The rigorous calculation of the universal density functional by the Lieb variation principle
|
|
|
- Reynard Ralph Baldwin
- 7 years ago
- Views:
Transcription
1 The rigorous calculation of the universal density functional by the Lieb variation principle Trygve Helgaker, Andy Teale, and Sonia Coriani Centre for Theoretical and Computational Chemistry (CTCC), Department of Chemistry, University of Oslo, Norway Dipartimento di Scienze Chimiche, Università degli Studi di Trieste, Trieste, Italy Quantum Monte Carlo meets Quantum Chemistry: new approaches for electron correlation CECAM-USI, Lugano, Switzerland, June 15 18, 2010 T. Helgaker (CTCC, University of Oslo) Calculation of the universal density functional CECAM 1 / 34
2 Outline 1 Lieb s formulation of density functional theory 2 Lieb maximization 3 The adiabatic connection 4 Dynamical correlation 5 Static correlation 6 Range separation 7 Summary Helgaker et al. (CTCC, University of Oslo) Overview CECAM 2 / 34
3 The universal density functional In 1979, Levy formulated DFT in terms of a constrained-search optimization E λ [v] = inf `Fλ [ρ] + (v ρ) the Hohenberg Kohn variation principle ρ F λ [ρ] = min Ψ T + λw Ψ the Levy variation principle Ψ ρ we have here introduced the interaction-strength parameter λ In 1983, Lieb gave a more symmetric but perhaps less intuitive formulation of DFT: E λ [v] = inf `Fλ [ρ] + (v ρ) the Hohenberg Kohn variation principle ρ F λ [ρ] = sup `Eλ [v] (v ρ) the Lieb variation principle v an unconstrained rather than constrained optimization of F λ [ρ] directly from E λ [v] equivalent to Levy s functional generalized to ensembles In Lieb s theory, E λ [v] and F λ [ρ] are related by Fenchel s inequality: E λ [v] F λ [ρ] + (v ρ) F λ [ρ] E λ [v] (v ρ) both variation principles attempt to sharpen this inequality into an equality these attempts are only successful for ground-state densities and potentials We shall apply the Lieb variation principle to standard ab-initio models of E λ [v] first application of Lieb s method to many-electron and molecular systems previous applications by Colonna and Savin (1999) to few-electron atoms Helgaker et al. (CTCC, University of Oslo) Lieb s universal density functional CECAM 3 / 34
4 Conjugate functionals E[v] F [ρ] The ground-state energy E[v] is concave in v by the Rayleigh Ritz variation principle it can therefore be exactly represented by its convex conjugate F [ρ]: E[v] F [ρ] F Ρ sup v E v v Ρ F Ρ F Ρ min Ρ v F Ρ Ρ min v max E v v Ρ E v E v inf Ρ F Ρ v Ρ E v max v Ρ v Ρ Each variation principle represents a Legendre Fenchel transformation the essential point is the concavity of E[v] rather than the details of the Schrödinger equation we may therefore construct F [v] also for approximate E[v] if these are concave Helgaker et al. (CTCC, University of Oslo) Lieb s universal density functional CECAM 4 / 34
5 The concave envelope E[v] F [ρ] co E[v] However, approximate E[v] may not be concave (not variationally minimized) it still generates a convex F [ρ], conjugate to the concave envelope co E[v] E[v] F Ρ sup v E v v Ρ F Ρ F Ρ Ρ min v max co E v E v v Ρ E v E v inf Ρ F Ρ v Ρ v Ρ The concave envelope co E[v] is the least concave upper bound to E[v] with this caveat, we may introduce all ab-initio levels of theory for E[v] into DFT as E[v] converges to the exact ground-state energy, so does co E[v] Helgaker et al. (CTCC, University of Oslo) Lieb s universal density functional CECAM 5 / 34
6 Lieb s theory for approximate energies Lieb s theory may be applied to any exact or approximate energy that is concave: Fλ mod [ρ] LF Eλ mod [v] examples: the lowest state of any spin symmetry examples: all variationally determined energies such as E HF FCI λ [v] and Eλ [v] However, F mod λ [ρ] encapsulates information only about the concave part of Eλ mod[v] Eλ mod [v] LF Fλ mod [ρ] LF co E mod λ [v] E mod [v] λ Nothing more is needed for approximate ground-state energies such as MP2 and CCSD the approximate energy co E MP2 MP2 λ [v] is just as good as Eλ [v] We cannot hope to represent excited-state energies by a universal density functional the nth excited state E n λ [v] is in general not concave however, the sum of electronic energies up to the nth excited state is concave nx E n λ [v] = E i λ [v] i=0 concave Helgaker et al. (CTCC, University of Oslo) Approximate energies CECAM 6 / 34
7 Lieb maximizations For a given density ρ(r) and chosen level of theory E λ [v], we wish to calculate F λ [ρ] = max v `Eλ [v] (v ρ) by maximizing the right-hand side with respect to variations in the potential v(r) this should be fairly easy given that concave functionals have at most one maximum The potential is parameterized as suggested by Wu and Yang 2003: where the three terms are v c(r) = v ext(r) + (1 λ)v ref (r) + X ct gt(r) t the physical, external potential v ext(r) the Fermi Amaldi (or other) reference potential to ensure correct asymptotic behaviour v ref (r) = 1 N 1 Z ρ(r ) r r dr an expansion in Gaussians g t(r) with coefficients c t we use large orbital basis sets, typically augmented with diffuse functions Implemented levels of theory: HF, MP2, CCD, CCSD, CCSD(T) All code is implemented in DALTON Teale, Coriani and Helgaker, J. Chem. Phys. 130, (2009); ibid. 132, (2010) Helgaker et al. (CTCC, University of Oslo) Lieb maximization CECAM 7 / 34
8 First- and second-order Lieb maximizations We determine v c(r) and hence F λ [ρ] by maximizing with respect to c t the quantity G λ,ρ (c) = E λ [v c] (v c ρ) v c(r) = v ext(r) + (1 λ)v ref (r) + P t ct gt(r) The quasi-newton method requires only the gradient of G λ,ρ (c): G λ,ρ (c) c t = (g t ρ λ,c ρ) here ρ λ,c is the density provided by E λ [v c] our convergence target is a gradient norm smaller than 10 6 convergence in iterations with BFGS update The Newton method requires also the Hessian 2 G λ,ρ (c) c t c u = ZZ g t(r)g u(r ) δρ(r) δv(r dr dr ) convergence in 5 10 iterations with exact, interacting Hessian convergence in 5 15 iterations with approximate, noninteracting Hessian There are no global convergence problems a concave functional such as E λ [v] (v ρ) has at most one maximum the potential v is more difficult to converge than F λ [ρ] in finite basis sets Helgaker et al. (CTCC, University of Oslo) Lieb maximization CECAM 8 / 34
9 The adiabatic connection We are going to consider the dependence of F λ [ρ] on λ: Ψ F λ [ρ] = min Ψ T + λw = Ψρ Ψ ρ λ T + λw Ψρ λ The functional F λ [ρ] increases monotonically with λ in a concave manner: F Λ Ρ W Λ Ρ F 0 Ρ F Λ Ρ F 0 Ρ It can be represented by a monotonically decreasing right-continuous integrand W λ [ρ]: Z λ F λ [ρ] = F 0 [ρ] + W µ[ρ] dµ 0 the integrand W λ [ρ] is discontinuous where F λ [ρ] is nondifferentiable Assuming adiabaticity, the integrand is taken to be continuous: W λ [ρ] = F λ [ρ] = Ψ ρ λ W λ Ψρ AC integrand at each λ, we shall calculate both F λ [ρ] and its derivative F λ [ρ] Helgaker et al. (CTCC, University of Oslo) The adiabatic connection CECAM 9 / 34
10 Computational overview For given E λ [v] and ρ, we calculate the universal functional and its λ derivative: F λ [ρ] = E λ [v max] (v max ρ) Lieb maximization F λ [ρ] = E λ [vmax] first-order property We next perform the Kohn Sham decomposition by introducing F 0 [ρ] and F 0 [ρ]: F 0 [ρ] = T s[ρ] noninteracting kinetic energy F 0 [ρ] = J[ρ] + Ex[ρ] Coulomb and exchange energies F λ [ρ] = T s[ρ] + λj[ρ] + λe x[ρ] + E c,λ [ρ] correlation energy The correlation energy is the only term that depends on λ in a nontrivial manner: F λ [ρ] = J[ρ] + Ex[ρ] + E c,λ [ρ] AC integrand The correlation energy is negative and concave in λ, here illustrated for the neon atom: area Ec Ρ Ec, Λ Ρ dec, Λ Ρ dλ area Tc Ρ 0.35 Helgaker et al. (CTCC, University of Oslo) The adiabatic connection CECAM 10 / 34
11 Basis-set convergence of the AC integrand Helium atom at the FCI/aug-cc-pVXZ levels of theory E XC aug cc pvdz a.u. E XC aug cc pvdz FCI a.u a.u. WΛ Λ Helgaker et al. (CTCC, University of Oslo) Dynamical correlation CECAM 11 / 34
