Allium Ureteral Stent (URS)
|
|
|
- Allan Norris
- 10 years ago
- Views:
Transcription
1 Allium Ureteral Stent (URS) Instructions For Use Manufactured by Allium Ltd.
2 DEVICE NAME: ALLIUM Ureteral Stent (URS) is intended to be inserted into the lower ureter to allow free flow of urine from the kidney to the bladder by supporting the obstructed area of the ureteral lumen, keep it open and prevent its re-obstruction. The ALLIUM Ureteral Stent is intended for endoscopic (retrograde), percutaneous (antegrade) insertion or combined antegrade/retrograde insertion (under cystoscopic and fluoroscopic guidance) into the ureter of patients diagnosed with chronic ureteral obstruction caused by benign or malignant causes. It is intended to remain in the ureter up to 1 year. DEVICE DESCRIPTION: The ALLIUM Ureteral Stent are offered in 2 configurations The URS stent equipped with an anchor The URS-O-R stent without an anchor. Both stents have a tubular shape body with a high radial force segment to keep open the stenosed part after its dilation, and low radial force upstream end for reducing friction between the upper end of the stent and the ureter. The aim of the low radial force downstream end is to adapt to the shape and function of the intramural ureteral segment and prevent or reduce vesicoureteral reflux.the URS anchoring intravesical segment is for preventing upward migration. Radiopaque Markers Length Radiopaque Markers Diameter Low radial force end segment Low radial force end segment The URS has 3 radiopaque markers at each end and 1 at the anchor. Anchor The URS O-R has 3 radiopaque markers at each end. The ALLIUM Ureteral Stent system is composed of 2 main elements: 1. The stent 2. Deployment device The stent has a metal structure with self-radial-expanding design (24 or 30 Fr in expanded diameter), completely covered with a thin layer of polymeric material and is easy to remove. The Ureteral stents have radiopaque markers to enhance their fluoroscopic visibility: The URS-O-R stent has 3 radiopaque markers at both ends and the URS stent have another single radiopaque marker on the anchor. Once inserted into the occluded ureter using the specially designed 10 Fr deployment device the stent is released for self-expansion in the occluded part of the ureter. The stent does not change its length during deployment. Following deployment the deployment device is then carefully removed from the body. URS Insertion system The deployment device for retrograde insertion has a black outer visual marker (OVM) on the overtube and beneath it 4 yellow inner visual markers () on the inner tube, both indicating the place of the wire connecting the anchor to the body of the stent. D URS-R-Retrograde System OVM 2
3 The deployment device for antegrade insertion has a black outer visual marker (OVM) on the overtube and beneath it a yellow inner visual marker () on the inner tube, both indicating the place of the wire connecting the anchor to the body of the stent. D OVM URS-A-Antegrade System Allium Ureteral Stent (URS) Ordering Information Retrograde Delivery System with anchor-80 cm Antegrade Delivery System with anchor -80 cm Order Number Stent Stent Body Order Number Stent Stent Body Diameter Diameter Length Length URS-R mm 100 mm URS-A mm 100 mm URS-R mm 120 mm URS-A mm 120 mm URS-R mm 100 mm URS-A mm 100 mm URS-R mm 120 mm URS-A mm 120 mm URS-O-R Insertion system R- A radiopaque marker on the overtube that mark the beginning of the high radial force segmet of the stent. OVM- A black outer visual marker on the overtube mark the distal end of the high radial force segment. - 4 yellow inner visual markers on the inner tube, indicating the distal end of the stent. RM URS-O-R-Retrograde System OVM Allium Ureteral Stent (URS-O-R) Ordering Information Retrograde Delivery System Without Anchor-80 cm Stent Stent Order Number Diamet Body er Length URS-O-R mm 100 mm URS-O-R mm 120 mm URS-O-R mm 100 mm URS-O-R mm 120 mm 3
4 INDICATIONS FOR USE: ALLIUM Ureteral Stents are indicated for use in malignant or benign ureteral occlusions necessitating long-term or chronic ureteral stenting (i.e. patients indicated for inserting double-j stents for 6 months or longer): Pelvic malignancies compressing the ureter Non-operable, occluding, primary or infiltrative ureteral malignancies Uretero-intestinal anastomotic strictures Latrogenic benign strictures of the ureter CONTRAINDICATIONS The insertion of ALLIUM Ureteral Stent is contraindicated in patients who Have an active urinary tract infection (increased WBC count, fever, chills etc) or has had two diagnosed acute urinary tract infections in the past year accompanied with increased WBC count, fever, chills. Have a hematuria that has not been previously evaluated and treated. Cannot tolerate any form of antibiotic treatment. Have a post-surgical anatomy that precludes cystoscopic or percutaneous approach. Currently are receiving any anticoagulation therapy (patients should stop it at least a week before stent insertion). Have a history of allergy to iodine preparations. Have a history of illness, medication or surgery that may affect the efficacy of the stent. POTENTIAL COMPLICATIONS Potential complications associated with insertion of the Allium Ureteral Stents (URS) are similar to all other ureteral stents. They may include, but are not limited to the following: Failure to access the obstructed site, pain/discomfort, perforation of the ureter, bleeding, urinary frequency or urgency, stent misplacement or migration, stent obstruction by tissue or stone, infection, sepsis, allergic reaction to the nickel-titanium alloy irritation. Mild hematuria is possibly to occur and related to device insertion, particularly during the first few days after insertion. If the symptoms persist patients should be instructed to contact their physician. PRECAUTIONS Checking the device: The device should be visually inspected for any damage prior to use. If any damage to the product or to its sterile packaging is observed THE DEVICE SHOULD NOT BE USED. Training: Proper training is required to position and deploy the Allium Ureteral Stents. Prior to its use; the technical information supplied with this device should be carefully reviewed. Stent positioning: Manipulation of the deployment device and stent positioning should be done using high-quality endoscopic and fluoroscopic equipment. WARNINGS General: The Allium Ureteral Stents (URS) are indented to be used in the ureter when the occlusion is not involving the intramural ureter or the ureteral orifice. Its design allows inserting the main body into the occluded ureter with its lower soft segment at the intramural part. At the end of the delivery, the anchoring segment should be situated in the bladder. If the intramural part or the ureteral orifice is involved in the occlusion it is recommended to use the URS-O-R and leave 1 cm of the main body (the high radial force segment) protruding through the orifice into the bladder. Stents used in this localization have a higher tendency for migration toward the bladder. The Allium Ureteral Stents are not intended for definitive treatment of ureteral diseases or of the complications of ureteral diseases. The Allium Ureteral Stents and their delivery mechanism should not come in contact with organic solvents at any time before their use. Allium Ureteral Stents should not be used in blood vessels. Device Related: Note that the mounting of the Allium Ureteral Stent (URS) to the deployment systems for retrograde and antegrade insertion are different. The "Retrograde System" cannot be 4
5 used for antegrade insertion and the "Antegrade System" cannot be used for retrograde insertion. Single use device: The Allium Ureteral Stent (URS) is intended for Single Use Only- DO NOT RESTERILIZE. Reuse, reprocessing, resterilization or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, resterilization or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, transmission of infectious diseases from one patient to another. Contamination of the device may lead to injury, illness or death of the patient or end user. The device should not be used if the package is open or damaged or if the device has been contaminated prior to insertion. The constricted stent and delivery system should be visually inspected for damage prior to use. Re-mounting an expanded stent into the delivery system should not be attempted. Reuse of the stent should not be attempted. This can seriously damage the patient s health. The stent should only be placed under cystoscopic visualization (retrograde insertion) or under direct fluoroscopic visualization combined with cystoscopic guidance (antegrade insertion). Catheterization through an implanted stent is not recommended. Introduction and passage of a catheter through the stented ureter into the kidney may dislodge the stent and/or damage the cover. Transureteral instrumentation while the stent is in place is not recommended. Longitudinal compression of the stent by instrumentation could dislodge the stent. The stent may migrate during and after placement: If this occurs, the stent should be removed and a new one may be considered for insertion instead. DIRECTIONS FOR USE Pre-procedural preparation The antibiotic prophylaxis for each patient is a broad spectrum oral or intravenous antibiotic to be started at least 3 hours before the procedure and continued according to protocols used by the hospital for percutaneous or endoscopic transurethral double-j stent insertion procedures. Choosing the appropriate URS Components of the Deployment Device A -Inner tube with a channel for passing the guide-wire, ending with a Luer connector covered with a vented cap B -Locking knob of the Y-connector C -Irrigation port of the Y-connector D - Over-tube covering the stent OVM -Black visual marker on the over-tube - 4 Yellow visual markers on the inner tube C D B A D URS-R- Retrograde System OVM 5
