Title: Basic Principles of Risk Management for Medical Device Design
|
|
|
- Constance Nancy Ross
- 10 years ago
- Views:
Transcription
1 Title: Basic Principles of Risk Management for Medical Device Design WHITE PAPER Author: Ganeshkumar Palanichamy
2 Abstract Medical devices developed for human application are used for diagnostic or treatment purposes. They may either be an instrument, an apparatus or a material. Moreover, these devices can be used for daily patient care as well as for medical scientific purposes. Researchers in charge to develop new medical devices are faced with the complex task of making a medical device safe for human use. This implies that the device should be safe and effective. Risk management involves the identification, understand, control, and prevent failures that can result in hazards when people use medical devices. Risk Analysis plays a key role in the development of medical devices design. Risk analysis, or hazard analysis, is a structured tool for the evaluation of potential problems which could be encountered in connection with the use of taking a drug, or using a medical device. Manufacturers are expected to identify possible hazards associated with the design in both normal and fault conditions. If any risk is judged unacceptable, it should be reduced to acceptable levels by appropriate means. The purpose of this paper is to describe the importance of Risk Analysis, Risk Management Process, Application of Risk Management tools, and the benefit of the Risk Management Analysis. The goal is to minimize use-related hazards, assure that intended users are able to use medical devices safely and effectively throughout the product life cycle, and to facilitate review of new device submissions and design control documentation. Wipro Technologies Page 2 of 20
3 Contents 1. Introduction Overview of Risk Management Hazard Analysis Procedure Analysis ISO 14971: Conclusion References About the Author About Wipro Technologies Wipro Technologies Page 3 of 20
4 1. Introduction The globalization of the medical device marketplace, combined with the growth of medical device usage, has led to a significant increase the complex task of making a medical device safe for human use activity among device manufacturers. Risk Management has become an important competitive tool to gain access to foreign markets. As clinicians, patients, regulators, and litigators become more sensitive to safety issues related to human factors, the importance of appropriate translation and safety controls will increase. Risk management is necessary to ensure device usability, safety, and regulatory compliance. In some cases, critical human factors and risk management decisions are made that depend on specific language in the user interface or labeling. For instance, hazardous situations can arise based on improper interpretation of date/time information or units of measurement displayed. Mitigation of such risks is typically a key focus during initial device development for the initial locale (e.g., the United States); however, the impact of translation and localization on these items is often not as carefully identified, controlled, verified, and validated. FDA's quality system regulation is intended to give manufacturers "the flexibility to determine the controls that are necessary to be commensurate with risk." FDA sees risk analysis as an essential requirement of the regulation but gives little guidance on specific risk analysis approaches and procedures such as fault tree analysis (FTA) or failure mode and effects analysis (FMEA). As medical device companies review and update their approaches to risk analysis, they may find value in what other industries including chemical, aerospace, and defense have learned about using it to reduce risk. Companies can manage and reduce risk more effectively by including risk thinking as early as possible in device or process development and revisiting those issues systematically throughout the development process. Wipro Technologies Page 4 of 20
5 2. Overview of Risk Management Risk management involves the identification, understand, control, and prevent failures that can result in hazards when people use medical devices. Manufacturers are expected to identify possible hazards associated with the design in both normal and fault conditions. The risks associated with the hazards, including those resulting from user error, should be calculated in both normal and fault conditions. If any risk is judged unacceptable, it should be reduced to acceptable levels by appropriate means. 2.1 Why should we perform Risk Management? Risk analysis is now required by law. Identification of device design problems prior to distribution eliminates costs associated with recalls. It offers a measure of protection from product liability damage awards. Regulatory submissions checklists used by the FDA now call for inclusion of risk analysis. It is the right thing to do. Product Liability. To ensure safety of the device. To ensure that any unsafe device that do reach the market are promptly identified and efficiently corrected. Risk management system demonstrates that the manufacture providing safe device. An overall risk management process involves the essential steps in Figure 1. In order to manage risk, hazards must first be identified. By evaluating the potential consequences of hazards and their likelihood, a measure of risk can be estimated. This value is compared to the company's risk-acceptability criteria and, if it is too high, the risk needs to be mitigated. Wipro Technologies Page 5 of 20
6 Identify All Hazards For Each Hazard Apply Mitigation Determine Severity and Frequency of Exposure Next Hazard Unacceptable Assign Risk Level Acceptable Figure 1. Risk Management Flow Chart Because risk cannot be completely eliminated, the risk that remains must be managed. The following steps can be used in a risk management program Develop written definitions of what needs to be done and how to do it. Define responsibilities and accountability. Define what needs authorization and who is responsible for handling it. Define the skills and knowledge necessary to implement the system and a provision for training those who do not possess these skills. Develop and maintain written documentation to demonstrate conformance to policies and procedures. Incorporate measures to cross-check and verify that procedures are followed. Verify that systems are in place and functioning properly. 2.2 Risk Control Process through which decisions are reached and protective measures are implemented for reducing or maintaining risk within the specified level. 2.3 Risk control and monitoring activities Actions intended to eliminate or reduce each risk to meet the previously determined risk acceptability criteria. One or more risk control measures may be incorporated. Wipro Technologies Page 6 of 20
7 Risk controls may begin as early as design input and continue over the medical device life time. Some regulatory schemes prescribe a fixed hierarchy of risk controls that should be examined in the following order: Inherent safety by design Protective measures in the device or its manufacture Information for safety, such as warnings, maintenance schedules, etc. Throughout the life-cycle of the device the manufacturer monitors whether the risks continue to remain acceptable and whether any new hazards or risks are discovered. An effective and well defined Quality Management System is key Information typically obtained from the quality management system, for example, production, complaints, customer feedback, should be used as part of this monitoring. 2.4 Risk Control Measures Protective measures, e.g. default operating modes Information for safety, e.g., warnings in labeling Many measures require intervention The correct response for the circumstances, e.g. a patient-specific response Timeliness 2.5 Safety Risk Zone Identifies the residual safety risk for each hazard in order to make a determination of its acceptability. Hazards that fall into Safety Risk Zone 1 (R=1) are considered generally acceptable or minimal and require no further analysis. Additional mitigations are optional for hazards that fall into Safety Risk Zone 1. Hazards that fall into Safety Risk Zone 2 (R=2) are considered conditionally acceptable but require analysis and mitigation. These are considered acceptable only when adequate mitigations are identified. Hazards that fall into Safety Risk Zone 3 (R=3) are generally unacceptable. If hazards in Safety Risk Zone 3 cannot be further mitigated to the point of falling into an acceptable Risk Zone (R=1 or 2), a formal risk/ benefit analysis shall be performed and documented. Wipro Technologies Page 7 of 20
8 Figure 2. Safety Risk Zone Severity Criteria Catastrophic Death or system loss. Critical Severe injury or major system damage. Marginal Injury requiring medical attention or system damage. Negligible Possible minor injury or minor system damage. Wipro Technologies Page 8 of 20
9 Probability WHITE PAPER Basic Principles of Risk Management for Medical Device Design Occurance Frequent Probable Occasional Remote Improbable Criteria Expected to occur frequently. Will occur several times in the life of an item. Likely to occur sometime in the life of an item. Unlikely, but possible to occur in the life of an item. So unlikely, it can be assumed occurrence may not be experienced. Severity Catastrophic (4) Critical (3) Marginal (2) Negligible (1) Frequent (4) Probable (3) Occasional (2) Remote (1) Figure 3. Risk Assessment Matrix 2.6 Mitigation Mitigation means: What are you going to do about the situation? 1st Line of Defense Avoid or eliminate failure causes 2nd Line of Defense Identify or detect the failure earlier 3rd Line of Defense Reduce the impacts/consequences of failure Identifies the safety risk control measures that have been implemented to effectively reduce the likelihood of the hazard or minimize the severity of the resulting harm. It could include actions taken in the design, processes, and/or labeling. The use of labeling as a sole mitigation was minimized. Wipro Technologies Page 9 of 20
10 When mitigations were added to the Hazard Dictionary, they were peer reviewed and the Safety Risk section was reevaluated in order to identify whether any new hazards were introduced or the safety risk of any existing hazards was increased. Wipro Technologies Page 10 of 20
