Bone-Anchored (BAHA) and Semi Implantable Hearing Aids
|
|
|
- Avice Hunter
- 9 years ago
- Views:
Transcription
1 Bone-Anchored (BAHA) and Semi Implantable Hearing Aids Policy Number: 2016M0023B Effective Date: February 1, 2016 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION 2 Cochlear Implants, 2013M0037A COVERAGE RATIONALE/CLINICAL CONSIDERATIONS 2 BACKGROUND 4 REGULATORY STATUS 5 CLINICAL EVIDENCE 8 APPLICABLE CODES 8 REFERENCES 10 POLICY HISTORY/REVISION INFORMATION 13 INSTRUCTIONS: Medical Policy assists in administering UCare benefits when making coverage determinations for members under our health benefit plans. When deciding coverage, all reviewers must first identify enrollee eligibility, federal and state legislation or regulatory guidance regarding benefit mandates, and the member specific Evidence of Coverage (EOC) document must be referenced prior to using the medical policies. In the event of a conflict, the enrollee's specific benefit document and federal and state legislation and regulatory guidance supersede this Medical Policy. In the absence of benefit mandates or regulatory guidance that govern the service, procedure or treatment, or when the member s EOC document is silent or not specific, medical policies help to clarify which healthcare services may or may not be covered. This Medical Policy is provided for informational purposes and does not constitute medical advice. In addition to medical policies, UCare also uses tools developed by third parties, such as the InterQual Guidelines, to assist us in administering health benefits. The InterQual Guidelines are intended to be used in connection with the independent professional medical judgment of a qualified health care provider and do not constitute the practice of medicine or medical advice. Other Policies and Coverage Determination Guidelines may also apply. UCare reserves the right, in its sole discretion, to modify its Policies and Guidelines as necessary and to provide benefits otherwise excluded by medical policies when necessitated by operational considerations. Page. 1 of 13
2 POLICY DESCRIPTION: This policy provides information about bone-anchored and semi-implantable hearing aids and their recommended use. Bone-anchored hearing aids (BAHAs) transmit sound vibrations to the inner ear by direct bone transmission through the skull. A titanium screw implanted into the skull allows removable coupling of the sound processor to the bone. Semi-implantable hearing aids consist of an external audio processor that is worn behind the ear or in the ear canal, a receiver that may be housed with the audio processor or implanted, and an electromagnetic transducer that is implanted so its tip contacts the ossicles or is close to a magnet implanted on the ossicles. Both devices are intended to improve hearing precision in individuals who have moderate to severe conductive or mixed hearing loss and who cannot use or are dissatisfied with the level of sound perception or quality of sound provided by standard air conduction hearing aids (ACHAs). COVERAGE RATIONALE / CLINICAL CONSIDERATIONS: A. Unilateral or bilateral osseointegraded implants, bone-anchored and semi implantable hearing aid(s) devices, may be considered MEDICALLY NECESSARY as an alternative to an air-conduction hearing aid for individuals five years of age and older with a conductive or mixed hearing loss who have one of the following medical conditions and meet at least one audiology criteria: Medical Conditions (at least one): Congenital or surgically induced malformations (e.g., atresia) of the external or middle ear canal Severe chronic external otitis or otitis media Tumors of the external ear canal and/or tympanic cavity Dermatitis of the external ear canal Other anatomic or medical conditions that contraindicate the use of an air conduction hearing aid Audiologic criteria (at least one): A pure tone average bone-conduction threshold measured at 0.5, 1, 2, and 3 khz of better than or equal to 45 db (OBC and BP100 devices), 55 db (Intenso device), or 65 db (Cordele II device). For bilateral implantation, patients should meet the above audiologic criteria and have a symmetrically conductive or mixed hearing loss as defined by a difference between left and right side bone conduction threshold of less than 10 db on average measured at 0.5, 1, 2 and 3 khz (4 khz for OBC and Ponto Pro), or less than 15 db at individual frequencies. B. A semi implantable bone-conduction (bone-anchored) hearing aid may be considered MEDICALLY NECESSARY as an alternative to an air-conduction CROS hearing aid in patients 5 years of age and older with single-sided sensorineural deafness and normal hearing in the other ear. The pure tone average air conduction threshold of the normal ear should be better than 20 db measured at 0.5, 1, 2, and 3 khz. C. Replacement parts or upgrades to existing BAHA components (e.g., batteries, processor) are considered not medically necessary when the criteria specified in (A) or (B) above are not met or when requested for convenience or to upgrade to newer technology when the current components remain functional. Page. 2 of 13
3 D. Partially implantable bone conduction hearing systems using magnetic coupling for acoustic transmission (e.g., Otomag Alpha 1 [M]) are considered EXPERIMENTAL OR INVESTIGATIONAL. E. Other uses of implantable bone-conduction (bone-anchored) hearing aids, including use in patients with bilateral sensorineural hearing loss, are considered EXPERIMENTAL OR INVESTIGATIONAL. BENEFIT NOTE: Bone-Anchored (BAHA) and Semi Implantable Hearing Aid devices are referred to as Hearing Aid, Bone Conduction in the U.S. Food and Drug Administration (FDA) label. The FDA also indicates that these devices have substantially equivalent technology as air-conduction hearing aids with digital sound processing. However, Medicare and Medicaid Services (CMS) consider these devices as prosthetics. Therefore, osseointegrated devices that produce perception of sound by replacing the function of the middle ear, cochlea or auditory nerve are payable by Medicare Advantage as prosthetic devices. Thus, Plan needs to review contract language in making decisions about classification. Benefit limitations regarding hearing aids may apply in the Exchange and other products. Clinical Considerations: BAHAs utilize an FDA-approved, bone-anchored, bone conduction hearing aid, specifically indicated for individuals five years of age and older. The most successful outcomes are obtained when used in adults who suffer from chronic otitis media and mastoids and were unable to tolerate traditional air conduction hearing aids or in whom these were contraindicated due to either recurrent otorrhoea, otitis externa or aural stenosis, and in pediatrics with congenital aural atresia. BAHAs avoid the several potential complications of surgical reconstruction of congenital atresia, an operation fraught with difficulty in young children with acceptable results only in expert hands. Current techniques of fixture implantation and osseointegration are associated with minimal complication rates. However, the health of the titanium implant and the ultimate success of the BAHA depend heavily upon meticulous surgical care and cleaning of the abutment. However, complications of BAHAs can be considered in two categories: intra-operative and post-operative complications. Intraoperative complications are more common in children because most of them have craniofacial abnormalities. Magnetic resonance imaging (MRI) is contraindicated in patients using Semi-Implantable Electromagnetic Hearing Aids devices because it produces linear and rotational forces that may dislodge the implant and damage surrounding tissue. The FDA recommends removing the implant if MRI is medically necessary. Electrosurgery near the implant, diathermy over the implant, or electroconvulsive therapy in a patient with the implant are also not advised due to potential damage to the implant or the patient s hearing. In patients being considered for implantable bone-conduction (bone-anchored) hearing aid(s), skull bone quality and thickness should be assessed for adequacy to ensure implant stability. Additionally, patients (or caregivers) must be able to perform proper hygiene to prevent infection and ensure the stability of the implants and percutaneous abutments. Page. 3 of 13
4 BACKGROUND: More than 28 million Americans suffer from congenital or acquired hearing loss. Hearing loss can be classified as conductive, sensorineural, or mixed hearing loss. Conductive hearing loss involves the external and middle ear and is due to mechanical or physical blockage of sound as a result of excessive cerumen, a punctured eardrum, birth/congenital defects such as congenital aural atresia (CAA), ear infections or heredity. In sensorineural or "nerve" hearing loss, the auditory cranial nerve or part of the bone of the inner ear is damaged due to birth-related condition, long-term viral or bacterial infections, trauma, exposure to loud noises, the use of certain drugs, fluid buildup in the middle ear, or a benign tumor in the inner ear (acoustic neuroma). Mixed hearing loss is conductive hearing loss coupled with sensorineural hearing loss. Normal range or no impairment of hearing occurs at 0 to 20 db threshold. The American Speech-Language-Hearing Association (ASLHA, 2010) defines the degree (severity) of hearing loss (HL) as: mild (20 to 40 db), moderate (40 to 60 db), severe (60 to 80 db), and profound (greater than or equal to 80 db) Permanent hearing loss is often treated with hearing aids. Conventional external hearing aids consist of a microphone to detect sounds in the environment, a battery-powered amplifier to increase sound volume, and a small-diameter tube that functions as a sound pressure conduit. Sound received in the microphone is processed by the amplifier and transmitted by the sound pressure conduit into the ear canal as sound pressure, which vibrates the eardrum, ossicles, and inner ear fluids. Over the last decade, conventional hearing aids have evolved with improvements in analog and digital processing. Despite improvements, conventional hearing aids continue to be limited by problems that lead to nonuse including occlusive effects, acoustic feedback, sound distortion, infection, chronic irritation, discomfort, frequent battery changes and maintenance, lifestyle restrictions, and