6 OVM D URS A-Antegrade System RM OVM URS-O-R Retrograde System Figure 4 - Allium Ureteral Stents Deployment Device MRI INFO URS MRI Information MR Conditional The Biliary Stent was determined to be MR-conditional. Non-clinical testing demonstrated that the Biliary Stent is MR Conditional. A patient with this device can be scanned safely, immediately after placement under the following conditions: Static Magnetic Field -Static magnetic field of 3-Tesla or less -Maximum spatial gradient magnetic field of 720-Gauss/cm or less MRI-Related Heating In non-clinical testing, the Biliary Stent produced the following temperature rises during MRI performed for 15-min of scanning (i.e., per pulse sequence) in 1.5-Tesla/64-MHz (Magnetom, Siemens Medical Solutions, Malvern, PA. Software Numaris/4, Version Syngo MR 2002B DHHS Active-shielded, horizontal field scanner) and 3-Tesla (3-Tesla/128-MHz, Excite, HDx, Software 14X.M5, General Electric Healthcare, Milwaukee, WI) MR systems: 1.5-Tesla 3-Tesla MR system reported, whole body averaged SAR 2.9-W/kg 2.9-W/kg Calorimetry measured values, whole body averaged SAR 2.1-W/kg 2.7-W/kg Highest temperature change +2.7 C +3.5 C These temperature changes will not pose a hazard to a human subject under the conditions indicated above. Artifact Information MR image quality may be compromised if the area of interest is in the exact same area or relatively close to the position of the Biliary Stent. Therefore, optimization of MR imaging parameters to compensate for the presence of this device may be necessary. The maximum artifact size (i.e., as seen on the gradient echo pulse sequence) extends approximately 5-mm relative to the size and shape of this implant. 6
7 Pulse Sequence T1-SE T1-SE GRE GRE Signal Void Size 1,263-mm2 68-mm mm2 116-mm2 Plane Orientation Parallel Perpendicular Parallel Perpendicular System Preparation: Prior to insertion of the stent and as part of its preparation the deployment device should be flushed as following: 1. Ensure that the Y-connector knob (B) is closed tightly. 2. Fill a syringe with 5-10 ml irrigation water/saline solution. 3. Connect the syringe to the irrigation port (C) of the Y connector. 4. Slowly flush the system while making sure that the water is coming out between the over-tube tip and the conical tip (D). Flushing the system is a must for easy and smooth deployment of the stent. 5. After irrigation, open the Y-connector knob (B) and ensure that it remains open completely during deployment of the stent. Identifying, Measuring and Dilation of the Ureteral obstruction and Stent Insertion: Instructions for URS Retrograde Insertion: Perform a retrograde ureterography to identify, mark and measure the site and length of the occlusion and its distance from the ureteral orifice. This is done by marking the landmarks using external radiopaque markers applied to the skin. If the patient has a nephrostomy catheter the ureterography can be performed antegradely. Measuring is done under a combination of endoscopy and fluoroscopy using a ureteral catheter. Use a cystoscope (21 Fr or larger) with a single working channel allowing passage to Fr instruments. Choose the "Retrograde System" with the appropriate stent length (the upstream end of the stent should be at least 10 mm above the upper end of the occlusion). Pass a guide-wire through the occlusion. Under fluoroscopy perform dilatation of the stenosis to at least 14 Fr. Leaving the guide wire in place insert the stent mounted on its 10 Fr deployment device through the working channel of the cystoscope into the ureter and advance it until the black outer visual marker (OVM) is at the ureteral orifice. Holding the stent in position, verify that the knob (B) of the Y-connector is open. Pull the Y-connector towards the rear Luer (A) carefully, using a constant force while holding the rear Luer (A) firmly fixed. This pulling will move backward the black outer visual marker (OVM). Ensure the correct positioning of the stent by identifying the yellow inner visual markers () at the ureteral orifice. At least 3 of the 4 markers should remain below the orifice during the entire deployment procedure. Under fluoroscopy, follow the expansion of the stent (expansion of the 3 radiopaque markers at the upstream end). Continue pulling the over-tube until the anchoring segment is released completely and is seen in the bladder. After complete expansion of the stent, verify that the stent has been completely released from the deployment system by gently pushing it in and out. Under fluoroscopic and/or endoscopic control carefully remove the deployment device, taking care not to dislodge the stent. After removing the entire deployment device remove the guide wire. Instructions for URS-O-R Retrograde Insertion: If the intramural part or the ureteral orifice is involved in the occlusion, perform a retrograde ureterography to identify, mark and measure the site and length of the occlusion and especially the existence of aditional proximal strictures. This is done by marking the landmarks using external radiopaque markers applied to the skin. If the patient has a nephrostomy catheter, the ureterography can be performed antegradely. Measuring is done under a combination of endoscopy and fluoroscopy using a ureteral catheter. Use a cystoscope (21 Fr. or larger) with a single working channel allowing passage of Fr instruments. 7
8 Choose the "URS-O-R Retrograde System" with the appropriate stent length (the upstream end of the stent should be at least 1 cm above the upper end of the occlusion and at least 1.5 cm of stent body protruding into the bladder). Pass a guide-wire through the occlusion. Under fluoroscopy perform dilatation of the stenosis to at least 14 Fr. Leaving the guide wire in place, insert the guide wire into the tip of the10 Fr deployment device. Carefully advance deployment device over the guide wire through the working channel of the cystoscope. Using fluoroscopy guidance, advance the deployment device over the guide wire into the ureter until the black outer visual marker (OVM) is at the ureteral orifice. Holding the stent in position, verify that the knob (B) of the Y-connector is open. Pull the Y-connector towards the rear Luer (A) carefully, using a constant force while holding the rear Luer (A) firmly fixed. This pulling will move backward the black outer visual marker (OVM). Ensure the correct positioning of the stent by identifying that all the yellow inner visual markers () are 1 cm from the ureteral orifice during the entire deployment procedure. Under fluoroscopy, follow the expansion of the stent (expansion of the 3 radiopaque markers at the upstream end). Continue pulling the over-tube until the distal segment is released completely and is seen in the bladder by the cystoscope. After complete expansion of the stent, verify that the stent has been completely released from the deployment system by gently pushing it in and out. Under fluoroscopic and/or endoscopic control, carefully remove the deployment device, taking care not to dislodge the stent. After removing the entire deployment device remove the guide wire. Instructions for Antegrade Insertion under Endoscopic and Fluoroscopic Guidance: Insert a nephrostomy catheter to the kidney. Under fluoroscopy perform an antegrade ureterography through the nephrostomy catheter. Identify the obstructed area and mark the target area for the stent using external radiopaque markers applied to the beginning and the end of the obstruction. Measure the distance between the upstream end of the obstruction and the ureteral orifice by retrogradely inserting a ureteral catheter. Insert antegradely a 0.035" guide-wire toward the bladder passing the occlusion. Verify that the distal end of the guide-wire starts to loop in the bladder. Pull the bladder end of the guide wire until it comes out from the urethral meatus. If a standard length guide wire is used hold firmly its proximal and distal ends with a strong forceps to prevent its accidental entering into the nephrostomy tract or the uretrhra. Dilate the obstructed area using a dilatation balloon inserted either antegradely or retrogradely to at least 14 Fr. Choose the "Antegrade System" with the appropriate stent length (the upstream end of the stent should be at least 10 mm above the upper end of the occlusion) Make sure that 100 cm of the guide-wire is out of the nephrostome. Over the guide-wire insert antegradely the stent mounted on its deployment device into the ureter and advance it to pass the occlusion area. Follow passage of the stent through the occlusion by fluoroscopy and entering of the tip of the deployment system into the bladder using a flexible cystoscope. Verify that the black outer visual marker (OVM) enters the bladder. Holding the stent in position, verify that the knob (B) of the Y-connector is open. Pull the Y-connector towards the rear Luer (A) carefully, using a constant force while holding the rear Luer (A) firmly fixed. This pulling will move backward the black outer visual marker (OVM) which will enter into the orifice. Ensure the correct positioning of the stent by identifying at least 3 of the yellow inner visual markers () are at the ureteral orifice. This marker should remain below the orifice during the entire deployment procedure. Under fluoroscopy, follow the expansion of the stent. 8