12 Basis-set convergence of the AC integrand Helium atom at the FCI/aug-cc-pVXZ levels of theory E XC aug cc pvtz a.u. E XC aug cc pvtz FCI a.u a.u. WΛ Λ Helgaker et al. (CTCC, University of Oslo) Dynamical correlation CECAM 12 / 34
13 Basis-set convergence of the AC integrand Helium atom at the FCI/aug-cc-pVXZ levels of theory 1.02 E XC aug cc pvqz a.u. E XC aug cc pvqz FCI a.u WΛ Λ Helgaker et al. (CTCC, University of Oslo) Dynamical correlation CECAM 13 / 34
14 Basis-set convergence of the AC integrand Helium atom at the FCI/aug-cc-pVXZ levels of theory 1.02 E XC aug cc pv5z a.u. E XC aug cc pv5z FCI a.u WΛ Λ Helgaker et al. (CTCC, University of Oslo) Dynamical correlation CECAM 14 / 34
15 Basis-set convergence of the AC integrand Helium atom at the FCI/aug-cc-pVXZ levels of theory 1.02 E XC aug cc pv6z a.u. E XC aug cc pv6z FCI a.u WΛ Λ Helgaker et al. (CTCC, University of Oslo) Dynamical correlation CECAM 15 / 34
16 Basis-set convergence of the AC integrand Helium atom at the FCI/aug-cc-pVXZ levels of theory 1.02 E XC Extrap a.u. E XC Extrap FCI a.u WΛ Λ Helgaker et al. (CTCC, University of Oslo) Dynamical correlation CECAM 16 / 34
17 Hartree Fock DFT The HF energy has a tiny correlation contribution ( 2.1 mh for water) the orbitals change with increasing λ but always provide the same density at λ = 0, the kinetic energy is minimized; at λ = 1, the total energy is minimized the associated orbital-relaxation energy is proportional to λ E c Ρ WXC,Λ a.u T c Ρ Λ The AC correlation curve is therefore very nearly a straight line in HF-OEP theory, the energy is 2.2 mh higher Helgaker et al. (CTCC, University of Oslo) Dynamical correlation CECAM 17 / 34
18 The HOMO LUMO gap In HF theory, the wave function is one-determinantal at all 0 λ 1 the KS operator changes smoothly into the Fock operator, reproducing the HF density at all λ: ˆf λ = ˆf KS + λ ˆˆkx v x(r) v c,λ (r) The HOMO LUMO gap increases with increasing λ LUMO Eigenvalue a.u p s p LUMO 1 LUMO HOMO 3s p p s HOMO 1 2s Λ virtual orbitals increase in energy as nonlocal exchange replaces local exchange occupied orbitals decrease in energy because of relaxation Helgaker et al. (CTCC, University of Oslo) Dynamical correlation CECAM 18 / 34
19 The MP2 and CCSD correlation energies The MP2 and CCSD curves of water are not straight but bend slightly upwards the MP2 curve lies below the CCSD curve E c Ρ WXC,Λ a.u T c Ρ Λ A linear curve would reflect a λ 2 dependence of the correlation energy why does the curve bend? Helgaker et al. (CTCC, University of Oslo) Dynamical correlation CECAM 19 / 34
20 Dynamical correlation: doubles contribution Second-order perturbation theory suggests the following model: E MP2 (λ) = P ijab λ 2 ij ab 2 ε a(λ)+ε b (λ) ε i (λ) ε j (λ) λ2 w 2 h+λg = λw λw h+λg quadratic dependence damped by an increasing HOMO LUMO gap We obtain a two-parameter AC model by differentiation and setting w = g we adjust h and g to reproduce exactly initial slope and end point CCSD water WΛ a.u CCSD neon Λ For λ 0, the AC curve becomes horizontal the wave function can no longer adjust: the electrons are as far apart as allowed by the density Helgaker et al. (CTCC, University of Oslo) Dynamical correlation CECAM 20 / 34
21 Dynamical correlation: triples contribution The CCSD(T) curve for water lies slightly below the CCSD curve the difference increases with increasing λ WXC,Λ a.u T c Ρ E c Ρ CCSD W CCSD T Ρ W CCSD Ρ a.u W CCSD T Ρ W CCSD Ρ 9.5 CCSD T Λ Λ An inspection of the triples energy correction suggests the following form E T (λ) = λ3 w 3 (h + λg) 2 E T (λ) = cλ2 + O(λ 3 ) λ 2 an almost perfect fit with a one-parameter quadratic curve for λ 0, the triples AC integrand becomes horizontal Helgaker et al. (CTCC, University of Oslo) Dynamical correlation CECAM 21 / 34
22 From dynamical to static correlation: dissociation of H 2 Static correlation energy arises from (near) degeneracy of electronic configurations it is proportional to the interaction constant λ the corresponding AC curve is therefore horizontal As H 2 dissociates, correlation changes from dynamical to static purely dynamical correlation at equilibrium curvature increases as HOMO LUMO gap decreases with increasing bond length purely static correlation at dissociation bohr bohr bohr We here (and in the following) assume spin-restricted theory Helgaker et al. (CTCC, University of Oslo) Static correlation CECAM 22 / 34
23 A two-parameter model for the H 2 molecule From a two-level CI model, we obtain the ground-state energy E CI (λ) = 1 2 E p 1 2 E 2 + 4w 2 λ 2, E = h + gλ Differentiation gives a simple two-parameter model for the AC integrand with w = g E CI (λ) = 1 2 g g(h + gλ) + 4w 2 λ 2 p (h + gλ) 2 + 4w 2 λ 2 good least-square fits (full lines) and fits to initial gradient and end point (dashed lines) works for both dynamical and static correlation 0.00 Wc,Λ a.u R 0.7 a.u. R 1.4 a.u. R 3.0 a.u R 5.0 a.u. R 7.0 a.u R 10.0 a.u. Λ Helgaker et al. (CTCC, University of Oslo) Static correlation CECAM 23 / 34
24 The design of DFT functionals from AC models There has been much work on modeling AC integrands: Ernzerhof (1996), Burke et al. (1997), Seidl et al. (2000), Mori-Sánchez et al. (2007) In the absence of accurate data, there is little to differentiate the proposed AC forms such data is provided by the Lieb variation principle none of the suggested forms reproduce our data Part of the motivation for studying the AC is to develop new functionals simple forms can be turned directly into functionals by integration (Becke HH) All such AC forms are only as good as their input data we must also provide a recipe for producing input data We shall now consider one such simple model, based on strongly interacting electrons Helgaker et al. (CTCC, University of Oslo) Static correlation CECAM 24 / 34
25 Strongly interacting electrons (λ > 1) The AC curve of the CI model contains two adjustable parameters: E (λ) = 1 2 g g(h+gλ)+4g2 λ 2 (h+gλ), E(1) = R g 2 λ 2 0 E (µ) dµ Initial slope is twice the second-order Görling Levy perturbation theory E (0) = 2E0 GL2 [ρ] Görling Levy theory is an order-by-order expansion in λ for fixed density similar to bare-nucleus perturbation theory for fixed potential The point-charge-plus-continuum (PC) model yields the end point E ( ) = W PC [ρ] developed by Seidl, Perdew and Kurth (2000) for strictly correlated electrons λ = used in the interaction-strength-interpolation (ISI) model of the AC integrand Fixing h and g to reproduce 2E0 GL2 [ρ] and W PC [ρ], we obtain the dashed AC curves below Helgaker et al. (CTCC, University of Oslo) Static correlation CECAM 25 / 34
26 Attractive electrons (λ < 0) Attractive electrons have recently been considered by Seidl and Gori-Giorgi (2010) we have calculated AC curves for the physical H 2 density in the range 1 λ R 10.0 a.u WΛ W0 a.u Λ Repulsive electrons (λ > 0): all AC curves become horizontal as the electrons become strictly correlated (for fixed density) covalent H 2 density Attractive electrons (λ < 0): all AC curves become equally sloped as the electrons move together (for fixed density) ionic H 2 density Helgaker et al. (CTCC, University of Oslo) Static correlation CECAM 26 / 34
27 Benchmarking explicit exchange correlation functionals Accurate studies of F λ [ρ] gives information about the exact functional this information may be used to benchmark approximate functionals example: comparison of the BLYP functional with the exact one Dissociation of H 2 at the RHF, BLYP and FCI levels of theory the BLYP functional improves considerably on HF theory for dissociation what is the reason for this improvement? HF BLYP FCI Helgaker et al. (CTCC, University of Oslo) Static correlation CECAM 27 / 34
28 BLYP and FCI correlation curves for H 2 The BLYP functional treats correlation as dynamical at all bond distances it was designed for spin-unrestricted theory but is used here in a spin-restricted manner it hence ignores static correlation BLYP FCI BLYP FCI R 1.4 bohr R 3.0 bohr BLYP BLYP R 5.0 bohr FCI R 10.0 bohr FCI Helgaker et al. (CTCC, University of Oslo) Static correlation CECAM 28 / 34
