HER2 FISH pharmdx. Assay Kit
|
|
|
- Dennis O’Connor’
- 10 years ago
- Views:
Transcription
1 PATHOLOGY HER2 FISH pharmdx Assay Kit
2 HER2 FISH pharmdx Clarity You Can Count On Reliable Results at a Glance The robust HER2 FISH pharmdx assay offers bright and distinct signals for easy and fast reading of slides, yielding consistent counting accuracy. HER2 FISH pharmdx kit is a direct fluorescence in situ hybridization (FISH) assay designed to quantitatively determine HER2 gene amplification in formalin-fixed, paraffin-embedded breast cancer tissue specimens. The HER2 FISH pharmdx kit is FDA approved as an aid in the assessment of patients when Herceptin (trastuzumab) treatment is being considered. IHC and FISH Predictor of Clinical Benefit from Herceptin Accurate assessment of a patient s HER2 status is vital for correctly identifying women likely to benefit from Herceptin. Patients most likely to benefit are those whose tumors have high HER2 receptor overexpression and/or amplification of the HER2 gene.1 Herceptin increases the benefits of chemotherapy in HER2 positive breast cancers.2,3,4 Initial trials treating women with tumors scoring IHC 2+ and IHC 3+ demonstrated clinical benefit from Herceptin. Among those patients, those who tested IHC 3+ or FISH-positive showed the greatest benefit.4 60 FISH + IHC3+ IHC2+/ CT Alone Herceptin + Chemotherapy (CT) by HER2+ Status Overall response rate (%) CT + Herceptin 2,3
3 Most clinical guidelines recommend HER2 testing with IHC and/or FISH at the time of initial breast cancer diagnosis and confirmation of IHC 2+ results with FISH. 5,6,7 Dako s HercepTest is a semi-quantitative immunohistochemical (IHC) assay to determine HER2 protein overexpression. It is FDA approved as an aid in assessing patients considered for Herceptin therapy. HercepTest, in combination with HER2 FISH pharmdx, delivers a complimentary IVD HER2 assay package to aid in id entifying these patients. Use of HER2 FISH pharmdx in a HER2 Testing Algorithm 5,6,7 Patient Tumor Sample HercepTest HER2 FISH pharmdx SCORE 0 1+ Negative Negative 2+ Weakly Positive (Equivocal) 3+ Positive NEGATIVE Non-amplified (Score <2) POSITIVE Amplified (Score 2) HER2 FISH pharmdx NEGATIVE Non-amplified (Score <2) POSITIVE Amplified (Score 2) Report to Oncologist for Herceptin Consideration HercepTest Highly Concordant to FISH Breast Cancer Specimen IHC Status Percentage of All Cases 6 Concordance IHC/FISH 8 (HercepTest/PathVysion) (n = 426 from 37 Centers) Reliability of HER2 Status 9 0/ % Reliably Negative % Weakly Positive (Equivocal), Require Confirmatory Testing %* Reliably Positive * Of the 102 IHC 3+ tumors, 6 were found to be FISH negative, 5 of these had FISH scores between 1.75 and 2.00 and 1 had a higher number of copies of chromosome 17. PharmacoDiagnostic and pharmdx are registered trademarks of Dako A/S. HercepTest and Herceptin are registered trademarks of Genentech, Inc., subject to licenses held by Dako and F. Hoffmann-La Roche Ltd.
4 Clearly Effective from a Scientific View HER2 Testing: Biology The HER2 gene is located on chromosome 17 and encodes the HER2 protein (p185 HER2). It is present in two copies in all normal diploid cells. In some breast cancer tumors the HER2 gene amplifies as part of the malignant transformation. HER2 Testing: IHC and FISH Immunohistochemistry (IHC) measures the level of HER2 receptor overexpression, while fluorescence in situ hybridization (FISH) quantifies the level of HER2 gene amplification. Together they are the most commonly used methods of determining HER2 status in routine diagnostic settings.6,10 IHC and FISH Targets for HER2 Testing FISH HER2 Receptors DNA IHC mrna Transcript Ligand Amplified result, Score >2. Breast cancer specimen stained with HER2 FISH pharmdx. Breast Cancer Cell Positive result, Score 3+. Breast cancer specimen stained with HercepTest. HER2 gene amplification is the underlying biological change that results in HER2 overexpression. HER2 FISH pharmdx Assay HER2 FISH pharmdx quantitatively determines interphase HER2 gene amplification in formalin-fixed, paraffin-embedded (FFPE) breast cancer tissue. HER2 FISH pharmdx uses a probe mix to quantitatively determine HER2 gene amplification. Dako s HER2 FISH pharmdx includes a chromosome 17 reference probe to correct for HER2 signal number in 11 the event of chromosome 17 aneusomy.
5 Cytogenetic Ideogram CEN-17 HER HER2 gene, amplified copy number, ratio HER2/CEN CEN-17 PNA probe directly labeled with fluorescein (FITC) targets the centromeric region of the chromosome (green signals). HER2 DNA probe directly labeled with Texas Red fluorochrome targets the HER2 amplicon (red signals). Results are expressed as a ratio of HER2 gene copies (red signals) per number of chromosome 17 copies (green signals). HER2 FISH pharmdx Technique Probe DNA labeled with fluorescent dye Denaturation of probe and target nucleic acid Labeled probe hybridizes to HER2 gene on chromosome 17 Denature and hybridize Fluorescence In Situ Hybridization Hybridization signals observed using fluorescent microscope
6 The Solution You ve Been Looking For Set your sights on great counting accuracy and productivity with the HER2 FISH pharmdx kit. Featuring a distinct, enhanced signal and improved assay robustness for results you can count on. HER2 FISH pharmdx Demonstrates Proven Results Amplified Accuracy In a multicenter comparison of FFPE breast cancer samples reflecting the natural range of amplification (ratios ; ; and ) tested in three separate runs, the sites involved were in complete agreement with the classification of HER2 status as HER2 negative or positive. 12 Specimen Non-amplified Altered but non-amplified Low-level amplification High-level amplification HER2 Negative HER2 Positive HER2 Testing. 100% accurate in the classification of HER2 status.
