RELAY Plus Thoracic Stent-Graft System with Transport Delivery System
|
|
|
- Elmer Underwood
- 8 years ago
- Views:
Transcription
1 RELAY Plus Thoracic Stent-Graft System with Transport Delivery System TABLE OF CONTENTS Section Page DEVICE DESCRIPTION 8 RADIOPAQUE MARKER BANDS 8 RELAY PLUS TRANSPORT DELIVERY SYSTEM 8 INDICATIONS FOR USE 8 CONTRAINDICATIONS 9 WARNINGS AND PRECAUTIONS 9 HOW PRODUCT IS SUPPLIED 10 DEVICE SELECTION 10 CASE PREPLANNING / INDIVIDUALIZATION OF TREATMENT 11 EQUIPMENT REQUIREMENTS 11 THE IMPLANTATION PROCEDURE 12 MAGNETIC RESONANCE SAFETY INFORMATION 15 ADVERSE EVENTS 16 SYMBOLS/DEFINITIONS AND COMPANY ADDRESSES 16
2 DEVICE DESCRIPTION The RELAY Plus Endoluminal Stent-Graft System is an endovascular device intended to treat thoracic aortic pathologies. The RELAY Stent-Graft is comprised of Duralloy, self-expanding nitinol, stents sutured to polyester vascular graft fabric. The stent scaffold is a series of serpentine springs stacked in a tubular configuration. These stents are spaced along the length of the graft fabric. Longitudinal support for the stentgraft is provided by a curved nitinol wire called Spiral Support Strut. Additionally, radiopaque markers are placed on the stent-graft to aid visualization and accurate placement. RELAY Stent-Graft end configuration: One proximal end configuration is available: The SAFEX end configuration (Fig 1) consists of uncovered, sinusoidal nitinol wires of varying heights; circumferentially projecting above the fabric end of the graft. These uncovered wires when in position, expand to the vessel wall anchoring the device in place and aid in creating a seal zone oriented to the vasculature. Secondly, this design is placed across vessels maintaining patency, i.e. left subclavian/ left common carotid arteries, and increasing the landing zone area. One distal configuration is also available. The STRAIGHT end configuration (Fig 1) consists of fabric covering the nitinol springs evenly about the circumference of the stent-graft. This configuration is present distally on all Stent-Grafts Proximal Marker 2. Spiral Support Strut Marker 3. Spiral Support Strut 4. Distal Marker 5. Safex Stent 6. Safex End Configuration 7. Freeflex 8. Straight End Configuration RADIOPAQUE MARKER BANDS All stent stent-grafts will have platinum/iridium radiopaque marker bands (See Fig 1) which will indicate the fabric edge and will also serve as guides for positioning the spiral support strut. RELAY PLUS TRANSPORT DELIVERY SYSTEM The RELAY Plus Transport delivery system consists of a series of coaxially arranged sheaths and catheters, along with a tubular handle control system. The delivery mechanism consists of two stages. The first stage consists of a hydrophilically coated introducer (Outer Primary Sheath), which is used to advance and track over a guidewire. The tip of the Outer Primary Sheath contains a preformed curve. Within the first stage is the second stage. The second stage is a flexible sheath in which the stent-graft is compressed. The flexibility of the second stage permits tracking through tortuous and curved portions of the thoracic aorta. The stent-graft is self-expandable and the delivery system is withdrawn after deployment. INDICATIONS FOR USE The RELAY stent-graft is intended for the treatment of thoracic aortic pathologies such as aneurysms, pseudoaneurysms, dissections, penetrating ulcers and intramural hematoma, in adult patients (as defined by local statutes). Anatomical/Sizing features specified in Tables 1, 2 and 3 should be observed. 8
3 Delivery System Tip 2. Apex Holder 3. Inner Secondary Sheath 4. Outer Primary Sheath 5. Radiopaque Marker 6. Stationary Grip 7. Shipping Retainer 8. Main Body 9. Flush Port/Stopcock 10. Deployment Grip 11. Controller 12. Stainless Steel Rod 13. Apex Release Retainer 14. Apex Release Grip 15. Guidewire Luer 16. Flushport 17. Handle Body Mark CONTRAINDICATIONS OF USE FOR THE RELAY PLUS SYSTEM The RELAY Plus System is contraindicated when patients present with any of the following characteristics/ conditions: Pregnancy/lactation. Aneurysm/lesion location not accessible to the delivery system and stent placement. Arterial access size insufficient for delivery system entrance. Treatment of lesion that would require a delivery system with usable length greater than 90 cm. Excessive arterial disease precluding delivery system entrance or passage. Untreatable allergy or history of allergic reaction to radiographic contrast media (x-ray dye). Untreatable allergy or history of allergic reaction to anticoagulants. Systemic infection. Arterial tortuosity not allowing passage of the delivery system. Arterial or aneurysm/lesion size incompatible with stent graft. Has congenital connective tissue disease rendering the aneurysm/lesion untreatable. Mycotic aneurysm/lesions. Aortic inner diameter that cannot accommodate the expanded Inner Secondary Sheath outer diameter of approximately 12mm. Thoracoabdominal aneurysms. Hypersensitivity to polyester or nitinol. Massive thrombus. Bleeding diathesis. WARNINGS AND PRECAUTIONS Placement of stent grafts in the thoracic aorta often requires proximity to the great vessels perfusing the brain increasing the possibility of thrombus or embolization proximally. Care should be taken to ensure air has been purged from the system. Proximal and distal required landing zones vary with stent-graft size (See Table 2). Excessive aortic tortuosity may result in not being able to properly position the stent graft or stent graft kinking with thrombus formation. If balloon modeling is desired, use a compliant balloon equal in size to the largest diameter stent graft used. Balloon inflation should not exceed 1 atm. Do not use power/pressure injections through the Transport delivery system. Care should be taken with respect to occlusion of dominant intercostals/spinal cord arteries. Care should be taken when treating morbidly obese patients as visualization of imaging may be compromised. Careful consideration should be given to treating patients where tracking through a previously placed endovascular or surgical prosthesis is required. Given the contraindication for pregnant or lactating women, care should be taken when treating women of childbearing potential. Consideration should be given to treating patients with significant comorbidities/high risk for open surgical repair. * In selected circumstances, the benefits of this procedure may outweigh possible risks. 9
4 HOW PRODUCT IS SUPPLIED The RELAY Plus Thoracic Stent-Graft System with Transport delivery system is provided STERILE. DO NOT RESTERILIZE-SINGLE USE ONLY. Re-sterilization of the device for re-use will result in loss of component integrity (e.g., reduction of stent-graft radial force, component cracking or discoloration, etc.). Do not reuse, reprocess, clean/disinfect, or re-sterilize. Reuse, reprocessing, cleaning/disinfection, or resterilization may compromise the structural integrity of the device and/or lead to device failure which, in turn, may result in deterioration of health or death of patients or users. Reuse, reprocessing or re-sterilization may also create a risk of contamination of the device and/or cause patient infection or cross-infection, including, but not limited to the transmission of infections disease(s) from one patient to another. Contamination of the device may lead to injury, illness or death of the patient end-user. Also, each single-use device carries specific labeling instructions relative to storage, use and handling to minimize exposure to conditions, which could compromise the product, the patient or the user. These conditions cannot be assured once the packaging is opened and discarded. The RELAY endoluminal straight thoracic stent-grafts are available in 4 approximate lengths: 100 mm 150 mm 200 mm 0 mm The straight grafts are available in 2 mm incremented diameters ranging from 22 mm to 46 mm. Tapered stent-grafts are available with proximal diameters ranging from 28 mm to 46 mm, decreasing incrementally by 4 mm over the length of the stent-graft. The product is supplied with the following model designation identified on the label. DEVICE SELECTION Tables 1 & 3 address the selection of the appropriate stent-graft diameters for the RELAY : Table 2 addresses the recommended healthy landing zone length depending on stent-graft diameter selected. STRAIGHT STENT-GRAFTS (Table 1.) *Special order 10
