EZValidation Online Tool. The easy way to apply guidelines. The EZValidation Online Tool. The implementation of new tests
|
|
|
- Aldous Lambert
- 8 years ago
- Views:
Transcription
1 The implementation of new tests The EZValidation Online Tool Since 999, AcroMetrix has provided quality control requires validation or verification studies that meet is a comprehensive tool specifically for assisting in the materials with the very best support possible to our guidelines established by governmental agencies or validation and verification of molecular tests. It provides molecular diagnostics customers. AcroMetrix became international institutions. The interpretation of guidelines, standard guideline choices for the laboratory to follow, part of Life Technologies in 200 and is organized under especially in regards to new testing techniques such as calculates the number of reactions and quality control the Applied Biosystems family of products. molecular diagnostic methods, can be very challenging. materials required, and applies statistical analysis Access to standardized protocols that meet or exceed to generate a final report to support user validation regulations, coupled with the appropriate control materials and verification of molecular tests. Contact your local and software solutions to aid in data analysis, is what sales representative or perform a free test drive of our laboratory managers look for. online tool. Over the coming months, you will notice changes to the AcroMetrix website and product packaging as we migrate to the Applied Biosystems brand. We re confident that this rebranding process will not only help us to continue to grow as a company, but that it will enable us to better assist you. For more information, please go to EZValidation Online Tool The EZValidation Online Tool is a tool intended to assist laboratories in meeting their verification and validation requirements. Each laboratory is responsible for ensuring compliance with applicable international, national, and local clinical laboratory regulations and other specific accreditations requirements. 200 Life Technologies Corporation. All rights reserved. The trademarks mentioned herein are the property of Life Technologies Corporation or their respective owners. Printed in the USA. CO AcroMetrix by Life Technologies 600 Egret Ct. Benicia, CA 9450 USA Phone Toll Free International Sales For our office locations please call the division headquarters or refer to our website at The easy way to apply guidelines
2 The implementation of new tests The EZValidation Online Tool Since 999, AcroMetrix has provided quality control requires validation or verification studies that meet is a comprehensive tool specifically for assisting in the materials with the very best support possible to our guidelines established by governmental agencies or validation and verification of molecular tests. It provides molecular diagnostics customers. AcroMetrix became international institutions. The interpretation of guidelines, standard guideline choices for the laboratory to follow, part of Life Technologies in 200 and is organized under especially in regards to new testing techniques such as calculates the number of reactions and quality control the Applied Biosystems family of products. molecular diagnostic methods, can be very challenging. materials required, and applies statistical analysis Access to standardized protocols that meet or exceed to generate a final report to support user validation regulations, coupled with the appropriate control materials and verification of molecular tests. Contact your local and software solutions to aid in data analysis, is what sales representative or perform a free test drive of our laboratory managers look for. online tool. Over the coming months, you will notice changes to the AcroMetrix website and product packaging as we migrate to the Applied Biosystems brand. We re confident that this rebranding process will not only help us to continue to grow as a company, but that it will enable us to better assist you. For more information, please go to EZValidation Online Tool The EZValidation Online Tool is a tool intended to assist laboratories in meeting their verification and validation requirements. Each laboratory is responsible for ensuring compliance with applicable international, national, and local clinical laboratory regulations and other specific accreditations requirements. 200 Life Technologies Corporation. All rights reserved. The trademarks mentioned herein are the property of Life Technologies Corporation or their respective owners. Printed in the USA. CO AcroMetrix by Life Technologies 600 Egret Ct. Benicia, CA 9450 USA Phone Toll Free International Sales For our office locations please call the division headquarters or refer to our website at The easy way to apply guidelines
3 How much time does validation of a molecular test take? It typically takes 58 working days, on average, to perform validation or verification of a molecular test, according to a recent survey of US laboratory managers and supervisors performing molecular testing. Some of the challenges reported were: The EZValidation Online Tool is designed specifically for Difficulty in determining the requirements necessary for a validation study Relying on software used in other laboratories that does not account for molecular methods Creating statistical analyses in Excel spreadsheets Keeping organized when working with multiple people on a validation The EZValidation Online Tool is the first to provide these molecular assay validation and verification features: Standard and customizable protocol options for the laboratory to follow Automatic calculation of the number of reactions and quality materials required Customizable plate mapping based upon your amplification detection system The ability to upload data for automated statistical analysis Validation or verification protocol and final report for printing or storage as a PDF file molecular diagnostic laboratories performing quantitative validation or verification studies. Days to Perform Molecular Validation Steps 30 Take a free test drive In 4 easy steps, the EZValidation Online Tool will calculate the number of reactions, panels, and standards needed. Visit our site at for a free test drive! Identify guideline (ISO, CLIA, or custom) Enter lab and assay info, calculate reagent needs Set up other users, create and print plate maps Enter and review data Generate reports Figure 3. Drop-down driven choices of packaged assays to choose from. Figure 4. A choice of ISO 589 or CAP/CLIA/NY State guidelines and the list of protocols which can be chosen or de-selected. Figure 5. Confirmation of your instruments, list of controls, and extraction volume needed. Figure 6. Easy-to-read order summary containing the number of total reactions and the standards and controls needed for your validation or verification. Options to save your study (if you log in) and request a quote are provided. Figure 2. Typical molecular assay validation workflow Research Guidelines and Create Protocols Set up Statistical Analysis and Create the Final Validation/ Templates Verification Report Figure. Typical number of working days required to perform different steps during the validation of a molecular test (excluding time to execute protocols). With the EZValidation Online Tool, you have a convenient, intuitive application to guide you through: Translating regulatory guidelines into user-defined validation or verification prot ocols Calculating the amount of reagents and quality materials needed to run the protocol Consolidating studies (i.e., accuracy, precision, LOD) across runs and days to minimize time and reagents Selecting and performing statistical analyses Combining results and data analysis into a single validation report for signature Source: Molecular verification and validation survey of US laboratory managers and supervisors. Performed September 200. Standard guideline choices are just the beginning AcroMetrix is focused on quality control products, services, and systems for molecular diagnostics. The EZValidation Online Tool offers a choice of protocols based on ISO 589 or CAP/CLIA/New York State validation and verification guidelines for setting up your studies. In addition, we ve designed the online tool so that you can create your own protocols. Keep up with changing guidelines As a leader in molecular quality materials, AcroMetrix closely monitors changing requirements for clinical laboratories as new standards emerge. Because the EZValidation Online Tool is webbased, you have access to new protocols as guidelines change. Designed for AcroMetrix, but compatible with any While we encourage the use of AcroMetrix quality materials for your validations and verifications, we realize your need for flexibility to accommodate new tests. The EZValidation Online Tool is designed for use with or without AcroMetrix quality control materials. Year subscriptions or pay per use are available. Inquire with your local sales representative for more information.
