Dermovate Cream / Dermovate Ointment
|
|
|
- Jeffry Hunter
- 7 years ago
- Views:
Transcription
1 The format of this leaflet as determined by the Ministry of Health and its content was checked and approved in August 2007 Dermovate Cream / Dermovate Ointment TITLE Clobetasol propionate SCOPE Trade Names DERMOVATE OINTMENT DERMOVATE CREAM Formulation and Strength Clobetasol propionate 0.05 % w/w Excipients DERMOVATE OINTMENT Propylene Glycol Sorbitan Sesquioleate White Soft Paraffin DERMOVATE CREAM Cetostearyl Alcohol Glyceryl Monostearate Arlacel 165 Beeswax substitute 6621 Propylene Glycol Chlorocresol Sodium citrate Citric Acid Monohydrate Purified Water CLINICAL INFORMATION Indications Resistant Dermatoses responsive to potent corticosteroid therapy Dosage and Administration Apply sparingly to the affected area once or twice daily. Therapy should be discontinued when control is achieved. Treatment should not be continued for more than four weeks without the patients condition being Page 1 of 5
2 reviewed. Repeated short courses of Dermovate may be used to control exacerbations. If continuous steroid treatment is necessary, a less potent preparation should be used. In very resistant lesions, especially where there is hyperkeratosis, the anti-inflammatory effect of Dermovate can be enhanced, if necessary, by occluding the treatment area with polythene film. Overnight occlusion only is usually adequate to bring about a satisfactory response. Thereafter improvement can usually be maintained by application without occlusion. Contraindications - Hypersensitivity to the preparation. - Rosacea. - Acne vulgaris. - Perioral dermatitis. - Perianal and genital pruritus. - Primary cutaneous viral infections (e.g., herpes simplex, chickenpox). - The use of Dermovate skin preparations is not indicated in the treatment of primary infected skin lesions caused by infection with fungi or bacteria; dermatoses in children under one year of age, including dermatitis and napkin eruptions. Warnings and Precautions Topical steroids may be hazardous in psoriasis for a number of reasons including rebound relapses, development of tolerance, risk of generalised pustular psoriasis and development of local or systemic toxicity due to impaired barrier function of the skin. If used in psoriasis careful patient supervision is important. Appropriate antimicrobial therapy should be used whenever treating inflammatory lesions which have become infected. Any spread of infection requires withdrawal of topical corticosteroid therapy and systemic administration of antimicrobial agents. Bacterial infection is encouraged by the warm, moist conditions induced by occlusive dressings, and the skin should be cleansed before a fresh dressing is applied. Long-term continuous therapy should be avoided where possible, particularly in infants and children, as adrenal suppression can occur even without occlusion. If Dermovate is required for use in children, it is recommended that the treatment should be reviewed weekly. It should be noted that the infant's napkin may act as an occlusive dressing. The face, more than other areas of the body, may exhibit atrophic changes after prolonged treatment with potent topical corticosteroids. This must be borne in mind when treating such conditions as psoriasis, discoid lupus erythematosus and severe eczema. If applied to the eyelids, care is needed to ensure that the preparation does not enter the eye, as glaucoma might result. Interactions None reported. Page 2 of 5
3 Pregnancy and Lactation Pregnancy Topical administration of corticosteroids to pregnant animals can cause abnormalities of foetal development. The relevance of this finding to human beings has not been established, however, topical steroids should not be used extensively in pregnancy, i.e., in large amounts for prolonged periods. Lactation The safe use of clobetasol propionate during lactation has not been established. Ability to perform tasks that require judgement, motor or cognitive skills Clobetasol propionate is not expected to have any effects. Adverse Reactions Immune system disorders Very rare: Hypersensitivity Local hypersensitivity reactions such as erythema, rash, pruritus, urticaria, local skin burning and allergic contact dermatitis may occur at the site of application and may resemble symptoms of the condition under treatment. If signs of hypersensitivity appear, application should be stopped immediately. Endocrine disorders Very rare: Features of Hypercortisolism As with other topical corticosteroids, prolonged use of large amounts, or treatment of extensive areas can result in sufficient systemic absorption to produce the features of hypercortisolism. This effect is more likely to occur in infants and children, and if occlusive dressings are used. In infants, the napkin may act as an occlusive dressing. Provided the weekly dosage is less than 50g in adults, any suppression of the HPA axis is likely to be transient with a rapid return to normal values once the short course of steroid therapy has ceased. Vascular disorders Uncommon: Dilatation of the superficial blood vessels Prolonged and intensive treatment with highly-active corticosteroid preparations may cause dilatation of the superficial blood vessels, particularly when occlusive dressings are used, or when skin folds are involved. Skin and subcutaneous tissue disorders Uncommon: Local atrophy, striae Very rare: Thinning, pigmentation changes, hypertrichosis, exacerbation of underlying symptoms, pustular psoriasis. Prolonged and intensive treatment with highly-active corticosteroid preparations may cause local atrophic changes, such as thinning and striae (see also Vascular Disorders MedDRA System Organ Class subheading), particularly when occlusive dressings are used, or when skin folds are involved. In very rare instances, treatment of psoriasis with corticosteroids (or its withdrawal) is thought to have provoked the pustular form of the disease. Page 3 of 5
