Medical images such as X-rays, computed tomography (CT) images,
|
|
|
- Jeremy Armstrong
- 8 years ago
- Views:
Transcription
1 Performance Metrics in Clinical trials To be confident about making decisions based on medical images for individuals and for clinical trials, medical professionals can use metrics to develop adequate assurance that the images were appropriately acquired and analyzed. Home Study article Learning Objective After reading this article, participants should be able to describe how an imaging core lab partners with sponsors to use metrics to ensure the collection of quality imaging endpoint data for clinical research studies. Disclosures Hui Jing Yu, PhD, Colin G. Miller, PhD, and Dawn Flitcraft are employees of and stockholders in BioClinica, Inc. Hui Jing Yu, PhD Colin G. Miller, PhD Dawn Flitcraft Metrics in Medical Imaging Changing the Picture Medical images such as X-rays, computed tomography (CT) images, positron emission tomography (PET) images, dual energy X-ray absorptiometry (DXA), and magnetic resonance images (MRIs) are essential tools for diagnosing and monitoring diseases and directing treatments. The medical decisions based on these images are vitally important for individual patients and clinical trials as a whole. To be confident about making these decisions, radiologists and other medical professionals must have adequate assurance that the images were appropriately acquired and analyzed. Medical imaging plays a growing role in clinical trials due to increased use of technology and improved computing power. 1 In clinical trials, medical imaging is used primarily to evaluate efficacy endpoints, and, more and more frequently, for safety evaluations and/or eligibility criteria. Background In multicenter clinical trials, the images will be obtained at multiple clinical sites, each with its own standard operating procedures, technologists, procedural protocols, and equipment. The experience of the technologists, the customization of each protocol, and the make and models of the equipment used may vary significantly from one site to another. Additionally, many of these trials take place over periods of time ranging from weeks to years, during which changes of personnel and equipment often occur. Image quality control is required to minimize both inter- and intra-site data variance and to ensure delivery of more precise results. An imaging core lab (ICL) offers a full suite of medical image management solutions for the lifecycle of a trial and for a wide range of imaging modalities. Table 1 lists typical services provided by ICLs. The goal of an ICL is to unify all the essential image data in a standardized format, in order to expedite the central review of the images and data export 2 4 (see Figure 1 on generic imaging workflow). A method to track and uphold rigorous standards, as related to high image quality in a clinical trial context, is required to ensure the endpoints are clearly met. The use of imaging performance metrics to monitor image quality so that the targets assigned to each metric are met has therefore allowed appropriate levels of control for both the ICL and sponsors, thereby enhancing trial performance and quality. Essentially, there are two major types of imaging from a quality control (QC) viewpoint: 36 x
2 Table 1 Comparison of Image Core Lab Services Study Initiation and Startup Collection Management Independent Review Identify expert readers and consultants Assign project team Engage study startup team Design imaging protocol Communication plans Project-specific work instructions Develop imaging review charter Deploy site surveys Attend investigator meetings Provide imaging study kits Perform site visits Conduct web-based training Collect image data Query sites for missing data Translate/digitize image data Image quality assurance/ quality control Image data query resolution Archive for long-term storage Analyze images Design independent read system Develop imaging review charter Provide reader training Conduct independent read Monitor independent read Monitor inter-/intrareader variability Export data Figure 1 Imaging Core Lab Process Workflow Hospital or Investigator Site Takes Image and Sends to Core Lab two-dimensional (2-D) (e.g., plain film X-ray, DXA, and ultrasound) and three-dimensional (3-D) or tomographic techniques (e.g., CT, MRI, and PET). The QC for 2-D imaging is more critical on positioning, since slight rotation or incorrect positioning may hide important anatomic features. The 3-D techniques tend to need more QC on the acquisition settings and review of patient motion, since the acquisition times are longer. Image QC primarily consists of checks on correct positioning, complete anatomical positioning, lack of patient motion, and a check on the correct acquisition or instrument settings, such as the scan mode (e.g., T1 or T2, etc.) in MRI or scan thickness and coning in CT. Core Lab Reviews, Archives, and Makes Images Available to Readers Image Quality Metrics Readers Readers Within the lifecycle of an imaging trial, trial performance can be tracked using four types of metrics: cycle time, timeliness, quality, and efficiency/cost (Figure 2 and Table 2). Quality metrics Figure 2 Imaging Performance Flowchart Site Startup Review Completed and Report Delivered Imaging Guidelines Sent to Site Independent Review Process Site Query Resolution can be further tracked as image quality (metrics as determined by reader or independent reviewer, although images are checked for quality at the technologists level before sending to the reader), image queries sent to sites, missing imaging visits, and adherence to acquisition protocol. A key first step to ensure that highquality imaging endpoint data are collected for studies is to have standardization of image acquisition between sites. This can usually be accomplished by providing training to each site via a group location, telephone, Web conference, etc. Occasionally, site visits (visits to educate the technologists) are performed if the protocol is deemed to be more challenging than the standard-of-care procedure, or if the sites are not adhering to the imaging guidelines. Imaging guidelines are provided to the site simply to communicate and document the image-related expectations and requirements for a trial. On an ongoing basis, data arrive at the ICL and are inspected for image quality, usually by radiological technologists, prior being sent for the radiological evaluation or read. The reader can then determine the presence or absence of necessary imaging and the associated image quality. Image quality metrics can be calculated based on the percentage of images that are readable (evaluable), suboptimal (readable but not optimal), Image Acquired at Site QC Process Queries to Sites Image Sent to Core Lab Image Received at Core Lab x 37
3 Table 2 Imaging Core Lab Performance Metrics Metric Category Metric Title Unit of Measure Reporting Frequency Target 1 Project Startup Average number of days study kit sent Turnaround time - Days Monthly 3 2 Project Startup Completion of site qualification/training Percentage (%) Monthly 95% 3 Project Startup Independent review charter Turnaround time - Days Monthly 5 days from receipt of latest draft/final protocol 4a Image Acquisition Average number of days from image time point acquisition to receipt 4b Image Acquisition Average number of days from image time point acquisition to receipt 5a Image Acquisition Average number of days from image receipt to initial feedback sent to site 5b Image Acquisition Average number of days from image receipt to initial feedback sent to site 6 Image Processing Average number of days from image receipt to ready for independent review 7 Image Processing Average number of days from when the image is designated for review to completion of the review, excluding images which have outstanding queries 8 Quality Percentage of non-evaluable images vs. total images received 9 Quality Percentage of non-evaluable/missing baseline images Turnaround time - Days Monthly 3 (electronic transfer) Turnaround time - Days Monthly 7 (traditional transfer) Turnaround time - Monthly 24 hours Hours (eligibility/safety) Turnaround time - Days Monthly 3 days Turnaround time - Days Monthly 3 days Turnaround time - Days Monthly Variable Percentage (%) Monthly 3% Percentage (%) Monthly 2% 10 Quality Quality of data export Percentage (%) Monthly 99% 11 On-Time Delivery On-time delivery of read report(s) Percentage (%) Monthly 98% 12 On-Time Delivery On-time delivery of data export(s) Percentage (%) Monthly 98% 13 On-Time Delivery On-time delivery of FINAL data export Percentage (%) Monthly 99.9% 14 Image Queries Percentage of Queries Percentage (%) Monthly < 10% 15 Image Queries Average number of days queries outstanding Turnaround time - Days Monthly 7 or not readable both by the technologist and the readers. This can obviously be evaluated on the study level, but also on country- and site-specific levels. If there are issues, a query can be generated and sent to the site for immediate resolution. The percentage of site queries is a performance metric that captures rate of issue as an indication of whether or not the site training addressed the necessary key points for acquisition and how closely the protocol was being followed. When a query is unresolved, or imaging cannot or could not be per- formed, or protocol is not followed, the result is missing imaging data for either the baseline visits or nonbaseline visits. Such metrics can be defined and tracked throughout the trial, allowing for early escalation of potential site performance or study protocol design issues. Lastly, the number of image acquisition technique related amendments, upon the agreement between the ICL and sponsors, could be incorporated as a metric as an indirect measurement of image quality (e.g., the greater the number of amendments, the lesser the robustness of acquisition protocol and quality). Two Case Studies What follows are two case studies presented as examples of how ICLs use metrics to ensure that high-quality imaging data are collecting for studies (see Table 3 for a summary). Case 1 Many ICLs have started to include imaging performance metrics as part of their standard reports. Implementing a set of standardized metrics can allow the early escalation of potential core lab or site performance issues that require immediate remediation and identification of any need to retrain 38 x
