Artificial Intervertebral Disc: Cervical Spine
|
|
|
- Carmella Bruce
- 10 years ago
- Views:
Transcription
1 dapplies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided in the applicable contract. Medical technology is constantly evolving, and we reserve the right to review and update Medical Policy periodically. Note: Artificial Intervertebral Disc: Lumbar Spine is addressed in medical policy number When Services May Be Eligible for Coverage Coverage for eligible medical treatments or procedures, drugs, devices or biological products may be provided only if: Benefits are available in the member s contract/certificate, and Medical necessity criteria and guidelines are met. Based on review of available data, the Company may consider artificial intervertebral cervical discs to be eligible for coverage. Patient Selection Criteria Coverage eligibility will be considered when all of the following criteria are met: The device is approved by the U.S. Food and Drug Administration (FDA); and Replacement is performed at either one level or two contiguous levels from C3-C7; and Patient has failed at least six weeks of non-surgical therapy; and Patient has intractable radiculopathy and/or myelopathy due to herniated disc or osteophyte formation with symptomatic nerve root and/or spinal cord compression documented by ALL the following: o Neck and/or arm pain; and o Functional and/or neurological deficit; and o Radiographic imaging (e.g., computed tomography (CT), magnetic resonance imaging (MRI), x- rays). When Services Are Considered Investigational Coverage is not available for investigational medical treatments or procedures, drugs, devices or biological products. The use of artificial intervertebral cervical discs when patient selection criteria are not met is considered to be investigational.* Background/Overview Several prosthetic devices are currently available for artificial intervertebral disc arthroplasty (AIDA) of the cervical spine. AIDA is proposed as an alternative to anterior cervical discectomy and fusion (ACDF) for patients with symptomatic cervical degenerative disc disease (DDD). DDD is a manifestation of spinal spondylosis that causes deterioration of the intervertebral discs of the cervical spine. Symptoms of cervical DDD include arm pain, weakness, and paresthesias associated with cervical radiculopathy. Disc herniation, osteophytes, kyphosis, or instability that compress the spinal cord Page 1 of 7
2 result in myelopathy, which is manifested by subtle changes in gait or balance, weakness in the arms or legs, and numbness of the arms or hands, in severe cases. The prevalence of DDD secondary to cervical spondylosis increases with age. An estimated 60% of individuals older than 40 years have radiographic evidence of cervical DDD. By age 65, some 95% of men and 70% of women have at least one degenerative change evident at radiographic examination. It is estimated that approximately five million adults in the United States are disabled to an extent by spine-related disorders, although only a small fraction of those are clear candidates for spinal surgery. Cervical DDD is initially treated conservatively using noninvasive measures (e.g., rest, heat, ice, analgesics, anti-inflammatory agents, exercise). If symptoms do not improve or resolve after six weeks or more, or if they progress, surgical intervention may be indicated. Candidates for surgical intervention have chronic pain or neurologic symptoms secondary to cervical DDD and no contraindications for the procedure. ACDF is currently considered the definitive surgical treatment for symptomatic DDD of the cervical spine. The goals of ACDF are to relieve pressure on the spinal nerves (decompression) and to restore spinal column alignment and stability. Resolution of pain and neurologic symptoms may be expected in 80% to 100% of ACDF patients. ACDF involves an anterolateral surgical approach, decompression of the affected spinal level, discectomy, and emplacement of either autograft or allograft bone in the prepared intervertebral space to stimulate healing and eventual fusion between the vertebral s. A metal anterior cervical plate is attached to the adjoining vertebral bodies to stabilize the fusion site, maintain neck lordosis, and reduce the need for prolonged postoperative brace application that is needed following ACDF without an anterior plate. The choice of bone material for interbody fusion in ACDF has important clinical implications. Allograft bone has several drawbacks, including a small (albeit, unproven) risk of infectious disease transmission; possible immunologic reaction to the allograft, and possible limited commercial availability of appropriate graft material. In contrast, the use of autograft bone in ACDF has potentially substantial morbidities at the harvest site, generally the iliac crest. These morbidities include moderate-tosevere, sometimes prolonged pain; deep infection; adjacent nerve and artery damage; and increased risk of stress fracture. Although there may be slight differences between autograft and allograft sources in the postoperative rate of union, clinical studies demonstrate similar rates of postoperative fusion (90 100%) and satisfactory outcomes for single-level, anterior-plated ACDF, using either bone source. Thus, the choice of graft material involves a trade-off between the risks specific to autograft harvest versus those specific to use of allograft material. Biomechanical modeling studies have suggested that altered adjacent segment kinematics following fusion may lead to adjacent-level DDD; however, the clinical relevance of these changes has not been established. AIDA is proposed as an alternative to ACDF for patients with symptomatic cervical DDD. In AIDA, an artificial disc device is secured in the prepared intervertebral space rather than in bone. An anterior plate is not placed to stabilize the adjacent vertebrae, and postsurgical external orthosis is usually not required. It is hypothesized that AIDA will maintain anatomical disk space height, normal segmental lordosis, and physiological motion patterns at the index and adjacent cervical levels. The potential to reduce the risk of adjacent-level DDD above or below a fusion site has been the major rationale driving device development and use. Page 2 of 7
3 Disc arthroplasty and ACDF for single-level disease have very similar surgical indications, primarily unremitting pain due to radiculopathy or myelopathy, weakness in the extremities, or paresthesia. However, the chief complaint in AIDA candidates should be radicular or myelopathic symptoms in the absence of significant spondylosis. Patients with advanced spondylosis or hard disc herniations have a separate pathologic condition and require a different surgical approach. FDA or Other Governmental Regulatory Approval U.S. Food and Drug Administration (FDA) The Prestige ST Cervical Disc (Medtronic) received U.S. FDA premarket application (PMA) approval as a Class III device on July 16, The Prestige ST Cervical Disc is composed of stainless steel and is indicated in skeletally mature patients for reconstruction of the disc from C3-C7 following single-level discectomy. The device is implanted via an open anterior approach. Intractable radiculopathy and/or myelopathy should be present, with at least one of the following items producing symptomatic nerve root and/or spinal cord compression as documented by patient history (e.g., pain [neck and/or arm pain], functional deficit, and/or neurologic deficit) and radiographic studies (e.g., computed tomography [CT], magnetic resonance imaging [MRI], x-rays): herniated disc and/or osteophyte formation. The FDA has required the Prestige disc manufacturer to conduct a 7-year post-approval clinical study of the safety and function of the device and a 5-year enhanced surveillance study of the disc to more fully characterize adverse events in a broader patient population. Another disc arthroplasty product, the ProDisc-C (Synthes Spine) received FDA PMA approval in December As with the Prestige ST Cervical Disc, the FDA approval of ProDisc-C is conditional on 7- year follow-up of the 209 subjects included in the noninferiority trial (discussed in Rationale section), 7-year follow-up on 99 continued access subjects, and a 5-year enhanced surveillance study to more fully characterize adverse events when the device is used under general conditions of use. The post-approval study reports are to be delivered to the FDA annually. The Bryan Cervical Disc (Medtronic Sofamor Danek) consists of 2 titanium-alloy shells encasing a polyurethane nucleus and has been available outside of the United States since The Bryan Cervical Disc was approved by the FDA in May 2009 for treatment using an anterior approach of single-level cervical DDD defined as any combination of the following: disc herniation with radiculopathy, spondylotic radiculopathy, disc herniation with myelopathy, or spondylotic myelopathy resulting in impaired function and at least one clinical neurologic sign associated with the cervical level to be treated, and necessitating surgery as demonstrated using CT, myelography and CT, and/or MRI. Patients receiving the Bryan cervical disc should have failed at least 6 weeks of non-operative treatment prior to implantation of the Bryan cervical disc. As a condition for approval of this device, the FDA required the manufacturer to extend its follow-up of enrolled subjects to 10 years after surgery. The study will involve the investigational and control patients from the pivotal investigational device exemption (IDE) study arm, as well as the patients who received the device as part of the continued access study arm. In addition, the manufacturer must perform a 5-year enhanced surveillance study of the BRYAN Cervical Disc to more fully characterize adverse events when the device is used in a broader patient population. Page 3 of 7
