AURA Clinical Trial FP Guidelines
|
|
|
- Ambrose Sparks
- 8 years ago
- Views:
Transcription
1 AURA Clinical Trial FP Guidelines Table of Contents Overview 2 What is a Clinical Trial? Guidelines Acronym/ Abbreviations/ Key Codes FP Workflow.. Step 1: PC-O create CT.. 3 Step 2: OCR/ URA-M advance review & PC-O uploads final budget Step 3: URA-M route BAFO for unit review... 6 Step 4: PI concurrence and COI.. 6 Step 5: PC-O complete AURA budget grid, Update IRB, Route BAFO for unit approval... 7 Step 6: UA review & approve. 8 Step 7: OCR BAFO review... 8 Step 8: PC-O make changes for OCR 9 Step 9: OCR BAFO approval Step 10: URA-M BAFO review Step 11: PC-O makes changes for URA-M Step 12: URA-M endorsement, notice of award, budget period update Guidelines by Role PC-O.. 11 OCR 15 URA-M.. 16 PI.. 18 UA
2 Overview: This document outlines the Clinical Trial (CT) non-system-to-system (i.e. non grants.gov) funding submission process to assist users in pre-award proposal development. Use bookmarks to your left to navigate; view the entire CT process or jump to requirements by role. Below are acronyms/ key codes used throughout the CT process guidelines. Note: When submitting system-to-system CT s, Question 1.3 will be marked as research and does not follow this workflow.. What is a Clinical Trial? A clinical trial is as a prospective study involving human subjects designed to answer specific questions about the effects of biomedical or behavioral interventions. Such studies may include drugs, treatments, devices, or behavioral or nutritional strategies. Behavioral clinical trials include studies of interventions to increase behaviors (e.g. cancer screening, physical activity, fruits and vegetable intake), eliminate/ reduce behaviors (e.g. smoking, sun exposure) or improve coping, quality of life, and decrease negative side effects of treatment. Observational studies and those that do not test interventions are not considered clinical trials. In the area of molecular or imaging diagnostics, a study is a clinical trial if it uses the information from the diagnostic test in a manner that somehow affects medical decision-making for the study subject. In this way the information from the diagnostic test may have an impact on some aspect of outcome, and assessment of this outcome may be a key goal of the trial. By contrast, studies that do not use information from the diagnostic test in any manner that can affect the outcome of study subjects, but whose objective is only gathering data on the characteristics of a new diagnostic approach, are not clinical trials and are not covered by this DSM policy, unless performing the diagnostic test itself imposes some risk on study subjects. Guidelines Acronym/ Abbreviations/ Key Codes Acronym/Abbreviation URA OCR FP PC-O URA-M PI UA BAFO COI CRO IB ICF Title University Research Administration Office of Clinical Research Funding Proposal Proposal Creator Owner University Research Administration Manager Principal Investigator Unit Approver Best and Final Offer Conflict of Interest Contract Research Organization Investigator s Brochure Informed Consent Form Blue Green Orange Purple Maroon Key Code PC-O OCR URA-M PI UA 2
3 Step 1: (PC-O) Create CT and route to OCR/ URA-M for advance review Click "New Funding Submission" button in the green navigation bar on the left side of the screen 1.1 Enter full protocol title or your preferred abbreviation 1.2 Enter full protocol title 1.3 Select "Clinical Trial" from dropdown menu 1.4 Click "Add" button Edit UC_ReportingCreditUnit Select PC's department Edit UC_ReportingCreditUnit Enter "100" Edit UC_ReportingCreditUnit Select "Yes" radio button Edit UC_ReportingCreditUnit Select funding source (not the CRO, not the institution giving us a subaward) 1.8 Select "Grant," "Contract," or "Subaward" radio button, as appropriate 1.9 *Only complete if 1.8 is "Subaward"* Select Prime Grantee (agency that is giving a subaward to the University) 1.10 Enter full protocol title or your preferred language for use on Current & Pending Support forms. 1.0 Click "Continue" button 2.1a Select PI 2.2a Select "No" radio button 2.0a Select "3.0 Agency/Sponsor Details" on the "Jump To" dropdown menu in the top blue navigation bar 3.1 Select "No" radio button 3.3 Enter "n/a" 3.12 Select "Yes" or "No" radio button, as appropriate 3.0 Click "Continue" button 7.1 Select "No" radio button 7.2 Select PC (yourself) 7.3 *Only complete if you would like to assign Read or Edit access* click "Add" button Edit Choose individual UCFP_SupportPerson7.3.1 Edit Choose type of access for both FP and Budget UCFP_SupportPerson7.3.2 Edit UCFP_Support Person 7.3 if done, click "OK and Add Another" to add another individual 7.4 Choose a PID 7.5 Select URA Manager to review from dropdown menu 7.6 Enter "2/2/2222" 7.8 Select "URA" radio button 7.0 Click "Continue" button 9.1 Select "Yes" radio button 9.5 Select "Yes" or "No" radio button, as appropriate 9.9 Select "Yes" or "No" radio button, as appropriate 9.0 Click "Continue" button 10.3 Select "Yes" radio button 10.0 Click "Continue" button 3
4 Step 1 Cont d: 11.1 *View 11.0 will only appear if 9.5 was "Yes"* Click "Add" button Edit UCFP_Subawardee Select outgoing subaward organization. If unknown, select "PLACEHOLDER" and AURA-Help@uchicago.edu for assistance. Edit UCFP_Subawardee 11.1 Select "OK" button if done, select "OK and Add Another to add more outgoing suabwardees." 11.0 Click "Continue" button 13.1 Select phase, if evident from the protocol title. Otherwise select "Other" radio button *Only complete if 13.1 is "Other"* Enter relevant information. Ex. registry, chart review, n/a, unknown 13.3 Select "Yes" or "No" radio button, as appropriate 13.4 *Only complete if 13.3 is "Yes"* Enter IND number 13.5 Select "Yes" or "No" radio button, as appropriate 13.6 *Only complete if 13.5 is "Yes"* Enter IDE number 13.7 Select "Yes" or "No" radio button, as appropriate 13.8 Select "Yes" or "No" radio button, as appropriate Select "Yes" or "No" radio button, as appropriate *Only complete if is "Yes"* Enter CRO name Enter CRO (or, if no CRO, Sponsor) contact name for contract negotiations Enter CRO (or, if no CRO, Sponsor) contact for contract negotiation Upload all documents required for advance review, clearly labeled, using upload date in file name for version control 13.0 Click "Continue" button 15.1 Skipping the radio buttons, insert date of IRB meeting intended for review in "Date Project Starts." 15.1 Return to radio buttons and select the "Yes" radio button 15.1 Enter date of IRB meeting intended for review in the "Start" date field of the first budget period row 15.0 Select "18.0 Internal Documents" from "Jump To" dropdown menu in the top blue navigation bar 18.1 Select "Yes" or "No" radio button, as appropriate 18.0 Click "Save" button, then "Exit" button in top blue navigation bar Funding Proposal summary page CT - Route Concurrence 1.0 CT - Route Concurrence 3.0 CT - Route Concurrence When ready to route for OCR and URA advance review, click "CT - Route Concurrence" button under "My Activities" in the left green navigation bar enter comments, if any system "In CT Advance Review" to OCR and URA PC-O routes CT concurrence to OCR & URA-M OCR/ URA-M receive system notification 4
5 Step 2: (OCR, URA-M, PC-O ) Advance review & PC-O uploads final budget OCR, URA-M, and PC-O complete review accordingly by role. FP Printer Version FP Printer Version Click on My Funding Proposals tab Locate the project by "Filter by" "ID" and entering "%" and the FP number (found in the "In CT Advanced Review" ) and clicking the "Go" button. Select the project by clicking on the Name Click on "Printer Version" button on left green navigation bar Complete advance review, including documents in 13.17, and provide comments to PC and URA via non-aura When done reviewing, click "Close" button in top right hand corner OCR Click on "URA Inbox" tab Click on "Printer Version" button on left green navigation bar FP Printer Version CT - Ready to Negotiate 1.0 CT - Ready to Negotiate 2.0/3.0 CT - Ready to Negotiate FP Summary page PC-O 1.0 PC-O Complete advance review, including documents in 13.17, and provide comments and contract markup to PC and OCR via When ready to negotiate, click "CT - Ready to Negotiate" button in left green navigation bar Upload markup in 2.0. If negotiation is not necessary, enter "n/a" in 3.0 If negotiations are not required, or when negotiations are complete, click " PC-O" button on left green navigation bar Enter "contract is final" system " ed PC-O" *Complete this activity only when budget negotiations have been completed outside of AURA* Locate proposal by "Filter by" "ID" and entering "%" and the FP number in the search field, and click "Go" button Select the project by clicking on the Name Click on "Edit Funding Proposal" button in left green navigation bar 1.0 Select "13.0 Clinical Trials" on the "Jump to" dropdown menu Upload final internal and external budgets, using "BAFO" and the upload date in the file name for version control 13.0 Click "Save" button, then "Exit" button in the top blue navigation bar Click on "URA-M" button in left green navigation bar URA-M 1.0 Enter "budget is final" URA-M system " ed URA-M" stating "budget is final" to URA-M URA-M URA-M PC-O informing contract is final PC-O PC-O URA-M informing budget is final 5
6 Step 3: (URA-M) Route BAFO for unit review CT- Forward BAFO for Unit Review 1.0 CT - Forward BAFO for Unit Review 2.0 CT - Forward BAFO for Unit Review 3.0 CT - Forward BAFO for Unit Review *Complete this step only once PC has " ed URA-M" that the budget is final and URA-M has " ed PC-O" that the contract is final* Log in with CNET ID Click on URA Inbox tab Click "Route BAFO for Unit Review" button in left green navigation bar Upload final contract Enter instructions, if any, for routing (ex. hard copies, PI signature required, okay) system "CT in PC-PI Approval" PI & PC-O receive system notification to complete concurrence and COI Step 4: (PI) Concurrence and COI Note: PC-O is not permitted to complete COI on PI s behalf. Click on "PI Inbox" tab Click "Printer Version" button in left green navigation bar Printer Version Review clinical trial information and documents in and Printer Version Click "Close" button in top right corner when done reviewing Click "CT - PI Concurrence and COI" button in left green navigation bar CT - PI Concurrence and COI Answer all required questions CT - PI Concurrence and COI Note: Currently no system in place to notify PC-O that Step 5 is complete. PC-O should periodically check CT to confirm PI concurrence/ COI 6