4 Table 3 Case Study and Metrics Summary Case Study Category Metric Title Target sites or redesign image acquisition guidelines. One example of this approach is the case where a client considered an X-ray procedure to be so simple and straightforward that site training was not requested, and it was assumed that a paper manual would suffice. 5 Unfortunately, that decision resulted in a data clarification form (DCF) rate (site queries) of approximately 75%, which caused both a lack of precision and loss of time due to requests for repeat procedures, and directly translated into poor data and increased costs. The sponsor quickly produced a CDbased training program 6 for this study, including a test to ensure understanding of the material, and requested that the sites have the appropriate personnel take the program. The result after sites completed the CD training was a 90% decrease in the DCF rate to less than 7%. This training format provides an excellent, cost-effective way to ensure protocol compliance while improving the precision of study data. This, in turn, either improves overall statistics and/or shortens the time required to detect sig- Figure 3 Case 1 Exhibit: Post-Training Result in Reduction of Site Queries 90% 60% 30% 0% 1 Image Queries Percentage of site queries < 15% 2 Image Quality Percentage of non-evaluable baseline images < 2% 2 Image Quality Percentage of non-evaluable images (non-baseline) < 2% 75% No Training 90% reduction in site queries 7% Post Training nificant change, thus reducing overall cost for sponsors. Case 2 The integration of the ICL as part of clinical trials in all therapeutic areas where medical images are collected is particularly important to harmonize data quality across sites. For example, in an oncology trial involving more than 30 countries and 200 sites across the globe, it was challenging to obtain high-quality, standardized data due to varying technical capabilities at hospitals and imaging facilities. Other sources of variations included study duration and the imaging modalities involved. In this case, the study lasted for six years, with CT and MRI data being collected for all time points. At screening (baseline), all subjects were required to have a bone scan (nuclear medicine image), which was to be repeated at follow-up time points if disease was present at baseline or if clinically indicated. Because an ICL was involved in the study, the sites received standardized instructions (image guidelines) at the start of the study and, for the most part, the imaging quality was comparable across sites. However, many imaging protocols, including the one for this study, provide high-level requirements for imaging and do not include the necessary level of detail that is realistically needed. In this study, 95% of total data submitted for the study was digital, with only 5% of film data submission. Of the film data submitted, 90% were bone scans, because it was challenging for some sites to provide technically adequate digital images in the correct format. Instead, images submitted digitally were in JPEG format with improper leveling and windowing, resulting in images with a lack of details. Considering that bone scans were required for all subjects at screening and these images were used to determine subject progression, the problems encountered with film data could have resulted in a much lower than ideal rate of readable images for follow-up imaging time points. However, through tracking of the relevant metrics, the ICL was able identify these issues early and, together with the sponsor, worked with the regional monitors and sites to find locations where subjects could receive bone scans that were acceptable and usable for the study. Furthermore, the ICL provided extra training to optimize contrast for screenshot images. Such implementation resulted in excellent submission turnouts as measured by image quality metrics. Out of 789 baseline time points, only one was not readable (i.e., 99.87% baseline images readable), and out of 4,810 follow-up time points, only 40 were not readable (i.e., 99.17% non-baseline images readable). Most of the scans that were considered not readable were caused by missing anatomy. Thus, if the ICL is not involved at the start and standardized guidelines are not provided, then studies can run into data quality issues that might otherwise be avoided. Electronic Image Submission Overall, submission of images via electronic means reduces the transit time from the site by greater than 80% from traditional means (courier). This is achieved by avoiding customs involvement when moving the package in and out of countries, as well as any other human or weather involvement, which could delay the shipment from the site to the ICL. Electronic submission is the quickest way to submit images to the ICL and enables the site to mask the image data x 39
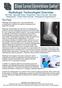
5 Figure 4 Case 2 Exhibit: Good Quality Bone Scan (Left Set) vs. Poor Quality Bone Scan (Right Set) Lesions Not Visible on Poor Quality Scan 2. Miller CG, Noever K Taking care of your subject s image: the role of medical imaging core laboratories. Good Clinical Practices Journal 10(9). 3. Miller CG Medical imaging core laboratories. Applied Clinical Trials October Miller C, Noever K Scanning data for clinical information. Good Clinical Practices Journal 10(9): Pearson D, Miller CG Clinical Trials in Osteoporosis. Springer Verlag. 6. Miller CG, Wertz K Education alternatives for imaging techniques in clinical trials. World Pharma Network July: van Meurs P, Miller CG Image conscious: new FDA guidance. Samedan Ltd December: before transmitting to the ICL. Also, electronic submission is spotlighted in the training materials and at the investigator meetings because this is a great solution to a constant challenge for all clinical trials. Even with minimal setup required for electronic image transmission programs, some sites continue to send image data via courier. This may be out of habit because firewalls and other technology hurdles are typically not an issue at the sites. The ICLs should work very closely with the CRO to encourage sites to use electronic image transmission programs. Conclusion The use of medical imaging in clinical trials has developed from the early days of passively collecting images and having them evaluated on light (film) boxes by radiologists. Improvements in the related technology over time have greatly increased the ability of medical experts to use imaging as a critical biomarker, whether for eligibility, safety, or efficacy. The practice now stands as its own major scientific pursuit as well as a focus for operational logistics management. 7 The critical use of metrics has helped empower this progress; metrics in their own right are of little value, unless they can effect change to a process. The examples provided here have demonstrated the value of metrics for contributing to the ICL operational capabilities and, ultimately, for providing improved study outcomes through decreased variability in data, leading to greater statistical confidence in study findings. Greater statistical confidence will ultimately lead to a decreased number of patients in future trials. Finally, the ethical and financial implications of using appropriate metrics should not be underestimated. References 1. Reiber H, van Kuijk C, Schwarz L Medical imaging and its use in clinical trials. European Pharmaceutical Contractor Autumn 2005: Hui Jing Yu, PhD, is a medical affairs scientist at Bio- Clinica, Inc., where she provides scientific and medical support to the pharmaceutical industry on the use of imaging biomarkers in clinical trials. She also provides support to internal business development, marketing, and operations teams. She holds a bachelor s degree in biomedical and electrical engineering, an MSc degree focused on physiology and biophysics research, and a PhD in biomedical engineering from Stony Brook University in New York. She has written and coauthored several scientific publications and, as the primary author, she drafted and reviewed this manuscript. She can be reached at huijing.yu@bioclinica.com. Colin G. Miller, PhD, is senior vice president for medical affairs at BioClinica, Inc., where he is responsible for medical and scientific consulting. He joined BioClinica (formerly Bio-Imaging Technologies, Inc.) in 1999 as vice president of business development. He has also served as director of clinical services at Bona Fide (a company he started in 1994 that was later acquired by Bio-Imaging Technologies, Inc.); and as the head of the physical measurements team for Europe at Procter & Gamble Pharmaceuticals. A fellow of the Institute of Clinical Research, he also is an associate member of the Radiological Society of North America, a member of the American Society of Bone and Mineral Research, and a member of the Metrics Champion Consortium. He has written and coauthored more than 40 scientific publications. He received his bachelor s degree in physiology and zoology from the University of Sheffield and a PhD from the University of Hull, both in the U.K. As a coauthor, he critically reviewed this manuscript. Dawn Flitcraft is senior vice president for client services at BioClinica, Inc., where she oversees the project management, imaging core lab, and clinical operations departments of the Medical Imaging Solutions Division and is responsible for overall client relations. She joined BioClinica as director of project management when it acquired Quintiles Intelligent Imaging in She held several positions at Quintiles, including image processing specialist, senior manager, and finally director of clinical research and development. She holds a bachelor s degree in biology and nuclear medicine from Cedar Crest College in Allentown, Pa.; has certifications from the Nuclear Medicine Technology Certification Board, the American Registry of Radiologic Technologists (Nuclear), and the American Registry for Diagnostic Medical Sonography; and is a member of the Steering Committee for the Metrics Champion Consortium. As a coauthor, she critically reviewed this manuscript. 40 x