11 3. Hazard Analysis Even before a final design has been developed, a preliminary hazard analysis can be conducted to establish the baseline hazards associated with a device. In essence, the analysis consists of listing the major components and operating requirements of the device and evaluating their potential hazards. The components and operating requirements could include raw materials and wastes, hardware, monitoring and control systems, human-device interfaces, services, and the operating environment. Some potential hazards that may need to be evaluated include toxicity, flammability, and reactivity of raw materials and wastes; sensitivity to environmental factors such as temperature and humidity; mechanical or electronic hazards; and human factors associated with the operator-device interface. The patient-device interface can also be hazardous because of unsafe or ineffective delivery of energy, administration of drugs, or control of lifesustaining functions. Also, incorrect information could lead to a misdiagnosis or wrong treatment or therapy being ordered. When conducting a preliminary hazard analysis, use a what-if or brainstorming approach to identify possible failures, evaluate potential consequences, and develop risk management strategies. These strategies lead to an improved, lower-cost design. Generally, failure scenarios can be prioritized by the severity of each hazard. At this stage, there is often insufficient detail to evaluate hazard likelihood accurately. However, comparisons may be made with similar devices and their histories in the medical device reports. An evaluation revealing severe hazard potential may prompt a radical change in the conceptual design. The goal is to eliminate all high-severity hazards and reduce as many medium- and lowseverity hazards as possible. There is considerable flexibility at this early design stage. Major changes can make the device inherently safer at minimal cost. For example, if use of a chemical was determined to be a significant hazard, other less-toxic chemicals or a diluted form of the original chemical might be a reasonable mitigating measure. During prototype development, more detailed hazard and risk analysis can be performed. At this stage of design, process and mechanical drawings are available, and the basic process operations have been defined. The device and its operation can be reviewed by a number of analysis techniques, including topdown and bottom-up approaches. A hazard and operability (HAZOP) study is a bottom-up approach ideal for new or complex designs involving a number of processing steps. A HAZOP is conducted on individual steps, each of which has design intent. Wipro Technologies Page 11 of 20
12 If the deviation defined by the combination of a design parameter and guide word (e.g., more flow or less flow) can result in a hazard, potential causes and any existing controls are identified. The risk level can be evaluated using a risk matrix in which consequence and frequency ranges have been established according to a company's internal risk-acceptability criteria (Figure 2). When a device contains many mechanical components, an FMEA should be considered. However, an FMEA is time-consuming and is generally applied only to Class III devices or to the safety critical portions of devices. For those devices that contain many electrical components, an FMEA is also a desirable methodology. This is another bottom-up approach that focuses on a particular component of a medical device and explores the various failure modes that can occur. For each failure mode that results in an undesirable consequence, potential causes and existing controls are evaluated, and the level of risk can be determined by using a risk matrix. An FTA is an effective top-down approach. The team starts with the undesired consequence or top event and identifies the initiating and contributing events that must occur to produce it. These events are combined using logic gates. A logic gate is the point at which two or more independent events are combined in order to produce a higher-level event. The logic gate determines whether the sub event probabilities or frequencies should be multiplied, for an and gate, or added, for an or gate. If all events under a gate are necessary for the higher event to occur, an and gate is used. If each of the events is sufficient to produce the higher event on its own, an or gate is used. Both mechanical failures and human errors can readily be included in a fault tree. An example of a partial fault tree for a pacemaker is shown in Figure 4. Wipro Technologies Page 12 of 20
13 Injury from Pacemaker Installation/Operation Operator injury Patient injury Device contaminated with biohazard Operator not wearing Gloves Mechanical Failure of Pacemaker Electrical Failure of Pacemaker Figure 4. A Partial Fault Tree Analysis for a Pacemaker The top event is an injury resulting from installation or operation of the device. Below the top event are two sub events labeled operator injury and patient injury. Since either could produce the top event, they are combined using an or gate. Under the operator injury branch, one potential scenario has been identified that involves having the device contaminated with a biohazard such as blood (the initiating event) and the operator not wearing gloves (contributing event). Since both the initiating and contributing events must occur for an injury to take place, these events are combined using an and gate. If failure rates for each event on a fault tree are available or can be estimated from generic data, the top-event frequency can be calculated and compared to a company's internal risk-acceptability criteria. A fault tree is a powerful riskanalysis tool, but its greatest limitation is the availability of relevant failure data. Therefore, fault trees are generally best used to compare risks of various alternatives. The greatest benefit of a fault tree is that events that contribute most frequently to the top event can readily be identified, and mitigating measures can be focused on reducing the frequency of these events. Wipro Technologies Page 13 of 20
14 4. Procedure Analysis Although HAZOP, FMEA, and FTA allow evaluation of human errors in design, operation, and maintenance of medical devices, it is often desirable to conduct a separate analysis focused on procedures. Typically, a what-if approach is used for this type of analysis. Procedures are grouped into process steps similar to those study sections used with HAZOP. Each process step is evaluated to determine if an undesirable consequence could result from incorrect procedures. Checklists are the simplest tools for conducting design reviews but are generally not sufficient. The true benefit of checklists is to support the other techniques described previously. For example, a checklist of potential hazards identified in previous reviews or from incidents associated with similar devices would be useful during a design review. After completion of the review, the checklist can be examined to ensure that the study evaluated all previously identified potential hazards. For example, during a HAZOP, possible human errors are evaluated; however, as a final check, a human-factors checklist is often used. The risk analysis should include any risks associated with the manufacture and delivery of the device to its intended location. For devices that involve solutions or components that can be degraded by environmental factors (e.g., heat, humidity, cold, or light), storage and transportation methods need to be reviewed. Identified problems could lead to changes in packaging or warnings on storage or packaging containers. It is important that any changes made during the design process be reviewed to ensure that safety hazards are not being introduced into the design. Small changes are generally reviewed using a what-if approach, whereas larger changes may require a HAZOP or FMEA. A final design or prestart-up review should be conducted before starting production. Extensive checklists ensure that all design specifications have been met and all previous design review recommendations have been addressed. The final design review should also include a physical inspection of the device in its intended workspace (e.g., laboratory, hospital, doctor's office) to identify any issues not readily apparent from looking at drawings, such as location of vents and drains, accessibility for maintenance, pinch points, and sharp edges. A punchlist (findings or observations developed during a safety or design review) of final action items is typically generated and prioritized into items that need to be completed prior to start of production and others that can be incorporated into the next model. Software used to control or monitor a medical device also needs to be reviewed. Software can be grouped into its primary functions (e.g., start-up, treatment, diagnostics, and maintenance) just as procedures can be grouped into process steps. Three generic sub functions are evaluated for each primary function: Wipro Technologies Page 14 of 20
15 Function the software component does not perform its intended function correctly per its original design intent. Timing the software component performs its function at the wrong time. Data the software component performs its function using incorrect or corrupt data. Software errors can produce unexpected consequences, particularly those that involve corrupt data or false alarms. It is important to have a means of detecting software errors or a means to detect the effects of software errors on a device. For example, a software error resulting in a failure of the alarm notification system would disable all alarm systems. Separate redundant alarms or interlocks on critical aspects of a device need to be considered. Wipro Technologies Page 15 of 20
16 5. ISO 14971:2007 It is the specified standard for risk management used to demonstrate compliance with the Risk Management requirements of the Medical Devices Directive (MDD). The standard addresses risk management to patient, operator, other parties, external equipment and/or the environment. Risk Management Process ISO requires the manufacturer to establish, document and maintain a risk management process for: Reviewing the intended use (intended purpose) of the medical device Identification of hazards (known and foreseeable) Estimation of the probability of occurrence of harm Estimation of the severity of each hazard and its harm Evaluation of associated risks (decision making) Control of these risks Monitoring of the effectiveness of these controls throughout the whole lifecycle of a medical device. The risk management process does not end with the design and manufacturing process but also includes applicable sterilization, packaging, labeling, storage, handling/ transport, distribution and market surveillance. The manufacturer shall apply risk management from the initial conception until the ultimate decommissioning and disposal of the product. Therefore, the gathering of postproduction information is a required part of the process. The latest version of ISO 14971:2007 ( Medical devices Application of risk management to medical devices ) was approved on 5 December 2006 by the Association for the Advancement of Medical Instrumentation (AAMI) and on 1 February 2007 by the American National Standards Institute (ANSI). Finally published in May 2007 as ANSI/AAMI/ISO 14971:2007 Wipro Technologies Page 16 of 20
