Potelligent CHOK1SV. European Antibody Congress 30 Nov - 02 Dec, Geneva, Switzerland. Alison Porter, 2009 Lonza Biologics plc, Slough, UK
|
|
|
- Anthony Burke
- 7 years ago
- Views:
Transcription
1 Potelligent European Antibody Congress 30 Nov - 02 Dec, Geneva, Switzerland Alison Porter, 2009 Lonza Biologics plc, Slough, UK
2 Disclaimer Certain matters discussed in this presentation may constitute forward-looking statements. These statements are based on current expectations and estimates of Lonza Group Ltd, although Lonza Group Ltd can give no assurance that these expectations and estimates will be achieved. The actual results may differ materially in the future from the forward-looking statements included in this presentation due to various factors. Furthermore, Lonza Group Ltd has no obligation to update the statements contained in this presentation. Note: All slides are incomplete without verbal comments. slide 2
3 Scope of Talk POTELLIGENT technology Introduction Benefits GS Gene Expression System and Introduction Benefits Combining technologies: Potelligent Performance of the Potelligent GS-CHO technology Growth and productivity characteristics Product characteristics ADCC slide 3
4 POTELLIGENT Technology
5 POTELLIGENT Technology Technology that applies an intelligent approach to creating more potent antibodies 100% fucose-free antibodies uses a proprietary fucosyltransferase-knockout (FUT8-KO) CHO cell line as a production cell Fucose-free mabs have markedly higher antibody-dependent cellular cytotoxicity (ADCC) than fucosylated mabs Eight POTELLIGENT mabs in clinic Licensed by BioWa Intellectual properties of POTELLIGENT technology: Title PCT US Method of Modulating The Activity of Functional Immunological Molecules WO00/61739 A1 (Priority date 04/09/99) US7,214,775 (US2005/ ) EP A1 covers composition of matter of fucose-free Mabs Antibody composition-producing cell WO02/31140 A1 US6,946,292 covers FUT8-KO mammalian cells (US2003/ ) EP A1 slide 5
6 POTELLIGENT mabs in Clinical Studies Compounds Target Ag Indication(s) Preclinical Phase-1 Phase-2 BIW-8405/MEDI-563 (KHK/MedImmune) KW-0761 (KHK/Amgen) IL-5R Asthma Immunology CCR4 Allergy/ Blood tumor Immunology / Oncology MDX-1401 (Medarex) CD30 Lymphoma MDX-1342 (Medarex) CD19 RA/CLL MDX-1411 (Medarex) CD70 RCC Oncology Immunology / Oncology Oncology Undisclosed - - Undisclosed - - BIW-8962 (KHK) GM2 Blood tumors Oncology slide 6
7 Summary of Clinical Data for KW-0761 and MEDI-563/BIW-8405 Antibody Molecule Target Cell Dose KW-0761 CCR4 ( /cell) Th2 cell ATLL/PTCL mg/kg mg/kg MEDI-563/ BIW8405 IL5Rα (<10 3 /cell) Eosinophil mg/kg Not immunogenic No adverse events relating to POTELLIGENT technology Highly active High antigen expression will not be required Active in blood, bone marrow, lymph nodes, and skin slide 7
8 POTELLIGENT and ADCC : N-acetylglucosamine : bisec GlcNAc : Mannose : Galactose : Sialic acid : Fucose Fucose Asn 297 Relationship between ADCC and structure of N-linked oligosaccharide of antibody 1. Boyd et al. Mol.Immunol.1995: Sialic acid No effect 2. Kumpel et al. Hum. Antib. Hybrid. 1994: (+) Gal 2 to 3-fold increase of ADCC 3. Umana et al. Nat. Biotech. 1999: (+) BisecGlcNAc 10-fold increase of ADCC 4. Shinkawa et al. J.B.C. 2003: POTELLIGENT (-) Fuc more potent ADCC than (+) Gal or (+) BisecGlcNAc slide 8
9 POTELLIGENT and ADCC Target Cell Antibody IgG1 ADCC FcγRIIIa NK Cell Enhanced ADCC has been confirmed by multiple antibodies Rituximab anti-t cell Mab Cytotoxicity (%) mab concentration (µg/ml) POTELLIGENT mab Fucosylated mab Effector: human PBMC 37 C, 4hr incubation, LDH or Cr 51 release assay slide 9
10 Why Use the POTELLIGENT Technology Dramatically enhanced potency and efficacy of mabs Clinical results with POTELLIGENT -enhanced mabs expected to show higher efficacy in human patients when compared with nonenhanced antibodies Increased Fc receptor binding Overcomes the problem of low clinical responses due to genetic differences in the Fc receptor Lowered effective therapeutic dose of your antibodies Potential to deliver much more with much less mabs that are expected to be proven safe and well tolerated, with no immunogenic concerns Requires no change in current manufacturing process BioWa s proprietary technology works seamlessly with systems and processes already in place slide 10
11 GS and
12 Industry-leading GS Gene Expression System Owned and licensed by Lonza A mammalian gene expression system For therapeutic proteins and monoclonal antibodies GS-CHO and GS-NS0 cell lines Consistent and reliable means of generating high yielding cell lines Speed Amplification not usually necessary Stable cell lines Widely accepted Over 100 companies, 75 academic institutions Over 50 products in the clinic 100s of high-yielding cell lines created (last 15 years) Regulatory acceptance 7 Licensed products slide 12
13 Adaptation of CHO cells to single cell suspension culture and to proteinfree medium can be problematic and time consuming One solution to this is to pre-adapt the host cell line to the desired culture conditions is a suspension variant of CHO-K1 which has been preadapted to chemically-defined, animal component-free (CDACF) medium CDACF media can be used throughout all stages of the cell line construction process Compatible with single-cell cloning by FACS or other automated methods Cell lines derived from exhibit excellent growth characteristics Reach high maximum viable cell concentrations Able to maintain cultures at high viability slide 13
14 Comparison of Growth of Recombinant Cell Lines Derived From Different CHO Host Cell Lines 120 Viable Viable cell cell concentration ( /ml) / ml) Time (h) 22H11 LB01 22H11 created using CHO-K1 LB01 created using Evaluated same fed-batch CDACF process in 10 L fed-batch bioreactor LB01 growth profile representative of -derived cell lines slide 14
15 Comparison of Product Concentration from and CHO-K1 Derived Cell Lines in 10 L Fed-batch Bioreactor Host cell line Xv max IVC Qp mab 10 6 cells /ml 10 6 cells /ml.h pg/cell.h mg/l CHO-K Switch from CHO-K1 to gave 3 to 4-fold increase in product concentration by increasing both Qp and IVC Process improvements have given a further 4-5 fold increase in product concentration Product concentrations up to 9.5 g/l seen for alternative recombinant GS-CHO cell lines in latest process slide 15
16 Combining Technologies: Potelligent
17 Potelligent Potelligent combining: BioWa s POTELLIGENT technology to generate more potent mabs through elimination of core fucose - With Lonza s GS Gene Expression System for robust manufacture of therapeutic antibodies Potelligent is available for licensing through a threeparty agreement among BioWa, Lonza and licensee Potelligent will be offered as part of Lonza's development services in combination with Lonza's GS Gene Expression System slide 17
18 Developing Potelligent Three derived host cell lines, whose alleles of FUT8 genes were completely disrupted, established Host cell lines were transfected with GS antibody vector As expected, antibodies produced from transfectants derived from the host cell lines show enhanced ADCC activity Cytotoxicity (%) Ab concentration (μg/ml) Effector: human PBMC Target: B-cell lymphoma Raji E/T ratio = 20:1 37 C, 4hr incubation LDH release assay Potelligent A Potelligent B Potelligent C slide 18
19 Developing Potelligent - Continued One host cell line selected for further development Potelligent C Based on growth and productivity data following transfection of the 3 host cell lines Number of Cell Lines Assessed in 24-well plates Product Concentration (mg/ L) Assessed in batch shake-flask culture Number of Cell Lines Product Concentration (mg/ L) Potelligent A Potelligent B Potelligent C Potelligent A Potelligent B Potelligent C Approximately 60 cell lines from each host Approximately 30 cell lines from each host slide 19
