Failure of the ocular surface epithelia occurs in many clinical
|
|
|
- Jeffrey Daniels
- 8 years ago
- Views:
Transcription
1 CLINICAL SCIENCE Efficacy of Standardized and Quality-Controlled Cord Blood Serum Eye Drop Therapy in the Healing of Severe Corneal Epithelial Damage in Dry Eye Piera Versura, BSD,* Vincenzo Profazio, MD,* Marina Buzzi, BSD, Alessandra Stancari, PharmD, Mario Arpinati, MD, Nazzarena Malavolta, MD, and Emilio C. Campos, MD* Purpose: We standardized quality-controlled cord blood serum (CBS) based eye drops and evaluated the efficacy of 1-month CBS treatment in the healing of diseased corneal epithelium in severe dry eye (DE) patients. Methods: Seventeen graft-versus-host disease (GVHD) and 13 Sjogren syndrome patients with severe persistent corneal defects were enrolled in the framework of a registered clinical trial (ClinicalTrials.gov NCT ). Sterile CBS eye drops were prepared to supply 0.15 ng per eye per day epithelial growth factor and administered for 1 month in a 1-day dose dispensing. The extent of epithelial defect was evaluated in square millimeters area, and subjective symptom score (Ocular Surface Disease Index score), Schirmer test I, break-up time, tear osmolarity, corneal esthesiometry (Cochet Bonnet esthesiometer), conjunctival scraping, and imprint cytology with goblet cell count were performed at baseline (V0) and after 15 (V1) and 30 (V2, endpoint) days of treatment. Satisfaction and tolerability questionnaires were evaluated at V1 and V2. Results: A significant reduction was shown at the endpoint versus baseline in corneal epithelial damage (mean ± SD, 16.1 ± 13.7 vs ± 30 mm 2 /area, respectively), discomfort symptoms (Ocular Surface Disease Index score, 22.3 ± 10.3 vs ± 16.9), scraping cytology score (3.8 ± 1.2 vs. 6.6 ± 2.1), and tear osmolarity (312.5 ± 7 vs. 322 ± 9.1 mosm/l), whereas a significant improvement was shown in corneal esthesiometry (48.2 ± 2.1 vs ± 2.1 nylon/mm/ length, P, 0.05). All patients reported a high degree of satisfaction upon drop instillation. Conclusions: Heterologous CBS-based eye drops represent a promising therapeutic approach in the healing of severely injured corneal epithelium and in subjective symptom relief. These drops can be obtained as readily available and quality-controlled blood derivative from cord blood banks on a routine basis. Received for publication January 11, 2012; revision received March 22, 2012; accepted March 29, From the *Ophthalmology Unit; Cord Blood Bank Transfusion Service; Pharmacy Service; Hematology Unit; and Reumatology Service, University of Bologna and S. Orsola-Malpighi Hospital, Bologna, Italy. Supported in part by a grant from Fondazione Cassa di Risparmio di Bologna to EC Campos. The authors declare no conflicts of interest. Reprints: Piera Versura, Ophthalmology Unit, University of Bologna, Policlinico S. Orsola-Malpighi, Pad. 1 Palagi, Via Palagi, Bologna, Italy ( piera.versura@unibo.it). Copyright 2012 by Lippincott Williams & Wilkins Key Words: cord blood, corneal epithelium damage, EGF, graft-versus-host disease, Sjogren syndrome (Cornea 2012;0:1 7) Failure of the ocular surface epithelia occurs in many clinical conditions with differing pathogeneses; the core of medical management is to stabilize or promote new growth of healthy conjunctival and corneal epithelia. A range of therapeutic approaches has been introduced and considerable advances have been achieved in recent years with the administration of topical products based upon blood preparations. These include eye drops prepared either from the patients own blood such as autologous serum (AS) 1,2 plasma rich in growth factors 3 and platelet lysate 4 or from donors such as umbilical cord blood serum (CBS). 5 The rationale for the use of all these stands upon their content of biologically active components and in particular of growth factors, shown to be higher than in patients tears. 6 CBS, in particular, contains various growth factors at a very high concentration and offers several advantages over the other blood-derived preparations, primarily because a large amount of serum can be obtained from the umbilical vein at one time so that many patients can benefit from this sampling and repeated blood collection from patients are avoided. In addition, the difficulty of obtaining blood from patients with poor general condition or blood dyscrasia and from those with infectious diseases is also avoided. Finally, the unsuitable application of eye drops containing proinflammatory cytokines, as may occur in eye drop preparations from AS in some diseases such as graft-versus-host disease (GVHD) 7 or Sjogren syndrome (SS), 8 may be prevented. CBS eye drops were found to be more effective than AS in healing epithelial defects, 9 and their efficacy has been successfully demonstrated in various conditions, from severe dry eye (DE) with or without primary Sjogren syndrome (SS-I) 9 to ocular GVHD, 10 persistent epithelial defects, 11 recurrent corneal erosions, 12 chemical burns, 13 and neurotrophic keratitis. 14 Interestingly, the epitheliotropic capacity of CBS in promoting limbal stem cell activity has been recently demonstrated also in corneal cell culture models. 15 Despite CBS therapeutic potentials, details about the real growth factor content in CBS eye drops used to treat patients were not reported in those previous articles nor were CBS Cornea Volume 0, Number 0, Month
2 Versura et al Cornea Volume 0, Number 0, Month 2012 donor selection, Good Manufacturing Practice preparation procedure, serological and microbiological quality assurance controls, treatment duration, or patient satisfaction. The purpose of this study was to standardize the protocol and procedure of CBS eye drop use to evaluate its efficacy in the healing of corneal epithelial defects and amelioration of painful subjective symptoms. MATERIALS AND METHODS Patients The study was conducted under the local Institutional Review Board/Ethics Committee approval (EudraCT: ; ClinicalTrials.gov NCT ) and the protocol followed the guidelines of the Declaration of Helsinki. Seventeen GVHD (11 men, 6 women, mean age, 38.8 ± 5.8 years, 33 eyes) and 13 SS-I (all women, mean age, 59.6 ± 13.1 years, 26 eyes) patients exhibiting severe corneal involvement at the time of enrollment, graded as grade 4 to 5 according to the Oxford grading level, 16 were included in the study. Eight of 17 GVHD patients and 5 of 13 SS patients had experienced AS therapy not administered by our unit; therefore, details could not be recorded. Informed consent was obtained from each enrolled subject. The patients were diagnosed as having severe DE according to the Dry Eye Workshop (DEWS) severity level. 17 Exclusion criteria were previous ocular surgery performed in the year leading up to the visit, wearing contact lenses, punctual plug placement or cauterization, and ocular allergy. Subjective symptoms of DE were graded on the basis of the DE discomfort symptoms questionnaire of the Ocular Surface Disease Index (OSDI). 18 Upon arrival for our evaluation, 24 of 30 patients were using various types of tear substitutes, gels, and ointments, whereas the remaining 6 were using antiinflammatory topical drugs. To normalize the study population to an identical regimen of eye drops/medication use, subjects who met the inclusion criteria of the study were dispensed hyaluronic acid based eye drops, to be used in both eyes for 4 days (washout period). Methods A slit-lamp evaluation and subjective symptom score were performed at baseline (V0) and after 2 (V1) and 4 (V2, endpoint) weeks of treatment. The following tests were performed at V0 and V2. Corneal sensitivity was measured using a Cochet Bonnet esthesiometer (Luneau, Chartres, France) and recorded in millimeters per nylon filament length. A measurement of less than 50 mm was considered low corneal sensitivity. 19 Schirmer test I and break-up time (BUT) were performed according to the DEWS guidelines. 20 Tear osmolarity was performed with TearLab Osmolarity System as described elsewhere 21 ; the cutoff value for severe DE was considered as more than 324 mosm/l. 21 To evaluate inflammation, scrapings were performed in both eyes at the lower and upper tarsal conjunctiva, and smears were read following the Scraping Cytology Score System described elsewhere, 22 pathological values of score more than 4 of 12. Impression cytology samples were obtained from the lower nasal bulbar conjunctiva using strips of polyethersulfone membrane filters, 0.20 mm (Supor-200) as described. 20 The degree of squamous metaplasia was divided into grades (0 to 3) using the grading scheme of Tseng, 23 and periodic acid-schiff positive goblet cell (GC) density was calculated as the number of cells per square millimeter (media of magnification consecutive visual fields). 24 Corneal epithelial damage was evaluated in square millimeter area at V0, V1, and V2. Briefly, corneal photography was taken after the administration of a 2 ml volume of 1% fluorescein dye, at a magnification of 16 using a camera attached to a slit-lamp microscope equipped with a 7503 Boston yellow filter kit (equivalent to Kodak Wratten 12) to enhance staining details. Gel Pro Image Analyser Software (Media Cybernetics, Bethesda, MD) was used to estimate the area of damaged corneal epithelium on acquired digital photographs, matched in comparison with a standard calibration area. Visual Analog Scale 25 (VAS) satisfaction and tolerability questionnaires were provided to patients after 2 (V1) and 4 (V2, endpoint) weeks of CBS treatment. These were compared with a VAS satisfaction questionnaire provided to patients at enrollment, concerning their opinion about the last therapy administered before the study. Treatment Preparation of CBS Eye Drops Total quality system and good manufacturing practice facilities were used. The umbilical cord blood was obtained from mothers with vaginal or cesarean section delivery after informed consent. Tests to detect the presence of infection (hepatitis B and C virus, human immunodeficiency virus, TPHA, TOXO, CMV, HIV/HBV/HCV-NAT, HTLVI/II) were performed at delivery and after the window period of 6 months (quarantine) during which infections cannot be detected but the donor may be infectious, according to European Medicines Agency regulations. In Food and Drug Administration regulations, NAT tests are recommended and not mandatory. A mean volume of 80 ml of the umbilical cord blood was collected from the umbilical vein, and blood was clotted for 2 hours at room temperature. After centrifugation at 3000 rpm for 15 minutes, the serum was carefully isolated under a laminar flow hood (BacT/Alert; Biomerieux; sterility tests was performed in each batch). Serum was frozen at 280 C for 6 months (quarantine period). To assess whether the procedure and storage could reduce growth factor content, levels of epithelial growth factor (EGF) and transforming growth factor (TGF)-b1 were tested in 7 CBS preparations, in the different procedure step points (Elisa Kit Quantikine; R&D Systems, Abingdon, UK). Screening of CBS to be used as the eye drop preparation 2 Ó 2012 Lippincott Williams & Wilkins