cosmesis. Implantable hearing devices, such as cochlear implants (CIs), bone-anchored hearing aids (BAHAs), and middle ear implants (MEIs), may offer alternatives for individuals who will not use or do not derive sufficient benefit from conventional hearing aids. These devices employ different designs to treat a specific type and degree of hearing loss. BAHAs transmit sound directly to the skull or ossicles instead of through an amplifier as in conventional hearing aids. BAHAs consist of a titanium fixture which is implanted behind the ear in the temporal bone and protrudes through the skin, and an external sound processor connects to the implanted fixture. The microphone of the sound processor detects sound vibrations and transfers them to the cochlea by direct bone conduction. The difference between the standard bone conduction hearing aid and the boneanchored hearing aid is direct stimulation of the bone instead of stimulation through the skin. MEIs generally have been used in adults with moderate to severe SNHL who are noncompliant with, or do not obtain sufficient benefit from, conventional hearing aids. MEIs are broadly divided into semiimplantable or fully implantable piezoelectric or electromagnetic hearing systems. Despite different designs, all MEIs work by stimulating the ossicles, thereby enhancing signals received by cochlear hair cells. Semi-implantable electromagnetic hearing aids consist of an audio processor unit that is worn behind the ear or in the ear canal and houses a microphone, sound processor, and battery; a receiver unit that may be housed with the audio processor or implanted; and an electromagnetic transducer that is implanted so that Page. 4 of 13
5 its tip contacts the ossicles or is close to a magnet implanted on the ossicles. The microphone detects, amplifies, and converts sounds into electric currents which are successively, manipulated by the sound processor, transmitted to the receiver coil, and conveyed to the electromagnetic transducer. This transducer converts the electric currents into a magnetic field that vibrates the ossicles, either by direct contact with the ossicles or by acting on (attracting and repelling) a magnet implanted on the ossicles. Currently, three semi-implantable electromagnetic hearing devices are commercially available in the United States and/or Europe. These are the Vibrant Soundbridge System (Med-El GmbH); the Maxum System (Ototronix), originally marketed under the name Soundtec Direct System (Ototronix); and the Semi- Implantable Middle Ear Transducer (MET) Ossicular Stimulator System (Otologics LLC). REGULATORY STATUS: 1. U.S. FOOD AND DRUG ADMINISTRATION (FDA): Bone-Anchored Hearing Aids (BAHAs): There are four BAHA sound processors for use with the BAHA auditory osseointegrated implant system manufactured by Cochlear Americas (Englewood, CO) that have received 510(k) clearance from the U.S. Food and Drug Administration (FDA): o BAHA Cordelle II o BAHA Divino o BAHA Intenso (digital signal processing) o BAHA BP100 The U.S. Food and Drug Administration (FDA) approved the BAHA system for the following indications: o Patients who have conductive or mixed hearing loss and can still benefit from sound amplification o Patients with bilaterally symmetric conductive or mixed hearing loss, may be implanted bilaterally o Patients with sensorineural deafness in one ear and normal hearing in the other (i.e., singlesided deafness, SSD) o Patients who are candidates for an air-conduction contralateral routing of signals (AC CROS) hearing aid but who cannot or will not wear an AC CROS device. In 1995, the FDA granted approval to Nobelpharm USA to market the BRÅNEMARK Bone-Anchored Hearing Aid (BAHA ) System, which is manufactured by Entific Medical Systems for adult patients with malformations of the external ear, chronically draining ear, a pure tone threshold hearing loss of 45 decibels (db), and/or inability or unwillingness to use an air conduction hearing aid. In 1999, this approval was extended for use in children 5 years of age or older. In 2002, the BAHA System was approved for single-sided deafness due to sensorineural deafness (Entific Medical Systems, 2005; FDA, 2005). Another BAHA, the Xomed Audiant Bone Conductor (Xomed, Inc.), was approved by the FDA in 1986 but is no longer on the market (FDA, 2005). In May 2011, the FDA cleared a modified sound processor, the BAHA BP110 Power as a substantially equivalent sound processor to the predicate BAHA Intenso. The Headband/Softband for BAHA received FDA 510(k) clearance in October 2000 as substantially Page. 5 of 13
6 equivalent to devices already on the market. With this application, there is no implantation surgery. The sound processor is attached to the head using either a hard or soft headband. The amplified sound is transmitted transcutaneously to the bones of the skull for transmission to the cochlea. The BAHA Softband received FDA clearance in 2002 for use in children younger than the age of 5 years. As this application has no implanted components, it is not addressed in the policy. In November 2008, the device OBC Bone Anchored Hearing Aid System (Oticon Medical, Kongebakken, Denmark) was cleared by the U.S. Food and Drug Administration (FDA) for marketing through the 510(k) process. Subsequently, additional bone conduction hearing systems have received 510(k) marketing clearance from the FDA including Otomag (Sophono, Inc., Boulder, CO) and Ponto (Oticon Medical). The Ponto Pro processor can be used with the Oticon or BAHA implants. In May 2011, Sophono, Inc. and Oticon Medical partnered to receive 510(k) marketing clearance from the FDA for the Otomag Alpha 1(M), a partially implantable bone conduction hearing system. All of these devices were determined to be substantially equivalent to existing devices (e.g., the Xomed Audiant, which was FDA cleared for marketing in 1986 but is no longer available). They share similar indications as the Cochlear Americas BAHA devices. Semi-Implantable Electromagnetic Hearing Aid Devices: Currently, there are only two semi-implantable electromagnetic hearing aid devices (product code, MPV) approved by the FDA. Vibrant Soundbridge System (Premarket Approval [PMA] number, P990052), which was approved on August 31, Maxum System (PMA number, ), which was approved by the FDA on September 7, Both systems have consistently been indicated for treating moderate to severe sensorineural hearing loss (SNHL) in adults (age 18 years) who desire an alternative to acoustic hearing aids, particularly those who have had prior experience with appropriately fitted acoustic hearing aids (CDRH, 2011). The Vibrant Soundbridge System is approved for use in Europe in children age 3 years, but is not approved in the United States (Med-El, 2010). The semi-implantable Middle Ear Transducer (MET) Ossicular Stimulator System (Otologics) is commercially available in Europe for treating moderate to severe SNHL in adults (Tringali et al., 2010) but is not approved by the FDA (CDRH, 2011). 2. CENTERS FOR MEDICARE AND MEDICAID SERVICES (CMS): Hearing Aids and Auditory Implants Section 1862(a)(7) of the Social Security Act states that no payment may be made under part A or part B for any expenses incurred for items or services where such expenses are for... hearing aids or examinations therefore.... This policy is further reiterated at 42 CFR (d) which specifically states that hearing aids or examination for the purpose of prescribing, fitting, or changing hearing aids are excluded from coverage. Hearing aids are amplifying devices that compensate for impaired hearing. Hearing aids include air conduction devices that provide acoustic energy to the cochlea via stimulation of the tympanic membrane with amplified sound. They also include bone conduction devices that provide mechanical energy to the cochlea via stimulation of the scalp with amplified mechanical vibration or by direct contact with the tympanic membrane or middle ear ossicles. Page. 6 of 13
7 Certain devices that produce perception of sound by replacing the function of the middle ear, cochlea or auditory nerve are payable by Medicare as prosthetic devices. These devices are indicated only when hearing aids are medically inappropriate or cannot be utilized due to congenital malformations, chronic disease, severe sensorineural hearing loss or surgery. The following are prosthetic devices: Cochlear implants and auditory brainstem implants, i.e., devices that replace the function of cochlear structures or auditory nerve and provide electrical energy to auditory nerve fibers and other neural tissue via implanted electrode arrays. Osseointegrated implants, i.e., devices implanted in the skull that replace the function of the middle ear and provide mechanical energy to the cochlea via a mechanical transducer. 3. MINNESOTA DEPARTMENT OF HUMAN SERVICES (DHS): Hearing Aid Services are an MHCP Covered Service. Hearing aid services are an MHCP covered service. Authorization is required for all hearing systems. When systems such as FM systems, vibrotactile devices, cochlear implants or personal communicators (e.g., pocket talkers) are requested, justification is needed, just as for non-contract aids. The audiologist must also address each of the following points: Why the person cannot use personal hearing aids (e.g., person's unique inabilities to use auditory information provided via hearing aids). Documentation of expectation of person's ability to recognize and use vibrotactile information, specific to vibrotactile instruments (e.g., response to environmental vibratory information or low frequency bone conducted vibratory information). All hearing aids must be purchased directly from manufacturers participating in the Hearing Aid Volume Purchase Contracts. Hearing aid service providers are paid the contract price plus a dispensing fee. Hearing aids must: Be new, current production models and be complete instruments, including all necessary equipment to make it fully functional, Use standard commercial batteries and battery sizes, Be accompanied by a live performance graph and invoice at the contracted price, Have a minimum 24-month manufacturer warranty covering parts and labor (warranty is exclusive of the ear piece, cord, and batteries), Have a one-year loss and damage warranty. Hearing aids that do not prove satisfactory to a user are to be returned to the manufacturer within 90 days from the date the hearing aid is provided to the recipient at no cost to DHS/MHCP or the hearing aid dealer. The hearing aid volume purchase contract requires that: The contract price for a hearing aid cannot be further reduced or altered, Orders for DHS contracted hearing aids may not be used to obtain, or grant, additional commercial discounts, The manufacturer may not charge for packaging, postage, insurance, or handling. Hearing aids do not include ear molds and accessories not included in the cost of the hearing aid but that are necessary to the recipient s use of the hearing aid: Page. 7 of 13