7 Step 5: (PC-O) Complete AURA budget grid, Update IRB, Route BAFO for unit approval FP Summary page Budget *Complete this step after PI Concurrence is completed* Log in with CNET ID Click on "PC Inbox" tab Select "Budget Workspaces" tab Select project budget Select "Grid: Inflation and F&A Rates" link on left green navigation bar Grid: Inflation and F&A Rates Change rate percentage from 40% to 25% Grid: Inflation and F&A Rates Click "Apply" button, then "OK" button in top blue navigation bar Budget General Budget Information 4.0 General Budget Information Personnel Costs Personnel Costs Personnel Costs General Costs General Costs General Costs Click "Edit Budget" button in left green navigation bar Select appropriate answer Click "Continue" button in top blue navigation bar Add 1 row to the grid Select PI from "Person" dropdown menu, enter "0%" in Effort and SalReq fields Select "3.7 General Costs - Grid" from "Jump to" dropdown menu Add 2 rows to the grid In one row, select "Clin Trial - Start-Up Fees" from the "Cost Type" dropdown menu, enter the start-up costs (direct costs only) in the "Unit Cost" field, enter "1" in "# of Units" field In the second row, select "Clin Trial - Per-Patient Fees" from the "Cost Type" dropdown menu, enter the per-patient amount (direct costs only) in the "Unit Cost" field, enter anticipated number of subjects in the "# Units" field General Costs Budget Click "Save" button, then "Exit" button in top blue navigation bar Click the project breadcrumb in the top gray navigation bar Click "Edit Funding Proposal" button in left green navigation bar 1.0 Select "10 - Compliance" from the "Jump to" dropdown menu Update protocol number and status (if approved, include approval date and upload a copy of the IRB approval letter) 10.0 Click "Save" button, then "Exit" button in top blue navigation bar Click "CT - Forward BAFO for Unit Approval" button in left green navigation bar CT - Forward BAFO for Unit Approval 1.0 CT - Forward BAFO for Unit Select your Unit Approver Approval 2.0 CT - Forward BAFO for Unit Approval system "In CT Unit Approval State" to UA PC-O route to Unit Approver (UA) UA receives system notification 7
8 Step 6: (UA) Review and approval My Home page Click on "UA Inbox" tab My Home page Click "Printer Version" button in left green navigation bar Printer Version Review clinical trial information and documents in and Printer Version Click "Close" button in top right corner when done reviewing Click "Unit Approval" button in left green navigation bar Unit Approval 1.0 Select "I approve without additional comments" radio button Unit Approval system "In CT OCR Review State" to OCR OCR receives system notification Step 7: (OCR) BAFO review My Home page My Home page Activity Details ( CT - PI Concurrence Completed) 1.0, 2.0a, and 2.0b Click on "OCR Inbox" tab Under "History" tab, select the link to "PI Concurrence Completed" Review PI's answers Activity Details ( CT - PI Click breadcrumb for project in top gray navigation bar Concurrence Completed) Click "Printer Version" button in left green navigation bar Printer Version Review clinical trial information and documents in and Printer Version Click "Close" button in top right corner when done reviewing *Complete this activity only if changes are required* Click "CT - Request Changes" button in the left green navigation bar CT - Request Changes 1.0 CT - Request Changes 2.0 Select "No" -- If PI needs to redo COI and concurrence, note that in section 3.0 of the activity and check the History tab for re-"pi Concurrence and COI" activity CT - Request Changes 3.0 Describe required changes CT - Request Changes system "In CT OCR Requested Changes State" to PC Note: If changes are not required, skip to Step 9 PC-O receives system notification 8
9 Step 8: (PC-O) Make changes for OCR CT - Changes Made 1.0 CT- Changes Made Click on "PC Inbox" tab Locate and select project Click "Edit Funding Proposal" button in left green navigation bar, make changes When changes are completed, click "CT - Changes Made" button in the left green navigation bar system "CT - OCR Review" to OCR OCR receives system notification for approval Step 9: (OCR) BAFO approval Click "OCR Approval" button in left green navigation bar Unit Approval 1.0 Unit Approval system "In CT Pending Institutional Endorsement State" to URA-M and PC PC & URA-M receive system notification for approval Step 10: (URA-M) BAFO review Click on "URA Inbox" tab Under "History" tab, select the link to "PI Concurrence Completed" Activity Details ( CT - PI Concurrence Completed) 1.0, Confirm that a COI Assurance is on file for the current year, and check answers Activity Details ( CT - PI Click breadcrumb for project in top gray navigation bar Concurrence Completed) Click "Printer Version" button in left green navigation bar Printer Version Review clinical trial information and documents in and Printer Version Click "Close" button in top right corner when done reviewing *Complete this activity only if changes are required* Click " PC-O" button in left green navigation screen PC-O 1.0 Describe required changes PC-O system " PC-O" to PC Note: If changes are not required, skip to Step 12 and URA-M completes process URA-M PC-O requesting changes 9
10 Step 11: (PC-O) Make changes for URA-M URA-M 1.0 URA-M Locate the project by "Filter by" "ID" and entering "%" and the FP number (found in the "In CT Advanced Review" ) and clicking the "Go" button. Select the project by clicking on the Name Select project by clicking on the Name Click on "Edit Funding Proposal" in left green navigation bar and make requested changes When changes are completed, click " URA-M" in left green navigation bar Enter "changes made" system " ed URA-M" PC-O URA-M for final review Step 12: (URA-M) Endorsement, notice of award, budget period update When contract has been signed, click "CT - Institutional Endorsement" button in left green navigation bar CT - Institutional Endorsement 1.0 CT - Institutional *Only complete this step if the contract is partially executed (i.e. not yet Endorsement 2.0 fully-executed)* Upload a copy of the partially-executed contract CT - Institutional Endorsement system "In CT Waiting for Fully Executed Endorsement State" to PC When contract is fully-executed, click "CT - Notice of Award" button in left green navigation bar CT - Notice of Award 1.0 CT - Notice of Award 2.0 Upload a copy of the fully-executed contract CT - Notice of Award 3.0 *Only complete this step if IRB approval is not yet shown in View 10 of the FP* Enter "This project does not have an approved protocol from the Institutional Review Board (IRB). Any work with Human Subjects may not begin on this project until the protocol has been approved by the appropriate Institutional Review Board." PC-O receives system notification CT - Notice of Award system "Clinical Trial Notice of Award Sent" to PC, PI, OCR Click "Edit Funding Proposal" button in left green navigation bar 1.0 Select " Budget Periods" in the "Jump to" dropdown menu 15.0 Update Start and End date fields, based on the term of the contract. If there is no date-specific End date, leave End date field blank 15.0 Click "Save" button, then "Exit" button in top blue navigation bar 10
11 Guidelines by Role PC-O Create CT and route to OCR/ URA-M for advance review Click "New Funding Submission" button in the green navigation bar on the left side of the screen 1.1 Enter full protocol title or your preferred abbreviation 1.2 Enter full protocol title 1.3 Select "Clinical Trial" from dropdown menu 1.4 Click "Add" button Edit UC_ReportingCreditUnit Select PC's department Edit UC_ReportingCreditUnit Enter "100" Edit UC_ReportingCreditUnit Select "Yes" radio button Edit UC_ReportingCreditUnit Select funding source (not the CRO, not the institution giving us a subaward) 1.8 Select "Grant," "Contract," or "Subaward" radio button, as appropriate 1.9 *Only complete if 1.8 is "Subaward"* Select Prime Grantee (agency that is giving a subaward to the University) 1.10 Enter full protocol title or your preferred language for use on Current & Pending Support forms. 1.0 Click "Continue" button 2.1a Select PI 2.2a Select "No" radio button 2.0a Select "3.0 Agency/Sponsor Details" on the "Jump To" dropdown menu in the top blue navigation bar 3.1 Select "No" radio button 3.3 Enter "n/a" 3.12 Select "Yes" or "No" radio button, as appropriate 3.0 Click "Continue" button 7.1 Select "No" radio button 7.2 Select PC (yourself) 7.3 *Only complete if you would like to assign Read or Edit access* click "Add" button Edit Choose individual UCFP_SupportPerson7.3.1 Edit Choose type of access for both FP and Budget UCFP_SupportPerson7.3.2 Edit UCFP_Support Person 7.3 if done, click "OK and Add Another" to add another individual 7.4 Choose a PID 7.5 Select URA Manager to review from dropdown menu 7.6 Enter "2/2/2222" 7.8 Select "URA" radio button 7.0 Click "Continue" button 9.1 Select "Yes" radio button 9.5 Select "Yes" or "No" radio button, as appropriate 9.9 Select "Yes" or "No" radio button, as appropriate 9.0 Click "Continue" button 10.3 Select "Yes" radio button 10.0 Click "Continue" button 11
12 11.1 *View 11.0 will only appear if 9.5 was "Yes"* Click "Add" button Edit UCFP_Subawardee Select outgoing subaward organization. If unknown, select "PLACEHOLDER" and for assistance. Edit UCFP_Subawardee 11.1 Select "OK" button if done, select "OK and Add Another to add more outgoing suabwardees." 11.0 Click "Continue" button 13.1 Select phase, if evident from the protocol title. Otherwise select "Other" radio button *Only complete if 13.1 is "Other"* Enter relevant information. Ex. registry, chart review, n/a, unknown 13.3 Select "Yes" or "No" radio button, as appropriate 13.4 *Only complete if 13.3 is "Yes"* Enter IND number 13.5 Select "Yes" or "No" radio button, as appropriate 13.6 *Only complete if 13.5 is "Yes"* Enter IDE number 13.7 Select "Yes" or "No" radio button, as appropriate 13.8 Select "Yes" or "No" radio button, as appropriate Select "Yes" or "No" radio button, as appropriate *Only complete if is "Yes"* Enter CRO name Enter CRO (or, if no CRO, Sponsor) contact name for contract negotiations Enter CRO (or, if no CRO, Sponsor) contact for contract negotiation Upload all documents required for advance review, clearly labeled, using upload date in file name for version control 13.0 Click "Continue" button 15.1 Skipping the radio buttons, insert date of IRB meeting intended for review in "Date Project Starts." 15.1 Return to radio buttons and select the "Yes" radio button 15.1 Enter date of IRB meeting intended for review in the "Start" date field of the first budget period row 15.0 Select "18.0 Internal Documents" from "Jump To" dropdown menu in the top blue navigation bar 18.1 Select "Yes" or "No" radio button, as appropriate 18.0 Click "Save" button, then "Exit" button in top blue navigation bar Funding Proposal summary page CT - Route Concurrence 1.0 CT - Route Concurrence 3.0 CT - Route Concurrence When ready to route for OCR and URA advance review, click "CT - Route Concurrence" button under "My Activities" in the left green navigation bar enter comments, if any system "In CT Advance Review" to OCR and URA 12
