Bone Morphogenetic Proteins & Spinal Surgery for Degenarative Disc Disease
|
|
|
- Doreen James
- 8 years ago
- Views:
Transcription
1 Ontario Health Technology Assessment Series 2004; Vol. 4, No. 4 Bone Morphogenetic Proteins & Spinal Surgery for Degenarative Disc Disease An Evidence Based Analysis March 2004 Medical Advisory Secretariat Ministry of Health and Long Term Care
2 Suggested Citation This report should be cited as follows: Medical Advisory Secretariat. Bone morphogenetic proteins & spinal surgery for degenerative disc disease: an evidence based analysis. Ontario Health Technology Assessment Series 2004;4(4). Permission Requests All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to How to Obtain Issues in the Ontario Health Technology Assessment Series All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: Print copies can be obtained by contacting Conflict of Interest Statement All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence based assessment process. There are no competing interests or conflicts of interest to declare. Peer Review All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit Contact Information The Medical Advisory Secretariat Ministry of Health and Long Term Care 20 Dundas Street West, 10th floor Toronto, Ontario CANADA M5G 2N6 MASinfo.moh@ontario.ca Telephone: ISSN (Online) ISBN (PDF) Bone Morphogenetic Proteins for DDD Ontario Health Technology Assessment Series 2004;4(4) 2
3 About the Medical Advisory Secretariat The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes. The Medical Advisory Secretariat also provides a secretariat function and evidence based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC). The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series. About the Ontario Health Technology Assessment Series To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted. The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology s diffusion into current practice and input from practicing medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes. If you are aware of any current additional evidence to inform an existing evidence based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit Disclaimer This evidence based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidencebased analysis is current to the date of publication. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence based analyses: Bone Morphogenetic Proteins for DDD Ontario Health Technology Assessment Series 2004;4(4) 3
4 Table of Contents Table of Contents... 4 Executive Summary... 5 Objective... 5 Bone Morphogenetic Proteins... 5 Review Strategy... 5 Summary of Findings... 5 Background... 6 Clinical Need... 6 Bone Morphogenetic Proteins... 6 Regulatory Status... 7 Literature Review... 8 Objective... 8 Questions to Be Answered... 8 Methods... 8 Results of the Literature Review... 8 Safety... 8 Clinical Outcomes... 9 Summary of Medical Advisory Secretariat Review Summary of Evidence Economic Analysis References Bone Morphogenetic Proteins for DDD Ontario Health Technology Assessment Series 2004;4(4) 4
5 Executive Summary Objective This review is intended to summarize the evidence of safety and effectiveness of a recently licensed spinal fusion surgery device that includes a recombinant human bone morphogenetic protein (BMP). Bone Morphogenetic Proteins BMP are proteins that occur naturally in the matrix of human bone and stimulate bone growth. For spinal surgery, BMP must be delivered to the vertebral level where bone growth is desired. The BMP device currently available in Canada comprises a specific cage device and a bovine collagen sponge that delivers BMP. This sponge must be implanted in combination with this specific cage device from the same manufacturer. The device is marketed under the trade name INFUSE TM and provides an alternative to autologous bone graft in spinal fusion. Review Strategy Published literature identified through searches of MEDLINE and EMBASE was supplemented with material submitted by the device manufacturer as part of regulatory approval in the United States and available through the public access area of the website of the Food and Drug Administration. Summary of Findings Evidence submitted to regulatory bodies in the USA and Canada indicates that the INFUSE! device appears safe. The largest number of spinal fusion cases using BMP devices has been for anterior lumbar interbody fusion. Although radiologic fusion occurs at a consistently faster rate among recipients of the BMP device than among recipients of autologous bone grafts, clinical outcomes (pain and disability) appear no different. Regardless of technique, improvements in pain and disability are reported by similar proportions of participants in all the arms of all the trials. BMP devices for cervical fusion have yet to be approved in Canada but one small scale trial has reported radiologic fusion in all participants in both BMP and autologous bone graft arms and improvement in neck pain scores for all participants. BMP devices for lumbar fusion are licensed, safe and appear equivalent to autologous bone graft procedures for spinal fusion in terms of patient outcomes with the notable exception that patients undergoing autologous bone graft report pain at the donor site. Laparoscopic approaches yield reductions in postoperative length of stay compared to conventional open approaches. Bone Morphogenetic Proteins for DDD Ontario Health Technology Assessment Series 2004;4(4) 5
6 Objective This review is intended to summarize the evidence of safety and effectiveness of a recently licensed spinal fusion surgery device that includes a recombinant human bone morphogenetic protein (BMP). Background Clinical Need Degenerative disc disease (DDD) is defined in terms of anatomic, biomechanical, radiological and clinical changes, most frequently noted in the lumbar or cervical spine. DDD is somewhat of a misnomer as it is not a disease per se, but rather a degenerative process which, in some people, may cause clinicallyevident symptoms. The symptom most commonly reported by patients is pain, exacerbated by activities such as sitting that increase loads on the intervertebral discs. With the growth of magnetic resonance imaging, radiologic evidence of DDD has been described in rough proportion to age i.e. 40% of people at age 40 rising to over 80% among people over age 80. While imaging techniques are a potentially valuable tool, their use has added to diagnostic uncertainty due to false positive findings, since not all persons with radiologic DDD will report pain or other symptoms. The literature on management of DDD encompasses a wide range of surgical and non-surgical techniques. In the United States, the numbers of person undergoing surgical treatment has expanded rapidly over the last decade. 1 Surgical management of DDD encompasses a range of procedures intended to provide additional support at the level of the degenerated disc. These can be categorized as spinal fusion, which uses various instrumentation coupled with bone tissue from the patient or a cadaveric donor to link the vertebrae at the level of the degenerated disc to adjacent vertebrae, or spinal arthroplasty. The second of these is addressed in a separate review. Based on population-based physician claims data, approximately 2000 spinal fusion procedures are performed in Ontario annually. Most are lumbar fusions. This number is expected to grow in the coming decades as the population of older Ontarians grows and thus, the number of surviving people with clinically significant DDD increases. In Ontario, spinal fusion surgery is overwhelmingly performed by physicians trained in either orthopaedics or neurosurgery. Bone Morphogenetic Proteins Bone morphogenetic proteins (BMP) are a family of naturally-occurring substances involved in tissue growth and development. Despite their name, BMP are also active in tissues other than bone. In bone, BMP stimulate new bone formation. BMP were first described in 1965, 2 isolated in 1988, 3 and are now available for therapeutic uses via recombinant production. Seven BMP have been identified and described. 4 Commercial clinical use of BMP has advanced most in the area of spinal fusion surgery products for both lumbar and cervical spine indications. Bone Morphogenetic Proteins for DDD Ontario Health Technology Assessment Series 2004;4(4) 6
7 Prior to the introduction of BMPs and various artificial disc technologies (see accompanying review), the surgical approach to clinically-significant DDD focused on fusion of adjacent spinal segments using bone graft with or without instrumentation, typically pedicle screws inserted posteriorly. Rates of fusion varied widely in reported case series, from 45% to 100% in one review. 5 The remaining patients would have no fusion, creating a pseudarthrosis, and these patients were also likely to incur a later complication of transitional syndrome or adjacent segment disease due to increased workload of segments adjacent to the fusion, even if successful. Over the last two decades, interbody cage devices have come to market that are intended both to support new bone growth and provide mechanical support. These devices are surgically inserted into the spine, using an anterior or posterior approach, and provide a scaffold for autologous bone, typically taken from the patient s iliac crest or allograft bone. 6 Some procedures have combined interbody devices with pedicle screws. For some patients, autologous bone graft procedures appear to confer improved post-operative function when compared to non-surgical alternatives, but also yield pain at the site of bone harvest and incision-related risks, albeit low, at the harvest site. In the context of spinal surgery, BMP must be delivered to the spine at the vertebral level where repair is desired. In the product currently available, BMP is delivered to the spine via a bovine collagen sponge and this vehicle must be implanted in combination with a specific cage device from the same manufacturer. Research into alternative delivery vehicles, particularly gene therapy, has yet to yield commercially-available products. Regulatory Status Two BMP-associated products have been approved for clinical use in Canada. Following Health Canada approval on February 13, 2002, Stryker Biotech markets a product containing BMP-7 (also named OP-1) for use following non-union of long bone fractures. Pilot studies of BMP-7/OP-1 for use in spinal fusion have been reported in the literature. 7,8 However, regulatory approval has yet to occur for this product for spinal fusion use. Following Health Canada approval on November 1, 2002, Medtronic markets a product containing BMP- 2 (INFUSE!). This product was approved for the indication of spinal fusion in skeletally mature patients with degenerative disc disease at one level from L4-S1. The patients may have up to Grade 1 spondylolithesis (slippage of one vertebrae in relation to another; grade 1 is least severe) at the involved level. Patients should have had at least six months of nonoperative treatment prior to treatment with the device. The device may be implanted via an anterior open or an anterior laparoscopic approach. The Canadian approval was based on the same information submitted for Food and Drug Administration (FDA) approval in the United States. 9 INFUSE! must be used with the LT-CAGE! device. The LT-CAGE! product used with autologous bone graft is approved for use in the United States and was used as the comparison intervention in the randomized trial of equivalence of INFUSE TM that was the basis for marketing approval in both countries. Bone Morphogenetic Proteins for DDD Ontario Health Technology Assessment Series 2004;4(4) 7
