Fingolimod (Gilenya ) Drug Review
|
|
|
- Suzan Todd
- 8 years ago
- Views:
Transcription
1 Fingolimod (Gilenya ) Drug Review AHFS 92:20, Biologic Response Modifiers Final Report March 2013 Review prepared by: Melissa Archer, PharmD, Clinical Pharmacist Gary Oderda, PharmD, MPH, Professor University of Utah College of Pharmacy Copyright 2013 by University of Utah College of Pharmacy Salt Lake City, Utah. All rights reserved.
2 Table of Contents Executive Summary... 3 Introduction... 4 Disease Overview... 4 Table 1. Comparison of Disease Modifying Agents used in the Treatment of Multiple Sclerosis... 6 Pharmacology... 7 Pharmacokinetics... 7 Methods... 7 Clinical Efficacy... 7 Adverse Drug Reactions Summary References
3 Executive Summary Introduction: Fingolimod (Gilenya ) is an oral disease-modifying agent indicated in the treatment of relapsing forms of multiple sclerosis (MS). Other disease-modifying agents indicated in the treatment of MS include: glatiramer (Copaxone ), interferon beta-1a (Avonex, Rebif ), interferon beta-1b (Betaseron, Extavia ), mitoxantrone (Novantrone ), natalizumab (Tysabri ), and teriflunomide (Aubagio ). Treatment guidelines for MS recommend a biologic or interferon agent as first-line therapy, but do not recommend one agent over another. Fingolimod may also be an effective first-line treatment option. Fingolimod is a sphingosine 1- phosphate receptor modulator, dose once daily, which is thought to reduce CNS inflammation in patients with MS. Clinical Efficacy: Clinical data evaluating the efficacy of fingolimod in the treatment of MS are limited. In comparative clinical trials, relapse rate is significantly improved with fingolimod compared to either interferon beta or placebo. No clinical benefit was demonstrated with fingolimod at doses greater than 0.5 mg. Data from follow-up trials indicate sustained efficacy and no increases in adverse events for 36 months. Limited data from a subgroup analysis indicate efficacy of fingolimod treatment across patients with a wide range of clinical features. Special Populations: Fingolimod was not studied in pregnant or lactating women, children, or adults over 65 years of age. One clinical trial evaluating fingolimod use in Japanese patients confirmed efficacy of fingolimod in this patient population. Adverse Drug Reactions: Fingolimod has relatively good tolerability. The most common adverse events reported with fingolimod therapy include headache, elevated liver enzymes, and diarrhea. Serious adverse events associated with fingolimod therapy include cardiovascular abnormalities (bradycardia and atrioventricular (AV) block). Fingolimod therapy is associated with first-dose bradycardia occurring within 6 hours of the first dose and is only available through specialty pharmacies or the manufacturers First Dose Program. Fingolimod is metabolized by the CYP4F2 isoenzyme and has not demonstrated induction or inhibition of any of the major CYP P450 enzymes or interactions with any of the major P-glycoproteins or other transporters. Summary: Fingolimod was the first oral disease-modifying agent approved for the treatment of relapsing multiple sclerosis. Limited comparative clinical evidence suggests fingolimod is more effective than placebo and has similar efficacy as interferon beta. Fingolimod has numerous adverse effects but they are usually only mild to moderate. Fingolimod is only available through specialty pharmacies or the manufacturers First Dose Program. 3
4 Introduction Fingolimod (Gilenya ) is an oral disease-modifying agent indicated in the treatment of relapsing forms of multiple sclerosis (MS). 1 Fingolimod was approved by the FDA in September 2010 and was the first oral disease-modifying medication approved for treatment of multiple sclerosis. 1 An additional oral agent was recently approved for the treatment of multiple sclerosis: teriflunomide (Aubagio ). 2 Many injectable disease modifying agents are available for the treatment of MS including glatiramer (Copaxone ), interferon beta-1a (Avonex, Rebif ), interferon beta-1b (Betaseron, Extavia ), mitoxantrone (Novantrone ), and natalizumab (Tysabri ). 3, 4 Table 1 compares the available disease-modifying agents. This review will focus on the safety and efficacy of fingolimod in the treatment of relapsing forms of multiple sclerosis. Disease Overview Demyelinating diseases are neurological disorders defined by the destruction of central nervous system (CNS) tissue and are typically immune-mediated conditions. 3, 4 Multiple sclerosis (MS) is the most common demyelinating disorder and is characterized by inflammation, demyelination, scarring, and neuronal loss. 3, 4 Patients with MS can exhibit benign illness to a debilitating disease resulting in significant changes to one s lifestyle. 3, 4 Multiple sclerosis affects nearly 400,000 individuals in the United States and 2.5 million individuals worldwide. 5-7 The average estimated lifetime cost of illness from MS is estimated to be $1.2 million. 5-8 Prevalence is higher in women than men and the disease is usually diagnosed between the ages of 20 and 50 5, 6, 8 years. The cause of multiple sclerosis is not known. 3, 4 Both genetic (race and gender) and environmental factors (geographical location, exposure to the sun, birth month) are linked to the disease Immunology also plays a role; MS is thought to be an auto-immune disease mediated by T-cells that compromise the blood brain barrier and allow inflammatory mediators to enter and attack the CNS. Diagnosis of MS is based on clinical symptoms in combination with evidence of lesions on magnetic resonance imaging (MRI). Symptoms vary depending on the location and severity of the CNS lesions and may include sensory loss, optic neuritis, weakness, parasthesias, ataxia, tremor, fatigue, cognitive changes, and bladder dysfunction. 3, 4 Multiple sclerosis (MS) is a chronic disease that can progress intermittently or continuously and is divided into four disease courses: relapsing-remitting multiple sclerosis (RRMS), primary-progressive multiple sclerosis (PPMS), secondary-progressive multiple sclerosis (SPMS), and progressive-relapsing multiple sclerosis (PRMS). 3, 4 Relapsing-remitting multiple sclerosis is the most common form of MS and is characterized by exacerbations of neurological dysfunction followed by remissions. 12 RRMS may eventually develop into secondary progressive multiple sclerosis which is characterized by a neurologic deterioration with or without relapses. Primary progressive multiple sclerosis occurs in 10-15% of patients with MS and is characterized by disease progression with some minor improvements and without any exacerbations. 11, 13 Progressive relapsing multiple sclerosis affects less than 5% of patients and is characterized by disease progression with acute relapses. Most medications used in the treatment of MS are indicated in the treatment of RRMS or SPMS; there are currently no medications labeled for use in PPMS. 3, 4 4
5 Treatment of MS varies depending on the clinical subset of MS present and individual patient characteristics. 3, 4, 6, 8 In general, treatment may include disease modifying agents in combination with symptomatic treatment. 3, 4 Symptomatic treatments include glucocorticoid therapy, benzodiazepines, muscle relaxers, anticonvulsants, antidepressants, and medications used to treat urinary disorders. 3, 4 Currently, no curative medication therapies are available in the treatment of MS. 6, 8 Disease-modifying agents provide symptomatic relief and reduced disease progression.24 Many injectable disease-modifying agents are available for use in MS and two oral agents are available. Treatment guidelines for multiple sclerosis cite many of the biologic and interferon agents as effective treatment options, but do not recommend one therapy over another The interferon agents and glatiramer demonstrate reduced relapse rates and slowed disease progression and may be considered first-line therapy in RRMS. 16 Natalizumab and mitoxantrone may be considered in progressive patients who do not respond to or tolerate other disease-modifying therapies. 16 Evidence evaluating fingolimod, the oral sphingosine-1- phosphate (S1P) inhibitor, suggests fingolimod is safe and effective in reducing acute MS attacks 1, 6, 8 and may be a more convenient treatment option for patients. 5
