RH de Boer 1, IE Smith 1, U Pastorino 2, MER O Brien 1, F Ramage 1, S Ashley 1 and P Goldstraw 2
|
|
|
- Amberlynn Anthony
- 8 years ago
- Views:
Transcription
1 British Journal of Cancer (1999) 79(9/10), Cancer Research Campaign Article no. bjoc Pre-operative chemotherapy in early stage resectable non-small-cell lung cancer: a randomized feasibility study justifying a multicentre phase III trial RH de Boer 1, IE Smith 1, U Pastorino 2, MER O Brien 1, F Ramage 1, S Ashley 1 and P Goldstraw 2 1 Department of Medicine, Royal Marsden NHS Trust, Fulham Rd, London SW3 6JJ; and 2 Department of Thoracic Surgery, Royal Brompton Hospital, Sydney St, London SW3 6JJ, UK Summary Surgical resection offers the best chance for cure for early stage non-small-cell lung cancer (NSCLC, stage I, II, IIIA), but the 5- year survival rates are only moderate, with systemic relapse being the major cause of death. Pre-operative (neo-adjuvant) chemotherapy has shown promise in small trials restricted to stage IIIA patients. We believe similar trials are now appropriate in all stages of operable lung cancer. A feasibility study was performed in 22 patients with early stage (IB, II, IIIA) resectable NSCLC; randomized to either three cycles of chemotherapy [mitomycin-c 8 mg m 2, vinblastine 6 mg m 2 and cisplatin 50 mg m 2 (MVP)] followed by surgery (n = 11), or to surgery alone. Of 40 eligible patients, 22 agreed to participate (feasibility 55%) and all complied with the full treatment schedule. All symptomatic patients achieved either complete (50%) or partial (50%) relief of tumour-related symptoms with pre-operative chemotherapy. Fifty-five per cent achieved objective tumour response, and a further 27% minor tumour shrinkage; none had progressive disease. Partial pathological response was seen in 50%. No severe (WHO grade III IV) toxicities occurred. No significant deterioration in quality of life was detected during chemotherapy. Pre-operative MVP chemotherapy is feasible in early stage NSCLC, and this study has now been initiated as a UK-wide Medical Research Council phase III trial. Keywords: non-small-cell lung cancer; pre-operative chemotherapy; mitomycin-c; vinblastine; cisplatin Received 26 June 1998 Revised 28 August 1998 Accepted 7 October 1998 Correspondence to: I E Smith Surgery offers the best chance of cure for patients with NSCLC, although no more than 20% are operable. Overall surgical 5-year survival rates are 55% for stage I, 25% for stage II, and 20% for stage III disease (Mountain, 1977); patients with completely resected T3 tumours without mediastinal node involvement have a slightly better outlook with approximately 40% 5-year survival (McCormack et al, 1987). These results can at best be described as moderate and it is likely that many surgical patients would accept the option of additional treatment to try to improve their outlook (Slevin et al, 1990). Randomized trials of post-operative adjuvant chemotherapy in NSCLC have shown some prolongation of disease-free survival with cisplatin-containing schedules (Holmes et al, 1986; Lad et al, 1988; Niiranen et al, 1992). A meta-analysis of eight such trials has shown a 13% reduction in the risk of death, corresponding to an absolute survival benefit of around 5% at 5 years (Non-small Cell Lung Cancer Collaborative Group, 1995). A value judgement is required on the clinical benefit of such treatment, and it seems unlikely that significant further progress will be made with postoperative therapy using currently available drugs. Pre-operative chemotherapy is currently being investigated in several tumour types including NSCLC, breast cancer and gastric cancer. In NSCLC, most pre-operative chemotherapy studies have been carried out in patients with stage IIIA disease. In nonrandomized studies, response rates have ranged from 50% to 75% with occasional (10%) complete remissions; subsequent resectability rates of 65 90% and 3 5-year survival rates of 17 40% have been reported (Faber et al, 1989; Skarin et al, 1989; Weiden et al, 1991; Burkes et al, 1992; Strauss et al, 1992; Martini et al, 1993). The heterogeneity of entry criteria and the lack of data from randomized trials make these results difficult to interpret, but they make the important point that the majority of such patients appear to have chemosensitive disease prior to surgery. Two small randomized trials have compared surgery with or without preoperative chemotherapy in patients with stage IIIA disease, each with similar and strikingly positive results in favour of chemotherapy. In the first study, median survival was 26 months for patients treated with pre-operative chemotherapy, compared with 8 months for patients treated with surgery alone (Rosell et al, 1994). The second study reported respective median survivals of 64 months vs 11 months (Roth et al, 1994). These results, although encouraging, must be interpreted with caution because of the small number of patients randomized. They emphasize the need for a large multi-centre randomized trial, and we felt that this should involve all patients with operable lung cancer. Such an approach is novel and is associated with several uncertainties. These include the question of acceptability of preoperative chemotherapy to both patient and doctor, the potential for unacceptable chemotherapy-induced toxicity prior to surgery, and the possibility of disease progression during chemotherapy. We therefore decided to carry out a small randomized pilot feasibility study to address these issues, using three pre-operative cycles of MVP (mitomycin-c, vinblastine and cisplatin). This is a 1514
2 Pre-operative chemotherapy in early stage NSCLC 1515 schedule which we have already shown to be active and well tolerated in advanced/metastatic NSCLC (Ellis et al, 1995). The primary aim of this pilot study was to assess feasibility of, and compliance with, pre-operative chemotherapy. The secondary aims were to assess response, symptom control, performance status, resectability and extent of surgery, side effects, and any technical difficulties that might arise with surgery following chemotherapy. We were also interested in assessing the quality of life of patients during the treatment schedule. We report our results here. MATERIALS AND METHODS Patients Patients with previously untreated, histologically proven, NSCLC were invited to enter the study. Patient entry criteria were: a tumour considered to be resectable (i.e. stage I, II, or IIIA); a World Health Organization (WHO) performance status of 0 1; fitness for both the chemotherapy regimen and the proposed thoracic surgery and no history of prior malignancy. A haematological and biochemical screen was performed and renal function was assessed by creatinine clearance. The clearance was measured either by ethylenediamine tetra-acetic acid (EDTA) scan, or by the Cockcroft and Gault formula (Cockcroft and Gault, 1976). Radiological assessment of the tumour was required by both chest X-ray (CXR) and chest computed tomography (CT) scan. If there was radiological evidence of mediastinal node involvement (N 2 ), or chest wall plus hilar disease (T 3 N 1 ), or if radiological staging was unclear, then mediastinoscopy and biopsy was performed. A bone scan was performed to rule out bony metastases. Patients accepting entry were required to sign a written consent form which had been approved by the ethics committee at each participating institution. Assessment of toxicity and response Chemotherapy-related toxicity was assessed and recorded after each cycle of treatment. Toxicity was measured using the WHO guidelines (Miller et al, 