Adverse Health Event Management: Some Lessons from the Krever Inquiry
|
|
|
- Antonia Walker
- 9 years ago
- Views:
Transcription
1 Adverse Health Event Management: Some Lessons from the Krever Inquiry Heather Hume, MD, FRCPC Executive Medical Director, Transfusion Medicine Canadian Blood Services NF Provincial Forum on Adverse Health Event Management St. John s NL May 26, 2008
2 Legacy - born in an environment of failure and scandal
3 The Commission of Inquiry on the Blood System in Canada Federal Federal government government determines determines need need for for a commission commission of of inquiry inquiry Justice Justice Horace Horace Krever, Krever, appointed appointed by by Order Order in in Council, Council, October October 4 th th to to review review and and report report on on the the mandate, mandate, organization, organization, management, management, operations, operations, financing financing and and regulation regulation of of all all activities activities of of the the blood blood system system in in Canada Canada Justice Justice Krever Krever tables tables an an Interim Interim Report Report February February th th recommendations recommendations focused focused on on operational, operational, technical technical and and clinical clinical aspects aspects of of blood blood system system at at the the time time Work Work on on final final report report continues continues days days of of hearings hearings from from individuals individuals phase phase I I input input from from those those infected infected with with HIV HIV or or HCV HCV phase phase II II national national issues issues concerning concerning historical historical actions actions and and relationships relationships phase phase III III organization organization of of the the blood blood system system at at the the time time appeals appeals under under Section Section of of the the Inquiries Inquiries Act Act caused caused significant significant delays delays final final report report released released November November th th,,
4 Canadian Blood Services In September 1998, Canadian Blood Services and Héma-Québec established Canadian Blood Services operates in 9 provinces and all 3 territories Population served ~25,000,000 (HQ ~7,700,000) Collect ~880,000 units/year Must recruit ~86,000 new donors each year Support ~750 health care facilities Funded by provincial/territorial MOH (excluding QC) Operations occur at arms-length from funders Independent Board of Directors Members appointed by the provincial MOH 4
5 Quotes from Krever - Foreword In the pages that follow, an account is given of a public health disaster that was unprecedented in Canada, and if we have learned from it, one that will never occur again. 5
6 Quotes from Krever Precautionary Principle When there was reasonable evidence that serious infectious diseases could be transmitted by blood, the principal actors in the blood supply system in Canada refrained from taking essential preventative measures until causation had been proved with scientific certainty. The result was a national public health disaster. 6
7 Quotes from Krever Balancing risks & benefits and partial measures The balancing of the risks and benefits of taking action should be dependent not only on the likelihood of the risk materializing but also on the severity of the effect if the risk does materialize, on the number of persons who could be affected, and on the ease of implementing protective or preventative measures. The more severe the potential effect, the lower the threshold should be for taking action.... If there are no measures that will entirely prevent the harm, measures that may only partially prevent transmission should be undertaken. 7
8 The Commission of Inquiry on the Blood System in Canada Final report (Nov 1997) 50 recommendations #1 Compensation #2 The Canadian blood system basic principles #3-28 The operator: a national blood service #29-45 The regulator: the health protection branch #46-50 Public health 8
9 Krever recommendation # 6 that the blood supply system be operated in an open and accessible manner. the current lack of confidence in the blood system results, in no small measure, from the absence of public participation in the decision-making process that, until now, has characterized the system. 9
10 Krever recommendation # 14..that the following standing committees be created to facilitate the work of the national blood service: a safety committee a technical and scientific committee a liaison committee All committees should have as member representatives from consumer groups and the public. 10
11 Krever recommendation # 22 that there be an effective exchange of information between the national blood service and all hospitals that supply blood components and blood products 11
12 Krever recommendation # 28 that, on learning of potential risks to the safety of blood components or blood products, the national blood service cause recipients to be informed. 12
13 Krever recommendation # 40 that there be an active program of postmarket surveillance for blood components and blood products 13
14 Krever recommendation # 28 that, on learning of potential risks to the safety of blood components or blood products, the national blood service cause recipients to be informed. 14
15 Definitions Lookback Lookback - The process of identifying and initiating testing of recipients who received blood components from a donor who, on subsequent testing, is confirmed positive for a transfusiontransmissible infectious agent. from a regulatory point of view is similar to a recall 15
16 Definitions Traceback Traceback - The process of investigating donors who contributed blood component(s) for a transfusion to a patient who developed a bloodtransmissible infection. from a regulatory point of view is similar to an adverse reaction reporting requirement for adverse reactions 24 hr if life-threatening or fatal 15 days for all others 16
17 Difficulties with lookback & resolving those difficulties Not begun in a timely manner Poor records lack of traceability Many patients did not know that they had received a transfusion Policies/SOPs/resources Blood supply, hospitals Requirement for tracking vein to vein Patient information Informed consent Notification of transfusion 17
18 Canadian Blood Services ENHANCED LOOKBACK OUTCOMES ON COMPLETED INVESTIGATIONS From: To: HIV HTLV HCV HBV Total Cases # % # % # % # % Initiated through Centre Screening % % % % Initiated through Traceback % 4 1.0% % 9 3.0% Initiated through Other* % % % % Initiated through SSP** 1 0.1% 0 0.0% % 0 0.0% Cases Open % 7 1.8% % % Cases Completed % % % % First-time Donors % % % % Repeated Donors % % % % Not available % % % % Total # of Recipients Afftected*** % % % % Recipients (+) % % % % Recipients (-) % % % % Recipients Not Found; Status Unknown % % % % *Information provided by donor, donor's physician, Public Health **Stored Sample Project ***Estimated total # of transfused components = total # number of recipients affected
19 West Nile Virus (WNV) and the blood supply Applying lessons from Krever Changes in the basic structure of the Canadian blood suppliers Also a conscious effort to apply the precautionary principle implement partial measures assure an adequate exchange of information 19
20 Early HIV Transfusion-Related Milestones Date Event Comment June cases of P.carinii pneumonia in homosexual men Initial report July 1982 Initial cases in 3 persons with hemophilia Possibility of tainted blood supply Dec 1982 Initial transfusion-related case, in an infant Further evidence of tainted blood supply May 1983 Montagnier isolates the virus causing AIDS Later also isolated by Gallo in the USA Jan 1984 Report of 18 cases of transfusionrelated AIDS The debate is over... Nov 1985 Blood donor HIV antibody screening fully implemented in Canada In USA implemented in March 1985
21 WNV milestones Summer 1999 Aug 2002 Sept 2002 Oct 2002 July st documented cases of human WNV infection in North America (eastern USA) Publication estimating theoretical risk of transmission of WNV through transfusion WNV transmission through solid organ transplantation USA CDC confirm 6 cases of transmission-transmitted WNV Blood donor screening for WNV RNA implemented in Canada and USA
22 HIV/WNV Comparisons HIV (1982) WNV (2002) Agent Unknown Known PCR technology for blood donor viral screening Blood operator access to independent funding Non-existent No In place for HCV and HIV for several years Yes 22
23 HIV/WNV Comparisons Communication among health care players Precautionary principle Public and stakeholder expectations Public scrutiny HIV Very limited WNV Fairly well developed 23