4 Overdosage Symptoms and Signs Acute overdosage is very unlikely to occur, however, in the case of chronic overdosage or misuse the features of hypercortisolism may appear. Treatment In this situation topical steroids should be reduced or discontinued gradually under medical supervision because of the risk of adrenal insufficiency. CLINICAL PHARMACOLOGY Pharmacodynamics Mechanism of Action The major effect of clobetasol propionate on skin is a non-specific anti-inflammatory response, as a result of vasoconstriction and decrease in collagen synthesis. Pharmacokinetics Absorption Percutaneous penetration of clobetasol propionate varies among individuals and can be increased by the use of occlusive dressings, or when the skin is inflamed or diseased. Distribution Mean peak plasma clobetasol propionate concentrations of 0.63 ng/ml occurred in one study eight hours after the second application (13 hours after an initial application) of 30 g clobetasol propionate 0.05 % ointment to normal individuals with healthy skin. Following the application of a second dose of 30 g clobetasol propionate cream 0.05 % mean peak plasma concentrations were slightly higher than the ointment and occurred 10 hours after application. In a separate study, mean peak plasma concentrations of approximately 2.3 ng/ml and 4.6 ng/ml occurred respectively in patients with psoriasis and eczema three hours after a single application of 25 g clobetasol propionate 0.05 % ointment. Metabolism Following percutaneous absorption of clobetasol propionate the drug probably follows the metabolic pathway of systemically administered corticosteroids. However, systemic metabolism of clobetasol has never been fully characterised or quantified. NON-CLINICAL INFORMATION See Pregnancy and Lactation. Page 4 of 5
5 PHARMACEUTICAL INFORMATION Shelf Life 2 years. Storage Store below 30 C. Nature and Contents of Container Cream: Clobetasol propionate Cream is packed in collapsible aluminium tubes, internally coated with epoxy-resin lacquer, with a polypropylene closure. Ointment: Clobetasol propionate Ointment is packed in collapsible aluminium tubes, either internally coated with epoxy-resin lacquer or uncoated, with a polypropylene closure. Incompatibilities None reported. Use and Handling Patients should be advised to wash their hands after applying clobetasol propionate, unless it is the hands that are being treated. Cream: Do not dilute clobetasol propionate Cream. Ointment: No special instructions. Manufacturer: License Holder: Glaxo Operations UK Ltd.Barnard Castle, Durham, UK GLAXOSMITH KLINE (ISRAEL) LTD 25 Basel St., Petach-Tikva License Number: DERMOVATE OINTMENT DERMOVATE CREAM Page 5 of 5
NEW ZEALAND DATA SHEET BETNOVATE -C CREAM and OINTMENT
 NEW ZEALAND DATA SHEET BETNOVATE -C CREAM and OINTMENT Name of each active ingredient Betamethasone valerate 0.1% w/w and clioquinol 3% w/w Presentations BETNOVATE-C Cream is a smooth, straw-coloured water-miscible
NEW ZEALAND DATA SHEET BETNOVATE -C CREAM and OINTMENT Name of each active ingredient Betamethasone valerate 0.1% w/w and clioquinol 3% w/w Presentations BETNOVATE-C Cream is a smooth, straw-coloured water-miscible
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
1g cream or ointment contains 1 mg methylprednisolone aceponate.
 CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement
 Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
Betnovate -C 0.1% / 3% w/w Cream Betamethasone (as valerate) and Clioquinol
 [GSK Logo] Package Leaflet: Information for the User Betnovate -C 0.1% / 3% w/w Cream Betamethasone (as valerate) and Clioquinol Read all of this leaflet carefully before you start using this medicine
[GSK Logo] Package Leaflet: Information for the User Betnovate -C 0.1% / 3% w/w Cream Betamethasone (as valerate) and Clioquinol Read all of this leaflet carefully before you start using this medicine
NERIDERM Cream, Ointment, Fatty Ointment
 PRESCRIBING INFORMATION NERIDERM Cream, Ointment, Fatty Ointment Qualitative and Quantitive Composition 1 g Neriderm contains 1 mg (0.1%) diflucortolone valerate. Pharmaceutical Form Cream, Ointment, Fatty
PRESCRIBING INFORMATION NERIDERM Cream, Ointment, Fatty Ointment Qualitative and Quantitive Composition 1 g Neriderm contains 1 mg (0.1%) diflucortolone valerate. Pharmaceutical Form Cream, Ointment, Fatty
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
For Mild to Moderate Plaque Psoriasis and Moderate to Severe Atopic Dermatitis
 For Mild to Moderate Plaque Psoriasis and Moderate to Severe Atopic Dermatitis OLUX-E (clobetasol propionate) Foam, 0.05% Please see Important Safety Information on back page and accompanying Full Prescribing
For Mild to Moderate Plaque Psoriasis and Moderate to Severe Atopic Dermatitis OLUX-E (clobetasol propionate) Foam, 0.05% Please see Important Safety Information on back page and accompanying Full Prescribing
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
PRODUCT MONOGRAPH. HydroVal. (Hydrocortisone Valerate USP) Cream & Ointment 0.2 % Topical Corticosteroid
 PRODUCT MONOGRAPH HydroVal (Hydrocortisone Valerate USP) Cream & Ointment 0.2 % Topical Corticosteroid TaroPharma Preparation Date: A Division of Taro Pharmaceuticals Inc. September 02, 2003 130 East Drive
PRODUCT MONOGRAPH HydroVal (Hydrocortisone Valerate USP) Cream & Ointment 0.2 % Topical Corticosteroid TaroPharma Preparation Date: A Division of Taro Pharmaceuticals Inc. September 02, 2003 130 East Drive
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel
 Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
PACKAGE LEAFLET: INFORMATION FOR THE USER. Elidel 10 mg/g Cream. pimecrolimus
 PACKAGE LEAFLET: INFORMATION FOR THE USER Elidel 10 mg/g Cream pimecrolimus Read all of this leaflet carefully before you start using Elidel cream Keep this leaflet. You may need to read it again. If you
PACKAGE LEAFLET: INFORMATION FOR THE USER Elidel 10 mg/g Cream pimecrolimus Read all of this leaflet carefully before you start using Elidel cream Keep this leaflet. You may need to read it again. If you
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013.
