The following list consists of a few tips and tricks to use when navigating eirb.
|
|
|
- Bryce Blake
- 10 years ago
- Views:
Transcription
1 TIPS/TRICKS The following list consists of a few tips and tricks to use when navigating eirb. GENERAL Submitting applications Only the PI can submit applications. However, the Study Coordinator can submit Continuing Reviews, Amendments, Reportable Events, and Requested Revisions. The Co- Investigator will have the ability to create and edit study applications and subprojects; however, he/she cannot submit new applications to the USF IRB. It is important to note that selecting Finish at the end of an application does not submit it for review. In order to submit the initial application, continuing review, amendment or reportable event, you must select Submit on the respective workspace page. To verify your application submission, please check the current state, which you can find by looking at the red rectangle in the top left corner of the workspace screen. If the red rectangle still says Pre-Submission or Changes Requested by, your application has not been submitted for review to the USF IRB. You can also check your history tab, which will indicate the date and time an application has been submitted, as well as by whom. Withdrawing applications Only the PI or USF IRB has the ability to withdraw applications. After an application has been created, the PI has the ability to withdraw the application during the Pre- Submission stage. Withdrawal by the PI can also take place at the Department Review and/or Affiliate Review stages (as long as the application is in the study staff s inbox). Once the application reaches the IRB, he/she will need to notify the assigned IRB Staff Reviewer to withdraw the application. Back button on application form When completing the study application in eirb, you will use the Continue and the Back buttons, to navigate the application. One thing to note is the back button within the eirb application takes you back to the previous page visited, not the previous application page. Also, make certain to Save before clicking the Back button, or all information added and changes made to that particular page will be lost. Required Reviews (section 2.2 of Study Application) If the H. Lee Moffitt Cancer Center & Research Institute (MCC) is selected as a USF affiliate Site on question 1.8.2, it will not appear on section 2.2 for Required Reviews however Tampa General Hospital (TGH) and James A. Haley Veterans Hospital (VA) will. This is okay and you will not be asked to correct it. If you are a student and do not have a specific department affiliation, choose the department to which your faculty advisor is assigned. v.1_12may2011 1
2 CV/Team Member Education Certification Everyone on the study team, regardless of assigned tasks, should have current Human Subjects Protections Certification and a CV/resume uploaded to the arc system. If the study team uploads education into eirb, the account will continue to show the education as certification not current until the IRB Staff verifies and enters the dates during the IRB Staff Review. The certification not current education status will not prevent you from submitting an application, nor will it prevent the study from being reviewed. Just keep in mind, the study will not be approved by IRB until it all study team member have current education. Amendments After a study has been approved, any changes made to the study must be submitted via an Amendment. It is helpful if the study team provides as much detail and justification as possible as to what is being amended. In addition to uploading the new clean documents (i.e. protocol and consent forms) adding a track change version of the amended documents or a summary description of the changes is also helpful. Without track changes or a summary description of the changes, the IRB Staff reviewer does not know what the revisions are. When informed consent documents are revised and approved via an Amendment, you can find the approved Consent forms either by clicking the links in the Amendment approval letter or under the Attachments tab on the study s main workspace. Continuing Reviews When submitting a continuing review application, PI s are asked to provide copies of the 2 most recently signed informed consent documents, unless they are not obtaining signed informed consent because they were granted a waiver of documentation. When submitting the consent documents, please redact any identifiable information related to the subject from the signed forms. Converting to eirb (submitting final reports) Only convert from paper to eirb if your study will be active beyond January 1, If you are only completing a final report, do not convert your study to do so. Submit your final report on paper forms which can be found at Requested Revisions - When receiving requested revisions from a reviewer (Department, Affiliate, or the IRB), in addition to making all the necessary revisions requested by the reviewer, you must also respond to all reviewer notes. The revisions should not be placed in the Response Required comment box, but instead directly in the application. Doing so will result in the application being returned to you to put in the appropriate section, thus prolonging the review process. Responding to concerns is a three-step process. First you must respond to the concerns within the application and make relevant changes on the applicable forms. Second, you must click submit revisions in order for the reviewer to receive your responses. If you are unsure whether your application has been submitted, check the state of the study and the history tab. As long as the current state says, Changes requested by, your study is still in your inbox and has not been sent back to the reviewer for further review. Department Review - A member of the study team cannot issue department approval (or affiliate approval) for their own study, due to the conflict of interest this presents. If department approval has been issued by a member of the study team, the IRB cannot review the application until it has been reviewed by an alternate department approver who is not on the study. v.1_12may2011 2