4 In more recent years, continued FDA approval requires completion of 2 post-approval studies. One study provides extended follow-up of the pre-market pivotal cohort out to 7 years. The second study provides 10- year enhanced surveillance of adverse event data. Continued approval is contingent on submission of annual reports, which include the number of devices sold, heterotopic ossification, device malfunction, device removal, or other serious device-related complications, and analysis of all explanted discs. The following have received FDA approval: The PCM [porous-coated motion] Cervical Disc (NuVasive) received FDA approval in 2012 (P100012). The PCM is a semi-constrained device consisting of 2 metal (cobalt-chromium alloy) s and a polyethylene insert that fits between the s. Secure -C (Globus Medical) was approved in 2012 (P100003). The Secure-C is a 3 piece semiconstrained device with 2 metal (cobalt chromium molybdenum alloy) s and a polyethelene insert. The Mobi-C (LDR Spine) received FDA approval in Mobi-C is 3 piece semiconstrained device with metal (cobalt-chromium alloy) s and a polyethylene insert. The Mobi-C is approved for 1 (P110002) or 2 level (P110009) disc replacement. A number of other devices are under study in trials in the United States. Cervical Disc Prostheses Under Investigation in the U.S. Prosthesis (Manufacturer) Implant Composition Articulation Design Bearing Surface Bearing Constraint Fixation FDA Status Prestige LP (Medtronic) Titanium-ceramic composite Ellipsoid saucer MoM Semiconstrained Primary dual rails enrollment Kineflex C Cervical Artificial Disc Implant (Spinal Motion) Cobalt-chromiummolybdenum Three piece, metal core MoM Unconstrained Primary central keel CerviCore Intervertebral Disc (Stryker) Cobalt-chromiummolybdenum Saddle MoM Unconstrained Primary dual rails Status unknown Discover (DePuy) Titanium-onpolyethelene Three piece, polyethelene core MoP Unconstrained Primary Spike fixation enrollment Page 4 of 7
5 NeoDisc (NuVasive) Freedom Cervical Disc (AxioMed) trial Recruiting M6-C (Spinal Kinetics) Titanium s and polymer core Seven-piece, with s and a nucleus, fibrous annulus and sheath trial withdrawn prior to enrollment Centers for Medicare and Medicaid Services (CMS) A search of the Medicare National Database ( identified a national coverage decision on artificial intervertebral discs for the lumbar spine. There is no national coverage decision on artificial intervertebral discs for the cervical spine. Rationale/Source This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, Blue Cross and Blue Shield Association technology assessment program (TEC) and other non-affiliated technology evaluation centers, reference to federal regulations, other plan medical policies, and accredited national guidelines. References 1. Blue Cross and Blue Shield Association, Medical Policy Reference Manual, Artificial Intervertebral Disc: Cervical Spine, , 01: Boselie TF, Willems PC, van Mameren H et al. Arthroplasty versus fusion in single-level cervical degenerative disc disease: a cochrane review. Spine (Phila Pa 1976) 2013; 38(17):E U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health. Summary of Safety and Effectiveness Data: Mobi-C Available online at: Last accessed November, Davis RJ, Kim KD, Hisey MS et al. Cervical total disc replacement with the Mobi-C cervical artificial disc compared with anterior discectomy and fusion for treatment of 2-level symptomatic degenerative disc disease: a prospective, randomized, controlled multicenter. J Neurosurg Spine 2013; 19(5): U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health. Summary of Safety and Effectiveness Data: SECURE-C Available online at: Last accessed November, Coric D, Kim PK, Clemente JD et al. Prospective randomized study of cervical arthroplasty and anterior cervical discectomy and fusion with long-term follow-up: results in 74 patients from a single site. J Neurosurg Spine 2013; 18(1): Policy History 02/13/2008 Medical Director review 02/20/2008 Medical Policy Committee approval. 02/04/2009 Medical Director review Page 5 of 7
6 02/19/2009 Medical Policy Committee approval. No change to coverage. 02/04/2009 Medical Policy Committee review 02/17/2009 Medical Policy Implementation Committee approval. No change to coverage. 02/03/2011 Medical Policy Committee review 02/16/2011 Medical Policy Implementation Committee approval. No change to coverage. 02/02/2012 Medical Policy Committee review 02/15/2012 Medical Policy Implementation Committee approval. No change to coverage. 02/07/2013 Medical Policy Committee review 02/20/2013 Medical Policy Implementation Committee approval. Coverage changed from investigational to eligible with criteria. 12/12/2013 Medical Policy Committee review 12/18/2013 Medical Policy Implementation Committee approval. Criteria revised to include two contiguous levels from C3 to C7 as eligible for coverage. FDA information updated. 03/05/2015 Medical Policy Committee review 03/20/2015 Medical Policy Implementation Committee approval. No change to coverage. 08/03/2015 Coding update: ICD10 Diagnosis code section added; ICD9 Procedure code section removed. Next Scheduled Review Date: 03/2016 Coding The five character codes included in the Blue Cross Blue Shield of Louisiana Medical Policy Coverage Guidelines are obtained from Current Procedural Terminology (CPT ), copyright 2014 by the American Medical Association (AMA). CPT is developed by the AMA as a listing of descriptive terms and five character identifying codes and modifiers for reporting medical services and procedures performed by physician. The responsibility for the content of Blue Cross Blue Shield of Louisiana Medical Policy Coverage Guidelines is with Blue Cross and Blue Shield of Louisiana and no endorsement by the AMA is intended or should be implied. The AMA disclaims responsibility for any consequences or liability attributable or related to any use, nonuse or interpretation of information contained in Blue Cross Blue Shield of Louisiana Medical Policy Coverage Guidelines. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The AMA assumes no liability for data contained or not contained herein. Any use of CPT outside of Blue Cross Blue Shield of Louisiana Medical Policy Coverage Guidelines should refer to the most current Current Procedural Terminology which contains the and most current listing of CPT codes and descriptive terms. Applicable FARS/DFARS apply. CPT is a registered trademark of the American Medical Association. Codes used to identify services associated with this policy may include (but may not be limited to) the following: Code Type Code CPT 0095T, 0098T, 0375T, 22856, 22858, 22861, HCPCS ICD-9 Diagnosis ICD-10 Diagnosis No codes All related diagnoses All related diagnoses Page 6 of 7
7 *Investigational A medical treatment, procedure, drug, device, or biological product is Investigational if the effectiveness has not been clearly tested and it has not been incorporated into standard medical practice. Any determination we make that a medical treatment, procedure, drug, device, or biological product is Investigational will be based on a consideration of the following: A. Whether the medical treatment, procedure, drug, device, or biological product can be lawfully marketed without approval of the U.S. FDA and whether such approval has been granted at the time the medical treatment, procedure, drug, device, or biological product is sought to be furnished; or B. Whether the medical treatment, procedure, drug, device, or biological product requires further studies or s to determine its maximum tolerated dose, toxicity, safety, effectiveness, or effectiveness as compared with the standard means of treatment or diagnosis, must improve health outcomes, according to the consensus of opinion among experts as shown by reliable evidence, including: 1. Consultation with the Blue Cross and Blue Shield Association technology assessment program (TEC) or other nonaffiliated technology evaluation center(s); 2. Credible scientific evidence published in peer-reviewed medical literature generally recognized by the relevant medical community; or 3. Reference to federal regulations. **Medically Necessary (or Medical Necessity ) - Health care services, treatment, procedures, equipment, drugs, devices, items or supplies that a Provider, exercising prudent clinical judgment, would provide to a patient for the purpose of preventing, evaluating, diagnosing or treating an illness, injury, disease or its symptoms, and that are: A. In accordance with nationally accepted standards of medical practice; B. Clinically appropriate, in terms of type, frequency, extent, level of care, site and duration, and considered effective for the patient's illness, injury or disease; and C. Not primarily for the personal comfort or convenience of the patient, physician or other health care provider, and not more costly than an alternative service or sequence of services at least as likely to produce equivalent therapeutic or diagnostic results as to the diagnosis or treatment of that patient's illness, injury or disease. For these purposes, nationally accepted standards of medical practice means standards that are based on credible scientific evidence published in peer-reviewed medical literature generally recognized by the relevant medical community, Physician Specialty Society recommendations and the views of Physicians practicing in relevant clinical areas and any other relevant factors. Indicated trademarks are the registered trademarks of their respective owners. NOTICE: Medical Policies are scientific based opinions, provided solely for coverage and informational purposes. Medical Policies should not be construed to suggest that the Company recommends, advocates, requires, encourages, or discourages any particular treatment, procedure, or service, or any particular course of treatment, procedure, or service. Page 7 of 7
Protocol. Artificial Intervertebral Disc: Cervical Spine
 Protocol Artificial Intervertebral Disc: Cervical Spine (701108) Medical Benefit Effective Date: 10/01/15 Next Review Date: 07/16 Preauthorization Yes Review Dates: 06/07, 07/08, 05/09, 05/10, 03/11, 03/12,
Protocol Artificial Intervertebral Disc: Cervical Spine (701108) Medical Benefit Effective Date: 10/01/15 Next Review Date: 07/16 Preauthorization Yes Review Dates: 06/07, 07/08, 05/09, 05/10, 03/11, 03/12,
White Paper: Cervical Disc Replacement: When is the Mobi-C Cervical Disc Medically Necessary?
