Subepithelial mass lesions in the stomach are relatively
|
|
|
- Camilla Lyons
- 7 years ago
- Views:
Transcription
1 GASTROENTEROLOGY 2006;130: American Gastroenterological Association Institute Technical Review on the Management of Gastric Subepithelial Masses This literature review and the recommendations therein were prepared for the American Gastroenterological Association Institute Clinical Practice and Economics Committee. The paper was approved by the Committee on January 19, 2006, and by the AGA Institute Governing Board on April 20, See CME Quiz on page Subepithelial mass lesions in the stomach are relatively common findings in patients undergoing upper gastrointestinal endoscopy. While such masses are often referred to as submucosal, these lesions are more correctly termed subepithelial because they may arise from layers of the gastric wall other than the histologic submucosa or from extrinsic compression of the stomach by a number of normal or abnormal intra-abdominal structures. In practice, the diagnostic evaluation and subsequent management of subepithelial masses varies considerably because approaches to these lesions are currently in evolution. The goal of the present review is to examine available evidence concerning the diagnosis and management of gastric subepithelial masses. A literature review was conducted to identify all English-language articles relating to gastric subepithelial masses published between 1980 and A search of MEDLINE and PubMed was performed using the following key words: subepithelial tumor, subepithelial mass, submucosal tumor, or submucosal mass. The following terms were also searched to identify additional relevant articles: gastrointestinal stromal tumor, carcinoid, pancreatic rest, glomus tumor, inclusion cyst, duplication cyst, leiomyoma, leiomyosarcoma, lymphoma, lipoma, inflammatory fibroid polyp, and extraluminal compression. The reference lists of the articles identified in this manner were then manually searched to identify any additional references. References published only in abstract form were excluded. The present review concerns gastric subepithelial masses, and therefore articles concerned solely with subepithelial masses in other parts of the gastrointestinal tract were also excluded. Epidemiology The endoscopic appearance of a subepithelial lesion in the stomach is that of a mass, bulge, or impression visible within the gastric lumen that is covered by normal-appearing epithelium. While every endoscopist has encountered subepithelial lesions during endoscopy, the frequency of finding such lesions is likely to vary according to the size and location of the mass as well as the care taken during the endoscopic examination. The prevalence of subepithelial gastric masses on routine endoscopies is uncertain, although one retrospective study reported a prevalence of 0.36% during upper endoscopy performed between 1976 and No recent studies have identified the prevalence of gastric subepithelial masses on a population basis. Diagnostic Techniques Endoscopy The evaluation of subepithelial masses begins with the initial endoscopy. Features of subepithelial masses that can be assessed during endoscopy include an estimate of the size, shape, mobility, consistency (pillow sign, firm, cystic), pulsation, color, and mucosal appearance. In general, subepithelial masses have normal-appearing mucosa overlying the lesion, although erythema or inflammation on histologic examination of mucosal biopsy specimens unrelated to the underlying mass can be present. Furthermore, subepithelial lesions usually appear smooth with tapered margins along the edge of the lesion. However, it can be difficult to differentiate an intramural lesion from extramural compression with endoscopy alone. Two prospective studies have shown that the sensitivity and specificity of endoscopy to correctly differentiate an intramural lesion from extramural compression were between 89% and 98% and between 29% and 64%, respectively. 2,3 Probing of the subepithelial mass can be performed with closed biopsy forceps to determine its consistency. If there is any concern that the lesion is vascular or cystic, Abbreviations used in this paper: CT, computed tomography; ESD, endoscopic submucosal dissection; ESMR, endoscopic submucosal resection; EUS, endoscopic ultrasonography; FNA, fine-needle aspiration; GIST, gastrointestinal stromal tumor; MRI, magnetic resonance imaging by the American Gastroenterological Association Institute /06/$32.00 doi: /j.gastro
2 2218 AMERICAN GASTROENTEROLOGICAL ASSOCIATION INSTITUTE GASTROENTEROLOGY Vol. 130, No. 7 no biopsy specimens should be taken until the lesion has been evaluated with endoscopic ultrasonography (EUS). However, the consistency of the mass can suggest a diagnosis. For example, a mobile mass that is soft and indents when depressed using biopsy forceps (pillow sign) is highly suggestive of a lipoma. 2 Further evaluation is suggested for lesions that are firm and not consistent with either a cyst or a lipoma. Endosonography EUS can reliably differentiate intramural lesions from extrinsic compression. Furthermore, EUS will often result in a specific diagnosis, especially if the lesion is due to extrinsic compression. If an intramural lesion is identified, EUS can be used to ascertain the exact size and layer of origin, as well as additional morphologic features that can suggest the diagnosis. On EUS, the mass can be either homogeneous or heterogeneous and can be hyperechoic, hypoechoic, or anechoic. Anechoic masses can also be interrogated with Doppler to assess for blood flow. EUS is also used to evaluate the margins of the mass to determine if they are smooth or irregular and whether the mass disrupts or distorts adjacent layers or organs. Small, well-circumscribed lesions are typically benign, whereas a lesion with irregular margins that invades into other layers or structures is more likely to represent a malignant process. 4,5 The features identified on EUS can then be used to determine if further tests such as endoscopic resection, fine-needle aspiration (FNA), or core biopsies are required. EUS imaging of the gastrointestinal tract wall typically exhibits 5 distinct layers but can also exhibit 7 or 9 layers, depending on the region of the gastrointestinal tract being examined and the frequency of the ultrasound transducer. Initial interpretation of the EUS images assumed direct correspondence of the layers seen on EUS to those seen on histology. However, it was later proven that this was an incorrect interpretation of the EUS images of the gastrointestinal tract wall. 6 In fact, it was shown that the 5 distinct layers seen on EUS imaging corresponded to the following: layer 1, interface echo between the superficial mucosa and the acoustic coupling medium; layer 2, deep mucosa; layer 3, submucosa plus the acoustic interface between the submucosa and muscularis propria; layer 4, muscularis propria minus the acoustic interface between the submucosa and muscularis propria; and layer 5, serosa and subserosal fat. Cross-sectional Imaging (Computed Tomography/Magnetic Resonance Imaging) Transabdominal ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI) findings in patients with subepithelial masses have been the subjects of case reports and small case series. These imaging studies are most useful for defining the origin and extent of large extramural masses. Large lipomas can be detected on both CT and MRI by the characteristic density and magnetic resonance properties of fatty tissue. 7 CT and MRI can image large gastrointestinal stromal tumors (GISTs) and are especially helpful in the evaluation for metastatic spread of malignant GISTs. 8,9 Unlike EUS, CT and MRI cannot identify the histologic layers of the gut wall and are therefore of limited value in distinguishing between the different causes of intramural masses. Tissue Diagnosis Initial evaluation of subepithelial lesions with EUS helps identify those lesions where tissue diagnosis may be necessary. If a subepithelial lesion is found to be a hypoechoic mass in the third or fourth echo layer on EUS examination, then tissue sampling should be strongly considered to establish the diagnosis because malignant or potentially malignant lesions are included in the differential diagnosis and EUS findings have been shown to be insufficient to correctly establish the diagnosis. 2 A preoperative tissue diagnosis may not be necessary for large and/or symptomatic lesions that require surgery irrespective of histology. The need to obtain tissue from small ( 1 cm) hypoechoic masses of the gastric wall is controversial and has not been adequately studied but should be considered given the potential for malignant behavior of such lesions. EUS-guided FNA. EUS-guided FNA is commonly used to confirm the presence of malignancy in lymph nodes or organs adjacent to the gastrointestinal tract. EUS-guided FNA (typically with a 22-gauge needle) can be used to obtain a specimen for cytologic examination, and occasionally core tissue specimens, by directing the needle into the area of interest under direct ultrasound guidance. 10,11 Complications from EUS-guided FNA are rare but include perforation, infection, or hemorrhage. 10,12 16 An exception may be cyst aspiration, where infection has been reported to occur in up to 15% of cases, justifying the use of preprocedural antibiotic prophylaxis. 17,18 In reports in which prophylactic antibiotics were administered before aspiration of cysts, fewer infectious complications have been reported. 14,19 Cytology is most useful for distinguishing benign from malignant lesions but is less useful for determining the type of benign lesion present. Thus, the sensitivity, specificity, and accuracy of cytologic evaluation of intramural lesions are low. 11,12,20 The yield of EUS-guided FNA in the diagnosis of hypoechoic fourth-layer masses
3 June 2006 AMERICAN GASTROENTEROLOGICAL ASSOCIATION INSTITUTE 2219 such as GISTs may be improved with the application of immunohistochemical analysis. 11,21 23 Common markers used to evaluate hypoechoic intramural masses are CD117 (c-kit), CD34, smooth muscle actin, and S ,24 The c-kit protein is a transmembrane receptor with tyrosine kinase activity that is highly sensitive and specific for GISTs. CD34 is also expressed in approximately 80% of GISTs. Positive staining for smooth muscle actin suggests the presence of a leiomyoma or glomus tumor, and the presence of S100 suggests a neural origin or Schwannoma. A preliminary study by Ando et al 21 suggested that immunohistochemical staining for Ki-67, a marker of cell proliferation, improved the ability to diagnose malignant GISTs. If this finding is confirmed, it would be a significant advance because the inability of EUS-guided FNA to accurately predict which GISTs have high potential for malignant behavior is currently a major limitation of this procedure. EUS-guided core needle biopsy. EUS-guided core needle biopsy using a 19-gauge Trucut needle (Quick- Core; Wilson-Cook, Inc, Winston-Salem, NC) has been proposed as a method for obtaining sufficient tissue for histologic evaluation. 25,26 The Trucut needle provides a core of tissue that can be examined histologically for changes in tissue architecture in addition to the individual cell morphology scrutinized in the cytologic evaluation of FNA specimens. Initial experience using the Trucut biopsy needle in intramural lesions yielded the correct diagnosis in 4 of 5 cases compared with 1 of 5 cases using EUS-guided FNA. 25 However, additional prospective studies are required to determine if EUSguided core needle biopsy results in greater accuracy than EUS-guided FNA and to determine if complications are more frequent with this technique. Stacked forceps biopsy. Standard biopsy forceps are designed to sample primarily the mucosa, although submucosa is sometimes obtained. By taking multiple biopsy specimens of the same site using a jumbo forceps (interchangeably referred to as stacked, bite-on-bite, or tunneled biopsies), tissue from deeper layers of the gastric wall can be obtained. This technique appears to be safe with a relatively low risk of bleeding, although the diagnostic yield also appears to be limited. In one study, the diagnostic yield of stacked biopsies was 42% (15/36 lesions) and the complication rate was 2.8% (1/36 cases complicated by bleeding). 27 Endoscopic submucosal resection and dissection. The use of endoscopic submucosal resection (ESMR) or endoscopic submucosal dissection (ESD) to resect submucosal lesions is another technique to obtain tissue specimens for accurate histologic diagnosis This technique provides greater yield than stacked biopsies but has not been compared with either EUS-guided FNA or EUS-guided core needle biopsy. ESMR and ESD are usually reserved for lesions that are confined to the submucosal or deep mucosal layers due to the increased risk of perforation associated with endoscopic resection of lesions from the muscularis propria; however, there are some small case series in the literature of endoscopic resection of lesions arising from the muscularis propria with no associated perforations. 27,28 Given that the perforation rate for ESMR of submucosal lesions is reported to be 2% 3%, it is likely that endoscopic resection of lesions from the muscularis propria will be similar if not higher. 22 Bleeding, both during the procedure and delayed, is a significant complication that can occur with all endoscopic resection techniques. Differential Diagnosis The differential diagnosis of a gastric subepithelial mass depends on whether the lesion represents compression from a normal or abnormal structure adjacent to the gastric wall or if it originates from the wall itself. A brief description of lesions in the differential diagnosis of subepithelial lesions is presented with a description of the corresponding findings on EUS examination and additional methods that can be used to obtain tissue to establish the diagnosis. The differential diagnoses for intramural causes of gastric subepithelial masses are presented as benign lesions or lesions that are malignant or have malignant potential (Table 1). Extramural Lesions The most common source of extraluminal compression in the stomach is from the spleen and splenic vessels. 2,3,32 Other sources of extraluminal compression include normal abdominal structures such as the left lobe of the liver, gallbladder, colon, and pancreas. In addition, pathologic lesions such as tumors, abscesses, pancreatic pseudocysts, renal cysts, aneurysms, and enlarged lymph nodes can appear as gastric subepithelial lesions on endoscopy. Benign Intramural Lesions The differential diagnosis for intramural causes of gastric subepithelial masses can be divided into benign lesions or lesions that are malignant or have malignant potential. Lipoma. Gastric lipomas are a rare cause of gastric subepithelial masses, accounting for 1% of gastric intramural lesions. 33 Gastric lipomas are benign, slow-growing lesions; however, they can cause significant clinical symptoms such as bleeding caused by ulceration, obstruction