29 BLYP and FCI exchange correlation curves for H 2 The improved BLYP performance arises from an overestimation of exchange error cancellation between exchange and correlation reduces total error to about one third R 1.4 bohr R 3.0 bohr FCI exchangehf BLYP BLYP exchange FCI BLYP correlation HF BLYP FCI FCI correlation R 5.0 bohr R 10.0 bohr Helgaker et al. (CTCC, University of Oslo) Static correlation CECAM 29 / 34
30 Generalized, range-dependent adiabatic connection Up to now, we have studied the AC with a uniformly scaled two-electron interaction We shall now consider range-dependent adiabatic interactions λ erf w g λ (r 1 λ r ij ij ) = 2 «λ exp 1 «λ 2 «r 2 r ij π ij 1 λ 3 1 λ Λ Λ Λ Λ As λ increases, we build up the full interaction from the outer to inner region this provides an alternative view of correlation such ACs are of particular relevance for range-dependent methods Savin et al. 1996, Yang 1998, Toulouse et al Helgaker et al. (CTCC, University of Oslo) Range separation CECAM 30 / 34
31 AC correlation curves for the He isoelectronic series Curves reveal increasing compactness with increasing Z Z Z Z Z Note: long-range interactions to the left; short-range interactions to the right Helgaker et al. (CTCC, University of Oslo) Range separation CECAM 31 / 34
32 Range separation: dissociation of H 2 We consider first the total AC curve, including Coulomb, exchange and correlation it moves towards small λ values with increasing separation at full separation, all total interactions are interatomic bohr bohr bohr bohr Note: long-range interactions to the left; short-range interactions to the right Helgaker et al. (CTCC, University of Oslo) Range separation CECAM 32 / 34
33 Range separation: dissociation of H 2 We consider next the correlation-only AC curve at short bond distance, the interactions are predominantly short-ranged at long distances, short- and long-ranged interactions partially cancel bohr bohr bohr bohr Note: long-range interactions to the left; short-range interactions to the right Helgaker et al. (CTCC, University of Oslo) Range separation CECAM 33 / 34
34 Summary and acknowledgments We have calculated the universal density functional by Lieb maximization Newton and quasi-newton methods standard levels of theory: HF, MP2, CCSD, CCSD(T), BLYP many-electron atoms and molecules We have presented accurate adiabatic-connection curves and discussed their shape dynamical and static correlation accurate modeling of AC curves repulsive and attractive electrons benchmarking of approximate functionals range dependence An interesting project is to study the current dependence of F [ρ, j] we have code for gauge-origin-invariant calculations of E[v, A] in finite magnetic fields a generalized Legendre Fenchel transform will provide F [ρ, j] with charge and current densities this will help in benchmarking and developing approximate E xc[ρ, j] We would like to thank Andreas Savin and Paola Gori-Giorgi for discussions This work was supported by the Norwegian Research Council through Grant No (A.M.T) Grant No /V30 Centre for Theoretical and Computational Chemistry (CTCC) Helgaker et al. (CTCC, University of Oslo) Summary and acknowledgments CECAM 34 / 34
Lecture 5 Principal Minors and the Hessian
 Lecture 5 Principal Minors and the Hessian Eivind Eriksen BI Norwegian School of Management Department of Economics October 01, 2010 Eivind Eriksen (BI Dept of Economics) Lecture 5 Principal Minors and
Lecture 5 Principal Minors and the Hessian Eivind Eriksen BI Norwegian School of Management Department of Economics October 01, 2010 Eivind Eriksen (BI Dept of Economics) Lecture 5 Principal Minors and
5.61 Physical Chemistry 25 Helium Atom page 1 HELIUM ATOM
 5.6 Physical Chemistry 5 Helium Atom page HELIUM ATOM Now that we have treated the Hydrogen like atoms in some detail, we now proceed to discuss the next simplest system: the Helium atom. In this situation,
5.6 Physical Chemistry 5 Helium Atom page HELIUM ATOM Now that we have treated the Hydrogen like atoms in some detail, we now proceed to discuss the next simplest system: the Helium atom. In this situation,
Electric Dipole moments as probes of physics beyond the Standard Model
 Electric Dipole moments as probes of physics beyond the Standard Model K. V. P. Latha Non-Accelerator Particle Physics Group Indian Institute of Astrophysics Plan of the Talk Parity (P) and Time-reversal
Electric Dipole moments as probes of physics beyond the Standard Model K. V. P. Latha Non-Accelerator Particle Physics Group Indian Institute of Astrophysics Plan of the Talk Parity (P) and Time-reversal
An Introduction to Hartree-Fock Molecular Orbital Theory
 An Introduction to Hartree-Fock Molecular Orbital Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology June 2000 1 Introduction Hartree-Fock theory is fundamental
An Introduction to Hartree-Fock Molecular Orbital Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology June 2000 1 Introduction Hartree-Fock theory is fundamental
Lesson 3. Chemical Bonding. Molecular Orbital Theory
 Lesson 3 Chemical Bonding Molecular Orbital Theory 1 Why Do Bonds Form? An energy diagram shows that a bond forms between two atoms if the overall energy of the system is lowered when the two atoms approach
Lesson 3 Chemical Bonding Molecular Orbital Theory 1 Why Do Bonds Form? An energy diagram shows that a bond forms between two atoms if the overall energy of the system is lowered when the two atoms approach
Free Electron Fermi Gas (Kittel Ch. 6)
 Free Electron Fermi Gas (Kittel Ch. 6) Role of Electrons in Solids Electrons are responsible for binding of crystals -- they are the glue that hold the nuclei together Types of binding (see next slide)
Free Electron Fermi Gas (Kittel Ch. 6) Role of Electrons in Solids Electrons are responsible for binding of crystals -- they are the glue that hold the nuclei together Types of binding (see next slide)
Implicit and Explicit Coverage of Multi-reference Effects by Density Functional Theory
 Int. J. Mol. Sci. 2002, 3, 604-638 International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.org/ijms/ Implicit and Explicit Coverage of Multi-reference Effects by Density Functional Theory Dieter
Int. J. Mol. Sci. 2002, 3, 604-638 International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.org/ijms/ Implicit and Explicit Coverage of Multi-reference Effects by Density Functional Theory Dieter
Section 3: Crystal Binding
 Physics 97 Interatomic forces Section 3: rystal Binding Solids are stable structures, and therefore there exist interactions holding atoms in a crystal together. For example a crystal of sodium chloride
Physics 97 Interatomic forces Section 3: rystal Binding Solids are stable structures, and therefore there exist interactions holding atoms in a crystal together. For example a crystal of sodium chloride
CHEM6085: Density Functional Theory Lecture 2. Hamiltonian operators for molecules
 CHEM6085: Density Functional Theory Lecture 2 Hamiltonian operators for molecules C.-K. Skylaris 1 The (time-independent) Schrödinger equation is an eigenvalue equation operator for property A eigenfunction
CHEM6085: Density Functional Theory Lecture 2 Hamiltonian operators for molecules C.-K. Skylaris 1 The (time-independent) Schrödinger equation is an eigenvalue equation operator for property A eigenfunction
Q-Chem: Quantum Chemistry Software for Large Systems. Peter M.W. Gill. Q-Chem, Inc. Four Triangle Drive Export, PA 15632, USA. and
 Q-Chem: Quantum Chemistry Software for Large Systems Peter M.W. Gill Q-Chem, Inc. Four Triangle Drive Export, PA 15632, USA and Department of Chemistry University of Cambridge Cambridge, CB2 1EW, England
Q-Chem: Quantum Chemistry Software for Large Systems Peter M.W. Gill Q-Chem, Inc. Four Triangle Drive Export, PA 15632, USA and Department of Chemistry University of Cambridge Cambridge, CB2 1EW, England