8 Literature Review Objective This literature review addresses two objectives: to summarize information on the safety of the INFUSE! product, and to summarize information on the efficacy of this product in comparison to alternatives for spinal fusion. Questions to Be Answered 1) Given that INFUSE! is a recombinant biological potentially active at tissue sites other than bone, is there evidence of clinically significant antibody formation or tissue reactions to either the BMP component or the collagen sponge delivery vehicle? 2) How do outcomes with INFUSE! compare to those with alternative approaches, particularly autologous bone grafts? Methods A search of the literature from Medline and Embase covering the period 1966 through November, 2003 yielded 244 citations. Most of these were basic science papers or pre-clinical animal studies. In addition, the public access area of the Food and Drug Administration (FDA) website includes the premarketing approval submission made for INFUSE!, which provided an overview of safety and efficacy data. 10 For question 1, the safety data submitted by the manufacturer to the FDA provide the largest case series, for they include patients in both published and unpublished series. These safety data are summarized in the results section For question 2, randomized controlled trials (RCT) and case series comparing the INFUSE! product to autologous bone graft were identified. A total of 8 studies were reviewed and are summarized in section 2) below. Results of the Literature Review Safety The summary of effectiveness data submitted to the FDA included results on 349 persons who received the INFUSE! device and 183 controls. Bone Morphogenetic Proteins for DDD Ontario Health Technology Assessment Series 2004;4(4) 8
9 Table 1: Summary of effectiveness data submitted to FDA regarding the INFUSE! device INFUSE! Recipients Control Device Recipients Anti-rhBMP-2 antibodies 2/349 (0.6%) 1/183 (0.5%) Anti-bovine Type 1 collagen 18.1% 14.2% antibodies Anti-human Type 1 collagen antibodies None None Although the submission does not comment on this, the difference between the proportions of the two groups with antibodies to bovine collagen does not reach statistical significance (p>0.25). In light of the relatively high rate of bovine collagen antibodies among control participants, (who did not receive the bovine collagen used in the INFUSE! device), it may well be that the antibody assay also detects crossreacting antibodies. The incremental occurrence of antibodies to bovine type 1 collagen is 3.9%, which translates into 1 per 25 INFUSE! recipients. The clinical significance of these antibodies is not known. In addition, no cases of ectopic, heterotopic or undesirable exuberant bone formation were reported. In summary, within the limitations of relatively short follow-up and a few hundred cases, the INFUSE! product appears to be safe. Both Health Canada and the FDA have directed the manufacturer to complete longer-term follow-up safety studies among patients in whom this device is implanted. Clinical Outcomes At this time in Canada, BMP-related devices are approved for lumbar surgery only and thus the review focused on studies of lumbar spinal surgery. One trial reporting use of a BMP device in cervical spinal surgery is also briefly summarized. Regulatory approval for this indication may occur within the next months. Overall, surgery for DDD remains a technique about which there is little consensus. A Cochrane review of studies published through December 31, 1999 identified 16 randomized or quasi-randomized trials and concluded there is no scientific evidence about the effectiveness of any form of surgical decompression or fusion for degenerative lumbar spondylosis compared with natural history, placebo, or conservative treatment. 11 Since that review, investigators leading a reasonably well-designed Swedish study concluded that lumbar fusion by any of three techniques (posterolateral fusion, posterolateral fusion with internal screw fixation, and posterolateral fusion with internal screw fixation and interbody fusion) yielded statistically significant reductions in patient-reported back pain and disability compared to conservative treatment consisting of physiotherapy. Results among the various surgical groups did not differ significantly, suggesting that no particular approach was superior. 12 In addition, social insurance records were used to assess work disability and the net back to work rate was 36% among surgical patients compared to 13% among the non-surgical patients. 13 Even in this study, however, the authors stress the importance of careful selection of patients for surgery, a view that is widespread in the literature on surgical management of DDD. In addition, the literature is marked by a lack of consensus on the selection of outcome instruments. Performance analysis of the most widely used instrument, the Oswestry Disability Index, (ODI) has been reported, but estimates of the minimum clinically significant change (MCSC) range almost threefold from 5.2 to The ODI asks patients to rate their performance for each of ten items (e.g. sitting, travelling) on a six point ordinal scale, yielding a maximum possible total score of Bone Morphogenetic Proteins for DDD Ontario Health Technology Assessment Series 2004;4(4) 9
10 The authors of the Swedish randomized trial compared ODI results with those of a global assessment by patients and concluded that the MCSC was 10 points. However, this was noted to be within the 95% tolerance interval of 10 points which the authors calculate as a measure of the standard error of the ODI. 14 As a practical matter then, a change of at least 20 points would appear to represent a clinically significant change and encompass all published estimates of MCSC. In this context, the relevant question becomes one of demonstrating, at a minimum, the equivalence of surgery with the BMP product to prevailing surgical methods. For policy purposes, any relative outcome improvement for the BMP product over prevailing practice is important as an input to comparing costeffectiveness of the various approaches to surgical management of DDD. Summary of Medical Advisory Secretariat Review Following the format of the Medical Advisory Secretariat, the table below summarizes the provenance of the literature describing studies of effectiveness of human BMP-associated devices for spinal fusion surgery. Table 2: Level of evidence of effectiveness on bone morphogenetic proteins, to November 2003 Level of Evidence Study Design Number of Eligible Studies 1 Large randomized controlled trial, systematic reviews of 1 RCTs 1(g) Large randomized controlled trial unpublished but reported to an international scientific meeting 2 Small randomized controlled trial 4 2(g) Small randomized controlled trial unpublished but 2 reported to an international scientific meeting 3a Nonrandomized study with contemporaneous controls 3b Nonrandomized study with historical controls 3(g) Nonrandomized study presented at international conference 4a Surveillance (database or register) 4b Case series (multi-site) 1 4c Case series (single site) 4d Retrospective review, modelling 4(g) Case series presented at international conference The tables below summarize the results of these 8 studies. Outcomes have been classified as radiologic (typically assessed by the presence of fusion on computerized tomography (CT) images), patient (pain, disability), and health system & societal (length of stay, rehabilitation costs, return to work). Bone Morphogenetic Proteins for DDD Ontario Health Technology Assessment Series 2004;4(4) 10
11 Table 3: Summary of randomized controlled trial (RCT) literature on bone morphogenetic proteins (anterior lumbar interbody fusion) for degenerative disc disease, to November 2003 Bone Morphogenetic Proteins for DDD Ontario Health Technology Assessment Series 2004;4(4) 11
12 Table 4: Summary of studies on bone morphogenetic proteins (posterior lumbar and posterolateral lumbar fusion) for degenerative disc disease, to November 2003 Bone Morphogenetic Proteins for DDD Ontario Health Technology Assessment Series 2004;4(4) 12
13 Summary of Evidence! Evidence submitted to regulatory bodies in the USA and Canada indicates that the INFUSE! device appears safe. Long-term safety can only be assessed after additional follow-up as has been mandated by regulatory bodies in both countries.! The greatest experience with BMP devices for spinal surgery is for anterior lumbar interbody fusion. For this procedure, radiologic fusion occurs at a consistently faster rate among recipients of the BMP device than among recipients of autologous bone grafts. However, clinical outcomes (pain and disability) appear no different. Regardless of the particular technique, improvements in pain and disability are reported by similar proportions of participants in all the arms of all the trials. Reports of health system and societal outcomes are sparse.! Posterolateral fusion results from animal studies with BMP have been disappointing. New bone formation appears to be impeded by muscular compression of the collagen sponge carrier. These poor results in animal studies for posterolateral fusion have fuelled an ongoing search for a compression-resistant carrier for the BMP. 24 Small scale trials of BMP devices for posterior and posterolateral lumbar fusion report results consistent with anterior fusion trials, namely high rates of radiologic fusion and improvement in patient reports of pain and disability for all techniques.! BMP devices for cervical fusion have yet to be approved in Canada but one small scale trial has reported radiologic fusion in all participants in both BMP and autologous bone graft arms and improvement in neck pain scores for all participants.! Taken together, BMP devices for lumbar fusion are licensed, safe and appear equivalent to autologous bone graft procedures for spinal fusion in terms of patient outcomes with the notable exception that patients undergoing autologous bone graft report pain at the donor site. Laparoscopic approaches yield reductions in postoperative length of stay compared to open approaches.! Excepting the absence of donor site pain, evidence that the INFUSE! device is superior to autologous bone graft has yet to emerge. Thus, relative costs of BMP devices compared to alternatives may be an important consideration in decisions about the role of BMP devices for lumbar fusion at this time. Bone Morphogenetic Proteins for DDD Ontario Health Technology Assessment Series 2004;4(4) 13