3 If your department is designated as a department (or affiliate) approver (question or 2.2.3) in error, please send the application back to the study team, by choosing the submit requested revisions or information activity. Make certain to include a reviewer note, asking the study team to remove your department as an approver. If you are study staff and realize you have you made errors or need to add information to the application after it has been submitted, please contact the department approver (outside of eirb) and request that they send the application back to you to make the necessary changes. After you have made your revisions, make certain to Submit to route your application back through the review process. addresses addresses are one way that the IRB ensures each person has only one eirb account (a unique ID). When registering, it is imperative that you do not list anyone else s address, and that both the primary and secondary address belong to you, the registrant. If you list another person s address and that person decides to create his or her own account using the same address, s/he may experience a delay in the registration process, as we determine which address belongs to whom. We may also need to obtain alternate addresses, reset the password, and/or deactivate the existing account, all of which take time to process. Inbox vs. IRB tabs (determining if the study requires your attention) If you are unsure whether a study requires your attention, make note of where the study is located. If the study is in your INBOX, then it means the study requires that the study team work on the application and Submit. If you find the application is under the IRB tab, it is an application you are listed on as a study team member. This does not mean you are required to act on anything. If the study is not in the study team s INBOX, then you will not be able to make any revisions to the application at that time. However, you can make revisions to an application, submit a continuing review, and process reportable events to studies appearing in your IRB tab. COMPLETING THE STUDY APPLICATION If you are presented with a question in the application, that question should be answered. If you feel it does not apply to your particular research, explain why the question does not apply (i.e. N/A this is a records review study where subjects will not be recruited). With an N/A, RCA s will request less revisions or clarification, thus shortening the length of the review process. Be consistent with your application (protocol should match application, consents, flyers and other material). Spell out acronyms (i.e. MRI, CT) and explain medical terms in lay terms, especially in the informed consent document. Question Please only list one role per study team member. If you are the PI on the study, it is not necessary to list yourself as Co Investigator or Study Coordinator. Question All study personnel must have a current Resume/CV and Human Subjects Protections Education certification uploaded. Question 1.8 A Letter of Support is required if you are using a Non-USF or Non-Affiliate Site to conduct research. v.1_12may2011 3
4 Question Please include a clearly stated hypothesis. Question Please upload an appropriate protocol. It is strongly advised to write protocols in accordance with the IRB Protocol Guidelines. If you need assistance, please visit and click on "Protocol Guidelines." Sections 3 and 4 Although this information may already be included in the protocol, please write detailed responses in these sections or copy and paste the information from your protocol. If you copy and paste information from Protocols or Investigator s Brochures into the application, please be sure the information is relevant to what is being asked and proofread what you paste to ensure it is understandable as a response and covers all questions asked. Question Upload survey instrument(s) if included in your research. Section 5 All research data must be kept for a minimum of 5 years after the Final Report is approved by the IRB. If collected research data is on tape, you can destroy the tapes if the information has been transcribed, still ensuring the transcriptions are kept for the minimum timeframe. Section 6 If you are using any form of recruitment script, , invitation, etc., attach in Section 6.1c.3 as this must be reviewed and approved by the USF IRB. Section 7 It is strongly advised that Informed Consent documents are based off the IRB templates and contain all required elements in the IRB consent templates (Exceptions to this include templates created by MCC and VA). If requesting a waiver of Informed Consent, do not put the consent form in section (this is only for researchers obtaining a signed consent form). Instead, attach the document(s) in section Question Studies with non-english speaking subjects must have the translated informed consent document and verification that the document has been back-translated uploaded into eirb. In this document, please have the person translating the non-english informed consent document state that they take responsibility for the document's accurate translation. You may also wait until the English version of the informed consent document is approved to translate the form into another language. Once this is completed, the translated version of the form must be submitted to the USF IRB for review and approval via an amendment. Question This section addresses the participants understanding of the research. In this section, indicate if the participant will be asked open-ended questions that will require them to respond in such a way that the study personnel will be able to judge the participant s comprehension of the research study. If the potential participants will have legally authorized representative (LAR) who will be consenting for the subject s participation in the research, please indicate if this individual will be asked open-ended questions that will require them to respond in such a way that study personnel will be able to judge their comprehension of the research study. v.1_12may2011 4
5 Section 9 State who will have access to the participants confidential and private information, as well as where and how this information will be stored. Privacy responses should be different from Confidentiality responses. Privacy refers to participants and their interests in controlling the access of others to themselves and to information about themselves. Privacy also refers to how the study is presented to individuals (e.g. in private rooms), and should address the ways in which study data are collected (e.g. is study data collected in a private place). Confidentiality responses, on the other hand, should address how the data, source documents, and informed consent documents will be kept confidential and secure during data collection, analysis and storage. If any member of the study team is going to have access to Protected Health Information (PHI) then you must answer yes to question If medical records are being reviewed, then PHI is being accessed. The PI or Coordinator will need to respond to all HIPAA questions and in most cases apply for a HIPAA waiver if the Informed Consent process or documentation is being waived (applicable only to covered entities). Question 9.1b.2 -Data that includes medical record numbers and/or dates associated with a procedure or illness are considered identifiers according to the HIPAA Privacy Rule. Therefore, datasets that include this information would not be considered de-identified. Question Data Safety Monitoring refers to your plan for ensuring the integrity of the data collected, including how often you plan to monitor the data. (e.g. We will review the data, as it becomes available, with the PI to assure that the data is accurate. All data will be reviewed and initialed by the PI. All data will be compared to the participant's record and to their diary to ensure data was recorded accurately. Data integrity is monitored by the PI, the study staff members and the sponsor by verifying that study documents/data entry with the source and patient s medical charts. ) v.1_12may2011 5
