Post-PET Restaging Cancer Form National Oncologic PET Registry
|
|
|
- Ada Ellis
- 7 years ago
- Views:
Transcription
1 Post-PET Restaging Cancer Form National Oncologic PET Registry Facility ID #: Registry Case Number: Patient Name: Your patient had a PET scan on: mm/dd/yyyy. The PET scan was done for restaging of (cancer type). (auto fill cancer type from Pre-PET Form). After reviewing the PET report, please complete the following questions and return the form to the PET Facility. This form must be completed and entered into the NOPR database within 30 days of the PET scan. 1. Compared to your Pre-PET assessment, your impression of the overall extent of disease is? (choose one) More extensive No change Less extensive 2. Did the PET scan show evidence of cancer activity that was not previously documented? Yes No a. If yes, is some type of tissue biopsy planned of the area? Yes No 3. Your Post-PET working clinical staging is: (select only one) No evidence of disease / In remission Low probability of local recurrence (including regional lymph nodes) or metastases Local recurrence (including regional lymph nodes) Metastatic disease with single site Metastatic disease with multiple sites 4. Did the PET scan enable you to avoid more tests or procedures? Yes No 5. In light of the PET findings, which of the following management strategies are you now planning or have you already undertaken? (you must check only one) Observation (with close follow-up) Additional Imaging (CT, MRI) or other non-invasive diagnostic tests Tissue Biopsy (surgical, percutaneous, or endoscopic). Note: If concurrent biopsy and total surgical resection are planned, then mark surgical treatment below. Treatment (see additional required responses below) Treatment Goal: (check one) Curative Palliative Type(s): (check all that apply) Surgical Chemotherapy (including biologic modifiers) Radiation Other Supportive care Will treatment be directly provided by you? (check one) Yes No
2 6. I have read the Referring Physician Information Statement and: I do give my consent for the inclusion of data collected for this patient in NOPR research. I do NOT give my consent for the inclusion of data collected for this patient in NOPR research. 7. NAME OF PERSON WHO COMPLETED THE PAPER FORM First Name: Last Name: Date PHYSICIAN ATTESTATION OF DATA ACCURACY By signing below I verify that, to the best of my knowledge, the information on this form is accurate. Physician Signature: Date Printed Name of Physician: PRA Disclosure Statement According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is The time required to complete this information collection is estimated to average five (5) minutes per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: CMS, 7500 Security Boulevard, Attn: PRA Reports Clearance Officer, Mail Stop C , Baltimore, Maryland
3 Post-PET Suspected Cancer Recurrence Form National Oncologic PET Registry Facility ID #: Registry Case Number: Patient Name: Your patient had a PET scan on: mm/dd/yyyy. The PET scan was done for a suspected recurrence of (cancer type). (auto fill cancer type from Pre-PET Form). After reviewing the PET report, please complete the following questions and return the form to the PET Facility. This form must be completed and entered into the NOPR database within 30 days of the PET scan. 1. Compared to your Pre-PET assessment, your impression of the overall extent of disease is: (choose one) More extensive No change Less extensive 2. Did the PET scan show evidence of cancer activity that was not previously documented? Yes No If yes, is some type of tissue biopsy planned of the area? Yes No 3. Your Post-PET working clinical summary staging is: (select only one) No evidence of disease / In remission Low probability of local recurrence (including regional lymph nodes) or metastases Local recurrence (including regional lymph nodes) Metastatic disease with single site Metastatic disease with multiple sites 4. Did the PET scan enable you to avoid more tests or procedures? Yes No 5. In light of the PET findings, which of the following management strategies are you now planning or have you already undertaken? (you must check only one) Observation (with close follow-up) Additional Imaging (CT, MRI) or other non-invasive diagnostic tests Tissue Biopsy (surgical, percutaneous, or endoscopic). Note: If concurrent biopsy and total surgical resection are planned, then mark surgical treatment below. Treatment (see additional required responses below) Treatment Goal: (check one) Curative Palliative Type(s): (check all that apply) Surgical Chemotherapy (including biologic modifiers) Radiation Other Supportive care Will treatment be directly provided by you? (check one) Yes No
4 6. I have read the Referring Physician Information Statement and: I do give my consent for the inclusion of data collected for this patient in NOPR research. I do NOT give my consent for the inclusion of data collected for this patient in NOPR research. 7. NAME OF PERSON WHO COMPLETED THE PAPER FORM First Name: Last Name: Date PHYSICIAN ATTESTATION OF DATA ACCURACY By signing below I verify that, to the best of my knowledge, the information on this form is accurate. Physician Signature: Date Printed Name of Physician: PRA Disclosure Statement According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is The time required to complete this information collection is estimated to average five (5) minutes per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: CMS, 7500 Security Boulevard, Attn: PRA Reports Clearance Officer, Mail Stop C , Baltimore, Maryland
5 Post-PET Treatment Monitoring Form National Oncologic PET Registry Facility ID #: Registry Case Number: Patient Name: Your patient had a PET scan on: mm/dd/yyyy. The PET scan was done for treatment response monitoring of (cancer type) to chemo/radiation/or other therapy (auto fill from Pre-PET data form the cancer type and treatment type). After reviewing the PET report, please complete the following questions and return the form to the PET Facility. This form must be completed and entered into the NOPR database within 30 days of the PET scan. 1. In light of the PET findings, which of the following management strategies are you now planning or have you already undertaken? (you must check only one) Observation (with close follow-up) Additional Imaging (CT, MRI) or other non-invasive diagnostic tests Tissue Biopsy (surgical, percutaneous, or endoscopic). Note: If concurrent biopsy and total surgical resection are planned, then mark surgical treatment below. Treatment (see additional required responses below) Treatment Goal: (check one) Curative Palliative Type(s): (check all that apply) Surgical Chemotherapy (including biologic modifiers) Radiation Other Supportive care Will treatment be directly provided by you? (check one) Yes No 2. What is your current impression (in light of the PET findings) of your patient s response to currently ongoing therapy? (check one) Clearly responding Partial response No response or stable disease Progressive disease 3. Please indicate if and how you will modify your therapeutic plan in light of the PET findings. (You must check only one) Continue and complete currently ongoing therapy Modify dose or schedule of currently ongoing therapy Switch to another therapy or add another mode of therapy Stop therapy and switch to supportive care
6 4. If PET were not available, would you have done some type of alternative assessment at this time? Yes No 5. Did the PET scan enable you to avoid more tests or procedures? Yes No 6. In light of the PET results, how has the prognosis for your patient changed? (check one) Better No change Worse 7. I have read the Referring Physician Information Statement and: I do give my consent for the inclusion of data collected for this patient in NOPR research. I do NOT give my consent for the inclusion of data collected for this patient in NOPR research. 8. NAME OF PERSON WHO COMPLETED THE PAPER FORM First Name: Last Name: Date PHYSICIAN ATTESTATION OF DATA ACCURACY By signing below I verify that, to the best of my knowledge, the information on this form is accurate. Physician Signature: Date Printed Name of Physician: PRA Disclosure Statement According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is The time required to complete this information collection is estimated to average five (5) minutes per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: CMS, 7500 Security Boulevard, Attn: PRA Reports Clearance Officer, Mail Stop C , Baltimore, Maryland
