SUMMARY OF PRODUCT CHARACTERISTICS
|
|
|
- Steven Fields
- 9 years ago
- Views:
Transcription
1 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Plus 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 100 g of solution contains: Erythromycin 4.0 g, Tretinoin g. For a full list of excipients, see section PHARMACEUTICAL FORM Solution for application to the skin. Aknemycin Plus is a clear, light yellow solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications All forms of acne, both non-inflammatory forms with comedones and inflammatory forms with papules and pustules, particularly in the case of fat-rich skin. 4.2 Posology and method of administration For application to the skin. Unless otherwise prescribed, Aknemycin Plus is applied once or twice daily to the cleansed skin. Aknemycin Plus is applied directly from the special applicator bottle on to the skin. This enables the product to be applied simply, hygienically and sparingly. The applicator is constructed in such a way that no particles of dirt can be transferred from the skin to the solution. The duration of application depends on the condition of the skin, but should not exceed twelve weeks. Consistent application makes a significant contribution to the success of the therapy. 4.3 Contraindications Hypersensitivity to erythromycin and tretinoin or to any of the excipients. The preparation may not be used in acute eczemas, rosacea, acute inflammations of the skin (e.g., sunburn), particularly in the area of the mouth (perioral dermatitis). 4.4 Special warnings and precautions for use
2 Aknemycin Plus must not be allowed to come into contact with the eyes. Should this nevertheless occur, thorough rinsing with water is recommended. Aknemycin Plus should not be applied too close to the lips or nostrils. The hands should be thoroughly washed if Aknemycin Plus comes into contact with the fingers in spite of the use of the applicator. Do not light a cigarette or expose yourself to flame until the medicine has dried completely For use during pregnancy and the lactation period, see section Interaction with other medicinal products and other forms of interaction Photosensitivity may occur during therapy. The skin irritation described in the section "Undesirable effects" may be exacerbated by ultraviolet radiation (natural sunlight, sunlamps, solaria) and x-rays, as well as bathing in water containing chlorine or salts. This applies in particular to individuals who are exposed to sunlight for prolonged periods due to their profession, as well as to patients who are particularly sensitive to light. During therapy with Aknemycin Plus patients should avoid exposure to direct sunlight or other ultraviolet rays including sunlamps or sun beds. Any sunburn should be allowed to heal before the start of treatment with Aknemycin Plus. The simultaneous application of other skin preparations should be avoided, as this may exacerbate any skin irritation that is present. 4.6 Pregnancy and lactation For safety reasons Aknemycin Plus must not be used during the first three months of pregnancy. In order to avoid direct contact with the infant, Aknemycin Plus must not be used in the area of the breast during the lactation period. 4.7 Effects on ability to drive and use machines No particular precautions are required. 4.8 Undesirable effects The following frequency categories are used for the evaluation of undesirable effects: Very common ( 1/10) Common ( 1/100 to <1/10) Uncommon ( 1/1,000 to <1/100) Rare ( 1/10,000 to <1/1,000) Very rare (<1/10,000) Not known (frequency cannot be estimated from the available data) There may be rare cases of reduced pigmentation of the skin, as well as skin irritation in the form of reddening, burning, drying out and desquamation. In very rare cases the above symptoms may also be an expression of a hypersensitivity reaction (allergic contact eczema). At the beginning of treatment the acne may appear to worsen, with an increase in the number of inflammatory symptoms; this is a sign that the medicine is beginning to act and is mostly of a temporary nature. Treatment with Aknemycin Plus should therefore not be discontinued. However, the frequency of application can be reduced temporarily. 4.9 Overdose Page 2 of 6
3 No case of overdose has been reported. 5. PHARMACOLOGICAL PROPERTIES 5.1 Pharmacodynamic properties Pharmacotherapeutic group: Anti-acne preparations for topical use, anti-infectives, erythromycin in combination with tretinoin. ATC code: D10AF52 Erythromycin possesses a strong antibacterial effect with respect to Propionibacterium acnes, a skin germ to which a significant role in the pathogenesis of acne is attributed. Clinical trials have shown that after topical application erythromycin penetrates into the sebaceous gland ducts, where its bacteriostatic effect unfolds. The anti-microbial activity corresponds to that of penicillin, comprising gram-positive and some gram-negative germs. Erythromycin is reversibly bound to the 50 S subunit of the ribosomes of the bacteria, thus inducing inhibition of their protein biosynthesis. Restriction of the enzymatic activity of the bacterium inhibits lipolysis of the skin surface lipids, reducing the concentration of free fatty acids. Erythromycin results in clinical improvement to the acne efflorescences; the local antibiotic therapy is comparable in terms of its efficacy to the systemic administration of the antibiotic. Furthermore, erythromycin also has a direct anti-inflammatory effect. The alcoholic base of Aknemycin Plus supports the antibacterial effect of erythromycin, while at the same time removing the skin sebum. Tretinoin (vitamin A acid) is a deep-acting keratolytic agent. Tretinoin belongs to the group of retinoids and has a stimulating effect on the proliferation of the cornified cells of the skin. It binds to a cellular protein (CRABP) and in this way infiltrates the cell nucleus. The adhesiveness of the cornified cells formed under the influence of tretinoin is reduced and the development of cornified cell plugs in the acroinfundibulum of the follicle channel prevented. Tretinoin leads to comedolysis and a reduction in the size of the sebaceous glands. Open comedones are loosened and ejected, while closed comedones are transformed more rapidly into open ones and eliminated. During application with tretinoin the local effect is initially accompanied by irritation and an increase in the blood supply to the skin. Comedones that are not clinically visible are transformed into inflammatory ones and ejected, while persistent papules and nodes are removed more quickly. The skin irritation caused by tretinoin is largely attenuated by the combination with erythromycin. Aknemycin Plus is well tolerated by the skin; seborrhoeic skin conditions are normalised. In the hypersensitivity test according to Magnusson-Kligman there were no indications of hypersensitivity caused by Aknemycin Plus. 5.2 Pharmacokinetic properties After the topical application of erythromycin the substance cannot be detected in the serum; absorption therefore only occurs in quantities that are not detectable, if at all. Details on the absorption of tretinoin fluctuate between 0.5 % and a maximum of 24 %. The substance is rapidly degraded to polar metabolites and excreted via the intestines and kidneys; there is no storage in the liver. Systemic pharmacodynamic effects as a consequence of local treatment are not to be expected, particularly in view of the concentration used in the preparation. Page 3 of 6
