational therapy, physical therapy, massage, and speech therapy Standard pharmacologic therapy compared to appropriate non-pharmacologic therapy Nutritional supplements Mind and body approaches, such as mindfulness meditation, relaxation exercises, yoga, and tai chi 6
7 Original (refined) questions 3.1 In people with relapse-remitting MS who are already receiving disease modifying therapies (DMT), compare the effectiveness of DMT alone or DMT plus physical rehabilitation interventions for decreasing the rate and accumulation of disability. Rehabilitation Interventions can include an integrated, comprehensive team approach that can be defined and measured, or can compare specific physical interventions designed to address impairments, mobility and function. 3.2 In people with progressive forms of MS, compare the effectiveness of low, moderate, and high intensity exercise on health measures, prevention of disability and restoration of function. 3.3 Compare the effectiveness of standard therapy (pharma, CAM, PT, OT) for symptoms and/or relapse intensity and/or frequency in R/R MS across ethnic and socio-economic groups. 3.4 Compare the effectiveness of standard therapy (pharma, CAM, PT, OT) for progression to secondary progressive MS across ethnic and socio-economic groups. 3.5 What are the comparative benefits and risks of occupational or physical therapy vs. pharmaceutical treatments for patients with primary progressive MS? 3.6 What are the comparative benefits and risks of nutritional supplements vs. not using nutritional supplements in patients with primary progressive MS? 3.7 What are the harms and benefits of the disease modifying therapies with and without adjunctive treatments of OT, PT, ST, and exercise therapy to reduce de-conditioning in patients in the more progressive stages of MS? How does the form or stage of MS affect these results? 3.8 What is the comparative effectiveness of complementary and integrative approaches in particular massage, manipulation, or physical therapy for MS patients with chronic pain? 3.9 What are the comparative benefits and risks of mind and body approaches (mindfulness meditation, relaxation exercises, yoga, tai chi, etc.) interventions on emotional health and quality of life of MS patients? 3.10 What are the comparative benefits and risks of early wellness interventions (mind and body approaches, diet and exercise) on mental health outcomes (depression, anxiety) of MS patients? 7
8 3.11 In patients with MS, what are the best screening tools to evaluate the presence and severity of MS-related cognitive impairment? 3.12 Among MS experiencing depression, what are the comparative benefits and risks of treatment with antidepressants with or without counseling? 3.13 What are the comparative benefits and risks of pharmacological and non-pharmacological treatments for depression in people with a diagnosis of MS? Target population: diagnosis of MS, between the ages of 18 55, depression determined by BDI) score >13. Primary outcomes: change in BDI score from baseline to 3,6,12 months, comparison of side effects, comparison of risk perception of the various interventions 3.14 For persons with MS who have moderate to severe fatigue (average score of 4 or higher on Fatigue Severity Scale), compare strategies for fatigue management education (individual, group, online, medication vs aerobic exercise to counteract deconditioning) for their effects on Fatigue Impact (U-FIS), Quality of Life (FACT-G or SF-36), Self-Efficacy (SEPESCA) and energy conservation behavioral change (ECSS) For MS patients with specific symptoms (pain, spasticity, partial or complete paralysis, muscle weakness, fatigue, cognitive dysfunction, etc.) compare strategies to reduce these symptoms, both short and long term? 3.16 What are the comparative benefits and risks of individual fatigue management education, fatigue medication, and aerobic exercise for persons with MS and fatigue? 3.17 What is the comparative effectiveness of therapies other than DMTs (e.g., exercise therapy, CAM therapies, non-dmt pharmacologic therapies) on MS-related symptoms in patients with MS? Does this effectiveness differ for different subgroups of MS patients (i.e. those with relapsing, remitting MS, those with secondary progressive MS, or those with primary progressive MS)? 3.18 How does the use of therapies other than DMTs for patients with MS impact the effectiveness of DMTs in patients with MS? 3.19 In patients with MS who are experiencing cognitive impairment, what are the most effective pharmacotherapeutic and nonpharmacotherapeutic treatments to alleviate cognitive impairment? 3.20 How effective is movement or exercise in reducing symptoms and complications, including urinary tract infections, body sores, respiratory infections, spasticity, and de-conditioning/muscle loss/weakening? 8
9 3.21 In patients with relapsing remitting multiple sclerosis and significant fatigue, compare pharmaceutical approaches to reduce the level of fatigue What are the comparative benefits and risks of individual and online individual format fatigue management education for persons with MS and fatigue? 9