13 Upon receipt of URA-M confirming contract is final, upload final budget *Complete this activity only when budget negotiations have been completed outside of AURA* Locate proposal by "Filter by" "ID" and entering "%" and the FP number in the search field, and click "Go" button Select the project by clicking on the Name Click on "Edit Funding Proposal" button in left green navigation bar 1.0 Select "13.0 Clinical Trials" on the "Jump to" dropdown menu Upload final internal and external budgets, using "BAFO" and the upload date in the file name for version control 13.0 Click "Save" button, then "Exit" button in the top blue navigation bar Click on "URA-M" button in left green navigation bar URA-M 1.0 Enter "budget is final" URA-M system " ed URA-M" stating "budget is final" to URA-M After URA-M routes BAFO for unit review, PI completes concurrence, THEN FP Summary page Budget *Complete this step after PI Concurrence is completed* Log in with CNET ID Click on "PC Inbox" tab Select "Budget Workspaces" tab Select project budget Select "Grid: Inflation and F&A Rates" link on left green navigation bar Grid: Inflation and F&A Rates Change rate percentage from 40% to 25% Grid: Inflation and F&A Rates Click "Apply" button, then "OK" button in top blue navigation bar Budget General Budget Information 4.0 General Budget Information Personnel Costs Personnel Costs Personnel Costs General Costs General Costs General Costs General Costs Budget Click "Edit Budget" button in left green navigation bar Select appropriate answer Click "Continue" button in top blue navigation bar Add 1 row to the grid Select PI from "Person" dropdown menu, enter "0%" in Effort and SalReq fields Select "3.7 General Costs - Grid" from "Jump to" dropdown menu Add 2 rows to the grid In one row, select "Clin Trial - Start-Up Fees" from the "Cost Type" dropdown menu, enter the start-up costs (direct costs only) in the "Unit Cost" field, enter "1" in "# of Units" field In the second row, select "Clin Trial - Per-Patient Fees" from the "Cost Type" dropdown menu, enter the per-patient amount (direct costs only) in the "Unit Cost" field, enter anticipated number of subjects in the "# Units" field Click "Save" button, then "Exit" button in top blue navigation bar Click the project breadcrumb in the top gray navigation bar 13
14 Click "Edit Funding Proposal" button in left green navigation bar 1.0 Select "10 - Compliance" from the "Jump to" dropdown menu Update protocol number and status (if approved, include approval date and upload a copy of the IRB approval letter) 10.0 Click "Save" button, then "Exit" button in top blue navigation bar Click "CT - Forward BAFO for Unit Approval" button in left green navigation bar CT - Forward BAFO for Unit Approval 1.0 CT - Forward BAFO for Unit Select your Unit Approver Approval 2.0 CT - Forward BAFO for Unit Approval system "In CT Unit Approval State" to UA UA will review/ approve, OCR reviews/ approves BAFO Upon receipt of system notification for OCR change request proceed with the following/ IF no changes skip this step & PC-O role is complete CT - Changes Made 1.0 CT- Changes Made Click on "PC Inbox" tab Locate and select project Click "Edit Funding Proposal" button in left green navigation bar, make changes When changes are completed, click "CT - Changes Made" button in the left green navigation bar system "CT - OCR Review" to OCR OCR & URA-M review/ approves changes Upon receipt of from URA-M for change request proceed with the following/ IF no changes skip this step & PC-O role is complete URA-M 1.0 URA-M Locate the project by "Filter by" "ID" and entering "%" and the FP number (found in the "In CT Advanced Review" ) and clicking the "Go" button. Select the project by clicking on the Name Select project by clicking on the Name Click on "Edit Funding Proposal" in left green navigation bar and make requested changes When changes are completed, click " URA-M" in left green navigation bar Enter "changes made" system " ed URA-M" **Note: To view complete workflow (all roles in the process) see pages 3-10** 14
15 OCR Upon receipt of system notification that CT is ready for advance review, THEN FP Printer Version FP Printer Version Click on My Funding Proposals tab Locate the project by "Filter by" "ID" and entering "%" and the FP number (found in the "In CT Advanced Review" ) and clicking the "Go" button. Select the project by clicking on the Name Click on "Printer Version" button on left green navigation bar Complete advance review, including documents in 13.17, and provide comments to PC and URA via non-aura When done reviewing, click "Close" button in top right hand corner Upon receipt of system notification confirming UA approval, THEN My Home page My Home page Activity Details ( CT - PI Concurrence Completed) 1.0, 2.0a, and 2.0b Click on "OCR Inbox" tab Under "History" tab, select the link to "PI Concurrence Completed" Review PI's answers Activity Details ( CT - PI Click breadcrumb for project in top gray navigation bar Concurrence Completed) Click "Printer Version" button in left green navigation bar Printer Version Review clinical trial information and documents in and Printer Version Click "Close" button in top right corner when done reviewing *Complete this activity only if changes are required* Click "CT - Request Changes" button in the left green navigation bar CT - Request Changes 1.0 CT - Request Changes 2.0 Select "No" -- If PI needs to redo COI and concurrence, note that in section 3.0 of the activity and check the History tab for re-"pi Concurrence and COI" activity CT - Request Changes 3.0 Describe required changes CT - Request Changes system "In CT OCR Requested Changes State" to PC IF changes are required, PC-O will make edits and forward back for review/ approval, THEN Click "OCR Approval" button in left green navigation bar Unit Approval 1.0 Unit Approval system "In CT Pending Institutional Endorsement State" to URA-M and PC IF no changes skip this step and OCR role is complete 15
16 URA-M Upon receipt of system notification that proposal is ready for advance review, THEN Click on "URA Inbox" tab Click on "Printer Version" button on left green navigation bar FP Printer Version Complete advance review, including documents in 13.17, and provide comments and contract markup to PC and OCR via When ready to negotiate, click "CT - Ready to Negotiate" button in left green navigation bar CT - Ready to Negotiate 1.0 CT - Ready to Negotiate Upload markup in 2.0. If negotiation is not necessary, enter "n/a" in /3.0 CT - Ready to Negotiate FP Summary page If negotiations are not required, or when negotiations are complete, click " PC-O" button on left green navigation bar PC-O 1.0 Enter "contract is final" PC-O system " ed PC-O" PC-O reviews/ updates budget Upon receipt of from PC-O that budget has been finalized, THEN CT- Forward BAFO for Unit Review 1.0 CT - Forward BAFO for Unit Review 2.0 CT - Forward BAFO for Unit Review 3.0 CT - Forward BAFO for Unit Review *Complete this step only once PC has " ed URA-M" that the budget is final and URA-M has " ed PC-O" that the contract is final* Log in with CNET ID Click on URA Inbox tab Click "Route BAFO for Unit Review" button in left green navigation bar Upload final contract Enter instructions, if any, for routing (ex. hard copies, PI signature required, okay) system "CT in PC-PI Approval" PI completes concurrence, UA reviews/ approve, OCR review/ approve 16
17 IF OCR requires changes, URA-M receives notification for review and approval, THEN Click on "URA Inbox" tab Under "History" tab, select the link to "PI Concurrence Completed" Activity Details ( CT - PI Concurrence Completed) 1.0, Confirm that a COI Assurance is on file for the current year, and check answers Activity Details ( CT - PI Click breadcrumb for project in top gray navigation bar Concurrence Completed) Click "Printer Version" button in left green navigation bar Printer Version Review clinical trial information and documents in and Printer Version Click "Close" button in top right corner when done reviewing *Complete this activity only if changes are required* Click " PC-O" button in left green navigation screen PC-O 1.0 Describe required changes PC-O system " PC-O" to PC PC-O makes changes and URA-M for final review URA-M Endorsement, notice of award, budget period update When contract has been signed, click "CT - Institutional Endorsement" button in left green navigation bar CT - Institutional Endorsement 1.0 CT - Institutional *Only complete this step if the contract is partially executed (i.e. not yet Endorsement 2.0 fully-executed)* Upload a copy of the partially-executed contract CT - Institutional Endorsement system "In CT Waiting for Fully Executed Endorsement State" to PC When contract is fully-executed, click "CT - Notice of Award" button in left green navigation bar CT - Notice of Award 1.0 CT - Notice of Award 2.0 Upload a copy of the fully-executed contract CT - Notice of Award 3.0 *Only complete this step if IRB approval is not yet shown in View 10 of the FP* Enter "This project does not have an approved protocol from the Institutional Review Board (IRB). Any work with Human Subjects may not begin on this project until the proposal submitted to the agency has been reviewed by the appropriate Institutional Review Board. It is imperative that you provide URA with a copy of this approval or amendment upon receipt." CT - Notice of Award system "Clinical Trial Notice of Award Sent" to PC, PI, OCR Click "Edit Funding Proposal" button in left green navigation bar 1.0 Select " Budget Periods" in the "Jump to" dropdown menu 15.0 Update Start and End date fields, based on the term of the contract. If there is no date-specific End date, leave End date field blank 15.0 Click "Save" button, then "Exit" button in top blue navigation bar **Note: To view complete workflow (all roles in the process) see pages 3-10** 17
18 PI Upon receipt of system notification to complete PI concurrence & COI, THEN Note: PC-O is not permitted to complete COI on PI s behalf. Click on "PI Inbox" tab Click "Printer Version" button in left green navigation bar Printer Version Review clinical trial information and documents in and Printer Version Click "Close" button in top right corner when done reviewing Click "CT - PI Concurrence and COI" button in left green navigation bar CT - PI Concurrence and COI Answer all required questions CT - PI Concurrence and COI PI role complete **Note: To view complete workflow (all roles in the process) see pages 3-10** 18
19 UA Upon receipt of system notification that proposal is ready for approval, THEN My Home page Click on "UA Inbox" tab My Home page Click "Printer Version" button in left green navigation bar Printer Version Review clinical trial information and documents in and Printer Version Click "Close" button in top right corner when done reviewing Click "Unit Approval" button in left green navigation bar Unit Approval 1.0 Select "I approve without additional comments" radio button Unit Approval system "In CT OCR Review State" to OCR UA role complete **Note: To view complete workflow (all roles in the process) see pages 3-10** 19
Children s Research Management System (CRMS) Version 3.0. Children s Hospital Colorado Research Institute Training Guide April 2015
 Children s Research Management System (CRMS) Version 3.0 Children s Hospital Colorado Research Institute Training Guide April 2015 Table of Contents Operational Needs Assessment (ONA) 3 Visit Schedules
Children s Research Management System (CRMS) Version 3.0 Children s Hospital Colorado Research Institute Training Guide April 2015 Table of Contents Operational Needs Assessment (ONA) 3 Visit Schedules
The following list consists of a few tips and tricks to use when navigating eirb.