14 Economic Analysis Given the apparent equivalence of spinal fusion with the INFUSE TM device and spinal fusion with autologous bone graft, one of the challenges in economic analysis is trading off patient preferences for avoiding the pain of the iliac crest bone harvest against the higher cost of the device. At this time, the availability of this device does not appear to expand the spectrum of indications, nor does it appear to free up substantial resources for increasing surgical case volumes. Contrary to the United States where surplus hospital bed and operating room capacity, coupled with relatively generous Medicare reimbursement for persons over 65 and a higher ratio of neurosurgeons to population appear to be driving rates of surgery, the Canadian context does not have appreciable amounts of hospital or operating room capacity available. One published cost-effectiveness analysis of the INFUSE TM was identified which stated that the cost of the BMP device was US$3380. These authors, who state they have received grants and other forms of remuneration from the manufacturer of the device, conclude that this is likely to be offset to a significant extent by savings related to other medical resources. The evidence supporting this conclusion does not appear particularly robust. 25 At this time, given the device s relatively low sales in Canada, price data are likely to overstate costs, since volume purchasing would be expected to lower per-device costs. In addition, laparoscopic approaches, while reportedly shortening hospital stay, may have other instrumentation costs that need to be assessed. In summary, definitive evidence of a per-procedure cost increment or savings over current approaches to spinal fusion is not available. Data on negotiated device prices could be a useful input to a costeffectiveness analysis, ideally to be completed within the context of a broader review of surgical management of DDD. Bone Morphogenetic Proteins for DDD Ontario Health Technology Assessment Series 2004;4(4) 14
15 References 1. Abelson R, Petersen M. An operation to ease back pain bolsters the bottom line, too. New York Times 31 December, Urist MR. Bone: formation by autoinduction. Science, 1965; 150: Wang EA, Rosen V, Cordes P, et al. Purification and characterization of other distinct boneinducing factors. Proc Natl Acad Sci USA, 1988; 85: Accessed December 10, Boden SD, Kang J, Sandhu H et al. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans. Spine 2002; 27: Blumenthal SL, Ohnmeiss DD. Intervertebral cages for degenerative spinal diseases. Spine J 2003; 3: Johnsson R, Stromqvist B, Aspenberg P. Randomized radiostereometric study comparing osteogenic protein-1 (BMP-7) and autograft bone in human noninstrumental posterolateral lumbar fusion. Spine 2002; 27: Vaccaro AR, Patel T, Fischgrund J, et al. A pilot safety and efficacy study of OP-1 putty (rhbmp-7) as an adjunct to iliac crest autograft in posterolateral lumbar fusions. Eur Spine J 2003; 12: Personal Communication. Nancy Shadeed, Health Canada. December 17, Available at Gibson JNA, Waddell G, Grant IC. Surgery for degenerative lumbar spondylosis (Cochrane Methodology Review). In: The Cochrane Library, Issue 4, Chichester, UK: John Wiley & Sons, Ltd. 12. Fritzell P, Hagg O, Wessberg P, et al. Chronic low back pain and fusion: a comparison of three surgical techniques: a prospective multicenter randomized study from the Swedish lumbar spine study group. Spine 2002; 27: Fritzell P, Hagg O, Wessberg P, et al. Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish lumbar spine study group. Spine 2001; 26: Hagg O, Fritzell P, Nordwall A. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J 2003; 12: Fairbank JC, Couper J, Davies JB, et al. The Oswestry low back pain disability questionnaire. Physiotherapy 1980; 66: Bone Morphogenetic Proteins for DDD Ontario Health Technology Assessment Series 2004;4(4) 15
16 16. Burkus JK, Heim SE, Gornet MF, et al. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech 2003; 16: Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhbmp-2 with tapered interbody cages. J Spinal Disord Tech 2002; 15: Sandhu HS. Bone morphogenetic proteins and spinal surgery. Spine 2003; 28:15S:S64-S Boden SD, Zdeblick TA, Sandhu HS, et al. The use of rhbmp-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine 2000; 25: Burkus JK, Transfeldt EE, Kitchel SH, et al. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine 2002; 27: Alexander JT, Branch Jr. CL, Haid Jr. RW, et al. An analysis of the use of rhbmp-2 in PLIF constructs: clinical and radiographic outcomes. Cited in Sandhu HS. Bone morphogenetic proteins and spinal surgery. Spine 2003; 28:15S:S64-S Luque E. Latest clinical results using demineralized bone materials and rhbmp-2: the Mexican experience. Cited in Sandhu HS, Khan SN. Recombinant human bone morphogenetic protein-2: use in spinal fusion applications. J Bone Joint Surg 2003; 85A(S3): Baskin DS, Ryan P, Sonntag V et al. A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the Cornerstone-SR TM allograft ring and the Atlantis TM anterior cervical plate. Spine 2003; 28: Sandhu HS, Khan SN. Recombinant human bone morphogenetic protein-2: use in spinal fusion applications. J Bone Joint Surg 2003; 85A(S3): Polly Jr DW, Ackerman SJ, Shaffrey CI, et al. A cost analysis of bone morphogenetic protein versus autogenous iliac crest bone graft in single-level anterior lumbar fusion. Orthopedics 2003; 26: Bone Morphogenetic Proteins for DDD Ontario Health Technology Assessment Series 2004;4(4) 16
Low Back Pain (LBP) Prevalence. Low Back Pain (LBP) Prevalence. Lumbar Fusion: Where is the Evidence?
 15 th Annual Cleveland Clinic Pain Management Symposium Sarasota, Florida Lumbar Fusion: Where is the Evidence? Gordon R. Bell, M.D. Director, Cleveland Clinic Low Back Pain (LBP) Prevalence Lifetime prevalence:
15 th Annual Cleveland Clinic Pain Management Symposium Sarasota, Florida Lumbar Fusion: Where is the Evidence? Gordon R. Bell, M.D. Director, Cleveland Clinic Low Back Pain (LBP) Prevalence Lifetime prevalence:
ANTERIOR LUMBAR INTERBODY FUSION (ALIF) Basic Anatomical Landmarks: Anterior Lumbar Spine
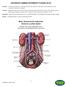 (ALIF) Anterior In human anatomy, referring to the front surface of the body or the position of one structure relative to another Lumbar Relating to the loins or the section of the back and sides between
(ALIF) Anterior In human anatomy, referring to the front surface of the body or the position of one structure relative to another Lumbar Relating to the loins or the section of the back and sides between
ISPI Newsletter Archive Lumbar Spine Surgery
 ISPI Newsletter Archive Lumbar Spine Surgery January 2005 Effects of Charite Artificial Disc on the Implanted and Adjacent Spinal Segments Mechanics Using a Hybrid Testing Protocol Spine. 30(24):2755-2764,
ISPI Newsletter Archive Lumbar Spine Surgery January 2005 Effects of Charite Artificial Disc on the Implanted and Adjacent Spinal Segments Mechanics Using a Hybrid Testing Protocol Spine. 30(24):2755-2764,
BONE OR SOFT TISSUE HEALING AND FUSION ENHANCEMENT PRODUCTS
 #1 MEDICAL POLICY BONE OR SOFT TISSUE HEALING AND FUSION ENHANCEMENT PRODUCTS Policy Number: 2016T0410P Effective Date: February 1, 2016 Table of Contents BENEFIT CONSIDERATIONS COVERAGE RATIONALE DEFINITIONS...
#1 MEDICAL POLICY BONE OR SOFT TISSUE HEALING AND FUSION ENHANCEMENT PRODUCTS Policy Number: 2016T0410P Effective Date: February 1, 2016 Table of Contents BENEFIT CONSIDERATIONS COVERAGE RATIONALE DEFINITIONS...
visualized. The correct level is then identified again. With the use of a microscope and
 SURGERY FOR SPINAL STENOSIS Laminectomy A one inch (or longer for extensive stenosis) incision is made in the middle of the back over the effected region of the spine. The muscles over the bone are moved
SURGERY FOR SPINAL STENOSIS Laminectomy A one inch (or longer for extensive stenosis) incision is made in the middle of the back over the effected region of the spine. The muscles over the bone are moved
Update to the Treatment of Degenerative Cervical Disc Disease
 Update to the Treatment of Degenerative Cervical Disc Disease Michael Lynn, MD Neurosurgeon, Southeastern Neurosurgical & Spine Institute Adjunct Assistant Clinical Professor of Bioengineering, Clemson
Update to the Treatment of Degenerative Cervical Disc Disease Michael Lynn, MD Neurosurgeon, Southeastern Neurosurgical & Spine Institute Adjunct Assistant Clinical Professor of Bioengineering, Clemson
SPINAL FUSION. North American Spine Society Public Education Series
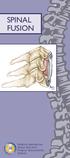 SPINAL FUSION North American Spine Society Public Education Series WHAT IS SPINAL FUSION? The spine is made up of a series of bones called vertebrae ; between each vertebra are strong connective tissues
SPINAL FUSION North American Spine Society Public Education Series WHAT IS SPINAL FUSION? The spine is made up of a series of bones called vertebrae ; between each vertebra are strong connective tissues
Clinical Commissioning Policy Proposition: Bone morphogenetic protein-2 in spinal fusion
 DRAFT FOR PUBLIC CONSULTATION Clinical Commissioning Policy Proposition: Bone morphogenetic protein-2 in spinal fusion Reference: NHS England D14X01/01 DRAFT FOR PUBLIC CONSULTATION Information Reader
DRAFT FOR PUBLIC CONSULTATION Clinical Commissioning Policy Proposition: Bone morphogenetic protein-2 in spinal fusion Reference: NHS England D14X01/01 DRAFT FOR PUBLIC CONSULTATION Information Reader
Artificial Intervertebral Disc: Cervical Spine
 dapplies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
dapplies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF).
 Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Interspinous Fixation (Fusion) Devices Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Interspinous Fixation (Fusion) Devices Lumbar Spine
Interspinous Fixation (Fusion) Devices Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Interspinous Fixation (Fusion) Devices Lumbar Spine
Evidence Based Medicine in Spinal Surgery
 Evidence Based Medicine in Spinal Surgery Roger Härtl, MD Associate Professor of Neurosurgery Chief of Spinal Surgery Neurosurgeon to the GIANTS Football team Brain & Spine Center Weill Cornell Medical
Evidence Based Medicine in Spinal Surgery Roger Härtl, MD Associate Professor of Neurosurgery Chief of Spinal Surgery Neurosurgeon to the GIANTS Football team Brain & Spine Center Weill Cornell Medical
Options for Cervical Disc Degeneration A Guide to the Fusion Arm of the M6 -C Artificial Disc Study
 Options for Cervical Disc Degeneration A Guide to the Fusion Arm of the M6 -C Artificial Disc Study Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine
Options for Cervical Disc Degeneration A Guide to the Fusion Arm of the M6 -C Artificial Disc Study Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine
Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF).
 Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra,
Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra,
White Paper: Cervical Disc Replacement: When is the Mobi-C Cervical Disc Medically Necessary?
 White Paper: Cervical Disc Replacement: When is the Mobi-C Cervical Disc Medically Necessary? For Health Plans, Medical Management Organizations and TPAs Cervical Disc Disease: An Overview The cervical
White Paper: Cervical Disc Replacement: When is the Mobi-C Cervical Disc Medically Necessary? For Health Plans, Medical Management Organizations and TPAs Cervical Disc Disease: An Overview The cervical
Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization
 APPROVED IRN10389-1.1-04 Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization Because it involves accessing the spine through the patient s side, the Direct Lateral approach
APPROVED IRN10389-1.1-04 Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization Because it involves accessing the spine through the patient s side, the Direct Lateral approach
White Paper: Reducing Utilization Concerns Regarding Spinal Fusion and Artificial Disc Implants
 White Paper: Reducing Utilization Concerns Regarding Spinal Fusion and Artificial Disc Implants For Health Plans, Medical Management Organizations and TPAs Executive Summary Back pain is one of the most
White Paper: Reducing Utilization Concerns Regarding Spinal Fusion and Artificial Disc Implants For Health Plans, Medical Management Organizations and TPAs Executive Summary Back pain is one of the most
Technology Breakthrough in Spinal Implants (Technical Insights)
 Technology Breakthrough in Spinal Implants (Technical Insights) Biomaterial innovations is a growth factor for spinal implant market June 2014 Table of Contents Section Page Number Executive Summary 4
Technology Breakthrough in Spinal Implants (Technical Insights) Biomaterial innovations is a growth factor for spinal implant market June 2014 Table of Contents Section Page Number Executive Summary 4
Dental Bone Grafting Options. A review of bone grafting options for patients needing more bone to place dental implants
 Dental Bone Grafting Options A review of bone grafting options for patients needing more bone to place dental implants Dental Bone Grafting Options What is bone grafting? Bone grafting options Bone from
Dental Bone Grafting Options A review of bone grafting options for patients needing more bone to place dental implants Dental Bone Grafting Options What is bone grafting? Bone grafting options Bone from
Spine Clinic Neurospine Specialists, Orthopaedics and Neurosurgery
 Spine Clinic Neurospine Specialists, Orthopaedics and Neurosurgery REVISION SPINE SURGERY Revision surgery is a very complex field which requires experience, training and evaluation in a very individual
Spine Clinic Neurospine Specialists, Orthopaedics and Neurosurgery REVISION SPINE SURGERY Revision surgery is a very complex field which requires experience, training and evaluation in a very individual
Spinal Surgery Functional Status and Quality of Life Outcome Specifications 2015 (01/01/2013 to 12/31/2013 Dates of Procedure) September 2014
 Description Methodology For patients ages 18 years and older who undergo a lumbar discectomy/laminotomy or lumbar spinal fusion procedure during the measurement year, the following measures will be calculated:
Description Methodology For patients ages 18 years and older who undergo a lumbar discectomy/laminotomy or lumbar spinal fusion procedure during the measurement year, the following measures will be calculated:
CERVICAL PROCEDURES PHYSICIAN CODING
 CERVICAL PROCEDURES PHYSICIAN CODING Anterior Cervical Discectomy with Interbody Fusion (ACDF) Anterior interbody fusion, with discectomy and decompression; cervical below C2 22551 first interspace 22552
CERVICAL PROCEDURES PHYSICIAN CODING Anterior Cervical Discectomy with Interbody Fusion (ACDF) Anterior interbody fusion, with discectomy and decompression; cervical below C2 22551 first interspace 22552
Surgical Procedures of the Spine
 Surgical Procedures of the Spine Jaideep Chunduri, M.D. Orthopaedic Spine Surgeon Beacon Orthopaedics and Sports Medicine Beacon Spine Center Objectives Discuss the 4 most common procedures performed in
Surgical Procedures of the Spine Jaideep Chunduri, M.D. Orthopaedic Spine Surgeon Beacon Orthopaedics and Sports Medicine Beacon Spine Center Objectives Discuss the 4 most common procedures performed in
Born January 13, 1959 in Ponce, Puerto Rico Fluent in English and Spanish
 Page 1 of 6 6160 Windhaven Pkwy Ste 200 Plano, TX 75093 Luis A. Mignucci, M.D. Phone (972)378-6908 Fax (972)378-6586 Personal Born January 13, 1959 in Ponce, Puerto Rico Fluent in English and Spanish Surgical
Page 1 of 6 6160 Windhaven Pkwy Ste 200 Plano, TX 75093 Luis A. Mignucci, M.D. Phone (972)378-6908 Fax (972)378-6586 Personal Born January 13, 1959 in Ponce, Puerto Rico Fluent in English and Spanish Surgical
ANTERIOR CERVICAL DISCECTOMY AND FUSION. Basic Anatomical Landmarks: Anterior Cervical Spine
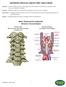 Anterior In the human anatomy, referring to the front surface of the body or position of one structure relative to another Cervical Relating to the neck, in the spine relating to the first seven vertebrae
Anterior In the human anatomy, referring to the front surface of the body or position of one structure relative to another Cervical Relating to the neck, in the spine relating to the first seven vertebrae
DUKE ORTHOPAEDIC SURGERY GOALS AND OBJECTIVES SPINE SERVICE
 GOALS AND OBJECTIVES PATIENT CARE Able to perform a complete musculoskeletal and neurologic examination on the patient including cervical spine, thoracic spine, and lumbar spine. The neurologic examination
GOALS AND OBJECTIVES PATIENT CARE Able to perform a complete musculoskeletal and neurologic examination on the patient including cervical spine, thoracic spine, and lumbar spine. The neurologic examination
Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, The Cervical Spine. What is the Cervical Spine?
 Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause pain and numbness in the neck, shoulders, arms, and even hands. This patient
Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause pain and numbness in the neck, shoulders, arms, and even hands. This patient
Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances?
 Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances? Do you experience weakness, tingling, numbness, stiffness, or cramping in your legs, buttocks or
Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances? Do you experience weakness, tingling, numbness, stiffness, or cramping in your legs, buttocks or
THE MEDICAL TREATMENT GUIDELINES
 THE MEDICAL TREATMENT GUIDELINES I. INTRODUCTION A. About the Medical Treatment Guidelines. On December 1, 2010, the NYS Workers' Compensation Board is implementing new regulations and Medical Treatment
THE MEDICAL TREATMENT GUIDELINES I. INTRODUCTION A. About the Medical Treatment Guidelines. On December 1, 2010, the NYS Workers' Compensation Board is implementing new regulations and Medical Treatment
BONE OR SOFT TISSUE HEALING AND FUSION ENHANCEMENT PRODUCTS
 CLINICAL POLICY BONE OR SOFT TISSUE HEALING AND FUSION ENHANCEMENT PRODUCTS Policy Number: SURGERY 056.10 T2 Effective Date: May 1, 2013 Table of Contents CONDITIONS OF COVERAGE... COVERAGE RATIONALE BACKGROUND...
CLINICAL POLICY BONE OR SOFT TISSUE HEALING AND FUSION ENHANCEMENT PRODUCTS Policy Number: SURGERY 056.10 T2 Effective Date: May 1, 2013 Table of Contents CONDITIONS OF COVERAGE... COVERAGE RATIONALE BACKGROUND...