Duality in General Programs. Ryan Tibshirani Convex Optimization 10-725/36-725
 Duality in General Programs Ryan Tibshirani Convex Optimization 10-725/36-725 1 Last time: duality in linear programs Given c R n, A R m n, b R m, G R r n, h R r : min x R n c T x max u R m, v R r b T
Duality in General Programs Ryan Tibshirani Convex Optimization 10-725/36-725 1 Last time: duality in linear programs Given c R n, A R m n, b R m, G R r n, h R r : min x R n c T x max u R m, v R r b T
Several Views of Support Vector Machines
 Several Views of Support Vector Machines Ryan M. Rifkin Honda Research Institute USA, Inc. Human Intention Understanding Group 2007 Tikhonov Regularization We are considering algorithms of the form min
Several Views of Support Vector Machines Ryan M. Rifkin Honda Research Institute USA, Inc. Human Intention Understanding Group 2007 Tikhonov Regularization We are considering algorithms of the form min
Computer lab: Density functional perturbation theory. theory for lattice dynamics
 Computer lab: density functional perturbation theory for lattice dynamics SISSA and DEMOCRITOS Trieste (Italy) Outline 1 The dynamical matrix 2 3 4 5 Dynamical matrix We want to write a small computer
Computer lab: density functional perturbation theory for lattice dynamics SISSA and DEMOCRITOS Trieste (Italy) Outline 1 The dynamical matrix 2 3 4 5 Dynamical matrix We want to write a small computer
Nonlinear Optimization: Algorithms 3: Interior-point methods
 Nonlinear Optimization: Algorithms 3: Interior-point methods INSEAD, Spring 2006 Jean-Philippe Vert Ecole des Mines de Paris Jean-Philippe.Vert@mines.org Nonlinear optimization c 2006 Jean-Philippe Vert,
Nonlinear Optimization: Algorithms 3: Interior-point methods INSEAD, Spring 2006 Jean-Philippe Vert Ecole des Mines de Paris Jean-Philippe.Vert@mines.org Nonlinear optimization c 2006 Jean-Philippe Vert,
1. Degenerate Pressure
 . Degenerate Pressure We next consider a Fermion gas in quite a different context: the interior of a white dwarf star. Like other stars, white dwarfs have fully ionized plasma interiors. The positively
. Degenerate Pressure We next consider a Fermion gas in quite a different context: the interior of a white dwarf star. Like other stars, white dwarfs have fully ionized plasma interiors. The positively
8. Average product reaches a maximum when labor equals A) 100 B) 200 C) 300 D) 400
 Ch. 6 1. The production function represents A) the quantity of inputs necessary to produce a given level of output. B) the various recipes for producing a given level of output. C) the minimum amounts
Ch. 6 1. The production function represents A) the quantity of inputs necessary to produce a given level of output. B) the various recipes for producing a given level of output. C) the minimum amounts
Mulliken suggested to split the shared density 50:50. Then the electrons associated with the atom k are given by:
 1 17. Population Analysis Population analysis is the study of charge distribution within molecules. The intention is to accurately model partial charge magnitude and location within a molecule. This can
1 17. Population Analysis Population analysis is the study of charge distribution within molecules. The intention is to accurately model partial charge magnitude and location within a molecule. This can
NMR and IR spectra & vibrational analysis
 Lab 5: NMR and IR spectra & vibrational analysis A brief theoretical background 1 Some of the available chemical quantum methods for calculating NMR chemical shifts are based on the Hartree-Fock self-consistent
Lab 5: NMR and IR spectra & vibrational analysis A brief theoretical background 1 Some of the available chemical quantum methods for calculating NMR chemical shifts are based on the Hartree-Fock self-consistent
Patrick S. Vogt, Institut für physikalische Chemie der Universität Basel 1. An introduction to Molpro 2000 by Patrick S. Vogt 21.08.
 Patrick S. Vogt, Institut für physikalische Chemie der Universität Basel 1 An introduction to Molpro 2000 by Patrick S. Vogt 21.08.2000 Patrick S. Vogt, Institut für physikalische Chemie der Universität
Patrick S. Vogt, Institut für physikalische Chemie der Universität Basel 1 An introduction to Molpro 2000 by Patrick S. Vogt 21.08.2000 Patrick S. Vogt, Institut für physikalische Chemie der Universität
Multi-variable Calculus and Optimization
 Multi-variable Calculus and Optimization Dudley Cooke Trinity College Dublin Dudley Cooke (Trinity College Dublin) Multi-variable Calculus and Optimization 1 / 51 EC2040 Topic 3 - Multi-variable Calculus
Multi-variable Calculus and Optimization Dudley Cooke Trinity College Dublin Dudley Cooke (Trinity College Dublin) Multi-variable Calculus and Optimization 1 / 51 EC2040 Topic 3 - Multi-variable Calculus
CHAPTER 26 ELECTROSTATIC ENERGY AND CAPACITORS
 CHAPTER 6 ELECTROSTATIC ENERGY AND CAPACITORS. Three point charges, each of +q, are moved from infinity to the vertices of an equilateral triangle of side l. How much work is required? The sentence preceding
CHAPTER 6 ELECTROSTATIC ENERGY AND CAPACITORS. Three point charges, each of +q, are moved from infinity to the vertices of an equilateral triangle of side l. How much work is required? The sentence preceding
FLAP P11.2 The quantum harmonic oscillator
 F L E X I B L E L E A R N I N G A P P R O A C H T O P H Y S I C S Module P. Opening items. Module introduction. Fast track questions.3 Ready to study? The harmonic oscillator. Classical description of
F L E X I B L E L E A R N I N G A P P R O A C H T O P H Y S I C S Module P. Opening items. Module introduction. Fast track questions.3 Ready to study? The harmonic oscillator. Classical description of
Basis Sets in Quantum Chemistry C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology
 Basis Sets in Quantum Chemistry C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Basis Sets Generically, a basis set is a collection of vectors which spans (defines)
Basis Sets in Quantum Chemistry C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Basis Sets Generically, a basis set is a collection of vectors which spans (defines)
= N 2 = 3π2 n = k 3 F. The kinetic energy of the uniform system is given by: 4πk 2 dk h2 k 2 2m. (2π) 3 0
 Chapter 1 Thomas-Fermi Theory The Thomas-Fermi theory provides a functional form for the kinetic energy of a non-interacting electron gas in some known external potential V (r) (usually due to impurities)
Chapter 1 Thomas-Fermi Theory The Thomas-Fermi theory provides a functional form for the kinetic energy of a non-interacting electron gas in some known external potential V (r) (usually due to impurities)
CHAPTER 9 ATOMIC STRUCTURE AND THE PERIODIC LAW
 CHAPTER 9 ATOMIC STRUCTURE AND THE PERIODIC LAW Quantum mechanics can account for the periodic structure of the elements, by any measure a major conceptual accomplishment for any theory. Although accurate
CHAPTER 9 ATOMIC STRUCTURE AND THE PERIODIC LAW Quantum mechanics can account for the periodic structure of the elements, by any measure a major conceptual accomplishment for any theory. Although accurate
Orbits of the Lennard-Jones Potential
 Orbits of the Lennard-Jones Potential Prashanth S. Venkataram July 28, 2012 1 Introduction The Lennard-Jones potential describes weak interactions between neutral atoms and molecules. Unlike the potentials
Orbits of the Lennard-Jones Potential Prashanth S. Venkataram July 28, 2012 1 Introduction The Lennard-Jones potential describes weak interactions between neutral atoms and molecules. Unlike the potentials
3) Of the following, radiation has the shortest wavelength. A) X-ray B) radio C) microwave D) ultraviolet E) infrared Answer: A
 1) Which one of the following is correct? A) ν + λ = c B) ν λ = c C) ν = cλ D) λ = c ν E) νλ = c Answer: E 2) The wavelength of light emitted from a traffic light having a frequency of 5.75 1014 Hz is.