9 After complete release of the stent pull backward the deployment device until it reaches the upper ureter and perform an antegrade ureterography by injecting contrast through the irrigation port (C) to verify accurate stent placement. Carefully remove the entire deployment device through the nephrostomy tract Remove the guide wire. Leaving a nephostomy catheter after stent insertion for a few days is optional. Instructions for Combined Antegrade-Retrograde insertion: An alternative technique for inserting the Allium Ureteral Stent (URS) is the combined antegrade-retrograde insertion. In such a combined approach dilation of the occlusion can be done either retrogradely or antegradely. Pass antegradely a double-length guide-wire into the bladder through the nephrostomy tube. Turn the patient to a lithotomy position. Pull the bladder end of the guide wire at least 100 cm out of the urethral meatus. Insert the stent mounted on a "Retrograde System" following the Instructions for Retrograde Insertion described above. Suggested Patient Follow Up: Patients implanted with Allium Ureteral Stent (URS) should be followed like the patients inserted a double-j ureteral stent. Stent Removal Steps The Allium Ureteral Stent (URS) is a temporary device and is designed to be easily removed from the ureter. The Allium Ureteral Stent (URS) can be removed under vision (endoscopically). Removal of stent should be done under sedation, general or regional anesthesia. Under cystoscopic vision the anchoring segment is identified and engaged with a foreign body forceps. Pulling the engaged stent outward together with the working element may initiate unraveling of the stent. All the elements together with the cystoscope are pulled outward. For verifying that the entire stent came out the second small wire loop at the up-stream end of the stent should be seen on the removed stent. DISCLAIMER OF WARRANTIES Allium, Ltd. warrants that reasonable care has been used in the manufacture of this device. This warranty is exclusive and in lieu of all other warranties whether expressed, implied, written or oral, including but not limited to any warranties of merchantability of fitness for a particular purpose. As a result of biological differences in individuals, no product is 100% effective under all circumstances. Because of this fact, and since Allium, Ltd. has no control over the conditions under which the device is used, diagnosis of the patient, methods of administration or its handling after the device leaves our possession, Allium, Ltd. does not warrant either a good effect or against any ill effect following its use. Allium, Ltd. shall not be liable for any incidental or consequential loss, damage, or expense arising directly or indirectly from the use of this device. Allium, Ltd. will replace any device that we feel was defective at the time of shipment. No representative of Allium, Ltd. may change any of the foregoing or assume any additional liability or responsibility with this device. 9
10 Labeling Information Symbol This Symbol Means Do Not Reuse Use By Batch Code Sterilization Using Ethylene Oxide Catalog Number Caution, Consult Accompanying Documents Manufacturer Authorized Representative in the European Committee Consult Instructions for Use Store in Dry Place at Room Temperature Do Not Use if Package is Damaged For further questions or information, please contact the manufacturer: Allium, Ltd. Ha-Eshel 2 P.O.BOX 3081 Caesarea Industrial Park Israel Phone: Fax: [email protected] REF: AUTHORIZED REPRESENTATIVE MEDNET GmbH Borkstrasse 10 D Munster Germany 10
Allium Triangular Prostatic Urethral Stent System (TPS) Instructions For Use
 Allium Triangular Prostatic Urethral Stent System (TPS) Instructions For Use Manufactured by Allium Ltd. Device Name: Allium Triangular Prostatic Urethral Stent (TPS) Allium Triangular Prostatic Urethral
Allium Triangular Prostatic Urethral Stent System (TPS) Instructions For Use Manufactured by Allium Ltd. Device Name: Allium Triangular Prostatic Urethral Stent (TPS) Allium Triangular Prostatic Urethral
LifeStent XL Biliary Stent System
 B05688 Rev.2/07-13 LifeStent XL Biliary Stent System Recommended Guidewire Length Table Catheter Working Length Recommended Guidewire Length 130 cm 300 cm 80 cm 260 cm 130 cm & 80 cm 160 cm & 110 cm Figure
B05688 Rev.2/07-13 LifeStent XL Biliary Stent System Recommended Guidewire Length Table Catheter Working Length Recommended Guidewire Length 130 cm 300 cm 80 cm 260 cm 130 cm & 80 cm 160 cm & 110 cm Figure
Bard LifeStar Biliary Stent System
 Bard LifeStar Biliary Stent System Instructions For Use (IFU) Bard LifeStar Biliary Stent System Delivery System Diagram B05686 Vers.1/08-11 1 Information for use Read the Bard LifeStar Biliary Stent System
Bard LifeStar Biliary Stent System Instructions For Use (IFU) Bard LifeStar Biliary Stent System Delivery System Diagram B05686 Vers.1/08-11 1 Information for use Read the Bard LifeStar Biliary Stent System
Contraindications: Malign or benign strictures in the upper part of esophagus close to the cricopharyngeal muscle.
 Manufactured by: ELLA CS, s.r.o. Milady Horákové 504 500 06 Hradec Králové 6 Czech Republic Phone: +420 49 527 91 11 Fax: +420 49 526 56 55 E-mail: [email protected] Instructions for Use FerX-ELLA Esophageal
Manufactured by: ELLA CS, s.r.o. Milady Horákové 504 500 06 Hradec Králové 6 Czech Republic Phone: +420 49 527 91 11 Fax: +420 49 526 56 55 E-mail: [email protected] Instructions for Use FerX-ELLA Esophageal
Instructions for Use. Device Description The AMPLATZER Vascular Plug II is a self-expandable nitinol mesh occlusion device (see Figure 1).
 Vascular Plug II Instructions for Use Device Description The AMPLATZER Vascular Plug II is a self-expandable nitinol mesh occlusion device (see Figure 1). A B Figure 1. AMPLATZER Vascular Plug II A. Nitinol
Vascular Plug II Instructions for Use Device Description The AMPLATZER Vascular Plug II is a self-expandable nitinol mesh occlusion device (see Figure 1). A B Figure 1. AMPLATZER Vascular Plug II A. Nitinol
AERO. Tracheobronchial Stent Technology System
 AERO Tracheobronchial Stent Technology System Review Instructions For Use Before Using This System. Single Use Non-sterile MR Conditional CONTENTS Instructions For Use.............................. 3 IMPORTANT
AERO Tracheobronchial Stent Technology System Review Instructions For Use Before Using This System. Single Use Non-sterile MR Conditional CONTENTS Instructions For Use.............................. 3 IMPORTANT
HELPFUL STEP-BY-STEP ILLUSTRATIONS ARE LOCATED IN THIS BOOKLET
 HELPFUL STEP-BY-STEP ILLUSTRATIONS ARE LOCATED IN THIS BOOKLET DESCRIPTION The Vet Sent Ureter comes as a sterile stent system comprised of two components: 1) the radioopaque, double pigtail multi-fenestrated
HELPFUL STEP-BY-STEP ILLUSTRATIONS ARE LOCATED IN THIS BOOKLET DESCRIPTION The Vet Sent Ureter comes as a sterile stent system comprised of two components: 1) the radioopaque, double pigtail multi-fenestrated
Aspira* Peritoneal Drainage Catheter
 Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
A Guide for Patients Living with a Biliary Metal Stent
 A Guide for Patients Living with a Biliary Metal Stent What is a biliary metal stent? A biliary metal stent (also known as a bile duct stent ) is a flexible metallic tube specially designed to hold your
A Guide for Patients Living with a Biliary Metal Stent What is a biliary metal stent? A biliary metal stent (also known as a bile duct stent ) is a flexible metallic tube specially designed to hold your
WallFlex Biliary RX Stent. Fully, Partially and Uncovered Self-Expanding Metal Stents
 WallFlex Biliary RX Stent Fully, Partially and Uncovered Self-Expanding Metal Stents WallFlex Biliary RX Stent Fully, Partially and Uncovered Self-Expanding Metal Stents The WallFlex Biliary RX Stent is
WallFlex Biliary RX Stent Fully, Partially and Uncovered Self-Expanding Metal Stents WallFlex Biliary RX Stent Fully, Partially and Uncovered Self-Expanding Metal Stents The WallFlex Biliary RX Stent is
Aspira* Pleural Drainage Catheter
 Aspira* Pleural Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Pleural Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid from the
Aspira* Pleural Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Pleural Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid from the
surg urin Surgery: Urinary System 1
 Surgery: Urinary System 1 This section contains information to assist providers in billing for surgical procedures related to the urinary system. Extracorporeal Shock Wave Lithotripsy Medi-Cal covers Extracorporeal
Surgery: Urinary System 1 This section contains information to assist providers in billing for surgical procedures related to the urinary system. Extracorporeal Shock Wave Lithotripsy Medi-Cal covers Extracorporeal
PERCUTANEOUS PD CATHETER IMPLANTATION SYSTEM
 Place on Patient s Cranial Border of the Pubic Symphysis IMPLANTATION STENCIL Classic Exit Cuff Site PERCUTANEOUS PD CATHETER IMPLANTATION SYSTEM INSTRUCTIONS FOR USE VP 511 and VP-511M Implantation System
Place on Patient s Cranial Border of the Pubic Symphysis IMPLANTATION STENCIL Classic Exit Cuff Site PERCUTANEOUS PD CATHETER IMPLANTATION SYSTEM INSTRUCTIONS FOR USE VP 511 and VP-511M Implantation System
