The National Clinical Lung Cancer Audit (LUCADA)
|
|
|
- Vernon McDowell
- 10 years ago
- Views:
Transcription
1 The National Clinical Lung Cancer Audit (LUCADA) DATA MANUAL Title: Version: Date: September 2013
2 LUCADA Lung Cancer Audit VERSION HISTORY Version Date Issued Brief Summary of Change Owner s Name 1.3 Sept /10/2005 Released after extensive rewrite LUCADA Implementation Group /02/07 Re-written to incorporate software update LUCADA Project Team /07/07 Minor amendment to LCNS fields LUCADA Project Team /07/10 Updated to include UICC 7 staging LUCADA Project Team /10/10 Minor correction to UICC 7 staging LUCADA Project Team /11/2012 Minor update to field 7.10 to allow collection of Radiofrequency ablation LUCADA Project Team For more information on the status of this document, please contact: Health and Social Care Information Centre Clinical Audit Support Unit (CASU) 1 Trevelyan Square Leeds LS1 6AE Internet: [email protected] Date of Issue November 2012 Crown Copyright 2007 Ref:
3 TABLE OF CONTENTS INTRODUCTION... 4 Preface... 4 How to use this Manual... 4 Organisation s... 4 PATIENT REFERRAL... 5 Demographics... 5 Referral... 6 CARE PLAN / MDT... 8 Diagnosis... 8 Pre-treatment Staging Cancer care plan / MDT details Co-morbidity KEY INVESTIGATIONS NURSING CARE TREATMENT Planned Treatment Clinical trial Surgery Post surgery pathology Chemotherapy Teletherapy (external beam radiotherapy) Brachytherapy Palliative care Active monitoring OUTCOME Death Prophylactic cranial irradiation Treatment plan APPENDIX A: HOW TO OBTAIN A LIST OF HOSPITAL SITE CODES APPENDIX B: NOTES ON CODING CLASSIFICATIONS International Statistical Classification of Diseases and Related Health Problems (ICD-10) International Classification of Diseases for Oncology, 2nd edition (ICD-O-2) Morphology codes SNOMED CT OPCS-4 - operation codes UICC Coding Page 3 of 40
4 INTRODUCTION Preface The LUCADA dataset is a subset of the National Cancer Dataset (NCDS Version 4.5) plus a number of additional data items. The LUCADA dataset was developed and agreed by the Royal College of Physicians Intercollegiate Lung Cancer Group during the period September 2001 to May 2002 and was updated by the LUCADA user group during The manual describes each element of the dataset. It details the content of each data item, including its definition and purpose. For items from the National cancer data set this manual takes information from the more comprehensive Cancer. Users are welcome to refer to this manual which includes many other data items relating to cancer. The national cancer data manual is designed to support the full National Cancer Dataset and is a very large and comprehensive document. In order to facilitate direct data entry, the organisation of this document corresponds with the order of screens and data items in the web-based LUCADA system. Users who upload data via CSV or XML files should read this manual in conjunction with the relevant CSV / XML specification document, downloadable from How to use this Manual The organisation of this manual corresponds with the order of screens and fields in the LUCADA system. Each section starts with a table listing the data items, reference numbers and the source of the definition. Data items with reference number beginning with L are part of the LUCADA dataset. All others are from the National Cancer Dataset (NCDS). After the table, each data item is listed with its format and specific guidance on its completion. Where the format is a Drop Down List, this is indicated using the acronym DDL. Text in round brackets after data item description denotes the LUCADA screen names for the data items. Organisation s Several fields in LUCADA are used to record the unique five character organisation code of the unit at which a patient is seen or treated. See the NHS Data Dictionary under Supporting Information, Administrative s, for a description of Organisation s. There are codes for private organisations (independent providers). Once identified, local provider codes can be permanently held by systems and each provider should therefore only need to look up its code once and then make this permanently available to those staff responsible for compiling and reporting datasets. See Appendix A for information on how to obtain the national list of site codes. Page 4 of 40
5 PATIENT REFERRAL Demographics Data Item Dataset Ref. Source NHS NUMBER 1.1 NHS Data Dictionary (NHS Number) PERSON FAMILY NAME 1.5 Automatically populated (Surname) PERSON NAME GIVEN (Forenames) 1.6 Automatically populated POSTCODE OF USUAL ADDRESS (AT 1.8 Automatically populated DIAGNOSIS) (Postcode) SEX 1.9 Automatically populated (Sex) BIRTH DATE (Date of Birth) 1.10 Automatically populated 1.1 NHS NUMBER (NHS Number) 10 digit numeric code Enter the patient s unique NHS Number. This is a required field. Upon entering the NHS number, the remaining data items in this section will be retrieved from the Open Exeter database. These data items cannot be edited through LUCADA. If the NHS number is not available for a patient it can be accessed via the NHS Tracing Service. This need only be done once for each patient as this is a permanent lifetime number which will not change. The following data items are populated automatically. Users should cross-check them to ensure that it is the correct patient:- 1.5 PERSON FAMILY NAME (Surname) 1.6 PERSON NAME GIVEN (Forenames) 1.8 POSTCODE OF USUAL ADDRESS (AT DIAGNOSIS) (Postcode) If the patient changes postcode after diagnosis, do not change the postcode. 1.9 SEX (Sex) The patient s sex will be recorded as:- Patient s Sex Not known 0 Male 1 Female 2 Not specified ambiguous or indeterminate BIRTH DATE (Date of birth) Record the patient s date of birth, in date format. For example, Page 5 of 40
6 Referral This section is used to record details about referral to a lung cancer specialist / team. All data items in this section should be completed with reference to the lung cancer episode. If the patient is referred under 2 week wait rules, complete cancer referral decision date also called date of decision to refer (section 2.5) but for non 2 week wait patients use lung cancer specialist referral date (section L24) field instead. If the cancer is discovered only at post mortem, then do not record any referrals information. Data Item Dataset Ref. Source SOURCE OF REFERRAL (FOR CANCER) 2.1 NCDS (Source of referral) CANCER REFERRAL DECISION DATE 2.5 NCDS (Date of decision to refer) DATE FIRST SEEN 2.9 NCDS (Date first seen) Lung Cancer Specialist Referral Date L24 LUCADA dataset ORGANISATION CODE (PROVIDER FIRST SEEN) (Place first seen) 1.3 NHS Data Dictionary, Supporting Information, Administrative s, NHS Trust 2.1 SOURCE OF REFERRAL FOR CANCER (Source of referral) Drop Down List Record the route of referral of the patient. Choose from following drop down menu options. Please note that when a patient has been admitted to hospital via a GP as an emergency or through A&E they may then go on to be referred to a lung cancer specialist. In this scenario either code 01or 05 could apply. Where possible, use code 05 unless the admission was straight to the lung cancer specialist team in which case use code 01. (Note that this data item is referring to the source of referral to the lung cancer team and this is not necessarily the same as the source of referral to the hospital) Source of referral Following an emergency admission (includes all acute admissions via A&E, Medical Admissions Unit, etc) Following a domiciliary visit 02 Referral from General Medical Practitioner (for out-patient or other nonemergency referrals) Referral from a consultant, other than in an A&E department. If the diagnosis took place within the screening services, then this code applies. Self-referral (i.e. the patient was not seen previously by a GP) 06 Other source of referral (will include referrals from Private Healthcare) 08 Following an A&E attendance ( i.e. an out-patient clinic attendance after an A&E visit) General Dental Practitioner 92 Community Dental Service 93 Not known (default) Page 6 of 40
7 2.5 CANCER REFERRAL DECISION DATE (Date of decision to refer) DD/MM/YYYY This data item should be collected for 2 week wait patients only Record the date on which the decision to refer to a lung cancer specialist was made, for example; The date on the letter, proforma or from the referring GP Where there is no date on the letter, proforma or from the referring GP the date of transmission of the fax or the postmark should be used Do not include patients referred with suspicion of another cancer that later turns out to be lung cancer e.g. two week wait referral was to the upper GI services but the cancer was later found to be a lung cancer. L24 LUNG CANCER SPECIALIST REFERRAL DATE DD/MM/YYYY This date should be recorded for all non 2 week wait patients The date of the letter, proforma, or referral note written in the patient s case notes for a referral from another hospital department (for lung cancer). For patients admitted as an emergency under the lung cancer team this date is the date of admission to hospital The date of the first out-patient appointment (for lung cancer) if the referral was a self referral. Please note that X-rays are not referrals so the date of the referral document should be used, not the X-ray date. 2.9 DATE FIRST SEEN (Date first seen) DD/MM/YYYY Record the date of the patient s first contact with the person or group in referred to above, or the first contact with a member of the lung cancer specialist team: date of first outpatient appointment (for lung cancer) date of outpatient visit when a diagnosis of (lung) cancer was first considered date the patient is first seen by the (lung cancer) specialist team in hospital for within-hospital referrals date of first booked diagnostic procedure (for lung cancer) if this precedes the first outpatient appointment date seen as an emergency, if the patient was first seen as an emergency (within a lung cancer pathway) Record this date in date format. 1.3 ORGANISATION CODE (PROVIDER FIRST SEEN) (Place first seen) 5 DIGIT ALPHA NUMERIC CODE Record the organisation code of the Unit at which the patient was first seen. This is a unique fivecharacter code (see Appendix A for how to obtain organisation codes). Page 7 of 40
8 CARE PLAN / MDT Diagnosis This section should be used to record details of the lung cancer diagnosis. If the malignancy is discovered only at autopsy, or via details from a death certificate, then this section should be completed with as much information as is available. Whenever possible these data items should be collected at the MDT meeting immediately prior to the patient commencing treatment. Record the following details, which will provide a definitive description of the tumour based on the information available at the time the items were completed. Data Item Dataset Ref. Source DIAGNOSIS DATE (CANCER) (Date of diagnosis) 4.1 European Network of Cancer Registries Site code (Place of diagnosis) L26 NHS Data Dictionary, Supporting Information, Administrative s, NHS Trust PRIMARY DIAGNOSIS (ICD) 4.2 ICD-10 coding (Primary site diagnosis) TUMOUR LATERALITY (Laterality) 4.3 NCDS BASIS OF DIAGNOSIS (CANCER) (Basis of diagnosis) 4.4 European Network of Cancer Registries 4.1 DIAGNOSIS DATE (Lung cancer pre-treatment) (Date of diagnosis) DD/MM/YYYY This field records the date of diagnosis of the tumour. The LUCADA hierarchy for diagnosis definition maps to the European Network of Cancer Registries (ENCR) definitions and the date recorded should follow these hierarchy rules Order of declining priority for LUCADA: 1. Date of first histological or cytological confirmation of this malignancy (with the exception of histology or cytology at autopsy). This date should be, in the following order: a. date when the specimen was taken b. or date of receipt by the pathologist c. or date of the pathology report 2. Date of diagnosis, other than 3, 4, 5 or 6. such as imaging from a CT, PET scan or other form of clinical diagnosis 3. Date of death, if no information is available other than the fact that the patient has died because of malignancy. 4. Date of death, if the malignancy is discovered at autopsy. 5. Date of admission to hospital because of this malignancy. 6. When evaluated at an out-patient clinic only: date of first consultation at the out-patient clinic because of this malignancy. L26 Site code (place of diagnosis) 5 DIGIT ALPHA NUMERIC CODE Record the organisation code of the place where the patient was diagnosed with lung cancer. This is a unique five-character code (See appendix A for how to obtain missing organisation codes). Page 8 of 40
