FULL PRESCRIBING INFORMATION: CONTENTS*
|
|
|
- Elmer Watson
- 9 years ago
- Views:
Transcription
1 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PHOTREXA VISCOUS and PHOTREXA safely and effectively. See full prescribing information for PHOTREXA VISCOUS and PHOTREXA. PHOTREXA VISCOUS (riboflavin 5 -phosphate in 20% dextran ophthalmic solution) 0.146% for topical ophthalmic use PHOTREXA (riboflavin 5 -phosphate ophthalmic solution) 0.146% for topical ophthalmic use For use with the KXL System Initial U.S. Approval: 2016 INDICATIONS AND USAGE PHOTREXA VISCOUS and PHOTREXA are photoenhancers indicated for use with the KXL System in corneal collagen cross-linking for the treatment of progressive keratoconus (1) DOSAGE AND ADMINISTRATION Debride the epithelium using standard aseptic technique using topical anesthesia. Then instill 1 drop of PHOTREXA VISCOUS topically on the eye every 2 minutes for 30 minutes. After 30 minutes, examine the eye under slit lamp for presence of a yellow flare in the anterior chamber. If flare is not detected, instill 1 drop of PHOTREXA VISCOUS every 2 minutes for an additional 2 to 3 drops and recheck for yellow flare. Repeat as necessary. Once flare is observed, perform ultrasound pachymetry. If corneal thickness is less than 400 microns, instill 2 drops of PHOTREXA every 5 to 10 seconds until the corneal thickness increases to at least 400 microns. Irradiation should not be performed unless this 400 micron threshold is met and the yellow flare is seen. Irradiate the eye for 30 minutes at 3mW/cm 2 using the KXL System as per the instructions in the KXL manual. During irradiation, continue topical instillation of PHOTREXA VISCOUS onto the eye every 2 minutes for the 30 minute irradiation period. Refer to the KXL Operator s manual for specific device instructions. DOSAGE FORMS AND STRENGTHS PHOTREXA VISCOUS in a 3 ml glass syringe containing sterile 1.46 mg/ml riboflavin 5 -phosphate in 20% dextran ophthalmic solution (3.1) PHOTREXA in a 3 ml glass syringe containing sterile 1.46 mg/ml riboflavin 5 -phosphate ophthalmic solution (3.2) CONTRAINDICATIONS None (4) WARNINGS AND PRECAUTIONS Ulcerative keratitis can occur. Monitor for resolution of epithelial defects (5) ADVERSE REACTIONS The most common ocular adverse reactions in any CXL-treated eye were corneal opacity (haze), punctate keratitis, corneal striae, corneal epithelium defect, eye pain, reduced visual acuity, and blurred vision (6.1). To report SUSPECTED ADVERSE REACTIONS, contact Avedro at or FDA at FDA-1088 or See 17 for PATIENT COUNSELING INFORMATION Revised: 4/2016 FULL PRESCRIBING INFORMATION: CONTENTS* 1. INDICATIONS AND USAGE Progressive Keratoconus 2. DOSAGE AND ADMINISTRATION 3. DOSAGE FORMS AND STRENGTHS 3.1 Photrexa Viscous 3.2 Photrexa 4. CONTRAINDICATIONS 5. WARNINGS AND PRECAUTIONS 6. ADVERSE REACTIONS 8. USE IN SPECIFIC POPULATIONS 8.1. Pregnancy 8.2. Lactation 8.4. Pediatric Use 8.5. Geriatric Use 11. DESCRIPTION 12. CLINICAL PHARMACOLOGY Mechanism of Action 13. NONCLINICAL TOXICOLOGY Carcinogenesis, Mutagenesis, Impairment of Fertility 14. CLINICAL STUDIES 16. HOW SUPPLIED/STORAGE AND HANDLING 17. PATIENT COUNSELING INFORMATION *Sections or subsections omitted from the full prescribing information are not listed. 1
2 FULL PRESCRIBING INFORMATION 1. INDICATIONS AND USAGE PHOTREXA VISCOUS and PHOTREXA are indicated for use in corneal collagen cross-linking in combination with the KXL System for the treatment of progressive keratoconus. 2. DOSAGE AND ADMINISTRATION Using topical anesthesia, debride the epithelium to a diameter of approximately 9 mm using standard aseptic technique. Post epithelial debridement, instill 1 drop of Photrexa Viscous topically on the eye every 2 minutes for 30 minutes. At the end of the 30 minute soaking period, examine the eye under the slit lamp for the presence of a yellow flare in the anterior chamber. If the yellow flare is not detected, instill 1 drop of Photrexa Viscous every 2 minutes for an additional 2 to 3 drops and recheck for the presence of a yellow flare. This process can be repeated as necessary. Once the yellow flare is observed, perform ultrasound pachymetry. If corneal thickness is less than 400 microns, instill 2 drops of PHOTREXA every 5 to 10 seconds until the corneal thickness increases to at least 400 microns. Irradiation should not be performed unless this 400 micron threshold is met and the yellow flare is seen. Irradiate the eye for 30 continuous minutes at 3mW/cm 2 at a wavelength of 365 nm, centered over the cornea, using the KXL System as per the instructions in the KXL manual. During irradiation, continue topical instillation of PHOTREXA VISCOUS onto the eye every 2 minutes for the 30 minute irradiation period. For topical ophthalmic use. Do not inject. Single use PHOTREXA VISCOUS and PHOTREXA only. Discard syringe(s) after use. PHOTREXA VISCOUS and PHOTREXA are for use with the KXL System only. PLEASE REFER TO THE KXL OPERATOR S MANUAL FOR SPECIFIC DEVICE INSTRUCTIONS. 3. DOSAGE FORMS AND STRENGTHS 3.1 PHOTREXA VISCOUS PHOTREXA VISCOUS in a 3 ml glass syringe containing sterile 1.46 mg/ml riboflavin 5 -phosphate in 20% dextran ophthalmic solution for topical administration. 3.2 PHOTREXA PHOTREXA in a 3 ml glass syringe containing sterile 1.46 mg/ml riboflavin 5 -phosphate ophthalmic solution for topical administration. 4. CONTRAINDICATIONS None. 2
3 5. WARNINGS AND PRECAUTIONS Ulcerative keratitis can occur. Monitor for resolution of epithelial defects. [See Adverse Reactions (6)]. 6. ADVERSE REACTIONS The following clinically significant adverse reactions are described elsewhere in the labeling: Ulcerative keratitis [Warnings and Precautions (5)] 6.1 Clinical Trials Experience: Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The safety of the corneal collagen cross-linking procedure was evaluated in 3 randomized, parallel-group, openlabel, sham-controlled trials; patients were followed up for 12 months. In each study, only one eye of subjects was designated as the study eye. Study eyes were randomized to receive one of the two study treatments (CXL or sham) at the baseline visit and were followed up at Day 1, Week 1, and Months 1, 3, 6, and 12. At Month 3 or later, sham study eyes and non-study eyes had the option of receiving CXL treatment, and were followed-up for 12 months from the time of receiving CXL treatment. Each CXL treated eye received a single course of CXL treatment only. Safety data were obtained from 193 randomized CXL study eyes, 191 control