 White Paper: Cervical Disc Replacement: When is the Mobi-C Cervical Disc Medically Necessary? For Health Plans, Medical Management Organizations and TPAs Cervical Disc Disease: An Overview The cervical
White Paper: Cervical Disc Replacement: When is the Mobi-C Cervical Disc Medically Necessary? For Health Plans, Medical Management Organizations and TPAs Cervical Disc Disease: An Overview The cervical
Options for Cervical Disc Degeneration A Guide to the Fusion Arm of the M6 -C Artificial Disc Study
 Options for Cervical Disc Degeneration A Guide to the Fusion Arm of the M6 -C Artificial Disc Study Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine
Options for Cervical Disc Degeneration A Guide to the Fusion Arm of the M6 -C Artificial Disc Study Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine
Options for Cervical Disc Degeneration A Guide to the M6-C. clinical study
 Options for Cervical Disc Degeneration A Guide to the M6-C clinical study Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause
Options for Cervical Disc Degeneration A Guide to the M6-C clinical study Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause
A Patient s Guide to Artificial Cervical Disc Replacement
 A Patient s Guide to Artificial Cervical Disc Replacement Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause pain and numbness
A Patient s Guide to Artificial Cervical Disc Replacement Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause pain and numbness
Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, The Cervical Spine. What is the Cervical Spine?
 Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause pain and numbness in the neck, shoulders, arms, and even hands. This patient
Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause pain and numbness in the neck, shoulders, arms, and even hands. This patient
BRYAN. Cervical Disc System. Patient Information
 BRYAN Cervical Disc System Patient Information 3 BRYAN Cervical Disc System PATIENT INFORMATION BRYAN Cervical Disc System PATIENT INFORMATION 1 BRYAN Cervical Disc System This patient information brochure
BRYAN Cervical Disc System Patient Information 3 BRYAN Cervical Disc System PATIENT INFORMATION BRYAN Cervical Disc System PATIENT INFORMATION 1 BRYAN Cervical Disc System This patient information brochure
Update to the Treatment of Degenerative Cervical Disc Disease
 Update to the Treatment of Degenerative Cervical Disc Disease Michael Lynn, MD Neurosurgeon, Southeastern Neurosurgical & Spine Institute Adjunct Assistant Clinical Professor of Bioengineering, Clemson
Update to the Treatment of Degenerative Cervical Disc Disease Michael Lynn, MD Neurosurgeon, Southeastern Neurosurgical & Spine Institute Adjunct Assistant Clinical Professor of Bioengineering, Clemson
A Patient s Guide to the Disorders of the Cervical and Upper Thoracic Spine
 A Patient s Guide to the Disorders of the Cervical and Upper Thoracic Spine General Anatomy of the Spine The spine can be divided into four regions based on vertebral shape and sagittal plane curve.» CERVICAL:
A Patient s Guide to the Disorders of the Cervical and Upper Thoracic Spine General Anatomy of the Spine The spine can be divided into four regions based on vertebral shape and sagittal plane curve.» CERVICAL:
PRESTIGE LP. Cervical Disc System. Patient Information
 PRESTIGE LP Cervical Disc System Patient Information 3 PRESTIGE LP Cervical Disc System Patient Information This patient information brochure is designed to help you understand one treatment option for
PRESTIGE LP Cervical Disc System Patient Information 3 PRESTIGE LP Cervical Disc System Patient Information This patient information brochure is designed to help you understand one treatment option for
Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF).
 Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra,
Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra,
CERVICAL PROCEDURES PHYSICIAN CODING
 CERVICAL PROCEDURES PHYSICIAN CODING Anterior Cervical Discectomy with Interbody Fusion (ACDF) Anterior interbody fusion, with discectomy and decompression; cervical below C2 22551 first interspace 22552
CERVICAL PROCEDURES PHYSICIAN CODING Anterior Cervical Discectomy with Interbody Fusion (ACDF) Anterior interbody fusion, with discectomy and decompression; cervical below C2 22551 first interspace 22552
Nonoperative Management of Herniated Cervical Intervertebral Disc With Radiculopathy. Spine Volume 21(16) August 15, 1996, pp 1877-1883
 Nonoperative Management of Herniated Cervical Intervertebral Disc With Radiculopathy 1 Spine Volume 21(16) August 15, 1996, pp 1877-1883 Saal, Joel S. MD; Saal, Jeffrey A. MD; Yurth, Elizabeth F. MD FROM
Nonoperative Management of Herniated Cervical Intervertebral Disc With Radiculopathy 1 Spine Volume 21(16) August 15, 1996, pp 1877-1883 Saal, Joel S. MD; Saal, Jeffrey A. MD; Yurth, Elizabeth F. MD FROM
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF).
 Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
Cardiopulmonary Exercise Stress Test (CPET) Archived Medical Policy
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
1 REVISOR 5223.0070. (4) Pain associated with rigidity (loss of motion or postural abnormality) or
 1 REVISOR 5223.0070 5223.0070 MUSCULOSKELETAL SCHEDULE; BACK. Subpart 1. Lumbar spine. The spine rating is inclusive of leg symptoms except for gross motor weakness, bladder or bowel dysfunction, or sexual
1 REVISOR 5223.0070 5223.0070 MUSCULOSKELETAL SCHEDULE; BACK. Subpart 1. Lumbar spine. The spine rating is inclusive of leg symptoms except for gross motor weakness, bladder or bowel dysfunction, or sexual
White Paper: Reducing Utilization Concerns Regarding Spinal Fusion and Artificial Disc Implants
 White Paper: Reducing Utilization Concerns Regarding Spinal Fusion and Artificial Disc Implants For Health Plans, Medical Management Organizations and TPAs Executive Summary Back pain is one of the most
White Paper: Reducing Utilization Concerns Regarding Spinal Fusion and Artificial Disc Implants For Health Plans, Medical Management Organizations and TPAs Executive Summary Back pain is one of the most
Mobi-C Cervical Artificial Disc Patient Education
 Mobi-C Cervical Artificial Disc Patient Education This patient education book explains: How the neck works. What happens when a cervical disc wears out. What are the risks and benefits of cervical artificial
Mobi-C Cervical Artificial Disc Patient Education This patient education book explains: How the neck works. What happens when a cervical disc wears out. What are the risks and benefits of cervical artificial
Do you have Back Pain? Associated with:
 Do you have Back Pain? Associated with: Herniated Discs? Protruding Discs? Degenerative Disk Disease? Posterior Facet Syndrome? Sciatica? You may be a candidate for Decompression Therapy The Dynatronics
Do you have Back Pain? Associated with: Herniated Discs? Protruding Discs? Degenerative Disk Disease? Posterior Facet Syndrome? Sciatica? You may be a candidate for Decompression Therapy The Dynatronics
TABLE OF CONTENTS. Surgical Technique 2. Indications 4. Product Information 5. 1. Patient Positioning and Approach 2
 Surgical Technique TABLE OF CONTENTS Surgical Technique 2 1. Patient Positioning and Approach 2 2. Intervertebral Device Implanted 2 3. Buttress Plate Selection 2 4. Awl Insertion 2 5. Screw Insertion
Surgical Technique TABLE OF CONTENTS Surgical Technique 2 1. Patient Positioning and Approach 2 2. Intervertebral Device Implanted 2 3. Buttress Plate Selection 2 4. Awl Insertion 2 5. Screw Insertion
Cervical Spondylosis (Arthritis of the Neck)
 Copyright 2009 American Academy of Orthopaedic Surgeons Cervical Spondylosis (Arthritis of the Neck) Neck pain is extremely common. It can be caused by many things, and is most often related to getting
Copyright 2009 American Academy of Orthopaedic Surgeons Cervical Spondylosis (Arthritis of the Neck) Neck pain is extremely common. It can be caused by many things, and is most often related to getting
Sample Treatment Protocol
 Sample Treatment Protocol 1 Adults with acute episode of LBP Definition: Acute episode Back pain lasting
Sample Treatment Protocol 1 Adults with acute episode of LBP Definition: Acute episode Back pain lasting
Herniated Cervical Disc
 Herniated Cervical Disc North American Spine Society Public Education Series What Is a Herniated Disc? The backbone, or spine, is composed of a series of connected bones called vertebrae. The vertebrae
Herniated Cervical Disc North American Spine Society Public Education Series What Is a Herniated Disc? The backbone, or spine, is composed of a series of connected bones called vertebrae. The vertebrae
Temple Physical Therapy
 Temple Physical Therapy A General Overview of Common Neck Injuries For current information on Temple Physical Therapy related news and for a healthy and safe return to work, sport and recreation Like Us
Temple Physical Therapy A General Overview of Common Neck Injuries For current information on Temple Physical Therapy related news and for a healthy and safe return to work, sport and recreation Like Us
Information for the Patient About Surgical
 Information for the Patient About Surgical Decompression and Stabilization of the Spine Aging and the Spine Daily wear and tear, along with disc degeneration due to aging and injury, are common causes
Information for the Patient About Surgical Decompression and Stabilization of the Spine Aging and the Spine Daily wear and tear, along with disc degeneration due to aging and injury, are common causes
SPINE ANATOMY AND PROCEDURES. Tulsa Spine & Specialty Hospital 6901 S. Olympia Avenue Tulsa, Oklahoma 74132
 SPINE ANATOMY AND PROCEDURES Tulsa Spine & Specialty Hospital 6901 S. Olympia Avenue Tulsa, Oklahoma 74132 SPINE ANATOMY The spine consists of 33 bones called vertebrae. The top 7 are cervical, or neck
SPINE ANATOMY AND PROCEDURES Tulsa Spine & Specialty Hospital 6901 S. Olympia Avenue Tulsa, Oklahoma 74132 SPINE ANATOMY The spine consists of 33 bones called vertebrae. The top 7 are cervical, or neck
Minimally Invasive Spine Surgery For Your Patients
 Minimally Invasive Spine Surgery For Your Patients Lukas P. Zebala, M.D. Assistant Professor Orthopaedic and Neurological Spine Surgery Department of Orthopaedic Surgery Washington University School of
Minimally Invasive Spine Surgery For Your Patients Lukas P. Zebala, M.D. Assistant Professor Orthopaedic and Neurological Spine Surgery Department of Orthopaedic Surgery Washington University School of
Patient Guide to Neck Surgery
 The following is a sampling of products offered by Zimmer Spine for use in Anterior Cervical Fusion procedures. Patient Guide to Neck Surgery Anterior Cervical Fusion Trinica Select With the Trinica and
The following is a sampling of products offered by Zimmer Spine for use in Anterior Cervical Fusion procedures. Patient Guide to Neck Surgery Anterior Cervical Fusion Trinica Select With the Trinica and
ANTERIOR CERVICAL DISCECTOMY AND FUSION. Basic Anatomical Landmarks: Anterior Cervical Spine
 Anterior In the human anatomy, referring to the front surface of the body or position of one structure relative to another Cervical Relating to the neck, in the spine relating to the first seven vertebrae
Anterior In the human anatomy, referring to the front surface of the body or position of one structure relative to another Cervical Relating to the neck, in the spine relating to the first seven vertebrae
Contents. Introduction 1. Anatomy of the Spine 1. 2. Spinal Imaging 7. 3. Spinal Biomechanics 23. 4. History and Physical Examination of the Spine 33
 Contents Introduction 1. Anatomy of the Spine 1 Vertebrae 1 Ligaments 3 Intervertebral Disk 4 Intervertebral Foramen 5 2. Spinal Imaging 7 Imaging Modalities 7 Conventional Radiographs 7 Myelography 9
Contents Introduction 1. Anatomy of the Spine 1 Vertebrae 1 Ligaments 3 Intervertebral Disk 4 Intervertebral Foramen 5 2. Spinal Imaging 7 Imaging Modalities 7 Conventional Radiographs 7 Myelography 9
A Patient s Guide to Diffuse Idiopathic Skeletal Hyperostosis (DISH)
 A Patient s Guide to Diffuse Idiopathic Skeletal Hyperostosis (DISH) Introduction Diffuse Idiopathic Skeletal Hyperostosis (DISH) is a phenomenon that more commonly affects older males. It is associated
A Patient s Guide to Diffuse Idiopathic Skeletal Hyperostosis (DISH) Introduction Diffuse Idiopathic Skeletal Hyperostosis (DISH) is a phenomenon that more commonly affects older males. It is associated
Cervical Disc Replacement vs ACDF
 Guest Discussants: Michael E. Janssen, DO Spine Education and Research Institute Denver, CO Alan S. Hilibrand, MD Jefferson Medical College/Rothman Institute Philadelphia, PA SpineLine Section Editor:
Guest Discussants: Michael E. Janssen, DO Spine Education and Research Institute Denver, CO Alan S. Hilibrand, MD Jefferson Medical College/Rothman Institute Philadelphia, PA SpineLine Section Editor:
If you or a loved one have suffered because of a negligent error during spinal surgery, you will be going through a difficult time.
 If you or a loved one have suffered because of a negligent error during spinal surgery, you will be going through a difficult time. You may be worried about your future, both in respect of finances and
If you or a loved one have suffered because of a negligent error during spinal surgery, you will be going through a difficult time. You may be worried about your future, both in respect of finances and
Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances?
 Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances? Do you experience weakness, tingling, numbness, stiffness, or cramping in your legs, buttocks or
Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances? Do you experience weakness, tingling, numbness, stiffness, or cramping in your legs, buttocks or
ATLANTIS Anterior Cervical Plate System ATLANTIS Translational Plate. 510(k) Summary. February 2007
 rk,1 10{2 ATLANTIS Anterior Cervical Plate System ATLANTIS Translational Plate 510(k) Summary FEB 2 3 2[007 February 2007 I. Company: Medtronic Sofamor Danek USA 1800 Pyramid Place Memphis, Tennessee 38132
rk,1 10{2 ATLANTIS Anterior Cervical Plate System ATLANTIS Translational Plate 510(k) Summary FEB 2 3 2[007 February 2007 I. Company: Medtronic Sofamor Danek USA 1800 Pyramid Place Memphis, Tennessee 38132
CERVICAL SPONDYLOSIS
 CERVICAL SPONDYLOSIS Dr. Sahni B.S Dy. Chief Medical Officer, ONGC Hospital Panvel-410221,Navi Mumbai,India Introduction The cervical spine consists of the top 7 vertebrae of the spine. These are referred
CERVICAL SPONDYLOSIS Dr. Sahni B.S Dy. Chief Medical Officer, ONGC Hospital Panvel-410221,Navi Mumbai,India Introduction The cervical spine consists of the top 7 vertebrae of the spine. These are referred
X Stop Spinal Stenosis Decompression
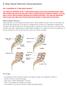 X Stop Spinal Stenosis Decompression Am I a candidate for X Stop spinal surgery? You may be a candidate for the X Stop spinal surgery if you have primarily leg pain rather than mostly back pain and your
X Stop Spinal Stenosis Decompression Am I a candidate for X Stop spinal surgery? You may be a candidate for the X Stop spinal surgery if you have primarily leg pain rather than mostly back pain and your
Spinal Surgery Functional Status and Quality of Life Outcome Specifications 2015 (01/01/2013 to 12/31/2013 Dates of Procedure) September 2014
 Description Methodology For patients ages 18 years and older who undergo a lumbar discectomy/laminotomy or lumbar spinal fusion procedure during the measurement year, the following measures will be calculated:
Description Methodology For patients ages 18 years and older who undergo a lumbar discectomy/laminotomy or lumbar spinal fusion procedure during the measurement year, the following measures will be calculated:
Advances In Spine Care. James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery
 Advances In Spine Care James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery Introduction The Spine - A common source of problems Back pain is the #2 presenting
Advances In Spine Care James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery Introduction The Spine - A common source of problems Back pain is the #2 presenting
ISPI Newsletter Archive Lumbar Spine Surgery
 ISPI Newsletter Archive Lumbar Spine Surgery January 2005 Effects of Charite Artificial Disc on the Implanted and Adjacent Spinal Segments Mechanics Using a Hybrid Testing Protocol Spine. 30(24):2755-2764,
ISPI Newsletter Archive Lumbar Spine Surgery January 2005 Effects of Charite Artificial Disc on the Implanted and Adjacent Spinal Segments Mechanics Using a Hybrid Testing Protocol Spine. 30(24):2755-2764,
Technology Breakthrough in Spinal Implants (Technical Insights)
 Technology Breakthrough in Spinal Implants (Technical Insights) Biomaterial innovations is a growth factor for spinal implant market June 2014 Table of Contents Section Page Number Executive Summary 4
Technology Breakthrough in Spinal Implants (Technical Insights) Biomaterial innovations is a growth factor for spinal implant market June 2014 Table of Contents Section Page Number Executive Summary 4
Subject: Implanted Devices for Spinal Stenosis Policy #: SURG.00092 Current Effective Date: 07/13/2011 Status: Reviewed Last Review Date: 05/19/2011
 1 of 5 6/18/2012 11:08 AM Medical Policy Subject: Implanted Devices for Spinal Stenosis Policy #: SURG.00092 Current Effective Date: 07/13/2011 Status: Reviewed Last Review Date: 05/19/2011 Description/Scope
1 of 5 6/18/2012 11:08 AM Medical Policy Subject: Implanted Devices for Spinal Stenosis Policy #: SURG.00092 Current Effective Date: 07/13/2011 Status: Reviewed Last Review Date: 05/19/2011 Description/Scope
SPINAL FUSION. North American Spine Society Public Education Series
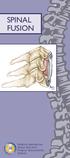 SPINAL FUSION North American Spine Society Public Education Series WHAT IS SPINAL FUSION? The spine is made up of a series of bones called vertebrae ; between each vertebra are strong connective tissues
SPINAL FUSION North American Spine Society Public Education Series WHAT IS SPINAL FUSION? The spine is made up of a series of bones called vertebrae ; between each vertebra are strong connective tissues
The Petrylaw Lawsuits Settlements and Injury Settlement Report
 The Petrylaw Lawsuits Settlements and Injury Settlement Report BACK INJURIES How Minnesota Juries Decide the Value of Pain and Suffering in Back Injury Cases The Petrylaw Lawsuits Settlements and Injury
The Petrylaw Lawsuits Settlements and Injury Settlement Report BACK INJURIES How Minnesota Juries Decide the Value of Pain and Suffering in Back Injury Cases The Petrylaw Lawsuits Settlements and Injury
SPINE SERVICE ROTATION ROTATION SPECIFIC OBJECTIVES (RSO) DEPT. OF ORTHOPEDICS AND PHYSICAL REHABILITATION UNIVERSITY OF MASSACHUSETTS
 SPINE SERVICE ROTATION ROTATION SPECIFIC OBJECTIVES (RSO) DEPT. OF ORTHOPEDICS AND PHYSICAL REHABILITATION UNIVERSITY OF MASSACHUSETTS The purpose of this RSO is to outline and clarify the objectives of
SPINE SERVICE ROTATION ROTATION SPECIFIC OBJECTIVES (RSO) DEPT. OF ORTHOPEDICS AND PHYSICAL REHABILITATION UNIVERSITY OF MASSACHUSETTS The purpose of this RSO is to outline and clarify the objectives of
Orthopaedic Spine Center. Anterior Cervical Discectomy and Fusion (ACDF) Normal Discs
 Orthopaedic Spine Center Graham Calvert MD James Woodall MD PhD Anterior Cervical Discectomy and Fusion (ACDF) Normal Discs The cervical spine consists of the bony vertebrae, discs, nerves and other structures.
Orthopaedic Spine Center Graham Calvert MD James Woodall MD PhD Anterior Cervical Discectomy and Fusion (ACDF) Normal Discs The cervical spine consists of the bony vertebrae, discs, nerves and other structures.