3 Cornea Volume 0, Number 0, Month 2012 CBS Eye Drop Therapy in Dry Eye source was achieved by EGF content analysis before CBS storage (EGF content.1.5 ng/ml was selected as threshold). After the quarantine, the preselected sera were thawed and pooled to obtain the needed amount of serum to treat all patients, diluted to 20% with refrigerated sterile phosphatebuffered saline with aseptic technique, and filtered (Millex HV 0.4 mm). The preparation was then aliquoted into luerlock cap 1-mL sterile syringes. Filled syringes were sealed and packed in single labeled envelopes for delivery to patients. A single syringe was used for the daily supply of CBS preparation. In 5 of 30 patients, the COL-20 medical device (Biomed, Modena, Italy) was used to prepare monodose vials, packed, frozen, and stored as above. Patients were instructed to administer one drop per eye for 8 times a day, after having thawed 1 syringe or vial the evening before the day of use. Statistical Evaluations Statistical evaluation was performed by using MedCalc and SPSS 14 software and applying the Wilcoxon signedrank test to determine the significance of changes after CBS eye drop therapy (P, 0.05 was considered to be statistically significant). Pearson (r) or Spearman (r) correlation coefficients were applied when appropriate; correlations were considered statistically significant at P, 0.05 (small correlation strength, ; medium ; large, ). Descriptive statistics for tests and variables analyzed in subjects are reported as mean ± SD. RESULTS The preparation of CBS eye drops is explained in Figure 1. Data from EGF and TGF-b1 determinations in CBS are shown in Figure 2; the content of these factors did not show any significant variation throughout the procedure. EGF content was found to be well correlated to CD34+ cell content (Pearson r = 0.54, P, 0.001). EGF supply was estimated as 0.15 ng per eye per day in the final preparation. All patients administered CBS eye drops for the entire study period and were fully compliant with the treatment regimen. Neither patient dropout nor any adverse event was registered. Patients successfully and homogeneously responded to CBS treatment, with no appreciable difference between those affected by GVHD and those suffering from SS-I. All parameters evaluated were found to have changed to a statistically significant extent from baseline to endpoint in all patients. Subjective symptoms of ocular surface discomfort significantly decreased after 2 weeks of treatment, and further at endpoint (Table 1). Corneal sensitivity, Schirmer test I, and BUT recovered with respect to baseline and all significantly increased at endpoint (Table 1). Tear osmolarity, inflammatory score evaluated by scraping cytology score, and conjunctival epithelium squamous metaplasia evaluated by imprint cytology score significantly decreased at endpoint versus baseline (Table 1). Periodic acid-schiff positive GC density did not show any increase in 14 of 30 patients; in these patients GC absence had been found in imprint samples at baseline. In the remaining 16 patients, GC density significantly increased from baseline to endpoint (number of GCs per square millimeter, mean ± SD; 57.6 ± 51.9 vs ± 79.8/mm 2, respectively, P, 0.001). A highly significant reduction of corneal epithelial damage was shown in all eyes from baseline as early as 2 weeks of treatment, which was further confirmed at endpoint (Fig. 3A). Only 2 of 59 eyes healed completely after 4 weeks of CBS treatment; in these eyes, the corneal epithelial damage at baseline had been estimated below 10 mm 2. The extent of initial corneal damage correlated with the magnitude of the corneal responses, measured by the area of injured epithelium at endpoint (Pearson r = 0.7, P, ). Baseline epithelial damage less than 25 mm 2 healed almost completely after 1 month of CBS treatment (Fig. 3B). No correlation was found between corneal responses and duration of epithelial defects or of systemic disease diagnosis nor between SS and GVHD patients. Pre post variations (ie, endpoint minus baseline value) for all parameters were calculated and correlated with each other. A significant correlation was found (r = 0.41, P = 0.02) between amelioration of subjective symptoms and inflammation score reduction in the whole population; the extent of initial corneal damage greater than 25 mm 2 increased the correlation strength (r = 0.62, P, 0.01). A significant difference was observed with the overall satisfaction questions estimated by a VAS score, already after 2 weeks of CBS treatment and at the endpoint visit (Fig. 4). The E point columns in the figure show the answers given by patients at enrollment, which refer to their feeling about the last topical therapy administered. DISCUSSION The central core of DE is now thought of as a downward spiral in which cascading inflammatory processes are initiated by tear hyperosmolarity, and the final stage comprises loss of compensatory mechanisms 26 ; the process can lead to ocular surface tissue damage with effects varying from mild to severe. Current treatment options are targeted to the disease severity level. 27 At severity levels 3 and 4 (the most severe), AS therapy is recommended because of its supplement in growth factors and other nutrients needed for proliferation, migration, and differentiation of the corneal and conjunctival epithelium. These natural components may support the healing of injured ocular surface epithelia, in cases of diminished tear growth factor content, as may occur in DE disease. 28 The use of AS in the ophthalmic setting dates back at least 3 decades, but despite its proved efficacy there is no standard procedure of preparation, quality control, storage, and administration. 29,30 In more recent years, the platelet lysate obtained from nongelified autologous platelet-rich plasma has attracted increasing interest 31 because platelet-rich plasma is also a source of a variety of growth factors with an important role in the wound-healing process in many tissues. To overcome the need for peripheral blood sampling from each single patient, heterologous blood sources have been proposed, with the advantage that they can be obtained as readily available and quality-controlled blood derivatives Ó 2012 Lippincott Williams & Wilkins 3
4 Versura et al Cornea Volume 0, Number 0, Month 2012 FIGURE 1. CBS preparation procedure is summarized from donor suitability to delivery. All steps were conducted in UNI EN ISO 9001:2008 certified laboratories. indicates the points of the procedure where determination of EGF and TGF-b1 were carried out, numbered 1 to 6 to match data summarized in Figure 2. indicates the points where sterility controls were performed. from blood banks on a routine basis. In this perspective, CBS eye drops may represent a powerful nonimmunogenic biological supplement source because the preparation contains an elevated growth factor content, higher than in peripheral blood serum 6,9,10 and natural antiinflammatory cytokines. 32 In the present article, we showed that there is a certain biological variability in mother donors with respect to the presence of growth factor in CBS, and optimizing donor selection and collection strategies is a matter of ongoing research. In the present study, we showed a strong correlation between EGF and CD34+ cell content, but not with the obstetric factors including gestational age, parity of the mother, sex and weight at birth of the newborn, weight of the placenta, and mode of delivery. Because CD34+ cell content is routinely estimated by blood banks, particular efforts should be made to collect CBS from donors with a high CD34+ cell content to preselect the serum and reduce preparation time and costs. The efficacy of CBS in the healing of persistent corneal epithelial defects has been reported in the past literature 5,9 14 and evaluated by the clinical evidence of injured area reduction after treatment. The results had been related mainly to growth factor supplementation, but recent in vitro data demonstrated that CBS-supplemented culture medium supports not only the proliferation but also the differentiation of human conjunctival and limbal epithelial cells. 15 However, previous articles did not report any details in terms of the selection of the donor, standardized protocol for microbiological quality assurance, or growth factor content in the final preparation. The present study examined these issues and the data obtained showed that EGF and TGF-b1 content did not change significantly through the various preparation steps, quarantine and storage periods. The eye drop solution used in the present study was prepared to achieve a standardized EGF content estimated as 0.15 ng per eye per day in the final preparation, and this allowed us to avoid bias of response related to composition variability. 4 Ó 2012 Lippincott Williams & Wilkins
5 Cornea Volume 0, Number 0, Month 2012 CBS Eye Drop Therapy in Dry Eye FIGURE 2. Evaluation of EGF and TGF-b1 content in different step points of the preparation procedure of CBS eye drops, preliminarily carried out on 7 (A through G) samples. Steps were numbered 1 to 6, as detailed in Figure 1 and specifically as follows: 1: freshly collected CBS; 2: after the quarantine period; 3: after dilution; 4: after filtration; 5: after 1 month of freezing; 6: after 2 months of freezing. A considerable biological variability was shown in both EGF ( pg/ml) and TGF-b1 ( ng/ml) over the samples analyzed, but no significant variation was observed throughout the whole process, suggesting that the procedure does not affect or reduce the content of both growth factors. Because of cost limitations, growth factor content control was restricted to EGF only, although we recognize that a wider range would be recommended. EGF, a cytokine secreted by the lacrimal gland, was arbitrarily selected as the biomarker to standardize the final product for its mitogenic effects and role in ocular surface homeostasis. 28,33 However, the introduction of pooling collection could be a reasonable compromise in the standardization of the final product, in contrast to the one donor one patient basis used in previous works. In this study, we demonstrated that 1 month of CBS treatment significantly reduced injured corneal epithelium area and improved painful disease sensation in all enrolled patients. Complete epithelial healing was achieved only in 2 of 59 eyes, whereas in another 23 eyes a nearly complete resolution was achieved; the extent of initial corneal damage but not duration of epithelial defects correlated with response, suggesting that CBS treatment should be initiated at the earliest and attempted also in those cases with a long history of corneal epithelium damages. Systemic diseases did not apparently affect the response to the treatment, although the relatively small number of patients did not make it possible to statistically analyze data for stratification. The ocular surface is considered as a functional unit and several parameters were shown to be improved as a consequence of epithelial healing in this study. BUT and Schirmer test results significantly increased at endpoint, in agreement with previous reports, 10,11 although a normal value was never detected in any eye after 1 month of CBS treatment. Conjunctival epithelial metaplasia was also shown to be significantly improved, confirming the in vitro data 15 on the role of CBS in promoting human conjunctival epithelial cell proliferation. Inhomogeneous results were obtained with respect to conjunctival GC recovery after CBS treatment; an increase in GC density was demonstrated in agreement with previous studies, 11 but no treatment response was observed in eyes showing GC loss at baseline. Perhaps this is related to the duration of the CBS therapy, and we could speculate that a longer period of treatment is needed to promote GC differentiation. More likely, CBS eye drops for this study were prepared by checking EGF content only, and it is feasible that other suppliers more specific for GC promotion 34 were insufficient in the final product. Preselection of CBS from donors based on more growth factor content (in addition to EGF) is planned for future studies. TABLE 1. Descriptive Statistics for Tests and Variables Analyzed in Patients From Baseline (V0) to Endpoint (V2) After CBS Treatment Test Measure V0 V1 V2 P OSDI Score 39.3 ± ± ± 10.3 V0 vs. V1, P, V0 vs. V2, P, V1 vs. V2, P, Corneal mm/nylon filament ± ± 2.42, esthesiometry length Schirmer test mm/wet strip after ± ± inch BUT Seconds 5.1 ± ± 3, Tear osmolarity mosm/l 322 ± ± 7, Scraping cytology Score 6.6 ± ± 1.2, Imprint cytology Score 1.89 ± ± Results are summarized (mean ± SD) from baseline to endpoint for subjective symptom reduction (OSDI score), corneal esthesiometry recovery, Schirmer test increase, BUT increase, tear osmolarity decrease, ocular surface inflammation reduction (scraping cytology), and conjunctival epithelium metaplasia decrease (imprint cytology). All data were shown to be statistically significant. P, OSDI, Ocular Surface Disease Index; V1, intermediate step point after 15 days of CBS treatment. Ó 2012 Lippincott Williams & Wilkins 5
