Quality Risk Management in Pharmaceutical Industry: A Review
|
|
|
- Ashlynn Montgomery
- 9 years ago
- Views:
Transcription
1 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : Vol.6, No.3, pp , July-Aug 2014 Quality Risk Management in Pharmaceutical Industry: A Review V Vijayakumar Reddy*, N Vishal Gupta, H V Raghunandan, U Nitin Kashyap Pharmaceutical Quality Assurance, Department of Pharmaceutics JSS College of Pharmacy, JSS University, Sri ShivarathreeshwaraNagara, Mysore, Karnataka, India *Corres. Author: [email protected] Abstract: This article aims to provide principles and examples of tools for Quality Risk Management (QRM) that can be applied to different aspects of pharmaceutical quality. These aspects include development, manufacturing, distribution, inspection and submission of review processes throughout the lifecycle of drug substances, drug products and biological products.quality Risk Management is an overall and continuing process of minimizing risks to product quality throughout its life-cycle in order to optimize its benefit and balance the risk. It is a systematic process for the evaluation, control, communication and review of risks to the quality of the medicinal product. The basic QRM process should include the level of effort, formality and documentation of QRM process which should commensurate with level of risk. QRM principles are utilized effectively in many areas of business and government including business, insurance, work-related safety, public health, pharmacovigilance and by agencies regulating these industries. The risk assessment process should be carried out by analyzing, identifying and evaluating the risk and the QRM plans should be reviewed after their follow up. The QRM implementation provides documented, clear and reproducible methods to accomplish steps of QRM process based on current knowledge about assessing the probability, severity and sometimes detectability of the risk. Using QRM tools, the pharmaceutical industry and regulators can evaluate, control, communicate and review the risks. Effective QRM implementation can facilitate better and well-versed decisions which can provide regulators with greater assurance of a company s ability to deal with possible risks. Keywords: Quality Risk Management,Risk- based approach, Patient safety, Product quality. Introduction Since a couple of years Quality Risk Management (QRM) has become a mandatory regulatory requirement towards healthcare organizations. QRM is an overall and continuing process of minimizing risks to product quality throughout its life-cycle in order to optimize its benefit and balance the risk. It is a systematic process for the evaluation, control, communication and review of risks to the quality of the medicinal product. It supports science based and practical decisions when integrated into quality systems, examples of quality systems include Validation, Quality Defects - Investigation, Auditing, Inspection, Documentation, Training etc. Quality Risk Management principles are effectively utilized in many areas including business, insurance, work related safety, public health, pharmacovigilance, and by agencies regulating these industries. Even though there are some examples of the use of quality risk management in the pharmaceutical industry, today they are limited and do not represent the full contributions that risk management has to offer. In relation to pharmaceuticals, though there are a variety of stakeholders, including medical practitioners and patients as well as government and industry, the safety of the patient by managing the risk to quality should be considered prime importance. The manufacturing and use of a drug product, including its components, necessarily involve some degree of risk. An effective QRM approach can further ensure the high quality of the drug product to the patient by
2 V Vijayakumar Reddy et al /Int.J. PharmTech Res.2014,6(3),pp identify and control potential quality issues during development and manufacturing. Use of QRM can improve the decision making if a quality problem arises. Effective QRM implementation can facilitate better and wellversed decisions which can provide regulators with greater assurance of a company s ability to deal with possible risks. [ 1,3] Principles of Quality Risk Management [1,2] Four primary principles of QRM are: The assessment of the risk to quality should be based on scientific knowledge and ultimately link to the protection of the patient. QRM should be dynamic, iterative and responsive to change. The level of effort, formality and documentation of the QRM process should be commensurate with the level of risk The capability for continual development and enhancement should be embedded in the QRM process. General Quality Risk Management Process Quality Risk Management is a systematic process for evaluation, control, communication and review of risks to the quality of the drug product across the product lifecycle. Risk can be defined as the combination of the probability of occurrence of harm and the severity of that harm. [4,5] Figure 1: Overview of a typical Quality Risk Management process [1] Initiating a Quality Risk Management Process Quality Risk Management should include systematic processes designed to organize, facilitate and improve science-based decision making with respect to risk. Steps used to initiate and plan a quality risk management process might include the following: [1] Define the problem and/or risk question, including relevant assumptions identify the potential for risk. Assemble background information and/or data on the potential hazard, harm or human health impact applicable to the risk assessment. Specify a timeline, and appropriate level of decision making for the risk management process.
3 V Vijayakumar Reddy et al /Int.J. PharmTech Res.2014,6(3),pp Risk Assessment Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards. It includes risk identification, risk analysis and risk evaluation. [5] Three fundamental questions are often helpful. 1. What might go wrong? 2. What is the possibility that it will go wrong? 3. What are the consequences? Risk identification is a organized use of information to identify hazards referring to the risk. Information can include historical data, theoretical analysis, and the concerns of stakeholders. Risk identification addresses the What might go wrong? question, including identifying the possible consequences. This provides the basis for further steps in the quality risk management process. Risk analysis is the estimation of the risk associated with the identified hazards. It is the qualitative or quantitative process of linking the likelihood of occurrence and severity of harms. In some risk management tools, the ability to detect the harm (detectability) also factors in the estimation of risk. Risk evaluation compares the identified and analyzed risk against given risk criteria. Risk evaluations consider the strength of evidence for all three of the fundamental questions. Different Steps Involved In the Risk Assessment Are [5,6] 1. Collect & organize the information. Gathering relevant information, reviewing appropriate references & identifying assumptions. Tools can be used to categorize the information. Define the limits of the QRM exercise.e 2. Formulate the Risk Question: It is the starting point of the QRM exercise, high level statement outlining the issue & purpose for conducting the QRM exercise including risk factors, the scope of the issue and any related limits or constraints 3. Choose Tool different tools include- Basic risk management facilitation methods (flowcharts, check sheets etc). Failure Mode Effects Analysis and Failure Mode Effects and Criticality Analysis. Fault Tree Analysis. Hazard Analysis and Critical Control Points. Hazard & Operability Analysis. Preliminary Hazard Analysis. Risk Ranking & Filtering. Supporting statistical tools. 4. Identify Risks Factors and Related Hazards A hazard is a failure that could cause potential harm to the patient. Once the hazards are recognized, they can then be categorized into one of five areas: Operator, Environment, System, Reagents, or Specimen. These categories will make it easier to later identify types of controls necessary to reduce unwanted risk.. 5. Define the Risk Components &Scales [4] RISK = PRIORITY * DETECTABILITY * SEVERITY Where, Severity- Criticality of the product.
4 V Vijayakumar Reddy et al /Int.J. PharmTech Res.2014,6(3),pp Priority- Complexity of the site (multi-product). Detection- Audit history. 6. Evaluate the risk for each hazard. This is the step where you decide how often that a failure will occur. 7. Determine acceptability of risks [6,7] Once the risks are assigned, the next step is to look at severity and probability of harm to determine whether the risks are acceptable. 8. Determine Action Threshold A level or value above which an action will take place and below which it will not. 9. Apply the tool Analyze the detailed risks and quantify those risks using the scales for severity, probability and detection to provide a risk score. conclude what actions are required based on the threshold for action. Risk Control Risk control includes decision making to reduce and/or accept risks. The intention of risk control is to reduce the risk to an acceptable level. The amount of effort used for risk control should be proportional to the significance of the risk [12,13] Risk control might focus on the following questions: Is the risk above an acceptable level? What actions might take to reduce or eliminate risks? What is the appropriate balance among benefits, risks and resources? Are new risks introduced as a result of the identified risks being controlled?
