FREQUENTLY ASKED QUESTIONS
|
|
|
- Miles Higgins
- 8 years ago
- Views:
Transcription
1 NATIONAL SCIENCE ADVISORY BOARD FOR BIOSECURITY FREQUENTLY ASKED QUESTIONS Establishment of the NSABB 1. What is the Administration s policy on biosecurity in life sciences research? 2. Why was the NSABB created? 3. Was the NSABB created in response to the National Academies/National Research Council report Biotechnology Research in an Age of Terrorism: Confronting the Dual Use Dilemma? 4. What did the NRC report say? 5. How long will the NSABB exist? Administration/Functions of the NSABB 6. What is the role of the NSABB? 7. What are the specific functions of the NSABB? 8. Who serves on the NSABB? How long do members serve? 9. How are NSABB members selected? 10. What federal agencies are represented on the NSABB? 11. How often does the NSABB meet? Are the meetings open to the public? 12. Who manages and staffs the NSABB? 13. How can I contact the NSABB? Oversight of Dual Use Research 14. What is dual use research? 15. Does the NSABB review or approve all dual use research? 16. What is the relationship between research involving Select Agents and dual use research? Is there a relationship between the oversight systems for these two areas of research? 17. How have the roles and responsibilities of Institutional Biosafety Committees (IBCs) changed with the announcement of new Federal biosecurity initiatives, including the establishment of the NSABB and a proposed role for IBCs in the review of dual use research?
2 Establishment of the NSABB 1. What is the Administration s policy on biosecurity in life sciences research? In addressing national biosecurity needs, the Administration is carefully balancing the importance of continued progress in the life sciences against the need to minimize the risk of misuse of research information to pose a threat to public health and other aspects of national security. This is resulting in a number of proposed policies and activities that collectively aim to enhance biosecurity in life sciences research. The initiatives underway include (1) implementation of the Public Health Security and Bioterrorism Preparedness and Response Act through the Select Agent Program, (2) promotion of research into development of countermeasures for biological threats, (3) establishment of the National Science Advisory Board for Biosecurity (NSABB) to provide guidance on biosecurity in life science research, and (4) a series of biosecurity initiatives on which the NSABB is advising. These initiatives include: Development of a framework for the review and oversight of dual use research of concern at the federal and institutional levels; Criteria for defining dual use research of concern; Guidelines for the publication, public presentation, and public communication of potentially sensitive research; Guidance on developing a code of conduct for scientists and laboratory workers engaged in research activities with dual use potential; Fostering international collaboration on the oversight of dual use biological research; Programs for education and training on the issue of dual use research and related biosecurity measures. 2. Why was the NSABB created? The advisory board was created to provide advice and guidance to the federal government regarding biological research yielding information and technologies with the potential to be misused to pose a biologic threat to public health or national security (i.e., dual use research). The NSABB advises on and recommends specific strategies for the efficient and effective oversight of federally conducted or supported dual use biological research, taking into consideration national security concerns and the needs of the research community. 3. Was the NSABB created in response to the National Academies/National Research Council report Biotechnology Research in an Age of Terrorism: Confronting the Dual Use Dilemma? The National Research Council (NRC) report made an important contribution to the development of biosecurity policy, although it was not the only stimulus for these actions. Prior to the release of the NRC report, the Administration had already initiated an examination of security issues in biological research. This executive branch evaluation was in progress when the report was published. The NRC report recommended a series of actions to improve biosecurity in life science research, one of which was the creation of an advisory body. The NSABB, as it is chartered, differs somewhat from the panel proposed by the NRC report, but has aims similar to those envisioned by the NRC committee.
3 4. What did the NRC report say? The NRC report discussed concerns related to the potential for biotechnology research to be used inappropriately for purposes of biowarfare or bioterrorism. For example, the report addressed a very significant issue facing life scientists, research administrators, policymakers, national security experts, and scientific publishers, namely, how to minimize the potential for misuse of biotechnology without hindering the progress of science and the important benefits that it yields. The report also recommended a series of actions to protect public health and improve research biosecurity. The Administration agrees that there is a need for improved biosecurity within the life sciences research community. The NSABB was established to recommend strategies to avoid unnecessary restrictions on critical life sciences research while addressing national security concerns. 5. How long will the NSABB exist? As with all federal advisory committees, the NSABB is chartered for two year intervals and will continue its work pending biannual renewals of the charter by the Department of Health and Human Services (HHS). Administration/Functions of the NSABB 6. What is the role of the NSABB? The NSABB advises all federal departments and agencies with an interest in life sciences research. The Board recommends specific strategies for the efficient and effective oversight of dual use biological research, including the development of guidelines for institutional review of dual use research. The Board considers both national security concerns and the needs of the research community when providing guidance and recommendations to the federal government. 7. What are the specific functions of the NSABB? The initial tasks of the Board included development of criteria for the identification of dual use research; tools for the communication of dual use research results and technologies; and guidance on developing a code of conduct for scientists and laboratory workers that can be adopted by federal agencies, as well as by professional organizations and institutions engaged in conducting life science research. In addition, the NSABB will advise on a number of other issues, including oversight of dual use research, biosecurity training and education for scientists; strategies to foster international dialogue on issues pertinent to dual use biological research; and any other issues as directed by the Secretary of HHS.
4 8. Who serves on the NSABB? How long do members serve? The NSABB has 25 voting members representing key stakeholder perspectives and 18 ex officio members representing various federal agencies. Members serve for overlapping terms of one to four years. The NSABB members provide expertise in areas such as molecular biology, microbiology, infectious diseases, laboratory biosafety and biosecurity, public health/epidemiology, health physics, pharmaceutical production, veterinary medicine, plant health, food production, bioethics, national security, biodefense, intelligence, law and law enforcement, scientific publishing, institutional biosafety committees, recombinant DNA, and export control. 9. How are NSABB members selected? The NSABB members are appointed by the Secretary of HHS in consultation with other federal departments and agencies with an interest in life sciences research. The public can submit nominations for future NSABB membership through the ex officio agencies or directly to the Office of Biotechnology Activities (OBA) at 10. What federal agencies are represented on the NSABB? The Board includes nonvoting ex officio members from relevant federal departments and agencies with an interest in life sciences research. These include: Executive Office of the President Department of Health and Human Services Department of Energy Department of Homeland Security Department of Veterans Affairs Department of Defense Department of the Interior Environmental Protection Agency U.S. Department of Agriculture National Science Foundation Department of Justice Department of State Department of Commerce National Aeronautics and Space Administration Intelligence Community Others as appropriate
5 11. How often does the NSABB meet? Are the meetings open to the public? The NSABB meets at least twice a year, and may also be convened on an as-needed basis. All meetings of the NSABB are announced in the Federal Register and on the NSABB web site at NSABB meetings are open to the public, except as determined otherwise by the Secretary of HHS, in accordance with the Sunshine Act (5 U.S.C. 552b(c)) and the Federal Advisory Committee Act. 12. Who manages and staffs the NSABB? The HHS Secretary designated the National Institutes of Health (NIH) to provide management and support services for the NSABB. The NSABB staff is located in the Office of Biotechnology Activities (OBA) within the Office of the Director, NIH. 13. How can I contact the NSABB? You may contact NSABB through the NIH Office of Biotechnology Activities at or by at nsabb@od.nih.gov. General information about NSABB, such as its membership and upcoming meeting dates, can be accessed on the NSABB Web site at Oversight of Dual Use Research 14. What is dual use research? The NSABB has proposed defining dual use research of concern as research that, based on current understanding, can be reasonably anticipated to provide knowledge, products, or technologies that could be directly misapplied by others to pose a threat to public health, agriculture, plants, animals, the environment, or materiel. The NSABB has also proposed a series of experimental outcomes that should be given special consideration for their dual use potential. The public will be invited to comment on this definition, and then the U.S. government will consider whether and how to adopt this definition as formal policy. 15. Does the NSABB review or approve all dual use research? No. The NSABB advises on the development of national policies governing institutional review and approval of dual use biological research. The Board will also develop criteria and processes for institutions to refer classes of research or specific experiments to the NSABB for guidance. Upon referral, the NSABB may review and provide guidance on experiments that exemplify a notable or novel category of dual use research requiring additional guidance from the Board. The NSABB will also advise on the interpretation and application of federal guidelines on dual use research in instances where the institution seeks additional advice. The NSABB does not approve the conduct of specific experiments.