Medical Imaging and Electronic Data Capture in Clinical Trials: the Future Paradigm
 Medical Imaging and Electronic Data Capture in Clinical Trials: the Future Paradigm Medical Imaging and Electronic Data Capture in Clinical Trials: the Future Paradigm All clinical trial data are ultimately
Medical Imaging and Electronic Data Capture in Clinical Trials: the Future Paradigm Medical Imaging and Electronic Data Capture in Clinical Trials: the Future Paradigm All clinical trial data are ultimately
www.bioclinica.com Imaging Core Lab Solutions Electronic Data Capture Clinical Trial Supply Planning IWR/IVRS
 Global clinical trial solutions. Real-world results. Imaging Core Lab Solutions Electronic Data Capture Clinical Trial Supply Planning IWR/IVRS Clinical Trial Management Clinical Data Management www.bioclinica.com
Global clinical trial solutions. Real-world results. Imaging Core Lab Solutions Electronic Data Capture Clinical Trial Supply Planning IWR/IVRS Clinical Trial Management Clinical Data Management www.bioclinica.com
Whitepapers on Imaging Infrastructure for Research Paper 1. General Workflow Considerations
 Whitepapers on Imaging Infrastructure for Research Paper 1. General Workflow Considerations Bradley J Erickson, Tony Pan, Daniel J Marcus, CTSA Imaging Informatics Working Group Introduction The use of
Whitepapers on Imaging Infrastructure for Research Paper 1. General Workflow Considerations Bradley J Erickson, Tony Pan, Daniel J Marcus, CTSA Imaging Informatics Working Group Introduction The use of
There are 2 types of clinical trials that are of interest to the. The Clinical Trials Network of the Society of Nuclear Medicine
 The Clinical Trials Network of the Society of Nuclear Medicine Michael M. Graham, PhD, MD The Clinical Trials Network of the Society of Nuclear Medicine was formed to provide quality assurance of both
The Clinical Trials Network of the Society of Nuclear Medicine Michael M. Graham, PhD, MD The Clinical Trials Network of the Society of Nuclear Medicine was formed to provide quality assurance of both
Prepublication Requirements
 Issued Prepublication Requirements The Joint Commission has approved the following revisions for prepublication. While revised requirements are published in the semiannual updates to the print manuals
Issued Prepublication Requirements The Joint Commission has approved the following revisions for prepublication. While revised requirements are published in the semiannual updates to the print manuals
Bon Secours St. Mary s Hospital School of Medical Imaging Course Descriptions by Semester 18 Month Program
 Bon Secours St. Mary s Hospital School of Medical Imaging Course Descriptions by Semester 18 Month Program First Semester RAD 1101 Patient Care, Ethics, Law and Diversity Credits This 16 week course prepares
Bon Secours St. Mary s Hospital School of Medical Imaging Course Descriptions by Semester 18 Month Program First Semester RAD 1101 Patient Care, Ethics, Law and Diversity Credits This 16 week course prepares
Clinical Data Management is involved in all aspects of processing the clinical data, working with a range of computer applications / database systems
 Clinical Data Management is involved in all aspects of processing the clinical data, working with a range of computer applications / database systems to support collection, cleaning and management of subject
Clinical Data Management is involved in all aspects of processing the clinical data, working with a range of computer applications / database systems to support collection, cleaning and management of subject
Presentation Objective: Benefits to New Employee. Goal for Participants. Benefits to the Administration
 Imaging Department Quick Step How to Create and Implement a Rapid Integration Orientation New Employee Orientation builds a bridge between the old job and the new job for better quality in health care
Imaging Department Quick Step How to Create and Implement a Rapid Integration Orientation New Employee Orientation builds a bridge between the old job and the new job for better quality in health care
The Field. Radiologic technologists take x-rays and administer nonradioactive materials into patients' bloodstreams for diagnostic purposes.
 Radiologic Technologist Overview The Field - Specialty Areas - Preparation - Day in the Life - Earnings - Employment - Career Path Forecast - Professional Organizations The Field Radiologic technologists
Radiologic Technologist Overview The Field - Specialty Areas - Preparation - Day in the Life - Earnings - Employment - Career Path Forecast - Professional Organizations The Field Radiologic technologists
Practical Image Management for
 Practical Image Management for Pharma Experiences and Directions. Use of Open Source Stefan Baumann, Head of Imaging Infrastructure, Novartis Agenda Introduction Drug Development, Imaging Trial Overview
Practical Image Management for Pharma Experiences and Directions. Use of Open Source Stefan Baumann, Head of Imaging Infrastructure, Novartis Agenda Introduction Drug Development, Imaging Trial Overview
The Importance of Following the PROTOCOL in Clinical Trials
 The Importance of Following the PROTOCOL in Clinical Trials Presentation Objectives: Upon completion of this presentation, participants will be able to: Describe the following terms: Protocol, Protocol
The Importance of Following the PROTOCOL in Clinical Trials Presentation Objectives: Upon completion of this presentation, participants will be able to: Describe the following terms: Protocol, Protocol
Needs, Providing Solutions
 Identifying Needs, Providing Solutions 1 I n d u s t r y The growth of medical research and the countless innovations coming from the pharmaceutical, biotechnology and medical device industry, has improved
Identifying Needs, Providing Solutions 1 I n d u s t r y The growth of medical research and the countless innovations coming from the pharmaceutical, biotechnology and medical device industry, has improved
INSERT COMPANY LOGO HERE BEST PRACTICES RESEARCH
 INSERT COMPANY LOGO HERE BEST PRACTICES RESEARCH Background and Company Performance Industry Challenges esource: Electronic Clinical Trial Solution Clinical trial sponsors and clinical research organizations
INSERT COMPANY LOGO HERE BEST PRACTICES RESEARCH Background and Company Performance Industry Challenges esource: Electronic Clinical Trial Solution Clinical trial sponsors and clinical research organizations
Consultation. Review of the scopes of practice for registration in the profession of medical radiation technology
 Consultation Review of the scopes of practice for registration in the profession of medical radiation technology CONTENTS Page Consultation Information... 3 Context of the Consultation... 4-7 Legislative
Consultation Review of the scopes of practice for registration in the profession of medical radiation technology CONTENTS Page Consultation Information... 3 Context of the Consultation... 4-7 Legislative
Teleradiology: The Present Perspective
 Teleradiology: The Present Perspective P. K. Bhuyan Department of Radio-Diagnosis, MIMER Medical College, Talegaon Dabhade, Pune 410507. Introduction Telemedicine is a broad term encompassing all methods,
Teleradiology: The Present Perspective P. K. Bhuyan Department of Radio-Diagnosis, MIMER Medical College, Talegaon Dabhade, Pune 410507. Introduction Telemedicine is a broad term encompassing all methods,
U.S. Bureau of Labor Statistics. Radiology Tech
 From the: U.S. Bureau of Labor Statistics Radiology Tech What They Do Radiologic technologists (RTs) perform diagnostic imaging examinations, such as x rays, on patients. Duties RTs typically do the following:
From the: U.S. Bureau of Labor Statistics Radiology Tech What They Do Radiologic technologists (RTs) perform diagnostic imaging examinations, such as x rays, on patients. Duties RTs typically do the following:
Brochure More information from http://www.researchandmarkets.com/reports/3422625/
 Brochure More information from http://www.researchandmarkets.com/reports/3422625/ Clinical Trials Imaging Market by Modality (CT, MRI, PET, Ultrasound, Echocardiography, X-rays), End User (Pharmaceutical
Brochure More information from http://www.researchandmarkets.com/reports/3422625/ Clinical Trials Imaging Market by Modality (CT, MRI, PET, Ultrasound, Echocardiography, X-rays), End User (Pharmaceutical
What is Clinical Data Management
 What is Clinical Data Management Clinical Data Management is involved in all aspects of processing the clinical data, working with a range of computer applications, database systems to support collection,
What is Clinical Data Management Clinical Data Management is involved in all aspects of processing the clinical data, working with a range of computer applications, database systems to support collection,
clinical Trials - A Need for Organisational Change
 Contents 42 Research in India: Tomorrow and Beyond India has been a popular destination for clinical trials and, as there is an increasing acceptance of Indian data, Viraj Rajadhyaksha stresses that there
Contents 42 Research in India: Tomorrow and Beyond India has been a popular destination for clinical trials and, as there is an increasing acceptance of Indian data, Viraj Rajadhyaksha stresses that there
NEW HYBRID IMAGING TECHNOLOGY MAY HAVE BIG POTENTIAL FOR IMPROVING DIAGNOSIS OF PROSTATE CANCER
 Media Release April 7, 2009 For Immediate Release NEW HYBRID IMAGING TECHNOLOGY MAY HAVE BIG POTENTIAL FOR IMPROVING DIAGNOSIS OF PROSTATE CANCER London, Ontario Improved hybrid imaging techniques developed
Media Release April 7, 2009 For Immediate Release NEW HYBRID IMAGING TECHNOLOGY MAY HAVE BIG POTENTIAL FOR IMPROVING DIAGNOSIS OF PROSTATE CANCER London, Ontario Improved hybrid imaging techniques developed
Operational aspects of a clinical trial
 Operational aspects of a clinical trial Carlo Tomino Pharm.D. Coordinator Pre-authorization Department Head of Research and Clinical Trial Italian Medicines Agency Mwanza (Tanzania), June 11, 2012 1 Declaration
Operational aspects of a clinical trial Carlo Tomino Pharm.D. Coordinator Pre-authorization Department Head of Research and Clinical Trial Italian Medicines Agency Mwanza (Tanzania), June 11, 2012 1 Declaration
Image Area. View Point. Medical Imaging. Advanced Imaging Solutions for Diagnosis, Localization, Treatment Planning and Monitoring. www.infosys.