2. Does the patient have one of the following appropriate indications for placing indwelling urinary catheters?
 A. Decision to Insert a Urinary Catheter: 1. Before placing an indwelling catheter, please consider if these alternatives would be more appropriate: Bladder scanner: to assess and confirm urinary retention,
A. Decision to Insert a Urinary Catheter: 1. Before placing an indwelling catheter, please consider if these alternatives would be more appropriate: Bladder scanner: to assess and confirm urinary retention,
Tracheal Stent System
 nt Tracheal Stent System Available sizes: 08mm x 20mm NEW 08mm x 30mm 08mmx 50 mm 08mm x 60mm 08mm x 70mm 08mm x 80mm 08mm x 90mm 10mm x 20mm NEW 10 mm x 30 mm 10 mm x 50 mm 10 mm x 60 mm 10 mm x 70 mm
nt Tracheal Stent System Available sizes: 08mm x 20mm NEW 08mm x 30mm 08mmx 50 mm 08mm x 60mm 08mm x 70mm 08mm x 80mm 08mm x 90mm 10mm x 20mm NEW 10 mm x 30 mm 10 mm x 50 mm 10 mm x 60 mm 10 mm x 70 mm
Cook Celect and Günther Tulip Vena Cava Filter Patient Guide
 Cook Celect and Günther Tulip Vena Cava Filter Patient Guide www.cookmedical.com Filter patient guide Contents Pulmonary embolism 4 5 What is pulmonary embolism? What causes it? What are the symptoms of
Cook Celect and Günther Tulip Vena Cava Filter Patient Guide www.cookmedical.com Filter patient guide Contents Pulmonary embolism 4 5 What is pulmonary embolism? What causes it? What are the symptoms of
Ureteral Stenting and Nephrostomy
 Scan for mobile link. Ureteral Stenting and Nephrostomy Ureteral stenting and nephrostomy help restore urine flow through blocked ureters and return the kidney to normal function. Ureters are long, narrow
Scan for mobile link. Ureteral Stenting and Nephrostomy Ureteral stenting and nephrostomy help restore urine flow through blocked ureters and return the kidney to normal function. Ureters are long, narrow
BARD MEDICAL DIVISION UROLOGICAL DRAINAGE. Foley Catheter Care & Maintenance. Patient Education Guide
 BARD MEDICAL DIVISION Foley Catheter Care & Maintenance Patient Education Guide WHAT IS A FOLEY CATHETER? Because of your medical problem, your body is having trouble completely emptying your bladder of
BARD MEDICAL DIVISION Foley Catheter Care & Maintenance Patient Education Guide WHAT IS A FOLEY CATHETER? Because of your medical problem, your body is having trouble completely emptying your bladder of
Patient Information Booklet. Endovascular Stent Grafts: A Treatment for Abdominal Aortic Aneurysms
 Patient Information Booklet Endovascular Stent Grafts: A Treatment for Abdominal Aortic Aneurysms TABLE OF CONTENTS Introduction 1 Glossary 2 Abdominal Aorta 4 Abdominal Aortic Aneurysm 5 Causes 6 Symptoms
Patient Information Booklet Endovascular Stent Grafts: A Treatment for Abdominal Aortic Aneurysms TABLE OF CONTENTS Introduction 1 Glossary 2 Abdominal Aorta 4 Abdominal Aortic Aneurysm 5 Causes 6 Symptoms
Simple Urinary Techniques to Diagnose and Treat Complex Urinary Conditions
 Simple Urinary Techniques to Diagnose and Treat Complex Urinary Conditions Jody P. Lulich, DVM,PhD, The Minnesota Urolith Center, St Paul MN 55108 USA NONSURGICAL STONE REMOVAL: VOIDING UROHYDROPROPULSION
Simple Urinary Techniques to Diagnose and Treat Complex Urinary Conditions Jody P. Lulich, DVM,PhD, The Minnesota Urolith Center, St Paul MN 55108 USA NONSURGICAL STONE REMOVAL: VOIDING UROHYDROPROPULSION
URINARY CATHETER CARE
 URINARY CATHETER CARE INTRODUCTION Urinary catheter care is a very important skill, and it is a skill that many certified nursing assistants (CNAs) must know. Competence at providing urinary catheter care
URINARY CATHETER CARE INTRODUCTION Urinary catheter care is a very important skill, and it is a skill that many certified nursing assistants (CNAs) must know. Competence at providing urinary catheter care
Hemostasis Solutions Boston Scientific is committed to improving patient care in the management of gastrointestinal bleeding.
 Hemostasis Solutions Boston Scientific is committed to improving patient care in the management of gastrointestinal bleeding. Through innovation and continuous educational support, we offer a wide range
Hemostasis Solutions Boston Scientific is committed to improving patient care in the management of gastrointestinal bleeding. Through innovation and continuous educational support, we offer a wide range
A PRINTED copy of this guideline may not be the most recent version. The OFFICIAL version is located on IHNET at the Policies & Procedures Home Page
 A PRINTED copy of this guideline may not be the most recent version. The OFFICIAL version is located on IHNET at the Policies & Procedures Home Page IX0200: Prevention & Control of Catheter Associated
A PRINTED copy of this guideline may not be the most recent version. The OFFICIAL version is located on IHNET at the Policies & Procedures Home Page IX0200: Prevention & Control of Catheter Associated
Foley Catheter Placement
 Foley Catheter Placement Indications for a Foley Catheter Retention of urine leading to urinary hesitancy, straining to urinate, decrease in size and force of the urinary stream, interruption of urinary
Foley Catheter Placement Indications for a Foley Catheter Retention of urine leading to urinary hesitancy, straining to urinate, decrease in size and force of the urinary stream, interruption of urinary
USE OF STENTS FOR UPPER GI DISASTERS. Michael Talbot. The St George Hospital, Sydney
 USE OF STENTS FOR UPPER GI DISASTERS Michael Talbot. The St George Hospital, Sydney Disclosures Educational grants by Coviden, Applied Medical, Endogastric Solutions and Allergan in the last 3 years Clinical
USE OF STENTS FOR UPPER GI DISASTERS Michael Talbot. The St George Hospital, Sydney Disclosures Educational grants by Coviden, Applied Medical, Endogastric Solutions and Allergan in the last 3 years Clinical
Percutaneous Abscess Drainage
 Scan for mobile link. Percutaneous Abscess Drainage An abscess is an infected fluid collection within the body. Percutaneous abscess drainage uses imaging guidance to place a thin needle through the skin
Scan for mobile link. Percutaneous Abscess Drainage An abscess is an infected fluid collection within the body. Percutaneous abscess drainage uses imaging guidance to place a thin needle through the skin
Placement of an indwelling urinary catheter in female dogs
 Female Dog Urinary Catheterization 1 of 6 Placement of an indwelling urinary catheter in female dogs Bernie Hansen DVM MS North Carolina State University College of Veterinary Medicine Materials Needed
Female Dog Urinary Catheterization 1 of 6 Placement of an indwelling urinary catheter in female dogs Bernie Hansen DVM MS North Carolina State University College of Veterinary Medicine Materials Needed
PICC & Midline Catheters Patient Information Guide
 PICC & Midline Catheters Patient Information Guide medcompnet.com 1 table of contents Introduction 4 What is a PICC or Midline Catheter? 4 How is the PICC or Midline Catheter Inserted? 6 Catheter Care
PICC & Midline Catheters Patient Information Guide medcompnet.com 1 table of contents Introduction 4 What is a PICC or Midline Catheter? 4 How is the PICC or Midline Catheter Inserted? 6 Catheter Care
Patient Information Guide Morpheus CT Peripherally Inserted Central Catheter
 Patient Information Guide Morpheus CT Peripherally Inserted Central Catheter IC 192 Rev C A measure of flexibility and strength. Table of Contents 1. Introduction 2. What is the Morpheus CT PICC? 3. What
Patient Information Guide Morpheus CT Peripherally Inserted Central Catheter IC 192 Rev C A measure of flexibility and strength. Table of Contents 1. Introduction 2. What is the Morpheus CT PICC? 3. What
Palm Beach Obstetrics & Gynecology, PA
 Palm Beach Obstetrics & Gynecology, PA 4671 S. Congress Avenue, Lake Worth, FL 33461 561.434.0111 4631 N. Congress Avenue, Suite 102, West Palm Beach, FL 33407 Urinary Tract Infection About one of every
Palm Beach Obstetrics & Gynecology, PA 4671 S. Congress Avenue, Lake Worth, FL 33461 561.434.0111 4631 N. Congress Avenue, Suite 102, West Palm Beach, FL 33407 Urinary Tract Infection About one of every
Instructions for Use
 Pleural Effusion Shunt with External Pump Chamber Catalog No. 42-9005 Instructions for Use Denver Biomedical, Inc. Table of Contents Description 2 Indications 2 Contraindications 2 Warnings 4 Cautions
Pleural Effusion Shunt with External Pump Chamber Catalog No. 42-9005 Instructions for Use Denver Biomedical, Inc. Table of Contents Description 2 Indications 2 Contraindications 2 Warnings 4 Cautions
Subject: Magnetic Resonance Imaging (MRI) Information on Sorin Group Heart Valve Prostheses and Annuloplasty Devices
 Saluggia, 18 December 2013 Subject: Magnetic Resonance Imaging (MRI) Information on Sorin Group Heart Prostheses and Annuloplasty Devices To whom it may concern This letter summarizes the required MRI
Saluggia, 18 December 2013 Subject: Magnetic Resonance Imaging (MRI) Information on Sorin Group Heart Prostheses and Annuloplasty Devices To whom it may concern This letter summarizes the required MRI
150640_Brochure_B 4/12/07 2:58 PM Page 2. Patient Information. Freedom From an Enlarged Prostate
 150640_Brochure_B 4/12/07 2:58 PM Page 2 Patient Information Freedom From an Enlarged Prostate 150640_Brochure_B 4/12/07 2:58 PM Page 3 GreenLight Laser Therapy 1 150640_Brochure_B 4/12/07 2:58 PM Page
150640_Brochure_B 4/12/07 2:58 PM Page 2 Patient Information Freedom From an Enlarged Prostate 150640_Brochure_B 4/12/07 2:58 PM Page 3 GreenLight Laser Therapy 1 150640_Brochure_B 4/12/07 2:58 PM Page
Location: Clinical Practice Manual
 Subject: Area: Classification: Relevant to: Bladder Management Clinical Practice All Clinical Staff Implementation Date: March 2001 Review Date: March 2004 Responsible for Review: Approved by: Distribution:
Subject: Area: Classification: Relevant to: Bladder Management Clinical Practice All Clinical Staff Implementation Date: March 2001 Review Date: March 2004 Responsible for Review: Approved by: Distribution:
X-Plain Subclavian Inserted Central Catheter (SICC Line) Reference Summary
 X-Plain Subclavian Inserted Central Catheter (SICC Line) Reference Summary Introduction A Subclavian Inserted Central Catheter, or subclavian line, is a long thin hollow tube inserted in a vein under the
X-Plain Subclavian Inserted Central Catheter (SICC Line) Reference Summary Introduction A Subclavian Inserted Central Catheter, or subclavian line, is a long thin hollow tube inserted in a vein under the
53 X-rays and Diagnostic Radiology
 Learning Outcomes 53.1 Explain how x-rays are used for diagnostic and therapeutic purposes. 53-2 CHAPTER 53 X-rays and Diagnostic Radiology 53.2 Compare invasive and noninvasive diagnostic procedures.
