Corporate Medical Policy Electrical Bone Growth Stimulation
|
|
|
- Belinda Nichols
- 7 years ago
- Views:
Transcription
1 Corporate Medical Policy Electrical Bone Growth Stimulation File Name: Origination: Last CAP Review: Next CAP Review: Last Review: electrical_bone_growth_stimulation 4/1981 6/2016 6/2017 6/2016 Description of Procedure or Service Electrical bone growth stimulation is a medical technique to promote bone growth in difficult to heal fractures by applying a low electrical current to the fracture site. A variety of invasive and noninvasive interventions are used to treat fracture non-union including immobilization, casting, open or closed surgical reduction, pins, screw fixation, intramedullary rods and bone grafting. Bone growth stimulators, which may be non-invasive or invasive, may be used instead of, or in addition to, other interventions to promote bone healing. Electrical and electromagnetic fields can be generated and applied to bones through the following methods: 1. Surgical implantation of a cathode at the fracture site with the production of direct current (DC) electrical stimulation. Invasive devices require surgical implantation of a current generator in an intramuscular or subcutaneous space, while an electrode is implanted within the fragments of bone graft at the fusion site. The implantable device typically remains functional for 6 to 9 months after implantation, and, although the current generator is removed in a second surgical procedure when stimulation is completed, the electrode may or may not be removed. Implantable electrodes provide constant stimulation at the nonunion or fracture site, but carry increased risks associated with implantable leads. 2. Noninvasive electrical bone growth stimulators generate a weak electrical current within the target site using either pulsed electromagnetic fields, capacitive coupling, or combined magnetic fields. In capacitive coupling, small skin pads/electrodes are placed on either side of the fusion site and worn for 24 hours per day until healing occurs or up to 9 months. In contrast, pulsed electromagnetic fields are delivered via treatment coils that are placed into a back brace or directly onto the skin and are worn for 6 8 hours per day for 3 to 6 months. Combined magnetic fields deliver a time-varying magnetic field by superimposing the time-varying magnetic field onto an additional static magnetic field. This device involves a 30-minute treatment per day for 9 months. Patient compliance may be an issue with externally worn devices. 3. Semi-invasive (semi-implantable) stimulators use percutaneous electrodes and an external power supply obviating the need for a surgical procedure to remove the generator when treatment is finished. Regulatory Status The following implantable device was approved by the U.S. Food and Drug Administration (FDA) through the premarket approval process: The OsteoStim (Electro-Biology), which may also be marketed under the trade name SPF (Biomet). The noninvasive OrthoPak Bone Growth Stimulator (BioElectron) received U.S. Food and Drug Page 1 of 9
2 Policy Administration (FDA) premarket approval in 1984 for treatment of fracture nonunion. Pulsed electromagnetic field systems with FDA premarket approval (all noninvasive devices) include Physio - Stim from Orthofix Inc., first approved in 1986, and OrthoLogic 1000, approved in 1997, both indicated for treatment of established nonunion secondary to trauma, excluding vertebrae and all flat bones, in which the width of the nonunion defect is less than one-half the width of the bone to be treated; and the EBI Bone Healing System from Electrobiology, Inc., which was first approved in 1979 and indicated for nonunions, failed fusions, and congenital pseudoarthroses. No distinction was made between long and short bones. The FDA has approved labeling changes for electrical bone growth stimulators that remove any timeframe for the diagnosis. No semi-invasive electrical bone growth stimulator devices with FDA approval or clearance were identified. This policy discusses the use of bone growth stimulators on the appendicular skeleton and spine. Related Policies: Ultrasound Accelerated Fracture Healing Devices Bone Morphogenetic Protein Lumbar Spine Fusion Surgery ***Note: This Medical Policy is complex and technical. For questions concerning the technical language and/or specific clinical indications for its use, please consult your physician. BCBSNC will provide coverage for Electrical Bone Growth Stimulation when it is considered medically necessary because the medical criteria and guidelines shown below are met. Benefits Application This medical policy relates only to the services or supplies described herein. Please refer to the Member's Benefit Booklet for availability of benefits. Member's benefits may vary according to benefit design; therefore member benefit language should be reviewed before applying the terms of this medical policy. When Electrical Bone Growth Stimulation is covered 1. Non-invasive electrical bone growth stimulation may be considered medically necessary as treatment of fracture non-unions or congenital pseudoarthroses in the appendicular skeleton. The diagnosis of fracture non-union must meet ALL of the following criteria: a. at least 3 months have passed since the date of the fracture; b. serial radiographs for the preceding 3 month period have confirmed that no progressive signs of healing have occurred; c. the fracture gap is one centimeter or less; and d. the patient can be adequately immobilized and is of an age likely to comply with non - weight bearing for fractures of the pelvis and lower extremities. 2. Invasive methods of bone growth stimulation may be considered medically necessary when used as an adjunct to surgical treatment of non-union of major long bone fractures. Page 2 of 9
3 3. Either invasive or non-invasive methods of electrical bone growth stimulation may be considered medically necessary as an adjunct to lumbar spinal fusion surgery for patients with any of the following risk factors for subsequent failed fusion: a. one or more previous failed spinal fusion(s); b. grade III or worse spondylolisthesis; c. fusion to be performed at more than one level; d. current tobacco use; e. diabetes; f. renal disease; g. alcoholism; h. steroid use. 4. Non-invasive electrical bone stimulation may be considered medically necessary as a treatment for patients with failed lumbar spinal fusion. Failed spinal fusion is defined as a spinal fusion which has not healed at a minimum of 6 months after the original surgery, as evidenced by serial X-rays over a course of 3 months. When Electrical Bone Growth Stimulation is not covered 1. For any conditions or medical criteria other than those cited above. 2. Investigational applications of electrical bone growth stimulation in the appendicular skeleton include, but are not limited to: immediate post-surgical treatment; treatment of fresh fractures, delayed union arthrodesis failed arthrodesis stress fractures 3. Investigational applications of electrical bone growth stimulation in the spine include, but are not limited to: Semi-invasive electrical bone growth stimulators as an adjunct to lumbar fusion and for failed lumbar fusion. Invasive, semi-invasive, and noninvasive electrical stimulation as an adjunct to cervical fusion surgery and for failed cervical spine fusion. Policy Guidelines Fresh Fractures: A fracture is most commonly defined as fresh for 7 days after the fracture occurs. Most fresh closed fractures heal without complications with the use of standard fracture care, i.e., closed reduction and cast immobilization. Delayed Union: Delayed union is defined as a decelerating healing process as determined by serial x-rays, together with a lack of clinical and radiologic evidence of union, bony continuity or bone reaction at the fracture site. Page 3 of 9