4 2220 AMERICAN GASTROENTEROLOGICAL ASSOCIATION INSTITUTE GASTROENTEROLOGY Vol. 130, No. 7 Table 1. Differential Diagnosis of Intramural Gastric Subepithelial Masses Based on EUS Features Subepithelial lesion EUS layer a Echogenicity Benign Leiomyoma 2, 3 or 4 Hypoechoic Neural origin tumors Schwannoma 3 or 4 Hypoechoic Neuroma Neurofibroma Lipoma 3 Intensely hyperechoic Duplication cyst Any or extramural Anechoic Pancreatic rest 2 or 3 Hypoechoic Inflammatory 3 or 4 Hyperechoic fibroid polyp Granular cell 2 or 3 Hypoechoic tumor Varices 2 or 3 Anechoic Malignant or with malignant potential GIST 4 (rarely 2 or 3) Hypoechoic Lymphoma 2, 3 or 4 Hypoechoic Carcinoid 2 or 3 Hypoechoic Metastatic Any Hypoechoic carcinoma Glomus tumor 3 or 4 Hypoechoic a Layer 1 is the interface of luminal fluid and mucosa, layer 2 represents the deep mucosa, layer 3 is largely due to the submucosa, layer 4 represents the muscularis propria, and layer 5 is adventitia or serosa with adjacent fatty or fibrous tissue. from intussusception into the pylorus or duodenal bulb, and abdominal pain. 7,33,34 Lipomas present typically as solitary lesions; however, multiple lipomas of the stomach and duodenum have been reported. 35 On endoscopy, lipomas can have a yellow hue, often exhibit a pillow sign when probed with closed biopsy forceps, and may also exhibit some mobility. A recent study showed that the pillow sign had 98% specificity but only 40% sensitivity in identifying lipomas. 2 The finding of an intensely hyperechoic wellcircumscribed mass arising from the submucosal wall layer on EUS examination is essentially diagnostic for a lipoma, and no further evaluation is needed in such cases. Leiomyoma. Leiomyomas are smooth muscle tumors that arise from either the muscularis mucosae or muscularis propria within the wall of the gastrointestinal tract and are benign tumors composed of well-differentiated smooth muscle cells. True leiomyomas are rarely found in the stomach, although case reports are abundant in the older literature because tumors that are now classified as GISTs were often misclassified as leiomyomas or leiomyosarcomas. With new immunohistochemical methods, it is clear that tumors of smooth muscle origin, as identified by positive staining for smooth muscle actin and desmin and negative staining for CD117, CD34, and S100 protein, are rare in the stomach. A recent study that examined archived histologic slides of more than 500 gastrointestinal mesenchymal tumors previously classified as smooth muscle tumors showed that most were actually GISTs with the exception of masses in the esophagus, where true leiomyomas were more common. 36 In this relatively large study, no gastric leiomyomas were identified. In another retrospective study of gastric spindle cell tumors, 2 of 17 tumors were identified as leiomyomas. 21 Leiomyomas appear as hypoechoic, well-circumscribed masses arising from the muscularis propria or the muscularis mucosae on EUS examination. Varices. Gastric varices can have the appearance of a subepithelial mass or large gastric fold on endoscopy. 37 Gastric varices should be suspected in patients with portal hypertension (end-stage liver disease, Budd Chiari syndrome, portal vein thrombosis, and so on) and in patients with splenic vein thrombosis, typically due to pancreatitis, that result in isolated gastric varices. Endoscopic examination may reveal the presence of portal hypertensive gastropathy, and probing with closed biopsy forceps may reveal a soft consistency to the lesion. Closely examining the color of the lesion for a blue hue can also suggest that the lesion may be a varix. If these features are present, biopsy specimens should not be obtained and EUS examination should be performed to confirm that the lesion is a varix. EUS examination will show a round or tubular hypoechoic or anechoic structure located in the submucosa (third layer), and Doppler examination will demonstrate flow within the structure. Pancreatic rest. A pancreatic rest or heterotopic pancreatic tissue is the presence of ectopic pancreatic tissue within the wall of the stomach, typically in the gastric antrum, and usually within the submucosal layer. 38 Although they generally do not cause symptoms, pancreatic rests have been reported to present with nausea, epigastric pain, weight loss, hematemesis, and gastric outlet obstruction. 38,39 In addition, there are rare case reports of heterotopic pancreatic tissue that have undergone malignant change On endoscopy, a pancreatic rest commonly exhibits an umbilication on the surface of the nodule, although the specificity of this finding is not known. On EUS examination, a pancreatic rest will appear hypoechoic relative to the surrounding submucosal layer with a heterogeneous echotexture. Because the differential diagnosis for hypoechoic third-layer lesions includes potentially malignant lesions, tissue sampling to establish the diagnosis is necessary. Cap-assisted ESMR has been reported as a potential endoscopic method of obtaining an adequate histologic sample to diagnose a pancreatic rest. 45
5 June 2006 AMERICAN GASTROENTEROLOGICAL ASSOCIATION INSTITUTE 2221 Duplication cyst. Gastrointestinal duplication cysts are benign lesions that result from an error in the embryonic development of the foregut and are primarily diagnosed in the pediatric population. 46,47 Duplication cysts in adults are often found incidentally and are usually asymptomatic. The cysts are located either within or adjacent to the wall of the gastrointestinal tract and are lined with gastrointestinal epithelium. 48 Because duplication cysts generally do not communicate with the gastrointestinal lumen, they can enlarge, resulting in mass effect, rupture, or bleeding. 46,49,50 The diagnosis of a gastric duplication cyst can easily be made using EUS, which will show an anechoic, smooth, spherical, or tubular structure with a well-defined wall. 51,52 If the cystic structure is adjacent to the pancreas, EUS/FNA can be performed to sample the fluid contents of the cyst to rule out a pancreatic pseudocyst or a pancreatic cystic neoplasm. 53 Inflammatory fibroid polyp. Gastric inflammatory fibroid polyps are rare benign lesions in the stomach that are characterized histologically by fibrous tissue that is not encapsulated. In addition, there are commonly many small blood vessels and an eosinophilic inflammatory infiltrate. 54,55 On EUS examination, the polyps are located in the deep mucosal or submucosal layers without involvement of the muscularis propria. 55 They are typically hypoechoic with a homogeneous echotexture and indistinct margins. Occasionally they will appear slightly hyperechoic, which corresponds histologically with multiple small penetrating vessels within the fibrous stroma of the polyp. 55 Malignant and Potentially Malignant Intramural Lesions GIST. GISTs are the most commonly identified intramural subepithelial mass in the upper gastrointestinal tract, and some controversy exists regarding the diagnosis and management of these tumors. It is believed that new cases of GIST are diagnosed each year, with 10% 30% being malignant. 56 Most patients are in their fifth or sixth decade of life at the time of diagnosis, and the most common location of GISTs is the stomach. 24,56 GISTs were once believed to represent smooth muscle tumors (leiomyomas and leiomyosarcomas); however, they are now believed to arise from the interstitial cells of Cajal and can be identified using immunohistochemistry for expression of CD117, which is also known as c-kit protein (a cell membrane receptor with tyrosine kinase activity). Due to this recent change in classification, the older literature may be confusing and must be considered in light of this new information. A study of archived histologic slides of masses previously classified as smooth muscle tumors showed that most were actually GISTs with the exception of masses in the esophagus, where true leiomyomas were more common. 36 GISTs most commonly arise from the muscularis propria and are usually asymptomatic until the tumor becomes large or ulcerates, resulting in bleeding. EUS examination of a GIST shows a hypoechoic mass with a homogeneous echotexture that is contiguous with the muscularis propria (fourth EUS layer). Findings on EUS of a mass diameter 3 cm, irregular extraluminal border, cystic spaces, echogenic foci (heterogenous echotexture), and adjacent malignant-appearing lymph nodes are features that can suggest malignancy 4,5 ; however, even small GISTs have malignant potential and have been reported to metastasize Unfortunately, EUS findings do not accurately predict the malignant potential of a small GIST and histologic examination is necessary. There are reports of new techniques using EUS-guided FNA or core needle biopsies that may be helpful in determining the malignant potential of a GIST without having to perform surgical resection; however, studies examining their clinical utility in large numbers of patients have yet to be reported. 10,21,60 Carcinoid tumor. Gastric carcinoid tumors are neuroendocrine tumors that originate from enterochromaffin-like cells located in the gastric mucosa. Carcinoid tumors can be solitary or multiple, the latter usually occurring in the setting of hypergastrinemia due to gastrinoma or autoimmune atrophic gastritis. Carcinoid tumors originate in the mucosal layer and can invade into deeper structures of the gastrointestinal tract wall. 61 The biologic behavior appears to be different between solitary gastric carcinoids and multiple gastric carcinoids due to hypergastrinemia; solitary gastric carcinoids exhibit a greater potential for malignancy and metastasis to local lymph nodes and the liver On endoscopy, carcinoids typically appear as polypoid lesions with normal-appearing overlying mucosa. 65 EUS examination of a carcinoid tumor will show a hypoechoic lesion, typically originating from the deep mucosa or submucosa (second or third EUS layer), and can be useful in identifying the depth of invasion of the carcinoid tumor. 66 Furthermore, EUS can be used to assess for possible local lymph node metastasis. Lymphoma. Primary gastric lymphomas are typically either diffuse large B-cell lymphomas or low-grade B-cell mucosa-associated lymphoid tissue lymphomas. 67,68 In addition, disseminated nodal disease can secondarily involve the gastrointestinal tract. 69 Endoscopically, gastric lymphomas can present as an ulcerated polypoid mass, thickened gastric folds, or subepithelial
6 2222 AMERICAN GASTROENTEROLOGICAL ASSOCIATION INSTITUTE GASTROENTEROLOGY Vol. 130, No. 7 mass. Because gastric lymphomas typically involve the deep mucosa, standard biopsy specimens usually provide sufficient tissue to make the diagnosis. EUS of primary gastric lymphoma typically shows a hypoechoic lesion that can be localized to the second and third layers of the gastric wall or extend through the entire wall. 70 EUS can also be used to assess for local lymph node involvement, and EUS-guided FNA can be used to obtain tissue for flow cytometry to establish the diagnosis. 71,72 Glomus tumor. Glomus tumors originate from modified vascular smooth muscle cells and usually occur in peripheral soft tissue but can also occur in the gastrointestinal tract, typically the stomach. 73,74 These lesions are usually benign, but they have potential for malignant behavior and can also present with ulceration and hemorrhage. In a retrospective study of 31 gastric glomus tumors, Miettinen et al reported that all but one tumor exhibited benign behavior; however, one patient died due to liver metastases of the glomus tumor. 73 EUS will show a hypoechoic, well-circumscribed mass located in the third and/or fourth EUS layer; however, EUS findings are insufficient to establish the diagnosis and cannot be used to predict the malignant potential of the tumor. 75 EUS-guided FNA has been reported to successfully diagnose glomus tumors using cytologic and immunohistochemical analysis. Immunohistochemical staining will show positivity for smooth muscle actin and vimentin while being negative for CD117 (c-kit) staining, which helps to differentiate these lesions from GISTs. 73,76 Metastasis. Metastatic spread of malignancies to the gastric wall is extremely rare; however, various malignancies have potential to metastasize to the gastric wall, including malignant melanoma as well as carcinomas of the breast, lung, kidney, and ovaries EUS examination of most metastatic lesions will show a hypoechoic lesion that can be in any layer of the gastric wall. EUS-guided FNA can be performed to obtain tissue to establish the diagnosis of metastasis to the gastric wall. 81 Management The management of gastric subepithelial lesions depends on the diagnosis determined using the findings at endoscopy, EUS, and biopsy. Many benign gastric subepithelial masses, including lipomas, varices, pancreatic rests, duplication cysts, and extramural compression from normal structures, require no further evaluation or follow-up. The management of frankly malignant lesions is also straightforward in that all such lesions require complete removal by either surgical or endoscopic resection. However, many gastric subepithelial masses, including GISTs and some carcinoid tumors, may not be malignant at diagnosis but have potential for malignant behavior. The management of this category of gastric subepithelial lesions is controversial and often based on limited data and/or clinical experience. While a comprehensive review of the management of all the lesions that can present as a gastric subepithelial mass is beyond the scope of this review, strategies for observation versus surgical or endoscopic resection are discussed, with particular attention to GISTs and carcinoid tumors. Management of GISTs The clinical behavior of GISTs is quite variable and can be difficult to predict on the basis of available clinical and histologic features. Nonetheless, a consensus conference has proposed a strategy for predicting malignant behavior of GISTs based on size ( 2 cm, 2 5 cm, 5 10 cm, 10 cm) and mitotic count on histology ( 5, 6 10, or 10 per 50 high-power field), with the understanding that no GIST can be defined as benign on the basis of currently available diagnostic testing. 24 It should be noted that this method for predicting the malignant potential of GISTs requires a surgical specimen to determine the mitotic count and therefore cannot be determined preoperatively. EUS criteria, including size ( 3 cm), irregularity of the extraluminal border, and the presence of cystic spaces, echogenic foci, and heterogeneity, have also been associated with an increased likelihood of malignant behavior of GISTs, although the sensitivity and specificity of these criteria are imperfect and there is also considerable interobserver variability in interpretation of these findings. 4,5 Moreover, the natural history of GISTs is incompletely defined, and the potential for malignant behavior of even small lesions exists. 21,57 59 The optimal management of these lesions remains controversial. All GISTs have malignant potential according to the classification system proposed by the National Institutes of Health Consensus Conference 24 ; however, the absolute potential for malignancy and metastasis appears to be very low, especially for small ( 3 cm) lesions. Because surgery is the current standard of care for resecting GIST lesions, there is debate as to whether the potential morbidity and mortality associated with surgical resection are acceptable for removing a lesion with low potential for malignancy. Given the lack of sufficient evidence to guide management, we recommend that this decision be made on an individual basis, taking into consideration the clinical presentation (eg, symptoms, comorbidities), endoscopic and EUS evaluation, histologic evaluation (if obtained), and patient preference. Only prospective studies with long-term fol-
