CLINICAL PHARMACOLOGY
|
|
|
- Chloe Shepherd
- 9 years ago
- Views:
Transcription
1 FOR SUBCUTANEOUS USE ONLY DESCRIPTION Ganirelix Acetate Injection is a synthetic decapeptide with high antagonistic activity against naturally occurring gonadotropin-releasing hormone (GnRH). Ganirelix Acetate is derived from native GnRH with substitutions of amino acids at positions 1, 2, 3, 6, 8, and 10 to form the following molecular formula of the peptide: N-acetyl-3-(2-naphthyl)-D-alanyl-4-chloro-Dphenylalanyl-3-(3-pyridyl)-D-alanyl-L-seryl-L-tyrosyl-N 9,N 10 -diethyl-d-homoarginyl-l-leucyl- N 9,N 10 -diethyl-l-homoarginyl-l-prolyl-d-alanylamide acetate. The molecular weight for Ganirelix Acetate is as an anhydrous free base. The structural formula is as follows: Ganirelix Acetate Ganirelix Acetate Injection is supplied as a colorless, sterile, ready-to-use, aqueous solution intended for SUBCUTANEOUS administration only. Each sterile, prefilled syringe contains 250 mcg/0.5 ml of Ganirelix Acetate, 0.1 mg glacial acetic acid, 23.5 mg mannitol, and water for injection adjusted to ph 5.0 with acetic acid, NF and/or sodium hydroxide, NF. CLINICAL PHARMACOLOGY The pulsatile release of GnRH stimulates the synthesis and secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The frequency of LH pulses in the mid and late follicular phase is approximately 1 pulse per hour. These pulses can be detected as transient rises in serum LH. At midcycle, a large increase in GnRH release results in an LH surge. The midcycle LH surge initiates several physiologic actions including: ovulation, resumption of meiosis in the oocyte, and luteinization. Luteinization results in a rise in serum progesterone with an accompanying decrease in estradiol levels. 1
2 Ganirelix Acetate acts by competitively blocking the GnRH receptors on the pituitary gonadotroph and subsequent transduction pathway. It induces a rapid, reversible suppression of gonadotropin secretion. The suppression of pituitary LH secretion by Ganirelix Acetate is more pronounced than that of FSH. An initial release of endogenous gonadotropins has not been detected with Ganirelix Acetate, which is consistent with an antagonist effect. Upon discontinuation of Ganirelix Acetate, pituitary LH and FSH levels are fully recovered within 48 hours. Pharmacokinetics The pharmacokinetic parameters of single and multiple injections of Ganirelix Acetate Injection in healthy adult females are summarized in Table I. Steady-state serum concentrations are reached after 3 days of treatment. The pharmacokinetics of Ganirelix Acetate are doseproportional in the dose range of 125 to 500 mcg. TABLE I: Mean (SD) pharmacokinetic parameters of 250 mcg of Ganirelix Acetate following a single subcutaneous (SC) injection (n=15) and daily SC injections (n=15) for seven days. t max h t 1/2 h C max ng/ml AUC ng h/ml CL/F L/h V d /F L Ganirelix Acetate 1.1 (0.3) 12.8 (4.3) 14.8 (3.2) 96 (12) 2.4 (0.2) 43.7 (11.4) single dose Ganirelix Acetate 1.1 (0.2) 16.2 (1.6) 11.2 (2.4) 77.1 (9.8) 3.3 (0.4) 76.5 (10.3) multiple dose t max Time to maximum concentration t 1/2 Elimination half-life C max Maximum serum concentration AUC Area under the curve; Single dose: AUC 0 ; multiple dose: AUC 0 24 V d Volume of distribution Based on intravenous administration CL Clearance = Dose/AUC 0 F Absolute bioavailability Absorption Ganirelix Acetate is rapidly absorbed following subcutaneous injection with maximum serum concentrations reached approximately one hour after dosing. The mean absolute bioavailability of Ganirelix Acetate following a single 250 mcg subcutaneous injection to healthy female volunteers is 91.1%. Distribution The mean (SD) volume of distribution of Ganirelix Acetate in healthy females following intravenous administration of a single 250-mcg dose is 43.7 (11.4) liters (L). In vitro protein binding to human plasma is 81.9%. Metabolism Following single-dose intravenous administration of radiolabeled Ganirelix Acetate to healthy female volunteers, Ganirelix Acetate is the major compound present in the plasma (50 70% of total radioactivity in the plasma) up to 4 hours and urine ( % of administered dose) up to 24 hours. Ganirelix Acetate is not found in the feces. The 1 4 peptide and 1 6 peptide of Ganirelix Acetate are the primary metabolites observed in the feces. 2
3 Excretion On average, 97.2% of the total radiolabeled Ganirelix Acetate dose is recovered in the feces and urine (75.1% and 22.1%, respectively) over 288 h following intravenous single dose administration of 1 mg [ 14 C]-Ganirelix Acetate. Urinary excretion is virtually complete in 24 h, whereas fecal excretion starts to plateau 192 h after dosing. Special Populations The pharmacokinetics of Ganirelix Acetate Injection have not been determined in special populations such as geriatric, pediatric, renally impaired and hepatically impaired patients (see PRECAUTIONS). Drug-Drug Interactions Formal in vivo or in vitro drug-drug interaction studies have not been conducted (see PRECAUTIONS). Since Ganirelix Acetate can suppress the secretion of pituitary gonadotropins, dose adjustments of exogenous gonadotropins may be necessary when used during controlled ovarian hyperstimulation (COH). Clinical Studies The efficacy of Ganirelix Acetate Injection was established in two adequate and well-controlled clinical studies which included women with normal endocrine and pelvic ultrasound parameters. The studies intended to exclude subjects with polycystic ovary syndrome (PCOS) and subjects with low or no ovarian reserve. One cycle of study medication was administered to each randomized subject. For both studies, the administration of exogenous recombinant FSH [Follistim (follitropin beta for injection)] 150 IU daily was initiated on the morning of Day 2 or 3 of a natural menstrual cycle. Ganirelix Acetate Injection was administered on the morning of Day 7 or 8 (Day 6 of recombinant FSH administration). The dose of recombinant FSH administered was adjusted according to individual responses starting on the day of initiation of Ganirelix Acetate. Both recombinant FSH and Ganirelix Acetate were continued daily until at least three follicles were 17 mm or greater in diameter at which time hcg [Pregnyl (chorionic gonadotropin for injection, USP)] was administered. Following hcg administration, Ganirelix Acetate and recombinant FSH administration were discontinued. Oocyte retrieval, followed by in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI), was subsequently performed. In a multicenter, double-blind, randomized, dose-finding study, the safety and efficacy of Ganirelix Acetate Injection were evaluated for the prevention of LH surges in women undergoing COH with recombinant FSH. Ganirelix Acetate Injection doses ranging from 62.5 mcg to 2000 mcg and recombinant FSH were administered to 332 patients undergoing COH for IVF (see TABLE II). Median serum LH on the day of hcg administration decreased with increasing doses of Ganirelix Acetate. Median serum E 2 (17 -estradiol) on the day of hcg administration was 1475, 1110, and 1160 pg/ml for the 62.5-, 125-, and 250-mcg doses, respectively. Lower peak serum E 2 levels of 823, 703, and 441 pg/ml were seen at higher doses of Ganirelix Acetate 500, 1000, and 2000 mcg, respectively. The highest pregnancy and implantation rates were achieved with the 250-mcg dose of Ganirelix Acetate Injection as summarized in Table II. 3
