BONE-GRAFT SUBSTITUTES: FACTS, FICTIONS & APPLICATIONS
|
|
|
- Thomasina Miller
- 9 years ago
- Views:
Transcription
1 BONE-GRAFT SUBSTITUTES: FACTS, FICTIONS & APPLICATIONS AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 70th Annual Meeting February 5-9, 2003 New Orleans, Louisiana COMMITTEE ON BIOLOGICAL IMPLANTS Prepared by: Acknowledgements: A. Seth Greenwald, D.Phil.(Oxon) Exactech, Inc. Scott D. Boden, M.D. GenSci OrthoBiologics, Inc. Victor M. Goldberg, M.D. Interpore Cross International Yusuf Khan, M.S. Medtronic Sofamor Danek Cato T. Laurencin, M.D., Ph.D. Osteotech, Inc. Randy N. Rosier, M.D. Regeneration Technolgies, Inc. Stryker Biotech Synthes USA Wright Medical Technology, Inc. Zimmer, Inc. Presented with the permission of The Journal of Bone and Joint Surgery. This material was first published, in slightly different form, in J Bone Joint Surg Am 83(Suppl. 2):98-103, 2001.
2 A REALITY CHECK It is estimated that more than 500,000 bone-grafting procedures are performed annually in the United States, with approximately half of these procedures related to spine fusion. These numbers easily double on a global basis and indicate a shortage in the availability of musculoskeletal donor tissue traditionally used in these reconstructions. (Figure 1) Donors ,142 5,188 10,004 12,000 14,030 16,692 21,000 22, U.S. Sales ($millions) BMP Bone substitutes/ Platelet helpers Bone dowels Demineralized bone matrix products Allograft bone Figure 1: U.S. trends in musculoskeletal tissue donors Source: United Network for Organ Sharing & MTF Figure 2: U.S. sales of bone graft and bone substitutes Source: Orthopedic Network News, industry estimates This reality has stimulated a proliferation of corporate interest in supplying what is seen as a growing market in bonesubstitute materials. (Figure 2) These graft alternatives are subjected to varying degrees of regulatory scrutiny, and thus their true safety and effectiveness in patients may not be known prior to their use by orthopaedic surgeons. It is thus important to gain insight into this emerging class of bone-substitute alternatives. THE PHYSIOLOGY OF BONE GRAFTING The biology of bone grafts and their substitutes is appreciated from an understanding of the bone formation processes of Osteogenesis, Osteoinduction and Osteoconduction. Graft Osteogenesis: The cellular elements within a donor graft, which survive transplantation and synthesize new bone at the recipient site. Graft Osteoinduction: New bone realized through the active recruitment of host mesenchymal stem cells from the surrounding tissue, which differentiate into bone-forming osteoblasts. This process is facilitated by the presence of growth factors within the graft, principally bone morphogenetic proteins (BMPs). Graft Osteoconduction: The facilitation of blood-vessel incursion and new-bone formation into a defined passive trellis structure. All bone graft and bone-graft-substitute materials can be described through these processes. BONE AUTOGRAFTS Fresh autogenous cancellous and, to a lesser degree, cortical bone are benchmark graft materials that allograft and bone substitutes attempt to match in in vivo performance. They incorporate all of the above properties, are harvested at both primary and secondary surgical sites, and have no associated risk of viral transmission. Furthermore, they offer structural support to implanted devices and, ultimately, become mechanically efficient structures as they are incorporated into surrounding bone through creeping substitution. The availability of autografts is, however, limited and harvest is often associated with donor-site morbidity.
3 BONE ALLOGRAFTS The advantages of bone allograft harvested from cadaver sources include its ready availability in various shapes and sizes, avoidance of the need to sacrifice host structures and no donor-site morbidity. Bone allografts are distributed through regional tissue banks. Still, the grafts are not without controversy, particularly regarding their association with the transmission of infectious agents, a concern virtually eliminated through tissue-processing and sterilization. However, both freezing and irradiation modify the processes of graft incorporation and affect structural strength. A comparison of the properties of allograft and autograft bone is shown in Figure 3. Often, in complex surgical reconstructions, these materials are used in tandem with implants and fixation devices. (Figure 4) Bone Graft Autograft Cancellous Cortical Allograft Cancellous Frozen Freeze-Dry Cortical Frozen Freeze-Dry Demineralized Allogeneic Cancellous Chips Structural Strength Osteo- Conduction Osteo- Induction Figure 3: Comparative properties of bone grafts Osteogenesis (a) (b) (c) (d) Figure 4: (a) A 17-year old patient with osteosarcoma of the distal part of the femur with no extraosseous extension or metastatic disease. Following chemotherapy, (b) limb salvage with wide resection was performed. Femoral reconstruction with the use of an autogenous cortical fibular graft, iliac crest bone chips, morselized cancellous autograft and structural allograft combined with internal fixation. (c) Graft incorporation and remodeling are seen at 3 years. (d) Limb restoration is noted at 10 years following resection. (The intramedullary rod was removed at 5 years.) BONE GRAFT SUBSTITUTES The ideal bone-graft substitute is biocompatible, bioresorbable, osteoconductive, osteoinductive, structurally similar to bone, easy to use and cost-effective. Within these parameters a growing number of bone alternatives are commercially available for orthopaedic applications, including reconstruction of cavitary bone deficiency and augmentation in situations of segmental bone loss and interbody spine fusion. They are variable in their composition and their claimed mechanisms of action. Figure 5 shows a sampling of bone-graft substitute materials. Those containing growth factors in their composition inclusive of rhbmp-2 and rhbmp-7 (OP-1) demonstrate osteoinduction in clinical application, while the remainder are predominantly osteoconductive in their claims. All offer minimal structural integrity. A series of case examples demonstrate their mechanisms of action through the healing process. (Figures 6, 7 and 8)
4 Company Exactech, Inc. GenSci OrthoBiologics Interpore Cross International Medtronic Sofamor Danek Commercially available product Opteform Optefil TM OrthoBlast TM DynaGraft Composition DBM and cortical cancellous chips in gelatin carrier DBM suspended in gelatin carrier Heat sensitive copolymer with cancellous bone chips and DBM Heat sensitive copolymer with DBM Commercially available forms Formable putty in circular disks or syringeable cylinders Injectable bone paste or powdered form Injectable paste or putty Injectable gel, matrix or putty ProOsteon 500R Coral HA composite Granular or block InFuse TM rhbmp-2 protein with absorbable collagen sponge MTF/Synthes DBX DBM in a sodium hyaluronate carrier Osteotech Grafton DBM combined with Glycerol Regeneration Technologies Stryker Biotech Synthes Wright Medical Technology OSTEOFIL / REGENAFIL OP-1 Implant DBM combined with non-toxic natural gelatin carrier rhbmp-7 with type 1 bone collagen Freeze-dried powder and sponge in several sizes Claimed mechanisms of action sponge Osteoinduction Injectable paste, putty and corticocancellous mix Pellets, plugs, formable putty and injectable gel Injectable paste, injectable putty, strips and blocks with cortical cancellous chips Lyophilized powder reconstituted to form wet paste rian SRS Calcium phosphate Injectable paste Calceon 6 Calcium sulfate Pellets OSTEOSET AlloMatrix TM Surgical grade calcium sulfate DBM with surgical grade calcium sulfate powder Zimmer Collagraft TM hydroxyapatite, tricalcium phosphate Mixture of and bovine collagen Various sized pellets Injectable or formable putty Strip configurations Resorbable collagen scaffold Osteoinduction Compressive strength: 50 MPa when mixed with bone marrow Figure 5: Summary of typical bone-graft substitutes that are commercially available Burdens of proof Every lot tested in vivo for osteoinduction Every lot tested in vivo for osteoinduction Every lot tested in vivo for osteoinduction FDA status PMA approved for fusion with spinal cage HDE approval for long bone nonunions PMA approved (a) (b) (c) Figure 6: (a) A 60-year old female with a comminuted depressed fracture of the lateral tibial plateau. (b) Three weeks after ORIF with filling of the resulting defect with OSTEOSET (Wright Medical Technology, Inc., Arlington, TN) pellets. (c) At 7 months post-op, restoration of trabecular bone with complete dissolution of the graft material is noted.