9 4.2 PRIMARY DIAGNOSIS (ICD) (Primary site) Record the ICD_10 code that best describes the anatomical site of the primary cancer reported in this record. Choose from the following list of anatomical site codes. ICD-10 s for Primary Site C34 Malignant neoplasm of bronchus or lung C34.0 Main bronchus, Carina, Hilus of lung C34.1 Upper lobe, bronchus or lung C34.2 Middle lobe, bronchus or lung C34.3 Lower lobe, bronchus or lung C34.8 Overlapping lesion of bronchus and lung C34.9 Bronchus or lung, unspecified C33 Trachea C38 Malignant neoplasm of heart, mediastinum and pleura C38.4 Pleura C38.3 Mediastinum, part unspecified C38.8 Overlapping lesion of heart, mediastinum and pleura C45 Mesothelioma C45.0 Mesothelioma of pleura 4.3 TUMOUR LATERALITY (Laterality) DDL Record the side of the primary cancer; this is used to differentiate tumours in paired organs. Note that for sites where laterality is not required (i.e. mediastinum), record 8 for Not applicable. Tumour Laterality Left L Right R Midline M Bilateral B Not applicable 8 Not known BASIS OF DIAGNOSIS (CANCER) (Basis of diagnosis) DDL This field records the basis on which the lung cancer diagnosis was made. It is therefore an indicator of data quality, with microscopic histological verification being viewed as the gold standard diagnosis. The definition provided conforms with the international requirements specified by the European Network of Cancer Registries (ENCR). Page 9 of 40
10 Non-microscopic 0 (Death Certificate) Information only available from a death certificate 1 (Clinical) Diagnosis made before death, but without the benefit of any of the following (2-7) 2 (Clinical Investigation) Diagnosis made with the aid of diagnostic techniques (e.g. X-rays, endoscopy, imaging, ultrasound, exploratory surgery and autopsy) without a tissue diagnosis 4 (Specific tumour markers) Diagnosis made with the aid of biochemical and/or immunological markers, which are specific for a tumour site Microscopic 5 (Cytology) Diagnosis made by examination of cells, whether from a primary or secondary site, including fluids aspirated using endoscopes or needles. Also including microscopic examination of peripheral blood films and bone marrow aspirates. 6 (Histology of a metastases) Diagnosis made by histological examination of tissues from a metastasis, including autopsy specimens 7 (Histology of a primary tumour) Diagnosis made by histological examination of tissue from the primary tumour, however obtained, including all cutting and bone marrow biopsies. Also includes autopsy specimens of a primary tumour Unknown 9 (Unknown) Unknown: where there is no information on how the diagnosis has been made (e.g. PAS or HISS record only) Validation rule If basis of diagnosis equals 5, 6 or 7 a histology code (data item 4.5) must be also be completed. If primary site coding (4.2) indicates mesothelioma, the histology coding (4.5) should also be a mesothelioma unless the diagnosis is a clinical one. Pre-treatment Staging The TNM staging data items record the definitive pre-treatment TNM stage. TNM refers to the International Union Against Cancer s coding system (6 th edition 1997, 7 th edition 2009). This staging is usually agreed at the MDT meeting or cancer care plan meeting prior to treatment and based on the clinical and pathological data available. Such evidence arises from physical examination, imaging, endoscopy, biopsy, surgical exploration and other relevant examinations. As a result of the changes in the UICC staging manual version 7, TNM staging of small cell carcinoma of the lung is now the preferred method and should be recorded wherever possible. However, the old staging classification of Limited disease or Extensive disease (US Veterans Administration) is acceptable, if preferred. There is no accepted clinical classification for mesothelioma. Use of a surgical staging classification may be used at the discretion of individual organisations, but the data will not be used in any analysis. If the malignancy is discovered only at autopsy, or via a death certificate, then no pre-treatment TNM stage should be recorded. If a staging procedure other than imaging was undertaken this should be recorded. However, only staging resulting from surgery undertaken for diagnostic purposes only should be recorded here. If the surgery is undertaken with curative intent, the staging should be recorded in the treatment section. Page 10 of 40
11 Data Item Dataset Ref. Source TNM Classification Version Number L52 LUCADA T CATEGORY (FINAL PRE-TREATMENT) 6.1 UICC coding N CATEGORY (FINAL PRE-TREATMENT) 6.3 UICC coding M CATEGORY (FINAL PRE-TREATMENT) 6.5 UICC coding TNM CATEGORY (FINAL PRE-TREATMENT) (Pre-treatment stage group) 6.7 UICC coding Calculated by system SITE-SPECIFIC STAGING CLASSIFICATION 6.9 Site-specific coding (Site-specific classification) Pre Treatment Histology 4.5 SNOMED Did the patient have a staging procedure L43 LUCADA dataset Did patient have mediastinal sampling L22 LUCADA dataset Did the patient have an FNA L44 LUCADA dataset Did the patient have any other staging procedure L45 LUCADA dataset Staging procedure performed but method unknown L46 LUCADA dataset L52 TNM Classification Version Number Record the version of TNM used for staging. This applies to both pre -treatment and pathological stage. A patient must be staged throughout using UICC 6 or UICC 7, the two cannot be mixed within a single patient record Staging Classification 6 (Version 6 of TNM) 7 (Version 7 of TNM) 6.1 T CATEGORY (FINAL PRE-TREATMENT) (Final pre-treatment T category) DDL The tumour, T, component of the clinical TNM stage describes the size and location of the tumour at the time of diagnosis. This item is usually recorded pre-treatment. 6.3 N CATEGORY (FINAL PRE-TREATMENT) (Final pre-treatment N category) DDL The lymph node, N, component of the clinical TNM stage indicates any involvement of lymph nodes from the primary tumour described in T. Note that micro-metastases should be considered to be positive. 6.5 M CATEGORY (FINAL PRE-TREATMENT) (Final pre-treatment M category) DDL The metastasis, M, component of the clinical TNM stage indicates the presence or absence of metastasis. 6.7 TNM CATEGORY (FINAL PRE-TREATMENT) (Overall pre-treatment stage group) CALC The pre-treatment stage is calculated from the TNM description of the cancer. This is calculated by using the calculate button. A stage is automatically assigned using the criteria in the tables below Page 11 of 40
12 Version 6 Stage T N M Stage IA T1 N0 M0 Stage IB T2 N0 M0 Stage IIA T1 N1 M0 Stage IIB T2 N1 M0 T3 N0 M0 Stage IIIA T1 N2 M0 T2 N2 M0 T3 N1, N2 M0 Stage IIIB Any T N3 M0 T4 Any N M0 Stage IV Any T Any N M1 Version 7 Stage T N M Stage IA T1A N0 M0 Stage IA T1B N0 M0 Stage IB T2A N0 M0 Stage IIA T2B N0 M0 Stage IIA T1A N1 M0 Stage IIA T1B N1 M0 Stage IIA T2A N1 M0 Stage IIB T2B N1 M0 Stage IIB T3 N0 M0 Stage IIIA T1a N2 M0 Stage IIIA T1b N2 M0 Stage IIIA T2A N2 M0 Stage IIIA T2B N2 M0 Stage IIIA T3 N1 M0 Stage IIIA T3 N2 M0 Stage IIIA T4 N0 M0 Stage IIIA T4 N1 M0 Stage IIIB T4 N2 M0 Stage IIIB Any T N3 M0 Stage IV Any T Any N M1 (A or B) Uncertain Any T Nx M0 Stage IV Any T Nx M1 (A or B) Occult Carcinoma Tx N0 M0 4.5 HISTOLOGY (SNOMED) (Histology) Record the cell type, histology, of the primary cancer. LUCADA uses the SNOMED codings for this. Note that there may be a number of pathology reports contributing to the diagnosis. SNOMed s for Histology (Pathology) (SNOMed III (1993)/ICD-O-2 (1990) unless stated) LUCADA Mapping M 8010/2 Carcinoma in situ NSCLC M 8041/3 Small cell carcinoma SCLC M 8046/3 Non-small cell carcinoma (ICD-O-3) NSCLC (includes adenosquamous carcinoma) Page 12 of 40
13 M 8070/3 Squamous cell carcinoma NOS NSCLC M 8140/3 Adenocarcinoma NOS NSCLC (Adenocarcinoma without alveolar cell features) M 8250/3 Bronchio-alveolar cell carcinoma NSCLC (Adenocarcinoma with alveolar cell features) M 8012/3 Large cell Carcinoma NOS NSCLC M 8020/3 Large cell undifferentiated NSCLC M 8013/3 Large cell neuroendocrine (ICD-O-3) NSCLC M 8240/3 Carcinoid tumour NOS Separate category (includes atypical carcinoid) M 8980/3 Carcinosarcoma NOS NSCLC M 9050/3 Malignant Mesothelioma NOS Mesothelioma M 9052/3 Mesothelioma (epithelioid) Mesothelioma M 9051/3 Mesothelioma (sarcomatoid) (ICD-O-3) Mesothelioma M9053/3 Mesothelioma (biphasic) Mesothelioma M 8940/3 Mixed tumour (malignant) NSCLC M 9999/9 Other NSCLC 6.9 SITE-SPECIFIC STAGING CLASSIFICATION (Site-specific classification) RADIO As a result of the changes in the UICC staging manual version 7, TNM staging of small cell carcinoma of the lung is now the preferred method and should be recorded wherever possible. However, the old staging classification of Limited disease or Extensive disease (US Veterans Administration) is acceptable, if preferred.. (See also section L23 page 26) Stage Category Stage unknown X Limited 1 Extensive 2 Limited disease Disease confined to one hemithorax including involvement of ipsi-and/or contralateral hilar, mediastinal or supraclavicular lymph nodes. Patients with ipsilateral pleural effusion, regardless or pleural cytology, should be included in this group. Extensive disease Any disease beyond the definition of limited stage. L43 Did the patient have a staging procedure Record whether the patient underwent a procedure to stage the lung cancer (excluding imaging). If the staging was undertaken at the same time as a curative procedure this must be recorded in the treatment section. L22 Did the patient have mediastinal sampling Record if the patient had a mediastinoscopy mediastinotomy, open mediastinal sampling or other type of mediastinal biopsy L44 Did the patient have an FNA (This includes trucut needle biopsies) Record if the patient had an FNA staging procedure (Note needle biopsy of the primary lesion is recorded elsewhere) L45 Did the patient have any other staging procedure Record if the patient had a staging procedure other than mediastinal sampling or an FNA Page 13 of 40
14 L46 Staging procedure performed but method unknown Record if the patient had a staging procedure but the nature of this procedure is unknown or not recorded Cancer care plan / MDT details Following a diagnosis of cancer, a cancer care plan will be drawn up for the patient s care. The cancer care plan will relate to this particular diagnosis. It is appreciated that a cancer care plan can change as the patient s disease progresses or as more information about the stage of the disease is ascertained. Data items under this section would usually be recorded at the MDT meeting immediately prior to the start of any treatment. If the malignancy is discovered only at autopsy, or via a death certificate, then there will be no care plan and no details under this section will need to be recorded. Data Item Dataset Ref. Source MDT DISCUSSION INDICATOR 5.1 NCDS (Was this cancer care plan discussed at an MDT meeting?) MULTIDISCIPLINARY TEAM DISCUSSION DATE 5.2 NCDS (The date of the MDT meeting at which this cancer care plan was discussed) CANCER CARE PLAN INTENT 5.5 NCDS (Cancer care plan intent) FEV1 ABSOLUTE AMOUNT L5 LUCADA dataset (FEV1 (Absolute)) FEV1 PERCENTAGE L6 LUCADA dataset (FEV1 (Percentage of predicted)) PERFORMANCE STATUS (ADULT) (Performance status) 5.10 World Health Organisation coding 5.1 MDT DISCUSSION INDICATOR RADIO (Was the patient discussed at an MDT meeting?) Record whether the patient was discussed at an MDT meeting. If the patient was not discussed at an MDT meeting, then record no. If unknown, then record unknown. 5.2 MULTIDISCIPLINARY TEAM DISCUSSION DATE DD/MM/YYYY (The date of the MDT meeting at which the patient was discussed) If the patient was discussed at multiple MDT meetings, record the date of the MDT closest to the first treatment. If the patient was not discussed at an MDT meeting, then leave this field blank. Page 14 of 40