4 5.3 Preclinical safety data With the exception of a hypersensitivity test (see Local tolerance), no additional toxicological studies have been performed on animals with Aknemycin Plus. Acute toxicity Numerous animal trials have shown that erythromycin is a substance with a low level of acute toxicity. Investigations into the acute toxicity of tretinoin in rats and mice produced LD 50 values of approx. 2.1 g/kg when the substance was administered orally and 0.8 g/kg in the case of intraperitoneal administration. Tretinoin therefore displays low levels of acute toxicity. Subchronic and chronic toxicity Investigations into the chronic toxicity of the substance when administered orally to two species of animal showed no changes caused by the substance. The toxic doses are approximately 2,000 20,000 times higher than the therapeutic quantities of the active substance administered to humans. See "Local tolerance". Mutagenic and carcinogenic potential Erythromycin has no mutagenic effect; there are no indications of carcinogenic activity. An in vitro analysis using several test systems did not show any indications of mutagenic properties for erythromycin. Feeding trials over a period of two years on rats and mice provided no indications of carcinogenic potential. Dose-dependent granulomas occurred in the livers of male and female rats. Tretinoin has so far only been tested on bacteria with respect to its mutagenic effects. These trials produced a negative result. Long-term investigations into any carcinogenic potential have not been carried out. The results of the photocarcinogenesis and cocarcinogenesis observed in animal experiments which are occasionally a subject of discussion cannot be transferred automatically to human skin. Local tolerance In the maximisation test performed on guinea pigs the combination preparation did not display any hypersensitivity of the skin. Erythromycin is well tolerated when applied locally. The substance does not have a photosensitising effect. The sensitisation potential of erythromycin is considered to be very low. After the topical application of tretinoin (0.05% gel) no systemic intolerance is to be expected. Reversible skin irritation may occur locally with erythema, oedema, the rejection of superficial layers and epithelium proliferation. Occasionally, dose-dependent lightening of the skin may Page 4 of 6
5 occur as a consequence of the inflammatory reaction; however, no reduction in pigment is to be expected with the concentrations that are normally applied. The rabbit eye showed no irritation after the local application of tretinoin; the mucous membranes reacted less sensitively than the normal cornified skin. Reproduction toxicity Fertility and teratogenicity studies do not provide any indication that erythromycin leads to reproductive disturbances or damage when applied locally to humans. In rats and rabbits to which the substance was administered topically in doses of up to 1 mg/kg a day there were no teratogenic or other embryotoxic findings if oral intake of the active substance by the mothers was prevented. The plasma levels for tretinoin and effective metabolites lay within the range of endogenous concentrations and did not give any indications of appreciable percutaneous absorption. Tretinoin administered systemically (orally, subcutaneously) caused deformities in all species investigated (rats, mice, hamsters, rabbits, monkeys). The lowest teratogenic dose for the most sensitive species (mouse) is between 1 3 mg a day for subcutaneous administration. There have been individual reports of birth defects in children whose mothers had been treated topically with tretinoin during pregnancy. A cohort study involving children whose mothers were exposed to topical tretinoin treatment during the first trimester of pregnancy did not produce any accumulation of deformities in comparison to a group of women who had not been exposed. No controlled prospective studies have been performed on pregnant women. The tretinoin blood levels that produce teratogenic effects are not known. 6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients Ethanol 100 %; glycerol 85 %; copovidone. 6.2 Incompatibilities Light, oxidising agents. 6.3 Shelf life 2 years Shelf life after first opening the bottle: 6 months 6.4 Special precautions for storage Store below 25 C. 6.5 Nature and contents of container Solution for application to the skin: 1 applicator bottle of 25 ml N 1 2 applicator bottles of 25 ml each N 2 Clinic pack: 2 applicator bottles of 25 ml each Page 5 of 6
6 6.6 Special precautions for disposal No special requirements. 7. EUROPEAN MARKETING AUTHORISATION HOLDER Almirall Hermal GmbH, Germany Registration holder: Neopharm Ltd. P.O. Box 7063 Petach Tiqva Israel 8. MARKETING AUTHORISATION NUMBER(S) DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION 5 January 1994 / 25 January 1999 / 10 October DATE OF REVISION OF THE TEXT January GENERAL CLASSIFICATION FOR SUPPLY Medicinal product subject to medical prescription. The format of this leaflet was determined by the Ministry of Health and its content was checked and approved January 2011 Page 6 of 6
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON HYPERICUM PERFORATUM L., HERBA (TRADITIONAL USE)
 European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMEA/HMPC/745582/2009 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH
European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMEA/HMPC/745582/2009 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH
Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel)
 ISOTREX Gel Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel) Excipients Butylated hydroxytoluene Hydroxypropylcellulose Ethanol CLINICAL INFORMATION Indications
ISOTREX Gel Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel) Excipients Butylated hydroxytoluene Hydroxypropylcellulose Ethanol CLINICAL INFORMATION Indications
RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd.
 RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd. Table of Contents 1. Name of the product...2 2. Qualitative and quantitative composition...2
RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd. Table of Contents 1. Name of the product...2 2. Qualitative and quantitative composition...2
NEUROTONE THR 00904/0005 UKPAR
 NEUROTONE THR 00904/0005 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Product Information Leaflet
NEUROTONE THR 00904/0005 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Product Information Leaflet
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON AVENA SATIVA L., FRUCTUS
 European Medicines Agency Evaluation of Medicines for Human Use London, 4 September 2008 Doc. Ref. EMEA/HMPC/368600/2007 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
European Medicines Agency Evaluation of Medicines for Human Use London, 4 September 2008 Doc. Ref. EMEA/HMPC/368600/2007 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
1. ACNE 1. Lisa Schmidt, MPH, Eve A. Kerr, MD, and Kenneth Clark, MD
 1. ACNE 1 Lisa Schmidt, MPH, Eve A. Kerr, MD, and Kenneth Clark, MD The general approach to summarizing the key literature on acne was to review relevant sections of two medical text books (Vernon and
1. ACNE 1 Lisa Schmidt, MPH, Eve A. Kerr, MD, and Kenneth Clark, MD The general approach to summarizing the key literature on acne was to review relevant sections of two medical text books (Vernon and
Acne (Acne Vulgaris) A common type of bacteria that lives on the skin, known as Propionibacterium acnes, sometimes
 Acne (Acne Vulgaris) Acne, clinically known as acne vulgaris, is the most common skin disease. It affects 85% of teenagers, some as young as 12, and often continues into adulthood. It is also called pimples,
Acne (Acne Vulgaris) Acne, clinically known as acne vulgaris, is the most common skin disease. It affects 85% of teenagers, some as young as 12, and often continues into adulthood. It is also called pimples,
PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w
 NAME OF THE MEDICINE Stieva-A Cream PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w DESCRIPTION Chemical names: 3, 7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexene-1-yl)-2,4,6,8-non-tetraenoic
NAME OF THE MEDICINE Stieva-A Cream PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w DESCRIPTION Chemical names: 3, 7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexene-1-yl)-2,4,6,8-non-tetraenoic
Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006
 Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON HYPERICUM PERFORATUM L., HERBA (WELL-ESTABLISHED MEDICINAL USE)
 European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMA/HMPC/101304/2008 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMA/HMPC/101304/2008 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Dechra Veterinary Products Limited (A business unit of Dechra Pharmaceuticals PLC) Sansaw Business Park Hadnall, Shrewsbury Shropshire SY4 4AS
 Dechra Veterinary Products Limited (A business unit of Dechra Pharmaceuticals PLC) Sansaw Business Park Hadnall, Shrewsbury Shropshire SY4 4AS Tel: 01939 211200 Fax: 01939 211201 Email: [email protected]
Dechra Veterinary Products Limited (A business unit of Dechra Pharmaceuticals PLC) Sansaw Business Park Hadnall, Shrewsbury Shropshire SY4 4AS Tel: 01939 211200 Fax: 01939 211201 Email: [email protected]
1g cream or ointment contains 1 mg methylprednisolone aceponate.
 CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
1. NAME OF THE MEDICINAL PRODUCT. Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION. 1 capsule contains:
 1. NAME OF THE MEDICINAL PRODUCT Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 capsule contains: Impavido 10 mg capsules 10 mg Miltefosine. Impavido 50 mg
1. NAME OF THE MEDICINAL PRODUCT Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 capsule contains: Impavido 10 mg capsules 10 mg Miltefosine. Impavido 50 mg
FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE PUBLICLY AVAILABLE ASSESSMENT REPORT FOR A VETERINARY MEDICINAL PRODUCT
 FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE FOR A VETERINARY MEDICINAL PRODUCT EMEDOG, 1 mg/ml, solution for injection for dogs Date: 20/08/2015 French agency for food, environnemental
FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE FOR A VETERINARY MEDICINAL PRODUCT EMEDOG, 1 mg/ml, solution for injection for dogs Date: 20/08/2015 French agency for food, environnemental
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT ACEGON, 50 microgram/ml, solution for injection for cattle. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT ACEGON, 50 microgram/ml, solution for injection for cattle. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active
acne Dr. M. Goeteyn Dermatologie AZ Sint-Jan Brugge-Oostende AV [email protected]
 acne Dr. M. Goeteyn Dermatologie AZ Sint-Jan Brugge-Oostende AV [email protected] Why should you treat acne? -Impact on the quality of life -scarring What is acne? Acne is an inflammatory disease
acne Dr. M. Goeteyn Dermatologie AZ Sint-Jan Brugge-Oostende AV [email protected] Why should you treat acne? -Impact on the quality of life -scarring What is acne? Acne is an inflammatory disease
PACKAGE LEAFLET: INFORMATION FOR THE USER. VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution. Cyanocobalamin
 PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
Mucodyne-Clear 250 mg/5 ml Syrup PL 04425/0665
 Mucodyne-Clear 250 mg/5 ml Syrup PL 04425/0665 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
Mucodyne-Clear 250 mg/5 ml Syrup PL 04425/0665 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel
 Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
A.C.N.E. H.E.L.P. Advanced Comedolytic & Normalizing Effect by Heat, Electricity & Light for Prevention & Treatment of Acne.
 A.C.N.E. H.E.L.P. Advanced Comedolytic & Normalizing Effect by Heat, Electricity & Light for Prevention & Treatment of Acne. The acne More than 80% of the world population suffers at least once in a lifetime
A.C.N.E. H.E.L.P. Advanced Comedolytic & Normalizing Effect by Heat, Electricity & Light for Prevention & Treatment of Acne. The acne More than 80% of the world population suffers at least once in a lifetime
PACKAGE LEAFLET. CLINDAMYCIN capsules Clidamycin. One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride).
 PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
One vial contains 500 U* of C1-inhibitor** After reconstitution the product contains 500 U/5 ml which correspond to a concentration of 100 U/ml.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cetor 100 U/ml powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 500 U* of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cetor 100 U/ml powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 500 U* of
Community herbal monograph on Commiphora molmol Engler, gummi-resina
 12 July 2011 EMA/HMPC/96911/2010 Committee on Herbal Medicinal Products (HMPC) Community herbal monograph on Commiphora molmol Engler, gummi-resina Final Discussion in Working Party on Community monographs
12 July 2011 EMA/HMPC/96911/2010 Committee on Herbal Medicinal Products (HMPC) Community herbal monograph on Commiphora molmol Engler, gummi-resina Final Discussion in Working Party on Community monographs
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
Smoothbeam Laser Treatment of Acne Vulgaris. Emerging Applications
 Smoothbeam Laser Treatment of Acne Vulgaris Emerging Applications About Acne Vulgaris Very common - Affects 80% of population Almost every person experiences acne Most common reason to visit dermatologist
Smoothbeam Laser Treatment of Acne Vulgaris Emerging Applications About Acne Vulgaris Very common - Affects 80% of population Almost every person experiences acne Most common reason to visit dermatologist
ACNE. Nisakorn Saewan, Ph.D.
 ACNE, Ph.D. Contents Definition Cause of acne Type of acne Acne grades Acne treatments Objectives To explain the definition of acne To elucidate cause of acne To identify type and grade of acne To explain
ACNE, Ph.D. Contents Definition Cause of acne Type of acne Acne grades Acne treatments Objectives To explain the definition of acne To elucidate cause of acne To identify type and grade of acne To explain
Agencia Española de Medicamentos y Productos Sanitarios C/Campezo 1, Edificio 8 28022 Madrid España (Reference Member State)
 DEPARTAMENTO DE MEDICAMENTOS VETERINARIOS Medicamentos y Productos C/Campezo 1, Edificio 8 28022 Madrid España (Reference Member State) DECENTRALISED PROCEDURE PUBLICLY AVAILABLE ASSESSMENT REPORT FOR
DEPARTAMENTO DE MEDICAMENTOS VETERINARIOS Medicamentos y Productos C/Campezo 1, Edificio 8 28022 Madrid España (Reference Member State) DECENTRALISED PROCEDURE PUBLICLY AVAILABLE ASSESSMENT REPORT FOR
Acne Vulgaris Medicines Consultation Toolkit
 Introduction Acne is a very common skin condition, which affects more than 80% of people at some point in their life. 1 Furthermore, it is a chronic condition, which along with eczema and psoriasis, accounts
Introduction Acne is a very common skin condition, which affects more than 80% of people at some point in their life. 1 Furthermore, it is a chronic condition, which along with eczema and psoriasis, accounts
SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product)
 PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
Having regard to the Treaty establishing the European Economic Community, and in particular Article 100 thereof;
 DIRECTIVE 65/65/EEC Council Directive 65/65/EEC of 26 January 1965 on the approximation of provisions laid down by law, regulation or administrative action relating to medicinal products (OJ L No 22 of
DIRECTIVE 65/65/EEC Council Directive 65/65/EEC of 26 January 1965 on the approximation of provisions laid down by law, regulation or administrative action relating to medicinal products (OJ L No 22 of
The Cause of Acne. Caring for Acne
 Sebaceous (oil) glands generate oil. The nose area is typically affected by acne because the sebaceous glands are larger and more active in this vicinity than in any other area of the face. In order to
Sebaceous (oil) glands generate oil. The nose area is typically affected by acne because the sebaceous glands are larger and more active in this vicinity than in any other area of the face. In order to
Summary Acne vulgaris is a chronic, treatable disease but response to treatment may be delayed.