7 Clinical Confidence The clinical utility of the Dako HER2 FISH pharmdx kit was investigated using specimens obtained from cases enrolled in the Danish Breast Cancer Cooperative Group (DBCG) (89-D) study. The study included 200 archived specimens from patients diagnosed with grade II-III breast cancer. Evaluable specimens (190) were successfully hybridized and scored. Number of Observations HER2 FISH pharmdx (-) Amplified HER2 FISH pharmdx (+) Amplified Total PathVysion (-) amplified PathVysion (+) amplified Total Concordance = 93.68% (CI95: 90-97%) Concordance of HER2 FISH pharmdx to Vysis PathVysion Test results included 12 discrepancies between HER2 FISH pharmdx kit and Vysis PathVysion HER2 test. The tested population was enriched with HercepTest 2+ specimens. 12 Alternative Interpretation Saves Time and Maintains Concordance Extensive microscopy makes FISH time consuming. Therefore, timesaving counting methods were used in a comparison of HER2 FISH pharmdx kit to Vysis PathVysion HER-2 DNA Probe kit. Concordance remained unchanged for all investigated counting methods. 12 HER2 FISH pharmdx Results based on counting 10 nuclei 20 nuclei* 30 nuclei 60 nuclei PathVysion (-) Amplified n = 97 (-) (+) Amplified Amplified PathVysion (+) Amplified n = 48 Concordance 95% CI (-) (+) Amplified Amplified % % % % *Number of nuclei recommended
8 Noticeable Advantages at Every Step Timesaving Protocol for Optimal Results The HER2 FISH pharmdx kit protocol is simply a better choice compared to other commercially available kits. HER2 FISH pharmdx has fewer procedural steps, so it s faster and easier to use with a lower probability of error. It also uses fewer toxic materials such as formalin and formamide decreasing risk to laboratory personnel. Three simple steps for clear results with HER2 FISH pharmdx: Specimen pre-treatment Pre-treat specimens with Pre-Treatment Solution. Apply Ready-to-Use Pepsin enzyme to prepare slides for hybridization. Hybridization Apply Ready-to-Use, dual-color, HER2/CEN-17 Probe Mix to the specimen. Denature the target and probe nucleic acid. Allow the probe and target nucleic acid to hybridize. Use the Hybridizer for automated procedure. Wash the slides in Stringent Wash Buffer to remove incompletely hybridized nucleic acid hybrids. Interpretation Using a fluorescence microscope and appropriate filters, enumerate the probe signals and calculate ratio. Simplified Procedure Compared to Other HER2 FISH Assays Total assay time is 1.2 hours less than conventional FISH. Fewer steps No post-fixation step following pre-treatment. Only one phase for denaturation. Room temperature incubation of RTU Pepsin For In Vitro Diagnostic Use. FDA approved as an aid in the assessment of patients for whom Herceptin (trastuzumab) treatment is being considered.
9 Fast and Accurate Quantitative Interpretation Filter requirements Count signals with a fluorescence microscope equipped with a 100-watt mercury lamp. Use a specific DAPI filter and either a high quality Texas Red/FITC double filter or specific Texas Red and FITC single filters. Adequate filters are crucial for correct interpretation. Fluorochrome FITC Texas Red Excitation Wavelength 495 nm 596 nm Emission Wavelength 520 nm 615 nm HER2 FISH pharmdx signals Enhanced signals are bright, distinct and easy to evaluate. The centromeric probe (CEN-17) serves as a control for proper hybridization and allows differentiation of chromosome 17 aneusomy from true amplification. Scan path for signal counting. Non-amplified result breast carcinoma stained with HER2 FISH pharmdx. Amplified result breast carcinoma stained with HER2 FISH pharmdx.
10 Scoring Guide Assessable Tissue Score only invasive component of carcinoma. Avoid necrotic areas and cells with ambiguous borders. Disregard nuclei with weak signal intensity and non-specific or high background staining. Signal Location Locate the tumor within the context of an H&E stained slide and evaluate the same area on the FISH-stained slide. Scan several areas of the tumor to account for possible heterogeneity. Select distinct tumor areas for assessment. Begin analysis in upper left quadrant of selected area. Scan from left to right, counting signals in each tumor nucleus. Adjust microscope focus to locate all signals in individual nuclei. Signal Enumeration Count HER2 (red) and CEN-17 (green) signals in 20 nuclei in representative tumor areas. Calculate the HER2/CEN-17 ratio by dividing the total number of HER2 signals by the total number of CEN-17 signals. Two signals of the same size, separated by a distance equal or less than the diameter of one signal, are counted as one signal. Nuclei with high levels of HER2 gene amplification (red) may exhibit formation of signal clusters. Estimate the HER2 signal. HER2 clusters may obscure CEN-17 (green) signals; check using specific FITC filter. Nuclei exhibiting signals of only one color should not be scored. Do not score nuclei demonstrating over-digestion. Ratio of HER2/ CEN-17 Signals <2 2 HER2 Gene Status Non-amplified Amplified Result Negative Positive Results at or near the cut-off ( ) should be interpreted with caution. In those cases, count an additional 20 nuclei and recalculate. To download a PDF of the Scoring Sheet go to:
11 Counting Guide One green signal (split) indicates the presence of one copy of chromosome 17.* One red signal indicates the presence of one copy of the HER2 gene. The ratio of HER2 to CEN-17 is 1/1 = 1; non-amplified. Three green signals (one out of focus) indicate the presence of three copies of chromosome 17. Three red signals indicate the presence of three copies of the HER2 gene. The ratio of HER2 to CEN-17 is 3/3 = 1; non-amplified. Two green signals indicate the presence of two copies of chromosome 17. Three red signals (two split signals) indicate the presence of three copies of the HER2 gene.* The ratio of HER2 to CEN-17 is 3/2 = 1.5; non-amplified. One green signal indicates the presence of one copy of chromosome 17. Five red signals indicate the presence of five copies of the HER2 gene. The ratio of HER2 to CEN-17 is 5/1 = 5; amplified. Three green signals indicate the presence of three copies of chromosome 17. Approximately 12 red signals indicate the presence of 12 copies of the HER2 gene (cluster estimation). The ratio of HER2 to CEN-17 is 12/3 = 4; amplified. Do not score (nuclei are overlapping, not all areas of nuclei are visible). Do not score nuclei with signals of only one color (two green signals). Do not score (over-digested nuclei). Cluster of red signals hiding green signals. Check the green signals with a specific FITC filter, or do not score. * Two signals of the same size, separated by a distance equal to or less than the diameter of one signal, are counted as one signal.