15 5.5 CANCER CARE PLAN INTENT (Cancer Care Plan Intent) Record the intention of the treatment which is planned for the patient at this point in time. Intent Curative. Include here patients whose treatment includes: Surgical resection (including open and close but excluding all surgery on mesothelioma patients) Radical radiotherapy to primary site with potential for cure Chemotherapy in Limited Stage and/or Good Prognosis Small Cell Carcinoma (e.g. using Manchester prognostic score) Adjuvant (or neo-adjuvant) chemotherapy (combined with surgery) in Non-Small Cell Carcinoma Adjuvant radiotherapy in Small Cell carcinoma Prophylactic Cranial Irradiation Palliative. Include here patients whose treatment includes: Specialist Palliative Care Chemotherapy in Non-Small Cell Carcinoma (excepting Adjuvant and Neo-adjuvant therapy) Radiotherapy to primary tumour with palliative intent Radiotherapy to site of secondary cancer Brachytherapy (Endobronchial radiotherapy) Other endobronchial treatments (e.g. laser therapy, diathermy, cryotherapy, insertion of bronchial stent) Chemotherapy in Extensive Stage and/or Poor Prognosis Small Cell Carcinoma (e.g. using the Manchester prognostic score) Surgery for mesothelioma (EPP, debulking surgery and pleurodesis) Surgical Pleurodesis Superior Vena Caval Stenting Palliative - supportive care only. This item implies follow up with the aim of identifying symptoms and treating these if and when appropriate. Follow up could be through primary care, secondary care and includes both doctors, nurses and Macmillan staff. The plan implies that no immediate treatment is to be given but does not exclude the patient from having treatment at a future date if the need arose. No specific anti-cancer treatment Unknown (Note: it is highly unlikely that any treatment plan would be drawn up, where the intention of the treatment is Unknown. The use of this code should be carefully monitored) Plan C P S N 9 L.5 FEV1 ABSOLUTE AMOUNT (FEV1 (Absolute)) Numeric field Record the absolute value of the patient s Forced Expiratory Volume in first second in litres. This is particularly important for patients with COPD. L.6 FEV1 PERCENT (FEV1 percentage of predicted) % Numeric field Record the patient s Forced Expiratory Volume in first second as a percentage of the predicted value. Page 15 of 40
16 5.10 PERFORMANCE STATUS (ADULT) (Performance Status) Use the coding as shown in the table below. This uses World Health Organisation (WHO) coding, with the addition of code 5 Not recorded. Performance Status Able to carry out all normal activity without restriction 0 Restricted in physically strenuous activity but able to walk and do light work 1 Able to walk and capable of all self care but unable to carry out any work. Up and about 2 more than 50% of waking hours Capable of only limited self care, confined to bed or chair more than 50% of waking 3 hours Completely disabled. Cannot carry on any self care. Totally confined to bed or chair 4 Not recorded 5 Significant Co-morbidity / Treatment of Choice If a patient did not receive the treatment of choice the reason should be recorded here. If comorbidity (03) is selected as the reason why the patient did not receive the treatment of choice then the nature of the comorbidity must be recorded For the purpose of the audit, significant co-morbidity is defined as any co-morbidity which in the opinion of the Lung Cancer specialist teams is of sufficient severity to contra-indicate referral for a therapy that would otherwise be the preferred option. Data Item Dataset Ref. Source Was there any reason why the patient did L32 LUCADA dataset not receive the first choice of treatment L32 Did the patient receive the treatment of choice (DDL) Record why the patient did not receive the treatment of choice Data Item Was there any reason why the patient did not receive the first choice treatment 01 Died 03 Co-morbidity precluding treatment 06 Refused L4 COPD Co-morbidity If co-morbidity (03) is selected as the reason why the patient did not receive the treatment of choice, the nature of the co-morbidity should be recorded Data Item Dataset Ref. Source Dementia/Cerebrovascular disease L10 LUCADA dataset Cardiovascular disease L9 LUCADA dataset Renal failure L11 LUCADA dataset Other malignancy L12 LUCADA dataset Severe weight loss L13 LUCADA dataset Other significant co-morbidity L14 LUCADA dataset Page 16 of 40
17 Key Investigations This section describes the key investigations used in establishing a diagnosis of lung cancer. Record all procedures used even if the result was negative. The purpose of this section is to establish what diagnostic techniques are being used, in what order and whether there are delays in accessing certain services Data Item Dataset Ref. Source Was a CT scan performed 3.3 (2A or 2B) NCDS Date of CT scan 3.2 NCDS Was a PET / PET CT scan performed 3.3(4) NCDS Date of PET / PET CT scan 3.2 NCDS Was a bronchoscopy performed L33 LUCADA dataset Date of bronchoscopy L34 LUCADA dataset Was a CT guided biopsy performed L37 LUCADA dataset Date of CT guided biopsy L38 LUCADA dataset Was an other diagnostic biopsy performed L35 LUCADA dataset Date of other diagnostic biopsy L36 LUCADA dataset Record details of all key investigations used to establish the lung cancer diagnosis and stage. Record the date of any procedures undertaken 3.3 Was a CT Scan performed DDL Record if the patient underwent a CT scan Data Item 3.3 Was a CT scan performed Y Yes N No 9 - Unknown 3.2 Record the date the CT scan was undertaken DD/MM/YYYY This field records the date of the CT scan 3.3 Was a PET / PET-CT scan performed DDL Record if the patient underwent a PET / PET-CT scan Data Item 3.3 Was a PET / PET-CT scan performed Y Yes N No 9 - Unknown 3.2 Record the date the PET / PET-CT Scan was undertaken DD/MM/YYYY This field records the date of the PET / PET-CT scan L33 Was a bronchoscopy performed DDL Record if the patient underwent a bronchoscopy Page 17 of 40
18 Data Item L33 Was a bronchoscopy performed 1- Standard bronchoscopy only 2- EBUS only 3- Standard bronchoscopy and EBUS N -No 9 -Unknown L34 Record the date the bronchoscopy was undertaken DD/MM/YYYY This field records the date of the bronchoscopy L37 Was a CT guided biopsy performed DDL Record if the patient underwent a CT guided biopsy Data Item L37 Was a CT guided biopsy performed Y Yes N No 9 Unknown L38 Record the date the CT guided biopsy was undertaken DD/MM/YYYY This field records the date of the CT guided biopsy L35 Was any other biopsy performed DDL Record if the patient underwent any other biopsy Data Item L35 Was any other biopsy performed Y Yes N No 9 - Unknown L36 Record the date any other biopsy was undertaken DD/MM/YYYY This field records the date any other biopsy was taken Page 18 of 40
19 NURSING CARE This section is about the input of the Lung Cancer Nurse Specialist into the lung cancer patient journey and refers to any specialist nursing input that occurs between the patient being referred to the hospital to the point at which the patient receives their primary treatment. It is not intended to record follow up information after treatment. For the purposes of the audit a Lung Cancer Nurse Specialist is defined below A Lung Cancer Nurse Specialist is a first level nurse, locally recognised as part of the specialist lung cancer multidisciplinary team and designated (i.e. part of the formal job description) as a specialist in lung cancer. The nurse should spend at least 50% of his or her time caring for lung cancer patients. Patient assessment for the purposes of the nursing care section of the audit is defined as:- Any face to face contact with the patient or a telephone call during which the patients needs are discussed. This does not include interactions with the sole intention of introduction, providing contact details or MDT discussion. Data Item Dataset Ref. Source PATIENT ASSESSED BY LUNG CANCER NURSE SPECIALIST L47 DATE FIRST ASSESSMENT BY LUNG L48 CANCER NURSE SPECIALIST HOW WAS PATIENT FIRST ASSESSED L49 BY LUNG CANCER NURSE SPECIALIST AT WHAT STAGE(S) IN THE PATIENT L50 JOURNEY WAS THE PATIENT ASSESSED BY THE LUNG CANCER NURSE SPECIALIST LUNG CANCER NURSE SPECIALIST L51 PRESENT WHEN THE PATIENT RECEIVED THEIR DIAGNOSIS LUCADA dataset LUCADA dataset LUCADA dataset LUCADA dataset LUCADA dataset L47 Was the patient assessed by a Lung Cancer Nurse Specialist? Record here if the patient was assessed by a Lung Cancer Nurse Specialist DDL Data Item Was the patient assessed by a Lung Cancer Nurse Specialist L48 Date of assessment by Lung Cancer Nurse Specialist? Y N 9 Unknown 99 Not recorded DD/MM/YYYY Page 19 of 40
20 If the patient was assessed by a Lung Cancer Nurse Specialist record the date of the assessment Page 20 of 40
21 L49 How was the patient first assessed by a Lung Cancer Nurse Specialist? DDL If the patient was assessed by a Lung Cancer Nurse Specialist record how the assessment took place Data Item How was the patient first assessed by a Lung Cancer Nurse Specialist 1 In clinic 2 Home visit 3 Ward Visit 4 Telephone 8 Other 9 Unknown 99 Not recorded L50 At what stages in the patient journey was the patient assessed by the Lung Cancer Nurse Specialist? DDL If the patient was assessed by a Lung Cancer Nurse Specialist record at what point in the patient journey this occurred. The purpose of this audit field is to identify at which point in the patient journey patients are reviewed. This field does not record how many times a patient was seen, just the timings. Data Item At what stages in the patient journey was the patient assessed by the Lung Cancer Nurse Specialist 1 Before diagnosis 2 At diagnosis only 3 After diagnosis 4 Before and after diagnosis 9 Unknown 99 Not recorded L51 Was the Lung Cancer Nurse Specialist present when the patient received their diagnosis? DDL Record whether of not the lung cancer nurse specialist was present when the patient received their diagnosis of lung cancer. This field will automatically be set to Y if at diagnosis only is selected in L50 Data Item Lung Cancer Nurse Specialist present when the patient received their diagnosis Y N 9 Unknown 99 Not recorded Page 21 of 40
22 TREATMENT Treatment for lung cancer can involve single modality or combination treatment. The database allows the entry of single modality treatment or combination treatment. For the online reports the patient will be allocated to a treatment group by the earliest treatment date entered. Planned Treatment Planned multi-modality treatment will only be recorded if it is entered using the multiple modality option. If the individual modalities are entered using the single modality option, then the earliest treatment date entry will count as the treatment the patient received. Entry of more that one single modality treatment, for example, second line treatment because of disease progression, is at the user s discretion. If combination treatment is planned but not given, the relevant outcome section fields L30 and L31 must be completed explaining why. Clinical trial Use this section to record whether or not the patient is eligible for entry into a clinical trial Data Item Dataset Ref. Source PATIENT TRIAL STATUS (CANCER) 13.1 NCDS (Clinical Trial Status) 13.1 PATIENT TRIAL STATUS (CANCER) (Clinical Trial Status) RADIO Record the status of the clinical trial entry for the patient. Patient Trail Status Patient eligible, consented to and entered trial Y Patient not entered into clinical trial N Clinical trial status unknown 9 L28 Planned treatment type DDL Select whether the patient is to have single modality or multi modality treatment Data Item Repeating? Source of definition L28 Planned treatment type No 01Single Modality 02 Multiple Modality 9 Unknown L29 Treatment planned DDL Record the type of treatment regimen planned Page 22 of 40