 TREATMENT OF ACNE VULGARIS Summary Acne vulgaris is a chronic, treatable disease but response to treatment may be delayed. Topical agents are useful as monotherapy for mild acne and in combination with
TREATMENT OF ACNE VULGARIS Summary Acne vulgaris is a chronic, treatable disease but response to treatment may be delayed. Topical agents are useful as monotherapy for mild acne and in combination with
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement
 Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
It can therefore be bought without a prescription but should be used correctly to ensure its efficacy and to reduce side effects.
 140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
SUMMARYOF PRODUCT CHARACTERISTICS
 SUMMARYOF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Excis 10 mg/ml concentrate for solution for fish treatment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: Cypermethrin
SUMMARYOF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Excis 10 mg/ml concentrate for solution for fish treatment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: Cypermethrin
SUMMARY OF PRODUCT CHARACTERISTICS. Albuman 200 g/l is a solution containing 200 g/l (20%) of total protein of which at least 95% is human albumin.
 Albuman 200 g/l SPC 01 December 2015 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 200 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 200 g/l
Albuman 200 g/l SPC 01 December 2015 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 200 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 200 g/l
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES
 RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES Guideline Title Radiopharmaceuticals based on Monoclonal Antibodies Legislative basis Directives 65/65/EEC, 75/318/EEC as amended, Directive 89/343/EEC
RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES Guideline Title Radiopharmaceuticals based on Monoclonal Antibodies Legislative basis Directives 65/65/EEC, 75/318/EEC as amended, Directive 89/343/EEC
SAFETY DATA SHEET. according to 1907/2006/EC, Article 31. Zenith 6B Toilet Cleaner (072200)
 SAFETY DATA SHEET according to 190/2006/EC, Article 31 Page 1/5 Zenith 6B Toilet Cleaner (02200) SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier
SAFETY DATA SHEET according to 190/2006/EC, Article 31 Page 1/5 Zenith 6B Toilet Cleaner (02200) SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier
MicroSilver BG TM. The innovative agent for beautiful, healthy skin.
 The innovative agent for beautiful, healthy skin. Inhalt Why MicroSilver BG TM? 3 What is MicroSilver BG TM? 3 How does MicroSilver BG TM work? 3 Products and usage 4 MicroSilver BG TM still used today
The innovative agent for beautiful, healthy skin. Inhalt Why MicroSilver BG TM? 3 What is MicroSilver BG TM? 3 How does MicroSilver BG TM work? 3 Products and usage 4 MicroSilver BG TM still used today
Kalms Tablets THR 01074/0235 UKPAR
 Kalms Tablets THR 01074/0235 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Patient Information Leaflet
Kalms Tablets THR 01074/0235 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Patient Information Leaflet
ACNE AND ROSACEA. Disclosure. Distribution of Acne 6/12/2014. NJAFP 2014 Summer Celebration & Scientific Assembly 1
 ACNE AND ROSACEA Everett Schlam, MD Assistant Director, Mountainside Hospital Family Medicine Residency Program, Verona, NJ Disclosure Dr. Schlam has indicated that he has nothing to disclose relevant
ACNE AND ROSACEA Everett Schlam, MD Assistant Director, Mountainside Hospital Family Medicine Residency Program, Verona, NJ Disclosure Dr. Schlam has indicated that he has nothing to disclose relevant
EMEA PUBLIC STATEMENT ON LEFLUNOMIDE (ARAVA) - SEVERE AND SERIOUS HEPATIC REACTIONS -
 The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
Public Assessment Report. Decentralised Procedure
 Public Assessment Report Decentralised Procedure Linezolid 2 mg/ml solution for infusion Linezolid 600mg film coated tablets Linezolid 100mg/5ml granules for oral suspension Procedure No: UK Licence No:
Public Assessment Report Decentralised Procedure Linezolid 2 mg/ml solution for infusion Linezolid 600mg film coated tablets Linezolid 100mg/5ml granules for oral suspension Procedure No: UK Licence No:
MATERIAL SAFETY DATA SHEET
 Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid
 Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
UNIVERSIDAD DE GUAYAQUIL DEPARTMENT OF CHEMICAL SCIENCES
 CODE: 38/05 TITLE: Establishment of the potential anti-inflammatory effect of the product known as CUMANDA, originating from NutraMedix Laboratories, LLC, Florida OBJECTIVES: To study the possible anti-inflammatory
CODE: 38/05 TITLE: Establishment of the potential anti-inflammatory effect of the product known as CUMANDA, originating from NutraMedix Laboratories, LLC, Florida OBJECTIVES: To study the possible anti-inflammatory