 Image Area View Point Medical Imaging Advanced Imaging Solutions for Diagnosis, Localization, Treatment Planning and Monitoring www.infosys.com Over the years, medical imaging has become vital in the early
Image Area View Point Medical Imaging Advanced Imaging Solutions for Diagnosis, Localization, Treatment Planning and Monitoring www.infosys.com Over the years, medical imaging has become vital in the early
NHS Imaging and Radiodiagnostic activity in England. 2012/13 Release. August 2013
 NHS Imaging and Radiodiagnostic activity in England 2012/13 Release August 2013 Commentary This National Statistics release covers Imaging and Radiodiagnostic examinations or tests carried out in the NHS
NHS Imaging and Radiodiagnostic activity in England 2012/13 Release August 2013 Commentary This National Statistics release covers Imaging and Radiodiagnostic examinations or tests carried out in the NHS
Teleradiology Overview
 Teleradiology Services Teleradiology Overview Why teleradiology services benefits the community, the clinician and the radiologist: Recent years have seen an increasing global shortage of radiologists
Teleradiology Services Teleradiology Overview Why teleradiology services benefits the community, the clinician and the radiologist: Recent years have seen an increasing global shortage of radiologists
A Second Opinion for Subjective Endpoints: The Case for an Endpoint Assessment Committee
 A Second Opinion for Subjective Endpoints: The Case for an Endpoint Assessment Committee Introduction to EAC The FDA s recent guidance on imaging standards and increased frequency of requests for a Blinded
A Second Opinion for Subjective Endpoints: The Case for an Endpoint Assessment Committee Introduction to EAC The FDA s recent guidance on imaging standards and increased frequency of requests for a Blinded
ACR AAPM TECHNICAL STANDARD FOR DIAGNOSTIC MEDICAL PHYSICS PERFORMANCE MONITORING OF MAGNETIC RESONANCE IMAGING (MRI) EQUIPMENT
 The American College of Radiology, with more than 30,000 members, is the principal organization of radiologists, radiation oncologists, and clinical medical physicists in the United States. The College
The American College of Radiology, with more than 30,000 members, is the principal organization of radiologists, radiation oncologists, and clinical medical physicists in the United States. The College
through advances in risk-based
 Insight brief Quintiles is a market leader with >100 risk-based monitoring studies Quintiles developed solutions that bring as much as 25% cost reduction over traditional trial execution approaches Transform
Insight brief Quintiles is a market leader with >100 risk-based monitoring studies Quintiles developed solutions that bring as much as 25% cost reduction over traditional trial execution approaches Transform
FSH Society s 2014 Biennial FSHD Connect Meeting: Natural History Studies
 FSH Society s 2014 Biennial FSHD Connect Meeting: Natural History Studies Raymond A. Huml, MS, DVM, RAC Executive Director, Head, Global Biosimilars Business Development and Strategic Planning, Quintiles
FSH Society s 2014 Biennial FSHD Connect Meeting: Natural History Studies Raymond A. Huml, MS, DVM, RAC Executive Director, Head, Global Biosimilars Business Development and Strategic Planning, Quintiles
it s all about Choices School of Health Related Professions Diagnostic Imaging Technologies
 D E P A R T M E N T O F D I A G N O S T I C I M A G I N G T E C H N O L O G Y it s all Choices about S H R P School of Health Related Professions Diagnostic Imaging Technologies D I A G N O S T I C M E
D E P A R T M E N T O F D I A G N O S T I C I M A G I N G T E C H N O L O G Y it s all Choices about S H R P School of Health Related Professions Diagnostic Imaging Technologies D I A G N O S T I C M E
WHERE IN THE WORLD JILL LIPOTI?
 WHERE IN THE WORLD IS JILL LIPOTI? HELLO FROM NEW JERSEY CRCPD - National Symposium on Fusion Imaging and Multimodalities February 18-20, 2004 Kansas City, Missouri New Jersey s Requirements As They Pertain
WHERE IN THE WORLD IS JILL LIPOTI? HELLO FROM NEW JERSEY CRCPD - National Symposium on Fusion Imaging and Multimodalities February 18-20, 2004 Kansas City, Missouri New Jersey s Requirements As They Pertain
Radiologic Technology (RAD, CLE) courses are open only to Radiologic Technology majors.