5 ENG TARGET LANDING ZONE (Table 2.) Stent-graft Diameter Proximal Length mm mm mm 15 mm 20 mm mm TAPERED STENT-GRAFTS (Table 3.) Stent-graft Diameter mm mm Distal Length 15 mm mm Fever Thoracic Vessel Percent of Stent- Transient ischemic Attack Outer Primary Sheath Thoracic Stent-Graft Covered Length Stent-Graft Total Length Size mm Graft Oversize (%) French Size (O.D.) Taper Stent- Post Implantation Syndrome Graft size 150 mm 200 mm Cerebral 0 mm 150 vascular mm 200 accident mm 0 mm (stroke) 150 mm 200 mm 0 mm (mm) Proximal Distal Proximal Distal Stent- Stent- Stent- Stent- Stent- Stent- Stent- Stent- Stent- Hematoma Graft Graft Congestive Graft Graft heart Graft failure Graft Graft Graft Graft 28 Blood x Loss % 14-20% 155 mm 195 mm Paralysis/Paresthesia/Paraparesis 0 mm 170 mm 210 mm 5 mm 22 Fr 22 Fr 23 Fr 30 Hemorrhage x % 13-18% 155 mm 200 mm Death 0 mm 171 mm 216 mm 6 mm 22 Fr 23 Fr 23 Fr 32 Infection x % 12-17% 155 mm 200 mm Incision 0 mm Site 172 mm Complications 217 mm 7 mm 22 Fr 23 Fr Fr 34 Cardiac x 30 events % 11-15% 145 mm 200 mm Limb 0 mm ischemia 162 mm 217 mm 7 mm 23 Fr Fr Fr 36 x Anaphylaxsis % 10-14% 145 mm 190 mm 0 mm 163 mm 208 mm 8 mm Delivery system failure Fr Fr Fr 38 x Vessel Dissection 11% 9-13% 145 mm 190 mm 0 mm 164 mm 209 mm 9 mm Access failure Fr Fr Fr 40 x % Vessel Occlusion/Thrombosis 9-12% 145 mm 195 mm 0 mm 165 mm 215 mm 270 mm Stent graft misplacement Fr Fr Fr 42 x % 11% 150 mm 195 mm 0 mm 170 mm 215 mm 270 mm Fr Fr Fr Vessel Damage Endoleak 44 x % 11-14% 155 mm 200 mm 0 mm 176 mm 221 mm 271 mm Fr Fr Fr Emboli 46 x % 10-13% 155 mm Stent graft migration 200 mm 0 mm 176 mm 221 mm 271 mm Fr Fr Fr Hepatic failure Stent graft failure Ischemia (spinal cord, perfusion pathways) Wire form fractures CASE-PREPLANNING/INDIVIDUALIZATION OF TREATMENT Renal failure or Complications Suture fracture Practitioners Aneurysm using / Lesion the Rupture RELAY Plus Transport delivery Deployment device should failure have a thorough understanding of endovascular procedures and techniques. In particular, the RELAY Plus device should only be used by Arteriovenous fistula / aorto-esophageal fistula Perforation physicians and teams with experience and training in vascular interventional techniques, including, but not limited Radiation to, training overexposure on the use or of reaction the RELAY Plus Transport Device delivery dehiscence system. This will include practitioners with Pseudoaneurysm formal education/training in vascular surgery, interventional Stent-graft tearing/wear radiology, cardiothoracic surgery and interventional cardiology. Selecting the proper stent-graft with the appropriate length and diameter is paramount to the successful Product Device Design Stent Stent Stent Delivery Delivery exclusion of the indicated thoracic aortic pathologies. Measure all parameters needed for proper sizing ID Type Modification Diameter Covered Diameter System System Device usable Designation of the stent-graft carefully. Number Bolton Proximal Medical recommends Length evaluation Distal French of all Size imaging Length studies available, i.e., angiograms, CT scans, MRI scans, MRA scans and plain radiographs. Each imaging modality S: Standard offers 28 M: Main 3 XX XXX XX XX XX additional information to the sizing process. The physical characteristics of the vessel should Catalog be evaluated Product in addition to its size. Factors such as stenosis, atherosclerotic disease, ectasia and tortuosity may affect stent-graft selection and placement strategy. The final stent-graft selection will be the responsibility of the physician. Thoracic Thoracic Vessel Percent of Stent- Stent-graft Covered Length Outer Primary Sheath Taper Size (mm) Graft Oversize (%) French Size (O.D.) Practitioner must ensure that the access vessel diameter is compatible with the selected delivery system s Stent- Outer Primary Proximal Sheath Distal French Proximal size. The Distal physician 150mm and patient 200mm (and/or 0mm family) 150mm should review 200mm the risks 0mm and Graft Size benefits when discussing this endovascular device Stent- and the need Stent- for compliance Stent- with Stent- follow Stent- ups. Any pertinent Stent- (mm) Graft Graft Graft Graft Graft Graft actions to be avoided or precautions to be taken should also be discussed. (FR) (FR) (FR) 28x x EQUIPMENT REQUIREMENTS x Fluoroscopic 34x equipment -27 including 9-13 a high resolution 154 image intensifier 209 on 9 a freely angled 23 C-arm which can be ceiling or pedestal mounted or portable will be needed for the procedure. It is desirable if the image intensifier 36x has a complete range of motion to achieve AP projections to lateral projections. Its capabilities should include: 38x x Digital Subtraction Angiography 42x High resolution angiography 44x Roadmapping 46x
6 Supportive/supplementary equipment:.035 (0,89 mm)/300 cm Meier guidewire.035 (0,89 mm)/0 cm or 300 cm Lunderquist guidewire Guidewire torque device Inflation device with pressure gauge Aortic occlusion balloons Compliant stent graft modeling balloons of the appropriate size Arterial puncture needles 18G or 19G Nitinol goose neck snare (10-15 mm diameter) Assortment of vascular stents Assortment of angiographic and graduated pigtail catheters Anticoagulation and antiplatelet therapies are performed at the discretion of the physician. Similarly, blood pressure adjustment and spinal cord protection measures are also at the discretion of the physician. PREPARATION (Steps 1 through 7) THE IMPLANTATION PROCEDURE Position the patient on the surgical table where standard aseptic preparation of the surgical site is conducted. (Ensure that a compliant stent graft modeling balloon of the appropriate size is available if needed. Do not exceed 1 atm pressure with balloon). If a radiopaque ruler is to be used, place it underneath the patient at this time. Drape the patient with sterile surgical drapes leaving exposed the bilateral groin access sites. 1. Verify devices are correct for the patient. 2. Inspect the system packaging for visible tears, breaks or openings. 3. Take the Transport delivery system from the sterile packaging and bring it to the surgical table. Examine the Transport delivery system for structural integrity. DO NOT USE the system if defects are noted. 4. Perform a percutaneous needle puncture of the contralateral common femoral artery. Using the Seldinger technique, place a guidewire well into the abdominal aorta. Remove the needle and place a vascular introducer over the guidewire into the artery. Advance a 5FR (1,7mm) pigtail angiographic catheter over the guidewire to the level of the aortic arch. Remove the guidewire. Perform an arteriotomy of the ipsilateral common femoral artery using umbilical ties or surgical vessel loops for hemostatic control. Introduce a.035 (0,89mm) guidewire into the artery and advance it to the aortic arch. NOTE: Ensure that the controller in the 1 position, if it is not change it to the 1 position (aligned with the arrow in Fig 3). To change position, push Controller toward the Main Body Handle and rotate to desired position, then release. Check the distal end of the delivery system to ensure that the Delivery System Tip is properly seated in the Outer Primary Sheath (Fig. 4). If not, correct by moving the Deployment Grip until the Delivery System Tip is properly seated as shown in Fig. 4. Ensure that the tip side hole is not covered by the Outer Primary Sheath (Fig. 4). 5. Keep the controller in the 1 position to prevent premature deployment of the stent graft. Check that the Shipping Retainer covers the delivery system Main Body. The Shipping Retainer aids in preventing premature release of the stent graft from the outer sheath. WARNING: Do not remove the Shipping Retainer until the inner secondary sheath is to be advanced out of the outer primary sheath!!! 12
7 6. Remove silicone tubing from the Flush Port (Fig. 5a). Flush the delivery system with heparinized saline through the Flush Port (Fig 5b) to purge air from the coaxially situated sheaths. Ensure that a continuous stream of saline exits the tip side hole (Fig 4). It may be necessary to elevate the distal end of the system to different positions to bring air to the highest point for purging. The flush port valve must be closed under pressure to prevent air from re-entering the system. Visually inspect it for remaining air and repeat if necessary. Then flush through the guidewire luer and Flush Port extension tubing (Fig 5c). Remove extension tubing after flushing. 7. Important: Activate the hydrophilic coating by wetting the Tip and Introducer Sheath with saline. INTRODUCTION/ADVANCEMENT (Steps 8 through 17) 8. While holding and directing the introducer sheath with one hand and holding the distal handle grip with the other hand, advance the introducer sheath into the artery over the guidewire. The guidewire should always remain in the delivery system while inside the patient. 9. Under fluoroscopic control, advance the Outer Primary Sheath until the Delivery System Tip is just below the intended distal landing zone. If the aorta presents tight tortuosity, the tip should be advanced past the tight curvature(s) of the descending aorta to facilitate navigation of the secondary stage/inner sheath. Do not advance the outer primary sheath into the thoracic arch. If the Outer Primary Sheath cannot be advanced beyond the region of tight curvatures the delivery system should be removed from the patient and an alternate procedure be considered. 10. To advance the secondary inner sheath out of the outer primary sheath, remove the Shipping Retainer from the main body by grasping the fabric tab and pulling it away from the handle body (Fig. 5d). Once the secondary inner sheath is advanced, the user will be committed to implant the graft. CAUTION: The Controller must be in the 1 position. 11. While holding the black Stationary Grip so that the Main Body remains stationary, push the gray Deployment Grip forward (towards the Stationary Grip) until the stent-graft s proximal markers reach the proximal landing zone. Verify that the gray Deployment Grip has reached or passed the black Handle Body mark on the main handle body (Fig. 6b). This will ensure that the Inner Secondary Sheath has completely exited the Outer Primary Sheath (Fig 6a). Also, the distal stent marker bands will be seen approximately 2cm out of Primary Outer Sheath. If the gray Deployment Grip has not reached or passed the black Handle Body mark while the controller is still in position 1, hold the gray Deployment Grip stationary while pulling the Stationary Grip distally until the gray Deployment Grip reaches or passes the black Handle Body mark, this will ensure that the secondary inner sheath is fully out of the outer sheath. 12. As the Inner Secondary Sheath is advanced out of the Outer Primary Sheath, note the alignment of the Spiral Support Strut by locating the Spiral Support Strut markers under fluoroscopy. 13. Place the C-Arm DSA system into a left anterior oblique position in preparation for the initial angiogram. Check the patient image for the possibility of image corruption such as parallax or image distortion by the fluoroscopic x-ray beam divergence. The central ray should be perpendicular to the area of interest. If the device is to be implanted in a curved section of the aorta, verify that the D-shaped marker on the inner secondary sheath and the Spiral Support Strut marker(s) face the greatest curvature. If radial adjustment is needed, retract the deployment grip to bring the stent-graft to a straight portion of the vessel. When retracting the gray deployment grip, ensure that the distal end of the stent-graft is not pulled into the outer primary sheath (the black Handle Body mark can be used as a reference). It may be necessary to withdraw the whole device a few centimeters to bring the stent-graft to a straight position. 13