7 Post-PET Suspected Leukemia Form National Oncologic PET Registry Facility ID #: Registry Case Number: Patient Name: Your patient had a PET scan on: mm/dd/yyyy. You previously indicated that the PET scan was done for assessing whether a suspicious lesion is leukemia. After reviewing the PET report, please complete the following questions and return the form to the PET Facility. This form must be entered into the database within 30 days of the PET scan. 2. Has a tissue biopsy been performed of a suspicious site? Yes No 3. Did the PET scan enable you to avoid any tests or procedures? Yes No 4. In light of the PET findings, which of the following management strategies are you now planning or have you already undertaken? (you must check only one) Observation (with close follow-up) Additional Imaging (CT, MRI) or other non-invasive diagnostic tests Tissue Biopsy (surgical, percutaneous, or endoscopic). Note: If concurrent biopsy and total surgical resection are planned, then mark surgical treatment listed below. Treatment (see additional required responses below) Treatment Goal: (check one) Curative Palliative Type(s): (all that apply) Surgical Chemotherapy (including biologic modifiers) Radiation Other Supportive care Will treatment be directly provided by you? (check one) Yes No 4. I have read the Referring Physician Information Statement and: I do give my consent for the inclusion of data collected for this patient in NOPR research. I do NOT give my consent for the inclusion of data collected for this patient in NOPR research. 5. NAME OF PERSON WHO COMPLETED THE PAPER FORM: First Name: Last Name: Date:
8 PHYSICIAN ATTESTATION OF DATA ACCURACY By signing below I verify that, to the best of my knowledge, the information on this form is accurate. Physician Signature: Date Printed Name of Physician: PRA Disclosure Statement According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is The time required to complete this information collection is estimated to average five (5) minutes per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: CMS, 7500 Security Boulevard, Attn: PRA Reports Clearance Officer, Mail Stop C , Baltimore, Maryland
9 Post-PET Paraneoplastic Syndrome Form National Oncologic PET Registry Facility ID #: Registry Case Number: Patient Name: Your patient had a PET scan on: mm/dd/yyyy. You previously indicated that the PET scan was done to detect occult leukemia in a patient with a suspected paraneoplastic syndrome. (auto fill reason from Pre-PET Form) After reviewing the PET report, please complete the following questions and return the form to the PET Facility. This form must be entered into the database within 30 days of the PET scan. 1. Was leukemia (or another primary cancer site) identified by PET? Yes No 2. Was a tissue biopsy or surgical excision performed of a suspected tumor? Yes No 3. Did the PET scan enable you to avoid any tests or procedures? Yes No 4. In light of the PET findings, which of the following management strategies are you now planning or have you already undertaken? (you must check only one) Observation (with close follow-up) Additional Imaging (CT, MRI) or other non-invasive diagnostic tests Tissue Biopsy (surgical, percutaneous, or endoscopic). Note: If concurrent biopsy and total surgical resection are planned, then mark surgical treatment listed below. Treatment (see additional required responses below) Treatment Goal: (check one) Curative Palliative Type(s): (check all that apply) Surgical Chemotherapy (including biologic modifiers) Radiation Other Supportive care Will treatment be directly provided by you? (check one) Yes No 5. I have read the Referring Physician Information Statement and: I Do give my consent for the inclusion of data collected for this patient in NOPR research. I DO NOT give my consent for the inclusion of data collected for this patient in NOPR research.
10 6. NAME OF PERSON WHO COMPLETED THE PAPER FORM: First Name: Last Name: Date: PHYSICIAN ATTESTATION OF DATA ACCURACY By signing below I verify that, to the best of my knowledge, the information on this form is accurate. Physician Signature: Date Printed Name of Physician: PRA Disclosure Statement According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is The time required to complete this information collection is estimated to average five (5) minutes per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: CMS, 7500 Security Boulevard, Attn: PRA Reports Clearance Officer, Mail Stop C , Baltimore, Maryland
11 Post-PET Initial Staging Form National Oncologic PET Registry Facility ID #: Registry Case Number: Patient Name: Your patient had a PET scan on: mm/dd/yyyy. The PET scan was done for initial staging of leukemia. After reviewing the PET report, please complete the following questions and return the form to the PET Facility. This form must be entered into the database within 30 days of the PET scan. 1. Compared to your Pre-PET assessment, your impression of the extent of the patient s leukemia is? (check one) More extensive No change Less extensive 2. Did the PET scan, show evidence of leukemic involvement that was not previously documented? Yes No a. If yes, is some type of tissue biopsy planned of the area? Yes No 3. Are any more tests or imaging or biopsies planned before starting treatment? Yes No 4. Did the PET scan enable you to avoid any tests or procedures? Yes No 5. Your Post-PET working clinical summary staging is? (you must check only one) No evidence of disease / In remission Localized disease only Systemic disease Unknown or uncertain 6. In light of the PET findings, which of the following management strategies are you now planning or have you already undertaken? (you must choose only one) Observation (with close follow-up) Additional Imaging (CT, MRI) or other non-invasive diagnostic tests Tissue Biopsy (surgical, percutaneous, or endoscopic). Note: If concurrent biopsy and total surgical resection are planned, then mark surgical treatment listed below. Treatment (see additional required responses below) Treatment Goal: (check one) Curative Palliative Type(s): (check all that apply) Surgical Chemotherapy (including biologic modifiers) Radiation Other Supportive care Will treatment be directly provided by you? (check one) Yes No
12 7. I have read the Referring Physician Information Statement and: I do give my consent for the inclusion of data collected for this patient in NOPR research. I do NOT give my consent for the inclusion of data collected for this patient in NOPR research. 8. NAME OF PERSON WHO COMPLETED THE PAPER FORM: First Name: Last Name: Date: PHYSICIAN ATTESTATION OF DATA ACCURACY By signing below I verify that, to the best of my knowledge, the information on this form is accurate. Physician Signature: Date Printed Name of Physician: PRA Disclosure Statement According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is The time required to complete this information collection is estimated to average five (5) minutes per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: CMS, 7500 Security Boulevard, Attn: PRA Reports Clearance Officer, Mail Stop C , Baltimore, Maryland
Rotation Specific Goals & Objectives: University Health Network-Princess Margaret Hospital/ Sunnybrook Breast/Melanoma
 Rotation Specific Goals & Objectives: University Health Network-Princess Margaret Hospital/ Sunnybrook Breast/Melanoma Medical Expert: Breast Rotation Specific Competencies/Objectives 1.0 Medical History
Rotation Specific Goals & Objectives: University Health Network-Princess Margaret Hospital/ Sunnybrook Breast/Melanoma Medical Expert: Breast Rotation Specific Competencies/Objectives 1.0 Medical History
The National Oncologic PET Registry (NOPR): B. Operations Manual for. NOPR (NaF-PET)
 B1 The National Oncologic PET Registry (NOPR): B. Operations Manual for NOPR (NaF-PET) Sponsored by: Academy of Molecular Imaging Managed by: American College of Radiology American College of Radiology
B1 The National Oncologic PET Registry (NOPR): B. Operations Manual for NOPR (NaF-PET) Sponsored by: Academy of Molecular Imaging Managed by: American College of Radiology American College of Radiology
Lung Cancer Treatment Guidelines
 Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
The Di Bella Method (DBM) improves Survival, Objective Response and Performance Status in Breast Cancer
 BIT's 4th World Cancer Congress 2011 People s Republic of China Dalian The Di Bella Method (DBM) improves Survival, Objective Response and Performance Status in treated with DBM therapy Retrospective observational
BIT's 4th World Cancer Congress 2011 People s Republic of China Dalian The Di Bella Method (DBM) improves Survival, Objective Response and Performance Status in treated with DBM therapy Retrospective observational
MEDICARE PARTICIPATING PHYSICIAN OR SUPPLIER AGREEMENT
 DEPARTMENT OF HEALTH AND HUMAN SERVICES CENTERS FOR MEDICARE & MEDICAID SERVICES MEDICARE PARTICIPATING PHYSICIAN OR SUPPLIER AGREEMENT FORM APPROVED OMB NO. 0938-0373 Name(s) and Address of Participant*
DEPARTMENT OF HEALTH AND HUMAN SERVICES CENTERS FOR MEDICARE & MEDICAID SERVICES MEDICARE PARTICIPATING PHYSICIAN OR SUPPLIER AGREEMENT FORM APPROVED OMB NO. 0938-0373 Name(s) and Address of Participant*
RESEARCH EDUCATE ADVOCATE. Just Diagnosed with Melanoma Now What?