 TIPS/TRICKS The following list consists of a few tips and tricks to use when navigating eirb. GENERAL Submitting applications Only the PI can submit applications. However, the Study Coordinator can submit
TIPS/TRICKS The following list consists of a few tips and tricks to use when navigating eirb. GENERAL Submitting applications Only the PI can submit applications. However, the Study Coordinator can submit
Howard University. version 1 created: 11/2014. R e s e a r c h A d m i n i s t r a t i v e S e r v i c e s
 Howard University version 1 created: 11/2014 R e s e a r c h A d m i n i s t r a t i v e S e r v i c e s CONTENTS DocuSign Overview... 2 Account Setup... 2 Getting Started... 2 The Submission Routing Structure...
Howard University version 1 created: 11/2014 R e s e a r c h A d m i n i s t r a t i v e S e r v i c e s CONTENTS DocuSign Overview... 2 Account Setup... 2 Getting Started... 2 The Submission Routing Structure...
Introduction to ilab Solutions for VUMC Users
 Introduction to ilab Solutions for VUMC Users (VUMC PIs, Lab Managers, and Lab Members) Table of Contents Account Access & Login Credentials... 2 Account Registration for First-time Users... 2 Accessing
Introduction to ilab Solutions for VUMC Users (VUMC PIs, Lab Managers, and Lab Members) Table of Contents Account Access & Login Credentials... 2 Account Registration for First-time Users... 2 Accessing
proposalcentral Prepare and Submit a Proposal.
 proposalcentral Prepare and Submit a Proposal. If you need assistance, contact Customer Service by email at pcsupport@altum.com or by phone at 1-800-875-2562 1 Recommended Software proposalcentral Recommends
proposalcentral Prepare and Submit a Proposal. If you need assistance, contact Customer Service by email at pcsupport@altum.com or by phone at 1-800-875-2562 1 Recommended Software proposalcentral Recommends
1. From the left navigation menu, click the Key Personnel panel. This will open the Key Personnel subpanel, click on the Personnel link.
 KC: Investigator Certifications Non-NIH/PHS Sponsor Proposals Quick Reference Card Overview: Individuals listed as Principal Investigator or Co-Investigator on the Key Persons/Personnel screen are required
KC: Investigator Certifications Non-NIH/PHS Sponsor Proposals Quick Reference Card Overview: Individuals listed as Principal Investigator or Co-Investigator on the Key Persons/Personnel screen are required
How to Create a New Clinical Research Study
 How to Create a New Clinical Research Study 1 Contents 1. Create a Submission for an Existing eirb Study... 3 2. Create a New Submission in eirb... 4 3. CRMS Clinical Research Study... 5 4. Uploading a
How to Create a New Clinical Research Study 1 Contents 1. Create a Submission for an Existing eirb Study... 3 2. Create a New Submission in eirb... 4 3. CRMS Clinical Research Study... 5 4. Uploading a
Electronic Signature and Approval Process
 Howard University Electronic Signature and Approval Process Submitting a Proposal Package Electronically version 3 created: 05/2013 R e s e a r c h A d m i n i s t r a t i v e S e r v i c e s CONTENTS
Howard University Electronic Signature and Approval Process Submitting a Proposal Package Electronically version 3 created: 05/2013 R e s e a r c h A d m i n i s t r a t i v e S e r v i c e s CONTENTS
Proposal Development Guide
 Proposal Development Guide Contents The Proposal Tab... 3 Document Overview Panel... 3 Required Fields for Saving Document Panel... 3 Institutional Fields (Conditionally Required)... 9 Sponsor & Program
Proposal Development Guide Contents The Proposal Tab... 3 Document Overview Panel... 3 Required Fields for Saving Document Panel... 3 Institutional Fields (Conditionally Required)... 9 Sponsor & Program
The NIHMS System User s Guide to Submitting a Manuscript
 On May 2, 2005, The National Institutes of Health (NIH) Public Access Policy went into effect for NIH-funded researchers to submit their peer-reviewed manuscripts only those that have been accepted for
On May 2, 2005, The National Institutes of Health (NIH) Public Access Policy went into effect for NIH-funded researchers to submit their peer-reviewed manuscripts only those that have been accepted for
Creating Proposals and Managing Grants
 Creating Proposals and Managing Grants Page 1 of 41 Table of Contents Lesson 1 Grants Overview... 1 Grants Overview... 3 Pre-Award... 3 Post-Award... 4 Proposal Creation... 4 Proposal Projects... 4 Lesson
Creating Proposals and Managing Grants Page 1 of 41 Table of Contents Lesson 1 Grants Overview... 1 Grants Overview... 3 Pre-Award... 3 Post-Award... 4 Proposal Creation... 4 Proposal Projects... 4 Lesson
Howard University. DocuSign User Guide. Signing Documents Electronically. R e s e a r c h A d m i n i s t r a t i v e S e r v i c e s
 Howard University DocuSign User Guide Signing Documents Electronically 2012 R e s e a r c h A d m i n i s t r a t i v e S e r v i c e s CONTENTS Signing basics... 2 Account Setup... 2 Getting Started...
Howard University DocuSign User Guide Signing Documents Electronically 2012 R e s e a r c h A d m i n i s t r a t i v e S e r v i c e s CONTENTS Signing basics... 2 Account Setup... 2 Getting Started...