PACKAGE LEAFLET: INFORMATION FOR THE USER. Dalacin C 150 mg Capsules. clindamycin hydrochloride. Dalacin C 150mg Capsules clindamycin hydrochloride
 PACKAGE LEAFLET: INFORMATION FOR THE USER PFIZER Dalacin C 150 mg Capsules clindamycin hydrochloride Dalacin C 150mg Capsules clindamycin hydrochloride PFIZER Read all of this leaflet carefully before
PACKAGE LEAFLET: INFORMATION FOR THE USER PFIZER Dalacin C 150 mg Capsules clindamycin hydrochloride Dalacin C 150mg Capsules clindamycin hydrochloride PFIZER Read all of this leaflet carefully before
X-Plain Acne Reference Summary
 X-Plain Acne Reference Summary Introduction Nearly 17 million people in the United States have acne, making it one of the most common skin diseases in the USA. Although acne is not a serious health threat,
X-Plain Acne Reference Summary Introduction Nearly 17 million people in the United States have acne, making it one of the most common skin diseases in the USA. Although acne is not a serious health threat,
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Losartan Potassium and Hydrochlorothiazide Tablets 100 mg/12.5 mg Lupin Limited Goa 403 722
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Losartan Potassium and Hydrochlorothiazide Tablets 100 mg/12.5 mg Lupin Limited Goa 403 722
Acne. Sofia Chaudhry M.D. Histology of an inflamed comedo. Bolognia, 2008.
 Acne Sofia Chaudhry M.D. Histology of an inflamed comedo. Bolognia, 2008. Acne Vulgaris (Common Acne) Multifactorial disorder of pilosebaceous unit Affects 40-50 million individuals annually in U.S. alone
Acne Sofia Chaudhry M.D. Histology of an inflamed comedo. Bolognia, 2008. Acne Vulgaris (Common Acne) Multifactorial disorder of pilosebaceous unit Affects 40-50 million individuals annually in U.S. alone
Section 4. Toxicology
 Section 4 Toxicology Occupational Health Any chemical you use incorrectly can result in over-exposure leading to adverse health effects immediately or in the future. All hazardous materials can be handled
Section 4 Toxicology Occupational Health Any chemical you use incorrectly can result in over-exposure leading to adverse health effects immediately or in the future. All hazardous materials can be handled
UNIVERSIDAD DE GUAYAQUIL DEPARTMENT OF CHEMICAL SCIENCES
 CODE: 38/05 TITLE: Establishment of the potential anti-inflammatory effect of the product known as SAMENTO, originating from NutraMedix Laboratories, LLC, Florida OBJECTIVES: To study the possible anti-inflammatory
CODE: 38/05 TITLE: Establishment of the potential anti-inflammatory effect of the product known as SAMENTO, originating from NutraMedix Laboratories, LLC, Florida OBJECTIVES: To study the possible anti-inflammatory
MATERIAL SAFETY DATA SHEET
 Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Acne. Normal skin. What is acne?
 Acne Acne is extremely common in varying degrees of severity. About 9 out of 10 teenagers develop some degree of acne. However, this does not mean it should be ignored. Acne is a distressing condition
Acne Acne is extremely common in varying degrees of severity. About 9 out of 10 teenagers develop some degree of acne. However, this does not mean it should be ignored. Acne is a distressing condition
Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you.
 leaflet: Information for the user Macrogol 4000 10 g powder for oral solution in sachet Macrogol 4000
leaflet: Information for the user Macrogol 4000 10 g powder for oral solution in sachet Macrogol 4000
UKPAR Goodnight THR 00904/0003. Goodnight THR 00904/0003 UKPAR TABLE OF CONTENTS. Lay Summary Page 2. Scientific discussion Page 3
 Goodnight THR 00904/0003 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 12 Steps taken after authorisation Page 13 Summary of Product Characteristics
Goodnight THR 00904/0003 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 12 Steps taken after authorisation Page 13 Summary of Product Characteristics
Acne Vulgaris: Pathophysiology, diagnosis, and treatment of a common dermatologic condition
 Acne Vulgaris: Pathophysiology, diagnosis, and treatment of a common dermatologic condition Latasha Weeks, B.A., B.S. Doctor of Pharmacy Candidate, 2007 University of Maryland School of Pharmacy Learning
Acne Vulgaris: Pathophysiology, diagnosis, and treatment of a common dermatologic condition Latasha Weeks, B.A., B.S. Doctor of Pharmacy Candidate, 2007 University of Maryland School of Pharmacy Learning
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 Temporary Core Product Information Inde- (13May2013) Page 1 / 7 1 NAME OF THE MEDICINAL PRODUCT XYLONOR SPRAY, oromucosal spray, solution. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram contains:
Temporary Core Product Information Inde- (13May2013) Page 1 / 7 1 NAME OF THE MEDICINAL PRODUCT XYLONOR SPRAY, oromucosal spray, solution. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram contains:
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013.