4 TABLE II: Results from the multicenter, double-blind, randomized, dose-finding study to assess the efficacy of Ganirelix Acetate Injection to prevent premature LH surges in women undergoing COH with recombinant FSH. Daily dose (mcg) of Ganirelix Acetate Injection 62.5 mcg 125 mcg 250 mcg 500 mcg 1000 mcg 2000 mcg No. subjects receiving Ganirelix Acetate No. subjects with ET No. of subjects with LH rise 10 miu/ml * Serum LH (miu/ml) on day of hcg 5 th 95 th percentiles Serum E 2 (pg/ml) on day of hcg 5 th 95 th percentiles < < < Vital pregnancy rate per attempt, n (%) 7 (22.6) 17 (25.8) 25 (35.7) 8 (11.6) 9 (13.6) 2 (6.7) per transfer, n (%) 7 (25.9) 17 (27.9) 25 (40.3) 8 (14.8) 9 (14.8) 2 (7.4) Implantation rate (%) 14.2 (26.8) 16.3 (30.5) 21.9 (30.6) 9.0 (23.7) 8.5 (21.7) 4.9 (20.1) (Protocol 38602) * Following initiation of Ganirelix Acetate therapy. Includes subjects who have complied with daily injections Median values Mean (standard deviation) ET: Embryo Transfer As evidenced by ultrasound at 5 6 weeks following ET Transient LH rises alone were not deleterious to achieving pregnancy with Ganirelix Acetate at doses of 125 mcg (3/6 subjects) and 250 mcg (1/1 subjects). In addition, none of the subjects with LH rises 10 miu/ml had premature luteinization indicated by a serum progesterone above 2 ng/ml. A multicenter, open-label, randomized study was conducted to assess the efficacy and safety of Ganirelix Acetate Injection in women undergoing COH. Follicular phase treatment with Ganirelix Acetate 250 mcg was studied using a luteal phase GnRH agonist as a reference treatment. A total of 463 subjects were treated with Ganirelix Acetate by subcutaneous injection once daily starting on Day 6 of recombinant FSH treatment. Recombinant FSH was maintained at 150 IU for the first 5 days of ovarian stimulation and was then adjusted by the investigator on the sixth day of gonadotropin use according to individual responses. The results for the Ganirelix Acetate arm are summarized in Table III. 4
5 TABLE III: Results from the multicenter, open-label, randomized study to assess the efficacy and safety of Ganirelix Acetate Injection in women undergoing COH. Ganirelix Acetate 250 mcg No. subjects treated 463 Duration of GnRH analog (days) 5.4 (2.0) Duration of recombinant FSH (days) 9.6 (2.0) Serum E 2 (pg/ml) on day of hcg 5 th 95 th percentiles Serum LH (miu/ml) on day of hcg 5 th 95 th percentiles No. of subjects with LH rise 10 miu/ml * 13 No. of follicles > 11 mm 10.7 (5.3) No. of subjects with oocyte retrieval 440 No. of oocytes 8.7 (5.6) Fertilization rate 62.1% No. subjects with ET 399 No. of embryos transferred 2.2 (0.6) No. of embryos 6.0 (4.5) Ongoing pregnancy rate per attempt, n (%) 94 (20.3) per transfer, n (%) 93 (23.3) Implantation rate (%) 15.7 (29) (Protocol 38607) * Following initiation of Ganirelix Acetate therapy Median values Restricted to subjects with hcg injection Mean (standard deviation) ET: Embryo Transfer As evidenced by ultrasound at weeks following ET Includes one patient who achieved pregnancy with intrauterine induction Some centers were limited to the transfer of 2 embryos based on local practice standards The mean number of days of Ganirelix Acetate treatment was 5.4 (2 14). LH Surges The midcycle LH surge initiates several physiologic actions including: ovulation, resumption of meiosis in the oocyte, and luteinization. In 463 subjects administered Ganirelix Acetate Injection 250 mcg, a premature LH surge prior to hcg administration, (LH rise 10 miu/ml with a significant rise in serum progesterone > 2 ng/ml, or a significant decline in serum estradiol) occurred in less than 1% of subjects. INDICATIONS AND USAGE Ganirelix Acetate Injection is indicated for the inhibition of premature LH surges in women undergoing controlled ovarian hyperstimulation. CONTRAINDICATIONS Ganirelix Acetate Injection is contraindicated under the following conditions: Known hypersensitivity to Ganirelix Acetate or to any of its components. Known hypersensitivity to GnRH or any other GnRH analog. Known or suspected pregnancy (see PRECAUTIONS). 5
6 WARNINGS Ganirelix Acetate Injection should be prescribed by physicians who are experienced in infertility treatment. Before starting treatment with Ganirelix Acetate, pregnancy must be excluded. Safe use of Ganirelix Acetate during pregnancy has not been established (see CONTRAINDICATIONS and PRECAUTIONS). PRECAUTIONS General Special care should be taken in women with signs and symptoms of active allergic conditions. Cases of hypersensitivity reactions, including anaphylactoid reactions, have been reported, as early as with the first dose, during post-marketing surveillance (see ADVERSE REACTIONS). In the absence of clinical experience, Ganirelix Acetate treatment is not advised in women with severe allergic conditions. The packaging of this product contains natural rubber latex which may cause allergic reactions (see HOW SUPPLIED). Information for Patients Prior to therapy with Ganirelix Acetate Injection, patients should be informed of the duration of treatment and monitoring procedures that will be required. The risk of possible adverse reactions should be discussed (see ADVERSE REACTIONS). Ganirelix Acetate should not be prescribed if the patient is pregnant. Laboratory Tests A neutrophil count 8.3 ( x 10 9 /L) was noted in 11.9% (up to 16.8 x 10 9 /L) of all subjects treated within the adequate and well-controlled clinical trials. In addition, downward shifts within the Ganirelix Acetate Injection group were observed for hematocrit and total bilirubin. The clinical significance of these findings was not determined. Drug Interactions No formal drug-drug interaction studies have been performed. Carcinogenesis and Mutagenesis, Impairment of Fertility Long-term toxicity studies in animals have not been performed with Ganirelix Acetate Injection to evaluate the carcinogenic potential of the drug. Ganirelix Acetate did not induce a mutagenic response in the Ames test (S. typhimurium and E. coli) or produce chromosomal aberrations in in vitro assay using Chinese Hamster Ovary cells. Pregnancy Ganirelix Acetate Injection is contraindicated in pregnant women. When administered from Day 7 to near term to pregnant rats and rabbits at doses up to 10 and 30 mcg/day (approximately 0.4 to 3.2 times the human dose based on body surface area), Ganirelix Acetate increased the incidence of litter resorption. There was no increase in fetal abnormalities. No treatment-related changes in fertility, physical, or behavioral characteristics were observed in the offspring of female rats treated with Ganirelix Acetate during pregnancy and lactation. 6