24 HIV/WNV Comparisons Communication among health care players Precautionary principle Public and stakeholder expectations Public scrutiny HIV Very limited Not considered WNV Fairly well developed Guiding principle 24
25 HIV/WNV Comparisons Communication among health care players Precautionary principle Public and stakeholder expectations Public scrutiny HIV Very limited Not considered Relatively limited until about 1984 WNV Fairly well developed Guiding principle Post Krever very high expectations 25
26 HIV/WNV Comparisons Communication among health care players Precautionary principle Public and stakeholder expectations Public scrutiny HIV Very limited Not considered Relatively limited until about 1984 Relatively limited until about 1984 WNV Fairly well developed Guiding principle Post Krever very high expectations Intense media and stakeholder scrutiny 26
27 Precautionary Principle and Partial Measures: Inventory Management Dec 2002: withdrawal of frozen blood components collected in Ontario in summer 2002 Jan-May 2003: stockpiling of frozen components for use in the summer 2003 May-June 2003 increased RBC collections for use in July 2003 Sept 2003 withdrew components collected in Saskatchewan in Aug 2003 Summer 2003 blood clinics were not held in areas with significant number of human WNV cases 27
28 Communications Communications to hospitals Frequent Dear Colleague Letters Bulletin in the CMAJ Regular conference calls with stakeholders 2 blood suppliers, Health Canada, provincial MOH & public health officials International communications American colleagues Media Nov-Dec media interviews! 28
29 Krever recommendation # 6 that the blood supply system be operated in an open and accessible manner. the current lack of confidence in the blood system results, in no small measure, from the absence of public participation in the decision-making process that, until now, has characterized the system. 29
30 Krever recommendation # 14..that the following standing committees be created to facilitate the work of the national blood service: a safety committee a technical and scientific committee a liaison committee All committees should have as member representatives from consumer groups and the public. 30
31 Canadian Blood Services Advisory Committees National & Regional Liaison Committees NLC representation from 16 stakeholder groups RLC (7) total of 120 members Hospital Advisory Panel Hospital regional & functional representation National Advisory Committee on Blood & Blood Products Medical representation from all provinces served by Canadian Blood Services Scientific & Research Advisory Committee National & international membership 31
32 Example of public participation in decision- making: the MSM deferral policy 32
33 Current Donor Screening Each donor is asked ~85 different items related to health, medication, travel, lifestyle Every unit of blood collected is tested for transmissible diseases Blood Safety Donor Selection Donor Testing Good Manufacturing Practices (GMP) 33
34 MSM Deferral Policy Men who have had sex with men (even once) since 1977 are indefinitely ineligible to donate blood Policy implemented in mid-1980s Given the highly sensitive blood donor screening tests that we currently use to detect HIV (and the time that has passed) is this question still necessary? Could it be revised or removed? Decision to review the policy in 2006/07 Two overriding principles: Primary basis for donor deferral rests on the assessment and estimation of the various types of risks to health associated with donated blood Any changes to existing policies on donor deferral must result in an improved or equivalent level of safety by comparison to what now exists 34
35 Steps in determining whether or not to modify the MSM deferral criteria Internal medical point of view literature review analysis of surveillance data Assessment of international MSM policies Independent risk assessment by McLaughlin Centre for Population Health Risk Assessment Stakeholder consultation 35
36 Stakeholder Consultation Strategy Face-to-face sessions to gather insights of student associations, equality advocates, healthcare professionals and patient groups Independent facilitator with a structured process and agenda Presentation by Canadian Blood Services Medical, Scientific and Research Affairs Presentation by the McLaughlin Centre on the risk assessment Board member representatives present at meeting Provided opportunity for input into decision-making What would you/your organization like Canadian Blood Services to take into consideration in reviewing the MSM policy? Final report communicated to participants for comment prior to submission to the Board Always clear that the final decision-makers would be the members of the Board of Directors 36
37 Decision on the MSM Deferral Policy Canadian Blood Services will simultaneously maintain the current policy while actively gathering knowledge to close the gaps in information that were identified through the risk assessment and consultations: Better understanding of emerging pathogens Examining the risks and benefits of behavioural-based questions Monitoring the experiences of blood agencies that have modified or changed their own MSM deferral policies 37
38 Benefits of stakeholder consultations Decision-makers are able to make an informed decision - Participants provided valuable insights and input that might not otherwise have been brought to the Board Increases acceptance of the decision No public outcry Positive recognition of process even from those who did not agree with the final decision Increases mutual understanding All parties gained appreciation for each other s issues and challenges Enhanced existing relationships Built new relationships 38
39 Krever recommendation # 40 that there be an active program of postmarket surveillance for blood components and blood products 39
40 Adverse Event Surveillance Public Health Agency of Canada Transfusion-transmitted injury surveillance system (TTISS) Voluntary surveillance system for capturing moderate and severe transfusion reactions Collaboration with the blood suppliers agreement on definitions of reactions, severity, imputability agreement on what reactions need to be reported to the blood supplier as well as to TTISS reconciliation of data reported to each agency Provided by Public Health Agency of Canada (PHAC)
41 Infrastructure for National TTISS Reporting HOSPITALS Reportable Diseases Public Health Reportable Diseases Community Clinicians Volunteer Reporting Plasma Manufacturers Blood Manufacturers Provincial/Territorial Blood Offices Adverse Events Acute Delayed Health Canada Regulatory Mandatory Reporting Death = 24 hrs Severe = 15 days National Transfusion Transmitted Injuries Surveillance System (TTISS) Public Health Agency of Canada Provided by Public Health Agency of Canada (PHAC)
42 Provided by Public Health Agency of Canada (PHAC)
43 70% of transfusion activity in Canada Provided by Public Health Agency of Canada (PHAC) 43
44 Provided by Public Health Agency of Canada (PHAC)
45 Provided by Public Health Agency of Canada (PHAC)
46 Adverse Event Surveillance Public Health Agency of Canada Transfusion error surveillance system (TESS) pilot project in 4 provinces/11 hospitals begun in 2005 non-punitive, anonymous web-based reporting system of errors related to hospital-based transfusion activities groundwork for a national system that can be integrated with TTISS Provided by Public Health Agency of Canada (PHAC)
47 That That men men do do not not learn learn very very much much from from the the lessons of of history history is is the the most most important lesson lesson of of history history Aldous Huxley 47
BLOOD DONOR TESTING & LOOKBACK STUDIES Shan Yuan, MD Last Updated 2/8/2011. 1. ABO Typing: Performed each time with each donation
 Testing Performed: BLOOD DONOR TESTING & LOOKBACK STUDIES Shan Yuan, MD Last Updated 2/8/2011 1. ABO Typing: Performed each time with each donation 2. Rh Typing: o Performed along with ABO typing to determine
Testing Performed: BLOOD DONOR TESTING & LOOKBACK STUDIES Shan Yuan, MD Last Updated 2/8/2011 1. ABO Typing: Performed each time with each donation 2. Rh Typing: o Performed along with ABO typing to determine
PositionStatement EMERGENCY PREPAREDNESS AND RESPONSE CNA POSITION
 PositionStatement EMERGENCY PREPAREDNESS AND RESPONSE CNA POSITION The nursing profession 1 plays an integral role in all aspects of emergencies, including mitigation, preparedness, response and recovery.
