First-principle calculation of MgH 2 and LiH for hydrogen storage
|
|
|
- Pearl Lewis
- 10 years ago
- Views:
Transcription
1 Revue des Energies Renouvelables Vol.10 N 4 (2007) First-principle calculation of MgH 2 and LiH for hydrogen storage Y. Bouhadda *, A. Rabehi and S. Bezzari-Tahar-Chaouche Unité de Recherche Appliquée en Energies Renouvelables, B.P. 88, Garet Etaam, Ghardaïa (reçu le 20 Octobre 2007 accepté le 25 Décembre 2007) Abstract - First-principles calculation has been performed on the simple hydrides LiH and MgH 2 using the full-potential linearized augmented waves (FP-LAPW). The electronic structure and structural stability are studied. The formation energy has been investigated on these promising candidates for hydrogen storage applications. Our calculated results generally are in good agreement with experimental data. The differences were discussed in this paper. Résumé - Dans ce travail, nous avons étudié deux simples hydrures LiH et MgH 2 par le calcul du premier principe en utilisant la méthode de linéarisation LAPW. Les structures électroniques et cristallines d équilibre ont été étudiées, ainsi que l énergie de formation de ces deux hydrures prometteurs dans les applications de stockage solide d hydrogène. Nos résultats du calcul sont confrontés aux données expérimentales. Keywords: Hydrides FP-LAPW - Formation energy Hydrogen storage. 1. INTRODUCTION The large scale utilization of hydrogen as a fuel crucially depends on the development of compact storage materials with a high mass content of hydrogen [1]. One of the major problems encountered when utilizing metal hydrides for hydrogen storage is the low hydrogen weight capacity of these compounds. This disadvantage is due to the low atomic mass ratio of hydrogen to most of the metals and alloys. There are, however, certain additional requirements imposed on a hydrogen-storing material, e.g. moderate stability (ability to release hydrogen in the ambient temperature range), fast kinetics of absorption and desorption and resistance towards hydrogenation poisons; these requirements preclude the use of light metals such as lithium or magnesium for hydrogen storage purposes [2]. The ideal hydrogen storage material should have a high gravimetric hydrogen density, which requires the use of light metals. Moreover, the formation energy of such a material has to be such that it is stable at room temperature, yet it has to decompose at low temperature to release its hydrogen. In principle a large variety of hydride (ex: alanates and boranates) can be synthesized by changing the metal cations, which can be used to tune the formation energy [3]. Since synthesis is a very time consuming effort, there is a need for a materials specific theory with a predictive power for the Formation Energy (FE). At present the state of the art is formed by first principles calculations based upon Density Functional Theory (DFT). Several papers have been dedicated to trends in the DFT Fees of hydrides (alanates and boranates [3-7]). There exists a surprising variety of crystal structures among these compounds. In DFT calculations the crystal structure with the lowest energy has to be searched for each compound, and the cell parameters and the atomic positions have to be optimized. In this work we try to show how the first-principles calculations can be useful to understand the most important properties of hydrides. So we study here two simple hydrides: a - LiH is the simplest in term of its low molecular weight. b - MgH 2 one of the most important for the reversible storage of hydrogen because of the high percentage of this element that it can contain (the theoretical hydrogen storage capacity of pure Mg is up to 7.6 % of Hydrogen); also for its light weight and low cost. * [email protected] 545
2 546 Y. Bouhadda et al. 2. COMPUTATIONAL METHODS The present calculations have been performed using the full-potential linearized augmented plane-wave method (FP-LAPW) [8]. This is an implementation of density functional theory with different possible approximation for the exchange and correlation potential. The Kohn-Sham equations are solved using a basis of linearized plane wave [9]. The generalized gradient approximation (GGA) parameterized by Perdew and al. [10] is adopted for the exchangecorrelation energy. In all our calculations we have used the tetrahedron method on a grid of 1000 k points in the whole Brillouin zone. Muffin-tin radius of 1.8, 1.7 and 1.1 Bohr are used for Mg, Li and H respectively. 3. STRUCTURAL DETAILS LiH has a simple rock salt structure. It have a like NaCl-type structure with Li (0, 0, 0) and H (1/2, 1/2, 1/2) and a lattice parameter of Å [11]. The hydride MgH 2 crystallized in the tetragonal structure (space group P4 2 /mnm N 136) [12]. The Wyckoff position of Mg and H are 2a (0, 0, 0) and 4f (0.304, 0.304, 0) respectively. The lattice constants used as input for our calculation are the experimental values a = Å and c = 3.01 Å [12]. Also the lattice constants of hcp Mg and bcc Li are taken from reference [11]. 4. RESULT AND DISCUSSION 4.1 Structural optimization We have optimized the structural parameters for LiH and MgH 2 by fixing the experimental atomic position and varying the cell volume by ± 15, ± 10 and ± 5 % of the experimental volume V 0 ; and we have calculated total energy versus volume (Fig. 1 and Fig. 2). We have optimized the MgH 2, structure and calculated the total energy as function of unit cell volume (Fig. 1). Fig. 1: Calculated free energy versus unit-cell volume for MgH 2 ' The structure parameters, the bulk modulus B 0 and the pressure derivative B 0 listed in Table 1 were giving by fitting the total energies to Murnaghan equation of state [13]. Table 1 show that the calculated values are in good agreement with those obtained in previous works [12, 14-16].
3 First-principle calculation of MgH 2 and LiH for hydrogen storage 547 Fig. 2: Calculated free energy versus unit-cell volume for LiH Table 1: Optimized structural parameters, bulk modulus ( B 0 ) ' and its pressure derivative ( B 0 ) of MgH 2 a (Å) c (Å) B 0 (GPa) ' B [12] The same procedure was adopted for LiH (Fig. 2) and the Table 2 gives our results. Our calculated values are in good agreement with the experimental values. DFT and GGA can be used widely to predict the structural and the elastic proprieties of hydrides for hydrogen storage application. Table 2: Optimized structural parameters, bulk modulus ( B 0 ) ' and its pressure derivative ( B 0 ) of LiH a (Å) B 0 (GPa) ' B [11] [12] [14], 50 [15], 51 [16] [17], [18] (3,45 [16]) 4.2 The formation energy (formation heat) The reaction related to the formation of the hydride MgH 2 is: Mg + H 2 MgH 2 (1) To calculate the formation heat of the reaction (1) we subtracted the total energies of the pure element Mg and the hydrogen molecule from its hydride MgH 2 : H (MgH2 ) = E (MgH2 ) E (Mg) E (H2 ) (2) The same idea is used to evaluate the formation energy of LiH via the reaction: 1 Li + H2 LiH (3) 2 In Table 3, we summarized the total energy and the formation enthalpy computed. For MgH 2 and LiH ; we used the total energies obtained with the optimized structure of MgH 2 LiH Mg and Li. The total energy of the hydrogen molecule is Ry [19, 20].