1) Which one of the following is correct? A) ν + λ = c B) ν λ = c C) ν = cλ D) λ = c ν E) νλ = c Answer: E 2) The wavelength of light emitted from a traffic light having a frequency of 5.75 1014 Hz is.
Lecture 3: Linear methods for classification
 Lecture 3: Linear methods for classification Rafael A. Irizarry and Hector Corrada Bravo February, 2010 Today we describe four specific algorithms useful for classification problems: linear regression,
Lecture 3: Linear methods for classification Rafael A. Irizarry and Hector Corrada Bravo February, 2010 Today we describe four specific algorithms useful for classification problems: linear regression,
TOPIC 4: DERIVATIVES
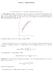 TOPIC 4: DERIVATIVES 1. The derivative of a function. Differentiation rules 1.1. The slope of a curve. The slope of a curve at a point P is a measure of the steepness of the curve. If Q is a point on the
TOPIC 4: DERIVATIVES 1. The derivative of a function. Differentiation rules 1.1. The slope of a curve. The slope of a curve at a point P is a measure of the steepness of the curve. If Q is a point on the
Multi-electron atoms
 Multi-electron atoms Today: Using hydrogen as a model. The Periodic Table HWK 13 available online. Please fill out the online participation survey. Worth 10points on HWK 13. Final Exam is Monday, Dec.
Multi-electron atoms Today: Using hydrogen as a model. The Periodic Table HWK 13 available online. Please fill out the online participation survey. Worth 10points on HWK 13. Final Exam is Monday, Dec.
Potential Energy Surfaces C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology
 Potential Energy Surfaces C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Potential Energy Surfaces A potential energy surface is a mathematical function that gives
Potential Energy Surfaces C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Potential Energy Surfaces A potential energy surface is a mathematical function that gives
The Limited Expansion of Diatomic Overlap Density Functional Theory (LEDO-DFT):
 The Limited Expansion of Diatomic Overlap Density Functional Theory (LEDO-DFT): Development and Implementation of Algorithms, Optimization of Auxiliary Orbitals and Benchmark Calculations Den Naturwissenschaftlichen
The Limited Expansion of Diatomic Overlap Density Functional Theory (LEDO-DFT): Development and Implementation of Algorithms, Optimization of Auxiliary Orbitals and Benchmark Calculations Den Naturwissenschaftlichen
A pure covalent bond is an equal sharing of shared electron pair(s) in a bond. A polar covalent bond is an unequal sharing.
 CHAPTER EIGHT BNDING: GENERAL CNCEPT or Review 1. Electronegativity is the ability of an atom in a molecule to attract electrons to itself. Electronegativity is a bonding term. Electron affinity is the
CHAPTER EIGHT BNDING: GENERAL CNCEPT or Review 1. Electronegativity is the ability of an atom in a molecule to attract electrons to itself. Electronegativity is a bonding term. Electron affinity is the
Envelope Theorem. Kevin Wainwright. Mar 22, 2004
 Envelope Theorem Kevin Wainwright Mar 22, 2004 1 Maximum Value Functions A maximum (or minimum) value function is an objective function where the choice variables have been assigned their optimal values.
Envelope Theorem Kevin Wainwright Mar 22, 2004 1 Maximum Value Functions A maximum (or minimum) value function is an objective function where the choice variables have been assigned their optimal values.
The Cobb-Douglas Production Function
 171 10 The Cobb-Douglas Production Function This chapter describes in detail the most famous of all production functions used to represent production processes both in and out of agriculture. First used
171 10 The Cobb-Douglas Production Function This chapter describes in detail the most famous of all production functions used to represent production processes both in and out of agriculture. First used
Study the following diagrams of the States of Matter. Label the names of the Changes of State between the different states.
 Describe the strength of attractive forces between particles. Describe the amount of space between particles. Can the particles in this state be compressed? Do the particles in this state have a definite
Describe the strength of attractive forces between particles. Describe the amount of space between particles. Can the particles in this state be compressed? Do the particles in this state have a definite
Graduate Macro Theory II: Notes on Investment
 Graduate Macro Theory II: Notes on Investment Eric Sims University of Notre Dame Spring 2011 1 Introduction These notes introduce and discuss modern theories of firm investment. While much of this is done
Graduate Macro Theory II: Notes on Investment Eric Sims University of Notre Dame Spring 2011 1 Introduction These notes introduce and discuss modern theories of firm investment. While much of this is done
0.1 Phase Estimation Technique
 Phase Estimation In this lecture we will describe Kitaev s phase estimation algorithm, and use it to obtain an alternate derivation of a quantum factoring algorithm We will also use this technique to design
Phase Estimation In this lecture we will describe Kitaev s phase estimation algorithm, and use it to obtain an alternate derivation of a quantum factoring algorithm We will also use this technique to design
1 Error in Euler s Method
 1 Error in Euler s Method Experience with Euler s 1 method raises some interesting questions about numerical approximations for the solutions of differential equations. 1. What determines the amount of
1 Error in Euler s Method Experience with Euler s 1 method raises some interesting questions about numerical approximations for the solutions of differential equations. 1. What determines the amount of
Linear Threshold Units
 Linear Threshold Units w x hx (... w n x n w We assume that each feature x j and each weight w j is a real number (we will relax this later) We will study three different algorithms for learning linear
Linear Threshold Units w x hx (... w n x n w We assume that each feature x j and each weight w j is a real number (we will relax this later) We will study three different algorithms for learning linear
Section 5 Molecular Electronic Spectroscopy (lecture 9 ish)
 Section 5 Molecular Electronic Spectroscopy (lecture 9 ish) Previously: Quantum theory of atoms / molecules Quantum Mechanics Vl Valence Molecular Electronic Spectroscopy Classification of electronic states
Section 5 Molecular Electronic Spectroscopy (lecture 9 ish) Previously: Quantum theory of atoms / molecules Quantum Mechanics Vl Valence Molecular Electronic Spectroscopy Classification of electronic states
1 The EOQ and Extensions
 IEOR4000: Production Management Lecture 2 Professor Guillermo Gallego September 9, 2004 Lecture Plan 1. The EOQ and Extensions 2. Multi-Item EOQ Model 1 The EOQ and Extensions This section is devoted to
IEOR4000: Production Management Lecture 2 Professor Guillermo Gallego September 9, 2004 Lecture Plan 1. The EOQ and Extensions 2. Multi-Item EOQ Model 1 The EOQ and Extensions This section is devoted to
Determining the Structure of an Organic Compound
 Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants In the 19 th and early 20 th
Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants In the 19 th and early 20 th
SECOND DERIVATIVE TEST FOR CONSTRAINED EXTREMA
 SECOND DERIVATIVE TEST FOR CONSTRAINED EXTREMA This handout presents the second derivative test for a local extrema of a Lagrange multiplier problem. The Section 1 presents a geometric motivation for the
SECOND DERIVATIVE TEST FOR CONSTRAINED EXTREMA This handout presents the second derivative test for a local extrema of a Lagrange multiplier problem. The Section 1 presents a geometric motivation for the
U = x 1 2. 1 x 1 4. 2 x 1 4. What are the equilibrium relative prices of the three goods? traders has members who are best off?