7 The effects on fetal resorption are logical consequences of the alteration in hormonal levels brought about by the antigonadotropic properties of this drug and could result in fetal loss in humans. Therefore, this drug should not be used in pregnant women (see CONTRAINDICATIONS). Nursing Mothers Ganirelix Acetate Injection should not be used by lactating women. It is not known whether this drug is excreted in human milk. Geriatric Use Clinical studies with Ganirelix Acetate Injection did not include a sufficient number of subjects aged 65 and over. ADVERSE REACTIONS The safety of Ganirelix Acetate Injection was evaluated in two randomized, parallel-group, multicenter controlled clinical studies. Treatment duration for Ganirelix Acetate ranged from 1 to 14 days. Table IV represents adverse events (AEs) from first day of Ganirelix Acetate administration until confirmation of pregnancy by ultrasound at an incidence of 1% in Ganirelix Acetate-treated subjects without regard to causality. TABLE IV: Incidence of common adverse events (Incidence 1% in Ganirelix Acetate-treated subjects). Completed controlled clinical studies (All-subjects-treated group). Adverse Events Occurring in 1% Ganirelix Acetate N=794 % (n) Abdominal Pain (gynecological) 4.8 (38) Death Fetal 3.7 (29) Headache 3.0 (24) Ovarian Hyperstimulation Syndrome 2.4 (19) Vaginal Bleeding 1.8 (14) Injection Site Reaction 1.1 (9) Nausea 1.1 (9) Abdominal Pain (gastrointestinal) 1.0 (8) During post-marketing surveillance, rare cases of hypersensitivity reactions, including anaphylactoid reactions, have been reported, as early as with the first dose (see PRECAUTIONS). Congenital Anomalies Clinical follow-up studies of 283 newborns of women administered Ganirelix Acetate Injection were reviewed. There were three neonates with major congenital anomalies and 18 neonates with minor congenital anomalies. The major congenital anomalies were: hydrocephalus/meningocele, omphalocele, and Beckwith-Wiedemann Syndrome. The minor congenital anomalies were: nevus, skin tags, sacral sinus, hemangioma, torticollis/asymmetric skull, talipes, supernumerary digit finger, hip subluxation, torticollis/high palate, occiput/abnormal hand crease, hernia umbilicalis, hernia inguinalis, hydrocele, undescended testis, and hydronephrosis. A subsequent analysis from an observational study in more than 1000 newborns compared the incidence of congenital anomalies in newborns of women administered Ganirelix to historical 7
8 controls of a GnRH agonist. This analysis included the 283 newborns in the original studies. This study demonstrated that the incidence of congenital anomalies in children born after COH treatment in women using Ganirelix was comparable with that reported after a COH treatment cycle using a GnRH agonist. The causal relationship between these congenital anomalies and Ganirelix Acetate is unknown. The incidence of congenital malformations after Assisted Reproductive Technology (ART) may be slightly higher than after spontaneous conceptions. This slightly higher incidence is thought to be related to differences in parental characteristics (e.g., maternal age, sperm characteristics) and to the higher incidence of multiple gestations after ART. OVERDOSAGE There have been no reports of overdosage with Ganirelix Acetate Injection in humans. DOSAGE AND ADMINISTRATION After initiating FSH therapy on Day 2 or 3 of the cycle, Ganirelix Acetate Injection 250 mcg may be administered subcutaneously once daily during the mid to late portion of the follicular phase. By taking advantage of endogenous pituitary FSH secretion, the requirement for exogenously administered FSH may be reduced. Treatment with Ganirelix Acetate should be continued daily until the day of hcg administration. When a sufficient number of follicles of adequate size are present, as assessed by ultrasound, final maturation of follicles is induced by administering hcg. The administration of hcg should be withheld in cases where the ovaries are abnormally enlarged on the last day of FSH therapy to reduce the chance of developing OHSS (Ovarian Hyperstimulation Syndrome). Directions for Using Ganirelix Acetate Injection 1. Ganirelix Acetate Injection is supplied in a sterile, prefilled syringe and is intended for SUBCUTANEOUS administration only. 2. Wash hands thoroughly with soap and water. 3. The most convenient sites for SUBCUTANEOUS injection are in the abdomen around the navel or upper thigh. 4. The injection site should be swabbed with a disinfectant to remove any surface bacteria. Clean about two inches around the point where the needle will be inserted and let the disinfectant dry for at least one minute before proceeding. 5. With syringe held upward, remove needle cover. 6. Pinch up a large area of skin between the finger and thumb. Vary the injection site a little with each injection. 7. The needle should be inserted at the base of the pinched-up skin at an angle of to the skin surface. 8. When the needle is correctly positioned, it will be difficult to draw back on the plunger. If any blood is drawn into the syringe, the needle tip has penetrated a vein or artery. If this happens, withdraw the needle slightly and reposition the needle without removing it from the skin. Alternatively, remove the needle and use a new, sterile, prefilled syringe. Cover the injection site with a swab containing disinfectant and apply pressure; the site should stop bleeding within one or two minutes. 8
9 9. Once the needle is correctly placed, depress the plunger slowly and steadily, so the solution is correctly injected and the skin is not damaged. 10. Pull the syringe out quickly and apply pressure to the site with a swab containing disinfectant. 11. Use the sterile, prefilled syringe only once and dispose of it properly. HOW SUPPLIED Ganirelix Acetate Injection is supplied in: Disposable, sterile, ready for use, prefilled 1 ml glass syringes containing 250 mcg/0.5 ml aqueous solution of Ganirelix Acetate closed with a rubber piston that does not contain latex. Each Ganirelix Acetate sterile, prefilled syringe is affixed with a 27 gauge x 1 / 2 -inch needle closed by a needle shield of natural rubber latex. (See PRECAUTIONS, General.) Single syringe NDC Storage Store at 25 C (77 F); excursions permitted to C (59 86 F) [see USP Controlled Room Temperature]. Protect from light. Manufactured by: Vetter Pharma-Fertigung GmbH & Co. KG, Ravensburg, Germany For patent information: Copyright 1999, 2008 Merck Sharp & Dohme B.V., a subsidiary of Merck & Co., Inc. All rights reserved. Revised: 03/2016 uspi-mk8761-soi-1603r005 Rx only 9
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT ACEGON, 50 microgram/ml, solution for injection for cattle. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT ACEGON, 50 microgram/ml, solution for injection for cattle. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active
The objectives of this chapter are: To provide an understanding of the various stimulation protocols used in IVF To enable the student to understand
 1 The objectives of this chapter are: To provide an understanding of the various stimulation protocols used in IVF To enable the student to understand the factors affecting the choice of protocol based
1 The objectives of this chapter are: To provide an understanding of the various stimulation protocols used in IVF To enable the student to understand the factors affecting the choice of protocol based
Welcome to chapter 8. The following chapter is called "Monitoring IVF Cycle & Oocyte Retrieval". The author is Professor Jie Qiao.
 Welcome to chapter 8. The following chapter is called "Monitoring IVF Cycle & Oocyte Retrieval". The author is Professor Jie Qiao. The learning objectives of this chapter are 2 fold. The first section
Welcome to chapter 8. The following chapter is called "Monitoring IVF Cycle & Oocyte Retrieval". The author is Professor Jie Qiao. The learning objectives of this chapter are 2 fold. The first section
How do fertility drugs work?
