Physical and Chemical Changes
|
|
|
- Melinda Paul
- 9 years ago
- Views:
Transcription
1 Physical and Chemical Changes Jana Barrow West Point Jr. High 2775 W 550 N West Point, UT [email protected] Eighth Grade Integrated Science Standard I: Students will understand the nature of changes in matter. Objective 2: Observe and evaluate evidence of chemical and physical change. a. Identify observable evidence of a physical change (e.g., change in shape, size, phase). b. Identify observable evidence of a chemical change (e.g., color change, heat or light given off, change in odor, gas given off). Intended Learning Outcomes (ILOs) 1. Use Science Process and Thinking Skills a. Observe objects and events for patterns and record both qualitative and quantitative information. e. When given a problem, plan and conduct experiments in which they: Select appropriate format (e.g., graph, chart, diagram) to summarize data obtained. 3. b. Distinguish between examples and non-examples of concepts that have been taught. Time Needed to Complete Inquiry: 45 minutes Inquiry: Students will be using a structured inquiry method to determine the following: What observations help to determine if a chemical or physical has occurred? What are some evidences that a chemical reaction has occurred? Prior Knowledge Needed: Students should have a working knowledge of how to operate in a laboratory situation, including proper behaviors and safety precautions. Students should also have a basic knowledge of what a chemical change is versus a physical change. Students need to know that when a chemical change occurs a new substance has been created. A physical change means that the substance has only changed its appearance and is still the same material.
2 Introduction: Most changes that occur can be classified as physical or chemical changes. Physical changes occur without any changes in composition. In other words, no new substances appear as a result of the change. This could be demonstrated by the crushing of rock salt or ripping paper into pieces. On the other hand, when chemical changes take place, a change in composition also occurs. Basically, one or more new substances appear as a result of the change. This could be demonstrated with adding food coloring to water and then adding bleach drop-wise to the mixture until the dye is de-colorized. Materials for the changes the students will perform are located at different stations in the room. Simple instructions for the students to follow at each station are included. The students should go to each station, not necessarily in order, record the name of the change, follow the instructions and record their observations and their conclusions about the type of change involved. Safety Precautions: Do NOT mix any items unless instructed. DO NOT taste any of the materials; some of them are poisonous. Wear goggles and aprons at all times. Wash your hands after completing the experiments. Materials / Resources Needed for the Investigation: Vinegar (Plain), baking soda, potato, iodine solution (can be obtained at the local drugstore Tincture of Iodine), effervescent tablets (like Alka-Seltzer), salt, water, small pieces of ice, matches, household ammonia, vinegar solution (iron acetate) that had very fine steel wool soaking in it for a week (cover to prevent evaporation, and stir periodically to release bubbles), calcium chloride (ice melt, but check labels), universal indicator or phenol red solution, cabbage juice, test tubes, small beakers, popsicle sticks or small craft sticks (the handle of a plastic spoon works too), sandwich size zip top baggies, salt water ice baths (make with ice and water with salt stirred in within a thermos/insulated container). Procedures of the Investigation: Put students in small groups and have them perform the following experiments. The last one is optional, since it is a little time consuming and sometimes strange things happen for no particular reason. This might be something to do for a demonstration. Depending on time and resources, you may decide to have the students do all of these investigations, or only a part of them.
3 Station #1 Vinegar and Baking Soda chemical change gas production Put about 1 cm of vinegar into a test tube. Then use a stick to put a SMALL (about ½ cm long on the stick) amount of baking soda into the tube. After your observations are complete, pour the test tube contents into the sink, and rinse the test tube with water before putting it back into the rack. Station #2 Iodine on a Potato chemical change color change Cut a SMALL fresh slice from the potato. Use a dropper to put 3-4 drops of iodine solution on the potato slice. After your observations are complete, throw away the used slice of potato into the trash can! NOTE: iodine solution can be obtained at a drugstore in the first aid section as Tincture of Iodine Station #3 Salt physical change dissolving of a solid Fill a test tube about ¼ full with tap water. Use a spoon handle to add ½ cm (long on the stick) of salt to the water. Shake the test tube gently until the salt dissolves. When you have completed your observations, pour the test tube contents into the sink, and rinse the test tube with water before putting it back into the rack. NOTE: You may want to evaporate the water in a demonstration to show the salt remains, or have them taste the water to prove the salt is still present. Station #4 Seltzer Tablet chemical change gas production Fill a test tube about ¼ full with tap water. Put a small piece of crushed seltzer tablet into the water in the test tube. When you have completed your observations, pour the test tube contents into the sink, and rinse the test tube with water before putting it back into the rack. Station #5 Ice physical change phase change Put 2 or 3 small pieces of ice into a test tube and observe what happens as the solid ice turns to a liquid. When you have completed your observations, pour the test tube contents into the sink and rinse it before returning it back to the rack. Station #6 Matches chemical change gas production, light, heat Strike a match and watch it as it burns. Blow the match out before it burns your fingers. When your observations are completed, rinse the used match in tap water and dispose of it in the waste basket.
4 Station #7 PH Indicators chemical change color change Fill a test tube about ¼ full of cabbage juice. Add 3-4 drops of the vinegar provided, and gently shake the test tube. When your observations are completed, discard the contents and rinse the test tube in the sink before returning it to the rack. NOTE: Prepare cabbage juice by boiling red cabbage (until the cabbage is cooked). Remove the cabbage and refrigerate the juice, because it can grow some great mold and other forms of science experiments! Station #8 Baggie Experiment chemical change color change; gas production; heat production Place one small spoonful of calcium chloride into a plastic sealable bag and the same amount of BAKING SODA. Seal the bag, shake it. Observe (physical change mixture of solids). Measure 2 small teaspoons (about 10 ml) of phenol red indicator solution into the small cup (or beaker). Carefully put the beaker into the bag. Seal it. GENTLY tilt the bag to tip the small cup (beaker) over. Observe. Hold the bag in your palm while making observations Clean the beaker with water and throw the baggie away. NOTE: to prepare the phenol red solution, add 1 g of phenol red to 1 gallon of water Station #9 Two Clear Liquids chemical change precipitate formed Take full dropper of the vinegar solution and squirt its contents into a 50 ml beaker. Take a full dropper of the ammonia and add it drop wise into the 50 ml beaker. Make sure you don t mix up the droppers!! When your observations are completed, pour the contents down the drain and clean the beaker with water. NOTE: To prepare the iron acetate (vinegar) solution, use the following: Cover very fine steel wool (size #0000) with vinegar in a beaker/jar (if the wool isn t covered, it will rust). You only need to use a small portion of the pad, not the whole piece of steel wool. Cover with a lid or plastic wrap to prevent evaporation. Let it sit for about a week, stirring periodically to release bubbles. Station #10 Water Baths - physical change phase change Fill a test tube ¼ or less with tap water. Place it in the salt-water ice bath and stir it around gently and constantly for 2-3 minutes (possibly more) without removing it. Take the test tube out. Record your observations.