6 16. What is the relationship between research involving Select Agents and dual use research? Is there a relationship between the oversight systems for these two areas of research? The oversight system for dual use research is likely to be complementary to that of Select Agents in that it will be based on principles and practices for reducing the likelihood that biological organisms, knowledge, and technologies are intentionally misused to pose a risk to public health and national security. Select Agent research is a very specific subset of life sciences research; it involves only those microorganisms and toxins specifically identified in DHHS and USDA regulations as having the potential to pose a severe threat to human, animal, or plant health, or to animal and plant products. All research conducted in the U.S. involving Select Agents, regardless of funding source, is subject to the Select Agent regulations promulgated by DHHS and USDA. These regulations establish requirements regarding the possession, use, and transfer of Select Agents and toxins, including requirements for registration, security risk assessments, safety plans, security plans, training, transfers, record keeping, inspections, and notifications. The criteria for dual use research apply to a much broader category of life sciences research, although they may incorporate certain research projects that involve Select Agents, depending on the nature of the particular experiments and the potential for misuse of the results and/or technology. 17. How have the roles and responsibilities of Institutional Biosafety Committees (IBCs) changed with the announcement of new Federal biosecurity initiatives, including the establishment of the NSABB and a proposed role for IBCs in the review of dual use research? At this time, the roles and responsibilities of IBCs have not changed. IBCs should continue to carry out the duties outlined in the NIH Guidelines for Research Involving Recombinant DNA Molecules. The NSABB has developed draft criteria to distinguish dual use research and will embark upon a process of developing guidelines that may eventually define a role for local review groups, such as IBCs, in the oversight of dual use research. IBCs and other stakeholders will have a voice in the development of these guidelines. The IBC community will be notified directly of any future changes in their responsibilities. Also, any relevant developments will be posted on the IBC pages of the Web site of the NIH Office of Biotechnology Activities:
How To Set Up A National Biological Laboratory Safety And Security Monitoring Program
 Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science Volume 10, Number 4, 2012 ª Mary Ann Liebert, Inc. DOI: 10.1089/bsp.2012.0054 Establishing a National Biological Laboratory Safety
Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science Volume 10, Number 4, 2012 ª Mary Ann Liebert, Inc. DOI: 10.1089/bsp.2012.0054 Establishing a National Biological Laboratory Safety
Administrative Procedure Chapter 12, Research Administrative Procedure: AP 12.217, Procedure for Institutional Oversight of Life
 Administrative Procedure, AP 12.217 Procedure for Institutional Oversight of Life Sciences Dual Use Research of Concern Page 1 of 15 Administrative Procedure Chapter 12, Research Administrative Procedure:
Administrative Procedure, AP 12.217 Procedure for Institutional Oversight of Life Sciences Dual Use Research of Concern Page 1 of 15 Administrative Procedure Chapter 12, Research Administrative Procedure:
United States Government Policy for Institutional Oversight of Life Sciences Dual Use Research of Concern
 United States Government Policy for Institutional Oversight of Life Sciences Dual Use Research of Concern Contents Section 1. Introduction... 2 Section 2. Purpose... 4 Section 3. Guiding Principles for
United States Government Policy for Institutional Oversight of Life Sciences Dual Use Research of Concern Contents Section 1. Introduction... 2 Section 2. Purpose... 4 Section 3. Guiding Principles for
NIH POLICY MANUAL. 1340 - NIH Occupational Safety and Health Management Issuing Office: ORS/DOHS 301-496-2960 Release Date: 2/27/06
 NIH POLICY MANUAL 1340 - NIH Occupational Safety and Health Management Issuing Office: ORS/DOHS 301-496-2960 Release Date: 2/27/06 1. Explanation of Material Transmitted: This chapter establishes the scope
NIH POLICY MANUAL 1340 - NIH Occupational Safety and Health Management Issuing Office: ORS/DOHS 301-496-2960 Release Date: 2/27/06 1. Explanation of Material Transmitted: This chapter establishes the scope
University of Nevada, Reno Environmental Health and Safety Policy
 University of Nevada, Reno Environmental Health and Safety Policy Title: Institutional Oversight of Dual Use Research of Concern Date: September 24, 2015 Revision: 0 Page: Page 1 of 7 POLICY: The University
University of Nevada, Reno Environmental Health and Safety Policy Title: Institutional Oversight of Dual Use Research of Concern Date: September 24, 2015 Revision: 0 Page: Page 1 of 7 POLICY: The University
Caltech Policy for Institutional Oversight of Life Sciences Dual Use Research of Concern
 Caltech Policy for Institutional Oversight of Life Sciences Dual Use Research of Concern Purpose The purpose of this Policy is to strengthen regular institutional review and oversight of certain life sciences
Caltech Policy for Institutional Oversight of Life Sciences Dual Use Research of Concern Purpose The purpose of this Policy is to strengthen regular institutional review and oversight of certain life sciences
BOSTON UNIVERSITY Dual Use Research of Concern ( DURC ) Policy
 1. Purpose BOSTON UNIVERSITY Dual Use Research of Concern ( DURC ) Policy The purpose of this policy is to outline Boston University s ( BU ) institutional oversight of Dual Use Research of Concern according
1. Purpose BOSTON UNIVERSITY Dual Use Research of Concern ( DURC ) Policy The purpose of this policy is to outline Boston University s ( BU ) institutional oversight of Dual Use Research of Concern according
The Johns Hopkins Hospital and The Johns Hopkins University Health, Safety and Environment Manual Biological Safety:
 Page 1 of 7 Keywords: Table of Contents Page Number I. POLICY 1 II. SUMMARY 1 III. REVIEW CYCLE 7 Appendix A: Process for Institutional Review of Life Sciences Research within the Scope of the Click Here
Page 1 of 7 Keywords: Table of Contents Page Number I. POLICY 1 II. SUMMARY 1 III. REVIEW CYCLE 7 Appendix A: Process for Institutional Review of Life Sciences Research within the Scope of the Click Here
Federal Office of Small and Disadvantaged Business Utilization (OSDBU) Directors Interagency Council. CHARTER
 Federal Office of Small and Disadvantaged Business Utilization (OSDBU) Directors Interagency Council. CHARTER MISSION: The mission of the Federal Office of Small and Disadvantaged Business Utilization
Federal Office of Small and Disadvantaged Business Utilization (OSDBU) Directors Interagency Council. CHARTER MISSION: The mission of the Federal Office of Small and Disadvantaged Business Utilization
FRAMEWORK FOR CONDUCTING RISK AND BENEFIT ASSESSMENTS OF GAIN-OF-FUNCTION RESEARCH: RECOMMENDATIONS OF THE NSABB WORKING GROUP
 1 2 3 FRAMEWORK FOR CONDUCTING RISK AND BENEFIT ASSESSMENTS OF GAIN-OF-FUNCTION RESEARCH: RECOMMENDATIONS OF THE NSABB WORKING GROUP 4 5 TABLE OF CONTENTS Background and Introduction 2 The Charge to the
1 2 3 FRAMEWORK FOR CONDUCTING RISK AND BENEFIT ASSESSMENTS OF GAIN-OF-FUNCTION RESEARCH: RECOMMENDATIONS OF THE NSABB WORKING GROUP 4 5 TABLE OF CONTENTS Background and Introduction 2 The Charge to the
Homeland Security Presidential Directive/HSPD-9 Subject: Defense of United States Agriculture and Food January 30, 2004
 For Immediate Release Office of the Press Secretary February 3, 2004 Homeland Security Presidential Directive/HSPD-9 Subject: Defense of United States Agriculture and Food January 30, 2004 Purpose (1)
For Immediate Release Office of the Press Secretary February 3, 2004 Homeland Security Presidential Directive/HSPD-9 Subject: Defense of United States Agriculture and Food January 30, 2004 Purpose (1)
UC Irvine Environmental Health & Safety SECTION: 2.07 INITIATOR: Campus Biosafety Officer
 UC Irvine Environmental Health & Safety SECTION: 2.07 INITIATOR: Campus Biosafety Officer TITLE: Institutional Biosafety Committee REVISION DATE : 03/16/2016 1. Program Description 2. Mission, Purpose,
UC Irvine Environmental Health & Safety SECTION: 2.07 INITIATOR: Campus Biosafety Officer TITLE: Institutional Biosafety Committee REVISION DATE : 03/16/2016 1. Program Description 2. Mission, Purpose,
Section VI Principles of Laboratory Biosecurity
 Section VI Principles of Laboratory Biosecurity Since the publication of the 4th edition of BMBL in 1999, significant events have brought national and international scrutiny to the area of laboratory security.