 How do fertility drugs work? Under normal circumstances, ovulation occurs once a month when a ripened egg which is ready to be fertilised is released from the ovaries. For couples who are trying to conceive,
How do fertility drugs work? Under normal circumstances, ovulation occurs once a month when a ripened egg which is ready to be fertilised is released from the ovaries. For couples who are trying to conceive,
Fertility care for women diagnosed with cancer
 Saint Mary s Hospital Department of Reproductive Medicine Fertility care for women diagnosed with cancer Information For Patients INF/DRM/NUR/16 V1/01/11/2013 1 2 Contents Page Overview 4 Our Service 4
Saint Mary s Hospital Department of Reproductive Medicine Fertility care for women diagnosed with cancer Information For Patients INF/DRM/NUR/16 V1/01/11/2013 1 2 Contents Page Overview 4 Our Service 4
Endocrinology of the Female Reproductive Axis
 Endocrinology of the Female Reproductive Axis girlontheriver.com Geralyn Lambert-Messerlian, PhD, FACB Professor Women and Infants Hospital Alpert Medical School at Brown University Women & Infants BROWN
Endocrinology of the Female Reproductive Axis girlontheriver.com Geralyn Lambert-Messerlian, PhD, FACB Professor Women and Infants Hospital Alpert Medical School at Brown University Women & Infants BROWN
GONAL-f (follitropin alfa for injection) For subcutaneous injection DESCRIPTION
 GONAL-f (follitropin alfa for injection) For subcutaneous injection DESCRIPTION Gonal-f (follitropin alfa for injection) is a human follicle stimulating hormone (FSH) preparation of recombinant DNA origin,
GONAL-f (follitropin alfa for injection) For subcutaneous injection DESCRIPTION Gonal-f (follitropin alfa for injection) is a human follicle stimulating hormone (FSH) preparation of recombinant DNA origin,
In Vitro Fertilization
 Patient Education In Vitro Fertilization What to expect This handout describes how to prepare for and what to expect when you have in vitro fertilization. It provides written information about this process,
Patient Education In Vitro Fertilization What to expect This handout describes how to prepare for and what to expect when you have in vitro fertilization. It provides written information about this process,
Drug Therapy Guidelines: Injectable Fertility Medications
 Drug Therapy Guidelines: Injectable Fertility Medications Effective Date: 11/20/07 Committee Review Date: 7/12/00, 5/8/01, 1/15/02, 5/6/0, 12/16/0, 6/8/04, 12/16/05, 2/1/06, 10/15/06, 7/20/07, 11/5/07
Drug Therapy Guidelines: Injectable Fertility Medications Effective Date: 11/20/07 Committee Review Date: 7/12/00, 5/8/01, 1/15/02, 5/6/0, 12/16/0, 6/8/04, 12/16/05, 2/1/06, 10/15/06, 7/20/07, 11/5/07
Artificial insemination with donor sperm
 Artificial insemination with donor sperm Ref. 123 / 2009 Reproductive Medicine Unit Servicio de Medicina de la Reproducción Gran Vía Carlos III 71-75 08028 Barcelona Tel. (+34) 93 227 47 00 Fax. (+34)
Artificial insemination with donor sperm Ref. 123 / 2009 Reproductive Medicine Unit Servicio de Medicina de la Reproducción Gran Vía Carlos III 71-75 08028 Barcelona Tel. (+34) 93 227 47 00 Fax. (+34)
PACKAGE INSERT PREGNYL. (chorionic gonadotropin for injection, USP) 10,000 units/vial. Human Gonadotropin
 PACKAGE INSERT PREGNYL (chorionic gonadotropin for injection, USP) 10,000 units/vial Human Gonadotropin Merck Canada Inc. 16750 route Transcanadienne Kirkland QC Canada H9H 4M7 Date of Revision: February
PACKAGE INSERT PREGNYL (chorionic gonadotropin for injection, USP) 10,000 units/vial Human Gonadotropin Merck Canada Inc. 16750 route Transcanadienne Kirkland QC Canada H9H 4M7 Date of Revision: February
In - Vitro Fertilization Handbook
 In - Vitro Fertilization Handbook William F. Ziegler, D.O. Medical Director Scott Kratka, ELD, TS Embryology Laboratory Director Lauren F. Lucas, P.A.-C, M.S. Physician Assistant Frances Cerniak, R.N.
In - Vitro Fertilization Handbook William F. Ziegler, D.O. Medical Director Scott Kratka, ELD, TS Embryology Laboratory Director Lauren F. Lucas, P.A.-C, M.S. Physician Assistant Frances Cerniak, R.N.
Consent for In Vitro Fertilization
 Consent for In Vitro Fertilization Print Patient s Name Print Partner s Name We (I), the undersigned, request, authorize and consent to the procedure of In Vitro Fertilization (IVF) and Embryo Transfer
Consent for In Vitro Fertilization Print Patient s Name Print Partner s Name We (I), the undersigned, request, authorize and consent to the procedure of In Vitro Fertilization (IVF) and Embryo Transfer
FERTILITY AND AGE. Introduction. Fertility in the later 30's and 40's. Am I fertile?
 FERTILITY AND AGE Introduction Delaying pregnancy is a common choice for women in today's society. The number of women in their late 30s and 40s attempting pregnancy and having babies has increased in
FERTILITY AND AGE Introduction Delaying pregnancy is a common choice for women in today's society. The number of women in their late 30s and 40s attempting pregnancy and having babies has increased in
Artificial insemination
 Artificial insemination What is involved? Artificial insemination is an assisted reproduction technique that consists of inserting laboratory-treated spermatozoa into the woman s uterus or cervical canal.
Artificial insemination What is involved? Artificial insemination is an assisted reproduction technique that consists of inserting laboratory-treated spermatozoa into the woman s uterus or cervical canal.
Hormonal Oral Contraceptives: An Overview By Kelsie Court. A variety of methods of contraception are currently available, giving men and
 Hormonal Oral Contraceptives: An Overview By Kelsie Court A variety of methods of contraception are currently available, giving men and women plenty of options in choosing a method suitable to his or her
Hormonal Oral Contraceptives: An Overview By Kelsie Court A variety of methods of contraception are currently available, giving men and women plenty of options in choosing a method suitable to his or her
(choriogonadotropin alfa injection) FOR SUBCUTANEOUS USE DESCRIPTION
 (choriogonadotropin alfa injection) FOR SUBCUTANEOUS USE DESCRIPTION Ovidrel PreFilled Syringe (choriogonadotropin alfa injection) is a sterile liquid preparation of choriogonadotropin alfa (recombinant
(choriogonadotropin alfa injection) FOR SUBCUTANEOUS USE DESCRIPTION Ovidrel PreFilled Syringe (choriogonadotropin alfa injection) is a sterile liquid preparation of choriogonadotropin alfa (recombinant
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
IMPORTANT DRUG WARNING Regarding Mycophenolate-Containing Products
 Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief
 DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
Reference ID: 3482803
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use EVZIO safely and effectively. See full prescribing information for EVZIO. EVZIO (naloxone hydrochloride
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use EVZIO safely and effectively. See full prescribing information for EVZIO. EVZIO (naloxone hydrochloride
CONSENT TO PARTICIPATE IN THE IN VITRO FERTILIZATION-EMBRYO TRANSFER PROGRAM