5 V Vijayakumar Reddy et al /Int.J. PharmTech Res.2014,6(3),pp Risk reduction focuses on processes for mitigation or avoidance of quality risk when it exceeds a specified level. Risk reduction might include actions taken to mitigate the severity and probability of harm. The implementation of risk reduction measures can introduce new risks into the system or increase the significance of other existing risks. Hence, it might be appropriate to revisit the risk assessment to identify and evaluate any possible change in risk after implementing a risk reduction process. Consider measures/actions that could: [6] 1. Decrease the severity Stop failure before significant consequences, reject, recall 2. Decrease the probability Inspect defect out of batch 3. Increase the detection Move from manual to machine inspection 4. Reapply the tool taking the mitigating measures into consideration 5. Determine if the mitigations/actions have introduced new risks Risk acceptance is a decision to accept risk. For some types of harms, even the best quality risk management practices might not entirely eliminate risk. In these circumstances, it might be agreed that an appropriate quality risk management strategy has been applied and that quality risk is reduced to a specified (acceptable) level. This (specified) acceptable level will depend on many parameters and should be decided on a case-by-case basis. Risk Review is theoutput/results of the risk management process should be reviewed to take into account new knowledge and experience. Once a quality risk management process has been initiated, that process should continue to be utilized for events that might impact the original quality risk management decision. Risk review might include reconsideration of risk acceptance decisions Risk Communication is the sharing of information about risk and risk management between the decision makers and others. The output/result of the quality risk management process should be appropriately communicated and documented. The included information might relate to the existence, nature, form, probability, severity, acceptability, control, treatment, detectability or other aspects of risks to quality. The approach described can be used to [14] : Thoroughly analyze products and processes to ensure the best scientific rationale is in place to improve the probability of success Identify important knowledge gaps coupled with processes that need to be understood to properly identify risks Provide a communication process that will best interface with all relevant parties involved in the Risk Management Plan Make possible to transfer process knowledge and product development history to ease product progression and to supplement generic corporate knowledge Enable the pharmaceutical industry to adopt a risk-based approach to development as described in external regulatory guidance. The Risk Management outputs will potentially very as reference documents to support product development and control strategy discussions in regulatory filings.
6 V Vijayakumar Reddy et al /Int.J. PharmTech Res.2014,6(3),pp Discussion Table 1: Common Risk Management Tools [2] Risk management tool Description/attributes Potential applications Diagram analysis Flowcharts Check sheets Process mapping Cause/effect diagrams Risk ranking and filtering Fault tree analysis (FTA) [8] Hazard operability analysis (HAZOP) [9] Hazards analysis and critical control points (HACCP) [11] Failure modes effects analysis (FMEA) [10] Basic tools Simple techniques that are commonly used to gather and organize data, structure RM processes and facilitate decision making Method to compare and rank risks Typically involves evaluation of multiple diverse quantitative and qualitative factors for each risk, and weighting factors and risk scores Advanced tools Method used to identify all root causes of an assumed failure or problem Used to evaluate system or sub-system failures one at a time, but can combine multiple causes of failure by identifying causal chains Relies heavily on full process understanding to identify causal factors Tool assumes that risk events are caused by deviations from the design and operating intentions Uses a systematic technique to help identify potential deviations from normal use or design intentions Identify and implement process controls that consistently and effectively prevent hazard conditions from occurring Bottom-up approach that considers how to prevent hazards from occurring and/or propagating Emphasizes strength of preventative controls rather than ability to detect Assumes comprehensive understanding of the process and that critical process parameters (CPPs) have been defined prior to initiating the assessment. Tool ensures that CPPs will be met. Assesses potential failure modes for processes and the probable effect on outcomesand/or product performance Once failure modes are known, risk reduction actions can be applied to eliminate, reduce, or control potential failures Compilation of observations, trends or other empirical information to support a variety of less complex deviations, complaints, defaults or other circumstances Prioritize operating areas or sites for audit/assessment Useful for situations when the risks and underlying consequences are diverse and difficult to compare using a single tool Investigate product complaints Evaluate deviations Access manufacturing processes, facilities and equipment Commonly used to evaluate process safety hazards Better for preventative applications rather than reactive Great precursor or complement to process validation Assessment of the efficacyof CPPs and the ability to consistently execute them for any process Evaluate equipment and facilities; analyze a manufacturing process to identify high risk stepsand/or critical parameters
7 V Vijayakumar Reddy et al /Int.J. PharmTech Res.2014,6(3),pp Highly dependent upon strong understanding of product, process and/or facility under evaluation Output is a relative riskscore for each failure mode Conclusion Quality Risk Management is a systematic process for evaluation, control, communication and review of risks to the quality of the drug product across the product lifecycle. Effective Quality Risk Management can facilitate better and more informed decisions, can provide regulators with greater assurance of a company s ability to deal with potential risks, and might affect the extent and level of direct regulatory oversight. References: 1. Guidance for industry Q9 Quality Risk Management by US department of Health and Human Services, Food and drug Administration, Center for Drug and Evaluation Research, June WHO Guideline on Quality Risk Management A draft guidance August Implementation of ICH Q9 in the pharmaceutical field an example of methodology from PIC/S prashantkumarkatiyar-international journal of pharma world research(an international quarterly pharmaceutical research journal) risk analysis and risk management in pharmaceuticalindustry prashantkumarkatiyarvol Risk assessment and quality control by By Max Williams, MPA, November Quality Risk Management- the pharmaceutical experience ann'omahony 11 november kelvin CPharmaceutical engeneeringvol 28 no 3 GAMP 5- A Quality Risk Management Approach may/june Health and Safety Briefing, Fault Tree Analysis, Pg. No. 26, Dec IEEE Xplore, Data Management Tool for Aiding the Hazard and Operability AnalysisProcess, Page(s):1 4, Aug Failure Mode and Effect Analysis: FMEA from Theory to Execution, D. H. Stamatis.2nd Edition WHO Technical Report Series No. 908, 2003, Application of Hazard Analysis andcritical Control Point (HACCP) methodology to pharmaceuticals. 12. Risk Management: Guidelines and Best Practices Missouri Information Technology Advisory Board Project Management Committee Risk Management Subcommittee November Risk Management - Vocabulary - Guidelines for Use in Standards, Internationalorganization for standardization, Jan 1, International Organization for Standardization. ISO 14971:2007 Medical devices - application of risk management to medical devices. *****
Quality Risk Management The Pharmaceutical Experience Ann O Mahony Quality Assurance Specialist Pfizer Biotech Grange Castle
 Quality Risk Management 11 November 2011 Galway, Ireland Quality Risk Management The Pharmaceutical Experience Ann O Mahony Quality Assurance Specialist Pfizer Biotech Grange Castle Overview Regulatory
Quality Risk Management 11 November 2011 Galway, Ireland Quality Risk Management The Pharmaceutical Experience Ann O Mahony Quality Assurance Specialist Pfizer Biotech Grange Castle Overview Regulatory
QUALITY RISK MANAGEMENT (QRM): A REVIEW
 Lotlikar et al Journal of Drug Delivery & Therapeutics; 2013, 3(2), 149-154 149 Available online at http://jddtonline.info REVIEW ARTICLE QUALITY RISK MANAGEMENT (QRM): A REVIEW Lotlikar MV Head Corporate
Lotlikar et al Journal of Drug Delivery & Therapeutics; 2013, 3(2), 149-154 149 Available online at http://jddtonline.info REVIEW ARTICLE QUALITY RISK MANAGEMENT (QRM): A REVIEW Lotlikar MV Head Corporate
Quality Risk Management Tools Quality Risk Management Tool Selection When to Select FMEA: QRM Tool Selection Matrix
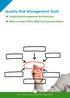 Quality Risk Management Tools Quality Risk Management Tool Selection When to Select FMEA: QRM Tool Selection Matrix 26 Quality Risk Management Tools The ICH Q9 guideline, Quality Risk Management, provides
Quality Risk Management Tools Quality Risk Management Tool Selection When to Select FMEA: QRM Tool Selection Matrix 26 Quality Risk Management Tools The ICH Q9 guideline, Quality Risk Management, provides
Quality Risk Management Principles and Industry Case Studies
 Final Draft Rev. December 28, 2008 Quality Risk Management Principles and Industry Case Studies T. Frank 1, S. Brooks 2, R. Creekmore 3, B. Hasselbalch 4, K. Murray 5, K. Obeng 6, S. Reich 5, E. Sanchez
Final Draft Rev. December 28, 2008 Quality Risk Management Principles and Industry Case Studies T. Frank 1, S. Brooks 2, R. Creekmore 3, B. Hasselbalch 4, K. Murray 5, K. Obeng 6, S. Reich 5, E. Sanchez
QUALITY RISK MANAGEMENT
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE QUALITY RISK MANAGEMENT Q9 Current Step 4 version
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE QUALITY RISK MANAGEMENT Q9 Current Step 4 version
Guidance for Industry
 Guidance for Industry Q9 Quality Risk Management U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation
Guidance for Industry Q9 Quality Risk Management U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation
Risk Assessment and Management. Allen L. Burgenson Manager, Regulatory Affairs Lonza Walkersville Inc.