5 Rinse the inside AND outside of the test tube well with water and return to the test tube rack. Data Collection: Students will create a single data table that shows the station number, station name, their observations, and whether each station is an example of a chemical change or a physical change. Data Analysis: Students will analyze their observations to first differentiate whether a physical or chemical change has occurred (is the original substance still present after the change has occurred). STOP the students at this time to see if they correctly identified the chemical and physical changes that occurred. Then, looking at their data for chemical reactions that occurred students will determine evidences that a chemical reaction has occurred. Students will also analyze their observations to look for evidences that a physical change has occurred. Assessment: After performing the experiments, students will create individual data tables and answer the questions in the data analysis as well as writing a welldeveloped conclusion.
6 Student Sheet Name Date Station Changes Introduction: Most changes that occur can be classified as physical or chemical changes. Physical changes occur without any changes in composition. In other words, no new substances appear as a result of the change. On the other hand, when chemical changes take place, a change in composition also occurs. Basically, one or more new substances appear as a result of the change. Materials for the changes you will perform are located at different stations in the room. Simple instructions for you to follow at each station are included. You should go to each station, not necessarily in order, record the name of the change, follow the instructions and record your observations and your conclusions about the type of change involved. Safety Precautions: Do NOT mix any items unless instructed. DO NOT taste any of the materials; some of them are poisonous. Wear goggles and aprons at all times. Wash your hands after completing the experiments. Procedures: Station #1 Vinegar and Baking Soda Put about 1 cm of vinegar into a test tube. Then use a stick to put a SMALL (about ½ cm long on the stick) amount of baking soda into the tube. After your observations are complete, pour the test tube contents into the sink, and rinse the test tube with water before putting it back into the rack. Station #2 Iodine on a Potato Cut a SMALL fresh slice from the potato. Use a dropper to put 3-4 drops of iodine solution on the potato slice. After your observations are complete, throw away the used slice of potato into the trash can! Station #3 Salt Fill a test tube about ¼ full with tap water. Use a stick to add ½ cm (long on the stick) of salt to the water. Cover the mouth of the test tube with your thumb and vigorously shake the tube. Observe what happens to the salt. When you have completed your observations, pour the test tube contents into the sink, and rinse the test tube with water before putting it back into the rack. Station #4 Seltzer Tablet Fill a test tube about ¼ full with tap water. Put a small piece of crushed seltzer tablet into the water in the test tube. When you have completed your observations, pour the test tube contents into the sink, and rinse the test tube with water before putting it back into the rack.
7 Station #5 Ice Put 2 or 3 small pieces of ice into a test tube and observe what happens to the solid ice for one minute while holding the test tube cupped in the palm of your hand. When you have completed your observations, pour the test tube contents into the sink and rinse it before returning it back to the rack. Station #6 Matches Strike a match and watch it as it burns. Blow the match out before it burns your fingers. When your observations are completed, rinse the used match in tap water and dispose of it in the waste basket. Station #7 PH Indicators Fill a test tube about ¼ full of cabbage juice. Add 3-4 drops of the vinegar provided, and gently shake the test tube. When your observations are completed, discard the contents and rinse the test tube in the sink before returning it to the rack. Station #8 Baggie Experiment Place one small spoonful of calcium chloride into a plastic sealable bag and the same amount of BAKING SODA. Seal the bag, shake it. Observe. Measure 2 small teaspoons (about 10 ml) of indicator solution into the small cup (or beaker). Carefully put the small cup into the bag. Seal it. GENTLY tilt the bag to tip the small cup over. Observe. Hold the baggie in the palm of your hand while making your observations. Clean the small cup with water and throw the baggie away. Station #9 Two Clear (or mostly) Liquids Take full dropper of the vinegar solution and squirt its contents into a 50 ml beaker. Take a full dropper of the ammonia and add it drop wise into the 50 ml beaker. Make sure you don t mix up the droppers!! When your observations are completed, pour the contents down the drain and clean the beaker with water. Station #10 Water Baths Fill a test tube ¼ or less with tap water. Place it in the salt water ice bath and stir it around gently and constantly for 2-3 minutes (possibly more) without removing it. Take the test tube out. Record your observations. Rinse the inside AND outside of the test tube well with water and return to the test tube rack. Data Collection: On your own paper, create a single data table that shows the station number, station name, your observations, and whether each station is an example of a chemical change or a physical change. Make sure your observations are SUPERcomplete write down EVERYTHING that you observe! Data Analysis: answer the following questions on your own paper 1. List all of the chemical changes you observed. 2. List all of the physical changes you observed. 3. What were some of the observations that indicated a physical change had occurred?
8 4. What were some of the observations that indicated a chemical reaction had occurred? Conclusion: On your own paper, write a well developed conclusion (at least 4 sentences) about what was learned and what types of observations (evidences) can be used to tell whether a physical or chemical change has occurred.
Household Acids and Bases
 Household Acids and Bases GRADE LEVEL INDICATORS Experiment Demonstrate that the ph scale (0-14) is used to measure acidity and classify substances or solutions as acidic, basic, or neutral. 21 Develop
Household Acids and Bases GRADE LEVEL INDICATORS Experiment Demonstrate that the ph scale (0-14) is used to measure acidity and classify substances or solutions as acidic, basic, or neutral. 21 Develop
CHM 130LL: ph, Buffers, and Indicators
 CHM 130LL: ph, Buffers, and Indicators Many substances can be classified as acidic or basic. Acidic substances contain hydrogen ions, H +, while basic substances contain hydroxide ions, OH. The relative
CHM 130LL: ph, Buffers, and Indicators Many substances can be classified as acidic or basic. Acidic substances contain hydrogen ions, H +, while basic substances contain hydroxide ions, OH. The relative
ACIDS AND BASES SAFETY PRECAUTIONS
 ACIDS AND BASES Mild acids and bases are used in cooking (their reaction makes biscuits and bread rise). Acids such as those in our stomachs eat away at food or digest it. Strong acids and bases are used
ACIDS AND BASES Mild acids and bases are used in cooking (their reaction makes biscuits and bread rise). Acids such as those in our stomachs eat away at food or digest it. Strong acids and bases are used
SNEAK PEAK inside ACTIVITY. ADVANCE PREPARATION see next page for more details Dilute alcohol with water Set out plastic cups, etc.