eyes, and 319 nonrandomized CXL non-study eyes. Overall, 512 eyes in 364 patients received CXL treatment. In progressive keratoconus subjects, the most common ocular adverse reactions in any CXL-treated eye were corneal opacity (haze), punctate keratitis, corneal striae, corneal epithelium defect, eye pain, reduced visual acuity, and blurred vision (Table 1). Adverse events reported in non-study, non-randomized CXL treated were similar in in terms of preferred terms and frequency to those seen in randomized study eyes. The majority of adverse events reported resolved during the first month, while events such as corneal epithelium defect, corneal striae, punctate keratitis, photophobia, dry eye and eye pain, and decreased visual acuity took up to 6 months to resolve and corneal opacity or haze took up to 12 months to resolve. In 1-2% of patients, corneal epithelium defect, corneal edema, corneal opacity and corneal scar continued to be observed at 12 months. Table 1: Most Common ( 1%) Ocular Adverse Reactions in CXL-Treated Study Eye in the Pooled Randomized Safety Population N (%) Progressive Keratoconus Studies Other Clinical Experience CXL Control CXL Control Group Group Group Group Preferred Term (N=102) 1 (N=103) 1 (N=91) 1 (N=88) 1 Anterior chamber cell 2 (2) 0 2 (2) 1 (1) Anterior chamber 4 (4) 0 5 (6) 2 (2) flare Asthenopia 1 (1) 1 (1) 2 (2) 0 Blepharitis (1) Corneal disorder 3 (3) 1 (1) 3 (3) 0 3
4 Preferred Term Corneal epithelium defect Progressive Keratoconus Studies CXL Group (N=102) 1 Control Group (N=103) 1 CXL Group (N=91) 1 Other Clinical Experience Control Group (N=88) 1 24 (24) 1 (1) 26 (28) 3 (3) Corneal oedema 3 (3) 0 3 (3) 0 Corneal opacity 2 65 (64) 9 (9) 65 (71) 8 (9) Corneal striae 24 (24) 12 (12) 8 (9) 6 (7) Corneal thinning 1 (1) 2 (2) 0 0 Diplopia 2 (2) 1 (1) 1 (1) 0 Dry eye 6 (6) 2 (2) 13 (14) 4 (5) Eye complication associated with device 2 (2) 0 1 (1) 0 Eye discharge 2 (2) 1 (1) 0 0 Eye oedema 7 (7) Eye pain 17 (17) 3 (3) 24 (26) 0 Eye pruritus 2 (2) Eyelid oedema 5 (5) 0 5 (6) 1 (1) Foreign body sensation in eyes 15 (15) 1 (1) 13 (14) 2 (2) Glare 4 (4) 1 (1) 2 (2) 0 Halo vision 1 (1) 0 2 (2) 0 Keratitis 1 (1) 0 3 (3) 0 Lacrimation increased 5 (5) 0 9 (10) 1 (1) Meibomian gland dysfunction 1 (1) 1 (1) 3 (3) 2 (2) Ocular discomfort (9) 0 Ocular hyperaemia 14 (14) 2 (2) 7 (8) 4 (5) Photophobia 11 (11) 0 17 (19) 0 Punctate keratitis 25 (25) 8 (8) 18 (20) 3 (3) Vision blurred 16 (16) 2 (2) 15 (17) 4 (5) Visual acuity reduced 10 (10) 9 (9) 10 (11) 1 (1) Visual impairment 3 (3) 2 (2) 4 (4) 1 (1) Vitreous detachment 2 (2) ) Results are presented as the number (%) of subjects with an event from baseline to Month 3. 2) Almost all cases of corneal opacity were reported as haze. Headache was reported in between 4 to 8% of treated patients. 4
5 8. USE IN SPECIFIC POPULATIONS 8.1. Pregnancy Risk Summary Animal development and reproduction studies have not been conducted with the PHOTREXA VISCOUS/PHOTREXA /KXL system. Since it is not known whether the corneal collagen cross-linking procedure can cause fetal harm or affect reproduction capacity, it should not be performed on pregnant women Lactation Risk Summary There are no data on the presence of PHOTREXA VISCOUS or PHOTREXA in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered, along with the mother s clinical need for the PHOTREXA/KXL corneal collagen crosslinking procedure and any potential adverse effects on the breastfed child from the PHOTREXA/KXL corneal collagen cross-linking procedure or from the underlying maternal condition Pediatric Use The safety and effectiveness of corneal collagen cross-linking has not been established in pediatric patients below the age of Geriatric Use No subjects enrolled in the clinical studies were 65 years of age or older. 11. DESCRIPTION PHOTREXA VISCOUS (riboflavin 5 -phosphate in 20% dextran ophthalmic solution) 0.146% and PHOTREXA (riboflavin 5 -phosphate ophthalmic solution) 0.146% are intended for topical ophthalmic administration as part of corneal collagen cross-linking with the KXL System. PHOTREXA VISCOUS and PHOTREXA are supplied as: PHOTREXA VISCOUS in a 3 ml glass syringe containing sterile 1.46 mg/ml riboflavin 5 -phosphate in 20% dextran ophthalmic solution for topical administration. PHOTREXA in a 3 ml glass syringe containing sterile 1.46 mg/ml riboflavin 5 -phosphate ophthalmic solution for topical administration. PHOTREXA VISCOUS (riboflavin 5 -phosphate in 20% dextran ophthalmic solution) 0.146% is a yellow sterile buffered viscous solution containing 1.46 mg/ml riboflavin 5 -phosphate and 20% dextran 500. The ph of the solution is approximately 7.1 and the osmolality is mosm/kg. Each 1 ml of solution contains 1.53 mg of riboflavin 5 -phosphate sodium (equivalent to 1.20 mg riboflavin). Riboflavin 5 -phosphate sodium USP is a mixture of the sodium salts of riboflavin, riboflavin monophosphates, and riboflavin diphosphates. The inactive ingredients are dibasic sodium phosphate, dextran, monobasic sodium phosphate, sodium chloride, and water for injection. PHOTREXA (riboflavin 5 -phosphate ophthalmic solution) 0.146% is a yellow sterile buffered solution containing 1.46 mg/ml riboflavin 5 -phosphate. The ph of the solution is approximately 7.1 and the osmolality is mosm/kg. Each 1 ml of solution contains 1.53 mg of riboflavin 5 -phosphate sodium (equivalent to 1.20 mg riboflavin). Riboflavin 5 -phosphate sodium USP is a mixture of the sodium salts of riboflavin, 5
6 riboflavin monophosphates, and riboflavin diphosphates. The inactive ingredients are dibasic sodium phosphate, monobasic sodium phosphate, sodium chloride, and water for injection. The chemical formula for riboflavin 5 -phosphate sodium (Vitamin B2) is C 17 H 20 N 4 NaO 9 P with a molecular mass of g/mol. O N N H N N O OH OH HO OH O P O O - Na+ Please refer to the KXL System Operator s Manual for a specific device description and instructions. 12. CLINICAL PHARMACOLOGY Mechanism of Action Riboflavin 5 -phosphate sodium (Vitamin B2) is the precursor of two coenzymes, flavin adenine dinucleotide and flavin mononucleotide, which catalyze oxidation/reduction reactions involved in a number of metabolic pathways. Under the conditions used for corneal collagen cross-linking, riboflavin 5 -phosphate functions as a photoenhancer and generates singlet oxygen which is responsible for the cross-linking. 13. NONCLINICAL TOXICOLOGY Carcinogenesis, Mutagenesis, Impairment of Fertility Animal studies have not been conducted to determine the carcinogenic potential of photoexcited riboflavin. Photoexcited riboflavin has been shown to be genotoxic in the Ames Salmonella reverse mutation assay and in the SOS/umu test system. The genotoxicity of riboflavin, in the absence of photoexcitation has been examined in vitro in bacterial reverse mutation assays, sister chromatid exchange assay, chromosomal aberration assays and in vivo in a mouse micronucleus study. The overall weight of evidence indicates that riboflavin, in the absence of photoexcitation, is not genotoxic. Animal studies to determine the effects of the PHOTREXA/KXL corneal collagen cross-linking procedure on fertility were not conducted. 6