Section VI Principles of Laboratory Biosecurity Since the publication of the 4th edition of BMBL in 1999, significant events have brought national and international scrutiny to the area of laboratory security.
DEPARTMENT OF HEALTH AND HUMAN SERVICES. Health Resources and Services Administration. Advisory Council on Blood Stem Cell Transplantation
 This document is scheduled to be published in the Federal Register on 01/21/2016 and available online at http://federalregister.gov/a/2016-01127, and on FDsys.gov Billing Code 4165-15 DEPARTMENT OF HEALTH
This document is scheduled to be published in the Federal Register on 01/21/2016 and available online at http://federalregister.gov/a/2016-01127, and on FDsys.gov Billing Code 4165-15 DEPARTMENT OF HEALTH
Dual-Use Bioethics / Biosecurity Online Learning Train-the-Trainer Program
 Dual-Use Bioethics / Biosecurity Online Learning Train-the-Trainer Program Simon Whitby 31st Workshop of the Pugwash Study Group on the Implementation of the Chemical and Biological Weapons Conventions:
Dual-Use Bioethics / Biosecurity Online Learning Train-the-Trainer Program Simon Whitby 31st Workshop of the Pugwash Study Group on the Implementation of the Chemical and Biological Weapons Conventions:
S. ll. To prevent the proliferation of weapons of mass destruction, to prepare for attacks using weapons of mass destruction, and for other purposes.
 TH CONGRESS ST SESSION S. ll To prevent the proliferation of weapons of mass destruction, to prepare for attacks using weapons of mass destruction, and for other purposes. IN THE SENATE OF THE UNITED STATES
TH CONGRESS ST SESSION S. ll To prevent the proliferation of weapons of mass destruction, to prepare for attacks using weapons of mass destruction, and for other purposes. IN THE SENATE OF THE UNITED STATES
Centre for Research Ethics & Bioethics
 Moral obligations for synthetic biology research Stefan Eriksson, Associate Professor in research ethics The worry Dual use: malicious uses such as using a pathogen for terrorism. Cf. the Swedish security
Moral obligations for synthetic biology research Stefan Eriksson, Associate Professor in research ethics The worry Dual use: malicious uses such as using a pathogen for terrorism. Cf. the Swedish security
Policy of the National Institute of Nursing Research for Data and Safety Monitoring of Extramural Clinical Trials
 Policy of the National Institute of Nursing Research for Data and Safety Monitoring of Extramural Clinical Trials Purpose and Scope This policy sets forth the National Institute of Nursing Research (NINR)
Policy of the National Institute of Nursing Research for Data and Safety Monitoring of Extramural Clinical Trials Purpose and Scope This policy sets forth the National Institute of Nursing Research (NINR)
Responsibilities of the Biosafety Professional and Institution
 Responsibilities of the Biosafety Professional and Institution Debra L. Hunt, DrPH, CBSP Director, Biological Safety; Responsible Official (Select Agents) Assistant Professor Duke University / Duke Medicine
Responsibilities of the Biosafety Professional and Institution Debra L. Hunt, DrPH, CBSP Director, Biological Safety; Responsible Official (Select Agents) Assistant Professor Duke University / Duke Medicine
Regulatory Risk Analysis in the European Union, United States, and Canada
 Regulatory Risk Analysis in the European Union, United States, and Canada A Joint Presentation by: Takis Daskaleros, European Commission Nancy Beck, United States, Office of the Management of the Budget
Regulatory Risk Analysis in the European Union, United States, and Canada A Joint Presentation by: Takis Daskaleros, European Commission Nancy Beck, United States, Office of the Management of the Budget
Guidance for Industry Independent Consultants for Biotechnology Clinical Trial Protocols
 Guidance for Industry Independent Consultants for Biotechnology Clinical Trial Protocols For questions on the content of this document contact Leonard Wilson, CBER at 301-827-0373 or Susan Johnson, CDER
Guidance for Industry Independent Consultants for Biotechnology Clinical Trial Protocols For questions on the content of this document contact Leonard Wilson, CBER at 301-827-0373 or Susan Johnson, CDER
1851 (d) RULE OF CONSTRUCTION. Nothing in this section shall be construed to (1) require a State to report data under subsection
 U:\REPT\OMNI\FinalOmni\CPRT--HPRT-RU00-SAHR-AMNT.xml 0 (d) RULE OF CONSTRUCTION. Nothing in this section shall be construed to () require a State to report data under subsection (a); or () require a non-federal
U:\REPT\OMNI\FinalOmni\CPRT--HPRT-RU00-SAHR-AMNT.xml 0 (d) RULE OF CONSTRUCTION. Nothing in this section shall be construed to () require a State to report data under subsection (a); or () require a non-federal
Commonwealth of Australia 2003
 Import Risk Analysis Handbook Canberra, 2003 Commonwealth of Australia 2003 This work is copyright. You may download, display, print and reproduce this material in unaltered form only (retaining this notice)
Import Risk Analysis Handbook Canberra, 2003 Commonwealth of Australia 2003 This work is copyright. You may download, display, print and reproduce this material in unaltered form only (retaining this notice)
REGULATION REGULATION ON THE WORKING PROCEDURES AND PRINCIPLES OF RISK ASSESSMENT COMMITTEES AND COMMISSIONS SECTION ONE
 24 December 2011 SATURDAY Official Gazette No.: 28152 REGULATION From the Ministry of Food, Agriculture and Livestock: REGULATION ON THE WORKING PROCEDURES AND PRINCIPLES OF RISK ASSESSMENT COMMITTEES
24 December 2011 SATURDAY Official Gazette No.: 28152 REGULATION From the Ministry of Food, Agriculture and Livestock: REGULATION ON THE WORKING PROCEDURES AND PRINCIPLES OF RISK ASSESSMENT COMMITTEES
Doctoral Program Department of Microbiology and Immunology
 Doctoral Program Department of Microbiology and Immunology (05/12/2011 revised) Graduate studies in the Department of Microbiology and Immunology are designed to provide the doctoral student with a broad
Doctoral Program Department of Microbiology and Immunology (05/12/2011 revised) Graduate studies in the Department of Microbiology and Immunology are designed to provide the doctoral student with a broad
BIOSAFETY POLICY. Vice-President, Services PURPOSE
 Effective Date: March 4, 2016 Supersedes/Amends: May 2006 Originating Office: Office of the Vice-President, Services Policy Number: VPS-52 PURPOSE Biological materials have the potential, if not managed
Effective Date: March 4, 2016 Supersedes/Amends: May 2006 Originating Office: Office of the Vice-President, Services Policy Number: VPS-52 PURPOSE Biological materials have the potential, if not managed
Governance Structure
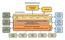 Secretariat -1 Chairs of Standing Committees - 3 Governance Structure Management Committee Secretariat Community and Municipal Consultative and Advisory Forum Board of Directors Principal Investigator
Secretariat -1 Chairs of Standing Committees - 3 Governance Structure Management Committee Secretariat Community and Municipal Consultative and Advisory Forum Board of Directors Principal Investigator
BYLAWS of the Graduate School of Biomedical Sciences