Learning Outcomes 53.1 Explain how x-rays are used for diagnostic and therapeutic purposes. 53-2 CHAPTER 53 X-rays and Diagnostic Radiology 53.2 Compare invasive and noninvasive diagnostic procedures.
Ureteroscopy with Laser Lithotripsy
 Ureteroscopy with Laser Lithotripsy Introduction Kidney stones are fairly common. Although kidney stones can be very painful, they are treatable, and in many cases preventable. Your doctor may recommend
Ureteroscopy with Laser Lithotripsy Introduction Kidney stones are fairly common. Although kidney stones can be very painful, they are treatable, and in many cases preventable. Your doctor may recommend
Policies & Procedures. I.D. Number: 1073
 Policies & Procedures Title:: CENTRAL VENOUS CATHETERS INSERTION ASSISTING I.D. Number: 1073 Authorization [] Pharmacy Nursing Committee [] MAC Motion #: [x] SHR Nursing Practice Committee Source: Nursing
Policies & Procedures Title:: CENTRAL VENOUS CATHETERS INSERTION ASSISTING I.D. Number: 1073 Authorization [] Pharmacy Nursing Committee [] MAC Motion #: [x] SHR Nursing Practice Committee Source: Nursing
Refer to Coaptite Injectable Implant Instructions for Use provided with product for complete instructions for use.
 Questions for my Doctor Refer to Coaptite Injectable Implant Instructions for Use provided with product for complete instructions for use. INDICATIONS: Coaptite Injectable Implant is indicated for soft
Questions for my Doctor Refer to Coaptite Injectable Implant Instructions for Use provided with product for complete instructions for use. INDICATIONS: Coaptite Injectable Implant is indicated for soft
RELAY Plus Thoracic Stent-Graft System with Transport Delivery System
 RELAY Plus Thoracic Stent-Graft System with Transport Delivery System TABLE OF CONTENTS Section Page DEVICE DESCRIPTION 8 RADIOPAQUE MARKER BANDS 8 RELAY PLUS TRANSPORT DELIVERY SYSTEM 8 INDICATIONS FOR
RELAY Plus Thoracic Stent-Graft System with Transport Delivery System TABLE OF CONTENTS Section Page DEVICE DESCRIPTION 8 RADIOPAQUE MARKER BANDS 8 RELAY PLUS TRANSPORT DELIVERY SYSTEM 8 INDICATIONS FOR
1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System
 1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System NEVRO CORP. All questions or concerns about Nevro products should be forwarded to: Nevro Corp. 1800 Bridge Parkway
1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System NEVRO CORP. All questions or concerns about Nevro products should be forwarded to: Nevro Corp. 1800 Bridge Parkway
PREVENTION OF CATHETER ASSOCIATED URINARY TRACT INFECTIONS. (CAUTIs)
 PREVENTION OF CATHETER ASSOCIATED URINARY TRACT INFECTIONS (CAUTIs) CAUTIs A UTI where an indwelling urinary catheter was in place for >2 calendar days on the date of event. OR If an indwelling urinary
PREVENTION OF CATHETER ASSOCIATED URINARY TRACT INFECTIONS (CAUTIs) CAUTIs A UTI where an indwelling urinary catheter was in place for >2 calendar days on the date of event. OR If an indwelling urinary
III-701 Urinary Catheterization/Bladder Irrigation Original Date: 3/1/1977 Last Review Date: 10/28/2004
 III-701 Urinary Catheterization/Bladder Irrigation Original Date: 3/1/1977 Last Review Date: 10/28/2004 Purpose A. Allow for precise measurement of urine output. B. Collect a sterile urine specimen. C.
III-701 Urinary Catheterization/Bladder Irrigation Original Date: 3/1/1977 Last Review Date: 10/28/2004 Purpose A. Allow for precise measurement of urine output. B. Collect a sterile urine specimen. C.
Placement of Epidural Catheter for Pain Management Shane Bateman DVM, DVSc, DACVECC
 Placement of Epidural Catheter for Pain Management Shane Bateman DVM, DVSc, DACVECC Indications: Patients with severe abdominal or pelvic origin pain that is poorly responsive to other analgesic modalities.
Placement of Epidural Catheter for Pain Management Shane Bateman DVM, DVSc, DACVECC Indications: Patients with severe abdominal or pelvic origin pain that is poorly responsive to other analgesic modalities.
Spinal Cord and Bladder Management Male: Intermittent Catheter
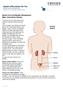 Spinal Cord and Bladder Management Male: Intermittent Catheter The 5 parts of the urinary system work together to get rid of waste and make urine. Urine is made in your kidneys and travels down 2 thin
Spinal Cord and Bladder Management Male: Intermittent Catheter The 5 parts of the urinary system work together to get rid of waste and make urine. Urine is made in your kidneys and travels down 2 thin
LOSS OF BLADDER CONTROL IS TREATABLE TAKE CONTROL AND RESTORE YOUR LIFESTYLE
 LOSS OF BLADDER CONTROL IS TREATABLE TAKE CONTROL AND RESTORE YOUR LIFESTYLE TALKING ABOUT STRESS INCONTINENCE (SUI) Millions of women suffer from stress incontinence (SUI). This condition results in accidental
LOSS OF BLADDER CONTROL IS TREATABLE TAKE CONTROL AND RESTORE YOUR LIFESTYLE TALKING ABOUT STRESS INCONTINENCE (SUI) Millions of women suffer from stress incontinence (SUI). This condition results in accidental
Urinary Diversion: Ileovesicostomy/Ileal Loop/Colon Loop
 Urinary Diversion: Ileovesicostomy/Ileal Loop/Colon Loop Why do I need this surgery? A urinary diversion is a surgical procedure that is performed to allow urine to safely pass from the kidneys into a
Urinary Diversion: Ileovesicostomy/Ileal Loop/Colon Loop Why do I need this surgery? A urinary diversion is a surgical procedure that is performed to allow urine to safely pass from the kidneys into a
To decrease and/or prevent the incidence of catheter associated infections and other complications associated with IUC.