10 Group 4 - Timing of therapy and study design 4.a In patients with primary progressive multiple sclerosis, what are the harms and benefits of continuing compared to discontinuing DMTs on symptoms, disability, and mortality? 4.b What are the harms and benefits of early vs. delayed (2-3 years) treatment with DMTs, in terms of symptoms and quality of life? 4.c Study designs What are the advantages and disadvantages of clinical trials that focus on a specific subset of populations, interventions, and outcomes vs. a larger, more comprehensive large observational study? Original (refined) questions 4.1 What are the comparative benefits and harms, in terms of both clinically-assessed outcomes and patient-reported outcomes, of treatment with each of the currently-approved disease modifying therapies (DMTs), as well as the case where they opt for no DMT at all, for people with relapsing-remitting multiple sclerosis (RRMS)? 4.2 In patients with SPMS, compare continuation vs. stopping immunomodulatory medications on outcomes. In patients with SPMS, compare continuation vs. stopping immunomodulatory medications on outcomes. 4.3 What are the harms and benefits of long-term vs. short-term or stopping or no DMT in terms of symptoms, disability, and mortality in patients with PPMS? 4.4 In patients who transition from relapse remitting MS to secondary progressive MS, what are the long term benefits and harms of prolonged treatment (more than 3 years) with DMTs vs. discontinuing DMTs? 4.5 Compare the outcomes (clinical and health) of patients with MS who begin treatment on a DMD early versus patients who delay treatment for 2-3 years. 4.6 In stable MS patients over the age of 5, compare clinically relevant outcomes in those who discontinue DMTs vs. continue DMTs to compare the risk of recurrent inflammatory MS activity, the risk of disability progression, and quality of life and other patient-reported outcomes (PROs). 10
11 4.7 Perform a longitudinal study, including data on age at diagnosis, gender, ethnicity, number of relapses/year, number of lesions, location of lesions, medication exposure (which drugs, treatment duration, include all drugs in analysis, failures, switches, etc.), exercise habits, co-morbidities, changes in mobility at six month increments to determine associations with outcomes, including cognitive function, performance of ADL s, employment, etc. 11
12 Cross-cutting issues C.1 How should the MS person s voice [e.g. Patient-reported outcome (PRO) measures] be used in a clinical trial aimed at slowing or stopping the progression of MS? C.2 How should MS persons input be leveraged to develop new clinician rated or performance based clinical outcome measures for MS? C.3 What outcomes should be used to determine the effectiveness of disease modifying drugs in clinical practice, and how should the MS person s voice be weighted? C.4 What are the best ways to inform patients with primary progressive MS about current research results and clinical trials? C.5 In patients with MS, what are the best screening tools to evaluate the presence and severity of MS-related fatigue? C.6 For MS patients experiencing a relapse, how do shared decision making aids impact patient satisfaction and patient reported outcomes? C.7 What are performance based assessments that can be utilized to assess cognitive function in outpatient clinical setting of patients with MS? C.8 What key patient reported outcomes could be used to support comparative effectiveness assessments for MS treatments and help MS patients making treatment decisions? C.9 How do the most frequently used MS outcomes (e.g., annualized relapse rate, disease progression, and disability) correlate with symptom severity, health-related quality of life, and other patient-centered outcomes that are meaningful to patients? C.10 What considerations are most important to patients and providers in deciding between various DMTs and/or other symptomatic treatment options? C.11 How do patient preferences influence the trade-offs between symptom relief and other treatment goals in MS? C.12 What factors impact decision making when it comes to motherhood and MS? C.13 In patients with MS and visual impairment, what is the effect on medication compliance of use of alternative prescribing instructions suitably for the visually impaired? 12
13 C.14 In patients with MS with mobility challenges, what is the effect on use of preventive screening and patient experience by providing accessible exam equipment, such as exam table scales, compared to usual care? C.15 Perform a longitudinal study, including data on age at diagnosis, gender, ethnicity, number of relapses/year, number of lesions, location of lesions, medication exposure (which drugs, treatment duration, include all drugs in analysis, failures, switches, etc.), exercise habits, co-morbidities, changes in mobility at six month increments to determine associations with outcomes, including cognitive function, performance of ADL s, employment, etc. 13
Progress in MS: Current and Emerging Therapies
 Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
What is Multiple Sclerosis? Gener al information
 What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