 Risk Assessment and Management Allen L. Burgenson Manager, Regulatory Affairs Lonza Walkersville Inc. Standard Disclaimer Standard Disclaimer: This presentation is the opinion of the presenter, and does
Risk Assessment and Management Allen L. Burgenson Manager, Regulatory Affairs Lonza Walkersville Inc. Standard Disclaimer Standard Disclaimer: This presentation is the opinion of the presenter, and does
Best Practice In A Change Management System
 Quality & Compliance Associates, LLC Best Practice In A Change Management System President Quality & Compliance Associates, LLC Change Control and Its Role in a Continuous Improvement Environment 3 Benefits
Quality & Compliance Associates, LLC Best Practice In A Change Management System President Quality & Compliance Associates, LLC Change Control and Its Role in a Continuous Improvement Environment 3 Benefits
Guidance for Industry: Quality Risk Management
 Guidance for Industry: Quality Risk Management Version 1.0 Drug Office Department of Health Contents 1. Introduction... 3 2. Purpose of this document... 3 3. Scope... 3 4. What is risk?... 4 5. Integrating
Guidance for Industry: Quality Risk Management Version 1.0 Drug Office Department of Health Contents 1. Introduction... 3 2. Purpose of this document... 3 3. Scope... 3 4. What is risk?... 4 5. Integrating
Quality Risk Management
 PS/INF 1/2010 * * Quality Risk Management Quality Risk Management Implementation of ICH Q9 in the pharmaceutical field an example of methodology from PIC/S Document > Authors: L. Viornery (AFSSAPS) Ph.
PS/INF 1/2010 * * Quality Risk Management Quality Risk Management Implementation of ICH Q9 in the pharmaceutical field an example of methodology from PIC/S Document > Authors: L. Viornery (AFSSAPS) Ph.
Improved Utilization of Self-Inspection Programs within the GMP Environment A Quality Risk Management Approach
 Improved Utilization of Self-Inspection Programs within the GMP Environment A Quality Risk Management Approach Barbara Jeroncic Self-inspection is a well-established and vital part of the pharmaceutical
Improved Utilization of Self-Inspection Programs within the GMP Environment A Quality Risk Management Approach Barbara Jeroncic Self-inspection is a well-established and vital part of the pharmaceutical
Application of Quality Risk Management to Pharmaceutical Operations. Eldon Henson, Vice President, Quality Operations
 Application of Quality Risk Management to Pharmaceutical Operations Eldon Henson, Vice President, Quality Operations Key Topics of Discussion Definition of Quality Risk Management (QRM) Overview of PDA
Application of Quality Risk Management to Pharmaceutical Operations Eldon Henson, Vice President, Quality Operations Key Topics of Discussion Definition of Quality Risk Management (QRM) Overview of PDA
Annex 2. WHO guidelines on quality risk management. 1. Introduction 62. 2. Glossary 67 3. Quality risk management process 70
 Annex 2 WHO guidelines on quality risk management 1. Introduction 62 1.1 Background and scope 62 1.2 Principles of quality risk management 64 2. Glossary 67 3. Quality risk management process 70 3.1 Initiating
Annex 2 WHO guidelines on quality risk management 1. Introduction 62 1.1 Background and scope 62 1.2 Principles of quality risk management 64 2. Glossary 67 3. Quality risk management process 70 3.1 Initiating
Quality Risk Management Understanding and Control the Risk in Pharmaceutical Manufacturing Industry
 International Journal of Pharmaceutical Science Invention ISSN (Online): 2319 6718, ISSN (Print): 2319 670X Volume 4 Issue 1 January 2015 PP.29-41 Quality Risk Management Understanding and Control the
International Journal of Pharmaceutical Science Invention ISSN (Online): 2319 6718, ISSN (Print): 2319 670X Volume 4 Issue 1 January 2015 PP.29-41 Quality Risk Management Understanding and Control the
Guidance for Industry. Q10 Pharmaceutical Quality System
 Guidance for Industry Q10 Pharmaceutical Quality System U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation
Guidance for Industry Q10 Pharmaceutical Quality System U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation
PHARMACEUTICAL QUALITY SYSTEM Q10
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE PHARMACEUTICAL QUALITY SYSTEM Q10 Current Step
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE PHARMACEUTICAL QUALITY SYSTEM Q10 Current Step
ICH Q9 Quality Risk Management - an industry view. Peter H. Gough, Eli Lilly and Company
 ICH Q9 Quality Risk Management - an industry view Peter H. Gough, Eli Lilly and Company Contents How did we get here? FDA 21 st Century GMP Initiative ICH activity Introduction to risk management Links
ICH Q9 Quality Risk Management - an industry view Peter H. Gough, Eli Lilly and Company Contents How did we get here? FDA 21 st Century GMP Initiative ICH activity Introduction to risk management Links
WHO GUIDELINE ON QUALITY RISK MANAGEMENT
 August 2010 RESTRICTED WHO GUIDELINE ON QUALITY RISK MANAGEMENT This guideline has been prepared by Dr Simon Mills, United Kingdom. Please address any comments on this proposal, by 1 October 2010 to Dr
August 2010 RESTRICTED WHO GUIDELINE ON QUALITY RISK MANAGEMENT This guideline has been prepared by Dr Simon Mills, United Kingdom. Please address any comments on this proposal, by 1 October 2010 to Dr
ICH guideline Q10 on pharmaceutical quality system
 September 2015 EMA/CHMP/ICH/214732/2007 Committee for Human Medicinal Products Step 5 Transmission to CHMP May 2007 Transmission to interested parties May 2007 Deadline for comments November 2007 Final
September 2015 EMA/CHMP/ICH/214732/2007 Committee for Human Medicinal Products Step 5 Transmission to CHMP May 2007 Transmission to interested parties May 2007 Deadline for comments November 2007 Final
Risk Assessment for Medical Devices. Linda Braddon, Ph.D. Bring your medical device to market faster 1
 Risk Assessment for Medical Devices Linda Braddon, Ph.D. Bring your medical device to market faster 1 My Perspective Work with start up medical device companies Goal: Making great ideas into profitable
Risk Assessment for Medical Devices Linda Braddon, Ph.D. Bring your medical device to market faster 1 My Perspective Work with start up medical device companies Goal: Making great ideas into profitable
Pharmaceutical Quality Management System: Current Concept
 Pharmaceutical Quality Management System: Current Concept Neetu Dubey 1, *, Himanshu Gupta 3, R.K. Sharma 2, Nitin Dubey 1, Nidhi Dubey 4 1. IPS Academy, Indore, Madhya Pradesh, India. 2. Prestige Institute
Pharmaceutical Quality Management System: Current Concept Neetu Dubey 1, *, Himanshu Gupta 3, R.K. Sharma 2, Nitin Dubey 1, Nidhi Dubey 4 1. IPS Academy, Indore, Madhya Pradesh, India. 2. Prestige Institute
Quality Risk Management ICH Q9 & ISO 14971. Presented by Michael Kerr 11 th November 2011
 Quality Risk Management ICH Q9 & ISO 14971 Presented by Michael Kerr 11 th November 2011 Agenda Risk Concept QRM Fundamentals Regulatory Expectations Warning Letters / Observations Application of QRM Introduction:
Quality Risk Management ICH Q9 & ISO 14971 Presented by Michael Kerr 11 th November 2011 Agenda Risk Concept QRM Fundamentals Regulatory Expectations Warning Letters / Observations Application of QRM Introduction:
Controlling Risks Risk Assessment
 Controlling Risks Risk Assessment Hazard/Risk Assessment Having identified the hazards, one must assess the risks by considering the severity and likelihood of bad outcomes. If the risks are not sufficiently
Controlling Risks Risk Assessment Hazard/Risk Assessment Having identified the hazards, one must assess the risks by considering the severity and likelihood of bad outcomes. If the risks are not sufficiently
Risk assessment is used as a
 FOCUS ON... COMPLIANCE Author Insights Online Exclusive www.bioprocessintl.com/bpiextra Quality Risk Management for Drug Products and Drug Substances by Thomas A. Little Risk assessment is used as a vetting
FOCUS ON... COMPLIANCE Author Insights Online Exclusive www.bioprocessintl.com/bpiextra Quality Risk Management for Drug Products and Drug Substances by Thomas A. Little Risk assessment is used as a vetting
ICH Q10 - Pharmaceutical Quality System
 WCC PDA Dinner Meeting Jan 2012 ICH Q10 - Pharmaceutical Quality System Neil Wilkinson NSF-DBA www.nsf-dba.com ICHQ10.1 WCC PDA Dinner Meeting Jan 2012 Your Presenter Partner at NSF-DBA USA Training, Consultancy,
WCC PDA Dinner Meeting Jan 2012 ICH Q10 - Pharmaceutical Quality System Neil Wilkinson NSF-DBA www.nsf-dba.com ICHQ10.1 WCC PDA Dinner Meeting Jan 2012 Your Presenter Partner at NSF-DBA USA Training, Consultancy,
ASSESSMENT OF QUALITY RISK MANAGEMENT IMPLEMENTATION
 PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PI 038-1 26 March 2012 AIDE-MEMOIRE ASSESSMENT OF QUALITY RISK MANAGEMENT IMPLEMENTATION PIC/S March 2012 Reproduction
PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PI 038-1 26 March 2012 AIDE-MEMOIRE ASSESSMENT OF QUALITY RISK MANAGEMENT IMPLEMENTATION PIC/S March 2012 Reproduction
4. Critical success factors/objectives of the activity/proposal/project being risk assessed
 ARTC Risk Management Work Instruction 2: 1. Conduct Risk Assessment Workshop This Work Instruction provides general guidelines for conducting a generic Risk Assessment workshop. The instructions supplement
ARTC Risk Management Work Instruction 2: 1. Conduct Risk Assessment Workshop This Work Instruction provides general guidelines for conducting a generic Risk Assessment workshop. The instructions supplement
With the introduction of GAMP 5, A
 Online Exclusive from PHARMACEUTICAL ENGINEERING The Official Magazine of ISPE January/February 2012, Vol. 32 No. 1 www.pharmaceuticalengineering.org Copyright ISPE 2012 This article presents the case
Online Exclusive from PHARMACEUTICAL ENGINEERING The Official Magazine of ISPE January/February 2012, Vol. 32 No. 1 www.pharmaceuticalengineering.org Copyright ISPE 2012 This article presents the case
QA GLP audits. Alain Piton. Antipolis,, France. [email protected]
 Risk based assessment applied to QA GLP audits Alain Piton Galderma R&D, Sophia-Antipolis Antipolis,, France [email protected] RISK BASED ASSESSMENT FOR GLP AUDITS INTRODUCTION Since the origin
Risk based assessment applied to QA GLP audits Alain Piton Galderma R&D, Sophia-Antipolis Antipolis,, France [email protected] RISK BASED ASSESSMENT FOR GLP AUDITS INTRODUCTION Since the origin
Impact Assessment in a Science & Risk Based Environment. R. Legland 11/04/11
 Impact Assessment in a Science & Risk Based Environment R. Legland 11/04/11 Background US GMP s EU GMP s Japan GMP s ICH Q8, Q9, Q10 Guidance ASTM Standard E2500-07 Science and Risk Based Approach to Determine
Impact Assessment in a Science & Risk Based Environment R. Legland 11/04/11 Background US GMP s EU GMP s Japan GMP s ICH Q8, Q9, Q10 Guidance ASTM Standard E2500-07 Science and Risk Based Approach to Determine
3.0 Risk Assessment and Analysis Techniques and Tools
 3.0 Risk Assessment and Analysis Techniques and Tools Risks are determined in terms of the likelihood that an uncontrolled event will occur and the consequences of that event occurring. Risk = Likelihood
3.0 Risk Assessment and Analysis Techniques and Tools Risks are determined in terms of the likelihood that an uncontrolled event will occur and the consequences of that event occurring. Risk = Likelihood
Software-based medical devices from defibrillators
 C O V E R F E A T U R E Coping with Defective Software in Medical Devices Steven R. Rakitin Software Quality Consulting Inc. Embedding defective software in medical devices increases safety risks. Given
C O V E R F E A T U R E Coping with Defective Software in Medical Devices Steven R. Rakitin Software Quality Consulting Inc. Embedding defective software in medical devices increases safety risks. Given
1 www.imarcresearch.com
 Risk Management in Clinical Research: PROCESS DEVELOPMENT & APPLICATION Introduction Recently, two key pieces of guidance were released from Food and Drug Administration (FDA) and European Medicines Agency
Risk Management in Clinical Research: PROCESS DEVELOPMENT & APPLICATION Introduction Recently, two key pieces of guidance were released from Food and Drug Administration (FDA) and European Medicines Agency
Commercial Manufacturing - Qualification & Validation-related GMP Deficiencies and Other Lifecycle Considerations
 Commercial Manufacturing - Qualification & Validation-related GMP Deficiencies and Other Lifecycle Considerations Kevin O Donnell PhD Market Compliance Manager, IMB PDA / FDA Conference Pharmaceutical
Commercial Manufacturing - Qualification & Validation-related GMP Deficiencies and Other Lifecycle Considerations Kevin O Donnell PhD Market Compliance Manager, IMB PDA / FDA Conference Pharmaceutical
Project Risk Management
 Project Risk Management Study Notes PMI, PMP, CAPM, PMBOK, PM Network and the PMI Registered Education Provider logo are registered marks of the Project Management Institute, Inc. Points to Note Risk Management
Project Risk Management Study Notes PMI, PMP, CAPM, PMBOK, PM Network and the PMI Registered Education Provider logo are registered marks of the Project Management Institute, Inc. Points to Note Risk Management
FMEA and FTA Analysis
 FMEA and FTA Analysis Why it is Coming to Your Hospital and Your Laboratory Tina A. Krenc Director, R&D Phase Systems Abbott Laboratories 1 Agenda Background on requirements for risk management Tools to
FMEA and FTA Analysis Why it is Coming to Your Hospital and Your Laboratory Tina A. Krenc Director, R&D Phase Systems Abbott Laboratories 1 Agenda Background on requirements for risk management Tools to
The use of risk assessment tools for microbiological assessment of cleanroom environments. by Tim Sandle
 The use of risk assessment tools for microbiological assessment of cleanroom environments by Tim Sandle Email: [email protected] / [email protected] Web: www.pharmig.blogspot.com Environmental
The use of risk assessment tools for microbiological assessment of cleanroom environments by Tim Sandle Email: [email protected] / [email protected] Web: www.pharmig.blogspot.com Environmental
Quality Assurance. Disclosure for Lilli Møller Andersen. No relevant financial relationships exist for any issue mentioned in this presentation
 Quality Assurance Disclosure for Lilli Møller Andersen No relevant financial relationships exist for any issue mentioned in this presentation Agenda Quality Assurance Quality Management System Quality