20 Developing Potelligent - Continued Lead host cell line named Potelligent Full cell line construction undertaken using Potelligent Including assessment of recombinant cell lines in lab-scale bioreactors ADCC assay on antibody samples from bioreactors cgmp Master Cell Bank (MCB) and Working Cell Bank (WCB) prepared Including regulatory data pack to support Potelligent slide 20
21 Performance of Potelligent GS-CHO Technology
22 Growth and Productivity Characteristics: Fed-batch Shake-flasks (Antibody 1) Individual Value Plot of Max. the Maximum Viable Cell Concentration Achieved in Fed-Batch Shake-Flasks Maximum 10 6 viable Xv (x10e6 cells/ml cells/ml) Potelligent IVC 10 9 (10E9 viable 6 cells.h/l) Individual Value Plot of the IVC IVC Achieved in Fed-Batch Shake-Flasks Potelligent Individual Value Plot of Product Concentration Achieved in Fed-Batch Shake-Flasks Product Concentration mg/l (mg/l) Potelligent Individual Value Plot of Specific Production Production Rate Achieved Rate in Fed-Batch Shake-Flasks Qp (pg/cell/h) Potelligent *Research with antibody 1 sponsored by KaloBios slide 22
23 Growth and Productivity Characteristics: Fed-batch Shake-flasks (Antibody 2) Individual Value Plot of Max. the Maximum Viable Cell Concentration Achieved in Fed-Batch Shake-Flasks Maximum 10 Xv 6 cells/ml (x10e6 cells/ml) Potelligent Individual Value Plot of Product Concentration Achieved in Fed-Batch Shake-Flasks Product Concentration mg/l (mg/l) Potelligent IVC 10 9 (10E9 viable cells.h/ml) cells.h/l Qp (pg/cell/h) IVC Individual Value Plot of the IVC Achieved in Fed-Batch Shake-Flasks Potelligent Specific Production Rate Individual Value Plot of Specific Production Rate Achieved in Fed-Batch SHake-Flasks Potelligent slide 23
24 Growth and Productivity Characteristics: 10 L Fed-batch Bioreactor Cultures (Antibody 2) Viable cell concentration (10 6 /ml) Growth Characteristics Antibody concentration (mg/l) Elapsed time (h) Elapsed time (h) Productivity Characteristics Key: Red line = Potelligent derived cell lines Blue line = derived cell lines slide 24
25 Growth and Productivity Characteristics: 10 L Fed-batch Bioreactor Cultures (Antibody 2) - Continued Host Cell Line IVC at Harvest 10 9 cell.h/l Viability at Harvest (%) Overall Production Rate (Qp) pg/cell/day Antibody Concentration at Harvest mg/l Potelligent Successful identification of Potelligent derived cell lines, with suitable growth and productivity characteristic, for use in a manufacturing process slide 25
26 Product Characteristics: Reduced + Non-reduced SDS PAGE and IEF 10 L Fed-batch Bioreactor Cultures (Antibody 2) Reduced Non-reduced Reduced and non-reduced SDS PAGE Lanes 4 to 7: Potelligent Lanes 8 to 9: Comparable band profiles for all samples IEF Gel on left, lanes 4 to 7: Potelligent Gel on right, lanes 2 to 3: Comparable band profiles for all samples slide 26
27 Product Characteristics: GP-HPLC 10 L Fed-batch Bioreactor Cultures (Antibody 2) Relative Peak Intensity (%) Host Cell Line Total Aggregate Monomer Fragment Potelligent < <0.1 GP-HPLC Aggregate levels in acceptable range (0 5%) Comparable aggregate and monomer levels slide 27
28 ADCC Activity of Antibody 2 Produced from 10 L Fed-Batch Bioreactor Cultures Effector: human PBMC Target: B-cell lymphoma Raji 37 C, 4hr incubation, LDH release assay Potelligent Potelligent produced antibodies with enhanced ADCC as expected slide 28
29 Summary Introduction of a new host cell line, combining Lonza and BioWa owned technologies Potelligent cgmp MCB and WCB available Recombinant cell lines created using Potelligent have shown Enhanced ADCC Growth suitable for a production process Further optimisation possible High product concentration levels in a platform process Further optimisation possible Work seamlessly with current manufacturing process Potelligent is available for licensing through a threeparty agreement among BioWa, Lonza and licensee Potelligent will be offered as part of Lonza's development services in combination with Lonza's GS Gene Expression System slide 29
30 Acknowledgements Lonza Samantha Vieira Tarik Senussi Alison Mastrangelo Gavin Edwards Andy Racher BioWa/Kyowa Hakko Kirin Naoko Yamane-Ohnuki Shigeru Iida Mitsuo Satoh Kenya Shitara Masamichi Koike slide 30
Improving GS-CHO Cell Line Selection: Reducing Time to Clinic
 Cell Line Development and Engineering 2-6 March 2009, Berlin Improving GS-CHO Cell Line Selection: Reducing Time to Clinic Adrian Haines, 2009 Lonza Biologics plc, Slough, UK Disclaimer Certain matters
Cell Line Development and Engineering 2-6 March 2009, Berlin Improving GS-CHO Cell Line Selection: Reducing Time to Clinic Adrian Haines, 2009 Lonza Biologics plc, Slough, UK Disclaimer Certain matters
Accelerating Antibody Process Development: Exploring the Synergies Between Engineered Host Cells and Process Development
 Pharma&Biotech Accelerating Antibody Process Development: Exploring the Synergies Between Engineered Host Cells and Process Development James Rance PhD Head of Development Services Singapore Lonza Biologics
Pharma&Biotech Accelerating Antibody Process Development: Exploring the Synergies Between Engineered Host Cells and Process Development James Rance PhD Head of Development Services Singapore Lonza Biologics
EMABling Antibody Production Platform
 EMABling Antibody Production Platform An industrial solution for the production of therapeutic antibodies with high cytotoxic activity B IOMANUFACTURING EMABling : A fully integrated development platform
EMABling Antibody Production Platform An industrial solution for the production of therapeutic antibodies with high cytotoxic activity B IOMANUFACTURING EMABling : A fully integrated development platform
The Importance of Developing a High Yield of Product
 European Antibody Congress Lyon, 3 rd November 2005 The Importance of Developing a High Yield of Product John Birch, Lonza Biologics plc Monoclonal Antibodies A Success Story Fastest growing segment of
European Antibody Congress Lyon, 3 rd November 2005 The Importance of Developing a High Yield of Product John Birch, Lonza Biologics plc Monoclonal Antibodies A Success Story Fastest growing segment of
Selection of Cell Lines for Manufacturing Therapeutic Antibodies by Flow Cytometric Cell Sorting. Andrew Racher Lonza Biologics
 Selection of Cell Lines for Manufacturing Therapeutic Antibodies by Flow Cytometric Cell Sorting Andrew Racher Lonza Biologics Structure of Talk 1. The Problem 2. Possible Solutions 3. Solution Chosen
Selection of Cell Lines for Manufacturing Therapeutic Antibodies by Flow Cytometric Cell Sorting Andrew Racher Lonza Biologics Structure of Talk 1. The Problem 2. Possible Solutions 3. Solution Chosen
ESTABLISHMENT OF CELL LINES FOR MANUFACTURING RECOMBINANT ANTIBODIES. Dr Andrew Racher
 ESTABLISHMENT OF CELL LINES FOR MANUFACTURING RECOMBINANT ANTIBODIES Dr Andrew Racher Lonza Biologics plc, 2004 Structure of talk Issues Selection of a high producing cell line Selection for process compatibility
ESTABLISHMENT OF CELL LINES FOR MANUFACTURING RECOMBINANT ANTIBODIES Dr Andrew Racher Lonza Biologics plc, 2004 Structure of talk Issues Selection of a high producing cell line Selection for process compatibility
How To Develop A Cell Line
 Advances in The Development of Biopharmaceuticals The application of modern technologies and services to the development of Biopharmaceutical processes Dr Jonathan H Dempsey Process Science Fellow Invitrogen
Advances in The Development of Biopharmaceuticals The application of modern technologies and services to the development of Biopharmaceutical processes Dr Jonathan H Dempsey Process Science Fellow Invitrogen
A customizable ADCC assay service for antibodies & fusion proteins.