5 (a) (b) Figure 7: (a) AP and Lateral radiographs, 67-year old female with depressed fracture of the lateral tibial plateau. (b) AP and Lateral radiographs 12 months after ORIF with filling the defect with rian SRS (Synthes USA, Paoli, PA). loss of reduction of the plateau surface is noted, fracture completely healed. (a) (b) (c) (d) Figure 8: (a) A 23-year old male with an open, comminuted, grade II fracture of the left tibia. Prior treatments included autograft, skin flap and multiple irrigation and debridement to treat infection. Amputation was scheduled after failure of these treatments. (b) Six months following treatment with IM rod fixation and OP-1 Implant (Stryker Biotech, Hopkinton, MA). He was full weight bearing and pain free 9 months post-operative. (c) Five years post-operative. (d) Ten years post-operative. BURDEN OF PROOF It is reasonable to assume that not all bone-substitute products will perform analogously. Thus, a quandary of choice confronts the orthopaedic surgeon. As a first principle, it is important to appreciate that different healing environments (e.g., a metaphyseal defect, a long-bone fracture, an interbody spine fusion, or a posterolateral spine fusion) have different levels of difficulty in forming new bone. For example, a metaphyseal defect will permit the successful use of many purely osteoconductive materials. In contrast, a posterolateral spine fusion will not succeed if purely osteoconductive materials are used as a stand-alone substitute. Thus, validation of any bone-graft substitute in one clinical site may not necessarily predict its performance in another location. A second principle is to seek the highest burden of proof reported from preclinical studies to justify the use of an osteoinductive graft material or the choice of one brand over another. Whether it is more difficult to make bone in humans than it is in cell-culture or rodent models, with a progressive hierarchy of difficulty in more complex species, has not been clearly determined. Only human trials can determine the efficacy of bone-graft substitutes in humans as well as their site-specific effectiveness.
6 BURDEN OF PROOF (Cont d.) A third principle requiring burden of proof specifically pertains to products that are not subjected to high levels of regulatory scrutiny, such as demineralized bone matrix (DBM) or platelet gels containing autologous growth factors. Such products are considered to involve minimal manipulation of cells or tissue and are thus regulated as tissue rather than as devices, unless they are configured with an additive and then require 510(k) clearance. As a result, there is no standardized level of proof of safety and effectiveness required before these products are marketed and are used in patients. While these products may satisfy the technical definition of minimal manipulation, there is a risk that they will not produce the expected results in humans when there has been little or no testing in relevant animal models. FUTURE Recent FDA approvals include the use of rhbmp-2 for assisted spinal fusion and rhbmp-7 (OP-1) as an autograft substitute for tibial non-unions. The FDA Orthopaedic Device Advisory Panel has also recommended extending the indication for rhbmp-2, in conjunction with a collagen sponge, for the treatment of long bone fractures. These clinical applications demonstrate impressive osteoinductive capacity and pave the way for broader clinical applications. Their methods of administration include direct placement in the surgical site, but results have been more promising when the growth factors have been administered in combination with substrates to facilitate timed-release delivery and/or provide a material scaffold for bone formation. FDA regulatory imperatives will continue to determine their availability. Their cost/benefit ratio will ultimately influence clinical use. Further advances in tissue-engineering, the integration of the biological, physical and engineering sciences, will create new carrier constructs that regenerate and restore tissue to its functional state. These constructs are likely to encompass additional families of growth factors, evolving biological scaffolds and incorporation of mesenchymal stem cells. Ultimately, the development of ex vivo bioreactors capable of bone manufacture with the appropriate biomechanical cues will provide tissue-engineered constructs for direct use in the skeletal system. TAKE HOME MESSAGE The increasing number of bone-grafting procedures performed annually in the U.S. has created a shortage of cadaver allograft material and a need to increase musculoskeletal tissue donation. This has stimulated corporate interest in developing and supplying a rapidly expanding number of bone substitutes, the makeup of which includes natural, synthetic, human and animal-derived materials. Fresh autogenous cancellous and, to a lesser degree, cortical bone are the benchmark graft materials that, ideally both allograft and bone substitutes should match in in vivo performance. Their shortcomings include limited availability and donor-site morbidity. The advantages of allograft bone include availability in various sizes and shapes as well as avoidance of hoststructure sacrifice and donor-site morbidity. Tissue-processing, however, modifies graft incorporation as well as structural strength. Transmission of infection, particularly the human immunodeficiency virus (HIV) has been virtually eliminated as a concern. The ideal bone-graft substitute is biocompatible, bioresorbable, osteoconductive, osteoinductive, structurally similar to bone, easy to use and cost-effective. Currently marketed products are variable in their composition and their claimed mechanisms of action. It is reasonable that not all bone-substitute products will perform the same. Recent FDA approvals for specific uses of recombinant human growth factors (rhbmp-2 and rhbmp-7 (OP-1)) are based on demonstrated osteoinductive capacity in human trials. Other applications will likely emerge. A quandary of choice confronts the orthopaedic surgeon. Caveat emptor! Selection should be based on reasoned burdens of proof. These include examination of the product claims and whether they are supported by preclinical and human studies in site-specific locations where they are to be utilized in surgery.