6 1, 17, 18 Table 1. Comparison of Disease Modifying Agents used in the Treatment of Multiple Sclerosis Product Drug class Administration Labeled uses Unlabeled uses Dosing Generic Fingolimod (Gilenya) Glatiramer (Copaxone) Interferon beta- 1a (Avenox) Interferon beta- 1a (Rebif) Interferon beta- 1b (Betaseron, Extavia) Mitoxantrone (Novantrone) Natalizumab (Tysabri) Teriflunomide (Aubagio) Sphingosine 1- Phosphate (S1P) Receptor Modulator Biological Interferon Interferon Interferon Anthracenedione antineoplastic agent Monoclonal Antibody, Selective Adhesion-Molecule Inhibitor Pyrimidine Synthesis Inhibitor Oral; patient selfadministered Subcutaneous; patient selfadministered Intramuscular; patient selfadministered or in physician s office Subcutaneous; patient selfadministered Subcutaneous; patient selfadministered Intravenous; in clinic or hospital setting capable of administering chemotherapy Intravenous; in clinic or hospital setting Oral; patient selfadministered Treatment of relapsing forms of multiple sclerosis (MS) to reduce the frequency of clinical exacerbations and delay disability progression Management of relapsing-remitting type multiple sclerosis, including patients with a first clinical episode with MRI features consistent with multiple sclerosis Treatment of relapsing forms of multiple sclerosis (MS); clinical isolated syndrome Treatment of relapsing forms of multiple sclerosis (MS) Treatment of relapsing forms of multiple sclerosis (MS); treatment of first clinical episode with MRI features consistent with MS Initial treatment of acute nonlymphocytic leukemias (ANLL [includes myelogenous, promyelocytic, monocytic and erythroid leukemias]); treatment of advanced hormone-refractory prostate cancer; secondary progressive or relapsing-remitting multiple sclerosis (MS) Monotherapy for the treatment of relapsing forms of multiple sclerosis; treatment of moderately- to severelyactive Crohn s disease Treatment of relapsing forms of multiple sclerosis (MS) N/A 0.5mg once daily; doses >0.5mg/day associated with increased adverse events and no additional benefit N/A 20mg daily No Treatment of secondary progressive forms of multiple sclerosis (MS); to decrease the number and volume of active brain lesions, decrease overall disease burden, and delay onset of clinically definite MS in patients who have experienced a single demyelinating event. Treatment of secondary progressive forms of multiple sclerosis (MS); clinical isolated syndrome Treatment of secondary-progressive MS Treatment of Hodgkin lymphoma (refractory), non- Hodgkin lymphomas (NHL), acute lymphocytic leukemia (ALL), relapsed acute myeloid leukemia (AML), breast cancer (metastatic), pediatric acute myelogenous leukemia (AML), pediatric acute promyelocytic leukemia (APL); part of a conditioning regimen for autologous hematopoietic stem cell transplantation (HSCT), metastatic breast cancer, relapsed leukemia (adults), lymphoma, hepatocellular carcinoma, Combination use with glatiramer or interferon beta for relapsing forms of multiple sclerosis N/A 30mcg weekly 22-44mcg three times weekly, with gradual dose titration mcg every other day, with gradual dose titration 12mg/m 2 every 3 months (maximum lifetime cumulative dose: 140 mg/m 2 ) 300mg every 4 weeks 7 mg or 14 mg once daily Available No No No No Yes No No 6
7 Pharmacology Fingolimod is a sphingosine 1-phosphate (S1P) receptor modulator that works by binding to S1P receptors and altering their activity in inflammatory processes. 3, 4 In multiple sclerosis, fingolimod appears to decrease lymphocyte movement into the CNS and results in reduced CNS inflammation. 3, 4 According to clinical data, lymphocyte count decreases up to 60% within 6 hours of the first dose of fingolimod and will return to normal 1-2 months after discontinuing the drug. Fingolimod therapy also results in decreased neutrophil count but does not reduce monocyte count. Pharmacokinetics Fingolimod is dosed once daily with or without food, is slowly absorbed over 6 hours and reaches maximum plasma concentrations by hours. 1, After administration, fingolimod is largely distributed into red blood cells, has a large volume of distribution (1,200 L), is highly plasma protein bound and is primarily metabolized by hepatic CYP4F2. 22 Fingolimod is renally excreted and no dosage adjustments are 1, 17, 18 required for patients with renal or hepatic dysfunction. Methods A literature search was conducted to identify articles addressing each key question, searching the MEDLINE database ( ), the Cochrane Library, and reference lists of review articles. For the clinical efficacy section, only clinical trials published in English and indexed on MEDLINE 1/2011 to 2/2013, evaluating efficacy of fingolimod with reduction of symptoms or improvement in disease state as an endpoint are included. Trials evaluating fingolimod as monotherapy or combination therapy where adjunctive medications remained constant throughout the trial are included. Trials comparing monotherapy with combination regimens were excluded. The following reports were excluded (note: some were excluded for more than 1 reason): Individual clinical trials which evaluated endpoints other than reduction of symptoms, such as lymphocyte count 23-25, cost-effectiveness 26, quality of life 27, or immune response 28, 29. Individual trials comparing fingolimod in dose-finding studies or in healthy 19-22, volunteers. Individual clinical trials without access to the full article. Clinical Efficacy Seven clinical trials are available evaluating the efficacy of fingolimod in the treatment of relapsing multiple sclerosis Three randomized controlled trials compared fingolimod to placebo or interferon beta, 37, 39, 40 three non-comparative 7
8 extension trials evaluated the long-term efficacy of fingolimod, and one sub-group analysis assessed relapse and disability outcomes. 38, 41 The studies evaluated patients (predominantly Caucasian, adult women) with symptoms and a magnetic resonance imaging (MRI) scan indicative of relapsing multiple sclerosis. Patients with a score of 0-6 out of 10 on the Expanded Disability Status Scale (EDSS; higher scores indicate greater disability) were included. Patients received fingolimod doses of mg once daily for up to 36 months. All trials evaluated the relapse rate, defined as a new or worsening symptom and an increase in EDSS score of 0.5 or more. Cohen et al 37 evaluated 1292 patients randomized to receive fingolimod 0.5 mg or 1.25 mg once daily or intramuscular interferon beta-1a (Avonex ) 30 mcg once weekly for 12 months. Relapse rates were significantly lower in the fingolimod treatment groups ( ) compared to the interferon beta treatment group (0.33, p<0.001). The number of new or enlarged lesions on MRI was significantly higher in the interferon beta group compared to the fingolimod groups (p<0.005) at 12 months. No significant differences in relapse rate or number of lesions occurred between the fingolimod treatment groups. No significant difference in progression of disability occurred in any of the treatment groups. Adverse events were mild to moderate and similar across treatment groups. Willing patients from the trial by Cohen et al 37 enrolled in a follow-up study for up to 2 years. 