8 After the stent-graft is in the straight position, while holding the stationary grip, rotate the gray deployment grip to manually align the Spiral Support Strut markers toward the greatest curvature of the aorta. The D-shaped marker can be used to aid in this placement (Fig 7). If the round portion of the D-shaped marker is facing the greater curvature, the gray deployment grip should be turned clockwise. If the round portion is facing the lesser curvature the turn should be counterclockwise. One to three handle revolutions maybe required before the stent-graft begins rotating. 14. Perform an angiogram of the area of interest and mark the target area. 15. Ensure that the surgical table and patient are locked into position. 16. Finalize the longitudinal placement of the stent-graft in relation to the proximal landing zone by adjusting the gray deployment grip as necessary. Confirm the position of the proximal and distal marker bands as well as the Spiral Support Strut markers. If the gray Deployment Grip reaches its maximum travel before the stent graft reaches the landing zone, the whole device should be advanced. Before advancing the whole device, the gray Deployment Grip should be retracted so as to re-capture the distal stent within the outer primary sheath. At this point, the entire delivery system is advanced as needed. Since the distal stent is captured within the outer primary sheath, the gray deployment grip should be advanced once more until the stent graft reaches the proximal landing zone and the distal stent is out of the outer primary sheath. Ensure the gray deployment grip has reached or passed the black Handle Body mark. DEPLOYMENT (Steps 18 through 20) 17. With the stent graft in the desired deployment position, turn the Controller to the 2 position (Fig. 8). CAUTION: Verify that the Controller is in the 2 position during steps 18 through While holding the Stationary Grip fixed, retract the Deployment Grip (Fig 9a) by pulling down the Inner Secondary Sheath and exposing the bare stent and the first covered stent. NOTE: The Inner Secondary Sheath has the D-Shaped radiopaque marker (See Fig 2, Transport drawing) located at its distal end that can be used to visualize its movement under fluoroscopy. 19. Make any final linear position adjustments (proximally or distally) by first changing the controller to position 1. Then using the gray Deployment Grip, move the stent graft proximally or distally to the desired location. After the stent graft is in the desired location, move the controller setting back to position 2. RELEASE (Steps 20 through 23) 20. To release and deploy the stent graft, completely retract the Inner Secondary Sheath by holding the Stationary Grip fixed and retracting the deployment grip with one continuous motion without stopping until the stent graft is fully deployed (Fig 9b). CAUTION: Failure to promptly deploy the stent graft will cause blood pressure to increase and may result in distal migration of the device during deployment. 21. Maintain attachment of the proximal end of the stent graft by the Apex Holder. To release the stent graft from the Apex Holder, go to the thumbscrew on the Apex Release Retainer. Loosen the thumbscrew by rotating counterclockwise 2-3 turns (Fig 10). 22. Lift and remove the Apex Release Retainer. 23. Under fluoroscopic control, release the Safex stent (bare spring apexes) by pulling the Apex Release Grip (Number 3, Fig 11) towards the Guidewire Luer until it reaches the end of the Stainless Steel Rod. The stent graft is now in final position (Fig 11). 14
9 CONCLUSION AND REMOVAL (Steps through 31) ENG. Place the Controller in the 4 position.. Retract the Stainless Steel Rod by pulling it completely distal, allowing the tip to rejoin the outer sheath (Fig 12). CAUTION: Perform this step carefully and under fluoroscopic control, monitoring the travel of the Delivery System Tip through the deployed stent-graft so that stent graft s position is not affected. If the tip does not reseat easily, apply slightly greater force until the tip seats properly.. Withdraw the entire system from the patient. 27. Perform a final angiogram to assess for any endoleaks and/or migration. Confirm successful aneurysm/ lesion exclusion. 28. If an endoleak is detected, consider balloon modeling to correct the leak. CAUTION: Do not exceed 1 atm. Balloon pressure. Always recheck position of stent graft following ballooning. 29. Straighten the angiographic pigtail catheter and remove the catheter and sheath from the percutaneous puncture site. 30. Perform standard surgical closure of the arteriotomy site. 31. Assess blood flow to the distal extremities. MAGNETIC RESONANCE SAFETY INFORMATION The RELAY stent-graft was determined to be MR-conditional. Specifically, when present in a patient undergoing a magnetic resonance imaging (MRI) procedure at 3-Tesla or less will not create an additional hazard or risk to the patient under the conditions used for the testing. Magnetic resonance imaging (MRI) procedures must be performed according to the following guidelines: A patient with the RELAY stent-graft may safely undergo an MRI procedure using an MR system with a static magnetic field of 3-Tesla or less. The RELAY stent-graft system does not exhibit magnetic field interactions with respect to translational attraction (tested at a maximum spatial gradient, 3.3-Tesla/ meter) and showed no torque during exposure to a 3-Tesla MR system. Therefore, there is no additional risk to a patient with the RELAY stent-graft with respect to movement or dislodgement using an MR system with a static magnetic field of 3-Tesla or less. In addition, because of the lack of magnetic field interactions at 3-Tesla, an MRI procedure may be performed on a patient immediately after implantation of the RELAY stent-graft. MRI procedures must not exceed exposures to radiofrequency (RF) fields greater than a whole-bodyaveraged specific absorption rate (SAR) of 2.0-W/kg for 15 minutes at 3-Tesla in a patient with the RELAY stent-graft. Safety information for magnetic resonance imaging (MRI) procedures pertains to the use of MR systems with static magnetic fields of 3-Tesla or less (maximum spatial gradient 3.3-Tesla/m) and a whole body averaged specific absorption rate (SAR) of 2.0W/kg for 15 minutes of MR imaging. The effects of performing MRI procedures using MR systems with static magnetic fields greater than 3-Tesla and other conditions have not been determined. 15
10 Stent-graft Diameter Proximal Length Stent-graft Diameter Distal Length mm 15 mm mm 15 mm mm 20 mm ADVERSE EVENTS40-46 mm mm mm mm Adverse events that may occur in conjunction with endovascular procedures include, but are not limited to those listed in the following section. Fever Post Implantation Syndrome Hematoma Blood Loss Hemorrhage Infection Cardiac events Anaphylaxsis Vessel Dissection Vessel Occlusion/Thrombosis Vessel Damage Emboli Hepatic failure Ischemia (spinal cord, perfusion pathways) Renal failure or Complications Aneurysm / Lesion Rupture Arteriovenous fistula / aorto-esophageal fistula Radiation overexposure or reaction Pseudoaneurysm Transient ischemic Attack Cerebral vascular accident (stroke) Congestive heart failure Paralysis/Paresthesia/Paraparesis Death Incision Site Complications Limb ischemia Delivery system failure Access failure Stent graft misplacement Endoleak Stent graft migration Stent graft failure Wire form fractures Suture fracture Deployment failure Perforation Device dehiscence Stent-graft tearing/wear - In the event of surgical removal or post-mortem examination, please contact Bolton Medical for guidance on removal and disposal of implant. Product Device Design Stent Stent Stent Delivery Delivery ID Type Modification Diameter Covered Diameter System System Device SYMBOLS/DEFINITIONS usable Designation Number Proximal Length Distal French Size Length S: Standard 28Do not M: Main Re-use 3 XX XXX Date of XXManufacture XX Catalog Product Do not Re-Sterilize Manufacturer Thoracic Thoracic Vessel Percent of Stent- Stent-graft Covered Length Taper Model/Catalogue Size (mm) Number Graft Oversize (%) Lot Number Stent- Proximal Distal Proximal Distal 150mm 200mm 0mm 150mm Graft Size Use By Stent- Store Stent- in a cool, Stent- Stent- (mm) Graftdry place Graft Graft Graft (FR) 28x Caution Do not 204 use if package x is damaged x28 MR Conditional x Sterilized 209 by Irradiation x32 Consult Instructions x34for use x MANUFACTURER: 42x LOCATION: BOLTON 44x40 MEDICAL ESPAÑA, S.L.U BOLTON 209MEDICAL, 9 INC C/NEWTON, 46x INTERNATIONAL PARKWAY SANT ESTEVE SESROVIRES SUNRISE, FLORIDA 333 USA BARCELONA, SPAIN TELEPHONE: TELEPHONE: FACSIMILE: FACSIMILE: Outer Primary Sheath French Size (O.D.) 200mm Stent- Graft (FR) mm Stent- Graft (FR)
Instructions for Use. Device Description The AMPLATZER Vascular Plug II is a self-expandable nitinol mesh occlusion device (see Figure 1).