1981). Peri-operative and post-operative complications were documented by the surgical team. Patients were assessed both clinically and with CXR prior to each cycle of treatment. Symptomatic response and WHO performance status were recorded. Symptomatic response was graded as complete, partial, no change, or progressive according to the patient s own assessment. A chest CT scan was performed following the last cycle of chemotherapy to determine the radiological response. Responses were graded according to standard International Union Against Cancer criteria (Hayward et al, 1977). In addition, a minor response was recorded if there was a reduction in size of the index lesion of between 25% and 50% of the sum of the products of the maximum diameter and a perpendicular diameter. Any patients with evidence of clinical and/or radiological progression were to be removed from study and to undergo immediate thoracic surgery. Pathological response was assessed by the on-site histopathologist. Pathological complete response was defined as absence of any tumour in the surgical specimen. Partial response was defined as the presence of only small residual foci of tumour cells, or the presence of significant areas of tumour necrosis throughout the surgical specimen. No change was defined as the presence of large areas of identifiable tumour cells in the surgical specimen with minimal evidence of tumour necrosis. All patients receiving pre-operative chemotherapy were asked to complete European Organisation for Research and Treatment of Cancer (EORTC) QL Core 30 Quality of Life questionnaires prior to chemotherapy, after completion of the three cycles, and then at 3 months post-surgery. Patients in the surgery-only arm were also asked to complete EORTC QL Core 30 Quality of Life questionnaires prior to surgery, and at 1 and 3 months after surgery. Chemotherapy regimen The chemotherapy regimen consisted of mitomycin-c 8 mg m 2, vinblastine 6 mg m 2 and cisplatin 50 mg m 2 given every 3 weeks for three cycles (the mitomycin-c was only given in cycles 1 and 2). A strict hydration regime was followed consisting of 1 l of normal saline plus 10 mmol of MgSO 4 plus 40 mg of frusemide prior to chemotherapy, with a further 1.5 l of normal saline with a total of 15 mmol of MgSO 4 and 30 mmol of KCl as postchemotherapy hydration. Anti-emetic prophylaxis consisted of 8 mg of dexamethasone with 3 mg of granisetron given i.v. prior to chemotherapy with oral dexamethasone and oral domperidone to take home. Chemotherapy was only administered if adequate haematological recovery had occurred (neutrophil count > 1500 mm 3 and platelet count > mm 3 ). If recovery had not occurred, treatment was delayed for a week. If there was a 2-week or more delay, than a 25% dose reduction was required. Creatinine clearance was calculated prior to each cycle, with the cisplatin dose adjusted if the clearance fell by more than 50% but remained greater than 60 ml min 1. If the clearance fell by more than 50% of the starting value, or to less than 60 ml min 1, then cisplatin was to be substituted by carboplatin; the dose determined by the Calvert formula (Calvert et al, 1989) with a desired area under curve (AUC) of 4. RESULTS Between July 1995 and May 1997, 96 patients were assessed for entry to this study; 56 were excluded because of poor performance status, comorbidity, previous cancers, or were found to be inoperable at mediastinoscopy. Of the 40 eligible patients, 22 (55%) consented to participate and 11 were randomized to each arm of the study. Of the remaining 18 patients, 17 gave as the reason for refusal to participate their concerns over further delay, and their preference for immediate surgery. One patient refused on the basis of a relative s bad experience with chemotherapy. Patient demographics are shown in Table 1. All 11 patients randomized to the chemotherapy arm received the planned three cycles of treatment. The median time between randomization and surgery for the chemotherapy-treated patients was 76.5 days (69 85 days), and for surgery-alone patients was 10 days (1 25 days). One patient, initially assessed as operable and entered into the study, was considered on pre-operative evaluation to have been inoperable from presentation. Thus, the patient did not proceed to surgery. The patient had been randomized to, and received, three cycles of chemotherapy, and had a minor radiological response. This patient was included in the assessment of toxicity, performance status, radiological response and follow-up. Cancer Research Campaign 1999 British Journal of Cancer (1999) 79(9/10),
3 1516 RH de Boer et al Table 1 Patient characteristics by treatment group Characteristic Chemotherapy Surgery alone Sex Male 9 8 Female 2 3 Median age (years) 67 (44 77) 60 (51 77) TNM Stage IA 0 0 IB 9 10 IIA 0 0 IIB 1 1 IIIA 1 0 Performance status (WHO) Weight loss Not significant > 5% of pre-illness weight 1 0 Histology Squamous cell carcinoma 7 5 Adenocarcinoma 2 4 Other (carcinoma/anaplastic/necrotic 2 2 mixed) Toxicity Significant chemotherapy-related toxicity was minimal. There were no WHO grade 3 or grade 4 level toxicities. No admissions were required due to effects of the treatment and there were no chemotherapy dose reductions or dose delays. The most frequently noted toxicities were all of grade 1 level: nausea and vomiting (45% of patients), lethargy (36%), and alopecia and constipation (27%). Haematological toxicity did not appear to be a problem with no episodes of febrile neutropenia. Nephrotoxicity did not occur. Symptomatic responses; change in performance status There was a good symptomatic response to MVP chemotherapy (Table 2). Of the 11 patients who were randomized to receive chemotherapy, three were asymptomatic at the start of treatment and remained that way throughout the three cycles. The other eight patients initially had a variety of symptoms including dyspnoea, cough, haemoptysis and pain. All eight had symptomatic improvement: four had a complete symptomatic response, and the other four had a partial response. No patient deteriorated. Two of 11 patients experienced an improvement in performance Table 2 Responses in pre-operative chemotherapy patients (n = 11) Response Symptomatic Radiological Complete response 4 (36%) 0 Partial response 4 (36%) 6 (55%) Minor response 0 3 (28%) Asymptomatic/no change 3 (28%) 2 (17%) Progressive disease 0 0 status, both patients improving from PS 1 to PS 0. In both cases this improvement occurred between cycles 2 and 3. The other nine patients were stable throughout the three cycles. No deterioration in performance status occurred. Objective responses Of the 11 patients receiving chemotherapy, six (55%) had a partial response (Table 2). The percentage reduction in the product of perpendicular diameters ranged from 50% to 82%. Three patients had a minor response (percentage reduction 29% to 46%), and two patients demonstrated no change in the size of their tumour. No patients had evidence of progressive disease during the treatment. Of the six responding patients, three had squamous carcinoma, two had adenocarcinoma and one had undifferentiated carcinoma. All six were stage IB prior to treatment. The two patients who had no response to chemotherapy had squamous cell histology, one being stage IB and the other stage IIB. Surgical results Of the 11 patients receiving immediate surgery, three had a single lobectomy, two had a bilobectomy, four had pneumonectomies and two had a lobectomy with resection of chest wall. Of the 10 patients who had surgery following chemotherapy, five had single lobectomies, two had