43 Patients in the fingolimod treatment group continued the same treatment and patients originally randomized to receive interferon beta-1a were randomly reassigned to receive fingolimod 0.5 mg or 1.25 mg once daily. Patients in the continuous fingolimod treatment group demonstrated persistent benefits in relapse rates and number of lesions over 24 months and reported lower relapse rates and number of lesions compared to patients switched from interferon to fingolimod (p<0.05) at 24 months. Relapse rate and the number of new or enlarged lesions on MRI were significantly improved in the interferon switched to fingolimod treatment groups at 24 months (p<0.05). No change in disability progression was reported in any of the treatment groups. Adverse event rates were similar across treatment groups at 12 and 24 months. Kappos et al (2010) 40 evaluated 1,272 patients randomized to receive fingolimod 0.5 mg or 1.25 mg or placebo once daily for 24 months. A total of 1,272 patients were randomized and 1,033 patients (81.2%) completed the study with 945 patients (74.3%) still receiving fingolimod at the end of the trial. Relapse rates were significantly lower in the fingolimod treatment groups ( ) compared to placebo (0.40, p<0.001). The number of new or enlarged lesions on MRI was significantly higher in the placebo group compared to the fingolimod groups (p<0.001) at 24 months. Adverse events were similar across treatment groups; however, adverse event discontinuation rate was higher in the fingolimod 1.25 mg treatment group (14.2%) compared to the fingolimod 0.5 mg (7.5%) or placebo (7.7%) treatment groups. A sub-group analysis of patients enrolled in the Kappos et al (2010) 40 trial is available evaluating the efficacy of fingolimod in subgroups of patients with different baseline characteristics. 42 Subgroups included sex, age, baseline disability scores and lesion parameters, and response to previous therapy. Relapse rates were significantly 8
9 lower than placebo across all subgroups except for in patients aged over 40 years. This limited evidence suggests patients with relapsing-remitting MS with a wide spectrum of clinical and MRI features may benefit from fingolimod treatment. Kappos et al (2006) 39 evaluated patients with both relapsing-remitting multiple sclerosis and secondary progressive multiple sclerosis randomized to receive fingolimod 1.25 mg or 5 mg or placebo for 6-12 months. The total number lesions on MRI was significantly higher in the placebo group (14.8) compared to the fingolimod groups ( , p<0.001) at 6 months. The study was not powered to find a difference in relapse rate between treatment groups. Adverse event rates were higher in the fingolimod 5 mg treatment group (96%) compared to placebo (82%, p<0.05). No differences in adverse event rates were reported between the fingolimod 1.25 mg treatment group and placebo. Liver enzymes were significantly elevated in the fingolimod treatment groups (10-12%) compared to placebo (1%) at 6 months. Willing patients from the trial by Kappos et al (2006) 39 enrolled in a follow-up study for an additional 1-2 years. 38, 41 Patients originally randomized to receive fingolimod 5 mg were reassigned to receive fingolimod 1.25 mg because an increase in adverse effects with no added clinical benefit occurred with the 5 mg dose. At 24 months, improvements in number of lesions and relapse rate were sustained and similar to results at 12 months. 41 The majority of patients continued to be lesion free (88-89%) and relapse free (68-73%) for up to 36 months. 38 No significant increases in adverse event rate were reported over the 36 months. Clinical data evaluating the efficacy of fingolimod in the treatment of MS are limited. In comparative clinical trials, relapse rate was significantly improved with fingolimod ( ) compared to either interferon beta (0.33) or placebo (0.4) with 1-2 years of treatment. No clinical benefit was demonstrated with fingolimod at doses greater than 0.5 mg. Data from follow-up trials indicate sustained efficacy and no increases in adverse events with long-term fingolimod treatment. Limited data from a subgroup analysis indicate efficacy of fingolimod treatment across patients with a wide range of clinical features. Overall, fingolimod is an efficacious treatment option for patients with relapsing multiple sclerosis. Special Populations One randomized clinical trial evaluating the safety and efficacy of fingolimod treatment in 171 Japanese patients with relapsing MS is available. 44 Patients were randomized to receive fingolimod 0.5 mg or 1.25 mg daily or placebo for six months. Higher rates of lesion free MRIs were reported in the fingolimod treatment groups (70-86%) compared to placebo (40%, p<0.05). No differences in relapse rates were reported. Total adverse events, serious adverse events, and adverse event discontinuation rates were reported more frequently in the fingolimod treatment groups compared to placebo. 9
10 Fingolimod was not studied in pregnant or lactating women, children, or adults over 65 years of age. 1, 17, 18 No pharmacokinetic differences were reported between males and females treated with fingolimod. Animal data suggest there may be increased risk of fetal birth defects or death. No fingolimod dosage adjustment is recommended in patients with renal impairment or hepatic insufficiency. 1, 17, 18 In a trial evaluating the effects of severe hepatic impairment on fingolimod, overall exposure doubled, the half-life was increased by 50% and the authors of the trial recommend reducing the fingolimod maintenance dose by 50% in patients with severe hepatic impairment. 33 Adverse Drug Reactions Although fingolimod has numerous adverse effects, it has good tolerability relative to alternative MS therapeutic agents. Adverse effects associated with fingolimod are frequent but usually only mild to moderate. The most common adverse events reported with fingolimod therapy include headache (25%), elevated liver enzymes (14%), diarrhea (12%), and cough (10%). 1, 17, 18 Serious adverse events associated with fingolimod therapy include cardiovascular abnormalities (bradycardia (4%) and atrioventricular (AV) block) and patients most commonly discontinued therapy due to elevated liver enzymes (up to three times the normal value). 1, 17, 18 Fingolimod therapy is associated with first-dose bradycardia (reduction of about beats/min) within 6 1, 17, 18 hours of the first dose and resolving within 24 hours. Because of this, fingolimod is only available through specialty pharmacies or the manufacturers First Dose Program. 17, 18 In a 3 year clinical trial, fingolimod was generally well tolerated. Upper respiratory infection, nasopharyngitis, headache, dyspnea, fatigue, and influenza were the most frequently reported adverse events. 38 Asymptomatic liver enzyme elevation, increased blood pressure, and bradycardia and heart block occurred throughout the 36 month period. 38 Fingolimod is metabolized by the CYP4F2 isoenzyme which primarily metabolizes endogenous substances. 1, 17, 18 Fingolimod has not demonstrated induction or inhibition of any of the major CYP P450 enzymes or interactions with any of the major P-glycoproteins or other transporters. Although drug interactions may occur, clinical studies have failed to demonstrate any significant drug-drug interactions. 22, 30 Theoretical or additive effects may occur with coadministration of fingolimod and ketoconazole, beta-blockers, and antiarrhythmic agents. 22, 30 Administration of fingolimod in combination with immunosuppressant agents may result in increased 1, 17, 18 immunosuppression. Summary Fingolimod is a sphingosine 1-phosphate receptor modulator indicated in the treatment of relapsing-remitting multiple sclerosis. Fingolimod is dosed once daily and was the first oral disease-modifying agent approved for use in MS. Higher than recommended doses produce increased side effects without increased benefit. The exact 10