 Vascular Plug II Instructions for Use Device Description The AMPLATZER Vascular Plug II is a self-expandable nitinol mesh occlusion device (see Figure 1). A B Figure 1. AMPLATZER Vascular Plug II A. Nitinol
Vascular Plug II Instructions for Use Device Description The AMPLATZER Vascular Plug II is a self-expandable nitinol mesh occlusion device (see Figure 1). A B Figure 1. AMPLATZER Vascular Plug II A. Nitinol
LifeStent XL Biliary Stent System
 B05688 Rev.2/07-13 LifeStent XL Biliary Stent System Recommended Guidewire Length Table Catheter Working Length Recommended Guidewire Length 130 cm 300 cm 80 cm 260 cm 130 cm & 80 cm 160 cm & 110 cm Figure
B05688 Rev.2/07-13 LifeStent XL Biliary Stent System Recommended Guidewire Length Table Catheter Working Length Recommended Guidewire Length 130 cm 300 cm 80 cm 260 cm 130 cm & 80 cm 160 cm & 110 cm Figure
Imaging of Thoracic Endovascular Stent-Grafts
 Imaging of Thoracic Endovascular Stent-Grafts Tariq Hameed, M.D. Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, Indiana Disclosures: No relevant financial
Imaging of Thoracic Endovascular Stent-Grafts Tariq Hameed, M.D. Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, Indiana Disclosures: No relevant financial
Talent Thoracic Stent Graft with THE Xcelerant Delivery System. Expanding the Indications for TEVAR
 Talent Thoracic with THE Xcelerant Delivery System Expanding the Indications for TEVAR Talent Thoracic Precise placement 1 Broad patient applicability 1 Excellent clinical outcomes 1, a + Xcelerant Delivery
Talent Thoracic with THE Xcelerant Delivery System Expanding the Indications for TEVAR Talent Thoracic Precise placement 1 Broad patient applicability 1 Excellent clinical outcomes 1, a + Xcelerant Delivery
Bard LifeStar Biliary Stent System
 Bard LifeStar Biliary Stent System Instructions For Use (IFU) Bard LifeStar Biliary Stent System Delivery System Diagram B05686 Vers.1/08-11 1 Information for use Read the Bard LifeStar Biliary Stent System
Bard LifeStar Biliary Stent System Instructions For Use (IFU) Bard LifeStar Biliary Stent System Delivery System Diagram B05686 Vers.1/08-11 1 Information for use Read the Bard LifeStar Biliary Stent System
A Patient s Guide to Minimally Invasive Abdominal Aortic Aneurysm Repair
 A Patient s Guide to Minimally Invasive Abdominal Aortic Aneurysm Repair Table of Contents The AFX Endovascular AAA System............................................ 1 What is an Abdominal Aortic Aneurysm
A Patient s Guide to Minimally Invasive Abdominal Aortic Aneurysm Repair Table of Contents The AFX Endovascular AAA System............................................ 1 What is an Abdominal Aortic Aneurysm
2009/JUL/20 at 9:18 a.m. Doc number: M710950B001 [Uni+] VALIANT THORACIC STENT GRAFT WITH THE CAPTIVIA DELIVERY SYSTEM Instructions for Use
![2009/JUL/20 at 9:18 a.m. Doc number: M710950B001 [Uni+] VALIANT THORACIC STENT GRAFT WITH THE CAPTIVIA DELIVERY SYSTEM Instructions for Use 2009/JUL/20 at 9:18 a.m. Doc number: M710950B001 [Uni+] VALIANT THORACIC STENT GRAFT WITH THE CAPTIVIA DELIVERY SYSTEM Instructions for Use](/thumbs/40/21239668.jpg) VALIANT THORACIC STENT GRAFT WITH THE CAPTIVIA DELIVERY SYSTEM Instructions for Use 0086 EXPLANATION OF SYMBOLS Explanation of symbols that may appear on product labeling. Consult instructions for use
VALIANT THORACIC STENT GRAFT WITH THE CAPTIVIA DELIVERY SYSTEM Instructions for Use 0086 EXPLANATION OF SYMBOLS Explanation of symbols that may appear on product labeling. Consult instructions for use
Patient Information Booklet. Endovascular Stent Grafts: A Treatment for Abdominal Aortic Aneurysms
 Patient Information Booklet Endovascular Stent Grafts: A Treatment for Abdominal Aortic Aneurysms TABLE OF CONTENTS Introduction 1 Glossary 2 Abdominal Aorta 4 Abdominal Aortic Aneurysm 5 Causes 6 Symptoms
Patient Information Booklet Endovascular Stent Grafts: A Treatment for Abdominal Aortic Aneurysms TABLE OF CONTENTS Introduction 1 Glossary 2 Abdominal Aorta 4 Abdominal Aortic Aneurysm 5 Causes 6 Symptoms
Instructions For Use Aorfix AAA Flexible Stent Graft System
 Instructions For Use AAA Flexible Stent Graft System With AorFlex Delivery Device Caution: Federal law (USA) restricts this implant to sale by or on the order of a physician. Page 1 of 62 PN02114 Issue
Instructions For Use AAA Flexible Stent Graft System With AorFlex Delivery Device Caution: Federal law (USA) restricts this implant to sale by or on the order of a physician. Page 1 of 62 PN02114 Issue
Contraindications: Malign or benign strictures in the upper part of esophagus close to the cricopharyngeal muscle.
 Manufactured by: ELLA CS, s.r.o. Milady Horákové 504 500 06 Hradec Králové 6 Czech Republic Phone: +420 49 527 91 11 Fax: +420 49 526 56 55 E-mail: volenec@ellacs.cz Instructions for Use FerX-ELLA Esophageal
Manufactured by: ELLA CS, s.r.o. Milady Horákové 504 500 06 Hradec Králové 6 Czech Republic Phone: +420 49 527 91 11 Fax: +420 49 526 56 55 E-mail: volenec@ellacs.cz Instructions for Use FerX-ELLA Esophageal
Endovascular Repair of an Axillary Artery Aneurysm: A Novel Approach
 Endovascular Repair of an Axillary Artery Aneurysm: A Novel Approach Bao- Thuy D. Hoang, MD 1, Jonathan- Hien Vu, MD 2, Jerry Matteo, MD 3 1 Department of Surgery, University of Florida College of Medicine,
Endovascular Repair of an Axillary Artery Aneurysm: A Novel Approach Bao- Thuy D. Hoang, MD 1, Jonathan- Hien Vu, MD 2, Jerry Matteo, MD 3 1 Department of Surgery, University of Florida College of Medicine,
Wingspan Stent System with Gateway PTA Balloon Catheter
 Wingspan System with Gateway PTA Balloon Catheter Directions for Use AUS Australian Sponsor Address Boston Scientific (Australia) Pty Ltd PO Box 332 BOTANY NSW 1455 Australia Free Phone 1800 676 133 Free
Wingspan System with Gateway PTA Balloon Catheter Directions for Use AUS Australian Sponsor Address Boston Scientific (Australia) Pty Ltd PO Box 332 BOTANY NSW 1455 Australia Free Phone 1800 676 133 Free
Understanding your Renal Stent Procedure. A patient Guide (COVER PAGE) TABLE OF CONTENTS (inside front page)
 Understanding your Renal Stent Procedure. A patient Guide (COVER PAGE) TABLE OF CONTENTS (inside front page) The Kidney and the Renal Arteries... 1 Renal Artery Disease... 2 Diagnosis of Renal.Artery Disease...
Understanding your Renal Stent Procedure. A patient Guide (COVER PAGE) TABLE OF CONTENTS (inside front page) The Kidney and the Renal Arteries... 1 Renal Artery Disease... 2 Diagnosis of Renal.Artery Disease...
REPORTING STENT PLACEMENT FOR NONOCCLUSIVE VASCULAR DISEASE IN LOWER EXTREMITIES
 REPORTING STENT PLACEMENT FOR NONOCCLUSIVE VASCULAR DISEASE IN LOWER EXTREMITIES Effective January 1, 2015, there was a change in CPT that affects reporting specific endovascular services provided in the
REPORTING STENT PLACEMENT FOR NONOCCLUSIVE VASCULAR DISEASE IN LOWER EXTREMITIES Effective January 1, 2015, there was a change in CPT that affects reporting specific endovascular services provided in the
Allium Ureteral Stent (URS)
 Allium Ureteral Stent (URS) Instructions For Use Manufactured by Allium Ltd. DEVICE NAME: ALLIUM Ureteral Stent (URS) is intended to be inserted into the lower ureter to allow free flow of urine from the
Allium Ureteral Stent (URS) Instructions For Use Manufactured by Allium Ltd. DEVICE NAME: ALLIUM Ureteral Stent (URS) is intended to be inserted into the lower ureter to allow free flow of urine from the
PATIENT INFORMATION BOOKLET
 PATIENT INFORMATION BOOKLET Wingspan Stent System with Gateway PTA Balloon Catheter TABLE OF CONTENTS Definitions... 2 What is the Purpose of This Booklet?... 3 What is an Intracranial Lesion?... 3 Who
PATIENT INFORMATION BOOKLET Wingspan Stent System with Gateway PTA Balloon Catheter TABLE OF CONTENTS Definitions... 2 What is the Purpose of This Booklet?... 3 What is an Intracranial Lesion?... 3 Who
RADIOLOGY 2014 CPT Codes
 RADIOLOGY 2014 CPT Codes Radiology 2014 CPT Codes CMS has issued 36 new procedure codes (one is a radiation therapy code) for CY 2014 that directly pertain to radiology with 26 of those codes the result
RADIOLOGY 2014 CPT Codes Radiology 2014 CPT Codes CMS has issued 36 new procedure codes (one is a radiation therapy code) for CY 2014 that directly pertain to radiology with 26 of those codes the result
AERO. Tracheobronchial Stent Technology System
 AERO Tracheobronchial Stent Technology System Review Instructions For Use Before Using This System. Single Use Non-sterile MR Conditional CONTENTS Instructions For Use.............................. 3 IMPORTANT
AERO Tracheobronchial Stent Technology System Review Instructions For Use Before Using This System. Single Use Non-sterile MR Conditional CONTENTS Instructions For Use.............................. 3 IMPORTANT
Cook Celect and Günther Tulip Vena Cava Filter Patient Guide
 Cook Celect and Günther Tulip Vena Cava Filter Patient Guide www.cookmedical.com Filter patient guide Contents Pulmonary embolism 4 5 What is pulmonary embolism? What causes it? What are the symptoms of
Cook Celect and Günther Tulip Vena Cava Filter Patient Guide www.cookmedical.com Filter patient guide Contents Pulmonary embolism 4 5 What is pulmonary embolism? What causes it? What are the symptoms of
Aspira* Peritoneal Drainage Catheter
 Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Aspira* Peritoneal Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Peritoneal Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid
Local Coverage Article: Endovascular Repair of Aortic Aneurysms (A53124)
 Local Coverage Article: Endovascular Repair of Aortic Aneurysms (A53124) Contractor Information Contractor Name Novitas Solutions, Inc. Article Information General Information Article ID A53124 Original