23 Data Item Repeating? Source of definition L29 Planned treatment No 01 Surgery 02 Teletherapy / Radiotherapy 03 Chemotherapy 04 Brachytherapy 05 Palliative care 06 Active Monitoring 07 Sequential chemotherapy and radiotherapy 08 Concurrent chemotherapy and radiotherapy 09 Induction chemotherapy to downstage before surgery 10 Neo-adjuvent chemotherapy and surgery 11 Surgery followed by adjuvant chemotherapy The details of the planned treatments must be recorded. Specialist palliative interventions including endobronchial laser treatment, bronchial stenting, IVC stenting drainage of a pleural effusion and medical pleurodesis. (Medical pleurodesis refers to a pleurodesis performed via a chest drain on a ward post drainage of a pleural effusion). These interventions should be recorded in the interventional palliative care window (L41) Surgery For Lung Cancer patients (including clinical diagnoses) Record here any surgical procedure under taken with the intention of curing the patient. Do not record here any procedures undertaken for purely diagnostic purposes these should be entered in the Care Plan / MDT section. Note that Radiofrequency ablation will be counted as a separate catgory. For Mesothelioma Patients It is accepted that all surgical procedures in this group of patients are palliative. Please enter any therapeutic procedures as surgery other (E57.8). Do not record here any procedures undertaken for purely diagnostic purposes these should be entered in the Care Plan / MDT section. The information for this section should be collected as soon as possible after the procedure has taken place. Data Item Dataset Ref. Source SITE CODE (OF SURGERY) (Hospital) 7.1 NHS Data Dictionary, Supporting Information, Administrative s, NHS Trust Site DECISION TO TREAT DATE (SURGERY) 7.5 (Date of decision to operate) PROCEDURE DATE 7.9 (Date of surgery) PRIMARY PROCEDURE (OPCS) 7.10 OPCS Page 23 of 40
24 (Main surgical procedure) Page 24 of 40
25 7.1 SITE CODE (OF SURGERY) (Hospital) Five character alphanumeric Record the organisation code of the hospital where the procedure took place. 7.5 DECISION TO TREAT DATE (SURGERY) (Date of decision to operate) DD/MM/YYYY Record the date on which it was decided that this patient should receive surgery. This is the date that the consultation between the patient and the surgeon took place and a treatment plan for surgery was agreed. Very often this is the date the patient was added to the surgical waiting list. 7.9 PROCEDURE DATE (Date of surgery) DD/MM/YYYY Record the date on which the procedure took place. Please note that this definition is different from that used for Cancer Waiting Times data which requires collection of the clinical intervention date (first diagnostic test) PRIMARY PROCEDURE (OPCS) (Main surgical procedure) Alphanumeric Record the main operative procedure carried out. See below for a list of procedures (which map to OPCS-4). OPCS/Read s for main Surgical Procedures Surgical Procedure Wedge resection of lesion of lung (segment) E54.4A Multiple wedges resected E54.8A Segmental resection E55.4B Sleeve resection E54.8B Lung resection with resection of chest wall (not identifying which E T01 lobe resection) Carinal resection E44.1 Lobectomy E54.3 Pneumonectomy E54.1 Bilobectomy E54.2 Open operation on lung (open and close) (Incision of lung nec) E57.4 Other open operation on lung E57.8 Lung radio frequency ablation E59.5 Extrapleural pneumonectomy 01 Debulking pleurectomy 02 Pleurodesis 03 Post surgery pathology Recorded here are details relating to the completeness of the surgical resection and the post operative staging based on the TNM system. Note that post-operative histology data is recorded in the histology window and not in the surgical treatment window. Page 25 of 40
26 Data Item Dataset Ref Source EXCISION MARGIN L15 UICC Coding (Excision margins R Classification ) T CATEGORY (PATHOLOGICAL) 8.16 UICC Coding (Pathological T category) N CATEGORY (PATHOLOGICAL) 8.17 UICC Coding (Pathological N category) M CATEGORY (PATHOLOGICAL) 8.18 UICC Coding (Pathological M category) TNM CATEGORY (PATHOLOGICAL) (Overall Pathological TNM Stage grouping) 8.19 UICC Coding L15 EXCISION MARGIN (Excision margins R Classification) DDL Record the absence or presence of residual tumour after surgery as per the table below. Excision Margin Presence of residual tumour cannot be assessd No residual tumour Microscopic residual tumour Macroscopic residual tumour RX R0 R1 R T CATEGORY (PATHOLOGICAL) (Pathological T category) DDL This field records the extent of the primary tumour after excision based on the evidence from pathological examination N CATEGORY (PATHOLOGICAL) (Pathological N category) This field records the absence or presence and extent of regional lymph node involvement with the lung cancer. It is based on histological examination of lymph nodes removed at the time of surgery M CATEGORY (PATHOLOGICAL) (Pathological M category) This field records the histological evidence of the absence or presence of distant metastases. Distant metastases cannot be assessed microscopically as it is frequently impossible for pathologists to assess their presence or absence. It is recognised that data item 8.18 (M Category Pathological) may not always be available. Because of this there are certain rules around the calculation of TNM CATEGORY (PATHOLOGICAL) (Data item 8.19). These rules are different for version 6 and version 7 of the classification. Version 6 When calculating the TNM category (pathological) the LUCADA system assumes that MX is equivalent to M0 unless M1 is recorded for final pre-treatment M category M (Data item 6.5). Version 7 If no value is recorded for data item 8.18, then the TNM Category (Pathological) is calculated using the value recorded for pre-treatment M Category (6.5) in combination with the pathological category values for T and N (data items 8.16 and 8.17) TNM CATEGORY (PATHOLOGICAL) (Overall pathological TNM stage grouping) CALC The pathological stage is populated using the calculate button. A stage is automatically assigned using the criteria in the tables below Page 26 of 40
27 Stage T N M Stage IA T1 N0 M0 Stage IB T2 N0 M0 Stage IIA T1 N1 M0 Stage IIB T2 N1 M0 T3 N0 M0 Stage IIIA T1 N2 M0 T2 N2 M0 T3 N1, N2 M0 Stage IIIB Any T N3 M0 T4 Any N M0 Stage IV Any T Any N M1 Version 7 Stage T N M Stage IA T1A N0 M0 Stage IA T1B N0 M0 Stage IB T2A N0 M0 Stage IIA T2B N0 M0 Stage IIA T1A N1 M0 Stage IIA T1B N1 M0 Stage IIA T2A N1 M0 Stage IIB T2B N1 M0 Stage IIB T3 N0 M0 Stage IIIA T1A N2 M0 Stage IIIA T1B N2 M0 Stage IIIA T2A N2 M0 Stage IIIA T2B N2 M0 Stage IIIA T3 N1 M0 Stage IIIA T3 N2 M0 Stage IIIA T4 N0 M0 Stage IIIA T4 N1 M0 Stage IIIB T4 N2 M0 Stage IIIB Any T N3 M0 Stage IV Any T Any N M1 (A or B) Uncertain Any T Nx M0 Stage IV Any T Nx M1 (A or B) Occult Carcinoma Tx N0 M0 Histology (Post Surgery) LUCADA implementation note This section is completed for all patients undergoing surgery where the surgery was performed as a curative procedure. It should not be completed for any other patient group. INVESTIGATION RESULT DATE (Date Specimen Reported) HISTOLOGY (SNOMED) (Histology) POST TREATMENT: SITE-SPECIFIC STAGING CLASSIFICATION SNOMED L23 Site-specific coding Page 27 of 40
28 (Site-specific classification) 8.3 INVESTIGATION RESULT DATE (Date specimen reported) DD/MM/YYYY The date of the histology report for the post-operative surgical specimen HISTOLOGY (SNOMED)(Histology) For Lung Cancer patients Record here any histology from specimens obtained during a surgical procedure where the surgery was performed with the intention of curing the patient. Do not record here any histology results from surgical procedures undertaken for purely diagnostic purposes these should be entered in the Care Plan / MDT section. For Mesothelioma Patients It is accepted that all surgical procedures in this group of patients are palliative. Please record any histology obtained from such procedures Do not record here any histology procedures undertaken for purely diagnostic purposes these should be entered in the Care Plan / MDT section. SNOMed s for Histology (Pathology) (SNOMed III (1993)/ICD-O-2 (1990) unless stated) LUCADA Mapping M 8010/2 Carcinoma in situ NSCLC M 8041/3 Small cell carcinoma SCLC M 8046/3 Non-small cell carcinoma (ICD-O-3) NSCLC (includes adenosquamous carcinoma) M 8070/3 Squamous cell carcinoma NOS NSCLC M 8140/3 Adenocarcinoma NOS NSCLC (Adenocarcinoma without alveolar cell features) M 8250/3 Bronchio-alveolar cell carcinoma NSCLC (Adenocarcinoma with alveolar cell features) M 8012/3 Large cell Carcinoma NOS NSCLC M 8020/3 Large cell undifferentiated NSCLC M 8013/3 Large cell neuroendocrine (ICD-O-3) NSCLC M 8240/3 Carcinoid tumour NOS Separate category (includes atypical carcinoid) M 8980/3 Carcinosarcoma NOS NSCLC M 9050/3 Malignant Mesothelioma NOS Mesothelioma M 9052/3 Mesothelioma (epithelioid) Mesothelioma M 9051/3 Mesothelioma (sarcomatoid) (ICD-O-3) Mesothelioma M 8940/3 Mixed tumour (malignant) NSCLC M 9999/9 Other NSCLC L23 SITE-SPECIFIC STAGING CLASSIFICATION (Site-specific classification) RADIO As a result of the changes in the UICC staging manual version 7, TNM staging of small cell carcinoma of the lung is now the preferred method and should be recorded wherever possible. However, the old staging classification of Limited disease or Extensive disease (US Veterans Administration) is acceptable, if preferred. Site Specific Stage Stage unknown X Limited 1 Extensive 2 Page 28 of 40
29 Page 29 of 40
30 Limited disease Disease confined to one hemithorax including involvement of ipsi-and/or contralateral hilar, mediastinal or supraclavicular lymph nodes. Patients with ipsilateral pleural effusion, regardless or pleural cytology, should be included in this group. Extensive disease Any disease beyond the definition of limited stage. Chemotherapy Data Item Dataset Ref. Source SITE CODE (OF CANCER DRUG TREATMENT) (Hospital) 9.1 NHS Data Dictionary, Supporting Information, Administrative s, NHS Trust Site DECISION TO TREAT DATE (ANTI- 9.4 CANCER DRUG REGIMEN) (Date of decision to treat) START DATE (ANTI-CANCER DRUG 9.10 REGIMEN) (Drug treatment start date) CHEMOTHERAPY TREATMENT GIVEN L27 01 Chemotherapy alone 02 Neo-adjuvent chemotherapy before surgery 03 Part of a chemotherapy / radiotherapy treatment plan 04 Adjuvent chemotherapy post surgery 05 Induction chemotherapy to down stage before surgery 9.1 SITE CODE (OF CANCER DRUG TREATMENT) (Hospital) Five character alphanumeric Record the unique five figure organisation code of the Hospital where the patient is receiving their chemotherapy. 9.4 DECISION TO TREAT DATE DD/MM/YYYY (ANTI-CANCER DRUG REGIMEN) (Date of decision to treat) Record the date on which it was decided that this patient should receive chemotherapy drug treatment. This is the date that the consultation between the patient and the clinician took place and a treatment plan for chemotherapy was agreed START DATE DD/MM/YYYY (ANTI-CANCER DRUG REGIMEN) (Drug treatment start date) Record the date on which the first dose of the chemotherapy drug treatment is administered to the patient Page 30 of 40
31 L27 Chemotherapy treatment given Record the reason that the drug treatment is being carried out. Chemotherapy alone 01 Neo-adjuvent chemotherapy before surgery 02 Part of a chemotherapy / radiotherapy treatment plan 03 Adjuvent chemotherapy post surgery 04 Induction chemotherapy to downstage prior to surgery 05 Teletherapy (external beam radiotherapy). This section is used to record the initial teletherapy treatment. Data Item Dataset Ref. Source SITE CODE (OF TELETHERAPY) 10.1 NHS Data Dictionary, Supporting (Hospital) Information, Administrative s, NHS Trust Site DECISION TO TREAT DATE 10.3 (TELETHERAPY TREATMENT COURSE) (Date of decision to treat) RADIOTHERAPY TREATMENT GIVEN L26 LUCADA DATASET RADIOTHERAPY ANATOMICAL 10.7 OPCS Z s TREATMENT SITE (Anatomical treatment site) START DATE (TELETHERAPY TREATMENT COURSE) (Teletherapy start date) SITE CODE (OF TELETHERAPY) (Hospital) Five character alphanumeric Record the organisation code of the hospital providing the teletherapy to the patient DECISION TO TREAT DATE DD/MM/YYYY (TELETHERAPY TREATMENT COURSE) (Date of decision to treat) Record the date on which it was decided that this patient should receive teletherapy. This is the date that the consultation between the patient and the clinician took place and a treatment plan for teletherapy was agreed. Page 31 of 40