 Reaction: Yes or No? Learning Objectives: Students will list and identify three characteristics of chemical reactions. GRADE LEVEL 3 8 SCIENCE TOPICS Solutions and Mixtures Chemical Reactions PROCESS SKILLS
Reaction: Yes or No? Learning Objectives: Students will list and identify three characteristics of chemical reactions. GRADE LEVEL 3 8 SCIENCE TOPICS Solutions and Mixtures Chemical Reactions PROCESS SKILLS
Reaction in a Bag. Scientific Method Demonstrations
 elearning 2009 Introduction Reaction in a Bag Scientific Method Demonstrations Publication No. 91419 Careful observation is the foundation of science, leading to questions about what we have observed how,
elearning 2009 Introduction Reaction in a Bag Scientific Method Demonstrations Publication No. 91419 Careful observation is the foundation of science, leading to questions about what we have observed how,
Dry Ice Color Show Dry Ice Demonstrations
 elearning 2009 Introduction Dry Ice Color Show Dry Ice Demonstrations Publication No. 95016 Add a small piece of solid carbon dioxide to a colored indicator solution and watch as the solution immediately
elearning 2009 Introduction Dry Ice Color Show Dry Ice Demonstrations Publication No. 95016 Add a small piece of solid carbon dioxide to a colored indicator solution and watch as the solution immediately
Chem 100 Lab Experiment #9 - ACID/BASE INDICATORS
 Lab #9 Chem 100 Lab Experiment #9 - ACID/BASE INDICATORS Name: Purpose: In this laboratory we will investigate how indicators can be used to test for the presence of acids or bases in a number of common
Lab #9 Chem 100 Lab Experiment #9 - ACID/BASE INDICATORS Name: Purpose: In this laboratory we will investigate how indicators can be used to test for the presence of acids or bases in a number of common
Chemistry 101. Chemistry Experiments for the Home Acidity Determination Using Indicators
 Chemistry 101 Chemistry Experiments for the Home Acidity Determination Using Indicators I. Objective: To determine the acidity of a variety of common substances by the use of indicators. To prepare your
Chemistry 101 Chemistry Experiments for the Home Acidity Determination Using Indicators I. Objective: To determine the acidity of a variety of common substances by the use of indicators. To prepare your
Hands-On Labs SM-1 Lab Manual
 EXPERIMENT 4: Separation of a Mixture of Solids Read the entire experiment and organize time, materials, and work space before beginning. Remember to review the safety sections and wear goggles when appropriate.
EXPERIMENT 4: Separation of a Mixture of Solids Read the entire experiment and organize time, materials, and work space before beginning. Remember to review the safety sections and wear goggles when appropriate.
Physical and Chemical Properties and Changes
 Physical and Chemical Properties and Changes An understanding of material things requires an understanding of the physical and chemical characteristics of matter. A few planned experiments can help you
Physical and Chemical Properties and Changes An understanding of material things requires an understanding of the physical and chemical characteristics of matter. A few planned experiments can help you
Acids and Bases. AND a widemouth container of the following solids:
 Acids and Bases GOAL To introduce students to acids and bases. MATERIALS: 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz. bottle of water - labeled Water 1 4 oz. bottle of
Acids and Bases GOAL To introduce students to acids and bases. MATERIALS: 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz. bottle of water - labeled Water 1 4 oz. bottle of
Chemquest: Physical Changes or Chemical Reactions
 Chemquest: Physical Changes or Chemical Reactions Erik Misner May 9, 2005 Background: This lesson is designed to be an interactive and fun way to learn the difference between physical changes and chemical
Chemquest: Physical Changes or Chemical Reactions Erik Misner May 9, 2005 Background: This lesson is designed to be an interactive and fun way to learn the difference between physical changes and chemical
Experiment 9: Acids and Bases Adapted from: Chemistry, Experimental Foundations, 4th Ed. Laboratory Manual, by Merrill, Parry & Bassow.
 Chem 121 Lab Clark College Experiment 9: Acids and Bases Adapted from: Chemistry, Experimental Foundations, 4th Ed. Laboratory Manual, by Merrill, Parry & Bassow. Content Goals: Increase understanding
Chem 121 Lab Clark College Experiment 9: Acids and Bases Adapted from: Chemistry, Experimental Foundations, 4th Ed. Laboratory Manual, by Merrill, Parry & Bassow. Content Goals: Increase understanding
Household Acids and Bases
 Household Acids and Bases Computer 28 Many common household solutions contain acids and bases. Acid-base indicators, such as litmus and red cabbage juice, turn different colors in acidic and basic solutions.
Household Acids and Bases Computer 28 Many common household solutions contain acids and bases. Acid-base indicators, such as litmus and red cabbage juice, turn different colors in acidic and basic solutions.
CHEMICAL DETERMINATION OF EVERYDAY HOUSEHOLD CHEMICALS
 CHEMICAL DETERMINATION OF EVERYDAY HOUSEHOLD CHEMICALS Purpose: It is important for chemists to be able to determine the composition of unknown chemicals. This can often be done by way of chemical tests.
CHEMICAL DETERMINATION OF EVERYDAY HOUSEHOLD CHEMICALS Purpose: It is important for chemists to be able to determine the composition of unknown chemicals. This can often be done by way of chemical tests.
Acids & Bases Around the House Use a ph indicator to find acids and bases
 Use a ph indicator to find acids and bases Description: Visitors predict whether various household solutions are acids or bases, and test their hypotheses using a universal ph indicator. Then, visitors
Use a ph indicator to find acids and bases Description: Visitors predict whether various household solutions are acids or bases, and test their hypotheses using a universal ph indicator. Then, visitors
Properties of Acids and Bases
 Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? What
Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? What
ph Measurements of Common Substances
 Chem 100 Section Experiment 10 Name Partner s Name Introduction ph Measurements of Common Substances The concentration of an acid or base is frequently expressed as ph. Historically, ph stands for the
Chem 100 Section Experiment 10 Name Partner s Name Introduction ph Measurements of Common Substances The concentration of an acid or base is frequently expressed as ph. Historically, ph stands for the
Introduction. ph = log [H + ]
![Introduction. ph = log [H + ] Introduction. ph = log [H + ]](/thumbs/40/21282159.jpg) Visualizing ph 2010, 1992 by David A. Katz. All rights reserved. Permission granted for classroom use. All reproductions must include original copyright. David A. Katz Chemist, Educator, Science Communicator,
Visualizing ph 2010, 1992 by David A. Katz. All rights reserved. Permission granted for classroom use. All reproductions must include original copyright. David A. Katz Chemist, Educator, Science Communicator,
III. BACKGROUND KNOWLEDGE
 Chemical Bonds and Reactions (Water) Grade Level: 7 (Applied Technology) Presented by: James Rubright, Three Oaks Middle School, Fort Myers, Florida Length of Unit: Ten Daily Lessons and Activities I.
Chemical Bonds and Reactions (Water) Grade Level: 7 (Applied Technology) Presented by: James Rubright, Three Oaks Middle School, Fort Myers, Florida Length of Unit: Ten Daily Lessons and Activities I.
Taking Apart the Pieces
 Lab 4 Taking Apart the Pieces How does starting your morning out right relate to relief from a headache? I t is a lazy Saturday morning and you ve just awakened to your favorite cereal Morning Trails and
Lab 4 Taking Apart the Pieces How does starting your morning out right relate to relief from a headache? I t is a lazy Saturday morning and you ve just awakened to your favorite cereal Morning Trails and
Lesson Plan: How Do We Know What is Healthy Water?