bilobectomies, one had a pneumonectomy, one had a lobectomy with chest wall resection, and one underwent a sleeve resection of the main bronchus in addition to a lobectomy. The surgery was tolerated well. There appeared to be no additional technical difficulties in the surgery performed on the patients who had received chemotherapy. There were no significant differences in the peri-operative and post-operative problems seen in the patients from each of the two arms. However, one patient in the chemotherapy arm did develop adult respiratory distress syndrome (ARDS) post-operatively and required a prolonged admission in intensive care before recovering. He had continuing problems with empyema post-discharge. Pathological findings No patient had a pathological complete response. A partial pathological response was seen in five of 10 assessable patients (Table 3): three of these five were found to have minimal residual tumour, two having only small single nodules of tumour tissue remaining; the other two patients with a partial response had large areas of tumour necrosis. Quality of life Quality of life analysis in patients receiving pre-operative chemotherapy showed that mean scores in four of the five func- Table 3 Pathological response post-chemotherapy (n = 10) Pathological complete response 0 Pathological partial response Minimal residual tumour 3 Large areas of necrosis 2 No apparent change 5 British Journal of Cancer (1999) 79(9/10), Cancer Research Campaign 1999
4 Pre-operative chemotherapy in early stage NSCLC 1517 Table 4 The average change in the mean scores of the functioning Quality of Life (QOL) scales between pre- and post-chemotherapy, and between prechemotherapy and 3 months post-surgery QOL scales Change from P-value* Change from P-value* pre- to post- pre-chemotherapy chemotherapy to post-surgery Physical No change Role No change Emotional Cognitive Social *Paired t-test. tioning QOL scales had either improved slightly (Emotional and Cognitive ), or remained stable (Physical and Role ), following chemotherapy. None of the changes reached statistical significance. There was a small, but non-significant, decrease in Social (Table 4). In the same patients, following surgery, there was a decrease in all the functioning QOL scale scores, with the largest decrease in the Social scale; again, none of these decreases reached significance (Table 4). Although an attempt was made to measure quality of life in the surgery-only patients, poor patient compliance with post-surgery questionnaires meant that meaningful analysis of this group was not possible. DISCUSSION This study has confirmed that the administration of three cycles of MVP chemotherapy prior to surgery in patients with early stage NSCLC (IB and II as well as IIIA) is feasible, with 55% of eligible patients agreeing to participate. All patients randomized complied with the full treatment schedule. As previously shown in patients with stage IV NSCLC (Ellis et al, 1995), the MVP regimen proved to be easy to administer, and had minimal toxicity. There were no grade III/IV toxicities, and the most common toxicities were minor (grade I) nausea and lethargy. There were no dose reductions required for either haematological or nephro-toxicity. There were also no treatment delays, an important issue in a potentially curable tumour, with surgery proceeding as planned in all appropriate cases. Although potential concerns have been voiced about delaying surgery during pre-operative chemotherapy, we found no evidence of disease progression during chemotherapy. Indeed, there was a suggestion that those patients receiving pre-operative chemotherapy required less extensive operations than those proceeding directly to surgery. This suggests that the benefit of chemotherapy-induced tumour regression outweighs the theoretical risk of tumour progression, a key point for confirmation in a phase III trial. In this context, five patients showed pathological evidence of tumour regression, with two of the patients having only small residual nodules of tumour present in the surgical specimen. In this small series, no complete pathological remissions were seen, but these have been reported in other series. Martini et al (1993) found a 14% pathological complete response rate in 136 patients with N2 disease following two to three cycles of MVP chemotherapy. Rosell et al (1994) reported one complete response, and four cases with only residual microscopic tumour foci in 30 stage IIIA patients treated with three cycles of pre-operative mitomycin-c, ifosfamide and cisplatin. A further useful benefit for pre-operative chemotherapy was the improvement in cancer-related symptoms prior to surgery. All patients who had symptoms at the time of randomization responded to chemotherapy, with half of them having a complete response. Two patients had improvement in their performance status, whilst the other nine remained stable. Patients receiving chemotherapy therefore arrived for surgery at least as fit as those proceeding directly to surgery, and sometimes fitter. We have also reported good symptomatic benefit from MVP in patients receiving chemotherapy for advanced, metastatic NSCLC (Ellis et al, 1995). Quality of life data on the Physical, Role, Emotional, Cognitive and Social scales likewise suggested no significant deterioration during chemotherapy. It was of interest that surgery appeared to have a greater detrimental effect than chemotherapy, and this needs formal testing in a phase III trial. A theoretical concern of pre-operative chemotherapy is increased peri-operative complications secondary to potential toxic effects of the drugs on pulmonary tissue. This particularly applies to the use of mitomycin-c, which has known pulmonary toxicity, but nearly always at larger cumulative doses than used here (Doyle et al, 1984; Linette et al, 1992). Although the numbers in this pilot study are too small to make definite conclusions, there did not appear to be a significant increase in peri-operative complications in the patients in the chemotherapy-treatment arm. Other randomized trials of preoperative chemotherapy have also not found a significant increase in surgical complications in the chemotherapy arm (Rosell et al, 1994; Roth et al, 1994). In conclusion, the results of this small pilot randomized phase II study have shown that this approach is acceptable to more than 50% of eligible patients; the MVP chemotherapy regimen is well tolerated and brings patients to surgery with improved symptom control and, in some cases, improved performance status. We did not encounter disease progression or other adverse events during the chemotherapy or at surgery. The study therefore justifies a large multi-centre phase III trial in all patients with operable NSCLC to see whether the survival benefit suggested with preoperative chemotherapy in the small randomised stage IIIA NSCLC trials can be confirmed. Such a trial is now underway under the auspices of the Medical Research Council Lung Group, and the data will be included in the currently running UK Big Lung Trial. Lung cancer specialists are invited to join. ACKNOWLEDGEMENTS The authors would like to thank Cathy Ratcliffe for her assistance in data collection, and the resident medical and nursing staff who were involved in the treatment and care of the patients. REFERENCES Burkes RL, Ginsberg RJ, Shepherd FA, Blackstein ME, Goldberg ME, Waters PF, Patterson GA, Todd T, Pearson FG, Cooper JD, Jones D and Lockwood G Cancer Research Campaign 1999 British Journal of Cancer (1999) 79(9/10),