32 L26 RADIOTHERAPY TREATMENT GIVEN DDL Record the reason that the teletherapy is being carried out. Treatment Given Curative (radical) radiotherapy 01 Curative (CHART / CHARTWEL) 02 Part of a chemotherapy / radiotherapy treatment plan 03 Adjuvent following surgical treatment 04 Palliative Radiotherapy RADIOTHERAPY ANATOMICAL TREATMENT SITE (Anatomical treatment site) DDL Record the actual site, which is the part of the body to which the prescription is administered. This is coded using OPCS4 Z codes from the following list. Where no other anatomical site has been mentioned, Z24.6 (lung) should be selected rather than leaving this field blank. Anatomical Site Tissue of brain (nec) Z01.9 Trachea Z24.3 Lung Z24.6 Mediastinum Z24.7 Skin of other (nec) Z50.9 Chest wall (nec) Z52.9 Bone (nec) Z87.1 Mesothelioma drain site 01 Other Region of Body (nec) Z START DATE DD/MM/YYYY (TELETHERAPY TREATMENT COURSE) (Teletherapy start date) Record the date on which the first treatment of teletherapy is administered to the patient. Brachytherapy Data Item Dataset Ref. Source SITE CODE (OF BRACHYTHERAPY) 11.1 NHS Data Dictionary, Supporting (Hospital) Information, Administrative DECISION TO TREAT DATE (BRACHYTHERAPY TREATMENT COURSE) (Date of decision to treat) START DATE (BRACHYTHERAPY TREATMENT COURSE) (Brachytherapy start date) 11.3 s, NHS Trust Site 11.9 NCDS 11.1 SITE CODE (OF BRACHYTHERAPY) (Hospital) Five character alphanumeric Record the five figure organisation code of the hospital providing the brachytherapy to the patient. Page 32 of 40
33 11.3 DECISION TO TREAT DATE DD/MM/YYYY (BRACHYTHERAPY TREATMENT COURSE) (Date of decision to treat) Record the date on which it was decided that this patient should receive brachytherapy. This is the date that the consultation between the patient and the clinician took place and a treatment plan for brachytherapy was agreed START DATE DD/MM/YYYY (BRACHYTHERAPY TREATMENT COURSE) (Brachytherapy start date) Record the date on which the first fraction of brachytherapy for this prescription is administered to the patient. Palliative care Data Item Dataset Ref. Source SITE CODE (SPECIALIST PALLIATIVE TREATMENT COURSE) 12.n1 NHS Data Dictionary, Supporting Information, Administrative s, NHS Trust Site PALLIATIVE CARE PROVIDER TYPE L39 LUCADA dataset PALLIATIVE CARE COMMUNITY L40 LUCADA dataset PROVIDER TYPE DECISION TO TREAT DATE (SPECIALIST 12.n2 PALLIATIVE TREATMENT COURSE) (Date of decision to treat) START DATE (SPECIALIST PALLIATIVE 12.n3 TREATMENT COURSE) (Specialist Palliative Care Start Date) PALLIATIVE CARE INTERVENTIONAL L41 LUCADA dataset TREATMENT GIVEN DATE INTERVENTIONAL TREATMENT GIVEN L42 LUCADA dataset Specialist palliative care is defined as care being administered by any member of a specialist palliative care team (e.g. Consultant in Palliative Care, Hospice Staff and Macmillan Specialist Palliative Care Nurses). It also includes specialist palliative procedures such as pleurodesis and the insertion of bronchial of superior vena caval stents. Details of such palliative procedures should be recorded under L41 and L n1 SITE CODE Five character alphanumeric (SPECIALIST PALLIATIVE TREATMENT COURSE) (Hospital) Record the five figure organisation code of the Unit providing the specialist palliative care to the patient. L39 PALLIATIVE CARE PROVIDER TYPE Enter whether the palliative care was given in hospital or the community L39 Palliative Care Provider Type 01 Hospital 02 Community L40 PALLIATIVE CARE COMMUNITY PROVIDER TYPE If the palliative care was given in the community indicate where this care was provided Page 33 of 40
34 L40 Palliative care community provider type 01 Hospice 02 Nursing Home 03 Home care 08 Other 09 Unknown 12.n2 DECISION TO TREAT DATE DD/MM/YYYY (SPECIALIST PALLIATIVE TREATMENT COURSE) (Date of decision to treat) Record the date on which it was decided that this patient should receive specialist palliative care. This is the date that the consultation between the patient and the clinician took place and a treatment plan for specialist palliative care was agreed. 12.n3 START DATE DD/MM/YYYY (SPECIALIST PALLIATIVE TREATMENT COURSE) (Specialist Palliative Care Start Date). Record the date of the first treatment/support from specialist palliative care. L41 PALLIATIVE CARE INTERVENTIONAL TREATMENT GIVEN (Radio) Record if the patient underwent a palliative care procedure (e.g. bronchial stenting, SVC stenting pleurodesis etc.) L42 PALLIATIVE CARE INTERVENTIONAL TREATMENT DATE Record the date that the palliative care procedure was undertaken DD/MM/YYYY Active monitoring Data Item Repeating? Source of definition L25 SITE CODE (ACTIVE MONITORING) No NCDS (Hospital) 16.9 DECISION TO TREAT DATE (ACTIVE MONITORING) No Date format L25 SITE CODE (ACTIVE MONITORING) (Hospital) Five character alphanumeric Record the five figure organisation code of the Unit where it was decided that the patient should be actively monitored DECISION TO TREAT DATE (ACTIVE MONITORING) DD/MM/YYYY If the first treatment is active monitoring, record the date on which it was decided that the patient should be actively monitored. Page 34 of 40
35 Outcome Death Record here details of the patients death. Also, complete L21 for all patients who received prophylactic cranial irradiation Data Item Repeating? Source of definition 15.1 PERSON DEATH DATE (Date of No NCDS death) L20 Was Death Related to Treatment? No LUCADA dataset 14.7 TREATMENT TYPE (CANCER No NCDS MORBIDITY) (Treatment Related Morbidity Type L21 Did patient received PCI?) No LUCADA dataset L30 Was the original treatment plan No LUCADA dataset carried out L31 Reason why original treatment plan failed No LUCADA dataset (Note 14.7 also cross references to NCDS 14.8, 14.10,14.11 & 14.12) 15.1 PERSON DEATH DATE (Date of death) Record the date the patient died, in date format. Completion of this field is optional as this will be automatically updated from ONS L 20 WAS DEATH RELATED TO TREATMENT? Record whether or not the patient death was related to treatment. Yes Y No N Unknown TREATMENT TYPE (CANCER MORBIDITY) (Treatment Related Morbidity Type) Record here the details of any treatment morbidity severe enough to result in death If death was related to treatment record treatment type to which death was related using the DDL below. Treatment Type Surgery 1 Chemotherapy 2 Radiotherapy 3 Combination 4 Page 35 of 40
36 Prophylactic cranial irradiation L 21 DID THE PATIENT RECEIVE PROPHYLACTIC CRANIAL IRRADIATION? Use the DDL to indicate whether patients received prophylactic cranial irradiation. This is particularly important for patients with limited stage small cell lung cancer Yes Y No N Unknown 9 Treatment plan L30 WAS THE ORIGINAL TREATMENT PLAN CARRIED OUT RADIO Record here if the original treatment plan (L29) was carried out Yes Y No N Unknown 9 L31 ORIGINAL TREATMENT PLAN FAILURE REASON DDL If the treatment plan (L29) was not followed or completed, record the reason why. L31 Reason why original treatment plan failed 01 Cancer progressed through treatment such that a new treatment plan required 02 Patient choice 03 Patient died 04 Treatment toxicity 05 Disease progression Page 36 of 40
37 Appendix A: How to obtain a list of hospital site codes The National Administrative s Service (NACS) is a Department of Health funded service that provides nationally agreed reference data to the NHS and other interested parties. The reference data covers not just healthcare organisations but also practitioners, postcodes and other administrative details. The National Administrative s Service provides information via NHS net, via a quarterly release of data on CD-ROM and on the Internet. To download a file containing hospital site codes Access the National Administrative s Service web site: On this page, use the Data Downloads link On this page, use the Downloads Index link. On this page, use the NHS Trust sites link. On this page, use the etrust.zip link against NHS Trusts and Trust sites. To obtain a particular NHS Trust, NHS Trust site or non-nhs Organisation Access the National Administrative s Service web site: nww.connectingforhealth.nhs.uk/nacs On this page, use the Online enquiries link. On this page, use the Search using name, address or postcode link. Page 37 of 40
38 Appendix B: Notes on coding classifications International Statistical Classification of Diseases and Related Health Problems (ICD-10) The classification of neoplasms is broken down into categories based on their point of origin, and behaviour Sites for malignant neoplasms are prefixed by C C00 - C75 covers specified primary sites (but excludes lymphoid, haematopoietic and related tissues) C76 - C80 cover ill-defined, secondary and unspecified sites C81 - C96 are malignant neoplasms of lymphoid, haematopoietic and related issues C97 are malignant neoplasms of multiple independent primary sites Sites for in situ and benign neoplasms are prefixed by D, but grouped into much broader categories D00 - D09 relate to in situ neoplasms D10 - D36 relate to benign neoplasms D37 - D48 relate to neoplasms of uncertain or unknown behaviour For each anatomical site the initial three alphanumeric code is supplemented by a decimal point and an additional digit to identify sub-site 0 to 7, or an overlapping site 8 (unless specifically indexed see below), or unspecified sub-site 9. Example C34 Malignant neoplasm of bronchus and lung C34.0 Main bronchus Carina Hilus (of lung) C34.1 Upper lobe, bronchus or lung C34.2 Middle lobe, bronchus or lung C34.3 Lower lobe, bronchus or lung C34.8 Overlapping lesion of bronchus and lung C34.8 Bronchus or lung, unspecified An in situ tumour of the bronchus and lung would be coded D02.2, where D02 are carcinoma in situ of middle ear and respiratory system, sub-site.2 bronchus and lung. A benign tumour of the bronchus and lung would be coded D14.3, where D14 are benign neoplasms of middle ear and respiratory system, with sub-site.3 for bronchus and lung. A tumour of uncertain or unknown behaviour would be coded as D38.1 where D38 are neoplasms of uncertain or unknown behaviour of middle ear and respiratory and intrathoracic organs, sub-site.1 for trachea, bronchus and lung. International Classification of Diseases for Oncology, 2nd edition (ICD-O-2) Morphology codes ICD-10 includes a copy of this coding system to allow it to be used in conjunction with the above site codes, to identify histological type. Morphology codes are prefixed by M to identify morphology. Four identity digits for the histological type of neoplasm, and a slash, then a one-digit behaviour code follow this as follows: Page 38 of 40
39 /0 benign /1 uncertain benign or malignant /2 carcinoma in situ /3 malignant, primary site /6 malignant metastatic, or secondary site Example: M8070 describes squamous carcinoma M8070/2 squamous cell carcinoma in-situ M8070/3 squamous cell carcinoma (Not Otherwise Specified) M8070/6 squamous cell carcinoma metastatic (i.e. in a node with an unknown primary) There is also a /9 behaviour code for malignant uncertain whether primary or metastatic site, but this is not applicable for use in conjunction with the ICD 'C' site codes as all malignant neoplasms are presumed to be primary /3 or secondary /6 It should be noted that some histological types of neoplasm are specific to certain sites or types of tissue and that the 'C' and 'D' site codes are shown after these. e.g. M8972/3 Pulmonary blastoma (C34.-) identifies that the morphology only relates to tumours occurring in the lungs. SNOMED CT SNOMED CT is a clinical terminology - the Systematised Nomenclature of Medicine. It is a common computerised language that will eventually be used by all computers in the NHS to facilitate communications between healthcare professionals. The morphology section of SNOMED CT is identical to ICD O, but it also gives a broader description of pathological terminology to aid coding. OPCS-4 - operation codes The basic structure of the classification comprises anatomically based chapters, each of which is given an alphabetic code. The next two digits describe the operation group, based on the largest operation first, descending to the smallest, followed by a decimal point and a fourth digit to identify the procedure itself. If the procedure described does not match the categories above then provided the procedure is recorded a.8 can be added, and where unspecified a.9 can be added. Example: E54 Excision of lung E54.1 Total pneumonectomy E54.2 Bibobectomy of lung E54.3 Lobectomy of lung E54.4 Excision of segment of lung E54.5 Partial lobectomy of lung nec E54.8 Other specified E54.9 Unspecified UICC Coding This refers to the International Union Against Cancer s TNM coding system (6th edition2002, 7 th edition 2009) the global standard in cancer staging. Page 39 of 40