Relapsing-remitting multiple sclerosis Ambulatory with or without aid
 AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Study Support Materials Cover Sheet
 Study Support Materials Cover Sheet Document Title ESCALATE Patient Brochure Intended Audience This brochure is designed to be given to potentially eligible patients as a take-home summary of key information
Study Support Materials Cover Sheet Document Title ESCALATE Patient Brochure Intended Audience This brochure is designed to be given to potentially eligible patients as a take-home summary of key information
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Multiple Sclerosis Update. Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center
 Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Medication Policy Manual. Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014
 Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Wellness for People with MS: What do we know about Diet, Exercise and Mood And what do we still need to learn? March 2015
 Wellness for People with MS: What do we know about Diet, Exercise and Mood And what do we still need to learn? March 2015 Introduction Wellness and the strategies needed to achieve it is a high priority
Wellness for People with MS: What do we know about Diet, Exercise and Mood And what do we still need to learn? March 2015 Introduction Wellness and the strategies needed to achieve it is a high priority
Disease Modifying Therapies (DMTs) in Multiple Sclerosis
 Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
SECTION 2. Section 2 Multiple Sclerosis (MS) Drug Coverage
 SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
A blood sample will be collected annually for up to 2 years for JCV antibody testing.
 Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
NHS BOURNEMOUTH AND POOLE AND NHS DORSET
 NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
Mellen Center for Multiple Sclerosis
 Mellen Center Cleveland Clinic Marie Namey, RN, MSN, MSCN Mellen Center Cleveland Clinic Cleveland, OH Home of. Mellen Center for Multiple Sclerosis Mellen Center Mission The Mellen Center remains committed
Mellen Center Cleveland Clinic Marie Namey, RN, MSN, MSCN Mellen Center Cleveland Clinic Cleveland, OH Home of. Mellen Center for Multiple Sclerosis Mellen Center Mission The Mellen Center remains committed
Multiple Sclerosis (MS) Class Update
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON
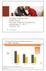 Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Clinical Commissioning Policy: Disease Modifying Therapies For patients With Multiple Sclerosis (MS) December 2012. Reference : NHSCB/D4/c/1
 Clinical Commissioning Policy: Disease Modifying Therapies For patients With Multiple Sclerosis (MS) December 2012 Reference : NHSCB/D4/c/1 NHS Commissioning Board Clinical Commissioning Policy: Disease
Clinical Commissioning Policy: Disease Modifying Therapies For patients With Multiple Sclerosis (MS) December 2012 Reference : NHSCB/D4/c/1 NHS Commissioning Board Clinical Commissioning Policy: Disease
Which injectable medication should I take for relapsing-remitting multiple sclerosis?
 Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Medication Policy Manual. Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004
 Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Version History. Previous Versions. Drugs for MS.Drug facts box fingolimod Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Medication Policy Manual. Topic: Gilenya, fingolimod Date of Origin: November 22, 2010
 Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Biogen Global Medical Grants Office Multiple Sclerosis: Areas of Interest
 Biogen Global Medical Grants Office Multiple Sclerosis: Areas of Interest Cycle C June 2, 2015 1 Introduction The landscape for the treatment of relapsing Multiple Sclerosis (MS) has quickly evolved over
Biogen Global Medical Grants Office Multiple Sclerosis: Areas of Interest Cycle C June 2, 2015 1 Introduction The landscape for the treatment of relapsing Multiple Sclerosis (MS) has quickly evolved over
Issues Regarding Use of Placebo in MS Drug Trials. Peter Scott Chin, MD Novartis Pharmaceuticals Corporation
 Issues Regarding Use of Placebo in MS Drug Trials Peter Scott Chin, MD Novartis Pharmaceuticals Corporation Context of the Guidance The draft EMA Guidance mentions placebo as a comparator for superiority
Issues Regarding Use of Placebo in MS Drug Trials Peter Scott Chin, MD Novartis Pharmaceuticals Corporation Context of the Guidance The draft EMA Guidance mentions placebo as a comparator for superiority
06/06/2012. The Impact of Multiple Sclerosis in the Pacific Northwest. James Bowen, MD. Swedish Neuroscience Institute
 The Impact of Multiple Sclerosis in the Pacific Northwest James Bowen, MD Multiple Sclerosis Center Multiple Sclerosis Center Swedish Neuroscience Institute 1 2 Motor Symptoms of MS Weakness Spasticity
The Impact of Multiple Sclerosis in the Pacific Northwest James Bowen, MD Multiple Sclerosis Center Multiple Sclerosis Center Swedish Neuroscience Institute 1 2 Motor Symptoms of MS Weakness Spasticity
A neurologist would assess your eligibility and suitability for the DMTs.
 Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
MSTAC Initial Application
 MSTAC Initial Application Please send applications to: Facsimile 04 916 7571 Further Contact Details: Address The Co-ordinator MSTAC PHARMAC P O Box 10-254 WELLINGTON Phone 04 460 4990 Email [email protected]
MSTAC Initial Application Please send applications to: Facsimile 04 916 7571 Further Contact Details: Address The Co-ordinator MSTAC PHARMAC P O Box 10-254 WELLINGTON Phone 04 460 4990 Email [email protected]
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
Multiple Sclerosis: What You Need To Know. For Professionals
 Multiple Sclerosis: What You Need To Know For Professionals What will I learn today? The Basics: What is MS? Living with MS: A Family Affair We Can Help: The National MS Society What MS Is: MS is thought
Multiple Sclerosis: What You Need To Know For Professionals What will I learn today? The Basics: What is MS? Living with MS: A Family Affair We Can Help: The National MS Society What MS Is: MS is thought
Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy
 Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy Klaus Schmierer, PhD FRCP Blizard Institute, Barts and The London School of Medicine & Dentistry Barts Health
Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy Klaus Schmierer, PhD FRCP Blizard Institute, Barts and The London School of Medicine & Dentistry Barts Health
Lemtrada (alemtuzumab)
 Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market
 A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market Launch of Several Pipeline Oral Products Could Diminish the Market for Injectable Therapies NEC3-52 November 2014 Contents
A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market Launch of Several Pipeline Oral Products Could Diminish the Market for Injectable Therapies NEC3-52 November 2014 Contents
1. Comparative effectiveness of alemtuzumab
 Cost-effectiveness of alemtuzumab (Lemtrada ) for the treatment of adult patients with relapsing remitting multiple sclerosis with active disease defined by clinical or imaging features The NCPE has issued
Cost-effectiveness of alemtuzumab (Lemtrada ) for the treatment of adult patients with relapsing remitting multiple sclerosis with active disease defined by clinical or imaging features The NCPE has issued
Survey of 267 Patients Using Low Dose Naltrexone for Multiple Sclerosis
 Survey of 267 Patients Using Low Dose Naltrexone for Multiple Sclerosis Summary In order to stimulate interest among other academic researchers in LDN trials for MS, an online patient tracking system has
Survey of 267 Patients Using Low Dose Naltrexone for Multiple Sclerosis Summary In order to stimulate interest among other academic researchers in LDN trials for MS, an online patient tracking system has
How to S.E.A.R.C.H. SM for the Right MS Therapy For You!
 How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
Review Date: March 2012. Issue Status: Approved Issue No: 2 Issue Date: March 2010
 Title: Multiple Sclerosis guidelines for the use of beta-interferon, glatiramer acetate, natalizumab, mitoxantrone and other disease Authors Name: Dr P Talbot Contact Name: Dr Paul Talbot Contact Phone
Title: Multiple Sclerosis guidelines for the use of beta-interferon, glatiramer acetate, natalizumab, mitoxantrone and other disease Authors Name: Dr P Talbot Contact Name: Dr Paul Talbot Contact Phone
Multiple Sclerosis Drug Discoveries - What the Future Holds
 Brochure More information from http://www.researchandmarkets.com/reports/1408035/ Multiple Sclerosis Drug Discoveries - What the Future Holds Description: The recent approval in the US of Novartis' orally
Brochure More information from http://www.researchandmarkets.com/reports/1408035/ Multiple Sclerosis Drug Discoveries - What the Future Holds Description: The recent approval in the US of Novartis' orally
Patient Group Input to CADTH
 Patient Group Input to CADTH Section 1 General Information Name of the drug CADTH is reviewing and indication(s) of interest Name of patient group/author of submission Patient group s contact information:
Patient Group Input to CADTH Section 1 General Information Name of the drug CADTH is reviewing and indication(s) of interest Name of patient group/author of submission Patient group s contact information:
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS?
 Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
A Letter From the MS Coalition
 0 A Letter From the MS Coalition The treatment of multiple sclerosis (MS) requires a comprehensive management strategy. One important component of that strategy is modifying the disease course. When deciding
0 A Letter From the MS Coalition The treatment of multiple sclerosis (MS) requires a comprehensive management strategy. One important component of that strategy is modifying the disease course. When deciding
Summary HTA. Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C. HTA-Report Summary
 Summary HTA HTA-Report Summary Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C Health policy background Multiple sclerosis (MS) is a chronic inflammatory disease
Summary HTA HTA-Report Summary Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C Health policy background Multiple sclerosis (MS) is a chronic inflammatory disease
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
Supporting MS-Related Disability Claims to Private Insurers: The Physician s Role
 Supporting MS-Related Disability Claims to Private Insurers: The Physician s Role What Is This Guide? This guide was compiled by the National Multiple Sclerosis Society as an aid to health care professionals
Supporting MS-Related Disability Claims to Private Insurers: The Physician s Role What Is This Guide? This guide was compiled by the National Multiple Sclerosis Society as an aid to health care professionals
Information about medicines for multiple sclerosis
 Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information about medicines for multiple sclerosis Information about medicines for multiple sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Understanding. Multiple Sclerosis. Tim, diagnosed in 2004.
 Understanding Multiple Sclerosis Tim, diagnosed in 2004. What Is Multiple Sclerosis? Multiple sclerosis (MS) is a neurologic disorder that affects the central nervous system (CNS). The CNS includes the
Understanding Multiple Sclerosis Tim, diagnosed in 2004. What Is Multiple Sclerosis? Multiple sclerosis (MS) is a neurologic disorder that affects the central nervous system (CNS). The CNS includes the
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
ISSUE DATE May 11, 2015 SUBJECT EFFECTIVE DATE May 18, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Multiple Sclerosis Agents Pharmacy Service Leesa M. Allen, Deputy Secretary
Fatigue in MS: 2005 update B. Colombo University of Milan - HSR
 Fatigue in MS: 2005 update B. Colombo University of Milan - HSR Fatigue in MS One of the more disabling symptoms Affects about 75/90 % of the patients May be the onset symptom Transient or chronic May
Fatigue in MS: 2005 update B. Colombo University of Milan - HSR Fatigue in MS One of the more disabling symptoms Affects about 75/90 % of the patients May be the onset symptom Transient or chronic May
EMD Serono Presents New Data on Rebif (Interferon beta-1a) and Multiple Sclerosis Pipeline at Joint ACTRIMS-ECTRIMS Meeting in Boston
 Erin-Marie Beals Phone 1-781-681-2850 September 9, 2014 EMD Serono Presents New Data on Rebif (Interferon beta-1a) and Multiple Sclerosis Pipeline at Joint ACTRIMS-ECTRIMS Meeting in Boston Data include
Erin-Marie Beals Phone 1-781-681-2850 September 9, 2014 EMD Serono Presents New Data on Rebif (Interferon beta-1a) and Multiple Sclerosis Pipeline at Joint ACTRIMS-ECTRIMS Meeting in Boston Data include
Multiple Sclerosis: An imaging review and update on new treatments.
 Multiple Sclerosis: An imaging review and update on new treatments. Dr Marcus Likeman Consultant Neuroradiologist North Bristol NHS Trust Bristol Royal Hospital for Children MRI appearances - White Matter
Multiple Sclerosis: An imaging review and update on new treatments. Dr Marcus Likeman Consultant Neuroradiologist North Bristol NHS Trust Bristol Royal Hospital for Children MRI appearances - White Matter
Growth in revenue from MS drugs has been driven largely by price increases over the last several years.