 Antibody Dependent Cell- Mediated Cytotoxicity (ADCC) Assay A customizable ADCC assay service for antibodies & fusion proteins. Our ADCC assay service accurately detects cell lysis based on LDH-release.
Antibody Dependent Cell- Mediated Cytotoxicity (ADCC) Assay A customizable ADCC assay service for antibodies & fusion proteins. Our ADCC assay service accurately detects cell lysis based on LDH-release.
LFB GROUP & SANOFI combine their bioproduction capabilities to provide integrated CMO services for biopharmaceuticals
 LFB GROUP & SANOFI combine their bioproduction capabilities to provide integrated CMO services for biopharmaceuticals MAbLaunch TM is a joint bioproduction platform combining LFB Biomanufacturing (LFB
LFB GROUP & SANOFI combine their bioproduction capabilities to provide integrated CMO services for biopharmaceuticals MAbLaunch TM is a joint bioproduction platform combining LFB Biomanufacturing (LFB
LFB GROUP & SANOFI combine their bioproduction capabilities to provide integrated CMO services for biopharmaceuticals
 LFB GROUP & SANOFI combine their bioproduction capabilities to provide integrated CMO services for biopharmaceuticals MAbLaunch TM is a joint bioproduction platform combining LFB Biomanufacturing (LFB
LFB GROUP & SANOFI combine their bioproduction capabilities to provide integrated CMO services for biopharmaceuticals MAbLaunch TM is a joint bioproduction platform combining LFB Biomanufacturing (LFB
Anti-CD38 anti-cd3 bispecific antibody in multiple myeloma
 Anti-CD38 anti-cd3 bispecific antibody in multiple myeloma David E. Szymkowski Senior Director, Biotherapeutics Proteins by Design 1960s...1980s...2000s... Where are the bispecific antibody drugs? J Exp.
Anti-CD38 anti-cd3 bispecific antibody in multiple myeloma David E. Szymkowski Senior Director, Biotherapeutics Proteins by Design 1960s...1980s...2000s... Where are the bispecific antibody drugs? J Exp.
Challenges in the cgmp Manufacturing of hescs: Lessons Learned from Monoclonal Antibodies
 Challenges in the cgmp Manufacturing of hescs: Lessons Learned from Monoclonal Antibodies ISCT 2011 Annual Meeting Rotterdam, The Netherlands May 18 21, 2011 BioProcess Technology Consultants www.bptc.com
Challenges in the cgmp Manufacturing of hescs: Lessons Learned from Monoclonal Antibodies ISCT 2011 Annual Meeting Rotterdam, The Netherlands May 18 21, 2011 BioProcess Technology Consultants www.bptc.com
Exciting Trends in Bioprocessing
 Exciting Trends in Bioprocessing Alfred Doig and Susan Dana Jones, Ph.D. April 20, 2015 BioProcess Technology Consultants, Inc. 12 Gill Street, Suite 5450 Woburn, MA 01801 Exciting Trends in Bioprocessing
Exciting Trends in Bioprocessing Alfred Doig and Susan Dana Jones, Ph.D. April 20, 2015 BioProcess Technology Consultants, Inc. 12 Gill Street, Suite 5450 Woburn, MA 01801 Exciting Trends in Bioprocessing
Literature Review: CHO versus HEK Cell Glycosylation
 Literature Review: CHO versus HEK Cell Glycosylation Contributing Authors: Krista Steger, Ph.D., James Brady, Ph.D., Meg Duskin, Karen Donato, Ph.D. FLOW TRANSFEC TION SY STEMS Abstract Choosing a host
Literature Review: CHO versus HEK Cell Glycosylation Contributing Authors: Krista Steger, Ph.D., James Brady, Ph.D., Meg Duskin, Karen Donato, Ph.D. FLOW TRANSFEC TION SY STEMS Abstract Choosing a host
High yield antibody production in a disposable WAVE Bioreactor
 High yield antibody production in a Process intensification using perfusion culture Christian Kaisermayer 1, Jianjun Yang 2 1 GE Healthcare Europe GmbH, Vienna, Austria (Christian.Kaisermayer@ge.com),
High yield antibody production in a Process intensification using perfusion culture Christian Kaisermayer 1, Jianjun Yang 2 1 GE Healthcare Europe GmbH, Vienna, Austria (Christian.Kaisermayer@ge.com),
Luca Romagnoli, Ph.D. Business Development Manager
 Modelli innovativi di produzione per lo sviluppo di un processo altamente qualitativo di farmaci biologici Luca Romagnoli, Ph.D. Business Development Manager BIOLOGICAL DRUGS - SOURCES Monoclonal antibodies
Modelli innovativi di produzione per lo sviluppo di un processo altamente qualitativo di farmaci biologici Luca Romagnoli, Ph.D. Business Development Manager BIOLOGICAL DRUGS - SOURCES Monoclonal antibodies
Accelerating Antibody Drug Development Using Novel ADCC Reporter Bioassays. Terry Surowy, PhD Research Manager
 Accelerating Antibody Drug Development Using Novel ADCC Reporter Bioassays Terry Surowy, PhD Research Manager Outline Antibody drugs and mechanisms of action (MOA) Introduction to ADCC and ADCC Reporter
Accelerating Antibody Drug Development Using Novel ADCC Reporter Bioassays Terry Surowy, PhD Research Manager Outline Antibody drugs and mechanisms of action (MOA) Introduction to ADCC and ADCC Reporter
Genes to Proteins to Antibodies
 Genes to Proteins to Antibodies About Us Fusion Antibodies is a CRO established in 2001 as a spin-out from Queen s University Belfast. The company building is situated in a charming area of Springbank
Genes to Proteins to Antibodies About Us Fusion Antibodies is a CRO established in 2001 as a spin-out from Queen s University Belfast. The company building is situated in a charming area of Springbank
Technologies for Monoclonal Antibody Production
 Technologies for Monoclonal Antibody Production First we help you bridge the distance between upstream and downstream. Then we help you shorten it. Monoclonal antibodies for therapeutic, diagnostic and
Technologies for Monoclonal Antibody Production First we help you bridge the distance between upstream and downstream. Then we help you shorten it. Monoclonal antibodies for therapeutic, diagnostic and
Production of proteins for medical use in TG silkworms
 Production of proteins for medical use in TG silkworms Development of animal therapeutic drugs Collaborative development of influenza vaccines with Institute of Biological Resource Collaborative development
Production of proteins for medical use in TG silkworms Development of animal therapeutic drugs Collaborative development of influenza vaccines with Institute of Biological Resource Collaborative development
From cells to therapeuticsvivalis. Humalex Fully human antibody discovery platform An integrated therapeutic development offering
 From cells to therapeuticsvivalis Humalex Fully human antibody discovery platform An integrated therapeutic development offering Humalex from bench-top to clinical development The Humalex platform enables
From cells to therapeuticsvivalis Humalex Fully human antibody discovery platform An integrated therapeutic development offering Humalex from bench-top to clinical development The Humalex platform enables
Catalent Biologics & Clinical Supplies The SMART Solution
 Catalent Biologics & Clinical Supplies The SMART Solution Advanced Technology and Integrated Solutions From DNA to Clinical Supply & Cold Chain Distribution Dr. Florian Schwaak Account Manager Germany