4 How much time does validation of a molecular test take? It typically takes 58 working days, on average, to perform validation or verification of a molecular test, according to a recent survey of US laboratory managers and supervisors performing molecular testing. Some of the challenges reported were: The EZValidation Online Tool is designed specifically for Difficulty in determining the requirements necessary for a validation study Relying on software used in other laboratories that does not account for molecular methods Creating statistical analyses in Excel spreadsheets Keeping organized when working with multiple people on a validation The EZValidation Online Tool is the first to provide these molecular assay validation and verification features: Standard and customizable protocol options for the laboratory to follow Automatic calculation of the number of reactions and quality materials required Customizable plate mapping based upon your amplification detection system The ability to upload data for automated statistical analysis Validation or verification protocol and final report for printing or storage as a PDF file molecular diagnostic laboratories performing quantitative validation or verification studies. Days to Perform Molecular Validation Steps 30 Take a free test drive In 4 easy steps, the EZValidation Online Tool will calculate the number of reactions, panels, and standards needed. Visit our site at for a free test drive! Identify guideline (ISO, CLIA, or custom) Enter lab and assay info, calculate reagent needs Set up other users, create and print plate maps Enter and review data Generate reports Figure 3. Drop-down driven choices of packaged assays to choose from. Figure 4. A choice of ISO 589 or CAP/CLIA/NY State guidelines and the list of protocols which can be chosen or de-selected. Figure 5. Confirmation of your instruments, list of controls, and extraction volume needed. Figure 6. Easy-to-read order summary containing the number of total reactions and the standards and controls needed for your validation or verification. Options to save your study (if you log in) and request a quote are provided. Figure 2. Typical molecular assay validation workflow Research Guidelines and Create Protocols Set up Statistical Analysis and Create the Final Validation/ Templates Verification Report Figure. Typical number of working days required to perform different steps during the validation of a molecular test (excluding time to execute protocols). With the EZValidation Online Tool, you have a convenient, intuitive application to guide you through: Translating regulatory guidelines into user-defined validation or verification prot ocols Calculating the amount of reagents and quality materials needed to run the protocol Consolidating studies (i.e., accuracy, precision, LOD) across runs and days to minimize time and reagents Selecting and performing statistical analyses Combining results and data analysis into a single validation report for signature Source: Molecular verification and validation survey of US laboratory managers and supervisors. Performed September 200. Standard guideline choices are just the beginning AcroMetrix is focused on quality control products, services, and systems for molecular diagnostics. The EZValidation Online Tool offers a choice of protocols based on ISO 589 or CAP/CLIA/New York State validation and verification guidelines for setting up your studies. In addition, we ve designed the online tool so that you can create your own protocols. Keep up with changing guidelines As a leader in molecular quality materials, AcroMetrix closely monitors changing requirements for clinical laboratories as new standards emerge. Because the EZValidation Online Tool is webbased, you have access to new protocols as guidelines change. Designed for AcroMetrix, but compatible with any While we encourage the use of AcroMetrix quality materials for your validations and verifications, we realize your need for flexibility to accommodate new tests. The EZValidation Online Tool is designed for use with or without AcroMetrix quality control materials. Year subscriptions or pay per use are available. Inquire with your local sales representative for more information.
5 How much time does validation of a molecular test take? It typically takes 58 working days, on average, to perform validation or verification of a molecular test, according to a recent survey of US laboratory managers and supervisors performing molecular testing. Some of the challenges reported were: The EZValidation Online Tool is designed specifically for Difficulty in determining the requirements necessary for a validation study Relying on software used in other laboratories that does not account for molecular methods Creating statistical analyses in Excel spreadsheets Keeping organized when working with multiple people on a validation The EZValidation Online Tool is the first to provide these molecular assay validation and verification features: Standard and customizable protocol options for the laboratory to follow Automatic calculation of the number of reactions and quality materials required Customizable plate mapping based upon your amplification detection system The ability to upload data for automated statistical analysis Validation or verification protocol and final report for printing or storage as a PDF file molecular diagnostic laboratories performing quantitative validation or verification studies. Days to Perform Molecular Validation Steps 30 Take a free test drive In 4 easy steps, the EZValidation Online Tool will calculate the number of reactions, panels, and standards needed. Visit our site at for a free test drive! Identify guideline (ISO, CLIA, or custom) Enter lab and assay info, calculate reagent needs Set up other users, create and print plate maps Enter and review data Generate reports Figure 3. Drop-down driven choices of packaged assays to choose from. Figure 4. A choice of ISO 589 or CAP/CLIA/NY State guidelines and the list of protocols which can be chosen or de-selected. Figure 5. Confirmation of your instruments, list of controls, and extraction volume needed. Figure 6. Easy-to-read order summary containing the number of total reactions and the standards and controls needed for your validation or verification. Options to save your study (if you log in) and request a quote are provided. Figure 2. Typical molecular assay validation workflow Research Guidelines and Create Protocols Set up Statistical Analysis and Create the Final Validation/ Templates Verification Report Figure. Typical number of working days required to perform different steps during the validation of a molecular test (excluding time to execute protocols). With the EZValidation Online Tool, you have a convenient, intuitive application to guide you through: Translating regulatory guidelines into user-defined validation or verification prot ocols Calculating the amount of reagents and quality materials needed to run the protocol Consolidating studies (i.e., accuracy, precision, LOD) across runs and days to minimize time and reagents Selecting and performing statistical analyses Combining results and data analysis into a single validation report for signature Source: Molecular verification and validation survey of US laboratory managers and supervisors. Performed September 200. Standard guideline choices are just the beginning AcroMetrix is focused on quality control products, services, and systems for molecular diagnostics. The EZValidation Online Tool offers a choice of protocols based on ISO 589 or CAP/CLIA/New York State validation and verification guidelines for setting up your studies. In addition, we ve designed the online tool so that you can create your own protocols. Keep up with changing guidelines As a leader in molecular quality materials, AcroMetrix closely monitors changing requirements for clinical laboratories as new standards emerge. Because the EZValidation Online Tool is webbased, you have access to new protocols as guidelines change. Designed for AcroMetrix, but compatible with any While we encourage the use of AcroMetrix quality materials for your validations and verifications, we realize your need for flexibility to accommodate new tests. The EZValidation Online Tool is designed for use with or without AcroMetrix quality control materials. Year subscriptions or pay per use are available. Inquire with your local sales representative for more information.