6 Versura et al Cornea Volume 0, Number 0, Month 2012 FIGURE 3. Corneal epithelial damage estimated as square millimeter per area (mean ± SD) at baseline and after 2 weeks (V1) and 1 month (endpoint, V2) of CBS treatment. Data from the whole population showed that epithelial damage was significantly reduced as early as 2 weeks of treatment and further at endpoint (A). Data from the population stratified by initial epithelial damage showed that baseline damage less than 25 mm 2 healed nearly completely after 1 month of CBS treatment (B). Subjective discomfort symptoms as measured by Ocular Surface Disease Index were demonstrated to be significantly reduced already after 2 weeks of CBS treatment. This amelioration did not occur as a consequence of a decrease of the sensitivity threshold as occurs in injured eyes. On the contrary, corneal sensitivity was shown to be significantly increased after CBS treatment, which suggests the occurrence of neural fiber promotion during epithelial healing, but we were not able to confirm this result in vivo by confocal analysis. Discomfort symptom reduction was demonstrated to be in correlation to a significant decrease of inflammatory score after CBS treatment in all subjects. The relationship between subjective symptoms and ocular surface inflammation has been debated in the literature 22,35 and correlated to the increase of interleukin (IL)-6 in tears of DE patients. In addition, the presence of natural antiinflammatory cytokines such as IL-10 has been documented in cord blood 36 ; the plasma concentrations of this protective cytokine reaches far FIGURE 4. Subjects satisfaction analyzed with a VAS score system, with the improvement of subjective symptoms at 2 weeks (V1) and at the endpoint (V2) visit after CBS treatment. The E point columns in the figure show the answers given by patients at enrollment, which refer to their feeling about the last topical therapy administered. higher levels in infants than adults. We did not evaluate the IL-10 content in the final CBS eye drop solution in the present study; the appropriateness of estimating its content in the serum preselection phase is now strongly recommended for future preparations. The role of tear hyperosmolarity in determining pain sensation has recently been hypothesized and demonstrated by our group in DE patients and in an in vitro human conjunctival primary cell culture model, 37 and in analogy with these results, we suggest that the subjective symptom decrease after CBS treatment could also benefit from the reduction in tear osmolarity shown in this study. The level of satisfaction with the treatment was very high for all patients, who homogeneously reported a significant improvement of their discomfort as early as 2 weeks, reaching full satisfaction at endpoint. DE associated with epithelial corneal impairment considerably affects patients quality of life and involves an increasing burden to the patient as the disease becomes more severe. We are currently evaluating whether discomfort symptom relief lasts longer and how long in the patients follow-up after CBS treatment. 6 Ó 2012 Lippincott Williams & Wilkins
7 Cornea Volume 0, Number 0, Month 2012 CBS Eye Drop Therapy in Dry Eye In conclusion, in this work we confirmed that CBS eye drops are a promising therapy for the healing of persistent epithelial defects in severe cases of DE (DEWS severity levels 3 and 4). BUT, Schirmer test, tear osmolarity, corneal esthesiometry, and conjunctival epithelial metaplasia significantly improved after 1 month of treatment. Subjective symptoms of pain and discomfort were satisfactorily relieved after 1 month as a possible consequence of inflammation decrease. The extent of area of corneal injury rather than its duration is the main factor determining the early response and repair process. CBS eye drops need to be prepared by well-equipped and trained laboratory staff. Preparation steps include efficacy and microbiology controls for a reproducible quality of the final product that can be prepared in advance in 1-day doses and stored at 220 C ready to be dispensed. ACKNOWLEDGMENTS The authors wish to thank Dr Primoz Juric, Investigational Drug Service of our hospital, for planning and collaboration, along with Chiara Coslovi, Cristiana Vaselli, and Adriana Terzi for their skilful technical help. REFERENCES 1. Ogawa Y, Okamoto S, Mori T, et al. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2003;31: Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjögren s syndrome. Br J Ophthalmol. 1999;83: López-Plandolit S, Morales MC, Freire V, et al. Plasma rich in growth factors as a therapeutic agent for persistent corneal epithelial defects. Cornea. 2010;29: Sandri G, Bonferoni MC, Rossi S, et al. Platelet lysate formulations based on mucoadhesive polymers for the treatment of corneal lesions. J Pharm Pharmacol. 2011;63: Vajpayee RB, Mukerji N, Tandon R, et al. Evaluation of umbilical cord serum therapy for persistent corneal epithelial defects. Br J Ophthalmol. 2003;87: Shen EP, Hu FR, Lo SC, et al. Comparison of corneal epitheliotrophic capacity among different human blood-derived preparations. Cornea. 2011;30: Coghill JM, Sarantopoulos S, Moran TP, et al. Effector CD4+ T cells, the cytokines they generate, and GVHD: something old and something new. Blood. 2011;117: Baturone R, Soto MJ, Márquez M, et al. Health-related quality of life in patients with primary Sjögren s syndrome: relationship with serum levels of proinflammatory cytokines. Scand J Rheumatol. 2009;38: Yoon KC, Heo H, Im SK, et al. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. Am J Ophthalmol. 2007;144: Yoon KC, Jeong IY, Im SK, et al. Therapeutic effect of umbilical cord serum eyedrops for the treatment of dry eye associated with graftversus-host disease. Bone Marrow Transplant. 2007;39: Yoon KC, Im SK, Park YG, et al. Application of umbilical cord serum eyedrops for the treatment of dry eye syndrome. Cornea. 2006; 25: Yoon KC, Choi J, You IC. Application of umbilical cord serum eyedrops for recurrent corneal erosions. Cornea. 2011;30: Sharma N, Goel M, Velpandian T, et al. Evaluation of umbilical cord serum therapy in acute ocular chemical burns. Invest Ophthalmol Vis Sci. 2011;52: Yoon KC, You IC, Im SK, et al. Application of umbilical cord serum eyedrops for the treatment of neurotrophic keratitis. Ophthalmology. 2007;114: Ang LP, Do TP, Thein ZM, et al. Ex vivo expansion of conjunctival and limbal epithelial cells using cord blood serum-supplemented culture medium. Invest Ophthalmol Vis Sci. 2011;52: Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22: The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Work- Shop (2007). Ocul Surf. 2007;5: Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000; 118: Xu KP, Yagi Y, Tsubota K. Decrease in corneal sensitivity and change in tear function in dry eye. Cornea. 1996;15: Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5: Versura P, Profazio V, Campos EC. Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res. 2010;35: Versura P, Profazio V, Fresina M, et al. A novel scraping cytology score system (SCSS) grades inflammation in dry eye patients. Curr Eye Res. 2009;34: Tseng SC. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology. 1985;92: Lopin E, Deveney T, Asbell PA. Impression cytology: recent advances and applications in dry eye disease. Ocul Surf. 2009;7: Slomovic AR, Caffery B, Gordon KD, Beck R. The CARES Study: a community-based evaluation of a new dry eye therapy. Clin Refract Optometry. 2006;6: Bron AJ, Yokoi N, Gafney E, et al. Predicted phenotypes of dry eye: proposed consequences of its natural history. Ocul Surf. 2009;7: Management and therapy of dry eye disease. Report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5: Lam H, Bleiden L, de Paiva CS, et al. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147: Liu L, Hartwig D, Harloff S, et al. An optimised protocol for the production of autologous serum eyedrops. Graefes Arch Clin Exp Ophthalmol. 2005;243: Yamada C, King KE, Ness PM. Autologous serum eyedrops: literature review and implications for transfusion medicine specialists. Transfusion. 2008;48: Hartwig D, Harloff S, Liu L, et al. Epitheliotrophic capacity of a growth factor preparation produced from platelet concentrates on corneal epithelial cells: a potential agent for the treatment of ocular surface defects? Transfusion. 2004;44: Rainsford E, Reen DJ. Interleukin 10, produced in abundance by human newborn T cells, may be the regulator of increased tolerance associated with cord blood stem cell transplantation. Br J Haematol. 2002;116: Pflugfelder SC, Jones D, Ji Z, et al. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19: Lambiase A, Micera A, Pellegrini G, et al. In vitro evidence of nerve growth factor effects on human conjunctival epithelial cell differentiation and mucin gene expression. Invest Ophthalmol Vis Sci. 2009;50: Enríquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16: Seghaye MC, Heyl W, Grabitz RG, et al. The production of pro- and anti-inflammatory cytokines in neonates assessed by stimulated whole cord blood culture and by plasma levels at birth. Biol Neonate. 1998;73: Versura P, Profazio V, Schiavi C, et al. Hyperosmolar stress upregulates HLA-DR expression in human conjunctival epithelium in dry eye patients and in vitro models. Invest Ophthalmol Vis Sci. 2011;52: Ó 2012 Lippincott Williams & Wilkins 7
A Life that is born makes Life Grow. programmes
 A Life that is born makes Life Grow Cord Blood: current experiences and future programmes Biological eye drops Marina Buzzi Emilia-Romagna Cord Blood Bank SIMT A.M. BO. Policlinico S.Orsola- Malpighi -
A Life that is born makes Life Grow Cord Blood: current experiences and future programmes Biological eye drops Marina Buzzi Emilia-Romagna Cord Blood Bank SIMT A.M. BO. Policlinico S.Orsola- Malpighi -
Treating the Eye with Autologous and Allogeneic Serum Administration. Clarke D. Newman, OD, FAAO
 Treating the Eye with Autologous and Allogeneic Serum Administration cdnewman@ earthlink.net Abstract: This one-hour course will detail the use of blood serum in the treatment of anterior ocular surface
Treating the Eye with Autologous and Allogeneic Serum Administration cdnewman@ earthlink.net Abstract: This one-hour course will detail the use of blood serum in the treatment of anterior ocular surface
Ocular Surface Diseases Course 2016 MIAMI,FLORIDA. Bascom Palmer Eye Institute. Sponsored by the University of Miami Miller School of Medicine
 Ocular Surface Diseases Course 2016 Bascom Palmer Eye Institute Sponsored by the University of Miami Miller School of Medicine MIAMI,FLORIDA April 16, 2016 BASCOM PALMER EYE INSTITUTE Ocular Surface Diseases
Ocular Surface Diseases Course 2016 Bascom Palmer Eye Institute Sponsored by the University of Miami Miller School of Medicine MIAMI,FLORIDA April 16, 2016 BASCOM PALMER EYE INSTITUTE Ocular Surface Diseases
There Are Millions of People at Risk For Dry Eye Who Is Likely to Develop This Irritating Condition?