 Patient Care Manual Standardized Procedure Number: PC-SP.115 Latest Revision Date: 01/27/2015 Effective Date: 10/10/2011 Standardized Procedure: Urethral Catheter (IUC), Adult, Discontinuance of FUNCTION
Patient Care Manual Standardized Procedure Number: PC-SP.115 Latest Revision Date: 01/27/2015 Effective Date: 10/10/2011 Standardized Procedure: Urethral Catheter (IUC), Adult, Discontinuance of FUNCTION
PATIENT INFORMATION BOOKLET
 PATIENT INFORMATION BOOKLET Wingspan Stent System with Gateway PTA Balloon Catheter TABLE OF CONTENTS Definitions... 2 What is the Purpose of This Booklet?... 3 What is an Intracranial Lesion?... 3 Who
PATIENT INFORMATION BOOKLET Wingspan Stent System with Gateway PTA Balloon Catheter TABLE OF CONTENTS Definitions... 2 What is the Purpose of This Booklet?... 3 What is an Intracranial Lesion?... 3 Who
Inferior Vena Cava filter and removal
 Inferior Vena Cava filter and removal What is Inferior Vena Cava Filter Placement and Removal? An inferior vena cava filter placement procedure involves an interventional radiologist (a specialist doctor)
Inferior Vena Cava filter and removal What is Inferior Vena Cava Filter Placement and Removal? An inferior vena cava filter placement procedure involves an interventional radiologist (a specialist doctor)
Prevention of catheter associated urinary tract infections
 Prevention of catheter associated urinary tract infections Dr. Suzan Sanavi, Nephrologist, M.D University of Social Welfare and Rehabilitation Akhavan Physical Spine Center INTRODUCTION Urinary bladder
Prevention of catheter associated urinary tract infections Dr. Suzan Sanavi, Nephrologist, M.D University of Social Welfare and Rehabilitation Akhavan Physical Spine Center INTRODUCTION Urinary bladder
Biliary Drain. What is a biliary drain?
 Biliary Drain What is a biliary drain? A biliary drain is a tube to drain bile from your liver. It is put in by a doctor called an Interventional Radiologist. The tube or catheter is placed through your
Biliary Drain What is a biliary drain? A biliary drain is a tube to drain bile from your liver. It is put in by a doctor called an Interventional Radiologist. The tube or catheter is placed through your
Care of the Catheterised Patient and Urinalysis
 Care of the Catheterised Patient and Urinalysis Male Pelvic Anatomy Female Pelvic Anatomy What does a urinary catheter do? Urinary Catheters Urinary Catheters Urinary Catheters Why do patients have catheters?
Care of the Catheterised Patient and Urinalysis Male Pelvic Anatomy Female Pelvic Anatomy What does a urinary catheter do? Urinary Catheters Urinary Catheters Urinary Catheters Why do patients have catheters?
Arrow NextStep and Arrow Cannon II Plus Chronic Hemodialysis Catheters
 Retrograde-Tunneling Insertion Poster Arrow NextStep and Arrow Cannon II Plus Chronic Hemodialysis Catheters Arrow Retrograde-Tunneling catheters Luer Lock caps Color-coded Luer Lock caps Arterial port
Retrograde-Tunneling Insertion Poster Arrow NextStep and Arrow Cannon II Plus Chronic Hemodialysis Catheters Arrow Retrograde-Tunneling catheters Luer Lock caps Color-coded Luer Lock caps Arterial port
A Patient s Guide to Minimally Invasive Abdominal Aortic Aneurysm Repair
 A Patient s Guide to Minimally Invasive Abdominal Aortic Aneurysm Repair Table of Contents The AFX Endovascular AAA System............................................ 1 What is an Abdominal Aortic Aneurysm
A Patient s Guide to Minimally Invasive Abdominal Aortic Aneurysm Repair Table of Contents The AFX Endovascular AAA System............................................ 1 What is an Abdominal Aortic Aneurysm
Normal bladder function requires a coordinated effort between the brain, spinal cord, and the bladder.
 .. Urinary Incontinence Urinary incontinence is not an inevitable part of aging, and it is not a disease. The loss of bladder control - called urinary incontinence - affects between 13 and 17 million adult
.. Urinary Incontinence Urinary incontinence is not an inevitable part of aging, and it is not a disease. The loss of bladder control - called urinary incontinence - affects between 13 and 17 million adult
Inferior Vena Cava Filter Placement and Removal
 Scan for mobile link. Inferior Vena Cava Filter Placement and Removal What is Inferior Vena Cava Filter Placement and Removal? In an inferior vena cava filter placement procedure, interventional radiologists
Scan for mobile link. Inferior Vena Cava Filter Placement and Removal What is Inferior Vena Cava Filter Placement and Removal? In an inferior vena cava filter placement procedure, interventional radiologists
X-Plain Foley Catheter Male Reference Summary
 X-Plain Foley Catheter Male Reference Summary Introduction A Foley catheter is a tube that is put through the urinary opening and into your bladder to drain urine. Your doctor may have placed or may ask
X-Plain Foley Catheter Male Reference Summary Introduction A Foley catheter is a tube that is put through the urinary opening and into your bladder to drain urine. Your doctor may have placed or may ask
When Procedural Support really matters. Navien TM A+ Intracranial Support Catheter
 When Procedural Support really matters Navien TM A+ Intracranial Support Catheter Coils / Liquid Embolics DURABLE ovalization resistance, Maintains patent lumen in tortuosity for smooth micro catheter
When Procedural Support really matters Navien TM A+ Intracranial Support Catheter Coils / Liquid Embolics DURABLE ovalization resistance, Maintains patent lumen in tortuosity for smooth micro catheter
Urinary Tract Infections
 1 Infections in the urinary tract are relatively common. These infections are often referred to as bladder infections. They are also known as UTI s or urinary tract infections. When an infection is confined
1 Infections in the urinary tract are relatively common. These infections are often referred to as bladder infections. They are also known as UTI s or urinary tract infections. When an infection is confined
Bard LifeStar Vascular Stent System
 Bard LifeStar Vascular Stent System Instructions For Use (IFU) Bard LifeStar Vascular Stent System Delivery System Diagram B05685 Vers.1/08-11 1 Instructions for use Read the Bard LifeStar Vascular Stent
Bard LifeStar Vascular Stent System Instructions For Use (IFU) Bard LifeStar Vascular Stent System Delivery System Diagram B05685 Vers.1/08-11 1 Instructions for use Read the Bard LifeStar Vascular Stent
Urinary tract and perineum
 9 Urinary tract and perineum Key Points 9.1 9.1 THE URINARY BLADDER URINARY RETENTION Acute retention of urine is an indication for emergency drainage of the bladder The common causes of acute retention
9 Urinary tract and perineum Key Points 9.1 9.1 THE URINARY BLADDER URINARY RETENTION Acute retention of urine is an indication for emergency drainage of the bladder The common causes of acute retention
PATIENT CARE MANUAL PROCEDURE
 PATIENT CARE MANUAL PROCEDURE NUMBER VII-E-5 PAGE 1 OF 7 APPROVED BY: CATEGORY: Tri-site Nursing Policy and Procedures Review Committee Body Systems; Genitourinary 1.0 GOALS To influence patient care providers
PATIENT CARE MANUAL PROCEDURE NUMBER VII-E-5 PAGE 1 OF 7 APPROVED BY: CATEGORY: Tri-site Nursing Policy and Procedures Review Committee Body Systems; Genitourinary 1.0 GOALS To influence patient care providers
INTERDISCIPLINARY CLINICAL MANUAL Practice Guideline
 INTERDISCIPLINARY CLINICAL MANUAL Practice Guideline TITLE: Insertion of a curved tip Urinary Catheter (Coude/Tiemann) NUMBER: CC 50-013 Effective Date: August 2015 Page 1 of 5 Applies To: All Preamble:
INTERDISCIPLINARY CLINICAL MANUAL Practice Guideline TITLE: Insertion of a curved tip Urinary Catheter (Coude/Tiemann) NUMBER: CC 50-013 Effective Date: August 2015 Page 1 of 5 Applies To: All Preamble:
PERIPHERALLY INSERTED CENTRAL CATHETERS (PICC) Fong So Kwan APN, Haematology unit Medical Department, QMH
 PERIPHERALLY INSERTED CENTRAL CATHETERS (PICC) Fong So Kwan APN, Haematology unit Medical Department, QMH 1 What is a PICC catheter? Primary vascular access device since their introduction in the mid-1970s,
PERIPHERALLY INSERTED CENTRAL CATHETERS (PICC) Fong So Kwan APN, Haematology unit Medical Department, QMH 1 What is a PICC catheter? Primary vascular access device since their introduction in the mid-1970s,