Quality Assurance Disclosure for Lilli Møller Andersen No relevant financial relationships exist for any issue mentioned in this presentation Agenda Quality Assurance Quality Management System Quality
Deviation Handling and Quality Risk Management
 Deviation Handling and Quality Risk Management A note for guidance for the manufacture of prequalified vaccines for supply to United Nations agencies July, 2013 Vaccine Quality and Regulations (VQR), Essential
Deviation Handling and Quality Risk Management A note for guidance for the manufacture of prequalified vaccines for supply to United Nations agencies July, 2013 Vaccine Quality and Regulations (VQR), Essential
A Risk Management Standard
 A Risk Management Standard Introduction This Risk Management Standard is the result of work by a team drawn from the major risk management organisations in the UK, including the Institute of Risk management
A Risk Management Standard Introduction This Risk Management Standard is the result of work by a team drawn from the major risk management organisations in the UK, including the Institute of Risk management
Managing Clinical Trial Risk: It's a Tough Job, But One Person Has To Do It
 Managing Clinical Trial Risk: It's a Tough Job, But One Person Has To Do It Michael Macri, Director, Strategic Services, inventiv Health Clinical Sherry Merrifield, Director, Clinical Monitoring, inventiv
Managing Clinical Trial Risk: It's a Tough Job, But One Person Has To Do It Michael Macri, Director, Strategic Services, inventiv Health Clinical Sherry Merrifield, Director, Clinical Monitoring, inventiv
WHO GUIDELINE ON QUALITY RISK MANAGEMENT
 August 2012 RESTRICTED WHO GUIDELINE ON QUALITY RISK MANAGEMENT REVISED DRAFT FOR COMMENT Please address any comments on this proposal, by 5 October 2012 to Dr S. Kopp, Medicines Quality Assurance Programme,
August 2012 RESTRICTED WHO GUIDELINE ON QUALITY RISK MANAGEMENT REVISED DRAFT FOR COMMENT Please address any comments on this proposal, by 5 October 2012 to Dr S. Kopp, Medicines Quality Assurance Programme,
ISO 14971: Overview of the standard
 FDA Medical Device Industry Coalition ISO 14971: Overview of the standard Risk Management Through Product Life Cycle: An Educational Forum William A. Hyman Department of Biomedical Engineering Texas A&M
FDA Medical Device Industry Coalition ISO 14971: Overview of the standard Risk Management Through Product Life Cycle: An Educational Forum William A. Hyman Department of Biomedical Engineering Texas A&M
STANDARD. Risk Assessment. Supply Chain Risk Management: A Compilation of Best Practices
 A S I S I N T E R N A T I O N A L Supply Chain Risk Management: Risk Assessment A Compilation of Best Practices ANSI/ASIS/RIMS SCRM.1-2014 RA.1-2015 STANDARD The worldwide leader in security standards
A S I S I N T E R N A T I O N A L Supply Chain Risk Management: Risk Assessment A Compilation of Best Practices ANSI/ASIS/RIMS SCRM.1-2014 RA.1-2015 STANDARD The worldwide leader in security standards
White paper. Corrective action: The closed-loop system
 White paper Corrective action: The closed-loop system Contents Summary How corrective action works The steps 1 - Identify non-conformities - Opening a corrective action 6 - Responding to a corrective action
White paper Corrective action: The closed-loop system Contents Summary How corrective action works The steps 1 - Identify non-conformities - Opening a corrective action 6 - Responding to a corrective action
FINAL DOCUMENT. Global Harmonization Task Force
 FINAL DOCUMENT Global Harmonization Task Force Title: Quality management system Medical Devices Guidance on corrective action and preventive action and related QMS processes Authoring Group: Study Group
FINAL DOCUMENT Global Harmonization Task Force Title: Quality management system Medical Devices Guidance on corrective action and preventive action and related QMS processes Authoring Group: Study Group
Quality Risk Management - The Medical Device Experience. Niamh Nolan Principal Design Assurance Engineer Boston Scientific
 Quality Risk Management - The Medical Device Experience Niamh Nolan Principal Design Assurance Engineer Boston Scientific Agenda Intent of Risk Management (RM) and Associated Regulations Overview of RM
Quality Risk Management - The Medical Device Experience Niamh Nolan Principal Design Assurance Engineer Boston Scientific Agenda Intent of Risk Management (RM) and Associated Regulations Overview of RM
Standard Operating Procedure Title: Quality Risk Management Techniques
 Department Quality Management Document no QMS-135 Title Quality Risk Management Techniques Prepared by: Date: Supersedes: Checked by: Date: Date Issued: Approved by: Date: Review Date: 1.0 Purpose This
Department Quality Management Document no QMS-135 Title Quality Risk Management Techniques Prepared by: Date: Supersedes: Checked by: Date: Date Issued: Approved by: Date: Review Date: 1.0 Purpose This
Occupational safety risk management in Australian mining
 IN-DEPTH REVIEW Occupational Medicine 2004;54:311 315 doi:10.1093/occmed/kqh074 Occupational safety risk management in Australian mining J. Joy Abstract Key words In the past 15 years, there has been a
IN-DEPTH REVIEW Occupational Medicine 2004;54:311 315 doi:10.1093/occmed/kqh074 Occupational safety risk management in Australian mining J. Joy Abstract Key words In the past 15 years, there has been a
PROPOSED UPDATED TEXT FOR WHO GOOD MANUFACTURING PRACTICES FOR PHARMACEUTICAL PRODUCTS: MAIN PRINCIPLES (JANUARY 2013)
 January 2013 RESTRICTED PROPOSED UPDATED TEXT FOR WHO GOOD MANUFACTURING PRACTICES FOR PHARMACEUTICAL PRODUCTS: MAIN PRINCIPLES (JANUARY 2013) DRAFT FOR COMMENTS Please address any comments on this proposal
January 2013 RESTRICTED PROPOSED UPDATED TEXT FOR WHO GOOD MANUFACTURING PRACTICES FOR PHARMACEUTICAL PRODUCTS: MAIN PRINCIPLES (JANUARY 2013) DRAFT FOR COMMENTS Please address any comments on this proposal
ICH Q10 Pharmaceutical Quality System (PQS)
 ICH Q10 Pharmaceutical Quality System (PQS) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH Q10 PQS Guideline Background Objectives
ICH Q10 Pharmaceutical Quality System (PQS) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH Q10 PQS Guideline Background Objectives
Risk Management Primer
 Risk Management Primer Purpose: To obtain strong project outcomes by implementing an appropriate risk management process Audience: Project managers, project sponsors, team members and other key stakeholders
Risk Management Primer Purpose: To obtain strong project outcomes by implementing an appropriate risk management process Audience: Project managers, project sponsors, team members and other key stakeholders
Risk Management for Aseptic Processing
 Risk Management for Aseptic Processing Ed White Welcome to The Aseptic Core. This column discusses scientific and regulatory aspects of aseptic processing, with an emphasis on aseptic formulation and filling.
Risk Management for Aseptic Processing Ed White Welcome to The Aseptic Core. This column discusses scientific and regulatory aspects of aseptic processing, with an emphasis on aseptic formulation and filling.