PositionStatement EMERGENCY PREPAREDNESS AND RESPONSE CNA POSITION The nursing profession 1 plays an integral role in all aspects of emergencies, including mitigation, preparedness, response and recovery.
A Real Time Lab for Pan Canadian Innovation Leveraging Canadian Blood Services Model for Better Value to Health care Systems
 A Real Time Lab for Pan Canadian Innovation Leveraging Canadian Blood Services Model for Better Value to Health care Systems November 14, 2014 Submission to the Advisory Panel on Health Care Innovation
A Real Time Lab for Pan Canadian Innovation Leveraging Canadian Blood Services Model for Better Value to Health care Systems November 14, 2014 Submission to the Advisory Panel on Health Care Innovation
JUST 5 EASY STEPS FOR CORD BLOOD DONATION...
 JUST 5 EASY STEPS FOR CORD BLOOD DONATION... STEP 1 Read this Information Kit. STEP 2 Print and fill in the forms. STEP 3 Give completed forms to your healthcare provider or to your hospital RN on day
JUST 5 EASY STEPS FOR CORD BLOOD DONATION... STEP 1 Read this Information Kit. STEP 2 Print and fill in the forms. STEP 3 Give completed forms to your healthcare provider or to your hospital RN on day
Lancet Device Incident Investigation Report - 2012
 Lancet Device Incident Investigation Report - 2012 Summary On May 16, 2012 the Winnipeg Regional Health Authority (WRHA) received notification from the University of Manitoba (U of M) of an incident at
Lancet Device Incident Investigation Report - 2012 Summary On May 16, 2012 the Winnipeg Regional Health Authority (WRHA) received notification from the University of Manitoba (U of M) of an incident at
BLOOD TRANSFUSION SAFETY
 BLOOD TRANSFUSION SAFETY Estimated use of red cell transfusion in developed countries 35% 15% 6 3 7% 34% Pregnancy-related 6% Children 3% Surgery 34% Trauma 7% Medical 35% Haematological 15% blood saves
BLOOD TRANSFUSION SAFETY Estimated use of red cell transfusion in developed countries 35% 15% 6 3 7% 34% Pregnancy-related 6% Children 3% Surgery 34% Trauma 7% Medical 35% Haematological 15% blood saves
*6816* 6816 CONSENT FOR DECEASED KIDNEY DONOR ORGAN OPTIONS
 The shortage of kidney donors and the ever-increasing waiting list has prompted the transplant community to look at different types of organ donors to meet the needs of our patients on the waiting list.
The shortage of kidney donors and the ever-increasing waiting list has prompted the transplant community to look at different types of organ donors to meet the needs of our patients on the waiting list.
2013-14 Report on Plans and Priorities Additional Information for Sub-programs and Sub-sub-programs
 2013-14 Report on Plans and Priorities Additional Information for Sub-programs and Sub-sub-programs Strategic Outcome: Protecting Canadians and empowering them to improve their health Program 1.1 Public
2013-14 Report on Plans and Priorities Additional Information for Sub-programs and Sub-sub-programs Strategic Outcome: Protecting Canadians and empowering them to improve their health Program 1.1 Public
Risk Profiling Toolkit DEVELOPING A CORPORATE RISK PROFILE FOR YOUR ORGANIZATION
 Risk Profiling Toolkit DEVELOPING A CORPORATE RISK PROFILE FOR YOUR ORGANIZATION I Background Under the TBS Risk Management Policy, departments and agencies must identify the potential perils, factors
Risk Profiling Toolkit DEVELOPING A CORPORATE RISK PROFILE FOR YOUR ORGANIZATION I Background Under the TBS Risk Management Policy, departments and agencies must identify the potential perils, factors
Briefing Note: Hepatitis B & Hepatitis C. Summary:
 Briefing Note: Hepatitis B & Hepatitis C Summary: In Canada, hepatitis B and hepatitis C infections remain serious public health concerns due to high prevalence rates, high health care expenditures and
Briefing Note: Hepatitis B & Hepatitis C Summary: In Canada, hepatitis B and hepatitis C infections remain serious public health concerns due to high prevalence rates, high health care expenditures and
Canadian Regulations Safety of Human Cells, Tissues and Organs (CTO) for Transplantation
 Canadian Regulations Safety of Human Cells, Tissues and Organs (CTO) for Transplantation Presentation to the American Association of Tissue Banks Savannah, Georgia March 2008 Health Canada Update Marianne
Canadian Regulations Safety of Human Cells, Tissues and Organs (CTO) for Transplantation Presentation to the American Association of Tissue Banks Savannah, Georgia March 2008 Health Canada Update Marianne
Global Database on Blood Safety
 Global Database on Blood Safety Summary Report 2011 1 Key facts Global Blood Collection: Around 92 million blood donations are collected annually from all types of blood donors (voluntary unpaid, family/replacement
Global Database on Blood Safety Summary Report 2011 1 Key facts Global Blood Collection: Around 92 million blood donations are collected annually from all types of blood donors (voluntary unpaid, family/replacement
Executive summary. Executive summary 8
 Executive summary Q fever is a zoonosis an infectious disease that can be transmitted from animals to humans caused by the bacterium Coxiella burnetii (C. burnetii). Until 2006, Q fever was a rare disease
Executive summary Q fever is a zoonosis an infectious disease that can be transmitted from animals to humans caused by the bacterium Coxiella burnetii (C. burnetii). Until 2006, Q fever was a rare disease
Case Finding for Hepatitis B and Hepatitis C
 Case Finding for Hepatitis B and Hepatitis C John W. Ward, M.D. Division of Viral Hepatitis Centers for Disease Control and Prevention Atlanta, Georgia, USA Division of Viral Hepatitis National Center
Case Finding for Hepatitis B and Hepatitis C John W. Ward, M.D. Division of Viral Hepatitis Centers for Disease Control and Prevention Atlanta, Georgia, USA Division of Viral Hepatitis National Center
Public Health in Canada: A Difficult History
 Public Health in Canada: A Difficult History COMMENTARY David L. Mowat, MBChB, MPH, FRCPC Deputy Chief Public Health Officer Public Health Practice and Regional Operations Public Health Agency of Canada
Public Health in Canada: A Difficult History COMMENTARY David L. Mowat, MBChB, MPH, FRCPC Deputy Chief Public Health Officer Public Health Practice and Regional Operations Public Health Agency of Canada
National Health Burden of CLD in Italy
 National Health Burden of CLD in Italy 11,000 deaths due to liver cirrhosis or HCC in 2006 Direct costs for the National Health System for treating CLD patients: 420 M / year for hospital care 164 M /