7 14. CLINICAL STUDIES Three prospective, randomized, parallel-group, open-label, sham-controlled trials were conducted to evaluate the safety and effectiveness of riboflavin ophthalmic solution/uva irradiation for performing corneal collagen cross-linking. These trials were sham-controlled for the first 3 months and had a total duration of 12 months for safety and efficacy evaluations. Study 1 enrolled 58 patients with progressive keratoconus. Study 2 enrolled 147 patients with progressive keratoconus. In each study, patients had one eye designated as the study eye and were randomized to receive one of two study treatments (CXL or sham) in their study eye at the baseline visit. The patients were evaluated at Day 1, Week 1, and Months 1, 3, 6, and 12. At Month 3 or later, patients had the option of receiving CXL treatment in both the sham study eyes and non-study eyes and were followed-up for 12 months from the time of receiving CXL treatment. Approximately 56% and 89% of the sham study eyes in patients with progressive keratoconus received CXL treatment by Month 3 and Month 6, respectively. The average age of keratoconus patients was 33 years. The average baseline K max value was 61 diopters. In each study, the maximum corneal curvature (K max ) was assessed at baseline, Months 1, 3, and 12. The CXLtreated eyes showed increasing improvement in K max from Month 3 through Month 12 (Figure 1). Progressive keratoconus patients had an average K max reduction of 1.4 diopter in Study 1 and 1.7 diopter in Study 2 at Month 12 in the CXL-treated eyes while the sham eyes had an average increase of 0.5 diopter in Study 1 and 0.6 diopter in Study 2 at Month 12; the difference (95% CI) between the CXL and sham groups in the mean change from baseline K max were -1.9 (-3.4, -0.3) diopters in Study 1 and -2.3 (-3.5, -1.0) diopters in Study 2. Figure 1: Mean (SD) (Diopter) Baseline Kmax and Change from Baseline Kmax Post-baseline missing data were imputed using last available K max value. For the sham study eyes that received CXL treatment after baseline, the last K max measurement recorded prior to receiving CXL treatment was used in the analysis for later time points. 16. HOW SUPPLIED/STORAGE AND HANDLING PHOTREXA VISCOUS and PHOTREXA are provided in a bulk pack of 10 (ten), single-use foil pouches. Each foil pouch contains a 3 ml glass syringe of PHOTREXA VISCOUS or PHOTREXA contained within a Tyvek pouch. The entire bulk pack should be stored at C (59-77 F) and care should be taken to minimize exposure of the syringe to light once removed from its protective packaging. Discard syringe after use. For topical ophthalmic use. PHOTREXA VISCOUS and PHOTREXA should be used with the KXL System only. 7
8 17. PATIENT COUNSELING INFORMATION Patients should be advised not to rub their eyes for the first five days after their procedure. Patients may be sensitive to light and have a foreign body sensation. Patients should be advised that there may be discomfort in the treated eye and that sunglasses may help with light sensitivity. If patients experience severe pain in the eye or any sudden decrease in their vision, they should be advised to contact their physician immediately. If the bandage contact lens that was placed on the patient s eye on the day of treatment falls out or becomes dislodged, the patient should be advised not to replace it and to contact their physician immediately. PHOTREXA VISCOUS (riboflavin 5 -phosphate in 20% dextran ophthalmic solution) 0.146%, PHOTREXA (riboflavin 5 -phosphate ophthalmic solution) 0.146% and the KXL System are marketed by: Avedro, 230 Third Avenue, Waltham, MA, April
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief
 DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
Selected Requirements of Prescribing Information
 The Selected Requirements of Prescribing Information (SRPI) is a checklist of 42 important format prescribing information (PI) items based on labeling regulations [21 CFR 201.56(d) and 201.57] and guidances.
The Selected Requirements of Prescribing Information (SRPI) is a checklist of 42 important format prescribing information (PI) items based on labeling regulations [21 CFR 201.56(d) and 201.57] and guidances.
Revised: 9/2014. LUMIGAN (bimatoprost ophthalmic solution) 0.01% for topical ophthalmic use. Initial U.S. Approval: 2001
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN (bimatoprost ophthalmic solution) 0.01% safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN (bimatoprost ophthalmic solution) 0.01% safely and effectively. See full prescribing information
Opening Remarks. David Muller, Ph.D. President and Chief Executive Officer Avedro, Inc.
 Corneal Collagen Cross-linking with Photrexa Viscous (riboflavin ophthalmic solution) 20% dextran, Photrexa (riboflavin ophthalmic solution)/kxl System NDA 203324 Joint Meeting of the Dermatologic and
Corneal Collagen Cross-linking with Photrexa Viscous (riboflavin ophthalmic solution) 20% dextran, Photrexa (riboflavin ophthalmic solution)/kxl System NDA 203324 Joint Meeting of the Dermatologic and
See 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2016 FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK OR
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
Corneal Collagen Crosslinking: An Introduction to the New Paradigm in Keratoconus. Michael Waggoner DO McDonald Eye Associates Fayetteville AR
 Corneal Collagen Crosslinking: An Introduction to the New Paradigm in Keratoconus Michael Waggoner DO McDonald Eye Associates Fayetteville AR Learning Objectives Gain knowledge in: The history of the development
Corneal Collagen Crosslinking: An Introduction to the New Paradigm in Keratoconus Michael Waggoner DO McDonald Eye Associates Fayetteville AR Learning Objectives Gain knowledge in: The history of the development
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate.
 NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
DOSAGE FORMS AND STRENGTHS Intravitreal implant containing dexamethasone 0.7 mg in the NOVADUR solid polymer drug delivery system.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OZURDEX safely and effectively. See full prescribing information for OZURDEX. OZURDEX (dexamethasone
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OZURDEX safely and effectively. See full prescribing information for OZURDEX. OZURDEX (dexamethasone
UPDATE ON AVEDRO CROSSLINKING STUDIES
 UPDATE ON AVEDRO CROSSLINKING STUDIES Peter S. Hersh, M.D. Director, Cornea & Laser Eye Institute CLEI Center for Keratoconus Clinical Professor of Ophthalmology, Rutgers Medical School Visiting Researcher,
UPDATE ON AVEDRO CROSSLINKING STUDIES Peter S. Hersh, M.D. Director, Cornea & Laser Eye Institute CLEI Center for Keratoconus Clinical Professor of Ophthalmology, Rutgers Medical School Visiting Researcher,
Transepithelial Crosslinking vs. Corneal Pocket Crosslinking. Christoph Kranemann MD Anna Yu OD
 Transepithelial Crosslinking vs. Corneal Pocket Crosslinking Christoph Kranemann MD Anna Yu OD Rome 2013 We have no financial interests in this presentation. Corneal collagen cross linking Creates new
Transepithelial Crosslinking vs. Corneal Pocket Crosslinking Christoph Kranemann MD Anna Yu OD Rome 2013 We have no financial interests in this presentation. Corneal collagen cross linking Creates new
Learn More About Product Labeling
 Learn More About Product Labeling Product label The product label is developed during the formal process of review and approval by regulatory agencies of any medicine or medical product. There are specific
Learn More About Product Labeling Product label The product label is developed during the formal process of review and approval by regulatory agencies of any medicine or medical product. There are specific
Keratoconus. Progressive bilateral ectasia. Onset puberty. Prevalence 1:2000. 20% progress to transplantation. Pathogenesis unclear
 Keratoconus Progressive bilateral ectasia Onset puberty Prevalence 1:2000 20% progress to transplantation Pathogenesis unclear Increased pepsin and catalase Decreased collagen crosslinking cf normal Conventional
Keratoconus Progressive bilateral ectasia Onset puberty Prevalence 1:2000 20% progress to transplantation Pathogenesis unclear Increased pepsin and catalase Decreased collagen crosslinking cf normal Conventional
PACKAGE LEAFLET: INFORMATION FOR THE USER. VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution. Cyanocobalamin
 PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
Trans-epithelial cross-linking with riboflavin solution: two-year clinical results
 Trans-epithelial cross-linking with riboflavin solution: two-year clinical results Authors: Ciro Caruso, M.D. 1,2, Luigi Pacente, M.D. 1, Robert L Epstein 5, Salvatore Troisi, M.D. 3, Gaetano Barbaro,
Trans-epithelial cross-linking with riboflavin solution: two-year clinical results Authors: Ciro Caruso, M.D. 1,2, Luigi Pacente, M.D. 1, Robert L Epstein 5, Salvatore Troisi, M.D. 3, Gaetano Barbaro,
Corneal Collagen Cross-Linking (CXL) With Riboflavin
 Dr. Paul J. Dubord, MD, FRCSC Clinical Professor Department of Ophthalmology and Visual Sciences University of British Columbia Patient Information Guide Corneal Collagen Cross-Linking (CXL) With Riboflavin
Dr. Paul J. Dubord, MD, FRCSC Clinical Professor Department of Ophthalmology and Visual Sciences University of British Columbia Patient Information Guide Corneal Collagen Cross-Linking (CXL) With Riboflavin
VISX Wavefront-Guided LASIK for Correction of Myopic Astigmatism, Hyperopic Astigmatism and Mixed Astigmatism (CustomVue LASIK Laser Treatment)
 CustomVue Advantage Patient Information Sheet VISX Wavefront-Guided LASIK for Correction of Myopic Astigmatism, Hyperopic Astigmatism and Mixed Astigmatism (CustomVue LASIK Laser Treatment) Statements
CustomVue Advantage Patient Information Sheet VISX Wavefront-Guided LASIK for Correction of Myopic Astigmatism, Hyperopic Astigmatism and Mixed Astigmatism (CustomVue LASIK Laser Treatment) Statements
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
2 DOSAGE AND ADMINISTRATION 2.1 Dosing and Dose Adjustment
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Auryxia safely and effectively. See full prescribing information for Auryxia. Auryxia (ferric citrate)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Auryxia safely and effectively. See full prescribing information for Auryxia. Auryxia (ferric citrate)
Surgical Advances in Keratoconus. Keratoconus. Innovations in Ophthalmology. New Surgical Advances. Diagnosis of Keratoconus. Scheimpflug imaging
 Surgical Advances in Keratoconus Keratoconus Ectatic disorder 1 in 1,000 individuals Starts in adolescence & early adulthood Uncertain cause 20% require corneal transplant Innovations in Ophthalmology
Surgical Advances in Keratoconus Keratoconus Ectatic disorder 1 in 1,000 individuals Starts in adolescence & early adulthood Uncertain cause 20% require corneal transplant Innovations in Ophthalmology
The pinnacle of refractive performance.
 Introducing! The pinnacle of refractive performance. REFRACTIVE SURGERY sets a new standard in LASIK outcomes More than 98% of patients would choose it again. 1 It even outperformed glasses and contacts
Introducing! The pinnacle of refractive performance. REFRACTIVE SURGERY sets a new standard in LASIK outcomes More than 98% of patients would choose it again. 1 It even outperformed glasses and contacts
Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006
 Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
PATIENT CONSENT FOR LASER IN-SITU KERATOMILEUSIS (LASIK)
 INTRODUCTION: You have been diagnosed with myopia (nearsightedness) or hyperopia (farsightedness) with or without astigmatism, or astigmatism alone. Myopia is a result of light entering the eye and focusing
INTRODUCTION: You have been diagnosed with myopia (nearsightedness) or hyperopia (farsightedness) with or without astigmatism, or astigmatism alone. Myopia is a result of light entering the eye and focusing
0.9% Sodium Chloride Injection, USP In VIAFLEX Plastic Container
 Page 1 of 8 PRESCRIBING INFORMATION 0.9% Sodium Chloride Injection, USP In VIAFLEX Plastic Container IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of
Page 1 of 8 PRESCRIBING INFORMATION 0.9% Sodium Chloride Injection, USP In VIAFLEX Plastic Container IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of
Congratulations! You have just joined the thousands of people who are enjoying the benefits of laser vision correction.