Catalent Biologics & Clinical Supplies The SMART Solution Advanced Technology and Integrated Solutions From DNA to Clinical Supply & Cold Chain Distribution Dr. Florian Schwaak Account Manager Germany
Eden Biodesign ebook Monoclonal Antibody Production: Building the Platform
 Eden Biodesign ebook Monoclonal Antibody Production: Building the Platform CHAPTER 1: Overview CHAPTER 2: Challenges CHAPTER 3: Purification Methodology CHAPTER 4: Results CHAPTER 5: About Eden Biodesign
Eden Biodesign ebook Monoclonal Antibody Production: Building the Platform CHAPTER 1: Overview CHAPTER 2: Challenges CHAPTER 3: Purification Methodology CHAPTER 4: Results CHAPTER 5: About Eden Biodesign
Microbiology AN INTRODUCTION EIGHTH EDITION
 TORTORA FUNKE CASE Microbiology AN INTRODUCTION EIGHTH EDITION Differentiate between innate and acquired immunity. Chapter 17 Specific Defenses of the Host: The Immune Response B.E Pruitt & Jane J. Stein
TORTORA FUNKE CASE Microbiology AN INTRODUCTION EIGHTH EDITION Differentiate between innate and acquired immunity. Chapter 17 Specific Defenses of the Host: The Immune Response B.E Pruitt & Jane J. Stein
BIOSIMILARS A COMPLETE DEVELOPMENT PLATFORM
 BIOSIMILARS A COMPLETE DEVELOPMENT PLATFORM FROM ORIGINATOR THROUGH COMPARABILITY TO MARKET STREAMLINE YOUR R&D, REGULATORY, ANALYTICAL, AND CLINICAL NEEDS WITH ONE PROVIDER The development pathway of
BIOSIMILARS A COMPLETE DEVELOPMENT PLATFORM FROM ORIGINATOR THROUGH COMPARABILITY TO MARKET STREAMLINE YOUR R&D, REGULATORY, ANALYTICAL, AND CLINICAL NEEDS WITH ONE PROVIDER The development pathway of
Testing Services for Large Molecule Drug Development
 Testing Services for Large Molecule Drug Development Our mission is to extend our clients capabilities by combining scientific knowledge, capacity, regulatory expertise and flexibility to provide the trusted,
Testing Services for Large Molecule Drug Development Our mission is to extend our clients capabilities by combining scientific knowledge, capacity, regulatory expertise and flexibility to provide the trusted,
HuCAL Custom Monoclonal Antibodies
 HuCAL Custom Monoclonal Antibodies Highly Specific Monoclonal Antibodies in just 8 Weeks PROVEN, HIGHLY SPECIFIC, HIGH AFFINITY ANTIBODIES IN 8 WEEKS WITHOUT HuCAL PLATINUM IMMUNIZATION (Human Combinatorial
HuCAL Custom Monoclonal Antibodies Highly Specific Monoclonal Antibodies in just 8 Weeks PROVEN, HIGHLY SPECIFIC, HIGH AFFINITY ANTIBODIES IN 8 WEEKS WITHOUT HuCAL PLATINUM IMMUNIZATION (Human Combinatorial
2) Macrophages function to engulf and present antigen to other immune cells.
 Immunology The immune system has specificity and memory. It specifically recognizes different antigens and has memory for these same antigens the next time they are encountered. The Cellular Components
Immunology The immune system has specificity and memory. It specifically recognizes different antigens and has memory for these same antigens the next time they are encountered. The Cellular Components
Chapter 43: The Immune System
 Name Period Our students consider this chapter to be a particularly challenging and important one. Expect to work your way slowly through the first three concepts. Take particular care with Concepts 43.2
Name Period Our students consider this chapter to be a particularly challenging and important one. Expect to work your way slowly through the first three concepts. Take particular care with Concepts 43.2
MAB Solut. MABSolys Génopole Campus 1 5 rue Henri Desbruères 91030 Evry Cedex. www.mabsolut.com. is involved at each stage of your project
 Mabsolus-2015-UK:Mise en page 1 03/07/15 14:13 Page1 Services provider Department of MABSolys from conception to validation MAB Solut is involved at each stage of your project Creation of antibodies Production
Mabsolus-2015-UK:Mise en page 1 03/07/15 14:13 Page1 Services provider Department of MABSolys from conception to validation MAB Solut is involved at each stage of your project Creation of antibodies Production
Company Update. March 2011
 Company Update March 2011 Safe Harbour This presentation includes forward-looking statements. Actual results could differ materially from those included in the forward-looking statements due to various
Company Update March 2011 Safe Harbour This presentation includes forward-looking statements. Actual results could differ materially from those included in the forward-looking statements due to various
Why is FTO important?
 Antibodies, Patents and Freedom to Operate: The Monoclonal Maze Timothy J. Shea, Jr. Director Tracy Muller Associate Sterne, Kessler, Goldstein & Fox P.L.L.C. 4 th Annual Antibody Therapeutics Conference
Antibodies, Patents and Freedom to Operate: The Monoclonal Maze Timothy J. Shea, Jr. Director Tracy Muller Associate Sterne, Kessler, Goldstein & Fox P.L.L.C. 4 th Annual Antibody Therapeutics Conference
Types, production of antibodies and Antibody/antigen interaction
 Types, production of antibodies and Antibody/antigen interaction Antibodies Secreted by B lymphocytes Great diversity and specificity: >109 different antibodies; can distinguish between very similar molecules
Types, production of antibodies and Antibody/antigen interaction Antibodies Secreted by B lymphocytes Great diversity and specificity: >109 different antibodies; can distinguish between very similar molecules
Chapter 18: Applications of Immunology
 Chapter 18: Applications of Immunology 1. Vaccinations 2. Monoclonal vs Polyclonal Ab 3. Diagnostic Immunology 1. Vaccinations What is Vaccination? A method of inducing artificial immunity by exposing
Chapter 18: Applications of Immunology 1. Vaccinations 2. Monoclonal vs Polyclonal Ab 3. Diagnostic Immunology 1. Vaccinations What is Vaccination? A method of inducing artificial immunity by exposing
Important Topics in the Expression of Recombinant Antibodies from CHO Cells
 Cell Line Development and Engineering Workshop, Prague, March 2008 Important Topics in the Expression of Recombinant Antibodies from CHO Cells Robert Young/Lonza Biologics/Mar 2008 Disclaimer Certain matters
Cell Line Development and Engineering Workshop, Prague, March 2008 Important Topics in the Expression of Recombinant Antibodies from CHO Cells Robert Young/Lonza Biologics/Mar 2008 Disclaimer Certain matters
Kempen & Co 4 th Healthcare/Life Sciences Conference. Brussels March 29, 2011
 Kempen & Co 4 th Healthcare/Life Sciences Conference Brussels March 29, 2011 Safe Harbour This presentation includes forward-looking statements. Actual results could differ materially from those included
Kempen & Co 4 th Healthcare/Life Sciences Conference Brussels March 29, 2011 Safe Harbour This presentation includes forward-looking statements. Actual results could differ materially from those included
Transgenic technology in the production of therapeutic proteins
 Transgenic technology in the production of therapeutic proteins Transgenic technology represents a new generation of biopharmaceutical production system to meet the medical needs of the new millennium.