4 548 Y. Bouhadda et al. Table 3: Calculated heat of formation of MgH 2 and LiH (f.u: formula unit) Elements Total Energy (Ry/f.u) Formation Heat (kj/mol H 2 ) Mg Li H 2 MgH 2 LiH ( ± 9.2 [21]) (162 [22], [23], 157 [24]) The calculated heat of formation of MgH 2 is kj/mol. H 2, and is in good agreement with the corresponding measured experimental value ± 9. 2 kj/mol. H 2 [21]. Our heat of formation of LiH is acceptable. The discrepancy can be explained by the zeropoint energy due to the vibration motion [22]. If we calculate reaction enthalpies from total energy differences only, we neglect the contributions from atomic vibrations. Such contributions are negligible for heavy elements (magnesium), whereas they may be significant for hydrogen and lithium [22]. 4.3 Electronic structure In this section, the lattice parameters were fixed at the calculated values (a = Å and c = Å). The total and the partial densities of state for MgH 2 are plotted in Fig. 3. There is hybridization between the H and the Mg states in the valence bands which have a dominant hydrogen character. The electronic structure is non-metallic with the energy gap of 3.60 ev. This value is in excellent agreement with the theoretical findings which reported a band gap around 3.4 ev [14, 15] and close to the value (4.2 ev) calculated by Vajeeston and al. [16]. Fig. 3: Total and partial densities of states (DOS) for MgH 2, the Fermi level is set as zero energy and marked by the vertical lines An experimental UV absorption study gave an absorption edge at 5.16 ev [15]. The divergence between the theoretical and the experimental studies is very clear, it is due to the accuracy obtained by the DFT theory for semiconductors and insulators where the DFT calculations severely underestimate the gap originate from the use of GGA exchange correlation functional [25]. In Fig. 4 we give DOS of LiH : it is non metallic and our calculated band gap is about 3.2 ev and as have been said above, DFT severely underestimate the experimental gap (4.99 ev for LiH at T = 4.2 K [26, 27]).
5 First-principle calculation of MgH 2 and LiH for hydrogen storage 549 Fig. 3: Total and partial densities of states (DOS) for LiH, the Fermi level is set as zero energy and marked by the vertical lines 5. CONCLUSION In this work the electronic structure of the simplest hydrides LiH and MgH 2 has been studied by the first-principles calculations. The equilibrium structures of these compounds are obtained from DFT/GGA total energy minimization. The enthalpy of formation was calculated theoretically and this quantity is very important to choose the hydrides for hydrogen storage application. The results were close to the experience and we can say that the first principles calculation is an important tool for modelling materials, hydrides and nanotubes for hydrogen storage applications. Acknowledgments: The authors would like to thank Pr. Y. Boudouma and Pr A.C. Chami (Professors at USTHB) for valuable discussions and helpful observations. Special thanks to Dr N. Fenineche (LERMPS, Université de Belfort-Montbéliard, France) for all advices and discussions about hydrogen storage. REFERENCES [1] F. Schüth, B. Bogdanovic and M. Felderhoff, Chem. Commun. 20, 2249, [2] M.H. Mintz; Z. Gavra; G. Kimmel and Z. Hadari, Journal of the Less-Common Metals, 74, pp , [3] Y. Nakamori et al., Correlation between thermo dynamical stabilities of metal borohydrides and cation electronegativites: First-principles calculations and experiments, Physical Review B 74, , [4] S.C. Chung and H. Morioka, Thermochemistry and Crystal Structures of Lithium, Sodium and Potassium Alanates as Determined by ab Initio Simulations, Journal of Alloys and Compounds, Vol. 372, pp , [5] P. Vajeeston, P. Ravindran, R. Vidya, H. Fjellvag and A. Kjekshus, Cryst. Growth Des., Vol. 4, pp. 471, [6] O.M. Løvvik, O. Swang, and S.M. Opalka, J. Mater. Res. 20, pp. 3199, [7] P. Vajeeston, P. Ravindran, A. Kjekshus and H. Fjellvag, Structural Stability of Alkali Boron Tetrahydrides ABH 4 (A = Li, Na, K, Rb, Cs) from First Principle Calculation, Journal of Alloys and Compounds, Vol. 387, N 1-2, pp , 2005.
6 550 Y. Bouhadda et al. [8] P. Blaha, K. Schwarz and J. Luitz, WIEN 97, Vienna, University of Technology, Improved and updated UNIX version of the original copyrighted WIEN code, Published by P. Blaha, K. Schwarz, P. Soratin and S.B. Trickey, in Comput. Phys. Commun., Vol. 59, pp. 399, [9] D. Singh, Plane Waves, Pseudopotentials, and the LAPW Methods, New York, Kluwer Academic, [10] J.P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., Vol. 77, N 18, pp , [11] C. Kittel, Introduction to Solid State Physics, Wiley, New York, [12] M. Bortz, B. Bertheville, G. Bottger and K. Yvon, Structure of the High Pressure Phase gamma-mgh 2 by Neutron Powder Diffraction, Journal of Alloys and Compounds, Vol. 287, N L4-L6, [13] F.D. Murnaghan, Proc. Natl. Acad. Sci. USA Vol. 30, pp. 244, [14] B. Pfrommer, C. Elasässer and M. Fähnle, Physical Review B 50, p. 5089, [15] R. Yu and P.K. Lam, Physical Review B 37, p. 8730, [16] P. Ravindran, P. Vajestoon, R. Vidya, A. Kjekshus, H. Fjellvåg, Violation of the 2Å Rule for Metal Hydrides, Physical Review Letters, Vol. 89, N 17, , [17] D.R. Stephens and E.M. Lilley, Compressions of Isotopic Lithium Hydrides, Journal of Applied Physics, Vol. 39, N 1, pp , [18] D. Gerlich and C.S. Smith, The Pressure and Temperature Derivatives of the Elastic Moduli of Lithium Hydride, Journal of Physics and Chemistry of Solids, Vol. 35, N 12, pp , [19] H. Nakamura, D. Nguyen-Manh and D.G. Pettifor, Electronic Structure and Energetics of LaNi 5, α- La 2 Ni 10 H and β-la 2 Ni 10 H 14, Journal of Alloys and Compounds, Vol. 281, N 2, pp , [20] C.X. Shang, M. Bououdina, Y. Song and Z.X. Guo, Mechanical Alloying and Electronic Simulations of (MgH2+M) Systems (M = Al, Ti, Fe, Ni, Cu and Nb) for H Storage. International Journal of Hydrogen Energy, Vol. 29, pp , [21] M. Yamagychi, E. Akiba, In R.W. Cahn, P. Haasen and E.J. Kramer (Eds), Materials Science and Technology, Vol. 3B. pp. 333, New York, VCH, [22] K. Miwa, N. Ohba and S. Towata, Physical Review, B69, , [23] J.F. Herbst and L.G. Hector Jr, Energetics of the Li Amide / Li Imide Hydrogen Storage Reaction, Physical Review, B72, , [24] Y. Fukaï, The Metal-Hydrogen System : Basic Bulk Properties, Vol. 21, 2nd Ed., Series in Materials Science, Springer-Verlag, Berlin, [25] R.M. Martin, Electronic Structure Basic Theory and Practical Methods, Cambridge University Press, Cambridge, England, [26] G.S. Zavt, K.A. Kalder, I.L. Kuusman, Ch.B. Lushichik, V.G. Plekhanov, S.O. Cholakh and P.A. E varestov, Sov. Phys. Solid State, Vol. 18, pp. 1588, [27] Y. Kondo and K. Asaumi, Effect of Pressure on the Direct Energy Gap of LiH, Journal of the Physical Society of Japan, Vol. 57, N 1, pp , 1988.
PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes.
 1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry
 Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry Why? Chemists are concerned with mass relationships in chemical reactions, usually run on a macroscopic scale (grams, kilograms, etc.). To deal with
Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry Why? Chemists are concerned with mass relationships in chemical reactions, usually run on a macroscopic scale (grams, kilograms, etc.). To deal with
Thermodynamic database of the phase diagrams in copper base alloy systems
 Journal of Physics and Chemistry of Solids 66 (2005) 256 260 www.elsevier.com/locate/jpcs Thermodynamic database of the phase diagrams in copper base alloy systems C.P. Wang a, X.J. Liu b, M. Jiang b,
Journal of Physics and Chemistry of Solids 66 (2005) 256 260 www.elsevier.com/locate/jpcs Thermodynamic database of the phase diagrams in copper base alloy systems C.P. Wang a, X.J. Liu b, M. Jiang b,
Untitled Document. 1. Which of the following best describes an atom? 4. Which statement best describes the density of an atom s nucleus?
 Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Name: Date: 1. Which of the following best describes an atom? A. protons and electrons grouped together in a random pattern B. protons and electrons grouped together in an alternating pattern C. a core
Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry Answers
 Key Questions & Exercises Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry Answers 1. The atomic weight of carbon is 12.0107 u, so a mole of carbon has a mass of 12.0107 g. Why doesn t a mole of
Key Questions & Exercises Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry Answers 1. The atomic weight of carbon is 12.0107 u, so a mole of carbon has a mass of 12.0107 g. Why doesn t a mole of
Name Class Date. In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question.
 Assessment Chapter Test A Chapter: States of Matter In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. The kinetic-molecular
Assessment Chapter Test A Chapter: States of Matter In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. The kinetic-molecular
TDS. Dirk Rosenthal Department of Inorganic Chemistry Fritz-Haber-Institut der MPG Faradayweg 4-6, DE 14195 Berlin [email protected].
 Modern Methods in Heterogeneous Catalysis Research TDS Dirk Rosenthal Department of Inorganic Chemistry Fritz-Haber-Institut der MPG Faradayweg 4-6, DE 14195 Berlin [email protected] TDS = TPD
Modern Methods in Heterogeneous Catalysis Research TDS Dirk Rosenthal Department of Inorganic Chemistry Fritz-Haber-Institut der MPG Faradayweg 4-6, DE 14195 Berlin [email protected] TDS = TPD
Name period AP chemistry Unit 2 worksheet Practice problems
 Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Summer Holidays Questions
 Summer Holidays Questions Chapter 1 1) Barium hydroxide reacts with hydrochloric acid. The initial concentration of the 1 st solution its 0.1M and the volume is 100ml. The initial concentration of the
Summer Holidays Questions Chapter 1 1) Barium hydroxide reacts with hydrochloric acid. The initial concentration of the 1 st solution its 0.1M and the volume is 100ml. The initial concentration of the
The Periodic Table: Periodic trends
 Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Unit 1 The Periodic Table: Periodic trends There are over one hundred different chemical elements. Some of these elements are familiar to you such as hydrogen, oxygen, nitrogen and carbon. Each one has
Chem 106 Thursday Feb. 3, 2011
 Chem 106 Thursday Feb. 3, 2011 Chapter 13: -The Chemistry of Solids -Phase Diagrams - (no Born-Haber cycle) 2/3/2011 1 Approx surface area (Å 2 ) 253 258 Which C 5 H 12 alkane do you think has the highest
Chem 106 Thursday Feb. 3, 2011 Chapter 13: -The Chemistry of Solids -Phase Diagrams - (no Born-Haber cycle) 2/3/2011 1 Approx surface area (Å 2 ) 253 258 Which C 5 H 12 alkane do you think has the highest
3. What would you predict for the intensity and binding energy for the 3p orbital for that of sulfur?
 PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
1 The water molecule and hydrogen bonds in water
 The Physics and Chemistry of Water 1 The water molecule and hydrogen bonds in water Stoichiometric composition H 2 O the average lifetime of a molecule is 1 ms due to proton exchange (catalysed by acids
The Physics and Chemistry of Water 1 The water molecule and hydrogen bonds in water Stoichiometric composition H 2 O the average lifetime of a molecule is 1 ms due to proton exchange (catalysed by acids
Matter, Materials, Crystal Structure and Bonding. Chris J. Pickard
 Matter, Materials, Crystal Structure and Bonding Chris J. Pickard Why should a theorist care? Where the atoms are determines what they do Where the atoms can be determines what we can do Overview of Structure
Matter, Materials, Crystal Structure and Bonding Chris J. Pickard Why should a theorist care? Where the atoms are determines what they do Where the atoms can be determines what we can do Overview of Structure
MOLECULAR DYNAMICS INVESTIGATION OF DEFORMATION RESPONSE OF THIN-FILM METALLIC NANOSTRUCTURES UNDER HEATING
 NANOSYSTEMS: PHYSICS, CHEMISTRY, MATHEMATICS, 2011, 2 (2), P. 76 83 UDC 538.97 MOLECULAR DYNAMICS INVESTIGATION OF DEFORMATION RESPONSE OF THIN-FILM METALLIC NANOSTRUCTURES UNDER HEATING I. S. Konovalenko
NANOSYSTEMS: PHYSICS, CHEMISTRY, MATHEMATICS, 2011, 2 (2), P. 76 83 UDC 538.97 MOLECULAR DYNAMICS INVESTIGATION OF DEFORMATION RESPONSE OF THIN-FILM METALLIC NANOSTRUCTURES UNDER HEATING I. S. Konovalenko
We shall first regard the dense sphere packing model. 1.1. Draw a two dimensional pattern of dense packing spheres. Identify the twodimensional