 CONSENT TO PARTICIPATE IN THE IN VITRO FERTILIZATION-EMBRYO TRANSFER PROGRAM I, after consultation with my physician, request to participate in the In Vitro Fertilization (IVF)-Embryo Transfer (ET) procedures
CONSENT TO PARTICIPATE IN THE IN VITRO FERTILIZATION-EMBRYO TRANSFER PROGRAM I, after consultation with my physician, request to participate in the In Vitro Fertilization (IVF)-Embryo Transfer (ET) procedures
Research Article Association of ABO Blood Type and Ovarian Stimulation Response in Oocyte Donors
 Cronicon OPEN ACCESS Nigel Pereira 1 *, Anne P Hutchinson 2, Jovana P Lekovich 1, Rony T Elias 1, Zev Rosenwaks 1 and Steven D Spandorfer 1 1 Ronald O Perelman and Claudia Cohen Center for Reproductive
Cronicon OPEN ACCESS Nigel Pereira 1 *, Anne P Hutchinson 2, Jovana P Lekovich 1, Rony T Elias 1, Zev Rosenwaks 1 and Steven D Spandorfer 1 1 Ronald O Perelman and Claudia Cohen Center for Reproductive
The cost-effectiveness of IVF in the UK: a comparison of three gonadotrophin treatments Sykes D, Out H J, Palmer S J, van Loon J
 The cost-effectiveness of IVF in the UK: a comparison of three gonadotrophin treatments Sykes D, Out H J, Palmer S J, van Loon J Record Status This is a critical abstract of an economic evaluation that
The cost-effectiveness of IVF in the UK: a comparison of three gonadotrophin treatments Sykes D, Out H J, Palmer S J, van Loon J Record Status This is a critical abstract of an economic evaluation that
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
CYCLE EVALUATION. Please review this guide carefully. I. Early In Cycle. A. Selection of the Dominant Follicle (~ Day 3)
 CYCLE EVALUATION In order to evaluate how well you ovulate, we will see you on three days during your menstrual cycle. Early in the cycle you select a dominant follicle, on or about the third day of your
CYCLE EVALUATION In order to evaluate how well you ovulate, we will see you on three days during your menstrual cycle. Early in the cycle you select a dominant follicle, on or about the third day of your
Anatomy and Physiology of Human Reproduction. Module 10a
 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
In Vitro Fertilization (IVF) Page 1 of 11
 In Vitro Fertilization (IVF) Page 1 of 11 This document is a part of your informed consent process. Both partners should read the entire document carefully. In vitro fertilization (IVF) is a treatment
In Vitro Fertilization (IVF) Page 1 of 11 This document is a part of your informed consent process. Both partners should read the entire document carefully. In vitro fertilization (IVF) is a treatment
THE CENTER FOR ADVANCED REPRODUCTIVE SERVICES (CARS) (The Center) CONSENT FOR IN VITRO FERTILIZATION AND EMBRYO TRANSFER
 THE CENTER FOR ADVANCED REPRODUCTIVE SERVICES (CARS) (The Center) CONSENT FOR IN VITRO FERTILIZATION AND EMBRYO TRANSFER Partner #1 Last Name (Surname): Partner #1 First Name: Partner #1 Last 5 Digits
THE CENTER FOR ADVANCED REPRODUCTIVE SERVICES (CARS) (The Center) CONSENT FOR IN VITRO FERTILIZATION AND EMBRYO TRANSFER Partner #1 Last Name (Surname): Partner #1 First Name: Partner #1 Last 5 Digits
Medications for Inducing Ovulation
 AMERICAN SOCIETY FOR REPRODUCTIVE MEDICINE Medications for Inducing Ovulation A Guide for Patients PATIENT INFORMATION SERIES Published by the American Society for Reproductive Medicine under the direction
AMERICAN SOCIETY FOR REPRODUCTIVE MEDICINE Medications for Inducing Ovulation A Guide for Patients PATIENT INFORMATION SERIES Published by the American Society for Reproductive Medicine under the direction
The Menstrual Cycle. Model 1: Ovarian Cycle follicular cells
 The Menstrual Cycle REVIEW questions to complete before starting this POGIL activity 1. Gonads produce both gametes and sex steroid hormones. For the female, name the: A. gonads ovaries B. gametes oocyte/ovum/egg
The Menstrual Cycle REVIEW questions to complete before starting this POGIL activity 1. Gonads produce both gametes and sex steroid hormones. For the female, name the: A. gonads ovaries B. gametes oocyte/ovum/egg
PLEGRIDY (peginterferon beta-1a) injection, for subcutaneous injection Initial U.S. Approval: 2014
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PLEGRIDY safely and effectively. See full prescribing information for PLEGRIDY. PLEGRIDY (peginterferon
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PLEGRIDY safely and effectively. See full prescribing information for PLEGRIDY. PLEGRIDY (peginterferon
Director, IVF Program, Division of Reproductive Endocrinology & Infertility
 Director, IVF Program, Division of Reproductive Endocrinology & Infertility Date: January 17, 2006 To: From: RE: All IVF candidates Chief, Reproductive Endocrinology & Infertility Criteria for IVF program
Director, IVF Program, Division of Reproductive Endocrinology & Infertility Date: January 17, 2006 To: From: RE: All IVF candidates Chief, Reproductive Endocrinology & Infertility Criteria for IVF program
Systematic review by: Dr. Ashraf Ahmed ElDaly, M.Sc., M.D.
 Role of luteinizing hormone supplementation in the follicular phase and pregnancy, during controlled ovarian hyperstimulation in in vitro fertilization Systematic review by: Dr. Ashraf Ahmed ElDaly, M.Sc.,
Role of luteinizing hormone supplementation in the follicular phase and pregnancy, during controlled ovarian hyperstimulation in in vitro fertilization Systematic review by: Dr. Ashraf Ahmed ElDaly, M.Sc.,
From Menses to Menopause: How Hormones Can Affect Blood Glucose Levels. Christine Day, RN, MS, CNS-BC Lake Superior College
 From Menses to Menopause: How Hormones Can Affect Blood Glucose Levels Christine Day, RN, MS, CNS-BC Lake Superior College Overview Will review hormonal changes over the female lifespan Discuss the effects
From Menses to Menopause: How Hormones Can Affect Blood Glucose Levels Christine Day, RN, MS, CNS-BC Lake Superior College Overview Will review hormonal changes over the female lifespan Discuss the effects
Understanding Blood Tests - Pregnancy/Fertility Monitoring by Beth Anne Ary M.D
 Understanding Blood Tests - Pregnancy/Fertility Monitoring by Beth Anne Ary M.D Blood tests are the most common and most important method of monitoring pregnancy-- both assisted pregnancies, and unassisted.
Understanding Blood Tests - Pregnancy/Fertility Monitoring by Beth Anne Ary M.D Blood tests are the most common and most important method of monitoring pregnancy-- both assisted pregnancies, and unassisted.
See 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2016 FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK OR
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
Acupuncture Treatment For Infertile Women Undergoing Intracytoplasmic Sperm injection
 Acupuncture Treatment For Infertile Women Undergoing Intracytoplasmic Sperm injection Sandra L. Emmons, MD Phillip Patton, MD Source: Medical Acupuncture, A Journal For Physicians By Physicians Spring
Acupuncture Treatment For Infertile Women Undergoing Intracytoplasmic Sperm injection Sandra L. Emmons, MD Phillip Patton, MD Source: Medical Acupuncture, A Journal For Physicians By Physicians Spring
IVF CLASS. IVF NURSE CONTACT INFORMATION: Darshana 301-400-2151, [email protected] Nicole 301-400-2146, nicole.l.sobers.ctr@health.