 RESEARCH EDUCATE ADVOCATE Just Diagnosed with Melanoma Now What? INTRODUCTION If you are reading this, you have undergone a biopsy (either of a skin lesion or a lymph node) or have had other tests in which
RESEARCH EDUCATE ADVOCATE Just Diagnosed with Melanoma Now What? INTRODUCTION If you are reading this, you have undergone a biopsy (either of a skin lesion or a lymph node) or have had other tests in which
HERC Coverage Guidance Advanced Imaging for Staging of Prostate Cancer Disposition of Public Comments
 Table of Contents Commenters... 1 Public Comments... 2 References Provided by Commenters... 6 Commenters Identification Stakeholder A Medical Imaging & Technology Alliance (MITA), Arlington, VA [Submitted
Table of Contents Commenters... 1 Public Comments... 2 References Provided by Commenters... 6 Commenters Identification Stakeholder A Medical Imaging & Technology Alliance (MITA), Arlington, VA [Submitted
SERVICES OFFERED: Yearly Comprehensive Medication Review (CMR) Quarterly Targeted Medication Review (TMR)
 MEDICATION THERAPY MANAGEMENT (MTM) PROGRAM 2015 plan year This document contains information about the MTM Program for plan year 2015. Our goal is to help you get the best results from your medications
MEDICATION THERAPY MANAGEMENT (MTM) PROGRAM 2015 plan year This document contains information about the MTM Program for plan year 2015. Our goal is to help you get the best results from your medications
PET Coding & Coverage: Including NOPR Sequel April 27, 2009. Presented by: Denise A. Merlino, MBA, CNMT, FSNMTS,CPC. Agenda
 Presented by: Barry Siegel, MD Mallinckrodt Institute of Radiology PET Coding & Coverage: Including NOPR Sequel April 27, 2009 Presented by: Denise A. Merlino, MBA, CNMT, FSNMTS,CPC President, Merlino
Presented by: Barry Siegel, MD Mallinckrodt Institute of Radiology PET Coding & Coverage: Including NOPR Sequel April 27, 2009 Presented by: Denise A. Merlino, MBA, CNMT, FSNMTS,CPC President, Merlino
Metastatic Renal Cell Carcinoma: Staging and Prognosis of Three Separate Cases.
 Metastatic Renal Cell Carcinoma: Staging and Prognosis of Three Separate Cases. Abstract This paper describes the staging, imaging, treatment, and prognosis of renal cell carcinoma. Three case studies
Metastatic Renal Cell Carcinoma: Staging and Prognosis of Three Separate Cases. Abstract This paper describes the staging, imaging, treatment, and prognosis of renal cell carcinoma. Three case studies
This form is used to advise Medicare of the person or persons you have chosen to have access to your personal health information.
 Medicare Beneficiary Services:1-800-MEDICARE (1-800-633-4227) TTY/ TDD:1-877-486-2048 This form is used to advise Medicare of the person or persons you have chosen to have access to your personal health
Medicare Beneficiary Services:1-800-MEDICARE (1-800-633-4227) TTY/ TDD:1-877-486-2048 This form is used to advise Medicare of the person or persons you have chosen to have access to your personal health
Probe: Could you tell me about when?
 PERIODIC ASSESSMENT OF TREATMENT AND VITAL/DISEASE STATUS Periodic Assessment of Cancer Treatment and Disease Status (To be administered to patient at 3 months and reviewed at 6, 9 and 12 months) Instructions:
PERIODIC ASSESSMENT OF TREATMENT AND VITAL/DISEASE STATUS Periodic Assessment of Cancer Treatment and Disease Status (To be administered to patient at 3 months and reviewed at 6, 9 and 12 months) Instructions:
D. FREQUENTLY ASKED QUESTIONS
 ACR BI-RADS ATLAS D. FREQUENTLY ASKED QUESTIONS 1. Under MQSA, is it necessary to include a numeric assessment code (i.e., 0, 1, 2, 3, 4, 5, or 6) in addition to the assessment category in all mammography
ACR BI-RADS ATLAS D. FREQUENTLY ASKED QUESTIONS 1. Under MQSA, is it necessary to include a numeric assessment code (i.e., 0, 1, 2, 3, 4, 5, or 6) in addition to the assessment category in all mammography
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
Smoking and misuse of certain pain medicines can affect the risk of developing renal cell cancer.
 Renal cell cancer Renal cell cancer is a disease in which malignant (cancer) cells form in tubules of the kidney. Renal cell cancer (also called kidney cancer or renal adenocarcinoma) is a disease in which
Renal cell cancer Renal cell cancer is a disease in which malignant (cancer) cells form in tubules of the kidney. Renal cell cancer (also called kidney cancer or renal adenocarcinoma) is a disease in which
The Need for Accurate Lung Cancer Staging
 The Need for Accurate Lung Cancer Staging Peter Baik, DO Thoracic Surgery Cancer Treatment Centers of America Oklahoma Osteopathic Association 115th Annual Convention Financial Disclosures: None 2 Objectives
The Need for Accurate Lung Cancer Staging Peter Baik, DO Thoracic Surgery Cancer Treatment Centers of America Oklahoma Osteopathic Association 115th Annual Convention Financial Disclosures: None 2 Objectives
Hand & Orthopedic Physical Therapy Associates, P.C.
 Patient Name: Hand & Orthopedic Physical Therapy Associates, P.C. Date of Birth: ADVANCE BENEFICIARY NOTICE OF NONCOVERAGE (ABN) NOTE: If Medicare doesn t pay for items listed below, you may have to pay.
Patient Name: Hand & Orthopedic Physical Therapy Associates, P.C. Date of Birth: ADVANCE BENEFICIARY NOTICE OF NONCOVERAGE (ABN) NOTE: If Medicare doesn t pay for items listed below, you may have to pay.