 There Are Millions of People at Risk For Dry Eye Who Is Likely to Develop This Irritating Condition? Dry eye can be a temporary or chronic condition and occurs when the eye does not produce tears properly,
There Are Millions of People at Risk For Dry Eye Who Is Likely to Develop This Irritating Condition? Dry eye can be a temporary or chronic condition and occurs when the eye does not produce tears properly,
IDENTIFY DRY EYE AN IN-OFFICE TEST TO AID IN DRY EYE DIAGNOSIS. 85% Sensitivity, 94% Specificity 1. This brochure is for use in Canada only.
 IDENTIFY DRY EYE AN IN-OFFICE TEST TO AID IN DRY EYE DIAGNOSIS 85% Sensitivity, 94% Specificity 1 IDENTIFY DRY EYE WITH INFLAMMADRY InflammaDry is the first and only rapid, in-office test that detects
IDENTIFY DRY EYE AN IN-OFFICE TEST TO AID IN DRY EYE DIAGNOSIS 85% Sensitivity, 94% Specificity 1 IDENTIFY DRY EYE WITH INFLAMMADRY InflammaDry is the first and only rapid, in-office test that detects
Grading staining: Oxford Schema The scheme is used to estimate surface damage in dry eye.
 DEWS DRY EYE: DIAGNOSTIC TEST TEMPLATE RAPPORTEUR A.J.Bron 21 st Oct 2004 TEST TO DIAGNOSE VERSION of TEST DESCRIPTION NATURE of STUDY CONDUCT of TESTS Grading staining: Oxford Schema The scheme is used
DEWS DRY EYE: DIAGNOSTIC TEST TEMPLATE RAPPORTEUR A.J.Bron 21 st Oct 2004 TEST TO DIAGNOSE VERSION of TEST DESCRIPTION NATURE of STUDY CONDUCT of TESTS Grading staining: Oxford Schema The scheme is used
5 Frequently Asked Questions About Adult Stem Cell Research
 5 Frequently Asked Questions About Adult Stem Cell Research Stem cells are often referred to in the sociopolitical realm with some level of controversy and beyond that, some level of confusion. Many researchers
5 Frequently Asked Questions About Adult Stem Cell Research Stem cells are often referred to in the sociopolitical realm with some level of controversy and beyond that, some level of confusion. Many researchers
UMBILICAL CORD BLOOD, STEM CELL BANKING
 UMBILICAL CORD BLOOD, STEM CELL BANKING Dr.Sharad Jain MD Blood Transfusion officer, & I/C Transfusion Medicine NSCB Medical College. Jabalpur.MP. Introduction: Every parent during childbirth DREAMS the
UMBILICAL CORD BLOOD, STEM CELL BANKING Dr.Sharad Jain MD Blood Transfusion officer, & I/C Transfusion Medicine NSCB Medical College. Jabalpur.MP. Introduction: Every parent during childbirth DREAMS the
AUTOGENEIC SERUM EYE DROPS
 New York State Council on Human Blood and Transfusion Services GUIDELINES FOR AUTOGENEIC SERUM EYE DROPS 2012 New York State Council on Human Blood and Transfusion Services Wadsworth Center Empire State
New York State Council on Human Blood and Transfusion Services GUIDELINES FOR AUTOGENEIC SERUM EYE DROPS 2012 New York State Council on Human Blood and Transfusion Services Wadsworth Center Empire State
The Definition & Classification of Dry Eye Disease
 Issue: April 2008 The Definition & Classification of Dry Eye Disease Guidelines from the 2007 International Dry Eye Workshop BY MICHAEL A. LEMP, M. D. AND GARY N. FOULKS, M. D., F.A.C.S. The diagnosis
Issue: April 2008 The Definition & Classification of Dry Eye Disease Guidelines from the 2007 International Dry Eye Workshop BY MICHAEL A. LEMP, M. D. AND GARY N. FOULKS, M. D., F.A.C.S. The diagnosis
U.S. Food and Drug Administration
 U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
Cord Blood Collections for the Texas Cord Blood Bank. Obstetrical Providers Training Module
 Cord Blood Collections for the Texas Cord Blood Bank Obstetrical Providers Training Module The Texas Cord Blood Bank The Texas Cord Blood Bank is a network of maternity hospitals and a central laboratory
Cord Blood Collections for the Texas Cord Blood Bank Obstetrical Providers Training Module The Texas Cord Blood Bank The Texas Cord Blood Bank is a network of maternity hospitals and a central laboratory
CORD BLOOD BANKING FAQ
 CORD BLOOD BANKING FAQ Cord Blood & Stem Cells Q: What is umbilical cord blood (UCB)? A: Bone marrow, peripheral blood and UCB constitute the three primary sources of stem cells. Cord blood, which, until
CORD BLOOD BANKING FAQ Cord Blood & Stem Cells Q: What is umbilical cord blood (UCB)? A: Bone marrow, peripheral blood and UCB constitute the three primary sources of stem cells. Cord blood, which, until
Diagnosing dry eye: It s now a fine art
 Article Date: 4/1/2016 Diagnosing dry eye: It s now a fine art Research has led to better, more diverse ways to reach a diagnosis, let alone more knowledge about DED itself. BY MARK B. ABELSON, MD, CM,
Article Date: 4/1/2016 Diagnosing dry eye: It s now a fine art Research has led to better, more diverse ways to reach a diagnosis, let alone more knowledge about DED itself. BY MARK B. ABELSON, MD, CM,
Lacrimal gland. Tear duct into nose
 Dry eye syndrome The aim of this information sheet is to answer any questions you may have about dry eye syndrome. If you have any further questions or concerns, please ask a doctor or nurse caring for
Dry eye syndrome The aim of this information sheet is to answer any questions you may have about dry eye syndrome. If you have any further questions or concerns, please ask a doctor or nurse caring for
A normal eye is protected by a layer of natural tears.
 A normal eye is protected by a layer of natural tears. If you need to use eye drops several times a day to make your eyes comfortably moist, you should consult your eye doctor. Helps increase tear production
A normal eye is protected by a layer of natural tears. If you need to use eye drops several times a day to make your eyes comfortably moist, you should consult your eye doctor. Helps increase tear production
CLIENT AGREEMENT. Copyright 2014 MiracleCord Inc. All rights reserved.
 CLIENT AGREEMENT MiracleCord, Inc. is a provider of services for the collecting, testing, processing, cryopreserving and storing of cells collected from a newbornʼs umbilical cord blood ( Cord Blood )
CLIENT AGREEMENT MiracleCord, Inc. is a provider of services for the collecting, testing, processing, cryopreserving and storing of cells collected from a newbornʼs umbilical cord blood ( Cord Blood )
UMBILICAL CORD BLOOD TRANSPLANTATION: KFSH EXPERIENCE
 UMBILICAL CORD BLOOD TRANSPLANTATION: KFSH EXPERIENCE HIND AL HUMAIDAN, MD,FRCPA Director, Blood Bank (Donor & Transfusion Services) and Stem Cell Cord Blood Bank Consultant Hematopathologist INTRODUCTION
UMBILICAL CORD BLOOD TRANSPLANTATION: KFSH EXPERIENCE HIND AL HUMAIDAN, MD,FRCPA Director, Blood Bank (Donor & Transfusion Services) and Stem Cell Cord Blood Bank Consultant Hematopathologist INTRODUCTION
Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual
 Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.03 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+ Progenitor Cells, cryopreserved Cryopreserved human umbilical
Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.03 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+ Progenitor Cells, cryopreserved Cryopreserved human umbilical
se.bonini@gmail.com Modena March 3, 2009
 A novel molecular marker of severe asthma Sergio Bonini Professor of Medicine, Second University of Naples INMM-CNR, ARTOV, Rome se.bonini@gmail.com i@ il Modena March 3, 2009 Circulating nerve growth
A novel molecular marker of severe asthma Sergio Bonini Professor of Medicine, Second University of Naples INMM-CNR, ARTOV, Rome se.bonini@gmail.com i@ il Modena March 3, 2009 Circulating nerve growth
UMBILICAL CORD BLOOD COLLECTION
 UMBILICAL CORD BLOOD COLLECTION by Frances Verter, PhD Founder & Director, Parent's Guide to Cord Blood Foundation info@parentsguidecordblood.org and Kim Petrella, RN Department of Obstetrics and Gynecology
UMBILICAL CORD BLOOD COLLECTION by Frances Verter, PhD Founder & Director, Parent's Guide to Cord Blood Foundation info@parentsguidecordblood.org and Kim Petrella, RN Department of Obstetrics and Gynecology
Use of Nepafenac in Lasek Johnny L. Gayton, MD
 Use of Nepafenac in Lasek Johnny L. Gayton, MD Kathrine Jackson, COA Eyesight Associates, Warner Robins, GA Analgesic Effect NSAIDs provide analgesic effect 1-3 Minimize pain and discomfort following cataract
Use of Nepafenac in Lasek Johnny L. Gayton, MD Kathrine Jackson, COA Eyesight Associates, Warner Robins, GA Analgesic Effect NSAIDs provide analgesic effect 1-3 Minimize pain and discomfort following cataract
Keeping Your Eyes Healthy after Treatment for Childhood Cancer
 Keeping Your Eyes Healthy after Treatment for Childhood Cancer High doses of radiation to the brain, eye, or eye socket (orbit) during treatment for childhood cancer can have a long-lasting affect on the
Keeping Your Eyes Healthy after Treatment for Childhood Cancer High doses of radiation to the brain, eye, or eye socket (orbit) during treatment for childhood cancer can have a long-lasting affect on the
Dry Eye: Natural History, Diagnosis and Treatment By Jeffrey P. Gilbard, M.D.