6 The implementation of new tests The EZValidation Online Tool Since 999, AcroMetrix has provided quality control requires validation or verification studies that meet is a comprehensive tool specifically for assisting in the materials with the very best support possible to our guidelines established by governmental agencies or validation and verification of molecular tests. It provides molecular diagnostics customers. AcroMetrix became international institutions. The interpretation of guidelines, standard guideline choices for the laboratory to follow, part of Life Technologies in 200 and is organized under especially in regards to new testing techniques such as calculates the number of reactions and quality control the Applied Biosystems family of products. molecular diagnostic methods, can be very challenging. materials required, and applies statistical analysis Access to standardized protocols that meet or exceed to generate a final report to support user validation regulations, coupled with the appropriate control materials and verification of molecular tests. Contact your local and software solutions to aid in data analysis, is what sales representative or perform a free test drive of our laboratory managers look for. online tool. Over the coming months, you will notice changes to the AcroMetrix website and product packaging as we migrate to the Applied Biosystems brand. We re confident that this rebranding process will not only help us to continue to grow as a company, but that it will enable us to better assist you. For more information, please go to EZValidation Online Tool The EZValidation Online Tool is a tool intended to assist laboratories in meeting their verification and validation requirements. Each laboratory is responsible for ensuring compliance with applicable international, national, and local clinical laboratory regulations and other specific accreditations requirements. 200 Life Technologies Corporation. All rights reserved. The trademarks mentioned herein are the property of Life Technologies Corporation or their respective owners. Printed in the USA. CO AcroMetrix by Life Technologies 600 Egret Ct. Benicia, CA 9450 USA Phone Toll Free International Sales For our office locations please call the division headquarters or refer to our website at The easy way to apply guidelines
WinKQCL 5 Endotoxin Detection and Analysis Software We Analyze Endotoxin Data Every Day
 Pharma&Biotech WinKQCL 5 Endotoxin Detection and Analysis Software We Analyze Endotoxin Data Every Day For Endotoxin Detection Pharma&Biotech WinKQCL 5 Software for Endotoxin Detection and Analysis WinKQCL
Pharma&Biotech WinKQCL 5 Endotoxin Detection and Analysis Software We Analyze Endotoxin Data Every Day For Endotoxin Detection Pharma&Biotech WinKQCL 5 Software for Endotoxin Detection and Analysis WinKQCL
The GeneAmp PCR System 9700. Results you can trust. A PCR platform you can grow with. GeneAmp. PCR System 9700
 The GeneAmp PCR System 9700 Results you can trust. A PCR platform you can grow with. GeneAmp PCR System 9700 The GeneAmp PCR System 9700 fits your lab bench, your applications and your budget. The GeneAmp
The GeneAmp PCR System 9700 Results you can trust. A PCR platform you can grow with. GeneAmp PCR System 9700 The GeneAmp PCR System 9700 fits your lab bench, your applications and your budget. The GeneAmp
Cliquid ChemoView 3.0 Software Simple automated analysis, from sample to report
 PRODUCT BULLETIN Cliquid ChemoView 3.0 Software for Routine Screening and Quantitation Cliquid ChemoView 3.0 Software Simple automated analysis, from sample to report KEY FEATURES Secure user login that
PRODUCT BULLETIN Cliquid ChemoView 3.0 Software for Routine Screening and Quantitation Cliquid ChemoView 3.0 Software Simple automated analysis, from sample to report KEY FEATURES Secure user login that
Quantifiler Human DNA Quantification Kit Quantifiler Y Human Male DNA Quantification Kit
 Product Bulletin Human Identification Quantifiler Human DNA Quantification Kit Quantifiler Y Human Male DNA Quantification Kit The Quantifiler kits produce reliable and reproducible results, helping to
Product Bulletin Human Identification Quantifiler Human DNA Quantification Kit Quantifiler Y Human Male DNA Quantification Kit The Quantifiler kits produce reliable and reproducible results, helping to
Picis Anesthesia. Completely Integrated Anesthesia Management System
 Picis Anesthesia Completely Integrated Anesthesia Management System 1 2 Picis Anesthesia Completely Integrated Anesthesia Information Management System Picis Anesthesia Information Management System (AIMS)
Picis Anesthesia Completely Integrated Anesthesia Management System 1 2 Picis Anesthesia Completely Integrated Anesthesia Information Management System Picis Anesthesia Information Management System (AIMS)
Automated Lab Management for Illumina SeqLab
 Automated Lab Management for Illumina SeqLab INTRODUCTION Whole genome sequencing holds the promise of understanding genetic variation and disease better than ever before. In response, Illumina developed
Automated Lab Management for Illumina SeqLab INTRODUCTION Whole genome sequencing holds the promise of understanding genetic variation and disease better than ever before. In response, Illumina developed
Real-time PCR: Understanding C t
 APPLICATION NOTE Real-Time PCR Real-time PCR: Understanding C t Real-time PCR, also called quantitative PCR or qpcr, can provide a simple and elegant method for determining the amount of a target sequence
APPLICATION NOTE Real-Time PCR Real-time PCR: Understanding C t Real-time PCR, also called quantitative PCR or qpcr, can provide a simple and elegant method for determining the amount of a target sequence
Financial Management TRANSACTION CONTROL AND APPROVAL
 Financial Management In today s complex, global, and regulated environment, organizations face numerous challenges in trying to meet deadlines, comply with local regulations and multiple reporting requirements,
Financial Management In today s complex, global, and regulated environment, organizations face numerous challenges in trying to meet deadlines, comply with local regulations and multiple reporting requirements,
TruSeq Custom Amplicon v1.5
 Data Sheet: Targeted Resequencing TruSeq Custom Amplicon v1.5 A new and improved amplicon sequencing solution for interrogating custom regions of interest. Highlights Figure 1: TruSeq Custom Amplicon Workflow
Data Sheet: Targeted Resequencing TruSeq Custom Amplicon v1.5 A new and improved amplicon sequencing solution for interrogating custom regions of interest. Highlights Figure 1: TruSeq Custom Amplicon Workflow
Optum Anesthesia. Completely integrated anesthesia information management system
 Optum Anesthesia Completely integrated anesthesia information management system 2 Completely integrated anesthesia information management system Optum Anesthesia Information Management System (AIMS) helps
Optum Anesthesia Completely integrated anesthesia information management system 2 Completely integrated anesthesia information management system Optum Anesthesia Information Management System (AIMS) helps
Welcome to the fully integrated autoimmune laboratory. Saves time by reducing hands-on functions NOVA Lite Barcoded IFA Slides
 The Integrated Lab Connected Welcome to the fully integrated autoimmune laboratory. Saves time by reducing hands-on functions NOVA Lite Barcoded IFA Slides NOVA View Reduces costs with automation efficiencies
The Integrated Lab Connected Welcome to the fully integrated autoimmune laboratory. Saves time by reducing hands-on functions NOVA Lite Barcoded IFA Slides NOVA View Reduces costs with automation efficiencies
Research Coordinator - PI s who have research coordinators or secretarial support can designate individuals to manage their IRB protocols in Mentor.
 Mentor Online IRB System IRB s require lots of documentation and managing this process can get to be a burden for both investigators and the IRB committee and administrator. The Mentor IRB system is designed
Mentor Online IRB System IRB s require lots of documentation and managing this process can get to be a burden for both investigators and the IRB committee and administrator. The Mentor IRB system is designed
TaqMan Genotyper Software v1.0.1 TaqMan Genotyping Data Analysis Software
 TaqMan Genotyper Software v1.0.1 TaqMan Genotyping Data Analysis Software March 2011 Product Overview TaqMan Genotyper Software TaqMan Genotyper Software Standalone data analysis software for Applied Biosystems
TaqMan Genotyper Software v1.0.1 TaqMan Genotyping Data Analysis Software March 2011 Product Overview TaqMan Genotyper Software TaqMan Genotyper Software Standalone data analysis software for Applied Biosystems
TotalChrom. Chromatography Data Systems. Streamlining your laboratory workflow
 TotalChrom Chromatography Data Systems Streamlining your laboratory workflow maximize productivity with TotalChrom CDS Acquiring, processing, reporting, reviewing and approving data is a streamlined series
TotalChrom Chromatography Data Systems Streamlining your laboratory workflow maximize productivity with TotalChrom CDS Acquiring, processing, reporting, reviewing and approving data is a streamlined series