Kidney Cancer OVERVIEW
 Kidney Cancer OVERVIEW Kidney cancer is the third most common genitourinary cancer in adults. There are approximately 54,000 new cancer cases each year in the United States, and the incidence of kidney
Kidney Cancer OVERVIEW Kidney cancer is the third most common genitourinary cancer in adults. There are approximately 54,000 new cancer cases each year in the United States, and the incidence of kidney
Breast Cancer Treatment Guidelines
 Breast Cancer Treatment Guidelines DCIS Stage 0 TisN0M0 Tamoxifen for 5 years for patients with ER positive tumors treated with: -Breast conservative therapy (lumpectomy) and radiation therapy -Excision
Breast Cancer Treatment Guidelines DCIS Stage 0 TisN0M0 Tamoxifen for 5 years for patients with ER positive tumors treated with: -Breast conservative therapy (lumpectomy) and radiation therapy -Excision
IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN
 + IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
+ IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
Interview with David Djang, MD On PET Scan in Oncology: Principles and Practice
 Interview with David Djang, MD On PET Scan in Oncology: Principles and Practice By Howard (Jack) West, MD May, 2009 Hello and welcome to the GRACE audio podcast on PET scanning. This one is with Dr. David
Interview with David Djang, MD On PET Scan in Oncology: Principles and Practice By Howard (Jack) West, MD May, 2009 Hello and welcome to the GRACE audio podcast on PET scanning. This one is with Dr. David
Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines
 Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines April 2008 (presented at 6/12/08 cancer committee meeting) By Shelly Smits, RHIT, CCS, CTR Conclusions by Dr. Ian Thompson, MD Dr. James
Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines April 2008 (presented at 6/12/08 cancer committee meeting) By Shelly Smits, RHIT, CCS, CTR Conclusions by Dr. Ian Thompson, MD Dr. James
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof.
 Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Diagnosis and Prognosis of Pancreatic Cancer
 Main Page Risk Factors Reducing Your Risk Screening Symptoms Diagnosis Treatment Overview Chemotherapy Radiation Therapy Surgical Procedures Lifestyle Changes Managing Side Effects Talking to Your Doctor
Main Page Risk Factors Reducing Your Risk Screening Symptoms Diagnosis Treatment Overview Chemotherapy Radiation Therapy Surgical Procedures Lifestyle Changes Managing Side Effects Talking to Your Doctor
Medication Therapy Management (MTM) Program
 Medication Therapy Management (MTM) Program Regence offers a Medication Therapy Management (MTM) program to ensure you are receiving the most effective medications, while also helping to reduce the risk
Medication Therapy Management (MTM) Program Regence offers a Medication Therapy Management (MTM) program to ensure you are receiving the most effective medications, while also helping to reduce the risk
Recommendations for cross-sectional imaging in cancer management, Second edition
 www.rcr.ac.uk Recommendations for cross-sectional imaging in cancer management, Second edition Breast cancer Faculty of Clinical Radiology www.rcr.ac.uk Contents Breast cancer 2 Clinical background 2 Who
www.rcr.ac.uk Recommendations for cross-sectional imaging in cancer management, Second edition Breast cancer Faculty of Clinical Radiology www.rcr.ac.uk Contents Breast cancer 2 Clinical background 2 Who
Breast Cancer. CSC Cancer Experience Registry Member, breast cancer
 ESSENTIALS Breast Cancer Take things one step at a time. Try not to be overwhelmed by the tidal wave of technical information coming your way. Finally you know your body best; you have to be your own advocate.
ESSENTIALS Breast Cancer Take things one step at a time. Try not to be overwhelmed by the tidal wave of technical information coming your way. Finally you know your body best; you have to be your own advocate.
Lung cancer forms in tissues of the lung, usually in the cells lining air passages.
 Scan for mobile link. Lung Cancer Lung cancer usually forms in the tissue cells lining the air passages within the lungs. The two main types are small-cell lung cancer (usually found in cigarette smokers)
Scan for mobile link. Lung Cancer Lung cancer usually forms in the tissue cells lining the air passages within the lungs. The two main types are small-cell lung cancer (usually found in cigarette smokers)
ESRD Application Access Form Previously known as Part B of the QualityNet Identity Management System (QIMS) Account Form
 Previously known as Part B of the QualityNet Identity Management System (QIMS) Account Form You must have a QIMS account in order to access (1) CROWNWeb and/or (2) ESRD Quality Incentive Program (QIP)
Previously known as Part B of the QualityNet Identity Management System (QIMS) Account Form You must have a QIMS account in order to access (1) CROWNWeb and/or (2) ESRD Quality Incentive Program (QIP)
Cancer Care Delivered Locally by Physicians You Know and Trust
 West Florida Physician Office Building Johnson Ave. University Pkwy. Olive Road N. Davis Hwy. For more information on West Florida Cancer Center: 850-494-5404 2130 East Johnson Avenue Pensacola, Florida
West Florida Physician Office Building Johnson Ave. University Pkwy. Olive Road N. Davis Hwy. For more information on West Florida Cancer Center: 850-494-5404 2130 East Johnson Avenue Pensacola, Florida
Oncology. Objectives. Cancer Nomenclature. Cancer is a disease of the cell Cancer develops when certain cells begin to grow out of control
 Oncology Objectives Describe the etiology and pathophysiological mechanisms of cancer Discuss medical and family history findings relevant to cancer Identify general signs and symptoms associated with
Oncology Objectives Describe the etiology and pathophysiological mechanisms of cancer Discuss medical and family history findings relevant to cancer Identify general signs and symptoms associated with
Management of spinal cord compression
 Management of spinal cord compression (SUMMARY) Main points a) On diagnosis, all patients should receive dexamethasone 10mg IV one dose, then 4mg every 6h. then switched to oral dose and tapered as tolerated
Management of spinal cord compression (SUMMARY) Main points a) On diagnosis, all patients should receive dexamethasone 10mg IV one dose, then 4mg every 6h. then switched to oral dose and tapered as tolerated
Appendix IV HIPAA Compliance and IRB Approval for the NOPR
 Appendix IV HIPAA Compliance and IRB Approval for the NOPR HIPAA Compliance and Participation in the NOPR The Health Insurance Portability and Accountability Act of 1996 (HIPAA) requires that providers
Appendix IV HIPAA Compliance and IRB Approval for the NOPR HIPAA Compliance and Participation in the NOPR The Health Insurance Portability and Accountability Act of 1996 (HIPAA) requires that providers
PET/CT in Lung Cancer
 PET/CT in Lung Cancer Rodolfo Núñez Miller, M.D. Nuclear Medicine and Diagnostic Imaging Section Division of Human Health International Atomic Energy Agency Vienna, Austria GLOBOCAN 2012 #1 #3 FDG-PET/CT
PET/CT in Lung Cancer Rodolfo Núñez Miller, M.D. Nuclear Medicine and Diagnostic Imaging Section Division of Human Health International Atomic Energy Agency Vienna, Austria GLOBOCAN 2012 #1 #3 FDG-PET/CT
Disease/Illness GUIDE TO ASBESTOS LUNG CANCER. What Is Asbestos Lung Cancer? www.simpsonmillar.co.uk Telephone 0844 858 3200
 GUIDE TO ASBESTOS LUNG CANCER What Is Asbestos Lung Cancer? Like tobacco smoking, exposure to asbestos can result in the development of lung cancer. Similarly, the risk of developing asbestos induced lung
GUIDE TO ASBESTOS LUNG CANCER What Is Asbestos Lung Cancer? Like tobacco smoking, exposure to asbestos can result in the development of lung cancer. Similarly, the risk of developing asbestos induced lung
Small cell lung cancer
 Small cell lung cancer Small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing organs that are found within
Small cell lung cancer Small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing organs that are found within
Mesothelioma. 1. Introduction. 1.1 General Information and Aetiology
 Mesothelioma 1. Introduction 1.1 General Information and Aetiology Mesotheliomas are tumours that arise from the mesothelial cells of the pleura, peritoneum, pericardium or tunica vaginalis [1]. Most are
Mesothelioma 1. Introduction 1.1 General Information and Aetiology Mesotheliomas are tumours that arise from the mesothelial cells of the pleura, peritoneum, pericardium or tunica vaginalis [1]. Most are
Colorectal Cancer Treatment
 Scan for mobile link. Colorectal Cancer Treatment Colorectal cancer overview Colorectal cancer, also called large bowel cancer, is the term used to describe malignant tumors found in the colon and rectum.