12 HER2 FISH pharmdx Complete Kit Includes: Pre-treatment solution (20x concentrated) Pepsin, ready-to-use HER2/CEN-17 probe mix Stringent wash buffer (20x concentrated) Fluorescence mounting medium, containing DAPI Wash buffer (20x concentrated) Coverslip sealant HER2 FISH pharmdx Product Name Size Code HER2 FISH pharmdx Kit 20 tests K5331 HercepTest (for manual use) 35 tests K5204 HercepTest for the Dako Autostainer/Autostainer Plus 50 tests K5207 HercepTest for Automated Link Platforms 50 tests SK001 Hybridizer 120V S2450 Hybridizer 240V S2451 Supporting Literature For information about supporting literature, contact your local Dako representative or visit 1. Bilous M, Dowsett M, Hanna W, Isola J, Lebeau A, Moreno A, et al. Current perspectives on HER2 testing: a review of national testing guidelines. Mod Pathol 2003;16: Slamon DJ Study # H0648g- Response Rate (%) Newly defined population. Correlating HER2 Testing. Methodologies with Response: IHC versus FISH. ASCO presentation at 36th Annual Meeting. May 19, Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344: Herceptin (Trastuzumab) Full Prescribing Information. Genentech, Inc., USA. November Wolff A, Hammond E, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Arch Pathol Lab Med Jan; 131: Bilous M, Ades C, Armes J, Bishop J, Brown R, Cooke B, et al. Predicting the HER2 status of breast cancer from basic histopathology data: an analysis of 1500 breast cancers as part of the HER2000 International Study. The Breast 2003;12: Zarbo, R, Hammond, E. Conference Summary, Strategic Science Symposium, Her2/neu of Breast Cancer Patients in Clinical Practice, Archives of Pathology & Laboratory Medicine, Arch Pathol Lab Med, 2003;127: Dowsett M, Bartlett J, Ellis IO, Salter J, Hills M, Mallon E, et al. Correlation between immunohistochemistry (HercepTest) and fluorescence in situ hybridization (FISH) for HER2 in 426 breast carcinomas from 37 centres. J Pathol 2003;199: Di Leo A, Dowsett M, Horten B, Penault-Llorca F. Current status of HER2 testing. Oncology 2002;63 Suppl 1:S Bell R. What can we learn from Herceptin trials in metastatic breast cancer. Oncology 2002;63 Suppl 1:S39-S Ellis IO, Dowsett M, Bartlett J, Walker R, Cooke T, Gullick W, et al. Recommendations for HER2 testing in the UK. J Clin Pathol 2000;53: HER2 FISH pharmdx Kit Package Insert. Dako Denmark A/S May 2005 For In Vitro Diagnostic Use. FDA approved as an aid in the assessment of patients for whom Herceptin (trastuzumab) treatment is being considered. Corporate Headquarters Denmark Distributors in more than 60 countries Australia Austria Belgium +32 (0) Brazil Canada China Denmark Finland France Germany Ireland Italy Japan The Netherlands Norway Poland Spain Sweden Switzerland United Kingdom +44 (0) United States of America MAY10
PATHOLOGY. HercepTestTM. Product Information
 PATHOLOGY HercepTestTM Product Information CLINICAL TRIALS HercepTest The First and Foremost Dako s pharmdx HercepTest was the first FDA-approved assay developed exclusively to aid physicians in identifying
PATHOLOGY HercepTestTM Product Information CLINICAL TRIALS HercepTest The First and Foremost Dako s pharmdx HercepTest was the first FDA-approved assay developed exclusively to aid physicians in identifying
HER2. Improved Quality and Efficiency FISH. HER2 IQFISH pharmdx
 WHI T E PAP E R FISH HER2 IQFISH pharmdx HER2 IQFISH pharmdx HER2 IQFISH pharmdx Improved Quality and Efficiency Improved Quality and Efficiency Debra S. Cohen, BS, CG(ASCAP)CM & Sharon Alsobrook, CG(ASCP)CM,
WHI T E PAP E R FISH HER2 IQFISH pharmdx HER2 IQFISH pharmdx HER2 IQFISH pharmdx Improved Quality and Efficiency Improved Quality and Efficiency Debra S. Cohen, BS, CG(ASCAP)CM & Sharon Alsobrook, CG(ASCP)CM,
HER2 Testing in Breast Cancer
 HER2 Testing in Breast Cancer GAIL H. VANCE, M.D. AGT MEETING JUNE 13, 2014 LOUISVILLE, KENTUCKY No Conflict of Interest to Report Human Epidermal Growth Factor Receptor 2-HER2 Human epidermal growth factor
HER2 Testing in Breast Cancer GAIL H. VANCE, M.D. AGT MEETING JUNE 13, 2014 LOUISVILLE, KENTUCKY No Conflict of Interest to Report Human Epidermal Growth Factor Receptor 2-HER2 Human epidermal growth factor
MEDICAL POLICY EFFECTIVE DATE: 07/20/06 REVISED DATE: 10/18/07, 10/23/08, 10/29/09, 10/28/10, 03/17/11, 02/16/12, 01/17/13, 01/16/14
 MEDICAL POLICY SUBJECT: HER-2 TESTING IN INVASIVE BREAST OR PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases,
MEDICAL POLICY SUBJECT: HER-2 TESTING IN INVASIVE BREAST OR PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases,
Laboratory Testing for Her2 Status in Breast Cancer
 Laboratory Testing for Her2 Status in Breast Cancer Katherine Geiersbach, M.D. Assistant Professor, Department of Pathology May 28, 2015 Overview Clinical relevance of Her2 status for treatment of breast
Laboratory Testing for Her2 Status in Breast Cancer Katherine Geiersbach, M.D. Assistant Professor, Department of Pathology May 28, 2015 Overview Clinical relevance of Her2 status for treatment of breast
EDUCATION. HercepTest TM. Interpretation Manual Breast Cancer
 EDUCATION HercepTest TM Interpretation Manual Breast Cancer EDUCATION HercepTest TM Table of Contents Contents 5 Introduction 6 HER2 Overview 6 HER2 Protein and HER2 Family 6 HER2 Testing IHC and FISH
EDUCATION HercepTest TM Interpretation Manual Breast Cancer EDUCATION HercepTest TM Table of Contents Contents 5 Introduction 6 HER2 Overview 6 HER2 Protein and HER2 Family 6 HER2 Testing IHC and FISH
Measurement of HER2. Daniel F. Hayes, MD Clinical Director, Breast Oncology Program University of Michigan Comprehensive Cancer Center
 Measurement of HER2 Daniel F. Hayes, MD Clinical Director, Breast Oncology Program University of Michigan Comprehensive Cancer Center Trastuzumab Metastatic Adjuvant Lapatinib Metastatic Trials Why Test
Measurement of HER2 Daniel F. Hayes, MD Clinical Director, Breast Oncology Program University of Michigan Comprehensive Cancer Center Trastuzumab Metastatic Adjuvant Lapatinib Metastatic Trials Why Test
March 19, 2014. Dear Dr. Duvall, Dr. Hambrick, and Ms. Smith,
 Dr. Daniel Duvall, Medical Officer Center for Medicare, Hospital and Ambulatory Policy Group Centers for Medicare and Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244 Dr. Edith Hambrick,
Dr. Daniel Duvall, Medical Officer Center for Medicare, Hospital and Ambulatory Policy Group Centers for Medicare and Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244 Dr. Edith Hambrick,
ab176915 StayBlue/AP Plus Stain Kit Instructions for Use An immunohistochemical chromogen substrate for staining tissue sections
 ab176915 StayBlue/AP Plus Stain Kit Instructions for Use An immunohistochemical chromogen substrate for staining tissue sections This product is for research use only and is not intended for diagnostic
ab176915 StayBlue/AP Plus Stain Kit Instructions for Use An immunohistochemical chromogen substrate for staining tissue sections This product is for research use only and is not intended for diagnostic
Immunohistochemical Visualization of Molecular Tests
 Part II: The Potentials and Pitfalls Chapter 13 Immunohistochemical Visualization of Molecular Tests Jens Mollerup, PhD, MSc Jan Trøst Jørgensen, PhD, MSc Mo le cu lar test (n.) The analysis of human DNA,
Part II: The Potentials and Pitfalls Chapter 13 Immunohistochemical Visualization of Molecular Tests Jens Mollerup, PhD, MSc Jan Trøst Jørgensen, PhD, MSc Mo le cu lar test (n.) The analysis of human DNA,
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer
 Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Published Ahead of Print on August 9, 2016 as 10.1200/JCO.2015.61.8983. J Clin Oncol 34. 2016 by American Society of Clinical Oncology INTRODUCTION
 Published Ahead of Print on August 9, 2016 as 10.1200/JCO.2015.61.8983 The latest version is at http://jco.ascopubs.org/cgi/doi/10.1200/jco.2015.61.8983 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E
Published Ahead of Print on August 9, 2016 as 10.1200/JCO.2015.61.8983 The latest version is at http://jco.ascopubs.org/cgi/doi/10.1200/jco.2015.61.8983 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E
HER2 Status: What is the Difference Between Breast and Gastric Cancer?