TargetQuan 3 Software. Leading the way in regulatory. POPs quantification. Bullet Bullet Bullet
 TargetQuan 3 Software Leading the way in regulatory POPs quantification Bullet Bullet Bullet Leading the way in regulatory POPs quantification Analyse Samples Open Sequence Process Sequence Analyse Samples
TargetQuan 3 Software Leading the way in regulatory POPs quantification Bullet Bullet Bullet Leading the way in regulatory POPs quantification Analyse Samples Open Sequence Process Sequence Analyse Samples
Best Practices for Maintaining Quality in Molecular Diagnostics Gyorgy Abel, MD, PhD
 Best Practices for Maintaining Quality in Molecular Diagnostics Gyorgy Abel, MD, PhD Director, Clinical Chemistry Molecular Diagnostics / Immunology Department of Laboratory Medicine Lahey Clinic Medical
Best Practices for Maintaining Quality in Molecular Diagnostics Gyorgy Abel, MD, PhD Director, Clinical Chemistry Molecular Diagnostics / Immunology Department of Laboratory Medicine Lahey Clinic Medical
OpenArray Sample Tracker Software
 QUICK REFERENCE OpenArray Sample Tracker Software For QuantStudio 12K Flex OpenArray Sample Block and the OpenArray Real-Time PCR System Publication Part Number 4460657 Rev. C Revision Date May 2012 Contents
QUICK REFERENCE OpenArray Sample Tracker Software For QuantStudio 12K Flex OpenArray Sample Block and the OpenArray Real-Time PCR System Publication Part Number 4460657 Rev. C Revision Date May 2012 Contents
Clinical trial for 510(k) clearance
 CAPILLARY ELECTROPHORESIS Clinical trial for 510(k) clearance The 3500 and 3500xL Dx Genetic Analyzers CS2 CO19459 Molokai- Instrument prelaunch brochure.indd 1 The next step in molecular medicine 3500
CAPILLARY ELECTROPHORESIS Clinical trial for 510(k) clearance The 3500 and 3500xL Dx Genetic Analyzers CS2 CO19459 Molokai- Instrument prelaunch brochure.indd 1 The next step in molecular medicine 3500
Data Analysis on the ABI PRISM 7700 Sequence Detection System: Setting Baselines and Thresholds. Overview. Data Analysis Tutorial
 Data Analysis on the ABI PRISM 7700 Sequence Detection System: Setting Baselines and Thresholds Overview In order for accuracy and precision to be optimal, the assay must be properly evaluated and a few
Data Analysis on the ABI PRISM 7700 Sequence Detection System: Setting Baselines and Thresholds Overview In order for accuracy and precision to be optimal, the assay must be properly evaluated and a few
Document Change Control
 Document Change Control for Quality & Regulatory Compliance Quality and Compliance Solutions Document Control Software for Microsoft Windows Document Change Control Improve efficiency and enforce consistency
Document Change Control for Quality & Regulatory Compliance Quality and Compliance Solutions Document Control Software for Microsoft Windows Document Change Control Improve efficiency and enforce consistency
SeqScape Software Version 2.5 Comprehensive Analysis Solution for Resequencing Applications
 Product Bulletin Sequencing Software SeqScape Software Version 2.5 Comprehensive Analysis Solution for Resequencing Applications Comprehensive reference sequence handling Helps interpret the role of each
Product Bulletin Sequencing Software SeqScape Software Version 2.5 Comprehensive Analysis Solution for Resequencing Applications Comprehensive reference sequence handling Helps interpret the role of each
MultiQuant Software 2.0 for Targeted Protein / Peptide Quantification
 MultiQuant Software 2.0 for Targeted Protein / Peptide Quantification Gold Standard for Quantitative Data Processing Because of the sensitivity, selectivity, speed and throughput at which MRM assays can
MultiQuant Software 2.0 for Targeted Protein / Peptide Quantification Gold Standard for Quantitative Data Processing Because of the sensitivity, selectivity, speed and throughput at which MRM assays can
Vmax Vyntus SPIRO. Advance your lung function testing experience
 Vmax Vyntus SPIRO Advance your lung function testing experience The Vmax Vyntus SPIRO with SentrySuite software is designed to advance the lung function testing experience for both the patient and the
Vmax Vyntus SPIRO Advance your lung function testing experience The Vmax Vyntus SPIRO with SentrySuite software is designed to advance the lung function testing experience for both the patient and the
What is PDF Share Forms? PDF Share Forms is a web-based solution for PDF forms integration with SharePoint
 PDF Share Forms company Multiple awards winner Replaces InfoPath with PDF forms (ISO 32000 standard) Automatically extracts data from form fields into SharePoint columns Integrates with SharePoint, Microsoft
PDF Share Forms company Multiple awards winner Replaces InfoPath with PDF forms (ISO 32000 standard) Automatically extracts data from form fields into SharePoint columns Integrates with SharePoint, Microsoft
Real-Time PCR Vs. Traditional PCR
 Real-Time PCR Vs. Traditional PCR Description This tutorial will discuss the evolution of traditional PCR methods towards the use of Real-Time chemistry and instrumentation for accurate quantitation. Objectives
Real-Time PCR Vs. Traditional PCR Description This tutorial will discuss the evolution of traditional PCR methods towards the use of Real-Time chemistry and instrumentation for accurate quantitation. Objectives
Validating Methods using Waters Empower TM 2 Method. Validation. Manager
 Validating Methods using Waters Empower TM 2 Method Validation Manager (MVM) Anders Janesten Nordic Informatics Sales Specialist 2008 Waters Corporation Decision-centric centric method development information
Validating Methods using Waters Empower TM 2 Method Validation Manager (MVM) Anders Janesten Nordic Informatics Sales Specialist 2008 Waters Corporation Decision-centric centric method development information
Infopoint Financial Control System (FCS)
 Infopoint Financial Control System (FCS) November 2010 Copyright 2010 Infor. All rights reserved. All trademarks, trade names, service marks, and logos referenced herein belong to their respective companies.
Infopoint Financial Control System (FCS) November 2010 Copyright 2010 Infor. All rights reserved. All trademarks, trade names, service marks, and logos referenced herein belong to their respective companies.
NETWRIX EVENT LOG MANAGER
 NETWRIX EVENT LOG MANAGER ADMINISTRATOR S GUIDE Product Version: 4.0 July/2012. Legal Notice The information in this publication is furnished for information use only, and does not constitute a commitment
NETWRIX EVENT LOG MANAGER ADMINISTRATOR S GUIDE Product Version: 4.0 July/2012. Legal Notice The information in this publication is furnished for information use only, and does not constitute a commitment
Document Control Management System
 Document Control Management System DocXellent is a leading provider of electronic document control software and quality software applications with over 30 years of experience. We design our products to
Document Control Management System DocXellent is a leading provider of electronic document control software and quality software applications with over 30 years of experience. We design our products to
ComplianceSP TM on SharePoint. Complete Document & Process Management for Life Sciences on SharePoint 2010 & 2013
 TM ComplianceSP TM on SharePoint Complete Document & Process Management for Life Sciences on SharePoint 2010 & 2013 Overview With increasing pressure on costs and margins across Life Sciences, the industry
TM ComplianceSP TM on SharePoint Complete Document & Process Management for Life Sciences on SharePoint 2010 & 2013 Overview With increasing pressure on costs and margins across Life Sciences, the industry
2.500 Threshold. 2.000 1000e - 001. Threshold. Exponential phase. Cycle Number
 application note Real-Time PCR: Understanding C T Real-Time PCR: Understanding C T 4.500 3.500 1000e + 001 4.000 3.000 1000e + 000 3.500 2.500 Threshold 3.000 2.000 1000e - 001 Rn 2500 Rn 1500 Rn 2000
application note Real-Time PCR: Understanding C T Real-Time PCR: Understanding C T 4.500 3.500 1000e + 001 4.000 3.000 1000e + 000 3.500 2.500 Threshold 3.000 2.000 1000e - 001 Rn 2500 Rn 1500 Rn 2000
Optimizing Automation of Internal Controls for GRC and General Business Process Compliance
 Optimizing Automation of Internal s for GRC and General Business Process Compliance Whitepaper Compliancy Software, Inc. www.compliancysoftware.com Telephone: +1.919.342.6212 Email: info@compliancysoftware.com
Optimizing Automation of Internal s for GRC and General Business Process Compliance Whitepaper Compliancy Software, Inc. www.compliancysoftware.com Telephone: +1.919.342.6212 Email: info@compliancysoftware.com
QA Software. a new way to view qa. Centralized, cloud-based data for easy management of TG-142 reporting. QA Pilot
 QA Software a new way to view qa Centralized, cloud-based data for easy management of TG-142 reporting QA Pilot All clinics are tasked with recording, reporting and archiving vast amounts of data to document
QA Software a new way to view qa Centralized, cloud-based data for easy management of TG-142 reporting QA Pilot All clinics are tasked with recording, reporting and archiving vast amounts of data to document
LanguageDesk is the web-based project management tool from Milengo that takes the hassle out of managing translation projects.