 Dry Eye: Natural History, Diagnosis and Treatment By Jeffrey P. Gilbard, M.D. Our understanding of dry eye disorders has improved dramatically over the past several years, enhancing our ability to diagnose
Dry Eye: Natural History, Diagnosis and Treatment By Jeffrey P. Gilbard, M.D. Our understanding of dry eye disorders has improved dramatically over the past several years, enhancing our ability to diagnose
How To Treat Leukaemia With Cord Blood Stem Cell
 Cord blood for the treatment of acute lymphoblastic leukemia in young children By Caitlin McGreevy Kiara Paramjothy Pass with Merit RESEARCH PAPER BASED ON PATHOLOGY LECTURES AT MEDLINK 2011 1 Abstract:
Cord blood for the treatment of acute lymphoblastic leukemia in young children By Caitlin McGreevy Kiara Paramjothy Pass with Merit RESEARCH PAPER BASED ON PATHOLOGY LECTURES AT MEDLINK 2011 1 Abstract:
Stem Cell Quick Guide: Stem Cell Basics
 Stem Cell Quick Guide: Stem Cell Basics What is a Stem Cell? Stem cells are the starting point from which the rest of the body grows. The adult human body is made up of hundreds of millions of different
Stem Cell Quick Guide: Stem Cell Basics What is a Stem Cell? Stem cells are the starting point from which the rest of the body grows. The adult human body is made up of hundreds of millions of different
Future Health Technologies. From Academia to Industry The Stem Cell Challenge. Roger J. Dainty MBE FIScT UK Managing Director
 Future Health Technologies From Academia to Industry The Stem Cell Challenge Roger J. Dainty MBE FIScT UK Managing Director How Did I Get Here? Working as a Research Medical Technician (Birmingham University
Future Health Technologies From Academia to Industry The Stem Cell Challenge Roger J. Dainty MBE FIScT UK Managing Director How Did I Get Here? Working as a Research Medical Technician (Birmingham University
A Cure for Sickle Cell Anemia and Thalassemia
 IV Simpósio Internacional de Hemoglobinopatias A Cure for Sickle Cell Anemia and Thalassemia Bertram Lubin, MD and Mark Walters, MD 4 September 2007 Topics to be covered Cord blood: Importance and biology
IV Simpósio Internacional de Hemoglobinopatias A Cure for Sickle Cell Anemia and Thalassemia Bertram Lubin, MD and Mark Walters, MD 4 September 2007 Topics to be covered Cord blood: Importance and biology
VITAMIN C AND INFECTIOUS DISEASE: A REVIEW OF THE LITERATURE AND THE RESULTS OF A RANDOMIZED, DOUBLE-BLIND, PROSPECTIVE STUDY OVER 8 YEARS
 39 Chapter 3 VITAMIN C AND INFECTIOUS DISEASE: A REVIEW OF THE LITERATURE AND THE RESULTS OF A RANDOMIZED, DOUBLE-BLIND, PROSPECTIVE STUDY OVER 8 YEARS Maxine Briggs TABLE OF CONTENTS I. Review of the
39 Chapter 3 VITAMIN C AND INFECTIOUS DISEASE: A REVIEW OF THE LITERATURE AND THE RESULTS OF A RANDOMIZED, DOUBLE-BLIND, PROSPECTIVE STUDY OVER 8 YEARS Maxine Briggs TABLE OF CONTENTS I. Review of the
Bringing Objectivity to Dry Eye Diagnosis and Assessment
 Bringing Objectivity to Dry Eye Diagnosis and Assessment Tear Osmolarity Testing Helps Solve the Dilemma of Identifying Dry Eye by Jay S. Pepose, MD, PHD as published online in Ophthalmology Management
Bringing Objectivity to Dry Eye Diagnosis and Assessment Tear Osmolarity Testing Helps Solve the Dilemma of Identifying Dry Eye by Jay S. Pepose, MD, PHD as published online in Ophthalmology Management
Acknowledgements. Dry Eye Update. Dry Eye. Dry Eye. Dry Eye. 2014 Monterey Symposium
 2014 Monterey Symposium Update Jimmy Jackson, OD, FAAO President InSight Lasik Acknowledgements I have received honoraria from Alcon Laboratories, Inc for speaking engagements. I have received research
2014 Monterey Symposium Update Jimmy Jackson, OD, FAAO President InSight Lasik Acknowledgements I have received honoraria from Alcon Laboratories, Inc for speaking engagements. I have received research
Hematopoiesis i starts from. HPC differentiates t to sequentially more mature. HPC circulate in very low
 Upstate Cord Blood Bank Hematopoietic Progenitor Cells Hematopoiesis i starts from self renewing HPC HPC differentiates t to sequentially more mature blood cells HPC circulate in very low numbers in adults
Upstate Cord Blood Bank Hematopoietic Progenitor Cells Hematopoiesis i starts from self renewing HPC HPC differentiates t to sequentially more mature blood cells HPC circulate in very low numbers in adults
Eye Manifestations of Lupus And Sjogren s Syndrome
 Eye Manifestations of Lupus And Sjogren s Syndrome Based on a presentation by Dr. N. Kevin Wade at the BC Lupus Society Symposium held October 22, 2005. Background Dr. Wade completed his MD in 1998 at
Eye Manifestations of Lupus And Sjogren s Syndrome Based on a presentation by Dr. N. Kevin Wade at the BC Lupus Society Symposium held October 22, 2005. Background Dr. Wade completed his MD in 1998 at
JOINT COMMISSION INTERNATIONAL ACCREDITATION STANDARDS FOR. 2nd Edition
 JOINT COMMISSION INTERNATIONAL ACCREDITATION STANDARDS FOR CliniCAl laboratories 2nd Edition Effective 1 April 2010 International Patient Safety Goals (IPSG) Goals The following is a list of all goals.
JOINT COMMISSION INTERNATIONAL ACCREDITATION STANDARDS FOR CliniCAl laboratories 2nd Edition Effective 1 April 2010 International Patient Safety Goals (IPSG) Goals The following is a list of all goals.
STANDARD BLOOD PRODUCTS AND SERVICES
 STANDARD BLOOD PRODUCTS AND SERVICES Policy NHP reimburses contracted providers for the medically necessary administration (transfusion) of blood and standard blood products. Prerequisites Authorization,
STANDARD BLOOD PRODUCTS AND SERVICES Policy NHP reimburses contracted providers for the medically necessary administration (transfusion) of blood and standard blood products. Prerequisites Authorization,
A Genetic Analysis of Rheumatoid Arthritis
 A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
Laser-Assisted In Situ Keratomileusis for Patients With Dry Eye
 CLINICAL SCIENCES Laser-Assisted In Situ Keratomileusis for Patients With Dry Eye Ikuko Toda, MD; Naoko Asano-Kato, MD; Yoshiko Hori-Komai, MD; Kazuo Tsubota, MD Objective: To evaluate the efficacy and
CLINICAL SCIENCES Laser-Assisted In Situ Keratomileusis for Patients With Dry Eye Ikuko Toda, MD; Naoko Asano-Kato, MD; Yoshiko Hori-Komai, MD; Kazuo Tsubota, MD Objective: To evaluate the efficacy and
Not All Stem Cells are the Same
 Cord Blood Banking and Transplantation Jennifer Willert, M.D. Hematology/Oncology Blood and Marrow Transplant Rady Children s Hospital San Diego Clinical Professor UCSD Not All Stem Cells are the Same
Cord Blood Banking and Transplantation Jennifer Willert, M.D. Hematology/Oncology Blood and Marrow Transplant Rady Children s Hospital San Diego Clinical Professor UCSD Not All Stem Cells are the Same
MOST FREQUENTLY ASKED CLIENT QUESTIONS
 6/2 Victor Crescent Narre Warren VIC 3805 Ph: (03) 8794 8255 Fax: (03) 8794 8256 www.ortho-k.com.au Sleep your way to great vision MOST FREQUENTLY ASKED CLIENT QUESTIONS What is ortho-k? Ortho-k (Orthokeratology)
6/2 Victor Crescent Narre Warren VIC 3805 Ph: (03) 8794 8255 Fax: (03) 8794 8256 www.ortho-k.com.au Sleep your way to great vision MOST FREQUENTLY ASKED CLIENT QUESTIONS What is ortho-k? Ortho-k (Orthokeratology)
The Prospect of Stem Cell Therapy in Multiple Sclerosis. Multiple sclerosis is a multifocal inflammatory disease of the central
 The Prospect of Stem Cell Therapy in Multiple Sclerosis Multiple sclerosis is a multifocal inflammatory disease of the central nervous system that generally affects young individuals, causing paralysis
The Prospect of Stem Cell Therapy in Multiple Sclerosis Multiple sclerosis is a multifocal inflammatory disease of the central nervous system that generally affects young individuals, causing paralysis
Patient-Reported Outcomes with LASIK (PROWL-1) Results
 Patient-Reported Outcomes with LASIK (PROWL-1) Results Elizabeth M. Hofmeister, MD CAPT, MC, USN Naval Medical Center San Diego Refractive Surgery Advisor for Navy Ophthalmology Assistant Professor of
Patient-Reported Outcomes with LASIK (PROWL-1) Results Elizabeth M. Hofmeister, MD CAPT, MC, USN Naval Medical Center San Diego Refractive Surgery Advisor for Navy Ophthalmology Assistant Professor of
C-30078 Diagnosis and Management of Dry Eyes Part 1. Dr Colm McAlinden, BSc (Hons), MSc, PhD and Dr Eirini Skiadaresi, MD.
