U.S. Food and Drug Administration
|
|
|
- Debra Russell
- 8 years ago
- Views:
Transcription
1 U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained and may be outdated.
2 C-1 Rejena (Sodium Hyaluronate Ophthalmic Solution, 0.18%) United States Food and Drug Administration Dermatologic and Ophthalmic Drugs Advisory Committee June 26, 2009
3 C-2 Rejena (Sodium Hyaluronate Ophthalmic Solution, 0.18%) Safety and Efficacy Review River Plate Biotechnology, Inc. Presenters: Russell Ellison, MD Roger Vogel, MD
4 C-3 Sponsor Attendees Russell Ellison, MD, MS - Chairman Luis Molina, PhD - President/CSO Terry W. Laliberte, BS - Director of Development Roger Vogel, MD - Medical Monitor, Consultant Christine Miller, PharmD - Regulatory, Consultant Gary Koch, PhD - Statistics, Consultant Steve Crockett, PhD - Statistics, Consultant
5 C-4 River Plate Biotechnology Presentation Introduction Rejena Efficacy and Safety Russell Ellison, MD Roger Vogel, MD
6 Dry Eye Disease Affects 5 Million Patients C-5 In the United States, an estimated 3.2 million women and 1.7 million men, a total of 4.9 million people are affected by dry eye disease. (Tsubota, 1992; Craig, 1997) Dry eye symptoms (dry-, gritty-, or scratchyfeeling eyes, itching, redness, blurred vision, and sensation of foreign body in the eye) are the leading cause of ophthalmology visits.
7 Efficacy Criteria for the Treatment of Dry Eye Disease C-6 Significant improvement in an objective sign of dry eye disease (ie, does drug have an effect on the disease) For example: Corneal erosion (fluorescein or lissamine green staining) Tear break up time (TBUT) Tear production (Schirmer s test) and Significant improvement in a symptom of dry eye (PRO) (ie, does drug have a clinical benefit perceived by patient) Reasonable, pragmatic, and rational criteria but very hard to meet
8 C-7 Dry Eye Study Challenges Variability of assessments Effectiveness of placebo or vehicle Signs and symptoms are poorly correlated Time course of signs and symptoms resolution can be different
9 C-8 Current Therapies Many products have been tested in clinical trials, but No prescription product is currently approved for the treatment of the signs and symptoms of dry eye disease Artificial tears (OTC) Provide only transient relief and are formulated with preservatives, which are irritants Certain products can aggravate the condition; tears with decongestants/antihistamines can decrease production of the tear film Cyclosporine ophthalmic emulsion (Restasis ) Topical immunosuppressant approved to increase tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with KCS Slow onset of action (months)
10 Rejena - Sodium Hyaluronate Ophthalmic Solution, 0.18% C-9 Preservative free Marketed in Europe and parts of Asia since January 1998 in 27 countries Vismed Vislube Hylovis ~ 9.5 million boxes (20 units/box) sold through Dec 31, 2008
11 Rejena Clinical Development Overview C subjects in 10 clinical trials with Rejena a 8 supportive clinical studies 5 studies against artificial tear products, all showing improvement over comparator 3 open label studies 2 adequate and well-controlled studies Baudouin 2005 study RP-001 study a There are several earlier studies using different formulations.
12 Baudouin 2005 Clinical Study Report Errata C-11 Sponsor recalculated all endpoints presented in briefing document resolving inconsistencies identified by FDA analysis Transcription errors from analysis files into summary tables appear in CSR and were carried forward into briefing document Primary endpoint: VAS summed score Descriptive statistics transcribed from PP population instead of ITT/LOCF P-value generated from ITT/LOCF as specified Secondary endpoints: Lissamine green staining and symptom frequency score P-values actually transcribed from analysis of absolute change from baseline whereas protocol specified percent change from baseline
13 Rejena Clinical Development Hypothesis Generation and Confirmation, and Replication C-12 Hypothesis generation Early studies positive for signs and/or symptoms vs artificial tears Rolando 1994 Rapisarda 1994 Baudouin 2001 Adequate and well-controlled study: Baudouin 2005 Did not meet primary endpoints 2 secondary endpoints showed an effect - selected for the design and powering of confirmatory study (RP-001) Lissamine green staining (sign) Symptom frequency score (symptom)
14 C-13 Rejena Clinical Development Hypothesis: Rejena is superior to vehicle (gold standard for comparator) for improvement in a sign (lissamine green staining) and a symptom (global symptom frequency score) in the same study Hypothesis confirmation: RP-001 Rejena was statistically significantly superior to vehicle for these 2 co-primary endpoints Replication: analysis of Baudouin 2005 using RP-001 analytical methodology
15 C-14 Efficacy and Safety of Rejena (Sodium Hyaluronate Ophthalmic Solution 0.18%) Roger Vogel, MD
16 C-15 Efficacy and Safety of Rejena Sodium hyaluronate Efficacy endpoints Dose selection rationale Baudouin 2005 RP-001 Integrated analysis Safety results Summary and conclusions
17 C-16 Sodium Hyaluronate Ubiquitous molecule Occurs naturally in all vertebrates in the vitreous body of the eye, the extracellular matrix of the skin, and synovial fluid Viscoelastic properties similar to natural tears Rejena sodium hyaluronate is obtained by bacterial fermentation High degree of purity No appreciable toxicity or immunosensitizing activity
18 C-17 Sodium Hyaluronate Extremely wide safety margin Sodium hyaluronate (SH) used Intraocularly as a viscoelastic during cataract extraction to protect corneal endothelium Intra-articularly as injections for synovial fluid replacement As a dermal filler
19 Sodium Hyaluronate Ophthalmic Solution 0.18% C-18 Under shear stress (during blinks) At rest between blinks Solution is elastic, nonviscous, and spreads easily over the ocular surface Solution is less elastic, more viscous
20 Actions of Sodium Hyaluronate Ophthalmic Solution 0.18% C-19 Stabilizes the precorneal tear film Restores tear film and ocular surface homeostatic states Allows recovery of goblet cells Entraps water and hydrates and lubricates the ocular surface As a result improves ocular comfort and quality of life
21 C-20 Efficacy and Safety of Rejena Sodium hyaluronate Efficacy endpoints Dose selection rationale Baudouin 2005 RP-001 Integrated analysis Safety results Summary and conclusions
22 6/19 3:30 pm C-21 Dry Eye Efficacy Endpoints - Signs Inflammatory suppression of tear secretion Decreased tear production Tear film instability Hyperosmolarity Decreased mucin production Loss of goblet cells Conjunctival & corneal epithelial damage Tear film break-up time (TBUT) Time required for the eye to lose its wet surface after a blink Normal eye: 10 to 12 seconds Dry eye: Less than 10 seconds
23 C-22 Dry Eye Efficacy Endpoints - Signs Inflammatory suppression of tear secretion Decreased tear production Tear film instability Hyperosmolarity Decreased mucin production Loss of goblet cells Conjunctival & corneal epithelial damage Corneal and conjunctival staining Fluorescein (corneal) stains the area between surface cells (extent to which epithelial cells are drying and shrinking) Lissamine green (corneal and conjunctival) stains keratinized and devitalized epithelial cells, goblet cells, mucous, and epithelial filaments (more sensitive dye than fluorescein)
24 Rejena Clinical Studies Efficacy Endpoints - Signs C-23 3 dose-finding studies used TBUT Baudouin 2005 Primary endpoint: fluorescein staining Secondary endpoints: lissamine green staining (also TBUT, Schirmer s test, tear prism height) RP-001 Primary endpoint: lissamine green staining
25 Rejena Clinical Studies Efficacy Endpoints - Symptoms C-24 Symptoms assessed Dryness Soreness Scratchiness Grittiness Burning Scoring Symptom intensity Symptom frequency Composite symptom Global symptom frequency
26 C-25 Efficacy and Safety of Rejena Sodium hyaluronate Efficacy endpoints Dose selection rationale Baudouin 2005 RP-001 Integrated analysis Safety results Summary and conclusions
27 C-26 Dose Selection Rationale: Concentration 3 clinical dose-finding studies Effects of sodium hyaluronate in solutions ranging from 0% (vehicle/placebo) to 0.3% on the prolongation of TBUT Higher concentrations more effective than lower concentrations 0.18% concentration was highest concentration that could be feasibly formulated
28 C-27 Clinical Program Objective Demonstrate that sodium hyaluronate ophthalmic solution 0.18% is safe and effective for the treatment of the signs and symptoms of dry eye disease
29 C-28 Rejena Clinical Development Hypothesis generating Early studies a Rapisarda 1994, Rolando 1994, and Baudouin 2001 all showed differences in signs and symptoms vs artificial tears Baudouin 2005 a 2 additional phase 4 studies with similar results, Prabhasawat 2007 and Johnson 2008.