5 1518 RH de Boer et al (1992) Induction chemotherapy with mitomycin, vindesine and cisplatin for stage III unresectable non-small-cell lung cancer: results of the Toronto phase II trial. J Clin Oncol 10: Calvert AH, Newell DR, Gumbrell LA, O Reilly S, Burnell M, Boxall FE, Siddick ZH, Judson IR, Gore ME and Wiltshaw E (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7: Cockcroft DW and Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16: Doyle LA, Ihde DC, Carney DN, Bunn PA, Cohen MH, Matthews MJ, Puffenbarger R, Cordes RS and Minna JD (1984) Combination chemotherapy with doxorubicin and mitomycin C in non-small-cell bronchogenic carcinoma. Severe pulmonary toxicity from q3 weekly mitomycin C. Am J Clin Oncol 7: Ellis PA, Smith IE, Hardy JR, Nicolson MC, Talbot DC, Ashley SE and Priest K (1995) Symptom relief with MVP (mitomycin-c, vinblastine and cisplatin) chemotherapy in advanced non-small-cell lung cancer. Br J Cancer 71: Faber LP, Kittle CF, Warren WH, Bonomi PD, Taylor SG IV, Reddy S and Lee MS (1989) Preoperative chemotherapy and radiation for stage III non-small cell lung cancer. Ann Thorac Surg 47: Hayward JL, Carbone PP, Heuson J-C, Kumaoka S, Segaloff A and Rubens RD (1977) Assessment of response to therapy in advanced breast cancer. Br J Cancer 35: Holmes EC and Gail M (1986) Surgical adjuvant therapy for stage II and stage III adenocarcinoma and large cell undifferentiated carcinoma. J Clin Oncol 4: Lad T, Rubinstein L and Sadeghi A (1988) The benefit of adjuvant treatment for resected locally advanced non-small-cell lung cancer. J Clin Oncol 6: 9 17 Linette DC, McGee KH and McFarland JA (1992) Mitomycin-induced pulmonary toxicity: case report and review of the literature. Ann Pharmacother 26: Martini N, Kris MG, Flehinger BJ, Gralla RJ, Bains MS, Burt ME, Heelan R, McCormack PM, Pisters KMW, Rigas JR, Rausch VW and Ginsberg RJ (1993) Preoperative chemotherapy for stage IIIA (N2) lung cancer: the Sloan- Kettering experience with 136 patients. Ann Thorac Surg 55: McCormack PM, Bains MS, Martini N, Burt ME and Kaiser LR (1987) Methods of skeletal reconstruction following resection of lung cancer invading the chest wall. Surg Clin North Am 67: Miller AB, Hoogstraten B, Staquet M and Winkler A (1981) Reporting results of cancer treatment. Cancer 47: Mountain CF (1977) Assessment of the role of surgery for control of lung cancer. Ann Thorac Surg 24: Niiranen A, Niitamo-Korhonen S, Kouri M, Assendelft A, Mattson K and Pyrhonen S (1992) Adjuvant chemotherapy after radical surgery for non-small-cell lung cancer: a randomised study. J Clin Oncol 10: Non-small Cell Lung Cancer Collaborative Group (1995) Chemotherapy in nonsmall cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Br Med J 311: Rosell R, Gomez-Codina J, Camps C, Maestre J, Padille J, Canto A, Mate JL, Li S, Roig J, Olazabal A, Canela M, Ariza A, Skacel Z, Morera-Prat J and Abad A (1994) A randomised trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med 330: Roth JA, Fossella F, Komaki R, Ryan MB, Putnam JB Jr, Lee JS, Dhingra H, De Caro L, Chasen M, McGavran M, Atkinson EN and Hong WK (1994) A randomised trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 86: Skarin A, Jochelson M, Sheldon T, Malcolm A, Oliynyk P, Overholt R, Hunt M and Frei E (1989) Neoadjuvant chemotherapy in marginally resectable stage III M0 non-small cell lung cancer: long term follow up in 41 patients. J Surg Oncol 40: Slevin ML, Stubbs L, Plant HJ, Wilson P, Gregory WM, Armes PJ and Downer SM (1990) Attitudes to chemotherapy: comparing views of patients with cancer with those of doctors, nurses and general public. Br Med J 300: Strauss GM, Herndon JE, Sherman DD, Mathisen DJ, Carey RW, Choi NC, Rege VB, Modeas C and Green MR (1992) Neoadjuvant chemotherapy and radiotherapy followed by surgery in stage IIIA non-small-cell carcinoma of the lung: report of a Cancer and Leukemia Group B phase II study. J Clin Oncol 10: Weiden PL and Piantadosi S (1991) Preoperative chemotherapy (cisplatin and fluorouracil) and radiation therapy in stage III non-small-cell lung cancer: a phase II study of the Lung Cancer Study Group. J Natl Cancer Inst 83: British Journal of Cancer (1999) 79(9/10), Cancer Research Campaign 1999
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer
 Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Survey on the treatment of non-small cell lung cancer (NSCLC) in England and Wales
 Eur Respir J 1997; 10: 1552 1558 DOI: 10.1183/09031936.97.10071552 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1997 European Respiratory Journal ISSN 0903-1936 Survey on the treatment
Eur Respir J 1997; 10: 1552 1558 DOI: 10.1183/09031936.97.10071552 Printed in UK - all rights reserved Copyright ERS Journals Ltd 1997 European Respiratory Journal ISSN 0903-1936 Survey on the treatment
Scottish Medicines Consortium
 Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
Schedule: Drug Dose iv/infusion/oral q Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8
 Carboplatin/Gemcitabine Lung Cancer (non-small cell) - Advanced Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8 Cycle frequency: Every three weeks Total
Carboplatin/Gemcitabine Lung Cancer (non-small cell) - Advanced Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8 Cycle frequency: Every three weeks Total
Good symptom relief with palliative MVP (mitomycin-c, vinblastine and cisplatin) chemotherapy in malignant mesothelioma
 Annals of Oncology 9: 9-7, 998. 998 Khmer Academic Publishers. Printed in the Netherlands Original article Good symptom relief with palliative MVP (mitomycin-c, vinblastine and cisplatin) chemotherapy
Annals of Oncology 9: 9-7, 998. 998 Khmer Academic Publishers. Printed in the Netherlands Original article Good symptom relief with palliative MVP (mitomycin-c, vinblastine and cisplatin) chemotherapy
non-small cell lung cancer
 Thorax 1995;50(Suppl 1):S25-S30 Neoadjuvant chemotherapy in stage lla non-small cell lung cancer Robert Milroy, Fergus Macbeth* Department of Respiratory Medicine, Stobhill Hospital NHS Trust, Glasgow,
Thorax 1995;50(Suppl 1):S25-S30 Neoadjuvant chemotherapy in stage lla non-small cell lung cancer Robert Milroy, Fergus Macbeth* Department of Respiratory Medicine, Stobhill Hospital NHS Trust, Glasgow,
NCCN Non-Small Cell Lung Cancer V.1.2011 Update Meeting 07/09/10
 Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof.
 Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Klaus Junker, MD; Kathrin Langner, MD; Folker Klinke, MD; Ulrich Bosse, MD; and Michael Thomas, MD
 Grading of Tumor Regression in Non-small Cell Lung Cancer* Morphology and Prognosis Klaus Junker, MD; Kathrin Langner, MD; Folker Klinke, MD; Ulrich Bosse, MD; and Michael Thomas, MD Objective: Different
Grading of Tumor Regression in Non-small Cell Lung Cancer* Morphology and Prognosis Klaus Junker, MD; Kathrin Langner, MD; Folker Klinke, MD; Ulrich Bosse, MD; and Michael Thomas, MD Objective: Different
Lung Cancer Treatment Guidelines
 Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
Activity of pemetrexed in thoracic malignancies
 Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
CANCER PULMON: ESTADIOS INICIALES POSTMUNDIAL PULMON DENVER 2015. 8-10-2015.Manuel Cobo Dols S. Oncología Médica HU Málaga Regional y VV
 CANCER PULMON: ESTADIOS INICIALES POSTMUNDIAL PULMON DENVER 2015 8-10-2015.Manuel Cobo Dols S. Oncología Médica HU Málaga Regional y VV Meta-analisis LACE: adyuvancia vs no adyuvancia Pignon JP, et al.
CANCER PULMON: ESTADIOS INICIALES POSTMUNDIAL PULMON DENVER 2015 8-10-2015.Manuel Cobo Dols S. Oncología Médica HU Málaga Regional y VV Meta-analisis LACE: adyuvancia vs no adyuvancia Pignon JP, et al.
Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence
 Post- survival in completely resected stage I non-small cell lung cancer with local J-J Hung, 1,2,3 W-H Hsu, 3 C-C Hsieh, 3 B-S Huang, 3 M-H Huang, 3 J-S Liu, 2 Y-C Wu 3 See Editorial, p 185 c A supplementary
Post- survival in completely resected stage I non-small cell lung cancer with local J-J Hung, 1,2,3 W-H Hsu, 3 C-C Hsieh, 3 B-S Huang, 3 M-H Huang, 3 J-S Liu, 2 Y-C Wu 3 See Editorial, p 185 c A supplementary
Radiotherapy in locally advanced & metastatic NSC lung cancer
 Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
Radiation Therapy in the Treatment of
 Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases
 Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Cycle frequency: Every three weeks Total number of cycles: 6
 Carboplatin/Paclitaxel Ovarian Cancer Adjuvant, Advanced Paclitaxel 175mg/m 2 500mls 5% dex/3hrs Day 1 Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Anti-emetic group - Moderately high Paclitaxel given first
Carboplatin/Paclitaxel Ovarian Cancer Adjuvant, Advanced Paclitaxel 175mg/m 2 500mls 5% dex/3hrs Day 1 Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Anti-emetic group - Moderately high Paclitaxel given first
Emerging Drug List GEFITINIB
 Generic (Trade Name): Manufacturer: Gefitinib (Iressa ) formerly referred to as ZD1839 AstraZeneca NO. 52 JANUARY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence:
Generic (Trade Name): Manufacturer: Gefitinib (Iressa ) formerly referred to as ZD1839 AstraZeneca NO. 52 JANUARY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence:
The lungs What is lung cancer? How common is it? Risks & symptoms Diagnosis & treatment options
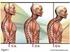 Why We re Here The lungs What is lung cancer? How common is it? Risks & symptoms Diagnosis & treatment options What Are Lungs? What Do They Do? 1 Located in the chest Allow you to breathe Provide oxygen
Why We re Here The lungs What is lung cancer? How common is it? Risks & symptoms Diagnosis & treatment options What Are Lungs? What Do They Do? 1 Located in the chest Allow you to breathe Provide oxygen
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
the standard of care 2009 5/1/2009 Mesothelioma: The standard of care take home messages PILC 2006 Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009
 Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
West of Scotland Cancer Network Chemotherapy Protocol. Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021)
 West of Scotland Cancer Network Chemotherapy Protocol Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021) Indication Palliative chemotherapy for malignant mesothelioma of the pleura Eligibility
West of Scotland Cancer Network Chemotherapy Protocol Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021) Indication Palliative chemotherapy for malignant mesothelioma of the pleura Eligibility
Adjuvant Therapy Non Small Cell Lung Cancer. Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015
 Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
A Practical Guide to Advances in Staging and Treatment of NSCLC
 A Practical Guide to Advances in Staging and Treatment of NSCLC Robert J. Korst, M.D. Director, Thoracic Surgery Medical Director, The Blumenthal Cancer Center The Valley Hospital Objectives Revised staging
A Practical Guide to Advances in Staging and Treatment of NSCLC Robert J. Korst, M.D. Director, Thoracic Surgery Medical Director, The Blumenthal Cancer Center The Valley Hospital Objectives Revised staging
Table of Contents. Data Supplement 1: Summary of ASTRO Guideline Statements. Data Supplement 2: Definition of Terms
 Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
Cycle frequency: Every three weeks Total number of cycles: 3 or 4
 BEP 3-day (Bleomycin/Etoposide/Cisplatin) Germ cell tumours Bleomycin 30,000iu 200mls N. Saline/30mins Days 2, 8 & 15 Etoposide 165mg/m 2 1L N. Saline/1hr Days 1, 2 & 3 Cisplatin 50mg/m 2 1L N. Saline/4hrs
BEP 3-day (Bleomycin/Etoposide/Cisplatin) Germ cell tumours Bleomycin 30,000iu 200mls N. Saline/30mins Days 2, 8 & 15 Etoposide 165mg/m 2 1L N. Saline/1hr Days 1, 2 & 3 Cisplatin 50mg/m 2 1L N. Saline/4hrs
The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy
 17 (Supplement 10): x108 x112, 2006 doi:10.1093/annonc/mdl247 The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy E. Felip & E. Vilar Oncology Department, Vall d
17 (Supplement 10): x108 x112, 2006 doi:10.1093/annonc/mdl247 The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy E. Felip & E. Vilar Oncology Department, Vall d
Adiuwantowe i neoadiuwantowe leczenie chorych na zaawansowanego raka żołądka
 Adiuwantowe i neoadiuwantowe leczenie chorych na zaawansowanego raka żołądka Neoadiuvant and adiuvant therapy for advanced gastric cancer Franco Roviello, IT Neoadjuvant and adjuvant therapy for advanced
Adiuwantowe i neoadiuwantowe leczenie chorych na zaawansowanego raka żołądka Neoadiuvant and adiuvant therapy for advanced gastric cancer Franco Roviello, IT Neoadjuvant and adjuvant therapy for advanced
Results of Surgical Treatment for Small Cell Lung Cancers
 NAOSITE: Nagasaki University's Ac Title Author(s) Results of Surgical Treatment for S Ayabe, Hiroyoshi; Nakamura, Akihiro Hara, Shinsuke; Tagawa, Yutaka; Kaw Citation Acta medica Nagasakiensia. 1994, 39
NAOSITE: Nagasaki University's Ac Title Author(s) Results of Surgical Treatment for S Ayabe, Hiroyoshi; Nakamura, Akihiro Hara, Shinsuke; Tagawa, Yutaka; Kaw Citation Acta medica Nagasakiensia. 1994, 39
L Lang-Lazdunski, A Bille, S Marshall, R Lal, D Landau, J Spicer
 Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine and systemic chemotherapy in malignant pleural mesothelioma. A 10-year experience. L Lang-Lazdunski, A Bille, S Marshall, R Lal,
Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine and systemic chemotherapy in malignant pleural mesothelioma. A 10-year experience. L Lang-Lazdunski, A Bille, S Marshall, R Lal,
Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
 Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Survival analysis of 220 patients with completely resected stage II non small cell lung cancer
 窑 Original Article 窑 Chinese Journal of Cancer Survival analysis of 22 patients with completely resected stage II non small cell lung cancer Yun Dai,2,3, Xiao Dong Su,2,3, Hao Long,2,3, Peng Lin,2,3, Jian
窑 Original Article 窑 Chinese Journal of Cancer Survival analysis of 22 patients with completely resected stage II non small cell lung cancer Yun Dai,2,3, Xiao Dong Su,2,3, Hao Long,2,3, Peng Lin,2,3, Jian
Stage IIIB disease includes patients with T4 tumors,
 Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines
 Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines April 2008 (presented at 6/12/08 cancer committee meeting) By Shelly Smits, RHIT, CCS, CTR Conclusions by Dr. Ian Thompson, MD Dr. James
Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines April 2008 (presented at 6/12/08 cancer committee meeting) By Shelly Smits, RHIT, CCS, CTR Conclusions by Dr. Ian Thompson, MD Dr. James
Small Cell Lung Cancer
 Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Regular follow-up after curative resection of nonsmall cell lung cancer: a real benefit for patients?