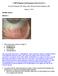 C-30078 Diagnosis and Management of Dry Eyes Part 1 Dr Colm McAlinden, BSc (Hons), MSc, PhD and Dr Eirini Skiadaresi, MD January 11 2013 Detailed Answers IMAGE A Image Courtesy of Professor Giuseppe Ravalico
C-30078 Diagnosis and Management of Dry Eyes Part 1 Dr Colm McAlinden, BSc (Hons), MSc, PhD and Dr Eirini Skiadaresi, MD January 11 2013 Detailed Answers IMAGE A Image Courtesy of Professor Giuseppe Ravalico
OPHTHALMOLOGIST PERCEPTIONS REGARDING TREATMENT OF MODERATE TO SEVERE DRY EYE: RESULTS OF A PHYSICIAN SURVEY
 OPHTHALMOLOGIST PERCEPTIONS REGARDING TREATMENT OF MODERATE TO SEVERE DRY EYE: RESULTS OF A PHYSICIAN SURVEY By Penny A. Asbell MD MBA* and Scott Spiegel PhD ABSTRACT Purpose: To understand ophthalmologists
OPHTHALMOLOGIST PERCEPTIONS REGARDING TREATMENT OF MODERATE TO SEVERE DRY EYE: RESULTS OF A PHYSICIAN SURVEY By Penny A. Asbell MD MBA* and Scott Spiegel PhD ABSTRACT Purpose: To understand ophthalmologists
Medicare and Corneal Surgery: Cosmetic versus Functional
 Medicare and Corneal Surgery: Cosmetic versus Functional Riva Lee Asbell INTRODUCTION With the introduction of several new CPT (Current Procedural Terminology) codes for cornea, corneal coding is in the
Medicare and Corneal Surgery: Cosmetic versus Functional Riva Lee Asbell INTRODUCTION With the introduction of several new CPT (Current Procedural Terminology) codes for cornea, corneal coding is in the
OVERVIEW OF CALIFORNIA DPA/TPA LAWS
 OVERVIEW OF CALIFORNIA DPA/TPA LAWS Robert E. Dister, O.D., J.D., M.S., F.A.A.O. Michael G. Harris, O.D., J.D., M.S., F.A.A.O. U.C. Berkeley School of Optometry CALIFORNIA OPTOMETRY DRUG PRIVILEGES DPA
OVERVIEW OF CALIFORNIA DPA/TPA LAWS Robert E. Dister, O.D., J.D., M.S., F.A.A.O. Michael G. Harris, O.D., J.D., M.S., F.A.A.O. U.C. Berkeley School of Optometry CALIFORNIA OPTOMETRY DRUG PRIVILEGES DPA
APPENDIX B SAMPLE INFORMED CONSENT FORM
 APPENDIX B SAMPLE INFORMED CONSENT FORM B.1 INFORMED CONSENT TO PARTICIPATE IN A RESEARCH STUDY Umbilical Cord Blood Banking for Transplantation You are being asked to take part in a research study that
APPENDIX B SAMPLE INFORMED CONSENT FORM B.1 INFORMED CONSENT TO PARTICIPATE IN A RESEARCH STUDY Umbilical Cord Blood Banking for Transplantation You are being asked to take part in a research study that
BSM Connection elearning Course
 BSM Connection elearning Course Scope of the Eye Care Practice 2008, BSM Consulting All Rights Reserved. Table of Contents OVERVIEW...1 THREE O S IN EYE CARE...1 ROUTINE VS. MEDICAL EXAMS...2 CONTACT LENSES/GLASSES...2
BSM Connection elearning Course Scope of the Eye Care Practice 2008, BSM Consulting All Rights Reserved. Table of Contents OVERVIEW...1 THREE O S IN EYE CARE...1 ROUTINE VS. MEDICAL EXAMS...2 CONTACT LENSES/GLASSES...2
A Gift for life. HTA Licence No. 22653
 A Gift for life HTA Licence No. 22653 2 3 the best possible gift for your new born child. 4 6 8 9 10 12 14 16 19 what is cord blood current treatments services contents properties for treatment process
A Gift for life HTA Licence No. 22653 2 3 the best possible gift for your new born child. 4 6 8 9 10 12 14 16 19 what is cord blood current treatments services contents properties for treatment process
Autologus serum in treatment of dry eye disorder: An evaluation
 ISSN: 2231-3354 Received on: 15-05-2012 Revised on: 28-05-2012 Accepted on: 03-06-2012 DOI: 10.7324/JAPS.2012.2627 Autologus serum in treatment of dry eye disorder: An evaluation Rashmi Singh, Ashish Gangwar,
ISSN: 2231-3354 Received on: 15-05-2012 Revised on: 28-05-2012 Accepted on: 03-06-2012 DOI: 10.7324/JAPS.2012.2627 Autologus serum in treatment of dry eye disorder: An evaluation Rashmi Singh, Ashish Gangwar,
EU Marie Skłodowska-Curie Initial Training Network!! EDEN
 ! EU Marie Skłodowska-Curie Initial Training Network!! EDEN Title: European Dry Eye Network! Call: HORIZON 2020-MSCA-ITN-2014 Proposal Number: 642760 Type of position: Early Stage Researcher (ESR)/ PhD
! EU Marie Skłodowska-Curie Initial Training Network!! EDEN Title: European Dry Eye Network! Call: HORIZON 2020-MSCA-ITN-2014 Proposal Number: 642760 Type of position: Early Stage Researcher (ESR)/ PhD
SMF Awareness Seminar 2014
 SMF Awareness Seminar 2014 Clinical Evaluation for In Vitro Diagnostic Medical Devices Dr Jiang Naxin Health Sciences Authority Medical Device Branch 1 In vitro diagnostic product means Definition of IVD
SMF Awareness Seminar 2014 Clinical Evaluation for In Vitro Diagnostic Medical Devices Dr Jiang Naxin Health Sciences Authority Medical Device Branch 1 In vitro diagnostic product means Definition of IVD
Class Im MD A novel device for Automated Analysis of Tear Film Dynamics. Michael Ring, DI(FH) Upper Austria University of Applied Sciences
 Class Im MD A novel device for Automated Analysis of Tear Film Dynamics Michael Ring, DI(FH) Upper Austria University of Applied Sciences Aim of the Research Develop a method to give information on the
Class Im MD A novel device for Automated Analysis of Tear Film Dynamics Michael Ring, DI(FH) Upper Austria University of Applied Sciences Aim of the Research Develop a method to give information on the
SAVE A LIFE... BY GIVING LIFE!
 SAVE A LIFE... BY GIVING LIFE! FOLLOW US ON: HÉMA-QUÉBEC PUBLIC CORD BLOOD BANK www.hema-quebec.qc.ca Scan this code with your smart phone to access the page Register to the Public Cord Blood Bank on the
SAVE A LIFE... BY GIVING LIFE! FOLLOW US ON: HÉMA-QUÉBEC PUBLIC CORD BLOOD BANK www.hema-quebec.qc.ca Scan this code with your smart phone to access the page Register to the Public Cord Blood Bank on the
Public Cord Blood Tissue Bank Committee on Health Care Services and Representative Peaden
 HOUSE OF REPRESENTATIVES COMMITTEE ON HEALTH CARE SERVICES ANALYSIS BILL #: HB 2337 (PCB HCS 00-07) RELATING TO: SPONSOR(S): TIED BILL(S): Public Cord Blood Tissue Bank Committee on Health Care Services
HOUSE OF REPRESENTATIVES COMMITTEE ON HEALTH CARE SERVICES ANALYSIS BILL #: HB 2337 (PCB HCS 00-07) RELATING TO: SPONSOR(S): TIED BILL(S): Public Cord Blood Tissue Bank Committee on Health Care Services
GT-020 Phase 1 Clinical Trial: Results of Second Cohort
 GT-020 Phase 1 Clinical Trial: Results of Second Cohort July 29, 2014 NASDAQ: GALT www.galectintherapeutics.com 2014 Galectin Therapeutics inc. Forward-Looking Statement This presentation contains, in
GT-020 Phase 1 Clinical Trial: Results of Second Cohort July 29, 2014 NASDAQ: GALT www.galectintherapeutics.com 2014 Galectin Therapeutics inc. Forward-Looking Statement This presentation contains, in
DEPARTMENT OF BONE MARROW AND STEM CELL TRANSPLANT
 www.narayanahealth.org DEPARTMENT OF BONE MARROW AND STEM CELL TRANSPLANT About Narayana Health City Narayana Health, one of India's largest and the world's most economical healthcare service providers
www.narayanahealth.org DEPARTMENT OF BONE MARROW AND STEM CELL TRANSPLANT About Narayana Health City Narayana Health, one of India's largest and the world's most economical healthcare service providers
Bone Marrow (Stem Cell) Transplant for Sickle Cell Disease
 Bone Marrow (Stem Cell) Transplant for Sickle Cell Disease Bone Marrow (Stem Cell) Transplant for Sickle Cell Disease 1 Produced by St. Jude Children s Research Hospital Departments of Hematology, Patient
Bone Marrow (Stem Cell) Transplant for Sickle Cell Disease Bone Marrow (Stem Cell) Transplant for Sickle Cell Disease 1 Produced by St. Jude Children s Research Hospital Departments of Hematology, Patient
Specific Standards of Accreditation for Residency Programs in Pediatric Hematology/Oncology
 Specific Standards of Accreditation for Residency Programs in Pediatric Hematology/Oncology INTRODUCTION 2009 A university wishing to have an accredited program in Pediatric Hematology/Oncology must also
Specific Standards of Accreditation for Residency Programs in Pediatric Hematology/Oncology INTRODUCTION 2009 A university wishing to have an accredited program in Pediatric Hematology/Oncology must also
Dry eyes holding you back? Ask your doctor about LipiFlow.