30 C-29 Efficacy and Safety of Rejena Sodium hyaluronate Efficacy endpoints Dose selection rationale Baudouin 2005 RP-001 Integrated analysis Safety results Summary and conclusions
31 Hypothesis Generating: Baudouin 2005 C-30 Rejena (n = 74) vs 0.9% saline (n = 77) Subjects with 3 months history of bilateral moderate dry eye disease or moderate dry eye disease due to Sjögren s syndrome Primary endpoints Objective endpoint: Percent change from baseline of the fluorescein staining summed score (average of the sum of the total scores over both eyes) at Day 28 Subjective endpoint: Percent change from baseline of the VAS summed intensity score (average of the sum of five VAS symptom scales for soreness, scratchiness, dryness, grittiness, and burning) at Day 28
32 Baudouin 2005 Study Conduct C-31 Sponsored by TRB Chemedica, SA (manufacturer in Europe) Study management and monitoring by Clirophtha, an experienced CRO in Europe 100% source document validation Study conducted under GCP according to ICH Experienced, well-published, principal investigator (who served as a principal investigator for Restasis development)
33 Baudouin 2005 Data Management & Statistical Analysis C-32 Data entry and management and statistical analysis performed by MD Research (CRO in Germany) Data listings and statistical tables and figures were forwarded to the EU sponsor, TRB Chemedica, SA, who prepared the clinical study report Abstract and poster authored by Dr. Baudouin, based on the CSR, presented in ARVO 2004
34 Baudouin Primary Endpoints (% Change From Baseline) C-33 Measure Visit Study drug Mean (SD) 1-sided P-value Fluorescein staining of cornea VAS summed score Day 7 Active (38.36) Saline (38.26) Day 28 Active (47.21) Saline (44.75) Day 7 Active (32.75) a Saline (33.55) Day 28 Active (31.68) a Saline (43.17) ITT = Intent to treat; SD = Standard deviation; VAS = Visual analogue scale a Calculated in ITT population, LOCF (correct analysis).
35 Hypothesis Generating: Baudouin 2005 Secondary Endpoints (% Change from Baseline, 1-sided P-values) C-34 Measure Visit Study drug Mean (SD) b CSR a P-value Lissamine green staining Symptom frequency score P-value per SAP (recalculated by Sponsor) b Day 7 Rejena (34.48) Saline (41.81) Day 28 Rejena (31.24) Saline (39.60) Day 7 Rejena (33.03) Saline (30.60) Day 28 Rejena (26.38) Saline (34.68) a Calculated from absolute change from baseline (transcription error). b Calculated from percent change from baseline.
36 Baudouin 2005 Summary C-35 Baudouin 2005 showed positive results in secondary endpoint for sign (lissamine green staining) and symptom (symptom frequency score) at Days 7 and 28 On agreement with FDA, Study RP-001 adopted a similar study design and these 2 endpoints as co-primary
37 C-36 Efficacy and Safety of Rejena Sodium hyaluronate Efficacy endpoints Dose selection rationale Baudouin 2005 RP-001 Integrated analysis Safety results Summary and conclusions
38 Hypothesis Confirmation: RP-001 C-37 Hypothesis: Rejena is significantly superior to vehicle for improvement in a sign and a symptom in the same study Agreed with FDA under a Special Protocol Assessment (SPA) Sign: absolute change from baseline in lissamine green staining (co-primary endpoint) Symptom: absolute change from baseline in global symptom frequency score (co-primary endpoint) Wilcoxon rank sum test primary analysis method: t-test secondary α = sided (α = sided)
39 Hypothesis Confirmation: RP-001 Sample Size Calculation C-38 Rejena (n = 221) vs vehicle (n = 223) RP-001 sample size based on 2-sided α = 0.05 Used difference in means and SD based upon subjects in Baudouin 2005 matching the entry criteria for RP-001 to determine the sample size for a power of 80% Prespecified masked interim analysis showed increased variance requiring sample size increase from 300 to 440
40 Inclusion Criteria RP-001 C-39 Subjects with 3 months history of diagnosed KCS or dry eye due to Sjögren s syndrome Experienced in the same eye: At least 2 symptoms of dry eye Rated as 2 (often) Scored as 50 mm on VAS intensity Objective parameters of dry eye: Corneal fluorescein staining total score of 3 Lissamine green staining total score of 3
41 RP-001 Change From Baseline at Day 7 Primary Endpoints (ITT, LOCF) C-40 Primary endpoint Visit Study drug Mean (SD) Lissamine green staining Global symptom frequency Day 0 Rejena 5.71 (2.42) Vehicle 5.52 (2.36) Wilcoxon rank sum test a 2-sided P-values van Elteren Student s t-test Day 7 Rejena 1.1 (2.01) Vehicle 0.7 (1.79) Day 0 Rejena 8.33 (2.23) Vehicle 8.22 (2.47) Day 7 Rejena 1.7 (2.78) Vehicle 1.1 (2.62) a Primary analytical method.
42 RP-001 Global Impact of Dry Eye on Daily Life C-41 Study drug % reporting improvement by 1 or more grades at Day 7 Rejena 40.3% Vehicle 30.4% Scale is 0 to 3, absent to severe
43 C-42 Efficacy and Safety of Rejena Sodium hyaluronate Efficacy endpoints Dose selection rationale Baudouin 2005 RP-001 Integrated analysis Safety results Summary and conclusions
44 C-43 Replication: Steps 1. Baudouin 2005 is supportive of efficacy Results suitable for integrated analysis (side-by-side comparison) 2. RP-001 confirmed results from Baudouin 2005 Efficacy demonstrated for same selected sign and symptom as those achieved in Baudouin Replication Side-by-side comparative analysis was conducted to evaluate how Baudouin 2005 and RP-001 replicate each other using RP-001 analytical methodology agreed upon with FDA
45 RP-001 Endpoints Used for Comparative Analysis C-44 Absolute change from baseline in lissamine green staining score Absolute change from baseline in global symptom frequency score Primary endpoint: Day 7 RP-001 time points: Days 7 and 14 Baudouin 2005 time points: Days 7 and 28
46 C-45 RP-001 Statistical Methodology for Comparative Analysis Reanalysis of Baudouin 2005 using analytical methodology for RP-001 that was agreed upon with FDA Selected study eye for lissamine green staining score Eye with lower baseline Schirmer s I test score ITT population (LOCF) defined as all randomized subjects: baseline observation carried forward Wilcoxon rank sum test (primary) Student s t-test (supportive)
47 Efficacy: Lissamine Green Staining Score Change from Baseline (ITT, LOCF) C-46 Baudouin 2005 Study RP sided P-values 2-sided P-values Wilcoxon Wilcoxon Visit Study drug Mean (SD) rank sum t-test Mean (SD) rank sum t-test Day 0 Rejena 4.03 (2.12) 5.71 (2.42) Control 3.83 (2.28) 5.52 (2.36) Day 7 Rejena 1.1 (1.51) (2.01) Control 0.6 (1.38) 0.7 (1.79)
48 Efficacy: Global Symptom Frequency Scores Change from Baseline (ITT, LOCF) C-47 Visit Study drug Mean (SD) Baudouin sided P-values Wilcoxon rank sum t-test Mean (SD) Day 0 Rejena 8.35 (2.27) 8.33 (2.23) Control 8.04 (1.76) 8.22 (2.47) Study RP sided P-values Wilcoxon rank sum t-test Day 7 Rejena 2.0 (2.44) Control 1.2 (2.58) (2.78) (2.62)