National Health Burden of CLD in Italy 11,000 deaths due to liver cirrhosis or HCC in 2006 Direct costs for the National Health System for treating CLD patients: 420 M / year for hospital care 164 M /
THE PREPARATION OF SINGLE DONOR CRYOPRECIPITATE
 FACTS AND FIGURES JUNE 2004 NO 2 THE PREPARATION OF SINGLE DONOR CRYOPRECIPITATE Revised Edition Shân Lloyd National Blood Transfusion Service Zimbabwe Published by the World Federation of Hemophilia (WFH);
FACTS AND FIGURES JUNE 2004 NO 2 THE PREPARATION OF SINGLE DONOR CRYOPRECIPITATE Revised Edition Shân Lloyd National Blood Transfusion Service Zimbabwe Published by the World Federation of Hemophilia (WFH);
Yale New Haven Health System Center for Healthcare Solutions
 Table of Contents Education and Training Yale New Haven Health System Center for Healthcare Solutions 2009-2010 Fall/Winter Course Guide TOPICS [email protected] www.yalenewhavenhealth.org/healthcaresolutions
Table of Contents Education and Training Yale New Haven Health System Center for Healthcare Solutions 2009-2010 Fall/Winter Course Guide TOPICS [email protected] www.yalenewhavenhealth.org/healthcaresolutions
Patient Information Sheet
 Healthcare Worker exposure to a patient s blood What is a healthcare worker exposure? Patient Information Sheet Occasionally, health care workers come into contact with the blood or body fluids of their
Healthcare Worker exposure to a patient s blood What is a healthcare worker exposure? Patient Information Sheet Occasionally, health care workers come into contact with the blood or body fluids of their
Canadian Blood Services National Public Cord Blood Bank Give Life Twice Transfusion Medicine Residents
 Canadian Blood Services National Public Cord Blood Bank Give Life Twice Transfusion Medicine Residents Eileen Quinlan Collection Supervisor, Brampton (GTA) 2015-11-10 History One Match Stem Cell and Marrow
Canadian Blood Services National Public Cord Blood Bank Give Life Twice Transfusion Medicine Residents Eileen Quinlan Collection Supervisor, Brampton (GTA) 2015-11-10 History One Match Stem Cell and Marrow
How to effectively report to SABRE and SHOT. Richard Haggas Transfusion Quality Manager [email protected]
 How to effectively report to SABRE and SHOT Richard Haggas Transfusion Quality Manager [email protected] Remit To explain how to successfully report to both MHRA and SHOT through the SABRE
How to effectively report to SABRE and SHOT Richard Haggas Transfusion Quality Manager [email protected] Remit To explain how to successfully report to both MHRA and SHOT through the SABRE
SOGC recommendation on ZIKA virus exposure for clinicians caring for pregnant women and those who intend to get pregnant
 SOGC recommendation on ZIKA virus exposure for clinicians caring for pregnant women and those who intend to get pregnant Foreword The rapid emergence of Zika virus as a potential causative agent for fetal
SOGC recommendation on ZIKA virus exposure for clinicians caring for pregnant women and those who intend to get pregnant Foreword The rapid emergence of Zika virus as a potential causative agent for fetal
Health Care Worker Health and Safety: Preventing Needlestick Injury and Occupational Exposure to Bloodborne Pathogens
 Health Care Worker Health and Safety: Preventing Needlestick Injury and Occupational Exposure to Bloodborne Pathogens World Health Organization International Council of Nurses WHO-ICN Project Preventing
Health Care Worker Health and Safety: Preventing Needlestick Injury and Occupational Exposure to Bloodborne Pathogens World Health Organization International Council of Nurses WHO-ICN Project Preventing
Determining Donor Eligibility Blood Donor vs. Stem Cell Donor. Wanda Koetz, RN, HPC Clinical Nurse Lead, Memorial Blood Centers ASFA - May 7, 2015
 Determining Donor Eligibility Blood Donor vs. Stem Cell Donor Wanda Koetz, RN, HPC Clinical Nurse Lead, Memorial Blood Centers ASFA - May 7, 2015 Objectives The learner will be able to: Define donor eligibility
Determining Donor Eligibility Blood Donor vs. Stem Cell Donor Wanda Koetz, RN, HPC Clinical Nurse Lead, Memorial Blood Centers ASFA - May 7, 2015 Objectives The learner will be able to: Define donor eligibility
William Atkinson, MD, MPH Hepatitis B Vaccine Issues June 16, 2016
 William Atkinson, MD, MPH Hepatitis B Vaccine Issues June 16, 2016 Advisory Committee on Immunization Practices (ACIP) The recommendations to be discussed are primarily those of the ACIP composed of 15
William Atkinson, MD, MPH Hepatitis B Vaccine Issues June 16, 2016 Advisory Committee on Immunization Practices (ACIP) The recommendations to be discussed are primarily those of the ACIP composed of 15
SIXTY-SEVENTH WORLD HEALTH ASSEMBLY. Agenda item 12.3 24 May 2014. Hepatitis
 SIXTY-SEVENTH WORLD HEALTH ASSEMBLY WHA67.6 Agenda item 12.3 24 May 2014 Hepatitis The Sixty-seventh World Health Assembly, Having considered the report on hepatitis; 1 Reaffirming resolution WHA63.18,
SIXTY-SEVENTH WORLD HEALTH ASSEMBLY WHA67.6 Agenda item 12.3 24 May 2014 Hepatitis The Sixty-seventh World Health Assembly, Having considered the report on hepatitis; 1 Reaffirming resolution WHA63.18,
Mother s blood test to check her unborn baby s blood group
 Mother s blood test to check her unborn baby s blood group This leaflet explains why it is important to have a blood test to check the baby s blood group, so that only those who need it, receive anti-d
Mother s blood test to check her unborn baby s blood group This leaflet explains why it is important to have a blood test to check the baby s blood group, so that only those who need it, receive anti-d
Planning for an Influenza Pandemic
 Overview It is unlikely that a new pandemic influenza strain will first emerge within Elgin County. The World Health Organization (WHO) uses a series of six phases, as outlined below, of pandemic alert
Overview It is unlikely that a new pandemic influenza strain will first emerge within Elgin County. The World Health Organization (WHO) uses a series of six phases, as outlined below, of pandemic alert
THE E-HEALTH JOURNEY. Ministry of Health Jamaica. Optimizing the use of ICT Applications in Health and Patient Care
 THE E-HEALTH JOURNEY Ministry of Health Jamaica Optimizing the use of ICT Applications in Health and Patient Care 8 th Caribbean Conference on Health Financing Initiatives Presenter: Mr. Arnold Cooper
THE E-HEALTH JOURNEY Ministry of Health Jamaica Optimizing the use of ICT Applications in Health and Patient Care 8 th Caribbean Conference on Health Financing Initiatives Presenter: Mr. Arnold Cooper
Version 4 SPECIAL LEAVE POLICY. A Policy and procedure giving guidance on the Special Leave provisions within the Trust.