7 June 2006 AMERICAN GASTROENTEROLOGICAL ASSOCIATION INSTITUTE 2223 low-up will identify the appropriate management strategy for GISTs. Management of Carcinoid Tumors Gastric carcinoids are believed to arise from enterochromaffin-like cells in the gastric mucosa, and clinical behavior can vary considerably. On the basis of clinical behavior and pathologic features, 3 distinct types of gastric carcinoids have been proposed. 63 Type 1 carcinoids are the most common, encompassing approximately 65% of gastric carcinoids, and are associated with hypergastrinemia in the setting of chronic atrophic gastritis. 83 Type 2 carcinoids arise in the setting of hypergastrinemia associated with a gastrinoma in patients with Zollinger Ellison syndrome and multiple endocrine neoplasia type 1. Gastric carcinoids that arise in the setting of hypergastrinemia (type 1 and type 2) are generally believed to have a favorable prognosis and rarely become locally invasive or metastatic. This is particularly true for type 1 lesions, where 5-year survival exceeds 95%. 83 Type 3 or sporadic carcinoids are less frequently identified (21% of gastric carcinoids) and arise in the setting of normal serum gastrin levels. 63 In contrast to type 1 carcinoids, up to one half of type 3 carcinoids will become locally invasive or metastatic. While the risk of metastasis is believed to be associated with size, there are reports of even small gastric carcinoids metastasizing to lymph nodes. 84 Therefore, recommended management of type 3 carcinoids in an otherwise healthy individual is aggressive surgical resection. 83 More conservative management of type 1 and 2 carcinoids can be considered, although many authorities recommend endoscopic excision of small ( 1 2 cm) gastric carcinoids if feasible followed by endoscopic surveillance. 85 Others have reported that reduction in antral G-cell mass via antrectomy can result in regression of carcinoids associated with hypergastrinemia and may be the optimal treatment for multiple large carcinoids that are symptomatic due to bleeding or obstruction. 86 Management Strategies Surveillance. When a subepithelial lesion is believed to be neither benign nor clearly malignant or premalignant or if a patient with a premalignant lesion is deemed to be at excessive operative risk, surveillance may be indicated. This is often the case with third- and fourth-layer hypoechoic masses where tissue sampling (ESMR or FNA) is not technically feasible or is not diagnostic. Such lesions are most likely GISTs. Moreover, if such lesions are small ( 3 cm) and no concerning endosonographic features are present, such as irregularity of the extraluminal border or presence of cystic spaces, echogenic foci, or heterogeneity, then surveillance may be considered with the understanding that no GIST can be confirmed to be benign. Only one small series of endosonographic follow-up in this setting has been published. 87 In this series, which included follow-up for a mean of 19 months, 24 of 25 lesions (96%) remained stable with regard to both size and echo features, whereas one lesion increased from 30 to 38 mm and became irregular and inhomogeneous during follow-up. This lesion was subsequently identified to be a GIST with high malignant potential at resection. Therefore, if surveillance of a premalignant lesion is undertaken, it should be done so after thorough discussion with the patient. If surveillance is selected, there may be alternatives to performing EUS to evaluate for a change in the lesions. Several articles have reported on the use of transabdominal ultrasonography for the examination of lesions arising in the gastric wall While transabdominal ultrasonography is less invasive than EUS, it must be emphasized that not all lesions arising in the gastric wall can be visualized by transabdominal ultrasonography; published series have reported that rates of adequate visualization for surveillance range from 69% to 93%. Polkowski et al have reported than visualization with transabdominal ultrasonography in this setting is inversely proportional to the size of the mass in the gastric wall, with only 61% and 50% visualization for lesions 30 mm and 10 mm, respectively. 90 Therefore, those lesions that are most likely to be considered for surveillance are also those that may be most difficult to visualize with transabdominal ultrasonography. Another alternative to surveillance with EUS may be to follow lesion size alone using gastroscopy with comparison to a size reference such as a biopsy forceps. Hwang et al have reported good correlation between endoscopic evaluation of size with a standard reference (open biopsy forceps) compared with EUS measurement of size. 2 This may have particular utility in settings where EUS is not readily available. Surgical resection. Treatment of GISTs 3 cm, selected gastric carcinoids, and other malignant or premalignant lesions should be complete removal of the lesion. In addition, lesions in which the malignant potential cannot be determined by less invasive means may also require surgical excision for diagnosis. While a complete review of surgical approaches to subepithelial masses in the stomach is beyond the scope of this review, indications and an overview of techniques are briefly discussed. For GISTs, local resection with clear margins appears to be adequate and neither wide histologic margins nor lymph node dissection appear to be necessary. 91 Simi-
8 2224 AMERICAN GASTROENTEROLOGICAL ASSOCIATION INSTITUTE GASTROENTEROLOGY Vol. 130, No. 7 larly, local excision appears to be adequate for removal of type 1 and type 2 carcinoid tumors, although the need for resection of these lesions is somewhat uncertain. 83,85 In contrast, type 3 gastric carcinoids appear to have much greater potential for aggressive behavior, and partial or total gastrectomy with lymph node dissection is required in such cases. 85 Traditionally, surgical resection of most malignant and premalignant subepithelial gastric masses has been accomplished by complete local resection using an open surgical approach. However, there are now numerous case series describing the use of laparoscopic or combined laparoscopic/endoscopic 99,100 approaches for resection of gastric subepithelial lesions. These series report high success rates usually in excess of 90% among well-selected patients with low rates of complications and shorter hospital stays compared with traditional open resections. However, it must be noted that the length of follow-up in many of the studies of laparoscopic resection is limited, and rates of local recurrence in reported case series of laparoscopic resection of GISTs range from 0% to 30%. Whether the rate of local recurrence differs between minimally invasive and open approaches cannot be determined with certainty from the available literature because no prospective, randomized comparison has been published to date. Despite this, minimally invasive approaches are becoming the standard of care for the surgical resection of GISTs and other mass lesions arising in the gastric wall. Endoscopic resection. Endoscopic resection of mucosal lesions is a common practice among gastroenterologists, but resection of lesions arising in deeper layers of the gastric wall is less commonly practiced owing to the more technically demanding nature of such procedures and the higher rate of complications, including bleeding and perforation. 27 However, endoscopic resection of lesions arising in both the submucosa and muscularis propria has been described and is increasingly performed, particularly in Asian centers. It must be emphasized that characterization of the layer of origin with EUS is necessary before proceeding with attempted endoscopic resection because the risk of the procedure is directly related to the depth of the tumor within the gastric wall. Case reports of endoscopic resection of lesions in the submucosal layer date back to the early 1990s. 101,102 Early series of endoscopic submucosal resection primarily included esophageal, small intestinal, and colonic lesions, although more recent series have included increasing numbers of gastric lesions. One early series consisted of 62 patients with esophageal submucosal lesions ranging in size from 0.6 to 7.5 cm. 28 In this series, lesions 2 cm in diameter were removed by standard snare polypectomy and those 2 cm were removed by stripping of the overlying mucosa followed by enucleation with a snare and electrocoagulation electrode. The investigators reported complete excision of the esophageal submucosal lesions with no perforations and 3 patients who required endoscopic therapy for bleeding. Another series included 45 patients with submucosal tumors in various parts of the gastrointestinal tract that were resected with a polypectomy snare with or without assistance of a grasping forceps using a double-channel endoscope. This series reported complete resection of all lesions with no perforations and only 5 patients with mild or moderate bleeding that was controlled endoscopically. 103 Kojima et al reported their experience with endoscopic submucosal resection of 31 submucosal tumors (14 gastric) with snare electrocautery and a grasping forceps via doublechannel endoscope with no perforations and a 9% bleeding rate. 29 In this same series, forceps biopsy of lesions arising in the submucosa was accomplished following resection of the overlying mucosa and submucosa by the same technique. Another series included 27 submucosal lesions (4 gastric) in which attempted removal with either snare and grasping forceps technique or cap-assisted technique was successful in 93% of cases with no complications. 30 One case report has reported the adjunctive use of band ligation before submucosal resection. 104 Endoscopic resection of lesions arising in the muscularis propria has also been reported in several small case series. Spinelli et al reported on the endoscopic resection of 7 gastric leiomyomas using a polypectomy snare with no major complications. 105 Sun et al reported a series of endoscopic resections of subepithelial lesions, including several lesions arising in the muscularis propria. 31 In this series, EUS was first used to inject saline into the submucosa. Lesions arising in the submucosa were then directly removed with a polypectomy snare, whereas tumors arising in the muscularis propria were removed via enucleation with a polypectomy snare after the overlying mucosa and submucosa were removed with a needle knife. Six submucosal tumors and 9 tumors in the muscularis mucosae were removed, with no perforations and 2 patients requiring endoscopic hemostasis for bleeding. Most lesions removed in this series were classified as leiomyomas, with no distinction made between GISTs and leiomyomas. One recent study has also reported the use of band ligation for the treatment of lesions arising in the muscularis propria after EUS with FNA or stacked forceps biopsy specimens were used to make a histologic diagnosis. 106 In this study of 64 upper gastrointestinal leiomyomas (14 gastric), bands were placed and lesions
9 June 2006 AMERICAN GASTROENTEROLOGICAL ASSOCIATION INSTITUTE 2225 allowed to slough over time with success rates of 100% in the esophagus and duodenum and 75% in the stomach. It should be noted that this study did not evaluate these fourth-layer hypoechoic masses for c-kit, although it is likely that many of these lesions were GISTs. Most of the lesions treated in this series were small ( 1 2 cm), and a disadvantage of this technique is that complete histologic evaluation of the lesions was not possible because the masses were not removed but rather allowed to slough into the lumen and pass through the gastrointestinal tract. A newer technique for endoscopic resection of subepithelial tumors involves the use of an insulated-tip electrosurgical knife, which was initially developed for the endoscopic resection of early-stage gastric cancer. Park et al reported the use of the insulated-tip electrosurgical knife for the resection of 5 esophageal and 10 gastric tumors, including 11 cases in which the tumor arose from the muscularis propria. 107 Complications included bleeding in one patient and perforation in one patient, both of which were managed with placement of endoscopic clips. Rösch et al have recently reported their experience with the combined use of suction cap ESMR and insulated-tip electrosurgical knife resection in 12 patients with tumors localized to the submucosa by EUS. 108 In this series, the complete removal rate was reported to be 79%, with one perforation and one clinically significant bleeding event occurring in these patients. In all case series of endoscopic resection of gastric subepithelial lesions, it must be emphasized that follow-up was limited and studies have not included examination of surgical or autopsy specimens to confirm completeness of resection. The clinical importance of complete resection of gastric subepithelial masses is currently unknown, although the premalignant nature of many of these lesions should warrant complete removal until data to the contrary are available. Moreover, while reported complication rates are relatively low, they are significantly greater than the risks associated with other endoscopic procedures such as polypectomy and complication rates are likely to be higher among less experienced operators. Ablation. A single case report has described the use of 95% ethanol to ablate a gastric GIST. 109 In this report, injection of ethanol into a 4-cm GIST under EUS guidance resulted in successful ablation of the tumor with no evidence of residual GIST at follow-up EUS 2 years after treatment. Whether the use of ethanol or other ablative therapies can be used for successful ablation of premalignant subepithelial lesions requires prospective study. Conclusions Identifying a subepithelial mass during endoscopy is common. Further evaluation with EUS allows for improved characterization of subepithelial lesions, aiding the clinician in narrowing the differential diagnosis; however, the specificity of EUS imaging findings alone has been disappointing. It must be emphasized that despite the lack of specificity of EUS in diagnosing the etiology of subepithelial lesions, it remains the best test for determining the need for further evaluation, endoscopic resection, or surgery. Obtaining tissue using EUSguided FNA or endoscopic submucosal resection is often necessary to establish the diagnosis and direct further patient management. The literature is abundant with case reports on endoscopic management of subepithelial masses; however, further investigation is needed to better define the optimal management of patients with GISTs and those with indeterminate lesions. JOO HA HWANG STEPHEN D. RULYAK MICHAEL B. KIMMEY Division of Gastroenterology University of Washington Seattle, Washington References 1. Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc 1991;5: Hwang JH, Saunders MD, Rulyak SJ, Shaw S, Nietsch H, Kimmey MB. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc 2005;62: Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, Ulm K. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol 2002;37: Chak A, Canto MI, Rösch T, Dittel HJ, Hawes RH, Tio TL, Lightdale CJ, Boyce HW, Scheiman J, Carpenter SL, Van DJ, Kochman ML, Sivak MV Jr. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc 1997;45: Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut 2000;46: Kimmey MB, Martin RW, Haggitt RC, Wang KY, Franklin DW, Silverstein FE. Histologic correlates of gastrointestinal ultrasound images. Gastroenterology 1989;96: Thompson WM, Kende AI, Levy AD. Imaging characteristics of gastric lipomas in 16 adult and pediatric patients. AJR Am J Roentgenol 2003;181: Hasegawa S, Semelka RC, Noone TC, Woosley JT, Marcos HB, Kenney PJ, Siegelman ES. Gastric stromal sarcomas: correlation of MR imaging and histopathologic findings in nine patients. Radiology 1998;208: Scatarige JC, Fishman EK, Jones B, Cameron JL, Sanders RC, Siegelman SS. Gastric leiomyosarcoma: CT observations. J Comput Assist Tomogr 1985;9:
10 2226 AMERICAN GASTROENTEROLOGICAL ASSOCIATION INSTITUTE GASTROENTEROLOGY Vol. 130, No Matsui M, Goto H, Niwa Y, Arisawa T, Hirooka Y, Hayakawa T. Preliminary results of the needle aspiration biopsy histology in upper gastrointestinal submucosal tumors. Endoscopy 1998; 30: Gu M, Ghafari S, Nguyen PT, Lin F. Cytologic diagnosis of gastrointestinal stromal tumors of the stomach by endoscopic ultrasound-guided fine-needle aspiration biopsy: cytomorphologic and immunohistochemical study of 12 cases. Diagn Cytopathol 2001;25: Williams DB, Sahai AV, Aabakken L, Penman ID, Van VA, Webb J, Wilson M, Hoffman BJ, Hawes RH. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut 1999;44: Affi A, Vazquez-Sequeiros E, Norton ID, Clain JE, Wiersema MJ. Acute extraluminal hemorrhage associated with EUS-guided fine needle aspiration: frequency and clinical significance. Gastrointest Endosc 2001;53: O Toole D, Palazzo L, Arotcarena R, Dancour A, Aubert A, Hammel P, Amaris J, Ruszniewski P. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc 2001; 53: Barawi M, Gottlieb K, Cunha B, Portis M, Gress F. A prospective evaluation of the incidence of bacteremia associated with EUSguided fine-needle aspiration. Gastrointest Endosc 2001;53: Levy MJ, Norton ID, Wiersema MJ, Schwartz DA, Clain JE, Vazquez-Sequeiros E, Wilson WR, Zinsmeister AR, Jondal ML. Prospective risk assessment of bacteremia and other infectious complications in patients undergoing EUS-guided FNA. Gastrointest Endosc 2003;57: Wildi SM, Hoda RS, Fickling W, Schmulewitz N, Varadarajuluy S, Roberts SS, Ferguson B, Hoffman BJ, Hawes RH, Wallace MB. Diagnosis of benign cysts of the mediastinum: the role and risks of EUS and FNA. Gastrointest Endosc 2003;58: Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 1997;112: Lee LS, Saltzman JR, Bounds BC, Poneros JM, Brugge WR, Thompson CC. EUS-guided fine needle aspiration of pancreatic cysts: a retrospective analysis of complications and their predictors. Clin Gastroenterol Hepatol 2005;3: Wiersema MJ, Wiersema LM, Khusro Q, Cramer HM, Tao LC. Combined endosonography and fine-needle aspiration cytology in the evaluation of gastrointestinal lesions. Gastrointest Endosc 1994;40: Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, Nagasaka T, Hayakawa T. The diagnosis of GI stromal tumors with EUSguided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc 2002;55: Hunt GC, Rader AE, Faigel DO. A comparison of EUS features between CD-117 positive GI stromal tumors and CD-117 negative GI spindle cell tumors. Gastrointest Endosc 2003;57: Stelow EB, Stanley MW, Mallery S, Lai R, Linzie BM, Bardales RH. Endoscopic ultrasound-guided fine-needle aspiration findings of gastrointestinal leiomyomas and gastrointestinal stromal tumors. Am J Clin Pathol 2003;119: Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 2002;33: Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc 2003;57: Wiersema MJ, Levy MJ, Harewood GC, Vazquez-Sequeiros E, Jondal ML, Wiersema LM. Initial experience with EUS-guided trucut needle biopsies of perigastric organs. Gastrointest Endosc 2002;56: Hunt GC, Smith PP, Faigel DO. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc 2003;57: Hyun JH, Jeen YT, Chun HJ, Lee HS, Lee SW, Song CW, Choi JH, Um SH, Kim CD, Ryu HS. Endoscopic resection of submucosal tumor of the esophagus: results in 62 patients. Endoscopy 1997;29: Kojima T, Takahashi H, Parra-Blanco A, Kohsen K, Fujita R. Diagnosis of submucosal tumor of the upper GI tract by endoscopic resection. Gastrointest Endosc 1999;50: Waxman I, Saitoh Y, Raju GS, Watari J, Yokota K, Reeves AL, Kohgo Y. High-frequency probe EUS-assisted endoscopic mucosal resection: a therapeutic strategy for submucosal tumors of the GI tract. Gastrointest Endosc 2002;55: Sun S, Wang M, Sun S. Use of endoscopic ultrasound-guided injection in endoscopic resection of solid submucosal tumors. Endoscopy 2002;34: Motoo Y, Okai T, Ohta H, Satomura Y, Watanabe H, Yamakawa O, Yamaguchi Y, Mouri I, Sawabu N. Endoscopic ultrasonography in the diagnosis of extraluminal compressions mimicking gastric submucosal tumors. Endoscopy 1994;26: Fernandez MJ, Davis RP, Nora PF. Gastrointestinal lipomas. Arch Surg 1983;118: Maderal F, Hunter F, Fuselier G, Gonzales-Rogue P, Torres O. Gastric lipomas an update of clinical presentation, diagnosis, and treatment. Am J Gastroenterol 1984;79: Deeths TM, Madden PN, Dodds WJ. Multiple lipomas of the stomach and duodenum. Am J Dig Dis 1975;20: Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol 2000; 13: Mendis RE, Gerdes H, Lightdale CJ, Botet JF. Large gastric folds: a diagnostic approach using endoscopic ultrasonography. Gastrointest Endosc 1994;40: Thoeni RF, Gedgaudas RK. Ectopic pancreas: usual and unusual features. Gastrointest Radiol 1980;5: Lai EC, Tompkins RK. Heterotopic pancreas. Review of a 26 year experience. Am J Surg 1986;151: Goldfarb WB, Bennett D, Monafo W. Carcinoma in a heterotopic gastric pancreas. Ann Surg 1963;158: Tanimura A, Yamamoto H, Shibata H, Sano E. Carcinoma in heterotopic gastric pancreas. Acta Pathol Jpn 1979;29: Hickman DM, Frey CF, Carson JW. Adenocarcinoma arising in gastric heterotopic pancreas. West J Med 1981;135: Jeng KS, Yang KC, Kuo S. Malignant degeneration of heterotopic pancreas. Gastrointest Endosc 1991;37: Ura H, Denno R, Hirata K, Saeki A, Hirata K, Natori H. Carcinoma arising from ectopic pancreas in the stomach: endosonographic detection of malignant change. J Clin Ultrasound 1998;26: Faigel DO, Gopal D, Weeks DA, Corless C. Cap-assisted endoscopic submucosal resection of a pancreatic rest. Gastrointest Endosc 2001;54: Lamont AC, Starinsky R, Cremin BJ. Ultrasonic diagnosis of duplication cysts in children. Br J Radiol 1984;57: Hulnick DH, Balthazar EJ. Gastric duplication cyst: GI series and CT correlation. Gastrointest Radiol 1987;12: Mathur M, Gupta SD, Bajpai M, Rohatagi M. Histochemical pattern in alimentary tract duplications of children. Am J Gastroenterol 1991;86: Ott DJ, Wolfman NT, Wu WC, Chen MY, White WL. Endoscopic ultrasonography of benign esophageal cyst simulating leiomyoma. J Clin Gastroenterol 1992;15:85 87.
11 June 2006 AMERICAN GASTROENTEROLOGICAL ASSOCIATION INSTITUTE Stringer MD, Dinwiddie R, Hall CM, Spitz L. Foregut duplication cysts: a diagnostic challenge. J R Soc Med 1993;86: Geller A, Wang KK, DiMagno EP. Diagnosis of foregut duplication cysts by endoscopic ultrasonography. Gastroenterology 1995;109: Faigel DO, Burke A, Ginsberg GG, Stotland BR, Kadish SL, Kochman ML. The role of endoscopic ultrasound in the evaluation and management of foregut duplications. Gastrointest Endosc 1997;45: Van Dam J, Rice TW, Sivak MV Jr. Endoscopic ultrasonography and endoscopically guided needle aspiration for the diagnosis of upper gastrointestinal tract foregut cysts. Am J Gastroenterol 1992;87: Kim YI, Kim WH. Inflammatory fibroid polyps of gastrointestinal tract. Evolution of histologic patterns. Am J Clin Pathol 1988; 89: Matsushita M, Hajiro K, Okazaki K, Takakuwa H. Gastric inflammatory fibroid polyps: endoscopic ultrasonographic analysis in comparison with the histology. Gastrointest Endosc 1997;46: Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol 1999;30: Meesters B, Pauwels PAA, Pijnenburg AM, Vlasveld LT, Repelaer van Driel OJ. Metastasis in a benign duodenal stromal tumour. Eur J Surg Oncol 1998;24: Trupiano JK, Stewart RE, Misick C, Appelman HD, Goldblum JR. Gastric stromal tumors: a clinicopathologic study of 77 cases with correlation of features with nonaggressive and aggressive clinical behaviors. Am J Surg Pathol 2002;26: Evans HL. Smooth muscle tumors of the gastrointestinal tract. A study of 56 cases followed for a minimum of 10 years. Cancer 1985;56: Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correction. Radiographics 2003;23: Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer 2005;103: Rindi G, Azzoni C, La Rosa S, Klersy C, Paolotti D, Rappel S, Stolte M, Capella C, Bordi C, Solcia E. ECL cell tumor and poorly differentiated endocrine carcinoma of the stomach: prognostic evaluation by pathological analysis. Gastroenterology 1999; 116: Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology 1993;104: Soga J. Gastric carcinoids: a statistical evaluation of 1,094 cases collected from the literature. Surg Today 1997;27: Nakamura S, Lida M, Yao T, Fujishima M. Endoscopic features of gastric carcinoids. Gastrointest Endosc 1991;37: Matsumoto T, Lida M, Suekane H, Tominaga M, Yao T, Fujishima M. Endoscopic ultrasonography in rectal carcinoid tumors: contribution to selection of therapy. Gastrointest Endosc 1991;37: Brooks JJ, Enterline HT. Primary gastric lymphomas. A clinicopathologic study of 58 cases with long-term follow-up and literature review. Cancer 1983;51: Lewin KJ, Ranchod M, Dorfman RF. Lymphomas of the gastrointestinal tract: a study of 117 cases presenting with gastrointestinal disease. Cancer 1978;42: Kolve M, Fischbach W, Greiner A, Wilms K. Differences in endoscopic and clinicopathological features of primary and secondary gastric non-hodgkin s lymphoma. German Gastrointestinal Lymphoma Study Group. Gastrointest Endosc 1999;49: Bolondi L, Casanova P, Caletti GC, Grigioni W, Zani L, Barbara L. Primary gastric lymphoma versus gastric carcinoma: endoscopic US evaluation. Radiology 1987;165: Tio TL, den Hartog Jager FC, Tijtgat GN. Endoscopic ultrasonography of non-hodgkin lymphoma of the stomach. Gastroenterology 1986;91: Vander Noot MR III, Eloubeidi MA, Chen VK, Eltoum I, Jhala D, Jhala N, Syed S, Chhieng DC. Diagnosis of gastrointestinal tract lesions by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer 2004;102: Miettinen M, Paal E, Lasota J, Sobin LH. Gastrointestinal glomus tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol 2002; 26: Tsuneyoshi M, Enjoji M. Glomus tumor: a clinicopathologic and electron microscopic study. Cancer 1982;50: Imamura A, Tochihara M, Natsui K, Murashima Y, Suga T, Yaosaka T, Fujinaga A, Koito K, Miyakawa H, Higashino K. Glomus tumor of the stomach: endoscopic ultrasonographic findings. Am J Gastroenterol 1994;89: Debol SM, Stanley MW, Mallery S, Sawinski E, Bardales RH. Glomus tumor of the stomach: cytologic diagnosis by endoscopic ultrasound-guided fine-needle aspiration. Diagn Cytopathol 2003;28: Panagiotou I, Brountzos EN, Bafaloukos D, Stoupis C, Brestas P, Kelekis DA. Malignant melanoma metastatic to the gastrointestinal tract. Melanoma Res 2002;12: Kadakia SC, Parker A, Canales L. Metastatic tumors to the upper gastrointestinal tract: endoscopic experience. Am J Gastroenterol 1992;87: Saunders NJ. Haematemesis due to gastric involvement by metastatic ovarian carcinoma 30 years after removal of the primary tumour. Br J Clin Pract 1986;40: Taylor RR, Phillips WS, O Connor DM, Harrison CR. Unusual intramural gastric metastasis of recurrent epithelial ovarian carcinoma. Gynecol Oncol 1994;55: Sangha S, Gergeos F, Freter P, Paiva L, Jacobson B. Diagnosis of ovarian cancer metastatic to the stomach by EUS-guided FNA. Gastrointest Endosc 2003;58: Taal BG, Peterse H, Boot H. Clinical presentation, endoscopic features, and treatment of gastric metastases from breast carcinoma. Cancer 2000;89: Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology 2005; 128: Kumashiro R, Naitoh H, Teshima K, Sakai T, Inutsuka S. Minute gastric carcinoid tumor with regional lymph node metastasis. Int Surg 1989;74; Gilligan CJ, Lawton GP, Tang LH, West AB, Modlin IM. Gastric carcinoid tumors: the biology and therapy of an enigmatic and controversial lesion. Am J Gastroenterol 1995;90: Hirschowitz BI, Griffith J, Pellegrin D, Cummings OW. Rapid regression of enterochromaffinlike cell gastric carcinoids in pernicious anemia after antrectomy. Gastroenterology 1992;102: Melzer E, Fidder H. The natural course of upper gastrointestinal submucosal tumors: an endoscopic ultrasound survey. Isr Med Assoc J 2000;2: Tsai TL, Changchien CS, Hu TH, Hsiaw CM. Demonstration of gastric submucosal lesions by high-resolution transabdominal sonography. J Clin Ultrasound 2000;28: Futagami K, Hata J, Haruma K, Yamashita N, Yoshida S, Tanaka S, Chayama K. Extracorporeal ultrasound is an effective diagnostic alternative to endoscopic ultrasound for gastric submucosal tumours. Scand J Gastroenterol 2001;36:
12 2228 AMERICAN GASTROENTEROLOGICAL ASSOCIATION INSTITUTE GASTROENTEROLOGY Vol. 130, No Polkowski M, Palucki J, Butruk E. Transabdominal ultrasound for visualizing gastric submucosal tumors diagnosed by endosonography: can surveillance be simplified? Endoscopy 2002;34: DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231: Choi YV, Oh ST. Laparoscopy in the management of gastric submucosal tumors. Surg Endosc 2005;14: Matthews BD, Walsh RM, Kercher KW, Sing RF, Pratt BL, Answini GA, Heniford BT. Laparoscopic vs open resection of gastric stromal tumors. Surg Endosc 2002;16: Hindmarsh A, Koo B, Lewis MP, Rhodes M. Laparoscopic resection of gastric gastrointestinal stromal tumors. Surg Endosc 2005;19: Tagaya N, Mikami H, Kogure H, Kubota K, Hosoya Y, Nagai H. Laparoscopic intragastric stapled resection of gastric submucosal tumors located near the esophagogastric junction. Surg Endosc 2002;16: Nguyen NT, Jim J, Nguyen A, Lee J, Chang K. Laparaoscopic resection of gastric stromal tumor: a tailored approach. Am Surg 2003;69: Basso N, Rosato P, De Leo A, Picconi T, Tretino P, Fantini A, Silecchia G. Laparoscopic treatment of gastric stromal tumors. Surg Endosc 2000;14: Buyske J, McDonald M, Fernandez C, Munson JL, Sanders LE, Tsao J, Birkett DH. Minimally invasive management of low-grade and benign gastric tumors. Surg Endosc 1997;11: Walsh RM, Ponsky J, Brody F, Matthews BD, Heniford BT. Combined endoscopic laparoscopic intragastric resection of gastric stromal tumors. J Gastrointest Surg 2003;7: Ludwig K, Wilhelm L, Scharlau U, Amtsberg G, Bernhardt J. Laparoscopic-endoscopic rendezvous resection of gastric tumors. Surg Endosc 2002;16: Takahashi H, Fujita R. Endoscopic diathermic resection of gastric submucosal tumors. Gastrointest Endosc 1991;33: Yu J, Luo HS, Wang XZ. Endoscopic treatment of submucosal lesions of the gastrointestinal tract. Endoscopy 1992;24: Kawamoto K, Yamada Y, Furukawa N, Utsonomiya T, Haraguchi Y, Mizuguchi M, Oiwa T, Takano H, Masuda K. Endoscopic submucosal tumorectomy for gastrointestinal submucosal tumors restricted to the submucosa: a new form of endoscopic minimal surgery. Gastrointest Endosc 1997;46: Chang K, Yoshinaka R, Nguyen P. Endoscopic ultrasound-assisted band ligation: a new technique for resection of submucosal tumors. Gastrointest Endosc 1996;44: Spinelli P, Carrai FG, Cambareri AR, Meroni E, Pizzetti P. Twostep endoscopic resection of gastric leiomyomas. Surg Endosc 1993;7: Sun S, Jin Y, Chang G, Wang C, Li X, Wang Z. Endoscopic band ligation without electrosurgery: a new technique for excision of small upper-gi leiomyoma. Gastrointest Endosc 2004;60: Park Y, Park S, Kim T, Song S, Choi E, Chung J, Kang J. Endoscopic enucleation of upper-gi submucosal tumors by using an insulated-tip electrosurgical knife. Gastrointest Endosc 2004;59: Rösch T, Sarbia M, Schumacher B, Deinert K, Frimberger E, Toermer T, Stolte M, Neuhaus H. Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulatedtip knives: a pilot series. Endoscopy 2004;36: Gunter E, Lingenfeiser T, Eitelbach F, Muller H, Ell C. EUSguided ethanol injection for treatment of a GI stromal tumor. Gastrointest Endosc 2003;57: Address requests for reprints to: Chair, Clinical Practice and Economics Committee, AGA Institute National Office, c/o Membership Department, 4930 Del Ray Avenue, Bethesda, Maryland Fax: (301) The Clinical Practice and Economics Committee acknowledges the following individuals whose critiques of this review paper provided valuable guidance to the authors: Manoop Bhutani, MD, Doug Faigel, MD, and Michael B. Wallace, MD.