 4 LanguageDesk Cloud-based translation LanguageDesk is the web-based project management tool from Milengo that takes the hassle out of managing translation projects. BENEFITS 24/7 availability Intuitive
4 LanguageDesk Cloud-based translation LanguageDesk is the web-based project management tool from Milengo that takes the hassle out of managing translation projects. BENEFITS 24/7 availability Intuitive
Unique Software Tools to Enable Quick Screening and Identification of Residues and Contaminants in Food Samples using Accurate Mass LC-MS/MS
 Unique Software Tools to Enable Quick Screening and Identification of Residues and Contaminants in Food Samples using Accurate Mass LC-MS/MS Using PeakView Software with the XIC Manager to Get the Answers
Unique Software Tools to Enable Quick Screening and Identification of Residues and Contaminants in Food Samples using Accurate Mass LC-MS/MS Using PeakView Software with the XIC Manager to Get the Answers
Put all your results in one basket. Orchard Pathology simplifies the complexities of clinical, molecular, and pathology testing and reporting.
 Put all your results in one basket. Orchard Pathology simplifies the complexities of clinical, molecular, and pathology testing and reporting. Designed for the Comprehensive Pathology Laboratory Orchard
Put all your results in one basket. Orchard Pathology simplifies the complexities of clinical, molecular, and pathology testing and reporting. Designed for the Comprehensive Pathology Laboratory Orchard
SERVICE PLANS. More Productivity. Less Worry. SERVICE PLANS
 SERVICE PLANS More Productivity. Less Worry. SERVICE PLANS Flexible service plans to help you maximize the value of your technology investment. Let us help you improve your laboratory productivity and
SERVICE PLANS More Productivity. Less Worry. SERVICE PLANS Flexible service plans to help you maximize the value of your technology investment. Let us help you improve your laboratory productivity and
Document Control for Control Freaks. Kristina Martin, MLS(ASCP) CM,MS
 Document Control for Control Freaks Kristina Martin, MLS(ASCP) CM,MS Objectives 1. Define document control 2. List components of document control 3. Identify regulatory and standards associated with document
Document Control for Control Freaks Kristina Martin, MLS(ASCP) CM,MS Objectives 1. Define document control 2. List components of document control 3. Identify regulatory and standards associated with document
Institutional Partnership Program
 GENEWIZ Outsourcing Services Institutional Partnership Program Solid Science. Superior Service. DNA Sequencing Partners to Fuel Your Success Institutions whose success depends on significant life science
GENEWIZ Outsourcing Services Institutional Partnership Program Solid Science. Superior Service. DNA Sequencing Partners to Fuel Your Success Institutions whose success depends on significant life science
CentraLink Data Management System
 www.siemens.com/diagnostics CentraLink Data Management System Delivering quality lab results faster for better patient care Answers for life. CentraLink Data Management System Delivering quality lab results
www.siemens.com/diagnostics CentraLink Data Management System Delivering quality lab results faster for better patient care Answers for life. CentraLink Data Management System Delivering quality lab results
User Guide Package Exception Management
 User Guide Package Exception Management 70-3262-4.6 PRECISION Applications 2012 September 2012 2012 Precision Software, a division of QAD Inc. Precision Software products are copyrighted and all rights
User Guide Package Exception Management 70-3262-4.6 PRECISION Applications 2012 September 2012 2012 Precision Software, a division of QAD Inc. Precision Software products are copyrighted and all rights
MET/TEAM Test Equipment Asset Management Software
 MET/TEAM Test Equipment Asset Management Software NEW, easy-to-use, browser-based calibration asset management software Modular, browser-based calibration asset management solution MET/TEAM software is
MET/TEAM Test Equipment Asset Management Software NEW, easy-to-use, browser-based calibration asset management software Modular, browser-based calibration asset management solution MET/TEAM software is
LogInspect 5 Product Features Robust. Dynamic. Unparalleled.
 LogInspect 5 Product Features Robust. Dynamic. Unparalleled. Enjoy ultra fast search capabilities in simple and complex modes optimized for Big Data Easily filter and display relevant topics, eg: Top 10
LogInspect 5 Product Features Robust. Dynamic. Unparalleled. Enjoy ultra fast search capabilities in simple and complex modes optimized for Big Data Easily filter and display relevant topics, eg: Top 10
Automated Library Preparation for Next-Generation Sequencing
 Buyer s Guide: Automated Library Preparation for Next-Generation Sequencing What to consider as you evaluate options for automating library preparation. Yes, success can be automated. Next-generation sequencing
Buyer s Guide: Automated Library Preparation for Next-Generation Sequencing What to consider as you evaluate options for automating library preparation. Yes, success can be automated. Next-generation sequencing
Business Online Banking User Guide
 Welcome to online business banking at Lewiston State Bank. If you have not enrolled for online business banking yet, please visit one of our locations or contact customer service toll free at (800) 233
Welcome to online business banking at Lewiston State Bank. If you have not enrolled for online business banking yet, please visit one of our locations or contact customer service toll free at (800) 233
LogPoint 5.1 Product Features Robust. Dynamic. Unparalleled.
 LogPoint 5.1 Product Features Robust. Dynamic. Unparalleled. LOGPOINT Enjoy ultra fast search capabilities in simple and complex modes optimized for Big Data Easily filter and display relevant topics,
LogPoint 5.1 Product Features Robust. Dynamic. Unparalleled. LOGPOINT Enjoy ultra fast search capabilities in simple and complex modes optimized for Big Data Easily filter and display relevant topics,
QuantStudio 12K Flex Real-Time PCR System. The all-in-one qpcr instrument
 QuantStudio 12K Flex Real-Time PCR System The all-in-one qpcr instrument Expand the boundaries of your research Life Technologies is taking qpcr to the next level. Designed for maximum throughput, flexibility,
QuantStudio 12K Flex Real-Time PCR System The all-in-one qpcr instrument Expand the boundaries of your research Life Technologies is taking qpcr to the next level. Designed for maximum throughput, flexibility,
Thermo Scientific ClinQuan MD Software For In Vitro Diagnostic Use. Confidence in Results With Data Integrity
 Thermo Scientific ClinQuan MD Software For In Vitro Diagnostic Use Confidence in Results With Data Integrity 2 Make the World Healthier With the LC-MS Tests You Run Confidence in Test Results With Data
Thermo Scientific ClinQuan MD Software For In Vitro Diagnostic Use Confidence in Results With Data Integrity 2 Make the World Healthier With the LC-MS Tests You Run Confidence in Test Results With Data
SAP SuccessFactors Onboarding Technical and Functional Specifications
 SAP SuccessFactors Onboarding Technical and Functional Specifications This specifications document describes key features and functionalities of SAP SuccessFactors Onboarding. s and Functionalities: Forms
SAP SuccessFactors Onboarding Technical and Functional Specifications This specifications document describes key features and functionalities of SAP SuccessFactors Onboarding. s and Functionalities: Forms
The Pension Portal. Helping you take your pension business into the paperless age
 The Pension Portal Helping you take your pension business into the paperless age When you ve been helping pension professionals implement client portals for as long as we have, you understand that the
The Pension Portal Helping you take your pension business into the paperless age When you ve been helping pension professionals implement client portals for as long as we have, you understand that the
Laboratory Director Responsibilities
 Clinical Laboratory Improvement Amendments (CLIA) Laboratory Director Responsibilities What Are My Responsibilities As A Laboratory Director NOTE: Congress passed the Clinical Laboratory Improvement Amendments
Clinical Laboratory Improvement Amendments (CLIA) Laboratory Director Responsibilities What Are My Responsibilities As A Laboratory Director NOTE: Congress passed the Clinical Laboratory Improvement Amendments
Major Process Future State Process Gap Technology Gap
 Outreach and Community- Based Services: Conduct education, training, legislative activity, screening and communication within the community and build appropriate partnerships and coalitions to promote
Outreach and Community- Based Services: Conduct education, training, legislative activity, screening and communication within the community and build appropriate partnerships and coalitions to promote
MATCH IT! Antibody Software Quick Reference Guide
 MATCH IT! Antibody Software Quick Reference Guide For Use with IMMUCOR LIFECODES Antibody Assays For In Vitro Diagnostic Use 1 LC1456.1 (09/15) This manual was produced for use with the MIAB MATCH IT!