Transgenic technology in the production of therapeutic proteins Transgenic technology represents a new generation of biopharmaceutical production system to meet the medical needs of the new millennium.
KMS-Specialist & Customized Biosimilar Service
 KMS-Specialist & Customized Biosimilar Service 1. Polyclonal Antibody Development Service KMS offering a variety of Polyclonal Antibody Services to fit your research and production needs. we develop polyclonal
KMS-Specialist & Customized Biosimilar Service 1. Polyclonal Antibody Development Service KMS offering a variety of Polyclonal Antibody Services to fit your research and production needs. we develop polyclonal
The use of FACS for the selection of cell lines with superior productivity characteristics
 ESACT-UK, Cambridge 2007 The use of FACS for the selection of cell lines with superior productivity characteristics Jonathan Welsh Synopsis Why use FACS in bioprocess development Issues regarding use of
ESACT-UK, Cambridge 2007 The use of FACS for the selection of cell lines with superior productivity characteristics Jonathan Welsh Synopsis Why use FACS in bioprocess development Issues regarding use of
Facility construction and start up for commercial scale manufacturing of monoclonal antibodies - A case study
 24 th Interphex, Japan, Technical Conference June 29 th, 2011 Michael Brown Facility construction and start up for commercial scale manufacturing of monoclonal antibodies - A case study Lonza AG Financially
24 th Interphex, Japan, Technical Conference June 29 th, 2011 Michael Brown Facility construction and start up for commercial scale manufacturing of monoclonal antibodies - A case study Lonza AG Financially
Advanced BioDesign Outlines Solutions. Antibody Overview. by Advanced BioDesign. Project Start. Immunogenicity. Selecting Your Antigen
 Advanced BioDesign Outlines Solutions by Advanced BioDesign Antibody Overview Launching an immunisation programme is an important experimental step that needs care. With Advanced BioDesign, you may develop
Advanced BioDesign Outlines Solutions by Advanced BioDesign Antibody Overview Launching an immunisation programme is an important experimental step that needs care. With Advanced BioDesign, you may develop
Basics of Immunology
 Basics of Immunology 2 Basics of Immunology What is the immune system? Biological mechanism for identifying and destroying pathogens within a larger organism. Pathogens: agents that cause disease Bacteria,
Basics of Immunology 2 Basics of Immunology What is the immune system? Biological mechanism for identifying and destroying pathogens within a larger organism. Pathogens: agents that cause disease Bacteria,
Accelerating drug development to FTIH: Potential of new expression technologies
 Accelerating drug development to FTIH: Potential of new expression technologies Lekan Daramola Associate Director Biopharmaceutical Development, Cell Culture & Fermentation Sciences CMC Strategy Forum
Accelerating drug development to FTIH: Potential of new expression technologies Lekan Daramola Associate Director Biopharmaceutical Development, Cell Culture & Fermentation Sciences CMC Strategy Forum
Application Note USD3013. Monoclonal Antibody Production in Suspension CHO Culture Using Single-Use PadReactor Mini Bioreactor
 Application Note USD313 Monoclonal Antibody Production in Suspension CHO Culture Using Single-Use PadReactor Mini Bioreactor Introduction Single use solutions are found in many applications as they offer
Application Note USD313 Monoclonal Antibody Production in Suspension CHO Culture Using Single-Use PadReactor Mini Bioreactor Introduction Single use solutions are found in many applications as they offer
The Body s Defenses CHAPTER 24
 CHAPTER 24 The Body s Defenses PowerPoint Lectures for Essential Biology, Third Edition Neil Campbell, Jane Reece, and Eric Simon Essential Biology with Physiology, Second Edition Neil Campbell, Jane Reece,
CHAPTER 24 The Body s Defenses PowerPoint Lectures for Essential Biology, Third Edition Neil Campbell, Jane Reece, and Eric Simon Essential Biology with Physiology, Second Edition Neil Campbell, Jane Reece,
Autoimmunity and immunemediated. FOCiS. Lecture outline
 1 Autoimmunity and immunemediated inflammatory diseases Abul K. Abbas, MD UCSF FOCiS 2 Lecture outline Pathogenesis of autoimmunity: why selftolerance fails Genetics of autoimmune diseases Therapeutic
1 Autoimmunity and immunemediated inflammatory diseases Abul K. Abbas, MD UCSF FOCiS 2 Lecture outline Pathogenesis of autoimmunity: why selftolerance fails Genetics of autoimmune diseases Therapeutic
GenScript Antibody Services
 GenScript Antibody Services Scientific experts, innovative technologies, proven performance We specialize in custom antibody production to empower your research Custom Polyclonal Antibody Production Custom
GenScript Antibody Services Scientific experts, innovative technologies, proven performance We specialize in custom antibody production to empower your research Custom Polyclonal Antibody Production Custom
Analyzing antibody sequence for recombinant antibody expression. Hangxing Yu, Ph.D Senior Scientist, GenScript May 20, 2015
 Analyzing antibody sequence for recombinant antibody expression Hangxing Yu, Ph.D Senior Scientist, GenScript May 20, 2015 Presentation Outline 1 2 3 4 Antibody basics, structure and function Antibody
Analyzing antibody sequence for recombinant antibody expression Hangxing Yu, Ph.D Senior Scientist, GenScript May 20, 2015 Presentation Outline 1 2 3 4 Antibody basics, structure and function Antibody
A disease and antibody biology approach to antibody drug discovery
 A disease and antibody biology approach to antibody drug discovery Björn Frendéus, PhD VP, Preclinical research Presenter: Björn Frendéus Date: 2011-11-08 1 Antibodies have revolutionized Cancer Treatment!
A disease and antibody biology approach to antibody drug discovery Björn Frendéus, PhD VP, Preclinical research Presenter: Björn Frendéus Date: 2011-11-08 1 Antibodies have revolutionized Cancer Treatment!
The Immune System: A Tutorial
 The Immune System: A Tutorial Modeling and Simulation of Biological Systems 21-366B Shlomo Ta asan Images taken from http://rex.nci.nih.gov/behindthenews/uis/uisframe.htm http://copewithcytokines.de/ The
The Immune System: A Tutorial Modeling and Simulation of Biological Systems 21-366B Shlomo Ta asan Images taken from http://rex.nci.nih.gov/behindthenews/uis/uisframe.htm http://copewithcytokines.de/ The
Monoclonal Antibody Production: Building the Platform. Andrew Clutterbuck Eden Biodesign Ltd.