8 Ear molds and ear impressions are billable for BTE aids, Accessories including chest harnesses, tone and ear hooks, carrying cases, T-coils, audio boots, neck loops, etc., are billable when not included in the price of the hearing aid (check the contract for hearing aid features included in the price). No extra charge may be made for: Casing color choice, Hypo-allergenic cases, Soft canal casing or other shell treatments, Conventional or screw-set volume control. Manufacturers will not process hearing aid orders unless all authorization requirements are met. CLINICAL EVIDENCE SUMMARY: Bone-Anchored Hearing Aids: The available evidence for unilateral or bilateral implantable boneconduction (bone-anchored) hearing aid(s) consists of observational studies that report prepostdifferences in hearing parameters after treatment with BAHA. While this evidence is not ideal, it is sufficient to demonstrate improved net health outcome for patients 5 years of age or older in certain situations. The evidence supports the use of these devices in patients with conductive or mixed hearing loss who meet other medical and audiologic criteria. For patients with single-sided sensorineural deafness, a binaural hearing benefit may be provided by way of contralateral routing of signals to the hearing ear. There is evidence that bilateral devices improve hearing to a greater degree than do unilateral devices. Bone-anchored hearing aids may be considered as an alternative to air-conduction devices in these patients and therefore, these devices may be considered medically necessary in these situations. Semi-Implantable Electromagnetic Hearing Aid Devices: The available evidence from the studies indicate that, compared with unaided hearing, the Vibrant Soundbridge System improves hearing thresholds and speech perception. This device appears to improve patient satisfaction; however, evidence is conflicting about the impact on hearing thresholds and speech perception compared with conventional hearing aids. APPLICABLE CODES: The Current Procedural Terminology (CPT ) codes and HCPCS codes listed in this policy are for reference purposes only. Listing of a service or device code in this policy does not imply that the service described by this code is a covered or non-covered health service. The inclusion of a code does not imply any right to reimbursement or guarantee claims payment. Other medical policies and coverage determination guidelines may apply. HCPCS Codes S9128 S2230 V508-V5299 L8690 Description Speech therapy, in the home, per diem Implantation of magnetic component of semi-implantable hearing device on ossicles in middle ear Hearing Services Auditory osseointegrated device, includes all internal and external components Page. 8 of 13
9 L8691 L8692 L8693 G0153 ICD-9 Codes Auditory osseointegrated device, external sound processor, replacement Auditory osseointegrated device, external sound processor, used without osseointegration, body worn, includes headband or other means of external attachment [excluded under plans that exclude coverage of hearing aids] Auditory osseointegrated device abutment, any length, replacement only Services performed by a qualified speech-language pathologist in the home health or hospice setting, each 15 minutes Description Malignant neoplasm of auditory tube, middle ear, and mastoid air cells Malignant neoplasm of head, face and neck Malignant neoplasm of skin of ear and external auditory canal Benign neoplasm of nasal cavities, middle ear, and accessory sinuses Benign neoplasm of head, face, and neck Benign neoplasm of ear and external auditory canal Carcinoma in situ of ear and external auditory canal Hunter s Syndrome Acquired deformities of auricle or pinna [surgically induced malformations of external ear canal or middle ear] Chronic serous otitis media, simple or unspecified [severe] Other and unspecified chronic nonsuppurative otitis media [severe] Chronic atticoantral suppurative otitis media Unspecified chronic suppurative otitis media Unspecified otitis media Otosclerosis [causing hearing loss in persons who cannot undergo stapedectomy] Conductive hearing loss Sensorineural hearing loss, unspecified Sensory hearing loss Neural hearing loss Central hearing loss Sensorineural hearing loss, unilateral Sensorineural hearing loss of combined types Mixed conductive and sensorineural hearing loss Other atopic dermatitis and related conditions , Contact dermatitis and other eczema [external ear/hypersensitivity reactions] Other anomalies of external ear with impairment of hearing [congenital malformations of external ear canal] Anomaly of middle ear, except ossicles [congenital malformations of middle ear] Anomalies of ear ossicles [congenital malformations of middle ear] Unspecified anomaly of ear [congenital malformations of external ear canal or middle ear] Hemifacial microsomi 755.1x* Syndactylia Pfeiffer s disease Crouzon s disease 756.0* Treacher Collins Thalamoid embryopathy * The x represents a range of codes; it is dependent on the specific diagnosis. Page. 9 of 13
10 ICD-10 Codes Description H90.3 Sensorineural hearing loss, bilateral H90.4 Sensorineural hearing loss, unilateral with unrestricted hearing on the contralateral side H90.5 Sensorineural hearing loss, unspecified H90.6 Mixed conductive and sensorineural hearing loss, bilateral H90.7 Mixed conductive and sensorineural hearing loss, unilateral with unrestricted hearing on the contralateral side H90.8 Mixed conductive and sensorineural hearing loss, unspecified CPT Codes Description Implantation or replacement of electromagnetic bone conduction hearing device in temporal bone Removal or repair of electromagnetic bone conduction hearing device in temporal bone Implantation, osseointegrated implant, temporal bone, with percutaneous attachment to external speech processor/cochlear stimulator; without mastoidectomy Implantation, osseointegrated implant, temporal bone, with percutaneous attachment to external speech processor/cochlear stimulator; with mastoidectomy CPT is a registered trademark of the American Medical Association. REFERENCES: 1. Baguley DM, Bird J, Humphriss R, Prevost A. The evidence base for the application of contralateral bone anchored hearing aids in acquired unilateral sensorineural hearing loss in adults. Clin Otolaryngol. 2006;31(1): Bance M, Abel SM, Papsin BC, et al. A comparison of the audiometric performance of bone anchored hearing aids and air conduction hearing aids. Otol Neurotol. 2002;23(6): Bergeron F. Bone-anchored hearing aid. AETMIS Summary. Montreal, QC: Agence D Evaluation des Technologies et des Modes D Intervention en Santé (AETMIS); May Briggs RJ, Luxford WM. Chronic ear surgery: a historical review. Am J Otol. 1994;15(4): Browning GG, Gatehouse S. Estimation of the benefit of bone-anchored hearing aids. Ann Otol Rhinol Laryngol. 1994;103(11): Burrell SP, Cooper HC, Proops DW. The bone anchored hearing aid--the third option for otosclerosis. J Laryngol Otol Suppl. 1996;21: Centers for Medicare & Medicaid Services (CMS). Hearing aids and auditory implants. Medicare Benefit Policy Manual, Ch General Exclusions from Coverage, Sec Available at: Accessed November 12, Centers for Medicare & Medicaid Services (CMS). Your Medicare Benefits. Revised Jan Available at: Accessed November 12, Christensen L, Richter GT, Dornhoffer JL. Update on bone-anchored hearing aids in pediatric patients with profound unilateral sensorineural hearing loss. Arch Otolaryngol Head Neck Surg. 2010;136(2): Clark M, Mierzwinski-Urban M. Bone anchored hearing aid in adults. Health Technology Inquiry Service. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health (CADTH); September 28, Cochlear Corporation. Bone Conduction Implants Available at: Accessed November 12, Colquitt JL, Jones J, Harris P, et al. Bone-anchored hearing aids (BAHAs) for people who are bilaterally deaf: A systematic review and economic evaluation. Health Technol Assess. 2011;15(26):1-200, iii-iv. 13. Colquitt JL, Loveman E, Baguley DM, et al. Bone-anchored hearing aids for people with bilateral hearing impairment: A systematic review. Clin Otolaryngol. 2011;36(5): Combs JT. Office screening for hearing loss. Contemp Pediatr. 1995;12(10): Cooper HR, Burrell SP, Powell RH, et al. The Birmingham bone anchored hearing aid programme: referrals, Page. 10 of 13