49 Phase 3 Efficacy Analysis Conclusion C-48 Hypothesis generated: early studies and Baudouin 2005 provided basis for generating hypothesis that Rejena was superior to vehicle in reducing lissamine green staining and global symptom frequency score Hypothesis confirmed: RP-001 statistically significant for these 2 co-primary endpoints Replication achieved: analysis of Baudouin 2005 with RP-001 analytical methodology
50 C-49 Summary of Efficacy 10 clinical studies assessed efficacy (957 subjects) for up to 2 months and were consistently positive 5 studies showed improvement over artificial tears 3 exploratory studies show improvement in ocular surface disease 2 Phase 3 studies: reduction in lissamine green staining and improvement in global symptom frequency score versus both saline and vehicle Rejena is effective for the treatment of the signs and symptoms of dry eye
51 C-50 Efficacy and Safety of Rejena Sodium hyaluronate Efficacy endpoints Dose selection rationale Baudouin 2005 RP-001 Integrated analysis Safety results Summary and conclusions
52 C-51 Safety Results 3 studies form basis of safety profile (n = 305) Phase 3 studies (Baudouin 2005, RP-001) Phase 2 study (Baudouin 2001) Safety evaluations included Slit lamp examination Best-corrected visual acuity (BCVA) Ophthalmic examination Collection of AEs Intraocular pressure (RP-001)
53 C-52 Safety Studies SH ophthalmic solution 0.18% compared with Dose regimen Average exposure Baudouin 2001 Baudouin 2005 RP-001 Carboxymethyl- Saline 0.9% Vehicle cellulose 1% 1 drop 3 times/ day or as needed, for up to 56 days 3.8 daily instillations 1 drop at least 3 and up to 8 times/day or as needed, for up to 28 days 3.7 daily instillations 1 to 2 drops at least 3 and up to 6 times/day or as needed, for up to 14 days 3.7 units used daily
54 C-53 Adverse Events Overview Patients, n (%) Baudouin 2005 RP-001 Rejena N = 74 Saline N = 77 Rejena N = 221 Vehicle N = 222 Any AE 10 (13.5) 9 (11.7) 57 (25.8) 48 (21.6) Any SAE (0.5) 1 (0.5) AE leading to 1 (1.4) 2 (2.6) 2 (0.9) 1 (0.5) withdrawal Death No AEs were reported in Baudouin 2001 (N = 10) No ocular SAEs reported in the 3 safety studies
55 Ocular Treatment-Emergent AEs in 1% of Subjects C-54 Adverse event Baudouin 2005 Rejena N = 74 Patients, n (%) Saline N = 77 Rejena N = 221 RP-001 Vehicle N = 222 Dry eye a (8.1) 14 (6.3) Eye pain a (5.9) 7 (3.2) Eye irritation a 2 (2.7) 0 4 (1.8) 5 (2.3) Foreign body sensation in eyes (2.3) 7 (3.2) Visual acuity reduced (1.8) 6 (2.7) Eye pruritis (1.8) 4 (1.8) Vision blurred a (1.8) 0 Ocular hyperaemia (1.4) 3 (1.4) Eyelid margin crusting a (1.4) 1 (0.5) a TEAEs that occurred at a greater frequency in Rejena-treated subjects.
56 C-55 Worldwide Marketing Experience Approximately 9.5 million boxes (20 units each) sold (Jan 1998 to Dec 31, 2008) Estimated 2.8 million patients have used Rejena 39 reports of post-marketing medical complaints reported (through 30 April 2009)
57 Spontaneous Complaint Reports Marketed Product Surveillance C-56 Adverse event Total complaints, n Burning sensation 16 Hypersensitivity/intolerance 13 Eye reddening 5 Foreign body sensation 1 Eye injury a 1 Local swelling 1 Blurred vision 1 Other 1 Total 39 a This complaint was due to incorrect handling of the delivery device.
58 C-57 Summary of Clinical Safety Rejena has an acceptable safety profile for the treatment of the signs and symptoms of dry eye No ocular SAEs Low incidence of AEs 14.4% of subjects experienced 1 AE Most were mild in intensity Ocular TEAEs No ocular AE occurred at a frequency greater than 8.1% Only 3 Rejena-treated subjects withdrew due to an ocular adverse event
59 C-58 Efficacy and Safety of Rejena Sodium hyaluronate Efficacy endpoints Dose selection rationale Baudouin 2005 RP-001 Integrated analysis Safety results Summary and conclusions
60 C-59 Summary and Conclusions RP-001 (Rejena /vehicle) confirms and replicates positive endpoints in Baudouin 2005 (Rejena/saline) Rejena is currently widely marketed outside the US; the safety profile is well established Clinical trial data demonstrate that Rejena is safe and well tolerated Clinical data demonstrate that Rejena is safe and effective for the treatment of dry eye disease
A normal eye is protected by a layer of natural tears.
 A normal eye is protected by a layer of natural tears. If you need to use eye drops several times a day to make your eyes comfortably moist, you should consult your eye doctor. Helps increase tear production
A normal eye is protected by a layer of natural tears. If you need to use eye drops several times a day to make your eyes comfortably moist, you should consult your eye doctor. Helps increase tear production
There Are Millions of People at Risk For Dry Eye Who Is Likely to Develop This Irritating Condition?
 There Are Millions of People at Risk For Dry Eye Who Is Likely to Develop This Irritating Condition? Dry eye can be a temporary or chronic condition and occurs when the eye does not produce tears properly,
There Are Millions of People at Risk For Dry Eye Who Is Likely to Develop This Irritating Condition? Dry eye can be a temporary or chronic condition and occurs when the eye does not produce tears properly,
WHAT TO DO IF YOU HAVE DRY EYES...
 WHAT TO DO IF YOU HAVE DRY EYES... Frank J. Holly, PhD Dry Eye Institute Yantis, Texas Based on a presentation given in Spanish at the patient session of the VIIIth Triennial Meeting of the International
WHAT TO DO IF YOU HAVE DRY EYES... Frank J. Holly, PhD Dry Eye Institute Yantis, Texas Based on a presentation given in Spanish at the patient session of the VIIIth Triennial Meeting of the International
Thyroid Eye Disease. Anatomy: There are 6 muscles that move your eye.
 Thyroid Eye Disease Your doctor thinks you have thyroid orbitopathy. This is an autoimmune condition where your body's immune system is producing factors that stimulate enlargement of the muscles that
Thyroid Eye Disease Your doctor thinks you have thyroid orbitopathy. This is an autoimmune condition where your body's immune system is producing factors that stimulate enlargement of the muscles that
Lacrimal gland. Tear duct into nose
 Dry eye syndrome The aim of this information sheet is to answer any questions you may have about dry eye syndrome. If you have any further questions or concerns, please ask a doctor or nurse caring for
Dry eye syndrome The aim of this information sheet is to answer any questions you may have about dry eye syndrome. If you have any further questions or concerns, please ask a doctor or nurse caring for
C-30078 Diagnosis and Management of Dry Eyes Part 1. Dr Colm McAlinden, BSc (Hons), MSc, PhD and Dr Eirini Skiadaresi, MD.