End User Training Guide
 End User Training Guide October 2013 2005-2013 ExpenseWire LLC. All rights reserved. 1 expensewire.com Use of this user documentation is subject to the terms and conditions of the applicable End- User
End User Training Guide October 2013 2005-2013 ExpenseWire LLC. All rights reserved. 1 expensewire.com Use of this user documentation is subject to the terms and conditions of the applicable End- User
Add Title. Electronic Services Verification Instructions
 Add Title Electronic Services Verification Instructions Electronic Services Verification Instructions Access CHAMPS Enter Daily Tasks What to do if the Client is Not in the Home Log services for Multiple
Add Title Electronic Services Verification Instructions Electronic Services Verification Instructions Access CHAMPS Enter Daily Tasks What to do if the Client is Not in the Home Log services for Multiple
SCHOOL OF MEDICINE OFFICE OF RESEARCH ADMINISTRATION
 SCHOOL OF MEDICINE OFFICE OF RESEARCH ADMINISTRATION Step By Step Guide for: Coeus Proposal Development (Coeus PD) (in place of eis) for Paper (Hard) Copy and Electronic (non Grants.gov) submission Effective
SCHOOL OF MEDICINE OFFICE OF RESEARCH ADMINISTRATION Step By Step Guide for: Coeus Proposal Development (Coeus PD) (in place of eis) for Paper (Hard) Copy and Electronic (non Grants.gov) submission Effective
UTRGV PeopleAdmin Applicant Tracking System
 UTRGV PeopleAdmin Applicant Tracking System Quick Links: How do I access PeopleAdmin? How do I navigate through PeopleAdmin? How do I login to PeopleAdmin? How do I perform as a Search Committee Member
UTRGV PeopleAdmin Applicant Tracking System Quick Links: How do I access PeopleAdmin? How do I navigate through PeopleAdmin? How do I login to PeopleAdmin? How do I perform as a Search Committee Member
CONFIGURING VIRTUAL TERMINAL: This is the screen you will see when you first open Virtual Terminal
 CONFIGURING VIRTUAL TERMINAL: This is the screen you will see when you first open Virtual Terminal Before you begin you must configure the Options for Virtual Terminal. Click on the Options drop down menu
CONFIGURING VIRTUAL TERMINAL: This is the screen you will see when you first open Virtual Terminal Before you begin you must configure the Options for Virtual Terminal. Click on the Options drop down menu
Patient Portal Users Guide
 e-mds Solution Series Patient Portal Users Guide Version 7.0 How to Use the Patient Portal CHARTING THE FUTURE OF HEALTHCARE e-mds 9900 Spectrum Drive. Austin, TX 78717 Phone 512.257.5200 Fax 512.335.4375
e-mds Solution Series Patient Portal Users Guide Version 7.0 How to Use the Patient Portal CHARTING THE FUTURE OF HEALTHCARE e-mds 9900 Spectrum Drive. Austin, TX 78717 Phone 512.257.5200 Fax 512.335.4375
Integrated Research Application System (IRAS)
 Integrated Research Application System (IRAS) Guidance on electronic submission of supporting documentation with applications and storing files in IRAS. New functionality is being phased in to IRAS to
Integrated Research Application System (IRAS) Guidance on electronic submission of supporting documentation with applications and storing files in IRAS. New functionality is being phased in to IRAS to
UFIRST Training Manual
 UFIRST Training Manual Division of Sponsored Programs UF Training & Organizational Development Page 1 TABLE OF CONTENTS UFIRST 5 INTRODUCTION 5 ADDITIONAL RESOURCES 5 HOW TO USE THIS MANUAL 5 ACKNOWLEDGEMENTS
UFIRST Training Manual Division of Sponsored Programs UF Training & Organizational Development Page 1 TABLE OF CONTENTS UFIRST 5 INTRODUCTION 5 ADDITIONAL RESOURCES 5 HOW TO USE THIS MANUAL 5 ACKNOWLEDGEMENTS
Form 2: Change Request & Amendments. Revised: May 2014
 Form 2: Change Request & Amendments 2014 Revised: Submitting a Form 2: Change Request/Amendment via imedris How to Submit a Form 2: Change Request/Amendment via imedris 1. Open your web browser. 2. Go
Form 2: Change Request & Amendments 2014 Revised: Submitting a Form 2: Change Request/Amendment via imedris How to Submit a Form 2: Change Request/Amendment via imedris 1. Open your web browser. 2. Go
INSTRUCTIONS FOR USE: OA-RX
 P a g e 1 INSTRUCTIONS FOR USE: OA-RX Welcome to Office Ally s Electronic Prescription module, called OA-Rx. This web-based program has been designed to integrate into Office Ally s Practice Mate and EHR
P a g e 1 INSTRUCTIONS FOR USE: OA-RX Welcome to Office Ally s Electronic Prescription module, called OA-Rx. This web-based program has been designed to integrate into Office Ally s Practice Mate and EHR
Creating and Routing a New Clinical Trial Project
 Creating and Routing a New Clinical Trial Project To create a new clinical trial project in InfoEd, click My Proposals in the left sidebar and click. Step 0: Confirm the Intended Principal Investigator
Creating and Routing a New Clinical Trial Project To create a new clinical trial project in InfoEd, click My Proposals in the left sidebar and click. Step 0: Confirm the Intended Principal Investigator
System-to-System FAQs User Role(s): Central, Department, Principal Investigator Last Updated: 06/16/2015
 GMAS System-to-System FAQs Please note that similar issues below could be resulting from other issues, and resolutions may not work. In those scenarios contact the helpdesk (contactgmas@harvard.edu) with
GMAS System-to-System FAQs Please note that similar issues below could be resulting from other issues, and resolutions may not work. In those scenarios contact the helpdesk (contactgmas@harvard.edu) with
Online Scheduling Instructions
 Online Scheduling Instructions 1. First, you will need to create a user account. Go to: https://emsweb.ad.siu.edu/virtualems/ a. Click on My Account, then scroll down to create an account. b. Fill out
Online Scheduling Instructions 1. First, you will need to create a user account. Go to: https://emsweb.ad.siu.edu/virtualems/ a. Click on My Account, then scroll down to create an account. b. Fill out
Conflict of Interest Electronic Document Quick Reference Guide
 Conflict of Interest Electronic Document Quick Reference Guide The following quick reference provides guidance for using the Electronic Document Signature (EDS) system for the Conflict of Interest (COI)
Conflict of Interest Electronic Document Quick Reference Guide The following quick reference provides guidance for using the Electronic Document Signature (EDS) system for the Conflict of Interest (COI)
CDMRP ereceipt System User Guide
 CDMRP ereceipt System User Guide Congressionally Directed Medical Research Programs (CDMRP) Last Updated: January 2011 Table of Contents Overview... 3 Purpose... 3 Help Desk Contact Information... 3 System
CDMRP ereceipt System User Guide Congressionally Directed Medical Research Programs (CDMRP) Last Updated: January 2011 Table of Contents Overview... 3 Purpose... 3 Help Desk Contact Information... 3 System
Clinical Trials Reporting Program (CTRP) and Clinicaltrials.gov (CT.gov) Helpful tips and guidance
 Clinical Trials Reporting Program (CTRP) and Clinicaltrials.gov (CT.gov) Helpful tips and guidance Objectives NCI s Clinical Trials Reporting Program (CTRP) Overview CTRP What is it? CTRP is an NCI mandated
Clinical Trials Reporting Program (CTRP) and Clinicaltrials.gov (CT.gov) Helpful tips and guidance Objectives NCI s Clinical Trials Reporting Program (CTRP) Overview CTRP What is it? CTRP is an NCI mandated
Verified Volunteers. System User Guide 10/2014. For assistance while navigating through the system, please contact Client Services at:
 Verified Volunteers System User Guide 10/2014 For assistance while navigating through the system, please contact Client Services at: RCAN@verifiedvolunteers.com - (855) 326-1860 - Option 1 Welcome to Verified
Verified Volunteers System User Guide 10/2014 For assistance while navigating through the system, please contact Client Services at: RCAN@verifiedvolunteers.com - (855) 326-1860 - Option 1 Welcome to Verified
External Account Creation and Upload Instructions for the Local Government (LG) Audit Report Collection System
 External Account Creation and Upload Instructions for the Local Government (LG) Audit Report Collection System In order to submit data for any Department of Audits and Accounts (DOAA) web application,
External Account Creation and Upload Instructions for the Local Government (LG) Audit Report Collection System In order to submit data for any Department of Audits and Accounts (DOAA) web application,
QUICK REFERENCE GUIDE
 QUICK REFERENCE GUIDE Signing Contracts within SWIFT External Revised Apr. 26, 2013; Jan. 10, 2013 Introduction After a Contract Document is approved by the state, it is routed to the Vendor Contact for
QUICK REFERENCE GUIDE Signing Contracts within SWIFT External Revised Apr. 26, 2013; Jan. 10, 2013 Introduction After a Contract Document is approved by the state, it is routed to the Vendor Contact for
Customer ACH Guide. Creating an ACH File in Online Banking
 Customer ACH Guide Customer ACH Guide ACH transactions include payroll files, debiting single/multiple account(s) (Ex: A gym collecting money for a gym membership), and crediting single/multiple account(s)
Customer ACH Guide Customer ACH Guide ACH transactions include payroll files, debiting single/multiple account(s) (Ex: A gym collecting money for a gym membership), and crediting single/multiple account(s)
Student Manager s Guide to the Talent Management System
 Department of Human Resources 50 Student Manager s Guide to the Talent Management System 1 Table of Contents Topic Page SYSTEM INTRODUCTION... 3 GETTING STARTED... 4 NAVIGATION WITHIN THE TALENT MANAGEMENT
Department of Human Resources 50 Student Manager s Guide to the Talent Management System 1 Table of Contents Topic Page SYSTEM INTRODUCTION... 3 GETTING STARTED... 4 NAVIGATION WITHIN THE TALENT MANAGEMENT
Research Coordinator - PI s who have research coordinators or secretarial support can designate individuals to manage their IRB protocols in Mentor.
 Mentor Online IRB System IRB s require lots of documentation and managing this process can get to be a burden for both investigators and the IRB committee and administrator. The Mentor IRB system is designed
Mentor Online IRB System IRB s require lots of documentation and managing this process can get to be a burden for both investigators and the IRB committee and administrator. The Mentor IRB system is designed
RelayClinical Service Feature Guide RelayClinical Notify
 RelayClinical Service Feature Guide RelayClinical Notify Release 15.11 November 2015 Health Connections Brought to Life Table of Contents Overview... 3 Benefits... 3 Models... 3 Alternate Deployment Option...
RelayClinical Service Feature Guide RelayClinical Notify Release 15.11 November 2015 Health Connections Brought to Life Table of Contents Overview... 3 Benefits... 3 Models... 3 Alternate Deployment Option...