4 Nonunions: The FDA labeling simply suggests that nonunion is considered established when the fracture site shows no visibly progressive signs of healing, without giving any guidance regarding the time frame of observation. However, it is suggested that a reasonable time period for lack of visible signs o f healing is 3 months. The following patient selection criteria are suggested, consistent with those proposed for electrical stimulation as a treatment of nonunions: At least 3 months have passed since the date of the fracture, AND Serial radiographs have confirmed that no progressive signs of healing have occurred, AND The fracture gap is 1cm or less, AND The patient can be adequately immobilized and is of an age when he/she is likely to comply with non-weight bearing. The evidence for noninvasive electrical bone growth stimulation in individuals who have fracture nonunion includes randomized controlled trials (RCTs) and systematic reviews of clinical trials. Relevant outcomes are symptoms, change in disease status, and functional outcomes. There is evidence from these studies that noninvasive electrical stimulators improve fracture healing for patients with fracture nonunion. However, the evidence is not from high-quality RCTs, and the systematic reviews provide only qualified support for this conclusion. The U.S. Food and Drug Administration has approved noninvasive electrical bone growth stimulation for the indications of fracture nonunions and congenital pseudoarthroses in the appendicular skeleton, and it is acknowledged that there are limited other options in this population. The evidence is sufficient to determine qualitatively that the technology results in a meaningful improvement in the net health outcome. The evidence for noninvasive electrical bone growth stimulation in individuals who have delayed union, fresh or stress fractures, or who have had surgery of the appendicular skeleton includes RCTs. Relevant outcomes are symptoms, change in disease status, and functional outcomes. Two RCTs found no benefit of electrical bone growth stimulation for fresh fractures. RCTs on delayed union of the other types of fractures were limited by small sample size and did not show a significant difference between study groups. The evidence is insufficient to determine the effects of the technology on health outcomes. The evidence for invasive or noninvasive electrical bone growth stimulation in individuals who are at high risk of lumbar spinal fusion failure includes a TEC Assessment and randomized controlled t rials (RCTs) Relevant outcomes are symptoms, change in disease status, and functional outcomes. Results from these trials indicate that in patients with risk factors for failed fusion, either invasive or noninvasive electrical bone stimulation increases the fusion rate. The evidence is sufficient to determine qualitatively that the technology results in a meaningful improvement in the net health outcome. The evidence for noninvasive electrical bone growth stimulation in individuals who are at normal risk for lumbar spinal fusion failure includes RCTs. Relevant outcomes are symptoms, change in disease status, and functional outcomes. Several RCTs have shown increased fusion rates with noninvasive bone stimulation in patient populations that include both high risk and normal risk groups. No studies were identified that studied a population of patients who were all at normal risk for fusion failure. The evidence is insufficient to determine the effects of the technology on health outcomes. The evidence for noninvasive electrical bone growth stimulation in individuals who have failed lumbar spinal fusion includes a TEC Assessment and studies where patients served as their own controls. Relevant outcomes are symptoms, change in disease status, and functional outcomes. Data indicate that noninvasive electrical stimulation improves the fusion rate in this population. The evidence is sufficient to determine qualitatively that the technology results in a meaningful improvement in the net health outcome. The evidence for invasive or noninvasive electrical bone growth stimulation in individuals who are undergoing cervical spinal fusion surgery or who have failed cervical spine fusion includes an RCT. Page 4 of 9
5 Relevant outcomes are symptoms, change in disease status, and functional outcomes. The only controlled trial published to date had methodologic limitations and the efficacy of electrical stimulation in the cervical spine has not been established. The evidence is insufficient to determine the effects of the technology on health outcomes. NOTE: Patients with implanted electronic devices should consult their physician before using these electrical bone growth stimulation devices. Billing/Coding/Physician Documentation Information This policy may apply to the following codes. Inclusion of a code in this section does not guarantee that it will be reimbursed. For further information on reimbursement guidelines, please see Administrative Policies on the Blue Cross Blue Shield of North Carolina web site at They are listed in the Category Search on the Medical Policy search page. Applicable codes: 20974, 20975, E0747, E0748, E0749 BCBSNC may request medical records for determination of medical necessity. When medical records are requested, letters of support and/or explanation are often useful, but are not sufficient documentation unless all specific information needed to make a medical necessity determination is included. Scientific Background and Reference Sources Consultant Review - 8/94 BCBSA Medical Policy Reference Manual - 12/95 FDA Approval letter dated 6/12/98 BCBSA Medical Policy Reference Manual - 9/23/98 Consultant Review - 3/99 Medical Policy Advisory Group - 5/99 Specialty Matched Consultant Advisory Panel - 11/1999 Medical Policy Advisory Group - 12/2/1999 Specialty Matched Consultant Advisory Panel - 8/2001 BCBSA Medical Policy Reference Manual - 12/18/2002; Specialty Matched Consultant Advisory Panel - 5/2003 BCBSA Medical Policy Reference Manual [Electronic Version] , 12/17/03 BCBSA Medical Policy Reference Manual [Electronic Version] , 02/25/04 BCBSA Medical Policy Reference Manual [Electronic Version] , 3/07/06 BCBSA Medical Policy Reference Manual [Electronic Version] , 3/15/05 Agency for Healthcare Research and Quality (AHRQ) Technology Assessment Program. The Role of Bone Growth Stimulating Devices and Orthobiologics in Healing Nonunion Fractures (September Page 5 of 9
6 2005). Retrieved 2/16/07 from Centers for Medicare and Medicaid Services. NCD for Osteogenic Stimulators, Manual Section Number Retrieved 2/19/07 from ncd%3a150%2e2% 3A2%3AOsteogenic+Stimulators BCBSA Medical Policy Reference Manual [Electronic Version] , 2/14/08 BCBSA Medical Policy Reference Manual [Electronic Version] , 8/14/08 Kooistra BW, Jain A, Hanson BP. (April 2009). Electrical Stimulation: Non-Unions. Indian J Orthop, 43(2), Retrieved from Foley KT, Mroz TE, Arnold PM et al. Randomized, prospective, and controlled clinical trial of pulsed electromagnetic field stimulation for cervical fusion. Spine J 2008; 8(3): BCBSA Medical Policy Reference Manual [Electronic Version] , 9/10/09 BCBSA Medical Policy Reference Manual [Electronic Version] , 9/10/09 Specialty Matched Consultant Advisory Panel review 7/2010 BCBSA Medical Policy Reference Manual [Electronic Version] , 9/16/10 BCBSA Medical Policy Reference Manual [Electronic Version] , 9/16/10 Griffin XL, Costa ML, Parsons N, Smith N. Electromagnetic field stimulation for treating delayed union or non-union of long bone fractures in adults. Cochrane Database of Systematic Reviews 2011, Abstract. Retrieved on June 20, 2011 from Specialty Matched Consultant Advisory Panel review 7/2011 BCBSA Medical Policy Reference Manual [Electronic Version] , 9/1/11 Specialty Matched Consultant Advisory Panel review 7/2012 BCBSA Medical Policy Reference Manual [Electronic Version] , 9/13/12 BCBSA Medical Policy Reference Manual [Electronic Version] , 10/11/12 Medical Director review 10/2012 Specialty Matched Consultant Advisory Panel review 7/2013 Medical Director review 7/2013 BCBSA Medical Policy Reference Manual [Electronic Version] , 10/10/13 Shi HF, Xiong J, Chen YX et al. Early application of pulsed electromagnetic field in the treatment of postoperative delayed union of long-bone fractures: a prospective randomized controlled study. BMC Musculoskelet Disord 2013; 14:35. Beck BR, Matheson GO, Bergman G et al. Do capacitively coupled electric fields accelerate tibial stress fracture healing? A randomized controlled trial. Am J Sports Med 2008; 36(3): Page 6 of 9