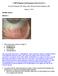 C-30078 Diagnosis and Management of Dry Eyes Part 1 Dr Colm McAlinden, BSc (Hons), MSc, PhD and Dr Eirini Skiadaresi, MD January 11 2013 Detailed Answers IMAGE A Image Courtesy of Professor Giuseppe Ravalico
C-30078 Diagnosis and Management of Dry Eyes Part 1 Dr Colm McAlinden, BSc (Hons), MSc, PhD and Dr Eirini Skiadaresi, MD January 11 2013 Detailed Answers IMAGE A Image Courtesy of Professor Giuseppe Ravalico
Patient-Reported Outcomes with LASIK (PROWL-1) Results
 Patient-Reported Outcomes with LASIK (PROWL-1) Results Elizabeth M. Hofmeister, MD CAPT, MC, USN Naval Medical Center San Diego Refractive Surgery Advisor for Navy Ophthalmology Assistant Professor of
Patient-Reported Outcomes with LASIK (PROWL-1) Results Elizabeth M. Hofmeister, MD CAPT, MC, USN Naval Medical Center San Diego Refractive Surgery Advisor for Navy Ophthalmology Assistant Professor of
Bringing Objectivity to Dry Eye Diagnosis and Assessment
 Bringing Objectivity to Dry Eye Diagnosis and Assessment Tear Osmolarity Testing Helps Solve the Dilemma of Identifying Dry Eye by Jay S. Pepose, MD, PHD as published online in Ophthalmology Management
Bringing Objectivity to Dry Eye Diagnosis and Assessment Tear Osmolarity Testing Helps Solve the Dilemma of Identifying Dry Eye by Jay S. Pepose, MD, PHD as published online in Ophthalmology Management
Re: LUMIGAN 0.03% (bimatoprost ophthalmic solution 0.03%) and LUMIGAN 0.01% (bimatoprost ophthalmic solution 0.01%)
 Allergan Ltd, 1st Floor, Marlow International, Parkway, Marlow, Bucks SL7 1YL Tel: (01628) 494444 Facsimile: (01628) 494449 Ref No: UK15-000677 Mandeep Allingham NW Surrey CCG February 18, 2015 Dear Ms
Allergan Ltd, 1st Floor, Marlow International, Parkway, Marlow, Bucks SL7 1YL Tel: (01628) 494444 Facsimile: (01628) 494449 Ref No: UK15-000677 Mandeep Allingham NW Surrey CCG February 18, 2015 Dear Ms
OPHTHALMOLOGIST PERCEPTIONS REGARDING TREATMENT OF MODERATE TO SEVERE DRY EYE: RESULTS OF A PHYSICIAN SURVEY
 OPHTHALMOLOGIST PERCEPTIONS REGARDING TREATMENT OF MODERATE TO SEVERE DRY EYE: RESULTS OF A PHYSICIAN SURVEY By Penny A. Asbell MD MBA* and Scott Spiegel PhD ABSTRACT Purpose: To understand ophthalmologists
OPHTHALMOLOGIST PERCEPTIONS REGARDING TREATMENT OF MODERATE TO SEVERE DRY EYE: RESULTS OF A PHYSICIAN SURVEY By Penny A. Asbell MD MBA* and Scott Spiegel PhD ABSTRACT Purpose: To understand ophthalmologists
IR Conference Call on EYLEA (aflibercept) Injection in Diabetic Macular Edema. September 28, 2013
 IR Conference Call on EYLEA (aflibercept) Injection in Diabetic Macular Edema September 28, 2013 Safe Harbor Statement This presentation includes forward-looking statements that involve risks and uncertainties
IR Conference Call on EYLEA (aflibercept) Injection in Diabetic Macular Edema September 28, 2013 Safe Harbor Statement This presentation includes forward-looking statements that involve risks and uncertainties
Study Design. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D. Ellis Unger, M.D. Ghanshyam Gupta, Ph.D. Chief, Therapeutics Evaluation Branch
 BLA: STN 103471 Betaseron (Interferon β-1b) for the treatment of secondary progressive multiple sclerosis. Submission dated June 29, 1998. Chiron Corp. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D.
BLA: STN 103471 Betaseron (Interferon β-1b) for the treatment of secondary progressive multiple sclerosis. Submission dated June 29, 1998. Chiron Corp. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D.
Dry eyes holding you back? Ask your doctor about LipiFlow.
 Dry eyes holding you back? Ask your doctor about LipiFlow. Think you may have dry eye? Take the quiz below to find out. Do you experience sensitivity to light, blurred vision, a burning sensation, or discomfort
Dry eyes holding you back? Ask your doctor about LipiFlow. Think you may have dry eye? Take the quiz below to find out. Do you experience sensitivity to light, blurred vision, a burning sensation, or discomfort
Use of Nepafenac in Lasek Johnny L. Gayton, MD
 Use of Nepafenac in Lasek Johnny L. Gayton, MD Kathrine Jackson, COA Eyesight Associates, Warner Robins, GA Analgesic Effect NSAIDs provide analgesic effect 1-3 Minimize pain and discomfort following cataract
Use of Nepafenac in Lasek Johnny L. Gayton, MD Kathrine Jackson, COA Eyesight Associates, Warner Robins, GA Analgesic Effect NSAIDs provide analgesic effect 1-3 Minimize pain and discomfort following cataract
Get Your Eyes Examined
 1 Vision Changes You may notice vision changes with aging. Many changes are common and can often be corrected. As you get older, you are at higher risk of age-related eye diseases and conditions. Get Your
1 Vision Changes You may notice vision changes with aging. Many changes are common and can often be corrected. As you get older, you are at higher risk of age-related eye diseases and conditions. Get Your
Class Im MD A novel device for Automated Analysis of Tear Film Dynamics. Michael Ring, DI(FH) Upper Austria University of Applied Sciences
 Class Im MD A novel device for Automated Analysis of Tear Film Dynamics Michael Ring, DI(FH) Upper Austria University of Applied Sciences Aim of the Research Develop a method to give information on the
Class Im MD A novel device for Automated Analysis of Tear Film Dynamics Michael Ring, DI(FH) Upper Austria University of Applied Sciences Aim of the Research Develop a method to give information on the
Grading staining: Oxford Schema The scheme is used to estimate surface damage in dry eye.
 DEWS DRY EYE: DIAGNOSTIC TEST TEMPLATE RAPPORTEUR A.J.Bron 21 st Oct 2004 TEST TO DIAGNOSE VERSION of TEST DESCRIPTION NATURE of STUDY CONDUCT of TESTS Grading staining: Oxford Schema The scheme is used
DEWS DRY EYE: DIAGNOSTIC TEST TEMPLATE RAPPORTEUR A.J.Bron 21 st Oct 2004 TEST TO DIAGNOSE VERSION of TEST DESCRIPTION NATURE of STUDY CONDUCT of TESTS Grading staining: Oxford Schema The scheme is used
TUTORIAL on ICH E9 and Other Statistical Regulatory Guidance. Session 1: ICH E9 and E10. PSI Conference, May 2011
 TUTORIAL on ICH E9 and Other Statistical Regulatory Guidance Session 1: PSI Conference, May 2011 Kerry Gordon, Quintiles 1 E9, and how to locate it 2 ICH E9 Statistical Principles for Clinical Trials (Issued
TUTORIAL on ICH E9 and Other Statistical Regulatory Guidance Session 1: PSI Conference, May 2011 Kerry Gordon, Quintiles 1 E9, and how to locate it 2 ICH E9 Statistical Principles for Clinical Trials (Issued
Age-Related Eye Diseases and Conditions. See Well for a Lifetime
 Age-Related Eye Diseases and Conditions See Well for a Lifetime Vision Changes You may notice vision changes with aging. Many changes are common and can often be corrected. As you get older, you are at
Age-Related Eye Diseases and Conditions See Well for a Lifetime Vision Changes You may notice vision changes with aging. Many changes are common and can often be corrected. As you get older, you are at
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 18 December 2002 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 18 December 2002 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE
Institute of Ophthalmology. Thyroid Eye Disease. aka Thyroid Associated Ophthalmopathy
 Institute of Ophthalmology Thyroid Eye Disease aka Thyroid Associated Ophthalmopathy Causes TED/TAO is an eye disease associated with disease of the thyroid gland Most commonly, it occurs with an overactive
Institute of Ophthalmology Thyroid Eye Disease aka Thyroid Associated Ophthalmopathy Causes TED/TAO is an eye disease associated with disease of the thyroid gland Most commonly, it occurs with an overactive
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Evaluation of Dryness of Eyes after Manual Small Incision Cataract Surgery with Corneoscleral Tunnel Incision
 ID: JCDR/2012/4461:2304 Opthalmology Section Original Article Evaluation of Dryness of Eyes after Manual Small Incision Cataract Surgery with Corneoscleral Tunnel Incision Kavitha Chikkanayakanahalli Venugopal,
ID: JCDR/2012/4461:2304 Opthalmology Section Original Article Evaluation of Dryness of Eyes after Manual Small Incision Cataract Surgery with Corneoscleral Tunnel Incision Kavitha Chikkanayakanahalli Venugopal,
Week 12 study results
 Week 12 study results 15 April 2015 Copyright 2015 Galapagos NV Disclaimer This document may contain certain statements, including forward-looking statements, such as statements concerning the safety and
Week 12 study results 15 April 2015 Copyright 2015 Galapagos NV Disclaimer This document may contain certain statements, including forward-looking statements, such as statements concerning the safety and
How To Treat Eye Problems With A Laser
 1550 Oak St., Suite 5 1515 Oak St., St Eugene, OR 97401 Eugene, OR 97401 (541) 687-2110 (541) 344-2010 INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK) This information is to help you make an informed
1550 Oak St., Suite 5 1515 Oak St., St Eugene, OR 97401 Eugene, OR 97401 (541) 687-2110 (541) 344-2010 INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK) This information is to help you make an informed
SYNOPSIS. Risperidone: Clinical Study Report CR003274
 SYNOPSIS Protocol No: CR003274 Title of Study: An Open-Label, Long-Term Trial of Risperidone Long-Acting Microspheres in the Treatment of Subjects Diagnosed with Schizophrenia Coordinating Investigator:
SYNOPSIS Protocol No: CR003274 Title of Study: An Open-Label, Long-Term Trial of Risperidone Long-Acting Microspheres in the Treatment of Subjects Diagnosed with Schizophrenia Coordinating Investigator:
Dry Eye: Natural History, Diagnosis and Treatment By Jeffrey P. Gilbard, M.D.