How to Create a New Clinical Research Study
 How to Create a New Clinical Research Study 1 Contents 1. Create a Submission for an Existing eirb Study... 3 2. Create a New Submission in eirb... 4 3. CRMS Clinical Research Study... 5 4. Uploading a
How to Create a New Clinical Research Study 1 Contents 1. Create a Submission for an Existing eirb Study... 3 2. Create a New Submission in eirb... 4 3. CRMS Clinical Research Study... 5 4. Uploading a
Human Subjects Research at OSU
 Office of Responsible Research Practices 300 Research Foundation 1960 Kenny Road Columbus, OH 43210-1063 Human Subjects Research at OSU Phone (614) 688-8457 Fax (614) 688-0366 www.orrp.osu.edu Behavioral
Office of Responsible Research Practices 300 Research Foundation 1960 Kenny Road Columbus, OH 43210-1063 Human Subjects Research at OSU Phone (614) 688-8457 Fax (614) 688-0366 www.orrp.osu.edu Behavioral
Documentation of the Informed Consent Process. USC Office for the Protection of Research Subjects (OPRS)
 Documentation of the Informed Consent Process USC Office for the Protection of Research Subjects (OPRS) Session Overview Highlights: Purpose of Informed Consent (IC) IC Process and Documentation Witness
Documentation of the Informed Consent Process USC Office for the Protection of Research Subjects (OPRS) Session Overview Highlights: Purpose of Informed Consent (IC) IC Process and Documentation Witness
IRB Principal Investigator Quick Reference
 Navigation and Basic Tasks... Create Study and Submission Approvals... Submit a Study... 5 Assign PI Proxy... 5 Respond to Clarification Requests... 6 Change Study Documents... 7 Create and Submit a CR
Navigation and Basic Tasks... Create Study and Submission Approvals... Submit a Study... 5 Assign PI Proxy... 5 Respond to Clarification Requests... 6 Change Study Documents... 7 Create and Submit a CR
CGIRB Connexus Tips. Version 1.1 July 2013 Copernicus Group IRB
 CGIRB Connexus Tips Version 1.1 July 2013 Copernicus Group IRB Study-Level Management in Connexus Thank you for selecting CGIRB Connexus as your shipment method for receiving copies of site approval documents
CGIRB Connexus Tips Version 1.1 July 2013 Copernicus Group IRB Study-Level Management in Connexus Thank you for selecting CGIRB Connexus as your shipment method for receiving copies of site approval documents
Frequently Asked Questions
 Frequently Asked Questions What is an electronic health record? Borgess has transitioned from paper-based medical records to electronic health records (EHRs). An EHR is an electronic version of your medical
Frequently Asked Questions What is an electronic health record? Borgess has transitioned from paper-based medical records to electronic health records (EHRs). An EHR is an electronic version of your medical
Application for an Off-Site Tissue Banking Waiver at a Non-Profit or Academic Institution
 Application for an Off-Site Tissue Banking Waiver at a Non-Profit or Academic Institution INSTRUCTIONS This form may be filled in and saved using Adobe Reader version 7.0 or higher. The full version of
Application for an Off-Site Tissue Banking Waiver at a Non-Profit or Academic Institution INSTRUCTIONS This form may be filled in and saved using Adobe Reader version 7.0 or higher. The full version of
Breast Cancer Registry of Greater Cincinnati (BCRGC) APPLICATION for ACCESS to INFORMATION/DATA
 Breast Cancer Registry of Greater Cincinnati (BCRGC) APPLICATION for ACCESS to INFORMATION/DATA Application Date: / / 20 Applicant's Name: LAST FIRST MIDDLE INITIAL Study Title: Institutional Affiliation(s)
Breast Cancer Registry of Greater Cincinnati (BCRGC) APPLICATION for ACCESS to INFORMATION/DATA Application Date: / / 20 Applicant's Name: LAST FIRST MIDDLE INITIAL Study Title: Institutional Affiliation(s)
www.etenders.gov.ie Electronic Tender Management System Quick User Guide Supplier
 Electronic Tender Management System Quick User Guide Supplier Page 1 Contents 1. Using the system 1.1 1.2 Supplier registration Finding published tenders 2. Responding to a public advertisement / notice
Electronic Tender Management System Quick User Guide Supplier Page 1 Contents 1. Using the system 1.1 1.2 Supplier registration Finding published tenders 2. Responding to a public advertisement / notice
Instructions for Form: Application for Claim of Exemption
 Instructions for Form: Application for Claim of Exemption In order to decide whether your activity involves research that may be reviewed and approved at the exempt level, review the following information.
Instructions for Form: Application for Claim of Exemption In order to decide whether your activity involves research that may be reviewed and approved at the exempt level, review the following information.
What is Covered by HIPAA at VCU?
 What is Covered by HIPAA at VCU? The Privacy Rule was designed to protect private health information from incidental disclosures. The regulations specifically apply to health care providers, health plans,
What is Covered by HIPAA at VCU? The Privacy Rule was designed to protect private health information from incidental disclosures. The regulations specifically apply to health care providers, health plans,
Electronic approvals for forms FAQs
 Click on any of the boxes below to explore more detail, including answers to frequently asked questions, video quick links, and more. Advisor experience Client experience Security Electronic approvals
Click on any of the boxes below to explore more detail, including answers to frequently asked questions, video quick links, and more. Advisor experience Client experience Security Electronic approvals
Web Application Access
 This guide provides information on: Establishing a WAM account for those individuals who do not have an EPA LAN account Logging on to Web Application Access (WAA) Viewing and entering your communities
This guide provides information on: Establishing a WAM account for those individuals who do not have an EPA LAN account Logging on to Web Application Access (WAA) Viewing and entering your communities
Online Employment System User Manual
 Troy University Online Employment System User Manual People Admin Version 7 Human Resources April 2014 Table of Contents Introduction... 2 User Types and Access... 2 Creating a User Account... 3 Homepage...
Troy University Online Employment System User Manual People Admin Version 7 Human Resources April 2014 Table of Contents Introduction... 2 User Types and Access... 2 Creating a User Account... 3 Homepage...
Revised March 2015. Online Hiring Center Job Aid
 Revised March 2015 Online Hiring Center Job Aid Section Title Page(s) I. Recruitment Life Cycle Overview................... 1,2 System Roles............................... 3 II. Create a Requisition (OHC)......................
Revised March 2015 Online Hiring Center Job Aid Section Title Page(s) I. Recruitment Life Cycle Overview................... 1,2 System Roles............................... 3 II. Create a Requisition (OHC)......................