11 selection, rehabilitation, philosophy and adult results. J Laryngol Otol Suppl. 1996;110(suppl 21): Cooper T, Tomlinson J, Sutton J, et al. The use of bone anchored hearing aids. Guidance Note for Purchasers. Trent Institute for Health Services Research, Universities of Leicester, Nottingham and Sheffield; Davids T, Gordon KA, Clutton D, Papsin BC. Bone-anchored hearing aids in infants and children younger than 5 years. Arch Otolaryngol Head Neck Surg. 2007;133(1): de Wolf MJ, Hendrix S, Cremers CW, Snik AF. Better performance with bone-anchored hearing aid than acoustic devices in patients with severe air-bone gap. Laryngoscope. 2011(a);121(3): de Wolf M, Hol M, Mylanus E, et al. Benefit and quality of life after bone-anchored hearing aid fitting in children with unilateral or bilateral hearing impairment. Arch Otolaryngol Head Neck Surg. 2011(b);137(2): Dutt SN, McDermott AL, Jelbert A, et al. Day to day use and service-related issues with the bone-anchored hearing aid: the Entific Medical Systems questionnaire. J Laryngol Otol. 2002b;116(suppl 28): Dutt SN, McDermott AL, Jelbert A, et al. The Glasgow benefit inventory in the evaluation of patient satisfaction with the bone-anchored hearing aid: quality of life issues. J Laryngol Otol. 2002a;116(suppl 28): Foster JR, Haggard MP. FAAF An efficient analytical test of speech perception. In: Proceedings of the Institute of Acoustics; MRC Institute of Hearing Research, Nottingham, England; 1979: Granström G, Berström K, Odersjö M, Tjellström A. Osseointegrated implants in children: experience from our first 100 patients. Otolaryngol Head Neck Surg. 2001;125(1): Granström G, Tjellström A. The bone-anchored hearing aid (BAHA) in children with auricular malformations. Ear Nose Throat J. 1997;76(4):238-40,242, Hakansson B, Liden G, Tjellström A, et al. Ten years of experience with the Swedish bone-anchored hearing system. Ann Otol Rhinol Laryngol Suppl. 1990;151: Hakansson BE, Carlsson PU, Tjellstrom A, et al. The bone-anchored hearing aid: Principal design and audiometric results. Ear Nose Throat J. 1994;73(9): Hayes, Inc. Medical Technology Directory. Bone-Anchored Hearing Aids. June 3, Reviewed Sept 4, Hayes, Inc. Medical Technology Directory. Semi-Implantable Electromagnetic Hearing Aids. April 8, Reviewed July 10, Health Technology Inquiry Service (HTIS). Completely-in-the-canal and bone anchored hearing aids: A review of the clinical effectiveness and cost-effectiveness. Health Technology Assessment (HTA). Ottawa, ON: Canadian Agency for Drugs and Technologies in Health (CADTH); March 4, Hol MK, Kunst SJ, Snik AF, Cremers CW. Pilot study on the effectiveness of the conventional CROS, the transcranial CROS and the BAHA transcranial CROS in adults with unilateral inner ear deafness. Eur Arch Otorhinolaryngol. 2010;267(6): Hol MK, Snik AF, Mylanus EA, Cremers CW. Does the bone-anchored hearing aid have a complementary effect on audiological and subjective outcomes in patients with unilateral conductive hearing loss? Audiol Neurootol. 2005;10(3): Macnamara M, Phillips D, Proops DW. The bone anchored hearing aid (BAHA) in chronic suppurative otitis media (CSOM). J Laryngol Otol Suppl. 1996;21: McLarnon CM, Davison T, Johnson IJ. Bone-anchored hearing aid: Comparison of benefit by patient subgroups. Laryngoscope. 2004;114(5): Minnesota Department of Human Services. Hearing Aid Services. Revised Available at: atestreleased&ddocname=id_ Accessed November 12, Mylanus EA, van der Pouw KC, Snik AF, et al. Intraindividual comparison of the bone-anchored hearing aid and airconduction hearing aids. Arch Otolaryngol Head Neck Surg. 1998;124(3): National Deaf Children's Society (NDCS). Quality standards in bone anchored hearing aids for children and young people. London, UK: NDCS; July Niehaus HH, Helms J, Muller J. Are implantable hearing devices really necessary? Ear Nose Throat J. 1995;74(4): , Niparko JK, Cox KM, Lustig LR. Comparison of the bone anchored hearing aid implantable hearing device with Page. 11 of 13
12 contralateral routing of offside signal amplification in the rehabilitation of unilateral deafness. Otol Neurotol. 2003;24(1): Olsen SØ, Glad H, Nielsen LH. Comparison of two bone anchored hearing instruments: BP100 and Ponto Pro. Int J Audiol. 2011;50(12): Ontario Ministry of Health and Long-Term Care, Medical Advisory Secretariat. Bone anchored hearing aid (BAHA). Health Technology Scientific Literature Review. Ontario Ministry of Health and Long-Term Care; September Parrella A, Mundy L. Bone anchored hearing aid (BAHA). Horizon Scanning Prioritising Summary - Volume 8. Adelaide, SA: Adelaide Health Technology Assessment (AHTA) on behalf of National Horizon Scanning Unit (HealthPACT and MSAC); Pichon-Riviere A, Augustovski F, Garcia Marti S, et al. BAHA devices in hypoacusia [summary]. IRR No Buenos Aires, Argentina: Institute for Clinical Effectiveness and Health Policy (IECS); Powell RH, Burrell SP, Cooper HR, et al. The Birmingham bone anchored hearing aid programme: Paediatric experience and results. J Laryngol Otol Suppl. 1996;21: Priwin C, Granstrom G. The bone-anchored hearing aid in children: A surgical and questionnaire follow-up study. Otolaryngol Head Neck Surg. 2005;132(4): Priwin C, Jönsson R, Hultcrantz M, et al. BAHA in children and adolescents with unilateral or bilateral conductive hearing loss: A study of outcome. Int J Pediatr Otorhinolaryngol. 2007;71(1): Snik AF, Mylanus EA, Cremers CW. Bone-anchored hearing aids in patients with sensorineural hearing loss and persistent otitis externa. Clin Otolaryngol. 1995;20(1): Spitzer JB, Soha NG, Wazen JJ. Evolving applications in the use of bone-anchored hearing aids. Am J Audiology. 2002;11: Stenfelt S, Hakansson B, Jonsson R, Granstrom G. A bone-anchored hearing aid for patients with pure sensorineural hearing impairment: A pilot study. Scand Audiol. 2000;29(3): Tjellstrom A, Hakansson B, Granstrom G. Bone-anchored hearing aids: Current status in adults and children. Otolaryngol Clin North Am. 2001;34(2): U.S. Food and Drug Administration. 510(k) Bilateral fitting of BAHA. Summary of Safety and Effectiveness. Search 510(k) database. No. K Available at: Accessed November 12, U.S. Food and Drug Administration. Branemark Bone Anchored Hearing Aid (BAHA) System. Summary of Safety and Effectiveness. 510(k)No. K U.S. Food and Drug Administration. BAHA BP100. Summary of Safety and Effectiveness. 510(k) No. K U.S. Food and Drug Administration. BAHA Cordelle II. Summary of Safety and Effectiveness. 510(k) No. K U.S. Food and Drug Administration. BAHA Divino. Summary of Safety and Effectiveness. 510(k) No. K U.S. Food and Drug Administration. BAHA Intenso. Summary of Safety and Effectiveness. 510(k) No. K U.S. Food and Drug Administration. Branemark Bone Anchored Hearing Aid (BAHA). Summary of Safety and Effectiveness. 510(k) No. K UK National Health Service, Cambridgeshire and Peterborough Public Health Network. Bone anchored hearing aids (BAHAs). Policy. Cambridgeshire Health Authority Board; February 27, Verhaegen VJ, Mulder JJ, Mylanus EA, et al. Profound mixed hearing loss: Bone-anchored hearing aid system or cochlear implant? Ann Otol Rhinol Laryngol. 2009;118(10): Wazem JJ, Spitzer JB, Ghossaini SN, et al. Transcranial contralateral cochlear stimulation in unilateral deafness. Otolaryngol Head Neck Surg. 2003;129: Wazen JJ, Caruso M, Tjellstrom A. Long-term results with the titanium bone-anchored hearing aid: The U.S. experience. Am J Otol. 1998;19(6): Wazen JJ, Spitzer J, Ghossaini SN, et al. Results of the bone-anchored hearing aid in unilateral hearing loss. Laryngoscope. 2001;111(6): Page. 12 of 13
13 POLICY HISTORY: DATE ACTION/DESCRIPTION 07/10/2013 New Policy 2013M0023A. Reviewed by Interim Medical Policy Committee. 07/25/2013 Reviewed and approved by the Quality Improvement Advisory and Credentialing Council (QIACC). 11/15/2013 Published to UCare.org. 12/7/2015 Updated. Reviewed and approved by Medical Policy Committee. Policy number revised to 2016M0023B. 12/17/2015 Reviewed and approved by the Quality Improvement Advisory and Credentialing Council (QIACC). 01/01/2016 Published to UCare.org. Page. 13 of 13
CONVENTIONAL AND DIGITAL HEARING AIDS
 CONVENTIONAL AND DIGITAL HEARING AIDS Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical
CONVENTIONAL AND DIGITAL HEARING AIDS Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical
Implantable Bone-Conduction and Bone-Anchored Hearing Aids. Original Policy Date
 MP 7.01.02 Implantable Bone-Conduction and Bone-Anchored Hearing Aids Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013
MP 7.01.02 Implantable Bone-Conduction and Bone-Anchored Hearing Aids Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013
SEMI-IMPLANTABLE AND FULLY IMPLANTABLE MIDDLE EAR HEARING AIDS
 Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline must be read in its
Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline must be read in its
Cochlear Implant, Bone Anchored Hearing Aids, and Auditory Brainstem Implant
 Origination: 06/23/08 Revised: 10/13/14 Annual Review: 11/12/15 Purpose: To provide cochlear implant, bone anchored hearing aids, and auditory brainstem implant guidelines for the Medical Department staff
Origination: 06/23/08 Revised: 10/13/14 Annual Review: 11/12/15 Purpose: To provide cochlear implant, bone anchored hearing aids, and auditory brainstem implant guidelines for the Medical Department staff
Clinical Policy Bulletin: Bone-Anchored Hearing Aid
 Bone-Anchored Hearing Aid Page 1 of 20 Aetna Better Health 2000 Market Suite Ste. 850 Philadelphia, PA 19103 AETNA BETTER HEALTH Clinical Policy Bulletin: Bone-Anchored Hearing Aid Number: 0403 Policy
Bone-Anchored Hearing Aid Page 1 of 20 Aetna Better Health 2000 Market Suite Ste. 850 Philadelphia, PA 19103 AETNA BETTER HEALTH Clinical Policy Bulletin: Bone-Anchored Hearing Aid Number: 0403 Policy
Clinical Commissioning Policy: Bone Anchored Hearing Aids. April 2013. Reference: NHSCB/ D09/P/a
 Clinical Commissioning Policy: Bone Anchored Hearing Aids April 2013 Reference: NHSCB/ D09/P/a NHS Commissioning Board Clinical Commissioning Policy: Bone Anchored Hearing Aids First published: April 2013
Clinical Commissioning Policy: Bone Anchored Hearing Aids April 2013 Reference: NHSCB/ D09/P/a NHS Commissioning Board Clinical Commissioning Policy: Bone Anchored Hearing Aids First published: April 2013
Comparison of Traditional Bone-Conduction Hearing Aids with the Baha â System DOI: 10.3766/jaaa.21.4.5
 J Am Acad Audiol 21:267 273 (2010) Comparison of Traditional Bone-Conduction Hearing Aids with the Baha â System DOI: 10.3766/jaaa.21.4.5 Lisa Christensen* Laura Smith-Olinde Jillian Kimberlain* Gresham
J Am Acad Audiol 21:267 273 (2010) Comparison of Traditional Bone-Conduction Hearing Aids with the Baha â System DOI: 10.3766/jaaa.21.4.5 Lisa Christensen* Laura Smith-Olinde Jillian Kimberlain* Gresham
Bone Anchored Hearing Aids (BAHA)
 B-ENT, 2007, 3, Suppl. 6, 45-50 Bone Anchored Hearing Aids (BAHA) G. E. J. Forton* and P. H. Van de Heyning** *E.N.T. department, Heilig Hart General Hospital, Roeselare; **University E.N.T. department,
B-ENT, 2007, 3, Suppl. 6, 45-50 Bone Anchored Hearing Aids (BAHA) G. E. J. Forton* and P. H. Van de Heyning** *E.N.T. department, Heilig Hart General Hospital, Roeselare; **University E.N.T. department,
Protocol. Implantable Bone-Conduction and Bone-Anchored Hearing Aids
 Protocol Implantable Bone-Conduction and Bone-Anchored Hearing Aids (70103) Medical Benefit Effective Date: 04/01/13 Next Review Date: 01/17 Preauthorization Yes Review Dates: 11/07, 07/08, 05/09, 03/10,
Protocol Implantable Bone-Conduction and Bone-Anchored Hearing Aids (70103) Medical Benefit Effective Date: 04/01/13 Next Review Date: 01/17 Preauthorization Yes Review Dates: 11/07, 07/08, 05/09, 03/10,
Implantable Bone Conduction Clinical Coverage Policy No: 1A-36 Hearing Aids (BAHA) Amended Date: October 1, 2015.