17 6. Conclusion All of the techniques described above have been successfully used in design reviews of medical devices. FTA is being used by pacemaker manufacturers based on FDA guidance for software aspects of 510(k) notification submissions for medical devices. Other computer-controlled medical devices will also need to be reviewed using FTA as a primary risk analysis tool. For mechanical devices that are used away from the patient, such as plasma and blood viral inactivation devices, as well as devices for preparing intravenous solutions, an FMEA is a reasonable choice. However, for associated activities such as preparation of disposables, which are manual operations, a what-if approach is preferred. The key to successful risk management in medical device design is to start early. As soon as conceptual designs are available, the risk management process can begin. A preliminary hazard analysis can be useful in selecting the concept with the highest level of inherent safety. Later, as the design is developed, design reviews at key points in the development process will allow changes to be made without significantly affecting the project schedule. The further along in the design process that changes are identified, the fewer choices are available to mitigate hazards without significant schedule implications. Generally, risk management activities will identify opportunities to improve device performance. The benefits of conducting risk analysis during medical device design can be significant and can be used to offset some or all of the cost of implementing risk-mitigating measures. There is always a trade-off in how to manage risk. Hardware or software controls are generally viewed as more effective since they are more reliable than humans. However, since there is need for human interaction in the operation of all medical devices, the element of risk needs to be adequately evaluated. Minimizing the level of routine human intervention will reduce risk and improve efficiency. Such risk reduction must be weighed against the cost of automating tasks that can be performed by individuals. Wipro Technologies Page 17 of 20
18 References 1. Mosenkis, R., in Grutting, C., Medical devices: international perspectives on health and safety. Amsterdam: Elsevier; Sawyer, C., Do It By Design: An Introduction to Human Factors in Medical Devices. FDA; Julian H. Braybook (ed.). Biocompatibility assessment of medical devices and materials. John Wiley & Sons, Wipro Technologies Page 18 of 20
19 About the Author Ganeshkumar Palanichamy is working at Wipro Technologies since September 2007 as a Senior Project Engineer, having 5 years of working experience in the CAD/CAM domain with expertise in Pressure Die Casting and Plastic Injection Molding. He is currently doing PhD in Plastic Injection Molding. Wipro Technologies Page 19 of 20
20 About Wipro Technologies Wipro is the first PCMM Level 5 and SEI CMMi Level 5 certified R & D, IT and Enterprise Services Company globally. Wipro provides comprehensive IT solutions and services (including systems integration, IS outsourcing, package implementation, software application development and maintenance) and Research & Development services (hardware and software design, development and implementation) to corporations globally. Wipro's unique value proposition is further delivered through our pioneering Offshore Outsourcing Model and stringent Quality Processes of SEI and Six Sigma. Copyright Wipro Technologies. All rights reserved. No part of this document may be reproduced, stored in a retrieval system, transmitted in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without express written permission from Wipro Technologies. Specifications subject to change without notice. All other trademarks mentioned herein are the property of their respective owners. Specifications subject to change without notice. Wipro Technologies Page 20 of 20
Risk Assessment for Medical Devices. Linda Braddon, Ph.D. Bring your medical device to market faster 1
 Risk Assessment for Medical Devices Linda Braddon, Ph.D. Bring your medical device to market faster 1 My Perspective Work with start up medical device companies Goal: Making great ideas into profitable
Risk Assessment for Medical Devices Linda Braddon, Ph.D. Bring your medical device to market faster 1 My Perspective Work with start up medical device companies Goal: Making great ideas into profitable
Controlling Risks Risk Assessment
 Controlling Risks Risk Assessment Hazard/Risk Assessment Having identified the hazards, one must assess the risks by considering the severity and likelihood of bad outcomes. If the risks are not sufficiently
Controlling Risks Risk Assessment Hazard/Risk Assessment Having identified the hazards, one must assess the risks by considering the severity and likelihood of bad outcomes. If the risks are not sufficiently
Software-based medical devices from defibrillators
 C O V E R F E A T U R E Coping with Defective Software in Medical Devices Steven R. Rakitin Software Quality Consulting Inc. Embedding defective software in medical devices increases safety risks. Given
C O V E R F E A T U R E Coping with Defective Software in Medical Devices Steven R. Rakitin Software Quality Consulting Inc. Embedding defective software in medical devices increases safety risks. Given
WHITEPAPER: SOFTWARE APPS AS MEDICAL DEVICES THE REGULATORY LANDSCAPE
 WHITEPAPER: SOFTWARE APPS AS MEDICAL DEVICES THE REGULATORY LANDSCAPE White paper produced by Maetrics For more information, please contact global sales +1 610 458 9312 +1 877 623 8742 [email protected]
WHITEPAPER: SOFTWARE APPS AS MEDICAL DEVICES THE REGULATORY LANDSCAPE White paper produced by Maetrics For more information, please contact global sales +1 610 458 9312 +1 877 623 8742 [email protected]
Designing an Effective Risk Matrix
 Designing an Effective Risk Matrix HENRY OZOG INTRODUCTION Risk assessment is an effective means of identifying process safety risks and determining the most cost-effective means to reduce risk. Many organizations
Designing an Effective Risk Matrix HENRY OZOG INTRODUCTION Risk assessment is an effective means of identifying process safety risks and determining the most cost-effective means to reduce risk. Many organizations
ISO 14971: Overview of the standard
 FDA Medical Device Industry Coalition ISO 14971: Overview of the standard Risk Management Through Product Life Cycle: An Educational Forum William A. Hyman Department of Biomedical Engineering Texas A&M
FDA Medical Device Industry Coalition ISO 14971: Overview of the standard Risk Management Through Product Life Cycle: An Educational Forum William A. Hyman Department of Biomedical Engineering Texas A&M
University of Paderborn Software Engineering Group II-25. Dr. Holger Giese. University of Paderborn Software Engineering Group. External facilities
 II.2 Life Cycle and Safety Safety Life Cycle: The necessary activities involving safety-related systems, occurring during a period of time that starts at the concept phase of a project and finishes when
II.2 Life Cycle and Safety Safety Life Cycle: The necessary activities involving safety-related systems, occurring during a period of time that starts at the concept phase of a project and finishes when
Quality Risk Management The Pharmaceutical Experience Ann O Mahony Quality Assurance Specialist Pfizer Biotech Grange Castle
 Quality Risk Management 11 November 2011 Galway, Ireland Quality Risk Management The Pharmaceutical Experience Ann O Mahony Quality Assurance Specialist Pfizer Biotech Grange Castle Overview Regulatory
Quality Risk Management 11 November 2011 Galway, Ireland Quality Risk Management The Pharmaceutical Experience Ann O Mahony Quality Assurance Specialist Pfizer Biotech Grange Castle Overview Regulatory
3.4.4 Description of risk management plan Unofficial Translation Only the Thai version of the text is legally binding.
 - 1 - Regulation of Department of Industrial Works Re: Criteria for hazard identification, risk assessment, and establishment of risk management plan B.E. 2543 (2000) ---------------------------- Pursuant
- 1 - Regulation of Department of Industrial Works Re: Criteria for hazard identification, risk assessment, and establishment of risk management plan B.E. 2543 (2000) ---------------------------- Pursuant
On-Site Risk Management Audit Checklist for Program Level 3 Process
 On-Site Risk Management Audit Checklist for Program Level 3 Process Auditor name: Date: I. Facility Information: Facility name: Facility location: County: Contact name: RMP Facility I.D. Phone Number:
On-Site Risk Management Audit Checklist for Program Level 3 Process Auditor name: Date: I. Facility Information: Facility name: Facility location: County: Contact name: RMP Facility I.D. Phone Number:
FINAL DOCUMENT. Implementation of risk management principles and activities within a Quality Management System. The Global Harmonization Task Force
 GHTF/SG3/N15R8 FINAL DOCUMENT Title: Implementation of risk management principles and activities within a Quality Management System Authoring Group: GHTF Study Group 3 Endorsed by: The Global Harmonization
GHTF/SG3/N15R8 FINAL DOCUMENT Title: Implementation of risk management principles and activities within a Quality Management System Authoring Group: GHTF Study Group 3 Endorsed by: The Global Harmonization
Hazard/Risk Identification and Control Procedure
 Hazard/Risk Identification and Control Procedure Introduction Hazard identification and the steps taken to minimize the risks associated with identified hazards are a critical component of working safely.
Hazard/Risk Identification and Control Procedure Introduction Hazard identification and the steps taken to minimize the risks associated with identified hazards are a critical component of working safely.