visualized. The correct level is then identified again. With the use of a microscope and
 SURGERY FOR SPINAL STENOSIS Laminectomy A one inch (or longer for extensive stenosis) incision is made in the middle of the back over the effected region of the spine. The muscles over the bone are moved
SURGERY FOR SPINAL STENOSIS Laminectomy A one inch (or longer for extensive stenosis) incision is made in the middle of the back over the effected region of the spine. The muscles over the bone are moved
Herniated Lumbar Disc
 Herniated Lumbar Disc North American Spine Society Public Education Series What Is a Herniated Disc? The spine is made up of a series of connected bones called vertebrae. The disc is a combination of strong
Herniated Lumbar Disc North American Spine Society Public Education Series What Is a Herniated Disc? The spine is made up of a series of connected bones called vertebrae. The disc is a combination of strong
Local Coverage Determination (LCD): Spinal Cord Stimulation (Dorsal Column Stimulation) (L34705)
 Local Coverage Determination (LCD): Spinal Cord Stimulation (Dorsal Column Stimulation) (L34705) Contractor Information Contractor Name Novitas Solutions, Inc. LCD Information Document Information LCD
Local Coverage Determination (LCD): Spinal Cord Stimulation (Dorsal Column Stimulation) (L34705) Contractor Information Contractor Name Novitas Solutions, Inc. LCD Information Document Information LCD
American Chiropractic Association. Commentary on Centers for Medicare and Medicaid Services (CMS)/PART. Clinical Documentation Guidelines
 American Chiropractic Association Commentary on Centers for Medicare and Medicaid Services (CMS)/PART Clinical Documentation Guidelines DISCLAIMER The American Chiropractic Association provides this commentary
American Chiropractic Association Commentary on Centers for Medicare and Medicaid Services (CMS)/PART Clinical Documentation Guidelines DISCLAIMER The American Chiropractic Association provides this commentary
THE LUMBAR SPINE (BACK)
 THE LUMBAR SPINE (BACK) At a glance Chronic back pain, especially in the area of the lumbar spine (lower back), is a widespread condition. It can be assumed that 75 % of all people have it sometimes or
THE LUMBAR SPINE (BACK) At a glance Chronic back pain, especially in the area of the lumbar spine (lower back), is a widespread condition. It can be assumed that 75 % of all people have it sometimes or
Surgery for cervical disc prolapse or cervical osteophyte
 Mr Paul S. D Urso MBBS(Hons), PhD, FRACS Neurosurgeon Provider Nº: 081161DY Epworth Centre Suite 6.1 32 Erin Street Richmond 3121 Tel: 03 9421 5844 Fax: 03 9421 4186 AH: 03 9483 4040 email: [email protected]
Mr Paul S. D Urso MBBS(Hons), PhD, FRACS Neurosurgeon Provider Nº: 081161DY Epworth Centre Suite 6.1 32 Erin Street Richmond 3121 Tel: 03 9421 5844 Fax: 03 9421 4186 AH: 03 9483 4040 email: [email protected]
Surgical Procedures of the Spine
 Surgical Procedures of the Spine Jaideep Chunduri, M.D. Orthopaedic Spine Surgeon Beacon Orthopaedics and Sports Medicine Beacon Spine Center Objectives Discuss the 4 most common procedures performed in
Surgical Procedures of the Spine Jaideep Chunduri, M.D. Orthopaedic Spine Surgeon Beacon Orthopaedics and Sports Medicine Beacon Spine Center Objectives Discuss the 4 most common procedures performed in
Patient Guide to Lower Back Surgery
 The following is a sampling of products offered by Zimmer Spine for use in Open Lumbar Fusion procedures. Patient Guide to Lower Back Surgery Open Lumbar Fusion Dynesys The Dynesys Dynamic Stabilization
The following is a sampling of products offered by Zimmer Spine for use in Open Lumbar Fusion procedures. Patient Guide to Lower Back Surgery Open Lumbar Fusion Dynesys The Dynesys Dynamic Stabilization
Cervical Conditions: Diagnosis and Treatments
 Cervical Conditions: Diagnosis and Treatments Mark R Mikles, M.D. Cervical Conditions: Diagnosis and Treatment Cervical conditions Neck Pain Radiculopathy Myelopathy 1 Cervical Conditions: Diagnosis and
Cervical Conditions: Diagnosis and Treatments Mark R Mikles, M.D. Cervical Conditions: Diagnosis and Treatment Cervical conditions Neck Pain Radiculopathy Myelopathy 1 Cervical Conditions: Diagnosis and
Magnetic Resonance Imaging
 Magnetic Resonance Imaging North American Spine Society Public Education Series What Is Magnetic Resonance Imaging (MRI)? Magnetic resonance imaging (MRI) is a valuable diagnostic study that has been used
Magnetic Resonance Imaging North American Spine Society Public Education Series What Is Magnetic Resonance Imaging (MRI)? Magnetic resonance imaging (MRI) is a valuable diagnostic study that has been used
KYPHON. Reimbursement Guide. Physician Reimbursement. Balloon Kyphoplasty Procedure. ICD-9-CM Diagnosis Codes. CPT Codes and Payment
 KYPHON Balloon Kyphoplasty Procedure Reimbursement Guide ICD-9-CM Diagnosis Codes Providers should report the ICD-9-CM diagnosis code that most accurately describes the patient s condition. Please refer
KYPHON Balloon Kyphoplasty Procedure Reimbursement Guide ICD-9-CM Diagnosis Codes Providers should report the ICD-9-CM diagnosis code that most accurately describes the patient s condition. Please refer
DUKE ORTHOPAEDIC SURGERY GOALS AND OBJECTIVES SPINE SERVICE
 GOALS AND OBJECTIVES PATIENT CARE Able to perform a complete musculoskeletal and neurologic examination on the patient including cervical spine, thoracic spine, and lumbar spine. The neurologic examination
GOALS AND OBJECTIVES PATIENT CARE Able to perform a complete musculoskeletal and neurologic examination on the patient including cervical spine, thoracic spine, and lumbar spine. The neurologic examination
Spine Trauma: When to Transfer. Alexander Ching, MD Director, Orthopaedic Spine Trauma OHSU
 Spine Trauma: When to Transfer Alexander Ching, MD Director, Orthopaedic Spine Trauma OHSU Disclosures Depuy Spine Consultant (teaching and courses) Department education and research funds Atlas Spine
Spine Trauma: When to Transfer Alexander Ching, MD Director, Orthopaedic Spine Trauma OHSU Disclosures Depuy Spine Consultant (teaching and courses) Department education and research funds Atlas Spine
How To Get An Mri Of The Lumbar Spine W/O Contrast
 Date notice sent to all parties: May 27, 2014 IRO CASE #: ReviewTex, Inc. 1818 Mountjoy Drive San Antonio, TX 78232 (phone) 210-598-9381 (fax) 210-598-9382 [email protected] Notice of Independent Review
Date notice sent to all parties: May 27, 2014 IRO CASE #: ReviewTex, Inc. 1818 Mountjoy Drive San Antonio, TX 78232 (phone) 210-598-9381 (fax) 210-598-9382 [email protected] Notice of Independent Review
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Interspinous Fixation (Fusion) Devices Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Interspinous Fixation (Fusion) Devices Lumbar Spine
Interspinous Fixation (Fusion) Devices Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Interspinous Fixation (Fusion) Devices Lumbar Spine
Lumbar Spinal Stenosis
 Lumbar Spinal Stenosis North American Spine Society Public Education Series What Is Lumbar Spinal Stenosis? The vertebrae are the bones that make up the lumbar spine (low back). The spinal canal runs through
Lumbar Spinal Stenosis North American Spine Society Public Education Series What Is Lumbar Spinal Stenosis? The vertebrae are the bones that make up the lumbar spine (low back). The spinal canal runs through
Cervical Stenosis & Myelopathy
 Cervical Stenosis & Myelopathy North American Spine Society Public Education Series What Are Cervical Stenosis and Myelopathy? The cervical spine (neck) is made up of a series of connected bones called
Cervical Stenosis & Myelopathy North American Spine Society Public Education Series What Are Cervical Stenosis and Myelopathy? The cervical spine (neck) is made up of a series of connected bones called
Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization
 APPROVED IRN10389-1.1-04 Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization Because it involves accessing the spine through the patient s side, the Direct Lateral approach
APPROVED IRN10389-1.1-04 Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization Because it involves accessing the spine through the patient s side, the Direct Lateral approach
Measure Title X RAY PRIOR TO MRI OR CAT SCAN IN THE EVAULATION OF LOWER BACK PAIN Disease State Back pain Indicator Classification Utilization
 Client HMSA: PQSR 2009 Measure Title X RAY PRIOR TO MRI OR CAT SCAN IN THE EVAULATION OF LOWER BACK PAIN Disease State Back pain Indicator Classification Utilization Strength of Recommendation Organizations
Client HMSA: PQSR 2009 Measure Title X RAY PRIOR TO MRI OR CAT SCAN IN THE EVAULATION OF LOWER BACK PAIN Disease State Back pain Indicator Classification Utilization Strength of Recommendation Organizations
Spinal Decompression
 Spinal Decompression Spinal decompression is just one more tool we have to treat radiculopathy. With appropriate education and exercises, this modality has been proven to assist in the resolution of symptoms
Spinal Decompression Spinal decompression is just one more tool we have to treat radiculopathy. With appropriate education and exercises, this modality has been proven to assist in the resolution of symptoms
Spine University s Guide to Kinetic MRIs Detect Disc Herniations
 Spine University s Guide to Kinetic MRIs Detect Disc Herniations 2 Introduction Traditionally, doctors use a procedure called magnetic resonance imaging (MRI) to diagnose disc injuries. Kinetic magnetic
Spine University s Guide to Kinetic MRIs Detect Disc Herniations 2 Introduction Traditionally, doctors use a procedure called magnetic resonance imaging (MRI) to diagnose disc injuries. Kinetic magnetic
LUMBAR SPINAL STENOSIS OBSERVATIONS, EVIDENCE, AND TRENDS FULILLING THE UNMET CLINICAL NEED WRITTEN BY: HALLETT MATHEWS, MD, MBA
 LUMBAR SPINAL STENOSIS OBSERVATIONS, EVIDENCE, AND TRENDS FULILLING THE UNMET CLINICAL NEED WRITTEN BY: HALLETT MATHEWS, MD, MBA Overview of Lumbar Spinal Stenosis Spine stabilization, which has equated
LUMBAR SPINAL STENOSIS OBSERVATIONS, EVIDENCE, AND TRENDS FULILLING THE UNMET CLINICAL NEED WRITTEN BY: HALLETT MATHEWS, MD, MBA Overview of Lumbar Spinal Stenosis Spine stabilization, which has equated
Low Back Pain (LBP) Prevalence. Low Back Pain (LBP) Prevalence. Lumbar Fusion: Where is the Evidence?