 Version 4 SPECIAL LEAVE POLICY A Policy and procedure giving guidance on the Special Leave provisions within the Trust. Authorised by: TEG Date authorised: December 2005 Next review date: 30 April 2016
Version 4 SPECIAL LEAVE POLICY A Policy and procedure giving guidance on the Special Leave provisions within the Trust. Authorised by: TEG Date authorised: December 2005 Next review date: 30 April 2016
GLOBAL DATABASE ON BLOOD SAFETY
 GLOBAL DATABASE ON BLOOD SAFETY Summary Report 1998 1999 World Health Organization Blood Transfusion Safety 1211 Geneva 27, Switzerland Tel: +41 22 791 4385 Fax: +41 22 791 4836 http://www.who.int/bct/bts
GLOBAL DATABASE ON BLOOD SAFETY Summary Report 1998 1999 World Health Organization Blood Transfusion Safety 1211 Geneva 27, Switzerland Tel: +41 22 791 4385 Fax: +41 22 791 4836 http://www.who.int/bct/bts
I thank them for their openness, transparency, and willingness to work with WHO to address this newly emerging infection.
 I N F L U E N Z A - L I K E I L L N E S S O U T B R E A K I N T H E U S A N D M E X I C O T r a n s c r i p t o f G L O B AL T E L E P H O N E N E W S C O N F E R E N C E w i t h D r M a r g a r e t C
I N F L U E N Z A - L I K E I L L N E S S O U T B R E A K I N T H E U S A N D M E X I C O T r a n s c r i p t o f G L O B AL T E L E P H O N E N E W S C O N F E R E N C E w i t h D r M a r g a r e t C
Viral hepatitis. Report by the Secretariat
 SIXTY-THIRD WORLD HEALTH ASSEMBLY A63/15 Provisional agenda item 11.12 25 March 2010 Viral hepatitis Report by the Secretariat THE DISEASES AND BURDEN 1. The group of viruses (hepatitis A, B, C, D and
SIXTY-THIRD WORLD HEALTH ASSEMBLY A63/15 Provisional agenda item 11.12 25 March 2010 Viral hepatitis Report by the Secretariat THE DISEASES AND BURDEN 1. The group of viruses (hepatitis A, B, C, D and
HIV/AIDS policy. Introduction
 HIV/AIDS policy Introduction The International Federation of Red Cross and Red Crescent Societies (International Federation) has a long tradition of working in the area of health and care. National Red
HIV/AIDS policy Introduction The International Federation of Red Cross and Red Crescent Societies (International Federation) has a long tradition of working in the area of health and care. National Red
Guidelines for Viral Hepatitis CTR Services
 Guidelines for Viral Hepatitis CTR Services During the 2007 North Dakota Legislative Assembly, legislation that called for the creation of a viral hepatitis program was introduced and approved. The North
Guidelines for Viral Hepatitis CTR Services During the 2007 North Dakota Legislative Assembly, legislation that called for the creation of a viral hepatitis program was introduced and approved. The North
Canadian Public Health Laboratory Network. Core Functions of Canadian Public Health Laboratories
 Canadian Public Health Laboratory Network Core Functions of Canadian Public Health Laboratories Canadian Public Health Laboratory Network The CPHLN Core Functions of Canadian Public Health Laboratories
Canadian Public Health Laboratory Network Core Functions of Canadian Public Health Laboratories Canadian Public Health Laboratory Network The CPHLN Core Functions of Canadian Public Health Laboratories
EPIDEMIOLOGY OF HEPATITIS B IN IRELAND
 EPIDEMIOLOGY OF HEPATITIS B IN IRELAND Table of Contents Acknowledgements 3 Summary 4 Introduction 5 Case Definitions 6 Materials and Methods 7 Results 8 Discussion 11 References 12 Epidemiology of Hepatitis
EPIDEMIOLOGY OF HEPATITIS B IN IRELAND Table of Contents Acknowledgements 3 Summary 4 Introduction 5 Case Definitions 6 Materials and Methods 7 Results 8 Discussion 11 References 12 Epidemiology of Hepatitis
OCCUPATIONAL HEALTH, DISABILITY AND LEAVE SECTOR MEASURES TO MINIMIZE EXPOSURE TO BLOODBORNE PATHOGENS AND POST-EXPOSURE PROPHYLAXIS POLICY
 UNIVERSITY OF OTTAWA OCCUPATIONAL HEALTH, DISABILITY AND LEAVE SECTOR MEASURES TO MINIMIZE EXPOSURE TO BLOODBORNE PATHOGENS AND POST-EXPOSURE PROPHYLAXIS POLICY Prepared by the Occupational Health, Disability
UNIVERSITY OF OTTAWA OCCUPATIONAL HEALTH, DISABILITY AND LEAVE SECTOR MEASURES TO MINIMIZE EXPOSURE TO BLOODBORNE PATHOGENS AND POST-EXPOSURE PROPHYLAXIS POLICY Prepared by the Occupational Health, Disability
Risk Management Plan (RMP) Guidance (Draft)
 Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare Translated by Pharmaceuticals and Medical Devices Agency Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour
Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare Translated by Pharmaceuticals and Medical Devices Agency Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour
Ontario Pandemic Influenza Plan for Continuity of Electricity Operations
 Planning Guideline GDE-162 Ontario Pandemic Influenza Plan for Continuity of Electricity Operations Planning Guideline Issue 4.0 October 13, 2015 Emergency Preparedness Task Force This planning guide provides
Planning Guideline GDE-162 Ontario Pandemic Influenza Plan for Continuity of Electricity Operations Planning Guideline Issue 4.0 October 13, 2015 Emergency Preparedness Task Force This planning guide provides
City of Cardiff Council Improving Scrutiny Project: Project Brief, January 2015
 APPENDIX A City of Cardiff Council Improving Scrutiny Project: Project Brief, January 2015 Project Purpose Cardiff is proud of its scrutiny arrangements and the important part scrutiny plays in Council
APPENDIX A City of Cardiff Council Improving Scrutiny Project: Project Brief, January 2015 Project Purpose Cardiff is proud of its scrutiny arrangements and the important part scrutiny plays in Council
AUTOLOGOUS BLOOD DONATION
 AUTOLOGOUS BLOOD DONATION UCLA Blood and Platelet Center 1045 Gayley Avenue Los Angeles, California 90024-3401 phone: 310.794.7207 website: www.gotblood.ucla.edu ----------------------------------------------------------------------------------------------------------------------------