THE EVOLVING ROLE OF BONE-GRAFT SUBSTITUTES
 THE EVOLVING ROLE OF BONE-GRAFT SUBSTITUTES AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 77TH ANNUAL MEETING MARCH 9-13, 2010 NEW ORLEANS, LOUISIANA ORTHOPAEDIC DEVICE FORUM PREPARED BY: A. SETH GREENWALD,
THE EVOLVING ROLE OF BONE-GRAFT SUBSTITUTES AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 77TH ANNUAL MEETING MARCH 9-13, 2010 NEW ORLEANS, LOUISIANA ORTHOPAEDIC DEVICE FORUM PREPARED BY: A. SETH GREENWALD,
BONE-GRAFT SUBSTITUTES: FACTS, FICTIONS & APPLICATIONS
 BONE-GRAFT SUBSTITUTES: FACTS, FICTIONS & APPLICATIONS AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 73rd Annual Meeting March 22-26, 2006 Chicago, Illinois ORTHOPAEDIC DEVICE FORUM Prepared by: A. Seth Greenwald,
BONE-GRAFT SUBSTITUTES: FACTS, FICTIONS & APPLICATIONS AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 73rd Annual Meeting March 22-26, 2006 Chicago, Illinois ORTHOPAEDIC DEVICE FORUM Prepared by: A. Seth Greenwald,
Summary of typical bone-graft substitutes that are commercially available - 2010
 Summary of typical bone-graft substitutes that are commercially available - 2010 Company AlloSource AlloFuse TM Heat sensitive copolymer with DBM Injectable gel and putty Cell culture Biomet Osteobiologics
Summary of typical bone-graft substitutes that are commercially available - 2010 Company AlloSource AlloFuse TM Heat sensitive copolymer with DBM Injectable gel and putty Cell culture Biomet Osteobiologics
Dental Bone Grafting Options. A review of bone grafting options for patients needing more bone to place dental implants
 Dental Bone Grafting Options A review of bone grafting options for patients needing more bone to place dental implants Dental Bone Grafting Options What is bone grafting? Bone grafting options Bone from
Dental Bone Grafting Options A review of bone grafting options for patients needing more bone to place dental implants Dental Bone Grafting Options What is bone grafting? Bone grafting options Bone from
Choosing Bone Graft Substitutes: Filling the Gaps on Evidence and Pricing. September 24, 2014
 Choosing Bone Graft Substitutes: Filling the Gaps on Evidence and Pricing September 24, 2014 Choosing Bone Graft Substitutes: Filling the Gaps on Evidence and Pricing ECRI Institute Webinar Presented by:
Choosing Bone Graft Substitutes: Filling the Gaps on Evidence and Pricing September 24, 2014 Choosing Bone Graft Substitutes: Filling the Gaps on Evidence and Pricing ECRI Institute Webinar Presented by:
ORTHOPEDICS BONE GRAFT & SYNTHETIC BONE. Gabelli & Company, Inc. January 27, 2004. Companies Highlighted
 One Corporate Center Rye, NY 10580-1422 Tel (914) 921-3700 Fax (914) 921-5098 www.gabelli.com January 27, 2004 Gabelli & Company, Inc. ORTHOPEDICS BONE GRAFT & SYNTHETIC BONE MD Series Threaded Bone Dowels
One Corporate Center Rye, NY 10580-1422 Tel (914) 921-3700 Fax (914) 921-5098 www.gabelli.com January 27, 2004 Gabelli & Company, Inc. ORTHOPEDICS BONE GRAFT & SYNTHETIC BONE MD Series Threaded Bone Dowels
S YN T H E T I C C A N C E L LO U S B O N E
 S YN T H E T I C C A N C E L LO U S B O N E CELLPLEX TCP Graft is a synthetic cancellous scaffold that provides an optimum environment for cellular infiltration. Composed of tricalcium phosphate, the scaffold
S YN T H E T I C C A N C E L LO U S B O N E CELLPLEX TCP Graft is a synthetic cancellous scaffold that provides an optimum environment for cellular infiltration. Composed of tricalcium phosphate, the scaffold
it will set at body temperature, and it will grow. UNIQUE FORMULATIONS
 BETTER BONE GRAFT FORMULATION. SUPERIOR BONE GRAFT HANDLING. When choosing a Demineralized Bone Matrix, remember that not all products are created equal. For excellent clinical performance and patient
BETTER BONE GRAFT FORMULATION. SUPERIOR BONE GRAFT HANDLING. When choosing a Demineralized Bone Matrix, remember that not all products are created equal. For excellent clinical performance and patient
chronos BOne VOid Filler Beta-Tricalcium Phosphate (b-tcp) bone graft substitute
 chronos BOne VOid Filler Beta-Tricalcium Phosphate (b-tcp) bone graft substitute chronos Bone Void Filler Osteoconductive Resorbable Synthetic chronos Granules and Preforms are synthetic, porous, osteoconductive,
chronos BOne VOid Filler Beta-Tricalcium Phosphate (b-tcp) bone graft substitute chronos Bone Void Filler Osteoconductive Resorbable Synthetic chronos Granules and Preforms are synthetic, porous, osteoconductive,
INFUSE Bone Graft (rhbmp-2/acs)
 1 INFUSE Bone Graft (rhbmp-2/acs) For patients who need more bone to place dental implants Enjoy living with INFUSE Bone Graft. www.medtronic.com Medtronic Spinal and Biologics Business Worldwide Headquarters
1 INFUSE Bone Graft (rhbmp-2/acs) For patients who need more bone to place dental implants Enjoy living with INFUSE Bone Graft. www.medtronic.com Medtronic Spinal and Biologics Business Worldwide Headquarters
DBX. Bone Graft Substitute. The osteoinductive power.
 DBX. Bone Graft Substitute. The osteoinductive power. Osteoinductivity for challenging bony fusion Excellent handling properties Certified safety The answer to bone voids Brochure for use outside of the
DBX. Bone Graft Substitute. The osteoinductive power. Osteoinductivity for challenging bony fusion Excellent handling properties Certified safety The answer to bone voids Brochure for use outside of the
BONE OR SOFT TISSUE HEALING AND FUSION ENHANCEMENT PRODUCTS
 #1 MEDICAL POLICY BONE OR SOFT TISSUE HEALING AND FUSION ENHANCEMENT PRODUCTS Policy Number: 2016T0410P Effective Date: February 1, 2016 Table of Contents BENEFIT CONSIDERATIONS COVERAGE RATIONALE DEFINITIONS...
#1 MEDICAL POLICY BONE OR SOFT TISSUE HEALING AND FUSION ENHANCEMENT PRODUCTS Policy Number: 2016T0410P Effective Date: February 1, 2016 Table of Contents BENEFIT CONSIDERATIONS COVERAGE RATIONALE DEFINITIONS...
INFUSE Bone Graft. Patient Information Brochure
 INFUSE Bone Graft Patient Information Brochure This Patient Guide is designed to help you decide whether or not to have surgery using INFUSE Bone Graft to treat your broken tibia (lower leg). There are
INFUSE Bone Graft Patient Information Brochure This Patient Guide is designed to help you decide whether or not to have surgery using INFUSE Bone Graft to treat your broken tibia (lower leg). There are
Bone Morphogenetic Proteins & Spinal Surgery for Degenarative Disc Disease
 Ontario Health Technology Assessment Series 2004; Vol. 4, No. 4 Bone Morphogenetic Proteins & Spinal Surgery for Degenarative Disc Disease An Evidence Based Analysis March 2004 Medical Advisory Secretariat
Ontario Health Technology Assessment Series 2004; Vol. 4, No. 4 Bone Morphogenetic Proteins & Spinal Surgery for Degenarative Disc Disease An Evidence Based Analysis March 2004 Medical Advisory Secretariat
Bone Graft Substitutes
 Medical Coverage Policy Page: 1 of 21 Change Summary: Updated Description, Coverage Limitations, Background, Provider Claims Codes, Medical Terms, References Disclaimer Description Coverage Determination
Medical Coverage Policy Page: 1 of 21 Change Summary: Updated Description, Coverage Limitations, Background, Provider Claims Codes, Medical Terms, References Disclaimer Description Coverage Determination
BONE OR SOFT TISSUE HEALING AND FUSION ENHANCEMENT PRODUCTS
 CLINICAL POLICY BONE OR SOFT TISSUE HEALING AND FUSION ENHANCEMENT PRODUCTS Policy Number: SURGERY 056.10 T2 Effective Date: May 1, 2013 Table of Contents CONDITIONS OF COVERAGE... COVERAGE RATIONALE BACKGROUND...
CLINICAL POLICY BONE OR SOFT TISSUE HEALING AND FUSION ENHANCEMENT PRODUCTS Policy Number: SURGERY 056.10 T2 Effective Date: May 1, 2013 Table of Contents CONDITIONS OF COVERAGE... COVERAGE RATIONALE BACKGROUND...
