Retinal assessment using optical coherence tomography $
|
|
|
- Easter Maxwell
- 9 years ago
- Views:
Transcription
1 Progress in Retinal and Eye Research 25 (2006) Retinal assessment using optical coherence tomography $ Roge rio A. Costa a,, Mirian Skaf b, Luiz A.S. Melo Jr. b, Daniela Calucci a, Jose A. Cardillo a, Jarbas C. Castro c, David Huang d, Maciej Wojtkowski e a U.D.A.T. Retina Diagnostic and Treatment Division, Hospital de Olhos de Araraquara, Rua Padre Duarte 989 ap 172, Araraquara, SP , Brazil b Glaucoma Section, Hospital de Olhos de Araraquara, Araraquara, SP, Brazil c Instituto de Física de São Carlos USP, São Carlos-SP, Brazil d Department of Ophthalmology, University of Southern California, Los Angeles, CA, USA e Institute of Physics, Nicolaus Copernicus University, Torun, Poland Abstract Over the 15 years since the original description, optical coherence tomography (OCT) has become one of the key diagnostic technologies in the ophthalmic subspecialty areas of retinal diseases and glaucoma. The reason for the widespread adoption of this technology originates from at least two properties of the OCT results: on the one hand, the results are accessible to the non-specialist where microscopic retinal abnormalities are grossly and easily noticeable; on the other hand, results are reproducible and exceedingly quantitative in the hands of the specialist. However, as in any other imaging technique in ophthalmology, some artifacts are expected to occur. Understanding of the basic principles of image acquisition and data processing as well as recognition of OCT limitations are crucial issues to using this equipment with cleverness. Herein, we took a brief look in the past of OCT and have explained the key basic physical principles of this imaging technology. In addition, each of the several steps encompassing a third generation OCT evaluation of retinal tissues has been addressed in details. A comprehensive explanation about next generation OCT systems has also been provided and, to conclude, we have commented on the future directions of this exceptional technique. r 2006 Elsevier Ltd. All rights reserved. Keywords: Artifacts; Cross-sectional; Fourier domain; Glaucoma; Interferometer; Macula; Macular map; Measurement; Nerve fiber layer; Optic disc; Optical coherence tomography (OCT); Photoreceptor; Retinal boundary; Retinal thickness; Spectral Contents 1. History of optical coherence tomography (OCT): conception of the idea Basic physical principles OCT in clinical setting Retina (and retinal diseases) Image acquisition Qualitative analysis Quantitative analysis RNFL and glaucoma RNFL thickness protocols Abbreviations: A-scan(s), axial scan(s); HRL, highly reflective layer; OCT, optical coherence tomography; RNFL, retinal nerve fiber layer; RPE, retinal pigment epithelium; RTA, retinal thickness analyzer; SLD, superluminescent diode $ Supported in part by Fundac-ão de Amparo à Pesquisa do Estado de Sa o Paulo, FAPESP Grant no.: 98/ , and by Grant no.: KBN 4T11E Corresponding author. Tel./fax: address: [email protected] (R.A. Costa) /$ - see front matter r 2006 Elsevier Ltd. All rights reserved. doi: /j.preteyeres
2 326 ARTICLE IN PRESS R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Reproducibility Diagnostic capability and progression evaluation Normative database Future directions Additional considerations Next generation OCT devices Spectral OCT instrument using Fourier domain detection Standard-resolution retinal imaging with high-speed spectral OCT High-resolution retinal imaging with high-speed spectral OCT Additional considerations Concluding remarks Acknowledgments References History of optical coherence tomography (OCT): conception of the idea OCT was first developed by David Huang and colleagues in James Fujimoto s laboratory at the Massachusetts Institute of Technology (MIT) and published in a 1991 Science article (Huang et al., 1991). The Fujimoto s laboratory was specialized in femtosecond lasers at the time. These are lasers that emit pulses of only several tens of femtosecond (million billionth of a second) and can be used to measure the delay of light reflected from tissue structures with near micron precision. Because femtosecond lasers were too bulky and expensive for routine clinical use, Huang worked on an interferometer system that could use a cheap and compact diode light source to measure the time-of-flight of light with the same precision. He realized then that this technique, called optical coherence domain reflectometry, could be the basis of a new imaging technology with unprecedented potential for non-invasive imaging of retina and other tissues with micron resolution. This new technique was coined optical coherence tomography because it relied on measuring the coherence of light reflected from tissue structures and generates cross-sectional images, or tomographs. The initial retinal OCT experiment that Huang conducted with Joel Schuman, an ophthalmologist then at Harvard, took several hours to acquire a single image. To improve the imaging speed, Fujimoto recruited Eric Swanson, then working on optical communications at MIT Lincoln Laboratory. With Swanson s crucial assistance, it was first developed an efficient fiber-optic OCT system that was fast enough for clinical testing (Swanson et al., 1993). The first clinical tests of retinal scanning were conducted by Carmen Puliafito s group that was then at the Massachusetts Eye and Ear Infirmary, Harvard Medical School. The encouraging results lead to commercialization of the technology in the mid-1990s by Humphrey Instruments, Inc. (since acquired by Carl Zeiss Meditec, Inc). The latest Zeiss Stratus OCT system (third generation OCT or OCT3) is now used by thousands of ophthalmologists for the management of macular diseases and glaucoma. 2. Basic physical principles Clinical examination using the slit lamp has been used for several years as the main instrument for retinal structural assessment. Meanwhile, many other imaging techniques have been developed to examine cross-sectional retinal morphology. The confocal scanning laser ophthalmoscope (cslo) forms retinal images by sequentially collecting reflections from laterally and longitudinally well-defined retinal volumes. Several cslo images taken with sequential focal depths can generate three-dimensional information on the distribution of retinal reflectivity for topographic and tomographic assessments (Huang, 1999). The longitudinal resolution of a cslo, however, is limited to 300 mm due to the available numerical aperture through the pupil and ocular aberrations (Bartsch and Freeman, 1994). Cross-sectional measurements of the retina can be achieved in the clinical setting as well by the instrument coined retinal thickness analyzer (RTA). The RTA employs the principle of optical triangulation to provide direct measurement of the retinal thickness with an estimated accuracy of mm (Zeimer et al., 1989). The instrument projects a narrow slit of 543 nm He Ne laser light onto the retina and calculates the distance between the reflections that correspond to the vitreoretinal and chorioretinal interfaces. Although recent advances in this instrumentation have enabled rapid multiple optical sectioning of neighboring retinal regions to generate a retinal thickness map (Zeimer et al., 1996), information is restricted to fundus (macular) regions of 2 2 mm and limited qualitative data can be extracted from such imaging methodology. Optical coherence tomography (OCT) is based on the imaging of reflected light. But unlike a simple camera image that only has transverse dimensions (left/right, up/down), it resolves depth. The depth resolution of OCT is extremely fine, typically on the order of 0.01 mm or 0.4 thousandth of an inch. This provides cross-sectional views (tomography) of internal tissue structures similar to tissue sections under a microscope, without disturbing the tissue as in histology. Thus, OCT has been described as a method for non-invasive tissue biopsy.
3 R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Fig. 1. The optical coherence tomography beam is scanned across the retina (1). The delay of a superficial reflection (2) is shorter than that of a deeper reflection (3). Reprinted by courtesy of Elsevier (Huang et al., in progress). Fig. 3. An optical coherence tomography cross-sectional image (grayscale image) is built up from many A-scans (red plot lines). Reprinted by courtesy of Elsevier (Huang et al., in progress). Fig. 2. An axial scan (A-scan) of the retina. The amplitude of reflection on a decibel (db) scale is plotted against depth. Reprinted by courtesy of Elsevier (Huang et al., in progress). In OCT, a beam of light (typically nm wavelength in the near infrared) is scanned across the tissue sample. The OCT system then collects the reflected light and measures its time-of-flight delay. Light reflected from deeper layers have longer propagation delays than that reflected from more superficial layers (Fig. 1). The amplitude of reflected light can be plotted against delay (Fig. 2) to demonstrate tissue reflectivity at successively deeper levels of tissue penetration along the axis of beam propagation. This is called an axial scan (A-scan). As the OCT probe beam is scanned across a sample, many A-scans are acquired to form an image (Fig. 3). A color or gray-scale is used to represent the signal amplitude. Ultrasound imaging and RADAR are also reflectometry-based imaging methods. Because OCT employs light, several advantages are gained. The wavelength of light (0.001 mm) is shorter than that of ultrasound (0.1 mm) and radio wave (410 mm). Therefore the spatial resolution of OCT is much higher. And unlike ultrasound imaging, OCT does not require probe-tissue contact or an immersion fluid since light passes through the air tissue interface easily. Because light travels very rapidly ( m/s), it is not possible to directly measure the time-of-flight delay on a small spatial scale. The micron-scale resolution of OCT is achieved by comparing the delays of sample reflections with the known delay of a reference reflection in an interferometer. The classic OCT system (Fig. 4) employs a low-coherence fiber-optic Michelson interferometer (Huang et al., 1991). Interferometry measures the effect of combining 2 light waves. Low coherence means that the system employs a wide range of wavelengths. We will explain the concepts of interferometry and coherence separately. The interferometer (Fig. 4) has source, sample, reference, and detector arms all centered on a 50/50 fiber coupler. Output of the superluminescent diode (SLD) light source is launched into the source arm fiber and split by the coupler into the sample and reference arms. Sample and reference reflections are recombined at the coupler and produce interference. This interferometric signal is converted from light to electrical current by a photodetector, processed electronically, and transferred to computer memory. To understand how the Michelson interferometer works, let us start with the simple case (Fig. 5) where the sample arm reflection comes from a simple mirror surface and the light source emits only one wavelength. Think of the sample and reference reflections as 2 waves of light. The coupler partially transfers theses waves to the detector arm,
4 328 ARTICLE IN PRESS R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Fig. 4. Schematic of the classic optical coherence tomography system. Reprinted by courtesy of Elsevier (Huang et al., in progress). Fig. 6. Combining interference signals from a range of wavelengths (left) produces a pulse (right). The width of this pulse determines the axial resolution of optical coherence tomography. Reprinted by courtesy of Elsevier (Huang et al., in progress). Fig. 5. In a single-wavelength Michelson interferometer, varying the reference delay produces a sinusoidal oscillation of optical power in the detector arm. Reprinted by courtesy of Elsevier (Huang et al., in progress). where they combine and produce an interference signal. Interference can be thought of as the addition of the amplitude of two waves. When the two waves are in phase (lined up peak to peak), they interfere constructively, forming a peak in the interference waveform. When the 2 waves are exactly out of phase (lined up peak to trough), they interfere destructively, forming a trough in the interference waveform. As the reference mirror is moved, the phase of the reference wave changes, producing a sinusoidal interference signal. As the reference mirror moves through 1 2 cycle of the source wavelength, the roundtrip delay of the reference wave varies by one wavelength and the interference signal goes through one cycle of sinusoidal oscillation. To resolve the delay of sample reflections, the OCT system uses a light source that has a wide range of wavelengths (low coherence). When the interference signals are added together over the range of wavelengths, the interferometric oscillation fades as the delay mismatch between the reference and sample reflections increases (Fig. 6). To understand why this occurs, look carefully at the phase relationship between the wavelength components shown schematically on the left panel of Fig. 6. When the delay mismatch is near zero (center of waveforms), the interference signals from all wavelength components have the same phase (peaks and troughs lined up) or are coherent. This adds up to large interferometric modulation (see large peaks and troughs in the center of the rightside waveform). When the mismatch is large (away from the center of the waveforms), the interference waveforms vary widely in phase over the wavelength range (peaks and troughs not lined up) and add up to near a flat line. The summed interference signal (Fig. 6, right) forms a wave pulse. In an OCT system, the pulse is demodulated electronically to extract the pulse envelope (shape of the pulse without the sinusoidal oscillation). The width of the pulse envelope is the coherence length, which determines the axial resolution of the OCT system. The coherence length is inversely proportional with the wavelength range or bandwidth. When the OCT system is used to image an actual tissue sample, there are many reflections at different depths. As the reference mirror is scanned, each sample reflection gives rise to a signal pulse when the reference delay matches it. The plot of the demodulated interferometric signal is the axial scan (Fig. 2) waveform, which represents amplitude of reflection vs. depth. All clinical commercial available OCT systems to date employ SLD light sources. SLDs are similar to the diode lasers inside the common compact disc (CD) player, but are made to emit over a wider range of wavelengths. SLDs are used because they are economical, compact, longlasting, and emit high quality beams that couples efficiently with an optical fiber. The resolution of clinical OCT is basically limited by the state of SLD technology. Early retinal OCT systems typically employ SLDs emitting around an 820 nm center wavelength over a bandwidth of 20 nm full-width half-maximum (FWHM) (Swanson et al., 1993; Hee et al., 1995a). This limits the axial resolution to roughly 15 mm FWHM in air and 11 mm in tissue. The Stratus OCT System (Carl Zeiss Meditec, Inc., Dublin, CA, USA) has 9 10 mm axial resolution in tissue.
5 R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) OCT in clinical setting 3.1. Retina (and retinal diseases) Definitive inclusion of OCT in the diagnostic arsenal of the retina specialist occurred with the availability of third generation devices. Indeed, OCT has turned out to be the ancillary exam of choice to assist the diagnosis of several chorioretinal diseases (Haouchine et al., 2004; Jorge et al., 2004; Browning et al., 2004; Johnson, 2005; Niwa et al., 2005; Sandhu and Talks, 2005; Catier et al., 2005; Costa et al., 2005; Gaucher et al., 2005; Montero and Ruiz- Moreno, 2005; Meirelles et al., 2005). Additionally, the role of OCT to monitor morphologic retinal changes over time has turned out to be apparent, particularly given the latest concept of disease modulation associated with new treatment modalities and the contemporary pharmacological era of alternative management of chorioretinal diseases (Costa et al., 2002, 2003a c; Campochiaro et al., 2004; Michels et al., 2005; Eter and Spaide, 2005; Gross, 2005; Salinas-Alaman et al., 2005; Hillenkamp et al., 2005; Sahni et al., 2005; Cardillo et al., 2005; Bonini-Filho et al., 2005). Two representative examples of drugs fitting in such modern treatment concept might be triamcinolone and pegaptanib. It has been demonstrated that disease control using intravitreal drug injections may be preferable to photothermal destruction of pathological tissues in some instances. Borne out from several studies over the past decade, this premise has been to a great extent based on the results of OCT evaluation, which has provided incontestable evidence of favorable macular remodeling after treatment. While the initial reports about the alternative use of intravitreal injection of triamcinolone for the treatment of macular edema of a variety of causes may represent the launch of the concept of disease modulation for the management of chorioretinal diseases (Jonas and Sofker, 2001; Antcliff et al., 2001; Martidis et al., 2001, 2002; Jonas et al., 2002; Greenberg et al., 2002), the recent FDA approval of pegaptanib (Macugen s, Pfizer) for the treatment of neovascular age-related macular degeneration serves as the ultimate frontier that a new treatment era has definitively began (Gragoudas et al., 2004; Doggrell, 2005). Presently, several drugs and alternative laser treatments for the management of macular diseases are under evaluation in randomized clinical trials, and there is growing common sense that OCT may be preferable to angiographic studies to monitor the retinal response in such setting, particularly because of the theoretical possibility of quantitative analysis of the induced changes in a micrometer scale (Fig. 7). However, as commonly seen in other techniques of retinal imaging, one must bear in mind that artifacts are expected to occur in the several steps encompassing an OCT evaluation. Understanding of the basic principles of image acquisition and data processing as well as recognition of OCT limitations may help us to use this imaging technique with cleverness. For Fig. 7. Quantitative assessment by third generation optical coherence tomography of morphologic retinal changes induced by treatments in a patient with diffuse diabetic macular edema. (A) At baseline, retina is diffusely thickened due to intra- and sub-retinal fluid. (B) Four weeks after high-density grid laser photocoagulation, favorable macular remodeling is disclosed by OCT. At the right side of the figure, quantitative analysis (micrometer scale) of the changes in the average retinal thickness from baseline in nine different macular sub-fields is disclosed. scholarship purposes, we have divided this section into 3 parts: (a) image acquisition; (b) qualitative analysis; and (c) quantitative analysis Image acquisition Part of the artifacts generated during OCT data processing may be attenuated given that optimal image acquisition has been performed. For such, the examiner should be aware of as well as should follow all guidelines presented in the device s user manual. In this segment, some guidelines are emphasized and additional tips to minimize bias generated by the examiner are presented Scanning centralization. When acquiring macular scans for retinal thickness measurements (macular thickness map and fast macular thickness map scan acquisition protocols), it is imperative that the examiner centers all 6 scans at the fovea. This is relatively easy for cooperative patients with relative good vision. However, the profile of patients with chorioretinal diseases submitted to OCT evaluation at our institution is quite different. In general, patients are aged 50 or more, and have poor fixation due to impaired visual acuity. Additionally, the examiner frequently faces a situation in which precise identification of the foveal center is problematic because of distortions of the macular architecture associated with the disease. Therefore, the examiner not only must be capable to identify the location of the fovea in abnormal maculas but also has to be extremely serene to correctly place all 6 scans at the presumed foveal center. While the former will be sole dependent on examiner expertize, the latter task may be facilitated by switching on the center line tool of the OCT3 software. Right-click anywhere in the scan acquisition window and a vertical line appears in the center of the scan image. This feature facilitates macular scans centralization and shortens the acquisition time of optimal scans for macular maps. To deactivate the center line, right-click another time in the scan acquisition window (Fig. 8).
6 330 ARTICLE IN PRESS R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Data verification and validation. Immediately at the end of one scanning session for macular thickness maps using either standard (512 A-scans/image) or fast (128 A-scans/image) acquisition protocols, some actions should be performed by the examiner prior to data processing to generate the macular maps. Initially, the examiner should verify possible artifacts in the delineation of the retinal boundaries. For such, each image (B-scan) should be processed in separate using the retinal thickness (single eye) analysis protocol and accuracy of automatic delineation confirmed. If delineation errors were verified in any of the 6 B-scans, a new complete scanning session should be performed (Fig. 9). Once adequate images have been acquired, the examiner should then verify centralization of the 6 scans (in respect to the foveal center). By means of data processing using the retinal map (single eye) or retinal thickness/volume (OU) analyze protocols, the software automatically calculates the average (7SD) retinal thickness at the fovea, named foveal thickness or foveal height (Fig. 10). The more central A-scan of each one of the 6 B-scans acquired is used to calculate foveal height. Since all 6 scans are to be centered at the same point (fovea), in theory, in a perfect scan acquisition session, the central A-scan should be the same for all 6 B-scans (intersecting point). Therefore, in this hypothetical situation, the SD of the average foveal height is to be equal to zero. Depending on the macular status, SD values higher than 30 mm are highly suggestive that at least one of the 6 scans is not correctly centered at the fovea, and a new complete scan acquisition session should be performed Manual raster scanning. At the end of a scanning session for macular map, it is highly advisable to the examiner to perform a manual raster scanning throughout the macular area to minimize the chance of missing morphological details in the adjacencies of the macular Fig. 8. (A) During fundus scanning, right-click anywhere in the scan acquisition window (arrowhead), and a vertical line appears in the center of the scan image (B). This feature facilitates macular scans centralization (C and D), and shortens the acquisition time of adequate scans for macular maps. Fig. 10. Checking of scans displacement in respect to the foveal center (after verification of the automatic delineation of retinal boundaries [Fig. 9]). (A) By using the retinal map (single eye) or retinal thickness/ volume (OU) analyze protocols, the software automatically calculates the average (7SD) retinal thickness at the fovea, named foveal thickness, center or foveal height. In spite of delineation flaws observed in first scanning (Scan 5 Fig. 9(A)), which induced a 10% difference in average RT in two subfields (between red lines) (B), note that the SD of the foveal thickness was o30 mm in both scanning acquisition sessions (A and B), indicating good centralization of the 6 scans in both settings. Fig. 9. Verifying in separate the automatic delineation of the retinal boundaries in each image (B-scan) to be used to macular maps. (A) At the end of one scanning session for macular thickness maps, analysis of the automatic retinal boundaries delineation using the retinal thickness (single eye) analysis protocol revealed one major delineation error in scan 5 (red dashed line/asterisk). (B) A new complete scanning session has solved the issue (red dashed line/asterisk).