 Set 3: Task 1 and 2 considers many of the examples that are given in the compendium. Crystal structures derived from sphere packing models may be used to describe metals (see task 2), ionical compounds
Set 3: Task 1 and 2 considers many of the examples that are given in the compendium. Crystal structures derived from sphere packing models may be used to describe metals (see task 2), ionical compounds
SUGGESTION ANSWER SCHEME CHAPTER 8: THERMOCHEMISTRY. 1 (a) Use the data in the table below to answer the following questions:
 SUGGESTION ANSWER SCHEME CHAPTER 8: THERMOCHEMISTRY ANSWER SCHEME UPS 2004/2005 SK027 1 (a) Use the data in the table below to answer the following questions: Enthalpy change ΔH (kj/mol) Atomization energy
SUGGESTION ANSWER SCHEME CHAPTER 8: THERMOCHEMISTRY ANSWER SCHEME UPS 2004/2005 SK027 1 (a) Use the data in the table below to answer the following questions: Enthalpy change ΔH (kj/mol) Atomization energy
Plate waves in phononic crystals slabs
 Acoustics 8 Paris Plate waves in phononic crystals slabs J.-J. Chen and B. Bonello CNRS and Paris VI University, INSP - 14 rue de Lourmel, 7515 Paris, France [email protected] 41 Acoustics 8 Paris We
Acoustics 8 Paris Plate waves in phononic crystals slabs J.-J. Chen and B. Bonello CNRS and Paris VI University, INSP - 14 rue de Lourmel, 7515 Paris, France [email protected] 41 Acoustics 8 Paris We
NORGES TEKNISK- NATURVITENSKAPELIGE UNIVERSITET INSTITUTT FOR FYSIKK. Eksamen i Emne TFY4220 Faste Stoffers Fysikk
 Page of 5 NORGES TEKNISK- NATURVITENSKAPELIGE UNIVERSITET INSTITUTT FOR FYSIKK Fagleg kontakt under eksamen: Institutt for fysikk, Gløshaugen Professor Steinar Raaen, 4896758 Eksamen i Emne TFY40 Faste
Page of 5 NORGES TEKNISK- NATURVITENSKAPELIGE UNIVERSITET INSTITUTT FOR FYSIKK Fagleg kontakt under eksamen: Institutt for fysikk, Gløshaugen Professor Steinar Raaen, 4896758 Eksamen i Emne TFY40 Faste
Electrons in Atoms & Periodic Table Chapter 13 & 14 Assignment & Problem Set
 Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
Electrons in Atoms & Periodic Table Name Warm-Ups (Show your work for credit) Date 1. Date 2. Date 3. Date 4. Date 5. Date 6. Date 7. Date 8. Electrons in Atoms & Periodic Table 2 Study Guide: Things You
High Open Circuit Voltage of MQW Amorphous Silicon Photovoltaic Structures
 High Open Circuit Voltage of MQW Amorphous Silicon Photovoltaic Structures ARGYRIOS C. VARONIDES Physics and EE Department University of Scranton 800 Linden Street, Scranton PA, 18510 United States Abstract:
High Open Circuit Voltage of MQW Amorphous Silicon Photovoltaic Structures ARGYRIOS C. VARONIDES Physics and EE Department University of Scranton 800 Linden Street, Scranton PA, 18510 United States Abstract:
Chemistry Diagnostic Questions
 Chemistry Diagnostic Questions Answer these 40 multiple choice questions and then check your answers, located at the end of this document. If you correctly answered less than 25 questions, you need to
Chemistry Diagnostic Questions Answer these 40 multiple choice questions and then check your answers, located at the end of this document. If you correctly answered less than 25 questions, you need to
The Application of Density Functional Theory in Materials Science
 The Application of Density Functional Theory in Materials Science Slide 1 Outline Atomistic Modelling Group at MUL Density Functional Theory Numerical Details HPC Cluster at the MU Leoben Applications
The Application of Density Functional Theory in Materials Science Slide 1 Outline Atomistic Modelling Group at MUL Density Functional Theory Numerical Details HPC Cluster at the MU Leoben Applications
Bohr s Model of the Atom
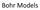 Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
Bohr Models Bohr s Model of the Atom Focuses on electrons and their arrangement. Bohr stated that electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Bohr
HYDROGEN STORAGE AND MICROSTRUCTURE INVESTIGATIONS OF La 0.7- Av. Prof. Lineu Prestes, 2242, ZIP 05508-000, São Paulo, Brazil.
 HYDROGEN STORAGE AND MICROSTRUCTURE INVESTIGATIONS OF La 0.7- x Pr x ALLOYS G. S. Galdino 1,a, J. C. S. Casini 1, E. A. Ferreira 1, R. N. Faria 1, H. Takiishi 1 a [email protected] 1 Department of Metallurgy,
HYDROGEN STORAGE AND MICROSTRUCTURE INVESTIGATIONS OF La 0.7- x Pr x ALLOYS G. S. Galdino 1,a, J. C. S. Casini 1, E. A. Ferreira 1, R. N. Faria 1, H. Takiishi 1 a [email protected] 1 Department of Metallurgy,
B) atomic number C) both the solid and the liquid phase D) Au C) Sn, Si, C A) metal C) O, S, Se C) In D) tin D) methane D) bismuth B) Group 2 metal
 1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts
 AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
Thermodynamics of Crystal Formation
 Thermodynamics of Crystal Formation! All stable ionic crystals have negative standard enthalpies of formation, ΔH o f, and negative standard free energies of formation, ΔG o f. Na(s) + ½ Cl 2 (g) NaCl(s)
Thermodynamics of Crystal Formation! All stable ionic crystals have negative standard enthalpies of formation, ΔH o f, and negative standard free energies of formation, ΔG o f. Na(s) + ½ Cl 2 (g) NaCl(s)
Thermodynamics. Thermodynamics 1
 Thermodynamics 1 Thermodynamics Some Important Topics First Law of Thermodynamics Internal Energy U ( or E) Enthalpy H Second Law of Thermodynamics Entropy S Third law of Thermodynamics Absolute Entropy
Thermodynamics 1 Thermodynamics Some Important Topics First Law of Thermodynamics Internal Energy U ( or E) Enthalpy H Second Law of Thermodynamics Entropy S Third law of Thermodynamics Absolute Entropy
Find a pair of elements in the periodic table with atomic numbers less than 20 that are an exception to the original periodic law.