Jobs4Saints User Guide: How to Post a Position
 Jobs4Saints User Guide: How to Post a Position Jobs4Saints can be accessed from the following site: https://www.myinterfase.com/marymount/employer/ Below is the log-in screen for employers. Please log
Jobs4Saints User Guide: How to Post a Position Jobs4Saints can be accessed from the following site: https://www.myinterfase.com/marymount/employer/ Below is the log-in screen for employers. Please log
IRB Submissions and Human Subjects Research Compliance. Georgia Health Sciences University
 IRB Submissions and Human Subjects Research Compliance Offi f H R hp t ti Office of Human Research Protection Georgia Health Sciences University Objectives Identify the steps required to submit a protocol
IRB Submissions and Human Subjects Research Compliance Offi f H R hp t ti Office of Human Research Protection Georgia Health Sciences University Objectives Identify the steps required to submit a protocol
RelayClinical Service Feature Guide Results Templates
 RelayClinical Service Feature Guide Results Templates Release 15.8 August 2015 Health Connections Brought to Life Table of Contents Overview... 3 What s Inside... 3 How Can We Help?... 3 Navigating Results
RelayClinical Service Feature Guide Results Templates Release 15.8 August 2015 Health Connections Brought to Life Table of Contents Overview... 3 What s Inside... 3 How Can We Help?... 3 Navigating Results
IRB 101: HUMAN SUBJECTS PROTECTION PROGRAM
 1 IRB 101: HUMAN SUBJECTS PROTECTION PROGRAM Wednesday, April 23, 2014 Cohen Lounge Hila Berger, MPH, CIP Research Compliance Administrator Amy Krenzer, CIP IRB Coordinator 2 Objectives What is the IRB
1 IRB 101: HUMAN SUBJECTS PROTECTION PROGRAM Wednesday, April 23, 2014 Cohen Lounge Hila Berger, MPH, CIP Research Compliance Administrator Amy Krenzer, CIP IRB Coordinator 2 Objectives What is the IRB
WHEN I WANT TO: I NEED TO SUBMIT: {for CIRB studies, see the specific FAQ}
 IRB forms and materials to be submitted WHEN I WANT TO: I NEED TO SUBMIT: {for CIRB studies, see the specific FAQ} 1 2 Request a determination as to whether AHC is the appropriate IRB of record (i.e. is
IRB forms and materials to be submitted WHEN I WANT TO: I NEED TO SUBMIT: {for CIRB studies, see the specific FAQ} 1 2 Request a determination as to whether AHC is the appropriate IRB of record (i.e. is
University of Hawai i Human Studies Program. Guidelines for Developing a Clinical Research Protocol
 University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
PeopleAdmin. Creating a Posting for HR Users
 PeopleAdmin Creating a Posting for HR Users Applicant Tracking This guide provides instructions on Creating a Posting utilized to post a vacant position. The position description serves as the basis for
PeopleAdmin Creating a Posting for HR Users Applicant Tracking This guide provides instructions on Creating a Posting utilized to post a vacant position. The position description serves as the basis for
Creating Your PALS Online Account for New Teachers Navigate to the PALS Online homepage
 Creating Your PALS Online Account for New Teachers Navigate to the PALS Online homepage Type www.wi.palsk8.com into the address bar of your internet browser. New or Returning? To create a new teacher account,
Creating Your PALS Online Account for New Teachers Navigate to the PALS Online homepage Type www.wi.palsk8.com into the address bar of your internet browser. New or Returning? To create a new teacher account,
Glen Ellyn School District 41 Online Registration Process Help Document
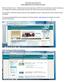 Glen Ellyn School District 41 Welcome to Family Access. In Family Access you may register your child for the next school year; schedule conferences; see messages from teachers; pay fees/place money on
Glen Ellyn School District 41 Welcome to Family Access. In Family Access you may register your child for the next school year; schedule conferences; see messages from teachers; pay fees/place money on
HPTN 067 Qualitative Manual
 HPTN 067 Qualitative Manual Qualitative Manual Version 2.0 April 11, 2012 TABLE OF CONTENTS 1 Overview... 3 2 Study Timeline... 7 3 Recruitment... 7 3.1 Informed Consent Procedures and Documentation (All
HPTN 067 Qualitative Manual Qualitative Manual Version 2.0 April 11, 2012 TABLE OF CONTENTS 1 Overview... 3 2 Study Timeline... 7 3 Recruitment... 7 3.1 Informed Consent Procedures and Documentation (All
Create a Basic Skype* Account. Intel Easy Steps 1 2012 Intel Corporation All rights reserved.