MATCH IT! Antibody Software Quick Reference Guide For Use with IMMUCOR LIFECODES Antibody Assays For In Vitro Diagnostic Use 1 LC1456.1 (09/15) This manual was produced for use with the MIAB MATCH IT!
Our solutions reduce costs, streamline process and increase efficiencies. Facilities Management Litigation and Document Solutions
 Discover Possibilities With Pitney Bowes Legal Solutions Our suite of solutions is the differentiator in today s complex legal environment. We manage the business of law Pitney Bowes is the market leader
Discover Possibilities With Pitney Bowes Legal Solutions Our suite of solutions is the differentiator in today s complex legal environment. We manage the business of law Pitney Bowes is the market leader
8 Key Requirements of an IT Governance, Risk and Compliance Solution
 8 Key Requirements of an IT Governance, Risk and Compliance Solution White Paper: IT Compliance 8 Key Requirements of an IT Governance, Risk and Compliance Solution Contents Introduction............................................................................................
8 Key Requirements of an IT Governance, Risk and Compliance Solution White Paper: IT Compliance 8 Key Requirements of an IT Governance, Risk and Compliance Solution Contents Introduction............................................................................................
Bio-Rad Laboratories. QC data management solutions. Introduce Your Laboratory to a Whole New World of Unity Data Management Solutions
 Bio-Rad Laboratories QC data management solutions QC Data Management Solutions Introduce Your Laboratory to a Whole New World of Unity Data Management Solutions Bio-Rad Laboratories QC data management
Bio-Rad Laboratories QC data management solutions QC Data Management Solutions Introduce Your Laboratory to a Whole New World of Unity Data Management Solutions Bio-Rad Laboratories QC data management
How To Create A Help Desk For A System Center System Manager
 System Center Service Manager Vision and Planned Capabilities Microsoft Corporation Published: April 2008 Executive Summary The Service Desk function is the primary point of contact between end users and
System Center Service Manager Vision and Planned Capabilities Microsoft Corporation Published: April 2008 Executive Summary The Service Desk function is the primary point of contact between end users and
Residential Lending and Asset Management Solution for Investor-Owned Properties
 & Residential ending and Asset Management Solution for Investor-Owned Properties www.therockportgroup.com/sfr Complete transaction lifecycle oversight for investor-owned residential lending and asset management.
& Residential ending and Asset Management Solution for Investor-Owned Properties www.therockportgroup.com/sfr Complete transaction lifecycle oversight for investor-owned residential lending and asset management.
Deploying the Workspace Application for Microsoft SharePoint Online
 Microsoft Dynamics GP Deploying the Workspace Application for Microsoft SharePoint Online Microsoft Dynamics GP Workspace is a method to enable Microsoft Excel-based dashboards for SharePoint Online. This
Microsoft Dynamics GP Deploying the Workspace Application for Microsoft SharePoint Online Microsoft Dynamics GP Workspace is a method to enable Microsoft Excel-based dashboards for SharePoint Online. This
Adding Clinical Services to a Research Core Lab at the UW-Madison. Josh Hyman, Director UWBC DNA Sequencing Facility
 Adding Clinical Services to a Research Core Lab at the UW-Madison Josh Hyman, Director UWBC DNA Sequencing Facility Impetus UW researchers/clinicians becoming interested in moving more discoveries from
Adding Clinical Services to a Research Core Lab at the UW-Madison Josh Hyman, Director UWBC DNA Sequencing Facility Impetus UW researchers/clinicians becoming interested in moving more discoveries from
our group Mission Overview Offering
 ABOUT OUR COMPANY Mission Infrahealth is dedicated to offering cost-effective infrastructure solutions tailored to achieve maximum results for healthcare professionals. We are focused on delivering medical
ABOUT OUR COMPANY Mission Infrahealth is dedicated to offering cost-effective infrastructure solutions tailored to achieve maximum results for healthcare professionals. We are focused on delivering medical
GeneMapper ID-X Software Version 1.0
 Administrator s Guide GeneMapper ID-X Software Version 1.0 Note: To improve the clarity of graphics in this PDF file, use the zoom tool to increase magnification to 150% or greater. Administrator s Guide
Administrator s Guide GeneMapper ID-X Software Version 1.0 Note: To improve the clarity of graphics in this PDF file, use the zoom tool to increase magnification to 150% or greater. Administrator s Guide
User Bulletin. GeneMapper Software Version 4.0. Installation Options. In This User Bulletin. Overview
 User Bulletin Software Version 4.0 February 2006 SUBJECT: Installation Options In This User Bulletin Overview This user bulletin covers:............................... 2 Installation Options for the........
User Bulletin Software Version 4.0 February 2006 SUBJECT: Installation Options In This User Bulletin Overview This user bulletin covers:............................... 2 Installation Options for the........
billingplatform.com Case Study for Bizcel do Brazil Enterprise Billing for Cellular Providers
 Case Study for Bizcel do Brazil Enterprise Billing for Cellular Providers billingplatform.com 1. 2. Company profile Founded in 1994, Bizcel is one of the leading telecommunications companies in Brazil
Case Study for Bizcel do Brazil Enterprise Billing for Cellular Providers billingplatform.com 1. 2. Company profile Founded in 1994, Bizcel is one of the leading telecommunications companies in Brazil
Adding Management. www.starlims.com
 Adding Management to Your LIS Given the dynamic nature of their operations, today s clinical laboratories need to be nimble and agile. They need to cope with ever changing requirements brought about by
Adding Management to Your LIS Given the dynamic nature of their operations, today s clinical laboratories need to be nimble and agile. They need to cope with ever changing requirements brought about by
Estimating and Vendor Quotes An Estimating and Vendor Quote Workflow Guide
 Estimating and Vendor Quotes An Estimating and Vendor Quote Workflow Guide Production Estimating What can it do for you? Estimate Usage The Estimating application is used to prepare quotes for production
Estimating and Vendor Quotes An Estimating and Vendor Quote Workflow Guide Production Estimating What can it do for you? Estimate Usage The Estimating application is used to prepare quotes for production
Volunteer Software Feature Comparison
 Thank you for your interest in our Better software! This document has been prepared to list the functions of Better software and, if helpful, to assist you in comparing it to other software you might be
Thank you for your interest in our Better software! This document has been prepared to list the functions of Better software and, if helpful, to assist you in comparing it to other software you might be
THE PREMIER INTEGRATED IT SERVICE MANAGEMENT TOOL
 By choosing 2 ENTERPRISE, you become more competitive by investing time and energy on what really matters, your business. THE PREMIER INTEGRATED IT SERVIE MANAGEMENT TOOL 2 ENTERPRISE is the premier ITSM
By choosing 2 ENTERPRISE, you become more competitive by investing time and energy on what really matters, your business. THE PREMIER INTEGRATED IT SERVIE MANAGEMENT TOOL 2 ENTERPRISE is the premier ITSM
SUBJECT: New Features in Version 5.3
 User Bulletin Sequencing Analysis Software v5.3 July 2007 SUBJECT: New Features in Version 5.3 This user bulletin includes the following topics: New Features in v5.3.....................................