 Example Exercise 6.1 Periodic Law Find the two elements in the fifth row of the periodic table that violate the original periodic law proposed by Mendeleev. Mendeleev proposed that elements be arranged
Example Exercise 6.1 Periodic Law Find the two elements in the fifth row of the periodic table that violate the original periodic law proposed by Mendeleev. Mendeleev proposed that elements be arranged
Ch. 4: Imperfections in Solids Part 1. Dr. Feras Fraige
 Ch. 4: Imperfections in Solids Part 1 Dr. Feras Fraige Outline Defects in Solids 0D, Point defects vacancies Interstitials impurities, weight and atomic composition 1D, Dislocations edge screw 2D, Grain
Ch. 4: Imperfections in Solids Part 1 Dr. Feras Fraige Outline Defects in Solids 0D, Point defects vacancies Interstitials impurities, weight and atomic composition 1D, Dislocations edge screw 2D, Grain
Module #17. Work/Strain Hardening. READING LIST DIETER: Ch. 4, pp. 138-143; Ch. 6 in Dieter
 Module #17 Work/Strain Hardening READING LIST DIETER: Ch. 4, pp. 138-143; Ch. 6 in Dieter D. Kuhlmann-Wilsdorf, Trans. AIME, v. 224 (1962) pp. 1047-1061 Work Hardening RECALL: During plastic deformation,
Module #17 Work/Strain Hardening READING LIST DIETER: Ch. 4, pp. 138-143; Ch. 6 in Dieter D. Kuhlmann-Wilsdorf, Trans. AIME, v. 224 (1962) pp. 1047-1061 Work Hardening RECALL: During plastic deformation,
Free Electron Fermi Gas (Kittel Ch. 6)
 Free Electron Fermi Gas (Kittel Ch. 6) Role of Electrons in Solids Electrons are responsible for binding of crystals -- they are the glue that hold the nuclei together Types of binding (see next slide)
Free Electron Fermi Gas (Kittel Ch. 6) Role of Electrons in Solids Electrons are responsible for binding of crystals -- they are the glue that hold the nuclei together Types of binding (see next slide)
Bonds. Bond Length. Forces that hold groups of atoms together and make them function as a unit. Bond Energy. Chapter 8. Bonding: General Concepts
 Bonds hapter 8 Bonding: General oncepts Forces that hold groups of atoms together and make them function as a unit. Bond Energy Bond Length It is the energy required to break a bond. The distance where
Bonds hapter 8 Bonding: General oncepts Forces that hold groups of atoms together and make them function as a unit. Bond Energy Bond Length It is the energy required to break a bond. The distance where
Hydrogen Storage in Ammonia and Aminoborane Complexes
 ydrogen Storage in Ammonia and Aminoborane Complexes Ali Raissi Florida Solar Energy Center University of Central Florida ydrogen Program Annual Review Session: ydrogen Storage Carbon & Other Berkeley,
ydrogen Storage in Ammonia and Aminoborane Complexes Ali Raissi Florida Solar Energy Center University of Central Florida ydrogen Program Annual Review Session: ydrogen Storage Carbon & Other Berkeley,
Nuclear ZPE Tapping. Horace Heffner May 2007
 ENERGY FROM UNCERTAINTY The uncertainty of momentum for a particle constrained by distance Δx is given, according to Heisenberg, by: Δmv = h/(2 π Δx) but since KE = (1/2) m v 2 = (1/(2 m) ) (Δmv) 2 ΔKE
ENERGY FROM UNCERTAINTY The uncertainty of momentum for a particle constrained by distance Δx is given, according to Heisenberg, by: Δmv = h/(2 π Δx) but since KE = (1/2) m v 2 = (1/(2 m) ) (Δmv) 2 ΔKE
Amount of Substance. http://www.avogadro.co.uk/definitions/elemcompmix.htm
 Page 1 of 14 Amount of Substance Key terms in this chapter are: Element Compound Mixture Atom Molecule Ion Relative Atomic Mass Avogadro constant Mole Isotope Relative Isotopic Mass Relative Molecular
Page 1 of 14 Amount of Substance Key terms in this chapter are: Element Compound Mixture Atom Molecule Ion Relative Atomic Mass Avogadro constant Mole Isotope Relative Isotopic Mass Relative Molecular
AP Chemistry Semester One Study Guide
 AP Chemistry Semester One Study Guide Unit One: General Chemistry Review Unit Two: Organic Nomenclature Unit Three: Reactions Unit Four: Thermochemistry Unit Five: Electronic Structure of the Atom Unit
AP Chemistry Semester One Study Guide Unit One: General Chemistry Review Unit Two: Organic Nomenclature Unit Three: Reactions Unit Four: Thermochemistry Unit Five: Electronic Structure of the Atom Unit
CLASS TEST GRADE 11. PHYSICAL SCIENCES: CHEMISTRY Test 6: Chemical change
 CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 6: Chemical change MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You may
CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 6: Chemical change MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You may
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING
 CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
IEA/DOE/SNL HYDRIDE DATABASES (Excel Versions)
 IEA/DOE/SNL HYDRIDE DATABASES (Excel Versions) INTRODUCTION This is an introduction to the Excel versions of databases on metal-hydrogen systems, their properties, applications and literature sources.
IEA/DOE/SNL HYDRIDE DATABASES (Excel Versions) INTRODUCTION This is an introduction to the Excel versions of databases on metal-hydrogen systems, their properties, applications and literature sources.
ANSWER KEY : BUILD AN ATOM PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom )
 ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
ANSWER KEY : PART I: ATOM SCREEN Build an Atom simulation ( http://phet.colorado.edu/en/simulation/build an atom ) 1. Explore the Build an Atom simulation with your group. As you explore, talk about what
Mg 2 FeH 6 -BASED NANOCOMPOSITES WITH HIGH CAPACITY OF HYDROGEN STORAGE PROCESSED BY REACTIVE MILLING
 Mg 2 FeH 6 -BASED NANOCOMPOSITES WITH HIGH CAPACITY OF HYDROGEN STORAGE PROCESSED BY REACTIVE MILLING A. A. C. Asselli (1)*, C. S. Kiminami (2), A. M. Jorge Jr. (2), T.T. Ishikawa (2), and W. J. Botta
Mg 2 FeH 6 -BASED NANOCOMPOSITES WITH HIGH CAPACITY OF HYDROGEN STORAGE PROCESSED BY REACTIVE MILLING A. A. C. Asselli (1)*, C. S. Kiminami (2), A. M. Jorge Jr. (2), T.T. Ishikawa (2), and W. J. Botta
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni
 SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
SCPS Chemistry Worksheet Periodicity A. Periodic table 1. Which are metals? Circle your answers: C, Na, F, Cs, Ba, Ni Which metal in the list above has the most metallic character? Explain. Cesium as the
100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals.
 2.21 Ionic Bonding 100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals. Forming ions Metal atoms lose electrons to form +ve ions. Non-metal
2.21 Ionic Bonding 100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals. Forming ions Metal atoms lose electrons to form +ve ions. Non-metal
Indiana's Academic Standards 2010 ICP Indiana's Academic Standards 2016 ICP. map) that describe the relationship acceleration, velocity and distance.
 .1.1 Measure the motion of objects to understand.1.1 Develop graphical, the relationships among distance, velocity and mathematical, and pictorial acceleration. Develop deeper understanding through representations
.1.1 Measure the motion of objects to understand.1.1 Develop graphical, the relationships among distance, velocity and mathematical, and pictorial acceleration. Develop deeper understanding through representations
Chapter 7 Periodic Properties of the Elements
 Chapter 7 Periodic Properties of the Elements 1. Elements in the modern version of the periodic table are arranged in order of increasing. (a). oxidation number (b). atomic mass (c). average atomic mass
Chapter 7 Periodic Properties of the Elements 1. Elements in the modern version of the periodic table are arranged in order of increasing. (a). oxidation number (b). atomic mass (c). average atomic mass
MOLECULAR DYNAMICS SIMULATION OF DYNAMIC RESPONSE OF BERYLLIUM
 MOLECULAR DYNAMICS SIMULATION OF DYNAMIC RESPONSE OF BERYLLIUM Aidan P. Thompson, J. Matthew D. Lane and Michael P. Desjarlais Sandia National Laboratories, Albuquerque, New Mexico 87185, USA Abstract.
MOLECULAR DYNAMICS SIMULATION OF DYNAMIC RESPONSE OF BERYLLIUM Aidan P. Thompson, J. Matthew D. Lane and Michael P. Desjarlais Sandia National Laboratories, Albuquerque, New Mexico 87185, USA Abstract.
MODERN ATOMIC THEORY AND THE PERIODIC TABLE
 CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
Modification of Pd-H 2 and Pd-D 2 thin films processed by He-Ne laser
 Modification of Pd-H 2 and Pd-D 2 thin films processed by He-Ne laser V.Nassisi #, G.Caretto #, A. Lorusso #, D.Manno %, L.Famà %, G.Buccolieri %, A.Buccolieri %, U.Mastromatteo* # Laboratory of Applied
Modification of Pd-H 2 and Pd-D 2 thin films processed by He-Ne laser V.Nassisi #, G.Caretto #, A. Lorusso #, D.Manno %, L.Famà %, G.Buccolieri %, A.Buccolieri %, U.Mastromatteo* # Laboratory of Applied
Chem 1A Exam 2 Review Problems
 Chem 1A Exam 2 Review Problems 1. At 0.967 atm, the height of mercury in a barometer is 0.735 m. If the mercury were replaced with water, what height of water (in meters) would be supported at this pressure?
Chem 1A Exam 2 Review Problems 1. At 0.967 atm, the height of mercury in a barometer is 0.735 m. If the mercury were replaced with water, what height of water (in meters) would be supported at this pressure?
(1) e.g. H hydrogen that has lost 1 electron c. anion - negatively charged atoms that gain electrons 16-2. (1) e.g. HCO 3 bicarbonate anion
 GS106 Chemical Bonds and Chemistry of Water c:wou:gs106:sp2002:chem.wpd I. Introduction A. Hierarchy of chemical substances 1. atoms of elements - smallest particles of matter with unique physical and
GS106 Chemical Bonds and Chemistry of Water c:wou:gs106:sp2002:chem.wpd I. Introduction A. Hierarchy of chemical substances 1. atoms of elements - smallest particles of matter with unique physical and
= 1.038 atm. 760 mm Hg. = 0.989 atm. d. 767 torr = 767 mm Hg. = 1.01 atm
 Chapter 13 Gases 1. Solids and liquids have essentially fixed volumes and are not able to be compressed easily. Gases have volumes that depend on their conditions, and can be compressed or expanded by
Chapter 13 Gases 1. Solids and liquids have essentially fixed volumes and are not able to be compressed easily. Gases have volumes that depend on their conditions, and can be compressed or expanded by
The Advanced Placement Examination in Chemistry. Part I Multiple Choice Questions Part II Free Response Questions Selected Questions from1970 to 2010
 The Advanced Placement Examination in Chemistry Part I Multiple Choice Questions Part II Free Response Questions Selected Questions from1970 to 2010 Atomic Theory and Periodicity Part I 1984 1. Which of
The Advanced Placement Examination in Chemistry Part I Multiple Choice Questions Part II Free Response Questions Selected Questions from1970 to 2010 Atomic Theory and Periodicity Part I 1984 1. Which of
DO PHYSICS ONLINE FROM QUANTA TO QUARKS QUANTUM (WAVE) MECHANICS
 DO PHYSICS ONLINE FROM QUANTA TO QUARKS QUANTUM (WAVE) MECHANICS Quantum Mechanics or wave mechanics is the best mathematical theory used today to describe and predict the behaviour of particles and waves.
DO PHYSICS ONLINE FROM QUANTA TO QUARKS QUANTUM (WAVE) MECHANICS Quantum Mechanics or wave mechanics is the best mathematical theory used today to describe and predict the behaviour of particles and waves.
Section 1: Arranging the Elements Pages 106-112
 Study Guide Chapter 5 Periodic Table Section 1: Arranging the Elements Pages 106-112 DISCOVERING A PATTERN 1. How did Mendeleev arrange the elements? a. by increasing density b. by increasing melting point
Study Guide Chapter 5 Periodic Table Section 1: Arranging the Elements Pages 106-112 DISCOVERING A PATTERN 1. How did Mendeleev arrange the elements? a. by increasing density b. by increasing melting point
Heat transfer efficiency improvement in high-pressure metal hydride by modeling approach
 International Journal of Smart Grid and Clean Energy Heat transfer efficiency improvement in high-pressure metal hydride by modeling approach Nur Aisyah Jalalludin a*, Noboru Katayama b, Sumio Kogoshi
International Journal of Smart Grid and Clean Energy Heat transfer efficiency improvement in high-pressure metal hydride by modeling approach Nur Aisyah Jalalludin a*, Noboru Katayama b, Sumio Kogoshi
The Mole Concept. The Mole. Masses of molecules
 The Mole Concept Ron Robertson r2 c:\files\courses\1110-20\2010 final slides for web\mole concept.docx The Mole The mole is a unit of measurement equal to 6.022 x 10 23 things (to 4 sf) just like there
The Mole Concept Ron Robertson r2 c:\files\courses\1110-20\2010 final slides for web\mole concept.docx The Mole The mole is a unit of measurement equal to 6.022 x 10 23 things (to 4 sf) just like there
Candidate Style Answer
 Candidate Style Answer Chemistry A Unit F321 Atoms, Bonds and Groups High banded response This Support Material booklet is designed to accompany the OCR GCE Chemistry A Specimen Paper F321 for teaching
Candidate Style Answer Chemistry A Unit F321 Atoms, Bonds and Groups High banded response This Support Material booklet is designed to accompany the OCR GCE Chemistry A Specimen Paper F321 for teaching
CHAPTER 6 Chemical Bonding
 CHAPTER 6 Chemical Bonding SECTION 1 Introduction to Chemical Bonding OBJECTIVES 1. Define Chemical bond. 2. Explain why most atoms form chemical bonds. 3. Describe ionic and covalent bonding.. 4. Explain
CHAPTER 6 Chemical Bonding SECTION 1 Introduction to Chemical Bonding OBJECTIVES 1. Define Chemical bond. 2. Explain why most atoms form chemical bonds. 3. Describe ionic and covalent bonding.. 4. Explain
Chapter 6 Assessment. Name: Class: Date: ID: A. Multiple Choice Identify the choice that best completes the statement or answers the question.
 Name: Class: Date: ID: A Chapter 6 Assessment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. When an atom loses an electron, it forms a(n) a. anion. c.
Name: Class: Date: ID: A Chapter 6 Assessment Multiple Choice Identify the choice that best completes the statement or answers the question. 1. When an atom loses an electron, it forms a(n) a. anion. c.
The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = V S V C. = 2(sphere volume) = 2 = V C = 4R
 3.5 Show that the atomic packing factor for BCC is 0.68. The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = V S V C Since there are two spheres associated
3.5 Show that the atomic packing factor for BCC is 0.68. The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = V S V C Since there are two spheres associated
Unit 12 Practice Test
 Name: Class: Date: ID: A Unit 12 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1) A solid has a very high melting point, great hardness, and
Name: Class: Date: ID: A Unit 12 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1) A solid has a very high melting point, great hardness, and
Atoms and Elements. Outline Atoms Orbitals and Energy Levels Periodic Properties Homework
 Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
Unit 6 The Mole Concept
 Chemistry Form 3 Page 62 Ms. R. Buttigieg Unit 6 The Mole Concept See Chemistry for You Chapter 28 pg. 352-363 See GCSE Chemistry Chapter 5 pg. 70-79 6.1 Relative atomic mass. The relative atomic mass
Chemistry Form 3 Page 62 Ms. R. Buttigieg Unit 6 The Mole Concept See Chemistry for You Chapter 28 pg. 352-363 See GCSE Chemistry Chapter 5 pg. 70-79 6.1 Relative atomic mass. The relative atomic mass
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Unit 1 Chemical Changes and Structure Revision Notes
 Unit 1 Revision Notes Rates of reaction The rate of reaction can be increased by: increasing the concentration of a solution decreasing the particle size of a solid increasing the temperature adding a
Unit 1 Revision Notes Rates of reaction The rate of reaction can be increased by: increasing the concentration of a solution decreasing the particle size of a solid increasing the temperature adding a
Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras
 Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras Module - 2 Lecture - 2 Part 2 of 2 Review of Atomic Bonding II We will continue
Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras Module - 2 Lecture - 2 Part 2 of 2 Review of Atomic Bonding II We will continue
All answers must use the correct number of significant figures, and must show units!
 CHEM 10113, Quiz 2 September 7, 2011 Name (please print) All answers must use the correct number of significant figures, and must show units! IA Periodic Table of the Elements VIIIA (1) (18) 1 2 1 H IIA
CHEM 10113, Quiz 2 September 7, 2011 Name (please print) All answers must use the correct number of significant figures, and must show units! IA Periodic Table of the Elements VIIIA (1) (18) 1 2 1 H IIA
CHAPTER 6 REVIEW. Chemical Bonding. Answer the following questions in the space provided.
 Name Date lass APTER 6 REVIEW hemical Bonding SETIN 1 SRT ANSWER Answer the following questions in the space provided. 1. a A chemical bond between atoms results from the attraction between the valence
Name Date lass APTER 6 REVIEW hemical Bonding SETIN 1 SRT ANSWER Answer the following questions in the space provided. 1. a A chemical bond between atoms results from the attraction between the valence
Science Standard Articulated by Grade Level Strand 5: Physical Science
 Concept 1: Properties of Objects and Materials Classify objects and materials by their observable properties. Kindergarten Grade 1 Grade 2 Grade 3 Grade 4 PO 1. Identify the following observable properties
Concept 1: Properties of Objects and Materials Classify objects and materials by their observable properties. Kindergarten Grade 1 Grade 2 Grade 3 Grade 4 PO 1. Identify the following observable properties
Molecular Dynamics Simulations
 Molecular Dynamics Simulations Yaoquan Tu Division of Theoretical Chemistry and Biology, Royal Institute of Technology (KTH) 2011-06 1 Outline I. Introduction II. Molecular Mechanics Force Field III. Molecular
Molecular Dynamics Simulations Yaoquan Tu Division of Theoretical Chemistry and Biology, Royal Institute of Technology (KTH) 2011-06 1 Outline I. Introduction II. Molecular Mechanics Force Field III. Molecular
Unit 2: Quantities in Chemistry
 Mass, Moles, & Molar Mass Relative quantities of isotopes in a natural occurring element (%) E.g. Carbon has 2 isotopes C-12 and C-13. Of Carbon s two isotopes, there is 98.9% C-12 and 11.1% C-13. Find
Mass, Moles, & Molar Mass Relative quantities of isotopes in a natural occurring element (%) E.g. Carbon has 2 isotopes C-12 and C-13. Of Carbon s two isotopes, there is 98.9% C-12 and 11.1% C-13. Find
Controlling Gold Nanoparticles with Atomic Precision: Synthesis and Structure Determination
 Controlling Gold Nanoparticles with Atomic Precision: Synthesis and Structure Determination Huifeng Qian Department of Chemistry, Carnegie Mellon University, USA Advisor: Prof. Rongchao Jin Background
Controlling Gold Nanoparticles with Atomic Precision: Synthesis and Structure Determination Huifeng Qian Department of Chemistry, Carnegie Mellon University, USA Advisor: Prof. Rongchao Jin Background
INTI COLLEGE MALAYSIA A? LEVEL PROGRAMME CHM 111: CHEMISTRY MOCK EXAMINATION: DECEMBER 2000 SESSION. 37 74 20 40 60 80 m/e
 CHM111(M)/Page 1 of 5 INTI COLLEGE MALAYSIA A? LEVEL PROGRAMME CHM 111: CHEMISTRY MOCK EXAMINATION: DECEMBER 2000 SESSION SECTION A Answer ALL EIGHT questions. (52 marks) 1. The following is the mass spectrum
CHM111(M)/Page 1 of 5 INTI COLLEGE MALAYSIA A? LEVEL PROGRAMME CHM 111: CHEMISTRY MOCK EXAMINATION: DECEMBER 2000 SESSION SECTION A Answer ALL EIGHT questions. (52 marks) 1. The following is the mass spectrum
Lecture: 33. Solidification of Weld Metal
 Lecture: 33 Solidification of Weld Metal This chapter presents common solidification mechanisms observed in weld metal and different modes of solidification. Influence of welding speed and heat input on
Lecture: 33 Solidification of Weld Metal This chapter presents common solidification mechanisms observed in weld metal and different modes of solidification. Influence of welding speed and heat input on
EXPERIMENT 4 The Periodic Table - Atoms and Elements
 EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
Atoms, Elements, and the Periodic Table (Chapter 2)
 Atoms, Elements, and the Periodic Table (Chapter 2) Atomic Structure 1. Historical View - Dalton's Atomic Theory Based on empirical observations, formulated as Laws of: Conservation of Mass Definite Proportions
Atoms, Elements, and the Periodic Table (Chapter 2) Atomic Structure 1. Historical View - Dalton's Atomic Theory Based on empirical observations, formulated as Laws of: Conservation of Mass Definite Proportions
A Beginner s Guide to Materials Studio and DFT Calculations with Castep. P. Hasnip ([email protected])
 A Beginner s Guide to Materials Studio and DFT Calculations with Castep P. Hasnip ([email protected]) September 18, 2007 Materials Studio collects all of its files into Projects. We ll start by creating
A Beginner s Guide to Materials Studio and DFT Calculations with Castep P. Hasnip ([email protected]) September 18, 2007 Materials Studio collects all of its files into Projects. We ll start by creating
4.1 Studying Atom. Early evidence used to develop models of atoms.
 4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
4.1 Studying Atom Early evidence used to develop models of atoms. Democritus said that all matter consisted of extremely small particles that could NOT be divided called these particles atoms from the
Crystal Structure of High Temperature Superconductors. Marie Nelson East Orange Campus High School NJIT Professor: Trevor Tyson
 Crystal Structure of High Temperature Superconductors Marie Nelson East Orange Campus High School NJIT Professor: Trevor Tyson Introduction History of Superconductors Superconductors are material which
Crystal Structure of High Temperature Superconductors Marie Nelson East Orange Campus High School NJIT Professor: Trevor Tyson Introduction History of Superconductors Superconductors are material which
SCH 4C1 Unit 2 Problem Set Questions taken from Frank Mustoe et all, "Chemistry 11", McGraw-Hill Ryerson, 2001
 SCH 4C1 Unit 2 Problem Set Questions taken from Frank Mustoe et all, "Chemistry 11", McGraw-Hill Ryerson, 2001 1. A small pin contains 0.0178 mol of iron. How many atoms of iron are in the pin? 2. A sample
SCH 4C1 Unit 2 Problem Set Questions taken from Frank Mustoe et all, "Chemistry 11", McGraw-Hill Ryerson, 2001 1. A small pin contains 0.0178 mol of iron. How many atoms of iron are in the pin? 2. A sample
10. Calculate the mass percent nitrogen in (NH 4 ) 2 CO 3 (molar mass = 96.09 g/mol). a. 29.1 % c. 17.9 % e. 14.6 % b. 35.9 % d. 0.292 % f. 96.