 Monoclonal Antibody Production: Building the Platform Andrew Clutterbuck Eden Biodesign Ltd. Questions Questions are encouraged throughout the presentation and can be asked by using the email address provided
Monoclonal Antibody Production: Building the Platform Andrew Clutterbuck Eden Biodesign Ltd. Questions Questions are encouraged throughout the presentation and can be asked by using the email address provided
specific B cells Humoral immunity lymphocytes antibodies B cells bone marrow Cell-mediated immunity: T cells antibodies proteins
 Adaptive Immunity Chapter 17: Adaptive (specific) Immunity Bio 139 Dr. Amy Rogers Host defenses that are specific to a particular infectious agent Can be innate or genetic for humans as a group: most microbes
Adaptive Immunity Chapter 17: Adaptive (specific) Immunity Bio 139 Dr. Amy Rogers Host defenses that are specific to a particular infectious agent Can be innate or genetic for humans as a group: most microbes
Custom Antibody Services
 Custom Antibody Services Custom service offerings DNA sequence Plasmid Peptide Structure Protein Peptide Small molecule Cells Spleen Lymphocytes Antigen Preparation Immunization Fusion & Subcloning Expansion
Custom Antibody Services Custom service offerings DNA sequence Plasmid Peptide Structure Protein Peptide Small molecule Cells Spleen Lymphocytes Antigen Preparation Immunization Fusion & Subcloning Expansion
Activation and effector functions of HMI
 Activation and effector functions of HMI Hathairat Thananchai, DPhil Department of Microbiology Faculty of Medicine Chiang Mai University 25 August 2015 ว ตถ ประสงค หล งจากช วโมงบรรยายน แล วน กศ กษาสามารถ
Activation and effector functions of HMI Hathairat Thananchai, DPhil Department of Microbiology Faculty of Medicine Chiang Mai University 25 August 2015 ว ตถ ประสงค หล งจากช วโมงบรรยายน แล วน กศ กษาสามารถ
BioProcessing J O U R N A L. Trends and Developments in BioProcess Technology. Volume 9 Issue 1 ISSN 1538-8786
 A Production of ISBioTech Summer 2010 Issue BioProcessing J O U R N A L Trends and Developments in BioProcess Technology Volume 9 Issue 1 ISSN 1538-8786 Trends & Developments in BioProcess Technology Novel
A Production of ISBioTech Summer 2010 Issue BioProcessing J O U R N A L Trends and Developments in BioProcess Technology Volume 9 Issue 1 ISSN 1538-8786 Trends & Developments in BioProcess Technology Novel
Overview of Upstream and Downstream Processing of Biopharmaceuticals
 Overview of Upstream and Downstream Processing of Biopharmaceuticals Ian Marison Professor of Bioprocess Engineering and Head of School of Biotechnology, Dublin City University, Glasnevin, Dublin 9, Ireland
Overview of Upstream and Downstream Processing of Biopharmaceuticals Ian Marison Professor of Bioprocess Engineering and Head of School of Biotechnology, Dublin City University, Glasnevin, Dublin 9, Ireland
Name (print) Name (signature) Period. (Total 30 points)
 AP Biology Worksheet Chapter 43 The Immune System Lambdin April 4, 2011 Due Date: Thurs. April 7, 2011 You may use the following: Text Notes Power point Internet One other person in class "On my honor,
AP Biology Worksheet Chapter 43 The Immune System Lambdin April 4, 2011 Due Date: Thurs. April 7, 2011 You may use the following: Text Notes Power point Internet One other person in class "On my honor,
Guidance for Industry
 Guidance for Industry Interpreting Sameness of Monoclonal Antibody Products Under the Orphan Drug Regulations U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance for Industry Interpreting Sameness of Monoclonal Antibody Products Under the Orphan Drug Regulations U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
CD22 Antigen Is Broadly Expressed on Lung Cancer Cells and Is a Target for Antibody-Based Therapy
 CD22 Antigen Is Broadly Expressed on Lung Cancer Cells and Is a Target for Antibody-Based Therapy Joseph M. Tuscano, Jason Kato, David Pearson, Chengyi Xiong, Laura Newell, Yunpeng Ma, David R. Gandara,
CD22 Antigen Is Broadly Expressed on Lung Cancer Cells and Is a Target for Antibody-Based Therapy Joseph M. Tuscano, Jason Kato, David Pearson, Chengyi Xiong, Laura Newell, Yunpeng Ma, David R. Gandara,
IMPROVEMENT OF RECOMBINANT PROTEIN S COST OF GOODS & AFFORDABILITY
 IMPROVEMENT OF RECOMBINANT PROTEIN S COST OF GOODS & AFFORDABILITY Li How Chen PhD Senior Director, revo Biologics Inc. USA Sami CHTOUROU PhD Senior Vice President Innovation & Scientific Affairs LFB Biotechnologies
IMPROVEMENT OF RECOMBINANT PROTEIN S COST OF GOODS & AFFORDABILITY Li How Chen PhD Senior Director, revo Biologics Inc. USA Sami CHTOUROU PhD Senior Vice President Innovation & Scientific Affairs LFB Biotechnologies
1.Gene Synthesis. 2.Peptide & Phospho-P. Assembly PCR. Design & Synthesis. Advantages. Specifications. Advantages
 1.Gene Synthesis Assembly PCR Looking for a cdna for your research but could not fish out the gene through traditional cloning methods or a supplier? Abnova provides a gene synthesis service via assembly
1.Gene Synthesis Assembly PCR Looking for a cdna for your research but could not fish out the gene through traditional cloning methods or a supplier? Abnova provides a gene synthesis service via assembly
Making the switch to a safer CAR-T cell therapy
 Making the switch to a safer CAR-T cell therapy HaemaLogiX 2015 Technical Journal Club May 24 th 2016 Christina Müller - chimeric antigen receptor = CAR - CAR T cells are generated by lentiviral transduction
Making the switch to a safer CAR-T cell therapy HaemaLogiX 2015 Technical Journal Club May 24 th 2016 Christina Müller - chimeric antigen receptor = CAR - CAR T cells are generated by lentiviral transduction
Thermo Scientific PepFinder Software A New Paradigm for Peptide Mapping
 Thermo Scientific PepFinder Software A New Paradigm for Peptide Mapping For Conclusive Characterization of Biologics Deep Protein Characterization Is Crucial Pharmaceuticals have historically been small
Thermo Scientific PepFinder Software A New Paradigm for Peptide Mapping For Conclusive Characterization of Biologics Deep Protein Characterization Is Crucial Pharmaceuticals have historically been small
Company Presentation June 2011 Biotest AG 0
 Company Presentation June 2011 0 Disclaimer This document contains forward-looking statements on overall economic development as well as on the business, earnings, financial and asset situation of and
Company Presentation June 2011 0 Disclaimer This document contains forward-looking statements on overall economic development as well as on the business, earnings, financial and asset situation of and
Towards Well-Defined ADCs (Antibody Drug Conjugates)
 Towards Well-Defined ADCs (Antibody Drug Conjugates) Next generation approach June 10, 2015 Dr. Yong Zu Kim, CEO and President LegoChem Biosciences, Inc. Antibody Drug Conjugates ADC binds to Antigen Endocytosis
Towards Well-Defined ADCs (Antibody Drug Conjugates) Next generation approach June 10, 2015 Dr. Yong Zu Kim, CEO and President LegoChem Biosciences, Inc. Antibody Drug Conjugates ADC binds to Antigen Endocytosis
Antibody Function & Structure
 Antibody Function & Structure Specifically bind to antigens in both the recognition phase (cellular receptors) and during the effector phase (synthesis and secretion) of humoral immunity Serology: the
Antibody Function & Structure Specifically bind to antigens in both the recognition phase (cellular receptors) and during the effector phase (synthesis and secretion) of humoral immunity Serology: the
The immune system. Bone marrow. Thymus. Spleen. Bone marrow. NK cell. B-cell. T-cell. Basophil Neutrophil. Eosinophil. Myeloid progenitor
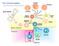 The immune system Basophil Neutrophil Bone marrow Eosinophil Myeloid progenitor Dendritic cell Pluripotent Stem cell Lymphoid progenitor Platelets Bone marrow Thymus NK cell T-cell B-cell Spleen Cancer
The immune system Basophil Neutrophil Bone marrow Eosinophil Myeloid progenitor Dendritic cell Pluripotent Stem cell Lymphoid progenitor Platelets Bone marrow Thymus NK cell T-cell B-cell Spleen Cancer
PX Therapeutics : the partner for early stage biotherapeutics development Biotuesday, May 5 2009
 PX Therapeutics : the partner for early stage biotherapeutics development Biotuesday, May 5 2009 Christelle Dagoneau, PhD Business Development Director Company Profile Protein expert incorporated in 2000
PX Therapeutics : the partner for early stage biotherapeutics development Biotuesday, May 5 2009 Christelle Dagoneau, PhD Business Development Director Company Profile Protein expert incorporated in 2000
Validated Cell-Based Assays for Rapid Screening and Functional Characterization of Therapeutic Monoclonal Antibodies
 Validated Cell-Based Assays for Rapid Screening and Functional Characterization of Therapeutic Monoclonal Antibodies Abhishek Saharia, Ph. D. Senior Product Manager Validation Assays Drug Discovery Evolution
Validated Cell-Based Assays for Rapid Screening and Functional Characterization of Therapeutic Monoclonal Antibodies Abhishek Saharia, Ph. D. Senior Product Manager Validation Assays Drug Discovery Evolution
This presentation may contain forward-looking statements, which reflect Trillium's current expectation regarding future events. These forward-looking
 Q1/2016 This presentation may contain forward-looking statements, which reflect Trillium's current expectation regarding future events. These forward-looking statements involve risks and uncertainties
Q1/2016 This presentation may contain forward-looking statements, which reflect Trillium's current expectation regarding future events. These forward-looking statements involve risks and uncertainties
Cell Culture Technology for Pharmaceutical and Cellular Therapies
 Cell Culture Technology for Pharmaceutical and Cellular Therapies Sadettin S. Ozturk, Ph.D. Centocor Inc. 200 Great Valley Parkway, Malvern, PA 19355 Outline 1. Introduction to Monoclonal Antibodies 2.