 RADIOLOGIC TECHNOLOGY (A.A.S Degree) Director: Prof. Virginia Mishkin, M.S., R.T. (R) (M) (QM) A radiologic technologist is a skilled professional who provides a specialized health care service. This rewarding
RADIOLOGIC TECHNOLOGY (A.A.S Degree) Director: Prof. Virginia Mishkin, M.S., R.T. (R) (M) (QM) A radiologic technologist is a skilled professional who provides a specialized health care service. This rewarding
August 2011. www.ppdi.com
 Innovative Technology Provides Seamless Data Integration Linking Clinical Trial Patient Data to Central Laboratory Data Via an Oracle -Based Exchange Platform August 2011 www.ppdi.com Introduction Drug
Innovative Technology Provides Seamless Data Integration Linking Clinical Trial Patient Data to Central Laboratory Data Via an Oracle -Based Exchange Platform August 2011 www.ppdi.com Introduction Drug
EMA Clinical Laboratory Guidance - Key points and Clarifications PAUL STEWART
 EMA Clinical Laboratory Guidance - Key points and Clarifications PAUL STEWART Framework Labs generate data that are used to make decisions on the safety and efficacy of medicinal products; consequently,
EMA Clinical Laboratory Guidance - Key points and Clarifications PAUL STEWART Framework Labs generate data that are used to make decisions on the safety and efficacy of medicinal products; consequently,
Introduction. The Evolution of the Data Management Role: The Clinical Data Liaison
 Introduction The CDL is a new role that will become a standard in the industry for companies that want to make more efficient use of limited resources: time and money. A CDL is key in that he or she conducts
Introduction The CDL is a new role that will become a standard in the industry for companies that want to make more efficient use of limited resources: time and money. A CDL is key in that he or she conducts
GCP INSPECTORS WORKING GROUP <DRAFT> REFLECTION PAPER ON EXPECTATIONS FOR ELECTRONIC SOURCE DOCUMENTS USED IN CLINICAL TRIALS
 European Medicines Agency London, 17 October 2007 Doc. Ref. EMEA/505620/2007 GCP INSPECTORS WORKING GROUP REFLECTION PAPER ON EXPECTATIONS FOR ELECTRONIC SOURCE DOCUMENTS USED IN CLINICAL TRIALS
European Medicines Agency London, 17 October 2007 Doc. Ref. EMEA/505620/2007 GCP INSPECTORS WORKING GROUP REFLECTION PAPER ON EXPECTATIONS FOR ELECTRONIC SOURCE DOCUMENTS USED IN CLINICAL TRIALS
The role, duties and responsibilities of clinical trials personnel Monitoring: rules and recommendations
 The role, duties and responsibilities of clinical trials personnel Monitoring: rules and recommendations Maria Luisa Paoloni OPBG Clinical & Research Services Monitoring and Responsible of monitoring:
The role, duties and responsibilities of clinical trials personnel Monitoring: rules and recommendations Maria Luisa Paoloni OPBG Clinical & Research Services Monitoring and Responsible of monitoring:
Section 1 Project Management, Project Communication/Process Design, Mgmt, Documentation, Definition & Scope /CRO-Sponsor Partnership
 Section 1 Project Management, Project Communication/Process Design, Mgmt, Documentation, Definition & Scope /CRO-Sponsor Partnership PROJECT MANAGEMENT - SCOPE DEFINITION AND MANAGEMENT Understands the
Section 1 Project Management, Project Communication/Process Design, Mgmt, Documentation, Definition & Scope /CRO-Sponsor Partnership PROJECT MANAGEMENT - SCOPE DEFINITION AND MANAGEMENT Understands the
Highmark Provider Privileging Requirements
 Highmark Provider Privileging Requirements Copyright 2008 by Highmark Inc. All rights reserved. No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or
Highmark Provider Privileging Requirements Copyright 2008 by Highmark Inc. All rights reserved. No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or
DAIDS Bethesda, MD USA POLICY
 Overview NIH policy requiring independent data and safety monitoring boards (DSMB) for all multicenter Phase III trials has existed since 1979; the most recent restatement was issued in 1998 (NIH Policy
Overview NIH policy requiring independent data and safety monitoring boards (DSMB) for all multicenter Phase III trials has existed since 1979; the most recent restatement was issued in 1998 (NIH Policy
SOP #: Revision #: Current Version Implementation Date: Page #: Page 1 of 10 Last Reviewed/Update Date: Expiration
 Implementation Page #: Page 1 of 10 Last Reviewed/Update 1. Purpose and Scope The purpose of this document is to describe the Medical Physics and Radiation Safety program at Boston University (BU) and
Implementation Page #: Page 1 of 10 Last Reviewed/Update 1. Purpose and Scope The purpose of this document is to describe the Medical Physics and Radiation Safety program at Boston University (BU) and
Department of Veterans Affairs VHA DIRECTIVE 2011-005 Veterans Health Administration Washington, DC 20420 February 8, 2011
 Department of Veterans Affairs VHA DIRECTIVE 2011-005 Veterans Health Administration Washington, DC 20420 RADIOLOGY PICTURE ARCHIVING AND COMMUNICATION SYSTEMS (PACS) 1. PURPOSE: This Veterans Health Administration
Department of Veterans Affairs VHA DIRECTIVE 2011-005 Veterans Health Administration Washington, DC 20420 RADIOLOGY PICTURE ARCHIVING AND COMMUNICATION SYSTEMS (PACS) 1. PURPOSE: This Veterans Health Administration
The Practice Standards for Medical Imaging and Radiation Therapy. Quality Management Practice Standards
 The Practice Standards for Medical Imaging and Radiation Therapy Quality Management Practice Standards 2015 American Society of Radiologic Technologists. All rights reserved. Reprinting all or part of
The Practice Standards for Medical Imaging and Radiation Therapy Quality Management Practice Standards 2015 American Society of Radiologic Technologists. All rights reserved. Reprinting all or part of
M E D I C AL D I AG N O S T I C S P E C I AL I S T Schematic Code 14251 (31000081)
 I. DESCRIPTION OF WORK M E D I C AL D I AG N O S T I C S P E C I AL I S T Schematic Code 14251 (31000081) Positions in this banded class perform skilled technical work in the administration of specialized
I. DESCRIPTION OF WORK M E D I C AL D I AG N O S T I C S P E C I AL I S T Schematic Code 14251 (31000081) Positions in this banded class perform skilled technical work in the administration of specialized
SITE IMAGING MANUAL ACRIN 6698
 SITE IMAGING MANUAL ACRIN 6698 Diffusion Weighted MR Imaging Biomarkers for Assessment of Breast Cancer Response to Neoadjuvant Treatment: A sub-study of the I-SPY 2 TRIAL Version: 1.0 Date: May 28, 2012
SITE IMAGING MANUAL ACRIN 6698 Diffusion Weighted MR Imaging Biomarkers for Assessment of Breast Cancer Response to Neoadjuvant Treatment: A sub-study of the I-SPY 2 TRIAL Version: 1.0 Date: May 28, 2012
Program. College of Health Sciences. Diagnostic imaging is a form of medical imaging which is performed with the purpose of diagnosing disease.
 College of Health Sciences DIAGNOSTIC MEDICAL SONOGRAPHY Program Diagnostic Medical Sonography (DMS) is offered by the CHS Department of Health Sciences as one of six sub majors under the BioMedical Sciences
College of Health Sciences DIAGNOSTIC MEDICAL SONOGRAPHY Program Diagnostic Medical Sonography (DMS) is offered by the CHS Department of Health Sciences as one of six sub majors under the BioMedical Sciences
Decision Support for Ordering Appropriate High-Tech Diagnostic Imaging Scans at the Point-of-Order
 Decision Support for Ordering Appropriate High-Tech Diagnostic Imaging Scans at the Point-of-Order Abstract The use of high-tech diagnostic imaging (HTDI) procedures saw double-digit increases in the decade
Decision Support for Ordering Appropriate High-Tech Diagnostic Imaging Scans at the Point-of-Order Abstract The use of high-tech diagnostic imaging (HTDI) procedures saw double-digit increases in the decade
PET and PET/CT in Clinical Trials
 PET and PET/CT in Clinical Trials Nathan C. Hall, M.D., Ph.D. The Ohio State University Medical Center CALGB Imaging Core Lab CALGB Imaging Committee Outline Introduction to PET Positron Emitter Physics
PET and PET/CT in Clinical Trials Nathan C. Hall, M.D., Ph.D. The Ohio State University Medical Center CALGB Imaging Core Lab CALGB Imaging Committee Outline Introduction to PET Positron Emitter Physics