Risk-Based Validation of Commercial Off-the-Shelf Computer Systems
 Risk-Based Validation of Commercial Off-the-Shelf Computer Systems Published by Advanstar Communications in Journal of Validation Technology May 2005, Vol. 11, No. 3 Supplied by (*) www.labcompliance.com
Risk-Based Validation of Commercial Off-the-Shelf Computer Systems Published by Advanstar Communications in Journal of Validation Technology May 2005, Vol. 11, No. 3 Supplied by (*) www.labcompliance.com
Hazard Identification and Risk Assessment in Foundry
 Hazard Identification and Risk Assessment in Foundry M.SaravanaKumar 1, Dr.P.SenthilKumar 2 1 (Industrial Safety Engineering, K.S.R, College of Engineering / Anna University, Chennai, India) 2 (Department
Hazard Identification and Risk Assessment in Foundry M.SaravanaKumar 1, Dr.P.SenthilKumar 2 1 (Industrial Safety Engineering, K.S.R, College of Engineering / Anna University, Chennai, India) 2 (Department
Upon completion of this activity, the participant should be able to:
 The Utility of Root Cause Analysis and Failure Mode and Effects Analysis in the Hospital Setting Learning objectives: Upon completion of this activity, the participant should be able to: 1. Discuss the
The Utility of Root Cause Analysis and Failure Mode and Effects Analysis in the Hospital Setting Learning objectives: Upon completion of this activity, the participant should be able to: 1. Discuss the
Quality Metrics An FDA Perspective PDA Dinner and Dialogue. Melissa Seymour VP, Corporate Quality DRAFT
 Quality Metrics An FDA Perspective PDA Dinner and Dialogue Melissa Seymour VP, Corporate Quality DRAFT Agenda How does industry use Metrics? FDA Challenges and Requirements and Use Complexities of Implementation
Quality Metrics An FDA Perspective PDA Dinner and Dialogue Melissa Seymour VP, Corporate Quality DRAFT Agenda How does industry use Metrics? FDA Challenges and Requirements and Use Complexities of Implementation
Corrective and Preventive Action Background & Examples Presented by:
 Corrective and Preventive Action Background & Examples Presented by: Kimberly Lewandowski-Walker Food and Drug Administration Division of Domestic Field Investigations Office of Regulatory Affairs Overview
Corrective and Preventive Action Background & Examples Presented by: Kimberly Lewandowski-Walker Food and Drug Administration Division of Domestic Field Investigations Office of Regulatory Affairs Overview
ENTERPRISE RISK MANAGEMENT FRAMEWORK
 ENTERPRISE RISK MANAGEMENT FRAMEWORK COVENANT HEALTH LEGAL & RISK MANAGEMENT CONTENTS 1.0 PURPOSE OF THE DOCUMENT... 3 2.0 INTRODUCTION AND OVERVIEW... 4 3.0 GOVERNANCE STRUCTURE AND ACCOUNTABILITY...
ENTERPRISE RISK MANAGEMENT FRAMEWORK COVENANT HEALTH LEGAL & RISK MANAGEMENT CONTENTS 1.0 PURPOSE OF THE DOCUMENT... 3 2.0 INTRODUCTION AND OVERVIEW... 4 3.0 GOVERNANCE STRUCTURE AND ACCOUNTABILITY...
RISK MANAGEMENT FOR INFRASTRUCTURE
 RISK MANAGEMENT FOR INFRASTRUCTURE CONTENTS 1.0 PURPOSE & SCOPE 2.0 DEFINITIONS 3.0 FLOWCHART 4.0 PROCEDURAL TEXT 5.0 REFERENCES 6.0 ATTACHMENTS This document is the property of Thiess Infraco and all
RISK MANAGEMENT FOR INFRASTRUCTURE CONTENTS 1.0 PURPOSE & SCOPE 2.0 DEFINITIONS 3.0 FLOWCHART 4.0 PROCEDURAL TEXT 5.0 REFERENCES 6.0 ATTACHMENTS This document is the property of Thiess Infraco and all
Software Development for Medical Devices
 Overcoming the Challenges of Compliance, Quality and Cost An MKS White Paper Introduction Software is fast becoming the differentiator for manufacturers of medical devices. The rewards available from software
Overcoming the Challenges of Compliance, Quality and Cost An MKS White Paper Introduction Software is fast becoming the differentiator for manufacturers of medical devices. The rewards available from software
Hazard Analysis and Critical Control Points (HACCP) 1 Overview
 Manufacturing Technology Committee Risk Management Working Group Risk Management Training Guides Hazard Analysis and Critical Control Points (HACCP) 1 Overview Hazard Analysis and Critical Control Point
Manufacturing Technology Committee Risk Management Working Group Risk Management Training Guides Hazard Analysis and Critical Control Points (HACCP) 1 Overview Hazard Analysis and Critical Control Point
PMI Risk Management Professional (PMI-RMP) Exam Content Outline
 PMI Risk Management Professional (PMI-RMP) Exam Content Outline Project Management Institute PMI Risk Management Professional (PMI-RMP) Exam Content Outline Published by: Project Management Institute,
PMI Risk Management Professional (PMI-RMP) Exam Content Outline Project Management Institute PMI Risk Management Professional (PMI-RMP) Exam Content Outline Published by: Project Management Institute,
Quality Thinking in other Industries. Dominic Parry Inspired Pharma Training. WEB www.inspiredpharma.com GMP BLOG inspiredpharmablog.
 Quality Thinking in other Industries Dominic Parry Inspired Pharma Training WEB www.inspiredpharma.com GMP BLOG inspiredpharmablog.com Welcome The traditional focus on quality Quality in the eyes of GMP
Quality Thinking in other Industries Dominic Parry Inspired Pharma Training WEB www.inspiredpharma.com GMP BLOG inspiredpharmablog.com Welcome The traditional focus on quality Quality in the eyes of GMP
In accordance with risk management best practices, below describes the standard process for enterprise risk management (ERM), including:
 Enterprise Risk Management Process and Procedures Scope In accordance with risk management best practices, below describes the standard process for enterprise risk management (ERM), including: Risk identification
Enterprise Risk Management Process and Procedures Scope In accordance with risk management best practices, below describes the standard process for enterprise risk management (ERM), including: Risk identification
Guideline on Process Validation
 1 2 3 4 29 March 2012 EMA/CHMP/CVMP/QWP/70278/2012-Rev1 Committee for Medicinal Products for Human Use (CHMP) Committee for Medicinal Products for Veterinary Use (CVMP) 5 6 Draft Draft Agreed by CHMP /
1 2 3 4 29 March 2012 EMA/CHMP/CVMP/QWP/70278/2012-Rev1 Committee for Medicinal Products for Human Use (CHMP) Committee for Medicinal Products for Veterinary Use (CVMP) 5 6 Draft Draft Agreed by CHMP /
Risk Assessment Tools for Identifying Hazards and Evaluating Risks Associated with IVD Assays
 Risk Assessment Tools for Identifying Hazards and Evaluating Risks Associated with IVD Assays Robert C. Menson, PhD AACC Annual Meeting Philadelphia, PA 22 July 2003 What Risks Must Be Managed? Risk to
Risk Assessment Tools for Identifying Hazards and Evaluating Risks Associated with IVD Assays Robert C. Menson, PhD AACC Annual Meeting Philadelphia, PA 22 July 2003 What Risks Must Be Managed? Risk to
Failure Analysis Methods What, Why and How. MEEG 466 Special Topics in Design Jim Glancey Spring, 2006
 Failure Analysis Methods What, Why and How MEEG 466 Special Topics in Design Jim Glancey Spring, 2006 Failure Analysis Methods Every product or process has modes of failure. An analysis of potential failures
Failure Analysis Methods What, Why and How MEEG 466 Special Topics in Design Jim Glancey Spring, 2006 Failure Analysis Methods Every product or process has modes of failure. An analysis of potential failures
Linking Risk Management to Business Strategy, Processes, Operations and Reporting
 Linking Risk Management to Business Strategy, Processes, Operations and Reporting Financial Management Institute of Canada February 17 th, 2010 KPMG LLP Agenda 1. Leading Practice Risk Management Principles