 Chapter 7 General Equilibrium Exercise 7. Suppose there are 00 traders in a market all of whom behave as price takers. Suppose there are three goods and the traders own initially the following quantities:
Chapter 7 General Equilibrium Exercise 7. Suppose there are 00 traders in a market all of whom behave as price takers. Suppose there are three goods and the traders own initially the following quantities:
Pathways of Li + ion mobility in superionic perovskites by ab initio simulations
 Pathways of Li + ion mobility in superionic perovskites by ab initio simulations Michele Catti Dipartimento di Scienza dei Materiali, Università di Milano Bicocca, Milano, Italy (catti@mater.unimib.it)
Pathways of Li + ion mobility in superionic perovskites by ab initio simulations Michele Catti Dipartimento di Scienza dei Materiali, Università di Milano Bicocca, Milano, Italy (catti@mater.unimib.it)
CHAPTER 24 GAUSS S LAW
 CHAPTER 4 GAUSS S LAW 4. The net charge shown in Fig. 4-40 is Q. Identify each of the charges A, B, C shown. A B C FIGURE 4-40 4. From the direction of the lines of force (away from positive and toward
CHAPTER 4 GAUSS S LAW 4. The net charge shown in Fig. 4-40 is Q. Identify each of the charges A, B, C shown. A B C FIGURE 4-40 4. From the direction of the lines of force (away from positive and toward
PHY4604 Introduction to Quantum Mechanics Fall 2004 Practice Test 3 November 22, 2004
 PHY464 Introduction to Quantum Mechanics Fall 4 Practice Test 3 November, 4 These problems are similar but not identical to the actual test. One or two parts will actually show up.. Short answer. (a) Recall
PHY464 Introduction to Quantum Mechanics Fall 4 Practice Test 3 November, 4 These problems are similar but not identical to the actual test. One or two parts will actually show up.. Short answer. (a) Recall
SENSITIVITY ANALYSIS AND INFERENCE. Lecture 12
 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
Group Theory and Chemistry
 Group Theory and Chemistry Outline: Raman and infra-red spectroscopy Symmetry operations Point Groups and Schoenflies symbols Function space and matrix representation Reducible and irreducible representation
Group Theory and Chemistry Outline: Raman and infra-red spectroscopy Symmetry operations Point Groups and Schoenflies symbols Function space and matrix representation Reducible and irreducible representation
Chapter 27: Taxation. 27.1: Introduction. 27.2: The Two Prices with a Tax. 27.2: The Pre-Tax Position
 Chapter 27: Taxation 27.1: Introduction We consider the effect of taxation on some good on the market for that good. We ask the questions: who pays the tax? what effect does it have on the equilibrium
Chapter 27: Taxation 27.1: Introduction We consider the effect of taxation on some good on the market for that good. We ask the questions: who pays the tax? what effect does it have on the equilibrium
1 Variational calculation of a 1D bound state
 TEORETISK FYSIK, KTH TENTAMEN I KVANTMEKANIK FÖRDJUPNINGSKURS EXAMINATION IN ADVANCED QUANTUM MECHAN- ICS Kvantmekanik fördjupningskurs SI38 för F4 Thursday December, 7, 8. 13. Write on each page: Name,
TEORETISK FYSIK, KTH TENTAMEN I KVANTMEKANIK FÖRDJUPNINGSKURS EXAMINATION IN ADVANCED QUANTUM MECHAN- ICS Kvantmekanik fördjupningskurs SI38 för F4 Thursday December, 7, 8. 13. Write on each page: Name,
A Detailed Price Discrimination Example
 A Detailed Price Discrimination Example Suppose that there are two different types of customers for a monopolist s product. Customers of type 1 have demand curves as follows. These demand curves include
A Detailed Price Discrimination Example Suppose that there are two different types of customers for a monopolist s product. Customers of type 1 have demand curves as follows. These demand curves include
or, put slightly differently, the profit maximizing condition is for marginal revenue to equal marginal cost:
 Chapter 9 Lecture Notes 1 Economics 35: Intermediate Microeconomics Notes and Sample Questions Chapter 9: Profit Maximization Profit Maximization The basic assumption here is that firms are profit maximizing.
Chapter 9 Lecture Notes 1 Economics 35: Intermediate Microeconomics Notes and Sample Questions Chapter 9: Profit Maximization Profit Maximization The basic assumption here is that firms are profit maximizing.
Heidi H. Falden, Kasper R. Falster-Hansen, Keld L. Bak, Sten Rettrup, and Stephan P. A. Sauer*,
 J. Phys. Chem. A 2009, 113, 11995 12012 11995 Benchmarking Second Order Methods for the Calculation of Vertical Electronic Excitation Energies: Valence and Rydberg States in Polycyclic Aromatic Hydrocarbons
J. Phys. Chem. A 2009, 113, 11995 12012 11995 Benchmarking Second Order Methods for the Calculation of Vertical Electronic Excitation Energies: Valence and Rydberg States in Polycyclic Aromatic Hydrocarbons
TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES. PHYS 3650, Exam 2 Section 1 Version 1 October 31, 2005 Total Weight: 100 points
 TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES PHYS 3650, Exam 2 Section 1 Version 1 October 31, 2005 Total Weight: 100 points 1. Check your examination for completeness prior to starting.
TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES PHYS 3650, Exam 2 Section 1 Version 1 October 31, 2005 Total Weight: 100 points 1. Check your examination for completeness prior to starting.
The Application of Density Functional Theory in Materials Science
 The Application of Density Functional Theory in Materials Science Slide 1 Outline Atomistic Modelling Group at MUL Density Functional Theory Numerical Details HPC Cluster at the MU Leoben Applications
The Application of Density Functional Theory in Materials Science Slide 1 Outline Atomistic Modelling Group at MUL Density Functional Theory Numerical Details HPC Cluster at the MU Leoben Applications
The Steepest Descent Algorithm for Unconstrained Optimization and a Bisection Line-search Method
 The Steepest Descent Algorithm for Unconstrained Optimization and a Bisection Line-search Method Robert M. Freund February, 004 004 Massachusetts Institute of Technology. 1 1 The Algorithm The problem
The Steepest Descent Algorithm for Unconstrained Optimization and a Bisection Line-search Method Robert M. Freund February, 004 004 Massachusetts Institute of Technology. 1 1 The Algorithm The problem
Chemistry Workbook 2: Problems For Exam 2
 Chem 1A Dr. White Updated /5/1 1 Chemistry Workbook 2: Problems For Exam 2 Section 2-1: Covalent Bonding 1. On a potential energy diagram, the most stable state has the highest/lowest potential energy.
Chem 1A Dr. White Updated /5/1 1 Chemistry Workbook 2: Problems For Exam 2 Section 2-1: Covalent Bonding 1. On a potential energy diagram, the most stable state has the highest/lowest potential energy.