 The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
LOOK, FEEL AND LIVE BETTER. Topical Skin Care
 LOOK, FEEL AND LIVE BETTER Topical Skin Care Pycnogenol in Topical Skin Care Pycnogenol is widely used in topical and oral applications for various dermatological indications. A unique combination of pharmacological
LOOK, FEEL AND LIVE BETTER Topical Skin Care Pycnogenol in Topical Skin Care Pycnogenol is widely used in topical and oral applications for various dermatological indications. A unique combination of pharmacological
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate.
 NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B
 PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B A. EXPLANATORY NOTE For the purpose of this General Introduction the following nymbering applies:
PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B A. EXPLANATORY NOTE For the purpose of this General Introduction the following nymbering applies:
Public Assessment Report Scientific discussion. Tenofovir disoproxil Teva (tenofovir disoproxil) SE/H/1432/01/DC
 Public Assessment Report Scientific discussion Tenofovir disoproxil Teva (tenofovir disoproxil) SE/H/1432/01/DC This module reflects the scientific discussion for the approval of Tenofovir disoproxil Teva.
Public Assessment Report Scientific discussion Tenofovir disoproxil Teva (tenofovir disoproxil) SE/H/1432/01/DC This module reflects the scientific discussion for the approval of Tenofovir disoproxil Teva.
PACKAGE LEAFLET: INFORMATION FOR THE USER. Elidel 10 mg/g Cream. pimecrolimus
 PACKAGE LEAFLET: INFORMATION FOR THE USER Elidel 10 mg/g Cream pimecrolimus Read all of this leaflet carefully before you start using Elidel cream Keep this leaflet. You may need to read it again. If you
PACKAGE LEAFLET: INFORMATION FOR THE USER Elidel 10 mg/g Cream pimecrolimus Read all of this leaflet carefully before you start using Elidel cream Keep this leaflet. You may need to read it again. If you
Neutral Superoxidized solution vs. benzoyl peroxide gel 5% in the treatment of. Superoxidized solution (SOS) is an electrochemically processed aqueous
 Neutral Superoxidized solution vs. benzoyl peroxide gel 5% in the treatment of acne vulgaris: a randomized open-label clinical trial. Summary. Superoxidized solution (SOS) is an electrochemically processed
Neutral Superoxidized solution vs. benzoyl peroxide gel 5% in the treatment of acne vulgaris: a randomized open-label clinical trial. Summary. Superoxidized solution (SOS) is an electrochemically processed
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
F r e q u e n t l y A s k e d Q u e s t i o n s
 Acne who specializes in treating skin problems) about how you can help prevent acne and if treatment would help you. Q: What is acne? A: Acne is a disorder that causes outbreaks of skin lesions commonly
Acne who specializes in treating skin problems) about how you can help prevent acne and if treatment would help you. Q: What is acne? A: Acne is a disorder that causes outbreaks of skin lesions commonly
OFF! DEEP WOODS SPORTSMEN INSECT REPELLENT I (PUMP SPRAY)
 1. PRODUCT AND COMPANY IDENTIFICATION Product information Trade name : OFF! DEEP WOODS SPORTSMEN INSECT REPELLENT I (PUMP Use of the : Insect Repellent Substance/Preparation Company : S.C. Johnson and
1. PRODUCT AND COMPANY IDENTIFICATION Product information Trade name : OFF! DEEP WOODS SPORTSMEN INSECT REPELLENT I (PUMP Use of the : Insect Repellent Substance/Preparation Company : S.C. Johnson and
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL EVALUATION OF ANTICANCER MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 23 July 1998 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL
The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 23 July 1998 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL
Sodium Sulphite Anhydrous
 Revision date 08-04-2016 1.1 Product identifier Sodium Sulphite Anhydrous Section 1: Identification of the substance/ mixture and of the company/ undertaking. Product name Other names Sodium Sulphite Anhydrous
Revision date 08-04-2016 1.1 Product identifier Sodium Sulphite Anhydrous Section 1: Identification of the substance/ mixture and of the company/ undertaking. Product name Other names Sodium Sulphite Anhydrous
before it starts getting better. Do not give up! We will continue to work with you to get your acne better.