Bone Grafts, Bone Morphogenetic Proteins, and Bone Substitutes
 Chapter 6 Bone Grafts, Bone Morphogenetic Proteins, and Bone Substitutes Hyun Bae, MD Neil Bhamb, MD Linda E.A. Kanim, MA Justin S. Field, MD I. Bone Healing A. Primary bone healing Occurs with constructs
Chapter 6 Bone Grafts, Bone Morphogenetic Proteins, and Bone Substitutes Hyun Bae, MD Neil Bhamb, MD Linda E.A. Kanim, MA Justin S. Field, MD I. Bone Healing A. Primary bone healing Occurs with constructs
Technology Breakthrough in Spinal Implants (Technical Insights)
 Technology Breakthrough in Spinal Implants (Technical Insights) Biomaterial innovations is a growth factor for spinal implant market June 2014 Table of Contents Section Page Number Executive Summary 4
Technology Breakthrough in Spinal Implants (Technical Insights) Biomaterial innovations is a growth factor for spinal implant market June 2014 Table of Contents Section Page Number Executive Summary 4
Patellofemoral Chondrosis
 Patellofemoral Chondrosis What is PF chondrosis? PF chondrosis (cartilage deterioration) is the softening or loss of smooth cartilage, most frequently that which covers the back of the kneecap, but the
Patellofemoral Chondrosis What is PF chondrosis? PF chondrosis (cartilage deterioration) is the softening or loss of smooth cartilage, most frequently that which covers the back of the kneecap, but the
LifeNet Health Presented by James Clagett, PhD., Chief Science Officer LifeNet Health - The Best Keep Secret in Regenerative Medicine
 Presented by James Clagett, PhD., Chief Science Officer - The Best Keep Secret in Regenerative Medicine Corporate Headquarters Virginia Beach, VA Who is? helps save lives and restore health for thousands
Presented by James Clagett, PhD., Chief Science Officer - The Best Keep Secret in Regenerative Medicine Corporate Headquarters Virginia Beach, VA Who is? helps save lives and restore health for thousands
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF).
 Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
INFUSE Bone Graft Oral Maxillofacial Bone Grafting Procedures
 INFUSE Bone Graft Oral Maxillofacial Bone Grafting Procedures Dental Products Advisory Panel Committee Ed Chin, DPh Group Director, Regulatory Affairs Medtronic Spinal and Biologics 1 INFUSE Bone Graft
INFUSE Bone Graft Oral Maxillofacial Bone Grafting Procedures Dental Products Advisory Panel Committee Ed Chin, DPh Group Director, Regulatory Affairs Medtronic Spinal and Biologics 1 INFUSE Bone Graft
Technique Guide. Norian SRS Fast Set Putty. Calcium phosphate bone void filler.
 Technique Guide Norian SRS Fast Set Putty. Calcium phosphate bone void filler. TableofContents Introduction Norian SRS Fast Set Putty 2 Indications and Contraindications 3 Basic Science 4 Surgical Technique
Technique Guide Norian SRS Fast Set Putty. Calcium phosphate bone void filler. TableofContents Introduction Norian SRS Fast Set Putty 2 Indications and Contraindications 3 Basic Science 4 Surgical Technique
CORNERSTONE TISSUE PARALLEL TISSUE OFFERINGS
 CORNERSTONE TISSUE PARALLEL TISSUE OFFERINGS Precision Machined Tissue Medtronic Sofamor Danek established itself as a leader in the machined allograft tissue market with the introduction of the CORNERSTONE-SR
CORNERSTONE TISSUE PARALLEL TISSUE OFFERINGS Precision Machined Tissue Medtronic Sofamor Danek established itself as a leader in the machined allograft tissue market with the introduction of the CORNERSTONE-SR
Bone graft substitutes currently available in orthopaedic practice
 INSTRUCTIONAL REVIEW: TRAUMA Bone graft substitutes currently available in orthopaedic practice THE EVIDENCE FOR THEIR USE T. Kurien, R. G. Pearson, B. E. Scammell From Queen s Medical Centre, University
INSTRUCTIONAL REVIEW: TRAUMA Bone graft substitutes currently available in orthopaedic practice THE EVIDENCE FOR THEIR USE T. Kurien, R. G. Pearson, B. E. Scammell From Queen s Medical Centre, University
SUPERIOR COURT OF THE STATE OF CALIFORNIA FOR THE COUNTY OF LOS ANGELES. 21 COMES NOW Plaintiff APRIL CHRISTINE CABANA who alleges as follows:
 RonaldL.M. Goldman, Esq., Bar Number Bijan Esfandiari, Esq., Bar Number 1 A. Ilyas Akbari, Esq., Bar Number 1 BAUM HEDLUND ARISTEI & GOLDMAN, P.C. 1 Wilshire Blvd., Suite 0 Los Angeles, CA 00. Tel: ()-
RonaldL.M. Goldman, Esq., Bar Number Bijan Esfandiari, Esq., Bar Number 1 A. Ilyas Akbari, Esq., Bar Number 1 BAUM HEDLUND ARISTEI & GOLDMAN, P.C. 1 Wilshire Blvd., Suite 0 Los Angeles, CA 00. Tel: ()-
Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF).
 Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra,
Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra,
Mitchell S. Fourman M.Phil Eugene Borst BS Eric Bogner, MD S. Robert Rozbruch MD Austin T. Fragomen, MD
 Post-Operative CT Measurements Accurately Predict the Need for Clinical Intervention Retrospective Findings to Support Prospective Clinical Trial Protocol Mitchell S. Fourman M.Phil Eugene Borst BS Eric
Post-Operative CT Measurements Accurately Predict the Need for Clinical Intervention Retrospective Findings to Support Prospective Clinical Trial Protocol Mitchell S. Fourman M.Phil Eugene Borst BS Eric
Spinal Arthrodesis Group Exercises
 Spinal Arthrodesis Group Exercises 1. Two surgeons work together to perform an arthrodesis. Dr. Bonet, a general surgeon, makes the anterior incision to gain access to the spine for the arthrodesis procedure.
Spinal Arthrodesis Group Exercises 1. Two surgeons work together to perform an arthrodesis. Dr. Bonet, a general surgeon, makes the anterior incision to gain access to the spine for the arthrodesis procedure.
Innovation Across Borders Forum VBO-FEB Innovation Case Preparation Form
 Innovation Across Borders Forum VBO-FEB Innovation Case Preparation Form WHO Welke onderneming(en) werd(en) hierbij betrokken? (grootte, bedrijfssector, )? Met welke partner(s) (clusters, O&O-centrum,
Innovation Across Borders Forum VBO-FEB Innovation Case Preparation Form WHO Welke onderneming(en) werd(en) hierbij betrokken? (grootte, bedrijfssector, )? Met welke partner(s) (clusters, O&O-centrum,
Bone graft materials in fixation of orthopaedic implants
 PHD THESIS DANISH MEDICAL JOURNAL Bone graft materials in fixation of orthopaedic implants in sheep Hassan Babiker This review has been accepted as a thesis together with 3 previously published papers
PHD THESIS DANISH MEDICAL JOURNAL Bone graft materials in fixation of orthopaedic implants in sheep Hassan Babiker This review has been accepted as a thesis together with 3 previously published papers
PART B - Human Tissue List February 2015
 PART B - Human Tissue List February 2015 Billing Code CARDIO-THORACIC Conduit - Aortic TBV56 Donor Tissue Bank of Victoria $1,505.00 Conduit - Aortic + Valve QHV09 The Prince Charles Hospital - Queensland
PART B - Human Tissue List February 2015 Billing Code CARDIO-THORACIC Conduit - Aortic TBV56 Donor Tissue Bank of Victoria $1,505.00 Conduit - Aortic + Valve QHV09 The Prince Charles Hospital - Queensland
August 21, 2015. TCM Associates Ltd Mr. Iain Alligan Technical Director 3 Hillgrove Business Park Nazeing Road Essex EN9 2HB United Kingdom
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 Mr. Iain Alligan Technical
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 Mr. Iain Alligan Technical
ANTERIOR LUMBAR INTERBODY FUSION (ALIF) Basic Anatomical Landmarks: Anterior Lumbar Spine
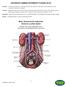 (ALIF) Anterior In human anatomy, referring to the front surface of the body or the position of one structure relative to another Lumbar Relating to the loins or the section of the back and sides between
(ALIF) Anterior In human anatomy, referring to the front surface of the body or the position of one structure relative to another Lumbar Relating to the loins or the section of the back and sides between
chronos. Bone Graft Substitute. Osteoconductive, resorbable, synthetic.