 BYLAWS of the Graduate School of Biomedical Sciences SECTION 1. Programs of the Graduate School of Biomedical Sciences The Graduate School of Biomedical Sciences (GSBS) of the Texas Tech University Health
BYLAWS of the Graduate School of Biomedical Sciences SECTION 1. Programs of the Graduate School of Biomedical Sciences The Graduate School of Biomedical Sciences (GSBS) of the Texas Tech University Health
One Hundred Seventh Congress of the United States of America
 H. R. 3448 One Hundred Seventh Congress of the United States of America AT THE SECOND SESSION Begun and held at the City of Washington on Wednesday, the twenty-third day of January, two thousand and two
H. R. 3448 One Hundred Seventh Congress of the United States of America AT THE SECOND SESSION Begun and held at the City of Washington on Wednesday, the twenty-third day of January, two thousand and two
Department of Defense INSTRUCTION
 Department of Defense INSTRUCTION NUMBER 6200.02 February 27, 2008 SUBJECT: Application of Food and Drug Administration (FDA) Rules to Department of Defense Force Health Protection Programs USD(P&R) References:
Department of Defense INSTRUCTION NUMBER 6200.02 February 27, 2008 SUBJECT: Application of Food and Drug Administration (FDA) Rules to Department of Defense Force Health Protection Programs USD(P&R) References:
INFORMATION MANAGEMENT
 United States Government Accountability Office Report to the Committee on Homeland Security and Governmental Affairs, U.S. Senate May 2015 INFORMATION MANAGEMENT Additional Actions Are Needed to Meet Requirements
United States Government Accountability Office Report to the Committee on Homeland Security and Governmental Affairs, U.S. Senate May 2015 INFORMATION MANAGEMENT Additional Actions Are Needed to Meet Requirements
Canadian Public Health Laboratory Network. Core Functions of Canadian Public Health Laboratories
 Canadian Public Health Laboratory Network Core Functions of Canadian Public Health Laboratories Canadian Public Health Laboratory Network The CPHLN Core Functions of Canadian Public Health Laboratories
Canadian Public Health Laboratory Network Core Functions of Canadian Public Health Laboratories Canadian Public Health Laboratory Network The CPHLN Core Functions of Canadian Public Health Laboratories
Emergency Management
 Emergency Management Health Portfolio Presented by Lise Gauthier Regional Coordinator Emergency Management Health Portfolio / Quebec Region Conference on Emergency Preparedness for First Nations in Québec
Emergency Management Health Portfolio Presented by Lise Gauthier Regional Coordinator Emergency Management Health Portfolio / Quebec Region Conference on Emergency Preparedness for First Nations in Québec
THE WHITE HOUSE. Office of the Press Secretary. For Immediate Release June 26, 2013 EXECUTIVE ORDER - - - - - - -
 THE WHITE HOUSE Office of the Press Secretary For Immediate Release June 26, 2013 EXECUTIVE ORDER - - - - - - - ESTABLISHING THE WHITE HOUSE COUNCIL ON NATIVE AMERICAN AFFAIRS By the authority vested in
THE WHITE HOUSE Office of the Press Secretary For Immediate Release June 26, 2013 EXECUTIVE ORDER - - - - - - - ESTABLISHING THE WHITE HOUSE COUNCIL ON NATIVE AMERICAN AFFAIRS By the authority vested in
FACILITY SAFETY PLAN INSTRUCTIONS
 FACILITY SAFETY PLAN INSTRUCTIONS Approval of the Facility Safety Plan is granted on an institution basis rather than on a proposal basis. Facility Safety Plan approvals are granted for a 5-year period
FACILITY SAFETY PLAN INSTRUCTIONS Approval of the Facility Safety Plan is granted on an institution basis rather than on a proposal basis. Facility Safety Plan approvals are granted for a 5-year period
Appendix -- Homeland Security Mission Funding by Agency and Budget Account (budget authority in millions of dollars)
 Department of Agriculture 508.8-528.6-603.6 Agricultural Research Service/ Buildings and Facilities/005-18-1401 - - 45.0-113.7 Protecting Critical Infrastructure and Key Assets - - 45.0-113.7 Agricultural
Department of Agriculture 508.8-528.6-603.6 Agricultural Research Service/ Buildings and Facilities/005-18-1401 - - 45.0-113.7 Protecting Critical Infrastructure and Key Assets - - 45.0-113.7 Agricultural
No. 29 February 12, 2016. The President
 Vol. 81 Friday, No. 29 February 12, 2016 Part IV The President Executive Order 13719 Establishment of the Federal Privacy Council VerDate Sep2014 20:00 Feb 11, 2016 Jkt 238001 PO 00000 Frm 00001 Fmt
Vol. 81 Friday, No. 29 February 12, 2016 Part IV The President Executive Order 13719 Establishment of the Federal Privacy Council VerDate Sep2014 20:00 Feb 11, 2016 Jkt 238001 PO 00000 Frm 00001 Fmt
Major Public Health Threat
 GAO United States General Accounting Office Testimony Before the Committee on Government Reform, House of Representatives For Release on Delivery Expected at 10:00 a.m. EST Thursday, February 12, 2004
GAO United States General Accounting Office Testimony Before the Committee on Government Reform, House of Representatives For Release on Delivery Expected at 10:00 a.m. EST Thursday, February 12, 2004
PROGRAM ASSESSMENT PLAN Program Outcomes and Learning Assessment Criteria
 Master of Science in Biotechnology Studies (BIOT) Information and Technology Systems Department PROGRAM ASSESSMENT PLAN Program Outcomes and Learning Assessment Criteria Summer 2007 TABLE OF CONTENTS Program
Master of Science in Biotechnology Studies (BIOT) Information and Technology Systems Department PROGRAM ASSESSMENT PLAN Program Outcomes and Learning Assessment Criteria Summer 2007 TABLE OF CONTENTS Program
1. Program Title Master of Science Program in Biochemistry (International Program)
 1 Program Structure and Specification Master of Science Program in Biochemistry (International Program) Curriculum Last Revised in 2012 for Students Entering in Academic Year 2016 -----------------------------------------
1 Program Structure and Specification Master of Science Program in Biochemistry (International Program) Curriculum Last Revised in 2012 for Students Entering in Academic Year 2016 -----------------------------------------
Texas Tech University Health Sciences Center. Recombinant DNA Biosafety Committee (RDBC) POLICIES AND PROCEDURES MANUAL. Effective Date: May 15, 2013
 Texas Tech University Health Sciences Center Recombinant DNA Biosafety Committee (RDBC) POLICIES AND PROCEDURES MANUAL Effective Date: May 15, 2013-1 - 1.0 Introduction This Texas Tech University Health
Texas Tech University Health Sciences Center Recombinant DNA Biosafety Committee (RDBC) POLICIES AND PROCEDURES MANUAL Effective Date: May 15, 2013-1 - 1.0 Introduction This Texas Tech University Health
REPORT TO CONGRESS ON THE IMPLEMENTATION OF THE FY 2003 INTEROPERABLE COMMUNICATIONS EQUIPMENT GRANT PROGRAM
 REPORT TO CONGRESS ON THE IMPLEMENTATION OF THE FY 2003 INTEROPERABLE COMMUNICATIONS EQUIPMENT GRANT PROGRAM Department of Homeland Security Emergency Preparedness & Response Directorate May 2003 Table
REPORT TO CONGRESS ON THE IMPLEMENTATION OF THE FY 2003 INTEROPERABLE COMMUNICATIONS EQUIPMENT GRANT PROGRAM Department of Homeland Security Emergency Preparedness & Response Directorate May 2003 Table
The Center for Medicare, CMS, shall provide management and support services. ESTIMATED ANNUAL OPERATING COSTS AND STAFF YEARS
 CHARTER ADVISORY PANEL ON AMBULATORY PAYMENT CLASSIFICATION GROUPS AUTHORITY Section 1833(t)(9)(A) of the Social Security Act (42 U.S.C. 1395l(t)(9)(A)). The Advisory Panel on Ambulatory Payment Classification
CHARTER ADVISORY PANEL ON AMBULATORY PAYMENT CLASSIFICATION GROUPS AUTHORITY Section 1833(t)(9)(A) of the Social Security Act (42 U.S.C. 1395l(t)(9)(A)). The Advisory Panel on Ambulatory Payment Classification
Big Data for Patients (BD4P) Program Overview
 Big Data for Patients (BD4P) Program Overview [Updated August 5, 2015] Proposal: Big Data for Patients (BD4P) Training Program Page 1 Background The new and emerging field of data science ( Big Data )
Big Data for Patients (BD4P) Program Overview [Updated August 5, 2015] Proposal: Big Data for Patients (BD4P) Training Program Page 1 Background The new and emerging field of data science ( Big Data )
Title V Preventing Fraud and Abuse. Subtitle A- Establishment of New Health and Human Services and Department of Justice Health Care Fraud Positions
 Title V Preventing Fraud and Abuse Subtitle A- Establishment of New Health and Human Services and Department of Justice Health Care Fraud Positions Sec. 501. Health and Human Services Senior Advisor There
Title V Preventing Fraud and Abuse Subtitle A- Establishment of New Health and Human Services and Department of Justice Health Care Fraud Positions Sec. 501. Health and Human Services Senior Advisor There
BACHELOR OF SCIENCE IN MICROBIOLOGY
 BACHELOR OF SCIENCE IN MICROBIOLOGY The goal of the B.S. Microbiology program at Albany College of Pharmacy and Health Sciences is to prepare graduates for employment or advanced study in fields requiring
BACHELOR OF SCIENCE IN MICROBIOLOGY The goal of the B.S. Microbiology program at Albany College of Pharmacy and Health Sciences is to prepare graduates for employment or advanced study in fields requiring
How To Write A Book On Risk Management
 National Center for Risk and Economic Analysis of Terrorism Events CREATE FY2015 (Year 11) Call for White Papers CREATE, the DHS-sponsored Center of Excellence at the University of Southern California,
National Center for Risk and Economic Analysis of Terrorism Events CREATE FY2015 (Year 11) Call for White Papers CREATE, the DHS-sponsored Center of Excellence at the University of Southern California,
EMERGENCY SUPPORT FUNCTION ANNEXES: INTRODUCTION
 EMERGENCY SUPPORT FUNCTION ANNEXES: INTRODUCTION Purpose This section provides an overview of the Emergency Support Function (ESF) structure, common elements of each of the ESFs, and the basic content
EMERGENCY SUPPORT FUNCTION ANNEXES: INTRODUCTION Purpose This section provides an overview of the Emergency Support Function (ESF) structure, common elements of each of the ESFs, and the basic content
BIOTERRORISM RISK ASSESSMENT GROUP (BRAG) FEDERAL BUREAU OF INVESTIGATION CRIMINAL JUSTICE INFORMATION SERVICES DIVISION CLARKSBURG, WEST VIRGINIA
 BIOTERRORISM RISK ASSESSMENT GROUP (BRAG) FEDERAL BUREAU OF INVESTIGATION CRIMINAL JUSTICE INFORMATION SERVICES DIVISION CLARKSBURG, WEST VIRGINIA MISSION To enhance national security and public safety
BIOTERRORISM RISK ASSESSMENT GROUP (BRAG) FEDERAL BUREAU OF INVESTIGATION CRIMINAL JUSTICE INFORMATION SERVICES DIVISION CLARKSBURG, WEST VIRGINIA MISSION To enhance national security and public safety
STT ENVIRO CORP. (the Company ) CHARTER OF THE CORPORATE GOVERNANCE AND NOMINATING COMMITTEE. As amended by the Board of Directors on May 10, 2012
 STT ENVIRO CORP. (the Company ) CHARTER OF THE CORPORATE GOVERNANCE AND NOMINATING COMMITTEE PURPOSE AND SCOPE As amended by the Board of Directors on May 10, 2012 The primary function of the Committee
STT ENVIRO CORP. (the Company ) CHARTER OF THE CORPORATE GOVERNANCE AND NOMINATING COMMITTEE PURPOSE AND SCOPE As amended by the Board of Directors on May 10, 2012 The primary function of the Committee
significantly to the Gross Domestic Product (GDP) GDP is over a trillion dollars/yr
 Agricultural Emergency Response Training (AgERT) Auburn University College of Veterinary Medicine Julie A. Gard, BS, DVM, PhD, DACT (presented by Donna Angarano) Why a concern? ce Agriculture contributes
Agricultural Emergency Response Training (AgERT) Auburn University College of Veterinary Medicine Julie A. Gard, BS, DVM, PhD, DACT (presented by Donna Angarano) Why a concern? ce Agriculture contributes
FEDERAL DATA CENTER CONSOLIDATION TASK FORCE CHARTER
 FEDERAL DATA CENTER CONSOLIDATION TASK FORCE CHARTER PURPOSE The charter establishes the Data Center Consolidation Task Force (Task Force) and consigns that Task Force work in support of the Federal Data
FEDERAL DATA CENTER CONSOLIDATION TASK FORCE CHARTER PURPOSE The charter establishes the Data Center Consolidation Task Force (Task Force) and consigns that Task Force work in support of the Federal Data
No. 30 February 16, 2016. The President
 Vol. 81 Tuesday, No. 30 February 16, 2016 Part IV The President Executive Order 13719 Establishment of the Federal Privacy Council: Republication VerDate Sep2014 16:34 Feb 12, 2016 Jkt 238001 PO 00000
Vol. 81 Tuesday, No. 30 February 16, 2016 Part IV The President Executive Order 13719 Establishment of the Federal Privacy Council: Republication VerDate Sep2014 16:34 Feb 12, 2016 Jkt 238001 PO 00000
INSPECTOR GENERAL DEPARTMENT OF DEFENSE 4800 MARK CENTER DRIVE ALEXANDRIA, VIRGINIA 22350-1500
 INSPECTOR GENERAL DEPARTMENT OF DEFENSE 4800 MARK CENTER DRIVE ALEXANDRIA, VIRGINIA 22350-1500 MAY 2 8 2014 MEMORANDUM FOR DISTRIBUTION SUBJECT: Government Accountability Office (GAO) Weekly Activity Repati
INSPECTOR GENERAL DEPARTMENT OF DEFENSE 4800 MARK CENTER DRIVE ALEXANDRIA, VIRGINIA 22350-1500 MAY 2 8 2014 MEMORANDUM FOR DISTRIBUTION SUBJECT: Government Accountability Office (GAO) Weekly Activity Repati
a GAO-09-687 GAO NATIONAL INSTITUTES OF HEALTH Completion of Comprehensive Risk Management Program Essential to Effective Oversight
 GAO United States Government Accountability Office Report to the Ranking Member, Committee on Finance, U.S. Senate September 2009 NATIONAL INSTITUTES OF HEALTH Completion of Comprehensive Risk Management
GAO United States Government Accountability Office Report to the Ranking Member, Committee on Finance, U.S. Senate September 2009 NATIONAL INSTITUTES OF HEALTH Completion of Comprehensive Risk Management
GAO COMBATING TERRORISM. Observations on Options to Improve the Federal Response. Testimony
 GAO For Release on Delivery Expected at 3:00 p.m. Tuesday, April 24, 2001 United States General Accounting Office Testimony Before the Subcommittee on Economic Development, Public Buildings, and Emergency
GAO For Release on Delivery Expected at 3:00 p.m. Tuesday, April 24, 2001 United States General Accounting Office Testimony Before the Subcommittee on Economic Development, Public Buildings, and Emergency
Article I. Objective. Article II. Membership. A. Criteria for Membership
 Public Health Graduate Program Bylaws Administrative Home: Department of Public Health Sciences, School of Medicine Revised: 1/25/07; 8/27/10 Graduate Council s Approval Date: June 16, 2011 Article I.