ATLANTIS Anterior Cervical Plate System ATLANTIS Translational Plate. 510(k) Summary. February 2007
 rk,1 10{2 ATLANTIS Anterior Cervical Plate System ATLANTIS Translational Plate 510(k) Summary FEB 2 3 2[007 February 2007 I. Company: Medtronic Sofamor Danek USA 1800 Pyramid Place Memphis, Tennessee 38132
rk,1 10{2 ATLANTIS Anterior Cervical Plate System ATLANTIS Translational Plate 510(k) Summary FEB 2 3 2[007 February 2007 I. Company: Medtronic Sofamor Danek USA 1800 Pyramid Place Memphis, Tennessee 38132
Minimally Invasive Spine Surgery What is it and how will it benefit patients?
 Minimally Invasive Spine Surgery What is it and how will it benefit patients? Dr Raoul Pope MBChB, FRACS, Neurosurgeon and Minimally Invasive Spine Surgeon Concord Hospital and Mater Private Hospital Sydney
Minimally Invasive Spine Surgery What is it and how will it benefit patients? Dr Raoul Pope MBChB, FRACS, Neurosurgeon and Minimally Invasive Spine Surgeon Concord Hospital and Mater Private Hospital Sydney
ARTIFICIAL DISC REPLACEMENT FOR DEGENERATIVE DISC DISEASE OF THE LUMBAR SPINE. A Technology Assessment
 ARTIFICIAL DISC REPLACEMENT FOR DEGENERATIVE DISC DISEASE OF THE LUMBAR SPINE A Technology Assessment INTRODUCTION The California Technology Assessment Forum has been asked to update its review of the
ARTIFICIAL DISC REPLACEMENT FOR DEGENERATIVE DISC DISEASE OF THE LUMBAR SPINE A Technology Assessment INTRODUCTION The California Technology Assessment Forum has been asked to update its review of the
Patient Guide to Neck Surgery
 The following is a sampling of products offered by Zimmer Spine for use in Anterior Cervical Fusion procedures. Patient Guide to Neck Surgery Anterior Cervical Fusion Trinica Select With the Trinica and
The following is a sampling of products offered by Zimmer Spine for use in Anterior Cervical Fusion procedures. Patient Guide to Neck Surgery Anterior Cervical Fusion Trinica Select With the Trinica and
2013 North American SSL Certificate
 2013 2014 INSERT COMPANY LOGO HERE 2014 North American 2013 North American SSL Certificate Minimally Invasive Spinal Surgical Solutions Product Product Line Leadership Strategy Leadership Award Award Background
2013 2014 INSERT COMPANY LOGO HERE 2014 North American 2013 North American SSL Certificate Minimally Invasive Spinal Surgical Solutions Product Product Line Leadership Strategy Leadership Award Award Background
LUMBAR SPINAL STENOSIS OBSERVATIONS, EVIDENCE, AND TRENDS FULILLING THE UNMET CLINICAL NEED WRITTEN BY: HALLETT MATHEWS, MD, MBA
 LUMBAR SPINAL STENOSIS OBSERVATIONS, EVIDENCE, AND TRENDS FULILLING THE UNMET CLINICAL NEED WRITTEN BY: HALLETT MATHEWS, MD, MBA Overview of Lumbar Spinal Stenosis Spine stabilization, which has equated
LUMBAR SPINAL STENOSIS OBSERVATIONS, EVIDENCE, AND TRENDS FULILLING THE UNMET CLINICAL NEED WRITTEN BY: HALLETT MATHEWS, MD, MBA Overview of Lumbar Spinal Stenosis Spine stabilization, which has equated
Minimally Invasive Spine Surgery For Your Patients
 Minimally Invasive Spine Surgery For Your Patients Lukas P. Zebala, M.D. Assistant Professor Orthopaedic and Neurological Spine Surgery Department of Orthopaedic Surgery Washington University School of
Minimally Invasive Spine Surgery For Your Patients Lukas P. Zebala, M.D. Assistant Professor Orthopaedic and Neurological Spine Surgery Department of Orthopaedic Surgery Washington University School of
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): 6/14/2004 Most Recent Review Date (Revised): 9/29/2015 Effective Date: 12/1/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
Original Issue Date (Created): 6/14/2004 Most Recent Review Date (Revised): 9/29/2015 Effective Date: 12/1/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
Evidence-based lumbar spine surgery
 34 Acta Orthop Scand (Suppl 5) 02; 73 Evidence-based lumbar spine surgery The role of national registration Björn Strömqvist Department of Orthopedics, Lund University Hospital, SE-221 85 Lund, Sweden,
34 Acta Orthop Scand (Suppl 5) 02; 73 Evidence-based lumbar spine surgery The role of national registration Björn Strömqvist Department of Orthopedics, Lund University Hospital, SE-221 85 Lund, Sweden,
Options for Cervical Disc Degeneration A Guide to the M6-C. clinical study
 Options for Cervical Disc Degeneration A Guide to the M6-C clinical study Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause
Options for Cervical Disc Degeneration A Guide to the M6-C clinical study Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause
BONE-GRAFT SUBSTITUTES: FACTS, FICTIONS & APPLICATIONS
 BONE-GRAFT SUBSTITUTES: FACTS, FICTIONS & APPLICATIONS AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 70th Annual Meeting February 5-9, 2003 New Orleans, Louisiana COMMITTEE ON BIOLOGICAL IMPLANTS Prepared by:
BONE-GRAFT SUBSTITUTES: FACTS, FICTIONS & APPLICATIONS AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 70th Annual Meeting February 5-9, 2003 New Orleans, Louisiana COMMITTEE ON BIOLOGICAL IMPLANTS Prepared by:
Curriculum Vitae (Updated 06/09)
 Curriculum Vitae (Updated 06/09) JOHN M. SMALL, M.D. Business Address Florida Orthopaedic Institute 13020 N. Telecom Parkway Temple Terrace, FL 33637 Business Phone 813-978-9700 : March 27, 1958 Medical
Curriculum Vitae (Updated 06/09) JOHN M. SMALL, M.D. Business Address Florida Orthopaedic Institute 13020 N. Telecom Parkway Temple Terrace, FL 33637 Business Phone 813-978-9700 : March 27, 1958 Medical
Degenerative Spine Solutions
 Degenerative Spine Solutions The Backbone for Your Surgical Needs Aesculap Spine Backbone for Your Degenerative Spine Needs Comprehensive operative solutions, unique product technology and world-class
Degenerative Spine Solutions The Backbone for Your Surgical Needs Aesculap Spine Backbone for Your Degenerative Spine Needs Comprehensive operative solutions, unique product technology and world-class
The MOVIE. Currently VP, Regulatory Affairs, MCRA Former Orthopedic Devices Branch Chief (2006)
 FDA played by Glenn Stiegman Currently VP, Regulatory Affairs, MCRA Former Orthopedic Devices Branch Chief (2006) Managed Orthopedic and Spine groups (Branch split in 2007) Former Orthopedic Reviewer (2000-2005)
FDA played by Glenn Stiegman Currently VP, Regulatory Affairs, MCRA Former Orthopedic Devices Branch Chief (2006) Managed Orthopedic and Spine groups (Branch split in 2007) Former Orthopedic Reviewer (2000-2005)
Choosing Bone Graft Substitutes: Filling the Gaps on Evidence and Pricing. September 24, 2014
 Choosing Bone Graft Substitutes: Filling the Gaps on Evidence and Pricing September 24, 2014 Choosing Bone Graft Substitutes: Filling the Gaps on Evidence and Pricing ECRI Institute Webinar Presented by:
Choosing Bone Graft Substitutes: Filling the Gaps on Evidence and Pricing September 24, 2014 Choosing Bone Graft Substitutes: Filling the Gaps on Evidence and Pricing ECRI Institute Webinar Presented by:
ICD-10-PCS Documentation and Coding for Spinal Procedures October 22, 2015
 Questions and Answers 1. We have a question regarding a spinal surgical procedure. The diagnosis was bilateral lateral recess stenosis and central stenosis from L2-L5. The procedure was an open lumbar
Questions and Answers 1. We have a question regarding a spinal surgical procedure. The diagnosis was bilateral lateral recess stenosis and central stenosis from L2-L5. The procedure was an open lumbar
SUPERIOR COURT OF THE STATE OF CALIFORNIA FOR THE COUNTY OF LOS ANGELES. 21 COMES NOW Plaintiff APRIL CHRISTINE CABANA who alleges as follows:
 RonaldL.M. Goldman, Esq., Bar Number Bijan Esfandiari, Esq., Bar Number 1 A. Ilyas Akbari, Esq., Bar Number 1 BAUM HEDLUND ARISTEI & GOLDMAN, P.C. 1 Wilshire Blvd., Suite 0 Los Angeles, CA 00. Tel: ()-
RonaldL.M. Goldman, Esq., Bar Number Bijan Esfandiari, Esq., Bar Number 1 A. Ilyas Akbari, Esq., Bar Number 1 BAUM HEDLUND ARISTEI & GOLDMAN, P.C. 1 Wilshire Blvd., Suite 0 Los Angeles, CA 00. Tel: ()-
Advances In Spine Care. James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery
 Advances In Spine Care James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery Introduction The Spine - A common source of problems Back pain is the #2 presenting