National Institutes of Health/Office of Extramural Research. esnap User Guide
 National Institutes of Health/Office of Extramural Research esnap User Guide Version 2.2.3.0 August 01, 2003 Table of Contents Introduction...4 Logging In to esnap...4 Logging In...4 Logging Out...5 Username
National Institutes of Health/Office of Extramural Research esnap User Guide Version 2.2.3.0 August 01, 2003 Table of Contents Introduction...4 Logging In to esnap...4 Logging In...4 Logging Out...5 Username
RAMSeS Document Workflow Management
 RAMSeS has an internal tracking system that allows Sponsored Programs/Research, and in some cases, Contracts and Grants staff to track work items. The system is called the Document Workflow Management
RAMSeS has an internal tracking system that allows Sponsored Programs/Research, and in some cases, Contracts and Grants staff to track work items. The system is called the Document Workflow Management
Introduction to ilab Solutions for VU Core Users
 Introduction to ilab Solutions for VU Core Users Table of Contents Account Access & Login Credentials... 2 Account Registration for First-time Users... 2 Accessing VUMC Cores... 3 Billing Numbers for VU
Introduction to ilab Solutions for VU Core Users Table of Contents Account Access & Login Credentials... 2 Account Registration for First-time Users... 2 Accessing VUMC Cores... 3 Billing Numbers for VU
Lewis & Clark College. Posting In PeopleAdmin
 Lewis & Clark College Posting In PeopleAdmin What is PeopleAdmin? PeopleAdmin is an applicant tracking system which you can use to post your work-study positions. Students can apply for the position directly
Lewis & Clark College Posting In PeopleAdmin What is PeopleAdmin? PeopleAdmin is an applicant tracking system which you can use to post your work-study positions. Students can apply for the position directly
FAST Travel System. Guide: Creating Expense Reports
 FAST Travel System Guide: Creating Expense Reports Purpose: To provide instructions on how to create an Expense Report (ER) in FAST 9.2 Travel module. Definition: Expense Report submission entered into
FAST Travel System Guide: Creating Expense Reports Purpose: To provide instructions on how to create an Expense Report (ER) in FAST 9.2 Travel module. Definition: Expense Report submission entered into
Document Services Online Customer Guide
 Document Services Online Customer Guide Logging in... 3 Registering an Account... 3 Navigating DSO... 4 Basic Orders... 5 Getting Started... 5 Attaching Files & Print Options... 7 Advanced Print Options
Document Services Online Customer Guide Logging in... 3 Registering an Account... 3 Navigating DSO... 4 Basic Orders... 5 Getting Started... 5 Attaching Files & Print Options... 7 Advanced Print Options
Covered Entities Guide for Public Users. Registering a Contract Pharmacy
 Registering a Contract Pharmacy Major Sections in This Guide: To jump to a specific section in this guide, click one of these links: Searching for a Covered Entity (page 4) Terminating an Existing Contract
Registering a Contract Pharmacy Major Sections in This Guide: To jump to a specific section in this guide, click one of these links: Searching for a Covered Entity (page 4) Terminating an Existing Contract
Section 8(e) Notice User Guide Primary Support
 Primary Support Environmental Protection Agency Office of Pollution Prevention and Toxics Manage Toxic Substances Table of Contents 1 Introduction... 1 1.1 Overview... 1 1.2 Labeling Confidential Information...
Primary Support Environmental Protection Agency Office of Pollution Prevention and Toxics Manage Toxic Substances Table of Contents 1 Introduction... 1 1.1 Overview... 1 1.2 Labeling Confidential Information...
NYS OCFS CMS Manual CHAPTER 1...1-1 CHAPTER 2...2-1 CHAPTER 3...3-1 CHAPTER 4...4-1. Contract Management System
 NYS OCFS CMS Manual C O N T E N T S CHAPTER 1...1-1 Chapter 1: Introduction to the Contract Management System...1-2 Using the Contract Management System... 1-2 Accessing the Contract Management System...
NYS OCFS CMS Manual C O N T E N T S CHAPTER 1...1-1 Chapter 1: Introduction to the Contract Management System...1-2 Using the Contract Management System... 1-2 Accessing the Contract Management System...
Electronic Signature and Routing Process
 Howard University Electronic Signature and Routing Process Submitting a Proposal Package Electronically 2012 R e s e a r c h A d m i n i s t r a t i v e S e r v i c e s CONTENTS DocuSign Overview... 2
Howard University Electronic Signature and Routing Process Submitting a Proposal Package Electronically 2012 R e s e a r c h A d m i n i s t r a t i v e S e r v i c e s CONTENTS DocuSign Overview... 2
Santa Cruz Contracts End User Guide
 Santa Cruz Contracts End User Guide SunGard Public Sector 1000 Business Center Drive Lake Mary, Florida 32746 Phone: (800) 695-6915 Fax: (407) 304-1005 Web site: http://www.sungardps.com 2011 SunGard Public
Santa Cruz Contracts End User Guide SunGard Public Sector 1000 Business Center Drive Lake Mary, Florida 32746 Phone: (800) 695-6915 Fax: (407) 304-1005 Web site: http://www.sungardps.com 2011 SunGard Public
Federal Program Office (FPO) User Manual
 Federal Program Office (FPO) User Manual System Navigation NOAA Grants Online Program Management Office August 2015 Table of Contents Overview... 5 Accessing Grants Online... 5 Grants Online Navigation
Federal Program Office (FPO) User Manual System Navigation NOAA Grants Online Program Management Office August 2015 Table of Contents Overview... 5 Accessing Grants Online... 5 Grants Online Navigation
Managing Submissions via ExpressO: A Guide for Law Review Editors
 : A Guide for Law Review Editors Table of Contents List of Figures... 3 Welcome to ExpressO... 4 Contacting bepress Consulting Services... 4 Accessing ExpressO... 5 Editorial Privileges... 5 Editor Tools:
: A Guide for Law Review Editors Table of Contents List of Figures... 3 Welcome to ExpressO... 4 Contacting bepress Consulting Services... 4 Accessing ExpressO... 5 Editorial Privileges... 5 Editor Tools:
Contents Overview... 3 The flow of a ticket... 3 Getting help... 3 K2 Partner and Customer Portal... 3 K2 Knowledge Base... 4 K2 Community site...
 Support Ticket Help Contents Overview... 3 The flow of a ticket... 3 Getting help... 3 K2 Partner and Customer Portal... 3 K2 Knowledge Base... 4 K2 Community site... 4 K2 Help Files... 4 Asking for help...
Support Ticket Help Contents Overview... 3 The flow of a ticket... 3 Getting help... 3 K2 Partner and Customer Portal... 3 K2 Knowledge Base... 4 K2 Community site... 4 K2 Help Files... 4 Asking for help...
Online Employment System User Manual
 Troy University Online Employment System User Manual People Admin Version 7 Human Resources April 2014 Table of Contents Introduction... 2 User Types and Access... 2 Creating a User Account... 3 Homepage...
Troy University Online Employment System User Manual People Admin Version 7 Human Resources April 2014 Table of Contents Introduction... 2 User Types and Access... 2 Creating a User Account... 3 Homepage...
Annual Review (for Managers)
 Annual Review (for Managers) How Does the Annual Review Process Work? You and your employees will use the online forms contained in SuccessFactors to conduct their annual reviews. In this guide, learn
Annual Review (for Managers) How Does the Annual Review Process Work? You and your employees will use the online forms contained in SuccessFactors to conduct their annual reviews. In this guide, learn
Rochester Institute of Technology. Finance and Administration. Drupal 7 Training Documentation
 Rochester Institute of Technology Finance and Administration Drupal 7 Training Documentation Written by: Enterprise Web Applications Team CONTENTS Workflow... 4 Example of how the workflow works... 4 Login
Rochester Institute of Technology Finance and Administration Drupal 7 Training Documentation Written by: Enterprise Web Applications Team CONTENTS Workflow... 4 Example of how the workflow works... 4 Login
HHS Accelerator: Invoices and Payments
 HHS Accelerator: Invoices and Payments Table of Contents Monitoring the Status of an Invoice.....3 View Invoices Listed in HHS Accelerator 3 Stages of an Invoice.. 4 Filter Invoices..... 5 Submit an Invoice....6
HHS Accelerator: Invoices and Payments Table of Contents Monitoring the Status of an Invoice.....3 View Invoices Listed in HHS Accelerator 3 Stages of an Invoice.. 4 Filter Invoices..... 5 Submit an Invoice....6
To Access Signature Manager/ScanDocs from Patient Screen
 1. Highlight a patient's name to activate both icons 2. The "Signature Manager" icon is on the tool bar This is where you sign your Documents and Orders 3. The "ScanDocs" tab is where you sign scanned
1. Highlight a patient's name to activate both icons 2. The "Signature Manager" icon is on the tool bar This is where you sign your Documents and Orders 3. The "ScanDocs" tab is where you sign scanned
Please use the following index links to quickly access the information you are looking for:
 Please use the following index links to quickly access the information you are looking for: Create a new My PHLY account Registration for Agents Auto ID Web Edit Profile Logout instructions Online Bill
Please use the following index links to quickly access the information you are looking for: Create a new My PHLY account Registration for Agents Auto ID Web Edit Profile Logout instructions Online Bill
Course Overrides. Introduction. Accessing Course Overrides
 Introduction Course overrides, or registration permits, allow students to register for a class past certain requirements or class capacity. Assigning a course override to a student does not register them
Introduction Course overrides, or registration permits, allow students to register for a class past certain requirements or class capacity. Assigning a course override to a student does not register them
Physician Quality Reporting System (PQRS) Physician Portal
 The American College of Radiology Physician Quality Reporting System (PQRS) Physician Portal User Guide January 29, 2016 American College of Radiology 1891 Preston White Drive Reston, VA 20191-4397 Copyright
The American College of Radiology Physician Quality Reporting System (PQRS) Physician Portal User Guide January 29, 2016 American College of Radiology 1891 Preston White Drive Reston, VA 20191-4397 Copyright
For new users to the Grants Management System, please visit the Document Library, found in the main menu of the New GME.