 Create a Basic Skype* Account Intel Easy Steps 1 2012 Intel Corporation Using Skype* to Communicate: Create and use a Basic Skype Account There are different ways of communicating and sharing through the
Create a Basic Skype* Account Intel Easy Steps 1 2012 Intel Corporation Using Skype* to Communicate: Create and use a Basic Skype Account There are different ways of communicating and sharing through the
Register as a New User
 ed Internet Browsers Getting Started Register as a New User 1. From http://connect.att.jobs, click Log In under Returning Job Seekers. 2. On the Login page, click New User. 3. On the New User Registration
ed Internet Browsers Getting Started Register as a New User 1. From http://connect.att.jobs, click Log In under Returning Job Seekers. 2. On the Login page, click New User. 3. On the New User Registration
Study Protocol Template
 Study Protocol Template (Chart Reviews) Instructions: This protocol template is a tool to facilitate the development of a study protocol specifically designed for the investigator initiated studies. It
Study Protocol Template (Chart Reviews) Instructions: This protocol template is a tool to facilitate the development of a study protocol specifically designed for the investigator initiated studies. It
UFIRST Training Manual
 UFIRST Training Manual Division of Sponsored Programs UF Training & Organizational Development Page 1 TABLE OF CONTENTS UFIRST 5 INTRODUCTION 5 ADDITIONAL RESOURCES 5 HOW TO USE THIS MANUAL 5 ACKNOWLEDGEMENTS
UFIRST Training Manual Division of Sponsored Programs UF Training & Organizational Development Page 1 TABLE OF CONTENTS UFIRST 5 INTRODUCTION 5 ADDITIONAL RESOURCES 5 HOW TO USE THIS MANUAL 5 ACKNOWLEDGEMENTS
Banner Employee Self-Service Web Time Entry. Student Workers User s Guide
 Banner Employee Self-Service Web Time Entry Student Workers User s Guide Table of Contents Introduction to Web Time Entry (WTE)... 1 Timeframe and Deadlines...1 Logging On....3 Access Time Sheet...5 Time
Banner Employee Self-Service Web Time Entry Student Workers User s Guide Table of Contents Introduction to Web Time Entry (WTE)... 1 Timeframe and Deadlines...1 Logging On....3 Access Time Sheet...5 Time
WebFile Guide For Claimants
 COMMONWEALTH OF VIRGINIA WORKERS COMPENSATION COMMISSION WebFile Guide For Claimants Instructional Guide for WebFile System SEPTEMBER 2009 EDITION PREFACE This Guide is designed to assist Claimants with
COMMONWEALTH OF VIRGINIA WORKERS COMPENSATION COMMISSION WebFile Guide For Claimants Instructional Guide for WebFile System SEPTEMBER 2009 EDITION PREFACE This Guide is designed to assist Claimants with
Wordware Family Website Instructions
 Registering on the Family Website Wordware Family Website Instructions Important: You must fully complete registration in one session in order to guarantee that there are no problems with activating your
Registering on the Family Website Wordware Family Website Instructions Important: You must fully complete registration in one session in order to guarantee that there are no problems with activating your
A Guide to the British Academy Electronic Submission System (e-gap2)
 INTERNAL USER GUIDE A Guide to the British Academy Electronic Submission System (e-gap2) A Quick Guide for Applicants applying for funding using the e-gap2 System The British Academy web page: http://www.britac.ac.uk/
INTERNAL USER GUIDE A Guide to the British Academy Electronic Submission System (e-gap2) A Quick Guide for Applicants applying for funding using the e-gap2 System The British Academy web page: http://www.britac.ac.uk/
Institutional Review Board
 Institutional Review Board Ethical Principles of Informed Consent Informed Consent Guidelines The principle of respect for persons requires that people be given the opportunity to choose what will or will
Institutional Review Board Ethical Principles of Informed Consent Informed Consent Guidelines The principle of respect for persons requires that people be given the opportunity to choose what will or will
Module 1 Getting Started
 Module 1 Getting Started Introduction Talent Connect Job Seeker User Guide The goal of the Pure Michigan Talent Connect website is to provide a centralized location for Employers and Job Seekers to connect.
Module 1 Getting Started Introduction Talent Connect Job Seeker User Guide The goal of the Pure Michigan Talent Connect website is to provide a centralized location for Employers and Job Seekers to connect.
Frequently Asked Questions (FAQs)
 Candidate Help Frequently Asked Questions (FAQs) Job Search Your Candidate Profile Hiring Process How to Apply Related Information Job Search What is a basic search? The Basic Search feature allows you
Candidate Help Frequently Asked Questions (FAQs) Job Search Your Candidate Profile Hiring Process How to Apply Related Information Job Search What is a basic search? The Basic Search feature allows you
Faculty Introduction to Self-Service
 Faculty Introduction to Self-Service This user guide focuses on how faculty members can use Self-Service to access and update their information. Using a Web browser, faculty members can enter student grades,
Faculty Introduction to Self-Service This user guide focuses on how faculty members can use Self-Service to access and update their information. Using a Web browser, faculty members can enter student grades,
CAQH ProView. Practice Manager Module User Guide
 CAQH ProView Practice Manager Module User Guide Table of Contents Chapter 1: Introduction... 1 CAQH ProView Overview... 1 System Security... 2 Chapter 2: Registration... 3 Existing Practice Managers...
CAQH ProView Practice Manager Module User Guide Table of Contents Chapter 1: Introduction... 1 CAQH ProView Overview... 1 System Security... 2 Chapter 2: Registration... 3 Existing Practice Managers...
Getting Started ONLINE APPLICATION. Access the online certification application system
 Online Application This instruction guide is for currently certified firms seeking renewal and firms applying for the first time. The information presented is drawn from example scenarios and may not exactly
Online Application This instruction guide is for currently certified firms seeking renewal and firms applying for the first time. The information presented is drawn from example scenarios and may not exactly
Vanderbilt University
 Vanderbilt University Performance Evaluation System Supervisor s Guide Provided by Vanderbilt Human Resources Table of Contents Overview... 3 VPES Evaluation Types... 3 VPES User Types... 4 Need Help?...
Vanderbilt University Performance Evaluation System Supervisor s Guide Provided by Vanderbilt Human Resources Table of Contents Overview... 3 VPES Evaluation Types... 3 VPES User Types... 4 Need Help?...
Determining who the investigators are and who the principal investigator is:
 Responsibilities of the IRBs of the Health System and University for Research Conducted by Investigators from a Combination of the Following Institutions: the Health System, University, and Medical School
Responsibilities of the IRBs of the Health System and University for Research Conducted by Investigators from a Combination of the Following Institutions: the Health System, University, and Medical School
CDMRP ereceipt System User Guide
 CDMRP ereceipt System User Guide Congressionally Directed Medical Research Programs (CDMRP) Last Updated: January 2011 Table of Contents Overview... 3 Purpose... 3 Help Desk Contact Information... 3 System
CDMRP ereceipt System User Guide Congressionally Directed Medical Research Programs (CDMRP) Last Updated: January 2011 Table of Contents Overview... 3 Purpose... 3 Help Desk Contact Information... 3 System
Document Services Online Customer Guide
 Document Services Online Customer Guide Logging in... 3 Registering an Account... 3 Navigating DSO... 4 Basic Orders... 5 Getting Started... 5 Attaching Files & Print Options... 7 Advanced Print Options
Document Services Online Customer Guide Logging in... 3 Registering an Account... 3 Navigating DSO... 4 Basic Orders... 5 Getting Started... 5 Attaching Files & Print Options... 7 Advanced Print Options
Frequently asked questions.