7 R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Fig. 11. Manual raster scanning. (A) Perifoveal detachment of the posterior hyaloid and presumed foveal split were seen (impending macular hole); detailed raster scanning revealed that in fact the pseudocyst had already extended posteriorly (A 0 ), and there was an eccentric opening of its roof (arrowhead) (stage 2 macular hole). (B) Incomplete vitreofoveal separation and a tomographic appearance that gives the impression that a lamellar hole is to be formed; however, detailed scanning demonstrates an already established full-thickness macular hole (B 0 ). center. This maneuver enables an enhanced view of the macular status. It is not rare to find new findings such as abnormal vitreoretinal adhesions. The use of 8 mm in length scans for manual raster scanning may also facilitate the detection of vitreomacular adhesions (Fig. 11) Scan review software tool. Occasionally during scanning of ocular fundus, the examiner misses the exact timing to freeze the desired image or some computer delay occurs after the freeze button has been pressed, and the subsequent tomogram is now frozen in the scan acquisition window computer screen. By pressing the review button, the examiner is allowed to retrieve all tomograms acquired in that session (scan review window). If an optimal tomogram exists amongst recovered images, it can be selected and saved by the examiner (Fig. 12) Bimanual technique. A simple change proposed by one of the authors (D.C.) in the disposition of the mouse within the device s table may also facilitate immensely OCT evaluation in patients with poor collaboration. By changing the mouse position to the left side of the keyboard, the examiner will be able to control simultaneously the alignment of the scanner unit (patient module) with the right hand (joystick), and all the screen settings (such as Z-offset, polarization, and fixation LED) with the left hand (mouse). In addition, to shorten acquisition time, the examiner may keep the freeze (with or without flash) button of the scan acquisition window pressed during scanning; when an optimal tomogram is displayed in the screen, the image is rapidly frozen by just releasing the button (Fig. 13). A short period of adaptation may be needed until the examiner get used to this alternative mouse disposition Additional considerations. The third generation of OCT instruments offers basically two modes of image acquisition for macular thickness measurements. In the fast mode, each tomogram (B-scan) consists of 128 A-scans Fig. 12. Review software tool. After activating the scanning mode (A), the examiner may freeze the image at any desired time (B). By pressing the review button (C), the examiner is allowed to retrieve all tomograms acquired in that session (scan review window). If an optimal tomogram exists amongst recovered images, it can be selected (D) and saved by the examiner (E). Fig. 13. Alternative mouse disposition within the table of third generation OCT. The examiner may keep one of freeze (with or without flash) buttons of the scan acquisition window pressed during scanning to speed up image acquirement. while in standard mode each tomogram consists of 512 A-scans. In both modes, a total of 6 tomograms oriented at 301 intervals are acquired. Topographic macular maps are ultimately derived from each individual A-scan per OCT study (768 A-scans in fast mode; 3072 A-scans in standard mode) (Fig. 14). The equivalence of retinal maps generated by standard and fast modes in clinical scenarios other than diabetic macular edema remains to be determined. Additionally one must bear in mind that, although quite convenient for the patient and presumably less
8 332 ARTICLE IN PRESS R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) time-consuming for the examiner, the fast mode acquires all six tomograms in sequence at one single scanning session, increasing the chance for an undesirable misalignment of one or more of the tomograms in relation to the foveal center (Fig. 8(D)). Since macular maps are generally used to evaluate possible changes in the macular status over time, it is highly advisable to verify the capability of the examiner to generate reproducible maps (Fig. 15) Qualitative analysis Third generation OCT is an established strategy that improves first generations OCT technology and achieves extraordinary in vivo visualization of retinal features and disease. OCT3 exceeds first generations OCT imaging by obtaining superior axial-image resolution and higher pixeldensity images, and therefore offers better recognition of the intraretinal layers. Improved acquisition speed (400 A-scans/s) allows, within 1 2 s, the visualization of the retinal morphology approaching a level of structural differentiation obtainable only with histopathology (Fig. 16). Fig. 14. Fast and standard macular thickness maps. (A) In the fast mode, each tomogram (B-scan) consists of 128 A-scans while in standard mode (B) each tomogram consists of 512 A-scans. In both modes, a total of 6 tomograms oriented at 301 intervals are acquired Defining inner and outer HRL. Ever since the initial images obtained using first generation OCT became available, several studies have focused on the precise interpretation of retinal reflective signals and their correlation with retinal morphology. From the first generation tomograms of normal retinas it was demonstrated that a clear highly reflective layer (HRL) exists at the outer aspect of the neuro-sensory retina. Based on clinical and tomographical correlation studies, it had been suggested that the outer most reflective layer might correspond to a complex formed by the RPE and choriocapillaris, and such layer was then coined RPE-choriocapillaris Fig. 15. (A D) Four scanning sessions performed in 5-min intervals using the fast macular thickness map acquisition protocol in a patient with central serous chorioretinopathy. (A 0 D 0 ) Data processing revealed optimal centralization of the scans in all four scanning sessions and good correspondence of topographic macular maps.
9 R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Fig. 16. Differences in image quality related to A-scan density (normal macula). Note that image quality is increases as the number of A-scans/ image multiplies (128 [A], 256 [B], and 512 [C]). hyper-reflective complex. In 1998, Huang et al. first proposed an alternative interpretation of such hyperreflective complex (Huang et al., 1998). Based on an OCT study with histological and pathological correlation in normal and rd chickens, they suggested that the outer most hyper-reflective complex might correspond to photoreceptors inner and outer segments, RPE, and anterior choroidal pigmentation (they termed it outer retina choroid complex). At that time, they have also demonstrated that in the most severely affected rd chickens, which lacked photoreceptor layer, the total thickness of the outer most hyper-reflective complex is reduced, suggesting missing the highly reflective peak derived from the photoreceptor IS/OS (Huang et al., 1998). Supportive data to Huang s hypothesis occurred as soon as third generation and ultrahigh-resolution OCT clinical studies became available (Montero et al., 2003; Drexler et al., 2003; Jorge et al., 2004; Costa et al., 2004). The single outer hyper-reflective layer seen by first generations OCT was then visualized as two parallel, highly reflective (red/white) layers separated from each other by one thin layer of moderate reflectivity (green/yellow) at the level of the posterior boundary of the retina (Fig. 17). The inner HRL, which most likely corresponds to the junction between the inner and outer segments of the photoreceptors, assumes a forward bowshaped configuration in the center of the macula consistent with the well-known increase in length of the outer segments of the cones in such region. The outer HRL, which appeared approximately two times thicker than the inner HRL, have been described to be equivalent to the RPE-choriocapillaris hyper-reflective complex seen on first generations OCT. However, the precise interpretation of the outer HRL remains to be established. Is choriocapillaris signaling contributing to its formation? Such question is to be answered as soon as additional ultrahigh-resolution OCT data becomes available. Fig. 17. Third generation optical coherence tomography appearance of one normal macula. (A) Two, well-defined, linear, highly reflective layers (HRL) are seen at the outer aspect of the neural retina. (B) The inner HRL (corresponding to the photoreceptors IS/OS junction) is thinner than the outer, and is characterized by mild forward bow-shaped configuration at the foveal center. The outer HRL may correspond to a hyper-reflective complex formed by RPE [and choriocapillaris] Outer retina. Particular analysis of the inner HRL tomographic appearance has provided new insights for better comprehension of diseases affecting the macula. The integrity of the inner HRL has been correlated to some extent with visual acuity. Eyes with vitreomacular traction syndrome or macular edema and extraordinary good visual acuity levels represent a good example of the value of such particular analysis (Fig. 18A,B). In unilateral resolved central serous chorioretinopathy as well as in eyes presenting visual acuity deterioration due to photic maculopathy the same rationale may be used (Eandi et al., 2005; Jorge et al., 2004). Third generation OCT evaluation has demonstrated abnormal reflectivity at the level of the outer foveal retina, such as intense fragmentation or complete interruption of the inner HRL. It has been suggested that visual acuity may be more severely distorted in eyes presenting full-thickness involvement of the photoreceptors reflective layer (Jorge et al., 2004). Analysis of the inner HRL has also demonstrated usefulness in the evaluation and better understanding of the outcomes of operated macular holes. It has been demonstrated that in spite of anatomical success and recovery of the macular shape, the postoperative visual acuity and improvement of visual acuity were not directly related to the morphological results. Outer retinal features appear to be more important to determine postoperative visual function as irregularities at the level of the inner HRL after macular hole surgery may prevent visual acuity improvement (Uemoto et al., 2002; Kitaya et al., 2004; Villate et al., 2005) (Fig. 18C,D). New and unexpected findings have also been demonstrated by studies using OCT in several hereditary retinopathies (Aleman et al., 2002; Jacobson et al., 1998, 2000, 2003, 2004, 2005; Milam et al., 2003; Pianta et al., 2003). These scientific results include: localization of the missing photoreceptor component in retinitis pigmentosa (Jacobson et al., 1998, 2000); prediction of sub-retinal and sub-rpe deposits in cone-rod dystrophy and in Best macular dystrophy (Aleman et al., 2002; Pianta et al.,
10 334 ARTICLE IN PRESS R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Fig. 18. Tomographic appearance of outer foveal retina and visual acuity (VA). (A) Typical inner HRL appearance in normal macula (VA ¼ 20/20). (B) Macular edema in branch retinal vein occlusion and VA ¼ 20/25; note at the fovea, the inner HRL is well preserved. Intense fragmentation of the inner HRL in one patient with outer macular hole and VA ¼ 20/50 1 (C), and in patient with photic maculopathy and VA ¼ 20/60 (D). Fig. 19. Vitreomacular status and diabetic macular edema. From perifoveal posterior vitreous detachment (A and B) to vitreofoveal traction (and increased foveal high) (C), and finally to spontaneous complete vitreofoveal separation (and favorable macular remodeling). 2003); unexpected retinal lamination abnormalities (Jacobson et al., 2003, 2004); and, definition of the relationship between outer nuclear layer thickness and visual function (Jacobson et al., 2005) Vitreoretinal interface. Unparalleled characterization of the vitreoretinal interface has been possible with the advent of third generation OCT. Studies focused on the analysis of the vitreomacular interface have led to new insights about our understanding and management of several maculopathies, such as idiopathic macular hole and diabetic macular edema. For example, a high prevalence of perifoveal posterior vitreous detachment with incomplete vitreofoveal separation has been demonstrated in diabetic patients with macular edema, suggesting a possible additional role of the vitreomacular interface status in the pathogenesis of diabetic macular edema (Gaucher et al., 2005). Anecdotal reports of favorable macular remodeling in eyes with diabetic macular edema after spontaneous vitreofoveal separation, as well as in the early post- Nd:YAG laser capsulotomy period, may provide additional supportive data of the possible influence of the vitreomacular interface status in such scenario (Watanabe et al., 2000; Yamaguchi et al., 2003) (Fig. 19). Fig. 20. Third generation optical coherence tomography evaluation of the left eye of a 57-year-old woman with idiopathic full-thickness macular hole in the right eye. (A) At presentation a full-thickness macular hole was seen in patient s right eye, and OCT3 scan of the left eye revealed complete detachment of the posterior hyaloid in the macular region with hyperreflective signals in the plane of the detached posterior hyaloid over the foveal region (arrow). Despite minimal irregularities of the inner foveal contour, macular architecture was relatively well preserved at presentation, and visual acuity was 20/20. (B) Five months later, visual acuity decreased to 20/25 2 and an epiretinal membrane (grade 1) was seen in her left eye associated with the development of a full-thickness macular hole. Oblique scans revealed complete interruption of the foveal retina of approximately 99 mm associated with perifoveal intraretinal fluid accumulation and attenuation of the inner HRL suggesting edema of the outer perifoveal neural retina. Tornambe s retinal hydration theory may explain macular hole formation in eyes presenting complete vitreofoveal separation and associated disruption of the inner fovea. Presently, there are more than 80 published OCT studies about macular holes. Old concepts have been revisited and new findings and conjectures, such as the influence of oblique vitreofoveal tracional forces, the demonstration of intrafoveal split as well as the retinal hydration theory, have been possible due to enhanced appreciation of the vitreomacular interface status and macular morphologic features in eyes with macular holes (Hee et al., 1995b; Gaudric et al., 1999; Haouchine et al., 2001; Tornambe, 2003) (Fig. 20) Additional considerations. An extraordinary qualitative analysis of the macular morphologic features has been enabled by third generation OCT technology. However,
11 R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Fig. 21. Young patient with pathologic myopia complaining of distortion and acute loss of vision. (A) At presentation OCT evaluation suggested the presence of a true abnormal hyper-reflective formation (arrowheads) such as choroidal neovascularization. (B) Six weeks latter, the macula contour was practically normal. The patient had serohemorrhagic complications related to one small lacquer crack located juxtafoveally, without associated neovascularization. one should bear in mind that optimal interpretation of OCT3 findings in clinical setting is highly dependent on adequate clinical correlation and, whenever suitable, clinical and angiographical correlation may be preferable. Third generation OCT evaluation in eyes presenting early stages (grade 0 and 1) epiretinal membranes and in patients with serohemorrhagic macular complications due to choroidal neovascularization are good examples of entities in which interpretation bias may occur in the absence of clinical/clinical and angiographical correlation. The occurrence of normal cross sectional images (with no apparent abnormal finding) is not rare in patients with early stage epiretinal membranes. In the same view, one should be caution in the interpretation of tomographic findings in exudative maculopathies, given that outer retina edema as well as blood may eventually simulate the tomographic appearance of one virtually abnormal formation (Fig. 21). Finally, an important aspect of OCT data that has been available since the first generation technology, but is very rarely studied, is the intensity changes resulting from intraretinal abnormalities in backscatter. In terms of future work, this rich data source, which is available in each scan performed nowadays, requires careful attention in the future. Quantitative analysis of intra-retinal OCT intensity changes have been demonstrated experimentally by Cideciyan et al. in dogs (Cideciyan et al., 2005). Also relevant to this subject, is one of the very few clinical examples of changes in intra-retinal light scattering, reported by Lerche et al. (2001), including 20 patients with retinal venous occlusive disease Quantitative analysis Cross sectional retinal imaging provides a unique opportunity to quantify the overall thickness of the retina in vivo (Huang et al., 1991; Hee et al., 1995a; Schuman et al., 1995; Puliafito et al., 1996; Zeimer et al., 1996; Podoleanu et al., 1998; Bartsch and Freeman, 1994). Indubitably, one of the most attractive features of third generation OCT is the theoretical possibility of attaining reproducible and accurate measurements of ocular fundus tissues. In clinical settings, it has significant potential both as a diagnostic tool and particularly as a way to monitor objectively subtle retinal changes induced by therapeutic interventions. Understanding of the basic principles of automatic retinal thickness measurements by OCT3 as well as recognition of the software limitations are crucial steps to facilitating users to extract tomographic data as real as possible Fundamentals of OCT automatic retinal thickness measurement. At first moment, it is very difficult to understand why the OCT3 software erroneously delineates the retinal boundaries in an optimal tomogram as such exemplified in Fig. 22. Initially, one should bear in mind that automatic retinal thickness measurements are generated in essence by means of a mathematical calculation (algorithm). The algorithm identifies differences in the image reflectance patterns in each A-scan (up to 512 A-scans in OCT 3) that compose one tomogram (B-scan), and assumes that the distance between two relatively high reflective structures represents the retinal thickness at that A-scan. As a result, the OCT software locates the presumed inner retina boundary at the vitreoretinal interface (first high reflective structure) and the presumed outer retina boundary at the retinal pigment epithelial-photoreceptor outer segment interface (second high reflective structure). The algorithm also compares the shape of one A-scan to adjacent A-scans, once great differences in shape are not Fig. 22. Automatic retinal thickness measurement. Although quite good scans were acquired (blue-dashed box), the OCT 3 software algorithm was not able to delineate correctly the outer retinal boundaries (red dashed boxes, arrows).
12 336 ARTICLE IN PRESS R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) expected to occur in side-by-side A-scans. The software then places a line on the inner vitreoretinal interface and another on the retinal pigment epithelium (RPE)-outer retinal interface and determines retinal thickness as the distance between these lines at each measurement point along the scan s x-axis. Therefore, even in fine-looking B-scans, errors in retinal boundaries delineation may occur Software delineation of outer neuro-sensory retinal boundary. It was believed in the past that scans of normal eyes did not have inner and outer retina boundaries misidentification artifacts and only had artifact related to examiner error. Therefore, under optimal scan acquisition, measurement of the retinal thickness is expected to be perfect, basically depending on the ability of the OCT 3 software to recognize both interfaces at each A-scan that composes the tomogram. However, it has been recently demonstrated that built-in OCT3 software has encountering severe problems in recognizing the outer boundaries of the neurosensory retina in optimal OCT3 scans (Costa et al., 2004). Of particular concern was the finding that such recognition was invariably incorrect in normal eyes (Costa et al., 2004). As explained above, the so-called RPE-choriocapillaris reflective complex seen in first generations OCT now is disclosed as two well-defined HRL at the level of the outer retina in the macular region of healthy subjects in third generation tomograms; the inner HRL corresponding to the junction of the inner and outer segments of the photoreceptors while the outer one most likely corresponding to the retinal pigment epithelium or a reflective complex formed by RPE and choriocapillaris. A similar tomographic appearance has been also evidenced in non-affected macular regions of patients with selected eye diseases (idiopathic macular hole, central serous chorioretinopathy, and macular edema), whereas affected regions generally demonstrated a single-layer high reflective appearance with disappearance of the inner HRL (Costa et al., 2004). The use of the OCT3 automated retinal thickness measurement tool (software versions 1.0, 2.0, and 3.0) has generating erroneous values due to incorrect interpretation of the inner HRL as the outer neural retina boundary (Costa et al., 2004; Pons and Garcia-Valenzuela, 2005) (Fig. 23). Measurements using the automated tool of the OCT3 software (version 3.0) in comparison to manual caliper-assisted technique, in which the outer HRL was interpreted as the outer boundary, demonstrated that a significant difference existed in the generated values for retinal thickness at specific macular regions in healthy subjects caused by such misalignment. Manual caliperassisted retinal thickness measurements at specific macular regions differed from those automatically generated by 9.9% from up to 38% (Costa et al., 2004) Topographic macular maps. Built-in software of third generation OCT performs measurements of macular thickness using 6 intersecting 6-mm-long OCT images oriented in a radial pattern centered on the fovea (Hee et al., 1998). Six images of 512 A-scans (transverse pixels) each can be acquired in approximately 8 10 s using macular thickness map or radial lines acquisition protocols, or 6 images of 128 A-scans each can be acquired in approximately 2 s using the fast macular thickness map. The radial scanning protocol was designed to concentrate measurements in the central fovea, where high sampling density is most important. The 6 OCT images are segmented to detect the retinal thickness, which is displayed as a false-color topographic map divided into 9 regions: one central (central macular thickness [CMT]) plus 8 Early Treatment Diabetic Retinopathy Studyfashion sub-fields, and the average thickness value for each region is displayed in separate. Because the radial pattern of 6 OCT images samples the macular thickness along clock hours, the retinal thickness in the wedges between each image is interpolated. Therefore, this imaging protocol may miss focal peculiarities in a span of o1 clock hour, or 301. In addition, misidentification of retinal Fig. 23. Automated retinal thickness measurement was obtained from corresponding A-scan at the fovea (left). Manual caliper-assisted measurement of retinal thickness at the fovea using the automated delineation for the inner retinal boundary by the software as one point (inner caliper cross) and positioning the outer caliper cross just above the outer HRL demonstrated a difference of 51 mm.