Application of Quality Risk Management to Pharmaceutical Operations. Eldon Henson, Vice President, Quality Operations
 Application of Quality Risk Management to Pharmaceutical Operations Eldon Henson, Vice President, Quality Operations Key Topics of Discussion Definition of Quality Risk Management (QRM) Overview of PDA
Application of Quality Risk Management to Pharmaceutical Operations Eldon Henson, Vice President, Quality Operations Key Topics of Discussion Definition of Quality Risk Management (QRM) Overview of PDA
Risk Assessment Tools for Identifying Hazards and Evaluating Risks Associated with IVD Assays
 Risk Assessment Tools for Identifying Hazards and Evaluating Risks Associated with IVD Assays Robert C. Menson, PhD AACC Annual Meeting Philadelphia, PA 22 July 2003 What Risks Must Be Managed? Risk to
Risk Assessment Tools for Identifying Hazards and Evaluating Risks Associated with IVD Assays Robert C. Menson, PhD AACC Annual Meeting Philadelphia, PA 22 July 2003 What Risks Must Be Managed? Risk to
Medical Software Development. International standards requirements and practice
 Medical Software Development International standards requirements and practice Food and Drug Administration What? A public health agency Why? Protect American consumers How? By enforcing the Federal Food,
Medical Software Development International standards requirements and practice Food and Drug Administration What? A public health agency Why? Protect American consumers How? By enforcing the Federal Food,
LSST Hazard Analysis Plan
 LSST Hazard Analysis Plan Large Synoptic Survey Telescope 950 N. Cherry Avenue Tucson, AZ 85719 www.lsst.org 1. REVISION SUMMARY: Contents 1 Introduction... 5 2 Definition of Terms... 5 2.1 System... 5
LSST Hazard Analysis Plan Large Synoptic Survey Telescope 950 N. Cherry Avenue Tucson, AZ 85719 www.lsst.org 1. REVISION SUMMARY: Contents 1 Introduction... 5 2 Definition of Terms... 5 2.1 System... 5
Hazard Analysis and Critical Control Points (HACCP) 1 Overview
 Manufacturing Technology Committee Risk Management Working Group Risk Management Training Guides Hazard Analysis and Critical Control Points (HACCP) 1 Overview Hazard Analysis and Critical Control Point
Manufacturing Technology Committee Risk Management Working Group Risk Management Training Guides Hazard Analysis and Critical Control Points (HACCP) 1 Overview Hazard Analysis and Critical Control Point
RISK MANAGEMENT FOR INFRASTRUCTURE
 RISK MANAGEMENT FOR INFRASTRUCTURE CONTENTS 1.0 PURPOSE & SCOPE 2.0 DEFINITIONS 3.0 FLOWCHART 4.0 PROCEDURAL TEXT 5.0 REFERENCES 6.0 ATTACHMENTS This document is the property of Thiess Infraco and all
RISK MANAGEMENT FOR INFRASTRUCTURE CONTENTS 1.0 PURPOSE & SCOPE 2.0 DEFINITIONS 3.0 FLOWCHART 4.0 PROCEDURAL TEXT 5.0 REFERENCES 6.0 ATTACHMENTS This document is the property of Thiess Infraco and all
A System-safety process for by-wire automotive systems
 A System-safety process for by-wire automotive systems Steer-by-wire and other by-wire systems (as defined in this article) offer many passive and active safety advantages. To help ensure these advantages
A System-safety process for by-wire automotive systems Steer-by-wire and other by-wire systems (as defined in this article) offer many passive and active safety advantages. To help ensure these advantages
A Risk Management Capability Model for use in Medical Device Companies
 A Risk Management Capability Model for use in Medical Device Companies John Burton Vitalograph Ltd. Gort Rd. Business Park Ennis Ireland 353.65.686471 [email protected] Fergal Mc Caffery Lero
A Risk Management Capability Model for use in Medical Device Companies John Burton Vitalograph Ltd. Gort Rd. Business Park Ennis Ireland 353.65.686471 [email protected] Fergal Mc Caffery Lero
A Risk Management Standard
 A Risk Management Standard Introduction This Risk Management Standard is the result of work by a team drawn from the major risk management organisations in the UK, including the Institute of Risk management
A Risk Management Standard Introduction This Risk Management Standard is the result of work by a team drawn from the major risk management organisations in the UK, including the Institute of Risk management
Risk Assessment and Management. Allen L. Burgenson Manager, Regulatory Affairs Lonza Walkersville Inc.
 Risk Assessment and Management Allen L. Burgenson Manager, Regulatory Affairs Lonza Walkersville Inc. Standard Disclaimer Standard Disclaimer: This presentation is the opinion of the presenter, and does
Risk Assessment and Management Allen L. Burgenson Manager, Regulatory Affairs Lonza Walkersville Inc. Standard Disclaimer Standard Disclaimer: This presentation is the opinion of the presenter, and does
With the introduction of GAMP 5, A
 Online Exclusive from PHARMACEUTICAL ENGINEERING The Official Magazine of ISPE January/February 2012, Vol. 32 No. 1 www.pharmaceuticalengineering.org Copyright ISPE 2012 This article presents the case
Online Exclusive from PHARMACEUTICAL ENGINEERING The Official Magazine of ISPE January/February 2012, Vol. 32 No. 1 www.pharmaceuticalengineering.org Copyright ISPE 2012 This article presents the case
Quality Risk Management - The Medical Device Experience. Niamh Nolan Principal Design Assurance Engineer Boston Scientific
 Quality Risk Management - The Medical Device Experience Niamh Nolan Principal Design Assurance Engineer Boston Scientific Agenda Intent of Risk Management (RM) and Associated Regulations Overview of RM
Quality Risk Management - The Medical Device Experience Niamh Nolan Principal Design Assurance Engineer Boston Scientific Agenda Intent of Risk Management (RM) and Associated Regulations Overview of RM
QUALITY RISK MANAGEMENT (QRM): A REVIEW
 Lotlikar et al Journal of Drug Delivery & Therapeutics; 2013, 3(2), 149-154 149 Available online at http://jddtonline.info REVIEW ARTICLE QUALITY RISK MANAGEMENT (QRM): A REVIEW Lotlikar MV Head Corporate
Lotlikar et al Journal of Drug Delivery & Therapeutics; 2013, 3(2), 149-154 149 Available online at http://jddtonline.info REVIEW ARTICLE QUALITY RISK MANAGEMENT (QRM): A REVIEW Lotlikar MV Head Corporate
SUPPLIER QUALITY MANAGEMENT SYSTEM QUESTIONNAIRE
 Company Name Street Address City, State, Zip code Phone Number Fax Company Website Email Address ORGANIZATION NAME PHONE NUMBER EMAIL ADDRESS President/CEO General Manager Engineering Manager Production
Company Name Street Address City, State, Zip code Phone Number Fax Company Website Email Address ORGANIZATION NAME PHONE NUMBER EMAIL ADDRESS President/CEO General Manager Engineering Manager Production
AEROSPACE STANDARD. Quality Management Systems - Requirements for Aviation, Space and Defense Organizations RATIONALE
 AEROSPACE STANDARD AS9100C Issued 1999-11 Revised 2009-01 Superseding AS9100B Quality Management Systems - Requirements for Aviation, Space and Defense Organizations RATIONALE This standard has been revised
AEROSPACE STANDARD AS9100C Issued 1999-11 Revised 2009-01 Superseding AS9100B Quality Management Systems - Requirements for Aviation, Space and Defense Organizations RATIONALE This standard has been revised
Media fills Periodic performance qualification (Re-Validation)
 Media fills Periodic performance qualification (Re-Validation) Minimum number of Simulations Number of units Contaminated Units Action a Two per Year (Retrospective & Prospective Validation) < 5000 5000
Media fills Periodic performance qualification (Re-Validation) Minimum number of Simulations Number of units Contaminated Units Action a Two per Year (Retrospective & Prospective Validation) < 5000 5000
3.0 Risk Assessment and Analysis Techniques and Tools
 3.0 Risk Assessment and Analysis Techniques and Tools Risks are determined in terms of the likelihood that an uncontrolled event will occur and the consequences of that event occurring. Risk = Likelihood
3.0 Risk Assessment and Analysis Techniques and Tools Risks are determined in terms of the likelihood that an uncontrolled event will occur and the consequences of that event occurring. Risk = Likelihood
FINAL DOCUMENT. Global Harmonization Task Force
 FINAL DOCUMENT Global Harmonization Task Force Title: Quality management system Medical Devices Guidance on corrective action and preventive action and related QMS processes Authoring Group: Study Group