 ACNE Acne is a common condition in teenagers and adults. It affects up to 80-90% of teenagers. Developing an acne bump is multifactorial, meaning that several processes lead to the formation of the acne
ACNE Acne is a common condition in teenagers and adults. It affects up to 80-90% of teenagers. Developing an acne bump is multifactorial, meaning that several processes lead to the formation of the acne
VOLTAREN OPHTHA EYE DROPS
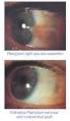 פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
Safety Data Sheet. according to WHMIS. SECTION 1: Identification of the substance/mixture and of the company/undertaking
 Print date: 03.02.2016 Product code: 00625-0004_US_GHS Page 1 of 7 SECTION 1: Identification of the substance/mixture and of the company/undertaking Product identifier Relevant identified uses of the substance
Print date: 03.02.2016 Product code: 00625-0004_US_GHS Page 1 of 7 SECTION 1: Identification of the substance/mixture and of the company/undertaking Product identifier Relevant identified uses of the substance
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Maintenance of abstinence in alcohol dependence
 Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Acne breakouts vulgaris is one of the commonest skin conditions, which dermatologists treat and it
 Introduction Acne breakouts vulgaris is one of the commonest skin conditions, which dermatologists treat and it mostly impacts teens, though it might appear at any sort of age. Acne breakouts cause necessarily
Introduction Acne breakouts vulgaris is one of the commonest skin conditions, which dermatologists treat and it mostly impacts teens, though it might appear at any sort of age. Acne breakouts cause necessarily
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE S1A. Current Step 4 version
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals. Step 5
 European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
Annex II. Scientific conclusions and grounds for refusal presented by the European Medicines Agency
 Annex II Scientific conclusions and grounds for refusal presented by the European Medicines Agency 5 Scientific conclusions Overall summary of the scientific evaluation of Ethinylestradiol-Drospirenone
Annex II Scientific conclusions and grounds for refusal presented by the European Medicines Agency 5 Scientific conclusions Overall summary of the scientific evaluation of Ethinylestradiol-Drospirenone
PRODUCT MONOGRAPH. isotretinoin capsules USP, 10, 40mg. Nodular/Inflammatory and Conglobate Acne Therapy
 PRODUCT MONOGRAPH Pr ACCUTANE ROCHE isotretinoin capsules USP, 10, 40mg Nodular/Inflammatory and Conglobate Acne Therapy Hoffmann-La Roche Limited 7070 Mississauga Road Mississauga, ON L5N 5M8 Date of
PRODUCT MONOGRAPH Pr ACCUTANE ROCHE isotretinoin capsules USP, 10, 40mg Nodular/Inflammatory and Conglobate Acne Therapy Hoffmann-La Roche Limited 7070 Mississauga Road Mississauga, ON L5N 5M8 Date of
CORE SmPC FOR RADIOPHARMACEUTICALS
 CORE SmPC FOR RADIOPHARMACEUTICALS The QRD Product Information template with explanatory notes and the convention to be followed for QRD templates provide general guidance on format and text and should
CORE SmPC FOR RADIOPHARMACEUTICALS The QRD Product Information template with explanatory notes and the convention to be followed for QRD templates provide general guidance on format and text and should
Acne Vulgaris: Pathophysiology, diagnosis, and treatment of a common dermatologic condition
 Acne Vulgaris: Pathophysiology, diagnosis, and treatment of a common dermatologic condition Dr. Mohamed Emam Lecturer in Clinical Pharmacy Department Faculty of Pharmacy Beni Suef University Hair Pilosebaceous
Acne Vulgaris: Pathophysiology, diagnosis, and treatment of a common dermatologic condition Dr. Mohamed Emam Lecturer in Clinical Pharmacy Department Faculty of Pharmacy Beni Suef University Hair Pilosebaceous
Safety Data Sheet Per GHS Standard Format
 SDS Date: May, 2015 Safety Data Sheet Per GHS Standard Format SECTION 1: CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Identifier Product Name: ShockWave Hydrogen Peroxide Disinfectant & Cleaner
SDS Date: May, 2015 Safety Data Sheet Per GHS Standard Format SECTION 1: CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Identifier Product Name: ShockWave Hydrogen Peroxide Disinfectant & Cleaner
ALLERGENIC EXTRACT. Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE. U.S. Government License No.
 ALLERGENIC EXTRACT Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE U.S. Government License No. 308 Revised 07/04 PO Box 800 Lenoir, NC 28645 USA DESCRIPTION This set
ALLERGENIC EXTRACT Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE U.S. Government License No. 308 Revised 07/04 PO Box 800 Lenoir, NC 28645 USA DESCRIPTION This set
Pathogenesis 10/04/2015. Acne
 Deepani Rathnayake MBBS, MD, FACD Acne Affects >80% of adolescents >40% of adults Associated with Disfigurement Loss of confidence Depression Affects quality of life Pathogenesis i) increased sebum production,
Deepani Rathnayake MBBS, MD, FACD Acne Affects >80% of adolescents >40% of adults Associated with Disfigurement Loss of confidence Depression Affects quality of life Pathogenesis i) increased sebum production,
VOLTAREN OPHTHA EYE DROPS
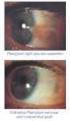 2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
MRP-No. DE/H/0279/001/P/002 Dr. Scheffler Vitamin C, 1000mg, effervescent tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER Dr. Scheffler Vitamin C, 1000 mg, effervescent tablets Ascorbic Acid Read all of this leaflet carefully because it contains important information for you. This
PACKAGE LEAFLET: INFORMATION FOR THE USER Dr. Scheffler Vitamin C, 1000 mg, effervescent tablets Ascorbic Acid Read all of this leaflet carefully because it contains important information for you. This
There is a risk of renal impairment in dehydrated children and adolescents.
 PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