 The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
PRODUCT INFORMATION. Silver sulfadiazine is a sulfonamide and has broad antimicrobial activity against both Grampositive and Gram-negative organisms.
 PRODUCT INFORMATION Name of the Medicine: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
PRODUCT INFORMATION Name of the Medicine: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
Public Assessment Report. Pharmacy to General Sales List Reclassification. Pirinase Hayfever Relief for Adults 0.05% Nasal Spray.
 Public Assessment Report Pharmacy to General Sales List Reclassification Pirinase Hayfever Relief for Adults 0.05% Nasal Spray (Fluticasone) PL 00079/0688 Glaxo Wellcome UK Limited TABLE OF CONTENTS Introduction
Public Assessment Report Pharmacy to General Sales List Reclassification Pirinase Hayfever Relief for Adults 0.05% Nasal Spray (Fluticasone) PL 00079/0688 Glaxo Wellcome UK Limited TABLE OF CONTENTS Introduction
Hydrozole Cream Hydrocortisone (microfine) 1% w/w and Clotrimazole 1% w/w
 CONSUMER MEDICINE INFORMATION What is in this leaflet? This leaflet answers some common questions about Hydrozole It does not contain all the available information. It does not take the place of talking
CONSUMER MEDICINE INFORMATION What is in this leaflet? This leaflet answers some common questions about Hydrozole It does not contain all the available information. It does not take the place of talking
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate.
 NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
FLAMAZINE CREAM 1.0% w/w
 PRODUCT INFORMATION NAME OF THE MEDICINE: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
PRODUCT INFORMATION NAME OF THE MEDICINE: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel)
 ISOTREX Gel Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel) Excipients Butylated hydroxytoluene Hydroxypropylcellulose Ethanol CLINICAL INFORMATION Indications
ISOTREX Gel Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel) Excipients Butylated hydroxytoluene Hydroxypropylcellulose Ethanol CLINICAL INFORMATION Indications
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Plus 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 100 g of solution contains: Erythromycin 4.0 g, Tretinoin 0.025 g. For a full
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Plus 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 100 g of solution contains: Erythromycin 4.0 g, Tretinoin 0.025 g. For a full
3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP
 PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
TOPICAL THERAPY. ADVANTAGES - increased dose of medication to affected area. - reduced systemic side effects and toxicity
 TOPICAL THERAPY ADVANTAGES - increased dose of medication to affected area. - reduced systemic side effects and toxicity DISADVANTAGES - takes time - may be greasy or messy - may have different preparations
TOPICAL THERAPY ADVANTAGES - increased dose of medication to affected area. - reduced systemic side effects and toxicity DISADVANTAGES - takes time - may be greasy or messy - may have different preparations
Mucodyne-Clear 250 mg/5 ml Syrup PL 04425/0665
 Mucodyne-Clear 250 mg/5 ml Syrup PL 04425/0665 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
Mucodyne-Clear 250 mg/5 ml Syrup PL 04425/0665 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
D.RIGOPOULOS, D.IOANNIDES,* D.KALOGEROMITROS, S.GREGORIOU AND A.KATSAMBAS
 British Journal of Dermatology 24; 151: 171 175. DOI: 1.1111/j.1365-2133.24.628.x Therapeutics Pimecrolimus cream 1% vs. betamethasone 17-valerate Æ1% cream in the treatment of seborrhoeic dermatitis.
British Journal of Dermatology 24; 151: 171 175. DOI: 1.1111/j.1365-2133.24.628.x Therapeutics Pimecrolimus cream 1% vs. betamethasone 17-valerate Æ1% cream in the treatment of seborrhoeic dermatitis.
ALLERGENIC EXTRACT. Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE. U.S. Government License No.