Local Coverage Article: Endovascular Repair of Aortic Aneurysms (A53124) Contractor Information Contractor Name Novitas Solutions, Inc. Article Information General Information Article ID A53124 Original
PERCUTANEOUS PD CATHETER IMPLANTATION SYSTEM
 Place on Patient s Cranial Border of the Pubic Symphysis IMPLANTATION STENCIL Classic Exit Cuff Site PERCUTANEOUS PD CATHETER IMPLANTATION SYSTEM INSTRUCTIONS FOR USE VP 511 and VP-511M Implantation System
Place on Patient s Cranial Border of the Pubic Symphysis IMPLANTATION STENCIL Classic Exit Cuff Site PERCUTANEOUS PD CATHETER IMPLANTATION SYSTEM INSTRUCTIONS FOR USE VP 511 and VP-511M Implantation System
When Procedural Support really matters. Navien TM A+ Intracranial Support Catheter
 When Procedural Support really matters Navien TM A+ Intracranial Support Catheter Coils / Liquid Embolics DURABLE ovalization resistance, Maintains patent lumen in tortuosity for smooth micro catheter
When Procedural Support really matters Navien TM A+ Intracranial Support Catheter Coils / Liquid Embolics DURABLE ovalization resistance, Maintains patent lumen in tortuosity for smooth micro catheter
XIENCE V and Magnetic Resonance Imaging Examination
 XIENCE V and Magnetic Resonance Imaging Examination To Whom it May Concern: Thank you for your inquiry regarding magnetic resonance imaging (MRI) examinations of patients implanted with the Abbott Vascular
XIENCE V and Magnetic Resonance Imaging Examination To Whom it May Concern: Thank you for your inquiry regarding magnetic resonance imaging (MRI) examinations of patients implanted with the Abbott Vascular
Ultrasound in Vascular Surgery. Torbjørn Dahl
 Ultrasound in Vascular Surgery Torbjørn Dahl 1 The field of vascular surgery Veins dilatation and obstruction (varicose veins and valve dysfunction) Arteries dilatation and narrowing (aneurysms and atherosclerosis)
Ultrasound in Vascular Surgery Torbjørn Dahl 1 The field of vascular surgery Veins dilatation and obstruction (varicose veins and valve dysfunction) Arteries dilatation and narrowing (aneurysms and atherosclerosis)
Bard LifeStar Vascular Stent System
 Bard LifeStar Vascular Stent System Instructions For Use (IFU) Bard LifeStar Vascular Stent System Delivery System Diagram B05685 Vers.1/08-11 1 Instructions for use Read the Bard LifeStar Vascular Stent
Bard LifeStar Vascular Stent System Instructions For Use (IFU) Bard LifeStar Vascular Stent System Delivery System Diagram B05685 Vers.1/08-11 1 Instructions for use Read the Bard LifeStar Vascular Stent
Complications of Femoral Catheterization. Daniel Kaufman, MD University Hospital of Brooklyn December 16, 2005
 Complications of Femoral Catheterization Daniel Kaufman, MD University Hospital of Brooklyn December 16, 2005 Case Presentation xx yr old female presents with fever, chills, and painful swelling of R groin
Complications of Femoral Catheterization Daniel Kaufman, MD University Hospital of Brooklyn December 16, 2005 Case Presentation xx yr old female presents with fever, chills, and painful swelling of R groin
Achilles Tendon Repair, Operative Technique
 *smith&nephew ANKLE TECHNIQUE GUIDE Achilles Tendon Repair, Operative Technique Prepared in Consultation with: C. Niek van Dijk, MD, PhD KNEE HIP SHOULDER EXTREMITIES Achilles Tendon Repair, Operative
*smith&nephew ANKLE TECHNIQUE GUIDE Achilles Tendon Repair, Operative Technique Prepared in Consultation with: C. Niek van Dijk, MD, PhD KNEE HIP SHOULDER EXTREMITIES Achilles Tendon Repair, Operative
ON-Q * Catheters and Introducers
 Instructions For Use ON-Q * Catheters and Introducers MANUFACTURED BY: Kimberly-Clark 1400 Holcomb Bridge Road Roswell, GA 30076 USA Kimberly-Clark N.V. Da Vincilaan 1 1935 Zaventem, Belgium Figure 1 4
Instructions For Use ON-Q * Catheters and Introducers MANUFACTURED BY: Kimberly-Clark 1400 Holcomb Bridge Road Roswell, GA 30076 USA Kimberly-Clark N.V. Da Vincilaan 1 1935 Zaventem, Belgium Figure 1 4
Hemostasis Solutions Boston Scientific is committed to improving patient care in the management of gastrointestinal bleeding.
 Hemostasis Solutions Boston Scientific is committed to improving patient care in the management of gastrointestinal bleeding. Through innovation and continuous educational support, we offer a wide range
Hemostasis Solutions Boston Scientific is committed to improving patient care in the management of gastrointestinal bleeding. Through innovation and continuous educational support, we offer a wide range
Understanding Coronary Artery Disease, Cardiac Catheterization, and Treatment Options. A Guide for Patients
 Understanding Coronary Artery Disease, Cardiac Catheterization, and Treatment Options A Guide for Patients Coronary Artery Disease If you or a member of your family has been diagnosed with coronary artery
Understanding Coronary Artery Disease, Cardiac Catheterization, and Treatment Options A Guide for Patients Coronary Artery Disease If you or a member of your family has been diagnosed with coronary artery
Instructions For Use
 Instructions For Use B05667 Rev.4/05-14 INFORMATION FOR USE Caution: Federal law (U.S.) restricts the use of this device to sale by or on the order of a physician. Device Description The Fluency Plus
Instructions For Use B05667 Rev.4/05-14 INFORMATION FOR USE Caution: Federal law (U.S.) restricts the use of this device to sale by or on the order of a physician. Device Description The Fluency Plus
GRAFTMASTER RX Coronary Stent Graft System PPL2082232 (2/1/13)
 GRAFTMASTER RX Coronary Stent Graft System PPL2082232 (2/1/13) Graphical Symbols for Medical Device Labeling Manufacturer Sterilized using ethylene oxide Catalogue number Batch code French size Outer diameter
GRAFTMASTER RX Coronary Stent Graft System PPL2082232 (2/1/13) Graphical Symbols for Medical Device Labeling Manufacturer Sterilized using ethylene oxide Catalogue number Batch code French size Outer diameter
The treatment of peripheral vascular disease has
 Covered s in Peripheral Vascular Aneurysms and Emergencies Reviewing current covered stent selection and use through case study and discussion. By Alberto Posabella, MD; Raffaele Rosso, MD, FACS, FRCS;
Covered s in Peripheral Vascular Aneurysms and Emergencies Reviewing current covered stent selection and use through case study and discussion. By Alberto Posabella, MD; Raffaele Rosso, MD, FACS, FRCS;
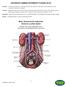
Renovascular Disease. Renal Artery and Arteriosclerosis
 Other names: Renal Artery Stenosis (RAS) Renal Vascular Hypertension (RVH) Renal Artery Aneurysm (RAA) How does the normal kidney work? The blood passes through the kidneys to remove the body s waste.
Other names: Renal Artery Stenosis (RAS) Renal Vascular Hypertension (RVH) Renal Artery Aneurysm (RAA) How does the normal kidney work? The blood passes through the kidneys to remove the body s waste.
Joint Working Group to produce guidance on delivering an Endovascular Aneurysm Repair (EVAR) Service.
 Joint Working Group to produce guidance on delivering an Endovascular Aneurysm Repair (EVAR) Service. Royal College of Radiologists British Society of Interventional Radiology The Vascular Society of Great
Joint Working Group to produce guidance on delivering an Endovascular Aneurysm Repair (EVAR) Service. Royal College of Radiologists British Society of Interventional Radiology The Vascular Society of Great
Note to Teachers about The Virtual Stroke Lab Student Handout
 Note to Teachers about The Virtual Stroke Lab Student Handout This document contains a handout that can be distributed for students to fill out as they complete The Virtual Stroke Lab, a free online Virtual
Note to Teachers about The Virtual Stroke Lab Student Handout This document contains a handout that can be distributed for students to fill out as they complete The Virtual Stroke Lab, a free online Virtual
Overview of Newer Stent Devices for Aneurysm Treatment
 Overview of Newer Stent Devices for Aneurysm Treatment Randall C. Edgell, M.D. Associate Professor Vascular and Interventional Neurology Saint Louis University Disclosure Outcome adjudication for THERAPY,
Overview of Newer Stent Devices for Aneurysm Treatment Randall C. Edgell, M.D. Associate Professor Vascular and Interventional Neurology Saint Louis University Disclosure Outcome adjudication for THERAPY,
What You Should Know About Cerebral Aneurysms
 What You Should Know About Cerebral Aneurysms From the Cerebrovascular Imaging and Interventions Committee of the American Heart Association Cardiovascular Radiology Council Randall T. Higashida, M.D.,
What You Should Know About Cerebral Aneurysms From the Cerebrovascular Imaging and Interventions Committee of the American Heart Association Cardiovascular Radiology Council Randall T. Higashida, M.D.,
STONY BROOK UNIVERSITY HOSPITAL VASCULAR CENTER CREDENTIALING POLICY
 STONY BROOK UNIVERSITY HOSPITAL VASCULAR CENTER CREDENTIALING POLICY Per Medical Board decision March 18, 2008: These credentialing standards do NOT apply to peripheral angiography performed in the context
STONY BROOK UNIVERSITY HOSPITAL VASCULAR CENTER CREDENTIALING POLICY Per Medical Board decision March 18, 2008: These credentialing standards do NOT apply to peripheral angiography performed in the context
BRYAN. Cervical Disc System. Patient Information
 BRYAN Cervical Disc System Patient Information 3 BRYAN Cervical Disc System PATIENT INFORMATION BRYAN Cervical Disc System PATIENT INFORMATION 1 BRYAN Cervical Disc System This patient information brochure
BRYAN Cervical Disc System Patient Information 3 BRYAN Cervical Disc System PATIENT INFORMATION BRYAN Cervical Disc System PATIENT INFORMATION 1 BRYAN Cervical Disc System This patient information brochure