 chronos. Bone Graft Substitute. Osteoconductive, resorbable, synthetic. Optimized osteoconductive matrix Enhancement with biological factors Fast remodeling within 6 to 18 months The answer to bone voids
chronos. Bone Graft Substitute. Osteoconductive, resorbable, synthetic. Optimized osteoconductive matrix Enhancement with biological factors Fast remodeling within 6 to 18 months The answer to bone voids
WRIGHT MEDICAL GROUP INC
 WRIGHT MEDICAL GROUP INC FORM 10-K (Annual Report) Filed 02/27/14 for the Period Ending 12/31/13 Address 5677 AIRLINE ROAD ARLINGTON, TN 38002 Telephone 9018679971 CIK 0001137861 SIC Code 3842 - Orthopedic,
WRIGHT MEDICAL GROUP INC FORM 10-K (Annual Report) Filed 02/27/14 for the Period Ending 12/31/13 Address 5677 AIRLINE ROAD ARLINGTON, TN 38002 Telephone 9018679971 CIK 0001137861 SIC Code 3842 - Orthopedic,
CPT Codes for Bone Marrow Transplant January 2015 James L. Gajewski, MD
 The blood and marrow transplant field has 15 dedicated CPT codes. These CPT codes can be categorized into three groups: 1. Collection Codes 2. Cell Processing Codes 3. Cell Infusion Codes Collection Codes
The blood and marrow transplant field has 15 dedicated CPT codes. These CPT codes can be categorized into three groups: 1. Collection Codes 2. Cell Processing Codes 3. Cell Infusion Codes Collection Codes
Demineralized Bone Matrix: Development of a Process for Optimal Performance. the better approach
 Demineralized Bone Matrix: Development of a Process for Optimal Performance the better approach the better approach Introduction MTF is a non-profit organization founded in 1987 by academic orthopaedic
Demineralized Bone Matrix: Development of a Process for Optimal Performance the better approach the better approach Introduction MTF is a non-profit organization founded in 1987 by academic orthopaedic
Orthopedic Applications of Stem Cell Therapy
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Don t Let Life Pass You By Because Of Oral Bone Loss
 Don t Let Life Pass You By Because Of Oral Bone Loss Ask For Dental Implant Solutions From BIOMET 3i Scan With Your Smartphone! In order to scan QR codes, your mobile device must have a QR code reader
Don t Let Life Pass You By Because Of Oral Bone Loss Ask For Dental Implant Solutions From BIOMET 3i Scan With Your Smartphone! In order to scan QR codes, your mobile device must have a QR code reader
Anatomy and Physiology 101 for Attorneys
 Knee Injuries Anatomy and Physiology 101 for Attorneys Phil Davidson, MD Heiden-Davidson Orthopedics Salt Lake City, UT May 2011 Introduction Dr. Phil Davidson Park City and SLC clinics Education: Harvard,
Knee Injuries Anatomy and Physiology 101 for Attorneys Phil Davidson, MD Heiden-Davidson Orthopedics Salt Lake City, UT May 2011 Introduction Dr. Phil Davidson Park City and SLC clinics Education: Harvard,
Curriculum Vitae (Updated 06/09)
 Curriculum Vitae (Updated 06/09) JOHN M. SMALL, M.D. Business Address Florida Orthopaedic Institute 13020 N. Telecom Parkway Temple Terrace, FL 33637 Business Phone 813-978-9700 : March 27, 1958 Medical
Curriculum Vitae (Updated 06/09) JOHN M. SMALL, M.D. Business Address Florida Orthopaedic Institute 13020 N. Telecom Parkway Temple Terrace, FL 33637 Business Phone 813-978-9700 : March 27, 1958 Medical
US Regulations for Import and Export of Cell Therapy Products
 US Regulations for Import and Export of Cell Therapy Products Kurt Gunter, MD U Minnesota UCB Transplants (2001-2007)* Total units: 790 Number US banks: 19 Number non-us banks: 12 % units from non-us banks:
US Regulations for Import and Export of Cell Therapy Products Kurt Gunter, MD U Minnesota UCB Transplants (2001-2007)* Total units: 790 Number US banks: 19 Number non-us banks: 12 % units from non-us banks:
ICD-10-PCS Documentation and Coding for Spinal Procedures October 22, 2015
 Questions and Answers 1. We have a question regarding a spinal surgical procedure. The diagnosis was bilateral lateral recess stenosis and central stenosis from L2-L5. The procedure was an open lumbar
Questions and Answers 1. We have a question regarding a spinal surgical procedure. The diagnosis was bilateral lateral recess stenosis and central stenosis from L2-L5. The procedure was an open lumbar
Healthcare Industry: US Orthopedic Implants and Devices Market
 Healthcare Industry: US Orthopedic Implants and Devices Market by KenResearch - Tuesday, March 11, 2014 http://www.kenresearch.com/blog/2014/03/us-orthopedic-implants-and-devices-market/ The Replacement
Healthcare Industry: US Orthopedic Implants and Devices Market by KenResearch - Tuesday, March 11, 2014 http://www.kenresearch.com/blog/2014/03/us-orthopedic-implants-and-devices-market/ The Replacement
Spine Clinic Neurospine Specialists, Orthopaedics and Neurosurgery
 Spine Clinic Neurospine Specialists, Orthopaedics and Neurosurgery REVISION SPINE SURGERY Revision surgery is a very complex field which requires experience, training and evaluation in a very individual
Spine Clinic Neurospine Specialists, Orthopaedics and Neurosurgery REVISION SPINE SURGERY Revision surgery is a very complex field which requires experience, training and evaluation in a very individual
THE CARTILAGE IMPLANT FOR BIOLOGICAL CARTILAGE REPAIR
 THE CARTILAGE IMPLANT FOR BIOLOGICAL CARTILAGE REPAIR 02/13 Content chondrotissue (p.4) Scientific Background (p.5) Preclinical Outcomes (p.6) Clinical Outcomes: MRI Evaluation (p.7) Clinical Outcomes:
THE CARTILAGE IMPLANT FOR BIOLOGICAL CARTILAGE REPAIR 02/13 Content chondrotissue (p.4) Scientific Background (p.5) Preclinical Outcomes (p.6) Clinical Outcomes: MRI Evaluation (p.7) Clinical Outcomes:
Bone Regeneration and Repair: Current and Future Aspects
 Bone Regeneration and Repair: Current and Future Aspects Dr. Madhukar Rajaram Wagh 1, Dr. Dilip Ravalia 2 1 Assistant Professor, Department of Surgery, Gujarat Adani Institute of Medical Sciences, Bhuj,
Bone Regeneration and Repair: Current and Future Aspects Dr. Madhukar Rajaram Wagh 1, Dr. Dilip Ravalia 2 1 Assistant Professor, Department of Surgery, Gujarat Adani Institute of Medical Sciences, Bhuj,
Fracture Care Coding September 28, 2011
 Fracture Care Coding September 28, 2011 Julie Edens Leu, CPC, CPCO, CPMA, CPC-I 1 Disclaimer Every reasonable effort has been made to ensure that the educational material provided today is accurate and
Fracture Care Coding September 28, 2011 Julie Edens Leu, CPC, CPCO, CPMA, CPC-I 1 Disclaimer Every reasonable effort has been made to ensure that the educational material provided today is accurate and
The MOVIE. Currently VP, Regulatory Affairs, MCRA Former Orthopedic Devices Branch Chief (2006)
 FDA played by Glenn Stiegman Currently VP, Regulatory Affairs, MCRA Former Orthopedic Devices Branch Chief (2006) Managed Orthopedic and Spine groups (Branch split in 2007) Former Orthopedic Reviewer (2000-2005)
FDA played by Glenn Stiegman Currently VP, Regulatory Affairs, MCRA Former Orthopedic Devices Branch Chief (2006) Managed Orthopedic and Spine groups (Branch split in 2007) Former Orthopedic Reviewer (2000-2005)
Norbert Weber, Lilly Ostrovsky. Musculoskeletal Transplant Foundation (MTF), Edison, NJ, USA. Disclosures: N. Weber: None. L. Ostrovsky: None.