 15 th Annual Cleveland Clinic Pain Management Symposium Sarasota, Florida Lumbar Fusion: Where is the Evidence? Gordon R. Bell, M.D. Director, Cleveland Clinic Low Back Pain (LBP) Prevalence Lifetime prevalence:
15 th Annual Cleveland Clinic Pain Management Symposium Sarasota, Florida Lumbar Fusion: Where is the Evidence? Gordon R. Bell, M.D. Director, Cleveland Clinic Low Back Pain (LBP) Prevalence Lifetime prevalence:
Issued and entered this _6th_ day of October 2010 by Ken Ross Commissioner ORDER I PROCEDURAL BACKGROUND
 STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX Petitioner
STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX Petitioner
.org. Cervical Spondylosis (Arthritis of the Neck) Anatomy. Cause
 Cervical Spondylosis (Arthritis of the Neck) Page ( 1 ) Neck pain can be caused by many things but is most often related to getting older. Like the rest of the body, the disks and joints in the neck (cervical
Cervical Spondylosis (Arthritis of the Neck) Page ( 1 ) Neck pain can be caused by many things but is most often related to getting older. Like the rest of the body, the disks and joints in the neck (cervical
Cervical Spine Surgery. Orthopaedic Nursing Seminar. Dr Michelle Atkinson. Friday October 21 st 2011. Cervical Disc Herniation
 Cervical Spine Surgery Dr Michelle Atkinson The Sydney and Dalcross Adventist Hospitals Orthopaedic Nursing Seminar Friday October 21 st 2011 Cervical disc herniation The most frequently treated surgical
Cervical Spine Surgery Dr Michelle Atkinson The Sydney and Dalcross Adventist Hospitals Orthopaedic Nursing Seminar Friday October 21 st 2011 Cervical disc herniation The most frequently treated surgical
Artificial Intervertebral Disc: Lumbar Spine
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Artificial Intervertebral Disc: Lumbar Spine Number 7.01.87 Effective
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Artificial Intervertebral Disc: Lumbar Spine Number 7.01.87 Effective
Treating Bulging Discs & Sciatica. Alexander Ching, MD
 Treating Bulging Discs & Sciatica Alexander Ching, MD Disclosures Depuy Spine Teaching and courses K2 Spine Complex Spine Study Group Disclosures Take 2 I am a spine surgeon I like spine surgery I believe
Treating Bulging Discs & Sciatica Alexander Ching, MD Disclosures Depuy Spine Teaching and courses K2 Spine Complex Spine Study Group Disclosures Take 2 I am a spine surgeon I like spine surgery I believe
A review of spinal problems
 Dr Ulrich R Hähnle MD, FCS Orthopaedic Surgeon, Wits Facharzt für Orthopädie, Berlin Phone: +27 11 485 3236 Fax: +27 11 485 2446 Suite 102, Medical Centre, Linksfield Park Clinic P.O. Box 949, Johannesburg
Dr Ulrich R Hähnle MD, FCS Orthopaedic Surgeon, Wits Facharzt für Orthopädie, Berlin Phone: +27 11 485 3236 Fax: +27 11 485 2446 Suite 102, Medical Centre, Linksfield Park Clinic P.O. Box 949, Johannesburg
Chiropractor Compliance Summary Documentation Compliance Criteria for Chiropractic Claims Submitted to the Funds
 Chiropractor Compliance Summary Documentation Compliance Criteria for Chiropractic Claims Submitted to the Funds Date: April 23, 2012 Source Information: Medicare Policy Purpose The United Mine Workers
Chiropractor Compliance Summary Documentation Compliance Criteria for Chiropractic Claims Submitted to the Funds Date: April 23, 2012 Source Information: Medicare Policy Purpose The United Mine Workers
ANTERIOR LUMBAR INTERBODY FUSION (ALIF) Basic Anatomical Landmarks: Anterior Lumbar Spine
 (ALIF) Anterior In human anatomy, referring to the front surface of the body or the position of one structure relative to another Lumbar Relating to the loins or the section of the back and sides between
(ALIF) Anterior In human anatomy, referring to the front surface of the body or the position of one structure relative to another Lumbar Relating to the loins or the section of the back and sides between
Minimally Invasive Spine Surgery What is it and how will it benefit patients?
 Minimally Invasive Spine Surgery What is it and how will it benefit patients? Dr Raoul Pope MBChB, FRACS, Neurosurgeon and Minimally Invasive Spine Surgeon Concord Hospital and Mater Private Hospital Sydney
Minimally Invasive Spine Surgery What is it and how will it benefit patients? Dr Raoul Pope MBChB, FRACS, Neurosurgeon and Minimally Invasive Spine Surgeon Concord Hospital and Mater Private Hospital Sydney
The indications for lumbar and cervical disc replacement
 The Spine Journal 4 (2004) 177S 181S The indications for lumbar and cervical disc replacement Paul C. McAfee, MD St. Joseph Hospital, Scoliosis and Spine Center, O Dea Medical Building 104, 7505 Osler
The Spine Journal 4 (2004) 177S 181S The indications for lumbar and cervical disc replacement Paul C. McAfee, MD St. Joseph Hospital, Scoliosis and Spine Center, O Dea Medical Building 104, 7505 Osler
THE MEDICAL TREATMENT GUIDELINES
 THE MEDICAL TREATMENT GUIDELINES I. INTRODUCTION A. About the Medical Treatment Guidelines. On December 1, 2010, the NYS Workers' Compensation Board is implementing new regulations and Medical Treatment
THE MEDICAL TREATMENT GUIDELINES I. INTRODUCTION A. About the Medical Treatment Guidelines. On December 1, 2010, the NYS Workers' Compensation Board is implementing new regulations and Medical Treatment
Herniated Disk. This reference summary explains herniated disks. It discusses symptoms and causes of the condition, as well as treatment options.