 Quick Reference Guide Fresh Fruit and Vegetable Program FY16 Funding Application Fresh Fruit and Vegetable Program Period 1 (FY16) Fresh Fruit and Vegetable Program Period 2 (FY16) For new users to the
Quick Reference Guide Fresh Fruit and Vegetable Program FY16 Funding Application Fresh Fruit and Vegetable Program Period 1 (FY16) Fresh Fruit and Vegetable Program Period 2 (FY16) For new users to the
Selection Manager: Quick Start Guide
 VERSION 1103 01.24.12 SELECTION MANAGER GUIDE Selection Manager: Quick Start Guide PART 1: GENERAL INFORMATION Selection Manager is the hiring official s interface for USA Staffing. In Selection Manager,
VERSION 1103 01.24.12 SELECTION MANAGER GUIDE Selection Manager: Quick Start Guide PART 1: GENERAL INFORMATION Selection Manager is the hiring official s interface for USA Staffing. In Selection Manager,
STAFF HIRING PROCESS ******************************************* Posting a Position
 Hiring Manager creates a job posting on-line. STAFF HIRING PROCESS ******************************************* Posting a Position 1. From the www.cmich.edu webpage, select CentralLink in the upper right
Hiring Manager creates a job posting on-line. STAFF HIRING PROCESS ******************************************* Posting a Position 1. From the www.cmich.edu webpage, select CentralLink in the upper right
E-Commerce Manual. A brief overview of your website s E-commerce system with screenshots. E-commerce Manual
 E-Commerce A brief overview of your website s system with screenshots. 1 Contents Categories:...3 Products:...4 Adding a New Product:...5 Adding an Image to a Product:...7 Creating a New Attribute:...8
E-Commerce A brief overview of your website s system with screenshots. 1 Contents Categories:...3 Products:...4 Adding a New Product:...5 Adding an Image to a Product:...7 Creating a New Attribute:...8
USING THE ELECTRONIC REQUEST FOR PROPOSAL APPROVAL AND SUBMISSION (E-ROUTING) FORM
 USING THE ELECTRONIC REQUEST FOR PROPOSAL APPROVAL AND SUBMISSION (E-ROUTING) FORM This electronic version of the routing sheet is very similar to the paper version ORSP has used for years with minor changes.
USING THE ELECTRONIC REQUEST FOR PROPOSAL APPROVAL AND SUBMISSION (E-ROUTING) FORM This electronic version of the routing sheet is very similar to the paper version ORSP has used for years with minor changes.
Criminal Attorney Online Vouchers. 18b Web application www.nyc.gov/18b. Training Guide Last Updated: March 2014 (v1.05)
 Department of Finance Fiscal Services & Purchasing Division Assigned Counsel Plan Criminal Attorney Online Vouchers 18b Web application www.nyc.gov/18b Training Guide Last Updated: March 2014 (v1.05) TABLE
Department of Finance Fiscal Services & Purchasing Division Assigned Counsel Plan Criminal Attorney Online Vouchers 18b Web application www.nyc.gov/18b Training Guide Last Updated: March 2014 (v1.05) TABLE
IRB Principal Investigator Quick Reference
 Navigation and Basic Tasks... Create Study and Submission Approvals... Submit a Study... 5 Assign PI Proxy... 5 Respond to Clarification Requests... 6 Change Study Documents... 7 Create and Submit a CR
Navigation and Basic Tasks... Create Study and Submission Approvals... Submit a Study... 5 Assign PI Proxy... 5 Respond to Clarification Requests... 6 Change Study Documents... 7 Create and Submit a CR
Perform this procedure when you need to add a recurring payment option, or when you need to change or withdraw it.
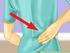 Purpose Use this procedure to add, change, or remove recurring payments. Trigger Perform this procedure when you need to add a recurring payment option, or when you need to change or withdraw it. Prerequisites
Purpose Use this procedure to add, change, or remove recurring payments. Trigger Perform this procedure when you need to add a recurring payment option, or when you need to change or withdraw it. Prerequisites
HiCAMS User Guide. Chapter 3: Contract Adjustments. Section 1: Review Claims
 HiCAMS User Guide Chapter 3: Contract Adjustments Section 1: Review Claims Contents About Claims Entering and Approving Claims Sending Notifications Changing or Correcting Issues Processing Claims Window
HiCAMS User Guide Chapter 3: Contract Adjustments Section 1: Review Claims Contents About Claims Entering and Approving Claims Sending Notifications Changing or Correcting Issues Processing Claims Window
University of Alaska Statewide Financial Systems User Documentation. BANNER TRAVEL AND EXPENSE MANAGEMENT TEM (Quick)
 University of Alaska Statewide Financial Systems User Documentation BANNER TRAVEL AND EXPENSE MANAGEMENT TEM (Quick) Travel and Expense Management Table of Contents 2 Table of Contents Table of Contents...
University of Alaska Statewide Financial Systems User Documentation BANNER TRAVEL AND EXPENSE MANAGEMENT TEM (Quick) Travel and Expense Management Table of Contents 2 Table of Contents Table of Contents...
Topia Technology, Inc. 1119 Pacific Avenue, Suite 200, Tacoma WA 98402 253.572.9712 www.secrata.com
 SECRATA Enterprise File Sync and Share Application Version 4.7 Topia Technology, Inc. 1119 Pacific Avenue, Suite 200, Tacoma WA 98402 253.572.9712 www.secrata.com Table of Contents TABLE OF CONTENTS...
SECRATA Enterprise File Sync and Share Application Version 4.7 Topia Technology, Inc. 1119 Pacific Avenue, Suite 200, Tacoma WA 98402 253.572.9712 www.secrata.com Table of Contents TABLE OF CONTENTS...
Comprehensive Study Documents List (Biomedical Studies)
 Comprehensive Study Documents List (Biomedical Studies) Investigators conducting human subjects research must maintain study documents in adherence to federal and state regulations, USC policies, and good
Comprehensive Study Documents List (Biomedical Studies) Investigators conducting human subjects research must maintain study documents in adherence to federal and state regulations, USC policies, and good
Table of Contents Install a Printer Driver... 2 Print a Test Label... 9 Uninstall a Printer and Driver... 10
 Table of Contents Install a Printer Driver... 2 Print a Test Label... 9 Uninstall a Printer and Driver... 10 Copyright 2016, United Parcel Service of America, Inc. All rights reserved. 1 Install a Printer
Table of Contents Install a Printer Driver... 2 Print a Test Label... 9 Uninstall a Printer and Driver... 10 Copyright 2016, United Parcel Service of America, Inc. All rights reserved. 1 Install a Printer
AC4S Employee s User Guide for Deltek Time & Expense 9. Expense Reports
 AC4S Employee s User Guide for Deltek Time & Expense 9 Expense Reports Open Expense Report To record expenses, click on Expense on the top menu bar. Next, click on Record Expenses, then Expense Report
AC4S Employee s User Guide for Deltek Time & Expense 9 Expense Reports Open Expense Report To record expenses, click on Expense on the top menu bar. Next, click on Record Expenses, then Expense Report
User Guide for Evolve Simulation Learning System: Electronic Medical Record (Fundamentals)
 User Guide for Evolve Simulation Learning System: Electronic Medical Record (Fundamentals) Introduction The Simulation Learning System s Electronic Medical Record (EMR) allows you to document the treatment
User Guide for Evolve Simulation Learning System: Electronic Medical Record (Fundamentals) Introduction The Simulation Learning System s Electronic Medical Record (EMR) allows you to document the treatment
Scanning Documents into OneSite The Preiss Company
 Scanning Documents into OneSite The Preiss Company Scanning Single Documents (i.e. lease only) For a tutorial on scanning multiple documents at once (i.e. lease & application) skip to the section titled
Scanning Documents into OneSite The Preiss Company Scanning Single Documents (i.e. lease only) For a tutorial on scanning multiple documents at once (i.e. lease & application) skip to the section titled
Rensselaer County. Contract Management System
 Rensselaer County Contract Management System VENDOR REFERENCE MANUAL Version 2.0 Table of Contents Getting Started and Logging In...4 The Contract Dashboard Tab...6 Vendor Profile Tab...9 Contract Overview
Rensselaer County Contract Management System VENDOR REFERENCE MANUAL Version 2.0 Table of Contents Getting Started and Logging In...4 The Contract Dashboard Tab...6 Vendor Profile Tab...9 Contract Overview
USER S GUIDE TO THE CHANGE OF MAJOR PROCESS USING PORTAL WORKFLOW (eforms)
 USER S GUIDE TO THE CHANGE OF MAJOR PROCESS USING PORTAL WORKFLOW (eforms) Office of the Registrar April, 2011 Introduction: To support the implementation of Cal Poly s new change of major policy, the
USER S GUIDE TO THE CHANGE OF MAJOR PROCESS USING PORTAL WORKFLOW (eforms) Office of the Registrar April, 2011 Introduction: To support the implementation of Cal Poly s new change of major policy, the
BEST / Act 230 Funding
 BEST / Act 230 Funding GRANTIUM APPLICATION INSTRUCTIONS FOR FY 16 (2015 2016 Academic Year) Table of Contents Logging into Grantium and Changing Your Password... 3 Forgot Your Password?... 4 How to Get
BEST / Act 230 Funding GRANTIUM APPLICATION INSTRUCTIONS FOR FY 16 (2015 2016 Academic Year) Table of Contents Logging into Grantium and Changing Your Password... 3 Forgot Your Password?... 4 How to Get
Quick Start Guide for Online Medical Certifiers and Online Support Staff
 Quick Start Guide for Online Medical Certifiers and Online Support Staff Vitals Information Partnership (VIP) Electronic Death Registration System (EDRS) Revision July 1, 2014 Notes Notes Use this page
Quick Start Guide for Online Medical Certifiers and Online Support Staff Vitals Information Partnership (VIP) Electronic Death Registration System (EDRS) Revision July 1, 2014 Notes Notes Use this page
CGIRB Connexus Tips. Version 1.1 July 2013 Copernicus Group IRB
 CGIRB Connexus Tips Version 1.1 July 2013 Copernicus Group IRB Study-Level Management in Connexus Thank you for selecting CGIRB Connexus as your shipment method for receiving copies of site approval documents
CGIRB Connexus Tips Version 1.1 July 2013 Copernicus Group IRB Study-Level Management in Connexus Thank you for selecting CGIRB Connexus as your shipment method for receiving copies of site approval documents
Scheduling Document Creation
 Scheduling Document Creation What is a Schedule in epm? Schedules The epm Schedule application is an integral part of the Portfolio Management suite of applications. Start and end dates for each summary
Scheduling Document Creation What is a Schedule in epm? Schedules The epm Schedule application is an integral part of the Portfolio Management suite of applications. Start and end dates for each summary
TRAVEL AND EXPENSE CENTER REPORTS
 TRAVEL AND EXPENSE CENTER REPORTS NOTE: This document is designed to be used online and has a number of embedded links to processes and additional information. We discourage the printing of manuals as
TRAVEL AND EXPENSE CENTER REPORTS NOTE: This document is designed to be used online and has a number of embedded links to processes and additional information. We discourage the printing of manuals as
CONTINUING EDUCATION ONLINE REGISTRATION TUTORIAL
 CONTINUING EDUCATION SEARCHING FOR A COURSE BASIC SEARCH Click on the drop-down menu marked All terms and select Fall Semester 0 - Conservatory. Type the course reference number, course name or key words:
CONTINUING EDUCATION SEARCHING FOR A COURSE BASIC SEARCH Click on the drop-down menu marked All terms and select Fall Semester 0 - Conservatory. Type the course reference number, course name or key words:
Outlook Web Access 2003 New Features Guide
 Global Information Services Outlook Web Access 2003 New Features Guide Table of Contents After following a link, click on the toolbar to return to the Table of Contents. OWA 2003 2 Log in Screen 2 OWA
Global Information Services Outlook Web Access 2003 New Features Guide Table of Contents After following a link, click on the toolbar to return to the Table of Contents. OWA 2003 2 Log in Screen 2 OWA
Non-Profit Solution for Microsoft Dynamics CRM
 Non-Profit Solution for Microsoft Dynamics CRM 1 Non-Profit Solution for Microsoft Dynamics CRM Users Guide Table of Contents Introduction... 2 Overview... 2 Default Objects... 2 Screen Elements... 3 Required
Non-Profit Solution for Microsoft Dynamics CRM 1 Non-Profit Solution for Microsoft Dynamics CRM Users Guide Table of Contents Introduction... 2 Overview... 2 Default Objects... 2 Screen Elements... 3 Required
Vaccine Ordering Guide for Provider Staff
 Purpose: This guide demonstrates the steps needed for provider staff to place and receive vaccine orders using the Immunization Information System. Order/Transfers Menu Heading - What it will allow you
Purpose: This guide demonstrates the steps needed for provider staff to place and receive vaccine orders using the Immunization Information System. Order/Transfers Menu Heading - What it will allow you
ARIBA Contract Management System. User Guide to Accompany Training
 ARIBA Contract Management System User Guide to Accompany Training Technical Training Team 6/29/2010 Table of Contents How to use this Guide... 4 Contract Management Process... 5 ARIBA- Getting Started...