 Lesson Plan: How Do We Know What is Healthy Water? Estimated Time: 1-3 days ph /Chlorine / Hardness State Standards taught and addressed Grade 8: Standards Taught (and evaluated at end of lesson) Science
Lesson Plan: How Do We Know What is Healthy Water? Estimated Time: 1-3 days ph /Chlorine / Hardness State Standards taught and addressed Grade 8: Standards Taught (and evaluated at end of lesson) Science
Experiment 3: Extraction: Separation of an Acidic, a Basic and a Neutral Substance
 1 Experiment 3: Extraction: Separation of an Acidic, a Basic and a Neutral Substance Read pp 142-155, 161-162, Chapter 10 and pp 163-173, Chapter 11, in LTOC. View the videos: 4.2 Extraction (Macroscale);
1 Experiment 3: Extraction: Separation of an Acidic, a Basic and a Neutral Substance Read pp 142-155, 161-162, Chapter 10 and pp 163-173, Chapter 11, in LTOC. View the videos: 4.2 Extraction (Macroscale);
Return to Lab Menu. Acids and Bases in Your House
 Return to Lab Menu Acids and Bases in Your House OBJECTIVES Isolate a natural acid-base indicator. Determine the acid-base properties of common household solutions. INTRODUCTION Acids and bases are among
Return to Lab Menu Acids and Bases in Your House OBJECTIVES Isolate a natural acid-base indicator. Determine the acid-base properties of common household solutions. INTRODUCTION Acids and bases are among
Syllabus OC18 Use litmus or a universal indicator to test a variety of solutions, and classify these as acidic, basic or neutral
 Chemistry: 9. Acids and Bases Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC18 Use litmus or a universal indicator to test a variety
Chemistry: 9. Acids and Bases Please remember to photocopy 4 pages onto one sheet by going A3 A4 and using back to back on the photocopier Syllabus OC18 Use litmus or a universal indicator to test a variety
Enzyme Pre-Lab. Using the Enzyme worksheet and Enzyme lab handout answer the Pre-Lab questions the pre-lab must be complete before beginning the lab.
 Enzyme Pre-Lab Using the Enzyme worksheet and Enzyme lab handout answer the Pre-Lab questions the pre-lab must be complete before beginning the lab. Background: In this investigation, you will study several
Enzyme Pre-Lab Using the Enzyme worksheet and Enzyme lab handout answer the Pre-Lab questions the pre-lab must be complete before beginning the lab. Background: In this investigation, you will study several
Polarity and Properties Lab PURPOSE: To investigate polar and non-polar molecules and the affect of polarity on molecular properties.
 Name!!!! date Polarity and Properties Lab PURPOSE: To investigate polar and non-polar molecules and the affect of polarity on molecular properties. STATION 1: Oil and water do not mix. We all know that.
Name!!!! date Polarity and Properties Lab PURPOSE: To investigate polar and non-polar molecules and the affect of polarity on molecular properties. STATION 1: Oil and water do not mix. We all know that.
Mixtures and Pure Substances
 Unit 2 Mixtures and Pure Substances Matter can be classified into two groups: mixtures and pure substances. Mixtures are the most common form of matter and consist of mixtures of pure substances. They
Unit 2 Mixtures and Pure Substances Matter can be classified into two groups: mixtures and pure substances. Mixtures are the most common form of matter and consist of mixtures of pure substances. They
THE ACTIVITY OF LACTASE
 THE ACTIVITY OF LACTASE Lab VIS-8 From Juniata College Science in Motion Enzymes are protein molecules which act to catalyze the chemical reactions in living things. These chemical reactions make up the
THE ACTIVITY OF LACTASE Lab VIS-8 From Juniata College Science in Motion Enzymes are protein molecules which act to catalyze the chemical reactions in living things. These chemical reactions make up the
In this experiment, we will use three properties to identify a liquid substance: solubility, density and boiling point..
 Identification of a Substance by Physical Properties 2009 by David A. Katz. All rights reserved. Permission for academic use provided the original copyright is included Every substance has a unique set
Identification of a Substance by Physical Properties 2009 by David A. Katz. All rights reserved. Permission for academic use provided the original copyright is included Every substance has a unique set
IDENTIFICATION OF POLYMERS 1998 by David A. Katz. All rights reserved
 IDENTIFICATION OF POLYMERS 1998 by David A. Katz. All rights reserved David A. Katz Chemist, Educator, Science Communicator, and Consultant 133 N. Desert Stream Dr., Tucson, AZ 85745 Voice/Fax: 520-624-2207
IDENTIFICATION OF POLYMERS 1998 by David A. Katz. All rights reserved David A. Katz Chemist, Educator, Science Communicator, and Consultant 133 N. Desert Stream Dr., Tucson, AZ 85745 Voice/Fax: 520-624-2207
Kool Demo for Acid-Base Reactions
 Kool Demo for Acid-Base Reactions Kool Demo for Acid-Base Reactions Adapted from : http://www.stevespanglerscience.com/lab/experiments/color-changing-milk-of-magnesia Materials: Red cabbage juice indicator
Kool Demo for Acid-Base Reactions Kool Demo for Acid-Base Reactions Adapted from : http://www.stevespanglerscience.com/lab/experiments/color-changing-milk-of-magnesia Materials: Red cabbage juice indicator
Name. Lab 3: ENZYMES. In this lab, you ll investigate some of the properties of enzymes.
 Name Lab 3: ENZYMES In this lab, you ll investigate some of the properties of enzymes. So what are enzymes? Enzymes are large protein molecules (macromolecules) They catalyze or speed up chemical reactions
Name Lab 3: ENZYMES In this lab, you ll investigate some of the properties of enzymes. So what are enzymes? Enzymes are large protein molecules (macromolecules) They catalyze or speed up chemical reactions
Chemical versus Physical Changes
 Chemical versus Physical Changes Permission to Copy - This document may be reproduced for non-commercial educational purposes Copyright 2009 General Electric Company What are physical and chemical changes?
Chemical versus Physical Changes Permission to Copy - This document may be reproduced for non-commercial educational purposes Copyright 2009 General Electric Company What are physical and chemical changes?
Unit A: Studying Materials Scientifically
 ITEM BANKS Unit A: Studying Materials Scientifically Multiple choice: Circle the best answer. 1. What safety rules should you always follow while doing a science laboratory? a. Wear safety goggles at all
ITEM BANKS Unit A: Studying Materials Scientifically Multiple choice: Circle the best answer. 1. What safety rules should you always follow while doing a science laboratory? a. Wear safety goggles at all
Review and apply Investigation 5. Let s review Pages 311-312
 Review and apply Investigation 5 Let s review Pages 311-312 1. After you tested all the known powders with all the test liquids, describe what you did to identify the unknown powder. Students should have
Review and apply Investigation 5 Let s review Pages 311-312 1. After you tested all the known powders with all the test liquids, describe what you did to identify the unknown powder. Students should have
Experiment 12- Classification of Matter Experiment
 Experiment 12- Classification of Matter Experiment Matter can be classified into two groups: mixtures and pure substances. Mixtures are the most common form of matter and consist of mixtures of pure substances.
Experiment 12- Classification of Matter Experiment Matter can be classified into two groups: mixtures and pure substances. Mixtures are the most common form of matter and consist of mixtures of pure substances.
VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE. Acids and Bases. Fall 2012
 VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE Acids and Bases Fall 2012 GOAL: To introduce students to acids and bases. MATERIALS 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz.
VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE Acids and Bases Fall 2012 GOAL: To introduce students to acids and bases. MATERIALS 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz.