 Eur Respir J 2002; 19: 464 468 DOI: 10.1183/09031936.02.00231802 Printed in UK all rights reserved Copyright #ERS Journals Ltd 2002 European Respiratory Journal ISSN 0903-1936 Regular follow-up after curative
Eur Respir J 2002; 19: 464 468 DOI: 10.1183/09031936.02.00231802 Printed in UK all rights reserved Copyright #ERS Journals Ltd 2002 European Respiratory Journal ISSN 0903-1936 Regular follow-up after curative
Controversies in Management of. Inoperable NSCLC. Inoperable NSCLC. Introduction:
 Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Management of Non-Small Cell Lung Cancer Guide for General Practitioners
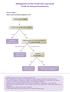 Management of n-small Cell Lung Cancer Guide for General Practitioners Clinical Stage I Cancer only in one lobe of lung and
Management of n-small Cell Lung Cancer Guide for General Practitioners Clinical Stage I Cancer only in one lobe of lung and
How To Treat Lung Cancer At Cleveland Clinic
 Treatment Guide Lung Cancer Management The Chest Cancer Center at Cleveland Clinic, which includes specialists from the Respiratory Institute, Taussig Cancer Institute and Miller Family Heart & Vascular
Treatment Guide Lung Cancer Management The Chest Cancer Center at Cleveland Clinic, which includes specialists from the Respiratory Institute, Taussig Cancer Institute and Miller Family Heart & Vascular
Active centers: 2. Number of patients/subjects: Planned: 20 Randomized: Treated: 20 Evaluated: Efficacy: 13 Safety: 20
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
Lung Cancer and Mesothelioma
 Lung Cancer and Mesothelioma Robert Kratzke, M.D. John C. Skoglund Professor of Lung Cancer Research Section of Heme/Onc/Transplant Department of Medicine University of Minnesota Medical School Malignant
Lung Cancer and Mesothelioma Robert Kratzke, M.D. John C. Skoglund Professor of Lung Cancer Research Section of Heme/Onc/Transplant Department of Medicine University of Minnesota Medical School Malignant
Lung Pathway Group Pemetrexed and Cisplatin in Non-Small Cell Lung Cancer (NSCLC)
 Indication: NICE TA181 First line treatment option in advanced or metastatic non-squamous NSCLC (histology confirmed as adenocarcinoma or large cell carcinoma) Performance status 0-1 Regimen details: Pemetrexed
Indication: NICE TA181 First line treatment option in advanced or metastatic non-squamous NSCLC (histology confirmed as adenocarcinoma or large cell carcinoma) Performance status 0-1 Regimen details: Pemetrexed
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer
 Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
3.0 With final Comments for presentation at Sub Group Meeting 24. 24.11.10
 Guideline for the Treatment of Lung Cancer Version History 2.0 Endorsed by the Governance Committee as treatment of lung cancer 27.07.09 with radiotherapy and chemotherapy. 2.1 Re-written to include the
Guideline for the Treatment of Lung Cancer Version History 2.0 Endorsed by the Governance Committee as treatment of lung cancer 27.07.09 with radiotherapy and chemotherapy. 2.1 Re-written to include the
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
London Cancer. Mesothelioma Lung Protocols
 London Cancer Mesothelioma Lung Protocols Version 0.9 Contents 1. Staging... 3 2. Mesothelioma Summary of Chemotherapy Protocols... 4 3. Mesothelioma Chemotherapy Protocols... 7 3.1. Pemetrexed (Alimta
London Cancer Mesothelioma Lung Protocols Version 0.9 Contents 1. Staging... 3 2. Mesothelioma Summary of Chemotherapy Protocols... 4 3. Mesothelioma Chemotherapy Protocols... 7 3.1. Pemetrexed (Alimta
Postoperative Adjuvant Chemotherapy, with or without Radiotherapy, in Completely Resected Non-Small Cell Lung Cancer
 EBS 7-1-2 EDUCATION AND INFORMATION 2013 Evidence-based Series 7-1-2: EDUCATION AND INFORMATION- 2013 Postoperative Adjuvant Chemotherapy, with or without Radiotherapy, in Completely Resected Non-Small
EBS 7-1-2 EDUCATION AND INFORMATION 2013 Evidence-based Series 7-1-2: EDUCATION AND INFORMATION- 2013 Postoperative Adjuvant Chemotherapy, with or without Radiotherapy, in Completely Resected Non-Small
Small Cell Lung Cancer
 Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Management of stage III A-B of NSCLC. Hamed ALHusaini Medical Oncologist
 Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Integrating Chemotherapy and Liver Surgery for the Management of Colorectal Metastases
 I Congresso de Oncologia D Or July 5-6, 2013 Integrating Chemotherapy and Liver Surgery for the Management of Colorectal Metastases Michael A. Choti, MD, MBA, FACS Department of Surgery Johns Hopkins University
I Congresso de Oncologia D Or July 5-6, 2013 Integrating Chemotherapy and Liver Surgery for the Management of Colorectal Metastases Michael A. Choti, MD, MBA, FACS Department of Surgery Johns Hopkins University
Out-Patient Chemotherapy for Lung Cancer
 Lung Cancer Out-Patient Chemotherapy for Lung Cancer Principles and practice JMAJ 46(12): 542 546, 2003 Shuichi YONEDA Director, Department of Pulmonary Medicine, Saitama Cancer Center Abstract: Recent
Lung Cancer Out-Patient Chemotherapy for Lung Cancer Principles and practice JMAJ 46(12): 542 546, 2003 Shuichi YONEDA Director, Department of Pulmonary Medicine, Saitama Cancer Center Abstract: Recent
Effects of Chemotherapy on Quality of Life for Patients with Lung Cancer
 CLINICAL STUDY Effects of Chemotherapy on Quality of Life for Patients with Lung Cancer Ahmet Bircan, MD 1 ; M. Bahad r Berktafl, MD 1 ; Hülya Bay z, MD 1 ; Nihal Baflay, MD 1 ; Sema Bircan, MD 2 ; Mine
CLINICAL STUDY Effects of Chemotherapy on Quality of Life for Patients with Lung Cancer Ahmet Bircan, MD 1 ; M. Bahad r Berktafl, MD 1 ; Hülya Bay z, MD 1 ; Nihal Baflay, MD 1 ; Sema Bircan, MD 2 ; Mine
Adjuvant Chemotherapy After Complete Resection of Non-Small Cell Lung Cancer
 REVIEW ARTICLE Adjuvant Chemotherapy After Complete Resection of Non-Small Cell Lung Cancer Eckart Laack, Carsten Bokemeyer, Dieter Kurt Hossfeld SUMMARY Introduction: In non-small cell lung cancer (NSCLC)
REVIEW ARTICLE Adjuvant Chemotherapy After Complete Resection of Non-Small Cell Lung Cancer Eckart Laack, Carsten Bokemeyer, Dieter Kurt Hossfeld SUMMARY Introduction: In non-small cell lung cancer (NSCLC)
KIDNEY FUNCTION RELATION TO SIZE OF THE TUMOR IN RENAL CELL CANCINOMA
 KIDNEY FUNCTION RELATION TO SIZE OF THE TUMOR IN RENAL CELL CANCINOMA O.E. Stakhvoskyi, E.O. Stakhovsky, Y.V. Vitruk, O.A. Voylenko, P.S. Vukalovich, V.A. Kotov, O.M. Gavriluk National Canсer Institute,
KIDNEY FUNCTION RELATION TO SIZE OF THE TUMOR IN RENAL CELL CANCINOMA O.E. Stakhvoskyi, E.O. Stakhovsky, Y.V. Vitruk, O.A. Voylenko, P.S. Vukalovich, V.A. Kotov, O.M. Gavriluk National Canсer Institute,
7. Prostate cancer in PSA relapse
 7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
Mesothelioma. Malignant Pleural Mesothelioma
 Mesothelioma William G. Richards, PhD Brigham and Women s Hospital Malignant Pleural Mesothelioma 2,000-3,000 cases per year (USA) Increasing incidence Asbestos (50-80%, decreasing) 30-40 year latency
Mesothelioma William G. Richards, PhD Brigham and Women s Hospital Malignant Pleural Mesothelioma 2,000-3,000 cases per year (USA) Increasing incidence Asbestos (50-80%, decreasing) 30-40 year latency
Clinical Indications and Results Following Chest Wall Resection
 Clinical Indications and Results Following Chest Wall Resection for Recurrent Malignant Pleural Mesothelioma Ali SO, Burt BM, Groth SS, DaSilva MC, Yeap BY, Richards WG, Baldini EH and Sugarbaker DJ. Division
Clinical Indications and Results Following Chest Wall Resection for Recurrent Malignant Pleural Mesothelioma Ali SO, Burt BM, Groth SS, DaSilva MC, Yeap BY, Richards WG, Baldini EH and Sugarbaker DJ. Division
Stage I Non-Small Cell Lung Cancer: Recurrence Patterns, Prognostic Factors and Survival
 16 Stage I Non-Small Cell Lung Cancer: Recurrence Patterns, Prognostic Factors and Survival Jung-Jyh Hung and Yu-Chung Wu Division of Thoracic Surgery, Department of Surgery, Taipei Veterans General Hospital
16 Stage I Non-Small Cell Lung Cancer: Recurrence Patterns, Prognostic Factors and Survival Jung-Jyh Hung and Yu-Chung Wu Division of Thoracic Surgery, Department of Surgery, Taipei Veterans General Hospital
Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study
 Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Epidemiology, Staging and Treatment of Lung Cancer. Mark A. Socinski, MD
 Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
The National Clinical Lung Cancer Audit (LUCADA)
 The National Clinical Lung Cancer Audit (LUCADA) DATA MANUAL Title: Version: 3.1.5 Date: September 2013 LUCADA Lung Cancer Audit VERSION HISTORY Version Date Issued Brief Summary of Change Owner s Name
The National Clinical Lung Cancer Audit (LUCADA) DATA MANUAL Title: Version: 3.1.5 Date: September 2013 LUCADA Lung Cancer Audit VERSION HISTORY Version Date Issued Brief Summary of Change Owner s Name
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians
 Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
PET/CT in Lung Cancer
 PET/CT in Lung Cancer Rodolfo Núñez Miller, M.D. Nuclear Medicine and Diagnostic Imaging Section Division of Human Health International Atomic Energy Agency Vienna, Austria GLOBOCAN 2012 #1 #3 FDG-PET/CT
PET/CT in Lung Cancer Rodolfo Núñez Miller, M.D. Nuclear Medicine and Diagnostic Imaging Section Division of Human Health International Atomic Energy Agency Vienna, Austria GLOBOCAN 2012 #1 #3 FDG-PET/CT
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Avastin: Glossary of key terms
 Avastin: Glossary of key terms Adenocarcinoma Adenoma Adjuvant therapy Angiogenesis Anti-angiogenics Antibody Antigen Avastin (bevacizumab) Benign A form of carcinoma that originates in glandular tissue.