Learning Luncheon 7: Endoscopic Mucosal Resection: When, Where and How?
 Endoscopic Mucosal Resection (EMR): When, Where, and Charles J. Lightdale, MD Columbia University New York, NY Endoscopic Mucosal Resection (EMR) EMR developed for removal of sessile or flat neoplasms
Endoscopic Mucosal Resection (EMR): When, Where, and Charles J. Lightdale, MD Columbia University New York, NY Endoscopic Mucosal Resection (EMR) EMR developed for removal of sessile or flat neoplasms
EMR Can anyone do this?
 EMR Can anyone do this? Norio Fukami, MD University of Colorado Piecemeal resection? 1 Endoscopic mucosal resection (EMR) and Endoscopic submucosal dissection (ESD) Endoscopic removal of premalignant or
EMR Can anyone do this? Norio Fukami, MD University of Colorado Piecemeal resection? 1 Endoscopic mucosal resection (EMR) and Endoscopic submucosal dissection (ESD) Endoscopic removal of premalignant or
WHAT S WRONG WITH MY GALL BLADDER? GALL BLADDER POLYPS
 WHAT S WRONG WITH MY GALL BLADDER? GALL BLADDER POLYPS This is a patient information booklet providing specific practical information about gall bladder polyps in brief. Its aim is to provide the patient
WHAT S WRONG WITH MY GALL BLADDER? GALL BLADDER POLYPS This is a patient information booklet providing specific practical information about gall bladder polyps in brief. Its aim is to provide the patient
How to report Upper GI EMR/ESD specimens
 Section of Pathology and Tumour Biology How to report Upper GI EMR/ESD specimens Dr.H.Grabsch Warning. Most of the criteria, methodologies, evidence presented in this talk are based on studies in early
Section of Pathology and Tumour Biology How to report Upper GI EMR/ESD specimens Dr.H.Grabsch Warning. Most of the criteria, methodologies, evidence presented in this talk are based on studies in early
Thursday, November 3, 2005
 Thursday, November 3, 2005 8:30-10:30 a. m. Gastric Tumors, Session 1 Chairman: P. Ruszniewski, Clichy, France 9:00-9:30 a. m. Working Group Sessions Pathology and Genetics Group leaders: G. Rindi, Parma,
Thursday, November 3, 2005 8:30-10:30 a. m. Gastric Tumors, Session 1 Chairman: P. Ruszniewski, Clichy, France 9:00-9:30 a. m. Working Group Sessions Pathology and Genetics Group leaders: G. Rindi, Parma,
Captivator EMR Device
 Device Clinical Article and Abstract Summary Endoscopic Mucosal Bergman et al: EMR Training Tips Bergman et al: EMR Learning Curve ASGE: EMR & ESD Guidelines Bergman et al: Captivator EMR vs Cook Duette
Device Clinical Article and Abstract Summary Endoscopic Mucosal Bergman et al: EMR Training Tips Bergman et al: EMR Learning Curve ASGE: EMR & ESD Guidelines Bergman et al: Captivator EMR vs Cook Duette
These parameters cannot, at the present time, be determined by non-invasive imaging techniques.
 Endoscopic Mucosal Resection for Upper Gastrointestinal Lesions Kenneth K. Wang, M.D. Chairman, WEO Publication and Guidelines Committee Professor of Medicine, Mayo Clinic Rochester, Minnesota Upper gastrointestinal
Endoscopic Mucosal Resection for Upper Gastrointestinal Lesions Kenneth K. Wang, M.D. Chairman, WEO Publication and Guidelines Committee Professor of Medicine, Mayo Clinic Rochester, Minnesota Upper gastrointestinal
The utility of endoscopic ultrasonography and endoscopy in the endoscopic mucosal resection of early gastric cancer
 Gut 1999;45:599 604 599 The utility of endoscopic ultrasonography and endoscopy in the endoscopic mucosal resection of early gastric cancer S Ohashi, K Segawa, S Okamura, M Mitake, H Urano, M Shimodaira,
Gut 1999;45:599 604 599 The utility of endoscopic ultrasonography and endoscopy in the endoscopic mucosal resection of early gastric cancer S Ohashi, K Segawa, S Okamura, M Mitake, H Urano, M Shimodaira,
Endoscopic Submucosal Dissection (E.S.D.) vs. Endoscopic Mucosal Resection (E.M.R.) in Colombia. Advocating E.M.R.
 Controversies in Gastroenterology Endoscopic Submucosal Dissection (E.S.D.) vs. Endoscopic Mucosal Resection (E.M.R.) in Colombia. Advocating E.M.R. Raúl Cañadas Garrido, MD. 1 1 Internist-Gastroenterologist.
Controversies in Gastroenterology Endoscopic Submucosal Dissection (E.S.D.) vs. Endoscopic Mucosal Resection (E.M.R.) in Colombia. Advocating E.M.R. Raúl Cañadas Garrido, MD. 1 1 Internist-Gastroenterologist.
Evolution of Barrett s esophagus
 Endoscopic Treatment and Surveillance of Esophageal Cancer: GI Perspective Charles J. Lightdale, MD Columbia University New York, NY Evolution of Barrett s esophagus Squamous esophagus Chronic inflammation
Endoscopic Treatment and Surveillance of Esophageal Cancer: GI Perspective Charles J. Lightdale, MD Columbia University New York, NY Evolution of Barrett s esophagus Squamous esophagus Chronic inflammation
Kidney Cancer OVERVIEW
 Kidney Cancer OVERVIEW Kidney cancer is the third most common genitourinary cancer in adults. There are approximately 54,000 new cancer cases each year in the United States, and the incidence of kidney
Kidney Cancer OVERVIEW Kidney cancer is the third most common genitourinary cancer in adults. There are approximately 54,000 new cancer cases each year in the United States, and the incidence of kidney
Hyung Hun Kim, 1 Gwang Ha Kim, 2 Ji Hyun Kim, 3 Myung-Gyu Choi, 1 Geun Am Song, 2 and Sung Eun Kim 4. 1. Introduction
 Gastroenterology Research and Practice, Article ID 253860, 7 pages http://dx.doi.org/10.1155/2014/253860 Clinical Study The Efficacy of Endoscopic Submucosal Dissection of Type I Gastric Carcinoid Tumors
Gastroenterology Research and Practice, Article ID 253860, 7 pages http://dx.doi.org/10.1155/2014/253860 Clinical Study The Efficacy of Endoscopic Submucosal Dissection of Type I Gastric Carcinoid Tumors
Billing Guideline. Subject: Colorectal Cancer Screening Exams (Invasive Procedures) Effective Date: 1/1/2012 Last Update Effective: 4/16
 Subject: Colorectal Cancer Screening Exams (Invasive Procedures) Effective Date: 1/1/2012 Last Update Effective: 4/16 Billing Guideline Background Health First administers benefit packages with full coverage
Subject: Colorectal Cancer Screening Exams (Invasive Procedures) Effective Date: 1/1/2012 Last Update Effective: 4/16 Billing Guideline Background Health First administers benefit packages with full coverage
Cystic Neoplasms of the Pancreas: A multidisciplinary approach to the prevention and early detection of invasive pancreatic cancer.
 This lecture is drawn from the continuing medical education program Finding Hope: Prevention, Early Detection and Treatment of Pancreatic Cancer, Nov, 2011. Robert P. Jury, MD Cystic Neoplasms of the Pancreas:
This lecture is drawn from the continuing medical education program Finding Hope: Prevention, Early Detection and Treatment of Pancreatic Cancer, Nov, 2011. Robert P. Jury, MD Cystic Neoplasms of the Pancreas:
OBJECTIVES By the end of this segment, the community participant will be able to:
 Cancer 101: Cancer Diagnosis and Staging Linda U. Krebs, RN, PhD, AOCN, FAAN OCEAN Native Navigators and the Cancer Continuum (NNACC) (NCMHD R24MD002811) Cancer 101: Diagnosis & Staging (Watanabe-Galloway
Cancer 101: Cancer Diagnosis and Staging Linda U. Krebs, RN, PhD, AOCN, FAAN OCEAN Native Navigators and the Cancer Continuum (NNACC) (NCMHD R24MD002811) Cancer 101: Diagnosis & Staging (Watanabe-Galloway
Endoscopic Resection for Barrett s Esophagus and Early Cancer 2014 Masters of Minimally Invasive Surgery
 Endoscopic Resection for Barrett s Esophagus and Early Cancer 2014 Masters of Minimally Invasive Surgery Matthew Hartwig, M.D. Duke Cancer Institute Case Presentation: Patient ER 51 y/o man with schizophrenia
Endoscopic Resection for Barrett s Esophagus and Early Cancer 2014 Masters of Minimally Invasive Surgery Matthew Hartwig, M.D. Duke Cancer Institute Case Presentation: Patient ER 51 y/o man with schizophrenia
PROTOCOL OF THE RITA DATA QUALITY STUDY
 PROTOCOL OF THE RITA DATA QUALITY STUDY INTRODUCTION The RITA project is aimed at estimating the burden of rare malignant tumours in Italy using the population based cancer registries (CRs) data. One of
PROTOCOL OF THE RITA DATA QUALITY STUDY INTRODUCTION The RITA project is aimed at estimating the burden of rare malignant tumours in Italy using the population based cancer registries (CRs) data. One of
Endoscopy and infection: Prevention of infection during endoscopy Treatment of infection by endoscopy. M. Arvanitakis SRBG June 2009
 Endoscopy and infection: Prevention of infection during endoscopy Treatment of infection by endoscopy M. Arvanitakis SRBG June 2009 Outline Antibiotic prophylaxis during endoscopy Upper GI endoscopy Lower
Endoscopy and infection: Prevention of infection during endoscopy Treatment of infection by endoscopy M. Arvanitakis SRBG June 2009 Outline Antibiotic prophylaxis during endoscopy Upper GI endoscopy Lower
ESD for colorectal lesions I am in favour. Alessandro Repici, MD Digestive Endoscopy Unit IRCCS Istituto Clinico Humanitas Milano, Italy
 ESD for colorectal lesions I am in favour Alessandro Repici, MD Digestive Endoscopy Unit IRCCS Istituto Clinico Humanitas Milano, Italy Surgery for early colonic lesions 51 pts referred for lap colectomy
ESD for colorectal lesions I am in favour Alessandro Repici, MD Digestive Endoscopy Unit IRCCS Istituto Clinico Humanitas Milano, Italy Surgery for early colonic lesions 51 pts referred for lap colectomy
Neoplasia gastrica cistica: GIST o leiomiosarcoma? Sebastiano Cacciaguerra U. O. Chirurgia Pediatrica Ospedale Garibaldi Catania
 Neoplasia gastrica cistica: GIST o leiomiosarcoma? Sebastiano Cacciaguerra U. O. Chirurgia Pediatrica Ospedale Garibaldi Neoplasia gastrica cistica: GIST o leiomiosarcoma? Aims of presentation Atypical
Neoplasia gastrica cistica: GIST o leiomiosarcoma? Sebastiano Cacciaguerra U. O. Chirurgia Pediatrica Ospedale Garibaldi Neoplasia gastrica cistica: GIST o leiomiosarcoma? Aims of presentation Atypical
CHAPTER 2. Neoplasms (C00-D49) March 2014. 2014 MVP Health Care, Inc.