Lesson 4. Temperature change
 54 Lesson 4 Temperature change T E A C H E R G U I D E Lesson summary Students meet scientist Jason Williams, an industrial chemist who designs the materials and processes for making solar cells. He explains
54 Lesson 4 Temperature change T E A C H E R G U I D E Lesson summary Students meet scientist Jason Williams, an industrial chemist who designs the materials and processes for making solar cells. He explains
Luminol Test PROCESS SKILLS SCIENCE TOPICS VOCABULARY
 EXPERIMENT: LUMINOL TEST Luminol Test Visitors mix a solution of luminol with fake blood (hydrogen peroxide) to produce a reaction that gives off blue light. OBJECTIVES: Visitors learn that some chemical
EXPERIMENT: LUMINOL TEST Luminol Test Visitors mix a solution of luminol with fake blood (hydrogen peroxide) to produce a reaction that gives off blue light. OBJECTIVES: Visitors learn that some chemical
OSMOSIS AND DIALYSIS 2003 BY Wendy Weeks-Galindo with modifications by David A. Katz
 OSMOSIS AND DIALYSIS 2003 BY Wendy Weeks-Galindo with modifications by David A. Katz OSMOSIS Osmosis is the reason that a fresh water fish placed in the ocean desiccates and dies. Osmosis is the reason
OSMOSIS AND DIALYSIS 2003 BY Wendy Weeks-Galindo with modifications by David A. Katz OSMOSIS Osmosis is the reason that a fresh water fish placed in the ocean desiccates and dies. Osmosis is the reason
Basic Toxicology Lab Stations. Modified By Stefani D. Hines, M.A., M.S. Station 1: SEPUP Determining Threshold Limits: Taste Test for Salt Solution
 Basic Toxicology Lab Stations Modified By Stefani D. Hines, M.A., M.S. University of Arizona, Southwest Environmental Health Sciences Center, funded by the National Institute of Environmental Health Sciences
Basic Toxicology Lab Stations Modified By Stefani D. Hines, M.A., M.S. University of Arizona, Southwest Environmental Health Sciences Center, funded by the National Institute of Environmental Health Sciences
Experiment 16-Acids, Bases and ph
 Definitions acid-an ionic compound that releases or reacts with water to form hydrogen ion (H + ) in aqueous solution. They taste sour and turn litmus red. Acids react with certain metals such as zinc,
Definitions acid-an ionic compound that releases or reacts with water to form hydrogen ion (H + ) in aqueous solution. They taste sour and turn litmus red. Acids react with certain metals such as zinc,
This compound, which contains two carbon atoms with a C-OH structure on one end of the molecule is ethanol, commonly called ethyl alcohol.
 ESTERS An Introduction to rganic hemistry Reactions 2006, 1990, 1982 by David A. Katz. All rights reserved. Reproduction permitted for educationa use provided original copyright is included. In contrast
ESTERS An Introduction to rganic hemistry Reactions 2006, 1990, 1982 by David A. Katz. All rights reserved. Reproduction permitted for educationa use provided original copyright is included. In contrast
The Acid Test Grade Nine
 Ohio Standards Connection: Physical Sciences Benchmark B Explain how atoms react with each other to form other substances and how molecules react with each other or other atoms to form even different substances.
Ohio Standards Connection: Physical Sciences Benchmark B Explain how atoms react with each other to form other substances and how molecules react with each other or other atoms to form even different substances.
Acids & Bases: Using Purple Cabbage as a ph indicator. Grade 9 Activity Plan
 Acids & Bases: Using Purple Cabbage as a ph indicator Grade 9 Activity Plan 1 Acids, Bases & Purple Cabbage Objectives: 1. To demonstrate the basic physical and chemical properties of acids and bases.
Acids & Bases: Using Purple Cabbage as a ph indicator Grade 9 Activity Plan 1 Acids, Bases & Purple Cabbage Objectives: 1. To demonstrate the basic physical and chemical properties of acids and bases.
Experiment 10 Enzymes
 Experiment 10 Enzymes Enzymes are proteins that act as catalysts for biological reactions. Enzymes, like all catalysts, speed up reactions without being used up themselves. They do this by lowering the
Experiment 10 Enzymes Enzymes are proteins that act as catalysts for biological reactions. Enzymes, like all catalysts, speed up reactions without being used up themselves. They do this by lowering the
Teacher Demo: Turning Water into Wine into Milk into Beer
 SNC2D/2P Chemical Reactions/Chemical Reactions and their Practical Applications Teacher Demo: Turning Water into Wine into Milk into Beer Topics evidence of chemical change types of chemical reactions
SNC2D/2P Chemical Reactions/Chemical Reactions and their Practical Applications Teacher Demo: Turning Water into Wine into Milk into Beer Topics evidence of chemical change types of chemical reactions
Letter to the Student... 5 Test-Taking Checklist... 6 Next Generation Sunshine State Standards Correlation Chart... 7
 Table of Contents Letter to the Student..................................... 5 Test-Taking Checklist.................................... 6 Next Generation Sunshine State Standards Correlation Chart...
Table of Contents Letter to the Student..................................... 5 Test-Taking Checklist.................................... 6 Next Generation Sunshine State Standards Correlation Chart...
Film Canister ROCKETS. An activity of reaction rates and the scientific method
 Film Canister ROCKETS An activity of reaction rates and the scientific method Developed by: Elisabeth Mills, UCLA NSF GK-12 Fellow Title of Lesson: Film Canister Rockets Grade level: 8 th Grade Subject(s):
Film Canister ROCKETS An activity of reaction rates and the scientific method Developed by: Elisabeth Mills, UCLA NSF GK-12 Fellow Title of Lesson: Film Canister Rockets Grade level: 8 th Grade Subject(s):
Ice Cream Lab & Application Questions
 Deep Freeze 1 Ice Cream Lab & Application Questions Name: Period: Date: Overview Have you ever wondered what it is about throwing salt on ice that makes it melt? And just why does it melt? Where does the
Deep Freeze 1 Ice Cream Lab & Application Questions Name: Period: Date: Overview Have you ever wondered what it is about throwing salt on ice that makes it melt? And just why does it melt? Where does the
PREPARATION AND PROPERTIES OF A SOAP
 (adapted from Blackburn et al., Laboratory Manual to Accompany World of Chemistry, 2 nd ed., (1996) Saunders College Publishing: Fort Worth) Purpose: To prepare a sample of soap and to examine its properties.
(adapted from Blackburn et al., Laboratory Manual to Accompany World of Chemistry, 2 nd ed., (1996) Saunders College Publishing: Fort Worth) Purpose: To prepare a sample of soap and to examine its properties.