 ALLERGENIC EXTRACT Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE U.S. Government License No. 308 Revised 07/04 PO Box 800 Lenoir, NC 28645 USA DESCRIPTION This set
ALLERGENIC EXTRACT Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE U.S. Government License No. 308 Revised 07/04 PO Box 800 Lenoir, NC 28645 USA DESCRIPTION This set
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
Foam: A Unique Delivery Vehicle for Topically Applied Formulations
 : A Unique Delivery Vehicle for Topically Applied Formulations Dov Tamarkin, PhD ix Ltd. Key Words:, Emulsion, Lipophilic Emulsion, Nanoemulsion, Aqueous, Hydroethanolic, Potent-Solvent, Suspension, Ointment,
: A Unique Delivery Vehicle for Topically Applied Formulations Dov Tamarkin, PhD ix Ltd. Key Words:, Emulsion, Lipophilic Emulsion, Nanoemulsion, Aqueous, Hydroethanolic, Potent-Solvent, Suspension, Ointment,
Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006
 Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd.
 RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd. Table of Contents 1. Name of the product...2 2. Qualitative and quantitative composition...2
RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd. Table of Contents 1. Name of the product...2 2. Qualitative and quantitative composition...2
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
A t f e t r e r th t is s lec e t c u t re r e t h t e e st s u t den e t t sh s ould b e e a b a le e t o t :
 Dermatopharmacology Prof Werner Sinclair Department of Dermatology University of the Free State Outcomes for this Lecture After this lecture the student should be able to: Name the most important characteristics
Dermatopharmacology Prof Werner Sinclair Department of Dermatology University of the Free State Outcomes for this Lecture After this lecture the student should be able to: Name the most important characteristics
Leukocytoclastic Vasculitis and Stasis Dermatitis With Id Reaction
 Id Reaction December 01, 2007 By David L. Kaplan, MD [1] A Photo Quiz to Hone Dermatologic Skills Case 1: A slightly pruritic eruption developed on the lower legs of a 39-year-old woman after she had an
Id Reaction December 01, 2007 By David L. Kaplan, MD [1] A Photo Quiz to Hone Dermatologic Skills Case 1: A slightly pruritic eruption developed on the lower legs of a 39-year-old woman after she had an
White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other.
 Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
PACKAGE LEAFLET: INFORMATION FOR THE USER. VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution. Cyanocobalamin
 PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Eskazole Tablets 400 mg 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albendazole 400 mg Excipients with known effect: Lactose, sunset yellow
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Eskazole Tablets 400 mg 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albendazole 400 mg Excipients with known effect: Lactose, sunset yellow
NEUROTONE THR 00904/0005 UKPAR
 NEUROTONE THR 00904/0005 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Product Information Leaflet
NEUROTONE THR 00904/0005 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Product Information Leaflet
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in September 2008 HAVRIX 1440
 The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in September 2008 HAVRIX 1440 HAVRIX 720 Junior Monodose TITLE Hepatitis A (inactivated) vaccine
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in September 2008 HAVRIX 1440 HAVRIX 720 Junior Monodose TITLE Hepatitis A (inactivated) vaccine
PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w
 NAME OF THE MEDICINE Stieva-A Cream PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w DESCRIPTION Chemical names: 3, 7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexene-1-yl)-2,4,6,8-non-tetraenoic
NAME OF THE MEDICINE Stieva-A Cream PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w DESCRIPTION Chemical names: 3, 7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexene-1-yl)-2,4,6,8-non-tetraenoic
The clinical studies have been performed in children, adolescents and adults, from 4 years up to 55 years of age.
 NAME OF THE MEDICINE TdaP-Booster. Diphtheria, tetanus and pertussis (acellular mono-component) vaccine (adsorbed, reduced antigen content). DESCRIPTION TdaP-Booster is a suspension for injection in pre-filled
NAME OF THE MEDICINE TdaP-Booster. Diphtheria, tetanus and pertussis (acellular mono-component) vaccine (adsorbed, reduced antigen content). DESCRIPTION TdaP-Booster is a suspension for injection in pre-filled
I B2.4. Design of the patient information leaflet for VariQuin
 (English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
(English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
X-Plain Psoriasis Reference Summary
 X-Plain Psoriasis Reference Summary Introduction Psoriasis is a long-lasting skin disease that causes the skin to become inflamed. Patches of thick, red skin are covered with silvery scales. It affects
X-Plain Psoriasis Reference Summary Introduction Psoriasis is a long-lasting skin disease that causes the skin to become inflamed. Patches of thick, red skin are covered with silvery scales. It affects
CONTRAINDICATIONS. None (4).
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Taclonex Ointment safely and effectively. See Full Prescribing Information for Taclonex Ointment.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Taclonex Ointment safely and effectively. See Full Prescribing Information for Taclonex Ointment.
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension
 VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
Professor Andrew Wright,
 Professor Andrew Wright, Systemic treatments are drugs taken as tablets or injections that travel through the bloodstream, dampening down the immune system to reach and treat eczema all over the body.
Professor Andrew Wright, Systemic treatments are drugs taken as tablets or injections that travel through the bloodstream, dampening down the immune system to reach and treat eczema all over the body.
Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid
 Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
See 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2016 FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK OR
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
Beclometasone Dipropionate 50 micrograms per spray Aqueous Nasal Spray PL 16431/0178
 Beclometasone Dipropionate 50 micrograms per spray Aqueous Nasal Spray PL 16431/0178 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 5 Steps Taken for Assessment Page 11 Steps Taken
Beclometasone Dipropionate 50 micrograms per spray Aqueous Nasal Spray PL 16431/0178 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 5 Steps Taken for Assessment Page 11 Steps Taken
PRODUCT INFORMATION OINTMENT, CREAM & LOTION
 PRDUCT INFRMATIN ADVANTAN INTMENT, FATTY INTMENT, CREAM & LTIN NAME F THE DRUG The chemical name is 21-acetoxy-11β-hydroxy-6α-methyl-17-propionyloxy-1, 4- pregnadiene-3, 20-dione. The chemical structure
PRDUCT INFRMATIN ADVANTAN INTMENT, FATTY INTMENT, CREAM & LTIN NAME F THE DRUG The chemical name is 21-acetoxy-11β-hydroxy-6α-methyl-17-propionyloxy-1, 4- pregnadiene-3, 20-dione. The chemical structure
160S01105, Page 1 of 7. Human Hepatitis B Immunoglobulin, solution for intramuscular injection.
 160S01105, Page 1 of 7 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF
160S01105, Page 1 of 7 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF
VOLTAREN OPHTHA EYE DROPS
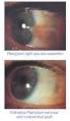 פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
skin and soft tissue infections (skinfold pyoderma, impetigo, folliculitis, furunculosis, cellulitis) caused by susceptible strains of organisms.
 Part II SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL P 5 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredient Marbofloxacin 5mg per tablet
Part II SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL P 5 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredient Marbofloxacin 5mg per tablet
FLAMAZINE CREAM MSDS No. 185
 SECTION 1 IDENTIFICATION OF THE MATERIAL AND SUPPLIER Product Product Name FLAMAZINE CREAM Other Names - Manufacturer s Product Code 66800531, 66800532, 66150153, 66150155 Use Topical antibacterial cream,
SECTION 1 IDENTIFICATION OF THE MATERIAL AND SUPPLIER Product Product Name FLAMAZINE CREAM Other Names - Manufacturer s Product Code 66800531, 66800532, 66150153, 66150155 Use Topical antibacterial cream,
SUMMARY OF PRODUCT CHARACTERISTICS
 Registration No. : 2C 22/47 (N) Importer / Manufacturer: Sanofi Pasteur Ltd., Thailand/Sanofi Pasteur S.A., France SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICAL PRODUCT : PENTAXIM (Diphtheria,
Registration No. : 2C 22/47 (N) Importer / Manufacturer: Sanofi Pasteur Ltd., Thailand/Sanofi Pasteur S.A., France SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICAL PRODUCT : PENTAXIM (Diphtheria,
Dechra Veterinary Products Limited (A business unit of Dechra Pharmaceuticals PLC) Sansaw Business Park Hadnall, Shrewsbury Shropshire SY4 4AS
 Dechra Veterinary Products Limited (A business unit of Dechra Pharmaceuticals PLC) Sansaw Business Park Hadnall, Shrewsbury Shropshire SY4 4AS Tel: 01939 211200 Fax: 01939 211201 Email: info.uk@dechra.com
Dechra Veterinary Products Limited (A business unit of Dechra Pharmaceuticals PLC) Sansaw Business Park Hadnall, Shrewsbury Shropshire SY4 4AS Tel: 01939 211200 Fax: 01939 211201 Email: info.uk@dechra.com
Felimazole 5 mg Coated Tablet
 .. CARTON TEXT - FRONT PANEL PRESCRIPTION ANIMAL REMEDY READ SAFETY DIRECTIONS BEFORE OPENING OR USING KEEP OUT OF REACH OF CHILDREN FOR ANIMAL TREATMENT ONLY RLP Approved Felimazole 5 mg Coated Tablet
.. CARTON TEXT - FRONT PANEL PRESCRIPTION ANIMAL REMEDY READ SAFETY DIRECTIONS BEFORE OPENING OR USING KEEP OUT OF REACH OF CHILDREN FOR ANIMAL TREATMENT ONLY RLP Approved Felimazole 5 mg Coated Tablet
SUMMARY OF PRODUCT CHARACTERISTICS. Albuman 200 g/l is a solution containing 200 g/l (20%) of total protein of which at least 95% is human albumin.
 Albuman 200 g/l SPC 01 December 2015 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 200 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 200 g/l
Albuman 200 g/l SPC 01 December 2015 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 200 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 200 g/l
FUCIBET LIPID CREAM (FUSIDIC ACID 2%, BETAMETHASONE 0.1%) PL 00043/0218 UKPAR TABLE OF CONTENTS
 FUCIBET LIPID CREAM (FUSIDIC ACID 2%, BETAMETHASONE 0.1%) PL 00043/0218 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 23 Summary of Product Characteristics
FUCIBET LIPID CREAM (FUSIDIC ACID 2%, BETAMETHASONE 0.1%) PL 00043/0218 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 23 Summary of Product Characteristics
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON HYPERICUM PERFORATUM L., HERBA (TRADITIONAL USE)
 European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMEA/HMPC/745582/2009 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH
European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMEA/HMPC/745582/2009 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH
0.9% Sodium Chloride Injection, USP In VIAFLEX Plastic Container
 Page 1 of 8 PRESCRIBING INFORMATION 0.9% Sodium Chloride Injection, USP In VIAFLEX Plastic Container IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of
Page 1 of 8 PRESCRIBING INFORMATION 0.9% Sodium Chloride Injection, USP In VIAFLEX Plastic Container IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of
PHENYLEPHRINE HYDROCHLORIDE INJECTION USP
 PRESCRIBING INFORMATION PHENYLEPHRINE HYDROCHLORIDE INJECTION USP 10 mg/ml Sandoz Canada Inc. Date of Preparation: September 1992 145 Jules-Léger Date of Revision : January 13, 2011 Boucherville, QC, Canada
PRESCRIBING INFORMATION PHENYLEPHRINE HYDROCHLORIDE INJECTION USP 10 mg/ml Sandoz Canada Inc. Date of Preparation: September 1992 145 Jules-Léger Date of Revision : January 13, 2011 Boucherville, QC, Canada
There is a risk of renal impairment in dehydrated children and adolescents.
 PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
D E R M A T O L O G Y
 We customize individual prescriptions for the specific needs of our patients. J A N U A R Y 2 0 1 3 I N S I D E T H I S I S S U E : Warts 2 Melasma 3 P R E S C R I P T I O N C O M P O U N D I N G F O R
We customize individual prescriptions for the specific needs of our patients. J A N U A R Y 2 0 1 3 I N S I D E T H I S I S S U E : Warts 2 Melasma 3 P R E S C R I P T I O N C O M P O U N D I N G F O R
It can therefore be bought without a prescription but should be used correctly to ensure its efficacy and to reduce side effects.
 140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
P AC K AG E L E AF L E T: INFORMAT I ON FO R THE USER. 500 mg, film-coated tablet Active substance: metformin hydrochloride
 P AC K AG E L E AF L E T: INFORMAT I ON FO R THE USER Siofor 500 500 mg, film-coated tablet Active substance: metformin hydrochloride For use in children above 10 years and adults Read all of this leaflet
P AC K AG E L E AF L E T: INFORMAT I ON FO R THE USER Siofor 500 500 mg, film-coated tablet Active substance: metformin hydrochloride For use in children above 10 years and adults Read all of this leaflet
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]
![READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]](/thumbs/35/17235501.jpg) READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
MEDICATION GUIDE. PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1%
![MEDICATION GUIDE. PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1% MEDICATION GUIDE. PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1%](/thumbs/27/10084771.jpg) MEDICATION GUIDE PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1% Read the Medication Guide every time you or a family member gets PROTOPIC Ointment. There may be new information. This Medication
MEDICATION GUIDE PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1% Read the Medication Guide every time you or a family member gets PROTOPIC Ointment. There may be new information. This Medication
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT ACEGON, 50 microgram/ml, solution for injection for cattle. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT ACEGON, 50 microgram/ml, solution for injection for cattle. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active
Salbutamol 1mg/ml Nebuliser Solution. Salbutamol 2mg/ml Nebuliser Solution PL 36390/0035 PL 36390/0036
 Salbutamol 1mg/ml Nebuliser Solution Salbutamol 2mg/ml Nebuliser Solution PL 36390/0035 PL 36390/0036 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment
Salbutamol 1mg/ml Nebuliser Solution Salbutamol 2mg/ml Nebuliser Solution PL 36390/0035 PL 36390/0036 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment
Neutral Superoxidized solution vs. benzoyl peroxide gel 5% in the treatment of. Superoxidized solution (SOS) is an electrochemically processed aqueous
 Neutral Superoxidized solution vs. benzoyl peroxide gel 5% in the treatment of acne vulgaris: a randomized open-label clinical trial. Summary. Superoxidized solution (SOS) is an electrochemically processed
Neutral Superoxidized solution vs. benzoyl peroxide gel 5% in the treatment of acne vulgaris: a randomized open-label clinical trial. Summary. Superoxidized solution (SOS) is an electrochemically processed
SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product)
 PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
4 Clinical Particulars
 SUMMARY OF PRODUCT CHARACTERISTICS 1 Name of the Medicinal Product Procyclidine Syrup 5mg/5ml 2. Qualitative and Quantitative Composition Each 5ml dose contains 5mg Procyclidine Hydrochloride BP. 3. Pharmaceutical
SUMMARY OF PRODUCT CHARACTERISTICS 1 Name of the Medicinal Product Procyclidine Syrup 5mg/5ml 2. Qualitative and Quantitative Composition Each 5ml dose contains 5mg Procyclidine Hydrochloride BP. 3. Pharmaceutical
CHLORHEXIDINE ACETATE WITH CETRIMIDE (%) ANTISEPTIC SOLUTION
 CHLORHEXIDINE ACETATE WITH CETRIMIDE (%) ANTISEPTIC SOLUTION NAME OF THE MEDICINE Proprietary name: Non-proprietary name: Chemical Name: Molecular formula: Baxter Chlorhexidine Cetrimide Antiseptic Solution
CHLORHEXIDINE ACETATE WITH CETRIMIDE (%) ANTISEPTIC SOLUTION NAME OF THE MEDICINE Proprietary name: Non-proprietary name: Chemical Name: Molecular formula: Baxter Chlorhexidine Cetrimide Antiseptic Solution
PowerLight LED Light Therapy. The FUTURE of corrective skin
 PowerLight LED Light Therapy The FUTURE of corrective skin care TODAY LED facial treatments Effective when used with correct protocols Non thermal stimulation of collagen Increases circulation and lymphatic
PowerLight LED Light Therapy The FUTURE of corrective skin care TODAY LED facial treatments Effective when used with correct protocols Non thermal stimulation of collagen Increases circulation and lymphatic
1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT POLLINEX Trees Extension Course, 2000 Standardised Units (SU)/0.5ml, suspension for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT POLLINEX Trees Extension Course, 2000 Standardised Units (SU)/0.5ml, suspension for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Dalacin C 150 mg Hard Capsules Clindamycin (as clindamycin hydrochloride)
 Package leaflet: Information for the patient Dalacin C 150 mg Hard Capsules Clindamycin (as clindamycin hydrochloride) Read all of this leaflet carefully before you start taking this medicine because it
Package leaflet: Information for the patient Dalacin C 150 mg Hard Capsules Clindamycin (as clindamycin hydrochloride) Read all of this leaflet carefully before you start taking this medicine because it
PACKAGE LEAFLET. CLINDAMYCIN capsules Clidamycin. One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride).
 PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you.
 leaflet: Information for the user Macrogol 4000 10 g powder for oral solution in sachet Macrogol 4000
leaflet: Information for the user Macrogol 4000 10 g powder for oral solution in sachet Macrogol 4000
Department of Dermatology, Churchill Hospital PUVA Treatment
 Oxford University Hospitals NHS Trust Department of Dermatology, Churchill Hospital PUVA Treatment information for patients CONTENTS What is PUVA 3 What conditions are treated with PUVA? 3 How is PUVA
Oxford University Hospitals NHS Trust Department of Dermatology, Churchill Hospital PUVA Treatment information for patients CONTENTS What is PUVA 3 What conditions are treated with PUVA? 3 How is PUVA
Public Assessment Report Scientific discussion. Tostrex (Testosterone) SE/H/571/01
 Public Assessment Report Scientific discussion Tostrex (Testosterone) SE/H/571/01 This module reflects the scientific discussion for the approval of Tostrex. The procedure was finalised at 2006-04-07.
Public Assessment Report Scientific discussion Tostrex (Testosterone) SE/H/571/01 This module reflects the scientific discussion for the approval of Tostrex. The procedure was finalised at 2006-04-07.
Results of the first clinical study on a new treatment for Psori asis: the T.M.S.T. method
 MINERAL MOR LTD Results of the first clinical study on a new treatment for Psori asis: the TMST method Marco Harari MD 12122012 Conclusions: 1 No side effects were recorded during the 5week study period
MINERAL MOR LTD Results of the first clinical study on a new treatment for Psori asis: the TMST method Marco Harari MD 12122012 Conclusions: 1 No side effects were recorded during the 5week study period
Public Assessment Report. Decentralised Procedure. Cefuroxime 250mg and 500mg film-coated tablets. Cefuroxime 500mg film-coated tablets
 Public Assessment Report Decentralised Procedure Cefuroxime 250mg film-coated tablets Cefuroxime 500mg film-coated tablets Procedure No: UK Licence No: PL 35646/0020-0021 Alkem Pharma GmbH 1 LAY SUMMARY
Public Assessment Report Decentralised Procedure Cefuroxime 250mg film-coated tablets Cefuroxime 500mg film-coated tablets Procedure No: UK Licence No: PL 35646/0020-0021 Alkem Pharma GmbH 1 LAY SUMMARY
Treating your skin condition with narrowband ultraviolet B radiation (NB-UVB)
 Treating your skin condition with narrowband ultraviolet B radiation (NB-UVB) Your doctor has referred you to the Dowling Day Treatment Centre for a course of narrow band ultraviolet treatment for your
Treating your skin condition with narrowband ultraviolet B radiation (NB-UVB) Your doctor has referred you to the Dowling Day Treatment Centre for a course of narrow band ultraviolet treatment for your
Stepping toward a different treatment option LEARN WHAT ACTHAR CAN DO FOR YOU
 FOR MS RELAPSES Stepping toward a different treatment option LEARN WHAT ACTHAR CAN DO FOR YOU As a person with multiple sclerosis (MS), you know firsthand the profound impact MS relapses can have on your
FOR MS RELAPSES Stepping toward a different treatment option LEARN WHAT ACTHAR CAN DO FOR YOU As a person with multiple sclerosis (MS), you know firsthand the profound impact MS relapses can have on your
Agencia Española de Medicamentos y Productos Sanitarios C/Campezo 1, Edificio 8 28022 Madrid España (Reference Member State)
 DEPARTAMENTO DE MEDICAMENTOS VETERINARIOS Medicamentos y Productos C/Campezo 1, Edificio 8 28022 Madrid España (Reference Member State) DECENTRALISED PROCEDURE PUBLICLY AVAILABLE ASSESSMENT REPORT FOR
DEPARTAMENTO DE MEDICAMENTOS VETERINARIOS Medicamentos y Productos C/Campezo 1, Edificio 8 28022 Madrid España (Reference Member State) DECENTRALISED PROCEDURE PUBLICLY AVAILABLE ASSESSMENT REPORT FOR
Summary of the risk management plan (RMP) for Otezla (apremilast)
 EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
Importer / Manufacturer: MSD (THAILAND) LTD./ Merck & Co.,Inc., West Point, Pennsylvania 19486 SUMMARY OF PRODUCT CHARACTERISTICS
 Registration No. 1C 68/47(NC) Importer / Manufacturer: MSD (THAILAND) LTD./ Merck & Co.,Inc., West Point, Pennsylvania 19486 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICAL PRODUCT VARIVAX [Varicella
Registration No. 1C 68/47(NC) Importer / Manufacturer: MSD (THAILAND) LTD./ Merck & Co.,Inc., West Point, Pennsylvania 19486 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICAL PRODUCT VARIVAX [Varicella
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON AVENA SATIVA L., FRUCTUS