 Dear Valued Patient, Thank you for choosing Shady Grove Ophthalmology for your laser vision correction procedure. Our excellent staff is committed to offering you the highest quality eye care using state
Dear Valued Patient, Thank you for choosing Shady Grove Ophthalmology for your laser vision correction procedure. Our excellent staff is committed to offering you the highest quality eye care using state
8.5 Geriatric Use * Sections or subsections omitted from the full prescribing information are not listed.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Injectafer safely and effectively. See full prescribing information for Injectafer. INJECTAFER (ferric
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Injectafer safely and effectively. See full prescribing information for Injectafer. INJECTAFER (ferric
Accelerated Refractive Performance
 Accelerated Refractive Performance Get There at the Speed of WaveLight Designed to accommodate your refractive technology goals now and into the future, the WaveLight Workstation is a faster way to get
Accelerated Refractive Performance Get There at the Speed of WaveLight Designed to accommodate your refractive technology goals now and into the future, the WaveLight Workstation is a faster way to get
How To Treat Eye Problems With A Laser
 1550 Oak St., Suite 5 1515 Oak St., St Eugene, OR 97401 Eugene, OR 97401 (541) 687-2110 (541) 344-2010 INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK) This information is to help you make an informed
1550 Oak St., Suite 5 1515 Oak St., St Eugene, OR 97401 Eugene, OR 97401 (541) 687-2110 (541) 344-2010 INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK) This information is to help you make an informed
Excimer Laser Eye Surgery
 Excimer Laser Eye Surgery This booklet contains general information that is not specific to you. If you have any questions after reading this, ask your own physician or health care worker. They know you
Excimer Laser Eye Surgery This booklet contains general information that is not specific to you. If you have any questions after reading this, ask your own physician or health care worker. They know you
What We Do and Don t Know about Corneal Crosslinking. Roy Rubinfeld, MD
 What We Do and Don t Know about Corneal Crosslinking Roy Rubinfeld, MD Washington Eye Physicians and Surgeons Chevy Chase, MD Georgetown University Medical Center, Washington Hospital Center, Washington,
What We Do and Don t Know about Corneal Crosslinking Roy Rubinfeld, MD Washington Eye Physicians and Surgeons Chevy Chase, MD Georgetown University Medical Center, Washington Hospital Center, Washington,
INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK)
 INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK) This information and the Patient Information booklet must be reviewed so you can make an informed decision regarding Photorefractive Keratectomy (PRK)
INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK) This information and the Patient Information booklet must be reviewed so you can make an informed decision regarding Photorefractive Keratectomy (PRK)
Revised 04/2015 FULL PRESCRIBING INFORMATION: CONTENTS*
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use KYBELLA safely and effectively. See full prescribing information for KYBELLA. KYBELLA (deoxycholic
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use KYBELLA safely and effectively. See full prescribing information for KYBELLA. KYBELLA (deoxycholic
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
Collagen Cross Linking in the limelight: The Universal Dream of Keratoconus Treatment
 Collagen Cross Linking in the limelight: The Universal Dream of Keratoconus Treatment Andrew S. Morgenstern, OD FAAO Booz Allen Hamilton Contractor at The Vision Center of Excellence Who We Work For My
Collagen Cross Linking in the limelight: The Universal Dream of Keratoconus Treatment Andrew S. Morgenstern, OD FAAO Booz Allen Hamilton Contractor at The Vision Center of Excellence Who We Work For My
REFRACTIVE ERROR AND SURGERIES IN THE UNITED STATES
 Introduction REFRACTIVE ERROR AND SURGERIES IN THE UNITED STATES 150 million wear eyeglasses or contact lenses 2.3 million refractive surgeries performed between 1995 and 2001 Introduction REFRACTIVE SURGERY:
Introduction REFRACTIVE ERROR AND SURGERIES IN THE UNITED STATES 150 million wear eyeglasses or contact lenses 2.3 million refractive surgeries performed between 1995 and 2001 Introduction REFRACTIVE SURGERY:
9/15/2013. Naturally-existing Corneal Pathology Forme Fruste Keratoconus Keratoconus Pellucid Marginal Degeneration
 Bill Tullo, OD, FAAO Diplomate Vice-President of Clinical Services TLC Vision 33386-PO 1 Hour Bill Tullo, OD has no financial interests in any of the products or companies discussed in this program Naturally-existing
Bill Tullo, OD, FAAO Diplomate Vice-President of Clinical Services TLC Vision 33386-PO 1 Hour Bill Tullo, OD has no financial interests in any of the products or companies discussed in this program Naturally-existing
Overview of Refractive Surgery
 Overview of Refractive Surgery Michael N. Wiggins, MD Assistant Professor, College of Health Related Professions and College of Medicine, Department of Ophthalmology Jones Eye Institute University of Arkansas
Overview of Refractive Surgery Michael N. Wiggins, MD Assistant Professor, College of Health Related Professions and College of Medicine, Department of Ophthalmology Jones Eye Institute University of Arkansas
VOLTAREN OPHTHA EYE DROPS
 פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
RELEX SMILE AND SMILE EXTRA.. OUR 1 YEAR RESULTS AND PATIENTS SURVEY
 RELEX SMILE AND SMILE EXTRA.. OUR 1 YEAR RESULTS AND PATIENTS SURVEY DR SANDIP MITRA MD FRCS CORNEA AND REFRACTIVE FELLOW (ROYAL VICTORIA EYE AND EAR HOSPITAL, AUSTRALIA) RELEX SMILE UNIT AT THE ALZAHRA
RELEX SMILE AND SMILE EXTRA.. OUR 1 YEAR RESULTS AND PATIENTS SURVEY DR SANDIP MITRA MD FRCS CORNEA AND REFRACTIVE FELLOW (ROYAL VICTORIA EYE AND EAR HOSPITAL, AUSTRALIA) RELEX SMILE UNIT AT THE ALZAHRA
Billing and Coding Guide
 Billing and Coding Guide INDICATIONS Injectafer is an iron replacement product indicated for the treatment of iron deficiency anemia (IDA) in adult patients: who have intolerance to or have had unsatisfactory
Billing and Coding Guide INDICATIONS Injectafer is an iron replacement product indicated for the treatment of iron deficiency anemia (IDA) in adult patients: who have intolerance to or have had unsatisfactory
TABLE OF CONTENTS: LASER EYE SURGERY CONSENT FORM
 1 BoydVision TABLE OF CONTENTS: LASER EYE SURGERY CONSENT FORM Risks and Side Effects... 2 Risks Specific to PRK... 3 Risks Specific to LASIK... 4 Patient Statement of Consent... 5 Consent for Laser Eye
1 BoydVision TABLE OF CONTENTS: LASER EYE SURGERY CONSENT FORM Risks and Side Effects... 2 Risks Specific to PRK... 3 Risks Specific to LASIK... 4 Patient Statement of Consent... 5 Consent for Laser Eye
Managing Challenging Cases in Refractive Surgery
 Managing Challenging Cases in Refractive Surgery Missouri Optometric Association Stephen A. Wexler, MD Eric E. Polk, OD, FAAO Outline The presenters will review challenging cases they have managed in refractive
Managing Challenging Cases in Refractive Surgery Missouri Optometric Association Stephen A. Wexler, MD Eric E. Polk, OD, FAAO Outline The presenters will review challenging cases they have managed in refractive
I have read and understood this page. Patient Initials
 INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK) AND ADVANCE SURFACE ABLATION (ASA) This information and the Patient Information booklet must be reviewed so you can make an informed decision regarding
INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK) AND ADVANCE SURFACE ABLATION (ASA) This information and the Patient Information booklet must be reviewed so you can make an informed decision regarding
CENTRO OFTALMOLOGICO GUSTAVO TAMAYO BOGOTA COLOMBIA LASIK XTRA GUSTAVO TAMAYO MD CLAUDIA CASTELL MD PILAR VARGAS MD
 CENTRO OFTALMOLOGICO GUSTAVO TAMAYO BOGOTA COLOMBIA LASIK XTRA GUSTAVO TAMAYO MD CLAUDIA CASTELL MD PILAR VARGAS MD From: BOGOTA LASER REFRACTIVE INSTITUTE Bogota, Colombia DISCLOSURES FOR GUSTAVO TAMAYO
CENTRO OFTALMOLOGICO GUSTAVO TAMAYO BOGOTA COLOMBIA LASIK XTRA GUSTAVO TAMAYO MD CLAUDIA CASTELL MD PILAR VARGAS MD From: BOGOTA LASER REFRACTIVE INSTITUTE Bogota, Colombia DISCLOSURES FOR GUSTAVO TAMAYO
Short and long term complications of combined. Protocol) in 412 keratoconus eyes (2 7 years follow up)
 Short and long term complications of combined topography guided PRK and CXL (the Athens Protocol) in 412 keratoconus eyes (2 7 years follow up) Anastasios John Kanellopoulos, MD Director, Laservision.gr
Short and long term complications of combined topography guided PRK and CXL (the Athens Protocol) in 412 keratoconus eyes (2 7 years follow up) Anastasios John Kanellopoulos, MD Director, Laservision.gr
Verisyse Phakic IOL. Facts You Need to Know About Implantation of the Verisyse Phakic IOL (-5 to -20 D) for the Correction of Myopia (Nearsightedness)
 Verisyse Phakic IOL Facts You Need to Know About Implantation of the Verisyse Phakic IOL (-5 to -20 D) for the Correction of Myopia (Nearsightedness) Patient Information Brochure This brochure is designed
Verisyse Phakic IOL Facts You Need to Know About Implantation of the Verisyse Phakic IOL (-5 to -20 D) for the Correction of Myopia (Nearsightedness) Patient Information Brochure This brochure is designed
Sharjah: Al Zahra Private Hospital, Al Zahra square Tel: 06 5619999, Appointments: 06 5167080, 06 5167081
 Sharjah: Al Zahra Private Hospital, Al Zahra square Tel: 06 5619999, Appointments: 06 5167080, 06 5167081 Email: [email protected] Dubai: Al Zahra Medical Centre, Sheikh Zayed Road Tel: 04 3315000, Appointments:
Sharjah: Al Zahra Private Hospital, Al Zahra square Tel: 06 5619999, Appointments: 06 5167080, 06 5167081 Email: [email protected] Dubai: Al Zahra Medical Centre, Sheikh Zayed Road Tel: 04 3315000, Appointments:
3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP
 PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
Original Article Corneal Collagen Cross Linking Pak Armed Forces Med J 2015; 65(1): 105-9
 Original Article Corneal Collagen Cross Linking Pak Armed Forces Med J 2015; 65(1): 105-9 COMPARISON OF RAPID AND CONVENTIONAL CORNEAL COLLAGEN CROSS LINKING IN PATIENTS HAVING KERATOCONUS Intisar ul Haq,
Original Article Corneal Collagen Cross Linking Pak Armed Forces Med J 2015; 65(1): 105-9 COMPARISON OF RAPID AND CONVENTIONAL CORNEAL COLLAGEN CROSS LINKING IN PATIENTS HAVING KERATOCONUS Intisar ul Haq,
1-1 INDIAN OIL CORPORATION - REFRACTIVE SURGERY CENTRE ( LASIK) SANKARA NETHRALAYA (JKCN BRANCH) NO 21, PYGROFTS GARDEN ROAD, CHENNAI 6
 1-1 1-2 How do we see? Eye Structure (Normal) The eye is like a camera. In a camera, light passes through a lens system back onto the film. The cornea and lens are at the front of the eye (anterior chamber)
1-1 1-2 How do we see? Eye Structure (Normal) The eye is like a camera. In a camera, light passes through a lens system back onto the film. The cornea and lens are at the front of the eye (anterior chamber)
used to treat inflammation, corneal injury and bacterial infections in the external part of the eye.
 TOBRADEX * Eye Drops Tobramycin and Dexamethasone CONSUMER MEDICINE INFORMATION What is in this Leaflet This leaflet answers some common questions about TOBRADEX* Eye Drops. It does not contain all the
TOBRADEX * Eye Drops Tobramycin and Dexamethasone CONSUMER MEDICINE INFORMATION What is in this Leaflet This leaflet answers some common questions about TOBRADEX* Eye Drops. It does not contain all the
Ultraviolet (UV) Radiation Safety
 Ultraviolet (UV) Radiation Safety April 2005 Compiled by Myung Chul Jo Environmental Health and Safety University of Nevada Reno Page 1 of 8 Table of Contents 1. UVRadiation 3 2. Common sources of UV radiation
Ultraviolet (UV) Radiation Safety April 2005 Compiled by Myung Chul Jo Environmental Health and Safety University of Nevada Reno Page 1 of 8 Table of Contents 1. UVRadiation 3 2. Common sources of UV radiation
Reference ID: 3482803
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use EVZIO safely and effectively. See full prescribing information for EVZIO. EVZIO (naloxone hydrochloride
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use EVZIO safely and effectively. See full prescribing information for EVZIO. EVZIO (naloxone hydrochloride
RAGWITEK TM (Short Ragweed Pollen Allergen Extract) Tablet for Sublingual Use Initial U.S. Approval: 2014
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use RAGWITEK safely and effectively. See full prescribing information for RAGWITEK. RAGWITEK TM (Short
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use RAGWITEK safely and effectively. See full prescribing information for RAGWITEK. RAGWITEK TM (Short
Corniche Road [email protected] Bld. #4WB - Office #443 +971 (2) 671 2020 +971 (4) 260 2444 EYE SURGERY
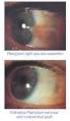
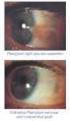
 EYE SURGERY 1. CATARACT This is the loss of transparency of the lens within the eye, which is transparent in healthy individuals. Vision will be impaired if the lens within the eye gets cloudy. Normal
EYE SURGERY 1. CATARACT This is the loss of transparency of the lens within the eye, which is transparent in healthy individuals. Vision will be impaired if the lens within the eye gets cloudy. Normal
Consent for LASIK (Laser In Situ Keratomileusis) Retreatment
 Consent for LASIK (Laser In Situ Keratomileusis) Retreatment Please read the following consent form very carefully. Please initial at the bottom of each page where indicated. Do not sign this form unless
Consent for LASIK (Laser In Situ Keratomileusis) Retreatment Please read the following consent form very carefully. Please initial at the bottom of each page where indicated. Do not sign this form unless
Corporate Medical Policy Implantation of Intrastromal Corneal Ring Segments
 Corporate Medical Policy Implantation of Intrastromal Corneal Ring Segments File Name: Origination: Last CAP Review: Next CAP Review: Last Review: implantation_of_intrastromal_corneal_ring_segments 8/2008
Corporate Medical Policy Implantation of Intrastromal Corneal Ring Segments File Name: Origination: Last CAP Review: Next CAP Review: Last Review: implantation_of_intrastromal_corneal_ring_segments 8/2008
Keratoconus and Riboflavin UV-Crosslinking of the Cornea
 Keratoconus and Riboflavin UV-Crosslinking of the Cornea Institut für Refraktive und Ophthalmo-Chirurgie Dear patient, A keratoconus has been diagnosed on your cornea. In this disease, which affects 1
Keratoconus and Riboflavin UV-Crosslinking of the Cornea Institut für Refraktive und Ophthalmo-Chirurgie Dear patient, A keratoconus has been diagnosed on your cornea. In this disease, which affects 1
MOST FREQUENTLY ASKED CLIENT QUESTIONS
 6/2 Victor Crescent Narre Warren VIC 3805 Ph: (03) 8794 8255 Fax: (03) 8794 8256 www.ortho-k.com.au Sleep your way to great vision MOST FREQUENTLY ASKED CLIENT QUESTIONS What is ortho-k? Ortho-k (Orthokeratology)
6/2 Victor Crescent Narre Warren VIC 3805 Ph: (03) 8794 8255 Fax: (03) 8794 8256 www.ortho-k.com.au Sleep your way to great vision MOST FREQUENTLY ASKED CLIENT QUESTIONS What is ortho-k? Ortho-k (Orthokeratology)
160S01105, Page 1 of 7. Human Hepatitis B Immunoglobulin, solution for intramuscular injection.