 IVF CLASS IVF NURSE CONTACT INFORMATION: Darshana 301-400-2151, [email protected] Nicole 301-400-2146, [email protected] PLEASE NOTE: If you do not have medications for the next
IVF CLASS IVF NURSE CONTACT INFORMATION: Darshana 301-400-2151, [email protected] Nicole 301-400-2146, [email protected] PLEASE NOTE: If you do not have medications for the next
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
The Menstrual Cycle, Hormones and Fertility Treatment
 The Menstrual Cycle, Hormones and Fertility Treatment How many of us understand how our monthly cycle works? Every 28 days (or thereabouts), between the ages of around 13 and 51, a woman will release a
The Menstrual Cycle, Hormones and Fertility Treatment How many of us understand how our monthly cycle works? Every 28 days (or thereabouts), between the ages of around 13 and 51, a woman will release a
IN VITRO FERTILISATION IVF and ICSI
 IN VITRO FERTILISATION IVF and ICSI Page 1 of 7 WHAT ARE IVF and ICSI? IVF is short for in vitro fertilisation which means fertilisation outside the body. It usually involves stimulation of the ovaries
IN VITRO FERTILISATION IVF and ICSI Page 1 of 7 WHAT ARE IVF and ICSI? IVF is short for in vitro fertilisation which means fertilisation outside the body. It usually involves stimulation of the ovaries
, hereby agree to a form of treatment known
 Patient Consent for Therapy Human In Vitro Fertilization and Embryo Transfer This is to certify that I, as In Vitro Fertilization and Embryo Transfer., hereby agree to a form of treatment known I have
Patient Consent for Therapy Human In Vitro Fertilization and Embryo Transfer This is to certify that I, as In Vitro Fertilization and Embryo Transfer., hereby agree to a form of treatment known I have
Egg Donation Process, Risks, Consent and Agreement
 THE CENTER FOR HUMAN REPRODUCTION (CHR) 21 East 69 th Street, New York, NY 10021 T: 212-994-4400; F: 212-994-4499 Egg Donation Process, Risks, Consent and Agreement Updated on: 10/8/2014 Date: Egg Donor
THE CENTER FOR HUMAN REPRODUCTION (CHR) 21 East 69 th Street, New York, NY 10021 T: 212-994-4400; F: 212-994-4499 Egg Donation Process, Risks, Consent and Agreement Updated on: 10/8/2014 Date: Egg Donor
Assisted Reproductive Technologies at IGO
 9339 Genesee Avenue, Suite 220 San Diego, CA 92121 858 455 7520 Assisted Reproductive Technologies at IGO Although IGO no longer operates an IVF laboratory or program as such, we work closely with area
9339 Genesee Avenue, Suite 220 San Diego, CA 92121 858 455 7520 Assisted Reproductive Technologies at IGO Although IGO no longer operates an IVF laboratory or program as such, we work closely with area
Medications for Inducing Ovulation
 AMERICAN SOCIETY FOR REPRODUCTIVE MEDICINE Medications for Inducing Ovulation A Guide for Patients PATIENT INFORMATION SERIES Published by the American Society for Reproductive Medicine under the direction
AMERICAN SOCIETY FOR REPRODUCTIVE MEDICINE Medications for Inducing Ovulation A Guide for Patients PATIENT INFORMATION SERIES Published by the American Society for Reproductive Medicine under the direction
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
Symposium on RECENT ADVANCES IN ASSISTED REPRODUCTIVE TECHNOLOGY
 Symposium on RECENT ADVANCES IN ASSISTED REPRODUCTIVE TECHNOLOGY Dr Niel Senewirathne Senior Consultant of Obstetrician & Gynaecologist De zoyza Maternity Hospita 1 ART - IVF & ICSI 2 Infertility No pregnancy
Symposium on RECENT ADVANCES IN ASSISTED REPRODUCTIVE TECHNOLOGY Dr Niel Senewirathne Senior Consultant of Obstetrician & Gynaecologist De zoyza Maternity Hospita 1 ART - IVF & ICSI 2 Infertility No pregnancy
Rationale for replacing IVIG with Intralipid (IL) for immunological pregnancy loss
 Rationale for replacing IVIG with Intralipid (IL) for immunological pregnancy loss Recurrent Pregnancy Loss The reason that an embryo may not implant successfully is either because there is something intrinsically
Rationale for replacing IVIG with Intralipid (IL) for immunological pregnancy loss Recurrent Pregnancy Loss The reason that an embryo may not implant successfully is either because there is something intrinsically
Revised: 9/2014. LUMIGAN (bimatoprost ophthalmic solution) 0.01% for topical ophthalmic use. Initial U.S. Approval: 2001
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN (bimatoprost ophthalmic solution) 0.01% safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN (bimatoprost ophthalmic solution) 0.01% safely and effectively. See full prescribing information
Informed Consent Packet - In Vitro Fertilization (IVF)
 Center for Reproductive Medicine (CRM) Informed Consent Packet - In Vitro Fertilization (IVF) This packet contains the required IVF treatment consent documents. Please read, consider and, if you agree,
Center for Reproductive Medicine (CRM) Informed Consent Packet - In Vitro Fertilization (IVF) This packet contains the required IVF treatment consent documents. Please read, consider and, if you agree,
HOW IS OVARIAN RESERVE ASSESSED?
 HOW IS OVARIAN RESERVE ASSESSED? The majority of indicators we have to assess OR assess egg quantity rather than egg quality and these two do not always go hand in hand. No individual test is a perfect
HOW IS OVARIAN RESERVE ASSESSED? The majority of indicators we have to assess OR assess egg quantity rather than egg quality and these two do not always go hand in hand. No individual test is a perfect
INJECTION TECHNIQUE. IVF NURSING OFFICE: (301) 400-2151 Darshana (301) 400-2146 Nicole
 IVF NURSING OFFICE: (301) 400-2151 Darshana (301) 400-2146 Nicole PLEASE NOTE: If you do not have medications for the next day s dose, you MUST go to the clinic that morning at 6:30 AM for more medications.
IVF NURSING OFFICE: (301) 400-2151 Darshana (301) 400-2146 Nicole PLEASE NOTE: If you do not have medications for the next day s dose, you MUST go to the clinic that morning at 6:30 AM for more medications.
IN-VITRO FERTILIZATION BASICS
 IN-VITRO FERTILIZATION BASICS IVF HANDBOOK Table of Contents Page Pre-treatment Recommendations 1 Medication Overview 1-7 Cycle Monitoring 7-8 Cycle Cancellation 8 Pre-egg Retrieval Instructions 9 Day
IN-VITRO FERTILIZATION BASICS IVF HANDBOOK Table of Contents Page Pre-treatment Recommendations 1 Medication Overview 1-7 Cycle Monitoring 7-8 Cycle Cancellation 8 Pre-egg Retrieval Instructions 9 Day
INFERTILITY/POLYCYSTIC OVARIAN SYNDROME. Ovulatory Dysfunction: Polycystic ovarian syndrome (PCOS)
 Introduction Infertility is defined as the absence of pregnancy following 12 months of unprotected intercourse. Infertility may be caused by Ovulatory Dysfunction, Blocked Fallopian Tubes, Male Factor
Introduction Infertility is defined as the absence of pregnancy following 12 months of unprotected intercourse. Infertility may be caused by Ovulatory Dysfunction, Blocked Fallopian Tubes, Male Factor
Polycystic Ovarian Syndrome
 Polycystic Ovarian Syndrome What is Polycystic Ovarian Syndrome? Polycystic ovary syndrome (or PCOS) is a common condition affecting 3 to 5% of women of reproductive age. It is linked with hormonal imbalances,
Polycystic Ovarian Syndrome What is Polycystic Ovarian Syndrome? Polycystic ovary syndrome (or PCOS) is a common condition affecting 3 to 5% of women of reproductive age. It is linked with hormonal imbalances,
Risks and complications of assisted conception
 Risks and complications of assisted conception August 005 Richard Kennedy British Fertility Society Factsheet www.fertility.org.uk No medical treatment is entirely free from risk and infertility treatment
Risks and complications of assisted conception August 005 Richard Kennedy British Fertility Society Factsheet www.fertility.org.uk No medical treatment is entirely free from risk and infertility treatment
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
2 DOSAGE AND ADMINISTRATION 2.1 Dosing and Dose Adjustment
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Auryxia safely and effectively. See full prescribing information for Auryxia. Auryxia (ferric citrate)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Auryxia safely and effectively. See full prescribing information for Auryxia. Auryxia (ferric citrate)
In vitro fertilisation (IVF) & intra-cytoplasmic sperm injection (ICSI)
 In vitro fertilisation (IVF) & intra-cytoplasmic sperm injection (ICSI) Inside: The stages of ivf & icsi What to expect from treatment Coping with stress Part of the Pathways to Parenthood booklet series
In vitro fertilisation (IVF) & intra-cytoplasmic sperm injection (ICSI) Inside: The stages of ivf & icsi What to expect from treatment Coping with stress Part of the Pathways to Parenthood booklet series
POLYCYSTIC OVARY SYNDROME
 POLYCYSTIC OVARY SYNDROME Information Leaflet Your Health. Our Priority. Page 2 of 6 What is polycystic ovary syndrome? (PCOS) Polycystic ovary syndrome (PCOS) is the most common hormonal disorder in women
POLYCYSTIC OVARY SYNDROME Information Leaflet Your Health. Our Priority. Page 2 of 6 What is polycystic ovary syndrome? (PCOS) Polycystic ovary syndrome (PCOS) is the most common hormonal disorder in women
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
 SOMAVERT pegvisomant for injection PATIENT INFORMATION SOMAVERT (SOM-ah-vert) (pegvisomant for injection) Read the patient information that comes with SOMAVERT before you start using it and each time you
SOMAVERT pegvisomant for injection PATIENT INFORMATION SOMAVERT (SOM-ah-vert) (pegvisomant for injection) Read the patient information that comes with SOMAVERT before you start using it and each time you
Hull & East Riding Prescribing Committee
 Hull & East Riding Prescribing Committee Guideline on Prescribing of Gonadorelin (GnRH) Analogues and Progesterone Receptor Modulators in treatment of Endometriosis or prior to Endometrial Ablation, or
Hull & East Riding Prescribing Committee Guideline on Prescribing of Gonadorelin (GnRH) Analogues and Progesterone Receptor Modulators in treatment of Endometriosis or prior to Endometrial Ablation, or
Age and Fertility. A Guide for Patients PATIENT INFORMATION SERIES
 Age and Fertility A Guide for Patients PATIENT INFORMATION SERIES Published by the American Society for Reproductive Medicine under the direction of the Patient Education Committee and the Publications
Age and Fertility A Guide for Patients PATIENT INFORMATION SERIES Published by the American Society for Reproductive Medicine under the direction of the Patient Education Committee and the Publications
ALLERGENIC EXTRACT. Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE. U.S. Government License No.