LCAO-MO Correlation Diagrams
 LCAO-MO Correlation Diagrams (Linear Combination of Atomic Orbitals to yield Molecular Orbitals) For (Second Row) Homonuclear Diatomic Molecules (X 2 ) - the following LCAO-MO s are generated: LCAO MO
LCAO-MO Correlation Diagrams (Linear Combination of Atomic Orbitals to yield Molecular Orbitals) For (Second Row) Homonuclear Diatomic Molecules (X 2 ) - the following LCAO-MO s are generated: LCAO MO
3. Reaction Diffusion Equations Consider the following ODE model for population growth
 3. Reaction Diffusion Equations Consider the following ODE model for population growth u t a u t u t, u 0 u 0 where u t denotes the population size at time t, and a u plays the role of the population dependent
3. Reaction Diffusion Equations Consider the following ODE model for population growth u t a u t u t, u 0 u 0 where u t denotes the population size at time t, and a u plays the role of the population dependent
Infrared Spectroscopy: Theory
 u Chapter 15 Infrared Spectroscopy: Theory An important tool of the organic chemist is Infrared Spectroscopy, or IR. IR spectra are acquired on a special instrument, called an IR spectrometer. IR is used
u Chapter 15 Infrared Spectroscopy: Theory An important tool of the organic chemist is Infrared Spectroscopy, or IR. IR spectra are acquired on a special instrument, called an IR spectrometer. IR is used
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts
 AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
Chapter 2. Atomic Structure and Interatomic Bonding
 Chapter 2. Atomic Structure and Interatomic Bonding Interatomic Bonding Bonding forces and energies Primary interatomic bonds Secondary bonding Molecules Bonding Forces and Energies Considering the interaction
Chapter 2. Atomic Structure and Interatomic Bonding Interatomic Bonding Bonding forces and energies Primary interatomic bonds Secondary bonding Molecules Bonding Forces and Energies Considering the interaction
Electron Arrangements
 Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
Section 3.4 Electron Arrangements Objectives Express the arrangement of electrons in atoms using electron configurations and Lewis valence electron dot structures New Vocabulary Heisenberg uncertainty
Numerical Methods for Differential Equations
 Numerical Methods for Differential Equations Chapter 1: Initial value problems in ODEs Gustaf Söderlind and Carmen Arévalo Numerical Analysis, Lund University Textbooks: A First Course in the Numerical
Numerical Methods for Differential Equations Chapter 1: Initial value problems in ODEs Gustaf Söderlind and Carmen Arévalo Numerical Analysis, Lund University Textbooks: A First Course in the Numerical
3/5/2014. iclicker Participation Question: A. MgS < AlP < NaCl B. MgS < NaCl < AlP C. NaCl < AlP < MgS D. NaCl < MgS < AlP
 Today: Ionic Bonding vs. Covalent Bonding Strengths of Covalent Bonds: Bond Energy Diagrams Bond Polarities: Nonpolar Covalent vs. Polar Covalent vs. Ionic Electronegativity Differences Dipole Moments
Today: Ionic Bonding vs. Covalent Bonding Strengths of Covalent Bonds: Bond Energy Diagrams Bond Polarities: Nonpolar Covalent vs. Polar Covalent vs. Ionic Electronegativity Differences Dipole Moments
Lecture 2: The SVM classifier
 Lecture 2: The SVM classifier C19 Machine Learning Hilary 2015 A. Zisserman Review of linear classifiers Linear separability Perceptron Support Vector Machine (SVM) classifier Wide margin Cost function
Lecture 2: The SVM classifier C19 Machine Learning Hilary 2015 A. Zisserman Review of linear classifiers Linear separability Perceptron Support Vector Machine (SVM) classifier Wide margin Cost function
Bohr Model Calculations for Atoms and Ions
 Bohr Model Calculations for Atoms and Ions Frank Riou Department of Chemistry College of St. nedict St. Johnʹs University St. Joseph, MN 56374 Abstract A debroglie Bohr model is described that can be used
Bohr Model Calculations for Atoms and Ions Frank Riou Department of Chemistry College of St. nedict St. Johnʹs University St. Joseph, MN 56374 Abstract A debroglie Bohr model is described that can be used
3. Convex functions. basic properties and examples. operations that preserve convexity. the conjugate function. quasiconvex functions
 3. Convex functions Convex Optimization Boyd & Vandenberghe basic properties and examples operations that preserve convexity the conjugate function quasiconvex functions log-concave and log-convex functions
3. Convex functions Convex Optimization Boyd & Vandenberghe basic properties and examples operations that preserve convexity the conjugate function quasiconvex functions log-concave and log-convex functions
Cost Minimization and the Cost Function
 Cost Minimization and the Cost Function Juan Manuel Puerta October 5, 2009 So far we focused on profit maximization, we could look at a different problem, that is the cost minimization problem. This is
Cost Minimization and the Cost Function Juan Manuel Puerta October 5, 2009 So far we focused on profit maximization, we could look at a different problem, that is the cost minimization problem. This is
CHEM 101/105 BONDING (continued) Lect-16
 CHEM 0/05 BONDING (continued) Lect6 A Second covalent bonding theory, MOLECULAR ORBITAL THEORY accounts for covalent bonding by... before looking at MO, return for a moment to the individual unbonded atom
CHEM 0/05 BONDING (continued) Lect6 A Second covalent bonding theory, MOLECULAR ORBITAL THEORY accounts for covalent bonding by... before looking at MO, return for a moment to the individual unbonded atom
Numerical methods for American options
 Lecture 9 Numerical methods for American options Lecture Notes by Andrzej Palczewski Computational Finance p. 1 American options The holder of an American option has the right to exercise it at any moment
Lecture 9 Numerical methods for American options Lecture Notes by Andrzej Palczewski Computational Finance p. 1 American options The holder of an American option has the right to exercise it at any moment
Bindel, Spring 2012 Intro to Scientific Computing (CS 3220) Week 3: Wednesday, Feb 8
 Spaces and bases Week 3: Wednesday, Feb 8 I have two favorite vector spaces 1 : R n and the space P d of polynomials of degree at most d. For R n, we have a canonical basis: R n = span{e 1, e 2,..., e
Spaces and bases Week 3: Wednesday, Feb 8 I have two favorite vector spaces 1 : R n and the space P d of polynomials of degree at most d. For R n, we have a canonical basis: R n = span{e 1, e 2,..., e
arxiv:quant-ph/9607009v1 11 Jul 1996
 Distillability of Inseparable Quantum Systems Micha l Horodecki Department of Mathematics and Physics University of Gdańsk, 80 952 Gdańsk, Poland arxiv:quant-ph/9607009v1 11 Jul 1996 Pawe l Horodecki Faculty
Distillability of Inseparable Quantum Systems Micha l Horodecki Department of Mathematics and Physics University of Gdańsk, 80 952 Gdańsk, Poland arxiv:quant-ph/9607009v1 11 Jul 1996 Pawe l Horodecki Faculty
Elementary Gradient-Based Parameter Estimation
 Elementary Gradient-Based Parameter Estimation Julius O. Smith III Center for Computer Research in Music and Acoustics (CCRMA Department of Music, Stanford University, Stanford, California 94305 USA Abstract
Elementary Gradient-Based Parameter Estimation Julius O. Smith III Center for Computer Research in Music and Acoustics (CCRMA Department of Music, Stanford University, Stanford, California 94305 USA Abstract
Chapter 10. Key Ideas Correlation, Correlation Coefficient (r),
 Chapter 0 Key Ideas Correlation, Correlation Coefficient (r), Section 0-: Overview We have already explored the basics of describing single variable data sets. However, when two quantitative variables
Chapter 0 Key Ideas Correlation, Correlation Coefficient (r), Section 0-: Overview We have already explored the basics of describing single variable data sets. However, when two quantitative variables
DO PHYSICS ONLINE FROM QUANTA TO QUARKS QUANTUM (WAVE) MECHANICS
 DO PHYSICS ONLINE FROM QUANTA TO QUARKS QUANTUM (WAVE) MECHANICS Quantum Mechanics or wave mechanics is the best mathematical theory used today to describe and predict the behaviour of particles and waves.
DO PHYSICS ONLINE FROM QUANTA TO QUARKS QUANTUM (WAVE) MECHANICS Quantum Mechanics or wave mechanics is the best mathematical theory used today to describe and predict the behaviour of particles and waves.
Lecture 3: Optical Properties of Bulk and Nano. 5 nm
 Lecture 3: Optical Properties of Bulk and Nano 5 nm The Previous Lecture Origin frequency dependence of χ in real materials Lorentz model (harmonic oscillator model) 0 e - n( ) n' n '' n ' = 1 + Nucleus
Lecture 3: Optical Properties of Bulk and Nano 5 nm The Previous Lecture Origin frequency dependence of χ in real materials Lorentz model (harmonic oscillator model) 0 e - n( ) n' n '' n ' = 1 + Nucleus
Testing the kinetic energy functional: Kinetic energy density as a density functional
 JOURAL OF CHEMICAL PHYSICS VOLUME 118, UMBER 18 8 MAY 2003 Testing the kinetic energy functional: Kinetic energy density as a density functional Eunji Sim, Joe Larkin, and Kieron Burke Department of Chemistry
JOURAL OF CHEMICAL PHYSICS VOLUME 118, UMBER 18 8 MAY 2003 Testing the kinetic energy functional: Kinetic energy density as a density functional Eunji Sim, Joe Larkin, and Kieron Burke Department of Chemistry
Molecular-Orbital Theory
 Molecular-Orbital Theory 1 Introduction Orbitals in molecules are not necessarily localized on atoms or between atoms as suggested in the valence bond theory. Molecular orbitals can also be formed the
Molecular-Orbital Theory 1 Introduction Orbitals in molecules are not necessarily localized on atoms or between atoms as suggested in the valence bond theory. Molecular orbitals can also be formed the
Laboratory 11: Molecular Compounds and Lewis Structures
 Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
2.3 Convex Constrained Optimization Problems
 42 CHAPTER 2. FUNDAMENTAL CONCEPTS IN CONVEX OPTIMIZATION Theorem 15 Let f : R n R and h : R R. Consider g(x) = h(f(x)) for all x R n. The function g is convex if either of the following two conditions
42 CHAPTER 2. FUNDAMENTAL CONCEPTS IN CONVEX OPTIMIZATION Theorem 15 Let f : R n R and h : R R. Consider g(x) = h(f(x)) for all x R n. The function g is convex if either of the following two conditions
CHEMISTRY BONDING REVIEW
 Answer the following questions. CHEMISTRY BONDING REVIEW 1. What are the three kinds of bonds which can form between atoms? The three types of Bonds are Covalent, Ionic and Metallic. Name Date Block 2.