Cell Culture Technology for Pharmaceutical and Cellular Therapies Sadettin S. Ozturk, Ph.D. Centocor Inc. 200 Great Valley Parkway, Malvern, PA 19355 Outline 1. Introduction to Monoclonal Antibodies 2.
Introduction to Bioprocessing
 Introduction to Bioprocessing Cambridge Healthtech Institute Peptalk Palm Springs, CA Presented by Susan Dana Jones and Sheila Magil BioProcess Technology Consultants www.bptc.com BioProcess Technology
Introduction to Bioprocessing Cambridge Healthtech Institute Peptalk Palm Springs, CA Presented by Susan Dana Jones and Sheila Magil BioProcess Technology Consultants www.bptc.com BioProcess Technology
argen-x Reports Fourth Quarter Business Update And Full Year 2014 Financial Results
 argen-x Reports Fourth Quarter Business Update And Full Year 2014 Financial Results Management to host conference call today at 6 pm CET / 1 pm EDT 18 March 2015 Breda, the Netherlands / Ghent, Belgium
argen-x Reports Fourth Quarter Business Update And Full Year 2014 Financial Results Management to host conference call today at 6 pm CET / 1 pm EDT 18 March 2015 Breda, the Netherlands / Ghent, Belgium
12. November 2013 Jan Endell. From library to bedside: Potential of the anti-cd38 antibody MOR202 in combination therapy of multiple myeloma
 12. November 2013 Jan Endell From library to bedside: Potential of the anti-cd38 antibody MOR202 in combination therapy of multiple myeloma The MorphoSys Pipeline 21 Clinical Programs, 82 Total Program
12. November 2013 Jan Endell From library to bedside: Potential of the anti-cd38 antibody MOR202 in combination therapy of multiple myeloma The MorphoSys Pipeline 21 Clinical Programs, 82 Total Program
Scientific Challenges for Development of Biosimilar Monoclonal Antibodies. Rafiqul Islam Director, Global Bioanalytical Services Celerion
 Scientific Challenges for Development of Biosimilar Monoclonal Antibodies Rafiqul Islam Director, Global Bioanalytical Services Celerion Presentation outline Biosimilars Definitions and Concepts Regulatory
Scientific Challenges for Development of Biosimilar Monoclonal Antibodies Rafiqul Islam Director, Global Bioanalytical Services Celerion Presentation outline Biosimilars Definitions and Concepts Regulatory
From Research Services and Process Development to GMP Manufacturing
 From Research Services and Process Development to GMP Manufacturing P a r ag o n B i o s e r v i c e s, I n c. A contract research and GMP manufacturing organization (CMO) with a focus on the development
From Research Services and Process Development to GMP Manufacturing P a r ag o n B i o s e r v i c e s, I n c. A contract research and GMP manufacturing organization (CMO) with a focus on the development
3 months 1.5 months 1.5 months. 1 month
 Rabbit monoclonal antibody (Mab) is secreted by the plasma B-cell of the rabbit. Traditional generation of rabbit Mab relies on a rabbit myeloma for B- cell fusion (
Rabbit monoclonal antibody (Mab) is secreted by the plasma B-cell of the rabbit. Traditional generation of rabbit Mab relies on a rabbit myeloma for B- cell fusion (
Amaxa Mouse T Cell Nucleofector Kit
 Amaxa Mouse T Cell Nucleofector Kit For T cells isolated from C57BL/6 & BALB/c mice Evaluated for murine T cells isolated from C57BL/6 & BALB/c mice This protocol is designed for murine lymphocytes or
Amaxa Mouse T Cell Nucleofector Kit For T cells isolated from C57BL/6 & BALB/c mice Evaluated for murine T cells isolated from C57BL/6 & BALB/c mice This protocol is designed for murine lymphocytes or
Applications of Ab Molecules. Chapter 4 Monoclonal Ab (p.99) Chapter 5 Ab genes and Ab Engineering (p.128)
 Applications of Ab Molecules Chapter 4 Monoclonal Ab (p.99) Chapter 5 Ab genes and Ab Engineering (p.128) Monoclonal Antibodies Clonal Selection of B Lymphocytes Hybridoma Köhler and Milsten (1975) - continuous
Applications of Ab Molecules Chapter 4 Monoclonal Ab (p.99) Chapter 5 Ab genes and Ab Engineering (p.128) Monoclonal Antibodies Clonal Selection of B Lymphocytes Hybridoma Köhler and Milsten (1975) - continuous
DARATUMUMAB, A CD38 MONOCLONAL ANTIBODY IN PATIENTS WITH MULTIPLE MYELOMA - DATA FROM A DOSE- ESCALATION PHASE I/II STUDY
 DARATUMUMAB, A CD38 MONOCLONAL ANTIBODY IN PATIENTS WITH MULTIPLE MYELOMA - DATA FROM A DOSE- ESCALATION PHASE I/II STUDY Torben Plesner, Henk Lokhorst, Peter Gimsing, Hareth Nahi, Steen Lisby, Paul Richardson
DARATUMUMAB, A CD38 MONOCLONAL ANTIBODY IN PATIENTS WITH MULTIPLE MYELOMA - DATA FROM A DOSE- ESCALATION PHASE I/II STUDY Torben Plesner, Henk Lokhorst, Peter Gimsing, Hareth Nahi, Steen Lisby, Paul Richardson
Application Note. Purifying common light-chain bispecific antibodies using MCSGP. Summary
 Application Note Purifying common light-chain bispecific antibodies using MCSGP Category Matrix Method Keywords Analytes ID Continuous chromatography, biochromatography Antibodies MCSGP Bispecific antibody,
Application Note Purifying common light-chain bispecific antibodies using MCSGP Category Matrix Method Keywords Analytes ID Continuous chromatography, biochromatography Antibodies MCSGP Bispecific antibody,
Monoclonal Antibodies in Cancer. Ralph Schwall, PhD Associate Director, Translational Oncology Genentech, Inc.