40 Copyright for Lung Cancer Copyright 2010, The Information Centre, Clinical Audit Support Unit (CASU). All rights reserved. This work remains the sole and exclusive property of The Information Centre and may only be reproduced where there is explicit reference to the ownership of The Information Centre. This work may be re-used by NHS and government organisations without permission. Commercial reuse of this work must be granted by The Information Centre. Page 40 of 40
Please see the LUCADA data manual v3.1.3, available in the downloads section
 National Lung Cancer Audit Frequently Asked Questions What dataset should be used? Please see the LUCADA data manual v3.1.3, available in the downloads section What does LUCADA stand for? LUCADA stands
National Lung Cancer Audit Frequently Asked Questions What dataset should be used? Please see the LUCADA data manual v3.1.3, available in the downloads section What does LUCADA stand for? LUCADA stands
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians
 Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
A Practical Guide to Advances in Staging and Treatment of NSCLC
 A Practical Guide to Advances in Staging and Treatment of NSCLC Robert J. Korst, M.D. Director, Thoracic Surgery Medical Director, The Blumenthal Cancer Center The Valley Hospital Objectives Revised staging
A Practical Guide to Advances in Staging and Treatment of NSCLC Robert J. Korst, M.D. Director, Thoracic Surgery Medical Director, The Blumenthal Cancer Center The Valley Hospital Objectives Revised staging
Lung Cancer Treatment Guidelines
 Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
General Information About Non-Small Cell Lung Cancer
 General Information About Non-Small Cell Lung Cancer Non-small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing
General Information About Non-Small Cell Lung Cancer Non-small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing
Small Cell Lung Cancer
 Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Management of Non-Small Cell Lung Cancer Guide for General Practitioners
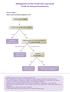 Management of n-small Cell Lung Cancer Guide for General Practitioners Clinical Stage I Cancer only in one lobe of lung and
Management of n-small Cell Lung Cancer Guide for General Practitioners Clinical Stage I Cancer only in one lobe of lung and
INTERNATIONAL ASSOCIATION FOR THE STUDY OF LUNG CANCER Prospective Mesothelioma Staging Project
 INTERNATIONAL ASSOCIATION FOR THE STUDY OF LUNG CANCER Prospective Mesothelioma Staging Project Data Forms and Fields in CRAB Electronic Data Capture System - Reduced Set - Pivotal data elements for developing
INTERNATIONAL ASSOCIATION FOR THE STUDY OF LUNG CANCER Prospective Mesothelioma Staging Project Data Forms and Fields in CRAB Electronic Data Capture System - Reduced Set - Pivotal data elements for developing
Surgery. Wedge resection only part of the lung, not. not a lobe, is removed. Cancer Council NSW
 The treatment you receive will depend on your lung cancer type, for example, whether you have a non-small cell lung cancer Adenocarcinoma or Squamous cell carcinoma, and if this is a sub-type with a mutation.
The treatment you receive will depend on your lung cancer type, for example, whether you have a non-small cell lung cancer Adenocarcinoma or Squamous cell carcinoma, and if this is a sub-type with a mutation.
Radiation Therapy in the Treatment of
 Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Lung Cancer: Diagnosis, Staging and Treatment
 PATIENT EDUCATION patienteducation.osumc.edu Lung Cancer: Diagnosis, Staging and Treatment Cancer begins in our cells. Cells are the building blocks of our tissues. Tissues make up the organs of the body.
PATIENT EDUCATION patienteducation.osumc.edu Lung Cancer: Diagnosis, Staging and Treatment Cancer begins in our cells. Cells are the building blocks of our tissues. Tissues make up the organs of the body.
Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines
 Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines April 2008 (presented at 6/12/08 cancer committee meeting) By Shelly Smits, RHIT, CCS, CTR Conclusions by Dr. Ian Thompson, MD Dr. James
Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines April 2008 (presented at 6/12/08 cancer committee meeting) By Shelly Smits, RHIT, CCS, CTR Conclusions by Dr. Ian Thompson, MD Dr. James
Non-Small Cell Lung Cancer
 Non-Small Cell Lung Cancer John delcharco, MD (Statistics based on CVMC data 2009-2013) Statistics Lung cancer is the leading cause of cancer deaths in the United States. The American Cancer Society estimates
Non-Small Cell Lung Cancer John delcharco, MD (Statistics based on CVMC data 2009-2013) Statistics Lung cancer is the leading cause of cancer deaths in the United States. The American Cancer Society estimates
How To Prepare A Meeting For A Health Care Conference
 THORACIC ONCOLOGY MULTIDISCIPLINARY TEAM MEETINGS: OPERATIONAL POLICY EXECUTIVE SUMMARY 1. All patients in Lothian with thoracic malignancies should be discussed at designated times in pathway (see App
THORACIC ONCOLOGY MULTIDISCIPLINARY TEAM MEETINGS: OPERATIONAL POLICY EXECUTIVE SUMMARY 1. All patients in Lothian with thoracic malignancies should be discussed at designated times in pathway (see App
National Bowel Cancer Audit Report 2008 Public and Executive Summary
 National Bowel Cancer Audit Report 2008 Public and Executive Summary Prepared in association with: Healthcare Quality Improvement Partnership HQIP Association of Coloproctology of Great Britain and Ireland
National Bowel Cancer Audit Report 2008 Public and Executive Summary Prepared in association with: Healthcare Quality Improvement Partnership HQIP Association of Coloproctology of Great Britain and Ireland
This factsheet aims to outline the characteristics of some rare lung cancers, and highlight where each type of lung cancer may be different.
 There are several different kinds of lung cancer, often referred to as lung cancer subtypes. Some of these occur more often than others. In this factsheet we will specifically look at the subtypes of cancers
There are several different kinds of lung cancer, often referred to as lung cancer subtypes. Some of these occur more often than others. In this factsheet we will specifically look at the subtypes of cancers
Understanding Pleural Mesothelioma
 Understanding Pleural Mesothelioma UHN Information for patients and families Read this booklet to learn about: What is pleural mesothelioma? What causes it? What are the symptoms? What tests are done to
Understanding Pleural Mesothelioma UHN Information for patients and families Read this booklet to learn about: What is pleural mesothelioma? What causes it? What are the symptoms? What tests are done to
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); [email protected] Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); [email protected] Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
Objectives. Mylene T. Truong, MD. Malignant Pleural Mesothelioma Background
 Imaging of Pleural Tumors Mylene T. Truong, MD Imaging of Pleural Tumours Mylene T. Truong, M. D. University of Texas M.D. Anderson Cancer Center, Houston, TX Objectives To review tumors involving the
Imaging of Pleural Tumors Mylene T. Truong, MD Imaging of Pleural Tumours Mylene T. Truong, M. D. University of Texas M.D. Anderson Cancer Center, Houston, TX Objectives To review tumors involving the
2015/16 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL)-
 2015/16 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL)- SECTION B PART 1 - SERVICE SPECIFICATION FOR LUNG CANCER Service specification
2015/16 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL)- SECTION B PART 1 - SERVICE SPECIFICATION FOR LUNG CANCER Service specification
Lung cancer forms in tissues of the lung, usually in the cells lining air passages.
 Scan for mobile link. Lung Cancer Lung cancer usually forms in the tissue cells lining the air passages within the lungs. The two main types are small-cell lung cancer (usually found in cigarette smokers)
Scan for mobile link. Lung Cancer Lung cancer usually forms in the tissue cells lining the air passages within the lungs. The two main types are small-cell lung cancer (usually found in cigarette smokers)
How To Treat Lung Cancer At Cleveland Clinic
 Treatment Guide Lung Cancer Management The Chest Cancer Center at Cleveland Clinic, which includes specialists from the Respiratory Institute, Taussig Cancer Institute and Miller Family Heart & Vascular
Treatment Guide Lung Cancer Management The Chest Cancer Center at Cleveland Clinic, which includes specialists from the Respiratory Institute, Taussig Cancer Institute and Miller Family Heart & Vascular
Rotation Specific Goals & Objectives: University Health Network-Princess Margaret Hospital/ Sunnybrook Breast/Melanoma
 Rotation Specific Goals & Objectives: University Health Network-Princess Margaret Hospital/ Sunnybrook Breast/Melanoma Medical Expert: Breast Rotation Specific Competencies/Objectives 1.0 Medical History
Rotation Specific Goals & Objectives: University Health Network-Princess Margaret Hospital/ Sunnybrook Breast/Melanoma Medical Expert: Breast Rotation Specific Competencies/Objectives 1.0 Medical History
Small cell lung cancer
 Small cell lung cancer Small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing organs that are found within
Small cell lung cancer Small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing organs that are found within
Lung Cancer & Mesothelioma 2011-2015
 Lung Cancer & Mesothelioma 2011-2015 Annex G Mesothelioma 1. The vision for mesothelioma services is set out in the Mesothelioma Framework issued by DH on 26 February 2007 (supported by the British Thoracic
Lung Cancer & Mesothelioma 2011-2015 Annex G Mesothelioma 1. The vision for mesothelioma services is set out in the Mesothelioma Framework issued by DH on 26 February 2007 (supported by the British Thoracic
Neoplasms of the LUNG and PLEURA
 Neoplasms of the LUNG and PLEURA 2015-2016 FCDS Educational Webcast Series Steven Peace, BS, CTR September 19, 2015 2015 Focus o Anatomy o SSS 2000 o MPH Rules o AJCC TNM 1 Case 1 Case Vignette HISTORY:
Neoplasms of the LUNG and PLEURA 2015-2016 FCDS Educational Webcast Series Steven Peace, BS, CTR September 19, 2015 2015 Focus o Anatomy o SSS 2000 o MPH Rules o AJCC TNM 1 Case 1 Case Vignette HISTORY:
Malignant Mesothelioma
 Malignant Malignant mesothelioma is a tumour originating from mesothelial cells. 85 95% of mesotheliomas are caused by asbestos exposure. It occurs much more commonly in the chest (malignant pleural mesothelioma)
Malignant Malignant mesothelioma is a tumour originating from mesothelial cells. 85 95% of mesotheliomas are caused by asbestos exposure. It occurs much more commonly in the chest (malignant pleural mesothelioma)
Malignant Mesothelioma
 Malignant mesothelioma is a tumour originating from mesothelial cells. 85 95% of mesotheliomas are caused by asbestos exposure. It occurs much more commonly in the chest (malignant pleural mesothelioma)
Malignant mesothelioma is a tumour originating from mesothelial cells. 85 95% of mesotheliomas are caused by asbestos exposure. It occurs much more commonly in the chest (malignant pleural mesothelioma)
B. Dingle MD, FRCPC, Brian Yaremko MD,FRCPC, R. Ash, MD, FRCPC, P. Truong, MD, FRCPC
 Lung Cancer B. Dingle MD, FRCPC, Brian Yaremko MD,FRCPC, R. Ash, MD, FRCPC, P. Truong, MD, FRCPC EPIDEMIOLOGY The estimated incidence of lung cancer in Canada for 2007 is 23,300 with 12,400 occurring in
Lung Cancer B. Dingle MD, FRCPC, Brian Yaremko MD,FRCPC, R. Ash, MD, FRCPC, P. Truong, MD, FRCPC EPIDEMIOLOGY The estimated incidence of lung cancer in Canada for 2007 is 23,300 with 12,400 occurring in
Non-Small Cell Lung Cancer
 Non-Small Cell Lung Cancer About Your Lungs and Lung Cancer How do your lungs work? To understand lung cancer it is helpful to understand your lungs. Your lungs put oxygen into the blood, which the heart
Non-Small Cell Lung Cancer About Your Lungs and Lung Cancer How do your lungs work? To understand lung cancer it is helpful to understand your lungs. Your lungs put oxygen into the blood, which the heart
III. EXTENT OF DISEASE
 Advanced Abstracting Lung Cancer III. EXTENT OF DISEASE Staging Systems and Documentation 1 Source: AJCC Cancer Staging Illustrations from the AJCC Cancer Staging Atlas. Springer, 2007. Used with permission.