Scan for mobile link. Colorectal Cancer Treatment Colorectal cancer overview Colorectal cancer, also called large bowel cancer, is the term used to describe malignant tumors found in the colon and rectum.
REHABILITATION HOSPITAL CRITERIA WORK SHEET
 DEPARTMENT OF HEALTH AND HUMAN SERVICES CENTERS FOR MEDICARE & MEDICAID SERVICES FORM APPROVED OMB NO. 0938-0986 REHABILITATION HOSPITAL CRITERIA WORK SHEET RELATED MEDICARE PROVIDER NUMBER ROOM NUMBERS
DEPARTMENT OF HEALTH AND HUMAN SERVICES CENTERS FOR MEDICARE & MEDICAID SERVICES FORM APPROVED OMB NO. 0938-0986 REHABILITATION HOSPITAL CRITERIA WORK SHEET RELATED MEDICARE PROVIDER NUMBER ROOM NUMBERS
GENERAL CODING. When you review old cases that were coded to unknown, make corrections based on guidelines in effect at the time of diagnosis.
 GENERAL CODING When you review old cases that were coded to unknown, make corrections based on guidelines in effect at the time of diagnosis. Exception: You must review and revise EOD coding for prostate
GENERAL CODING When you review old cases that were coded to unknown, make corrections based on guidelines in effect at the time of diagnosis. Exception: You must review and revise EOD coding for prostate
Objectives. Mylene T. Truong, MD. Malignant Pleural Mesothelioma Background
 Imaging of Pleural Tumors Mylene T. Truong, MD Imaging of Pleural Tumours Mylene T. Truong, M. D. University of Texas M.D. Anderson Cancer Center, Houston, TX Objectives To review tumors involving the
Imaging of Pleural Tumors Mylene T. Truong, MD Imaging of Pleural Tumours Mylene T. Truong, M. D. University of Texas M.D. Anderson Cancer Center, Houston, TX Objectives To review tumors involving the
Carcinoma of the Cervix. Kathleen M. Schmeler, MD Associate Professor Department of Gynecologic Oncology
 Carcinoma of the Cervix Kathleen M. Schmeler, MD Associate Professor Department of Gynecologic Oncology Cervical Cancer Treatment Treatment Microinvasive (Stage IA1): Simple (extrafascial) hysterectomy/cone
Carcinoma of the Cervix Kathleen M. Schmeler, MD Associate Professor Department of Gynecologic Oncology Cervical Cancer Treatment Treatment Microinvasive (Stage IA1): Simple (extrafascial) hysterectomy/cone
Non-Small Cell Lung Cancer Therapies
 Non-Small Cell Lung Cancer Therapies Guest Expert: Roy, MD, PhD Assistant Professor of Therapeutic Radiology Scott, MD Assistant Professor of Medical Oncology www.wnpr.org www.yalecancercenter.org Welcome
Non-Small Cell Lung Cancer Therapies Guest Expert: Roy, MD, PhD Assistant Professor of Therapeutic Radiology Scott, MD Assistant Professor of Medical Oncology www.wnpr.org www.yalecancercenter.org Welcome
Oncology Program. MedStarGoodSam.org. 2012 Annual Report 2011 Statistical Data Breast Cancer Focus
 MedStarGoodSam.org Oncology Program 2012 Annual Report 2011 Statistical Data Breast Cancer Focus 5601 Loch Raven Blvd. Baltimore, MD 21239 443-444-8000 PHONE 13-MGSH-0168.052013 Message from Our Chairman
MedStarGoodSam.org Oncology Program 2012 Annual Report 2011 Statistical Data Breast Cancer Focus 5601 Loch Raven Blvd. Baltimore, MD 21239 443-444-8000 PHONE 13-MGSH-0168.052013 Message from Our Chairman
Us TOO University Presents: Understanding Diagnostic Testing
 Us TOO University Presents: Understanding Diagnostic Testing for Prostate Cancer Patients Today s speaker is Manish Bhandari, MD Program moderator is Pam Barrett, Us TOO International Made possible by
Us TOO University Presents: Understanding Diagnostic Testing for Prostate Cancer Patients Today s speaker is Manish Bhandari, MD Program moderator is Pam Barrett, Us TOO International Made possible by
Current Status and Perspectives of Radiation Therapy for Breast Cancer
 Breast Cancer Current Status and Perspectives of Radiation Therapy for Breast Cancer JMAJ 45(10): 434 439, 2002 Masahiro HIRAOKA, Masaki KOKUBO, Chikako YAMAMOTO and Michihide MITSUMORI Department of Therapeutic
Breast Cancer Current Status and Perspectives of Radiation Therapy for Breast Cancer JMAJ 45(10): 434 439, 2002 Masahiro HIRAOKA, Masaki KOKUBO, Chikako YAMAMOTO and Michihide MITSUMORI Department of Therapeutic
Small Cell Lung Cancer
 Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Case Number: RT2009-124(M) Potential Audiences: Intent Doctor, Oncology Special Nurse, Resident Doctor
 Renal Cell Carcinoma of the Left Kidney Post Radical Surgery with pt4 Classification with Multiple Lung and Single Brain Metastases: the Role and Treatment Consideration of Radiotherapy Case Number: RT2009-124(M)
Renal Cell Carcinoma of the Left Kidney Post Radical Surgery with pt4 Classification with Multiple Lung and Single Brain Metastases: the Role and Treatment Consideration of Radiotherapy Case Number: RT2009-124(M)
The TV Series. www.healthybodyhealthymind.com INFORMATION TELEVISION NETWORK
 The TV Series www.healthybodyhealthymind.com Produced By: INFORMATION TELEVISION NETWORK ONE PARK PLACE 621 NW 53RD ST BOCA RATON, FL 33428 1-800-INFO-ITV www.itvisus.com 2005 Information Television Network.
The TV Series www.healthybodyhealthymind.com Produced By: INFORMATION TELEVISION NETWORK ONE PARK PLACE 621 NW 53RD ST BOCA RATON, FL 33428 1-800-INFO-ITV www.itvisus.com 2005 Information Television Network.