Angioplasty and Stent Education Guide
 Angioplasty and Stent Education Guide Table of Contents Treating coronary artery disease...2 What is coronary artery disease...3 Coronary artery disease treatment options...4 What are coronary artery
Angioplasty and Stent Education Guide Table of Contents Treating coronary artery disease...2 What is coronary artery disease...3 Coronary artery disease treatment options...4 What are coronary artery
Tracheal Stent System
 nt Tracheal Stent System Available sizes: 08mm x 20mm NEW 08mm x 30mm 08mmx 50 mm 08mm x 60mm 08mm x 70mm 08mm x 80mm 08mm x 90mm 10mm x 20mm NEW 10 mm x 30 mm 10 mm x 50 mm 10 mm x 60 mm 10 mm x 70 mm
nt Tracheal Stent System Available sizes: 08mm x 20mm NEW 08mm x 30mm 08mmx 50 mm 08mm x 60mm 08mm x 70mm 08mm x 80mm 08mm x 90mm 10mm x 20mm NEW 10 mm x 30 mm 10 mm x 50 mm 10 mm x 60 mm 10 mm x 70 mm
Minimally Invasive Spine Surgery
 Chapter 1 Minimally Invasive Spine Surgery 1 H.M. Mayer Primum non nocere First do no harm In the long history of surgery it always has been a basic principle to restrict the iatrogenic trauma done to
Chapter 1 Minimally Invasive Spine Surgery 1 H.M. Mayer Primum non nocere First do no harm In the long history of surgery it always has been a basic principle to restrict the iatrogenic trauma done to
X-Plain Subclavian Inserted Central Catheter (SICC Line) Reference Summary
 X-Plain Subclavian Inserted Central Catheter (SICC Line) Reference Summary Introduction A Subclavian Inserted Central Catheter, or subclavian line, is a long thin hollow tube inserted in a vein under the
X-Plain Subclavian Inserted Central Catheter (SICC Line) Reference Summary Introduction A Subclavian Inserted Central Catheter, or subclavian line, is a long thin hollow tube inserted in a vein under the
Inferior Vena Cava filter and removal
 Inferior Vena Cava filter and removal What is Inferior Vena Cava Filter Placement and Removal? An inferior vena cava filter placement procedure involves an interventional radiologist (a specialist doctor)
Inferior Vena Cava filter and removal What is Inferior Vena Cava Filter Placement and Removal? An inferior vena cava filter placement procedure involves an interventional radiologist (a specialist doctor)
TAXUS Express 2. Paclitaxel-Eluting Coronary Stent System. Patient Information Guide
 TAXUS Express 2 Paclitaxel-Eluting Coronary Stent System Patient Information Guide 1 Table of Contents Coronary Artery Disease...2 Who Is at Risk?...3 Diagnosis of Coronary Artery Disease...3 Treatment
TAXUS Express 2 Paclitaxel-Eluting Coronary Stent System Patient Information Guide 1 Table of Contents Coronary Artery Disease...2 Who Is at Risk?...3 Diagnosis of Coronary Artery Disease...3 Treatment
PROMUS Element Plus. Everolimus-Eluting Platinum Chromium Coronary Stent System. Patient Information Guide
 PROMUS Element Plus Patient Information Guide PROMUS Element Plus Patient Information Guide You have recently had a PROMUS Element drug-coated stent implanted in the coronary arteries of your heart. The
PROMUS Element Plus Patient Information Guide PROMUS Element Plus Patient Information Guide You have recently had a PROMUS Element drug-coated stent implanted in the coronary arteries of your heart. The
IN.PACT ADMIRAL DRUG-COATED BALLOON NEW TECHNOLOGY ADD-ON PAYMENT (NTAP)
 IN.PACT ADMIRAL DRUG-COATED BALLOON NEW TECHNOLOGY ADD-ON PAYMENT (NTAP) New Technology Add-on (NTAP) for DCB OVERVIEW Effective October 1, 2015, hospital inpatient cases using a drug-coated balloon (DCB)
IN.PACT ADMIRAL DRUG-COATED BALLOON NEW TECHNOLOGY ADD-ON PAYMENT (NTAP) New Technology Add-on (NTAP) for DCB OVERVIEW Effective October 1, 2015, hospital inpatient cases using a drug-coated balloon (DCB)
4052 Slimplicity Tech final_layout 1 6/29/15 3:29 PM Page 2 Surgical Technique
 Surgical Technique TABLE OF CONTENTS Slimplicity Anterior Cervical Plate System Overview 2 Indications 2 Implants 3 Instruments 4 Surgical Technique 6 1. Patient Positioning and Approach 6 2. Plate Selection
Surgical Technique TABLE OF CONTENTS Slimplicity Anterior Cervical Plate System Overview 2 Indications 2 Implants 3 Instruments 4 Surgical Technique 6 1. Patient Positioning and Approach 6 2. Plate Selection
Technique Guide. Reamer/Irrigator/Aspirator (RIA). For intramedullary reaming and bone harvesting.
 Technique Guide Reamer/Irrigator/Aspirator (RIA). For intramedullary reaming and bone harvesting. Table of Contents Introduction Reamer/ Irrigator/ Aspirator (RIA) 2 Indications 4 Surgical Technique Preparation
Technique Guide Reamer/Irrigator/Aspirator (RIA). For intramedullary reaming and bone harvesting. Table of Contents Introduction Reamer/ Irrigator/ Aspirator (RIA) 2 Indications 4 Surgical Technique Preparation
Instructions for Use Cordis PRECISE Nitinol Stent System (5.5F and 6F) 10368453-2
 Instructions for Use Cordis PRECISE Nitinol Stent System (5.5F and 6F) 10368453-2 Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. Read all instructions carefully.
Instructions for Use Cordis PRECISE Nitinol Stent System (5.5F and 6F) 10368453-2 Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. Read all instructions carefully.
Instructions for Use
 Pleural Effusion Shunt with External Pump Chamber Catalog No. 42-9005 Instructions for Use Denver Biomedical, Inc. Table of Contents Description 2 Indications 2 Contraindications 2 Warnings 4 Cautions
Pleural Effusion Shunt with External Pump Chamber Catalog No. 42-9005 Instructions for Use Denver Biomedical, Inc. Table of Contents Description 2 Indications 2 Contraindications 2 Warnings 4 Cautions
The Electronic Medical Record in its entirety can be found on pages 39-54. The EMR is the reading tool that students will use as they complete The
 The Electronic Medical Record in its entirety can be found on pages 39-54. The EMR is the reading tool that students will use as they complete The Virtual Stroke Lab. The idea of the EMR was that students
The Electronic Medical Record in its entirety can be found on pages 39-54. The EMR is the reading tool that students will use as they complete The Virtual Stroke Lab. The idea of the EMR was that students
(English) NEXUS SPINE SPACER SYSTEM
 (English) NEXUS SPINE SPACER SYSTEM INDICATIONS FOR USE NEXUS Spine Spacer System, a GEO Structure is indicated for use in the thoraco-lumbar spine (i.e., T1 to L5) to replace a diseased vertebral body
(English) NEXUS SPINE SPACER SYSTEM INDICATIONS FOR USE NEXUS Spine Spacer System, a GEO Structure is indicated for use in the thoraco-lumbar spine (i.e., T1 to L5) to replace a diseased vertebral body
TAXUS Express2 Paclitaxel-Eluting Coronary Stent System TAXUS Liberté Paclitaxel-Eluting Coronary Stent System. A Patient s Guide
 TAXUS Express2 Paclitaxel-Eluting Coronary Stent System TAXUS Liberté Paclitaxel-Eluting Coronary Stent System A Patient s Guide Table of Contents Coronary Artery Disease... 2 Who Is at Risk?... 3 Diagnosis
TAXUS Express2 Paclitaxel-Eluting Coronary Stent System TAXUS Liberté Paclitaxel-Eluting Coronary Stent System A Patient s Guide Table of Contents Coronary Artery Disease... 2 Who Is at Risk?... 3 Diagnosis
Policies & Procedures. I.D. Number: 1073
 Policies & Procedures Title:: CENTRAL VENOUS CATHETERS INSERTION ASSISTING I.D. Number: 1073 Authorization [] Pharmacy Nursing Committee [] MAC Motion #: [x] SHR Nursing Practice Committee Source: Nursing
Policies & Procedures Title:: CENTRAL VENOUS CATHETERS INSERTION ASSISTING I.D. Number: 1073 Authorization [] Pharmacy Nursing Committee [] MAC Motion #: [x] SHR Nursing Practice Committee Source: Nursing
PERIPHERALLY INSERTED CENTRAL CATHETERS (PICC) Fong So Kwan APN, Haematology unit Medical Department, QMH
 PERIPHERALLY INSERTED CENTRAL CATHETERS (PICC) Fong So Kwan APN, Haematology unit Medical Department, QMH 1 What is a PICC catheter? Primary vascular access device since their introduction in the mid-1970s,
PERIPHERALLY INSERTED CENTRAL CATHETERS (PICC) Fong So Kwan APN, Haematology unit Medical Department, QMH 1 What is a PICC catheter? Primary vascular access device since their introduction in the mid-1970s,
100000002022.2. Instructions for Use. Cordis S.M.A.R.T. CONTROL Vascular Stent System
 100000002022.2 Instructions for Use Cordis S.M.A.R.T. CONTROL Vascular Stent System Symbols Catalog Number LOT NonPyrogenic Lot Number Store in cool, dark, dry place Use By Date Sterilized with ethylene
100000002022.2 Instructions for Use Cordis S.M.A.R.T. CONTROL Vascular Stent System Symbols Catalog Number LOT NonPyrogenic Lot Number Store in cool, dark, dry place Use By Date Sterilized with ethylene
Imaging of Acute Stroke. Noam Eshkar, M.D New Jersey Neuroscience Institute JFK Medical Center Edison Radiology Group
 Imaging of Acute Stroke Noam Eshkar, M.D New Jersey Neuroscience Institute JFK Medical Center Edison Radiology Group Modalities Non Contrast CT (NCCT) Contrast CT Angiography MRI MR Angiography Perfusion
Imaging of Acute Stroke Noam Eshkar, M.D New Jersey Neuroscience Institute JFK Medical Center Edison Radiology Group Modalities Non Contrast CT (NCCT) Contrast CT Angiography MRI MR Angiography Perfusion
WallFlex Biliary RX Stent. Fully, Partially and Uncovered Self-Expanding Metal Stents
 WallFlex Biliary RX Stent Fully, Partially and Uncovered Self-Expanding Metal Stents WallFlex Biliary RX Stent Fully, Partially and Uncovered Self-Expanding Metal Stents The WallFlex Biliary RX Stent is
WallFlex Biliary RX Stent Fully, Partially and Uncovered Self-Expanding Metal Stents WallFlex Biliary RX Stent Fully, Partially and Uncovered Self-Expanding Metal Stents The WallFlex Biliary RX Stent is
Scout Vessel Guard. A cover for vessels during anterior lumbar spine surgery.