11 mechanism of action of fingolimod is not known but it is thought to alter lymphocyte activity in inflammatory processes and reduce CNS inflammation. Treatment guidelines for multiple sclerosis recommend many of the biologic and interferon agents as effective treatment options, but do not recommend one therapy over another. Limited comparative clinical evidence is available for fingolimod and of the available disease modifying agents, comparative evidence is only available for fingolimod versus the interferon products. In the available comparative trials, fingolimod demonstrated similar efficacy as interferon beta. In placebo-controlled trials, fingolimod therapy significantly reduced disability progression at 3 and 6 months compared to placebo and the benefits of fingolimod therapy were demonstrated for up to 36 months. Fingolimod has numerous adverse effects but they are usually only mild to moderate. The most common adverse events reported with fingolimod therapy include headache, elevated liver enzymes, and diarrhea. The most serious adverse events associated with fingolimod therapy are cardiovascular abnormalities and first-dose bradycardia. Fingolimod is only available through specialty pharmacies or the manufacturers First Dose Program. 11
12 References 1. Novartis. Gilenya (fingolimod) oral capsules [package insert]. In. East Hanover, NJ: Novartis Pharmaceuticals Corporation; Sanofi. Aubagio (teriflunomide) oral tablets [package insert]. In. Cambridge, MA: Genzyme Corporation; Goodin DS, Hauser SL. Chapter 380. Multiple Sclerosis and Other Demyelinating Diseases. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine. 18th ed. New York: McGraw-Hill; Ropper AH, Samuels MA. Chapter 36. Multiple Sclerosis and Allied Demyelinating Diseases. In: Ropper AH, Samuels MA, eds. Adams and Victor's Principles of Neurology. 9th ed. New York: New York; Kantarci O, Wingerchuk D. Epidemiology and natural history of multiple sclerosis: new insights. Curr Opin Neurol 2006;19: New drugs for relapsing multiple sclerosis. Med Lett Drugs Ther;54: Killestein J, Rudick RA, Polman CH. Oral treatment for multiple sclerosis. Lancet Neurol;10: Gold R. Oral therapies for multiple sclerosis: a review of agents in phase III development or recently approved. CNS Drugs;25: Compston A, Coles A. Multiple sclerosis. Lancet 2008;372: Fox RJ, Bethoux F, Goldman MD, Cohen JA. Multiple sclerosis: advances in understanding, diagnosing, and treating the underlying disease. Cleve Clin J Med 2006;73: Keegan BM, Noseworthy JH. Multiple sclerosis. Annu Rev Med 2002;53: Siva A. The spectrum of multiple sclerosis and treatment decisions. Clin Neurol Neurosurg 2006;108: Weinstock-Guttman B, Ramanathan M. Multiple sclerosis in 2011: Advances in therapy, imaging and risk factors in MS. Nat Rev Neurol;8: Goodin DS, Frohman EM, Garmany GP, Jr., et al. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology 2002;58: National Multiple Sclerosis Society. Expert opinion paper: National CLinical Advisory Board of the National Multiple Sclerosis Society - treatment recommendations for physicians, disease management consensus statement. In: Society NMS, ed. New York, NY; Goodin DS, Arnason BG, Coyle PK, Frohman EM, Paty DW. The use of mitoxantrone (Novantrone) for the treatment of multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2003;61: Lexi-Comp I, ed. Drug Information Handbook. 21st ed. Hudson, OH: Lexi-Comp; McEvoy GK, Snow EK, Kester L, Litvak K, Miller J, Welsh OH, eds. AHFS 2011 Drug Information. Bethesda, MD: American Society of Health-System Pharmacists; Kovarik JM, Hartmann S, Bartlett M, et al. Oral-intravenous crossover study of fingolimod pharmacokinetics, lymphocyte responses and cardiac effects. Biopharm Drug Dispos 2007;28: Kovarik JM, Schmouder R, Barilla D, Riviere GJ, Wang Y, Hunt T. Multiple-dose FTY720: tolerability, pharmacokinetics, and lymphocyte responses in healthy subjects. J Clin Pharmacol 2004;44: Kovarik JM, Schmouder R, Barilla D, Wang Y, Kraus G. Single-dose FTY720 pharmacokinetics, food effect, and pharmacological responses in healthy subjects. Br J Clin Pharmacol 2004;57: Kovarik JM, Dole K, Riviere GJ, et al. Ketoconazole increases fingolimod blood levels in a drug interaction via CYP4F2 inhibition. J Clin Pharmacol 2009;49: Johnson TA, Evans BL, Durafourt BA, et al. Reduction of the peripheral blood CD56(bright) NK lymphocyte subset in FTY720-treated multiple sclerosis patients. J Immunol;187: Johnson TA, Lapierre Y, Bar-Or A, Antel JP. Distinct properties of circulating CD8+ T cells in FTY720- treated patients with multiple sclerosis. Arch Neurol;67: Johnson TA, Shames I, Keezer M, et al. Reconstitution of circulating lymphocyte counts in FTY720- treated MS patients. Clin Immunol;137: Agashivala N, Kim E. Cost-effectiveness of early initiation of fingolimod versus delayed initiation after 1 year of intramuscular interferon beta-1a in patients with multiple sclerosis. Clin Ther;34:
13 27. Montalban X, Comi G, O'Connor P, et al. Oral fingolimod (FTY720) in relapsing multiple sclerosis: impact on health-related quality of life in a phase II study. Mult Scler;17: Mehling M, Hilbert P, Fritz S, et al. Antigen-specific adaptive immune responses in fingolimod-treated multiple sclerosis patients. Ann Neurol;69: Kowarik MC, Pellkofer HL, Cepok S, et al. Differential effects of fingolimod (FTY720) on immune cells in the CSF and blood of patients with MS. Neurology;76: Kovarik JM, Lu M, Riviere GJ, et al. The effect on heart rate of combining single-dose fingolimod with steady-state atenolol or diltiazem in healthy subjects. Eur J Clin Pharmacol 2008;64: Kovarik JM, Riviere GJ, Neddermann D, Maton S, Hunt TL, Schmouder RL. A mechanistic study to assess whether isoproterenol can reverse the negative chronotropic effect of fingolimod. J Clin Pharmacol 2008;48: Kovarik JM, Schmouder RL, Barilla D, et al. FTY720 and cyclosporine: evaluation for a pharmacokinetic interaction. Ann Pharmacother 2004;38: Kovarik JM, Schmouder RL, Hartmann S, et al. Fingolimod (FTY720) in severe hepatic impairment: pharmacokinetics and relationship to markers of liver function. J Clin Pharmacol 2006;46: Kovarik JM, Slade A, Riviere GJ, et al. The ability of atropine to prevent and reverse the negative chronotropic effect of fingolimod in healthy subjects. Br J Clin Pharmacol 2008;66: Kovarik JM, Slade A, Voss B, et al. Ethnic sensitivity study of fingolimod in white and Asian subjects. Int J Clin Pharmacol Ther 2007;45: Kovarik JM, Tedesco-Silva H, Lorber MI, Foster C. Exposure-efficacy relationships of a fingolimodeverolimus regimen in kidney transplant patients at risk for delayed graft function. Transplant Proc 2006;38: Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med;362: Comi G, O'Connor P, Montalban X, et al. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3-year results. Mult Scler;16: Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 2006;355: Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med;362: O'Connor P, Comi G, Montalban X, et al. Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a phase II extension study. Neurology 2009;72: Devonshire V, Havrdova E, Radue EW, et al. Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol;11: Khatri B, Barkhof F, Comi G, et al. Comparison of fingolimod with interferon beta-1a in relapsingremitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol;10: Saida T, Kikuchi S, Itoyama Y, et al. A randomized, controlled trial of fingolimod (FTY720) in Japanese patients with multiple sclerosis. Mult Scler;18:
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Teriflunomide (Aubagio ) Drug Review
 Teriflunomide (Aubagio ) Drug Review AHFS 92:20, Biologic Response Modifiers Final Report March 2013 Review prepared by: Melissa Archer, PharmD, Clinical Pharmacist Gary Oderda, PharmD, MPH, Professor
Teriflunomide (Aubagio ) Drug Review AHFS 92:20, Biologic Response Modifiers Final Report March 2013 Review prepared by: Melissa Archer, PharmD, Clinical Pharmacist Gary Oderda, PharmD, MPH, Professor
Medication Policy Manual. Topic: Gilenya, fingolimod Date of Origin: November 22, 2010
 Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual. Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014
 Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual. Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004
 Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Version History. Previous Versions. Drugs for MS.Drug facts box fingolimod Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Conflict of Interest Declaration. Overview of New Medications for Multiple Sclerosis. Assessment Question. Objectives 4/1/2011
 Conflict of Interest Declaration Overview of New Medications for Multiple Sclerosis I or my spouse have no actual or potential conflict of interest in relation to this activity. Crystal Obering, Pharm.D.,
Conflict of Interest Declaration Overview of New Medications for Multiple Sclerosis I or my spouse have no actual or potential conflict of interest in relation to this activity. Crystal Obering, Pharm.D.,
Lemtrada (alemtuzumab)
 Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Lemtrada (alemtuzumab) Policy Number: 5.02.517 Last Review: 08/2015 Origination: 08/2015 Next Review: 08/2016 Policy BCBSKC will provide coverage for Lemtrada (alemtuzumab) when it is determined to be
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
New Treatment Options for MS Patients: Understanding risks versus benefits
 New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
New Treatment Options for MS Patients: Understanding risks versus benefits By Michael A. Meyer, MD Department of Neurology, Sisters Hospital, Buffalo, NY Objectives: 1. to understand fundamentals of MS
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Relapsing-remitting multiple sclerosis Ambulatory with or without aid
 AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
Progress in MS: Current and Emerging Therapies
 Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
National Multiple Sclerosis Society. Disease Modification in Multiple Sclerosis. Current as of January 2, 2013
 National Multiple Sclerosis Society Disease Modification in Multiple Sclerosis Current as of January 2, 2013 Since 1993, the U.S. Food and Drug Administration (FDA) has approved several medications for
National Multiple Sclerosis Society Disease Modification in Multiple Sclerosis Current as of January 2, 2013 Since 1993, the U.S. Food and Drug Administration (FDA) has approved several medications for
fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK
 fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK 08 August 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK 08 August 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
Disclosures. Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
 Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Drug Class Review. Disease-modifying Drugs for Multiple Sclerosis. Single Drug Addendum: Fingolimod
 Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Single Drug Addendum: Fingolimod Final Original Report February 2011 The Agency for Healthcare Research and Quality has not yet seen or
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON
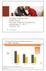 Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis (MS) Class Update
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
What is Multiple Sclerosis? Gener al information
 What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook
 National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
Integrating New Treatments: A Case Based Approach
 Integrating New Treatments: A Case Based Approach JILL CONWAY, MD, MA, MSCE DIRECTOR, MS CENTER DIRECTOR, NEUROLOGY CLERKSHIP AT UNCSOM- CHARLOTTE CAMPUS CAROLINAS HEALTHCARE CENTER Objectives Provide
Integrating New Treatments: A Case Based Approach JILL CONWAY, MD, MA, MSCE DIRECTOR, MS CENTER DIRECTOR, NEUROLOGY CLERKSHIP AT UNCSOM- CHARLOTTE CAMPUS CAROLINAS HEALTHCARE CENTER Objectives Provide
Which injectable medication should I take for relapsing-remitting multiple sclerosis?
 Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Department of Health. Rheynn Slaynt. Clinical Recommendations Committee
 Recommendation 06/13 Department of Health Rheynn Slaynt Clinical Recommendations Committee The Isle of Man Department of Health recommend Gilenya (fingolimod) as a HIGH PRIORITY - as an option for the
Recommendation 06/13 Department of Health Rheynn Slaynt Clinical Recommendations Committee The Isle of Man Department of Health recommend Gilenya (fingolimod) as a HIGH PRIORITY - as an option for the
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents
 New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
Personalised Medicine in MS
 Personalised Medicine in MS Supportive Evidence from Therapeutic Trials Ludwig Kappos Neurology and Department of Biomedicine University Hospital CH-4031 Basel LKappos@uhbs.ch Established partially effective
Personalised Medicine in MS Supportive Evidence from Therapeutic Trials Ludwig Kappos Neurology and Department of Biomedicine University Hospital CH-4031 Basel LKappos@uhbs.ch Established partially effective
Multiple Sclerosis Update. Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center
 Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Multiple Sclerosis Update Bridget A. Bagert, MD, MPH Director, Ochsner Multiple Sclerosis Center None Disclosures First of All. Why is my talk in the Neurodegenerative hour? I respectfully object! Case
Treatments for MS: Immunotherapy. Gilenya (fingolimod) Glatiramer acetate (Copaxone )
 Treatments for MS: Immunotherapy There are currently several disease-modifying therapies approved for people with MS in Australia. These therapies, called immunotherapies, work to reduce disease activity
Treatments for MS: Immunotherapy There are currently several disease-modifying therapies approved for people with MS in Australia. These therapies, called immunotherapies, work to reduce disease activity
Multiple Sclerosis - Relapsing and Remissioning
 DISEASE-MODIFYING THERAPIES IN RELAPSING-REMITTING MULTIPLE SCLEROSIS* Benjamin M. Greenberg, MD, MHS ABSTRACT Four major disease-modifying therapies are discussed within the context of relapsing and remitting
DISEASE-MODIFYING THERAPIES IN RELAPSING-REMITTING MULTIPLE SCLEROSIS* Benjamin M. Greenberg, MD, MHS ABSTRACT Four major disease-modifying therapies are discussed within the context of relapsing and remitting
CNS DEMYLINATING DISORDERS
 CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
CNS DEMYLINATING DISORDERS Multiple sclerosis A Dutch saint named Lidwina, who died in 1433, may have been one of the first known MS patients. After she fell while ice skating, she developed symptoms such
A neurologist would assess your eligibility and suitability for the DMTs.
 Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Choices Disease Modifying Treatments Disease modifying treatments (DMTs) are medications which modify the disease course. They target inflammation and are designed to reduce the damage caused by relapses.