Continuing Medical Education Article Imaging of Multiple Myeloma and Related Plasma Cell Dyscrasias JNM, July 2012, Volume 53, Number 7
 Continuing Medical Education Article Imaging of Multiple Myeloma and Related Plasma Cell Dyscrasias JNM, July 2012, Volume 53, Number 7 Authors Ronald C. Walker 1,2, Tracy L. Brown 3, Laurie B. Jones-Jackson
Continuing Medical Education Article Imaging of Multiple Myeloma and Related Plasma Cell Dyscrasias JNM, July 2012, Volume 53, Number 7 Authors Ronald C. Walker 1,2, Tracy L. Brown 3, Laurie B. Jones-Jackson
Bristol Hospital Cancer Care Center 2015 Annual Report
 Bristol Hospital Cancer Care Center 2015 Annual Report 2015 Annual Report Cancer Care Center At every point along the path, our team is there, keeping the focus on the most important team member - the
Bristol Hospital Cancer Care Center 2015 Annual Report 2015 Annual Report Cancer Care Center At every point along the path, our team is there, keeping the focus on the most important team member - the
Recruiting now. Could you help by joining this study?
 Non-Small Cell Lung Cancer Recruiting now AstraZeneca is looking for men and women with locally advanced or metastatic non-small cell lung cancer (NSCLC) to join ATLANTIC, a clinical study to help investigate
Non-Small Cell Lung Cancer Recruiting now AstraZeneca is looking for men and women with locally advanced or metastatic non-small cell lung cancer (NSCLC) to join ATLANTIC, a clinical study to help investigate
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer
 Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
Anaplastic Thyroid Cancer:
 1 Anaplastic Thyroid Cancer: A Doctor s Perspective for Patients and Families Living with the Disease By Maria E. Cabanillas, M.D., F.A.C.E. Associate Professor and Faculty Director of Clinical Research
1 Anaplastic Thyroid Cancer: A Doctor s Perspective for Patients and Families Living with the Disease By Maria E. Cabanillas, M.D., F.A.C.E. Associate Professor and Faculty Director of Clinical Research
HAVE YOU BEEN NEWLY DIAGNOSED with DCIS?
 HAVE YOU BEEN NEWLY DIAGNOSED with DCIS? Jen D. Mother and volunteer. Diagnosed with DCIS breast cancer in 2012. An educational guide prepared by Genomic Health This guide is designed to educate women
HAVE YOU BEEN NEWLY DIAGNOSED with DCIS? Jen D. Mother and volunteer. Diagnosed with DCIS breast cancer in 2012. An educational guide prepared by Genomic Health This guide is designed to educate women
Breast. Patient information. cancer clinical pathway
 Breast Patient information cancer clinical pathway This leaflet was written to properly inform people following breast cancer treatment plan. It doesn t replace the dialogue with healthcare staff; it rather
Breast Patient information cancer clinical pathway This leaflet was written to properly inform people following breast cancer treatment plan. It doesn t replace the dialogue with healthcare staff; it rather
A SAFE, NON-INVASIVE TREATMENT OPTION: GAMMA KNIFE PERFEXION
 A SAFE, NON-INVASIVE TREATMENT OPTION: GAMMA KNIFE PERFEXION Not actually a knife, the Gamma Knife Perfexion is an advanced radiosurgery device which uses extremely precise beams of radiation to treat
A SAFE, NON-INVASIVE TREATMENT OPTION: GAMMA KNIFE PERFEXION Not actually a knife, the Gamma Knife Perfexion is an advanced radiosurgery device which uses extremely precise beams of radiation to treat
Brain Tumor Treatment
 Scan for mobile link. Brain Tumor Treatment Brain Tumors Overview A brain tumor is a group of abnormal cells that grows in or around the brain. Tumors can directly destroy healthy brain cells. They can
Scan for mobile link. Brain Tumor Treatment Brain Tumors Overview A brain tumor is a group of abnormal cells that grows in or around the brain. Tumors can directly destroy healthy brain cells. They can
OBJECTIVES By the end of this segment, the community participant will be able to:
 Cancer 101: Cancer Diagnosis and Staging Linda U. Krebs, RN, PhD, AOCN, FAAN OCEAN Native Navigators and the Cancer Continuum (NNACC) (NCMHD R24MD002811) Cancer 101: Diagnosis & Staging (Watanabe-Galloway
Cancer 101: Cancer Diagnosis and Staging Linda U. Krebs, RN, PhD, AOCN, FAAN OCEAN Native Navigators and the Cancer Continuum (NNACC) (NCMHD R24MD002811) Cancer 101: Diagnosis & Staging (Watanabe-Galloway
A Practical Guide to Advances in Staging and Treatment of NSCLC
 A Practical Guide to Advances in Staging and Treatment of NSCLC Robert J. Korst, M.D. Director, Thoracic Surgery Medical Director, The Blumenthal Cancer Center The Valley Hospital Objectives Revised staging
A Practical Guide to Advances in Staging and Treatment of NSCLC Robert J. Korst, M.D. Director, Thoracic Surgery Medical Director, The Blumenthal Cancer Center The Valley Hospital Objectives Revised staging
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians
 Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
The Center for Prostate Cancer. Personalized Treatment. Clinical Excellence.
 The Center for Prostate Cancer Personalized Treatment. Clinical Excellence. The Center for Prostate Cancer Leaders in Prostate Cancer Treatment and Research The Center for Prostate Cancer at the North
The Center for Prostate Cancer Personalized Treatment. Clinical Excellence. The Center for Prostate Cancer Leaders in Prostate Cancer Treatment and Research The Center for Prostate Cancer at the North
Small Business Health Options Program (SHOP) Health coverage application for employers
 Small Business Health Options Program (SHOP) Health coverage application for employers Maryland Health Connection s Small Business Health Options Program (SHOP) is open to all eligible small business owners.
Small Business Health Options Program (SHOP) Health coverage application for employers Maryland Health Connection s Small Business Health Options Program (SHOP) is open to all eligible small business owners.
Hosts. New Methods for Treating Colorectal Cancer
 Hosts Anees Chagpar MD Associate Professor of Surgical Oncology Francine MD Professor of Medical Oncology New Methods for Treating Colorectal Cancer Guest Expert: Scott, MD Associate Professor in the Department
Hosts Anees Chagpar MD Associate Professor of Surgical Oncology Francine MD Professor of Medical Oncology New Methods for Treating Colorectal Cancer Guest Expert: Scott, MD Associate Professor in the Department
Analysis of Prostate Cancer at Easter Connecticut Health Network Using Cancer Registry Data
 The 2014 Cancer Program Annual Public Reporting of Outcomes/Annual Site Analysis Statistical Data from 2013 More than 70 percent of all newly diagnosed cancer patients are treated in the more than 1,500
The 2014 Cancer Program Annual Public Reporting of Outcomes/Annual Site Analysis Statistical Data from 2013 More than 70 percent of all newly diagnosed cancer patients are treated in the more than 1,500
CHAPTER 2. Neoplasms (C00-D49) March 2014. 2014 MVP Health Care, Inc.