Advanced Abstracting Lung Cancer III. EXTENT OF DISEASE Staging Systems and Documentation 1 Source: AJCC Cancer Staging Illustrations from the AJCC Cancer Staging Atlas. Springer, 2007. Used with permission.
Lung cancer case study
 Change Presentation title and date in Footer dd.mm.yyyy Lung cancer case study Dr Jaishree Bhosle Consultant Medical Oncologist Change Presentation title and date in Footer dd.mm.yyyy 1 2 Part One Initial
Change Presentation title and date in Footer dd.mm.yyyy Lung cancer case study Dr Jaishree Bhosle Consultant Medical Oncologist Change Presentation title and date in Footer dd.mm.yyyy 1 2 Part One Initial
Lung Cancer. This reference summary will help you better understand lung cancer and the treatment options that are available.
 Lung Cancer Introduction Lung cancer is the number one cancer killer of men and women. Over 165,000 people die of lung cancer every year in the United States. Most cases of lung cancer are related to cigarette
Lung Cancer Introduction Lung cancer is the number one cancer killer of men and women. Over 165,000 people die of lung cancer every year in the United States. Most cases of lung cancer are related to cigarette
Stage I, II Non Small Cell Lung Cancer
 Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
Salisbury Lung Cancer Service (1 of 5)
 Salisbury Lung Cancer Service (1 of 5) i If you need this information in another language or medium (audio, large print, etc) please contact Customer Care on 0800 374 208 email: customercare@ salisbury.nhs.uk.
Salisbury Lung Cancer Service (1 of 5) i If you need this information in another language or medium (audio, large print, etc) please contact Customer Care on 0800 374 208 email: customercare@ salisbury.nhs.uk.
Table of Contents. Data Supplement 1: Summary of ASTRO Guideline Statements. Data Supplement 2: Definition of Terms
 Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
Treating Mesothelioma - A Quick Guide
 Treating Mesothelioma - A Quick Guide Contents This is a brief summary of the information on Treating mesothelioma from CancerHelp UK. You will find more detailed information on the website. In this information
Treating Mesothelioma - A Quick Guide Contents This is a brief summary of the information on Treating mesothelioma from CancerHelp UK. You will find more detailed information on the website. In this information
Pathology of lung cancer
 Pathology of lung cancer EASO COURSE ON LUNG CANCER AND MESOTHELIOMA DAMASCUS (SYRIA), MAY 3-4, 2007 Gérard ABADJIAN MD Pathologist Associate Professor, Saint Joseph University Pathology Dept. Hôtel-Dieu
Pathology of lung cancer EASO COURSE ON LUNG CANCER AND MESOTHELIOMA DAMASCUS (SYRIA), MAY 3-4, 2007 Gérard ABADJIAN MD Pathologist Associate Professor, Saint Joseph University Pathology Dept. Hôtel-Dieu
National Lung Cancer Audit Report
 National Lung Cancer Audit Report 2013 Report for the audit period 2012 Copyright 2013, Health and Social Care Information Centre, National Lung Cancer Audit. All rights reserved. 1 Prepared in partnership
National Lung Cancer Audit Report 2013 Report for the audit period 2012 Copyright 2013, Health and Social Care Information Centre, National Lung Cancer Audit. All rights reserved. 1 Prepared in partnership
Avastin: Glossary of key terms
 Avastin: Glossary of key terms Adenocarcinoma Adenoma Adjuvant therapy Angiogenesis Anti-angiogenics Antibody Antigen Avastin (bevacizumab) Benign A form of carcinoma that originates in glandular tissue.
Avastin: Glossary of key terms Adenocarcinoma Adenoma Adjuvant therapy Angiogenesis Anti-angiogenics Antibody Antigen Avastin (bevacizumab) Benign A form of carcinoma that originates in glandular tissue.
Male. Female. Death rates from lung cancer in USA
 Male Female Death rates from lung cancer in USA Smoking represents an interesting combination of an entrenched industry and a clearly drug-induced cancer Tobacco Use in the US, 1900-2000 5000 100 Per Capita
Male Female Death rates from lung cancer in USA Smoking represents an interesting combination of an entrenched industry and a clearly drug-induced cancer Tobacco Use in the US, 1900-2000 5000 100 Per Capita
Chapter 13. The hospital-based cancer registry
 Chapter 13. The hospital-based cancer registry J.L. Young California Tumor Registry, 1812 14th Street, Suite 200, Sacramento, CA 95814, USA Introduction The purposes of a hospital-based cancer registry
Chapter 13. The hospital-based cancer registry J.L. Young California Tumor Registry, 1812 14th Street, Suite 200, Sacramento, CA 95814, USA Introduction The purposes of a hospital-based cancer registry
Epidemiology, Staging and Treatment of Lung Cancer. Mark A. Socinski, MD
 Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
How treatment is planned Giving your consent The benefits and disadvantages of treatment Second opinion
 Treatment overview for lung cancer This information is an extract from the booklet Understanding lung cancer. You may find the full booklet helpful. We can send you a free copy see page 5. Contents How
Treatment overview for lung cancer This information is an extract from the booklet Understanding lung cancer. You may find the full booklet helpful. We can send you a free copy see page 5. Contents How
Disease/Illness GUIDE TO ASBESTOS LUNG CANCER. What Is Asbestos Lung Cancer? www.simpsonmillar.co.uk Telephone 0844 858 3200
 GUIDE TO ASBESTOS LUNG CANCER What Is Asbestos Lung Cancer? Like tobacco smoking, exposure to asbestos can result in the development of lung cancer. Similarly, the risk of developing asbestos induced lung
GUIDE TO ASBESTOS LUNG CANCER What Is Asbestos Lung Cancer? Like tobacco smoking, exposure to asbestos can result in the development of lung cancer. Similarly, the risk of developing asbestos induced lung
Sternotomy and removal of the tumor
 Sternotomy and removal of the tumor All thymomas originate from epithelial thymic cells 4% of them consist of a pure population of epithelial cells Most have mixed populations of lymphoid cells to a
Sternotomy and removal of the tumor All thymomas originate from epithelial thymic cells 4% of them consist of a pure population of epithelial cells Most have mixed populations of lymphoid cells to a
Surgical guidelines for the management of breast cancer
 Available online at www.sciencedirect.com EJSO xx (2009) S1eS22 www.ejso.com Guidelines Surgical guidelines for the management of breast cancer Contents Association of Breast Surgery at BASO 2009 Introduction...
Available online at www.sciencedirect.com EJSO xx (2009) S1eS22 www.ejso.com Guidelines Surgical guidelines for the management of breast cancer Contents Association of Breast Surgery at BASO 2009 Introduction...