 Ask the Experts HER2 Status: What is the Difference Between Breast and Gastric Cancer? Bharat Jasani MBChB, PhD, FRCPath Marco Novelli MBChB, PhD, FRCPath Josef Rüschoff, MD Robert Y. Osamura, MD, FIAC
Ask the Experts HER2 Status: What is the Difference Between Breast and Gastric Cancer? Bharat Jasani MBChB, PhD, FRCPath Marco Novelli MBChB, PhD, FRCPath Josef Rüschoff, MD Robert Y. Osamura, MD, FIAC
VENTANA Digital Pathology. Reliable. Efficient. Comprehensive.
 VENTANA Digital Pathology Reliable. Efficient. Comprehensive. In the quest to improve the lives of all patients afflicted with cancer, every second counts. Fast, informed clinical decisions are essential
VENTANA Digital Pathology Reliable. Efficient. Comprehensive. In the quest to improve the lives of all patients afflicted with cancer, every second counts. Fast, informed clinical decisions are essential
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA
 CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
BioPATH: A Study of Biomarker Profiles in Asia Pacific HER2 Breast Cancer Patients Treated with Lapatinib and Other Anti-HER2 Therapy
 BioPATH: A Study of Biomarker Profiles in Asia Pacific HER2 Breast Cancer Patients Treated with Lapatinib and Other Anti-HER2 Therapy Soonmyung Paik 1 ; Gyungyub Gong 2 ; Yap Yoon Sim 3 ; Tae- You Kim
BioPATH: A Study of Biomarker Profiles in Asia Pacific HER2 Breast Cancer Patients Treated with Lapatinib and Other Anti-HER2 Therapy Soonmyung Paik 1 ; Gyungyub Gong 2 ; Yap Yoon Sim 3 ; Tae- You Kim
Outline. 1. Experiment. 2. Sample analysis and storage. 3. Image analysis and presenting data. 4. Probemaker
 Tips and tricks Note: this is just an informative document with general recommendations. Please contact [email protected] should you have any queries. Document last reviewed 2011-11-17 Outline 1. Experiment
Tips and tricks Note: this is just an informative document with general recommendations. Please contact [email protected] should you have any queries. Document last reviewed 2011-11-17 Outline 1. Experiment
J Clin Oncol 24:3032-3038. 2006 by American Society of Clinical Oncology INTRODUCTION
 VOLUME 24 NUMBER 19 JULY 1 2006 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T HER2 Testing by Local, Central, and Reference Laboratories in Specimens From the North Central Cancer Treatment
VOLUME 24 NUMBER 19 JULY 1 2006 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T HER2 Testing by Local, Central, and Reference Laboratories in Specimens From the North Central Cancer Treatment
Monoclonal Antibodies in Cancer. Ralph Schwall, PhD Associate Director, Translational Oncology Genentech, Inc.
 Monoclonal Antibodies in Cancer Ralph Schwall, PhD Associate Director, Translational Oncology Genentech, Inc. Disclaimer I had nothing to do with Herceptin Using lessons learned in new antibody projects
Monoclonal Antibodies in Cancer Ralph Schwall, PhD Associate Director, Translational Oncology Genentech, Inc. Disclaimer I had nothing to do with Herceptin Using lessons learned in new antibody projects
HER2 Testing in Gastric and Esophageal Adenocarcinoma: Emerging Therapeutic Options and Diagnostic Challenges
 Technical Articles HER2 Testing in Gastric and Esophageal Adenocarcinoma: Emerging Therapeutic Options and Diagnostic Challenges Christa L. Whitney-Miller, MD David G. Hicks, MD Department of Pathology
Technical Articles HER2 Testing in Gastric and Esophageal Adenocarcinoma: Emerging Therapeutic Options and Diagnostic Challenges Christa L. Whitney-Miller, MD David G. Hicks, MD Department of Pathology
PROTOCOL. Immunocytochemistry (ICC) MATERIALS AND EQUIPMENT REQUIRED
 PROTOCOL Immunocytochemistry (ICC) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 11-07 MATERIALS AND EQUIPMENT REQUIRED Materials: MitoSciences primary monoclonal antibody/antibodies Fluorophore-conjugated
PROTOCOL Immunocytochemistry (ICC) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 11-07 MATERIALS AND EQUIPMENT REQUIRED Materials: MitoSciences primary monoclonal antibody/antibodies Fluorophore-conjugated
Prognostic and Predictive Factors in Breast Cancer Kyle T. Bradley, MD, MS CAP Cancer Committee
 Prognostic and Predictive Factors in Breast Cancer Kyle T. Bradley, MD, MS CAP Cancer Committee Breast cancer is the most common malignant tumor in American women and is second only to lung cancer as a
Prognostic and Predictive Factors in Breast Cancer Kyle T. Bradley, MD, MS CAP Cancer Committee Breast cancer is the most common malignant tumor in American women and is second only to lung cancer as a
micrornas Non protein coding, endogenous RNAs of 21-22nt length Evolutionarily conserved
 microrna 2 micrornas Non protein coding, endogenous RNAs of 21-22nt length Evolutionarily conserved Regulate gene expression by binding complementary regions at 3 regions of target mrnas Act as negative
microrna 2 micrornas Non protein coding, endogenous RNAs of 21-22nt length Evolutionarily conserved Regulate gene expression by binding complementary regions at 3 regions of target mrnas Act as negative
Avastin in breast cancer: Summary of clinical data
 Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Local Coverage Determination (LCD): MolDX: Breast Cancer Assay: Prosigna (L36125)
 Local Coverage Determination (LCD): MolDX: Breast Cancer Assay: Prosigna (L36125) Contractor Information Contractor Name Palmetto GBA LCD Information Document Information LCD ID L36125 Original ICD-9 LCD
Local Coverage Determination (LCD): MolDX: Breast Cancer Assay: Prosigna (L36125) Contractor Information Contractor Name Palmetto GBA LCD Information Document Information LCD ID L36125 Original ICD-9 LCD
PREPARED FOR: U.S. Army Medical Research and Materiel CommandFort Detrick, Maryland 21702 5012
 AD Award Number: W81XWH 07 1 0542 TITLE: Is Nuclear Structure Altered in Breast Cancer Cells? PRINCIPAL INVESTIGATOR: Han Htun, Ph.D. CONTRACTING ORGANIZATION: University of California Los Angeles, CA