 Dry Eye: Natural History, Diagnosis and Treatment By Jeffrey P. Gilbard, M.D. Our understanding of dry eye disorders has improved dramatically over the past several years, enhancing our ability to diagnose
Dry Eye: Natural History, Diagnosis and Treatment By Jeffrey P. Gilbard, M.D. Our understanding of dry eye disorders has improved dramatically over the past several years, enhancing our ability to diagnose
Eye Manifestations of Lupus And Sjogren s Syndrome
 Eye Manifestations of Lupus And Sjogren s Syndrome Based on a presentation by Dr. N. Kevin Wade at the BC Lupus Society Symposium held October 22, 2005. Background Dr. Wade completed his MD in 1998 at
Eye Manifestations of Lupus And Sjogren s Syndrome Based on a presentation by Dr. N. Kevin Wade at the BC Lupus Society Symposium held October 22, 2005. Background Dr. Wade completed his MD in 1998 at
Tear Osmolarity in Refractive Surgery: Its Impact on Clinical Outcomes
 Tear Osmolarity in Refractive Surgery: Its Impact on Clinical Outcomes Hawaiian Eye 2013 Marguerite McDonald, MD, FACS Clinical Professor of Ophthalmology NYU Langone Medical Center New York, New York
Tear Osmolarity in Refractive Surgery: Its Impact on Clinical Outcomes Hawaiian Eye 2013 Marguerite McDonald, MD, FACS Clinical Professor of Ophthalmology NYU Langone Medical Center New York, New York
The Consequences of Missing Data in the ATLAS ACS 2-TIMI 51 Trial
 The Consequences of Missing Data in the ATLAS ACS 2-TIMI 51 Trial In this white paper, we will explore the consequences of missing data in the ATLAS ACS 2-TIMI 51 Trial and consider if an alternative approach
The Consequences of Missing Data in the ATLAS ACS 2-TIMI 51 Trial In this white paper, we will explore the consequences of missing data in the ATLAS ACS 2-TIMI 51 Trial and consider if an alternative approach
The Product Review Life Cycle A Brief Overview
 Stat & Quant Mthds Pharm Reg (Spring 2, 2014) Lecture 2,Week 1 1 The review process developed over a 40 year period and has been influenced by 5 Prescription User Fee Act renewals Time frames for review
Stat & Quant Mthds Pharm Reg (Spring 2, 2014) Lecture 2,Week 1 1 The review process developed over a 40 year period and has been influenced by 5 Prescription User Fee Act renewals Time frames for review
MANAGEMENT OF OPHTHALMIC PATIENTS PRESENTING WITH WELDERS ARC EYE / FLASHBURN, BY OPHTHALMIC NURSE PRACTITIONERS
 Birmingham and Midland Eye Centre Ophthalmic Guideline MANAGEMENT OF OPHTHALMIC PATIENTS PRESENTING WITH WELDERS ARC EYE / FLASHBURN, BY OPHTHALMIC NURSE PRACTITIONERS Policy author Accountable Executive
Birmingham and Midland Eye Centre Ophthalmic Guideline MANAGEMENT OF OPHTHALMIC PATIENTS PRESENTING WITH WELDERS ARC EYE / FLASHBURN, BY OPHTHALMIC NURSE PRACTITIONERS Policy author Accountable Executive
Congratulations! You have just joined the thousands of people who are enjoying the benefits of laser vision correction.
 Dear Valued Patient, Thank you for choosing Shady Grove Ophthalmology for your laser vision correction procedure. Our excellent staff is committed to offering you the highest quality eye care using state
Dear Valued Patient, Thank you for choosing Shady Grove Ophthalmology for your laser vision correction procedure. Our excellent staff is committed to offering you the highest quality eye care using state
Eye Diseases. 1995-2014, The Patient Education Institute, Inc. www.x-plain.com otf30101 Last reviewed: 05/21/2014 1
 Eye Diseases Introduction Some eye problems are minor and fleeting. But some lead to a permanent loss of vision. There are many diseases that can affect the eyes. The symptoms of eye diseases vary widely,
Eye Diseases Introduction Some eye problems are minor and fleeting. But some lead to a permanent loss of vision. There are many diseases that can affect the eyes. The symptoms of eye diseases vary widely,
Diagnosing dry eye: It s now a fine art
 Article Date: 4/1/2016 Diagnosing dry eye: It s now a fine art Research has led to better, more diverse ways to reach a diagnosis, let alone more knowledge about DED itself. BY MARK B. ABELSON, MD, CM,
Article Date: 4/1/2016 Diagnosing dry eye: It s now a fine art Research has led to better, more diverse ways to reach a diagnosis, let alone more knowledge about DED itself. BY MARK B. ABELSON, MD, CM,
A survey of eye-related complaints among call-center agents in Metro Manila
 VOL. 35 NO. 2 PHILIPPINE JOURNAL OF Ophthalmology JULY ORIGINAL ARTICLE DECEMBER 2010 S. Richard G. Cabrera III, MD Ruben Lim-Bon-Siong, MD International Eye Institute St. Luke s Medical Center Quezon
VOL. 35 NO. 2 PHILIPPINE JOURNAL OF Ophthalmology JULY ORIGINAL ARTICLE DECEMBER 2010 S. Richard G. Cabrera III, MD Ruben Lim-Bon-Siong, MD International Eye Institute St. Luke s Medical Center Quezon
A 3-PRODUCT SALICYLIC ACID REGIMEN EFFECTIVELY TREATS ACNE VULGARIS IN POST- ADOLESCENT WOMEN
 A 3-PRODUCT SALICYLIC ACID REGIMEN EFFECTIVELY TREATS ACNE VULGARIS IN POST- ADOLESCENT WOMEN J. R. Kaczvinsky 1, C. E. Mack 1, L. L. Griffin 1, J. Li 1, R. A. Rose-Mansfield 1, S. C. Weitz 1, M. J. Marmor
A 3-PRODUCT SALICYLIC ACID REGIMEN EFFECTIVELY TREATS ACNE VULGARIS IN POST- ADOLESCENT WOMEN J. R. Kaczvinsky 1, C. E. Mack 1, L. L. Griffin 1, J. Li 1, R. A. Rose-Mansfield 1, S. C. Weitz 1, M. J. Marmor
IDENTIFY DRY EYE AN IN-OFFICE TEST TO AID IN DRY EYE DIAGNOSIS. 85% Sensitivity, 94% Specificity 1. This brochure is for use in Canada only.