Radiotherapy in locally advanced & metastatic NSC lung cancer
 Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
Lung Cancer and Mesothelioma
 Lung Cancer and Mesothelioma Robert Kratzke, M.D. John C. Skoglund Professor of Lung Cancer Research Section of Heme/Onc/Transplant Department of Medicine University of Minnesota Medical School Malignant
Lung Cancer and Mesothelioma Robert Kratzke, M.D. John C. Skoglund Professor of Lung Cancer Research Section of Heme/Onc/Transplant Department of Medicine University of Minnesota Medical School Malignant
Lung Cancer Understanding your diagnosis
 Lung Cancer Understanding your diagnosis Lung Cancer Understanding your diagnosis When you first hear that you have cancer you may feel alone and afraid. You may be overwhelmed by the large amount of information
Lung Cancer Understanding your diagnosis Lung Cancer Understanding your diagnosis When you first hear that you have cancer you may feel alone and afraid. You may be overwhelmed by the large amount of information
LUNG CANCER. FCDS 2011 Educational Webcast Series November 17, 2011
 LUNG CANCER FCDS 2011 Educational Webcast Series November 17, 2011 Judy Bonner, RN, MS, CTR and Lynne Pearson, CTR, LHRM Steven Peace, CTR Updated for 2011 Requirements and CSv02.03.02 1 Presentation Outline
LUNG CANCER FCDS 2011 Educational Webcast Series November 17, 2011 Judy Bonner, RN, MS, CTR and Lynne Pearson, CTR, LHRM Steven Peace, CTR Updated for 2011 Requirements and CSv02.03.02 1 Presentation Outline
Extrapleural Pneumonectomy for Malignant Mesothelioma: Pro. Joon H. Lee 9/17/2012
 Extrapleural Pneumonectomy for Malignant Mesothelioma: Pro Joon H. Lee 9/17/2012 Malignant Pleural Mesothelioma (Epidemiology) Incidence: 7/mil (Japan) to 40/mil (Australia) Attributed secondary to asbestos
Extrapleural Pneumonectomy for Malignant Mesothelioma: Pro Joon H. Lee 9/17/2012 Malignant Pleural Mesothelioma (Epidemiology) Incidence: 7/mil (Japan) to 40/mil (Australia) Attributed secondary to asbestos
Lung Cancer. Understanding your diagnosis
 Lung Cancer Understanding your diagnosis Lung Cancer Understanding your diagnosis When you first hear that you have cancer, you may feel alone and afraid. You may be overwhelmed by the large amount of
Lung Cancer Understanding your diagnosis Lung Cancer Understanding your diagnosis When you first hear that you have cancer, you may feel alone and afraid. You may be overwhelmed by the large amount of
An Overview of Lung Cancer Symptoms, Pathophysiology, And Treatment Linda H. Yoder
 CE Objectives and Evaluation Form appear on page 235. An Overview of Lung Cancer Symptoms, Pathophysiology, And Treatment Linda H. Yoder Patients with lung cancer can provide treatment challenges for even
CE Objectives and Evaluation Form appear on page 235. An Overview of Lung Cancer Symptoms, Pathophysiology, And Treatment Linda H. Yoder Patients with lung cancer can provide treatment challenges for even
General Rules SEER Summary Stage 2000. Objectives. What is Staging? 5/8/2014
 General Rules SEER Summary Stage 2000 Linda Mulvihill Public Health Advisor NCRA Annual Meeting May 2014 National Center for Chronic Disease Prevention and Health Promotion Division of Cancer Prevention
General Rules SEER Summary Stage 2000 Linda Mulvihill Public Health Advisor NCRA Annual Meeting May 2014 National Center for Chronic Disease Prevention and Health Promotion Division of Cancer Prevention
Guideline for the Imaging of Patients Presenting with Breast Symptoms incorporating the guideline for the use of MRI in breast cancer
 Guideline for the Imaging of Patients Presenting with Breast Symptoms incorporating the guideline for the use of MRI in breast cancer Version History Version Date Summary of Change/Process 0.1 09.01.11
Guideline for the Imaging of Patients Presenting with Breast Symptoms incorporating the guideline for the use of MRI in breast cancer Version History Version Date Summary of Change/Process 0.1 09.01.11
Survival analysis of 220 patients with completely resected stage II non small cell lung cancer
 窑 Original Article 窑 Chinese Journal of Cancer Survival analysis of 22 patients with completely resected stage II non small cell lung cancer Yun Dai,2,3, Xiao Dong Su,2,3, Hao Long,2,3, Peng Lin,2,3, Jian
窑 Original Article 窑 Chinese Journal of Cancer Survival analysis of 22 patients with completely resected stage II non small cell lung cancer Yun Dai,2,3, Xiao Dong Su,2,3, Hao Long,2,3, Peng Lin,2,3, Jian
A918: Prostate: adenocarcinoma
 A918: Prostate: adenocarcinoma General facts of prostate cancer The prostate is about the size of a walnut. It is just below the bladder and in front of the rectum. The tube that carries urine (the urethra)
A918: Prostate: adenocarcinoma General facts of prostate cancer The prostate is about the size of a walnut. It is just below the bladder and in front of the rectum. The tube that carries urine (the urethra)
How To Treat Mesothelioma
 Mesothelioma in the UK Liz Darlison Mesothelioma UK The National Macmillan Mesothelioma Resource Centre June 2010 Mesothelioma Applied Research Foundation - 2010 www.curemeso.org Contents Size of Mesothelioma
Mesothelioma in the UK Liz Darlison Mesothelioma UK The National Macmillan Mesothelioma Resource Centre June 2010 Mesothelioma Applied Research Foundation - 2010 www.curemeso.org Contents Size of Mesothelioma
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Updates in Mesothelioma By Samieh Amer, MD Professor of Cardiothoracic Surgery Faculty of Medicine, Cairo University History Wagner and his colleagues (1960) 33 cases of mesothelioma
بسم هللا الرحمن الرحيم Updates in Mesothelioma By Samieh Amer, MD Professor of Cardiothoracic Surgery Faculty of Medicine, Cairo University History Wagner and his colleagues (1960) 33 cases of mesothelioma
Primary -Benign - Malignant Secondary
 TUMOURS OF THE LUNG Primary -Benign - Malignant Secondary The incidence of lung cancer has been increasing almost logarithmically and is now reaching epidemic levels. The overall cure rate is very low
TUMOURS OF THE LUNG Primary -Benign - Malignant Secondary The incidence of lung cancer has been increasing almost logarithmically and is now reaching epidemic levels. The overall cure rate is very low
How To Know When To Stage Lung Cancer
 WHITE PAPER - SBRT for Non Small Cell Lung Cancer I. Introduction This white paper will focus on non-small cell lung carcinoma with sections one though six comprising a general review of lung cancer from
WHITE PAPER - SBRT for Non Small Cell Lung Cancer I. Introduction This white paper will focus on non-small cell lung carcinoma with sections one though six comprising a general review of lung cancer from
Treatment Part Two 1 FLORIDA CANCER DATA SYSTEM Treatment - Part Two
 Treatment Part Two 1 Prerequisites 2 Completion of FCDS, Introduction to Abstracting module Completion of FCDS, Treatment Part One Learning Objectives 3 Recognize cancer treatment modalities Acquire a
Treatment Part Two 1 Prerequisites 2 Completion of FCDS, Introduction to Abstracting module Completion of FCDS, Treatment Part One Learning Objectives 3 Recognize cancer treatment modalities Acquire a
Lung cancer LUNG CANCER. Box 1 Clinical signs
 22 LUNG CANCER Lung cancer Bronchial carcinoma refers to two distinct clinical entities small cell and non-small cell carcinoma. Although these conditions have much in common, with broadly similar presenting
22 LUNG CANCER Lung cancer Bronchial carcinoma refers to two distinct clinical entities small cell and non-small cell carcinoma. Although these conditions have much in common, with broadly similar presenting
Tricia Cox on 7/18/2012 at Oncology Center. Sarah Randolf. Female
 SAMPLE This Survivorship Care Plan will facilitate cancer care following active treatment. It may include important contact information, a treatment summary, recommendations for follow-up care testing,
SAMPLE This Survivorship Care Plan will facilitate cancer care following active treatment. It may include important contact information, a treatment summary, recommendations for follow-up care testing,
CANCER WAITING TARGETS A GUIDE (VERSION 5)
 National Cancer Waits Project CANCER WAITING TARGETS A GUIDE (VERSION 5) Cancer Action Team Amendments from version 4 to version 5 The waiting times guide has been updated from version 4. Sections which
National Cancer Waits Project CANCER WAITING TARGETS A GUIDE (VERSION 5) Cancer Action Team Amendments from version 4 to version 5 The waiting times guide has been updated from version 4. Sections which
a commitment in their practice to the roles and responsibilities of clinical oncologists X
 CLINICAL ONCOLOGY BLUEPRINT OF ASSESSMENT SYSTEM Good Medical Practice areas of curriculum - ST5 (Intermediate) Standards GMP criterion Workplace based assessment By the end of the third year of training
CLINICAL ONCOLOGY BLUEPRINT OF ASSESSMENT SYSTEM Good Medical Practice areas of curriculum - ST5 (Intermediate) Standards GMP criterion Workplace based assessment By the end of the third year of training
PRIMARY LUNG CANCER TREATMENT
 PRIMARY LUNG CANCER TREATMENT Cancer Care Pathways Directorate Tailored Information in Cancer Care (TICC) Sir Anthony Mamo Oncology Centre December 2014 Contents About this booklet 1 Types of Lung Cancer
PRIMARY LUNG CANCER TREATMENT Cancer Care Pathways Directorate Tailored Information in Cancer Care (TICC) Sir Anthony Mamo Oncology Centre December 2014 Contents About this booklet 1 Types of Lung Cancer
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer
 Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
How To Write Lung Cancer Data Standards For Nhs Scotland
 For reference only Do Not Use For more information contact: [email protected] Lung Cancer Pathology Data Standards June 2008 National Clinical Dataset Development Programme (NCDDP) Support Team Information
For reference only Do Not Use For more information contact: [email protected] Lung Cancer Pathology Data Standards June 2008 National Clinical Dataset Development Programme (NCDDP) Support Team Information
B10/S/a Cancer: Malignant Mesothelioma (Adult)
 B10/S/a 2013/14 NHS STANDARD CONTRACT FOR CANCER: MALIGNANT MESOTHELIOMA (ADULT) SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider Lead Period Date
B10/S/a 2013/14 NHS STANDARD CONTRACT FOR CANCER: MALIGNANT MESOTHELIOMA (ADULT) SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider Lead Period Date
Diagnosis and Prognosis of Pancreatic Cancer
 Main Page Risk Factors Reducing Your Risk Screening Symptoms Diagnosis Treatment Overview Chemotherapy Radiation Therapy Surgical Procedures Lifestyle Changes Managing Side Effects Talking to Your Doctor
Main Page Risk Factors Reducing Your Risk Screening Symptoms Diagnosis Treatment Overview Chemotherapy Radiation Therapy Surgical Procedures Lifestyle Changes Managing Side Effects Talking to Your Doctor
Chapter 2 Staging of Breast Cancer
 Chapter 2 Staging of Breast Cancer Zeynep Ozsaran and Senem Demirci Alanyalı 2.1 Introduction Five decades ago, Denoix et al. proposed classification system (tumor node metastasis [TNM]) based on the dissemination
Chapter 2 Staging of Breast Cancer Zeynep Ozsaran and Senem Demirci Alanyalı 2.1 Introduction Five decades ago, Denoix et al. proposed classification system (tumor node metastasis [TNM]) based on the dissemination
Malignant Mesothelioma Current Approaches to a Difficult Problem. Raja M Flores, MD Thoracic Surgery Memorial Sloan-Kettering Cancer Center
 Malignant Mesothelioma Current Approaches to a Difficult Problem Raja M Flores, MD Thoracic Surgery Memorial Sloan-Kettering Cancer Center Malignant Pleural Mesothelioma Clinical Presentation Insidious
Malignant Mesothelioma Current Approaches to a Difficult Problem Raja M Flores, MD Thoracic Surgery Memorial Sloan-Kettering Cancer Center Malignant Pleural Mesothelioma Clinical Presentation Insidious
The Need for Accurate Lung Cancer Staging
 The Need for Accurate Lung Cancer Staging Peter Baik, DO Thoracic Surgery Cancer Treatment Centers of America Oklahoma Osteopathic Association 115th Annual Convention Financial Disclosures: None 2 Objectives
The Need for Accurate Lung Cancer Staging Peter Baik, DO Thoracic Surgery Cancer Treatment Centers of America Oklahoma Osteopathic Association 115th Annual Convention Financial Disclosures: None 2 Objectives
Key findings about the quality of care for people with Lung Cancer in England incorporating headline and completeness data from Wales
 National Lung Cancer Audit Key findings about the quality of care for people with Lung Cancer in England incorporating headline and completeness data from Wales Report for the audit period 2005 Prepared
National Lung Cancer Audit Key findings about the quality of care for people with Lung Cancer in England incorporating headline and completeness data from Wales Report for the audit period 2005 Prepared
Understanding Lung Cancer
 Lung Understanding Lung Cancer Information for individuals with lung cancer and their families Your Primary Team at the JCC Medical Oncologist Primary Nurse Radiation Oncologist Primary Nurse Other Oncology
Lung Understanding Lung Cancer Information for individuals with lung cancer and their families Your Primary Team at the JCC Medical Oncologist Primary Nurse Radiation Oncologist Primary Nurse Other Oncology
Mesothelioma. 1995-2013, The Patient Education Institute, Inc. www.x-plain.com ocft0101 Last reviewed: 03/21/2013 1
 Mesothelioma Introduction Mesothelioma is a type of cancer. It starts in the tissue that lines your lungs, stomach, heart, and other organs. This tissue is called mesothelium. Most people who get this
Mesothelioma Introduction Mesothelioma is a type of cancer. It starts in the tissue that lines your lungs, stomach, heart, and other organs. This tissue is called mesothelium. Most people who get this
Guideline for the Referral, Diagnosis and Staging of Patients with Lung Cancer
 Guideline for the Referral, Diagnosis and Staging of Patients with Lung Cancer Incorporating: Guideline for the diagnosis and staging of patients with lung cancer detected within secondary care, guidelines
Guideline for the Referral, Diagnosis and Staging of Patients with Lung Cancer Incorporating: Guideline for the diagnosis and staging of patients with lung cancer detected within secondary care, guidelines
Understanding Metastatic Disease
 Supported by an unrestricted educational grant from Pfizer Understanding Metastatic Disease Metastatic disease or metastases are phrases that mean the same as Secondary cancer. This means that the cancer
Supported by an unrestricted educational grant from Pfizer Understanding Metastatic Disease Metastatic disease or metastases are phrases that mean the same as Secondary cancer. This means that the cancer