 Monoclonal Antibodies in Cancer Ralph Schwall, PhD Associate Director, Translational Oncology Genentech, Inc. Disclaimer I had nothing to do with Herceptin Using lessons learned in new antibody projects
Monoclonal Antibodies in Cancer Ralph Schwall, PhD Associate Director, Translational Oncology Genentech, Inc. Disclaimer I had nothing to do with Herceptin Using lessons learned in new antibody projects
Triskel: a strategic consulting firm for biopharmaceutical companies
 BioStart : Manufacturing and operations in Biotech Strategic stakes and technology evolution in recombinant protein production APFL May 14 th, 2009 François LAWNY Triskel Integrated Services Geneva, Switzerland
BioStart : Manufacturing and operations in Biotech Strategic stakes and technology evolution in recombinant protein production APFL May 14 th, 2009 François LAWNY Triskel Integrated Services Geneva, Switzerland
Development and validation of neutralising anti-drug antibody (Nabs) assays
 Development and validation of neutralising anti-drug antibody (Nabs) assays Clare Kingsley Sector Manager, Bioanalytical Sciences, Quotient Bioresearch EBF 2012 Overview Anti-drug antibody and neutralising
Development and validation of neutralising anti-drug antibody (Nabs) assays Clare Kingsley Sector Manager, Bioanalytical Sciences, Quotient Bioresearch EBF 2012 Overview Anti-drug antibody and neutralising
Integrated Protein Services
 Integrated Protein Services Custom protein expression & purification Version DC04-0012 Expression strategy The first step in the recombinant protein generation process is to design an appropriate expression
Integrated Protein Services Custom protein expression & purification Version DC04-0012 Expression strategy The first step in the recombinant protein generation process is to design an appropriate expression
Can receive blood from: * I A I A and I A i o Type A Yes No A or AB A or O I B I B and I B i o Type B No Yes B or AB B or O
 Genetics of the ABO Blood Groups written by J. D. Hendrix Learning Objectives Upon completing the exercise, each student should be able: to explain the concept of blood group antigens; to list the genotypes
Genetics of the ABO Blood Groups written by J. D. Hendrix Learning Objectives Upon completing the exercise, each student should be able: to explain the concept of blood group antigens; to list the genotypes
CCR Biology - Chapter 9 Practice Test - Summer 2012
 Name: Class: Date: CCR Biology - Chapter 9 Practice Test - Summer 2012 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Genetic engineering is possible
Name: Class: Date: CCR Biology - Chapter 9 Practice Test - Summer 2012 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Genetic engineering is possible
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Evaluation of Medicines for Human Use London, 10 October 2007 Doc. Ref. COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON POTENCY TESTING OF CELL BASED IMMUNOTHERAPY
European Medicines Agency Evaluation of Medicines for Human Use London, 10 October 2007 Doc. Ref. COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON POTENCY TESTING OF CELL BASED IMMUNOTHERAPY
Chapter 5: Organization and Expression of Immunoglobulin Genes
 Chapter 5: Organization and Expression of Immunoglobulin Genes I. Genetic Model Compatible with Ig Structure A. Two models for Ab structure diversity 1. Germ-line theory: maintained that the genome contributed
Chapter 5: Organization and Expression of Immunoglobulin Genes I. Genetic Model Compatible with Ig Structure A. Two models for Ab structure diversity 1. Germ-line theory: maintained that the genome contributed
Geniron. Custom Antibody Services. Serum services Antibody Monoclonal. Purification Antibody Mono Y Genetic Immunization Genbody Polyclonal Antibody
 Geniron Custom Antibody Services Serum services Antibody Monoclonal Purification Antibody Mono Y Genetic Immunization Genbody Polyclonal Antibody Geniron Poly Y WE PROVIDE OUR SERVICES TO With Expertise
Geniron Custom Antibody Services Serum services Antibody Monoclonal Purification Antibody Mono Y Genetic Immunization Genbody Polyclonal Antibody Geniron Poly Y WE PROVIDE OUR SERVICES TO With Expertise
A Genetic Analysis of Rheumatoid Arthritis
 A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
A Risk Assessment of Pre-Licensure Manufacturing Changes
 A Risk Assessment of Pre-Licensure Manufacturing Changes Patrick G. Swann, Ph.D. Deputy Director Division of Monoclonal Antibodies Office of Biotechnology Products Office of Pharmaceutical Science FDA-CDER
A Risk Assessment of Pre-Licensure Manufacturing Changes Patrick G. Swann, Ph.D. Deputy Director Division of Monoclonal Antibodies Office of Biotechnology Products Office of Pharmaceutical Science FDA-CDER
Technology Transfer of CMC Activities for MAb Manufacturing. 2010 ge healthcare (www.gelifesciences.com)
 M a n u f a c t u r i n g OPERATIONS Technology Transfer of CMC Activities for MAb Manufacturing by Patricia Seymour, Susan Dana Jones, Howard L. Levine With combined 2009 revenues estimated to be over
M a n u f a c t u r i n g OPERATIONS Technology Transfer of CMC Activities for MAb Manufacturing by Patricia Seymour, Susan Dana Jones, Howard L. Levine With combined 2009 revenues estimated to be over
Evaluation of an alkali stable Protein A matrix versus Protein A Sepharose Fast Flow and considerations on process scale-up to 20,000L.
 Evaluation of an alkali stable Protein A matrix versus Protein A Sepharose Fast Flow and considerations on process scale-up to 20,000L. Simon C.B. Jones & Martin P. Smith. LONZA, 224 Bath Road, Slough,
Evaluation of an alkali stable Protein A matrix versus Protein A Sepharose Fast Flow and considerations on process scale-up to 20,000L. Simon C.B. Jones & Martin P. Smith. LONZA, 224 Bath Road, Slough,
Engineering Chinese Hamster Ovary Cells to Maximize Effector Function of Produced Antibodies Using FUT8 sirna
 Engineering Chinese Hamster Ovary Cells to Maximize Effector Function of Produced Antibodies Using FUT8 sirna Katsuhiro Mori, Reiko Kuni-Kamochi, Naoko Yamane-Ohnuki, Masako Wakitani, Kazuya Yamano, Harue
Engineering Chinese Hamster Ovary Cells to Maximize Effector Function of Produced Antibodies Using FUT8 sirna Katsuhiro Mori, Reiko Kuni-Kamochi, Naoko Yamane-Ohnuki, Masako Wakitani, Kazuya Yamano, Harue
Valentina Gualato, Ph.D. Process Development Scientist
 COMPANY PRESENTATION Quality and Innovation Valentina Gualato, Ph.D. Process Development Scientist MISSION areta international is a biotech company dedicated to the contract development and manufacturing
COMPANY PRESENTATION Quality and Innovation Valentina Gualato, Ph.D. Process Development Scientist MISSION areta international is a biotech company dedicated to the contract development and manufacturing
TransIT -2020 Transfection Reagent
 Quick Reference Protocol, MSDS and Certificate of Analysis available at mirusbio.com/5400 INTRODUCTION TransIT -2020 Transfection Reagent is a high-performance, animal-origin free, broad spectrum reagent
Quick Reference Protocol, MSDS and Certificate of Analysis available at mirusbio.com/5400 INTRODUCTION TransIT -2020 Transfection Reagent is a high-performance, animal-origin free, broad spectrum reagent
B Cells and Antibodies
 B Cells and Antibodies Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School Lecture outline Functions of antibodies B cell activation; the role of helper T cells in antibody production
B Cells and Antibodies Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School Lecture outline Functions of antibodies B cell activation; the role of helper T cells in antibody production
Breakthrough Cancer Therapies: Directing the Immune System to Eliminate Tumor Cells. Corporate Presentation May 2015
 Breakthrough Cancer Therapies: Directing the Immune System to Eliminate Tumor Cells Corporate Presentation May 2015 Forward-looking statements / safe harbor This presentation and the accompanying oral
Breakthrough Cancer Therapies: Directing the Immune System to Eliminate Tumor Cells Corporate Presentation May 2015 Forward-looking statements / safe harbor This presentation and the accompanying oral
Manufacturing process of biologics
 Manufacturing process of biologics K. Ho Afssaps, France 2011 ICH International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use 2011 ICH 1 Disclaimer:
Manufacturing process of biologics K. Ho Afssaps, France 2011 ICH International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use 2011 ICH 1 Disclaimer:
