Design method for multi-component distillation system based on quasi-binary method*
|
|
|
- Frederica Watts
- 7 years ago
- Views:
Transcription
1 Korean J. Chem. Eng., 24(4), (2007) SHORT COMMUNICATION Design method for multi-component distillation system based on quasi-binary method* Wu Lianying, Hu Yangdong and Wen Ya College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao, , China (Received 30 June 2006 accepted 1 December 2006) Abstract A novel design method for a multi-component distillation system based on the quasi-binary model is presented. The quasi-binary method, which converts the multi-component system to a quasi-binary system, could simplify the design process of multi-component distillation. Subsequently the software integration method is introduced to the distillation design and an automatic calculation program is developed by using Visual C++ language. The design of multi-component distillation, in which the minimum reflux ratio R min or liquid-vapor ratio (L/V) min and the minimum numbers of stage N min is determined easily and quicly, is automatically performed by the technology of software integration. Three examples are solved to demonstrate the feasibility and effectiveness of the presented method for the multi-component distillation. Key words: Distillation Design, Quasi-Binary Equilibrium, Multi-Component, Software Integration INTRODUCTION Distillation is a widely used separation process that dominates all separations in the chemical and petroleum industries, and it is the largest energy consumer among process unit operations. Thus, the tas of optimal design and synthesis of distillation processes is an important and challenging issue, especially for multi-component separation processes. The design techniques of distillation have attracted more and more attention in order to reduce the energy demand. Shortcut design and rigorous design methods are discussed in detail in the literature for different cases. The most popular and easy to use shortcut technique for designing binary system distillation is the McCabe-Thiele method. This simple method allows one not only to quantitatively predict minimum reflux conditions and minimum number of stages, but also to get a clear illustration and a pictorial understanding of these and other concepts, such as that of pinch conditions. Nothing of the ind is available for multi-component distillation, where the need for a multi-variant description of vapor-liquid equilibrium maes it very difficult to visualize the column design procedure. Hence, stage-by-stage models are used, which have a mathematically simple structure but also a serious drawbac. To overcome this limitation, classical stage equations are analyzed [1] through a dynamical systems approach and the distillation column is regarded as a continuous countercurrent contactor with negligible axial dispersion. These are more complex than the stage-by-stage equations, but small in number and therefore can be solved by using convenient mathematical tools which allow one to reach a deep insight into both steady state and dynamical column behavior. The Underwood method [2] has been available for a long time as a suitable technique for the minimum energy demand calculation of ideal mixtures. In recent years, a shortcut method has been used for non-ideal mixtures by To whom correspondence should be addressed. wulianying@mail.ouc.edu.cn *Supported by the National Nature Science Fund Program of China (No ). the modified Underwood equation [3-8]. Although the short-cut method and its extensions have shown satisfactory design results, it is possible that such solutions are not accurate enough in the design of a distillation process because of the existence of several assumptions, such as constant molar overflow, constant relativity volatility. A rigorous design method [9-11] is adopted to reduce the energy consumption or the capital cost for the heat integration distillation process. However, due to complexity, most past research [12,13] on complex distillation configurations was restricted to three component mixtures, and only a few promising flowsheets were constructed for ternary mixtures. The design method of four or more component mixtures has been proposed by some scholars [14-17], but the corresponding applicability is also limited due to the combinatorial problem of the possible configurations for multi-component separations. Moreover, there is a lac of quic and simple design procedure as well as modeling and synthesis methods for these types of distillation schemes. In consequence, the optimal design of multicomponent distillation processes is usually sought in a search space, which excludes considerations of complex distillation flowsheets due to the lac of experience and available nowledge. In this paper, a novel design method of multi-component distillation process, which is based on the quasi-binary model, is presented. First, the quasi-binary equilibrium, which converts the multicomponent system to quasi-binary system using congregated component, is introduced to simplify the multi-component distillation process. Second, the design process is automatically performed by the technology of software integration, of which the minimum reflux ratio R min or liquid-vapor ratio (L/V) min and the minimum numbers of stage N min is determined easily and quicly. Three examples of the multi-component distillation for ideal and non-ideal mixtures are given as well. PROBLEM STATEMENT The problem addressed in this paper can be stated as follows: Given are a multi-component feed composition, flow rate and specified product. The relativity volatility of all components is not con- 556
2 Design method for multi-component distillation system based on quasi-binary method 557 stant and varies along the column. The feeds, side streams and heat flows are located at the fitful point. The problem then consists of determining the minimum number of stages and the minimum liquid-vapor ratio (or reflux ratio). QUASI-BINARY EQUILIBRIUM OF MULTI-COMPONENT (QBEMC) The vapor-liquid equilibrium relationship is the foundation for the design of a distillation column. For a simple binary system, there are four variables and two degrees of freedom in the system based on the phase rule. If one variable is fixed, only one variable among the others can be changed independently. But the multi-component situation is more complex than that of binary system. There are N variables that can be changed independently for the N components case. The equilibrium diagram of multi-component is very difficult to formulate accurately, which is the main difficulty and has not been solved satisfactorily so far. To solve the problem, the QBEMC is put forward and described as follows. 1. The Concept of Congregated Component Unlie a binary system, the product composition of the bottom and distillate are not determined directly for multi-component distillation. Instead, the composition of one component is generally subject to a low value in the distillate, and the composition of another component is subject to a low value in the bottom as well. The other components are not specified except the specified components which are called ey components. One of the ey components, for which the value of volatile is high, is called the light ey component; the other is called heavy ey component. In this paper, the congregated component on the basis of the ey components is presented to describe the multi-component system. The congregated component could be attained by the following steps: firstly the components are raned according to their relative volatility by a descending order, that is, for the sequence ABCDE of the feed mixture, A and E represent the most volatile component and the least volatile component, respectively. Secondly, according to the separation tas, the two ey components are determined. For example, the tas is to separate the components C and D. The component C is called the light ey component, while the component D is called heavy ey component. Thirdly, the components (A, B, C) are congregated to one component - the light congregated component (LCC); in turn the other components (D, E) are called the heavy congregated components (HCC). Then the multi-component system is converted to a quasi-binary system. The design method of a distillation column for a binary system could be applied to the quasi-binary system. Since the determination of light congregated component and heavy congregated component is based on the separation specification not on the volatility, the volatility difference could not affect the design method, but it could affect calculation time. If the volatility difference is large in the light congregated component or heavy congregated component, the design of the column, which follows the separation column of light congregated component and heavy congregated component, would be easy or considered as the ideal mixture. Then the calculation would require short time. On the contrary, a long time would be required for the calculation. 2. Phase Equilibrium of QBEMC For a quasi-binary system, the equilibrium relationship of congregated component could be derived from that of the individual component, which could be formulated as follows: For the j stage: y j1 = j1 x j1 (1) y j2 = j2 x j2 (2) y ji = ji x ji (3) X j = Y j = x ji = x j1 + x j2 + Λ + x j ji x ji = y j1 + y j2 + Λ + y j Y j =K j X j (6) K j = x i i x i Here, Y j, X j are vapor and liquid composition of the congregated component, respectively. The K j, equilibrium constant of the quasibinary system, is a function of temperature, pressure, and composition and varies along the column. Eqs. (4) and (5) show that there are numerous combinations of x ji (y ji ), which could be summed equal to the same value of X j (Y j ). So the real K j along the stages must be determined according as the separation tas, which is important to the design process and very difficult in multi-component distillation. Here the simulation software, such as PROII, HYSYS, and AS- PENPLUS could be employed to simulate multi-component distillation processes. In this paper, ASPENPLUS is adopted to obtain the equilibrium of QBEMC. For a given value of reflux ratio and number of stages, the composition of the individual component on each stage would be obtained subsequently. Then the Y j -X j relationship along the column would be described in a two-dimensional diagram easily or as an equation by curve-fitting. But the relationship of Y j -X j would be limited because it corresponds to a certain stage number and reflux ratio. Therefore, a series of simulation processes for different stage number and reflux ratio are performed (in Fig. 1, Fig. 1. Quasi-binary equilibrium of cyclohexane-water-isopropanol system. (4) (5) (7) Korean J. Chem. Eng.(Vol. 24, No. 4)
3 558 W. Lianying et al. the three profiles represent the number of stages, 15, 20 and 25, respectively). The result shows that the profiles of Y j -X j are very similar and the difference between them can almost be neglected. Thus, the localization of Y j -X j equilibrium could be overcome by using the average of a series of results and then the Y j -X j equilibrium relationship could serve for the design of distillation. QUASI-BINARY METHOD (QBM) FOR COLUMN DESIGN With multi-component treated as a quasi-binary system, a column separating a multi-component mixture can be treated as a column separating LCC and HCC mixtures. According to the separation tas, quasi-binary equilibrium is attained by using the simulation software. The minimum reflux ratio is calculated by the Lagrange quadratic interpolation, and the minimum stage number is calculated stage by stage. The total design process is automatically performed by the technology of software integration. The program schematic is shown as Fig. 2. The detailed design steps are described in the following sections. 1. Determining of Phase Equilibrium The value of the liquid-vapor ratio and the number of stages of the distillation separation process is affected deeply by the equilibrium relationship of the system. So the quasi-binary equilibrium of a multi-component system should be described before one calculates the value of the liquid-vapor ratio and the number of stages. The reflux ratio R and stage number N must be initialized according to the property of the mixture before being simulated by calling the software. The Fense and Underwood equations can be used to calculate the R min and N min when the relative volatilities are constant or do not change significantly along the column. Then the R min and N min are multiplied by a constant and the R and N are attained. For example, R=3R min, N=4N min. Subsequently, the R and N are input to simulation software as the initialization. While the relative volatility is not treated as a constant, the R and N could be set at a large number according to experience and are input to the simulation software as the initialization. Then the composition of each component Fig. 2. The program schematic. of liquid and vapor along the column would be received by rigorous simulation. According to the separation tas, the LCC and HCC must be determined. Sequentially, the composition of LCC and HCC would be attained along the column by Eqs. (1)-(5). Afterward, the quasi-binary equilibrium equation could be obtained by using Eqs. (6) and (8). 2. Calculation of Minimum Reflux Ratio R min or Liquid-vapor Ratio (L/V) min When the composition data of each congregated component along the column has been obtained, the minimum reflux ratio can be calculated as follows. First, the composition of LCC and HCC for the feed point is calculated. For example, four components with the molar composition are set as x f1, x f2, x f3, x f4, respectively. The target is to separate the second and third components. That is, the first and second components are the light congregated components and the third and fourth components are height congregated components. Then the X f, liquid composition of LCC, equals the sum of x f1 and x f2. The Y F could be calculated by using the Lagrange quadratic interpolation method. For example, among the stochastic three points (X 0, Y 0 ), (X 1, Y 1 ), (X 1, Y 1 ) on the equilibrium line, the interpolation method is described as the following interpolation function equations. Y F =Y 0 l 0 (X F )+Y 1 l 1 (X F )+Y 2 l 2 (X F ) (8) l 0 ( X F ) = l 1 ( X F ) = l 2 ( X F ) = ( X F X 1 )( X F X 2 ) ( X 0 X 1 )( X 0 X 2 ) ( X F X 0 )( X F X 2 ) ( X 1 X 0 )( X 1 X 2 ) ( X F X 0 )( X F X 1 ) ( X 2 X 0 )( X 2 X 1 ) (9) (10) (11) Then the composition of liquid and vapor for each stage along the column is received. Second, it is important to estimate if there are concave points a- mong the composition points. The slope method is adopted to accomplish this wor. The composition of the top point and bottom point is specified, (X d, X d ) and (X q, X q ), respectively. Connecting the top point (or bottom point) and feed point would draw a line which slope given as a=(x D Y F )/(X D X F ). The equation of the line is described as Y'=aX+b. For each liquid composition X j, the Y j ' could be calculated. If Y j '>Y j, there is concave point among the composition points. Otherwise, the concave point is not subsistent. Third, the minimum reflux ratio could be calculated, respectively, for a concave situation and non-concave situation. For a non-concave situation, the minimum reflux ratio could be calculated by the slope of the line which connects the top point (or bottom point) and feed point. For the concave situation, the Lagrange quadratic interpolation method and golden section method are adopted to find the point of tangency which exists between the feed point and top point. Then drawing a line from the point of tangency to feed point or top point of column, the slope of the line could be used to calculate the minimum reflux ratio R min. 3. Calculation of Minimum Number of Stages The stage to stage calculation method is adopted to calculate the minimum number of stages. The operation line is Y=X for infinite reflux. The top point of the column (X d, X d ) is set as the beginning July, 2007
4 Design method for multi-component distillation system based on quasi-binary method 559 point of the operation line. Then Y 1 =X d. The X 1 is calculated by the chord position method. Then Y 2 =X 1 ; the rest may be deduced by analogy until X n <X q. The minimum number of stages is n. The iterative equation is described as follows: X ' = X j X j 1 X j+1 fx ( j 1 ) f( X j+1 ) Here fx ( j 1 ) ( ) j=1, 2 (12) f(x j+1 )=g(x j+1 ) Y j 1 j=1, 2 (13) f(x j 1 )=g(x j 1 ) Y j 1 j=1, 2 (14) The g(x) is the polynomial of Lagrange quadratic interpolation, which is given above by Eqs. (8)-(11). f(x ' ) g(x ' ) Y j 1 j=1, 2 (15) The iterative calculation goes on with the following steps: (1) Given composition point: (X j 1, Y j 1 ), (X j,y j ), (X j+1, Y j+1 ) (2) Calculating the g(x j 1 ), g(x j+1 ), f(x j 1 ), f(x j+1 ), X' and f(x') (3) If f(x j 1 ) <ε, then X=X', End. Otherwise go step (4). (4) X j 1 =X j+1, X j+1 =X'. go step (2). 4. Calculation of the Operation Reflux Ratio R and Number of Stages N Once the minimum reflux ratio and minimum numbers of stages of a column separating the multi-component mixture have been determined, the following two methods could be used to calculate the operation reflux ratio and number of stages. In the first method, the operation reflux ratio is set times the minimum reflux ratio. Then the number of stages is calculated using stage to stage. In another method, the operation number of stages could be obtained by multiplying a factor of the minimum number of stages. Then the actual reflux ratio is calculated in turn. In this paper, the former method is adopted. The ratio factor between the operation reflux ratio and minimum reflux ratio is set to 1.2 (R=1.2R min ) in order to compare the result with that of the reference [18]. But the factor is not limited only to 1.2. Other values, such as 1.3 or 1.5, could be adopted to accomplish the multi-component distillation by QBM. The vapor and liquid composition of feed could be calculated, which is mentioned above in section 5.2. The number of stages is calculated from the distillate point and bottom point to the feed point, respectively, by the chord position method, as mentioned above. The total number of stages is the sum of the number of stages of rectifying section and stripping section. At the same time, the feed location is determined. 5. Verification of the R min and N min The calculation results of R min and N min are verified in this section to demonstrate the design method presented in this paper. The number of stages is set to a large number (N=300) to rigorously simulate. If the specified separation is not satisfied by adjusting the reflux ratio and the feed location, the value of R min calculated by the above method would be accurate and the design method is feasible. Similarly, the N min could be verified. The following examples would give further explanation. EXAMPLES Example 1: The feed of methanol, ethanol and 1-propanol with Table 1. Comparison of shortcut design and QBM Shortcut design QBM X F (mole fraction: 0.3/0.1/ /0.1/0.6 methanol/ethanol/1-propanol) α methanol /α ethanol /α 1-propanol 1.805/1/ /1/0.488 Nmin R min /(L/V) min 2.78/ /0.741 N R Feed stage mole fraction of 0.3, 0.1 and 0.6 is considered. The desired separation is methanol from the other two components. The relative volatilities are nearly constant and are 1.805, 1 and 0.488, respectively. The product specifications were 1% recovery of the heavy and light eys component in the distillate and bottoms. The results are summarized in Table 1. From Table 1, it can be seen that the results obtained by the two methods of column design have little error for the prediction of the minimum stage number and minimum reflux ratio (R min ) or liquidvapor ratio ((L/V) min ) at the same feed composition and product specification. It illustrates the validity of the QBM for column design. Noticeably, N min, N, R, R min and the feed location are automatically calculated by using QBM, which is extremely quic and simple because iteration is avoided, especially compared to the other available methods, such as the boundary value method [19] or the use of manifolds [20]. Example 2: Consider the four-column distillation sequence separating the quaternary mixture with azeotropic point of acetone, chloroform, benzene and toluene. The feed data for this example are given in Table 2 [18] and the separation sequence is shown in Fig. 3. The results are summarized in Table 2. From Table 2, it can be seen that there exists larger error among the FUG, HYSYS and the QBM in the calculation result of N min and R min or (L/V) min. The reason is that there are several assumptions in the FUG method for prediction of N min and R min or (L/V) min. So it is not accurate to column design especially for a non-ideal mixture. Numerous other examples studied gave the same general results. The reason for the error between HYSYS and the QBM could be the proportion factor, which is that the ratio of R and R min, is different. For the HYSYS method, the proportion factors adopted in the four columns are different. But the same proportion factor is set to the four columns for the QBM. To illustrate the results of QBM method presented in this paper, a rigorous simulation using ASPEN- PLUS is adopted. The specification of distillate and bottom could be reached in the following two conditions: first, the stage number is set to a smaller value than N min given by FUG or HYSYS; the production specification could be attained while the R or (L/V) increases larger. In the same manner, the R min or (L/V) min is set to a smaller value than that of FUG or HYSYS; the production specification also could be obtained while the stage number becomes larger. But if the stage number (or reflux ratio) is smaller than N min (or R min ) given by QBM, the production specification always cannot be satisfied. Thus, the result of QBM is accurate and the QBM is effective for the multi- Korean J. Chem. Eng.(Vol. 24, No. 4)
5 560 W. Lianying et al. Table 2. Comparison of the QBM and the FUG shortcut design Splits Column I Column II Column III Column IV X F 0.26/0.32/0.21/ /0.39/0.26/ /0.83/0.01/0 0/0.02/0.48/0.50 X D 0.93/0.04/0.03/ /0.83/0.01/0 0/1.0/0/0 0/0.02/0.98/0 X B 0.09/0.39/0.26/0.26 0/0.02/0.48/ /0.67/0.02/0 0/0/0/1.0 D/F Shortcut method (FUG) R min N min R N HYSYS R min N min R N QBM (L/V) min /R min 0.787/ / / /1.324 N min (L/V)/R 0.816/ / / /1.59 N Feed stage Fig. 3. Four column distillation sequence separating the quaternary mixture of acetone, chloroform, benzene and toluene. component non-ideal mixture too, which could accurately predict the N min and R min or (L/V) min. Example 3: In this example the azeotropic distillation for separating the mixture isopropanol/water is analyzed. The mole fraction of feed mixture is 0.41/0.59 and the cyclohexane is employed as entrainer. The separation flowsheet is shown in Fig. 4, which comprises two distillation columns--concentrating column and dehydration column. The concentrating column is a general column which is designed easily. The dehydration column is a complex azeotropic distillation column and will be discussed in detail. The product specifications were 99.5% recovery of isopropanol at the bottom and less than 0.01% residual quantity of isopropanol at the distillate. Because the relative volatilities of the azeotropic mixture vary significantly along the column, the initial values of R and N would Fig. 4. The flowsheet of azeotropic distillation for separating the mixture isopropanol/water. Table 3. Design results of the azeotropic distillation of isopropanolwater system Column I Column II Nmin (L/V)min N L/V be given a relatively large number according to the experience of calling the simulation software. Subsequently, the automatic calculation program would be performed to accomplish the process design. The results are shown in Table 3. The liquid-vapor ratio is adopted to replace the reflux ratio as there is not a condenser for the dehydration column. Afterward, the obtained L/V and N are input to AS- July, 2007
6 Design method for multi-component distillation system based on quasi-binary method 561 PENPLUS software to simulate the separation process. The product specification is satisfied. It indicates that the QBM is feasible for distillation design of non-ideal mixtures and complex systems. CONCLUSION The design of distillation for multi-component separation has been studied. The concept of congregated components, which converts a multi-component system to quasi-binary system using light congregated components and heavy congregated components, has been put forward to simplify multi-component distillation processes. Then the design method based on the quasi-binary, which is automatically performed by the technology of software integration, is presented to complete the design of multi-component distillation. The results of three examples are shown: the proposed method is valid for multi-component distillation processes, which is not only applied to ideal mixtures but also non-ideal mixtures. ACKNOWLEDGMENTS Financial support from the National Nature Science Fund Program of China and the National Fundamental Research Development Program of China is gratefully acnowledged. NOMENCLATURE A, B, C, D, E : pure component of mixture K : phase balance constant of integrated component : phase balance constant of pure component L : mole flow rate of liquid V : mole flow rate of vapor y : mole fraction of pure component in the vapor phase x : mole fraction of pure component in the liquid phase N : number of stages in a column R : reflux ratio X : mole fraction of integrated component in liquid phase Y : mole fraction of integrated component in vapor phase α : relative volatility of component Subscripts F : feed of the column D, d : distillate product B, b : bottom product Min : minimum i : the i-th component n : the n-th stage Acronyms FUG : Fense-Underwood-Gilliland method MCS : Model of Column Section VLE : Vapor-Liquid Equilibrium LCC : Light congregated component HCC : Heavy congregated component QBM : Quasi-Binary Method REFERENCES 1. B. Neri, M. Mazzotti, G. Storti and M. Morbidelli, Ind. Eng. Chem. Res., 37, 2250 (1998). 2. A. Underwood, Chem. Eng. Prog., 44, 603 (1948). 3. J. Cho and J.-K. Jeon, Korean J. Chem. Eng., 23, 1 (2006). 4. V. Jula and M. F. Doherty, Chem. Eng. Sci., 45, 1801 (1990). 5. J. Bausa, R. V. Watzdrof and W. Marquardt, AIChE J., 44, 2181 (1998). 6. J. Bausa, R. V. Watzdrof and W. Marquardt, AIChE J., 45, 1615 (1999). 7. J. S. Logsdon, U. M. Diwear and L. T. Biegler, Chemical Engineering Research and Design, 68, 434 (1990). 8. U. M. Diwear and K. P. Madhavan, Ind. Eng. Chem. Res., 30, 713 (1991). 9. F. V. Petlyu, V. M. Platonov and D. M. Slavinsii, Int. Chem. Eng., 5, 555 (1965). 10. R. Smith, Chemical process design, McGraw-Hill (1995). 11. X. Yuan and A. Weizhong, Chinese Journal of Chemical Engineering, 10, 495 (2002). 12. D. W. Tedder and D. F. Rudd, AIChE J., 24, 303 (1978). 13. C. J. King, Separation processes, 2nd ed., McGraw-Hill Boo Co., New Yor (1980). 14. R. Agrawal, Ind. Eng. Chem. Res., 35, 1059 (1996). 15. R. Ben-Guang, A. Kraslawsi and L. Nystriim, Comput. Chem. Eng., 25, 807 (2001). 16. Y. H. Kim, M. Naaiwa and K. S. Kwang, Korean J. Chem. Eng., 19, 383 (2002). 17. G. Liu, M. Jobson, R. Smith and M. W. Oliver, Ind. Eng. Chem. Res., 43, 908 (2004). 18. Y. H. Kim, Chemical Engineering Journal, 89, 89 (2002). 19. S. G. Levy, D. B. Van Dongen and M. F. Doherty, Ind. Eng. Chem. Fundam., 24, 463 (1985). 20. D. Y. C. Thong and M. Jobson, Chem. Eng. Sci., 56, 4392 (2001). Korean J. Chem. Eng.(Vol. 24, No. 4)
Dynamic Models Towards Operator and Engineer Training: Virtual Environment
 European Symposium on Computer Arded Aided Process Engineering 15 L. Puigjaner and A. Espuña (Editors) 2005 Elsevier Science B.V. All rights reserved. Dynamic Models Towards Operator and Engineer Training:
European Symposium on Computer Arded Aided Process Engineering 15 L. Puigjaner and A. Espuña (Editors) 2005 Elsevier Science B.V. All rights reserved. Dynamic Models Towards Operator and Engineer Training:
Figure 56. Simple mixing process with process specification for the outlet stream.
 Flowsheet Analysis One of the most useful functions of process simulators is the ability to manipulate and analyze the different design variables to determine the required value or study its effect on
Flowsheet Analysis One of the most useful functions of process simulators is the ability to manipulate and analyze the different design variables to determine the required value or study its effect on
Minimum Reflux in Liquid Liquid Extraction
 17 th European Symposium on Computer Aided Process Engineering ESCAPE17 V. Plesu and P.S. Agachi (Editors) 2007 Elsevier B.V. All rights reserved. 1 Minimum Reflux in Liquid Liquid Extraction Santanu Bandyopadhyay
17 th European Symposium on Computer Aided Process Engineering ESCAPE17 V. Plesu and P.S. Agachi (Editors) 2007 Elsevier B.V. All rights reserved. 1 Minimum Reflux in Liquid Liquid Extraction Santanu Bandyopadhyay
More than 80 years ago, McCabe and Thiele
 isualizing the McCabe-Thiele Diagram Paul M. Mathias Fluor Corp. Use this spreadsheet-based visualization and interactive analysis of the McCabe-Thiele diagram to understand the foundations of distillation
isualizing the McCabe-Thiele Diagram Paul M. Mathias Fluor Corp. Use this spreadsheet-based visualization and interactive analysis of the McCabe-Thiele diagram to understand the foundations of distillation
Lecture 9 Solving Material Balances Problems Involving Non-Reactive Processes
 CHE 31. INTRODUCTION TO CHEMICAL ENGINEERING CALCULATIONS Lecture 9 Solving Material Balances Problems Involving Non-Reactive Processes Component and Overall Material Balances Consider a steady-state distillation
CHE 31. INTRODUCTION TO CHEMICAL ENGINEERING CALCULATIONS Lecture 9 Solving Material Balances Problems Involving Non-Reactive Processes Component and Overall Material Balances Consider a steady-state distillation
Distillation Principles
 Distillation Principles Definition of distillation, Types of columns, Simple Distillation methods (Flash, batch, Steam), Basic distillation Equipment and operation, Column internal, Reboilers, Distillation
Distillation Principles Definition of distillation, Types of columns, Simple Distillation methods (Flash, batch, Steam), Basic distillation Equipment and operation, Column internal, Reboilers, Distillation
Performing Multi - Phase Mass and Energy Balances
 Performing Multi-Phase Mass and Energy Balances (Separations) Performing Multi - Phase Mass and Energy Balances Using thermodynamics in mass / energy balance problems means that additional equations are
Performing Multi-Phase Mass and Energy Balances (Separations) Performing Multi - Phase Mass and Energy Balances Using thermodynamics in mass / energy balance problems means that additional equations are
Optimization of Natural Gas Processing Plants Including Business Aspects
 Page 1 of 12 Optimization of Natural Gas Processing Plants Including Business Aspects KEITH A. BULLIN, Bryan Research & Engineering, Inc., Bryan, Texas KENNETH R. HALL, Texas A&M University, College Station,
Page 1 of 12 Optimization of Natural Gas Processing Plants Including Business Aspects KEITH A. BULLIN, Bryan Research & Engineering, Inc., Bryan, Texas KENNETH R. HALL, Texas A&M University, College Station,
ORGANIC LABORATORY TECHNIQUES 10 10.1. NEVER distill the distillation flask to dryness as there is a risk of explosion and fire.
 ORGANIC LABORATORY TECHNIQUES 10 10.1 DISTILLATION NEVER distill the distillation flask to dryness as there is a risk of explosion and fire. The most common methods of distillation are simple distillation
ORGANIC LABORATORY TECHNIQUES 10 10.1 DISTILLATION NEVER distill the distillation flask to dryness as there is a risk of explosion and fire. The most common methods of distillation are simple distillation
Chemical Process Simulation
 Chemical Process Simulation The objective of this course is to provide the background needed by the chemical engineers to carry out computer-aided analyses of large-scale chemical processes. Major concern
Chemical Process Simulation The objective of this course is to provide the background needed by the chemical engineers to carry out computer-aided analyses of large-scale chemical processes. Major concern
Dortmund Data Bank (DDB) DDB Software Package (DDBSP)
 Showcase on Technology Dortmund Data Bank (DDB) DDB Software Package (DDBSP) Practical Application of Distillation Synthesis for NOx Reduction, Energy Cost Savings, & Improved Environmental Compliance
Showcase on Technology Dortmund Data Bank (DDB) DDB Software Package (DDBSP) Practical Application of Distillation Synthesis for NOx Reduction, Energy Cost Savings, & Improved Environmental Compliance
STEADY-STATE AND DYNAMIC SIMULATION OF CRUDE OIL DISTILLATION USING ASPEN PLUS AND ASPEN DYNAMICS
 Petroleum & Coal ISSN 1337-7027 Available online at www.vurup.sk/pc Petroleum & Coal 51 (2) 100-109, 2009 STEADY-STATE AND DYNAMIC SIMULATION OF CRUDE OIL DISTILLATION USING ASPEN PLUS AND ASPEN DYNAMICS
Petroleum & Coal ISSN 1337-7027 Available online at www.vurup.sk/pc Petroleum & Coal 51 (2) 100-109, 2009 STEADY-STATE AND DYNAMIC SIMULATION OF CRUDE OIL DISTILLATION USING ASPEN PLUS AND ASPEN DYNAMICS
Liquid-Liquid Extraction (LLX)
 Liquid-Liquid Extraction (LLX) Extraction is a liquid-liquid operation. It is a process of transferring a solute from one liquid phase to another immiscible or partially miscible liquid in contact with
Liquid-Liquid Extraction (LLX) Extraction is a liquid-liquid operation. It is a process of transferring a solute from one liquid phase to another immiscible or partially miscible liquid in contact with
Simulation of Multistage Countercurrent Liquid-Liquid Extraction
 Leonardo Journal of Sciences ISSN 1583-0233 Issue 20, January-June 2011 p. 79-94 Simulation of Multistage Countercurrent Liquid-Liquid Extraction Annasaheb WARADE 1*, Ravindra GAIKWAD 1, Rajiv SAPKAL 2
Leonardo Journal of Sciences ISSN 1583-0233 Issue 20, January-June 2011 p. 79-94 Simulation of Multistage Countercurrent Liquid-Liquid Extraction Annasaheb WARADE 1*, Ravindra GAIKWAD 1, Rajiv SAPKAL 2
Introduction to Process Optimization
 Chapter 1 Introduction to Process Optimization Most things can be improved, so engineers and scientists optimize. While designing systems and products requires a deep understanding of influences that achieve
Chapter 1 Introduction to Process Optimization Most things can be improved, so engineers and scientists optimize. While designing systems and products requires a deep understanding of influences that achieve
Process Heat Integration between Distillation Columns for Ethylene Hydration Process
 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 35, 013 Guest Editors: Petar Varbanov, Jiří Klemeš, Panos Seferlis, Athanasios I. Papadopoulos, Spyros Voutetakis Copyright 013, AIDIC Servizi S.r.l.,
A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 35, 013 Guest Editors: Petar Varbanov, Jiří Klemeš, Panos Seferlis, Athanasios I. Papadopoulos, Spyros Voutetakis Copyright 013, AIDIC Servizi S.r.l.,
RECTIFIER DESIGN FOR FUEL ETHANOL PLANTS
 RECTIFIER DESIGN FOR FUEL ETHANOL PLANTS By Daniel R. Summers, P.E. SULZER CHEMTECH USA, Inc. Presented at the AIChE Annual Meeting Advances in Distillation Equipment and Applications Paper 264b November
RECTIFIER DESIGN FOR FUEL ETHANOL PLANTS By Daniel R. Summers, P.E. SULZER CHEMTECH USA, Inc. Presented at the AIChE Annual Meeting Advances in Distillation Equipment and Applications Paper 264b November
Design and Control Degrees of Freedom
 2204 Ind. Eng. Chem. Res. 1996, 35, 2204-2214 Design and Control Degrees of Freedom William L. Luyben* Department of Chemical Engineering, Lehigh University, Iacocca Hall, Bethlehem, Pennsylvania 18015
2204 Ind. Eng. Chem. Res. 1996, 35, 2204-2214 Design and Control Degrees of Freedom William L. Luyben* Department of Chemical Engineering, Lehigh University, Iacocca Hall, Bethlehem, Pennsylvania 18015
Thermodynamics of Mixing
 Thermodynamics of Mixing Dependence of Gibbs energy on mixture composition is G = n A µ A + n B µ B and at constant T and p, systems tend towards a lower Gibbs energy The simplest example of mixing: What
Thermodynamics of Mixing Dependence of Gibbs energy on mixture composition is G = n A µ A + n B µ B and at constant T and p, systems tend towards a lower Gibbs energy The simplest example of mixing: What
Everest. Leaders in Vacuum Booster Technology
 This article has been compiled to understand the process of Solvent Recovery process generally carried out at low temperatures and vacuum. In many chemical processes solute is to be concentrated to high
This article has been compiled to understand the process of Solvent Recovery process generally carried out at low temperatures and vacuum. In many chemical processes solute is to be concentrated to high
The Basics of FEA Procedure
 CHAPTER 2 The Basics of FEA Procedure 2.1 Introduction This chapter discusses the spring element, especially for the purpose of introducing various concepts involved in use of the FEA technique. A spring
CHAPTER 2 The Basics of FEA Procedure 2.1 Introduction This chapter discusses the spring element, especially for the purpose of introducing various concepts involved in use of the FEA technique. A spring
Università degli Studi di Milano, Dipartimento di Chimica, via Golgi 19, 20133 Milano, Italy b
 A publication of 1897 CHEMICAL ENGINEERING TRANSACTIONS VOL. 32, 2013 Chief Editors: Sauro Pierucci, Jiří J. Klemeš Copyright 2013, AIDIC Servizi S.r.l., ISBN 978-88-95608-23-5; ISSN 1974-9791 The Italian
A publication of 1897 CHEMICAL ENGINEERING TRANSACTIONS VOL. 32, 2013 Chief Editors: Sauro Pierucci, Jiří J. Klemeš Copyright 2013, AIDIC Servizi S.r.l., ISBN 978-88-95608-23-5; ISSN 1974-9791 The Italian
Distillation vaporization sublimation. vapor pressure normal boiling point.
 Distillation Distillation is an important commercial process that is used in the purification of a large variety of materials. However, before we begin a discussion of distillation, it would probably be
Distillation Distillation is an important commercial process that is used in the purification of a large variety of materials. However, before we begin a discussion of distillation, it would probably be
Conceptual Design. Davide Manca. Lesson 2 of Process Systems Engineering Master Degree in Chemical Engineering Politecnico di Milano
 Conceptual Design Davide Manca Lesson 2 of Process Systems Engineering Master Degree in Chemical Engineering Politecnico di Milano Introduction The purpose of engineering is to create new material wealth.
Conceptual Design Davide Manca Lesson 2 of Process Systems Engineering Master Degree in Chemical Engineering Politecnico di Milano Introduction The purpose of engineering is to create new material wealth.
μ α =μ β = μ γ = =μ ω μ α =μ β =μ γ = =μ ω Thus for c components, the number of additional constraints is c(p 1) ( ) ( )
 Phase Diagrams 1 Gibbs Phase Rule The Gibbs phase rule describes the degrees of freedom available to describe a particular system with various phases and substances. To derive the phase rule, let us begin
Phase Diagrams 1 Gibbs Phase Rule The Gibbs phase rule describes the degrees of freedom available to describe a particular system with various phases and substances. To derive the phase rule, let us begin
Chemical Equilibrium by Gibbs Energy Minimization on Spreadsheets*
 Int. J. Engng Ed. Vol. 16, No. 4, pp. 335±339, 2000 0949-149X/91 $3.00+0.00 Printed in Great Britain. # 2000 TEMPUS Publications. Chemical Equilibrium by Gibbs Energy Minimization on Spreadsheets* Y. LWIN
Int. J. Engng Ed. Vol. 16, No. 4, pp. 335±339, 2000 0949-149X/91 $3.00+0.00 Printed in Great Britain. # 2000 TEMPUS Publications. Chemical Equilibrium by Gibbs Energy Minimization on Spreadsheets* Y. LWIN
Experiment 5: Phase diagram for a three-component system (Dated: April 12, 2010)
 Experiment 5: Phase diagram for a three-component system (Dated: April 12, 2010) I. INTRODUCTION It is sometimes necessary to know the mutual solubilities of liquids in a two-phase system. For example,
Experiment 5: Phase diagram for a three-component system (Dated: April 12, 2010) I. INTRODUCTION It is sometimes necessary to know the mutual solubilities of liquids in a two-phase system. For example,
Phase Equilibrium: Fugacity and Equilibrium Calculations. Fugacity
 Phase Equilibrium: Fugacity and Equilibrium Calculations (FEC) Phase Equilibrium: Fugacity and Equilibrium Calculations Relate the fugacity and the chemical potential (or the partial molar Gibbs free energy)
Phase Equilibrium: Fugacity and Equilibrium Calculations (FEC) Phase Equilibrium: Fugacity and Equilibrium Calculations Relate the fugacity and the chemical potential (or the partial molar Gibbs free energy)
Chem 420/523 Chemical Thermodynamics Homework Assignment # 6
 Chem 420/523 Chemical hermodynamics Homework Assignment # 6 1. * Solid monoclinic sulfur (S α ) spontaneously converts to solid rhombic sulfur (S β ) at 298.15 K and 0.101 MPa pressure. For the conversion
Chem 420/523 Chemical hermodynamics Homework Assignment # 6 1. * Solid monoclinic sulfur (S α ) spontaneously converts to solid rhombic sulfur (S β ) at 298.15 K and 0.101 MPa pressure. For the conversion
Lecture 3: Models of Solutions
 Materials Science & Metallurgy Master of Philosophy, Materials Modelling, Course MP4, Thermodynamics and Phase Diagrams, H. K. D. H. Bhadeshia Lecture 3: Models of Solutions List of Symbols Symbol G M
Materials Science & Metallurgy Master of Philosophy, Materials Modelling, Course MP4, Thermodynamics and Phase Diagrams, H. K. D. H. Bhadeshia Lecture 3: Models of Solutions List of Symbols Symbol G M
vap H = RT 1T 2 = 30.850 kj mol 1 100 kpa = 341 K
 Thermodynamics: Examples for chapter 6. 1. The boiling point of hexane at 1 atm is 68.7 C. What is the boiling point at 1 bar? The vapor pressure of hexane at 49.6 C is 53.32 kpa. Assume that the vapor
Thermodynamics: Examples for chapter 6. 1. The boiling point of hexane at 1 atm is 68.7 C. What is the boiling point at 1 bar? The vapor pressure of hexane at 49.6 C is 53.32 kpa. Assume that the vapor
Distillation of Alcohol
 CHEM 121L General Chemistry Laboratory Revision 1.6 Distillation of Alcohol To learn about the separation of substances. To learn about the separation technique of distillation. To learn how to characterize
CHEM 121L General Chemistry Laboratory Revision 1.6 Distillation of Alcohol To learn about the separation of substances. To learn about the separation technique of distillation. To learn how to characterize
The Effect Of Implementing Thermally Coupled Distillation Sequences On Snowball Effects For Reaction-Separation-Recycle Systems
 20 th European Symposium on Computer Aided Process Engineering ESCAPE20 S. Pierucci and G. Buzzi Ferraris (Editors) 2010 Elsevier B.V. All rights reserved. The Effect Of Implementing Thermally Coupled
20 th European Symposium on Computer Aided Process Engineering ESCAPE20 S. Pierucci and G. Buzzi Ferraris (Editors) 2010 Elsevier B.V. All rights reserved. The Effect Of Implementing Thermally Coupled
Refinery Process Design
 Refinery Process Design (Lecture Notes) Dr. Ramgopal Uppaluri ramgopalu@iitg.ernet.in Department of Chemical Engineering Indian Institute of Technology Guwahati North Guwahati 781039, Assam, India March
Refinery Process Design (Lecture Notes) Dr. Ramgopal Uppaluri ramgopalu@iitg.ernet.in Department of Chemical Engineering Indian Institute of Technology Guwahati North Guwahati 781039, Assam, India March
Absorption with chemical reaction: evaluation of rate promoters effect on CO 2 absorption in hot potassium carbonate solutions
 17 th European Symposium on Computer Aided Process Engineering ESCAPE17 V. Plesu and P.S. Agachi (Editors) 007 Elsevier B.V. All rights reserved. 1 Absorption with chemical reaction: evaluation of rate
17 th European Symposium on Computer Aided Process Engineering ESCAPE17 V. Plesu and P.S. Agachi (Editors) 007 Elsevier B.V. All rights reserved. 1 Absorption with chemical reaction: evaluation of rate
The Ideal Solution. ChemActivity T15
 ChemActivity T15 The Ideal Solution Focus Question: An equi-molar mixture of benzene and toluene is prepared. What will be the composition of the vapor in equilibrium with this solution? Model 1: Benzene
ChemActivity T15 The Ideal Solution Focus Question: An equi-molar mixture of benzene and toluene is prepared. What will be the composition of the vapor in equilibrium with this solution? Model 1: Benzene
Sample Test 1 SAMPLE TEST 1. CHAPTER 12
 13 Sample Test 1 SAMPLE TEST 1. CHAPTER 12 1. The molality of a solution is defined as a. moles of solute per liter of solution. b. grams of solute per liter of solution. c. moles of solute per kilogram
13 Sample Test 1 SAMPLE TEST 1. CHAPTER 12 1. The molality of a solution is defined as a. moles of solute per liter of solution. b. grams of solute per liter of solution. c. moles of solute per kilogram
A simple and unified algorithm to solve fluid phase. equilibria using either the gamma-phi or the phi-phi. approach for binary and ternary mixtures
 A simple and unified algorithm to solve fluid phase equilibria using either the gamma-phi or the phi-phi approach for binary and ternary mixtures Romain PRIVAT,a, Jean-Noël JAUBERT a and Yannick PRIVAT
A simple and unified algorithm to solve fluid phase equilibria using either the gamma-phi or the phi-phi approach for binary and ternary mixtures Romain PRIVAT,a, Jean-Noël JAUBERT a and Yannick PRIVAT
Experiment 446.1 SURFACE TENSION OF LIQUIDS. Experiment 1, page 1 Version of June 17, 2016
 Experiment 1, page 1 Version of June 17, 2016 Experiment 446.1 SURFACE TENSION OF LIQUIDS Theory To create a surface requires work that changes the Gibbs energy, G, of a thermodynamic system. dg = SdT
Experiment 1, page 1 Version of June 17, 2016 Experiment 446.1 SURFACE TENSION OF LIQUIDS Theory To create a surface requires work that changes the Gibbs energy, G, of a thermodynamic system. dg = SdT
CHEM 120 Online Chapter 7
 CHEM 120 Online Chapter 7 Date: 1. Which of the following statements is not a part of kinetic molecular theory? A) Matter is composed of particles that are in constant motion. B) Particle velocity increases
CHEM 120 Online Chapter 7 Date: 1. Which of the following statements is not a part of kinetic molecular theory? A) Matter is composed of particles that are in constant motion. B) Particle velocity increases
Microeconomic Theory: Basic Math Concepts
 Microeconomic Theory: Basic Math Concepts Matt Van Essen University of Alabama Van Essen (U of A) Basic Math Concepts 1 / 66 Basic Math Concepts In this lecture we will review some basic mathematical concepts
Microeconomic Theory: Basic Math Concepts Matt Van Essen University of Alabama Van Essen (U of A) Basic Math Concepts 1 / 66 Basic Math Concepts In this lecture we will review some basic mathematical concepts
6. 2. Unit 6: Physical chemistry of spectroscopy, surfaces and chemical and phase equilibria
 6. 2 Phase equilibria Many industrial processes involve several phases in equilibrium gases, liquids, solids and even different crystalline forms of the solid state. Predicting the number of phases present
6. 2 Phase equilibria Many industrial processes involve several phases in equilibrium gases, liquids, solids and even different crystalline forms of the solid state. Predicting the number of phases present
MOLECULAR WEIGHT BY BOILING POINT ELEVATION
 MOLECULAR WEIGHT BY BOILING POINT ELEVATION BACKGROUND This experiment demonstrates the use of colligative properties. The goal is to measure the molecular weight of a non-volatile solute by determining
MOLECULAR WEIGHT BY BOILING POINT ELEVATION BACKGROUND This experiment demonstrates the use of colligative properties. The goal is to measure the molecular weight of a non-volatile solute by determining
Developed Equation for fitting ASTM Distillation curves. Dr. Khalid Farhod Chasib Chemical Engineering Department - University of technology
 Developed Equation for fitting ASTM Distillation curves Dr. Khalid Farhod Chasib Chemical Engineering Department - University of technology Ukhalid_farhod@uotechnology.edu.iqU ABSTRACT The present work
Developed Equation for fitting ASTM Distillation curves Dr. Khalid Farhod Chasib Chemical Engineering Department - University of technology Ukhalid_farhod@uotechnology.edu.iqU ABSTRACT The present work
Setting your session preferences
 What is Aspen? 7 Basic Steps 1 Setting your session preferences 2 Building the simulation 3 Entering the simulation environment 4 Using the workbook 5 Installing Unit Operations 6 Run Your Simulation 7
What is Aspen? 7 Basic Steps 1 Setting your session preferences 2 Building the simulation 3 Entering the simulation environment 4 Using the workbook 5 Installing Unit Operations 6 Run Your Simulation 7
Creating, Solving, and Graphing Systems of Linear Equations and Linear Inequalities
 Algebra 1, Quarter 2, Unit 2.1 Creating, Solving, and Graphing Systems of Linear Equations and Linear Inequalities Overview Number of instructional days: 15 (1 day = 45 60 minutes) Content to be learned
Algebra 1, Quarter 2, Unit 2.1 Creating, Solving, and Graphing Systems of Linear Equations and Linear Inequalities Overview Number of instructional days: 15 (1 day = 45 60 minutes) Content to be learned
Journal Of Petoleum Research & Studies. Developed Equation For Fitting ASTM Distillation Curves. Dr. Khalid Farhod Chasib
 Developed Equation For Fitting ASTM Distillation Curves Dr. Khalid Farhod Chasib Chemical Engineering Department - University Of Technology Abstract Introduction The present work deals with fitting literature
Developed Equation For Fitting ASTM Distillation Curves Dr. Khalid Farhod Chasib Chemical Engineering Department - University Of Technology Abstract Introduction The present work deals with fitting literature
STEADY STATE MODELING AND SIMULATION OF HYDROCRACKING REACTOR
 Petroleum & Coal ISSN 1337-7027 Available online at www.vurup.sk/petroleum-coal Petroleum & Coal 54 (1) 59-64, 2012 STEADY STATE MODELING AND SIMULATION OF HYDROCRACKING REACTOR Abhinanyu Kumar, Shishir
Petroleum & Coal ISSN 1337-7027 Available online at www.vurup.sk/petroleum-coal Petroleum & Coal 54 (1) 59-64, 2012 STEADY STATE MODELING AND SIMULATION OF HYDROCRACKING REACTOR Abhinanyu Kumar, Shishir
Modelling and Simulation of the Freezing Systems and Heat Pumps Using Unisim Design
 Modelling and Simulation of the Freezing Systems and Heat Pumps Using Unisim Design C. Patrascioiu Abstract The paper describes the modeling and simulation of the heat pumps domain processes. The main
Modelling and Simulation of the Freezing Systems and Heat Pumps Using Unisim Design C. Patrascioiu Abstract The paper describes the modeling and simulation of the heat pumps domain processes. The main
A three point formula for finding roots of equations by the method of least squares
 A three point formula for finding roots of equations by the method of least squares Ababu Teklemariam Tiruneh 1 ; William N. Ndlela 1 ; Stanley J. Nkambule 1 1 Lecturer, Department of Environmental Health
A three point formula for finding roots of equations by the method of least squares Ababu Teklemariam Tiruneh 1 ; William N. Ndlela 1 ; Stanley J. Nkambule 1 1 Lecturer, Department of Environmental Health
Chemistry 13: States of Matter
 Chemistry 13: States of Matter Name: Period: Date: Chemistry Content Standard: Gases and Their Properties The kinetic molecular theory describes the motion of atoms and molecules and explains the properties
Chemistry 13: States of Matter Name: Period: Date: Chemistry Content Standard: Gases and Their Properties The kinetic molecular theory describes the motion of atoms and molecules and explains the properties
CHEM 101/105 Numbers and mass / Counting and weighing Lect-03
 CHEM 101/105 Numbers and mass / Counting and weighing Lect-03 Interpretation of Elemental Chemical Symbols, Chemical Formulas, and Chemical Equations Interpretation of an element's chemical symbol depends
CHEM 101/105 Numbers and mass / Counting and weighing Lect-03 Interpretation of Elemental Chemical Symbols, Chemical Formulas, and Chemical Equations Interpretation of an element's chemical symbol depends
4. How many integers between 2004 and 4002 are perfect squares?
 5 is 0% of what number? What is the value of + 3 4 + 99 00? (alternating signs) 3 A frog is at the bottom of a well 0 feet deep It climbs up 3 feet every day, but slides back feet each night If it started
5 is 0% of what number? What is the value of + 3 4 + 99 00? (alternating signs) 3 A frog is at the bottom of a well 0 feet deep It climbs up 3 feet every day, but slides back feet each night If it started
Optimal Model-Based Production Planning for Refinery Operation
 Optimal Model-Based Production Planning for Refinery Operation Abdulrahman Alattas Advisor: Ignacio E. Grossmann Chemical Engineering Department Carnegie Mellon University 1 Presentation Outline Introduction
Optimal Model-Based Production Planning for Refinery Operation Abdulrahman Alattas Advisor: Ignacio E. Grossmann Chemical Engineering Department Carnegie Mellon University 1 Presentation Outline Introduction
TOPIC 4: DERIVATIVES
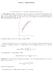 TOPIC 4: DERIVATIVES 1. The derivative of a function. Differentiation rules 1.1. The slope of a curve. The slope of a curve at a point P is a measure of the steepness of the curve. If Q is a point on the
TOPIC 4: DERIVATIVES 1. The derivative of a function. Differentiation rules 1.1. The slope of a curve. The slope of a curve at a point P is a measure of the steepness of the curve. If Q is a point on the
Calorimetry: Heat of Vaporization
 Calorimetry: Heat of Vaporization OBJECTIVES INTRODUCTION - Learn what is meant by the heat of vaporization of a liquid or solid. - Discuss the connection between heat of vaporization and intermolecular
Calorimetry: Heat of Vaporization OBJECTIVES INTRODUCTION - Learn what is meant by the heat of vaporization of a liquid or solid. - Discuss the connection between heat of vaporization and intermolecular
Modeling, Simulation & Experimentation of Separation Processes for CO2 Removal from Natural Gas
 1 Modeling, Simulation & Experimentation of Separation Processes for CO2 Removal from Natural Gas High Pressure Membrane Units High Pressure Membrane Contactors High Pressure Absorption with Amines José
1 Modeling, Simulation & Experimentation of Separation Processes for CO2 Removal from Natural Gas High Pressure Membrane Units High Pressure Membrane Contactors High Pressure Absorption with Amines José
is identically equal to x 2 +3x +2
 Partial fractions 3.6 Introduction It is often helpful to break down a complicated algebraic fraction into a sum of simpler fractions. 4x+7 For example it can be shown that has the same value as 1 + 3
Partial fractions 3.6 Introduction It is often helpful to break down a complicated algebraic fraction into a sum of simpler fractions. 4x+7 For example it can be shown that has the same value as 1 + 3
Experiment 12E LIQUID-VAPOR EQUILIBRIUM OF WATER 1
 Experiment 12E LIQUID-VAPOR EQUILIBRIUM OF WATER 1 FV 6/26/13 MATERIALS: PURPOSE: 1000 ml tall-form beaker, 10 ml graduated cylinder, -10 to 110 o C thermometer, thermometer clamp, plastic pipet, long
Experiment 12E LIQUID-VAPOR EQUILIBRIUM OF WATER 1 FV 6/26/13 MATERIALS: PURPOSE: 1000 ml tall-form beaker, 10 ml graduated cylinder, -10 to 110 o C thermometer, thermometer clamp, plastic pipet, long
molecular aggregates would then be present in the water: e.g., linear chains containing
 VISCOSITY MEASUREMENTS OF ALCOHOL-WATER MIXTURES AND THE STRUCTURE OF WATER BY M. AGENO AND C. FRONTALI PHYSICS LABORATORY, ISTITUTO SUPERIORE DI SANITA, ROME, ITALY Communicated by Emilio Segr', February
VISCOSITY MEASUREMENTS OF ALCOHOL-WATER MIXTURES AND THE STRUCTURE OF WATER BY M. AGENO AND C. FRONTALI PHYSICS LABORATORY, ISTITUTO SUPERIORE DI SANITA, ROME, ITALY Communicated by Emilio Segr', February
AM-08-14 Cost Effective Solutions for Reduction of Benzene in Gasoline
 Annual Meeting March 9-11, 2008 Manchester Grand Hyatt San Diego, CA Cost Effective Solutions for Reduction of Benzene in Gasoline Presented By: Kerry Rock Director, Technology Commercialization CDTECH
Annual Meeting March 9-11, 2008 Manchester Grand Hyatt San Diego, CA Cost Effective Solutions for Reduction of Benzene in Gasoline Presented By: Kerry Rock Director, Technology Commercialization CDTECH
COMP 250 Fall 2012 lecture 2 binary representations Sept. 11, 2012
 Binary numbers The reason humans represent numbers using decimal (the ten digits from 0,1,... 9) is that we have ten fingers. There is no other reason than that. There is nothing special otherwise about