AUTOLOGOUS BLOOD DONATION UCLA Blood and Platelet Center 1045 Gayley Avenue Los Angeles, California 90024-3401 phone: 310.794.7207 website: www.gotblood.ucla.edu ----------------------------------------------------------------------------------------------------------------------------
Viral Safety of Plasma-Derived Products
 Viral Safety of Plasma-Derived Products SLIDE 1 This presentation will cover viral validation studies for plasma-derived products. FDA requires that the manufacturing process for biopharmaceutical products
Viral Safety of Plasma-Derived Products SLIDE 1 This presentation will cover viral validation studies for plasma-derived products. FDA requires that the manufacturing process for biopharmaceutical products
CONSENT FORM. Cord Blood Banking for Transplantation
 Cord Blood Program Northwest Tissue Center/Puget Sound Blood Center 921 Terry Avenue Seattle, WA 98104 (206) 292-1896 Swedish Medical Center 747 Broadway Seattle, WA 98122 (206) 386-6000 CONSENT FORM Cord
Cord Blood Program Northwest Tissue Center/Puget Sound Blood Center 921 Terry Avenue Seattle, WA 98104 (206) 292-1896 Swedish Medical Center 747 Broadway Seattle, WA 98122 (206) 386-6000 CONSENT FORM Cord
Red Blood Cell Transfusions for Sickle Cell Disease
 Red Blood Cell Transfusions for Sickle Cell Disease Red Blood Cell Transfusions for Sickle Cell Disease 1 Produced by St. Jude Children s Research Hospital, Departments of Hematology, Patient Education,
Red Blood Cell Transfusions for Sickle Cell Disease Red Blood Cell Transfusions for Sickle Cell Disease 1 Produced by St. Jude Children s Research Hospital, Departments of Hematology, Patient Education,
Public Health Workforce Development in Canada
 Health Canada Santé Canada Public Health Workforce Development in Canada PAHO Regional Forum Antigua, Guatemala July 21, 2004 Brent Moloughney MD, MSc, FRCPC Public Health Consultant to Health Canada Overview
Health Canada Santé Canada Public Health Workforce Development in Canada PAHO Regional Forum Antigua, Guatemala July 21, 2004 Brent Moloughney MD, MSc, FRCPC Public Health Consultant to Health Canada Overview
West Nile virus National Surveillance Report English Edition September 8 to September 14, 2013 (Report Week 37)
 West Nile virus National Surveillance Report English Edition September to September, (Report Week ) Canada Humans: During surveillance week, the Public Health Agency of Canada (the Agency) was informed
West Nile virus National Surveillance Report English Edition September to September, (Report Week ) Canada Humans: During surveillance week, the Public Health Agency of Canada (the Agency) was informed
Q and A for PARTNER Studies: Interim analysis results presented at CROI 2014. CROI presentation can be found at www.chip.dk
 Q and A for PARTNER Studies: Interim analysis results presented at CROI 2014 STATUS: Results embargoed until 4 March 2014, 18:30 Central European Time (CET). CROI presentation can be found at www.chip.dk
Q and A for PARTNER Studies: Interim analysis results presented at CROI 2014 STATUS: Results embargoed until 4 March 2014, 18:30 Central European Time (CET). CROI presentation can be found at www.chip.dk
Coding and Billing for HIV Services in Healthcare Facilities
 P a g e 1 Coding and Billing for HIV Services in Healthcare Facilities The Hawai i State Department of Health STD/AIDS Prevention Branch is pleased to provide you information on billing and reimbursement
P a g e 1 Coding and Billing for HIV Services in Healthcare Facilities The Hawai i State Department of Health STD/AIDS Prevention Branch is pleased to provide you information on billing and reimbursement
The Ontario Cancer Research Ethics Board Overview
 The Ontario Cancer Research Ethics Board Overview Research Ethics Research ethics review is vital to the advancement of ethically sound research. Before individuals can be enrolled in a research study,
The Ontario Cancer Research Ethics Board Overview Research Ethics Research ethics review is vital to the advancement of ethically sound research. Before individuals can be enrolled in a research study,
University of Colorado Hospital Policy and Procedure Transplant and Implant Tissue Storage and Issuance
 University of Colorado Hospital Policy and Procedure Transplant and Implant Tissue Storage and Issuance Related Policies and Procedures: Temperature Controlled Storage Unit Monitoring Hospital Infection
University of Colorado Hospital Policy and Procedure Transplant and Implant Tissue Storage and Issuance Related Policies and Procedures: Temperature Controlled Storage Unit Monitoring Hospital Infection
Nurse Aide Training Program Application Checklist
 Nurse Aide Training Program Application Checklist The following checklist must be completed before enrolling in the Nurse Aide Training course: Complete, sign, and date the Application Form Have the physical
Nurse Aide Training Program Application Checklist The following checklist must be completed before enrolling in the Nurse Aide Training course: Complete, sign, and date the Application Form Have the physical
Blood Donor Counselling
 Blood Donor Counselling Implementation Guidelines In collaboration with: Blood Donor Counselling Implementation Guidelines In collaboration with: WHO Library Cataloguing-in-Publication Data Blood donor
Blood Donor Counselling Implementation Guidelines In collaboration with: Blood Donor Counselling Implementation Guidelines In collaboration with: WHO Library Cataloguing-in-Publication Data Blood donor
Content Introduction. Pag 3. Introduction. Pag 4. The Global Fund in Zimbabwe. Pag 5. The Global Fund Grant Portfolio in Zimbabwe.