 Immunogenicity of Biopharmaceuticals in Large Animal Models: Timedependent Formation of Antibodies and Detection of TNF-alpha in Serum and Synovial Fluid Norbert Weber, Lilly Ostrovsky. Musculoskeletal
Immunogenicity of Biopharmaceuticals in Large Animal Models: Timedependent Formation of Antibodies and Detection of TNF-alpha in Serum and Synovial Fluid Norbert Weber, Lilly Ostrovsky. Musculoskeletal
Do you have anything to add? If so, I d love to hear from you! Jessica Robinson Conference Manager Life Sciences @jessbiopharma
 1 Who is the most influential figure in cord blood around the world? What is the biggest challenge to overcome in the use of cord blood as a source of stem cells? We asked 10 leading experts in the cord
1 Who is the most influential figure in cord blood around the world? What is the biggest challenge to overcome in the use of cord blood as a source of stem cells? We asked 10 leading experts in the cord
March 6-7, 2015. (Friday & Saturday) COURSE DIRECTORS. Course will be held at the BIOMET Skills Academy Miami, Florida
 NEW THREE DIMENSIONAL RIDGE AUGMENTATION & ADVANCED DENTAL IMPLANT COURSE Robert E. Marx, DDS Professor of Surgery and Chief March 6-7, 2015 (Friday & Saturday) COURSE DIRECTORS Course will be held at
NEW THREE DIMENSIONAL RIDGE AUGMENTATION & ADVANCED DENTAL IMPLANT COURSE Robert E. Marx, DDS Professor of Surgery and Chief March 6-7, 2015 (Friday & Saturday) COURSE DIRECTORS Course will be held at
HyStem. Hydrogels CELLULAR MATRICES FOR TRANSLATIONAL RESEARCH. esibio.com
 HyStem Hydrogels CELLULAR MATRICES FOR TRANSLATIONAL RESEARCH HyStem Hydrogel Extracellular Matrices Chemically-defined For use in 2D and 3D formats in vitro and in vivo applications Customizable for many
HyStem Hydrogels CELLULAR MATRICES FOR TRANSLATIONAL RESEARCH HyStem Hydrogel Extracellular Matrices Chemically-defined For use in 2D and 3D formats in vitro and in vivo applications Customizable for many
Born January 13, 1959 in Ponce, Puerto Rico Fluent in English and Spanish
 Page 1 of 6 6160 Windhaven Pkwy Ste 200 Plano, TX 75093 Luis A. Mignucci, M.D. Phone (972)378-6908 Fax (972)378-6586 Personal Born January 13, 1959 in Ponce, Puerto Rico Fluent in English and Spanish Surgical
Page 1 of 6 6160 Windhaven Pkwy Ste 200 Plano, TX 75093 Luis A. Mignucci, M.D. Phone (972)378-6908 Fax (972)378-6586 Personal Born January 13, 1959 in Ponce, Puerto Rico Fluent in English and Spanish Surgical
CURRICULUM VITAE DAVID B. HAHN, M.D.
 CURRICULUM VITAE DAVID B. HAHN, M.D. Orthopedic Surgeon Colorado Limb Consultants 1601 East 19 th Avenue, Suite 3300 Denver, Colorado 80218 (303) 837-0072 FAX (303) 837-0075 Orthopedic Surgeon Presbyterian/St.
CURRICULUM VITAE DAVID B. HAHN, M.D. Orthopedic Surgeon Colorado Limb Consultants 1601 East 19 th Avenue, Suite 3300 Denver, Colorado 80218 (303) 837-0072 FAX (303) 837-0075 Orthopedic Surgeon Presbyterian/St.
vitality meets bone volume around dental implants Straumann BoneCeramic
 vitality meets bone volume around dental implants Straumann BoneCeramic The vitality you need around your implants Bone volume restoration and preservation for the desired esthetic outcome Image by PD
vitality meets bone volume around dental implants Straumann BoneCeramic The vitality you need around your implants Bone volume restoration and preservation for the desired esthetic outcome Image by PD
The Smart Dentin Grinder. Business Opportunity 2014
 The Smart Dentin Grinder Business Opportunity 2014 2 The Market Grafting Materials for Dental Reconstruction Currently a $550M global market An upcoming market, with 5.7% annual growth Tissue and Bone
The Smart Dentin Grinder Business Opportunity 2014 2 The Market Grafting Materials for Dental Reconstruction Currently a $550M global market An upcoming market, with 5.7% annual growth Tissue and Bone
Low Back Pain (LBP) Prevalence. Low Back Pain (LBP) Prevalence. Lumbar Fusion: Where is the Evidence?