ARIBA Contract Management System User Guide to Accompany Training Technical Training Team 6/29/2010 Table of Contents How to use this Guide... 4 Contract Management Process... 5 ARIBA- Getting Started...
Employer Online Access Documentation
 Employer Online Access Documentation BBCS Payroll Services Online Portal The following has been provided as a brief introduction to the Online Access Portal for BBCS Payroll Customers. It is to help you
Employer Online Access Documentation BBCS Payroll Services Online Portal The following has been provided as a brief introduction to the Online Access Portal for BBCS Payroll Customers. It is to help you
Health Indicators Advancing Healthy Aging in Your Community. Database Instructions for Managers
 Health Indicators Advancing Healthy Aging in Your Community Database Instructions for Managers Getting to the Database Website You can access the Health Indicators online database in two different ways.
Health Indicators Advancing Healthy Aging in Your Community Database Instructions for Managers Getting to the Database Website You can access the Health Indicators online database in two different ways.
A Step-by-Step Guide in Preparing Donor Report Using the Query GM_RCARE_969
 CARE USA Shared Services Center A Step-by-Step Guide in Preparing Donor Report I. INTRODUCTION The GM_RCARE_969 is a PeopleSoft Query report that extracts information from the Project Costing module, which
CARE USA Shared Services Center A Step-by-Step Guide in Preparing Donor Report I. INTRODUCTION The GM_RCARE_969 is a PeopleSoft Query report that extracts information from the Project Costing module, which
BLACKBOARD CONTENT COLLECTION FACULTY TRAINING GUIDE
 BLACKBOARD CONTENT COLLECTION FACULTY TRAINING GUIDE Table of Contents About the Guide... 1 Overview... 2 Navigating the Content Collection... 3 Accessing the Content Collection... 3 Content Collection
BLACKBOARD CONTENT COLLECTION FACULTY TRAINING GUIDE Table of Contents About the Guide... 1 Overview... 2 Navigating the Content Collection... 3 Accessing the Content Collection... 3 Content Collection
University of Alaska Statewide Financial Systems User Documentation. BANNER TRAVEL AND EXPENSE MANAGEMENT TEM (Detail)
 University of Alaska Statewide Financial Systems User Documentation BANNER TRAVEL AND EXPENSE MANAGEMENT TEM (Detail) Travel and Expense Management Table of Contents 2 Table of Contents Table of Contents...
University of Alaska Statewide Financial Systems User Documentation BANNER TRAVEL AND EXPENSE MANAGEMENT TEM (Detail) Travel and Expense Management Table of Contents 2 Table of Contents Table of Contents...
REGISTER OF COMPANIES, ENTERPRISES AND BUSINESS
 RWANDA BUSINESS REGISTRY PROJECT REGISTER OF COMPANIES, ENTERPRISES AND BUSINESS NAMES USER MANUAL Online user Version: 1.0 March 9 th 2010 NORWAY REGISTERS DEVELOPMENT AS TABLE OF CONTENTS 1 INTRODUCTION...
RWANDA BUSINESS REGISTRY PROJECT REGISTER OF COMPANIES, ENTERPRISES AND BUSINESS NAMES USER MANUAL Online user Version: 1.0 March 9 th 2010 NORWAY REGISTERS DEVELOPMENT AS TABLE OF CONTENTS 1 INTRODUCTION...
Travel and Expenses Expense Reports
 Travel and Expenses Expense Reports Agenda Expense Report Process (travel & non-travel) Interim Approval Process (travel & non-travel) Entering Expense Reports (travel & non-travel) Searching for Expense
Travel and Expenses Expense Reports Agenda Expense Report Process (travel & non-travel) Interim Approval Process (travel & non-travel) Entering Expense Reports (travel & non-travel) Searching for Expense
Accounts Payable. Vendors and Vouchers
 Accounts Payable Vendors and Vouchers 1 Agenda Searching for Vendors Entering Vouchers Budget Checking Submitting for Approval Viewing Workflow Viewing Voucher Status Approving Vouchers Denying (returning)
Accounts Payable Vendors and Vouchers 1 Agenda Searching for Vendors Entering Vouchers Budget Checking Submitting for Approval Viewing Workflow Viewing Voucher Status Approving Vouchers Denying (returning)
PISA 2015 MS Online School Questionnaire: User s Manual
 OECD Programme for International Student Assessment 2015 PISA 2015 MS Online School Questionnaire: User s Manual Doc: CY6_CBA_SCQ_MSPrincipalManual.docx September 2014 Produced by ETS, Core 2 Contractor
OECD Programme for International Student Assessment 2015 PISA 2015 MS Online School Questionnaire: User s Manual Doc: CY6_CBA_SCQ_MSPrincipalManual.docx September 2014 Produced by ETS, Core 2 Contractor
Wisconsin Medicaid Electronic Health Record Incentive Program for Eligible Professionals
 P- Wisconsin Medicaid Electronic Health Record Incentive Program for Eligible Professionals June 15,2015 User Guide i Table of Contents 1 Introduction... 1 2 Before You Begin... 2 2.1 Register
P- Wisconsin Medicaid Electronic Health Record Incentive Program for Eligible Professionals June 15,2015 User Guide i Table of Contents 1 Introduction... 1 2 Before You Begin... 2 2.1 Register
Web Forms. Step One: Review Simple Contact Form
 Web Forms Follow these instructions to create a Simple Contact Form to place in your email signature or the body of an email. Keep reading to create a form specifically for an agent. Step One: Review Simple
Web Forms Follow these instructions to create a Simple Contact Form to place in your email signature or the body of an email. Keep reading to create a form specifically for an agent. Step One: Review Simple
Initial Setup of Mozilla Thunderbird with IMAP for Windows 7
 Initial Setup of Mozilla Thunderbird Concept This document describes the procedures for setting up the Mozilla Thunderbird email client to download messages from Google Mail using Internet Message Access
Initial Setup of Mozilla Thunderbird Concept This document describes the procedures for setting up the Mozilla Thunderbird email client to download messages from Google Mail using Internet Message Access
Colorado Medical Assistance Program Web Portal Dental Claims User Guide
 Colorado Medical Assistance Program Web Portal Dental Claims User Guide The Dental Claim Lookup screen (Figure 1) is the main screen from which to manage Dental claims. It consists of different sections
Colorado Medical Assistance Program Web Portal Dental Claims User Guide The Dental Claim Lookup screen (Figure 1) is the main screen from which to manage Dental claims. It consists of different sections
Kuali Coeus Research Management (KCRM) User Guide: Create a new Budget document for a Child Award
 Kuali Coeus Research Management (KCRM) User Guide: Create a new Budget document for a Child Award Version 3.0 November 27, 2013 Prepared By: K. Foster/E. Serrano Purpose: To create detailed or modular
Kuali Coeus Research Management (KCRM) User Guide: Create a new Budget document for a Child Award Version 3.0 November 27, 2013 Prepared By: K. Foster/E. Serrano Purpose: To create detailed or modular
SpringCM Integration Guide. for Salesforce
 SpringCM Integration Guide for Salesforce January 2013 Introduction You are minutes away from fully integrating SpringCM into your Salesforce account. The SpringCM Open Cloud Connector will allow you to
SpringCM Integration Guide for Salesforce January 2013 Introduction You are minutes away from fully integrating SpringCM into your Salesforce account. The SpringCM Open Cloud Connector will allow you to