 160S01105, Page 1 of 7 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF
160S01105, Page 1 of 7 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid
 Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
INFORMED CONSENT FOR PHAKIC IMPLANT SURGERY
 INFORMED CONSENT FOR PHAKIC IMPLANT SURGERY INTRODUCTION This information is being provided to you so that you can make an informed decision about having eye surgery to reduce or eliminate your nearsightedness.
INFORMED CONSENT FOR PHAKIC IMPLANT SURGERY INTRODUCTION This information is being provided to you so that you can make an informed decision about having eye surgery to reduce or eliminate your nearsightedness.
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Annex II. Scientific conclusions and grounds for revocation of the marketing authorisations
 Annex II Scientific conclusions and grounds for revocation of the marketing authorisations 4 Scientific conclusions and grounds for revocation of the marketing authorisations Overall summary of the scientific
Annex II Scientific conclusions and grounds for revocation of the marketing authorisations 4 Scientific conclusions and grounds for revocation of the marketing authorisations Overall summary of the scientific
SLADE AND BAKER VISION CENTER INFORMED CONSENT FOR LASER VISION CORRECTION (LVC)
 SLADE AND BAKER VISION CENTER INFORMED CONSENT FOR LASER VISION CORRECTION (LVC) PLEASE READ THE FOLLOWING PAGES CAREFULLY AND INITIAL AND SIGN WHERE INDICATED. PLEASE DO NOT SIGN ANY SECTION THAT YOU
SLADE AND BAKER VISION CENTER INFORMED CONSENT FOR LASER VISION CORRECTION (LVC) PLEASE READ THE FOLLOWING PAGES CAREFULLY AND INITIAL AND SIGN WHERE INDICATED. PLEASE DO NOT SIGN ANY SECTION THAT YOU
Kensington Eye Center 4701 Randolph Road, #G-2 Rockville, MD 20852 (301) 881-5701 www.keceyes.com
 Kensington Eye Center 4701 Randolph Road, #G-2 Rockville, MD 20852 (301) 881-5701 www.keceyes.com Natasha L. Herz, MD INFORMED CONSENT FOR DESCEMET S STRIPPING and AUTOMATED ENDOTHELIAL KERATOPLASTY (DSAEK)
Kensington Eye Center 4701 Randolph Road, #G-2 Rockville, MD 20852 (301) 881-5701 www.keceyes.com Natasha L. Herz, MD INFORMED CONSENT FOR DESCEMET S STRIPPING and AUTOMATED ENDOTHELIAL KERATOPLASTY (DSAEK)
5/24/2013 ESOIRS 2013. Moderator: Alaa Ghaith, MD. Faculty: Ahmed El Masri, MD Mohamed Shafik, MD Mohamed El Kateb, MD
 ESOIRS 2013 Moderator: Alaa Ghaith, MD Faculty: Ahmed El Masri, MD Mohamed Shafik, MD Mohamed El Kateb, MD 1 A systematic approach to the management of Keratoconus through the presentation of different
ESOIRS 2013 Moderator: Alaa Ghaith, MD Faculty: Ahmed El Masri, MD Mohamed Shafik, MD Mohamed El Kateb, MD 1 A systematic approach to the management of Keratoconus through the presentation of different
Laser Vision Correction
 How will Laser Vision Correction affect my Lifestyle? Your Guide to Laser Vision Correction The Gift of Better Vision A few things to note after your surgery. As you enjoy your new-and-improved eyesight,
How will Laser Vision Correction affect my Lifestyle? Your Guide to Laser Vision Correction The Gift of Better Vision A few things to note after your surgery. As you enjoy your new-and-improved eyesight,
INFORMED CONSENT FOR LASIK AND PRK SURGERY
 INFORMED CONSENT FOR LASIK AND PRK SURGERY Please read the following very carefully. Please initial each page where indicated. Do not sign this form unless you read and understand each page. Patient s
INFORMED CONSENT FOR LASIK AND PRK SURGERY Please read the following very carefully. Please initial each page where indicated. Do not sign this form unless you read and understand each page. Patient s
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]
![READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]](/thumbs/35/17235501.jpg) READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
INFORMED CONSENT TO HAVE LASIK
 A Division of Scott & Christie and Associates INFORMED CONSENT TO HAVE LASIK This information is to help you make an informed decision about having Laser Assisted Intrastromal Keratomileusis (LASIK), an
A Division of Scott & Christie and Associates INFORMED CONSENT TO HAVE LASIK This information is to help you make an informed decision about having Laser Assisted Intrastromal Keratomileusis (LASIK), an
The Visian ICL Advantages
 The Visian ICL Advantages Many vision correction procedures promise an improved level of vision, but few vision correction alternatives offer the quality and features found with the Visian ICL. These include:
The Visian ICL Advantages Many vision correction procedures promise an improved level of vision, but few vision correction alternatives offer the quality and features found with the Visian ICL. These include:
Hioxifilcon D SOFT CONTACT LENS FOR DAILY WEAR
 1 of 5 PACKAGE INSERT FOR Hioxifilcon D SOFT CONTACT LENS FOR DAILY WEAR CAUTION: FEDERAL LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A LICENSED PRACTITIONER Important: Please read carefully
1 of 5 PACKAGE INSERT FOR Hioxifilcon D SOFT CONTACT LENS FOR DAILY WEAR CAUTION: FEDERAL LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A LICENSED PRACTITIONER Important: Please read carefully
Welcome to the Verisyse Seminar
 Patient Seminar Welcome to the Verisyse Seminar Today we ll answer some of the most common questions about the Verisyse Phakic Intraocular Lens (IOL) including: Who is a candidate How the procedure is
Patient Seminar Welcome to the Verisyse Seminar Today we ll answer some of the most common questions about the Verisyse Phakic Intraocular Lens (IOL) including: Who is a candidate How the procedure is
VOLTAREN OPHTHA EYE DROPS
 2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
Package leaflet: Information for the patient. Dorzolamide Mylan 20 mg/ml Eye drops, Solution Dorzolamide
 Package leaflet: Information for the patient Dorzolamide Mylan 20 mg/ml Eye drops, Solution Dorzolamide Read all of this leaflet carefully before you start using this medicine because it contains important
Package leaflet: Information for the patient Dorzolamide Mylan 20 mg/ml Eye drops, Solution Dorzolamide Read all of this leaflet carefully before you start using this medicine because it contains important
Medical Director, Shinagawa LASIK Center, Tokyo, Japan Adjunct Professor, Department of Ophthalmology, Wenzhou Medical College, Wenzhou, China
 Medical Director,, Tokyo, Japan Adjunct Professor, Department of Ophthalmology, Wenzhou Medical College, Wenzhou, China Financial disclosure: Ziemer Group AG, Switzerland AcuFocus, CA Schwind Eye-Tech-Solutions,
Medical Director,, Tokyo, Japan Adjunct Professor, Department of Ophthalmology, Wenzhou Medical College, Wenzhou, China Financial disclosure: Ziemer Group AG, Switzerland AcuFocus, CA Schwind Eye-Tech-Solutions,
There Are Millions of People at Risk For Dry Eye Who Is Likely to Develop This Irritating Condition?