Avastin: Glossary of key terms Adenocarcinoma Adenoma Adjuvant therapy Angiogenesis Anti-angiogenics Antibody Antigen Avastin (bevacizumab) Benign A form of carcinoma that originates in glandular tissue.
Tricia Cox on 7/18/2012 at Oncology Center. Sarah Randolf. Female
 SAMPLE This Survivorship Care Plan will facilitate cancer care following active treatment. It may include important contact information, a treatment summary, recommendations for follow-up care testing,
SAMPLE This Survivorship Care Plan will facilitate cancer care following active treatment. It may include important contact information, a treatment summary, recommendations for follow-up care testing,
Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma
 Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma Marc de Perrot, Ronald Feld, Natasha B Leighl, Andrew Hope, Thomas K Waddell, Shaf Keshavjee,
Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma Marc de Perrot, Ronald Feld, Natasha B Leighl, Andrew Hope, Thomas K Waddell, Shaf Keshavjee,
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Updates in Mesothelioma By Samieh Amer, MD Professor of Cardiothoracic Surgery Faculty of Medicine, Cairo University History Wagner and his colleagues (1960) 33 cases of mesothelioma
بسم هللا الرحمن الرحيم Updates in Mesothelioma By Samieh Amer, MD Professor of Cardiothoracic Surgery Faculty of Medicine, Cairo University History Wagner and his colleagues (1960) 33 cases of mesothelioma
Metastatic Breast Cancer 201. Carolyn B. Hendricks, MD October 29, 2011
 Metastatic Breast Cancer 201 Carolyn B. Hendricks, MD October 29, 2011 Overview Is rebiopsy necessary at the time of recurrence or progression of disease? How dose a very aggressive treatment upfront compare
Metastatic Breast Cancer 201 Carolyn B. Hendricks, MD October 29, 2011 Overview Is rebiopsy necessary at the time of recurrence or progression of disease? How dose a very aggressive treatment upfront compare
Targeted Therapy What the Surgeon Needs to Know
 Targeted Therapy What the Surgeon Needs to Know AATS Focus in Thoracic Surgery 2014 David R. Jones, M.D. Professor & Chief, Thoracic Surgery Memorial Sloan Kettering Cancer Center I have no disclosures
Targeted Therapy What the Surgeon Needs to Know AATS Focus in Thoracic Surgery 2014 David R. Jones, M.D. Professor & Chief, Thoracic Surgery Memorial Sloan Kettering Cancer Center I have no disclosures
ORIGINAL ARTICLE THORACIC ONCOLOGY
 Ann Surg Oncol (2013) 20:1934 1940 DOI 10.1245/s10434-012-2800-x ORIGINAL ARTICLE THORACIC ONCOLOGY Predictors for Locoregional Recurrence for Clinical Stage III-N2 Non-small Cell Lung Cancer with Nodal
Ann Surg Oncol (2013) 20:1934 1940 DOI 10.1245/s10434-012-2800-x ORIGINAL ARTICLE THORACIC ONCOLOGY Predictors for Locoregional Recurrence for Clinical Stage III-N2 Non-small Cell Lung Cancer with Nodal
Lung Cancer. Public Outcomes Report. Submitted by Omar A. Majid, MD
 Public Outcomes Report Lung Cancer Submitted by Omar A. Majid, MD Lung cancer is the most common cancer-related cause of death among men and women. It has been estimated that there will be 226,1 new cases
Public Outcomes Report Lung Cancer Submitted by Omar A. Majid, MD Lung cancer is the most common cancer-related cause of death among men and women. It has been estimated that there will be 226,1 new cases
Lung cancer (non-small-cell)
 Patient information from the BMJ Group Lung cancer (non-small-cell) It can be devastating to find out that you or someone close to you has lung cancer. You will have to make some important decisions about
Patient information from the BMJ Group Lung cancer (non-small-cell) It can be devastating to find out that you or someone close to you has lung cancer. You will have to make some important decisions about
Stage I, II Non Small Cell Lung Cancer
 Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
Disease/Illness GUIDE TO ASBESTOS LUNG CANCER. What Is Asbestos Lung Cancer? www.simpsonmillar.co.uk Telephone 0844 858 3200
 GUIDE TO ASBESTOS LUNG CANCER What Is Asbestos Lung Cancer? Like tobacco smoking, exposure to asbestos can result in the development of lung cancer. Similarly, the risk of developing asbestos induced lung
GUIDE TO ASBESTOS LUNG CANCER What Is Asbestos Lung Cancer? Like tobacco smoking, exposure to asbestos can result in the development of lung cancer. Similarly, the risk of developing asbestos induced lung
SAKK Lung Cancer Group. Current activities and future projects
 SAKK Lung Cancer Group Current activities and future projects SAKK Lung Cancer Group Open group of physicians interested in lung cancer Mostly Medical Oncologists, but also Thoracic Surgeons Radiation
SAKK Lung Cancer Group Current activities and future projects SAKK Lung Cancer Group Open group of physicians interested in lung cancer Mostly Medical Oncologists, but also Thoracic Surgeons Radiation
The Di Bella Method (DBM) improves Survival, Objective Response and Performance Status in Breast Cancer
 BIT's 4th World Cancer Congress 2011 People s Republic of China Dalian The Di Bella Method (DBM) improves Survival, Objective Response and Performance Status in treated with DBM therapy Retrospective observational
BIT's 4th World Cancer Congress 2011 People s Republic of China Dalian The Di Bella Method (DBM) improves Survival, Objective Response and Performance Status in treated with DBM therapy Retrospective observational
POLICY A. INDICATIONS
 Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
Non-Small Cell Lung Cancer
 Non-Small Cell Lung Cancer John delcharco, MD (Statistics based on CVMC data 2009-2013) Statistics Lung cancer is the leading cause of cancer deaths in the United States. The American Cancer Society estimates
Non-Small Cell Lung Cancer John delcharco, MD (Statistics based on CVMC data 2009-2013) Statistics Lung cancer is the leading cause of cancer deaths in the United States. The American Cancer Society estimates
Update on Small Cell Lung Cancer
 Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
The Need for Accurate Lung Cancer Staging
 The Need for Accurate Lung Cancer Staging Peter Baik, DO Thoracic Surgery Cancer Treatment Centers of America Oklahoma Osteopathic Association 115th Annual Convention Financial Disclosures: None 2 Objectives
The Need for Accurate Lung Cancer Staging Peter Baik, DO Thoracic Surgery Cancer Treatment Centers of America Oklahoma Osteopathic Association 115th Annual Convention Financial Disclosures: None 2 Objectives
Summary of treatment benefits
 Risk Management Plan PEMETREXED Powder for concentrate for Solution for infusion Pemetrexed is also indicated as monotherapy for the maintenance treatment of locally advanced or metastatic non small cell
Risk Management Plan PEMETREXED Powder for concentrate for Solution for infusion Pemetrexed is also indicated as monotherapy for the maintenance treatment of locally advanced or metastatic non small cell
Osteosarcoma: treatment beyond surgery
 Osteosarcoma: treatment beyond surgery Eric Chow, DVM DACVS Sue Downing, DVM DACVIM-Oncology Providing the best quality care and service for the patient, the client, and the referring veterinarian. Osteosarcoma
Osteosarcoma: treatment beyond surgery Eric Chow, DVM DACVS Sue Downing, DVM DACVIM-Oncology Providing the best quality care and service for the patient, the client, and the referring veterinarian. Osteosarcoma
Surgery. Wedge resection only part of the lung, not. not a lobe, is removed. Cancer Council NSW
 The treatment you receive will depend on your lung cancer type, for example, whether you have a non-small cell lung cancer Adenocarcinoma or Squamous cell carcinoma, and if this is a sub-type with a mutation.