User Bulletin Sequencing Analysis Software v5.3 July 2007 SUBJECT: New Features in Version 5.3 This user bulletin includes the following topics: New Features in v5.3.....................................
e-solutions by Thermo Fisher Scientific
 e-solutions by Thermo Fisher Scientific You need a partner that s committed to delivering online and on-site solutions that meet your unique requirements our e-solutions are designed to do just that. thermofisher.com
e-solutions by Thermo Fisher Scientific You need a partner that s committed to delivering online and on-site solutions that meet your unique requirements our e-solutions are designed to do just that. thermofisher.com
QuantStudio 3D AnalysisSuite Software
 USER GUIDE QuantStudio 3D AnalysisSuite Software for use with QuantStudio 3D Digital PCR System Publication Number MAN0008161 Revision 1.0 For Research Use Only. Not for use in diagnostic procedures. For
USER GUIDE QuantStudio 3D AnalysisSuite Software for use with QuantStudio 3D Digital PCR System Publication Number MAN0008161 Revision 1.0 For Research Use Only. Not for use in diagnostic procedures. For
Automating Anesthesia at Meditech Hospitals: Removing the Risk
 White Paper Automating Anesthesia at Meditech Hospitals: Removing the Risk Direct integration with Meditech, the largest installed base in the industry, plus, powerful decision support capabilities, make
White Paper Automating Anesthesia at Meditech Hospitals: Removing the Risk Direct integration with Meditech, the largest installed base in the industry, plus, powerful decision support capabilities, make
Centricity Enterprise Provider Tools
 GE Healthcare Centricity Enterprise Provider Tools The clinical information system that gives you critical data when and where you need it. As a healthcare provider, your patients rely on you to deliver
GE Healthcare Centricity Enterprise Provider Tools The clinical information system that gives you critical data when and where you need it. As a healthcare provider, your patients rely on you to deliver
ClinPlus. Clinical Trial Management System (CTMS) Technology Consulting Outsourcing. Expedite trial design and study set-up
 Technology Consulting Outsourcing ClinPlus Clinical Trial Management System (CTMS) Expedite trial design and study set-up Efficiently collect and manage patient and trial administration data Benefit from
Technology Consulting Outsourcing ClinPlus Clinical Trial Management System (CTMS) Expedite trial design and study set-up Efficiently collect and manage patient and trial administration data Benefit from
BD Affirm VPIII. Microbial Identification System
 BD Affirm VPIII Microbial Identification System The Only Diagnostic Test that Differentiates and Identifies 3 Vaginitis Pathogens from a Single Sample, with DNA Certainty. Translating the power and the
BD Affirm VPIII Microbial Identification System The Only Diagnostic Test that Differentiates and Identifies 3 Vaginitis Pathogens from a Single Sample, with DNA Certainty. Translating the power and the
Hematology, Chemistry, Microbiology. Clinical Pathology and Anatomic Pathology. Hospital Information System or Practice Management System
 Take your laboratory to market and stay competitive. Harvest the power of laboratory outreach and connectivity to your clients EMRs with Orchard Copia. Today, for laboratory outreach, EMR integration and
Take your laboratory to market and stay competitive. Harvest the power of laboratory outreach and connectivity to your clients EMRs with Orchard Copia. Today, for laboratory outreach, EMR integration and
Resource Management for the Oil and Gas Industry
 SAP Brief SAP Business Suite SAP Workforce Scheduling and Optimization by ClickSoftware Objectives Resource Management for the Oil and Gas Industry Optimized workforce scheduling with SAP software Optimized
SAP Brief SAP Business Suite SAP Workforce Scheduling and Optimization by ClickSoftware Objectives Resource Management for the Oil and Gas Industry Optimized workforce scheduling with SAP software Optimized
MATCH IT! DNA Software Quick Reference Guide
 MATCH IT! DNA Software Quick Reference Guide For Use with IMMUCOR LIFECODES HLA SSO Assays For In Vitro Diagnostic Use 1 LC1497.1 (08/15) This manual was produced for use with the MIDNA MATCH IT! DNA Software
MATCH IT! DNA Software Quick Reference Guide For Use with IMMUCOR LIFECODES HLA SSO Assays For In Vitro Diagnostic Use 1 LC1497.1 (08/15) This manual was produced for use with the MIDNA MATCH IT! DNA Software
User Guide for the 21 CFR Part 11 Module in SDS Software v1.4
 Applied Biosystems 7500/7500 Fast Real-Time PCR System User Guide for the 21 CFR Part 11 Module in SDS Software v1.4 Introduction Installation Logging in to the SDS Software Configuring the 21CFR11 Module
Applied Biosystems 7500/7500 Fast Real-Time PCR System User Guide for the 21 CFR Part 11 Module in SDS Software v1.4 Introduction Installation Logging in to the SDS Software Configuring the 21CFR11 Module
Protect the Past, Secure the Future
 Enterprise Content Management for Higher Education Protect the Past, Secure the Future Improve information access, protect records and promote strategic planning Learn More Inside Improve administrative
Enterprise Content Management for Higher Education Protect the Past, Secure the Future Improve information access, protect records and promote strategic planning Learn More Inside Improve administrative
MRMPilot Software: Accelerating MRM Assay Development for Targeted Quantitative Proteomics
 MRMPilot Software: Accelerating MRM Assay Development for Targeted Quantitative Proteomics With Unique QTRAP and TripleTOF 5600 System Technology Targeted peptide quantification is a rapidly growing application
MRMPilot Software: Accelerating MRM Assay Development for Targeted Quantitative Proteomics With Unique QTRAP and TripleTOF 5600 System Technology Targeted peptide quantification is a rapidly growing application
Mayo Central Laboratory for Clinical Trials A Division within Mayo Clinical Trial Services
 Mayo Central Laboratory for Clinical Trials A Division within Mayo Clinical Trial Services LABORATORY TESTING SERVICES YOUR PARTNER IN LABORATORY MEDICINE The key note of progress in the 20th century is
Mayo Central Laboratory for Clinical Trials A Division within Mayo Clinical Trial Services LABORATORY TESTING SERVICES YOUR PARTNER IN LABORATORY MEDICINE The key note of progress in the 20th century is
FCAP Array v3.0 Software: A New Tool to Analyze BD Cytometric Bead Array (CBA) Data. Monisha Sundarrajan, PhD BD Biosciences
 FCAP Array v3.0 Software: A New Tool to Analyze BD Cytometric Bead Array (CBA) Data Monisha Sundarrajan, PhD BD Biosciences 23 13795 00 Agenda Introduction Overview of BD CBA bead based immunoassays FCAP
FCAP Array v3.0 Software: A New Tool to Analyze BD Cytometric Bead Array (CBA) Data Monisha Sundarrajan, PhD BD Biosciences 23 13795 00 Agenda Introduction Overview of BD CBA bead based immunoassays FCAP
LabChip GX/GXII with LabChip GxP Software
 Regulatory Compliance LabChip GX/GXII with LabChip GxP Software Supporting Regulatory Compliance Caliper LabChip GX/GXII suite of instruments provides automated electrophoresis to analyze quality, size,
Regulatory Compliance LabChip GX/GXII with LabChip GxP Software Supporting Regulatory Compliance Caliper LabChip GX/GXII suite of instruments provides automated electrophoresis to analyze quality, size,
MOVING THE CLINICAL ANALYTICAL ENVIRONMENT INTO THE CLOUD