 March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
SammyJo s MS-LDN Timeline
 SammyJo s MS-LDN Timeline Better 1 Stop all other meds 4/4 2 EDSS Disability Scale 3 3 3.5 3.5 3.5 3.5 4 4 4 4 4.5 5 5_ 6 7 8 Start LDN 2/4 6.5 7 9 Worse 1 Steroids Copaxone Novan trone Avonex Steroid
SammyJo s MS-LDN Timeline Better 1 Stop all other meds 4/4 2 EDSS Disability Scale 3 3 3.5 3.5 3.5 3.5 4 4 4 4 4.5 5 5_ 6 7 8 Start LDN 2/4 6.5 7 9 Worse 1 Steroids Copaxone Novan trone Avonex Steroid
Information About Medicines for Multiple Sclerosis
 Information About Medicines for Multiple Sclerosis Information About Medicines for Multiple Sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information About Medicines for Multiple Sclerosis Information About Medicines for Multiple Sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK
 fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK 08 August 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK 08 August 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
Multiple Sclerosis. Current and Future Players. GDHC1009FPR/ Published March 2013
 Multiple Sclerosis Current and Future Players GDHC1009FPR/ Published March 2013 Executive Summary Moderate Growth in the Multiple Sclerosis Market is Expected from 2012 2022 GlobalData estimates the 2012
Multiple Sclerosis Current and Future Players GDHC1009FPR/ Published March 2013 Executive Summary Moderate Growth in the Multiple Sclerosis Market is Expected from 2012 2022 GlobalData estimates the 2012
Treatment in Relapsing MS: Choosing Among the Options. Donald Negroski, MD
 Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
New perception of disability including cognition, fatigue, pain and other impairments related to MS
 New perception of disability including cognition, fatigue, pain and other impairments related to MS Diego Cadavid, MD Director, MS Clinical Development Biogen Idec 1 Need for clarity on terminology for
New perception of disability including cognition, fatigue, pain and other impairments related to MS Diego Cadavid, MD Director, MS Clinical Development Biogen Idec 1 Need for clarity on terminology for
Multiple sclerosis disease-modifying drugs second line treatments
 Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple Sclerosis
 Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents
 New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
How To Use A Drug In Multiple Sclerosis
 Revised (2009) guidelines for prescribing in multiple sclerosis INTRODUCTION In January 2001, the (ABN) first published guidelines for the use of licensed disease modifying treatments (ß-interferon and
Revised (2009) guidelines for prescribing in multiple sclerosis INTRODUCTION In January 2001, the (ABN) first published guidelines for the use of licensed disease modifying treatments (ß-interferon and
Drug Class Review. Disease-modifying Drugs for Multiple Sclerosis. Single Drug Addendum: Fingolimod
 Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
Multiple Sclerosis. Global Drug Forecast and Market Analysis to 2022 Event-Driven Update. GDHC34PIDR / Published April 2013
 Multiple Sclerosis Global Drug Forecast and Market Analysis to 2022 Event-Driven Update GDHC34PIDR / Published April 2013 Executive Summary The table below presents the key metrics for multiple sclerosis
Multiple Sclerosis Global Drug Forecast and Market Analysis to 2022 Event-Driven Update GDHC34PIDR / Published April 2013 Executive Summary The table below presents the key metrics for multiple sclerosis
Dimethyl fumarate for treating relapsing remitting multiple sclerosis
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Final appraisal determination Dimethyl fumarate for treating relapsing remitting multiple sclerosis This guidance was developed using the single technology
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Final appraisal determination Dimethyl fumarate for treating relapsing remitting multiple sclerosis This guidance was developed using the single technology
Decisions relating to Multiple Sclerosis treatments
 10 October 2014 Decisions relating to Multiple Sclerosis treatments PHARMAC is pleased to announce that, from 1 November 2014, it will: list fingolimod (Gilenya); list natalizumab (Tysabri); and change
10 October 2014 Decisions relating to Multiple Sclerosis treatments PHARMAC is pleased to announce that, from 1 November 2014, it will: list fingolimod (Gilenya); list natalizumab (Tysabri); and change
Multiple Sclerosis. Multiple Sclerosis. In addition to help nursing professional to understand the signs and
 How to Receive Your CE Credits Read your selected course Completed the quiz at the end of the course with a 70% or greater. Complete the evaluation for your selected course. Print your Certificate CE s
How to Receive Your CE Credits Read your selected course Completed the quiz at the end of the course with a 70% or greater. Complete the evaluation for your selected course. Print your Certificate CE s
Disclosures. Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
 Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Insight. Drug Class. Background, new developments, key strategies. Introduction. Faulty signals. 400,000 patients in the US 1
 Drug Class Insight Multiple Sclerosis Background, new developments, key strategies Introduction 400,000 patients in the US 1 Most people diagnosed between age 20 50 2 2-3x more women than men get MS 2
Drug Class Insight Multiple Sclerosis Background, new developments, key strategies Introduction 400,000 patients in the US 1 Most people diagnosed between age 20 50 2 2-3x more women than men get MS 2
OHTAC Recommendation
 OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
Depression: Facility Assessment Checklists
 Depression: Facility Assessment Checklists A facility system assessment is a starting point for a quality improvement project. The checklists included in this booklet will be most useful if you take a
Depression: Facility Assessment Checklists A facility system assessment is a starting point for a quality improvement project. The checklists included in this booklet will be most useful if you take a
Clinical Study Synopsis
 Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
Major Depressive Disorders Questions submitted for consideration by workshop participants
 Major Depressive Disorders Questions submitted for consideration by workshop participants Prioritizing Comparative Effectiveness Research Questions: PCORI Stakeholder Workshops June 9, 2015 Patient-Centered
Major Depressive Disorders Questions submitted for consideration by workshop participants Prioritizing Comparative Effectiveness Research Questions: PCORI Stakeholder Workshops June 9, 2015 Patient-Centered
Managing Relapsing Remitting MS Risks & benefits of emerging therapies. Dr Mike Boggild The Walton Centre
 Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
Interferons (Avonex, Betaferon, Rebif ) for relapsing remitting multiple sclerosis (RRMS)
 Interferons (Avonex, Betaferon, Rebif ) for relapsing remitting multiple sclerosis (RRMS) Review Question: What happens when people with RRMS take interferons? The short answer: This review found that
Interferons (Avonex, Betaferon, Rebif ) for relapsing remitting multiple sclerosis (RRMS) Review Question: What happens when people with RRMS take interferons? The short answer: This review found that
The submission positioned dimethyl fumarate as a first-line treatment option.
 Product: Dimethyl Fumarate, capsules, 120 mg and 240 mg, Tecfidera Sponsor: Biogen Idec Australia Pty Ltd Date of PBAC Consideration: July 2013 1. Purpose of Application The major submission sought an
Product: Dimethyl Fumarate, capsules, 120 mg and 240 mg, Tecfidera Sponsor: Biogen Idec Australia Pty Ltd Date of PBAC Consideration: July 2013 1. Purpose of Application The major submission sought an
Multiple Sclerosis (MS) is a disease of the central nervous system (including the brain and spinal cord) in which the nerves degenerate.
 What is Multiple Sclerosis? Multiple Sclerosis (MS) is a disease of the central nervous system (including the brain and spinal cord) in which the nerves degenerate. A disease of the central nervous system
What is Multiple Sclerosis? Multiple Sclerosis (MS) is a disease of the central nervous system (including the brain and spinal cord) in which the nerves degenerate. A disease of the central nervous system
Understanding your Tecfidera treatment
 Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
Understanding your Tecfidera treatment
 Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis
 Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness
Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness
Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis
 Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness of teriflunomide
Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness of teriflunomide
MEDICAL ASSISTANCE HANDBOOK PRIOR AUTHORIZATION OF PHARMACEUTICAL SERVICES I. Requirements for Prior Authorization of Tysabri
 MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF I. Requirements for Prior Authorization of Tysabri A. Prescriptions That Require Prior Authorization All prescriptions for Tysabri must be prior authorized.
MEDICAL ASSISTANCE HBOOK PRI AUTHIZATION OF I. Requirements for Prior Authorization of Tysabri A. Prescriptions That Require Prior Authorization All prescriptions for Tysabri must be prior authorized.
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru381 Topic: Lemtrada TM, alemtuzumab Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru381 Topic: Lemtrada TM, alemtuzumab Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Multiple Sclerosis & MS Ireland Media Fact Sheet
 Multiple Sclerosis & MS Ireland Media Fact Sheet This fact sheets gives a summary of the main facts and issues relating to Multiple Sclerosis and gives an overview of the services offered by MS Ireland.
Multiple Sclerosis & MS Ireland Media Fact Sheet This fact sheets gives a summary of the main facts and issues relating to Multiple Sclerosis and gives an overview of the services offered by MS Ireland.