 IDENTIFY DRY EYE AN IN-OFFICE TEST TO AID IN DRY EYE DIAGNOSIS 85% Sensitivity, 94% Specificity 1 IDENTIFY DRY EYE WITH INFLAMMADRY InflammaDry is the first and only rapid, in-office test that detects
IDENTIFY DRY EYE AN IN-OFFICE TEST TO AID IN DRY EYE DIAGNOSIS 85% Sensitivity, 94% Specificity 1 IDENTIFY DRY EYE WITH INFLAMMADRY InflammaDry is the first and only rapid, in-office test that detects
INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK)
 INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK) This information and the Patient Information booklet must be reviewed so you can make an informed decision regarding Photorefractive Keratectomy (PRK)
INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK) This information and the Patient Information booklet must be reviewed so you can make an informed decision regarding Photorefractive Keratectomy (PRK)
MOST FREQUENTLY ASKED CLIENT QUESTIONS
 6/2 Victor Crescent Narre Warren VIC 3805 Ph: (03) 8794 8255 Fax: (03) 8794 8256 www.ortho-k.com.au Sleep your way to great vision MOST FREQUENTLY ASKED CLIENT QUESTIONS What is ortho-k? Ortho-k (Orthokeratology)
6/2 Victor Crescent Narre Warren VIC 3805 Ph: (03) 8794 8255 Fax: (03) 8794 8256 www.ortho-k.com.au Sleep your way to great vision MOST FREQUENTLY ASKED CLIENT QUESTIONS What is ortho-k? Ortho-k (Orthokeratology)
Ocular Surface Diseases Course 2016 MIAMI,FLORIDA. Bascom Palmer Eye Institute. Sponsored by the University of Miami Miller School of Medicine
 Ocular Surface Diseases Course 2016 Bascom Palmer Eye Institute Sponsored by the University of Miami Miller School of Medicine MIAMI,FLORIDA April 16, 2016 BASCOM PALMER EYE INSTITUTE Ocular Surface Diseases
Ocular Surface Diseases Course 2016 Bascom Palmer Eye Institute Sponsored by the University of Miami Miller School of Medicine MIAMI,FLORIDA April 16, 2016 BASCOM PALMER EYE INSTITUTE Ocular Surface Diseases
Subject: No. Page PROTOCOL AND CASE REPORT FORM DEVELOPMENT AND REVIEW Standard Operating Procedure
 703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain
 P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
DRY EYE & Carboxymethyl Cellulose
 DRY EYE & Carboxymethyl Cellulose DR.SHIKHA DUBEY, DOMS (Nagpur University), Short-term fellow retina, Phaco-fellow. SHANTI EYE CARE CENTRE Dinner session sponsored by Cipla Foresight, held on 19-03-05
DRY EYE & Carboxymethyl Cellulose DR.SHIKHA DUBEY, DOMS (Nagpur University), Short-term fellow retina, Phaco-fellow. SHANTI EYE CARE CENTRE Dinner session sponsored by Cipla Foresight, held on 19-03-05
Psoriasis, Incidence, Quality of Life, Psoriatic Arthritis, Prevalence
 1.0 Abstract Title Prevalence and Incidence of Articular Symptoms and Signs Related to Psoriatic Arthritis in Patients with Psoriasis Severe or Moderate with Adalimumab Treatment (TOGETHER). Keywords Psoriasis,
1.0 Abstract Title Prevalence and Incidence of Articular Symptoms and Signs Related to Psoriatic Arthritis in Patients with Psoriasis Severe or Moderate with Adalimumab Treatment (TOGETHER). Keywords Psoriasis,
ALTERNATIVES TO LASIK
 EYE PHYSICIANS OF NORTH HOUSTON 845 FM 1960 WEST, SUITE 101, Houston, TX 77090 Office: 281 893 1760 Fax: 281 893 4037 INFORMED CONSENT FOR LASER IN-SITU KERATOMILEUSIS (LASIK) INTRODUCTION This information
EYE PHYSICIANS OF NORTH HOUSTON 845 FM 1960 WEST, SUITE 101, Houston, TX 77090 Office: 281 893 1760 Fax: 281 893 4037 INFORMED CONSENT FOR LASER IN-SITU KERATOMILEUSIS (LASIK) INTRODUCTION This information
The Definition & Classification of Dry Eye Disease
 Issue: April 2008 The Definition & Classification of Dry Eye Disease Guidelines from the 2007 International Dry Eye Workshop BY MICHAEL A. LEMP, M. D. AND GARY N. FOULKS, M. D., F.A.C.S. The diagnosis
Issue: April 2008 The Definition & Classification of Dry Eye Disease Guidelines from the 2007 International Dry Eye Workshop BY MICHAEL A. LEMP, M. D. AND GARY N. FOULKS, M. D., F.A.C.S. The diagnosis
Laurie Shaker-Irwin, Ph.D., M.S. Co-Leader, Regulatory Knowledge and Research Ethics UCLA Clinical and Translational Science Institute
 Laurie Shaker-Irwin, Ph.D., M.S. Co-Leader, Regulatory Knowledge and Research Ethics UCLA Clinical and Translational Science Institute Understand the protocol completely Recognize institutional polices
Laurie Shaker-Irwin, Ph.D., M.S. Co-Leader, Regulatory Knowledge and Research Ethics UCLA Clinical and Translational Science Institute Understand the protocol completely Recognize institutional polices
Facts about diabetic macular oedema
 Patient information medical retina services Facts about diabetic macular oedema What is diabetic macular oedema? Diabetic eye disease is a leading cause of blindness registration among working age adults
Patient information medical retina services Facts about diabetic macular oedema What is diabetic macular oedema? Diabetic eye disease is a leading cause of blindness registration among working age adults
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Laser-Assisted In Situ Keratomileusis for Patients With Dry Eye
 CLINICAL SCIENCES Laser-Assisted In Situ Keratomileusis for Patients With Dry Eye Ikuko Toda, MD; Naoko Asano-Kato, MD; Yoshiko Hori-Komai, MD; Kazuo Tsubota, MD Objective: To evaluate the efficacy and
CLINICAL SCIENCES Laser-Assisted In Situ Keratomileusis for Patients With Dry Eye Ikuko Toda, MD; Naoko Asano-Kato, MD; Yoshiko Hori-Komai, MD; Kazuo Tsubota, MD Objective: To evaluate the efficacy and
TEMPLATE DATA MANAGEMENT PLAN
 TEMPLATE DATA MANAGEMENT PLAN ICRIN (QM sub group) Version: XX Date: XXXXXXX Page 1 of 6 1.0 Document Ownership The Data Management Plan (DMP) will be initiated and subsequently owned by the Data Manager
TEMPLATE DATA MANAGEMENT PLAN ICRIN (QM sub group) Version: XX Date: XXXXXXX Page 1 of 6 1.0 Document Ownership The Data Management Plan (DMP) will be initiated and subsequently owned by the Data Manager
Not Just for Dry Eyes Off Label Use of Cyclosporine
 Not Just for Dry Eyes Off Label Use of Cyclosporine William D. Townsend, O.D., F.A.A.O Advanced Eye Care Canyon, TX Adjunct Professor- UHCO Houston, TX drbilltownsend@gmail.com TOA Convention Austin, TX
Not Just for Dry Eyes Off Label Use of Cyclosporine William D. Townsend, O.D., F.A.A.O Advanced Eye Care Canyon, TX Adjunct Professor- UHCO Houston, TX drbilltownsend@gmail.com TOA Convention Austin, TX
Consent for Bilateral Simultaneous Refractive Surgery PRK
 Consent for Bilateral Simultaneous Refractive Surgery PRK Please sign and return Patient Copy While many patients choose to have both eyes treated at the same surgical setting, there may be risks associated
Consent for Bilateral Simultaneous Refractive Surgery PRK Please sign and return Patient Copy While many patients choose to have both eyes treated at the same surgical setting, there may be risks associated
SF-DCT INFORMATION FOR PRIMARY SJOGREN S SYNDROME (PSS) CLAIMS OPTION 1. (PSS claims are not eligible for Disease Option 2)
 SF-DCT INFORMATION FOR PRIMARY SJOGREN S SYNDROME (PSS) CLAIMS OPTION 1 (PSS claims are not eligible for Disease Option 2) 1 Primary Sjogren s Syndrome (PSS) Sjogren s Syndrome (SHOW-grins) is an autoimmune
SF-DCT INFORMATION FOR PRIMARY SJOGREN S SYNDROME (PSS) CLAIMS OPTION 1 (PSS claims are not eligible for Disease Option 2) 1 Primary Sjogren s Syndrome (PSS) Sjogren s Syndrome (SHOW-grins) is an autoimmune
Sheffield Kidney Institute. Planning a Clinical Trial
 Planning a Clinical Trial Clinical Trials Testing a new drug Ethical Issues Liability and Indemnity Trial Design Trial Protocol Statistical analysis Clinical Trials Phase I: Phase II: Phase III: Phase
Planning a Clinical Trial Clinical Trials Testing a new drug Ethical Issues Liability and Indemnity Trial Design Trial Protocol Statistical analysis Clinical Trials Phase I: Phase II: Phase III: Phase
Immunex Corporation Novantrone (Mitoxantrone HCL) P&CNS Advisory Committee Briefing Document. Page 020
 Page 020 4.0 Efficacy of Mitoxantrone in Multiple Sclerosis The efficacy of mitoxantrone in MS was demonstrated in two well-designed, randomized trials: Studies 901 and 902. The study design and efficacy
Page 020 4.0 Efficacy of Mitoxantrone in Multiple Sclerosis The efficacy of mitoxantrone in MS was demonstrated in two well-designed, randomized trials: Studies 901 and 902. The study design and efficacy
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
NCT00272090. sanofi-aventis HOE901_3507. insulin glargine
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
Patient Information. Name: Soc Security #: Date of Birth: Age: Male / Female. LOCAL Address: Street City State Zip. Phone: Home: Cell / Work:
 Patient Information PERSONAL INFORMATION (Please Print Clearly) Name: Soc Security #: Date of Birth: Age: Male / Female LOCAL Address: Street City State Zip Phone: Home: Cell / Work: Email Address: Out
Patient Information PERSONAL INFORMATION (Please Print Clearly) Name: Soc Security #: Date of Birth: Age: Male / Female LOCAL Address: Street City State Zip Phone: Home: Cell / Work: Email Address: Out
COPYRIGHTED MATERIAL. What Is Dry Eye Syndrome, and Who Gets It?