 Neoplasms (C00-D49) March 2014 2014 MVP Health Care, Inc. CHAPTER SPECIFIC CATEGORY CODE BLOCKS C00-C14 Malignant neoplasms of lip, oral cavity and pharynx C15-C26 Malignant neoplasms of digestive organs
Neoplasms (C00-D49) March 2014 2014 MVP Health Care, Inc. CHAPTER SPECIFIC CATEGORY CODE BLOCKS C00-C14 Malignant neoplasms of lip, oral cavity and pharynx C15-C26 Malignant neoplasms of digestive organs
Small Business Health Options Program (SHOP) Health coverage application for employers
 Small Business Health Options Program (SHOP) Health coverage application for employers Form Approved OMB No. 0938-1193 The SHOP is open to all eligible small business owners. It should take about 15 minutes
Small Business Health Options Program (SHOP) Health coverage application for employers Form Approved OMB No. 0938-1193 The SHOP is open to all eligible small business owners. It should take about 15 minutes
Florida Breast Health Specialists Breast Cancer Information and Facts
 Definition Breast cancer is a cancer that starts in the tissues of the breast. There are two main types of breast cancer: Ductal carcinoma starts in the tubes (ducts) that move milk from the breast to
Definition Breast cancer is a cancer that starts in the tissues of the breast. There are two main types of breast cancer: Ductal carcinoma starts in the tubes (ducts) that move milk from the breast to
9. Discuss guidelines for follow-up post-thyroidectomy for cancer (labs/tests) HH
 9. Discuss guidelines for follow-up post-thyroidectomy for cancer (labs/tests) HH Differentiated thyroid cancer expresses the TSH receptor on the cell membrane and responds to TSH stimulation by increasing
9. Discuss guidelines for follow-up post-thyroidectomy for cancer (labs/tests) HH Differentiated thyroid cancer expresses the TSH receptor on the cell membrane and responds to TSH stimulation by increasing
Cancer Association of South Africa (CANSA)
 Cancer Association of South Africa (CANSA) Fact Sheet on Metastatic Breast Cancer Introduction Metastatic breast cancer is cancer that has spread beyond the breast and lymph nodes under the arm. It occurs
Cancer Association of South Africa (CANSA) Fact Sheet on Metastatic Breast Cancer Introduction Metastatic breast cancer is cancer that has spread beyond the breast and lymph nodes under the arm. It occurs
Thyroid Cancer Diagnosis and Management. Jerome Hershman, M.D. Internal Medicine Grand Rounds University of Missouri, Columbia October 21, 2010
 Thyroid Cancer Diagnosis and Management Jerome Hershman, M.D. Internal Medicine Grand Rounds University of Missouri, Columbia October 21, 2010 DISCLOSURE NOTHING TO DISCLOSE in regard to financial conflict
Thyroid Cancer Diagnosis and Management Jerome Hershman, M.D. Internal Medicine Grand Rounds University of Missouri, Columbia October 21, 2010 DISCLOSURE NOTHING TO DISCLOSE in regard to financial conflict
Sternotomy and removal of the tumor
 Sternotomy and removal of the tumor All thymomas originate from epithelial thymic cells 4% of them consist of a pure population of epithelial cells Most have mixed populations of lymphoid cells to a
Sternotomy and removal of the tumor All thymomas originate from epithelial thymic cells 4% of them consist of a pure population of epithelial cells Most have mixed populations of lymphoid cells to a
Guidelines for Management of Renal Cancer
 Guidelines for Management of Renal Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Section 5 updated bullets 5.3 and 5.4 Section 6 updated
Guidelines for Management of Renal Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Section 5 updated bullets 5.3 and 5.4 Section 6 updated
portable x-ray survey report
 department of HealtH and Human services centers for medicare & medicaid services form approved omb no. 0938-0027 portable x-ray survey report provider number H1 date surveyed (1) initial (2) survey W H2
department of HealtH and Human services centers for medicare & medicaid services form approved omb no. 0938-0027 portable x-ray survey report provider number H1 date surveyed (1) initial (2) survey W H2
Management of Non-Small Cell Lung Cancer Guide for General Practitioners
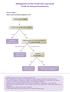 Management of n-small Cell Lung Cancer Guide for General Practitioners Clinical Stage I Cancer only in one lobe of lung and
Management of n-small Cell Lung Cancer Guide for General Practitioners Clinical Stage I Cancer only in one lobe of lung and
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline
 Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Breast Health Program
 Breast Health Program Working together, for your health. Breast Health Program The Breast Health Program at The University of Arizona Cancer Center offers patients a personalized approach to breast cancer,
Breast Health Program Working together, for your health. Breast Health Program The Breast Health Program at The University of Arizona Cancer Center offers patients a personalized approach to breast cancer,
Treatment and Surveillance of Non- Muscle Invasive Bladder Cancer
 Treatment and Surveillance of Non- Muscle Invasive Bladder Cancer David Josephson, MD FACS Fellowship Director, Urologic Oncology and Robotic Surgery Program Staging Most important in risk assessment and
Treatment and Surveillance of Non- Muscle Invasive Bladder Cancer David Josephson, MD FACS Fellowship Director, Urologic Oncology and Robotic Surgery Program Staging Most important in risk assessment and
Thyroid Cancer: Resection, Dissection, Surveillance and Recurrence. Cord Sturgeon, MD
 Thyroid Cancer: Resection, Dissection, Surveillance and Recurrence Cord Sturgeon, MD Associate Professor of Surgery Northwestern University Feinberg School of Medicine Director of Endocrine Surgery Chicago,
Thyroid Cancer: Resection, Dissection, Surveillance and Recurrence Cord Sturgeon, MD Associate Professor of Surgery Northwestern University Feinberg School of Medicine Director of Endocrine Surgery Chicago,
Goals and Objectives: Breast Cancer Service Department of Radiation Oncology
 Goals and Objectives: Breast Cancer Service Department of Radiation Oncology The breast cancer service provides training in the diagnosis, management, treatment, and follow-up of breast malignancies, including
Goals and Objectives: Breast Cancer Service Department of Radiation Oncology The breast cancer service provides training in the diagnosis, management, treatment, and follow-up of breast malignancies, including
LYMPHOMA IN DOGS. Diagnosis/Initial evaluation. Treatment and Prognosis
 LYMPHOMA IN DOGS Lymphoma is a relatively common cancer in dogs. It is a cancer of lymphocytes (a type of white blood cell) and lymphoid tissues. Lymphoid tissue is normally present in many places in the
LYMPHOMA IN DOGS Lymphoma is a relatively common cancer in dogs. It is a cancer of lymphocytes (a type of white blood cell) and lymphoid tissues. Lymphoid tissue is normally present in many places in the
People Living with Cancer
 Patient Guide ASCOInformation for People Living with Cancer ADVANCED LUNG CANCER TREATMENT Recommendations of the American Society of Clinical Oncology Welcome The American Society of Clinical Oncology
Patient Guide ASCOInformation for People Living with Cancer ADVANCED LUNG CANCER TREATMENT Recommendations of the American Society of Clinical Oncology Welcome The American Society of Clinical Oncology
Colorectal Cancer Care A Cancer Care Map for Patients