 Chem 171-2-3: Final Exam Review Multiple Choice Problems 1. What is the molar mass of barium perchlorate, Ba(ClO 4 ) 2? a. 189.90 g/mol c. 272.24 g/mol e. 336.20 g/mol b. 240.24 g/mol d. 304.24 g/mol f.
Chem 171-2-3: Final Exam Review Multiple Choice Problems 1. What is the molar mass of barium perchlorate, Ba(ClO 4 ) 2? a. 189.90 g/mol c. 272.24 g/mol e. 336.20 g/mol b. 240.24 g/mol d. 304.24 g/mol f.
6.5 Periodic Variations in Element Properties
 324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
324 Chapter 6 Electronic Structure and Periodic Properties of Elements 6.5 Periodic Variations in Element Properties By the end of this section, you will be able to: Describe and explain the observed trends
Periodic Table, Valency and Formula
 Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
Chapter 1 The Atomic Nature of Matter
 Chapter 1 The Atomic Nature of Matter 6. Substances that cannot be decomposed into two or more simpler substances by chemical means are called a. pure substances. b. compounds. c. molecules. d. elements.
Chapter 1 The Atomic Nature of Matter 6. Substances that cannot be decomposed into two or more simpler substances by chemical means are called a. pure substances. b. compounds. c. molecules. d. elements.
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE
 ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
ATOMS AND THE PERIODIC TABLE CHAPTER 3 PHYSICAL SCIENCE Chapter 3 Vocabulary Words (27 words) Nucleus Atomic number Proton Mass number Neutron Isotopes Electron Atomic mass unit (amu) Energy level Average
Section 3: Crystal Binding
 Physics 97 Interatomic forces Section 3: rystal Binding Solids are stable structures, and therefore there exist interactions holding atoms in a crystal together. For example a crystal of sodium chloride
Physics 97 Interatomic forces Section 3: rystal Binding Solids are stable structures, and therefore there exist interactions holding atoms in a crystal together. For example a crystal of sodium chloride
Go/No-Go Decision: Pure, Undoped Single Walled Carbon Nanotubes for Vehicular Hydrogen Storage. October 2006. United States Department of Energy
 Go/No-Go Decision: Pure, Undoped Single Walled Carbon Nanotubes for Vehicular Hydrogen Storage October 2006.. United States Department of Energy Go/No-Go Decision: Pure, Undoped Single Walled Carbon Nanotubes
Go/No-Go Decision: Pure, Undoped Single Walled Carbon Nanotubes for Vehicular Hydrogen Storage October 2006.. United States Department of Energy Go/No-Go Decision: Pure, Undoped Single Walled Carbon Nanotubes
Periodic Table Questions
 Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
Periodic Table Questions 1. The elements characterized as nonmetals are located in the periodic table at the (1) far left; (2) bottom; (3) center; (4) top right. 2. An element that is a liquid at STP is
SEMICONDUCTOR I: Doping, semiconductor statistics (REF: Sze, McKelvey, and Kittel)
 SEMICONDUCTOR I: Doping, semiconductor statistics (REF: Sze, McKelvey, and Kittel) Introduction Based on known band structures of Si, Ge, and GaAs, we will begin to focus on specific properties of semiconductors,
SEMICONDUCTOR I: Doping, semiconductor statistics (REF: Sze, McKelvey, and Kittel) Introduction Based on known band structures of Si, Ge, and GaAs, we will begin to focus on specific properties of semiconductors,
Boyle s law - For calculating changes in pressure or volume: P 1 V 1 = P 2 V 2. Charles law - For calculating temperature or volume changes: V 1 T 1
 Common Equations Used in Chemistry Equation for density: d= m v Converting F to C: C = ( F - 32) x 5 9 Converting C to F: F = C x 9 5 + 32 Converting C to K: K = ( C + 273.15) n x molar mass of element
Common Equations Used in Chemistry Equation for density: d= m v Converting F to C: C = ( F - 32) x 5 9 Converting C to F: F = C x 9 5 + 32 Converting C to K: K = ( C + 273.15) n x molar mass of element
Unit 2 Periodic Behavior and Ionic Bonding
 Unit 2 Periodic Behavior and Ionic Bonding 6.1 Organizing the Elements I. The Periodic Law A. The physical and chemical properties of the elements are periodic functions of their atomic numbers B. Elements
Unit 2 Periodic Behavior and Ionic Bonding 6.1 Organizing the Elements I. The Periodic Law A. The physical and chemical properties of the elements are periodic functions of their atomic numbers B. Elements
Chapter 5 TEST: The Periodic Table name
 Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
Chapter 5 TEST: The Periodic Table name HPS # date: Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The order of elements in the periodic table is based
UNIT (2) ATOMS AND ELEMENTS
 UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
STATE UNIVERSITY OF NEW YORK COLLEGE OF TECHNOLOGY CANTON, NEW YORK COURSE OUTLINE CHEM 150 - COLLEGE CHEMISTRY I
 STATE UNIVERSITY OF NEW YORK COLLEGE OF TECHNOLOGY CANTON, NEW YORK COURSE OUTLINE CHEM 150 - COLLEGE CHEMISTRY I PREPARED BY: NICOLE HELDT SCHOOL OF SCIENCE, HEALTH, AND PROFESSIONAL STUDIES SCIENCE DEPARTMENT
STATE UNIVERSITY OF NEW YORK COLLEGE OF TECHNOLOGY CANTON, NEW YORK COURSE OUTLINE CHEM 150 - COLLEGE CHEMISTRY I PREPARED BY: NICOLE HELDT SCHOOL OF SCIENCE, HEALTH, AND PROFESSIONAL STUDIES SCIENCE DEPARTMENT
Forensic Science Standards and Benchmarks
 Forensic Science Standards and Standard 1: Understands and applies principles of scientific inquiry Power : Identifies questions and concepts that guide science investigations Uses technology and mathematics
Forensic Science Standards and Standard 1: Understands and applies principles of scientific inquiry Power : Identifies questions and concepts that guide science investigations Uses technology and mathematics