 C H A P T E R 1 What Is Dry Eye Syndrome, and Who Gets It? Myth Dry eye syndrome is not a serious disease. Fact If dry eye syndrome is not treated properly, it can lead to severe eye problems, including
C H A P T E R 1 What Is Dry Eye Syndrome, and Who Gets It? Myth Dry eye syndrome is not a serious disease. Fact If dry eye syndrome is not treated properly, it can lead to severe eye problems, including
Overview of Drug Development: the Regulatory Process
 Overview of Drug Development: the Regulatory Process Roger D. Nolan, PhD Director, Project Operations Calvert Research Institute November, 2006 Adapted from course taught by Cato Research Background: Roger
Overview of Drug Development: the Regulatory Process Roger D. Nolan, PhD Director, Project Operations Calvert Research Institute November, 2006 Adapted from course taught by Cato Research Background: Roger
Are you interested in Laser Vision Correction/ LASIK? Yes / No
 Peter J. Cornell, M.D. Stuart B. Stoll, M.D. 450 North Bedford Drive, Suite 101 Beverly Hills, CA 90210 P: (310) 274 9205 F: (310) 274-7229 www.bhlasik.com Name Last First Middle Date of Birth Age_ Sex:
Peter J. Cornell, M.D. Stuart B. Stoll, M.D. 450 North Bedford Drive, Suite 101 Beverly Hills, CA 90210 P: (310) 274 9205 F: (310) 274-7229 www.bhlasik.com Name Last First Middle Date of Birth Age_ Sex:
BSM Connection elearning Course
 BSM Connection elearning Course Scope of the Eye Care Practice 2008, BSM Consulting All Rights Reserved. Table of Contents OVERVIEW...1 THREE O S IN EYE CARE...1 ROUTINE VS. MEDICAL EXAMS...2 CONTACT LENSES/GLASSES...2
BSM Connection elearning Course Scope of the Eye Care Practice 2008, BSM Consulting All Rights Reserved. Table of Contents OVERVIEW...1 THREE O S IN EYE CARE...1 ROUTINE VS. MEDICAL EXAMS...2 CONTACT LENSES/GLASSES...2
Blepharoplasty - Eyelid Surgery
 Blepharoplasty - Eyelid Surgery Introduction Eyelid surgery repairs sagging or drooping eyelids. The surgery is also known as blepharoplasty, or an eyelid lift. Sagging or drooping eyelids happen naturally
Blepharoplasty - Eyelid Surgery Introduction Eyelid surgery repairs sagging or drooping eyelids. The surgery is also known as blepharoplasty, or an eyelid lift. Sagging or drooping eyelids happen naturally
Acknowledgements. Dry Eye Update. Dry Eye. Dry Eye. Dry Eye. 2014 Monterey Symposium
 2014 Monterey Symposium Update Jimmy Jackson, OD, FAAO President InSight Lasik Acknowledgements I have received honoraria from Alcon Laboratories, Inc for speaking engagements. I have received research
2014 Monterey Symposium Update Jimmy Jackson, OD, FAAO President InSight Lasik Acknowledgements I have received honoraria from Alcon Laboratories, Inc for speaking engagements. I have received research
Ohr Pharmaceutical Reports Fiscal Year 2015 Financial and Business Results
 December 10, 2015 Ohr Pharmaceutical Reports Fiscal Year 2015 Financial and Business Results OHR-102 Phase 3 Program in Wet-AMD to be Initiated Upon Completion of SPA; Enroll Patients in Q1 2016 Conference
December 10, 2015 Ohr Pharmaceutical Reports Fiscal Year 2015 Financial and Business Results OHR-102 Phase 3 Program in Wet-AMD to be Initiated Upon Completion of SPA; Enroll Patients in Q1 2016 Conference
Eyetube Roundtable. Blepharitis in the United States, 2009: A Perspective on Prevalence. Marguerite McDonald, MD, FACS
 Eyetube Roundtable Blepharitis in the United States, 2009: A Perspective on Prevalence Marguerite McDonald, MD, FACS Clinical Professor of Ophthalmology NYU School of Medicine New York, New York Adjunct
Eyetube Roundtable Blepharitis in the United States, 2009: A Perspective on Prevalence Marguerite McDonald, MD, FACS Clinical Professor of Ophthalmology NYU School of Medicine New York, New York Adjunct
Ocular Surface Syndrome after LASIK
 Alaa Atef Ghaith, MD Professor of Ophthalmology Alexandria University Ocular Surface Syndrome after LASIK Up to 33% of eyes Multifactorial entity which causes distress to the patients and physicians 1
Alaa Atef Ghaith, MD Professor of Ophthalmology Alexandria University Ocular Surface Syndrome after LASIK Up to 33% of eyes Multifactorial entity which causes distress to the patients and physicians 1
Minor Lid Surgery. Information for patients
 Minor Lid Surgery Information for patients This leaflet has been produced to give you information about the problems you have been having with your eyelid. If you have any questions or require further
Minor Lid Surgery Information for patients This leaflet has been produced to give you information about the problems you have been having with your eyelid. If you have any questions or require further
Drugs for MS.Drug fact box cannabis extract (Sativex) Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug fact box cannabis extract (Sativex) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further
Version History Policy Title Drugs for MS.Drug fact box cannabis extract (Sativex) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further
Administrative Code. Title 23: Medicaid Part 217 Vision Services
 Title 23: Medicaid Administrative Code Title 23: Medicaid Part 217 Vision Services Table of Contents Table of Contents Title 23: Medicaid... 1 Table of Contents... 1 Title 23: Division of Medicaid... 1
Title 23: Medicaid Administrative Code Title 23: Medicaid Part 217 Vision Services Table of Contents Table of Contents Title 23: Medicaid... 1 Table of Contents... 1 Title 23: Division of Medicaid... 1
Glaucoma filtration surgery (Tube surgery)
 Oxford Eye Hospital Glaucoma filtration surgery (Tube surgery) Information for patients page 2 This leaflet gives you information that will help you decide whether to have glaucoma tube surgery. You might
Oxford Eye Hospital Glaucoma filtration surgery (Tube surgery) Information for patients page 2 This leaflet gives you information that will help you decide whether to have glaucoma tube surgery. You might
GT-020 Phase 1 Clinical Trial: Results of Second Cohort
 GT-020 Phase 1 Clinical Trial: Results of Second Cohort July 29, 2014 NASDAQ: GALT www.galectintherapeutics.com 2014 Galectin Therapeutics inc. Forward-Looking Statement This presentation contains, in
GT-020 Phase 1 Clinical Trial: Results of Second Cohort July 29, 2014 NASDAQ: GALT www.galectintherapeutics.com 2014 Galectin Therapeutics inc. Forward-Looking Statement This presentation contains, in
Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple Sclerosis
 Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Ophthalmic Consultants of Long Island
 Case History Improving Cataract and Refractive Surgery Outcomes Through Ocular Surface Optimization 59 year old healthy white female History increased IOP Mother has history of glaucoma Presents for refractive
Case History Improving Cataract and Refractive Surgery Outcomes Through Ocular Surface Optimization 59 year old healthy white female History increased IOP Mother has history of glaucoma Presents for refractive
dry eye formula Address Dry Eye Symptoms from the Inside Out The most complete eye vitamin for the relief of dry eye discomfort
 I started using your product three weeks ago. I d often use artificial tear drops 6 times a day. Now I barely have to use those drops at all. My eyes feel great and moist, and I see much better. Thank
I started using your product three weeks ago. I d often use artificial tear drops 6 times a day. Now I barely have to use those drops at all. My eyes feel great and moist, and I see much better. Thank
Thyroid eye disease (TED)
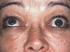 Thyroid eye disease (TED) Mr David H Verity, MD MA FRCOphth Consultant Ophthalmic Surgeon Synonyms: Graves ophthalmopathy, thyroid ophthalmopathy, thyroid associated ophthalmopathy This information leaflet
Thyroid eye disease (TED) Mr David H Verity, MD MA FRCOphth Consultant Ophthalmic Surgeon Synonyms: Graves ophthalmopathy, thyroid ophthalmopathy, thyroid associated ophthalmopathy This information leaflet
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
1.0 Abstract. Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA. Keywords. Rationale and Background:
 1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
Your one stop vision centre Our ophthalmic centre offers comprehensive eye management, which includes medical,
 sight see OLYMPIA EYE & LASER CENTRE Your one stop vision centre Our ophthalmic centre offers comprehensive eye management, which includes medical, At the Olympia Eye & Laser Centre, our vision is to improve
sight see OLYMPIA EYE & LASER CENTRE Your one stop vision centre Our ophthalmic centre offers comprehensive eye management, which includes medical, At the Olympia Eye & Laser Centre, our vision is to improve
9/3/2015. Stephanie L. Woo, O.D., F.A.A.O., F.S.L.S.