FINAL DOCUMENT Global Harmonization Task Force Title: Quality management system Medical Devices Guidance on corrective action and preventive action and related QMS processes Authoring Group: Study Group
Risk Management for Medical Devices
 Risk Management for Medical Devices CĂLIN CORCIOVĂ, MARIUS TURNEA, RADU CIORAP Department Biomedical Science Faculty of Medical Bioengineering, Grigore T. Popa University of Medicine and Pharmacy - Iasi
Risk Management for Medical Devices CĂLIN CORCIOVĂ, MARIUS TURNEA, RADU CIORAP Department Biomedical Science Faculty of Medical Bioengineering, Grigore T. Popa University of Medicine and Pharmacy - Iasi
FDA Perspectives on Human Factors in Device Development
 FDA Perspectives on in Device Development Molly Follette Story, PhD FDA /CDRH / ODE Understanding Regulatory Requirements for Usability Testing RAPS Webinar June 7, 2012 Overview What are human factors
FDA Perspectives on in Device Development Molly Follette Story, PhD FDA /CDRH / ODE Understanding Regulatory Requirements for Usability Testing RAPS Webinar June 7, 2012 Overview What are human factors
Empowering the Quality and Regulatory Compliance Functions
 Empowering the Quality and Regulatory Compliance Functions Management must take steps to ensure that regulatory and quality compliance is everyone s responsibility. By: J. Glenn George, Kenneth Imler,
Empowering the Quality and Regulatory Compliance Functions Management must take steps to ensure that regulatory and quality compliance is everyone s responsibility. By: J. Glenn George, Kenneth Imler,
Failure Modes & Effects Analysis
 The Failure Modes and Effects Analysis (FMEA), also known as Failure Modes, Effects, and Criticality Analysis (FMECA), is a systematic method by which potential failures of a product or process design
The Failure Modes and Effects Analysis (FMEA), also known as Failure Modes, Effects, and Criticality Analysis (FMECA), is a systematic method by which potential failures of a product or process design
Basic Fundamentals Of Safety Instrumented Systems
 September 2005 DVC6000 SIS Training Course 1 Basic Fundamentals Of Safety Instrumented Systems Overview Definitions of basic terms Basics of safety and layers of protection Basics of Safety Instrumented
September 2005 DVC6000 SIS Training Course 1 Basic Fundamentals Of Safety Instrumented Systems Overview Definitions of basic terms Basics of safety and layers of protection Basics of Safety Instrumented
Risk Management in IEC 60601-1 3 rd Edition. Presented by Alberto Paduanelli Medical Devices Lead Auditor, MHS-UK, TÜV SÜD Product Service
 Risk Management in IEC 60601-1 3 rd Edition Presented by Alberto Paduanelli Medical Devices Lead Auditor, MHS-UK, TÜV SÜD Product Service General Information Time of presentation: 50-60 min. Questions
Risk Management in IEC 60601-1 3 rd Edition Presented by Alberto Paduanelli Medical Devices Lead Auditor, MHS-UK, TÜV SÜD Product Service General Information Time of presentation: 50-60 min. Questions
Occupational safety risk management in Australian mining
 IN-DEPTH REVIEW Occupational Medicine 2004;54:311 315 doi:10.1093/occmed/kqh074 Occupational safety risk management in Australian mining J. Joy Abstract Key words In the past 15 years, there has been a
IN-DEPTH REVIEW Occupational Medicine 2004;54:311 315 doi:10.1093/occmed/kqh074 Occupational safety risk management in Australian mining J. Joy Abstract Key words In the past 15 years, there has been a
Quality Risk Management Tools Quality Risk Management Tool Selection When to Select FMEA: QRM Tool Selection Matrix
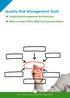 Quality Risk Management Tools Quality Risk Management Tool Selection When to Select FMEA: QRM Tool Selection Matrix 26 Quality Risk Management Tools The ICH Q9 guideline, Quality Risk Management, provides
Quality Risk Management Tools Quality Risk Management Tool Selection When to Select FMEA: QRM Tool Selection Matrix 26 Quality Risk Management Tools The ICH Q9 guideline, Quality Risk Management, provides
Guidance for Industry: Quality Risk Management
 Guidance for Industry: Quality Risk Management Version 1.0 Drug Office Department of Health Contents 1. Introduction... 3 2. Purpose of this document... 3 3. Scope... 3 4. What is risk?... 4 5. Integrating
Guidance for Industry: Quality Risk Management Version 1.0 Drug Office Department of Health Contents 1. Introduction... 3 2. Purpose of this document... 3 3. Scope... 3 4. What is risk?... 4 5. Integrating
Risk Management and the Impact of EN ISO 14971:2012 Annex Z
 Risk Management and the Impact of EN ISO 14971:2012 Annex Z BSI 2014 Medical Device Mini-Roadshow Ibim Tariah Ph.D Technical Director, Healthcare Solutions Copyright 2014 BSI. All rights reserved. 1 Risk
Risk Management and the Impact of EN ISO 14971:2012 Annex Z BSI 2014 Medical Device Mini-Roadshow Ibim Tariah Ph.D Technical Director, Healthcare Solutions Copyright 2014 BSI. All rights reserved. 1 Risk
Quality Risk Management ICH Q9 & ISO 14971. Presented by Michael Kerr 11 th November 2011
 Quality Risk Management ICH Q9 & ISO 14971 Presented by Michael Kerr 11 th November 2011 Agenda Risk Concept QRM Fundamentals Regulatory Expectations Warning Letters / Observations Application of QRM Introduction:
Quality Risk Management ICH Q9 & ISO 14971 Presented by Michael Kerr 11 th November 2011 Agenda Risk Concept QRM Fundamentals Regulatory Expectations Warning Letters / Observations Application of QRM Introduction:
Your Software Quality is Our Business. INDEPENDENT VERIFICATION AND VALIDATION (IV&V) WHITE PAPER Prepared by Adnet, Inc.
 INDEPENDENT VERIFICATION AND VALIDATION (IV&V) WHITE PAPER Prepared by Adnet, Inc. February 2013 1 Executive Summary Adnet is pleased to provide this white paper, describing our approach to performing
INDEPENDENT VERIFICATION AND VALIDATION (IV&V) WHITE PAPER Prepared by Adnet, Inc. February 2013 1 Executive Summary Adnet is pleased to provide this white paper, describing our approach to performing
Guidance for Industry
 Guidance for Industry Q9 Quality Risk Management U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation
Guidance for Industry Q9 Quality Risk Management U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation
Improved Utilization of Self-Inspection Programs within the GMP Environment A Quality Risk Management Approach
 Improved Utilization of Self-Inspection Programs within the GMP Environment A Quality Risk Management Approach Barbara Jeroncic Self-inspection is a well-established and vital part of the pharmaceutical
Improved Utilization of Self-Inspection Programs within the GMP Environment A Quality Risk Management Approach Barbara Jeroncic Self-inspection is a well-established and vital part of the pharmaceutical
How To Improve Safety In The Nhs
 PS035 Original research completed in 2010 Briefing Paper Introduction: Principal Investigator: Professor John Clarkson Should the NHS adopt a new system for predicting possible risks to patient safety?
PS035 Original research completed in 2010 Briefing Paper Introduction: Principal Investigator: Professor John Clarkson Should the NHS adopt a new system for predicting possible risks to patient safety?
Best Practice In A Change Management System
 Quality & Compliance Associates, LLC Best Practice In A Change Management System President Quality & Compliance Associates, LLC Change Control and Its Role in a Continuous Improvement Environment 3 Benefits
Quality & Compliance Associates, LLC Best Practice In A Change Management System President Quality & Compliance Associates, LLC Change Control and Its Role in a Continuous Improvement Environment 3 Benefits
Design Verification The Case for Verification, Not Validation
 Overview: The FDA requires medical device companies to verify that all the design outputs meet the design inputs. The FDA also requires that the final medical device must be validated to the user needs.
Overview: The FDA requires medical device companies to verify that all the design outputs meet the design inputs. The FDA also requires that the final medical device must be validated to the user needs.