7 Patel DS, Roth M, Kapil N. Stress fractures: diagnosis, treatment, and prevention. Am Fam Physician Jan 1;83(1): BCBSA Medical Policy Reference Manual [Electronic Version] , 1/9/14 Specialty Matched Consultant Advisory Panel review 7/2014 Medical Director review 7/2014 BCBSA Medical Policy Reference Manual [Electronic Version] , 10/9/14 BCBSA Medical Policy Reference Manual [Electronic Version] , 12/11/14 Specialty Matched Consultant Advisory Panel review 6/2015 BCBSA Medical Policy Reference Manual [Electronic Version] , 4/14/2016 BCBSA Medical Policy Reference Manual [Electronic Version] , 4/14/2016 Specialty Matched Consultant Advisory Panel 6/2016 Policy Implementation/Update Information 4/81 Original Policy 11/81 Reaffirmed 12/83 Reaffirmed 5/99 Medical Policy Advisory Group 7/99 Reformatted, Description of Procedure or Service changed, Medical Term Definitions added. 12/99 Reaffirmed, Medical Policy Advisory Group 2/01 System coding changes. 6/01 Removed "The presence of iron-containing internal fixation devices in the area to be stimulated." from When Electrical Bone Growth Stimulation is Not Covered section of the policy. Coding format change. E0760 removed from coding. 10/01 Specialty Matched Consultant Advisory Panel - 8/01. No changes. 5/03 Specialty Matched Consultant Advisory Panel review. Revised under "when it is covered" section, number 1.b., changed the term "non-progressive" to "no progressive". Code E0760 removed from the Billing/Coding section. 3/04 Benefits Application and Billing/Coding sections updated for consistency. 8/26/04 Code descriptions removed. References added. Definition for non-union corrected in Medical Terms and definition for delayed union added. 6/2/2005 Specialty Matched Consultant Advisory Panel review on 5/23/2005. No changes made to the policy statement. SUR6240 added as key word. Page 7 of 9
8 6/18/07 Routine biennial review. Information added to Description for clarity. Specialty Matched Consultant Advisory Panel review 5/18/07. No changes to policy coverage criteria. (adn) 7/20/09 Routine biennial review. Specialty Matched Consultant Advisory Panel review 5/21/09. No change to policy statement. (adn) 8/17/10 Specialty Matched Consultant Advisory Panel review 7/2010. Removed Medical Policy number. Updated references. Updated the Policy Guidelines section. Existing medically necessary policy statements modified by adding lumbar (spine) to the statements. Steroid use added as another high-risk condition for non-fusion. Added the following criteria to When Not Covered section: 3. Implantable and semi-invasive electrical bone growth stimulators are considered investigational. 4. Invasive, semi-invasive, and noninvasive electrical stimulation are considered investigational as an adjunct to cervical fusion surgery and for failed cervical spine fusion. (mco) 8/16/11 Description section updated. References updated. Specialty Matched Consultant Advisory Panel review 7/2011. No changes to policy statements. (mco) 11/8/11 References updated. No changes to Policy Statements. (mco) 8/7/12 Specialty Matched Consultant Advisory Panel review 7/2012. No changes to Policy Statements. (mco) 11/27/12 Added related policy to Description section. Revised the following statement in the When not Covered section: 2.Investigational applications of electrical bone growth stimulation in the appendicular skeleton include, but are not limited to, immediate post-surgical treatment., and treatment of fresh fractures, delayed union or failed arthrodesis. Delayed union is defined as a decelerating fracture healing process, as identified by serial x-rays. 3. Semi-invasive electrical bone growth stimulators are considered investigational as an adjunct to lumbar fusion and for failed lumbar fusion. References updated. Medical Director review. Policy noticed on 11/27/12 for effective date 2/26/13. (mco) 7/30/13 Specialty Matched Consultant Advisory Panel review 7/2013. Medical Director review 7/2013. No changes to Policy Statements. (mco) 11/26/13 References updated. No changes to Policy Statements. (mco) 2/25/14 Description section updated. Policy Guidelines and References updated. Use of electrical bone growth stimulation for stress fractures and arthrodesis added to When not Covered section. When Covered section updated to include the following criterion: The patient can be adequately immobilized and is of an age likely to comply with non-weight bearing for fractures of the pelvis and lower extremities. Medical Director review 2/2014. Policy noticed on 2/25/14 for effective date 4/29/14. (mco) 8/12/14 Specialty Matched Consultant Advisory Panel review 7/2014. Medical Director review 7/2014. No changes to Policy Statements. (mco) 1/27/15 Reference added. Related policies added. (sk) 2/24/15 Reference added. (sk) 7/28/15 Specialty Matched Consultant Advisory Panel review 6/24/2015. (sk) 5/31/16 References added. Policy Guidelines updated. (sk) 7/26/16 Specialty Matched Consultant Advisory Panel review 6/29/2016. (sk) Page 8 of 9
9 Medical policy is not an authorization, certification, explanation of benefits or a contract. Benefits and eligibility are determined before medical guidelines and payment guidelines are applied. Benefits are determined by the group contract and subscriber certificate that is in effect at the time services are rendered. This document is solely provided for informational purposes only and is based on research of current medical literature and review of common medical practices in the treatment and diagnosis of disease. Medical practices and knowledge are constantly changing and BCBSNC reserves the right to review and revise its medical policies periodically. Page 9 of 9
Corporate Medical Policy
 Corporate Medical Policy Diagnosis and Treatment of Sacroiliac Joint Pain File Name: Origination: Last CAP Review: Next CAP Review: Last Review: diagnosis_and_treatment_of_sacroiliac_joint_pain 8/2010
Corporate Medical Policy Diagnosis and Treatment of Sacroiliac Joint Pain File Name: Origination: Last CAP Review: Next CAP Review: Last Review: diagnosis_and_treatment_of_sacroiliac_joint_pain 8/2010
Corporate Medical Policy Continuous Passive Motion in the Home Setting
 Corporate Medical Policy Continuous Passive Motion in the Home Setting File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_passive_motion_in_the_home_setting 9/1993 6/2016
Corporate Medical Policy Continuous Passive Motion in the Home Setting File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_passive_motion_in_the_home_setting 9/1993 6/2016
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: spinal_cord_stimulation 3/1980 10/2015 10/2016 10/2015 Description of Procedure or Service Spinal cord stimulation
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: spinal_cord_stimulation 3/1980 10/2015 10/2016 10/2015 Description of Procedure or Service Spinal cord stimulation
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: vagus_nerve_stimulation 6/1998 5/2016 5/2017 5/2016 Description of Procedure or Service Stimulation of the
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: vagus_nerve_stimulation 6/1998 5/2016 5/2017 5/2016 Description of Procedure or Service Stimulation of the
Corporate Medical Policy
 Corporate Medical Policy Electrical Stimulation for the Treatment of Arthritis File Name: Origination: Last CAP Review: Next CAP Review: Last Review: electrical_stimulation_for_the_treatment_of_arthritis
Corporate Medical Policy Electrical Stimulation for the Treatment of Arthritis File Name: Origination: Last CAP Review: Next CAP Review: Last Review: electrical_stimulation_for_the_treatment_of_arthritis
Corporate Medical Policy
 Corporate Medical Policy Proteomics-based Testing Related to Ovarian Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteomics_based_testing_related_to_ovarian_cancer 7/2010