AD Award Number: W81XWH 07 1 0542 TITLE: Is Nuclear Structure Altered in Breast Cancer Cells? PRINCIPAL INVESTIGATOR: Han Htun, Ph.D. CONTRACTING ORGANIZATION: University of California Los Angeles, CA
Chapter 12 Filters for FISH Imaging
 Chapter 12 Filters for FISH Imaging Dan Osborn The application of in situ hybridization (ISH) has advanced from short lived, non-specific isotopic methods, to very specific, long lived, multiple color
Chapter 12 Filters for FISH Imaging Dan Osborn The application of in situ hybridization (ISH) has advanced from short lived, non-specific isotopic methods, to very specific, long lived, multiple color
FDA Regulation of Whole Slide Imaging (WSI) Devices: Current Thoughts
 FDA Regulation of Whole Slide Imaging (WSI) Devices: Current Thoughts Clinical Laboratory Improvement Advisory Committee Meeting Centers for Disease Control and Prevention February 15, 2012 Tremel A. Faison,
FDA Regulation of Whole Slide Imaging (WSI) Devices: Current Thoughts Clinical Laboratory Improvement Advisory Committee Meeting Centers for Disease Control and Prevention February 15, 2012 Tremel A. Faison,
Changes in Breast Cancer Reports After Second Opinion. Dr. Vicente Marco Department of Pathology Hospital Quiron Barcelona. Spain
 Changes in Breast Cancer Reports After Second Opinion Dr. Vicente Marco Department of Pathology Hospital Quiron Barcelona. Spain Second Opinion in Breast Pathology Usually requested when a patient is referred
Changes in Breast Cancer Reports After Second Opinion Dr. Vicente Marco Department of Pathology Hospital Quiron Barcelona. Spain Second Opinion in Breast Pathology Usually requested when a patient is referred
How To Get A Cell Print
 QUICK CELL CAPTURE AND CHARACTERIZATION GUIDE FOR CELLSEARCH CUSTOMERS CellSave EDTA Blood sample Rare cell capture Enumeration Single protein marker Cell capture for molecular characterization CELLSEARCH
QUICK CELL CAPTURE AND CHARACTERIZATION GUIDE FOR CELLSEARCH CUSTOMERS CellSave EDTA Blood sample Rare cell capture Enumeration Single protein marker Cell capture for molecular characterization CELLSEARCH
amplification tech Optical Design of CFX96 Real-Time PCR Detection System Eliminates the Requirement of a Passive Reference Dye
 amplification tech note 6047 Optical Design of CFX96 Real-Time PCR Detection System Eliminates the Requirement of a Passive Reference Dye Liz Jordan and Richard Kurtz, Gene Expression Division, io-rad
amplification tech note 6047 Optical Design of CFX96 Real-Time PCR Detection System Eliminates the Requirement of a Passive Reference Dye Liz Jordan and Richard Kurtz, Gene Expression Division, io-rad
http://www.springer.com/3-540-22006-2
 http://www.springer.com/3-540-22006-2 1 Molecular Pathology Laboratory of the Future Christopher A. Moskaluk 1.1 The Past The integration of laboratory analysis with human medicine has traditionally been
http://www.springer.com/3-540-22006-2 1 Molecular Pathology Laboratory of the Future Christopher A. Moskaluk 1.1 The Past The integration of laboratory analysis with human medicine has traditionally been
QIAsymphony RGQ Protocol Sheet
 QIAsymphony RGQ Protocol Sheet Settings to run the artus CT/NG QS-RGQ Kit (Rotor-Gene Q software.) Check availability of new electronic labeling revisions at www.qiagen.com/products/artusctngqsrgqkitce.aspx
QIAsymphony RGQ Protocol Sheet Settings to run the artus CT/NG QS-RGQ Kit (Rotor-Gene Q software.) Check availability of new electronic labeling revisions at www.qiagen.com/products/artusctngqsrgqkitce.aspx
Thermo Scientific ClinQuan MD Software For In Vitro Diagnostic Use. Confidence in Results With Data Integrity
 Thermo Scientific ClinQuan MD Software For In Vitro Diagnostic Use Confidence in Results With Data Integrity 2 Make the World Healthier With the LC-MS Tests You Run Confidence in Test Results With Data
Thermo Scientific ClinQuan MD Software For In Vitro Diagnostic Use Confidence in Results With Data Integrity 2 Make the World Healthier With the LC-MS Tests You Run Confidence in Test Results With Data
Digital Pathology Image Analysis with Definiens
 Understanding Images DEEPER INSIGHTS FASTER RESULTS BETTER DECISIONS Digital Pathology Image Analysis with Definiens 2,05 0,012 coexpression 10000 circularity infiltration biomarker intensity 48 mitotic
Understanding Images DEEPER INSIGHTS FASTER RESULTS BETTER DECISIONS Digital Pathology Image Analysis with Definiens 2,05 0,012 coexpression 10000 circularity infiltration biomarker intensity 48 mitotic
BREAST CANCER PATHOLOGY
 BREAST CANCER PATHOLOGY FACT SHEET Version 4, Aug 2013 This fact sheet was produced by Breast Cancer Network Australia with input from The Royal College of Pathologists of Australasia I m a nurse and know
BREAST CANCER PATHOLOGY FACT SHEET Version 4, Aug 2013 This fact sheet was produced by Breast Cancer Network Australia with input from The Royal College of Pathologists of Australasia I m a nurse and know
PNA BRAF Mutation Detection Kit
 - PNA BRAF Mutation Detection Kit Catalog Number KA2102 50 tests/kit Version: 01 Intended for research use only www.abnova.com Introduction and Background Intended use The PNA BRAF Mutation Detection Kit
- PNA BRAF Mutation Detection Kit Catalog Number KA2102 50 tests/kit Version: 01 Intended for research use only www.abnova.com Introduction and Background Intended use The PNA BRAF Mutation Detection Kit
FULLY AUTOMATED PARALLEL DUAL STAINING DETECTION SYSTEM. ChromoPlex 1 Dual Detection for BOND
 ChromoPlex 1 Dual Detection for BOND FULLY AUTOMATED PARALLEL DUAL STAINING DETECTION SYSTEM View multiple antibodies on a single slide to deliver a comprehensive clinical result. 1 MULTIPLY YOUR CAPABILITIES
ChromoPlex 1 Dual Detection for BOND FULLY AUTOMATED PARALLEL DUAL STAINING DETECTION SYSTEM View multiple antibodies on a single slide to deliver a comprehensive clinical result. 1 MULTIPLY YOUR CAPABILITIES
HAVE YOU BEEN NEWLY DIAGNOSED with DCIS?