 Implantable Bone Conduction Clinical Coverage Policy No: 1A-36 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Conductive Hearing Loss... 1 1.2 Sensorineural Hearing Loss...
Implantable Bone Conduction Clinical Coverage Policy No: 1A-36 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Conductive Hearing Loss... 1 1.2 Sensorineural Hearing Loss...
Vibrant Soundbridge Implantable Hearing System
 Vibrant Soundbridge Implantable Hearing System Kristin M. Avitabile, MS, CCC-A Clinical Manager, Southeastern U.S. MED-EL Corporation Hearing Technology Hearing Aids Mild to severe HL Problems with feedback
Vibrant Soundbridge Implantable Hearing System Kristin M. Avitabile, MS, CCC-A Clinical Manager, Southeastern U.S. MED-EL Corporation Hearing Technology Hearing Aids Mild to severe HL Problems with feedback
HEARING AIDS AND DEVICES INCLUDING WEARABLE, BONE-ANCHORED AND SEMI- IMPLANTABLE
 MEDICAL POLICY HEARING AIDS AND DEVICES INCLUDING WEARABLE, BONE-ANCHORED AND SEMI- IMPLANTABLE Policy Number: 2015T0396O Effective Date: December 1, 2015 Table of Contents BENEFIT CONSIDERATIONS COVERAGE
MEDICAL POLICY HEARING AIDS AND DEVICES INCLUDING WEARABLE, BONE-ANCHORED AND SEMI- IMPLANTABLE Policy Number: 2015T0396O Effective Date: December 1, 2015 Table of Contents BENEFIT CONSIDERATIONS COVERAGE
Hearing Aids - Adult HEARING AIDS - ADULT HS-159. Policy Number: HS-159. Original Effective Date: 3/18/2010. Revised Date(s): 3/18/2011; 3/1/2012
 Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
BONE-CONDUCTION HEARING AIDS
 BONE-CONDUCTION HEARING AIDS Introduction Conventional hearing aids fit in the ear canal and amplify sounds, which the hearing aid user then hears in the normal way. However, these hearing aids are not
BONE-CONDUCTION HEARING AIDS Introduction Conventional hearing aids fit in the ear canal and amplify sounds, which the hearing aid user then hears in the normal way. However, these hearing aids are not
How To Know If A Cochlear Implant Is Right For You
 MEDICAL POLICY SUBJECT: COCHLEAR IMPLANTS AND PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
MEDICAL POLICY SUBJECT: COCHLEAR IMPLANTS AND PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
KANSAS MEDICAL ASSISTANCE PROGRAM PROVIDER MANUAL. Audiology
 KANSAS MEDICAL ASSISTANCE PROGRAM PROVIDER MANUAL Audiology PART II Introduction Section BILLING INSTRUCTIONS Page 7000 Audiology Billing Instructions............... 7-1 Submission of Claim................
KANSAS MEDICAL ASSISTANCE PROGRAM PROVIDER MANUAL Audiology PART II Introduction Section BILLING INSTRUCTIONS Page 7000 Audiology Billing Instructions............... 7-1 Submission of Claim................
Ponto The bone anchored hearing system from Oticon Medical. Audiological Manual. Ponto, Ponto Pro & Ponto Pro Power
 Ponto The bone anchored hearing system from Oticon Medical Audiological Manual US Ponto, Ponto Pro & Ponto Pro Power Contents Introduction...3 Patient selection...4 Pre-operative evaluation and counseling...
Ponto The bone anchored hearing system from Oticon Medical Audiological Manual US Ponto, Ponto Pro & Ponto Pro Power Contents Introduction...3 Patient selection...4 Pre-operative evaluation and counseling...
Candidacy Guide. Ponto TM The Bone Anchored Hearing System
 Candidacy Guide Ponto TM The Bone Anchored Hearing System Contents Identifying patients Introduction Introduction... 3 Identifying patients for a bone anchored solution... 5 Conductive or mixed hearing
Candidacy Guide Ponto TM The Bone Anchored Hearing System Contents Identifying patients Introduction Introduction... 3 Identifying patients for a bone anchored solution... 5 Conductive or mixed hearing
Changing the Landscape for Auditory Bone Conduction Device Recipients. Michael Scott, AuD., CCC/A Cincinnati Children s Hospital ISHA: 4/16/2016
 Changing the Landscape for Auditory Bone Conduction Device Recipients Michael Scott, AuD., CCC/A Cincinnati Children s Hospital ISHA: 4/16/2016 Disclosures I am an employee of Cincinnati Children s Hospital.
Changing the Landscape for Auditory Bone Conduction Device Recipients Michael Scott, AuD., CCC/A Cincinnati Children s Hospital ISHA: 4/16/2016 Disclosures I am an employee of Cincinnati Children s Hospital.
Bone Anchored Hearing Aids B.A.H.A
 Bone Anchored Hearing Aids B.A.H.A Dr. Abdulrahman Hagr MBBS FRCS(c) Assistant Professor King Saud University Otolaryngology Consultant Otologist, Neurotologist & Skull Base Surgeon King Abdulaziz Hospital
Bone Anchored Hearing Aids B.A.H.A Dr. Abdulrahman Hagr MBBS FRCS(c) Assistant Professor King Saud University Otolaryngology Consultant Otologist, Neurotologist & Skull Base Surgeon King Abdulaziz Hospital
Middle Ear Implants for the Treatment of Hearing Loss
 Middle Ear Implants for the Treatment of Hearing Loss FINAL STE REPORT December 2011 Submitted to: The Alberta Health Technologies Decision Process Health Technologies and Services Policy Unit Clinical
Middle Ear Implants for the Treatment of Hearing Loss FINAL STE REPORT December 2011 Submitted to: The Alberta Health Technologies Decision Process Health Technologies and Services Policy Unit Clinical
Bone Conduction Implants Recent Changes in Surgical Techniques, Hearing Technology & Implant Options
 2 0 1 5 A G B e l l L S L S y m p o s i u m Bone Conduction Implants Recent Changes in Surgical Techniques, Hearing Technology & Implant Options J u l y 1 0, 2 0 1 5 The Oticon Medical company Part of
2 0 1 5 A G B e l l L S L S y m p o s i u m Bone Conduction Implants Recent Changes in Surgical Techniques, Hearing Technology & Implant Options J u l y 1 0, 2 0 1 5 The Oticon Medical company Part of
Florida Medicaid. Hearing Services Coverage Policy
 Florida Medicaid Agency for Health Care Administration June 2016 Table of Contents 1.0 Introduction... 1 1.1 Description... 1 1.2 Legal Authority... 1 1.3 Definitions... 1 2.0 Eligible Recipient... 2 2.1
Florida Medicaid Agency for Health Care Administration June 2016 Table of Contents 1.0 Introduction... 1 1.1 Description... 1 1.2 Legal Authority... 1 1.3 Definitions... 1 2.0 Eligible Recipient... 2 2.1
So, how do we hear? outer middle ear inner ear
 The ability to hear is critical to understanding the world around us. The human ear is a fully developed part of our bodies at birth and responds to sounds that are very faint as well as sounds that are
The ability to hear is critical to understanding the world around us. The human ear is a fully developed part of our bodies at birth and responds to sounds that are very faint as well as sounds that are
GUIDELINES DEFINITIONS:
 U n i t e d H e a l t h C a r e G u i d e l i n e Division Departments Community Plan Products State :Arizona UnitedHealthcare Children s Rehabilitative Services Title: CRS IMPLANTABLE BONE CONDUCTION
U n i t e d H e a l t h C a r e G u i d e l i n e Division Departments Community Plan Products State :Arizona UnitedHealthcare Children s Rehabilitative Services Title: CRS IMPLANTABLE BONE CONDUCTION
Bone Anchored Hearing Aid
 Ontario Health Technology Assessment Series 2002; Vol. 2, No. 3 Bone Anchored Hearing Aid An Evidence Based Analysis September 2002 Medical Advisory Secretariat Ministry of Health and Long Term Care Suggested
Ontario Health Technology Assessment Series 2002; Vol. 2, No. 3 Bone Anchored Hearing Aid An Evidence Based Analysis September 2002 Medical Advisory Secretariat Ministry of Health and Long Term Care Suggested
Hearing Aids. What Is a Hearing Aid? How Common Is Hearing Loss and What Causes It? How Do We Hear?