Linking Risk Management to Business Strategy, Processes, Operations and Reporting Financial Management Institute of Canada February 17 th, 2010 KPMG LLP Agenda 1. Leading Practice Risk Management Principles
Software Development for Medical Devices
 Software Development for Medical Devices Overcoming the Challenges of Compliance, Quality and Cost Software is fast becoming the differentiator for manufacturers of medical devices. The rewards of software
Software Development for Medical Devices Overcoming the Challenges of Compliance, Quality and Cost Software is fast becoming the differentiator for manufacturers of medical devices. The rewards of software
Aligning Quality Management Processes to Compliance Goals
 Aligning Quality Management Processes to Compliance Goals MetricStream.com Smart Consulting Group Joint Webinar February 23 rd 2012 Nigel J. Smart, Ph.D. Smart Consulting Group 20 E. Market Street West
Aligning Quality Management Processes to Compliance Goals MetricStream.com Smart Consulting Group Joint Webinar February 23 rd 2012 Nigel J. Smart, Ph.D. Smart Consulting Group 20 E. Market Street West
Industry Implications of Pharmaceutical Quality ICH Guidelines
 EIPG General Assembly Industry Implications of Pharmaceutical Quality ICH Guidelines 20 th April 2008 Pharmaceutical Quality Develop a harmonised pharmaceutical quality system applicable across the lifecycle
EIPG General Assembly Industry Implications of Pharmaceutical Quality ICH Guidelines 20 th April 2008 Pharmaceutical Quality Develop a harmonised pharmaceutical quality system applicable across the lifecycle
PMI Risk Management Professional (PMI-RMP ) - Practice Standard and Certification Overview
 PMI Risk Management Professional (PMI-RMP ) - Practice Standard and Certification Overview Sante Torino PMI-RMP, IPMA Level B Head of Risk Management Major Programmes, Selex ES / Land&Naval Systems Division
PMI Risk Management Professional (PMI-RMP ) - Practice Standard and Certification Overview Sante Torino PMI-RMP, IPMA Level B Head of Risk Management Major Programmes, Selex ES / Land&Naval Systems Division
The FDA recently announced a significant
 This article illustrates the risk analysis guidance discussed in GAMP 4. 5 By applying GAMP s risk analysis method to three generic classes of software systems, this article acts as both an introduction
This article illustrates the risk analysis guidance discussed in GAMP 4. 5 By applying GAMP s risk analysis method to three generic classes of software systems, this article acts as both an introduction
DRAFT RESEARCH SUPPORT BUILDING AND INFRASTRUCTURE MODERNIZATION RISK MANAGEMENT PLAN. April 2009 SLAC I 050 07010 002
 DRAFT RESEARCH SUPPORT BUILDING AND INFRASTRUCTURE MODERNIZATION RISK MANAGEMENT PLAN April 2009 SLAC I 050 07010 002 Risk Management Plan Contents 1.0 INTRODUCTION... 1 1.1 Scope... 1 2.0 MANAGEMENT
DRAFT RESEARCH SUPPORT BUILDING AND INFRASTRUCTURE MODERNIZATION RISK MANAGEMENT PLAN April 2009 SLAC I 050 07010 002 Risk Management Plan Contents 1.0 INTRODUCTION... 1 1.1 Scope... 1 2.0 MANAGEMENT
ISCT Cell Therapy Liaison Meeting AABB Headquarters in Bethesda, MD. Regulatory Considerations for the Use of Software for Manufacturing HCT/P
 ISCT Cell Therapy Liaison Meeting AABB Headquarters in Bethesda, MD September 10, 2009 David Doleski, Team Leader, Branch 2 Division of Manufacturing and Product Quality (DMPQ) Office of Compliance and
ISCT Cell Therapy Liaison Meeting AABB Headquarters in Bethesda, MD September 10, 2009 David Doleski, Team Leader, Branch 2 Division of Manufacturing and Product Quality (DMPQ) Office of Compliance and
Design Verification The Case for Verification, Not Validation
 Overview: The FDA requires medical device companies to verify that all the design outputs meet the design inputs. The FDA also requires that the final medical device must be validated to the user needs.
Overview: The FDA requires medical device companies to verify that all the design outputs meet the design inputs. The FDA also requires that the final medical device must be validated to the user needs.
Project Management Challenges in Software Development
 Abstract Research Journal of Management Sciences ISSN 2319 1171 Project Management Challenges in Software Development Uma Sankar S.S. 1 and R. Jubi 2 1 Research and Development Centre, Bharathiar University,
Abstract Research Journal of Management Sciences ISSN 2319 1171 Project Management Challenges in Software Development Uma Sankar S.S. 1 and R. Jubi 2 1 Research and Development Centre, Bharathiar University,
Strengthening Risk Evaluation and Mitigation Strategies (REMS) Through Systematic Analysis, Standardized Design, and Evidence-Based Assessment
 September 25, 2013 Discussion Guide Strengthening Risk Evaluation and Mitigation Strategies (REMS) Through Systematic Analysis, Standardized Design, and Evidence-Based Assessment Background With the passage
September 25, 2013 Discussion Guide Strengthening Risk Evaluation and Mitigation Strategies (REMS) Through Systematic Analysis, Standardized Design, and Evidence-Based Assessment Background With the passage
Optimization of Software Quality using Management and Technical Review Techniques
 Optimization of Software Quality using Management and Technical Review Techniques Inibehe Emmanuel Akpannah Post Graduate Student (MSc. Information Technology), SRM University, Chennai, India Abstract
Optimization of Software Quality using Management and Technical Review Techniques Inibehe Emmanuel Akpannah Post Graduate Student (MSc. Information Technology), SRM University, Chennai, India Abstract
WHITEPAPER: SOFTWARE APPS AS MEDICAL DEVICES THE REGULATORY LANDSCAPE
 WHITEPAPER: SOFTWARE APPS AS MEDICAL DEVICES THE REGULATORY LANDSCAPE White paper produced by Maetrics For more information, please contact global sales +1 610 458 9312 +1 877 623 8742 [email protected]
WHITEPAPER: SOFTWARE APPS AS MEDICAL DEVICES THE REGULATORY LANDSCAPE White paper produced by Maetrics For more information, please contact global sales +1 610 458 9312 +1 877 623 8742 [email protected]
Process Validation: Practical Aspects of the New FDA Guidance
 Process Validation: Practical Aspects of the New FDA Guidance ISPE Boston Chapter Meeting April 18, 2013 Rusty Morrison Commissioning Agents, Inc. Objectives / Summary What is Process Validation? Regulatory
Process Validation: Practical Aspects of the New FDA Guidance ISPE Boston Chapter Meeting April 18, 2013 Rusty Morrison Commissioning Agents, Inc. Objectives / Summary What is Process Validation? Regulatory
Pharmaceutical Quality Systems: US Perspective
 Pharmaceutical Quality Systems: US Perspective Rick Friedman Associate Director, Office of Manufacturing and Product Quality Center for Drug Evaluation and Research Topics Background: The ICH Q10 Pharmaceutical
Pharmaceutical Quality Systems: US Perspective Rick Friedman Associate Director, Office of Manufacturing and Product Quality Center for Drug Evaluation and Research Topics Background: The ICH Q10 Pharmaceutical
Media fills Periodic performance qualification (Re-Validation)
 Media fills Periodic performance qualification (Re-Validation) Minimum number of Simulations Number of units Contaminated Units Action a Two per Year (Retrospective & Prospective Validation) < 5000 5000
Media fills Periodic performance qualification (Re-Validation) Minimum number of Simulations Number of units Contaminated Units Action a Two per Year (Retrospective & Prospective Validation) < 5000 5000