 Neoplasms (C00-D49) March 2014 2014 MVP Health Care, Inc. CHAPTER SPECIFIC CATEGORY CODE BLOCKS C00-C14 Malignant neoplasms of lip, oral cavity and pharynx C15-C26 Malignant neoplasms of digestive organs
Neoplasms (C00-D49) March 2014 2014 MVP Health Care, Inc. CHAPTER SPECIFIC CATEGORY CODE BLOCKS C00-C14 Malignant neoplasms of lip, oral cavity and pharynx C15-C26 Malignant neoplasms of digestive organs
Endoscopic mucosal resection for treatment of early gastric cancer
 Gut 2001;48:225 229 225 Endoscopic mucosal resection for treatment of early gastric cancer H Ono, H Kondo, T Gotoda, K Shirao, H Yamaguchi, D Saito, K Hosokawa, T Shimoda, S Yoshida Department of Endoscopy
Gut 2001;48:225 229 225 Endoscopic mucosal resection for treatment of early gastric cancer H Ono, H Kondo, T Gotoda, K Shirao, H Yamaguchi, D Saito, K Hosokawa, T Shimoda, S Yoshida Department of Endoscopy
Center for Endoscopic Research & Therapeutics
 Center for Endoscopic Research & Therapeutics 5758 South Maryland Avenue (MC9028) Chicago, Illinois 60637 (773) 702-1459 www.uchospitals.edu Center for Endoscopic Research & Therapeutics To refer a patient
Center for Endoscopic Research & Therapeutics 5758 South Maryland Avenue (MC9028) Chicago, Illinois 60637 (773) 702-1459 www.uchospitals.edu Center for Endoscopic Research & Therapeutics To refer a patient
Diagnosis and Prognosis of Pancreatic Cancer
 Main Page Risk Factors Reducing Your Risk Screening Symptoms Diagnosis Treatment Overview Chemotherapy Radiation Therapy Surgical Procedures Lifestyle Changes Managing Side Effects Talking to Your Doctor
Main Page Risk Factors Reducing Your Risk Screening Symptoms Diagnosis Treatment Overview Chemotherapy Radiation Therapy Surgical Procedures Lifestyle Changes Managing Side Effects Talking to Your Doctor
Lenox Hill Hospital Department of Surgery General Surgery Goals and Objectives
 Lenox Hill Hospital Department of Surgery General Surgery Goals and Objectives Medical Knowledge and Patient Care: Residents must demonstrate knowledge and application of the pathophysiology and epidemiology
Lenox Hill Hospital Department of Surgery General Surgery Goals and Objectives Medical Knowledge and Patient Care: Residents must demonstrate knowledge and application of the pathophysiology and epidemiology
Cancer of the Cardia/GE Junction: Surgical Options
 Cancer of the Cardia/GE Junction: Surgical Options Michael A Smith, MD Associate Chief Thoracic Surgery Center for Thoracic Disease St Joseph s Hospital and Medical Center Phoenix, AZ Michael Smith, MD
Cancer of the Cardia/GE Junction: Surgical Options Michael A Smith, MD Associate Chief Thoracic Surgery Center for Thoracic Disease St Joseph s Hospital and Medical Center Phoenix, AZ Michael Smith, MD
Pancreatic masses: What is there besides cancer
 Pancreatic masses: What is there besides cancer Poster No.: C-0201 Congress: ECR 2010 Type: Educational Exhibit Topic: Abdominal Viscera (Solid Organs) Authors: M. A. Portilha, C. Ruivo, I. Santiago, M.
Pancreatic masses: What is there besides cancer Poster No.: C-0201 Congress: ECR 2010 Type: Educational Exhibit Topic: Abdominal Viscera (Solid Organs) Authors: M. A. Portilha, C. Ruivo, I. Santiago, M.
Rotation Specific Goals & Objectives: University Health Network-Princess Margaret Hospital/ Sunnybrook Breast/Melanoma
 Rotation Specific Goals & Objectives: University Health Network-Princess Margaret Hospital/ Sunnybrook Breast/Melanoma Medical Expert: Breast Rotation Specific Competencies/Objectives 1.0 Medical History
Rotation Specific Goals & Objectives: University Health Network-Princess Margaret Hospital/ Sunnybrook Breast/Melanoma Medical Expert: Breast Rotation Specific Competencies/Objectives 1.0 Medical History
Challenges in gastric, appendiceal and rectal NETs Leuven, 29.11.2014
 Challenges in gastric, appendiceal and rectal NETs Leuven, 29.11.2014 Prof. Dr. Chris Verslype, Leuven Prof. Dr. Aurel Perren, Bern Menue Challenges: 1. Gastric NET 2. Appendiceal NET 3. Rectal NET SEER,
Challenges in gastric, appendiceal and rectal NETs Leuven, 29.11.2014 Prof. Dr. Chris Verslype, Leuven Prof. Dr. Aurel Perren, Bern Menue Challenges: 1. Gastric NET 2. Appendiceal NET 3. Rectal NET SEER,
The digestive system. Medicine and technology. Normal structure and function Diagnostic methods Example diseases and therapies
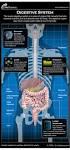 The digestive system Medicine and technology Normal structure and function Diagnostic methods Example diseases and therapies The digestive system An overview (1) Oesophagus Liver (hepar) Biliary system
The digestive system Medicine and technology Normal structure and function Diagnostic methods Example diseases and therapies The digestive system An overview (1) Oesophagus Liver (hepar) Biliary system
LYMPHOMA IN DOGS. Diagnosis/Initial evaluation. Treatment and Prognosis
 LYMPHOMA IN DOGS Lymphoma is a relatively common cancer in dogs. It is a cancer of lymphocytes (a type of white blood cell) and lymphoid tissues. Lymphoid tissue is normally present in many places in the
LYMPHOMA IN DOGS Lymphoma is a relatively common cancer in dogs. It is a cancer of lymphocytes (a type of white blood cell) and lymphoid tissues. Lymphoid tissue is normally present in many places in the
Breast Ultrasound: Benign vs. Malignant Lesions
 October 25-November 19, 2004 Breast Ultrasound: Benign vs. Malignant Lesions Jill Steinkeler,, Tufts University School of Medicine IV Breast Anatomy Case Presentation-Patient 1 62 year old woman with a
October 25-November 19, 2004 Breast Ultrasound: Benign vs. Malignant Lesions Jill Steinkeler,, Tufts University School of Medicine IV Breast Anatomy Case Presentation-Patient 1 62 year old woman with a
How to treat early gastric cancer. Surgery
 How to treat early gastric cancer Surgery Mark I. van Berge Henegouwen Department of Surgery, AMC, Amsterdam Director upper GI surgical unit Academic Medical Center Upper GI surgery at AMC 100 oesophagectomies
How to treat early gastric cancer Surgery Mark I. van Berge Henegouwen Department of Surgery, AMC, Amsterdam Director upper GI surgical unit Academic Medical Center Upper GI surgery at AMC 100 oesophagectomies
CASE OF THE MONTH AUGUST-2015 DR. GURUDUTT GUPTA HEAD HISTOPATHOLOGY
 CASE OF THE MONTH AUGUST-2015 DR. GURUDUTT GUPTA HEAD HISTOPATHOLOGY CASE HISTORY 52Y MALE RIGHT RADICAL NEPHERECTOMY Case of right renal mass with IVC thrombus. History of surgery and RT for right occipital
CASE OF THE MONTH AUGUST-2015 DR. GURUDUTT GUPTA HEAD HISTOPATHOLOGY CASE HISTORY 52Y MALE RIGHT RADICAL NEPHERECTOMY Case of right renal mass with IVC thrombus. History of surgery and RT for right occipital
Renal Cysts What should I do now?
 Renal Cysts What should I do now? Dr Edmund Chiong Asst. Professor & Consultant Department of Urology National University Hospital What are renal cysts? Fluid-filled structures in the kidney that are not
Renal Cysts What should I do now? Dr Edmund Chiong Asst. Professor & Consultant Department of Urology National University Hospital What are renal cysts? Fluid-filled structures in the kidney that are not
Case Report Concurrent Esophageal Dysplasia and Leiomyoma
 Case Reports in Gastrointestinal Medicine, Article ID 804175, 5 pages http://dx.doi.org/10.1155/2014/804175 Case Report Concurrent Esophageal Dysplasia and Leiomyoma Asim Shuja 1 and Khalid A. Alkimawi
Case Reports in Gastrointestinal Medicine, Article ID 804175, 5 pages http://dx.doi.org/10.1155/2014/804175 Case Report Concurrent Esophageal Dysplasia and Leiomyoma Asim Shuja 1 and Khalid A. Alkimawi
THYROID CANCER. I. Introduction
 THYROID CANCER I. Introduction There are over 11,000 new cases of thyroid cancer each year in the US. Females are more likely to have thyroid cancer than men by a ratio of 3:1, and it is more common in
THYROID CANCER I. Introduction There are over 11,000 new cases of thyroid cancer each year in the US. Females are more likely to have thyroid cancer than men by a ratio of 3:1, and it is more common in
Characterization of small renal lesions: Problem solving with MRI Gary Israel, MD
 Characterization of small renal lesions: Problem solving with MRI Gary Israel, MD With the widespread use of cross-sectional imaging, many renal masses are incidentally found. These need to be accurately
Characterization of small renal lesions: Problem solving with MRI Gary Israel, MD With the widespread use of cross-sectional imaging, many renal masses are incidentally found. These need to be accurately
LIVER CANCER AND TUMOURS
 LIVER CANCER AND TUMOURS LIVER CANCER AND TUMOURS Healthy Liver Cirrhotic Liver Tumour What causes liver cancer? Many factors may play a role in the development of cancer. Because the liver filters blood
LIVER CANCER AND TUMOURS LIVER CANCER AND TUMOURS Healthy Liver Cirrhotic Liver Tumour What causes liver cancer? Many factors may play a role in the development of cancer. Because the liver filters blood
Colocutaneous Fistula. Disclosures
 Colocutaneous Fistula Madhulika G. Varma MD Associate Professor Chief, Colorectal Surgery University of California, San Francisco Honoraria Applied Medical Covidien Disclosures 1 Colocutaneous Fistula
Colocutaneous Fistula Madhulika G. Varma MD Associate Professor Chief, Colorectal Surgery University of California, San Francisco Honoraria Applied Medical Covidien Disclosures 1 Colocutaneous Fistula
Bridging Techniques. What s between EMR and Traditional Surgery? Elisabeth C. McLemore, MD, FACS, FASCRS
 Bridging Techniques What s between EMR and Traditional Surgery? Elisabeth C. McLemore, MD, FACS, FASCRS Associate Professor of Surgery Assistant Program Director, General Surgery Residency Disclosures
Bridging Techniques What s between EMR and Traditional Surgery? Elisabeth C. McLemore, MD, FACS, FASCRS Associate Professor of Surgery Assistant Program Director, General Surgery Residency Disclosures
Post-DDW OAG Course - Therapeutic Endoscopy
 Post-DDW OAG Course - Therapeutic Endoscopy June 13, 2015 Jeffrey Mosko Division of Gastroenterology St. Michael's Hospital University of Toronto moskoj@smh.ca Program Name: Post-DDW OAG course CanMEDS
Post-DDW OAG Course - Therapeutic Endoscopy June 13, 2015 Jeffrey Mosko Division of Gastroenterology St. Michael's Hospital University of Toronto moskoj@smh.ca Program Name: Post-DDW OAG course CanMEDS
Gastrointestinal Bleeding
 Gastrointestinal Bleeding Introduction Gastrointestinal bleeding is a symptom of many diseases rather than a disease itself. A number of different conditions can cause gastrointestinal bleeding. Some causes
Gastrointestinal Bleeding Introduction Gastrointestinal bleeding is a symptom of many diseases rather than a disease itself. A number of different conditions can cause gastrointestinal bleeding. Some causes
Something Old, Something New.
 Something Old, Something New. Michelle A. Fajardo, D.O. Loma Linda University Medical Center Clinical Presentation 6 year old boy, presented with hematuria Renal mass demonstrated by ultrasound & CT scan
Something Old, Something New. Michelle A. Fajardo, D.O. Loma Linda University Medical Center Clinical Presentation 6 year old boy, presented with hematuria Renal mass demonstrated by ultrasound & CT scan
Objectives. Mylene T. Truong, MD. Malignant Pleural Mesothelioma Background
 Imaging of Pleural Tumors Mylene T. Truong, MD Imaging of Pleural Tumours Mylene T. Truong, M. D. University of Texas M.D. Anderson Cancer Center, Houston, TX Objectives To review tumors involving the
Imaging of Pleural Tumors Mylene T. Truong, MD Imaging of Pleural Tumours Mylene T. Truong, M. D. University of Texas M.D. Anderson Cancer Center, Houston, TX Objectives To review tumors involving the
What is Barrett s esophagus? How does Barrett s esophagus develop?