 Hearing Aids What Is a Hearing Aid? A hearing aid is an electronic, battery-operated device that amplifies and changes sound to allow for improved communication. Hearing aids receive sound through a microphone,
Hearing Aids What Is a Hearing Aid? A hearing aid is an electronic, battery-operated device that amplifies and changes sound to allow for improved communication. Hearing aids receive sound through a microphone,
Mississippi Medicaid. Provider Reference Guide. For Part 203. Physician Services
 Mississippi Medicaid Provider Reference Guide For Part 203 Physician Services This is a companion document to the Mississippi Administrative Code Title 23 and must be utilized as a reference only. January
Mississippi Medicaid Provider Reference Guide For Part 203 Physician Services This is a companion document to the Mississippi Administrative Code Title 23 and must be utilized as a reference only. January
Hearing with Bone-anchored Hearing Aid (BAHA)
 REVIEW ARTICLE Hearing with Bone-anchored Hearing Aid (BAHA) AIJOC Hearing with Bone-anchored Hearing Aid (BAHA) 1 Gauri Mankekar, 2 Payal Bhattacharya Chitranshi, 3 MV Kirtane 1 ENT Consultant, PD Hinduja
REVIEW ARTICLE Hearing with Bone-anchored Hearing Aid (BAHA) AIJOC Hearing with Bone-anchored Hearing Aid (BAHA) 1 Gauri Mankekar, 2 Payal Bhattacharya Chitranshi, 3 MV Kirtane 1 ENT Consultant, PD Hinduja
Diseases of the middle ear
 Diseases of the middle ear Acute Suppurative Otitis Media: Acute suppurative otitis media may be viral or bacterial and is accompanied by signs of pain, pressure sensation, diminished hearing and occasional
Diseases of the middle ear Acute Suppurative Otitis Media: Acute suppurative otitis media may be viral or bacterial and is accompanied by signs of pain, pressure sensation, diminished hearing and occasional
HCPCS CODING REFERENCE CHART
 HCPCS CODES HCPCS CODING REFERENCE CHART HCPCS CODE DESCRIPTIONS COMMENTS L7510 Repair of prosthetic device, repair or replace minor parts This code covers parts for bone anchored hearing aid that are
HCPCS CODES HCPCS CODING REFERENCE CHART HCPCS CODE DESCRIPTIONS COMMENTS L7510 Repair of prosthetic device, repair or replace minor parts This code covers parts for bone anchored hearing aid that are
Audiology Services. Carolyn Dando Audiology Services Manager South Warwickshire NHS
 Audiology Services Carolyn Dando Audiology Services Manager South Warwickshire NHS What are we going to cover today? General overview of the ear Hearing loss Hearing assessments, results Hearing aids Paediatric
Audiology Services Carolyn Dando Audiology Services Manager South Warwickshire NHS What are we going to cover today? General overview of the ear Hearing loss Hearing assessments, results Hearing aids Paediatric
Unilateral (Hearing Loss in One Ear) Hearing Loss Guidance
 Unilateral (Hearing Loss in One Ear) Hearing Loss Guidance Indiana s Early Hearing Detection and Intervention Program Before universal newborn hearing screening, most children with unilateral hearing loss
Unilateral (Hearing Loss in One Ear) Hearing Loss Guidance Indiana s Early Hearing Detection and Intervention Program Before universal newborn hearing screening, most children with unilateral hearing loss
HEARING & HEARING LOSS. Dr I Butler 2015
 HEARING & HEARING LOSS Dr I Butler 2015 DISCLOSURE Sponsorship to attend local and international workshops Cochlear (Southern ENT) Med el TOPICS Anatomy Classification of hearing loss Congenital hearing
HEARING & HEARING LOSS Dr I Butler 2015 DISCLOSURE Sponsorship to attend local and international workshops Cochlear (Southern ENT) Med el TOPICS Anatomy Classification of hearing loss Congenital hearing
GONCA SENNAROĞLU PhD LEVENT SENNAROĞLU MD. Department of Otolaryngology Hacettepe University ANKARA, TURKEY
 GONCA SENNAROĞLU PhD LEVENT SENNAROĞLU MD Department of Otolaryngology Hacettepe University ANKARA, TURKEY To present the audiological findings and rehabilitative outcomes of CI in children with cochlear
GONCA SENNAROĞLU PhD LEVENT SENNAROĞLU MD Department of Otolaryngology Hacettepe University ANKARA, TURKEY To present the audiological findings and rehabilitative outcomes of CI in children with cochlear
Treatment Guide Understanding Hearing Loss. Cleveland Clinic Hearing Specialists. Choosing Care for Hearing Loss
 Treatment Guide Understanding Hearing Loss Good hearing is part of a full and active life. Let us help you achieve a world of better hearing and improve your quality of life. Choosing Care for Hearing
Treatment Guide Understanding Hearing Loss Good hearing is part of a full and active life. Let us help you achieve a world of better hearing and improve your quality of life. Choosing Care for Hearing
Hearing Loss in Geriatric Primary Care Mary Ann Forciea MD Josh Uy MD
 Hearing Loss in Geriatric Primary Care Mary Ann Forciea MD Josh Uy MD Q: In my office practice, I screen for hearing loss with A Level of difficulty in office conversation Questionnaire Hand held hldaudiometer
Hearing Loss in Geriatric Primary Care Mary Ann Forciea MD Josh Uy MD Q: In my office practice, I screen for hearing loss with A Level of difficulty in office conversation Questionnaire Hand held hldaudiometer
Introduction Bone Anchored Implants (BAI), Candidacy and Pre-Operative Testing for Adult Patients
 Introduction Bone Anchored Implants (BAI), Candidacy and Pre-Operative Testing for Adult Patients Developed by Hakanssonand his colleagues in Sweden in the late 1970s 3 Components Sound Processor (#1)
Introduction Bone Anchored Implants (BAI), Candidacy and Pre-Operative Testing for Adult Patients Developed by Hakanssonand his colleagues in Sweden in the late 1970s 3 Components Sound Processor (#1)
NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES PROCEDURE CODES
 NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES PROCEDURE CODES Table of Contents GENERAL INFORMATION AND INSTRUCTIONS----------------------------------------------- 2 A. DIAGNOSTIC SERVICES
NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES PROCEDURE CODES Table of Contents GENERAL INFORMATION AND INSTRUCTIONS----------------------------------------------- 2 A. DIAGNOSTIC SERVICES
NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES PROCEDURE CODES
 NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES PROCEDURE CODES Table of Contents WHAT S NEW FOR THE 2016 MANUAL? --------------------------------------------------------------------------------
NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES PROCEDURE CODES Table of Contents WHAT S NEW FOR THE 2016 MANUAL? --------------------------------------------------------------------------------
Prevalence of otological disorders in diabetic patients with hearing loss
 Prevalence of otological disorders in diabetic patients with hearing loss Manche Santoshi Kumari *, Jangala Madhavi *, Koralla Raja Meganadh *, Akka Jyothy Institute of Genetics and Hospital for Genetic
Prevalence of otological disorders in diabetic patients with hearing loss Manche Santoshi Kumari *, Jangala Madhavi *, Koralla Raja Meganadh *, Akka Jyothy Institute of Genetics and Hospital for Genetic
- Review ear anatomy. Evaluation of Hearing. - Specific causes of hearing loss
 Hearing Loss in Primary Care Aaron C. Moberly, MD Otolaryngologist Department of Otorhinolaryngology The Ohio State University Wexner Medical Center Overview - Review ear anatomy - Evaluation of hearing
Hearing Loss in Primary Care Aaron C. Moberly, MD Otolaryngologist Department of Otorhinolaryngology The Ohio State University Wexner Medical Center Overview - Review ear anatomy - Evaluation of hearing
Understanding Hearing Loss 404.591.1884. www.childrensent.com
 Understanding Hearing Loss 404.591.1884 www.childrensent.com You just found out your child has a hearing loss. You know what the Audiologist explained to you, but it is hard to keep track of all the new
Understanding Hearing Loss 404.591.1884 www.childrensent.com You just found out your child has a hearing loss. You know what the Audiologist explained to you, but it is hard to keep track of all the new
Light wear for a powerful hearing. Bone Conduction Headset
 Light wear for a powerful hearing Bone Conduction Headset 2 Light wear for a powerful hearing Melody Flex, the new bone conduction headset is AUTEL s solution to improve hearing quality of those affected
Light wear for a powerful hearing Bone Conduction Headset 2 Light wear for a powerful hearing Melody Flex, the new bone conduction headset is AUTEL s solution to improve hearing quality of those affected
Wisconsin Department of Health & Family Services Division of Disability and Elder Services Bureau of Aging & Long Term Care Resources
 Waiver Wise Technical Assistance for the Community Options Program Waiver COP-W Wisconsin Department of Health & Family Services Division of Disability and Elder Services Bureau of Aging & Long Term Care
Waiver Wise Technical Assistance for the Community Options Program Waiver COP-W Wisconsin Department of Health & Family Services Division of Disability and Elder Services Bureau of Aging & Long Term Care
Middle ear conditions
 Middle ear conditions Middle ear conditions This factsheet is part of our Ears and ear problems range. It is written for people who have been diagnosed with a condition that affects the middle ear. Read
Middle ear conditions Middle ear conditions This factsheet is part of our Ears and ear problems range. It is written for people who have been diagnosed with a condition that affects the middle ear. Read
Hearing Aid Styles. Siemens Phonak Widex Starkey. Sonic Oticon Unitron. Resound
 Hearing Aid Styles The purpose of this paper is to assist you in becoming an educated consumer. It should not be construed that this is a definitive paper. Rather, it is merely a starting point, an overview.
Hearing Aid Styles The purpose of this paper is to assist you in becoming an educated consumer. It should not be construed that this is a definitive paper. Rather, it is merely a starting point, an overview.
Hearing Screening Coding Fact Sheet for Primary Care Pediatricians
 Hearing Screening Coding Fact Sheet for Primary Care Pediatricians While coding for hearing screening is relatively straightforward, ensuring that appropriate payment is received for such services is a
Hearing Screening Coding Fact Sheet for Primary Care Pediatricians While coding for hearing screening is relatively straightforward, ensuring that appropriate payment is received for such services is a
ORIGINAL ARTICLE. attitude toward the new hearing (REPRINTED) ARCH OTOLARYNGOL HEAD NECK SURG/ VOL 130, APR 2004 394
 The Bone-Anchored Hearing Aid Quality-of-Life Assessment ORIGINAL ARTICLE Myrthe K. S. Hol, MD; Marian A. Spath, MSc; Paul F. M. Krabbe, PhD; Catharina T. M. van der Pouw, MD, PhD; Ad F. M. Snik, PhD;
The Bone-Anchored Hearing Aid Quality-of-Life Assessment ORIGINAL ARTICLE Myrthe K. S. Hol, MD; Marian A. Spath, MSc; Paul F. M. Krabbe, PhD; Catharina T. M. van der Pouw, MD, PhD; Ad F. M. Snik, PhD;
Hearing Devices Policy and Administration Manual
 Hearing Devices Policy and Administration Manual Assistive Devices Program, Ministry of Health and Long-Term Care Table of Amendments This page will list all substantive changes to policies and procedures
Hearing Devices Policy and Administration Manual Assistive Devices Program, Ministry of Health and Long-Term Care Table of Amendments This page will list all substantive changes to policies and procedures
6/18/2015. Bone Conduction Implants Recent Changes in Technology, Implant Options and Surgical Techniques Rebecca Cihocki AuD Clinical Trainer
 Bone Conduction Implants Recent Changes in Technology, Implant Options and Surgical Techniques Rebecca Cihocki AuD Clinical Trainer Our Company The Oticon Medical Company Part of William Demant Global
Bone Conduction Implants Recent Changes in Technology, Implant Options and Surgical Techniques Rebecca Cihocki AuD Clinical Trainer Our Company The Oticon Medical Company Part of William Demant Global
ICD-10 Codes Utilized by Audiologists
 ICD-10 Codes Utilized by Audiologists Introduction Beginning with the first claim filed to all payers on or after October 1, 2015, the ICD-10 codes must be utilized in box 21 A-L on the CMS 1500 claim
ICD-10 Codes Utilized by Audiologists Introduction Beginning with the first claim filed to all payers on or after October 1, 2015, the ICD-10 codes must be utilized in box 21 A-L on the CMS 1500 claim
Cochlear Implants: A Communication Choice. Cochlear Implants: A Communication Tool. www.cochlear.com
 Cochlear Ltd ABN 96 002 618 073 14 Mars Road, PO Box 629 Lane Cove NSW 2066 Australia Tel: 61 2 9428 6555 Fax: 61 2 9428 6353 Cochlear Americas 400 Inverness Parkway Suite 400 Englewood CO 80112 USA Tel:
Cochlear Ltd ABN 96 002 618 073 14 Mars Road, PO Box 629 Lane Cove NSW 2066 Australia Tel: 61 2 9428 6555 Fax: 61 2 9428 6353 Cochlear Americas 400 Inverness Parkway Suite 400 Englewood CO 80112 USA Tel:
Chapter 8. Hearing and Ear Abnormalities in Fanconi Anemia. Introduction. Anatomy and Function of the Ear
 Chapter 8 Hearing and Ear Abnormalities in Fanconi Anemia H. Jeffrey Kim, MD, FACS, Christopher Zalewski, MA, and Carmen C. Brewer, PhD Introduction In 1927, Guido Fanconi, MD, noticed an association between
Chapter 8 Hearing and Ear Abnormalities in Fanconi Anemia H. Jeffrey Kim, MD, FACS, Christopher Zalewski, MA, and Carmen C. Brewer, PhD Introduction In 1927, Guido Fanconi, MD, noticed an association between
Coding Fact Sheet for Primary Care Pediatricians
 1/1/2015 Hearing Testing Coding Fact Sheet Coding Fact Sheet for Primary Care Pediatricians While coding for hearing screening is relatively straightforward, ensuring that appropriate payment is received
1/1/2015 Hearing Testing Coding Fact Sheet Coding Fact Sheet for Primary Care Pediatricians While coding for hearing screening is relatively straightforward, ensuring that appropriate payment is received
Pediatric Hearing Assessment
 Pediatric Hearing Assessment Stanton Jones Key Points This chapter outlines the methods of hearing assessment that are appropriate for children from birth to adolescence. The importance of timely referral
Pediatric Hearing Assessment Stanton Jones Key Points This chapter outlines the methods of hearing assessment that are appropriate for children from birth to adolescence. The importance of timely referral
Getting a Cochlear Implant
 Cochlear Implants Getting a Cochlear Implant the journey to better hearing 1 All of life s journeys depend on taking the first step determining how to get from where you are to where you want to go. This
Cochlear Implants Getting a Cochlear Implant the journey to better hearing 1 All of life s journeys depend on taking the first step determining how to get from where you are to where you want to go. This
1/26/2011. 50% of deafness and hearing impairment is avoidable through prevention, early diagnosis, and management.
 Hearing Impairment Roseann Mulligan, DDS, MS Herman Ostrow School of Dentistry of the University of Southern California 1 JAMA, July 4, 2007 Vol 298, No. 1 2 278 million - moderate to profound bilateral
Hearing Impairment Roseann Mulligan, DDS, MS Herman Ostrow School of Dentistry of the University of Southern California 1 JAMA, July 4, 2007 Vol 298, No. 1 2 278 million - moderate to profound bilateral
8.Audiological Evaluation
 8. A U D I O L O G I C A L E V A L U A T I O N 8.Audiological Evaluation The external ear of the child with Progeria Behavioral testing for assessing hearing thresholds Objective electrophysiologic tests
8. A U D I O L O G I C A L E V A L U A T I O N 8.Audiological Evaluation The external ear of the child with Progeria Behavioral testing for assessing hearing thresholds Objective electrophysiologic tests
Occupational Noise Induced Hearing Loss
 Occupational Noise Induced Hearing Loss M Baxter FRACS SISA Adelaide June 2014 ENT in Personal Injury Claims EAR Hearing Loss -main, Dizziness Nose Injuries ->cosmesis,breathing: Loss of sense of smell:
Occupational Noise Induced Hearing Loss M Baxter FRACS SISA Adelaide June 2014 ENT in Personal Injury Claims EAR Hearing Loss -main, Dizziness Nose Injuries ->cosmesis,breathing: Loss of sense of smell:
Your Hearing ILLUMINATED
 Your Hearing ILLUMINATED INFORMATION FROM YOUR HEARING CARE PROFESSIONAL REDISCOVER your hearing and reconnect 1 with the important things you might have been missing. Your sense of hearing is a vital
Your Hearing ILLUMINATED INFORMATION FROM YOUR HEARING CARE PROFESSIONAL REDISCOVER your hearing and reconnect 1 with the important things you might have been missing. Your sense of hearing is a vital
Hearing Tests for Children with Multiple or Developmental Disabilities by Susan Agrawal
 www.complexchild.com Hearing Tests for Children with Multiple or Developmental Disabilities by Susan Agrawal Hearing impairment is a common problem in children with developmental disabilities or who have
www.complexchild.com Hearing Tests for Children with Multiple or Developmental Disabilities by Susan Agrawal Hearing impairment is a common problem in children with developmental disabilities or who have
The new wide Ponto implant design clinical and surgical aspects
 The new wide Ponto implant design clinical and surgical aspects Partik Westerkull, MSc. Eng. Ph., Senior Research Consultant to Oticon Medical Lars Jinton, Director of Engineering, Oticon Medical With
The new wide Ponto implant design clinical and surgical aspects Partik Westerkull, MSc. Eng. Ph., Senior Research Consultant to Oticon Medical Lars Jinton, Director of Engineering, Oticon Medical With
The benefit and success of the Bone Anchored Hearing Aid. Ann-Louise McDermott
 The benefit and success of the Bone Anchored Hearing Aid Ann-Louise McDermott Print: Lay-out: Cover-design: Print Partners Ipskamp Nijmegen Diny Helsper Mr. Adam Field. Department of Medical Illustration,
The benefit and success of the Bone Anchored Hearing Aid Ann-Louise McDermott Print: Lay-out: Cover-design: Print Partners Ipskamp Nijmegen Diny Helsper Mr. Adam Field. Department of Medical Illustration,
Position Paper on Cochlear Implants in Children
 Position Paper on Cochlear Implants in Children Position: The Canadian Association of Speech-Language Pathologists and Audiologists (CASLPA) supports cochlear implantation in children where appropriate
Position Paper on Cochlear Implants in Children Position: The Canadian Association of Speech-Language Pathologists and Audiologists (CASLPA) supports cochlear implantation in children where appropriate
Questions and Answers for Parents
 Questions and Answers for Parents There are simple, inexpensive tests available to detect hearing impairment in infants during the first days of life. In the past, most hearing deficits in children were
Questions and Answers for Parents There are simple, inexpensive tests available to detect hearing impairment in infants during the first days of life. In the past, most hearing deficits in children were
A PROFESSIONAL PRACTICE PROFILE
 A PROFESSIONAL PRACTICE PROFILE FOR HEARING HEALTH PROFESSIONALS The International Hearing Society has adopted the following practice profile as a comprehensive declaration of dispensing characteristics
A PROFESSIONAL PRACTICE PROFILE FOR HEARING HEALTH PROFESSIONALS The International Hearing Society has adopted the following practice profile as a comprehensive declaration of dispensing characteristics
Ear Disorders and Problems
 Ear Disorders and Problems Introduction Your ear has three main parts: outer, middle and inner. You use all of them to hear. There are many disorders and problems that can affect the ear. The symptoms
Ear Disorders and Problems Introduction Your ear has three main parts: outer, middle and inner. You use all of them to hear. There are many disorders and problems that can affect the ear. The symptoms
5th Congress of Alps-Adria Acoustics Association NOISE-INDUCED HEARING LOSS
 5th Congress of Alps-Adria Acoustics Association 12-14 September 2012, Petrčane, Croatia NOISE-INDUCED HEARING LOSS Davor Šušković, mag. ing. el. techn. inf. [email protected] Abstract: One of
5th Congress of Alps-Adria Acoustics Association 12-14 September 2012, Petrčane, Croatia NOISE-INDUCED HEARING LOSS Davor Šušković, mag. ing. el. techn. inf. [email protected] Abstract: One of
NEW YORK STATE MEDICAID PROGRAM HEARING AID/AUDIOLOGY MANUAL
 NEW YORK STATE MEDICAID PROGRAM HEARING AID/AUDIOLOGY MANUAL POLICY GUIDELINES Table of Contents SECTION I - REQUIREMENTS FOR PARTICIPATION IN MEDICAID...2 SERVICES PROVIDED TO PATIENTS UNDER 21 YEARS
NEW YORK STATE MEDICAID PROGRAM HEARING AID/AUDIOLOGY MANUAL POLICY GUIDELINES Table of Contents SECTION I - REQUIREMENTS FOR PARTICIPATION IN MEDICAID...2 SERVICES PROVIDED TO PATIENTS UNDER 21 YEARS
Hearcentres Guide to Hearing Aid Terminology
 Hearcentres Guide to Hearing Aid Terminology Sophisticated modern hearing aids use a number of complicated technologies and techniques to ensure great improvements in hearing. Not only are the terms used
Hearcentres Guide to Hearing Aid Terminology Sophisticated modern hearing aids use a number of complicated technologies and techniques to ensure great improvements in hearing. Not only are the terms used
Audiologist and Hearing Aid Dispenser. Provider Manual
 Audiologist and Hearing Aid Provider Manual Provider 1 TABLE OF CONTENTS Chapter I. General Program Policies Chapter II. Member Eligibility Chapter IV. Billing Iowa Medicaid Appendix III. Provider-Specific
Audiologist and Hearing Aid Provider Manual Provider 1 TABLE OF CONTENTS Chapter I. General Program Policies Chapter II. Member Eligibility Chapter IV. Billing Iowa Medicaid Appendix III. Provider-Specific
Image. 3.11.3 SW Review the anatomy of the EAC and how this plays a role in the spread of tumors.