EU GMP Requirements - Quality Systems - Bernd Boedecker GMP Inspectorate of Hannover / Germany at Turkish Ministry of Health Ankara, 20-21 Oct 2009
 EU GMP Requirements - Quality Systems - Bernd Boedecker GMP Inspectorate of Hannover / Germany at Turkish Ministry of Health Ankara, 20-21 Oct 2009 contact data Bernd Boedecker Staatliches Gewerbeaufsichtsamt
EU GMP Requirements - Quality Systems - Bernd Boedecker GMP Inspectorate of Hannover / Germany at Turkish Ministry of Health Ankara, 20-21 Oct 2009 contact data Bernd Boedecker Staatliches Gewerbeaufsichtsamt
4 Testing General and Automated Controls
 4 Testing General and Automated Controls Learning Objectives To understand the reasons for testing; To have an idea about Audit Planning and Testing; To discuss testing critical control points; To learn
4 Testing General and Automated Controls Learning Objectives To understand the reasons for testing; To have an idea about Audit Planning and Testing; To discuss testing critical control points; To learn
ICH Q 10 Pharmaceutical Quality System PQS DIA China Annual Meeting, May 2010
 ICH Q 10 Pharmaceutical Quality System PQS DIA China Annual Meeting, May 2010 Joseph C. Famulare Head of External Relations and Collaboration Pharma Global Technical Operations Global Quality, F. Hoffmann-La
ICH Q 10 Pharmaceutical Quality System PQS DIA China Annual Meeting, May 2010 Joseph C. Famulare Head of External Relations and Collaboration Pharma Global Technical Operations Global Quality, F. Hoffmann-La
Guideline on good pharmacovigilance practices (GVP)
 1 2 20 February 2012 EMA/541760/2011 3 4 Guideline on good pharmacovigilance practices (GVP) Module I Pharmacovigilance systems and their quality systems Draft finalised by the Agency in collaboration
1 2 20 February 2012 EMA/541760/2011 3 4 Guideline on good pharmacovigilance practices (GVP) Module I Pharmacovigilance systems and their quality systems Draft finalised by the Agency in collaboration
Content Sheet 16-1: Introduction to Documents & Records
 Content Sheet 16-1: Introduction to Documents & Records Role in quality management system The management of documents and records is one of the 12 essential elements of the quality system. The management
Content Sheet 16-1: Introduction to Documents & Records Role in quality management system The management of documents and records is one of the 12 essential elements of the quality system. The management
Risk-Based Environmental Monitoring. Marsha Stabler Hardiman Senior Consultant Concordia ValSource Wednesday September 17, 2014 FDA/PQRI
 Risk-Based Environmental Monitoring Marsha Stabler Hardiman Senior Consultant Concordia ValSource Wednesday September 17, 2014 FDA/PQRI Presenter Marsha Stabler Hardiman Over 20 years experience in the
Risk-Based Environmental Monitoring Marsha Stabler Hardiman Senior Consultant Concordia ValSource Wednesday September 17, 2014 FDA/PQRI Presenter Marsha Stabler Hardiman Over 20 years experience in the
Safety Regulation Group SAFETY MANAGEMENT SYSTEMS GUIDANCE TO ORGANISATIONS. April 2008 1
 Safety Regulation Group SAFETY MANAGEMENT SYSTEMS GUIDANCE TO ORGANISATIONS April 2008 1 Contents 1 Introduction 3 2 Management Systems 2.1 Management Systems Introduction 3 2.2 Quality Management System
Safety Regulation Group SAFETY MANAGEMENT SYSTEMS GUIDANCE TO ORGANISATIONS April 2008 1 Contents 1 Introduction 3 2 Management Systems 2.1 Management Systems Introduction 3 2.2 Quality Management System
Title: Basic Principles of Risk Management for Medical Device Design
 Title: Basic Principles of Risk Management for Medical Device Design WHITE PAPER Author: Ganeshkumar Palanichamy Abstract Medical devices developed for human application are used for diagnostic or treatment
Title: Basic Principles of Risk Management for Medical Device Design WHITE PAPER Author: Ganeshkumar Palanichamy Abstract Medical devices developed for human application are used for diagnostic or treatment
Annex 7 Application of Hazard Analysis and Critical Control Point (HACCP) methodology to pharmaceuticals
 World Health Organization WHO Technical Report Series, No. 908, 2003 Annex 7 Application of Hazard Analysis and Critical Control Point (HACCP) methodology to pharmaceuticals 1. Introduction Traditionally,
World Health Organization WHO Technical Report Series, No. 908, 2003 Annex 7 Application of Hazard Analysis and Critical Control Point (HACCP) methodology to pharmaceuticals 1. Introduction Traditionally,
Periodic risk assessment by internal audit
 Periodic risk assessment by internal audit I Introduction The Good Practice Internal Audit Manual Template, developed by the Internal Audit CoP of Pempal, defines the importance and the impact that an
Periodic risk assessment by internal audit I Introduction The Good Practice Internal Audit Manual Template, developed by the Internal Audit CoP of Pempal, defines the importance and the impact that an
The Total Quality Assurance Networking Model for Preventing Defects: Building an Effective Quality Assurance System using a Total QA Network
 The Total Quality Assurance Networking Model for Preventing Defects: Building an Effective Quality Assurance System using a Total QA Network TAKU KOJIMA 1, KAKURO AMASAKA 2 School of Science and Engineering
The Total Quality Assurance Networking Model for Preventing Defects: Building an Effective Quality Assurance System using a Total QA Network TAKU KOJIMA 1, KAKURO AMASAKA 2 School of Science and Engineering
The purpose of Capacity and Availability Management (CAM) is to plan and monitor the effective provision of resources to support service requirements.
 CAPACITY AND AVAILABILITY MANAGEMENT A Project Management Process Area at Maturity Level 3 Purpose The purpose of Capacity and Availability Management (CAM) is to plan and monitor the effective provision
CAPACITY AND AVAILABILITY MANAGEMENT A Project Management Process Area at Maturity Level 3 Purpose The purpose of Capacity and Availability Management (CAM) is to plan and monitor the effective provision
IVD Regulation Overview. Requirements to Assure Quality & Effectiveness
 IVD Regulation Overview Requirements to Assure Quality & Effectiveness CLIAC Jan. 2002 Statutory and Regulatory Requirements Statute: Food, Drug, and Cosmetic Act Food and Drugs Act of 1906 Food and Drug
IVD Regulation Overview Requirements to Assure Quality & Effectiveness CLIAC Jan. 2002 Statutory and Regulatory Requirements Statute: Food, Drug, and Cosmetic Act Food and Drugs Act of 1906 Food and Drug
Motivations. spm - 2014 adolfo villafiorita - introduction to software project management
 Risk Management Motivations When we looked at project selection we just took into account financial data In the scope management document we emphasized the importance of making our goals achievable, i.e.
Risk Management Motivations When we looked at project selection we just took into account financial data In the scope management document we emphasized the importance of making our goals achievable, i.e.
QUALITY MANUAL REVISION RECORD
 Page 2 of 31 REVISION RECORD Date Rev Description Jun 18, 2007 N/C Original Issue Sep 16, 2009 A Update to ISO 9001:2008 Standard. Feb 04, 2010 B Revised exclusions, removed (Except 7.3.7 from the exclusion
Page 2 of 31 REVISION RECORD Date Rev Description Jun 18, 2007 N/C Original Issue Sep 16, 2009 A Update to ISO 9001:2008 Standard. Feb 04, 2010 B Revised exclusions, removed (Except 7.3.7 from the exclusion