 Scout Vessel Guard. A cover for vessels during anterior lumbar spine surgery. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Scout Vessel Guard 2
Scout Vessel Guard. A cover for vessels during anterior lumbar spine surgery. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Scout Vessel Guard 2
SUCTION, ASPIRATING TUBES
 162300 536204 162345 162301 (shown twice size) Yankauer Suction Tubes YANKAUER SUCTION TUBES Standard adult size. 10 mm diameter. Tip has 2 mm central opening and four 2 mm side openings. 162300 12 1/2"
162300 536204 162345 162301 (shown twice size) Yankauer Suction Tubes YANKAUER SUCTION TUBES Standard adult size. 10 mm diameter. Tip has 2 mm central opening and four 2 mm side openings. 162300 12 1/2"
Supera Peripheral Stent System Instructions for Use
 Supera Peripheral Stent System Instructions for Use Table of Contents 1.0 DEVICE DESCRIPTION 2.0 HOW SUPPLIED 3.0 INDICATIONS 4.0 CONTRAINDICATIONS 5.0 WARNINGS 6.0 PRECAUTIONS 6.1 Stent Delivery System
Supera Peripheral Stent System Instructions for Use Table of Contents 1.0 DEVICE DESCRIPTION 2.0 HOW SUPPLIED 3.0 INDICATIONS 4.0 CONTRAINDICATIONS 5.0 WARNINGS 6.0 PRECAUTIONS 6.1 Stent Delivery System
What Is an Arteriovenous Malformation (AVM)?
 What Is an Arteriovenous Malformation (AVM)? From the Cerebrovascular Imaging and Intervention Committee of the American Heart Association Cardiovascular Council Randall T. Higashida, M.D., Chair 1 What
What Is an Arteriovenous Malformation (AVM)? From the Cerebrovascular Imaging and Intervention Committee of the American Heart Association Cardiovascular Council Randall T. Higashida, M.D., Chair 1 What
Vascular Stent System (6F, 20 100mm stents)
 100000000922.2 Instructions for Use Cordis S.M.A.R.T. CONTROL Vascular Stent System (6F, 20 100mm stents) Cordis S.M.A.R.T. Vascular Stent System (6F, 120 150mm stents) Symbols Catalog Number LOT NonPyrogenic
100000000922.2 Instructions for Use Cordis S.M.A.R.T. CONTROL Vascular Stent System (6F, 20 100mm stents) Cordis S.M.A.R.T. Vascular Stent System (6F, 120 150mm stents) Symbols Catalog Number LOT NonPyrogenic
Aspira* Pleural Drainage Catheter
 Aspira* Pleural Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Pleural Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid from the
Aspira* Pleural Drainage Catheter Instructions For Use Access Systems Product Description: The Aspira* Pleural Drainage Catheter is a tunneled, long-term catheter used to drain accumulated fluid from the
Lentur Cable System. Surgical Technique
 Lentur Cable System Surgical Technique Contents Introduction... Page 1 System Design Features And Benefits... Page 2 Implants... Page 3 Instrumentation... Page 4 Surgical Technique... Page 5 Single Cable...
Lentur Cable System Surgical Technique Contents Introduction... Page 1 System Design Features And Benefits... Page 2 Implants... Page 3 Instrumentation... Page 4 Surgical Technique... Page 5 Single Cable...
Jugular/Subclavian Vein Approach Instructions for Use
 Jugular/Subclavian Vein Approach Instructions for Use Instructions for Use For use in the Vena Cava Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. A. Device
Jugular/Subclavian Vein Approach Instructions for Use Instructions for Use For use in the Vena Cava Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. A. Device
ANTERIOR LUMBAR INTERBODY FUSION (ALIF) Basic Anatomical Landmarks: Anterior Lumbar Spine
 (ALIF) Anterior In human anatomy, referring to the front surface of the body or the position of one structure relative to another Lumbar Relating to the loins or the section of the back and sides between
(ALIF) Anterior In human anatomy, referring to the front surface of the body or the position of one structure relative to another Lumbar Relating to the loins or the section of the back and sides between
Orsiro Hybrid Drug Eluting Stent Industry's first hybrid DES
 Vascular Intervention Drug Eluting Stents Orsiro Orsiro Hybrid Drug Eluting Stent Industry's first hybrid DES Orsiro An ideal combination of passive and active components The Orsiro Hybrid Drug Eluting
Vascular Intervention Drug Eluting Stents Orsiro Orsiro Hybrid Drug Eluting Stent Industry's first hybrid DES Orsiro An ideal combination of passive and active components The Orsiro Hybrid Drug Eluting
WATCHMAN Left Atrial Appendage Closure Device
 WATCHMAN Left Atrial Appendage Closure Device Patient Information Guide WATCHMAN Left Atrial Appendage Closure Device PATIENT INFORMATION GUIDE Your doctor has recommended that you consider undergoing
WATCHMAN Left Atrial Appendage Closure Device Patient Information Guide WATCHMAN Left Atrial Appendage Closure Device PATIENT INFORMATION GUIDE Your doctor has recommended that you consider undergoing
ANGIO-SEAL EVOLUTION VASCULAR CLOSURE DEVICE INSTRUCTIONS FOR USE
 ANGIO-SEAL EVOLUTION VASCULAR CLOSURE DEVICE INSTRUCTIONS FOR USE TO ENSURE PROPER DEPLOYMENT AND USE OF THIS DEVICE AND TO PREVENT INJURY TO PATIENTS, READ ALL INFORMATION CONTAINED IN THESE INSTRUCTIONS
ANGIO-SEAL EVOLUTION VASCULAR CLOSURE DEVICE INSTRUCTIONS FOR USE TO ENSURE PROPER DEPLOYMENT AND USE OF THIS DEVICE AND TO PREVENT INJURY TO PATIENTS, READ ALL INFORMATION CONTAINED IN THESE INSTRUCTIONS
Policies and Procedures. Related to. IABP Therapy
 Policies and Procedures Related to IABP Therapy Courtesy of Datascope Corp. Clinical Support Services The following policies and procedures are intended to serve as guidelines for developing hospital policy.
Policies and Procedures Related to IABP Therapy Courtesy of Datascope Corp. Clinical Support Services The following policies and procedures are intended to serve as guidelines for developing hospital policy.
Placement of Epidural Catheter for Pain Management Shane Bateman DVM, DVSc, DACVECC
 Placement of Epidural Catheter for Pain Management Shane Bateman DVM, DVSc, DACVECC Indications: Patients with severe abdominal or pelvic origin pain that is poorly responsive to other analgesic modalities.
Placement of Epidural Catheter for Pain Management Shane Bateman DVM, DVSc, DACVECC Indications: Patients with severe abdominal or pelvic origin pain that is poorly responsive to other analgesic modalities.
Mini TightRope CMC Surgical Technique
 Mini TightRope CMC Surgical Technique Mini TightRope CMC Mini TightRope CMC Fixation The Mini TightRope provides a unique means to suspend the thumb metacarpal after partial or complete trapezial resection
Mini TightRope CMC Surgical Technique Mini TightRope CMC Mini TightRope CMC Fixation The Mini TightRope provides a unique means to suspend the thumb metacarpal after partial or complete trapezial resection
Percutaneous Transluminal Angioplasty (PTA) and Stenting For PVS Patients
 Percutaneous Transluminal Angioplasty (PTA) and Stenting For PVS Patients There are two types of blood vessels in the body arteries and veins. Arteries carry blood rich in oxygen from the heart to all
Percutaneous Transluminal Angioplasty (PTA) and Stenting For PVS Patients There are two types of blood vessels in the body arteries and veins. Arteries carry blood rich in oxygen from the heart to all
Posterior Referencing. Surgical Technique
 Posterior Referencing Surgical Technique Posterior Referencing Surgical Technique INTRO Introduction Instrumentation Successful total knee arthroplasty depends in part on re-establishment of normal lower
Posterior Referencing Surgical Technique Posterior Referencing Surgical Technique INTRO Introduction Instrumentation Successful total knee arthroplasty depends in part on re-establishment of normal lower
USE OF SIMULATION TO IMPROVE CARDIOVASCULAR STENT DEVELOPMENT
 USE OF SIMULATION TO IMPROVE CARDIOVASCULAR STENT DEVELOPMENT V. Astier, S. Bidal, V. Garitey, F. Mouret Abstract : A stent is a wire metal mesh tube placed into an artery or blood vessel to hold the structure
USE OF SIMULATION TO IMPROVE CARDIOVASCULAR STENT DEVELOPMENT V. Astier, S. Bidal, V. Garitey, F. Mouret Abstract : A stent is a wire metal mesh tube placed into an artery or blood vessel to hold the structure
SCORM. For more patient education, please visit www.cypherusa.com
 Attach Label Understanding Coronary Artery Disease, Cardiac Catheterization, and Treatment Options Stent Implant Card CYPHER Sirolimus-eluting Coronary Stent SCORM P.O. Box 025700 Miami, FL 33102-5700,
Attach Label Understanding Coronary Artery Disease, Cardiac Catheterization, and Treatment Options Stent Implant Card CYPHER Sirolimus-eluting Coronary Stent SCORM P.O. Box 025700 Miami, FL 33102-5700,
Since 1991, when Parodi et al1 described a minimally
 Aptus Endovascular AAA Repair System Report of the 1-year follow-up in a first-in-man study. BY TAKAO OHKI, MD; DAVID H. DEATON, MD; AND JOSÉ ANTONIO CONDADO, MD Since 1991, when Parodi et al1 described
Aptus Endovascular AAA Repair System Report of the 1-year follow-up in a first-in-man study. BY TAKAO OHKI, MD; DAVID H. DEATON, MD; AND JOSÉ ANTONIO CONDADO, MD Since 1991, when Parodi et al1 described
UW MEDICINE PATIENT EDUCATION. Aortic Stenosis. What is heart valve disease? What is aortic stenosis?