Should Costing Version 1.1
 Should Costing Identify should cost elements early in the design phase, and enable cost down initiatives Version 1.1 August, 2010 WHITE PAPER Copyright Notice Geometric Limited. All rights reserved. No
Should Costing Identify should cost elements early in the design phase, and enable cost down initiatives Version 1.1 August, 2010 WHITE PAPER Copyright Notice Geometric Limited. All rights reserved. No
Effective Software Verification for Medical Devices
 STERLINGTECH AND KLOCWORK WHITE PAPER NOVEMBER 2009 Effective Software Verification for Medical Devices Achieving compliance and meeting productivity goals with static analysis In addition to producing
STERLINGTECH AND KLOCWORK WHITE PAPER NOVEMBER 2009 Effective Software Verification for Medical Devices Achieving compliance and meeting productivity goals with static analysis In addition to producing
Title: OHS Risk Management Procedure
 Issue Date: July 2011 Review Date: July 2013 Page Number: 1 of 9 1. Purpose: To outline the methodology by which Department of Education and Early Childhood Development (DEECD) identifies, assesses, controls
Issue Date: July 2011 Review Date: July 2013 Page Number: 1 of 9 1. Purpose: To outline the methodology by which Department of Education and Early Childhood Development (DEECD) identifies, assesses, controls
The FDA Perspective on Human Factors in Medical Device Software Development
 The FDA Perspective on in Medical Device Development Molly Follette Story, PhD FDA /CDRH / ODE 2012 IQPC Design for Medical Devices Europe Munich, Germany Februar y1, 2012 Overview Guidance for FDA premarket
The FDA Perspective on in Medical Device Development Molly Follette Story, PhD FDA /CDRH / ODE 2012 IQPC Design for Medical Devices Europe Munich, Germany Februar y1, 2012 Overview Guidance for FDA premarket
Standardization in the Outsourcing Industry
 Standardization in the Outsourcing Industry November 2010 Outsourcing provides rapid business transformation and cost reductions through labor arbitrage and consolidation of business processes spread across
Standardization in the Outsourcing Industry November 2010 Outsourcing provides rapid business transformation and cost reductions through labor arbitrage and consolidation of business processes spread across
ELECTRICAL SAFETY RISK ASSESSMENT
 ELECTRICAL SAFETY RISK ASSESSMENT The intent of this procedure is to perform a risk assessment, which includes a review of the electrical hazards, the associated foreseeable tasks, and the protective measures
ELECTRICAL SAFETY RISK ASSESSMENT The intent of this procedure is to perform a risk assessment, which includes a review of the electrical hazards, the associated foreseeable tasks, and the protective measures
Quality Risk Management Principles and Industry Case Studies
 Final Draft Rev. December 28, 2008 Quality Risk Management Principles and Industry Case Studies T. Frank 1, S. Brooks 2, R. Creekmore 3, B. Hasselbalch 4, K. Murray 5, K. Obeng 6, S. Reich 5, E. Sanchez
Final Draft Rev. December 28, 2008 Quality Risk Management Principles and Industry Case Studies T. Frank 1, S. Brooks 2, R. Creekmore 3, B. Hasselbalch 4, K. Murray 5, K. Obeng 6, S. Reich 5, E. Sanchez
identify hazards, analyze or evaluate the risk associated with that hazard, and determine appropriate ways to eliminate or control the hazard.
 What is a risk assessment? Risk assessment is the process where you: identify hazards, analyze or evaluate the risk associated with that hazard, and determine appropriate ways to eliminate or control the
What is a risk assessment? Risk assessment is the process where you: identify hazards, analyze or evaluate the risk associated with that hazard, and determine appropriate ways to eliminate or control the
Guidance for Industry. Q10 Pharmaceutical Quality System
 Guidance for Industry Q10 Pharmaceutical Quality System U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation
Guidance for Industry Q10 Pharmaceutical Quality System U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation
CONCEPTS OF FOOD SAFETY QUALITY MANAGEMENT SYSTEMS. Mrs. Malini Rajendran
 CONCEPTS OF FOOD SAFETY AND QUALITY MANAGEMENT SYSTEMS Mrs. Malini Rajendran Brief background 1963 - The Codex Alimentarius Commission was created by FAO and WHO to develop food standards, guidelines and
CONCEPTS OF FOOD SAFETY AND QUALITY MANAGEMENT SYSTEMS Mrs. Malini Rajendran Brief background 1963 - The Codex Alimentarius Commission was created by FAO and WHO to develop food standards, guidelines and
Overview of Medical Device Design Controls in the US. By Nandini Murthy, MS, RAC
 Overview of Medical Device Controls in the US By Nandini Murthy, MS, RAC 18 controls are a regulatory requirement for medical devices. In the US, compliance with the design controls section of 21 Code
Overview of Medical Device Controls in the US By Nandini Murthy, MS, RAC 18 controls are a regulatory requirement for medical devices. In the US, compliance with the design controls section of 21 Code
Overview of International Medical Device Human Factors Standards. Ed Israelski PhD, Director of Human Factors Abbott Abbott Park, IL, USA
 Overview of International Medical Device Human Factors Standards Ed Israelski PhD, Director of Human Factors Abbott Abbott Park, IL, USA Outline History of Medical Device Human Factors Standards Summary
Overview of International Medical Device Human Factors Standards Ed Israelski PhD, Director of Human Factors Abbott Abbott Park, IL, USA Outline History of Medical Device Human Factors Standards Summary
Quality Risk Management Understanding and Control the Risk in Pharmaceutical Manufacturing Industry
 International Journal of Pharmaceutical Science Invention ISSN (Online): 2319 6718, ISSN (Print): 2319 670X Volume 4 Issue 1 January 2015 PP.29-41 Quality Risk Management Understanding and Control the
International Journal of Pharmaceutical Science Invention ISSN (Online): 2319 6718, ISSN (Print): 2319 670X Volume 4 Issue 1 January 2015 PP.29-41 Quality Risk Management Understanding and Control the
FMEA Failure Risk Scoring Schemes
 FMEA Failure Risk Scoring Schemes 1-10 Scoring for Severity, Occurrence and Detection CATEGORY Severity 10 9 8 7 6 5 3 2 1 Hazardous, without warning Hazardous, with warning Very High High Moderate Low
FMEA Failure Risk Scoring Schemes 1-10 Scoring for Severity, Occurrence and Detection CATEGORY Severity 10 9 8 7 6 5 3 2 1 Hazardous, without warning Hazardous, with warning Very High High Moderate Low
SAFETY LIFECYCLE WORKBOOK FOR THE PROCESS INDUSTRY SECTOR
 SAFETY LIFECYCLE WORKBOOK FOR THE PROCESS INDUSTRY SECTOR SAFETY LIFECYCLE WORKBOOK FOR THE PROCESS INDUSTRY SECTOR The information and any recommendations that may be provided herein are not intended
SAFETY LIFECYCLE WORKBOOK FOR THE PROCESS INDUSTRY SECTOR SAFETY LIFECYCLE WORKBOOK FOR THE PROCESS INDUSTRY SECTOR The information and any recommendations that may be provided herein are not intended
a Medical Device Privacy Consortium White Paper
 a Medical Device Privacy Consortium White Paper Introduction The Medical Device Privacy Consortium (MDPC) is a group of leading companies addressing health privacy and security issues affecting the medical
a Medical Device Privacy Consortium White Paper Introduction The Medical Device Privacy Consortium (MDPC) is a group of leading companies addressing health privacy and security issues affecting the medical
The purpose of this Supplier Quality Standard is to communicate the expectations and requirements of Baxter Healthcare Corporation to its suppliers.
 Supplier Quality Standard 1.0 Purpose The purpose of this Supplier Quality Standard is to communicate the expectations and requirements of Baxter Healthcare Corporation to its suppliers. These expectations
Supplier Quality Standard 1.0 Purpose The purpose of this Supplier Quality Standard is to communicate the expectations and requirements of Baxter Healthcare Corporation to its suppliers. These expectations
How To Write Software
 1 Medical Device Software - Software Life Cycle Processes IEC 62304 2 Credits John F. Murray Software Compliance Expert U.S. Food and Drug Administration Marcie R. Williams Medical Device Fellow Ph.D.
1 Medical Device Software - Software Life Cycle Processes IEC 62304 2 Credits John F. Murray Software Compliance Expert U.S. Food and Drug Administration Marcie R. Williams Medical Device Fellow Ph.D.