 Neoplasms of the Ear and Lateral Skull Base Image 3.11.1 SW What are the three most common neoplasms of the auricle? 3.11.2 SW What are the four most common neoplasms of the external auditory canal (EAC)
Neoplasms of the Ear and Lateral Skull Base Image 3.11.1 SW What are the three most common neoplasms of the auricle? 3.11.2 SW What are the four most common neoplasms of the external auditory canal (EAC)
Surgery for Conductive Hearing Loss
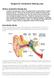 Surgery for Conductive Hearing Loss What is conductive hearing loss Conductive hearing loss is a form of hearing loss due to abnormalities in mobile portions of the ear. Theses are the movable parts (including
Surgery for Conductive Hearing Loss What is conductive hearing loss Conductive hearing loss is a form of hearing loss due to abnormalities in mobile portions of the ear. Theses are the movable parts (including
Cochlear implants for children and adults with severe to profound deafness
 Issue date: January 2009 Review date: February 2011 Cochlear implants for children and adults with severe to profound deafness National Institute for Health and Clinical Excellence Page 1 of 41 Final appraisal
Issue date: January 2009 Review date: February 2011 Cochlear implants for children and adults with severe to profound deafness National Institute for Health and Clinical Excellence Page 1 of 41 Final appraisal
MODEL SUPERBILL for AUDIOLOGY
 MODEL SUPERBILL for AUDIOLOGY The following is a model of a superbill which could be used by an audiology practice when billing private health plans. This sample is not meant to dictate which services
MODEL SUPERBILL for AUDIOLOGY The following is a model of a superbill which could be used by an audiology practice when billing private health plans. This sample is not meant to dictate which services
NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES FEE SCHEDULE
 NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES FEE SCHEDULE Table of Contents GENERAL INFORMATION AND INSTRUCTIONS----------------------------------------------- 2 CODES -------------------------------------------------------------------------------------------------------
NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES FEE SCHEDULE Table of Contents GENERAL INFORMATION AND INSTRUCTIONS----------------------------------------------- 2 CODES -------------------------------------------------------------------------------------------------------
Pure Tone Hearing Screening in Schools: Revised Notes on Main Video. IMPORTANT: A hearing screening does not diagnose a hearing loss.
 Pure Tone Hearing Screening in Schools: Revised Notes on Main Video (Notes are also available for Video segments: Common Mistakes and FAQs) IMPORTANT: A hearing screening does not diagnose a hearing loss.
Pure Tone Hearing Screening in Schools: Revised Notes on Main Video (Notes are also available for Video segments: Common Mistakes and FAQs) IMPORTANT: A hearing screening does not diagnose a hearing loss.
PURE TONE AUDIOMETRY Andrew P. McGrath, AuD
 PURE TONE AUDIOMETRY Andrew P. McGrath, AuD Pure tone audiometry is the standard behavioral assessment of an individual s hearing. The results of pure tone audiometry are recorded on a chart or form called
PURE TONE AUDIOMETRY Andrew P. McGrath, AuD Pure tone audiometry is the standard behavioral assessment of an individual s hearing. The results of pure tone audiometry are recorded on a chart or form called
COCHLEAR NERVE APLASIA : THE AUDIOLOGIC PERSPECTIVE A CASE REPORT. Eva Orzan, MD Pediatric Audiology University Hospital of Padova, Italy
 COCHLEAR NERVE APLASIA : THE AUDIOLOGIC PERSPECTIVE A CASE REPORT Eva Orzan, MD Pediatric Audiology University Hospital of Padova, Italy Congenital absence or underdevelopment of the cochlear nerve has
COCHLEAR NERVE APLASIA : THE AUDIOLOGIC PERSPECTIVE A CASE REPORT Eva Orzan, MD Pediatric Audiology University Hospital of Padova, Italy Congenital absence or underdevelopment of the cochlear nerve has
A diagram of the ear s structure. The outer ear includes the portion of the ear that we see the pinna/auricle and the ear canal.
 A diagram of the ear s structure THE OUTER EAR The outer ear includes the portion of the ear that we see the pinna/auricle and the ear canal. The pinna or auricle is a concave cartilaginous structure,
A diagram of the ear s structure THE OUTER EAR The outer ear includes the portion of the ear that we see the pinna/auricle and the ear canal. The pinna or auricle is a concave cartilaginous structure,
NIHL - understanding audiograms and medical causation
 NIHL - understanding audiograms and medical causation Darrell Smith Partner, BLM Manchester Birmingham Cardiff Leeds Liverpool London Manchester Southampton Stockton-on-Tees How the ear works The human
NIHL - understanding audiograms and medical causation Darrell Smith Partner, BLM Manchester Birmingham Cardiff Leeds Liverpool London Manchester Southampton Stockton-on-Tees How the ear works The human
Getting Started Kei Te Timata
 Getting Started Kei Te Timata AN INTRODUCTION FOR THE FAMILIES AND WHANAU OF CHILDREN DIAGNOSED WITH A HEARING LOSS. THIS IS A JOINT PROJECT BY DEAF EDUCATION AOTEAROA NEW ZEALAND AND THE NATIONAL AUDIOLOGY
Getting Started Kei Te Timata AN INTRODUCTION FOR THE FAMILIES AND WHANAU OF CHILDREN DIAGNOSED WITH A HEARING LOSS. THIS IS A JOINT PROJECT BY DEAF EDUCATION AOTEAROA NEW ZEALAND AND THE NATIONAL AUDIOLOGY
2015 ICD-10-CM Diagnosis Codes Related to Hearing and Vestibular Disorders
 2015 ICD-10-CM Diagnosis Codes Related to Hearing and Vestibular Disorders The codes in ICD-10 are not valid for any purpose or use in the United States until October 1, 2015. General Information This
2015 ICD-10-CM Diagnosis Codes Related to Hearing and Vestibular Disorders The codes in ICD-10 are not valid for any purpose or use in the United States until October 1, 2015. General Information This
130 CMR: DIVISION OF MEDICAL ASSISTANCE. 130 CMR 416.000: HEARING INSTRUMENT SPECIALIST SERVICES Section
 130 CMR 416.000: HEARING INSTRUMENT SPECIALIST SERVICES Section 416.401: 416.402: 416.403: 416.404: 416.405: 416.406: 416.407: 416.408: 416.409: 416.410: 416.414: 416.415: 416.416: 416.417: 416.418: 416.419:
130 CMR 416.000: HEARING INSTRUMENT SPECIALIST SERVICES Section 416.401: 416.402: 416.403: 416.404: 416.405: 416.406: 416.407: 416.408: 416.409: 416.410: 416.414: 416.415: 416.416: 416.417: 416.418: 416.419:
The Disability Tax Credit Certificate Tip sheet for Audiologists
 The Disability Tax Credit Certificate Tip sheet for Audiologists Developed by: The Canadian Academy of Audiology (CAA) & Speech- Language and Audiology Canada (SAC) Purpose of This Document The Canada
The Disability Tax Credit Certificate Tip sheet for Audiologists Developed by: The Canadian Academy of Audiology (CAA) & Speech- Language and Audiology Canada (SAC) Purpose of This Document The Canada
Guidance on professional practice for Hearing Aid Audiologists
 Guidance on professional practice for Hearing Aid Audiologists Assuring High Quality Professional Hearing Care Introduction This booklet is intended to be guidance on good professional practices for Registered
Guidance on professional practice for Hearing Aid Audiologists Assuring High Quality Professional Hearing Care Introduction This booklet is intended to be guidance on good professional practices for Registered
Anatomy and Physiology of Hearing (added 09/06)
 Anatomy and Physiology of Hearing (added 09/06) 1. Briefly review the anatomy of the cochlea. What is the cochlear blood supply? SW 2. Discuss the effects of the pinna, head and ear canal on the transmission
Anatomy and Physiology of Hearing (added 09/06) 1. Briefly review the anatomy of the cochlea. What is the cochlear blood supply? SW 2. Discuss the effects of the pinna, head and ear canal on the transmission