Metrics 101. Produced by the TMF Reference Model Metrics and Reporting Sub-team. 1 August 2014
 Metrics 101 Produced by the TMF Reference Model Metrics and Reporting Sub-team 1 August 2014 Agenda Why a metrics program? Goals of a metrics program Types of metrics Further analytics on metrics Metrics
Metrics 101 Produced by the TMF Reference Model Metrics and Reporting Sub-team 1 August 2014 Agenda Why a metrics program? Goals of a metrics program Types of metrics Further analytics on metrics Metrics
Workshop: Defining the Medical Imaging Requirements for a Health Center. Teleradiology and Networking
 Workshop: Defining the Medical Imaging Requirements for a Health Center April 17 2011 E-health and Telemedicine ehealth is the use, in the health sector, of digital data - transmitted, stored and retrieved
Workshop: Defining the Medical Imaging Requirements for a Health Center April 17 2011 E-health and Telemedicine ehealth is the use, in the health sector, of digital data - transmitted, stored and retrieved
STUDY PLAN FOR THE CERTIFICATE OF THE HIGHER SPECIALIZATION IN ( Diagnostic Radiology)
 STUDY PLAN FOR THE CERTIFICATE OF THE HIGHER SPECIALIZATION IN ( Diagnostic Radiology) plan number :15/11/97/NT I-GENERAL RULES AND CONDITIONS: 1- This plan conforms to the regulations of granting the
STUDY PLAN FOR THE CERTIFICATE OF THE HIGHER SPECIALIZATION IN ( Diagnostic Radiology) plan number :15/11/97/NT I-GENERAL RULES AND CONDITIONS: 1- This plan conforms to the regulations of granting the
Bon Secours St. Mary s School of Medical Imaging Course Descriptions by Semester Class of 2009. CRS 101 Clinical Radiation Science 3
 Bon Secours St. Mary s School of Medical Imaging Course Descriptions by Semester Class of 2009 First Year Fall REV.5/07 Credits CRS 101 Clinical Radiation Science 3 A clinical education course designed
Bon Secours St. Mary s School of Medical Imaging Course Descriptions by Semester Class of 2009 First Year Fall REV.5/07 Credits CRS 101 Clinical Radiation Science 3 A clinical education course designed
European Academy of DentoMaxilloFacial Radiology
 European Academy of DentoMaxilloFacial Radiology Framework for Specialist Training in Dental and Maxillofacial Radiology Background The scope of DentoMaxilloFacial Radiology DMFR (Dental and Maxillofacial
European Academy of DentoMaxilloFacial Radiology Framework for Specialist Training in Dental and Maxillofacial Radiology Background The scope of DentoMaxilloFacial Radiology DMFR (Dental and Maxillofacial
Hollie Goddard Sr. IRB Coordinator McKesson Specialty Health
 Hollie Goddard Sr. IRB Coordinator McKesson Specialty Health We are responsible for acquiring, analyzing, and protecting medical information vital to providing quality patient care HIM professionals ensure
Hollie Goddard Sr. IRB Coordinator McKesson Specialty Health We are responsible for acquiring, analyzing, and protecting medical information vital to providing quality patient care HIM professionals ensure
The New EU Clinical Trials Regulation: The Good, the Bad, the Ugly
 A Full-Service International CRO The New EU Clinical Trials Regulation: The Good, the Bad, the Ugly Dr. Martine Dehlinger-Kremer Vice President, Global Medical and Regulatory Affairs The original intent
A Full-Service International CRO The New EU Clinical Trials Regulation: The Good, the Bad, the Ugly Dr. Martine Dehlinger-Kremer Vice President, Global Medical and Regulatory Affairs The original intent
CLINICAL TRIAL FEASIBILITY AND CAPACITY PLANNING FORM
 1 CLINICAL TRIAL FEASIBILITY AND CAPACITY PLANNING FORM Clinical trials that run into difficulties often have problems that could have been spotted in advance. An early feasibility assessment helps determine
1 CLINICAL TRIAL FEASIBILITY AND CAPACITY PLANNING FORM Clinical trials that run into difficulties often have problems that could have been spotted in advance. An early feasibility assessment helps determine
Health Sciences Degrees
 CAYUGA COMMUNITY COLLEGE Health Sciences Degrees Liberal Arts and Science: Health Science A.S. Cardiovascular Perfusion Chiropractic Medicine Medical Imaging/Radiography Medical Technology & Medical Biotechnology
CAYUGA COMMUNITY COLLEGE Health Sciences Degrees Liberal Arts and Science: Health Science A.S. Cardiovascular Perfusion Chiropractic Medicine Medical Imaging/Radiography Medical Technology & Medical Biotechnology
Streamlining the Flow of Clinical Trial Data: EHR to EDC to Sponsor
 Streamlining the Flow of Clinical Trial : EHR to EDC to Sponsor Landen Bain Liaison to Healthcare CDISC Interchange Standards Consortium) Jane Griffin, RPh Director, Pharmaceutical Research Cerner Corporation
Streamlining the Flow of Clinical Trial : EHR to EDC to Sponsor Landen Bain Liaison to Healthcare CDISC Interchange Standards Consortium) Jane Griffin, RPh Director, Pharmaceutical Research Cerner Corporation
Regulatory Writing Clinical Project Management Strategic Communications
 Regulatory Writing Clinical Project Management Strategic Communications FLEXIBLE. STRATEGIC. INFORMED. COMPLIANT. For over 15 years, Niche Science and Technology has been providing outstanding medical
Regulatory Writing Clinical Project Management Strategic Communications FLEXIBLE. STRATEGIC. INFORMED. COMPLIANT. For over 15 years, Niche Science and Technology has been providing outstanding medical
Performance Profile. Clinical Study Physician. www.celyad.com 1
 Performance Profile Clinical Study Physician www.celyad.com 1 Role: Provide medical guidance and leadership throughout the development and conduct of clinical trials. You will be responsible for overseeing
Performance Profile Clinical Study Physician www.celyad.com 1 Role: Provide medical guidance and leadership throughout the development and conduct of clinical trials. You will be responsible for overseeing
Sharon H. Johnson, BS, MS 123 Main Street Capital City, VA 00000 Phone: 434-555-1234 Email: shjohnson@email.com
 SAMPLE CRA CV Sharon H. Johnson, BS, MS 123 Main Street Capital City, VA 00000 Phone: 434-555-1234 Email: shjohnson@email.com Education: Masters of Science, Healthcare Administration, Capital City University,
SAMPLE CRA CV Sharon H. Johnson, BS, MS 123 Main Street Capital City, VA 00000 Phone: 434-555-1234 Email: shjohnson@email.com Education: Masters of Science, Healthcare Administration, Capital City University,
Imaging Technology. Diagnostic Medical Sonographer, Dosimetrist, Nuclear Medicine Technologist, Radiation Therapist, Radiologic Technologist
 Imaging Technology Diagnostic Medical Sonographer, Dosimetrist, Nuclear Medicine Technologist, Radiation Therapist, Radiologic Technologist Diagnostic Medical Sonographer, Dosimetrist Diagnostic Medical
Imaging Technology Diagnostic Medical Sonographer, Dosimetrist, Nuclear Medicine Technologist, Radiation Therapist, Radiologic Technologist Diagnostic Medical Sonographer, Dosimetrist Diagnostic Medical
Patient Prep Information
 Stereotactic Breast Biopsy Patient Prep Information Imaging Services Cannon Memorial Hospital Watauga Medical Center Table Weight Limits for each facility Cannon Memorial Hospital Watauga Medical Center
Stereotactic Breast Biopsy Patient Prep Information Imaging Services Cannon Memorial Hospital Watauga Medical Center Table Weight Limits for each facility Cannon Memorial Hospital Watauga Medical Center
First floor, Main Hospital North Services provided 24/7 365 days per year
 First floor, Main Hospital North Services provided 24/7 365 days per year General Radiology (X-ray) Fluoroscopy Ultrasound (Sonography) Nuclear Medicine P.E.T. imaging Computed Tomography (CT scan) Magnetic
First floor, Main Hospital North Services provided 24/7 365 days per year General Radiology (X-ray) Fluoroscopy Ultrasound (Sonography) Nuclear Medicine P.E.T. imaging Computed Tomography (CT scan) Magnetic
Tasmanian Department of Health and Human Service
 Tasmanian Department of Health and Human Service Agency Health Professional Reference Group Allied Health Professional Workforce Planning Group Allied Health Professional Workforce Planning Project Audiology