Public Health Graduate Program Bylaws Administrative Home: Department of Public Health Sciences, School of Medicine Revised: 1/25/07; 8/27/10 Graduate Council s Approval Date: June 16, 2011 Article I.
HOUSE BILL No. 2388. By Committee on Corrections and Juvenile Justice 2-9
 Session of 00 HOUSE BILL No. By Committee on Corrections and Juvenile Justice - 0 0 0 AN ACT concerning crimes, punishment and criminal procedure; relating to racial disproportionality in the juvenile
Session of 00 HOUSE BILL No. By Committee on Corrections and Juvenile Justice - 0 0 0 AN ACT concerning crimes, punishment and criminal procedure; relating to racial disproportionality in the juvenile
NSA Briefing: Security Risk Assessments for Possession, Use and Transfer of Select Agents
 NSABB Briefing: Security Risk Assessments for Possession, Use, and Transfer of Select Agents Robbin S. Weyant Division of Select Agents and Toxins Coordinating Office for Terrorism Preparedness and Emergency
NSABB Briefing: Security Risk Assessments for Possession, Use, and Transfer of Select Agents Robbin S. Weyant Division of Select Agents and Toxins Coordinating Office for Terrorism Preparedness and Emergency
REPUBLIC OF KENYA VACANCY IN THE MINISTRY OF INTERIOR AND CO-ORDINATION OF NATIONAL GOVERNMENT
 REPUBLIC OF KENYA PUBLIC SERVICE COMMISSION Our Vision To be the lead service commission in the provision, management and development of competent human resource for the Public Service. Our Mission To
REPUBLIC OF KENYA PUBLIC SERVICE COMMISSION Our Vision To be the lead service commission in the provision, management and development of competent human resource for the Public Service. Our Mission To
Degree Charter Bachelor Degree Programme Life Sciences. Academic year 2015 2016. Education Committee Regulations
 Degree Charter Bachelor Degree Programme Life Sciences Academic year 2015 2016 Education Committee Regulations Contents Content Article 1 Status and definitions... 3 Article 2 Joint Education Committees...
Degree Charter Bachelor Degree Programme Life Sciences Academic year 2015 2016 Education Committee Regulations Contents Content Article 1 Status and definitions... 3 Article 2 Joint Education Committees...
HOMELAND SECURITY MARKET DEVELOPMENT IN ILLINOIS
 HOMELAND SECURITY MARKET DEVELOPMENT IN ILLINOIS The Role of Community Colleges in Workforce Education ICCB Homeland Security Conference 2/21/2007 Present by: Michael Brody, Assistant Deputy Director The
HOMELAND SECURITY MARKET DEVELOPMENT IN ILLINOIS The Role of Community Colleges in Workforce Education ICCB Homeland Security Conference 2/21/2007 Present by: Michael Brody, Assistant Deputy Director The
Pathways to progress One Health expertise in Australia:
 Pathways to progress One Health expertise in Australia: A Public Health Association of Australia (PHAA) and National Centre for Epidemiology and Population Health (NCEPH) national expert consultation Introduction
Pathways to progress One Health expertise in Australia: A Public Health Association of Australia (PHAA) and National Centre for Epidemiology and Population Health (NCEPH) national expert consultation Introduction
Our connection to the South Australian Strategic Plan and Economic Priorities
 General information Title: Principal Biosecurity Officer, Weeds Classification: PO4 Division: Biosecurity SA Type of appointment: Branch: NRM Biosecurity Ongoing Business NRM Biosecurity Term contract
General information Title: Principal Biosecurity Officer, Weeds Classification: PO4 Division: Biosecurity SA Type of appointment: Branch: NRM Biosecurity Ongoing Business NRM Biosecurity Term contract
Quality Assurance Guidelines for Laboratories Performing Microbial Forensic Work
 for Laboratories Performing Microbial Forensic Work Produced by the Members of the Scientific Working Group on Microbial Genetics and Forensics (SWGMGF) Contact: Bruce Budowle, Chair, FBI Laboratory, 2501
for Laboratories Performing Microbial Forensic Work Produced by the Members of the Scientific Working Group on Microbial Genetics and Forensics (SWGMGF) Contact: Bruce Budowle, Chair, FBI Laboratory, 2501
FACULTY OF MEDICAL SCIENCE
 Doctor of Philosophy Program in Microbiology FACULTY OF MEDICAL SCIENCE Naresuan University 171 Doctor of Philosophy Program in Microbiology The time is critical now for graduate education and research
Doctor of Philosophy Program in Microbiology FACULTY OF MEDICAL SCIENCE Naresuan University 171 Doctor of Philosophy Program in Microbiology The time is critical now for graduate education and research
Federal Sponsors/GCO. Agency for International Development Columbia Warren. Corporation for National and Community Service Columbia Warren
 Federal Sponsors/GCO Agency for International Development Columbia Warren Corporation for National and Community Service Columbia Warren Department of Commerce Linda Griswold Bureau of Economic Analysis
Federal Sponsors/GCO Agency for International Development Columbia Warren Corporation for National and Community Service Columbia Warren Department of Commerce Linda Griswold Bureau of Economic Analysis
THE FEDERAL DEMONSTRATION PARTNERSHIP February 4, 2002 SOLICITATION TO PARTICIPATE IN PHASE IV
 THE FEDERAL DEMONSTRATION PARTNERSHIP February 4, 2002 SOLICITATION TO PARTICIPATE IN PHASE IV [NOTE: THIS IS A REVISED EDITION OF THE FEBRUARY 4, 2002 SOLICITATION WITH ADDITIONAL AND UPDATED INFORMATION
THE FEDERAL DEMONSTRATION PARTNERSHIP February 4, 2002 SOLICITATION TO PARTICIPATE IN PHASE IV [NOTE: THIS IS A REVISED EDITION OF THE FEBRUARY 4, 2002 SOLICITATION WITH ADDITIONAL AND UPDATED INFORMATION
POLICIES AND PROCEDURES FOR THE HOWARD UNIVERSITY INSTITUTIONAL BIOSAFETY COMMITTEE (IBC)