Summary HTA. Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C. HTA-Report Summary
 Summary HTA HTA-Report Summary Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C Health policy background Multiple sclerosis (MS) is a chronic inflammatory disease
Summary HTA HTA-Report Summary Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C Health policy background Multiple sclerosis (MS) is a chronic inflammatory disease
PCORI Workshop on Treatment for Multiple Sclerosis. Breakout Group Topics and Questions Draft 3-27-15
 PCORI Workshop on Treatment for Multiple Sclerosis Breakout Group Topics and Questions Draft 3-27-15 Group 1 - Comparison across DMTs, including differential effects in subgroups Consolidated straw man
PCORI Workshop on Treatment for Multiple Sclerosis Breakout Group Topics and Questions Draft 3-27-15 Group 1 - Comparison across DMTs, including differential effects in subgroups Consolidated straw man
SECTION 2. Section 2 Multiple Sclerosis (MS) Drug Coverage
 SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
SECTION 2 Multiple Sclerosis (MS) Drug Coverage Section 2 Multiple Sclerosis (MS) Drug Coverage ALBERTA HEALTH AND WELLNESS DRUG BENEFIT LIST Selected Drug Products used in the treatment of patients with
Current and future options of MS treatment Prof. Dr. Karl Vass, AKH Wien
 Current and future options of MS treatment Prof. Dr. Karl Vass, AKH Wien European Health Forum, Gastein 6 th October 2010 Multiple Sclerosis is the most common neurological disorder in young Caucasian
Current and future options of MS treatment Prof. Dr. Karl Vass, AKH Wien European Health Forum, Gastein 6 th October 2010 Multiple Sclerosis is the most common neurological disorder in young Caucasian
How to S.E.A.R.C.H. SM for the Right MS Therapy For You!
 How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
How to S.E.A.R.C.H. SM for the Right MS Therapy For You! The Changing Landscape The first treatment for relapsing-remitting multiple sclerosis (RRMS) was approved by the United States Food and Drug Administration
New Developments in the Treatment and Management of Multiple Sclerosis
 New Developments in the Treatment and Management of Multiple Sclerosis Myla D. Goldman, MD, MS For a CME/CEU version of this article, please go to www.namcp.org/cmeonline.htm, and then click the activity
New Developments in the Treatment and Management of Multiple Sclerosis Myla D. Goldman, MD, MS For a CME/CEU version of this article, please go to www.namcp.org/cmeonline.htm, and then click the activity
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
Therapeutic Class Overview Multiple Sclerosis Agents
 Therapeutic Class Overview Multiple Sclerosis Agents Therapeutic Class Overview/Summary: Several biologic response modifiers are Food and Drug Administration (FDA)- approved for the treatment of relapsing-remitting
Therapeutic Class Overview Multiple Sclerosis Agents Therapeutic Class Overview/Summary: Several biologic response modifiers are Food and Drug Administration (FDA)- approved for the treatment of relapsing-remitting
Drug Use Research & Management Program Phone Fax Generic Name: Brand Name (Manufacturer): PDL Class: Comparator Therapies: Dossier received:
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-945-5220 Fax 503-947-1119 Generic Name: Fingolimod Brand Name (Manufacturer):
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-945-5220 Fax 503-947-1119 Generic Name: Fingolimod Brand Name (Manufacturer):
MEDICAL POLICY STATEMENT
 MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 10/01/2013 10/1/2015 08/25/2015 Policy Name Policy Number Multiple Sclerosis Therapy Class SRx-0022
MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 10/01/2013 10/1/2015 08/25/2015 Policy Name Policy Number Multiple Sclerosis Therapy Class SRx-0022
Compositional Changes of B and T Cell Subtypes during Fingolimod Treatment in Multiple Sclerosis Patients: A 12-Month Follow-Up Study
 Compositional Changes of B and T Cell Subtypes during Fingolimod Treatment in Multiple Sclerosis Patients: A 12-Month Follow-Up Study Nele Claes 1 *; Tessa Dhaeze 1 *; Judith Fraussen PhD 1 ; Bieke Broux
Compositional Changes of B and T Cell Subtypes during Fingolimod Treatment in Multiple Sclerosis Patients: A 12-Month Follow-Up Study Nele Claes 1 *; Tessa Dhaeze 1 *; Judith Fraussen PhD 1 ; Bieke Broux
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru381 Topic: Lemtrada TM, alemtuzumab Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru381 Topic: Lemtrada TM, alemtuzumab Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Multiple Sclerosis: What You Need To Know. For Professionals
 Multiple Sclerosis: What You Need To Know For Professionals What will I learn today? The Basics: What is MS? Living with MS: A Family Affair We Can Help: The National MS Society What MS Is: MS is thought
Multiple Sclerosis: What You Need To Know For Professionals What will I learn today? The Basics: What is MS? Living with MS: A Family Affair We Can Help: The National MS Society What MS Is: MS is thought
Fingolimod (Gilenya ) National Drug Monograph
 Fingolimod (Gilenya ) National Drug Monograph February 211 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives The purpose of VA PBM Services drug monographs
Fingolimod (Gilenya ) National Drug Monograph February 211 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives The purpose of VA PBM Services drug monographs
A Letter From the MS Coalition
 0 A Letter From the MS Coalition The treatment of multiple sclerosis (MS) requires a comprehensive management strategy. One important component of that strategy is modifying the disease course. When deciding
0 A Letter From the MS Coalition The treatment of multiple sclerosis (MS) requires a comprehensive management strategy. One important component of that strategy is modifying the disease course. When deciding
The MS Disease- Modifying Drugs. Gener al information
 The MS Disease- Modifying Drugs Gener al information Current as of October 30, 2009. This online version is updated as breaking news requires. If you have downloaded and printed a copy from the web, please
The MS Disease- Modifying Drugs Gener al information Current as of October 30, 2009. This online version is updated as breaking news requires. If you have downloaded and printed a copy from the web, please
teriflunomide, 14mg, film-coated tablets (Aubagio ) SMC No. (940/14) Genzyme Ltd.
 teriflunomide, 14mg, film-coated tablets (Aubagio ) SMC No. (940/14) Genzyme Ltd. 10 January 2014 (Issued 07 February 2014) The Scottish Medicines Consortium (SMC) has completed its assessment of the above
teriflunomide, 14mg, film-coated tablets (Aubagio ) SMC No. (940/14) Genzyme Ltd. 10 January 2014 (Issued 07 February 2014) The Scottish Medicines Consortium (SMC) has completed its assessment of the above
AUBAGIO (teriflunomide) oral tablet
 AUBAGIO (teriflunomide) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
AUBAGIO (teriflunomide) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
Brand Name: Gilenya. Generic Name: Fingolimod. Manufacturer 1 : Novartis Pharmaceutical Corporation
 Brand Name: Gilenya Generic Name: Fingolimod Manufacturer 1 : Novartis Pharmaceutical Corporation Drug Class 1,2 : Sphingosine 1-phosphate receptor modulator Uses: Labeled Uses 1,2,3,4,5 : Relapsing forms
Brand Name: Gilenya Generic Name: Fingolimod Manufacturer 1 : Novartis Pharmaceutical Corporation Drug Class 1,2 : Sphingosine 1-phosphate receptor modulator Uses: Labeled Uses 1,2,3,4,5 : Relapsing forms
fingolimod (as hydrochloride), 0.5mg hard capsules (Gilenya ) SMC No. (763/12) Novartis Pharmaceuticals UK Ltd
 fingolimod (as hydrochloride), 0.5mg hard capsules (Gilenya ) SMC No. (763/12) Novartis Pharmaceuticals UK Ltd 10 February 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the
fingolimod (as hydrochloride), 0.5mg hard capsules (Gilenya ) SMC No. (763/12) Novartis Pharmaceuticals UK Ltd 10 February 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio
 ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
A blood sample will be collected annually for up to 2 years for JCV antibody testing.
 Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Treatment in Relapsing MS: Choosing Among the Options. Donald Negroski, MD
 Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
Version History. Previous Versions. Policy Title. Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
J.P. Morgan Cazenove Therapeutic Seminar
 Jannan, MS J.P. Morgan Cazenove Therapeutic Seminar David Meeker - CEO, Genzyme June 25, 2012 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private
Jannan, MS J.P. Morgan Cazenove Therapeutic Seminar David Meeker - CEO, Genzyme June 25, 2012 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private
How To Use A Drug In Multiple Sclerosis
 Revised (2009) guidelines for prescribing in multiple sclerosis INTRODUCTION In January 2001, the (ABN) first published guidelines for the use of licensed disease modifying treatments (ß-interferon and
Revised (2009) guidelines for prescribing in multiple sclerosis INTRODUCTION In January 2001, the (ABN) first published guidelines for the use of licensed disease modifying treatments (ß-interferon and
ß-interferon and. ABN Guidelines for 2007 Treatment of Multiple Sclerosis with. Glatiramer Acetate
 ABN Guidelines for 2007 Treatment of Multiple Sclerosis with ß-interferon and Glatiramer Acetate Published by the Association of British Neurologists Ormond House, 27 Boswell Street, London WC1N 3JZ Contents
ABN Guidelines for 2007 Treatment of Multiple Sclerosis with ß-interferon and Glatiramer Acetate Published by the Association of British Neurologists Ormond House, 27 Boswell Street, London WC1N 3JZ Contents
Laquinimod Polman, C. et al. Neurology 2005;64:987-991
 Laquinimod Polman, C. et al. Neurology 2005;64:987-991 Multicenter, double-blind, randomized trial, patients with RR MS received 0.1 mg or 0.3 mg laquinimod or placebo as three daily tablets for 24 weeks
Laquinimod Polman, C. et al. Neurology 2005;64:987-991 Multicenter, double-blind, randomized trial, patients with RR MS received 0.1 mg or 0.3 mg laquinimod or placebo as three daily tablets for 24 weeks
acquired chronic immune-mediated inflammatory condition of CNS. MS in children: 10% +secondary progressive MS: rare +primary progressive MS: rare
 Immunomodulatory Therapies in Pediatric MS Vuong Chinh Quyen Neurology Department Medscape Mar 8, 2013 Multiple Sclerosis in Children. Iran J Child Neurol. 2013 Spring Introduction acquired chronic immune-mediated
Immunomodulatory Therapies in Pediatric MS Vuong Chinh Quyen Neurology Department Medscape Mar 8, 2013 Multiple Sclerosis in Children. Iran J Child Neurol. 2013 Spring Introduction acquired chronic immune-mediated
- Patients treated with alemtuzumab in CARE-MS II were more than twice as likely to experience disability improvement compared to Rebif -
 PRESS RELEASE Significant Improvement in Disability Scores Observed in Multiple Sclerosis Patients Who Received Lemtrada TM* (Alemtuzumab) Compared With Rebif in Phase lll Trial - Patients treated with
PRESS RELEASE Significant Improvement in Disability Scores Observed in Multiple Sclerosis Patients Who Received Lemtrada TM* (Alemtuzumab) Compared With Rebif in Phase lll Trial - Patients treated with
Progress in the field: therapeutic improvements for all patients?
 Progress in the field: therapeutic improvements for all patients? Krzysztof Selmaj, Department of Neurology, Medical University of Lodz, PL Warsaw 15 May, 2015 Main features of MS Inflammation Demyelination
Progress in the field: therapeutic improvements for all patients? Krzysztof Selmaj, Department of Neurology, Medical University of Lodz, PL Warsaw 15 May, 2015 Main features of MS Inflammation Demyelination
Growth in revenue from MS drugs has been driven largely by price increases over the last several years.
 March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
March 4, 2013 Ben Weintraub, PhD Are Injectable MS Drugs Finished? Market Ready for Tecfidera Companies: Biogen (BIIB) Sanofi (SNY) Teva (TEVA) Novartis (NVS) Merck Serono Bayer Schering Products: Tecfidera
NHS BOURNEMOUTH AND POOLE AND NHS DORSET
 NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
NHS BOURNEMOUTH AND POOLE AND NHS DORSET COMMISSIONING STATEMENT ON THE USE OF BETA-INTERFERON IN RELAPSING-REMITTING MULTIPLE SCLEROSIS OR SECONDARY PROGRESSIVE MULTIPLE SCLEROSIS, WHERE RELAPSES ARE
Pharmacotherapy of Multiple Sclerosis
 PHARMACY / MEDICAL POLICY 5.01.565 Pharmacotherapy of Multiple Sclerosis Effective Date: July 1, 2016 Last Revised: June 14, 2016 Replaces: Extracted from 5.01.550 RELATED MEDICAL POLICIES: 5.01.556 Rituximab:
PHARMACY / MEDICAL POLICY 5.01.565 Pharmacotherapy of Multiple Sclerosis Effective Date: July 1, 2016 Last Revised: June 14, 2016 Replaces: Extracted from 5.01.550 RELATED MEDICAL POLICIES: 5.01.556 Rituximab:
The MS Disease- Modifying Medications GENERAL INFORMATION
 The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
The MS Disease- Modifying Medications GENERAL INFORMATION Current as of March 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication, please
How to S.E.A.R.C.H. for the Right MS Therapy for You!