Advances In Spine Care James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery Introduction The Spine - A common source of problems Back pain is the #2 presenting
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: lumbar_spine_fusion_surgery 9/2010 5/2015 5/2016 5/2015 Description of Procedure or Service Low back pain
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: lumbar_spine_fusion_surgery 9/2010 5/2015 5/2016 5/2015 Description of Procedure or Service Low back pain
THE EVOLVING ROLE OF BONE-GRAFT SUBSTITUTES
 THE EVOLVING ROLE OF BONE-GRAFT SUBSTITUTES AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 77TH ANNUAL MEETING MARCH 9-13, 2010 NEW ORLEANS, LOUISIANA ORTHOPAEDIC DEVICE FORUM PREPARED BY: A. SETH GREENWALD,
THE EVOLVING ROLE OF BONE-GRAFT SUBSTITUTES AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 77TH ANNUAL MEETING MARCH 9-13, 2010 NEW ORLEANS, LOUISIANA ORTHOPAEDIC DEVICE FORUM PREPARED BY: A. SETH GREENWALD,
John E. O Toole, Marjorie C. Wang, and Michael G. Kaiser
 Hypothermia and Human Spinal Cord Injury: Updated Position Statement and Evidence Based Recommendations from the AANS/CNS Joint Sections on Disorders of the Spine & Peripheral Nerves and Neurotrauma &
Hypothermia and Human Spinal Cord Injury: Updated Position Statement and Evidence Based Recommendations from the AANS/CNS Joint Sections on Disorders of the Spine & Peripheral Nerves and Neurotrauma &
SPINE SERVICE ROTATION ROTATION SPECIFIC OBJECTIVES (RSO) DEPT. OF ORTHOPEDICS AND PHYSICAL REHABILITATION UNIVERSITY OF MASSACHUSETTS
 SPINE SERVICE ROTATION ROTATION SPECIFIC OBJECTIVES (RSO) DEPT. OF ORTHOPEDICS AND PHYSICAL REHABILITATION UNIVERSITY OF MASSACHUSETTS The purpose of this RSO is to outline and clarify the objectives of
SPINE SERVICE ROTATION ROTATION SPECIFIC OBJECTIVES (RSO) DEPT. OF ORTHOPEDICS AND PHYSICAL REHABILITATION UNIVERSITY OF MASSACHUSETTS The purpose of this RSO is to outline and clarify the objectives of
Patient Guide to Lower Back Surgery
 The following is a sampling of products offered by Zimmer Spine for use in Open Lumbar Fusion procedures. Patient Guide to Lower Back Surgery Open Lumbar Fusion Dynesys The Dynesys Dynamic Stabilization
The following is a sampling of products offered by Zimmer Spine for use in Open Lumbar Fusion procedures. Patient Guide to Lower Back Surgery Open Lumbar Fusion Dynesys The Dynesys Dynamic Stabilization
Reimbursement Overview Supporting Adjunctive Use of the coflex-f Implant During Lumbar Fusion Procedures
 Interlaminar Technology Reimbursement Overview Supporting Adjunctive Use of the coflex-f Implant During Lumbar Fusion Procedures Coding Recommendations Overview Implant Description & Device Type Differentiation
Interlaminar Technology Reimbursement Overview Supporting Adjunctive Use of the coflex-f Implant During Lumbar Fusion Procedures Coding Recommendations Overview Implant Description & Device Type Differentiation
4052 Slimplicity Tech final_layout 1 6/29/15 3:29 PM Page 2 Surgical Technique
 Surgical Technique TABLE OF CONTENTS Slimplicity Anterior Cervical Plate System Overview 2 Indications 2 Implants 3 Instruments 4 Surgical Technique 6 1. Patient Positioning and Approach 6 2. Plate Selection
Surgical Technique TABLE OF CONTENTS Slimplicity Anterior Cervical Plate System Overview 2 Indications 2 Implants 3 Instruments 4 Surgical Technique 6 1. Patient Positioning and Approach 6 2. Plate Selection
Information for the Patient About Surgical
 Information for the Patient About Surgical Decompression and Stabilization of the Spine Aging and the Spine Daily wear and tear, along with disc degeneration due to aging and injury, are common causes
Information for the Patient About Surgical Decompression and Stabilization of the Spine Aging and the Spine Daily wear and tear, along with disc degeneration due to aging and injury, are common causes
INFUSE Bone Graft Oral Maxillofacial Bone Grafting Procedures
 INFUSE Bone Graft Oral Maxillofacial Bone Grafting Procedures Dental Products Advisory Panel Committee Ed Chin, DPh Group Director, Regulatory Affairs Medtronic Spinal and Biologics 1 INFUSE Bone Graft
INFUSE Bone Graft Oral Maxillofacial Bone Grafting Procedures Dental Products Advisory Panel Committee Ed Chin, DPh Group Director, Regulatory Affairs Medtronic Spinal and Biologics 1 INFUSE Bone Graft
INFUSE Bone Graft (rhbmp-2/acs)
 1 INFUSE Bone Graft (rhbmp-2/acs) For patients who need more bone to place dental implants Enjoy living with INFUSE Bone Graft. www.medtronic.com Medtronic Spinal and Biologics Business Worldwide Headquarters
1 INFUSE Bone Graft (rhbmp-2/acs) For patients who need more bone to place dental implants Enjoy living with INFUSE Bone Graft. www.medtronic.com Medtronic Spinal and Biologics Business Worldwide Headquarters
A Patient s Guide to Artificial Cervical Disc Replacement
 A Patient s Guide to Artificial Cervical Disc Replacement Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause pain and numbness
A Patient s Guide to Artificial Cervical Disc Replacement Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause pain and numbness
Evidence-based Guidelines for the Performance of Lumbar Fusion
 CHAPTER 31 Evidence-based Guidelines for the Performance of Lumbar Fusion Daniel K. Resnick, M.D. INTRODUCTION The number of lumbar fusions procedures performed in the United States has increased substantially
CHAPTER 31 Evidence-based Guidelines for the Performance of Lumbar Fusion Daniel K. Resnick, M.D. INTRODUCTION The number of lumbar fusions procedures performed in the United States has increased substantially
Instrumented in situ posterolateral fusion for low-grade lytic spondylolisthesis in adults
 Acta Orthop. Belg., 2005, 71, 83-87 ORIGINAL STUDY Instrumented in situ posterolateral fusion for low-grade lytic spondylolisthesis in adults Mohamed A. EL MASRY, Walaa I. EL ASSUITY, Youssry K. EL HAWARY,
Acta Orthop. Belg., 2005, 71, 83-87 ORIGINAL STUDY Instrumented in situ posterolateral fusion for low-grade lytic spondylolisthesis in adults Mohamed A. EL MASRY, Walaa I. EL ASSUITY, Youssry K. EL HAWARY,
Combined interbody cage and anterior plating in the surgical treatment of cervical disc disease
 Acta Orthop. Belg., 2004, 70, 461-465 ORIGINAL STUDY Combined interbody cage and anterior plating in the surgical treatment of cervical disc disease Anastasios CHRISTODOULOU, Avraam PLOUMIS, Ioannis TERZIDIS,
Acta Orthop. Belg., 2004, 70, 461-465 ORIGINAL STUDY Combined interbody cage and anterior plating in the surgical treatment of cervical disc disease Anastasios CHRISTODOULOU, Avraam PLOUMIS, Ioannis TERZIDIS,
Chronic low back pain (LBP), defined as pain lasting
 SPINE Volume 38, Number 7, pp E409 E422 2013, Lippincott Williams & Wilkins LITERATURE REVIEW Lumbar Spine Fusion for Chronic Low Due to Degenerative Disc Disease A Systematic Review Frank M. Phillips,
SPINE Volume 38, Number 7, pp E409 E422 2013, Lippincott Williams & Wilkins LITERATURE REVIEW Lumbar Spine Fusion for Chronic Low Due to Degenerative Disc Disease A Systematic Review Frank M. Phillips,
Value Analysis Brief MIS Lateral Approach to Interbody Fusion
 Value Analysis Brief MIS Lateral Approach to Interbody Fusion Methods This value analysis brief presents information on the clinical and economic benefits of the minimally invasive lateral approach to
Value Analysis Brief MIS Lateral Approach to Interbody Fusion Methods This value analysis brief presents information on the clinical and economic benefits of the minimally invasive lateral approach to
Spinal Decompression: Measurement of Treatment Outcomes. William D. Grant, EdD. Catherine E. Saxton, BS
 1 Spinal Decompression: Measurement of Treatment Outcomes. William D. Grant, EdD Catherine E. Saxton, BS Correspondence to: William D. Grant, EdD SUNY Upstate Medical University IHP 3302 750 E. Adams St.
1 Spinal Decompression: Measurement of Treatment Outcomes. William D. Grant, EdD Catherine E. Saxton, BS Correspondence to: William D. Grant, EdD SUNY Upstate Medical University IHP 3302 750 E. Adams St.