The treatment you receive will depend on your lung cancer type, for example, whether you have a non-small cell lung cancer Adenocarcinoma or Squamous cell carcinoma, and if this is a sub-type with a mutation.
Metastatic Renal Cell Carcinoma: Staging and Prognosis of Three Separate Cases.
 Metastatic Renal Cell Carcinoma: Staging and Prognosis of Three Separate Cases. Abstract This paper describes the staging, imaging, treatment, and prognosis of renal cell carcinoma. Three case studies
Metastatic Renal Cell Carcinoma: Staging and Prognosis of Three Separate Cases. Abstract This paper describes the staging, imaging, treatment, and prognosis of renal cell carcinoma. Three case studies
Carcinoma of the vagina is a relatively uncommon disease, affecting only about 2,000 women in
 EVERYONE S GUIDE FOR CANCER THERAPY Malin Dollinger, MD, Ernest H. Rosenbaum, MD, Margaret Tempero, MD, and Sean Mulvihill, MD 4 th Edition, 2001 Vagina Jeffrey L. Stern, MD Carcinoma of the vagina is
EVERYONE S GUIDE FOR CANCER THERAPY Malin Dollinger, MD, Ernest H. Rosenbaum, MD, Margaret Tempero, MD, and Sean Mulvihill, MD 4 th Edition, 2001 Vagina Jeffrey L. Stern, MD Carcinoma of the vagina is
Mesothelioma. 1. Introduction. 1.1 General Information and Aetiology
 Mesothelioma 1. Introduction 1.1 General Information and Aetiology Mesotheliomas are tumours that arise from the mesothelial cells of the pleura, peritoneum, pericardium or tunica vaginalis [1]. Most are
Mesothelioma 1. Introduction 1.1 General Information and Aetiology Mesotheliomas are tumours that arise from the mesothelial cells of the pleura, peritoneum, pericardium or tunica vaginalis [1]. Most are
Chapter 7: Lung Cancer
 Chapter 7: Lung Cancer Contents Chapter 7: Lung Cancer... 1 Small Cell... 2 Good PS + Limited stage... 2 Cisplatin/etoposide... 2 Concurrent chemotherapy + XRT... 2 Good / Intermediate PS... 2 Carboplatin
Chapter 7: Lung Cancer Contents Chapter 7: Lung Cancer... 1 Small Cell... 2 Good PS + Limited stage... 2 Cisplatin/etoposide... 2 Concurrent chemotherapy + XRT... 2 Good / Intermediate PS... 2 Carboplatin
PET. Can we afford PET-CT. Positron annihilation. PET-CT scanner. PET detection
 PET-CT Can we afford PET-CT John Buscombe New technology Combines functional information-pet anatomical information-ct Machine able to perform both studies in single imaging episode PET imaging depends
PET-CT Can we afford PET-CT John Buscombe New technology Combines functional information-pet anatomical information-ct Machine able to perform both studies in single imaging episode PET imaging depends
Pulmonary function. Is patient potentially operable? Yes. tests 3. Yes. Pulmonary function. tests 3, if clinically indicated. Yes
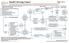 INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
Adjuvant cisplatin-based chemotherapy in non-small-cell lung cancer: new insights into
 Annals of Oncology Advance Access published September 5, 2014 1 Adjuvant cisplatin-based chemotherapy in non-small-cell lung cancer: new insights into the effect on failure type via a multistate approach
Annals of Oncology Advance Access published September 5, 2014 1 Adjuvant cisplatin-based chemotherapy in non-small-cell lung cancer: new insights into the effect on failure type via a multistate approach
Principal Investigator: Valerie W. Rusch, MD, FACS, Chief, Thoracic Surgery Memorial Sloan-Kettering Cancer Center
 Protocol 1101-1088 Phase I study of intra-pleural administration of GL-ONC1 in patients with malignant pleural effusion: primary, metastases and mesothelioma Principal Investigator: Valerie W. Rusch, MD,
Protocol 1101-1088 Phase I study of intra-pleural administration of GL-ONC1 in patients with malignant pleural effusion: primary, metastases and mesothelioma Principal Investigator: Valerie W. Rusch, MD,
THE SECRETS OF OUR SUCCESS
 THE SECRETS OF OUR SUCCESS QUALITY OF LIFE STUDIES OF THE NCIC Andrea Bezjak, MDCM, MSc,, FRCPC Chair, NCIC CTG QOL Committee Outline of the Presentation Can we consider NCIC CTG QOL activities a success?
THE SECRETS OF OUR SUCCESS QUALITY OF LIFE STUDIES OF THE NCIC Andrea Bezjak, MDCM, MSc,, FRCPC Chair, NCIC CTG QOL Committee Outline of the Presentation Can we consider NCIC CTG QOL activities a success?
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer
 Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin
 COMPENDIA TRANSPARENCY TRACKING FORM DRUG: Docetaxel INDICATION: Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin COMPENDIA TRANSPARENCY REQUIREMENTS 1 Provide
COMPENDIA TRANSPARENCY TRACKING FORM DRUG: Docetaxel INDICATION: Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin COMPENDIA TRANSPARENCY REQUIREMENTS 1 Provide
a commitment in their practice to the roles and responsibilities of clinical oncologists X
 CLINICAL ONCOLOGY BLUEPRINT OF ASSESSMENT SYSTEM Good Medical Practice areas of curriculum - ST5 (Intermediate) Standards GMP criterion Workplace based assessment By the end of the third year of training
CLINICAL ONCOLOGY BLUEPRINT OF ASSESSMENT SYSTEM Good Medical Practice areas of curriculum - ST5 (Intermediate) Standards GMP criterion Workplace based assessment By the end of the third year of training