 There Are Millions of People at Risk For Dry Eye Who Is Likely to Develop This Irritating Condition? Dry eye can be a temporary or chronic condition and occurs when the eye does not produce tears properly,
There Are Millions of People at Risk For Dry Eye Who Is Likely to Develop This Irritating Condition? Dry eye can be a temporary or chronic condition and occurs when the eye does not produce tears properly,
ALLERGENIC EXTRACT. Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE. U.S. Government License No.
 ALLERGENIC EXTRACT Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE U.S. Government License No. 308 Revised 07/04 PO Box 800 Lenoir, NC 28645 USA DESCRIPTION This set
ALLERGENIC EXTRACT Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE U.S. Government License No. 308 Revised 07/04 PO Box 800 Lenoir, NC 28645 USA DESCRIPTION This set
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION combined hepatitis A (inactivated) and hepatitis B (recombinant) vaccine This leaflet is part III of a three-part "Product Monograph" published when was approved for sale
PART III: CONSUMER INFORMATION combined hepatitis A (inactivated) and hepatitis B (recombinant) vaccine This leaflet is part III of a three-part "Product Monograph" published when was approved for sale
PACKAGE LEAFLET: INFORMATION FOR THE USER. PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion. Paracetamol
 PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
Risks and Limitations of LASIK Procedure
 Drs. Fine, Hoffman & Packer, LLC 1550 Oak Street, Suite #5 Eugene, OR 97401 541-687-2110 From Drs. Fine, Hoffman, & Packer Risks and Limitations of LASIK Procedure Infection, serious injury, or even death,
Drs. Fine, Hoffman & Packer, LLC 1550 Oak Street, Suite #5 Eugene, OR 97401 541-687-2110 From Drs. Fine, Hoffman, & Packer Risks and Limitations of LASIK Procedure Infection, serious injury, or even death,
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Consent for Bilateral Simultaneous Refractive Surgery PRK
 Consent for Bilateral Simultaneous Refractive Surgery PRK Please sign and return Patient Copy While many patients choose to have both eyes treated at the same surgical setting, there may be risks associated
Consent for Bilateral Simultaneous Refractive Surgery PRK Please sign and return Patient Copy While many patients choose to have both eyes treated at the same surgical setting, there may be risks associated
An Introduction to the Improved FDA Prescription Drug Labeling
 An Introduction to the Improved FDA Prescription Drug Labeling 1 Introduction Mary E. Kremzner, Pharm.D. CDR, U.S. Public Health Service Deputy Director, Division of Drug Information Center for Drug Evaluation
An Introduction to the Improved FDA Prescription Drug Labeling 1 Introduction Mary E. Kremzner, Pharm.D. CDR, U.S. Public Health Service Deputy Director, Division of Drug Information Center for Drug Evaluation
FITTING & PATIENT MANAGEMENT GUIDE
 89130_FIG 11/6/07 3:34 PM Page 1 FITTING & PATIENT MANAGEMENT GUIDE ACUVUE OASYS Brand Contact Lenses with HYDRACLEAR PLUS (senofilcon A) Visibility Tinted with UV Blocker 89130_FIG 11/6/07 3:34 PM Page
89130_FIG 11/6/07 3:34 PM Page 1 FITTING & PATIENT MANAGEMENT GUIDE ACUVUE OASYS Brand Contact Lenses with HYDRACLEAR PLUS (senofilcon A) Visibility Tinted with UV Blocker 89130_FIG 11/6/07 3:34 PM Page
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
For Educational Use Only - Not for Detailing or Distribution
 This document is intended for healthcare professionals practicing in the United States and may contain information that has not been approved by the FDA. It is supplied to you as a professional courtesy
This document is intended for healthcare professionals practicing in the United States and may contain information that has not been approved by the FDA. It is supplied to you as a professional courtesy
Ophthalmic Consultants of Long Island
 Case History Improving Cataract and Refractive Surgery Outcomes Through Ocular Surface Optimization 59 year old healthy white female History increased IOP Mother has history of glaucoma Presents for refractive
Case History Improving Cataract and Refractive Surgery Outcomes Through Ocular Surface Optimization 59 year old healthy white female History increased IOP Mother has history of glaucoma Presents for refractive
ORTHOKERATOLOGY CONTACT LENSES
 ORTHOKERATOLOGY CONTACT LENSES A Guide to Trouble-Free Contact lens Wear (Instructions for Use) Consult an Eye Care Professional for suitability of wear www.oculusgp.com Page 1 of 9 Product Type Contents
ORTHOKERATOLOGY CONTACT LENSES A Guide to Trouble-Free Contact lens Wear (Instructions for Use) Consult an Eye Care Professional for suitability of wear www.oculusgp.com Page 1 of 9 Product Type Contents
What is Refractive Error?
 Currently, about 55% of the civilian pilots in the United States must utilize some form of refractive correction to meet the vision requirements for medical certification. While spectacles are the most
Currently, about 55% of the civilian pilots in the United States must utilize some form of refractive correction to meet the vision requirements for medical certification. While spectacles are the most
Femto-LASIK. Pulsewidth: Ultrashort-pulse micro- machining can make sub- wavelength holes. micromachining
 All-laser laser LASIK (Femto( Femto-LASIK) Femto-LASIK 台 大 眼 科 王 一 中 IntraLase 2/1 Perfect Vision Ziemer (DaVinci) Carl Zeiss Meditec Pulsewidth: Femtosecond laser (Nd:Glass)) 153 nm (near infrared) Each
All-laser laser LASIK (Femto( Femto-LASIK) Femto-LASIK 台 大 眼 科 王 一 中 IntraLase 2/1 Perfect Vision Ziemer (DaVinci) Carl Zeiss Meditec Pulsewidth: Femtosecond laser (Nd:Glass)) 153 nm (near infrared) Each