Subject: Implanted Devices for Spinal Stenosis Policy #: SURG.00092 Current Effective Date: 07/13/2011 Status: Reviewed Last Review Date: 05/19/2011
 1 of 5 6/18/2012 11:08 AM Medical Policy Subject: Implanted Devices for Spinal Stenosis Policy #: SURG.00092 Current Effective Date: 07/13/2011 Status: Reviewed Last Review Date: 05/19/2011 Description/Scope
1 of 5 6/18/2012 11:08 AM Medical Policy Subject: Implanted Devices for Spinal Stenosis Policy #: SURG.00092 Current Effective Date: 07/13/2011 Status: Reviewed Last Review Date: 05/19/2011 Description/Scope
Lumbar Spinal Stenosis Materclass: Surgical management of lumbar spinal stenosis:
 Lumbar Spinal Stenosis Materclass: Surgical management of lumbar spinal stenosis: Presented By: Michelle Emsley Senior Spinal In-Patient Physiotherapist Learning objectives Indications Evidence Post operative
Lumbar Spinal Stenosis Materclass: Surgical management of lumbar spinal stenosis: Presented By: Michelle Emsley Senior Spinal In-Patient Physiotherapist Learning objectives Indications Evidence Post operative
.org. Fractures of the Thoracic and Lumbar Spine. Cause. Description
 Fractures of the Thoracic and Lumbar Spine Page ( 1 ) Spinal fractures can vary widely in severity. While some fractures are very serious injuries that require emergency treatment, other fractures can
Fractures of the Thoracic and Lumbar Spine Page ( 1 ) Spinal fractures can vary widely in severity. While some fractures are very serious injuries that require emergency treatment, other fractures can
Pulmonary Rehabilitation in Ontario: OHTAC Recommendation
 Pulmonary Rehabilitation in Ontario: OHTAC Recommendation ONTARIO HEALTH TECHNOLOGY ADVISORY COMMITTEE MARCH 2015 Pulmonary Rehabilitation in Ontario: OHTAC Recommendation. March 2015; pp. 1 13 Suggested
Pulmonary Rehabilitation in Ontario: OHTAC Recommendation ONTARIO HEALTH TECHNOLOGY ADVISORY COMMITTEE MARCH 2015 Pulmonary Rehabilitation in Ontario: OHTAC Recommendation. March 2015; pp. 1 13 Suggested
Magnetic Resonance Imaging
 Magnetic Resonance Imaging North American Spine Society Public Education Series What Is Magnetic Resonance Imaging (MRI)? Magnetic resonance imaging (MRI) is a valuable diagnostic study that has been used
Magnetic Resonance Imaging North American Spine Society Public Education Series What Is Magnetic Resonance Imaging (MRI)? Magnetic resonance imaging (MRI) is a valuable diagnostic study that has been used
INFUSE Bone Graft. Patient Information Brochure
 INFUSE Bone Graft Patient Information Brochure This Patient Guide is designed to help you decide whether or not to have surgery using INFUSE Bone Graft to treat your broken tibia (lower leg). There are
INFUSE Bone Graft Patient Information Brochure This Patient Guide is designed to help you decide whether or not to have surgery using INFUSE Bone Graft to treat your broken tibia (lower leg). There are
2001 Volvo Award Winner in Clinical Studies: Lumbar Fusion Versus Nonsurgical Treatment for Chronic Low Back Pain
 2001 Volvo Award Winner in Clinical Studies: Lumbar Fusion Versus Nonsurgical Treatment for Chronic Low Back Pain A Multicenter Randomized Controlled Trial From the Swedish Lumbar Spine Study Group Peter
2001 Volvo Award Winner in Clinical Studies: Lumbar Fusion Versus Nonsurgical Treatment for Chronic Low Back Pain A Multicenter Randomized Controlled Trial From the Swedish Lumbar Spine Study Group Peter
TABLE OF CONTENTS. Surgical Technique 2. Indications 4. Product Information 5. 1. Patient Positioning and Approach 2
 Surgical Technique TABLE OF CONTENTS Surgical Technique 2 1. Patient Positioning and Approach 2 2. Intervertebral Device Implanted 2 3. Buttress Plate Selection 2 4. Awl Insertion 2 5. Screw Insertion
Surgical Technique TABLE OF CONTENTS Surgical Technique 2 1. Patient Positioning and Approach 2 2. Intervertebral Device Implanted 2 3. Buttress Plate Selection 2 4. Awl Insertion 2 5. Screw Insertion
IN THE CIRCUIT COURT OF COOK COUNTY, ILLINOIS, COUNTY DEPARTMENT, LAW DIVISION
 IN THE CIRCUIT COURT OF COOK COUNTY, ILLINOIS, COUNTY DEPARTMENT, LAW DIVISION KARL L. SANDA, ) ) Plaintiff, ) ) w. ) ) Case No. : MEDTRONIC, INC, MEDTRONIC ) SOFAMOR DANEK USA, INC., ) NORTHWESTERN MEMORIAL
IN THE CIRCUIT COURT OF COOK COUNTY, ILLINOIS, COUNTY DEPARTMENT, LAW DIVISION KARL L. SANDA, ) ) Plaintiff, ) ) w. ) ) Case No. : MEDTRONIC, INC, MEDTRONIC ) SOFAMOR DANEK USA, INC., ) NORTHWESTERN MEMORIAL
On Cervical Zygapophysial Joint Pain After Whiplash. Spine December 1, 2011; Volume 36, Number 25S, pp S194 S199
 On Cervical Zygapophysial Joint Pain After Whiplash 1 Spine December 1, 2011; Volume 36, Number 25S, pp S194 S199 Nikolai Bogduk, MD, PhD FROM ABSTRACT Objective To summarize the evidence that implicates
On Cervical Zygapophysial Joint Pain After Whiplash 1 Spine December 1, 2011; Volume 36, Number 25S, pp S194 S199 Nikolai Bogduk, MD, PhD FROM ABSTRACT Objective To summarize the evidence that implicates
REPUTATION MANAGEMENT: SPINAL FUSION SURGERY
 REPUTATION MANAGEMENT: SPINAL FUSION SURGERY Meaningful and Positive Progress in Spinal Fusion Results: A Journey towards Excellence Paul J. Slosar, M.D. SpineCare Medical Group As a practicing spine surgeon,
REPUTATION MANAGEMENT: SPINAL FUSION SURGERY Meaningful and Positive Progress in Spinal Fusion Results: A Journey towards Excellence Paul J. Slosar, M.D. SpineCare Medical Group As a practicing spine surgeon,
1 REVISOR 5223.0070. (4) Pain associated with rigidity (loss of motion or postural abnormality) or
 1 REVISOR 5223.0070 5223.0070 MUSCULOSKELETAL SCHEDULE; BACK. Subpart 1. Lumbar spine. The spine rating is inclusive of leg symptoms except for gross motor weakness, bladder or bowel dysfunction, or sexual
1 REVISOR 5223.0070 5223.0070 MUSCULOSKELETAL SCHEDULE; BACK. Subpart 1. Lumbar spine. The spine rating is inclusive of leg symptoms except for gross motor weakness, bladder or bowel dysfunction, or sexual
Complications in Adult Deformity Surgery
 Complications in Adult Deformity Surgery Proximal Junctional Kyphosis: Thoracolumbar and Cervicothoracic Sigurd Berven, MD Professor in Residence UC San Francisco Disclosures Research/Institutional Support:
Complications in Adult Deformity Surgery Proximal Junctional Kyphosis: Thoracolumbar and Cervicothoracic Sigurd Berven, MD Professor in Residence UC San Francisco Disclosures Research/Institutional Support:
Issued and entered this _6th_ day of October 2010 by Ken Ross Commissioner ORDER I PROCEDURAL BACKGROUND
 STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX Petitioner
STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX Petitioner
A Patient s Guide to the Disorders of the Cervical and Upper Thoracic Spine
 A Patient s Guide to the Disorders of the Cervical and Upper Thoracic Spine General Anatomy of the Spine The spine can be divided into four regions based on vertebral shape and sagittal plane curve.» CERVICAL:
A Patient s Guide to the Disorders of the Cervical and Upper Thoracic Spine General Anatomy of the Spine The spine can be divided into four regions based on vertebral shape and sagittal plane curve.» CERVICAL:
Corporate Medical Policy Electrical Bone Growth Stimulation
 Corporate Medical Policy Electrical Bone Growth Stimulation File Name: Origination: Last CAP Review: Next CAP Review: Last Review: electrical_bone_growth_stimulation 4/1981 6/2016 6/2017 6/2016 Description
Corporate Medical Policy Electrical Bone Growth Stimulation File Name: Origination: Last CAP Review: Next CAP Review: Last Review: electrical_bone_growth_stimulation 4/1981 6/2016 6/2017 6/2016 Description
Contents. Introduction 1. Anatomy of the Spine 1. 2. Spinal Imaging 7. 3. Spinal Biomechanics 23. 4. History and Physical Examination of the Spine 33
 Contents Introduction 1. Anatomy of the Spine 1 Vertebrae 1 Ligaments 3 Intervertebral Disk 4 Intervertebral Foramen 5 2. Spinal Imaging 7 Imaging Modalities 7 Conventional Radiographs 7 Myelography 9
Contents Introduction 1. Anatomy of the Spine 1 Vertebrae 1 Ligaments 3 Intervertebral Disk 4 Intervertebral Foramen 5 2. Spinal Imaging 7 Imaging Modalities 7 Conventional Radiographs 7 Myelography 9
How to Overcome the 5 Biggest Reimbursement Challenges in Joint & Spine Coding
 How to Overcome the 5 Biggest Reimbursement Challenges in Joint & Spine Coding Presented by: Carolyn Neumann, CPC Senior Manager Coding and Coverage Access The opinions and codes denoted within are suggestions