V: Infusion Therapy. Alberta Licensed Practical Nurses Competency Profile 217
 V: Infusion Therapy Alberta Licensed Practical Nurses Competency Profile 217 Competency: V-1 Knowledge of Intravenous Therapy V-1-1 V-1-2 V-1-3 V-1-4 V-1-5 Demonstrate knowledge and ability to apply critical
V: Infusion Therapy Alberta Licensed Practical Nurses Competency Profile 217 Competency: V-1 Knowledge of Intravenous Therapy V-1-1 V-1-2 V-1-3 V-1-4 V-1-5 Demonstrate knowledge and ability to apply critical
PROCEDURE- SPECIFIC INFORMATION FOR PATIENTS
 The British Association of Urological Surgeons 35-43 Lincoln s Inn Fields London WC2A 3PE Phone: Fax: Website: E- mail: +44 (0)20 7869 6950 +44 (0)20 7404 5048 www.baus.org.uk [email protected] PROCEDURE-
The British Association of Urological Surgeons 35-43 Lincoln s Inn Fields London WC2A 3PE Phone: Fax: Website: E- mail: +44 (0)20 7869 6950 +44 (0)20 7404 5048 www.baus.org.uk [email protected] PROCEDURE-
KnifeLight. Carpal Tunnel Ligament Release. Operative Technique
 KnifeLight Carpal Tunnel Ligament Release Operative Technique Contents Page 1. Features & Benefits 3 Intended Use and Indications 3 Contraindications 3 Features & Benefits 3 2. Operative Technique 4 Antegrade
KnifeLight Carpal Tunnel Ligament Release Operative Technique Contents Page 1. Features & Benefits 3 Intended Use and Indications 3 Contraindications 3 Features & Benefits 3 2. Operative Technique 4 Antegrade
Malfunctioning PD Catheters
 Malfunctioning PD Catheters Introduction Migration reported to occur in between 5% to 35% of the implanted catheters. majority migrates to the right upper quadrant suggests that the problem bears a relationship
Malfunctioning PD Catheters Introduction Migration reported to occur in between 5% to 35% of the implanted catheters. majority migrates to the right upper quadrant suggests that the problem bears a relationship
Page 1 of 10 MC1482 Peripherally-Inserted Central Catheter. Peripherally-Inserted Central Catheter (PICC)
 Page 1 of 10 MC1482 Peripherally-Inserted Central Catheter Peripherally-Inserted Central Catheter (PICC) Page 2 of 10 MC1482 Peripherally-Inserted Central Catheter Introduction A peripherally-inserted
Page 1 of 10 MC1482 Peripherally-Inserted Central Catheter Peripherally-Inserted Central Catheter (PICC) Page 2 of 10 MC1482 Peripherally-Inserted Central Catheter Introduction A peripherally-inserted
BOUGIES/DILATORS. Dilators
 118 507505 Pilling offers a comprehensive line of laryngeal and esophageal dilators and bougies, including designs by Drs. Jackson, Tucker, and Maloney. Dilators JACKSON LARYNGEAL DILATORS Rigid, metallic,
118 507505 Pilling offers a comprehensive line of laryngeal and esophageal dilators and bougies, including designs by Drs. Jackson, Tucker, and Maloney. Dilators JACKSON LARYNGEAL DILATORS Rigid, metallic,
KwikPen Insulin delivery device
 USER MANUAL KwikPen Insulin delivery device PLEASE READ THESE INSTRUCTIONS BEFORE USE Lilly Introduction The KwikPen is designed for ease of use. It is a disposable pen containing 3 ml (300 units) of U-
USER MANUAL KwikPen Insulin delivery device PLEASE READ THESE INSTRUCTIONS BEFORE USE Lilly Introduction The KwikPen is designed for ease of use. It is a disposable pen containing 3 ml (300 units) of U-
Delivering nutrition, fluids & medication via a PEG. By Julia Pointer & Caroline Ross, Staff Nurses, Hospice Day Service
 Delivering nutrition, fluids & medication via a PEG By Julia Pointer & Caroline Ross, Staff Nurses, Hospice Day Service Aims of this session? To give an explanation of the theory and practice of a Percutaneous
Delivering nutrition, fluids & medication via a PEG By Julia Pointer & Caroline Ross, Staff Nurses, Hospice Day Service Aims of this session? To give an explanation of the theory and practice of a Percutaneous
NFC Communication Tray Model: HHX-IT3-Z
 INSTRUCTION MANUAL NFC Communication Tray Model: HHX-IT3-Z ENGLISH CONTENTS Introduction...3 Important safety information...4 Know your unit...6 Transferring data to the PC...7 Care and maintenance...8
INSTRUCTION MANUAL NFC Communication Tray Model: HHX-IT3-Z ENGLISH CONTENTS Introduction...3 Important safety information...4 Know your unit...6 Transferring data to the PC...7 Care and maintenance...8
VaPro Touch Free Hydrophilic Intermittent Catheter
 7202 What healthcare practitioners are saying about the catheter:4 Easy to explain to patients how to use the product. Medical Assistant 72102 72122 72142 7204 72104 72124 72144 724 73124 73144 734 Advance
7202 What healthcare practitioners are saying about the catheter:4 Easy to explain to patients how to use the product. Medical Assistant 72102 72122 72142 7204 72104 72124 72144 724 73124 73144 734 Advance
Guy s, King s and St Thomas Cancer Centre The Cancer Outpatient Clinic Central venous catheter: Peripherally inserted central catheter
 Guy s, King s and St Thomas Cancer Centre The Cancer Outpatient Clinic Central venous catheter: Peripherally inserted central catheter This information leaflet aims to help answer some of the questions
Guy s, King s and St Thomas Cancer Centre The Cancer Outpatient Clinic Central venous catheter: Peripherally inserted central catheter This information leaflet aims to help answer some of the questions
A GUIDE TO HAVING A URETERIC STENT INSERTED
 A GUIDE TO HAVING A URETERIC STENT INSERTED WHAT IS A URETERIC STENT? A ureteric stent is a thin plastic tube which can be inserted into your ureters (tubes that carry urine from your kidney to your bladder)
A GUIDE TO HAVING A URETERIC STENT INSERTED WHAT IS A URETERIC STENT? A ureteric stent is a thin plastic tube which can be inserted into your ureters (tubes that carry urine from your kidney to your bladder)
Operator s Manual. Pressure Injection Cell Model PC8500. Congratulations! Contents
 Operator s Manual Pressure Injection Cell Model PC8500 Congratulations! Congratulations on your purchase of a Next Advance Pressure Injection Cell. Please read this operator s manual which explains proper
Operator s Manual Pressure Injection Cell Model PC8500 Congratulations! Congratulations on your purchase of a Next Advance Pressure Injection Cell. Please read this operator s manual which explains proper
Bile Leaks After Laparoscopic Cholecystectomy. Kings County Hospital Center Eliana A. Soto, MD
 Bile Leaks After Laparoscopic Cholecystectomy Kings County Hospital Center Eliana A. Soto, MD Biliary Injuries during Cholecystectomy In the 1990s, high rate of biliary injury was due in part to learning
Bile Leaks After Laparoscopic Cholecystectomy Kings County Hospital Center Eliana A. Soto, MD Biliary Injuries during Cholecystectomy In the 1990s, high rate of biliary injury was due in part to learning
Childhood Urinary Tract Infections
 Childhood Urinary Tract Infections What is a UTI? Urinary tract infection (UTI) is one of the most common infections in childhood. It can cause distress to the child, concerns to the parents, and may
Childhood Urinary Tract Infections What is a UTI? Urinary tract infection (UTI) is one of the most common infections in childhood. It can cause distress to the child, concerns to the parents, and may
URETEROSCOPY (AND TREATMENT OF KIDNEY STONES)
 URETEROSCOPY (AND TREATMENT OF KIDNEY STONES) AN INFORMATION LEAFLET Written by: Department of Urology May 2011 Stockport: 0161 419 5698 Website: w w w. s t o c k p o r t. n h s. u k Tameside: 0161 922
URETEROSCOPY (AND TREATMENT OF KIDNEY STONES) AN INFORMATION LEAFLET Written by: Department of Urology May 2011 Stockport: 0161 419 5698 Website: w w w. s t o c k p o r t. n h s. u k Tameside: 0161 922
AMS Sphincter 800 Urinary Prosthesis
 AMS Sphincter 800 Urinary Prosthesis AMS Sphincter 800 AMS Sphincter 800 AMS Sphincter 800 The device is implanted in the body and cannot be seen. The cuff can be placed at the bulbous urethra or at the
AMS Sphincter 800 Urinary Prosthesis AMS Sphincter 800 AMS Sphincter 800 AMS Sphincter 800 The device is implanted in the body and cannot be seen. The cuff can be placed at the bulbous urethra or at the
Achieving Independence
 Bard: Intermittent Self-Catheterization A Guide to Self-Catheterization Achieving Independence Introduction This brochure is provided by Bard, a leading provider of urology products since 1907. The best