 ALLERGENIC EXTRACT Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE U.S. Government License No. 308 Revised 07/04 PO Box 800 Lenoir, NC 28645 USA DESCRIPTION This set
ALLERGENIC EXTRACT Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE U.S. Government License No. 308 Revised 07/04 PO Box 800 Lenoir, NC 28645 USA DESCRIPTION This set
Plan B (Levonorgestrel) Tablets, 0.75 mg
 Plan B (Levonorgestrel) Tablets, 0.75 mg Rx only for women age 17 and younger For women age 17 and younger, Plan B is a prescription only emergency contraceptive. Plan B is intended to prevent pregnancy
Plan B (Levonorgestrel) Tablets, 0.75 mg Rx only for women age 17 and younger For women age 17 and younger, Plan B is a prescription only emergency contraceptive. Plan B is intended to prevent pregnancy
Recent Progress in In Vitro Fertilization and Intracytoplasmic Sperm Injection Technologies in Japan
 Research and Reviews Recent Progress in In Vitro Fertilization and Intracytoplasmic Sperm Injection Technologies in Japan JMAJ 52(1): 29 33, 2009 Kaoru YANAGIDA* 1 Abstract The three basic pillars of fertility
Research and Reviews Recent Progress in In Vitro Fertilization and Intracytoplasmic Sperm Injection Technologies in Japan JMAJ 52(1): 29 33, 2009 Kaoru YANAGIDA* 1 Abstract The three basic pillars of fertility
AGE & FERTILITY: Effective Evaluation & Treatment I. LANE WONG, MD, FACOG. www.hopefertilitycenter.com www.hopeivf.com
 Page 1 of 6 AGE & FERTILITY: Effective Evaluation & Treatment I. LANE WONG, MD, FACOG. www.hopefertilitycenter.com www.hopeivf.com Age has a profound effect on female fertility. This is common knowledge,
Page 1 of 6 AGE & FERTILITY: Effective Evaluation & Treatment I. LANE WONG, MD, FACOG. www.hopefertilitycenter.com www.hopeivf.com Age has a profound effect on female fertility. This is common knowledge,
Reproduction and its Hormonal Control
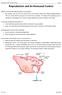 Reproduction and its Hormonal Control Page 1 Reproduction and its Hormonal Control Different mammals have different patterns of reproduction Eg mammals, rats and mice can breed all year round, whereas
Reproduction and its Hormonal Control Page 1 Reproduction and its Hormonal Control Different mammals have different patterns of reproduction Eg mammals, rats and mice can breed all year round, whereas
SO, WHAT IS A POOR RESPONDER?
 SO, WHAT IS A POOR RESPONDER? We now understand why ovarian reserve is important and how we assess it, but how is poor response defined? Unfortunately, there is no universally accepted definition for the
SO, WHAT IS A POOR RESPONDER? We now understand why ovarian reserve is important and how we assess it, but how is poor response defined? Unfortunately, there is no universally accepted definition for the
NIH Clinical Center Patient Education Materials Giving a subcutaneous injection
 NIH Clinical Center Patient Education Materials What is a subcutaenous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous injection into the fatty
NIH Clinical Center Patient Education Materials What is a subcutaenous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous injection into the fatty
In vitro fertilization for Orthodox Jewish couples: antagonist cycle modifications allowing for mikveh attendance before oocyte retrieval
 In vitro fertilization for Orthodox Jewish couples: antagonist cycle modifications allowing for mikveh attendance before oocyte retrieval David E. Reichman, M.D., Anate Aelion Brauer, M.D., Dan Goldschlag,
In vitro fertilization for Orthodox Jewish couples: antagonist cycle modifications allowing for mikveh attendance before oocyte retrieval David E. Reichman, M.D., Anate Aelion Brauer, M.D., Dan Goldschlag,
Reduced Ovarian Reserve Is there any hope for a bad egg?
 Reduced Ovarian Reserve Is there any hope for a bad egg? Dr. Phil Boyle Galway Clinic, 19 th March 2014 For more information on Low AMH see www.napro.ie Anti Mullerian Hormone AMH levels are commonly measured
Reduced Ovarian Reserve Is there any hope for a bad egg? Dr. Phil Boyle Galway Clinic, 19 th March 2014 For more information on Low AMH see www.napro.ie Anti Mullerian Hormone AMH levels are commonly measured
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension
 VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
1. AMOUNT OF FSH PRESENT
 The Menstrual Cycle Name Date Period PRE-LAB 1. Write down three facts you know about the menstrual cycle. A. B. C. FOLLICULAR PHASE Within the ovaries are located many egg cells. Each egg is enclosed
The Menstrual Cycle Name Date Period PRE-LAB 1. Write down three facts you know about the menstrual cycle. A. B. C. FOLLICULAR PHASE Within the ovaries are located many egg cells. Each egg is enclosed
Fast Track to IVF. Objectives
 Disclosure statement: Richard H. Reindollar, M.D. has no relevant financial relationships with any manufacturers of pharmaceuticals, laboratory supplies, or medical devices. Fast Track to IVF Richard H.
Disclosure statement: Richard H. Reindollar, M.D. has no relevant financial relationships with any manufacturers of pharmaceuticals, laboratory supplies, or medical devices. Fast Track to IVF Richard H.
Egg Donation Process, Risk, Consent and Agreement
 Department of Obstetrics and Gynecology Strong Fertility Center Kathleen Hoeger, MD, MPH Director Bala Bhagavath, MD Vivian Lewis, MD John T. Queenan, Jr., MD Wendy Vitek, MD Egg Donation Process, Risk,
Department of Obstetrics and Gynecology Strong Fertility Center Kathleen Hoeger, MD, MPH Director Bala Bhagavath, MD Vivian Lewis, MD John T. Queenan, Jr., MD Wendy Vitek, MD Egg Donation Process, Risk,
New Zealand Datasheet
 New Zealand Datasheet Name of Medicine PRAXBIND Idarucizumab 50 mg/ml solution for injection/infusion Presentation Idarucizumab is a humanized monoclonal antibody fragment (Fab) molecule derived from an
New Zealand Datasheet Name of Medicine PRAXBIND Idarucizumab 50 mg/ml solution for injection/infusion Presentation Idarucizumab is a humanized monoclonal antibody fragment (Fab) molecule derived from an
Objectives At the completion of this module, unlicensed assistive personnel (UAP) should be able to:
 Objectives At the completion of this module, unlicensed assistive personnel (UAP) should be able to: 1. administer medications by subcutaneous injections. 2. document medication administration in the client
Objectives At the completion of this module, unlicensed assistive personnel (UAP) should be able to: 1. administer medications by subcutaneous injections. 2. document medication administration in the client
MINISTRY OF HEALTH Quality and Service Administration. Fe r t i l i z at i o n. to I n - V i t r o. G u i d e. i n I s r a e l
 MINISTRY OF HEALTH Quality and Service Administration G u i d e to I n - V i t r o Fe r t i l i z at i o n i n I s r a e l Contents Introduction 3 The Natural Fertilization Process 4 In Vitro Fertilization
MINISTRY OF HEALTH Quality and Service Administration G u i d e to I n - V i t r o Fe r t i l i z at i o n i n I s r a e l Contents Introduction 3 The Natural Fertilization Process 4 In Vitro Fertilization
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013.