 Frequently asked questions. What is Bath Online? Bath Online is the online savings service from Bath Building Society that provides you with 24-hour access to view your existing savings accounts and the
Frequently asked questions. What is Bath Online? Bath Online is the online savings service from Bath Building Society that provides you with 24-hour access to view your existing savings accounts and the
OMDC Online Application Portal (OAP) - Quick Start Guide
 OMDC Online Application Portal (OAP) - Quick Start Guide 1. INTRODUCTION This guide offers the quickest way to get you started on the OMDC Online Application Portal (OAP). Before you begin, step through
OMDC Online Application Portal (OAP) - Quick Start Guide 1. INTRODUCTION This guide offers the quickest way to get you started on the OMDC Online Application Portal (OAP). Before you begin, step through
SYSTEM REQUIREMENTS... 5 FREE RESOURCES... 6 GETTING STARTED...
 Table of Contents ABOUT... 4 Authorized Use... 4 Questions and Contact Information... 4 SYSTEM REQUIREMENTS... 5 FREE RESOURCES... 6 GETTING STARTED... 7 Account Creation Overview and Types of Accounts...
Table of Contents ABOUT... 4 Authorized Use... 4 Questions and Contact Information... 4 SYSTEM REQUIREMENTS... 5 FREE RESOURCES... 6 GETTING STARTED... 7 Account Creation Overview and Types of Accounts...
Central Commissioning Facility Research Management Systems (RMS): User Guidance
 Central Commissioning Facility Research Management Systems (RMS): User Guidance Contents 1. How to login and register a new account... 2 2. How to accept an invitation to review... 8 3. How to submit a
Central Commissioning Facility Research Management Systems (RMS): User Guidance Contents 1. How to login and register a new account... 2 2. How to accept an invitation to review... 8 3. How to submit a
Nova Southeastern University IRB - Consent Form Checklist Version Date: 02/17/2010
 Nova Southeastern University IRB - Consent Form Checklist Version Date: 02/17/2010 This form is intended to assist researchers in creating consent and assent forms. Informed consent is one of the primary
Nova Southeastern University IRB - Consent Form Checklist Version Date: 02/17/2010 This form is intended to assist researchers in creating consent and assent forms. Informed consent is one of the primary
A Quick Guide to Using CommonHelp
 A Quick Guide to Using CommonHelp Introduction CommonHelp is the Commonwealth of Virginia s fast and easy way to apply online for many Virginia social services assistance programs. Through a single online
A Quick Guide to Using CommonHelp Introduction CommonHelp is the Commonwealth of Virginia s fast and easy way to apply online for many Virginia social services assistance programs. Through a single online
Self Service User Guide
 Self Service User Guide User Guide V 1.0 1 17/10/2011 VERSION HISTORY Version Date Change Summary 1.0 October 2011 N/A User Guide V 1.0 2 17/10/2011 Contents VERSION HISTORY... 2 INTRODUCTION... 5 DATA
Self Service User Guide User Guide V 1.0 1 17/10/2011 VERSION HISTORY Version Date Change Summary 1.0 October 2011 N/A User Guide V 1.0 2 17/10/2011 Contents VERSION HISTORY... 2 INTRODUCTION... 5 DATA
Quick Reference Guide. Vendor FAQ
 This Frequently Asked Questions guide is meant to assist vendor users of the new emaryland Marketplace. If you require additional support, the emaryland Marketplace Support Team can be reached at [email protected].
This Frequently Asked Questions guide is meant to assist vendor users of the new emaryland Marketplace. If you require additional support, the emaryland Marketplace Support Team can be reached at [email protected].
1. On the Careers page, click on the "Click here to Register" link. You will then be taken to the Register page.
 Recommended Browsers for the recruiting system: Internet Explorer 7, 8, or 9 on Microsoft Windows Firefox 3.x on Microsoft Windows, UNIX, Linux, and Mac OSX Apple Safari 3.6 on Mac OS X Document Resources
Recommended Browsers for the recruiting system: Internet Explorer 7, 8, or 9 on Microsoft Windows Firefox 3.x on Microsoft Windows, UNIX, Linux, and Mac OSX Apple Safari 3.6 on Mac OS X Document Resources
California Conservation Corps. Corpsmember Advisory Board. Handbook
 California Conservation Corps Corpsmember Advisory Board Handbook Last updated on January 26, 2010 Table of Contents Introduction..........................................................................
California Conservation Corps Corpsmember Advisory Board Handbook Last updated on January 26, 2010 Table of Contents Introduction..........................................................................