Answer the following questions. CHEMISTRY BONDING REVIEW 1. What are the three kinds of bonds which can form between atoms? The three types of Bonds are Covalent, Ionic and Metallic. Name Date Block 2.
Modern Optimization Methods for Big Data Problems MATH11146 The University of Edinburgh
 Modern Optimization Methods for Big Data Problems MATH11146 The University of Edinburgh Peter Richtárik Week 3 Randomized Coordinate Descent With Arbitrary Sampling January 27, 2016 1 / 30 The Problem
Modern Optimization Methods for Big Data Problems MATH11146 The University of Edinburgh Peter Richtárik Week 3 Randomized Coordinate Descent With Arbitrary Sampling January 27, 2016 1 / 30 The Problem
Chapter 9. Chemical reactivity of molecules depends on the nature of the bonds between the atoms as well on its 3D structure
 Chapter 9 Molecular Geometry & Bonding Theories I) Molecular Geometry (Shapes) Chemical reactivity of molecules depends on the nature of the bonds between the atoms as well on its 3D structure Molecular
Chapter 9 Molecular Geometry & Bonding Theories I) Molecular Geometry (Shapes) Chemical reactivity of molecules depends on the nature of the bonds between the atoms as well on its 3D structure Molecular
1 The Brownian bridge construction
 The Brownian bridge construction The Brownian bridge construction is a way to build a Brownian motion path by successively adding finer scale detail. This construction leads to a relatively easy proof
The Brownian bridge construction The Brownian bridge construction is a way to build a Brownian motion path by successively adding finer scale detail. This construction leads to a relatively easy proof
Lesson19: Comparing Predictive Accuracy of two Forecasts: Th. Diebold-Mariano Test
 Lesson19: Comparing Predictive Accuracy of two Forecasts: The Diebold-Mariano Test Dipartimento di Ingegneria e Scienze dell Informazione e Matematica Università dell Aquila, umberto.triacca@univaq.it
Lesson19: Comparing Predictive Accuracy of two Forecasts: The Diebold-Mariano Test Dipartimento di Ingegneria e Scienze dell Informazione e Matematica Università dell Aquila, umberto.triacca@univaq.it
Chapter 18: The Structure of the Atom
 Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
Review of Fundamental Mathematics
 Review of Fundamental Mathematics As explained in the Preface and in Chapter 1 of your textbook, managerial economics applies microeconomic theory to business decision making. The decision-making tools
Review of Fundamental Mathematics As explained in the Preface and in Chapter 1 of your textbook, managerial economics applies microeconomic theory to business decision making. The decision-making tools
The properties of an ideal Fermi gas are strongly determined by the Pauli principle. We shall consider the limit: µ >> k B T βµ >> 1,
 Chapter 3 Ideal Fermi gas The properties of an ideal Fermi gas are strongly determined by the Pauli principle. We shall consider the limit: µ >> k B T βµ >>, which defines the degenerate Fermi gas. In
Chapter 3 Ideal Fermi gas The properties of an ideal Fermi gas are strongly determined by the Pauli principle. We shall consider the limit: µ >> k B T βµ >>, which defines the degenerate Fermi gas. In
Probabilistic Linear Classification: Logistic Regression. Piyush Rai IIT Kanpur
 Probabilistic Linear Classification: Logistic Regression Piyush Rai IIT Kanpur Probabilistic Machine Learning (CS772A) Jan 18, 2016 Probabilistic Machine Learning (CS772A) Probabilistic Linear Classification:
Probabilistic Linear Classification: Logistic Regression Piyush Rai IIT Kanpur Probabilistic Machine Learning (CS772A) Jan 18, 2016 Probabilistic Machine Learning (CS772A) Probabilistic Linear Classification:
Introduction to Matrix Algebra
 Psychology 7291: Multivariate Statistics (Carey) 8/27/98 Matrix Algebra - 1 Introduction to Matrix Algebra Definitions: A matrix is a collection of numbers ordered by rows and columns. It is customary
Psychology 7291: Multivariate Statistics (Carey) 8/27/98 Matrix Algebra - 1 Introduction to Matrix Algebra Definitions: A matrix is a collection of numbers ordered by rows and columns. It is customary
Towards Online Recognition of Handwritten Mathematics
 Towards Online Recognition of Handwritten Mathematics Vadim Mazalov, joint work with Oleg Golubitsky and Stephen M. Watt Ontario Research Centre for Computer Algebra Department of Computer Science Western
Towards Online Recognition of Handwritten Mathematics Vadim Mazalov, joint work with Oleg Golubitsky and Stephen M. Watt Ontario Research Centre for Computer Algebra Department of Computer Science Western
Examples on Monopoly and Third Degree Price Discrimination
 1 Examples on Monopoly and Third Degree Price Discrimination This hand out contains two different parts. In the first, there are examples concerning the profit maximizing strategy for a firm with market
1 Examples on Monopoly and Third Degree Price Discrimination This hand out contains two different parts. In the first, there are examples concerning the profit maximizing strategy for a firm with market
Understanding Poles and Zeros
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY DEPARTMENT OF MECHANICAL ENGINEERING 2.14 Analysis and Design of Feedback Control Systems Understanding Poles and Zeros 1 System Poles and Zeros The transfer function
MASSACHUSETTS INSTITUTE OF TECHNOLOGY DEPARTMENT OF MECHANICAL ENGINEERING 2.14 Analysis and Design of Feedback Control Systems Understanding Poles and Zeros 1 System Poles and Zeros The transfer function
A FIRST COURSE IN OPTIMIZATION THEORY
 A FIRST COURSE IN OPTIMIZATION THEORY RANGARAJAN K. SUNDARAM New York University CAMBRIDGE UNIVERSITY PRESS Contents Preface Acknowledgements page xiii xvii 1 Mathematical Preliminaries 1 1.1 Notation
A FIRST COURSE IN OPTIMIZATION THEORY RANGARAJAN K. SUNDARAM New York University CAMBRIDGE UNIVERSITY PRESS Contents Preface Acknowledgements page xiii xvii 1 Mathematical Preliminaries 1 1.1 Notation
Increasing for all. Convex for all. ( ) Increasing for all (remember that the log function is only defined for ). ( ) Concave for all.
 1. Differentiation The first derivative of a function measures by how much changes in reaction to an infinitesimal shift in its argument. The largest the derivative (in absolute value), the faster is evolving.
1. Differentiation The first derivative of a function measures by how much changes in reaction to an infinitesimal shift in its argument. The largest the derivative (in absolute value), the faster is evolving.
The Characteristic Polynomial
 Physics 116A Winter 2011 The Characteristic Polynomial 1 Coefficients of the characteristic polynomial Consider the eigenvalue problem for an n n matrix A, A v = λ v, v 0 (1) The solution to this problem
Physics 116A Winter 2011 The Characteristic Polynomial 1 Coefficients of the characteristic polynomial Consider the eigenvalue problem for an n n matrix A, A v = λ v, v 0 (1) The solution to this problem
Differential Relations for Fluid Flow. Acceleration field of a fluid. The differential equation of mass conservation
 Differential Relations for Fluid Flow In this approach, we apply our four basic conservation laws to an infinitesimally small control volume. The differential approach provides point by point details of
Differential Relations for Fluid Flow In this approach, we apply our four basic conservation laws to an infinitesimally small control volume. The differential approach provides point by point details of