 Barrett s Esophagus What is Barrett s esophagus? Barrett s esophagus is a pre-cancerous condition affecting the lining of the esophagus, the swallowing tube that carries foods and liquids from the mouth
Barrett s Esophagus What is Barrett s esophagus? Barrett s esophagus is a pre-cancerous condition affecting the lining of the esophagus, the swallowing tube that carries foods and liquids from the mouth
AORTOENTERIC FISTULA. Mark H. Tseng MD Brooklyn VA Hospital February 11, 2005
 AORTOENTERIC FISTULA Mark H. Tseng MD Brooklyn VA Hospital February 11, 2005 AORTOENTERIC FISTULA diagnosis and management Mark H. Tseng MD Brooklyn VA Hospital February 11, 2005 AORTOENTERIC FISTULA Aortoenteric
AORTOENTERIC FISTULA Mark H. Tseng MD Brooklyn VA Hospital February 11, 2005 AORTOENTERIC FISTULA diagnosis and management Mark H. Tseng MD Brooklyn VA Hospital February 11, 2005 AORTOENTERIC FISTULA Aortoenteric
Guideline for the Imaging of Patients Presenting with Breast Symptoms incorporating the guideline for the use of MRI in breast cancer
 Guideline for the Imaging of Patients Presenting with Breast Symptoms incorporating the guideline for the use of MRI in breast cancer Version History Version Date Summary of Change/Process 0.1 09.01.11
Guideline for the Imaging of Patients Presenting with Breast Symptoms incorporating the guideline for the use of MRI in breast cancer Version History Version Date Summary of Change/Process 0.1 09.01.11
Format for ANSWERING REVIEWERS
 Format for ANSWERING REVIEWERS July 15, 2015 Dear Editor, Please find enclosed the edited manuscript in Word format (file name: 19935-revised manuscript). Title: Management and associated factors of delayed
Format for ANSWERING REVIEWERS July 15, 2015 Dear Editor, Please find enclosed the edited manuscript in Word format (file name: 19935-revised manuscript). Title: Management and associated factors of delayed
Immunohistochemistry of soft tissue tumors
 Immunohistochemistry of soft tissue tumors Immunohistochemistry Major advances : antigen retrieval techniques (HIER) sensitive detection systems numerous antibodies of good quality Standardization : automated
Immunohistochemistry of soft tissue tumors Immunohistochemistry Major advances : antigen retrieval techniques (HIER) sensitive detection systems numerous antibodies of good quality Standardization : automated
Gynecology Abnormal Pelvic Anatomy and Physiology: Cervix. Cervix. Nabothian cysts. cervical polyps. leiomyomas. Cervical stenosis
 Gynecology Abnormal Pelvic Anatomy and Physiology: (Effective February 2007) pediatric, reproductive, and perimenopausal/postmenopausal (24-28 %) Cervix Nabothian cysts result from chronic cervicitis most
Gynecology Abnormal Pelvic Anatomy and Physiology: (Effective February 2007) pediatric, reproductive, and perimenopausal/postmenopausal (24-28 %) Cervix Nabothian cysts result from chronic cervicitis most
Ultrasonography of the Adrenal Glands CVM 6105 Kari L. Anderson, DVM, Diplomate ACVR Associate Clinical Professor of Veterinary Radiology
 1: US of adrenal glands, KLA Ultrasonography of the Adrenal Glands CVM 6105 Kari L. Anderson, DVM, Diplomate ACVR Associate Clinical Professor of Veterinary Radiology Ultrasound has quickly become an important
1: US of adrenal glands, KLA Ultrasonography of the Adrenal Glands CVM 6105 Kari L. Anderson, DVM, Diplomate ACVR Associate Clinical Professor of Veterinary Radiology Ultrasound has quickly become an important
Introduction: Tumor Swelling / new growth / mass. Two types of growth disorders: Non-Neoplastic. Secondary / adaptation due to other cause.
 Disorders of Growth Introduction: Tumor Swelling / new growth / mass Two types of growth disorders: Non-Neoplastic Secondary / adaptation due to other cause. Neoplastic. Primary growth abnormality. Non-Neoplastic
Disorders of Growth Introduction: Tumor Swelling / new growth / mass Two types of growth disorders: Non-Neoplastic Secondary / adaptation due to other cause. Neoplastic. Primary growth abnormality. Non-Neoplastic
By Anne C. Travis, M.D., M.Sc. and John R. Saltzman, M.D., FACG Brigham and Women's Hospital Harvard Medical School Boston, MA
 SMALL BOWEL BLEEDING: CAUSES, DIAGNOSIS AND TREATMENT By Anne C. Travis, M.D., M.Sc. and John R. Saltzman, M.D., FACG Brigham and Women's Hospital Harvard Medical School Boston, MA 1. What is the small
SMALL BOWEL BLEEDING: CAUSES, DIAGNOSIS AND TREATMENT By Anne C. Travis, M.D., M.Sc. and John R. Saltzman, M.D., FACG Brigham and Women's Hospital Harvard Medical School Boston, MA 1. What is the small
Breast Cancer: from bedside and grossing room to diagnoses and beyond. Adriana Corben, M.D.
 Breast Cancer: from bedside and grossing room to diagnoses and beyond Adriana Corben, M.D. About breast anatomy Breasts are special organs that develop in women during puberty when female hormones are
Breast Cancer: from bedside and grossing room to diagnoses and beyond Adriana Corben, M.D. About breast anatomy Breasts are special organs that develop in women during puberty when female hormones are
Primary -Benign - Malignant Secondary
 TUMOURS OF THE LUNG Primary -Benign - Malignant Secondary The incidence of lung cancer has been increasing almost logarithmically and is now reaching epidemic levels. The overall cure rate is very low
TUMOURS OF THE LUNG Primary -Benign - Malignant Secondary The incidence of lung cancer has been increasing almost logarithmically and is now reaching epidemic levels. The overall cure rate is very low
BAISHIDENG PUBLISHING GROUP INC
 Reviewer s code: 01714224 Reviewer s country: Italy Date reviewed: 2015-01-30 20:36 [ Y] Grade A: Priority publishing [ ] Accept [ ] Grade C: Good [ Y] Grade D: Fair language [ Y] Major revision The article
Reviewer s code: 01714224 Reviewer s country: Italy Date reviewed: 2015-01-30 20:36 [ Y] Grade A: Priority publishing [ ] Accept [ ] Grade C: Good [ Y] Grade D: Fair language [ Y] Major revision The article
Use of stents in esophageal cancer" Hans Gerdes, M.D. Director, GI Endoscopy Unit Memorial Sloan-Kettering Cancer Center
 Use of stents in esophageal cancer" Hans Gerdes, M.D. Director, GI Endoscopy Unit Memorial Sloan-Kettering Cancer Center Features of esophageal cancer Esophageal cancer is an abnormal growth that arises
Use of stents in esophageal cancer" Hans Gerdes, M.D. Director, GI Endoscopy Unit Memorial Sloan-Kettering Cancer Center Features of esophageal cancer Esophageal cancer is an abnormal growth that arises
Recommendations for cross-sectional imaging in cancer management, Second edition
 www.rcr.ac.uk Recommendations for cross-sectional imaging in cancer management, Second edition Breast cancer Faculty of Clinical Radiology www.rcr.ac.uk Contents Breast cancer 2 Clinical background 2 Who
www.rcr.ac.uk Recommendations for cross-sectional imaging in cancer management, Second edition Breast cancer Faculty of Clinical Radiology www.rcr.ac.uk Contents Breast cancer 2 Clinical background 2 Who
What to Expect from Intestinal Ultrasonography
 261) What to Expect from Intestinal Ultrasonography Červenková J., Steyerová P. Charles University in Prague, First Faculty of Medicine and General Teaching Hospital, Department of Radiology, Prague, Czech
261) What to Expect from Intestinal Ultrasonography Červenková J., Steyerová P. Charles University in Prague, First Faculty of Medicine and General Teaching Hospital, Department of Radiology, Prague, Czech
General Rules SEER Summary Stage 2000. Objectives. What is Staging? 5/8/2014
 General Rules SEER Summary Stage 2000 Linda Mulvihill Public Health Advisor NCRA Annual Meeting May 2014 National Center for Chronic Disease Prevention and Health Promotion Division of Cancer Prevention
General Rules SEER Summary Stage 2000 Linda Mulvihill Public Health Advisor NCRA Annual Meeting May 2014 National Center for Chronic Disease Prevention and Health Promotion Division of Cancer Prevention
Metastatic Cervical Cancer s/p Radiation Therapy, Radical Hysterectomy and Attempted Modified Internal Hemipelvectomy
 Metastatic Cervical Cancer s/p Radiation Therapy, Radical Hysterectomy and Attempted Modified Internal Hemipelvectomy Sarah Hutto,, MSIV Marc Underhill, M.D. January 27, 2009 Past History 45 yo female
Metastatic Cervical Cancer s/p Radiation Therapy, Radical Hysterectomy and Attempted Modified Internal Hemipelvectomy Sarah Hutto,, MSIV Marc Underhill, M.D. January 27, 2009 Past History 45 yo female
SUNY DOWNSTATE MEDICAL CENTER SURGERY GRAND ROUNDS February 28, 2013 VERENA LIU, MD ROSEANNA LEE, MD
 SUNY DOWNSTATE MEDICAL CENTER SURGERY GRAND ROUNDS February 28, 2013 VERENA LIU, MD ROSEANNA LEE, MD Case Presentation 35 year old male referred from PMD with an asymptomatic palpable right neck mass PMH/PSH:
SUNY DOWNSTATE MEDICAL CENTER SURGERY GRAND ROUNDS February 28, 2013 VERENA LIU, MD ROSEANNA LEE, MD Case Presentation 35 year old male referred from PMD with an asymptomatic palpable right neck mass PMH/PSH:
The Diagnosis of Cancer in the Pathology Laboratory
 The Diagnosis of Cancer in the Pathology Laboratory Dr Edward Sheffield Christmas Select 74 Meeting, Queen s Hotel Cheltenham, 3 rd December 2014 Agenda Overview of the pathology of cancer How specimens
The Diagnosis of Cancer in the Pathology Laboratory Dr Edward Sheffield Christmas Select 74 Meeting, Queen s Hotel Cheltenham, 3 rd December 2014 Agenda Overview of the pathology of cancer How specimens
Current Status of Esophageal Cancer Treatment
 Cancer Current Status of Esophageal Cancer Treatment JMAJ 46(11): 497 503, 2003 Hiroyasu MAKUUCHI Professor and Chairman, Department of Surgery, Tokai University School of Medicine Abstract: The diagnosis
Cancer Current Status of Esophageal Cancer Treatment JMAJ 46(11): 497 503, 2003 Hiroyasu MAKUUCHI Professor and Chairman, Department of Surgery, Tokai University School of Medicine Abstract: The diagnosis
Metastatic Renal Cell Carcinoma: Staging and Prognosis of Three Separate Cases.
 Metastatic Renal Cell Carcinoma: Staging and Prognosis of Three Separate Cases. Abstract This paper describes the staging, imaging, treatment, and prognosis of renal cell carcinoma. Three case studies
Metastatic Renal Cell Carcinoma: Staging and Prognosis of Three Separate Cases. Abstract This paper describes the staging, imaging, treatment, and prognosis of renal cell carcinoma. Three case studies
D. FREQUENTLY ASKED QUESTIONS
 ACR BI-RADS ATLAS D. FREQUENTLY ASKED QUESTIONS 1. Under MQSA, is it necessary to include a numeric assessment code (i.e., 0, 1, 2, 3, 4, 5, or 6) in addition to the assessment category in all mammography
ACR BI-RADS ATLAS D. FREQUENTLY ASKED QUESTIONS 1. Under MQSA, is it necessary to include a numeric assessment code (i.e., 0, 1, 2, 3, 4, 5, or 6) in addition to the assessment category in all mammography
A912: Kidney, Renal cell carcinoma
 A912: Kidney, Renal cell carcinoma General facts of kidney cancer Renal cell carcinoma, a form of kidney cancer that involves cancerous changes in the cells of the renal tubule, is the most common type
A912: Kidney, Renal cell carcinoma General facts of kidney cancer Renal cell carcinoma, a form of kidney cancer that involves cancerous changes in the cells of the renal tubule, is the most common type
Detection and staging of recurrent prostate cancer is still one of the important clinical problems in prostate cancer. A rise in PSA or biochemical
 Summary. 111 Detection and staging of recurrent prostate cancer is still one of the important clinical problems in prostate cancer. A rise in PSA or biochemical recurrence (BCR) is the first sign of recurrent
Summary. 111 Detection and staging of recurrent prostate cancer is still one of the important clinical problems in prostate cancer. A rise in PSA or biochemical recurrence (BCR) is the first sign of recurrent
Gastrointestinal Stromal Tumor (GIST) What is cancer?
 Gastrointestinal Stromal Tumor (GIST) What is cancer? The body is made up of trillions of living cells. Normal body cells grow, divide to make new cells, and die in an orderly way. During the early years
Gastrointestinal Stromal Tumor (GIST) What is cancer? The body is made up of trillions of living cells. Normal body cells grow, divide to make new cells, and die in an orderly way. During the early years
Report series: General cancer information
 Fighting cancer with information Report series: General cancer information Eastern Cancer Registration and Information Centre ECRIC report series: General cancer information Cancer is a general term for
Fighting cancer with information Report series: General cancer information Eastern Cancer Registration and Information Centre ECRIC report series: General cancer information Cancer is a general term for
Evaluation and Management of the Breast Mass. Gary Dunnington,, M.D. Department of Surgery Internal Medicine Ambulatory Conference December 4, 2003
 Evaluation and Management of the Breast Mass Gary Dunnington,, M.D. Department of Surgery Internal Medicine Ambulatory Conference December 4, 2003 Common Presentations of Breast Disease Breast Mass Abnormal
Evaluation and Management of the Breast Mass Gary Dunnington,, M.D. Department of Surgery Internal Medicine Ambulatory Conference December 4, 2003 Common Presentations of Breast Disease Breast Mass Abnormal
Understanding. Pancreatic Cancer
 Understanding Pancreatic Cancer Understanding Pancreatic Cancer The Pancreas The pancreas is an organ that is about 6 inches long. It s located deep in your belly between your stomach and backbone. Your
Understanding Pancreatic Cancer Understanding Pancreatic Cancer The Pancreas The pancreas is an organ that is about 6 inches long. It s located deep in your belly between your stomach and backbone. Your
Colorectal cancer. A guide for journalists on colorectal cancer and its treatment
 Colorectal cancer A guide for journalists on colorectal cancer and its treatment Contents Contents 2 3 Section 1: Colorectal cancer 4 i. What is colorectal cancer? 4 ii. Causes and risk factors 4 iii.