13 R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) boundaries in the 6 B-scans that are used to generate retinal maps may interfere significantly in the average retinal thickness value displayed for each sub-field and CMT, as well as in values estimations for macular volume. Of concern, is the fact that CMT values have been used as the main tomographic outcome to monitor retinal changes by therapeutic interventions. Because of particular morphologic foveal features, retinal thickness measurements at this region are more sensible to the OCT3 software measurement flaws (Costa et al., 2004). Recent data from next generation OCT prototypes have been supportive to our concerns about the automatic delineation of the true retinal boundaries by OCT software. Comparison of retinal thickness maps obtained using OCT3 and 3D OCT data from one high speed ultrahigh-resolution OCT prototype, which enables differentiation of the junction between the inner and outer segments of the photoreceptors (inner HRL) as a distinct feature from the RPE (outer HRL), showed a 8 9% difference in retinal thickness values for the 8 macular map sub-fields and up to 16% for CMT, when the thickness map that measures the retinal thickness as the distance from the inner interface of the hyporeflective band corresponding to the RPE to the vitreal retinal interface was disclosed (Wojtkowski et al., 2005) Current third generation OCT software versions. As recently clarified (Hee, 2005), the original macular thickness algorithms (upon which the current OCT3 algorithms are based) were designed to determine the inner and outer retinal boundaries in diabetic macular edema, a condition leading to retinal thickening in which intraretinal fluid accumulation and hard exudates occur but the retinal pigment epithelium and inner limiting membrane remain intact (Hee et al., 1998); in such scenario, the inner HRL is frequently attenuated or absent due to intraretinal edema, and the OCT3 software correctly delineates the outer HRL as the outer boundary (Costa et al., 2004; Costa, 2005). Additionally, we should remember that the inner HRL was not so evident in first generation OCT. The enhanced resolution offered by OCT3 compared with first generations OCT provides images displaying more complex internal features that, paradoxically, require more refined boundary detection algorithms. Improved versions of the OCT3 software are constantly under development. The new software version (4.0) including innovative features to minimize problems during image acquisition has been just recently released. Normative reference values for retinal thickness measurements of the macula have also been included in such version. However, one must bear in mind that misidentification of the outer retinal boundary is still occurring in version 4.0, and, of particular concern, that normative reference values have been established according to OCT3 data in which the outer retinal boundary was considered to be the inner HRL, and not the outer HRL Additional considerations. Summing up, automatic OCT macular thickness is calculated by computer image-processing algorithms with several notable flaws. The most important flaw is that the software does not truly measure the anatomic macular thickness due to its inability to reliably discriminate the junction between the inner and outer segments of the photoreceptors (inner HRL) from the RPE, because both produce a high backscattering signal. Therefore, macular thickness is actually measured using the hyper-reflective layer corresponding to the junction between the photoreceptor inner and outer segment (inner HRL) as the outer retinal boundary, effectively truncating the outer segments in most subjects. Obviously, this is not an incapacitating limitation of the methodology, but this issue should be stressed because one may assume that these measurements are more anatomically meaningful than they truly are. By addressing these issues, we intent to promote a better comprehension of the actual limitations of the OCT3 software, and to assure researchers as well as manufacture s best efforts to overcome them as fast as possible. Recognition of the limitations of any particular device is the basic principle for its use with cleverness RNFL and glaucoma Interest in retinal nerve fiber layer (RNFL) analysis in glaucoma has been recorded as early as 1972, when Hoyt and Newman initially reported RNFL atrophy in patients with glaucoma (Hoyt and Newman, 1972), thus suggesting RNFL thinning as a possible sensitive indicator of glaucomatous damage. When glaucomatous damage begins, ganglion cell degeneration can occur in either diffuse or focal forms. Diffuse atrophy of the nerve fibers is more difficult to assess especially in early stages of the disease, but focal axonal degeneration causes characteristic changes in the appearance of the RNFL and is more easily recognized. Dark slits or grooves appear among the arcuate bundles approaching the optic disc superiorly or inferiorly (Hoyt et al., 1973; Sommer et al., 1991). Abnormal RNFL appearance may be sufficient evidence to initiate glaucoma therapy since at least 25 35% retinal ganglion cell loss is lost before detection of abnormalities in automated visual field testing (Kerrigan-Baumrind et al., 2000). Therefore, evaluation of RNFL has gained growing interest amongst glaucoma specialists in the past few decades. OCT is a relatively new technology that provides highresolution cross-sectional imaging of the RNFL. A built-in algorithm automatically calculates the RNFL thickness when interpreting data acquired using the several RNFL scan acquisition protocols RNFL thickness protocols There are innumerous ways to study the RNFL with OCT, but a fixed diameter circular scan around the optic disc has been used as standard for most of the investigators.
14 338 ARTICLE IN PRESS R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) The most used glaucoma scan acquisition protocols are the RNFL thickness (3.4) and the fast RNFL thickness (3.4). The former enables the acquirement of three circular scans of 3.46 mm diameter around the optic disc, which can then be averaged. The fast protocol acquires three circle images of lower resolution sequentially in one single-scan acquisition session (Fig. 24). While the RNFL thickness acquisition protocol is composed of one circle with 512 A-scans/image and requires 1.28 s of scanning time, the fast RNFL thickness comprises three circles of 256 A-scans/ image and requires 1.92 s. Proportional circle and the RNFL thickness (2.27 disc) acquisition protocols enable one to account for the Fig. 24. (A) Fundus image and corresponding B-scan of the RNFL thickness 3.4-mm (circle diameter) acquisition protocol in the regular mode (512 A-scans/image). (B) Fundus image and corresponding B-scans of the fast RNFL thickness 3.4-mm (circle diameter) acquisition protocol. Three images of 256 A-scans each are captured simultaneously (consecutively, at same scanning session). variability of the optic disc size by multiplying the optic disc radius by a factor to determine the final diameter of measurement scanning circle (Fig. 25), thus RNFL measurements taking place further away from disc margin in larger discs. Most investigators prefer fixed circles rather than proportional circles to analyze the RNFL thickness. RNFL thickness measurements are displayed by quadrant, clock hour and overall mean. Compared with first generations OCT systems, the third generation OCT allows high-density scanning protocols, which produces images with high transverse pixel density, thus resulting in better image quality. However, increase in the image acquisition time may lead to measurement artifacts due to eye motion. In a study comparing fast RNFL scan acquisition protocol (256 A-scans/image) with RNFL measurement using images of 512 A-scans, Leung et al. (2004) concluded that the latter (high-density protocol) has provided better sensitivity and stronger correlation with visual function Reproducibility Schuman et al. (1996) have suggested the use of 3.4-mm diameter circle as the standard for RNFL OCT evaluation after a reproducibility study involving 11 normal volunteers and 10 glaucomatous patients. Each subject underwent five repetitions of a series of scans on five separate occasions. Each series consisted of three circular scans around the optic nerve head (diameters of 2.9, 3.4 and 4.5 mm). Reproducibility was better in a given eye on a given visit than from visit to visit. In addition, the internal fixation was superior to external fixation regarding reproducibility. The 3.4-mm circle was then suggested for future studies because reproducibility was significantly better at this circle diameter than at 2.9 mm. Additionally, the 3.4-mm circle allowed measurement of NFL in a Fig. 25. Four different RNFL thickness circle acquisition protocols. (A) Fixed 3.4 mm diameter circle. (B D) After determining the disc radius (1 mm) three additional protocols enable to tailor de measurement circle accordingly: (B) nerve head circle (measurement circle was chosen to be 400 mm after the disc margin), (C) RNFL thickness (2.27 disc), and (D) proportional circle (1.5 was chosen as the multiplication factor).
15 R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) thicker area than 4.5 mm, what potentially permits a higher sensitivity to subtle NFL defects. Other reproducibility studies have demonstrated that OCT RNFL measurements are reproducible for both normal and glaucomatous eyes (Blumenthal et al., 2000; Carpineto et al., 2003; Paunescu et al., 2004; Budenz et al., 2005). Blumenthal et al. (2000) evaluated the reproducibility of OCT RNFL measurements in normal and glaucomatous patients for a prospective instrument validation study. Only a modest contribution to variability was found for session (1%), visit (5%), and operator (2%). Budenz et al. (2005) studying the reproducibility of standard and fast RNLF thickness scans found that both scan acquisition protocols yields reproducible and comparable measurements in glaucoma as well as in healthy individuals. The nasal quadrant showed more variations in the measurements than other sectors. According to Paunescu et al. (2004), the best reproducibility for RNFL measurements was found for dilated eyes and scanning rate of 256 A-scans per image acquired in the fast mode when compared to high-density scanning (512 A-scans per image) acquired in the regular acquisition mode. Although increased density may cause a reproducibility problem, higher-density scanning protocols have provided better diagnostic sensitivity in glaucoma detection and a stronger correlation with visual function according to Leung et al. (2004) Diagnostic capability and progression evaluation According to Schuman et al. (1995), RNFL measurements by OCT demonstrate a high degree of correlation with functional status, as measured by visual field examination. Neither cupping of the optic disc nor neural rim area were as strongly associated with visual field loss as was RNFL thickness in that study. RNFL, especially in the inferior quadrant, was significantly thinner in glaucomatous eyes than in normal eyes. Finally, it was found a decrease in RNFL thickness with aging, even when controlling for factors associated with the diagnosis of glaucoma. In a retrospective observational case series study that included 29 glaucoma patients, RNFL thickness measured with OCT was topographically correlated with glaucomatous visual field defects measured with short-wavelength automated perimetry (SWAP) (Sanchez-Galeana et al., 2004). In a paper by Pieroth et al. (1999), OCT-enabled focal defects detection with a sensitivity of 65% and a specificity of 81%. OCT analysis of RNFL thickness in eyes with focal defects showed good structural and functional correlation with clinical parameters and allowed identification of focal defects in the RNFL in early stages of glaucoma (Fig. 26). By evaluating 64 eyes of glaucoma or glaucoma-suspect individuals, Wollstein et al. (2005a) have shown a greater likelihood of detection of glaucomatous progression by the OCT in comparison to standard automated perimetry. In another study, the mean RNFL thickness of 28 ocular hypertensive eyes was compared with age-matched 30 normal and 29 glaucomatous eyes using 3.4-mm diameter scans (Bowd et al., 2000). Average RNFL thickness in temporal, superior, nasal, inferior quadrants and total average were obtained. Mean RNFL was significantly thinner in ocular hypertensive eyes than in normal eyes, more specifically in the inferior and nasal quadrants. RNFL was significantly thinner in glaucomatous eyes than in ocular hypertensive and normal eyes (total RNFL and all quadrants). In a study published in 2001, Bowd et al. (2001) compared the abilities of scanning laser polarimetry (SLP), OCT, SWAP, and frequency-doubling technology perimetry (FDT) to discriminate between healthy eyes and those with early glaucoma, classified based on standard automated perimetry and optic disc appearance. In general, areas under the receiver operating characteristics (ROC) curve were largest for OCT parameters, followed by FDT, SLP, and SWAP, regardless of the definition of glaucoma used. The most sensitive OCT and FDT parameters tended to be more sensitive than the most sensitive SWAP and SLP parameters at the specificities investigated, regardless of diagnostic criteria. Medeiros et al. (2004) using the current commercial available versions of SLP (GDx-VCC), third generation OCT, and Heidelberg retina tomograph (HRT II), have demonstrated similar sensitivity results among the best parameters of each equipment. OCT RNFL thickness decreases with increasing RNFL damage detected with red-free photography and visual field (Soliman et al., 2002). The global average OCT RNFL thickness correlated significantly with the photographic total RNFL score. This study suggests the validity of OCT measurements and its potential advantage for detection of early cases of glaucoma. Leung et al. (2005a) published a study evaluating the relationship between structure and function in glaucoma. In this study, the Advanced Glaucoma Intervention Study and the Collaborative Initial Glaucoma Treatment Study scores as well as, the mean deviation in decibel an unlogged 1/Lambert were used as measures of visual function. Better correlations were demonstrated between OCT RNLF measurements and visual function than between GDx-VCC measurements and visual function. The RNFL thickness has also been measured in children (Mrugacz and Bakunowicz-Lazarczyk, 2005; Hess et al., 2005). In a study including 26 normal eyes and 26 glaucomatous eyes, Mrugacz and Bakunowicz-Lazarczyk (2005) found that the mean RNFL thickness as well as the inferior quadrant measurements was statistically thinner in children with glaucoma than in healthy ones. In another study, Hess et al. (2005) showed that both macular and RNFL thickness were thinner in glaucomatous in comparison with healthy children. OCT was used to measure macular and nerve fiber layer thickness and to analyze their correlation with each other and with glaucoma status (Guedes et al., 2003). Both macular and RNFL thickness as measured by OCT showed statistically significant correlations with
16 340 ARTICLE IN PRESS R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Fig. 26. Fundus photography showing localized RNFL defects (arrows) in both eyes, which could also be identified by OCT using the RNFL thickness average protocol (with normative data analysis). Glaucomatous damage was mild in the right eye, and moderate in the left eye. glaucoma, although RNFL thickness showed a stronger association than macular thickness. Ishikawa et al. (2005) developed a software algorithm to obtain automated segmentation measurements of retinal layers in the macula. Four retinal layers were obtained and the algorithm was capable to discriminate between glaucomatous and normal eyes. In three layers (macular nerve fiber layer, inner retinal complex, and outer retinal complex), the measurements were statistically significantly thinner in glaucomatous eyes than in normal eyes. In another study involving macular measurements, Leung et al. (2005b) found macular thickness measurements significantly reduced in glaucomatous patients. However, peripapillary RNFL thickness measurements provided greater power to discriminate between normal, glaucoma-suspect and glaucoma eyes than macular measurements. Wollstein et al. (2005b) also found the RNFL measurement to provide better discrimination between glaucomatous and normal individuals than macular measurement. In another report, Wollstein et al. (2004) show that the peripapillary RNFL measurements have higher sensitivity and specificity than macular measurements. New strategies to evaluate macular thickness in relation to glaucoma detection, including macular symmetry testing (Bagga et al., 2005), are under development. More recently, investigators have been trying to increase the sensitivity and specificity of the OCT by adding measurement information that this device provides rather than analyzing isolated parameters (Medeiros et al., 2005; Huang and Chen, 2005; Chen and Huang, 2005; Burgansky-Eliash et al., 2005). Medeiros et al. (2005) combining selected optic nerve head and RNFL parameters obtained larger area under ROC curve than using single parameters. Huang and Chen (2005) compared several automated classifiers to differentiate normal from glaucomatous eyes. In this study, the Mahalanobis space showed better results than linear discriminant analysis and artificial neural network. In another study by Chen and Huang (2005), 21 parameters (optic nerve head and RNFL) were combined to obtain a linear discriminant function. The use of linear discriminant analysis increased the discrimination power to differentiate glaucomatous and healthy individuals in that study. Five classifier methods to discriminate between glaucoma and healthy subjects were studied by Burgansky-Eliash et al. (2005). In this study, the classifier methods studied were linear discriminant analysis, support vector machine, recursive partitioning and regression tree,
17 R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) generalized linear model and generalized additive model. The largest area under ROC curve was obtained using support vector machine, and the best discrimination between advanced and early glaucoma was provided by the generalized additive model Normative database In spite of the good reproducibility and potential to detect glaucomatous damage, the format of data presentation by initial versions of third generation OCT software and absence of a normative reference restricted OCT RNFL data interpretation and its use in clinical setting as a routinely diagnostic tool. To address such particular issues, a normative RNFL database to analyze patient s data has been made available within the last software package (version 4.0) of third generation OCT systems. Nevertheless, normative reference analysis software for glaucoma applications is not fully developed, and there is a scarcity of age, refractive error, and mainly, race-specific normative data upon which to compare eyes. The normative database is based on 3.4-mm diameter circular scan measurements. This might represent a potential source of error since a fixed diameter does not account for the distance between the measurement circle and the optic disc margin. Considering the progressive decrease of the RNFL thickness with increasing distance from disc margin (Varma et al, 1996), the disc size may interfere in RNFL thickness measurements (Fig. 27). By using third generation OCT, Savini et al. (2005) have demonstrated that RNFL thickness increased significantly with an increase in optic disc size, and this can be due to a shorter distance between the scan and optic disc margin. New strategies have to be developed to better evaluate RNFL thickness. These ideas are in accordance with the findings of Carpineto et al. (2003). These authors studied glaucomatous patients and a gender- and age-matched group of normal subjects with three different circle diameter nerve head programs: R ¼ 1.73 mm (3.4-mm diameter circle) as well as 1.5R and 2.0R (optic nerve head scanning is performed after positioning an aiming circle whose dimension can be adjusted to the optic disc size, and the actual scan radius is greater than the aiming circle radius by the designated R). For each option, variance components and intraclass correlation coefficients were determined. The total variance increased with circle diameter and the intersubject standard deviation showed a tendency to increase with radius in both groups. The RNFL thickness decreased with increasing circle radius. Multiple regression for intraclass correlation coefficient of RNFL thickness showed that intraclass correlation coefficient was higher for normal eyes and for scan protocol 1.5R than for R ¼ 1:73 and 2.0R. The 1.5R option allowed RNFL measurements in a thicker area than R ¼ 1:73 and 2.0R Future directions By the use of the 3.4-mm circle scan acquisition protocol, Williams et al. (2002) have defined a parameter called NFL (50), which is the RNFL thickness value at which there is a 50% likelihood of a visual field defect with either automated standard or FDT perimetries. RNFL layer thickness analysis using this parameter demonstrated that OCT might be clinically useful in identifying subjects who have visual field loss. However, the positive predictive value suggested that OCT might need higher resolution and better reproducibility to enhance its sensitivity and specificity for population screening. Increase of the axial resolution may be needed to improve OCT efficacy in detecting and following glaucomatous loss, but it is quite likely that refinements in the actual software algorithm may be sufficient to increase the specificity and sensitivity of this technology. According to Jones et al. (2001), OCT measurements close to disc margin underestimate RNFL thickness in approximately 37%. In a study performed by Skaf et al. (2005), the OCT algorithm to determine RNFL thickness is incorrect close to disc margin up to approximately 400 mm resulting in underestimated RNFL measurements. This might happen because retinal nerve fibers have a different orientation Fig. 27. Fixed 3.4-mm circle and large optic discs. The measurement is performed closer to disc margin and values for RNFL thickness tend to be overestimated.