Bay Area Scientists in Schools Presentation Plan
 Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Grade Level 5th The Chemical Workout/Blow it Up Chemistry Graduate Students from the Maimone Group at UC Berkeley Standards Connection(s):
Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Grade Level 5th The Chemical Workout/Blow it Up Chemistry Graduate Students from the Maimone Group at UC Berkeley Standards Connection(s):
Neutralizing an Acid and a Base
 Balancing Act Teacher Information Objectives In this activity, students neutralize a base with an acid. Students determine the point of neutralization of an acid mixed with a base while they: Recognize
Balancing Act Teacher Information Objectives In this activity, students neutralize a base with an acid. Students determine the point of neutralization of an acid mixed with a base while they: Recognize
Physical and Chemical Changes Pre Test Questions
 Pre Test Questions Name: Period: Date: 1. Which of the following is an example of physical change? a. Mixing baking soda and vinegar together, and this causes bubbles and foam. b. A glass cup falls from
Pre Test Questions Name: Period: Date: 1. Which of the following is an example of physical change? a. Mixing baking soda and vinegar together, and this causes bubbles and foam. b. A glass cup falls from
Acids, Bases, and ph
 CHAPTER 9 1 SECTION Acids, Bases, and Salts Acids, Bases, and ph KEY IDEAS As you read this section, keep these questions in mind: What properties do acids have? What properties do bases have? How can
CHAPTER 9 1 SECTION Acids, Bases, and Salts Acids, Bases, and ph KEY IDEAS As you read this section, keep these questions in mind: What properties do acids have? What properties do bases have? How can
The Amazing Elephant Toothpaste! Lesson Overview
 The Amazing Elephant Toothpaste! Lesson Overview Students will investigate chemical change. Suggested Grade Levels: 3-8 Standards for Lesson Content Standard A: Science as Inquiry Content Standard B: Physical
The Amazing Elephant Toothpaste! Lesson Overview Students will investigate chemical change. Suggested Grade Levels: 3-8 Standards for Lesson Content Standard A: Science as Inquiry Content Standard B: Physical
STANDARDIZATION OF A SODIUM HYDROXIDE SOLUTION EXPERIMENT 14
 STANDARDIZATION OF A SODIUM HYDROXIDE SOLUTION EXPERIMENT 14 OBJECTIVE The objective of this experiment will be the standardization of sodium hydroxide using potassium hydrogen phthalate by the titration
STANDARDIZATION OF A SODIUM HYDROXIDE SOLUTION EXPERIMENT 14 OBJECTIVE The objective of this experiment will be the standardization of sodium hydroxide using potassium hydrogen phthalate by the titration
Chapter 6, Lesson 4: Temperature and the Rate of a Chemical Reaction
 Chapter 6, Lesson 4: Temperature and the Rate of a Chemical Reaction Key Concepts Reactants must be moving fast enough and hit each other hard enough for a chemical reaction to take place. Increasing the
Chapter 6, Lesson 4: Temperature and the Rate of a Chemical Reaction Key Concepts Reactants must be moving fast enough and hit each other hard enough for a chemical reaction to take place. Increasing the
Sugar or Salt? Ionic and Covalent Bonds
 Lab 11 Sugar or Salt? Ionic and Covalent Bonds TN Standard 2.1: The student will investigate chemical bonding. Have you ever accidentally used salt instead of sugar? D rinking tea that has been sweetened
Lab 11 Sugar or Salt? Ionic and Covalent Bonds TN Standard 2.1: The student will investigate chemical bonding. Have you ever accidentally used salt instead of sugar? D rinking tea that has been sweetened
Amino Acids, Peptides, and Proteins
 1 Amino Acids, Peptides, and Proteins Introduction Amino Acids Amino acids are the building blocks of proteins. In class you learned the structures of the 20 common amino acids that make up proteins. All
1 Amino Acids, Peptides, and Proteins Introduction Amino Acids Amino acids are the building blocks of proteins. In class you learned the structures of the 20 common amino acids that make up proteins. All
EXPERIMENT 10: TITRATION AND STANDARDIZATION
 EXPERIMENT 10: TITRATION AND STANDARDIZATION PURPOSE To determine the molarity of a NaOH solution by titrating it with a standard HCl solution. To determine the molarity of acetic acid in vinegar using
EXPERIMENT 10: TITRATION AND STANDARDIZATION PURPOSE To determine the molarity of a NaOH solution by titrating it with a standard HCl solution. To determine the molarity of acetic acid in vinegar using
sciencemuseumoutreach Kitchen Science 1 Demonstrations to do at home
 sciencemuseumoutreach Kitchen Science 1 Demonstrations to do at home The Creative Canal Project (CCP) is part of the Science Museum s Outreach Department, which works with teachers, students, families
sciencemuseumoutreach Kitchen Science 1 Demonstrations to do at home The Creative Canal Project (CCP) is part of the Science Museum s Outreach Department, which works with teachers, students, families
Solids, Liquids, and Gases
 Solids, Liquids, and Gases nd Intended for Grade: 2 Grade Subject: Science Description: Activities to help students understand solids, liquids, gases, and the changes between these states. Objective: The
Solids, Liquids, and Gases nd Intended for Grade: 2 Grade Subject: Science Description: Activities to help students understand solids, liquids, gases, and the changes between these states. Objective: The
Science Crime Busters B & Forensics C. NC Event Only
 Science Crime Busters B & Forensics C NC Event Only Presenters Aarti Thakkar Student at Duke University Four-time state medalist in Forensics Competed in Forensics at National Science Olympiad Jessica
Science Crime Busters B & Forensics C NC Event Only Presenters Aarti Thakkar Student at Duke University Four-time state medalist in Forensics Competed in Forensics at National Science Olympiad Jessica
Determining the Quantity of Iron in a Vitamin Tablet. Evaluation copy
 Determining the Quantity of Iron in a Vitamin Tablet Computer 34 As biochemical research becomes more sophisticated, we are learning more about the role of metallic elements in the human body. For example,
Determining the Quantity of Iron in a Vitamin Tablet Computer 34 As biochemical research becomes more sophisticated, we are learning more about the role of metallic elements in the human body. For example,
Experiment 8 Preparation of Cyclohexanone by Hypochlorite Oxidation
 Experiment 8 Preparation of Cyclohexanone by ypochlorite xidation In this experiment we will prepare cyclohexanone from cyclohexanol using hypochlorite oxidation. We will use common household bleach that
Experiment 8 Preparation of Cyclohexanone by ypochlorite xidation In this experiment we will prepare cyclohexanone from cyclohexanol using hypochlorite oxidation. We will use common household bleach that
Lab: Properties of Polar and Nonpolar Substances
 Lab: Properties of Polar and Nonpolar Substances Purpose: To explain the interactions of matter in relation to polarity. Stations 1 and 2 - il and water do not mix As a metaphor, oil and water are often
Lab: Properties of Polar and Nonpolar Substances Purpose: To explain the interactions of matter in relation to polarity. Stations 1 and 2 - il and water do not mix As a metaphor, oil and water are often
Cooking A World of New Tastes
 Cooking A World of New Tastes S E G M E N T 2 Cooking With Moist Heat Steaming Boiling Glazing Segment 2 Cooking With Moist Heat 19 S E G M E N T 2 Cooking With Moist Heat Learning Objectives Apply steaming
Cooking A World of New Tastes S E G M E N T 2 Cooking With Moist Heat Steaming Boiling Glazing Segment 2 Cooking With Moist Heat 19 S E G M E N T 2 Cooking With Moist Heat Learning Objectives Apply steaming
Activity: How Do We Clean Up an Oil Spill?
 Activity: How Do We Clean Up an Oil Spill? Summary In this activity, students simulate an oil spill and test different materials abilities to clean the oil spill. Resource Type Activity Grade Level High
Activity: How Do We Clean Up an Oil Spill? Summary In this activity, students simulate an oil spill and test different materials abilities to clean the oil spill. Resource Type Activity Grade Level High
Safety Safety glasses or goggles must be worn in the laboratory at all times.