CAPA - the importance of data analysis
 CAPA - the importance of data analysis Presented by: Sue Jacobs QMS Consulting, Inc. 1 847 359 4456 [email protected] QMS Consulting, Inc. 2007 1 Topics Regulatory Requirements Design Controls and
CAPA - the importance of data analysis Presented by: Sue Jacobs QMS Consulting, Inc. 1 847 359 4456 [email protected] QMS Consulting, Inc. 2007 1 Topics Regulatory Requirements Design Controls and
GUIDANCE NOTE FOR MANUFACTURERS OF CUSTOM-MADE MEDICAL DEVICES
 GUIDANCE NOTE FOR MANUFACTURERS OF CUSTOM-MADE MEDICAL DEVICES Foreword This guidance document is informative and advisory and has no legal authority. Individual national enforcement authorities are bound
GUIDANCE NOTE FOR MANUFACTURERS OF CUSTOM-MADE MEDICAL DEVICES Foreword This guidance document is informative and advisory and has no legal authority. Individual national enforcement authorities are bound
Customer Interaction Analytics Speech Analytics The Next Frontier
 Customer Interaction Analytics Speech Analytics The Next Frontier www.wipro.com RAJESH SEHGAL & SHALABH SRIVASTAVA PROCESS LAB, MISSION QUALITY & OPERATIONAL EXCELLENCE, WIPRO BPO Table of Contents Customer
Customer Interaction Analytics Speech Analytics The Next Frontier www.wipro.com RAJESH SEHGAL & SHALABH SRIVASTAVA PROCESS LAB, MISSION QUALITY & OPERATIONAL EXCELLENCE, WIPRO BPO Table of Contents Customer
Accident Investigation
 Accident Investigation ACCIDENT INVESTIGATION/adentcvr.cdr/1-95 ThisdiscussionistakenfromtheU.S.Department oflabor,minesafetyandhealthadministration Safety Manual No. 10, Accident Investigation, Revised
Accident Investigation ACCIDENT INVESTIGATION/adentcvr.cdr/1-95 ThisdiscussionistakenfromtheU.S.Department oflabor,minesafetyandhealthadministration Safety Manual No. 10, Accident Investigation, Revised
PHARMACEUTICAL QUALITY SYSTEM Q10
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE PHARMACEUTICAL QUALITY SYSTEM Q10 Current Step
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE PHARMACEUTICAL QUALITY SYSTEM Q10 Current Step
IFS Food Safety and Quality Management System
 This is an ideal package for Food Manufacturers looking to meet the requirements of the IFS Food Standard for quality and food safety of food products. Ensure your Food Safety & Quality Management System
This is an ideal package for Food Manufacturers looking to meet the requirements of the IFS Food Standard for quality and food safety of food products. Ensure your Food Safety & Quality Management System
Introduction into IEC 62304 Software life cycle for medical devices
 Introduction into IEC 62304 Software life cycle for medical devices Christoph Gerber 4. September 2008 SPIQ 9/5/2008 1 Agenda Current Picture Regulatory requirements for medical device software IEC 62304
Introduction into IEC 62304 Software life cycle for medical devices Christoph Gerber 4. September 2008 SPIQ 9/5/2008 1 Agenda Current Picture Regulatory requirements for medical device software IEC 62304
FMEA and FTA Analysis
 FMEA and FTA Analysis Why it is Coming to Your Hospital and Your Laboratory Tina A. Krenc Director, R&D Phase Systems Abbott Laboratories 1 Agenda Background on requirements for risk management Tools to
FMEA and FTA Analysis Why it is Coming to Your Hospital and Your Laboratory Tina A. Krenc Director, R&D Phase Systems Abbott Laboratories 1 Agenda Background on requirements for risk management Tools to
Title: Rio Tinto management system
 Standard Rio Tinto management system December 2014 Group Title: Rio Tinto management system Document No: HSEC-B-01 Standard Function: Health, Safety, Environment and Communities (HSEC) No. of pages: 23
Standard Rio Tinto management system December 2014 Group Title: Rio Tinto management system Document No: HSEC-B-01 Standard Function: Health, Safety, Environment and Communities (HSEC) No. of pages: 23
Failure Analysis Methods What, Why and How. MEEG 466 Special Topics in Design Jim Glancey Spring, 2006
 Failure Analysis Methods What, Why and How MEEG 466 Special Topics in Design Jim Glancey Spring, 2006 Failure Analysis Methods Every product or process has modes of failure. An analysis of potential failures
Failure Analysis Methods What, Why and How MEEG 466 Special Topics in Design Jim Glancey Spring, 2006 Failure Analysis Methods Every product or process has modes of failure. An analysis of potential failures
Quality Management System Manual
 Quality Management System Manual Assurance ISO / AS Manual Quality Management System EXCEEDING ALL EXPECTATIONS Since our inception in 1965, Swiss-Tech has supplied the medical, aerospace, hydraulic, electronic
Quality Management System Manual Assurance ISO / AS Manual Quality Management System EXCEEDING ALL EXPECTATIONS Since our inception in 1965, Swiss-Tech has supplied the medical, aerospace, hydraulic, electronic
How To Manage Risk
 1. Purpose [Name of Program] [Year] Risk Management Plan The purpose of the Risk Management Program is to support the mission and vision of [Name of Program] as it pertains to clinical risk and consumer
1. Purpose [Name of Program] [Year] Risk Management Plan The purpose of the Risk Management Program is to support the mission and vision of [Name of Program] as it pertains to clinical risk and consumer
A System-Safety Process For By-Wire Automotive Systems
 SAE TECHNICAL PAPER SERIES 2000-01-1056 A System-Safety Process For By-Wire Automotive Systems Sanket Amberkar, Joseph G. D Ambrosio and Brian T. Murray Delphi Automotive Systems Joseph Wysocki HRL Laboratories
SAE TECHNICAL PAPER SERIES 2000-01-1056 A System-Safety Process For By-Wire Automotive Systems Sanket Amberkar, Joseph G. D Ambrosio and Brian T. Murray Delphi Automotive Systems Joseph Wysocki HRL Laboratories
Core Infrastructure Risk Management Plan
 SHIRE OF MOUNT MAGNET Roads and Buildings Core Infrastructure Risk Management Plan Version 1 May 2013 AM4SRRC Document Control Asset Management for Small, Rural or Remote Communities Document ID: 59_280_110211
SHIRE OF MOUNT MAGNET Roads and Buildings Core Infrastructure Risk Management Plan Version 1 May 2013 AM4SRRC Document Control Asset Management for Small, Rural or Remote Communities Document ID: 59_280_110211
Implementation of ANSI/AAMI/IEC 62304 Medical Device Software Lifecycle Processes.
 Implementation of ANSI/AAMI/IEC 62304 Medical Device Software Lifecycle Processes.. www.pharmout.net Page 1 of 15 Version-02 1. Scope 1.1. Purpose This paper reviews the implementation of the ANSI/AAMI/IEC
Implementation of ANSI/AAMI/IEC 62304 Medical Device Software Lifecycle Processes.. www.pharmout.net Page 1 of 15 Version-02 1. Scope 1.1. Purpose This paper reviews the implementation of the ANSI/AAMI/IEC
How to Use the Design Process to Manage Risk: Elements of Design Controls and Why It Matters
 environmental failure analysis & prevention health technology development How to Use the Design Process to Manage Risk: Elements of Design Controls and Why It Matters Kevin L. Ong, Ph.D., P.E. Managing
environmental failure analysis & prevention health technology development How to Use the Design Process to Manage Risk: Elements of Design Controls and Why It Matters Kevin L. Ong, Ph.D., P.E. Managing
Edwin Lindsay Principal Consultant. Compliance Solutions (Life Sciences) Ltd, Tel: + 44 (0) 7917134922 E-Mail: [email protected].