Binary numbers The reason humans represent numbers using decimal (the ten digits from 0,1,... 9) is that we have ten fingers. There is no other reason than that. There is nothing special otherwise about
THE BASICS Q: What is VOC? Q: What are flashing losses/voc emissions from hydrocarbon storage tanks? - 1 -
 Calculation of Flashing Losses/VOC Emissions from Hydrocarbon Storage Tanks THE BASICS Q: What is VOC? A: VOC is an acronym that stands for Volatile Organic Compounds. VOC are components of hydrocarbon
Calculation of Flashing Losses/VOC Emissions from Hydrocarbon Storage Tanks THE BASICS Q: What is VOC? A: VOC is an acronym that stands for Volatile Organic Compounds. VOC are components of hydrocarbon
Fractional Distillation and Gas Chromatography
 Fractional Distillation and Gas Chromatography Background Distillation The previous lab used distillation to separate a mixture of hexane and toluene based on a difference in boiling points. Hexane boils
Fractional Distillation and Gas Chromatography Background Distillation The previous lab used distillation to separate a mixture of hexane and toluene based on a difference in boiling points. Hexane boils
FUNDAMENTALS OF ENGINEERING THERMODYNAMICS
 FUNDAMENTALS OF ENGINEERING THERMODYNAMICS System: Quantity of matter (constant mass) or region in space (constant volume) chosen for study. Closed system: Can exchange energy but not mass; mass is constant
FUNDAMENTALS OF ENGINEERING THERMODYNAMICS System: Quantity of matter (constant mass) or region in space (constant volume) chosen for study. Closed system: Can exchange energy but not mass; mass is constant
48 Practice Problems for Ch. 17 - Chem 1C - Joseph
 48 Practice Problems for Ch. 17 - Chem 1C - Joseph 1. Which of the following concentration measures will change in value as the temperature of a solution changes? A) mass percent B) mole fraction C) molality
48 Practice Problems for Ch. 17 - Chem 1C - Joseph 1. Which of the following concentration measures will change in value as the temperature of a solution changes? A) mass percent B) mole fraction C) molality
ECONOMICAL OPTIONS FOR RECOVERING NGL / LPG AT LNG RECEIVING TERMINALS
 ECONOMICAL OPTIONS FOR RECOVERING NGL / LPG AT RECEIVING TERMINALS Presented at the 86 th Annual Convention of the Gas Processors Association March 13, 2007 San Antonio, Texas Kyle T. Cuellar Ortloff Engineers,
ECONOMICAL OPTIONS FOR RECOVERING NGL / LPG AT RECEIVING TERMINALS Presented at the 86 th Annual Convention of the Gas Processors Association March 13, 2007 San Antonio, Texas Kyle T. Cuellar Ortloff Engineers,
Introduction to Matrix Algebra
 Psychology 7291: Multivariate Statistics (Carey) 8/27/98 Matrix Algebra - 1 Introduction to Matrix Algebra Definitions: A matrix is a collection of numbers ordered by rows and columns. It is customary
Psychology 7291: Multivariate Statistics (Carey) 8/27/98 Matrix Algebra - 1 Introduction to Matrix Algebra Definitions: A matrix is a collection of numbers ordered by rows and columns. It is customary
Chapter Test B. Chapter: Measurements and Calculations
 Assessment Chapter Test B Chapter: Measurements and Calculations PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.
Assessment Chapter Test B Chapter: Measurements and Calculations PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.
In this experiment, we will use three properties to identify a liquid substance: solubility, density and boiling point..
 Identification of a Substance by Physical Properties 2009 by David A. Katz. All rights reserved. Permission for academic use provided the original copyright is included Every substance has a unique set
Identification of a Substance by Physical Properties 2009 by David A. Katz. All rights reserved. Permission for academic use provided the original copyright is included Every substance has a unique set
Distillation Experiment
 Distillation Experiment CHM226 Background The distillation process is a very important technique used to separate compounds based on their boiling points. A substance will boil only when the vapor pressure
Distillation Experiment CHM226 Background The distillation process is a very important technique used to separate compounds based on their boiling points. A substance will boil only when the vapor pressure
IB Chemistry. DP Chemistry Review
 DP Chemistry Review Topic 1: Quantitative chemistry 1.1 The mole concept and Avogadro s constant Assessment statement Apply the mole concept to substances. Determine the number of particles and the amount
DP Chemistry Review Topic 1: Quantitative chemistry 1.1 The mole concept and Avogadro s constant Assessment statement Apply the mole concept to substances. Determine the number of particles and the amount
A New Quantitative Behavioral Model for Financial Prediction
 2011 3rd International Conference on Information and Financial Engineering IPEDR vol.12 (2011) (2011) IACSIT Press, Singapore A New Quantitative Behavioral Model for Financial Prediction Thimmaraya Ramesh
2011 3rd International Conference on Information and Financial Engineering IPEDR vol.12 (2011) (2011) IACSIT Press, Singapore A New Quantitative Behavioral Model for Financial Prediction Thimmaraya Ramesh
Chapter 9. Systems of Linear Equations
 Chapter 9. Systems of Linear Equations 9.1. Solve Systems of Linear Equations by Graphing KYOTE Standards: CR 21; CA 13 In this section we discuss how to solve systems of two linear equations in two variables
Chapter 9. Systems of Linear Equations 9.1. Solve Systems of Linear Equations by Graphing KYOTE Standards: CR 21; CA 13 In this section we discuss how to solve systems of two linear equations in two variables
SPE 54005. Copyright 1999, Society of Petroleum Engineers Inc.
 SPE 54005 Volatile Oil. Determination of Reservoir Fluid Composition From a Non-Representative Fluid Sample Rafael H. Cobenas, SPE, I.T.B.A. and Marcelo A. Crotti, SPE, Inlab S.A. Copyright 1999, Society
SPE 54005 Volatile Oil. Determination of Reservoir Fluid Composition From a Non-Representative Fluid Sample Rafael H. Cobenas, SPE, I.T.B.A. and Marcelo A. Crotti, SPE, Inlab S.A. Copyright 1999, Society
ENGINEERING ECONOMICS AND FINANCE
 CHAPTER Risk Analysis in Engineering and Economics ENGINEERING ECONOMICS AND FINANCE A. J. Clark School of Engineering Department of Civil and Environmental Engineering 6a CHAPMAN HALL/CRC Risk Analysis
CHAPTER Risk Analysis in Engineering and Economics ENGINEERING ECONOMICS AND FINANCE A. J. Clark School of Engineering Department of Civil and Environmental Engineering 6a CHAPMAN HALL/CRC Risk Analysis
PROCESS DESIGN AND CONTROL. Consistent Inventory Control. Elvira Marie B. Aske and Sigurd Skogestad*
 10892 Ind. Eng. Chem. Res. 2009, 48, 10892 10902 PROCESS DESIGN AND CONTROL Consistent Inventory Control Elvira Marie B. Aske and Sigurd Skogestad* Department of Chemical Engineering, Norwegian UniVersity
10892 Ind. Eng. Chem. Res. 2009, 48, 10892 10902 PROCESS DESIGN AND CONTROL Consistent Inventory Control Elvira Marie B. Aske and Sigurd Skogestad* Department of Chemical Engineering, Norwegian UniVersity
EXAMINATION Luleå University of Technology
 EXAMINATION Luleå University of Technology Course: B0004K Course name: Unit Operations Date: 2013-01-14 Time: 9.00 15.00 Aid: Del A: Inga hjälpmedel (no help materials) Del B: Christie J Geankoplis, Transport
EXAMINATION Luleå University of Technology Course: B0004K Course name: Unit Operations Date: 2013-01-14 Time: 9.00 15.00 Aid: Del A: Inga hjälpmedel (no help materials) Del B: Christie J Geankoplis, Transport
Algebra Unpacked Content For the new Common Core standards that will be effective in all North Carolina schools in the 2012-13 school year.
 This document is designed to help North Carolina educators teach the Common Core (Standard Course of Study). NCDPI staff are continually updating and improving these tools to better serve teachers. Algebra
This document is designed to help North Carolina educators teach the Common Core (Standard Course of Study). NCDPI staff are continually updating and improving these tools to better serve teachers. Algebra
Melting Point, Boiling Point, and Index of Refraction
 Melting Point, Boiling Point, and Index of Refraction Melting points, boiling points, and index of refractions are easily measured physical properties of organic compounds useful in product characterization
Melting Point, Boiling Point, and Index of Refraction Melting points, boiling points, and index of refractions are easily measured physical properties of organic compounds useful in product characterization
Answer Key for California State Standards: Algebra I
 Algebra I: Symbolic reasoning and calculations with symbols are central in algebra. Through the study of algebra, a student develops an understanding of the symbolic language of mathematics and the sciences.
Algebra I: Symbolic reasoning and calculations with symbols are central in algebra. Through the study of algebra, a student develops an understanding of the symbolic language of mathematics and the sciences.