 Stephanie L. Woo, O.D., F.A.A.O., F.S.L.S. 1 This is a repeat from the lecture from GSLS, items may have changed since that date. I am not a billing EXPERT like them! 2 Bill a scleral lens fit with materials
Stephanie L. Woo, O.D., F.A.A.O., F.S.L.S. 1 This is a repeat from the lecture from GSLS, items may have changed since that date. I am not a billing EXPERT like them! 2 Bill a scleral lens fit with materials
Explanation of the Procedure
 Informed Consent Cataract Surgery with Intraocular Lens Implant Please initial below indicating that you have read and understand each section Introduction The internal lens of the eye can become cloudy
Informed Consent Cataract Surgery with Intraocular Lens Implant Please initial below indicating that you have read and understand each section Introduction The internal lens of the eye can become cloudy
Management of Dry Eyes in primary care
 UK Vision Strategy RCGP Royal College of General Practitioners Management of Dry Eyes in primary care Key learning points Dry eye is a very common condition presenting to GPs, and is probably underdiagnosed.
UK Vision Strategy RCGP Royal College of General Practitioners Management of Dry Eyes in primary care Key learning points Dry eye is a very common condition presenting to GPs, and is probably underdiagnosed.
PATIENT CONSENT FOR LASER IN-SITU KERATOMILEUSIS (LASIK)
 INTRODUCTION: You have been diagnosed with myopia (nearsightedness) or hyperopia (farsightedness) with or without astigmatism, or astigmatism alone. Myopia is a result of light entering the eye and focusing
INTRODUCTION: You have been diagnosed with myopia (nearsightedness) or hyperopia (farsightedness) with or without astigmatism, or astigmatism alone. Myopia is a result of light entering the eye and focusing
TABLE OF CONTENTS: LASER EYE SURGERY CONSENT FORM
 1 BoydVision TABLE OF CONTENTS: LASER EYE SURGERY CONSENT FORM Risks and Side Effects... 2 Risks Specific to PRK... 3 Risks Specific to LASIK... 4 Patient Statement of Consent... 5 Consent for Laser Eye
1 BoydVision TABLE OF CONTENTS: LASER EYE SURGERY CONSENT FORM Risks and Side Effects... 2 Risks Specific to PRK... 3 Risks Specific to LASIK... 4 Patient Statement of Consent... 5 Consent for Laser Eye
Guidance for Industry Migraine: Developing Drugs for Acute Treatment
 Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Clinical Study Synopsis
 Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
U.S. Food and Drug Administration
 U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
Comparative Evaluation of Dry Eye Following Cataract Surgery: A Study from North India
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 13, Issue 6 Ver. III (Jun. 2014), PP 13-18 Comparative Evaluation of Dry Eye Following Cataract Surgery:
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 13, Issue 6 Ver. III (Jun. 2014), PP 13-18 Comparative Evaluation of Dry Eye Following Cataract Surgery:
CONSENT FORM. Procedure: Descemet s Stripping Automated Endothelial Keratoplasty (DSAEK)
 CONSENT FORM Procedure: Descemet s Stripping Automated Endothelial Keratoplasty (DSAEK) Surgeon: Jeffrey W. Liu, M.D. Peninsula Laser Eye Medical Group 1174 Castro Street, Ste. 100 Mountain View, CA 94040
CONSENT FORM Procedure: Descemet s Stripping Automated Endothelial Keratoplasty (DSAEK) Surgeon: Jeffrey W. Liu, M.D. Peninsula Laser Eye Medical Group 1174 Castro Street, Ste. 100 Mountain View, CA 94040
Corneal Collagen Cross-Linking (CXL) With Riboflavin
 Dr. Paul J. Dubord, MD, FRCSC Clinical Professor Department of Ophthalmology and Visual Sciences University of British Columbia Patient Information Guide Corneal Collagen Cross-Linking (CXL) With Riboflavin
Dr. Paul J. Dubord, MD, FRCSC Clinical Professor Department of Ophthalmology and Visual Sciences University of British Columbia Patient Information Guide Corneal Collagen Cross-Linking (CXL) With Riboflavin
SMILE SURGERY A GUIDE FOR PATIENTS. Professional care for your eye health
 SMILE SURGERY A GUIDE FOR PATIENTS Professional care for your eye health Contents About Dr John Males... 1 Our Commitment to Our Patients... 2 COMMON QUESTIONS How Does an Eye Work?... 3 What is Myopia
SMILE SURGERY A GUIDE FOR PATIENTS Professional care for your eye health Contents About Dr John Males... 1 Our Commitment to Our Patients... 2 COMMON QUESTIONS How Does an Eye Work?... 3 What is Myopia
FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema
 Media Release Basel, 13 August 2012 FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema First major treatment advance in more than 25 years for sight-threatening condition
Media Release Basel, 13 August 2012 FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema First major treatment advance in more than 25 years for sight-threatening condition
Guidance for Industry Acne Vulgaris: Developing Drugs for Treatment
 Guidance for Industry Acne Vulgaris: Developing Drugs for Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document
Guidance for Industry Acne Vulgaris: Developing Drugs for Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document
PROSE Treatment Information for Patients and Doctors
 PROSE Treatment Information for Patients and Doctors prior to getting my PROSE devices, life was hard. Everything that I loved to do was slipping away...that all changed the day I got PROSE. PROSE patient
PROSE Treatment Information for Patients and Doctors prior to getting my PROSE devices, life was hard. Everything that I loved to do was slipping away...that all changed the day I got PROSE. PROSE patient
Comparison/Control Groups. Mary Foulkes, PhD Johns Hopkins University
 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
IntraLase and LASIK: Risks and Complications
 No surgery is without risks and possible complications and LASIK is no different in that respect. At Trusted LASIK Surgeons, we believe patients can minimize these risks by selecting a highly qualified
No surgery is without risks and possible complications and LASIK is no different in that respect. At Trusted LASIK Surgeons, we believe patients can minimize these risks by selecting a highly qualified
Kensington Eye Center 4701 Randolph Road, #G-2 Rockville, MD 20852 (301) 881-5701 www.keceyes.com
 Kensington Eye Center 4701 Randolph Road, #G-2 Rockville, MD 20852 (301) 881-5701 www.keceyes.com Natasha L. Herz, MD INFORMED CONSENT FOR DESCEMET S STRIPPING and AUTOMATED ENDOTHELIAL KERATOPLASTY (DSAEK)
Kensington Eye Center 4701 Randolph Road, #G-2 Rockville, MD 20852 (301) 881-5701 www.keceyes.com Natasha L. Herz, MD INFORMED CONSENT FOR DESCEMET S STRIPPING and AUTOMATED ENDOTHELIAL KERATOPLASTY (DSAEK)