Tasmanian Department of Health and Human Service Agency Health Professional Reference Group Allied Health Professional Workforce Planning Group Allied Health Professional Workforce Planning Project Audiology
http://www.bls.gov/oco/ocos104.htm
 http://www.bls.gov/oco/ocos104.htm Nuclear Medicine Technologists Nature of the Work Training, Other Qualifications, and Advancement Employment Job Outlook Projections Data Earnings OES Data Related Occupations
http://www.bls.gov/oco/ocos104.htm Nuclear Medicine Technologists Nature of the Work Training, Other Qualifications, and Advancement Employment Job Outlook Projections Data Earnings OES Data Related Occupations
Programme Guide PGDCDM
 Post Graduate Diploma in Clinical Data Management and Biostatistics with SAS Programme Guide PGDCDM School of Health Sciences Indira Gandhi National Open University WHY THIS PROGRAMME? The Post Graduate
Post Graduate Diploma in Clinical Data Management and Biostatistics with SAS Programme Guide PGDCDM School of Health Sciences Indira Gandhi National Open University WHY THIS PROGRAMME? The Post Graduate
Imaging in Indian Health:
 Imaging in Indian Health: Project Overview Document Version: 2.2 Issue Date: 081206 Prepared By: MCarroll MD Table of Contents Introduction to VistA Imaging Overview 3 Key Features 4 Frequently Asked Questions
Imaging in Indian Health: Project Overview Document Version: 2.2 Issue Date: 081206 Prepared By: MCarroll MD Table of Contents Introduction to VistA Imaging Overview 3 Key Features 4 Frequently Asked Questions
CLINICAL RESEARCH GENERIC TASK DESCRIPTIONS
 Purpose Purpose Purpose Primary responsibility for implementation, coordination, evaluation, communication and/or management of research studies. May involve more than one study or multiple sites within
Purpose Purpose Purpose Primary responsibility for implementation, coordination, evaluation, communication and/or management of research studies. May involve more than one study or multiple sites within
U.S. Food and Drug Administration
 U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
The role, responsibilities and status of the clinical medical physicist in AFOMP
 Australasian Physical & Engineering Sciences in Medicine Volume 32 Number 4, 2009 AFOMP POLICY STATEMENT N O 1 The role, responsibilities and status of the clinical medical physicist in AFOMP K. H. Ng*
Australasian Physical & Engineering Sciences in Medicine Volume 32 Number 4, 2009 AFOMP POLICY STATEMENT N O 1 The role, responsibilities and status of the clinical medical physicist in AFOMP K. H. Ng*
COLLEGE ON MEDICAL PHYSICS
 COLLEGE ON MEDICAL PHYSICS (30 August - 22 September 2004) Miramare - Trieste, Italy Directors: A. Benini, P. Sprawls and S. Tabakov Local Organizer: L. Bertocchi FINAL PROGRAMME (all lectures were held
COLLEGE ON MEDICAL PHYSICS (30 August - 22 September 2004) Miramare - Trieste, Italy Directors: A. Benini, P. Sprawls and S. Tabakov Local Organizer: L. Bertocchi FINAL PROGRAMME (all lectures were held
CLINICAL DEVELOPMENT OPTIMIZATION
 PAREXEL CLINICAL RESEARCH SERVICES CLINICAL DEVELOPMENT OPTIMIZATION Enhancing the clinical development process to achieve optimal results ADVANCED TECHNOLOGY COMBINED WITH INTELLIGENT THINKING CAN HELP
PAREXEL CLINICAL RESEARCH SERVICES CLINICAL DEVELOPMENT OPTIMIZATION Enhancing the clinical development process to achieve optimal results ADVANCED TECHNOLOGY COMBINED WITH INTELLIGENT THINKING CAN HELP
5 Health Care Pathways
 Health Care Careers 5 Health Care Pathways All Health Care occupations are organized into 5 pathways. THERAPEUTIC SERVICES DIAGNOSTIC SERVICES HEALTH INFORMATICS SUPPORT SERVICES BIOTECHNOLOGY RESEARCH
Health Care Careers 5 Health Care Pathways All Health Care occupations are organized into 5 pathways. THERAPEUTIC SERVICES DIAGNOSTIC SERVICES HEALTH INFORMATICS SUPPORT SERVICES BIOTECHNOLOGY RESEARCH
Education. October, 1985 GE Medical Systems Milwaukee, WI Signa Operators Training Program
 William H. Faulkner, Jr. B.S.,R.T.(R)(MR)(CT), FSMRT 1554 Sedgefield Dr. Ooltewah, TN 37363 (423) 894-7214(W) (423) 893-9422 (H) Fax: (615) 290-5229 E-mail: faulkner@t2star.com Internet: http://www.t2star.com
William H. Faulkner, Jr. B.S.,R.T.(R)(MR)(CT), FSMRT 1554 Sedgefield Dr. Ooltewah, TN 37363 (423) 894-7214(W) (423) 893-9422 (H) Fax: (615) 290-5229 E-mail: faulkner@t2star.com Internet: http://www.t2star.com
Health Management Information Systems: Medical Imaging Systems. Slide 1 Welcome to Health Management Information Systems, Medical Imaging Systems.
 Health Management Information Systems: Medical Imaging Systems Lecture 8 Audio Transcript Slide 1 Welcome to Health Management Information Systems, Medical Imaging Systems. The component, Health Management
Health Management Information Systems: Medical Imaging Systems Lecture 8 Audio Transcript Slide 1 Welcome to Health Management Information Systems, Medical Imaging Systems. The component, Health Management
Radiology Quality Initiative (RQI) Program Answers to Frequently Asked Questions
 Radiology Quality Initiative (RQI) Program Answers to Frequently Asked Questions Program Overview... 2 Program Requirements... 4 Claims... 7 Online Tools... 7 Standards for Imaging Guidelines... 8 Page
Radiology Quality Initiative (RQI) Program Answers to Frequently Asked Questions Program Overview... 2 Program Requirements... 4 Claims... 7 Online Tools... 7 Standards for Imaging Guidelines... 8 Page
Clarkson College and Central Community College (CCC)
 Articulation Agreement between Clarkson College and Central Community College (CCC) Effective Fall 2013 This agreement states the conditions under which Clarkson College will accept 18 semester hours of
Articulation Agreement between Clarkson College and Central Community College (CCC) Effective Fall 2013 This agreement states the conditions under which Clarkson College will accept 18 semester hours of
Education, Training, and Certification of Medical Physicist in Korea
 Education, Training, and Certification of Medical Physicist in Korea Rena Lee, Education Committee, KSMP Tae-Suk Suh, President, KSMP The 7th Asia-Oceania Congress of Medical Physics IOMP Symposium on
Education, Training, and Certification of Medical Physicist in Korea Rena Lee, Education Committee, KSMP Tae-Suk Suh, President, KSMP The 7th Asia-Oceania Congress of Medical Physics IOMP Symposium on
GE Healthcare. Centricity * PACS with Universal Viewer. Universal Viewer. Where it all comes together.
 GE Healthcare Centricity * PACS with Universal Viewer Universal Viewer. Where it all comes together. Where it all comes together Centricity PACS with Universal Viewer introduces an intuitive imaging application
GE Healthcare Centricity * PACS with Universal Viewer Universal Viewer. Where it all comes together. Where it all comes together Centricity PACS with Universal Viewer introduces an intuitive imaging application
it s all Choices about S H R P School of Health Related Professions Vascular Technology
 D E P A R T M E N T O F M E D I C A L I M A G I N G S E R V I C E S it s all Choices about S H R P School of Health Related Professions Vascular Technology Choices Students who choose UMDNJ s School of
D E P A R T M E N T O F M E D I C A L I M A G I N G S E R V I C E S it s all Choices about S H R P School of Health Related Professions Vascular Technology Choices Students who choose UMDNJ s School of
Radiography Frequently Asked Questions
 Frequently Asked Questions What is a radiographer? Radiographers, commonly referred to as X-ray techs or radiologic technologists, are medical personnel, who perform diagnostic imaging procedures. They
Frequently Asked Questions What is a radiographer? Radiographers, commonly referred to as X-ray techs or radiologic technologists, are medical personnel, who perform diagnostic imaging procedures. They
National curriculum and assessment guidelines in preparation for registration as a Medical Biological Scientist
 National curriculum and assessment guidelines in preparation for registration as a Medical Biological Scientist National curriculum Medical Biological Scientists Last Modified March 2013 Page 1 of 9 1.