Corporate Medical Policy Proteomics-based Testing Related to Ovarian Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteomics_based_testing_related_to_ovarian_cancer 7/2010
Bone Growth Stimulators
 Medical Coverage Policy Page: 1 of 15 Change Summary: Updated Coverage Determination, Coverage Limitations, Background, Medical Terms, References Disclaimer Description Coverage Determination Background
Medical Coverage Policy Page: 1 of 15 Change Summary: Updated Coverage Determination, Coverage Limitations, Background, Medical Terms, References Disclaimer Description Coverage Determination Background
Corporate Medical Policy
 Corporate Medical Policy Continuous Monitoring of Glucose in the Interstitial Fluid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_monitoring_of_glucose_in_the_interstitial_fluid
Corporate Medical Policy Continuous Monitoring of Glucose in the Interstitial Fluid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_monitoring_of_glucose_in_the_interstitial_fluid
Kidney Transplantation and Cancer - A Review
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: renal_kidney_transplantation 4/1980 4/2016 4/2017 4/2016 Description of Procedure or Service A kidney transplant
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: renal_kidney_transplantation 4/1980 4/2016 4/2017 4/2016 Description of Procedure or Service A kidney transplant
Corporate Reimbursement Policy
 Corporate Reimbursement Policy Co-Surgeon, Assistant Surgeon, Team Surgeon and Assistant-at-Surgery Guidelines File Name: Origination: Last Review: Next Review: co-surgeon_assistant_surgeon_and_assistant_at_surgery_guidelines
Corporate Reimbursement Policy Co-Surgeon, Assistant Surgeon, Team Surgeon and Assistant-at-Surgery Guidelines File Name: Origination: Last Review: Next Review: co-surgeon_assistant_surgeon_and_assistant_at_surgery_guidelines
Corporate Reimbursement Policy
 Corporate Reimbursement Policy Multiple Surgical Procedure Guidelines for Professional Providers File Name: Origination: Last Review: Next Review: multiple_surgical_procedure_guidelines_for_professional_providers
Corporate Reimbursement Policy Multiple Surgical Procedure Guidelines for Professional Providers File Name: Origination: Last Review: Next Review: multiple_surgical_procedure_guidelines_for_professional_providers
Corporate Medical Policy
 Corporate Medical Policy Quantitative Electroencephalography as a Diagnostic Aid for Attention File Name: Origination: Last CAP Review: Next CAP Review: Last Review: quantitative_electroencephalography_as_a_diagnostic_aid_for_adhd
Corporate Medical Policy Quantitative Electroencephalography as a Diagnostic Aid for Attention File Name: Origination: Last CAP Review: Next CAP Review: Last Review: quantitative_electroencephalography_as_a_diagnostic_aid_for_adhd
Corporate Medical Policy Implantation of Intrastromal Corneal Ring Segments
 Corporate Medical Policy Implantation of Intrastromal Corneal Ring Segments File Name: Origination: Last CAP Review: Next CAP Review: Last Review: implantation_of_intrastromal_corneal_ring_segments 8/2008
Corporate Medical Policy Implantation of Intrastromal Corneal Ring Segments File Name: Origination: Last CAP Review: Next CAP Review: Last Review: implantation_of_intrastromal_corneal_ring_segments 8/2008
Corporate Medical Policy Cord Blood as a Source of Stem Cells
 Corporate Medical Policy Cord Blood as a Source of Stem Cells File Name: Origination: Last CAP Review: Next CAP Review: Last Review cord_blood_as_a_source_of_stem_cells 2/2001 3/2015 3/2016 3/2015 Description
Corporate Medical Policy Cord Blood as a Source of Stem Cells File Name: Origination: Last CAP Review: Next CAP Review: Last Review cord_blood_as_a_source_of_stem_cells 2/2001 3/2015 3/2016 3/2015 Description
Corporate Medical Policy Spinal Manipulation under Anesthesia
 Corporate Medical Policy Spinal Manipulation under Anesthesia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: spinal_manipulation_under_anesthesia 5/1998 1/2015 1/2016 1/2015 Description
Corporate Medical Policy Spinal Manipulation under Anesthesia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: spinal_manipulation_under_anesthesia 5/1998 1/2015 1/2016 1/2015 Description
Corporate Medical Policy Orthotics
 Corporate Medical Policy Orthotics File Name: Origination: Last CAP Review: Next CAP Review: Last Review: orthotics 6/1990 2/2016 2/2017 2/2016 Description of Procedure or Service An orthotic (orthosis)
Corporate Medical Policy Orthotics File Name: Origination: Last CAP Review: Next CAP Review: Last Review: orthotics 6/1990 2/2016 2/2017 2/2016 Description of Procedure or Service An orthotic (orthosis)
Corporate Medical Policy Laser Treatment of Port Wine Stains
 Corporate Medical Policy Laser Treatment of Port Wine Stains File Name: Origination: Last CAP Review: Next CAP Review: Last Review: laser_treatment_of_port_wine_stains 9/2010 9/2015 9/2016 9/2015 Description
Corporate Medical Policy Laser Treatment of Port Wine Stains File Name: Origination: Last CAP Review: Next CAP Review: Last Review: laser_treatment_of_port_wine_stains 9/2010 9/2015 9/2016 9/2015 Description
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: skilled_nursing_facility_care 02/2008 2/2015 2/2016 2/2015 Description of Procedure or Service A skilled
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: skilled_nursing_facility_care 02/2008 2/2015 2/2016 2/2015 Description of Procedure or Service A skilled
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: testing_serum_vitamin_d_levels 9/2015 2/2016 2/2017 2/2016 Description of Procedure or Service Vitamin D,
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: testing_serum_vitamin_d_levels 9/2015 2/2016 2/2017 2/2016 Description of Procedure or Service Vitamin D,
Corporate Medical Policy Ambulatory Blood Pressure Monitoring
 Corporate Medical Policy Ambulatory Blood Pressure Monitoring File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ambulatory_blood_pressure_monitoring 7/1982 4/2016 4/2017 4/2016 Description
Corporate Medical Policy Ambulatory Blood Pressure Monitoring File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ambulatory_blood_pressure_monitoring 7/1982 4/2016 4/2017 4/2016 Description
Corporate Medical Policy Computer Assisted Surgical Navigational Orthopedic Procedures
 Corporate Medical Policy Computer Assisted Surgical Navigational Orthopedic File Name: Origination: Last CAP Review: Next CAP Review: Last Review: computer_assisted_surgical_navigational_orthopedic_procedures
Corporate Medical Policy Computer Assisted Surgical Navigational Orthopedic File Name: Origination: Last CAP Review: Next CAP Review: Last Review: computer_assisted_surgical_navigational_orthopedic_procedures
Corporate Medical Policy Saturation Biopsy for Diagnosis and Staging of Prostate Cancer
 Corporate Medical Policy Saturation Biopsy for Diagnosis and Staging of Prostate Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: saturation_biopsy_for_diagnosis_and_staging_of_prostate_cancer
Corporate Medical Policy Saturation Biopsy for Diagnosis and Staging of Prostate Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: saturation_biopsy_for_diagnosis_and_staging_of_prostate_cancer
Corporate Medical Policy
 Corporate Medical Policy Intensity Modulated Radiation Therapy (IMRT) of Head and Neck File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_head_and_neck
Corporate Medical Policy Intensity Modulated Radiation Therapy (IMRT) of Head and Neck File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_head_and_neck
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: private_duty_nursing_services 11/3/2005 2/2016 2/2017 2/2016 Description of Procedure or Service Private
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: private_duty_nursing_services 11/3/2005 2/2016 2/2017 2/2016 Description of Procedure or Service Private
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: immune_cell_function_assay 11/2009 3/2016 3/2017 3/2016 Description of Procedure or Service Careful monitoring
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: immune_cell_function_assay 11/2009 3/2016 3/2017 3/2016 Description of Procedure or Service Careful monitoring
Corporate Medical Policy
 Corporate Medical Policy Pelvic Floor Stimulation as a Treatment of Urinary and Fecal File Name: Origination: Last CAP Review: Next CAP Review: Last Review: pelvic_floor_stimulation_as_a_treatment_of_urinary_and_fecal_incontinence
Corporate Medical Policy Pelvic Floor Stimulation as a Treatment of Urinary and Fecal File Name: Origination: Last CAP Review: Next CAP Review: Last Review: pelvic_floor_stimulation_as_a_treatment_of_urinary_and_fecal_incontinence
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: microwave_tumor_ablation 12/2011 11/2015 11/2016 11/2015 Description of Procedure or Service Microwave ablation
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: microwave_tumor_ablation 12/2011 11/2015 11/2016 11/2015 Description of Procedure or Service Microwave ablation
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: adoptive_immunotherapy 11/1993 3/2016 3/2017 3/2016 Description of Procedure or Service The spontaneous regression
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: adoptive_immunotherapy 11/1993 3/2016 3/2017 3/2016 Description of Procedure or Service The spontaneous regression
Corporate Medical Policy Cord Blood as a Source of Stem Cells
 Corporate Medical Policy Cord Blood as a Source of Stem Cells File Name: Origination: Last CAP Review: Next CAP Review: Last Review cord_blood_as_a_source_of_stem_cells 2/2001 3/2015 3/2016 3/2015 Description
Corporate Medical Policy Cord Blood as a Source of Stem Cells File Name: Origination: Last CAP Review: Next CAP Review: Last Review cord_blood_as_a_source_of_stem_cells 2/2001 3/2015 3/2016 3/2015 Description
Corporate Reimbursement Policy
 Corporate Reimbursement Policy Guidelines for Global Maternity Reimbursement File Name: Origination: Last Review: Next Review: guidelines_for_global_maternity_reimbursement 10/2003 7/2016 7/2017 Description
Corporate Reimbursement Policy Guidelines for Global Maternity Reimbursement File Name: Origination: Last Review: Next Review: guidelines_for_global_maternity_reimbursement 10/2003 7/2016 7/2017 Description
Clinical Policy Guideline
 Clinical Policy Guideline Policy Title: Bone Density Testing Policy No: B0215A.00 Effective Date: 01/01/15 Date Reviewed: 03/25/15 I. DEFINITION/BACKGROUND Bone density testing is used to estimate the
Clinical Policy Guideline Policy Title: Bone Density Testing Policy No: B0215A.00 Effective Date: 01/01/15 Date Reviewed: 03/25/15 I. DEFINITION/BACKGROUND Bone density testing is used to estimate the
Corporate Medical Policy Septoplasty
 Corporate Medical Policy Septoplasty File Name: Origination: Last CAP Review: Next CAP Review: Last Review: septoplasty 4/1999 8/2015 8/2016 8/2015 Description of Procedure or Service There are many potential
Corporate Medical Policy Septoplasty File Name: Origination: Last CAP Review: Next CAP Review: Last Review: septoplasty 4/1999 8/2015 8/2016 8/2015 Description of Procedure or Service There are many potential
Corporate Medical Policy Intensity-Modulated Radiation Therapy (IMRT) of the Prostate
 Corporate Medical Policy Intensity-Modulated Radiation Therapy (IMRT) of the Prostate File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_the_prostate
Corporate Medical Policy Intensity-Modulated Radiation Therapy (IMRT) of the Prostate File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_the_prostate
Corporate Medical Policy Durable Medical Equipment (DME)
 Corporate Medical Policy Durable Medical Equipment (DME) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: durable_medical_equipment_(dme) 1/2000 9/2015 9/2016 9/2015 Description of
Corporate Medical Policy Durable Medical Equipment (DME) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: durable_medical_equipment_(dme) 1/2000 9/2015 9/2016 9/2015 Description of
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: infusion_therapy_in_the_home 3/1998 2/2016 2/2017 2/2016 Description of Procedure or Service Home infusion
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: infusion_therapy_in_the_home 3/1998 2/2016 2/2017 2/2016 Description of Procedure or Service Home infusion
Corporate Medical Policy Genetic Testing for Fanconi Anemia
 Corporate Medical Policy Genetic Testing for Fanconi Anemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_fanconi_anemia 03/2015 3/2016 3/2017 3/2016 Description
Corporate Medical Policy Genetic Testing for Fanconi Anemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_fanconi_anemia 03/2015 3/2016 3/2017 3/2016 Description
Corporate Medical Policy
 File Name: anesthesia_services Origination: 8/2007 Last CAP Review: 1/2016 Next CAP Review: 1/2017 Last Review: 1/2016 Corporate Medical Policy Description of Procedure or Service There are three main
File Name: anesthesia_services Origination: 8/2007 Last CAP Review: 1/2016 Next CAP Review: 1/2017 Last Review: 1/2016 Corporate Medical Policy Description of Procedure or Service There are three main
Corporate Medical Policy Genetic Testing for Alpha-1 Antitrypsin Deficiency
 Corporate Medical Policy Genetic Testing for Alpha-1 Antitrypsin Deficiency File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_alpha_1_antitrypsin_deficiency 5/2012
Corporate Medical Policy Genetic Testing for Alpha-1 Antitrypsin Deficiency File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_alpha_1_antitrypsin_deficiency 5/2012
Mitchell S. Fourman M.Phil Eugene Borst BS Eric Bogner, MD S. Robert Rozbruch MD Austin T. Fragomen, MD
 Post-Operative CT Measurements Accurately Predict the Need for Clinical Intervention Retrospective Findings to Support Prospective Clinical Trial Protocol Mitchell S. Fourman M.Phil Eugene Borst BS Eric
Post-Operative CT Measurements Accurately Predict the Need for Clinical Intervention Retrospective Findings to Support Prospective Clinical Trial Protocol Mitchell S. Fourman M.Phil Eugene Borst BS Eric
National Medical Policy
 National Medical Policy Subject: Bone Growth Stimulators, Electrical and Ultrasonic Policy Number: NMP194 Effective Date*: January 2005 Updated: May 2015 This National Medical Policy is subject to the
National Medical Policy Subject: Bone Growth Stimulators, Electrical and Ultrasonic Policy Number: NMP194 Effective Date*: January 2005 Updated: May 2015 This National Medical Policy is subject to the
Corporate Medical Policy Reconstructive Eyelid Surgery and Brow Lift
 Corporate Medical Policy Reconstructive Eyelid Surgery and Brow Lift File Name: Origination: Last CAP Review: Next CAP Review: Last Review: reconstructive_eyelid_surgery_and_brow_lift 1/2000 9/2015 9/2016
Corporate Medical Policy Reconstructive Eyelid Surgery and Brow Lift File Name: Origination: Last CAP Review: Next CAP Review: Last Review: reconstructive_eyelid_surgery_and_brow_lift 1/2000 9/2015 9/2016
Corporate Reimbursement Policy
 Corporate Reimbursement Policy Code Bundling Rules Not Addressed in ClaimCheck or Correct Coding Initiative File Name: code_bundling_rules_not_addressed_in_claim_check Origination: 6/2004 Last Review:
Corporate Reimbursement Policy Code Bundling Rules Not Addressed in ClaimCheck or Correct Coding Initiative File Name: code_bundling_rules_not_addressed_in_claim_check Origination: 6/2004 Last Review:
Corporate Medical Policy Electromagnetic Navigation Bronchoscopy
 Corporate Medical Policy Electromagnetic Navigation Bronchoscopy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: electromagnetic_navigation_bronchoscopy 1/2010 3/2016 3/2017 3/2016
Corporate Medical Policy Electromagnetic Navigation Bronchoscopy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: electromagnetic_navigation_bronchoscopy 1/2010 3/2016 3/2017 3/2016
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: lumbar_spine_fusion_surgery 9/2010 5/2015 5/2016 5/2015 Description of Procedure or Service Low back pain
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: lumbar_spine_fusion_surgery 9/2010 5/2015 5/2016 5/2015 Description of Procedure or Service Low back pain
intensity_modulated_radiation_therapy_imrt_of_abdomen_and_pelvis 11/2009 5/2016 5/2017 5/2016
 Corporate Medical Policy Intensity Modulated Radiation Therapy (IMRT) of Abdomen File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_abdomen_and_pelvis
Corporate Medical Policy Intensity Modulated Radiation Therapy (IMRT) of Abdomen File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_abdomen_and_pelvis
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cll_and_sll
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cll_and_sll
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hyperbaric_oxygen_therapy 4/1980 1/2016 1/2017 1/2016 Description of Procedure or Service Hyperbaric oxygen
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hyperbaric_oxygen_therapy 4/1980 1/2016 1/2017 1/2016 Description of Procedure or Service Hyperbaric oxygen
Corporate Medical Policy Allergy Immunotherapy (Desensitization)
 Corporate Medical Policy Allergy Immunotherapy (Desensitization) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: allergy_immunotherapy 7/1979 11/2014 11/2015 10/2015 Description
Corporate Medical Policy Allergy Immunotherapy (Desensitization) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: allergy_immunotherapy 7/1979 11/2014 11/2015 10/2015 Description
Information for the Patient About Surgical
 Information for the Patient About Surgical Decompression and Stabilization of the Spine Aging and the Spine Daily wear and tear, along with disc degeneration due to aging and injury, are common causes
Information for the Patient About Surgical Decompression and Stabilization of the Spine Aging and the Spine Daily wear and tear, along with disc degeneration due to aging and injury, are common causes
Fracture Care Coding September 28, 2011
 Fracture Care Coding September 28, 2011 Julie Edens Leu, CPC, CPCO, CPMA, CPC-I 1 Disclaimer Every reasonable effort has been made to ensure that the educational material provided today is accurate and
Fracture Care Coding September 28, 2011 Julie Edens Leu, CPC, CPCO, CPMA, CPC-I 1 Disclaimer Every reasonable effort has been made to ensure that the educational material provided today is accurate and
James A. Sanfilippo, M.D. CONSENT FOR SPINAL SURGERY PATIENT: DATE:
 James A. Sanfilippo, M.D. CONSENT FOR SPINAL SURGERY PATIENT: DATE: 1. I have been strongly advised to carefully read and consider this operative permit. I realize that it is important that I understand
James A. Sanfilippo, M.D. CONSENT FOR SPINAL SURGERY PATIENT: DATE: 1. I have been strongly advised to carefully read and consider this operative permit. I realize that it is important that I understand
Corporate Medical Policy Brachytherapy Treatment of Breast Cancer
 Corporate Medical Policy Brachytherapy Treatment of Breast Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: brachytherapy_treatment_of_breast_cancer 7/1996 5/2015 5/2016 5/2015
Corporate Medical Policy Brachytherapy Treatment of Breast Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: brachytherapy_treatment_of_breast_cancer 7/1996 5/2015 5/2016 5/2015
.org. Posterior Tibial Tendon Dysfunction. Anatomy. Cause. Symptoms
 Posterior Tibial Tendon Dysfunction Page ( 1 ) Posterior tibial tendon dysfunction is one of the most common problems of the foot and ankle. It occurs when the posterior tibial tendon becomes inflamed
Posterior Tibial Tendon Dysfunction Page ( 1 ) Posterior tibial tendon dysfunction is one of the most common problems of the foot and ankle. It occurs when the posterior tibial tendon becomes inflamed
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF).
 Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
DUKE ORTHOPAEDIC SURGERY GOALS AND OBJECTIVES SPINE SERVICE
 GOALS AND OBJECTIVES PATIENT CARE Able to perform a complete musculoskeletal and neurologic examination on the patient including cervical spine, thoracic spine, and lumbar spine. The neurologic examination
GOALS AND OBJECTIVES PATIENT CARE Able to perform a complete musculoskeletal and neurologic examination on the patient including cervical spine, thoracic spine, and lumbar spine. The neurologic examination
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Interspinous Fixation (Fusion) Devices Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Interspinous Fixation (Fusion) Devices Lumbar Spine
Interspinous Fixation (Fusion) Devices Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Interspinous Fixation (Fusion) Devices Lumbar Spine
(English) NEXUS SPINE SPACER SYSTEM
 (English) NEXUS SPINE SPACER SYSTEM INDICATIONS FOR USE NEXUS Spine Spacer System, a GEO Structure is indicated for use in the thoraco-lumbar spine (i.e., T1 to L5) to replace a diseased vertebral body
(English) NEXUS SPINE SPACER SYSTEM INDICATIONS FOR USE NEXUS Spine Spacer System, a GEO Structure is indicated for use in the thoraco-lumbar spine (i.e., T1 to L5) to replace a diseased vertebral body
Corporate Medical Policy
 Corporate Medical Policy Intensity Modulated Radiation Therapy for Tumors of the Central File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_for_tumors
Corporate Medical Policy Intensity Modulated Radiation Therapy for Tumors of the Central File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_for_tumors
REPUTATION MANAGEMENT: SPINAL FUSION SURGERY
 REPUTATION MANAGEMENT: SPINAL FUSION SURGERY Meaningful and Positive Progress in Spinal Fusion Results: A Journey towards Excellence Paul J. Slosar, M.D. SpineCare Medical Group As a practicing spine surgeon,
REPUTATION MANAGEMENT: SPINAL FUSION SURGERY Meaningful and Positive Progress in Spinal Fusion Results: A Journey towards Excellence Paul J. Slosar, M.D. SpineCare Medical Group As a practicing spine surgeon,
Name of Policy: Reconstructive versus Cosmetic Surgery
 Name of Policy: Reconstructive versus Cosmetic Surgery Policy #: 106 Latest Review Date: February 2010 Category: Administrative Policy Grade: Background/Definitions: As a general rule, benefits are payable
Name of Policy: Reconstructive versus Cosmetic Surgery Policy #: 106 Latest Review Date: February 2010 Category: Administrative Policy Grade: Background/Definitions: As a general rule, benefits are payable
KYPHON. Reimbursement Guide. Physician Reimbursement. Balloon Kyphoplasty Procedure. ICD-9-CM Diagnosis Codes. CPT Codes and Payment
 KYPHON Balloon Kyphoplasty Procedure Reimbursement Guide ICD-9-CM Diagnosis Codes Providers should report the ICD-9-CM diagnosis code that most accurately describes the patient s condition. Please refer
KYPHON Balloon Kyphoplasty Procedure Reimbursement Guide ICD-9-CM Diagnosis Codes Providers should report the ICD-9-CM diagnosis code that most accurately describes the patient s condition. Please refer
Corporate Medical Policy
 Corporate Medical Policy Computed Tomography to Detect Coronary Artery Calcification File Name: computed_tomography_to_detect_coronary_artery_calcification Origination: 3/1994 Last CAP Review 11/2014 Next
Corporate Medical Policy Computed Tomography to Detect Coronary Artery Calcification File Name: computed_tomography_to_detect_coronary_artery_calcification Origination: 3/1994 Last CAP Review 11/2014 Next
Ruby O Brochta-Woodward BSN, CPC, CCS-P, COSC, ACS-OR April 17, 2013 AAPC National Conference Orlando, FL
 Ruby O Brochta-Woodward BSN, CPC, CCS-P, COSC, ACS-OR April 17, 2013 AAPC National Conference Orlando, FL Fracture coding, what do you need to know? Types of fractures Types of treatment Fracture care
Ruby O Brochta-Woodward BSN, CPC, CCS-P, COSC, ACS-OR April 17, 2013 AAPC National Conference Orlando, FL Fracture coding, what do you need to know? Types of fractures Types of treatment Fracture care
Degenerative Spine Solutions
 Degenerative Spine Solutions The Backbone for Your Surgical Needs Aesculap Spine Backbone for Your Degenerative Spine Needs Comprehensive operative solutions, unique product technology and world-class
Degenerative Spine Solutions The Backbone for Your Surgical Needs Aesculap Spine Backbone for Your Degenerative Spine Needs Comprehensive operative solutions, unique product technology and world-class
Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF).
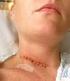 Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra,
Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra,
Using Predictive Analytics to Build a World Class Healthcare System
 Using Predictive Analytics to Build a World Class Healthcare System Swati Abbott CEO, Blue Health Intelligence Doug Porter SVP and CIO, Blue Cross/Blue Shield Association Using Predictive Analytics to
Using Predictive Analytics to Build a World Class Healthcare System Swati Abbott CEO, Blue Health Intelligence Doug Porter SVP and CIO, Blue Cross/Blue Shield Association Using Predictive Analytics to
Issued and entered this _6th_ day of October 2010 by Ken Ross Commissioner ORDER I PROCEDURAL BACKGROUND
 STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX Petitioner
STATE OF MICHIGAN DEPARTMENT OF ENERGY, LABOR & ECONOMIC GROWTH OFFICE OF FINANCIAL AND INSURANCE REGULATION Before the Commissioner of Financial and Insurance Regulation In the matter of XXXXX Petitioner
.org. Fractures of the Thoracic and Lumbar Spine. Cause. Description
 Fractures of the Thoracic and Lumbar Spine Page ( 1 ) Spinal fractures can vary widely in severity. While some fractures are very serious injuries that require emergency treatment, other fractures can
Fractures of the Thoracic and Lumbar Spine Page ( 1 ) Spinal fractures can vary widely in severity. While some fractures are very serious injuries that require emergency treatment, other fractures can
Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery
 Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery 221 Madison Ave Morristown, New Jersey 07960 (973) 538 4444 Fax (973) 538 0420 Patient
Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery 221 Madison Ave Morristown, New Jersey 07960 (973) 538 4444 Fax (973) 538 0420 Patient
Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances?
 Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances? Do you experience weakness, tingling, numbness, stiffness, or cramping in your legs, buttocks or
Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances? Do you experience weakness, tingling, numbness, stiffness, or cramping in your legs, buttocks or
Wrist and Hand. Patient Information Guide to Bone Fracture, Bone Reconstruction and Bone Fusion: Fractures of the Wrist and Hand: Carpal bones
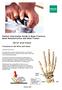 Patient Information Guide to Bone Fracture, Bone Reconstruction and Bone Fusion: Wrist and Hand Fractures of the Wrist and Hand: Fractures of the wrist The wrist joint is made up of the two bones in your
Patient Information Guide to Bone Fracture, Bone Reconstruction and Bone Fusion: Wrist and Hand Fractures of the Wrist and Hand: Fractures of the wrist The wrist joint is made up of the two bones in your
Corporate Medical Policy Urinary Tumor Markers for Bladder Cancer
 Corporate Medical Policy Urinary Tumor Markers for Bladder Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: urinary_tumor_markers_for_bladder_cancer 5/2011 11/2015 11/2016
Corporate Medical Policy Urinary Tumor Markers for Bladder Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: urinary_tumor_markers_for_bladder_cancer 5/2011 11/2015 11/2016
Osteoarthritis progresses slowly and the pain and stiffness it causes worsens over time.
 Arthritis of the Foot and Ankle Arthritis is the leading cause of disability in the United States. It can occur at any age, and literally means "pain within a joint." As a result, arthritis is a term used
Arthritis of the Foot and Ankle Arthritis is the leading cause of disability in the United States. It can occur at any age, and literally means "pain within a joint." As a result, arthritis is a term used
Ambulatory Surgery Center Coding and Payment Guide 2015
 Targeted Drug Delivery Ambulatory Surgery Center Coding and Payment Guide 2015 Flowonix Medical has compiled this coding information for your convenience. This information is gathered from third party
Targeted Drug Delivery Ambulatory Surgery Center Coding and Payment Guide 2015 Flowonix Medical has compiled this coding information for your convenience. This information is gathered from third party
ICD 10 CM IMPLEMENTATION DATE OCT 1, 2015
 Presented by: Teri Romano, RN, MBA, CPC, CMDP ICD 10 CM IMPLEMENTATION DATE OCT 1, 2015 Source: http://journal.ahima.org/2015/02/04/us house committee to hold hearing on icd 10 implementation/ 2 2015 Web_Non
Presented by: Teri Romano, RN, MBA, CPC, CMDP ICD 10 CM IMPLEMENTATION DATE OCT 1, 2015 Source: http://journal.ahima.org/2015/02/04/us house committee to hold hearing on icd 10 implementation/ 2 2015 Web_Non
testosterone_pellet_implantation_for_androgen_deficiency_in_men 10/2015 N/A 11/2016 10/2015 This policy is not effective until December 30, 2015
 Corporate Medical Policy Testosterone Pellet Implantation for Androgen Deficiency in File Name: Origination: Last CAP Review: Next CAP Review: Last Review: testosterone_pellet_implantation_for_androgen_deficiency_in_men
Corporate Medical Policy Testosterone Pellet Implantation for Androgen Deficiency in File Name: Origination: Last CAP Review: Next CAP Review: Last Review: testosterone_pellet_implantation_for_androgen_deficiency_in_men
September 25, 2014. Dear Ms. McCoy:
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 September 25, 2014 Biomet
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 September 25, 2014 Biomet
SPINAL FUSION. North American Spine Society Public Education Series
 SPINAL FUSION North American Spine Society Public Education Series WHAT IS SPINAL FUSION? The spine is made up of a series of bones called vertebrae ; between each vertebra are strong connective tissues
SPINAL FUSION North American Spine Society Public Education Series WHAT IS SPINAL FUSION? The spine is made up of a series of bones called vertebrae ; between each vertebra are strong connective tissues
Minimally Invasive Spine Surgery For Your Patients
 Minimally Invasive Spine Surgery For Your Patients Lukas P. Zebala, M.D. Assistant Professor Orthopaedic and Neurological Spine Surgery Department of Orthopaedic Surgery Washington University School of
Minimally Invasive Spine Surgery For Your Patients Lukas P. Zebala, M.D. Assistant Professor Orthopaedic and Neurological Spine Surgery Department of Orthopaedic Surgery Washington University School of
Adult Forearm Fractures
 Adult Forearm Fractures Your forearm is made up of two bones, the radius and ulna. In most cases of adult forearm fractures, both bones are broken. Fractures of the forearm can occur near the wrist at
Adult Forearm Fractures Your forearm is made up of two bones, the radius and ulna. In most cases of adult forearm fractures, both bones are broken. Fractures of the forearm can occur near the wrist at
Spinal Surgery Functional Status and Quality of Life Outcome Specifications 2015 (01/01/2013 to 12/31/2013 Dates of Procedure) September 2014
 Description Methodology For patients ages 18 years and older who undergo a lumbar discectomy/laminotomy or lumbar spinal fusion procedure during the measurement year, the following measures will be calculated:
Description Methodology For patients ages 18 years and older who undergo a lumbar discectomy/laminotomy or lumbar spinal fusion procedure during the measurement year, the following measures will be calculated:
Mississippi Medicaid. Provider Reference Guide. For Part 203. Physician Services
 Mississippi Medicaid Provider Reference Guide For Part 203 Physician Services This is a companion document to the Mississippi Administrative Code Title 23 and must be utilized as a reference only. January
Mississippi Medicaid Provider Reference Guide For Part 203 Physician Services This is a companion document to the Mississippi Administrative Code Title 23 and must be utilized as a reference only. January
Corporate Medical Policy
 Corporate Medical Policy Ado-Trastuzumab Emtansine (Trastuzumab-DM1) for Treatment of File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ado_trastuzumab_emtansine_(trastuzumab-dm1)_for_treatment_of_her-2_positivemalignancies
Corporate Medical Policy Ado-Trastuzumab Emtansine (Trastuzumab-DM1) for Treatment of File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ado_trastuzumab_emtansine_(trastuzumab-dm1)_for_treatment_of_her-2_positivemalignancies
MEDICAL POLICY POLICY TITLE DENTAL AND ORAL SURGERY SERVICES AFTER AN ACCIDENT POLICY NUMBER MP- 1.108
 Original Issue Date (Created): July 1, 2005 Most Recent Review Date (Revised): Effective Date: June 29, 2010 May 25, 2011- RETIRED I. POLICY II. Dental and/or oral surgery services (on a limited basis)
Original Issue Date (Created): July 1, 2005 Most Recent Review Date (Revised): Effective Date: June 29, 2010 May 25, 2011- RETIRED I. POLICY II. Dental and/or oral surgery services (on a limited basis)
4052 Slimplicity Tech final_layout 1 6/29/15 3:29 PM Page 2 Surgical Technique
 Surgical Technique TABLE OF CONTENTS Slimplicity Anterior Cervical Plate System Overview 2 Indications 2 Implants 3 Instruments 4 Surgical Technique 6 1. Patient Positioning and Approach 6 2. Plate Selection
Surgical Technique TABLE OF CONTENTS Slimplicity Anterior Cervical Plate System Overview 2 Indications 2 Implants 3 Instruments 4 Surgical Technique 6 1. Patient Positioning and Approach 6 2. Plate Selection
Magnetic Resonance Imaging
 Magnetic Resonance Imaging North American Spine Society Public Education Series What Is Magnetic Resonance Imaging (MRI)? Magnetic resonance imaging (MRI) is a valuable diagnostic study that has been used
Magnetic Resonance Imaging North American Spine Society Public Education Series What Is Magnetic Resonance Imaging (MRI)? Magnetic resonance imaging (MRI) is a valuable diagnostic study that has been used
Patient Guide to Lower Back Surgery
 The following is a sampling of products offered by Zimmer Spine for use in Open Lumbar Fusion procedures. Patient Guide to Lower Back Surgery Open Lumbar Fusion Dynesys The Dynesys Dynamic Stabilization
The following is a sampling of products offered by Zimmer Spine for use in Open Lumbar Fusion procedures. Patient Guide to Lower Back Surgery Open Lumbar Fusion Dynesys The Dynesys Dynamic Stabilization
Measure Title X RAY PRIOR TO MRI OR CAT SCAN IN THE EVAULATION OF LOWER BACK PAIN Disease State Back pain Indicator Classification Utilization
 Client HMSA: PQSR 2009 Measure Title X RAY PRIOR TO MRI OR CAT SCAN IN THE EVAULATION OF LOWER BACK PAIN Disease State Back pain Indicator Classification Utilization Strength of Recommendation Organizations
Client HMSA: PQSR 2009 Measure Title X RAY PRIOR TO MRI OR CAT SCAN IN THE EVAULATION OF LOWER BACK PAIN Disease State Back pain Indicator Classification Utilization Strength of Recommendation Organizations
How To Cover Occupational Therapy
 Guidelines for Medical Necessity Determination for Occupational Therapy These Guidelines for Medical Necessity Determination (Guidelines) identify the clinical information MassHealth needs to determine
Guidelines for Medical Necessity Determination for Occupational Therapy These Guidelines for Medical Necessity Determination (Guidelines) identify the clinical information MassHealth needs to determine
Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization
 APPROVED IRN10389-1.1-04 Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization Because it involves accessing the spine through the patient s side, the Direct Lateral approach
APPROVED IRN10389-1.1-04 Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization Because it involves accessing the spine through the patient s side, the Direct Lateral approach
visualized. The correct level is then identified again. With the use of a microscope and
 SURGERY FOR SPINAL STENOSIS Laminectomy A one inch (or longer for extensive stenosis) incision is made in the middle of the back over the effected region of the spine. The muscles over the bone are moved
SURGERY FOR SPINAL STENOSIS Laminectomy A one inch (or longer for extensive stenosis) incision is made in the middle of the back over the effected region of the spine. The muscles over the bone are moved
White Paper: Reducing Utilization Concerns Regarding Spinal Fusion and Artificial Disc Implants
 White Paper: Reducing Utilization Concerns Regarding Spinal Fusion and Artificial Disc Implants For Health Plans, Medical Management Organizations and TPAs Executive Summary Back pain is one of the most
White Paper: Reducing Utilization Concerns Regarding Spinal Fusion and Artificial Disc Implants For Health Plans, Medical Management Organizations and TPAs Executive Summary Back pain is one of the most
.org. Metastatic Bone Disease. Description
 Metastatic Bone Disease Page ( 1 ) Cancer that begins in an organ, such as the lungs, breast, or prostate, and then spreads to bone is called metastatic bone disease (MBD). More than 1.2 million new cancer
Metastatic Bone Disease Page ( 1 ) Cancer that begins in an organ, such as the lungs, breast, or prostate, and then spreads to bone is called metastatic bone disease (MBD). More than 1.2 million new cancer
CPT 76977, 77078, 77079, 77080, 77081, 77083, or HCPCS G0130:
 Bone Mass Measurements Coverage Information Noridian Administrative Services (NAS), LLC, is providing coverage information regarding Bone Mass Measurements (BMM) in response to multiple provider inquiries.
Bone Mass Measurements Coverage Information Noridian Administrative Services (NAS), LLC, is providing coverage information regarding Bone Mass Measurements (BMM) in response to multiple provider inquiries.
Is it time for a new drug development paradigm?
 Is it time for a new drug development paradigm? Robert McDonough, M.D. Senior Director, Clinical Policy Research and Development 1 The Aetna Way Our Cause To make quality health care more affordable and
Is it time for a new drug development paradigm? Robert McDonough, M.D. Senior Director, Clinical Policy Research and Development 1 The Aetna Way Our Cause To make quality health care more affordable and
Corporate Medical Policy Breast Surgeries
 Corporate Medical Policy Breast Surgeries File Name: Origination: Last CAP Review: Next CAP Review: Last Review: breast_surgeries 1/2000 9/2015 9/2016 9/2015 Description of Procedure or Service Policy
Corporate Medical Policy Breast Surgeries File Name: Origination: Last CAP Review: Next CAP Review: Last Review: breast_surgeries 1/2000 9/2015 9/2016 9/2015 Description of Procedure or Service Policy
PANCREAS, PANCREAS-KIDNEY, SEGMENT OF PANCREAS AND ISLET PANCREATIC TISSUE TRANSPLANTATION SUR703.013
 PANCREAS, PANCREAS-KIDNEY, SEGMENT OF PANCREAS AND ISLET PANCREATIC TISSUE TRANSPLANTATION SUR703.013 COVERAGE: A simultaneous or combined pancreas-kidney transplant (SPK) from a cadaver donor or a simultaneous
PANCREAS, PANCREAS-KIDNEY, SEGMENT OF PANCREAS AND ISLET PANCREATIC TISSUE TRANSPLANTATION SUR703.013 COVERAGE: A simultaneous or combined pancreas-kidney transplant (SPK) from a cadaver donor or a simultaneous
Health Benchmarks Program Clinical Quality Indicator Specification 2013
 Health Benchmarks Program Clinical Quality Indicator Specification 2013 Measure Title USE OF IMAGING STUDIES FOR LOW BACK PAIN Disease State Musculoskeletal Indicator Classification Utilization Strength
Health Benchmarks Program Clinical Quality Indicator Specification 2013 Measure Title USE OF IMAGING STUDIES FOR LOW BACK PAIN Disease State Musculoskeletal Indicator Classification Utilization Strength
External Insulin Pumps Corporate Medical Policy
 External Insulin Pumps Corporate Medical Policy File name: External Insulin Pumps File code: UM.DME.02 Origination: 4/2006 Last Review: 02/2014 (ICD-10 remediation only) Next Review: 10/2014 Effective
External Insulin Pumps Corporate Medical Policy File name: External Insulin Pumps File code: UM.DME.02 Origination: 4/2006 Last Review: 02/2014 (ICD-10 remediation only) Next Review: 10/2014 Effective