 Content Introduction The Global Fund in Zimbabwe The Global Fund Grant Portfolio in Zimbabwe Capacity Development 2009-2014 Capacity Development and Transition Planning 2014 Overview of the Capacity Development
Content Introduction The Global Fund in Zimbabwe The Global Fund Grant Portfolio in Zimbabwe Capacity Development 2009-2014 Capacity Development and Transition Planning 2014 Overview of the Capacity Development
Cord Blood - Public and Private Cord Blood Banking
 Policy Directive Ministry of Health, NSW 73 Miller Street North Sydney NSW 2060 Locked Mail Bag 961 North Sydney NSW 2059 Telephone (02) 9391 9000 Fax (02) 9391 9101 http://www.health.nsw.gov.au/policies/
Policy Directive Ministry of Health, NSW 73 Miller Street North Sydney NSW 2060 Locked Mail Bag 961 North Sydney NSW 2059 Telephone (02) 9391 9000 Fax (02) 9391 9101 http://www.health.nsw.gov.au/policies/
Governance Structure
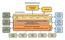 Secretariat -1 Chairs of Standing Committees - 3 Governance Structure Management Committee Secretariat Community and Municipal Consultative and Advisory Forum Board of Directors Principal Investigator
Secretariat -1 Chairs of Standing Committees - 3 Governance Structure Management Committee Secretariat Community and Municipal Consultative and Advisory Forum Board of Directors Principal Investigator
Human Resource Management
 Human Resource Management Module 18 Participate in this seminar to learn more about the board of education s role as an employer. Module 18 workshop and resource materials include these important topics:
Human Resource Management Module 18 Participate in this seminar to learn more about the board of education s role as an employer. Module 18 workshop and resource materials include these important topics:
(EMEA/CHMP/BWP/298390/2005)
 9 February 2006 Reference: EMEA 06004 PPTA s comments on the proposed Guidelines on Validation of immunoassays for the detection of Hepatitis B Virus surface antigen (HBsAg) (EMEA/CHMP/BWP/298390/2005)
9 February 2006 Reference: EMEA 06004 PPTA s comments on the proposed Guidelines on Validation of immunoassays for the detection of Hepatitis B Virus surface antigen (HBsAg) (EMEA/CHMP/BWP/298390/2005)
Approval Review Process: Baccalaureate Nursing Programs in New Brunswick
 Baccalaureate Nursing Programs in New Brunswick Mission The Nurses Association of New Brunswick is a professional regulatory organization that exists to protect the public and to support nurses by promoting
Baccalaureate Nursing Programs in New Brunswick Mission The Nurses Association of New Brunswick is a professional regulatory organization that exists to protect the public and to support nurses by promoting
FAO/WHO Regional Conference on Food Safety for the Americas and the Caribbean San José, Costa Rica, 6-9 December 2005
 Agenda Item 5 Conference Room Document 13 FAO/WHO Regional Conference on Food Safety for the Americas and the Caribbean San José, Costa Rica, 6-9 December 2005 THE FOOD SAFETY REGULATORY SYSTEM IN CANADA
Agenda Item 5 Conference Room Document 13 FAO/WHO Regional Conference on Food Safety for the Americas and the Caribbean San José, Costa Rica, 6-9 December 2005 THE FOOD SAFETY REGULATORY SYSTEM IN CANADA
COMMONWEALTH of VIRGINIA Department of Health
 COMMONWEALTH of VIRGINIA Department of Health MARISSA J. LEVINE, MD, MPH, FAAFP PO BOX 2448 TTY 7-1-1 OR STATE HEALTH COMMISSIONER RICHMOND, VA 23218 1-800-828-1120 Dear Colleague: Emerging Infections
COMMONWEALTH of VIRGINIA Department of Health MARISSA J. LEVINE, MD, MPH, FAAFP PO BOX 2448 TTY 7-1-1 OR STATE HEALTH COMMISSIONER RICHMOND, VA 23218 1-800-828-1120 Dear Colleague: Emerging Infections
IN THE SENATE OF THE UNITED STATES , 2003. M. introduced the following bill; which was referred to the Committee on A BILL
 DRAFT //0 0TH CONGRESS ST SESS. S. To direct the Secretary of Health and Human Services to establish, promote, and support a comprehensive program for education, prevention, and treatment of Hepatitis
DRAFT //0 0TH CONGRESS ST SESS. S. To direct the Secretary of Health and Human Services to establish, promote, and support a comprehensive program for education, prevention, and treatment of Hepatitis
Viral Hepatitis. 2009 APHL survey report
 Issues in Brief: viral hepatitis testing Association of Public Health Laboratories May Viral Hepatitis Testing 9 APHL survey report In order to characterize the role that the nation s public health laboratories
Issues in Brief: viral hepatitis testing Association of Public Health Laboratories May Viral Hepatitis Testing 9 APHL survey report In order to characterize the role that the nation s public health laboratories
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL. EudraLex. The Rules Governing Medicinal Products in the European Union
 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Public Health and Risk Assessment Pharmaceuticals Brussels, SANCO/C8/AM/an D(2010) 380358 EudraLex The Rules Governing Medicinal Products in
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Public Health and Risk Assessment Pharmaceuticals Brussels, SANCO/C8/AM/an D(2010) 380358 EudraLex The Rules Governing Medicinal Products in
The Danish Bone Marrow Donor Registry DBMDR
 The DBMDR Vision To achieve and maintain a position as an internationally recognized hematopoietic stem cell donor registry with respect to high quality of donor data base and HLA typing, individualized,
The DBMDR Vision To achieve and maintain a position as an internationally recognized hematopoietic stem cell donor registry with respect to high quality of donor data base and HLA typing, individualized,
Type of change. V02 Review Feb 13. V02.1 Update Jun 14 Section 6 NPSAS Alerts
 Document Title Reference Number Lead Officer Author(s) (name and designation) Ratified By Central Alerting System (CAS) Policy NTW(O)17 Medical Director Tony Gray Head of Safety and Patient Experience
Document Title Reference Number Lead Officer Author(s) (name and designation) Ratified By Central Alerting System (CAS) Policy NTW(O)17 Medical Director Tony Gray Head of Safety and Patient Experience
12/2/2015 HEPATITIS B AND HEPATITIS C BLOOD EXPOSURE OBJECTIVES VIRAL HEPATITIS