 European Medicines Agency Evaluation of Medicines for Human Use London, 4 September 2008 Doc. Ref. EMEA/HMPC/368600/2007 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
European Medicines Agency Evaluation of Medicines for Human Use London, 4 September 2008 Doc. Ref. EMEA/HMPC/368600/2007 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
Ascorbic Acid 50 mg Tablets. Ascorbic Acid 100 mg Tablets. Ascorbic Acid 200 mg Tablets. Ascorbic Acid 500 mg Tablets PL 20416/0286 PL 20416/0287
 Ascorbic Acid 50 mg Tablets Ascorbic Acid 100 mg Tablets Ascorbic Acid 200 mg Tablets Ascorbic Acid 500 mg Tablets PL 20416/0286 PL 20416/0287 PL 20416/0288 PL 20416/0289 UKPAR TABLE OF CONTENTS Lay Summary
Ascorbic Acid 50 mg Tablets Ascorbic Acid 100 mg Tablets Ascorbic Acid 200 mg Tablets Ascorbic Acid 500 mg Tablets PL 20416/0286 PL 20416/0287 PL 20416/0288 PL 20416/0289 UKPAR TABLE OF CONTENTS Lay Summary
ECZEMA: YOUR GP THE SECRETS WON T TELL YOU
 ECZEMA: THE SECRETS YOUR GP WON T TELL YOU As a sufferer of eczema or dry skin, it s likely that you ll understand the frustrations associated with trying various creams and lotions that are supposed to
ECZEMA: THE SECRETS YOUR GP WON T TELL YOU As a sufferer of eczema or dry skin, it s likely that you ll understand the frustrations associated with trying various creams and lotions that are supposed to
Sponsor Novartis. Generic Drug Name Secukinumab. Therapeutic Area of Trial Psoriasis. Approved Indication investigational
 Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Revised: 9/2014. LUMIGAN (bimatoprost ophthalmic solution) 0.01% for topical ophthalmic use. Initial U.S. Approval: 2001
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN (bimatoprost ophthalmic solution) 0.01% safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN (bimatoprost ophthalmic solution) 0.01% safely and effectively. See full prescribing information
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
VOLTAREN OPHTHA EYE DROPS
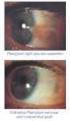 2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
Methotrexate Dose For Juvenile Rheumatoid Arthritis
 Methotrexate Dose For Juvenile Rheumatoid Arthritis should i take methotrexate for my ra methotrexate 50 mg/ml methotrexate sodium 2.5mg tablets what is the usual dosage of methotrexate for ra methotrexate
Methotrexate Dose For Juvenile Rheumatoid Arthritis should i take methotrexate for my ra methotrexate 50 mg/ml methotrexate sodium 2.5mg tablets what is the usual dosage of methotrexate for ra methotrexate
EBMT Education Day for Nurses and AHPs April 2012 Skin care: not every rash is GVHD
 EBMT Education Day for Nurses and AHPs April 2012 Skin care: not every rash is GVHD Eileen Parry Consultant Dermatologist Tameside Hospital Foundation Trust Overview How to assess a patient with a rash
EBMT Education Day for Nurses and AHPs April 2012 Skin care: not every rash is GVHD Eileen Parry Consultant Dermatologist Tameside Hospital Foundation Trust Overview How to assess a patient with a rash
1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT POLLINEX Trees, 300, 800 and 2000 Standardised Units (SU)/0.5ml, suspension for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION POLLINEX
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT POLLINEX Trees, 300, 800 and 2000 Standardised Units (SU)/0.5ml, suspension for injection 2 QUALITATIVE AND QUANTITATIVE COMPOSITION POLLINEX
Learn More About Product Labeling
 Learn More About Product Labeling Product label The product label is developed during the formal process of review and approval by regulatory agencies of any medicine or medical product. There are specific
Learn More About Product Labeling Product label The product label is developed during the formal process of review and approval by regulatory agencies of any medicine or medical product. There are specific
EFFIMET 1000 XR Metformin Hydrochloride extended release tablet
 BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
A guide for people with genital herpes
 A guide for people with genital herpes Contents Getting the facts 4 The key facts 6 What is genital herpes? 8 Genital herpes symptoms 10 Getting tested 12 The first outbreak 14 Recurrent outbreaks 16 Common
A guide for people with genital herpes Contents Getting the facts 4 The key facts 6 What is genital herpes? 8 Genital herpes symptoms 10 Getting tested 12 The first outbreak 14 Recurrent outbreaks 16 Common