 HAVE YOU BEEN NEWLY DIAGNOSED with DCIS? Jen D. Mother and volunteer. Diagnosed with DCIS breast cancer in 2012. An educational guide prepared by Genomic Health This guide is designed to educate women
HAVE YOU BEEN NEWLY DIAGNOSED with DCIS? Jen D. Mother and volunteer. Diagnosed with DCIS breast cancer in 2012. An educational guide prepared by Genomic Health This guide is designed to educate women
Understanding your pathology report
 Understanding your pathology report 2 Contents Contents Introduction 3 What is a pathology report? 3 Waiting for your results 4 What s in a pathology report? 4 Information about your breast cancer 5 What
Understanding your pathology report 2 Contents Contents Introduction 3 What is a pathology report? 3 Waiting for your results 4 What s in a pathology report? 4 Information about your breast cancer 5 What
Description of Procedure or Service. assays_of_genetic_expression_to_determine_prognosis_of_breast_cancer 11/2004 3/2015 3/2016 3/2015
 Corporate Medical Policy Assays of Genetic Expression to Determine Prognosis of Breast File Name: Origination: Last CAP Review: Next CAP Review: Last Review: assays_of_genetic_expression_to_determine_prognosis_of_breast_cancer
Corporate Medical Policy Assays of Genetic Expression to Determine Prognosis of Breast File Name: Origination: Last CAP Review: Next CAP Review: Last Review: assays_of_genetic_expression_to_determine_prognosis_of_breast_cancer
Annexin V-FITC Apoptosis Detection Kit
 ab14085 Annexin V-FITC Apoptosis Detection Kit Instructions for Use For the rapid, sensitive and accurate measurement of Apoptosis in living cells (adherent and suspension). This product is for research
ab14085 Annexin V-FITC Apoptosis Detection Kit Instructions for Use For the rapid, sensitive and accurate measurement of Apoptosis in living cells (adherent and suspension). This product is for research
Interpretation Guide for VENTANA anti-her2/neu (4B5)
 Interpretation Guide for VENTANA anti-her2/neu (4B5) Rabbit Monoclonal Primary Antibody Staining of Breast and Gastric Carcinoma E2451_1211C_HER2_4B5_Breast_and_Gastric_Interpretation_Guide-05.indd 1 Table
Interpretation Guide for VENTANA anti-her2/neu (4B5) Rabbit Monoclonal Primary Antibody Staining of Breast and Gastric Carcinoma E2451_1211C_HER2_4B5_Breast_and_Gastric_Interpretation_Guide-05.indd 1 Table
Application Guide... 2
 Protocol for GenomePlex Whole Genome Amplification from Formalin-Fixed Parrafin-Embedded (FFPE) tissue Application Guide... 2 I. Description... 2 II. Product Components... 2 III. Materials to be Supplied
Protocol for GenomePlex Whole Genome Amplification from Formalin-Fixed Parrafin-Embedded (FFPE) tissue Application Guide... 2 I. Description... 2 II. Product Components... 2 III. Materials to be Supplied
Application Note. Single Cell PCR Preparation
 Application Note Single Cell PCR Preparation From Automated Screening to the Molecular Analysis of Single Cells The AmpliGrid system is a highly sensitive tool for the analysis of single cells. In combination
Application Note Single Cell PCR Preparation From Automated Screening to the Molecular Analysis of Single Cells The AmpliGrid system is a highly sensitive tool for the analysis of single cells. In combination
Real-Time PCR Vs. Traditional PCR
 Real-Time PCR Vs. Traditional PCR Description This tutorial will discuss the evolution of traditional PCR methods towards the use of Real-Time chemistry and instrumentation for accurate quantitation. Objectives
Real-Time PCR Vs. Traditional PCR Description This tutorial will discuss the evolution of traditional PCR methods towards the use of Real-Time chemistry and instrumentation for accurate quantitation. Objectives
INSTRUCTION Probemaker
 INSTRUCTION Probemaker Instructions for Duolink In Situ Probemaker PLUS (Art. no. 92009-0020) and Duolink In Situ Probemaker MINUS (Art. no. 92010-0020) Table of content 1. Introduction 4 2. Applications
INSTRUCTION Probemaker Instructions for Duolink In Situ Probemaker PLUS (Art. no. 92009-0020) and Duolink In Situ Probemaker MINUS (Art. no. 92010-0020) Table of content 1. Introduction 4 2. Applications
Your Guide to the Breast Cancer Pathology Report
 Your Guide to the Breast Cancer Pathology Report Developed for you by Breastcancer.org is a nonprofit organization dedicated to providing education and information on breast health and breast cancer. The
Your Guide to the Breast Cancer Pathology Report Developed for you by Breastcancer.org is a nonprofit organization dedicated to providing education and information on breast health and breast cancer. The
Adjuvant treatment of breast cancer patients with trastuzumab
 doi:10.2478/v10019-007-0020-y review Adjuvant treatment of breast cancer patients with trastuzumab Erika Matos, Tanja Čufer Institute of Oncology Ljubljana, Department of Medical Oncology, Ljubljana, Slovenia
doi:10.2478/v10019-007-0020-y review Adjuvant treatment of breast cancer patients with trastuzumab Erika Matos, Tanja Čufer Institute of Oncology Ljubljana, Department of Medical Oncology, Ljubljana, Slovenia
PSA Testing 101. Stanley H. Weiss, MD. Professor, UMDNJ-New Jersey Medical School. Director & PI, Essex County Cancer Coalition. weiss@umdnj.
 PSA Testing 101 Stanley H. Weiss, MD Professor, UMDNJ-New Jersey Medical School Director & PI, Essex County Cancer Coalition [email protected] September 23, 2010 Screening: 3 tests for PCa A good screening
PSA Testing 101 Stanley H. Weiss, MD Professor, UMDNJ-New Jersey Medical School Director & PI, Essex County Cancer Coalition [email protected] September 23, 2010 Screening: 3 tests for PCa A good screening
IBCSG Tissue Bank Policy
 THE INTERNATIONAL BREAST CANCER STUDY GROUP IBCSG Tissue Bank Policy Accepted by the IBCSG Executive Committee on March 24, 2006 Accepted by the IBCSG Ethics Committee on March 24, 2006 Accepted by the
THE INTERNATIONAL BREAST CANCER STUDY GROUP IBCSG Tissue Bank Policy Accepted by the IBCSG Executive Committee on March 24, 2006 Accepted by the IBCSG Ethics Committee on March 24, 2006 Accepted by the
Chapter 11 Preparation of FFPE Tissue Slides for Solid Tumor FISH Analysis
 Chapter 11 Preparation of FFPE Tissue Slides for Solid Tumor FISH Analysis Sven Müller PhD, Steen H. Matthiesen PhD and Kirsten V. Nielsen MS Tumor tissue analysis by fluorescence in situ hybridization
Chapter 11 Preparation of FFPE Tissue Slides for Solid Tumor FISH Analysis Sven Müller PhD, Steen H. Matthiesen PhD and Kirsten V. Nielsen MS Tumor tissue analysis by fluorescence in situ hybridization
Avastin in breast cancer: Summary of clinical data
 Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Thermo Scientific PepFinder Software A New Paradigm for Peptide Mapping
 Thermo Scientific PepFinder Software A New Paradigm for Peptide Mapping For Conclusive Characterization of Biologics Deep Protein Characterization Is Crucial Pharmaceuticals have historically been small
Thermo Scientific PepFinder Software A New Paradigm for Peptide Mapping For Conclusive Characterization of Biologics Deep Protein Characterization Is Crucial Pharmaceuticals have historically been small
Appendix One. HER2-positive early breast cancer, its treatment and prognosis
 Appendix One. HER2-positive early breast cancer, its treatment and prognosis Breast cancer and HER2/neu over-expression Health need is one of PHARMAC s nine decision criteria (http://www.pharmac.govt.nz/pdf/231205.pdf
Appendix One. HER2-positive early breast cancer, its treatment and prognosis Breast cancer and HER2/neu over-expression Health need is one of PHARMAC s nine decision criteria (http://www.pharmac.govt.nz/pdf/231205.pdf