Information on the New Royalty Framework
 Alberta Department Energy Information on the New Royalty Framework For the October 30/31, 2008 Training Sessions Gas Royalty Calculation Unit 10/17/2008 1 TABLE OF CONTENTS Section 1 - Summary... 5 Section
Alberta Department Energy Information on the New Royalty Framework For the October 30/31, 2008 Training Sessions Gas Royalty Calculation Unit 10/17/2008 1 TABLE OF CONTENTS Section 1 - Summary... 5 Section
Lecture 2. Marginal Functions, Average Functions, Elasticity, the Marginal Principle, and Constrained Optimization
 Lecture 2. Marginal Functions, Average Functions, Elasticity, the Marginal Principle, and Constrained Optimization 2.1. Introduction Suppose that an economic relationship can be described by a real-valued
Lecture 2. Marginal Functions, Average Functions, Elasticity, the Marginal Principle, and Constrained Optimization 2.1. Introduction Suppose that an economic relationship can be described by a real-valued
Thermodynamics. Thermodynamics (Th)
 Thermodynamics (Th) Thermodynamics Thermodynamics can be used to make the number of necessary measurements smaller, or to make the type of measurement easier. It turns out that volumetric flowrates are
Thermodynamics (Th) Thermodynamics Thermodynamics can be used to make the number of necessary measurements smaller, or to make the type of measurement easier. It turns out that volumetric flowrates are
Physical Chemistry Laboratory I CHEM 445 Experiment 6 Vapor Pressure of a Pure Liquid (Revised, 01/09/06)
 1 Physical Chemistry Laboratory I CHEM 445 Experiment 6 Vapor Pressure of a Pure Liquid (Revised, 01/09/06) The vapor pressure of a pure liquid is an intensive property of the compound. That is, the vapor
1 Physical Chemistry Laboratory I CHEM 445 Experiment 6 Vapor Pressure of a Pure Liquid (Revised, 01/09/06) The vapor pressure of a pure liquid is an intensive property of the compound. That is, the vapor
Note on growth and growth accounting
 CHAPTER 0 Note on growth and growth accounting 1. Growth and the growth rate In this section aspects of the mathematical concept of the rate of growth used in growth models and in the empirical analysis
CHAPTER 0 Note on growth and growth accounting 1. Growth and the growth rate In this section aspects of the mathematical concept of the rate of growth used in growth models and in the empirical analysis
AC 2008-2205: SIMULATION-BASED LEARNING OF DISTILLATION PRINCIPLES IN HISTORICAL CONTEXT: FROM DA VINCI S ALEMBICS TO MODERN APPLICATIONS
 AC 2008-2205: SIMULATION-BASED LEARNING OF DISTILLATION PRINCIPLES IN HISTORICAL CONTEXT: FROM DA VINCI S ALEMBICS TO MODERN APPLICATIONS Yakov Cherner, ATeL, LLC Yakov E. Cherner, Ph.D. a Founder and
AC 2008-2205: SIMULATION-BASED LEARNING OF DISTILLATION PRINCIPLES IN HISTORICAL CONTEXT: FROM DA VINCI S ALEMBICS TO MODERN APPLICATIONS Yakov Cherner, ATeL, LLC Yakov E. Cherner, Ph.D. a Founder and
Solving Rational Equations
 Lesson M Lesson : Student Outcomes Students solve rational equations, monitoring for the creation of extraneous solutions. Lesson Notes In the preceding lessons, students learned to add, subtract, multiply,
Lesson M Lesson : Student Outcomes Students solve rational equations, monitoring for the creation of extraneous solutions. Lesson Notes In the preceding lessons, students learned to add, subtract, multiply,
Liquefied Natural Gas (LNG)
 Graduate Diploma in Petroleum Studies Major in Liquefied Natural Gas (LNG) INDUCTION Launching ceremony Week 39, 2012 Administration / Plant visit / Fundamentals of LNG and LNG main risks awareness Module
Graduate Diploma in Petroleum Studies Major in Liquefied Natural Gas (LNG) INDUCTION Launching ceremony Week 39, 2012 Administration / Plant visit / Fundamentals of LNG and LNG main risks awareness Module
9.2.3.7 Retention Parameters in Column Chromatography
 9.2.3.7 Retention Parameters in Column Chromatography Retention parameters may be measured in terms of chart distances or times, as well as mobile phase volumes; e.g., t R ' (time) is analogous to V R
9.2.3.7 Retention Parameters in Column Chromatography Retention parameters may be measured in terms of chart distances or times, as well as mobile phase volumes; e.g., t R ' (time) is analogous to V R
Polynomial and Rational Functions
 Polynomial and Rational Functions Quadratic Functions Overview of Objectives, students should be able to: 1. Recognize the characteristics of parabolas. 2. Find the intercepts a. x intercepts by solving
Polynomial and Rational Functions Quadratic Functions Overview of Objectives, students should be able to: 1. Recognize the characteristics of parabolas. 2. Find the intercepts a. x intercepts by solving
Refinery Equipment of Texas. Mini - Refinery Feasibility Overview
 Mini - Refinery Feasibility Overview Introduction This paper is intended to provide information, answer questions, and assist the owner or project developer in making informed buying decisions. A mini-refinery
Mini - Refinery Feasibility Overview Introduction This paper is intended to provide information, answer questions, and assist the owner or project developer in making informed buying decisions. A mini-refinery
CH3 Stoichiometry. The violent chemical reaction of bromine and phosphorus. P.76
 CH3 Stoichiometry The violent chemical reaction of bromine and phosphorus. P.76 Contents 3.1 Counting by Weighing 3.2 Atomic Masses 3.3 The Mole 3.4 Molar Mass 3.5 Percent Composition of Compounds 3.6
CH3 Stoichiometry The violent chemical reaction of bromine and phosphorus. P.76 Contents 3.1 Counting by Weighing 3.2 Atomic Masses 3.3 The Mole 3.4 Molar Mass 3.5 Percent Composition of Compounds 3.6
How to Design and Interpret a Multiple-Choice-Question Test: A Probabilistic Approach*
 Int. J. Engng Ed. Vol. 22, No. 6, pp. 1281±1286, 2006 0949-149X/91 $3.00+0.00 Printed in Great Britain. # 2006 TEMPUS Publications. How to Design and Interpret a Multiple-Choice-Question Test: A Probabilistic
Int. J. Engng Ed. Vol. 22, No. 6, pp. 1281±1286, 2006 0949-149X/91 $3.00+0.00 Printed in Great Britain. # 2006 TEMPUS Publications. How to Design and Interpret a Multiple-Choice-Question Test: A Probabilistic
Chemistry 112 Laboratory Experiment 6: The Reaction of Aluminum and Zinc with Hydrochloric Acid
 Chemistry 112 Laboratory Experiment 6: The Reaction of Aluminum and Zinc with Hydrochloric Acid Introduction Many metals react with acids to form hydrogen gas. In this experiment, you will use the reactions
Chemistry 112 Laboratory Experiment 6: The Reaction of Aluminum and Zinc with Hydrochloric Acid Introduction Many metals react with acids to form hydrogen gas. In this experiment, you will use the reactions
Counters and Decoders
 Physics 3330 Experiment #10 Fall 1999 Purpose Counters and Decoders In this experiment, you will design and construct a 4-bit ripple-through decade counter with a decimal read-out display. Such a counter
Physics 3330 Experiment #10 Fall 1999 Purpose Counters and Decoders In this experiment, you will design and construct a 4-bit ripple-through decade counter with a decimal read-out display. Such a counter
SECTION 10-2 Mathematical Induction
 73 0 Sequences and Series 6. Approximate e 0. using the first five terms of the series. Compare this approximation with your calculator evaluation of e 0.. 6. Approximate e 0.5 using the first five terms
73 0 Sequences and Series 6. Approximate e 0. using the first five terms of the series. Compare this approximation with your calculator evaluation of e 0.. 6. Approximate e 0.5 using the first five terms
Feasibility Analysis of Ternary Feed Mixtures of Methane with Oxygen, Steam, and Carbon Dioxide for the Production of Methanol Synthesis Gas
 1410 Ind. Eng. Chem. Res. 1998, 37, 1410-1421 Feasibility Analysis of Ternary Feed Mixtures of Methane with Oxygen, Steam, and Carbon Dioxide for the Production of Methanol Synthesis Gas George J. Tjatjopoulos*,
1410 Ind. Eng. Chem. Res. 1998, 37, 1410-1421 Feasibility Analysis of Ternary Feed Mixtures of Methane with Oxygen, Steam, and Carbon Dioxide for the Production of Methanol Synthesis Gas George J. Tjatjopoulos*,
CHEM 36 General Chemistry EXAM #1 February 13, 2002
 CHEM 36 General Chemistry EXAM #1 February 13, 2002 Name: Serkey, Anne INSTRUCTIONS: Read through the entire exam before you begin. Answer all of the questions. For questions involving calculations, show
CHEM 36 General Chemistry EXAM #1 February 13, 2002 Name: Serkey, Anne INSTRUCTIONS: Read through the entire exam before you begin. Answer all of the questions. For questions involving calculations, show
k 2f, k 2r C 2 H 5 + H C 2 H 6
 hemical Engineering HE 33 F pplied Reaction Kinetics Fall 04 Problem Set 4 Solution Problem. The following elementary steps are proposed for a gas phase reaction: Elementary Steps Rate constants H H f,
hemical Engineering HE 33 F pplied Reaction Kinetics Fall 04 Problem Set 4 Solution Problem. The following elementary steps are proposed for a gas phase reaction: Elementary Steps Rate constants H H f,