 MOVING THE CLINICAL ANALYTICAL ENVIRONMENT INTO THE CLOUD STIJN ROGIERS, SENIOR INDUSTRY CONSULTANT, LIFE SCIENCES/HEALTH CARE (EMEA/AP) SANDEEP JUNEJA CONSULTING MANAGER (SSOD) AGENDA Move towards cloud
MOVING THE CLINICAL ANALYTICAL ENVIRONMENT INTO THE CLOUD STIJN ROGIERS, SENIOR INDUSTRY CONSULTANT, LIFE SCIENCES/HEALTH CARE (EMEA/AP) SANDEEP JUNEJA CONSULTING MANAGER (SSOD) AGENDA Move towards cloud
Power Up Your Business
 Power Up Your Business Improve Overall Productivity & Performance This is not your average time and billing software. With a suite of dynamic business intelligence tools, BillQuick provides key metrics
Power Up Your Business Improve Overall Productivity & Performance This is not your average time and billing software. With a suite of dynamic business intelligence tools, BillQuick provides key metrics
End-to-End E-Clinical Coverage with Oracle Health Sciences InForm GTM
 End-to-End E-Clinical Coverage with InForm GTM A Complete Solution for Global Clinical Trials The broad market acceptance of electronic data capture (EDC) technology, coupled with an industry moving toward
End-to-End E-Clinical Coverage with InForm GTM A Complete Solution for Global Clinical Trials The broad market acceptance of electronic data capture (EDC) technology, coupled with an industry moving toward
Craig Hallum Conference Investor Presentation
 Craig Hallum Conference Investor Presentation Improving cancer outcomes with groundbreaking precision in molecular testing Paul Kinnon President and CEO Sept 2015 1 Forward-Looking Statements Certain statements
Craig Hallum Conference Investor Presentation Improving cancer outcomes with groundbreaking precision in molecular testing Paul Kinnon President and CEO Sept 2015 1 Forward-Looking Statements Certain statements
CONTRACTORS: GL RATER TRAINING GUIDE
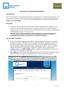 Getting Started: AUI is providing custom direct access rating and servicing solutions to III and Nationwide agencies. This unique end to end platform affords agents one of the quickest self-serve rating
Getting Started: AUI is providing custom direct access rating and servicing solutions to III and Nationwide agencies. This unique end to end platform affords agents one of the quickest self-serve rating
History of Innovation
 1 History of Innovation Only Immucor consistently delivers the next-generation of blood bank automation to labs worldwide ABS HV Galileo NEO 1998 1999 2004 2007 2010 ABS2000 ABS Precis Echo 2 Innovation
1 History of Innovation Only Immucor consistently delivers the next-generation of blood bank automation to labs worldwide ABS HV Galileo NEO 1998 1999 2004 2007 2010 ABS2000 ABS Precis Echo 2 Innovation
B i o s o l u t i o n s
 B i o s o l u t i o n s Implementation of pharmatracker TM : A case study Prepared by Ocimum Biosolutions Ocimumbio Solutions August 2006 All rights reserved. 2 B i o s o l u t i o n s an ISO 9001:2000
B i o s o l u t i o n s Implementation of pharmatracker TM : A case study Prepared by Ocimum Biosolutions Ocimumbio Solutions August 2006 All rights reserved. 2 B i o s o l u t i o n s an ISO 9001:2000
Software Getting Started Guide
 Software Getting Started Guide For Research Use Only. Not for use in diagnostic procedures. P/N 001-097-569-03 Copyright 2010-2013, Pacific Biosciences of California, Inc. All rights reserved. Information
Software Getting Started Guide For Research Use Only. Not for use in diagnostic procedures. P/N 001-097-569-03 Copyright 2010-2013, Pacific Biosciences of California, Inc. All rights reserved. Information
DRIVING SUCCESS 8 BEST PRACTICES FOR EASY EMPLOYEE EXPENSE TRACKING
 DRIVING SUCCESS 8 BEST PRACTICES FOR EASY EMPLOYEE EXPENSE TRACKING Introduction Maybe you re a project manager who needs to accurately capture employee expenses and allocate them to particular projects.
DRIVING SUCCESS 8 BEST PRACTICES FOR EASY EMPLOYEE EXPENSE TRACKING Introduction Maybe you re a project manager who needs to accurately capture employee expenses and allocate them to particular projects.
Well known electronics and manufacturing company based in Manchester, UK.
 Case Study Customer Well known electronics and manufacturing company based in Manchester, UK. Profile The business is a member of one of the biggest electronic group based in Japan. Their organization
Case Study Customer Well known electronics and manufacturing company based in Manchester, UK. Profile The business is a member of one of the biggest electronic group based in Japan. Their organization
HISTO SPOT SSO System. The most convenient automated HLA typing system. BAG Health Care the experts for HLA and blood group diagnostics
 HISTO SPOT SSO System The most convenient automated HLA typing system BAG Health Care the experts for HLA and blood group diagnostics HISTO SPOT SSO System for on call, high throughput and disease association
HISTO SPOT SSO System The most convenient automated HLA typing system BAG Health Care the experts for HLA and blood group diagnostics HISTO SPOT SSO System for on call, high throughput and disease association
Improve your equity research productivity
 Improve your equity research productivity Creating and updating company models Standardized Excel based company models ensure each analyst s work seamlessly integrates with research database and can be
Improve your equity research productivity Creating and updating company models Standardized Excel based company models ensure each analyst s work seamlessly integrates with research database and can be
Realistic? Content management initiatives can be a huge investment of time, money, and resources.
 Realistic? Content management initiatives can be a huge investment of time, money, and resources. Huge investment = Huge risk This session will describe a way to implement a practical content management
Realistic? Content management initiatives can be a huge investment of time, money, and resources. Huge investment = Huge risk This session will describe a way to implement a practical content management
Quality Assurance for the Clinical Laboratory
 EP Evaluator Quality Assurance for the Clinical Laboratory Simplified process, powerful results While we (clinical labs) are but a small component of the total healthcare system, our data control a large
EP Evaluator Quality Assurance for the Clinical Laboratory Simplified process, powerful results While we (clinical labs) are but a small component of the total healthcare system, our data control a large
Application Note # LCMS-62 Walk-Up Ion Trap Mass Spectrometer System in a Multi-User Environment Using Compass OpenAccess Software
 Application Note # LCMS-62 Walk-Up Ion Trap Mass Spectrometer System in a Multi-User Environment Using Compass OpenAccess Software Abstract Presented here is a case study of a walk-up liquid chromatography
Application Note # LCMS-62 Walk-Up Ion Trap Mass Spectrometer System in a Multi-User Environment Using Compass OpenAccess Software Abstract Presented here is a case study of a walk-up liquid chromatography
Smart. Scalable. Simple.
 Thermo Scientific Wireless Monitoring Solution Smart. Scalable. Simple. providing the protection you need with the simplicity you deserve Thermo Scientific Wireless Monitoring Solution Optimized Sample
Thermo Scientific Wireless Monitoring Solution Smart. Scalable. Simple. providing the protection you need with the simplicity you deserve Thermo Scientific Wireless Monitoring Solution Optimized Sample