 Dry eyes holding you back? Ask your doctor about LipiFlow. Think you may have dry eye? Take the quiz below to find out. Do you experience sensitivity to light, blurred vision, a burning sensation, or discomfort
Dry eyes holding you back? Ask your doctor about LipiFlow. Think you may have dry eye? Take the quiz below to find out. Do you experience sensitivity to light, blurred vision, a burning sensation, or discomfort
Discover the Possibilities Born With Your Baby
 Discover the Possibilities Born With Your Baby Your Simple Guide to Saving Cord Blood The Power and Promise The lifesaving power of cord blood and regenerative healing potential of cord blood and cord
Discover the Possibilities Born With Your Baby Your Simple Guide to Saving Cord Blood The Power and Promise The lifesaving power of cord blood and regenerative healing potential of cord blood and cord
Blood-Forming Stem Cell Transplants
 Blood-Forming Stem Cell Transplants What are bone marrow and hematopoietic stem cells? Bone marrow is the soft, sponge-like material found inside bones. It contains immature cells known as hematopoietic
Blood-Forming Stem Cell Transplants What are bone marrow and hematopoietic stem cells? Bone marrow is the soft, sponge-like material found inside bones. It contains immature cells known as hematopoietic
Managing Post-Operative Complications for LASIK and PRK
 Managing Post-Operative Complications for LASIK and PRK LASIK Flap Complications Epithelial defects o Cause Basement membrane dystrophy Recurrent erosion syndrome Dry eyes Trauma PRK as alternative Pre-treat
Managing Post-Operative Complications for LASIK and PRK LASIK Flap Complications Epithelial defects o Cause Basement membrane dystrophy Recurrent erosion syndrome Dry eyes Trauma PRK as alternative Pre-treat
your complete stem cell bank
 your complete stem cell bank HYDERABAD - 88985 000 888, WARANGAL - 8297 256 777 VISAKHAPATNAM - 7799 990 774 VIJAYAWADA AND GUNTUR - 7799 990 771 NELLORE - 7799 990 772, KADAPA - 8297 256 700 RAJAHMUNDRY
your complete stem cell bank HYDERABAD - 88985 000 888, WARANGAL - 8297 256 777 VISAKHAPATNAM - 7799 990 774 VIJAYAWADA AND GUNTUR - 7799 990 771 NELLORE - 7799 990 772, KADAPA - 8297 256 700 RAJAHMUNDRY
Support Program for Improving Graduate School Education Advanced Education Program for Integrated Clinical, Basic and Social Medicine
 Support Program for Improving Graduate School Education Advanced Education Program for Integrated Clinical, Basic and Social Medicine January 27, 2009 Dear Professors (representative) of departments, Subject:
Support Program for Improving Graduate School Education Advanced Education Program for Integrated Clinical, Basic and Social Medicine January 27, 2009 Dear Professors (representative) of departments, Subject:
Bone Marrow Transplantation and Peripheral Blood Stem Cell Transplantation: Questions and Answers. Key Points
 CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Bone Marrow Transplantation
CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Bone Marrow Transplantation
Cold as an adjunctive therapy for headache
 Cold as an adjunctive therapy for headache Seymour Diamond, MD Frederick G. Freltag, DO Preview Patients with acute headache are often so vexed by pain that they seek out numerous physicians and headache
Cold as an adjunctive therapy for headache Seymour Diamond, MD Frederick G. Freltag, DO Preview Patients with acute headache are often so vexed by pain that they seek out numerous physicians and headache
Stem Cell Transplantation
 Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
HAEMOPHILIA & UMBILICAL CORD BLOOD TRANSPLANT
 HAEMOPHILIA & UMBILICAL CORD BLOOD TRANSPLANT Haemostatic System in Body Blood vessels Platelets Plasma coagulation system Proteolytic or Fibrinolytic system How Bleeding Stops Vasoconstriction Platelet
HAEMOPHILIA & UMBILICAL CORD BLOOD TRANSPLANT Haemostatic System in Body Blood vessels Platelets Plasma coagulation system Proteolytic or Fibrinolytic system How Bleeding Stops Vasoconstriction Platelet
Cord Blood Bank Business Plan
 Cord Blood Bank Business Plan A sample of how to create a new program Produced by the AABB subsection: Cellular Therapy Business Management with major contributions by Nicole Omer TABLE OF CONTENTS Page
Cord Blood Bank Business Plan A sample of how to create a new program Produced by the AABB subsection: Cellular Therapy Business Management with major contributions by Nicole Omer TABLE OF CONTENTS Page
Stem cells will change medicine as we know it. Are you ready? STEM CELL BANKING
 Stem cells will change medicine as we know it. Are you ready? STEM CELL BANKING Stem cell banking with Store-A-Tooth is a unique investment in the health of your family. WHY BANK STEM CELLS? YOU PLAN FOR
Stem cells will change medicine as we know it. Are you ready? STEM CELL BANKING Stem cell banking with Store-A-Tooth is a unique investment in the health of your family. WHY BANK STEM CELLS? YOU PLAN FOR
Thank you in advance- your feedback and insights are invaluable to the BFS team. We really appreciate your ongoing support.
 Survey Questions SECTION 1: INTRODUCTION The Boston Foundation for Sight (BFS) is requesting your help in responding to a survey. We are trying to create social networking and support systems to better
Survey Questions SECTION 1: INTRODUCTION The Boston Foundation for Sight (BFS) is requesting your help in responding to a survey. We are trying to create social networking and support systems to better
Sepax. your child s cord blood is in safe hands. World leader in adult stem cell processing
 Sepax your child s cord blood is in safe hands World leader in adult stem cell processing Biosafe Experience Credibility Innovation Dear Customer, Innovation in cell processing is our credo and this is
Sepax your child s cord blood is in safe hands World leader in adult stem cell processing Biosafe Experience Credibility Innovation Dear Customer, Innovation in cell processing is our credo and this is
STATE OF NEBRASKA STATUTES RELATING TO OPTOMETRY PRACTICE ACT
 2014 STATE OF NEBRASKA STATUTES RELATING TO OPTOMETRY PRACTICE ACT Department of Health and Human Services Division of Public Health Licensure Unit 301 Centennial Mall South, Third Floor PO Box 94986 Lincoln,
2014 STATE OF NEBRASKA STATUTES RELATING TO OPTOMETRY PRACTICE ACT Department of Health and Human Services Division of Public Health Licensure Unit 301 Centennial Mall South, Third Floor PO Box 94986 Lincoln,
AssureImmune. Cord Blood: For Something That Precious, Bank with the Best. Important Facts for When You re Expecting. AssureImmune.
 AssureImmune AssureImmune Cord Blood: For Something That Precious, Bank with the Best Important Facts for When You re Expecting Banking your baby s umbilical cord blood could be a potentially lifesaving
AssureImmune AssureImmune Cord Blood: For Something That Precious, Bank with the Best Important Facts for When You re Expecting Banking your baby s umbilical cord blood could be a potentially lifesaving
Jamie Peregrine, MD, PGY-4 KU-Wichita, OB/GYN Wesley Medical Center
 Jamie Peregrine, MD, PGY-4 KU-Wichita, OB/GYN Wesley Medical Center Uses for umbilical cord stem cells Describe the indications and uses for umbilical cord stem cells. Counsel patients on the advantages
Jamie Peregrine, MD, PGY-4 KU-Wichita, OB/GYN Wesley Medical Center Uses for umbilical cord stem cells Describe the indications and uses for umbilical cord stem cells. Counsel patients on the advantages
STEM CELL THERAPEUTIC AND RESEARCH ACT OF 2005
 STEM CELL THERAPEUTIC AND RESEARCH ACT OF 2005 VerDate 14-DEC-2004 06:53 Jan 05, 2006 Jkt 039139 PO 00000 Frm 00001 Fmt 6579 Sfmt 6579 E:\PUBLAW\PUBL129.109 APPS10 PsN: PUBL129 119 STAT. 2550 PUBLIC LAW
STEM CELL THERAPEUTIC AND RESEARCH ACT OF 2005 VerDate 14-DEC-2004 06:53 Jan 05, 2006 Jkt 039139 PO 00000 Frm 00001 Fmt 6579 Sfmt 6579 E:\PUBLAW\PUBL129.109 APPS10 PsN: PUBL129 119 STAT. 2550 PUBLIC LAW
XIV ANNUAL MEETING OF NURSING
 Archive of Oncology 2000;8(Suppl 1):69. SESSION 5 XIV ANNUAL MEETING OF NURSING 69 Archive of Oncology 2000;8(Suppl 1):70. 70 UDC: 616.34-007.272:616-089.8:614.39 I. GAJI M. ULAFI Æ. MIHAJLOVI R. NE I
Archive of Oncology 2000;8(Suppl 1):69. SESSION 5 XIV ANNUAL MEETING OF NURSING 69 Archive of Oncology 2000;8(Suppl 1):70. 70 UDC: 616.34-007.272:616-089.8:614.39 I. GAJI M. ULAFI Æ. MIHAJLOVI R. NE I
HemaCare Corporation Company Overview. Comprehensive Product and Service Solutions for BioResearch and Cellular Therapy
 HemaCare Corporation Company Overview Comprehensive Product and Service Solutions for BioResearch and Cellular Therapy 2015 HemaCare Corporation Leading provider of apheresis products, human blood cells,
HemaCare Corporation Company Overview Comprehensive Product and Service Solutions for BioResearch and Cellular Therapy 2015 HemaCare Corporation Leading provider of apheresis products, human blood cells,
Cord Blood, Cord Life
 投 稿 類 別 : 英 文 寫 作 類 篇 名 : Cord Blood, Cord Life 作 者 : 詹 苡 廷 國 立 台 中 家 商 應 用 外 語 科 三 年 二 班 張 詠 婷 國 立 台 中 家 商 應 用 外 語 科 三 年 二 班 賴 雅 云 國 立 台 中 家 商 應 用 外 語 科 三 年 二 班 指 導 老 師 : 廖 明 珠 Abstract In the past few