UBC Engineering Co op Program Using EngCORE Employer Instructions
 UBC Engineering Co op Program Using EngCORE Employer Instructions Engineering Co-op Program Faculty of Applied Science 2385 East Mall Vancouver, BC Canada V6T 1Z4 Phone 604 822 3022 Fax 604 822 3449 [email protected]
UBC Engineering Co op Program Using EngCORE Employer Instructions Engineering Co-op Program Faculty of Applied Science 2385 East Mall Vancouver, BC Canada V6T 1Z4 Phone 604 822 3022 Fax 604 822 3449 [email protected]
Onboarding User Manual Version 9-20159
 Contents Hire (Companies using Hiring Manager + Onboarding)... 4 Hire (Companies using Onboarding only)... 5 Starting the Onboarding Process... 6 Complete at Home... 6 What If the Employee Can t Locate
Contents Hire (Companies using Hiring Manager + Onboarding)... 4 Hire (Companies using Onboarding only)... 5 Starting the Onboarding Process... 6 Complete at Home... 6 What If the Employee Can t Locate
Licensure Management & Application Online System
 Licensure Management & Application Online System School System User Guide March/April 2015 Created by: Iron Data with NCDPI/Licensure Section March, 2015 1 School System User Guide NC DPI Online Licensure
Licensure Management & Application Online System School System User Guide March/April 2015 Created by: Iron Data with NCDPI/Licensure Section March, 2015 1 School System User Guide NC DPI Online Licensure
Human Research Protection Program Good Clinical Practice Guidance for Investigators Investigator & Research Staff Responsibilities
 This Guidance Document is to ensure that investigators and research personnel recognize their responsibilities associated with the conduct of human subject research by outlining their responsibilities,
This Guidance Document is to ensure that investigators and research personnel recognize their responsibilities associated with the conduct of human subject research by outlining their responsibilities,
USAJOBS/CareerConnector Applicant User Guide. May 2010 Prepared by HCO ETS
 USAJOBS/CareerConnector Applicant User Guide May 2010 Prepared by HCO ETS USAJOBS/CareerConnector SIX STEPS TO APPLY FOR IRS JOBS ONLINE USING USAJOBS/CAREERCONNECTOR Step 1 - Create a USAJOBS account
USAJOBS/CareerConnector Applicant User Guide May 2010 Prepared by HCO ETS USAJOBS/CareerConnector SIX STEPS TO APPLY FOR IRS JOBS ONLINE USING USAJOBS/CAREERCONNECTOR Step 1 - Create a USAJOBS account
Banner Web Time Entry STUDENT Web Timekeeping Manual
 Banner Web Time Entry STUDENT Web Timekeeping Manual Introduction Web Time Entry (WTE) is a web-based time recording system designed to improve accuracy and eliminate loss or delays in processing paper
Banner Web Time Entry STUDENT Web Timekeeping Manual Introduction Web Time Entry (WTE) is a web-based time recording system designed to improve accuracy and eliminate loss or delays in processing paper
Cloud Web Portal User Guide Version 2.0
 Cloud Web Portal User Guide Version 2.0 Welcome to ncrypted Cloud! ncrypted Cloud is a Privacy, Security, and Collaboration application that uses Industry Standard Encryption Technology (AES-256 bit encryption)
Cloud Web Portal User Guide Version 2.0 Welcome to ncrypted Cloud! ncrypted Cloud is a Privacy, Security, and Collaboration application that uses Industry Standard Encryption Technology (AES-256 bit encryption)
Account to Account Transfer FAQ
 Account to Account Transfer FAQ Help -Registration and Signup. Why do I have to verify my external accounts? We do everything possible to protect your security and maintain the integrity of the payments
Account to Account Transfer FAQ Help -Registration and Signup. Why do I have to verify my external accounts? We do everything possible to protect your security and maintain the integrity of the payments
STEPS TO REGISTER YOUR PROFILE AND BOOK THE COUPON
 STEPS TO REGISTER YOUR PROFILE AND BOOK THE COUPON 1. Registering with Sabarimala Virtual Q Portal A user can register into the site after a few easy steps. A user needs to register with the site to be
STEPS TO REGISTER YOUR PROFILE AND BOOK THE COUPON 1. Registering with Sabarimala Virtual Q Portal A user can register into the site after a few easy steps. A user needs to register with the site to be
Patient Portal Training Manual
 Patient Portal Training Manual The Patient Portal is a secure website that will allow patients to access medical information from the Electronic Medical Record (EMR), send messages to their providers or
Patient Portal Training Manual The Patient Portal is a secure website that will allow patients to access medical information from the Electronic Medical Record (EMR), send messages to their providers or
Delegate Access. In Lync 2013
 Delegate Access 101613 Contents Delegate Defined... 3 Set up Delegate Access in Lync 2013... 3 Set up Delegate Access Using Outlook 2013... 4 Add a Mailbox... 6 Open a Delegated Mailbox... 8 Information
Delegate Access 101613 Contents Delegate Defined... 3 Set up Delegate Access in Lync 2013... 3 Set up Delegate Access Using Outlook 2013... 4 Add a Mailbox... 6 Open a Delegated Mailbox... 8 Information
Using My NCBI and My Bibliography Tools to Complete the NIH Research Project Progress Report (RPPR)
 Using My NCBI and My Bibliography Tools to Complete the NIH Research Project Progress Report (RPPR) Prior to using the My Bibliography tool within My NCBI, do the following: Obtain an NIH era Commons account
Using My NCBI and My Bibliography Tools to Complete the NIH Research Project Progress Report (RPPR) Prior to using the My Bibliography tool within My NCBI, do the following: Obtain an NIH era Commons account
Delegate Access. In Lync 2010
 Delegate Access 080713 Contents Delegate Defined... 3 Set up Delegate Access in Lync 2010... 3 Set up Delegate Access Using Outlook 2010... 4 Add a Mailbox... 6 Open a Delegated Mailbox... 7 Information
Delegate Access 080713 Contents Delegate Defined... 3 Set up Delegate Access in Lync 2010... 3 Set up Delegate Access Using Outlook 2010... 4 Add a Mailbox... 6 Open a Delegated Mailbox... 7 Information
FAFSA Verification and IRS Data Retrieval Tool Help and Tips
 FAFSA Verification and IRS Data Retrieval Tool Help and Tips What is verification? Each year students and their families apply for financial aid by submitting a Free Application for Federal Student Aid
FAFSA Verification and IRS Data Retrieval Tool Help and Tips What is verification? Each year students and their families apply for financial aid by submitting a Free Application for Federal Student Aid
SEC External Guide for Using the E-mail Encryption Solution
 Securities and Exchange Commission Office of Information Technology SEC External Guide for Using the E-mail Encryption Solution The Securities and Exchange Commission National Exam Program Hotline (202)551-3925
Securities and Exchange Commission Office of Information Technology SEC External Guide for Using the E-mail Encryption Solution The Securities and Exchange Commission National Exam Program Hotline (202)551-3925
THE QUICK GUIDE Your complete reference on how to complete your Pre-employment requirements.