 15 th Annual Cleveland Clinic Pain Management Symposium Sarasota, Florida Lumbar Fusion: Where is the Evidence? Gordon R. Bell, M.D. Director, Cleveland Clinic Low Back Pain (LBP) Prevalence Lifetime prevalence:
15 th Annual Cleveland Clinic Pain Management Symposium Sarasota, Florida Lumbar Fusion: Where is the Evidence? Gordon R. Bell, M.D. Director, Cleveland Clinic Low Back Pain (LBP) Prevalence Lifetime prevalence:
Corporate Medical Policy Cord Blood as a Source of Stem Cells
 Corporate Medical Policy Cord Blood as a Source of Stem Cells File Name: Origination: Last CAP Review: Next CAP Review: Last Review cord_blood_as_a_source_of_stem_cells 2/2001 3/2015 3/2016 3/2015 Description
Corporate Medical Policy Cord Blood as a Source of Stem Cells File Name: Origination: Last CAP Review: Next CAP Review: Last Review cord_blood_as_a_source_of_stem_cells 2/2001 3/2015 3/2016 3/2015 Description
Transplant Coordinator
 Have you signed your organ donor card? Hearts, kidneys, livers, and lungs can all be transplanted. When you die, one of your organs could help a critically ill patient live a longer life. What I do every
Have you signed your organ donor card? Hearts, kidneys, livers, and lungs can all be transplanted. When you die, one of your organs could help a critically ill patient live a longer life. What I do every
Combined interbody cage and anterior plating in the surgical treatment of cervical disc disease
 Acta Orthop. Belg., 2004, 70, 461-465 ORIGINAL STUDY Combined interbody cage and anterior plating in the surgical treatment of cervical disc disease Anastasios CHRISTODOULOU, Avraam PLOUMIS, Ioannis TERZIDIS,
Acta Orthop. Belg., 2004, 70, 461-465 ORIGINAL STUDY Combined interbody cage and anterior plating in the surgical treatment of cervical disc disease Anastasios CHRISTODOULOU, Avraam PLOUMIS, Ioannis TERZIDIS,
14.0 Stem Cell Laboratory Services
 Laboratory Services Contact Information: To inquire about assisting with surgical harvesting of bone marrow, cellular therapy (CT) product processing, cryopreservation, storage, or any other lab services,
Laboratory Services Contact Information: To inquire about assisting with surgical harvesting of bone marrow, cellular therapy (CT) product processing, cryopreservation, storage, or any other lab services,
SPINAL FUSION. North American Spine Society Public Education Series
 SPINAL FUSION North American Spine Society Public Education Series WHAT IS SPINAL FUSION? The spine is made up of a series of bones called vertebrae ; between each vertebra are strong connective tissues
SPINAL FUSION North American Spine Society Public Education Series WHAT IS SPINAL FUSION? The spine is made up of a series of bones called vertebrae ; between each vertebra are strong connective tissues
This is a licensed product of Ken Research and should not be copied
 TABLE OF CONTENTS 1. The US Orthopedic Implants and Devices Market Introduction 2. The US Orthopedic Implants and Devices Market Value Chain 3. The US Orthopedic Implants and Devices Market Size, 2008-2013
TABLE OF CONTENTS 1. The US Orthopedic Implants and Devices Market Introduction 2. The US Orthopedic Implants and Devices Market Value Chain 3. The US Orthopedic Implants and Devices Market Size, 2008-2013
Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization
 APPROVED IRN10389-1.1-04 Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization Because it involves accessing the spine through the patient s side, the Direct Lateral approach
APPROVED IRN10389-1.1-04 Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization Because it involves accessing the spine through the patient s side, the Direct Lateral approach
Advances In Spine Care. James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery
 Advances In Spine Care James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery Introduction The Spine - A common source of problems Back pain is the #2 presenting
Advances In Spine Care James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery Introduction The Spine - A common source of problems Back pain is the #2 presenting
IN THE CIRCUIT COURT OF COOK COUNTY, ILLINOIS, COUNTY DEPARTMENT, LAW DIVISION
 IN THE CIRCUIT COURT OF COOK COUNTY, ILLINOIS, COUNTY DEPARTMENT, LAW DIVISION KARL L. SANDA, ) ) Plaintiff, ) ) w. ) ) Case No. : MEDTRONIC, INC, MEDTRONIC ) SOFAMOR DANEK USA, INC., ) NORTHWESTERN MEMORIAL
IN THE CIRCUIT COURT OF COOK COUNTY, ILLINOIS, COUNTY DEPARTMENT, LAW DIVISION KARL L. SANDA, ) ) Plaintiff, ) ) w. ) ) Case No. : MEDTRONIC, INC, MEDTRONIC ) SOFAMOR DANEK USA, INC., ) NORTHWESTERN MEMORIAL
.org. Metastatic Bone Disease. Description
 Metastatic Bone Disease Page ( 1 ) Cancer that begins in an organ, such as the lungs, breast, or prostate, and then spreads to bone is called metastatic bone disease (MBD). More than 1.2 million new cancer
Metastatic Bone Disease Page ( 1 ) Cancer that begins in an organ, such as the lungs, breast, or prostate, and then spreads to bone is called metastatic bone disease (MBD). More than 1.2 million new cancer
. Soft Tissue Regeneration... Consultant. RTI Surgical...Consultant. OSSUR...Consultant. Arthrex...Consultant
 sl23lls 1 E ACL Graft Choice in the Elite Athlete and When Do I Let Them Return to Sport: Graft Ghoice: Allografts : @ Peter A. lndelicato MD Emeritus Professor Sports Medicine Department of OrthoPaedic
sl23lls 1 E ACL Graft Choice in the Elite Athlete and When Do I Let Them Return to Sport: Graft Ghoice: Allografts : @ Peter A. lndelicato MD Emeritus Professor Sports Medicine Department of OrthoPaedic
Technological Advancements in Spinal Fusion Implants: A Summary of the Current Scientific and Clinical Research on Titanium Engineered Surfaces
 Reprinted with permission of The Spinal Research Foundation. SPINAL RESEARCH FOUNDATION Technological Advancements in Spinal Fusion Implants: A Summary of the Current Scientific and Clinical Research on
Reprinted with permission of The Spinal Research Foundation. SPINAL RESEARCH FOUNDATION Technological Advancements in Spinal Fusion Implants: A Summary of the Current Scientific and Clinical Research on
coligne treatment technology
 coligne treatment technology coligne a strategy in spine Leadership begins before the patient enters the surgical theater, among the spine care professionals forming the coalliance. This may be a small
coligne treatment technology coligne a strategy in spine Leadership begins before the patient enters the surgical theater, among the spine care professionals forming the coalliance. This may be a small
STEM CELL TRANSPLANTS
 UAMS Information on STEM CELL TRANSPLANTS What is a Stem Cell Transplant? A stem cell transplant is an infusion of stem cells following high-dose chemotherapy. The infused cells effectively rescue the
UAMS Information on STEM CELL TRANSPLANTS What is a Stem Cell Transplant? A stem cell transplant is an infusion of stem cells following high-dose chemotherapy. The infused cells effectively rescue the
STIMULATION AND SUBSTITUTION OF BONE ALLOGRAFT AROUND NON- CEMENTED IMPLANTS
 STIMULATION AND SUBSTITUTION OF BONE ALLOGRAFT AROUND NON- CEMENTED IMPLANTS Ph.D. thesis Thomas Bo Jensen Faculty of Health Sciences University of Aarhus 2003 Side 1 af 40 Abbreviations...4 Abstract...6
STIMULATION AND SUBSTITUTION OF BONE ALLOGRAFT AROUND NON- CEMENTED IMPLANTS Ph.D. thesis Thomas Bo Jensen Faculty of Health Sciences University of Aarhus 2003 Side 1 af 40 Abbreviations...4 Abstract...6
Integumentary System Individual Exercises
 Integumentary System Individual Exercises 1. A physician performs an incision and drainage of a subcutaneous abscess in his office for a particularly uncooperative established patient. How should this
Integumentary System Individual Exercises 1. A physician performs an incision and drainage of a subcutaneous abscess in his office for a particularly uncooperative established patient. How should this
Osteoporosis Medicines and Jaw Problems
 Osteoporosis Medicines and Jaw Problems J. Michael Digney, D.D.S. Osteoporosis is a condition that affects over 10 million patients in this country, with the majority of those being post-menopausal women.
Osteoporosis Medicines and Jaw Problems J. Michael Digney, D.D.S. Osteoporosis is a condition that affects over 10 million patients in this country, with the majority of those being post-menopausal women.