投 稿 類 別 : 英 文 寫 作 類 篇 名 : Cord Blood, Cord Life 作 者 : 詹 苡 廷 國 立 台 中 家 商 應 用 外 語 科 三 年 二 班 張 詠 婷 國 立 台 中 家 商 應 用 外 語 科 三 年 二 班 賴 雅 云 國 立 台 中 家 商 應 用 外 語 科 三 年 二 班 指 導 老 師 : 廖 明 珠 Abstract In the past few
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
APPLICATION FOR SITE INSPECTION Eye Bank Association of America
 APPLICATION FOR SITE INSPECTION Eye Bank Association of America Complete this application to begin the process for inspection and accreditation by the Eye Bank Association of America (EBAA). See the EBAA
APPLICATION FOR SITE INSPECTION Eye Bank Association of America Complete this application to begin the process for inspection and accreditation by the Eye Bank Association of America (EBAA). See the EBAA
TRANSFUSION MEDICINE
 TRANSFUSION MEDICINE Transfusion medicine is a one-month per year rotation for a total of three months. During each rotation the resident is exposed to the basic concepts of transfusion medicine. Specific
TRANSFUSION MEDICINE Transfusion medicine is a one-month per year rotation for a total of three months. During each rotation the resident is exposed to the basic concepts of transfusion medicine. Specific
RealLine HCV PCR Qualitative - Uni-Format
 Instructions for use PCR KIT FOR EXTRACTION OF RNA AND REAL TIME PCR DETECTION KIT FOR HEPATITIS C VIRUS RNA Research Use Only Qualitative Uni Format VBD0798 48 tests valid from: December 2013 Rev11122013
Instructions for use PCR KIT FOR EXTRACTION OF RNA AND REAL TIME PCR DETECTION KIT FOR HEPATITIS C VIRUS RNA Research Use Only Qualitative Uni Format VBD0798 48 tests valid from: December 2013 Rev11122013
Bone Marrow/Stem Cell Transplant
 Blue Distinction Centers for Transplants Program Selection Criteria for 2010 Mid-Point Designations To qualify as a Blue Distinction Center for Transplants (), each facility must satisfy s quality based
Blue Distinction Centers for Transplants Program Selection Criteria for 2010 Mid-Point Designations To qualify as a Blue Distinction Center for Transplants (), each facility must satisfy s quality based
Collecting cord blood to save and improve lives. Rebecca Roberts
 Collecting cord blood to save and improve lives Rebecca Roberts Cells4Life Introduction to stem cells Why cord blood is important Legislation Cells4Life Cord blood collection What is a stem cell? Cells
Collecting cord blood to save and improve lives Rebecca Roberts Cells4Life Introduction to stem cells Why cord blood is important Legislation Cells4Life Cord blood collection What is a stem cell? Cells
Human Umbilical Cord-derived Multipotent Mesenchymal Stromal Cells (huc-msc) Handling Instructions
 Human Umbilical Cord-derived Multipotent Mesenchymal Stromal Cells (huc-msc) Order No.: 19401-005, 19401-010 Handling Instructions For preclinical ex vivo use. Not intended for therapeutic use. Table of
Human Umbilical Cord-derived Multipotent Mesenchymal Stromal Cells (huc-msc) Order No.: 19401-005, 19401-010 Handling Instructions For preclinical ex vivo use. Not intended for therapeutic use. Table of
Basic Science in Medicine
 Medical Journal of th e Islamic Republic of Iran Volume 18 Number 3 Fall 1383 November 2004 Basic Science in Medicine ] EXPANSION OF HUMAN CORD BLOOD PRIMITIVE PROGENITORS IN SERUM-FREE MEDIA USING HUMAN
Medical Journal of th e Islamic Republic of Iran Volume 18 Number 3 Fall 1383 November 2004 Basic Science in Medicine ] EXPANSION OF HUMAN CORD BLOOD PRIMITIVE PROGENITORS IN SERUM-FREE MEDIA USING HUMAN
Descemet s Stripping Endothelial Keratoplasty (DSEK)
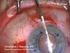 Descemet s Stripping Endothelial Keratoplasty (DSEK) Your doctor has decided that you will benefit from a corneal transplant operation. This handout will explain your options to you. It explains the differences
Descemet s Stripping Endothelial Keratoplasty (DSEK) Your doctor has decided that you will benefit from a corneal transplant operation. This handout will explain your options to you. It explains the differences
Cord Blood Stem Cell Transplantation
 LEUKEMIA LYMPHOMA MYELOMA FACTS Cord Blood Stem Cell Transplantation No. 2 in a series providing the latest information on blood cancers Highlights Umbilical cord blood, like bone marrow and peripheral
LEUKEMIA LYMPHOMA MYELOMA FACTS Cord Blood Stem Cell Transplantation No. 2 in a series providing the latest information on blood cancers Highlights Umbilical cord blood, like bone marrow and peripheral
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
Newborn Stem Cells from Cord Blood and the Brain: Repairing Injury and Improving Function
 BACKROUNDER: Newborn Stem Cells from Cord Blood and the Brain: Repairing Injury and Improving Function Introduction WITH ONGOING DEVELOPMENTS IN RESEARCH, it is estimated that 1 in every 3 people may benefit
BACKROUNDER: Newborn Stem Cells from Cord Blood and the Brain: Repairing Injury and Improving Function Introduction WITH ONGOING DEVELOPMENTS IN RESEARCH, it is estimated that 1 in every 3 people may benefit
Human Peripheral Blood Mononuclear Cell (PBMC) Manual
 Human Peripheral Blood Mononuclear Cell (PBMC) Manual INSTRUCTION MANUAL ZBM0063.04 SHIPPING CONDITIONS Human Peripheral Blood Mononuclear Cells, cryopreserved Cryopreserved human peripheral blood mononuclear
Human Peripheral Blood Mononuclear Cell (PBMC) Manual INSTRUCTION MANUAL ZBM0063.04 SHIPPING CONDITIONS Human Peripheral Blood Mononuclear Cells, cryopreserved Cryopreserved human peripheral blood mononuclear
INMA LABORATORY MANUAL
 INMA LABORATORY MANUAL NUTRITION BIOMARKERS Cohorte-VALENCIA This manual describes the collection, separation, storage and transport of blood samples for the INMA study. Careful collection of samples is
INMA LABORATORY MANUAL NUTRITION BIOMARKERS Cohorte-VALENCIA This manual describes the collection, separation, storage and transport of blood samples for the INMA study. Careful collection of samples is
Comparative Evaluation of Dry Eye Following Cataract Surgery: A Study from North India
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 13, Issue 6 Ver. III (Jun. 2014), PP 13-18 Comparative Evaluation of Dry Eye Following Cataract Surgery:
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 13, Issue 6 Ver. III (Jun. 2014), PP 13-18 Comparative Evaluation of Dry Eye Following Cataract Surgery:
Dr Saeed Al Amoudi. Consultant Hematologist King s college hospital Director of Regional Lab Jeddah
 Dr Saeed Al Amoudi Consultant Hematologist King s college hospital Director of Regional Lab Jeddah MAGIC BULLET Cord Blood Banking What is Cord Blood? Cord blood is the blood left in the placenta and umbilical
Dr Saeed Al Amoudi Consultant Hematologist King s college hospital Director of Regional Lab Jeddah MAGIC BULLET Cord Blood Banking What is Cord Blood? Cord blood is the blood left in the placenta and umbilical
ASSEMBLY, No. 2591 STATE OF NEW JERSEY. 212th LEGISLATURE INTRODUCED FEBRUARY 23, 2006
 ASSEMBLY, No. STATE OF NEW JERSEY th LEGISLATURE INTRODUCED FEBRUARY, 00 Sponsored by: Assemblywoman CHARLOTTE VANDERVALK District (Bergen) SYNOPSIS Requires hospitals to provide information to pregnant
ASSEMBLY, No. STATE OF NEW JERSEY th LEGISLATURE INTRODUCED FEBRUARY, 00 Sponsored by: Assemblywoman CHARLOTTE VANDERVALK District (Bergen) SYNOPSIS Requires hospitals to provide information to pregnant
Postrefractive surgery dry eye Guilherme G. Quinto, Walter Camacho and Ashley Behrens
 Postrefractive surgery dry eye Guilherme G. Quinto, Walter Camacho and Ashley Behrens The Wilmer Ophthalmological Institute, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA Correspondence
Postrefractive surgery dry eye Guilherme G. Quinto, Walter Camacho and Ashley Behrens The Wilmer Ophthalmological Institute, The Johns Hopkins University School of Medicine, Baltimore, Maryland, USA Correspondence
Blood-Based Cancer Diagnostics
 The Biotechnology Education Company Blood-Based Cancer Diagnostics EDVO-Kit 141 Store entire experiment at room temperature. EXPERIMENT OBJECTIVE: The objective of this experiment is to learn and understand
The Biotechnology Education Company Blood-Based Cancer Diagnostics EDVO-Kit 141 Store entire experiment at room temperature. EXPERIMENT OBJECTIVE: The objective of this experiment is to learn and understand
4. All cord blood banks should be subject to the same standards, regulations and accreditation requirements.
 WMDA Policy Statement on the Utility of Autologous or Family Cord Blood Unit Storage The WMDA Board adopted this policy on 25 th of May 2006. Policy updated _April 2011 The Cord Blood Working Group and
WMDA Policy Statement on the Utility of Autologous or Family Cord Blood Unit Storage The WMDA Board adopted this policy on 25 th of May 2006. Policy updated _April 2011 The Cord Blood Working Group and
Subcutaneous Testosterone-Anastrozole Therapy in Breast Cancer Survivors. 2010 ASCO Breast Cancer Symposium Abstract 221 Rebecca L. Glaser M.D.
 Subcutaneous Testosterone-Anastrozole Therapy in Breast Cancer Survivors 2010 ASCO Breast Cancer Symposium Abstract 221 Rebecca L. Glaser M.D., FACS Learning Objectives After reading and reviewing this
Subcutaneous Testosterone-Anastrozole Therapy in Breast Cancer Survivors 2010 ASCO Breast Cancer Symposium Abstract 221 Rebecca L. Glaser M.D., FACS Learning Objectives After reading and reviewing this