 June 2013 POLICIES AND PROCEDURES FOR THE HOWARD UNIVERSITY INSTITUTIONAL BIOSAFETY COMMITTEE (IBC) Page 1 of 19 CONTENTS SECTION 1: PURPOSE AND SCOPE OF POLICIES AND PROCEDURES --------------------------------
June 2013 POLICIES AND PROCEDURES FOR THE HOWARD UNIVERSITY INSTITUTIONAL BIOSAFETY COMMITTEE (IBC) Page 1 of 19 CONTENTS SECTION 1: PURPOSE AND SCOPE OF POLICIES AND PROCEDURES --------------------------------
RECOMMEND APPROVAL OF MASTER OF SCIENCE IN BIOMEDICAL SCIENCE AND BIOTECHNOLOGY AT UNIVERSITY OF COLORADO DENVER
 Page 1 of 5 TOPIC: PREPARED BY: RECOMMEND APPROVAL OF MASTER OF SCIENCE IN BIOMEDICAL SCIENCE AND BIOTECHNOLOGY AT UNIVERSITY OF COLORADO DENVER IAN MACGILLIVRAY, DIRECTOR OF ACADEMIC AFFAIRS I. SUMMARY
Page 1 of 5 TOPIC: PREPARED BY: RECOMMEND APPROVAL OF MASTER OF SCIENCE IN BIOMEDICAL SCIENCE AND BIOTECHNOLOGY AT UNIVERSITY OF COLORADO DENVER IAN MACGILLIVRAY, DIRECTOR OF ACADEMIC AFFAIRS I. SUMMARY
FIRST COAST HEALTH ALLIANCE, LLC CHARTER AUDIT, FINANCE, AND NETWORK CONTRACTS COMMITTEE
 AUDIT, FINANCE, AND NETWORK CONTRACTS COMMITTEE 1. Establishment and Purpose. The Audit, Finance, and Networks Contracts Committee is established by the Board for the purpose of overseeing the integrity
AUDIT, FINANCE, AND NETWORK CONTRACTS COMMITTEE 1. Establishment and Purpose. The Audit, Finance, and Networks Contracts Committee is established by the Board for the purpose of overseeing the integrity
Federal Experts Security Advisory Panel Recommendations and Future Activities
 U.S. Department of Health and Human Services U.S. Department of Agriculture Federal Experts Security Advisory Panel Recommendations and Future Activities June 23, 2011 Briefing for the National Science
U.S. Department of Health and Human Services U.S. Department of Agriculture Federal Experts Security Advisory Panel Recommendations and Future Activities June 23, 2011 Briefing for the National Science
2013-14 Report on Plans and Priorities Additional Information for Sub-programs and Sub-sub-programs
 2013-14 Report on Plans and Priorities Additional Information for Sub-programs and Sub-sub-programs Strategic Outcome: Protecting Canadians and empowering them to improve their health Program 1.1 Public
2013-14 Report on Plans and Priorities Additional Information for Sub-programs and Sub-sub-programs Strategic Outcome: Protecting Canadians and empowering them to improve their health Program 1.1 Public
BIOSCIENCES COURSE TITLE AWARD
 COURSE TITLE AWARD BIOSCIENCES As a Biosciences undergraduate student at the University of Westminster, you will benefit from some of the best teaching and facilities available. Our courses combine lecture,
COURSE TITLE AWARD BIOSCIENCES As a Biosciences undergraduate student at the University of Westminster, you will benefit from some of the best teaching and facilities available. Our courses combine lecture,
DEPARMTMENT OF HOMELAND SECURITY AUTHORIZATION BILL FOR FY 2008 AND FY 2009 SECTION-BY-SECTION
 DEPARMTMENT OF HOMELAND SECURITY AUTHORIZATION BILL FOR FY 2008 AND FY 2009 SECTION-BY-SECTION TITLE I: AUTHORIZATION OF APPROPRIATIONS Sec. 101. Authorization of Appropriations. This section authorizes
DEPARMTMENT OF HOMELAND SECURITY AUTHORIZATION BILL FOR FY 2008 AND FY 2009 SECTION-BY-SECTION TITLE I: AUTHORIZATION OF APPROPRIATIONS Sec. 101. Authorization of Appropriations. This section authorizes
Homeland Security Graduate Programs
 Homeland Security Graduate Programs TABLE OF CONTENTS Homeland Security Portfolio...3 Homeland Security Master s Degree Base Program and Graduate Certificate... 4 5 Public Health Preparedness... 6 7 Geospatial
Homeland Security Graduate Programs TABLE OF CONTENTS Homeland Security Portfolio...3 Homeland Security Master s Degree Base Program and Graduate Certificate... 4 5 Public Health Preparedness... 6 7 Geospatial
Department of Defense DIRECTIVE
 Department of Defense DIRECTIVE NUMBER 5400.11 October 29, 2014 DCMO SUBJECT: DoD Privacy Program References: See Enclosure 1 1. PURPOSE. This directive: a. Reissues DoD Directive (DoDD) 5400.11 (Reference
Department of Defense DIRECTIVE NUMBER 5400.11 October 29, 2014 DCMO SUBJECT: DoD Privacy Program References: See Enclosure 1 1. PURPOSE. This directive: a. Reissues DoD Directive (DoDD) 5400.11 (Reference
Federal Bureau of Investigation s Integrity and Compliance Program
 Evaluation and Inspection Division Federal Bureau of Investigation s Integrity and Compliance Program November 2011 I-2012-001 EXECUTIVE DIGEST In June 2007, the Federal Bureau of Investigation (FBI) established
Evaluation and Inspection Division Federal Bureau of Investigation s Integrity and Compliance Program November 2011 I-2012-001 EXECUTIVE DIGEST In June 2007, the Federal Bureau of Investigation (FBI) established
The Biotech Business Life Cycle. The Lawyer s Role as Counselor and Advisor
 The Biotech Business Life Cycle and The Lawyer s Role as Counselor and Advisor ASU February 15, 2010 1 BIOTECH BUSINESS CYCLE AN INTERDISCIPLINARY APPROACH TO LEGAL REPRESENTATION Intellectual Property
The Biotech Business Life Cycle and The Lawyer s Role as Counselor and Advisor ASU February 15, 2010 1 BIOTECH BUSINESS CYCLE AN INTERDISCIPLINARY APPROACH TO LEGAL REPRESENTATION Intellectual Property
UNIVERSITY OF CALIFORNIA. March 4, 2015 CHANCELLORS DIRECTOR ALIVISATOS MEDICAL CENTER CHIEF EXECUTIVE OFFICERS. Dear Colleagues:
 UNIVERSITY OF CALIFORNIA BERKELEY DAVIS IRVINE LOS ANGELES MERCED RIVERSIDE SAN DIEGO SAN FRANCISCO P1 A.DEiolj S.NTA BARBARA SANTA CRUZ CHANCELLORS DIRECTOR ALIVISATOS MEDICAL CENTER CHIEF EXECUTIVE OFFICERS
UNIVERSITY OF CALIFORNIA BERKELEY DAVIS IRVINE LOS ANGELES MERCED RIVERSIDE SAN DIEGO SAN FRANCISCO P1 A.DEiolj S.NTA BARBARA SANTA CRUZ CHANCELLORS DIRECTOR ALIVISATOS MEDICAL CENTER CHIEF EXECUTIVE OFFICERS
20, 1994. Service on Feb. 20, 1994.