Automating Cell Biology Annual general meeting, September 7, 2015. Phase Holographic Imaging www.phiab.se
 Automating Cell Biology Annual general meeting, September 7, 2015 www.phiab.se 1 PHASE HOLOGRAPHIC IMAGING (PHI) Began as a research project at Lund University, Sweden, in 2000 Founded in 2004 Sales in
Automating Cell Biology Annual general meeting, September 7, 2015 www.phiab.se 1 PHASE HOLOGRAPHIC IMAGING (PHI) Began as a research project at Lund University, Sweden, in 2000 Founded in 2004 Sales in
IHC Nuclear Image Analysis. User s Guide
 IHC Nuclear Image Analysis User s Guide Copyright 2007 Aperio Technologies, Inc. Part Number/Revision: MAN-0027, Revision B Date: January 2, 2007 This document applies to software versions Release 8.0
IHC Nuclear Image Analysis User s Guide Copyright 2007 Aperio Technologies, Inc. Part Number/Revision: MAN-0027, Revision B Date: January 2, 2007 This document applies to software versions Release 8.0
Corporate Medical Policy
 Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
ab185916 Hi-Fi cdna Synthesis Kit
 ab185916 Hi-Fi cdna Synthesis Kit Instructions for Use For cdna synthesis from various RNA samples This product is for research use only and is not intended for diagnostic use. Version 1 Last Updated 1
ab185916 Hi-Fi cdna Synthesis Kit Instructions for Use For cdna synthesis from various RNA samples This product is for research use only and is not intended for diagnostic use. Version 1 Last Updated 1
Breast and Lung Cancer Biomarker Research at ASCO: Changing Treatment Patterns
 July 2013 Edition Vol. 7, Issue 7 Breast and Lung Cancer Biomarker Research at ASCO: Changing Treatment Patterns By Julie Katz, MPH, MPhil Biomarkers played a prominent role in the research presented in
July 2013 Edition Vol. 7, Issue 7 Breast and Lung Cancer Biomarker Research at ASCO: Changing Treatment Patterns By Julie Katz, MPH, MPhil Biomarkers played a prominent role in the research presented in
Information Model Requirements of Post-Coordinated SNOMED CT Expressions for Structured Pathology Reports
 Information Model Requirements of Post-Coordinated SNOMED CT Expressions for Structured Pathology Reports W. Scott Campbell, Ph.D., MBA James R. Campbell, MD Acknowledgements Steven H. Hinrichs, MD Chairman
Information Model Requirements of Post-Coordinated SNOMED CT Expressions for Structured Pathology Reports W. Scott Campbell, Ph.D., MBA James R. Campbell, MD Acknowledgements Steven H. Hinrichs, MD Chairman
Real-time PCR: Understanding C t
 APPLICATION NOTE Real-Time PCR Real-time PCR: Understanding C t Real-time PCR, also called quantitative PCR or qpcr, can provide a simple and elegant method for determining the amount of a target sequence
APPLICATION NOTE Real-Time PCR Real-time PCR: Understanding C t Real-time PCR, also called quantitative PCR or qpcr, can provide a simple and elegant method for determining the amount of a target sequence
1.Gene Synthesis. 2.Peptide & Phospho-P. Assembly PCR. Design & Synthesis. Advantages. Specifications. Advantages
 1.Gene Synthesis Assembly PCR Looking for a cdna for your research but could not fish out the gene through traditional cloning methods or a supplier? Abnova provides a gene synthesis service via assembly
1.Gene Synthesis Assembly PCR Looking for a cdna for your research but could not fish out the gene through traditional cloning methods or a supplier? Abnova provides a gene synthesis service via assembly
Complimentary CME. Metastatic Breast Cancer: Monitoring Soluble HER2 Levels
 Complimentary CME Metastatic Breast Cancer: Monitoring Soluble HER2 Levels Program Description HER2/neu-positive tumors account for approximately 20% of all breast cancers and these tumors carry poor prognosis.
Complimentary CME Metastatic Breast Cancer: Monitoring Soluble HER2 Levels Program Description HER2/neu-positive tumors account for approximately 20% of all breast cancers and these tumors carry poor prognosis.
White paper Evaluation of BRAF (V600E) Mutation by Immunohistochemical Staining with anti-braf V600E (VE1) Antibody: A Comparison with Sanger
 White paper Evaluation of BRAF (V600E) Mutation by Immunohistochemical Staining with anti-braf V600E (VE1) Antibody: A Comparison with Sanger Sequencing 2 Evaluation of BRAF (V600E) Mutation by Immunohistochemical
White paper Evaluation of BRAF (V600E) Mutation by Immunohistochemical Staining with anti-braf V600E (VE1) Antibody: A Comparison with Sanger Sequencing 2 Evaluation of BRAF (V600E) Mutation by Immunohistochemical
I. THE IMPORTANCE OF HER2 IN BREAST CANCER
 I. THE IMPORTANCE OF HER2 IN BREAST CANCER The human epidermal growth factor receptors (HER), also known as ERBB receptors, are a family of signal transduction proteins. There are 4 family members in humans
I. THE IMPORTANCE OF HER2 IN BREAST CANCER The human epidermal growth factor receptors (HER), also known as ERBB receptors, are a family of signal transduction proteins. There are 4 family members in humans
Application of Automated Data Collection to Surface-Enhanced Raman Scattering (SERS)
 Application Note: 52020 Application of Automated Data Collection to Surface-Enhanced Raman Scattering (SERS) Timothy O. Deschaines, Ph.D., Thermo Fisher Scientific, Madison, WI, USA Key Words Array Automation
Application Note: 52020 Application of Automated Data Collection to Surface-Enhanced Raman Scattering (SERS) Timothy O. Deschaines, Ph.D., Thermo Fisher Scientific, Madison, WI, USA Key Words Array Automation
Corporate Medical Policy Urinary Tumor Markers for Bladder Cancer
 Corporate Medical Policy Urinary Tumor Markers for Bladder Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: urinary_tumor_markers_for_bladder_cancer 5/2011 11/2015 11/2016
Corporate Medical Policy Urinary Tumor Markers for Bladder Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: urinary_tumor_markers_for_bladder_cancer 5/2011 11/2015 11/2016
A 70-year old Man with Pleural Effusion
 Mesothelioma Diagnosis: Pitfalls and Latest Updates S Klebe and DW Henderson Recommendations Indisputable malignant cells on cytomorphological criteria which demonstrate a mesothelial phenotype, which
Mesothelioma Diagnosis: Pitfalls and Latest Updates S Klebe and DW Henderson Recommendations Indisputable malignant cells on cytomorphological criteria which demonstrate a mesothelial phenotype, which
How To Use A Breast Cancer Test To Help You Choose Chemotherapy
 Gene expression profiling and expanded immunohistochemistry tests for guiding adjuvant chemotherapy decisions in early breast cancer management: MammaPrint, Oncotype DX, IHC4 and Mammostrat Issued: September
Gene expression profiling and expanded immunohistochemistry tests for guiding adjuvant chemotherapy decisions in early breast cancer management: MammaPrint, Oncotype DX, IHC4 and Mammostrat Issued: September
What is special about a stain, and why do we call some stains
 Technical Articles Evolution of Use of Special Stains Alton D. Floyd, PhD Independent Consultant Edwardsburg, MI, USA What is special about a stain, and why do we call some stains special? We all think
Technical Articles Evolution of Use of Special Stains Alton D. Floyd, PhD Independent Consultant Edwardsburg, MI, USA What is special about a stain, and why do we call some stains special? We all think
inform Advanced Image Analysis Software For Accurately Quantifying Biomarkers in Tissue Sections
 P R O D U C T N O T E inform Advanced Image Analysis Key Features Quantitative analysis of biomarker epression in tissue sections and TMAs Separation of weakly epressing and overlapping markers Cellular