 APPLE BROWNING: A STUDY OF OXIDATION OF FOODS 2005, 1997 by David A. Katz. All rights reserved. Reproduction permitted for education use provided original copyright is included. You are preparing for a
APPLE BROWNING: A STUDY OF OXIDATION OF FOODS 2005, 1997 by David A. Katz. All rights reserved. Reproduction permitted for education use provided original copyright is included. You are preparing for a
2 MATTER. 2.1 Physical and Chemical Properties and Changes
 2 MATTER Matter is the material of which the universe is composed. It has two characteristics: It has mass; and It occupies space (i.e., it has a volume). Matter can be found in three generic states: Solid;
2 MATTER Matter is the material of which the universe is composed. It has two characteristics: It has mass; and It occupies space (i.e., it has a volume). Matter can be found in three generic states: Solid;
Table 1. Common esters used for flavors and fragrances
 ESTERS An Introduction to rganic hemistry Reactions 2012, 2006, 1990, 1982 by David A. Katz. All rights reserved. Reproduction permitted for educationa use provided original copyright is included. In contrast
ESTERS An Introduction to rganic hemistry Reactions 2012, 2006, 1990, 1982 by David A. Katz. All rights reserved. Reproduction permitted for educationa use provided original copyright is included. In contrast
Experiment #8 properties of Alcohols and Phenols
 Introduction Experiment #8 properties of Alcohols and Phenols As has been mentioned before, over 20 million organic compounds have been identified. If each substance had to be studied as an entity completely
Introduction Experiment #8 properties of Alcohols and Phenols As has been mentioned before, over 20 million organic compounds have been identified. If each substance had to be studied as an entity completely
Chemical Changes. Measuring a Chemical Reaction. Name(s)
 Chemical Changes Name(s) In the particle model of matter, individual atoms can be bound tightly to other atoms to form molecules. For example, water molecules are made up of two hydrogen atoms bound to
Chemical Changes Name(s) In the particle model of matter, individual atoms can be bound tightly to other atoms to form molecules. For example, water molecules are made up of two hydrogen atoms bound to
TEACHING CHEMISTRY ONLINE
 TEACHING CHEMISTRY ONLINE Karen Tobias and Dr. David D. Kumar, FAIC* Abstract This paper describes teaching chemistry online using examples from Broward Virtual, a franchise of Florida Virtual School.
TEACHING CHEMISTRY ONLINE Karen Tobias and Dr. David D. Kumar, FAIC* Abstract This paper describes teaching chemistry online using examples from Broward Virtual, a franchise of Florida Virtual School.
Exploring Energy. Third - Fifth TEKS. Vocabulary
 Exploring Energy Third - Fifth TEKS Third Grade: 3.5A, 3.5B, 3.5C, 3.6A Fourth Grade: 4.5A, 4.5B, 4.6A, 4.6B, 4.6C Fifth Grade: 5.5A, 5.6A, 5.6B Vocabulary conductor, convection, conversions, electrical,
Exploring Energy Third - Fifth TEKS Third Grade: 3.5A, 3.5B, 3.5C, 3.6A Fourth Grade: 4.5A, 4.5B, 4.6A, 4.6B, 4.6C Fifth Grade: 5.5A, 5.6A, 5.6B Vocabulary conductor, convection, conversions, electrical,
The Digestive System Grade 5
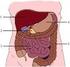 TEACHING LEARNING COLLABORATIVE (TLC) LIFE SCIENCE The Digestive System Grade 5 Created by: Shelly Bell (Kelseyville Elementary School), Bart Pontoni (Riviera Elementary School), Shane Lee (Pomo Elementary
TEACHING LEARNING COLLABORATIVE (TLC) LIFE SCIENCE The Digestive System Grade 5 Created by: Shelly Bell (Kelseyville Elementary School), Bart Pontoni (Riviera Elementary School), Shane Lee (Pomo Elementary
Target Mole Lab. Mole Relationships and the Balanced Equation. For each student group Hydrochloric acid solution, HCl, 3 M, 30 ml
 elearning 2009 Introduction Target Mole Lab Mole Relationships and the Balanced Equation Publication No. A common chemical reaction used in chemistry class is zinc and hydrochloric In this lab, students
elearning 2009 Introduction Target Mole Lab Mole Relationships and the Balanced Equation Publication No. A common chemical reaction used in chemistry class is zinc and hydrochloric In this lab, students
1. Qualitative Analysis of Chromium, Iron, and Copper
 1. Qualitative Analysis of Chromium, Iron, and Copper Introduction We have used copper and iron as basic materials since the Bronze and Iron Ages, but our extensive use of chromium began only after the
1. Qualitative Analysis of Chromium, Iron, and Copper Introduction We have used copper and iron as basic materials since the Bronze and Iron Ages, but our extensive use of chromium began only after the
Recovery of Elemental Copper from Copper (II) Nitrate
 Recovery of Elemental Copper from Copper (II) Nitrate Objectives: Challenge: Students should be able to - recognize evidence(s) of a chemical change - convert word equations into formula equations - perform
Recovery of Elemental Copper from Copper (II) Nitrate Objectives: Challenge: Students should be able to - recognize evidence(s) of a chemical change - convert word equations into formula equations - perform
WHAT S NEW, CO? Thanks for the opportunity to work with your students. Our goal is to teach developmentally TEACHER S GUIDE
 TEACHER S GUIDE WHAT S NEW, CO? GET TO KNOW A CHEMICAL REACTION 2 Thanks for the opportunity to work with your students. Our goal is to teach developmentally appropriate chemistry concepts that support
TEACHER S GUIDE WHAT S NEW, CO? GET TO KNOW A CHEMICAL REACTION 2 Thanks for the opportunity to work with your students. Our goal is to teach developmentally appropriate chemistry concepts that support
USING TECHNOLOGY FOR DATA COLLECTION
 USING TECHNOLOGY FOR DATA COLLECTION The advances in technology have provided a number of instruments for collecting and interpreting data. These include portable microscopes, electronic balances, ph meters,
USING TECHNOLOGY FOR DATA COLLECTION The advances in technology have provided a number of instruments for collecting and interpreting data. These include portable microscopes, electronic balances, ph meters,
PHYSICAL AND CHEMICAL PROPERTIES AND CHANGES
 PHYSICAL AND CHEMICAL PROPERTIES AND CHANGES Name Key PHYSICAL PROPERTY CHEMICAL PROPERTY 1. observed with senses 1. indicates how a substance 2. determined without destroying matter reacts with something
PHYSICAL AND CHEMICAL PROPERTIES AND CHANGES Name Key PHYSICAL PROPERTY CHEMICAL PROPERTY 1. observed with senses 1. indicates how a substance 2. determined without destroying matter reacts with something
First Grade Unit A: PHYSICAL SCIENCE Chapter 1: Observing Solids, Liquids and Gases Lessons 1 to 5
 First Grade Unit A: PHYSICAL SCIENCE Chapter 1: Observing Solids, Liquids and Gases Lessons 1 to 5 Physical Science Overview Materials (matter) come in different forms. Water can be rain falling (liquid)
First Grade Unit A: PHYSICAL SCIENCE Chapter 1: Observing Solids, Liquids and Gases Lessons 1 to 5 Physical Science Overview Materials (matter) come in different forms. Water can be rain falling (liquid)
Non-polar hydrocarbon chain
 THE SCIENCE OF SOAPS AND DETERGENTS 2000 by David A. Katz. All rights reserved Reproduction permitted for educational purposes as long as the original copyright is included. INTRODUCTION A soap is a salt
THE SCIENCE OF SOAPS AND DETERGENTS 2000 by David A. Katz. All rights reserved Reproduction permitted for educational purposes as long as the original copyright is included. INTRODUCTION A soap is a salt
Ontario Science and Technology Curriculum 1999 Strand: Matter and Materials Topic: Properties of Liquids and Solids Grade: 2
 Name: Ontario Science and Technology Curriculum 1999 Strand: Matter and Materials Topic: Properties of Liquids and Solids Grade: 2 All rights reserved Developed by T Tasker May be photocopied for classroom
Name: Ontario Science and Technology Curriculum 1999 Strand: Matter and Materials Topic: Properties of Liquids and Solids Grade: 2 All rights reserved Developed by T Tasker May be photocopied for classroom
ISOLATION OF CAFFEINE FROM TEA
 ISLATIN F CAFFEINE FRM TEA Introduction In this experiment, caffeine is isolated from tealeaves. The chief problem with the isolation is that caffeine does not exist alone in the tealeaves, but other natural
ISLATIN F CAFFEINE FRM TEA Introduction In this experiment, caffeine is isolated from tealeaves. The chief problem with the isolation is that caffeine does not exist alone in the tealeaves, but other natural
Ice Cream Lab- A Tasty Phase Change!