 Herniated Disk Introduction Your backbone, or spine, has 24 moveable vertebrae made of bone. Between the bones are soft disks filled with a jelly-like substance. These disks cushion the vertebrae and keep
Herniated Disk Introduction Your backbone, or spine, has 24 moveable vertebrae made of bone. Between the bones are soft disks filled with a jelly-like substance. These disks cushion the vertebrae and keep
How to Overcome the 5 Biggest Reimbursement Challenges in Joint & Spine Coding
 How to Overcome the 5 Biggest Reimbursement Challenges in Joint & Spine Coding Presented by: Carolyn Neumann, CPC Senior Manager Coding and Coverage Access The opinions and codes denoted within are suggestions
How to Overcome the 5 Biggest Reimbursement Challenges in Joint & Spine Coding Presented by: Carolyn Neumann, CPC Senior Manager Coding and Coverage Access The opinions and codes denoted within are suggestions
Jonathan R. Gottlieb, MD 7220 SW 127 Street Pinecrest, FL 33156 [email protected]
 Jonathan R. Gottlieb, MD 7220 SW 127 Street Pinecrest, FL 33156 [email protected] CURRENT PRACTICE September 2009-present University of Miami School of Medicine Assistant Professor, Department of
Jonathan R. Gottlieb, MD 7220 SW 127 Street Pinecrest, FL 33156 [email protected] CURRENT PRACTICE September 2009-present University of Miami School of Medicine Assistant Professor, Department of
Anterior Cervical Discectomy and Fusion
 A Patient s Guide to Anterior Cervical Discectomy and Fusion 651 Old Country Road Plainview, NY 11803 Phone: 5166818822 Fax: 5166813332 [email protected] DISCLAIMER: The information in this booklet is
A Patient s Guide to Anterior Cervical Discectomy and Fusion 651 Old Country Road Plainview, NY 11803 Phone: 5166818822 Fax: 5166813332 [email protected] DISCLAIMER: The information in this booklet is
LOW BACK PAIN; MECHANICAL
 1 ORTHO 16 LOW BACK PAIN; MECHANICAL Background This case definition was developed by the Armed Forces Health Surveillance Center (AFHSC) for the purpose of epidemiological surveillance of a condition
1 ORTHO 16 LOW BACK PAIN; MECHANICAL Background This case definition was developed by the Armed Forces Health Surveillance Center (AFHSC) for the purpose of epidemiological surveillance of a condition
Spine Clinic Neurospine Specialists, Orthopaedics and Neurosurgery
 Spine Clinic Neurospine Specialists, Orthopaedics and Neurosurgery REVISION SPINE SURGERY Revision surgery is a very complex field which requires experience, training and evaluation in a very individual
Spine Clinic Neurospine Specialists, Orthopaedics and Neurosurgery REVISION SPINE SURGERY Revision surgery is a very complex field which requires experience, training and evaluation in a very individual
Posterior Cervical Decompression
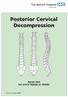 Posterior Cervical Decompression Spinal Unit Tel: 01473 702032 or 702097 Issue 2: January 2009 Following your recent MRI scan and consultation with your spinal surgeon, you have been diagnosed with a
Posterior Cervical Decompression Spinal Unit Tel: 01473 702032 or 702097 Issue 2: January 2009 Following your recent MRI scan and consultation with your spinal surgeon, you have been diagnosed with a
James A. Sanfilippo, M.D. CONSENT FOR SPINAL SURGERY PATIENT: DATE:
 James A. Sanfilippo, M.D. CONSENT FOR SPINAL SURGERY PATIENT: DATE: 1. I have been strongly advised to carefully read and consider this operative permit. I realize that it is important that I understand
James A. Sanfilippo, M.D. CONSENT FOR SPINAL SURGERY PATIENT: DATE: 1. I have been strongly advised to carefully read and consider this operative permit. I realize that it is important that I understand
Contractor Number 11302. Oversight Region Region IV
 Local Coverage Determination (LCD): Spinal Cord Stimulators for Chronic Pain (L32549) Contractor Information Contractor Name Palmetto GBA opens in new window Contractor Number 11302 Contractor Type MAC
Local Coverage Determination (LCD): Spinal Cord Stimulators for Chronic Pain (L32549) Contractor Information Contractor Name Palmetto GBA opens in new window Contractor Number 11302 Contractor Type MAC
The treatment of symptomatic cervical degenerative disk
 CHAPTER 12 Cervical Disk Arthroplasty: Patient Selection Dale Ding, MD, and Mark E. Shaffrey, MD The treatment of symptomatic cervical degenerative disk disease through an anterior transcervical retropharyngeal
CHAPTER 12 Cervical Disk Arthroplasty: Patient Selection Dale Ding, MD, and Mark E. Shaffrey, MD The treatment of symptomatic cervical degenerative disk disease through an anterior transcervical retropharyngeal
ICD 10 CM IMPLEMENTATION DATE OCT 1, 2015
 Presented by: Teri Romano, RN, MBA, CPC, CMDP ICD 10 CM IMPLEMENTATION DATE OCT 1, 2015 Source: http://journal.ahima.org/2015/02/04/us house committee to hold hearing on icd 10 implementation/ 2 2015 Web_Non
Presented by: Teri Romano, RN, MBA, CPC, CMDP ICD 10 CM IMPLEMENTATION DATE OCT 1, 2015 Source: http://journal.ahima.org/2015/02/04/us house committee to hold hearing on icd 10 implementation/ 2 2015 Web_Non
NON SURGICAL SPINAL DECOMPRESSION. Dr. Douglas A. VanderPloeg
 NON SURGICAL SPINAL DECOMPRESSION Dr. Douglas A. VanderPloeg CONTENTS I. Incidence of L.B.P. II. Anatomy Review III. IV. Disc Degeneration, Bulge, and Herniation Non-Surgical Spinal Decompression 1. History
NON SURGICAL SPINAL DECOMPRESSION Dr. Douglas A. VanderPloeg CONTENTS I. Incidence of L.B.P. II. Anatomy Review III. IV. Disc Degeneration, Bulge, and Herniation Non-Surgical Spinal Decompression 1. History
Status Active. Assistant Surgeons. This policy addresses reimbursement for assistant surgical procedures during the same operative session.
 Status Active Reimbursement Policy Section: Surgery/Interventional Procedure Policy Number: RP - Surgery/Interventional Procedure - 001 Assistant Surgeons Effective Date: June 1, 2015 Assistant Surgeons
Status Active Reimbursement Policy Section: Surgery/Interventional Procedure Policy Number: RP - Surgery/Interventional Procedure - 001 Assistant Surgeons Effective Date: June 1, 2015 Assistant Surgeons
DIFFERENTIAL DIAGNOSIS OF LOW BACK PAIN. Arnold J. Weil, M.D., M.B.A. Non-Surgical Orthopaedics, P.C. Atlanta, GA
 DIFFERENTIAL DIAGNOSIS OF LOW BACK PAIN Arnold J. Weil, M.D., M.B.A. Non-Surgical Orthopaedics, P.C. Atlanta, GA MEDICAL ALGORITHM OF REALITY LOWER BACK PAIN Yes Patient will never get better until case
DIFFERENTIAL DIAGNOSIS OF LOW BACK PAIN Arnold J. Weil, M.D., M.B.A. Non-Surgical Orthopaedics, P.C. Atlanta, GA MEDICAL ALGORITHM OF REALITY LOWER BACK PAIN Yes Patient will never get better until case
Born January 13, 1959 in Ponce, Puerto Rico Fluent in English and Spanish
 Page 1 of 6 6160 Windhaven Pkwy Ste 200 Plano, TX 75093 Luis A. Mignucci, M.D. Phone (972)378-6908 Fax (972)378-6586 Personal Born January 13, 1959 in Ponce, Puerto Rico Fluent in English and Spanish Surgical
Page 1 of 6 6160 Windhaven Pkwy Ste 200 Plano, TX 75093 Luis A. Mignucci, M.D. Phone (972)378-6908 Fax (972)378-6586 Personal Born January 13, 1959 in Ponce, Puerto Rico Fluent in English and Spanish Surgical