San Diego Stem Cell Treatment Center Frequently Asked Questions
 San Diego Stem Cell Treatment Center Frequently Asked Questions What is a Stem Cell? A stem cell is basically any cell that can replicate and differentiate. This means the cell can not only multiply, but
San Diego Stem Cell Treatment Center Frequently Asked Questions What is a Stem Cell? A stem cell is basically any cell that can replicate and differentiate. This means the cell can not only multiply, but
Bone Replacement Materials and Techniques Used for Achieving Vertical Alveolar Bone Augmentation
 Materials 2015, 8, 2953-2993; doi:10.3390/ma8062953 Article OPEN ACCESS materials ISSN 1996-1944 www.mdpi.com/journal/materials Bone Replacement Materials and Techniques Used for Achieving Vertical Alveolar
Materials 2015, 8, 2953-2993; doi:10.3390/ma8062953 Article OPEN ACCESS materials ISSN 1996-1944 www.mdpi.com/journal/materials Bone Replacement Materials and Techniques Used for Achieving Vertical Alveolar
CHS 06-07 BONES AND SKELETAL TISSUES
 CHS 06-07 BONES AND SKELETAL TISSUES This chapter provides a review of bone and skeletal tissue. The human skeleton is composed primarily of two connective tissues: (1) cartilage and (2) bone. CHARACTERISTICS
CHS 06-07 BONES AND SKELETAL TISSUES This chapter provides a review of bone and skeletal tissue. The human skeleton is composed primarily of two connective tissues: (1) cartilage and (2) bone. CHARACTERISTICS
TABLE OF CONTENTS. Surgical Technique 2. Indications 4. Product Information 5. 1. Patient Positioning and Approach 2
 Surgical Technique TABLE OF CONTENTS Surgical Technique 2 1. Patient Positioning and Approach 2 2. Intervertebral Device Implanted 2 3. Buttress Plate Selection 2 4. Awl Insertion 2 5. Screw Insertion
Surgical Technique TABLE OF CONTENTS Surgical Technique 2 1. Patient Positioning and Approach 2 2. Intervertebral Device Implanted 2 3. Buttress Plate Selection 2 4. Awl Insertion 2 5. Screw Insertion
Orthopedic Foot Instruments. Dedicated instruments for reconstructive foot surgery.
 Orthopedic Foot Instruments. Dedicated instruments for reconstructive foot surgery. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by
Orthopedic Foot Instruments. Dedicated instruments for reconstructive foot surgery. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by
CorMatrix ECM Technology
 CorMatrix ECM Technology Rethink the treatment of a damaged heart REMODEL. REGROW. RESTORE. CorMatrix ECM Technology provides a natural bioscaffold matrix that enables the body s own cells to repair and
CorMatrix ECM Technology Rethink the treatment of a damaged heart REMODEL. REGROW. RESTORE. CorMatrix ECM Technology provides a natural bioscaffold matrix that enables the body s own cells to repair and
Clinical Privileges Profile Plastic Surgery. Indu & Raj Soin Medical Center
 Printed Name Clinical Privileges Profile Plastic Surgery Indu & Raj Soin Medical Center Applicant: Check off the Requested box for each privilege requested. Applicants have the burden of producing information
Printed Name Clinical Privileges Profile Plastic Surgery Indu & Raj Soin Medical Center Applicant: Check off the Requested box for each privilege requested. Applicants have the burden of producing information
Technique Guide. Reamer/Irrigator/Aspirator (RIA). For intramedullary reaming and bone harvesting.
 Technique Guide Reamer/Irrigator/Aspirator (RIA). For intramedullary reaming and bone harvesting. Table of Contents Introduction Reamer/ Irrigator/ Aspirator (RIA) 2 Indications 4 Surgical Technique Preparation
Technique Guide Reamer/Irrigator/Aspirator (RIA). For intramedullary reaming and bone harvesting. Table of Contents Introduction Reamer/ Irrigator/ Aspirator (RIA) 2 Indications 4 Surgical Technique Preparation
Bone Remodeling using a novel, injectable bioceramic (CERAMENT ) in foot and ankle reconstruction with long term follow-up:
 Bone Remodeling using a novel, injectable bioceramic (CERAMENT ) in foot and ankle reconstruction with long term follow-up: A CASE SERIES REVIEW LAWRENCE A. DIDOMENICO, DPM Adjunct Professor, Ohio College
Bone Remodeling using a novel, injectable bioceramic (CERAMENT ) in foot and ankle reconstruction with long term follow-up: A CASE SERIES REVIEW LAWRENCE A. DIDOMENICO, DPM Adjunct Professor, Ohio College
High-Flex Solutions for the MIS Era. Zimmer Unicompartmental High Flex Knee System
 High-Flex Solutions for the MIS Era Zimmer Unicompartmental High Flex Knee System Zimmer Unicompartmental High Flex Knee Built On Success In today s health care environment, meeting patient demands means
High-Flex Solutions for the MIS Era Zimmer Unicompartmental High Flex Knee System Zimmer Unicompartmental High Flex Knee Built On Success In today s health care environment, meeting patient demands means
Howell ACL System. Howell 65 Tibial Guide EZLoc Femoral Fixation Device WasherLoc Tibial Fixation Device Graft Choice
 Howell ACL System Howell 65 Tibial Guide EZLoc Femoral Fixation Device WasherLoc Tibial Fixation Device Graft Choice Howell ACL System Howell 65 Tibial Guide EZLoc Femoral Fixation Device WasherLoc Tibial
Howell ACL System Howell 65 Tibial Guide EZLoc Femoral Fixation Device WasherLoc Tibial Fixation Device Graft Choice Howell ACL System Howell 65 Tibial Guide EZLoc Femoral Fixation Device WasherLoc Tibial
Zimmer Periarticular Elbow Locking Plate System
 Zimmer Periarticular Elbow Locking Plate System Surgical Technique The Right Solutions for Fractures Around the Elbow Disclaimer This document is intended exclusively for physicians and is not intended
Zimmer Periarticular Elbow Locking Plate System Surgical Technique The Right Solutions for Fractures Around the Elbow Disclaimer This document is intended exclusively for physicians and is not intended
Clarification of Medicare Benefits Schedule rules for the Transport Accident Commission and WorkSafe Victoria
 Clarification of Medicare Benefits Schedule rules for the Transport Accident Commission and WorkSafe Victoria MAY 2013 When paying the reasonable costs of medical services, the TAC and WorkSafe pay in
Clarification of Medicare Benefits Schedule rules for the Transport Accident Commission and WorkSafe Victoria MAY 2013 When paying the reasonable costs of medical services, the TAC and WorkSafe pay in
Guidance For Research Involving Human Embryonic Stem Cells, Germ Cells, And Cells Obtained From Cord Blood
 Guidance For Research Involving Human Embryonic Stem Cells, Germ Cells, And Cells Obtained From Cord Blood Supreme Council of Health Department of Research Guidance Regarding Research Involving Human Embryonic
Guidance For Research Involving Human Embryonic Stem Cells, Germ Cells, And Cells Obtained From Cord Blood Supreme Council of Health Department of Research Guidance Regarding Research Involving Human Embryonic
Stem Cell Therapy. Treat your pain, improve your lifestyle and look after your wellbeing. What are stem cells?
 (02) 9824 3044 LIVERPOOL NSW 2170 Stem Cell Therapy Treat your pain, improve your lifestyle and look after your wellbeing What are stem cells? Stem cells are cells that can differentiate into other types
(02) 9824 3044 LIVERPOOL NSW 2170 Stem Cell Therapy Treat your pain, improve your lifestyle and look after your wellbeing What are stem cells? Stem cells are cells that can differentiate into other types