 Colorectal Cancer Care A Cancer Care Map for Patients Understanding the process of care that a patient goes through in the diagnosis and treatment of colorectal cancer in BC. Colorectal Cancer Care Map
Colorectal Cancer Care A Cancer Care Map for Patients Understanding the process of care that a patient goes through in the diagnosis and treatment of colorectal cancer in BC. Colorectal Cancer Care Map
Cancer of the Cervix
 Cancer of the Cervix WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 A woman's cervix (the opening of the uterus) is lined with cells. Cancer of the cervix occurs when those cells change,
Cancer of the Cervix WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 A woman's cervix (the opening of the uterus) is lined with cells. Cancer of the cervix occurs when those cells change,
Cutaneous Lymphoma FAST FACTS
 Cutaneous Lymphoma FAST FACTS What is Cutaneous Lymphoma? Cutaneous lymphomas are types of non-hodgkin s lymphomas (NHL) that originate in the lymphocytes (white blood cells). Unlike most other types of
Cutaneous Lymphoma FAST FACTS What is Cutaneous Lymphoma? Cutaneous lymphomas are types of non-hodgkin s lymphomas (NHL) that originate in the lymphocytes (white blood cells). Unlike most other types of
Guideline for the Imaging of Patients Presenting with Breast Symptoms incorporating the guideline for the use of MRI in breast cancer
 Guideline for the Imaging of Patients Presenting with Breast Symptoms incorporating the guideline for the use of MRI in breast cancer Version History Version Date Summary of Change/Process 0.1 09.01.11
Guideline for the Imaging of Patients Presenting with Breast Symptoms incorporating the guideline for the use of MRI in breast cancer Version History Version Date Summary of Change/Process 0.1 09.01.11
FDG-PET/CT and SPECT for cancer staging. Executive summary INFORMES DE EVALUACIÓN DE TECNOLOGÍAS SANITARIAS AETSA INFORMES, ESTUDIOS E INVESTIGACIÓN
 FDG-PET/TC y SPECT para la estadificación oncológica Estadificación del cáncer colorrectal, identificación del nódulo pulmonar solitario y la re-estadificación del linfoma. Informe corto FDG-PET/CT and
FDG-PET/TC y SPECT para la estadificación oncológica Estadificación del cáncer colorrectal, identificación del nódulo pulmonar solitario y la re-estadificación del linfoma. Informe corto FDG-PET/CT and
General Information About Non-Small Cell Lung Cancer
 General Information About Non-Small Cell Lung Cancer Non-small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing
General Information About Non-Small Cell Lung Cancer Non-small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing
Understanding. Pancreatic Cancer
 Understanding Pancreatic Cancer Understanding Pancreatic Cancer The Pancreas The pancreas is an organ that is about 6 inches long. It s located deep in your belly between your stomach and backbone. Your
Understanding Pancreatic Cancer Understanding Pancreatic Cancer The Pancreas The pancreas is an organ that is about 6 inches long. It s located deep in your belly between your stomach and backbone. Your
Mesothelioma. 1995-2013, The Patient Education Institute, Inc. www.x-plain.com ocft0101 Last reviewed: 03/21/2013 1
 Mesothelioma Introduction Mesothelioma is a type of cancer. It starts in the tissue that lines your lungs, stomach, heart, and other organs. This tissue is called mesothelium. Most people who get this
Mesothelioma Introduction Mesothelioma is a type of cancer. It starts in the tissue that lines your lungs, stomach, heart, and other organs. This tissue is called mesothelium. Most people who get this
MEDICARE ENROLLMENT APPLICATION
 MEDICARE ENROLLMENT APPLICATION REASSIGNMENT OF MEDICARE BENEFITS CMS-855R SEE PAGE 2 FOR INFORMATION ON WHERE TO MAIL THIS APPLICATION. DEPARTMENT OF HEALTH AND HUMAN SERVICES CENTERS FOR MEDICARE & MEDICAID
MEDICARE ENROLLMENT APPLICATION REASSIGNMENT OF MEDICARE BENEFITS CMS-855R SEE PAGE 2 FOR INFORMATION ON WHERE TO MAIL THIS APPLICATION. DEPARTMENT OF HEALTH AND HUMAN SERVICES CENTERS FOR MEDICARE & MEDICAID
Chapter 13. The hospital-based cancer registry
 Chapter 13. The hospital-based cancer registry J.L. Young California Tumor Registry, 1812 14th Street, Suite 200, Sacramento, CA 95814, USA Introduction The purposes of a hospital-based cancer registry
Chapter 13. The hospital-based cancer registry J.L. Young California Tumor Registry, 1812 14th Street, Suite 200, Sacramento, CA 95814, USA Introduction The purposes of a hospital-based cancer registry
Update on thyroid cancer surveillance and management of recurrent disease. Minimally invasive thyroid surgery
 Update on thyroid cancer surveillance and management of recurrent disease Minimally invasive thyroid surgery July 2006 Michael W. Yeh, MD Program Director, Endocrine Surgery Assistant Professor, David
Update on thyroid cancer surveillance and management of recurrent disease Minimally invasive thyroid surgery July 2006 Michael W. Yeh, MD Program Director, Endocrine Surgery Assistant Professor, David
ADJUVANT TREATMENT CLINICAL EVALUATION NEOADJUVANT TREATMENT
 te: Consider Clinical Trials as treatment options for eligible patients. Referral to a center with both pediatric oncology and orthopedic surgery is essential. CLINICAL EVALUATION This practice algorithm
te: Consider Clinical Trials as treatment options for eligible patients. Referral to a center with both pediatric oncology and orthopedic surgery is essential. CLINICAL EVALUATION This practice algorithm
Changes in Breast Cancer Reports After Second Opinion. Dr. Vicente Marco Department of Pathology Hospital Quiron Barcelona. Spain
 Changes in Breast Cancer Reports After Second Opinion Dr. Vicente Marco Department of Pathology Hospital Quiron Barcelona. Spain Second Opinion in Breast Pathology Usually requested when a patient is referred
Changes in Breast Cancer Reports After Second Opinion Dr. Vicente Marco Department of Pathology Hospital Quiron Barcelona. Spain Second Opinion in Breast Pathology Usually requested when a patient is referred
Understanding brachytherapy
 Understanding brachytherapy Brachytherapy Cancer Treatment Legacy Cancer Institute Your health care provider has requested that you receive a type of radiation treatment called brachytherapy as part of
Understanding brachytherapy Brachytherapy Cancer Treatment Legacy Cancer Institute Your health care provider has requested that you receive a type of radiation treatment called brachytherapy as part of
THYROID CANCER. I. Introduction
 THYROID CANCER I. Introduction There are over 11,000 new cases of thyroid cancer each year in the US. Females are more likely to have thyroid cancer than men by a ratio of 3:1, and it is more common in
THYROID CANCER I. Introduction There are over 11,000 new cases of thyroid cancer each year in the US. Females are more likely to have thyroid cancer than men by a ratio of 3:1, and it is more common in
1400 Telegraph Bloomfield Hills, MI 48302 248-334-6877-Phone number/248-334-6877-fax Number CANCER TREATMENT
 1400 Telegraph Bloomfield Hills, MI 48302 248-334-6877-Phone number/248-334-6877-fax Number CANCER TREATMENT Learning that your pet has a diagnosis of cancer can be overwhelming. We realize that your pet
1400 Telegraph Bloomfield Hills, MI 48302 248-334-6877-Phone number/248-334-6877-fax Number CANCER TREATMENT Learning that your pet has a diagnosis of cancer can be overwhelming. We realize that your pet