18 342 ARTICLE IN PRESS R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) close to disc margin (fibers curve to form the optic nerve) and/or the software may not be prepared with proper landmarks for this region Additional considerations OCT has demonstrated good reproducibility of measurements and capability to detect early glaucomatous damage. A normative database has been incorporated to the last version of the commercial available third generation OCT system. Nevertheless, while the 3.4-mm fixed circle around the optic disc remains as the standard protocol to analyze RNFL thickness, improvement of the database is demanding. Some other new features such as disc size compensation as well as new measurement landmarks and scan positions may be needed. The belief that optimized acquisition protocols and an improved algorithm for RNFL measurements, coupled with the current axial resolution of third generation OCT systems, can offer an extraordinary tool to glaucoma diagnostic and follow-up seems quite reasonable. Third generation OCT has superior resolution (o10 mm) compared with other instruments currently available for the same purpose. Some next generation ultrahigh-resolution OCT prototypes have even more axial resolution (2 3 mm) (Wollstein et al., 2005c). In addition, the possibility to associate the ultrahigh-resolution with a spectral/fourier domain detection enables a dramatic increase in image acquisition speed (Wojtkowski et al., 2005) and potentially eliminates the motion problem previously associated to high-density images. 4. Next generation OCT devices Significant progress in the field of OCT retinal imaging has been made since the third generation of OCT instruments was introduced by Zeiss Meditec in Since then a new instrument, which combines OCT with scanning laser ophthalmoscopy has been introduced by OTI in 2004 (Podoleanu et al., 2004). Also a new way of scanning has been used in OCT instrumentation for threedimensional imaging presented by Laser Diagnostic Technologies (Hitzenberger et al., 2003). Novel tools and OCT measurement techniques have been developed in research laboratories. Some of them may have significant impact on the OCT retinal-imaging field in the future. The most important developments are probably new light sources enabling imaging with sub-micron axial resolution (Drexler et al., 1999, 2001, 2003; Kowalevicz et al., 2002; Fujimoto, 2003) and a novel high-speed OCT technique called spectral OCT (Fercher et al., 1995; Hausler and Linduer, 1998; Wojtkowski et al., 2002b). Spectral OCT is based on Fourier domain detection, which allows increasing the measurement speed more than 50 times comparing to commercial OCT instrument (Nassif et al., 2004; Wojtkowski et al., 2003). In principle OCT is able to provide three-dimensional information about the retinal morphology similar to other tomographic methods including CT and MRI. However, the speeds of commercially available third generation OCT and combined SLO-OCT OTI instruments are insufficient to measure full sets of three-dimensional data having a large number of pixels per image in vivo. The highest reported speeds for retinal OCT systems based on standard OCT detection have been achieved by transverse scanning with an acousto-optic modulator generating a highly stable carrier frequency (Hitzenberger et al., 2003). This system (Hitzenberger et al., 2003) can acquire cross-sectional images of the retina almost five times faster than OCT 3. The demonstrated system enables three-dimensional data acquisition with a fundus field of view up to 151, 64 points per axial scan, and 256 lines per cross-sectional image. Such a low number of pixels prevents exact analysis of cross-sectional information. In order to design threedimensional OCT instruments capable of collecting crosssectional images with pixel counts similar to third generation OCT, the acquisition speed should be increased by at least 50 times compared to the commercial unit. This can be realized only by a significant redesign of the OCT system. A recently demonstrated development is the novel application of Fourier domain detection to OCT technology (Hausler and Linduer, 1998; Wojtkowski et al., 2002b). This new method significantly improves the speed and sensitivity of OCT instruments (Choma et al., 2003a; de Boer et al., 2003; Leitgeb et al., 2003a). One of the most important considerations for OCT instruments imaging the laminar structure of the retina is the axial image resolution. This parameter is determined by the spectral bandwidth of the light source used in the OCT instrument (Drexler, 2004; Fercher et al., 2003). In order to improve the axial resolution, new broad bandwidth light sources have been constructed and applied to OCT systems (Drexler, 2004; Drexler et al., 1999). Application of these light sources to the novel high speed OCT instruments based on Fourier domain detection can provide a very powerful tool for ophthalmic diagnostics in the future Spectral OCT instrument using Fourier domain detection Recently developed Fourier domain OCT imaging techniques dramatically improve the sensitivity and imaging speed of OCT (Choma et al., 2003a; de Boer et al., 2003; Leitgeb et al., 2003a). In Fourier domain OCT the axial structure of an object (optical A-scan) is retrieved from the interferometric signal detected as a function of the optical frequency (spectral fringe pattern). Fourier domain OCT detection can be performed in two complementary ways: Spectral OCT (SOCT) using a spectrometer with a multi-channel detector (Fercher et al., 1995) or Swept Source OCT using a rapidly tunable laser source (Chinn et al., 1997; Lexer et al., 1997; Yun et al., 2003). Spectral OCT and swept source OCT are especially promising for ultrahigh-resolution imaging because they overcome the imaging speed limitations of standard OCT. Therefore, it is
19 R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) possible to use these techniques to form three-dimensional maps of the macula and the optic disc (Nassif et al., 2004; Wojtkowski et al., 2004a). In addition, the advantage of providing direct access to the spectral fringe pattern enables a wide range of novel applications. Direct access to the spectral information and phase of the interference fringes permits measurement of absorption (Leitgeb et al., 2000), numerical compensation of dispersion (Cense et al., 2004; Wojtkowski et al., 2004b), and numerical resolution improvement (Szkulmowski et al., 2005). It was also demonstrated that SOCT measurements have the advantage of high phase stability, which causes minimum detectable flow velocity. This is 25 times less than what has been measured using standard OCT and results in 100 mm/s 5 mm/s of measurable flow velocities (Leitgeb et al., 2003b, 2004b; White et al., 2003). The first ophthalmic application of single-scan spectral OCT was the measurement of eye length (Fercher et al., 1995). Demonstration of biomedical OCT imaging of human skin in vivo using Fourier domain detection was presented in 1996 (Hausler and Linduer, 1998). The first retinal and anterior chamber imaging using spectral OCT was reported in 2002 (Wojtkowski et al., 2002b). Continuation of this work resulted in the demonstration of the first high-speed retinal imaging obtained using a spectral OCT system with 10 mm axial resolution and acquisition time of 8 ms for a 128 transverse pixel image (Wojtkowski et al., 2003). Application of the line scan CCD camera, enabling high acquisition speeds of up to 29,000 axial scans per second (Nassif et al., 2004). This represents an approximately 70 improvement in imaging speed compared to third generation OCT, which only operates at 400 axial scans per second. One of the first experiments with combined Fourier domain and ultrahigh-resolution OCT was performed in 2003, and examples of ophthalmic imaging with 3 mm axial resolution were published in 2004 (Wojtkowski et al., 2004a). Other groups have also recently demonstrated ultrahigh-resolution imaging using SOCT. A resolution of 3.5 mm in the retina was achieved at acquisition rates of 15,000 axial scans per second (Cense et al., 2004). An image resolution of 2.5 mm was demonstrated at 10,000 axial scans per second (Leitgeb et al., 2004a). The best axial resolution in the retina to date, 2.1 mm, was achieved using high-speed acquisition rates of 16,000 axial scans per second by the group at MIT (Wojtkowski et al., 2004b). The first demonstration of ophthalmic ranging by swept source OCT was done in 1997 (Lexer et al., 1997). Recently, a group from Wellman Laboratories demonstrated a new high speed tunable laser operating with a central wavelength of 1310 nm enabling imaging at the rate of 15,600 A-scans per second (Yun et al., 2003). The absorption of water at 1310 nm prevents the application of this laser to retinal imaging. A swept source OCT system using 800 nm wavelength suitable for retinal imaging has not been reported to date. Therefore, in this contribution, the potential of the new Fourier domain OCT detection for retinal imaging will be demonstrated by a spectral OCT instrument Standard-resolution retinal imaging with high-speed spectral OCT The measurement speed of a third generation OCT instrument is less than 400 axial scans per second. Therefore, this instrument needs more than 1 s (approximately 1.92 s) to acquire an OCT image with 512 optical A-scans. One-second measurements by regular OCT suffer from motion artifacts in the obtained cross-sectional images (Fig. 28). These artifacts can be corrected by automated numerical alignment of adjacent optical A-scans. This procedure can generate errors in the presence of discontinuities in the retinal structure caused by pathological changes, or that are naturally existent in the region of the optic disc. Also, these methods flatten cross-sectional images and information about the true topography of the retina is automatically lost. Fig. 28 shows a comparison of cross-sectional images of normal macula taken by standard third generation OCT and the new spectral OCT instrument based on Fourier domain detection. In both cases the axial resolution is 10 mm. The motion artifacts in the presented third generation OCT image required numerical correction whereas the spectral OCT cross-sectional image, measured in only 0.17 s, did not require any motion correction. The speed advantage of the spectral OCT instrument enables the acquisition of cross-sectional images with many more optical A-scans. The cross-sectional image of the macula presented in Fig. 28(c) is reconstructed from 4000 A-scans. An increased number of A-scans and a slightly increased transverse resolution can dramatically improve the quality of SOCT images. Fig. 29(a) shows the cross sectional SOCT image of the retinal panorama measured horizontally from the fovea to the inferior part of the optic disc. Fig. 29(b) shows another cross-sectional image taken across the fovea of the same subject. Both of these images are reconstructed from 2500 A-scans with an axial resolution of 10 mm. Here the nerve fiber layer, ganglion cell layer, inner and outer plexiform layers, inner and outer nuclear layer, external limiting membrane, junction between the photoreceptor inner and outer segments, and retinal pigment epithelium are all well visualized and delineated more clearly than in standard third generation OCT. Another important advantage of high-speed imaging is the possibility of real time observation of cross-sectional images measured in vivo. This makes it more comfortable for the operator and can shorten the total examination time. It is also possible to choose the region of interest and the scanning range during the examination, which can help in imaging small focal pathologic changes present outside the macula or optic disc regions. Furthermore, the realtime imaging performed by the spectral OCT instrument enables observation of dynamic changes present in the retina. In Fig. 30 the set of real-time spectral OCT crosssectional images of the peripheral part of the human optic
20 344 ARTICLE IN PRESS R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Fig. 28. Comparison of standard-resolution third generation optical coherence tomography (OCT) (Stratus OCT) and spectral OCT images. (A) Stratus OCT image of macula contains 512 axial scans (A-scans) and was acquired in 1.3 s with axial resolution of 10 micrometers. (B) Stratus OCT image after numerical correction of motion artifacts. (C) spectral OCT image of macula contains 4000 A-scans and was acquired in 0.17 s with axial resolution of 10 mm. disc is presented. Each frame consists of 128 A-scans with 512 samples per A-scan. The exposure time is 32 ms per A-scan. The transverse scan was performed superiorly to the optic disc cup area in order to examine blood vessels. The region indicated by the arrow in the last frame varies during the measurement, whereas the rest of the image is stable. This is most likely caused by a pulsation of the blood vessels. The most important advantage of high-speed imaging with spectral OCT is that this technique enables threedimensional data collection similar to other tomographic techniques. Standard third generation OCT devices use specific imaging protocols in order to obtain quantitative information about full retinal thickness, retinal nerve fiber layer thickness, and optic nerve head parameters. Spectral OCT instruments enable the acquisition of all this information with the use of one scanning protocol the raster scan, which provides three-dimensional volumetric data of retinal structure (Nassif et al., 2004; Wojtkowski et al., 2004a). Additionally, raster scanning simplifies data processing and reconstruction of cross-sectional images rendered with arbitrary orientations. For spectral OCT, raster scanning also can provide combined profilometric and cross-sectional information that is important for quantitative analysis of optic nerve head parameters or nerve fiber layer thickness. Fig. 31 shows examples of the imaging and processing of three-dimensional retinal data. In Fig. 31(a), the volume rendering of the optic disc region is shown. Using software similar to that used for MRI enables segmentation of specific intraretinal
21 R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Fig. 29. Standard-resolution spectral optical coherence tomography retinal imaging with high quality: (A) cross-sectional image of the retinal panorama measured horizontally from temporal to nasal along the fovea to the inferior part of the optic disc, (B) cross-sectional image of the fovea measured in the same eye. Both images contain 2500 axial scans. Red square shows approximately the region where the bottom image was taken. layers from the three-dimensional data set (Fig. 31(b)). Three-dimensional imaging also has the advantage of reconstructing fundus view (Wojtkowski et al., 2004a) similar to this obtained by scanning laser ophthalmoscopy. The OCT fundus image is generated by summing the reflectivities of successive layers along the axial direction. The SOCT fundus image enables precise registration of OCT cross-sections with fundus photography. Fig. 32 shows the fundus image created from a three-dimensional data set acquired using standard-resolution spectral OCT. The pattern created by the blood vessels is clearly visible and can be used to correlate the OCT data with the fundus photograph High-resolution retinal imaging with high-speed spectral OCT The first demonstration of ultrahigh-resolution retinal imaging with 3-mm resolution using a standard OCT system was demonstrated in 1999 by Drexler et al. This represents a factor of five- to ten- fold resolution improvement over standard third generation OCT instruments, which has 10 mm axial resolution. This technology has already been implemented in two clinical systems constructed at MIT (Drexler et al., 2001; Ko et al., 2005) and the University of Vienna (Drexler et al., 2003). Both of these systems use femtosecond titanium:sapphire (Ti:Sa) lasers as light sources. Clinical results obtained with these instruments demonstrate that ultrahigh-resolution OCT can greatly improve the visualization of retinal architectural morphology, and promise to improve the accuracy of quantitative morphometric measurements. Ultrahigh-resolution OCT enables the detection of individual retinal layers such as the ganglion cell layer, inner and outer nuclear and plexiform layers, as well as the photoreceptor and RPE morphology, which are difficult to visualize with standard-resolution OCT (Drexler et al., 1999, 2001, 2003; Ko et al., 2004). In contrast to commercially available OCT instruments, ultrahigh-resolution OCT can reveal changes in retinal morphology associated with retinal disease, such as photoreceptor integrity or impairment (Drexler et al., 2003; Ko et al., 2004). Ultrahigh-resolution OCT has not yet been commercialized because of the high cost of femtosecond lasers. The recent introduction of compact broadband semiconductor light sources will enable widespread use of ultrahighresolution OCT instruments. The cost of the broadband superluminescent diode is approximately 5 times lower than the cost of the full Ti:Sa laser system. These light sources are based on two or more superluminescent diode modules combined by single mode fiber couplers. The application of broadband semiconductor light sources to OCT ophthalmic imaging has been demonstrated in 2004 (Ko et al., 2004). The high-quality retinal images obtained by a spectral OCT system using combined superluminescent diode modules has been also presented in 2004 (Cense et al., 2004).
22 346 ARTICLE IN PRESS R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Fig. 30. Real-time spectral optical coherence tomography observation of dynamic processes in the retina: (A) the set of cross-sectional images measured with 8 frames per second; (B) cross-sectional image of optic disc region with indicated region of interest. Fig. 33 shows the cross-sectional SOCT images of the macula and optic disc measured with a high axial resolution of 4.5 mm. The delineation of retinal layers in the presented image is much clearer than that of standardresolution imaging. The improvement is especially visible in the retinal pigment epithelium, ganglion cell layer, and photoreceptor layer. The high number of collected A-scans helps to decrease the noise level and improves the continuity of retinal layers. The performance of automated segmentation algorithms for thickness measurements and layer identification can also be improved. The collection of three-dimensional ultrahigh-resolution SOCT data enables the quantitative analysis of all major retinal layers, including photoreceptor layer details such as the external limiting membrane (ELM), junction between the inner and outer segments (IS/OS), and the RPE. Fig. 34 shows an example of the full thickness retinal map and a thickness map of the part of the photoreceptor layer from IS/OS to RPE. This analysis has great potential in objective measurements of progression in various macular diseases. Simultaneous increase of the coverage and resolution, guaranteed by three-dimensional ultrahigh-resolution highspeed SOCT, will enable analysis of small focal pathological changes that can be missed by standard OCT techniques. This can effectively improve the capability of OCT technology to diagnose early pathological changes
23 R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Fig. 31. Three-dimensional spectral optical coherence tomography imaging with standard resolution: (A) volume rendering of optic disc region, (B) segmentation of intraretinal layers in macular region; NFL-nerve fiber layer, OPL-outer plexiform layer, IS/OS-junction between inner and outer segments of photoreceptors, and RPE- retinal pigment epithelium. Fig. 32. Three-dimensional spectral optical coherence tomography (OCT) imaging with standard resolution: (A) fundus view (400 horizontal points, 300 vertical points) reconstructed from the spectral OCT data contains 300 cross-sectional images; (B) two cross-sectional images from the three-dimensional set of data. The location of each cross-sectional image is perfectly registered relative to the fundus view. and can help in understanding the pathogenesis of retinal diseases Additional considerations Combined ultrahigh resolution and Fourier domain detection techniques can give unprecedented improvement of the capability of OCT systems for retinal imaging. However, the advantages of the new spectral OCT technique cannot be easily realized as a commercial clinical instrument. Technical problems still exist, which impede the introduction of spectral OCT instruments to ophthalmology clinics. For example, images obtained by SOCT can suffer from coherent noise artifacts and conjugate images (Wojtkowski et al., 2002a, b), which decrease the effective axial measurement range and can lead to misinterpretation of resultant tomographic images. These artifacts cause folding of the OCT cross-sectional image if the optical distance of the structure to be imaged is higher than the effective measurement range. It has been shown that these unwanted effects can be eliminated by phase-shifting methods (Choma et al., 2003b; Targowski et al., 2004, 2005; Wojtkowski et al., 2002a). These techniques require the collection of multiple signals from
24 348 ARTICLE IN PRESS R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Fig. 33. High-resolution spectral optical coherence tomography imaging: (A) cross-sectional image of macula, and (B) cross-sectional image of optic disc. Both images contain axial scans and were acquired in 0.43 s with axial resolution of 4.5 mm. the same region taken with an additional selected phase shift in the reference arm, with the condition that the object is stationary between the measurements to within a fraction of micrometer. The latter condition makes these phaseshifting methods hard to apply for clinical practice. Another unsolved problem in spectral OCT is the drop of sensitivity with increase of imaging depth (Hausler and Linduer, 1998; Wojtkowski et al., 2002b). This effect is especially significant in ultrahigh-resolution SOCT imaging where the digitalization must be much finer than in standard-resolution OCT. This problem is fundamentally associated with the detection performed by a spectrometer (Hausler and Linduer, 1998) and it is much less severe in swept source OCT (Yun et al., 2003). Also the swept source OCT gives more flexibility in controlling of the axial scan parameters, which are fixed in spectral OCT (Wojtkowski et al., 2004b). The future of the clinical application of Fourier domain detection to retinal imaging will depend on how the problems mentioned above will be solved, as well as on the development of high-speed tunable laser technology, which will enable the application of swept source OCT to retinal imaging. 5. Concluding remarks The introduction of OCT in ophthalmology represents a definitive change in the way doctors understand and treat several diseases affecting the retina. It is quite likely as well, that the role of OCT as a method to diagnose and manage glaucoma will be further defined in the near future. Understanding of the basic principles in which OCT relays on is essential to understand its actual limitations and to use this technology with wisdom. We have already learned a lot with data provided by first generations of OCT, and there is much more to learn with forthcoming data from numerous ongoing studies worldwide that, presently, are using third generation OCT systems. A huge leap forward in improving OCT imaging performance is expected to occur within the next few years (or should we say months?) with the commercial availability of next generation OCT
25 R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Fig. 34. Three-dimensional high-resolution, spectral optical coherence tomography imaging: (A) reconstruction of the OCT fundus view, (B) crosssectional image with delineated automatically segmented layers, (C) full retinal thickness map, and (D) thickness map of outer segments of photoreceptors; ILM-inner limiting membrane, IS/OS-junction between inner and outer segments of photoreceptors, and RPE-retinal pigment epithelium. devices. One should say that three-dimensional, highspeed, ultrahigh-resolution retinal assessment is just around the corner. Acknowledgments M.W. thanks Prof. Andrzej Kowalczyk and members of Medical Physics Group from Nicolaus Copernicus University in Torun especially to Iwona Gorczynska and Anna Szkulmowska, and Prof. James G. Fujimoto from Massachusetts Institute of Technology, Cambridge, MA, USA and members of his group: Vikas Sharma, Aurea Zare, Vivek Srinivasan, Tony Ko and Mariana Carvalho. R.A.C. and M.W. includes special thank to Yijun Huang, and to Robert Huber and Robert Zawadzki, respectively, for creative discussions. References Aleman, T.S., Cideciyan, A.V., Volpe, N.J., Stevanin, G., Brice, A., Jacobson, S.G., Spinocerebellar ataxia type 7 (SCA7) shows a cone-rod dystrophy phenotype. Exp. Eye Res. 74, Antcliff, R.J., Spalton, D.J., Stanford, M.R., Graham, E.M., Fytche, T.J., Marshall, J., Intravitreal triamcinolone for uveitic cystoid macular edema: an optical coherence tomography study. Ophthalmology 108, Bagga, H., Greenfield, D.S., Knighton, R.W., Macular symmetry testing for glaucoma detection. J. Glaucoma 14, Bartsch, D-U., Freeman, W.R., Axial intensity distribution analysis of the human retina with a confocal scanning laser tomograph. Exp. Eye Res. 58, Blumenthal, E.Z., Williams, J.M., Weinreb, R.N., Girkin, C.A., Berry, C.C., Zangwill, L.M., Reproducibility of nerve fiber layer thickness measurements by use of optical coherence tomography. Ophthalmology 107, Bonini-Filho, M.A., Jorge, R., Barbosa, J.C., Calucci, D., Cardillo, J.A., Costa, R.A., Intravitreal injection versus sub-tenon s infusion of triamcinolone acetonide for refractory diabetic macular edema: a randomized clinical trial. Invest. Ophthalmol. Vis. Sci. 46, Bowd, C., Weinreb, R.N., Williams, J.M., Zangwill, L.M., The retinal nerve fiber layer thickness in ocular hypertensive, normal, and glaucomatous eyes with optical coherence tomography. Arch. Ophthalmol. 118, Bowd, C., Zangwill, L.M., Berry, C.C., Blumenthal, E.Z., Vasile, C., Sanchez-Galeana, C., Bosworth, C.F., Sample, P.A., Weinreb, R.N., Detecting early glaucoma by assessment of retinal nerve fiber layer thickness and visual function. Invest. Ophthalmol. Vis. Sci. 42,
26 350 ARTICLE IN PRESS R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Browning, D.J., McOwen, M.D., Bowen Jr., R.M., O Marah, T.L., Comparison of the clinical diagnosis of diabetic macular edema with diagnosis by optical coherence tomography. Ophthalmology 111, Budenz, D.L., Chang, R.T., Huang, X., Knighton, R.W., Tielsch, J.M., Reproducibility of retinal nerve fiber thickness measurements using the stratus OCT in normal and glaucomatous eyes. Invest. Ophthalmol. Vis. Sci. 46, Burgansky-Eliash, Z., Wollstein, G., Chu, T., Ramsey, J.D., Glymour, C., Noecker, R.J., Ishikawa, H., Schuman, J.S., Optical coherence tomography machine learning classifiers for glaucoma detection: a preliminary study. Invest. Ophthalmol. Vis. Sci. 46, Campochiaro, P.A., C99-PKC Study Group, Reduction of diabetic macular edema by oral administration of the kinase inhibitor PKC412. Invest. Ophthalmol. Vis. Sci. 45, Cardillo, J.A., Melo Jr., L.A., Costa, R.A., Skaf, M., Belfort Jr., R., Souza-Filho, A.A., Farah, M.E., Kuppermann, B.D., Comparison of intravitreal versus posterior sub-tenon s capsule injection of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology 112, Carpineto, P., Ciancaglini, M., Zuppardi, E., Falconio, G., Doronzo, E., Mastropasqua, L., Reliability of nerve fiber layer thickness measurements using optical coherence tomography in normal and glaucomatous eyes. Ophthalmology 110, Catier, A., Tadayoni, R., Paques, M., Erginay, A., Haouchine, B., Gaudric, A., Massin, P., Characterization of macular edema from various etiologies by optical coherence tomography. Am. J. Ophthalmol. 140, Cense, B., Nassif, N., Chen, T.C., Pierce, M.C., Yun, S., Park, B.H., Bouma, B., Tearney, G., de Boer, J.F., Ultrahigh-resolution high-speed retinal imaging using spectral-domain optical coherence tomography. Opt. Express 12, Chen, H.Y., Huang, M.L., Discrimination between normal and glaucomatous eyes using Stratus optical coherence tomography in Taiwan Chinese subjects. Graefes Arch. Clin. Exp. Ophthalmol. 243, Chinn, S.R., Swanson, E.A., Fujimoto, J.G., Optical coherence tomography using a frequency-tunable optical source. Opt. Lett. 22, Choma, M.A., Sarunic, M.V., Yang, C.H., Izatt, J.A., 2003a. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt. Express 11, Choma, M.A., Yang, C.H., Izatt, J.A., 2003b. Instantaneous quadrature low-coherence interferometry with 3 3 fiber-optic couplers. Opt. Lett. 28, Cideciyan, A.V., Jacobson, S.G., Aleman, T.S., et al., In vivo dynamics of retinal injury and repair in the rhodopsin mutant dog model of human retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 102, (Epub 2005 Mar 22). Costa, R.A., Evaluation of image artifact produced by optical coherence tomography of retinal pathology. Am. J. Ophthalmol. 140, Costa, R.A., Scapucin, L., Moraes, N.S., Calucci, D., Melo Jr., L.A., Cardillo, J.A., Farah, M.E., Indocyanine green-mediated photothrombosis as a new technique of treatment for persistent central serous chorioretinopathy. Curr. Eye Res. 25, Costa, R.A., Farah, M.E., Cardillo, J.A., Calucci, D., Williams, G.A., 2003a. Immediate indocyanine green angiography and optical coherence tomography evaluation after photodynamic therapy for subfoveal choroidal neovascularization. Retina 23, Costa, R.A., Calucci, D., Cardillo, J.A., Farah, M.E., 2003b. Selective occlusion of subfoveal choroidal neovascularization in angioid streaks by using a new technique of ingrowth site treatment. Ophthalmology 110, Costa, R.A., Calucci, D., Teixeira, L.F., Cardillo, J.A., Bonomo, P.P., 2003c. Selective occlusion of subfoveal choroidal neovascularization in pathologic myopia using a new technique of ingrowth site treatment. Am. J. Ophthalmol. 135, Costa, R.A., Calucci, D., Skaf, M., Cardillo, J.A., Castro, J.C., Melo Jr., L.A., Martins, M.C., Kaiser, P.K., Optical coherence tomography 3: automatic delineation of the outer neural retinal boundary and its influence on retinal thickness measurements. Invest. Ophthalmol. Vis. Sci. 45, Costa, R.A., Calucci, D., Paccola, L., Jorge, R., Cardillo, J.A., Castro, J.C., Scott, I.U., Occult chorioretinal anastomosis in age-related macular degeneration: a prospective study by optical coherence tomography. Am. J. Ophthalmol. 140, de Boer, J.F., Cense, B., Park, B.H., Pierce, M.C., Tearney, G.J., Bouma, B.E., Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Opt. Lett. 28, Doggrell, S.A., Pegaptanib: the first antiangiogenic agent approved for neovascular macular degeneration. Expert. Opin. Pharmacother. 6, Drexler, W., Ultrahigh-resolution optical coherence tomography. J. Biomed. Opt. 9, Drexler, W., Morgner, U., Kartner, F.X., Pitris, C., Boppart, S.A., Li, X.D., Ippen, E.P., Fujimoto, J.G., In vivo ultrahigh-resolution optical coherence tomography. Opt. Lett. 24, Drexler, W., Morgner, U., Ghanta, R.K., Kärtner, F.X., Schuman, J.S., Fujimoto, J.G., Ultrahigh-resolution ophthalmic optical coherence tomography. Nat. Med. 7, (Erratum in: Nat. Med. 7, 636). Drexler, W., Sattmann, H., Hermann, B., Ko, T.H., Stur, M., Unterhuber, A., Scholda, C., Findl, O., Wirtitsch, M., Fujimoto, J.G., Fercher, A.F., Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Arch. Ophthalmol. 121, Eandi, C.M., Chung, J.E., Cardillo-Piccolino, F., Spaide, R.F., Optical coherence tomography in unilateral resolved central serous chorioretinopathy. Retina 25, Eter, N., Spaide, R.F., Comparison of fluorescein angiography and optical coherence tomography for patients with choroidal neovascularization after photodynamic therapy. Retina 25, Fercher, A.F., Hitzenberger, C.K., Kamp, G., Elzaiat, S.Y., Measurement of intraocular distances by backscattering spectral interferometry. Opt. Commun. 117, Fercher, A.F., Drexler, W., Hitzenberger, C.K., Lasser, T., Optical coherence tomography-principles and applications. Reports Prog. Phys. 66, Fujimoto, J.G., Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat. Biotech. 21, Gaucher, D., Tadayoni, R., Erginay, A., Haouchine, B., Gaudric, A., Massin, P., Optical coherence tomography assessment of the vitreoretinal relationship in diabetic macular edema. Am. J. Ophthalmol. 139, Gaudric, A., Haouchine, B., Massin, P., Paques, M., Blain, P., Erginay, A., Macular hole formation: new data provided by optical coherence tomography. Arch. Ophthalmol. 117, Gragoudas, E.S., Adamis, A.P., Cunningham Jr., E.T., Feinsod, M., Guyer, D.R., VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group, Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 351, Greenberg, P.B., Martidis, A., Rogers, A.H., Duker, J.S., Reichel, E., Intravitreal triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Br. J. Ophthalmol. 86, Gross, J.G., Late reopening and spontaneous closure of previously repaired macular holes. Am. J. Ophthalmol. 140, Guedes, V., Schuman, J.S., Hertzmark, E., Wollstein, G., Correnti, A., Mancini, R., Lederer, D., Voskanian, S., Velazquez, L., Pakter, H.M., Pedut-Kloizman, T., Fujimoto, J.G., Mattox, C., Optical coherence tomography measurement of macular and nerve fiber layer thickness in normal and glaucomatous human eyes. Ophthalmology 110, Haouchine, B., Massin, P., Gaudric, A., Foveal pseudocyst as the first step in macular hole formation: a prospective study by optical coherence tomography. Ophthalmology 108,
27 R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Haouchine, B., Massin, P., Tadayoni, R., Erginay, A., Gaudric, A., Diagnosis of macular pseudoholes and lamellar macular holes by optical coherence tomography. Am. J. Ophthalmol. 138, Hausler, G., Linduer, M.W., Coherence radar and spectral radar new tools for dermatological diagnosis. J. Biomed. Opt. 3, Hee, M.R., Artifacts in optical coherence tomography topographic maps. Am. J. Ophthalmol. 139, Hee, M.R., Izatt, J.A., Swanson, E.A., Huang, D., Schuman, J.S., Lin, C.P., Puliafito, C.A., Fujimoto, J.G., 1995a. Optical coherence tomography of the human retina. Arch. Ophthalmol. 113, Hee, M.R., Puliafito, C.A., Wong, C., Duker, J.S., Reichel, E., Schuman, J.S., Swanson, E.A., Fujimoto, J.G., 1995b. Optical coherence tomography of macular holes. Ophthalmology 102, Hee, M.R., Puliafito, C.A., Duker, J.S., Reichel, E., Coker, J.G., Wilkins, J.R., Schuman, J.S., Swanson, E.A., Fujimoto, J.G., Topography of diabetic macular edema with optical coherence tomography. Ophthalmology 105, Hess, D.B., Asrani, S.G., Bhide, M.G., Enyedi, L.B., Stinnett, S.S., Freedman, S.F., Macular and retinal nerve fiber layer analysis of normal and glaucomatous eyes in children using optical coherence tomography. Am. J. Ophthalmol. 139, Hillenkamp, J., Saikia, P., Gora, F., Sachs, H.G., Lohmann, C.P., Roider, J., Baumler, W., Gabel, V.P., Macular function and morphology after peeling of idiopathic epiretinal membrane with and without the assistance of indocyanine green. Br. J. Ophthalmol. 89, Hitzenberger, C.K., Trost, P., Lo, P.W., Zhou, Q.Y., Threedimensional imaging of the human retina by high-speed optical coherence tomography. Opt. Expr. 11, Hoyt, W.F., Newman, N.M., The earliest observable defect in glaucoma? Lancet 1, Hoyt, W.F., Frisen, L., Newman, N.M., Fundoscopy of nerve fiber layer defects in glaucoma. Invest. Ophthalmol. 12, Huang, D., Swanson, E.A., Lin, C.P., et al., Optical coherence tomography. Science 254, Huang, D., Tan, O., Fujimoto, J.G., et al., Optical coherence tomography. In: Huang D, Kaiser PK, Lowder CY, Traboulsi E, (Eds.), Retinal Imaging, first ed., Elsevier; in progress. Huang, M.L., Chen, H.Y., Development and comparison of automated classifiers for glaucoma diagnosis using stratus optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 46, Huang, Y., Cideciyan, A.V., Papastergiou, G.I., Banin, E., Semple- Rowland, S.L., Milam, A.H., Jacobson, S.G., Relation of optical coherence tomography to microanatomy in normal and rd chickens. Invest. Ophthalmol. Vis. Sci. 39, Huang, Y., Optical coherence tomography (OCT) in hereditary retinal degenerations: layer-by-layer analyses in normal and diseased retinas. A dissertation in Bioengineering. Presented to the faculties of the University of Pennsylvania in partial fulfillment of the requirements for the degree of Doctor of Philosophy. Ishikawa, H., Stein, D.M., Wollstein, G., Beaton, S., Fujimoto, J.G., Schuman, J.S., Macular segmentation with optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 46, Jacobson, S.G., Cideciyan, A.V., Huang, Y., et al., Retinal degenerations with truncation mutations in the cone-rod homeobox (CRX) gene. Invest. Ophthalmol. Vis. Sci. 39, Jacobson, S.G., Cideciyan, A.V., Iannaccone, A., et al., Disease expression of RP1 mutations causing autosomal dominant retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 41, Jacobson, S.G., Cideciyan, A.V., Aleman, T.S., et al., Crumbs homolog 1 (CRB1) mutations result in a thick human retina with abnormal lamination. Hum. Mol. Genet. 12, Jacobson, S.G., Sumaroka, A., Aleman, T.S., et al., Nuclear receptor NR2E3 gene mutations distort human retinal laminar architecture and cause an unusual degeneration. Hum. Mol. Genet. 13, (Epub 30 June 2004). Jacobson, S.G., Aleman, T.S., Cideciyan, A.V., et al., Identifying photoreceptors in blind eyes caused by RPE65 mutations: prerequisite for human gene therapy success. Proc. Natl. Acad. Sci. USA 102, (Epub 18 April 2005). Johnson, M.W., Tractional cystoid macular edema: a subtle variant of the vitreomacular traction syndrome. Am. J. Ophthalmol. 140, Jones, A.L., Sheen, N.J., North, R.V., Morgan, J.E., The Humphrey optical coherence tomography scanner: quantitative analysis and reproducibility study of the normal human retinal nerve fibre layer. Br. J. Ophthalmol. 85, Jonas, J.B., Sofker, A., Intraocular injection of crystalline cortisone as adjunctive treatment of diabetic macular edema. Am. J. Ophthalmol. 132, Jonas, J.B., Kreissig, I., Degenring, R.F., Intravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusion. Graefes Arch. Clin. Exp. Ophthalmol. 240, (Epub 13 August 2002). Jorge, R., Costa, R.A., Quirino, L.S., Paques, M.W., Calucci, D., Cardillo, J.A., Scott, I.U., Optical coherence tomography findings in patients with late solar retinopathy. Am. J. Ophthalmol. 137, Kerrigan-Baumrind, L.A., Quigley, H.A., Pease, M.E., Kerrigan, D.F., Mitchell, R.S., Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest. Ophthalmol. Vis. Sci. 41, Kitaya, N., Hikichi, T., Kagokawa, H., Takamiya, A., Takahashi, A., Yoshida, A., Irregularity of photoreceptor layer after successful macular hole surgery prevents visual acuity improvement. Am. J. Ophthalmol. 138, Ko, T.H., Adler, D.C., Fujimoto, J.G., Mamedov, D., Prokhorov, V., Shidlovski, V., Yakubovich, S., Ultrahigh resolution optical coherence tomography imaging with a broadband superluminescent diode light source. Opt. Express 12, Ko, T.H., Fujimoto, J.G., Schuman, J.S., Paunescu, L.A., Kowalevicz, A.M., Hartl, I., Drexler, W., Wollstein, G., Duker, J.S., Ishikawa, H., Comparison of ultrahigh and standard resolution optical coherence tomography for imaging of macular pathology. Ophthalmology (22 September 2005, Epub ahead of print). Kowalevicz, A.M., Schibli, T.R., Kartner, F.X., Fujimoto, J.G., Ultralow-threshold Kerr-lens mode-locked Ti:Al2O3 laser. Opt. Lett. 27, Leitgeb, R., Wojtkowski, M., Kowalczyk, A., Hitzenberger, C.K., Sticker, M., Fercher, A.F., Spectral measurement of absorption by spectroscopic frequency-domain optical coherence tomography. Opt. Lett. 25, Leitgeb, R., Hitzenberger, C.K., Fercher, A.F., 2003a. Performance of Fourier domain vs. time domain optical coherence tomography. Opt. Express 11, Leitgeb, R.A., Schmetterer, L., Drexler, W., Fercher, A.F., Zawadzki, R.J., Bajraszewski, T., 2003b. Real-time assessment of retinal blood flow with ultrafast acquisition by color Doppler Fourier domain optical coherence tomography. Opt. Express 11, Leitgeb, R.A., Drexler, W., Unterhuber, A., Hermann, B., Bajraszewski, T., Le, T., Stingl, A., Fercher, A.F., 2004a. Ultrahigh resolution Fourier domain optical coherence tomography. Opt. Express 12, Leitgeb, R.A., Schmetterer, L., Hitzenberger, C.K., Fercher, A.F., Berisha, F., Wojtkowski, M., Bajraszewski, T., 2004b. Real-time measurement of in vitro flow by Fourier-domain color Doppler optical coherence tomography. Opt. Lett. 29, Lerche, R.C., Schaudig, U., Scholz, F., Walter, A., Richard, G., Structural changes of the retina in retinal vein occlusion: imaging and quantification with optical coherence tomography. Ophthal. Surg. Lasers 32, Leung, C.K., Yung, W.H., Ng, A.C., Woo, J., Tsang, M.K., Tse, K.K., Evaluation of scanning resolution on retinal nerve fiber layer measurement using optical coherence tomography in normal and glaucomatous eyes. J. Glaucoma 13, Leung, C.K., Chong, K.K., Chan, W.M., Yiu, C.K., Tso, M.Y., Woo, J., Tsang, M.K., Tse, K.K., Yung, W.H., 2005a. Comparative study of
28 352 ARTICLE IN PRESS R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) retinal nerve fiber layer measurement by Stratus OCT and GDx VCC, II: structure/function regression analysis in glaucoma. Invest. Ophthalmol. Vis. Sci. 46, Leung, C.K., Chan, W.M., Yung, W.H., Ng, A.C., Woo, J., Tsang, M.K., Tse, R.K., 2005b. Comparison of macular and peripapillary measurements for the detection of glaucoma: an optical coherence tomography study. Ophthalmology 112, Lexer, F., Hitzenberger, C.K., Fercher, A.F., Kulhavy, M., Wavelength-tuning interferometry of intraocular distances. Appl. Opt. 36, Martidis, A., Duker, J.S., Puliafito, C.A., Intravitreal triamcinolone for refractory cystoid macular edema secondary to birdshot retinochoroidopathy. Arch. Ophthalmol. 119, Martidis, A., Duker, J.S., Greenberg, P.B., Rogers, A.H., Puliafito, C.A., Reichel, E., Baumal, C., Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology 109, Medeiros, F.A., Zangwill, L.M., Bowd, C., Weinreb, R.N., Comparison of the GDx VCC scanning laser polarimeter, HRT II confocal scanning laser ophthalmoscope, and stratus OCT optical coherence tomograph for the detection of glaucoma. Arch. Ophthalmol. 122, Medeiros, F.A., Zangwill, L.M., Bowd, C., Vessani, R.M., Susanna Jr., R., Weinreb, R.N., Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am. J. Ophthalmol. 139, Meirelles, R.L., Aggio, F.B., Costa, R.A., Farah, M.E., STRATUS optical coherence tomography in unilateral colobomatous excavation of the optic disc and secondary retinoschisis. Graefes Arch. Clin. Exp. Ophthalmol. 243, (Epub 4 August 2004). Michels, S., Rosenfeld, P.J., Puliafito, C.A., Marcus, E.N., Venkatraman, A.S., Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology 112, Milam, A.H., Barakat, M.R., Gupta, N., et al., Clinicopathologic effects of mutant GUCY2D in Leber congenital amaurosis. Ophthalmology 110, Montero, J.A., Ruiz-Moreno, J.M., Optical coherence tomography characterisation of idiopathic central serous chorioretinopathy. Br. J. Ophthalmol. 89, Montero, J.A., Ruiz-Moreno, J.M., Tavolato, M., Follow-up of age-related macular degeneration patients treated by photodynamic therapy with optical coherence tomography 3. Graefes Arch. Clin. Exp. Ophthalmol. 241, Mrugacz, M., Bakunowicz-Lazarczyk, A., Optical coherence tomography measurement of the retinal nerve fiber layer in normal and juvenile glaucomatous eyes. Ophthalmologica 219, Nassif, N.A., Cense, B., Park, B.H., Pierce, M.C., Yun, S.H., Bouma, B.E., Tearney, G.J., Chen, T.C., de Boer, J.F., In vivo high-resolution video-rate spectral-domain optical coherence tomography of the human retina and optic nerve. Opt. Express 12, Niwa, H., Terasaki, H., Ito, Y., Miyake, Y., Macular hole development in fellow eyes of patients with unilateral macular hole. Am. J. Ophthalmol. 140, Paunescu, L.A., Schuman, J.S., Price, L.L., Stark, P.C., Beaton, S., Ishikawa, H., Wollstein, G., Fujimoto, J.G., Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using Stratus OCT. Invest. Ophthalmol. Vis. Sci. 45, Pianta, M.J., Aleman, T.S., Cideciyan, A.V., et al., In vivo micropathology of Best macular dystrophy with optical coherence tomography. Exp. Eye. Res. 76, Pieroth, L., Schuman, J.S., Hertzmark, E., Hee, M.R., Wilkins, J.R., Coker, J., Mattox, C., Pedut-Kloizman, R., Puliafito, C.A., Fujimoto, J.G., Swanson, E., Evaluation of focal defects of the nerve fiber layer using optical coherence tomography. Ophthalmology 106, Podoleanu, A.G., Seeger, M., Dobre, G.M., Webb, D.J., Jackson, D.A., Fitzke, F.W., Transversal and longitudinal images from the retina of the living eye using low coherence reflectometry. J. Biomed. Opt. 3, Podoleanu, A.G., Dobre, G.M., Cucu, R.G., Rosen, R., Garcia, P., Nieto, J., Will, D., Gentile, R., Muldoon, T., Walsh, J., Yannuzzi, L.A., Fisher, Y., Orlock, D., Weitz, R., Rogers, J.A., Dunne, S., Boxer, A., Combined multiplanar optical coherence tomography and confocal scanning ophthalmoscopy. J. Biomed. Opt. 9, Pons, M.E., Garcia-Valenzuela, E., Redefining the limit of the outer retina in optical coherence tomography scans. Ophthalmology 112, Puliafito, C.A., Hee, M.R., Schuman, J.S., Fujimoto, J.G., Optical Coherence Tomography of Ocular Diseases. Slack Inc., Thorafore, NJ. Sahni, J., Stanga, P., Wong, D., Harding, S., Optical coherence tomography in photodynamic therapy for subfoveal choroidal neovascularisation secondary to age related macular degeneration: a cross sectional study. Br. J. Ophthalmol. 89, Salinas-Alaman, A., Garcia-Layana, A., Maldonado, M.J., Sainz-Gomez, C., Alvarez-Vidal, A., Using optical coherence tomography to monitor photodynamic therapy in age related macular degeneration. Am. J. Ophthalmol. 140, Sanchez-Galeana, C.A., Bowd, C., Zangwill, L.M., Sample, P.A., Weinreb, R.N., Short-wavelength automated perimetry results are correlated with optical coherence tomography retinal nerve fiber layer thickness measurements in glaucomatous eyes. Ophthalmology 111, Sandhu, S.S., Talks, S.J., Correlation of optical coherence tomography, with or without additional colour fundus photography, with stereo fundus fluorescein angiography in diagnosing choroidal neovascular membranes. Br. J. Ophthalmol. 89, Savini, G., Zanini, M., Carelli, V., Sadun, A.A., Ross-Cisneros, F.N., Barboni, P., Correlation between retinal nerve fibre layer thickness and optic nerve head size: an optical coherence tomography study. Br. J. Ophthalmol. 89, Schuman, J.S., Hee, M.R., Puliafito, C.A., Wong, C., Pedut-Kloizman, T., Lin, C.P., Hertzmark, E., Izatt, J.A., Swanson, E.A., Fujimoto, J.G., Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch. Ophthalmol. 113, Schuman, J.S., Pedut-Kloizman, T., Hertzmark, E., Hee, M.R., Wilkins, J.R., Coker, J.G., Puliafito, C.A., Fujimoto, J.G., Swanson, E.A., Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography. Ophthalmology 103, Skaf, M., Bernardes, A.B., Cardillo, J.A., Costa, R.A., Melo, L.A., Castro, J.C., Varma, R., Retinal nerve fibre layer thickness profile in normal eyes using third-generation optical coherence tomography. Eye (22 July 2005, Epub ahead of print). Soliman, M.A., Van Den Berg, T.J., Ismaeil, A.A., De Jong, L.A., De Smet, M.D., Retinal nerve fiber layer analysis: relationship between optical coherence tomography and red-free photography. Am. J. Ophthalmol. 133, Sommer, A., Katz, J., Quigley, H.A., Miller, N.R., Robin, A.L., Richter, R.C., Witt, K.A., Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch. Ophthalmol. 109, Swanson, E.A., Izatt, J.A., Hee, M.R., et al., In vivo retinal imaging by optical coherence tomography. Opt. Lett. 18, Szkulmowski, M., Wojtkowski, M., Bajraszewski, T., Gorczynska, I., Targowski, P., Wasilewski, W., Kowalczyk, A., Radzewicz, C., Quality improvement for high resolution in vivo images by Spectral domain Optical Coherence Tomography with supercontinuum source. Opt. Commun., forthcoming. Targowski, P., Gorczynska, I., Szkulmowski, M., Cyganek, M., Wojtkowski, M., Kowalczyk, A., Improved complex spectral domain OCT for in vivo eye imaging. Opt. Commun., forthcoming. Targowski, P., Wojtkowski, M., Kowalczyk, A., Bajraszewski, T., Szkulmowski, M., Gorczynska, W., Complex spectral OCT in human eye imaging in vivo. Opt. Commun. 229,
29 R.A. Costa et al. / Progress in Retinal and Eye Research 25 (2006) Tornambe, P.E., Macular hole genesis: the hydration theory. Retina 23, Uemoto, R., Yamamoto, S., Aoki, T., Tsukahara, I., Yamamoto, T., Takeuchi, S., Macular configuration determined by optical coherence tomography after idiopathic macular hole surgery with or without internal limiting membrane peeling. Br. J. Ophthalmol. 86, Varma, R., Skaf, M., Barron, E., Retinal nerve fiber layer thickness in normal human eyes. Ophthalmology 103, Villate, N., Lee, J.E., Venkatraman, A., Smiddy, W.E., Photoreceptor layer features in eyes with closed macular holes: optical coherence tomography findings and correlation with visual outcomes. Am. J. Ophthalmol. 139, Watanabe, M., Oshima, Y., Emi, K., Optical cross-sectional observation of resolved diabetic macular edema associated with vitreomacular separation. Am. J. Ophthalmol. 129, White, B.R., Pierce, M.C., Nassif, N., Cense, B., Park, B.H., Tearney, G.J., Bouma, B.E., Chen, T.C., de Boer, J.F., In vivo dynamic human retinal blood flow imaging using ultra-high-speed spectral domain optical Doppler tomography. Opt. Express 11, Williams, Z.Y., Schuman, J.S., Gamell, L., Nemi, A., Hertzmark, E., Fujimoto, J.G., Mattox, C., Simpson, J., Wollstein, G., Optical coherence tomography measurement of nerve fiber layer thickness and the likelihood of a visual field defect. Am. J. Ophthalmol. 134, Wojtkowski, M., Kowalczyk, A., Leitgeb, R., Fercher, A.F., 2002a. Full range complex spectral optical coherence tomography technique in eye imaging. Opt. Lett. 27, Wojtkowski, M., Leitgeb, R., Kowalczyk, A., Bajraszewski, T., Fercher, A.F., 2002b. In vivo human retinal imaging by Fourier domain optical coherence tomography. J. Biomed. Opt. 7, Wojtkowski, M., Bajraszewski, T., Targowski, P., Kowalczyk, A., Real-time in vivo imaging by high-speed spectral optical coherence tomography. Opt. Lett. 28, Wojtkowski, M., Bajraszewski, T., Gorczynska, I., Targowski, P., Kowalczyk, A., Wasilewski, W., Radzewicz, C., 2004a. Ophthalmic imaging by spectral optical coherence tomography. Am. J. Ophthalmol. 138, Wojtkowski, M., Srinivasan, V.J., Ko, T.H., Fujimoto, J.G., Kowalevicz, A., Duker, J.S., 2004b. Ultrahigh resolution, high speed, Fourier domain optical coherence tomography and methods for dispersion compensation. Optics Express 12, Wojtkowski, M., Srinivasan, V., Fugimoto, J.G., Ko, T., Schuman, J.S., Kowalczyk, A., Duker, J.S., Three-dimension retinal imaging with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology 112, Wollstein, G., Schuman, J.S., Price, L.L., Aydin, A., Beaton, S.A., Stark, P.C., Fujimoto, J.G., Ishikawa, H., Optical coherence tomography (OCT) macular and peripapillary retinal nerve fiber layer measurements and automated visual fields. Am. J. Ophthalmol. 138, Wollstein, G., Schuman, J.S., Price, L.L., Aydin, A., Stark, P.C., Hertzmark, E., Lai, E., Ishikawa, H., Mattox, C., Fujimoto, J.G., Paunescu, L.A., 2005a. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch. Ophthalmol. 123, Wollstein, G., Ishikawa, H., Wang, J., Beaton, S.A., Schuman, J.S., 2005b. Comparison of three optical coherence tomography scanning areas for detection of glaucomatous damage. Am. J. Ophthalmol. 139, Wollstein, G., Paunescu, L.A., Ko, T.H., Fujimoto, J.G., Kowalevicz, A., Hartl, I., Beaton, S., Ishikawa, H., Mattox, C., Singh, O., Duker, J., Drexler, W., Schuman, J.S., 2005c. Ultrahigh-resolution optical coherence tomography in glaucoma. Ophthalmology 112, Yamaguchi, Y., Otani, T., Kishi, S., Resolution of diabetic cystoid macular edema associated with spontaneous vitreofoveal separation. Am. J. Ophthalmol. 135, Yun, S., Tearney, G., de Boer, J.F., Iftima, N., Bouma, B., Highspeed optical frequency-domain imaging. Opt. Express 11, Zeimer, R., Shahidi, M., Mori, M., Zou, S., Asrani, S., A new method for rapid mapping of the retinal thickness at the posterior pole. Invest. Ophthalmol. Vis. Sci. 37, Zeimer, R., Mori, M., Khoobehi, B., Feasibility test of a new method to measure retinal thickness noninvasively. Invest. Ophthalmol. Vis. Sci. 30,
SOCT Copernicus HR Specification
 Specification Light Source: SLED Central Wavelength: 850 nm Axial Resolution: 3 µm Transversal Resolution: 12-18 µm Scanning Speed: 52000 A-Scan per second A-Scan Resolution: 1024 points B-Scan Resolution:
Specification Light Source: SLED Central Wavelength: 850 nm Axial Resolution: 3 µm Transversal Resolution: 12-18 µm Scanning Speed: 52000 A-Scan per second A-Scan Resolution: 1024 points B-Scan Resolution:
The Nurse/Technician Role Within the Emerging Ophthalmic Technology - OCTs/B-Scan
 The Nurse/Technician Role Within the Emerging Ophthalmic Technology - OCTs/B-Scan Margie V. Wilson, COMT Chief Clinical Supervisor UCSD Shiley Eye Center Thanks, Carol! Unfortunately. I have not financial
The Nurse/Technician Role Within the Emerging Ophthalmic Technology - OCTs/B-Scan Margie V. Wilson, COMT Chief Clinical Supervisor UCSD Shiley Eye Center Thanks, Carol! Unfortunately. I have not financial
Trends in Ophthalmology a Market Perspective. Matthias Karl, Michael Kempe
 Trends in Ophthalmology a Market Perspective Matthias Karl, Michael Kempe 30.04.2013 Agenda 1 2 3 4 Role of Optical Technologies in Ophthalmology Demands of the Market - Trends in the Application Implications
Trends in Ophthalmology a Market Perspective Matthias Karl, Michael Kempe 30.04.2013 Agenda 1 2 3 4 Role of Optical Technologies in Ophthalmology Demands of the Market - Trends in the Application Implications
Spectral domain OCT used to view and quantify choroidal vascular congestion in new subretinal fluid following scleral buckling.
 Spectral domain OCT used to view and quantify choroidal vascular congestion in new subretinal fluid following scleral buckling. Robert Gizicki MD, Mohamed Haji, Flavio Rezende Retina Service, Hôpital Maisonneuve-Rosemont,
Spectral domain OCT used to view and quantify choroidal vascular congestion in new subretinal fluid following scleral buckling. Robert Gizicki MD, Mohamed Haji, Flavio Rezende Retina Service, Hôpital Maisonneuve-Rosemont,
When a Macular Hole Is Not A Macular Hole. Contents. OCT Anatomy. Vitreomacular Traction 3/1/2012
 When a Macular Hole Is Not A Macular Hole Salil Shukla, MD Omni Eye Specialists Spring Symposium March 3, 2012 Contents Vitreomacular Traction Epiretinal Membrane Lamellar Hole Macular Hole (Myopic macular
When a Macular Hole Is Not A Macular Hole Salil Shukla, MD Omni Eye Specialists Spring Symposium March 3, 2012 Contents Vitreomacular Traction Epiretinal Membrane Lamellar Hole Macular Hole (Myopic macular
Optic Nerve Imaging. Management of Glaucoma
 Optic Nerve Imaging in the Diagnosis and Management of Glaucoma Scott D. Smith, MD, MPH Associate Professor of Clinical Ophthalmology Director, Glaucoma Service Columbia University, New York, NY Glaucoma
Optic Nerve Imaging in the Diagnosis and Management of Glaucoma Scott D. Smith, MD, MPH Associate Professor of Clinical Ophthalmology Director, Glaucoma Service Columbia University, New York, NY Glaucoma
Comparison to other anterior segment imaging devices
 found helpful in a number of areas of ophthalmology. However, in the field of anterior segment tumors its usefulness is confined to a subset of tumors that are confined to the iris, hypo pigmented, and
found helpful in a number of areas of ophthalmology. However, in the field of anterior segment tumors its usefulness is confined to a subset of tumors that are confined to the iris, hypo pigmented, and
Ultrasonic Wave Propagation Review
 Ultrasonic Wave Propagation Review Presented by: Sami El-Ali 1 1. Introduction Ultrasonic refers to any study or application of sound waves that are higher frequency than the human audible range. Ultrasonic
Ultrasonic Wave Propagation Review Presented by: Sami El-Ali 1 1. Introduction Ultrasonic refers to any study or application of sound waves that are higher frequency than the human audible range. Ultrasonic
FTIR Instrumentation
 FTIR Instrumentation Adopted from the FTIR lab instruction by H.-N. Hsieh, New Jersey Institute of Technology: http://www-ec.njit.edu/~hsieh/ene669/ftir.html 1. IR Instrumentation Two types of instrumentation
FTIR Instrumentation Adopted from the FTIR lab instruction by H.-N. Hsieh, New Jersey Institute of Technology: http://www-ec.njit.edu/~hsieh/ene669/ftir.html 1. IR Instrumentation Two types of instrumentation
THE EYES IN MARFAN SYNDROME
 THE EYES IN MARFAN SYNDROME Marfan syndrome and some related disorders can affect the eyes in many ways, causing dislocated lenses and other eye problems that can affect your sight. Except for dislocated
THE EYES IN MARFAN SYNDROME Marfan syndrome and some related disorders can affect the eyes in many ways, causing dislocated lenses and other eye problems that can affect your sight. Except for dislocated
Clinical Applications of Optical Coherence Tomography in Ophthalmology
 11 Clinical Applications of Optical Coherence Tomography in Ophthalmology Upender K. Wali and Nadia Al Kharousi College of Medicine and Health Sciences, Sultan Qaboos University Oman In Ophthalmology,
11 Clinical Applications of Optical Coherence Tomography in Ophthalmology Upender K. Wali and Nadia Al Kharousi College of Medicine and Health Sciences, Sultan Qaboos University Oman In Ophthalmology,
Diabetic Eye Screening Revised Grading Definitions
 Diabetic Eye Screening Revised Grading Definitions Version 1.3, 1 November 2012 To provide guidance on revised grading definitions for the NHS Diabetic Eye Screening Programme Project/Category Document
Diabetic Eye Screening Revised Grading Definitions Version 1.3, 1 November 2012 To provide guidance on revised grading definitions for the NHS Diabetic Eye Screening Programme Project/Category Document
NEW. CIRRUS HD-OCT 5000/500 Advancing Smart OCT. Imaging Applications: Anterior Segment Glaucoma Retina. International Version
 NEW CIRRUS HD-OCT 5000/500 Advancing Smart OCT Imaging Applications: Anterior Segment Glaucoma Retina International Version NEW PanoMap Analysis Wide-field structural damage assessment for glaucoma NEW
NEW CIRRUS HD-OCT 5000/500 Advancing Smart OCT Imaging Applications: Anterior Segment Glaucoma Retina International Version NEW PanoMap Analysis Wide-field structural damage assessment for glaucoma NEW
Measure of Confidence. Glaucoma Module Premium Edition
 Measure of Confidence Glaucoma Module Premium Edition The Changing Face of Glaucoma Practice Literature 1 Leske et al., Arch Ophthalmol 1997; 115:1051-1057 2 Doughty et al., Surv Ophthalmol 2000; 44:367-408
Measure of Confidence Glaucoma Module Premium Edition The Changing Face of Glaucoma Practice Literature 1 Leske et al., Arch Ophthalmol 1997; 115:1051-1057 2 Doughty et al., Surv Ophthalmol 2000; 44:367-408
How To Understand The Current Of An Optic Nerve Fiber Layer
 A Practical Guide for Journal Interpretation of Current of Optical Glaucoma Coherence Practice, Tomography January-April Retinal 2009;3(1):9-13 Nerve Fiber Layer Measurement A Practical Guide for Interpretation
A Practical Guide for Journal Interpretation of Current of Optical Glaucoma Coherence Practice, Tomography January-April Retinal 2009;3(1):9-13 Nerve Fiber Layer Measurement A Practical Guide for Interpretation
EFFECT OF MYOPIC LASIK ON RETINAL NERVE FIBER LAYER THICKNESS- IS IT SAFE OR UNSAFE?
 24. Glaucoma: Imaging EFFECT OF MYOPIC LASIK ON RETINAL NERVE FIBER LAYER THICKNESS- IS IT SAFE OR UNSAFE? Chief Author: Dr. Amit porwal 1 Co-Authors: Dr. Kavita Porwal 2, Dr. Puja Rai 1 1. Choithram Netralaya,
24. Glaucoma: Imaging EFFECT OF MYOPIC LASIK ON RETINAL NERVE FIBER LAYER THICKNESS- IS IT SAFE OR UNSAFE? Chief Author: Dr. Amit porwal 1 Co-Authors: Dr. Kavita Porwal 2, Dr. Puja Rai 1 1. Choithram Netralaya,
ReLEx smile Minimally invasive vision correction Information for patients
 ReLEx smile Minimally invasive vision correction Information for patients Seeing is living Our eyes are our most important sensory organ. The human brain obtains over 80 % of its information via the sense
ReLEx smile Minimally invasive vision correction Information for patients Seeing is living Our eyes are our most important sensory organ. The human brain obtains over 80 % of its information via the sense
DETECTION OF SUBSURFACE DAMAGE IN OPTICAL TRANSPARENT MATERIALSS USING SHORT COHERENCE TOMOGRAPHY. Rainer Boerret, Dominik Wiedemann, Andreas Kelm
 URN (Paper): urn:nbn:de:gbv:ilm1-2014iwk-199:0 58 th ILMENAU SCIENTIFIC COLLOQUIUM Technische Universität Ilmenau, 08 12 September 2014 URN: urn:nbn:de:gbv:ilm1-2014iwk:3 DETECTION OF SUBSURFACE DAMAGE
URN (Paper): urn:nbn:de:gbv:ilm1-2014iwk-199:0 58 th ILMENAU SCIENTIFIC COLLOQUIUM Technische Universität Ilmenau, 08 12 September 2014 URN: urn:nbn:de:gbv:ilm1-2014iwk:3 DETECTION OF SUBSURFACE DAMAGE
Visual Disorders in Middle-Age and Elderly Patients with Diabetic Retinopathy
 Medical Care for the Elderly Visual Disorders in Middle-Age and Elderly Patients with Diabetic Retinopathy JMAJ 46(1): 27 32, 2003 Shigehiko KITANO Professor, Department of Ophthalmology, Diabetes Center,
Medical Care for the Elderly Visual Disorders in Middle-Age and Elderly Patients with Diabetic Retinopathy JMAJ 46(1): 27 32, 2003 Shigehiko KITANO Professor, Department of Ophthalmology, Diabetes Center,
Study of the Human Eye Working Principle: An impressive high angular resolution system with simple array detectors
 Study of the Human Eye Working Principle: An impressive high angular resolution system with simple array detectors Diego Betancourt and Carlos del Río Antenna Group, Public University of Navarra, Campus
Study of the Human Eye Working Principle: An impressive high angular resolution system with simple array detectors Diego Betancourt and Carlos del Río Antenna Group, Public University of Navarra, Campus
Optical Coherence Tomography OCT. 3D Imaging in Medical Technology and Quality Control
 Optical Coherence Tomography OCT 3D Imaging in Medical Technology and Quality Control SLN Seminar, EPMT Lausanne, 26. May. 2011 Ch. Meier www.optolab.ch 1 / 27 SLN/EPMT, Lausanne, 26.5.2011 Ch. Meier Outline
Optical Coherence Tomography OCT 3D Imaging in Medical Technology and Quality Control SLN Seminar, EPMT Lausanne, 26. May. 2011 Ch. Meier www.optolab.ch 1 / 27 SLN/EPMT, Lausanne, 26.5.2011 Ch. Meier Outline
INTERNATIONAL CLINICAL DIABETIC RETINOPATHY DISEASE SEVERITY SCALE DETAILED TABLE
 Proposed Disease Severity Level No apparent Mild Non- Proliferative Moderate Nonproliferative Severe Non- Proliferative Proliferative INTERNATIONAL CLINICAL DIABETIC RETINOPATHY DISEASE SEVERITY SCALE
Proposed Disease Severity Level No apparent Mild Non- Proliferative Moderate Nonproliferative Severe Non- Proliferative Proliferative INTERNATIONAL CLINICAL DIABETIC RETINOPATHY DISEASE SEVERITY SCALE
Retinal Imaging Biomarkers for Early Diagnosis of Alzheimer s Disease
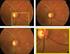 Retinal Imaging Biomarkers for Early Diagnosis of Alzheimer s Disease Eleonora (Nora) Lad, MD, PhD Assistant Professor of Ophthalmology, Vitreoretinal diseases Duke Center for Macular Diseases Duke University
Retinal Imaging Biomarkers for Early Diagnosis of Alzheimer s Disease Eleonora (Nora) Lad, MD, PhD Assistant Professor of Ophthalmology, Vitreoretinal diseases Duke Center for Macular Diseases Duke University
Principles of Diagnosis MEDICAL DECISION-MAKING IN OPTOMETRIC PRACTICE. Medical Decision-Making. Medical Decision-Making.
 MEDICAL DECISION-MAKING IN OPTOMETRIC PRACTICE Craig Thomas, O.D. 3900 West Wheatland Road Dallas, Texas 75237 972-780-7199 [email protected] Principles of Diagnosis The study of the art and science of
MEDICAL DECISION-MAKING IN OPTOMETRIC PRACTICE Craig Thomas, O.D. 3900 West Wheatland Road Dallas, Texas 75237 972-780-7199 [email protected] Principles of Diagnosis The study of the art and science of
CR-2 AF and CR-2 PLUS AF Non-Mydriatic cameras. Designed for the highest possible image quality. See the smallest details, don t miss pathology.
 CR-2 AF and CR-2 PLUS AF Non-Mydriatic cameras Designed for the highest possible image quality. See the smallest details, don t miss pathology. CR-2 AF Extremely compact and lightweight (just 15 kg) camera:
CR-2 AF and CR-2 PLUS AF Non-Mydriatic cameras Designed for the highest possible image quality. See the smallest details, don t miss pathology. CR-2 AF Extremely compact and lightweight (just 15 kg) camera:
Fundus Photograph Reading Center
 Modified 7-Standard Field Digital Color Fundus Photography (7M-D) 8010 Excelsior Drive, Suite 100, Madison WI 53717 Telephone: (608) 410-0560 Fax: (608) 410-0566 Table of Contents 1. 7M-D Overview... 2
Modified 7-Standard Field Digital Color Fundus Photography (7M-D) 8010 Excelsior Drive, Suite 100, Madison WI 53717 Telephone: (608) 410-0560 Fax: (608) 410-0566 Table of Contents 1. 7M-D Overview... 2
Three pearls in one shell. Heidelberg Retina Tomograph
 Three pearls in one shell Heidelberg Retina Tomograph HRT the practice builder HRT for every practice Milestones of a pioneer 1991 Heidelberg Retina Tomograph First scanning laser system for glaucoma exam.
Three pearls in one shell Heidelberg Retina Tomograph HRT the practice builder HRT for every practice Milestones of a pioneer 1991 Heidelberg Retina Tomograph First scanning laser system for glaucoma exam.
Scanning Acoustic Microscopy Training
 Scanning Acoustic Microscopy Training This presentation and images are copyrighted by Sonix, Inc. They may not be copied, reproduced, modified, published, uploaded, posted, transmitted, or distributed
Scanning Acoustic Microscopy Training This presentation and images are copyrighted by Sonix, Inc. They may not be copied, reproduced, modified, published, uploaded, posted, transmitted, or distributed
NC DIVISION OF SERVICES FOR THE BLIND POLICIES AND PROCEDURES VOCATIONAL REHABILITATION
 NC DIVISION OF SERVICES FOR THE BLIND POLICIES AND PROCEDURES VOCATIONAL REHABILITATION Section: E Revision History Revised 01/97; 05/03; 02/08; 04/08; 03/09; 05/09; 12/09; 01/11; 12/14 An individual is
NC DIVISION OF SERVICES FOR THE BLIND POLICIES AND PROCEDURES VOCATIONAL REHABILITATION Section: E Revision History Revised 01/97; 05/03; 02/08; 04/08; 03/09; 05/09; 12/09; 01/11; 12/14 An individual is
ICG IMAGING FOR EXUDATIVE ARMD
 ICG IMAGING FOR EXUDATIVE ARMD MARK H. NELSON, MD MBA WINSTON-SALEM, NC ASRS INSTRUCTION COURSE AUGUST 24, 2011 FINANCIAL DISCLOSURE Novartis Pharmaceutics, Incorporated Consultant, Grant Recipient Heidelberg
ICG IMAGING FOR EXUDATIVE ARMD MARK H. NELSON, MD MBA WINSTON-SALEM, NC ASRS INSTRUCTION COURSE AUGUST 24, 2011 FINANCIAL DISCLOSURE Novartis Pharmaceutics, Incorporated Consultant, Grant Recipient Heidelberg
Macular Hole. James L. Combs, M.D. Eleanore M. Ebert, M.D. Byron S. Ladd, M.D. George E. Sanborn, M.D. Jeffrey H. Slott, M.D.
 Macular Hole James L. Combs, M.D. Eleanore M. Ebert, M.D. Byron S. Ladd, M.D. George E. Sanborn, M.D. Jeffrey H. Slott, M.D. (804) 285-5300 or (804) 287-4200 www.vaeye.com Macular Hole In order to maintain
Macular Hole James L. Combs, M.D. Eleanore M. Ebert, M.D. Byron S. Ladd, M.D. George E. Sanborn, M.D. Jeffrey H. Slott, M.D. (804) 285-5300 or (804) 287-4200 www.vaeye.com Macular Hole In order to maintain
Top Medicare Audit problems. Retinal Imaging Technology. Optometric Medical Coding. Unilateral codes. Modifiers
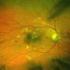 Top Medicare Audit problems Evaluation, Diagnosis, Coding and Reimbursement Associated with Medical Vitreo-Retinal Conditions Kim Castleberry, OD Plano Eye Associates Patient/staff initiated billing complaints!
Top Medicare Audit problems Evaluation, Diagnosis, Coding and Reimbursement Associated with Medical Vitreo-Retinal Conditions Kim Castleberry, OD Plano Eye Associates Patient/staff initiated billing complaints!
Cataract Testing. What a Patient undergoes prior to surgery
 Cataract Testing What a Patient undergoes prior to surgery FINANCIAL DISCLOSURE I have no financial interest or relationships to disclose What do most Technicians find to be the most mundane yet very important
Cataract Testing What a Patient undergoes prior to surgery FINANCIAL DISCLOSURE I have no financial interest or relationships to disclose What do most Technicians find to be the most mundane yet very important
Rediscover quality of life thanks to vision correction with technology from Carl Zeiss. Patient Information
 Rediscover quality of life thanks to vision correction with technology from Carl Zeiss Patient Information 5 2 It was really w Vision defects: Light that goes astray For clear vision the eyes, cornea and
Rediscover quality of life thanks to vision correction with technology from Carl Zeiss Patient Information 5 2 It was really w Vision defects: Light that goes astray For clear vision the eyes, cornea and
The Olympus stereology system. The Computer Assisted Stereological Toolbox
 The Olympus stereology system The Computer Assisted Stereological Toolbox CAST is a Computer Assisted Stereological Toolbox for PCs running Microsoft Windows TM. CAST is an interactive, user-friendly,
The Olympus stereology system The Computer Assisted Stereological Toolbox CAST is a Computer Assisted Stereological Toolbox for PCs running Microsoft Windows TM. CAST is an interactive, user-friendly,
Customized corneal ablation can be designed. Slit Skiascopic-guided Ablation Using the Nidek Laser. Scott MacRae, MD; Masanao Fujieda
 Slit Skiascopic-guided Ablation Using the Nidek Laser Scott MacRae, MD; Masanao Fujieda ABSTRACT PURPOSE: To present the approach of using a scanning slit refractometer (the ARK 10000) in conjunction with
Slit Skiascopic-guided Ablation Using the Nidek Laser Scott MacRae, MD; Masanao Fujieda ABSTRACT PURPOSE: To present the approach of using a scanning slit refractometer (the ARK 10000) in conjunction with
Femto-LASIK. Pulsewidth: Ultrashort-pulse micro- machining can make sub- wavelength holes. micromachining
 All-laser laser LASIK (Femto( Femto-LASIK) Femto-LASIK 台 大 眼 科 王 一 中 IntraLase 2/1 Perfect Vision Ziemer (DaVinci) Carl Zeiss Meditec Pulsewidth: Femtosecond laser (Nd:Glass)) 153 nm (near infrared) Each
All-laser laser LASIK (Femto( Femto-LASIK) Femto-LASIK 台 大 眼 科 王 一 中 IntraLase 2/1 Perfect Vision Ziemer (DaVinci) Carl Zeiss Meditec Pulsewidth: Femtosecond laser (Nd:Glass)) 153 nm (near infrared) Each
Experiment 5. Lasers and laser mode structure
 Northeastern University, PHYS5318 Spring 2014, 1 1. Introduction Experiment 5. Lasers and laser mode structure The laser is a very important optical tool that has found widespread use in science and industry,
Northeastern University, PHYS5318 Spring 2014, 1 1. Introduction Experiment 5. Lasers and laser mode structure The laser is a very important optical tool that has found widespread use in science and industry,
~Contributing to Eye Health~
 ~Contributing to Eye Health~ 26 Eye Care Market Trends FY2007 Retinal Camera market forecasts by eye disease FY 2010 Auto Keratorefractometer Retina 160 Refraction Retina 200 Refraction Auto Lens Edger
~Contributing to Eye Health~ 26 Eye Care Market Trends FY2007 Retinal Camera market forecasts by eye disease FY 2010 Auto Keratorefractometer Retina 160 Refraction Retina 200 Refraction Auto Lens Edger
Vitreo-Retinal and Macular Degeneration Frequently Asked Questions
 Vitreo-Retinal and Macular Degeneration Frequently Asked Questions What is a Vitreo-Retinal specialist? Retinal specialists are eye physicians and surgeons who focus on diseases in the back of the eye
Vitreo-Retinal and Macular Degeneration Frequently Asked Questions What is a Vitreo-Retinal specialist? Retinal specialists are eye physicians and surgeons who focus on diseases in the back of the eye
The Normative Database for the RTVue. Software version 4.0. Michael J. Sinai, PhD
 The Normative Database for the RTVue Software version 4.0 Michael J. Sinai, PhD Glaucoma is a multi-factorial optic neuropathy with characteristic structural changes and subsequent functional impairment.
The Normative Database for the RTVue Software version 4.0 Michael J. Sinai, PhD Glaucoma is a multi-factorial optic neuropathy with characteristic structural changes and subsequent functional impairment.
Application Report: Running µshape TM on a VF-20 Interferometer
 : Running µshape TM on a VF-20 Interferometer General This report describes how a fiber interferometer from Arden Photonics Ltd was used together with the µshape TM Generic software package. The VF-20
: Running µshape TM on a VF-20 Interferometer General This report describes how a fiber interferometer from Arden Photonics Ltd was used together with the µshape TM Generic software package. The VF-20
An Informational Guide to CENTRAL RETINAL VEIN OCCLUSION
 Science of CRVO www.scienceofcrvo.org An Informational Guide to CENTRAL RETINAL VEIN OCCLUSION This brochure will guide you in understanding CRVO and the treatment options available to prevent vision loss.
Science of CRVO www.scienceofcrvo.org An Informational Guide to CENTRAL RETINAL VEIN OCCLUSION This brochure will guide you in understanding CRVO and the treatment options available to prevent vision loss.
Module 13 : Measurements on Fiber Optic Systems
 Module 13 : Measurements on Fiber Optic Systems Lecture : Measurements on Fiber Optic Systems Objectives In this lecture you will learn the following Measurements on Fiber Optic Systems Attenuation (Loss)
Module 13 : Measurements on Fiber Optic Systems Lecture : Measurements on Fiber Optic Systems Objectives In this lecture you will learn the following Measurements on Fiber Optic Systems Attenuation (Loss)
Eye Diseases. 1995-2014, The Patient Education Institute, Inc. www.x-plain.com otf30101 Last reviewed: 05/21/2014 1
 Eye Diseases Introduction Some eye problems are minor and fleeting. But some lead to a permanent loss of vision. There are many diseases that can affect the eyes. The symptoms of eye diseases vary widely,
Eye Diseases Introduction Some eye problems are minor and fleeting. But some lead to a permanent loss of vision. There are many diseases that can affect the eyes. The symptoms of eye diseases vary widely,
Introduction to acoustic imaging
 Introduction to acoustic imaging Contents 1 Propagation of acoustic waves 3 1.1 Wave types.......................................... 3 1.2 Mathematical formulation.................................. 4 1.3
Introduction to acoustic imaging Contents 1 Propagation of acoustic waves 3 1.1 Wave types.......................................... 3 1.2 Mathematical formulation.................................. 4 1.3
Alexandria Fairfax Sterling Leesburg 703-931-9100 703-573-8080 703-430-4400 703-858-3170
 DIABETIC RETINOPATHY www.theeyecenter.com This pamphlet has been written to help people with diabetic retinopathy and their families and friends better understand the disease. It describes the cause, symptoms,
DIABETIC RETINOPATHY www.theeyecenter.com This pamphlet has been written to help people with diabetic retinopathy and their families and friends better understand the disease. It describes the cause, symptoms,
DIABETIC RETINOPATHY. Diabetes mellitus is an abnormality of the blood glucose metabolism due to altered
 DIABETIC RETINOPATHY Diabetes mellitus Diabetes mellitus is an abnormality of the blood glucose metabolism due to altered insulin production or activity. There are two types diabetes mellitus- Insulin
DIABETIC RETINOPATHY Diabetes mellitus Diabetes mellitus is an abnormality of the blood glucose metabolism due to altered insulin production or activity. There are two types diabetes mellitus- Insulin
Guide to Eye Surgery and Eye-related Claims
 If you or a loved one have suffered because of a negligent error during eye treatment or surgery, you may be worried about how you will manage in the future, particularly if your eyesight has been made
If you or a loved one have suffered because of a negligent error during eye treatment or surgery, you may be worried about how you will manage in the future, particularly if your eyesight has been made
iocutouchtm for ipad Contents of Videos and Still Images Anatomy 906. Normal Eye and Orbit - no labels 907. Normal Eye and Orbit - with labels
 iocutouchtm for ipad Contents of Videos and Still Images Anatomy 906. Normal Eye and Orbit - no labels 907. Normal Eye and Orbit - with labels Normal Eye 474. Normal Eye overview 476. Cornea - overview
iocutouchtm for ipad Contents of Videos and Still Images Anatomy 906. Normal Eye and Orbit - no labels 907. Normal Eye and Orbit - with labels Normal Eye 474. Normal Eye overview 476. Cornea - overview
This newest generation laser offers specific technological features and treatment options designed to substantially improve the retina laser therapy:
 1 The latest generation of Quantel Medical Multispot Laser (Supra Scan 577) offers new technological features and treatment methods for improving retina laser therapy. Q uantel Medical Lasers give excellent
1 The latest generation of Quantel Medical Multispot Laser (Supra Scan 577) offers new technological features and treatment methods for improving retina laser therapy. Q uantel Medical Lasers give excellent
Diabetic macular edema (DME) is the primary cause of
 Retina Fully Software for Retinal Thickness in Eyes With Diabetic Macular Edema From Images Acquired by Cirrus and Spectralis Systems Joo Yong Lee, 1,2 Stephanie J. Chiu, 3 Pratul P. Srinivasan, 3 Joseph
Retina Fully Software for Retinal Thickness in Eyes With Diabetic Macular Edema From Images Acquired by Cirrus and Spectralis Systems Joo Yong Lee, 1,2 Stephanie J. Chiu, 3 Pratul P. Srinivasan, 3 Joseph
The Evolution of the Optical Zone in Corneal Refractive Surgery. Bruce Drum, Ph.D.
 The Evolution of the Optical Zone in Corneal Refractive Surgery. Bruce Drum, Ph.D. FDA, Division of Ophthalmic and ENT Devices, Rockville, MD Disclaimer This presentation represents the professional opinion
The Evolution of the Optical Zone in Corneal Refractive Surgery. Bruce Drum, Ph.D. FDA, Division of Ophthalmic and ENT Devices, Rockville, MD Disclaimer This presentation represents the professional opinion
CLINICAL SCIENCES. Enhanced Depth Imaging Optical Coherence Tomography of Small Choroidal Melanoma
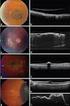 CLINICAL SCIENCES Enhanced Depth Imaging Optical Coherence Tomography of Small Choroidal Melanoma Comparison With Choroidal Nevus Carol L. Shields, MD; Swathi Kaliki, MD; Duangnate Rojanaporn, MD; Sandor
CLINICAL SCIENCES Enhanced Depth Imaging Optical Coherence Tomography of Small Choroidal Melanoma Comparison With Choroidal Nevus Carol L. Shields, MD; Swathi Kaliki, MD; Duangnate Rojanaporn, MD; Sandor
Femtosecond Lasers in LASIK Surgery
 Femtosecond Lasers in LASIK Surgery Dr Chan Tat Keong Senior Consultant Refractive Surgery Service Singapore National Eye Centre Disclosure Speaker has no financial interest in the products to be discussed
Femtosecond Lasers in LASIK Surgery Dr Chan Tat Keong Senior Consultant Refractive Surgery Service Singapore National Eye Centre Disclosure Speaker has no financial interest in the products to be discussed
Synthetic Sensing: Proximity / Distance Sensors
 Synthetic Sensing: Proximity / Distance Sensors MediaRobotics Lab, February 2010 Proximity detection is dependent on the object of interest. One size does not fit all For non-contact distance measurement,
Synthetic Sensing: Proximity / Distance Sensors MediaRobotics Lab, February 2010 Proximity detection is dependent on the object of interest. One size does not fit all For non-contact distance measurement,
Doppler. Doppler. Doppler shift. Doppler Frequency. Doppler shift. Doppler shift. Chapter 19
 Doppler Doppler Chapter 19 A moving train with a trumpet player holding the same tone for a very long time travels from your left to your right. The tone changes relative the motion of you (receiver) and
Doppler Doppler Chapter 19 A moving train with a trumpet player holding the same tone for a very long time travels from your left to your right. The tone changes relative the motion of you (receiver) and
Alexandria s Guide to LASIK
 Alexandria s Guide to LASIK A Community Service Project sponsored by: Wallace Laser Center Your Guide To A Successful LASIK Procedure The word LASIK is actually an acronym for Laser Assisted In-Situ Keratomileusis.
Alexandria s Guide to LASIK A Community Service Project sponsored by: Wallace Laser Center Your Guide To A Successful LASIK Procedure The word LASIK is actually an acronym for Laser Assisted In-Situ Keratomileusis.
Development of Optical Wave Microphone Measuring Sound Waves with No Diaphragm
 Progress In Electromagnetics Research Symposium Proceedings, Taipei, March 5 8, 3 359 Development of Optical Wave Microphone Measuring Sound Waves with No Diaphragm Yoshito Sonoda, Takashi Samatsu, and
Progress In Electromagnetics Research Symposium Proceedings, Taipei, March 5 8, 3 359 Development of Optical Wave Microphone Measuring Sound Waves with No Diaphragm Yoshito Sonoda, Takashi Samatsu, and
Introduction Houston Retina Associates
 1 2 Introduction This book was written by Houston Retina Associates to provide our patients with basic knowledge about retinal anatomy, an introduction to some of the diagnostic tests and treatments used
1 2 Introduction This book was written by Houston Retina Associates to provide our patients with basic knowledge about retinal anatomy, an introduction to some of the diagnostic tests and treatments used
Correction of motion artifacts and scanning beam distortions in 3D ophthalmic optical coherence tomography imaging
 Correction of motion artifacts and scanning beam distortions in 3D ophthalmic optical coherence tomography imaging Robert J. Zawadzki* a, Alfred R. Fuller b, Stacey S. Choi a, David F. Wiley b,c, Bernd
Correction of motion artifacts and scanning beam distortions in 3D ophthalmic optical coherence tomography imaging Robert J. Zawadzki* a, Alfred R. Fuller b, Stacey S. Choi a, David F. Wiley b,c, Bernd
Encoded Phased Array Bridge Pin Inspection
 Encoded Phased Array Bridge Pin Inspection James S. Doyle Baker Testing Services, Inc. 22 Reservoir Park Dr. Rockland, MA 02370 (781) 871-4458; fax (781) 871-0123; e-mail [email protected] Product
Encoded Phased Array Bridge Pin Inspection James S. Doyle Baker Testing Services, Inc. 22 Reservoir Park Dr. Rockland, MA 02370 (781) 871-4458; fax (781) 871-0123; e-mail [email protected] Product
OPTICAL COHERENCE TOMOGRAPHY (OCT) IS AN
 Thickness Profiles of Retinal Layers by Optical Coherence Tomography Image Segmentation AHMET MURAT BAGCI, MAHNAZ SHAHIDI, RASHID ANSARI, MICHAEL BLAIR, NORMAN PAUL BLAIR, AND RUTH ZELKHA PURPOSE: To report
Thickness Profiles of Retinal Layers by Optical Coherence Tomography Image Segmentation AHMET MURAT BAGCI, MAHNAZ SHAHIDI, RASHID ANSARI, MICHAEL BLAIR, NORMAN PAUL BLAIR, AND RUTH ZELKHA PURPOSE: To report
CHAPTER 3: DIGITAL IMAGING IN DIAGNOSTIC RADIOLOGY. 3.1 Basic Concepts of Digital Imaging
 Physics of Medical X-Ray Imaging (1) Chapter 3 CHAPTER 3: DIGITAL IMAGING IN DIAGNOSTIC RADIOLOGY 3.1 Basic Concepts of Digital Imaging Unlike conventional radiography that generates images on film through
Physics of Medical X-Ray Imaging (1) Chapter 3 CHAPTER 3: DIGITAL IMAGING IN DIAGNOSTIC RADIOLOGY 3.1 Basic Concepts of Digital Imaging Unlike conventional radiography that generates images on film through
Application Note #503 Comparing 3D Optical Microscopy Techniques for Metrology Applications
 Screw thread image generated by WLI Steep PSS angles WLI color imaging Application Note #503 Comparing 3D Optical Microscopy Techniques for Metrology Applications 3D optical microscopy is a mainstay metrology
Screw thread image generated by WLI Steep PSS angles WLI color imaging Application Note #503 Comparing 3D Optical Microscopy Techniques for Metrology Applications 3D optical microscopy is a mainstay metrology
Preparing for laser treatment for diabetic retinopathy and maculopathy
 Preparing for laser treatment for diabetic retinopathy and maculopathy This leaflet sets out to answer the questions people with diabetic retinopathy commonly ask about laser treatment. You might want
Preparing for laser treatment for diabetic retinopathy and maculopathy This leaflet sets out to answer the questions people with diabetic retinopathy commonly ask about laser treatment. You might want
3D TOPOGRAPHY & IMAGE OVERLAY OF PRINTED CIRCUIT BOARD ASSEMBLY
 3D TOPOGRAPHY & IMAGE OVERLAY OF PRINTED CIRCUIT BOARD ASSEMBLY Prepared by Duanjie Li, PhD & Andrea Novitsky 6 Morgan, Ste156, Irvine CA 92618 P: 949.461.9292 F: 949.461.9232 nanovea.com Today's standard
3D TOPOGRAPHY & IMAGE OVERLAY OF PRINTED CIRCUIT BOARD ASSEMBLY Prepared by Duanjie Li, PhD & Andrea Novitsky 6 Morgan, Ste156, Irvine CA 92618 P: 949.461.9292 F: 949.461.9232 nanovea.com Today's standard
Optical Fibres. Introduction. Safety precautions. For your safety. For the safety of the apparatus
 Please do not remove this manual from from the lab. It is available at www.cm.ph.bham.ac.uk/y2lab Optics Introduction Optical fibres are widely used for transmitting data at high speeds. In this experiment,
Please do not remove this manual from from the lab. It is available at www.cm.ph.bham.ac.uk/y2lab Optics Introduction Optical fibres are widely used for transmitting data at high speeds. In this experiment,
Avastin (Bevacizumab) Intravitreal Injection
 Avastin (Bevacizumab) Intravitreal Injection This handout describes how Avastin may be used to treat wet age related macular degeneration (AMD) or macular edema due to retinal vascular disease such as
Avastin (Bevacizumab) Intravitreal Injection This handout describes how Avastin may be used to treat wet age related macular degeneration (AMD) or macular edema due to retinal vascular disease such as
MASTER OCT LECTURE OUTLINE Ophthalmic Photographers Society November 16, 2013 New Orleans, LA
 MASTER OCT LECTURE OUTLINE Ophthalmic Photographers Society November 16, 2013 New Orleans, LA Authors: James B Soque, CRA COA and Pamela A Weber, MD Island Retina Shirley, New York OPS COURSE SA 3 B Financial
MASTER OCT LECTURE OUTLINE Ophthalmic Photographers Society November 16, 2013 New Orleans, LA Authors: James B Soque, CRA COA and Pamela A Weber, MD Island Retina Shirley, New York OPS COURSE SA 3 B Financial
Timing Errors and Jitter
 Timing Errors and Jitter Background Mike Story In a sampled (digital) system, samples have to be accurate in level and time. The digital system uses the two bits of information the signal was this big
Timing Errors and Jitter Background Mike Story In a sampled (digital) system, samples have to be accurate in level and time. The digital system uses the two bits of information the signal was this big
ENGINEERING METROLOGY
 ENGINEERING METROLOGY ACADEMIC YEAR 92-93, SEMESTER ONE COORDINATE MEASURING MACHINES OPTICAL MEASUREMENT SYSTEMS; DEPARTMENT OF MECHANICAL ENGINEERING ISFAHAN UNIVERSITY OF TECHNOLOGY Coordinate Measuring
ENGINEERING METROLOGY ACADEMIC YEAR 92-93, SEMESTER ONE COORDINATE MEASURING MACHINES OPTICAL MEASUREMENT SYSTEMS; DEPARTMENT OF MECHANICAL ENGINEERING ISFAHAN UNIVERSITY OF TECHNOLOGY Coordinate Measuring
SCHWIND CAM Perfect Planning wide range of applications
 SCHWIND CAM Perfect Planning wide range of applications ORK-CAM PresbyMAX PALK-CAM PTK-CAM 2 SCHWIND CAM the system solution The latest version of the modular SCHWIND CAM represents an even more efficient
SCHWIND CAM Perfect Planning wide range of applications ORK-CAM PresbyMAX PALK-CAM PTK-CAM 2 SCHWIND CAM the system solution The latest version of the modular SCHWIND CAM represents an even more efficient
Retinal Detachments Mark E. Hammer, M.D. Ivan J. Suñer, M.D. Marc C. Peden, M.D.
 Retinal Detachments Mark E. Hammer, M.D. Ivan J. Suñer, M.D. Marc C. Peden, M.D. What is a retinal detatchment? The retina is the light sensitive layer covering the inside of the back of the eye. It is
Retinal Detachments Mark E. Hammer, M.D. Ivan J. Suñer, M.D. Marc C. Peden, M.D. What is a retinal detatchment? The retina is the light sensitive layer covering the inside of the back of the eye. It is
Modern Classical Optics
 Modern Classical Optics GEOFFREY BROOKER Department of Physics University of Oxford OXPORD UNIVERSITY PRESS Contents 1 Electromagnetism and basic optics 1 1.1 Introduction 1 1.2 The Maxwell equations 1
Modern Classical Optics GEOFFREY BROOKER Department of Physics University of Oxford OXPORD UNIVERSITY PRESS Contents 1 Electromagnetism and basic optics 1 1.1 Introduction 1 1.2 The Maxwell equations 1
NEW HORIZONS IN CORNEAL SURGERY VERSATILE FEMTOSECOND LASER WORKSTATION WE FOCUS ON PERFECTION
 NEW HORIZONS VERSATILE FEMTOSECOND IN CORNEAL LASER WORKSTATION SURGERY WE FOCUS ON PERFECTION ADVANCED FEMTOSECOND LASER TECHNOLOGY COMMITTED TO VERSATILITY > ONE SYSTEM FOR ALL FEMTO-APPLICATIONS > ANATOMICALLY
NEW HORIZONS VERSATILE FEMTOSECOND IN CORNEAL LASER WORKSTATION SURGERY WE FOCUS ON PERFECTION ADVANCED FEMTOSECOND LASER TECHNOLOGY COMMITTED TO VERSATILITY > ONE SYSTEM FOR ALL FEMTO-APPLICATIONS > ANATOMICALLY
Color holographic 3D display unit with aperture field division
 Color holographic 3D display unit with aperture field division Weronika Zaperty, Tomasz Kozacki, Malgorzata Kujawinska, Grzegorz Finke Photonics Engineering Division, Faculty of Mechatronics Warsaw University
Color holographic 3D display unit with aperture field division Weronika Zaperty, Tomasz Kozacki, Malgorzata Kujawinska, Grzegorz Finke Photonics Engineering Division, Faculty of Mechatronics Warsaw University
Phased-Array ROWA-SPA: High-performance testing machine for combined, 100-percent automated testing of square and round bars
 11th European Conference on Non-Destructive Testing (ECNDT 2014), October 6-10, 2014, Prague, Czech Republic Phased-Array ROWA-SPA: High-performance testing machine for combined, 100-percent automated
11th European Conference on Non-Destructive Testing (ECNDT 2014), October 6-10, 2014, Prague, Czech Republic Phased-Array ROWA-SPA: High-performance testing machine for combined, 100-percent automated
Plastic Film Texture Measurement With 3D Profilometry
 Plastic Film Texture Measurement With 3D Profilometry Prepared by Jorge Ramirez 6 Morgan, Ste156, Irvine CA 92618 P: 949.461.9292 F: 949.461.9232 nanovea.com Today's standard for tomorrow's materials.
Plastic Film Texture Measurement With 3D Profilometry Prepared by Jorge Ramirez 6 Morgan, Ste156, Irvine CA 92618 P: 949.461.9292 F: 949.461.9232 nanovea.com Today's standard for tomorrow's materials.
Keratoconus Detection Using Corneal Topography
 Keratoconus Detection Using Corneal Topography Jack T. Holladay, MD, MSEE, FACS ABSTRACT PURPOSE: To review the topographic patterns associated with keratoconus suspects and provide criteria for keratoconus
Keratoconus Detection Using Corneal Topography Jack T. Holladay, MD, MSEE, FACS ABSTRACT PURPOSE: To review the topographic patterns associated with keratoconus suspects and provide criteria for keratoconus
We are on WHAT ARE THE RISK FACTORS FOR MACULAR DEGENERATION?
 We are on WHAT ARE THE RISK FACTORS FOR MACULAR DEGENERATION? Age Being 60 years of age and older Race Whites are much more likely to lose vision from AMD than Blacks Gender Women tend to be at greater
We are on WHAT ARE THE RISK FACTORS FOR MACULAR DEGENERATION? Age Being 60 years of age and older Race Whites are much more likely to lose vision from AMD than Blacks Gender Women tend to be at greater
Cabling & Test Considerations for 10 Gigabit Ethernet LAN
 Introduction Current communication data rates in local networks range from 10/100 megabits per second (Mbps) in Ethernet to 1 gigabit per second (Gbps) in fiber distributed data interface (FDDI) and Gigabit
Introduction Current communication data rates in local networks range from 10/100 megabits per second (Mbps) in Ethernet to 1 gigabit per second (Gbps) in fiber distributed data interface (FDDI) and Gigabit
Robot Perception Continued
 Robot Perception Continued 1 Visual Perception Visual Odometry Reconstruction Recognition CS 685 11 Range Sensing strategies Active range sensors Ultrasound Laser range sensor Slides adopted from Siegwart
Robot Perception Continued 1 Visual Perception Visual Odometry Reconstruction Recognition CS 685 11 Range Sensing strategies Active range sensors Ultrasound Laser range sensor Slides adopted from Siegwart
Seeing Beyond the Symptoms
 Seeing Beyond the Symptoms Cataracts are one of the leading causes of vision impairment in the United States. 1 However, because cataracts form slowly and over a long period of time, many people suffer
Seeing Beyond the Symptoms Cataracts are one of the leading causes of vision impairment in the United States. 1 However, because cataracts form slowly and over a long period of time, many people suffer
Optical Methods of Surface Measurement
 Optical Methods of Surface Measurement Ted Vorburger, Guest Researcher National Institute of Standards and Technology (NIST) Measurement Science and Standards in Forensic Firearms Analysis 2012 NIST, Gaithersburg,
Optical Methods of Surface Measurement Ted Vorburger, Guest Researcher National Institute of Standards and Technology (NIST) Measurement Science and Standards in Forensic Firearms Analysis 2012 NIST, Gaithersburg,
Sensori ottici e laser nelle applicazioni industriali
 Sensori ottici e laser nelle applicazioni industriali Guido GIULIANI Pavia [email protected] 1 Outline Optical sensors in industry: why? Types of optical sensors optical barriers distance measurement
Sensori ottici e laser nelle applicazioni industriali Guido GIULIANI Pavia [email protected] 1 Outline Optical sensors in industry: why? Types of optical sensors optical barriers distance measurement
Consumer s Guide to LASIK
 Consumer s Guide to LASIK A Community Service Project brought to you by Price Vision Group Your Guide To A Successful LASIK Procedure The purpose of this educational guide is to help prospective patients
Consumer s Guide to LASIK A Community Service Project brought to you by Price Vision Group Your Guide To A Successful LASIK Procedure The purpose of this educational guide is to help prospective patients
Which eye conditions can avastin injections be used for?
 What is avastin? Avastin is a drug that is licensed for the treatment of a certain type of colorectal cancer but can also be used to treat certain eye conditions by being injected into the eye. Although
What is avastin? Avastin is a drug that is licensed for the treatment of a certain type of colorectal cancer but can also be used to treat certain eye conditions by being injected into the eye. Although
Polarization of Light
 Polarization of Light References Halliday/Resnick/Walker Fundamentals of Physics, Chapter 33, 7 th ed. Wiley 005 PASCO EX997A and EX999 guide sheets (written by Ann Hanks) weight Exercises and weights
Polarization of Light References Halliday/Resnick/Walker Fundamentals of Physics, Chapter 33, 7 th ed. Wiley 005 PASCO EX997A and EX999 guide sheets (written by Ann Hanks) weight Exercises and weights
Do You STILL Need Technology? DO YOU STILL TECHNOLOGY? The Challenges in 2013. The Challenges in 2013. FEC s Production Reports 2012
 DO YOU STILL TECHNOLOGY? Craig Thomas, O.D. 3900 West Wheatland Road Dallas, Texas 75237 (972) 780-7199 [email protected] NEED Do You STILL Need Technology? Why do we need to buy technology like a $10,000
DO YOU STILL TECHNOLOGY? Craig Thomas, O.D. 3900 West Wheatland Road Dallas, Texas 75237 (972) 780-7199 [email protected] NEED Do You STILL Need Technology? Why do we need to buy technology like a $10,000
femtosecond laser platform Exceptional versatility without compromise
 Introducing the VICTUS femtosecond laser platform Exceptional versatility without compromise FEMTOSECOND TECHNOLOGY that empowers Introducing VICTUS the first femtosecond laser capable of exceptional performance
Introducing the VICTUS femtosecond laser platform Exceptional versatility without compromise FEMTOSECOND TECHNOLOGY that empowers Introducing VICTUS the first femtosecond laser capable of exceptional performance
BIOMEDICAL ULTRASOUND
 BIOMEDICAL ULTRASOUND Goals: To become familiar with: Ultrasound wave Wave propagation and Scattering Mechanisms of Tissue Damage Biomedical Ultrasound Transducers Biomedical Ultrasound Imaging Ultrasonic
BIOMEDICAL ULTRASOUND Goals: To become familiar with: Ultrasound wave Wave propagation and Scattering Mechanisms of Tissue Damage Biomedical Ultrasound Transducers Biomedical Ultrasound Imaging Ultrasonic
APRIL 8th 2016 Therapy
 APRIL 8th 2016 Therapy 09.00-10.00 SESSION 1: Age-related Macular Degeneration Moderators: S. Sadda, D. Sarraf, K. Bailey Freund, E. Souied 09.00-09.15 Prospective SD OCT Analysis of PED Associated with
APRIL 8th 2016 Therapy 09.00-10.00 SESSION 1: Age-related Macular Degeneration Moderators: S. Sadda, D. Sarraf, K. Bailey Freund, E. Souied 09.00-09.15 Prospective SD OCT Analysis of PED Associated with
Applications in Dermatology, Dentistry and LASIK Eye Surgery using LASERs
 Applications in Dermatology, Dentistry and LASIK Eye Surgery using LASERs http://www.medispainstitute.com/menu_laser_tattoo.html http://www.life123.com/bm.pix/bigstockphoto_close_up_of_eye_surgery_catar_2264267.s600x600.jpg
Applications in Dermatology, Dentistry and LASIK Eye Surgery using LASERs http://www.medispainstitute.com/menu_laser_tattoo.html http://www.life123.com/bm.pix/bigstockphoto_close_up_of_eye_surgery_catar_2264267.s600x600.jpg
Title:Clinical and Optic Coherence Tomography Findings of Focal Choroidal Excavation in Chinese Patients
 Author's response to reviews Title:Clinical and Optic Coherence Tomography Findings of Focal Choroidal Excavation in Chinese Patients Authors: Jie Guo ([email protected]) Lu Zhong ([email protected]) Chunhui
Author's response to reviews Title:Clinical and Optic Coherence Tomography Findings of Focal Choroidal Excavation in Chinese Patients Authors: Jie Guo ([email protected]) Lu Zhong ([email protected]) Chunhui
OCT-guided Femtosecond Laser for LASIK and Presbyopia Treatment
 Shinagawa LASIK Center OCT-guided Femtosecond Laser for LASIK and Presbyopia Treatment Minoru Tomita, MD, Ph.D 1) Executive Medical Director at Shinagawa LASIK Center, Tokyo, Japan 2) Clinical Professor
Shinagawa LASIK Center OCT-guided Femtosecond Laser for LASIK and Presbyopia Treatment Minoru Tomita, MD, Ph.D 1) Executive Medical Director at Shinagawa LASIK Center, Tokyo, Japan 2) Clinical Professor
Customized corneal ablation and super vision. Customized Corneal Ablation and Super Vision
 Customized Corneal Ablation and Super Vision Scott M. MacRae, MD; James Schwiegerling, PhD; Robert Snyder, MD, PhD ABSTRACT PURPOSE: To review the early development of new technologies that are becoming
Customized Corneal Ablation and Super Vision Scott M. MacRae, MD; James Schwiegerling, PhD; Robert Snyder, MD, PhD ABSTRACT PURPOSE: To review the early development of new technologies that are becoming
Understanding posterior vitreous detachment
 Understanding posterior vitreous detachment About posterior vitreous detachment Causes of PVD Symptoms and diagnosis Treatment PVD and other eye conditions Coping Useful contacts About posterior vitreous
Understanding posterior vitreous detachment About posterior vitreous detachment Causes of PVD Symptoms and diagnosis Treatment PVD and other eye conditions Coping Useful contacts About posterior vitreous