 Edwin Lindsay Principal Consultant, Tel: + 44 (0) 7917134922 E-Mail: [email protected] There were no guidelines/ regulations There was no training No Procedures No Inspectors Inform All staff of
Edwin Lindsay Principal Consultant, Tel: + 44 (0) 7917134922 E-Mail: [email protected] There were no guidelines/ regulations There was no training No Procedures No Inspectors Inform All staff of
June 2010 HEALTH, SAFETY, AND ENVIRONMENT MANAGEMENT SYSTEM (HSEMS)
 June 2010 HEALTH, SAFETY, AND ENVIRONMENT MANAGEMENT SYSTEM (HSEMS) TABLE OF CONTENTS PAGE PART I INTRODUCTION Corporate Health, Safety and Environment Policy.. 1 Purpose... 2 HSEMS Framework... 3 PART
June 2010 HEALTH, SAFETY, AND ENVIRONMENT MANAGEMENT SYSTEM (HSEMS) TABLE OF CONTENTS PAGE PART I INTRODUCTION Corporate Health, Safety and Environment Policy.. 1 Purpose... 2 HSEMS Framework... 3 PART
ALL PRODUCTS MFG & SUPPLY
 ALL PRODUCTS MFG & SUPPLY 618 ANDERSON DRIVE ROMEOVILLE, IL 60446 PHONE: 877-255-8700 FAX: 877-255-8701 WWW. APGASKET.COM QUALITY MANAGEMENT SYSTEM MANUAL DATE: 11/20/12 REVISION 9.1 UNCONTROLLED COPY
ALL PRODUCTS MFG & SUPPLY 618 ANDERSON DRIVE ROMEOVILLE, IL 60446 PHONE: 877-255-8700 FAX: 877-255-8701 WWW. APGASKET.COM QUALITY MANAGEMENT SYSTEM MANUAL DATE: 11/20/12 REVISION 9.1 UNCONTROLLED COPY
Revision Date Author Description of change. 10 07Jun13 Mark Benton Removed Admin. Manager from approval
 Page 2 of 15 Document Revision History Revision Date Author Description of change 10 07Jun13 Mark Benton Removed Admin. Manager from approval 12Feb13 Mark Benton 08 01Oct12 Mark Benton 07 8/30/2012 Refer
Page 2 of 15 Document Revision History Revision Date Author Description of change 10 07Jun13 Mark Benton Removed Admin. Manager from approval 12Feb13 Mark Benton 08 01Oct12 Mark Benton 07 8/30/2012 Refer
Quality Risk Management in Pharmaceutical Industry: A Review
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.6, No.3, pp 908-914, July-Aug 2014 Quality Risk Management in Pharmaceutical Industry: A Review V Vijayakumar Reddy*,
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.6, No.3, pp 908-914, July-Aug 2014 Quality Risk Management in Pharmaceutical Industry: A Review V Vijayakumar Reddy*,
Risk Management at Chevron
 Risk Management at Chevron Jean Bruney AIChE/SACHE Workshop Context for HES Risk Management Corporation Sets policies & expectations Centers of Expertise Establish processes & verify Oversight Level Global
Risk Management at Chevron Jean Bruney AIChE/SACHE Workshop Context for HES Risk Management Corporation Sets policies & expectations Centers of Expertise Establish processes & verify Oversight Level Global
The use of statistical problem solving methods for Risk Assessment
 The use of statistical problem solving methods for Risk Assessment Citti P., Delogu M., Giorgetti A. University of Florence. DMTI, Department of Mechanics and Industrial Technology Via Santa Marta, 3 50121
The use of statistical problem solving methods for Risk Assessment Citti P., Delogu M., Giorgetti A. University of Florence. DMTI, Department of Mechanics and Industrial Technology Via Santa Marta, 3 50121
Product Liability Risk Control Checklist
 Product Liability Risk Control Checklist Appendix C1 This material will help you evaluate your products liability exposure. It is designed to enable you to focus on areas that need to be addressed to safeguard
Product Liability Risk Control Checklist Appendix C1 This material will help you evaluate your products liability exposure. It is designed to enable you to focus on areas that need to be addressed to safeguard
Quality Risk Management
 PS/INF 1/2010 * * Quality Risk Management Quality Risk Management Implementation of ICH Q9 in the pharmaceutical field an example of methodology from PIC/S Document > Authors: L. Viornery (AFSSAPS) Ph.
PS/INF 1/2010 * * Quality Risk Management Quality Risk Management Implementation of ICH Q9 in the pharmaceutical field an example of methodology from PIC/S Document > Authors: L. Viornery (AFSSAPS) Ph.
IVD Regulation Overview. Requirements to Assure Quality & Effectiveness
 IVD Regulation Overview Requirements to Assure Quality & Effectiveness CLIAC Jan. 2002 Statutory and Regulatory Requirements Statute: Food, Drug, and Cosmetic Act Food and Drugs Act of 1906 Food and Drug
IVD Regulation Overview Requirements to Assure Quality & Effectiveness CLIAC Jan. 2002 Statutory and Regulatory Requirements Statute: Food, Drug, and Cosmetic Act Food and Drugs Act of 1906 Food and Drug
ORACLE CONSULTING GROUP
 ORACLE CONSULTING GROUP An Official United States Agent Firm for Foreign Establishments CONSULTING MEMORANDUM: DEALING WITH A MEDICAL DEVICE IN THE U.S. 5398 Golder Ranch Rd., Ste. 1 Tucson, Arizona 85739
ORACLE CONSULTING GROUP An Official United States Agent Firm for Foreign Establishments CONSULTING MEMORANDUM: DEALING WITH A MEDICAL DEVICE IN THE U.S. 5398 Golder Ranch Rd., Ste. 1 Tucson, Arizona 85739
Hazard Identification, Risk Assessment and Control Procedure
 Hazard Identification, Risk Assessment and Control Procedure 1. Purpose To ensure that there is a formal process for hazard identification, risk assessment and control to effectively manage workplace and
Hazard Identification, Risk Assessment and Control Procedure 1. Purpose To ensure that there is a formal process for hazard identification, risk assessment and control to effectively manage workplace and
MEDICAL DEVICE GUIDANCE
 May 2014 MEDICAL DEVICE GUIDANCE GN-23: Guidance on Labelling for Medical Devices PREFACE This document is intended to provide general guidance. Although we have tried to ensure that the information contained
May 2014 MEDICAL DEVICE GUIDANCE GN-23: Guidance on Labelling for Medical Devices PREFACE This document is intended to provide general guidance. Although we have tried to ensure that the information contained
Hazard Identification and Risk Assessment for the Use of Booster Fans in Underground Coal Mines
 Hazard Identification and Risk Assessment for the Use of Booster Fans in Underground Coal Mines Felipe Calizaya Michael G. Nelson Mahesh Shriwas Feb 26, 2013 Outline Introduction Booster Fans in U/G Coal
Hazard Identification and Risk Assessment for the Use of Booster Fans in Underground Coal Mines Felipe Calizaya Michael G. Nelson Mahesh Shriwas Feb 26, 2013 Outline Introduction Booster Fans in U/G Coal
Safety, Health and Environment Management System Overview
 PLAN EXCEL ACT CHECK Setting the PACE! Safety, Health and Environment Management System Overview 1 Nalco Management System Nalco's Safety, Health and Environment Sustainability Principles Nalco Company
PLAN EXCEL ACT CHECK Setting the PACE! Safety, Health and Environment Management System Overview 1 Nalco Management System Nalco's Safety, Health and Environment Sustainability Principles Nalco Company
Safety Regulation Group SAFETY MANAGEMENT SYSTEMS GUIDANCE TO ORGANISATIONS. April 2008 1
 Safety Regulation Group SAFETY MANAGEMENT SYSTEMS GUIDANCE TO ORGANISATIONS April 2008 1 Contents 1 Introduction 3 2 Management Systems 2.1 Management Systems Introduction 3 2.2 Quality Management System
Safety Regulation Group SAFETY MANAGEMENT SYSTEMS GUIDANCE TO ORGANISATIONS April 2008 1 Contents 1 Introduction 3 2 Management Systems 2.1 Management Systems Introduction 3 2.2 Quality Management System
Software Development for Medical Devices
 Overcoming the Challenges of Compliance, Quality and Cost An MKS White Paper Introduction Software is fast becoming the differentiator for manufacturers of medical devices. The rewards available from software
Overcoming the Challenges of Compliance, Quality and Cost An MKS White Paper Introduction Software is fast becoming the differentiator for manufacturers of medical devices. The rewards available from software
An introduction to designing reliable cloud services
 An introduction to designing reliable cloud services January 2014 Contents Overview 2 Cloud service reliability versus resiliency 4 Recovery-oriented computing 5 Planning for failure 7 Designing for and
An introduction to designing reliable cloud services January 2014 Contents Overview 2 Cloud service reliability versus resiliency 4 Recovery-oriented computing 5 Planning for failure 7 Designing for and