 DEPARTMENT OF AGRICULTURE Agricultural Cooperative Service Agricultural Marketing Service Agricultural Research Service Agricultural Stabilization & Conservation Service Animal & Plant Health Inspection
DEPARTMENT OF AGRICULTURE Agricultural Cooperative Service Agricultural Marketing Service Agricultural Research Service Agricultural Stabilization & Conservation Service Animal & Plant Health Inspection
a GAO-03-139 GAO BIOTERRORISM Information Technology Strategy Could Strengthen Federal Agencies Abilities to Respond to Public Health Emergencies
 GAO United States General Accounting Office Report to Congressional Requesters May 2003 BIOTERRORISM Information Technology Strategy Could Strengthen Federal Agencies Abilities to Respond to Public Health
GAO United States General Accounting Office Report to Congressional Requesters May 2003 BIOTERRORISM Information Technology Strategy Could Strengthen Federal Agencies Abilities to Respond to Public Health
DAIDS Bethesda, MD USA POLICY
 Overview NIH policy requiring independent data and safety monitoring boards (DSMB) for all multicenter Phase III trials has existed since 1979; the most recent restatement was issued in 1998 (NIH Policy
Overview NIH policy requiring independent data and safety monitoring boards (DSMB) for all multicenter Phase III trials has existed since 1979; the most recent restatement was issued in 1998 (NIH Policy
Dual-Use Bioethics/Biosecurity Training
 Dual-Use Bioethics/Biosecurity Training 2 nd Annual Conference BACAC Regional Biosafety and Biosecurity Collaboration Bishkek, Kyrgyz Republic 11-12 November 2010 Masamichi Minehata Bradford Disarmament
Dual-Use Bioethics/Biosecurity Training 2 nd Annual Conference BACAC Regional Biosafety and Biosecurity Collaboration Bishkek, Kyrgyz Republic 11-12 November 2010 Masamichi Minehata Bradford Disarmament
South Dakota Department of Public Safety Office of Homeland Security Senior Advisory Committee Charter
 1. Official Designation: South Dakota Department of Public Safety Office of Homeland Security Senior Advisory Committee Charter Homeland Security Senior Advisory Committee (HSSAC) 2. Authority: The South
1. Official Designation: South Dakota Department of Public Safety Office of Homeland Security Senior Advisory Committee Charter Homeland Security Senior Advisory Committee (HSSAC) 2. Authority: The South
TITLE VI NATIONAL EMERGENCY MANAGEMENT
 120 STAT. 1394 PUBLIC LAW 109 295 OCT. 4, 2006 Training, there is appropriated an additional $2,500,000, to remain available until expended for National Special Security Events. SEC. 560. Transfer authority
120 STAT. 1394 PUBLIC LAW 109 295 OCT. 4, 2006 Training, there is appropriated an additional $2,500,000, to remain available until expended for National Special Security Events. SEC. 560. Transfer authority
FAO/WHO Regional Conference on Food Safety for the Americas and the Caribbean San José, Costa Rica, 6-9 December 2005
 Agenda Item 5 Conference Room Document 13 FAO/WHO Regional Conference on Food Safety for the Americas and the Caribbean San José, Costa Rica, 6-9 December 2005 THE FOOD SAFETY REGULATORY SYSTEM IN CANADA
Agenda Item 5 Conference Room Document 13 FAO/WHO Regional Conference on Food Safety for the Americas and the Caribbean San José, Costa Rica, 6-9 December 2005 THE FOOD SAFETY REGULATORY SYSTEM IN CANADA
APPENDIX T: GUIDELINES FOR A THESIS RESEARCH PROPOSAL. Masters of Science Clinical Laboratory Sciences Program
 APPENDIX T: GUIDELINES FOR A THESIS RESEARCH PROPOSAL Masters of Science Clinical Laboratory Sciences Program Name of Candidate:..... Name of Thesis Director:. Track :... I. Topic of research proposal
APPENDIX T: GUIDELINES FOR A THESIS RESEARCH PROPOSAL Masters of Science Clinical Laboratory Sciences Program Name of Candidate:..... Name of Thesis Director:. Track :... I. Topic of research proposal
Bioterrorism & Emergency Readiness
 Bioterrorism & Emergency Readiness COMPETENCIES FOR ALL PUBLIC HEALTH WORKERS A Message from the Centers for Disease Control and Prevention Dear Public Health Colleague, A prepared workforce is an essential
Bioterrorism & Emergency Readiness COMPETENCIES FOR ALL PUBLIC HEALTH WORKERS A Message from the Centers for Disease Control and Prevention Dear Public Health Colleague, A prepared workforce is an essential
International Consortium for Harmonization of Clinical Laboratory Results. Operating Procedures
 International Consortium for Harmonization of Clinical Laboratory Results Operating Procedures Approved: February 11, 2014 Background Results from clinical laboratory measurement procedures should be comparable
International Consortium for Harmonization of Clinical Laboratory Results Operating Procedures Approved: February 11, 2014 Background Results from clinical laboratory measurement procedures should be comparable
Subject: National Preparedness
 For Immediate Release Office of the Press Secretary The White House December 17, 2003 Homeland Security Presidential Directive / HSPD-8 Subject: National Preparedness Purpose (1) This directive establishes
For Immediate Release Office of the Press Secretary The White House December 17, 2003 Homeland Security Presidential Directive / HSPD-8 Subject: National Preparedness Purpose (1) This directive establishes
Presidential Documents
 Federal Register Vol. 58, No. 190 Presidential Documents Monday, October 4, 1993 Title 3 The President Executive Order 12866 of September 30, 1993 Regulatory Planning and Review The American people deserve
Federal Register Vol. 58, No. 190 Presidential Documents Monday, October 4, 1993 Title 3 The President Executive Order 12866 of September 30, 1993 Regulatory Planning and Review The American people deserve
Three Branches of Government. Lesson 2
 Three Branches of Government The Executive Branch The President of the United States is the leader of the executive branch. The President s duties are to: Enforce federal laws and recommend new ones Serve
Three Branches of Government The Executive Branch The President of the United States is the leader of the executive branch. The President s duties are to: Enforce federal laws and recommend new ones Serve
Malcolm Dando. Landau Network Centro Volta. and University of Bradford
 European Biosecurity Awareness Raising Network Review Series on Policy, Ethics and Security Paper n 4 Biological Weapons and the Biological Weapons Convention: Bioethics and Dual-Use Malcolm Dando Landau
European Biosecurity Awareness Raising Network Review Series on Policy, Ethics and Security Paper n 4 Biological Weapons and the Biological Weapons Convention: Bioethics and Dual-Use Malcolm Dando Landau
Project GRACE 311957. Preliminary Author. List:
 DRAFT Networking and database technology FP7 Collaborative Project GRACE 311957 Preliminary Author List: S. Unger, S. Kecke, W. Craig Version 07/04/ /2013 2 Table of contents 1. Introduction... 5 2. Requirements
DRAFT Networking and database technology FP7 Collaborative Project GRACE 311957 Preliminary Author List: S. Unger, S. Kecke, W. Craig Version 07/04/ /2013 2 Table of contents 1. Introduction... 5 2. Requirements
River Valley Therapy & Sports Medicine, Inc. Notice of Privacy Practices
 River Valley Therapy & Sports Medicine, Inc. Notice of Privacy Practices This notice describes how medical information about you may be used and disclosed and how you can get access to this information.
River Valley Therapy & Sports Medicine, Inc. Notice of Privacy Practices This notice describes how medical information about you may be used and disclosed and how you can get access to this information.
Guide for the Care and Use of Laboratory Animals
 Guide for the Care and Use of Laboratory Animals Institute of Laboratory Animal Resources Commission on Life Sciences National Research Council National Academy Press Washington, D.C. Copyright 1996 by
Guide for the Care and Use of Laboratory Animals Institute of Laboratory Animal Resources Commission on Life Sciences National Research Council National Academy Press Washington, D.C. Copyright 1996 by
Laboratory. BIOSAFETY and BIOSECURITY in THAILAND
 Laboratory BIOSAFETY and BIOSECURITY in THAILAND Surang Dejsirilert National Institute of Health Department of Medical Sciences Ministry of Public Health http://www.dmsc dmsc.moph.go..go.th ional Institute
Laboratory BIOSAFETY and BIOSECURITY in THAILAND Surang Dejsirilert National Institute of Health Department of Medical Sciences Ministry of Public Health http://www.dmsc dmsc.moph.go..go.th ional Institute
Medical Science Building, Rm C696 185 South Orange Ave. Newark, New Jersey 07101. www.umdnj.edu/gsbsnweb
 Medical Science Building, Rm C696 185 South Orange Ave. Newark, New Jersey 07101 Newark Division www.umdnj.edu/gsbsnweb University of Medicine & Dentistry of New Jersey Graduate School of Biomedical Sciences
Medical Science Building, Rm C696 185 South Orange Ave. Newark, New Jersey 07101 Newark Division www.umdnj.edu/gsbsnweb University of Medicine & Dentistry of New Jersey Graduate School of Biomedical Sciences
NEPTUNE MARINE SERVICES LTD ACN 105 665 843. Charter of the Risk Management Committee
 NEPTUNE MARINE SERVICES LTD ACN 105 665 843 Charter of the Risk Management Committee 1. Introduction... 1 2. Objective... 1 3. Constitution of Committee... 1 4. Composition... 2 5. Chairperson... 2 6.
NEPTUNE MARINE SERVICES LTD ACN 105 665 843 Charter of the Risk Management Committee 1. Introduction... 1 2. Objective... 1 3. Constitution of Committee... 1 4. Composition... 2 5. Chairperson... 2 6.