 Ice Cream Lab- A Tasty! Name Date EN Class Purpose: To investigate the effects of heat transfer on phase changes. To investigate the effects of temperature changes on physical changes. Materials: ½ cup
Ice Cream Lab- A Tasty! Name Date EN Class Purpose: To investigate the effects of heat transfer on phase changes. To investigate the effects of temperature changes on physical changes. Materials: ½ cup
How to write a formal lab report correctly. This is based off a lab done in AP biology and all examples are taken from student lab write-ups.
 How to write a formal lab report correctly. This is based off a lab done in AP biology and all examples are taken from student lab write-ups. Title: Potato Catalase Enzyme Lab (1 point). * Objective, variables,
How to write a formal lab report correctly. This is based off a lab done in AP biology and all examples are taken from student lab write-ups. Title: Potato Catalase Enzyme Lab (1 point). * Objective, variables,
Unit 1 - Pure Substances and Mixtures Chapter 2: Solutions
 2.1 Solutes & Solvents Vocabulary: Unit 1 - Pure Substances and Mixtures Chapter 2: Solutions solvent the larger part of a solution - the part of a solution into which the solutes dissolve solute the smaller
2.1 Solutes & Solvents Vocabulary: Unit 1 - Pure Substances and Mixtures Chapter 2: Solutions solvent the larger part of a solution - the part of a solution into which the solutes dissolve solute the smaller
10-ml Graduated cylinder 40 ml 3% Hydrogen peroxide solution (found in stores) Straight-edged razor blade Scissors and Forceps (tweezers)
 Name: Class: Date: Objectives * Measure the effects of changes in temperature, ph, and enzyme concentration on reaction rates of an enzyme catalyzed reaction in a controlled experiment. * Explain how environmental
Name: Class: Date: Objectives * Measure the effects of changes in temperature, ph, and enzyme concentration on reaction rates of an enzyme catalyzed reaction in a controlled experiment. * Explain how environmental
5th Grade Lesson Plan: The Cell: The building blocks of life
 5th Grade Lesson Plan: The Cell: The building blocks of life Overview This series of lessons was designed to meet the needs of gifted children for extension beyond the standard curriculum with the greatest
5th Grade Lesson Plan: The Cell: The building blocks of life Overview This series of lessons was designed to meet the needs of gifted children for extension beyond the standard curriculum with the greatest
CLEANING WATER. Student Section
 National Aeronautics and Space Administration CLEANING WATER Student Section Student Name This lesson challenges you to create and test a water filtration system. During this lesson, you will design and
National Aeronautics and Space Administration CLEANING WATER Student Section Student Name This lesson challenges you to create and test a water filtration system. During this lesson, you will design and
Disinfecting Your Private Well
 TCEQ GENERAL INFORMATION Water Supply Division GI-432 August 2013 Disinfecting Your Private Well Is Your Well Flooded? Disinfect It Before You Drink It! If your private well is flooded, do not use water
TCEQ GENERAL INFORMATION Water Supply Division GI-432 August 2013 Disinfecting Your Private Well Is Your Well Flooded? Disinfect It Before You Drink It! If your private well is flooded, do not use water
EXPERIMENT 4 Acid Strength
 EXPERIMENT 4 Acid Strength Introduction Many common substances are either acids or bases. Some acids, like stomach acid are necessary for our health, while others, like sulfuric acid are dangerous and
EXPERIMENT 4 Acid Strength Introduction Many common substances are either acids or bases. Some acids, like stomach acid are necessary for our health, while others, like sulfuric acid are dangerous and
Fiber Analysis 2005, 2004, 2003, 2001, 1999 by David A. Katz. All rights reserved.
 Fiber Analysis 2005, 2004, 2003, 2001, 1999 by David A. Katz. All rights reserved. Fiber evidence can be found at crime scenes in a number of different ways. In personal contact between the clothing of
Fiber Analysis 2005, 2004, 2003, 2001, 1999 by David A. Katz. All rights reserved. Fiber evidence can be found at crime scenes in a number of different ways. In personal contact between the clothing of
ANALYSIS OF VITAMIN C
 Purpose To learn how to analyze food for vitamin C content and to examine various sources for vitamin C content. Caution Handle the glassware with caution to prevent breakage. When using a burner in the
Purpose To learn how to analyze food for vitamin C content and to examine various sources for vitamin C content. Caution Handle the glassware with caution to prevent breakage. When using a burner in the
DYES AND DYEING 2003 by David A. Katz. All rights reserved. Permission for classroom use provided original copyright is included.
 DYES AND DYEING 2003 by David A. Katz. All rights reserved. Permission for classroom use provided original copyright is included. Dyeing of textiles has been practiced for thousands of years with the first
DYES AND DYEING 2003 by David A. Katz. All rights reserved. Permission for classroom use provided original copyright is included. Dyeing of textiles has been practiced for thousands of years with the first
Leavener Lineup. Getting started. How do we use chemical reactions in the kitchen? Hands-on experiment. Year levels 4 5. Curriculum Links.
 rise and Shine: what Makes Bread Rise? Lesson 2 Leavener Lineup Year levels 4 5 Curriculum Links Science Science knowledge helps people to understand the effect of their actions (Yr 4, ACSHE062). Solids,
rise and Shine: what Makes Bread Rise? Lesson 2 Leavener Lineup Year levels 4 5 Curriculum Links Science Science knowledge helps people to understand the effect of their actions (Yr 4, ACSHE062). Solids,