Colorectal cancer A guide for journalists on colorectal cancer and its treatment Contents Contents 2 3 Section 1: Colorectal cancer 4 i. What is colorectal cancer? 4 ii. Causes and risk factors 4 iii.
Endoscopic Management of Barrett s High-Grade Dysplasia and Early Stage Esophageal Cancer
 VOLUME 10, ISSUE 2, YEAR 2011 Endoscopic Management of Barrett s High-Grade Dysplasia and Early Stage Esophageal Cancer James L. Wise, MD Duluth, MN. Introduction: In recent years there has been intense
VOLUME 10, ISSUE 2, YEAR 2011 Endoscopic Management of Barrett s High-Grade Dysplasia and Early Stage Esophageal Cancer James L. Wise, MD Duluth, MN. Introduction: In recent years there has been intense
Tumour Markers. What are Tumour Markers? How Are Tumour Markers Used?
 Dr. Anthony C.H. YING What are? Tumour markers are substances that can be found in the body when cancer is present. They are usually found in the blood or urine. They can be products of cancer cells or
Dr. Anthony C.H. YING What are? Tumour markers are substances that can be found in the body when cancer is present. They are usually found in the blood or urine. They can be products of cancer cells or
Cancer of the Cervix
 Cancer of the Cervix WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 A woman's cervix (the opening of the uterus) is lined with cells. Cancer of the cervix occurs when those cells change,
Cancer of the Cervix WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 A woman's cervix (the opening of the uterus) is lined with cells. Cancer of the cervix occurs when those cells change,
The Whipple Procedure. Sally Hodges, Ph.D.(c) Given the length and difficulty of the procedure, regardless of the diagnosis, certain
 The Whipple Procedure Sally Hodges, Ph.D.(c) Preoperative procedures Given the length and difficulty of the procedure, regardless of the diagnosis, certain assurances must occur prior to offering a patient
The Whipple Procedure Sally Hodges, Ph.D.(c) Preoperative procedures Given the length and difficulty of the procedure, regardless of the diagnosis, certain assurances must occur prior to offering a patient
Frequently Asked Questions About Ovarian Cancer
 Media Contact: Gerri Gomez Howard Cell: 303-748-3933 gerri@gomezhowardgroup.com Frequently Asked Questions About Ovarian Cancer What is ovarian cancer? Ovarian cancer is a cancer that forms in tissues
Media Contact: Gerri Gomez Howard Cell: 303-748-3933 gerri@gomezhowardgroup.com Frequently Asked Questions About Ovarian Cancer What is ovarian cancer? Ovarian cancer is a cancer that forms in tissues
Surveillance for Hepatocellular Carcinoma
 Surveillance for Hepatocellular Carcinoma Marion G. Peters, MD John V. Carbone, MD, Endowed Chair Professor of Medicine Chief of Hepatology Research University of California San Francisco Recorded on April
Surveillance for Hepatocellular Carcinoma Marion G. Peters, MD John V. Carbone, MD, Endowed Chair Professor of Medicine Chief of Hepatology Research University of California San Francisco Recorded on April
Laparoscopic Ultrasonography Assisted Retroperitoneal Lymph Node Sampling in Patients Evaluated for Stomach Cancer Recurrence
 Case Series Laparoscopic Ultrasonography Assisted Retroperitoneal Lymph Node Sampling in Patients Evaluated for Stomach Cancer Recurrence Honsoul Kim, MD, Woo Jin Hyung, MD, Joon Seok Lim, MD, Mi-Suk Park,
Case Series Laparoscopic Ultrasonography Assisted Retroperitoneal Lymph Node Sampling in Patients Evaluated for Stomach Cancer Recurrence Honsoul Kim, MD, Woo Jin Hyung, MD, Joon Seok Lim, MD, Mi-Suk Park,
Cytopathology Case Presentation #8
 Cytopathology Case Presentation #8 Emily E. Volk, MD William Beaumont Hospital, Troy, MI Jonathan H. Hughes, MD Laboratory Medicine Consultants, Las Vegas, Nevada Clinical History 44 year old woman presents
Cytopathology Case Presentation #8 Emily E. Volk, MD William Beaumont Hospital, Troy, MI Jonathan H. Hughes, MD Laboratory Medicine Consultants, Las Vegas, Nevada Clinical History 44 year old woman presents
Endo Conference: Large Polypectomy & EMR
 Endo Conference: Large Polypectomy & EMR Dr. Whang Feb 3, 2015 VOGELGRAM: genetic pathway of colorectal cancer & genes affected by point mutations Outline I. Baseline Colonoscopy II. Colon Polyps III.
Endo Conference: Large Polypectomy & EMR Dr. Whang Feb 3, 2015 VOGELGRAM: genetic pathway of colorectal cancer & genes affected by point mutations Outline I. Baseline Colonoscopy II. Colon Polyps III.
KIDNEY FUNCTION RELATION TO SIZE OF THE TUMOR IN RENAL CELL CANCINOMA
 KIDNEY FUNCTION RELATION TO SIZE OF THE TUMOR IN RENAL CELL CANCINOMA O.E. Stakhvoskyi, E.O. Stakhovsky, Y.V. Vitruk, O.A. Voylenko, P.S. Vukalovich, V.A. Kotov, O.M. Gavriluk National Canсer Institute,
KIDNEY FUNCTION RELATION TO SIZE OF THE TUMOR IN RENAL CELL CANCINOMA O.E. Stakhvoskyi, E.O. Stakhovsky, Y.V. Vitruk, O.A. Voylenko, P.S. Vukalovich, V.A. Kotov, O.M. Gavriluk National Canсer Institute,
VIRTUAL UPPER GASTROINTESTINAL ENDOSCOPY
 CLINICAL POLICY VIRTUAL UPPER GASTROINTESTINAL ENDOSCOPY Policy Number: DIAGNOSTIC 045.7 T Effective Date: December 1, 015 Table of Contents APPLICABLE LINES OF BUSINESS/PRODUCTS... BENEFIT CONSIDERATIONS...
CLINICAL POLICY VIRTUAL UPPER GASTROINTESTINAL ENDOSCOPY Policy Number: DIAGNOSTIC 045.7 T Effective Date: December 1, 015 Table of Contents APPLICABLE LINES OF BUSINESS/PRODUCTS... BENEFIT CONSIDERATIONS...
The Whipple Operation for Pancreatic Cancer: Optimism vs. Reality. Franklin Wright UCHSC Department of Surgery Grand Rounds September 11, 2006
 The Whipple Operation for Pancreatic Cancer: Optimism vs. Reality Franklin Wright UCHSC Department of Surgery Grand Rounds September 11, 2006 Overview Pancreatic ductal adenocarcinoma Pancreaticoduodenectomy
The Whipple Operation for Pancreatic Cancer: Optimism vs. Reality Franklin Wright UCHSC Department of Surgery Grand Rounds September 11, 2006 Overview Pancreatic ductal adenocarcinoma Pancreaticoduodenectomy
Carbohydrate antigen 19 9 (CA 19 9) (serum, plasma)
 Carbohydrate antigen 19 9 (CA 19 9) (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Carbohydrate antigen 19 9 (CA 19 9) 1.2 Alternative names Cancer antigen 19 9, cancer antigen GI
Carbohydrate antigen 19 9 (CA 19 9) (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Carbohydrate antigen 19 9 (CA 19 9) 1.2 Alternative names Cancer antigen 19 9, cancer antigen GI
.org. Metastatic Bone Disease. Description
 Metastatic Bone Disease Page ( 1 ) Cancer that begins in an organ, such as the lungs, breast, or prostate, and then spreads to bone is called metastatic bone disease (MBD). More than 1.2 million new cancer
Metastatic Bone Disease Page ( 1 ) Cancer that begins in an organ, such as the lungs, breast, or prostate, and then spreads to bone is called metastatic bone disease (MBD). More than 1.2 million new cancer
Update on Mesothelioma
 November 8, 2012 Update on Mesothelioma Intro incidence and nomenclature Update on Classification Diagnostic specimens Morphologic features Epithelioid Histology Biphasic Histology Immunohistochemical
November 8, 2012 Update on Mesothelioma Intro incidence and nomenclature Update on Classification Diagnostic specimens Morphologic features Epithelioid Histology Biphasic Histology Immunohistochemical
Laparoscopic Surgery of the Colon and Rectum (Large Intestine) A Simple Guide to Help Answer Your Questions
 Laparoscopic Surgery of the Colon and Rectum (Large Intestine) A Simple Guide to Help Answer Your Questions What are the Colon and Rectum? The colon and rectum together make up the large intestine. After
Laparoscopic Surgery of the Colon and Rectum (Large Intestine) A Simple Guide to Help Answer Your Questions What are the Colon and Rectum? The colon and rectum together make up the large intestine. After
NEOPLASMS C00 D49. Presented by Jan Halloran CCS
 NEOPLASMS C00 D49 Presented by Jan Halloran CCS 1 INTRODUCTION A neoplasm is a new or abnormal growth. In the ICD-10-CM classification system, neoplastic disease is classified in categories C00 through
NEOPLASMS C00 D49 Presented by Jan Halloran CCS 1 INTRODUCTION A neoplasm is a new or abnormal growth. In the ICD-10-CM classification system, neoplastic disease is classified in categories C00 through
Nicole Kounalakis, MD
 Breast Disease: Diagnosis and Management Nicole Kounalakis, MD Assistant Professor of Surgery Goal of Breast Evaluation The goal of breast evaluation is to classify findings as: normal physiologic variations
Breast Disease: Diagnosis and Management Nicole Kounalakis, MD Assistant Professor of Surgery Goal of Breast Evaluation The goal of breast evaluation is to classify findings as: normal physiologic variations
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: microwave_tumor_ablation 12/2011 11/2015 11/2016 11/2015 Description of Procedure or Service Microwave ablation
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: microwave_tumor_ablation 12/2011 11/2015 11/2016 11/2015 Description of Procedure or Service Microwave ablation
Spleen. Anatomy. (Effective February 2007) (1%-5%) Normal. Related Anatomy Anterior to spleen. Medial border. Posteriorly
 Spleen (Effective February 2007) (1%-5%) Anatomy Normal Intraperitoneal, except hilum Left hypochondrium Left hemidiaphragm superior generally considered to be ovoid, with a convex superior and a concave
Spleen (Effective February 2007) (1%-5%) Anatomy Normal Intraperitoneal, except hilum Left hypochondrium Left hemidiaphragm superior generally considered to be ovoid, with a convex superior and a concave
Disease/Illness GUIDE TO ASBESTOS LUNG CANCER. What Is Asbestos Lung Cancer? www.simpsonmillar.co.uk Telephone 0844 858 3200
 GUIDE TO ASBESTOS LUNG CANCER What Is Asbestos Lung Cancer? Like tobacco smoking, exposure to asbestos can result in the development of lung cancer. Similarly, the risk of developing asbestos induced lung
GUIDE TO ASBESTOS LUNG CANCER What Is Asbestos Lung Cancer? Like tobacco smoking, exposure to asbestos can result in the development of lung cancer. Similarly, the risk of developing asbestos induced lung
Abdominal CT scan findings in Acute Appendicitis
 Abdominal CT scan findings in Acute Appendicitis Pathophysiology of acute appendicitis. Acute appendicitis occurs when the lumen is obstructed, leading to fluid accumulation, luminal distention, inflammation
Abdominal CT scan findings in Acute Appendicitis Pathophysiology of acute appendicitis. Acute appendicitis occurs when the lumen is obstructed, leading to fluid accumulation, luminal distention, inflammation
To Whipple or Not to Whipple, that is the Question: Evaluating the Resectability of Pancreatic Adenocarcinoma
 August 2009 To Whipple or Not to Whipple, that is the Question: Evaluating the Resectability of Pancreatic Adenocarcinoma Christina Ramirez, Harvard Medical School Year III Gillian Lieberman, MD Agenda
August 2009 To Whipple or Not to Whipple, that is the Question: Evaluating the Resectability of Pancreatic Adenocarcinoma Christina Ramirez, Harvard Medical School Year III Gillian Lieberman, MD Agenda
ERBEJET 2. The versatility of waterjet surgery: ERBEJET 2 with hybrid instruments WATERJET SURGERY
 ERBEJET 2 The versatility of waterjet surgery: ERBEJET 2 with hybrid instruments WATERJET SURGERY Gentle interventions in surgery and endoscopy Waterjet surgery with hybrid technology Waterjet surgery
ERBEJET 2 The versatility of waterjet surgery: ERBEJET 2 with hybrid instruments WATERJET SURGERY Gentle interventions in surgery and endoscopy Waterjet surgery with hybrid technology Waterjet surgery
DESMOPLASTIC SMALL ROUND CELL TUMOR: A RARE PATHOLOGY PUZZLE
 DESMOPLASTIC SMALL ROUND CELL TUMOR: A RARE PATHOLOGY PUZZLE Ryan Granger University of Rhode Island Cytotechnology program May 2, 2015 ASCT Annual Meeting Nashville, Tennessee DESMOPLASTIC SMALL ROUND
DESMOPLASTIC SMALL ROUND CELL TUMOR: A RARE PATHOLOGY PUZZLE Ryan Granger University of Rhode Island Cytotechnology program May 2, 2015 ASCT Annual Meeting Nashville, Tennessee DESMOPLASTIC SMALL ROUND
Gastrointestinal Stromal Tumor (GIST)
 Gastrointestinal Stromal Tumor (GIST) What are gastrointestinal stromal tumors? Cancer starts when cells in the body begin to grow out of control. Cells in nearly any part of the body can become cancer,
Gastrointestinal Stromal Tumor (GIST) What are gastrointestinal stromal tumors? Cancer starts when cells in the body begin to grow out of control. Cells in nearly any part of the body can become cancer,