How to Overcome the 5 Biggest Reimbursement Challenges in Joint & Spine Coding Presented by: Carolyn Neumann, CPC Senior Manager Coding and Coverage Access The opinions and codes denoted within are suggestions
Artificial Intervertebral Disc: Lumbar Spine
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Artificial Intervertebral Disc: Lumbar Spine Number 7.01.87 Effective
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Artificial Intervertebral Disc: Lumbar Spine Number 7.01.87 Effective
Mitchell S. Fourman M.Phil Eugene Borst BS Eric Bogner, MD S. Robert Rozbruch MD Austin T. Fragomen, MD
 Post-Operative CT Measurements Accurately Predict the Need for Clinical Intervention Retrospective Findings to Support Prospective Clinical Trial Protocol Mitchell S. Fourman M.Phil Eugene Borst BS Eric
Post-Operative CT Measurements Accurately Predict the Need for Clinical Intervention Retrospective Findings to Support Prospective Clinical Trial Protocol Mitchell S. Fourman M.Phil Eugene Borst BS Eric
Nonoperative Management of Herniated Cervical Intervertebral Disc With Radiculopathy. Spine Volume 21(16) August 15, 1996, pp 1877-1883
 Nonoperative Management of Herniated Cervical Intervertebral Disc With Radiculopathy 1 Spine Volume 21(16) August 15, 1996, pp 1877-1883 Saal, Joel S. MD; Saal, Jeffrey A. MD; Yurth, Elizabeth F. MD FROM
Nonoperative Management of Herniated Cervical Intervertebral Disc With Radiculopathy 1 Spine Volume 21(16) August 15, 1996, pp 1877-1883 Saal, Joel S. MD; Saal, Jeffrey A. MD; Yurth, Elizabeth F. MD FROM
Surgery for cervical disc prolapse or cervical osteophyte
 Mr Paul S. D Urso MBBS(Hons), PhD, FRACS Neurosurgeon Provider Nº: 081161DY Epworth Centre Suite 6.1 32 Erin Street Richmond 3121 Tel: 03 9421 5844 Fax: 03 9421 4186 AH: 03 9483 4040 email: paul@pauldurso.com
Mr Paul S. D Urso MBBS(Hons), PhD, FRACS Neurosurgeon Provider Nº: 081161DY Epworth Centre Suite 6.1 32 Erin Street Richmond 3121 Tel: 03 9421 5844 Fax: 03 9421 4186 AH: 03 9483 4040 email: paul@pauldurso.com
Adult Spine Rotation Specific Evaluation Orthopaedic Surgery Training Program School of Medicine, Queen s University
 Adult Spine Rotation Specific Evaluation Orthopaedic Surgery Training Program School of Medicine, Queen s University CanMEDS Roles / Competencies Name: PGY Rotation Dates: s s Exceeds N/A Attending Staff:
Adult Spine Rotation Specific Evaluation Orthopaedic Surgery Training Program School of Medicine, Queen s University CanMEDS Roles / Competencies Name: PGY Rotation Dates: s s Exceeds N/A Attending Staff:
A Patient s Guide to Diffuse Idiopathic Skeletal Hyperostosis (DISH)
 A Patient s Guide to Diffuse Idiopathic Skeletal Hyperostosis (DISH) Introduction Diffuse Idiopathic Skeletal Hyperostosis (DISH) is a phenomenon that more commonly affects older males. It is associated
A Patient s Guide to Diffuse Idiopathic Skeletal Hyperostosis (DISH) Introduction Diffuse Idiopathic Skeletal Hyperostosis (DISH) is a phenomenon that more commonly affects older males. It is associated
Behavioral and Physical Treatments for Tension-type and Cervicogenic Headache
 Evidence Report: Behavioral and Physical Treatments for Tension-type and Cervicogenic Headache Douglas C. McCrory, MD, MHSc Donald B. Penzien, PhD Vic Hasselblad, PhD Rebecca N. Gray, DPhil Duke University
Evidence Report: Behavioral and Physical Treatments for Tension-type and Cervicogenic Headache Douglas C. McCrory, MD, MHSc Donald B. Penzien, PhD Vic Hasselblad, PhD Rebecca N. Gray, DPhil Duke University
Chronic Disease Management Systems for the Treatment and Management of Diabetes in Primary Health Care Practices in Ontario: OHTAC Recommendation
 Chronic Disease Management Systems for the Treatment and Management of Diabetes in Primary Health Care Practices in Ontario: OHTAC Recommendation Ontario Health Technology Advisory Committee April 2014
Chronic Disease Management Systems for the Treatment and Management of Diabetes in Primary Health Care Practices in Ontario: OHTAC Recommendation Ontario Health Technology Advisory Committee April 2014
Postoperative Rehabilitation following Lumbar Disc
 Postoperative Rehabilitation following Lumbar Disc Arthroplasty A Clinical Reasoning & Best Evidence Approach Outline Prevalence Indications for lumbar disc arthroplasty Surgical procedures Post-op rehabilitation
Postoperative Rehabilitation following Lumbar Disc Arthroplasty A Clinical Reasoning & Best Evidence Approach Outline Prevalence Indications for lumbar disc arthroplasty Surgical procedures Post-op rehabilitation
ARTICLES. Prevalence of Herniated Intervertebral Discs of the Cervical Spine in Asymptomatic Subjects Using MRI Scans: A Qualitative Systematic Review
 Please note that this electronic prepublication galley may contain typographical errors and may be missing artwork, such as charts, photographs, etc. Pagination in this version will differ from the published
Please note that this electronic prepublication galley may contain typographical errors and may be missing artwork, such as charts, photographs, etc. Pagination in this version will differ from the published
Risks of Spinal Surgery
 Risks of Spinal Surgery Infection One of the more common potential complications of any surgery is a wound infection. In spinal surgery this can be a very severe problem. It occurs in 1.5 percent to 3
Risks of Spinal Surgery Infection One of the more common potential complications of any surgery is a wound infection. In spinal surgery this can be a very severe problem. It occurs in 1.5 percent to 3
coligne treatment technology
 coligne treatment technology coligne a strategy in spine Leadership begins before the patient enters the surgical theater, among the spine care professionals forming the coalliance. This may be a small
coligne treatment technology coligne a strategy in spine Leadership begins before the patient enters the surgical theater, among the spine care professionals forming the coalliance. This may be a small
Jonathan R. Gottlieb, MD 7220 SW 127 Street Pinecrest, FL 33156 jonrgottlieb@gmail.com
 Jonathan R. Gottlieb, MD 7220 SW 127 Street Pinecrest, FL 33156 jonrgottlieb@gmail.com CURRENT PRACTICE September 2009-present University of Miami School of Medicine Assistant Professor, Department of
Jonathan R. Gottlieb, MD 7220 SW 127 Street Pinecrest, FL 33156 jonrgottlieb@gmail.com CURRENT PRACTICE September 2009-present University of Miami School of Medicine Assistant Professor, Department of
Design System review of Medline, CINAHL, AMED, PEDro, and Cochrane databases till August 2010.
 The McKenzie Institute International 2013 Vol. 2, No. 2 LITERATURE REVIEWS Summary and Perspective of Recent Literature Stephen May, PhD, MA, FCSP, Dip. MDT, MSc (UK) May S, Comer C. Is surgery more effective
The McKenzie Institute International 2013 Vol. 2, No. 2 LITERATURE REVIEWS Summary and Perspective of Recent Literature Stephen May, PhD, MA, FCSP, Dip. MDT, MSc (UK) May S, Comer C. Is surgery more effective
Oswestry Low Back Pain Disability Questionnaire Oswestry Disability Index
 Appendix D Oswestry Low Back Pain Disability Questionnaire Please complete this questionnaire. It is designed to tell us how your back pain affects your ability to function in every day life. I have Chronic
Appendix D Oswestry Low Back Pain Disability Questionnaire Please complete this questionnaire. It is designed to tell us how your back pain affects your ability to function in every day life. I have Chronic
Do you have Back Pain? Associated with:
 Do you have Back Pain? Associated with: Herniated Discs? Protruding Discs? Degenerative Disk Disease? Posterior Facet Syndrome? Sciatica? You may be a candidate for Decompression Therapy The Dynatronics
Do you have Back Pain? Associated with: Herniated Discs? Protruding Discs? Degenerative Disk Disease? Posterior Facet Syndrome? Sciatica? You may be a candidate for Decompression Therapy The Dynatronics
NON SURGICAL SPINAL DECOMPRESSION. Dr. Douglas A. VanderPloeg
 NON SURGICAL SPINAL DECOMPRESSION Dr. Douglas A. VanderPloeg CONTENTS I. Incidence of L.B.P. II. Anatomy Review III. IV. Disc Degeneration, Bulge, and Herniation Non-Surgical Spinal Decompression 1. History
NON SURGICAL SPINAL DECOMPRESSION Dr. Douglas A. VanderPloeg CONTENTS I. Incidence of L.B.P. II. Anatomy Review III. IV. Disc Degeneration, Bulge, and Herniation Non-Surgical Spinal Decompression 1. History