P R O D U C T N O T E inform Advanced Image Analysis Key Features Quantitative analysis of biomarker epression in tissue sections and TMAs Separation of weakly epressing and overlapping markers Cellular
Introduction To Real Time Quantitative PCR (qpcr)
 Introduction To Real Time Quantitative PCR (qpcr) SABiosciences, A QIAGEN Company www.sabiosciences.com The Seminar Topics The advantages of qpcr versus conventional PCR Work flow & applications Factors
Introduction To Real Time Quantitative PCR (qpcr) SABiosciences, A QIAGEN Company www.sabiosciences.com The Seminar Topics The advantages of qpcr versus conventional PCR Work flow & applications Factors
Fluorescent dyes for use with the
 Detection of Multiple Reporter Dyes in Real-time, On-line PCR Analysis with the LightCycler System Gregor Sagner, Cornelia Goldstein, and Rob van Miltenburg Roche Molecular Biochemicals, Penzberg, Germany
Detection of Multiple Reporter Dyes in Real-time, On-line PCR Analysis with the LightCycler System Gregor Sagner, Cornelia Goldstein, and Rob van Miltenburg Roche Molecular Biochemicals, Penzberg, Germany
18.5 Percent Overall Response Rate Observed in Pembrolizumab-Treated Patients with this Aggressive Form of Breast Cancer
 News Release Media Contacts: Annick Robinson Investor Contacts: Joseph Romanelli (514) 837-2550 (908) 740-1986 Stephanie Lyttle NATIONAL Public Relations (514) 843-2365 Justin Holko (908) 740-1879 Merck
News Release Media Contacts: Annick Robinson Investor Contacts: Joseph Romanelli (514) 837-2550 (908) 740-1986 Stephanie Lyttle NATIONAL Public Relations (514) 843-2365 Justin Holko (908) 740-1879 Merck
Image Lab Software How to Obtain Stain-Free Gel and Blot Images. Instructions
 Image Lab Software How to Obtain Stain-Free Gel and Blot Images Instructions Table of Contents Obtaining Stain-Free Gel images...1 5 Obtaining Stain-Free Blot Images...6 9 If the Gel Doc XR+ or ChemiDoc
Image Lab Software How to Obtain Stain-Free Gel and Blot Images Instructions Table of Contents Obtaining Stain-Free Gel images...1 5 Obtaining Stain-Free Blot Images...6 9 If the Gel Doc XR+ or ChemiDoc
10/06/2013. Commentary. The best way to achieve optimal treatment of today s patients is to ensure the availability of
 With main focus on the Estrogen Receptor Commentary The best way to achieve optimal treatment of today s patients is to ensure the availability of reliableand timelypathological th l i l assessment in
With main focus on the Estrogen Receptor Commentary The best way to achieve optimal treatment of today s patients is to ensure the availability of reliableand timelypathological th l i l assessment in
Technical Note. Roche Applied Science. No. LC 19/2004. Color Compensation
 Roche Applied Science Technical Note No. LC 19/2004 Purpose of this Note Color The LightCycler System is able to simultaneously detect and analyze more than one color in each capillary. Due to overlap
Roche Applied Science Technical Note No. LC 19/2004 Purpose of this Note Color The LightCycler System is able to simultaneously detect and analyze more than one color in each capillary. Due to overlap
Resolving Equivocal HER2 Status in Breast Cancer by Automated and Quantitative RNA Chromogenic In Situ Hybridization (CISH)
 Resolving Equivocal HER2 Status in Breast Cancer by Automated and Quantitative RNA Chromogenic In Situ Hybridization (CISH) USCAP, Vancouver, Canada, March 2012 Zhen Wang 1, Son Bui 2, Hongwei Wang 2,
Resolving Equivocal HER2 Status in Breast Cancer by Automated and Quantitative RNA Chromogenic In Situ Hybridization (CISH) USCAP, Vancouver, Canada, March 2012 Zhen Wang 1, Son Bui 2, Hongwei Wang 2,
Breast Cancer. CSC Cancer Experience Registry Member, breast cancer
 ESSENTIALS Breast Cancer Take things one step at a time. Try not to be overwhelmed by the tidal wave of technical information coming your way. Finally you know your body best; you have to be your own advocate.
ESSENTIALS Breast Cancer Take things one step at a time. Try not to be overwhelmed by the tidal wave of technical information coming your way. Finally you know your body best; you have to be your own advocate.
PATHOLOGY. HercepTest TM Interpretation Manual - Gastric Cancer
 PATHOLOGY HercepTest TM Interpretation Manual - Gastric Cancer Table of Contents Introduction... 2 HER2 Protein and HER2 Family... 3 HER2 Testing Algorithm... 4 The HercepTest TM Kit...5 HER2 FISH pharmdx
PATHOLOGY HercepTest TM Interpretation Manual - Gastric Cancer Table of Contents Introduction... 2 HER2 Protein and HER2 Family... 3 HER2 Testing Algorithm... 4 The HercepTest TM Kit...5 HER2 FISH pharmdx
Evaluating the Efficiency of Targeted Designs for Randomized Clinical Trials
 Vol. 0, 6759 6763, October 5, 004 Clinical Cancer Research 6759 Perspective Evaluating the Efficiency of Targeted Designs for Randomized Clinical Trials Richard Simon and Aboubakar Maitournam Biometric
Vol. 0, 6759 6763, October 5, 004 Clinical Cancer Research 6759 Perspective Evaluating the Efficiency of Targeted Designs for Randomized Clinical Trials Richard Simon and Aboubakar Maitournam Biometric
Chapter 10 Immunofluorescence
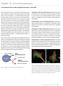 Chapter 10 Immunofluorescence J. Paul Robinson PhD, Jennifer Sturgis BS and George L. Kumar PhD Immunofluorescence (IF) is a common laboratory technique used in almost all aspects of biology. This technique
Chapter 10 Immunofluorescence J. Paul Robinson PhD, Jennifer Sturgis BS and George L. Kumar PhD Immunofluorescence (IF) is a common laboratory technique used in almost all aspects of biology. This technique
Make the invisible visible! SENSORS WITH EXCELLENT BACKGROUND SUPPRESSION
 Make the invisible visible! SENSORS WITH EXCELLENT BACKGROUND SUPPRESSION photoelectric sensors Thanks to its vision for innovation and technological enhancement, Contrinex keeps on setting new standards
Make the invisible visible! SENSORS WITH EXCELLENT BACKGROUND SUPPRESSION photoelectric sensors Thanks to its vision for innovation and technological enhancement, Contrinex keeps on setting new standards
Get the benefits of Norgren s unique range of Online services
 Get the benefits of Norgren s unique range of Online services Make your job easier and save time - everything you need to select, design and purchase Norgren pneumatics is in one convenient location, available
Get the benefits of Norgren s unique range of Online services Make your job easier and save time - everything you need to select, design and purchase Norgren pneumatics is in one convenient location, available
Genomic Medicine The Future of Cancer Care. Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America
 Genomic Medicine The Future of Cancer Care Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America Personalized Medicine Personalized health care is a broad term for interventions
Genomic Medicine The Future of Cancer Care Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America Personalized Medicine Personalized health care is a broad term for interventions
ab185915 Protein Sumoylation Assay Ultra Kit
 ab185915 Protein Sumoylation Assay Ultra Kit Instructions for Use For the measuring in vivo protein sumoylation in various samples This product is for research use only and is not intended for diagnostic
ab185915 Protein Sumoylation Assay Ultra Kit Instructions for Use For the measuring in vivo protein sumoylation in various samples This product is for research use only and is not intended for diagnostic