 How to S.E.A.R.C.H. for the Right MS Therapy for You! How to S.E.A.R.C.H. for the Right MS Therapy for You! Copyright Multiple Sclerosis Association of America, 2012. All rights reserved. This booklet
How to S.E.A.R.C.H. for the Right MS Therapy for You! How to S.E.A.R.C.H. for the Right MS Therapy for You! Copyright Multiple Sclerosis Association of America, 2012. All rights reserved. This booklet
Understanding How Existing and Emerging MS Therapies Work
 Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
Understanding How Existing and Emerging MS Therapies Work This is a promising and hopeful time in the field of multiple sclerosis (MS). Many new and different therapies are nearing the final stages of
06/06/2012. The Impact of Multiple Sclerosis in the Pacific Northwest. James Bowen, MD. Swedish Neuroscience Institute
 The Impact of Multiple Sclerosis in the Pacific Northwest James Bowen, MD Multiple Sclerosis Center Multiple Sclerosis Center Swedish Neuroscience Institute 1 2 Motor Symptoms of MS Weakness Spasticity
The Impact of Multiple Sclerosis in the Pacific Northwest James Bowen, MD Multiple Sclerosis Center Multiple Sclerosis Center Swedish Neuroscience Institute 1 2 Motor Symptoms of MS Weakness Spasticity
News on modifying diseases therapies. Michel CLANET CHU Toulouse France ECTRIMS
 News on modifying diseases therapies Michel CLANET CHU Toulouse France ECTRIMS Current treatment strategies Future oral treatments Future non oral treatments Drug safety and risks CIS at risk of MS Active
News on modifying diseases therapies Michel CLANET CHU Toulouse France ECTRIMS Current treatment strategies Future oral treatments Future non oral treatments Drug safety and risks CIS at risk of MS Active
What is MS? 1. disease that affects the central nervous. Is a disease that affects both white and gray matter
 What is MS? 1 Neuron Damaged myelin due to inflammation MS is a chronic immunemediated disease that affects the central nervous system (CNS) Is a disease that affects both white and gray matter Interrupted
What is MS? 1 Neuron Damaged myelin due to inflammation MS is a chronic immunemediated disease that affects the central nervous system (CNS) Is a disease that affects both white and gray matter Interrupted
5.07.09. Aubagio. Aubagio (teriflunomide) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Multiple Sclerosis Drug Discoveries - What the Future Holds
 Brochure More information from http://www.researchandmarkets.com/reports/1408035/ Multiple Sclerosis Drug Discoveries - What the Future Holds Description: The recent approval in the US of Novartis' orally
Brochure More information from http://www.researchandmarkets.com/reports/1408035/ Multiple Sclerosis Drug Discoveries - What the Future Holds Description: The recent approval in the US of Novartis' orally
Literature Scan: Oral Multiple Sclerosis Drugs
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Version History. Previous Versions. for secondary progressive MS (SPMS) Policy Title. Drugs for MS.Drug facts box Interferon beta 1b
 Version History Policy Title Drugs for MS.Drug facts box Interferon beta 1b for secondary progressive MS (SPMS) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review
Version History Policy Title Drugs for MS.Drug facts box Interferon beta 1b for secondary progressive MS (SPMS) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis
 Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Final Update 3 Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness
Drug Class Review Disease-modifying Drugs for Multiple Sclerosis Final Update 3 Report May 2016 The purpose of reports is to make available information regarding the comparative clinical effectiveness
Clinical Commissioning Policy: Disease Modifying Therapies For patients With Multiple Sclerosis (MS) December 2012. Reference : NHSCB/D4/c/1
 Clinical Commissioning Policy: Disease Modifying Therapies For patients With Multiple Sclerosis (MS) December 2012 Reference : NHSCB/D4/c/1 NHS Commissioning Board Clinical Commissioning Policy: Disease
Clinical Commissioning Policy: Disease Modifying Therapies For patients With Multiple Sclerosis (MS) December 2012 Reference : NHSCB/D4/c/1 NHS Commissioning Board Clinical Commissioning Policy: Disease
Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple Sclerosis
 Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Information About Medicines for Multiple Sclerosis
 Information About Medicines for Multiple Sclerosis Information About Medicines for Multiple Sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Information About Medicines for Multiple Sclerosis Information About Medicines for Multiple Sclerosis What is multiple sclerosis? 1 Multiple sclerosis (MS) is a lifelong disease that affects your brain
Ontario Reimburses CIS Indication for REBIF, a First-Line Treatment for Multiple Sclerosis
 May 25, 2015 Contact: Shikha Virdi 905-919-0200 ext. 5504 Ontario Reimburses CIS Indication for REBIF, a First-Line Treatment for Multiple Sclerosis Rebif now reimbursed under Ontario Drug Benefit Program
May 25, 2015 Contact: Shikha Virdi 905-919-0200 ext. 5504 Ontario Reimburses CIS Indication for REBIF, a First-Line Treatment for Multiple Sclerosis Rebif now reimbursed under Ontario Drug Benefit Program
Natalizumab (Tysabri)
 Natalizumab (Tysabri) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Natalizumab (Tysabri) Date of issue: July 2010 Review date: July 2011 Contents Section
Natalizumab (Tysabri) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Natalizumab (Tysabri) Date of issue: July 2010 Review date: July 2011 Contents Section
Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis
 Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness of teriflunomide
Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness of teriflunomide
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
RISK EVALUATION AND MITIGATION STRATEGY (REMS)
 Initial REMS Approval: 9/2010 Most Recent Modification: 05/2015 0.5mg capsules Sphingosine 1-phosphate Receptor Modulator Novartis Pharmaceuticals Corporation One Health Plaza East Hanover, NJ 07936 RISK
Initial REMS Approval: 9/2010 Most Recent Modification: 05/2015 0.5mg capsules Sphingosine 1-phosphate Receptor Modulator Novartis Pharmaceuticals Corporation One Health Plaza East Hanover, NJ 07936 RISK
Disease Modifying Therapies (DMTs) in Multiple Sclerosis
 Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
Disease Modifying Therapies (DMTs) in Multiple Sclerosis Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology Conflict of Interest Dr. Stobbe has no conflicts of
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS?
 Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
Novel therapeutic approaches in multiple sclerosis Neuroprotective and remyelinating agents, the future of clinical trials in MS? Marie Trad, M.D., Lynne Hughes, Cathy VanBelle, Amy Del Medico 3rd International
The MS Disease- Modifying Medications
 The MS Disease- Modifying Medications National MS Society 1 Current as of November 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
The MS Disease- Modifying Medications National MS Society 1 Current as of November 2014. This online brochure is updated with breaking news as required. If you have a printed a copy of this publication,
Managing Relapsing Remitting MS Risks & benefits of emerging therapies. Dr Mike Boggild The Walton Centre
 Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
Resources for the Primary Care Provider. Please print these out for reference
 Resources for the Primary Care Provider Please print these out for reference Resources for providers American Academy of Neurology www.aan.com Provides education and resources, such as guidelines for clinical
Resources for the Primary Care Provider Please print these out for reference Resources for providers American Academy of Neurology www.aan.com Provides education and resources, such as guidelines for clinical
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
this 7^ day of September 2014 by Randall S. Gregg Special Deputy Director
 r STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v File No. 143525-001 Health Alliance Plan of Michigan,
r STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v File No. 143525-001 Health Alliance Plan of Michigan,
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
New treatments in MS What s here and what s nearly here
 5 th MS Research Day, June 14 th 2014 New treatments in MS What s here and what s nearly here David Miller Queen Square MS Centre at UCL and UCLH Course of MS and its treatment Relapsing remitting Disability
5 th MS Research Day, June 14 th 2014 New treatments in MS What s here and what s nearly here David Miller Queen Square MS Centre at UCL and UCLH Course of MS and its treatment Relapsing remitting Disability
Resources for the Patient. Please print these out and give them to your patients with MS
 Resources for the Patient Please print these out and give them to your patients with MS An internet program for persons with MS on the FCM website Multiple Sclerosis: A Toolbox of Information and Resources
Resources for the Patient Please print these out and give them to your patients with MS An internet program for persons with MS on the FCM website Multiple Sclerosis: A Toolbox of Information and Resources
NHS Suffolk Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice)
 NHS Suffolk Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice) This drug has been reviewed because it is a product that may be prescribed in primary
NHS Suffolk Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice) This drug has been reviewed because it is a product that may be prescribed in primary
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Gold R, Giovannoni G, Selmaj K, et al, for
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Gold R, Giovannoni G, Selmaj K, et al, for
Case Report Early Bilateral Cystoid Macular Oedema Secondary to Fingolimod in Multiple Sclerosis
 Case Reports in Medicine Volume 2012, Article ID 134636, 4 pages doi:10.1155/2012/134636 Case Report Early Bilateral Cystoid Macular Oedema Secondary to Fingolimod in Multiple Sclerosis Lei Liu and Fiona
Case Reports in Medicine Volume 2012, Article ID 134636, 4 pages doi:10.1155/2012/134636 Case Report Early Bilateral Cystoid Macular Oedema Secondary to Fingolimod in Multiple Sclerosis Lei Liu and Fiona