Bard: Intermittent Self-Catheterization A Guide to Self-Catheterization Achieving Independence Introduction This brochure is provided by Bard, a leading provider of urology products since 1907. The best
Medical Malpractice in Endourology: Analysis of Closed Cases From the State of New York
 Medical Malpractice in Endourology: Analysis of Closed Cases From the State of New York Brian Duty,* Zhamshid Okhunov, Zeph Okeke and Arthur Smith From the Department of Urology, North Shore-Long Island
Medical Malpractice in Endourology: Analysis of Closed Cases From the State of New York Brian Duty,* Zhamshid Okhunov, Zeph Okeke and Arthur Smith From the Department of Urology, North Shore-Long Island
Patient Information. PORT-A-CATH Implantable Venous Access Systems
 Patient Information PORT-A-CATH Implantable Venous Access Systems Your doctor has prescribed treatment that requires the frequent administration of medications or other fluids directly into your bloodstream
Patient Information PORT-A-CATH Implantable Venous Access Systems Your doctor has prescribed treatment that requires the frequent administration of medications or other fluids directly into your bloodstream
URINARY CATHETER INSERTION - STRAIGHT OR INDWELLING CATHETER
 URINARY CATHETER INSERTION - STRAIGHT OR INDWELLING CATHETER PURPOSE To obtain a sterile urine specimen. To facilitate emptying bladder. To relieve bladder distention. To irrigate bladder. To measure residual
URINARY CATHETER INSERTION - STRAIGHT OR INDWELLING CATHETER PURPOSE To obtain a sterile urine specimen. To facilitate emptying bladder. To relieve bladder distention. To irrigate bladder. To measure residual
By Anne C. Travis, M.D., M.Sc. and John R. Saltzman, M.D., FACG Brigham and Women's Hospital Harvard Medical School Boston, MA
 SMALL BOWEL BLEEDING: CAUSES, DIAGNOSIS AND TREATMENT By Anne C. Travis, M.D., M.Sc. and John R. Saltzman, M.D., FACG Brigham and Women's Hospital Harvard Medical School Boston, MA 1. What is the small
SMALL BOWEL BLEEDING: CAUSES, DIAGNOSIS AND TREATMENT By Anne C. Travis, M.D., M.Sc. and John R. Saltzman, M.D., FACG Brigham and Women's Hospital Harvard Medical School Boston, MA 1. What is the small
USE OF SIMULATION TO IMPROVE CARDIOVASCULAR STENT DEVELOPMENT
 USE OF SIMULATION TO IMPROVE CARDIOVASCULAR STENT DEVELOPMENT V. Astier, S. Bidal, V. Garitey, F. Mouret Abstract : A stent is a wire metal mesh tube placed into an artery or blood vessel to hold the structure
USE OF SIMULATION TO IMPROVE CARDIOVASCULAR STENT DEVELOPMENT V. Astier, S. Bidal, V. Garitey, F. Mouret Abstract : A stent is a wire metal mesh tube placed into an artery or blood vessel to hold the structure
MR Information Date: March 11, 2014
 ii Heinz Kurz GmbH Medizintechnik Tuebinger Str. 3 72144 Dusslingen Germany Phone +49 (0)7072 9179-0 Fax +49 (0) 7072 91 79 79 Email: [email protected] Internet: www.kurzmed.com Kurz Medical, Inc. 5126 South
ii Heinz Kurz GmbH Medizintechnik Tuebinger Str. 3 72144 Dusslingen Germany Phone +49 (0)7072 9179-0 Fax +49 (0) 7072 91 79 79 Email: [email protected] Internet: www.kurzmed.com Kurz Medical, Inc. 5126 South
Medications or therapeutic solutions may be injected directly into the bloodstream
 Intravenous Therapy Medications or therapeutic solutions may be injected directly into the bloodstream for immediate circulation and use by the body. State practice acts designate which health care professionals
Intravenous Therapy Medications or therapeutic solutions may be injected directly into the bloodstream for immediate circulation and use by the body. State practice acts designate which health care professionals
Yukon Choice DES + Translumina - The polymer-free DES solution. www.translumina.de. Coronary Stent System for Drug Application
 Coronary Stent System for Drug Application Yukon Choice DES + new catheter polymer-free DES Translumina - The polymer-free DES solution www.translumina.de A First Class Stent The YUKON Choice DES+ is the
Coronary Stent System for Drug Application Yukon Choice DES + new catheter polymer-free DES Translumina - The polymer-free DES solution www.translumina.de A First Class Stent The YUKON Choice DES+ is the
MINIMALLY INVASIVE MANAGEMENT OF FELINE LOWER URINARY TRACT DISEASE:
 MINIMALLY INVASIVE MANAGEMENT OF FELINE LOWER URINARY TRACT DISEASE: LASERS, STENTS AND BALLOONS Dr. Allyson Berent, DVM, DACVIM The Animal Medical Center, New York, NY Feline lower urinary tract disease
MINIMALLY INVASIVE MANAGEMENT OF FELINE LOWER URINARY TRACT DISEASE: LASERS, STENTS AND BALLOONS Dr. Allyson Berent, DVM, DACVIM The Animal Medical Center, New York, NY Feline lower urinary tract disease
Flushing and Dressing a Peripherally Inserted Central Catheter (PICC Line): a Guide for Nurses
 Flushing and Dressing a Peripherally Inserted Central Catheter (PICC Line): a Guide for Nurses Information for Nurses Introduction This information is for community nursing staffs who have been asked to
Flushing and Dressing a Peripherally Inserted Central Catheter (PICC Line): a Guide for Nurses Information for Nurses Introduction This information is for community nursing staffs who have been asked to
Treatment of Maxillary Sinusitis Using the SinuSys Vent-Os Sinus Dilation System
 White PAPer Treatment of Maxillary Sinusitis Using the SinuSys Vent-Os Sinus Dilation System Jerome Hester, MD Chief Medical Officer SinuSys Corporation Palo Alto, California USA introduction Establishment
White PAPer Treatment of Maxillary Sinusitis Using the SinuSys Vent-Os Sinus Dilation System Jerome Hester, MD Chief Medical Officer SinuSys Corporation Palo Alto, California USA introduction Establishment
Get the Facts, Be Informed, Make YOUR Best Decision. Pelvic Organ Prolapse
 Pelvic Organ Prolapse ETHICON Women s Health & Urology, a division of ETHICON, INC., a Johnson & Johnson company, is dedicated to providing innovative solutions for common women s health problems and to
Pelvic Organ Prolapse ETHICON Women s Health & Urology, a division of ETHICON, INC., a Johnson & Johnson company, is dedicated to providing innovative solutions for common women s health problems and to
Policies & Procedures. Care of
 Policies & Procedures Title: SUPRAPUBIC CATHETER Care of Changing Removal Authorization: [x] SHR Nursing Practice Committee I.D. Number: 1021 Source: Nursing Date Revised: November 2014 Date Effective:
Policies & Procedures Title: SUPRAPUBIC CATHETER Care of Changing Removal Authorization: [x] SHR Nursing Practice Committee I.D. Number: 1021 Source: Nursing Date Revised: November 2014 Date Effective:
Gary M. Annuniziata, D.O., F.A.C.P. Anh T. Duong, M.D. Jonathan C. Lin, M.D., MPH. Preparation for EGD, ERCP, Peg Placement.
 Gary M. Annuniziata, D.O., F.A.C.P. Anh T. Duong, M.D. Jonathan C. Lin, M.D., MPH Phone- (760) 321-2500 Fax- (760) 321-5720 Preparation for EGD, ERCP, Peg Placement Patient Name- Procedure Date and Time-
Gary M. Annuniziata, D.O., F.A.C.P. Anh T. Duong, M.D. Jonathan C. Lin, M.D., MPH Phone- (760) 321-2500 Fax- (760) 321-5720 Preparation for EGD, ERCP, Peg Placement Patient Name- Procedure Date and Time-
A4.7 Management of a totally occluded central catheter and persistent withdrawal occlusion (PWO)
 A4.7 Management of a totally occluded central catheter and persistent withdrawal occlusion (PWO) Types of Catheter Related Thrombotic A catheter-related thrombus may be intraluminal (inside the catheter)
A4.7 Management of a totally occluded central catheter and persistent withdrawal occlusion (PWO) Types of Catheter Related Thrombotic A catheter-related thrombus may be intraluminal (inside the catheter)
Nephrostomy &/or Ureteric Stent Insertion PROCEDURAL CONSENT FORM. A. Interpreter / cultural needs. B. Procedure. C. Risks of the procedure
 DO NOT WRITE IN THIS BINDING MARGIN v2.00-03/2011 SW9231 Facility: Nephrostomy &/or Ureteric Stent Insertion A. Interpreter / cultural needs An Interpreter Service is required? Yes No If Yes, is a qualified
DO NOT WRITE IN THIS BINDING MARGIN v2.00-03/2011 SW9231 Facility: Nephrostomy &/or Ureteric Stent Insertion A. Interpreter / cultural needs An Interpreter Service is required? Yes No If Yes, is a qualified