 HEPATITIS B AND HEPATITIS C BLOOD EXPOSURE DISEASE 101 ONLINE CONFERENCE SARAH WENINGER, MPH VIRAL HEPATITIS.STD.HIV PREVENTION COORDINATOR DECEMBER 3, 2015 OBJECTIVES Describe the populations that should
HEPATITIS B AND HEPATITIS C BLOOD EXPOSURE DISEASE 101 ONLINE CONFERENCE SARAH WENINGER, MPH VIRAL HEPATITIS.STD.HIV PREVENTION COORDINATOR DECEMBER 3, 2015 OBJECTIVES Describe the populations that should
Guidelines for preparing Standard Operating Procedures (SOP) for Institutional Ethics Committee for Human Research
 Guidelines for preparing Standard Operating Procedures (SOP) for Institutional Ethics Committee for Human Research 1. Objective: The objective of this SOP is to contribute to the effective functioning
Guidelines for preparing Standard Operating Procedures (SOP) for Institutional Ethics Committee for Human Research 1. Objective: The objective of this SOP is to contribute to the effective functioning
Red blood cell transfusions in the Netherlands Towards (very) low use
 Red blood cell transfusions in the Netherlands Towards (very) low use Marian G.J. van Kraaij, MD PhD hematologist/ transfusion medicine specialist Director Dept Transfusion Medicine Sanquin Blood Supply,
Red blood cell transfusions in the Netherlands Towards (very) low use Marian G.J. van Kraaij, MD PhD hematologist/ transfusion medicine specialist Director Dept Transfusion Medicine Sanquin Blood Supply,
Personal Assessment Form for RN(NP) Practice for the SRNA Continuing Competence Program (CCP)
 Personal Assessment Form for RN(NP) Practice for the SRNA Continuing Competence Program (CCP) Completing a personal assessment is a mandatory component of the SRNA CCP. It allows a RN and RN(NP) to strategically
Personal Assessment Form for RN(NP) Practice for the SRNA Continuing Competence Program (CCP) Completing a personal assessment is a mandatory component of the SRNA CCP. It allows a RN and RN(NP) to strategically
BC Patient Safety & Learning System
 Presentation to Health Quality Network June 10 th, 2009 1 BC Patient Safety & Learning System Overview Background Project Timeline Pilot evaluation results Provincial implementation Concept Strategies
Presentation to Health Quality Network June 10 th, 2009 1 BC Patient Safety & Learning System Overview Background Project Timeline Pilot evaluation results Provincial implementation Concept Strategies
Government of Canada Transformation of Pay Administration Initiative. Presentation to Financial Management Institute
 Government of Canada Transformation of Pay Administration Initiative Presentation to Financial Management Institute Presented By: Brigitte Fortin, Associate Assistant Deputy Minister Accounting, Banking
Government of Canada Transformation of Pay Administration Initiative Presentation to Financial Management Institute Presented By: Brigitte Fortin, Associate Assistant Deputy Minister Accounting, Banking
Management Initiatives in Ontario School Boards: Supporting Student Achievement by Minimizing Distractors
 Management Initiatives in Ontario School Boards: Supporting Student Achievement by Minimizing Distractors Nancy Naylor Assistant Deputy Minister Business and Finance Division Ontario Ministry of Education
Management Initiatives in Ontario School Boards: Supporting Student Achievement by Minimizing Distractors Nancy Naylor Assistant Deputy Minister Business and Finance Division Ontario Ministry of Education
AABB Interorganizational Task Force on Pandemic Influenza and the Blood Supply
 AABB terorganizational Task Force on Pandemic fluenza and the Blood Supply BLOOD COLLECTION FACILITY AND TRANSFUSION SERVICE PANDEMIC INFLUENZA PLANNING CHECKLIST the event of pandemic influenza, blood
AABB terorganizational Task Force on Pandemic fluenza and the Blood Supply BLOOD COLLECTION FACILITY AND TRANSFUSION SERVICE PANDEMIC INFLUENZA PLANNING CHECKLIST the event of pandemic influenza, blood
Immigrant Settlement Support Funding Guidelines
 Immigrant Settlement Support Funding Guidelines Department of Post-Secondary Education, Training and Labour 2015-2016 This document is available online at www.gnb.ca/population For further information
Immigrant Settlement Support Funding Guidelines Department of Post-Secondary Education, Training and Labour 2015-2016 This document is available online at www.gnb.ca/population For further information
Pandemic Influenza Vaccines: Lessons Learned from the H1N1 Influenza Pandemic
 Pandemic Influenza Vaccines: Lessons Learned from the H1N1 Influenza Pandemic Nancy J. Cox, Ph.D. Director, Influenza Division Director WHO Collaborating Center for Influenza NCIRD, Centers for Disease
Pandemic Influenza Vaccines: Lessons Learned from the H1N1 Influenza Pandemic Nancy J. Cox, Ph.D. Director, Influenza Division Director WHO Collaborating Center for Influenza NCIRD, Centers for Disease
NHS LANARKSHIRE ACUTE DIVISION SUBSTANCE MISUSE NURSE LIAISON SERVICE ANNUAL REPORT 2009-2010
 NHS LANARKSHIRE ACUTE DIVISION SUBSTANCE MISUSE NURSE LIAISON SERVICE ANNUAL REPORT 2009-2010 PI/Annual Report 2009/10 1 CONTENTS Executive summary Background Partnership Working Brief Interventions Performance
NHS LANARKSHIRE ACUTE DIVISION SUBSTANCE MISUSE NURSE LIAISON SERVICE ANNUAL REPORT 2009-2010 PI/Annual Report 2009/10 1 CONTENTS Executive summary Background Partnership Working Brief Interventions Performance
Ebola Virus Disease Preparedness in Saskatchewan. Pacific Northwest Border Health Alliance Annual Workshop April 30, 2015
 Ebola Virus Disease Preparedness in Saskatchewan Pacific Northwest Border Health Alliance Annual Workshop April 30, 2015 Select Milestones Mar 23, 2014 Jul 28, 2014 Jul 29, 2014 Jul 31, 2014 Aug 6, 2014
Ebola Virus Disease Preparedness in Saskatchewan Pacific Northwest Border Health Alliance Annual Workshop April 30, 2015 Select Milestones Mar 23, 2014 Jul 28, 2014 Jul 29, 2014 Jul 31, 2014 Aug 6, 2014
Canadian Arts Data / Données sur les Arts au Canada. Request for Proposals to Provide Management Services
 ` Canadian Arts Data / Données sur les Arts au Canada Request for Proposals to Provide Management Services Closing date: 4PM (EDT), 20 September 2012 Please send your proposal to Chair, CADAC Policy, Planning
` Canadian Arts Data / Données sur les Arts au Canada Request for Proposals to Provide Management Services Closing date: 4PM (EDT), 20 September 2012 Please send your proposal to Chair, CADAC Policy, Planning