 The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
The following chapter is called "Follow-ups with a Positive or a Negative Pregnancy Test".
 Slide 1 Welcome to chapter 7. The following chapter is called "Follow-ups with a Positive or a Negative Pregnancy Test". The author is Professor Pasquale Patrizio. Slide 2 This chapter has the following
Slide 1 Welcome to chapter 7. The following chapter is called "Follow-ups with a Positive or a Negative Pregnancy Test". The author is Professor Pasquale Patrizio. Slide 2 This chapter has the following
Assisted reproductive technologies (ART) in Canada: 2011 results from the Canadian ART Register
 1 Assisted reproductive technologies (ART) in Canada: 2011 results from the Canadian ART Register Joanne Gunby, M.Sc. CARTR Co-ordinator Email: [email protected] Supported by the IVF Directors Group of
1 Assisted reproductive technologies (ART) in Canada: 2011 results from the Canadian ART Register Joanne Gunby, M.Sc. CARTR Co-ordinator Email: [email protected] Supported by the IVF Directors Group of
PACKAGE LEAFLET: INFORMATION FOR THE USER. PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion. Paracetamol
 PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
0.9% Sodium Chloride Injection, USP In VIAFLEX Plastic Container
 Page 1 of 8 PRESCRIBING INFORMATION 0.9% Sodium Chloride Injection, USP In VIAFLEX Plastic Container IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of
Page 1 of 8 PRESCRIBING INFORMATION 0.9% Sodium Chloride Injection, USP In VIAFLEX Plastic Container IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of
UW MEDICINE PATIENT EDUCATION. Using Insulin. Basic facts about insulin and self-injection. What is insulin? How does diabetes affect the body?
 UW MEDICINE PATIENT EDUCATION Using Insulin Basic facts about insulin and self-injection This handout explains what insulin is, the different types of insulin, how to store it, how to give an injection
UW MEDICINE PATIENT EDUCATION Using Insulin Basic facts about insulin and self-injection This handout explains what insulin is, the different types of insulin, how to store it, how to give an injection
OVULATION & INTRAUTERINE INSEMINATION (IUI)
 OVULATION & This handbook is intended to give you an overview of infertility treatment with IUI and ovulation induction and to better understand the medical circumstances that lead to this treatment option.
OVULATION & This handbook is intended to give you an overview of infertility treatment with IUI and ovulation induction and to better understand the medical circumstances that lead to this treatment option.
Department of Health Commencing insulin therapy
 Department of Health Commencing insulin therapy Great state. Great opportunity. State of Queensland (Queensland Health) 2008 2013 This work is licensed under a Creative Commons Attribution No Derivatives
Department of Health Commencing insulin therapy Great state. Great opportunity. State of Queensland (Queensland Health) 2008 2013 This work is licensed under a Creative Commons Attribution No Derivatives
Who are Levonorgestrel Tablets, 0.75mg for? Other things your patients should know: When are Levonorgestrel Tablets, 0.75mg effective?
 One-hour ACPE accredited continuing education program is available at no charge for pharmacists provided by the University of Illinois-Chicago College of Pharmacy at www.pharmacyce.uic.edu Accidents happen...
One-hour ACPE accredited continuing education program is available at no charge for pharmacists provided by the University of Illinois-Chicago College of Pharmacy at www.pharmacyce.uic.edu Accidents happen...
8.5 Geriatric Use * Sections or subsections omitted from the full prescribing information are not listed.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Injectafer safely and effectively. See full prescribing information for Injectafer. INJECTAFER (ferric
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Injectafer safely and effectively. See full prescribing information for Injectafer. INJECTAFER (ferric
Infertility Services Medical Policy For University of Vermont Medical Center and Central Vermont Medical Center employer groups
 Infertility Services Medical Policy For University of Vermont Medical Center and Central Vermont Medical Center employer groups File name: Infertility Services File code: UM.REPRO.01 Last Review: 02/2016
Infertility Services Medical Policy For University of Vermont Medical Center and Central Vermont Medical Center employer groups File name: Infertility Services File code: UM.REPRO.01 Last Review: 02/2016
Ovarian Cyst. Homoeopathy Clinic. Introduction. Types of Ovarian Cysts. Contents. Case Reports. 21 August 2002
 Case Reports 21 August 2002 Ovarian Cyst Homoeopathy Clinic Check Yourself If you have any of the following symptoms call your doctor. Sense of fullness or pressure or a dull ache in the abdomen Pain during
Case Reports 21 August 2002 Ovarian Cyst Homoeopathy Clinic Check Yourself If you have any of the following symptoms call your doctor. Sense of fullness or pressure or a dull ache in the abdomen Pain during
Prediction of Pregnancy Outcome Using HCG, CA125 and Progesterone in Cases of Habitual Abortions
 Prediction of Pregnancy Outcome Using HCG, CA125 and Progesterone in * (MBChB, FICMS, CABOG) **Sawsan Talib Salman (MBChB, FICMS, CABOG) ***Huda Khaleel Ibrahim (MBChB) Abstract Background: - Although
Prediction of Pregnancy Outcome Using HCG, CA125 and Progesterone in * (MBChB, FICMS, CABOG) **Sawsan Talib Salman (MBChB, FICMS, CABOG) ***Huda Khaleel Ibrahim (MBChB) Abstract Background: - Although
Forming families for over 20 years IN VITRO. www.ctfertility.com
 Forming families for over 20 years IN VITRO fertilization www.ctfertility.com Forming families for over 20 years Michael B. Doyle, M.D. Medical Director Introduction to IN VITRO fertilization Contents
Forming families for over 20 years IN VITRO fertilization www.ctfertility.com Forming families for over 20 years Michael B. Doyle, M.D. Medical Director Introduction to IN VITRO fertilization Contents
Instructions for Use PROCRIT (PRO KRIT) (epoetin alfa)
 Instructions for Use PROCRIT (PROKRIT) (epoetin alfa) Use these Instructions for Use if you or your caregiver has been trained to give PROCRIT injections at home. Do not give yourself the injection unless
Instructions for Use PROCRIT (PROKRIT) (epoetin alfa) Use these Instructions for Use if you or your caregiver has been trained to give PROCRIT injections at home. Do not give yourself the injection unless
FULL PRESCRIBING INFORMATION: CONTENTS*
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PHOTREXA VISCOUS and PHOTREXA safely and effectively. See full prescribing information for PHOTREXA
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PHOTREXA VISCOUS and PHOTREXA safely and effectively. See full prescribing information for PHOTREXA
One vial contains 500 U* of C1-inhibitor** After reconstitution the product contains 500 U/5 ml which correspond to a concentration of 100 U/ml.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cetor 100 U/ml powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 500 U* of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cetor 100 U/ml powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 500 U* of
טופס הסכמה לטיפולי הפרייה חוץ גופית
 טופס הסכמה לטיפולי הפרייה חוץ גופית CONSENT FORM: IN-VITRO FERTILIZATION (IVF) 1. General In-vitro fertilization is performed in cases of impaired fertility, which may be caused by the following: Obstruction
טופס הסכמה לטיפולי הפרייה חוץ גופית CONSENT FORM: IN-VITRO FERTILIZATION (IVF) 1. General In-vitro fertilization is performed in cases of impaired fertility, which may be caused by the following: Obstruction