 UW MEDICINE PATIENT EDUCATION Aortic Stenosis Causes, symptoms, diagnosis, and treatment This handout describes aortic stenosis, a narrowing of the aortic valve in your heart. It also explains how this
UW MEDICINE PATIENT EDUCATION Aortic Stenosis Causes, symptoms, diagnosis, and treatment This handout describes aortic stenosis, a narrowing of the aortic valve in your heart. It also explains how this
HELPFUL STEP-BY-STEP ILLUSTRATIONS ARE LOCATED IN THIS BOOKLET
 HELPFUL STEP-BY-STEP ILLUSTRATIONS ARE LOCATED IN THIS BOOKLET DESCRIPTION The Vet Sent Ureter comes as a sterile stent system comprised of two components: 1) the radioopaque, double pigtail multi-fenestrated
HELPFUL STEP-BY-STEP ILLUSTRATIONS ARE LOCATED IN THIS BOOKLET DESCRIPTION The Vet Sent Ureter comes as a sterile stent system comprised of two components: 1) the radioopaque, double pigtail multi-fenestrated
Zimmer Small Fragment Universal Locking System. Surgical Technique
 Zimmer Small Fragment Universal Locking System Surgical Technique Zimmer Small Fragment Universal Locking System 1 Zimmer Small Fragment Universal Locking System Surgical Technique Table of Contents Introduction
Zimmer Small Fragment Universal Locking System Surgical Technique Zimmer Small Fragment Universal Locking System 1 Zimmer Small Fragment Universal Locking System Surgical Technique Table of Contents Introduction
Choice of guiding catheters and guidewires
 Choice of guiding catheters and guidewires Dr. Ahmad Elsayed Yousef, MD Lecturer of Cardiology, Ain Shams University Werner Frossmann-1929 1 X-ray for angioraphy (1950-1970) Cine frame from the first selective
Choice of guiding catheters and guidewires Dr. Ahmad Elsayed Yousef, MD Lecturer of Cardiology, Ain Shams University Werner Frossmann-1929 1 X-ray for angioraphy (1950-1970) Cine frame from the first selective
Aneurysm Embolization System for the Treatment of Cerebral Aneurysms. Information for Patients and Families
 this booklet is provided for for any non emergency questions, contact us at Aneurysm Embolization System for the Treatment of Cerebral Aneurysms Information for Patients and Families 2 3 Information for
this booklet is provided for for any non emergency questions, contact us at Aneurysm Embolization System for the Treatment of Cerebral Aneurysms Information for Patients and Families 2 3 Information for
Vascular Technology (VT) Content Outline Anatomy & physiology 20% Cerebrovascular Cerebrovascular normal anatomy Evaluate the cerebrovascular vessels
 Vascular Technology (VT) Content Outline Anatomy & physiology 20% normal anatomy Evaluate the cerebrovascular vessels hemodynamics Evaluate the cerebrovascular vessels for normal perfusion normal anatomy
Vascular Technology (VT) Content Outline Anatomy & physiology 20% normal anatomy Evaluate the cerebrovascular vessels hemodynamics Evaluate the cerebrovascular vessels for normal perfusion normal anatomy
A Patient Information Guide for the CYPHER Sirolimus-eluting Coronary Stent
 bringing evidence to life Understanding Coronary Artery Disease, Cardiac Catheterization, and Treatment Options A Patient Information Guide for the CYPHER Sirolimus-eluting Coronary Stent Patient s Name
bringing evidence to life Understanding Coronary Artery Disease, Cardiac Catheterization, and Treatment Options A Patient Information Guide for the CYPHER Sirolimus-eluting Coronary Stent Patient s Name
Subclavian Steal Syndrome By Marta Thorup
 Subclavian Steal Syndrome By Marta Thorup Definition Subclavian steal syndrome (SSS), is a constellation of signs and symptoms that arise from retrograde flow of blood in the vertebral artery, due to proximal
Subclavian Steal Syndrome By Marta Thorup Definition Subclavian steal syndrome (SSS), is a constellation of signs and symptoms that arise from retrograde flow of blood in the vertebral artery, due to proximal
HUNTER TENDON IMPLANTS 127846-6
 HUNTER TENDON IMPLANTS 127846-6 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch) Türkçe
HUNTER TENDON IMPLANTS 127846-6 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch) Türkçe
A PATIENT S GUIDE TO CARDIAC CATHETERIZATION
 A PATIENT S GUIDE TO CARDIAC CATHETERIZATION The science of medicine. The compassion to heal. This teaching booklet is designed to introduce you to cardiac catheterization. In the following pages, we will
A PATIENT S GUIDE TO CARDIAC CATHETERIZATION The science of medicine. The compassion to heal. This teaching booklet is designed to introduce you to cardiac catheterization. In the following pages, we will
Dept. of Medical Imaging University of Ottawa
 ED Visits Related to Bariatric Surgery: Review of Normal Post-Surgical Anatomy as Well as Complications Dept. of Medical Imaging University of Ottawa Disclosures Background Roux-en-Y Gastric Bypass Surgery
ED Visits Related to Bariatric Surgery: Review of Normal Post-Surgical Anatomy as Well as Complications Dept. of Medical Imaging University of Ottawa Disclosures Background Roux-en-Y Gastric Bypass Surgery
An Unusual Huge Coronary Artery Aneurysm with Fistula
 Case Report Acta Cardiol Sin 2006;22:40 4 An Unusual Huge Coronary Artery Aneurysm with Fistula Chung-Chi Yang, 1 Yu-Wen Chen, Chien-Sung Tsai, 2 Cheng-Chung Cheng and Tien-Ping Tsao Coronary artery aneurysms
Case Report Acta Cardiol Sin 2006;22:40 4 An Unusual Huge Coronary Artery Aneurysm with Fistula Chung-Chi Yang, 1 Yu-Wen Chen, Chien-Sung Tsai, 2 Cheng-Chung Cheng and Tien-Ping Tsao Coronary artery aneurysms
Syringe. Product Usage Information: Precautions:
 Syringe Standard Draw Procedure In-Patient, Automated Retraction Non-Reusable. Prepare and give injection using aseptic technique according to institutional policy.. For injection into patients, continue
Syringe Standard Draw Procedure In-Patient, Automated Retraction Non-Reusable. Prepare and give injection using aseptic technique according to institutional policy.. For injection into patients, continue
Guidelines for the Management of Patients Following Endoluminal Vein Dilation Procedures for the Treatment of Multiple Sclerosis
 Guidelines for the Management of Patients Following Endoluminal Vein Dilation Procedures for the Treatment of Multiple Sclerosis Report Submitted to the Minister of Health and Long-Term Care By the Ontario
Guidelines for the Management of Patients Following Endoluminal Vein Dilation Procedures for the Treatment of Multiple Sclerosis Report Submitted to the Minister of Health and Long-Term Care By the Ontario
Biliary and Endovascular Product Catalog
 Biliary and Endovascular Product Catalog To navigate within this catalog: - Single click on a chapter or section letter - Type the product name or catalog number in the Find box on the Adobe tool bar Table
Biliary and Endovascular Product Catalog To navigate within this catalog: - Single click on a chapter or section letter - Type the product name or catalog number in the Find box on the Adobe tool bar Table
Vertebrobasilar Disease
 The Vascular Surgery team at the University of Michigan is dedicated to providing exceptional treatments for in the U-M Cardiovascular Center (CVC), our new state-of-the-art clinical facility. Treatment
The Vascular Surgery team at the University of Michigan is dedicated to providing exceptional treatments for in the U-M Cardiovascular Center (CVC), our new state-of-the-art clinical facility. Treatment
PROTEGE. Carotid Stent System INSTRUCTIONS FOR USE. CAUTION: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
 THE ENDOVASCULAR COMPANY PROTEGE RX Carotid Stent System INSTRUCTIONS FOR USE CAUTION: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. . DEVICE DESCRIPTION The PROTÉGÉ
THE ENDOVASCULAR COMPANY PROTEGE RX Carotid Stent System INSTRUCTIONS FOR USE CAUTION: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. . DEVICE DESCRIPTION The PROTÉGÉ
Table 1. Balloon and Stent Specifications. Nominal Pressure During Stent Deployment (atm/kpa) Stent Length (mm)
 90590772-01 ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning 2010-05 < EN > VeriFLEX (Liberté ) m o n o r a i l o v e r - t h e - w i r e Bare-Metal
90590772-01 ONLY Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. warning 2010-05 < EN > VeriFLEX (Liberté ) m o n o r a i l o v e r - t h e - w i r e Bare-Metal
Morpheus SMART PICC CT Insertion Kit
 Morpheus SMART PICC CT Insertion Kit Peripherally Inserted Central Catheter INSTRUCTIONS FOR USE ANGIODYNAMICS, INC. 603 QUEENSBURY AVE. QUEENSBURY, NY 12804 U.S.A. TOLL FREE: 800-772-6446 PHONE: 518-798-1215
Morpheus SMART PICC CT Insertion Kit Peripherally Inserted Central Catheter INSTRUCTIONS FOR USE ANGIODYNAMICS, INC. 603 QUEENSBURY AVE. QUEENSBURY, NY 12804 U.S.A. TOLL FREE: 800-772-6446 PHONE: 518-798-1215