 1 Completing your Onboarding Paperwork The onboarding paperwork are documents that formalize your employment with SYKES Home powered by Alpine Access. These documents include client, province, and company
1 Completing your Onboarding Paperwork The onboarding paperwork are documents that formalize your employment with SYKES Home powered by Alpine Access. These documents include client, province, and company
Electronic Selection of Consultants
 Electronic Selection of Consultants User Guide for Consulting Firms Version 3 This User Guide was produced by the Operations and Corporate Procurement Groups and the Information Solutions Group Global
Electronic Selection of Consultants User Guide for Consulting Firms Version 3 This User Guide was produced by the Operations and Corporate Procurement Groups and the Information Solutions Group Global
CLINICAL RESEARCH ROLES CLINICAL RESEARCH ROLESCLINICAL RESEARCH ROLES
 CLINICAL RESEARCH ROLESCLINICAL RESEARCH ROLES 1. Clinical Study Team Principal Investigator Co Principal Investigator (Co PI) Research Coordinator Study Monitor Data manage Sponsor Post Doctoral Scholar
CLINICAL RESEARCH ROLESCLINICAL RESEARCH ROLES 1. Clinical Study Team Principal Investigator Co Principal Investigator (Co PI) Research Coordinator Study Monitor Data manage Sponsor Post Doctoral Scholar
User Guide for Archie and Review Manager 5. The Cochrane Airways Group
 User Guide for Archie and Review Manager 5 The Cochrane Airways Group 1 Contents Introduction to User Guide... 3 Preliminary details... 4 Managing your contact details... 5 Principles of Review Manager
User Guide for Archie and Review Manager 5 The Cochrane Airways Group 1 Contents Introduction to User Guide... 3 Preliminary details... 4 Managing your contact details... 5 Principles of Review Manager
Verizon Business National Unified Messaging Service Enhanced Service Guide
 USER GUIDE Voice Verizon Business National Unified Messaging Service Enhanced Service Guide What Is Unified Messaging? Verizon Business National Unified Messaging Service is an interactive voicemail system
USER GUIDE Voice Verizon Business National Unified Messaging Service Enhanced Service Guide What Is Unified Messaging? Verizon Business National Unified Messaging Service is an interactive voicemail system
How do you organize the schools you are applying to?
 BMR COLLEGE APPLICATION PROCESS FOR NAVIANCE Included below are the procedures for sending applications and records to colleges from BMR High School. In order to insure that each student s applications
BMR COLLEGE APPLICATION PROCESS FOR NAVIANCE Included below are the procedures for sending applications and records to colleges from BMR High School. In order to insure that each student s applications
Microsoft Outlook 2007 Calendar Features
 Microsoft Outlook 2007 Calendar Features Participant Guide HR Training and Development For technical assistance, please call 257-1300 Copyright 2007 Microsoft Outlook 2007 Calendar Objectives After completing
Microsoft Outlook 2007 Calendar Features Participant Guide HR Training and Development For technical assistance, please call 257-1300 Copyright 2007 Microsoft Outlook 2007 Calendar Objectives After completing
E-FILE. Universal Service Administrative Company (USAC) Last Updated: September 2015
 E-FILE USER GUIDE This document providers E-File users with an overview of E-File account management, managing entitlements, and instructions on how to submit forms, such as the FCC Form 498, FCC Form
E-FILE USER GUIDE This document providers E-File users with an overview of E-File account management, managing entitlements, and instructions on how to submit forms, such as the FCC Form 498, FCC Form
How To Write A Health Care Plan
 USER GUIDE STEP UP PERFORMANCE MANAGEMENT SYSTEM Oklahoma State Department of Health Table of Contents Chapter 1 Getting Started... 2 Chapter 2 Overview and Public Health System Alignment... 5 Chapter
USER GUIDE STEP UP PERFORMANCE MANAGEMENT SYSTEM Oklahoma State Department of Health Table of Contents Chapter 1 Getting Started... 2 Chapter 2 Overview and Public Health System Alignment... 5 Chapter
GETTING STARTED WITH EDISS AND TOTAL ONBOARDING (TOB)
 GETTING STARTED WITH EDISS AND TOTAL ONBOARDING (TOB) Table of Contents What is an electronic transaction?...2 What forms will be required for EDISS registration now that TOB is effective for most lines
GETTING STARTED WITH EDISS AND TOTAL ONBOARDING (TOB) Table of Contents What is an electronic transaction?...2 What forms will be required for EDISS registration now that TOB is effective for most lines
Application Procedures: Rhodes Scholarship IMPORTANT DATES AND DEADLINES - Application Year 2015-16
 Application Procedures: Rhodes Scholarship IMPORTANT DATES AND DEADLINES - Application Year 2015-16 Step Description Date Due Complete 1 Schedule Initial Consultation and/or Attend Info Session Spring
Application Procedures: Rhodes Scholarship IMPORTANT DATES AND DEADLINES - Application Year 2015-16 Step Description Date Due Complete 1 Schedule Initial Consultation and/or Attend Info Session Spring
and forms from multiple folders simultaneously to send out for e signing as the system will automatically create a Queue as you select documents.
 This guide was prepared to show our members how to submit saved forms (and/or uploaded documents) out for e signature using our built in integration with Settleware s secure e signing services. Once you
This guide was prepared to show our members how to submit saved forms (and/or uploaded documents) out for e signature using our built in integration with Settleware s secure e signing services. Once you