National curriculum and assessment guidelines in preparation for registration as a Medical Biological Scientist National curriculum Medical Biological Scientists Last Modified March 2013 Page 1 of 9 1.
cro s - focus on laboratories
 ExEcutivE PanEl contract RESEaRcH ORGaniZatiOnS 18 cro s - focus on laboratories Bayer Healthcare s Global Alliance Manager Suzanne Besaw heads this discussion on the current trends in contract research
ExEcutivE PanEl contract RESEaRcH ORGaniZatiOnS 18 cro s - focus on laboratories Bayer Healthcare s Global Alliance Manager Suzanne Besaw heads this discussion on the current trends in contract research
Health Sciences Division. xxx
 83 xxx The offers programs and courses to meet the highest academic and industry standards and in consequence our graduates are highly sought after by employers. Health Sciences graduates can expect to
83 xxx The offers programs and courses to meet the highest academic and industry standards and in consequence our graduates are highly sought after by employers. Health Sciences graduates can expect to
Houston Cancer Institute
 Houston Cancer Institute A personal path to healing Memorial-West Houston Katy Northwest Houston Southeast Houston Sugar Land Convenience for Patients State of the Art Therapies and Diagnosis Real Support
Houston Cancer Institute A personal path to healing Memorial-West Houston Katy Northwest Houston Southeast Houston Sugar Land Convenience for Patients State of the Art Therapies and Diagnosis Real Support
The Practice Standards for Medical Imaging and Radiation Therapy. Sonography Practice Standards
 The Practice Standards for Medical Imaging and Radiation Therapy Sonography Practice Standards 2015 American Society of Radiologic Technologists. All rights reserved. Reprinting all or part of this document
The Practice Standards for Medical Imaging and Radiation Therapy Sonography Practice Standards 2015 American Society of Radiologic Technologists. All rights reserved. Reprinting all or part of this document
Guidance for CTUs on Assessing the Suitability of Laboratories Processing Research Samples
 Guidance for CTUs on Assessing the Suitability of Laboratories Processing Research Samples UKCRC Registered CTUs Network Guidance for CTUs on Assessing the Suitability of Laboratories Processing Research
Guidance for CTUs on Assessing the Suitability of Laboratories Processing Research Samples UKCRC Registered CTUs Network Guidance for CTUs on Assessing the Suitability of Laboratories Processing Research
High Country Nuclear Medicine Conference Clinical Decision Support, the Good, the Bad and the Ugly Sue Bunning, Director Health Policy and Regulatory
 High Country Nuclear Medicine Conference Clinical Decision Support, the Good, the Bad and the Ugly Sue Bunning, Director Health Policy and Regulatory Affairs March 2, 2015 SNMMI s History Founded in 1954
High Country Nuclear Medicine Conference Clinical Decision Support, the Good, the Bad and the Ugly Sue Bunning, Director Health Policy and Regulatory Affairs March 2, 2015 SNMMI s History Founded in 1954
HL7 and Meaningful Use
 HL7 and Meaningful Use Grant M. Wood HL7 Ambassador HIMSS14 2012 Health Level Seven International. All Rights Reserved. HL7 and Health Level Seven are registered trademarks of Health Level Seven International.
HL7 and Meaningful Use Grant M. Wood HL7 Ambassador HIMSS14 2012 Health Level Seven International. All Rights Reserved. HL7 and Health Level Seven are registered trademarks of Health Level Seven International.
The Practice Standards for Medical Imaging and Radiation Therapy. Radiography Practice Standards
 The Practice Standards for Medical Imaging and Radiation Therapy Radiography Practice Standards 2015 American Society of Radiologic Technologists. All rights reserved. Reprinting all or part of this document
The Practice Standards for Medical Imaging and Radiation Therapy Radiography Practice Standards 2015 American Society of Radiologic Technologists. All rights reserved. Reprinting all or part of this document
To Certify or Not to Certify
 To Certify or Not to Certify Sandra Halvorson, BA, CCRP Clinical Research Coordinator II CIBMTR Minneapolis Campus Sue Logan, BS, CCRP Clinical Research Coordinator II CIBMTR Minneapolis Campus November
To Certify or Not to Certify Sandra Halvorson, BA, CCRP Clinical Research Coordinator II CIBMTR Minneapolis Campus Sue Logan, BS, CCRP Clinical Research Coordinator II CIBMTR Minneapolis Campus November
To Certify or Not to Certify Sandra Halvorson, BA, CCRP
 To Certify or Not to Certify Sandra Halvorson, BA, CCRP Clinical Research Coordinator II CIBMTR Minneapolis Campus Sue Logan, BS, CCRP Clinical Research Coordinator II We have no financial relationships
To Certify or Not to Certify Sandra Halvorson, BA, CCRP Clinical Research Coordinator II CIBMTR Minneapolis Campus Sue Logan, BS, CCRP Clinical Research Coordinator II We have no financial relationships
University Children s Hospital Basel
 Cooperation Siemens International Reference Center Pediatric MRI www.siemens.com/skyra University Children s Hospital Basel Siemens International Reference Center Pediatric MRI Answers for life. Partnering
Cooperation Siemens International Reference Center Pediatric MRI www.siemens.com/skyra University Children s Hospital Basel Siemens International Reference Center Pediatric MRI Answers for life. Partnering
The Evolution of Data Management Job Models. in the Execution of Clinical Trials. info@kcr.com. www.kcrcro.com
 The Evolution of Data Management Job Models in the Execution of Clinical Trials info@kcr.com KCR S.A. Corporate Headquarters 6 Postepu str., 02-676 Warsaw Phone: +48 22 313 13 13 Fax: +48 22 313 13 14
The Evolution of Data Management Job Models in the Execution of Clinical Trials info@kcr.com KCR S.A. Corporate Headquarters 6 Postepu str., 02-676 Warsaw Phone: +48 22 313 13 13 Fax: +48 22 313 13 14
ABHES BULLETIN. Subject: Final Radiologic Technology Standards Effective January 1, 2013
 ACCREDITING BUREAU OF HEALTH EDUCATION SCHOOLS 7777 Leesburg Pike, Suite 314 N. Falls Church, Virginia 22043 Tel. 703/917.9503 Fax 703/917.4109 E-Mail: info@abhes.org ABHES BULLETIN To: From: ABHES-Accredited
ACCREDITING BUREAU OF HEALTH EDUCATION SCHOOLS 7777 Leesburg Pike, Suite 314 N. Falls Church, Virginia 22043 Tel. 703/917.9503 Fax 703/917.4109 E-Mail: info@abhes.org ABHES BULLETIN To: From: ABHES-Accredited
New Investigator Collaborations and Interactions: Regulatory
 Your Health and Safety... Our priority Votre santé et votre Securité notre priorité New Investigator Collaborations and Interactions: Regulatory NCIC Clinical Trials Group New Investigator Clinical Trials
Your Health and Safety... Our priority Votre santé et votre Securité notre priorité New Investigator Collaborations and Interactions: Regulatory NCIC Clinical Trials Group New Investigator Clinical Trials
Society of Nuclear Medicine 1850 Samuel Morse Drive Reston, VA 20190-5316 www.snm.org
 What is nuclear medicine? Nuclear medicine is a medical specialty that is used to diagnose and treat diseases in a safe and painless way. Nuclear medicine procedures permit the determination of medical
What is nuclear medicine? Nuclear medicine is a medical specialty that is used to diagnose and treat diseases in a safe and painless way. Nuclear medicine procedures permit the determination of medical
BUILDING QUALITY INTO CLINICAL TRIALS AN FDA PERSPECTIVE
 BUILDING QUALITY INTO CLINICAL TRIALS AN FDA PERSPECTIVE Jean Toth-Allen, Ph.D. Biophysicist Office of Good Clinical Practice Office of the Commissioner May 14, 2012 1 Overview I. Background why we are
BUILDING QUALITY INTO CLINICAL TRIALS AN FDA PERSPECTIVE Jean Toth-Allen, Ph.D. Biophysicist Office of Good Clinical Practice Office of the Commissioner May 14, 2012 1 Overview I. Background why we are
TransCelerate's Role in Transforming Pharmaceutical Trials Presentation to PCORNet
 TransCelerate's Role in Transforming Pharmaceutical Trials Presentation to PCORNet Dalvir Gill, PhD - Chief Executive Officer 17 October, 2014 Presentation Objectives + TransCelerate History + Participating
TransCelerate's Role in Transforming Pharmaceutical Trials Presentation to PCORNet Dalvir Gill, PhD - Chief Executive Officer 17 October, 2014 Presentation Objectives + TransCelerate History + Participating
An information platform that delivers clinical studies better, faster, safer and more cost effectively
 An information platform that delivers clinical studies better, faster, safer and more cost effectively Powering Process & Performance Proactively manage study start-up and execution Risk profile new sites
An information platform that delivers clinical studies better, faster, safer and more cost effectively Powering Process & Performance Proactively manage study start-up and execution Risk profile new sites
